
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Genet., 16 November 2018
Sec. Stem Cell Research
Volume 9 - 2018 | https://doi.org/10.3389/fgene.2018.00544
 Yaroslav R. Efremov1,2
Yaroslav R. Efremov1,2 Anastasia S. Proskurina1
Anastasia S. Proskurina1 Ekaterina A. Potter1
Ekaterina A. Potter1 Evgenia V. Dolgova1
Evgenia V. Dolgova1 Oksana V. Efremova2
Oksana V. Efremova2 Oleg S. Taranov3
Oleg S. Taranov3 Aleksandr A. Ostanin4
Aleksandr A. Ostanin4 Elena R. Chernykh4
Elena R. Chernykh4 Nikolay A. Kolchanov1
Nikolay A. Kolchanov1 Sergey S. Bogachev1*
Sergey S. Bogachev1*A functional analysis of 167 genes overexpressed in Krebs-2 tumor initiating cells was performed. In the first part of the study, the genes were analyzed for their belonging to one or more of the three groups, which represent the three major phenotypic manifestation of malignancy of cancer cells, namely (1) proliferative self-sufficiency, (2) invasive growth and metastasis, and (3) multiple drug resistance. 96 genes out of 167 were identified as possible contributors to at least one of these fundamental properties. It was also found that substantial part of these genes are also known as genes responsible for formation and/or maintenance of the stemness of normal pluri-/multipotent stem cells. These results suggest that the malignancy is simply the ability to maintain the stem cell specific genes expression profile, and, as a consequence, the stemness itself regardless of the controlling effect of stem niches. In the second part of the study, three stress factors combined into the single concept of “generalized cellular stress,” which are assumed to activate the expression of these genes, were defined. In addition, possible mechanisms for such activation were identified. The data obtained suggest the existence of a mechanism for the de novo formation of a pluripotent/stem phenotype in the subpopulation of “committed” tumor cells.
Malignant neoplasms have been known to medicine for several thousand years, and it can assuredly be stated that all this time science has tried to find and formulate the fundamental properties that determine the development of tumors in vivo. The evolution of our ideas regarding the processes of the onset and development of tumors has overcome a very long and difficult way. As the first steps, the primitive macroscopic anatomical descriptions had been made (Yelloly, 1809). With the progress in methodology and instruments development, they gave way to the similar microscopic ones (Creighton, 1882) and even later–to the first attempts to determine the functional properties of tumor cells in vitro (Carrel and Ebeling, 1928). At the late steps, we came to an understanding (well, at least we tend to think so) of the fundamental physiological and molecular-genetic processes of tumor development, which, finally, made it possible to formulate the “Hallmarks of Cancer.”
There are two main points of view on the significant signs of malignancy of cancer and its underlying unit–cancer cells. In the first case, it is asserted that the hallmarks of cancer comprise six biological capabilities acquired during the multistep development of tumors. The hallmarks constitute an organizing principle for rationalizing the complexities of neoplastic disease. They include (1) Self-Sufficiency in Growth Signals, (2) Insensitivity to Antigrowth Signals, (3) Evading Apoptosis, (4) Limitless Replicative Potential, (5) Sustained Angiogenesis, and (6) Tissue Invasion and Metastasis (Hanahan and Weinberg, 2000, 2011).
In the second case, the authors offer an alternative set of key characteristics that determine the malignancy of a cancerous tumor and cancer cells that form it. This variant includes (1) selective growth and proliferative advantages, (2) altered stress response favoring overall survival, (3) vascularization, (4) invasion and metastasis, (5) metabolic rewiring, (6) an abetting microenvironment, and (7) immune modulation (Fouad and Aanei, 2017).
It is easy to note that these two lists both quite clearly overlap, have also quite a fundamental difference. Thus, for example, the authors of the second model do not include immortalization in the list of significant properties that define the behavior of the tumor. This property, in fact, represents a fundamental, extra-hierarchical qualitative event, which, on the one hand, is itself not a manifestation of malignancy, yet, on the other hand, is indispensable for its development.
Since the hallmarks of cancer and cancer cells malignancy, as they are denoted by the authors cited above, seem to be excessively detailed, we in our scrutiny narrowed them down to three more general categories that define the malignant potential at the phenotypic level. The first is the proliferative self-sufficiency as a set of characteristics that provide uncontrolled tumor growth. It comprises both independence from external mitogenic stimuli and immunity to stimuli that cause cell cycle arrest or apoptosis. The second one is invasiveness. It combines such properties as the ability to lyse the basal membrane, an increased capacity for migration, and the ability to adapt to the tissue environment, which is initially uncharacteristic for the tumor cell. And the last, third category is multiple drug resistance. This one is, in fact, a part of a broader detoxification mechanism essential for the survival of cells under aggressive tumor conditions. We also excluded from nomenclature both immortalization (for the reason described above) and sustained angiogenesis (due to ultimate dependence on the tumor context–this feature is essential for solid forms only).
Along with the definition of the cancer cells malignancy hallmarks and understanding of the mechanisms of tumor progression, data on the high heterogeneity of the tumor cellular mass were accumulated. These data turned out to contradict, to a certain extent, the theory of clonal origin of tumors.
The clonal nature of tumors has been known for a long time: it was first shown for human lymphomas (Fialkow et al., 1967, 1970; Steele, 1970) and subsequently confirmed for other types of tumors (Baylin et al., 1976; Nowell, 1976). At approximately the same time, it was found that tumors are quite heterogeneous and consist of cells that differ, and sometimes to a great extent, both in phenotype, and in physiological, proliferative and tumor-initiating attributes. For glioblastomas, for example, it was shown that tumors contain variable proportions of actively proliferating and nonproliferating tumor cells and that up to 70% of the cells in these tumors are resting (nonproliferating) (Hoshino and Wilson, 1975). However, one of the most convincing and demonstrative essays in terms of evidence of the tumor cells population heterogeneity is the work of Lavrovsky et al. In this essay, multiple clones from several spontaneously established murine sarcomas of CBA, C3H, and Balb/c genotypes were obtained and described. The phenotype of these clones was shown to vary from highly tumorigenic to the so-called pseudonormal. The tumorigenic clones were characterized by rapid multilayer growth and almost complete independence of the serum content, while the pseudonormal ones demonstrated sensitivity to growth factors as well as contact inhibition and the ability to differentiate into adipocytes after prolonged arrest in G0 (Lavrovsky et al., 1992).
Thus, in the early 90s of the last century, a firm paradigm for tumor growth emerged in molecular oncology. It was claimed that tumor growth is driven by a small subpopulation of actively dividing cells, while the rest of the cellular mass, which constitutes the bulk of the tumor, is a kind of ballast formed as a result of high genetic instability of tumor cells (Pathak, 1990).
The further accumulation of knowledge on tumors development has revealed that the cellular composition of tumors is essentially determined by certain internal rules, similar to those for normal organs. As a logical consequence, the previous paradigm has been evolutionary updated. In accordance to this updated paradigm, the tumor is considered an aberrant organ, developing from a subpopulation of poorly differentiated tumor cells with an infinite proliferative potential. For such a type of cells the new term “cancer stem cell” (by analogy with normal stem cells) was invented. And despite the term first being used in 1980 (Mattox and Von Hoff, 1980), the targeted investigation of this phenomenon started only in this millennium, when the term acquired its final meaning as a definition for poorly differentiated cells with indefinite potential for self-renewal that drive tumorigenesis (Reya et al., 2001).
However, in contrast to normal stem cells with their quite objective and clearly formalized criteria of stemness, the definition of stemness for cancer cells remains generally problematic and the search for such criteria is one of the high priority tasks in molecular oncology.
Recently, it was found that a certain subpopulation of Krebs-2 ascites carcinoma cells has the inherent ability to internalize fragments of extracellular double-stranded DNA (hereinafter–TAMRA+ cells). This subpopulation also demonstrates such a fundamental property of cancer stem cells (CSCs) as the ability to induce upon transplantation the development of a new tumor with histological and cellular characteristics similar to the original one. Elimination of these cells leads either to the loss of the grafting potential by the transplant, or to the cure of mice from developed Krebs-2 ascites (Dolgova et al., 2012, 2013, 2014, 2016; Potter et al., 2016b, 2018). Thus, the ability to internalize extracellular double-stranded DNA can be referred to as a valid marker (or, at least, as one of) of cancer cells stemness.
The paradigm of CSC and aberrant organogenesis had resolved the issue of tumors heterogeneity in the context of their clonal origin. But a new question had arisen.
The clonal nature of tumors implies that the entire mass of the tumor is the progeny of a single cell. The rapid and extensive growth of a tumor mass inevitably should lead to a situation, when a progenitor cell, i.e., CSC, remains in the very center of a tumor. It, in turn, should apparently cause either the complete cessation of tumor growth, or such a slowing down of it that is, in fact, almost equivalent to cessation. The tumor growth observed both in experiments and in medical practice is possible only in the presence of an essentially large number of CSCs, more or less evenly distributed throughout the tumor volume. As it is shown by our numerous experiments on mice and cultures of human cancer cells, as well as by the results of other researchers, the content of CSCs in tumor tissue varies from a few hundredths of a percent to several percents, and they are dispersedly scattered throughout a tumor mass or in ascitic fluid (Dolgova et al., 2014; Potter et al., 2016a,b). This means that under regular conditions of tumor tissue development, one CSC ensures the existence and biological activity of about 100–1,000 tumor cells. Assuming all the above, the question is how such a pattern of the CSCs distribution is being formed during the tumor quasi-organ development from a single progenitor.
It is generally accepted that the source of new CSCs, as in the case of normal pluri-/multipotent stem cells, is symmetrical division, as a manifestation of one of the fundamental properties of stemness. The newly formed CSC easily leaves not only its original site of localization in the tumor, but also the formed tumor tissue itself and, without losing its malignant properties, can migrate either to other parts of the growing tumor quasi-organ or to distant parts of the body. In other words, symmetrical division of the progenitor provides a constant supply of new CSCs, which migrate from the primary niche to the periphery of the tumor, creating new growth foci there, and the utmost case of such migrations is metastases.
This hypothesis, which explains the ability of CSCs to increase their population by symmetrical division followed by migration, is supported, in part, by the results we obtained earlier. Daily we estimated the numbers of TAMRA+ cells in Krebs-2 ascites from its onset and until the death of the animals (14 days). A characteristic oscillation in the number of TAMRA+ cells within 3 days accompanied by an increase in the volume of ascitic fluid and the total mass of cancer cells was observed. Along this time span, the number of TAMRA+ cells increased 3-fold and then returned to the baseline. The following model was proposed to explain this observation. The first act of symmetrical division produces two equal CSCs. One of these new CSCs enters the second division producing two daughter cells that both still possess the ability to internalize the TAMRA-labeled DNA probe. After the third division, the progeny of CSC lose their ability to internalize DNA and the percentage of TAMRA+ cells returns to initial value (Potter et al., 2016a,b).
Nevertheless, there are numerous data that suggest the existence of another mechanism for the formation and maintenance of the CSCs population.
Thus, in the study cited above, we found a discrepancy that did not fit into the theory explaining the increase in the number of CSCs as a result of their symmetrical division. It was found that for the majority of the mice analyzed, days of a “peak value” were observed, when the amount of CSCs significantly exceeded the regular threshold values typical for the observed oscillation of the CSCs counts (Potter et al., 2016a,b).
In the also mentioned above work of Lavrovsky et al, the efficacy of tumor formation upon transplantation of the progeny of the obtained clones into syngeneic mice has been evaluated. It was shown that tumors develop both in the case of highly tumorigenic clones, with the properties of CSCs, and in the case of pseudonormal cells, which displayed properties of committed cells. The difference between tumorigenic and pseudonormal clones was only in the incidence of tumor formation and in the time lapse required for this (Lavrovsky et al., 1992).
It is also known that many of immortalized cell lines displaying a “normal” phenotype of committed cells, such as various 3T3 lines, for example, produce tumors upon transplantation into syngeneic or immunodeficient animals (Greig et al., 1985; Melchiori et al., 1992). In other words, the data presented suggest that upon transplantation of “committed” cells of 3T3 type, i.e. possessing an infinite proliferative potential, but not an undifferentiated phenotype, in vivo CSCs can emerge de novo, giving rise to a tumor. Recent evidences support such a model of “dynamic stemness” for, at least, melanomas. Melanoma cells might temporally acquire tumor-initiating properties or switch from a status of tumor-initiating cells to a more differentiated one depending on the tumor context (Tuccitto et al., 2016).
A number of other studies demonstrating the feasibility of tumor cells to transit in both directions from cells of stem-like phenotype to differentiated ones and back again have also been compiled and reviewed (ElShamy and Duhé, 2013; Campos-Sánchez and Cobaleda, 2015).
Numerous observations of “dynamic stemness” allow to hypothesize the emergent nature of, at least, a part of the CSCs population. Accordingly, it is logical to presume that their emergence is associated with certain conditions in the micro- and humoral-environment, leading to the activation of the signaling pathways required for the induction of pluripotent/stem phenotype. Such a hypothesis implies the possibility of a reversible switching of the malignant identity of tumor cells and explains the distribution pattern of CSCs throughout the tumor volume, including its distal regions.
Assuming all the above, it is CSCs that are obviously to be responsible for the implementation of the “tumorigenicity program” and thus have to evince the properties of malignancy to the highest extent, while the role of the remaining mass of tumor cells is still rather speculative.
Previously we have isolated the enriched population of TAMRA+ cells, which, as mentioned above, display all the principal properties of CSCs, and have identified 167 genes overexpressed in these cells relative to TAMRA− cells (see Additional Table 1) (Potter et al., 2017).
In accordance to the proposed model of malignancy that consists of proliferative self-sufficiency, invasiveness and multiple drug resistance, we analyzed all these 167 genes with regard to their possible roles in realization of these fundamental properties. The existing data mining revealed that the genes involved in the formation of TAMRA+ cells malignancy differed in their significance based on their contribution to the one or several attributes of malignancy simultaneously. It also turned out that besides their role as known CSCs markers, a significant part of genes from the list were also markers of stemness in normal pluri-/multipotent stem cells involved in maintaining their stem phenotype.
Upon identification of genes principal for formation and maintenance of the malignant/pluripotent properties of cancer cells, we have attempted to reveal the possible mechanisms of activation of these genes as well as to deduce the conditions essential for such an activation. Analysis of published data has revealed the plausible influence of stress factors on activation of both the identified genes and stem-like phenotype of tumor cells itself. The following analysis of ChIP-Seq data gave us a clue to possible mechanisms of activating effect of “generalized cellular stress.”
In the following parts of the article we describe a number of well known and generally accepted statements based on multiple experiments with a wide range of models including cellular in vitro models, experimental animals and clinically obtained tumor samples. To prove the majority of these statements we refer to review articles. In cases when the model represents an individual and unique one, we describe it in more details.
As already mentioned, we consider proliferative self-sufficiency as a complex property. On the one hand, it is defined as the ability of a cell to maintain proliferation under conditions of inaccessibility or deficiency in external mitogenic stimuli. On the other hand, it reflects the ability to keep viability and avoid apoptosis despite the presence of pro-apoptotic signals. It can be achieved by a rather large set of mechanisms, from autocrine synthesis and secretion of growth factors and components of the extracellular matrix (reviewed in Hoelzinger et al., 2007) to blocking the internal mechanisms of the apoptotic program (reviewed in Mallard and Tiralongo, 2017). The main problem we encountered in the analysis and selection of genes contributing to this property is the dependence of the functional properties of their protein products on the overall gene-protein context in each particular case. Often the same protein can act both as a tumor suppressor and as a tumor inducer depending on the type of cells or conditions. As an example, we can refer to the gene Perp, which was overexpressed in TAMRA+ cells, and which we, nevertheless, could not relate to any of the groups due to the lack of direct evidence of its functional effect on the formulated properties. It was shown that in the case of invasive squamous cell carcinoma, Perp functions as a tumor suppressor (Kong et al., 2013), while the Perp−/− mice were more resistant to papilloma development than those of the wild-type, that suggests its pro-oncogenic function (Marques et al., 2005). Moreover, it is a possible case when the protein product of a gene normally functions as a tumor suppressor, but upon the mutation its properties as a tumor suppressor are either lost or even inverted and it acquires pro-oncogenic function as it is shown, for example, for “gain-of-function” mutations of the p53 tumor suppressor gene (Vogiatzi et al., 2016). Since we did not have the opportunity to resolve all these of issues, we decided that the gene is to be included in a certain functional group if in principle there is evidence of its positive impact on the implementation of the corresponding property. As a result, we have selected 82 genes that one way or another participate in formation of the proliferative self-sufficiency of tumor cells (Table 1).
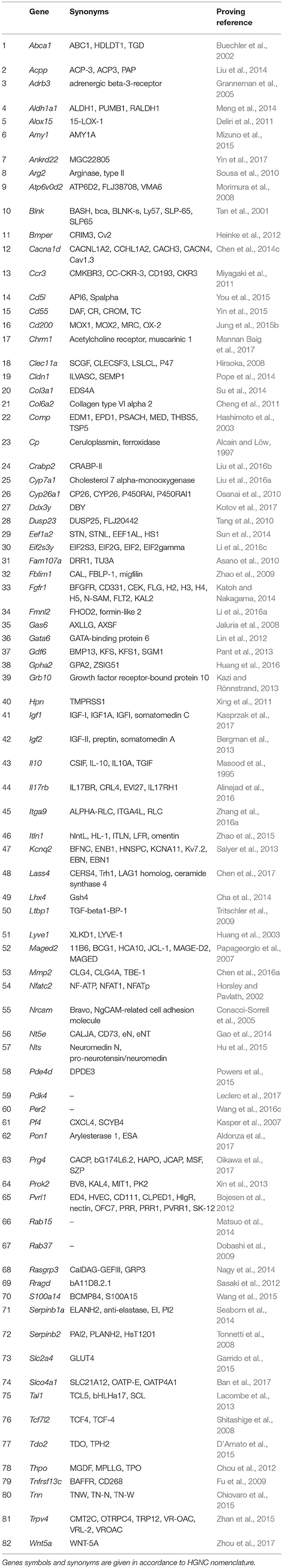
Table 1. Genes showing elevated expression in TAMRA+ Krebs-2 carcinoma cells relative to TAMRA− cells, the activation of which results in excessive proliferative activity or resistance to apoptosis.
Another fundamental property of malignant tumors is their ability of invasive growth and metastasis. This process commonly starts with proteolytic degradation of the basal membrane by metalloproteinases of various types, the increased expression of which is one of the main indicators of invasive tumor growth (reviewed in Jiang et al., 2015). Further, the metastasizing cell must have a number of specific properties. First, it should be able to exist in an unattached state while in the bloodstream or lymphatic vessel. This functional feature overlaps to a significant extent with the previous property to block the apoptosis, in this case–apoptosis caused by the detachment from matrix, the so-called anoykis (reviewed in Taddei et al., 2012). Second, metastasizing cell should be able to settle down and normally proliferate in the initially alien tissue environment, which can be attained through the increased expression of numerous molecules of cell adhesion, often specific for lymphoid cells (reviewed in Chong et al., 2012). And third, the cell should be able to avoid a tissue-specific immune response. This is usually being achieved, either, similarly to the previous case, by expressing specific surface markers, or by synthesizing and secreting immunosuppressive mediators and cytokines (reviewed in Kuol et al., 2017). Another important role in the invasion and metastasis is assigned to proteins that stimulate the migratory function of cells (reviewed in Bordeleau et al., 2014). This group was constituted of 64 genes promoting one or more of mentioned functional properties (Table 2).
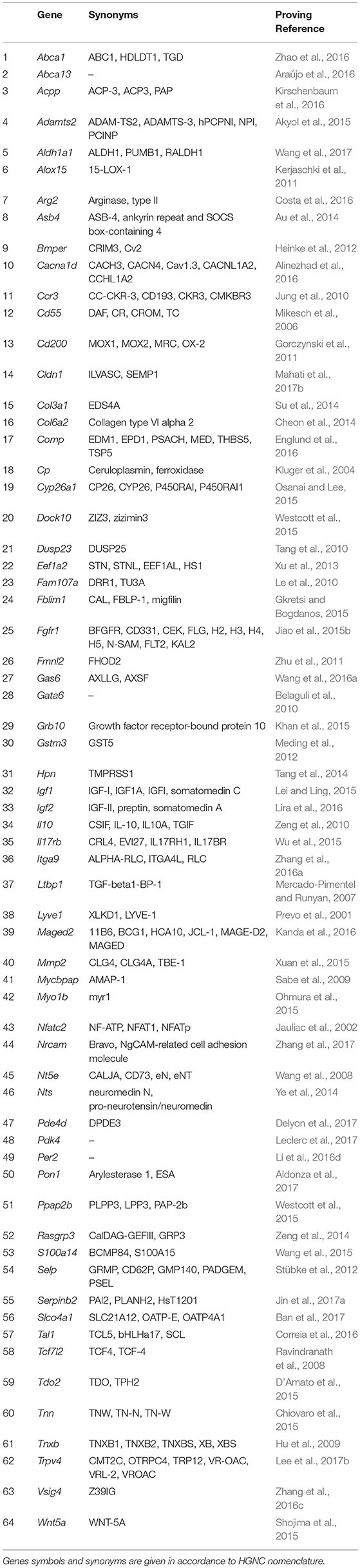
Table 2. Genes showing elevated expression in TAMRA+ Krebs-2 carcinoma cells relative to TAMRA− cells, the activation of which results in invasive growth and metastasis.
One of important, if not the most important, problems in clinical oncology is the resistance of tumors to antitumor drugs. When in the 50s of the last century this phenomenon had started to be actively investigated, the drug resistance was believed to be an adaptive response that develops as a result of tumor cells selection under long-term exposure to a certain drug. It was generally accepted to associate the drug resistance with an elevated level of expression of enzymes responsible for xenobiotics metabolism, such as P450 family oxygenases (reviewed in Harvey and Morgan, 2014), and specific transmembrane transport proteins providing efflux of xenobiotics and their metabolites (reviewed in Chen et al., 2016b). However, more recent observations have revealed that very often drug resistance is initially intrinsic to a certain subpopulation of tumor cells and is associated not only with the above-mentioned causes (reviewed in Gottesman, 2002). The main effect of antitumor drugs is known to be associated with their either cytostatic or cytotoxic properties, which in turn are mainly realized through DNA damage and should activate apoptotic processes. Accordingly, activation of mechanisms allowing to overcome G1/S arrest or blocking the realization of the apoptotic program, increases the resistance of tumor cells to chemotherapy (Volm, 1998). Moreover, the DNA-damaging effect of chemotherapeutic agents is neutralized by the cellular systems of antioxidative defense (reviewed in Victorino et al., 2014). And, finally, in the very end of the last century, another mechanism of tumors drug resistance—the so-called Cell-Adhesion Mediated Drug Resistance (CAM-DR) was discovered (reviewed in Dalton, 1999). This mechanism, in fact, represents a complex adaptive response that comprises the increased resistance to apoptosis due to anti-apoptotic signals from integrins (Damiano, 2002), reduced tumor permeability for chemotherapeutic agents (Kerbel et al., 1996; Grantab and Tannock, 2012), and formation of syncytium, which also leads to increased drug resistance (Lu and Kang, 2009; Nagler et al., 2011; Mittal et al., 2017). We found the evidences of anticancer therapy resistance inducing activity for 38 genes (Table 3).
