
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 27 March 2020
Sec. Livestock Genomics
Volume 11 - 2020 | https://doi.org/10.3389/fgene.2020.00139
This article is part of the Research Topic High-Throughput Phenotyping in the Genomic Improvement of Livestock View all 17 articles
 Qiuming Chen1†
Qiuming Chen1† Kaixing Qu2†
Kaixing Qu2† Zhijie Ma3
Zhijie Ma3 Jingxi Zhan2
Jingxi Zhan2 Fengwei Zhang1
Fengwei Zhang1 Jiafei Shen1
Jiafei Shen1 Qingqing Ning1
Qingqing Ning1 Peng Jia1
Peng Jia1 Jicai Zhang2
Jicai Zhang2 Ningbo Chen1
Ningbo Chen1 Hong Chen1
Hong Chen1 Bizhi Huang2*
Bizhi Huang2* Chuzhao Lei1*
Chuzhao Lei1*Abnormal neurotransmitter concentration is one of the factors that affect the health status, behavioral personality, and welfare level of animals, but the genetic basis of the abnormality is still largely unknown. The objective of this study is to identify putative genomic loci associated with neurotransmitter concentration in cattle. We measured serotonin (5HT), dopamine (DA), cortisol, glutamate (Glu), and ACTH concentrations in blood serum using double-antibody sandwich ELISA in 30 Brahman cattle and 127 Yunling cattle. Interestingly, we found that ACTH concentration was positively correlated with body weight, cannon circumference, and hip width (P < 0.05). Genome-wide association study (GWAS) was performed with mixed linear models using autosomal SNPs derived from the whole-genome sequence. We identified five, five, two, three, and five suggestive loci associated with 5HT, DA, cortisol, Glu, and ACTH concentration, respectively. These 20 associated loci implicated 18 candidate genes. For Glu concentration, the most significant association locus was assigned to MCHR1, a G-coupled receptor that could modulate glutamate release. For dopamine concentration, a very strong association locus was located in the intron of SLC18A2, which is a critical mediator of dopamine dynamics. However, for ACTH concentration, a very strong association locus was assigned to HTR1F, a G protein-coupled receptor that can influence the release of ACTH. Other candidate genes of interest identified for neurotransmitter concentration were PRMT6, GADD45A, PCCA, ANGPT1, ACCS, LOC100336971, TNR, GSDMA, CNTN3, CARMIL1, CDKAL1, RBFOX1, PCDH15, and LGALS12. Our findings will provide targets for the genetic improvement of neurotransmitter-related traits in domestic cattle and basic materials for studying the mechanism of neurotransmitter synthesis, release, and transport in human and animals.
Neurotransmitters are endogenous chemical substances that act as chemical messengers during synaptic transmission. Abnormal of neurotransmitter concentration predisposes to psychiatric and neurodegenerative disease in human. For example, after treatment with clomipramine, the whole-blood serotonin (5HT) content decreased in patients with obsessive-compulsive disorder (Hanna et al., 1993). In the early stages of Parkinson’s disease, the dopamine content was reduced in peripheral blood lymphocytes (Caronti et al., 1999). Therefore, investigation of neurotransmitter content in blood is helpful for understanding the mechanism of psychiatric and neurodegenerative disease in human.
In farm animals, neurotransmitter content in blood was often acted as an indicator correlating with physiological state, temperamental difference, and welfare level. In adult cow, the cortisol concentration in plasma increased after machine milking (Negrao et al., 2004). Previous behavioral experiments have demonstrated that excitable cattle exhibited higher cortisol concentration in plasma than moderate cattle (Curley et al., 2006; Cooke et al., 2019). In animal welfare, previous study has proved that minor corral changes and the adoption of good handling practices in Nellore cows can reduce the cortisol release of individuals (Lima et al., 2018). Experiments on slaughter and transportation have also demonstrated that the elevation of ACTH concentration in plasma is a response to physiological stress in cattle (Knights and Smith, 2007; Zulkifli et al., 2014). Although the revelation of genetic mechanisms underlying plasma neurotransmitter concentration will provide a genetic method to select individuals with a stable physiological state and moderate temperament to raise the welfare level and improve production efficiency in cattle, there are few studies that establish the links between neurotransmitter concentration and genetic variants.
Currently, with the decrease of sequencing cost, tens or hundreds of thousands of SNPs can be used to identify the relationship between important differential traits and genetic variants. In milk traits, a genome-wide association study (GWAS) proved that DGAT1 and SCD1 could affect fatty acid synthesis (Li et al., 2015). In temperament traits related to neurotransmitter concentration, previous studies have identified numerous QTLs explaining phenotypic difference (Valente et al., 2016; dos Santos et al., 2017). In fact, the number of SNPs for GWAS has risen to tens of millions. For example, based on 25.4 million imputed whole-genome sequence (WGS) variants, a meta-analysis of GWAS identified 163 genomic regions significantly associated with stature (Bouwman et al., 2018). However, no attempts have been made to identify the genomic loci associated with neurotransmitter concentrations.
