
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 27 November 2020
Sec. Genomics of Plants and the Phytoecosystem
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.572975
This article is part of the Research Topic Genetics and Genomics to Enhance Crop Production, Towards Food Security View all 36 articles
 Anjan Kumar Pradhan1†
Anjan Kumar Pradhan1† Sundeep Kumar1*†
Sundeep Kumar1*† Amit Kumar Singh1†
Amit Kumar Singh1† Neeraj Budhlakoti2
Neeraj Budhlakoti2 Dwijesh C. Mishra2
Dwijesh C. Mishra2 Divya Chauhan1
Divya Chauhan1 Shikha Mittal1
Shikha Mittal1 Monendra Grover2
Monendra Grover2 Suneel Kumar1
Suneel Kumar1 Om P. Gangwar3
Om P. Gangwar3 Subodh Kumar3
Subodh Kumar3 Arun Gupta4
Arun Gupta4 Subhash C. Bhardwaj3†
Subhash C. Bhardwaj3† Anil Rai2
Anil Rai2 Kuldeep Singh1
Kuldeep Singh1Resistance in modern wheat cultivars for stripe rust is not long lasting due to the narrow genetic base and periodical evolution of new pathogenic races. Though nearly 83 Yr genes conferring resistance to stripe rust have been cataloged so far, few of them have been mapped and utilized in breeding programs. Characterization of wheat germplasm for novel sources of resistance and their incorporation into elite cultivars is required to achieve durable resistance and thus to minimize the yield losses. Here, a genome-wide association study (GWAS) was performed on a set of 391 germplasm lines with the aim to identify quantitative trait loci (QTL) using 35K Axiom® array. Phenotypic evaluation disease severity against four stripe rust pathotypes, i.e., 46S119, 110S119, 238S119, and 47S103 (T) at the seedling stage in a greenhouse providing optimal conditions was carried out consecutively for 2 years (2018 and 2019 winter season). We identified, a total of 17 promising QTl which passed FDR criteria. Moreover these 17 QTL identified in the current study were mapped at different genomic locations i.e. 1B, 2A, 2B, 2D, 3A, 3B, 3D, 4B, 5B and 6B. These 17 QTLs identified in the present study might play a key role in marker-assisted breeding for developing stripe rust resistant wheat cultivars.
In wheat, stripe rust caused by Puccinia striiformis f. sp. tritici (Pst) is the most damaging and widely prevalent disease. It causes significant yield losses in almost every part of the world where cool and humid conditions persist during crop season. In India, stripe (yellow) rust is the major disease in North West Plain Zones (NWPZ) especially sub-mountainous parts of Punjab, Haryana, and Western Uttar Pradesh, the major wheat growing regions of India. Occurrence of stripe rust has also been observed frequently in Jammu & Kashmir, Himachal Pradesh, and tarai regions of Uttarakhand (Chen, 2005; Indu and Saharan, 2011). However, a major outbreak of stripe rust was observed in the North Western Plain Zone (NWPZ) and the Northern Hills Zone (NHZ) of India in 2006 and 2012–13 which caused heavy yield losses (Prashar et al., 2007; Saharan et al., 2013).
Yield losses due to this disease vary from 10 to 40% which depends upon various factors like severity level, susceptibility of cultivars, stage of infection, rate of disease development, and duration of disease. If infection takes place at the seedling stage and the conducive environment persists until maturity, then yield losses may go up to 100% (Afzal et al., 2007).
The presence of large number of pathotypes is the main reason for the epidemic of stripe rust. Out of 140 stripe rust pathotypes known globally, more than 28 pathotypes have been reported in India (Line and Qayoum, 1992; Chen et al., 2010; Bhardwaj et al., 2014; Tomar et al., 2014). As new pathotypes evolve fast, host plants require novel genes to encounter new pathotypes. Although there are more than 83 cataloged Yr resistance genes (McIntosh et al., 2017; Li et al., 2020) most of them are all-stage resistance genes with the exception of 22 genes, namely Yr11-14, Yr16, Yr18/Lr34/Sr57/Pm38/Ltn1, Yr29/Lr46/Sr58/Pm39/Ltn2, Yr30/Lr27/Sr2, Yr36, Yr39, Yr46/Lr67/Sr55/Pm46/Ltn3, Yr52, Yr59, Yr62, Yr68, Yr71, Yr75, Yr77-80, and Yr82 (McIntosh et al., 1995; Chen and Kang, 2017; Feng et al., 2018; Nsabiyera et al., 2018; Pakeerathan et al., 2019). In addition, several temporary Yr resistance genes and QTL have been identified and reviewed (Rosewarne et al., 2013; Chen and Kang, 2017).
Although rust can be controlled by good agronomical management and fungicides, still cultivation of resistant cultivars is one of the best and most economical options (Wellings, 2011). To develop resistant cultivars at regular intervals, new sources of resistance and new genes/QTLs need to be identified from virgin germplasm lines. GWAS have been found effective for the identification of novel genes/QTLs in germplasm lines, i.e., leaf rust (Kertho et al., 2015), stem rust (Yu et al., 2012; Letta et al., 2013; Laidò et al., 2015), and stripe rust resistance to verify the effect of previously discovered Yr genes and QTLs (Tadesse et al., 2013; Zegeye et al., 2014; Naruoka et al., 2015; Bulli et al., 2016; Pasam et al., 2017). Recently, many studies have reported QTLs for stripe rust resistance in wheat using GWAS (Liu et al., 2017a; Muleta et al., 2017a; Yao et al., 2019; Cheng et al., 2020). However, very few GWAS attempts have been made on Indian wheat germplasm lines for identification of stripe rust genes. Most of the landraces used in this study are unexplored and not used in any breeding program. Therefore, the present study was conducted with the aim to identify QTLs/defense genes associated with stripe rust seedling stage resistance through GWAS.
An association mapping panel of 391 wheat germplasm lines which includes 290 Indian landraces/ indigenous germplasm, 24 exotic, and 77 other germplasm lines including advanced breeding lines and some Indian varieties released during 1960 (Supplementary Table 1). The association mapping panel was evaluated against four virulent and predominant pathotypes of P. striiformis tritici in India. The landraces, indigenous and exotic germplasm lines were collected from the National Gene bank of India located at ICAR-NBPGR, New Delhi. The advanced wheat breeding lines used in this analysis were developed at ICAR-Indian Institute of Wheat and Barley Research (IIWBR) Karnal. All the 391 wheat germplasm lines were evaluated at the seedling stage using a mixture of Pst pathotypes prevalent in India over 2 years (2018 and 2019 winter season) under greenhouse conditions at IIWBR Regional Station, Shimla. Reference lines known to possess specific Yr gene/s commonly occurring in wheat were used to confirm the purity of pathotypes (Bhardwaj et al., 2012). A mixture of fresh garden soil and FYM (1:1 ratio), autoclaved at 60°C for 4 h was used for growing wheat plants in plastic pots and aluminum bread pans/trays. In an aluminum bread pan tray, 4–5 seeds of each line were clump planted. Each tray contained 18 lines and a susceptible check (A-9-30-1). In plastic pots 10–12 seedlings were raised. After seeding, the trays/pots were kept in the greenhouse at 22 ± 2°C with proper labels and provided with optimum conditions to ensure normal germination and growth of the seedling.
After a week, wheat seedlings were inoculated with each pathotype separately by atomizing the uredosporic inoculum suspended in non-phytotoxic isoparaffinic oil (commercially known as Soltrol 170 produced by Chevron Phillips Chemicals Asia Pvt. Ltd., Singapore). The inoculated plants were fine sprayed with water and incubated for 24 h in water saturated glass chambers. The plants were then transferred to the greenhouse and dusted with elemental dust powder of Sulfur to avoid the occurrence of powdery mildew infection. All the optimum greenhouse conditions for good plant growth and proper stripe rust infection were provided. The greenhouse temperature was maintained at 15 ± 1°C. The response of host-pathogen interactions was recorded in the form of infection type after 16–18 days of inoculation following Nayar et al. (1997). Disease severity was recorded on 10 plants for each germplasm line. Wheat-rust infection responses (low or high) were recorded on experimental material and differentials (to ascertain the purity of pathotypes) by following McNeal et al. (1971) using a 0–9 scale where, 0 indicates Immune (no observed visible infection), 1 means highly resistant (necrotic/chlorotic flecks appears without sporulation), 2 means resistant (there are necrotic/chlorotic stripes without sporulation) 3 shows moderately resistant (trace sporulation, necrotic/chlorotic stripes are there), 4 represents moderately resistant (light sporulation, necrotic/chlorotic stripes appears), 5 suggests moderately susceptible (intermediate sporulation, necrotic/chlorotic stripes appears on leaves), 6 specify highly moderate(moderate sporulation, necrotic/chlorotic stripes were termed as resistant), whereas 7 means moderately susceptible (abundant sporulation, necrotic/chlorotic stripes can be observed on the leaves), 8 means susceptible (abundant sporulation, with chlorosis), while 9 is highly susceptible (plant shows abundant sporulation, without chlorosis, were categorized as susceptible).
To determine genotypic and year variances among pathotypes, Analysis of variance (ANOVA) was performed using SAS v9.3 (software). Frequency distribution of genotypes for different pathotypes under study has also been generated showing the performance of phenotypes. Heritability of the pathotypes infection was estimated using the restricted maximum likelihood (REML) method. For the analysis of 2-years data, pathotype mean, variance, standard deviation, and ranges of each germplasm line was calculated.
Leaves from 15-days old seedlings were collected and standard CTAB protocol was followed for genomic DNA extraction (Doyle, 1990). A total of 391 germplasm lines were genotyped using the 35K Axiom® array (Affymetrix product ID 550524) for wheat having 35,143 SNPs. SNPs having low-quality clustering and minor allele frequency (MAF ≤ 10%), across all the genotypes were excluded from the analysis. To assign an exact physical location to each SNP on wheat chromosomes, SNP probe sequences were subjected to BLAST against wheat reference genome RefSeq v1.0 (https://wheat-urgi.versailles.inra.fr/Seq-Repository/Assemblies) following default parameters. A total of 19,090 polymorphic SNPs were assigned to an exact physical location on the wheat genome and used for further downstream analysis.
A set of 525 random SNP markers distributed across the 21 wheat chromosomes (25 markers per chromosome) were used to determine the population structure. The STRUCTURE v2.3.4 software based on admixture model with correlated allele frequency was applied to categorize sub-population in the current germplasm lines (Pritchard et al., 2000). For Structure, parameters like 20,000 iterations and 50,000 Monte Carlo Markov Chain (MCMC) replicates were set to determine K values in the range of 1–10. For each K value, ten independent structure runs were carried out and further the results were exported to Structure Harvester (http://taylor0.biology.ucla.edu/structureHarvester/) software for determining the most likely number of subpopulations in germplasm lines (Evanno et al., 2005). Further, fixation index (Fst) for subpopulations was estimated from various STRUCTURE runs. Principal components (PC) were also inferred using the Genomic Association and Prediction Integrated Tool (GAPIT) R package to further analyze population sub-structuring and a comparison was made from the results analyzed with STRUCTURE. To further determine the genetic structure of the lines, cluster analysis based on the neighbor joining (NJ) tree algorithm according to shared-allele distance was also performed in TASSELv5.0. The branching pattern in the NJ tree was assessed based on bootstrapping over loci with 1,000 replications, while the consensus bootstrap value was displayed with the help of ITOL program v5 (https://itol.embl.de/).
