
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 05 November 2020
Sec. Evolutionary and Population Genetics
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.568223
This article is part of the Research Topic Coping with Climate Change: A Genomic Perspective on Thermal Adaptation View all 11 articles
Several cyanobacterial species are dominant primary producers in hot spring microbial mats. To date, hot spring cyanobacterial taxonomy, as well as the evolution of their genomic adaptations to high temperatures, are poorly understood, with genomic information currently available for only a few dominant genera, including Fischerella and Synechococcus. To address this knowledge gap, the present study expands the genomic landscape of hot spring cyanobacteria and traces the phylum-wide genomic consequences of evolution in high temperature environments. From 21 globally distributed hot spring metagenomes, with temperatures between 32 and 75°C, 57 medium- and high-quality cyanobacterial metagenome-assembled genomes were recovered, representing taxonomic novelty for 1 order, 3 families, 15 genera and 36 species. Comparative genomics of 93 hot spring genomes (including the 57 metagenome-assembled genomes) and 66 non-thermal genomes, showed that the former have smaller genomes and a higher GC content, as well as shorter proteins that are more hydrophilic and basic, when compared to the non-thermal genomes. Additionally, the core accessory orthogroups from the hot spring genomes of some genera had a greater abundance of functional categories, such as inorganic ion metabolism, translation and post-translational modifications. Moreover, hot spring genomes showed increased abundances of inorganic ion transport and amino acid metabolism, as well as less replication and transcription functions in the protein coding sequences. Furthermore, they showed a higher dependence on the CRISPR-Cas defense system against exogenous nucleic acids, and a reduction in secondary metabolism biosynthetic gene clusters. This suggests differences in the cyanobacterial response to environment-specific microbial communities. This phylum-wide study provides new insights into cyanobacterial genomic adaptations to a specific niche where they are dominant, which could be essential to trace bacterial evolution pathways in a warmer world, such as the current global warming scenario.
Cyanobacteria are photosynthetic microorganisms that shaped the earth’s atmosphere during the Great Oxidation Event 2.6 billion years ago (Castenholz et al., 2001; Schirrmeister et al., 2015). They are morphologically diverse and thrive in most environments exposed to light, such as the ocean, lakes, soils, deserts and hot springs (Castenholz et al., 2001). Indeed, they are members of microbial mat communities from non-acidic hot springs, leading primary production and nitrogen fixation (Castenholz, 1969; Ward et al., 1998; Alcamán et al., 2015). Within these thermal microbial mats, which are found across all continents (Castenholz, 1969; Ionescu et al., 2010), the temperature boundary for cyanobacterial survival is approximately 73°C (Cox et al., 2011). In these environments, the temperature gradient shapes the microbial community (Klatt et al., 2011; Mackenzie et al., 2013), with some thermophilic cyanobacteria living at higher temperatures, and thermotolerant or mesophilic members living in the hot spring outflow or borders (Finsinger et al., 2008; Ward et al., 2012).
Over the last two centuries, several hot spring cyanobacterial species have been described (for descriptions, see Komárek, 1999, 2013; Komárek and Anagnostidis, 2005; Ward et al., 2012). Later, phylogenetic analyses revealed a wide biogeographical distribution of unicellular and filamentous thermal members (Papke et al., 2003; Miller et al., 2007; Ionescu et al., 2010; Sciuto and Moro, 2016), demonstrating that the ability to survive at high temperatures is polyphyletic within the phylum (Sanchez-Baracaldo et al., 2005; Uyeda et al., 2016). Nevertheless, the taxonomic information remains incomplete because most known thermophilic strains have only been assigned at the family or genus level, with newly obtained environmental 16S rRNA gene sequences classified based solely on these references (Ionescu et al., 2010; Mackenzie et al., 2013). This, together with the currently persisting conflict in cyanobacterial taxonomy and nomenclature (see Oren and Ventura, 2017, and references therein), prevents the high-resolution description of new cyanobacterial species and genera from environmental samples.
Three major morphological groups of early-described thermophilic cyanobacteria (Schwabe, 1837; Copeland, 1936) have been widely studied. The first group is the unicellular cyanobacteria that diverged and now specializes along the temperature gradient and vertical layers of hot spring microbial mats (Ward et al., 1998, 2006; Olsen et al., 2015). They survive up to the oxygenic photosynthesis temperature limit (Meeks and Castenholz, 1971; Cox et al., 2011), and are most represented by the genera Synechococcus and Thermosynechococcus, which comprise two very deep branches near the base of the phylum Cyanobacteria (Shih et al., 2013; Dagan et al., 2013). The second group is represented by filamentous non-heterocystous cyanobacteria, which are morphologically diverse as the Spirulina, Leptolyngbya and Phormidium genera (see Copeland, 1936). Some members have the potential to perform both oxygenic and anoxygenic photosynthesis (Momper et al., 2019). Furthermore, they also are globally distributed and dominant in hot springs (Sciuto and Moro, 2016; Yoon et al., 2017). The third and most studied hot spring morphological group is the filamentous heterocystous cyanobacteria, which includes the true-branching species Fischerella thermalis. This group has become a high-temperature model for different research topics, such as photosynthesis, nitrogen fixation, multicellularity and biogeography (Alcorta et al. (2019), and references therein).
Genomes from various hot spring strains have shown diverse adaptations to the thermal environment, such as different strategies for phosphate and nitrogen uptake; light responses at different temperatures and depths; and heterocyst envelope composition (Bhaya et al., 2007; Olsen et al., 2015; Alcorta et al., 2018; Sano et al., 2018). Genome reduction is a main evolutionary trend related to the hot spring environment, such as that observed for Synechococcus sp. OS-A and OS-B’, and Thermosynechococcus (Larsson et al., 2011). The negative correlation of genome size and protein length with increasing temperature is also a major evolutionary trend related to thermophilic bacteria (Sabath et al., 2013), as is differentiated nucleotide content, codon usage, amino acid composition (Vieille and Zeikus, 2001; Singer and Hickey, 2003; Zeldovich et al., 2007) and the prevalence of the CRISPR-Cas system (Weinberger et al., 2012). However, cyanobacterial genomes are not well represented in these studies; thus, the genomic features and functional categories that differentiate thermophilic cyanobacteria are still unknown.
To obtain new information on hot spring cyanobacterial genomic features, metagenomic reconstruction of complete or partial genomes, known as metagenome-assembled genomes (MAGs), was used to uncover an unprecedent diversity. From 21 globally distributed neutral-pH hot spring microbial mats, with temperatures between 32 and 75°C, 57 new cyanobacterial MAGs were obtained, some of which were classified into well-known genera and species, while others represent new taxa at the order to species levels. Comparative genomics corroborates thermophilic features prevalent in other bacteria, and also reveals new trends related to exclusive orthologs, abundances of protein functional categories and adaptative genes involved in the response to the microbial and viral hot spring community. These results highlight various consequences of the ecological speciation process on thermophilic cyanobacterial genomes. However, more studies are now required to reveal the initial colonization mechanism of these organisms to this extreme habitat.
Phototrophic microbial mat samples were taken from El Tatio geyser field (Atacama, Chile), Cahuelmó hot spring (Northern Patagonia, Chile) and Kroner Lake (Deception Island, Antarctica). The sample location, collection year and physicochemical parameters are listed in Supplementary Table 1. Temperature and pH were determined using a multiparameter instrument (model 35607-85; Oakton, Des Plaines, IL, United Stattes). For molecular analysis, 2 cm core samples were collected and frozen at −80°C until subsequent procedures. DNA was isolated using a bead-beating protocol with xanthogenate lysis buffer and a phenol–chloroform extraction, according to Alcorta et al. (2018). The quality and quantity of nucleic acids were checked using the Qubit (LifeTechnologies, Carlsbad, CA, United States) and Nanodrop (Thermo Fisher Scientific, Waltham, MA, United States) systems.
For metagenomic analysis, DNA samples were sequenced on the Illumina HiSeq platform (Illumina, San Diego, CA, United States) at the Research and Testing Laboratory (Lubbock, TX, United States). Briefly, the DNA was fragmented using the NEBNext dsDNA Fragmentase kit (New England Biolabs, Ipswich, MA, United States), followed by DNA clean up via column purification and library construction with the NEBUltra DNA Library Prep kit for Illumina (New England Biolabs). Methodology from Guajardo-Leiva et al. (2018) was followed for quality filtering using Cutadapt (Martin, 2011) with the parameters: paired-end mode, a perfect match of at least 10 bp (-O 10) against the standard Illumina adaptors, hard clipping of the first five leftmost bases (-u 5), 3′ end trimming for bases with a quality score below 28 (-q 28) and retaining only sequences longer than 30 bp (-m 30).
De novo assemblies of trimmed reads were generated using SPAdes v3.10.1 (Bankevich et al., 2012) with the –meta option. Contigs longer than 1,000 bp were grouped into MAGs using the metaWRAP binning module (Uritskiy et al., 2018), which incorporates the following three binning software: metaBAT 2 v2.12.1 (Kang et al., 2019), MaxBin2 (Wu et al., 2016) and CONCOCT v1.1.0 (Alneberg et al., 2014) with default parameters. Next, the bin_refinement module of metaWRAP (Uritskiy et al., 2018) was used to consolidate results from the three methods using the -c 50 and -x 10 options to obtain bins with over 50% completeness and less than 10% contamination according to the CheckM tool v1.0.18 (Parks et al., 2015), which also uses HMMER v3.2.11 (Eddy, 1998) as a third-party software. The refineM tool (Parks et al., 2017) was used to clean potential contig contamination with different genomic properties (tetranucleotide signature and coverage) via the scaffold_stats, outliers and filter_bin modules. The refineM tool (Parks et al., 2017) was also used to clean potential contamination based on taxonomic assignment with the following modules: call_genes, which uses Prodigal v.2.6.3 (Hyatt et al., 2010); taxon_profile, using the GTDB R80 custom protein database from the Genome Taxonomy Database (GTDB; Parks et al., 2018; available at2); and taxon_filter and ssu_erroneous, using the GTDB R80 custom SSU database2. The obtained MAGs were reassessed with the CheckM tool v1.0.18 (Parks et al., 2015), their tRNAs were predicted with the ARAGORN webserver (Laslett and Canback, 2004), and their ribosomal subunit sequences were searched with Barrnap v0.93. According to the Genomic Standards Consortium parameters, this information allowed us to classify the MAGs as high-, medium- or low-quality (Bowers et al., 2017). Taxonomic assignment was performed using GTDB-tk v0.3.2 software with version R89 (Chaumeil et al., 2020), which also uses pplacer as a third-party software (Matsen et al., 2010). Genomes belonging to the phylum Cyanobacteria were used for further analyses. The similarity between MAGs was assessed through all-vs-all comparisons of the average nucleotide identity (ANI) using fastANI software (Jain et al., 2018) and the average amino acid identity (AAI) using compareM software4. MAGs identified as possible new taxa were grouped according to thresholds of similarity for the ANI and AAI values stated by Konstantinidis et al. (2017).
Additionally, a locally built database of public metagenomes from hot springs was analyzed. SRA files were first downloaded from the NCBI database, and subsequently quality trimmed, assembled, binned and taxonomically classified as described above. The only procedural difference was that the SRR5581334, SRR7905023 and ERR372908 metagenomes were assembled using MEGAHIT 1.2.9 (Li et al., 2015), due to memory requirements. Details of these metagenomes are listed in Supplementary Table 1. High- and medium-quality MAGs assigned to the phylum Cyanobacteria were used for further analyses. All obtained cyanobacterial MAGs were submitted in FASTA format to the Figshare repository5 under 10.6084/m9.figshare.12400979. Meanwhile, 34 high- and medium-quality MAGs with > 95% completeness were deposited under NCBI BioProject numbers PRJNA635751 and PRJNA645256. The MAGs obtained using primary data from this study and from Alcamán-Arias et al. (2018) were submitted to the NCBI WGS database, while those obtained from Chan et al. (2015), Kaushal et al. (2018), Ward et al. (2019) and Roy et al. (2020) were submitted to the Third Party Annotation database6.
The abundance of the recovered MAGs was assessed through read mapping. Briefly, quality trimmed reads of each sample were mapped using BBMap v38.717 with a minimum identity of 99% (minid = 0.99 and idfilter = 0.97). A MAG was considered present in a sample when it had a coverage > 1x across 75% of its genome; otherwise, the abundance was considered zero. Absolute read counts of selected MAGs were normalized as the number of reads recruited per kilobase of MAG and gigabase of metagenome (RPKG), which allowed the direct comparison of genome abundances between metagenomes of different depths (Reji and Francis, 2020). Normalized read counts were used to calculate diversity metrics, as well as the similarity matrix for multivariate analyses with the “Vegan” package in R.