The Yunling cattle breed considered in this study is a composite of 1/2 Brahman cattle (Bos indicus), 1/4 Murray Grey cattle (Bos taurus), and 1/4 Yunnan indigenous cattle (Bos taurus × Bos indicus), respectively. Therefore, it is an excellent model for the identification of genomic loci explaining important differential phenotypic traits in domestic cattle. Here, we detected five neurotransmitter concentrations in blood serum using double-antibody sandwich ELISA in 29 Brahman cattle and 128 Yunling cattle and evaluated the effect of neurotransmitter concentrations on body measurement traits. We uncovered the genetic architecture for neurotransmitter concentration by performing GWAS using the whole-genome sequence. The findings provide genomic material for genetic improvement of neurotransmitter-related traits in domestic cattle and basic materials for exploring the mechanism of neurotransmitter synthesis, release, and transport in mammals.
The dataset came from Brahman cattle and Yunling cattle. All test individuals are multiparous cows. To ensure the consistency of reproductive status, the subjects were not within 2 weeks pre-calving, calving, or within 2 weeks post-calving. In terms of feeding management, the experimental animals consist of 36 pen-feeding individuals (five Brahman cattle and 31 Yunling cattle) and 121 free-grazing individuals (25 Brahman cattle and 96 Yunling cattle). All pen-feeding individuals are fed a total mixed ratio (TMR) of 65% coarse and 35% concentrated fodder. From June to November every year, the free-grazing individuals eat grass in the meadow, and from December to May every year, this is supplemented with proper TMR.
We encouraged the test individuals into a squeeze crush to perform body measurement and to collect blood and tissue samples. First, we determined 15 body measurement traits using a measuring tape and a measuring stick. These 15 body measurement traits comprised the withers height, hip cross height, body length, chest circumference, abdominal circumference, cannon circumference, chest width, chest depth, hip circumference, hip width, ischium width, head length, forehead size, rump length, and body length. Next, the whole blood was collected from a jugular vein to detect neurotransmitter concentration. Finally, ear tissue was collected to extract genomic DNA using an ear punch.
Serum was harvested from centrifuged whole blood samples (2000 g centrifugation for 20 min) and then stored at −80°C prior to neurotransmitter concentration determination. Neurotransmitter concentrations were determined by the double-antibody sandwich ELISA method according to the manufacturer’s manuals (MMBio, Jiangsu, China) by comparison of samples with standard curves generated with known concentrations. The absorbance was originally obtained from a Multiskan MS Primary EIA V. 1.5-0 reader at a wavelength of 450 nm. In addition, we checked for the effect of breed and feeding regime on neurotransmitter concentration using the general linear model in R software (“lm” function).
DNA extracted from ear tissues taken from Brahman cattle and Yunling cattle was used for this analysis. The standard phenol-chloroform protocol was used to isolate DNA (Green and Sambrook, 2012). Whole-genome sequencing was performed using the Illumina NovaSeq platform. The size of the insert fragment was 500 bp. Finally, ∼16.51 billion reads were generated. Pair-end sequence reads were mapped to the reference Bos taurus genome (ARS-UCD1.2) using BWA-MEM (Li and Durbin, 2009) with default parameters. Picard was used to exclude potential duplicate reads (“REMOVE_DUPLICATES = true”). We used the Genome Analysis Toolkit 3.8 (GATK) (Nekrutenko and Taylor, 2012) (“HaplotypeCaller,” “GenotypeGVCFs,” and “SelectVariants” modules) to call candidate SNPs. To filter SNPs and avoid possible false positives, the “VariantFiltration” module of GATK was adopted with the following options: (1) SNPs with QD (variant confidence/quality by depth) < 2 were filtered; (2) SNPs with FS (Phred-scaled P-value using Fisher’s exact test) > 60 were filtered; (3) SNPs with Mapping Quality Rank Sum < −12.5 were filtered; (4) SNPs with Read Pos RankSum < −8.0 were filtered; (5) SNPs with sequence depth (for all individuals) < 1/3x or >3x were filtered. Inference of haplotype phase and imputing of missing alleles were performed using Beagle (Browning and Browning, 2007). In addition, we performed principal component analysis using smartPCA in the EIGENSOFT v5.0 package (Patterson et al., 2006) to adjust population stratification.
To investigate the relationship between neurotransmitter concentrations and body measurement traits, partial Pearson’s correlation adjusting for ancestry (principal component 1), feeding regime, and breed was computed using the ppcor package (Kim, 2015) in R.
After filtering the SNPs with MAF > 0.1 or missing rate > 0.1, a total of 12983056 SNPs remained and were used to carry out GWAS analysis for neurotransmitter concentrations. The association analysis was carried out using the Genome-Wide Efficient Mixed-Model Association (GEMMA) software package (Zhou and Stephens, 2012). The mixed linear model assumed the following model:
where y is a vector of phenotypes, α is a vector of fixed effects representing marker effects, β is a vector of fixed effects representing non-marker effects, and μ is a vector of unknown random effect. X, S, and K represent the incidence matrices related to α, β, and μ, respectively, and ε represents a vector of random residual effects. The principal component 1 was defined as the S matrix. The kinship matrix calculated from nucleotide polymorphism was defined as the K matrix.
To estimate the correction required for multiple testing, the SNP data was subsequently pruned for linkage disequilibrium in PLINK (Purcell et al., 2007), using a 50 SNP sliding window with a five SNP increment between windows, retaining only SNPs with a pairwise r2 < 0.2. The number of LD-pruned SNPs (747,835) was defined as the effective number of independent SNPs. Therefore, the P-value thresholds were set at 6.7 × 10–8 (significant, 0.05/747835) and 1.3 × 10–6 (suggestive, 1/747835).