Linkage disequilibrium (LD) based on pairwise measures between SNP markers were estimated using TASSELv5.0 (Bradbury et al., 2007). LD can be estimated as squared allele frequency correlation (R2) between pairs of intra-chromosomal SNPs with known chromosomal position. The background LD in the wheat AM panel was calculated to identify critical distance for LD decay. The average pattern of genome-wide LD decay over physical distance was determined by constructing a scatterplot of R2 values against the corresponding physical distance among markers. Further, the extent of LD decay was also estimated using the Locally Weighted Scatter-plot Smoother (LOESS) model (Cleveland, 1979). The critical R2 value that shows the area beyond which LD is due to true physical linkage was determined using 95th percentile of the square root of transformed R2 data of unlinked markers (Breseghello and Sorrells, 2005). Further, the intersection of LD decay curve was observed at R2 = 0.156 and at 2.5 Mb distance. Therefore, all the significantly associated SNPs (clustered SNPs) falling within this distance were designated as single QTLs.
GWAS was conducted using a panel of 19,090 high quality polymorphic SNP markers and disease severity data against four different pathotypes 47S103, 238S119, 46S119, and 110S119 at seedling stage. This analysis was done using the phenotyping data from both the year (2018 and 2019) separately as well as for pooled data. We have presented the GWAS results for the marker using pooled data, however we have also highlighted the markers which were consistent in both years. Marker-trait associations (MTAs) were identified using the compressed mixed linear model (CMLM) (Yu et al., 2006; Zhang et al., 2010) implemented in GAPIT R package (Lipka et al., 2012). CMLM uses the additional information like Principal component (usually three components) and Kinship of population (K), hence it is also called PC3 + K CMLM model. CMLM is compressed form of mixed linear model. The general equation of MLM can be written as follows
Where, y = observed phenotypic vector, β = vector of marker fixed effects; u = vector of random additive genetic effects from individual lines; e = vector of residuals; X and Z are known design matrices.
P-value and R2 were used as parameter to identify significant marker-trait associations (MTAs). Significant MTAs were identified at the threshold of P < 0.001. In order to show the distribution of SNPs over the chromosome, Manhattan plots have also been generated.
Candidate genes and their corresponding molecular functions were retrieved from the wheat genome assembly IWGSC Ref-Sequence v1.1 using BLASTN function of BLAST program with default parameters. Associated SNPs were extracted along with their annotations for transcript located within the distance. The identified putative candidate genes were further validated using stripe rust disease resistance data from Sequence Read Archive (SRA) NCBI database (Bio Project—PRJNA613349).
The ANOVA results revealed highly significant variation (P < 0.0001) for germplasm lines and pathotypes (Table 1) while the genotype × year interaction effect was found to be non-significant. Further, the frequency distribution of the infection types (ITs) produced by the four different Pst races (a) 110S119, (b) 238S119, (c) 46S119, and (d) 47S103 in association mapping panel is shown in Figures 1A–D. Among 391 germplasm lines, 35 (10%), 40 (11%), 144 (37%), and 189 (49%) showed (Supplementary Table 2) seedling resistance to races 110S119, 238S119, 46S119, and 47S103, respectively in 1st year and 33 (9%), 37 (10%), 144 (37%), 181 (47%), respectively in 2nd year (Figure 2B). A higher percentage of germplasm lines were susceptible (IT = 8–9) to races 110S119 (60%), 238S119 (61%), 46S119 (29%), and 47S103 (23%) (Figure 2A) in first year as compared to the second year 110S119 (61%), 238S119 (47%), 46S119 (15%), and 47S103 (7%). Further, the heritability (h2) value for stripe rust infection type (IT) was 0.58 (Table 2).
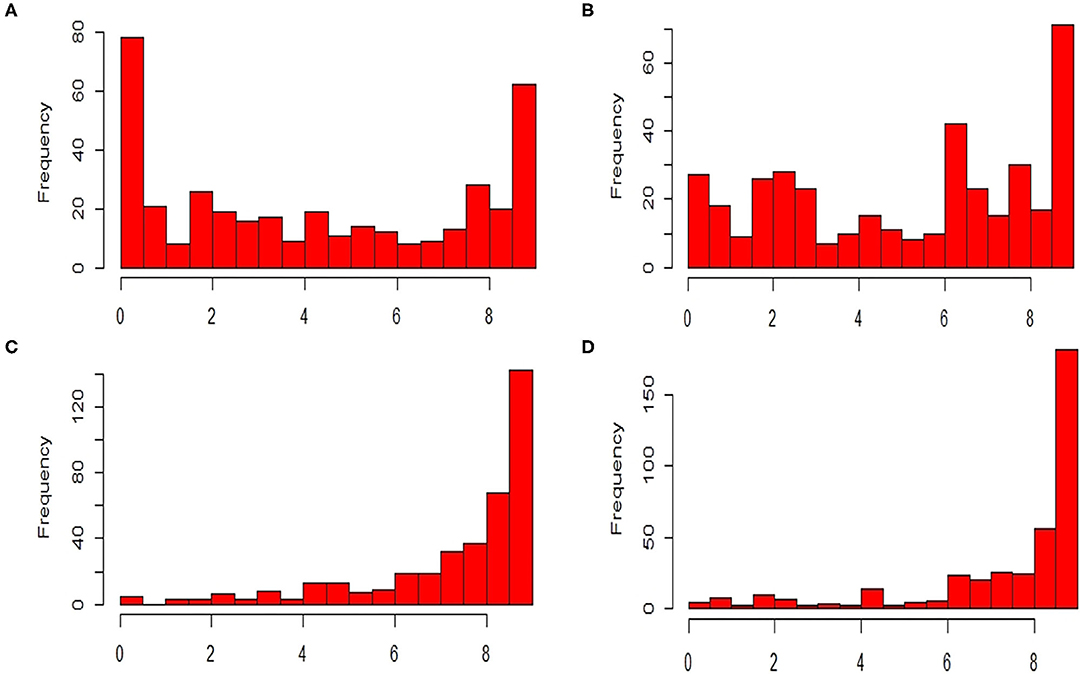
Figure 1. Frequency distributions of stripe rust resistant pathotypes (A) 110S119, (B) 238S119, (C) 46S119, and (D) 47S103.
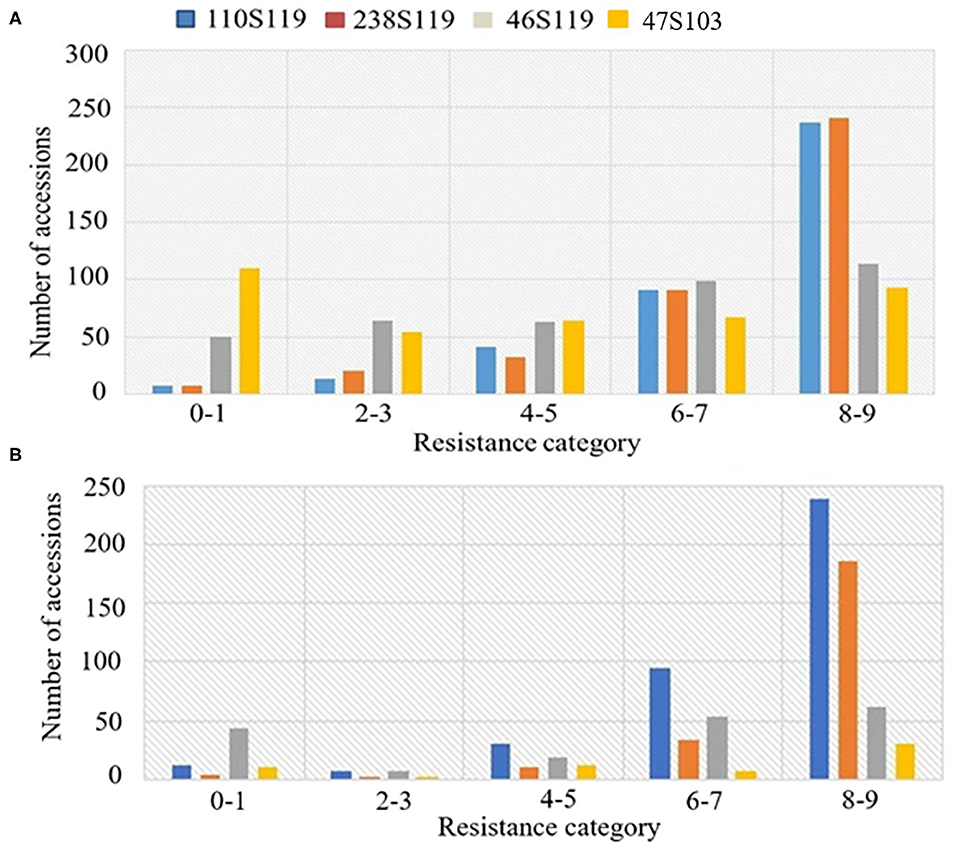
Figure 2. Frequency distributions of stripe rust resistance types produced by seedlings of the bread wheat genotypes (A) 2018, (B) 2019.
A total of 19,090 SNPs assigned to an exact physical location on the wheat genome were used for association mapping. Out of 19,090 SNPs, 6,083 were mapped on A sub-genome, 7,253 on B sub-genome and 5,754 on D sub-genome. The number of SNPs on individual chromosomes ranged from 378 on 4D to 1,315 on 2B. Chromosome level distribution of SNPs represented that A sub-genome possesses maximum SNPs on 2A (1,079), followed by 1A (1,053) and 7A (953). Whereas, in the B sub-genome, the maximum SNPs were on 2B (1,315), followed by 5B (1,257) and 1B (1,159). While, in the case of D sub-genome, maximum SNPs were on 2D (1,176) followed by 1D (1,039) and 5D (897) (Table 3).
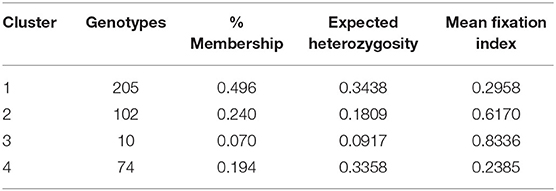
Table 3. Genetic clusters and their member genotypes, proportion of membership, expected heterozygosity, and the mean values of Fst observed from structure analysis of 391 Stripe rust resistance wheat genotypes.
Population structure analysis categorizes the 391 genotypes into four sub-populations (SP). Containing 205, 102, 10, and 74 genotypes, respectively. Sub-populations 1 (SP 1) was the largest among the four sub-populations, having 52.2% of the genotypes from the association mapping panel followed by sub-population 2 (SP 2), sub-population 4 (SP 4), and the smallest one sub-population 3 (SP 3), i.e., 2.5% (Table 4). Sub-populations 1 and 4 revealed the highest level of heterozygosity, i.e., 0.3438 and 0.3358, respectively. Individuals of each subpopulation were further categorized as pure and admixtures in type based on membership proportion. Genotypes that had a membership proportion of ≥0.8 were considered pure and genotypes <0.8 were considered admixtures. Based on this criterion, composition of four sub-populations was as follows; SP 1–60% pure and 40% admixtures; SP 2–36% pure and 64% admixtures; SP 3–40% pure and 60% admixtures and SP 4–37% pure and 63% admixtures (Figure 3A). Out of four subpopulations, SP 1 consists of a higher proportion of susceptible germplasm lines than others. In SP 1, most of the germplasm lines were susceptible to pathotype 238S119 and highly resistant to pathotype 46S119. On the other hand, in SP 2, most of the germplasm lines were susceptible to pathotype 110S119 and highly resistant to stripe rust pathotype 46S119. For the SP 4, maximum genotypes were resistant against 47S103 followed by 46S119, while susceptibility was for the 238S119 followed by 110S119.
