
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 08 February 2019
Sec. Systems Biology Archive
Volume 10 - 2019 | https://doi.org/10.3389/fgene.2019.00055
 Ping Li1,2†
Ping Li1,2† Hong Yang1,2†
Hong Yang1,2† Lu Wang1†
Lu Wang1† Haoju Liu3,4
Haoju Liu3,4 Heqiang Huo5
Heqiang Huo5 Chengjun Zhang1
Chengjun Zhang1 Aizhong Liu1
Aizhong Liu1 Andan Zhu1
Andan Zhu1 Jinyong Hu1
Jinyong Hu1 Yongjun Lin3
Yongjun Lin3 Li Liu1*
Li Liu1*In some plants, exposure to stress can induce a memory response, which appears to play an important role in adaptation to recurrent stress environments. However, whether rice exhibits drought stress memory and the molecular mechanisms that might underlie this process have remained unclear. Here, we ensured that rice drought memory was established after cycles of mild drought and re-watering treatment, and studied gene expression by whole-transcriptome strand-specific RNA sequencing (ssRNA-seq). We detected 6,885 transcripts and 238 lncRNAs involved in the drought memory response, grouped into 16 distinct patterns. Notably, the identified genes of dosage memory generally did not respond to the initial drought treatment. Our results demonstrate that stress memory can be developed in rice under appropriate water deficient stress, and lncRNA, DNA methylation and endogenous phytohormones (especially abscisic acid) participate in rice short-term drought memory, possibly acting as memory factors to activate drought-related memory transcripts in pathways such as photosynthesis and proline biosynthesis, to respond to the subsequent stresses.
Plants can experience recurrent environmental stresses throughout the whole life cycle (Avramova, 2015), and their responses to later instances of a stress often differ from the first response (Bruce et al., 2007; Ding et al., 2013). These different responses to similar stress conditions illustrate the concept of “stress memory” (Avramova, 2015), which is sometimes called plant priming (Wang et al., 2014; Li et al., 2015). Priming and memory are effective means by which plants enhance their resistance to stress, but the associated molecular mechanisms are unclear. Water stress can induce a decrease in leaf water potential and cause stomatal closing, which limits CO2 uptake and photosynthetic activity. Endogenous abscisic acid (ABA) is rapidly induced under drought stress, which can trigger a series of physiological responses and signaling transduction, such as the production of reactive oxygen species (ROS), an increase in cytosolic Ca2+, and the activation of signal transduction pathways (Osakabe et al., 2014).
Many drought resistance mechanisms have evolved under selective pressure imposed by the environment. For example, JA, ABA, and SA function in the response to drought stress in Medicago truncatula (Guo et al., 2016). ABA is a predominant hormone that regulates stomatal closure under drought stress. ABA-related genes such as the Arabidopsis thaliana transcription factor (TF) gene NAC016, the Fagopyrume sculentum DRE-Binding TF gene FeDREB1, Arabidopsis CALCIUM-DEPENDENTPROTEINKINASE8 (CPK8), CATALASE 3 (CAT3), Ubiquitin E3 ligase gene RZFP34/CHYR1, and SnRK2.6 function in the drought response (Fang et al., 2015; Linster et al., 2015; Sakuraba et al., 2015; Yang et al., 2015). Arabidopsis with over-expressed VaNAC26 showed increased drought tolerance via activating related genes in JA biosynthesis signaling pathway (Fang et al., 2016). However, these mechanisms were all verified in plant stress response.
Studies in model plants have revealed that chromatin states and signal transduction pathways, especially those related to phytohormones, as well as TFs are involve in the formation of short-term memory (Barrett and Wood, 2008; Julio Camarero et al., 2018). For instance, memory genes with functions in hormone [ABA and jasmonic acid (JA)]-regulated pathways were identified via RNA-Seq analysis after repeated drought stress in A. thaliana and Zea mays, implicating phytohormones in short-term drought stress memory (Ding et al., 2012, 2013, 2014; Virlouvet and Fromm, 2015). In addition, rice chilling tolerance is increased by salicylic acid (SA) priming, which reduces the chilling-induced electrolyte leakage from leaves (Kang and Saltveit, 2002).
Rice is a staple food for nearly one third of the world population, but requires high amounts of water to produce (Mohanty et al., 2016). Although plants have evolved a range of mechanisms to resist drought stress in the natural environment (Guo et al., 2016), drought stress remains one of the main limiting factors of rice. Dehydration memory behavior has been detected in switchgrass (Panicum virgatum L.) (Zhang et al., 2018a) and Zea mays (Virlouvet et al., 2018), but whether the physiological response is similar in rice and the underlying mechanism of such a rice drought ‘memory’ have remained unclear.
Here, we imposed cycles of mild drought and re-watering to examine drought memory in rice and found that short-term stress memory can be established in rice. We identified clusters of transcripts involved in “stress memory” and “transcription memory” through transcriptome analysis, including a large number of newly identified drought-related genes that did not respond to the initial drought treatment. Some key memory transcripts functioned in ABA signaling, pointing to pivotal roles of ABA in initiating drought stress memory in rice. Our results indicate that protective substances such as proline and processes such as photosynthesis and DNA methylation are important to rice drought memory formation and shed light on the mechanisms of short-term memory formation under repeated drought stress treatments.
Rice plants (Oryza sativa L. ssp. japonica cv. Zhonghua 11) were used for all experiments in this study. Seeds were germinated in petri dishes containing more than four layers of moistened filter paper at 30°C in the dark for 4 days. The germinated seeds were grown in culture vessels containing 1/4 modified Hoagland solution according to Jones and Pittman (1982). Plants were grown under a 12 h light/12 h dark photoperiod (180 μmol m-2 s-1 light intensity) at 28°C (Qian et al., 2015). Four-week-old seedlings (3.5 leaf stage) were moved out of the pots (used as the non-treatment control, R0) and then exposed to air drying for 80 min at 28°C (estimated water loss to 45%; used as the first drought stress treatment, S1). The drought stress-treated seedlings were fully re-watered for 22 h at 28°C (used as the first re-watering treatment, R1). Then, half of the R1 seedlings were cultured under the normal watering condition as the no-stress memory treatment control (C). Drought and re-watering treatments were repeated for two more rounds to enhance the stress memory. The physiological effect of drought treatment was estimated using the relative water content (RWC) according to the formula: RWC = (FW-DW)/(RW-DW) × 100% (Ding et al., 2014), where FW, DW, and RW represent the fresh weight of control plants, drought-treated plants, and re-watered plants, respectively. RWC was used as a parameter for monitoring drought stress (Gilbert and Medina, 2016), which can indicate the water retaining capacity and reveal the adaptive capacity. The leaves from R0, S1, R1, S2, R2, S3, R3, S4 seedlings (plants with repeated drought/re-watering treatments) and C1, C2 seedlings (recovered plants from R1, no-stress memory treatment control for R2 and R3, accordingly) were collected for analysis; some leaves were immediately frozen in liquid nitrogen, while others were used for physiological index measurements.
Photosynthetic parameters were measured using an IMAGING-PAM chlorophyll fluorometer and Imaging Win software (Walz; Effeltrich, Germany). After 20 min dark adaptation, the minimum fluorescence (F0), maximum fluorescence (Fm) and steady-state fluorescence (Fs) levels were determined according to the method of Su et al. (2015). Fv/Fm was calculated using the formula: Fv/Fm = (Fm-F0)/Fm (Woo et al., 2008). The maximum quantum yield of photosynthesis (Fv/Fm) is a photosynthetic parameter commonly used to indicate the growth potential of plants.
Phytohormones (JA and ABA) were extracted from 0.1 to 0.3 g of frozen leaves as described by Cai et al. (2015). JA (0.2 ng), ABA (0.6 ng) and stable isotope-labeled CKs (0.01 ng) were added to the sample extraction buffer. Phytohormones were extracted and the content was determined by HPLC using the method described by Lee et al. (2015).
Proline was extracted as described by Xin and Browse (1998). The free proline content was measured at 520 nm following the method described by Bates et al. (1973), using L-proline as the standard.
Total RNAs from R0, S1, R1, S2, R2, S3, R3, S4 treated samples (pooled samples from twenty to thirty individual plants were used for each treatment) were extracted using TRIzol following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, United States). R0, S1, R3, and S4 samples were collected for RNA sequencing (RNA-Seq). Three biological replicates were applied to all sequencing samples. The RNA libraries were sequenced on the Illumina sequencing platform by Genedenovo Biotechnology, Co., Ltd. (Guangzhou, China).
Transcript reconstruction was carried out with Cufflinks software (Trapnell et al., 2012) after comparison using TopHat2. Assembling reads partition merging was tested using Cuffmerge (Kim et al., 2013). The expression levels of each gene were calculated and normalized by the corresponding Fragments Per Kilobase of transcript per Million mapped reads (FPKM) values. The differentially expressed genes (DEGs) were screened with FPKM values according to edgeR’s general filtering criteria (log2| Fold Change| > 1&&FDR < 0.05).
Gene Ontology (GO) enrichment analysis was performed using the WEGO 2.01. Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was performed using the KOBAS 3.0 tools2.
Two software programs, CNCI (version 2) (Sun et al., 2013) and CPC (Kong et al., 2007), were used to assess the protein-coding potential of new transcripts using default parameters. LncRNAs reported by both methods were selected for further analysis and were classified based on secondary structures and sequence consensus. To reveal the interaction between antisense lncRNA and mRNA, RNAplex software (Tafer and Hofacker, 2008)3 was used to predict the complementary correlation of antisense lncRNAs and mRNAs.