After completing GWAS, we further narrowed down our findings to obtain corresponding candidate genes. Firstly, we calculated the pairwise linkage disequilibrium among SNPs associated with neurotransmitter concentration. The borders of associated loci were defined according to the LD value (r2 > 0.6). False-positive signals were filtered for by retaining the associated loci with ≥2 suggestive SNPs. The LD plots of suggestive SNPs for associated loci were visualized using the Haploview program (Barrett et al., 2005). Secondly, the SNP with the smallest Pwald value in an association locus with neurotransmitter concentrations was defined as the leading SNP. Finally, we performed functional annotation for suggestive SNPs associated with neurotransmitter concentrations using ANNOVAR (Wang et al., 2010) according to the Bos taurus reference genome (ARS-UCD1.2).
We measured five neurotransmitter concentrations, including serotonin (5HT), dopamine (DA), cortisol, glutamate (Glu), and ACTH in blood serum of Brahman cattle and Yunling cattle using the double-antibody sandwich ELISA method. The descriptive statistics of the five neurotransmitter concentrations are summarized in Table 1. The coefficients of variation of 5HT, DA, cortisol, Glu, and ACTH concentrations were 19.81, 18.42, 17.45, 17.23, and 18.87%, respectively.
We used a general linear model to test the influence of breed and feeding regime on the five neurotransmitter concentrations. The result showed that there was no significant difference between Brahman cattle and Yunling cattle or between free grazing and pen feeding. We also calculated the pairwise partial correlations among neurotransmitter concentrations (Figure 1). The result showed that there was no significant correlation among neurotransmitter concentrations. Meanwhile, the association between neurotransmitter concentrations and the 15 body measurement traits was also assessed using the above approach (Figure 1). It is remarkable that only ACTH concentration was positively correlated with body weight (rs = 0.251, P = 3.31 × 10–3), cannon circumference (rs = 0.163, P = 0.043), and hip width (rs = 0.168, P = 0.038).
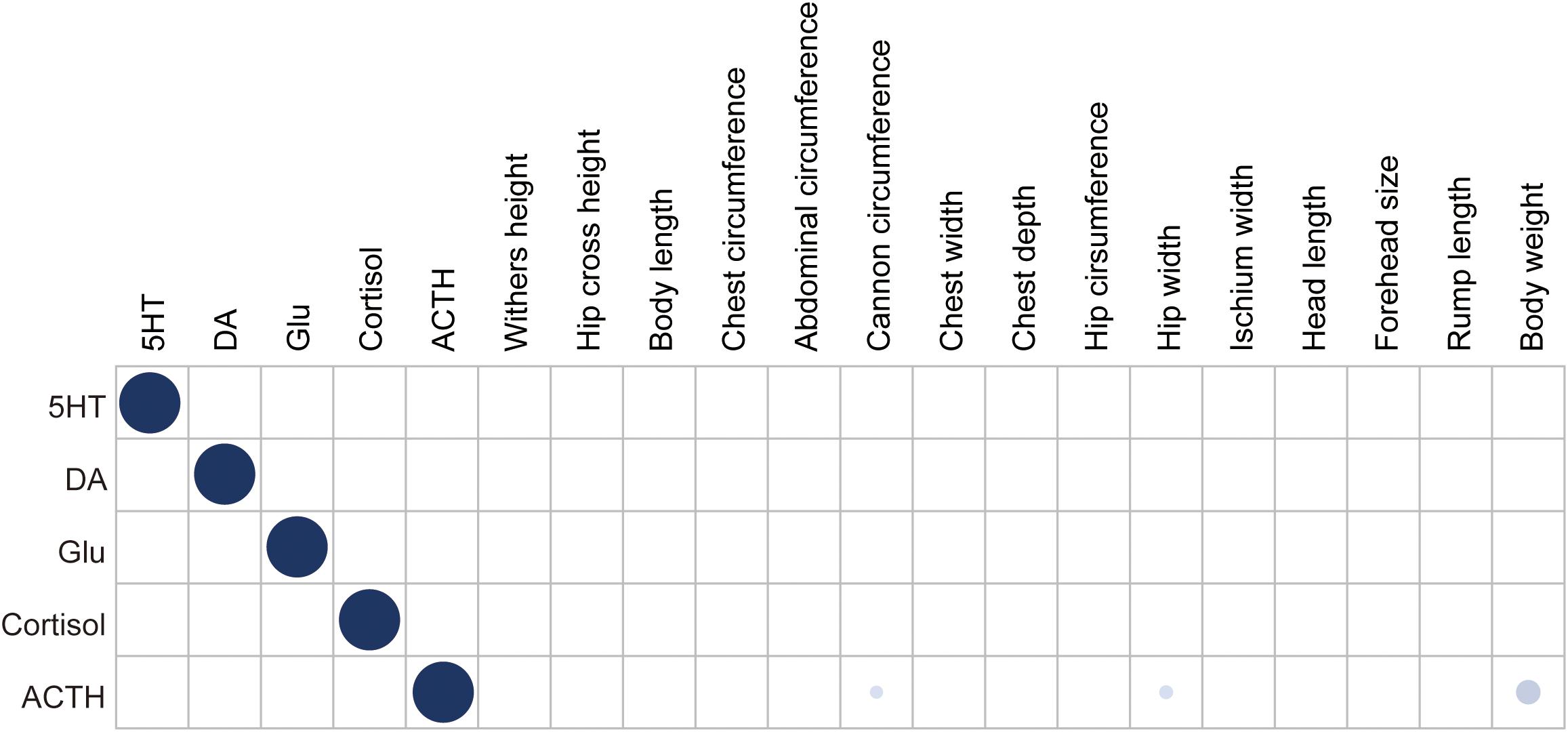
Figure 1. Heatmap depicting Pearson’s correlation coefficients between neurotransmitter concentration and body measurement traits. Dot size and color saturation represent the strength of the correlation. Significant correlations (P ≤ 0.05) are marked in blue.