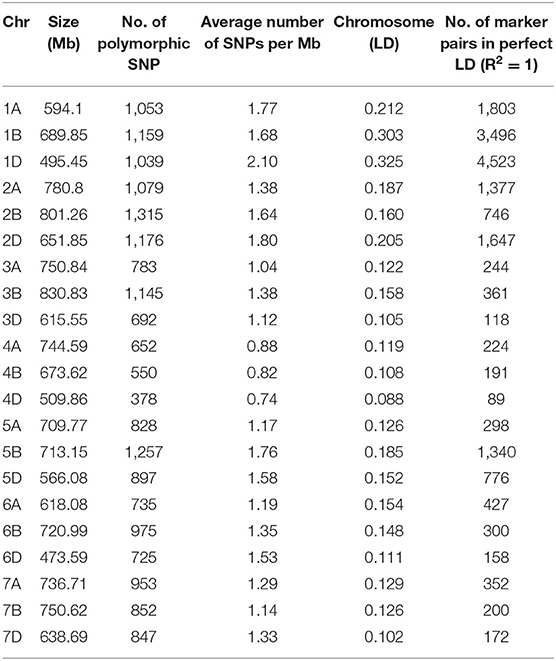
Table 4. Distribution of 19,090 SNPs in 21 chromosomes identified LD in 391 resistant of Stripe rust bread wheat genotypes.
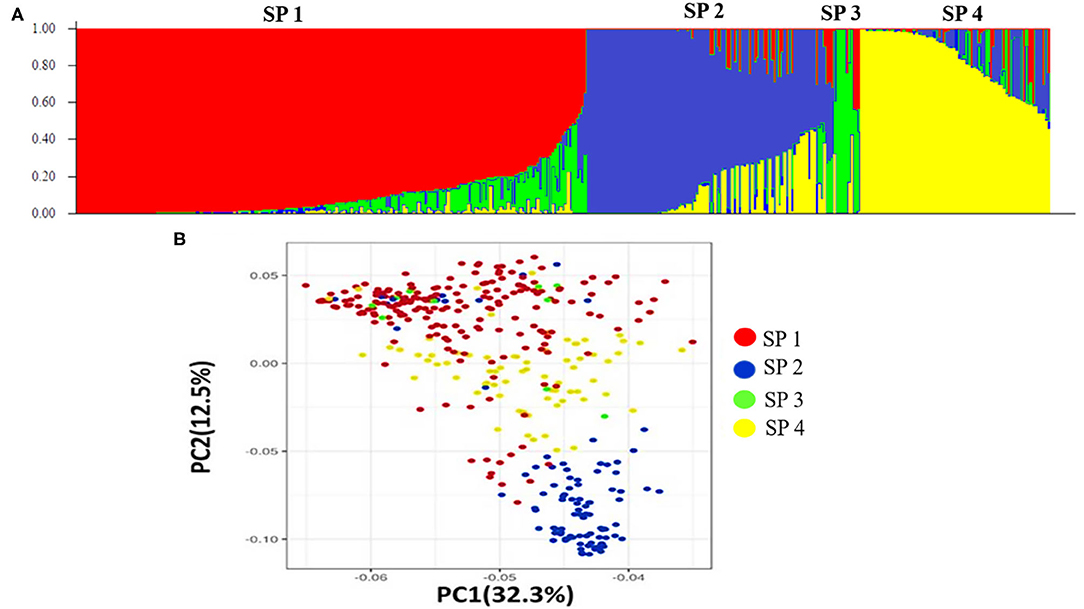
Figure 3. Population structure of the 391 stripe rust germplasm lines combined with markers. (A) Population structure of the bread wheat among 19,090 SNP polymorphism markers, (B) Principal component analysis.
Principal component analysis (PCA) was also performed using 391 genotypes to estimate population structure including 1st three PCs for further downstream analysis. PC1 and PC2 have explained 32.3 and 12.5% of the genetic variance, respectively. The PC analysis scatter plot (Figure 3B) also confirmed the results of population structure analysis as it showed that 1st and 2nd PCs were composed mainly by four sub-populations. Moreover, cluster analysis was carried out based on the neighbor-joining (NJ) algorithm that revealed four clusters in an association panel. Here, the neighbor-joining (NJ) tree of the stripe rust resistance wheat lines was evaluated by shared-allele genetic distance using high-density SNP markers (Figure 4).
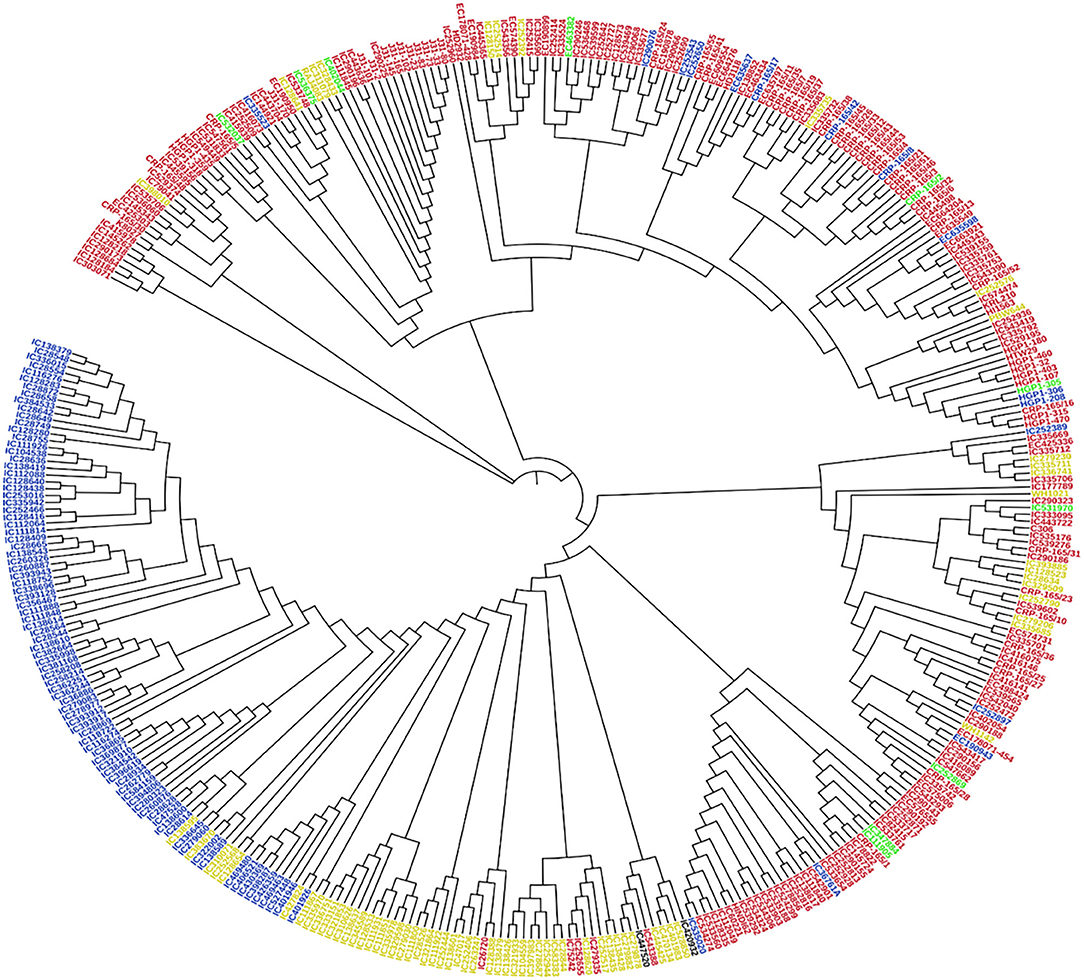
Figure 4. Cluster analysis was based on the neighbor-joining (NJ) algorithm. Genotypes were divided into four subpopulations using STRUCTURE (K = 4). Red, Blue, Green, and Yellow color represents SP 1, SP 2, SP 3, and SP 4, respectively.
The LD for each sub-genome, i.e., A, B, and D genome was estimated from all pairs of SNPs present over there. Individually the average R2 of genome wide LD was 0.14 for A sub-genome, 0.16 for B sub-genome, and 0.15 for D sub-genome. SNP markers, whose map positions were assigned, were further used to estimate intra-chromosomal LD. A total of 31.46% intra-chromosomal pairs loci were in significant LD (i.e., R2 of 0.2), while 18,842 SNP pairs were in a perfect LD (i.e., R2 = 1). The decay of LD across the genome is an important parameter that determines the number of significant markers required for performing GWAS analysis. The extent of LD distribution was graphically demonstrated by plotting intra-chromosomal R2 values for loci against their physical distance and a second-degree LOESS curve was also fitted for further exploration. The background LD in the analyzed AM panel was equal to 0.156 and taken as the threshold cut-off for estimating LD decay. In the selected wheat panel LD decayed the fastest in the A sub-genome comparison to B and D. In the A sub genome R2 value for the marker pairs reached 0.156 (used as a threshold) at 1.9 Mb as compared to 2.3 Mb in B and 2.9 Mb in D sub-genomes (Figures 5A–D).
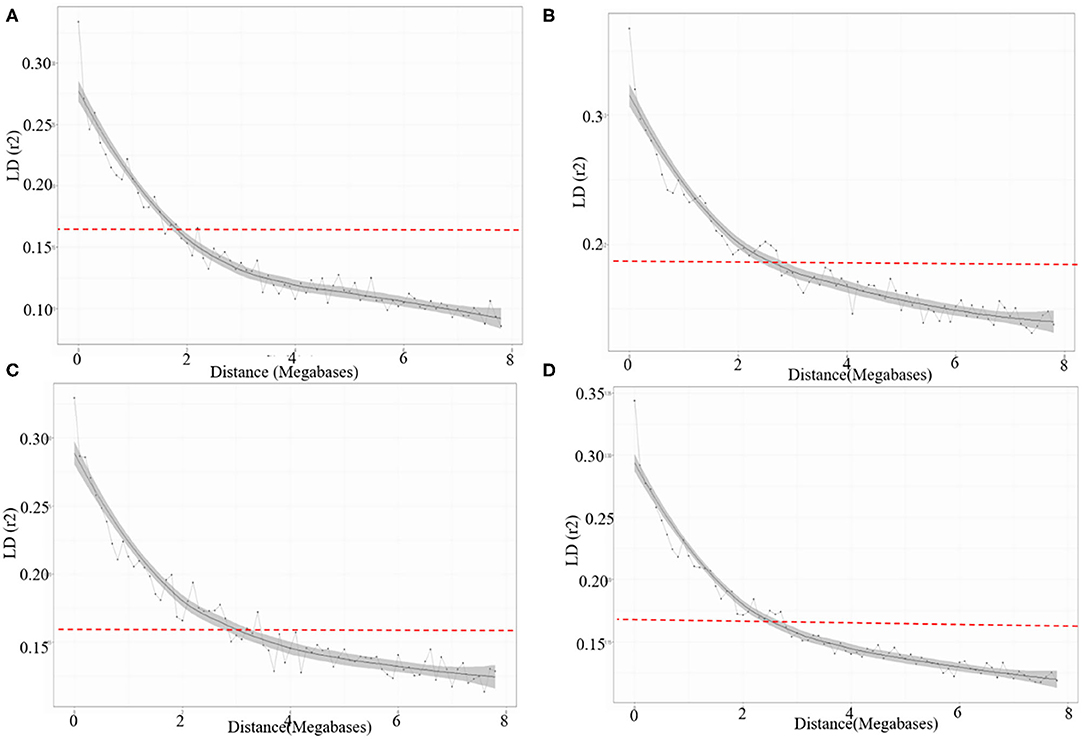
Figure 5. LD decay across the (A) A, (B) B, (C) D sub-genomes, and (D) whole genome of wheat. The values on the Y-axis represent the squared correlation coefficient (R2) and the values at X-axis represent physical distance (Mb).