To obtain memory profiles involving the DEGs and lncRNAs, the Short Time-series Expression Miner (STEM v1.3.8) program was used.
To identify potential miRNA precursors, lncRNA sequences were aligned to a miRBase (version 21), and those with sequence identity > 90% were selected. In addition, miRPara software (Wu et al., 2011), which is based on the SVM method, was also used to predict miRNA precursors. The plant microRNA database (PMRD4) was employed to predicted miRNA targets. The microRNA database (miRBase5) can also be used to search for sequence information.
RNA (1 μg) was treated with DNaseI and reverse-transcribed with oligo (dT) using a PrimeScriptTMRT reagent Kit (Takara, Japan). The relative expression levels of individual genes were measured with gene-specific primers by real-time quantitative PCR (qRT-PCR) analysis, which was carried out in a 20 μl reaction mix with 1 μl of diluted cDNA template and SYBR Premix Ex TaqII (Takara, Japan) with a Bio-Rad CFX96. The ELONGATION FACTOR1-ALPHA (EF-1α) gene (LOC_Os03g08020) served as the internal control (Wu et al., 2016).
The genomic DNA was extracted from treatment leaves using the CTAB method (Porebski et al., 1997). The DNA were then fragmented to 350 bp via sonication. The fragments were ligated with adapters and converted with bisulfite using EpiTech Bisulfite Kit (Qiagen, Valencia, CA, United States). The bisulfited fragments were sequenced by Illumina HisSeq TM2500. For data analysis, the clean reads were mapped to the rice reference genome using the BSMAP software, allowing up to four mismatches. The differentially methylated regions (DMRs) were then identified using the sliding-window approach with 200 bp window slide at 50 bp intervals. A Fisher’s exact test was performed for each window. P-values (≤0.05) from Fisher’s exact test were corrected for multiple tests with FDR (≤0.05) using Benjamini and Hochberg.
To investigate whether rice shows a drought memory effect, the RWC of the plants subjected to various “drought training treatments” were determined (Figure 1A and Supplementary Figure S1). For all treatment groups, the RWC declined as air-drying time increased (0-80 min). The RWC of S1 plants sharply dropped to 59.55% after 80 min of air drying. However, the RWC values of S2–S5 plants were significantly higher than those of S1 plants, and the water content loss was significantly slower after more than two treatments (P < 0.05). The significant changes in water loss during cycles of drought treatment indicated that rice exhibited drought memory after cycles of drought training.
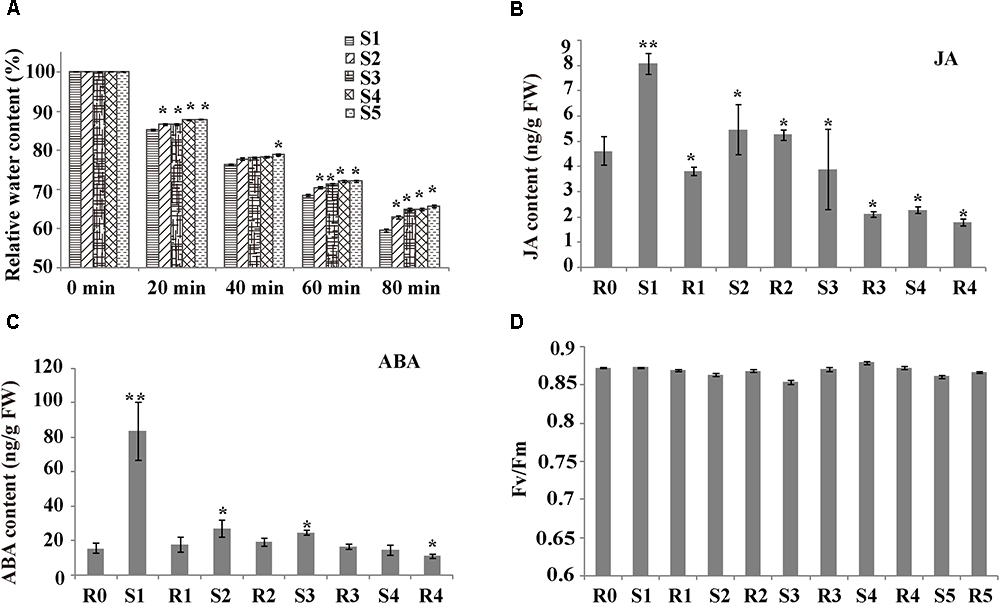
Figure 1. Plant response parameters during drought training treatment. (A) Relative water content (RWC) of the sampled leaves, after air drying for the indicated times. Values are the mean ± SD (n = 10). Within each water treatment, asterisks indicate significant differences by Tukey LSD test (∗P < 0.05). (B) Hormone levels during drought treatment. Levels of JA were measured at the indicated times. Values are the mean ± SD (n = 5). Within each water treatment, asterisks indicate significant differences by Tukey LSD test (∗P < 0.05, ∗∗P < 0.01). (C) Hormone levels during drought treatment. Levels of abscisic acid (ABA) were measured at the indicated times. Values are the mean ± SD (n = 5). Within each water treatment, asterisks indicate significant differences by Tukey LSD test (∗P < 0.05, ∗∗P < 0.01). (D) Photosynthesis rate of plants exposed to stress cycles during the initial watered (W) state and on recovery (R1–R5) from one or more stresses. Values are the mean ± SD (n = 10).
To check the growth of stress-treated rice and verify rice drought memory, we measured photosynthesis parameters and phytohormone levels. The Fv/Fm did not significantly differ among drought-stress treatments (Figure 1D), suggesting that the rice leaves recovered well after each drought/re-watering treatment. Stress can induce many signaling pathways, especially ABA and JA. To determine if ABA and JA are involved in rice drought memory response, we measured the endogenous contents of these phytohormones from R0 to R4 (Figures 1B,C). JA contents were 1.75-fold higher in S1 plants than in R0 plants (Figure 1B). Interestingly, after the first re-watering, JA levels decreased to less than 1/2 of those in S1 plants and were maintained at a relatively stable level after several cycles of drought treatments (Figure 1B). ABA levels had a similar alteration pattern to JAs. The endogenous ABA content increased after each drought stress period, decreased after re-watering, and remained stable after several rounds of treatments, with a value similar to that of R0 plants (Figure 1C). The different changes of JA and ABA after cycles of drought treatment indicated that rice drought memory responses possibly included maintaining homeostasis of endogenous JA and ABA levels.
To explore the mechanism of the drought memory formation in rice, we using ssRNA-Seq to identify drought memory-related gene expression with samples from the R0, S1, R3, and S4 treatments (Supplementary Figure S1). Memory genes are defined as those with transcript levels in subsequent stresses that are significantly different from their levels during the first stress period (Ding et al., 2012; Avramova, 2015; Liu et al., 2016). Accordingly, we defined memory genes based on this criterion: among genes that were responsive to the stress, those genes for which transcript levels in subsequent stress periods (S4) and re-watering treatment (R3) were significantly different from their levels during the initial stress period (S1) were considered to be memory genes.
When DEGs were clustered according their expression patterns using the STEM program, the DEGs (10,124 genes) and differentially expressed lncRNAs (376 lncRNAs) from the four libraries could be classified into 26 clusters (Supplementary Table S1). Sixteen of these clusters (profiles 0, 1, 3, 4, 7, 9, 10, 12, 13, 15, 16, 18, 21, 22, 24, and 25) represented memory transcripts: their expression levels in subsequent treatments (R3 and S4) were markedly different from that in the first treatment (S1) (Figures 2A,B and Supplementary Figures S2A,B). In all, we found 6885 memory-related transcripts (including 326 novel transcripts) and 238 memory-related lncRNAs (Supplementary Table S2).