Genomic DNA samples from 157 individuals were sequenced to ∼5.60 × genome coverage each. About 16.51 billion reads were aligned to the Bos taurus reference genome sequence ARS-UCD1.2, and the average alignment rate was 99.55% (Supplementary Table S1). After filtering raw SNPs, a total of ∼40.98 million SNPs were retained. In principal component analysis, principal component 1 explained 3.5% of the total variation and separated Brahman cattle from Yunling cattle (Figure 2).
The GWAS Manhattan plot for 5HT concentration in blood serum is shown in Figure 3A. In total, 36 SNPs were found to be suggestively associated with variation in 5HT concentration (Supplementary Table S2). After detecting the linkage disequilibrium of these SNPs, we identified five suggestive association loci for 5HT concentration in blood serum (Table 2). According to the annotation of ANNOVAR software, the most significant locus was observed on BTA15 and was located in gene desert (regions over 500 kb that are devoid of protein-coding genes). The second-ranked locus was observed on BTA4 and was also located in gene desert. The third-ranked locus was observed on BTA15, and its leading SNP was located in the intron of ACCS. The remaining association loci were observed on BTA19 and BTA22, and their candidate genes embodied SLC39A11 and CNTN3, respectively.
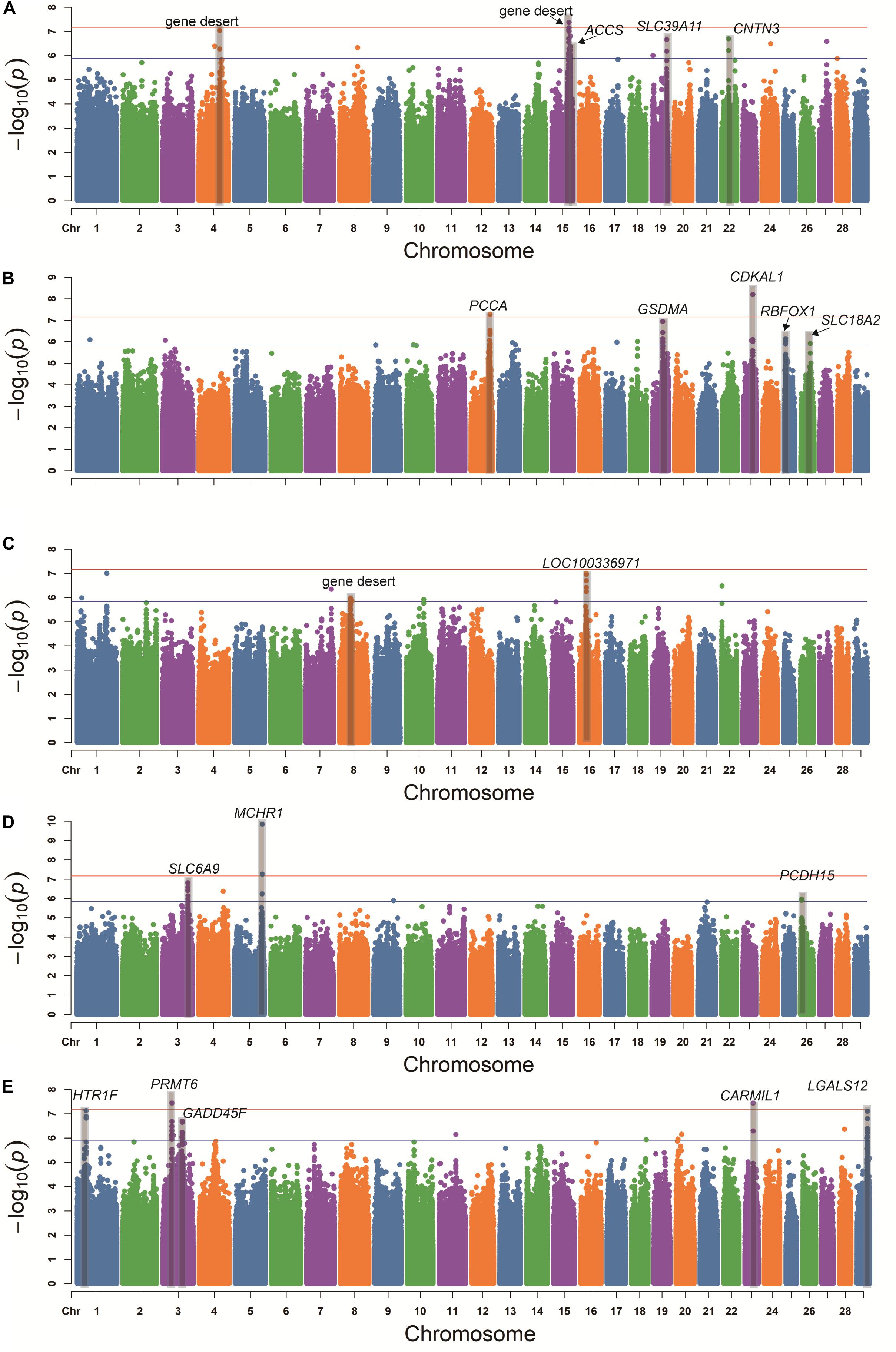
Figure 3. Genome-wide association study of the concentrations of 5HT (A), DA (B), cortisol (C), Glu (D), and ACTH (E) in blood serum using the mixed linear model. Red line and blue line indicate the significant threshold and suggestive threshold, respectively.
