
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 12 October 2018
Sec. Genetics of Common and Rare Diseases
Volume 9 - 2018 | https://doi.org/10.3389/fgene.2018.00400
 Tom E. J. Theunissen1,2
Tom E. J. Theunissen1,2 Minh Nguyen1,2
Minh Nguyen1,2 Rick Kamps1
Rick Kamps1 Alexandra T. Hendrickx1
Alexandra T. Hendrickx1 Suzanne C. E. H. Sallevelt1
Suzanne C. E. H. Sallevelt1 Ralph W. H. Gottschalk1
Ralph W. H. Gottschalk1 Chantal M. Calis1
Chantal M. Calis1 Alphons P. M. Stassen1
Alphons P. M. Stassen1 Bart de Koning1
Bart de Koning1 Elvira N. M. Mulder-Den Hartog3
Elvira N. M. Mulder-Den Hartog3 Kees Schoonderwoerd4
Kees Schoonderwoerd4 Sabine A. Fuchs5
Sabine A. Fuchs5 Yvonne Hilhorst-Hofstee6
Yvonne Hilhorst-Hofstee6 Marianne de Visser7
Marianne de Visser7 Jo Vanoevelen1
Jo Vanoevelen1 Radek Szklarczyk1,2
Radek Szklarczyk1,2 Mike Gerards1,8
Mike Gerards1,8 Irenaeus F. M. de Coo1,3†
Irenaeus F. M. de Coo1,3† Debby M. E. I. Hellebrekers1†
Debby M. E. I. Hellebrekers1† Hubert J. M. Smeets1,2*†
Hubert J. M. Smeets1,2*†Mitochondrial disorders, characterized by clinical symptoms and/or OXPHOS deficiencies, are caused by pathogenic variants in mitochondrial genes. However, pathogenic variants in some of these genes can lead to clinical manifestations which overlap with other neuromuscular diseases, which can be caused by pathogenic variants in non-mitochondrial genes as well. Mitochondrial pathogenic variants can be found in the mitochondrial DNA (mtDNA) or in any of the 1,500 nuclear genes with a mitochondrial function. We have performed a two-step next-generation sequencing approach in a cohort of 117 patients, mostly children, in whom a mitochondrial disease-cause could likely or possibly explain the phenotype. A total of 86 patients had a mitochondrial disorder, according to established clinical and biochemical criteria. The other 31 patients had neuromuscular symptoms, where in a minority a mitochondrial genetic cause is present, but a non-mitochondrial genetic cause is more likely. All patients were screened for pathogenic variants in the mtDNA and, if excluded, analyzed by whole exome sequencing (WES). Variants were filtered for being pathogenic and compatible with an autosomal or X-linked recessive mode of inheritance in families with multiple affected siblings and/or consanguineous parents. Non-consanguineous families with a single patient were additionally screened for autosomal and X-linked dominant mutations in a predefined gene-set. We identified causative pathogenic variants in the mtDNA in 20% of the patient-cohort, and in nuclear genes in 49%, implying an overall yield of 68%. We identified pathogenic variants in mitochondrial and non-mitochondrial genes in both groups with, obviously, a higher number of mitochondrial genes affected in mitochondrial disease patients. Furthermore, we show that 31% of the disease-causing genes in the mitochondrial patient group were not included in the MitoCarta database, and therefore would have been missed with MitoCarta based gene-panels. We conclude that WES is preferable to panel-based approaches for both groups of patients, as the mitochondrial gene-list is not complete and mitochondrial symptoms can be secondary. Also, clinically and genetically heterogeneous disorders would require sequential use of multiple different gene panels. We conclude that WES is a comprehensive and unbiased approach to establish a genetic diagnosis in these patients, able to resolve multi-genic disease-causes.
Mitochondrial disorders are clinically highly heterogeneous, with a broad variety of neurological and muscular symptoms involved and having significant clinical overlap with other neuromuscular disorders. Although, mitochondrial disorders are characterized by deficiencies in the oxidative phosphorylation (OXPHOS) and ATP production, biochemical deficiencies are not always detected in the lab. Besides, OXPHOS deficiencies can be a secondary phenomenon in neuromuscular or multi-system disorders with a non-mitochondrial cause (Pyle et al., 2015; Niyazov et al., 2016). Mitochondrial disorders are also genetically heterogeneous, as different gene defects can result in a similar phenotype and both the nuclear and mitochondrial genomes are involved (Rotig and Munnich, 2003; McFarland et al., 2010). These features complicate the establishment of a genetic diagnosis in mitochondrial patients (Koenig, 2008).
For pathogenic variants in the multi-copy mtDNA, which contains 37 genes and is exclusively maternally inherited, the mutation load of the so-called heteroplasmic pathogenic variants also affects the clinical presentation (Thorburn and Dahl, 2001; Hellebrekers et al., 2012). The estimated number of nuclear genes involved in mitochondrial function is around 1500 (Prokisch and Ahting, 2007; Calvo and Mootha, 2010), of which only > 250 genes have been shown to be involved in mitochondrial disease (Alston et al., 2017). In mitochondrial disorders (patients in group 1), OXPHOS deficiencies are often due to genetic defects in the OXPHOS complexes (subunits and assembly factors), or, more indirectly, in processes such as mitochondrial protein translation and degradation, mtDNA maintenance, fusion and fission, substrate transport, or phospholipid metabolism. Still, mitochondrial dysfunction can also be a more secondary defect in genetic syndromes or neuromuscular disease (Hui et al., 2006). Also, in patients with neuromuscular symptoms that are not specific for a mitochondrial disease (patients in group 2), pathological variants in mitochondrial genes have been reported in addition to other non-mitochondrial genetic causes.