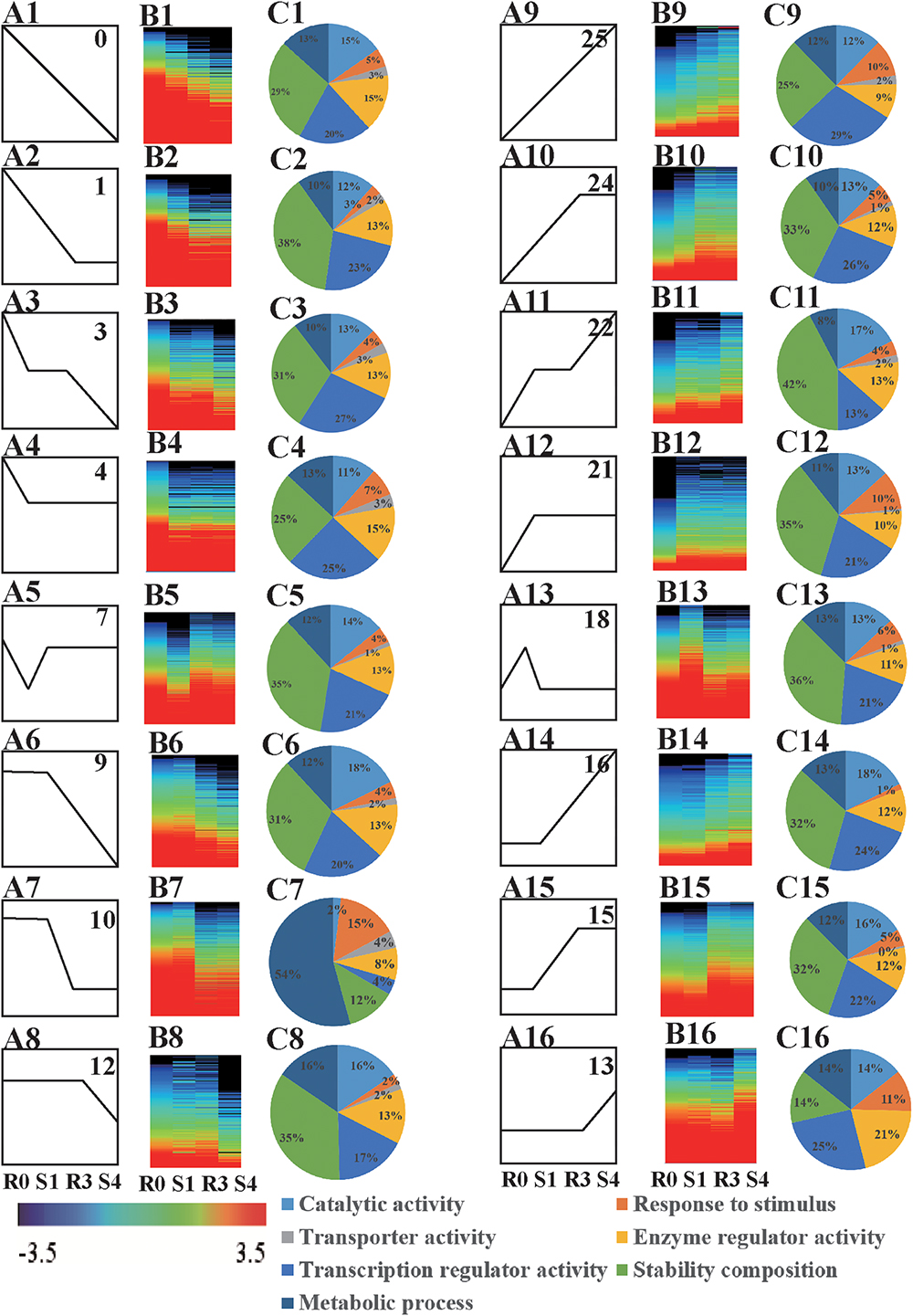
Figure 2. Analysis of memory genes. (A) Expression profiles of drought memory genes (A1–A16). (B) Heatmap of expression profiles indicated in (A) (B1–B16). (C) GO biological processes, cellular components, and molecular functions enriched (FDR < 0.05) among drought memory genes indicated in (A) (C1–C16).
The functions of the 16 clusters of memory genes were associated with metabolic process, response to stimulus, structure molecule activity, and catalytic activity (Figures 2C, 3 and Supplementary Table S3). Carbon fixation, carotenoid biosynthesis, and plant hormone signal transduction were significantly enriched in rice stress memory responses. These overrepresented memory DEGs suggested that the activation of pyruvate kinase (PK) provides a precursor and energy for rice drought response, which also activated malate dehydrogenase (MDH2) within the TCA cycle. The biosynthesis of carotenoid activated transduction of plant hormone (protein phosphatase, PP2C) and activity of TFs (MYC, WRKY), transmitted the signals to stress response genes (late embryogenesis abundant protein, LEA) and cell component to improve the stress resistance of rice (Figure 3).
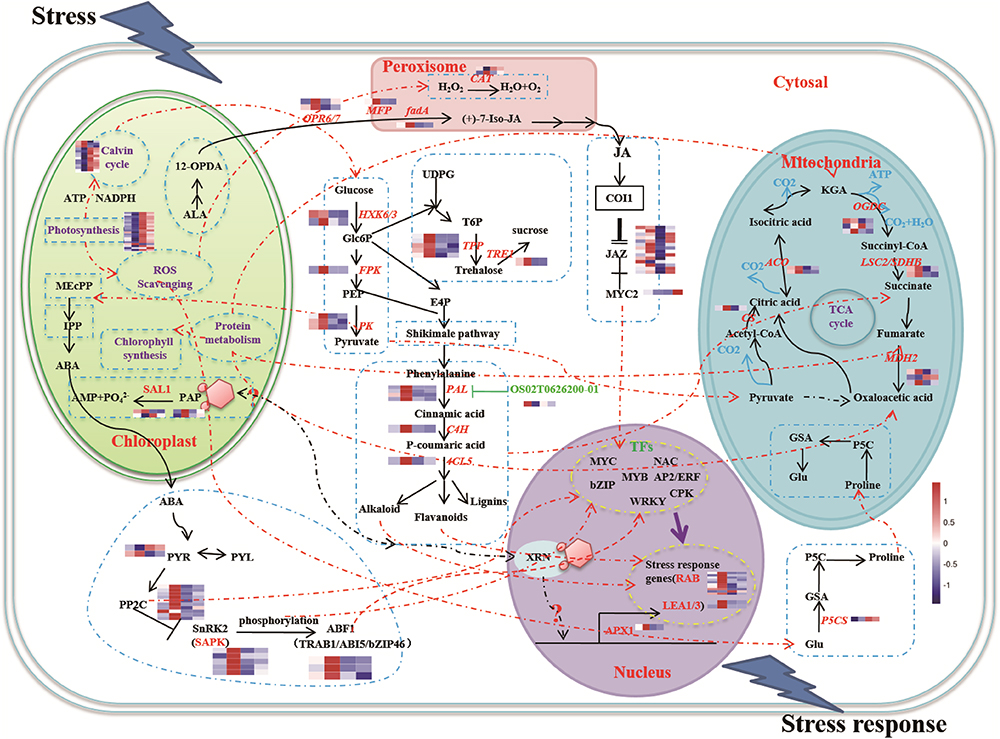
Figure 3. A schematic representation of main processes involved in rice drought memory response. The glycolysis pathway was actived under drought stress, the pyruvate generated was the precursor substance of pigment and signals of chloroplast, which activated upstream genes of ABA signaling. ABA signaling pathway played the central role signals to improve plant drought resistance by active the expression of TFs encoded in the nucleus, genes of energy metabolism in mitochondria, proline synthase and JAZs of JA signaling pathway. Broken lines indicate possible, but not confirmed, routes. Full lines represent the routes have been demonstrated in previous studies. Heatmap of memory transcripts involved in these pathways are shown.
Of the 16 memory-related gene patterns, transcripts belonging to profiles 13, 15, 16, 18, 21, 22, 24, and 25 reflected a positive response to rice drought/re-watering stress (Figures 2A–C), which could be divided into five general categories based on their expression patterns: accumulated memory, lineage memory, initial memory, stable memory, and dosage-dependent memory.
Transcripts of stable (profiles 21, 24), accumulated (profile 22), and lineage memory (profile 25) expression patterns were common types of variation, and were significantly increased at the initial and the subsequent drought treatment. Arginine and proline metabolism was one of the significantly enriched pathways in the stable memory profile (Supplementary Table S4). Transcripts belonging to profiles 7 and 18, which were initially induced significantly in S1, and finally maintained at a stable level despite subsequent re-watering (R3) or drought (S4), were considered likely to be involved in initial memory. Notably, these expression patterns were similar to the patterns of plant endogenous hormone levels, especially for ABA (Figures 1C, 2A). Plant hormone signal transduction, arginine and proline metabolism and photosynthesis pathways were also enriched among the stable memory transcripts (Supplementary Table S4).
Transcripts in profiles 9, 10, 12, 13, 15, and 16 did not change at the initial drought stress treatment, but showed significantly induced expression at R3 or S4, and were predicted to be involved in dosage-dependent memory (Figure 2A). These transcripts had not previously been classified as drought-response genes because of their unresponsiveness to the initial drought treatment. However, they should not be ignored, given their quantity (3396 transcripts) and important functions (Supplementary Table S2). In the dosage memory pattern, DEGs in the cytoplasm, the membrane and the organelle were the most enriched, which represented basic metabolic pathways for plant growth and stress responses (Supplementary Table S4). These results indicated that rice drought memory is likely associated with photosynthesis, phytohormone signaling, and proline metabolism.
Many metabolic pathways, in addition to photosynthesis, function in the chloroplast, which plays an important role in plant stress resistance (Gururani et al., 2015; Nazar et al., 2015). In the current study, we identified 780 chloroplast-related transcripts that function in the response to drought stress memory, including six novel transcripts. KEGG enrichment analysis showed that the chloroplast-related memory transcripts had the most enrichment in carotenoid biosynthesis, biosynthesis of amino acids and biosynthesis of secondary metabolites (Supplementary Table S5). There were 24 memory transcripts associated with photosynthesis. Most of them were assigned to initial memory, and were sharply reduced by the first drought treatment and maintained a stable level with the subsequent drought treatments; examples of these included those encoding subunits of the photosystem I reaction center and of ATP synthase (Supplementary Table S5). Interestingly, among the memory-related transcripts, we found several SAL1, 3′-phosphoadenosine 5′-phosphate (PAP) and ascorbate peroxidase (APX) genes, which are involved in the SAL1-PAP pathway for chloroplast retrograde signaling, suggesting that retrograde signaling and the drought memory response likely interact (Estavillo et al., 2011) (Supplementary Table S3).