In Figure 3B, the GWAS for DA concentration shows that 25 SNPs were found to be suggestively associated with the variation of DA concentration (Supplementary Table S3). After detecting the linkage disequilibrium of these SNPs, we identified five suggestive association loci for DA concentration (Table 2). Based on the annotation of ANNOVAR software, the most significant locus was observed on BTA23 and was located in the intron of CDKAL1. The second-ranked locus, which was located in the intron of PCCA, was observed on BTA12. The third-ranked locus was observed on BTA19, and its leading SNP was located in the intron of GSDMA. The remaining association loci were observed on BTA25 and BTA26, and their candidate genes included RBFOX1 and SLC18A2, respectively.
Figure 3C shows the GWAS Manhattan plot for cortisol concentration in blood serum. In total, 12 SNPs were found to be suggestively associated with variation in cortisol concentration (Supplementary Table S4). After detecting the linkage disequilibrium of these SNPs, we identified two suggestive association loci for cortisol concentration (Table 2). After annotation using ANNOVAR, the most significant locus was observed on BTA16, and its nearest gene was LOC100336971. Another associated locus was observed on BTA8 and was located in gene desert.
The GWAS Manhattan plot for Glu concentration in blood serum is shown in Figure 3D. In total, 11 SNPs were found to be suggestively associated with variation of Glu concentration (Supplementary Table S5). After detecting the linkage disequilibrium of these SNPs, we identified three suggestive association loci with Glu concentration (Table 2). By means of ANNOVAR annotation, we found that the most significant locus was observed on BTA5 and was located ∼8 Kb upstream of MCHR1. The second-ranked locus was observed on BTA3, and its leading SNP was located upstream of KLF17. The third-ranked locus was observed on BTA26 and located in the intron of PCDH15.
In Figure 3E, our result shows that 34 SNPs were found to be suggestively associated with the variation in ACTH concentration in blood serum (Supplementary Table S6). After detecting the linkage disequilibrium of these SNPs, we identified five suggestive association loci for ACTH concentration (Table 2). The most significant locus was observed on BTA3 and was located downstream of PRMT6. The second-ranked locus was observed on BTA23 and was located in the intron of CARMIL1. The third-ranked locus was observed on BTA1, and its leading SNP was located upstream of HTR1F. The remaining associated loci were observed on BTA3 and BTA29, and their candidate gene contained GADD45A and LGALS12, respectively.
The LD plots of 12 loci at which the number of suggestive SNPs associated with neurotransmitter concentration in blood serum was above 3 are presented in Figure 4. The results show that four loci had LD blocks (the 1:35945547-35945584 locus, 3:36525172-36526078 locus, 15:68085551-69989675 locus, and 16:28978311-28979773 locus) with a length of less than 1 kb. There were no LD blocks at the remaining eight loci.
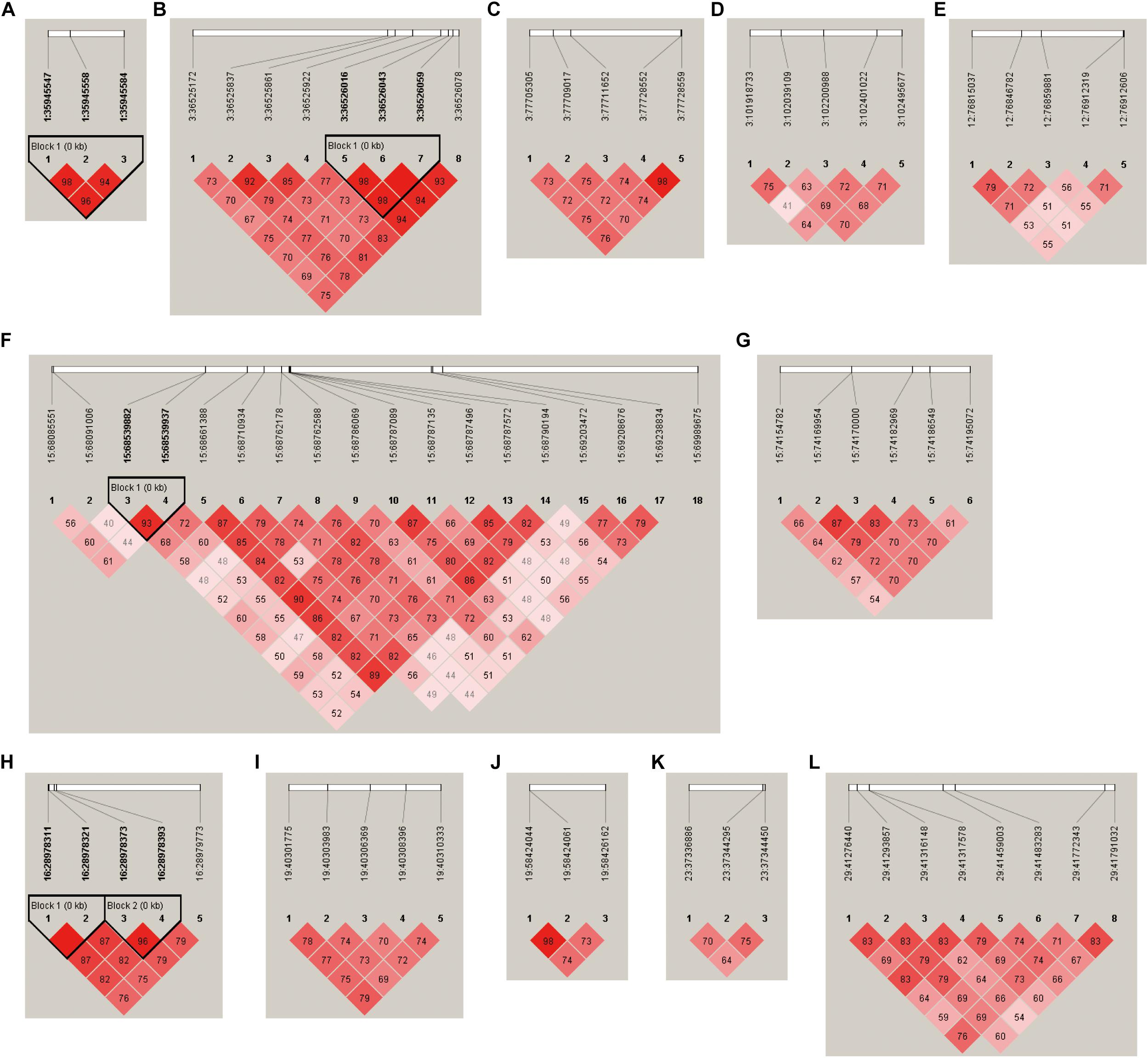
Figure 4. LD plots of suggestive SNPs on neurotransmitter concentration at the (A) 1:35945547–35945584 locus, (B) 3:36525172–36526078 locus, (C) 3:77705305–77728559 locus, (D) 3:101918733–102495677 locus, (E) 12:76815037–76912606 locus, (F) 15:68085551–69989675 locus, (G) 15:74154782–74195072 locus, (H) 16:28978311–28979773 locus, (I) 19:40301775–40310333 locus, (J) 19:58424044–58426162 locus, (K) 23:37336886–37344450 locus, and (L) 29:41276440–41791032 locus.
