- 1Department of Neurosurgery, Fujian Provincial Hospital South Branch, Fuzhou, China
- 2Department of Neurosurgery, Shengli Clinical Medical College of Fujian Medical University, Fuzhou, China
- 3Department of Neurosurgery, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 4Department of Neurosurgery, Shanghai General Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Glioblastoma (GBM) is one of the most common and fatal malignancies worldwide, while its prognostic biomarkers are still being explored. This study aims to identify potential genes with clinical and prognostic significance by integrating bioinformatics analysis and investigating their function in HNSCC. Based on the Single-cell RNA sequencing (scRNA-seq) results of H3K27M-glioma cells, computational bioinformatics methods were employed for selecting prognostic biomarker for GBM. The protein NPC2 (NPC Intracellular Cholesterol Transporter 2), which has been shown to be related to lipoprotein metabolism and innate immune system, was identified to be upregulated in GBM. NPC2 showed a relatively higher expression in GBM samples, and a negative correlation with tumor purity and tumor infiltrating immune cells. Additionally, NPC2 was knocked down in U87-MG and U251 cells line, and cell proliferation and migration capability were evaluated with CCK-8, scratch and transwell assay, respectively. Cytological experiments has shown that NPC2 overexpression inhibited GBM cells proliferation and migration, indicating its important role in GBM progression. This is the first investigation into the prognostic value of NPC2 interact with GBM. The potential molecular factor NPC2 have been identified as a prognostic biomarker for GBM.
Introduction
Glioblastoma (GBM) is the most common and lethal primary brain malignancy with standard therapeutic regimen including surgical resection, followed by radiotherapy, with concurrent chemotherapy (temozolomide) and maintenance therapy (temozolomide for 6−12 months) (Abdul et al., 2018; Santangelo et al., 2020). Despite an aggressive multimodal standard therapy, patients with GBM have a dramatically poor prognosis with a median overall survival of only 13−15 months after standard therapy (Geraldo et al., 2019; Albright et al., 2020). The complicated genetic and molecular changes within cancer cells lead to the complex biology of GBM. The Cancer Genome Atlas (TCGA) initiative has provided a higher-resolution picture of driven characterization of rapid advancements in GBM biomarker discovery with distinct expression signatures (Chen Z. et al., 2020). However, the prognosis for GBM patients is still poor. Hence, the discovery of more sensitive prognostic biomarkers for GBM remains crucial. Currently, age, metastasis, and tumor resection have been identified as prognostic factors for GBM (Lakomy et al., 2020; Li et al., 2020; Shen et al., 2020). What’s more, genetic researches have also promoted the disclosure of a few sporadic biomarkers for GBM (Mirchia and Richardson, 2020; Zhao et al., 2020).
The protein NPC2 (NPC Intracellular Cholesterol Transporter 2) is a protein coding gene containing a lipid recognition domain, which has been shown to be related to lipoprotein metabolism and innate immune system pathways. NPC2 plays an important role in regulating the transport of cholesterol through the late endosomal/lysosomal system. Mutations in this gene have been associated with Niemann-Pick disease, type C2, and frontal lobe atrophy (Infante et al., 2008; Gong et al., 2016; Li et al., 2016). In the past decades, more and more researchers have focused on the biological genetic functions of genes involved in the tumorigenesis of GBM with the development of high-throughput sequencing (Diehn et al., 2000; Diboun et al., 2006; Couturier et al., 2020). However, the link between NPC2 and prognosis of GBM has not been reported yet. This is the first investigation into the prognostic value of NPC2 interact with GBM.
The aim of this study is to explore the prognostic and carcinogenic potential of robust molecular signature that can improve the ability to predict GBM prognosis. We downloaded the single-cell RNA sequencing in 3,321 cells from six primary H3K27M-glioma and matched models profiling datasets about GBM tumor and normal tissues from the Gene Expression Omnibus (GEO). Eight overlapping differentially expressed genes between tumor and non-tumor tissues were explored, these novel potential prognostic markers were identified via multiple bioinformatics analysis, including biological process functional annotation and pathway enrichment analysis, as well as gene expression profiling interactive survival analysis. We have investigated the association between gene NPC2 expression and GBM tumorigenesis, and the prognosis role of NPC2 by integrating the GBM samples from the GEO dataset with Single-cell RNA sequencing (scRNA-seq) results of H3K27M-glioma cells. Differential expression, correlation with tumor infiltrates, prognostic significance, cell proliferation, and migration capability were evaluated with CCK-8, scratch wound healing assay, and transwell assay.
Materials and Methods
Data Preprocessing and Screening Differentially Expressed Genes
We downloaded the gene expression profiles of single-cell RNA sequencing in 3,321 cells from six primary H3K27M-glioma and matched models from the Gene Expression Omnibus (GEO)1 with accession number of GSE102130 (Filbin et al., 2018). GSE102130 dataset was composed of single-cell RNA sequencing from 2,458 single cells (K27M glioma patients) and from several models derived from BCH869—including patient derived xenograft (PDX), gliomaspheres (GS), and differentiated glioma cells (DGC). The differentially expressed genes (DEGs) were obtained by comparing the malignant cells with the non-malignant cells based on threshold of p-value <0.05 and absolute value of fold change >2 (Supplementary Table 1).
Comprehensive Analysis
GEPIA is a web server and an online database2. GEPIA adopts a standard processing pipeline to analyze the RNA-Seq expression data from GTEx and TCGA, which included 8,587 normal and 9,736 tumor samples (Subramanian et al., 2005). We obtained samples from TCGA and used GEPIA to analyze the connections between Overall Survival (OS) and Disease Free Survival (DFS) with NPC2 expression in GBM. Additionally, a boxplot using disease state as a variable was graphed to calculate differential expression of NPC2. A p-value <0.05 was considered statistically significant. Our study was in accordance with the publication guidelines provided by TCGA.
Correlation Analysis Between NPC2 and Immune Infiltration
TIMER database was used to detect the tumor immune infiltration using mRNA sequencing (mRNA-Seq) data from TCGA. TIMER applies deconvolution statistical methods previously published to infer the abundance of tumor infiltrating immune cells (TIICs) from gene expression profiles.
Cell Culturing
Human GBM cell lines U87-MG and U251 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cell lines were cultured in minimum essential medium-alpha with 10% heat-inactivated fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin. Humidified incubators were supplied with 95% air atmosphere and 5% carbon dioxide at 37°C.
Cell Transfection
U87-MG and U251 cells at exponential stage were used for transfection. Before transfection, 1 × 106 cells were cultured in 6−well plates with 2 mL complete medium for 24 h until they were 90% confluent. Lentivirus-mediated NPC2 (sh-NPC) and non-targeting shRNA (sh-NC) were obtained from GenePharma (Shanghai, China).
Cell Counting Kit-8 (CCK-8) Assay
Cellular viability was determined by the Cell Counting Kit-8 (Beyotime Biotechnology). After transfection, 100 μL of U87-MG or U251 cells per well were plated into 96-well plates at a density of 500 cells per well. 10 μL of CCK8 solution was incubated with cell medium daily to each well for another 2 h at 37°C. The absorbance of each well was detected at 570 nm by the Multiskan FC Microplate spectrophotometer. Data were presented as mean ± SD and comparisons were calculated by two-way RM ANOVA by the GraphPad Prism.
Scratch Wound Healing Assay
U251 and U87-MG cells were evenly planted in a 6-well plate with 1 × 106 cells per well. The plate was vertically scratched with a 10 μL sterile pipette tip when the cells covered 90% of the plate bottom area. After that, the culture medium in the plate was discarded and gently washed with PBS for three times, and the cell debris residue was rinsed off to make sure the visual field clear during photographing. The culture medium containing 1% FBS was added to the six-well plate. A 3 mm wound was introduced across the diameter of each plate. Cell migration was observed by microscopy at 24 h.
