- 1Key Lab of Agricultural Animal Genetics, Breeding and Reproduction of Ministry of Education, College of Animal Science and Technology, Huazhong Agricultural University, Wuhan, China
- 2Department of Animal Production, Faculty of Agriculture, Cairo University, Giza, Egypt
- 3Guangxi Provincial Key Laboratory of Buffalo Genetics, Breeding and Reproduction Technology, Buffalo Research Institute, Chinese Academy of Agricultural Sciences, Nanning, China
- 4National Institute for Biotechnology and Genetic Engineering (NIBGE), Faisalabad, Pakistan
- 5International Joint Research Centre for Animal Genetics, Breeding and Reproduction, Wuhan, China
- 6Hubei Province’s Engineering Research Center in Buffalo Breeding and Products, Wuhan, China
Bovine and buffalo are important livestock species that have contributed to human lives for more than 1000 years. Improving fertility is very important to reduce the cost of production. In the current review, we classified reproductive traits into three categories: ovulation, breeding, and calving related traits. We systematically summarized the heritability estimates, molecular markers, and genomic selection (GS) for reproductive traits of bovine and buffalo. This review aimed to compile the heritability and genome-wide association studies (GWASs) related to reproductive traits in both bovine and buffalos and tried to highlight the possible disciplines which should benefit buffalo breeding. The estimates of heritability of reproductive traits ranged were from 0 to 0.57 and there were wide differences between the populations. For some specific traits, such as age of puberty (AOP) and calving difficulty (CD), the majority beef population presents relatively higher heritability than dairy cattle. Compared to bovine, genetic studies for buffalo reproductive traits are limited for age at first calving and calving interval traits. Several quantitative trait loci (QTLs), candidate genes, and SNPs associated with bovine reproductive traits were screened and identified by candidate gene methods and/or GWASs. The IGF1 and LEP pathways in addition to non-coding RNAs are highlighted due to their crucial relevance with reproductive traits. The distribution of QTLs related to various traits showed a great differences. Few GWAS have been performed so far on buffalo age at first calving, calving interval, and days open traits. In addition, we summarized the GS studies on bovine and buffalo reproductive traits and compared the accuracy between different reports. Taken together, GWAS and candidate gene approaches can help to understand the molecular genetic mechanisms of complex traits. Recently, GS has been used extensively and can be performed on multiple traits to improve the accuracy of prediction even for traits with low heritability, and can be combined with multi-omics for further analysis.
Introduction
Reproductive traits are economically important for sustainable food production, especially for monotocous livestock, such as cattle and buffalo. Low reproductive capacity or infertility can be described as an extended duration between two calvings. This problem requires additional inseminations, more veterinary attention, and hormonal treatments, which consequently alters the current and subsequent lactations (Boichard, 1990). Also, additional costs are raised due to culling and replacing animals with fertility problems (Roxström and Strandberg, 2002). Enhancing fertility is the best choice not only to reduce the culling cost but also to save important genetic materials and increase farm profit (Dekkers, 1991). Several countries have included female reproductive traits in the breeding goals to emphasize the genetic aspects of reducing fertility costs (FCOST) in dairy cattle (Kadarmideen and Simm, 2002). Herein, we emphasize the recent literature about genetic parameters, genome-wide association study (GWAS), and genomic selection (GS) for reproductive traits in cattle and buffalo over the past 20 years for researchers, who can integrate these traits in cattle and buffalo breeding programs and achieve optimum fertility.
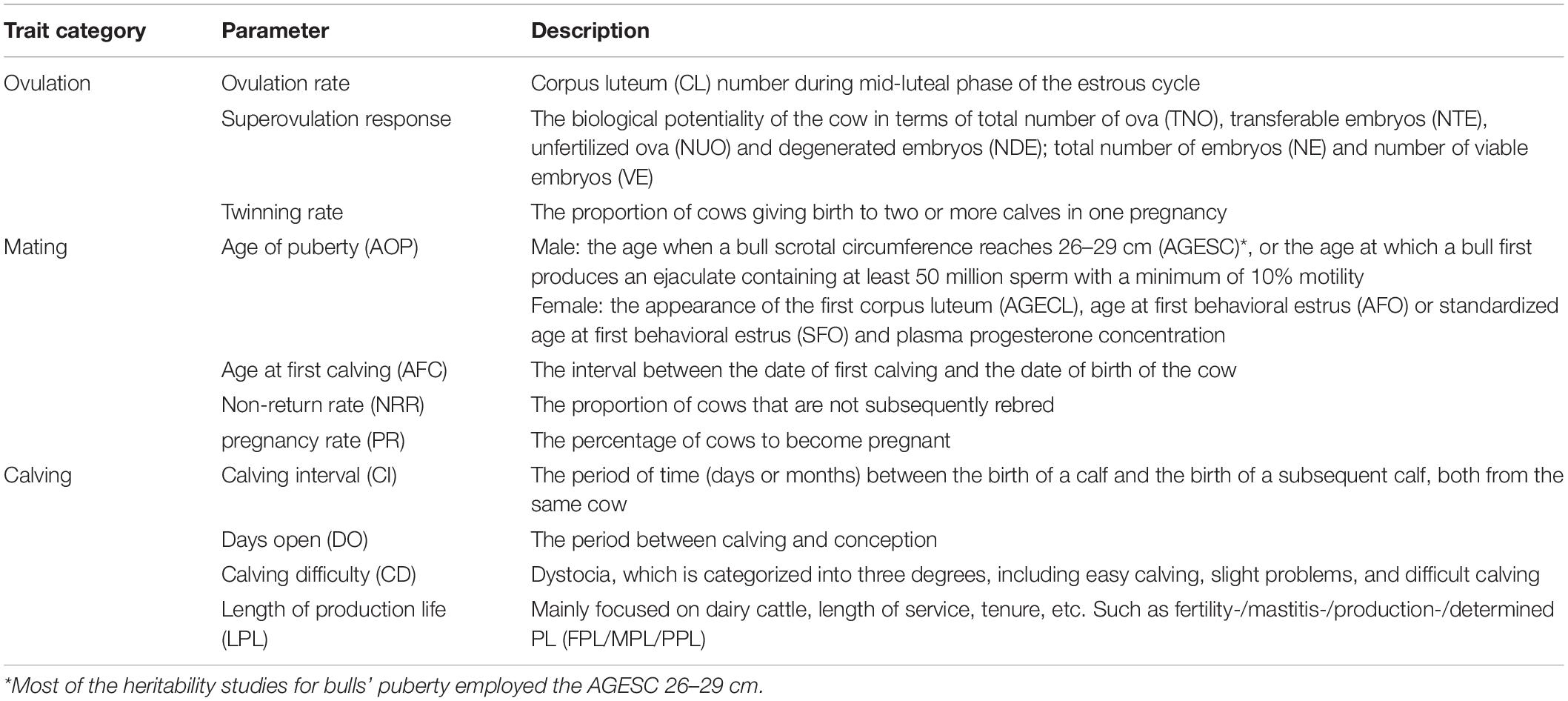
In the previous study, reproductive traits were divided into binary, interval, and continuous traits with respect to statistical distribution (Berry and Evans, 2014). To better understand and utilize reproductive traits in livestock and breeding programs, they are reclassified as ovulation, mating, and calving-related traits from the physiological viewpoint (Cammack et al., 2009; Table 1).