Previous studies on neurotransmitter concentration in blood have primarily focused on physiological difference under different conditions (Knights and Smith, 2007; Zulkifli et al., 2014; Lima et al., 2018) or analysis of its correlation with temperament in cattle (Curley et al., 2006; Cooke et al., 2019). In our present study, cattle with a higher ACTH concentration in blood serum tended to have better stature for production (i.e., higher body weight, higher cannon circumference, higher hip width). To our knowledge, this is the first correlative identification between ACTH concentration and body measurement traits. Although the correlation is only phenotypic, the positive correlation between ACTH concentration and body measurement traits suggests that improvement of ACTH concentration will have a positive impact on cattle production.
Subsequently, we implemented GWAS to identify the genomic loci explaining the phenotypic variance in neurotransmitter concentrations in blood serum using the whole-genome sequence. We identified five, five, two, three, and five suggestive loci associated with 5HT, DA, cortisol, Glu, and ACTH, respectively, suggesting that neurotransmitter concentration in blood serum is polygenetically controlled. To our knowledge, this is the first identification of genomic loci associated with neurotransmitter concentration in blood serum in cattle using GWAS. Moreover, we found that the LD level for the suggestive loci was very low, suggesting that the loci associated with neurotransmitter concentration were not the target of phenotypic selection or did not experience bottlenecks or gene drift. Although our sample size was smaller for GWAS, the variants with high frequency and large effect have been identified, and further GWAS with a larger sample size will result in the identification of additional variants with low frequency and small effect in future. In addition, our genome coverage was very low (∼5.60×), but a previous study has shown that very low-depth whole-genome sequencing is an efficient alternative to complex trait association studies (Schwartzentruber et al., 2018).
Among 20 suggestive loci, the most significant locus was associated with glutamate concentration in blood serum and was located 8 kb upstream of MCHR1, a G-coupled receptor for the neuropeptide melanin-concentrating hormone, which modulates glutamate release from presynaptic terminal (Gao and van den Pol, 2001). Knockout MCHR1 mice exhibited reduced anxiety-like behavior (Roy et al., 2006). Another strong locus associated with glutamate concentration was assigned to SLC6A9, encoding a glycine transporter. In previous studies, glycine has been found to act as an inhibitory neurotransmitter in the central nervous system (Alfadhel et al., 2016) and an obligatory co-agonist of glutamate involved in the regulation of glutamatergic neurotransmission (Johnson and Ascher, 1987), which suggested that SLC6A9 may participate in the glutamate transporter. For dopamine concentration in blood serum, a strong locus was located in the intron of SLC18A2, which is a critical mediator of dopamine dynamics in neuronal terminal (Lohr et al., 2015). Another strong locus associated with dopamine concentration was located in the intron of PCCA, which is the alpha subunit of the heterodimeric mitochondrial enzyme Propionyl-CoA carboxylase (Stankovics and Ledley, 1993). It has been demonstrated that dopa decarboxylase is involved in dopamine synthesis (Seifert et al., 1980), suggesting that PCCA may participate in the dopamine synthesis. In terms of ACTH concentration, the nearest gene of a leading SNP was HTR1F, a G protein-coupled receptor for 5HT, which can regulate the HPA axis by moderating the output of corticotropin-releasing hormone in the hypothalamus and further influence release of ACTH (Falkenberg and Rajeevan, 2010). The identification of these candidate genes may provide better opportunities for investigating the molecular mechanism of neurotransmitter synthesis, release, and transport in mammals.
