- Swammerdam Institute for Life Sciences (SILS), Molecular Plant Pathology, University of Amsterdam, Amsterdam, Netherlands
Specificity in the plant immune system is mediated by Resistance (R) proteins. Most R genes encode intracellular NLR-type immune receptors and these pathogen sensors require helper NLRs to activate immune signaling upon pathogen perception. Resistance conferred by many R genes is temperature sensitive and compromised above 28°C. Many Solanaceae R genes, including the potato NLR Rx1 conferring resistance to Potato Virus X (PVX), have been reported to be temperature labile. Rx1 activity, like many Solanaceae NLRs, depends on helper-NLRs called NRC’s. In this study, we investigated Rx1 resistance at elevated temperatures in potato and in Nicotiana benthamiana plants stably expressing Rx1 upon rub-inoculation with GFP-expressing PVX particles. In parallel, we used susceptible plants as a control to assess infectiousness of PVX at a range of different temperatures. Surprisingly, we found that Rx1 confers virus resistance in N. benthamiana up to 32°C, a temperature at which the PVX::GFP lost infectiousness. Furthermore, at 34°C, an Rx1-mediated hypersensitive response could still be triggered in N. benthamiana upon PVX Coat-Protein overexpression. As the Rx1-immune signaling pathway is not temperature compromised, this implies that at least one N. benthamiana helper NRC and its downstream signaling components are temperature tolerant. This finding suggests that the temperature sensitivity for Solanaceous resistances is likely attributable to the sensor NLR and not to its downstream signaling components.
Introduction
Plants have developed a multi-layered immune system activated by receptor proteins that detect pathogen-generated molecules. Immune receptors can be classified into two main groups: (i) the extracellular receptors, mainly Receptor like-Kinases (RLK) or -Proteins (RLP), commonly associated with either recognition of pathogens’ conserved features (microbial- or pathogen associated molecular patterns, MAMP or PAMP) or by pathogen inflicted damage (damage associated molecular patterns or DAMP) and (ii) Intracellular receptors. Members of this latter group often encode Nucleotide-binding domain and leucine-rich repeat (NLR) proteins that recognize specific pathogen encoded avirulence factors (Avr) (Dodds and Rathjen, 2010). NLRs can be divided into two major sub-groups according to their N-terminal domain, the TNLs, with a Toll/interleukin-1 receptor (TIR) and the CNLs, with a Coiled Coil (CC) domain (Monteiro and Nishimura, 2018). NLRs have been described as molecular switches that turn ON immune signaling after pathogen perception (Takken et al., 2006). NLR activation often triggers local cell death, the so-called hypersensitive response (HR) (Balint-Kurti, 2019).
In plant genomes, NLRs are typically encoded by a large gene family consisting of several hundreds of genes. NLRs can be categorized into two operative groups, the sensors (e.g., NLRs responsible for pathogen perception) and the transducers (or helpers). The latter group has recently been highlighted and is responsible for relaying or translating the upstream signal from the sensor NLR to the downstream signaling components (Wu et al., 2018). In Solanaceae, a phylogenetically related NLR family, consisting of NLR Required for Cell death (NRC) genes, have been described as helper NLRs (Adachi et al., 2019). Required by a large number of sensor NLRs that mediate resistance against diverse pathogens, they constitute a complex network of immune receptors (Wu et al., 2017). For example, the sensor NLRs Mi-1 from tomato, Rpi-blb2 and R1 from potato rely exclusively on NRC4 to trigger resistance responses, while the tomato NLR Prf and the potato NLR GPA2 can trigger HR via NRC2 or NRC3. Other NLRs such as Rx1 from potato or Bs2 from pepper can pair with either NRC2, NRC3, or NRC4 (Wu et al., 2017). Interestingly, the founder NRC, NRC1, has been initially identified to be required for resistance mediated by the non-NLR, Cf-4 that confers resistance toward the fungus Cladosporium fulvum in tomato (Gabriels et al., 2007). This finding suggests a potential role of these helpers to integrate immune signaling from both intra- and extracellular immune receptors (Leibman-Markus et al., 2018).
Although environmental conditions, such as temperature, have a crucial impact on the outcome of the diseases, this third component of the disease triangle (plant, pathogen, and environment) is often overlooked in plant-pathogen interaction studies. A temperature dependency of disease resistance has been reported in several cases involving different kind of pathogens, such as viruses, fungi, oomycetes, bacteria, or nematodes. For example, the tobacco NLR N is unable to confer resistance to the Tobacco mosaic virus (TMV) above 28°C (Whitham et al., 1996). Resistance to the nematode Meloidogyne incognita mediated by the NLR Mi-1 in tomato is compromised by exposure to 35°C for 3 h preceding inoculation (De Carvalho et al., 2015). Tsw-mediated resistance fails to trigger resistance to Tomato spotted wilt virus (TSWV) at 32°C and above in pepper plants (Moury et al., 1998). The NLR Bs2 from pepper, conferring resistance to the bacterium Xanthomonas axonopodis pv. vesicatoria, shows compromised resistance and HR at 32°C (Romero et al., 2002). Interestingly, non-NLR mediated resistance; such as resistance mediated by the transmembrane receptor like proteins Cf-4 and Cf-9 against C. fulvum is also impaired at elevated temperature (Cai et al., 2001; De Jong et al., 2002).