Heritability Estimates of Reproductive Traits
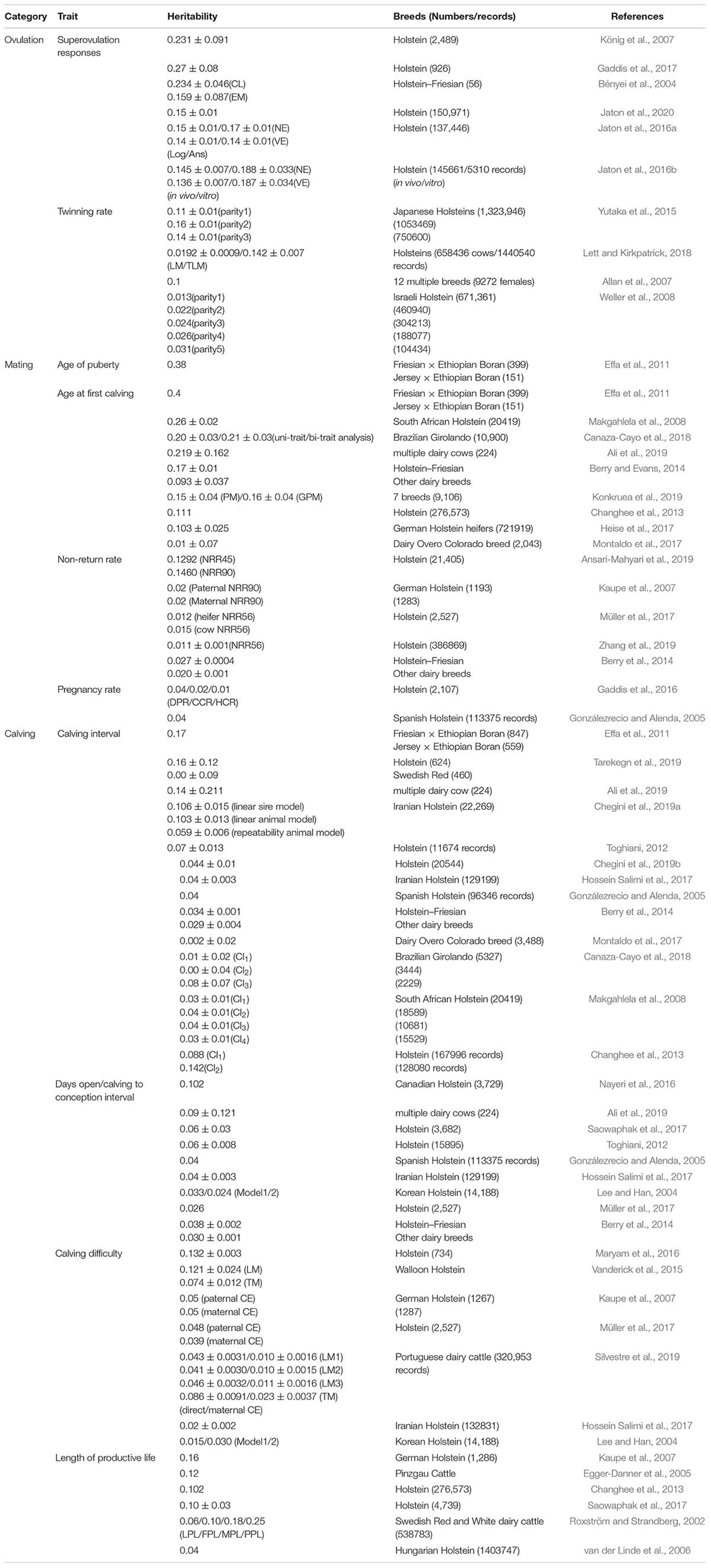
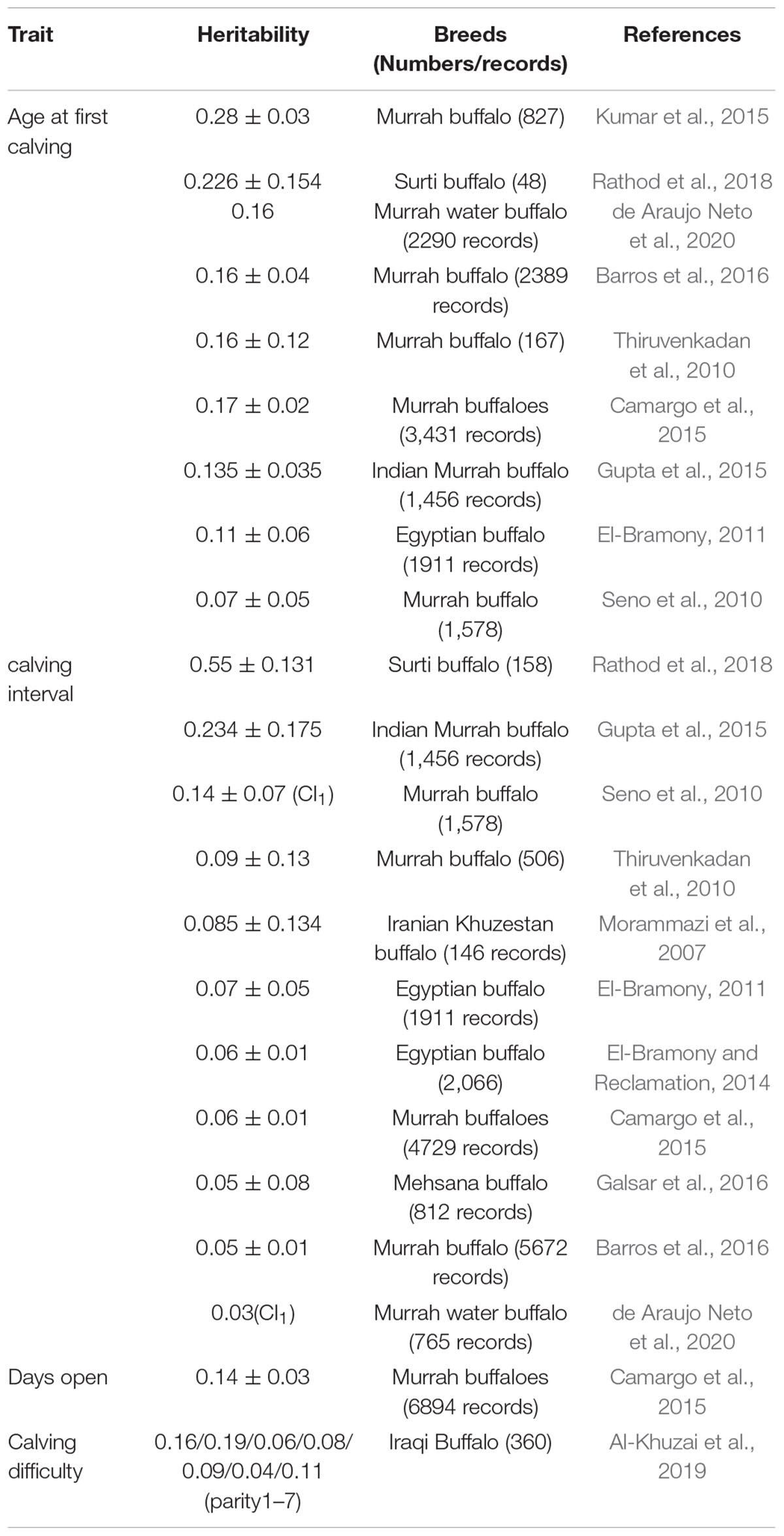
Genetic variation, which is a variability in breeding values within a population for a trait under selection, significantly affects the accuracy of genetic selection. Heritability measures how much of the phenotypic variation is attributed to genetic variation, and affects the rate of genetic improvement for a trait over generations. Over the past 20 years, several studies were conducted to estimate the heritability of different reproductive traits in dairy cattle (Table 2), beef cattle (Table 3), and buffalo cows (Table 4).
In dairy cattle, all ovulation-related traits range from low to moderate heritabilities (Table 2). The heritability estimate of the superovulation response was about 0.15 in Holstein cows (Jaton et al., 2020). Regarding mating-related traits, heritability estimates for age of puberty (AOP) and age at first calving (AFC) were moderate in most cattle populations, except for AFC in the Chile population (h2 = 0.01) (Montaldo et al., 2017). Likewise, the heritabilities of non-return rate (NRR) and pregnancy rate (PR) of Holstein dairy cows and Brown Swiss cattle were low (Gaddis et al., 2016; Tiezzi et al., 2018; Ansari-Mahyari et al., 2019; Zhang et al., 2019). Regarding the superovulation response and twinning rate, heritability was higher for superovulation, indicating a response to hormone treatment is more heritable than natural ovulation in dairy cows. Non-return and PR are directly related to reproductive outcomes. Unfortunately, the heritability estimates for these two traits were remarkably low. Besides, dairy cows’ calving-related traits, including calving interval, days open, calving difficulty (CD), and the length of the productive life, were all of low heritability. Therefore, management practices (reproductive management, balanced nutrition, etc.) and/or environmental factors are of significant importance for improving reproductive efficiency and preventing reproductive disorders in dairy cows. Thus, selection on dairy cows’ AOP, first calving, and superovulation response may gain more progression than other traits.
In beef cattle, the superovulation response had higher heritability than those of ovulation rate, and twinning rate was similar to those reported in dairy cattle (Table 3). Regarding mating-related traits, AOP had moderate to high heritability estimates in most beef populations; for example, the estimate reached 0.78 in the Brahman bull population (Fortes et al., 2012). The h2 for scrotal circumference was also reported to have moderate to high estimates. Excluding the Angus population (0.2) (Doyle et al., 2000) in beef cattle, the NRR and PR of heritability were low, as reported in dairy cattle. The heritabilities for calving difficulties in beef cattle had moderate to high estimates, unlike those reported in dairy cattle with low heritabilities. In comparison, other mating-related reproductive traits, such as DO, NRR, CI, and length of productive life had low heritabilities similar to dairy cattle. Taken together, selections on beef cow’s AOP, calving difficulties, DO, NRR, and CI traits may gain more progression due to the moderate to high estimates of heritabilities compared with other traits (Cassell, 2009).
The excellent milk quality and the limitation of buffalo milk yield contribute to the breeding selection focusing more on milk production traits in buffalo compared with reproductive traits. Currently, there are limited studies for estimating genetic parameters for reproductive traits in buffalo species, mainly for AFC and CI (Table 4). The heritability estimates of AFC in the buffalo population is close to Holstein cattle (Gupta et al., 2015; Kumar et al., 2015; Barros et al., 2016; Rathod et al., 2018). Most studies showed that the heritability of CI is low, mostly below 0.1 (Morammazi et al., 2007; Thiruvenkadan et al., 2010; El-Bramony and Reclamation, 2014; Camargo et al., 2015). However, the highest record for CI was 0.55 in Surti buffalo, which may be due to the limited numbers of lactation records and/or number of parities per sire monitored (Rathod et al., 2018). The heritabilities of DO (Camargo et al., 2015) and CD (Al-Khuzai et al., 2019) were similar to those reported in dairy cattle.
Comparing heritabilities between different traits in dairy and beef cattle along with buffalo, we found that:
(1) Most of the reproductive traits had low habitabilities, but not all. In the dairy and beef cattle, AOP showed high heritabilities. The heritability estimates for scrotal circumference of the beef bull were medium to high. Also, the superovulation response in dairy and beef cattle was worthy of notice. These moderate to high heritability traits could be applied to the selection and breeding system.
(2) The heritability estimates for calving intervals, NRR, days open, and length of reproductive life in most populations were very low, which indicated that these traits would be influenced and improved by proper management practices. The application of synchronization-timed AI protocol (Goodling et al., 2005), body composition control, reproductive disorder treatment, and culling on time would benefit the related performance.
(3) The heritability of the same trait varies greatly among different breeds. For instance, the heritability of age at first calving was as high as 0.4 in a crossbreed of dairy cows (Effa et al., 2011), while the Dairy Overo Colorado breed was as low as 0.01 (Montaldo et al., 2017). The heritability of CI reported in Surti buffalo is 0.55 (Rathod et al., 2018) compared to the Murrah buffalo cows near to 0.1 (Thiruvenkadan et al., 2010). Although heritability was estimated using paternal half-sib correlation methods in both studies, lactation records, number of buffaloes, and sired by bulls were higher for Murrah buffaloes. Even in the same breed, the different populations showed varied values, which may related to different management and performance.