The association of SNP markers with stripe rust resistance against four different pathotypes at seedling stages were determined by CMLM analysis using kinship (K) matrix and population structure (Q matrix) as a covariate. A total of forty QTLs (51 MTAs) in 19 genomic regions (1A, 1B, 2A, 2B, 2D, 3A, 3B, 3D, 4A, 4B, 4D, 5A, 5B, 5D, 6A, 6B, 6D, 7A, and 7D) were linked with resistance to four pathotypes (at P < 0.001, and out of these 40 QTLs, 17 QTLs were significant at FDR adjusted P < 0.30).
These QTLs were distributed as follows; 12 QTLs for 47S103, 12 QTLs for 46S119, 7 QTLs for 110S119, and 9 QTLs for 238S119. Coefficient of determination (R2) for each QTL was also determined. Phenotypic variance ranged from 6.6 to 28.8%. Ability of the GWAS model was tested using Manhattan plots and quantile-quantile-plots (Q-Q plots) between observed and expected P-values of association, that revealed a good fitting for the model with population structure and kinship (Table 5, Figures 6A–D), i.e., (a) 47S103, (b) 46S119, (c) 110S119, and (d) 238S119.
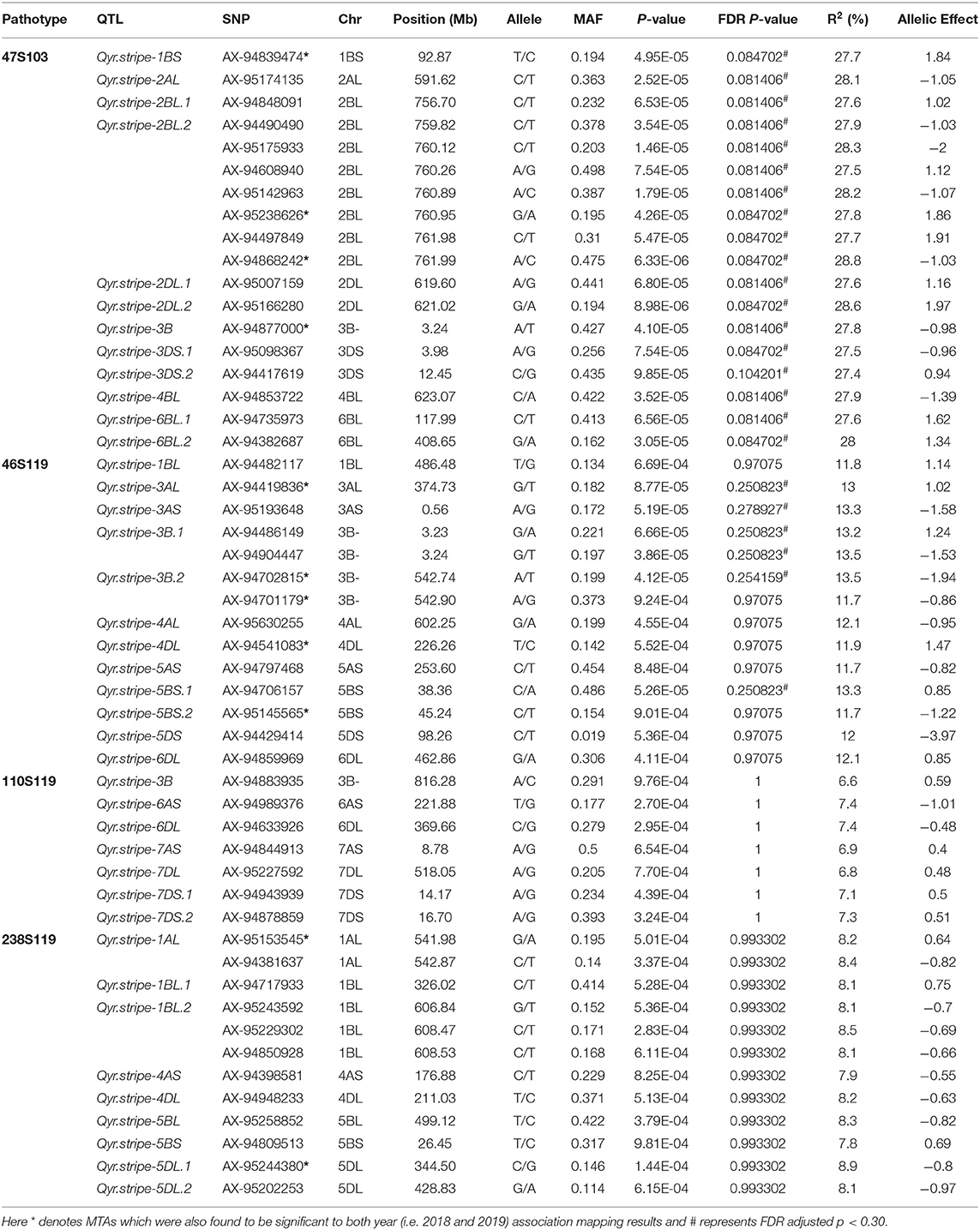
Table 5. Quantitative trait loci for stripe rust resistance identified at the seedling stage using pooled data.
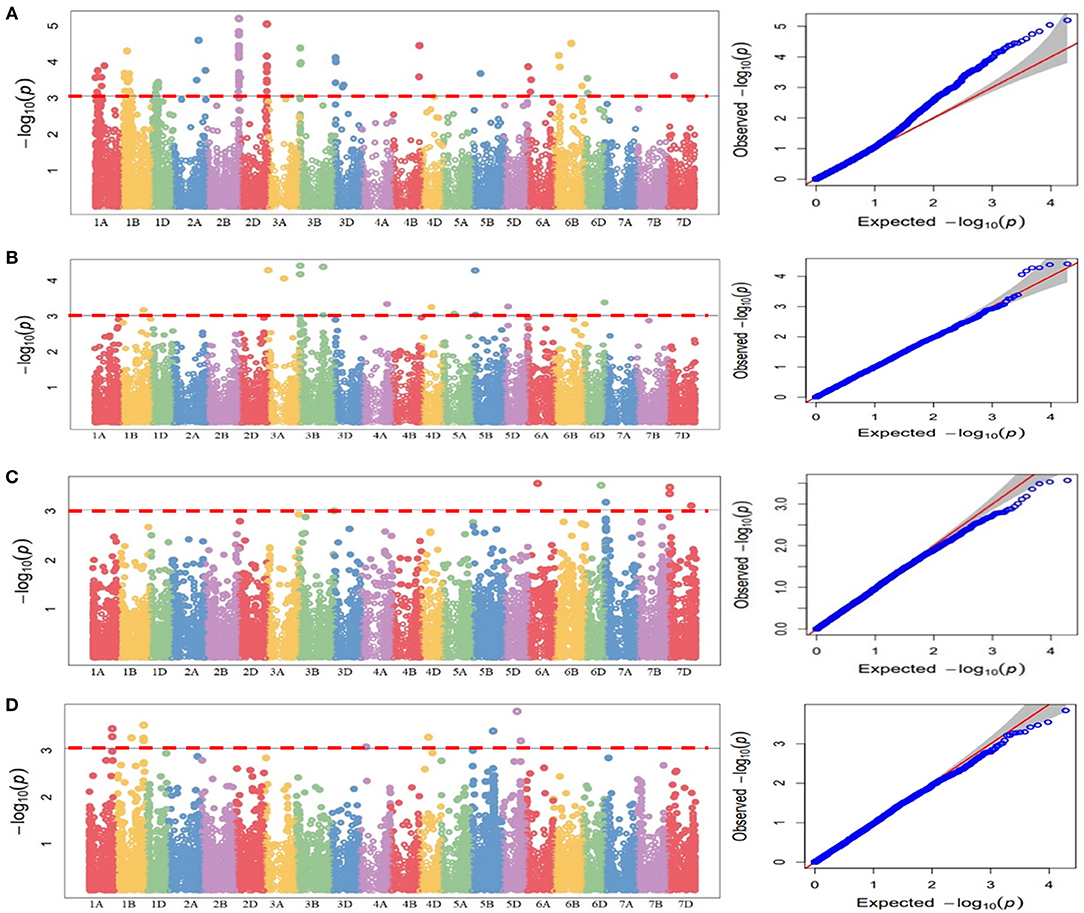
Figure 6. Manhattan plots for statistical significance P-values across 21 wheat chromosomes for SNP markers associated with stripe rust four pathotypes. (A) 47S103, (B) 46S119, (C) 110S119, (D) 238S119 and Quantile-Quantile plots indicating the normality of data four pathotypes. Horizontal line represents significant MTAs at threshold of P < 0.001.
Twelve QTLs for 47S103 were mapped on 8 different chromosomes (i.e., 1B, 2A, 2B, 2D, 3B, 3D, 4B, and 6B) and most of them were on B sub-genome. Only 1 QTL was mapped on the A sub-genome (Qyr.stripe-2AL with p-value 2.52E-05 and R2 = 0.28). Seven QTLs were mapped on B sub genome, out of which 2 QTLs, i.e., Qyr.stripe-2BL.1 and Qyr.stripe-2BL.2, were found on the long arm of the chromosome 2B between 760 and 762 Mb (2.17 Mb region). Four QTLs were mapped on D sub-genome (Qyr.stripe-2DL.1, Qyr.stripe-2DL.2, Qyr.stripe-3DS.1, Qyr.stripe-3DS.2) at different positions.
For pathotype 46S119, a total of 12 QTLs were identified which mapped on nine different chromosomes (1B, 3A, 3B, 4A, 4D, 5A, 5B, 5D, and 6D), most of them were mapped on B sub-genome. In these QTLs, R2 ranged from 11.7 to 13.5%. Four QTLs were mapped on A sub-genome (i.e., Qyr.stripe-3AL, Qyr.stripe-3AS, Qyr.stripe-4AL, Qyr.stripe-5AS) at various locations. Further, 5 QTLs were mapped on B sub-genome, 2 of them, i.e., Qyr.stripe-3B.1, Qyr.stripe-3B.2 were found on 3B at 3 Mb and 542 Mb location as a close neighbor at 0.01 and 0.16 Mb apart, respectively. Other 3 QTLs were mapped on D sub-genome (Qyr.stripe-4DL, Qyr.stripe-5DS, Qyr.stripe-6DL).
In the case of pathotype 110S119, 7 significant QTLs were mapped on five different chromosomes (3B, 6A, 6D, 7A, and 7D). The highest number of QTLs were mapped on D sub-genome, i.e., 4 QTLs (Qyr.stripe-6DL, Qyr.stripe-7DL, Qyr.stripe-7DS.1, Qyr.stripe-7DS.2) and 2 QTLs were mapped on A sub-genome (Qyr.stripe-6AS, Qyr.stripe-7AS), whereas only 1 QTL was found on B sub-genome (Qyr.stripe-3B with p-value 9.76E-04 and R2 = 0.07). Further for the pathotype 238S119, 9 QTLs were identified as significant. These QTLs were found on 6 different chromosomes at various locations (i.e., 1A, 4A, 1B, 5B, 4D, and 5D). Maximum number, i.e., 4 QTLs were found to be associated with B sub-genome (Qyr.stripe-1BL.1, Qyr.stripe-1BL.2, Qyr.stripe-5BL, Qyr.stripe-5BS) followed by D (Qyr.stripe-4DL, Qyr.stripe-5DL.1, Qyr.stripe-5DL.2) and A sub-genome (Qyr.stripe-1AL, Qyr.stripe-4AS).