Transwell Assay
Cells in logarithmic growth phase were seeded at the upper transwell chamber insert (Corning, United States) at a density of 2 × 104 cells per well. The chamber was placed in a 24-well plate in which the upper chamber contained serum-free cell culture medium and the lower chamber contained 10% FBS complete medium. The culture was continued for 24 h. The medium was discarded, and stained with a crystal violet solution to observe the number of migrated cells.
Statistical Analysis
Data were presented as mean ± SD and comparisons were calculated by Student’s t test (two sided, unpaired) or two-way RM ANOVA by the GraphPad Prism. Using multivariate Cox analysis, we evaluated NPC2 expression along with other pathological and clinical factors affected OS. A p-value <0.05 for NPC2 expression was set as the threshold. The correlations between clinical characteristics and NPC2 expression were analyzed using logistic regression.
Results
Overall Characteristics of the Cell Cluster by Reanalysis
In this research, GBM cortex of single cell RNA sequencing was sampled from 3,321 cells from six primary H3K27M-glioma and normal models in the GSE102130 dataset. Analyzed by using the Gene Expression Omnibus (GEO) database3, 14 cell clusters (clusters 1−14) were characterized. According to canonical cell biomarkers such as CD14 (Mathews et al., 2019), CX3CR1 (Murai et al., 2020), MBP (García-León et al., 2020), PLP1 (Guo et al., 2020), PROM1 (Bao et al., 2006), CD24 (Tiburcio et al., 2020), three tumor cell types were identified by unsupervised clustering and expression of lineage-specific markers following batch correction in 14 clusters, including microglia, oligodendrocytes, and glioma stem cells (Figure 1).
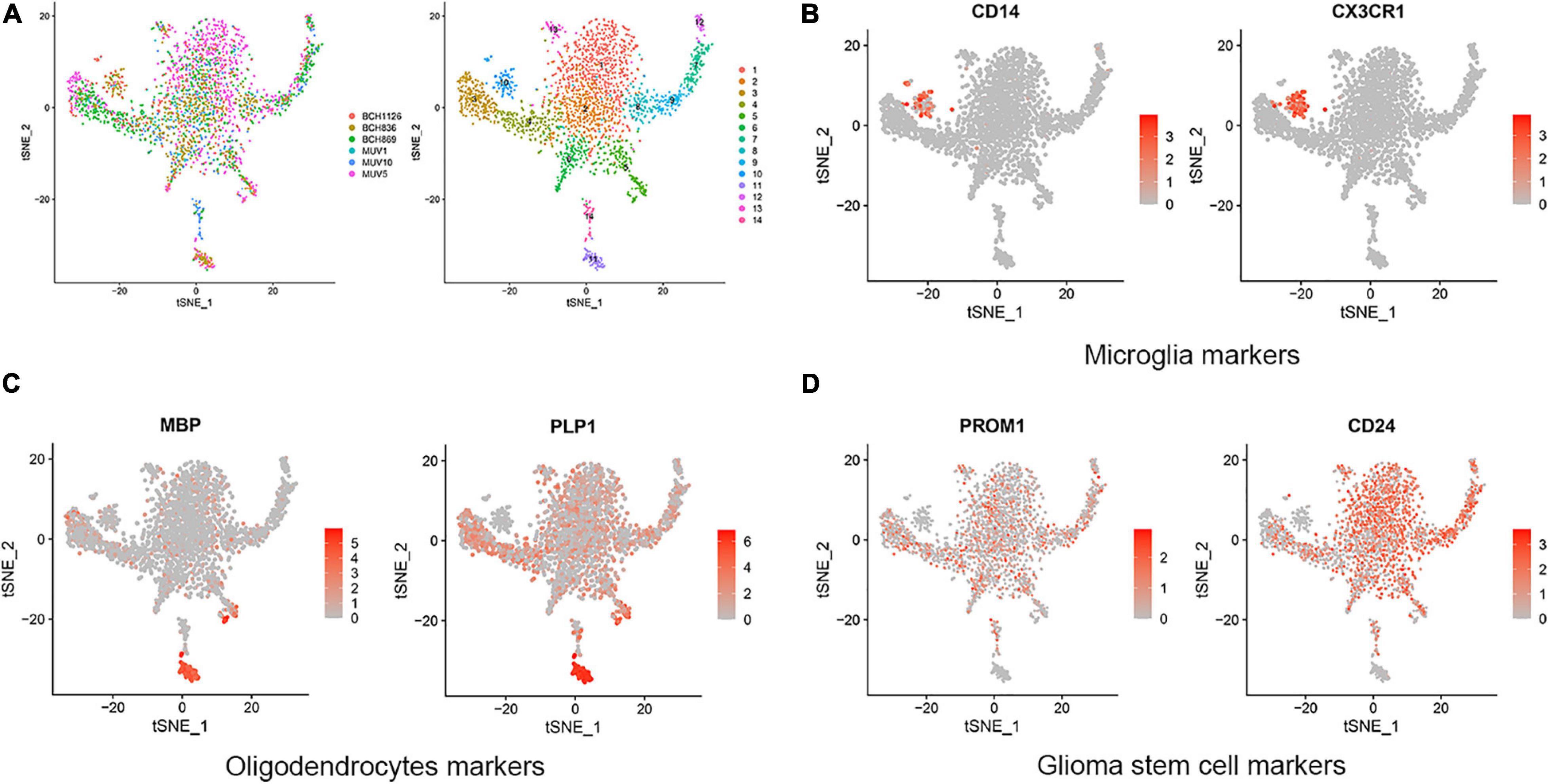
Figure 1. Cell clusters and cell types distribution in six GBM tissue sample. (A) A total of 14 cell clusters was identified and shown with t-SNE maps in six GBM tissue samples. (B–D) T-SNE map showing canonical markers for three cell types: microglia (B), oligodendrocytes (C), and glioma stem cells (D).
Common DEGs in a Single-Cell RNA Sequencing Reanalysis and TCGA Database of GBM
In order to determine the DEGs most highly associated with the prognosis GBM, we reanalyzed single-cell RNA sequencing based on our DEGs, TCGA cohort DEGs, and PRGs of GBM. As a result of which, 17 common DEGs were characterized, as shown in Figure 2A, including APLP1, SOX6, NPC2, ARPC1B, RHOG, IFI30, CYTH4, NNAT, CPQ, LY96, LSP1, TSPAN6, MAN2B1, TM4SF1, CD81, NMB, and MSTN. Then, we compared the OS and DFS maps of the 17 common DEGs and found that the expression trends of eight common DEGs were consistent totally. As shown in Figures 2B,C, including MAN2B1, LSP1, LY96, CPQ, CYTH4, IFI30, ARPC1B, and NPC2.