(4) For most of the reproductive traits, beef cattle had higher heritability estimates compared to those estimated in dairy cattle for the AOP and CD (Tables 2, 3). Either the genetic makeup or the fact that dairy cows are more susceptible to reproductive diseases, such as endometritis, vaginitis, ovarian cyst, and mastitis, due to high energy consumption for milk production may be the reason for this difference.
(5) The breeding progress of buffalo is slow compared to dairy and beef cattle, as a few studies have reported during the last decade. Further large-scale studies are required to accurately estimate the genetic parameters for different reproductive traits in buffalo populations.
Marker-Associated Studies for Bovine and Buffalo Reproductive Traits
Concerning the disadvantages of the long cycle and not up-to-mark efficiency of traditional breeding, several association analyses were performed to identify genomic loci associated with the trait variation to find the possible candidate genes or to detect causative mutations. This section summarized the GWAS and candidate gene studies for bovine and buffalo reproductive traits published in the past 20 years (2000–2020) (Supplementary Tables 1–3).
At present, there are few marker-assisted selection (MAS) studies on the reproductive traits of buffalo. In this regard, FSHR, INHA, LHCGR, and OPN were reported to have significant effects on the buffalo superovulation responses. So far, few GWAS have been performed on buffalo reproductive traits (Camargo et al., 2015; Li et al., 2018a, b; de Araujo Neto et al., 2020). Previous GWASs for reproductive traits (Camargo et al., 2015; Li et al., 2018a) were conducted using the bovine reference genome assembly, and the results are expressed for bovine autosomes (BTA). Camargo et al. (2015) reported some candidate genes (TPCN1, SCG5, and Fig 4) associated with reproductive traits such as AFC, CI, and DO in buffalo. Also, Li et al. (2018a; 2018b) found 25 SNPs in 13 genes related to reproductive traits by integrating RNA-seq and GWAS methods. They also described significant SNPs on BBU 6, 9, and 15 [corresponding to bovine chromosomes 3, 7, 14, and 8: equivalence presented by Cribiu et al. (2001)]. Recently, ssGBLUP was employed to identify genomic regions affecting AFC and first calving interval (FCI) in buffalo cows and select candidate loci and gene expression (de Araujo Neto et al., 2020). They reported that the observed candidate regions for both traits (CI, AFC; explaining a large proportion of variance for both traits) were located on BBU 3, 12, 21, and 22. Also, candidate regions were found on BBU 6, 7, 8, 9, and 15 for age at first calving and on BBU 4, 14, and 19 for FCI. The ROCK2, PMVK, ADCY2, MAP2K6, BMP10, and GFPT1 genes are the main candidates for reproductive traits in water dairy buffaloes, which may be used in the future for animal breeding programs or for gene expression studies of the species (de Araujo Neto et al., 2020). The GFPT1 and BMP10 genes are interesting because they have been detected for both traits, which may be related to a possible pleiotropic effect.
The candidate gene studies for bovine reproductive traits mostly used genes of hormones and/or growth factors and their receptors as candidates (Tang et al., 2011; Yang et al., 2013; Arslan et al., 2017). For example, polymorphisms in the GnRH, GnRHR, LEP, and LHCGR were studied for association with reproductive traits of buffalo bulls. Notably, genes involved in IGF1 and LEP pathways were reported to affect multiple reproductive traits. For example, IGF1 could affect a variety of ovulation- and mating-related traits. LEP and LEPR showed significant effects on both breeding- and calving-related traits. Moreover, long non-coding RNA and ribosomal RNA could be future research directions since non-coding RNAs (U6 spliceosomal RNA) were reported to affect reproductive traits (Fortes et al., 2013; Nascimento et al., 2016; Buzanskas et al., 2017). The combination of GWAS and other omics studies are becoming more useful, as they provide a broad space for exploring candidate gene functions and related mechanisms.
Further, we visualized the chromosomal distribution of quantitative trait loci (QTL) in cattle related to each reproductive trait using the Cattle Quantitative Trait Locus Database (Cattle QTLdb) (Hu et al., 2019) (Supplementary Figures 1–3). Only 11 QTL related to ovulation-related traits were identified, and four of these were located on chromosome 5, where the IGF1 gene is placed (Miller et al., 1992) (Supplementary Figure 1). The QTL for mating-related traits were spread throughout different chromosomes (Supplementary Figure 1A). The most abundant chromosome is BTX with 10237 QTL (96.4%) related to puberty. BTA2 (21QTLs, 19.6%) and BTA14 (15 QTLs, 14.0%) had the most associated loci for AFC (Supplementary Figure 1B). Most of the QTL for NRR were located on BTA17 (233421 QTLs, 94.7%). However, QTL for PR-related were scattered (Supplementary Figure 2). About 37.1% of QTL related to calving interval were enriched in BTA25 (17.5%) and BTA29 (19.6%). Whereas, BTA 21 enriched the most QTLs (44.8%) related to CD, and BTA18 had 30.7% of QTL related to the length of productive life.
Undoubtedly, these significantly enriched chromosomes (BTX related to puberty, BTA related to NRR, and BTA related to CD) could be directions for future research. Moreover, certain areas that affect multiple traits of different species also deserve further attention. For example, McClure et al. (2010) found one SNP related to CD at 49.1 Mb of BTA 20 in Angus cattle (McClure et al., 2010), while Ke et al. (2014) reported SNP in a similar region in dairy cattle affecting age at first calving. The relationship between these highly enriched chromosomal regions and various traits is worthy of further investigation.
Based on morphological and behavioral criteria, the domestic Asian water buffalo has two types (Macgregor, 1941). The two types have different chromosome numbers: river buffalo (Bubalus bubalis, 2n = 50) and swamp buffalo (Bubalus bubalis carabanesis, 2n = 48) (Ulbrich and Fischer, 1966). In addition, the chromosomal karyotype of hybrid buffalo is more complicated. Although presenting different species, buffalo and bovine share highly homologous chromosomes banding, as well as gene mapping (Amaral et al., 2008; Michelizzi et al., 2010; Kale et al., 2014). It is also reported that river buffalo and bovine chromosomes can be matched arm for arm at the cytogenetic level (Williams et al., 2017; Du et al., 2019). Despite the complicated genomic background of buffalo, candidate genes or their chromosome locations identified for the bovine reproductive traits could be considered as a valuable reference for buffalo.
Genomic Selection for Reproductive Traits in Bovine and Buffalo
Phenotypic records for a trait of individuals and their relatives are used to estimate breeding values by employing the best linear unbiased prediction (BLUP) to facilitate animal selection for economically important traits (Henderson, 1984). It is believed for genetic selection that information at the DNA level can quicken the genetic progression compared to phenotypic data alone. The sparse map of genetic markers can be used to detect QTL (Georges et al., 1995). Combining genetic marker information with BLUP (Fernando and Grossman, 1989) showed an increase in the genetic gain by 8–38% (Fernando and Grossman, 1989; Goddard, 1996). The effectiveness of sparse markers in outbreeding species was limited, as an establishment of linkage phase between a marker and QTL is necessary for every family in which the marker is to be used for selection (Meuwissen et al., 2001).
The total number of SNP estimated at millions and the advent of DNA Chip technology made genotyping of many animals for many of these markers feasible and cost-effective. However, a dense marker map improved precision for QTL mapping by traditional linkage analysis (Darvasi et al., 1993). Therefore, a search for a different approach to efficiently use all this marker information remained necessary.
Considering a denser marker map, not only could some markers be close to QTL but also, in linkage disequilibrium with it, it was anticipated that some markers could have a positive effect on the quantitative traits across all families and be used for selection without the need to establish a Linkage phase in each family. Close markers can also be combined into a haplotype. Chromosome bearing the rare marker haplotype is likely to be identical by descent and hence carry the same QTL allele. Meuwissen et al. (2001), estimated the effect of the quantitative trait of the small chromosome segment defined by the haplotype of the allele that they carry. They concluded that it’s possible to accurately estimate the breeding value of animals that have no phenotypic records by estimating a large number of haplotype effects. Using least squares, all haplotype effects could not be estimated simultaneously. Even when only the largest effects were included, they were overestimated and the accuracy of predicting breeding value was low. Methods that assumed prior distribution for the variance associated with each chromosome segment gave a more accurate prediction of breeding values even when the prior was not correct. Selection based on breeding values predicted from markers could substantially increase the rate of genetic gain in animals and plants, especially if combined with reproductive techniques to shorten the generation interval. Selection based on pedigree has played an important role in the selective breeding improvement in domestic animals.
Quantitative traits are usually affected by many genes and, consequently, the benefits from the MAS are limited by the proportion of the genetic variance explained by the QTL. Hence, it is warranted to utilize all the QTL affecting the traits in MAS. Nevertheless, a dense marker map defines a very large number of chromosome segments and so there will be many effects to be estimated, probably more than there are phenotypic data points from which to estimate them (Meuwissen et al., 2001).
With the emergence of high-density SNP chips, such as Illumina chips [BovineHD BeadChip SNP, BovineSNP50 chip, High-Density Bovine SNP chip (777K)] and Axiom® Buffalo Genotyping Array (90K), GS methods are improving livestock genetic evaluation systems. They have the advantages of high accuracy, short interval between generations, and rapid genetic progress.