Genetic diagnosis of mtDNA-disorders requires screening of all 37 mtDNA genes and determining the heteroplasmy levels of the variants, being either point mutations or large rearrangements. In addition, mtDNA copy-number is being tested to identify mtDNA depletions. mtDNA analysis in diagnostic setting commonly started with screening for a few relatively common point mutations using mutation-specific restriction enzymes or qPCR based methods (Fan et al., 2006; Wang et al., 2011). In case common pathogenic variants were not detected, the entire mitochondrial genome was analyzed by Sanger sequencing or chip-based methods (van Eijsden et al., 2006; Finsterer et al., 2009). However, these methods are non-quantitative, requiring a second molecular test to determine the mutation load. Besides, chip-based methods have difficulties in detecting small indels (Tang and Huang, 2010; Xie et al., 2011; McCormick et al., 2013). For the large mtDNA deletions, it was time-consuming to determine the exact breakpoints, which could be important for prognosis (He et al., 2002; Wong et al., 2003). The application of next-generation sequencing has greatly increased the possibilities for detecting, characterizing and quantifying point mutations, and rearrangements across the complete mitochondrial genome with one single technology (Li et al., 2010; Huang, 2011; Cui et al., 2013). Although, accurate determination of the heteroplasmy levels of large mtDNA deletions still requires quantitative PCR analysis. The first tissue tested is blood, but this is extended to muscle or urine in case the mtDNA could have been missed in blood (Koenig, 2008). If no pathogenic variants in the mtDNA were present, then moving into the analysis of nuclear genes traditionally relied on (stepwise) Sanger sequencing of nuclear candidate genes which were selected based on clinical and biochemical features or linkage/homozygosity mapping. Again next-generation sequencing methods have made this approach obsolete, as whole exome sequencing (WES), enabled the detection of the majority of the genetic variations in the coding part of the genome (Wortmann et al., 2015). An unbiased and complete genetic analysis is important, especially in heterogeneous mitochondrial disease, where genotype-phenotype relations can be indistinct and novel genes involved.
We have performed a complete next-generation sequencing strategy, analysing the mtDNA and exome in a cohort of 86 patients with mitochondrial disease (group 1) and 31 neuromuscular patients in whom a pathogenic variant in a mitochondrial gene could possibly be involved (group 2). The latter group includes heterogeneous neuromuscular patients with disease symptoms that are not specific for mitochondrial disease, but in which a mitochondrial genetic cause has been identified in a minority of cases. The results were obtained over a 4-year period from 2012 to 2016. Patients were tested for pathogenic variants in the mtDNA by next-generation sequencing and, if negative, further analyzed by WES. WES-variants were filtered according to the presumed genetic model of disease inheritance, allele frequencies, conservation, and the predicted effect of the variant. With this approach, we identified in 68% of our patients a causative pathogenic variant in new and known disease genes, which were either inherited or de novo.
One hundred and seventeen patients, from consanguineous and non-consanguineous families, in this study were under treatment at Erasmus MC, Maastricht UMC+, Leiden UMC, UMC Utrecht, AMC Amsterdam (The Netherlands). The patients had a clinical phenotype that could be caused by a mitochondrial defect. The age of disease onset in the cohort varied from birth to approximately 50 years old. As 77% of our patients was below 18 years at the age of diagnosis we used the mitochondrial disease criteria (MDC) for children (Morava et al., 2006). The MDC include clinical signs and symptoms (max 4 points), metabolic abnormalities and neuroimaging (max 4 points) and histologic anomalies (max 4 points). Score 1: mitochondrial disorder unlikely; score 2 to 4: possible mitochondrial disorder; score 5 to 7: probable mitochondrial disorder; score 8 to 12: definite mitochondrial disorder. In group I patients were included, in whom the diagnosis of a mitochondrial disease was probable or definite (MDC > 5). Patients not meeting these MD-criteria (MDC < 5, group 2) had neuromuscular symptoms that are not specific for mitochondrial disease, but in which a mitochondrial genetic cause has been identified in a minority of cases. All parents were unaffected. Subjects gave written informed consent for WES analysis in accordance with the Declaration of Helsinki. Research was prospectively reviewed and approved by the local ethical committee of the Maastricht University Medical Centre.
Sequencing of the mtDNA was performed using the Illumina MiSeq platform. Enrichment of the entire mtDNA was performed by a single long-range PCR using Phusion Hot Start DNA polymerase II kit (Thermoscientific) and 100 ng of total genomic DNA, according to the manufacturer's instructions. Library preparation was performed by the Illumina Nextera XT kit according to the manufacturer's instructions. Twelve indexed DNA libraries were equimolarly pooled and sequenced in a single lane of 1 MiSeq flow-cell with a 2 × 300 bp paired-end chemistry. Data demultiplexing was performed with Illumina CASAVA software (v.1.8.4.) and reads were aligned against the revised Cambridge mitochondrial reference sequence (but without the gap at position 3107) by BWA software (v.0.5.9.) (Li and Durbin, 2009). For both variant and small indel identification, Python 2.6.6., Python package pysam 0.7.8. and SAMTools 0.1.19 software were used (Li et al., 2009). Large deletions were identified by alignment with the Smith and Waterman algorithm and the EMBOSS water program (v.6.5.7.). Annotation and filtering of mtDNA variants and indels were performed using in-house build Perl tools and a MySQL annotation database. Calculation of the heteroplasmy level at any nucleotide position was performed by the read depth of the mutant vs. reference nucleotide. We have validated the detection and quantification accuracy of our NGS strategy by analysing DNA samples with varying heteroplasmy levels of different substitution variants, small indels and large deletions, as previously determined by MitoChip, RFLP and PCR/Southern blotting (Supplementary Data Tables S1–S5; SRA submission SUB4444333). The entire mtDNA, excluding the highly polymorphic D-loop, was analyzed using a 2% heteroplasmy cut-off for known pathogenic point mutations and a 5% cut-off for the remaining positions and small indels. If no mtDNA mutations were detected in blood of the MD-patient (group 1) and a mtDNA disease was highly likely, this was confirmed on muscle or urine DNA. To test for mtDNA depletions, qPCR quantification was performed on DNA extracted from available muscle biopsies, using SensiMix SYBR (Bioline), where mtDNA copy-number (based on the mitochondrial ND1 gene) was normalized to the nDNA copy-number (based on the nuclear B2M gene).