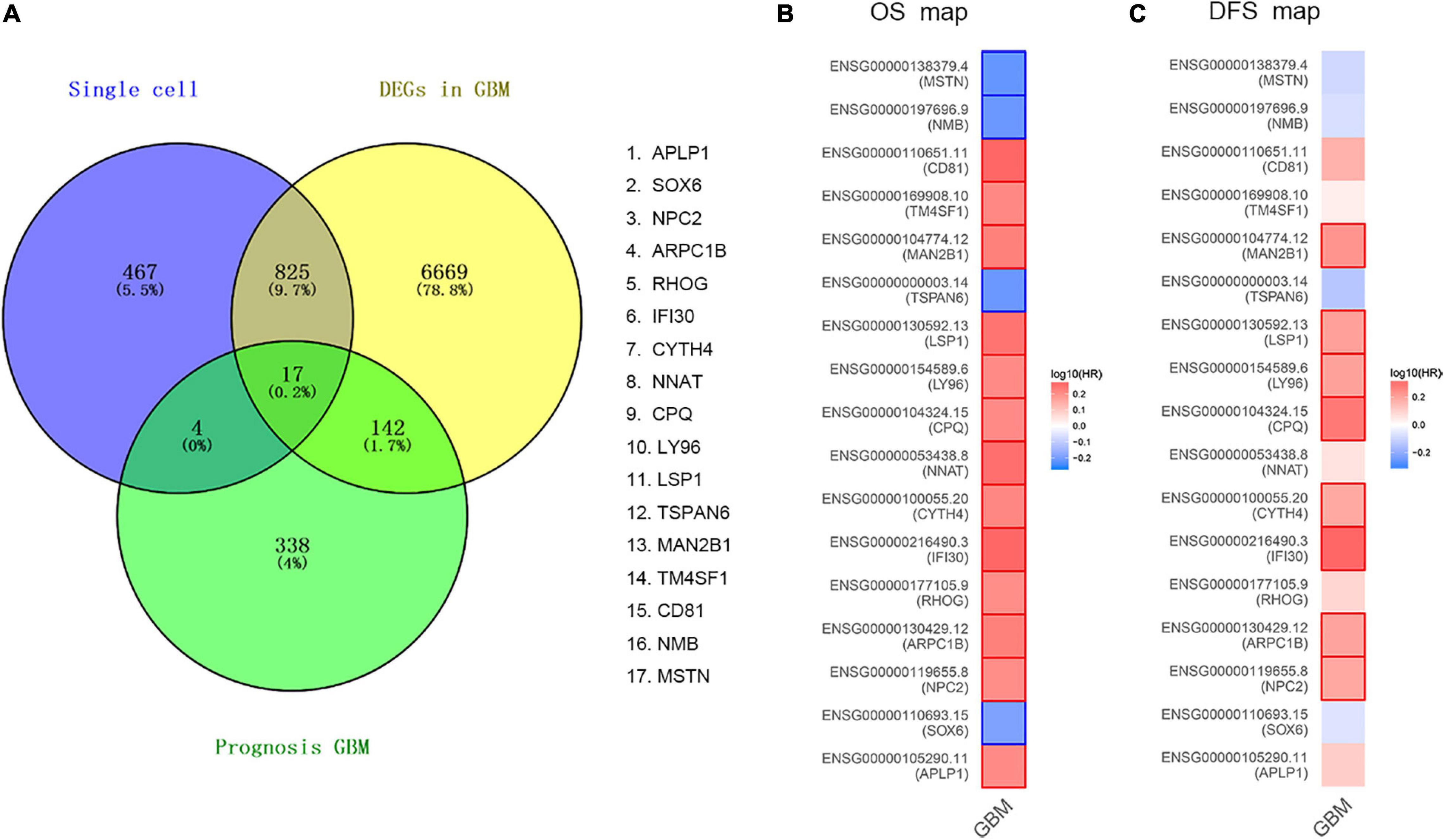
Figure 2. Validation of common DEGs. (A) The common genes between our DEGs, TCGA cohort DEGs, and PRGs of GBM. Different color areas represent different datasets. The cross areas showed the commonly changed DEGs. DEGs were identified with classical t test; statistically significant DEGs were defined with P < 0.001 and | log2 fold change| > 1 as the cut-off criteria. (B) Overall Survival (OS) map of common DEGs. (C) Disease Free Survival (DFS) map of common DEGs.
DEGs Identification
To further dig out the roles of these common DEGs in GBM, we analyzed the relative expression level expression level of the eight common DEGs in normal and GBM tissues based on GEPIA database. We found out that the eight common DEGs mRNA expression were also significantly increased in GBM when compared to the normal group using the GEPIA database (p-value <0.01, |Log2FC| > 1) (Figure 3). What’s more, a strong correlation exists between their mRNA levels and OS or DFS (p-value <0.001, Figure 4), including MAN2B1(OS, HR = 1.6, LogRank P = 0.0093; DFS, HR = 1.7,LogRank P = 0.012), LSP1(OS, HR = 1.7, LogRank P = 0.0028; DFS, HR = 1.6, LogRank P = 0.017), LY96(OS, HR = 1.7, LogRank P = 0.0028; DFS, HR = 1.6, LogRank P = 0.03), CPQ(OS, HR = 1.6, LogRank P = 0.0079; DFS, HR = 1.9, LogRank P = 0.0017), CYTH4(OS, HR = 1.7, LogRank P = 0.0045; DFS, HR = 1.5, LogRank P = 0.0043), IFI30(OS, HR = 1.9, LogRank P = 0.00062; DFS, HR = 2.1, LogRank P = 0.00057), ARPC1B(OS, HR = 1.7, LogRank P = 0.0032; DFS, HR = 1.6, LogRank P = 0.026), and NPC2(OS, HR = 1.7, LogRank P = 0.0046; DFS, HR = 1.6, LogRank P = 0.0032). GBM patients with a higher expression of these eight common DEGs showed a shorter survival rate, compared with patients with a lower gene expression, suggesting these eight genes as hazardous prognosticator in GBM patients.
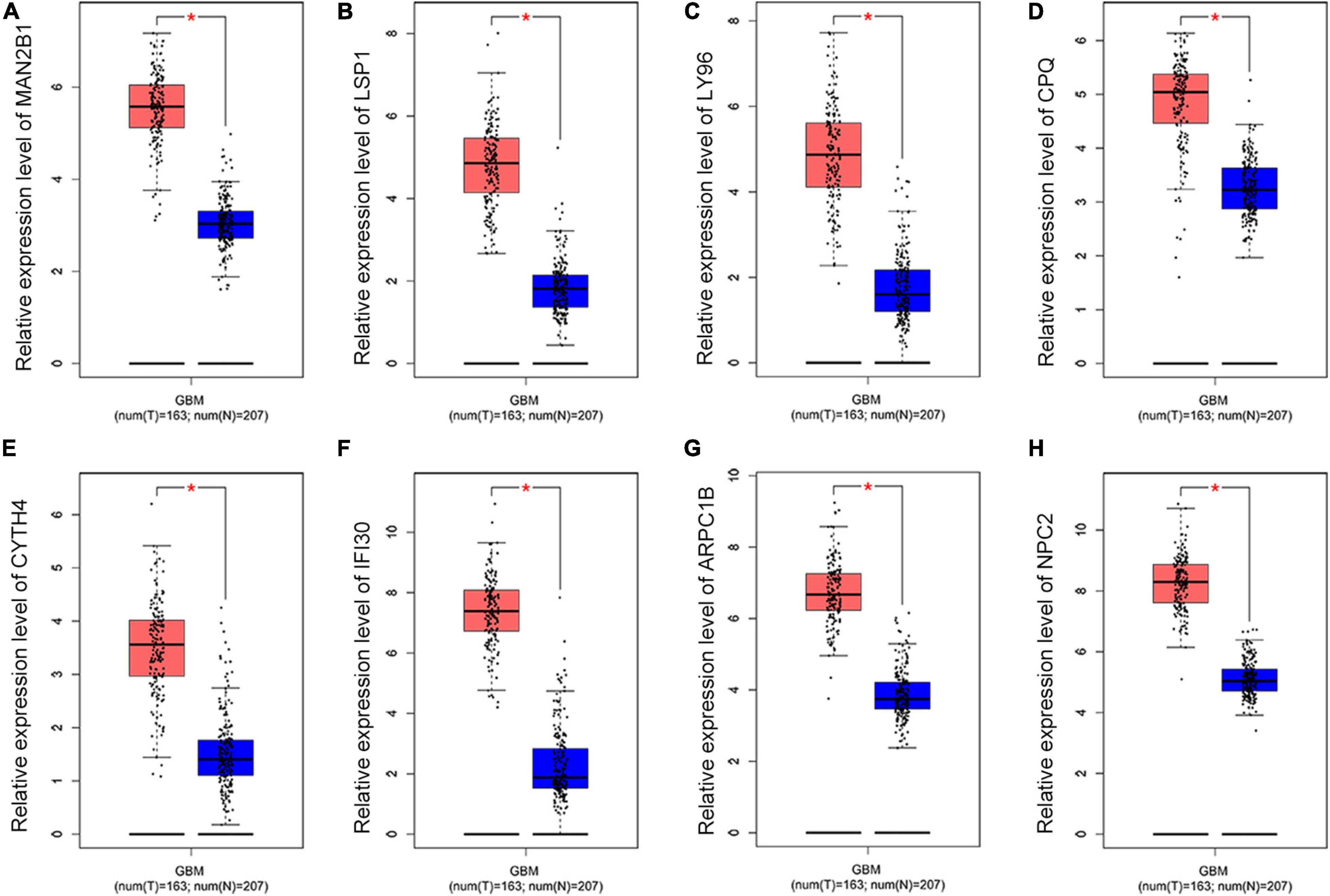
Figure 3. The relative expression level of eight common DEGs. Boxplots illustrating the relative expression level of (A) MAN2B1; (B) LSP1; (C) LY96; (D) CPQ; (E) CYTH4; (F) IFI30; (G) ARPC1B; (H) NPC2 in normal and GBM tissues based on the GEPIA database. *Significant difference, p < 0.05.