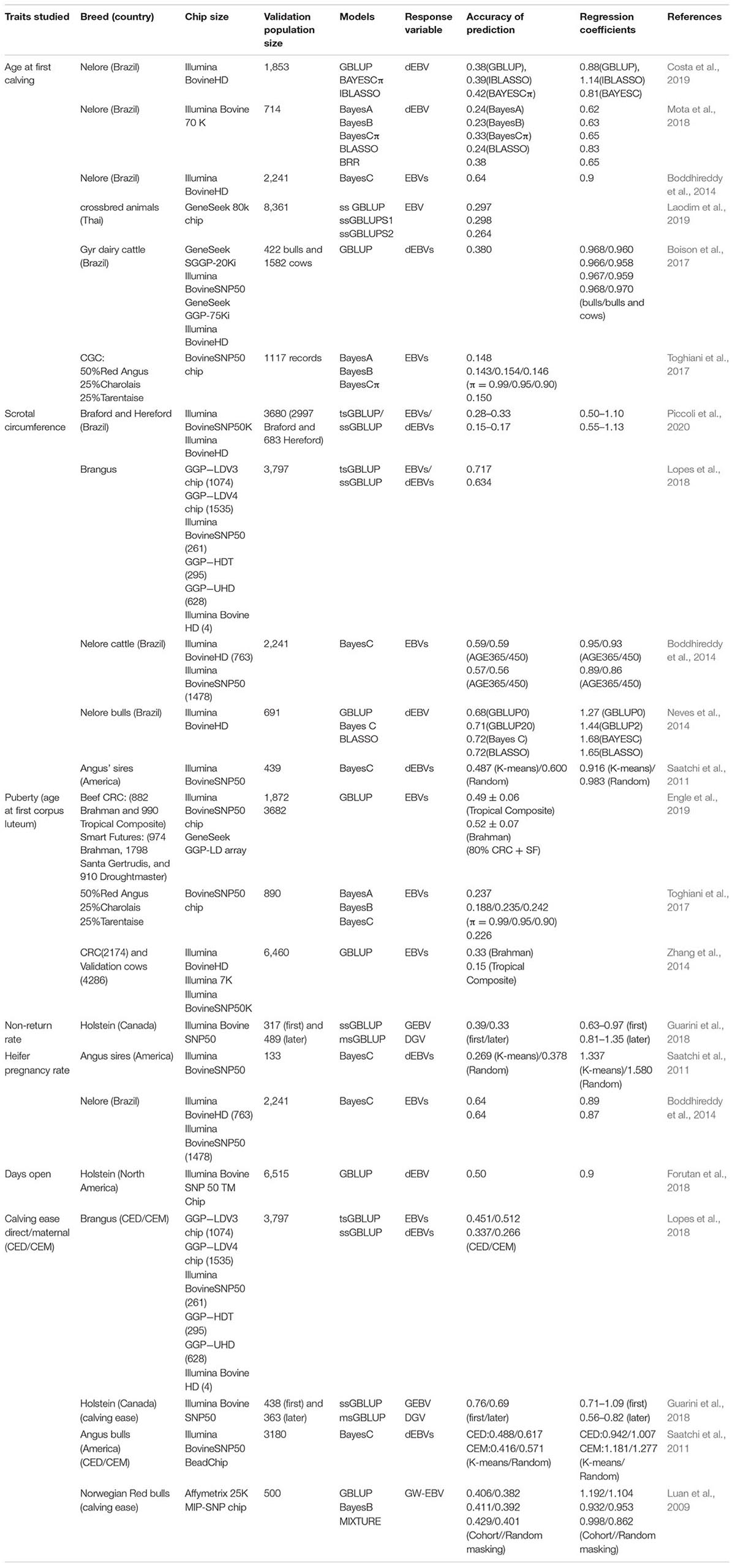
At present, GS has been applied in cattle on a large scale, but mainly focus on milk production and carcass traits (Silva et al., 2014; Weller et al., 2017). The GS studies on reproductive traits in dairy and beef cattle, including AFC, puberty, NRR, PR, days open, and CD, are listed on Table 5.
For AFC, the accuracy of genomic prediction was varied among different populations and methods. In the Nellore breed, the accuracy of prediction for AFC was 0.64 (Boddhireddy et al., 2014); however, another scholarly journal reported that the accuracy ranged between 0.38 and 0.42 by three different models (Costa et al., 2019). The prediction accuracy is around 0.23–0.33 in another Nellore cow population (Mota et al., 2018). Using the ssGBLUP model, the accuracy of prediction for AFC was 0.299 in the Thai native breed (Laodim et al., 2019), and was 0.56 in the Gyr dairy cattle breed (Boison et al., 2017).
Genomic selection studies on puberty (scrotal circumference and age at first corpus luteum) showed that the accuracy performance of different models is above 0.6 (Boddhireddy et al., 2014; Neves et al., 2014; Toghiani et al., 2017; Lopes et al., 2018; Engle et al., 2019). However, the accuracy was decreased dramatically in crossbred populations (Zhang et al., 2014; Piccoli et al., 2020). The limited reference population in the hybrid population and the general traits of the reference population have no direct counterpart in the validation population, which may be the reason for this decrease.
In the PR studies, the accuracy of prediction was 0.269 in the Angus population (Saatchi et al., 2011) and 0.64 in Nelore cattle (Boddhireddy et al., 2014). For CD, the highest accuracy was 0.516 in Brangus using GBLUP models (Lopes et al., 2018), and the prediction accuracy of different beef cattle breeds is around 0.45 among different models (Luan et al., 2009; Saatchi et al., 2011), while the accuracy in dairy cows was lower by 0.24–0.34 (Guarini et al., 2018).
Regarding buffalo studies, genomic evaluation reports are very limited either for productive or reproductive traits. There is only one published study for AFC and CI in buffalo (de Araujo Neto et al., 2020). Genomic evaluation studies in buffalo are still in the developing stage. The main limitation of applying genomic evaluation in buffalo is the lack of a well-structured reference population. Since the number of individuals with both genotypic and phenotypic information in each country is still limited, a multi-breed genomic evaluation would be the best alternative (Liu et al., 2018; Abdel-Shafy et al., 2020a, b).
Conclusion and Perspectives
Reproductive traits were depreciated during selection indexes to improve the genetic potential of livestock. Hence, the recently desired gains are being practiced to ensure that the all TMI (total merit index) traits show a positive response or, at the very least, no negative response. However, the statistical data from the Council on Dairy Cattle Breeding (CDCB)1 indicated that, without severely slowing genetic gain for milk production, the daughter PR has stabilized and the declining trend has been reversing since 2003. A similar trend has also been demonstrated by García-Ruiz et al. (2016). Moreover, several pregnancy-related SNPs with neutral associations with milk production in Holstein bulls were identified (Cochran et al., 2013). It elicits the possibility of increasing fertility without reducing productive performance during selection.
Unlike dairy and beef cattle, few studies have been performed so far for reproductive traits in buffalo. Methods such as GWAS and GS require a large group size, well-structured pedigree, and accurate phenotypic records, which are big challenges for buffalo populations. The first reference for buffalo genome sequencing was released in 2017 (Williams et al., 2017), lacking the sequence in the chromosome and genes annotation, which was completed and updated in 2019 (Low et al., 2019; Mintoo et al., 2019). It will quicken the GS research and be significantly helpful in promoting buffalo breeding.
Dissimilar to dairy production traits, GWAS for reproductive traits seems to be underpowered and has difficulty in finding major QTL. It still provides genetic variability across many genome-wide genes and intragenic regions for complex trait studies, which greatly increases the understanding of complex traits’ molecular genetic mechanisms.
For reproductive traits with low heritability, the genetic gain using GS is improved three to four times per year compared to traditional methods (García-Ruiz et al., 2016). However, GS is also facing some difficulties, especially for buffalo, such as lacking an optimum population structure with record and some species having no dense marker maps yet. Its accuracy is limited by the reference population’s size and SNP marker density, which is obvious in some hybrid populations. In developing countries, there is a lack of complete historical records, and the number of genotyped animals has limited the development of GS. Also, for those traits with low to high heritability (such as puberty, age at first calving, and CD), multivariate GS can performed on multiple traits to improve prediction accuracy. In addition, multi-breed genomic evaluation can be used for populations with limited size. Besides, multi-omics data integration and analysis are gaining more attention from fields such as genomics, transcriptomics, and epigenomics.
Author Contributions
GH contributed to the conception and design of the study. BS wrote the first draft of the manuscript and collected the data. CD, HS, MA, and YY wrote sections of the manuscript. NG, HA, SM, YZ, TD, LY, and SZ revised the manuscript and made profound suggestions. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (31872352), Fundamental Research Funds for the Central Universities (2662018PY037), and the Earmarked Fund for Modern Agro-Industry Technology Research System (CARS-36).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.617128/full#supplementary-material
Footnote
References
Abdel-Shafy, H., Awad, M. A. A., El-Regalaty, H., El-Assal, S. E.-D., and Abou-Bakr, S. (2020a). Prospecting genomic regions associated with milk production traits in Egyptian buffalo. J. Dairy Res. 87, 389–396. doi: 10.1017/s0022029920000953
Abdel-Shafy, H., Awad, M. A. A., El-Regalaty, H., Ismael, A., El-Assal, S. E.-D., and Abou-Bakr, S. (2020b). A single-step genomic evaluation for milk production in Egyptian buffalo. Livestock Sci. 234:103977. doi: 10.1016/j.livsci.2020.103977
Ahlberg, C. (2014). Genetic Parameter Estimates and Breed Effects for Calving Difficulty and Birth Weight in a Multi-Breed Population. Lincoln, NE: University of Nebraska.