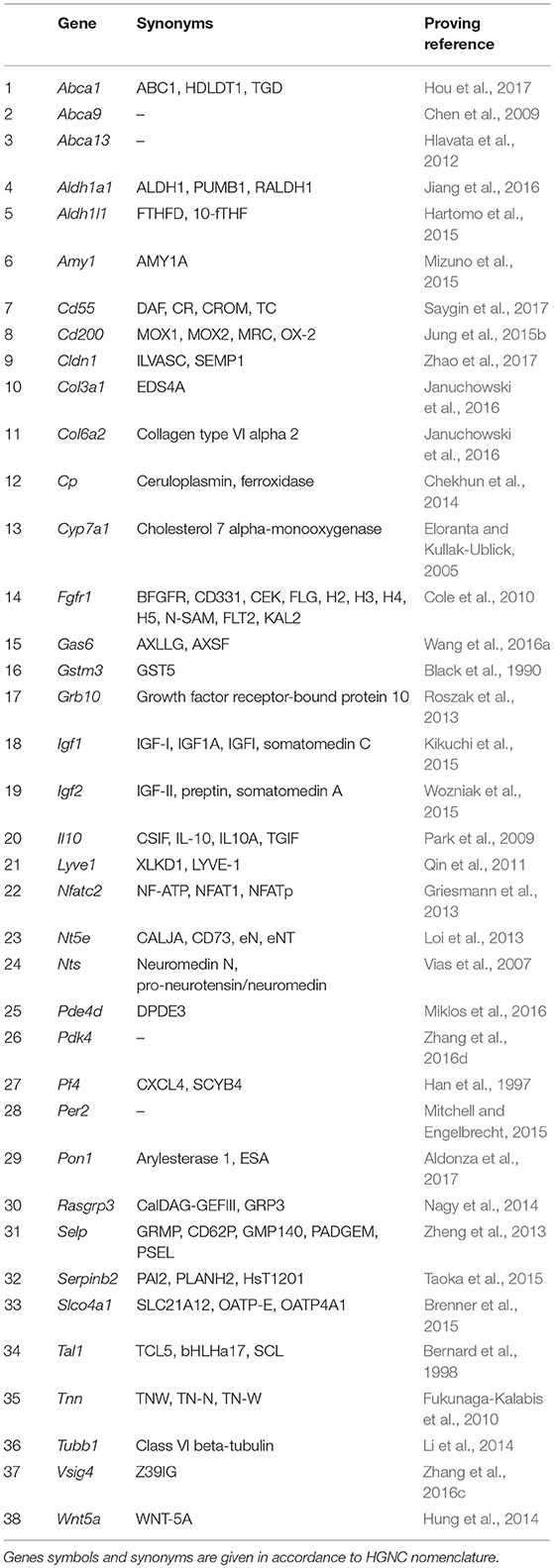
Table 3. Genes showing elevated expression in TAMRA+ Krebs-2 carcinoma cells relative to TAMRA− cells, the activation of which results in increased resistance of cells to xenobiotics and anti-tumor drugs.
The carried out data mining showed that out of 167 genes we tested, at least 96 belong to at least one of the three groups by their functional role in the formation of the tumorigenic phenotype. Herewith, all these genes in a completely natural way were dispensed into 7 additional groups. Group A (28 genes): Abca1, Aldh1a1, Cd55, Cd200, Cldn1, Col3a1, Col6a2, Cp, Fgfr1, Gas6, Grb10, Igf1, Igf2, Il10, Lyve1, Nfatc2, Nt5e, Nts, Pde4d, Pdk4, Per2, Pon1, Rasgrp3, Serpinb2, Slco4a1, Tal1, Tnn, Wnt5a—genes contributing to the formation of all three features. Group B (25 genes): Acpp, Alox15, Arg2, Bmper, Cacna1d, Ccr3, Comp, Cyp26a1, Dusp23, Eef1a2, Fam107a, Fblim1, Fmnl2, Gata6, Hpn, Il17rb, Itga9, Ltbp1, Maged2, Mmp2, Nrcam, S100a14, Tcf7l2, Tdo2, Trpv4—genes that simultaneously provide proliferative self-sufficiency and invasive growth/metastasis. Group C (4 genes): Abca13, Gstm3, Selp, Vsig4—genes that confer the drug resistance along with the metastatic phenotype. Group D (3 genes): Amy1, Cyp7a1, Pf4—genes responsible for proliferative self-sufficiency and drug resistance. Group E (26 genes): Adrb3, Ankrd22, Atp6v0d2, Blnk, Cd5l, Chrm1, Clec11a, Crabp2, Ddx3y, Eif2s3y, Gdf6, Gpha2, Itln1, Kcnq2, Lass4, Lhx4, Prok2, Prg4, Pvrl1, Rab15, Rab37, Rragd, Serpinb1a, Slc2a4, Thpo, Tnfrsf13c—genes responsible for proliferative self-sufficiency solely. Group F (7 genes): Adamts2, Asb4, Dock10, Mycbpap, Myo1b, Ppap2b, Tnxb—genes-inducers of invasive growth and metastasis. Group G (3 genes): Abca9, Aldh1l1, Tubb1—drug resistance genes (Figure 1).
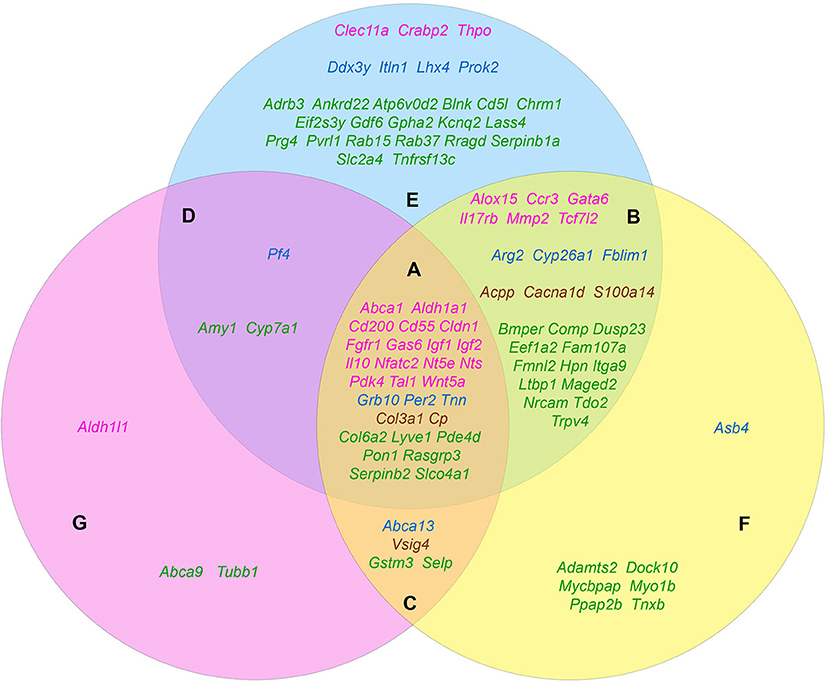
Figure 1. The distribution pattern of genes, overexpressed in TAMRA+ Krebs-2 carcinoma cells relative to TAMRA− cells, to functional groups. The blue area corresponds to the proliferative self-sufficiency, yellow, to invasiveness and metastasis; pink, to drug resistance. (A–F) Sections indicate the subgroups of the genes with regard to their multi-functionality: (A) the most multi-functional genes contributing to all three properties; (B) genes contributing to proliferative self-sufficiency and invasive growth/metastasis; (C) genes contributing to drug resistance and metastatic phenotype; (D) genes contributing to proliferative self-sufficiency and drug resistance; (E) genes contributing to proliferative self-sufficiency; (F) genes contributing to invasive growth and metastasis; (G) genes contributing to drug resistance. The genes symbols are indicated in different colors in accordance with their proven functional role in the formation of the stem or stem-like phenotype of normal pluri-/multipotent as well as tumor stem cells: pink denoting genes that are known markers of both normal pluri-/multipotent and tumor stem cells; blue, known markers of normal pluri-/multipotent stem cells; brown, known markers of tumor stem cells; green, genes for which their participation in the formation of stemness has not been proven at all.
Since the genes of the first four groups are “polyfunctional,” i.e., impact two or more properties simultaneously, it is logical to conclude that they contribute significantly greater (in comparison to the genes of the remaining three groups) to the formation of highly tumorigenic phenotype of the TAMRA+ cells. This makes them to be the most plausible candidates for the role of the main genetic markers of CSCs as well as malignancy itself. Moreover, the composed molecular-genetic “portrait,” emphasizing the differences of these cells from the bulk of tumor cells, gives additional reasons to believe that the main properties of tumor malignancy are determined precisely by CSCs.
Since the term “cancer stem cell” was introduced to designate a certain subpopulation of tumor cells on the basis of their phenotypic and functional similarity to normal pluri-/multipotent stem cells, it was initially assumed that there could be some common molecular-genetic mechanisms that provide such a similarity (Reya et al., 2001). Indeed, such stem cell-specific signaling pathways as, for example, Wnt-, Notch-, and Shh-dependent ones, have been demonstrated to be involved in development of various human and murine tumors (Ellisen et al., 1991; Henrique et al., 1997; Korinek et al., 1998; Chan et al., 1999; Wechsler-Reya and Scott, 1999, 2001; Zhang and Kalderon, 2001).
In this connection, we have considered it to be interesting to search the existing literature for evidences of the functional involvement of the identified genes-inducers of highly tumorigenic phenotype of the TAMRA+ cells in maintaining the stemness of normal pluri-/multipotent cells. In addition, we evaluated the involvement of these genes in the formation and maintenance of the stem-like phenotype of tumor cells.
Based on the screening results, 45 genes were assigned to the category of “stemness markers,” which makes up 46% of the analyzed and 27% of the total (167) genes differentially overexpressed in TAMRA+ cells of the Krebs-2 carcinoma. Herewith, more than half of these genes, namely 27 out of 45, are known to be implicated in maintenance and functional realization of stem properties of both tumor and normal pluripotent cells. However, four of these genes were included in this group with some reservations. Thus, for Cd55 and Il10, no direct contribution to the formation or maintenance of stemness of normal pluri-/multipotent cells was proved, but the essential role in the realization of the reparative functions of mesenchymal and autologous-induced pluripotent stem cells by dint of the immunosuppressive action of the protein products of these genes was demonstrated (Ardianto et al., 2010; Liu et al., 2012; de Almeida et al., 2014; Lee et al., 2015b). The role of Nts in the formation of the pluripotent phenotype has been proved only in the case of the so-called induced pluripotent cells (Cai et al., 2015). And, finally, for Crabp2 there was no direct evidence of its participation in the formation of stemness, but only demonstration of specific expression in normal human amniotic fluid-derived stem cells and in polycythemia vera-derived tumor stem cells (Steidl et al., 2005; Kim et al., 2010). In conjunction with its role in the metabolism of retinoic acids and their derivatives, this makes it to be attractive as a plausible marker of stemness for both tumor and normal stem cells.
Six more genes were identified as established markers of tumor-initiating stem cells. The remaining 12 genes were associated exclusively with normal pluripotent and multipotent stem cells, although, again, with some reservations. So, for Abca13 only specific expression in early human embryonic stem cells, decreasing during consecutive passages has been shown (Barbet et al., 2012), while for Arg2, as well as for the above Cd55 and Il10, its functional role is limited to the immunosuppressive effect necessary to overcome tissue-specific immunity by stem cells as it was shown for human pluripotent cells (Chen et al., 2015b). The results of the screening are summarized in Table 4.
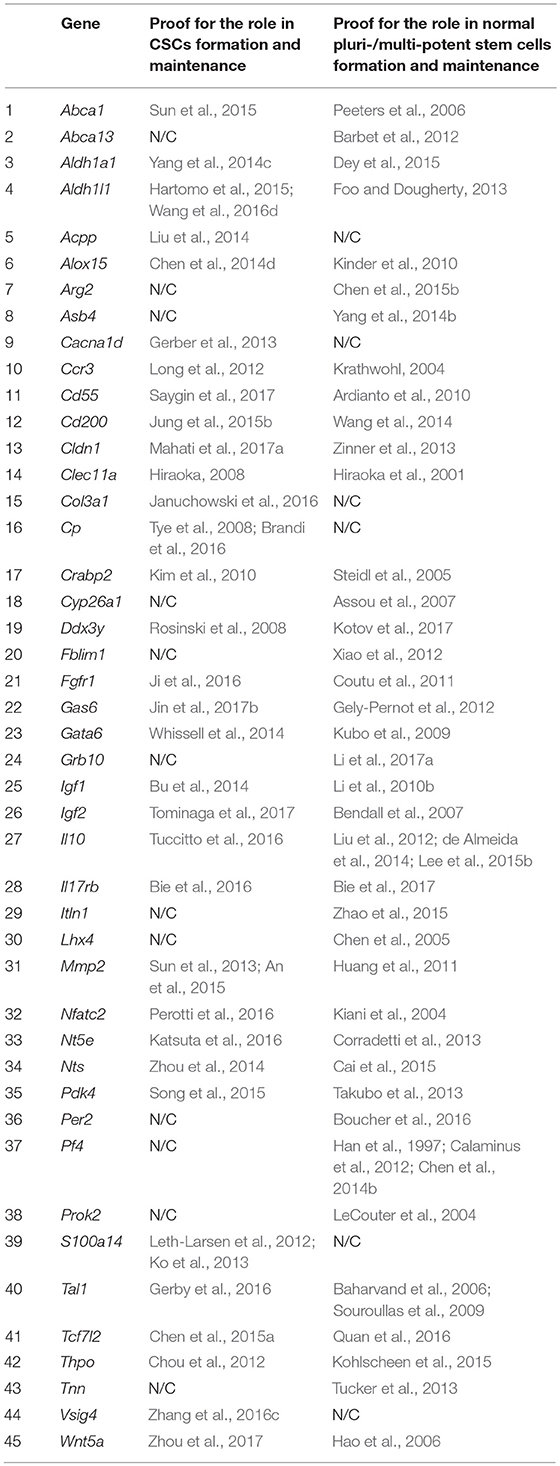
Table 4. Genes showing elevated expression in TAMRA+ Krebs-2 carcinoma cells relative to TAMRA− cells, and participating in formation and maintenance of stem properties of tumorigenic as well as normal pluri-/multipotent stem cells.
Identification of such an entity as a CSC has allowed to apply the principles of organogenesis to the development of tumors. From this point of view, the tumor is considered to be an aberrant organ, developing from a tumor cell possessing an infinite proliferative potential and a poorly differentiated stem-like phenotype (Reya et al., 2001). This approach implies the existence of functional analogies between normal stem cells involved in embryogenesis and tumor stem cells. Taking into account the functional purpose of normal pluri-/multipotent cells, their basic physiological properties can be deduced. First, it is obvious that the stem cell must possess a certain degree of proliferative autonomy and increased survival abilities to realize the function of the population self-maintenance. Second, the stem cell must evince active migratory and immunosuppressive functions, as well as the multiple tissue adherence to realize its genesis/reparative/regenerative functions. And third, the stem cell must have a well developed system of detoxification and resistance to xenobiotics, to keep the genome of both its own and the population as a whole intact. i.e., the attributes of stemness and the ones of malignancy, which we defined above, are the same, at least in the first approximation, and, respectively, the molecular-genetic mechanisms that determine these two characteristics can overlap to a significant degree.
Based on Table 4 and Figure 1 data, it can be noted that 21 of the 45 stemness marker genes got into Group A, which includes 28 genes that are most important for the formation of TAMRA+ cells malignancy. That is, this group substantially (75%) consists of the genes essential for the formation and maintenance of stem properties. At this, only two genes are identified as indicators of the stem-like phenotype of tumor cells, while the remaining 19 are necessary for the functioning of normal pluri-/multipotent stem cells. Another 12 genes were included into group B consisting of a total of 25 genes, while the rest were more or less evenly distributed over the remaining five groups.
Thus, the identity of genes determining the malignant properties of tumor-initiating cells and the stem properties of normal pluri-/multipotent stem cells has been revealed. Molecular-genetic identity of tumor-initiating and normal stem cells, as well as their morphophysiological one, gave us a reason to presume the identity of the very properties of malignancy and pluripotency themselves, that can be also designated as the properties of “independent behavior.” Up to the day, a significant number of evidences confirming the presumed behavioral identity of both types of cells has been presented. Thus, for example, it had been shown that transplantation of human embryonic stem cells, as well as of diploid and aneuploid pluripotent ones can lead to the development of tumors, most commonly identified as benign teratomas or malignant teratocarcinomas (Blum and Benvenisty, 2008, 2009). This property is postulated to be the hallmark of all pluripotent stem cell types, which demonstrates their potential to differentiate in all tissue types (reviewed in Dressel, 2011). On the other hand, classical experiments on the inoculation of teratocarcinomas cells into mouse embryos at the early stages of development have shown that, getting into the “right” conditions, malignant cells can differentiate into normal tissue, resulting in the development of a normal mosaic organism (Martin and Evans, 1975; Mintz and Illmensee, 1975; Illmensee and Mintz, 1976).
In other words, all these facts could mean that malignancy and stemness/pluripotency are one and the same entity, and the way this entity could be realized—malignancy or normal stemness—depends on the cellular microenvironment that provides the mentioned “right” location and conditions. And it is the stem cell niche that is apparently to be the appropriate location with appropriate conditions.
Initially, the term “stem cell niche” was proposed by Schofield in 1978 to describe a hypothetical cellular structure that provides conditions for the existence of a stem cell in which it is able to maintain its basic properties of self-renewal and maintenance of an undifferentiated or poorly differentiated state (Schofield, 1978). In its contemporary meaning, the role of the stem niche is dedicated to two basic functions. The first is to maintain the population of stem cells at a certain level by balancing pro-mitogenic and anti-mitogenic signals and providing a specific microenvironment necessary to maintain the undifferentiated state of stem cells (Schofield, 1983; Lin, 2002; Ohlstein et al., 2004; reviewed in Li and Neaves, 2006). The second is to act as a kind of “Maxwell's demon,” allowing niche exit to committed precursor cells, but not stem ones (reviewed in Marthiens et al., 2010). The last function has its reverse. The implication is that if a stem cell leaves the niche for any reason, it must either go back—the so-called “homing” known for hematopoietic stem cells, which can leave the stem niche for a while and then return (Whetton and Graham, 1999), or lose stemness and switch to a committed state, which, finally, ends with differentiation (Voog and Jones, 2010; O'Brien and Bilder, 2013). Simply stated, stem cells could not exist outside the stem cell niche. The main, as well as the only difference between CSCs and normal stem cells which is, in fact, the property of malignancy itself, is the ability to form and maintain stem/pluripotent properties outside a specific niche. This property comprises the defiance to morphogenetic signals from normal cellular and stromal environment and, as a consequence, the ability to form the tumorous stroma as well as the tumor itself in any tissue of the organism independently on the local environmental conditions.
Summarizing the section it should be said that the search for mechanisms providing such “independent behavior” of the CSCs is the principal priority in fundamental molecular oncology for now.
The hypothesis of “dynamic stemness” presumes the inducibility of stem-like phenotype in some subpopulation of “committed” tumor cells. It seems to be logical that such an induction and the following de novo appearance of CSCs occurs rather due to certain changes in cellular humoral or stromal environment. Thus, revealing the genes responsible for the stemness of TAMRA+ cells of the Krebs-2 carcinoma allows, in addition to the above, to deduce both the causes and mechanisms of induction of the stem-like phenotype in some part of the tumor cells.
It is well known that tumor growth and development is always accompanied by a number of stress factors. The first of them is the formation of hypoxia foci (Moulder and Rockwell, 1987; reviewed in Bertout et al., 2008). The second one is the oxidative stress, which develops due to various inflammatory and immune reactions (reviewed in Murr et al., 1999; Laviano et al., 2007). And, finally, an increased level of endogenous xenobiotics, such as, for example, kynurenine (Kurz et al., 2011), that are able to activate both AhR (Poormasjedi-Meibod et al., 2016) and other xenosensors. Accordingly, we decided to check the published data in order to find out how much these stress factors are capable of activating the stemness of tumor cells in general as well as the expression of selected “stemness genes” in particular (Figure 2).
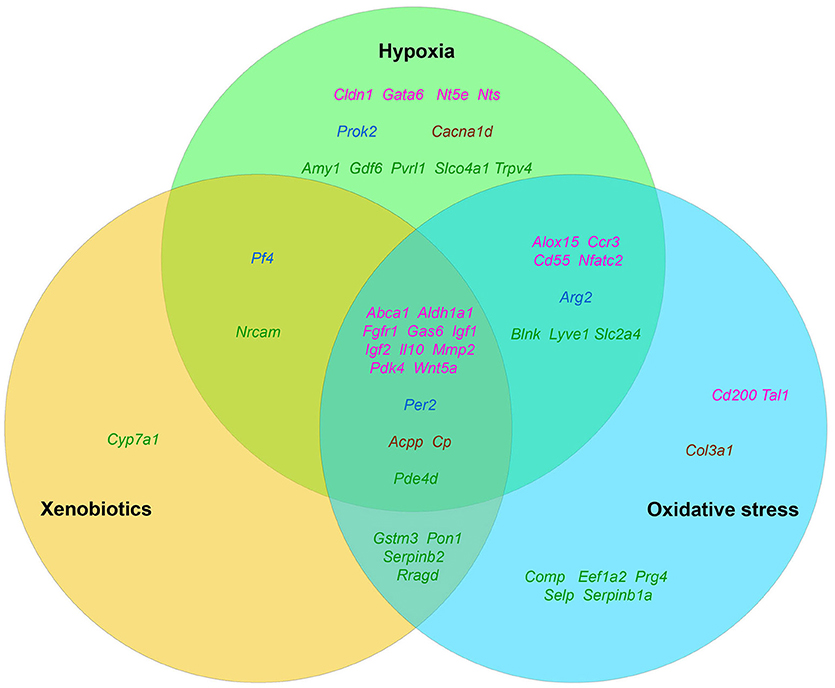
Figure 2. The distribution pattern of genes, overexpressed in TAMRA+ Krebs-2 carcinoma cells relative to TAMRA− cells, with regard to their activation in response to appropriate stimulus. The green area corresponds to hypoxia, blue–to oxidative stress, orange–to xenobiotics. The genes symbols are indicated in different colors in accordance with their proven functional role in the formation of the stem or stem-like phenotype of normal pluri-/multipotent as well as tumor stem cells: pink denoting genes that are known markers of both normal pluri-/multipotent and tumor stem cells; blue–known markers of normal pluri-/multipotent stem cells; brown–known markers of tumor stem cells; green–genes for which their participation in the formation of stemness has not been proven at all.
The fact that hypoxia is a strong stimulus that enhances the aggressive behavior of tumors had been known for a long time (reviewed in Bertout et al., 2008). More recent studies have shown that hypoxia is mandatory for the existence of normal embryonic and other pluri-/multipotent stem cells (Mohyeldin et al., 2010; López-Iglesias et al., 2015; Hammoud et al., 2016), and induces the stem-like phenotype in prostate cancer (Bae et al., 2016), human lung cancer (Iida et al., 2012) and other types of tumors (reviewed in Li and Rich, 2010; Seo et al., 2016). The existing data analysis has revealed that 35 of the 96 genes we have identified as essential for stemness are activated, one way or another, in conditions of local or systemic hypoxia (Table 5, Figure 2).
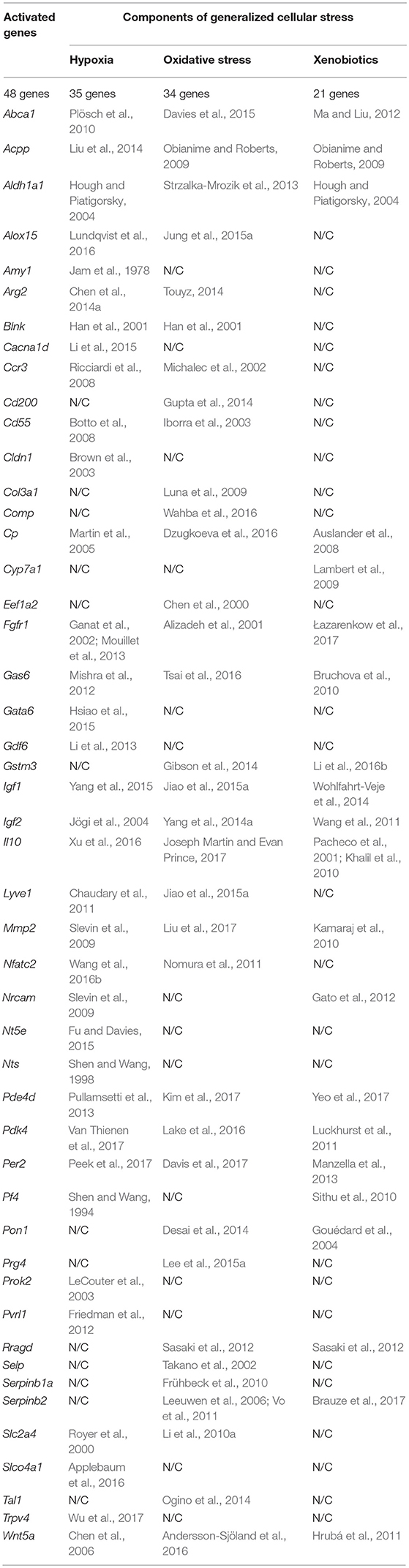
Table 5. The results of the analysis of published data on activating effect of the factors of generalized cellular stress for the tested genes that provide a malignant/pluripotent phenotype of the Krebs-2 CSCs.
Data on the role of oxidative stress in regulation of tumor cells stemness are in general quite contradictory. Numerous studies confirm suppression of the stem-like phenotype of tumor cells under oxidative stress conditions in, for example, in vitro experiments with SUM159 breast cancer cells and pancreatic CSCs of various origin (Cipak et al., 2010; Ma et al., 2017). On the other hand there are numerous quite convincing direct evidences of stemness induction in response to oxidative stress, as, for example, in MCF7 and ZR751 breast cancer cells (Gopal et al., 2016) or in lung cancer cells (Saijo et al., 2016) as well as in a number of other in vitro models (reviewed in Dayem et al., 2010). We found the evidences of oxidative stress activating effect for 34 genes of our list (Table 5, Figure 2).