While temperature sensitivity of resistance seems widespread, it is not trivial to study this aspect in many plant pathogen interactions. One reason is that pathogen fitness and virulence can also be affected by (elevated) temperatures (Velasquez et al., 2018), complicating identification of temperature sensitive components in an interaction. Therefore, as a proxy for immune activation at elevated temperatures, the capacity of R genes to trigger HR upon (over)expression of their corresponding Avr is often used. For example, in Wang et al. (2009) N- and Rx1-temperature sensitivity is assessed by monitoring loss of HR when co-expressed with the corresponding Avrs; p50 from TMV and the Coat Protein (CP) from Potato mosaic virus (PVX) at 28 and 30°C, respectively (Wang et al., 2009). However, HR is not always correlated with functional resistance. For example, HR triggered by the recognition of Pseudomonas syringae pv. tomato DC 3000 (PtoDC3000) HopZ1a or AvrRpt2 in Arabidopsis thaliana is suppressed at 30°C, while resistance to the bacteria is unaffected (Menna et al., 2015). Additionally, co-expression of R and or Avr gene(s) in heterologous systems often relies on Agrobacterium-mediated transient transformation assays (ATTA). A drawback of this system is the temperature sensitivity of T-DNA transfer by Agrobacterium tumefaciens, which makes plant transformation above 27°C highly inefficient (Dillen et al., 1997).
Many Solanaceae resistances, mediated by NLR- or non-NLR sensors that depend on NRC helpers, are reported to be compromised at elevated temperature. However, the temperature sensitive component of their molecular signaling pathways (sensor, helper, or downstream signaling) remains unknown. Since this temperature sensitivity concerns resistance triggered by different types of receptors (NLR and non-NLR, such as the RLPs Cf-4 and Cf-9), it is tempting to speculate that shared downstream signaling components, such as the helpers NRCs, could be the Achilles’ heel of the immune signaling at elevated temperatures. Rx1 is a well-studied NLR from potato and is a perfect model to challenge our hypothesis since it can signal via NRC2, NRC3, or NRC4 (Wu et al., 2017).
The NLR Rx1 triggers resistance to PVX upon recognition of its CP in potato and in N. benthamiana stably expressing Rx1 from its native promoter (Bendahmane et al., 1999). Rx1 confers a so called “extreme resistance” response that prevents viral replication without triggering cell death (Bendahmane et al., 1999). Overexpression of the avirulent CP (CP106) in an Rx1-expressing plant nonetheless can trigger HR in heterologous species such as N. benthamiana, whereas CP105, a CP variant of an Rx1 resistance breaking strain of PVX, does not (Goulden et al., 1993; Bendahmane et al., 1999). Rx1 has been reported to be temperature sensitive as it was unable to trigger HR at 30°C upon ATTA-mediated CP-expression in N. benthamiana (Wang et al., 2009). However, the capacity of Rx1 to mount resistance against PVX at elevated temperature is not known.
Here we investigate Rx1-mediated resistance to PVX in N. benthamiana plants stably expressing Rx1 from its native promoter and in potato. Infection with the GFP-expressing PVX particles (PVX::GFP) was done by rub inoculation and is independent of Agrobacterium transformation. We observed that, in N. benthamiana, Rx1 is preventing PVX::GFP replication up to temperatures above which PVX::GFP is no longer infectious. The temperature resilience of Rx1 in potato could not be assessed as the virus itself is not efficiently infecting potato at 30°C in our experimental set-up. Besides, we assessed the capacity of Rx1 to trigger HR at elevated temperatures using Rx1 N. benthamiana plants stably transformed with a dexamethasone (DEX)-inducible CP construct. We observed that Rx1-mediated HR can still be observed at temperatures up to 34°C. Altogether, our results imply that Rx1 is a thermotolerant R protein – as are its downstream signaling components – providing new insights in the mechanisms underlying thermosensitivity in plant immunity.
Materials and Methods
Plant Lines, N. benthamiana Transformation and Growing Conditions
Wild-type and transgenic Rx1:4xHA (Lu et al., 2003), expressing Rx1 under control of its native promoter, N. benthamiana plants were used for PVX bioassays. To monitor HR Rx1:4xHA+DEX::CP106 9-4 (referred to as Rx1D106, internal identifier #FP1807) and Rx1:4xHA+DEX::CP105 6-6 (referred to as Rx1D105, internal identifier #FP1810) stable transgenic lines were generated. For this, N. benthamiana Rx1:4xHA plants were transformed using the dexamethasone (DEX) inducible PVX-CP constructs, either DEX::CP106 or DEX::CP105 described in Knip et al. (2019), using Agrobacterium-mediated transformation as described in Sparkes et al. (2006). Briefly, A. tumefaciens infiltrated leaves were surface sterilized, cut into 2 cm2 diamond shape pieces, and placed on shoot-induction medium supplemented with 14.8 μg/ml hygromycin for selection. Shoots from putative transformants were transferred to root-induction medium containing 14.8 μg/ml hygromycin. Ten and seven candidate transformants for DEX::CP106 or DEX::CP105 constructs, respectively, were selected for seed production. Segregation for hygromycin resistance of the obtained T1 progeny was assessed on selective medium and seven and five lines with a single insertion were identified for DEX::CP106 or DEX::CP105 constructs, respectively. Homozygosity of T = 2 plants was evaluated by Real-Time PCR using gDNA by estimation of t-DNA copy number (by amplification of the Hygromycin resistance gene, using the oligonucleotides FP7722-HP ThygroFW: GTTCGGGGATTCCCAATACGAGGTC and FP7723-HPThygroRV: ATCGAAATTGCCGTCAACCAAGCTC) compared to a endogenous reference gene (NRG1, amplified using the oligonucleotides FP8254: GTGTCCGACCACTAAGCATGGAACTA and FP8255: CTGCTGGTGCATCCTTTCTGGAAATC). The Real-Time PCRs were performed in QuantStudioTM3 (Thermo Fisher Scientific). The 10 μL PCR contained 0.2 μM of each primer, 100 ng of gDNA, 0.05 μl of DNA polymerase (DreamTaq, Thermo Fisher Scientific), 1x Evagreen dye (Solis Biodyne), 1x ROX passive reference, dNTPs, and water. The cycling program was set to initial denaturation 2 min at 94°C, 40 cycles; denaturation for 15 s at 95°C, annealing for 20 s at 58°C, elongation for 30 s at 72°C, followed a melting curve analysis of 15 s at 95°C, 1 min at 60°C, 15 s at 95°C. The copy number analysis was performed using the online Thermo Fisher Scientific application. Plants being phenotypically similar to the parental Rx1:4xHA plants, and showing expression of the CP upon dexamethasone treatment were selected, resulting in three and two independent homozygous Rx1D106 and Rx1D105 lines, respectively. One out of the three Rx1D106 and one out of the two Rx1D105, were used for this study as the different Rx1D106 and Rx1D105 lines showed identical responses when DEX treated (data not shown).
