- 1Consortium for Translational Research on Aggression and Drug Abuse (ConTRADA), University of Kansas, Lawrence, KS, United States
- 2Clinical Child Psychology Program, University of Kansas, Lawrence, KS, United States
- 3Departments of Psychiatry and Behavioral Sciences and Pediatrics, University of Kansas Medical Center, Kansas City, KS, United States
- 4Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, UT, United States
Polysubstance use (PSU) is highly prevalent among college students. Recent evidence indicates that PSU is based on gene x environment (G×E) interactions, yet the specific biosocial factors underlying this problem remain elusive. We recently reported that lifetime use of tobacco and cannabis in college students is influenced by the interaction of the X-linked MAOA (monoamine oxidase A) gene and child maltreatment. Building on these premises, here we evaluated whether the same G×E interaction may also predict PSU in this population. Students of a large Midwestern university (n = 470; 50.9% females) took part in a computer survey for substance use, as well as childhood trauma exposure, using the Child Trauma Questionnaire (CTQ). DNA was extracted from their saliva samples and genotyped for MAOA variable-number of tandem repeat (VNTR) variants. Findings indicated that the highest number of substances were used by male students harboring low-activity MAOA alleles with a history of childhood emotional abuse. In contrast, female homozygous high-activity MAOA carriers with a history of emotional and physical abuse reported consumption of the greatest number of substances. Our results indicate that PSU among college students is influenced by the interaction of MAOA and child maltreatment in a sex-specific fashion. Further studies are warranted to understand the mechanisms of sex differences in the biosocial interplays underlying PSU in this at-risk group.
Introduction
Polysubstance use (PSU) is a major health concern that has garnered much attention from clinicians and researchers, due to its robust association with substance use disorders and other negative outcomes throughout the lifespan (McCabe et al., 2006; Trenz et al., 2012; Moss et al., 2014). Recent surveys have ascertained that PSU risk is particularly high among college students (Gledhill-Hoyt et al., 2000; Johnston et al., 2004; Mohler-Kuo et al., 2003; Barrett et al., 2006; National Center on Addiction and Substance Abuse at Columbia University, 2007; O'Grady et al., 2008) with alcohol, tobacco, and cannabis being the three most widely used substances in this population (Lipari and Jean-Francois, 2016). Indeed, these drugs share similar trajectories of use among emerging adults, with high rates of comorbidity (Jackson et al., 2008) and simultaneous consumption (Martin et al., 1992; Baggio et al., 2014).
Vulnerability to PSU, and more generally to substance use disorders and related behavioral phenotypes (including externalizing psychopathology), is strongly influenced by both genetic (Uhl et al., 2001; Dick et al., 2009) and environmental factors. Several genes implicated in the predisposition to substance use disorders have been shown to be related to monoamine neurotransmitters, such as serotonin, dopamine, and norepinephrine (Guo et al., 2007; Ducci and Goldman, 2012); these molecules are known to serve a pivotal role in the pathophysiology of drug abuse (Volkow et al., 2007; Fitzgerald, 2013; Müller and Homberg, 2015). Early-life adversity, and particularly child maltreatment is another well-known variable associated with high risk of PSU (Galaif et al., 2001; Leeb et al., 2008; Goldstein et al., 2013; Cohen et al., 2017). It has been estimated that ~70% of adolescents receiving substance abuse treatment have a history of trauma (Funk et al., 2003), and that maltreated children are 300% more likely to develop substance abuse (Kilpatrick et al., 2003). According to recent conceptual frameworks, the pathogenic influence of child maltreatment and other forms of early stress on PSU is moderated by genetic factors (Vink, 2016). However, only limited data are available on the specific interactions of heritable factors and child maltreatment with respect to PSU predisposition.
We recently showed that, among college students, tobacco and cannabis consumption is influenced by the interaction of child maltreatment and the gene MAOA, the X-linked gene encoding for monoamine oxidase A (Fite et al., 2018). In line with our report, Stogner and Gibson (2013) also documented that the interplay of this gene with lifetime stress increases the risk for initiation to alcohol and cannabis use in male adolescents. Monoamine oxidase A catalyzes the degradation of serotonin, norepinephrine and dopamine (Bortolato et al., 2008). The best-characterized MAOA functional polymorphism is a 30-bp variable number tandem repeat located in its promoter region (uVNTR) (Sabol et al., 1998). The six alleles of this genotype feature different numbers of repeats (2, 3, 3.5, 4, 5, and 6) (Huang et al., 2004), in association with different transcriptional efficiency and enzyme activity. The two- and three-repeat variants, which are associated with low activity (Sabol et al., 1998; Deckert et al., 1998; Denney et al., 1999), confer a greater risk for externalizing psychopathology in male carriers with a history of maltreatment (Caspi et al., 2002; Kim-Cohen et al., 2006; Williams et al., 2009; Fergusson et al., 2011).
A large body of evidence has documented that MAOA uVNTR variants exert a sex-dimorphic influence on the overall risk and specific clinical manifestations of alcohol use disorders, both per se and in interaction with early-life adversity (Samochowiec et al., 1999; Schmidt et al., 2000; Vanyukov et al., 2004; Guindalini et al., 2005; Herman et al., 2005; Ducci et al., 2008; Nilsson et al., 2011). Low-activity uVNTR alleles (hereafter designated as MAOA-L), for example, are associated with a younger age of onset of alcohol dependence (Vanyukov et al., 1995; Vanyukov et al., 2004) and antisocial alcoholism (Samochowiec et al., 1999) in males. A history of maltreatment predisposes female carriers of high-activity alleles (MAOA-H) or male MAOA-L carriers to a greater risk of alcohol use (Nilsson et al., 2011). In alignment with these findings, we found that greater lifetime tobacco use was predicted by the interaction of childhood maltreatment and MAOA-L variants in males and MAOA-H alleles in females (Fite et al., 2018).
Given these premises, the present study tested the hypothesis that the same gene x environment (G×E) interactions may predispose to PSU in college students and analyzed whether the influence of these biosocial interplays may follow a sex-dimorphic pattern.
Methods
Participants
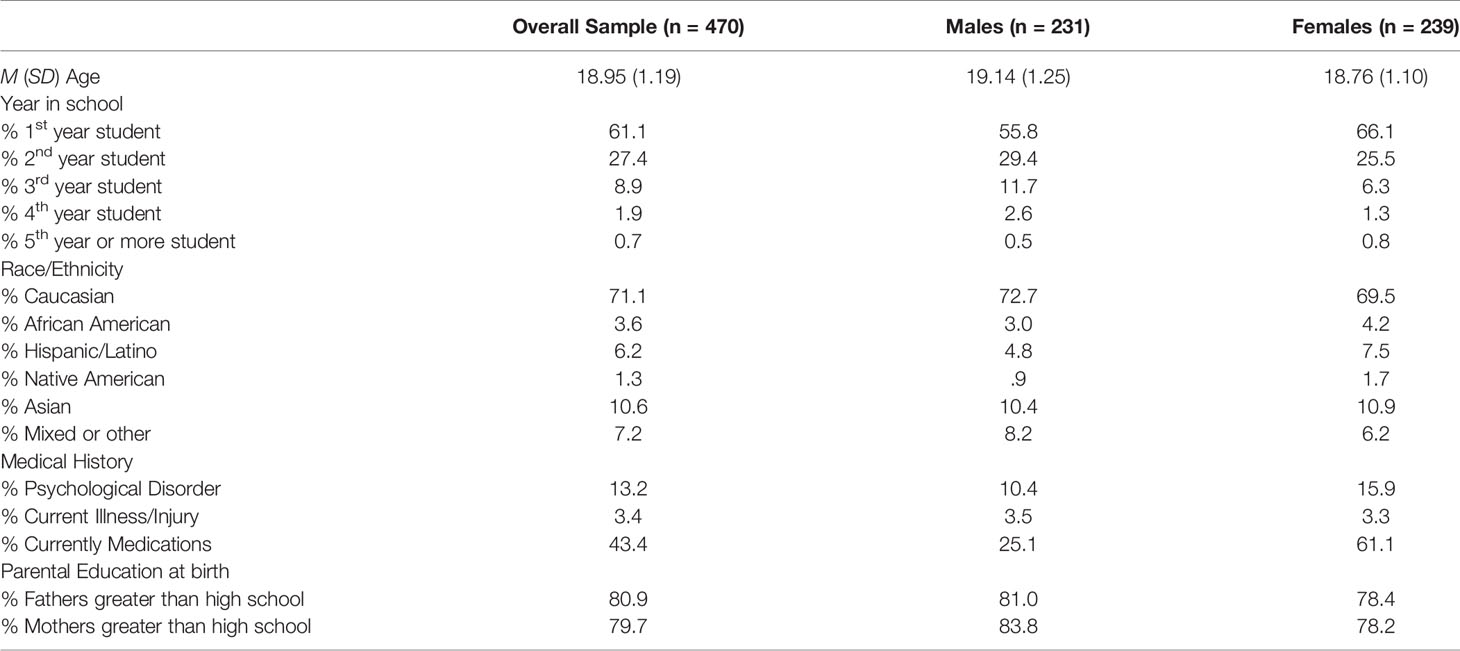
Participants were 470 students (239 females and 231 males; see Table 1) enrolled in undergraduate psychology courses at a large Midwestern university. Recruitment was based on SONA, an online system that allows students to electronically sign up to participate in active studies at the university. Most students (71.1%) identified as Caucasians, attended the first year of college (61.1%) and reported that their parents had a higher educational level than high school (80.9% of fathers and 79.7% of mothers).
