
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 15 April 2021
Sec. Evolutionary and Population Genetics
Volume 12 - 2021 | https://doi.org/10.3389/fgene.2021.628192
This article is part of the Research Topic Gene Regulation as a Driver of Adaptation and Speciation View all 11 articles
 Tao Wang1,2†
Tao Wang1,2† Yuanyuan Guo1,2†
Yuanyuan Guo1,2† Shengwei Liu1,2
Shengwei Liu1,2 Chaoxin Zhang1,2
Chaoxin Zhang1,2 Tongyan Cui1,2
Tongyan Cui1,2 Kun Ding3
Kun Ding3 Peng Wang4
Peng Wang4 Xibiao Wang1*
Xibiao Wang1* Zhipeng Wang1,2*
Zhipeng Wang1,2*Tibetan pigs are native mammalian species on the Tibetan Plateau that have evolved distinct physiological traits that allow them to tolerate high-altitude hypoxic environments. However, the genetic mechanism underlying this adaptation remains elusive. Here, based on multitissue transcriptional data from high-altitude Tibetan pigs and low-altitude Rongchang pigs, we performed a weighted correlation network analysis (WGCNA) and identified key modules related to these tissues. Complex network analysis and bioinformatics analysis were integrated to identify key genes and three-node network motifs. We found that among the six tissues (muscle, liver, heart, spleen, kidneys, and lungs), lung tissue may be the key organs for Tibetan pigs to adapt to hypoxic environment. In the lung tissue of Tibetan pigs, we identified KLF4, BCL6B, EGR1, EPAS1, SMAD6, SMAD7, KDR, ATOH8, and CCN1 genes as potential regulators of hypoxia adaption. We found that KLF4 and EGR1 genes might simultaneously regulate the BCL6B gene, forming a KLF4–EGR1–BCL6B complex. This complex, dominated by KLF4, may enhance the hypoxia tolerance of Tibetan pigs by mediating the TGF-β signaling pathway. The complex may also affect the PI3K-Akt signaling pathway, which plays an important role in angiogenesis caused by hypoxia. Therefore, we postulate that the KLF4–EGR1–BCL6B complex may be beneficial for Tibetan pigs to survive better in the hypoxia environments. Although further molecular experiments and independent large-scale studies are needed to verify our findings, these findings may provide new details of the regulatory architecture of hypoxia-adaptive genes and are valuable for understanding the genetic mechanism of hypoxic adaptation in mammals.
Hypoxia is a significant environmental characteristic of high altitude, which exerts a marked impact on biological organisms and imposes extreme physiological challenges in mammals. The Tibetan pig was originally distributed at altitudes of 2,900–4,300 m in the Tibetan Plateau (Ai et al., 2014). Physiological studies showed that Tibetan pigs have evolved physiological adaptations to high-altitude hypoxia, such as a thicker alveolar septum with more highly developed capillaries (Ma et al., 2019) and a larger and strong heart (Li et al., 2013). Therefore, they represent a suitable animal model for exploring the molecular mechanism of hypoxia adaptation in high-altitude organisms.
With the development of sequencing technology, the majority of studies have explored the genetic basis of hypoxic adaptation in Tibetan pigs from the perspective of selection signals (Li et al., 2013, 2016; Ai et al., 2014; Huang et al., 2019; Ma et al., 2019; Shang et al., 2020) or by using differential expression analysis between differential conditional gene expression in one tissue based on the transcriptome (Jia et al., 2016; Zhang B. et al., 2017) up to the present. Although previous studies have identified the EPAS1, HIF1A, EGLN1, RGCC KLF6, TGFB2, EGLN3, and ACE genes related to hypoxia, these genes may only explain a minority of genetic variance due to the case of the missing heritability. Therefore, the most detailed solution to the missing heritability problem would involve identifying all causal genetic variants (Young, 2019) and exploring related gene networks that have facilitated high-altitude adaptation of Tibetan pigs.
The adaptation of Tibetan pigs to hypoxia is a very complex biological process that may involve multiple genes and transcriptional regulation among genes. The gene network provides a systemic view of gene regulation by the coordinated activity of multiple genes and regulatory factors and serves as a medium for understanding the mechanism of gene regulation (Narang et al., 2015). Based on the gene expression profile, a gene network was constructed by quantitative modeling, which can be used for rational design of molecular approaches to target specific biological processes (Nishio et al., 2008) and infer new biological functions (Cheng and Gerstein, 2012; McLeay et al., 2012). Although gene expression status cannot completely determine gene function, constructing gene network based on gene expression profile may be a feasible method to explore the mechanism of hypoxia adaptation. Moreover, the gene network cannot only intuitively elucidate the regulatory relationship between genes but also identify important hub genes. These hub genes represent candidates for further experimental investigation and potential biomarkers for complex traits (Döhr et al., 2005; Buckingham and Rigby, 2014; Chen et al., 2016).
Transcription factors (TFs) and microRNAs (miRNAs) regulate gene expression at the transcriptional and posttranscriptional levels, respectively. They coordinately control the dynamics and output of gene transcription and tightly control spatial and temporal patterns of gene expression. Therefore, constructing a gene regulatory network involving TFs and miRNAs is helpful in understanding the regulatory mechanism of genes in adaptation to hypoxia.
Moreover, most cellular tasks are not performed by individual genes but by groups of functionally associated genes, generally referred to as modules. In a gene regulatory network, modules appear as groups of densely interconnected nodes, also called communities or clusters (Adamcsek et al., 2006). Among these clusters of gene regulatory networks, size-3 network motifs were suggested to be recurring circuit elements that carry out key information processing tasks. The three-node motif included 13 types of connected subgraphs, such as V-out, 3-Chain, feed forward loop (FFL), 3-Loop, and Clique. Among them, the FFL motif consists of two input regulators, A and B, and one output factor C, where regulators A and B regulate target factor C together, and A also regulates B, as shown in Figure 1. According to the regulation functions (activate or inhibit) between the three elements in FFL, it can be divided into two categories: coherent feed-forward loop and incoherent feed-forward loop (Mangan et al., 2003; Alon, 2007). In the coherent feed-forward loop, the regulators strengthen each other’s functions, and have the effect of controlling stability and resisting noise in biological networks (Le and Kwon, 2013). In the incoherent feed-forward loop, the regulator performs the opposite function to speed up the response and suppress the delay (Kim et al., 2008; Lan and Tu, 2013). FFL plays an important role in biological processing (Milo et al., 2002; Mangan and Alon, 2003), which appears in hundreds of gene systems in Escherichia coli, yeast, fruit fly, and humans. The FFL motif governs many aspects of normal cell functions, such as creating bistable switches of gene expression in developing tissues for spatial avoidance, controlling the time sequence of gene expression to create temporal avoidance, and minimizing expression fluctuation against noise (Shalgi et al., 2009).
The Tibetan pig and Rongchang pig are two indigenous pig breeds in China. Rongchang pigs are cross-fertile relatives of Tibetan pigs, living in geographically neighboring low-altitude regions (Tang et al., 2017). Ai et al. (2014) found that Tibetan pigs had a close genetic distance with Rongchang pigs through neighbor-joining (NJ) tree analysis. In this study, based on transcriptional data from six tissues in Tibetan pigs and Rongchang pigs, the key module of lung tissues was identified by constructing a gene network. By integrating complex network analysis and bioinformatics analysis, we identified key genes and size-3 network motifs and found that KLF4, a key regulator of the complex, may enhance the survival ability of Tibetan pigs by mediating the TGF-β signaling pathway in the hypoxia environments. This study provides a valuable clue to further understand the molecular mechanism of adaptability in high-altitude hypoxia.
The protein-encoding genes and miRNA expression profile data of six tissues (muscle, liver, heart, spleen, kidneys, and lungs) from three Tibetan pigs and three Rongchang pigs were obtained from the Gene Expression Omnibus (GEO) database at the National Center for Biotechnology Information (NCBI) under accession numbers GSE93855 (provided by Tang et al., 2017) and GSE124418 (uploaded by Long et al., 2019), respectively. For details about the experimental animals, please refer to the Supplementary Material. Gene list, expressed in each tissue, was updated based on the Sus scrofa 11.1 genome assembly. Taking into account that genes are with very low expression and are less reliable and indistinguishable from the sampling noise, we selected the top 50% of protein-encoding genes of the median absolute deviation (MAD) of expression level.
Network analysis was performed according to the protocol of the WGCNA R package (Langfelder and Horvath, 2008). We used the following criteria to identify the key module of each tissue: (1) the p-value of the correlation between the module and the tissue was less than 3.97×10−4 (0.05/126) using the Bonferroni correction method, and (2) the median of the gene significance (GS) value was greater than 0.8. In addition, we calculated the fundamental topology concepts of each key module, including density, mean cluster coefficient, centralization, and heterogeneity.
In this study, we used the Mfuzz package in R (Kumar and Futschik, 2007) to identify multitissue expression patterns of each gene in each key module. Based on the fuzzy c-means algorithm, this software implements soft clustering methods for microarray data analysis, which makes the clustering process less sensitive to noise and effectively reflects the strength of a gene’s association with a given cluster.
We used the tissue-specificity index (TSI, τ) (Yanai et al., 2005) to grade the scalar measure of the specificity of an expression profile, which ranged from 0 for housekeeping genes to 1 for strictly TS genes. According to Yanai et al. (2005), genes with TSI > 0.9 were considered TS genes.
We used the online software DAVID (v6.8) (Huang et al., 2009) to perform functional enrichment analysis of genes in each key module with all protein-encoding genes on pig genome as the background genes set, including gene ontology (GO) and KEGG pathway analysis.
We identified the hub genes in each key module according to the following criteria: (1) GS value of the gene ≥ 0.8, (2) module membership (MM) value of the gene ≥ 0.95, and (3) in each module, Kwithin ranked in the top 20% of the genes. The module membership (MM) is defined as the correlation of the module eigengenes and the gene expression profile. The Kwithin value represents the degree of connectivity of edges located under the same module as the gene.
We used the TFBSTools package in R (Tan and Lenhard, 2016) to predict the target genes of TFs in each key module of Tibetan pigs. The relScore value was set to 0.85, and other parameters were defaulted. Based on the miRanda tool (Enright et al., 2003), we predicted target genes of the miRNAs, and the Tot Score and Tot Energy were set to 140 and −20, respectively. The gene regulatory network in each Tibetan pig tissue was constructed by combining TFs, miRNAs, target genes, co-expressed genes, hub genes, and their interactions.
