
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 16 January 2020
Sec. Evolutionary and Population Genetics
Volume 10 - 2019 | https://doi.org/10.3389/fgene.2019.01166
This article is part of the Research Topic The Genetic and Environmental Basis for Diseases in Understudied Populations View all 21 articles
The World Health Organization in 2016 estimated that over 20% of the global disease burden and deaths were attributed to modifiable environmental factors. However, data clearly characterizing the impact of environmental exposures and health endpoints in African populations is limited. To describe recent progress and identify important research gaps, we reviewed literature on environmental health research in African populations over the last decade, as well as research incorporating both genomic and environmental factors. We queried PubMed for peer-reviewed research articles, reviews, or books examining environmental exposures and health outcomes in human populations in Africa. Searches utilized medical subheading (MeSH) terms for environmental exposure categories listed in the March 2018 US National Report on Human Exposure to Environmental Chemicals, which includes chemicals with worldwide distributions. Our search strategy retrieved 540 relevant publications, with studies evaluating health impacts of ambient air pollution (n=105), indoor air pollution (n = 166), heavy metals (n = 130), pesticides (n = 95), dietary mold (n = 61), indoor mold (n = 9), per- and polyfluoroalkyl substances (PFASs, n = 0), electronic waste (n = 9), environmental phenols (n = 4), flame retardants (n = 8), and phthalates (n = 3), where publications could belong to more than one exposure category. Only 23 publications characterized both environmental and genomic risk factors. Cardiovascular and respiratory health endpoints impacted by air pollution were comparable to observations in other countries. Air pollution exposures unique to Africa and some other resource limited settings were dust and specific occupational exposures. Literature describing harmful health effects of metals, pesticides, and dietary mold represented a context unique to Africa. Studies of exposures to phthalates, PFASs, phenols, and flame retardants were very limited. These results underscore the need for further focus on current and emerging environmental and chemical health risks as well as better integration of genomic and environmental factors in African research studies. Environmental exposures with distinct routes of exposure, unique co-exposures and co-morbidities, combined with the extensive genomic diversity in Africa may lead to the identification of novel mechanisms underlying complex disease and promising potential for translation to global public health.
A global assessment by the World Health Organization (WHO) in 2016 estimated that 24% of the global disease burden and 23% of all deaths were attributed to modifiable environmental factors, including physical, chemical, and biological hazards to human health (Prüss-Ustün et al., 2016). The highest number of deaths per capita attributable to the environment was reported for sub-Saharan Africa, primarily reflecting infectious diseases, but also noncommunicable diseases and injuries. Disease burden was highest (36%) among children. In modern Africa, there has been rapid industrial development in the absence of health and environmental safety guidelines that parallel those in the United States, Canada, or Europe (Organization, 2017). Heavy metals, pesticides, air pollution, water contaminants, and waste represent hazardous exposures increasing in Africa (Nweke and Sanders, 2009), but with limited research attention on the implications for human health. Many chemicals that pose health risks to exposed populations in Africa and around the world are known to be endocrine disrupting chemicals (EDCs). A meeting of scientists around this issue took place in South Africa in 2015, leading to a “call to action” to utilize available scientific knowledge to address the impact of EDCs on human as well as wildlife health in Africa (Bornman et al., 2017). This meeting report also called for a shift from reaction to prevention, with utilization of existing datasets, increased biomonitoring, and surveillance of environmental chemicals, as well as further research including the support of longitudinal studies (Bornman et al., 2017).
Often in parallel to environmental health research, genomic research related to The Human Genome Project has advanced our understanding of disease susceptibility with enormous productivity and ongoing promise. Initial research in genomics had limited participation from African study populations, despite the important genomic diversity represented by African populations. However, huge efforts to address this limitation took place in the last decade resulting in an ongoing genomic research revolution in Africa (Consortium et al., 2014). Much of that effort was enabled by investments from the African Society of Human Genetics, National Institutes of Health (NIH), and the Wellcome Trust through the Human Heredity and Health in Africa (H3Africa) consortium (www.h3africa.org). The H3Africa consortium began in June 2010 to support genomic and epidemiological research led by African scientists (Consortium et al., 2014). Genomic research in Africa is not limited to the bounds of this consortium, but it represents a research infrastructure that enables innovative science. For example, studies covering common diseases such as cardiovascular (Owolabi et al., 2014), neurological (Akinyemi et al., 2016), respiratory (Zar et al., 2016a; Zar et al., 2016b), kidney (Osafo et al., 2015), and other non-communicable diseases are represented in this consortium. Developments in pharmacogenomics (Warnich et al., 2011) and the human microbiome (Adebamowo et al., 2017) are also underway, and many studies incorporate information about HIV, malaria, tuberculosis, and other common infections in Africa. The H3Africa consortium also promotes opportunities for training in bioinformatics (Adoga et al., 2014; Oluwagbemi et al., 2014; Mulder et al., 2016), supports three biorepositories on the African continent, and facilitates policy and ethical recommendations (Consortium et al., 2014; Barchi and Little, 2016; Munung et al., 2016; de Vries and Pool, 2017).
Not only does Africa offer the richest genomic diversity in the world, it also has an extensive diversity of under-researched environmental exposures, including some exposures unique to the continent, which present important public health issues. Integration of genomic variants with environmental risk factors is vital to properly characterize disease risk in Africa. However, the starting point for incorporating genomic (G) and environmental (E) factors can be daunting. Important questions include: What environmental exposures are relevant to what African populations? What are the priorities? What has been studied and what are the relevant health outcomes? How do the exposures and health outcomes differ compared to populations in other regions of the world? How can genomics and environmental exposures be integrated?
The purpose of this review is to summarize and provide examples of the latest environmental health research and the G x E interactions that have been characterized this decade in Africa. In this paper we use the “G x E” terminology to broadly represent the integration of genomic and environmental data in a research project or study population. It can represent various statistical or data science methods for evaluating both genomic and environmental factors and is not strictly referring to the biological or statistical sense of the term interaction. Our review expands previous reviews describing the distribution of environmental exposures in selected African populations by focusing on the evaluated health outcomes related to environmental exposures and including all of Africa.
We queried the PubMed database to identify peer-reviewed research or review articles or books (referred to generally as publications) examining environmental exposures and health outcomes in human populations residing on the African continent. We searched for publications evaluating the following environmental exposure categories: Ambient air pollution, indoor air pollution, electronic waste, environmental phenols, flame retardants, dietary mold, indoor mold, pesticides, perfluoroalkyl substances (PFASs), phthalates, and heavy metals. All search strategies, which included keywords as well as Medical Subject Headings (MeSH), are provided in the Supplementary Text, pulling preliminary results. All African countries were represented in the query and no exclusions were made based on the language of publication. The date range searched was from January 1, 2010 to March 20, 2018. Research articles were excluded if they did not include a measure/data for the queried exposure(s) and/or any health outcome(s). For example, research articles describing biomonitoring efforts or surveillance of human exposure to chemicals were not included if they did not also measure at least one health endpoint in a study population. We further refined our search to examine a subset of research or review articles that incorporated genomics, representing G x E research articles.
Our literature search identified a total of 540 publications, representing 482 research articles, 57 reviews, and 1 book. A full list of the publications is provided in Supplementary Table 1. The results per exposure category are displayed in Table 1 and Figures 1 and 2, where publications could belong to more than one category. The largest number of publications identified in our search represented exposures to indoor air pollution (n = 166), heavy metals (n = 130), ambient air pollution (n = 105), pesticides (n = 95), and dietary mold (n = 61). Notably fewer publications were retrieved for the exposure categories perfluoroalkyl substances (n = 16 initially, 0 after restricted to only those evaluating health outcomes), electronic waste (n = 9), indoor mold (n = 9), flame retardants (n = 8), environmental phenols (n = 4), and phthalates (n = 3). When we further subset the overall results to publications also evaluating genomic susceptibility or G x E interactions, we identified only 23 publications (21 research articles, 2 reviews, and no books). To summarize the publications across exposure categories, we highlight the important health endpoints, diseases, or outcomes evaluated, some specific exposures measured (and when possible, how measured), important at risk or vulnerable populations, and current research/data gaps.
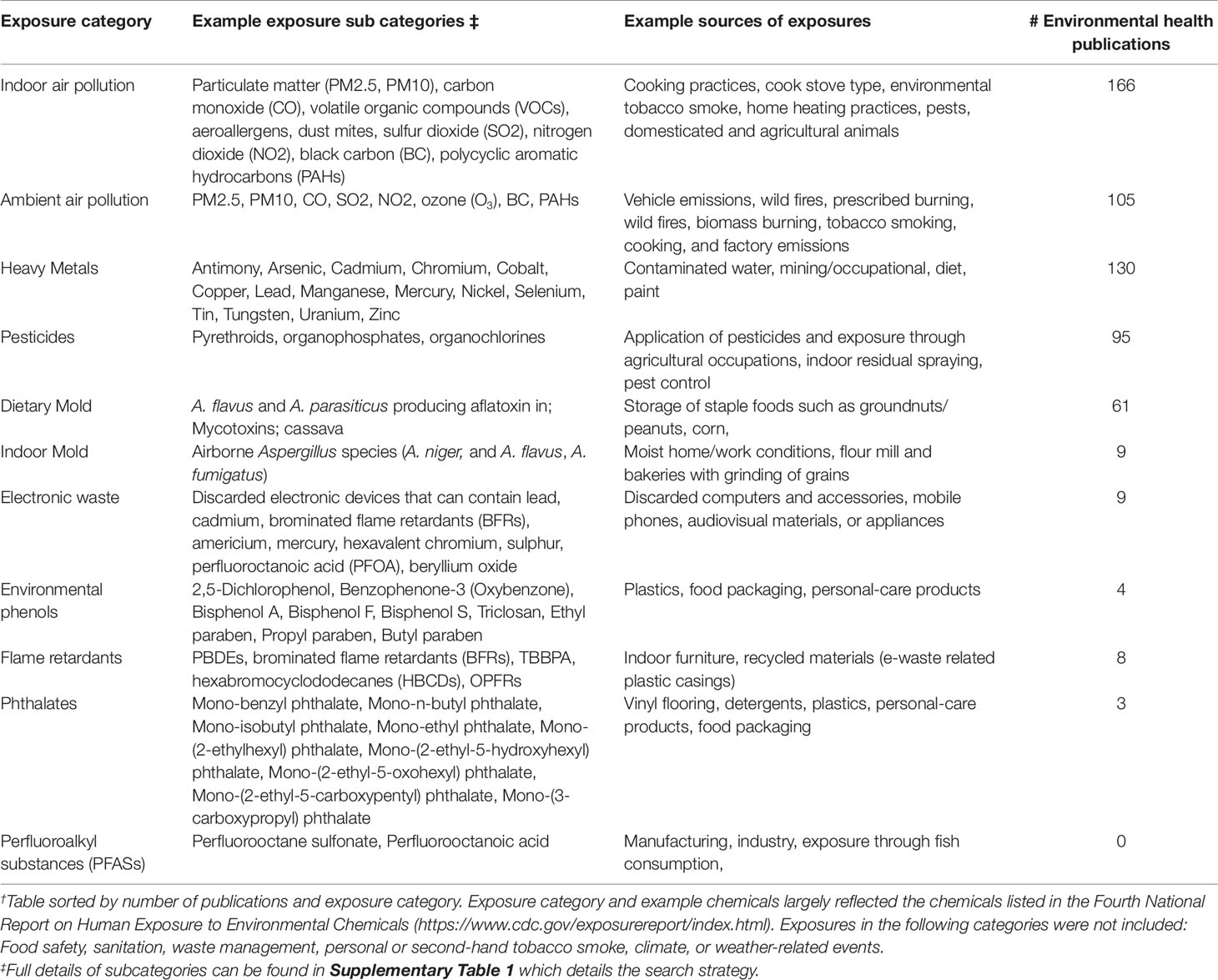
Table 1 Summary of literature search results: Landscape of environmental health research in African populations. †
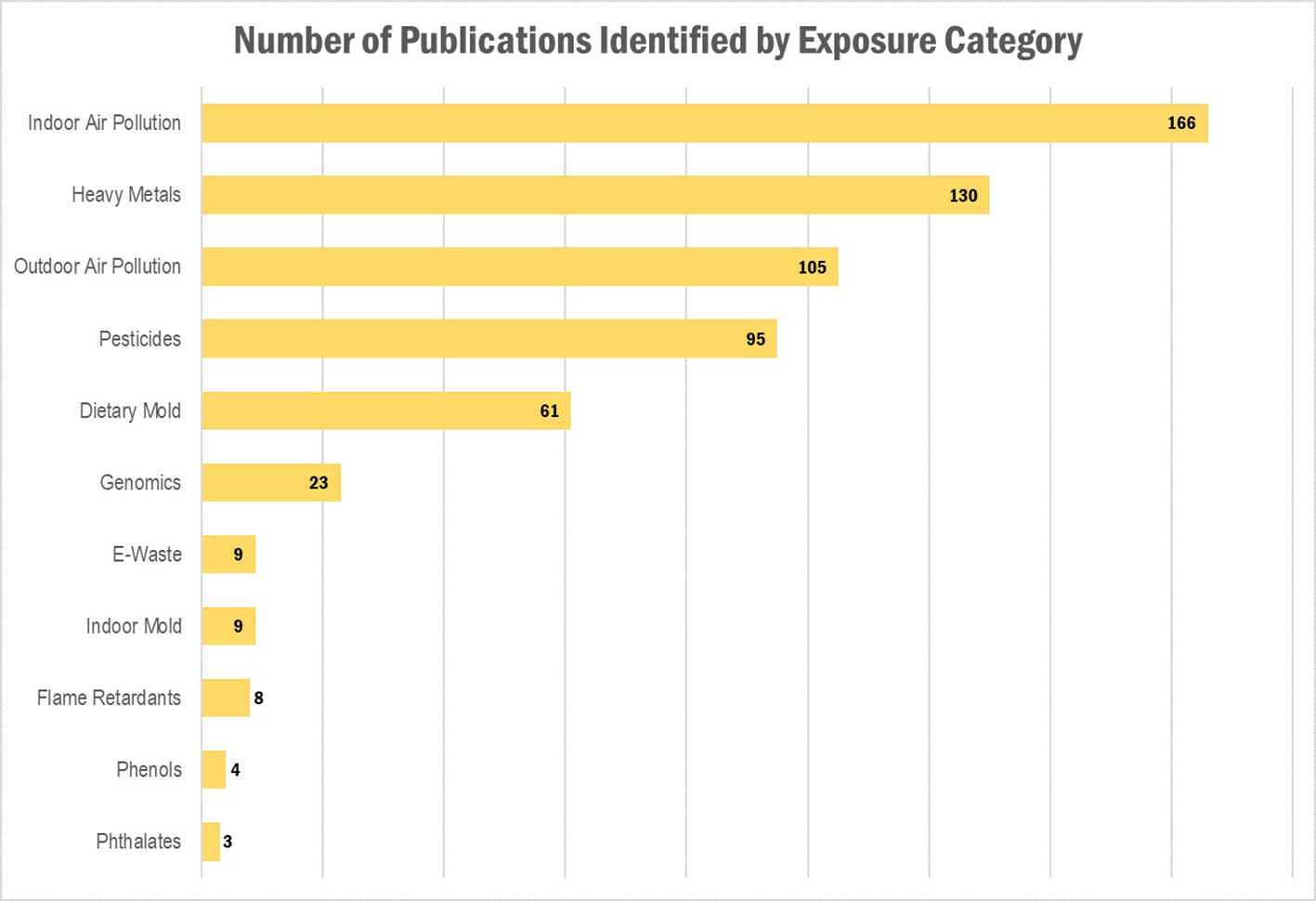
Figure 1 Results of the literature search: Number of publications identified, by exposure category. Publications could belong to more than one category.
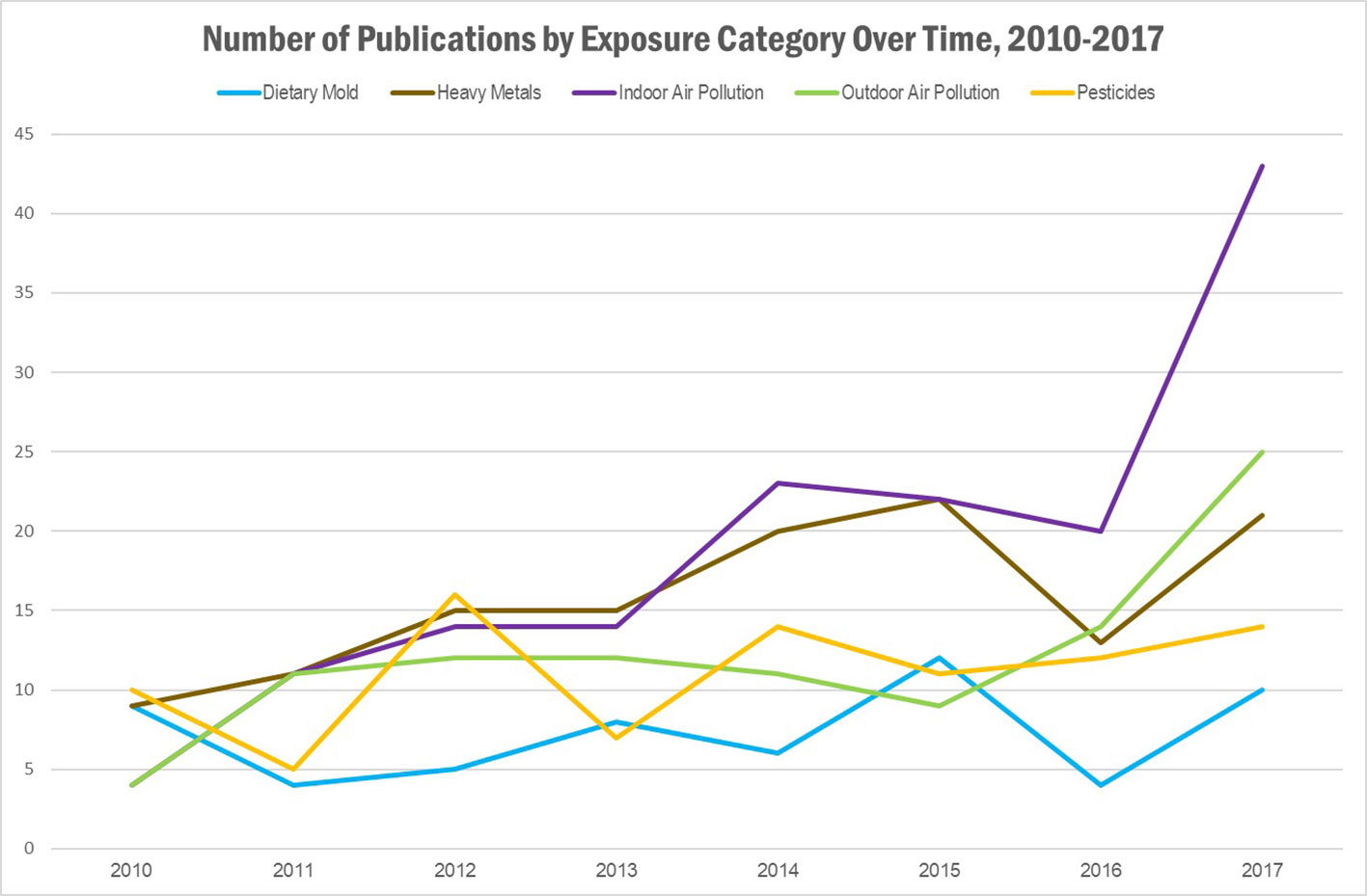
Figure 2 Results of literature search: Number of publications identified, by exposure category and year of publication. Publications could belong to more than one category.
We identified a total of 166 publications describing indoor air pollution and health endpoints across the African continent (Table 1). A 2016 Lancet review of 79 metabolic risk factors in a systematic analysis of the global burden of disease indicated that between 1990 and 2015, global exposure to household air pollution as well as unsafe sanitation, childhood underweight status, childhood stunting, and smoking, each decreased by more than 25% (GBD 2015 Risk Factors Collaborators, 2016). Household air pollution was listed as one of the top ten largest contributors to global disability-adjusted life-years (DALYs), representing 85.6 million (66.7 million to 106.1 million) global DALYs (2016).
Across the indoor air pollution articles identified in our literature review, a critical health outcome noted was cardiovascular disease. Cardiovascular morbidities related to household air pollution have been identified in other countries, such as in China, Bangladesh, and Pakistan, raising ongoing concern for these risks in Africa (Noubiap et al., 2015). Studies specific to African populations identified in our review evaluated the impact of indoor air pollution on cardiovascular endpoints, such as cardiac chamber structure and function (Agarwal et al., 2018), blood pressure (Quinn et al., 2016; Alexander et al., 2017; Quinn et al., 2017; Arku et al., 2018; Swart et al., 2018), and inflammatory biomarkers (Olopade et al., 2017). Five of these articles focused on exposures to cooking or biomass fuel use in the home (Quinn et al., 2016; Alexander et al., 2017; Olopade et al., 2017; Quinn et al., 2017; Arku et al., 2018). Respiratory disease represented another major health outcome impacted by indoor air pollution; evaluated as the primary outcome of interest or a relevant co-morbidity in 77 of the identified indoor air pollution articles. This included articles describing general child respiratory health (Albers et al., 2015), acute lower respiratory tract infections in children (Buchner and Rehfuess, 2015), shortness of breath (Das et al., 2017), and asthma. Asthma and related morbidities were characterized in 37 articles and included outcomes such as asthma diagnosis and severity (Oluwole et al., 2017), asthma control (Kuti et al., 2017), allergen sensitization (Mbatchou Ngahane et al., 2016), and atopy (Morcos et al., 2011). Indoor air pollution-related impairments on innate immunity were also noted in some studies. For example, Rylance et al. (2015) observed an association between household air pollution and inflammatory responses assessed with IL6 and IL8 production and altered phagocytosis in macrophages exposed in vitro to respirable sized particulates.