In conclusion, our study revealed that ACTH concentration in blood serum was significantly related to body measurement traits (body weight, cannon circumference, hip cross height, and hip width). We performed GWAS for neurotransmitter concentration in blood serum using autosomal SNPs derived from WGS and then identified five, five, two, three, and five suggestive loci associated with 5HT, DA, cortisol, Glu, and ACTH, respectively. These 20 loci implicated 17 candidate genes, including MCHR1 (a G-coupled receptor involved in glutamate release), SLC18A2 (a critical mediator of dopamine dynamics), and HTR1F (a G protein-coupled receptor involved in release of ACTH). The revelation of the genetic underpinnings of neurotransmitter concentration will provide theoretical guidance for the improvement of neurotransmitter concentration by genetic manipulation to reduce stress, elevate welfare level, and boost productivity in cattle. In addition, our findings are helpful for follow-up studies to identify causal variants of difference in neurotransmitter concentration in blood serum and investigate the molecular mechanism of neurotransmitter synthesis, release, and transport in mammals.
Sequences are available from GenBank with the BioProject accession number PRJNA555741.
This study was approved by the Institutional Animal Care and Use Committee of Northwest A&F University (Permit Number: NWAFAC1019).
CL and BH conceived and designed the project. QC, KQ, JZ, QN, PJ, JZ, NC, and HC carried out sampling. QC, FZ, and JS performed the analyses. QC wrote the manuscript. ZM revised the manuscript. All authors reviewed and approved the final manuscript.
This work was supported by the Program of National Beef Cattle and Yak Industrial Technology system (Grant No. CARS-37), the Program of Yunling Scholar, the Young and Middle-aged Academic Technology Leader Backup Talent Cultivation Program in Yunnan Province, China (No. 2018HB045), and Yunnan Provincial Major S&T Project (2019ZG007 and 2019ZG011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2020.00139/full#supplementary-material
TABLE S1 | Summary of sequencing data.
TABLE S2 | The suggestive SNPs associated with 5HT concentration in blood serum.
TABLE S3 | The suggestive SNPs associated with DA concentration in blood serum.
TABLE S4 | The suggestive SNPs associated with cortisol concentration in blood serum.
TABLE S5 | The suggestive SNPs associated with Glu concentration in blood serum.
TABLE S6 | The suggestive SNPs associated with ACTH concentration in blood serum.
Alfadhel, M., Nashabat, M., Al Qahtani, H., Alfares, A., Al Mutairi, F., Al Shaalan, H., et al. (2016). Mutation in SLC6A9 encoding a glycine transporter causes a novel form of non-ketotic hyperglycinemia in humans. Hum. Genet. 135, 1263–1268. doi: 10.1007/s00439-016-1719-x
Barrett, J. C., Fry, B., Maller, J., and Daly, M. J. (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265. doi: 10.1093/bioinformatics/bth457
Bouwman, A. C., Daetwyler, H. D., Chamberlain, A. J., Ponce, C. H., Sargolzaei, M., Schenkel, F. S., et al. (2018). Meta-analysis of genome-wide association studies for cattle stature identifies common genes that regulate body size in mammals. Nat. Genet. 50:362. doi: 10.1038/s41588-018-0056-5
Browning, S. R., and Browning, B. L. (2007). Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81, 1084–1097. doi: 10.1086/521987
Caronti, B., Tanda, G., Colosimo, C., Ruggieri, S., Calderaro, C., Palladini, G., et al. (1999). Reduced dopamine in peripheral blood lymphocytes in Parkinson’s disease. Neuroreport 10, 2907–2910. doi: 10.1097/00001756-199909290-00006
Cooke, R. F., Moriel, P., Cappellozza, B. I., Miranda, V. F. B., Batista, L. F. D., Colombo, E. A., et al. (2019). Effects of temperament on growth, plasma cortisol concentrations and puberty attainment in Nelore beef heifers. Animal 13, 1208–1213. doi: 10.1017/s1751731118002628
Curley, K. Jr., Paschal, J., Welsh, T. Jr., and Randel, R. (2006). Exit velocity as a measure of cattle temperament is repeatable and associated with serum concentration of cortisol in Brahman bulls. J. Anim. Sci. 84, 3100–3103. doi: 10.2527/jas.2006-055
dos Santos, F. C, Peixoto, M. G. C. D., de Souza Fonseca, P. A., Pires Md, F. Á, Ventura, R. V., Rosse , C., et al. (2017). Identification of candidate genes for reactivity in Guzerat (Bos indicus) cattle: a genome-wide association Study. PloS One 12:e0169163. doi: 10.1371/journal.pone.0169163
Falkenberg, V. R., and Rajeevan, M. S. (2010). Identification of a potential molecular link between the glucocorticoid and serotonergic signaling systems. J., Mol. Neurosci. 41, 322–327. doi: 10.1007/s12031-009-9320-6
Gao, X. B., and van den Pol, A. N. (2001). Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J. Physiol. 533, 237–252. doi: 10.1111/j.1469-7793.2001.0237b.x
Green, M. R., and Sambrook, J. (2012). Molecular Cloning. A Laboratory Manual 4th. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press.