The three-node motifs in the gene regulatory network of each tissue were obtained using mfinder1.2 (Kashtan et al., 2004). The number of random networks was set to 10,000. The Z score describes the significance of the difference between the frequency of motifs in the real network and that in the corresponding randomized network. The significance profile (SP) is the vector of Z scores normalized to length 1, describing the statistical significance of each motif in the network (Milo et al., 2004). We constructed the triad significance profile (TSP) of the six tissues from Tibetan pigs, which display certain relations between subgraph types.
We calculated the importance scores of each node, edge, and size-3 sub-graph in the lung-specific gene regulatory network. Each node was scored according to the connectivity, differential expression between different conditions, tissue-specific expression, and TF characteristics. The score of each edge was the weight value of the edge from WGCNA. The score of each candidate size-3 sub-graph was calculated by combining the node score and the edge score. The calculation formula and specific details are referred to the Supplementary Material.
The lung tissue expression profiles of three Tibetan sheep and three yaks were obtained from the GEO database (accession: GSE93855) (Tang et al., 2017), and the expression profiles in the lung tissue of four Diqing Tibetan pigs were collected from another dataset (accession: GSE84409) (Jia et al., 2016). We used WGCNA to perform module analysis. The Hmisc package in R was used to statistically test the correlation between genes.
In this study, we selected 5,723 protein-coding genes (top 50% of the MAD value) for subsequent analysis. Cluster analysis revealed that different samples from the same tissue of Tibetan pigs and Rongchang pigs clustered together, and no outlier samples were observed, as shown in Figure 2.
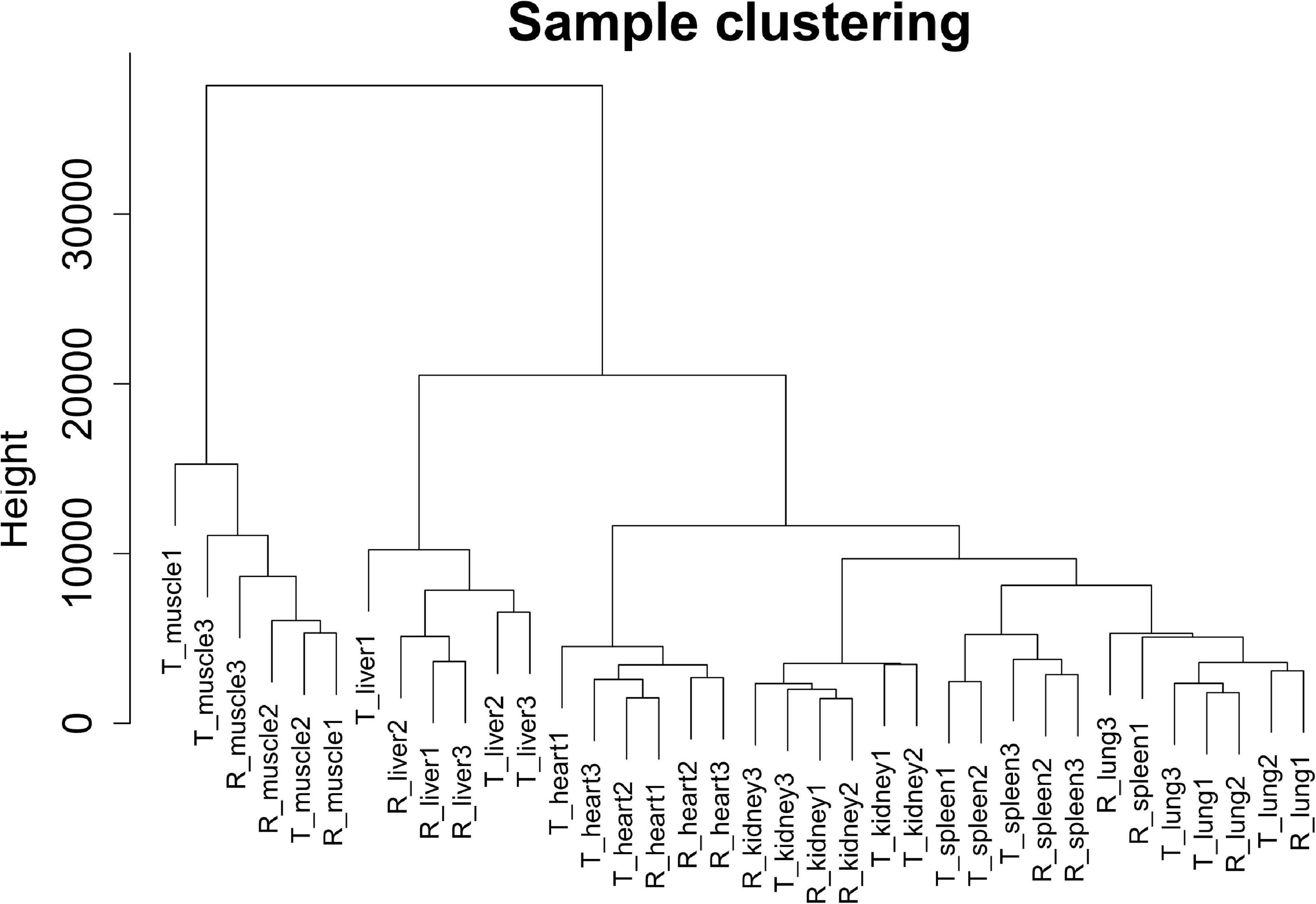
Figure 2. Clustering dendrogram of 36 tissue samples of Tibetan pigs and Rongchang pigs. The figure shows the clustering of a total of 36 tissue samples of Tibetan pigs and Rongchang pigs, where “T” represents Tibetan pigs, and “R” represents Rongchang pigs. For example, “T_muscle1” represents the muscle sample of the first individual Tibetan pig.
We constructed a co-expression network for Tibetan pigs and Rongchang pigs. To fulfill the criteria of approximate scale-free topology, the soft threshold power β was set to 20 [the scale-free topological index R2 = 0.85 for Tibetan pigs (Figure 3A) and R2 = 0.80 for Rongchang pigs (Supplementary Figure 1A)]. Through hierarchic clustering and dynamic branch cutting procedures, 21 and 20 merged modules were identified in the co-expression network of Tibetan pigs and Rongchang pigs, respectively. Clustering of the modules is shown in Figure 3B.
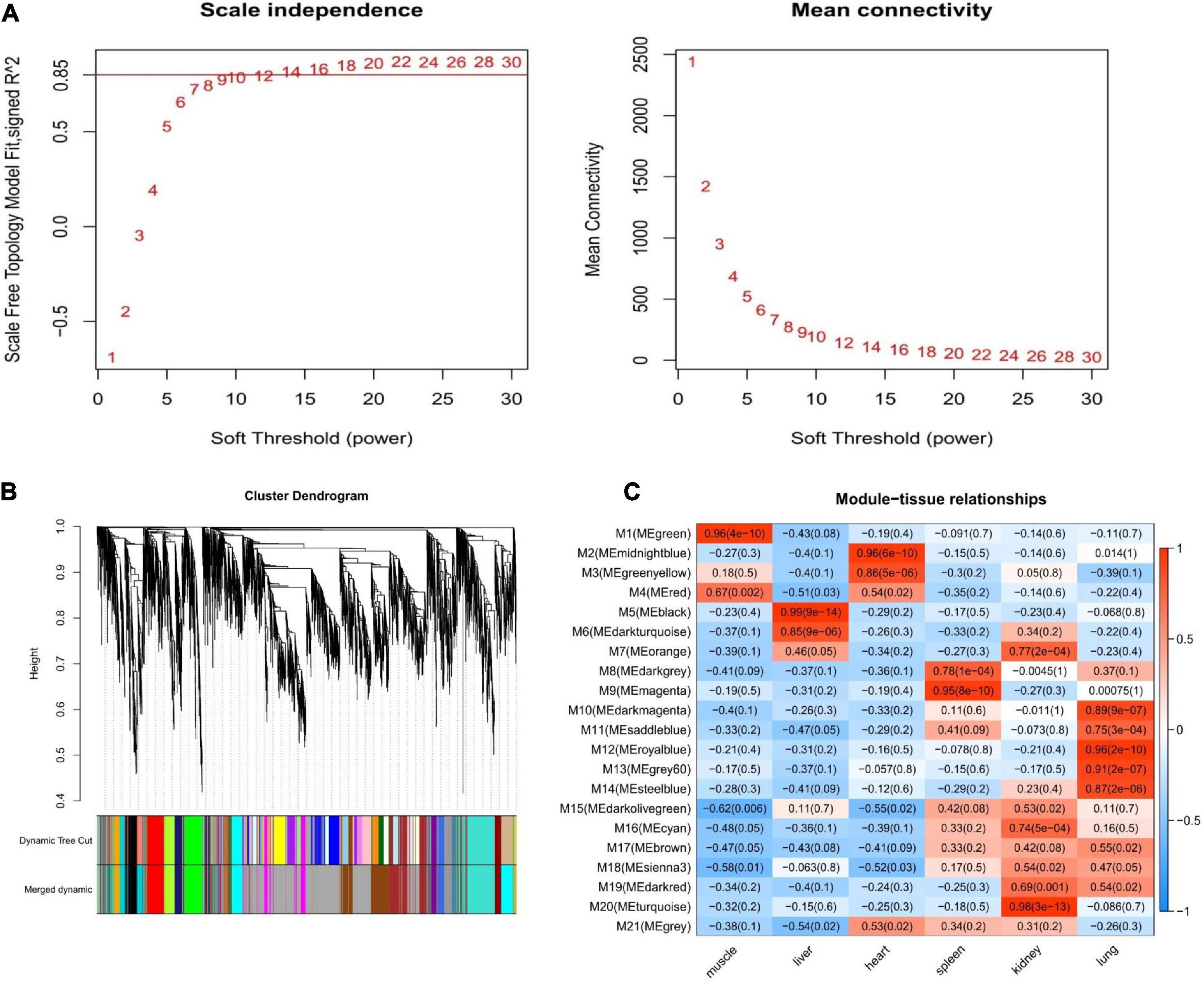
Figure 3. Weighted gene co-expression network analysis of Tibetan pigs. (A) Network topology of different soft-thresholding power of Tibetan pig co-expression network. The left panel displays the influence of soft-thresholding power (x-axis) on scale-free fit index (y-axis). The right panel shows the influence of soft-thresholding power (x-axis) on the mean connectivity (degree, y-axis). (B) Gene clustering module of Tibetan pig co-expression network. The dissimilarity was based on topological overlap. The “Merged dynamic” is the result of merging modules with a correlation higher than 0.9. The y-axis is the distance determined by the extent of topological overlap. (C) Heatmap of the correlation between module eigengenes and the six tissues of Tibetan pigs. The x-axis is the six tissues of Tibetan pigs, and the y-axis is the module eigengene (ME). In the heatmap, red represents high adjacency (positive correlation), and blue represents low adjacency (negative correlation). In brackets is the p-value of the correlation test.