Homozygosity mapping and CNV analysis was performed by HumanMapping 250K array (Affymetrix, Santa Clara, California) and Genotyping console 4.0 (Affymetrix). Homozygosity or hemizygosity regions were defined by the “Homozygosity” mapper (Seelow et al., 2009), with a cutoff of 5 MB. Exons were captured with SureSelect version 5 exome enrichment kit (Agilent Technologies, Santa Clara, California), including untranslated regions. Sequencing was performed on a HiSeq2000 platform (Illumina, San Diego, California), using a 2 × 100 bp paired-end setting. Bcl2fastq 1.8.4 (Illumina) allowed Basecalling and demultiplexing, and Burrows-Wheeler Aligner 0.5.9 (Broad Institute, Cambridge, Massachusetts) was used for read alignment against human reference genome hg19. Duplicate reads were removed by Picard software suite 1.77 (Broad Institute, Cambridge, Massachusetts) and variant calling was performed with GATK 2.1-8 (Broad Institute).
Exome data of consanguineous families or families consisting of > 1 patient was filtered for recessive homozygous, compound heterozygous, and X-linked (XLR) pathogenic variants. Variants with allele frequencies <1% (dbSNP151 Exome Aggregation Consortium) were evaluated, covering missense variants, indels, nonsense mutations, and splice variants. Non-annotated variants were maintained, unless allele frequencies exceeded 5% prevalence in our in-house patient database. Pathogenicity of nonsynonymous missense variants was estimated by Polymorphism Phenotyping-2 (PolyPhen-2; Harvard, Boston, Massachusetts), Sorting Intolerant From Tolerant (J. Craig Venter Institute, Rockville, Maryland), Protein Variation Effect Analyzer (PROVEAN; J. Craig Venter Institute), and MutationTaster (NeuroCure Cluster of Excellence/Berlin Institute of Health, Berlin, Germany). Nonsense, frameshift, and splice-site variations were maintained. WES data of single patients from non-consanguineous families were additionally filtered for heterozygous variants in known (OMIM (Online Mendelian Inheritance in Man) disease genes with known dominant and/or de novo pathogenic variants. All identified pathogenic variants were checked for their inclusion in the MitoCarta build 2.0 database for mitochondrial localized proteins.
All variants that are listed in this manuscript were assigned as “most probably disease causing” (class 4) or “disease causing” (class 5). “Disease causing” (class 5), based on previous publications reporting patients with a similar phenotype, possibly with functional prove, or, in case of unpublished variants, classified as “most probably disease causing” (class 4), according to the diagnostic standards and guidelines of the American College of Medical Genetics and Genomics (ACMG) (Richards et al., 2015). The ACMG criteria take into account, among others: allele frequencies in control databases (missense variants), amino-acid conservation, Grantham score, functional domains, and splice predictions and nucleotide conservation in case of intronic variants. Pathogenic variants were only included if these segregated correctly with the disease in the family.
A cohort of 117 patients from unaffected parents, was subject to a two-step next-generation sequencing approach (Figure 1). The age of disease onset in the cohort varied from birth to approximately 50 years old, 77% (90/117) were patients <18 years of age. We solved 20% of the cohort with a disease-causing mtDNA defect, involving 23 MD-patients from group 1 and none from group 2. Subsequent WES analysis solved an additional 49% (57 patients) of the cohort, consisting of 39 patients of group 1 and 18 patients from group 2. With this strategy, we achieved an overall diagnostic yield of 68%, with comparable yield in the group of inherited disease cases (69%) compared to the single patients from non-consanguineous families (68%).
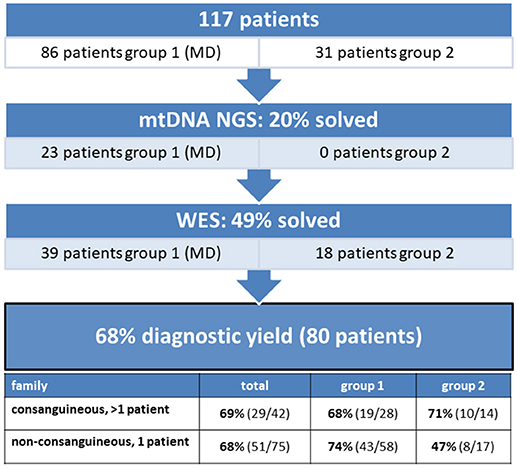
Figure 1. The diagnostic yield of mtDNA and whole exome sequencing in a patient cohort consisting of 117 patients. 20% of the patients were solved with a mtDNA defect and 49% with a nuclear DNA defect, implying an overall diagnostic yield of 68%.
mtDNA sequencing resulted in the identification of a disease-causing pathogenic variants in 20% (23/117) of the patient cohort (Figure 1), solving 5% (2/42) of the inherited disease cases (consanguineous, > 1 patient) and 28% (21/75) of the single patients from non-consanguineous families. As shown in Table 1, all cases with a mtDNA defect were MD-patients (group 1) and all 23 identified mtDNA defects involved known mtDNA mutations in typical mtDNA disorders, like LHON (Leber's hereditary optic neuropathy), MELAS (mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes)/MIDD (maternally inherited diabetes mellitus and deafness), Leigh syndrome and CPEO (chronic progressive external ophthalmoplegia)/Kearns-Sayre syndrome. 39% (9/23) of the mtDNA mutations were, mostly homoplasmic (8 out of 9), LHON mutations (m.11778G>A and m.14484T>C), clinically manifesting with optic atrophy. Only one LHON patient carried 80% heteroplasmy. The m.3243A>G mutation covered 30% (7/23) of the mtDNA defects, and explained both MELAS (2 patients) and MIDD (5 patients) phenotypes. Another mutation was identified in the ND5 gene (m.13513G>A), as a cause of Leigh syndrome. 26% (6/23) of the mtDNA defects were large single deletions, of which the 4,977 bp deletion (breakpoints 8482:13460) was detected in 4 patients. These patients had CPEO, sometimes in combination with additional symptoms of Kearns-Sayre syndrome (pigmentary retinopathy, cardiac conduction abnormalities). Patients with multiple mtDNA deletions or mtDNA depletion (not included in Table 1) were further analyzed by WES, as being suggestive of an underlying nuclear DNA defect in the mtDNA maintanance or replication genes.