Most of the studies evaluating indoor air pollution focused on cooking practices including biomass fuel burning in indoor stoves. A total of 24 of the indoor air pollution research articles described exposure to dust. For example, dust was noted as a trigger for allergic rhinitis (Adegbiji et al., 2018) and house dust/dust mite exposure was associated with asthma (Bardei et al., 2016; Flatin et al., 2018). Particulate matter was evaluated in 24 of the indoor air pollution research studies, most focusing on PM10 (Abou-Khadra, 2013; Ibhafidon et al., 2014; Makamure et al., 2016; Jafta et al., 2017; Nkhama et al., 2017; Nkosi et al., 2017; Mentz et al., 2018) and PM2.5 exposures (Oluwole et al., 2013; Chafe et al., 2014; Ibhafidon et al., 2014; Dutta et al., 2017; Lacey et al., 2017; Lin et al., 2017a; Malley et al., 2017; Nkhama et al., 2017; Wylie et al., 2017a; Wylie et al., 2017b; Mentz et al., 2018). Some studies also measured NO, NO2, SO2, CO, and O3 (Jafta et al., 2017; Wylie et al., 2017a). DDT and DDE contamination from indoor residual spraying was found in household undisturbed dust and associated with DDT and DDE metabolites in serum of residents (Gaspar et al., 2015).
Women conducting most of the household cooking and children helping or in proximity of cooking may be most impacted by indoor air pollution, depending on the family household practices.
Although Rylance et al. (2015) described impairments to the immune system with exposure to indoor air, the interaction between this impairment and susceptibility to infections such as HIV or other infections warrants further research. A review by El-Gamal et al. (2017) describes literature on a wide range of aeroallergens across Africa but data on indoor aeroallergens are not included in all regions. The authors note the importance of characterizing genetic susceptibility in the context of immunodeficiencies in Africa, which has not received sufficient research attention.
We identified 105 articles describing health impacts of ambient air pollution in Africa (Table 1). Nine of these represented review papers, covering outcomes such as chronic lung diseases among HIV positive individuals (Attia et al., 2017), children’s health such as pediatric asthma (Wolff et al., 2012; Jassal, 2015), biomarkers of genotoxicity (DeMarini, 2013), reproductive outcomes like preterm birth (Kumar et al., 2017; Malley et al., 2017), and severity of sickle cell disease (Tewari et al., 2015). Articles represented scientific depth and detail across the continent, covering key public health issues. Among all article types, notable endpoints evaluated were cardiovascular and cardiometabolic outcomes (Wichmann and Voyi, 2012; Benaissa et al., 2016), as well as broader burden of disease or life expectancy estimates (Berhane et al., 2016; Mokdad and GBD 2015 Eastern Mediterranean Region Lower Respiratory Infections Collaborators, 2018; Etchie et al., 2018). Some studies reported null findings. For example, an incremental life-time cancer risk was considered low in the context of exposure to PAHs from air pollution among city center residents of Kumasi, Ghana (Bortey-Sam et al., 2015). Additional outcomes evaluated included markers of oxidative stress, inflammatory cytokines, and chemokines (Cachon et al., 2014), chronic bronchitis from occupational exposures to dust (Hinson et al., 2016), elevated prostate specific antigen (PSA) among young men exposed occupationally to quarry pollutants (Ewenighi et al., 2017), chronic respiratory symptoms among limestone factory workers in Zambia (Bwalya et al., 2011), and allergic rhinitis in urban areas (Flatin et al., 2018). Exacerbation of silicosis due to higher doses of particulate matter exposure, impacts of exposure to prenatal air pollution on DNA methylation in the context of HIV status and antiretroviral treatment (Goodrich et al., 2016), asthma and asthma exacerbations, mortality, cerebrovascular outcomes, cardiovascular outcomes, and daily respiratory mortality were also evaluated.
Several studies evaluated both indoor and ambient air pollution exposures and articles covered both the urban and rural settings (Supplementary Table 1). Ambient air pollution exposure in urban areas was noted in 36 publications including a study of air pollution and sleep disorders in children living in Egypt (Abou-Khadra, 2013). Occupational exposures were another important source of ambient air pollution exposure. Activities included limestone processing in Zambia (Bwalya et al., 2011), exposure to desert dust in West Africa [reviewed by de Longueville et al. (2013)], traffic exhaust (DeMarini, 2013), dust and fumes in artisanal mining (Ekosse, 2011), city transit-related air pollution (Elenge et al., 2011; Elenge and De Brouwer, 2011; Ekpenyong et al., 2012), stone quarrying industry exposures including deposition of inhaled aerosol particles at an industrial site in Egypt (Furi et al., 2017), sulfur dioxide (SO2) emissions from platinum group metal (PGM) smelting in Zimbabwe (Gwimbi, 2017), and charcoal processing activities in Namibia, including exposure to charcoal dust (Hamatui et al., 2016). DNA adducts to measure air pollution exposure among urban and suburban residents was also implemented in some studies (Ayi-Fanou et al., 2011).
Across the articles evaluating air pollution exposure, occupationally exposed workers represented a critical population at risk. For example, exposure to pollutants through dust was mentioned in approximately one third of the ambient air pollution studies, half of which evaluated occupational exposures. Another study identified higher DNA adducts related to air pollution among taxi-motorbike drivers, roadside residents, street vendors, and gasoline sellers, compared to suburban and village inhabitants in Benin (Ayi-Fanou et al., 2011). Importantly, the impact of pollutant exposures correlating to occupation are not limited to impacts among workers. People living near work sites may also be affected. For example, Durban, South Africa represents one of Africa's busiest ports and the combination of industry, traffic, and biomass burning has led to substantial air pollution. A study of school children in Durban observed associations between air pollution exposures and respiratory symptoms, with notable burden on children with asthma (Mentz et al., 2018). These studies suggest that the impacts of occupational air pollution exposures are not limited to health endpoints in the workers alone. Immunocompromised individuals such as those living with HIV may also be more likely to experience chronic respiratory symptoms, abnormal spirometry, and chest radiographic abnormalities following air pollution exposures (Attia et al., 2017).
Ambient air pollution exposure has been well characterized as an issue across Africa and around the world. Health impacts comparable to what has been identified in other populations were particularly clear for respiratory outcomes. Given the unique occupational settings in some regions of Africa, very high levels of exposure are of ongoing concern as is the peripheral impact on children and immunocompromised individuals.
Reproductive outcomes have been associated with various high heavy metal exposures in Africa. For example, associations between impaired semen quality and possible infertility has been reported for higher levels of cadmium, lead, zinc, and selenium (Awadalla et al., 2011; Oluboyo et al., 2012; Abarikwu, 2013; Famurewa and Ugwuja, 2017). Elevated serum heavy metals (cadmium and lead) along with a reduction of essential micronutrients (zinc and copper) may also contribute to recurrent pregnancy loss (Ajayi et al., 2012). An association between lower maternal zinc, copper, and cadmium levels as well as cord copper levels with low birthweight newborns has also been observed (Abass et al., 2014; Rollin et al., 2015). Elevated lead and arsenic exposures may be associated with preterm birth and other birth outcomes in general (Kumar et al., 2017; Rollin et al., 2017) and cord blood mercury was significantly associated with birth weight, length, and head and chest circumference in a Nigerian study population (Obi et al., 2015). Several African countries have a high level of preeclampsia and significant associations between preeclampsia and serum levels of calcium and magnesium or excretion of high amounts of several toxic metals, especially lead, have been identified (Ikechukwu et al., 2012; Motawei et al., 2013; Elongi Moyene et al., 2016). Egypt has one of the highest incidences of intrauterine growth retardation, and this appears to be positively correlated with heavy metal toxicity (El-Baz et al., 2015).
Lead toxicity (sometimes in combination with high cadmium exposures) has been shown to be associated with renal function impairment (Alasia et al., 2010b). Occupationally lead-exposed subjects have been shown to have significantly higher blood lead levels, as well as serum urea, creatinine, and serum uric acid levels, and other renal biomarkers and markers of nephrotoxicity. Multiple studies suggest a higher risk for developing hyperuricemia and renal impairment with high lead exposure (Alasia et al., 2010a; Cabral et al., 2012; Cabral et al., 2015). Workers in a variety of occupations, including automobile technicians, e-waste workers, miners, and shooting-range workers are at risk for substantially high lead levels (Saliu et al., 2015; Obiri et al., 2016b; Mathee et al., 2017). Blood lead levels in school children have been associated with a variety of behavioral and cognitive outcomes, including: lower IQ, poorer school performance, anti-social or violent tendencies, hearing deficiencies, and delayed onset of puberty (Naicker et al., 2010; Tomoum et al., 2010; Abdel Rasoul et al., 2012; Naicker et al., 2012; Kashala-Abotnes et al., 2016; AbuShady et al., 2017; Nkomo et al., 2017).
A high prevalence of acute lead poisoning in children has been an ongoing issue in many African countries (Bouftini et al., 2015; Bose-O’Reilly et al., 2018), with the lead poisoning crisis in Zamfara State, Northern Nigeria noted as one of the worst such cases in modern history. More than 400 children have died in Zamfara as a result of ongoing lead intoxication since early in 2010, and this acute lead poisoning is believed to be related to artisanal gold mining (Moszynski, 2010; Dooyema et al., 2012; Bartrem et al., 2014). Younger children with high venous blood lead level thresholds during the first year of the Zamfara outbreak response displayed a variety of neurological outcomes and were at higher risk for encephalopathy (Greig et al., 2014). Another recent lead poisoning outbreak reportedly occurred from consumption of an ayurvedic medicine in South Africa (Mathee et al., 2015).
A variety of cancers have also been associated with heavy metal exposure (Fasinu and Orisakwe, 2013; Obiri et al., 2016a; Obiri et al., 2016b). Low levels of selenium was associated with the development of breast cancer (Alatise et al., 2013), as was higher levels of lead for infiltrating ductal breast carcinoma (Alatise and Schrauzer, 2010). Cadmium and arsenic were found to be synergistically associated with bladder cancer and both exposures are often also associated with smoking status (Feki-Tounsi et al., 2013a; Feki-Tounsi et al., 2013b; Feki-Tounsi et al., 2014). A higher serum selenium concentration and a deficiency of zinc and molybdenum was found to be associated with esophageal squamous dysplasia (Ray et al., 2012; Pritchett et al., 2017). A positive association between cadmium exposure and pediatric cancer may also be present (Sherief et al., 2015). High levels of some heavy metals (chromium, nickel, cadmium) were associated with head and neck cancer as well (Khlifi et al., 2013a; Khlifi et al., 2013b).
Many studies reported neurological outcomes associated with occupational exposure to mercury. Prominent symptoms among fluorescent lamp factory workers exposed to mercury included tremors, emotional lability, memory changes, neuromuscular changes, and performance deficits in tests of cognitive function (Al-Batanony et al., 2013). Neurological symptoms, memory disturbances, and anxiety and depression were found in dentists exposed to mercury. Bilateral and symmetric intentional tremor in both upper limbs were found in dentists exposed to particularly high levels of mercury (Chaari et al., 2015). Chronic mercury intoxication, with tremor, ataxia and other neurological symptoms, along with kidney dysfunction and immunotoxicity, have been identified in individuals with high body burdens of mercury living in or near artisanal small-scale mining communities. Exposed groups showed poorer results in different neuropsychological tests. Over half of amalgam burners (workers with highest mercury levels as a group) were found to have symptoms of mercury intoxication (Bose-O’Reilly et al., 2017), and a large proportion of small-scale gold miners have mercury exposures above occupational exposure limits (Tomicic et al., 2011; Gibb and O’Leary, 2014; Steckling et al., 2014; Mensah et al., 2016).
The early effects of methylmercury due to fish consumption and other possible sources of exposure have also been extensively studied. Some negative outcomes associated with growth and nervous system effects on fetuses and newborns, cognitive function, reproduction, and longer-lasting cardiovascular effects as adults have been observed (Karagas et al., 2012; Gonzalez-Estecha et al., 2014). However, other nutrients, particularly n-3 polyunsaturated fatty acids (PUFAs) in fish, may modify some of these health effects (Lynch et al., 2011; Gribble et al., 2015; Strain et al., 2015). For example, although an adverse association of educational measures with postnatal mercury exposure in males but not females was found in one study from the Seychelles Child Development Study (Davidson et al., 2010), a number of other studies from this cohort have found no significant associations between methyl mercury exposure (either through fish consumption or prenatal exposure to dental amalgams) and neurodevelopmental outcomes (Watson et al., 2011; Watson et al., 2012; Watson et al., 2013; van Wijngaarden et al., 2013; van Wijngaarden et al., 2017).
A limited number of other studies have assessed various heavy metals and trace elements in relation to health outcomes. Alterations of some essential trace metals may play a role in the development of diabetes mellitus and obesity in children and older adults (El Husseiny et al., 2011; Harani et al., 2012; Azab et al., 2014; Badran et al., 2016). Arsenic and lead appear to impact diabetes and cardiovascular outcomes but have been studied very little in the African context (Ettinger et al., 2014). Exposure to arsenic was significantly associated with increased odds of asthma and tachycardia in one report (Bortey-Sam et al., 2018). Neurocognitive and motor impairments observed in konzo, a motor neuron disease associated with cassava cyanogenic exposure in nutritionally challenged African children, may possibly be driven by the combined effects of cyanide toxicity and selenium deficiency (Bumoko et al., 2015). Selenium and a number of other trace elements may also influence goiter development and general thyroid metabolism (Kishosha et al., 2011; Maouche et al., 2015; El-Fadeli et al., 2016; Gashu et al., 2016). Liver function may be compromised in nickel-plating workers (El-Shafei, 2011). Chronic neuropathology appears to be associated with chronic manganese exposure in South African mine workers (Gonzalez-Cuyar et al., 2014). Some trace metals may also play a role in the development of anemia (Henriquez-Hernandez et al., 2017). Low serum zinc levels were associated with acute lower respiratory infections (Ibraheem et al., 2014). Elevated blood lead levels seem to be associated with increased asthma severity (Mohammed et al., 2015). Selenium deficiency may be a risk factor for peripartum cardiomyopathy as well as other vascular complications and the impact of this may vary based on race (Karaye et al., 2015; Swart et al., 2018). An association of some metals with the risk of nasosinusal polyposis disease were observed for some genetic variants involved in DNA repair pathways affecting susceptibility (Khlifi et al., 2015; Khlifi et al., 2017). High concentrations of some harmful elements in geophagic clays eaten in Africa may be associated with cardiovascular outcomes (Olatunji et al., 2014). Mineral imbalances and lead exposure may also be associated with elevated blood pressure (Rebacz-Maron et al., 2013; Were et al., 2014). Disturbances in copper have been implicated in one study of Parkinson’s disease as well (Younes-Mhenni et al., 2013).
A connection between autism and various metals has also been studied. Altered urinary porphyrins, biomarkers of mercury toxicity, were observed in Egyptian children with autism spectrum disorder (Khaled et al., 2016). Levels of mercury, lead, and aluminum in hair of autistic patients was significantly higher than controls in one study (Mohamed Fel et al., 2015). High exposures of some heavy metals, particularly lead and mercury, have been treated with chelating agents, which appeared to improve autistic symptoms (Yassa, 2014).
Mercury was sometimes determined by using a direct mercury analyzer, while most heavy metals were measured by atomic absorption spectrophotometer in blood and serum (and sometimes hair, nails, and air/soil samples) (Ojo et al., 2014; Were et al., 2014; Sherief et al., 2015; Iwegbue et al., 2017). The quantification of metals in various tissues was also assessed by atomic absorption spectroscopy (Feki-Tounsi et al., 2014). A variety of biomarkers were incorporated into various studies, especially to monitor kidney injury or dysfunction (Samir and Aref, 2011; Cabral et al., 2012; Cabral et al., 2015). Some heavy metals’ association with lipid peroxidation, DNA damage, oxidative stress, or apoptosis was assessed (El-Baz et al., 2015; Bortey-Sam et al., 2018) and the genotoxic impact of some occupational exposures was explored (El Shanawany et al., 2017).
A variety of occupations clearly pose high risks for substantial exposure to heavy metals. Industrial metals are presently contaminating the environment and the water supplies, and the lack of education of workers and personal protective equipment was reported (Alatise and Schrauzer, 2010; Mensah et al., 2016). Individuals living near landfills and e-waste sites, particularly children, are at risk for a variety of exposures as e-waste components/constituents with heavy metal contamination can accumulate, in soil and surrounding vegetation, to toxic and genotoxic levels that could induce adverse health effects in exposed individuals (Alabi et al., 2012; Cabral et al., 2012). The outbreaks related to the fatal childhood lead poisoning illustrate the extreme vulnerability for young children (Dooyema et al., 2012; Bartrem et al., 2014). Other studies demonstrated the more subtle health outcomes related to lead exposures and suggest that even in the absence of overt clinical manifestations of lead toxicity, knowledge of lead exposure may influence the diagnosis in children presenting with anemia, intellectual impairment, poor academic performance, hearing impairments, and other outcomes (Abdel Rasoul et al., 2012).
There are numerous studies suggesting evidence for a variety of interactions among multiple heavy metals and trace elements, and the impact of these interactions on health outcomes. The interaction between lead and selenium is one of many interesting interactions associated with some cancers as lead may abolish the natural inhibitory effect on carcinogenesis observed for selenium (Alatise and Schrauzer, 2010). A synergistic interaction between cadmium and arsenic is also associated with bladder cancer (Alatise and Schrauzer, 2010; Feki-Tounsi et al., 2013a; Feki-Tounsi et al., 2014). There was evidence that obese children may be at a greater risk of developing an imbalance (mainly deficiency) of trace elements, which may be playing an important role in the pathogenesis of obesity and related metabolic risk factors (Azab et al., 2014). The mechanistic interactions of many heavy metals and trace elements, and the impact of these complex co-exposures for a variety of health outcomes is a substantial research gap in our current understanding.
The lead poisoning in Zamfara is an extreme example of both lead and multiple heavy metal mortality and morbidity, but highlights the importance of environmental remediation, chelation therapy, public health education, and control of mining activities to prevent future outbreaks (Dooyema et al., 2012; Bartrem et al., 2014). Furthermore, the primary source of lead pollution responsible for the lead poisoning of children in Nigeria appeared not to come from official mining activities but mainly from small scale operations conducted by local villagers, suggesting that some governmental regulation may be warranted (Moszynski, 2010). The oral chelating agent 2,3-dimercaptosuccinic acid (DMSA, succimer) appeared to be pharmacodynamically effective for the treatment of severe childhood lead poisoning in a resource-limited setting (Thurtle et al., 2014); in a number of situations, blood lead level monitoring has been used to show lower lead levels in children following implementation of such interventions (Brown et al., 2010; Bouftini et al., 2015).
The relationship between many metals and antioxidant enzymes and the role of the oxidative stress and inflammation pathways needs to be further explored (Maouche et al., 2015). Molecular mechanisms of how oxidative stress acts as a driver for organ dysfunction and the impact of antioxidants to mediate the potential toxic effect of various metal exposures will be important research areas to continue to explore (Samir and Aref, 2011). As one example, strategies to prevent konzo have successfully included dietary supplementation with trace elements, preferentially those with antioxidant and cyanide-scavenging properties (Bumoko et al., 2015).
The relationship between heavy metals and many disease outcomes are in preliminary stages in African studies and elsewhere. Other associations between heavy metals and some diseases have been established in predominantly European populations but have not been extensively studied in the African context. The association of metals with autism, respiratory disease, and other health outcomes have been inconsistent and will require additional exploration. The impact of other nutrients in fish modifying methylmercury neurotoxicity is also an ongoing source of investigation (Lynch et al., 2011).
Pesticides, particularly the insecticide DDT and its breakdown product dichlorodiphenyl trichloroethylene (DDE) and other endocrine disrupting compounds, have been associated with numerous reproductive outcomes including male infertility, impaired semen quality, increased sperm defects, anogenital distance, mean penile length in baby boys, various urogenital malformations, and spontaneous miscarriages and infant deaths (Lubick, 2010; Naidoo et al., 2010; El-Helaly et al., 2011; English et al., 2012; Abarikwu, 2013; El Kholy et al., 2013; Bornman et al., 2017). One recent paper suggested decreased ovarian reserve associated with exposure to pyrethroid pesticides (Whitworth et al., 2015). Emerging evidence suggests that many endocrine-disrupting pesticides have effects on cardiometabolic outcomes (Azandjeme et al., 2013). For example, DDT concentration has been consistently and positively associated with body composition and body weight in young girls, and DDT and DDE were found to be associated with elevated risk of hypertensive disorders in pregnancy (Coker et al., 2018; Murray et al., 2018), while chronic exposure of non-diabetic farmers to organophosphorus malathion pesticides appears to induce insulin resistance (Raafat et al., 2012). One study examined a variety of biochemical effects of pesticides including hematological profile, lipid parameters, serum markers of nephrotoxicity and hepatotoxicity, as well as the activities of butyryl cholinesterase (BChE), acetylcholinesterase (AChE), and thiolactonase-paroxonase (PON). The study concluded that long-term exposure to pesticides may play an important role in the development of vascular diseases via metabolic disorders of lipoproteins, lipid peroxidation and oxidative stress, inhibition of BChE, and decrease in thiolactonase-PON levels (Wafa et al., 2013).
Neurological outcomes were the most commonly associated health outcomes reported for cumulative exposure to both organophosphorus and pyrethroid compounds. Pesticide applicators and farm workers (including adolescent and child workers) exposed to these compounds exhibit neurological/neurobehavioral symptoms, deficits in neurobehavior performance tests, and neuromuscular disorders. These symptoms are often associated with greater inhibition of serum BChE and acetylcholinesterase activity, effect biomarkers often associated with neurotoxicity and cumulative TCPy, which is a biomarker of the organophosphorus pesticide chlorpyrifos (Sosan et al., 2010; Khan et al., 2014; Rohlman et al., 2014; Singleton et al., 2015; Manyilizu et al., 2016; Rohlman et al., 2016; Ismail et al., 2017b; Negatu et al., 2018). Some evidence for possible neurodevelopmental effects related to DDT in children has also been suggested (Osunkentan and Evans, 2015). Some associations were found between pesticide exposure and increased risks to various cancer outcomes, including bladder cancer, breast cancer, colorectal cancer, non-Hodgkin’s lymphoma, and hepatocellular carcinoma (Lo et al., 2010; Awadelkarim et al., 2012; Amr et al., 2015; Arrebola et al., 2015; VoPham et al., 2017). Respiratory outcomes were also commonly associated with both cumulative and acute pesticide exposure, including associations with idiopathic pulmonary fibrosis, decreased lung function/increased wheeze, lower airway inflammation, chronic cough, and asthma (Awadalla et al., 2012; Callahan et al., 2014; Ndlovu et al., 2014; Okonya and Kroschel, 2015; Mamane et al., 2016; Quansah et al., 2016; Sankoh et al., 2016). Interestingly, a novel Hirmi Valley liver disease was identified in recent decades in Ethiopa, which may be partially caused by co-exposure of acetyllycopssamine and DDT (Robinson et al., 2014). Perhaps most striking is the substantial literature on acute pesticide poisoning, both accidental and intentional, with adolescents’ intent on suicide (generally with the use of organophosphorus compounds and carbamates) contributing to an alarming increase in recent years (Balme et al., 2012; Azab et al., 2016; da Silva et al., 2016). In one study looking at acute pesticide poisoning in Kampala hospitals, 63% of cases of acute pesticide poisoning were intentional (Ssemugabo et al., 2017). The most common symptoms associated with accidental acute pesticide poisoning included skin and eye irritation, headaches, vomiting, nausea, chest pain respiratory disorders, and blurred vision (Karunamoorthi et al., 2012; Okonya and Kroschel, 2015; da Silva et al., 2016; Sankoh et al., 2016; Manyilizu et al., 2017; Ssemugabo et al., 2017).