Next, the GS values of genes contained in these modules and the correlation between each module and different tissues in Tibetan pigs (Figure 3C) were calculated. According to the screening criteria, key modules from six tissues (muscle, liver, heart, spleen, kidneys, and lungs) in Tibetan pigs were determined. These modules contained 267, 215, 157, 201, 420, and 350 genes, respectively. The list of genes and the co-expression network for each tissue are shown in Supplementary Table 1 and Supplementary Figure 2, respectively. The gene list of each key module of Rongchang pigs are shown in Supplementary Table 1.
We calculated the network topology of the key module of each tissue from the Tibetan pigs and Rongchang pigs, including density, mean cluster coefficient, centralization, and heterogeneity. The results are shown in Table 1. We observed that the network density and the clustering coefficient of Tibetan pig lung and heart tissues were the lowest, while those of the spleen were the highest. These network concepts indicated that the key modules of the lungs and heart were a sparse network. The network topology of Rongchang pigs was similar to those of Tibetan pigs.
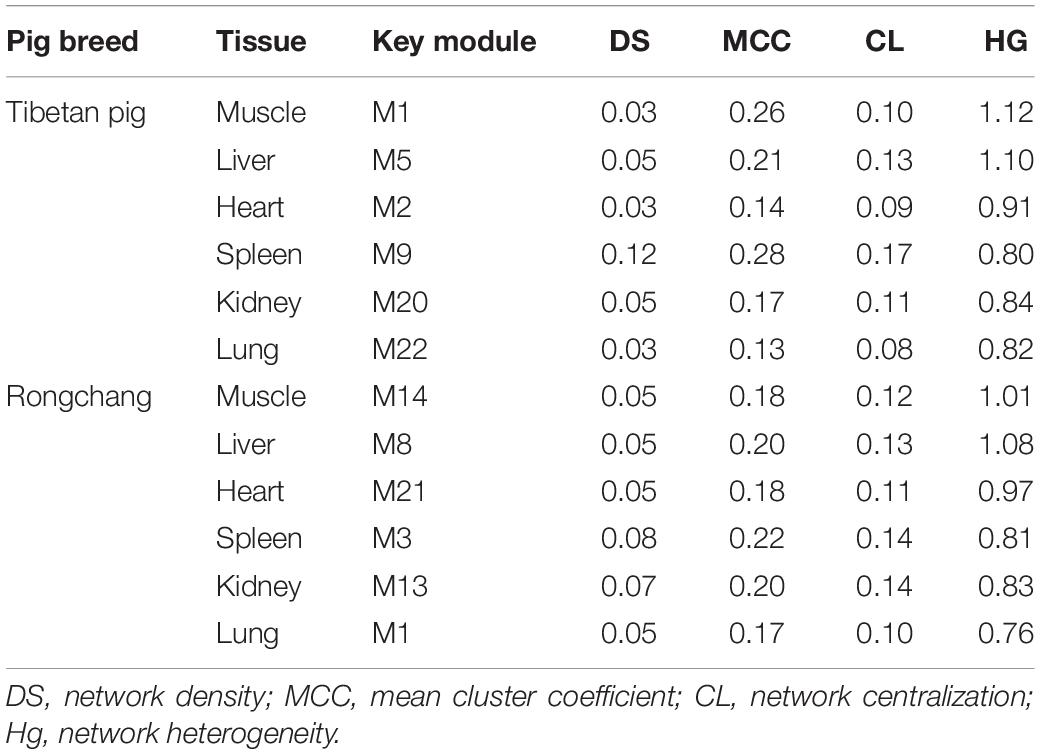
Table 1. The fundamental network topology concepts of key modules in Tibetan pig and Rongchang pig tissues.
According to the analysis of gene expression patterns, we found that compared with other tissues, Tibetan pigs and Rongchang pigs had the largest differences in gene expression patterns of the key modules of lung tissue. In the key module of lung tissue, gene expression patterns in multitissues could be divided into eight clusters. Compared with other tissues, the level of gene expression in the lung tissues of Tibetan pigs was the highest in all clusters (Figure 4A). However, the genes in the lung tissues of Rongchang pigs are expressed as the highest only in cluster 1, cluster 5, and cluster 6 (Figure 4B). The different gene expression patterns may be caused by physiological changes in the hypoxia environment of Tibetan pigs or interspecies differences between Tibetan pigs and Rongchang pigs.

Figure 4. Multitissue expression patterns of genes in key modules of lung tissue of two pig breeds. (A) Multitissue expression patterns of key module genes in Tibetan pig lung tissues. (B) Multitissue expression patterns of key module genes in Rongchang pig lung tissues. The 1, 2, 3, 4, 5, and 6 in the figure represent the muscle, liver, heart, spleen, kidneys, and lung tissues, respectively. The yellow- or green-colored lines correspond to genes with low membership value; the red- and purple-colored lines correspond to genes with high membership value.
A total of 210 and 206 genes were identified as tissue specific (τ > 0.9) in the key modules of Tibetan pigs and Rongchang pigs, respectively. For the gene details, see Supplementary Table 2. For Tibetan pigs, there were 32, 50, 23, 36, 47, and 22 TS genes in the key modules of the muscle, liver, heart, spleen, kidneys and lungs, respectively. There are more TS genes in the lung tissues of Tibetan pigs than in Rongchang pigs. Compared with the other five tissues, the number of TS genes in the lung has the largest difference between the two pig breeds.
To further understand the biological functions of genes in each key module in Tibetan pigs and Rongchang pigs, we conducted gene function enrichment analysis. After the Benjamini correction, we identified significant pathway enrichment in Tibetan pigs and Rongchang pigs, as shown in Supplementary Table 3. Compared with Rongchang pigs, there were 10, 4, 1, and 13 pathways in the muscle, lungs, heart, and spleen that were only significantly enriched in Tibetan pigs, as shown in Table 2. Pathways enriched only in Tibetan pig lungs, including regulated cell growth, proliferation, migration, and apoptosis include focal adhesion (ssc04510), ECM–receptor interaction (ssc04512), PI3K–Akt signaling pathway (ssc04151), and TGF-β signaling pathway (ssc04350). In addition, 30 pathways were significantly enriched only in Rongchang pigs (see Supplementary Table 4).
According to the screening criteria of hub genes, we have determined the hub genes of each key module of Tibetan pigs and Rongchang pigs. Compared with Rongchang pigs, Tibetan pigs had more hub genes in the liver, kidneys, and lung tissues. There was no hub gene overlap between the lung tissues of Tibetan pigs and Rongchang pigs. In addition, eight hub genes were TS gene in the lung tissue of Tibetan pigs, while Rongchang pigs only have one. Table 3 summarizes the hub gene information in Tibetan pigs and Rongchang pigs.
The gene regulatory network of Tibetan pig tissues was constructed by combining TFs, miRNAs, target genes, co-expression genes, and hub genes. There were 115, 80, 35, 117, 160, and 157 nodes (genes) and 986, 1,786, 298, 1,976, 5,315, and 1,075 edges (regulatory relationship) in the gene regulatory network of the muscle, liver, heart, spleen, kidneys, and lungs, respectively, as shown in Figure 5. There were 9, 3, 1, 3, 3, and 16 TFs, respectively, in the gene regulatory network of each tissue. In total, 35 TFs belonged to 10 TF families, among which 10 TFs were also hub genes. According to the PWM provided by the CIS-BP database, 20 TFs target genes were predicted. We found that these 20 TFs regulate 237 genes (94 genes are hub genes) in each tissue key modules, predicting a total of 408 regulatory relationships. Through the prediction of miRNA target genes, we found that genes in the key modules of the muscle, liver, heart, spleen, kidneys, and lungs were regulated by 8, 3, 3, 2, 4, and 6 miRNAs, respectively. Table 4 summarizes the information of TFs, miRNAs, target genes, and hub genes in the gene regulatory network of six tissues in Tibetan pigs.
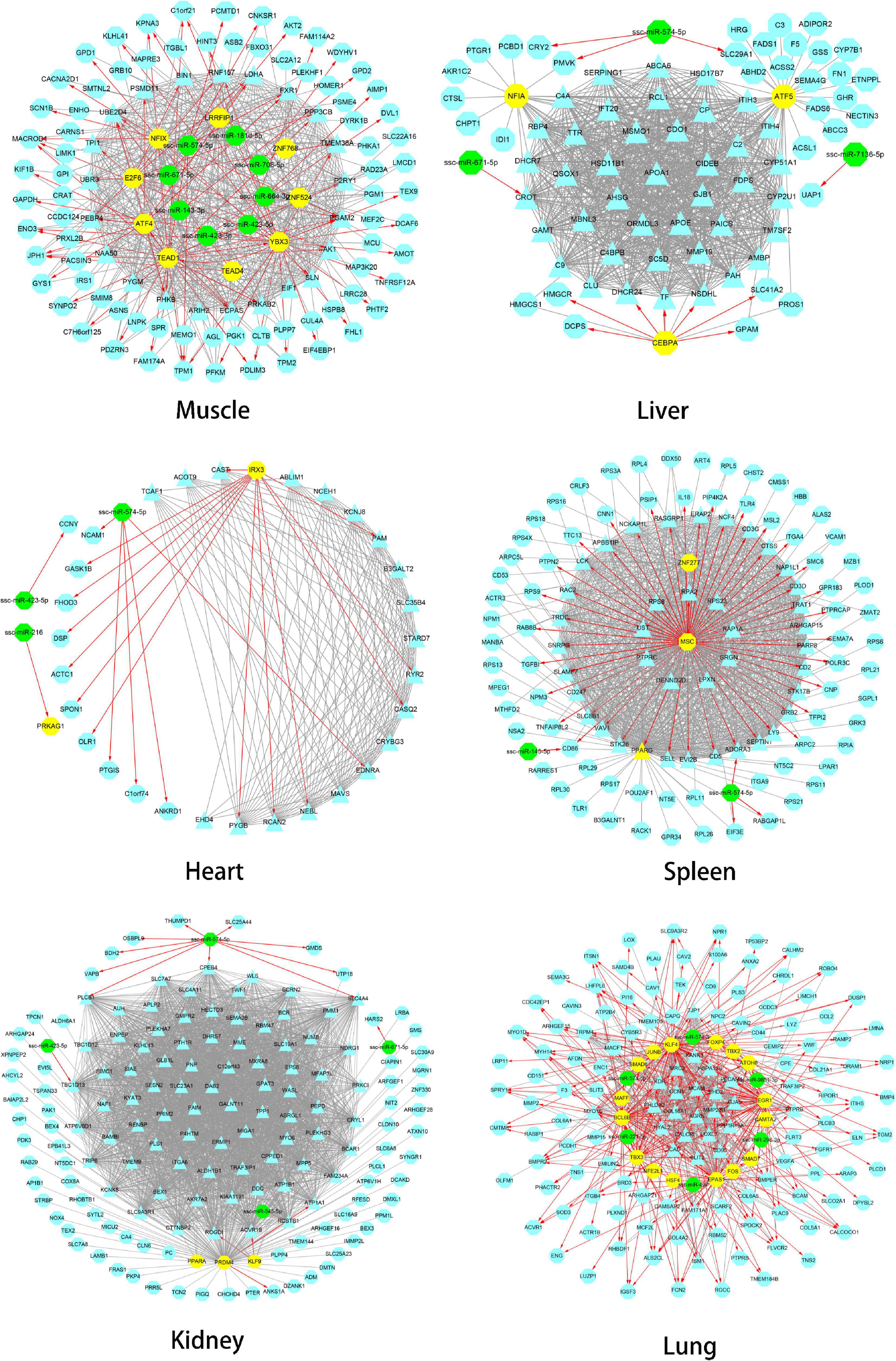
Figure 5. Gene regulatory network of six tissues of Tibetan pigs. In each network in the figure, the yellow dots represent TFs, the green dots represent miRNAs, and the hub genes are represented by triangles. The red edges with arrows represent the regulatory relationship between TFs and miRNAs and target genes. The gray edge indicates that there is only a co-expression relationship between the two genes.