N. benthamiana plants were grown under long-day conditions in a climate chamber (22°C, 70% humidity, 16 h/8 h light/dark) for 3–4 weeks. Potato tubers from two diploid potato genotypes RH 89-039-16 (RH) and SH82-93-488 (SH) (Van der Voort et al., 1997) were planted in soil and plants were grown for 6 weeks under the conditions described above. One day before treatment (either PVX::GFP rub-inoculation or dexamethasone treatment, see below), plants were transferred and incubated in a MD1400 MODULAR CLIMATE CHAMBER (Snijders Labs) at the indicated temperatures under a constant humidity of 80% at a 12/12h light/dark regime.
PVX::GFP Rub-Inoculation and in planta Virus Detection in N. benthamiana
To produce infectious PVX::GFP particles, leaves of 4 weeks old WT N. benthamiana plants were agroinfiltrated with an A. tumefaciens GV3101 strain containing the pJIC SA_Rep helper plasmid and the PVX::GFP construct (internal identifier BglFP#4081) according to Ma et al. (2012). PVX::GFP was obtained by inserting the LSS-msfGFP ORF1 after a duplicated CP promoter, into SgsI – NotI restriction sites of pGR106, a binary vector containing an infectious PVX clone (Jones et al., 1999). Two weeks after agroinfiltration, systemically infected leaves were either snap frozen with liquid nitrogen and stored at –80°C or directly used for rub-inoculation. PVX::GFP inoculum was made by grinding a fresh, or frozen, PVX::GFP infected leaf with a mortar in 4 mL 50 mM potassium phosphate buffer pH 7. The youngest fully expanded leaves of 3 weeks old N. benthamiana plants (WT or Rx1) were mechanically inoculated by rubbing the adaxial side with a piece of miracloth (Merck; pore size of 22–25 μm) soaked in the PVX::GFP inoculum using Carborundum as an abrasive. Five minutes post-inoculation the inoculated leaves were rinsed with tap water and excess water was removed using paper tissues. Ten days after rub-inoculation, plants were photographed using a Panasonic Lumix DMC-LX15 camera placed in a dark chamber (Extraneous Light Protector and RS 1 stand, Kaiser, Germany) illuminated with UV light (RB 5003 UV Lighting Unit code n°5591, Kaiser, Germany).
RNA Isolation and RT-PCR
For PVX::GFP RNA detection, systemic leaves from PVX::GFP rub-inoculated plants were sampled 10 days post-inoculation. To verify induction of PVX-CP transcription after dexamethasone treatment, leaves of Rx1D105 and Rx1D106 were sampled at 0, 2, and 4 h post-dexamethasone induction (hpdi). Total RNA was extracted using TRIzol LS reagent (Thermo Fisher Scientific, Waltham, MA, United States). The RNA was treated with DNase (Thermo Fischer Scientific) according to the supplier’s protocol and RNA concentrations were determined by measuring the absorbance at 260 nm on a NanoDrop (Thermo Fisher Scientific). cDNA was synthesized from 1.5 μg of total RNA using RevertAid H reverse transcriptase and Oligo-dT (Eurofins) in the presence of the RNAse inhibitor Ribolock (Thermo Fisher Scientific) following the supplier’s protocol and diluted 5 times in Milli-Q H2O.
Semi quantitative Reverse-Transcriptase (RT) PCR (35 cycles, annealing temperature of 60°C) was performed on 1 μl of diluted cDNA using DreamTaq DNA Polymerase (Thermo Fisher Scientific) following the supplier’s protocol, using CP specific primers FP8371-PVX-CP-F: CACTGCAGGCGCAACTCC and FP8372-PVX-CP-R: GTCGTTGGATTGYGCCCT or EF1α primers FP8391-NbEF1α-F: AGCTTTACCTCCCAAGTCATC and FP8392-NbEF1α-R: AGAACGCCTGTCAATCTTGG as a positive internal control.
Potato Inoculation and PVX Detection by ELISA and Western Blot
Six terminal leaflets from 6 weeks old potato plants SH and RH genotypes were rub-inoculated with PVX::GFP as described above. Plants were kept at either 20 or 30°C prior to analysis. One week after inoculation, a quarter of each inoculated leaflet was sampled and homogenized in 50 mM Sodium Phosphate buffer pH 7, using a Tissuelyser (QIAGEN) and three 3 mm steel beads at 30 Hz for twice 30 s. The virus concentration was determined by DAS-ELISA using PVX antibodies (Prime Diagnostics, Wageningen, The Netherlands). Plates (NUNC-Immuno Plates Maxisorp F96) were coated with a 1:1000 dilution of the PVX polyclonal antibody to bind the antigen. A second polyclonal PVX antibody, conjugated with alkaline phosphatase, was used for detection by monitoring the conversion of the p-nitrophenyl phosphate substrate. Absorbance of each well was recorded at 405 nm with a reference filter of 655 nm using a BioTek Synergy H1 Hybrid multi-mode microplate reader (BioTek).
Proteins were isolated from one centimeter of petiole of each inoculated leaf 1 week post-inoculation using the method described in Knip et al. (2019). PVX detection by Western blot was performed on these samples as described in Knip et al. (2019) using PVX-specific antibody (diluted 1 : 3000) (ref 110411, Bioreba, Reinach, Switzerland).
Rx1-Mediated HR Induction
One day after transfer of 3 weeks old Rx1D106 and Rx1D105 plants into the growth incubator at 20, 30, 32, 33, or 34°C, leaves were treated with 20 μM dexamethasone, 0.01% Silwet R-77 in Milli-Q H2O. The dexamethasone solution was applied on an ∼1 cm-diameter circle on the left side of each leaf. HR was assessed and pictures were taken 24 h post-dexamethasone application.