Procedures
All study procedures were approved by the researchers' Institutional Review Board. All participants were instructed to abstain from eating for 1 h before the study, and refrain from the use of any drug (including prescription medicines and caffeinated beverages) for at least 3 h before the study. Upon arrival, they were given a complete summary of the study and provided informed consent. Subsequently, participants rinsed their mouth with water and, ten minutes later, were instructed to give 2 ml of saliva in a tube for genetic analyses. Then, they provided demographic information, including their age and race/ethnicity, and completed a Qualtrics online survey in about 1 h. At the end of the study, participants were compensated with a $5 debit card for the saliva sample and 3 SONA credits for the survey. To keep the identity of participants anonymous, survey responses and saliva samples were assigned a unique ID without any identifying information.
Questionnaires
The survey included the following questionnaires:
1. The Child Trauma Questionnaire (CTQ), a standardized self-report instrument for the retrospective assessment of trauma exposure during childhood (Bernstein and Fink, 1998). The CTQ consists of 5 subscales of trauma (physical abuse, emotional abuse, sexual abuse, physical neglect, and emotional neglect) with multiple items based on a 5-point Likert scale format. Mean scores for each subscale, as well as an overall child maltreatment score, were calculated. The physical neglect subscale yielded the lowest reliability coefficient (α = 0.56) in the current sample; internal consistencies for the remaining four subscales were good (with all α's > 0.81);
2. A substance use questionnaire. based on three items from the Center for Substance Abuse Prevention (CSAP) Student Survey (Pentz et al., 1989), a self-report instrument assessing lifetime tobacco (i.e., “Have you ever smoked a cigarette, even just a few puffs, or used chewing tobacco, snuff, or dip), alcohol (i.e., “Have you ever had a drink of alcohol?”), and cannabis use (i.e., “Have you ever tried marijuana?”). The number of substances used by each participant was calculated (ranging from 0 to 3).
MAOA uVNTR Variants Genotyping
DNA extracted and MAOA-uVNTR genotyping were performed as previously described (Fite et al., 2018). All laboratory procedures were carried out by personnel blind to the demographic and psychological characteristics of the subject (other than gender). All genotype data of participants are shown in Table 2. Given that the MAOA gene is located on the X chromosome, males were designated as either low-activity (MAOA-L) or high-activity (MAOA-H) hemizygous, depending on the number of repeats of their allelic variant (2 and 3 vs 3.5 and 4, respectively). Conversely, females were either homozygous for either allele (MAOA-LL or MAOA-HH) or heterozygous carriers (MAOA-LH). In line with previous studies on MAOA (Byrd and Manuck, 2014), carriers of 5-repeat uVNTR alleles were excluded from the analyses, as the actual functional significance of this variant remains controversial (Sabol et al., 1998; Deckert et al., 1998). To allow for comparability between males and females, MAOA-LL and MAOA-LH female participants were combined (n = 165), in agreement with previous functional studies on sex-dimorphic effects of MAOA uVNTR variants (Fan et al., 2003; Meyer-Lindenberg et al., 2006; Frazzetto et al., 2007; Buckholtz et al., 2008; Dannlowski et al., 2009) The validity of this approach was confirmed by analyzing the interactions of MAOA genotype variants (MAOA-LL, MAOA-HH, and MAOA-LH) and maltreatment types in female participants. The results of these analyses indicated that MAOA-LH genotype operated consistent with the MAOA-LL genotype in its interaction with maltreatment types to predict PSU. All genotypic and phenotypic data are presented as Supplementary Materials.
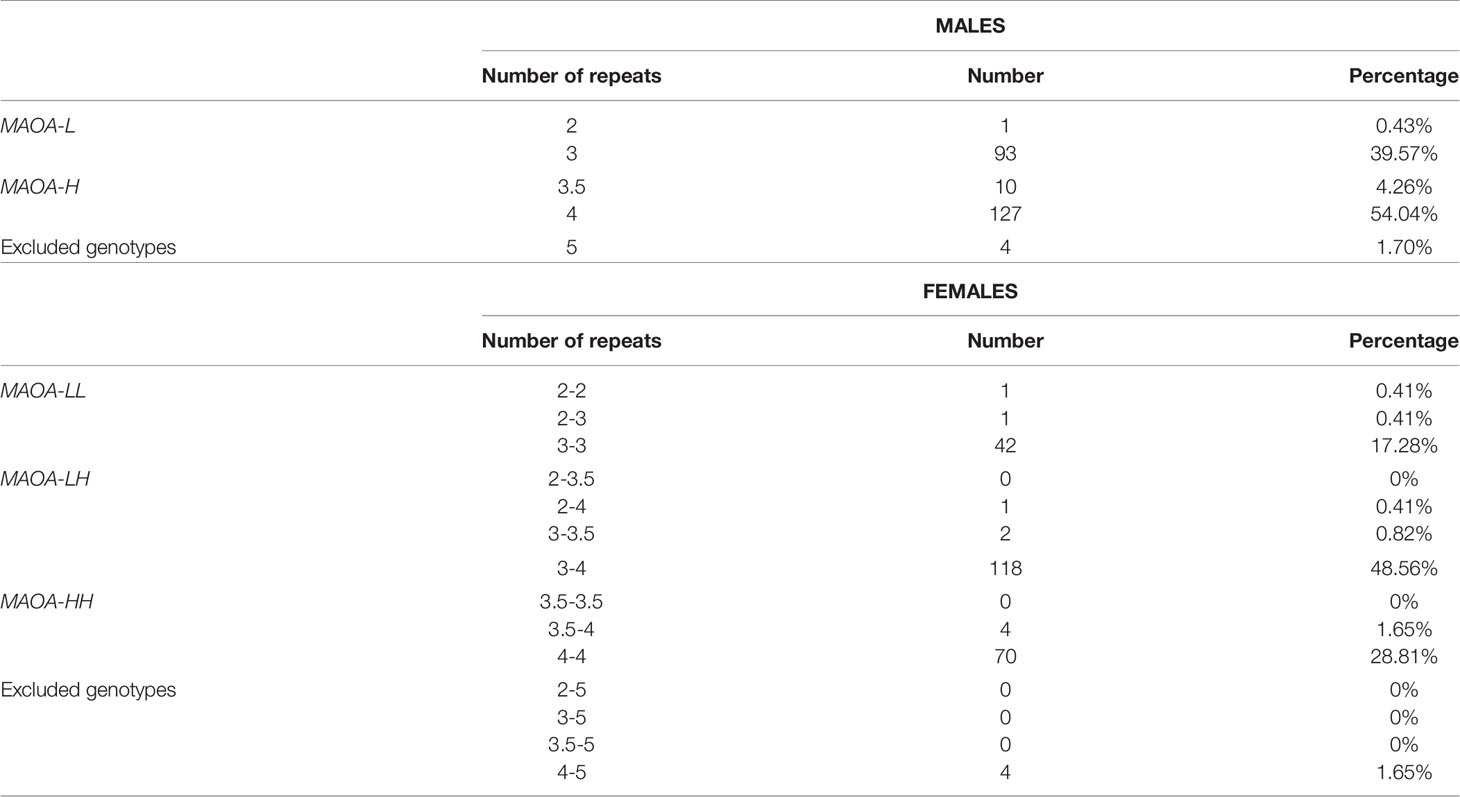
Table 2 Genotypic data of all participants. Genotypes containing 5-repeat variants were not included in either MAOA low-activity (MAOA-L) or high-activity (MAOA-H) allele groups. For more details, see text.