Furthermore, in order to identify consistent MTAs across the years, GWAS was separately performed for 2018 and 2019. The results are presented in Supplementary Tables 3, 4. A total of 9 QTLs were consistently present in 2018 and 2019, i.e., in agreement with the results of the pooled analysis. These include 3 QTLs for 47S103 (1BS: Qyr.stripe-1BS, 2BL: Qyr.stripe-2BL.2 and 3B: Qyr.stripe-3B), 4 QTLs for 46S119 (3AL: Qyr.stripe-3AL, 3B: Qyr.stripe-3B.2, 4DL: Qyr.stripe-4DL and 5BS: Qyr.stripe-5BS.2), and 2 QTLs for 238S119 (1AL: Qyr.stripe-1AL and 5DL: Qyr.stripe-5DL.1).
Candidate genes for pathotypes 47S103, 46S1119, 110S119, and 238S119 were identified by mapping the markers on wheat genome assembly. Seven prominent markers (47S103-2BL, i.e., for pathotype 47S103 on long arm of B sub-genome) mapped to 2.17 Mb interval ranging from 759.82 Mb (AX-94490490) to 761.99 Mb (AX-94868242) on chromosome 2BL. This interval contains 25 genes, of which 5 had a high enrichment score. Similarly, 238S119-1BL mapped to 1.69 Mb interval ranging from 606.84 Mb (AX-95243592) to 608.53 Mb (AX-94850928) on chromosome 1BL. This interval contains seven genes, of which two were had a high enrichment score. Interestingly, the two markers, i.e., AX-94877000 and AX-94904447 associated with 47S103 and 46S119 pathotypes, respectively were linked with a common gene (TraesCS3B02G005900) at 3.24 Mb on chromosome 3B. For pathotypes 46S119, 110S119, and 238S110 three of these genes, TraesCS6D02G384800, TraesCS7A02G021700, and TraesCS1B02G376000 mapped on chromosome 6DL, 7AS, and 1BL, respectively. These genes were annotated as leucine-rich repeat receptor-like protein kinases (LRR) and serine-threonine/tyrosine-protein kinase (STPK) which have key roles in pathogen recognition and disease resistance.
The annotation of associated SNPs revealed potential candidate genes (Supplementary Table 5 and Table 6). Maximum candidate genes were found for pathotype 47S103 which encodes various class of proteins and enzymes, such as Plant actin-related protein 8 (Arp complex) acts as a host response to pathogen infection (Qi et al., 2017), the heavy metal-associated domain plays an important role in the development of vascular plants and in plant responses to environmental changes. Phosphatidylinositol 3-/4-kinase (PI3K) acts as a catalytic domain and plays vital roles in the regulation of various cellular activities, including proliferation, differentiation, membrane ruffling and prevention of apoptosis (Cantley, 2002). Exocyst subunit Exo70 family protein (EXO70) describes the expression profiling of EXO70 genes from wheat (Zhao et al., 2019). Seven candidate genes were found for pathotype 46S119. Out of seven genes, TraesCS6D02G384800 gene on 6DL was annotated leucine-rich repeat receptor-like protein kinases and serine-threonine/tyrosine-protein kinase (STPK) which has key roles in pathogen recognition and disease resistance. Further, six candidate genes (Table 6) identified against pathotype 110S119 were annotated, and they encoded Isopenicillin N synthase-like, Agent domain plant type, Isopenicillin N synthase-like, bifunctional inhibitor/plant lipid transfer protein, Mitochondrial substrate/solute carrier and leucine-rich repeat receptor-like protein kinases, respectively. The candidate genes annotated for 238S119 revealed functions, such as amino acid pathway regulation, transcription regulation, DNA repair and metabolite transfer.
The putative candidate genes were further validated using expression data available in the public domain. The expression of candidate genes was checked against susceptible (PBW343) and resistant (FLW29) wheat cultivars and their expression has been represented through heat map (Figure 7 and Supplementary Table 7).
A total of 51 MTAs (40 QTLs) in relation to different pathotypes were identified. The number of favorable alleles ranged from 1 to 41 for studied germplasm lines. Alleles associated with a reduction in disease response were considered as favorable alleles at each locus of the respective SNP. Broadly we have considered infection type 1–4 as resistant, infection type 5–6 and 7–9 were considered to be moderate and susceptible, respectively. Disease severity can also be understood as percentage of infection (0–9, i.e., on 10 scale). A significant negative correlation (at p-value < 0.0001) was observed in case of all four pathotypes, i.e., 110S119 (−0.63), 238S119 (−0.70), 46S119 (−0.72), and 47S103 (−0.80), between the number of favorable alleles in each genotype to their respective disease severity value. The same fact has also been validated by fitting simple linear regression by considering disease severity as response and number of favorable alleles as an independent factor (Figures 8A–D). Model parameters were found to be significant (at p-value < 0.0001) with a good range of model R2 for all four pathotypes.
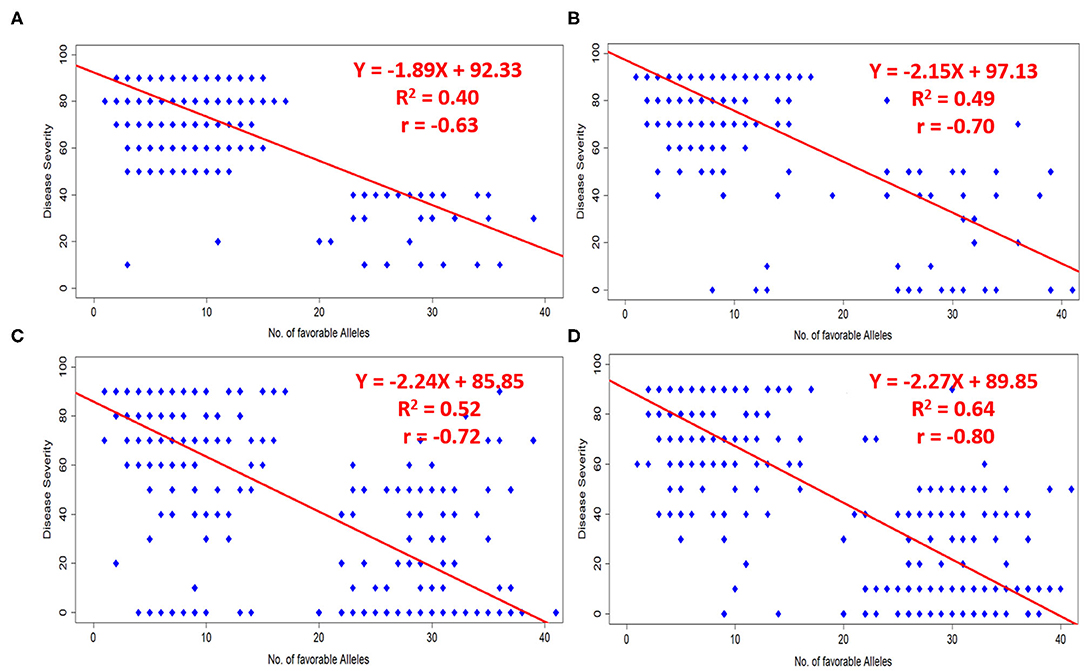
Figure 8. Effects of favorable alleles on response to four pathotypes (A) 110S119, (B) 238S119, (C) 46S119, and (D) 47S103.
The evolution of new virulent races of P. striiformis has always been a big challenge before wheat scientists. The new virulent races make the existing ruling varieties susceptible as most of the varieties have race specific resistance that can be overcome easily by new virulent pathogens. Heavy yield losses have been reported due to evolution of new pathotypes (Burdon et al., 2014; Hulbert and Pumphrey, 2014). Hence, there is a need for a constant search for new resistance sources for developing resistant cultivars with durable resistance at regular interval. Global as well as National level efforts have been made to explore new sources of resistance by exploring wheat germplasm collection maintained in gene banks (Gurung et al., 2014; Sehgal et al., 2015; Kumar et al., 2016). Most of the germplasm lines conserved in Gene banks have not been utilized in any breeding program therefore, chances of getting novel sources of resistance is comparatively higher in comparison to breeding lines which are already in use in breeding programs. Further, with the advancement of genotyping technology, these germplasm lines can be effectively characterized and utilized by breeding programs (Cavanagh et al., 2013; Wang et al., 2014). In India, prime focus is to evaluate the wheat lines at the seedling stage for resistance against three new, i.e., 110S119, 238S119, and 47S103 and two already known Yr9-virulent pathotypes, i.e., 46S119 and 78S84 (Gangwar et al., 2019).
Germplasm lines including landraces are the primary source of genes and a valuable source of resistance, until now very few landraces have been used in wheat breeding programs (Bajgain et al., 2015; Naruoka et al., 2015; Turner et al., 2017). In our study, significant phenotypic variation among the genotypes has been observed as depicted by ANOVA results. On the basis of IT scores, our results suggested 110S119 and 238S119 were the most virulent pathotypes in 2018 whereas 110S119 was the most virulent in 2019. Recently, a study of diverse spring wheat panel for stripe rust also showed similar results, i.e., 110S119, the most virulent pathotype (Kumar et al., 2020).
We identified novel resistance sources for all the studied pathotypes. Some of them were resistant against two of the pathotypes which includes IC111888, IC290156. In indigenous wheat germplasm lines including landraces for 2 consecutive years. Germplasm lines with higher levels of resistance against prevailing races of stripe rust can be utilized in breeding rust resistant cultivars in the future.
Most of the breeding lines and varieties included in our study showed resistance against the pathotype 47S103 and 46S119 and it could be because these pathotypes evolved in 1991 and 1996, respectively and most of the breeding programs were focusing on these pathotypes only (Bhardwaj et al., 2014). The other two pathotypes 110S119 and 238S119 came into existence during 2014.
Genetic diversity is the basic requirement of any breeding program. The determination of extent of genetic diversity and population structure are the foremost requirement for initiating and utilizing plant genetic resources in breeding programs and also for genetic studies (Atwell et al., 2010). We found the marker density of 19,090 SNPs on different chromosomes and identified that Genome B showed the highest marker density as compared to sub-genome A and D. Previous studies also revealed highest marker density of polymorphic SNPs on the B sub-genome (Kumar et al., 2020).
Population structure is an important factor which influences LD (Flint-Garcia et al., 2003). Our AM panel contains the genotypes from different geographic regions, out of 391 genotypes, 247 were from northern part of India, 86 were from other parts of India like the central zone (Rajasthan, Madhya Pradesh and Gujrat), or Southern part of India (Karnataka, Tamilnadu, Andhra Pradesh, Maharashtra) while 58 genotypes were exotic. Approximately 50% of genotypes were land races/or locally collected germplasm lines. Population structure determined by both STRUCTURE program and PCA based approach suggested four sub-populations (Kumar et al., 2020). A significant level of admixture may be due to the sharing of the germplasm across breeding programs. However, some collection regions have strong enrichment in specific genetic-based sub-populations, like SP 1 contains 76% of the germplasm lines of Indian origin either landraces or breeding lines and released varieties while 24% breeding lines were procured from the CIMMYT Mexico. Out of 102 genotypes from SP 2, only five lines were from CIMMYT Mexico, the rest were local land races (65) and old varieties (32). SP 3 contained only 10 genotypes, of which two were from CIMMYT and eight were from North Indian Origin. SP 4 contains 51 local land races and 22 breeding lines of Indian origin.