In the remaining 94 patients, prior to whole exome analysis, the DNA were subject to SNP-array analysis, using the Affymetrix GeneChip Human Mapping 250K, to detect copy number variations (CNV) and homozygosity regions. No CNVs were detected. In patients from consanguineous families, mapping of the homozygous regions provided an additional filtering criterion to select the most promising genetic variants from the exome data. When multiple affected siblings were present, the entire family, if available, was analyzed by means of SNP-arrays. This approach significantly increased the efficiency of interpreting WES results. WES identified a disease-causing gene defect in 49% (57/117) of the complete patient cohort (Figure 1). In an additional 7% of the cohort, WES analysis identified a genetic variant which could explain the patient's phenotype, but for which definite evidence is currently lacking. Further studies should reveal which of these pathogenic variants could be classified as disease-causing.
WES-data filtering was based on the presumed genetic mode of inheritance. We applied a variant selection strategy for autosomal recessive (AR) and recessive X-linked (XLR) disorders to the inherited disease cases (consanguinity, >1 patient). As shown in Table 2, this group consisted of 40 families, where WES identified of a genetic defect in 68% (27/40) of the cases, covering homozygous, compound heterozygous and X-linked pathogenic variants (Tables 3, 4). In group 1 (MD patients), 65% (17/26) of the cases were solved, and in group 2 a disease causing gene defect was found in 71% (10/14). As expected, a broad spectrum of genes with relatively few genes with a literature reported mitochondrial function [20% (2/10)] was identified in group 2 (Table 4) compared to the proportion of such genes in group 1 [76% (13/17)] (Table 3). In 54 non-consanguineous families with a single patient, we applied a variant selection strategy for autosomal recessive (AR), X-linked (XLR and XLD) and single heterozygous pathogenic variants, expected to be dominant de novo variants. In order to limit the huge amount of heterozygous variants for dominant disease-causing mutations, variant interpretation was restricted to genes that have previously been reported in dominant disease based on the OMIM database. With this approach, we unraveled the genetic cause in 56% (30/54) of these families (Table 2), 15% of which were single dominant pathogenic variants. Follow-up investigations in the parents revealed that the pathogenic variants either occurred de novo in the patients or were present in one of the parent, who displayed subclinical symptoms upon further investigation. The diagnostic yield was 59% (22/37) in patient group 1 and 47% (8/17) in group 2, where respectively, 86% (19/22) (Table 3) and 13% (1/8) (Table 4) of the defects were located in a gene with reported mitochondrial function.
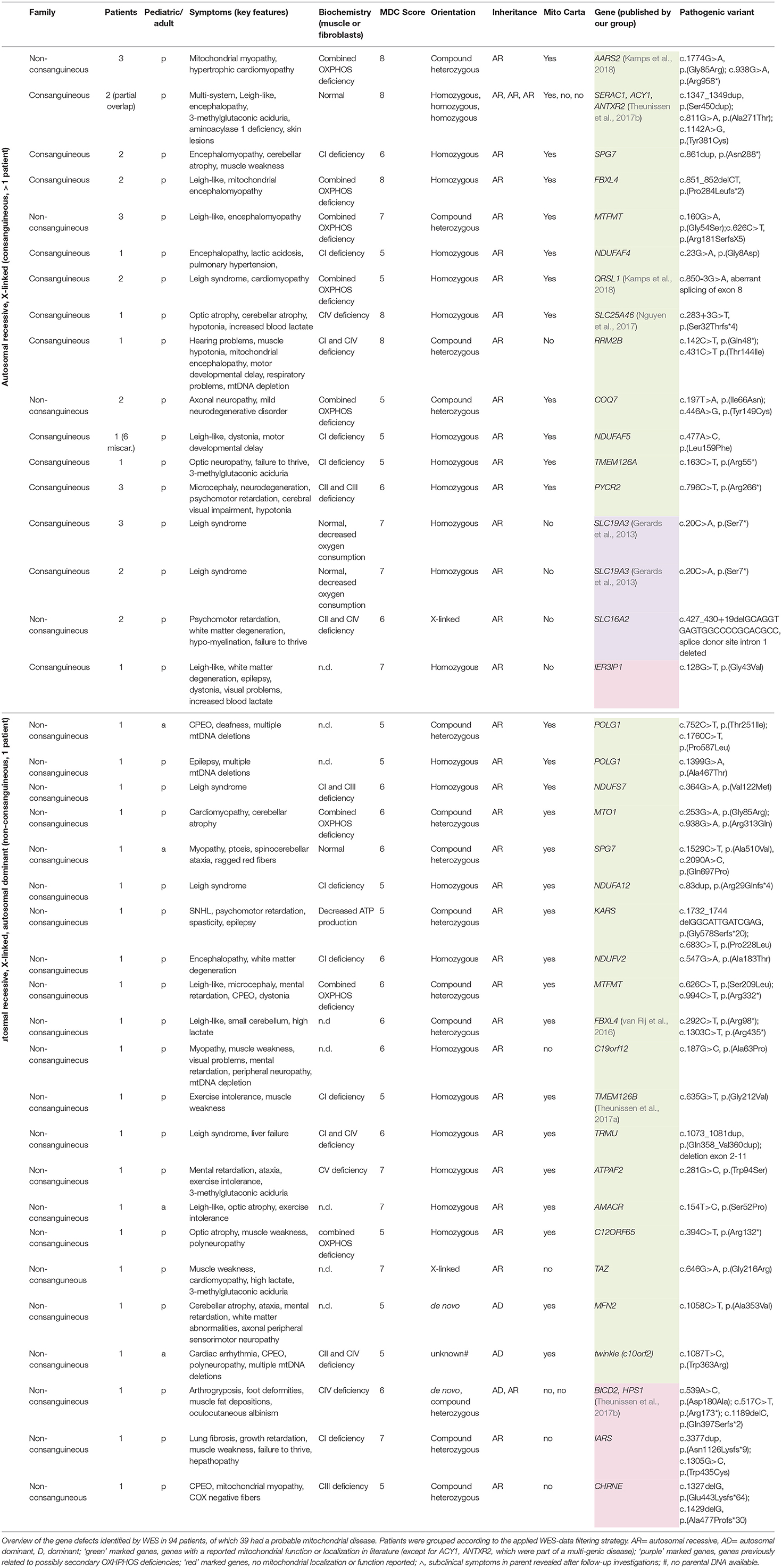
Table 3. Disease-causing pathogenic variants identified by WES in group 1, consisting of mitochondrial patients (MD).