Data Analysis
Of the original 500 students recruited for the study, MAOA genotyping could not be performed for 11 participants, while 11 participants were missing CTQ and/or substance use data. We further excluded 8 participants (4 males and 4 females) carrying 5-repeat uVNTR alleles. Based on power tables (Aiken and West, 1991), it was determined that the current sample had adequate power (α = 0.80) to detect moderate to large, but not small, MAOA × maltreatment interaction effects for males and females. No differences in sex or age (ps > 0.48) or in child maltreatment scores (ps > 0.16) were found in the comparison between the participants included in and excluded from the analyses. Multiple regression models were used to evaluate proposed associations. Substance use count was the dependent variable in each model, with sex, MAOA variant, and maltreatment types included as independent variables. All five maltreatment types were included in each model to evaluate unique associations. Three-way interactions (e.g., sex × MAOA variant × maltreatment type) were then evaluated one at a time to determine if child maltreatment-MAOA interactive effects depended on sex. All independent variables were mean centered prior to analyses to aid in the interpretation of interaction effects. Statistically significant interactions were probed based on sex (male vs. female) and for MAOA variants to determine the nature of the interactions, consistent with standard procedures (Aiken and West, 1991).
Results
Approximately 11.5% of the sample had not used any substance, 28.9% of the sample had used one substance, 23.2% had used two substances, and 36.4% of the sample had used three substances. Based on the clinical cutoff scores recommended by Bernstein and Fink (1998), ~46.5% of the sample reported at least low levels of one or more maltreatment types. This percentage is consistent with previous data on undergraduate, emerging adult samples (Reichert and Flannery-Schroeder, 2014).
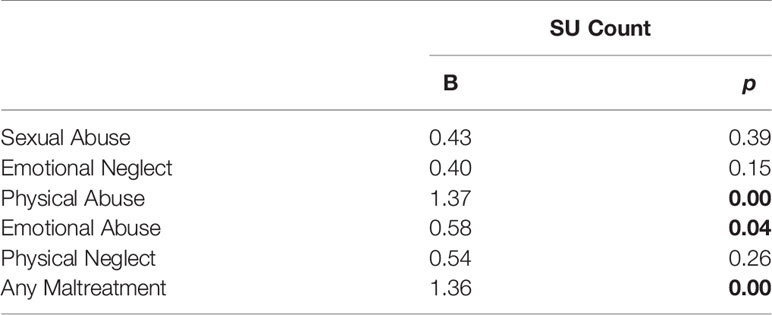
Regression analyses indicated a significant three-way interaction when examining any experience of maltreatment (B = 1.36, p = 0.00; see Table 3). Additionally, a significant three -way interaction was found for physical abuse (B = 1.37, p = 0.00) as well as emotional abuse (B = 0.58, p = 0.04). However, no significant three -way interactions were found for any other child maltreatment type: physical neglect (B = 0.54, p = 0.26), emotional neglect (B = 0.40, p = 0.15), or sexual abuse (B = 0.43, p = 0.39). Additionally, no significant two-way interactions between maltreatment variables and MAOA alleles were evident (ps > 0.12).
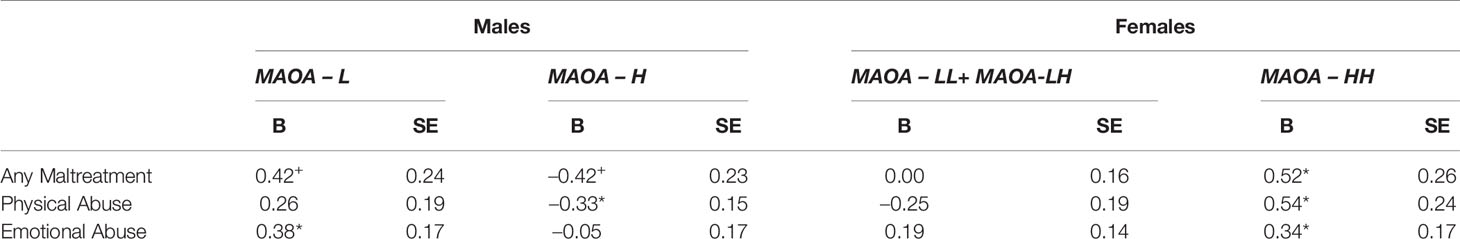
The statistically significant three -way interactions with any maltreatment type, physical abuse, and emotional abuse were further evaluated by conducting tests of the simple slopes (Table 4). Specifically, the models were conditioned at MAOA-H and MAOA-L for both males and females to determine the patterns of associations. For MAOA-L males, there was a marginally statistically trend for any maltreatment type (B = 0.42, p = 0.08) (Figure 1A) and statistically significant effect for and emotional abuse (B = 0.38, p = 0.03) (Figure 1C) to be positively associated with the number of substances used. However, an association between physical abuse and number of substances used was not found (B = 0.26, p = 0.17) (Figure 1B). For MAOA-H males, any maltreatment type was marginally statistically negatively associated (B = -0.42, p = 0.07) (Figure 1A) and physical abuse was statistically negatively associated (B = -0.33, p = 0.03) with the number of substances used (Figure 1B). Emotional abuse (B = -0.05, p = 0.77) was statistically unrelated to number of substances used for MAOA-H males (Figure 1C).