In gene networks, some motifs displayed much higher frequencies than expected in randomized networks (Ravasz et al., 2002; Shen-Orr et al., 2002), and these motifs were suggested to be recurring circuit elements that perform key information-processing tasks (Rosenfeld et al., 2002; Shen-Orr et al., 2002; Mangan and Alon, 2003). Among them, the motif composed of three nodes contains 13 kinds, including V-out, 3-Chain, FFL, 3-Loop, Clique, and so on. Using the mfinder1.2 software, we identified 8,894, 13,067, 993, 19,899, 78,959, and 14,692 motifs in the gene regulatory networks of the muscle, liver, heart, spleen, kidneys, and lung tissues in Tibetan pigs, respectively. There were significant differences in the distribution of motifs among the different gene regulatory networks (p < 2.2E-16). The motif information in the gene regulatory networks of the six tissues is shown in Table 5.
To analyze the statistical significance of each motif type, we generated 10,000 random networks representing a conservation rule. The distribution of TSP in the gene regulatory network of the lung tissues is shown in Figure 6. We found that the frequency of FFL, Regulated mutual, Regulating mutual, and Clique motifs in the lung tissue gene regulatory network was significantly different from that of random networks (p < 1E-04). In the muscle and heart tissue gene regulatory network, Regulated mutual and Clique motif were significant motif types, while V-out, Semi clique, and Clique motif were significant in the gene regulatory network of the kidneys.

Figure 6. The triad significance profile (TSP) of Tibetan pig lung gene regulatory network. The ordinate in the figure is the normalized Z value, and the abscissa is the 13 motif types. The point marked with “*” is that the frequency of the corresponding motif in the lung tissue gene regulatory network is significantly different from that of random networks (p < 1E-04). The motifs are FFL (7), Regulated mutual (9), Regulating mutual (10), and Clique (13) in that order.
We further analyzed FFL, Regulated mutual, and Regulated mutual motifs in the gene regulatory network of lung tissues. All FFL motifs in the gene regulatory network of lung tissues were TF1→TF2, including KLF4→EPAS1, KLF4→BCL6B, KLF4→FOS, EGR1→BCL6B, EGR1→EPAS1, BCL6B→EPAS1, TBX3→EPAS1, and TBX3→BCL6B. Then, the two TFs shared a target gene. As a result, 51 target genes were regulated, including four TFs, forming 13 FFLs, and 21 hub genes, forming 71 FFLs. In addition, three of these target genes were both TF and hub gene, forming eight FFLs.
There were two main types of Regulating mutual motifs. One includes two TFs regulating each other, including EGR1–KLF4, EGR1–TBX3, and KLF4–TBX3, and jointly regulating the same target gene. A total of 47 target genes were regulated, including six TFs, forming 12 complexes, and 27 hub genes, forming 49 complexes. Among these target genes, four target genes were both TF and hub genes, forming 10 complexes. The other type of Regulating mutual motifs includes two TFs that were co-expressed and shared a target gene. We found that FOS and JUNB co-expressed and co-regulated the DUSP1 gene.
In the Regulated mutual motif, one TF regulated two genes, and there was a co-expression relationship between the two target genes. It was composed of TFs, including EGR1, KLF4, EPAS1, BCL6B, and TBX3, and their regulated target genes, forming a total of 810 complexes. Of these complexes, there were eight in which both target genes are TFs and 593 in which both are hub genes. In the Clique motif, only the EGR1–KLF4–TBX3 motif was the mutual regulation of these three TFs, and the remaining motifs were co-expressed relationships among genes.
Formulas (8) and (10) were used to evaluate the importance of each gene and the three-node motif, including FFL, Regulating mutual, and Regulated mutual type motif, in the gene regulatory network of lung tissues. We found that the several important top genes were KLF4, BCL6B, EGR1, SMAD6, and EPAS1 transcription factor genes, which were also hub genes. The top 25% of the node importance scores in the Tibetan pig lung gene regulatory network are shown in Table 6.
The Regulating mutual motif formed by KLF4–EGR1–BCL6B was the most important motif based on the motif score. We call it the “KLF4–EGR1–BCL6B” complex. In this motif, the KLF4 and EGR1 genes regulate the same target gene, BCL6B. This complex preferred to synergistically regulate the EPAS1, KDR, SMAD6, SMAD7, CCN1, and ATOH8 genes (Figure 7), which comprised 18 motifs (Table 7). The “KLF4–EGR1–BCL6B” complex may coordinately regulate the SMAD6 and SMAD7 genes, which play an important role in the TGF-β signaling pathway. EPAS1 is an important hypoxia-inducible factor. This complex may also indirectly regulate SMAD6 and SMAD7 genes by regulating the EPAS1 gene. This complex may also regulate the KDR gene, which is involved in the PI3K–Akt signaling pathway. Both TGF-β and PI3K–Akt signaling pathways play an important role in hypoxia response (Chen et al., 2006; Ambalavanan et al., 2008; Jia et al., 2016; Qi et al., 2019).
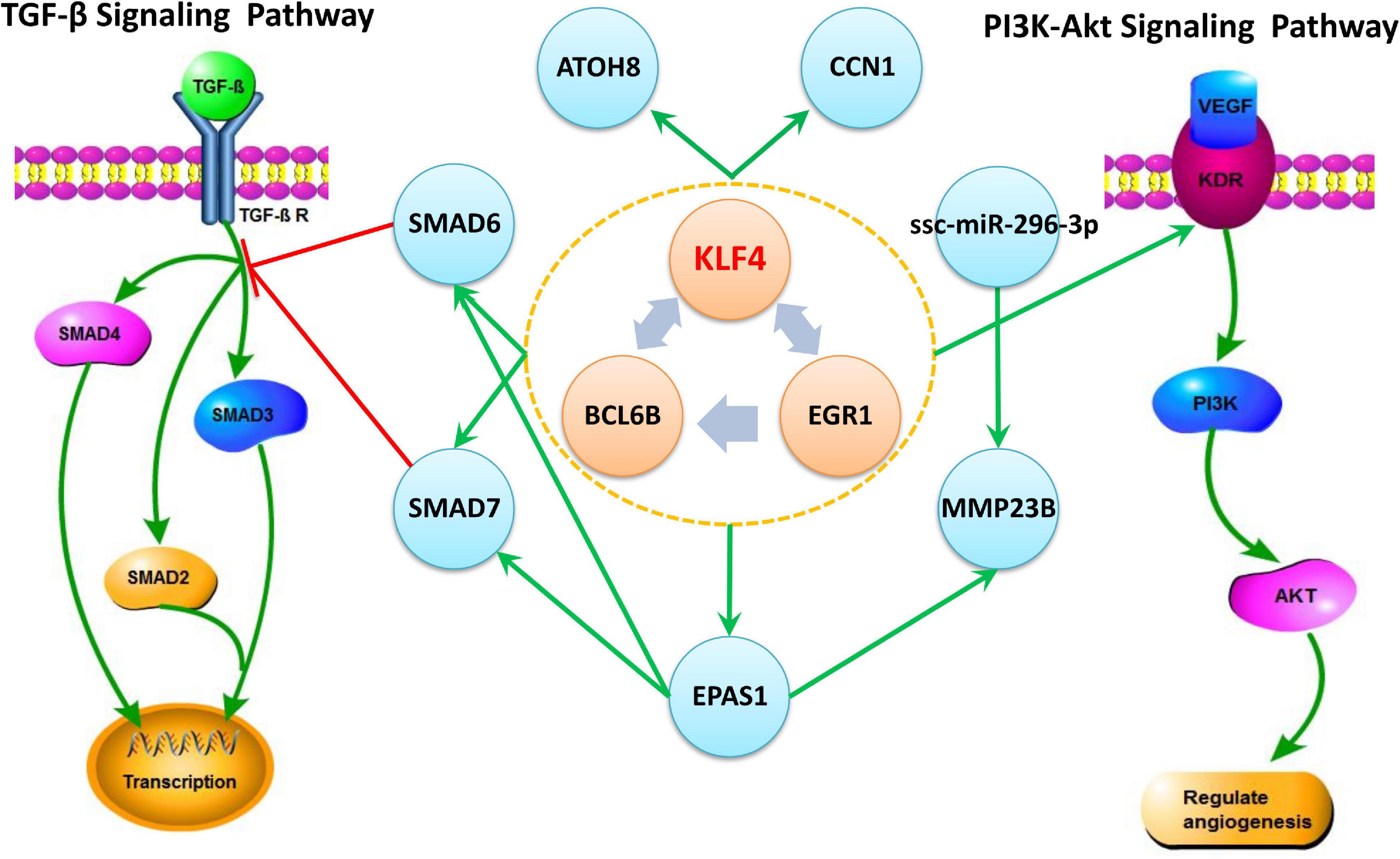
Figure 7. The “KLF4–EGR1–BCL6B” complex and their regulated genes in Tibetan pig lung tissues. The complex formed by KLF4–EGR1–BCL6B regulates the EPAS1, SMAD6, SMAD7, KDR, ATOH8, and CCN1 genes and mediates the TGF-β and PI3K-Akt signaling pathways by regulating SMAD6, SMAD7, and KDR genes, respectively. The green edge in the figure represents regulation, and the red edge represents inhibition.