Finally, we have found data, albeit not numerous, confirming that xenobiotics are also able to induce the stemness of tumor cells. This was shown, for example, for human bronchial epithelial cells (Liu et al., 2016c) and SUM149 inflammatory breast cancer cells (Stanford et al., 2016). As well, xenobiotics turned out to activate the expression of 21 out of 96 genes of stemness of TAMRA+ cells of the Krebs-2 tumor (Table 5, Figure 2).
Thus, the datamining analysis showed that 48 of the 96 genes we designated as potentially important for the formation of the poorly differentiated/stem-like phenotype of tumor cells are activated in response to at least 1 of 3 stress stimuli–hypoxia, oxidative stress, or xenobiotics. Moreover, 14 genes (Aldh1a1, Abca1, Igf1, Igf2, Il10, Gas6, Fgfr1, Wnt5a, Pdk4, Per2, Cp, Pde4d, Mmp2, Acpp) respond with increased expression to all 3 stimuli. It is easy to note that 12 of these 14 genes belong to group A (Figure 1), which contains genes most significant for maintaining stemness/malignancy. Moreover, visual representation of these results in Figure 2 signifies the multiplicity of inducing agents for the majority of stemness-specific genes (pink, blue, and brown denoted ones).
It is known that none of the mentioned stress stimuli exist separately in vivo, instead they are always inextricably linked to each other. So, hypoxia, as well as the presence of xenobiotics, lead to oxidative stress (Netzer et al., 2015; Pizzino et al., 2017). On the other hand, oxidative stress leads to a corruption in metabolism that, in turn, causes the formation of various endogenous xenobiotics such as kynurenine (Ramírez-Ortega et al., 2017; Wigner et al., 2018) or tryptamine-4,5-dione (Jiang et al., 1999; Suga et al., 2017). Therefore, we decided to combine these three stress factors into the single concept of “generalized cellular stress.”
It is quite obvious that the fact that we have not found any data on the impact of generalized cellular stress on the remaining 48 genes does not mean that there really is no such an influence. Our hypothesis on the role of stress in the activation of stemness could be to some extent supported by data on the presence of regulatory elements that provide the binding of transcription factors and transcriptional activation of these genes in response to factors of generalized cellular stress. To conduct such analysis, we used the open web resource “Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool”: http://amp.pharm.mssm.edu/Enrichr/ (Chen et al., 2013; Kuleshov et al., 2016). Databases of this tool contain an excessive compilation of a huge number of results obtained in the ChIP-Seq (Chromatin ImmunoPrecipitation-Sequencing) experiments. It allows to use the tool not only to determine the degree of sampling enrichment by the criterion of the presence of functional binding sites for certain transcription factors, but also in principle to determine the presence of such sites in the subject genes. So we used the “ChEA 2016” section of the tool to test all 96 “stemness genes” for the presence of binding sites for transcription factors established by experiments on ChIP-Seq analysis. One of the main outcomes of this analysis was that the 72 genes from our list contain binding sites for the SOX2 transcription factor, 59–OCT4/POU5F1, 54–NANOG, 45–KLF4, and 52–c-MYC (Table 6). In fact, only 7 genes out of 96 (Lyve1, Il17rb, Fam107a, Nrcam, Vsig4, Pf4, Amy1, Eif2s3y) contained no binding sites for any of the listed factors.
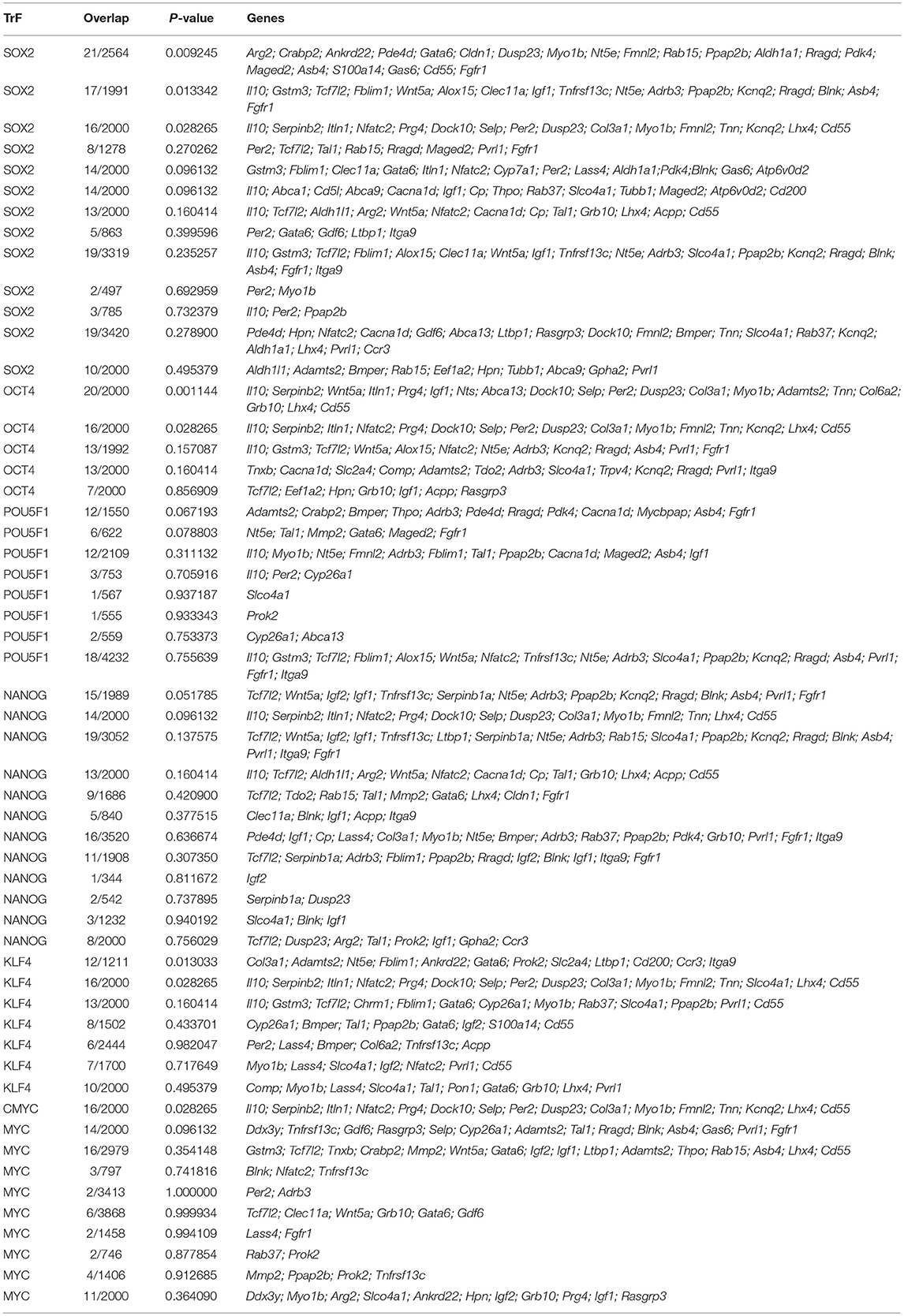
Table 6. Results of “ChEA 2016” analysis for 96 ≪stemness genes≫ showing elevated expression in TAMRA+ Krebs-2 carcinoma cells relative to TAMRA− cells, with regard to enrichment with SOX2/OCT4/POU5F1/NANOG/KLF4/c-MYC binding sites.
SOX2, OCT4/POU5F1, Nanog, KLF4 and c-Myc are known to be the five main transcription factors forming the transcriptional profile of stem cells. Activation of these factors is sufficient for reprogramming a normal somatic cell into a pluripotent/multipotent stem cell, as was first demonstrated on mouse embryonic and adult fibroblast cultures (Takahashi and Yamanaka, 2006; reviewed in Heng et al., 2010). These transcription factors are also shown to be activated under conditions of hypoxia (Li and Rich, 2010; Mathieu et al., 2011; Iida et al., 2012; Bae et al., 2016), oxidative stress (Cullingford et al., 2008; Kang et al., 2009; Kim et al., 2013; Chang et al., 2014; Balvan et al., 2015; Saijo et al., 2016) and in the presence of xenobiotics (Jang et al., 2014; Liu et al., 2016c; Stanford et al., 2016). Thus, the mechanism of formation of the tumor cells stemness can be proposed. This mechanism implies the activation of these key factors under conditions of generalized cellular stress that, in turn, leads to increased expression of specific targets, which probably include also the genes providing the stem-like phenotype of Krebs-2 cells.
In addition, we have decided to check for the possibility of an alternative mechanism of the “stem-genes” activation under conditions of generalized cellular stress independent of SOX2/OCT4/Nanog/KLF4/c-Myc pathway.
The main transcription factors that provide a cellular response to hypoxia are the proteins of the HIF family (hypoxia-inducible factor) (reviewed in Peet et al., 2017). However, the factors such as NFkB, CREB, AP-1, Egr-1, NF-IL6/C/EBPβ, RTEF-1, GATA2, STAT5, ETS1 (reviewed in Cummins and Taylor, 2005) as well as RUNX1 (Lee et al., 2017a) also take a direct part in the regulation of transcription under hypoxia/anoxia. ChIP-Seq enrichment analysis has revealed that 92 of 96 genes contain binding sites for at least one of these transcription factors with the following distribution: CREB1–25 genes, RELA (NFκB)−9 genes, cJUN (AP-1)−31 genes, MAF (AP-1)−15 genes, EGR1–42 genes, C/EBPβ-59 genes, ETS1–11 genes, STAT5–4 genes, GATA2–33 genes, RTEF-1/TEAD4–15 genes, RUNX1/AML1–45 genes (data not shown).
In addition, 88 genes contain a binding site(s) for such xenosensors or their intermediators, as PPARα/δ/γ (58 genes), NFE2L2/NRF2 (14 genes), AHR (6 genes), NR1I2/PXR (9 genes), FOXO1/3 (17 genes) (Klotz and Steinbrenner, 2017), MITF (25 genes) (Huang et al., 2013), EGR1 (42 genes) (Thiel and Cibelli, 2002; Sullivan et al., 2012), as well as for androgen receptor (AR) (55 genes). The last one had been shown to be activated not only by steroid hormones, but also by various xenobiotics, including endogenous ones as well (Araki et al., 2005) (data not shown).
Compared with other components of generalized cellular stress, oxidative stress activates the widest range of transcription factors, among them NFE2L2/NRF2, NFκB, cJUN, MAF, FOXO1/3, STAT1/3, ELK1, MEF2A (Zhang et al., 2016b; Klotz and Steinbrenner, 2017; Nemmiche, 2017; Sies et al., 2017), FLI1 and HOXB4 (Monzen et al., 2011), C/EBPα (Xu et al., 2009; Puri et al., 2012), C/EBPδ (Hour et al., 2010; Banerjee et al., 2016), MYB (Wan et al., 2005), GATA3 (Li et al., 2017b), and IRF8 (Li et al., 2017c; Sakai et al., 2017). It turned out that all 96 genes of our list contain site(s) for at least one of these transcription factors. The most represented factor was FLI1 (56 genes), followed by GATA3 (53 genes) and STAT3 (52 genes). Another 9 factors composed the group of average representation: cJUN−31 genes, IRF8–22 genes, C/EBPα-22 genes, C/EBPδ-21 genes, MYB−19 genes, MAF−15 genes, NFE2L2/NRF2–14 genes, FOXO1 and STAT1–12 genes for each. The remaining 5 factors were low-represented: NFκB/RELA−9 genes, ELK1–8 genes, FOXO3–7 genes, and, finally, HOXB4 and MEF2A−5 genes for each (data not shown).
We draw two principal conclusions from the results of “ChEA 2016” analysis. The first conclusion is that, in fact, all the genes, potentially implicated in maintaining stem-like phenotype of Krebs-2 TAMRA+ cells, can be activated under generalized cellular stress conditions. And the second one is that such an activation can be mediated both by induction of stemness by SOX2, OCT4/POU5F1, Nanog, KLF4, and c-Myc factors, and by direct action of specific mediators of cellular response to hypoxia-xenobiotics-oxidative stress. Yet the presence of binding sites for certain transcription factors does not necessarily ensure the transcriptional activation that depends significantly on general epigenetic/physiological context. It presumes the necessity of complex approach. As we already mentioned above, there are experimental evidences that a number of genes from our list are activated under stress conditions. The analysis of binding sites, respectively, suggests the possible mechanisms of such activation and allows us to extrapolate these mechanisms to other “stem genes.”
The issue of the mechanisms of CSCs origination as well as of means they use to self-maintain and increase their population in developing malignant neoplasms is one of the most important for modern oncology, as it is key for the development of methods of antitumor therapy.
The classical model for the formation of CSCs subpopulation is based on the ability of pluripotent cells to divide symmetrically, as the main way of self-renewal of the population (Franco et al., 2016; Rich, 2016). Moreover, CSCs possess the additional ability to retain their “pluripotent” properties outside of the “stem niche” conditions as well as the ability for amoeboid migration characteristic for most of poorly differentiated cells (Sakamoto et al., 2011). It ensures a uniform distribution of the initiating cells newly formed after symmetrical division throughout the tumor volume and, respectively, provides conditions for the continuous exponential growth of the tumor mass.
The model of stemness induction under conditions of generalized cellular stress we have proposed, complements the classical model and allows to resolve certain discrepancies in the available experimental data with the model “symmetrical division-migration.” At this, it should be emphasized that our concept of generalized cellular stress is not limited to the factors listed above and can be extended with such components as inflammation, ionizing radiation, heat shock, etc. Moreover, this model can also possibly explain the carcinogenic effect of chronic oxidative stress, inflammation and the action of carcinogenic xenobiotics through de novo induction of “pluripotency” followed by transformation into malignancy.
Simultaneous existence of two independent and complementary mechanisms for the formation and maintenance of CSCs subpopulation implies that there may be a third and a fourth variant(s). To complete the picture of possible mechanisms of CSCs origination, other hypotheses also need to be mentioned.
One of the hypotheses explains the phenomenon of CSCs de novo emergence due to genetic instability that is inherent characteristic of tumor cells. Formation of cells with a stem-like phenotype evenly dispersed throughout the volume of the tumor mass is believed to be the one of possible consequences of this instability (Lagasse, 2008). However, this explanation has a significant drawback, as it is barely consistent with the fact that tumors retain their histological and biochemical properties, and, accordingly, the overall transcriptional profile during development, as well as upon metastasis and transplantation into model animals (Franzén et al., 1997; Süsskind et al., 2017). This fact testifies to the persistence of a certain “genetic individuality” of cells that drive tumor growth, which to significant extent contradicts the stochastic model of the formation of a tumorigenic population due to genetic instability.
Another possible mechanism for the formation of a pluripotent phenotype in tumor cells could be the phenomenon of “genometastasis” (García-Casas et al., 2017). It is supposed that extracellular double-stranded DNA released from cells that have undergone apoptosis or necrosis, both primary and secondary, can be internalized by cancer cells that have passed the first stages of commitment/differentiation, but still retained such a basic feature of CSC as the ability to capture fragments of extracellular double-stranded DNA. The occurrence of DNA with certain genetic or structural features in internal compartments of such cells can lead to a restoration of the pluripotent potential of the committed cells and their reversible conversion into new CSCs. The proposed “reversive mechanism” does not contradict the proposed concept of the stemness induction under the generalized cellular stress, but, somehow, complements it, since the action of factors of generalized cellular stress is always accompanied by intensive death of cancer cells, which results in an excessive amount of extracellular double-stranded DNA (Wen et al., 2017). This hypothesis addresses the mechanism for retransformation of the early committed progeny of existing CSCs. The main disadvantage of this model, as well as of the previous one, based on genetic instability, is indeterminacy and randomness of the results of events occurring during the “genometastasis” (multiple mutations, random genetic composition of the internalized DNA etc).
Another intriguing model of CSC formation is the “Blebbishield emergency program.” It was found that cancer cells undergoing apoptosis can avoid cell death by evoking this program. During this process, one of the apoptotic bodies becomes a center of aggregation for other ones that results in the formation of so-called “Blebbishield” that, in turn, further transforms into a new CSC. Such a newly formed CSC demonstrates a more aggressive tumorigenic behavior and can even fuse with immunity cells. As a result of all these transformations, the new secondary tumor with significantly more aggressive characteristics arises (Jinesh and Kamat, 2016, 2017).
In general, all the hypotheses considered, starting with genetic instability and ending with the fusion of apoptotic bodies, describe the formation of pluripotent/stem phenotype of tumor cells as a probabilistic event with unpredictable results, somehow or other related to changes in their genetic material. The fluctuations in the percentage of CSCs we have observed in experimental tumors (Potter et al., 2016a) suggests that the main cause of “dynamic stemness” is not genomic but epigenetic changes.
The model we proposed for stemness induction in response to the components of generalized cellular stress, namely hypoxia, oxidative stress and the action of xenobiotics, apparently describes some basic mechanisms of the cellular response to stress. It can be presumed that CSCs serve as a kind of “Emergency service” for tumors, emerging de novo and ensuring their survival under unfavorable conditions. With all this, a number of questions remain, and the main one is why the proportion of CSCs relative to the entire mass of the tumor remains rather low despite the stress conditions? Moreover, it is not clear how long CSCs can sustain a stem-like phenotype, and whether stemness maintenance depends on external conditions or gradually fades regardless of the presence/absence of inducing agents?
Assuming all of the above, we have to admit that the significant majority of existing anti-tumor pharmaceutical and radiotherapy schemes lead to the formation of generalized cellular stress conditions, and, therefore, are likely to induce de novo formation of CSCs in the total mass of nonstem tumor cells (Chang, 2016). Perhaps this explains the fact that despite a certain progress, the overall effectiveness of cancer treatment remains extremely unsatisfactory, and cancer remains one of the leading causes of mortality in the world.
YE performed the analysis, interpreted the data, and drafted the manuscript. AP interpreted the data and drafted the manuscript. EP and ED interpreted the data. OE performed the analysis. OT, AO, and EC participated in the study design. NK coordinated all work. SB conceived the study, participated in its design, coordinated and drafted the manuscript. All authors read and approved the final manuscript.
This study was supported by the State scientific project N 0324-2018-0019 and by the Integration project of the Siberian Branch of the Russian Academy of Sciences Reconstruction, computer analysis and modeling of the structural and functional organization of biomedical-significant gene networks (project N 0324-2018-0021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are gratitude to Dr. Dmitriy Yu. Oshchepkov for critical comments.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00544/full#supplementary-material
CSC, cancer stem cell; TAMRA, carboxytetramethylrhodamine, fluorescent dye.
Akyol, S., Cömertoglu, I., Firat, R., Çakmak, Ö., Yukselten, Y., Erden, G., et al. (2015). Effect of insulin on the mRNA expression of procollagen N-proteinases in chondrosarcoma OUMS-27 cells. Oncol. Lett. 10, 1091–1096. doi: 10.3892/ol.2015.3317
Alcaín, F. J., and Löw, H. (1997). Ceruloplasmin releases pH-induced inhibition of cell proliferation stimulated by growth factors. Redox Rep. 3, 287–293. doi: 10.1080/13510002.1997.11747125
Aldonza, M. B. D., Son, Y. S., Sung, H.-J., Ahn, J. M., Choi, Y.-J., Kim, Y.-I., et al. (2017). Paraoxonase-1 (PON1) induces metastatic potential and apoptosis escape via its antioxidative function in lung cancer cells. Oncotarget 8, 42817–42835. doi: 10.18632/oncotarget.17069
Alinejad, V., Hossein Somi, M., Baradaran, B., Akbarzadeh, P., Atyabi, F., Kazerooni, H., et al. (2016). Co-delivery of IL17RB siRNA and doxorubicin by chitosan-based nanoparticles for enhanced anticancer efficacy in breast cancer cells. Biomed. Pharmacother. 83, 229–240. doi: 10.1016/j.biopha.2016.06.037
Alinezhad, S., Väänänen, R.-M., Mattsson, J., Li, Y., Tallgrén, T., Tong Ochoa, N., et al. (2016). Validation of novel biomarkers for prostate cancer progression by the combination of bioinformatics, clinical and functional studies. PLoS ONE 11:e0155901. doi: 10.1371/journal.pone.0155901
Alizadeh, M., Wada, M., Gelfman, C. M., Handa, J. T., and Hjelmeland, L. M. (2001). Downregulation of differentiation specific gene expression by oxidative stress in ARPE-19 cells. Invest. Ophthalmol. Vis. Sci. 42, 2706–2713.
An, H., Kim, J. Y., Oh, E., Lee, N., Cho, Y., and Seo, J. H. (2015). Salinomycin promotes anoikis and decreases the CD44+/CD24-stem-like population via inhibition of STAT3 activation in MDA-MB-231 cells. PLoS ONE 10:e0141919. doi: 10.1371/journal.pone.0141919
Andersson-Sjöland, A., Karlsson, J. C., and Rydell-Törmänen, K. (2016). ROS-induced endothelial stress contributes to pulmonary fibrosis through pericytes and Wnt signaling. Lab. Investig. 96, 206–217. doi: 10.1038/labinvest.2015.100
Applebaum, M. A., Jha, A. R., Kao, C., Hernandez, K. M., DeWane, G., Salwen, H. R., et al. (2016). Integrative genomics reveals hypoxia inducible genes that are associated with a poor prognosis in neuroblastoma patients. Oncotarget 7, 76816–76826. doi: 10.18632/oncotarget.12713
Araki, N., Ohno, K., Takeyoshi, M., and Iida, M. (2005). Evaluation of a rapid in vitro androgen receptor transcriptional activation assay using AR-EcoScreenTM cells. Toxicol. Vitr. 19, 335–352. doi: 10.1016/j.tiv.2004.10.008
Araújo, T. M., Seabra, A. D., Lima, E. M., Assumpção, P. P., Montenegro, R. C., Demachki, S., et al. (2016). Recurrent amplification of RTEL1 and ABCA13 and its synergistic effect associated with clinicopathological data of gastric adenocarcinoma. Mol. Cytogenet. 9:52. doi: 10.1186/s13039-016-0260-x
Ardianto, B., Sugimoto, T., Kawano, S., Kasagi, S., Jauharoh, S. N., Kurimoto, C., et al. (2010). The HPB-AML-I cell line possesses the properties of mesenchymal stem cells. J. Exp. Clin. Cancer Res. 29:163. doi: 10.1186/1756-9966-29-163
Asano, Y., Kishida, S., Mu, P., Sakamoto, K., Murohara, T., and Kadomatsu, K. (2010). DRR1 is expressed in the developing nervous system and downregulated during neuroblastoma carcinogenesis. Biochem. Biophys. Res. Commun. 394, 829–835. doi: 10.1016/j.bbrc.2010.03.085
Assou, S., Le Carrour, T., Tondeur, S., Ström, S., Gabelle, A., Marty, S., et al. (2007). A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells 25, 961–973. doi: 10.1634/stemcells.2006-0352
Au, V., Tsang, F. H., Man, K., Fan, S. T., Poon, R. T., and Lee, N. P. (2014). Expression of ankyrin repeat and SOCS box containing 4 (ASB4) confers migration and invasion properties of hepatocellular carcinoma cells. Biosci. Trends 8, 101–110. doi: 10.5582/bst.8.101
Auslander, M., Yudkovski, Y., Chalifa-Caspi, V., Herut, B., Ophir, R., Reinhardt, R., et al. (2008). Pollution-affected fish hepatic transcriptome and its expression patterns on exposure to cadmium. Mar. Biotechnol. 10, 250–261. doi: 10.1007/s10126-007-9060-y
Bae, K.-M., Dai, Y., Vieweg, J., and Siemann, D. W. (2016). Hypoxia regulates SOX2 expression to promote prostate cancer cell invasion and sphere formation. Am. J. Cancer Res. 6, 1078–1088.