LD is one of the most important factors that determine the power of association analysis. We estimated LD for all the chromosomes of three sub-genomes, i.e., A, B, and D. The critical R2 values for genome A, B, and D were 0.14, 0.16, and 0.15, respectively. We observed faster decay of LD in the sub-genome A than the other two sub-genomes (B and D). Many other studies have also showed rapid decay of LD in sub-genome A (Chao et al., 2010; Voss-Fels et al., 2015). Further, as in this analysis, the longest LD decay distance was observed for the sub-genome D, the same has been reported in previous studies (Chao et al., 2010; Voss-Fels et al., 2015; Liu et al., 2017b; Maulana et al., 2018; Qaseem et al., 2019). Since the LD decay is influenced by population composition, it thus may vary in different populations, but broadly the sub-genome D generally has a longer LD decay distance as compared to the other two sub-genomes.
Further, although GWAS enables high resolution mapping of traits, often it might also reveal false positive associations if the confounding factors like population structure and genetic-relatedness among genotypes of the association panel are not accounted for. Therefore, in our analysis, a CMLM based method which accounts for both of these factors was used (Wei et al., 2017). The population structure analysis using model based approach, N-J based phylogeny and PCA revealed four sub-populations in the AM panel. However, the clustering pattern observed in our analysis was not explainable on the basis of geographical origin/source of included germplasm lines. One of the possible reasons for this could be the extensive sharing of germplasm within the wheat breeding program of India in the past six decades.
Our study has demonstrated the power of the GWAS approach in uncovering genomic regions associated with stripe rust resistance. In total, 51 MTAs were identified for four studied stripe rust pathotypes. Based on LD decay distance, we classified these 51 MTAs into 40 QTLs (23 suggestive QTLs and 17 significantly associated QTLs) distributed on all the wheat chromosomes except Chr1D and Chr7B. These QTLs further need to be validated before it can be used in any future breeding programs. Previous studies have also identified Yr genes/QTLs distributed on almost all the wheat chromosomes as both major and minor genes are known to be responsible for conferring resistance against stripe rust pathogens (Zegeye et al., 2014; Liu et al., 2017b; Muleta et al., 2017a; Ye et al., 2019; xbib80). Zegeye et al. (2014) identified stripe rust resistance QTLs on 1B, 2A, 2B, 3B, 3D, 5A, 5B, 6D, and 7A. Muleta et al. (2017b) identified stripe rust resistance QTLs on 1B, 2A, 2B, 2D, 5B, and 7B. Whereas, Ye et al. (2019) identified 12 QTLs on the long arms of 1B, 3D, 5A, 5B, and 7B and the short arms of chromosomes 1A, 5A, 6A, 6B, and 7A.
Further, we have compared the location of identified QTLs in this study with that of the previous studies. For some of the QTLs, exact comparison across different studies was difficult due to the difference in the number of markers, mapping populations and the genotyping techniques (SSR or SNP) used in these studies. For such QTLs, chromosome arm location (short/long) seems to be a good criterion and was used in our analysis. The details of the previously identified QTLs are provided in Supplementary Table 6. Important novel and previously identified QTLs for stripe rust resistance identified in our analysis are discussed below.
For pathotype 47S103 (T), our analysis identified 2 QTLs (Qyr.stripe-2BL.1, Qyr.stripe2BL.2) on the long arm of 2B. Of these, Qyr.stripe-2BL.1 at 756.7 Mb explained 27.6% of phenotypic variation for disease severity, and coincided to Yr5 on 2BL, close to marker wsnp_Ex_c2153_4043746 (Zegeye et al., 2014). In fact, another stripe rust resistance gene is Yr7 located at 685Mb on 2BL was also very near to Qyr.stripe-2BL.1 identified in our analysis (Zhang et al., 2009). Another QTL on 2B, Qyr.stripe-2BL.2 QTL was associated with 7 markers (AX-94490490, AX-95175933, AX-94608940, AX-95142963, AX-95238626, AX-94497849, AX-94868242) and located in the interval 759.82-761.99 Mb and was near to SSR marker Xwmc361 that is close to stripe rust resistance QTL Naxos (QYr.cass-2BL) (Ren et al., 2012). Muhammad et al. (2020) had also reported two QTLs on 2BL (QYr.uaf.2BL.1 and QYr.uaf.2BL.2) spanning the genomic region containing three important genes Yr5, Yr43 and Yr54. Further, QTL identified on the small arm of 3BS, Qyr.stripe-3B (at 3.24 Mb) coincided with Qyr.ramp-3B.1 which was associated with infection caused by two stripe rust pathotypes Yr-47S103 and Yr-46S119 (Kumar et al., 2020). The QTL on 6BL, Qyr.stripe-6BL.2 at 408 Mb was close to Xwmc397 and Xwmc105b SSR markers associated with stripe rust resistance (Christiansen et al., 2006).
For pathotype 46S119, 12 QTLs were identified. Among these, six QTLs (Qyr.stripe-1BL, Qyr.stripe-3AS, Qyr.stripe-4AL, Qyr.stripe-5AS Qyr.stripe-5BS.2, Qyr.stripe-5DS) were located in the previously located regions and remaining were novel (Qyr.stripe-3AL, Qyr.stripe-3B.1 Qyr.stripe-3B.2, Qyr.stripe-4DL, Qyr.stripe-5BS.1 and Qyr.stripe-6DL). The QTL, Qyr.stripe-1BL identified on 1BL explained 11.8% of phenotypic variance for disease severity and coincided to the genomic region containing Yr29, independently identified by Lan et al. (2014) and Muhammad et al. (2020). The long arm of chromosome 1BL, is considered to be rich in Yr resistance genes (Muhammad et al., 2020) which was clearly evident from the detection of 3 QTLs in our analysis, one QTL for pathotype 46S119 and two QTLs for pathotype 238S119. The Qyr.stripe-4AL at 602.2 Mb explained 12.1% of phenotypic variance and was very near to Yr51 and Yr60 (Randhawa et al., 2014; Herrera-Foessel et al., 2015).
For pathotype 238S119, a total of nine genome regions were identified in our analysis. Among these, three QTLs (Qyr.stripe-1BL.1, Qyr.stripe-1BL.2 Qyr.stripe-5BL) were located in the previously identified genomic regions for stripe rust resistance and other six were novel (Qyr.stripe-1AL, Qyr.stripe-4AS, Qyr.stripe-4DL, Qyr.stripe-5BS, Qyr.stripe-5DL.1 and Qyr.stripe-5DL.2). Qyrstripe-1BL on long arm of 1BL at 326.58 Mb, defined up to 8.2% of phenotypic variance for disease severity and coincided to the genomic region containing Yr64 and Yr65 genes (Cheng et al., 2014). Besides this, there were other Yr genes, such as Yr10 and Yr29 in the vicinity of the identified QTL on Chr1BL (Liu et al., 2008; Lan et al., 2014; Muhammad et al., 2020). Moreover, we have also identified a haplotype block of 3 SNPs (AX-95243592, AX-95229302 and AX-94850928) on 1B in the interval 606–608 Mb. Since these 3 SNPs were in high LD, this region was considered just one locus, QTL Qyr.stripe-1BL.2. A previous study on stripe rust resistance in a durum wheat cultivar Wollaroi revealed Yr29 (Xgwm818, Xgwm259) on 1BL at 670 Mb, which was close to Qyrstripe-1BL.2 identified in our study (McIntosh et al., 1995, Bansal et al., 2014). The QTL Qyr.stripe-5BL was located at 499.12 Mb on chromosome 5BL. This QTL was found to be in the vicinity of QTL Qyr.sun-5B, that provided resistance to adult stage stripe resistance (Bariana et al., 2010). Besides the known QTLs, our study has revealed 5 novel QTLs for stripe rust resistance (pathotype 238S119) distributed on 4AS, 4DL, 5BS, and 5DL. These QTLs were at a large distance from the previously identified QTL on these chromosomes.
Similarly, for 110S119, 7 QTLs were identified which included two (Qyr.stripe-6AS and Qyr.stripe-6DL) that coincided with previously reported genomic regions and five were novel (Qyr.stripe-3B, Qyr.stripe-7AS, Qyr.stripe-7DL Qyr.stripe-7DS.1, and Qyr.stripe-7DS.2) (Muhammad et al., 2020). Further, all of them explained <10% phenotypic variance suggesting they were minor QTLs and could be important for providing durable resistance.
Identification of favorable alleles for stripe rust resistance is necessary to enhance the cultivars resistance. In the present study, the correlation between stripe rust resistance for different pathotypes and favorable alleles was highly significant and biologically meaningful. In our data, we identified some indigenous lines which were immune (i.e., having 0 disease severity index) to stripe rust resistance. For example, IC111888 (local germplasm) and IC290156 (local germplasm) from NBPGR, New Delhi was found to be immune in 2 races i.e., 238S119 and 46S119; 46S119 and 47S103, respectively. IC111888 and IC290156 had 41 and 37 favorable alleles. It could be concluded that these germplasm lines have relatively few or low identified resistance-associated favorable alleles and therefore showed high disease severity index. This finding will further provide insights for wheat breeders when choosing the diverse parents as a source of rust resistance to breed wheat for the 12 million hectares prone to this disease.
The results of the present investigation showed the value of our diverse genetic resources conserved in Indian National Gene bank. The germplasm lines/landraces found resistant in the present investigation are valuable sources of resistance and can be used to achieve durable and diverse resistance against stripe rust. In the present study, out of the 40 identified QTLs, 20 QTLs were potentially novel for stripe rust resistance. Further, three putative candidate genes associated with these QTLs are expected to play major roles in marker-assisted wheat breeding for stripe rust resistance in wheat. Further, Genomic regions identified in the present investigations have significant associations with stripe rust resistance in Indian wheat germplasm. However, identified QTLs need to be examined for favorable SNP alleles associated with resistance genes so that they can be used in breeding programs.
The datasets generated for this study can be found in Zenodo, doi: 10.5281/zenodo.4058520.
AKP: investigation, data recording, data analysis, writing—original draft, and writing-review and editing. SundK: conceptualization, data curation, formal analysis, investigation, writing—original draft, and writing-review and editing. AS: conceptualization, investigation, and writing-review and editing. NB: contributed in bioinformatics analysis. DM: contributed in bioinformatics analysis. DC: investigation and data recording. SM and MG: review and editing. SuneK: formal analysis, assisted in writing original draft, review, and editing. OG: phenotypic evaluation of association mapping panel against four different pathotypes of stripe rust. SubK: prepared the inoculum and created artificial epiphytotic conditions for all four pathotypes for 2 years in greenhouse. AG: germplasm curation and reviewing manuscript. SB: phenotypic evaluation of association mapping panel against four different pathotypes of stripe rust. AR and KS: writing—review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The financial support provided by IASRI-CABin for conducting this study was highly acknowledged. All the authors were thankful to Division of Germplasm Conservation and Division of Germplasm Evaluation for providing germplasm lines for this study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.572975/full#supplementary-material
Supplementary Table 1. Details of wheat germplasm lines and distribution of favorable alleles to different pathotypes among 391 wheat germplasm lines used in the present study.