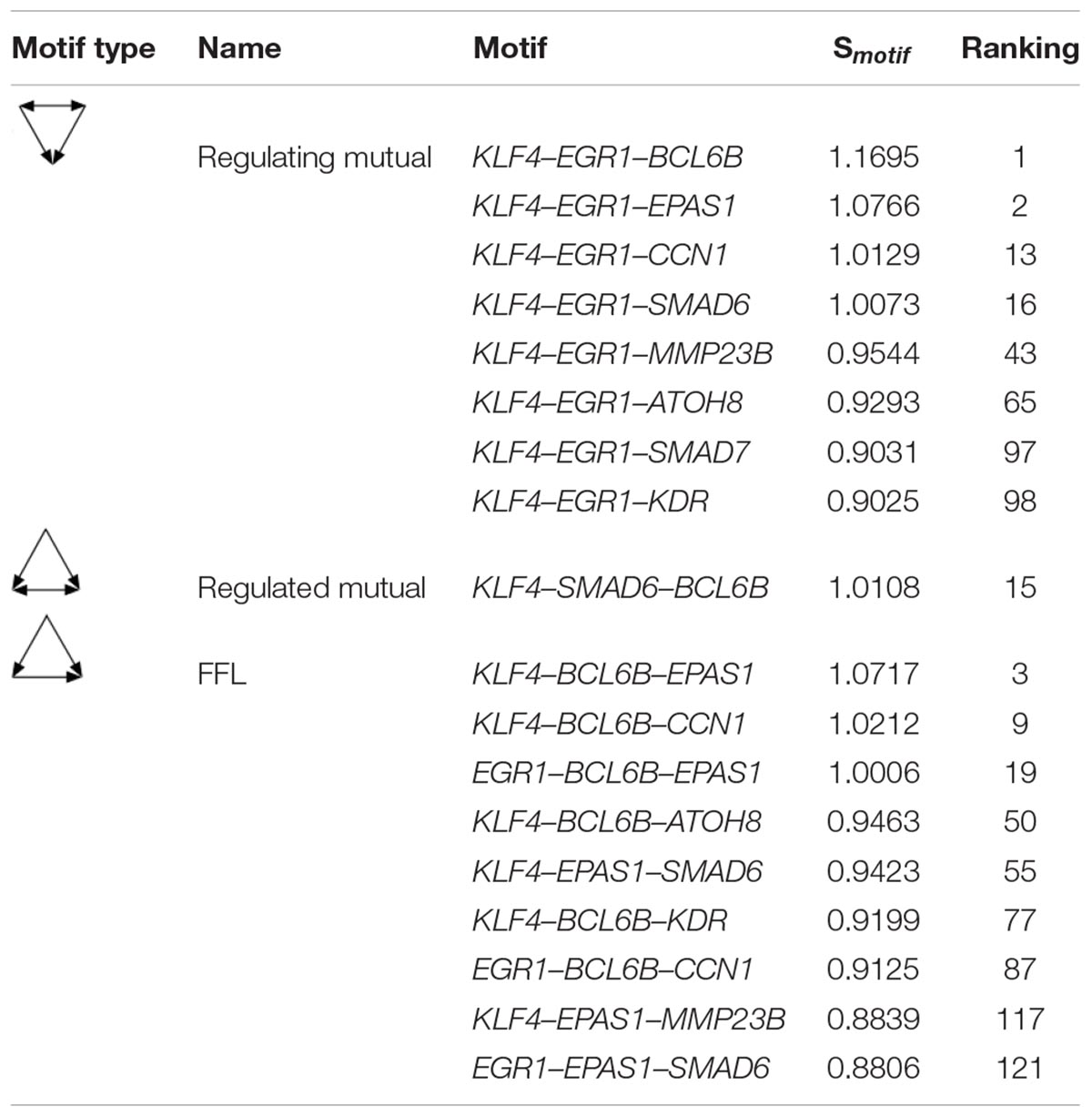
Table 7. The motifs formed between the “KLF4–EGR1–BCL6B” complex and its regulatory genes in the lung.
To confirm the relationship between KLF4, EGR1, BCL6B, SMAD6, EPAS1, KDR, SMAD7, CCN1, ATOH8, and MMP23B genes, we used lung tissue transcriptomic data from the Tibetan sheep, yak, and Diqing Tibetan pig population for validation via the co-expression network analysis. We identified the key module of the lung tissues for the three validation groups using WGCNA. The network topology of these key modules, including density, mean cluster coefficient, centralization, and heterogeneity, were similar to those of Songpan Tibetan pigs. There were 151, 150, and 73 overlap genes between gene sets of the lung key module for the three validation groups and Tibetan pigs, respectively.
Due to using commercially available Agilent Whole Porcine Genome Oligo (4 × 44 K) Microarrays for Diqing Tibetan pig, there was no probe annotation information for the BCL6B, CCN1, ATOH8, and MMP23B genes. In Diqing Tibetan pig lung tissues, six genes, including KLF4, EGR1, EPAS1, SMAD6, SMAD7, and KDR, were highly expressed and were significantly positively correlated between genes, except for between KDR gene and others (as shown in the Supplementary Table 5). Overall, there were 11 and nine edges (the co-expression relationship) among the above genes found in the Songpan Tibetan pig and Diqing Tibetan pig (Figure 8A), respectively. So, 81.82% (9/11) of the co-expression relationships in the above genes were confirmed. Based on the WGCNA for the Diqing Tibetan pig lung tissue, we found the KLF4, EGR1, and SMAD7 genes were clustered into one module, while EPAS1 and SMAD6 were clustered into the other module.
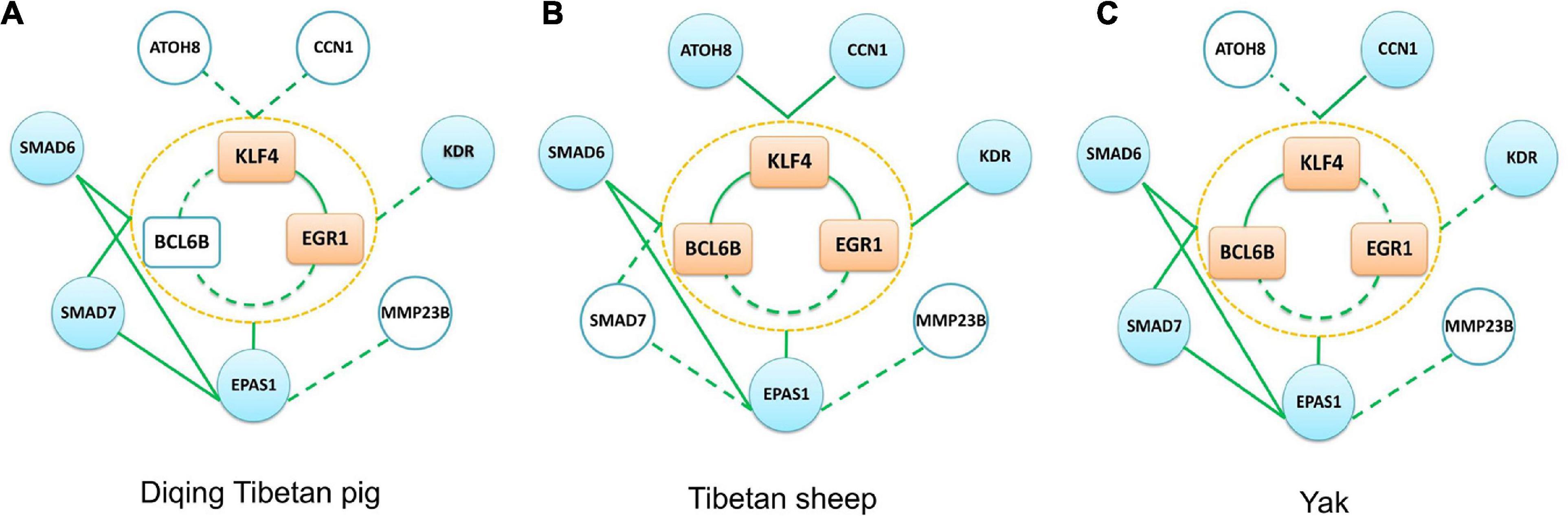
Figure 8. Three other groups (Diqing Tibetan pig, Tibetan sheep, and yak) verification network. (A–C) The verification results of the Diqing Tibetan pig, Tibetan sheep, and yak, respectively. The peach-colored rectangle in the middle part of each subgraph represents the KLF4–EGR1–BCL6B complex, the sky blue circle represents the target gene, and the white circle represents that this gene is not included in the data set. The solid green line in the figure represents the edge we verified, and the dotted line represents the unverified edge.
The KLF4, BCL6B, EPAS1, EGR1, SMAD6, KDR, CCN1, and ATOH8 genes had the highest expression in lung tissues compared with the other five tissues of Tibetan sheep (muscle, liver, heart, spleen, and kidneys). There were 14 and 24 significantly co-expression relationships among the above genes, which were identified in Tibetan sheep (see Figure 8B and the Supplementary Table 5) and Songpan Tibetan pig, respectively. In total, 58.33% (14/24) of the relationships between genes were validated. Except for EGR1 and ATOH8, the other genes were all clustered into the same key module related to the lung using WGCNA.
With the exception of ATOH8 and MMP23B, the other genes were most highly expressed in the lung tissues of yak compared with the other five tissues. We detected 12 and 24 significantly positive correlations in the above genes of Tibetan yak lung tissue (see Figure 8C and the Supplementary Table 5) and Songpan Tibetan pig. We successfully verified 50% (12/24) of the relationships among genes. After performing WGCNA, we found that KLF4, BCL6B, EPAS1, SMAD6, and SMAD7 were clustered into key modules related to the yak lungs.