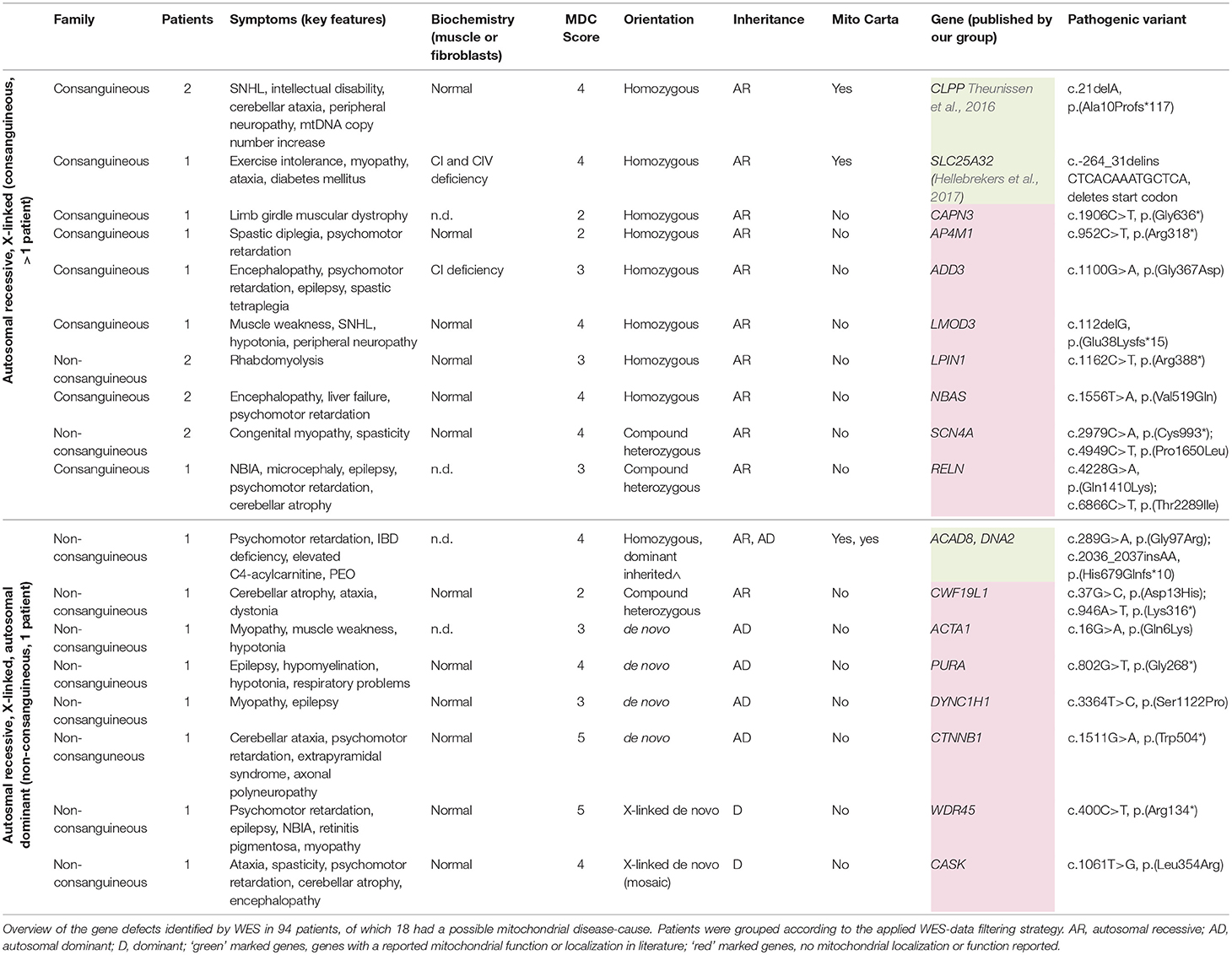
Table 4. Disease-causing pathogenic variants identified by WES in group 2, consisting of patients with a possible mitochondrial disorder.
Overall, we saw that 31% (13/42) of the disease-causing genes in the mitochondrial patients (group 1) were not included in the MitoCarta database for mitochondrial localized proteins at the time we identified the genetic defect (Table 3). Surprisingly, this included 3 genes with a literature reported function in mitochondrial metabolism, in which pathogenic variants are a well-known cause of mitochondrial disease (RRM2B, c19orf12, TAZ). Furthermore, we identified 2 different genes (SLC19A3 (2x), SLC16A2) for which a role in mitochondrial OXPHOS is still unclear. Although a mitochondrial function cannot be excluded, it has been suggested that the OXPHOS deficiencies in the corresponding patients, might be secondary (Niyazov et al., 2016; de Beaurepaire et al., 2018). Furthermore, 5 different genes with no reported function in mitochondrial metabolism (IER3IP1, IARS, CHRNE, BICD2, HPS1) have been identified. These genes require further functional testing to reveal a possible “novel” role in mitochondrial functioning or to demonstrate a secondary respiratory deficiency. In the patient with the multi-genic cause (BICD2 and HPS1), an additional, third, gene defect might have been missed. Two additional genes that were lacking in the MitoCarta database were not related to the mitochondrial symptoms of the patients, as these genes encode proteins with no mitochondrial function, but were together with a mitochondrial gene defect part of a complex multi-genic disease phenotype, in which more than a single gene defect is involved (ACY1, ANTXR2).