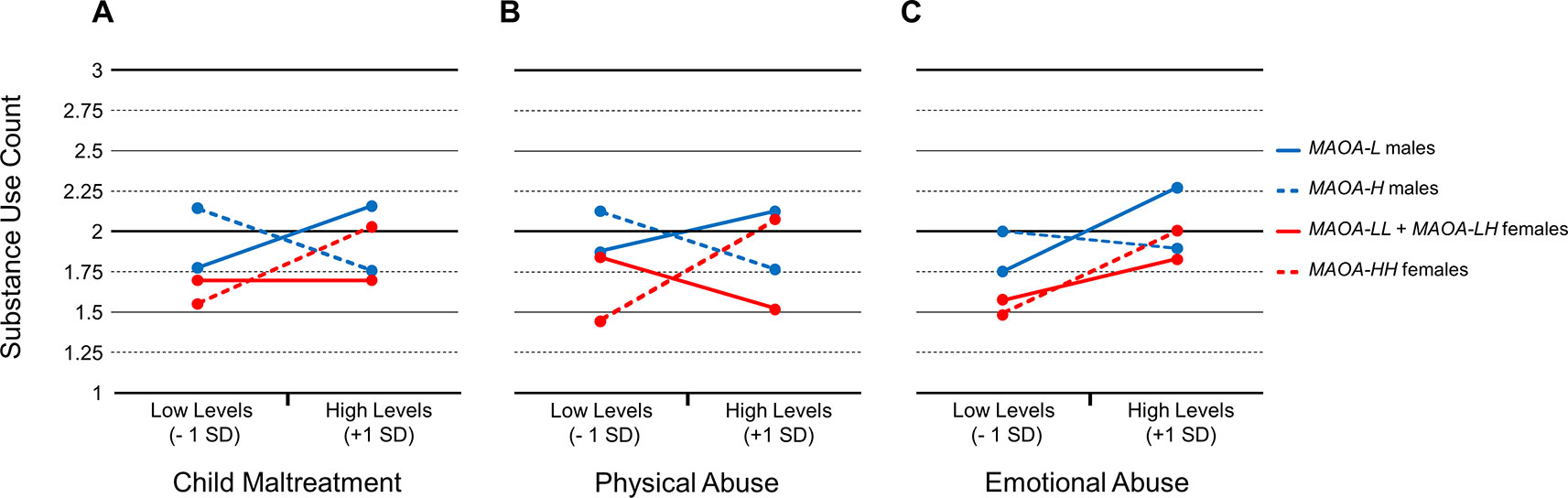
Figure 1 Associations between child maltreatment types and substance use count for male and female carriers of MAOA uVNTR variants. (A) Overall associations with child maltreatment scores. (B) Associations with physical abuse scores. (C) Associations with emotional abuse scores.
In contrast, for female carriers of low-activity MAOA variants (MAOA-LL and MAOA-LH), there was no association evident between any maltreatment type (B = 0.00, p = 0.99) (Figure 1A), physical abuse (B = -0.25, p = 0.18) (Figure 1B), or emotional abuse (B = 0.19, p = 0.17) (Figure 1C) and number of substances used. For homozygous MAOA-H females, there was a statistically significant positive association between any maltreatment type (B = 0.52, p = 0.04) (Figure 1A), and physical abuse (B = 0.54, p = 0.03) (Figure 1B), and emotional abuse (B = 0.34, p = 0.04) (Figure 1C) and number of substances used.
Discussion
The results of the current study showed that, in a sample of students enrolled in a large Midwestern university, PSU was predicted by the interaction of MAOA uVNTR allelic variants, sex, and specific child maltreatment types. The highest number of substances used was found in MAOA-L male and MAOA-HH female carriers with a history of emotional abuse (as well as physical abuse in women). To our knowledge, this is the first report documenting a key role of MAOA as a mediator of child maltreatment with respect to PSU. While previous studies have shown the importance of G×E interactions in PSU (Vaughn et al., 2009; Rende, 2011), the specific genetic factors implicated in such biosocial interplays remain mostly elusive; if confirmed by future studies, our results may point to MAOA as a key molecular basis for PSU.
The present findings extend our previous report of sex-dimorphic influences of G×E interactions in the lifetime use of tobacco (Fite et al., 2018) among college students. Furthermore, these results are consistent with previous evidence indicating sex differences in the interactive influence of these G×E interactions with respect to antisocial conduct (Nikulina et al., 2012; Stogner and Gibson, 2013; Byrd and Manuck, 2014; Harro and Oreland, 2016) and alcohol use (Nilsson et al., 2011). The interaction of MAOA alleles and child maltreatment can be interpreted from the perspective of the diathesis-stress model, which postulates that the predisposition to specific neurobehavioral deficits is the result of a synergistic combination of genetic and environmental untoward factors (Zuckerman, 1999). Another alternative interpretation follows the differential susceptibility hypothesis, which posits that specific genetic variables may sensitize to both the positive and the negative influence of early experiences (Ellis et al., 2011). This possibility is partially supported by Belsky and colleagues (Belsky et al., 2009; Belsky and Beaver, 2011), who have conceptualized that MAOA variants may act as plasticity factors in the predisposition to substance use and other psychopathological conditions.
In line with previous data (Armour et al., 2014), the current results highlight the importance of examining specific maltreatment types in relation to PSU. Our findings suggest that, although physical abuse and emotional abuse interact with MAOA variants to predict PSU even when statistically controlling for the other maltreatment types, no interaction effects were found for sexual abuse, physical neglect, or emotional neglect. This evidence is partially consistent with a previous study by Nikulina and colleagues (2012) suggesting that MAOA does not serve as a protective or risk factor for substance use outcomes among individuals who have experienced childhood sexual abuse. However, in contrast with our results, the results of that investigation showed that alcohol use was not predicted by the interaction of MAOA with either physical abuse or neglect. Given that the participants of that study ranged between 31 and 51 years of age, it is possible that the discrepancy with those results may reflect age differences; accordingly, the moderating effect of MAOA on child maltreatment and negative outcomes has been hypothesized to be age-dependent (Huizinga et al., 2006). Alternatively, these divergent findings may result from other differences between our studies, including the substance use outcomes (i.e., PSU vs alcohol abuse) and measurement of child maltreatment (i.e., self-report vs official records). Nevertheless, research shows that experiences of child maltreatment are associated with decreased propensity for reward selection, which could be due to lower reward sensitivity (Guyer et al., 2006). In turn, this dual risk might increase the risk of PSU. Thus, child maltreatment types, physical abuse and emotional abuse may be more saliently associated with blunted reward sensitivity.
The existence of sex-dimorphic G×E interactions involving MAOA uVNTR alleles has been attested in other psychopathological states. For example, male carriers of MAOA-L alleles with a history of child maltreatment have a significantly higher risk of antisocial, aggressive, and violent behavior (Caspi et al., 2002; Kim-Cohen et al., 2006; Beaver et al., 2010; Aslund et al., 2011; Fergusson et al., 2011; Fergusson et al., 2012; Byrd and Manuck, 2014; Godar et al., 2016). Notably, the same G×E interaction has been reproduced in mouse models, further supporting the biological nature of this biosocial interplay (Godar et al., 2019). Conversely, female carriers of MAOA-H alleles with a positive history for early-life adversity display a higher proclivity for antisocial and violent responses (Sjöberg et al., 2007; McGrath et al., 2012; Verhoeven et al., 2012). It has been hypothesized that this effect may reflect the enhancement of emotional reactivity during adolescence (Byrd et al., 2018). Furthermore, these effects may reflect sex- and genotype-specific differences in the effects of MAOA on monoamine metabolism (Jönsson et al., 2000; Aklillu et al., 2009). Notably, aggression and delinquency have been extensively linked to PSU, particularly in boys (McCormick and Smith, 1995; Mason and Windle, 2002; Martinotti et al., 2009). This concurrence strongly suggests that the G×E interaction of MAOA genotype and child maltreatment may predispose to a broad set of externalizing responses, ranging from antisocial personality to PSU propensity. In line with this interpretation, neuroimaging studies have pointed to MAOA as a key molecule to influence the function of the anterior cingulate cortex (ACC) (Passamonti et al., 2008). This region plays a major role in the regulation of self-regulation (Posner et al., 2007), the key domain implicated in the ontogeny of antisocial behavior (Gardner et al., 2008; Trentacosta and Shaw, 2009; Gillespie et al., 2018), as well as in the role of G×E interactions in PSU (Vaughn et al., 2009). The effects of MAOA on ACC activation patterns are sex-dimorphic; specifically, MAOA-L male and MAOA-H female carriers with a history of early stress display impairments in the activation of the ACC in response inhibition (Holz et al., 2016), a process directly related to self-regulation (Posner and Rothbart, 1998; Blair and Ursache, 2011; Hofmann et al., 2012). It should be noted that functional deficits of the ACC are associated with a reduction in inhibitory control (Bush et al., 2000; Chan et al., 2011), as well as a facilitation of ventral striatal responses to incentive stimuli, which in turn increases drug use propensity (Holmes et al., 2016; Koyama et al., 2017). Notably, these deficits may be particularly overt in young individuals (and therefore highly relevant in the age range of college students), due to their incomplete myelination of the ACC as well as the development of the dopaminergic system, which further exacerbates their proclivity to engage in impulsive and risky actions and heightens their reward sensitivity (Casey et al., 2008; Steinberg, 2008). At least in females, the presence of MAOA-H alleles may further reduce dopamine levels, ultimately promoting the ontogeny of reward deficiency syndrome (Blum, 2017; Blum et al., 2018). From this perspective, these results suggest that the interaction of MAOA-L alleles in males and MAOA-H in females and early-life maltreatment may interfere with the development of inhibitory control in emerging adulthood, ultimately increasing PSU risk.