Abscisic acid and JA are thought to be important signals in abiotic stress responses, as well as drought stress memory (Harb et al., 2010; Fleta-Soriano et al., 2015), but the expression patterns of ABA- and JA-related genes during these processes remain unclear in rice. We therefore performed qRT-PCR to examine the expression levels of these transcripts and found that they underwent significant changes in expression during the initial drought but showed divergent patterns in later stages (Figures 4, 5), which is consistent with our sequencing data (Table 1). 9-cis-epoxycarotenoid dioxygenase 3 (NCED3) (LOC_Os03g44380) and 9-cis-epoxycarotenoid dioxygenase 4 (NCED4) (LOC_Os07g05940) transcripts, which play key roles in ABA biosynthesis, accumulated in S1 and were maintained at stable low levels during the subsequent drought and re-watering treatment (Figure 4). The relative expression levels of key JA biosynthesis genes were quite different during the drought cycle. MFP (LOC_Os02g17390), glutaryl-CoA dehydrogenase (ACX) gene (LOC_Os05g07090), allene oxide synthase (AOS) gene (LOC_Os03g12500), allene oxide cyclase (AOC) gene (LOC_Os03g32314) and lipoxygenase (LOX) gene (LOC_Os08g39840) had similar expression patterns: they accumulated during S1 and maintained at low levels under subsequent treatments. The expression of 12-oxo-phytodienoic acid reductase (OPR) gene (LOC_Os06g11210) increased in S1, declined in R1, sharply increased in S2 and was maintained at a stable low level under subsequent treatment (Figure 5). These results indicate that genes directly involved in ABA and JA biosynthesis were induced by the first drought stress and maintained at stable expression levels after several rounds of treatment. Similarly, the expression levels of upstream JA and ABA biosynthesis genes were initially higher and sometimes fluctuated, subsequently exhibiting the memory effect.
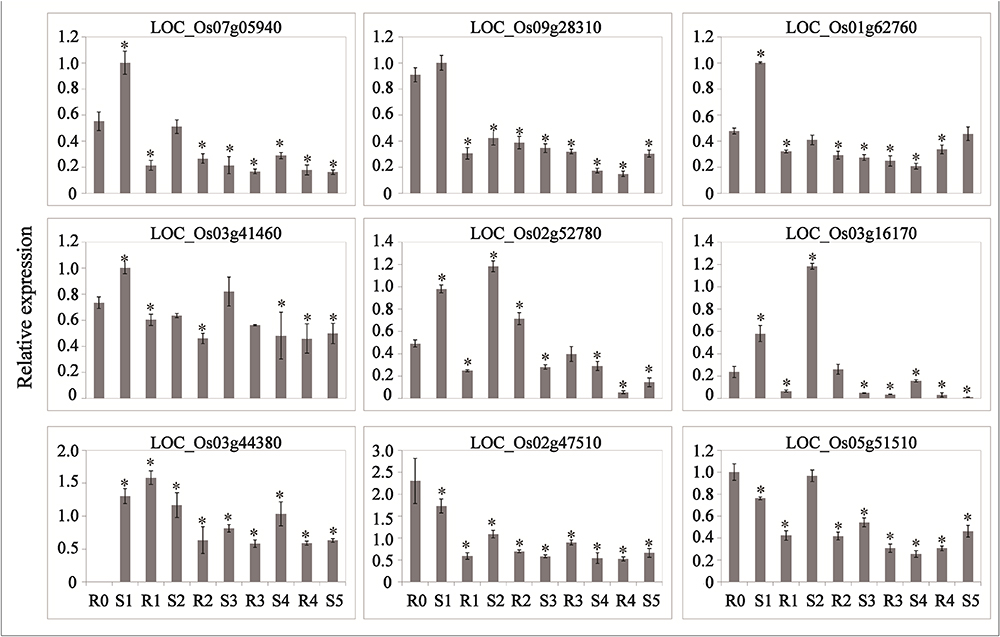
Figure 4. Real-time quantitative PCR analysis for stress memory transcripts involved in ABA metabolism and signaling pathways during the drought stress cycles. EF-1α (LOC_Os03g08020) was used as an internal control. Data are means of three biological replicates and error bars are ± SE from three independent experiments, each performed with 6–8 leaves from five separate plants. Asterisks indicate significant differences by Tukey LSD test (∗P < 0.05).
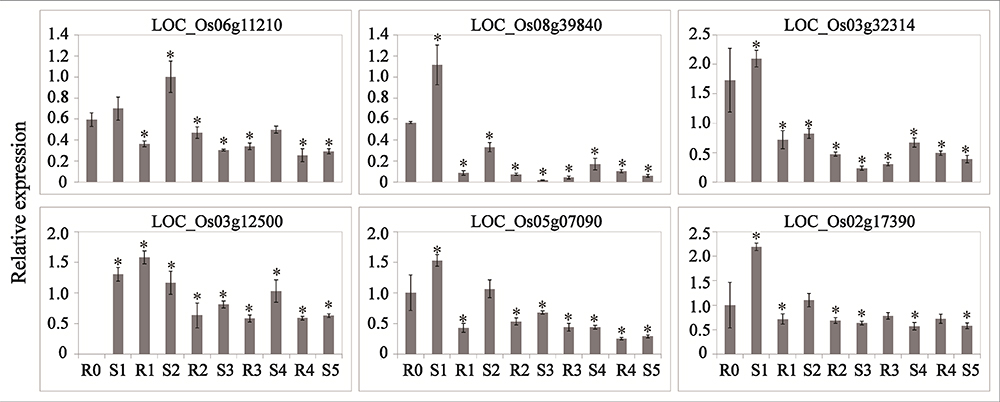
Figure 5. Real-time quantitative PCR analysis for stress memory transcripts involved in the JA synthesis pathway during the drought stress cycles. EF-1α (LOC_Os03g08020) was used as an internal control. Data are means of three biological replicates and error bars are ± SE from three independent experiments, each performed with 6–8 leaves from five separate plants. Asterisks indicate significant differences by Tukey LSD test (∗P < 0.05).
LncRNAs can function in the response to external stimuli and tissue development, and they can act as cis- or trans-regulators to control gene expression (Kim and Sung, 2012). We performed association analysis of the lncRNAs and mRNAs and found three memory-related mRNA transcripts involved in markedly different pathways associated with the lncRNAs (Table 2 and Supplementary Table S6). After aligning the predicted lncRNAs to the miRNA precursor database (miRBase), we discovered 12 lncRNAs that might be the precursors of miRNAs (Supplementary Table S7). TCONS_00028567, located downstream of SAPK10 (LOC_Os03g41460) (profile 18), was up-regulated after drought stress, with the highest relative expression levels detected after the second treatment, and the R3 and S4 treatments exhibited the memory effect, with lower expression levels than those detected in S2. The same expression pattern was observed for osa-MIR1428e (Supplementary Figure S3), which may be spliced from this lncRNA and function as a post-transcriptional regulator.
Proline has been considered to be a critical component of drought tolerance, and the gene encoding the proline-biosynthetic enzyme Δ1-pyrroline-5-carboxylate synthetase 1 (P5CS1) exhibits transcriptional memory after repeated salt stress (Feng et al., 2016). In our study, the expression of P5CS1 (LOC_Os01g62900) was rapidly induced after the first drought stress (S1), and reached a peak value at R2, after which it did not significantly vary (Figure 6A). Similarly the other P5CS1 gene (LOC_Os05g38150) was rapidly induced after the first drought stress (S1), and decreased at R2, after which it did not significantly vary in expression. The free proline content increased significantly to 2740.757 μg/g after the first drought treatment and diminished with the re-watering treatment. Consistent with the gene expression results, the proline content reached a second peak at R2, and then remained stable throughout the subsequent treatments (Figure 6B), suggesting that proline is also involved in rice drought memory response.
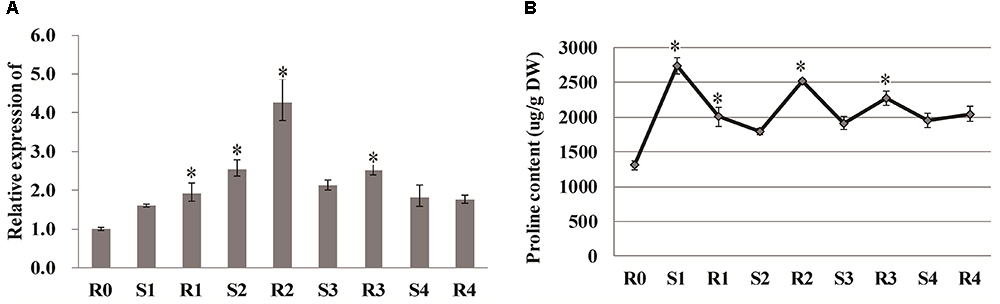
Figure 6. Analysis of proline and P5CS1. (A) qRT-PCR analysis of P5CS1 (LOC_Os01g62900). (B) Proline content under stress treatment. Data are means of three biological replicates and error bars are ± SE from three independent experiments, each performed with 6–8 leaves from five separate plants. Asterisks indicate significant differences by Tukey LSD test (∗P < 0.05).
DNA methylation, a common epigenetic change, plays crucial functions in development and stress responses in plants (Zhang et al., 2018b). Modulation of gene expression is one strategy for plant adaptation to the environment, which were directly regulated by DNA methylation (Chwialkowska et al., 2016). To explore the relationship between DNA methylation and drought memory, linkage analysis was conducted on differentially DNA methylated regions and the expression of memory genes. 5373 memory transcripts were identified as candidates to be regulated by DNA methylation, especially CHH methylation (4774 memory transcripts) (Figure 7 and Supplementary Table S8). The expression of 3064 memory transcripts were significantly associated with DNA methylation changes (Pearson correlation coefficient > 0.04 and < -0.04); these transcripts were related to the biosynthesis of secondary metabolites (osa01110), phenylpropanoid biosynthesis (osa00940) and plant hormone signal transduction (osa04075) (Supplementary Table S8). This evidence indicates that DNA methylation could regulate the expression of rice drought memory transcripts.