Baharvand, H., Ashtiani, S. K., Taee, A., Massumi, M., Valojerdi, M. R., Yazdi, P. E., et al. (2006). Generation of new human embryonic stem cell lines with diploid and triploid karyotypes. Dev. Growth Differ. 48, 117–128. doi: 10.1111/j.1440-169X.2006.00851.x
Balvan, J., Gumulec, J., Raudenska, M., Krizova, A., Stepka, P., Babula, P., et al. (2015). Oxidative stress resistance in metastatic prostate cancer: renewal by self-eating. PLoS ONE 10:e0145016. doi: 10.1371/journal.pone.0145016
Ban, M. J., Ji, S. H., Lee, C.-K., Bae, S. B., Kim, H. J., Ahn, T. S., et al. (2017). Solute carrier organic anion transporter family member 4A1 (SLCO4A1) as a prognosis marker of colorectal cancer. J. Cancer Res. Clin. Oncol. 143, 1437–1447. doi: 10.1007/s00432-017-2393-7
Banerjee, S., Aykin-Burns, N., Krager, K. J., Shah, S. K., Melnyk, S. B., Hauer-Jensen, M., et al. (2016). Loss of C/EBPδ enhances IR-induced cell death by promoting oxidative stress and mitochondrial dysfunction. Free Radic. Biol. Med. 99, 296–307. doi: 10.1016/j.freeradbiomed.2016.08.022
Barbet, R., Peiffer, I., Hutchins, J. R., Hatzfeld, A., Garrido, E., and Hatzfeld, J. A. (2012). Expression of the 49 human ATP binding cassette (ABC) genes in pluripotent embryonic stem cells and in early- and late-stage multipotent mesenchymal stem cells: possible role of ABC plasma membrane transporters in maintaining human stem cell pluripotency. Cell Cycle 11, 1611–1620. doi: 10.4161/cc.20023
Baylin, S. B., Gann, D. S., and Hsu, S. H. (1976). Clonal origin of inherited medullary thyroid carcinoma and pheochromocytoma. Science 193, 321–323. doi: 10.1126/science.935869
Belaguli, N. S., Aftab, M., Rigi, M., Zhang, M., Albo, D., and Berger, D. H. (2010). GATA6 promotes colon cancer cell invasion by regulating urokinase plasminogen activator gene expression. Neoplasia 12, 856–865. doi: 10.1593/neo.10224
Bendall, S. C., Stewart, M. H., Menendez, P., George, D., Vijayaragavan, K., Werbowetski-Ogilvie, T., et al. (2007). IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature 448, 1015–1021. doi: 10.1038/nature06027
Bergman, D., Halje, M., Nordin, M., and Engström, W. (2013). Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology 59, 240–249. doi: 10.1159/000343995
Bernard, M., Delabesse, E., Novault, S., Hermine, O., and Macintyre, E. A. (1998). Antiapoptotic effect of ectopic TAL1/SCL expression in a human leukemic T-cell line. Cancer Res. 58, 2680–2687.
Bertout, J. A., Patel, S. A., and Simon, M. C. (2008). The impact of O2 availability on human cancer. Nat. Rev. Cancer 8, 967–975. doi: 10.1038/nrc2540
Bie, Q., Sun, C., Gong, A., Li, C., Su, Z., Zheng, D., et al. (2016). Non-tumor tissue derived interleukin-17B activates IL-17RB/AKT/β-catenin pathway to enhance the stemness of gastric cancer. Sci. Rep. 6:25447. doi: 10.1038/srep25447
Bie, Q., Zhang, B., Sun, C., Ji, X., Barnie, P. A., Qi, C., et al. (2017). IL-17B activated mesenchymal stem cells enhance proliferation and migration of gastric cancer cells. Oncotarget 8, 18914–18923. doi: 10.18632/oncotarget.14835
Black, S. M., Beggs, J. D., Hayes, J. D., Bartoszek, A., Muramatsu, M., Sakai, M., et al. (1990). Expression of human glutathione S-transferases in Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. Biochem. J. 268, 309–315. doi: 10.1042/bj2680309
Blum, B., and Benvenisty, N. (2008). The tumorigenicity of human embryonic stem cells. Adv. Cancer Res. 100, 133–158. doi: 10.1016/S0065-230X(08)00005-5
Blum, B., and Benvenisty, N. (2009). The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle 8, 3822–3830. doi: 10.4161/cc.8.23.10067
Bojesen, K. B., Clausen, O., Rohde, K., Christensen, C., Zhang, L., Li, S., et al. (2012). Nectin-1 binds and signals through the fibroblast growth factor receptor. J. Biol. Chem. 287, 37420–37433. doi: 10.1074/jbc.M112.345215
Bordeleau, F., Alcoser, T. A., and Reinhart-King, C. A. (2014). Physical biology in cancer. 5. The rocky road of metastasis: the role of cytoskeletal mechanics in cell migratory response to 3D matrix topography. AJP Cell Physiol. 306, C110–C120. doi: 10.1152/ajpcell.00283.2013
Botto, L., Beretta, E., Bulbarelli, A., Rivolta, I., Lettiero, B., Leone, B. E., et al. (2008). Hypoxia-induced modifications in plasma membranes and lipid microdomains in A549 cells and primary human alveolar cells. J. Cell. Biochem. 105, 503–513. doi: 10.1002/jcb.21850
Boucher, H., Vanneaux, V., Domet, T., Parouchev, A., and Larghero, J. (2016). Circadian clock genes modulate human bone marrow mesenchymal stem cell differentiation, migration and cell cycle. PLoS ONE 11:e0146674. doi: 10.1371/journal.pone.0146674
Brandi, J., Dalla Pozza, E., Dando, I., Biondani, G., Robotti, E., Jenkins, R., et al. (2016). Secretome protein signature of human pancreatic cancer stem-like cells. J. Proteomics 136, 1–12. doi: 10.1016/j.jprot.2016.01.017
Brauze, D., Zawierucha, P., Kiwerska, K., Bednarek, K., Oleszak, M., Rydzanicz, M., et al. (2017). Induction of expression of aryl hydrocarbon receptor-dependent genes in human HepaRG cell line modified by shRNA and treated with β-naphthoflavone. Mol. Cell. Biochem. 425, 59–75. doi: 10.1007/s11010-016-2862-3
Brenner, S., Klameth, L., Riha, J., Schölm, M., Hamilton, G., Bajna, E., et al. (2015). Specific expression of OATPs in primary small cell lung cancer (SCLC) cells as novel biomarkers for diagnosis and therapy. Cancer Lett. 356, 517–524. doi: 10.1016/j.canlet.2014.09.025
Brown, R. C., Mark, K. S., Egleton, R. D., Huber, J. D., Burroughs, A. R., and Davis, T. P. (2003). Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NFkappaB. J. Cell Sci. 116, 693–700. doi: 10.1242/jcs.00264
Bruchova, H., Vasikova, A., Merkerova, M., Milcova, A., Topinka, J., Balascak, I., et al. (2010). Effect of maternal tobacco smoke exposure on the placental transcriptome. Placenta 31, 186–191. doi: 10.1016/j.placenta.2009.12.016
Bu, Y., Jia, Q. A., Ren, Z. G., Zhang, J. B., Jiang, X. M., Liang, L., et al. (2014). Maintenance of stemness in oxaliplatin-resistant hepatocellular carcinoma is associated with increased autocrine of IGF1. PLoS ONE 9:e89686. doi: 10.1371/journal.pone.0089686
Buechler, C., Bared, S. M., Aslanidis, C., Ritter, M., Drobnik, W., and Schmitz, G. (2002). Molecular and functional interaction of the ATP-binding cassette transporter A1 with Fas-associated death domain protein. J. Biol. Chem. 277, 41307–41310. doi: 10.1074/jbc.C200436200
Cai, Y. N., Dai, X. H., Zhang, Q. H., Hu, R., and Dai, Z. M. (2015). Gene expression profiling of somatic and pluripotent cells reveals novel pathways involved in reprogramming. Genet. Mol. Res. 14, 12085–12092. doi: 10.4238/2015.October.5.21
Calaminus, S. D. J., Guitart, A., Sinclair, A., Schachtner, H., Watson, S. P., Holyoake, T. L., et al. (2012). Lineage tracing of Pf4-Cre Marks hematopoietic stem cells and their progeny. PLoS ONE 7:e51361. doi: 10.1371/journal.pone.0051361
Campos-Sánchez, E., and Cobaleda, C. (2015). Tumoral reprogramming: plasticity takes a walk on the wild side. Biochim. Biophys. Acta 1849, 436–447. doi: 10.1016/j.bbagrm.2014.07.003
Carrel, A., and Ebeling, A. H. (1928). The fundamental properties of the fibroblast and the macrophage: III. The malignant fibroblast of sarcoma 10 of the Crocker foundation. J. Exp. Med. 48, 105–123. doi: 10.1084/jem.48.1.105
Cha, N., Liu, W., Yang, N., Xie, S., Gao, Y., Chen, X., et al. (2014). Oncogenicity of LHX4 in colorectal cancer through Wnt/β-catenin/TCF4 cascade. Tumor Biol. 35, 10319–10324. doi: 10.1007/s13277-014-2210-8
Chan, E. F., Gat, U., McNiff, J. M., and Fuchs, E. (1999). A common human skin tumour is caused by activating mutations in β- catenin. Nat. Genet. 21, 410–413. doi: 10.1038/7747
Chang, J. C. (2016). Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine 95, S20–S25. doi: 10.1097/MD.0000000000004766
Chang, Q., Chen, B., Thakur, C., Lu, Y., and Chen, F. (2014). Arsenic-induced sub-lethal stress reprograms human bronchial epithelial cells to CD61− cancer stem cells. Oncotarget 5, 1290–1303. doi: 10.18632/oncotarget.1789
Chaudary, N., Milosevic, M., and Hill, R. P. (2011). Suppression of vascular endothelial growth factor receptor 3 (VEGFR3) and vascular endothelial growth factor C (VEGFC) inhibits hypoxia-induced lymph node metastases in cervix cancer. Gynecol. Oncol. 123, 393–400. doi: 10.1016/j.ygyno.2011.07.006
Chekhun, V. F., Lozovska, Y. V., Burlaka, A. P., Lukyanova, N. Y., Todor, I. N., and Naleskina, L. A. (2014). Peculiarities of antioxidant system and iron metabolism in organism during development of tumor resistance to cisplatin. Exp. Oncol. 36, 196–201.
Chen, B., Xue, J., Meng, X., Slutzky, J. L., Calvert, A. E., and Chicoine, L. G. (2014a). Resveratrol prevents hypoxia-induced arginase II expression and proliferation of human pulmonary artery smooth muscle cells via Akt-dependent signaling. Am. J. Physiol. Cell. Mol. Physiol. 307, L317–L325. doi: 10.1152/ajplung.00285.2013
Chen, C., Cao, F., Bai, L., Liu, Y., Xie, J., Wang, W., et al. (2015a). IKKβ enforces a LIN28B/TCF7L2 positive feedback loop that promotes cancer cell stemness and metastasis. Cancer Res. 75, 1725–1735. doi: 10.1158/0008-5472.CAN-14-2111
Chen, E., Proestou, G., Bourbeau, D., and Wang, E. (2000). Rapid up-regulation of peptide elongation factor EF-1α protein levels is an immediate early event during oxidative stress-induced apoptosis. Exp. Cell Res. 259, 140–148. doi: 10.1006/excr.2000.4952
Chen, E. Y., Tan, C. M., Kou, Y., Duan, Q., Wang, Z., Meirelles, G., et al. (2013). Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14:128. doi: 10.1186/1471-2105-14-128
Chen, H.-F., Yu, C.-Y., Chen, M.-J., Chou, S.-H., Chiang, M.-S., Chou, W.-H., et al. (2015b). Characteristic expression of major histocompatibility complex and immune privilege genes in human pluripotent stem cells and their derivatives. Cell Transplant. 24, 845–864. doi: 10.3727/096368913X674639
Chen, J., Hersmus, N., Van Duppen, V., Caesens, P., Denef, C., and Vankelecom, H. (2005). The adult pituitary contains a cell population displaying stem/progenitor cell and early-embryonic characteristics. Endocrinology 146, 3985–3998. doi: 10.1210/en.2005-0185
Chen, J., Li, X., Ma, D., Liu, T., Tian, P., and Wu, C. (2017). Ceramide synthase-4 orchestrates the cell proliferation and tumor growth of liver cancer in vitro and in vivo through the nuclear factor-κB signaling pathway. Oncol. Lett. 14, 1477–1483. doi: 10.3892/ol.2017.6365
Chen, J.-J., Gao, Y., Tian, Q., Liang, Y.-M., and Yang, L. (2014b). Platelet factor 4 protects bone marrow mesenchymal stem cells from acute radiation injury. Br. J. Radiol. 87:20140184. doi: 10.1259/bjr.20140184
Chen, K. G., Valencia, J. C., Gillet, J.-P., Hearing, V. J., and Gottesman, M. M. (2009). Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res. 22, 740–749. doi: 10.1111/j.1755-148X.2009.00630.x
Chen, L., Fink, T., Ebbesen, P., and Zachar, V. (2006). Temporal transcriptome of mouse ATDC5 chondroprogenitors differentiating under hypoxic conditions. Exp. Cell Res. 312, 1727–1744. doi: 10.1016/j.yexcr.2006.02.013
Chen, R., Zeng, X., Zhang, R., Huang, J., Kuang, X., Yang, J., et al. (2014c). Cav1.3 channel α1Dprotein is overexpressed and modulates androgen receptor transactivation in prostate cancers. Urol. Oncol. Semin. Orig. Investig. 32, 524–536. doi: 10.1016/j.urolonc.2013.05.011
Chen, S. X., Yin, J. F., Lin, B. C., Su, H. F., Zheng, Z., Xie, C. Y, et al. (2016a). Upregulated expression of long noncoding RNA SNHG15 promotes cell proliferation and invasion through regulates MMP2/MMP9 in patients with GC. Tumor Biol. 37, 6801–6812. doi: 10.1007/s13277-015-4404-0
Chen, Y., Peng, C., Abraham, S. A., Shan, Y., Guo, Z., Desouza, N., et al. (2014d). Arachidonate 15-lipoxygenase is required for chronic myeloid leukemia stem cell survival. J. Clin. Invest. 124, 3847–3862. doi: 10.1172/JCI66129
Chen, Z., Shi, T., Zhang, L., Zhu, P., Deng, M., Huang, C., et al. (2016b). Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: a review of the past decade. Cancer Lett. 370, 153–164. doi: 10.1016/j.canlet.2015.10.010
Cheng, I. H., Lin, Y.-C., Hwang, E., Huang, H.-T., Chang, W.-H., Liu, Y.-L., et al. (2011). Collagen VI protects against neuronal apoptosis elicited by ultraviolet irradiation via an Akt/Phosphatidylinositol 3-kinase signaling pathway. Neuroscience 183, 178–188. doi: 10.1016/j.neuroscience.2011.03.057
Cheon, D.-J., Tong, Y., Sim, M.-S., Dering, J., Berel, D., Cui, X., et al. (2014). A collagen-remodeling gene signature regulated by TGF-β signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin. Cancer Res. 20, 711–723. doi: 10.1158/1078-0432.CCR-13-1256
Chiovaro, F., Martina, E., Bottos, A., Scherberich, A., Hynes, N. E., and Chiquet-Ehrismann, R. (2015). Transcriptional regulation of tenascin-W by TGF-beta signaling in the bone metastatic niche of breast cancer cells. Int. J. Cancer 137, 1842–1854. doi: 10.1002/ijc.29565
Chong, H. C., Tan, C. K., Huang, R. L., and Tan, N. S. (2012). Matricellular proteins: a sticky affair with cancers. J. Oncol. 2012, 1–17. doi: 10.1155/2012/351089
Chou, F.-S., Griesinger, A., Wunderlich, M., Lin, S., Link, K. A., Shrestha, M., et al. (2012). The thrombopoietin/MPL/Bcl-xL pathway is essential for survival and self-renewal in human preleukemia induced by AML1-ETO. Blood 120, 709–719. doi: 10.1182/blood-2012-01-403212
Cipak, A., Mrakovcic, L., Ciz, M., Lojek, A., Mihaylova, B., Goshev, I., et al. (2010). Growth suppression of human breast carcinoma stem cells by lipid peroxidation product 4-hydroxy-2-nonenal and hydroxyl radical-modified collagen. Acta Biochim. Pol. 57, 165–71.
Cole, C., Lau, S., Backen, A., Clamp, A., Rushton, G., Dive, C., et al. (2010). Inhibition of FGFR2 and FGFR1 increases cisplatin sensitivity in ovarian cancer. Cancer Biol. Ther. 10, 495–504. doi: 10.4161/cbt.10.5.12585
Conacci-Sorrell, M., Kaplan, A., Raveh, S., Gavert, N., Sakurai, T., and Ben-Ze'ev, A. (2005). The shed ectodomain of Nr-CAM stimulates cell proliferation and motility, and confers cell transformation. Cancer Res. 65, 11605–11612. doi: 10.1158/0008-5472.CAN-05-2647
Corradetti, B., Meucci, A., Bizzaro, D., Cremonesi, F., and Lange Consiglio, A. (2013). Mesenchymal stem cells from amnion and amniotic fluid in the bovine. Reproduction 145, 391–400. doi: 10.1530/REP-12-0437
Correia, N. C., Fragoso, R., Carvalho, T., Enguita, F. J., and Barata, J. T. (2016). MiR-146b negatively regulates migration and delays progression of T-cell acute lymphoblastic leukemia. Sci. Rep. 6:31894. doi: 10.1038/srep31894
Costa, H., Xu, X., Overbeek, G., Vasaikar, S., Patro, C. P., Kostopoulou, O. N., et al. (2016). Human cytomegalovirus may promote tumour progression by upregulating arginase-2. Oncotarget 7, 47221–47231. doi: 10.18632/oncotarget.9722
Coutu, D. L., François, M., and Galipeau, J. (2011). Inhibition of cellular senescence by developmentally regulated FGF receptors in mesenchymal stem cells. Blood 117, 6801–6812. doi: 10.1182/blood-2010-12-321539
Creighton, C. (1882). Three cases of tumour arising from skin-glands in the dog, showing the connection between disorder of the glandular structure and function, and cancerous invasion of the connective tissue. Med. Chir. Trans. 65, 53–70.3. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/20896600
Cullingford, T. E., Butler, M. J., Marshall, A. K., Tham, E. L., Sugden, P. H., and Clerk, A. (2008). Differential regulation of Krüppel-like factor family transcription factor expression in neonatal rat cardiac myocytes: effects of endothelin-1, oxidative stress and cytokines. Biochim. Biophys. Acta-Mol. Cell Res. 1783, 1229–1236. doi: 10.1016/j.bbamcr.2008.03.007
Cummins, E. P., and Taylor, C. T. (2005). Hypoxia-responsive transcription factors. Pflügers Arch.-Eur. J. Physiol. 450, 363–371. doi: 10.1007/s00424-005-1413-7
Dalton, W. S. (1999). The tumor microenvironment as a determinant of drug response and resistance. Drug Resist. Updat. 2, 285–288. doi: 10.1054/drup.1999.0097
D'Amato, N. C., Rogers, T. J., Gordon, M. A., Greene, L. I., Cochrane, D. R., Spoelstra, N. S., et al. (2015). A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 75, 4651–4664. doi: 10.1158/0008-5472.CAN-15-2011
Damiano, J. S. (2002). Integrins as novel drug targets for overcoming innate drug resistance. Curr. Cancer Drug Targets 2, 37–43. doi: 10.2174/1568009023334033
Davies, N. A., Watkeys, L., Butcher, L., Potter, S., Hughes, M. G., Moir, H., et al. (2015). The contributions of oxidative stress, oxidised lipoproteins and AMPK towards exercise-associated PPARγ signalling within human monocytic cells. Free Radic. Res. 49, 45–56. doi: 10.3109/10715762.2014.978311
Davis, B. T., Voigt, R. M., Shaikh, M., Forsyth, C. B., and Keshavarzian, A. (2017). CREB protein mediates alcohol-induced circadian disruption and intestinal permeability. Alcohol. Clin. Exp. Res. 41, 2007–2014. doi: 10.1111/acer.13513
Dayem, A. A., Choi, H.-Y., Kim, J.-H., and Cho, S.-G. (2010). Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers 2, 859–884. doi: 10.3390/cancers2020859
de Almeida, P., Meyer, E. H., Kooreman, N. G., Diecke, S., Dey, D., Sanchez-Freire, V., et al. (2014). Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nat. Commun. 5:3903. doi: 10.1038/ncomms4903
Deliri, H., Meller, N., Kadakkal, A., Malhotra, R., Brewster, J., Doran, A. C., et al. (2011). Increased 12/15-lipoxygenase enhances cell growth, fibronectin deposition, and neointimal formation in response to carotid injury. Arterioscler. Thromb. Vasc. Biol. 31, 110–116. doi: 10.1161/ATVBAHA.110.212068
Delyon, J., Servy, A., Laugier, F., André, J., Ortonne, N., Battistella, M., et al. (2017). PDE4D promotes FAK-mediated cell invasion in BRAF-mutated melanoma. Oncogene 36, 3252–3262. doi: 10.1038/onc.2016.469
Desai, S., Baker, S. S., Liu, W., Moya, D. A., Browne, R. W., Mastrandrea, L., et al. (2014). Paraoxonase 1 and oxidative stress in paediatric non-alcoholic steatohepatitis. Liver Int. 34, 110–117. doi: 10.1111/liv.12308
Dey, D., Pan, G., Varma, N. R., and Palaniyandi, S. S. (2015). Sca-1 + cells from fetal heart with high aldehyde dehydrogenase activity exhibit enhanced gene expression for self-renewal, proliferation, and survival. Oxid. Med. Cell. Longev. 2015, 1–8. doi: 10.1155/2015/730683
Dobashi, S., Katagiri, T., Hirota, E., Ashida, S., Daigo, Y., Shuin, T., et al. (2009). Involvement of TMEM22 overexpression in the growth of renal cell carcinoma cells. Oncol. Rep. 21, 305–312. doi: 10.3892/or_00000222
Dolgova, E. V., Alyamkina, E. A., Efremov, Y. R., Nikolin, V. P., Popova, N. A., Tyrinova, T. V., et al. (2014). Identification of cancer stem cells and a strategy for their elimination. Cancer Biol. Ther. 15, 1378–1394. doi: 10.4161/cbt.29854
Dolgova, E. V., Efremov, Y. R., Orishchenko, K. E., Andrushkevich, O. M., Alyamkina, E. A., Proskurina, A. S., et al. (2013). Delivery and processing of exogenous double-stranded DNA in mouse CD34+ hematopoietic progenitor cells and their cell cycle changes upon combined treatment with cyclophosphamide and double-stranded DNA. Gene 528, 74–83. doi: 10.1016/j.gene.2013.06.058
Dolgova, E. V., Proskurina, A. S., Nikolin, V. P., Popova, N. A., Alyamkina, E. A., Orishchenko, K. E., et al. (2012). “Delayed death” phenomenon: a synergistic action of cyclophosphamide and exogenous DNA. Gene 495, 134–145. doi: 10.1016/j.gene.2011.12.032
Dolgova, E. V., Shevela, E. Y., Tyrinova, T. V., Minkevich, A. M., Proskurina, A. S., Potter, E. A., et al. (2016). Nonadherent spheres with multiple myeloma surface markers contain cells that contribute to sphere formation and are capable of internalizing extracellular double-stranded DNA. Clin. Lymphoma Myeloma Leuk. 16, 563–576. doi: 10.1016/j.clml.2016.06.014
Dressel, R. (2011). Effects of histocompatibility and host immune responses on the tumorigenicity of pluripotent stem cells. Semin. Immunopathol. 33, 573–591. doi: 10.1007/s00281-011-0266-8
Dzugkoeva, F. S., Mozhaeva, I. V., Dzugkoev, S. G., Margieva, O. I., Tedtoeva, A. I., and Otiev, M. A. (2016). Oxidative stress and biochemical markers of endothelial dysfunction and organ damage under conditions of experimental nonferrous metal intoxication. Bull. Exp. Biol. Med. 162, 199–202. doi: 10.1007/s10517-016-3575-z
Ellisen, L. W., Bird, J., West, D. C., Soreng, A. L., Reynolds, T. C., Smith, S. D., et al. (1991). TAN-1, the human homolog of the drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66, 649–661. doi: 10.1016/0092-8674(91)90111-B
Eloranta, J. J., and Kullak-Ublick, G. A. (2005). Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch. Biochem. Biophys. 433, 397–412. doi: 10.1016/j.abb.2004.09.019
ElShamy, W. M., and Duhé, R. J. (2013). Overview: cellular plasticity, cancer stem cells and metastasis. Cancer Lett. 341, 2–8. doi: 10.1016/j.canlet.2013.06.020
Englund, E., Bartoschek, M., Reitsma, B., Jacobsson, L., Escudero-Esparza, A., Orimo, A., et al. (2016). Cartilage oligomeric matrix protein contributes to the development and metastasis of breast cancer. Oncogene 35, 5585–5596. doi: 10.1038/onc.2016.98
Fialkow, P. J., Gartler, S. M., and Yoshida, A. (1967). Clonal origin of chronic myelocytic leukemia in man. Proc. Natl. Acad. Sci. U.S.A. 58, 1468–1471. doi: 10.1073/pnas.58.4.1468
Fialkow, P. J., Klein, G., Gartler, S. M., and Clifford, P. (1970). Clonal origin for individual Burkitt tumours. Lancet 1, 384–386.
Foo, L. C., and Dougherty, J. D. (2013). Aldh1L1 is expressed by postnatal neural stem cells in vivo. Glia 61, 1533–1541. doi: 10.1002/glia.22539
Fouad, Y. A., and Aanei, C. (2017). Revisiting the hallmarks of cancer. Am. J. Cancer Res. 7, 1016–1036.