Many of the reviewed studies evaluated chronic pesticide exposure and alteration in serum enzymes associated with detoxification of pesticides, particularly inhibition of butyryl cholinesterase activity (Araoud et al., 2010; Araoud et al., 2011; Araoud et al., 2012). Biomarkers of exposures to the organophosphorus pesticides, chlorpyrifos (CPF) and Profenofos (PFF), were evaluated in some studies by measuring urinary levels of 3,5,6-trichloro-2-pyridinol (TCPy), a specific CPF metabolite and 4-bromo-2-chlorophenol (BCP), a specific PFF metabolite (Singleton et al., 2015). Inhibition of blood butyryl cholinesterase (BChE) and acetylcholinesterase (AChE) activities are effect biomarkers that were also evaluated in several of the reviewed studies (Ismail et al., 2010; Khan et al., 2014; Rohlman et al., 2014; Singleton et al., 2015; Rohlman et al., 2016; Ismail et al., 2017a; Ismail et al., 2017b). DDE/DDT was often assayed using ELISA (Bimenya et al., 2010).
The in utero and early childhood effects of various pesticides and impact on long-term health highlights early life as a key susceptible time window for pesticide exposure. Adolescents working seasonally or during certain periods on farms may have a higher risk of neurotoxic effects of pesticide exposure because of their rapidly developing brains and bodies (Ismail et al., 2010; Ismail et al., 2017a; Ismail et al., 2017b). Because of the high morbidity and mortality associated with childhood and adolescent poisoning with pesticides (sometimes intentional), targeted prevention initiatives should be a high priority (Balme et al., 2010; Balme et al., 2012).
The health effects of many pesticides have not been as extensively studied in African countries and may have different etiologies and patterns of exposure compared to other parts of the world. For example, the Sudan is experiencing a rapidly increasing cancer incidence, but little is known on tumor subtypes, epidemiology, or genetic or environmental cancer risk factors there or in other African countries (Awadelkarim et al., 2012).
Many of the reported agricultural pesticide studies in Africa were limited by exposure assessment methods (with many relying heavily on questionnaires alone to assess pesticide exposure and health risks). Future research could focus on improved pesticide exposure assessment methods, potentially incorporating multiple approaches and longitudinal studies to incorporate seasonal effects (VoPham et al., 2017). However, many opportunities exist now for comprehensive interventions to reduce both exposure and health risks associated with pesticide applications for both acute and cumulative exposures. 93% of farmers in rural Tanzania reported past lifetime pesticide poisoning (Lekei et al., 2014). Several reports have demonstrated acute pesticide poisoning to be associated with behaviors including lack of protective clothing, poor pesticide handling, not washing vegetables before eating, nozzle sucking, etc. (Magauzi et al., 2011; Oesterlund et al., 2014; Mekonen et al., 2015; da Silva et al., 2016; Sankoh et al., 2016; Manyilizu et al., 2017). One study from Sierra Leone reported most farmers having no knowledge about the safe handling of pesticides as 71% of them have never received any form of safety training (Sankoh et al., 2016). Comprehensive training and use of protective safety gear and clothing and safe handling practices may substantially reduce agricultural farmers’ health risks. In addition, given that chronic exposure to pesticides appears to affect several biochemical parameters, biomonitoring of effects in agricultural workers might be a useful way to assess the individual risk of handling pesticides. For example, BChE activity appears to be a useful indicator to monitor workers chronically exposed to pesticides as it is indicative of adverse effects of pesticides in agricultural workers and might detect the effects of pesticides before adverse clinical health effects occur (Araoud et al., 2011).
Important data are still needed to help policy makers perform risk-benefit analyses of the use of DDT and other pesticides in areas of Africa most heavily impacted by malaria (Thompson et al., 2018). A variety of indoor residual spraying of insecticides is associated with substantial decreased risk of developing malaria (Kigozi et al., 2012; Loha et al., 2012), and a recent study in South Africa reported DDT most effective for malaria control while acknowledging the detrimental health effects. Alternative prevention methods for controlling malaria are needed as well as more studies illustrating the long-term impacts of DDT on health (Hlongwana et al., 2013).
Mycotoxins, particularly aflatoxin and fumonisins, are natural toxins that many people in Africa are exposed to because they contaminate the staple diet of groundnuts, maize, and other cereals (Darwish et al., 2014). Aflatoxin in particular (which is produced by the fungi Aspergillus flavus and Aspergillus parasiticus) (Afum et al., 2016) is established as a cause of cirrhosis and human liver cancer (hepatocellular carcinoma-HCC) and growth faltering (perhaps due to micronutrient deficiencies) in young children (Obuseh et al., 2011; Bosetti et al., 2014; Shirima et al., 2015; Smith et al., 2015; Wirth et al., 2017). Adverse birth outcomes and anemia in pregnant women and acute aflatoxin poisoning in Africa are also concerns (Kimanya et al., 2010; Shuaib et al., 2010a; Shuaib et al., 2010b; Wild and Gong, 2010; Khlangwiset et al., 2011; Hoffmann et al., 2015). Several reports have investigated possible impaired semen quality (infertility) in men associated with aflatoxin (Abarikwu, 2013; Eze and Okonofua, 2015). There is potential association of zearalenone (a non-steroidal estrogenic mycotoxin) with breast cancer risk (Belhassen et al., 2015). Ergotism has been associated with several species of Claviceps that are in rye and other cereal grains (Belser-Ehrlich et al., 2013). Fumonisin B (1) is a mycotoxin produced by Fusarium spp. molds and it has been linked with primary liver cancer and esophageal cancer (Domijan, 2012). Fumonisins have also been associated with neural tube defects (Wild and Gong, 2010). Aflatoxin and other mycotoxins have been linked to possible neurotoxicological outcomes as well as chronic hepatomegaly (Gong et al., 2012). Ochratoxin A, a mycotoxin produced by several Aspergillus and Penicillium species, is associated with chronic interstitial nephropathy (Hmaissia Khlifa et al., 2012; Gil-Serna et al., 2018). Contaminated peanuts have been associated recently with growth faltering (Mupunga et al., 2017). Wheat handlers exposed to A. flavus may have elevated risks of liver cancer as well (Saad-Hussein et al., 2014). HIV positive and HBV/HCV positive individuals exposed to aflatoxin are at substantially increased disease risks due to the established synergistic action of aflatoxin with HIV and HBV/HCB infection (Kew, 2013).
Aflatoxin has been established as a potent liver carcinogen working through a genotoxic mechanism involving metabolic activation to an epoxide, formation of DNA adducts and, in humans, modification of the p53 gene. Extensive mechanistic research combined with molecular epidemiology has allowed quantitative risk assessment for aflatoxin to be measured. Molecular biomarkers to quantify aflatoxin exposure in individuals were essential to link aflatoxin exposure with liver cancer risk. Biomarkers were validated in populations with high HCC incidence including the Gambia, West Africa region (Wogan et al., 2012). Aflatoxin metabolite AFM(1) and other mycotoxin metabolites have been measured in breast milk (Adejumo et al., 2013) while aflatoxin-albumin (AF-alb) and AFB1-lysine have typically been measured in blood plasma or serum through a variety of methods (See Table 2) (Mitchell et al., 2017; McMillan et al., 2018). Correlations between urinary aflatoxin M1 (AFM1) and aflatoxin albumin adduct (Af-alb) have been established and suggest that urinary AFM1 is a good biomarker of aflatoxin. AFM1 appears to measure shorter-term exposure to aflatoxin whereas AF-alb measures longer term exposure (Chen et al., 2018). Serum levels of ochratoxin A might also serve as a useful biomarker of HCC risk (Matsuda et al., 2013).
Growth faltering makes young children particularly vulnerable to mycotoxins as fetal and early postnatal growth and development appear to be affected and because aflatoxin is known to cross the placental barrier (Castelino et al., 2014; Castelino et al., 2015). Interventions should focus on reducing mold exposures during critical periods of fetal and infant development, particularly for nursing infants having possible contaminated milk (Adejumo et al., 2013). HIV positive and HBV/HCV positive individuals are also at risk populations for the health effects related to aflatoxin exposure (Kew, 2013). Agricultural workers and rural populations, particularly subsistence farming communities, are important at risk populations as well.
Mycotoxin risk management has been successful in West Africa and other African countries, and this has substantially reduced disease attributable to aflatoxin (Liu et al., 2012; McGlynn et al., 2015). Many intervention/prevention efforts (including post-harvest storage measures) are now underway to reduce exposure to highly toxic and carcinogenic contaminants in staple diets in Africa, especially aflatoxin and fumonisins, which people are exposed to daily through grain and cereal staples in their diet. Aflatoxin biomarkers have also been used to show that primary prevention to reduce aflatoxin exposure can be achieved by low-technology approaches at the subsistence farm level in sub-Saharan Africa (Wogan et al., 2012). Daily urinary AFM1 levels have been shown to be useful as a biomarker of internal aflatoxin B1 exposure in short-term intervention trials to determine efficacy of interventions (Mitchell et al., 2013). Further application of knowledge to practice is currently underway with numerous intervention/prevention studies, clinical trials, and education (Wild and Gong, 2010; Hoffmann et al., 2015; Saleh et al., 2015). The comprehensive approach used to create many successful preventive interventions to reduce health risks associated with aflatoxin is a model for the development, validation, and application of biomarkers for other environmental exposures (Wogan et al., 2012).
There is evidence that maternal exposure to aflatoxin during the early stages of pregnancy is associated with differential DNA methylation patterns of infants, including in genes related to growth and immune function but how mycotoxin exposure in embryonic and fetal development may influence later disease risk needs to be explored (Hernandez-Vargas et al., 2015). The association between aflatoxin exposure and alteration in immune responses observed in humans suggest that aflatoxin could suppress the immune system and work synergistically with HIV to increase disease severity and progression to AIDS, but in general, the neurotoxicological and immunological/immunodepression aspects are not well understood (Jolly et al., 2015). While studies have shown synergism between aflatoxin and HBV in causing HCC, much less is known about whether aflatoxin and HCV synergize similarly (Palliyaguru and Wu, 2013). The relationship between HIV transmission frequency and fumonisin contamination also needs to be explored (Williams et al., 2010). Childhood immunizations for hepatitis B in many West African countries is still lagging behind many other countries, and this vaccination alone could substantially impact health risks (Ladep et al., 2014). Some findings of significant decrease in vitamin A associated with AF-ALB suggest that aflatoxin exposure compromises the micronutrient status of people who are immunocompromised, including people living with HIV (Obuseh et al., 2011). The interaction between aflatoxin and micronutrient deficiencies warrants more investigation (Watson et al., 2016; Watson et al., 2017).
Indoor fungal-related outbreaks were measured and found to be associated with mucormycosis, endophthalmitis, aspergillosis, as well as asthma exacerbation and other infections in a variety of Sub-Saharan African samples (Gharamah et al., 2012; El-Mahallawy et al., 2016).
Indoor mold was primarily measured as fungal spores present in airborne samples and measured in nasal swabs and sputum samples (Niare-Doumbo et al., 2014; Diongue et al., 2015) (Table 2).
At risk-populations that were examined included pediatric wards with leukemia patients and other immunocompromised or allergic patients, oncology wards, and ophthalmology operating rooms (Gharamah et al., 2012; Niare-Doumbo et al., 2014; Gheith et al., 2015). Occupational exposure to aflatoxin was found in textile workers and was associated with liver tumor biomarkers (Saad-Hussein et al., 2013). Airborne Aspergillus was associated with higher serum aflatoxin B1 and several liver enzymes among workers handling wheat flour (Saad-Hussein et al., 2016) as well, suggesting workers for several occupations may be at increased risk for indoor mold exposures.
Different sensitization rates have been observed in different classes of patients. Highest indoor mold counts in many studies were often associated with the rainy season but more research exploring sensitization rates and seasonal variations is needed (Hasnain et al., 2012). Protective gear and safety measures to reduce exposure for some occupations are needed.
The literature describing PFAS-related health outcomes in Africans was extremely limited. Although our review did not find research articles evaluating PFAS and health outcomes in African populations, there has been increasing attention to PFAS exposure, including studies measuring PFAS in non-humans [e.g. crocodiles, fish (Ahrens et al., 2016)]. One study evaluated PFAS in maternal serum and cord blood in South Africa (Hanssen et al., 2010) but did not evaluate specific health endpoints in the study population where PFAS was measured.
Ahrens et al. (2016) described a risk assessment strategy for evaluating potential human health outcomes related to the PFAS levels in different compartments of Ethiopia’s largest lake, Lake Tana. Their findings do not indicate any elevated health risks, but the authors note the potential for harmful effects with increasing levels over time.
Across the reviewed studies, perfluoroalkyl acids (PFAAs) were measured in water, sediment, and fish in Lake Tana, Ethiopia (Ahrens et al., 2016), in tilapia in South Africa (Bangma et al., 2017), and in wastewater and sludge from selected wastewater treatment plants in Kenya (Chirikona et al., 2015). Another study measured PFCs in maternal serum and cord blood of South African women-infant pairs. They did not report specific health outcomes but did note that the median maternal PFOS concentration was lower than has been reported in other studies, whereas the PFOA concentration was the same. The authors suggested that different exposure pathways (and sources) exist in this population compared to western-style study populations (Hanssen et al., 2010).
Individuals with high fish consumption (e.g. living near the lake, depending on the lake for food or occupation, etc.) are at higher risk of these exposures. Although the results in our review did not evaluate specific health outcomes, PFOS levels were reportedly increasing between 1978 and 2001 in a study population in Southern Sweden that included women from countries of origin within and outside of Sweden, including Africa. This study observed higher levels in women with Sweden as the country of origin, compared to women from the Middle East, North Africa, and sub-Saharan Africa (Ode et al., 2013). Ode et al. report that PFOS levels increased over time, whereas PFOA and PFNA levels were unchanged between 1978 and 2001 in their study population.
More research incorporating exposures and health endpoints measured in the same study population in Africa are needed. This gap may reflect potentially lower levels in African populations compared to U.S. and European populations where PFAS health studies have focused. However, as industrialization, urbanization, and globalization contribute to growing ubiquity of many environmental chemical exposures, we anticipate PFAS exposures may increase in African populations.
A variety of crude recycling operations in developing nations, including Africa, have been reported to lead to multiple health risks. In many cases, e-waste workers are exposed to highly contaminated fumes due to burning practices (Akormedi et al., 2013). Self-reported hearing difficulties and stress associated with potential cardiovascular disease symptoms (including elevated blood pressure levels) have been reported in electronic waste recycling workers (Were et al., 2014; Burns et al., 2016). Workers burning e-waste products have been reported as having very high blood lead levels and noise exposures often exceed recommended occupational and community noise exposure limits (Burns et al., 2016). Workers have reported moderate to prominent levels of perceived stress as measured via Cohen’s Perceived Stress Scale (Burns et al., 2016). Higher levels of a few chemicals related to e-waste recycling have also been associated with increased cancer risks (Obiri et al., 2016a).
Across the e-waste studies reviewed, levels of polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) were typically analyzed using gas chromatography/spectrophotometry. Heavy metals were measured using atomic absorption spectrophotometry, and DNA damage was assayed in human peripheral blood lymphocytes using an alkaline comet assay in soil and plant samples (Alabi et al., 2012). Lead, cadmium, chromium, copper, arsenic, tin, zinc, and cobalt via oral and dermal contact in bottom ash and soil were measured using random sampling techniques and analyzed using standard methods for chemical analysis prescribed by the American Water Works Association (Obiri et al., 2016a) (Table 2).
In general, e-waste workers in many African countries are a vulnerable at-risk population that may have a limited social safety net or legal protections. The chemical exposures reported in e-waste studies are relevant not just to e-waste workers but also to traders and residents, including children living in neighboring areas.
The exposures related to e-waste recycling is an understudied area but limited reported studies suggest clear health risks associated with this activity. Cleaner technologies and protective gear for workers as well as education efforts are needed. Several reports recognized the complicated e-waste infrastructure system in some African countries and the need to understand all stakeholders involved (Amankwaa et al., 2017). One review suggested approaching the e-waste crisis in sub-Saharan Africa with an ongoing health impact assessment that would address the health, environmental, and social aspects of the issue and where all the steps of the assessment are performed with input from local communities (Tetteh and Lengel, 2017).
Several recent African studies have quantified concentrations of a variety of flame retardants and attempted to associate exposure levels with different health outcomes. Elevated levels of concentrations of polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and some organochlorine pesticides (OCPs) were not found in colorectal cancer patients in Egypt, compared to controls (Abou-Elwafa Abdallah et al., 2017). Potential health concerns related to estimated lifetime cancer risk and other risks were suggested for levels of some organochlorine pesticides observed in soil samples (Sun et al., 2016), as well as DDT and PCBs from dietary fish exposure in one study (Ben Ameur et al., 2013). However, other studies did not show levels of flame retardants exceeding safety guidelines from dietary fish intake (Asante et al., 2013; El Megdiche et al., 2017). Concerns related to levels of PCBs, as well as brominated flame retardants such as polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecanes (HBCDs), hexabromobenzene (HBB), 2,3-dibromopropyl-2,4,6-tribromophenyl ether (DPTE), pentabromoethylbenzene (PBEB) and 2,3,4,5,6-pentabromotoluene (PBT), were also measured in breast milk in several studies and found to be unexpectedly high (with estimated hazard quotient values exceeding the threshold of 1 or the US EPA reference doses exceeded) (Asante et al., 2011; Muller et al., 2016).
The concentrations of polybrominated diphenyl ethers (PBDEs) were commonly measured in the reviewed studies by using gas chromatography electron impact ionization mass spectrometry (Akortia et al., 2017).
Potential health risks for children, particularly nursing infants, for a variety of flame retardants were observed. PCBs in dirty oils and obsolete equipment as well as new sources of DDT for malaria control in some countries in Africa were noted as potential sources of exposure (Asante et al., 2011; Sun et al., 2016).
Only four studies met the inclusion criteria for this review of measuring phenols in relation to health outcomes in Africa (Motsoeneng and Dalvie, 2015; Muller et al., 2016; Abou-Elwafa Abdallah et al., 2017; Kumar et al., 2017), one of which covered the topic in a recent review of environmental factors and global estimates of preterm birth (Kumar et al., 2017). Abou-Elwafa et al. (2017) measured polychlorinated biphenyls (PCBS), some organochlorine pesticides (OCPs), as well as polybrominated diphenyl ethers (PBDEs, see flame retardants section) in serum of study participants in Egypt. Notably, concentrations of these chemicals were much lower in this Egyptian study population compared to other published concentrations in populations around the world.
The health outcomes evaluated included colorectal cancer (Abou-Elwafa Abdallah et al., 2017), preterm birth (Kumar et al., 2017), birth weight and birth length (Muller et al., 2016), and neurological endpoints such as difficulty with buttoning, reading, or writing notes (Motsoeneng and Dalvie, 2015).
Across the studies, phenols were measured in serum, breast milk, and urine. Some of these studies also measured PCBs and OCPs and are discussed in greater detail in other sections.
Similar to other chemical exposure categories, high risk populations include pregnant women, nursing infants (early life exposures in general), and young children.
The limited publications describing phenols and health outcomes in Africa likely reflect the limited data of phenol use, distribution, and concentrations in human urine, serum, or blood. Despite the variability in the use of these compounds in some regions, the lipophilic and persistent characteristics of some chemicals enable bioaccumulation in the food chain. Most are listed as persistent organic pollutants under the United Nations Environment Programme (UNEP) Stockholm Convention (UNEP, 2009) (https://www.wipo.int/edocs/lexdocs/treaties/en/unep-pop/trt_unep_pop_2.pdf). There is very limited data for Africa evaluating health outcomes related to phenols. However, several studies document the existence of phenols in human samples such as methylated polybrominated diphenyl ethers in human milk from Bizerte, Tunisia (Ben Hassine et al., 2015), dust exposure in Egypt (Hassan and Shoeib, 2015), and urinary bisphenol A (not persistent) concentrations in girls in rural and urban Egypt (Nahar et al., 2012). The levels in Egypt were lower than NHANES age-matched American girls but the authors noted associations with food storage in plastic containers which may change over time in some Africa regions.
Data on the health effects of phthalates in Africa was also extremely limited—only three articles retrieved in our literature search evaluated the impact of exposure to phthalates and any health outcomes in an African study population (Colacino et al., 2011; Kumar et al., 2017; Van Zijl et al., 2017). Adverse health outcomes evaluated in these articles were preterm birth (Kumar et al., 2017) and estrogenic activity (Van Zijl et al., 2017). The third study focused on sources of exposure to phthalates among premenstrual girls in Egypt, reporting BMI, waist and hip circumference, and other anthropometric characteristics, comparing rural and urban study participants. The authors also compared the phthalate levels in this Egyptian population to the age-matched girls in U.S. NHANES data, identifying key sources of exposure (Colacino et al., 2011). Storage of food in plastic containers was a statistically significant predictor of mono-isobutyl phthalate (MiBP) measured in urine of premenstrual girls, suggesting an important dietary route of exposure. The urinary measurements of phthalates were similar between the US and Egyptian age-matched girls (Colacino et al., 2011). Kumar et al. (2017) reviewed potential contributing factors to preterm birth and suggested phthalates should be evaluated more extensively in Africa.