All genomes classified as Cyanobacteria (1,626 genomes as of September 2019) were downloaded from the NCBI database, and the GTDB taxonomy was assigned to each using GTDB-tk software (Chaumeil et al., 2020). The dRep v2.3.2 software (Olm et al., 2017) was used to dereplicate the entire set due to overrepresented species and low-quality genomes (e.g., only 1136 exhibited > 75% completeness), thus obtaining a final subset of 800 genomes (Supplementary Table 2). A total of 120 concatenated single-copy bacterial genes were recovered from the intermediate files of the GTDB-tk analysis (gtdbtk.bac120.msa.fasta files), and their sequences were subsequently aligned using MUSCLE v6.0.98 software (Edgar, 2004). Maximum-likelihood trees were generated using IQtree v.1.5.5 software with the TESTNEW option to choose the best substitution models, after which a non-parametric ultrafast bootstrap (-bb) support of 10,000 replicates was applied (Hoang et al., 2017). Node collapse and rooting of phylogenetic reconstructions were managed using the iTOL web server (Letunic and Bork, 2019).
Genomic features of the 57 MAGs obtained in the present study, as well as for the 800 cyanobacterial genomes from the NCBI database, were extracted from the CheckM summary results (see above, Supplementary Table 2). As the MAGs and NCBI genomes have different completeness levels, the expected genome size was calculated as EGS = (genome size ∗ 100)/(completeness). In conjunction with the MAGs, a subset of NCBI genomes that were taxonomically close to the MAGs (according to GTDB-tk), was used to compare the genome size, GC percentage and coding density between hot spring cyanobacteria (all 57 MAGs and 36 NCBI genomes) and non-thermal cyanobacteria (66 NCBI genomes). Together, these 159 genomes are hereafter referred to as the 159-subset. Additionally, the hydrophobicity, protein length, protein molecular weight, isoelectric point (pI) and amino acid usage were compared for the coding DNA sequences (CDSs) calculated with ProPAS v1.03 software (Wu and Zhu, 2012). Furthermore, genomes from both environmental groups were compared for the Thermosynechococcacceae, Elainellaceae and Oscillatoriaceae families, and for the Fischerella and Geminocystis genera (≥ 3 genomes for each environment) to identify differences at these specific taxonomic levels. The isolation environments and associated references for each cyanobacterial genome of the 159-subset are listed in Supplementary Table 3.
Additionally, the 159-subset orthogroups (orthologous gene clusters, see Supplementary Table 4) obtained with Orthofinder v2.3.3 software (Emms and Kelly, 2015), were used to identify the core and accessory orthogroups. Orthogroups present in > 97% of the subset (153 genomes) were considered the core genome (based on their distribution in the total 47,328 obtained orthogroups). Orthogroups sporadically present in both environmental groups were considered the phylum accessory set, while those exclusive to hot spring or non-thermal genomes were considered as the specific accessory orthogroups. This includes the singletons as well as the specific core accessory orthogroups if they have presence in all genomes an environmental group. This same classification was also performed for seven genera with genomes from both environmental groups (Calothrix, Cyanobacterium, Elainella, Fischerella, Geminocystis, Rivularia and Trichormus) and a heatmap of the relative abundance of COG categories was created with R software to make a hierarchical clustering (hclust) of the core and accessory categories. Orthogroups distributed in more than one order, family or genus in each environmental group, were further explored by searching for homologous sequences in the NCBI non-redundant protein database using BLASTP (Altschul et al., 1990). From the retrieved sequences, phylogenetic reconstructions were generated as explained above.
Functional annotation of the 159-subset genomes was done with eggnog-mapper software (Huerta-Cepas et al., 2017, 2019). First, the genomic percentage of COG categories in each genome was compared between hot spring and non-thermal cyanobacteria using STAMP v2.1.3 software (Parks et al., 2014) via the Welch’s t-test with Bonferroni multiple test correction. Next, specific annotations of complementary metabolism, defense systems and secondary metabolites were searched. Because many proteins are annotated with putative functions or as hypothetical proteins, the following specific terms were used to search within the annotated 159-subset: “nitrogenase,” “nitrate,” “nitrite,” “restriction,” “modification,” “capsid,” “dehydrogenase,” “CRISPR,” “virus,” “viral” and “capsid.” Orthogroups harboring protein sequences whose annotations indeed corresponded to these functions were then used for further analyses. Some orthogroups with ambiguous functions (different annotations within the orthogroup) were ignored. For the sulfide-quinone reductase protein, which is characterized as a possible switch between anoxygenic and oxygenic photosynthesis, BLASTP was used with the reference NCBI sequence (AF242368.1 or WP_071516517.1) to search the corresponding orthogroup. Secondary metabolite biosynthetic gene clusters (BGCs) were searched with antiSMASH v4.2.0 software (Blin et al., 2017) using the clusterblast and borderpredict options.
Correlation analyses between MAGs abundances and environmental parameters were calculated with the Mantel test implemented in the R package “Vegan,” only considering the variables temperature, pH and geographical location. Additional parameters that were not available for all samples, were excluded from the analyses. Wilcoxon’s paired test was used because the comparison of genome features was not balanced between hot spring and non-thermal genome groups. Correlation analyses were performed by determining the adjustment to the “normal” distribution of each variable with the Kolmogorov-Smirnov test and by using parametric Pearson’s or non-parametric Spearman’s tests, depending on the distribution of data. Correlation indexes were compared using the r-to-z Fischer transformation. For multiple analyses over the same dataset, Bonferroni and FDR corrections were applied to the obtained p-values. The R packages “Tidyverse,” “ggpubr,” “ggplot2,” “cocor,” and “maps” (R Core Team, 2017) were used for all statistical analyses and corrections, as well as most of the plots.
In this study, four new metagenomes from hot spring phototrophic microbial mats in Chile and Antarctica were analyzed along with 79 metagenomes already available in the NCBI database. The four new metagenomes altogether added up to 60.75 Gbp of total trimmed reads and 292,512 assembled contigs (> 1,000 bp), totaling 1.36 Gbp. For the locally built database, cyanobacterial sequences were identified in only 17 of the 79 hot spring metagenomes, which then were used for further analyses. Altogether these 21 cyanobacteria-containing metagenomes were distributed in North and South America, Asia and Antarctica (Figure 1), representing a temperature range between 32 and 75°C, and a pH range of 5.8 to 9.2. These comprised 359.8 Gbp of trimmed reads and 5.19 Gbp of assembled contigs (representing 1,393,425 contigs > 1,000 bp). A total of 1,152 medium- or high-quality MAGs were recovered from these 21 metagenomes. According to the GTDB-tk taxonomic assignment, most of these MAGs belong to the phyla Proteobacteria (16.1%), Chloroflexota (13.8%), Bacteroidota (12.9%), Planctomycetota (6.3%) and Cyanobacteria (4.9%) (Supplementary Table 5).
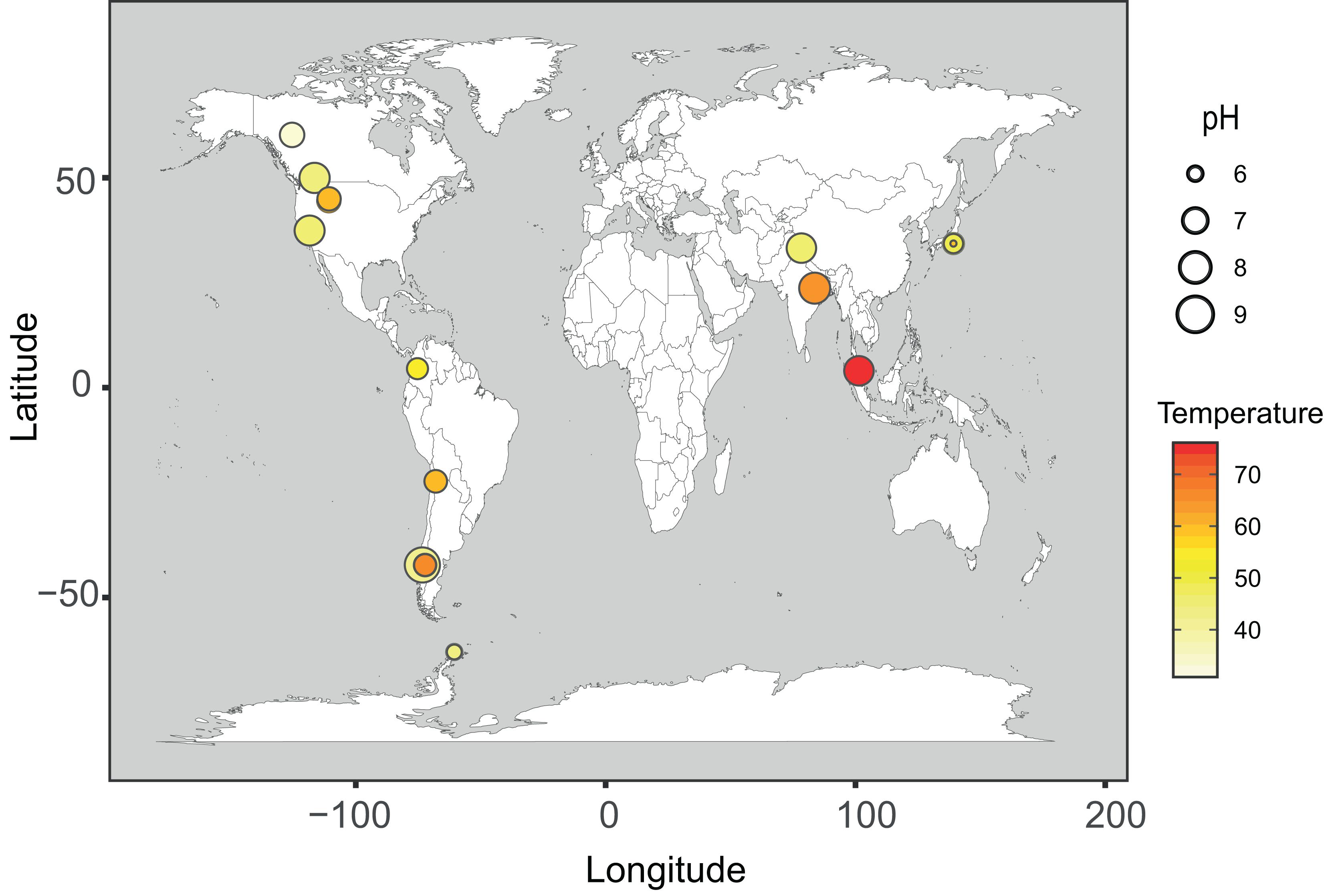
Figure 1. Geographical distribution of phototrophic hot spring metagenomes. Distribution map of the 21 hot spring microbial mat metagenomes used in the present study. Each circle represents one sample. The color scale for circles represents the temperature range from 32 to 75°C, and the circle size represents the pH range (from 5.8 to 9.2) of each sample.
A total of 20 cyanobacterial MAGs (phylum Cyanobacteria GTDB R89) were recovered from the four new metagenomes reported in the present study. Overall, 57 cyanobacterial MAGs with an average completeness of 88.5% (SD ± 14.7), were obtained from the entire set of 21 hot spring metagenomes. The average contamination of these MAGs was 1.2% (SD ± 1.1), with 4.8% being the highest value (Table 1). According to the GSC quality parameters (Bowers et al., 2017), only two MAGs were classified as high-quality (M3746_SRR7905025_W2019_013 and M46_SRR2626160_R2017_013); while 55 MAGs were medium-quality, from which 36 could be categorized as high-quality if their rRNA operon sequences were binned.
Several strategies have been used to recover partial genomes of hot spring cyanobacteria from metagenomes, including mapping contigs against reference genomes (Bhaya et al., 2007) and the use of binning tools (Thiel et al., 2017; Alcorta et al., 2018; Ward et al., 2019). Metagenomes from the binning-based studies were reanalyzed here, allowing for better genome recovery in terms of contig numbers and completeness. Furthermore, the ssu_erroneous module from refineM enabled removal of partial 16S rRNA gene sequences with incongruent taxonomy from other phyla. Reanalysis of previously published data is useful for maintaining confidence in public repositories of genomes (Shaiber and Eren, 2019) and to obtain genomes that fulfill acceptable standards (Bowers et al., 2017).
Cyanobacterial taxonomy and nomenclature have always been controversial (see Oren and Ventura, 2017, and references therein). Nowadays, applying a valid taxonomy based on the genomes of uncultured organisms is impossible (e.g., the rejection of the Whitman (2016) proposal), even when following good practice strategies (Konstantinidis et al., 2017; Chun et al., 2018). This study increased the percentage of available genomes within cyanobacterial taxa that have few cultured representatives that hopefully can be cultivated in the future.
As seen in Figure 2A, GTDB classification distributed the new 57 MAGs into 2 classes and 10 orders within the phylum Cyanobacteria (from 13 total orders in the class Cyanobacteriia and 2 orders in the class Sericytochromatia in GTDB R89). This dataset includes one MAG (medium-quality) classified as a new order in the Cyanobacteriia class (M55_SRR7473442_K2018_030: 76.3% completeness and 1.2% contamination). Only T60_TAT2020_004 was assigned to the non-photosynthetic Sericytochromatia class. ANI values between all MAGs distinguished 44 different species (Table 1), while GTDB-tk classified 14 MAGs to species with available genomes in the NCBI database (gANI > 95%; 9 different species when the MAGs were dereplicated; Table 1 and Figure 2B). Additionally, 21 MAGs were classified by topology or RED values as new species belonging to established GTDB genera. In summary, 43 MAGs represent taxonomic novelty as 1 new order, 3 new families, 15 new genera and 36 new species (Table 1).