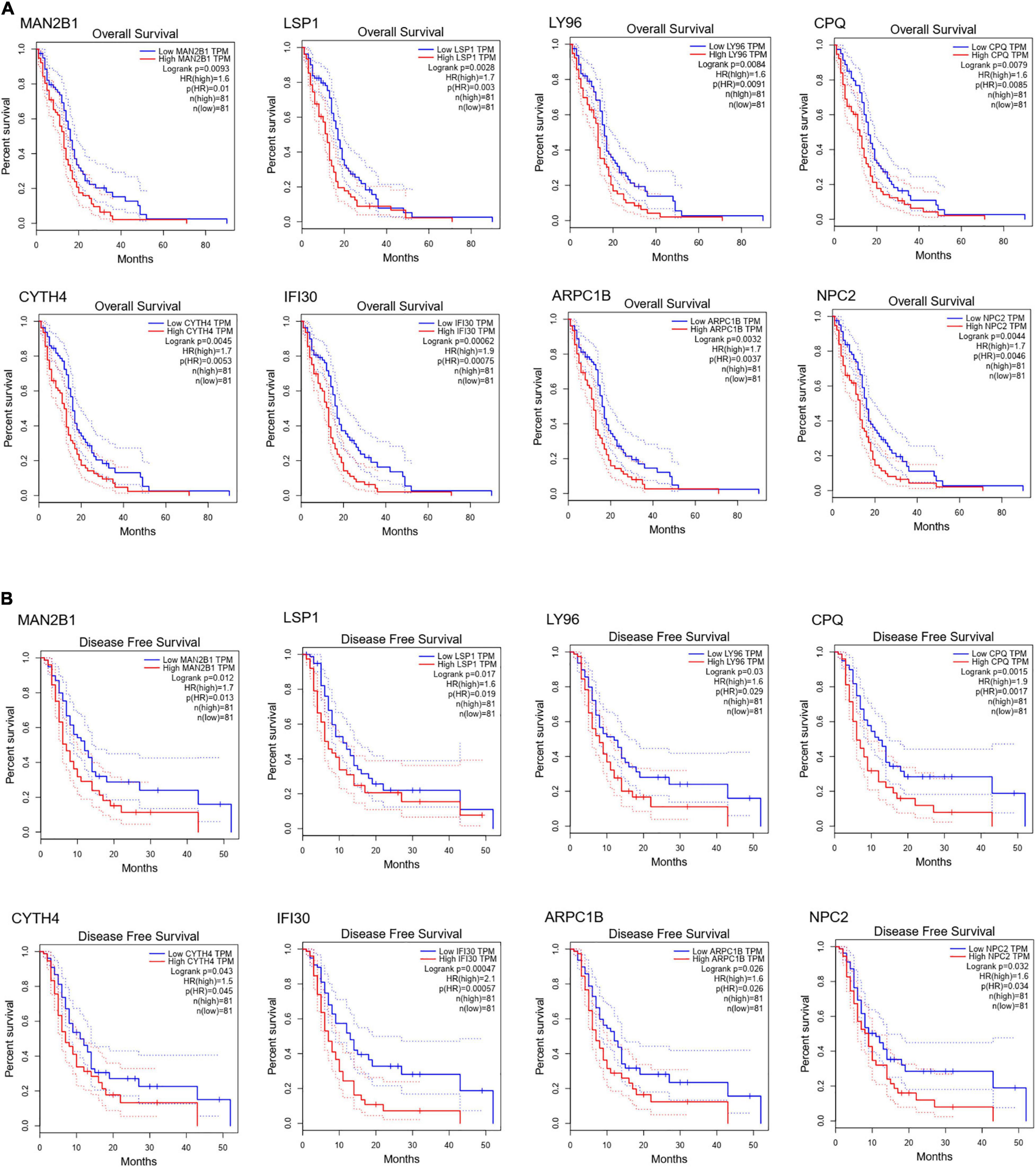
Figure 4. Survival curves of OS (A) and DFS (B) of eight common DEGs. Survival curves of OS (A) and DFS (B) of MAN2B1; LSP1; LY96; CPQ; CYTH4; IFI30; ARPC1B; NPC2 in normal and GBM tissues based on TCGA data in GEPIA. Red line indicates the samples with gene highly expressed, and blue line shows the samples with gene lowly expressed. HR, hazard ratio.