Phthalates were measured in urine using enzymatic deconjugation of the metabolites from their glucuronidated form, solid-phase extraction, separation with high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry as described previously (Silva et al., 2007; Van Zijl et al., 2017). Estrogenic activity was identified in drinking water from Pretoria and Cape Town that also contained detectable levels of estrogens, bisphenol-A, and phthalates. No harmful effects from these were detected in their study population—the health risk assessment revealed acceptable health and carcinogenic risks associated with the consumption of distribution point water.
Early life exposure is an important consideration in this group, impacting pregnant women and young children.
Much more work is needed to evaluate the health implications from exposure to phthalates in the African setting, as exposures may increase over time.
Only 23 of the identified studies in our literature review considered both genomic and environmental factors related to health outcomes in Africa. All of these articles are listed in Table 3. Although effects of PON1 genotype on organophosphorus pesticide chlorpyrifos (CPF) exposure effects for Egyptian agricultural workers were found to be minimal (Ellison et al., 2012), several other studies reported significant effects of genotype modification for various exposure risks. The GSTP1 genotype appeared to modify the effects of ambient air pollutants PM10 and SO2 on lung function in South African children (Reddy et al., 2012). Genetic polymorphisms in NAPH and SOD2 may modulate pesticide-associated risk for bladder cancer (Amr et al., 2015). The TNF-alpha 308 polymorphisms were associated with increased effects on lung function for several pollutants (SO2 and NO2) (Makamure et al., 2016). PON1 192RR and CYP2D6 1934A alleles were found to potentially alter susceptibility to organophosphate chronic toxicity in Egyptian agricultural workers as well (Tawfik Khattab et al., 2016). ERCC3 and ERCC2 polymorphisms impact the effect of cadmium exposure for nasal polyposis (Khlifi et al., 2017). Air pollution’s effect on cardiovascular risk factors may be modulated by the APOA5 1131 polymorphism (Lin et al., 2017b). The CD14 CT/TT genotype appears to be protective for increased exposure to some ambient air pollutants (Makamure et al., 2017). DNA variants in NAT2, PON1, and GSTM1 may also modify organophosphate neurotoxicity (Glass et al., 2018).
A variety of other DNA and genomic biomarkers were also explored in relation to the effect of various exposure health risks. Aflatoxin adducts are known to be carcinogenic and mutagenic and have been associated with induction of the arginine to serine mutation in p53, and act synergistically with the hepatitis B virus to cause liver cancer (Kew, 2013). Repeated exposure to alpha-CYP pesticides appears to lead to p53 gene mutations (El Okda et al., 2017). A genotoxic impact for occupationally exposed antimony trioxide individuals was also reported with DNA damage detected in the form of increased apurinic/apyrimidic sites (El Shanawany et al., 2017). Interindividual variation in adduct levels associated with benzene and PAHs may reflect genetic susceptibility as well (Ayi-Fanou et al., 2011). One review summarized a variety of studies looking at various genotoxic biomarkers (including cytogenetic endpoints, chromosomal aberrations, etc.), DNA damage markers (including comet assay and urinary 8-hydroxydeoxyguanosine), and genomic biomarkers (including leukocyte telomere length, gene expression, etc.) (DeMarini, 2013). These markers were often able to distinguish traffic-exposed individuals from controls but only one of the 63 papers from this review was from an African-based study (DeMarini, 2013). Prenatal exposure to air pollution and HIV status of mothers appeared to lead to differential methylation in infants particularly in certain biological pathways related to metabolic processes and viral regulation (Goodrich et al., 2016). Only one study evaluated epigenome-wide DNA methylation and this study found differential methylation in genes related to growth and immune function for infants of aflatoxin-exposed mothers (Hernandez-Vargas et al., 2015). Only one study explored the possible effects on the microbiome for a particular exposure, and this report described changes in lung microbiome with high levels of black carbon particulates (Rylance et al., 2016). No genome-wide association studies (GWAS) or whole genome sequencing or RNA sequencing studies were identified in this literature review.
In this review, we summarize environmental health research in Africa covering the last decade, highlighting exposures unique to Africa with important health implications. Substantial progress has been made in identifying a wide range of health effects related to hazardous environmental exposures. In general, indoor and ambient air pollution studies across Africa were well characterized and health impacts are comparable to what has been described in other regions around the world. Increased industrialization, traffic, and biomass fuel burning in parts of Africa will continue to contribute to substantial air pollution. Many industrial metals contaminating the environment in parts of Africa and health effects comparable to those observed elsewhere, particularly cancer and neurological outcomes. Several reproductive outcome associations with heavy metals may be of particular interest in the African context. For example, the high levels of preeclampsia described in several African countries and the unusually high incidence of intrauterine growth retardation in Egypt may possibly be driven by toxic metal concentrations (El-Helaly et al., 2011; Ikechukwu et al., 2012; Motawei et al., 2013; El-Baz et al., 2015; Elongi Moyene et al., 2016). The acute lead poisoning for children is an urgent ongoing issue in many African countries and prevention of exposure among children is critical. A variety of pesticide studies reported reproductive, neurological, respiratory, and cancer outcomes, with one novel liver disease reporting an association with DDT (Robinson et al., 2014). The acute pesticide poisoning of adolescents (some intentional) is alarming and may reflect ease of access to these chemicals in the African continent (Balme et al., 2012; Azab et al., 2016; da Silva et al., 2016; Ssemugabo et al., 2017). Extensive mechanistic research combined with human studies over many years have allowed aflatoxin and other mycotoxins to be accurately measured and has facilitated prevention and intervention strategies. The literature on PFOS, flame retardants, phenols, e-waste, and phthalates remains extremely limited.
A variety of research gaps across multiple exposure categories were identified. The role of the immune system and inflammation and how it interacts with various exposures is an area that warrants more research. The role of endocrine disrupting chemicals in general are evolving and expanding with studies around the world and the metabolic impacts of this class of compounds, particularly for obesity, diabetes, and cardiovascular outcomes, will need to be further explored. As industrialization, urbanization, and globalization continue to impact the African continent, many emerging exposures, including PFOS, flame retardants, phenols, e-waste products, and phthalates may increase over time and will need further study in Africa.
Key susceptible/at risk populations were similar across multiple exposure categories and these include: pregnant women, children (particularly in utero and early childhood stages), and workers in specific occupational settings (agricultural, mining, street vendors, taxi-motorbike drivers, waste workers, etc.), and people living near urban areas who may be more highly exposed to particulate air pollutants such as benzene and PAHs. Immunocompromised individuals (people with HIV or other infections, cancer patients, etc.) may be particularly vulnerable to the effects of toxicants. The combined effects of environmental exposures and infections need to be further examined in African studies.
In general, the limited number of African studies exploring any integration or interaction of genomic and environmental factors suggests a substantial research gap. Extremely limited epigenomics and other omics applications were reported. The impact of possible transgenerational effects of some exposures by epigenomic processes has yet to be examined (Kabasenche and Skinner, 2014). The exploration of the interaction of genetic and environmental factors for disease susceptibility may enable future preventive measures. For example, the potential for agricultural workers exposed to high levels of pesticides to be screened based on genotype would be a way to help target protective measures for high risk groups and reduce disease burden (Tawfik Khattab et al., 2016). A better understanding of the regulation of biosynthetic genes related to some mycotoxins may also lead to new ways to monitor the food chain for mycotoxin contamination (Gil-Serna et al., 2018). The genetic diversity in Africa, combined with unique exposures and co-morbidities, can lead to novel G x E findings that cannot be discovered elsewhere.
This review did not represent a systematic analysis of all findings reported in the literature. The purpose was to provide a broad scope of environmental health, including many complex exposure categories. Future systematic reviews could be implemented, focusing on one exposure category or a single or collection of chemicals. The greatest detail was provided for the G x E articles retrieved in this review, which, as has been noted, represented a critical research gap. Importantly, the WHO report in 2016 (Prüss-Ustün et al., 2016) stated that the current statistics related to many disease outcomes likely underestimate the true burden due to inadequate coverage in the literature, the challenge to capturing emerging risks, and the fact that many exposures take years to manifest into presentable symptoms or disease.
A number of exposures have received substantial research attention in Africa, which is encouraging, and some studies have provided unique insights that will allow further translational efforts to occur. Aflatoxin interventions and prevention efforts are a model for what could potentially be done with other exposures in a resource limited setting. Some reports were limited by exposure assessment methods (perhaps relying too heavily on questionnaires to assess exposure and health risks). Leveraging resources such as the Children’s Health Exposure Analysis Resource (CHEAR) or Human Health Exposure Analysis Resource (HHEAR) (Balshaw et al., 2017) may enable critical gains in environmental exposure measurements in biospecimens collected in African studies. Increased environmental data in coordination with genomic infrastructure such as that in the H3Africa consortium offers a strong platform for building G x E research in Africa, although collaborations should not be limited to these resources alone.
Another underrepresented area of research was geospatial methods and spatiotemporal modeling to evaluate health outcomes in African populations. The utilization of satellite data in combination with ground monitoring is challenged by inadequate coverage of ground monitoring in Africa. Involvement of data scientists and related experts is needed to leverage existing data to advance environmental health research in Africa. The application of these methods is increasingly important with ongoing and foreseeable changes in weather patterns, agriculture, industrial development, resource mining, drought, natural vegetation, and wildlife across Africa, all of which impact the habitats of vectors transmitting infectious diseases. Variability in nutrition, poverty, and infectious diseases that all impact immunity further emphasizes the importance of bolstering environmental health research capacity across the continent.
In the coming decade, we anticipate ongoing advancements in environmental health and genomics, in coordination rather than in parallel. Leveraging the resource infrastructures within Africa and the growing global collaborations that consortia and bottom up approaches are capable of, the future for G x E research in Africa is promising.
Conceived and designed the literature review: BJ, KM. Performed literature review and analysis of review results: SM. Wrote the paper: BJ, KM. Revised and approved the manuscript: BJ, KM, SM.
Author SM was employed by the company Vista Technology Services.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Alyse Owoc and Bronwyn Cox from the NIEHS library who assisted with EndNote library organization tasks. We are grateful for the thoughtful manuscript review by our NIEHS colleagues Thad Schug and Harriett Kinyamu. We particularly thank the H3Africa consortium scientists, funders, staff, and study participants for stimulating the need for this review.
BC, black carbon; CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PAHs, polycyclic aromatic hydrocarbons; PM, particulate matter; SO2, sulfur dioxide; VOCs, volatile organic compounds.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.01166/full#supplementary-material
Abarikwu, S. O. (2013). Causes and risk factors for male-factor infertility in Nigeria: a review. Afr. J. Reprod. Health 17 (4), 150–166.
Abass, R. M., Hamdan, H. Z., Elhassan, E. M., Hamdan, S. Z., Ali, N. I., Adam, I. (2014). Zinc and copper levels in low birth weight deliveries in Medani Hospital, Sudan. BMC Res. Notes 7, 386. doi: 10.1186/1756-0500-7-386
Abdel Rasoul, G. M., Al-Batanony, M. A., Mahrous, O. A., Abo-Salem, M. E., Gabr, H. M. (2012). Environmental lead exposure among primary school children in Shebin El-Kom District, Menoufiya Governorate, Egypt. Int. J. Occup. Environ. Med. 3 (4), 186–194.
Abou-Elwafa Abdallah, M., Zaky, A. H., Covaci, A. (2017). Levels and profiles of organohalogenated contaminants in human blood from Egypt. Chemosphere 176, 266–272. doi: 10.1016/j.chemosphere.2017.02.139
Abou-Khadra, M. K. (2013). Association between PM(1)(0) exposure and sleep of Egyptian school children. Sleep Breath 17 (2), 653–657. doi: 10.1007/s11325-012-0738-7
AbuShady, M. M., Fathy, H. A., Fathy, G. A., Fatah, S. A. E., Ali, A., Abbas, M. A. (2017). Blood lead levels in a group of children: the potential risk factors and health problems. J. Pediatr. (Rio J) 93 (6), 619–624. doi: 10.1016/j.jped.2016.12.006
Achour, A., Derouiche, A., Barhoumi, B., Kort, B., Cherif, D., Bouabdallah, S. (2017). Organochlorine pesticides and polychlorinated biphenyls in human adipose tissue from northern Tunisia: current extent of contamination and contributions of socio-demographic characteristics and dietary habits. Environ. Res. 156, 635–643. doi: 10.1016/j.envres.2017.04.021
Adebamowo, S. N., Dareng, E. O., Famooto, A. O., Offiong, R., Olaniyan, O., Obende, K. (2017). Cohort profile: African collaborative center for microbiome and genomics research’s (ACCME’s) human papillomavirus (HPV) and cervical cancer study. Int. J. Epidemiol. 46 (6), 1745–1745j. doi: 10.1093/ije/dyx050
Adegbiji, W. A., Olajide, G. T., Olajuyin, A. O., Aremu, S. K., Olusola, A. G. (2018). Pattern of allergic rhinitis among children in Ekiti, Nigeria. Int. J. Pediatr. Otorhinolaryngol. 106, 75–79. doi: 10.1016/j.ijporl.2018.01.014
Adejumo, O., Atanda, O., Raiola, A., Somorin, Y., Bandyopadhyay, R., Ritieni, A. (2013). Correlation between aflatoxin M1 content of breast milk, dietary exposure to aflatoxin B1 and socioeconomic status of lactating mothers in Ogun State, Nigeria. Food Chem. Toxicol. 56, 171–177. doi: 10.1016/j.fct.2013.02.027
Adoga, M. P., Fatumo, S. A., Agwale, S. M. (2014). H3Africa: a tipping point for a revolution in bioinformatics, genomics and health research in Africa. Source Code Biol. Med. 9, 10. doi: 10.1186/1751-0473-9-10
Afum, C., Cudjoe, L., Hills, J., Hunt, R., Padilla, L. A., Elmore, S. (2016). Association between Aflatoxin M(1) and Liver Disease in HBV/HCV Infected Persons in Ghana. Int. J. Environ. Res. Public Health 13 (4), 377. doi: 10.3390/ijerph13040377
Agarwal, A., Kirwa, K., Eliot, M. N., Alenezi, F., Menya, D., Mitter, S. S. (2018). Household air pollution is associated with altered cardiac function among women in kenya. Am. J. Respir. Crit. Care Med. 197 (7), 958–961. doi: 10.1164/rccm.201704-0832LE
Ahrens, L., Gashaw, H., Sjoholm, M., Gebrehiwot, S. G., Getahun, A., Derbe, E. (2016). Poly- and perfluoroalkylated substances (PFASs) in water, sediment and fish muscle tissue from Lake Tana, Ethiopia and implications for human exposure. Chemosphere 165, 352–357. doi: 10.1016/j.chemosphere.2016.09.007
Ajayi, O. O., Charles-Davies, M. A., Arinola, O. G. (2012). Progesterone, selected heavy metals and micronutrients in pregnant Nigerian women with a history of recurrent spontaneous abortion. Afr. Health Sci. 12 (2), 153–159. doi: 10.4314/ahs.v12i2.12
Akinyemi, J. O., Adedini, S. A., Wandera, S. O., Odimegwu, C. O. (2016). Independent and combined effects of maternal smoking and solid fuel on infant and child mortality in sub-Saharan Africa. Trop. Med. Int. Health 21 (12), 1572–1582. doi: 10.1111/tmi.12779
Akormedi, M., Asampong, E., Fobil, J. N. (2013). Working conditions and environmental exposures among electronic waste workers in Ghana. Int. J. Occup. Environ. Health 19 (4), 278–286. doi: 10.1179/2049396713y.0000000034
Akortia, E., Olukunle, O. I., Daso, A. P., Okonkwo, J. O. (2017). Soil concentrations of polybrominated diphenyl ethers and trace metals from an electronic waste dump site in the Greater Accra Region, Ghana: implications for human exposure. Ecotoxicol. Environ. Saf. 137, 247–255. doi: 10.1016/j.ecoenv.2016.12.008
Al-Batanony, M. A., Abdel-Rasul, G. M., Abu-Salem, M. A., Al-Dalatony, M. M., Allam, H. K. (2013). Occupational exposure to mercury among workers in a fluorescent lamp factory, Quisna Industrial Zone, Egypt. Int. J. Occup. Environ. Med. 4 (3), 149–156.
Alabi, O. A., Bakare, A. A., Xu, X., Li, B., Zhang, Y., Huo, X. (2012). Comparative evaluation of environmental contamination and DNA damage induced by electronic-waste in Nigeria and China. Sci. Total Environ. 423, 62–72. doi: 10.1016/j.scitotenv.2012.01.056
Alasia, D. D., Emem-Chioma, P. C., Wokoma, F. S. (2010a). Association of lead exposure, serum uric acid and parameters of renal function in Nigerian lead-exposed workers. Int. J. Occup. Environ. Med. 1 (4), 182–190.
Alasia, D. D., Emem-Chioma, P. C., Wokoma, F. S. (2010b). Occupational and environmental lead exposure in Port Harcourt, Nigeria: analysis of its association with renal function indices. Niger J. Med. 19 (4), 407–414.
Alatise, O. I., Babalola, O. O., Omoniyi-Esan, G. O., Lawal, O. O., Adesunkanmi, A. R., Agbakwuru, E. A. (2013). Selenium levels in neoplastic breast lesions. Niger Postgrad Med. J. 20 (2), 91–97.
Alatise, O. I., Schrauzer, G. N. (2010). Lead exposure: a contributing cause of the current breast cancer epidemic in Nigerian women. Biol. Trace Elem. Res. 136 (2), 127–139. doi: 10.1007/s12011-010-8608-2
Albers, P. N., Wright, C. Y., Voyi, K. V., Mathee, A. (2015). Household fuel use and child respiratory ill health in two towns in Mpumalanga, South Africa. S. Afr. Med. J. 105 (7), 573–577. doi: 10.7196/SAMJnew.7934
Alexander, D., Northcross, A., Wilson, N., Dutta, A., Pandya, R., Ibigbami, T. (2017). Randomized Controlled ethanol cookstove intervention and blood pressure in pregnant Nigerian women. Am. J. Respir. Crit. Care Med. 195 (12), 1629–1639. doi: 10.1164/rccm.201606-1177OC
Amankwaa, E. F., Adovor Tsikudo, K. A., Bowman, J. A. (2017). ‘Away’ is a place: the impact of electronic waste recycling on blood lead levels in Ghana. Sci. Total Environ. 601, 1566–1574. doi: 10.1016/j.scitotenv.2017.05.283
Amr, S., Dawson, R., Saleh, D. A., Magder, L. S., St George, D. M., El-Daly, M. (2015). Pesticides, gene polymorphisms, and bladder cancer among Egyptian agricultural workers. Arch. Environ. Occup. Health 70 (1), 19–26. doi: 10.1080/19338244.2013.853646
Araoud, M., Neffeti, F., Douki, W., Hfaiedh, H. B., Akrout, M., Hassine, M. (2012). Adverse effects of pesticides on biochemical and haematological parameters in Tunisian agricultural workers. J. Expo Sci. Environ. Epidemiol. 22 (3), 243–247. doi: 10.1038/jes.2012.11
Araoud, M., Neffeti, F., Douki, W., Hfaiedh, H. B., Akrout, M., Najjar, M. F. (2011). Factors influencing plasma butyrylcholinesterase activity in agricultural workers. Ann. Biol. Clin. (Paris) 69 (2), 159–166. doi: 10.1684/abc.2011.0531
Araoud, M., Neffeti, F., Douki, W., Najjar, M. F., Kenani, A. (2010). Paraoxonase 1 correlates with butyrylcholinesterase and gamma glutamyl transferase in workers chronically exposed to pesticides. J. Occup. Health 52 (6), 383–388.
Arku, R. E., Ezzati, M., Baumgartner, J., Fink, G., Zhou, B., Hystad, P. (2018). Elevated blood pressure and household solid fuel use in premenopausal women: analysis of 12 demographic and health surveys (DHS) from 10 countries. Environ. Res. 160, 499–505. doi: 10.1016/j.envres.2017.10.026
Arrebola, J. P., Belhassen, H., Artacho-Cordon, F., Ghali, R., Ghorbel, H., Boussen, H. (2015). Risk of female breast cancer and serum concentrations of organochlorine pesticides and polychlorinated biphenyls: a case-control study in Tunisia. Sci. Total Environ. 520, 106–113. doi: 10.1016/j.scitotenv.2015.03.045
Asante, K. A., Adu-Kumi, S., Nakahiro, K., Takahashi, S., Isobe, T., Sudaryanto, A. (2011). Human exposure to PCBs, PBDEs and HBCDs in Ghana: temporal variation, sources of exposure and estimation of daily intakes by infants. Environ. Int. 37 (5), 921–928. doi: 10.1016/j.envint.2011.03.011
Asante, K. A., Takahashi, S., Itai, T., Isobe, T., Devanathan, G., Muto, M. (2013). Occurrence of halogenated contaminants in inland and coastal fish from Ghana: levels, dietary exposure assessment and human health implications. Ecotoxicol. Environ. Saf. 94, 123–130. doi: 10.1016/j.ecoenv.2013.05.008
Attia, E. F., Miller, R. F., Ferrand, R. A. (2017). Bronchiectasis and other chronic lung diseases in adolescents living with HIV. Curr. Opin. Infect. Dis. 30 (1), 21–30.
Awadalla, N. J., El-Helaly, M., Gouida, M., Mandour, R., Mansour, M. (2011). Sperm chromatin structure, semen quality and lead in blood and seminal fluid of infertile men. Int. J. Occup. Environ. Med. 2 (1), 27–36.
Awadalla, N. J., Hegazy, A., Elmetwally, R. A., Wahby, I. (2012). Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Egypt: a multicenter case-control study. Int. J. Occup. Environ. Med. 3 (3), 107–116.