Genes identified in group 1 (MD-patients) were clustered according to their function (Table 5). Most genetic defects were detected in genes related to mitochondrial protein metabolism (protein translation and degradation) and OXPHOS function (ETC subunits, assembly factors, and cofactors). The first group mainly consisted of mitochondrial tRNA synthethases, transferases, and modification enzymes, where the resulting defects in mitochondrial protein synthesis (AARS2, MTFMT, MTO1, QRSL1, TRMU, C12ORF65) especially manifested with combined OXPHOS deficiencies (not measured for KARS). Among the group of OXPHOS-associated genes, defects in complex I subunits and assembly factors (NDUFA12, NDUFS7, NDUFV2, TMEM126A, TMEM126B, NDUFAF4, NDUFAF5) were most prevalent, all resulting in a significant complex I deficiency at the biochemical level. NDUFS7 resulted in an additional complex III deficiency. Defects in NDUFS7, NDUFA12 and NDUFAF5 caused Leigh-syndrome or a Leigh-like-phenotype. The third largest group represented genes involved in mtDNA metabolism, required for mtDNA maintenance, replication and nucleotide metabolism. The RRM2B defect resulted in mtDNA depletion, whereas pathogenic variants in the RMM2B interacting protein PYCR2 did not reveal any mtDNA abnormalities. Furthermore, pathogenic variants in POLG1, twinkle (c10orf2) and DNA2 displayed multiple mtDNA deletions in muscle. Interestingly, we found the mitochondrial localized, but functionally uncharacterized, c19orf12 gene to be associated with mtDNA depletion (mtDNA copy-number, normalized to nDNA, was 10–30% of healthy control samples). Among the mitochondrial patients (group 1), we identified pathogenic variants in 5 genes without a reported mitochondrial function or localization, but reported to result in a comparable phenotype as in our patient. 4 genes, functional in different cellular processes, including the transporter proteins SLC19A3 (thiamine) and SLC16A2 (thyroid hormones), tRNA-synthetase IARS, and acetylcholine receptor subunit CHRNE, were likely to underlie the OXPHOS deficiencies measured in these patients, although some of these genes have been related to possible secondary OXPHOS deficiency (SLC19A3 and SLC16A2). In an additional patient, the functionally uncharacterized IER3IP1 gene was likely to explain a seemingly mitochondrial disease phenotype, yet no OXPHOS measurement was performed in this patient. As indicated in Table 6, in patient's from group 2, 4 mitochondrial gene defects were identified, SLC25A32, CLPP, ACAD8, and DNA2 (multi-genic disease cause).
We used a next-generation sequencing strategy in a cohort of 86 patients with a likely mitochondrial disease (group 1), as these cases met the criteria for MD, and 31 patients, who did not meet the criteria for MD, but where a mitochondrial defect could possibly cause the disease symptoms. In 68% of the patients we identified a disease causing genetic defect, of which 20% was solved by mtDNA sequencing and 49% by subsequent WES analysis. Compared to conventional Sanger sequencing methods, which only solved 11% (Neveling et al., 2013a), primarily due to limitations on the number of genes being sequenced, this is a major step forward. Selective sequencing of targeted gene panels in two studies, including 1,598 and 1,034 mitochondrial disease genes, resulted in respectively 22% (102 suspected mitochondrial patients) (Lieber et al., 2013) and 24% (42 mitochondrial patients) (Calvo et al., 2012) diagnostic yield. This is less than in our study, which might in part be explained by an incompleteness of the panels (more details below). In a previous next-generation sequencing-based study, which included 113 pediatric patients with suspected mitochondrial disease, screening both the mtDNA and the exome, resulted in a diagnostic yield of 59% (Pronicka et al., 2016). Another study performing WES in 109 pediatric and young adult patients with suspected mitochondrial disease, established a genetic diagnosis in 39% of the patient cohort (after exclusion of mtDNA mutations) (Wortmann et al., 2015). Our pediatric patient group consisted of 90 patients, for which we could establish a genetic diagnosis in 66% (8 mtDNA and 51 nuclear gene mutations). Our higher yield could be caused by a complete analysis of both the mtDNA and the exome, with a strong selection for genetic cases, and follow-up investigations in case of an unknown gene. The relatively high number of patients from consanguineous parents could in part explain the high diagnostic yield, as a genetic cause is highly likely. As consanguineous parents have an increased risk of having children that suffer from multiple genetic diseases, WES data should always be completely analyzed and, preferably, parents should be offered preconception genetic testing. Different from previous studies on mitochondrial cohorts, we have included a more heterogeneous patient group (group 2), in which a relatively small portion is likely to be caused by a mitochondrial gene defect. In conclusion, our data shows that mtDNA sequencing followed by a complete whole exome analysis is the preferred strategy to identify the genetic basis in heterogeneous neuromuscular patients with a likely or possible mitochondrial disease cause.
As the mtDNA genome is relatively small, NGS allows cost effective and efficient testing of many patient samples within a single run, with sufficient sensitivity on blood DNA. Yet, as mtDNA mutations may disappear from blood during life, in cases where an mtDNA disorder is likely, DNA from muscle (or urine for the m.3243A>G) should be tested to prevent missing a diagnosis. Also for prognosis mutation levels in muscle are preferable. Where traditionally several complementary methods had to be used for detection and quantification of mtDNA mutations (e.g., RFLP, ARMS-qPCR, sanger sequencing, Mito-CHIP, Southern blot analysis), NGS can be used as a single method for identifying point mutations and indels, large deletion breakpoints and quantifying heteroplasmy levels of point mutations and small indels with high sensitivity and specificity (Tang and Huang, 2010). Only to accurately measure heteroplasmy levels of large mtDNA deletions and mtDNA depletions, additional qPCR based quantification is required. NGS of the mtDNA solved 20% of our patient cohort (all mitochondrial patients), mainly, but not only, consisting of patients with early disease onset (77% pediatric patients). In the pure pediatric subgroup with mitochondrial disease (group 1), mtDNA mutations accounted for 19% (8/43) of the genetic defects, which is in line with earlier reports that estimated involvement of a mtDNA defect in <20% of the pediatric mitochondrial patients (Schaefer et al., 2008). In our adult subgroup with mitochondrial disease (group 1), mtDNA defects accounted for 75% (12/16), which is comparable to the previously estimated 70–75% (Schaefer et al., 2008; Gorman et al., 2015). As shown in Table 1, most mtDNA patients carried LHON and MIDD/MELAS causing mutations, which was also likely based on the clinical presentation. Both MIDD and MELAS symptoms were caused by the m.3243A>G mutation, in line with what has been reported before (de Wit et al., 2012). Yet, as indicated by the heteroplasmy levels (24–41% in MELAS and 7–40% in MIDD), variation in symptom manifestations among these patients could not solely be explained from the differences in heteroplasmy level in blood, complicating prognostic predictions.