Franco, S. S., Szczesna, K., Iliou, M. S., Al-Qahtani, M., Mobasheri, A., Kobolák, J., et al. (2016). In vitro models of cancer stem cells and clinical applications. BMC Cancer 16:738. doi: 10.1186/s12885-016-2774-3
Franzén, B., Linder, S., Alaiya, A. A., Eriksson, E., Fujioka, K., Bergman, A.-C., et al. (1997). Analysis of polypeptide expression in benign and malignant human breast lesions. Electrophoresis 18, 582–587. doi: 10.1002/elps.1150180341
Friedman, G. K., Haas, M. C., Kelly, V. M., Markert, J. M., Gillespie, G. Y., and Cassady, K. A. (2012). Hypoxia moderates γ134.5-deleted herpes simplex virus oncolytic activity in human glioma xenoline primary cultures. Transl. Oncol. 5, 200–207. doi: 10.1593/tlo.12115
Fu, L., Lin-Lee, Y.-C., Pham, L. V., Tamayo, A. T., Yoshimura, L. C., and Ford, R. J. (2009). BAFF-R promotes cell proliferation and survival through interaction with IKKβ and NF-κB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood 113, 4627–4636. doi: 10.1182/blood-2008-10-183467
Fu, S., and Davies, K. P. (2015). Opiorphin-dependent upregulation of CD73 (a key enzyme in the adenosine signaling pathway) in corporal smooth muscle cells exposed to hypoxic conditions and in corporal tissue in pre-priapic sickle cell mice. Int. J. Impot. Res. 27, 140–145. doi: 10.1038/ijir.2015.5
Fukunaga-Kalabis, M., Martinez, G., Nguyen, T. K., Kim, D., Santiago-Walker, A., Roesch, A., et al. (2010). Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene 29, 6115–6124. doi: 10.1038/onc.2010.350
Ganat, Y., Soni, S., Chacon, M., Schwartz, M. L., and Vaccarino, F. M. (2002). Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience 112, 977–991. doi: 10.1016/S0306-4522(02)00060-X
Gao, Z. W., Dong, K., and Zhang, H. Z. (2014). The roles of CD73 in cancer. Biomed Res. Int. 2014, 1–9. doi: 10.1155/2014/460654
García-Casas, A., García-Olmo, D. C., and García-Olmo, D. (2017). Further the liquid biopsy: gathering pieces of the puzzle of genometastasis theory. World J. Clin. Oncol. 8, 378–388. doi: 10.5306/wjco.v8.i5.378
Garrido, P., Osorio, F. G., Morán, J., Cabello, E., Alonso, A., Freije, J. M., et al. (2015). Loss of GLUT4 induces metabolic reprogramming and impairs viability of breast cancer cells. J. Cell. Physiol. 230, 191–198. doi: 10.1002/jcp.24698
Gato, W. E., Hales, D. B., and Means, J. C. (2012). Hepatic gene expression analysis of 2-aminoanthracene exposed Fisher-344 rats reveal patterns indicative of liver carcinoma and type 2 diabetes. J. Toxicol. Sci. 37, 1001–1016. doi: 10.2131/jts.37.1001
Gely-Pernot, A., Coronas, V., Harnois, T., Prestoz, L., Mandairon, N., Didier, A., et al. (2012). An endogenous vitamin K-dependent mechanism regulates cell proliferation in the brain subventricular stem cell niche. Stem Cells 30, 719–731. doi: 10.1002/stem.1045
Gerber, J. M., Gucwa, J. L., Esopi, D., Gurel, M., Haffner, M. C., Vala, M., et al. (2013). Genome-wide comparison of the transcriptomes of highly enriched normal and chronic myeloid leukemia stem and progenitor cell populations. Oncotarget 4, 715–728. doi: 10.18632/oncotarget.990
Gerby, B., Veiga, D. F. T., Krosl, J., Nourreddine, S., Ouellette, J., Haman, A., et al. (2016). High-throughput screening in niche-based assay identifies compounds to target preleukemic stem cells. J. Clin. Invest. 126, 4569–4584. doi: 10.1172/JCI86489
Gibson, L. A., Lavoie, R. A., Bissegger, S., Campbell, L. M., and Langlois, V. S. (2014). A positive correlation between mercury and oxidative stress-related gene expression (GPX3 and GSTM3) is measured in female Double-crested Cormorant blood. Ecotoxicology 23, 1004–1014. doi: 10.1007/s10646-014-1243-5
Gkretsi, V., and Bogdanos, D. P. (2015). Experimental evidence of Migfilin as a new therapeutic target of hepatocellular carcinoma metastasis. Exp. Cell Res. 334, 219–227. doi: 10.1016/j.yexcr.2015.03.002
Gopal, K., Gupta, N., Zhang, H., Alshareef, A., Alqahtani, H., Bigras, G., et al. (2016). Oxidative stress induces the acquisition of cancer stem-like phenotype in breast cancer detectable by using a Sox2 regulatory region-2 (SRR2) reporter. Oncotarget 7, 3111–3127. doi: 10.18632/oncotarget.6630
Gorczynski, R. M., Clark, D. A., Erin, N., and Khatri, I. (2011). Role of CD200 expression in regulation of metastasis of EMT6 tumor cells in mice. Breast Cancer Res. Treat. 130, 49–60. doi: 10.1007/s10549-010-1259-3
Gottesman, M. M. (2002). Mechanisms of cancer drug resistance. Annu. Rev. Med. 53, 615–627. doi: 10.1146/annurev.med.53.082901.103929
Gouédard, C., Barouki, R., and Morel, Y. (2004). Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Mol. Cell. Biol. 24, 5209–5222. doi: 10.1128/MCB.24.12.5209-5222.2004
Granneman, J. G., Li, P., Zhu, Z., and Lu, Y. (2005). Metabolic and cellular plasticity in white adipose tissue I: effects of β 3 -adrenergic receptor activation. Am. J. Physiol. Metab. 289, E608–E616. doi: 10.1152/ajpendo.00009.2005
Grantab, R. H., and Tannock, I. F. (2012). Penetration of anticancer drugs through tumour tissue as a function of cellular packing density and interstitial fluid pressure and its modification by bortezomib. BMC Cancer 12:214. doi: 10.1186/1471-2407-12-214
Greig, R. G., Koestler, T. P., Trainer, D. L., Corwin, S. P., Miles, L., Kline, T., et al. (1985). Tumorigenic and metastatic properties of “normal” and ras-transfected NIH/3T3 cells. Proc. Natl. Acad. Sci. U.S.A. 82, 3698–3701.
Griesmann, H., Ripka, S., Pralle, M., Ellenrieder, V., Baumgart, S., Buchholz, M., et al. (2013). WNT5A-NFAT signaling mediates resistance to apoptosis in pancreatic cancer. Neoplasia 15, 11–22. doi: 10.1593/neo.121312
Gupta, S., Silva, T. S., Osizugbo, J. E., Tucker, L., Spratt, H. M., and Garg, N. J. (2014). Serum-mediated activation of macrophages reflects TcVac2 vaccine efficacy against chagas disease. Infect. Immun. 82, 1382–1389. doi: 10.1128/IAI.01186-13
Hammoud, A. A., Kirstein, N., Mournetas, V., Darracq, A., Broc, S., Blanchard, C., et al. (2016). Murine embryonic stem cell plasticity is regulated through klf5 and maintained by metalloproteinase mmp1 and hypoxia. PLoS ONE 11:e0146281. doi: 10.1371/journal.pone.0146281
Han, W., Takano, T., He, J., Ding, J., Gao, S., Noda, C., et al. (2001). Role of BLNK in oxidative stress signaling in B cells. Antioxidants Redox Signal. 3, 1065–1073. doi: 10.1089/152308601317203576
Han, Z. C., Lu, M., Li, J., Defard, M., Boval, B., Schlegel, N., et al. (1997). Platelet factor 4 and other CXC chemokines support the survival of normal hematopoietic cells and reduce the chemosensitivity of cells to cytotoxic agents. Blood 89, 2328–2335.
Hanahan, D., and Weinberg, R. A. (2000). The hallmarks of cancer. Cell 100, 57–70. doi: 10.1016/S0092-8674(00)81683-9
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646–674. doi: 10.1016/j.cell.2011.02.013
Hao, J., Li, T.-G., Qi, X., Zhao, D.-F., and Zhao, G.-Q. (2006). WNT/β-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev. Biol. 290, 81–91. doi: 10.1016/j.ydbio.2005.11.011
Hartomo, T. B., Van Huyen Pham, T., Yamamoto, N., Hirase, S., Hasegawa, D., Kosaka, Y., et al. (2015). Involvement of aldehyde dehydrogenase 1A2 in the regulation of cancer stem cell properties in neuroblastoma. Int. J. Oncol. 46, 1089–1098. doi: 10.3892/ijo.2014.2801
Harvey, R. D., and Morgan, E. T. (2014). Cancer, inflammation, and therapy: effects on cytochrome P450-mediated drug metabolism and implications for novel immunotherapeutic agents. Clin. Pharmacol. Ther. 96, 449–457. doi: 10.1038/clpt.2014.143
Hashimoto, Y., Tomiyama, T., Yamano, Y., and Mori, H. (2003). Mutation (D472Y) in the type 3 repeat domain of cartilage oligomeric matrix protein affects its early vesicle trafficking in endoplasmic reticulum and induces apoptosis. Am. J. Pathol. 163, 101–110. doi: 10.1016/S0002-9440(10)63634-6
Heinke, J., Kerber, M., Rahner, S., Mnich, L., Lassmann, S., Helbing, T., et al. (2012). Bone morphogenetic protein modulator BMPER is highly expressed in malignant tumors and controls invasive cell behavior. Oncogene 31, 2919–2930. doi: 10.1038/onc.2011.473
Heng, J. C., Orlov, Y. L., and Ng, H. H. (2010). Transcription factors for the modulation of pluripotency and reprogramming. Cold Spring Harb. Symp. Quant. Biol. 75, 237–244. doi: 10.1101/sqb.2010.75.003
Henrique, D., Hirsinger, E., Adam, J., Le Roux, I., Pourquié, O., Ish-Horowicz, D., et al. (1997). Maintenance of neuroepithelial progenitor cells by Delta–Notch signalling in the embryonic chick retina. Curr. Biol. 7, 661–670. doi: 10.1016/S0960-9822(06)00293-4
Hiraoka, A. (2008). Leukemia cell lines require self-secreted stem cell growth factor (SCGF) for their proliferation. Leuk. Res. 32, 1623–1625. doi: 10.1016/j.leukres.2008.01.003
Hiraoka, A., Yano Ki, K., Kagami, N., Takeshige, K., Mio, H., Anazawa, H., et al. (2001). Stem cell growth factor: in situ hybridization analysis on the gene expression, molecular characterization and in vitro proliferative activity of a recombinant preparation on primitive hematopoietic progenitor cells. Hematol. J. 2, 307–315. doi: 10.1038/sj.thj.6200118
Hlavata, I., Mohelnikova-Duchonova, B., Vaclavikova, R., Liska, V., Pitule, P., Novak, P., et al. (2012). The role of ABC transporters in progression and clinical outcome of colorectal cancer. Mutagenesis 27, 187–196. doi: 10.1093/mutage/ger075
Hoelzinger, D. B., Demuth, T., and Berens, M. E. (2007). Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J. Natl. Cancer Inst. 99, 1583–1593. doi: 10.1093/jnci/djm187
Horsley, V., and Pavlath, G. K. (2002). NFAT: ubiquitous regulator of cell differentiation and adaptation. J. Cell Biol. 156, 771–774. doi: 10.1083/jcb.200111073
Hoshino, T., and Wilson, C. B. (1975). Review of basic concepts of cell kinetics as applied to brain tumors. J. Neurosurg. 42, 123–131. doi: 10.3171/jns.1975.42.2.0123
Hou, H., Kang, Y., Li, Y., Zeng, Y., Ding, G., and Shang, J. (2017). miR-33a expression sensitizes Lgr5+ HCC-CSCs to doxorubicin via ABCA1. Neoplasma 64, 81–91. doi: 10.4149/neo_2017_110
Hough, R. B., and Piatigorsky, J. (2004). Preferential transcription of rabbit Aldh1a1 in the cornea: implication of hypoxia-related pathways. Mol. Cell. Biol. 24, 1324–1340. doi: 10.1128/MCB.24.3.1324-1340.2004
Hour, T.-C., Lai, Y.-L., Kuan, C.-I., Chou, C.-K., Wang, J.-M., Tu, H.-Y., et al. (2010). Transcriptional up-regulation of SOD1 by CEBPD: a potential target for cisplatin resistant human urothelial carcinoma cells. Biochem. Pharmacol. 80, 325–334. doi: 10.1016/j.bcp.2010.04.007
Hrubá, E., Vondrácek, J., Líbalová, H., Topinka, J., Bryja, V., Soucek, K., et al. (2011). Gene expression changes in human prostate carcinoma cells exposed to genotoxic and nongenotoxic aryl hydrocarbon receptor ligands. Toxicol. Lett. 206, 178–188. doi: 10.1016/j.toxlet.2011.07.011
Hsiao, K.-Y., Wu, M.-H., Chang, N., Yang, S.-H., Wu, C.-W., Sun, H. S., et al. (2015). Coordination of AUF1 and miR-148a destabilizes DNA methyltransferase 1 mRNA under hypoxia in endometriosis. Mol. Hum. Reprod. 21, 894–904. doi: 10.1093/molehr/gav054
Hu, H. R., Dong, Z., Yi, L., He, X. Y., Zhang, Y. L., Liu, Y. L., et al. (2015). Function and mechanism of neurotensin (NTS) and its receptor 1 (NTSR1) in occurrence and development of tumors. Zhongguo Zhong Yao Za Zhi 40, 2524–2536.
Hu, X., Zhang, Y., Zhang, A., Li, Y., Zhu, Z., Shao, Z., et al. (2009). Comparative serum proteome analysis of human lymph node negative/positive invasive ductal carcinoma of the breast and benign breast disease controls via label-free semiquantitative shotgun technology. OMICS 13, 291–300. doi: 10.1089/omi.2009.0016
Huang, G.-S., Dai, L.-G., Yen, B. L., and Hsu, S. H. (2011). Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 32, 6929–6945. doi: 10.1016/j.biomaterials.2011.05.092
Huang, L., Wang, C., Zhang, Y., Wu, M., and Zuo, Z. (2013). Phenanthrene causes ocular developmental toxicity in zebrafish embryos and the possible mechanisms involved. J. Hazard. Mater. 261, 172–180. doi: 10.1016/j.jhazmat.2013.07.030
Huang, S. S., Tang, F.-M., Huang, Y.-H., Liu, I.-H., Hsu, S.-C., Chen, S.-T., et al. (2003). Cloning, expression, characterization, and role in autocrine cell growth of cell surface retention sequence binding protein-1. J. Biol. Chem. 278, 43855–43869. doi: 10.1074/jbc.M306411200
Huang, W.-L., Li, Z., Lin, T.-Y., Wang, S.-W., Wu, F.-J., and Luo, C.-W. (2016). Thyrostimulin-TSHR signaling promotes the proliferation of NIH:OVCAR-3 ovarian cancer cells via trans-regulation of the EGFR pathway. Sci. Rep. 6:27471. doi: 10.1038/srep27471
Hung, T.-H., Hsu, S.-C., Cheng, C.-Y., Choo, K.-B., Tseng, C.-P., Chen, T.-C., et al. (2014). Wnt5A regulates ABCB1 expression in multidrug-resistant cancer cells through activation of the non-canonical PKA/β-catenin pathway. Oncotarget 5, 12273–12290. doi: 10.18632/oncotarget.2631
Iborra, A., Mayorga, M., Llobet, N., and Martínez, P. (2003). Expression of complement regulatory proteins [membrane cofactor protein (CD46), decay accelerating factor (CD55), and protectin (CD59)] in endometrial stressed cells. Cell. Immunol. 223, 46–51. doi: 10.1016/S0008-8749(03)00127-8
Iida, H., Suzuki, M., Goitsuka, R., and Ueno, H. (2012). Hypoxia induces CD133 expression in human lung cancer cells by up-regulation of OCT3/4 and SOX2. Int. J. Oncol. 40, 71–79. doi: 10.3892/ijo.2011.1207
Illmensee, K., and Mintz, B. (1976). Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc. Natl. Acad. Sci. U.S.A. 73, 549–553. doi: 10.1073/pnas.73.2.549
Jaluria, P., Konstantopoulos, K., Betenbaugh, M., and Shiloach, J. (2008). Egr1 andGas6 facilitate the adaptation of HEK-293 cells to serum-free media by conferring enhanced viability and higher growth rates. Biotechnol. Bioeng. 99, 1443–1452. doi: 10.1002/bit.21707
Jam, I., Shoham, M., Wolf, R. O., and Mishkin, S. (1978). Elevated serum amylase activity in the absence of clinical pancreatic or salivary gland disease: possible role of acute hypoxemia. Am. J. Gastroenterol. 70, 480–488.
Jang, J., Wang, Y., Kim, H. S., Lalli, M. A., and Kosik, K. S. (2014). Nrf2, a regulator of the proteasome, controls self-renewal and pluripotency in human embryonic stem cells. Stem Cells 32, 2616–2625. doi: 10.1002/stem.1764
Januchowski, R., Swierczewska, M., Sterzynska, K., Wojtowicz, K., Nowicki, M., and Zabel, M. (2016). Increased expression of several collagen genes is associated with drug resistance in ovarian cancer cell lines. J. Cancer 7, 1295–1310. doi: 10.7150/jca.15371
Jauliac, S., López-Rodriguez, C., Shaw, L. M., Brown, L. F., Rao, A., and Toker, A. (2002). The role of NFAT transcription factors in integrin-mediated carcinoma invasion. Nat. Cell Biol. 4, 540–544. doi: 10.1038/ncb816
Ji, W., Yu, Y., Li, Z., Wang, G., Li, F., Xia, W., et al. (2016). FGFR1 promotes the stem cell-like phenotype of FGFR1-amplified non-small cell lung cancer cells through the Hedgehog pathway. Oncotarget 7, 15118–15134. doi: 10.18632/oncotarget.7701
Jiang, J., Liu, Y., Tang, Y., Li, L., Zeng, R., Zeng, S., et al. (2016). ALDH1A1 induces resistance to CHOP in diffuse large B-cell lymphoma through activation of the JAK2/STAT3 pathway. Onco. Targets. Ther. Volume 9, 5349–5360. doi: 10.2147/OTT.S107957
Jiang, W. G., Sanders, A. J., Katoh, M., Ungefroren, H., Gieseler, F., Prince, M., et al. (2015). Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin. Cancer Biol. 35, S244–S275. doi: 10.1016/j.semcancer.2015.03.008
Jiang, X. R., Wrona, M. Z., and Dryhurst, G. (1999). Tryptamine-4,5-dione, a putative endotoxic metabolite of the superoxide- mediated oxidation of serotonin, is a mitochondrial toxin: possible implications in neurodegenerative brain disorders. Chem. Res. Toxicol. 12, 429–436. doi: 10.1021/tx9801615
Jiao, H., Natoli, R., Valter, K., Provis, J. M., and Rutar, M. (2015a). Spatiotemporal cadence of macrophage polarisation in a model of light-induced retinal degeneration. PLoS ONE 10:e0143952. doi: 10.1371/journal.pone.0143952
Jiao, J., Zhao, X., Liang, Y., Tang, D., and Pan, C. (2015b). FGF1–FGFR1 axis promotes tongue squamous cell carcinoma (TSCC) metastasis through epithelial–mesenchymal transition (EMT). Biochem. Biophys. Res. Commun. 466, 327–332. doi: 10.1016/j.bbrc.2015.09.021
Jin, T., Suk Kim, H., Choi, S., Hwang, E., Woo, J., Suk Ryu, H., et al. (2017a). microRNA-200c/141 upregulates SerpinB2 to promote breast cancer cell metastasis and reduce patient survival. Oncotarget 8, 32769–32782. doi: 10.18632/oncotarget.15680
Jin, Y., Nie, D., Li, J., Du, X., Lu, Y., Li, Y., et al. (2017b). Gas6/AXL signaling regulates self-renewal of chronic myelogenous leukemia stem cells by stabilizing β-catenin. Clin. Cancer Res. 23, 2842–2855. doi: 10.1158/1078-0432.CCR-16-1298
Jinesh, G. G., and Kamat, A. M. (2016). Blebbishield emergency program: an apoptotic route to cellular transformation. Cell Death Differ. 23, 757–758. doi: 10.1038/cdd.2016.26
Jinesh, G. G., and Kamat, A. M. (2017). The blebbishield emergency program overrides chromosomal instability and phagocytosis checkpoints in cancer stem cells. Cancer Res. 77, 6144–6156. doi: 10.1158/0008-5472.CAN-17-0522
Jögi, A., Vallon-Christersson, J., Holmquist, L., Axelson, H., Borg, A., and Påhlman, S. (2004). Human neuroblastoma cells exposed to hypoxia: induction of genes associated with growth, survival, and aggressive behavior. Exp. Cell Res. 295, 469–487. doi: 10.1016/j.yexcr.2004.01.013
Joseph Martin, S., and Evan Prince, S. (2017). Comparative modulation of levels of oxidative stress in the liver of anti-tuberculosis drug treated Wistar rats by vitamin B12, beta-carotene, and Spirulina fusiformis: role of NF-κB, iNOS, IL-6, and IL-10. J. Cell. Biochem. 118, 3825–3833. doi: 10.1002/jcb.26032
Jung, D.-W., Che, Z. M., Kim, J., Kim, K., Kim, K.-Y., Williams, D., et al. (2010). Tumor-stromal crosstalk in invasion of oral squamous cell carcinoma: a pivotal role of CCL7. Int. J. Cancer 127, 332–344. doi: 10.1002/ijc.25060
Jung, J. E., Karatas, H., Liu, Y., Yalcin, A., Montaner, J., Lo, E. H., et al. (2015a). STAT-dependent upregulation of 12/15-lipoxygenase contributes to neuronal injury after stroke. J. Cereb. Blood Flow Metab. 35, 2043–2051. doi: 10.1038/jcbfm.2015.169
Jung, Y.-S., Vermeer, P. D., Vermeer, D. W., Lee, S.-J., Goh, A. R., Ahn, H.-J., et al. (2015b). CD200: association with cancer stem cell features and response to chemoradiation in head and neck squamous cell carcinoma. Head Neck 37, 327–335. doi: 10.1002/hed.23608
Kamaraj, S., Anandakumar, P., Jagan, S., Ramakrishnan, G., and Devaki, T. (2010). Modulatory effect of hesperidin on benzo(a)pyrene induced experimental lung carcinogenesis with reference to COX-2, MMP-2 and MMP-9. Eur. J. Pharmacol. 649, 320–327. doi: 10.1016/j.ejphar.2010.09.017
Kanda, M., Nomoto, S., Oya, H., Takami, H., Shimizu, D., Hibino, S., et al. (2016). The expression of melanoma-associated antigen D2 both in surgically resected and serum samples serves as clinically relevant biomarker of gastric cancer progression. Ann. Surg. Oncol. 23, 214–221. doi: 10.1245/s10434-015-4457-8
Kang, J., Gemberling, M., Nakamura, M., Whitby, F. G., Handa, H., Fairbrother, W. G., et al. (2009). A general mechanism for transcription regulation by Oct1 and Oct4 in response to genotoxic and oxidative stress. Genes Dev. 23, 208–222. doi: 10.1101/gad.1750709
Kasper, B., Brandt, E., Brandau, S., and Petersen, F. (2007). Platelet factor 4 (CXC chemokine ligand 4) differentially regulates respiratory burst, survival, and cytokine expression of human monocytes by using distinct signaling pathways. J. Immunol. 179, 2584–2591. doi: 10.4049/jimmunol.179.4.2584
Kasprzak, A., Kwasniewski, W., Adamek, A., and Gozdzicka-Jozefiak, A. (2017). Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat. Res. Mutat. Res. 772, 78–104. doi: 10.1016/j.mrrev.2016.08.007
Katoh, M., and Nakagama, H. (2014). FGF receptors: cancer biology and therapeutics. Med. Res. Rev. 34, 280–300. doi: 10.1002/med.21288
Katsuta, E., Tanaka, S., Mogushi, K., Shimada, S., Akiyama, Y., Aihara, A., et al. (2016). CD73 as a therapeutic target for pancreatic neuroendocrine tumor stem cells. Int. J. Oncol. 48, 657–669. doi: 10.3892/ijo.2015.3299
Kazi, J. U., and Rönnstrand, L. (2013). FLT3 signals via the adapter protein Grb10 and overexpression of Grb10 leads to aberrant cell proliferation in acute myeloid leukemia. Mol. Oncol. 7, 402–418. doi: 10.1016/j.molonc.2012.11.003
Kerbel, R. S., St Croix, B., Florenes, V. A., and Rak, J. (1996). Induction and reversal of cell adhesion-dependent multicellular drug resistance in solid breast tumors. Hum. Cell 9, 257–264.