Awadelkarim, K. D., Mariani-Costantini, R., Elwali, N. E. (2012). Cancer in the Sudan: an overview of the current status of knowledge on tumor patterns and risk factors. Sci. Total Environ. 423, 214–228. doi: 10.1016/j.scitotenv.2010.09.010
Ayi-Fanou, L., Avogbe, P. H., Fayomi, B., Keith, G., Hountondji, C., Creppy, E. E. (2011). DNA-adducts in subjects exposed to urban air pollution by benzene and polycyclic aromatic hydrocarbons (PAHs) in Cotonou, Benin. Environ. Toxicol. 26 (1), 93–102. doi: 10.1002/tox.20533
Azab, S. F., Saleh, S. H., Elsaeed, W. F., Elshafie, M. A., Sherief, L. M., Esh, A. M. (2014). Serum trace elements in obese Egyptian children: a case-control study. Ital. J. Pediatr. 40, 20. doi: 10.1186/1824-7288-40-20
Azab, S. M., Hirshon, J. M., Hayes, B. D., El-Setouhy, M., Smith, G. S., Sakr, M. L. (2016). Epidemiology of acute poisoning in children presenting to the poisoning treatment center at Ain Shams University in Cairo, Egypt, 2009-2013. Clin. Toxicol. (Phila) 54 (1), 20–26. doi: 10.3109/15563650.2015.1112014
Azandjeme, C. S., Bouchard, M., Fayomi, B., Djrolo, F., Houinato, D., Delisle, H. (2013). Growing burden of diabetes in sub-saharan Africa: contribution of pesticides? Curr. Diabetes Rev. 9 (6), 437–449.
Badran, M., Morsy, R., Soliman, H., Elnimr, T. (2016). Assessment of trace elements levels in patients with Type 2 diabetes using multivariate statistical analysis. J. Trace Elem. Med. Biol. 33, 114–119. doi: 10.1016/j.jtemb.2015.10.006
Balme, K. H., Roberts, J. C., Glasstone, M., Curling, L., Mann, M. D. (2012). The changing trends of childhood poisoning at a tertiary children’s hospital in South Africa. S. Afr. Med. J. 102 (3 Pt 1), 142–146.
Balme, K. H., Roberts, J. C., Glasstone, M., Curling, L., Rother, H. A., London, L. (2010). Pesticide poisonings at a tertiary children’s hospital in South Africa: an increasing problem. Clin. Toxicol. (Phila) 48 (9), 928–934. doi: 10.3109/15563650.2010.534482
Balshaw, D. M., Collman, G. W., Gray, K. A., Thompson, C. L. (2017). The children’s health exposure analysis resource: enabling research into the environmental influences on children’s health outcomes. Curr. Opin. Pediatr. 29 (3), 385–389. doi: 10.1097/MOP.0000000000000491
Bangma, J. T., Reiner, J. L., Botha, H., Cantu, T. M., Gouws, M. A., Guillette, M. P. (2017). Tissue distribution of perfluoroalkyl acids and health status in wild Mozambique tilapia (Oreochromis mossambicus) from Loskop Dam, Mpumalanga, South Africa. J. Environ. Sci. (China) 61, 59–67. doi: 10.1016/j.jes.2017.03.041
Barchi, F., Little, M. T. (2016). National ethics guidance in Sub-Saharan Africa on the collection and use of human biological specimens: a systematic review. BMC Med. Ethics 17 (1), 64. doi: 10.1186/s12910-016-0146-9
Bardei, F., Bouziane, H., Kadiri, M., Rkiek, B., Tebay, A., Saoud, A. (2016). Skin sensitisation profiles to inhalant allergens for patients in Tetouan city (North West of Morocco). Rev. Pneumol. Clin. 72 (4), 221–227. doi: 10.1016/j.pneumo.2016.04.005
Bartrem, C., Tirima, S., von Lindern, I., von Braun, M., Worrell, M. C., Mohammad Anka, S. (2014). Unknown risk: co-exposure to lead and other heavy metals among children living in small-scale mining communities in Zamfara State, Nigeria. Int. J. Environ. Health Res. 24 (4), 304–319. doi: 10.1080/09603123.2013.835028
Belhassen, H., Jimenez-Diaz, I., Arrebola, J. P., Ghali, R., Ghorbel, H., Olea, N. (2015). Zearalenone and its metabolites in urine and breast cancer risk: a case-control study in Tunisia. Chemosphere 128, 1–6. doi: 10.1016/j.chemosphere.2014.12.055
Belser-Ehrlich, S., Harper, A., Hussey, J., Hallock, R. (2013). Human and cattle ergotism since 1900: symptoms, outbreaks, and regulations. Toxicol. Ind. Health 29 (4), 307–316. doi: 10.1177/0748233711432570
Ben Ameur, W., El Megdiche, Y., Eljarrat, E., Ben Hassine, S., Badreddine, B., Souad, T. (2013). Organochlorine and organobromine compounds in a benthic fish (Solea solea) from Bizerte Lagoon (northern Tunisia): implications for human exposure. Ecotoxicol. Environ. Saf. 88, 55–64. doi: 10.1016/j.ecoenv.2012.10.021
Ben Hassine, S., Ben Ameur, W., Eljarrat, E., Barcelo, D., Touil, S., Driss, M. R. (2015). Methoxylated polybrominated diphenyl ethers (MeO-PBDE) in human milk from Bizerte, Tunisia. Environ. Res. 138, 32–37. doi: 10.1016/j.envres.2015.01.016
Benaissa, F., Maesano, C. N., Alkama, R., Annesi-Maesano, I. (2016). Short-term health impact assessment of urban PM10 in Bejaia City (Algeria). Can. Respir. J. 2016, 8209485. doi: 10.1155/2016/8209485
Berhane, K., Kumie, A., Samet, J. (2016). Health effects of environmental exposures, occupational hazards and climate change in ethiopia: synthesis of situational analysis, needs assessment and the way forward. Ethiop. J. Health Dev. 30 (1 Spec Iss), 50–56.
Billet, S., Garcon, G., Dagher, Z., Verdin, A., Ledoux, F., Cazier, F. (2007). Ambient particulate matter (PM 2.5): physicochemical characterization and metabolic activation of the organic fraction in human lung epithelial cells (A549). Environ. Res. 105 (2), 212–223. doi: 10.1016/j.envres.2007.03.001
Bimenya, G. S., Harabulema, M., Okot, J. P., Francis, O., Lugemwa, M., Okwi, A. L. (2010). Plasma levels of DDT/DDE and liver function in malaria control personnel 6 months after indoor residual spraying with DDT in northern Uganda, 2008. S. Afr. Med. J. 100 (2), 118–121.
Bornman, M. S., Aneck-Hahn, N. H., de Jager, C., Wagenaar, G. M., Bouwman, H., Barnhoorn, I. E. J. (2017). Endocrine disruptors and health effects in Africa: a call for action. Environ. Health Perspect. 125 (8), 085005. doi: 10.1289/EHP1774
Bortey-Sam, N., Ikenaka, Y., Akoto, O., Nakayama, S. M., Yohannes, Y. B., Baidoo, E. (2015). Levels, potential sources and human health risk of polycyclic aromatic hydrocarbons (PAHs) in particulate matter (PM(10)) in Kumasi, Ghana. Environ. Sci. Pollut. Res. Int. 22 (13), 9658–9667. doi: 10.1007/s11356-014-4022-1
Bortey-Sam, N., Ikenaka, Y., Akoto, O., Nakayama, S. M. M., Asante, K. A., Baidoo, E. (2018). Association between human exposure to heavy metals/metalloid and occurrences of respiratory diseases, lipid peroxidation and DNA damage in Kumasi, Ghana. Environ. Pollut. 235, 163–170. doi: 10.1016/j.envpol.2017.12.005
Bose-O’Reilly, S., Bernaudat, L., Siebert, U., Roider, G., Nowak, D., Drasch, G. (2017). Signs and symptoms of mercury-exposed gold miners. Int. J. Occup. Med. Environ. Health 30 (2), 249–269. doi: 10.13075/ijomeh.1896.00715
Bose-O’Reilly, S., Yabe, J., Makumba, J., Schutzmeier, P., Ericson, B., Caravanos, J. (2018). Lead intoxicated children in Kabwe, Zambia. Environ. Res. 165, 420–424. doi: 10.1016/j.envres.2017.10.024
Bosetti, C., Turati, F., La Vecchia, C. (2014). Hepatocellular carcinoma epidemiology. Best Pract. Res. Clin. Gastroenterol. 28 (5), 753–770. doi: 10.1016/j.bpg.2014.08.007
Bouftini, S., Bahhou, J., Lelievre, B., de la Barca, J. M., Turcant, A., Diquet, B. (2015). Screening for childhood lead poisoning in the industrial region of Fez, Morocco. Arch. Environ. Contam. Toxicol. 68 (3), 442–450. doi: 10.1007/s00244-014-0108-5
Brown, M. J., McWeeney, G., Kim, R., Tahirukaj, A., Bulat, P., Syla, S. (2010). Lead poisoning among internally displaced Roma, Ashkali and Egyptian children in the United Nations-Administered Province of Kosovo. Eur. J. Public Health 20 (3), 288–292. doi: 10.1093/eurpub/ckp164
Buchner, H., Rehfuess, E. A. (2015). Cooking and season as risk factors for acute lower respiratory infections in African children: a cross-sectional multi-country analysis. PloS One 10 (6), e0128933. doi: 10.1371/journal.pone.0128933
Bumoko, G. M., Sadiki, N. H., Rwatambuga, A., Kayembe, K. P., Okitundu, D. L., Mumba Ngoyi, D. (2015). Lower serum levels of selenium, copper, and zinc are related to neuromotor impairments in children with konzo. J. Neurol. Sci. 349 (1-2), 149–153. doi: 10.1016/j.jns.2015.01.007
Burns, K. N., Sun, K., Fobil, J. N., Neitzel, R. L. (2016). Heart rate, stress, and occupational noise exposure among electronic waste recycling workers. Int. J. Environ. Res. Public Health 13 (1), 140. doi: 10.3390/ijerph13010140
Bwalya, D., Bratveit, M., Moen, B. E. (2011). Chronic respiratory symptoms among workers at a limestone factory in Zambia. Arch. Environ. Occup. Health 66 (1), 47–50. doi: 10.1080/19338244.2010.506498
Cabral, M., Dieme, D., Verdin, A., Garcon, G., Fall, M., Bouhsina, S. (2012). Low-level environmental exposure to lead and renal adverse effects: a cross-sectional study in the population of children bordering the Mbeubeuss landfill near Dakar, Senegal. Hum. Exp. Toxicol. 31 (12), 1280–1291. doi: 10.1177/0960327112446815
Cabral, M., Toure, A., Garcon, G., Diop, C., Bouhsina, S., Dewaele, D. (2015). Effects of environmental cadmium and lead exposure on adults neighboring a discharge: Evidences of adverse health effects. Environ. Pollut. 206, 247–255. doi: 10.1016/j.envpol.2015.06.032
Cachon, B. F., Firmin, S., Verdin, A., Ayi-Fanou, L., Billet, S., Cazier, F. (2014). Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM(2.5) and PM(> 2.5)) collected from Cotonou, Benin. Environ. Pollut. 185, 340–351. doi: 10.1016/j.envpol.2013.10.026
Callahan, C. L., Al-Batanony, M., Ismail, A. A., Abdel-Rasoul, G., Hendy, O., Olson, J. R. (2014). Chlorpyrifos exposure and respiratory health among adolescent agricultural workers. Int. J. Environ. Res. Public Health 11 (12), 13117–13129. doi: 10.3390/ijerph111213117
Castelino, J. M., Dominguez-Salas, P., Routledge, M. N., Prentice, A. M., Moore, S. E., Hennig, B. J. (2014). Seasonal and gestation stage associated differences in aflatoxin exposure in pregnant Gambian women. Trop. Med. Int. Health 19 (3), 348–354. doi: 10.1111/tmi.12250
Castelino, J. M., Routledge, M. N., Wilson, S., Dunne, D. W., Mwatha, J. K., Gachuhi, K. (2015). Aflatoxin exposure is inversely associated with IGF1 and IGFBP3 levels in vitro and in Kenyan schoolchildren. Mol. Nutr. Food Res. 59 (3), 574–581. doi: 10.1002/mnfr.201300619
Chaari, N., Chebel, S., Merchaoui, I., Kerkeni, A., Neffati, F., Najjar, F. (2015). Neuropsychological effects of mercury exposure among dentists in monastir City. Recent Pat. Inflammation Allergy Drug Discovery 9 (2), 151–158.
Chafe, Z. A., Brauer, M., Klimont, Z., Van Dingenen, R., Mehta, S., Rao, S. (2014). Household cooking with solid fuels contributes to ambient PM 2.5 air pollution and the burden of disease. Environ. Health Perspect. 122 (12), 1314–1320. doi: 10.1289/ehp.1206340
Chen, G., Gong, Y. Y., Kimanya, M. E., Shirima, C. P., Routledge, M. N. (2018). Comparison of urinary aflatoxin M1 and aflatoxin albumin adducts as biomarkers for assessing aflatoxin exposure in Tanzanian children. Biomarkers 23 (2), 131–136. doi: 10.1080/1354750x.2017.1285960
Chirikona, F., Filipovic, M., Ooko, S., Orata, F. (2015). Perfluoroalkyl acids in selected wastewater treatment plants and their discharge load within the Lake Victoria basin in Kenya. Environ. Monit. Assess. 187 (5), 238. doi: 10.1007/s10661-015-4425-6
Coker, E., Chevrier, J., Rauch, S., Bradman, A., Obida, M., Crause, M. (2018). Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ. Int. 113, 122–132. doi: 10.1016/j.envint.2018.01.016
Colacino, J. A., Soliman, A. S., Calafat, A. M., Nahar, M. S., Van Zomeren-Dohm, A., Hablas, A. (2011). Exposure to phthalates among premenstrual girls from rural and urban Gharbiah, Egypt: a pilot exposure assessment study. Environ. Health 10, 40. doi: 10.1186/1476-069x-10-40
Consortium, H. A., Rotimi, C., Abayomi, A., Abimiku, A., Adabayeri, V. M., Adebamowo, C. (2014). Research capacity. Enabling the genomic revolution in Africa. Sci. 344 (6190), 1346–1348. doi: 10.1126/science.1251546
da Silva, M., Stadlinger, N., Mmochi, A. J., Stalsby Lundborg, C., Marrone, G. (2016). Pesticide use and self-reported health symptoms among rice farmers in zanzibar. J. Agromedicine 21 (4), 335–344. doi: 10.1080/1059924x.2016.1211572
Darwish, W. S., Ikenaka, Y., Nakayama, S. M., Ishizuka, M. (2014). An overview on mycotoxin contamination of foods in Africa. J. Vet. Med. Sci. 76 (6), 789–797.
Das, I., Jagger, P., Yeatts, K. (2017). Biomass Cooking Fuels and Health Outcomes for Women in Malawi. Ecohealth 14 (1), 7–19. doi: 10.1007/s10393-016-1190-0
Davidson, P. W., Leste, A., Benstrong, E., Burns, C. M., Valentin, J., Sloane-Reeves, J. (2010). Fish consumption, mercury exposure, and their associations with scholastic achievement in the Seychelles Child Development Study. Neurotoxicology 31 (5), 439–447. doi: 10.1016/j.neuro.2010.05.010
de Longueville, F., Ozer, P., Doumbia, S., Henry, S. (2013). Desert dust impacts on human health: an alarming worldwide reality and a need for studies in West Africa. Int. J. Biometeorol. 57 (1), 1–19. doi: 10.1007/s00484-012-0541-y
de Vries, D. H., Pool, R. (2017). The influence of community health resources on effectiveness and sustainability of community and lay health worker programs in lower-income countries: a systematic review. PloS One 12 (1), e0170217. doi: 10.1371/journal.pone.0170217
DeMarini, D. M. (2013). Genotoxicity biomarkers associated with exposure to traffic and near-road atmospheres: a review. Mutagenesis 28 (5), 485–505. doi: 10.1093/mutage/get042
Diongue, K., Badiane, A. S., Seck, M. C., Ndiaye, M., Diallo, M. A., Diallo, S. (2015). Qualitative fungal composition of services at risk of nosocomial infections at Aristide Le Dantec Hospital (Dakar). J. Mycol. Med. 25 (1), e39–e43. doi: 10.1016/j.mycmed.2014.10.020
Domijan, A. M. (2012). Fumonisin B(1): a neurotoxic mycotoxin. Arh. Hig. Rada Toksikol. 63 (4), 531–544. doi: 10.2478/10004-1254-63-2012-2239
Dooyema, C. A., Neri, A., Lo, Y. C., Durant, J., Dargan, P. I., Swarthout, T. (2012). Outbreak of fatal childhood lead poisoning related to artisanal gold mining in northwestern Nigeria, 2010. Environ. Health Perspect. 120 (4), 601–607. doi: 10.1289/ehp.1103965
Dutta, A., Brito, K., Khramstova, G., Mueller, A., Chinthala, S., Alexander, D. (2017). Household air pollution and angiogenic factors in pregnant Nigerian women: A randomized controlled ethanol cookstove intervention. Sci. Total Environ. 599-600, 2175–2181. doi: 10.1016/j.scitotenv.2017.05.130
Ekosse, G. I. (2011). Health status within the precincts of a nickel-copper mining and smelting environment. Afr. Health Sci. 11 (1), 90–96.
Ekpenyong, C. E., Ettebong, E. O., Akpan, E. E., Samson, T. K., Daniel, N. E. (2012). Urban city transportation mode and respiratory health effect of air pollution: a cross-sectional study among transit and non-transit workers in Nigeria. BMJ Open 2 (5), e001253. doi: 10.1136/bmjopen-2012-001253
El-Baz, M. A., El-Deeb, T. S., El-Noweihi, A. M., Mohany, K. M., Shaaban, O. M., Abbas, A. M. (2015). Environmental factors and apoptotic indices in patients with intrauterine growth retardation: a nested case-control study. Environ. Toxicol. Pharmacol. 39 (2), 589–596. doi: 10.1016/j.etap.2015.01.009
El-Fadeli, S., Bouhouch, S., Skalny, A. V., Barkouch, Y., Pineau, A., Cherkaoui, M. (2016). Effects of imbalance in trace element on thyroid gland from moroccan Children. Biol. Trace Elem. Res. 170 (2), 288–293. doi: 10.1007/s12011-015-0485-2
El-Gamal, Y. M., Hossny, E. M., El-Sayed, Z. A., Reda, S. M. (2017). Allergy and immunology in Africa: challenges and unmet needs. J. Allergy Clin. Immunol. 140 (5), 1240–1243. doi: 10.1016/j.jaci.2017.09.004
El-Helaly, M., Abdel-Elah, K., Haussein, A., Shalaby, H. (2011). Paternal occupational exposures and the risk of congenital malformations–a case-control study. Int. J. Occup. Med. Environ. Health 24 (2), 218–227. doi: 10.2478/s13382-011-0019-x
El-Mahallawy, H. A., Khedr, R., Taha, H., Shalaby, L., Mostafa, A. (2016). Investigation and management of a rhizomucor outbreak in a pediatric cancer hospital in egypt. Pediatr. Blood Cancer 63 (1), 171–173. doi: 10.1002/pbc.25673
El-Shafei, H. M. (2011). Assessment of liver function among nickel-plating workers in Egypt. East Mediterr Health J. 17 (6), 490–494.
El Husseiny, N. M., Said, E. S., Shahat Mohamed, N., Othman, A. I. (2011). Impact of trace element changes on dehydroepiandrosterone sulfate in healthy and diabetic states among middle-age and elderly Egyptians. Biol. Trace Elem. Res. 143 (3), 1451–1460. doi: 10.1007/s12011-011-9012-2
El Kholy, M., Hamza, R. T., Saleh, M., Elsedfy, H. (2013). Penile length and genital anomalies in Egyptian male newborns: epidemiology and influence of endocrine disruptors. J. Pediatr. Endocrinol. Metab. 26 (5-6), 509–513. doi: 10.1515/jpem-2012-0350
El Megdiche, Y., Ameur, W. B., Bechir, H., Hassine, S. B., Badreddine, B., Touil, S. (2017). Anthropogenic (PBDE) and naturally-produced (MeO-PBDE) brominated compound levels in Bizerte Lagoon clams (Ruditapes decussatus): levels and human health risk assessment. Mar. Pollut. Bull. 125 (1-2), 176–185. doi: 10.1016/j.marpolbul.2017.08.005
El Okda, E. S., Abdel-Hamid, M. A., Hamdy, A. M. (2017). Immunological and genotoxic effects of occupational exposure to alpha-cypermethrin pesticide. Int. J. Occup. Med. Environ. Health 30 (4), 603–615. doi: 10.13075/ijomeh.1896.00810
El Shanawany, S., Foda, N., Hashad, D. I., Salama, N., Sobh, Z. (2017). The potential DNA toxic changes among workers exposed to antimony trioxide. Environ. Sci. Pollut. Res. Int. 24 (13), 12455–12461. doi: 10.1007/s11356-017-8805-z
Elenge, M. M., Aubry, J. C., Jacob, L., De Brouwer, C. (2011). Heavy metal in hair samples of 109 non-industrial (miners) population in Katanga. Sante 21 (1), 41–46. doi: 10.1684/san.2011.0229
Elenge, M. M., De Brouwer, C. (2011). Identification of hazards in the workplaces of Artisanal mining in Katanga. Int. J. Occup. Med. Environ. Health 24 (1), 57–66. doi: 10.2478/s13382-011-0012-4
Ellison, C. A., Crane, A. L., Bonner, M. R., Knaak, J. B., Browne, R. W., Lein, P. J. (2012). PON1 status does not influence cholinesterase activity in Egyptian agricultural workers exposed to chlorpyrifos. Toxicol. Appl. Pharmacol. 265 (3), 308–315. doi: 10.1016/j.taap.2012.08.031
Elongi Moyene, J. P., Scheers, H., Tandu-Umba, B., Haufroid, V., Buassa-Bu-Tsumbu, B., Verdonck, F. (2016). Preeclampsia and toxic metals: a case-control study in Kinshasa, DR Congo. Environ. Health 15, 48. doi: 10.1186/s12940-016-0132-1
English, R. G., Perry, M., Lee, M. M., Hoffman, E., Delport, S., Dalvie, M. A. (2012). Farm residence and reproductive health among boys in rural South Africa. Environ. Int. 47, 73–79. doi: 10.1016/j.envint.2012.06.006
Etchie, T. O., Etchie, A. T., Adewuyi, G. O., Pillarisetti, A., Sivanesan, S., Krishnamurthi, K. (2018). The gains in life expectancy by ambient PM 2.5 pollution reductions in localities in Nigeria. Environ. Pollut. 236, 146–157. doi: 10.1016/j.envpol.2018.01.034
Ettinger, A. S., Bovet, P., Plange-Rhule, J., Forrester, T. E., Lambert, E. V., Lupoli, N. (2014). Distribution of metals exposure and associations with cardiometabolic risk factors in the “Modeling the Epidemiologic Transition Study”. Environ. Health 13, 90. doi: 10.1186/1476-069x-13-90
Ewenighi, C. O., Dimkpa, U., Onyeanusi, J. C., Babtunde, A., Onoh, L. U. M., Onoh, G. O. (2017). Prostate-specific antigen and its derivatives in young adults occupationally exposed to quarry pollutants in southeastern Nigeria. Arch. Environ. Occup. Health 72 (5), 258–263. doi: 10.1080/19338244.2016.1207593
Eze, U. A., Okonofua, F. E. (2015). High prevalence of male infertility in africa: are mycotoxins to blame? Afr. J. Reprod. Health 19 (3), 9–17.