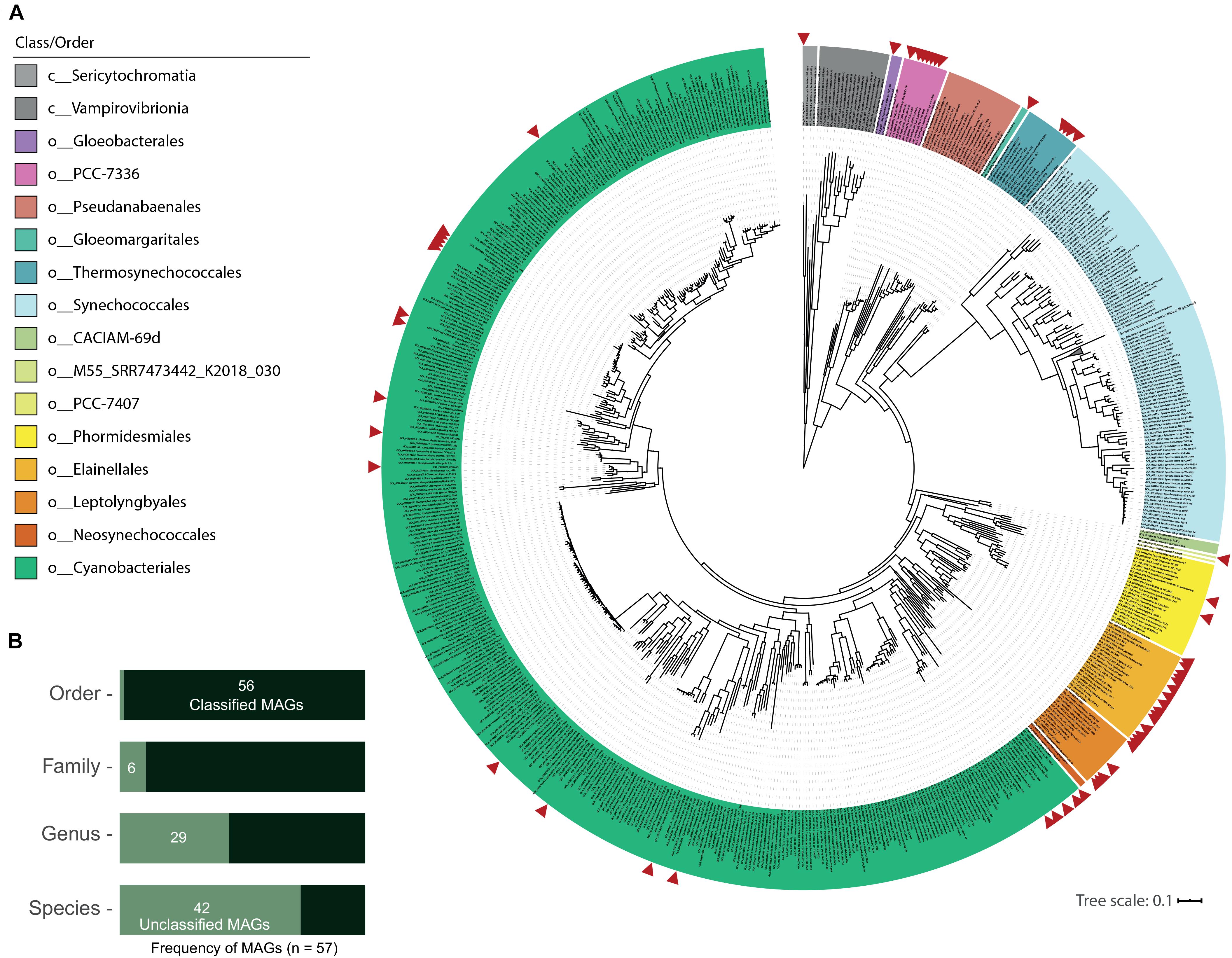
Figure 2. Novel hot spring cyanobacterial MAG phylogeny and taxonomic classification. (A) Phylogenomic reconstruction from multiple sequence alignment of 120 bacterial marker genes recovered from 800 NCBI cyanobacterial genomes and 57 new cyanobacterial MAGs from 21 hot spring metagenomes. Maximum likelihood tree reconstruction was performed with IQtree software using the LG + R10 model and a non-parametric UF-bootstrap support of 1,000 replicates. The labels are colored according to the GTDB order classification for c_Cyanobacteriia genomes, and class for Sericytochromatia and Vampirovibrionia members. The tree was rooted in the node between Sericytochromatia/Vambirovibrionia and Cyanobacteriia classes. 305 sequences in the Syn/Pro clade are collapsed in the tree. Red arrows indicate the position of hot spring MAGs in the tree that were distributed into 10 orders from a total of 15 in the cyanobacterial phylum. The new order retrieved in the present study is called M55_SRR7473442_K2018_030 in the legend. (B) Classification of MAGs according to the GTDB-tk. Unclassified MAGs correspond to those with no associated genome in the database at the specific taxonomic level.
This MAG classification is supported by a maximum likelihood phylogenomic reconstruction that incorporated 120 bacterial marker genes (Figure 2A) and included 800 NCBI cyanobacterial genomes (> 75% completeness; dereplicated from 1,626 genomes available in the NCBI assembly database as of September 2019). Distribution of the 57 MAGs in the GTDB R89 orders is as follows: o__ (new, 1), Cyanobacteriales (20), Elainellales (15), Gloeobacterales (1), Gloeomargaritales (1), Leptolyngbyales (5), PCC-7336 (7), Phormidesmiales (2), Thermosynechococcales (4) and UBA7694 (1, from the Sericytochromatia class). Some of these genomes form clades with known thermophilic cyanobacterial species from the following genera: Fischerella, Thermosynechococcus, Synechococcus (JA-3-3Ab), Chroogloeocystis, Chlorogloeopsis, Calothrix, Hydrococcus and O-77 (Thermoleptolyngbya). Others represent the first hot spring-associated genome reported within the given genus, specifically Rivularia (3 marine genomes), Alkalinema (1 freshwater genome), CCP2 (1 saltern genome) and Cyanobacterium (3 freshwater/saline lake genomes), as seen in Supplementary Table 3. The latter supports the concept that the ability to live at higher temperatures is a secondary specialization from primordial environments like marine or fresh water (Hammerschmidt et al., 2020), even in early-branching cyanobacteria, such as the Thermosynechococcales and PCC7336 orders, that now include non-thermal cyanobacterial members as revealed by GTDB classification (Supplementary Figure 1).
Cyanobacterial clades with few genomes are complemented by the group of MAGs that comprises new taxa at the genus or family levels, according to GTDB (see above). For instance, some of these new genomes are related to the recently described Gloeomargaritales order, whose members can accumulate intracellular carbonates (Moreira et al., 2017; Ponce-Toledo et al., 2017), as well as the early diverging non-thylakoid Gloeobacterales (Nakamura et al., 2003). Furthermore, the MAG from the non-photosynthetic Sericytochromatia class (UBA7694 order) further expands the described habitat of this taxon (Monchamp et al., 2019). This MAG also shows other features that are not currently associated with UBA7694 family genomes, such as the potential for H2 metabolism and a higher number of CRISPR-associated proteins (see below). Altogether, this data provides new insight into the diversity of thermophilic, thermotolerant (< 40°C) and hot spring-associated cyanobacteria, and hints at the yet-to-be-discovered taxonomic novelty in these extreme environments (e.g., the large numbers of unassigned cyanobacterial sequences from hot springs reported in Roeselers et al., 2007; Ionescu et al., 2010; Mackenzie et al., 2013; Momper et al., 2019; Uribe-Lorío et al., 2019, among others).
Although GTDB taxonomy solves several of the problems and rearrangements within the phylum, there are still unsolved issues. One of them is the use of placeholder names, such as the O-77 genus that should correspond to Thermoleptolyngbya (Sciuto and Moro, 2016; Yoon et al., 2017); the JSC-12 genus that has no generic assignment; and the JA-3-3Ab genus that is mostly known as Synechococcus OS-A and OS-B’ (Steunou et al., 2006; Bhaya et al., 2007). Moreover, the present phylogenomic analyses using GTDB R89 reproduced the polyphyly within the genera Chroogloeocystis (Brown et al., 2005), Geminocystis (Korelusova et al., 2009), Calothrix (Berrendero et al., 2008) and Synechococcus (Walter et al., 2017). Further actions should now focus on selecting and correctly updating the type species and type material to improve cyanobacterial taxonomy (Ramos et al., 2017). This will enable more taxonomically driven sequencing efforts like cyanoGEBA (Shih et al., 2013), as well as the inclusion of genomic type material from uncultured taxa (Chuvochina et al., 2019), such as the MAGs recovered in this study.
Taxonomic annotation is relevant for all cyanobacterial MAGs recovered here, but especially for those that may have a global or dominant distribution in hot springs. Accordingly, the proportion of MAG reads mapped against the entire set of metagenomes was determined and ranged from 0.1 to 60% (Figure 3A). The most represented orders (35 to 99% of the total cyanobacterial community) were Cyanobacteriales, Elainellales, Thermosynechococcales and PCC-7336 (Figure 3B and Supplementary Figure 3). The abundance of these MAGs in each hot spring was negatively correlated with the overall cyanobacterial alpha diversity in the respective metagenome (−0.490 rho Spearman’s correlation, p-value < 0.05; Figure 3A and Supplementary Figure 2A). The latter suggests that in low diversity systems, where cyanobacteria represent a major proportion of the microbial community, only a few MAGs outcompete other cyanobacterial species and successfully establish themselves as the dominant producers in the system. Whereas, in samples where non-cyanobacterial taxa dominate the community, a broader range of niches might be available for different groups of cyanobacteria to colonize.
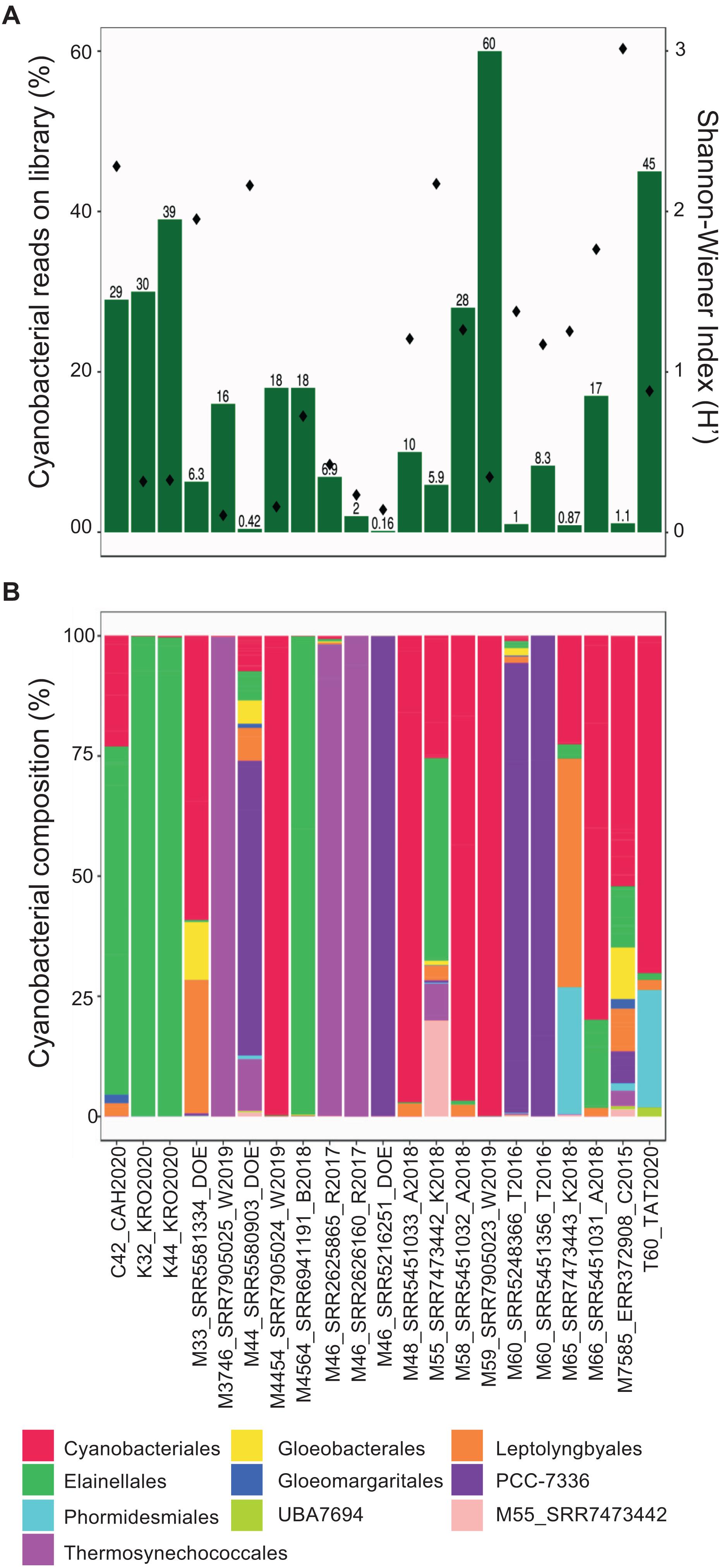
Figure 3. Diversity of hot spring cyanobacterial MAGs in metagenomes. (A) Left y-axis corresponds to the percentage of total mapped reads from each hot spring to a cyanobacterial MAG (green bars). Right y-axis corresponds to alpha-diversity values (dots) for each metagenome. (B) Normalized (relative) abundances of the reads assigned to each MAG at the order level in each sample. The possible new order is shown as M55_SRR7473442.