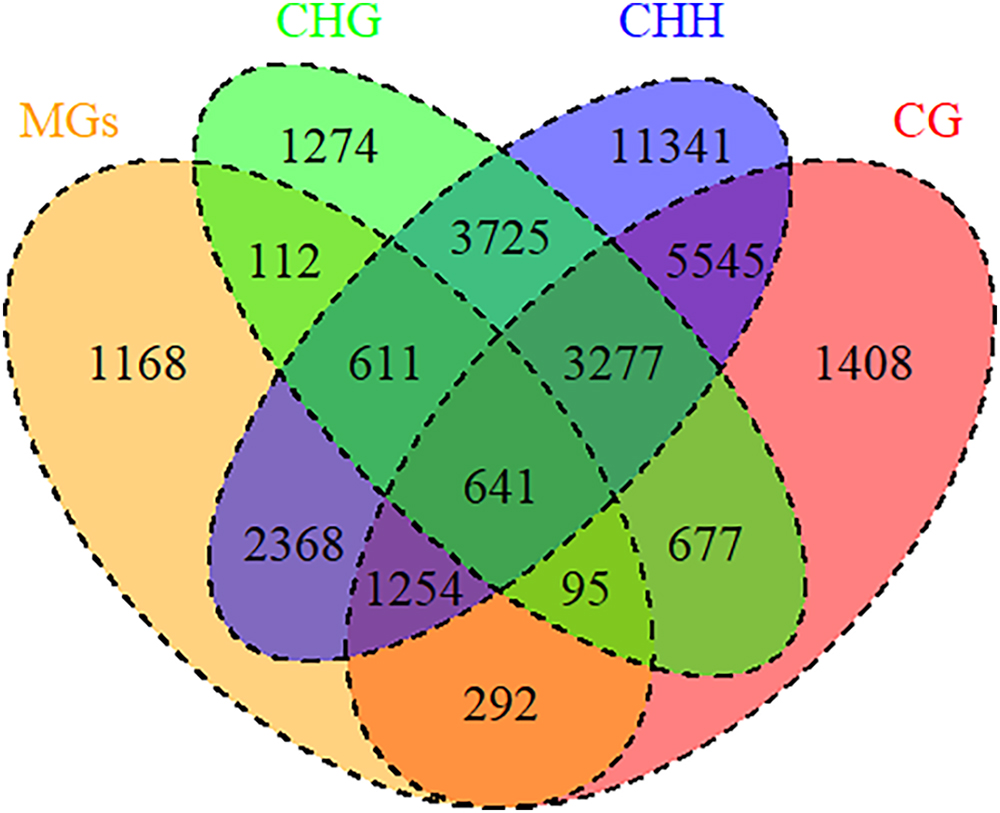
Figure 7. DNA methylation regulated memory genes. Venn diagrams of memory genes and DNA methylation. MGs: memory genes (orange); DNA methylation types: CG (red), CHG (green), CHH (blue).
Plants previously exposed to abiotic stresses may modify their responses to subsequent stresses (Bruce et al., 2007;Ding et al., 2012, 2013, 2014; Wang et al., 2014). A very recent analysis of a dryland genotype of rice (AN Cambara) revealed a memory effect induced by drought pre-treatment (Auler et al., 2017). Water stress can induce a decrease in leaf water (Osakabe et al., 2014). In order to reflect the ability of rice (Oryza sativa) memory, we examined the RWC as air-drying time increased (0–80 min). The slower loss of water, during the same time with the drought treatment cycles, might indicate the increase of the water retaining capacity and it revealed a better adaptive capacity to drought. There was no significant change of Fv/Fm under drought stress treatments, which ensured the normal activity of stress-treated rice plants. Endogenous ABA is rapidly produced during drought treatments, which can induce a cascade of physiological response and signaling transduction (Osakabe et al., 2014). In our study, ABA and JA were sharply induced by the first-time drought stress treatment (S1), which might trigger a series of plant drought response. JA maintained at a relatively stable low-level after the first drought stress, but ABA increased at every drought stress treatment, decreased at every re-watering treatment, and remained at a stable level with R0 after several rounds of treatment. The different changes in JA and ABA might indicate that the rice drought memory possibly includes maintaining homeostasis of endogenous JA and ABA levels. Whole-genome transcriptome analysis of Arabidopsis and maize have revealed the existence of various transcriptional memory response patterns (four memory response patterns), and more than 2,000 genes showed memory responses in Arabidopsis (Ding et al., 2012, 2013, 2014). Here, we also documented drought stress memory in rice, and identified a large number of memory transcripts from our ssRNA-Seq data. These memory transcripts displayed various expression patterns (16 memory response patterns) (Figure 2 and Supplementary Table S2). Together, our results indicate that drought memory could be established in rice and suggest that memory transcripts play different roles in rice drought memory formation.
Memory factors have been proposed to include not only key signaling metabolites and plant hormones but also proteins involved in their biosynthesis, such as kinases/phosphatases and TFs that regulate their activity (Conrath, 2011; Santos et al., 2011; D’Urso and Brickner, 2017; Lämke and Bäurle, 2017). Transcripts in profiles 21 and 24 encoded protection-associated proteins, such as tryptophan, arginine, proline, and cysteine. This pattern indicated that these proteins were important for responding to drought at the first stress treatment, which then activated downstream gene expression. Transcripts of profile 22 were important for initial drought resistance too, but they sharply increased during later drought treatment and afterward they maintained at a stable high expression level Functional analysis suggest that these transcripts may be responsible for RNA transport to spread the drought memory within the plant. Transcripts in profile 25 showed an overall trend of increasing accumulation, possibly underlying enzymatic reactions occurring throughout the plant drought response. By contrast, plant hormone signaling and energy metabolism genes of profile 18 responded positively in the first drought period, and then recovered to basal levels in the subsequent treatments. This pattern indicates that profile 18 transcripts acquired a stress imprint in the initial stress period, and we consider these transcripts to be strong candidates act as memory factors that activate protective transcripts to respond to subsequent drought stress. In brief, profile 18 transcripts may play mediator roles in rice drought memory formation (Supplementary Table S4).
Interestingly, half of the memory genes belong to dosage-dependent memory gene category (profiles 0, 15, 16). They encoded enzymes for porphyrin and chlorophyll metabolism, synthesis of secondary metabolites and base excision repair, which are important for damage repair of plant (Supplementary Table S4). In previous studies, drought-related genes were identified because their expression changed significantly during the drought. Compared to traditionally-defined drought-related genes, our dosage-dependent memory genes represent a large increase in the gene pool responsive to drought stress. We propose that dosage-dependent memory may play critical roles in memory formation, which would allow rice plants to successfully adapt to drought via repeated drought and re-watering.
Besides the dosage-dependent memory genes, transcripts in profiles 4 and 21 include drought memory responsive genes that may be newly identified due to the R3 data collected in our study. For instance, the non-memory genes identified in Arabidopsis (Ding et al., 2013) and maize (Ding et al., 2014) that showed +/ = or -/ = patterns based on only two drought and rewatering cycles would be divided among profiles 4 or 21, if their transcript levels in a third treatment R3 did not return to the basal levels.
Importantly, we found that many lncRNAs exhibited drought memory-related expression patterns. Our overall data set contained 271 novel lncRNAs and 3,488 known lncRNAs, including 168 lncNATs (Supplementary Table S1); 6.33% of these lncRNAs showed memory responsiveness (Supplementary Table S2). Although no previous evidence has shown an association of lncRNAs with short-term memory, lncRNAs have been reported to respond to salt-induced long-term memory (Wibowo et al., 2016). Thus, it is possible that lncRNAs represent a general regulation mechanism in stress memory.
Among the memory genes we identified, plant hormone signal transduction was the most significantly enriched pathway (Supplementary Table S3). Drought stress can induce many signaling pathways, especially the ABA signaling pathway, although there are few reports about the levels of ABA during cycles of drought (Fleta-Soriano and Munne-Bosch, 2016). MYC2-mediated JA signaling plays a key role in Arabidopsis drought memory formation, which exhibits a +/- memory pattern (Liu et al., 2016). By contrast, in our data MYC2 belonged to the dosage-dependent pattern (profiles 10, 13). This difference in expression pattern of MYC2 between Arabidopsis and rice suggests that the mechanism of drought memory formation in these two species might be different.
Arabidopsis repeatedly treated with high levels of ABA exhibits drought stress memory (Goh et al., 2003). Here, we found that endogenous ABA levels increased after drought stress and decreased after re-watering, but became stable after several rounds of treatment, reaching levels close to those detected before treatment (R0; Figure 1C). These results indicate that rice has a memory response to drought stress after more than two treatments, and this response is characterized by stable ABA levels, which is consistent with the finding that ABA is involved in short-term drought stress memory in other plants (Ding et al., 2012; Fleta-Soriano et al., 2015). Higher ABA contents appeared only in the S1 period, which implies that higher ABA levels were not sustained in the subsequent stress. In other words, ABA contents represented a memory effect induced during the initial stress but are not necessary for memory responses to subsequent stress episodes. At the same time, some transcripts (such as NCED and SAPKs) involved in ABA signal transduction and biosynthesis were memory-type transcripts, i.e., they showed different expression levels in the subsequent stress compared to the first stress (Figure 4 and Table 1). NCED catalyzes a key step in ABA biosynthesis, and a tight correlation between ABA content and NCED3/4 expression was detected (Figure 4). The changes in levels of ABA and key enzyme genes (NCED3/4) represent a stress imprint that can affect a plant’s response to subsequent stress. Our results partially supported a prediction that ABA plays a key regulatory role via its biosynthesis as a modulator (Fleta-Soriano and Munne-Bosch, 2016).The dehydrins and proteins related to drought (LEA, RAB) also displayed memory effects, which may be induced by ABA signaling for participation in drought responses (Figure 8 and Supplementary Table S9). SnRK2.2 and SnRK2.3 are important for implementing guard cell stress memory for the subsequent drought response (Virlouvet and Fromm, 2015). We found that SnRK2s (SAPK2, SAPK7, SAPK6, SAPK9, and SAPK10) (Table 1) are also likely to be important for drought stress memory in rice leaves. These genes are related to CYP76C2 and CYP707A3 in Arabidopsis, respectively, which are also thought to be memory-related genes.