Results
Virulence of PVX Is Compromised Above 32°C While Rx1-Mediated Resistance Is Unaffected at This Temperature
To determine a potential thermotolerance of Rx1-mediated resistance to PVX, Rx1 transgenic N. benthamiana plants were rub-inoculated with infectious PVX particles at 20, 30, 32, 33, or 34°C. To visualize infection and spread of the virus, a recombinant PVX::GFP strain was used that triggers production of green fluorescent protein in infected plant cells. As a positive control for infection, susceptible wildtype (WT) N. benthamiana were rub-inoculated with the virus and incubated at the indicated temperatures. Ten days after inoculation WT plants kept at 20°C displayed strong PVX symptoms (leaf deformations and stunting) that correlated with intense GFP fluorescence under UV light (Figure 1). Green fluorescence could be observed at infection foci on the inoculated leaf and its petioles, and around the vasculature of systemically infected leaves (Figure 1). As expected, no symptoms nor green fluorescence were observed in PVX::GFP-inoculated Rx1 plants at 20°C due to the resistance conferred by Rx1 (Figure 1). Instead, Rx1 plants appeared red under UV light due to chlorophyll autofluorescence. RT-PCR on systemic leaf material confirmed that viral transcripts were present in WT plants, but absent in Rx1 plants at 20°C (Figure 2). At 30°C and above, visual PVX symptoms could no longer be discerned in WT plants. However, GFP fluorescence could still be observed in systemic leaves of WT plants at 30°C, attesting the usefulness of a GFP reporter virus (Figure 1). In comparison, no GFP fluorescence was observed in Rx1 plants inoculated at 30°C (Figure 1). These differences were confirmed by semi quantitative RT-PCR revealing the presence of viral RNA in the WT plant, but not in the resistant Rx1 line (Figure 2). At 32°C, and above (data not shown), in addition to an absence of PVX symptoms, no GFP fluorescence was observed in WT or Rx1 plants (Figure 1). Plants inoculated at 33 and 34°C are phenotypically similar to those incubated at 32°C (data not shown). Only a very small amount of viral RNA could be detected in the systemic leaves of WT plants at 32°C, but no viral transcripts were observed at 33 and 34°C (Figure 2). These findings suggests that at temperatures above 32°C PVX::GFP is no longer able to infect and spread in N. benthamiana. In addition, as no viral RNAs were detected at 32°C or above in Rx1 plants, this suggests that Rx1 is able to confer resistance to PVX::GFP in N. benthamiana at least up to temperatures at which the virus is no longer infectious.
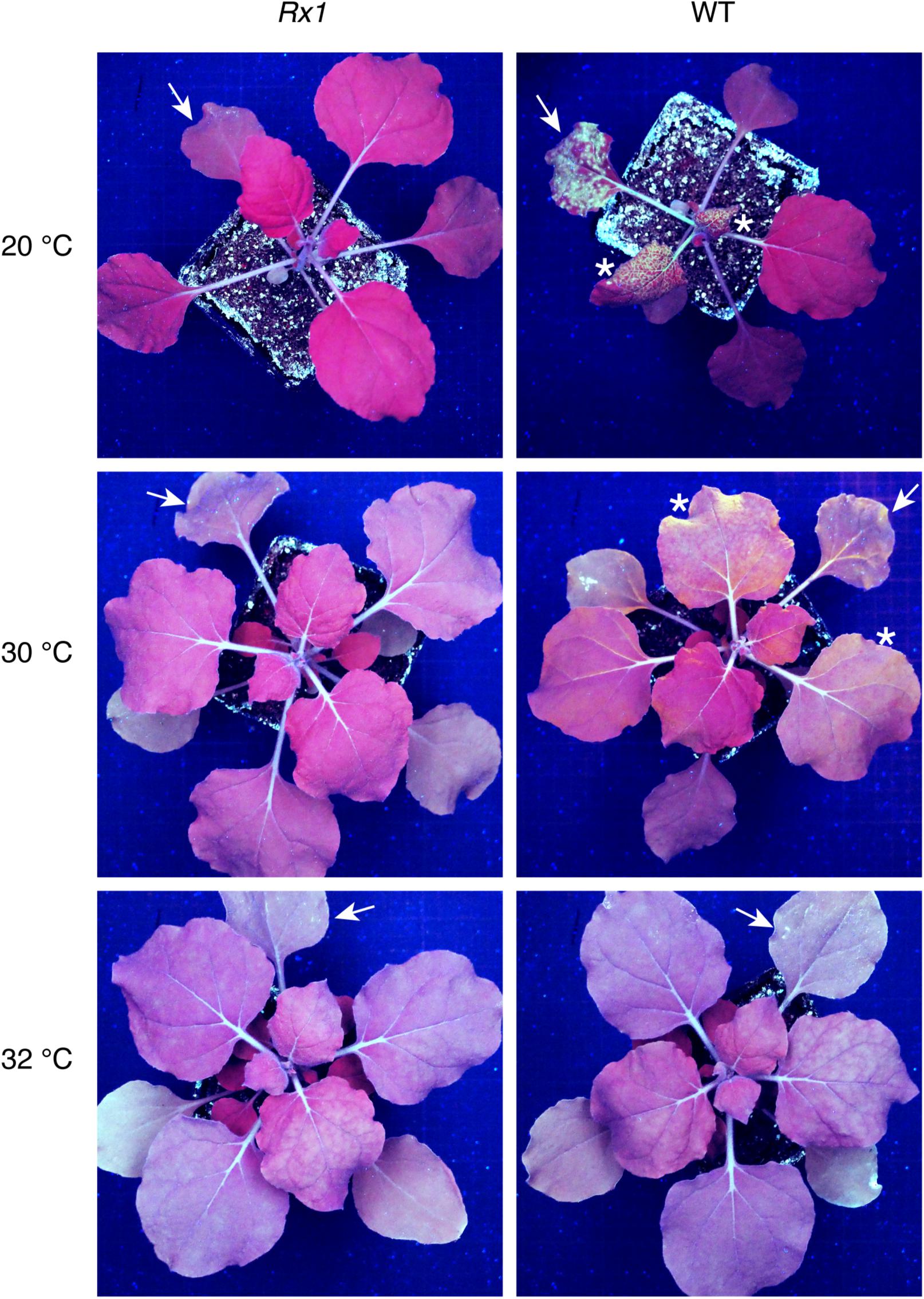
Figure 1. PVX::GFP fluorescence is observed in WT N. benthamiana plants up to 30°C but not in Rx1 plants at the indicated temperatures. Detection of green PVX::GFP fluorescence under UV light in Rx1 and WT N. benthamiana 10 days post-rub-inoculation. Arrows mark the rub-inoculated leaves and asterisks indicate systemic leaves emitting green fluorescence due to GFP accumulation.