Akanno, E. C., Plastow, G., Fitzsimmons, C., Miller, S. P., Baron, V., Ominski, K., et al. (2015). Genome-wide association for heifer reproduction and calf performance traits in beef cattle. Genome 58, 549–557. doi: 10.1139/gen-2015-0031
Ali, I., Muhammad Suhail, S., and Shafiq, M. (2019). Heritability estimates and genetic correlations of various production and reproductive traits of different grades of dairy cattle reared under subtropical condition. Reprod. Domest. Anim. 54, 1026–1033. doi: 10.1111/rda.13458
Al-Khuzai, H. M., Al-Dulaimi, M. K., and Zaini, Z. E. (2019). Estimation of heritability for reproductive traits and newborn mortality in iraqi buffaloes through the relationship of dams with daughters. Indian J. Public Health Res. Dev. 10, 660–663. doi: 10.5958/0976-5506.2019.02508.7
Allan, M., Kuehn, L., Cushman, R., Snelling, W., Echternkamp, S., and Thallman, R. (2014). Confirmation of quantitative trait loci using a low-density single nucleotide polymorphism map for twinning and ovulation rate on bovine chromosome 5. J. Anim. Sci. 87, 46–56. doi: 10.2527/jas.2008-0959
Allan, M., Thallman, R., Cushman, R., Echternkamp, S., White, S., Kuehn, L., et al. (2007). Association of a single nucleotide polymorphism in SPP1 with growth traits and twinning in a cattle population selected for twinning rate. J. Anim. Sci. 85, 341–347. doi: 10.2527/jas.2006-460
Amaral, M. E. J., Grant, J. R., Riggs, P. K., Stafuzza, N. B., Rodrigues Filho, E. A., Goldammer, T., et al. (2008). A first generation whole genome RH map of the river buffalo with comparison to domestic cattle. BMC Genomics 9:631. doi: 10.1186/1471-2164-9-631
Amaya-Martínez, A. A., Martínez, R., and Cerón-Muñoz, M. F. (2020). Genetic parameters for growth and reproduction in Simmental cattle from pedigree and genomic relationship. Rev. MVZ Córdoba 25:1520. doi: 10.21897/rmvz.1520
Ansari-Mahyari, S., Ojali, M. R., Forutan, M., Riasi, A., and Brito, L. F. (2019). Investigating the genetic architecture of conception and non-return rates in Holstein cattle under heat stress conditions. Trop. Anim. Health Prod. 51, 1847–1853. doi: 10.1007/s11250-019-01875-5
Arslan, K., Akyüz, B., Akçay, A., İlgar, E. G., Macun, H. C., and Çınar, M. U. (2017). Association of number of artificial inseminations per pregnancy in holstein dairy cows with polymorphism in luteinizing hormone receptor and follicle stimulating hormone receptor genes. Slovenian Vet. Res. 54, 91–98.
Barros, C. D. C., Aspilcueta-Borquis, R. R., Fraga, A. B., and Tonhati, H. (2016). Genetic parameter estimates for production and reproduction traits in dairy buffaloes. Rev. Caat. 29, 216–221. doi: 10.1590/1983-21252016v29n125rc
Bényei, B., Gáspárdy, A., Komlósi, I., and Pécsi, A. (2004). Repeatability and heritability of ovulation number and embryos in dam-daughters pairs in superovulated holstein–friesian cows. Reprod. Domest. Anim. 39, 99–102. doi: 10.1111/j.1439-0531.2004.00487.x
Berry, D. P., and Evans, R. (2014). Genetics of reproductive performance in seasonal calving beef cows and its association with performance traits. J. Anim. Sci. 92, 1412–1422. doi: 10.2527/jas.2013-6723
Berry, D. P., Wall, E., and Pryce, J. (2014). Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 8, 105–121. doi: 10.1017/s1751731114000743
Boddhireddy, P., Prayaga, K., Barros, P., Lôbo, R., and Denise, S. (2014). “Genomic predictions of economically important traits in Nelore cattle of Brazil,” in Proceedings of the 10th World Congr. Genet. Appl. Livest. Prod, Vancouver, BC.
Boichard, D. (1990). Estimation of the economic value of conception rate in dairy cattle. Livestock Prod. Sci. 24, 187–204. doi: 10.1016/0301-6226(90)90001-m
Boison, S., Utsunomiya, A., Santos, D., Neves, H., Carvalheiro, R., Mészáros, G., et al. (2017). Accuracy of genomic predictions in Gyr (Bos indicus) dairy cattle. J. Dairy Sci. 100, 5479–5490. doi: 10.3168/jds.2016-11811
Boligon, A., and Albuquerque, L. G. D. (2011). Genetic parameters and relationships of heifer pregnancy and age at first calving with weight gain, yearling and mature weight in Nelore cattle. Livestock Sci. 141, 12–16. doi: 10.1016/j.livsci.2011.04.009
Brzáková, M., Svitáková, A., Čítek, J., Veselá, Z., and Vostrý, L. (2019). Genetic parameters of longevity for improving profitability of beef cattle. J. Anim. Sci. 97, 19–28. doi: 10.1093/jas/sky390
Buzanskas, M. E., do Amaral Grossi, D., Ventura, R. V., Schenkel, F. S., Chud, T. C. S., Stafuzza, N. B., et al. (2017). Candidate genes for male and female reproductive traits in Canchim beef cattle. J. Anim. Sci. Biotechnol. 8:67.
Camargo, G. D., Aspilcueta-Borquis, R. R., Fortes, M., Porto-Neto, R., Cardoso, D. F., Santos, D., et al. (2015). Prospecting major genes in dairy buffaloes. BMC Genomics 16:872. doi: 10.1186/s12864-015-1986-2
Cammack, K., Thomas, M., and Enns, R. (2009). Reproductive traits and their heritabilities in beef cattle. Prof. Anim. Sci. 25, 517–528. doi: 10.15232/s1080-7446(15)30753-1
Canaza-Cayo, A. W., Lopes, P. S., Cobuci, J. A., Martins, M. F., and Silva, M. V. G. B. D. (2018). Genetic parameters of milk production and reproduction traits of Girolando cattle in Brazil. Ital. J. Anim. Sci. 17, 22–30. doi: 10.1080/1828051x.2017.1335180
Cassell, B. G. (2009). Using Heritability for Genetic Improvement. Blacksburg: Virginia Cooperative Extension.
Cervantes, I., Gutiérrez, J. P., Fernández, I., and Goyache, F. (2010). Genetic relationships among calving ease, gestation length, and calf survival to weaning in the Asturiana de los Valles beef cattle breed. J. Anim. Sci. 88, 96–101. doi: 10.2527/jas.2009-2066
Changhee, D., Nidarshani, W., Kwanghyun, C., Yunho, C., Taejeong, C., Byungho, P., et al. (2013). The effect of age at first calving and calving interval on productive life and lifetime profit in korean holsteins. Asian Austral. J. Anim. Sci. 26, 1511–1517. doi: 10.5713/ajas.2013.13105
Chegini, A., Hossein-Zadeh, N. G., Moghaddam, S. H. H., and Shadparvar, A. A. (2019a). Genetic aspects of some reproductive, udder health and energy status traits in Holstein cows. Theriogenology 130, 1–7. doi: 10.1016/j.theriogenology.2019.02.027
Chegini, A., Shadparvar, A. A., Hossein-Zadeh, N. G., and Mohammad-Nazari, B. (2019b). Genetic and environmental relationships among milk yield, persistency of milk yield, somatic cell count and calving interval in Holstein cows. Rev. Colomb. Ciencias Pecuarias 32, 81–89. doi: 10.17533/udea.rccp.v32n2a01
Cochran, S. D., Cole, J. B., Null, D. J., and Hansen, P. J. (2013). Discovery of single nucleotide polymorphisms in candidate genes associated with fertility and production traits in Holstein cattle. BMC Genet. 14:49. doi: 10.1186/1471-2156-14-49
Costa, E. V., Ventura, H. T., Veroneze, R., Silva, F. F., Pereira, M. A., and Lopes, P. S. (2020). Estimated genetic associations among reproductive traits in Nellore cattle using Bayesian analysis. Anim. Reprod. Sci. 214:106305. doi: 10.1016/j.anireprosci.2020.106305
Costa, R. B., Irano, N., Diaz, I. D. P. S., Takada, L., Da Costa Hermisdorff, I., Carvalheiro, R., et al. (2019). Prediction of genomic breeding values for reproductive traits in nellore heifers. Theriogenology 125, 12–17. doi: 10.1016/j.theriogenology.2018.10.014
Cribiu, E. P., Di Berardino, D., Di Meo, G. P., Eggen, A., Gallagher, D. S., Gustavsson, I., et al. (2001). International system for chromosome nomenclature of domestic Bovids (ISCNDB 2000). Cytogenet. Cell Genet. 92, 283–299. doi: 10.1159/000056917
Darvasi, A., Weinreb, A., Minke, V., Weller, J., and Soller, M. (1993). Detecting marker-QTL linkage and estimating QTL gene effect and map location using a saturated genetic map. Genetics 134, 943–951. doi: 10.1093/genetics/134.3.943
de Araujo Neto, F. R., Takada, L., Dos Santos, D. J. A., Aspilcueta-Borquis, R. R., Cardoso, D. F., Do Nascimento, A. V., et al. (2020). Identification of genomic regions related to age at first calving and first calving interval in water buffalo using single-step GBLUP. Reprod. Domest. Anim. 55, 1565–1572. doi: 10.1111/rda.13811
de Rezende, M. P. G., Malhado, C. H. M., Biffani, S., Carneiro, P. L. S., Carrillo, J. A., and Bozzi, R. (2020). Genotype-environment interaction for age at first calving in Limousine and Charolais cattle raised in Italy, employing reaction norm model. Livestock Sci. 232:103912. doi: 10.1016/j.livsci.2019.103912
Dekkers, J. (1991). Estimation of economic values for dairy cattle breeding goals: bias due to sub-optimal management policies. Livestock Prod. Sci. 29, 131–149. doi: 10.1016/0301-6226(91)90062-u
do Amaral Grossi, D., Berton, M. P., Buzanskas, M. E., Chud, T. C. S., Grupioni, N. V., De Paz, C. C. P., et al. (2016). Genetic analysis on accumulated productivity and calving intervals in Nelore cattle. Trop. Anim. Health Prod. 48, 207–210. doi: 10.1007/s11250-015-0915-3
Doyle, S., Golden, B., Green, R., and Brinks, J. (2000). Additive genetic parameter estimates for heifer pregnancy and subsequent reproduction in Angus females. J. Anim. Sci. 78, 2091–2098. doi: 10.2527/2000.7882091x
Du, C., Deng, T., Zhou, Y., Ye, T., Zhou, Z., Zhang, S., et al. (2019). Systematic analyses for candidate genes of milk production traits in water buffalo (Bubalus Bubalis). Anim. Genet. 50, 207–216. doi: 10.1111/age.12739
Effa, K., Wondatir, Z., Dessie, T., and Haile, A. (2011). Genetic and environmental trends in the long-term dairy cattle genetic improvement programmes in the central tropical highlands of Ethiopia. J. Cell Anim. Biol. 5, 96–104.