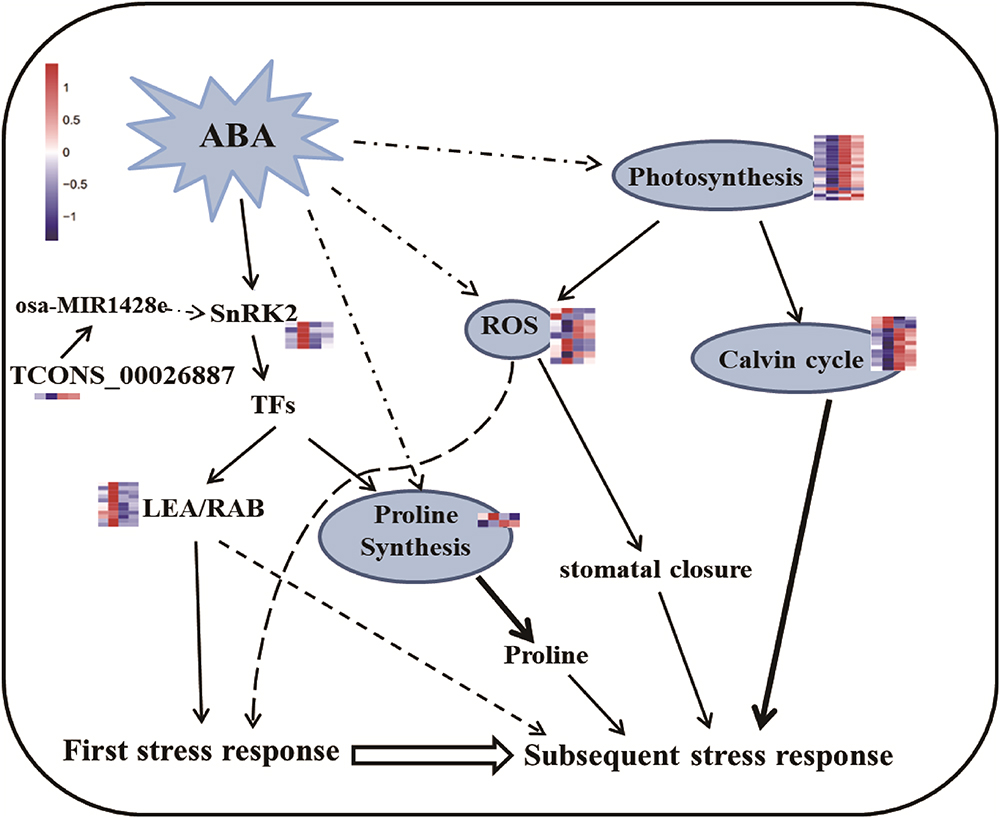
Figure 8. Predicted pathways for regulating rice drought memory response. The ABA/SnRK2-mediated pathway actives downstream genes in response to the first drought stress. ABA might act as memory factor to regulate genes related to photosynthesis, proline synthesis and ROS repair, result in accumulation of protective substances (such as proline) to respond to subsequent drought stress. During this process, TFs might also take part in regulating proline synthesis to accumulate proline. Broken lines indicate possible, but not confirmed, routes. Full lines represent the routes have been demonstrated in previous studies. Heatmap of memory transcripts involved in these pathways are shown.
These findings suggest that ABA-related pathways indeed participate in short-term drought memory, although there are some differences between these responses in maize and rice: the ABA-related memory genes in maize exhibit a [+/+] effect (their expression levels continue to increase throughout the drought re-watering process), while most ABA-related memory transcripts in rice showed a [+/-] memory effect (their expression levels increased sharply in the first drought treatment, but maintained in a stable low level in the subsequent treatment).
The likelihood of the involvement of stress-related lncRNAs in drought memory was discovered in our study(Raikwar et al., 2015). A novel lncRNA, TCONS_00028567, which may be the precursor of osa-MIR1428e, might regulate ABA signaling through its critical gene SAPK10 (Supplementary Figure S3). This finding suggests that a comprehensive regulation network engenders rice short-term drought memory and should help direct further investigations to understand the underlying molecular mechanisms.
It is generally recognized that proline is important for protecting cells from damage caused by drought stress and can scavenge ROS as well as acting as a molecular chaperone to stabilize protein structure (Szabados and Savoure, 2010; Auler et al., 2017). ABA can accelerate proline accumulation during plant exposure to drought stress (Savoure et al., 1997; Strizhov et al., 1997). However, molecular evidence was lacking in rice regarding whether proline plays critical roles in short-term drought memory formation. The activities of Δ1-pyrroline-5-carboxylate synthase (P5CS) and P5C reductase help increase proline biosynthesis, thereby helping plants combat abiotic stress (Hare and Cress, 1997). In the current study, P5CS expression did not significantly change between S1 and R2, but maintained at high levels at these two points (Figure 6), suggesting that proline might accumulate as the memory effect formed during the later treatment periods. Interestingly, salinity-induced proline accumulation is reported to exhibit a memory-related pattern (Feng et al., 2016), and this accumulation was similar to what we observed here related to drought stress memory. However, the expression of P5CS during the repeated stress treatment was different from that reported in other rice varieties (Auler et al., 2017), possibly due to the mild drought treatment used in our study. Overall, our results imply that the memory effect on proline might be influenced by ABA (Figure 8).
There are many studies on the relationship between ROS and proline metabolism; increased ROS can activate the biosynthesis of proline, which acts as a non-enzymatic antioxidant and influences plant redox homeostasis (Ben et al., 2014; Auler et al., 2017). ROS are important signaling molecules that help safeguard cellular components (Saeidnejad and Rajaei, 2015), but they can also be harmful to plants. Here, the major transcripts [APX1, monodehydroascorbate reductase (MDAR) and glutathione reductase (GR)] involved in ROS scavenging had similar expression patterns to those of NCED3/4 (profile 18), indicating that ROS scavenging is likely involved in the drought memory response in rice. The expression of APX1 showed a different profile from that of the proline biosynthetic gene P5CS, which indicates the complexity of drought memory formation and suggests the need for balance in cellular metabolic activities.
Recently, drought stress memory was shown to maintain ROS homeostasis and higher photosynthetic rates in subsequent stress treatments (Li et al., 2015; Wang et al., 2015). Interestingly, we found that most of the positive memory transcripts involved in photosynthesis belonged to profile 7 and the carbon fixation transcripts belonged to profile 15 (Supplementary Table S5). Bruce et al. (2007) found that the stress resistance response might function in reducing the efficiency of photosynthesis to compromise plant productivity in the short term, but increase tolerance to subsequent stress and promote productivity in the long term. Our results verified this prediction and suggested that photosynthesis efficiency decreased at the initial drought stress, but recovered and improved during the subsequent treatments.
Epigenetic, especially chromatin-based mechanisms played important functions during the stress adaption and memory in plants (Lämke and Bäurle, 2017). Garg et al. (2015) found that DNA methylation had had an influence on the transcription of rice (Oryza sativa) during the abiotic stress adaptation. But the research on the relationship of DNA methylation and drought transcript memory were few. Here, we found that the expression of the most memory genes could be directly regulated by DNA methylation, especially during biosynthesis of secondary metabolites (osa01110) and plant hormone signal transduction (osa04075) (Supplementary Table S8). It can be concluded that DNA methylation was involved in the formation and regulation of rice drought memory.
The drought memory of rice verified in this study provides new insight in rice drought resistance. The slower loss of water (RWC) ensured rice had better adaptation to drought. The maintaining homeostasis of endogenous JA and ABA levels may also participate in rice drought memory. The rice memory can be developed under suitable drought and re-watering treatment, and is largely dependent on the dosage memory genes, which were the new category in drought response, and recognized as non-responsive to drought stress in previous studies. Notably, we also found evidence that endogenous phytohormones (especially ABA), lncRNA and DNA methylation participate in rice short-term drought memory and might be important regulatory factors in rice drought memory formation. Novel information from this study shows that rice probably utilizes different mechanisms in drought stress memory to Arabidopsis, which were regulated by memory factors.
The datasets generated for this study can be found in Genome Sequence Archive (GSA), http://bigd.big.ac.cn/gsub/submit/gsa/subCRA001313/contents.
LL, PL, and LW acquisition of data, analysis of data, drafting or revising the article. HL plant culture and acquisition of data. AZ and LW analysis of gene expression data and computational analysis. CZ and HY experimentation. HH, YL, and AL drafting or revising the article. All authors read and approved the final manuscript.