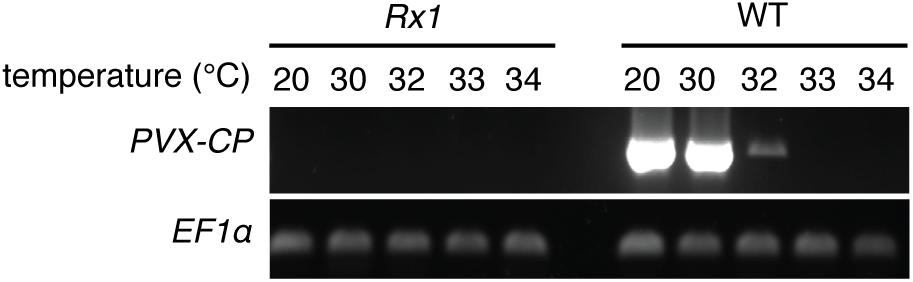
Figure 2. PVX-CP transcripts are detected in WT N. benthamiana plants up to 32°C, but not in inoculated Rx1 plants. RT-PCR mediated detection of PVX::GFP RNA in systemic leaves of Rx1 and WT N. benthamiana 10 days post-rub-inoculation. Specific amplification of PVX-CP transcripts and plant EF1α transcripts are presented in the top -and bottom-row, respectively.
The thermotolerance of Rx1-mediated resistance to PVX::GFP was investigated in potato plants with or without Rx1 resistance, the genotypes SH (Rx1) and RH (no Rx1), respectively. As expected, no virus was detected in the resistant SH genotype inoculated leaves or petioles at either 20 or 30°C (Supplementary Figures 1, 2). Notably, while PVX::GFP was detectable in sensitive RH potato inoculated leaves at 20°C, no virus could be detected in their petioles suggesting that the virus did not move systemically yet (Supplementary Figures 1, 2). Furthermore, no virus could be detected in susceptible RH inoculated leaves nor petioles at 30°C (Supplementary Figures 1, 2). This suggests that the capacity of PVX::GFP to infect at elevated temperature is determined by the host, as in N. benthamiana plants present in the same compartment high viral titers could be detected at 30°C (Supplementary Figures 1, 2). The inability of PVX::GFP to infect potato plants at elevated temperature in our experimental set-up prevents further investigation of the thermotolerance of Rx1 resistance in potato.
Rx1 Triggers HR Upon Recognition of the Avirulent PVX Coat Protein Variant at 34°C
Since PVX infection is fully abolished above 32°C, an alternative approach was used to monitor Rx1 activity at higher temperatures. Rx1 activation by the Coat Protein 106 variant (CP106) of PVX triggers a Hypersensitive Response in N. benthamiana that is visible as a necrotic sector. To express CP106, or CP105 as a negative control, we used the CESSNA system to enable inducible expression upon dexamethasone application (Knip et al., 2019). As Agrobacterium-mediated transformation is compromised at 27°C and higher (Dillen et al., 1997) we generated stable transgenic plants expressing Rx1 in combination with DEX::CP106 or DEX::CP105, referred to as Rx1D106 and Rx1D105, respectively). In the absence of dexamethasone, the generated Rx1D106 and Rx1D105 transgenic plants were phenotypically identical to WT plants (data not shown). Upon dexamethasone treatment, expression of CP105 and CP106 was observed in Rx1D105 and Rx1D106, respectively, within 2 h following application of dexamethasone (Supplementary Figure 3). As anticipated a clear HR was observed at the DEX treated sector in Rx1D106 but not in Rx1D105 leaves, as the latter does not trigger Rx1-mediated signaling (Figure 3). Using these hence validated transgenic lines, the capacity of Rx1 to trigger HR in response to CP106 at elevated temperature was examined. Dexamethasone was locally applied on the left side of one leaf of Rx1D106 and Rx1D105 plants placed at 20, 30, 32, 33, and 34°C. As shown in Figure 3, Rx1 triggered HR at all temperatures tested, but only upon dexamethasone mediated induction of CP106-, but not CP105-expression. These results show that the ability of Rx1 to trigger HR upon CP106 perception is not compromised at temperatures up to 34°C.