Interestingly, multivariate analyses, which were used to investigate the relationship between abundances, physico-chemical and geographic features, revealed that the cyanobacterial community composition was positively correlated with the hot spring location (Mantel statistic = 0.19, p-value = 9 × 10–4), but not with physicochemical parameters, such as temperature or pH (Mantel test, p-value > 0.01). For example, MAGs from the PCC-7336 order were more abundant in North American samples, while Thermosynechococcales MAGs were found mainly in Asian samples independent of hot spring conditions (Supplementary Figure 3), thereby corroborating the proposed biogeographical islands for these unicellular cyanobacteria (Papke et al., 2003; Cheng et al., 2020). The correlation between community composition and geographical location was also observed through non-metric multidimensional scaling and cluster analyses (Supplementary Figures 2B,C). Furthermore, the cyanobacterial community composition presented a similar pattern even within a specific hot spring, independent of the temperature and pH (Supplementary Figure 2C).
Another interesting result is that a single clade commonly dominates the cyanobacterial community within each hot spring, where a single order can make up to 99% of all cyanobacterial sequences (Figure 3B). Altogether, this information notices the possibility that different hot spring cyanobacteria occupy a similar niche in non-acidic thermal environments and that competitive exclusion between different cyanobacterial clades is a significant force driving the cyanobacterial community composition, as seen in saline freshwater systems (Roney et al., 2009). Accordingly, some hot springs have zones with different dominant cyanobacteria, sugesting successful competitive exclusion depending on the nitrogen compound availability, sulfide concentration, temperature resistance of each clade, and light adaptations in the thermal gradient (Ward et al., 2012). However, more studies are needed to test the competitive exclusion in these different cyanobacterial communities.
Large-scale comparisons have shown that there are common distinctive genomic features that differentiate thermophilic archaea and bacteria from their mesophilic counterparts; however, thermophilic cyanobacteria were underrepresented in these analyses (Singer and Hickey, 2003; Sabath et al., 2013). The cyanobacterial comparative genomics carried out in the present study, both at a general level (the 159-subset) and at specific taxonomic levels (families and genera), was useful to confirm previously detected genome trends of some cyanobacteria, such as genomic streamlining toward extreme environments (Larsson et al., 2011).
For the present study, the trend of an increasing optimum growth temperature with a decreasing genome size (Sabath et al., 2013) was also confirmed as a negative correlation between the source temperature of the metagenome and the expected genome size of the cyanobacterial MAGs (−0.355 Pearson’s correlation, p-value < 0.05). Differences in genome size and other features were compared between hot spring and non-thermal cyanobacterial genomes of the 159-subset, which comprised the 57 MAGs, 36 NCBI hot spring genomes and 66 NCBI non-thermal genomes, see section “MATERIALS AND METHODS” and Supplementary Table 3. Cyanobacterial MAGs showed a wide variation in genome size from 2.1 to 12.1 Mbp across the 10 different orders (Figure 4). However, hot spring cyanobacterial genomes (n = 93) were smaller and exhibited a higher GC percentage (Wilcoxon’s paired test, p-value < 0.05 for both analyses) than the non-thermal genomes (n = 66), while the coding density was similar between both groups (Wilcoxon’s paired test, p-value = 0.15) (Figure 5).
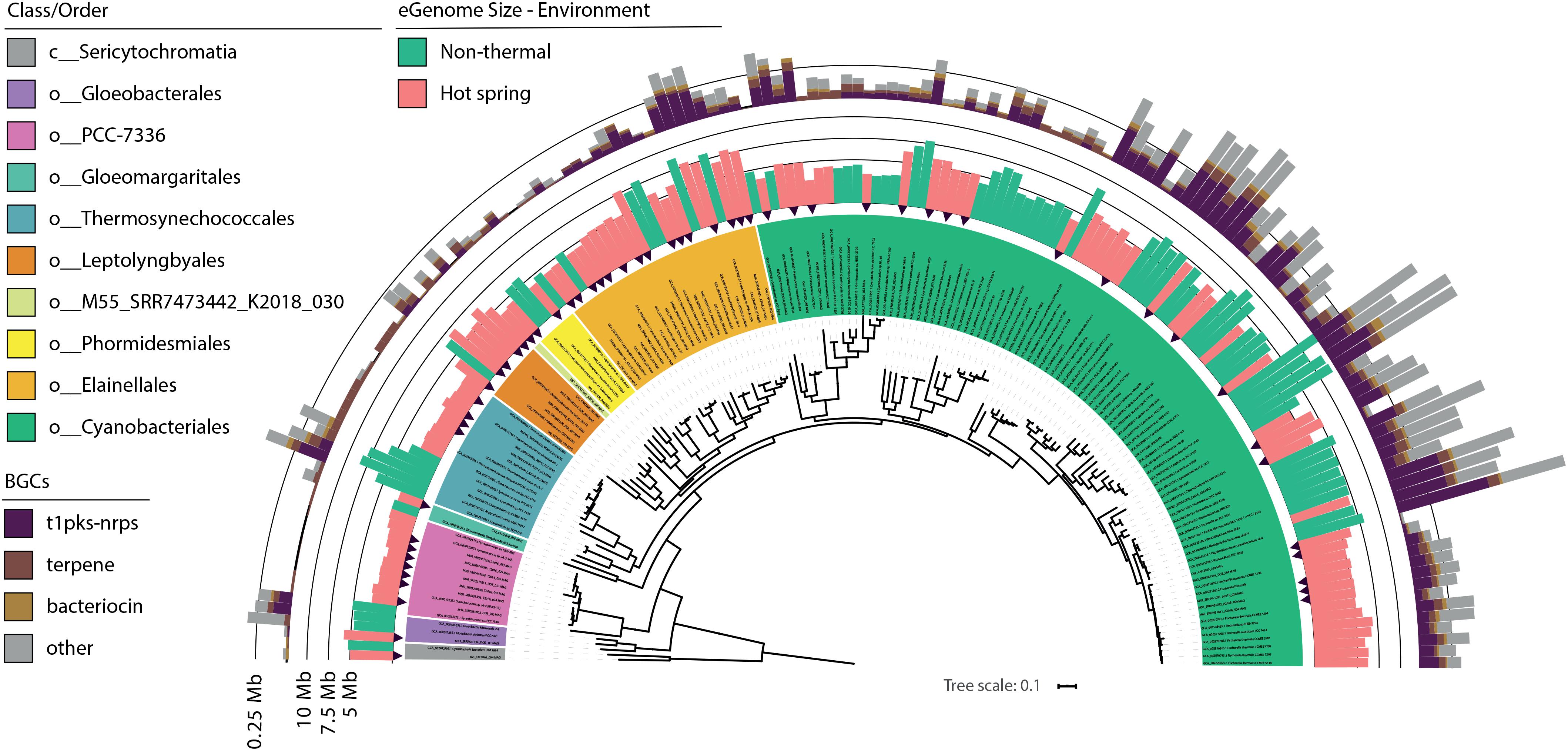
Figure 4. Genome size and secondary metabolite clusters of hot spring and close non-thermal cyanobacteria. Phylogenomic reconstruction from multiple sequence alignment of 120 bacterial marker genes recovered from 102 NCBI cyanobacterial genomes and 57 cyanobacterial MAGs from 21 hot spring metagenomes. Maximum likelihood tree reconstruction was done with IQtree software using the LG + R10 model and a non-parametric UF-bootstrap support of 1,000 replicates. The labels are colored according to the GTDB order classification for c_Cyanobacteriia genomes and class for Sericytochromatia members. The tree was rooted in the node between the Sericytochromatia and Cyanobacteriia classes. Black arrows indicate the position of hot spring MAGs in the tree. The inner ring indicates the estimated genome size (Mbp) for all 159 genomes and is colored according to the respective environmental origin (see Supplementary Table 3). The outer ring depicts the total size (Mbp) of secondary metabolite biosynthetic gene clusters (BGCs) detected for each genome using antiSMASH software.
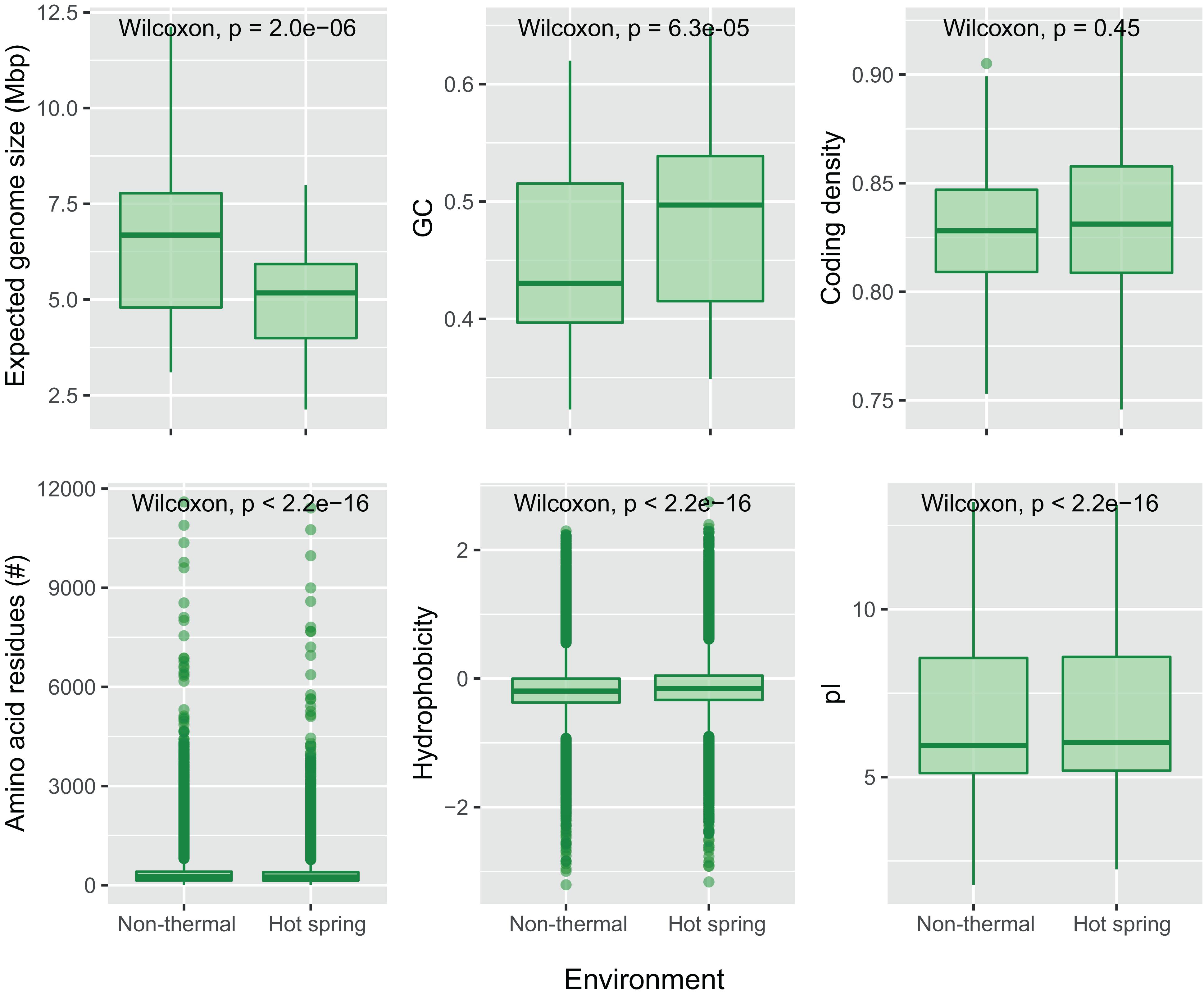
Figure 5. Comparison of genomic and protein properties between hot spring and non-thermal genomes. Boxplots for the “expected genomes size,” GC content and coding density were compared for the 159-subset genomes (93 from hot springs and 66 from non-thermal environments). The number of amino acid residues, hydrophobicity and isoelectric point (pI) were compared for the predicted CDSs from the 159-subset genomes from which 369,249 were from hot spring genomes and 348,018 from non-thermal genomes. Wilcoxon’s paired test was performed for each dataset, and p-values with Bonferroni’s multiple test correction are reported.