Tumor Infiltrates Correlation of NPC2 Expression
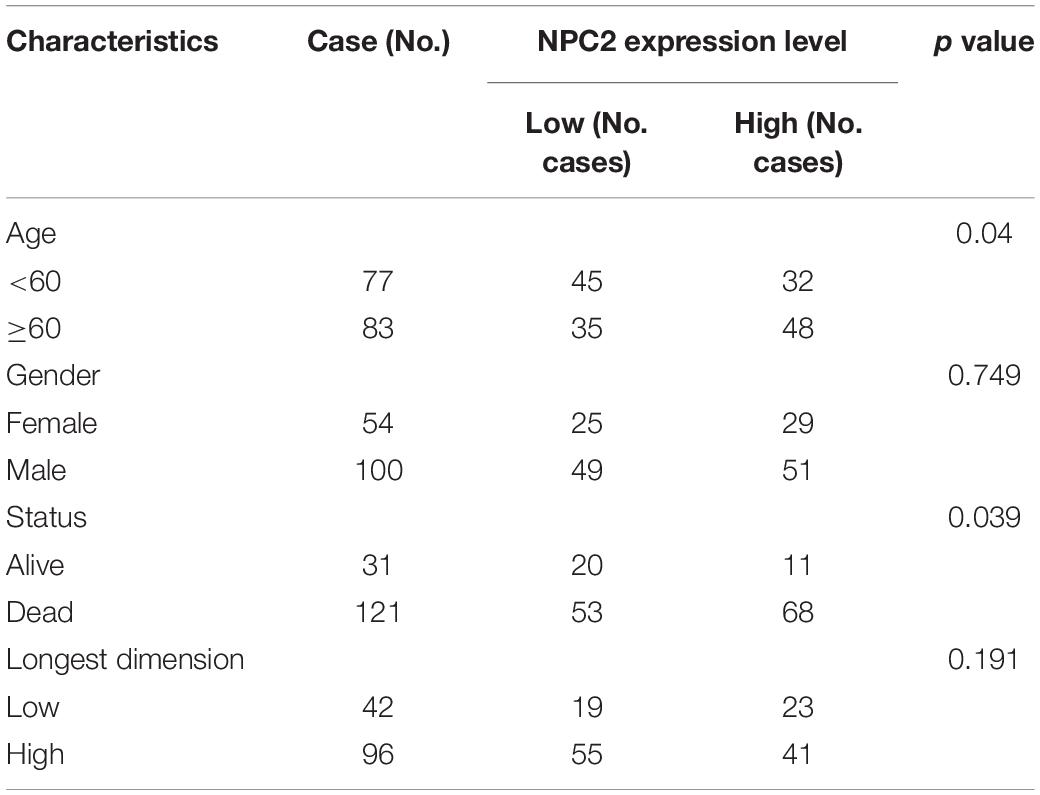
After searching in Pubmed, we found that the expression and function of LSP1 (Park et al., 2014), LY96 (Rajaraman et al., 2009), IFI30 (Liu et al., 2020; Zhu et al., 2020), and ARPC1B (Lauber et al., 2018) in GBM has been reported, and research about NPC2 in GBM are remain rescue. Hence, we focus on investing the clinical prognostic significance of the NPC2 genes in GBM. As shown in Table 1, by analyzing the NPC2 expression level and clinicopathological factors in 160 GBM patients, we found that NPC2 was correlated with patents’ age (p value = 0.04) and alive status (p value = 0.039). Additionally, considering the HR in OS and DFS maps were both higher than 1, we decided to choose NPC2 as the object to study in the following research. Then, we analyzed the correlation of NPC2 expression with tumor purity and tumor infiltrating immune cells. As expected, significant positive correlation of NPC2 expression with tumor purity was shown. What’s more, the correlation among NPC2 expression and tumor infiltrating B cell, CD8 + T cell, macrophages, neutrophils, and dendritic cells in GBM cancer samples was also obvious (Figure 5A). Furthermore, as the significant correlation in tumor infiltrating cell except CD4 + cell, correlation of B cell, T cell CD8+, M1 and M2 macrophages, neutrophil cells, and dendritic cells markers with NPC2 expression were analyzed. As exhibited in Figure 5B, the markers of B cell (CD19, CD79A), CD8 + T cell(CD8A, CD8B), M1 macrophages (PTGS2, IRF5), M2 macrophages (VSIG4, MS4A4A), neutrophil cells (ITGAM, CCR7), and myeloid dendritic cells (HLA-DPB1, HLA-DRA) all showed a significant positive correlation coefficient with NPC2, implicating potential role of NPC2 in tumor infiltration.
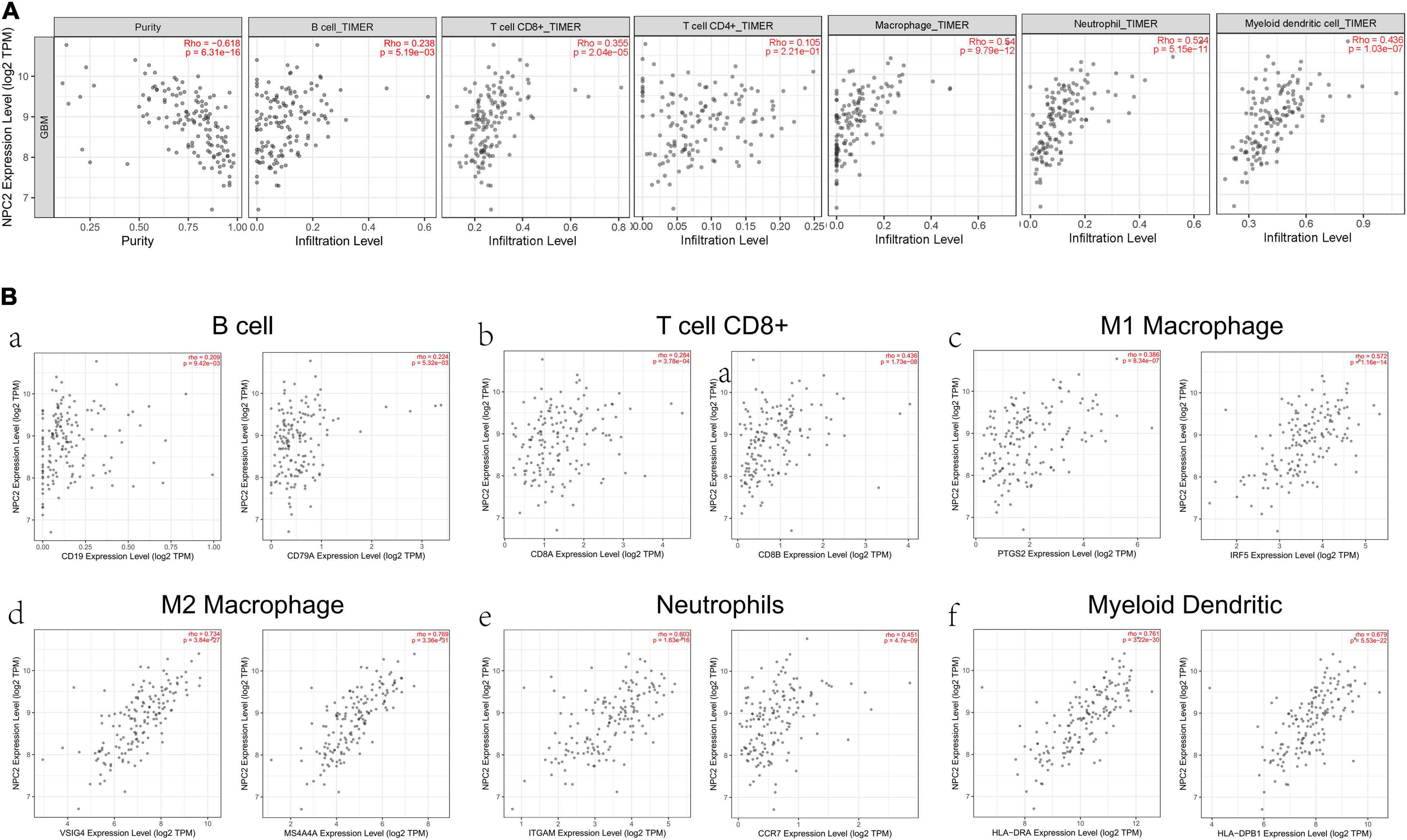
Figure 5. Correlation between immune cell and NPC2 expression. (A) The relationship between different functional immune cells, such as B cell, T cell CD8+, T cell CD4+, Macrophage, Neutrophil, and Myeloid dendritic cell with NPC2 expression in GBM. (B) The correlation NPC2 expression with canonical markers for six cell types: B cell, T cell CD8+, M1 Macrophage, M2 Macrophage Neutrophil, and Myeloid dendritic cell.
NPC2 Inhibits GBM Cancer Cell Proliferation, Migration, and Invasion
In order to verify the function of NPC2 in GBM, we silenced NPC2 in GBM cells. U87-MG and U251 are cell lines often used in the study of GBM (Gao et al., 2020; Tu et al., 2020; Xia et al., 2020; Zhang G. et al., 2020). CCK-8 assay has shown that cell proliferation was inhibited in U87-MG and U251 cells with Sh-NPC2 transfection compared with that in negative control (Sh-NC) (Figure 6A). Consistently, we performed the transwell migration assays to explore the potential effect of NPC2 on the on migration and invasion in U87-MG and U251 cells. As seen in Figure 6B, in transwell assays, the number of invading cells in the Sh-NPC2 group was less than that in the Sh-NC group. Similarly, wound healing assay showed that the metastasized cells were significantly reduced with about half of the wound closure potential when knockdown of NPC2 (Figure 6C). Together, the above results indicated that knockdown of NPC2 might inhibit growth, invasion, and migration abilities of U87-MG and U251 cells in GBM.