Supplementary Table 2. Disease severity data of 391 germplasm lines against four different pathotypes at seedling stage in 2 consecutive years.
Supplementary Table 3. List of significant MTAs associated to four pathotypes for year 2018.
Supplementary Table 4. List of significant MTAs associated to four pathotypes for year 2019.
Supplementary Table 5. Candidate genes identified within the interval region for chromosome 2BL (759.82–761.99 Mb) and 1BL (606.84–608.53 Mb).
Supplementary Table 6. Comparison of identified QTLs with previously published Yr genes or QTLs.
Supplementary Table 7. Expression of putative candidate genes against susceptible and resistant variety.
GWAS, Genome Wide Association Study; SNPs, Single Nucleotide Polymorphisms; MAS, Marker Assisted Selection; QTL, Quantitative Trait Locus; LD, Linkage Disequilibrium; QQ, Quantile-Quantile plot; PCA, Principal Component Analysis; GAPIT, Genomic Association and Prediction Integrated Tool; CMLM, Compressed Mixed Linear Model; MTA, Marker Trait Association; FDR, False Discovery Rate; YR, Yellow Rust; IWGSC, International Wheat Genome Sequencing Consortium; GO, Gene Ontology; BLAST, Basic Local Alignment Search Tool; ANOVA, Analysis of variance; REML, Restricted maximum Likelihood.
Afzal, S. N., Haque, M. I., Ahmedani, M. S., Bashir, S., and Rattu, A.R. (2007). Assessment of yield losses caused by Puccinia striiformis triggering stripe rust in the most common wheat varieties. Pak. J. Bot. 39, 2127–2134.
Atwell, S., Huang, Y. S., Vilhjálmsson, B. J., Willems, G., Horton, M., Li, Y., et al. (2010). Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465, 627–631. doi: 10.1038/nature08800
Bajgain, P., Rouse, M. N., Bulli, S., Bhavani, S., Gordon, T., Wanyera, R., et al. (2015). Association mapping of North American spring wheat breeding germplasm reveals loci conferring resistance to Ug99 and other African stem rust races. BMC Plant Biol. 15:249. doi: 10.1186/s12870-015-0628-9
Bansal, U. K., Kazi, A. G., Singh, B., Hare, R. A., and Bariana, H. S. (2014). Mapping of durable stripe rust resistance in a durum wheat cultivar Wollaroi. Mol. Breed. 33, 51–59. doi: 10.1007/s11032-013-9933-x
Bariana, H. S., Bansal, U. K., Schmidt, A., Lehmensiek, A., Kaur, J., Miah, H., et al. (2010). Molecular mapping of adult plant stripe rust resistance in wheat and identification of pyramided QTL genotypes. Euphytica 176, 251–260. doi: 10.1007/s10681-010-0240-x
Bhardwaj, S. C., Gangwar, O., Prasad, P., and Khan, H. (2014). Newsletter “Mehtaensis” (Flowerdale Shimla) of Indian Institute of Wheat and Barley Research. Mehtaensis 34, 1–23.
Bhardwaj, S. C., Gangwar, O., Singh, S. B., Saharan, M. S., and Sharma, S. (2012). Rust situation and pathotypes of Puccinia species in Leh Ladakh in relation to recurrence of wheat rusts in India. Indian Phytopath. 65, 230–232.
Bradbury, J., Zhang, Z., Kroon, D. E., Casstevens, T. M., Ramdoss, Y., and Buckler, E. S. (2007). TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics 23, 2633–2635. doi: 10.1093/bioinformatics/btm308
Breseghello, F., and Sorrells, F. M. (2005). Association mapping of kernel size and milling quality in wheat (Triticum aestivum L.) cultivars. Genetics 172, 1165–1177. doi: 10.1534/genetics.105.044586
Bulli, P., Zhang, J., Chao, S., Chen, X., and Pumphrey, M. (2016). Genetic architecture of resistance to stripe rust in a global winter wheat germplasm collection. G3 Genes Genomes Genet. 116:028407. doi: 10.1534/g3.116.028407
Burdon, J. J., Barrett, L. G., Rebetzke, G., and Thrall, H. (2014). Guiding deployment of resistance in cereals using evolutionary principles. Evol. Appl. 7, 609–624. doi: 10.1111/eva.12175
Cantley, L. C. (2002). The phosphoinositide 3-kinase pathway. Science 296, 1655–1657. doi: 10.1126/science.296.5573.1655
Cavanagh, C. R., Chao, S., Wang, S., Huang, B. E., Stephen, S., Kiani, S., et al. (2013). Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. U.S.A. 110, 8057–8062. doi: 10.1073/pnas.1217133110
Chao, S., Dubcovsky, J., Dvorak, J., Luo, M. C., Baenziger, S., Matnyazov, R., et al. (2010). Population-and genome-specific patterns of linkage disequilibrium and SNP variation in spring and winter wheat (Triticum aestivum L.). BMC Genomics 11:727 doi: 10.1186/1471-2164-11-727
Chen, X. M. (2005). Epidemiology and control of stripe rust [Puccinia striiformis f. s tritici] on wheat. Can. J. Plant Pathol. 27, 314–337. doi: 10.1080/07060660509507230
Chen, X. M., Penman, L., Wan, A. M., and Cheng, P. (2010). Virulence races of Puccinia striiformis f. s tritici in 2006 and 2007 and development of wheat stripe rust and distributions, dynamics, and evolutionary relationships of races from 2000 to 2007 in the United States. Can. J. Plant Pathol. 32, 315–323. doi: 10.1080/07060661.2010.499271
Cheng, B., Gao, X., Cao, N., Ding, Y., Gao, Y., Chen, T., et al. (2020). Genome-wide association analysis of stripe rust resistance loci in wheat genotypes from southwestern China. J. Appl. Genet. 61, 37–50. doi: 10.1007/s13353-019-00533-8
Cheng, P., Xu, L. S., Wang, M. N., See, D. R., and Chen, X. M. (2014). Molecular mapping of genes Yr64 and Yr65 for stripe rust resistance in hexaploidderivatives of durum wheat accessions PI 331260 and PI 480016. Theor. Appl. Genet. 127, 2267–2277. doi: 10.1007/s00122-014-2378-8
Christiansen, M. J., Feenstra, B., Skovgaard, I. M., and Andersen, S. B. (2006). Genetic analysis of resistance to yellow rust in hexaploid wheat using a mixture model for multiple crosses. Theor. Appl. Genet. 112, 581–591. doi: 10.1007/s00122-005-0128-7
Cleveland, W. S. (1979). Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 74, 829–836. doi: 10.1080/01621459.1979.10481038
Evanno, G., Regnaut, S., and Goudet, J. (2005). Detecting the number of clusters of individuals using the software structure: a simulation study. Mol. Ecol. 14, 2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x
Feng, J., Wang, M., See, D. R., Chao, S., Zheng, Y., and Chen, X. (2018). Characterization of novel gene Yr79 and four additional quantitative trait loci for all-stage and high-temperature adult-plant resistance to stripe rust in spring wheat PI 182103. Phytopathology 108, 737–747. doi: 10.1094/PHYTO-11-17-0375-R
Flint-Garcia, S. A., Thornsberry, J. M., and Buckler, E. S. (2003). Structure of linkage disequilibrium in plants. Ann. Rev. Plant Biol. 54, 357–374. doi: 10.1146/annurev.arplant.54.031902.134907
Gangwar, O., Kumar, S., Bhardwaj, S. C., Kashyap, L., Prasad, P., and Khan, H. (2019). Characterization of three new Yr9-virulences and identification of sources of resistance among recently developed Indian bread wheat germplasm. J. Plant Pathol. 101, 955–963. doi: 10.1007/s42161-019-00302-w
Gurung, S., Mamidi, S., Bonman, J. M., Xiong, M., Brown-Guedira, G., and Adhikari, T. B. (2014). Genome-wide association study reveals novel quantitative trait loci associated with resistance to multiple leaf spot diseases of spring wheat. PLoS ONE 9:e0108179. doi: 10.1371/journal.pone.0108179
Herrera-Foessel, S. A., Singh, R. P., Lan, C. X., Huerta-Espino, J., Calvo-Salazar, V., Bansal, U. K., et al. (2015). Yr60, a gene conferring moderate resistance to stripe rust in wheat. Plant Dis. 99, 508–511. doi: 10.1094/PDIS-08-14-0796-RE
Hulbert, S., and Pumphrey, M. (2014). A time for more booms and fewer busts? Unraveling cereal-rust interactions. Mol. Plant Microbe Interact. 27, 207–214. doi: 10.1094/MPMI-09-13-0295-FI
Indu, S., and Saharan, M. S. (2011). Status of wheat diseases in India with a special reference to stripe rust. Plant Dis. Res. 26:156.
Kertho, A., Mamidi, S., Bonman, J. M., McClean, P. E., and Acevedo, M. (2015). Genome-wide association mapping for resistance to leaf and stripe rust in winter-habit hexaploid wheat landraces. PLoS ONE 10:e0129580. doi: 10.1371/journal.pone.0129580
Kumar, D., Kumar, A., Chhokar, V., Gangwar, O., Bhardwaj, S. C., Sivasamy, M., et al. (2020). Genome-wide association studies in diverse spring wheat panel for stripe, stem and leaf rust resistance. Front. Plant Sci. 11:748. doi: 10.3389/fpls.2020.00748
Kumar, S., Archak, S., Tyagi, R. K., Kumar, J., Jacob, S. R., Srinivasan, K., et al. (2016). Evaluation of 19,460 wheat genotypes conserved in the Indian national genebank to identify new sources of resistance to rust and spot blotch diseases. PLoS ONE 11:e0167702. doi: 10.1371/journal.pone.0167702
Laidò, G., Panio, G., Marone, D., Russo, M. A., Ficco, D. B., Giovanniello, V., et al. (2015). Identification of new resistance loci to African stem rust race TTKSK in tetraploid wheats based on linkage and genome-wide association mapping. Front. Plant. Sci. 6:1033. doi: 10.3389/fpls.2015.01033
Lan, C., Singh, R., Huerta-Espino, J., Calvo-Salazar, V., and Herrera-Foessel, S. A. (2014). QTL characterization of resistance to leaf rust and stripe rust in the spring wheat line Francolin-1. Mol. Breed. 34, 789–803. doi: 10.1007/s11032-014-0075-6
Letta, T., Maccaferri, M., Badebo, A., Ammar, K., Ricci, A., Crossa, J., et al. (2013). Searching for novel sources of field resistance to Ug99 and Ethiopian stem rust races in durum wheat via association mapping. Theor. Appl. Genet. 126, 1237–1256. doi: 10.1007/s00122-013-2050-8
Li, J., Dundas, I., Dong, C., Li, G., Trethowan, R., Yang, Z., et al. (2020). Identification and characterization of a new stripe rust resistance gene Yr83 on rye chromosome 6R in wheat. Theor. Appl. Genet. 133, 1095–1107. doi: 10.1007/s00122-020-03534-y
Line, R. F., and Qayoum, A. (1992). Virulence, aggressiveness, evolution and distribution of races of Puccinia striiformis (the cause of stripe rust of wheat) in North America, 1968–87. US Dept. Agric. Tech. Bull. 1788, 1–44.