Additionally, whether or not some of these genome changes were reflected in the divergence of cyanobacterial genera and families with ≥ 3 genomes in each environmental group was investigated. For the genus Fischerella, hot spring genomes (n = 16) were 1.6 (± 0.7) Mbp smaller than the non-thermal genomes (n = 6) and had a higher GC content (Supplementary Figures 4, 5, Wilcoxon’s paired test, p-value < 0.05). Similarly, the families Elainellaceae and Thermosynechococcaceae exhibited smaller hot spring genomes (Wilcoxon’s paired test, p-value < 0.05; Supplementary Figure 4), with an increase in both the GC content and coding density for Thermosynechococcaceae (Wilcoxon’s paired test, p-value < 0.05; Supplementary Figures 5, 6). However, the low number of genomes for other genera and families in either environmental group allowed for only some trends to be observed. Alkalinema and Rivularia genomes from hot springs were > 2 Mbp smaller than those from non-thermal environments, and a similar trend was observed for the JA-3-3Ab order. Specifically, genomes of the JA-3-3Ab family (Synechococcus sp. OS-A and OS-B’ clade) were 2.4 (± 0.3) Mbp smaller than the marine Synechococcus sp. PCC 7336 genome from the same order.
Furthermore, differences in nucleotide content and protein properties that have been seen in other bacteria (Singer and Hickey, 2003; Sabath et al., 2013) were also found here. Analysis of the coding DNA sequences (Figure 5, Wilcoxon’s paired test, p-values < 0.05) showed that hot spring cyanobacterial proteins (n = 369,249) are not only shorter than those from non-thermal environments, but also lighter in molecular weight, more hydrophilic, and contain more basic isoelectric points. Indeed, amino acid composition analysis showed that hot spring cyanobacterial proteins have more basic amino acids, such as histidine and arginine, and less acidic amino acids, such as aspartate and glutamate. The aromatic tryptophan and the non-polar residues alanine, leucine, proline and valine were also more abundant in hot spring genomes. The increased alanine abundance (0.81%) and decreased asparagine (0.63%) and lysine (0.62%) abundances were the most notable differences (Wilcoxon’s paired test p-values < 0.05, Supplementary Figure 7), explaining the tendency toward more hydrophobic proteins in hot spring genomes. These amino acid frequencies agree with previous predictions of increased arginine and valine, and decreased serine and aspartate abundances in other thermophiles and hyperthermophiles (Vieille and Zeikus, 2001; Singer and Hickey, 2003), but disagree for the other amino acid frequencies observed here.
Analysis of orthologous CDSs was conducted in the 159-subset to determine if there were genes exclusively related to hot spring genomes. Of the 719,564 analyzed protein sequences, 691,501 (96.1%) were assigned into 47,328 orthogroups, 59.2% of which were singletons. Only 183 (0.4%) orthogroups had at least one ortholog sequence in ≥ 97% of the analyzed genomes, while 12,657 (26.7%) were sporadically present in both environments. The number of orthogroups with only orthologs (> 1, not singletons) in non-thermal genomes was 3,434 (7.3%, 66 genomes), while that from hot springs was 3,179 (6.7%, 93 genomes). Since environment-specific orthogroups with CDSs across taxa point to potential niche adaptation, CDSs present in more than one class, family or genus were searched. Several orthogroups were shared within 6 different orders or 7 families in hot spring genomes, while non-thermal genomes shared fewer orthogroups at these taxonomic levels (Supplementary Figure 8). Most of these widely distributed orthogroups from hot spring genomes were annotated as hypothetical proteins, but other annotated functions included a DsrE family protein and an SDR family oxidoreductase (Supplementary Table 6). Phylogenetic analysis of hypothetical protein orthogroups OG0008066 (shared between 5 families) and OG0006223 (shared between 7 genera) not only corroborates the close relationship between hot spring cyanobacteria, but also demonstrates an association with other common thermophilic bacteria, such as Chloroflexota, Deinococcota and Actinobacteriota (Supplementary Figures 9A,B). The distribution of these genes across diverse hot spring bacteria could be explained by horizontal gene transfer, as also found between other organisms living at high temperatures (Fuchsman et al., 2017).
Changes within the hot spring core and accessory genomes were determined for 7 genera (Calothrix, Cyanobacterium, Elainella, Fischerella, Geminocystis, Rivularia, and Trichormus) with both hot spring and non-thermal representatives. The core genome of each genus ranged from 2,007 to 3,488 CDSs, representing 24% (Calothrix) to 75% (Geminocystis and Cyanobacterium) of the CDSs in a genome. The orthogroups were classified according to whether they were present in all genomes (core), exclusively in hot spring or non-thermal genomes (specific accessory and specifc core accessory sets), or genus accessory if they were sporadically present in both environmental groups (Figure 6A and Supplementary Table 7). The COG distribution (Supplementary Figure 10) shows core genomes clustering, along with some of the accessory orthogroups from hot spring and non-thermal genomes. Core accessory orthogroups for all hot spring and non-thermal genera (except for Cyanobacterium) clustered together, suggesting common functions associated with these groups.
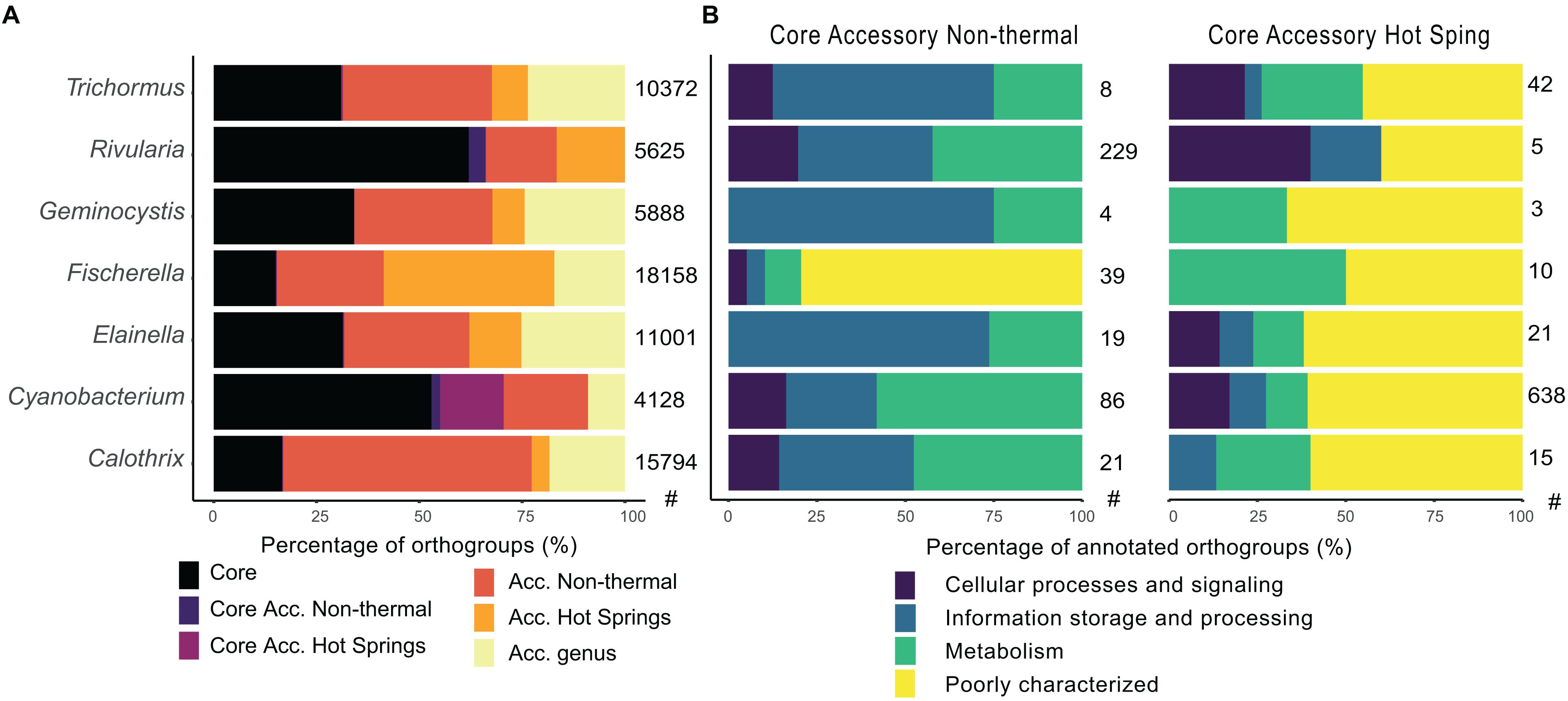
Figure 6. Comparison of core and accessory orthogroups from hot spring and non-thermal cyanobacterial genera. (A) The classification of orthogroup fractions according to their presence in all (core), in only hot spring or non-thermal genomes (specific accessory), or in both groups at the genus level (accessory genus). (B) COG characterization by eggnog mapper software of exclusive core accessory genes of hot spring and non-thermal environments for each compared genus. The categories were joined in the four main COG functional groups. The number of hot spring (HS) and non-thermal (NT) genomes used for these analyses is as follows: Trichormus (2 HS, 6 NT), Rivularia (1 HS, 3 NT), Geminocystis (3 HS, 3 NT), Fischerella (16 HS, 6 NT), Elainella (5 HS, 2 NT), Cyanobacterium (1 HS, 3NT) and Calothrix (2 HS, 8 NT). The number of orthogroups for each analysis is shown in the right side of each bar plot.
Regarding gene annotation (Figure 6B), non-thermal core accessory orthogroups had more COG function abundances in all genera that were classified as unknown (31, Poorly Char.); replication and repair (24, Information Stor.); energy production and metabolism (22, Metabolism); cell wall/membrane biogenesis (17, Cell Proc.); transcription (17, Information Stor.); and coenzyme metabolism (14, Metabolism) (except for the genus Rivularia, whose most represented function was transcription with 54 core accessory orthogroups in the non-thermal genomes). Nevertheless, core accessory genes of hot spring genomes did not exceed 42 orthogroups for each genus, with functions mostly classified in all genera as unknown (50, Poorly Char.); inorganic ion transport (8, Metabolism); amino acid metabolism and transport (6, Metabolism); and cell wall/membrane biogenesis (5, Cell Proc.). For the genus Cyanobacterium, which had only one available hot spring genome, most represented COGs in the hot spring core accessory orthogroups were related to unknown functions (388) (Figure 6B).
In general, similar patterns were found for the functional distribution of core genomes between cyanobacterial genera in both the hot spring and non-thermal environments, thereby corroborating previous analyses (Beck et al., 2012). However, these results also show a higher proportion of genes with unknown function in the core accessory genomes of hot spring cyanobacteria. The low number of identified core accessory genes might suggest a reductionist point of view for adaptation to high temperatures, as previously seen for the thermophilic unicellular red algae Galdieria sulphuraria, whose adaptation to this environment was mediated by horizontal gene transfer from bacteria and archaea (Schönknecht et al., 2013).
Several differences in COG categories were found between the 159-subset of hot spring and non-thermal genomes (Wilcoxon’s paired test, p-value < 0.05). The greatest difference was a decrease (∼1% relative abundance) in hot spring genomes for the L COG category (replication, recombination and repair). Additionally, higher abundances were detected in categories P (inorganic ion transport and metabolism), J (translation, ribosomal structure and biogenesis), H (coenzyme transport and metabolism), E (amino acid transport and metabolism) and O (post-translational modification, protein turnover and chaperones). Conversely, other COG categories, such as K (transcription), D (cell cycle control, cell division and chromosome partitioning) and Z (cytoskeleton) decreased in hot spring genomes (Figure 7).
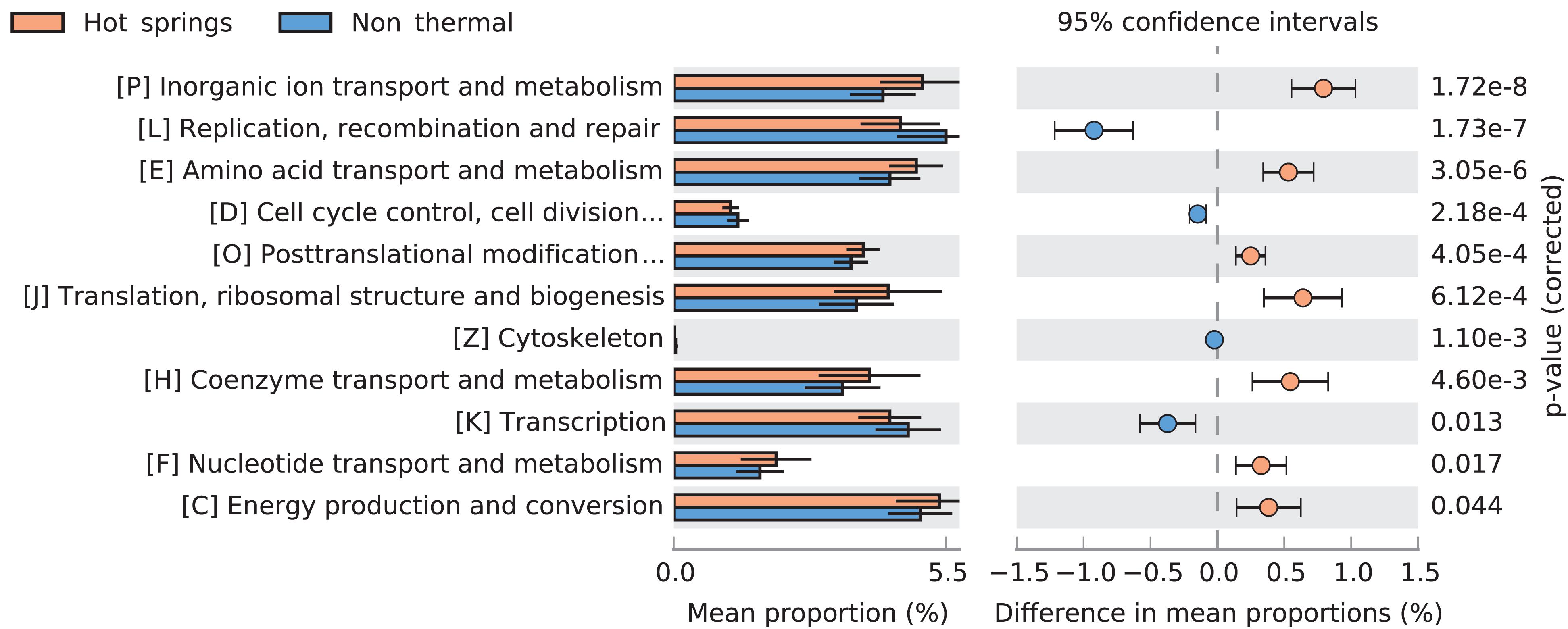
Figure 7. Comparison of COG categories between hot spring and non-thermal genomes. Extended error bar plot showing significantly different predicted COG categories between hot spring (n = 93) and non-thermal (n = 66) cyanobacterial genomes. Only corrected p-values < 0.05 are displayed.