Famurewa, A. C., Ugwuja, E. I. (2017). Association of blood and seminal plasma cadmium and lead levels with semen quality in non-occupationally exposed infertile men in Abakaliki, South East Nigeria. J. Family Reprod. Health 11 (2), 97–103.
Fasinu, P., Orisakwe, O. E. (2013). Heavy metal pollution in sub-Saharan Africa and possible implications in cancer epidemiology. Asian Pac. J. Cancer Prev. 14 (6), 3393–3402.
Feki-Tounsi, M., Olmedo, P., Gil, F., Khlifi, R., Mhiri, M. N., Rebai, A. (2013a). Cadmium in blood of Tunisian men and risk of bladder cancer: interactions with arsenic exposure and smoking. Environ. Sci. Pollut. Res. Int. 20 (10), 7204–7213. doi: 10.1007/s11356-013-1716-8
Feki-Tounsi, M., Olmedo, P., Gil, F., Khlifi, R., Mhiri, M. N., Rebai, A. (2013b). Low-level arsenic exposure is associated with bladder cancer risk and cigarette smoking: a case-control study among men in Tunisia. Environ. Sci. Pollut. Res. Int. 20 (6), 3923–3931. doi: 10.1007/s11356-012-1335-9
Feki-Tounsi, M., Olmedo, P., Gil, F., Mhiri, M. N., Rebai, A., Hamza-Chaffai, A. (2014). Trace metal quantification in bladder biopsies from tumoral lesions of Tunisian cancer and controls subjects. Environ. Sci. Pollut. Res. Int. 21 (19), 11433–11438. doi: 10.1007/s11356-014-3099-x
Flatin, M. C., Ade, S., Hounkpatin, S. H., Ametonou, B., Vodouhe, U. B., Adjibabi, W. (2018). Symptoms of allergic rhinitis in Parakou, Benin: Prevalence, severity and associated factors. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 135 (1), 33–36. doi: 10.1016/j.anorl.2017.07.003
Furi, P., Hofmann, W., Jokay, A., Balashazy, I., Moustafa, M., Czitrovszky, B. (2017). Comparison of airway deposition distributions of particles in healthy and diseased workers in an Egyptian industrial site. Inhal. Toxicol. 29 (4), 147–159. doi: 10.1080/08958378.2017.1326990
Gashu, D., Stoecker, B. J., Adish, A., Haki, G. D., Bougma, K., Aboud, F. E. (2016). Association of serum selenium with thyroxin in severely iodine-deficient young children from the Amhara region of Ethiopia. Eur. J. Clin. Nutr. 70 (8), 929–934. doi: 10.1038/ejcn.2016.27
Gaspar, F. W., Chevrier, J., Bornman, R., Crause, M., Obida, M., Barr, D. B. (2015). Undisturbed dust as a metric of long-term indoor insecticide exposure: Residential DDT contamination from indoor residual spraying and its association with serum levels in the VHEMBE cohort. Environ. Int. 85, 163–167. doi: 10.1016/j.envint.2015.09.014
GBD 2015 Risk Factors Collaborators (2016). Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388 (10053), 1659–1724. doi: 10.1016/s0140-6736(16)31679-8
Gharamah, A. A., Moharram, A. M., Ismail, M. A., Al-Hussaini, A. K. (2012). Bacterial and fungal endophthalmitis in upper Egypt: related species and risk factors. Asian Pac. J. Trop. BioMed. 2 (8), 655–659. doi: 10.1016/s2221-1691(12)60115-4
Gheith, S., Ranque, S., Bannour, W., Ben Youssef, Y., Khelif, A., Ben Said, M. (2015). Hospital environment fungal contamination and aspergillosis risk in acute leukaemia patients in Sousse (Tunisia). Mycoses 58 (6), 337–342. doi: 10.1111/myc.12320
Gibb, H., O’Leary, K. G. (2014). Mercury exposure and health impacts among individuals in the artisanal and small-scale gold mining community: a comprehensive review. Environ. Health Perspect. 122 (7), 667–672. doi: 10.1289/ehp.1307864
Gil-Serna, J., Garcia-Diaz, M., Gonzalez-Jaen, M. T., Vazquez, C., Patino, B. (2018). Description of an orthologous cluster of ochratoxin A biosynthetic genes in Aspergillus and Penicillium species. A comparative analysis. Int. J. Food Microbiol. 268, 35–43. doi: 10.1016/j.ijfoodmicro.2017.12.028
Glass, T., Dalvie, M. A., Holtman, Z., Vorster, A. A., Ramesar, R. S., London, L. (2018). DNA variants and organophosphate neurotoxicity among emerging farmers in the Western Cape of South Africa. Am. J. Ind. Med. 61 (1), 11–20. doi: 10.1002/ajim.22790
Gong, Y. Y., Wilson, S., Mwatha, J. K., Routledge, M. N., Castelino, J. M., Zhao, B. (2012). Aflatoxin exposure may contribute to chronic hepatomegaly in Kenyan school children. Environ. Health Perspect. 120 (6), 893–896. doi: 10.1289/ehp.1104357
Gonzalez-Cuyar, L. F., Nelson, G., Criswell, S. R., Ho, P., Lonzanida, J. A., Checkoway, H. (2014). Quantitative neuropathology associated with chronic manganese exposure in South African mine workers. Neurotoxicology 45, 260–266. doi: 10.1016/j.neuro.2013.12.008
Gonzalez-Estecha, M., Bodas-Pinedo, A., Rubio-Herrera, M. A., Martell-Claros, N., Trasobares-Iglesias, E. M., Ordonez-Iriarte, J. M. (2014). [The effects of methylmercury on health in children and adults; national and international studies]. Nutr. Hosp. 30 (5), 989–1007. doi: 10.3305/nh.2014.30.5.7728
Goodrich, J. M., Reddy, P., Naidoo, R. N., Asharam, K., Batterman, S., Dolinoy, D. C. (2016). Prenatal exposures and DNA methylation in newborns: a pilot study in Durban, South Africa. Environ. Sci. Process Impacts 18 (7), 908–917. doi: 10.1039/c6em00074f
Greig, J., Thurtle, N., Cooney, L., Ariti, C., Ahmed, A. O., Ashagre, T. (2014). Association of blood lead level with neurological features in 972 children affected by an acute severe lead poisoning outbreak in Zamfara State, northern Nigeria. PloS One 9 (4), e93716. doi: 10.1371/journal.pone.0093716
Gribble, M. O., Cheng, A., Berger, R. D., Rosman, L., Guallar, E. (2015). Mercury exposure and heart rate variability: a systematic review. Curr. Environ. Health Rep. 2 (3), 304–314. doi: 10.1007/s40572-015-0053-0
Gwimbi, P. (2017). Monitoring SO2emission trends and residents’ perceived health risks from PGM smelting at Selous Metallurgical Complex in Zimbabwe. Int. J. Equity Health 16 (1), 200. doi: 10.1186/s12939-017-0696-6
Hamatui, N., Naidoo, R. N., Kgabi, N. (2016). Respiratory health effects of occupational exposure to charcoal dust in Namibia. Int. J. Occup. Environ. Health 22 (3), 240–248. doi: 10.1080/10773525.2016.1214795
Hanssen, L., Rollin, H., Odland, J. O., Moe, M. K., Sandanger, T. M. (2010). Perfluorinated compounds in maternal serum and cord blood from selected areas of South Africa: results of a pilot study. J. Environ. Monit. 12 (6), 1355–1361. doi: 10.1039/b924420d
Harani, H., Otmane, A., Makrelouf, M., Ouadahi, N., Abdi, A., Berrah, A. (2012). Preliminary evaluation of the antioxidant trace elements in an Algerian patient with type 2 diabetes: special role of manganese and chromium. Ann. Biol. Clin. (Paris) 70 (6), 669–677. doi: 10.1684/abc.2012.0763
Hasnain, S. M., Al-Frayh, A. R., Subiza, J. L., Fernandez-Caldas, E., Casanovas, M., Geith, T. (2012). Sensitization to indigenous pollen and molds and other outdoor and indoor allergens in allergic patients from saudi arabia, United arab emirates, and Sudan. World Allergy Organ J. 5 (6), 59–65. doi: 10.1097/WOX.0b013e31825a73cd
Hassan, Y., Shoeib, T. (2015). Levels of polybrominated diphenyl ethers and novel flame retardants in microenvironment dust from Egypt: an assessment of human exposure. Sci. Total Environ. 505, 47–55. doi: 10.1016/j.scitotenv.2014.09.080
Henriquez-Hernandez, L. A., Boada, L. D., Carranza, C., Perez-Arellano, J. L., Gonzalez-Antuna, A., Camacho, M. (2017). Blood levels of toxic metals and rare earth elements commonly found in e-waste may exert subtle effects on hemoglobin concentration in sub-Saharan immigrants. Environ. Int. 109, 20–28. doi: 10.1016/j.envint.2017.08.023
Hernandez-Vargas, H., Castelino, J., Silver, M. J., Dominguez-Salas, P., Cros, M. P., Durand, G. (2015). Exposure to aflatoxin B1 in utero is associated with DNA methylation in white blood cells of infants in The Gambia. Int. J. Epidemiol. 44 (4), 1238–1248. doi: 10.1093/ije/dyv027
Hinson, A. V., Lokossou, V. K., Schlunssen, V., Agodokpessi, G., Sigsgaard, T., Fayomi, B. (2016). Cotton dust exposure and respiratory disorders among textile workers at a textile company in the southern part of benin. Int. J. Environ. Res. Public Health 13 (9), 895. doi: 10.3390/ijerph13090895
Hlongwana, K. W., Mavundza, E. J., Mohapi, E. P., Kruger, P., Urbach, J., Mukaratirwa, S. (2013). Vector-control personnel’s knowledge, perceptions and practices towards insecticides used for indoor residual spraying in Limpopo Province, South Africa. Parasit. Vectors 6, 118. doi: 10.1186/1756-3305-6-118
Hmaissia Khlifa, K., Ghali, R., Mazigh, C., Aouni, Z., Machgoul, S., Hedhili, A. (2012). Ochratoxin A levels in human serum and foods from nephropathy patients in Tunisia: where are you now? Exp. Toxicol. Pathol. 64 (5), 509–512. doi: 10.1016/j.etp.2010.11.006
Hoffmann, V., Jones, K., Leroy, J. (2015). Mitigating aflatoxin exposure to improve child growth in Eastern Kenya: study protocol for a randomized controlled trial. Trials 16, 552. doi: 10.1186/s13063-015-1064-8
Ibhafidon, L. I., Obaseki, D. O., Erhabor, G. E., Akor, A. A., Irabor, I., Obioh, I. (2014). Respiratory symptoms, lung function and particulate matter pollution in residential indoor environment in Ile-Ife, Nigeria. Niger Med. J. 55 (1), 48–53. doi: 10.4103/0300-1652.128164
Ibraheem, R. M., Johnson, A. B., Abdulkarim, A. A., Biliaminu, S. A. (2014). Serum zinc levels in hospitalized children with acute lower respiratory infections in the north-central region of Nigeria. Afr. Health Sci. 14 (1), 136–142. doi: 10.4314/ahs.v14i1.21
Ikechukwu, I. C., Ojareva, O. I., Ibhagbemien, A. J., Okhoaretor, O. F., Oluwatomi, O. B., Akhalufo, O. S. (2012). Blood lead, calcium, and phosphorus in women with preeclampsia in Edo State, Nigeria. Arch. Environ. Occup. Health 67 (3), 163–169. doi: 10.1080/19338244.2011.619212
Ismail, A. A., Bonner, M. R., Hendy, O., Abdel Rasoul, G., Wang, K., Olson, J. R. (2017a). Comparison of neurological health outcomes between two adolescent cohorts exposed to pesticides in Egypt. PloS One 12 (2), e0172696. doi: 10.1371/journal.pone.0172696
Ismail, A. A., Rohlman, D. S., Abdel Rasoul, G. M., Abou Salem, M. E., Hendy, O. M. (2010). Clinical and biochemical parameters of children and adolescents applying pesticides. Int. J. Occup. Environ. Med. 1 (3), 132–143.
Ismail, A. A., Wang, K., Olson, J. R., Bonner, M. R., Hendy, O., Abdel Rasoul, G. (2017b). The impact of repeated organophosphorus pesticide exposure on biomarkers and neurobehavioral outcomes among adolescent pesticide applicators. J. Toxicol. Environ. Health A 80 (10-12), 542–555. doi: 10.1080/15287394.2017.1362612
Iwegbue, C. M. A., Oliseyenum, E. C., Martincigh, B. S. (2017). Spatio-temporal distribution of metals in household dust from rural, semi-urban and urban environments in the Niger Delta, Nigeria. Environ. Sci. Pollut. Res. Int. 24 (16), 14040–14059. doi: 10.1007/s11356-017-8609-1
Jafta, N., Barregard, L., Jeena, P. M., Naidoo, R. N. (2017). Indoor air quality of low and middle income urban households in Durban, South Africa. Environ. Res. 156, 47–56. doi: 10.1016/j.envres.2017.03.008
Jassal, M. S. (2015). Pediatric asthma and ambient pollutant levels in industrializing nations. Int. Health 7 (1), 7–15. doi: 10.1093/inthealth/ihu081
Jolly, P. E., Akinyemiju, T. F., Jha, M., Aban, I., Gonzalez-Falero, A., Joseph, D. (2015). Temporal Variation and Association of Aflatoxin B(1) Albumin-Adduct Levels with Socio-Economic and Food Consumption Factors in HIV Positive Adults. Toxins (Basel) 7 (12), 5129–5140. doi: 10.3390/toxins7124868
Kabasenche, W. P., Skinner, M. K. (2014). DDT, epigenetic harm, and transgenerational environmental justice. Environ. Health 13, 62. doi: 10.1186/1476-069x-13-62
Kang, M. S., Nkurunziza, P., Muwanika, R., Qian, G., Tang, L., Song, X. (2015). Longitudinal evaluation of aflatoxin exposure in two cohorts in south-western Uganda. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 32 (8), 1322–1330. doi: 10.1080/19440049.2015.1048749
Karagas, M. R., Choi, A. L., Oken, E., Horvat, M., Schoeny, R., Kamai, E. (2012). Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 120 (6), 799–806. doi: 10.1289/ehp.1104494
Karaye, K. M., Yahaya, I. A., Lindmark, K., Henein, M. Y. (2015). Serum selenium and ceruloplasmin in nigerians with peripartum cardiomyopathy. Int. J. Mol. Sci. 16 (4), 7644–7654. doi: 10.3390/ijms16047644
Karunamoorthi, K., Mohammed, A., Jemal, Z. (2011). Peasant association member’s knowledge, attitudes, and practices towards safe use of pesticide management. Am. J. Ind. Med. 54 (12), 965–970. doi: 10.1002/ajim.21008
Karunamoorthi, K., Mohammed, M., Wassie, F. (2012). Knowledge and practices of farmers with reference to pesticide management: implications on human health. Arch. Environ. Occup. Health 67 (2), 109–116. doi: 10.1080/19338244.2011.598891
Kashala-Abotnes, E., Mumbere, P. P., Mishika, J. M., Ndjukendi, A. O., Mpaka, D. B., Bumoko, M. G. (2016). Lead exposure and early child neurodevelopment among children 12-24 months in Kinshasa, the Democratic Republic of Congo. Eur. Child Adolesc. Psychiatry 25 (12), 1361–1367. doi: 10.1007/s00787-016-0860-3
Kew, M. C. (2013). Aflatoxins as a cause of hepatocellular carcinoma. J. Gastrointestin Liver Dis. 22 (3), 305–310.
Khairy, M. A., Lohmann, R. (2013). Source apportionment and risk assessment of polycyclic aromatic hydrocarbons in the atmospheric environment of Alexandria, Egypt. Chemosphere 91 (7), 895–903. doi: 10.1016/j.chemosphere.2013.02.018
Khaled, E. M., Meguid, N. A., Bjorklund, G., Gouda, A., Bahary, M. H., Hashish, A. (2016). Altered urinary porphyrins and mercury exposure as biomarkers for autism severity in Egyptian children with autism spectrum disorder. Metab. Brain Dis. 31 (6), 1419–1426. doi: 10.1007/s11011-016-9870-6
Khan, K., Ismail, A. A., Abdel Rasoul, G., Bonner, M. R., Lasarev, M. R., Hendy, O. (2014). Longitudinal assessment of chlorpyrifos exposure and self-reported neurological symptoms in adolescent pesticide applicators. BMJ Open 4 (3), e004177. doi: 10.1136/bmjopen-2013-004177
Khlangwiset, P., Shephard, G. S., Wu, F. (2011). Aflatoxins and growth impairment: a review. Crit. Rev. Toxicol. 41 (9), 740–755. doi: 10.3109/10408444.2011.575766
Khlifi, R., Olmedo, P., Gil, F., Feki-Tounsi, M., Chakroun, A., Rebai, A. (2013a). Blood nickel and chromium levels in association with smoking and occupational exposure among head and neck cancer patients in Tunisia. Environ. Sci. Pollut. Res. Int. 20 (11), 8282–8294. doi: 10.1007/s11356-013-1466-7
Khlifi, R., Olmedo, P., Gil, F., Hammami, B., Chakroun, A., Rebai, A. (2013b). Arsenic, cadmium, chromium and nickel in cancerous and healthy tissues from patients with head and neck cancer. Sci. Total Environ. 452–453, 58–67. doi: 10.1016/j.scitotenv.2013.02.050
Khlifi, R., Olmedo, P., Gil, F., Hammami, B., Hamza-Chaffai, A. (2015). Cadmium and nickel in blood of Tunisian population and risk of nasosinusal polyposis disease. Environ. Sci. Pollut. Res. Int. 22 (5), 3586–3593. doi: 10.1007/s11356-014-3619-8
Khlifi, R., Olmedo, P., Gil, F., Hammami, B., Hamza-Chaffai, A., Rebai, A. (2017). Gene-environment interactions between ERCC2, ERCC3, XRCC1 and cadmium exposure in nasal polyposis disease. J. Appl. Genet. 58 (2), 221–229. doi: 10.1007/s13353-016-0375-0
Kigozi, R., Baxi, S. M., Gasasira, A., Sserwanga, A., Kakeeto, S., Nasr, S. (2012). Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PloS One 7 (8), e42857. doi: 10.1371/journal.pone.0042857
Kimanya, M. E., De Meulenaer, B., Roberfroid, D., Lachat, C., Kolsteren, P. (2010). Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 54 (11), 1659–1667. doi: 10.1002/mnfr.200900483
Kishosha, P. A., Galukande, M., Gakwaya, A. M. (2011). Selenium deficiency a factor in endemic goiter persistence in sub-Saharan Africa. World J. Surg. 35 (7), 1540–1545. doi: 10.1007/s00268-011-1096-5
Kumar, S., Sharma, S., Thaker, R. (2017). Occupational, environmental, and lifestyle factors and their contribution to preterm birth - an overview. Indian J. Occup. Environ. Med. 21 (1), 9–17. doi: 10.4103/ijoem.IJOEM_155_16
Kuti, B. P., Omole, K. O., Kuti, D. K. (2017). Factors associated with childhood asthma control in a resource-poor center. J. Family Med. Prim. Care 6 (2), 222–230. doi: 10.4103/jfmpc.jfmpc_271_16
Lacey, F. G., Henze, D. K., Lee, C. J., van Donkelaar, A., Martin, R. V. (2017). Transient climate and ambient health impacts due to national solid fuel cookstove emissions. Proc. Natl. Acad. Sci. U. S. A. 114 (6), 1269–1274. doi: 10.1073/pnas.1612430114
Ladep, N. G., Lesi, O. A., Mark, P., Lemoine, M., Onyekwere, C., Afihene, M. (2014). Problem of hepatocellular carcinoma in West Africa. World J. Hepatol. 6 (11), 783–792. doi: 10.4254/wjh.v6.i11.783
Lawin, H., Agodokpessi, G., Ayelo, P., Kagima, J., Sonoukon, R., Mbatchou Ngahane, B. H. (2016). A cross-sectional study with an improved methodology to assess occupational air pollution exposure and respiratory health in motorcycle taxi driving. Sci. Total Environ. 550, 1–5. doi: 10.1016/j.scitotenv.2016.01.068
Lawin, H., Ayi Fanou, L., Hinson, V., Wanjiku, J., Ukwaja, N. K., Gordon, S. B. (2017). Exhaled carbon monoxide: a non-invasive biomarker of short-term exposure to outdoor air pollution. BMC Public Health 17 (1), 320. doi: 10.1186/s12889-017-4243-6
Lekei, E. E., Ngowi, A. V., London, L. (2014). Farmers’ knowledge, practices and injuries associated with pesticide exposure in rural farming villages in Tanzania. BMC Public Health 14, 389. doi: 10.1186/1471-2458-14-389
Lin, H., Guo, Y., Di, Q., Zheng, Y., Kowal, P., Xiao, J. (2017a). Ambient PM 2.5 and stroke: effect modifiers and population attributable risk in six low- and middle-income countries. Stroke 48 (5), 1191–1197. doi: 10.1161/strokeaha.116.015739
Lin, Y. C., Nunez, V., Johns, R., Shiao, S. P. (2017b). APOA5 gene polymorphisms and cardiovascular diseases: metaprediction in global populations. Nurs. Res. 66 (2), 164–174. doi: 10.1097/nnr.0000000000000207
Liu, Y., Chang, C. C., Marsh, G. M., Wu, F. (2012). Population attributable risk of aflatoxin-related liver cancer: systematic review and meta-analysis. Eur. J. Cancer 48 (14), 2125–2136. doi: 10.1016/j.ejca.2012.02.009
Lo, A. C., Soliman, A. S., Khaled, H. M., Aboelyazid, A., Greenson, J. K. (2010). Lifestyle, occupational, and reproductive factors and risk of colorectal cancer. Dis. Colon Rectum 53 (5), 830–837. doi: 10.1007/DCR.0b013e3181d320b1
Loha, E., Lunde, T. M., Lindtjorn, B. (2012). Effect of bednets and indoor residual spraying on spatio-temporal clustering of malaria in a village in south Ethiopia: a longitudinal study. PloS One 7 (10), e47354. doi: 10.1371/journal.pone.0047354
Lubick, N. (2010). Examining DDT’s urogenital effects. Environ. Health Perspect. 118 (1), A18. doi: 10.1289/ehp.118-a18
Lynch, M. L., Huang, L. S., Cox, C., Strain, J. J., Myers, G. J., Bonham, M. P. (2011). Varying coefficient function models to explore interactions between maternal nutritional status and prenatal methylmercury toxicity in the Seychelles Child Development Nutrition Study. Environ. Res. 111 (1), 75–80. doi: 10.1016/j.envres.2010.09.005
Magauzi, R., Mabaera, B., Rusakaniko, S., Chimusoro, A., Ndlovu, N., Tshimanga, M. (2011). Health effects of agrochemicals among farm workers in commercial farms of Kwekwe district, Zimbabwe. Pan Afr. Med. J. 9, 26.