Egger-Danner, C., Kadlečík, O., Fuerst, C., and Kasarda, R. (2005). “Joint genetic evaluation for functional longevity for Pinzgau cattle,” in Proceedings of the 56th Annual Meeting of the European Association for Animal Production, Uppsala.
El-Bramony, M. M. (2011). Genetic and phenotypic parameters of milk yield and reproductive performance in the first three lactations of Egyptian buffalo. Egypt. J. Anim. Prod. 48, 1–10. doi: 10.21608/ejap.2011.94365
El-Bramony, M. M., and Reclamation, D. (2014). Estimation of genetic and phenotypic parameters for milk yield, lactation length, calving interval and body weight in the first lactation of Egyptian buffalo. Life Sci. J. 11, 1012–1019.
Elzo, M., Mateescu, R., Rae, D., Carr, C., Scheffler, T., Scheffler, J., et al. (2018). “Genomic-polygenic EBV for reproduction, ultrasound-carcass, and tenderness traits in the Florida multibreed Brahman-Angus population,” in Proceedings of the World Congress on Genetics Applied to Livestock Production, Rome, 3–7.
Engle, B. N., Corbet, N. J., Allen, J. M., Laing, A. R., Fordyce, G., Mcgowan, M. R., et al. (2019). Multivariate genomic predictions for age at puberty in tropically adapted beef heifers. J. Anim. Sci. 97, 90–100. doi: 10.1093/jas/sky428
Faraji-Arough, H., and Rokouei, M. (2016). Bayesian inference of genetic parameters for reproductive traits in Sistani native cows using Gibbs sampling. J. Livestock Sci. Technol. 4, 39–49.
Fernando, R., and Grossman, M. (1989). Marker assisted selection using best linear unbiased prediction. Genet. Select. Evol. 21, 1–11.
Forni, S., and Albuquerque, L. (2005). Estimates of genetic correlations between days to calving and reproductive and weight traits in Nelore cattle. J. Anim. Sci. 83, 1511–1515. doi: 10.2527/2005.8371511x
Fortes, M., Lehnert, S., Bolormaa, S., Reich, C., Fordyce, G., Corbet, N., et al. (2012). Finding genes for economically important traits: Brahman cattle puberty. Anim. Product. Sci. 52, 143–150. doi: 10.1071/an11165
Fortes, M. R., Reverter, A., Kelly, M., Mcculloch, R., and Lehnert, S. A. (2013). Genome-wide association study for inhibin, luteinizing hormone, insulin-like growth factor 1, testicular size and semen traits in bovine species. Andrology 1, 644–650. doi: 10.1111/j.2047-2927.2013.00101.x
Forutan, M., Mahyari, S., Schenkel, F., and Sargolzaei, M. (2018). Improving genomic evaluation of Holstein cattle using a haplotype-based relationship matrix. Iran. J. Anim. Sci. Res. 10, 393–402.
Gaddis, K. L. P., Null, D. J., and Cole, J. B. (2016). Explorations in genome-wide association studies and network analyses with dairy cattle fertility traits. J. Dairy Sci. 99, 6420–6435. doi: 10.3168/jds.2015-10444
Gaddis, K. P., Dikmen, S., Null, D., Cole, J., and Hansen, P. (2017). Evaluation of genetic components in traits related to superovulation, in vitro fertilization, and embryo transfer in Holstein cattle. J. Dairy Sci. 100, 2877–2891. doi: 10.3168/jds.2016-11907
Galsar, N. S., Shah, R., Pandey, J. P. D., and Prajapati, K. (2016). 7. ANALYSIS OF FIRST PRODUCTION AND REPRODUCTION TRAITS OF MEHSANA BUFFALOES MAINTAINED AT TROPICAL AND SEMI-ARID REGION OF GUJARAT, INDIA by NIRALI S. GALSAR 1, RR SHAH 2, JAY PRAKASH GUPTA 3, DP PANDEY 4, K. B. Life Sci. Leaflets 77, 65–75.
García-Ruiz, A., Cole, J. B., Vanraden, P. M., Wiggans, G. R., Ruiz-López, F. J., and Van Tassell, C. P. (2016). Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc. Natl. Acad. Sci. U.S.A. 113, E3995–E4004.
Georges, M., Nielsen, D., Mackinnon, M., Mishra, A., Okimoto, R., Pasquino, A. T., et al. (1995). Mapping quantitative trait loci controlling milk production in dairy cattle by exploiting progeny testing. Genetics 139, 907–920. doi: 10.1093/genetics/139.2.907
Goddard, M. (1996). The use of marker haplotypes in animal breeding schemes. Genet. Select. Evol. 28, 161–176. doi: 10.1186/1297-9686-28-2-161
Gonzálezrecio, O., and Alenda, R. (2005). Genetic parameters for female fertility traits and a fertility index in Spanish dairy cattle. J. Dairy Sci. 88, 3282–3289. doi: 10.3168/jds.s0022-0302(05)73011-3
Goodling, R. Jr., Shook, G., Weigel, K., and Zwald, N. (2005). The effect of synchronization on genetic parameters of reproductive traits in dairy cattle. J. Dairy Sci. 88, 2217–2225. doi: 10.3168/jds.s0022-0302(05)72897-6
Goyache, F., and Gutiérrez, J. P. (2001). Heritability of reproduetive traits in Asturiana de los Valles beef cattle breed. Arch. Anim. Breed. 44, 489–496. doi: 10.5194/aab-44-489-2001
Goyache, F., Gutiérrez, J. P., Fernández, I., Royo, L., and Álvarez, I. (2005). Genetic analysis of days open in beef cattle. Livestock Prod. Sci. 93, 283–289. doi: 10.1016/j.livprodsci.2004.10.002
Guarini, A., Lourenco, D., Brito, L., Sargolzaei, M., Baes, C. F., Miglior, F., et al. (2018). Comparison of genomic predictions for lowly heritable traits using multi-step and single-step genomic best linear unbiased predictor in Holstein cattle. J. Dairy Sci. 101, 8076–8086. doi: 10.3168/jds.2017-14193
Gupta, J. P., Sachdeva, G. K., Gandhi, R., and Chakaravarty, A. (2015). Developing multiple-trait prediction models using growth and production traits in Murrah buffalo. Buffalo Bull. 34, 347–355.
Gutiérrez, J. P., Álvarez, I., Fernández, I., Royo, L., Dıez, J., and Goyache, F. (2002). Genetic relationships between calving date, calving interval, age at first calving and type traits in beef cattle. Livestock Prod. Sci. 78, 215–222. doi: 10.1016/s0301-6226(02)00100-8
Heise, J., Stock, K. F., Reinhardt, F., Ha, N. T., and Simianer, H. (2017). Phenotypic and genetic relationships between age at first calving, its component traits, and survival of heifers up to second calving. J. Dairy Sci. 101:S0022030217309967.
Hossein Salimi, M., Hossein-Zadeh, N. G., Shadparvar, A. A., and Eghbal, A. R. (2017). Genetic evaluation of dystocia and its relationship with productive and reproductive traits in Holstein cows. Rev. Colomb. Ciencias Pecuarias 30, 126–137. doi: 10.17533/udea.rccp.v30n2a04
Hu, Z.-L., Park, C. A., and Reecy, J. M. (2019). Building a livestock genetic and genomic information knowledgebase through integrative developments of Animal QTLdb and CorrDB. Nucleic Acids Res. 47, D701–D710.
Jaton, C., Koeck, A., Sargolzaei, M., Malchiodi, F., Price, C., Schenkel, F., et al. (2016a). Genetic analysis of superovulatory response of Holstein cows in Canada. J. Dairy Sci. 99, 3612–3623. doi: 10.3168/jds.2015-10349
Jaton, C., Koeck, A., Sargolzaei, M., Price, C., Baes, C., Schenkel, F., et al. (2016b). Genetic correlations between number of embryos produced using in vivo and in vitro techniques in heifer and cow donors. J. Dairy Sci. 99, 8222–8226. doi: 10.3168/jds.2016-11356
Jaton, C., Schenkel, F., Chud, T., Malchiodi, F., Sargolzaei, M., Price, C., et al. (2020). Genetic and genomic analyses of embryo production in dairy cattle. Reprod. Fertil. Dev. 32, 50–55. doi: 10.1071/rd19275
Johnston, D., Barwick, S., Corbet, N., Fordyce, G., Holroyd, R., Williams, P., et al. (2009). Genetics of heifer puberty in two tropical beef genotypes in northern Australia and associations with heifer-and steer-production traits. Anim. Prod. Sci. 49, 399–412. doi: 10.1071/ea08276
Kadarmideen, H., and Simm, G. (2002). “Selection responses expected from index selection including disease resistance, fertility and longevity in dairy cattle,” in Proceedings of the 7th World Congress on Genetics Applied to Livestock Production, Montpellier, 01–19.
Kale, D., Yadav, B., and Prasad, J. (2014). DNA polymorphisms at candidate gene loci and their relation with milk production traits in Murrah Buffalo (Bubalus bubalis) Iran. J. Appl. Anim. Sci. 4, 39–43.