Using WES, we identified a disease-causing variant in an additional 49% of the patient cohort, where we applied a variant selection strategy fitting the presumed genetic mode of disease inheritance. In a group with likely inherited disease (consanguineous and/or multiple patients), filtering for recessive pathogenic variants solved 68% of the patients. A group of single patients from non-consanguineous parents was also screened for heterozygous variants in known (OMIM) AD disease genes, which resulted in an overall genetic diagnosis in 56% of the patients, of which 15% of the identified variants were de novo. To reduce the huge amount of potential dominant pathogenic variants, we have restricted our heterozygous variant selection to OMIM-reported dominant disease genes, and therefore might have missed some de novo cases. This could be overcome by WES trio-analysis. Additionally, the higher likelihood of an inherited genetic cause, the availability of homozygosity mapping data and of exome data of affected siblings might have contributed to a higher diagnostic yield in the first group. Our strategy did not result in large differences in diagnostic yield between patients from group 1 and patients from group 2. In an additional 7% of the WES cohort we found a genetic variant (variant of unknown significance) with a clear lead to the patient's phenotype (data not shown). For these cases, further laboratory testing or patient screening should reveal which genes can be classified as disease causing.
In 82% (32/39) of the likely mitochondrial patients (group 1), symptoms were caused by a gene with a reported mitochondrial function or localization. As expected, this was significantly lower in patient group 2 [17% (3/18)]. Our results showed that in patients with mitochondrial disease, most genetic defects were associated with mitochondrial protein metabolism and OXPHOS function, where a majority of the OXPHOS defects were found in complex I genes. In line with previously reported complex I deficient patients, we found that defects in NDUFS7, NDUFA12, and NDUFAF5 caused Leigh-syndrome or a Leigh-like-phenotype (Rahman and Thorburn, 1993). Although NDUFV2 defects have also been reported in association with Leigh syndrome (Cameron et al., 2015), our patient exclusively expressed symptoms of white matter degeneration (encephalopathy). Also, we identified TMEM126A as an underlying cause of isolated complex I deficiency, therewith supporting co-migration studies that characterized TMEM126A as an early assembly factor of complex I (Wessels et al., 2013). Patients with mtDNA abnormalities carried pathogenic variants in RRM2B, twinkle (c10orf2) and DNA2, which were all functionally related to mtDNA maintenance, and presented as the third largest group. Interestingly, we also found pathogenic variants in the c19orf12 gene in a patient with an mtDNA depletion. The link between this gene and the mtDNA depletion remains to be established.
In 6 mitochondrial patients (group 1), we identified a causal gene for which no direct mitochondrial function or localization has been reported. This included 6 genes involved in different cellular processes (SLC19A3, SLC16A2, IARS, CHRNE, BICD2, and HPS1). In two families with classical Leigh-syndrome we identified a defect in the thiamine transporter SLC19A3, where despite a decrease in mitochondrial oxygen consumption, complex activities seemed normal in muscle, and skin tissue (Gerards et al., 2013). Thiamine-diphosphate, the active form of thiamine, has been reported as an essential cofactor for several mitochondrial enzymes, including the pyruvate dehydrogenase complex (Ortigoza-Escobar et al., 2014). A second transmembrane transporter defect was found in the thyroid hormone (T3/T4) transporter SLC16A2, in a patient with Allan-Herndon Dudley syndrome, which resulted in a CII and CIV deficiency, where triiodothyronine (T3) has been suggested as an important regulator of mitochondrial activity (Wrutniak-Cabello et al., 2001). However, a role for SLC19A3, but also SLC16A2 as mitochondrial genes is under debate, as defects in these genes have been suggested to cause possible secondary respiratory chain deficiency (Niyazov et al., 2016; de Beaurepaire et al., 2018). In such case, patients might possibly get a false positive classification as mitochondrial (group 1) based on biochemical criteria Still, concerning SLC19A3, either being primary or secondary, this gene should be tested in patients with Leigh syndrome, and should be in all MD panels, because it is a treatable condition, even though, having a secondary effect, it may not have been included in the MitoCarta database. Furthermore, we found a pathogenic variant in the cytoplasmic located aminoacyl-tRNA synthetase IARS to cause complex I deficiency in skin and muscle tissue of a patient with a multi-system disorder, and a defect in the acetylcholine receptor subunit epsilon CHRNE associated with CII deficiency, COX negative muscle fibers and abnormally shaped muscle mitochondria in CPEO with myopathy. Also we measured a complex IV deficiency in a patient with a multi-genic cause, where either the identified BICD2 or HPS1 defect causes the deficiency, or a possible third defect was missed. In another patient, who was classified as mitochondrial based on the classical mitochondrial disease symptoms involved, the functionally uncharacterized IER3IP1 gene was identified. Although, no OXPHOS measurements were performed in this patient, knockdown of IER3IP1 was reported to affect mitochondrial function by decreasing the mitochondrial membrane potential and increasing cytochrome C release (Sun and Ren, 2017). The genetic defects in these patients need further functional characterization to prove or exclude a mitochondrial function, the involvement of secondary respiratory chain deficiencies, or perhaps the presence of an additional gene defect that has been missed by WES.