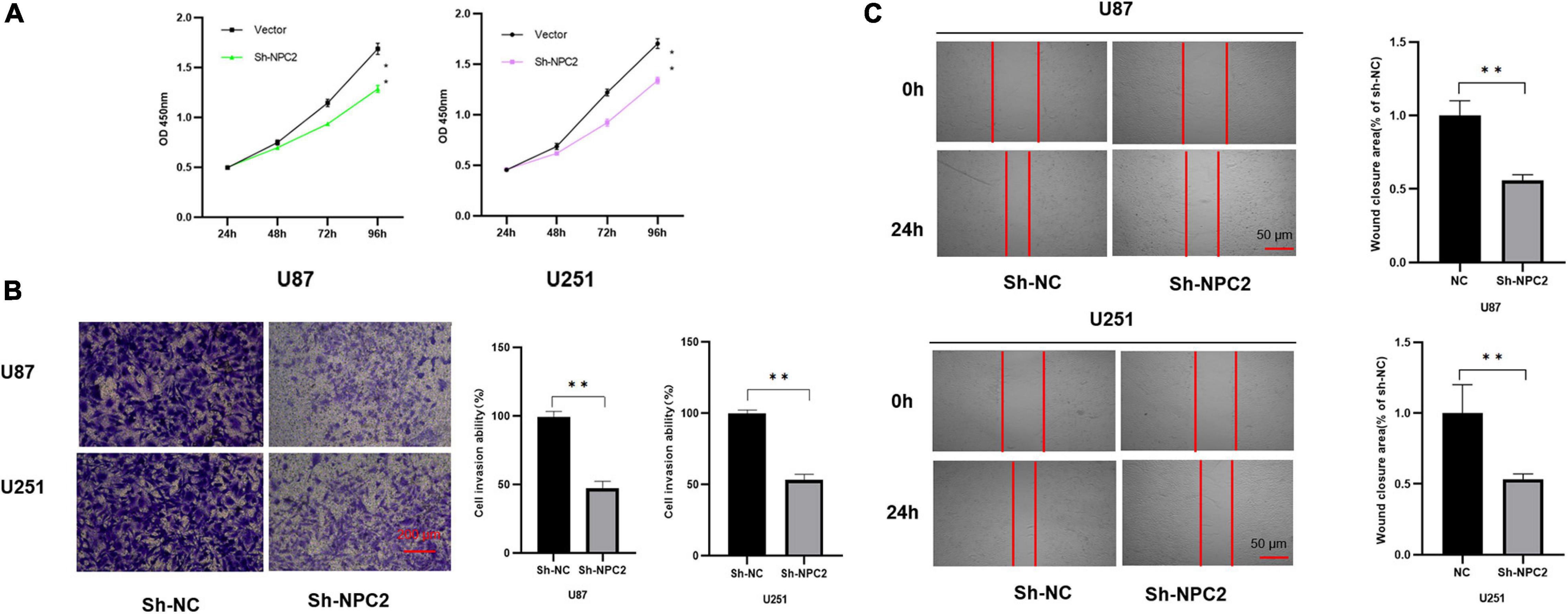
Figure 6. NPC2 inhibits U87-MG and U251 cells proliferation, migration, and invasion in vitro. (A) The effect of NPC2 knockdown on cell growth of U87-MG and U251cells detected by the CCK-8 assay. (B) The invasive and migration capacities were detected in U87-MG and U251 cells transfected with sh-NPC2 using transwell assays. (C) Silencing NPC2 attenuated wound closure corroborated in U87-MG and U251. The error bars indicate the mean ± SD (n = 3), and each experiment was repeated at least three times. *p < 0.05, **p < 0.01.
Discussion
GBM is one of the most commonly seen primary malignant brain tumor in adults with an overall poor prognosis (Ostrom et al., 2018; Zhang et al., 2019; Jin et al., 2020). The recurrence rate of GBM is particularly high. Almost all GBMs recur within the first year following diagnosis and second surgical resection (Ito et al., 2019). Accurate and effective prognosis assessment play an important role in individualized treatment and follow-up treatment of patients with GBM. GBM is usually diagnosed in the late stage by MRI with poor prognosis, therefore, a reliable prognostic marker is essential (Jiang et al., 2020). Indeed, effective prognostic care for GBM patients requires a better understanding of the molecular mechanism of GBM. With the rapid development of next generation sequencing and other “omics” profiling methods, we began to focus on single-cell RNA sequencing and genomic analysis of GBM cells to investigate the detailed or new causes of GBM pathogenesis. Genetic researches have also promoted the disclosure of a few sporadic biomarkers for GBM (Cao et al., 2020b). Trying to develop new prognostic markers for prognosis treatments of this cancer is necessary.
In this research, integrated bioinformatics analysis was performed to investigate the potential gene biomarker based on single-cell RNA sequencing dataset GSE102130, TCGA cohort DEGs, and PRGs of GBM. As a result, 14 cell clusters were identified in six GBM tissue samples and we found 17 common DEGs (Figures 1, 2A). Eight DEGs were identified to be the potential protective prognostic marker of GBM while patients with a higher expression of these eight common DEGs showed shorter survival rate (Figures 3, 4). After searching in Pubmed, we found out that the expression and function of LSP1 (Park et al., 2014), LY96 (Rajaraman et al., 2009), IFI30 (Liu et al., 2020; Zhu et al., 2020), and ARPC1B (Lauber et al., 2018) in GBM has been reported, while research about NPC2 in GBM remain rescue. Gao et al. identified LSP1 as an independent predictive factor for progressive malignancy in glioma (Cao et al., 2020a). Liu et al. found the expression of IFI30 to be high in glioblastomas and in gliomas with a mesenchymal subtype or wild-type isocitrate dehydrogenase, all of which indicated the malignancy and poor outcomes of glioma (Liu et al., 2020). Tumor infiltrating immune cells in tumor microenvironment were reported to be involved in GBM progression and prognosis prediction (Fu et al., 2020; Shu and Li, 2020; Zhang Y. et al., 2020). Immune cells such as microglia, peripheral macrophages, leukocytes, and myeloid-derived suppressor cells play an important role in infiltrating gliomas. They create an immunosuppressive microenvironment to facilitate GBM cell growth and invasion and were used for designing novel immunotherapeutic approaches (Domingues et al., 2016; Gieryng et al., 2017; Quail and Joyce, 2017). NPC2 is a protein coding gene which has been shown to be related to lipoprotein metabolism and innate immune system pathways. It plays a significant role in regulating the transport of cholesterol through the late endosomal/lysosomal system in the past researches (Lauber et al., 2018; Liu et al., 2020). Its predicting value of prognosis and treatment response in glioblastoma as a novel immune-related target was also verified (Zhu et al., 2020).
In this research, we found that NPC2 was negatively correlated with tumor purity and then were functionally investigated in tumor infiltrating immune cells (Figure 5). Our result also showed that NPC2 independently indicated unfavorable prognosis in glioma when adjusted for with patents’ age (p value = 0.04) and alive status (p value = 0.039) (Table 1), which revealed that NPC2 was a robust independent factor for predicting GBM survival. Considering that age has been identified as prognostic factors for GBM (Chen P. et al., 2020; Hao et al., 2020; Zheng et al., 2020), NPC2 might be a prognostic marker of GBM based on its significant positive correlation with this disease. Hence, we focus on investing the clinical prognostic significance of the NPC2 genes in GBM.