Kerjaschki, D., Bago-Horvath, Z., Rudas, M., Sexl, V., Schneckenleithner, C., Wolbank, S., et al. (2011). Lipoxygenase mediates invasion of intrametastatic lymphatic vessels and propagates lymph node metastasis of human mammary carcinoma xenografts in mouse. J. Clin. Invest. 121, 2000–2012. doi: 10.1172/JCI44751
Khalil, A., Villard, P.-H., Dao, M. A., Burcelin, R., Champion, S., Fouchier, F., et al. (2010). Polycyclic aromatic hydrocarbons potentiate high-fat diet effects on intestinal inflammation. Toxicol. Lett. 196, 161–167. doi: 10.1016/j.toxlet.2010.04.010
Khan, F. H., Pandian, V., Ramraj, S., Aravindan, S., Herman, T. S., and Aravindan, N. (2015). Reorganization of metastamiRs in the evolution of metastatic aggressive neuroblastoma cells. BMC Genomics 16:501. doi: 10.1186/s12864-015-1642-x
Kiani, A., Habermann, I., Haase, M., Feldmann, S., Boxberger, S., Sanchez-Fernandez, M. A., et al. (2004). Expression and regulation of NFAT (nuclear factors of activated T cells) in human CD34+ cells: down-regulation upon myeloid differentiation. J. Leukoc. Biol. 76, 1057–1065. doi: 10.1189/jlb.0404259
Kikuchi, J., Koyama, D., Wada, T., Izumi, T., Hofgaard, P. O., Bogen, B., et al. (2015). Phosphorylation-mediated EZH2 inactivation promotes drug resistance in multiple myeloma. J. Clin. Invest. 125, 4375–4390. doi: 10.1172/JCI80325
Kim, H. K., Hwang, S. H., and Abdi, S. (2017). Tempol ameliorates and prevents mechanical hyperalgesia in a rat model of chemotherapy-induced neuropathic pain. Front. Pharmacol. 7:532. doi: 10.3389/fphar.2016.00532
Kim, M.-C., Cui, F.-J., and Kim, Y. (2013). Hydrogen peroxide promotes epithelial to mesenchymal transition and stemness in human malignant mesothelioma cells. Asian Pac. J. Cancer Prev. 14, 3625–3630. doi: 10.7314/APJCP.2013.14.6.3625
Kim, Y. W., Kim, H.-J., Bae, S.-M., Kim, Y. J., Shin, J.-C., Chun, H.-J., et al. (2010). Time-course transcriptional profiling of human amniotic fluid-derived stem cells using microarray. Cancer Res. Treat. 42, 82–94. doi: 10.4143/crt.2010.42.2.82
Kinder, M., Wei, C., Shelat, S. G., Kundu, M., Zhao, L., Blair, I. A., et al. (2010). Hematopoietic stem cell function requires 12/15-lipoxygenase-dependent fatty acid metabolism. Blood 115, 5012–5022. doi: 10.1182/blood-2009-09-243139
Kirschenbaum, A., Izadmehr, S., Yao, S., O'Connor-Chapman, K. L., Huang, A., Gregoriades, E. M., et al. (2016). Prostatic acid phosphatase alters the RANKL/OPG system and induces osteoblastic prostate cancer bone metastases. Endocrinology 157, 4526–4533. doi: 10.1210/en.2016-1606
Klotz, L.-O., and Steinbrenner, H. (2017). Cellular adaptation to xenobiotics: interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 13, 646–654. doi: 10.1016/j.redox.2017.07.015
Kluger, H. M., Kluger, Y., Gilmore-Hebert, M., DiVito, K., Chang, J. T., Rodov, S., et al. (2004). cDNA microarray analysis of invasive and tumorigenic phenotypes in a breast cancer model. Lab. Investig. 84, 320–331. doi: 10.1038/labinvest.3700044
Ko, C. H., Cheng, C. F., Lai, C. P., Tzu, T. H., Chiu, C. W., Lin, M. W., et al. (2013). Differential proteomic analysis of cancer stem cell properties in hepatocellular carcinomas by isobaric tag labeling and mass spectrometry. J. Proteome Res. 12, 3573–3585. doi: 10.1021/pr4004294
Kohlscheen, S., Wintterle, S., Schwarzer, A., Kamp, C., Brugman, M. H., Breuer, D. C., et al. (2015). Inhibition of thrombopoietin/Mpl signaling in adult hematopoiesis identifies new candidates for hematopoietic stem cell maintenance. PLoS ONE 10:e0131866. doi: 10.1371/journal.pone.0131866
Kong, C. S., Cao, H., Kwok, S., Nguyen, C. M., Jordan, R. C., Beaudry, V. G., et al. (2013). Loss of the p53/p63 target PERP is an early event in oral carcinogenesis and correlates with higher rate of local relapse. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 115, 95–103. doi: 10.1016/j.oooo.2012.10.017
Korinek, V., Barker, N., Moerer, P., van Donselaar, E., Huls, G., Peters, P. J., et al. (1998). Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat. Genet. 19, 379–383. doi: 10.1038/1270
Kotov, A. A., Olenkina, O. M., Godneeva, B. K., Adashev, V. E., and Olenina, L. V. (2017). Progress in understanding the molecular functions of DDX3Y (DBY) in male germ cell development and maintenance. Biosci. Trends 11, 46–53. doi: 10.5582/bst.2016.01216
Krathwohl, M. D. (2004). Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells 22, 109–118. doi: 10.1634/stemcells.22-1-109
Kubo, H., Shimizu, M., Taya, Y., Kawamoto, T., Michida, M., Kaneko, E., et al. (2009). Identification of mesenchymal stem cell (MSC)-transcription factors by microarray and knockdown analyses, and signature molecule-marked MSC in bone marrow by immunohistochemistry. Genes to Cells 14, 407–424. doi: 10.1111/j.1365-2443.2009.01281.x
Kuleshov, M. V., Jones, M. R., Rouillard, A. D., Fernandez, N. F., Duan, Q., Wang, Z., et al. (2016). Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–W97. doi: 10.1093/nar/gkw377
Kuol, N., Stojanovska, L., Nurgali, K., and Apostolopoulos, V. (2017). The mechanisms tumor cells utilize to evade the host's immune system. Maturitas 105, 8–15.?doi: 10.1016/j.maturitas.2017.04.014
Kurz, K., Schroecksnadel, S., Weiss, G., and Fuchs, D. (2011). Association between increased tryptophan degradation and depression in cancer patients. Curr. Opin. Clin. Nutr. Metab. Care 14, 49–56. doi: 10.1097/MCO.0b013e328340d849
Lacombe, J., Krosl, G., Tremblay, M., Gerby, B., Martin, R., Aplan, P. D., et al. (2013). Genetic interaction between Kit and Scl. Blood 122, 1150–1161. doi: 10.1182/blood-2011-01-331819
Lagasse, E. (2008). Cancer stem cells with genetic instability: the best vehicle with the best engine for cancer. Gene Ther. 15, 136–142. doi: 10.1038/sj.gt.3303068
Lake, A. D., Wood, C. E., Bhat, V. S., Chorley, B. N., Carswell, G. K., Sey, Y. M., et al. (2016). Dose and effect thresholds for early key events in a PPARα-mediated mode of action. Toxicol. Sci. 149, 312–325. doi: 10.1093/toxsci/kfv236
Lambert, C. B., Spire, C., Claude, N., and Guillouzo, A. (2009). Dose- and time-dependent effects of phenobarbital on gene expression profiling in human hepatoma HepaRG cells. Toxicol. Appl. Pharmacol. 234, 345–360. doi: 10.1016/j.taap.2008.11.008
Laviano, A., Meguid, M. M., Preziosa, I., and Rossi Fanelli, F. (2007). Oxidative stress and wasting in cancer. Curr. Opin. Clin. Nutr. Metab. Care 10, 449–456. doi: 10.1097/MCO.0b013e328122db94
Lavrovsky, V. A., Guvakova, M. A., and Lavrovsky, Y. V. (1992). High frequency of tumour cell reversion to non-tumorigenic phenotype. Eur. J. Cancer 28, 17–21. doi: 10.1016/0959-8049(92)90375-C
Łazarenkow, A., Michalska, M., Mirowski, M., Słomiak, K., and Nawrot-Modranka, J. (2017). The effect of hydrazine derivatives of 3-formylchromones on angiogenic basic fibroblast growth factor and fibroblast growth factor receptor-1 in human melanoma cell line WM-115. Acta Biochim. Pol. 64, 585–590. doi: 10.18388/abp.2017_1565
Le, P. U., Angers-Loustau, A., de Oliveira, R. M., Ajlan, A., Brassard, C. L., Dudley, A., et al. (2010). DRR drives brain cancer invasion by regulating cytoskeletal-focal adhesion dynamics. Oncogene 29, 4636–4647. doi: 10.1038/onc.2010.216
Leclerc, D., Pham, D. N., Lévesque, N., Truongcao, M., Foulkes, W. D., Sapienza, C., et al. (2017). Oncogenic role of PDK4 in human colon cancer cells. Br. J. Cancer 116, 930–936. doi: 10.1038/bjc.2017.38
LeCouter, J., Lin, R., Tejada, M., Frantz, G., Peale, F., Hillan, K. J., et al. (2003). The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: localization of Bv8 receptors to endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 100, 2685–2690. doi: 10.1073/pnas.0337667100
LeCouter, J., Zlot, C., Tejada, M., Peale, F., and Ferrara, N. (2004). Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc. Natl. Acad. Sci. U.S.A. 101, 16813–16818. doi: 10.1073/pnas.0407697101
Lee, K.-Y., Feng, P.-H., Ho, S.-C., Chuang, K.-J., Chen, T.-T., Su, C.-L., et al. (2015a). Inter-alpha-trypsin inhibitor heavy chain 4: a novel biomarker for environmental exposure to particulate air pollution in patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 831–841. doi: 10.2147/COPD.S81611
Lee, S. H., Manandhar, S., and Lee, Y. M. (2017a). Roles of RUNX in hypoxia-induced responses and angiogenesis. Adv. Exp. Med. Biol. 962, 449–469. doi: 10.1007/978-981-10-3233-2_27
Lee, W.-J., Hah, Y.-S., Ock, S.-A., Lee, J.-H., Jeon, R.-H., Park, J.-S., et al. (2015b). Cell source-dependent in vivo immunosuppressive properties of mesenchymal stem cells derived from the bone marrow and synovial fluid of minipigs. Exp. Cell Res. 333, 273–288. doi: 10.1016/j.yexcr.2015.03.015
Leeuwen, D. M., van Agen, E., Gottschalk, R. W., Vlietinck, R., Gielen, M., van Herwijnen, M. H., et al. (2006). Cigarette smoke-induced differential gene expression in blood cells from monozygotic twin pairs. Carcinogenesis 28, 691–697. doi: 10.1093/carcin/bgl199
Lei, T., and Ling, X. (2015). IGF-1 promotes the growth and metastasis of hepatocellular carcinoma via the inhibition of proteasome-mediated cathepsin B degradation. World J. Gastroenterol. 21, 10137–10149. doi: 10.3748/wjg.v21.i35.10137
Leth-Larsen, R., Terp, M. G., Christensen, A. G., Elias, D., Kühlwein, T., Jensen, O. N., et al. (2012). Functional heterogeneity within the CD44 high human breast cancer stem cell-like compartment reveals a gene signature predictive of distant metastasis. Mol. Med. 18, 1109–1121. doi: 10.2119/molmed.2012.00091
Li, B., Xie, Z., Li, Z., Chen, S., and Li, B. (2016a). MicroRNA-613 targets FMNL2 and suppresses progression of colorectal cancer. Am. J. Transl. Res. 8, 5475–5484.
Li, C. Y., Renaud, H. J., Klaassen, C. D., and Cui, J. Y. (2016b). Age-specific regulation of drug-processing genes in mouse liver by ligands of xenobiotic-sensing transcription factors. Drug Metab. Dispos. 44, 1038–1049. doi: 10.1124/dmd.115.066639
Li, H., Gao, S., Huang, H., Liu, W., Huang, H., Liu, X., et al. (2017a). High throughput sequencing identifies an imprinted gene, Grb10, associated with the pluripotency state in nuclear transfer embryonic stem cells. Oncotarget 8, 47344–47355. doi: 10.18632/oncotarget.17185
Li, J., Zhao, L., Zhang, Y., Li, W., Duan, X., Chen, J., et al. (2017b). Imbalanced immune responses involving inflammatory molecules and immune-related pathways in the lung of acute and subchronic arsenic-exposed mice. Environ. Res. 159, 381–393. doi: 10.1016/j.envres.2017.08.036
Li, L., and Neaves, W. B. (2006). Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 66, 4553–4557. doi: 10.1158/0008-5472.CAN-05-3986
Li, M., Zhao, J., Hu, Y., Lu, H., and Guo, J. (2010a). Oxygen free radicals regulate energy metabolism via AMPK pathway following cerebral ischemia. Neurol. Res. 32, 779–784. doi: 10.1179/174313209X459174
Li, N., Mu, H., Zheng, L., Li, B., Wu, C., Niu, B., et al. (2016c). EIF2S3Y suppresses the pluripotency state and promotes the proliferation of mouse embryonic stem cells. Oncotarget 7, 11321–11331. doi: 10.18632/oncotarget.7187
Li, Q., Zhang, P., Yu, X., Zhao, Y., Li, Q., Zhang, Y., et al. (2017c). Lead transiently promotes granulocyte-macrophage progenitor differentiation and subsequently suppresses common myeloid progenitor differentiation. Toxicol. Sci. 160, 268–283. doi: 10.1093/toxsci/kfx176
Li, R., Wang, Y., Yang, Z., He, Y., Zhao, T., Fan, M., et al. (2015). Hypoxia-inducible factor-1α regulates the expression of L-type voltage-dependent Ca(2+) channels in PC12 cells under hypoxia. Cell Stress Chaperones 20, 507–516. doi: 10.1007/s12192-015-0575-2
Li, S., Qin, X., Chai, S., Qu, C., Wang, X., and Zhang, H. (2016d). Modulation of E-cadherin expression promotes migration ability of esophageal cancer cells. Sci. Rep. 6:21713. doi: 10.1038/srep21713
Li, T. S., Cheng, K., Lee, S. T., Matsushita, S., Davis, D., Malliaras, K., et al. (2010b). Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 28, 2088–2098. doi: 10.1002/stem.532
Li, W., Zhai, B., Zhi, H., Li, Y., Jia, L., Ding, C., et al. (2014). Association of ABCB1, β tubulin I, and III with multidrug resistance of MCF7/DOC subline from breast cancer cell line MCF7. Tumor Biol. 35, 8883–8891. doi: 10.1007/s13277-014-2101-z
Li, X., Yang, Y., Fang, J., and Zhang, H. (2013). FIZZ1 could enhance the angiogenic ability of rat aortic endothelial cells. Int. J. Clin. Exp. Pathol. 6, 1847–53.
Li, Z., and Rich, J. N. (2010). Hypoxia and hypoxia inducible factors in cancer stem cell maintenance. Curr. Top. Microbiol. Immunol. 345, 21–30. doi: 10.1007/82_2010_75
Lin, H. (2002). The stem-cell niche theory: lessons from flies. Nat. Rev. Genet. 3, 931–940. doi: 10.1038/nrg952
Lin, L., Bass, A. J., Lockwood, W. W., Wang, Z., Silvers, A. L., Thomas, D. G., et al. (2012). Activation of GATA binding protein 6 (GATA6) sustains oncogenic lineage-survival in esophageal adenocarcinoma. Proc. Natl. Acad. Sci. U.S.A. 109, 4251–4256. doi: 10.1073/pnas.1011989109
Liu, D., Zhang, R., Wu, J., Pu, Y., Yin, X., Cheng, Y., et al. (2017). Interleukin-17A promotes esophageal adenocarcinoma cell invasiveness through ROS-dependent, NF-κB-mediated MMP-2/9 activation. Oncol. Rep. 37, 1779–1785. doi: 10.3892/or.2017.5426
Liu, H., Pathak, P., Boehme, S., and Chiang, J. Y. (2016a). Cholesterol 7α-hydroxylase protects the liver from inflammation and fibrosis by maintaining cholesterol homeostasis. J. Lipid Res. 57, 1831–1844. doi: 10.1194/jlr.M069807
Liu, R.-Z., Li, S., Garcia, E., Glubrecht, D. D., Poon, H. Y., Easaw, J. C., et al. (2016b). Association between cytoplasmic CRABP2, altered retinoic acid signaling, and poor prognosis in glioblastoma. Glia 64, 963–976. doi: 10.1002/glia.22976
Liu, S., Yuan, M., Hou, K., Zhang, L., Zheng, X., Zhao, B., et al. (2012). Immune characterization of mesenchymal stem cells in human umbilical cord Wharton's jelly and derived cartilage cells. Cell. Immunol. 278, 35–44. doi: 10.1016/j.cellimm.2012.06.010
Liu, T. Z., Wang, X., Bai, Y. F., Liao, H. Z., Qiu, S. C., Yang, Y. Q., et al. (2014). The HIF-2alpha dependent induction of PAP and adenosine synthesis regulates glioblastoma stem cell function through the A2B adenosine receptor. Int. J. Biochem. Cell Biol. 49, 8–16. doi: 10.1016/j.biocel.2014.01.007
Liu, Y., Lu, R., Gu, J., Chen, Y., Zhang, X., Zhang, L., et al. (2016c). Aldehyde dehydrogenase 1A1 up-regulates stem cell markers in benzo[a]pyrene-induced malignant transformation of BEAS-2B cells. Environ. Toxicol. Pharmacol. 45, 241–250. doi: 10.1016/j.etap.2016.06.007
Loi, S., Pommey, S., Haibe-Kains, B., Beavis, P. A., Darcy, P. K., Smyth, M. J., et al. (2013). CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 11091–11096. doi: 10.1073/pnas.1222251110
Long, H., Xie, R., Xiang, T., Zhao, Z., Lin, S., Liang, Z., et al. (2012). Autocrine CCL5 signaling promotes invasion and migration of CD133+ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells 30, 2309–2319. doi: 10.1002/stem.1194
López-Iglesias, P., Alcaina, Y., Tapia, N., Sabour, D., Arauzo-Bravo, M. J., Sainz de la Maza, D., et al. (2015). Hypoxia induces pluripotency in primordial germ cells by HIF1α stabilization and Oct4 deregulation. Antioxid. Redox Signal. 22, 205–223. doi: 10.1089/ars.2014.5871
Lu, X., and Kang, Y. (2009). Cell fusion as a hidden force in tumor progression. Cancer Res. 69, 8536–8539. doi: 10.1158/0008-5472.CAN-09-2159
Luckhurst, C. A., Ratcliffe, M., Stein, L., Furber, M., Botterell, S., Laughton, D., et al. (2011). Synthesis and biological evaluation of N-alkylated 8-oxybenz[c]azepine derivatives as selective PPARδ agonists. Bioorg. Med. Chem. Lett. 21, 531–536. doi: 10.1016/j.bmcl.2010.10.083
Luna, C., Li, G., Qiu, J., Epstein, D. L., and Gonzalez, P. (2009). Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 15, 2488–2497. Available online at: http://www.molvis.org/molvis/v15/a266/
Lundqvist, A., Sandstedt, M., Sandstedt, J., Wickelgren, R., Hansson, G. I., Jeppsson, A., et al. (2016). The arachidonate 15-lipoxygenase enzyme product 15-HETE is present in heart tissue from patients with ischemic heart disease and enhances clot formation. PLoS ONE 11:e0161629. doi: 10.1371/journal.pone.0161629
Ma, Y., and Liu, D. (2012). Activation of pregnane X receptor by pregnenolone 16 α-carbonitrile prevents high-fat diet-induced obesity in AKR/J mice. PLoS ONE 7:e38734. doi: 10.1371/journal.pone.0038734
Ma, Y., Yu, W., Shrivastava, A., Alemi, F., Lankachandra, K., Srivastava, R. K., et al. (2017). Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway. Carcinogenesis 38, 1047–1056. doi: 10.1093/carcin/bgx070
Mahati, S., Bolati, D., Yang, Y., Mao, R., Zhang, H., and Bao, Y. (2017a). TMPRSS4 promotes cancer stem cell traits by regulating CLDN1 in hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 490, 906–912. doi: 10.1016/j.bbrc.2017.06.139
Mahati, S., Xiao, L., Yang, Y., Mao, R., and Bao, Y. (2017b). miR-29a suppresses growth and migration of hepatocellular carcinoma by regulating CLDN1. Biochem. Biophys. Res. Commun. 486, 732–737. doi: 10.1016/j.bbrc.2017.03.110
Mallard, B. W., and Tiralongo, J. (2017). Cancer stem cell marker glycosylation: nature, function and significance. Glycoconj. J. 34, 441–452. doi: 10.1007/s10719-017-9780-9
Mannan Baig, A., Khan, N. A., Effendi, V., Rana, Z., Ahmad, H. R., and Abbas, F. (2017). Differential receptor dependencies. Anticancer. Drugs 28, 75–87. doi: 10.1097/CAD.0000000000000432
Manzella, N., Bracci, M., Staffolani, S., Strafella, E., Rapisarda, V., Valentino, M., et al. (2013). Styrene altered clock gene expression in serum-shocked cultured human fibroblasts. Biosci. Biotechnol. Biochem. 77, 1296–1298. doi: 10.1271/bbb.120944
Marques, M. R., Horner, J. S., Ihrie, R. A., Bronson, R. T., and Attardi, L. D. (2005). Mice lacking the p53/p63 target gene Perp are resistant to papilloma development. Cancer Res. 65, 6551–6556. doi: 10.1158/0008-5472.CAN-05-0366
Marthiens, V., Kazanis, I., Moss, L., Long, K., and Ffrench-Constant, C. (2010). Adhesion molecules in the stem cell niche - more than just staying in shape? J. Cell Sci. 123, 1613–1622. doi: 10.1242/jcs.054312
Martin, F., Linden, T., Katschinski, D. M., Oehme, F., Flamme, I., Mukhopadhyay, C. K., et al. (2005). Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood 105, 4613–4619. doi: 10.1182/blood-2004-10-3980
Martin, G. R., and Evans, M. J. (1975). Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc. Natl. Acad. Sci. U.S.A. 72, 1441–1445. doi: 10.1073/pnas.72.4.1441
Masood, R., Zhang, Y., Bond, M., Scadden, D., Moudgil, T., Law, R., et al. (1995). Interleukin-10 is an autocrine growth factor for acquired immunodeficiency syndrome-related B-cell lymphoma. Blood 85, 3423–3430. Available online at: http://www.bloodjournal.org/content/85/12/3423.long?sso-checked=true
Mathieu, J., Zhang, Z., Zhou, W., Wang, A. J., Heddleston, J. M., Pinna, C. M., et al. (2011). HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 71, 4640–4652. doi: 10.1158/0008-5472.CAN-10-3320
Matsuo, T., Dat le, T., Komatsu, M., Yoshimaru, T., Daizumoto, K., Sone, S., et al. (2014). Early growth response 4 is involved in cell proliferation of small cell lung cancer through transcriptional activation of its downstream genes. PLoS ONE 9:e113606. doi: 10.1371/journal.pone.0113606
Mattox, D. E., and Von Hoff, D. D. (1980). Culture of human head and neck cancer stem cells using soft agar. Arch. Otolaryngol. 106, 672–674. doi: 10.1001/archotol.1980.00790350014005
Meding, S., Balluff, B., Elsner, M., Schöne, C., Rauser, S., Nitsche, U., et al. (2012). Tissue-based proteomics reveals FXYD3, S100A11 and GSTM3 as novel markers for regional lymph node metastasis in colon cancer. J. Pathol. 228, 459–470. doi: 10.1002/path.4021
Melchiori, A., Colacci, A., Lollini, P. L., De Giovanni, C., Carlone, S., Grilli, S., et al. (1992). Induction of invasive and experimental metastasis potential in BALB/c 3T3 cells by benzo(a)pyrene transformation. Invasion Metastasis 12, 1–11.