Lipka, A. E., Tian, F., Wang, Q., Peiffer, J., Li, M., and Bradbury, P. J. (2012). GAPIT: genome association and prediction integrated tool. Bioinformatics 28, 2397–2399. doi: 10.1093/bioinformatics/bts444
Liu, D., Xia, X. C., He, Z. H., and Xu, S. C. (2008). A novel homeobox-like gene associated with reaction to stripe rust and powdery mildew in common wheat. Phytopathology 98, 1291–1296. doi: 10.1094/PHYTO-98-12-1291
Liu, W., Maccaferri, M., Chen, X., Laghetti, G., Pignone, D., Pumphrey, M., et al. (2017a). Genome-wide association mapping reveals a rich genetic architecture of stripe rust resistance loci in emmer wheat (Triticum turgidum ss dicoccum). Theor. Appl. Genet. 130, 2249–2270. doi: 10.1007/s00122-017-2957-6
Liu, W., Maccaferri, M., Rynearson, S., Letta, T., Zegeye, H., Tuberosa, R., et al. (2017b). Novel sources of stripe rust resistance identified by genome-wide association mapping in Ethiopian durum wheat (Triticum turgidum ssp. durum). Front. Plant Sci. 8:774. doi: 10.3389/fpls.2017.00774
Maulana, F., Ayalew, H., Anderson, J. D., Kumssa, T. T., Huang, W., and Ma, X. F. (2018). Genome-wide association mapping of seedling heat tolerance in winter wheat. Front. Plant Sci. 9:1272 doi: 10.3389/fpls.2018.01272
McIntosh, R. A., Dubcovsky, J., Rogers, W. J., Morris, C., and Xia, X. C. (2017). Catalogue of Gene Symbols for Wheat.
McIntosh, R. A., Wellings, C. R., and Park, R. F. (1995). Wheat Rusts: An Atlas of Resistance Genes. CSIRO Publishing.
McNeal, F. H., Konzak, C. F., Smith, E., Tate, W. S., and Russell, T. S. (1971). A uniform system for recording and processing cereal research data. US Dept. Agric. Res. Serv. 34, 121–143.
Muhammad, S., Sajjad, M., Khan, S. H., Shahid, M., Zubair, M., Awan, F. S., et al. (2020). Genome-wide association analysis for stripe rust resistance in spring wheat (Triticum aestivum L.) germplasm. J. Integr. Agric. 19, 2035–2043. doi: 10.1016/S2095-3119(19)62841-8
Muleta, K. T., Bulli, P., Rynearson, S., Chen, X., and Pumphrey, M. (2017a). Loci associated with resistance to stripe rust (Puccinia striiformis f. s tritici) in a core collection of spring wheat (Triticum aestivum). PLoS ONE 12:e0179087. doi: 10.1371/journal.pone.0179087
Muleta, K. T., Rouse, M. N., Rynearson, S., Chen, X., Buta, B. G., and Pumphrey, M. O. (2017b). Characterization of molecular diversity and genome-wide mapping of loci associated with resistance to stripe rust and stem rust in Ethiopian bread wheat genotypes. BMC Plant Biol. 17:134. doi: 10.1186/s12870-017-1082-7
Naruoka, Y., Garland-Campbell, K., and Carter, A. (2015). Genome-wide association mapping for stripe rust (Puccinia striiformis f. s tritici) in US Pacific Northwest winter wheat (Triticum aestivum L.). Theor. Appl. Genet. 128, 1083–1101. doi: 10.1007/s00122-015-2492-2
Nayar, S. K., Prashar, M., and Bhardwaj, S. C. (1997). Manual of Current Techniques in Wheat Rusts. Research Bulletin No. 2: 32 Regional Station, Directorate of Wheat Research Flowerdale, Shimla.
Nsabiyera, V., Bariana, H. S., Qureshi, N., Wong, D., Hayden, M. J., and Bansal, U. K. (2018). Characterisation and mapping of adult plant stripe rust resistance in wheat line Aus27284. Theor. Appl. Genet. 131, 1459–1467. doi: 10.1007/s00122-018-3090-x
Pakeerathan, K., Bariana, H., Qureshi, N., Wong, D., Hayden, M., and Bansal, U. (2019). Identification of a new source of stripe rust resistance Yr82 in wheat. Theor. Appl. Genet. 132, 3169–3176. doi: 10.1007/s00122-019-03416-y
Pasam, R. K., Bansal, U., Daetwyler, H. D., Forrest, K. L., Wong, D., and Petkowski, J. (2017). Detection and validation of genomic regions associated with resistance to rust diseases in a worldwide hexaploid wheat landrace collection using BayesR and mixed linear model approaches. Theor. Appl. Genet. 130, 777–793. doi: 10.1007/s00122-016-2851-7
Prashar, M., Bhardwaj, S. C., Jain, S. K., and Datta, D. (2007). Pathotypic evolution in Puccinia striiformis in India during 1995–2004. Aust. J. Agric. Res. 58, 602–604. doi: 10.1071/AR07126
Pritchard, J. K., Stephens, M., and Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genet. Soc. Am. 155, 945–959.
Qaseem, M. F., Qureshi, R., Shaheen, H., and Shafqat, N. (2019). Genome-wide association analyses for yield and yield-related traits in bread wheat (Triticum aestivum L.) under pre-anthesis combined heat and drought stress in field conditions. PLoS ONE 14:e0213407. doi: 10.1371/journal.pone.0213407
Qi, T., Wang, J., Sun, Q., Day, B., Guo, J., and Ma, Q. (2017). TaARPC3, contributes to wheat resistance against the stripe rust fungus. Front. Plant Sci. 8:1245. doi: 10.3389/fpls.2017.01245
Randhawa, M., Bansal, U., Valárik, M., Klocová, B., Dolezel, J., and Bariana, H. (2014). Molecular mapping of stripe rust resistance gene Yr51 in chromosome 4AL of wheat. Theor. Appl. Genet. 127, 317–324. doi: 10.1007/s00122-013-2220-8
Ren, Y., He, Z., Li, J., Lillemo, M., Wu, L., Bai, B., et al. (2012). QTL mapping of adult-plant resistance to stripe rust in a population derived from common wheat cultivars Naxos and Shanghai 3/Catbird. Theor. Appl. Genet. 125, 1211–1221. doi: 10.1007/s00122-012-1907-6
Rosewarne, G. M., Herrera-Foessel, S. A., Singh, R., Huerta-Espino, J., Lan, C. X., and He, Z. H. (2013). Quantitative trait loci of stripe rust resistance in wheat. Theor. Appl. Genet. 126, 2427–2449. doi: 10.1007/s00122-013-2159-9
Sehgal, D., Vikram, P., Sansaloni, C., Ortiz, C., Saint Pierre, C., Payne, T., et al. (2015). Exploring and mobilizing the gene bank biodiversity for wheat improvement. PLoS ONE 10:e0132112. doi: 10.1371/journal.pone.0132112
Tadesse, W., Ogbonnaya, F., Jighly, A., Nazari, K., Rajaram, S., and Baum, M. (2013). Association mapping of resistance to yellow rust in winter wheat cultivars and elite genotypes. Crop Sci. 54, 607–616. doi: 10.2135/cropsci2013.05.0289
Tomar, S. M. S., Singh, S. K., Sivasamy, M., and Vinod, P. (2014). Wheat rusts in India: resistance breeding and gene deployment–a review. Indian J. Genet. Plant Breed. 74, 129–156. doi: 10.5958/0975-6906.2014.00150.3
Turner, M. K., Kolmer, J. A., Pumphrey, M. O., Bulli, P., Chao, S., and Anderson, J. A. (2017). Association mapping of leaf rust resistance loci in a spring wheat core collection. Theor. Appl. Genet. 130, 345–361. doi: 10.1007/s00122-016-2815-y
Voss-Fels, K., Frisch, M., Qian, L., Kontowski, S., Friedt, W., Gottwald, S., et al. (2015). Subgenomic diversity patterns caused by directional selection in bread wheat gene pools. Plant Genome 8, 1–13. doi: 10.3835/plantgenome2015.03.0013
Wang, X., Vignjevic, M., Jiang, D., Jacobsen, S., and Wollenweber, B. (2014). Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 65, 6441–6456. doi: 10.1093/jxb/eru362
Wei, W., Mesquita, A. C. O., Figueiró, A. D. A., Wu, X., Manjunatha, S., Wickland, D., et al. (2017). Genome-wide association mapping of resistance to a Brazilian isolate of Sclerotinia sclerotiorum in soybean genotypes mostly from Brazil. BMC Genomics 18:849. doi: 10.1186/s12864-017-4160-1
Wellings, C. R. (2011). Global status of stripe rust: a review of historical and current threats. Euphytica 179, 129–141. doi: 10.1007/s10681-011-0360-y
Yao, F., Zhang, X., Ye, X., Li, J., Long, L., Yu, C., et al. (2019). Characterization of molecular diversity and genome-wide association study of stripe rust resistance at the adult plant stage in Northern Chinese wheat landraces. BMC Genet. 20:38. doi: 10.1186/s12863-019-0736-x
Ye, X., Li, J., Cheng, Y., Yao, F., Long, L., Yu, C., et al. (2019). Genome-wide association study of resistance to stripe rust (Puccinia striiformis f. sp. tritici) in Sichuan wheat. BMC Plant Biol. 19:147. doi: 10.1186/s12870-019-1764-4
Yu, J., Pressoir, G., Briggs, W. H., Vroh Bi, I., Yamasaki, M., and Doebley, J. F. (2006). A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 38, 203–208. doi: 10.1038/ng1702
Yu, L. X., Morgounov, A., Wanyera, R., Keser, M., Singh, S., and Sorrells, M. (2012). Identification of Ug99 stem rust resistance loci in winter wheat germplasm using genome-wide association analysis. Theor. Appl. Genet. 125, 749–758. doi: 10.1007/s00122-012-1867-x
Zegeye, H., Rasheed, A., Makdis, F., Badebo, A., and Ogbonnaya, F. C. (2014). Genome-wide association mapping for seedling and adult plant resistance to stripe rust in synthetic hexaploid wheat. PLoS ONE 9:e105593. doi: 10.1371/journal.pone.0105593
Zhang, P., McIntosh, R. A., Hoxha, S., and Dong, C. (2009). Wheat stripe rust resistance genes Yr5 and Yr7 are allelic. Theor. Appl. Genet. 120, 25–29. doi: 10.1007/s00122-009-1156-5
Zhang, Z., Ersoz, E., Lai, C. Q., Todhunter, R. J., Tiwari, H. K., and Gore, M. A. (2010). Mixed linear model approach adapted for genome-wide association studies. Nat. Genet. 42, 355–60. doi: 10.1038/ng.546
Keywords: stripe rust, wheat, SNP, GWAS, GAPIT
Citation: Pradhan AK, Kumar S, Singh AK, Budhlakoti N, Mishra DC, Chauhan D, Mittal S, Grover M, Kumar S, Gangwar OP, Kumar S, Gupta A, Bhardwaj SC, Rai A and Singh K (2020) Identification of QTLs/Defense Genes Effective at Seedling Stage Against Prevailing Races of Wheat Stripe Rust in India. Front. Genet. 11:572975. doi: 10.3389/fgene.2020.572975
Received: 15 June 2020; Accepted: 30 September 2020;
Published: 27 November 2020.
Edited by:
Reyazul Rouf Mir, Sher-e-Kashmir University of Agricultural Sciences and Technology, IndiaReviewed by:
Vijay Gahlaut, University of Delhi, IndiaCopyright © 2020 Pradhan, Kumar, Singh, Budhlakoti, Mishra, Chauhan, Mittal, Grover, Kumar, Gangwar, Kumar, Gupta, Bhardwaj, Rai and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sundeep Kumar, c3VuZGVlcC5rdW1hckBpY2FyLmdvdi5pbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.