In the next step, we verified NPC2 as a prognostic biomarker in GBM through cytological experiments, which enabled us to determine its clinical value in GBM. As the results shown, cytological experiments including CCK-8 assay, transwell assay, and scratch healing illustrated that the knockdown of NPC2 might inhibit growth, invasion, proliferation, and migration abilities of U87-MG and U251 cells in GBM, supporting it as a prognostic biomarker for GBM. Currently, we have known a little about the role of NPC2 in GBM. It was the first time that reported NPC2 as a prognostic biomarker for GBM.
In conclusion, based on bioinformatics analysis and cytological experiments, we identified NPC2 as a novel biomarker with prognostic significance in GBM.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
DW, KL, and DK designed the research. SS, KL, and FL performed the experiments. FL, PZ, and SW visualized the results. DW, SS, and DK wrote the manuscript. DK and SS provided experimental resource. All authors reviewed and approved the final manuscript.
Funding
This study was funded by the Natural Science Foundation of Fujian Province (No. 2019J01500).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.611442/full#supplementary-material
Footnotes
References
Abdul, K. U., Houweling, M., Svensson, F., Narayan, R. S., Cornelissen, F. M. G., Kucukosmanoglu, A., et al. (2018). WINDOW consortium: a path towards increased therapy efficacy against glioblastoma. Drug Resist. Updat. 40, 17–24. doi: 10.1016/j.drup.2018.10.001
Albright, L. A. C., Farnham, J. M., Stevens, J., Teerlink, C. C., Palmer, C. A., Rowe, K., et al. (2020). Genome-wide analysis of high-risk primary brain cancer pedigrees identifies PDXDC1 as a candidate brain cancer predisposition gene. Neuro Oncol. 23, 277–283. doi: 10.1093/neuonc/noaa161
Bao, S., Wu, Q., McLendon, R., Hao, Y., Shi, Q., Hjelmeland, A., et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760. doi: 10.1038/nature05236
Cao, J., Guo, Q., Guan, G., Zhu, C., Zou, C., Zhang, L., et al. (2020a). Elevated lymphocyte specific protein 1 expression is involved in the regulation of leukocyte migration and immunosuppressive microenvironment in glioblastoma. Aging 12, 1656–1684. doi: 10.18632/aging.102706
Cao, J., Tang, Z., and Su, Z. (2020b). Long non-coding RNA LINC01426 facilitates glioblastoma progression via sponging miR-345-3p and upregulation of VAMP8. Cancer Cell Int. 20:327. doi: 10.1186/s12935-020-01416-3
Chen, P., Li, X., Ma, W., Li, H., Li, M., Yang, X., et al. (2020). Comprehensive transcriptomic analysis and experimental validation identify lncRNA HOXA-AS2/miR-184/COL6A2 as the critical cerna regulation involved in low-grade glioma recurrence. Onco Targets Ther. 13, 4999–5016. doi: 10.2147/OTT.S245896
Chen, Z., Herting, C. J., Ross, J. L., Gabanic, B., Puigdelloses Vallcorba, M., Szulzewsky, F., et al. (2020). Genetic driver mutations introduced in identical cell-of-origin in murine glioblastoma reveal distinct immune landscapes but similar response to checkpoint blockade. Glia 68, 2148–2166. doi: 10.1002/glia.23883
Couturier, C., Ayyadhury, S., Le, P., Nadaf, J., Monlong, J., Riva, G., et al. (2020). Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nat. Commun. 11:3406. doi: 10.1038/s41467-020-17186-5
Diboun, I., Wernisch, L., Orengo, C., and Koltzenburg, M. (2006). Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genomics 7:252. doi: 10.1186/1471-2164-7-252
Diehn, M., Eisen, M., Botstein, D., and Brown, P. (2000). Large-scale identification of secreted and membrane-associated gene products using DNA microarrays. Nat. Genet. 25, 58–62. doi: 10.1038/75603
Domingues, P., González-Tablas, M., Otero, Á, Pascual, D., Miranda, D., Ruiz, L., et al. (2016). Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav. Immun. 53, 1–15. doi: 10.1016/j.bbi.2015.07.019
Filbin, M., Tirosh, I., Hovestadt, V., Shaw, M., Escalante, L., Mathewson, N., et al. (2018). Developmental and oncogenic programs in H3K27M gliomas dissected by single-cell RNA-seq. Science (New York N. Y.) 360, 331–335. doi: 10.1126/science.aao4750
Fu, W., Wang, W., Li, H., Jiao, Y., Huo, R., Yan, Z., et al. (2020). Single-cell atlas reveals complexity of the immunosuppressive microenvironment of initial and recurrent glioblastoma. Front. Immunol. 11:835. doi: 10.3389/fimmu.2020.00835
Gao, L., Liu, J., Xu, P., Deng, G., Liu, B., Yuan, F., et al. (2020). AKT inhibitor SC66 inhibits proliferation and induces apoptosis in human glioblastoma through down-regulating AKT/β-catenin pathway. Front. Pharmacol. 11:1102. doi: 10.3389/fphar.2020.01102
García-León, J., García-Díaz, B., Eggermont, K., Cáceres-Palomo, L., Neyrinck, K., Madeiro da Costa, R., et al. (2020). Generation of oligodendrocytes and establishment of an all-human myelinating platform from human pluripotent stem cells. Nat. Protoc. 15, 3716–3744. doi: 10.1038/s41596-020-0395-4
Geraldo, L. H. M., Garcia, C., da Fonseca, A. C. C., Dubois, L. G. F., de Sampaio, E. S. T. C. L., Matias, D., et al. (2019). Glioblastoma therapy in the age of molecular medicine. Trends Cancer 5, 46–65. doi: 10.1016/j.trecan.2018.11.002
Gieryng, A., Pszczolkowska, D., Walentynowicz, K. A., Rajan, W. D., and Kaminska, B. (2017). Immune microenvironment of gliomas. Lab. Investig. 97:498. doi: 10.1038/labinvest.2017.19
Gong, X., Qian, H., Zhou, X., Wu, J., Wan, T., Cao, P., et al. (2016). Structural insights into the Niemann-Pick C1 (NPC1)-mediated cholesterol transfer and ebola infection. Cell 165, 1467–1478. doi: 10.1016/j.cell.2016.05.022
Guo, S., Wang, Y., and Wang, A. (2020). Identity and lineage fate of proteolipid protein 1 gene (Plp1)-expressing cells in the embryonic murine spinal cord. Dev. Dyn. 249, 946–960. doi: 10.1002/dvdy.184
Hao, C., Chen, G., Zhao, H., Li, Y., Chen, J., Zhang, H., et al. (2020). PD-L1 expression in glioblastoma, the clinical and prognostic significance: a systematic literature review and meta-analysis. Front. Oncol. 10:1015. doi: 10.3389/fonc.2020.01015
Infante, R. E., Wang, M. L., Radhakrishnan, A., Kwon, H. J., Brown, M. S., and Goldstein, J. L. (2008). NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. U.S.A. 105, 15287–15292. doi: 10.1073/pnas.0807328105
Ito, H., Nakashima, H., and Chiocca, E. (2019). Molecular responses to immune checkpoint blockade in glioblastoma. Nat. Med. 25, 359–361. doi: 10.1038/s41591-019-0385-7