This work was supported by the CAS Pioneer Hundred Talents Program (LL, JH, and CZ), the National Natural Science Foundation of China (31571262 to LL and 31570311 to JH), and the open funds of the National Key Laboratory of Crop Genetic Improvement.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Prof. Lizhong Xiong for the kind discussion and Prof. Qianwen Sun for data analysis.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2019.00055/full#supplementary-material
FIGURE S1 | Treatment and sequencing flow.
FIGURE S2 | Analysis of memory lncRNAs. (A) Expression profiles of drought memory lncRNAs (A1–A16). (B) Heatmap of expression profiles indicated in (A) (B1–B15).
FIGURE S3 | Real-time quantitative PCR analysis of SAPK10 (A) and MIR1428e (B) during the drought stress cycles. EF-1α (LOC_Os03g08020) was used as an internal control. Data are means of three biological replicates and error bars are ± SE from three independent experiments, each performed with 6–8 leaves from five separate plants. Asterisks indicate significant differences by Tukey LSD test (∗P < 0.05).
TABLE S1 | All DEGs and significantly differentially expressed lncRNAs (FDR < 0.05 and log2| fold change| > 1).
TABLE S2 | All memory transcripts and lncRNA memory transcripts involved in SAL1-PAP, proline synthesis, and carotenoid biosynthesis.
TABLE S3 | Kyoto Encyclopedia of Genes and Genomes enrichment analysis of all memory transcripts (P-value < 0.05 and q-value < 0.05 highlighted in red).
TABLE S4 | Kyoto Encyclopedia of Genes and Genomes analysis of memory transcripts clusters (P-value < 0.05 and q-value < 0.05 highlighted in red).
TABLE S5 | Kyoto Encyclopedia of Genes and Genomes analysis of all chloroplast-related memory transcripts (P-value < 0.05 and q-value < 0.05 highlighted in red) and memory transcripts involved in photosynthesis.
TABLE S6 | LncRNAs and mRNAs association analysis.
TABLE S7 | 12 miRNAs.
TABLE S8 | DNA methylation related memory transcripts.
TABLE S9 | All memory TFs.
Auler, P. A., Amaral, M. N. D., Rodrigues, G. D. S., Benitez, L. C., Maia, L. C. D., Souza, G. M., et al. (2017). Molecular responses to recurrent drought in two contrasting rice genotypes. Planta 246, 899–914. doi: 10.1007/s00425-017-2736-2
Avramova, Z. (2015). Transcriptional ‘memory’ of a stress: transient chromatin and memory (epigenetic) marks at stress-response genes. Plant J. 83, 149–159. doi: 10.1111/tpj.12832
Barrett, R. M., and Wood, M. A. (2008). Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn. Mem. 15, 460–467. doi: 10.1101/lm.917508
Bates, L. S., Waldren, R. P., and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1016/j.dental.2010.07.006
Ben, R. K., Abdelly, C., and Savouré, A. (2014). How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80, 278–284. doi: 10.1016/j.plaphy.2014.04.007
Bruce, T. J., Matthes, M. C., Napier, J. A., and Pickett, J. A. (2007). Stressful “memories” of plants: evidence and possible mechanisms. Plant Sci. 173, 603–608. doi: 10.1016/j.plantsci.2007.09.002
Cai, B. D., Ye, E. C., Yuan, B. F., and Feng, Y. Q. (2015). Sequential solvent induced phase transition extraction for profiling of endogenous phytohormones in plants by liquid chromatography-mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1004, 23–29. doi: 10.1016/j.jchromb.2015.09.031
Chwialkowska, K., Nowakowska, U., Mroziewicz, A., Szarejko, I., and Kwasniewski, M. (2016). Water-deficiency conditions differently modulate the methylome of roots and leaves in barley (Hordeum vulgare L.). J. Exp. Bot. 67, 1109–1121. doi: 10.1093/jxb/erv552
Conrath, U. (2011). Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531. doi: 10.1016/j.tplants.2011.06.004
Ding, Y., Fromm, M., and Avramova, Z. (2012). Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 3:740. doi: 10.1038/ncomms1732
Ding, Y., Liu, N., Virlouvet, L., Riethoven, J. J., Fromm, M., and Avramova, Z. (2013). Four distinct types of dehydration stress memory genes in Arabidopsis thaliana. BMC Plant Biol. 13:229. doi: 10.1186/1471-2229-13-229
Ding, Y., Virlouvet, L., Liu, N., Riethoven, J. J., Fromm, M., and Avramova, Z. (2014). Dehydration stress memory genes of Zea mays; comparison with Arabidopsis thaliana. BMC Plant Biol. 14:141. doi: 10.1186/1471-2229-14-141
D’Urso, A., and Brickner, J. H. (2017). Epigenetic transcriptional memory. Curr. Gene. 63, 435–439. doi: 10.1007/s00294-016-0661-8
Estavillo, G. M., Crisp, P. A., Pornsiriwong, W., Wirtz, M., Collinge, D., Carrie, C., et al. (2011). Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 23, 3992–4012. doi: 10.1105/tpc.111.091033
Fang, L., Su, L., Sun, X., Li, X., Sun, M., Karungo, S. K., et al. (2016). Expression of Vitis amurensis NAC26 in Arabidopsis enhances drought tolerance by modulating jasmonic acid synthesis. J. Exp. Bot. 67, 2829–2845. doi: 10.1093/jxb/erw122
Fang, Z., Zhang, X., Gao, J., Wang, P., Xu, X., Liu, Z., et al. (2015). A buckwheat (Fagopyrum esculentum) DRE-binding transcription factor gene, FeDREB1, enhances freezing and drought tolerance of transgenic Arabidopsis. Plant Mol. Biol. Rep. 33, 1510–1525. doi: 10.1007/s11105-015-0851-4
Feng, X. J., Li, J. R., Qi, S. L., Lin, Q. F., Jin, J. B., and Hua, X. J. (2016). Light affects salt stress-induced transcriptional memory of P5CS1 in Arabidopsis. Proc. Natl Acad. Sci. U. S. A. 113:E8335. doi: 10.1073/pnas.1610670114
Fleta-Soriano, E., and Munne-Bosch, S. (2016). Stress memory and the inevitable effects of drought: a physiological perspective. Front. Plant Sci. 7:143. doi: 10.3389/fpls.2016.00143
Fleta-Soriano, E., Pinto-Marijuan, M., and Munne-Bosch, S. (2015). Evidence of drought stress memory in the facultative CAM, Aptenia cordifolia: possible role of phytohormones. PLoS One 10:e0135391. doi: 10.1371/journal.pone.0135391
Garg, R., Narayana Chevala, V. V. S., Shankar, R., and Jain, M. (2015). Divergent DNA methylation patterns associated with gene expression in rice cultivars with contrasting drought and salinity stress response. Sci. Rep. 5:14922. doi: 10.1038/srep14922
Gilbert, M. E., and Medina, V. (2016). Drought adaptation mechanisms should guide experimental design. Trends plant sci. 21, 639–647. doi: 10.1016/j.tplants.2016.03.003
Goh, C. H., Nam, H. G., and Park, Y. S. (2003). Stress memory in plants: a negative regulation of stomatal response and transient induction of rd22 gene to light in abscisic acid-entrained Arabidopsis plants. Plant J. 36, 240–255. doi: 10.1046/j.1365-313X.2003.01872.x
Guo, H., Sun, Y., Peng, X., Wang, Q., Harris, M., and Ge, F. (2016). Up-regulation of abscisic acid signaling pathway facilitates aphid xylem absorption and osmoregulation under drought stress. J. Exp. Bot. 67, 681–693. doi: 10.1093/jxb/erv481
Gururani, M. A., Venkatesh, J., and Tran, L. S. P. (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 8, 1304–1320. doi: 10.1016/j.molp.2015.05.005
Harb, A., Krishnan, A., Ambavaram, M. M. R., and Pereira, A. (2010). Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 154, 1254–1271. doi: 10.1104/pp.110.161752
Hare, P. D., and Cress, W. A. (1997). Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 21, 79–102. doi: 10.1016/j.plaphy.2013.05.028
Jones, E. E., and Pittman, T. S. (1982). “Toward a general theory of strategic self-presentation” in Psychological Perspectives on the Self, Vol. 1, ed. J. Suls (Hillsdale: Erlbaum), 231–262.