Kaupe, B., Brandt, H., Prinzenberg, E., and Erhardt, G. (2007). Joint analysis of the influence of CYP11B1 and DGAT1 genetic variation on milk production, somatic cell score, conformation, reproduction, and productive lifespan in German Holstein cattle. J. Anim. Sci. 85, 11–21. doi: 10.2527/jas.2005-753
Ke, H., Iqbal, A., and Jj, K. (2014). A genome wide association study on age at first calving using high density single nucleotide polymorphism chips in Hanwoo (Bos taurus coreanae). Asian Austral. J. Anim. Sci. 27, 1406–1410. doi: 10.5713/ajas.2014.14273
Kluska, S., Olivieri, B. F., Bonamy, M., Chiaia, H. L. J., Feitosa, F. L. B., Berton, M. P., et al. (2018). Estimates of genetic parameters for growth, reproductive, and carcass traits in Nelore cattle using the single step genomic BLUP procedure. Livestock Sci. 216, 203–209. doi: 10.1016/j.livsci.2018.08.015
König, S., Bosselmann, F., and Von Borstel, U. H. S. (2007). Genetic analysis of traits affecting the success of embryo transfer in dairy cattle. J. Dairy Sci. 90, 3945–3954. doi: 10.3168/jds.2007-0089
Konkruea, T., Koonawootrittriron, S., Elzo, M. A., and Suwanasopee, T. (2019). Accuracy of genomic-polygenic and polygenic breeding values for age at first calving and milk yield in thai multibreed dairy cattle. Ann. Anim. Sci. 19, 633–645. doi: 10.2478/aoas-2019-0032
Kumar, V., Chakravarty, A., Patil, C., Valsalan, J., and Mahajan, A. (2015). Estimate of genetic and non-genetic parameters for age at first calving in Murrah buffalo. Indian J. Anim. Sci. 85, 84–85.
Laodim, T., Elzo, M. A., Koonawootrittriron, S., Suwanasopee, T., and Jattawa, D. (2019). Genomic-polygenic and polygenic predictions for milk yield, fat yield, and age at first calving in Thai multibreed dairy population using genic and functional sets of genotypes. Livestock Sci. 219, 17–24. doi: 10.1016/j.livsci.2018.11.008
Lázaro, S. F., Varona, L., Silva, F. F., Ventura, H. T., Veroneze, R., Brito, L. C., et al. (2019). Censored Bayesian models for genetic evaluation of age at first calving in Brazilian Brahman cattle. Livestock Sci. 221, 177–180. doi: 10.1016/j.livsci.2018.11.014
Lee, D., and Han, K. (2004). Genetic relationship between milk production, calving ease and days open at first parity in Holstein cows. Asian Austral. J. Anim. Sci. 17, 153–158. doi: 10.5713/ajas.2004.153
Lett, B. M., and Kirkpatrick, B. W. (2018). Short communication: heritability of twinning rate in Holstein cattle. J. Dairy Sci. 101, 4307-4311.
Li, J., Liu, J., Campanile, G., Plastow, G., Zhang, C., Wang, Z., et al. (2018a). Novel insights into the genetic basis of buffalo reproductive performance. BMC Genomics 19:814. doi: 10.1186/s12864-018-5208-6
Li, J., Liu, J., Liu, S., Plastow, G., Zhang, C., Wang, Z., et al. (2018b). Integrating RNA-seq and GWAS reveals novel genetic mutations for buffalo reproductive traits. Anim. Reprod. Sci. 197, 290–295. doi: 10.1016/j.anireprosci.2018.08.041
Liu, J. J., Liang, A. X., Campanile, G., Plastow, G., Zhang, C., Wang, Z., et al. (2018). Genome-wide association studies to identify quantitative trait loci affecting milk production traits in water buffalo. J. Dairy Sci. 101, 433–444. doi: 10.3168/jds.2017-13246
Lopes, F., Wu, X. L., Li, H., Xu, J., Perkins, T., Genho, J., et al. (2018). Improving accuracy of genomic prediction in Brangus cattle by adding animals with imputed low-density SNP genotypes. J. Anim. Breed. Genet. 135, 14–27. doi: 10.1111/jbg.12312
Lopez, B. I., Son, J.-H., Seo, K., and Lim, D. (2019). Estimation of genetic parameters for reproductive traits in Hanwoo (Korean Cattle). Animals 9:715. doi: 10.3390/ani9100715
Low, W. Y., Tearle, R., Bickhart, D. M., Rosen, B. D., Kingan, S. B., Swale, T., et al. (2019). Chromosome-level assembly of the water buffalo genome surpasses human and goat genomes in sequence contiguity. Nat. Commun. 10, 1–11.
Luan, T., Woolliams, J. A., Lien, S., Kent, M., Svendsen, M., and Meuwissen, T. H. (2009). The accuracy of genomic selection in Norwegian red cattle assessed by cross-validation. Genetics 183, 1119–1126. doi: 10.1534/genetics.109.107391
Macgregor, R. (1941). The domestic buffalo. Vet. Rec. 53, 443–450. doi: 10.7313/upo9781907284991.034
Makgahlela, M., Banga, C., Norris, D., Dzama, K., and Ngambi, J. (2008). Genetic analysis of age at first calving and calving interval in South African Holstein cattle. Asian J. Anim. Vet. Adv. 3, 197–205. doi: 10.3923/ajava.2008.197.205
Martínez-Velázquez, G., Ríos-Utrera, A., Román-Ponce, S., Baeza-Rodríguez, J., Arechavaleta-Velasco, M., Montaño-Bermúdez, M., et al. (2020). Genetic correlations between scrotal circumference, heifer fertility and stayability in Charolais–Charbray cattle. Livestock Sci. 232:103914. doi: 10.1016/j.livsci.2019.103914
Maryam, K., Beiginassiri, M., Nejad, A. N., Chaji, M., Roshanfekr, H., and Nazari, B. M. (2016). Genetic parameter estimation of dystocia variable in iranian holstein dairy cattle. Basrah J. Vet. Res. 15:4.
McClure, M., Morsci, N., Schnabel, R., Kim, J., Yao, P., Rolf, M., et al. (2010). A genome scan for quantitative trait loci influencing carcass, post-natal growth and reproductive traits in commercial Angus cattle. Anim. Genet. 41, 597–607. doi: 10.1111/j.1365-2052.2010.02063.x
Meuwissen, T. H., Hayes, B. J., and Goddard, M. E. (2001). Prediction of total genetic value using genome-wide dense marker maps. Genetics 157, 1819–1829.
Michelizzi, V. N., Dodson, M. V., Pan, Z., Amaral, M. E. J., Michal, J. J., Mclean, D. J., et al. (2010). Water buffalo genome science comes of age. Int. J. Biol. Sci. 6:333. doi: 10.7150/ijbs.6.333
Miller, J., Thomsen, P., Dixon, S., Tucker, E., Konfortov, B., and Harbitz, I. (1992). Synteny mapping of the bovine IGHG2, CRC and IGF1 genes. Anim. Genet. 23, 51–58. doi: 10.1111/j.1365-2052.1992.tb00017.x
Mintoo, A. A., Zhang, H., Chen, C., Moniruzzaman, M., Deng, T., Anam, M., et al. (2019). Draft genome of the river water buffalo. Ecol. Evol. 9, 3378–3388. doi: 10.1002/ece3.4965
Moioli, B., Steri, R., Marchitelli, C., Catillo, G., and Buttazzoni, L. (2017). Genetic parameters and genome-wide associations of twinning rate in a local breed, the Maremmana cattle. Animal 11, 1660–1666. doi: 10.1017/s1751731117000283
Montaldo, H., Trejo, C., and Lizana, C. (2017). Genetic parameters for milk yield and reproduction traits in the Chilean Dairy Overo Colorado cattle breed. Ciencia Investig. Agraria 44, 24–34.
Morammazi, S., Torshizi, R., Rouzbehan, Y., and Sayyadnejad, M. (2007). PosterEstimates of genetic parameters for production and reproduction traits in Khuzestan buffalos of Iran. Italian J. Anim. Sci. 6, 421–424. doi: 10.4081/ijas.2007.s2.421
Morris, C., Amyes, N., and Hickey, S. (2011). Responses of prolactin and hair growth to selection for age at puberty in Angus cattle. Animal 5, 198–201. doi: 10.1017/s1751731110001825
Morris, C., Wilson, J., Bennett, G., Cullen, N., Hickey, S., and Hunter, J. (2000). Genetic parameters for growth, puberty, and beef cow reproductive traits in a puberty selection experiment. N. Zeal. J. Agric. Res. 43, 83–91. doi: 10.1080/00288233.2000.9513411
Mota, R., Guimarães, S., Fortes, M., Hayes, B., Silva, F., Verardo, L., et al. (2017). Genome-wide association study and annotating candidate gene networks affecting age at first calving in Nellore cattle. J. Anim. Breed. Genet. 134, 484–492. doi: 10.1111/jbg.12299
Mota, R. R., E Silva, F. F., Guimarães, S. E. F., Hayes, B., Fortes, M. R. S., Kelly, M. J., et al. (2018). Benchmarking Bayesian genome enabled-prediction models for age at first calving in Nellore cows. Livestock Sci. 211, 75–79. doi: 10.1016/j.livsci.2018.03.009
Müller, M. P., Rothammer, S., Seichter, D., Russ, I., Hinrichs, D., Tetens, J., et al. (2017). Genome-wide mapping of 10 calving and fertility traits in Holstein dairy cattle with special regard to chromosome 18. J. Dairy Sci. 100, 1987–2006. doi: 10.3168/jds.2016-11506
Nascimento, A. V., Matos, M. C., Seno, L. O., Romero, A. R., Garcia, J. F., and Grisolia, A. B. (2016). Genome wide association study on early puberty in Bos indicus. Genet. Mol. Res. 15, 1–6.
Nayeri, S., Sargolzaei, M., Abo-Ismail, M. K., May, N., Miller, S. P., Schenkel, F., et al. (2016). Genome-wide association for milk production and female fertility traits in Canadian dairy Holstein cattle. BMC Genet. 17:75. doi: 10.1186/s12863-016-0386-1
Neves, H. H., Carvalheiro, R., O’brien, A. M. P., Utsunomiya, Y. T., Do Carmo, A. S., Schenkel, F. S., et al. (2014). Accuracy of genomic predictions in Bos indicus (Nellore) cattle. Genet. Select. Evol. 46:17. doi: 10.1186/1297-9686-46-17
Oyama, K., Katsuta, T., Anada, K., and Mukai, F. (2002). Heritability and repeatability estimates for reproductive traits of Japanese Black cows. Asian Austral. J. Anim. Sci. 15, 1680–1685. doi: 10.5713/ajas.2002.1680
Peixoto, M., Pereira, C., Bergmann, J., Penna, V., and Fonseca, C. (2004). Genetic parameters of multiple ovulation traits in Nellore females. Theriogenology 62, 1459–1464. doi: 10.1016/j.theriogenology.2004.02.019
Piccoli, M. L., Brito, L. F., Braccini, J., Oliveira, H. R., Cardoso, F. F., Roso, V. M., et al. (2020). Comparison of genomic prediction methods for evaluation of adaptation and productive efficiency traits in Braford and Hereford cattle. Livestock Sci. 231:103864. doi: 10.1016/j.livsci.2019.103864
Piper, L., Bindon, B., Swan, A., and Brewer, H. (2017). Genetic selection for litter size in cattle. Proc. Assoc. Advmt. Breed. Genet. 21, 101–105.