Several limitations of this study should be acknowledged. First, our analyses focused exclusively on MAOA polymorphisms, yet several studies point to the importance of many other genes in the vulnerability to PSU, such as those encoding for dopamine receptor 2 and 4 as well as dopamine and serotonin transporters (Blum et al., 2010); further studies are needed to evaluate the potential interaction of child maltreatment with these vulnerability factors. Second, although rich literature has documented that MAOA variants interact with childhood maltreatment to increase the propensity for externalizing behaviors, our findings need to be replicated in larger samples from multiple colleges and with less skewed ethnic distribution. Indeed, our sample comprised of predominantly Caucasian youth, which may limit the generalizability of current results. Second, this study relied solely on self-reports of constructs, with a low internal consistency associated with our measure of physical neglect. Future research examining associations in other samples (e.g., clinical and criminal) using multiple, psychometrically sound assessments of constructs would be useful for establishing generalizability of findings. Finally, our research combined two- and three-repeat variant carriers in the MAOA-L group; however, previous studies, however, have shown that, in males, two-repeat alleles resulted in much lower levels of promoter activity as well as stronger phenotypic effects than the three-repeat genotype (Sabol et al., 1998; Guo et al., 2008). Notably, two-repeat variants have shown to increase antisocial phenotypes, including the propensity to engage in particularly violent conduct (such as shooting and stabbing), in African-American males (Beaver et al., 2013; Beaver et al., 2014). Unfortunately, given that only one male participant was found to carry the two-repeat alleles, our analyses were not sufficiently powered to differentiate across specific genotypes; however, future studies will be needed to verify whether specific differences may be identified with respect to the interaction of specific variants with early maltreatment.
Despite these limitations, the current study contributes to the growing literature indicating sex differences in genetic risk of MAOA in addition to the importance of the interactive influences of genetic and environmental risk for PSU. Further, findings indicate the importance of evaluating specific maltreatment types to better understand MAOA and maltreatment interactive risks for substance use.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by IRB University of Kansas. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PF conceptualized the project, supervised data collection and analysis, and drafted the first draft of the manuscript. SB helped with data collection and analysis and helped draft the first version of the manuscript. WH performed genotyping analyses and drafted part of the method sections. AM and MGB contributed to the conceptualization of the study and reviewed genotyping data. MB conceptualized the project, reviewed data analysis, and wrote the final version of the manuscript. All authors have read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The present manuscript was supported by a University of Kansas Strategic Initiatives Grant (to PF and MB), the National Institute of Mental Health (NIH R01 MH104603 to MB), and the ALSAM Foundation (to MB).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.01314/full#supplementary-material
References
Aiken, L. S., West, S. G. (1991). Multiple regression: Testing and interpreting interactions (Newbury Park, CA: Sage Publication).
Aklillu, E., Karlsson, S., Zachrisson, O. O., Ozdemir, V., Agren, H. (2009). Association of MAOA gene functional promoter polymorphism with CSF dopamine turnover and atypical depression. Pharmacogenet. Genomics 19, 267–275. doi: 10.1097/FPC.0b013e328328d4d3
Armour, C., Shorter, G. W., Elhai, J. D., Elklit, A., Christoffersen, M. N. (2014). Polydrug use typologies and childhood maltreatment in a nationally representative survey of Danish young adults. J. Stud. Alcohol Drugs 75 (1), 170–178. doi: 10.15288/jsad.2014.75.170
Aslund, C., Nordquist, N., Comasco, E., Leppert, J., Oreland, L., Nilsson, K. W. (2011). Maltreatment, MAOA, and delinquency: sex differences in gene-environment interaction in a large population-based cohort of adolescents. Behav. Genet. 41, 262–272. doi: 10.1007/s10519-010-9356-y
Baggio, S., Deline, S., Studer, J., N'Goran, A., Mohler-Kuo, M., Daeppen, J. B., et al. (2014). Concurrent versus simultaneous use of alcohol and non-medical use of prescription drugs: is simultaneous use worse for mental, social, and health issues? J. Psychoactive Drugs 46, 334–339. doi: 10.1080/02791072.2014.921747
Barrett, S. P., Darredeau, C., Pihl, R. O. (2006). Patterns of simultaneous polysubstance use in drug using university students. Hum. Psychopharmacol. 21 (4), 255– 263. doi: 10.1002/hup.766
Beaver, K. M., DeLisi, M., Vaughn, M. G., Barnes, J. C. (2010). Monoamine oxidase a genotype is associated with gang membership and weapon use. Compr. Psychiatry 51, 130–134. doi: 10.1016/j.comppsych.2009.03.010
Beaver, K. M., Wright, J. P., Boutwell, B. B., Barnes, J. C., DeLisi, M., Vaughn, M. G. (2013). Exploring the association between the 2-repeat allele of the MAOA gene promoter polymorphism and psychopathic personality traits, arrests, incarceration, and lifetime antisocial behavior. Pers. Individ Dif. 54, 164–168. doi: 10.1016/j.paid.2012.08.014
Beaver, K. M., Barnes, J. C., Boutwell, B. B. (2014). The 2-repeat allele of the MAOA gene confers an increased risk for shooting and stabbing behaviors. Psychiatr. Q. 85, 257–265. doi: 10.1007/s11126-013-9287-x
Belsky, J., Beaver, K. M. (2011). Cumulative-genetic plasticity, parenting and adolescent self-regulation. J. Child Psychol. Psychiatry 52, 619–626. doi: 10.1111/j.1469-7610.2010.02327.x
Belsky, J., Jonassaint, C., Pluess, M., Stanton, M., Brummett, B., Williams, R. (2009). Vulnerability genes or plasticity genes? Mol. Psychiatry 14, 746–754. doi: 10.1038/mp.2009.44
Bernstein, D. P., Fink, L. (1998). Childhood trauma questionnaire: a retrospective self-report: manual. (San Antonio, TX: The Psychological Corporation).
Blair, C., Ursache, A. (2011). “A bidirectional model of executive functions and self-regulation,” in Handbook of self-regulation: Research, theory, and applications. Eds. Vohs, K. D., Baumeister, R. F. (New York, NY, US: Guilford Press), 300–320.
Blum, K., Giordano, J., Morse, S., Liu, Y., Tian, J., Bowirrat, A., et al. (2010). Genetic Addiction Risk Score (GARS) analysis: exploratory development of polymorphic risk alleles in poly-drug addicted males. Integr. Omics Appl. Biotechnol. 1, 1–14. doi: 10.5772/20067
Blum, K., Gondré-Lewis, M. C., Baron, D., Thanos, P. K., Braverman, E. R., Neary, J., et al. (2018). Introducing Precision Addiction Management of Reward Deficiency Syndrome, the Construct That Underpins All Addictive Behaviors. Front. Psychiatry 9, 548. doi: 10.3389/fpsyt.2018.00548
Blum, K. (2017). “Reward deficiency syndrome,” in The Sage Encyclopedia of Abnormal Clinical Psychology. Ed. Wenzel, A. (Newbury Park, CA: Sage Publication).