Meng, E., Mitra, A., Tripathi, K., Finan, M. A., Scalici, J., McClellan, S., et al. (2014). ALDH1A1 maintains ovarian cancer stem cell-like properties by altered regulation of cell cycle checkpoint and DNA repair network signaling. PLoS ONE 9:e107142. doi: 10.1371/journal.pone.0107142
Mercado-Pimentel, M. E., and Runyan, R. B. (2007). Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 185, 146–156. doi: 10.1159/000101315
Michalec, L., Choudhury, B. K., Postlethwait, E., Wild, J. S., Alam, R., Lett-Brown, M., et al. (2002). CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J. Immunol. 168, 846–52. doi: 10.4049/jimmunol.168.2.846
Mikesch, J.-H., Schier, K., Roetger, A., Simon, R., Buerger, H., and Brandt, B. (2006). The expression and action of decay-accelerating factor (CD55) in human malignancies and cancer therapy. Cell. Oncol. 28, 223–232. doi: 10.1155/2006/814816
Miklos, W., Heffeter, P., Pirker, C., Hager, S., Kowol, C., van Schoonhoven, S., et al. (2016). Loss of phosphodiesterase 4D mediates acquired triapine resistance via Epac-Rap1-Integrin signaling. Oncotarget 7, 84556–84574. doi: 10.18632/oncotarget.11821
Mintz, B., and Illmensee, K. (1975). Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 72, 3585–3589. doi: 10.1073/pnas.72.9.3585
Mishra, A., Wang, J., Shiozawa, Y., McGee, S., Kim, J., Jung, Y., et al. (2012). Hypoxia stabilizes GAS6/Axl signaling in metastatic prostate cancer. Mol. Cancer Res. 10, 703–712. doi: 10.1158/1541-7786.MCR-11-0569
Mitchell, M. I., and Engelbrecht, A. M. (2015). Circadian rhythms and breast cancer: the role of Per2 in doxorubicin-induced cell death. J. Toxicol. 2015:392360. doi: 10.1155/2015/392360
Mittal, K., Donthamsetty, S., Kaur, R., Yang, C., Gupta, M. V., Reid, M. D., et al. (2017). Multinucleated polyploidy drives resistance to Docetaxel chemotherapy in prostate cancer. Br. J. Cancer 116, 1186–1194. doi: 10.1038/bjc.2017.78
Miyagaki, T., Sugaya, M., Murakami, T., Asano, Y., Tada, Y., Kadono, T., et al. (2011). CCL11-CCR3 interactions promote survival of anaplastic large cell lymphoma cells via ERK1/2 activation. Cancer Res. 71, 2056–2065. doi: 10.1158/0008-5472.CAN-10-3764
Mizuno, S., Hanamura, I., Ota, A., Karnan, S., Narita, T., Ri, M., et al. (2015). Overexpression of salivary-type amylase reduces the sensitivity to bortezomib in multiple myeloma cells. Int. J. Hematol. 102, 569–578. doi: 10.1007/s12185-015-1859-0
Mohyeldin, A., Garzón-Muvdi, T., and Quiñones-Hinojosa, A. (2010). Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell 7, 150–161. doi: 10.1016/j.stem.2010.07.007
Monzen, S., Tashiro, E., and Kashiwakura, I. (2011). Megakaryocytopoiesis and thrombopoiesis in hematopoietic stem cells exposed to ionizing radiation. Radiat. Res. 176, 716–724. doi: 10.1667/RR2725.1
Morimura, T., Fujita, K., Akita, M., Nagashima, M., and Satomi, A. (2008). The proton pump inhibitor inhibits cell growth and induces apoptosis in human hepatoblastoma. Pediatr. Surg. Int. 24, 1087–1094. doi: 10.1007/s00383-008-2229-2
Mouillet, J.-F., Donker, R. B., Mishima, T., Cronqvist, T., Chu, T., and Sadovsky, Y. (2013). The unique expression and function of miR-424 in human placental trophoblasts1. Biol. Reprod. 89:25. doi: 10.1095/biolreprod.113.110049
Moulder, J. E., and Rockwell, S. (1987). Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev. 5, 313–341. doi: 10.1007/BF00055376
Murr, C., Fuith, L. C., Widner, B., Wirleitner, B., Baier-Bitterlich, G., and Fuchs, D. (1999). Increased neopterin concentrations in patients with cancer: indicator of oxidative stress? Anticancer Res. 19, 1721–1728.
Nagler, C., Hardt, C., Zänker, K. S., and Dittmar, T. (2011). Co-cultivation of murine BMDCs with 67NR mouse mammary carcinoma cells give rise to highly drug resistant cells. Cancer Cell Int. 11:21. doi: 10.1186/1475-2867-11-21
Nagy, Z., Kovács, I., Török, M., Tóth, D., Vereb, G., Buzás, K., et al. (2014). Function of RasGRP3 in the formation and progression of human breast cancer. Mol. Cancer 13, 96. doi: 10.1186/1476-4598-13-96
Nemmiche, S. (2017). Oxidative signaling response to cadmium exposure. Toxicol. Sci. 156, 4–10. doi: 10.1093/toxsci/kfw222
Netzer, N., Gatterer, H., Faulhaber, M., Burtscher, M., Pramsohler, S., and Pesta, D. (2015). Hypoxia, oxidative stress and fat. Biomolecules 5, 1143–1150. doi: 10.3390/biom5021143
Nomura, M., Yoshimura, Y., Kikuiri, T., Hasegawa, T., Taniguchi, Y., Deyama, Y., et al. (2011). Platinum nanoparticles suppress osteoclastogenesis through scavenging of reactive oxygen species produced in RAW264.7 cells. J. Pharmacol. Sci. 117, 243–252. doi: 10.1254/jphs.11099FP
Nowell, P. C. (1976). The clonal evolution of tumor cell populations. Science 194, 23–28. doi: 10.1126/science.959840
Obianime, A. W., and Roberts, I. I. (2009). Antioxidants, cadmium-induced toxicity, serum biochemical and the histological abnormalities of the kidney and testes of the male Wistar rats. Niger. J. Physiol. Sci. 24, 177–185.
O'Brien, L. E., and Bilder, D. (2013). Beyond the niche: tissue-level coordination of stem cell dynamics. Annu. Rev. Cell Dev. Biol. 29, 107–136. doi: 10.1146/annurev-cellbio-101512-122319
Ogino, T., Kobuchi, H., Fujita, H., Matsukawa, A., and Utsumi, K. (2014). Erythroid and megakaryocytic differentiation of K562 erythroleukemic cells by monochloramine. Free Radic. Res. 48, 292–302. doi: 10.3109/10715762.2013.865840
Ohlstein, B., Kai, T., Decotto, E., and Spradling, A. (2004). The stem cell niche: theme and variations. Curr. Opin. Cell Biol. 16, 693–699. doi: 10.1016/j.ceb.2004.09.003
Ohmura, G., Tsujikawa, T., Yaguchi, T., Kawamura, N., Mikami, S., Sugiyama, J., et al. (2015). Aberrant myosin 1b expression promotes cell migration and lymph node metastasis of HNSCC. Mol. Cancer Res. 13, 721–731. doi: 10.1158/1541-7786.MCR-14-0410
Oikawa, K., Mizusaki, A., Takanashi, M., Ozaki, T., Sato, F., Kuroda, M., et al. (2017). PRG4 expression in myxoid liposarcoma maintains tumor cell growth through suppression of an antitumor cytokine IL-24. Biochem. Biophys. Res. Commun. 485, 209–214. doi: 10.1016/j.bbrc.2017.02.055
Osanai, M., and Lee, G. H. (2015). The retinoic acid-metabolizing enzyme CYP26A1 upregulates fascin and promotes the malignant behavior of breast carcinoma cells. Oncol. Rep. 34, 850–858. doi: 10.3892/or.2015.4042
Osanai, M., Sawada, N., and Lee, G.-H. (2010). Oncogenic and cell survival properties of the retinoic acid metabolizing enzyme, CYP26A1. Oncogene 29, 1135–1144. doi: 10.1038/onc.2009.414
Pacheco, K. A., Tarkowski, M., Sterritt, C., Negri, J., Rosenwasser, L. J., and Borish, L. (2001). The influence of diesel exhaust particles on mononuclear phagocytic cell-derived cytokines: IL-10, TGF-beta and IL-1 beta. Clin. Exp. Immunol. 126, 374–383. doi: 10.1046/j.1365-2249.2001.01698.x
Pant, S. D., March, L. D., Famulski, J. K., French, C. R., Lehmann, O. J., and Waskiewicz, A. J. (2013). Molecular mechanisms regulating ocular apoptosis in zebrafish gdf6a mutants. Investig. Ophthalmol. Vis. Sci. 54, 5871–5879. doi: 10.1167/iovs.12-11315
Papageorgio, C., Brachmann, R., Zeng, J., Culverhouse, R., Zhang, W., and McLeod, H. (2007). MAGED2: a novel p53-dissociator. Int. J. Oncol. 31, 1205–1211. doi: 10.3892/ijo.31.5.1205
Park, Y. H., Sohn, S. K., Kim, J. G., Lee, M. H., Song, H. S., Kim, M. K., et al. (2009). Interaction between BCL2 and interleukin-10 gene polymorphisms alter outcomes of diffuse large B-cell lymphoma following rituximab plus CHOP chemotherapy. Clin. Cancer Res. 15, 2107–2115. doi: 10.1158/1078-0432.CCR-08-1588
Pathak, S. (1990). Cytogenetic abnormalities in cancer: with special emphasis on tumor heterogeneity. Cancer Metastasis Rev. 8, 299–318. doi: 10.1007/BF00052606
Peek, C. B., Levine, D. C., Cedernaes, J., Taguchi, A., Kobayashi, Y., Tsai, S. J., et al. (2017). Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 25, 86–92. doi: 10.1016/j.cmet.2016.09.010
Peet, D. J., Kittipassorn, T., Wood, J. P., Chidlow, G., and Casson, R. J. (2017). HIF signalling: the eyes have it. Exp. Cell Res. 356, 136–140. doi: 10.1016/j.yexcr.2017.03.030
Peeters, S. D., van der Kolk, D. M., de Haan, G., Bystrykh, L., Kuipers, F., de Vries, E. G., et al. (2006). Selective expression of cholesterol metabolism genes in normal CD34+CD38– cells with a heterogeneous expression pattern in AML cells. Exp. Hematol. 34, 622–630. doi: 10.1016/j.exphem.2006.01.020
Peixoto Lira, R. C., Fedatto, P. F., Marco Antonio, D. S., Leal, L. F., Martinelli, C. E., De Castro, M., et al. (2016). IGF2 and IGF1R in pediatric adrenocortical tumors: roles in metastasis and steroidogenesis. Endocr. Relat. Cancer 23, 481–493. doi: 10.1530/ERC-15-0426
Perotti, V., Baldassari, P., Molla, A., Vegetti, C., Bersani, I., Maurichi, A., et al. (2016). NFATc2 is an intrinsic regulator of melanoma dedifferentiation. Oncogene 35, 2862–2872. doi: 10.1038/onc.2015.355
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 1–13. doi: 10.1155/2017/8416763
Plösch, T., Gellhaus, A., Van Straten, E. M., Wolf, N., Huijkman, N. C., Schmidt, M., et al. (2010). The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: a reason for alterations in preeclampsia? Placenta 31, 910–918. doi: 10.1016/j.placenta.2010.07.009
Poormasjedi-Meibod, M. S., Salimi Elizei, S., Leung, V., Baradar Jalili, R., Ko, F., and Ghahary, A. (2016). Kynurenine modulates MMP-1 and type-I collagen expression via aryl hydrocarbon receptor activation in dermal fibroblasts. J. Cell. Physiol. 231, 2749–2760. doi: 10.1002/jcp.25383
Pope, J. L., Bhat, A. A., Sharma, A., Ahmad, R., Krishnan, M., Washington, M. K., et al. (2014). Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut 63, 622–634. doi: 10.1136/gutjnl-2012-304241
Potter, E. A., Dolgova, E. V., Proskurina, A. S., Efremov, Y. M., Minkevich, A. R., Rozanov, A. S., et al. (2017). Gene expression profiling of tumor-initiating stem cells from mouse Krebs-2 carcinoma using a novel marker of poorly differentiated cells. Oncotarget 8, 9425–9441. doi: 10.18632/oncotarget.14116
Potter, E. A., Dolgova, E. V., Proskurina, A. S., Efremov, Y. R., Taranov, O. S., Nikolin, V. P., et al. (2016a). Development of the therapeutic regimen based on the synergistic activity of cyclophosphamide and doublestranded DNA preparation which results in complete cure of mice engrafted with Krebs-2 ascites. Vavilov J. Genet. Breed. 20, 723–735. doi: 10.18699/VJ16.162
Potter, E. A., Dolgova, E. V., Proskurina, A. S., Minkevich, A. M., Efremov, Y. R., Taranov, O. S., et al. (2016b). A strategy to eradicate well-developed Krebs-2 ascites in mice. Oncotarget 7, 11580–11594. doi: 10.18632/oncotarget.7311
Potter, E. A., Proskurina, A. S., Ritter, G. S., Dolgova, E. V., Nikolin, V. P., Popova, N. A., et al. (2018). Efficacy of a new cancer treatment strategy based on eradication of tumor-initiating stem cells in a mouse model of Krebs-2 solid adenocarcinoma. Oncotarget 9, 28486–28499. doi: 10.18632/oncotarget.25503
Powers, G. L., Hammer, K. D., Domenech, M., Frantskevich, K., Malinowski, R. L., Bushman, W., et al. (2015). Phosphodiesterase 4D inhibitors limit prostate cancer growth potential. Mol. Cancer Res. 13, 149–160. doi: 10.1158/1541-7786.MCR-14-0110
Prevo, R., Banerji, S., Ferguson, D. J., Clasper, S., and Jackson, D. G. (2001). Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J. Biol. Chem. 276, 19420–19430. doi: 10.1074/jbc.M011004200
Pullamsetti, S. S., Banat, G. A., Schmall, A., Szibor, M., Pomagruk, D., Hänze, J., et al. (2013). Phosphodiesterase-4 promotes proliferation and angiogenesis of lung cancer by crosstalk with HIF. Oncogene 32, 1121–1134. doi: 10.1038/onc.2012.136
Puri, N., Sodhi, K., Haarstad, M., Kim, D. H., Bohinc, S., Foglio, E., et al. (2012). Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J. Cell. Biochem. 113, 1926–1935. doi: 10.1002/jcb.24061
Qin, Z., Dai, L., Bratoeva, M., Slomiany, M. G., Toole, B. P., and Parsons, C. (2011). Cooperative roles for emmprin and LYVE-1 in the regulation of chemoresistance for primary effusion lymphoma. Leukemia 25, 1598–1609. doi: 10.1038/leu.2011.144
Quan, Y., Zhang, X., Xu, S., Li, K., Zhu, F., Li, Q., et al. (2016). Tcf7l2 localization of putative stem/progenitor cells in mouse conjunctiva. Am. J. Physiol. Physiol. 311, C246–C254. doi: 10.1152/ajpcell.00014.2016
Ramírez-Ortega, D., Ramiro-Salazar, A., González-Esquivel, D., Ríos, C., Pineda, B., and Pérez de la Cruz, V. (2017). 3-Hydroxykynurenine and 3-hydroxyanthranilic acid enhance the toxicity induced by copper in rat astrocyte culture. Oxid. Med. Cell. Longev. 2017, 1–12. doi: 10.1155/2017/2371895
Ravindranath, A., O'Connell, A., Johnston, P. G., and El-Tanani, M. K. (2008). The role of LEF/TCF factors in neoplastic transformation. Curr. Mol. Med. 8, 38–50. doi: 10.2174/156652408783565559
Reya, T., Morrison, S. J., Clarke, M. F., and Weissman, I. L. (2001). Stem cells, cancer, and cancer stem cells. Nature 414, 105–111. doi: 10.1038/35102167
Ricciardi, A., Elia, A. R., Cappello, P., Puppo, M., Vanni, C., Fardin, P., et al. (2008). Transcriptome of hypoxic immature dendritic cells: modulation of chemokine/receptor expression. Mol. Cancer Res. 6, 175–185. doi: 10.1158/1541-7786.MCR-07-0391
Rich, J. N. (2016). Cancer stem cells: understanding tumor hierarchy and heterogeneity. Medicine 95, S2–S7. doi: 10.1097/MD.0000000000004764
Rosinski, K. V., Fujii, N., Mito, J. K., Koo, K. K. W., Xuereb, S. M., Sala-Torra, O., et al. (2008). DDX3Y encodes a class I MHC-restricted H-Y antigen that is expressed in leukemic stem cells. Blood 111, 4817–4826. doi: 10.1182/blood-2007-06-096313
Roszak, J., Smok-Pieniazek, A., Nocun, M., and Stepnik, M. (2013). Characterization of arsenic trioxide resistant clones derived from Jurkat leukemia T cell line: focus on PI3K/Akt signaling pathway. Chem. Biol. Interact. 205, 198–211. doi: 10.1016/j.cbi.2013.07.011
Royer, C., Lachuer, J., Crouzoulon, G., Roux, J., Peyronnet, J., Mamet, J., et al. (2000). Effects of gestational hypoxia on mRNA levels of Glut3 and Glut4 transporters, hypoxia inducible factor-1 and thyroid hormone receptors in developing rat brain. Brain Res. 856, 119–128. doi: 10.1016/S0006-8993(99)02365-3
Sabe, H., Hashimoto, S., Morishige, M., Ogawa, E., Hashimoto, A., Nam, J.-M., et al. (2009). The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast cancer invasion and metastasis. Traffic 10, 982–993. doi: 10.1111/j.1600-0854.2009.00917.x
Saijo, H., Hirohashi, Y., Torigoe, T., Horibe, R., Takaya, A., Murai, A., et al. (2016). Plasticity of lung cancer stem-like cells is regulated by the transcription factor HOXA5 that is induced by oxidative stress. Oncotarget 7, 50043–50056. doi: 10.18632/oncotarget.10571
Sáinz, N., Rodríguez, A., Catalán, V., Becerril, S., Ramírez, B., Gómez-Ambrosi, J., et al. (2010). Leptin administration downregulates the increased expression levels of genes related to oxidative stress and inflammation in the skeletal muscle of ob/ob mice. Mediators Inflamm. 2010:784343. doi: 10.1155/2010/784343
Sakai, E., Morita, M., Ohuchi, M., Kido, M. A., Fukuma, Y., Nishishita, K., et al. (2017). Effects of deficiency of Kelch-like ECH-associated protein 1 on skeletal organization: a mechanism for diminished nuclear factor of activated T cells cytoplasmic 1 during osteoclastogenesis. FASEB J. 31, 4011–4022. doi: 10.1096/fj.201700177R
Sakamoto, Y., Prudhomme, S., and Zaman, M. H. (2011). Viscoelastic gel-strip model for the simulation of migrating cells. Ann. Biomed. Eng. 39, 2735–2749. doi: 10.1007/s10439-011-0360-z
Salyer, S. A., Olberding, J. R., Distler, A. A., Lederer, E. D., Clark, B. J., Delamere, N. A., et al. (2013). Vacuolar ATPase driven potassium transport in highly metastatic breast cancer cells. Biochim. Biophys. Acta-Mol. Basis Dis. 1832, 1734–1743. doi: 10.1016/j.bbadis.2013.04.023
Sasaki, H., Shitara, M., Yokota, K., Hikosaka, Y., Moriyama, S., Yano, M., et al. (2012). RagD gene expression and NRF2 mutations in lung squamous cell carcinomas. Oncol. Lett. 4, 1167–1170. doi: 10.3892/ol.2012.938
Saygin, C., Wiechert, A., Rao, V. S., Alluri, R., Connor, E., Thiagarajan, P. S., et al. (2017). CD55 regulates self-renewal and cisplatin resistance in endometrioid tumors. J. Exp. Med. 214, 2715–2732. doi: 10.1084/jem.20170438
Schofield, R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25.
Seaborn, T., Ravni, A., Au, R., Chow, B. K., Fournier, A., Wurtz, O., et al. (2014). Induction of serpinb1a by PACAP or NGF is required for PC12 cells survival after serum withdrawal. J. Neurochem. 131, 21–32. doi: 10.1111/jnc.12780
Seo, E. J., Kim, D. K., Jang, I. H., Choi, E. J., Shin, S. H., Lee, S. I., et al. (2016). Hypoxia-NOTCH1-SOX2 signaling is important for maintaining cancer stem cells in ovarian cancer. Oncotarget 7, 55624–55638. doi: 10.18632/oncotarget.10954
Shen, D., and Wang, Y. (1994). Effects of hypoxia on platelet activation in pilots. Aviat. Space. Environ. Med. 65, 646–648.