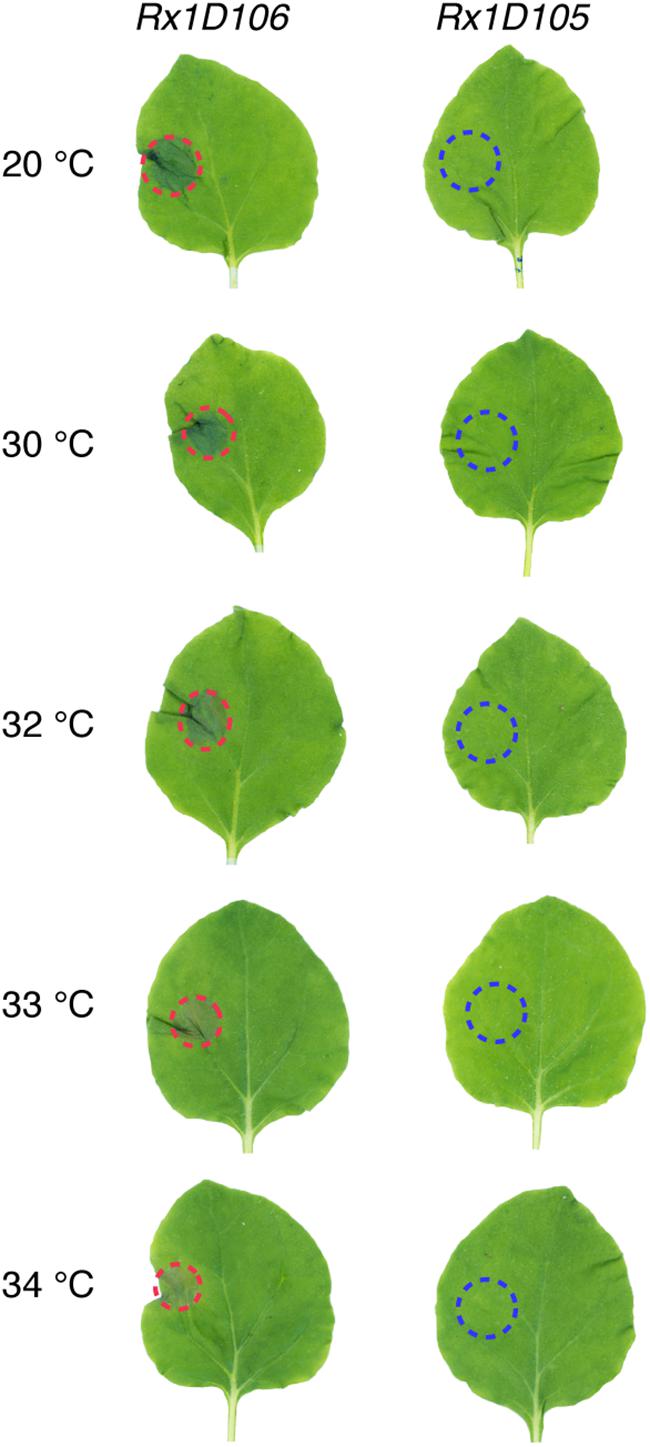
Figure 3. Rx1 triggers HR upon CP106 expression up to 34°C. Rx1-mediated HR in transgenic lines of N. benthamiana expressing both Rx1 and PVX-CP106 (Rx1D106) or PVX-CP105 Rx1D105) under the control of a dexamethasone-inducible promoter. CP expression was induced by spot application of dexamethasone on the left side of the leaf adaxial surface. HR, marked with red dotted lines, was visible in Rx1D106 lines at all temperatures tested while no cell death was observed in the Rx1D105 control.
Discussion
In this study, we show that Rx1-mediated resistance to PVX::GFP in N. benthamiana remains functional up to a temperature at which the virus was no longer infectious. We also show that the non-permissive temperatures for PVX::GFP infection differ between potato and N. benthamiana plants. Indeed, PVX::GFP could spread systemically up to 32°C in N. benthamiana, while at 30°C PVX::GFP multiplication in inoculated potato leaves was compromised (Supplementary Figure 1) and no systemically spreading virus could be detected in petioles of inoculated leaves (Supplementary Figure 2). In addition, Rx1-triggered HR was also observed at high temperatures (34°C) in N. benthamiana. This finding contrasts a previous study detailing that Rx1-mediated HR was abolished above 28°C (Wang et al., 2009). The main difference between both studies is the use of A. tumefaciens to express R and Avr constructs. In our study both genes are stably integrated in the plant genome and Avr expression is triggered by applying dexamethasone. In our system co-expressing Rx1 with the non-recognized CP105 variant did not trigger cell death, thereby ruling out the possibility that dexamethasone itself triggered cell death at elevated temperatures. Considering the fact that Agrobacterium-mediated transformation efficiency strongly decreases at increased temperatures, and was shown to be fully compromised at 29°C (Dillen et al., 1997), we propose that the absence of HR observed in Wang et al. (2009) could be attributed to a lack or a poor expression of the construct(s) used.
The decrease of PVX titer in local, and especially in systemic leaves, with increasing temperature has been previously reported in Nicotiana species (Ma et al., 2016). Furthermore, it is likely that PVX::GFP will have a slightly altered performance as compared to a natural strain. We observed that PVX::GFP infectiousness in potato was impaired at lower temperatures than in N. benthamiana. N. benthamiana is hypersusceptible to many RNA viruses due to a mutation in an RNA dependent RNA Polymerase that is important for antiviral defense based on RNA silencing (Yang et al., 2004). PVX replicates slower in potato than in N. benthamiana, which could explain the higher viral titers in N. benthamiana as compared to potato at 20°C (Supplementary Figure 1). At elevated temperature (e.g., 30°C) PVX::GFP was not detectable in potato 1 week after inoculation with the virus (Supplementary Figures 1, 2). We cannot exclude whether viral replication and spread is compromised in potato at the elevated temperature, but we could not prolong the incubation time as the plants started to collapse after 1 week. The mechanism underlying poor viral replication and spread is unknown, but could be related to the observation that the RNA silencing machinery in plants is more active at elevated temperatures (Szittya et al., 2003). Further study is required to resolve whether RNA silencing is responsible for impaired viral replication at elevated temperatures in potato.