Bortolato, M., Chen, K., Shih, J. C. (2008). Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 60, 1527–1533. doi: 10.1016/j.addr.2008.06.002
Buckholtz, J. W., Callicott, J. H., Kolachana, B., Hariri, A. R., Goldberg, T. E., Genderson, M., et al. (2008). Genetic variation in MAOA modulates ventromedial prefrontal circuitry mediating individual differences in human personality. Mol. Psychiatry 13, 313–324. doi: 10.1038/sj.mp.4002020
Bush, G., Luu, P., Posner, M. I. (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222. doi: 10.1016/s1364-6613(00)01483-2
Byrd, A. L., Manuck, S. B. (2014). MAOA, childhood maltreatment, and antisocial behavior: meta-analysis of a gene-environment interaction. Biol. Psychiatry 75, 9–17. doi: 10.1016/j.biopsych.2013.05.004
Byrd, A. L., Manuck, S. B., Hawes, S. W., Vebares, T. J., Nimgaonkar, V., Chowdari, K. V., et al. (2018). The interaction between monoamine oxidase A (MAOA) and childhood maltreatment as a predictor of personality pathology in females: Emotional reactivity as a potential mediating mechanism. Dev. Psychopathol. 22, 1–17. doi: 10.1017/S0954579417001900
Casey, B. J., Jones, R. M., Hare, T. A. (2008). The adolescent brain. Ann. N Y Acad. Sci. 1124, 111–126. doi: 10.1196/annals.1440.010
Caspi, A., McClay, J., Moffitt, T. E., Mill, J., Martin, J., Craig, I. W., et al. (2002). Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854. doi: 10.1126/science.1072290
Chan, A. S., Han, Y. M., Leung, W. W., Leung, C., Wong, V. C., Cheung, M. (2011). Abnormalities in the anterior cingulate cortex associated with attentional and inhibitory control deficits: a neurophysiological study on children with autism spectrum disorders. Res. Autism Spectr. Disord. 5, 254–266. doi: 10.1016/j.rasd.2010.04.007
Cohen, J. R., Menon, S. V., Shorey, R. C., Le, V. D., Temple, J. R. (2017). The distal consequences of physical and emotional neglect in emerging adults: a person-centered, multi-wave, longitudinal study. Child Abuse Negl. 63, 151–161. doi: 10.1016/j.chiabu.2016.11.030
Dannlowski, U., Ohrmann, P., Konrad, C., Domschke, K., Bauer, J., Kugel, H., et al. (2009). Reduced amygdala-prefrontal coupling in major depression: association with MAOA genotype and illness severity. Int. J. Neuropsychopharmacol. 12, 11–22. doi: 10.1017/S1461145708008973
Deckert, J., Catalano, M., Syagailo, Y. V., Bosi, M., Okladnova, O., Di Bella, D., et al. (1998). Excess of high activity monoamine oxidase a gene promoter alleles in female patients with panic disorder. Hum. Mol. Genet. 8, 621–624. doi: 10.1093/hmg/8.4.621
Denney, R. M., Koch, H., Craig, I. W. (1999). Association between monoamine oxidase A activity in human male skin fibroblasts and genotype of the MAOA promoter-associated variable number tandem repeat. Hum. Genet. 105, 542–551. doi: 10.1007/s004399900183
Dick, D. M., Prescott, C. A., McGue, M. (2009). “The genetics of substance use and substance use disorders,” in Handbook of Behavior Genetics, Ed. Kim, Y. K. (New York: Springer), 433–453.
Ducci, F., Goldman, D. (2012). The genetic basis of addictive disorders. Psychiatr. Clin. North Am. 35, 495–519.
Ducci, F., Enoch, M. A., Hodgkinson, C., Xu, K., Catena, M., Robin, R. W., et al. (2008). Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol. Psychiatry 13, 334–347.
Ellis, B. J., Boyce, W. T., Belsky, J., Bakermans-Kranenburg, M. J., van Ijzendoorn, M. H. (2011). Differential susceptibility to the environment: an evolutionary–neurodevelopmental theory. Dev. Psychopathol. 23, 7–28. doi: 10.1017/S0954579410000611
Fan, J., Fossella, J., Sommer, T., Wu, Y., Posner, M. I. (2003). Mapping the genetic variation of executive attention onto brain activity. Proc. Natl. Acad. Sci. U.S.A. 100, 7406–7411. doi: 10.1073/pnas.0732088100
Fergusson, D. M., Boden, J. M., Horwood, L. J., Miller, A. L., Kennedy, M. A. (2011). MAOA, abuse exposure and antisocial behaviour: 30-year longitudinal study. Br. J. Psychiatry 198, 457–463.
Fergusson, D. M., Boden, J. M., Horwood, L. J., Miller, A., Kennedy, M. A. (2012). Moderating role of the MAOA genotype in antisocial behaviour. Br. J. Psychiatry 200, 116–123. doi: 10.1192/bjp.bp.111.093328
Fite, P. J., Brown, S., Hossain, W., Manzardo, A., Butler, M. G., Bortolato, M. (2018). Tobacco and cannabis use in college students are predicted by sex-dimorphic interactions between MAOA genotype and child abuse. CNS Neurosci. Ther. 25 (1), 101–11. doi: 10.1111/cns.13002
Fitzgerald, P. J. (2013). Elevated norepinephrine may be a unifying etiological factor in the abuse of a broad range of substances: alcohol, nicotine, marijuana, heroin, cocaine, and caffeine. Subst. Abuse. 7, 171–183. doi: 10.4137/SART.S13019
Frazzetto, G., Di Lorenzo, G., Carola, V., Proietti, L., Sokolowska, E., Siracusano, A., et al. (2007). Early trauma and increased risk for physical aggression during adulthood: The moderating role of MAOA genotype. PloS One 2, e486. doi: 10.1371/journal.pone.0000486
Funk, R. R., McDermeit, M., Godley, S. H., Adams, L. (2003). Maltreatment issues by level of adolescent substance abuse treatment: the extent of the problem at intake and relationship to early outcomes. Child Maltreat 8, 36–45. doi: 10.1177/1077559502239607
Galaif, E. R., Stein, J. A., Newcomb, M. D., Bernstein, D. P. (2001). Gender differences in the prediction of problem alcohol use in adulthood: Exploring the influence of family factors and childhood maltreatment. J. @ Stud. Alcohol. 62 (4), 486–493. doi: 10.15288/jsa.2001.62.486
Gardner, T. W., Dishion, T. J., Connell, A. M. (2008). Adolescent self-regulation as resilience: resistance to antisocial behavior within the deviant peer context. J. Abnorm. Child Psychol. 36, 273–284. doi: 10.1007/s10802-007-9176-6
Gillespie, S. M., Brzozowski, A., Mitchell, I. J. (2018). Self-regulation and aggressive antisocial behaviour: insights from amygdala-prefrontal and heart-brain interactions. Psychol. Crime Law 24, 243–257.). doi: 10.1080/1068316X.2017.1414816
Gledhill-Hoyt, J., Lee, H., Strote, J., Wechsler, H. (2000). Increased use of marijuana and other illicit drugs at US colleges in the 1990s: results of three national surveys. Addiction 95, 1655–1667. 10.1046/j.1360-0443.2000.951116556.x
Godar, S. C., Fite, P. J., McFarlin, K. M., Bortolato, M. (2016). The role of monoamine oxidase a in aggression: current translational developments and future challenges. Prog. Neuropsychopharmacol. Biol. Psychiatry 69, 90–100. doi: 10.1016/j.pnpbp.2016.01.001
Godar, S. C., Mosher, L. J., Scheggi, S., Devoto, P., Moench, K. M., Strathman, H. J., et al. (2019). Gene-environment interactions in antisocial behavior are mediated by early-life 5-HT(2A) receptor activation. Neuropharmacol. 159, 107513. doi: 10.1016/j.neuropharm.2019.01.028
Goldstein, A. L., Faulkner, B., Wekerle, C. (2013). The relationship among internal resilience, smoking, alcohol use, and depression symptoms in emerging adults transitioning out of child welfare. Child Abuse Negl. 37 (1), 22–32. doi: 10.1016/j.chiabu.2012.08.007