Jiang, L., Zhong, M., Chen, T., Zhu, X., Yang, H., and Lv, K. (2020). Gene regulation network analysis reveals core genes associated with survival in glioblastoma multiforme. J. Cell. Mol. Med. 24, 10075–10087. doi: 10.1111/jcmm.15615
Jin, X., Xie, H., Liu, X., Shen, Q., Wang, Z., Hao, H., et al. (2020). RELL1, a novel oncogene, accelerates tumor progression and regulates immune infiltrates in glioma. Int. Immunopharmacol. 87, 106707. doi: 10.1016/j.intimp.2020.106707
Lakomy, R., Kazda, T., Selingerova, I., Poprach, A., Pospisil, P., Belanova, R., et al. (2020). Real-world evidence in glioblastoma: stupp’s regimen after a decade. Front. Oncol. 10:840. doi: 10.3389/fonc.2020.00840
Lauber, C., Klink, B., and Seifert, M. (2018). Comparative analysis of histologically classified oligodendrogliomas reveals characteristic molecular differences between subgroups. BMC Cancer 18:399. doi: 10.1186/s12885-018-4251-7
Li, G., Zhai, Y., Liu, H., Wang, Z., Huang, R., Jiang, H., et al. (2020). RPP30, a transcriptional regulator, is a potential pathogenic factor in glioblastoma. Aging 12, 16155–16171. doi: 10.18632/aging.103596
Li, X., Saha, P., Li, J., Blobel, G., and Pfeffer, S. R. (2016). Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proc. Natl. Acad. Sci. U.S.A. 113, 10079–10084. doi: 10.1073/pnas.1611956113
Liu, X., Song, C., Yang, S., Ji, Q., Chen, F., and Li, W. (2020). IFI30 expression is an independent unfavourable prognostic factor in glioma. J. Cell. Mol. Med. 24, 12433–12443. doi: 10.1111/jcmm.15758
Mathews, S., Branch Woods, A., Katano, I., Makarov, E., Thomas, M., Gendelman, H., et al. (2019). Human interleukin-34 facilitates microglia-like cell differentiation and persistent HIV-1 infection in humanized mice. Mol. Neurodegener. 14:12. doi: 10.1186/s13024-019-0311-y
Mirchia, K., and Richardson, T. (2020). Beyond IDH-mutation: emerging molecular diagnostic and prognostic features in adult diffuse gliomas. Cancers 12:1817. doi: 10.3390/cancers12071817
Murai, N., Mitalipova, M., and Jaenisch, R. (2020). Functional analysis of CX3CR1 in human induced pluripotent stem (iPS) cell-derived microglia-like cells. Eur. J. Neurosci. 52, 3667–3678. doi: 10.1111/ejn.14879
Ostrom, Q. T., Cote, D. J., Ascha, M., Kruchko, C., and Barnholtz-Sloan, J. S. (2018). Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 4, 1254–1262. doi: 10.1001/jamaoncol.2018.1789
Park, S., Fesinmeyer, M., Timofeeva, M., Caberto, C., Kocarnik, J., Han, Y., et al. (2014). Pleiotropic associations of risk variants identified for other cancers with lung cancer risk: the PAGE and TRICL consortia. J. Natl. Cancer Inst. 106:dju061. doi: 10.1093/jnci/dju061
Quail, D., and Joyce, J. (2017). The microenvironmental landscape of brain tumors. Cancer Cell 31, 326–341. doi: 10.1016/j.ccell.2017.02.009
Rajaraman, P., Brenner, A., Butler, M., Wang, S., Pfeiffer, R., Ruder, A., et al. (2009). Common variation in genes related to innate immunity and risk of adult glioma. Cancer Epidemiol. Biomark. Prevent. 18, 1651–1658. doi: 10.1158/1055-9965.EPI-08-1041
Santangelo, A., Rossato, M., Lombardi, G., Benfatto, S., Lavezzari, D., De Salvo, G. L., et al. (2020). A Molecular signature associated with prolonged survival in glioblastoma patients treated with regorafenib. Neuro Oncol. 23, 264–276. doi: 10.1093/neuonc/noaa156
Shen, C., Cheng, W., Lee, C., Dai, X., Chiao, M., Liang, Y., et al. (2020). Both p53 codon 72 Arg/Arg and pro/Arg genotypes in glioblastoma multiforme are associated with a better prognosis in bevacizumab treatment. BMC Cancer 20:709. doi: 10.1186/s12885-020-07210-8
Shu, C., and Li, Q. (2020). Current advances in PD-1/PD-L1 axis-related tumour-infiltrating immune cells and therapeutic regimens in glioblastoma. Crit. Rev. Oncol. Hematol. 151:102965. doi: 10.1016/j.critrevonc.2020.102965
Subramanian, A., Tamayo, P., Mootha, V., Mukherjee, S., Ebert, B., Gillette, M., et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550. doi: 10.1073/pnas.0506580102
Tiburcio, P., Locke, M., Bhaskara, S., Chandrasekharan, M., and Huang, L. (2020). The neural stem-cell marker CD24 is specifically upregulated in IDH-mutant glioma. Transl. Oncol. 13:100819.
Tu, G., Ho, Y., Ng, Z., Teo, K., Yeo, T., and Too, H. (2020). A facile and scalable in production non-viral gene engineered mesenchymal stem cells for effective suppression of temozolomide-resistant (TMZR) glioblastoma growth. Stem Cell Res. Ther. 11:391.
Xia, W., Zhu, J., Tang, Y., Wang, X., Wei, X., Zheng, X., et al. (2020). PD-L1 inhibitor regulates the miR-33a-5p/PTEN signaling pathway and can be targeted to sensitize glioblastomas to radiation. Front. Oncol. 10:821. doi: 10.3389/fonc.2020.00821
Zhang, B., Wu, Q., Xu, R., Hu, X., Sun, Y., Wang, Q., et al. (2019). The promising novel biomarkers and candidate small molecule drugs in lower-grade glioma: evidence from bioinformatics analysis of high-throughput data. J. Cell. Biochem. 120, 15106–15118.
Zhang, G., Tanaka, S., Jiapaer, S., Sabit, H., Tamai, S., Kinoshita, M., et al. (2020). RBPJ contributes to the malignancy of glioblastoma and induction of proneural-mesenchymal transition via IL-6-STAT3 pathway. Cancer Sci. 111, 4166–4176. doi: 10.1111/cas.14642
Zhang, Y., Mudgal, P., Wang, L., Wu, H., Huang, N., Alexander, P., et al. (2020). T cell receptor repertoire as a prognosis marker for heat shock protein peptide complex-96 vaccine trial against newly diagnosed glioblastoma. Oncoimmunology 9:1749476. doi: 10.1080/2162402X.2020.1749476
Zhao, H., Zhou, X., Yuan, G., Hou, Z., Sun, H., Zhai, N., et al. (2020). CDC6 is up-regulated and a poor prognostic signature in glioblastoma multiforme. Clin. Transl. Oncol. 4. doi: 10.1007/s12094-020-02449-w
Zheng, C., Berger, N., Li, L., and Xu, R. (2020). Epigenetic age acceleration and clinical outcomes in gliomas. PLoS One 15:e0236045. doi: 10.1371/journal.pone.0236045
Keywords: glioblastoma, prognostic biomarkers, NPC2, Gene Expression Omnibus (GEO), bioinformatics analysis
Citation: Wei D, Shen S, Lin K, Lu F, Zheng P, Wu S and Kang D (2021) NPC2 as a Prognostic Biomarker for Glioblastoma Based on Integrated Bioinformatics Analysis and Cytological Experiments. Front. Genet. 12:611442. doi: 10.3389/fgene.2021.611442
Received: 29 September 2020; Accepted: 08 February 2021;
Published: 11 March 2021.
Edited by:
Xiao Ke, Shenzhen University, ChinaCopyright © 2021 Wei, Shen, Lin, Lu, Zheng, Wu and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dezhi Kang, dmljdG9yd3h5QDEyNi5jb20=
†These authors have contributed equally to this work
 De Wei1,2,3†
De Wei1,2,3† Shanghang Shen
Shanghang Shen Dezhi Kang
Dezhi Kang