An additional comparison of specific functions related to complementary metabolisms and genes involved in microbial community interactions was performed. The results show no differences for the sulfide-quinone reductase gene (related to anoxygenic photosynthesis; Chi-square test p-value = 0.930) or for the nifHDK complex and accessory genes (related to nitrogen fixation; Wilcoxon’s paired test, p-value = 0.061). However, the potential acquisition of nitrate and its subsequent reduction to ammonium through the narB and nirA genes were less represented in hot spring genomes (Wilcoxon’s paired test, p-value < 0.05), as were the hox, hup, hyp, and hyb genes related to hydrogen metabolism (Wilcoxon’s paired test, p-value < 0.05), supporting the competitive exclusion trend by specific nutritional adaptations in the thermal gradient (Ward et al., 2012).
Genes related to the defense mechanisms against foreign nucleic acids and integrated viral proteins were also compared. The number of annotated viral protein orthogroups was 18, showing broad integration of phage CDSs, except for 15 of the hot spring genomes (including all JA-3-3Ab members). Restriction-modification CDSs were classified into 131 different orthogroups, while CRISPR-associated proteins (Cas) were classified into 73 clusters. Considering that genome streamlining was a primary difference between the environmental groups, the correlation between genome size and the number of CDSs annotated with these functions was analyzed. The correlation was higher for restriction-modification and viral proteins (0.778 Pearson’s correlation, p-value < 0.05; and 0.747 rho Spearman’s correlation, p-value < 0.05, respectively; Figure 8), when compared to the Cas proteins (0.360 Pearson’s correlation, p-value < 0.05). This scenario varies for Cas proteins when the genomes are separated into hot spring and non-thermal environments (Figure 8), in that the Pearson’s correlation for hot spring genomes was 0.477 (p-value < 0.05), while the non-thermal genomes showed a lower and non-significant Pearson’s correlation index of 0.182 (p-value = 0.142). Conversely, restriction-modification and viral proteins presented similar Pearson’s correlation indexes between both environmental groups (Fischer r-to-z transformation p-values > 0.05).
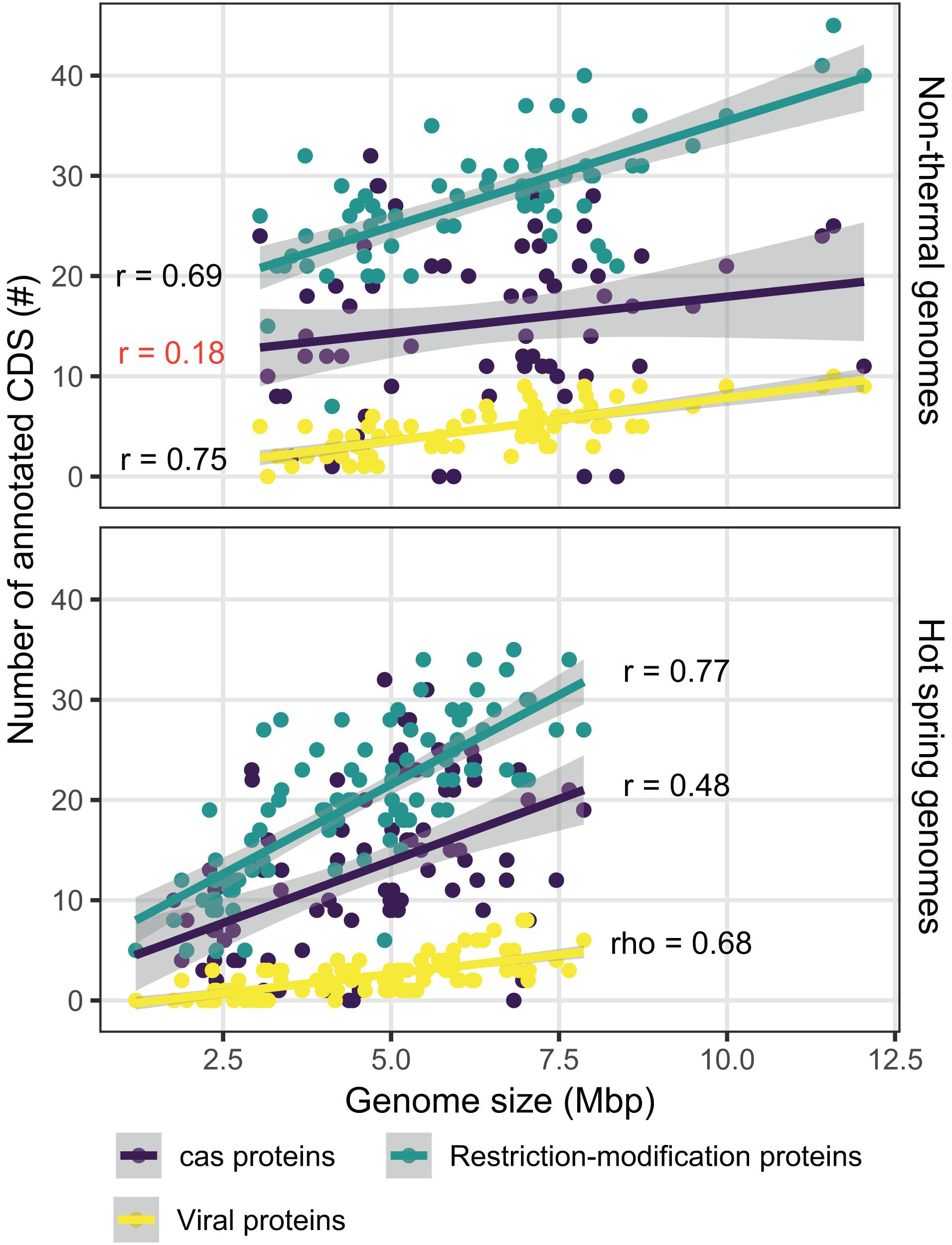
Figure 8. Comparison of defense mechanism-associated genes from hot spring and non-thermal genomes. Relationship between the number of CDSs determined for Cas proteins (purple), viral proteins (yellow) and restriction modification proteins (light blue), and the size of the 159 genomes used for hot spring/non-thermal comparisons. Upper panel shows non-thermal genomes (n = 66), while the bottom panel shows hot spring genomes (n = 93). Smooth linear regression was determined using R software with the ggplot package. Pearson’s r and Spearman’s rho correlations are provided for each protein category in the plot. Red represents correlations with a p-value > 0.05 and black represents those with p-values < 0.05.
Additionally, BGCs were analyzed because they have been found to be gained and lost during niche transitions (Kurmayer et al., 2015). A total of 6,773,751 bp representing secondary metabolite biosynthetic regions were identified for the 57 MAGs (varying from 0.08 to 11.15% of the total genome). The NRPS-PKS, bacteriocin and terpenes clusters were the most abundant (Figure 4), as has been previously seen for some hot spring cyanobacteria (Micallef et al., 2015). BGCs represented a smaller percentage in hot spring genomes (3.07 ± 2.6%, Wilcoxon’s paired test, p-value < 0.05) than in non-thermal genomes (5.18 ± 3.39%). For instance, Fischerella members show the greatest difference (0.5 ± 0.28 Mb; Wilcoxon’s paired test, p-value < 0.05; Supplementary Figure 11) in BGC genome percent between genomes from both environments.
For the 159-subset, the correlation between the number of BGC-dedicated base pairs per genome and genome size was higher (0.821 rho Spearman’s correlation, p-value < 0.05) than that predicted by Shih et al. (2013) (R2 = 0.3, p-value < 0.0001), but similar for the hot spring and non-thermal genomes (0.799 and 0.761, respectively). The latter suggests that the streamlining of hot spring genomes also involves a reduction in BGCs. An ecological explanation for BGC-loss in hot spring genomes could be the lower diversity of these microbial communities compared to other environments (Li et al., 2014). In hot spring communities, the role of secondary metabolites as weapons of inter-microbial warfare (O’Brien and Wright, 2011) could be diminished and susceptible to loss. Furthermore, viral and exogenous nucleic acids are less diverse in hot springs (Parmar et al., 2018), and cyanobacteria are subjected to strong viral predation and potential coevolution (Guajardo-Leiva et al., 2018), as also seen for other thermophilic organisms (Breitbart et al., 2004). Therefore, the prevalence of the CRISPR-Cas system in their genomes could be more important than in non-thermal genomes as seen in other phyla (Weinberger et al., 2012).
The ability to survive at higher temperatures has been gained and lost across bacteria and archaea (Pollo et al., 2015), and all genomic features common to thermophiles are not mandatory for all high-temperature organisms (Puigbò et al., 2008). This was also observed in this study, suggesting once again that different adaptation strategies exist. The polyphyly of this feature in cyanobacteria, seen here widespread in almost all orders, is explained as secondary adaptation (not basal) during the niche expansion stage of trait evolution for this phylum and is intimately related with the ability to form microbial mats (Hammerschmidt et al., 2020). Furthermore, the number of transitions to and from thermophily in cyanobacteria has shown a strong reduction between 0.9 and 0.8 Gya (during the cold temperatures of the Neoproterozoic Oxidation Event), and a slight increase in transitions over the last 0.3 Gya (warmer temperatures). The growing numbers of genera with both environmental groups here described increase the evidence of these recent transitions to thermophily. Altogether, it demonstrates the effect of global temperature changes during this niche expansion (Uyeda et al., 2016) and in the worldwide dispersal of thermophilic cyanobacteria, like Fischerella, during the global rise in air temperature 74 mya (Miller et al., 2007). Studying the pathways by which different groups of hot spring cyanobacteria became the autotrophic base for microbial mats in these environments will be the foundation for understanding how different organisms cope and proliferate at higher temperatures in a currently changing world.
Cyanobacteria are essential primary producers in hot spring phototrophic microbial mats. In this study, metagenomic binning was used to obtain 57 new cyanobacterial MAGs, subsequently revealing a wide distribution of new thermophilic cyanobacterial members across the phylum. How these cyanobacteria began to colonize these environments is still unknown; however, the adaptation to high temperatures have strong genomic consequences for cyanobacteria that currently live in hot springs. The transition from a non-thermal environment to hot springs is reflected in genomic properties that could be more advantageous to survival, such as small genomes and warm-adapted proteins, as well as a higher abundance of specific protein functional categories to cope with mineral water composition and new microbial and viral communities. This study demonstrates how adaptation to hot spring environments can be traced at the genus level, showing that recent divergences could be restricted to a small set of genes that can be shared by diverse members within the same niche. Under the current scenario of global change, it is important to understand the evolution of hot spring genomes as an example of selective pressure by warmer environments. Studying the information within MAGs from lower hot spring temperatures will allow us to predict changes in genomic features that many species may face in both present and future scenarios on Earth.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/,(NCBI BioProject numbers PRJNA635751 and PRJNA645256); https://figshare.com/, 10.6084/m9.figshare.1240097.
JA and BD conceived the study. JA, OS, and TA-S analyzed the metagenomic database and obtained the metagenome-assembled genomes. JA and TA-S performed the evolutionary and bioinformatic analyses under supervision of BD. JA and BD wrote the manuscript. All authors read, commented and approved the drafted manuscript.