Many previous studies primarily focused on identifying differentially expressed genes through gene expression profile analysis, but interactions between genes in different cell states may not have been fully considered (Kostka and Spang, 2004). Moreover, differences in gene expression are not equal to differences in gene action. Compared with expression level analysis, network-based analysis not only captures local patterns but also identifies global patterns in a biological context, revealing molecular regulation details of hub genes at the network level. At the same time, the hub genes related to biological processes identified through gene network analysis also provide clues for subsequent molecular studies.
In this study, we detected the gene regulatory network related to Tibetan pig lung tissues. An appropriate sample size is critical to the planning and interpretation of genetic studies, whether they are descriptive or analytical. The small sample sizes will result in imprecise estimates in a descriptive study and failure to achieve statistical significance in an analytic or comparative study (Weber and Hoo, 2018). Due to the sample size limitation of this study, this may have limited the generalizability of the results of the research, and further independent tests may be required to verify our findings. However, our research methods and results can provide some valuable clues for the study of the hypoxia adaptation mechanism of Tibetan pigs. Combining topological characteristics, differential expression, and tissue-specific expression, we identified a list of genes that may be related to hypoxia in Tibetan pig lung tissue, such as EPAS1, LOXL1, KLF4, EGR1, BCL6B, SMAD6, SMAD7, KDR, MMP23B, and miR-296.
The EPAS1 gene found in this study may be related to the adaptation to the hypoxic environment. The EPAS1 gene encodes one subunit of hypoxia-inducible factor (HIF), which show multifarious effects involved in complex oxygen sensing (Henderson et al., 2005) and regulation of angiogenesis, hemoglobin concentration, and erythrocytosis (Beall et al., 2010). In the Tibetan human population, the EPAS1 gene is involved in the chronic hypoxia response, and it has been shown to have a strong signature of selection (Bigham et al., 2010; Simonson et al., 2010; Peng et al., 2011; Xu et al., 2011). Moreover, Li et al. (2019) show that the mutant genotype frequencies of the rs13419896, rs1868092, and rs4953354 loci in the EPAS1 gene are significantly higher in the Tibetan population than in the plains population. Under plateau hypoxic conditions, the plains population was able to acclimate rapidly to hypoxia through increasing EPAS1 mRNA expression and changing the hemoglobin conformation. The EPAS1 gene also has obvious selection signature in other plateau animals, such as Tibetan horses (Liu et al., 2019) and Tibetan pigs (Ma et al., 2019) and has been identified as a key evolutionary molecule adapted to the plateau hypoxic environment.
Angiogenesis was an adaptive response to tissue hypoxia (Fong, 2008). A majority of the identified hub genes participated in the angiogenesis process, such as LOXL1, KLF4, and EGR1. The LOXL1 gene is essential for the stability and strength of elastic vessels and tissues (Li et al., 2020) and may play important roles in the enhanced angiogenesis promoted by hypoxia (Xie et al., 2017). The KLF4 gene tended to be pleiotropic. Not only does it promote pulmonary angiogenesis and blood transport (Ghaleb and Yang, 2017) and accelerate the acquisition and transport of oxygen but also it protects the lungs from hypoxia (Shatat et al., 2014). The EGR1 gene stimulates and induces the process of angiogenesis (Adamson and Mercola, 2002; Sheng et al., 2018). Angiogenesis is conducive to the increase in oxygen transport. Therefore, we infer that these genes might contribute to obtaining and transporting oxygen better in hypoxic environments, by involving in the angiogenesis process.
Based on the Tibetan pig lung tissue-specific gene network, we found that KLF4 and EGR1 simultaneously regulated the BCL6B gene, forming the KLF4–EGR1–BCL6B complex, which might be dominated by the KLF4 gene and affect the expression of EPAS1, SMAD6, SMAD7, CCN1, KDR, and ATOH8. These key genes and regulatory relationships were validated in the lung tissues of Tibetan pigs from Jia et al. (2016) and Tibetan sheep and yak from Tang et al. (2017). After a large literature review and verification of gene function annotation, we postulate that the KLF4–EGR1–BCL6B complex might be beneficial for Tibetan pigs to survive better in hypoxic environments.
The KLF4, EGR1, and BCL6B genes jointly regulate the SMAD6 and SMAD7 genes, which are important regulators of the TGF-β signaling pathway. In the TGF-β signaling pathway, the SMAD family genes are very important signal transduction and regulatory molecules. SMAD6 and SMAD7 are antagonists of the TGF-β gene family. High expression of SMAD7 inhibited the transcription of SMAD2 and SMAD3 gene induced by the TGF-β gene and antagonizes tissue fibrosis (Yan et al., 2009). Therefore, the KLF4–EGR1–BCL6B complex in Tibetan pig lungs may mediate the TGF-β signaling pathway by regulating the expression of SMAD6 and SMAD7, thereby enhancing the antifibrotic effect of the lungs.
Moreover, the KLF4–EGR1–BCL6B complex might regulate the KDR gene, which was primarily expressed in pulmonary vascular endothelial cells and has important proangiogenic activity (Melincovici et al., 2018). The KDR gene is an important regulator of the PI3K–Akt signaling pathway. Jia et al. (2016) and Qi et al. (2019) found that the PI3K–Akt signaling pathway was involved in hypoxia adaptation in both Tibetan pigs and yaks. Under hypoxic conditions, the combination of KDR and VEGF activates the downstream PI3K gene, thereby regulating proliferation and differentiation of neovascular endothelial cells and playing an important role in the development of angiogenesis (Graupera and Potente, 2013). Therefore, the KLF4–EGR1–BCL6B complex may act on the PI3K–Akt pathway by mediating the KDR gene and accelerating the acquisition and transportation of oxygen under hypoxic conditions.
In addition, the KLF4–EGR1–BCL6B complex also regulated the ATOH8, CCN1, and EPAS1 genes. High expression of CCN1 suppressed pulmonary vascular smooth muscle contraction in response to hypoxia (Lee et al., 2015). The ATOH8 gene participates in the ALK-1/SMAD/ATOH8 axis, which attenuated the hypoxic response in endothelial cells in the pulmonary circulation and might help prevent the development of pulmonary arterial hypertension (Morikawa et al., 2019). The MMP23B gene was a member of the MMP gene family, and MMP matrix metalloproteinases played an important role in tissue remodeling and angiogenesis (Białkowska et al., 2020). Moreover, MMP23B is regulated by EPAS1 and ssc-miR-296-3p. Studies had shown that miR-296 can regulate angiogenesis (Anand and Cheresh, 2011; Li et al., 2018).
The co-expression and network analysis were performed in three validation groups (Tibetan sheep, yak, and Diqing Tibetan pig). Comparing pigs, sheep, and cow living in normal oxygen content environments, some genes, such as KLF4, EGR1, EPAS1, SMAD6, and KDR genes, are overlapped in the key module of the Tibetan pig, Tibetan sheep, and yak lung tissues. As stated above, these genes improve the tolerance of Tibetan pigs to hypoxic environment through involving in angiogenesis and antagonizing lung tissue fibrosis.
Due to using porcine oligo microarrays for the Diqing Tibetan pig, some genes, such as BCL6B, do not have probe annotation information. We did not observe the KLF4–EGR1–BCL6B complex in the Diqing Tibetan pig lungs, but the KLF4 and EGR1 genes might jointly correlate with SMAD6, SMAD7, and EPAS1 genes. In the Tibetan sheep lung, the BCL6B gene did not significantly correlate with the EGR1 gene (p value = 0.0839), due to the limitation of sample size. So, further experiments in a large validation population, such as ChIP-seq, would help the demonstration of the regulation function of the complex KLF4–EGR1–BCL6B.
Although we identified the KLF4 gene as a key gene in the lung tissue of different species, and related to the SAMD6 and EPAS1 genes, there are some different co-expression relationships in the gene regulatory network of Tibetan pig, Tibetan sheep, and yak. We deem that the following reasons could have contributed to the observed differences. First, the study populations of different species have various genetic backgrounds. Many previous studies have also shown that there are different anatomical structures of tissues, physiological and biochemical indexes, and molecular mechanisms in the environment adaptation of plateau animals. Second, there are differences in the sampling methods and genetic drift events among studies on the same species. Third, gene regulatory programs display a wide range of characteristics, depending on where they are in the body and what stage in its life cycle. To control a cell’s behavior in different space and time, different gene expression profiles and regulation relationship will be observed.
In addition to the lung tissues, the heart also plays an important role in high-altitude hypoxia adaptation. Qi et al. (2019) showed that the heart and lung tissues were identified as the two key organs of yak hypoxia adaptation. In this study, we identified some specific expression genes related to hypoxia in the heart tissue of Songpan Tibetan pigs, such as EGLN3, RYR2, EDNRA, and EGLN3 (egl-9 family hypoxia inducible factor 3), that likely plays an important role in cellular adaptation to hypoxic conditions by participating in the HIL-1 signaling pathway. Zhang B. et al. (2017) used the transcriptomic and proteomic data of Tibetan pig heart tissues to identify the EGLN3 gene as important candidate genes for high-altitude adaptation. Moreover, the EGLN1 gene, a member of the same family of EGLN3, has been determined to be involved in the hypoxia adaptation of Tibetan humans (Bigham et al., 2010; Simonson et al., 2010; Xiang et al., 2013).
RYR2 and EDNRA may play crucial roles in heart development, heart rhythm stabilization, and signal transduction to cope with hypoxia. Zhang et al. (2014) found that the RYR2 gene was related to the hypoxia adaptability of Tibetan gray wolves. Low oxygen environment can increase EDNRA gene expression (Minchenko et al., 2019; Zhang Y. et al., 2017). However, Zhang B. et al. (2017) did not identify the RYR2 and EDNRA genes as hypoxia-related candidate genes by comparative mRNA and protein expression profiles in heart tissues of Tibetan and Yorkshine pigs, respectively. These inconsistencies may be a result of differences in the study population in the genetic backgrounds, population structure, developmental stages, and environmental factors. This also suggests that many key genes, in reducing hypoxia injury that exists within the Tibetan pig genome, have yet to be discovered.
In summary, through gene network analysis, we found that lung tissues may play an important role in hypoxia adaptation in Tibetan pigs. We comprehensively profiled the gene regulatory network of Tibetan pig lung tissues, identifying a series of candidate genes related to hypoxia and discovering that KLF4 is likely the core regulator of the KLF4–EGR1–BCL6B complex, which may mediate the TGF-β signaling pathway and improve the anti-hypoxic ability of Tibetan pigs. Although gene function is not entirely dependent on gene expression regulation, these findings may provide valuable clues and better understanding in exploring the underlying molecular mechanisms of hypoxia adaptation.