Shen, D., and Wang, Y. (1998). Changes of plasma level of neurotensin, somatostatin, and dynorphin A in pilots under acute hypoxia. Mil. Med. 163, 120–121. doi: 10.1093/milmed/163.2.120
Shitashige, M., Hirohashi, S., and Yamada, T. (2008). Wnt signaling inside the nucleus. Cancer Sci. 99, 631–637. doi: 10.1111/j.1349-7006.2007.00716.x
Shojima, K., Sato, A., Hanaki, H., Tsujimoto, I., Nakamura, M., Hattori, K., et al. (2015). Wnt5a promotes cancer cell invasion and proliferation by receptor-mediated endocytosis-dependent and -independent mechanisms, respectively. Sci. Rep. 5:8042. doi: 10.1038/srep08042
Sies, H., Berndt, C., and Jones, D. P. (2017). Oxidative stress. Annu. Rev. Biochem. 86, 715–748. doi: 10.1146/annurev-biochem-061516-045037
Sithu, S. D., Srivastava, S., Siddiqui, M. A., Vladykovskaya, E., Riggs, D. W., Conklin, D. J., et al. (2010). Exposure to acrolein by inhalation causes platelet activation. Toxicol. Appl. Pharmacol. 248, 100–110. doi: 10.1016/j.taap.2010.07.013
Slevin, M., Krupinski, J., Rovira, N., Turu, M., Luque, A., Baldellou, M., et al. (2009). Identification of pro-angiogenic markers in blood vessels from stroked-affected brain tissue using laser-capture microdissection. BMC Genomics 10:113. doi: 10.1186/1471-2164-10-113
Song, K., Kwon, H., Han, C., Zhang, J., Dash, S., Lim, K., et al. (2015). Active glycolytic metabolism in CD133(+) hepatocellular cancer stem cells: regulation by MIR-122. Oncotarget 6, 40822–40835. doi: 10.18632/oncotarget.5812
Souroullas, G. P., Salmon, J. M., Sablitzky, F., Curtis, D. J., and Goodell, M. A. (2009). Adult hematopoietic stem and progenitor cells require either Lyl1 or Scl for survival. Cell Stem Cell 4, 180–186. doi: 10.1016/j.stem.2009.01.001
Sousa, M. S., Latini, F. R., Monteiro, H. P., and Cerutti, J. M. (2010). Arginase 2 and nitric oxide synthase: pathways associated with the pathogenesis of thyroid tumors. Free Radic. Biol. Med. 49, 997–1007. doi: 10.1016/j.freeradbiomed.2010.06.006
Stanford, E. A., Wang, Z., Novikov, O., Mulas, F., Landesman-Bollag, E., Monti, S., et al. (2016). The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 14:20. doi: 10.1186/s12915-016-0240-y
Steidl, U., Schroeder, T., Steidl, C., Kobbe, G., Graef, T., Bork, S., et al. (2005). Distinct gene expression pattern of malignant hematopoietic stem and progenitor cells in polycythemia vera. Ann. N. Y. Acad. Sci. 1044, 94–108. doi: 10.1196/annals.1349.013
Strzalka-Mrozik, B., Prudlo, L., Kimsa, M. W., Kimsa, M. C., Kapral, M., Nita, M., et al. (2013). Quantitative analysis of SOD2, ALDH1A1 and MGST1 messenger ribonucleic acid in anterior lens epithelium of patients with pseudoexfoliation syndrome. Mol. Vis. 19, 1341–1349. Available online at: http://www.molvis.org/molvis/v19/1341/
Stübke, K., Wicklein, D., Herich, L., Schumacher, U., and Nehmann, N. (2012). Selectin-deficiency reduces the number of spontaneous metastases in a xenograft model of human breast cancer. Cancer Lett. 321, 89–99. doi: 10.1016/j.canlet.2012.02.019
Su, B., Zhao, W., Shi, B., Zhang, Z., Yu, X., Xie, F., et al. (2014). Let-7d suppresses growth, metastasis, and tumor macrophage infiltration in renal cell carcinoma by targeting COL3A1 and CCL7. Mol. Cancer 13:206. doi: 10.1186/1476-4598-13-206
Suga, N., Murakami, A., Nakamura, Y., Ishisaka, A., Kitamoto, N., Ito, M., et al. (2017). Cytotoxic and cytoprotective effects of tryptamine-4,5-dione on neuronal cells: a double-edged sword. Free Radic. Res. 51, 545–553. doi: 10.1080/10715762.2017.1331038
Sullivan, B. P., Cui, W., Copple, B. L., and Luyendyk, J. P. (2012). Early growth response factor-1 limits biliary fibrosis in a model of xenobiotic-induced cholestasis in mice. Toxicol. Sci. 126, 267–274. doi: 10.1093/toxsci/kfr311
Sun, D. X., Liao, G. J., Liu, K. G., and Jian, H. (2015). Endosialin-expressing bone sarcoma stem-like cells are highly tumor-initiating and invasive. Mol. Med. Rep. 12, 5665–5670. doi: 10.3892/mmr.2015.4218
Sun, M., Zhou, W., Zhang, Y. Y., Wang, D. L., and Wu, X. L. (2013). CD44+ gastric cancer cells with stemness properties are chemoradioresistant and highly invasive. Oncol. Lett. 5, 1793–1798. doi: 10.3892/ol.2013.1272
Sun, Y., Du, C., Wang, B., Zhang, Y., Liu, X., and Ren, G. (2014). Up-regulation of eEF1A2 promotes proliferation and inhibits apoptosis in prostate cancer. Biochem. Biophys. Res. Commun. 450, 1–6. doi: 10.1016/j.bbrc.2014.05.045
Süsskind, D., Hurst, J., Rohrbach, J. M., and Schnichels, S. (2017). Novel mouse model for primary uveal melanoma: a pilot study. Clin. Experiment. Ophthalmol. 45, 192–200. doi: 10.1111/ceo.12814
Taddei, M. L., Giannoni, E., Fiaschi, T., and Chiarugi, P. (2012). Anoikis: an emerging hallmark in health and diseases. J. Pathol. 226, 380–393. doi: 10.1002/path.3000
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. doi: 10.1016/j.cell.2006.07.024
Takano, M., Meneshian, A., Sheikh, E., Yamakawa, Y., Wilkins, K. B., Hopkins, E. A., et al. (2002). Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am. J. Physiol. Circ. Physiol. 283, H2054–H2061. doi: 10.1152/ajpheart.01001.2001
Takubo, K., Nagamatsu, G., Kobayashi, C. I., Nakamura-Ishizu, A., Kobayashi, H., Ikeda, E., et al. (2013). Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12, 49–61. doi: 10.1016/j.stem.2012.10.011
Tan, J. E., Wong, S. C., Gan, S. K., Xu, S., and Lam, K. P. (2001). The adaptor protein BLNK is required for b cell antigen receptor-induced activation of nuclear factor-kappa B and cell cycle entry and survival of B lymphocytes. J. Biol. Chem. 276, 20055–20063. doi: 10.1074/jbc.M010800200
Tang, J. P., Tan, C. P., Li, J., Siddique, M. M., Guo, K., Chan, S. W., et al. (2010). VHZ is a novel centrosomal phosphatase associated with cell growth and human primary cancers. Mol. Cancer 9:128. doi: 10.1186/1476-4598-9-128
Tang, X., Mahajan, S. S., Nguyen, L. T., Béliveau, F., Leduc, R., Simon, J. A., et al. (2014). Targeted inhibition of cell-surface serine protease Hepsin blocks prostate cancer bone metastasis. Oncotarget 5, 1352–1362. doi: 10.18632/oncotarget.1817
Taoka, Y., Matsumoto, K., Ohashi, K., Minamida, S., Hagiwara, M., Nagi, S., et al. (2015). Protein expression profile related to cisplatin resistance in bladder cancer cell lines detected by two-dimensional gel electrophoresis. Biomed. Res. 36, 253–261. doi: 10.2220/biomedres.36.253
Thiel, G., and Cibelli, G. (2002). Regulation of life and death by the zinc finger transcription factor Egr-1. J. Cell. Physiol. 193, 287–292. doi: 10.1002/jcp.10178
Tominaga, K., Shimamura, T., Kimura, N., Murayama, T., Matsubara, D., Kanauchi, H., et al. (2017). Addiction to the IGF2-ID1-IGF2 circuit for maintenance of the breast cancer stem-like cells. Oncogene 36, 1276–1286. doi: 10.1038/onc.2016.293
Tonnetti, L., Netzel-Arnett, S., Darnell, G. A., Hayes, T., Buzza, M. S., Anglin, I. E., et al. (2008). SerpinB2 protection of retinoblastoma protein from calpain enhances tumor cell survival. Cancer Res. 68, 5648–5657. doi: 10.1158/0008-5472.CAN-07-5850
Touyz, R. M. (2014). Linking LOX-1 and arginase II through mitochondria: a novel paradigm in endothelial dysfunction. Circ. Res. 115, 412–414. doi: 10.1161/CIRCRESAHA.114.304550
Tritschler, I., Gramatzki, D., Capper, D., Mittelbronn, M., Meyermann, R., Saharinen, J., et al. (2009). Modulation of TGF-β activity by latent TGF-β-binding protein 1 in human malignant glioma cells. Int. J. Cancer 125, 530–540. doi: 10.1002/ijc.24443
Tsai, W.-B., Long, Y., Park, J.-R., Chang, J. T., Liu, H., Rodriguez-Canales, J., et al. (2016). Gas6/Axl is the sensor of arginine-auxotrophic response in targeted chemotherapy with arginine-depleting agents. Oncogene 35, 1632–1642. doi: 10.1038/onc.2015.237
Tuccitto, A., Tazzari, M., Beretta, V., Rini, F., Miranda, C., Greco, A., et al. (2016). Immunomodulatory factors control the fate of melanoma tumor initiating cells. Stem Cells 34, 2449–2460. doi: 10.1002/stem.2413
Tucker, R. P., Ferralli, J., Schittny, J. C., and Chiquet-Ehrismann, R. (2013). Tenascin-C and tenascin-W in whisker follicle stem cell niches: possible roles in regulating stem cell proliferation and migration. J. Cell Sci. 126, 5111–5115. doi: 10.1242/jcs.134650
Tye, S. L., Gilg, A. G., Tolliver, L. B., Wheeler, W. G., Toole, B. P., and Maria, B. L. (2008). Hyaluronan regulates ceruloplasmin production by gliomas and their treatment-resistant multipotent progenitors. J. Child Neurol. 23, 1221–1230. doi: 10.1177/0883073808321066
van Lee, W. H., Choong, L. Y., Jin, T. H., Mon, N. N., Chong, S., Liew, C. S., et al. (2017b). TRPV4 plays a role in breast cancer cell migration via Ca2+-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis 6:e338. doi: 10.1038/oncsis.2017.39
Van Thienen, R., Masschelein, E., D'Hulst, G., Thomis, M., and Hespel, P. (2017). Twin resemblance in muscle HIF-1α responses to hypoxia and exercise. Front. Physiol. 7:676. doi: 10.3389/fphys.2016.00676
Vias, M., Burtt, G., Culig, Z., Veerakumarasivam, A., Neal, D. E., and Mills, I. G. (2007). A role for neurotensin in bicalutamide resistant prostate cancer cells. Prostate 67, 190–202. doi: 10.1002/pros.20518
Victorino, V. J., Pizzatti, L., Michelletti, P., and Panis, C. (2014). Oxidative stress, redox signaling and cancer chemoresistance: putting together the pieces of the puzzle. Curr. Med. Chem. 21, 3211–3226. doi: 10.2174/0929867321666140601164647
Vo, T. K. D., de Saint-Hubert, M., Morrhaye, G., Godard, P., Geenen, V., Martens, H. J., et al. (2011). Transcriptomic biomarkers of the response of hospitalized geriatric patients admitted with heart failure. Comparison to hospitalized geriatric patients with infectious diseases or hip fracture. Mech. Ageing Dev. 132, 131–139. doi: 10.1016/j.mad.2011.02.002
Vogiatzi, F., Brandt, D. T., Schneikert, J., Fuchs, J., Grikscheit, K., Wanzel, M., et al. (2016). Mutant p53 promotes tumor progression and metastasis by the endoplasmic reticulum UDPase ENTPD5. Proc. Natl. Acad. Sci. U.S.A. 113, E8433–E8442. doi: 10.1073/pnas.1612711114
Voog, J., and Jones, D. L. (2010). Stem cells and the niche: a dynamic duo. Cell Stem Cell 6, 103–115. doi: 10.1016/j.stem.2010.01.011
Wahba, M. G., Messiha, B. A., and Abo-Saif, A. A. (2016). Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. Pharm. Biol. 54, 1705–1715. doi: 10.3109/13880209.2015.1125931
Wan, J., Badham, H. J., and Winn, L. (2005). The role of c-MYB in benzene-initiated toxicity. Chem. Biol. Interact. 153–154, 171–178. doi: 10.1016/j.cbi.2005.03.037
Wang, C., Jin, H., Wang, N., Fan, S., Wang, Y., Zhang, Y., et al. (2016a). Gas6/Axl axis contributes to chemoresistance and metastasis in breast cancer through Akt/GSK-3β/β- catenin signaling. Theranostics 6, 1205–1219. doi: 10.7150/thno.15083
Wang, J., Liu, X., Li, T., Liu, C., and Zhao, Y. (2011). Increased hepatic Igf2 gene expression involves C/EBPβ in TCDD-induced teratogenesis in rats. Reprod. Toxicol. 32, 313–321. doi: 10.1016/j.reprotox.2011.06.117
Wang, J., Nikhil, K., Viccaro, K., Chang, L., White, J., and Shah, K. (2017). Phosphorylation-dependent regulation of ALDH1A1 by Aurora kinase A: insights on their synergistic relationship in pancreatic cancer. BMC Biol. 15:10. doi: 10.1186/s12915-016-0335-5
Wang, J., Zhu, Z., Huang, Y., Wang, P., Luo, Y., Gao, Y., et al. (2014). The subtype CD200-positive, chorionic mesenchymal stem cells from the placenta promote regeneration of human hepatocytes. Biotechnol. Lett. 36, 1335–1341. doi: 10.1007/s10529-014-1468-7
Wang, L., Zhou, X., Zhou, T., Ma, D., Chen, S., Zhi, X., et al. (2008). Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J. Cancer Res. Clin. Oncol. 134, 365–372. doi: 10.1007/s00432-007-0292-z
Wang, P., Xu, J., Hou, Z., Wang, F., Song, Y., Wang, J., et al. (2016b). miRNA-34a promotes proliferation of human pulmonary artery smooth muscle cells by targeting PDGFRA. Cell Prolif. 49, 484–493. doi: 10.1111/cpr.12265
Wang, Q., Ao, Y., Yang, K., Tang, H., and Chen, D. (2016c). Circadian clock gene Per2 plays an important role in cell proliferation, apoptosis and cell cycle progression in human oral squamous cell carcinoma. Oncol. Rep. 35, 3387–3394. doi: 10.3892/or.2016.4724
Wang, X., Yang, J., Qian, J., Liu, Z., Chen, H., and Cui, Z. (2015). S100A14, a mediator of epithelial-mesenchymal transition, regulates proliferation, migration and invasion of human cervical cancer cells. Am. J. Cancer Res. 5, 1484–1495.
Wang, Z., Li, K., Guo, X., Li, X., Bu, Y., Bai, X., et al. (2016d). The prognostic roles of ALDH1 isoenzymes in gastric cancer. Onco. Targets. Ther. 9, 3405–3414. doi: 10.2147/OTT.S102314
Wechsler-Reya, R., and Scott, M. P. (2001). The developmental biology of brain tumors. Annu. Rev. Neurosci. 24, 385–428. doi: 10.1146/annurev.neuro.24.1.385
Wechsler-Reya, R. J., and Scott, M. P. (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103–114. doi: 10.1016/S0896-6273(00)80682-0
Wen, F., Curlango-Rivera, G., Huskey, D. A., Xiong, Z., and Hawes, M. C. (2017). Visualization of extracellular DNA released during border cell separation from the root cap. Am. J. Bot. 104, 970–978. doi: 10.3732/ajb.1700142
Westcott, J. M., Prechtl, A. M., Maine, E. A., Dang, T. T., Esparza, M. A., Sun, H., et al. (2015). An epigenetically distinct breast cancer cell subpopulation promotes collective invasion. J. Clin. Invest. 125, 1927–1943. doi: 10.1172/JCI77767
Whetton, A. D., and Graham, G. J. (1999). Homing and mobilization in the stem cell niche. Trends Cell Biol. 9, 233–238. doi: 10.1016/S0962-8924(99)01559-7
Whissell, G., Montagni, E., Martinelli, P., Hernando-Momblona, X., Sevillano, M., Jung, P., et al. (2014). The transcription factor GATA6 enables self-renewal of colon adenoma stem cells by repressing BMP gene expression. Nat. Cell Biol. 16, 695–707. doi: 10.1038/ncb2992
Wigner, P., Czarny, P., Galecki, P., Su, K. P., and Sliwinski, T. (2018). The molecular aspects of oxidative and nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res. 262, 566–574. doi: 10.1016/j.psychres.2017.09.045
Wohlfahrt-Veje, C., Audouze, K., Brunak, S., Antignac, J. P., le Bizec, B., Juul, A., et al. (2014). Polychlorinated dibenzo-p-dioxins, furans, and biphenyls (PCDDs/PCDFs and PCBs) in breast milk and early childhood growth and IGF1. Reproduction 147, 391–399. doi: 10.1530/REP-13-0422
Wozniak, M., Duś-Szachniewicz, K., and Ziółkowski, P. (2015). Insulin-like growth factor-2 is induced following 5-aminolevulinic acid-mediated photodynamic therapy in SW620 human colon cancer cell line. Int. J. Mol. Sci. 16, 23615–23629. doi: 10.3390/ijms161023615
Wu, H.-H., Hwang-Verslues, W. W., Lee, W.-H., Huang, C.-K., Wei, P.-C., Chen, C.-L., et al. (2015). Targeting IL-17B–IL-17RB signaling with an anti–IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med. 212, 333–349. doi: 10.1084/jem.20141702
Wu, Q.-F., Qian, C., Zhao, N., Dong, Q., Li, J., Wang, B.-B., et al. (2017). Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes. Cell Death Dis. 8:e2828. doi: 10.1038/cddis.2017.227
Xiao, G., Cheng, H., Cao, H., Chen, K., Tu, Y., Yu, S., et al. (2012). Critical role of filamin-binding LIM protein 1 (FBLP-1)/migfilin in regulation of bone remodeling. J. Biol. Chem. 287, 21450–21460. doi: 10.1074/jbc.M111.331249
Xin, H., Lu, R., Lee, H., Zhang, W., Zhang, C., Deng, J., et al. (2013). G-protein-coupled receptor agonist BV8/prokineticin-2 and STAT3 protein form a feed-forward loop in both normal and malignant myeloid cells. J. Biol. Chem. 288, 13842–13849. doi: 10.1074/jbc.M113.450049
Xing, P., Li, J. G., Jin, F., Zhao, T. T., Liu, Q., Dong, H. T., et al. (2011). Clinical and biological significance of hepsin overexpression in breast cancer. J. Investig. Med. 59, 803–810. doi: 10.2310/JIM.0b013e31821451a1
Xu, C., Hu, D. M., and Zhu, Q. (2013). eEF1A2 promotes cell migration, invasion and metastasis in pancreatic cancer by upregulating MMP-9 expression through Akt activation. Clin. Exp. Metastasis 30, 933–944. doi: 10.1007/s10585-013-9593-6
Xu, L., Wang, X., Wang, J., Liu, D., Wang, Y., Huang, Z., et al. (2016). Hypoxia-induced secretion of IL-10 from adipose-derived mesenchymal stem cell promotes growth and cancer stem cell properties of Burkitt lymphoma. Tumor Biol. 37, 7835–7842. doi: 10.1007/s13277-015-4664-8
Xu, Y., Saegusa, C., Schehr, A., Grant, S., Whitsett, J. A., and Ikegami, M. (2009). C/EBPα is required for pulmonary cytoprotection during hyperoxia. Am. J. Physiol. Cell. Mol. Physiol. 297, L286–L298. doi: 10.1152/ajplung.00094.2009
Xuan, X., Li, S., Lou, X., Zheng, X., Li, Y., Wang, F., et al. (2015). Stat3 promotes invasion of esophageal squamous cell carcinoma through up-regulation of MMP2. Mol. Biol. Rep. 42, 907–915. doi: 10.1007/s11033-014-3828-8
Yang, B., Wagner, J., Damaschke, N., Yao, T., Wuerzberger-Davis, S. M., Lee, M. H., et al. (2014a). A novel pathway links oxidative stress to loss of insulin growth factor-2 (IGF2) imprinting through NF-κB activation. PLoS ONE 9:e88052. doi: 10.1371/journal.pone.0088052
Yang, C.-S., Chang, K.-Y., and Rana, T. M. (2014b). Genome-wide functional analysis reveals factors needed at the transition steps of induced reprogramming. Cell Rep. 8, 327–337. doi: 10.1016/j.celrep.2014.07.002
Yang, L., Ren, Y., Yu, X., Qian, F., Bian, B.-S., Xiao, H. L., et al. (2014c). ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod. Pathol. 27, 775–783. doi: 10.1038/modpathol.2013.189
Yang, Q., Sun, M., Ramchandran, R., and Raj, J. U. (2015). IGF-1 signaling in neonatal hypoxia-induced pulmonary hypertension: role of epigenetic regulation. Vascul. Pharmacol. 73, 20–31. doi: 10.1016/j.vph.2015.04.005
Ye, Y., Long, X., Zhang, L., Chen, J., Liu, P., Li, H., et al. (2014). NTS/NTR1 co-expression enhances epithelial-to-mesenchymal transition and promotes tumor metastasis by activating the Wnt/β-catenin signaling pathway in hepatocellular carcinoma. Oncotarget 7, 70303–70322. doi: 10.18632/oncotarget.11854
Yelloly, J. (1809). A case of tumour in the brain, with remarks on the propagation of nervous influence. Med. Chir. Trans. 1, 183–223. doi: 10.1177/095952870900100116
Yeo, C. D., Kim, Y. A., Lee, H. Y., Kim, J. W., Kim, S. J., Lee, S. H., et al. (2017). Roflumilast treatment inhibits lung carcinogenesis in benzo(a)pyrene-induced murine lung cancer model. Eur. J. Pharmacol. 812, 189–195. doi: 10.1016/j.ejphar.2017.07.004
Yin, H., Li, C., Wang, S., Guo, Q., Ren, X., and Jiang, G. (2015). Silencing of CD59 enhanced the sensitivity of HT29 cells to 5-Fluorouracil and Oxaliplatin. J. Infect. Chemother. 21, 8–15. doi: 10.1016/j.jiac.2014.08.020
Yin, J., Fu, W., Dai, L., Jiang, Z., Liao, H., Chen, W., et al. (2017). ANKRD22 promotes progression of non-small cell lung cancer through transcriptional up-regulation of E2F1. Sci. Rep. 7:4430. doi: 10.1038/s41598-017-04818-y
You, Q., Wu, Y., Yao, N., Shen, G., Zhang, Y., Xu, L., et al. (2015). Interaction of AIM with insulin-like growth factor-binding protein-4. Int. J. Mol. Med. 36, 833–838. doi: 10.3892/ijmm.2015.2262
Zeng, L., O'Connor, C., Zhang, J., Kaplan, A. M., and Cohen, D. A. (2010). IL-10 promotes resistance to apoptosis and metastatic potential in lung tumor cell lines. Cytokine 49, 294–302. doi: 10.1016/j.cyto.2009.11.015
Zeng, X., Hu, Z., Wang, Z., Tao, J., Lu, T., Yang, C., et al. (2014). Upregulation of RASGRP3 expression in prostate cancer correlates with aggressive capabilities and predicts biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 17, 119–125. doi: 10.1038/pcan.2013.51
Zhan, L., Yang, Y., Ma, T.-T., Huang, C., Meng, X.-M., Zhang, L., et al. (2015). Transient receptor potential vanilloid 4 inhibits rat HSC-T6 apoptosis through induction of autophagy. Mol. Cell. Biochem. 402, 9–22. doi: 10.1007/s11010-014-2298-6
Zhang, J., Na, S., Liu, C., Pan, S., Cai, J., and Qiu, J. (2016a). MicroRNA-125b suppresses the epithelial–mesenchymal transition and cell invasion by targeting ITGA9 in melanoma. Tumor Biol. 37, 5941–5949. doi: 10.1007/s13277-015-4409-8
Zhang, J., Wang, X., Vikash, V., Ye, Q., Wu, D., Liu, Y., et al. (2016b). ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 1–18. doi: 10.1155/2016/4350965
Zhang, X.-H., Qian, Y., Li, Z., Zhang, N.-N., and Xie, Y.-J. (2016c). Let-7g-5p inhibits epithelial-mesenchymal transition consistent with reduction of glioma stem cell phenotypes by targeting VSIG4 in glioblastoma. Oncol. Rep. 36, 2967–2975. doi: 10.3892/or.2016.5098
Zhang, Y., and Kalderon, D. (2001). Hedgehog acts as a somatic stem cell factor in the Drosophila ovary. Nature 410, 599–604. doi: 10.1038/35069099
Zhang, Y., Sui, F., Ma, J., Ren, X., Guan, H., Yang, Q., et al. (2017). Positive feedback loops between NrCAM and major signaling pathways contribute to thyroid tumorigenesis. J. Clin. Endocrinol. Metab. 102, 613–624. doi: 10.1210/jc.2016-1677
Zhang, Y., Zhang, Y., Geng, L., Yi, H., Huo, W., Talmon, G., et al. (2016d). Transforming growth factor β mediates drug resistance by regulating the expression of pyruvate dehydrogenase kinase 4 in colorectal cancer. J. Biol. Chem. 291, 17405–17416. doi: 10.1074/jbc.M116.713735
Zhao, J., Zhang, Y., Ithychanda, S. S., Tu, Y., Chen, K., Qin, J., et al. (2009). Migfilin interacts with Src and contributes to cell-matrix adhesion-mediated survival signaling. J. Biol. Chem. 284, 34308–34320. doi: 10.1074/jbc.M109.045021
Zhao, L.-R., Du, Y.-J., Chen, L., Liu, Z.-G., Jia, X.-Y., Pan, Y.-H., et al. (2015). Omentin-1 promotes the growth of neural stem cells via activation of Akt signaling. Mol. Med. Rep. 11, 1859–1864. doi: 10.3892/mmr.2014.2937
Zhao, W., Prijic, S., Urban, B. C., Tisza, M. J., Zuo, Y., Li, L., et al. (2016). Candidate antimetastasis drugs suppress the metastatic capacity of breast cancer cells by reducing membrane fluidity. Cancer Res. 76, 2037–2049. doi: 10.1158/0008-5472.CAN-15-1970
Zhao, Z., Li, J., Jiang, Y., Xu, W., Li, X., and Jing, W. (2017). CLDN1 increases grug resistance of non-small cell lung cancer by activating autophagy via up-regulation of ULK1 phosphorylation. Med. Sci. Monit. 23, 2906–2916. doi: 10.12659/MSM.904177
Zheng, Y., Yang, J., Qian, J., Qiu, P., Hanabuchi, S., Lu, Y., et al. (2013). PSGL-1/selectin and ICAM-1/CD18 interactions are involved in macrophage-induced drug resistance in myeloma. Leukemia 27, 702–710. doi: 10.1038/leu.2012.272
Zhou, J., Yi, L., Ouyang, Q., Xu, L., Cui, H., and Xu, M. (2014). Neurotensin signaling regulates stem-like traits of glioblastoma stem cells through activation of IL-8/CXCR1/STAT3 pathway. Cell. Signal. 26, 2896–2902. doi: 10.1016/j.cellsig.2014.08.027
Zhou, Y., Kipps, T. J., and Zhang, S. (2017). Wnt5a signaling in normal and cancer stem cells. Stem Cells Int. 2017:5295286. doi: 10.1155/2017/5295286
Zhu, X. L., Zeng, Y. F., Guan, J., Li, Y. F., Deng, Y. G., Bian, X. W., et al. (2011). FMNL2 is a positive regulator of cell motility and metastasis in colorectal carcinoma. J. Pathol. 224, 377–388. doi: 10.1002/path.2871
Keywords: cancer stem cell, TAMRA+ cells, induction of pluripotency, hypoxia, oxidative stress, xenobiotics, carcinogenesis, genes-markers of stemness
Citation: Efremov YR, Proskurina AS, Potter EA, Dolgova EV, Efremova OV, Taranov OS, Ostanin AA, Chernykh ER, Kolchanov NA and Bogachev SS (2018) Cancer Stem Cells: Emergent Nature of Tumor Emergency. Front. Genet. 9:544. doi: 10.3389/fgene.2018.00544
Received: 09 July 2018; Accepted: 26 October 2018;
Published: 16 November 2018.
Edited by:
Darius Widera, University of Reading, United KingdomReviewed by:
Pierfrancesco Pagella, University of Zurich, SwitzerlandCopyright © 2018 Efremov, Proskurina, Potter, Dolgova, Efremova, Taranov, Ostanin, Chernykh, Kolchanov and Bogachev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sergey S. Bogachev, bGFibW9sYmlvbEBtYWlsLnJ1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.