Guindalini, C., Scivoletto, S., Ferreira, R. G., Nishimura, A., Zilberman, M. L., Peluso, M. M., et al. (2005). Association of MAO A polymorphism and alcoholism in Brazilian females. Psychiatr. Genet. 15, 141–144. doi: 10.1097/00041444-200506000-00011
Guo, G., Wilhelmsen, K., Hamilton, N. (2007). Gene-lifecourse interaction for alcohol consumption in adolescence and young adulthood: five monoamine genes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B (4), 417–423. doi: 10.1002/ajmg.b.30340
Guo, G., Ou, X. M., Roettger, M., Shih, J. C. (2008). The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: associations and MAOA promoter activity. Eur. J. Hum. Genet. 16, 626–634. doi: 10.1038/sj.ejhg.5201999
Guyer, A. E., Nelson, E. E., Perez-Edgar, K., Hardin, M. G., Roberson-Nay, R., Monk, C. S., et al. (2006). Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J. Neurosci. 26, 6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006
Harro, J., Oreland, L. (2016). The role of MAO in personality and drug use. Prog. Neuropsychopharmacol. Biol. Psychiatry 69, 101–111. doi: 10.1016/j.pnpbp.2016.02.013
Herman., A. I., Kaiss, K. M., Ma, R., Philbeck, J. W., Hasan, A., Dasti, H., et al. (2005). Serotonin transporter promoter polymorphism and monoamine oxidase type A VNTR allelic variants together influence alcohol binge drinking risk in young women. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 133B, 74–78. doi: 10.1002/ajmg.b.30135
Hofmann, W., Schmeichel, B. J., Baddeley, A. D. (2012). Executive functions and self-regulation. Trends Cogn. Sci. 16, 174–180. doi: 10.1016/j.tics.2012.01.006
Holmes, A. J., Hollinshead, M. O., Roffman, J. L., Smoller, J. W., Buckner, R. L. (2016). Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. J. Neurosci. 36, 4038–4049. doi: 10.1523/JNEUROSCI.3206-15.2016
Holz, N., Boecker, R., Buchmann, A. F., Blomeyer, D., Baumeister, S., Hohmann, S., et al. (2016). Evidence for a sex-dependent maoa× childhood stress interaction in the neural circuitry of aggression. Cereb. Cortex 26, 904–914. doi: 10.1093/cercor/bhu249
Huang, Y. Y., Cate, S. P., Battistuzzi, C., Oquendo, M. A., Brent, D., Mann, J. J. (2004). An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology 29, 1498–1505. doi: 10.1038/sj.npp.1300455
Huizinga, D., Haberstick, B. C., Smolen, A., Menard, S., Young, S. E., Corley, R. P., et al. (2006). Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase a genotype. Biol. Psychiatry 60, 677–683. doi: 10.1016/j.biopsych.2005.12.022
Jönsson, E. G., Norton, N., Gustavsson, J. P., Oreland, L., Owen, M. J., Sedvall, G. C. (2000). A promoter polymorphism in the monoamine oxidase a gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J. Psychiatr. Res. 34, 239–244. doi: 10.1016/s0022-3956(00)00013-3
Jackson, K. M., Sher, K. J., Schulenberg, J. E. (2008). Conjoint developmental trajectories of young adult substance use. Alcohol Clin. Exp. Res. 32, 723–737. doi: 10.1111/j.1530-0277.2008.00643.x
Johnston, L. D., O'Malley, P. M., Bachman, J. G. (2004). Monitoring the future: National survey results on drug use, 1975-2002. Volume II, college students and adults. Ages 19–45. (Bethesda, MD: National Institute on Drug Abuse), 19–40.
Kilpatrick, D. G., Saunders, B. E., Smith, D. W. (2003). Youth Victimization: Prevalence and Implications [Electronic] (Office of Justice Program, National Institute of Justice: U.S. Department of Justice).
Kim-Cohen, J., Caspi, A., Taylor, A., Williams, B., Newcombe, R., Craig, I. W., et al. (2006). MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Mol. Psychiatry 11, 903–913. doi: 10.1038/sj.mp.4001851
Koyama, M. S., Parvaz, M. A., Goldstein, R. Z. (2017). The adolescent brain at risk for substance use disorders: a review of functional MRI research on motor response inhibition. Curr. Opin. Behav. Sci. 13, 186–195. doi: 10.1016/j.cobeha.2016.12.006
Leeb, R. T., Paulozzi, L., Melanson, C., Simon, T., Arias, I. (2008). Child maltreatment surveillance: Uniform definitions for public health and recommended data elements, Version 1.0 (Atlanta, GA): Centers for Disease Control and Prevention, National Center for Injury Prevention and Control).
Lipari, R. N., Jean-Francois, B. A. (2016). Day in the Life of College Students Aged 18 to 22, Substance use facts. The CBHSQ Report: May 26 (2016). Rockville, MD Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration.
Müller, C. P., Homberg, J. R. (2015). The role of serotonin in drug use and addiction. Behav. Brain Res. 277, 146–192. doi: 10.1016/j.bbr.2014.04.007
Martin, C. S., Clifford, P. R., Clapper, R. L. (1992). Patterns and predictors of simultaneous and concurrent use of alcohol, tobacco, marijuana, and hallucinogens in first-year college students. J. Subst. Abuse. 4 (3), 319–326. doi: 10.1016/0899-3289(92)90039-z
Martinotti, G., Carli, V., Tedeschi, D., Di Giannantonio, M., Roy, A., Janiri, L., et al. (2009). Mono- and polysubstance dependent subjects differ on social factors, childhood trauma, personality, suicidal behaviour, and comorbid Axis I diagnoses. Addict. Behav. 34, 790–793. doi: 10.1016/j.addbeh.2009.04.012
Mason, W. A., Windle, M. (2002). Reciprocal relations between adolescent substance use and delinquency: a longitudinal latent variable analysis. J. Abnorm. Psychol. 111, 63–76.
McCabe, S. E., Cranford, J. A., Morales, M., Young, A. (2006). Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. J. Stud. Alcohol. 67, 529–537. doi: 10.15288/jsa.2006.67.529
McCormick, R. A., Smith, M. (1995). Aggression and hostility in substance abusers: the relationship to abuse patterns, coping style, and relapse triggers. Addict. Behav. 20, 555–562. doi: 10.1016/0306-4603(95)00015-5
McGrath, L. M., Mustanski, B., Metzger, A., Pine, D. S., Kistner-Griffin, E., Cook, E., et al. (2012). A latent modeling approach to genotype-phenotype relationships: maternal problem behavior clusters, prenatal smoking, and MAOA genotype. Arch. Womens Ment. Health 15, 269–282. doi: 10.1007/s00737-012-0286-y
Meyer-Lindenberg, A., Buckholtz, J. W., Kolachana, B. R., Hariri, A., Pezawas, L., Blasi, G., et al. (2006). Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl. Acad. Sci. U.S.A. 103, 6269–6274. doi: 10.1073/pnas.0511311103
Mohler-Kuo, M., Lee, J. E., Wechsler, H. (2003). Trends in marijuana and other illicit drug use among college students: results from 4 Harvard School of Public Health College Alcohol Study surveys: 1993-2001. J. Am. Coll. Health 52, 17–24. doi: 10.1080/07448480309595719
Moss, H. B., Chen, C. M., Yi, H. (2014). Early adolescent patterns of alcohol, cigarette, and marijuana polysubstance use and young adulth substance use outcomes in a nationally representative sample. Drug Alcohol Dep. 136, 51–62. doi: 10.1016/j.drugalcdep.2013.12.011
National Center on Addiction and Substance Abuse at Columbia University (2007). Wasting the Best and the Brightest: Substance Abuse at America"s Colleges and Universities (New York: CASA).