Makamure, M. T., Reddy, P., Chuturgoon, A., Naidoo, R. N., Mentz, G., Batterman, S. (2016). Tumour necrosis factor alpha polymorphism (TNF-308alpha G/A) in association with asthma related phenotypes and air pollutants among children in KwaZulu-Natal. Asian Pac. J. Allergy Immunol. 34 (3), 217–222.
Makamure, M. T., Reddy, P., Chuturgoon, A., Naidoo, R. N., Mentz, G., Batterman, S. (2017). Interaction between ambient pollutant exposure, CD14 (-159) polymorphism and respiratory outcomes among children in Kwazulu-Natal, Durban. Hum. Exp. Toxicol. 36 (3), 238–246. doi: 10.1177/0960327116646620
Malley, C. S., Kuylenstierna, J. C., Vallack, H. W., Henze, D. K., Blencowe, H., Ashmore, M. R. (2017). Preterm birth associated with maternal fine particulate matter exposure: a global, regional and national assessment. Environ. Int. 101, 173–182. doi: 10.1016/j.envint.2017.01.023
Mamane, A., Tessier, J. F., Bouvier, G., Salamon, R., Lebailly, P., Raherison, C. (2016). Increase in the risk of respiratory disorders in adults and children related to crop-growing in Niger. J. Environ. Public Health 2016, 9848520. doi: 10.1155/2016/9848520
Manyilizu, W. B., Mdegela, R. H., Helleve, A., Skjerve, E., Kazwala, R., Nonga, H. (2017). Self-reported symptoms and pesticide use among farm workers in Arusha, Northern Tanzania: a cross sectional study. Toxics 5 (4), 25. doi: 10.3390/toxics5040024
Manyilizu, W. B., Mdegela, R. H., Kazwala, R., Nonga, H., Muller, M., Lie, E. (2016). Association of long-term pesticide exposure and biologic parameters in female farm workers in tanzania: a cross sectional study. Toxics 4 (4), 25. doi: 10.3390/toxics4040025
Maouche, N., Meskine, D., Alamir, B., Koceir, E. A. (2015). Trace elements profile is associated with insulin resistance syndrome and oxidative damage in thyroid disorders: Manganese and selenium interest in Algerian participants with dysthyroidism. J. Trace Elem. Med. Biol. 32, 112–121. doi: 10.1016/j.jtemb.2015.07.002
Mathee, A., de Jager, P., Naidoo, S., Naicker, N. (2017). Exposure to lead in South African shooting ranges. Environ. Res. 153, 93–98. doi: 10.1016/j.envres.2016.11.021
Mathee, A., Naicker, N., Teare, J. (2015). Retrospective investigation of a lead poisoning outbreak from the consumption of an ayurvedic medicine: Durban, South Africa. Int. J. Environ. Res. Public Health 12 (7), 7804–7813. doi: 10.3390/ijerph120707804
Matsuda, Y., Wakai, T., Kubota, M., Osawa, M., Sanpei, A., Fujimaki, S. (2013). Mycotoxins are conventional and novel risk biomarkers for hepatocellular carcinoma. World J. Gastroenterol. 19 (17), 2587–2590. doi: 10.3748/wjg.v19.i17.2587
Mbatchou Ngahane, B. H., Noah, D., Nganda Motto, M., Mapoure Njankouo, Y., Njock, L. R. (2016). Sensitization to common aeroallergens in a population of young adults in a sub-Saharan Africa setting: a cross-sectional study. Allergy Asthma Clin. Immunol. 12, 1. doi: 10.1186/s13223-015-0107-8
Mbelambela, E. P., Hirota, R., Eitoku, M., Muchanga, S. M. J., Kiyosawa, H., Yasumitsu-Lovell, K. (2017). Occupation exposed to road-traffic emissions and respiratory health among Congolese transit workers, particularly bus conductors, in Kinshasa: a cross-sectional study. Environ. Health Prev. Med. 22 (1), 11. doi: 10.1186/s12199-017-0608-9
McGlynn, K. A., Petrick, J. L., London, W. T. (2015). Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin. Liver Dis. 19 (2), 223–238. doi: 10.1016/j.cld.2015.01.001
McMillan, A., Renaud, J. B., Burgess, K. M. N., Orimadegun, A. E., Akinyinka, O. O., Allen, S. J. (2018). Aflatoxin exposure in Nigerian children with severe acute malnutrition. Food Chem. Toxicol. 111, 356–362. doi: 10.1016/j.fct.2017.11.030
Mekonen, S., Ambelu, A., Spanoghe, P. (2015). Effect of household coffee processing on pesticide residues as a means of ensuring consumers’ safety. J. Agric. Food Chem. 63 (38), 8568–8573. doi: 10.1021/acs.jafc.5b03327
Mensah, E. K., Afari, E., Wurapa, F., Sackey, S., Quainoo, A., Kenu, E. (2016). Exposure of small-scale gold miners in prestea to mercury, ghana, 2012. Pan Afr. Med. J. 25 (Suppl 1), 6. doi: 10.11604/pamj.supp.2016.25.1.6171
Mentz, G., Robins, T. G., Batterman, S., Naidoo, R. N. (2018). Acute respiratory symptoms associated with short term fluctuations in ambient pollutants among schoolchildren in Durban, South Africa. Environ. Pollut. 233, 529–539. doi: 10.1016/j.envpol.2017.10.108
Mitchell, N. J., Hsu, H. H., Chandyo, R. K., Shrestha, B., Bodhidatta, L., Tu, Y. K. (2017). Aflatoxin exposure during the first 36 months of life was not associated with impaired growth in Nepalese children: an extension of the MAL-ED study. PloS One 12 (2), e0172124. doi: 10.1371/journal.pone.0172124
Mitchell, N. J., Kumi, J., Johnson, N. M., Dotse, E., Marroquin-Cardona, A., Wang, J. S. (2013). Reduction in the urinary aflatoxin M1 biomarker as an early indicator of the efficacy of dietary interventions to reduce exposure to aflatoxins. Biomarkers 18 (5), 391–398. doi: 10.3109/1354750x.2013.798031
Mohamed Fel, B., Zaky, E. A., El-Sayed, A. B., Elhossieny, R. M., Zahra, S. S., Salah Eldin, W. (2015). Assessment of hair aluminum, lead, and mercury in a sample of autistic egyptian children: environmental risk factors of heavy metals in autism. Behav. Neurol. 2015, 545674. doi: 10.1155/2015/545674
Mohammed, A. A., Mohamed, F. Y., El-Okda el, S., Ahmed, A. B. (2015). Blood lead levels and childhood asthma. Indian Pediatr. 52 (4), 303–306.
Mokdad, A. H., GBD 2015 Eastern Mediterranean Region Lower Respiratory Infections Collaborators. (2018). Burden of lower respiratory infections in the Eastern Mediterranean Region between 1990 and 2015: findings from the Global Burden of Disease 2015 study. Int. J. Public Health63 (Suppl 1), S97-S108. doi: 10.1007/s00038-017-1007-0
Moolla, R., Curtis, C. J., Knight, J. (2015). Occupational exposure of diesel station workers to BTEX compounds at a bus depot. Int. J. Environ. Res. Public Health 12 (4), 4101–4115. doi: 10.3390/ijerph120404101
Morakinyo, O. M., Adebowale, A. S., Mokgobu, M. I., Mukhola, M. S. (2017). Health risk of inhalation exposure to sub-10 microm particulate matter and gaseous pollutants in an urban-industrial area in South Africa: an ecological study. BMJ Open 7 (3), e013941. doi: 10.1136/bmjopen-2016-013941
Morcos, M. M., Morcos, W. M., Ibrahim, M. A., Shaheen, M. A. (2011). Environmental exposure to endotoxin in rural and urban Egyptian school children and its relation to asthma and atopy. Minerva Pediatr. 63 (1), 19–26.
Moszynski, P. (2010). Lead poisoning in Nigeria causes “unprecedented” emergency. BMJ 341, c4031. doi: 10.1136/bmj.c4031
Motawei, S. M., Attalla, S. M., Gouda, H. E., El-Harouny, M. A., El-Mansoury, A. M. (2013). Lead level in pregnant women suffering from pre-eclampsia in Dakahlia, Egypt. Int. J. Occup. Environ. Med. 4 (1), 36–44.
Motsoeneng, P. M., Dalvie, M. A. (2015). Relationship between urinary pesticide residue levels and neurotoxic symptoms among women on farms in the Western Cape, South Africa. Int. J. Environ. Res. Public Health 12 (6), 6281–6299. doi: 10.3390/ijerph120606281
Mulder, N. J., Adebiyi, E., Alami, R., Benkahla, A., Brandful, J., Doumbia, S. (2016). H3ABioNet, a sustainable pan-African bioinformatics network for human heredity and health in Africa. Genome Res. 26 (2), 271–277. doi: 10.1101/gr.196295.115
Muller, M. H., Polder, A., Brynildsrud, O. B., Lie, E., Loken, K. B., Manyilizu, W. B. (2016). Brominated flame retardants (BFRs) in breast milk and associated health risks to nursing infants in Northern Tanzania. Environ. Int., 89–90, 38–47. doi: 10.1016/j.envint.2015.12.032
Munung, N. S., Marshall, P., Campbell, M., Littler, K., Masiye, F., Ouwe-Missi-Oukem-Boyer, O. (2016). Obtaining informed consent for genomics research in Africa: analysis of H3Africa consent documents. J. Med. Ethics 42 (2), 132–137. doi: 10.1136/medethics-2015-102796
Mupunga, I., Mngqawa, P., Katerere, D. R. (2017). Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients 9 (12), 1287. doi: 10.3390/nu9121287
Murray, J., Eskenazi, B., Bornman, R., Gaspar, F. W., Crause, M., Obida, M. (2018). Exposure to DDT and hypertensive disorders of pregnancy among South African women from an indoor residual spraying region: The VHEMBE study. Environ. Res. 162, 49–54. doi: 10.1016/j.envres.2017.12.006
Myers, J., London, L., Lucchini, R. G. (2014). Neurotoxicology and development: human, environmental, and social impacts. NeuroToxicology 45, 217–219.
Nahar, M. S., Soliman, A. S., Colacino, J. A., Calafat, A. M., Battige, K., Hablas, A. (2012). Urinary bisphenol A concentrations in girls from rural and urban Egypt: a pilot study. Environ. Health 11, 20. doi: 10.1186/1476-069x-11-20
Naicker, N., Norris, S. A., Mathee, A., Becker, P., Richter, L. (2010). Lead exposure is associated with a delay in the onset of puberty in South African adolescent females: findings from the Birth to Twenty cohort. Sci. Total Environ. 408 (21), 4949–4954. doi: 10.1016/j.scitotenv.2010.07.037
Naicker, N., Richter, L., Mathee, A., Becker, P., Norris, S. A. (2012). Environmental lead exposure and socio-behavioural adjustment in the early teens: the birth to twenty cohort. Sci. Total Environ. 414, 120–125. doi: 10.1016/j.scitotenv.2011.11.013
Naidoo, S., London, L., Rother, H. A., Burdorf, A., Naidoo, R. N., Kromhout, H. (2010). Pesticide safety training and practices in women working in small-scale agriculture in South Africa. Occup. Environ. Med. 67 (12), 823–828. doi: 10.1136/oem.2010.055863
Ndlovu, V., Dalvie, M. A., Jeebhay, M. F. (2014). Asthma associated with pesticide exposure among women in rural Western Cape of South Africa. Am. J. Ind. Med. 57 (12), 1331–1343. doi: 10.1002/ajim.22384
Negatu, B., Vermeulen, R., Mekonnen, Y., Kromhout, H. (2018). Neurobehavioural symptoms and acute pesticide poisoning: a cross-sectional study among male pesticide applicators selected from three commercial farming systems in Ethiopia. Occup. Environ. Med.75 (4), 283–289. doi: 10.1136/oemed-2017-104538
Niare-Doumbo, S., Normand, A. C., Diallo, Y. L., Dembele, A. K., Thera, M. A., Diallo, D. (2014). Preliminary study of the fungal ecology at the haematology and medical-oncology ward in Bamako, Mali. Mycopathologia 178 (1-2), 103–109. doi: 10.1007/s11046-014-9760-6
Nkhama, E., Ndhlovu, M., Dvonch, J. T., Lynam, M., Mentz, G., Siziya, S. (2017). Effects of airborne particulate matter on respiratory health in a community near a cement factory in chilanga, zambia: results from a panel study. Int. J. Environ. Res. Public Health 14 (11), 1351. doi: 10.3390/ijerph14111351
Nkomo, P., Mathee, A., Naicker, N., Galpin, J., Richter, L. M., Norris, S. A. (2017). The association between elevated blood lead levels and violent behavior during late adolescence: The South African Birth to Twenty Plus cohort. Environ. Int. 109, 136–145. doi: 10.1016/j.envint.2017.09.004
Nkosi, V., Wichmann, J., Voyi, K. (2017). Indoor and outdoor PM10 levels at schools located near mine dumps in Gauteng and North West Provinces, South Africa. BMC Public Health 17 (1), 42. doi: 10.1186/s12889-016-3950-8
Noubiap, J. J., Essouma, M., Bigna, J. J. (2015). Targeting Household Air Pollution for Curbing the Cardiovascular Disease Burden: A Health Priority in Sub-Saharan Africa. J. Clin. Hypertens. (Greenwich) 17 (10), 825–829. doi: 10.1111/jch.12610
Nweke, O. C., Sanders, W. H. (2009). Modern environmental health hazards: a public health issue of increasing significance in Africa. Environ. Health Perspect. 117 (6), 863–870. doi: 10.1289/ehp.0800126
Obaseki, D. O., Adeniyi, B., Jumbo, J., Oyewo, A., Irabor, I., Erhabor, G. E. (2014). Respiratory symptom, lung function and exhaled carbon monoxide among a sample of traffic workers in Lagos, Nigeria: a pilot survey. Niger Med. J. 55 (4), 306–309. doi: 10.4103/0300-1652.137190
Obi, E., Okafor, C., Igwebe, A., Ebenebe, J., Afonne, O. J., Ifediata, F. (2015). Elevated prenatal methylmercury exposure in Nigeria: evidence from maternal and cord blood. Chemosphere 119, 485–489. doi: 10.1016/j.chemosphere.2014.07.038
Obiri, S., Ansa-Asare, O. D., Mohammed, S., Darko, H. F., Dartey, A. G. (2016a). Exposure to toxicants in soil and bottom ash deposits in Agbogbloshie, Ghana: human health risk assessment. Environ. Monit. Assess. 188 (10), 583. doi: 10.1007/s10661-016-5575-x
Obiri, S., Cobbina, S. J., Armah, F. A., Luginaah, I. (2013). Assessment of cancer and noncancer health risks from exposure to PAHs in street dust in the Tamale Metropolis, Ghana. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 48 (4), 408–416. doi: 10.1080/10934529.2013.728914
Obiri, S., Yeboah, P. O., Osae, S., Adu-Kumi, S., Cobbina, S. J., Armah, F. A. (2016b). Human health risk assessment of artisanal miners exposed to toxic chemicals in water and sediments in the Prestea Huni Valley district of Ghana. Int. J. Environ. Res. Public Health 13 (1), 139. doi: 10.3390/ijerph13010139
Obuseh, F. A., Jolly, P. E., Kulczycki, A., Ehiri, J., Waterbor, J., Desmond, R. A. (2011). Aflatoxin levels, plasma vitamins A and E concentrations, and their association with HIV and hepatitis B virus infections in Ghanaians: a cross-sectional study. J. Int. AIDS Soc. 14, 53. doi: 10.1186/1758-2652-14-53
Ode, A., Rylander, L., Lindh, C. H., Kallen, K., Jonsson, B. A., Gustafsson, P. (2013). Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ. Sci. Pollut. Res. Int. 20 (11), 7970–7978. doi: 10.1007/s11356-013-1573-5
Oesterlund, A. H., Thomsen, J. F., Sekimpi, D. K., Maziina, J., Racheal, A., Jors, E. (2014). Pesticide knowledge, practice and attitude and how it affects the health of small-scale farmers in Uganda: a cross-sectional study. Afr. Health Sci. 14 (2), 420–433. doi: 10.4314/ahs.v14i2.19
Ogundele, L. T., Owoade, O. K., Hopke, P. K., Olise, F. S. (2017). Heavy metals in industrially emitted particulate matter in Ile-Ife, Nigeria. Environ. Res. 156, 320–325. doi: 10.1016/j.envres.2017.03.051
Ojo, J. O., Oketayo, O. O., Adesanmi, C. A., Horvat, M., Mazej, D., Tratnik, J. (2014). Influence of nutritional status on some toxic and essential elements in the blood of women exposed to vehicular pollution in Ile-Ife, Nigeria. Environ. Sci. Pollut. Res. Int. 21 (2), 1124–1132. doi: 10.1007/s11356-013-1951-z
Okonya, J. S., Kroschel, J. (2015). A cross-sectional study of pesticide use and knowledge of smallholder potato farmers in Uganda. BioMed. Res. Int. 2015, 759049. doi: 10.1155/2015/759049
Olatunji, A. S., Olajide-Kayode, J. O., Abimbola, A. F. (2014). Evaluation of geochemical characteristics and health effects of some geophagic clays southern Nigeria. Environ. Geochem. Health 36 (6), 1105–1114. doi: 10.1007/s10653-014-9619-2
Olopade, C. O., Frank, E., Bartlett, E., Alexander, D., Dutta, A., Ibigbami, T. (2017). Effect of a clean stove intervention on inflammatory biomarkers in pregnant women in Ibadan, Nigeria: A randomized controlled study. Environ. Int. 98, 181–190. doi: 10.1016/j.envint.2016.11.004
Oluboyo, A. O., Adijeh, R. U., Onyenekwe, C. C., Oluboyo, B. O., Mbaeri, T. C., Odiegwu, C. N. (2012). Relationship between serum levels of testosterone, zinc and selenium in infertile males attending fertility clinic in Nnewi, south east Nigeria. Afr. J. Med. Med. Sci. 41 Suppl, 51–54.
Oluwagbemi, O., Adebiyi, M., Fatumo, S., Macintyre, G. (2014). Paving the way towards a successful and fulfilling career in computational biology. PloS Comput. Biol. 10 (5), e1003593. doi: 10.1371/journal.pcbi.1003593
Oluwole, O., Arinola, G. O., Ana, G. R., Wiskel, T., Huo, D., Olopade, O. I. (2013). Relationship between household air pollution from biomass smoke exposure, and pulmonary dysfunction, oxidant-antioxidant imbalance and systemic inflammation in rural women and children in Nigeria. Glob J. Health Sci. 5 (4), 28–38. doi: 10.5539/gjhs.v5n4p28
Oluwole, O., Arinola, G. O., Huo, D., Olopade, C. O. (2017). Household biomass fuel use, asthma symptoms severity, and asthma underdiagnosis in rural schoolchildren in Nigeria: a cross-sectional observational study. BMC Pulm. Med. 17 (1), 3. doi: 10.1186/s12890-016-0352-8
Organization, W. H. (2017). Preventing noncommunicable diseases (NCDs) by reducing environmental risk factors. Geneva: World Health Organization.
Osafo, C., Raji, Y. R., Burke, D., Tayo, B. O., Tiffin, N., Moxey-Mims, M. M. (2015). Human heredity and health (H3) in africa kidney disease research network: a focus on methods in Sub-Saharan Africa. Clin. J. Am. Soc. Nephrol. 10 (12), 2279–2287. doi: 10.2215/CJN.11951214
Osunkentan, A. O., Evans, D. (2015). Chronic adverse effects of long-term exposure of children to dichlorodiphenyltrichloroethane (DDT) through indoor residual spraying: a systematic review. Rural Remote Health 15 (2), 2889.