NRCs are required for resistance mediated by both sensor CNLs and RLPs in Solanaceae (Gabriels et al., 2007; Wu et al., 2017). Several of these CNL- or RLP-mediated resistances are compromised at elevated temperatures (De Jong et al., 2002; Romero et al., 2002; De Carvalho et al., 2015), pointing to NRCs as potential suspects for thermosensitivity. However, our findings show that Rx1-mediated resistance against PVX::GFP is still efficient up to a temperature at which the virus is not infectious anymore. As Rx1 immunity is unaltered at elevated temperature, this means that Rx1 activation and its downstream signaling component are also functional. Rx1 has been shown to require NRC2, 3 or 4 downstream of its activation to trigger HR (Wu et al., 2017). Therefore, it is tempting to speculate that at least one of the Rx1-interacting NRCs functions at elevated temperature. Consequently, the immune sensors (sensor NLRs) are the most probable components that are affected in thermosensitive immune signaling Notably, a similar observation has been made for two TNLs, SNC1 from Arabidopsis and N from tobacco (Zhu et al., 2010). Through genetic screens and targeted mutagenesis, it was shown that SNC1 and N are the thermosensitive components in immune signaling. Together these observations suggest that for both CNL-, TNL-, or RLP- triggered immunity, the sensor is the temperature sensitive element. How a sensor NLR is affected by elevated temperature and loses its activity is unknown. Of note, several studies show that an elevated ambient temperature reduces accumulation of sensor NLR proteins, such as SNC1 or RPS4 and N in the nucleus (Wang et al., 2009; Zhu et al., 2010; Mang et al., 2012; Hua, 2013). For some NLRs a nuclear location has been shown to be crucial for triggering immunity, providing a potential mechanism (Qi and Innes, 2013).
The premise of these observations, that the sensors are thermosensitive while downstream immune signaling components do function at elevated temperatures, is relevant as it provides leads on how to improve thermotolerance of disease resistances by identifying the immune receptor itself as main target for mutagenesis.
Author Contributions
MR and FT designed the study and wrote the manuscript. MR, MK, TA, and MB performed experiments. MR analyzed the data and drafted all figures. All authors read and approved the final manuscript.
Funding
MR, MK, MB, and FT received funding from the NWO-Earth and Life Sciences-funded VICI project No. 865.14.003, and FT received funding from the European Union’s Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie Grant Agreement No. 676480 (Bestpass).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Marieke Mastop and Dr. Joachim Goedhart (University of Amsterdam) for providing lss-msf-GFP construct, Octavina Sukarta, and Dr. Aska Goverse (Wageningen University) for sharing the ELISA protocol and Dr. Nico Tintor for providing the potato tubers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.00417/full#supplementary-material
Footnotes
References
Adachi, H., Derevnina, L., and Kamoun, S. (2019). NLR singletons, pairs, and networks: evolution, assembly, and regulation of the intracellular immunoreceptor circuitry of plants. Curr. Opin. Plant Biol. 50, 121–131. doi: 10.1016/j.pbi.2019.04.007
Balint-Kurti, P. (2019). The plant hypersensitive response: concepts, control and consequences. Mol. Plant Pathol. 20, 1163–1178.
Bendahmane, A., Kanyuka, K., and Baulcombe, D. C. (1999). The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11, 781–791. doi: 10.1105/tpc.11.5.781
Cai, X. Z., Takken, F. L. W., Joosten, M., and De Wit, P. (2001). Specific recognition of AVR4 and AVR9 results in distinct patterns of hypersensitive cell death in tomato, but similar patterns of defence-related gene expression. Mol. Plant Pathol. 2, 77–86. doi: 10.1046/j.1364-3703.2001.00053.x
De Carvalho, L. M., Benda, N. D., Vaughan, M. M., Cabrera, A. R., Hung, K., Cox, T., et al. (2015). Mi-1-mediated nematode resistance in tomatoes is broken by short-term heat stress but recovers over time. J. Nematol. 47, 133–140.
De Jong, C. F., Takken, F. L. W., Cai, X. H., De Wit, P., and Joosten, M. (2002). Attenuation of Cf-mediated defense responses at elevated temperatures correlates with a decrease in elicitor-binding sites. Mol. Plant Microbe Interact. 15, 1040–1049. doi: 10.1094/mpmi.2002.15.10.1040
Dillen, W., De Clercq, J., Kapila, J., Zambre, M., Van Montagu, M., and Angenon, G. (1997). The effect of temperature on Agrobacterium tumefaciens-mediated gene transfer to plants. Plant J. 12, 1459–1463. doi: 10.1046/j.1365-313x.1997.12061459.x
Dodds, P. N., and Rathjen, J. P. (2010). Plant immunity: towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 11, 539–548. doi: 10.1038/nrg2812
Gabriels, S., Vossen, J. H., Ekengren, S. K., Van Ooijen, G., Abd-El-Haliem, A. M., Van Den Berg, G. C. M., et al. (2007). An NB-LRR protein required for HR signalling mediated by both extra- and intracellular resistance proteins. Plant J. 50, 14–28. doi: 10.1111/j.1365-313x.2007.03027.x
Goulden, M. G., Kohm, B. A., Cruz, S. S., Kavanagh, T. A., and Baulcombe, D. C. (1993). A feature of the coat protein of Potato Virus-X affects both induced virus-resistance in potato and viral fitness. Virology 197, 293–302. doi: 10.1006/viro.1993.1590
Hua, J. (2013). Modulation of plant immunity by light, circadian rhythm, and temperature. Curr. Opin. Plant Biol. 16, 406–413. doi: 10.1016/j.pbi.2013.06.017
Jones, L., Hamilton, A. J., Voinnet, O., Thomas, C. L., Maule, A. J., and Baulcombe, D. C. (1999). RNA-DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 11, 2291–2301. doi: 10.1105/tpc.11.12.2291
Knip, M., Richard, M. M. S., Oskam, L., Van Engelen, H. T. D., Aalders, T., and Takken, F. L. W. (2019). Activation of immune receptor Rx1 triggers distinct immune responses culminating in cell death after 4 hours. Mol. Plant Pathol. 20, 575–588. doi: 10.1111/mpp.12776
Leibman-Markus, M., Pizarro, L., Bar, M., Coaker, G., and Avni, A. (2018). NRC proteins – A critical node for pattern and effector mediated signaling. Plant Signal. Behav. 13:e1507404.