Publicly available datasets were analyzed in this study. This data can be found here: The data underlying this article are available in Gene Expression Omnibus (GEO) database at https://www.ncbi.nlm.nih.gov/geo/, and can be accessed with GSE93855, GSE124418, and GSE84409.
ZW, XW, and TW conceived the project. TW, YG, SL, and CZ performed the bioinformatics and data analysis. TW and ZW wrote the manuscript. TC, KD, and PW collected the samples and data. All authors read and approved the final manuscript.
This work was financially supported by the Natural Science Foundation of China (No. 32070571), the Academic Backbone Project of Northeast Agricultural University (No. 15XG14), and the NEAU Research Founding for Excellent Young Teachers (2010RCB29).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.628192/full#supplementary-material
Supplementary Figure 1 | Weighted gene co-expression network analysis of Rongchang pigs. (A) Analysis of network topology of Rongchang pig showed that it met the scale-free topology threshold of 0.8 when β = 20. The left panel shows the scale-free fit index as a function of the soft-threshold power. The right panel displays the mean connectivity as a function of the soft-threshold power. (B) The dissimilarity was based on topological overlap. The “Merged dynamic” is the result of merging modules with a correlation higher than 0.9. The y-axis is the distance determined by the extent of topological overlap. (C) Heatmap displaying the correlations and significant differences between gene modules and six tissues of Rongchang pigs. Red represents high adjacency (positive correlation) and blue represents low adjacency (negative correlation). In brackets is the p-value of the correlation test.
Supplementary Figure 2 | The co-expression network of six tissues key modules of Tibetan pig. The co-expression network of muscle, liver, heart, spleen, kidney and lung in the figure shows the co-expression relationship of weight above 0.35, 0.35, 0.25, 0.35, 0.35, and 0.25, respectively. The dark blue circles in the figure represent the hub genes of each network.
Supplementary Table 1 | List of genes in the key modules of the six tissues of Tibetan pigs and Rongchang pigs.
Supplementary Table 2 | List of tissue-specific genes for Tibetan pigs and Rongchang pigs. The first two columns in the table are the gene name and Ensembl ID. Columns 3–5 are the position coordinates of the gene. Columns 6–7 are information about the tissue-specific genes of Tibetan pigs, and the seventh column represents the TSI value of the Tibetan pig TS genes. Columns 8–9 are the information of Rongchang pig TS genes, and the ninth column represents the TSI value of the Rongchang pig TS genes.
Supplementary Table 3 | Significantly enriched signaling pathways in key modules of various tissues in Tibetan pigs and Rongchang pigs.
Supplementary Table 4 | Pathways that are only significantly enriched in Rongchang pig tissue modules.
Supplementary Table 5 | Correlation coefficients and p-values between candidate genes in the three validation groups (Diqing Tibetan pig, Tibetan sheep and yak).
Adamcsek, B., Palla, G., Farkas, I. J., Derényi, I., and Vicsek, T. (2006). CFinder: locating cliques and overlapping modules in biological networks. Bioinformatics 22, 1021–1023. doi: 10.1093/bioinformatics/btl039
Adamson, E. D., and Mercola, D. (2002). Egr1 transcription factor: multiple roles in prostate tumor cell growth and survival. Tumour Biol. 23, 93–102. doi: 10.1159/000059711
Ai, H., Yang, B., Li, J., Xie, X., Chen, H., and Ren, J. (2014). Population history and genomic signatures for high-altitude adaptation in Tibetan pigs. BMC Genomics 15:834. doi: 10.1186/1471-2164-15-834
Alon, U. (2007). Network motifs: theory and experimental approaches. Nat. Rev. Genet. 8, 450–461. doi: 10.1038/nrg2102
Ambalavanan, N., Nicola, T., Hagood, J., Bulger, A., Serra, R., Murphy-Ullrich, J., et al. (2008). Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am. J. Physiol. Lung. Cell. Mol. Physiol. 295, L86–L95. doi: 10.1152/ajplung.00534.2007
Anand, S., and Cheresh, D. A. (2011). MicroRNA-mediated regulation of the angiogenic switch. Curr. Opin. Hematol. 18, 171–176. doi: 10.1097/MOH.0b013e328345a180
Beall, C. M., Cavalleri, G. L., Deng, L., Elston, R. C., Gao, Y., Knight, J., et al. (2010). Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc. Natl. Acad. Sci. U.S.A. 107, 11459–11464. doi: 10.1073/pnas.1002443107
Białkowska, K., Marciniak, W., Muszyńska, M., Baszuk, P., Gupta, S., Jaworska-Bieniek, K., et al. (2020). Polymorphisms in MMP-1, MMP-2, MMP-7, MMP-13 and MT2A do not contribute to breast, lung and colon cancer risk in polish population. Hered. Cancer Clin. Pract. 18:16. doi: 10.1186/s13053-020-00147-w
Bigham, A., Bauchet, M., Pinto, D., Mao, X., Akey, J. M., Mei, R., et al. (2010). Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 6:e1001116. doi: 10.1371/journal.pgen.1001116
Buckingham, M., and Rigby, P. W. (2014). Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 28, 225–238. doi: 10.1016/j.devcel.2013.12.020
Chen, J., Alvarez, M. J., Talos, F., Dhruv, H., Rieckhof, G. E., and Iyer, A. (2016). Identification of causal genetic drivers of human disease through systems-level analysis of regulatory networks. Cell 166:1055. doi: 10.1016/j.cell.2016.07.036
Chen, Y., Feng, J., Li, P., Xing, D., Zhang, Y., Serra, R., et al. (2006). Dominant negative mutation of the TGF-beta receptor blocks hypoxia-induced pulmonary vascular remodeling. J. Appl. Physiol. (1985) 100, 564–571. doi: 10.1152/japplphysiol.00595.2005
Cheng, C., and Gerstein, M. (2012). Modeling the relative relationship of transcription factor binding and histone modifications to gene expression levels in mouse embryonic stem cells. Nucleic Acids Res. 40, 553–568. doi: 10.1093/nar/gkr752
Döhr, S., Klingenhoff, A., Maier, H., Hrabé de Angelis, M., Werner, T., and Schneider, R. (2005). Linking disease-associated genes to regulatory networks via promoter organization. Nucleic Acids Res. 33, 864–872. doi: 10.1093/nar/gki230
Enright, A. J., John, B., Gaul, U., Tuschl, T., Sander, C., and Marks, D. S. (2003). MicroRNA targets in Drosophila. Genome Biol. 5:R1. doi: 10.1186/gb-2003-5-1-r1
Fong, G. H. (2008). Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis 11, 121–140. doi: 10.1007/s10456-008-9107-3
Ghaleb, A. M., and Yang, V. (2017). Krüppel-like factor 4 (KLF4): What we currently know. Gene 611, 27–37. doi: 10.1016/j.gene.2017.02.025
Graupera, M., and Potente, M. (2013). Regulation of angiogenesis by PI3K signaling networks. Exp. Cell Res. 319, 1348–1355. doi: 10.1016/j.yexcr.2013.02.021
Henderson, J., Withford-Cave, J. M., Duffy, D. L., Cole, S. J., Sawyer, N. A., Gulbin, J. P., et al. (2005). The EPAS1 gene influences the aerobic-anaerobic contribution in elite endurance athletes. Hum. Genet. 118, 416–423. doi: 10.1007/s00439-005-0066-0
Huang, D. W., Sherman, B. T., and Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57. doi: 10.1038/nprot.2008.211
Huang, M., Yang, B., Chen, H., Zhang, H., Wu, Z., Ai, H., et al. (2019). The fine-scale genetic structure and selection signals of Chinese indigenous pigs. Evol. Appl. 13, 458–475. doi: 10.1111/eva.12887
Jia, C., Kong, X., Koltes, J. E., Gou, X., Yang, S., Yan, D., et al. (2016). Gene co-expression network analysis unraveling transcriptional regulation of high-altitude adaptation of Tibetan Pig. PLoS One 11:e0168161. doi: 10.1371/journal.pone.0168161
Kashtan, N., Itzkovitz, S., Milo, R., and Alon, U. (2004). Efficient sampling algorithm for estimating subgraph concentrations and detecting network motifs. Bioinformatics 20, 1746–1758. doi: 10.1093/bioinformatics/bth163
Kim, D., Kwon, Y. K., and Cho, K. H. (2008). The biphasic behavior of incoherent feed-forward loops in biomolecular regulatory networks. Bioessays 30, 1204–1211. doi: 10.1002/bies.20839
Kostka, D., and Spang, R. (2004). Finding disease specific alterations in the co-expression of genes. Bioinformatics 20, i194–i199. doi: 10.1093/bioinformatics/bth909
Kumar, L., and Futschik, M. E. (2007). Mfuzz: a software package for soft clustering of microarray data. Bioinformation 2, 5–7. doi: 10.6026/97320630002005
Lan, G., and Tu, Y. (2013). The cost of sensitive response and accurate adaptation in networks with an incoherent type-1 feed-forward loop. J. R. Soc. Interface 10:20130489. doi: 10.1098/rsif.2013.0489
Langfelder, P., Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559
Le, D. H., and Kwon, Y. K. (2013). A coherent feedforward loop design principle to sustain robustness of biological networks. Bioinformatics 29, 630–637. doi: 10.1093/bioinformatics/btt026
Lee, S. J., Zhang, M., Hu, K., Lin, L., Zhang, D., and Jin, Y. (2015). CCN1 suppresses pulmonary vascular smooth muscle contraction in response to hypoxia. Pulm. Circ. 5, 716–722. doi: 10.1086/683812
Li, C., Li, X., Xiao, J., Liu, J., Fan, X., Fan, F., et al. (2019). Genetic changes in the EPAS1 gene between tibetan and han ethnic groups and adaptation to the plateau hypoxic environment. PeerJ. 7:e7943. doi: 10.7717/peerj.7943
Li, G., Schmitt, H., Johnson, W. M., Lee, C., Navarro, I., Cui, J., et al. (2020). Integral role for lysyl oxidase-like-1 in conventional outflow tissue function and behavior. FASEB J. 34, 10762–10777. doi: 10.1096/fj.202000702RR
Li, H., Ouyang, X. P., Jiang, T., Zheng, X., He, P., and Zhao, G. (2018). MicroRNA-296: a promising target in the pathogenesis of atherosclerosis? Mol. Med. 24:12. doi: 10.1186/s10020-018-0012-y
Li, M., Jin, L., Ma, J., Tian, S., Li, R., and Li, X. (2016). Detecting mitochondrial signatures of selection in wild Tibetan pigs and domesticated pigs. Mitochondrial. DNA A DNA Mapp. Seq. Anal. 27, 747–752. doi: 10.3109/19401736.2014.913169
Li, M., Tian, S., Jin, L., Zhou, G., Li, Y., Zhang, Y., et al. (2013). Genomic analyses identify distinct patterns of selection in domesticated pigs and Tibetan wild boars. Nat. Genet. 45, 1431–1438. doi: 10.1038/ng.2811
Liu, X., Zhang, Y., Li, Y., Pan, J., Wang, D., Chen, W., et al. (2019). EPAS1 gain-of-function mutation contributes to high-altitude adaptation in Tibetan horses. Mol. Biol. Evol. 36, 2591–2603. doi: 10.1093/molbev/msz158
Long, K., Feng, S., Ma, J., Zhang, J., Jin, L., Tang, Q., et al. (2019). Small non-coding RNA transcriptome of four high-altitude vertebrates and their low-altitude relatives. Sci. Data 6:192. doi: 10.1038/s41597-019-0204-5
Ma, Y., Han, X., Huang, C., Zhong, L., Adeola, A. C., Irwin, D. M., et al. (2019). Population genomics analysis revealed origin and high-altitude adaptation of Tibetan pigs. Sci. Rep. 9:11463. doi: 10.1038/s41598-019-47711-6
Mangan, S., and Alon, U. (2003). Structure and function of the feed-forward loop network motif. Proc. Natl. Acad. Sci. U.S.A. 100, 11980–11985. doi: 10.1073/pnas.2133841100
Mangan, S., Zaslaver, A., and Alon, U. (2003). The coherent feedforward loop serves as a sign-sensitive delay element in transcription networks. J. Mol. Biol. 334, 197–204. doi: 10.1016/j.jmb.2003.09.049
McLeay, R. C., Lesluyes, T., Cuellar Partida, G., and Bailey, T. L. (2012). Genome-wide in silico prediction of gene expression. Bioinformatics 28, 2789–2796. doi: 10.1093/bioinformatics/bts529
Melincovici, C. S., Boşca, A. B., Şuşman, S., Mărginean, M., Mihu, C., Istrate, M., et al. (2018). Vascular endothelial growth factor (VEGF) – key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 59, 455–467.