Owolabi, M. O., Mensah, G. A., Kimmel, P. L., Adu, D., Ramsay, M., Waddy, S. P. (2014). Understanding the rise in cardiovascular diseases in Africa: harmonising H3Africa genomic epidemiological teams and tools. Cardiovasc. J. Afr. 25 (3), 134–136. doi: 10.5830/CVJA-2014-030
Oyedele, O. A., Ezekiel, C. N., Sulyok, M., Adetunji, M. C., Warth, B., Atanda, O. O. (2017). Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 251, 24–32. doi: 10.1016/j.ijfoodmicro.2017.03.020
Palliyaguru, D. L., Wu, F. (2013). Global geographical overlap of aflatoxin and hepatitis C: controlling risk factors for liver cancer worldwide. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 30 (3), 534–540. doi: 10.1080/19440049.2012.751630
Pritchett, N. R., Burgert, S. L., Murphy, G. A., Brockman, J. D., White, R. E., Lando, J. (2017). Cross sectional study of serum selenium concentration and esophageal squamous dysplasia in western Kenya. BMC Cancer 17 (1), 835. doi: 10.1186/s12885-017-3837-9
Prüss-Ustün, A., Wolf, J., Corvalán, C. F., Neville, T., Bos, R., Neira, M. (2016). Diseases due to unhealthy environments: an updated estimate of the global burden of disease attributable to environmental determinants of health. J. Public Health 39 (3), 464–475. doi: 10.1093/pubmed/fdw085
Quansah, R., Bend, J. R., Abdul-Rahaman, A., Armah, F. A., Luginaah, I., Essumang, D. K. (2016). Associations between pesticide use and respiratory symptoms: A cross-sectional study in Southern Ghana. Environ. Res. 150, 245–254. doi: 10.1016/j.envres.2016.06.013
Quinn, A. K., Ae-Ngibise, K. A., Jack, D. W., Boamah, E. A., Enuameh, Y., Mujtaba, M. N. (2016). Association of carbon monoxide exposure with blood pressure among pregnant women in rural Ghana: evidence from GRAPHS. Int. J. Hyg. Environ. Health 219 (2), 176–183. doi: 10.1016/j.ijheh.2015.10.004
Quinn, A. K., Ae-Ngibise, K. A., Kinney, P. L., Kaali, S., Wylie, B. J., Boamah, E. (2017). Ambulatory monitoring demonstrates an acute association between cookstove-related carbon monoxide and blood pressure in a Ghanaian cohort. Environ. Health 16 (1), 76. doi: 10.1186/s12940-017-0282-9
Raafat, N., Abass, M. A., Salem, H. M. (2012). Malathion exposure and insulin resistance among a group of farmers in Al-Sharkia governorate. Clin. Biochem. 45 (18), 1591–1595. doi: 10.1016/j.clinbiochem.2012.07.108
Ray, S. S., Das, D., Ghosh, T., Ghosh, A. K. (2012). The levels of zinc and molybdenum in hair and food grain in areas of high and low incidence of esophageal cancer: a comparative study. Glob J. Health Sci. 4 (4), 168–175. doi: 10.5539/gjhs.v4n4p168
Rebacz-Maron, E., Baranowska-Bosiacka, I., Gutowska, I., Chlubek, D. (2013). Blood pressure and levels of Fe, Ca, Mg, Zn, Cu, Na and K in the hair of young Bantu men from Tanzania. Biol. Trace Elem. Res. 151 (3), 350–359. doi: 10.1007/s12011-012-9578-3
Reddy, P., Naidoo, R. N., Robins, T. G., Mentz, G., Li, H., London, S. J. (2012). GSTM1 and GSTP1 gene variants and the effect of air pollutants on lung function measures in South African children. Am. J. Ind. Med. 55 (12), 1078–1086. doi: 10.1002/ajim.22012
Robinson, O., Want, E., Coen, M., Kennedy, R., van den Bosch, C., Gebrehawaria, Y. (2014). Hirmi Valley liver disease: a disease associated with exposure to pyrrolizidine alkaloids and DDT. J. Hepatol. 60 (1), 96–102. doi: 10.1016/j.jhep.2013.07.039
Rohlman, D. S., Ismail, A. A., Abdel-Rasoul, G., Lasarev, M., Hendy, O., Olson, J. R. (2014). Characterizing exposures and neurobehavioral performance in Egyptian adolescent pesticide applicators. Metab. Brain Dis. 29 (3), 845–855. doi: 10.1007/s11011-014-9565-9
Rohlman, D. S., Ismail, A. A., Rasoul, G. A., Bonner, M. R., Hendy, O., Mara, K. (2016). A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex 74, 383–395. doi: 10.1016/j.cortex.2015.09.011
Rohlman, D. S., Nuwayhid, I., Ismail, A., Saddik, B. (2012). Using epidemiology and neurotoxicology to reduce risks to young workers. Neurotoxicology 33 (4), 817–822. doi: 10.1016/j.neuro.2012.02.012
Rollin, H. B., Channa, K., Olutola, B. G., Odland, J. O. (2017). Evaluation of in utero exposure to arsenic in South Africa. Sci. Total Environ. 575, 338–346. doi: 10.1016/j.scitotenv.2016.10.044
Rollin, H. B., Kootbodien, T., Channa, K., Odland, J. O. (2015). Prenatal Exposure to Cadmium, Placental Permeability and Birth Outcomes in Coastal Populations of South Africa. PloS One 10 (11), e0142455. doi: 10.1371/journal.pone.0142455
Rylance, J., Fullerton, D. G., Scriven, J., Aljurayyan, A. N., Mzinza, D., Barrett, S. (2015). Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am. J. Respir. Cell Mol. Biol. 52 (5), 584–593. doi: 10.1165/rcmb.2014-0188OC
Rylance, J., Kankwatira, A., Nelson, D. E., Toh, E., Day, R. B., Lin, H. (2016). Household air pollution and the lung microbiome of healthy adults in Malawi: a cross-sectional study. BMC Microbiol. 16 (1), 182. doi: 10.1186/s12866-016-0803-7
Rylander, C., Phi, D. T., Odland, J. O., Sandanger, T. M. (2009). Perfluorinated compounds in delivering women from south central Vietnam. J. Environ. Monit. 11 (11), 2002–2008. doi: 10.1039/b908551c
Saad-Hussein, A., Beshir, S., Moubarz, G., Elserougy, S., Ibrahim, M. I. (2013). Effect of occupational exposure to aflatoxins on some liver tumor markers in textile workers. Am. J. Ind. Med. 56 (7), 818–824. doi: 10.1002/ajim.22162
Saad-Hussein, A., Taha, M. M., Beshir, S., Shahy, E. M., Shaheen, W., Elhamshary, M. (2014). Carcinogenic effects of aflatoxin B1 among wheat handlers. Int. J. Occup. Environ. Health 20 (3), 215–219. doi: 10.1179/2049396714y.0000000069
Saad-Hussein, A., Taha, M. M., Fadl, N. N., Awad, A. H., Mahdy-Abdallah, H., Moubarz, G. (2016). Effects of airborne Aspergillus on serum aflatoxin B1 and liver enzymes in workers handling wheat flour. Hum. Exp. Toxicol. 35 (1), 3–9. doi: 10.1177/0960327115573596
Saleh, D. A., Amr, S., Jillson, I. A., Wang, J. H., Crowell, N., Loffredo, C. A. (2015). Preventing hepatocellular carcinoma in Egypt: results of a Pilot Health Education Intervention Study. BMC Res. Notes 8, 384. doi: 10.1186/s13104-015-1351-1
Saliu, A., Adebayo, O., Kofoworola, O., Babatunde, O., Ismail, A. (2015). Comparative assessment of blood lead levels of automobile technicians in organised and roadside garages in Lagos, Nigeria. J. Environ. Public Health 2015, 976563. doi: 10.1155/2015/976563
Samir, A. M., Aref, W. M. (2011). Impact of occupational exposure to elemental mercury on some antioxidative enzymes among dental staff. Toxicol. Ind. Health 27 (9), 779–786. doi: 10.1177/0748233710397420
Sankoh, A. I., Whittle, R., Semple, K. T., Jones, K. C., Sweetman, A. J. (2016). An assessment of the impacts of pesticide use on the environment and health of rice farmers in Sierra Leone. Environ. Int. 94, 458–466. doi: 10.1016/j.envint.2016.05.034
Sherief, L. M., Abdelkhalek, E. R., Gharieb, A. F., Sherbiny, H. S., Usef, D. M., Almalky, M. A. (2015). Cadmium status among pediatric cancer patients in Egypt. Med. (Baltimore) 94 (20), e740. doi: 10.1097/md.0000000000000740
Shirima, C. P., Kimanya, M. E., Routledge, M. N., Srey, C., Kinabo, J. L., Humpf, H. U. (2015). A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environ. Health Perspect. 123 (2), 173–178. doi: 10.1289/ehp.1408097
Shuaib, F. M., Jolly, P. E., Ehiri, J. E., Jiang, Y., Ellis, W. O., Stiles, J. K. (2010a). Association between anemia and aflatoxin B1 biomarker levels among pregnant women in Kumasi, Ghana. Am. J. Trop. Med. Hyg. 83 (5), 1077–1083. doi: 10.4269/ajtmh.2010.09-0772
Shuaib, F. M., Jolly, P. E., Ehiri, J. E., Yatich, N., Jiang, Y., Funkhouser, E. (2010b). Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Trop. Med. Int. Health 15 (2), 160–167. doi: 10.1111/j.1365-3156.2009.02435.x
Silva, M. J., Samandar, E., Preau, J. L., Reidy, J. A., Needham, L. L., Calafat, A. M. (2007). Quantification of 22 phthalate metabolites in human urine. J. Chromatogr B Analyt. Technol. BioMed. Life Sci. 860 (1), 106–112. doi: 10.1016/j.jchromb.2007.10.023
Singleton, S. T., Lein, P. J., Dadson, O. A., McGarrigle, B. P., Farahat, F. M., Farahat, T. (2015). Longitudinal assessment of occupational exposures to the organophosphorous insecticides chlorpyrifos and profenofos in Egyptian cotton field workers. Int. J. Hyg. Environ. Health 218 (2), 203–211. doi: 10.1016/j.ijheh.2014.10.005
Singleton, S. T., Lein, P. J., Farahat, F. M., Farahat, T., Bonner, M. R., Knaak, J. B. (2014). Characterization of alpha-cypermethrin exposure in Egyptian agricultural workers. Int. J. Hyg. Environ. Health 217 (4-5), 538–545. doi: 10.1016/j.ijheh.2013.10.003
Smith, L. E., Prendergast, A. J., Turner, P. C., Mbuya, M. N., Mutasa, K., Kembo, G. (2015). The potential role of mycotoxins as a contributor to stunting in the SHINE trial. Clin. Infect. Dis. 61 Suppl 7, S733–S737. doi: 10.1093/cid/civ849
Sosan, M. B., Akingbohungbe, A. E., Durosinmi, M. A., Ojo, I. A. (2010). Erythrocyte cholinesterase enzyme activity and hemoglobin values in cacao farmers of southwestern Nigeria as related to insecticide exposure. Arch. Environ. Occup. Health 65 (1), 27–33. doi: 10.1080/19338240903390289
Ssemugabo, C., Halage, A. A., Neebye, R. M., Nabankema, V., Kasule, M. M., Ssekimpi, D. (2017). Prevalence, circumstances, and management of acute pesticide poisoning in hospitals in Kampala City, Uganda. Environ. Health Insights 11, 1178630217728924. doi: 10.1177/1178630217728924
Steckling, N., Bose-O’Reilly, S., Pinheiro, P., Plass, D., Shoko, D., Drasch, G. (2014). The burden of chronic mercury intoxication in artisanal small-scale gold mining in Zimbabwe: data availability and preliminary estimates. Environ. Health 13, 111. doi: 10.1186/1476-069x-13-111
Strain, J. J., Yeates, A. J., van Wijngaarden, E., Thurston, S. W., Mulhern, M. S., McSorley, E. M. (2015). Prenatal exposure to methyl mercury from fish consumption and polyunsaturated fatty acids: associations with child development at 20 mo of age in an observational study in the Republic of Seychelles. Am. J. Clin. Nutr. 101 (3), 530–537. doi: 10.3945/ajcn.114.100503
Sun, H., Qi, Y., Zhang, D., Li, Q. X., Wang, J. (2016). Concentrations, distribution, sources and risk assessment of organohalogenated contaminants in soils from Kenya, Eastern Africa. Environ. Pollut. 209, 177–185. doi: 10.1016/j.envpol.2015.11.040
Suter, M. K., Karr, C. J., John-Stewart, G. C., Gomez, L. A., Moraa, H., Nyatika, D. (2018). Implications of combined exposure to household air pollution and HIV on neurocognition in children. Int. J. Environ. Res. Public Health 15 (1), 163. doi: 10.3390/ijerph15010163
Swart, R., Schutte, A. E., van Rooyen, J. M., Mels, C. M. C. (2018). Serum selenium levels, the selenoprotein glutathione peroxidase and vascular protection: The SABPA study. Food Res. Int. 104, 69–76. doi: 10.1016/j.foodres.2017.06.054
Tawfik Khattab, A. M., Zayed, A. A., Ahmed, A. I., AbdelAal, A. G., Mekdad, A. A. (2016). The role of PON1 and CYP2D6 genes in susceptibility to organophosphorus chronic intoxication in Egyptian patients. Neurotoxicology 53, 102–107. doi: 10.1016/j.neuro.2015.12.015
Tetteh, D., Lengel, L. (2017). The urgent need for health impact assessment: proposing a transdisciplinary approach to the e-waste crisis in sub-Saharan Africa. Glob Health Promot. 24 (2), 35–42. doi: 10.1177/1757975916686926
Tewari, S., Brousse, V., Piel, F. B., Menzel, S., Rees, D. C. (2015). Environmental determinants of severity in sickle cell disease. Haematologica 100 (9), 1108–1116. doi: 10.3324/haematol.2014.120030
Thompson, L. A., Darwish, W. S., Ikenaka, Y., Nakayama, S. M., Mizukawa, H., Ishizuka, M. (2017). Organochlorine pesticide contamination of foods in Africa: incidence and public health significance. J. Vet. Med. Sci. 79 (4), 751–764. doi: 10.1292/jvms.16-0214
Thompson, L. A., Ikenaka, Y., Yohannes, Y. B., Ichise, T., Ito, G., Bortey-Sam, N. (2018). Human health risk from consumption of marine fish contaminated with DDT and its Mmetabolites in Maputo Bay, Mozambique. Bull. Environ. Contam. Toxicol. 100 (5), 672–676. doi: 10.1007/s00128-018-2323-7
Thurtle, N., Greig, J., Cooney, L., Amitai, Y., Ariti, C., Brown, M. J. (2014). Description of 3,180 courses of chelation with dimercaptosuccinic acid in children </ = 5 y with severe lead poisoning in Zamfara, Northern Nigeria: a retrospective analysis of programme data. PloS Med. 11 (10), e1001739. doi: 10.1371/journal.pmed.1001739
Tomicic, C., Vernez, D., Belem, T., Berode, M. (2011). Human mercury exposure associated with small-scale gold mining in Burkina Faso. Int. Arch. Occup. Environ. Health 84 (5), 539–546. doi: 10.1007/s00420-011-0615-x
Tomoum, H. Y., Mostafa, G. A., Ismail, N. A., Ahmed, S. M. (2010). Lead exposure and its association with pubertal development in school-age Egyptian children: pilot study. Pediatr. Int. 52 (1), 89–93. doi: 10.1111/j.1442-200X.2009.02893.x
van Wijngaarden, E., Thurston, S. W., Myers, G. J., Harrington, D., Cory-Slechta, D. A., Strain, J. J. (2017). Methyl mercury exposure and neurodevelopmental outcomes in the Seychelles Child Development Study Main cohort at age 22 and 24years. Neurotoxicol. Teratol. 59, 35–42. doi: 10.1016/j.ntt.2016.10.011
van Wijngaarden, E., Thurston, S. W., Myers, G. J., Strain, J. J., Weiss, B., Zarcone, T. (2013). Prenatal methyl mercury exposure in relation to neurodevelopment and behavior at 19 years of age in the Seychelles child development study. Neurotoxicol. Teratol. 39, 19–25. doi: 10.1016/j.ntt.2013.06.003
Van Zijl, M. C., Aneck-Hahn, N. H., Swart, P., Hayward, S., Genthe, B., De Jager, C. (2017). Estrogenic activity, chemical levels and health risk assessment of municipal distribution point water from Pretoria and Cape Town, South Africa. Chemosphere 186, 305–313. doi: 10.1016/j.chemosphere.2017.07.130
Vanker, A., Barnett, W., Nduru, P. M., Gie, R. P., Sly, P. D., Zar, H. J. (2015). Home environment and indoor air pollution exposure in an African birth cohort study. Sci. Total Environ. 536, 362–367. doi: 10.1016/j.scitotenv.2015.06.136
VoPham, T., Bertrand, K. A., Hart, J. E., Laden, F., Brooks, M. M., Yuan, J. M. (2017). Pesticide exposure and liver cancer: a review. Cancer Causes Control 28 (3), 177–190. doi: 10.1007/s10552-017-0854-6
Wafa, T., Nadia, K., Amel, N., Ikbal, C., Insaf, T., Asma, K. (2013). Oxidative stress, hematological and biochemical alterations in farmers exposed to pesticides. J. Environ. Sci. Health B 48 (12), 1058–1069. doi: 10.1080/03601234.2013.824285
Warnich, L., Drogemoller, B. I., Pepper, M. S., Dandara, C., Wright, G. E. (2011). Pharmacogenomic research in south africa: lessons learned and future opportunities in the rainbow nation. Curr. Pharmacogenomics Person Med. 9 (3), 191–207. doi: 10.2174/187569211796957575
Watson, G. E., Evans, K., Thurston, S. W., van Wijngaarden, E., Wallace, J. M., McSorley, E. M. (2012). Prenatal exposure to dental amalgam in the Seychelles Child development nutrition study: associations with neurodevelopmental outcomes at 9 and 30 months. Neurotoxicology 33 (6), 1511–1517. doi: 10.1016/j.neuro.2012.10.001
Watson, G. E., Lynch, M., Myers, G. J., Shamlaye, C. F., Thurston, S. W., Zareba, G. (2011). Prenatal exposure to dental amalgam: evidence from the Seychelles child development study main cohort. J. Am. Dent. Assoc. 142 (11), 1283–1294. doi: 10.14219/jada.archive.2011.0114
Watson, G. E., van Wijngaarden, E., Love, T. M., McSorley, E. M., Bonham, M. P., Mulhern, M. S. (2013). Neurodevelopmental outcomes at 5 years in children exposed prenatally to maternal dental amalgam: the Seychelles child development nutrition study. Neurotoxicol. Teratol. 39, 57–62. doi: 10.1016/j.ntt.2013.07.003
Watson, S., Chen, G., Sylla, A., Routledge, M. N., Gong, Y. Y. (2016). Dietary exposure to aflatoxin and micronutrient status among young children from Guinea. Mol. Nutr. Food Res. 60 (3), 511–518. doi: 10.1002/mnfr.201500382
Watson, S., Gong, Y. Y., Routledge, M. (2017). Interventions targeting child undernutrition in developing countries may be undermined by dietary exposure to aflatoxin. Crit. Rev. Food Sci. Nutr. 57 (9), 1963–1975. doi: 10.1080/10408398.2015.1040869
Were, F. H., Moturi, M. C., Gottesfeld, P., Wafula, G. A., Kamau, G. N., Shiundu, P. M. (2014). Lead exposure and blood pressure among workers in diverse industrial plants in Kenya. J. Occup. Environ. Hyg. 11 (11), 706–715. doi: 10.1080/15459624.2014.908258
Whitworth, K. W., Baird, D. D., Steiner, A. Z., Bornman, R. M., Travlos, G. S., Wilson, R. E. (2015). Anti-Mullerian hormone and lifestyle, reproductive, and environmental factors among women in rural South Africa. Epidemiol. 26 (3), 429–435. doi: 10.1097/ede.0000000000000265
Wichmann, J., Voyi, K. (2012). Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001-2006. Int. J. Environ. Res. Public Health 9 (11), 3978–4016. doi: 10.3390/ijerph9113978
Wild, C. P., Gong, Y. Y. (2010). Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis 31 (1), 71–82. doi: 10.1093/carcin/bgp264
Williams, J. H., Grubb, J. A., Davis, J. W., Wang, J. S., Jolly, P. E., Ankrah, N. A. (2010). HIV and hepatocellular and esophageal carcinomas related to consumption of mycotoxin-prone foods in sub-Saharan Africa. Am. J. Clin. Nutr. 92 (1), 154–160. doi: 10.3945/ajcn.2009.28761
Wirth, J. P., Rohner, F., Petry, N., Onyango, A. W., Matji, J., Bailes, A. (2017). Assessment of the WHO stunting framework using Ethiopia as a case study. Matern. Child Nutr. 13, e12310. doi: 10.1111/mcn.12310
Wogan, G. N., Kensler, T. W., Groopman, J. D. (2012). Present and future directions of translational research on aflatoxin and hepatocellular carcinoma. A review. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 29 (2), 249–257. doi: 10.1080/19440049.2011.563370
Wolff, P. T., Arison, L., Rahajamiakatra, A., Raserijaona, F., Niggemann, B. (2012). High asthma prevalence and associated factors in urban malagasy schoolchildren. J. Asthma 49 (6), 575–580. doi: 10.3109/02770903.2012.696170
Wylie, B. J., Kishashu, Y., Matechi, E., Zhou, Z., Coull, B., Abioye, A. I. (2017a). Maternal exposure to carbon monoxide and fine particulate matter during pregnancy in an urban Tanzanian cohort. Indoor Air 27 (1), 136–146. doi: 10.1111/ina.12289
Wylie, B. J., Matechi, E., Kishashu, Y., Fawzi, W., Premji, Z., Coull, B. A. (2017b). Placental pathology associated with household air pollution in a cohort of pregnant women from Dar es Salaam, Tanzania. Environ. Health Perspect. 125 (1), 134–140. doi: 10.1289/ehp256
Yassa, H. A. (2014). Autism: a form of lead and mercury toxicity. Environ. Toxicol. Pharmacol. 38 (3), 1016–1024. doi: 10.1016/j.etap.2014.10.005
Younes-Mhenni, S., Aissi, M., Mokni, N., Boughammoura-Bouatay, A., Chebel, S., Frih-Ayed, M. (2013). Serum copper, zinc and selenium levels in Tunisian patients with Parkinson’s disease. Tunis. Med. 91 (6), 402–405.
Zar, H. J., Barnett, W., Stadler, A., Gardner-Lubbe, S., Myer, L., Nicol, M. P. (2016a). Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. Lancet Respir. Med. 4 (6), 463–472. doi: 10.1016/S2213-2600(16)00096-5
Keywords: G x E, Africa, environmental, pesticides, metals, mold, air pollution
Citation: Joubert BR, Mantooth SN and McAllister KA (2020) Environmental Health Research in Africa: Important Progress and Promising Opportunities. Front. Genet. 10:1166. doi: 10.3389/fgene.2019.01166
Received: 17 November 2018; Accepted: 23 October 2019;
Published: 16 January 2020.
Edited by:
Mayowa Ojo Owolabi, University of Ibadan, NigeriaReviewed by:
Robinson Odong, Makerere University, UgandaCopyright © 2020 Joubert, Mantooth and McAllister. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bonnie R. Joubert, am91YmVydGJyQG5paC5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.