Julio Camarero, J., Gazol, A., Sangüesa-Barreda, G., Cantero, A., Sánchez-Salguero, R., Sánchez-Miranda, A., et al. (2018). Forest growth responses to drought at short- and long-term scales in spain: squeezing the stress memory from tree rings. Front. Ecolo. Evol. 6, 9.1–9.11. doi: 10.3389/fevo.2018.00009
Kang, H. M., and Saltveit, M. E. (2002). Chilling tolerance of maize, cucumber and rice seedling leaves and roots are differentially affected by salicylic acid. Physiol. Planta. 115, 571–576. doi: 10.1034/j.1399-3054.2002.1150411.x
Kim, D., Pertea, G., Trapnell, C., Pimentel, H., Kelley, R., and Salzberg, S. L. (2013). TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14:R36. doi: 10.1186/gb-2013-14-4-r36
Kim, E. D., and Sung, S. (2012). Long noncoding RNA: unveiling hidden layer of gene regulatory networks. Trends Plant Sci. 17, 16–21. doi: 10.1016/j.tplants.2011.10.008
Kong, L., Zhang, Y., Ye, Z. Q., Liu, X. Q., Zhao, S. Q., Wei, L., et al. (2007). CPC: assess the protein-coding potential of transcripts using sequence features and support vector machine. Nucleic Acids Res. 35, W345–W349. doi: 10.1093/nar/gkm391
Lämke, J., and Bäurle, I. (2017). Epigenetic and chromatin-based mechanisms in environmental stress adaptation and stress memory in plants. Genome Biol. 18:124. doi: 10.1186/s13059-017-1263-1266
Lee, S. M., Radhakrishnan, R., Kang, S. M., Kim, J. H., Lee, I. Y., Moon, B. K., et al. (2015). Phytotoxic mechanisms of bur cucumber seed extracts on lettuce with special reference to analysis of chloroplast proteins, phytohormones, and nutritional elements. Ecotoxicol. Environ. Safe. 122, 230–237. doi: 10.1016/j.ecoenv.2015.07.015
Li, X. N., Topbjerg, H. B., Jiang, D., and Liu, F. L. (2015). Drought priming at vegetative stage improves the antioxidant capacity and photosynthesis performance of wheat exposed to a short-term low temperature stress at jointing stage. Plant Soil 393, 307–318. doi: 10.1007/s11104-015-2499-0
Linster, E., Stephan, I., Bienvenut, W. V., Maple-Grødem, J., Myklebust, L. M., Huber, M., et al. (2015). Downregulation of N-terminal acetylation triggers ABA-mediated drought responses in Arabidopsis. Nat. commun. 6:7640. doi: 10.1038/ncomms8640
Liu, N., Staswick, P. E., and Avramova, Z. (2016). Memory responses of jasmonic acid-associated Arabidopsis genes to a repeated dehydration stress. Plant Cell Environ. 39, 2515–2529. doi: 10.1111/pce.12806
Mohanty, B., Kitazumi, A., Cheung, C. Y. M., Lakshmanan, M., de los Reyes, B. G., Jang, I.-C., et al. (2016). Identification of candidate network hubs involved in metabolic adjustments of rice under drought stress by integrating transcriptome data and genome-scale metabolic network. Plant Sci. 242, 224–239. doi: 10.1016/j.plantsci.2015.09.018
Nazar, R., Umar, S., Khan, N. A., and Sareer, O. (2015). Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. South Afr. J. Bot. 98, 84–94. doi: 10.1016/j.sajb.2015.02.005
Osakabe, Y., Osakabe, K., Shinozaki, K., and Tran, L.-S. (2014). Response of plants to water stress. Front. Plant Sci. 5:86. doi: 10.3389/fpls.2014.00086
Porebski, S., Bailey, L. G., and Baum, B. R. (1997). Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 15, 8–15. doi: 10.1007/bf02772108
Qian, B., Li, X., Liu, X., Chen, P., Ren, C., and Dai, C. (2015). Enhanced drought tolerance in transgenic rice overexpressing of maize C4 phosphoenolpyruvate carboxylase gene via NO and Ca 2+. J. plant physiol. 175, 9–20. doi: 10.1016/j.jplph.2014.09.019
Raikwar, S., Srivastava, V. K., Gill, S. S., Tuteja, R., and Tuteja, N. (2015). Emerging importance of helicases in plant stress tolerance: characterization of Oryza sativa repair helicase XPB2 promoter and its functional validation in tobacco under multiple stresses. Front. Plant Sci. 6:1094. doi: 10.3389/fpls.2015.01094
Saeidnejad, A. H., and Rajaei, P. (2015). Antioxidative responses to drought and salinity stress in plants, a comprehensive review. Int. J. Life Sci. 9, 1–8. doi: 10.3126/ijls.v9i2.12042
Sakuraba, Y., Kim, Y.-S., Han, S.-H., Lee, B.-D., and Paek, N.-C. (2015). The Arabidopsis transcription factor NAC016 promotes drought stress responses by repressing AREB1 transcription through a trifurcate feed-forward regulatory loop involving NAP. Plant Cell 27, 1771–1787. doi: 10.1105/tpc.15.00222
Santos, A. P., Serra, T., Figueiredo, D. D., Barros, P., Lourenco, T., Chander, S., et al. (2011). Transcription regulation of abiotic stress responses in rice: a combined action of transcription factors and epigenetic mechanisms. OMICS 15, 839–857. doi: 10.1089/omi.2011.0095
Savoure, A., Hua, X. J., Bertauche, N., VanMontagu, M., and Verbruggen, N. (1997). Abscisic acid-independent and abscisic acid-dependent regulation of proline biosynthesis following cold and osmotic stresses in Arabidopsis thaliana. Mol. General Genet. 254, 104–109. doi: 10.1007/s004380050397
Strizhov, N., Abraham, E., Okresz, L., Blickling, S., Zilberstein, A., Schell, J., et al. (1997). Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 12, 557–569. doi: 10.1046/j.1365-313X.1997.00557.x
Su, L. Y., Dai, Z. W., Li, S. H., and Xin, H. P. (2015). A novel system for evaluating drought-cold tolerance of grapevines using chlorophyll fluorescence. BMC Plant Biol. 15:82. doi: 10.1186/s12870-015-0459-8
Sun, L., Luo, H. T., Bu, D. C., Zhao, G. G., Yu, K. T., Zhang, C. H., et al. (2013). Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 41:e166. doi: 10.1093/nar/gkt646
Szabados, L., and Savoure, A. (2010). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. doi: 10.1016/j.tplants.2009.11.009
Tafer, H., and Hofacker, I. L. (2008). RNAplex: a fast tool for RNARNA interaction search. Bioinformatics 24, 2657–2663. doi: 10.1093/bioinformatics/btn193
Trapnell, C., Roberts, A., Goff, L., Pertea, G., Kim, D., Kelley, D. R., et al. (2012). Differential gene and transcript expression analysis of RNA-seq experiments with tophat and cufflinks. Nat. Protoc. 7, 562–578. doi: 10.1038/nprot.2012.016
Virlouvet, L., Avenson, T. J., Du, Q., Zhang, C., Liu, N., Fromm, M., et al. (2018). Dehydration stress memory: gene networks linked to physiological responses during repeated stresses of Zea mays. Front. Plant Sci. 9:1058. doi: 10.3389/fpls.2018.01058
Virlouvet, L., and Fromm, M. (2015). Physiological and transcriptional memory in guard cells during repetitive dehydration stress. New Phytol. 205, 596–607. doi: 10.1111/nph.13080
Wang, H., Chung, P. J., Liu, J., Jang, I.-C., Kean, M. J., Xu, J., et al. (2014). Genome-wide identification of long noncoding natural antisense transcripts and their responses to light in Arabidopsis. Genome Res. 24, 444–453. doi: 10.1101/gr.165555.113
Wang, X., Vignjevic, M., Liu, F. L., Jacobsen, S., Jiang, D., and Wollenweber, B. (2015). Drought priming at vegetative growth stages improves tolerance to drought and heat stresses occurring during grain filling in spring wheat. Plant Growth Regul. 75, 677–687. doi: 10.1007/s10725-014-9969-x
Wibowo, A., Becker, C., Marconi, G., Durr, J., Price, J., Hagmann, J., et al. (2016). Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. eLife 5:e13546. doi: 10.7554/eLife.13546
Woo, N. S., Badger, M. R., and Pogson, B. J. (2008). A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 4:27. doi: 10.1186/1746-4811-4-27
Wu, Y., Sun, Y., Wang, X. T., Lin, X. Y., Sun, S., Shen, K., et al. (2016). Transcriptome shock in an interspecific F1 triploid hybrid of Oryza revealed by RNA sequencing. J. Integr. Plant Bio. 58, 150–164. doi: 10.1111/jipb.12357
Wu, Y. G., Wei, B., Liu, H. Z., Li, T. X., and Rayner, S. (2011). MiRPara: a SVM-based software tool for prediction of most probable microRNA coding regions in genome scale sequences. BMC Bioinform. 12:107. doi: 10.1186/1471-2105-12-107
Xin, Z., and Browse, J. (1998). Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl. Acad. Sci. U. S. A. 95, 7799–7804. doi: 10.1073/pnas.95.13.7799
Yang, X., Wang, X., Ji, L., Yi, Z., Fu, C., Ran, J., et al. (2015). Overexpression of a Miscanthus lutarioriparius NAC gene MlNAC5 confers enhanced drought and cold tolerance in Arabidopsis. Plant cell Reports 34, 943–958. doi: 10.1007/s00299-015-1756-2
Zhang, C., Peng, X., Guo, X., Tang, G., Sun, F., Liu, S., et al. (2018a). Transcriptional and physiological data reveal the dehydration memory behavior in switchgrass (Panicum virgatum L.). Biotechnol. Biofuels 11:91. doi: 10.1186/s13068-018-1088-x
Keywords: ssRNA-Seq, short-term drought memory, ABA, photosynthesis, proline, memory factors
Citation: Li P, Yang H, Wang L, Liu H, Huo H, Zhang C, Liu A, Zhu A, Hu J, Lin Y and Liu L (2019) Physiological and Transcriptome Analyses Reveal Short-Term Responses and Formation of Memory Under Drought Stress in Rice. Front. Genet. 10:55. doi: 10.3389/fgene.2019.00055
Received: 31 October 2018; Accepted: 23 January 2019;
Published: 08 February 2019.
Edited by:
Marco Pellegrini, Italian National Research Council (CNR), ItalyReviewed by:
Chi Zhang, University of Nebraska–Lincoln, United StatesCopyright © 2019 Li, Yang, Wang, Liu, Huo, Zhang, Liu, Zhu, Hu, Lin and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Liu, bGl1bGlhQG1haWwua2liLmFjLmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.