Nikulina, V., Widom, C. S., Brzustowicz, L. M. (2012). Child abuse and neglect, MAOA, and mental health outcomes: a prospective examination. Biol. Psychiatry 71, 350–357. doi: 10.1016/j.biopsych.2011.09.008
Nilsson, K. W., Comasco, E., Åslund, C., Nordquist, N., Leppert, J., Oreland, L. (2011). MAOA genotype, family relations and sexual abuse in relation to adolescent alcohol consumption. Addict. Biol. 16, 347–355. doi: 10.1111/j.1369-1600.2010.00238.xf
O'Grady, K. E., Arria, A. M., Fitzelle, D. M., Wish, E. D. (2008). Heavy drinking and polydrug use among college students. J. Drug Issues 38, 445–466. doi: 10.1177/002204260803800204
Passamonti, L., Cerasa, A., Gioia, M. C., Magariello, A., Muglia, M., Quattrone, A., et al. (2008). Genetically dependent modulation of serotonergic inactivation in the human prefrontal cortex. Neuroimage 40, 1264–1273. doi: 10.1016/j.neuroimage.2007.12.028.
Pentz, M. A., Dwyer, J. H., MacKinnon, D. P., Flay, B. R., Hansen, W. B., Wang, E. Y., et al. (1989). A multicommunity trial for primary prevention of adolescent drug abuse: effects on drug use prevalence. J. Am. Med. Assoc. 262 (22), 3259–3266.
Posner, M. I., Rothbart, M. K. (1998). Attention, self-regulation and consciousness. Philos. Trans. R. Soc Lond. B. Biol. Sci. 353, 1915–1927. doi: 10.1098/rstb.1998.0344
Posner, M. I., Rothbart, M. K., Sheese, B. E., Tang, Y. (2007). The anterior cingulate gyrus and the mechanism of self-regulation. Cogn. Affect. Behav. Neurosci. 7, 391–395. doi: 10.3758/cabn.7.4.391
Reichert, E. L., Flannery-Schroeder, E. (2014). Posttraumatic cognitions as mediators between childhood maltreatment and poorer mental health among young adults. J. Child Adol. Trauma. 7, 153–162. doi: 10.1007/s40653-014-0021-0
Rende, R. (2011). Interaction of genes and environment in adolescent substance use: opportunities and implications for intervention. Mind Brain 2 (1), 50–55.
Sabol, S. Z., Hu, S., Hamer, D. (1998). A functional polymorphism in the monoamine oxidase a gene promoter. Hum. Genet. 103, 273–279. doi: 10.1007/s004390050816
Samochowiec, J., Lesch, K. P., Rottmann, M., Smolka, M., Syagailo, Y. V., Okladnova, O., et al. (1999). Association of a regulatory polymorphism in the promoter region of the monoamine oxidase a gene with antisocial alcoholism. Psychiatry Res. 86, 67–72. doi: 10.1016/s0165-1781(99)00020-7
Schmidt, L. G., Sander, T., Kuhn, S., Smolka, M., Rommelspacher, H., Samochowiec, J., et al. (2000). Different allele distribution of a regulatory MAOA gene promoter polymorphism in antisocial and anxious-depressive alcoholics. J. Neural Transm. 107, 681–689. doi: 10.1007/s007020070069
Sjöberg, R. L., Nilsson, K. W., Wargelius, H. L., Leppert, J., Lindström, L., Oreland, L. (2007). Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosocial factors. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 144B, 159–164. doi: 10.1002/ajmg.b.30360
Steinberg, L. (2008). A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 28, 78–106. doi: 10.1016/j.dr.2007.08.002
Stogner, J. M., Gibson, C. L. (2013). Stressful life events and adolescent drug use: moderating influences of the MAOA gene. J. Crim. Just. 41, 357–363. doi: 10.1016/j.jcrimjus.2013.06.003
Trentacosta, C. J., Shaw, D. S. (2009). Emotional self-regulation, peer rejection, and antisocial behavior: developmental associations from early childhood to early adolescence. J. Appl. Dev. Psychol. 30, 356–365. doi: 10.1016/j.appdev.2008.12.016
Trenz, R. C., Scherer, M., Harrell, P., Zur, J., Sinha, A., Latimer, W. (2012). Early onset of drug and polysubstance use as predictors of injection drug use among adult drug users. Addictive Beh. 37, 367–372. doi: 10.1016/j.addbeh.2011.11.011
Uhl, G. R., Liu, Q. R., Walther, D., Hess, J., Naiman, D. (2001). Polysubstance abuse-vulnerability genes: genome scans for association, using 1,004 subjects and 1,494 single-nucleotide polymorphisms. Am. J. Hum. Genet. 69, 1290–1300. doi: 10.1086/324467
Vanyukov, M. M., Moss, H. B., Yu, L. M., Tarter, R. E., Deka, R. (1995). Preliminary evidence for an association of a dinucleotide repeat polymorphism at the MAOA gene with early onset al.coholism/substance abuse. Am. J. Med. Genet. Neuropsychiat Genet. 60, 122–126. doi: 10.1002/ajmg.1320600207
Vanyukov, M. M., Maher, B. S., Devlin, B., Tarter, R. E., Kirillova, G. P., Yu, L. M., et al. (2004). Haplotypes of the monoamine oxidase genes and the risk for substance use disorders. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 125B, 120–125. doi: 10.1002/ajmg.b.20105
Vaughn, M. G., Beaver, K. M., DeLisi, M., Perron, B. E., Schelbe, L. (2009). Gene-environment interplay and the importance of self-control in predicting polydrug use and substance-related problems. Addict. Behav. 34, 112–116. doi: 10.1016/j.addbeh.2008.08.011
Verhoeven, F. E., Booij, L., Kruijt, A. W., Cerit, H., Antypa, N., Does, W. (2012). The effects of MAOA genotype, childhood trauma, and sex on trait and state-dependent aggression. Brain Behav. 2, 806–813. doi: 10.1002/brb3.96
Vink, J. M. (2016). Genetics of addiction: future focus on gene × environment interaction? J. Stud. Alcohol Drugs 77, 684–687. doi: 10.15288/jsad.2016.77.684
Volkow, N. D., Fowler, J. S., Wang, G. J., Swanson, J. M., Telang, F. (2007). Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch. Neurol. 64, 1575–1579. doi: 10.1001/archneur.64.11.1575
Williams, L. M., Gatt, J. M., Kuan, S. A., Dobson-Stone, C., Palmer, D. M., Paul, R. H., et al. (2009). A polymorphism of the MAOA gene is associated with emotional brain markers and personality traits on an antisocial index. Neuropsychopharmacology 34, 1797–1809. doi: 10.1038/npp.2009.1
Keywords: polysubstance use, MAOA, child maltreatment, sex differences, gene × environment interactions
Citation: Fite PJ, Brown S, Hossain WA, Manzardo A, Butler MG and Bortolato M (2020) Sex-Dimorphic Interactions of MAOA Genotype and Child Maltreatment Predispose College Students to Polysubstance Use. Front. Genet. 10:1314. doi: 10.3389/fgene.2019.01314
Received: 27 September 2019; Accepted: 02 December 2019;
Published: 17 January 2020.
Edited by:
Carla Cannizzaro, University of Palermo, ItalyReviewed by:
Nela Pivac, Rudjer Boskovic Institute, CroatiaYong-Kyu Kim, Howard Hughes Medical Institute (HHMI), United States
Copyright © 2020 Fite, Brown, Hossain, Manzardo, Butler and Bortolato. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paula J. Fite, cGZpdGVAa3UuZWR1; Marco Bortolato, bWFyY28uYm9ydG9sYXRvQHV0YWguZWR1
 Paula J. Fite
Paula J. Fite Shaquanna Brown1,2
Shaquanna Brown1,2 Merlin G. Butler
Merlin G. Butler Marco Bortolato
Marco Bortolato