Rathod, A., Vaidya, M., and Ali, S. S. (2018). Genetic studies of productive and reproductive attributes of surti buffalo in Maharashtra. Int. J. Livestock Res. 8, 309–314. doi: 10.5455/ijlr.20171016061752
Roxström, A., and Strandberg, E. (2002). Genetic analysis of functional, fertility-, mastitis-, and production-determined length of productive life in Swedish dairy cattle. Livestock Prod. Sci. 74, 125–135. doi: 10.1016/s0301-6226(01)00300-1
Saatchi, M., McClure, M. C., Mckay, S. D., Rolf, M. M., Kim, J., Decker, J. E., et al. (2011). Accuracies of genomic breeding values in American Angus beef cattle using K-means clustering for cross-validation. Genet. Select. Evol. 43:40. doi: 10.1186/1297-9686-43-40
Saowaphak, P., Duangjinda, M., Plaengkaeo, S., Suwannasing, R., and Boonkum, W. (2017). Genetic correlation and genome-wide association study (GWAS) of the length of productive life, days open, and 305-days milk yield in crossbred Holstein dairy cattle. Genet. Mol. Res. 16:16029091.
Schmidt, P., Campos, G., Roso, V., Souza, F., and Boligon, A. (2019). Genetic analysis of female reproductive efficiency, scrotal circumference and growth traits in Nelore cattle. Theriogenology 128, 47–53. doi: 10.1016/j.theriogenology.2019.01.032
Seno, L. O., Cardoso, V. L., El-Faro, L., Sesana, R. C., Aspilcueta-Borquis, R. R., Camargo, G. M. F. D., et al. (2010). Genetic parameters for milk yield, age at first calving and interval between first and second calving in milk buffaloes. Ital. J. Anim. Sci. 6, 397–400. doi: 10.4081/ijas.2007.s2.397
Setiaji, A., and Oikawa, T. (2019). Genetic parameters of reproductive traits from artificial insemination records of Japanese Black cows. Livestock Sci. 229, 85–89. doi: 10.1016/j.livsci.2019.09.018
Silva, M. V., Dos Santos, D. J., Boison, S. A., Utsunomiya, A. T., Carmo, A. S., Sonstegard, T. S., et al. (2014). The development of genomics applied to dairy breeding. Livestock Sci. 166, 66–75.
Silvestre, A., Martins, Â, Santos, V., and Colaço, J. (2019). Genetic parameters of calving ease in dairy cattle using threshold and linear models. Ital. J. Anim. Sci. 18, 80–87. doi: 10.1080/1828051x.2018.1482801
Tang, K.-Q., Li, S.-J., Yang, W.-C., Yu, J.-N., Han, L., Li, X., et al. (2011). An MspI polymorphism in the inhibin alpha gene and its associations with superovulation traits in Chinese Holstein cows. Mol. Biol. Rep. 38, 17–21. doi: 10.1007/s11033-010-0072-8
Tarekegn, G., Gullstrand, P., Strandberg, E., Båge, R., Rius-Vilarrasa, E., Christensen, J., et al. (2019). Genetic parameters of endocrine fertility traits based on in-line milk progesterone profiles in Swedish Red and Holstein dairy cows. J. Dairy Sci. 102, 11207–11216. doi: 10.3168/jds.2019-16691
Thiruvenkadan, A., Panneerselvam, S., Rajendran, R., and Murali, N. (2010). Analysis on the productive and reproductive traits of Murrah buffalo cows maintained in the coastal region of India. Appl. Anim. Husbandry Rural Dev. 3, 1–5. doi: 10.21608/jpd.2006.45174
Tiezzi, F., Arceo, M. E., Cole, J. B., and Maltecca, C. (2018). Including gene networks to predict calving difficulty in holstein, brown swiss and jersey cattle. BMC Genet. 19:20. doi: 10.1186/s12863-018-0606-y
Toghiani, S. (2012). Genetic relationships between production traits and reproductive performance in Holstein dairy cows. Arch. Anim. Breed. 55, 458–468. doi: 10.5194/aab-55-458-2012
Toghiani, S., Hay, E., Sumreddee, P., Geary, T., Rekaya, R., and Roberts, A. (2017). Genomic prediction of continuous and binary fertility traits of females in a composite beef cattle breed. J. Anim. Sci. 95, 4787–4795. doi: 10.2527/jas2017.1944
Tramonte, N. C., Grupioni, N. V., Stafuzza, N. B., Guidolin, D. G. F., Savegnago, R. P., Bezerra, L. A. F., et al. (2019). Genetic parameters, genetic trends, and principal component analysis for productive and reproductive traits of Guzera beef cattle. Rev. Bras. Zootecnia 48:34.
Ulbrich, F., and Fischer, H. (1966). The Chromosomes of the Asiatic Buffalo (Bubalus bubalis) and the African Buffalo (Cyncerus caffer). Hoboken, NJ: Wiley Online Library.
van der Linde, C., De Jong, G., Simai, S., Gombacsi, P., and Wellisch, P. (2006). Genetic evaluation for longevity in Hungary. Interbull. Bull. 3:35.
Vanderick, S., Troch, T., Gillon, A., Glorieux, G., and Gengler, N. (2015). Genetic parameters for direct and maternal calving ease in Walloon dairy cattle based on linear and threshold models. J. Anim. Breed. Genet. 131, 513–521. doi: 10.1111/jbg.12105
Vinothraj, S., Subramaniyan, A., Venkataramanan, R., Joseph, C., and Sivaselvam, S. (2016). Genetic evaluation of reproduction performance of Jersey× Red Sindhi crossbred cows. Vet. World 9:1012. doi: 10.14202/vetworld.2016.1012-1017
Warburton, C. L., Engle, B. N., Ross, E. M., Costilla, R., Moore, S. S., Corbet, N. J., et al. (2020). Use of whole-genome sequence data and novel genomic selection strategies to improve selection for age at puberty in tropically-adapted beef heifers. Genet. Select. Evol. 52, 1–13.
Weller, J., Ezra, E., and Ron, M. (2017). Invited review: A perspective on the future of genomic selection in dairy cattle. J. Dairy Sci. 100, 8633–8644. doi: 10.3168/jds.2017-12879
Weller, J. I., Golik, M., Seroussi, E., Ron, M., and Ezra, E. (2008). Detection of quantitative trait loci affecting twinning rate in Israeli Holsteins by the daughter design. J. Dairy Sci. 91, 2469–2474. doi: 10.3168/jds.2007-0915
Williams, J. L., Iamartino, D., Pruitt, K. D., Sonstegard, T., Smith, T. P., Low, W. Y., et al. (2017). Genome assembly and transcriptome resource for river buffalo, Bubalus bubalis (2 n= 50). Gigascience 6:gix088.
Yang, W. C., Yang, L. G., Riaz, H., Tang, K. Q., Chen, L., and Li, S. J. (2013). Effects in cattle of genetic variation within the IGF1R gene on the superovulation performance and pregnancy rates after embryo transfer. Anim. Reprod. Sci. 143, 24–29. doi: 10.1016/j.anireprosci.2013.10.008
Yutaka, M., Toshimi, B., and Mitsuyoshi, S. (2015). Genetic analysis of twinning rate and milk yield using a threshold-linear model in Japanese Holsteins. Anim. Sci. J. 86, 31–36. doi: 10.1111/asj.12236
Zhang, Y., Johnston, D., Bolormaa, S., Hawken, R., and Tier, B. (2014). Genomic selection for female reproduction in Australian tropically adapted beef cattle. Anim. Prod. Sci. 54, 16–24. doi: 10.1071/an13016
Keywords: reproduction, breeding, genetic improvement, heritability, GWAS
Citation: Shao B, Sun H, Ahmad MJ, Ghanem N, Abdel-Shafy H, Du C, Deng T, Mansoor S, Zhou Y, Yang Y, Zhang S, Yang L and Hua G (2021) Genetic Features of Reproductive Traits in Bovine and Buffalo: Lessons From Bovine to Buffalo. Front. Genet. 12:617128. doi: 10.3389/fgene.2021.617128
Received: 14 October 2020; Accepted: 25 February 2021;
Published: 23 March 2021.
Edited by:
Fabyano Fonseca Silva, Universidade Federal de Viçosa, BrazilReviewed by:
Sirlene Fernandes Lazaro, Purdue University, United StatesMahdi Mokhber, Urmia University, Iran
Copyright © 2021 Shao, Sun, Ahmad, Ghanem, Abdel-Shafy, Du, Deng, Mansoor, Zhou, Yang, Zhang, Yang and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohua Hua, aHVhZ3VvaHVhMDlAZ21haWwuY29t
†These authors have contributed equally to this work
 Baoshun Shao1†
Baoshun Shao1† Hui Sun
Hui Sun Muhammad Jamil Ahmad
Muhammad Jamil Ahmad Nasser Ghanem
Nasser Ghanem Hamdy Abdel-Shafy
Hamdy Abdel-Shafy Tingxian Deng
Tingxian Deng Shahid Mansoor
Shahid Mansoor Shujun Zhang
Shujun Zhang Liguo Yang
Liguo Yang Guohua Hua
Guohua Hua