Hanna, G. L., Yuwiler, A., and Cantwell, D. P. (1993). Whole-blood serotonin during clomipramine treatment of juvenile obsessive—compulsive disorder. J. Child Adoles. Psychopharmacol. 3, 223–229. doi: 10.1089/cap.1993.3.223
Johnson, J., and Ascher, P. (1987). Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325, 529. doi: 10.1038/325529a0
Kim, S. (2015). ppcor: an R package for a fast calculation to semi-partial correlation coefficients. Commun. Statist. Appl. Methods 22:665. doi: 10.5351/CSAM.2015.22.6.665
Knights, M., and Smith, G. W. (2007). Decreased ACTH secretion during prolonged transportation stress is associated with reduced pituitary responsiveness to tropic hormone stimulation in cattle. Domest. Anim. Endocrinol. 33, 442–450. doi: 10.1016/j.domaniend.2006.09.001
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, X., Buitenhuis, A. J., Lund, M. S., Li, C., Sun, D., Zhang, Q., et al. (2015). Joint genome-wide association study for milk fatty acid traits in Chinese and Danish Holstein populations. J. Dairy Sci. 98, 8152–8163. doi: 10.3168/jds.2015-9383
Lima, M. L. P., Negrão, J. A., de Paz, C. C. P., and Grandin, T. (2018). Minor corral changes and adoption of good handling practices can improve the behavior and reduce cortisol release in Nellore cows. Trop. Health Prod. 50, 525–530. doi: 10.1007/s11250-017-1463-9
Lohr, K. M., Stout, K. A., Dunn, A. R., Wang, M., Salahpour, A., Guillot, T. S., et al. (2015). Increased vesicular monoamine transporter 2 (VMAT2; Slc18a2) protects against methamphetamine toxicity. ACS Chem. Neurosci. 6, 790–799. doi: 10.1021/acschemneuro.5b00010
Negrao, J., Porcionato, M., De Passille, A., and Rushen, J. (2004). Cortisol in saliva and plasma of cattle after ACTH administration and milking. J. Dairy Sci. 87, 1713–1718. doi: 10.3168/jds.S0022-0302(04)73324-X
Nekrutenko, A., and Taylor, J. (2012). Next-generation sequencing data interpretation: enhancing reproducibility and accessibility. Nat. Rev. Genet. 13:667. doi: 10.1038/nrg3305
Patterson, N., Price, A. L., and Reich, D. (2006). Population structure and eigenanalysis. PLoS Genet. 2:e190. doi: 10.1371/journal.pgen.0020190
Purcell, S., Neale, B., Todd-Brown, K., Thomas, L., Ferreira, M. A., Bender, D., et al. (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Huma. Genet. 81, 559–575. doi: 10.1086/519795
Roy, M., David, N. K., Danao, J. V., Baribault, H., Tian, H., and Giorgetti, M. (2006). Genetic inactivation of melanin-concentrating hormone receptor subtype 1 (MCHR1) in mice exerts anxiolytic-like behavioral effects. Neuropsychopharmacology 31:112. doi: 10.1038/sj.npp.1300805
Schwartzentruber, P. D., Kilian, B., Pollard, M. O., Ge, X., Tsafantakis, E., Dedoussis, G., et al. (2018). Very low depth whole genome sequencing in complex trait association studies. Bioinformatics 35, 2555–2561. doi: 10.1093/bioinformatics/bty1032
Seifert, W. E. Jr., Foxx, J. L., and Butler, J. I (1980). Age effect on dopamine and serotonin metabolite levels in cerebrospinal fluid. Ann. Neurol. 8, 38–42. doi: 10.1002/ana.410080106
Stankovics, J., and Ledley, F. D. (1993). Cloning of functional alpha propionyl CoA carboxylase and correction of enzyme deficiency in pccA fibroblasts. Am. J. Hum. Genet. 52, 144.
Valente, T. S., Baldi, F., Sant’Anna, A. C., Albuquerque, L. G., and da Costa, M. J. R. P. (2016). Genome-Wide Association Study between single nucleotide polymorphisms and flight speed in Nellore cattle. PloS One 11:e0156956. doi: 10.1371/journal.pone.0156956
Wang, K., Li, M., and Hakonarson, H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164–e164. doi: 10.1093/nar/gkq603
Zhou, X., and Stephens, M. (2012). Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821. doi: 10.1038/ng.2310
Keywords: neurotransmitter concentration, genome-wide association study, candidate genes, Bos taurus, Bos indicus
Citation: Chen Q, Qu K, Ma Z, Zhan J, Zhang F, Shen J, Ning Q, Jia P, Zhang J, Chen N, Chen H, Huang B and Lei C (2020) Genome-Wide Association Study Identifies Genomic Loci Associated With Neurotransmitter Concentration in Cattle. Front. Genet. 11:139. doi: 10.3389/fgene.2020.00139
Received: 13 October 2019; Accepted: 06 February 2020;
Published: 27 March 2020.
Edited by:
Guilherme J. M. Rosa, University of Wisconsin–Madison, United StatesReviewed by:
Lingyang Xu, Chinese Academy of Agricultural Sciences, ChinaCopyright © 2020 Chen, Qu, Ma, Zhan, Zhang, Shen, Ning, Jia, Zhang, Chen, Chen, Huang and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bizhi Huang, aGJ6QHluYnAuY24=; Chuzhao Lei, bGVpY2h1emhhbzExMThAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.