This work was financially supported by FONDECYT regular project N° 1150171 and 1190998 (ANID) and ECOS-CONICYT (ANID) project ECOS160025. JA and OS were supported by Doctoral fellowships from CONICYT (ANID) N° 21191763 and 21172022, respectively. JA was also funded by Beca Gastos Operacionales 2020 CONICYT (ANID) N° 24200178. FONDAP-CONICYT (ANID) N°151110009 CR2 is also acknowledged for it support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Ph.D. Roy Mackenzie, Ph.D. Sergio Guajardo, Ph.D. Christina Ridley and Ph.D. (c) Pablo Vergara for their contributions to sampling, sequencing and editing help.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.568223/full#supplementary-material
Alcamán, M. E., Fernandez, C., Delgado, A., Bergman, B., and Díez, B. (2015). The cyanobacterium Mastigocladus fulfills the nitrogen demand of a terrestrial hot spring microbial mat. ISME J. 9, 2290–2303. doi: 10.1038/ismej.2015.63
Alcamán-Arias, M. E., Pedrós-Alió, C., Tamames, J., Fernández, C., Pérez-Pantoja, D., Vásquez, M., et al. (2018). Diurnal changes in active carbon and nitrogen pathways along the temperature gradient in Porcelana hot spring microbial mat. Front. Microbiol. 9:2353. doi: 10.3389/fmicb.2018.02353
Alcorta, J., Espinoza, S., Viver, T., Alcamán-Arias, M. E., Trefault, N., Rosselló-Móra, R., et al. (2018). Temperature modulates Fischerella thermalis ecotypes in Porcelana hot spring. Syst. Appl. Microbiol. 41, 531–543. doi: 10.1016/j.syapm.2018.05.006
Alcorta, J., Vergara-Barros, P., Antonaru, L. A., Alcamán-Arias, M. E., Nürnberg, D. J., and Díez, B. (2019). Fischerella thermalis: a model organism to study thermophilic diazotrophy, photosynthesis and multicellularity in cyanobacteria. Extremophiles 23, 635–647. doi: 10.1007/s00792-019-01125-4
Alneberg, J., Bjarnason, B. S., De Bruijn, I., Schirmer, M., Quick, J., Ijaz, U. Z., et al. (2014). Binning metagenomic contigs by coverage and composition. Nat. Methods 11, 1144–1146. doi: 10.1038/nmeth.3103
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Bankevich, A., Nurk, S., Antipov, D., Gurevich, A. A., Dvorkin, M., Kulikov, A. S., et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477. doi: 10.1089/cmb.2012.0021
Beck, C., Knoop, H., Axmann, I. M., and Steuer, R. (2012). The diversity of cyanobacterial metabolism: genome analysis of multiple phototrophic microorganisms. BMC Genomics 13:56. doi: 10.1186/1471-2164-13-56
Berrendero, E., Perona, E., and Mateo, P. (2008). Genetic and morphological characterization of Rivularia and Calothrix (Nostocales, Cyanobacteria) from running water. Int. J. Syst. Evol. Microbiol. 58, 447–460. doi: 10.1099/ijs.0.65273-0
Bhaya, D., Grossman, A. R., Steunou, A. S., Khuri, N., Cohan, F. M., Hamamura, N., et al. (2007). Population level functional diversity in a microbial community revealed by comparative genomic and metagenomic analyses. ISME J. 1, 703–713. doi: 10.1038/ismej.2007.46
Blin, K., Wolf, T., Chevrette, M. G., Lu, X., Schwalen, C. J., Kautsar, S. A., et al. (2017). antiSMASH 4.0—improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 45, W36–W41. doi: 10.1093/nar/gkx319
Bowers, R. M., Kyrpides, N. C., Stepanauskas, R., Harmon-Smith, M., Doud, D., Reddy, T. B. K., et al. (2017). Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat. Biotechnol. 35, 725–731. doi: 10.1038/nbt.3893
Breitbart, M., Wegley, L., Leeds, S., Schoenfeld, T., and Rohwer, F. (2004). Phage community dynamics in hot springs. Appl. Environ. Microbiol. 70, 1633–1640. doi: 10.1128/AEM.70.3.1633-1640.2004
Brown, I. I., Mummey, D., and Cooksey, K. E. (2005). A novel cyanobacterium exhibiting an elevated tolerance for iron. FEMS Microbiol. Ecol. 52, 307–314. doi: 10.1016/j.femsec.2004.11.020
Castenholz, R. W. (1969). Thermophilic blue-green algae and the thermal environment. Bacteriol. Rev. 33, 476–504. doi: 10.1128/mmbr.33.4.476-504.1969
Castenholz, R. W., Wilmotte, A., Herdman, M., Rippka, R., Waterbury, J. B., Iteman, I., et al. (2001). “Phylum BX. cyanobacteria,” in Bergey’s Manual§of Systematic Bacteriology, eds D. R. Boone, R. W. Castenholz, and G. M. Garrity (New York, NY: Springer), 473–599. doi: 10.1007/978-0-387-21609-6_27
Chan, C. S., Chan, K. G., Tay, Y. L., Chua, Y. H., and Goh, K. M. (2015). Diversity of thermophiles in a Malaysian hot spring determined using 16S rRNA and shotgun metagenome sequencing. Front. Microbiol. 6:177. doi: 10.3389/fmicb.2015.00177
Chaumeil, P. A., Mussig, A. J., Hugenholtz, P., and Parks, D. H. (2020). GTDB-Tk: a toolkit to classify genomes with the genome taxonomy database. Bioinformatics 36, 1925–1927. doi: 10.1093/bioinformatics/btz848
Cheng, Y. I., Chou, L., Chiu, Y. F., Hsueh, H. T., Kuo, C. H., and Chu, H. A. (2020). Comparative genomic analysis of a novel strain of Taiwan hot-spring cyanobacterium Thermosynechococcus sp. CL-1. Front. Microbiol. 11:82. doi: 10.3389/fmicb.2020.00082
Chun, J., Oren, A., Ventosa, A., Christensen, H., Arahal, D. R., da Costa, M. S., et al. (2018). Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 68, 461–466. doi: 10.1099/ijsem.0.002516
Chuvochina, M., Rinke, C., Parks, D. H., Rappé, M. S., Tyson, G. W., Yilmaz, P., et al. (2019). The importance of designating type material for uncultured taxa. Syst. Appl. Microbiol. 42, 15–21. doi: 10.1016/j.syapm.2018.07.003
Copeland, J. J. (1936). Yellowstone thermal myxophyceae. Ann. N. Y. Acad. Sci. 36, 4–223. doi: 10.1111/j.1749-6632.1936.tb56976.x
Cox, A., Shock, E. L., and Havig, J. R. (2011). The transition to microbial photosynthesis in hot spring ecosystems. Chem. Geol. 280, 344–351. doi: 10.1016/j.chemgeo.2010.11.022
Dagan, T., Roettger, M., Stucken, K., Landan, G., Koch, R., Major, P., et al. (2013). Genomes of Stigonematalean cyanobacteria (Subsection V) and the evolution of oxygenic photosynthesis from prokaryotes to plastids. Genome. Biol. Evol. 5, 31–44. doi: 10.1093/gbe/evs117
Eddy, S. R. (1998). Profile hidden Markov models. Bioinformatics 14, 755–763. doi: 10.1093/bioinformatics/14.9.755
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Emms, D. M., and Kelly, S. (2015). OrthoFinder: solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16:157. doi: 10.1186/s13059-015-0721-2
Finsinger, K., Scholz, I., Serrano, A., Morales, S., Uribe-Lorio, L., Mora, M., et al. (2008). Characterization of true−branching cyanobacteria from geothermal sites and hot springs of Costa Rica. Environ. Microbiol. 10, 460–473. doi: 10.1111/j.1462-2920.2007.01467.x
Fuchsman, C. A., Collins, R. E., Rocap, G., and Brazelton, W. J. (2017). Effect of the environment on horizontal gene transfer between bacteria and archaea. PeerJ 5:e3865. doi: 10.7717/peerj.3865
Guajardo-Leiva, S., Pedrós-Alió, C., Salgado, O., Pinto, F., and Díez, B. (2018). Active crossfire between Cyanobacteria and Cyanophages in phototrophic mat communities within hot springs. Front. Microbiol. 9:2039. doi: 10.3389/fmicb.2018.02039
Hammerschmidt, K., Landan, G., Tria, F. D. K., Alcorta, J., and Dagan, T. (2020). The order of trait emergence in the evolution of cyanobacterial multicellularity. bioRxiv [Preprint]. doi: 10.1101/570788
Hoang, D. T., Chernomor, O., Von Haeseler, A., Minh, B. Q., and Vinh, L. S. (2017). UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35, 518–522. doi: 10.1093/molbev/msx281
Huerta-Cepas, J., Forslund, K., Coelho, L. P., Szklarczyk, D., Jensen, L. J., Von Mering, C., et al. (2017). Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 34, 2115–2122. doi: 10.1093/molbev/msx148
Huerta-Cepas, J., Szklarczyk, D., Heller, D., Hernández-Plaza, A., Forslund, S. K., Cook, H., et al. (2019). eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 47, D309–D314. doi: 10.1093/nar/gky1085
Hyatt, D., Chen, G. L., LoCascio, P. F., Land, M. L., Larimer, F. W., and Hauser, L. J. (2010). Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11:119. doi: 10.1186/1471-2105-11-119
Ionescu, D., Hindiyeh, M., Malkawi, H., and Oren, A. (2010). Biogeography of thermophilic cyanobacteria: insights from the Zerka Ma’in hot springs (Jordan). FEMS Microbiol. Ecol. 72, 103–113. doi: 10.1111/j.1574-6941.2010.00835.x
Jain, C., Rodriguez-R, L. M., Phillippy, A. M., Konstantinidis, K. T., and Aluru, S. (2018). High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 9, 1–8. doi: 10.1038/s41467-018-07641-9
Kang, D. D., Li, F., Kirton, E., Thomas, A., Egan, R., An, H., et al. (2019). MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7:e7359. doi: 10.7717/peerj.7359
Kaushal, G., Kumar, J., Sangwan, R. S., and Singh, S. P. (2018). Metagenomic analysis of geothermal water reservoir sites exploring carbohydrate-related thermozymes. Int. J. Biol. Macromol. 119, 882–895. doi: 10.1016/j.ijbiomac.2018.07.196
Klatt, C. G., Wood, J. M., Rusch, D. B., Bateson, M. M., Hamamura, N., Heidelberg, J. F., et al. (2011). Community ecology of hot spring cyanobacterial mats: predominant populations and their functional potential. ISME J. 5, 1262–1278. doi: 10.1038/ismej.2011.73
Komárek, J. (1999). Cyanoprokaryota 1. Teil: Chroococcales. Subwasserflora von Mitteleuropa 19, 1–548. doi: 10.1127/algol_stud/103/2001/1
Komárek, J. (2013). Süsswasserflora Von Mitteleuropa: Cyanoprokaryota. Heterocytous Genera. Berlin: Springer.
Komárek, J., and Anagnostidis, K. (2005). Süßwasserflora von Mitteleuropa, bd. 19/2: Cyanoprokaryota: Oscillatoriales, Vol. 19. Heidelberg: Spektrum Akademischer Verlag.