Milo, R., Itzkovitz, S., Kashtan, N., Levitt, R., Shen-Orr, S., Ayzenshtat, I., et al. (2004). Superfamilies of evolved and designed networks. Science 303, 1538–1542. doi: 10.1126/science.1089167
Milo, R., Shen-Orr, S., Itzkovitz, S., Kashtan, N., Chklovskii, D., and Alon, U. (2002). Network motifs: simple building blocks of complex networks. Science 298, 824–827. doi: 10.1126/science.298.5594.824
Minchenko, D. O., Tsymbal, D. O., Riabovol, O. O., Viletska, Y. M., Lahanovska, Y. O., Sliusar, M. Y., et al. (2019). Hypoxic regulation of EDN1, EDNRA, EDNRB, and ECE1 gene expressions in ERN1 knockdown U87 glioma cells. Endocr. Regul. 53, 250–262. doi: 10.2478/enr-2019-0025
Morikawa, M., Mitani, Y., Holmborn, K., Kato, T., Koinuma, D., Maruyama, J., et al. (2019). The ALK-1/SMAD/ATOH8 axis attenuates hypoxic responses and protects against the development of pulmonary arterial hypertension. Sci. Signal. 12:eaay4430. doi: 10.1126/scisignal.aay4430
Narang, V., Ramli, M. A., Singhal, A., Kumar, P., de Libero, G., Poidinger, M., et al. (2015). Automated identification of core regulatory genes in human gene regulatory networks. PLoS Comput. Biol. 11:e1004504. doi: 10.1371/journal.pcbi.1004504
Nishio, Y., Usuda, Y., Matsui, K., and Kurata, H. (2008). Computer-aided rational design of the phosphotransferase system for enhanced glucose uptake in Escherichia. coli. Mol. Syst. Biol. 4:160. doi: 10.1038/msb4100201
Peng, Y., Yang, Z., Zhang, H., Cui, C., Qi, X., Luo, X., et al. (2011). Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol. Biol. Evol. 28, 1075–1081. doi: 10.1093/molbev/msq290
Qi, X., Zhang, Q., He, Y., Yang, L., Zhang, X., Shi, P., et al. (2019). The transcriptomic landscape of yaks reveals molecular pathways for high altitude adaptation. Genome Biol. Evol. 11, 72–85. doi: 10.1093/gbe/evy264
Ravasz, E., Somera, A. L., Mongru, D. A., Oltvai, Z. N., and Barabási, A. L. (2002). Hierarchical organization of modularity in metabolic networks. Science 297, 1551–1555. doi: 10.1126/science.1073374
Rosenfeld, N., Elowitz, M. B., and Alon, U. (2002). Negative autoregulation speeds the response times of transcription networks. J. Mol. Biol. 323, 785–793. doi: 10.1016/s0022-2836(02)00994-4
Shalgi, R., Brosh, R., Oren, M., Pilpel, Y., and Rotter, V. (2009). Coupling transcriptional and post-transcriptional miRNA regulation in the control of cell fate. Aging 1, 762–770. doi: 10.18632/aging.100085
Shang, P., Li, W., Tan, Z., Zhang, J., Dong, S., Wang, K., et al. (2020). Population genetic analysis of ten geographically isolated tibetan pig populations. Animals (Basel) 10:1297. doi: 10.3390/ani10081297
Shatat, M. A., Tian, H., Zhang, R., Tandon, G., Hale, A., Fritz, J. S., et al. (2014). Endothelial Krüppel-like factor 4 modulates pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 50, 647–653. doi: 10.1165/rcmb.2013-0135OC
Sheng, J., Liu, D., Kang, X., Chen, Y., Jiang, K., and Zheng, W. (2018). Egr-1 increases angiogenesis in cartilage via binding Netrin-1 receptor DCC promoter. J. Orthop. Surg. Res. 13:125. doi: 10.1186/s13018-018-0826-x
Shen-Orr, S. S., Milo, R., Mangan, S., and Alon, U. (2002). Network motifs in the transcriptional regulation network of Escherichia. coli. Nat. Genet. 31, 64–68. doi: 10.1038/ng881
Simonson, T. S., Yang, Y., Huff, C. D., Yun, H., Qin, G., Witherspoon, D. J., et al. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329, 72–75. doi: 10.1126/science.1189406
Tan, G., and Lenhard, B. (2016). TFBSTools: an R/bioconductor package for transcription factor binding site analysis. Bioinformatics 32, 1555–1556. doi: 10.1093/bioinformatics/btw024
Tang, Q., Gu, Y., Zhou, X., Jin, L., Guan, J., Liu, R., et al. (2017). Comparative transcriptomics of 5 high-altitude vertebrates and their low-altitude relatives. Gigascience 6, 1–9. doi: 10.1093/gigascience/gix105
Weber, E. J., and Hoo, Z. H. (2018). Why sample size estimates? Emerg. Med. J. 35, 755–756. doi: 10.1136/emermed-2018-207763
Xiang, K., Ouzhuluobu, Peng, Y., Yang, Z., Zhang, X., Cui, C., et al. (2013). Identification of a Tibetan-specific mutation in the hypoxic gene EGLN1 and its contribution to high-altitude adaptation. Mol. Biol. Evol. 30, 1889–1898. doi: 10.1093/molbev/mst090
Xie, Q., Xie, J., Tian, T., Ma, Q., Zhang, Q., Zhu, B., et al. (2017). Hypoxia triggers angiogenesis by increasing expression of LOX genes in 3-D culture of ASCs and ECs. Exp. Cell Res. 352, 157–163. doi: 10.1016/j.yexcr.2017.02.011
Xu, S., Li, S., Yang, Y., Tan, J., Lou, H., Jin, W., et al. (2011). A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol. Biol. Evol. 28, 1003–1011. doi: 10.1093/molbev/msq277
Yan, X., Liu, Z., and Chen, Y. (2009). Regulation of TGF-beta signaling by Smad7. Acta Biochim. Biophys. Sin. (Shanghai) 41, 263–272. doi: 10.1093/abbs/gmp018
Yanai, I., Benjamin, H., Shmoish, M., Chalifa-Caspi, V., Shklar, M., Ophir, R., et al. (2005). Genome-wide midrange transcription profiles reveal expression level relationships in human tissue specification. Bioinformatics 21, 650–659. doi: 10.1093/bioinformatics/bti042
Young, A. I. (2019). Solving the missing heritability problem. PLoS Genet. 15:e1008222. doi: 10.1371/journal.pgen.1008222
Zhang, B., Chamba, Y., Shang, P., Wang, Z., Ma, J., Wang, L., et al. (2017). Comparative transcriptomic and proteomic analyses provide insights into the key genes involved in high-altitude adaptation in the Tibetan pig. Sci. Rep. 7:3654. doi: 10.1038/s41598-017-03976-3
Zhang, W., Fan, Z., Han, E., Hou, R., Zhang, L., Galaverni, M., et al. (2014). Hypoxia adaptations in the grey wolf (Canis. lupus chanco) from Qinghai-Tibet Plateau. PLoS Genet. 10:e1004466. doi: 10.1371/journal.pgen.1004466
Keywords: Tibetan pig, multitissue, transcriptome, hypoxia adaptation, gene network
Citation: Wang T, Guo Y, Liu S, Zhang C, Cui T, Ding K, Wang P, Wang X and Wang Z (2021) KLF4, a Key Regulator of a Transitive Triplet, Acts on the TGF-β Signaling Pathway and Contributes to High-Altitude Adaptation of Tibetan Pigs. Front. Genet. 12:628192. doi: 10.3389/fgene.2021.628192
Received: 11 November 2020; Accepted: 10 March 2021;
Published: 15 April 2021.
Edited by:
Deborah A. Triant, University of Virginia, United StatesReviewed by:
Lorna Grindlay Moore, University of Colorado, United StatesCopyright © 2021 Wang, Guo, Liu, Zhang, Cui, Ding, Wang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xibiao Wang, d2FuZ3hpYmlhb0BuZWF1LmVkdS5jbg==; Zhipeng Wang, d2FuZ3poaXBlbmdAbmVhdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.