Lu, R., Malcuit, I., Moffett, P., Ruiz, M. T., Peart, J., Wu, A. J., et al. (2003). High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 22, 5690–5699. doi: 10.1093/emboj/cdg546
Ma, L., Huang, X., Yu, R., Jing, X. L., Xu, J., Wu, C. A., et al. (2016). Elevated ambient temperature differentially affects virus resistance in two tobacco species. Phytopathology 106, 94–100. doi: 10.1094/phyto-11-14-0300-r
Ma, L., Lukasik, E., Gawehns, F., and Takken, F. L. (2012). The use of agroinfiltration for transient expression of plant resistance and fungal effector proteins in Nicotiana benthamiana leaves. Methods Mol. Biol. (Clifton, N.J.) 835, 61–74. doi: 10.1007/978-1-61779-501-5_4
Mang, H.-G., Qian, W., Zhu, Y., Qian, J., Kang, H.-G., Klessig, D. F., et al. (2012). Abscisic acid deficiency antagonizes high-temperature inhibition of disease resistance through enhancing nuclear accumulation of resistance proteins SNC1 and RPS4 in Arabidopsis. Plant Cell 24, 1271–1284. doi: 10.1105/tpc.112.096198
Menna, A., Nguyen, D., Guttman, D. S., and Desveaux, D. (2015). Elevated temperature differentially influences effector-triggered immunity outputs in Arabidopsis. Front. Plant Sci. 6:995. doi: 10.3389/fpls.2015.00995
Monteiro, F., and Nishimura, M. T. (2018). “Structural, functional, and genomic diversity of plant NLR proteins: an evolved resource for rational engineering of plant immunity,” in Annual Review of Phytopathology, Vol. 56, eds J. E. Leach and S. E. Lindow, (Palo Alto, CA: Annual Reviews), 243–267. doi: 10.1146/annurev-phyto-080417-045817
Moury, B., Selassie, K. G., Marchoux, G., Daubeze, A. M., and Palloix, A. (1998). High temperature effects on hypersensitive resistance to Tomato Spotted wilt Tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur. J. Plant Pathol. 104, 489–498.
Qi, D., and Innes, R. W. (2013). Recent advances in plant NLR structure, function, localization, and signaling. Front. Immunol. 4:348. doi: 10.3389/fimmu.2013.00348
Romero, A. M., Kousik, C. S., and Ritchie, D. F. (2002). Temperature sensitivity of the hypersensitive response of bell pepper to Xanthomonas axonopodis pv. vesicatoria. Phytopathology 92, 197–203.
Sparkes, I. A., Runions, J., Kearns, A., and Hawes, C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat. Protoc. 1, 2019–2025. doi: 10.1038/nprot.2006.286
Szittya, G., Silhavy, D., Molnar, A., Havelda, Z., Lovas, A., Lakatos, L., et al. (2003). Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 22, 633–640. doi: 10.1093/emboj/cdg74
Takken, F. L. W., Albrecht, M., and Tameling, W. I. L. (2006). Resistance proteins: molecular switches of plant defence. Curr. Opin. Plant Biol. 9, 383–390. doi: 10.1016/j.pbi.2006.05.009
Van der Voort, J. N., Van Zandvoort, P., Van Eck, H. J., Folkertsma, R. T., Hutten, R. C. B., and Draaistra, J. (1997). Use of allele specificity of comigrating AFLP markers to align genetic maps from different potato genotypes. Mol. Gen. Genet. MGG 255, 438–447. doi: 10.1007/s004380050516
Velasquez, A. C., Castroverde, C. D. M., and He, S. Y. (2018). Plant-pathogen warfare under changing climate conditions. Curr. Biol. 28, R619–R634.
Wang, Y., Bao, Z. L., Zhu, Y., and Hua, J. (2009). Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant Microbe Interact. 22, 498–506. doi: 10.1094/mpmi-22-5-0498
Whitham, S., Mccormick, S., and Baker, B. (1996). The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc. Natl. Acad. Sci. U.S.A. 93, 8776–8781. doi: 10.1073/pnas.93.16.8776
Wu, C. H., Abd-El-Haliem, A., Bozkurt, T. O., Belhaj, K., Terauchi, R., Vossen, J. H., et al. (2017). NLR network mediates immunity to diverse plant pathogens. Proc. Natl. Acad. Sci. U.S.A. 114, 8113–8118. doi: 10.1073/pnas.1702041114
Wu, C. H., Derevnina, L., and Kamoun, S. (2018). Receptor networks underpin plant immunity. Science 360, 1300–1301. doi: 10.1126/science.aat2623
Yang, S. J., Carter, S. A., Cole, A. B., Cheng, N. H., and Nelson, R. S. (2004). A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana. Proc. Natl. Acad. Sci. U.S.A. 101, 6297–6302. doi: 10.1073/pnas.0304346101
Keywords: sensor NLR, helper NLR, plant immunity, temperature, virus, thermotolerance, disease triangle
Citation: Richard MMS, Knip M, Aalders T, Beijaert MS and Takken FLW (2020) Unlike Many Disease Resistances, Rx1-Mediated Immunity to Potato Virus X Is Not Compromised at Elevated Temperatures. Front. Genet. 11:417. doi: 10.3389/fgene.2020.00417
Received: 11 October 2019; Accepted: 02 April 2020;
Published: 24 April 2020.
Edited by:
Horacio Naveira, University of A Coruña, SpainReviewed by:
Dalia Gamil Aseel, Arid Lands Cultivation Research Institute (ALCRI), EgyptJacek Hennig, Institute of Biochemistry and Biophysics (PAN), Poland
Martin Cann, Durham University, United Kingdom
Copyright © 2020 Richard, Knip, Aalders, Beijaert and Takken. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank L. W. Takken, Zi5sLncudGFra2VuQHV2YS5ubA==
 Manon M. S. Richard
Manon M. S. Richard Marijn Knip
Marijn Knip Thomas Aalders
Thomas Aalders Machiel S. Beijaert
Machiel S. Beijaert Frank L. W. Takken
Frank L. W. Takken