Konstantinidis, K. T., Rosselló-Móra, R., and Amann, R. (2017). Uncultivated microbes in need of their own taxonomy. ISME J. 11, 2399–2406. doi: 10.1038/ismej.2017.113
Korelusova, J., Kaštovský, J., and Komárek, J. (2009). Heterogeneity of the cyanobacterial genus synechocystis and description of a new genus, geminocystis. J. Phycol. 45, 928–937. doi: 10.1111/j.1529-8817.2009.00701.x
Kurmayer, R., Blom, J. F., Deng, L., and Pernthaler, J. (2015). Integrating phylogeny, geographic niche partitioning and secondary metabolite synthesis in bloom-forming Planktothrix. ISME J. 9:909. doi: 10.1038/ismej.2014.189
Larsson, J., Nylander, J. A., and Bergman, B. (2011). Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol. Biol. 11:187. doi: 10.1186/1471-2148-11-187
Laslett, D., and Canback, B. (2004). ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 32, 11–16. doi: 10.1093/nar/gkh152
Letunic, I., and Bork, P. (2019). Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259. doi: 10.1093/nar/gkz239
Li, D., Liu, C. M., Luo, R., Sadakane, K., and Lam, T. W. (2015). MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676. doi: 10.1093/bioinformatics/btv033
Li, S. J., Hua, Z. S., Huang, L. N., Li, J., Shi, S. H., Chen, L. X., et al. (2014). Microbial communities evolve faster in extreme environments. Sci. Rep. 4:6205. doi: 10.1038/srep06205
Mackenzie, R., Pedrós-Alió, C., and Díez, B. (2013). Bacterial composition of microbial mats in hot springs in Northern Patagonia: variations with seasons and temperature. Extremophiles 17, 123–136. doi: 10.1007/s00792-012-0499-z
Martin, M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12. doi: 10.14806/ej.17.1.200
Matsen, F. A., Kodner, R. B., and Armbrust, E. V. (2010). pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics 11:538. doi: 10.1186/1471-2105-11-538
Meeks, J. C., and Castenholz, R. W. (1971). Growth and photosynthesis in an extreme thermophile, Synechococcus lividus (Cyanophyta). Arch. Mikrobiol. 78, 25–41. doi: 10.1007/BF00409086
Micallef, M. L., D’Agostino, P. M., Sharma, D., Viswanathan, R., and Moffitt, M. C. (2015). Genome mining for natural product biosynthetic gene clusters in the Subsection V cyanobacteria. BMC Genomics 16:669. doi: 10.1186/s12864-015-1855-z
Miller, S., Castenholz, R., and Pedersen, D. (2007). Phylogeography of the thermophilic cyanobacterium Mastigocladus laminosus. Appl. Environ. Microbiol. 73, 4751–4759. doi: 10.1128/AEM.02945-06
Momper, L., Hu, E., Moore, K. R., Skoog, E. J., Tyler, M., Evans, A. J., et al. (2019). Metabolic versatility in a modern lineage of cyanobacteria from terrestrial hot springs. Free Radical Biol. Med. 140, 224–232. doi: 10.1016/j.freeradbiomed.2019.05.036
Monchamp, M. E., Spaak, P., and Pomati, F. (2019). Long term diversity and distribution of non-photosynthetic cyanobacteria in peri-alpine lakes. Front. Microbiol. 9:3344. doi: 10.3389/fmicb.2018.03344
Moreira, D., Tavera, R., Benzerara, K., Skouri-Panet, F., Couradeau, E., Gérard, E., et al. (2017). Description of Gloeomargarita lithophora gen. nov., sp. nov., a thylakoid-bearing basal-branching cyanobacterium with intracellular carbonates, and proposal for Gloeomargaritales ord. nov. Int. J. Syst. Evol. Microbiol. 67:653. doi: 10.1099/ijsem.0.001679
Nakamura, Y., Kaneko, T., Sato, S., Mimuro, M., Miyashita, H., Tsuchiya, T., et al. (2003). Complete genome structure of Gloeobacter violaceus PCC 7421, a cyanobacterium that lacks thylakoids. DNA Res. 10, 137–145. doi: 10.1093/dnares/10.4.137
O’Brien, J., and Wright, G. D. (2011). An ecological perspective of microbial secondary metabolism. Curr. Opin. Biotechnol. 22, 552–558. doi: 10.1016/j.copbio.2011.03.010
Olm, M. R., Brown, C. T., Brooks, B., and Banfield, J. F. (2017). dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868. doi: 10.1038/ismej.2017.126
Olsen, M. T., Nowack, S., Wood, J. M., Becraft, E. D., LaButti, K., Lipzen, A., et al. (2015). The molecular dimension of microbial species: 3. Comparative genomics of Synechococcus strains with different light responses and in situ diel transcription patterns of associated putative ecotypes in the Mushroom Spring microbial mat. Front. Microbiol. 6:604. doi: 10.3389/fmicb.2015.00604
Oren, A., and Ventura, S. (2017). The current status of cyanobacterial nomenclature under the “prokaryotic” and the “botanical” code. Antonie Leeuwenhoek 110, 1257–1269. doi: 10.1007/s10482-017-0848-0
Papke, R. T., Ramsing, N. B., Bateson, M. M., and Ward, D. M. (2003). Geographical isolation in hot spring cyanobacteria. Environ. Microbiol. 5, 650–659. doi: 10.1046/j.1462-2920.2003.00460.x
Parks, D. H., Chuvochina, M., Waite, D. W., Rinke, C., Skarshewski, A., Chaumel, P., et al. (2018). A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat. Biotechnol. 36, 996–1004. doi: 10.1038/nbt.4229
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P., and Tyson, G. W. (2015). CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055. doi: 10.1101/gr.186072.114
Parks, D. H., Rinke, C., Chuvochina, M., Chaumeil, P. A., Woodcroft, B. J., Evans, P. N., et al. (2017). Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol. 2, 1533–1542. doi: 10.1038/s41564-017-0012-7
Parks, D. H., Tyson, G. W., Hugenholtz, P., and Beiko, R. G. (2014). STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30, 3123–3124. doi: 10.1093/bioinformatics/btu494
Parmar, K., Dafale, N., Pal, R., Tikariha, H., and Purohit, H. (2018). An insight into phage diversity at environmental habitats using comparative metagenomics approach. Curr. Microbiol. 75, 132–141. doi: 10.1007/s00284-017-1357-0
Pollo, S. M., Zhaxybayeva, O., and Nesbø, C. L. (2015). Insights into thermoadaptation and the evolution of mesophily from the bacterial phylum Thermotogae. Can. J. Microbiol. 61, 655–670. doi: 10.1139/cjm-2015-0073
Ponce-Toledo, R. I., Deschamps, P., López-García, P., Zivanovic, Y., Benzerara, K., and Moreira, D. (2017). An early-branching freshwater cyanobacterium at the origin of plastids. Curr. Biol. 27, 386–391. doi: 10.1016/j.cub.2016.11.056
Puigbò, P., Pasamontes, A., and Garcia-Vallve, S. (2008). Gaining and losing the thermophilic adaptation in prokaryotes. Trends Genet. 24, 10–14. doi: 10.1016/j.tig.2007.10.005
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Ramos, V., Morais, J., and Vasconcelos, V. M. (2017). A curated database of cyanobacterial strains relevant for modern taxonomy and phylogenetic studies. Sci. Data 4, 1–8. doi: 10.1038/sdata.2017.54
Reji, L., and Francis, C. A. (2020). Metagenome-assembled genomes reveal unique metabolic adaptations of a basal marine Thaumarchaeota lineage. ISME J. 14, 2105–2115. doi: 10.1038/s41396-020-0675-6
Roeselers, G., Norris, T. B., Castenholz, R. W., Rysgaard, S., Glud, R. N., Kühl, M., et al. (2007). Diversity of phototrophic bacteria in microbial mats from Arctic hot springs (Greenland). Environ. Microbiol. 9, 26–38. doi: 10.1111/j.1462-2920.2006.01103.x
Roney, H. C., Booth, G. M., and Cox, P. A. (2009). Competitive exclusion of cyanobacterial species in the Great Salt Lake. Extremophiles 13, 355–361. doi: 10.1007/s00792-008-0223-1
Roy, C., Rameez, M. J., Haldar, P. K., Peketi, A., Mondal, N., Bakshi, U., et al. (2020). Microbiome and ecology of a hot spring-microbialite system on the Trans-Himalayan Plateau. Sci. Rep. 10, 1–22. doi: 10.1038/s41598-020-62797-z
Sabath, N., Ferrada, E., Barve, A., and Wagner, A. (2013). Growth temperature and genome size in bacteria are negatively correlated, suggesting genomic streamlining during thermal adaptation. Genome Biol. Evol. 5, 966–977. doi: 10.1093/gbe/evt050
Sanchez-Baracaldo, P., Hayes, P. K., and Blank, C. E. (2005). Morphological and habitat evolution in the Cyanobacteria using a compartmentalization approach. Geobiology 3, 145–165. doi: 10.1111/j.1472-4669.2005.00050.x
Sano, E. B., Wall, C. A., Hutchins, P. R., and Miller, S. R. (2018). Ancient balancing selection on heterocyst function in a cosmopolitan cyanobacterium. Nat. Ecol. Evol. 2, 510–519. doi: 10.1038/s41559-017-0435-9
Schirrmeister, B. E., Gugger, M., and Donoghue, P. C. (2015). Cyanobacteria and the great oxidation event: evidence from genes and fossils. Palaeontology 58, 769–785. doi: 10.1111/pala.12178
Schönknecht, G., Chen, W. H., Ternes, C. M., Barbier, G. G., Shrestha, R. P., Stanke, M., et al. (2013). Gene transfer from bacteria and archaea facilitated evolution of an extremophilic eukaryote. Science 339, 1207–1210. doi: 10.1126/science.1231707
Sciuto, K., and Moro, I. (2016). Detection of the new cosmopolitan genus Thermoleptolyngbya (Cyanobacteria, Leptolyngbyaceae) using the 16S rRNA gene and 16S–23S ITS region. ıMol. Phylogenet. Evol. 105, 15–35. doi: 10.1016/j.ympev.2016.08.010
Shaiber, A., and Eren, A. M. (2019). Composite metagenome-assembled genomes reduce the quality of public genome repositories. mBio 10:e00725-19. doi: 10.1128/mBio.00725-19
Shih, P. M., Wu, D., Latifi, A., Axen, S. D., Fewer, D. P., Talla, E., et al. (2013). Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. U.S.A. 110, 1053–1058. doi: 10.1073/pnas.1217107110
Singer, G. A., and Hickey, D. A. (2003). Thermophilic prokaryotes have characteristic patterns of codon usage, amino acid composition and nucleotide content. Gene 317, 39–47. doi: 10.1016/S0378-1119(03)00660-7
Steunou, A. S., Bhaya, D., Bateson, M. M., Melendrez, M. C., Ward, D. M., Brecht, E., et al. (2006). In situ analysis of nitrogen fixation and metabolic switching in unicellular thermophilic cyanobacteria inhabiting hot spring microbial mats. Proc. Natl. Acad. Sci. U.S.A. 103, 2398–2403. doi: 10.1073/pnas.0507513103
Thiel, V., Hügler, M., Ward, D. M., and Bryant, D. A. (2017). The dark side of the mushroom spring microbial mat: life in the shadow of chlorophototrophs. II. Metabolic functions of abundant community members predicted from metagenomic analyses. Front. Microbiol. 8:943. doi: 10.3389/fmicb.2017.00943
Uribe-Lorío, L., Brenes-Guillén, L., Hernández-Ascencio, W., Mora-Amador, R., González, G., Ramírez-Umaña, C. J., et al. (2019). The influence of temperature and pH on bacterial community composition of microbial mats in hot springs from Costa Rica. MicrobiologyOpen 8:e893. doi: 10.1002/mbo3.893
Uritskiy, G. V., DiRuggiero, J., and Taylor, J. (2018). MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 6, 1–13. doi: 10.1186/s40168-018-0541-1
Uyeda, J. C., Harmon, L. J., and Blank, C. E. (2016). A comprehensive study of cyanobacterial morphological and ecological evolutionary dynamics through deep geologic time. PLoS One 11:e0162539. doi: 10.1371/journal.pone.0162539
Vieille, C., and Zeikus, G. J. (2001). Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 65, 1–43. doi: 10.1128/MMBR.65.1.1-43.2001
Walter, J. M., Coutinho, F. H., Dutilh, B. E., Swings, J., Thompson, F. L., and Thompson, C. C. (2017). Ecogenomics and taxonomy of Cyanobacteria phylum. Front. Microbiol. 8:2132. doi: 10.3389/fmicb.2017.02132
Ward, D. M., Bateson, M. M., Ferris, M. J., Kühl, M., Wieland, A., Koeppel, A., et al. (2006). Cyanobacterial ecotypes in the microbial mat community of Mushroom Spring (Yellowstone National Park, Wyoming) as species-like units linking microbial community composition, structure and function. Philos. Trans. R. Soc. B. 361, 1997–2008. doi: 10.1098/rstb.2006.1919
Ward, D. M., Castenholz, R. W., and Miller, S. R. (2012). Cyanobacteria in Geothermal Habitats. In Ecology of Cyanobacteria II. Dordrecht: Springer, 39–63. doi: 10.1007/978-94-007-3855-3_3
Ward, D. M., Ferris, M. J., Nold, S. C., and Bateson, M. M. (1998). A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol. Mol. Biol. Rev. 62, 1353–1370. doi: 10.1128/mmbr.62.4.1353-1370.1998
Ward, L. M., Idei, A., Nakagawa, M., Ueno, Y., Fischer, W. W., and McGlynn, S. E. (2019). Geochemical and metagenomic characterization of Jinata Onsen, a Proterozoic-analog hot spring, reveals novel microbial diversity including iron-tolerant phototrophs and thermophilic lithotrophs. Microbes Environ. 34, 278–292. doi: 10.1264/jsme2.ME19017
Weinberger, A. D., Wolf, Y. I., Lobkovsky, A. E., Gilmore, M. S., and Koonin, E. V. (2012). Viral diversity threshold for adaptive immunity in prokaryotes. mBio 3:e00456-12. doi: 10.1128/mBio.00456-12
Whitman, W. B. (2016). Modest proposals to expand the type material for naming of prokaryotes. Int. J. Syst. Evol. Microbiol. 66, 2108–2112. doi: 10.1099/ijsem.0.000980
Wu, S., and Zhu, Y. (2012). ProPAS: standalone software to analyze protein properties. Bioinformation 8:167. doi: 10.6026/97320630008167
Wu, Y. W., Simmons, B. A., and Singer, S. W. (2016). MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32, 605–607. doi: 10.1093/bioinformatics/btv638
Yoon, K. S., Nguyen, N. T., Tran, K. T., Tsuji, K., and Ogo, S. (2017). Nitrogen fixation genes and nitrogenase activity of the non-heterocystous cyanobacterium Thermoleptolyngbya sp. O-77. Microbes Environ. 32, 324–329. doi: 10.1264/jsme2.ME17015
Keywords: cyanobacteria, hot springs, metagenomes, thermophiles, taxonomy, MAGs
Citation: Alcorta J, Alarcón-Schumacher T, Salgado O and Díez B (2020) Taxonomic Novelty and Distinctive Genomic Features of Hot Spring Cyanobacteria. Front. Genet. 11:568223. doi: 10.3389/fgene.2020.568223
Received: 31 May 2020; Accepted: 15 October 2020;
Published: 05 November 2020.
Edited by:
Pedro Simões, University of Lisbon, PortugalReviewed by:
Francisco (Paco) Barona-Gomez, National Polytechnic Institute of Mexico (CINVESTAV), MexicoCopyright © 2020 Alcorta, Alarcón-Schumacher, Salgado and Díez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beatriz Díez, YmRpZXpAYmlvLnB1Yy5jbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.