31% of the disease-causing genes identified in our mitochondrial patients (group 1) were not included in the MitoCarta database at the moment of identification, and could therefore be missed with panel-based sequencing approaches that rely on the MitoCarta database. In addition to the 5 genes described above, this also involved some genes with a reported function in mitochondrial metabolism, indicating that it can be difficult to keep databases or panels up to date with recent discoveries. Also, the contribution of non-mitochondrial disease genes to complex multi-genic mitochondrial disease might be missed when applying targeted sequencing methods, as illustrated by two of the mitochondrial patient cases, in which defects in more than a single gene were involved. Our data shows that WES is the most suitable approach to characterize the causative gene defect in both groups, patients with most likely a mitochondrial disease, as according to the consensus criteria for MD (group 1), and patients in who a mitochondrial gene defect could possibly be involved (group 2). With respect to group 1, we show mitochondrial gene panels are still not complete, and that a strict preselection based on the consensus criteria for MD could result in the missing of mitochondrial gene defects (SLC25A32, CLPP, ACAD8, DNA2). Also in group 2 WES will be the preferred method, because of the genetic heterogeneity, targeted approaches can easily result in inefficient and sequential use of different gene panels, and still miss the complete picture in multi-genic disease. WES is a comprehensive and unbiased approach to establish a genetic diagnosis in heterogeneous mitochondrial disease, able to resolve complex multi-genic disease manifestations. Obviously, a disadvantage of WES in diagnostics settings will be the identification of novel, non-reported gene defects that often require extensive experimental setups to validate a possible role in mitochondrial disease. Embedding within or contact with a specialized research group should therefore be preferable. Identification of the genetic defect is not only important for diagnostics, but also for therapeutic interventions. Patients with SLC25A32, SLC19A3, and TMEM126B defects showed improvement upon treatment with respectively riboflavin, biotin/thiamine and high fat-diet (Gerards et al., 2013; Hellebrekers et al., 2017; Theunissen et al., 2017a). Yet, despite the significant improvements in the genetic diagnosis of mitochondrial disorders, the development of novel therapies is lagging behind.
TT, MN, RK, JV, DH, and MG were responsible for analysis and interpretation of patient WES data, and validation of the variants. RG, AH, and CC were involved in the handling of patient DNA and WES preparation. BdK, AS, and RS were responsible for the bioinformatics pipeline. KS performed biochemical measures on patient material. EM, SF, YH-H, MdV, IdC, and SS was responsible for patient contact and clinical analysis and diagnosis. IdC, DH, and HS were responsible for intellectual content and study setup.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CLA declared a past co-authorship with the authors SS and HS to the handling Editor.
This work was supported by the Princes Beatrix Spierfonds (grant no: W.OR11-24) and Stichting MetaKids. Funding sources had no involvement in study design; collection, analysis or interpretation of data; writing of the manuscript; or the decision to submit the article for publication.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2018.00400/full#supplementary-material
Acehan, D., Vaz, F., Houtkooper, R. H., James, J., Moore, V., Tokunaga, C., et al. (2011). Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J. Biol. Chem. 286, 899–908. doi: 10.1074/jbc.M110.171439
Alston, C. L., Rocha, M. C., Lax, N. Z., Turnbull, D. M., and Taylor, R. W. (2017). The genetics and pathology of mitochondrial disease. J. Pathol. 241, 236–250. doi: 10.1002/path.4809
Andrews, B., Carroll, J., Ding, S., Fearnley, I. M., and Walker, J. E. (2013). Assembly factors for the membrane arm of human complex I. Proc. Natl. Acad. Sci. U. S. A. 110, 18934–18939. doi: 10.1073/pnas.1319247110
Antonicka, H., Ostergaard, E., Sasarman, F., Weraarpachai, W., Wibrand, F., Pedersen, A. M., et al. (2010). Mutations in C12orf65 in patients with encephalomyopathy and a mitochondrial translation defect. Am. J. Hum. Genet. 87, 115–122. doi: 10.1016/j.ajhg.2010.06.004
Bonnen, P. E., Yarham, J. W., Besse, A., Wu, P., Faqeih, E. A., Al-Asmari, A. M., et al. (2013). Mutations in FBXL4 cause mitochondrial encephalopathy and a disorder of mitochondrial DNA maintenance. Am. J. Hum. Genet. 93, 471–481. doi: 10.1016/j.ajhg.2013.07.017
Bourdon, A., Minai, L., Serre, V., Jais, J. P., Sarzi, E., Aubert, S., et al. (2007). Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 39, 776–780. doi: 10.1038/ng2040
Calvo, S. E., Compton, A. G., Hershman, S. G., Lim, S. C., Lieber, D. S., Tucker, E. J., et al. (2012). Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 4:118ra110. doi: 10.1126/scitranslmed.3003310
Calvo, S. E., and Mootha, V. K. (2010). The mitochondrial proteome and human disease. Annu. Rev. Genom. Hum. Genet. 11, 25–44. doi: 10.1146/annurev-genom-082509-141720
Cameron, J. M., MacKay, N., Feigenbaum, A., Tarnopolsky, M., Blaser, S., Robinson, B. H., et al. (2015). Exome sequencing identifies complex I NDUFV2 mutations as a novel cause of Leigh syndrome. Eur. J. Paediatr. Neurol. 19, 525–532. doi: 10.1016/j.ejpn.2015.05.002
Cui, H., Li, F., Chen, D., Wang, G., Truong, C. K., Enns, G. M., et al. (2013). Comprehensive next-generation sequence analyses of the entire mitochondrial genome reveal new insights into the molecular diagnosis of mitochondrial DNA disorders. Genet. Med. 15, 388–394. doi: 10.1038/gim.2012.144
de Beaurepaire, I., Grevent, D., Rio, M., Desguerre, I., de Lonlay, P., Levy, R., et al. (2018). High predictive value of brain MRI imaging in primary mitochondrial respiratory chain deficiency. J. Med. Genet. 55, 378–383. doi: 10.1136/jmedgenet-2017-105094
de Coo, R. F., Buddiger, P., Smeets, H. J., and van Oost, B. A. (1997). Molecular cloning and characterization of the human mitochondrial NADH:oxidoreductase 10-kDa gene (NDUFV3). Genomics 45, 434–437. doi: 10.1006/geno.1997.4930
de Wit, H. M., Westeneng, H. J., van Engelen, B. G., and Mudde, A. H. (2012). MIDD or MELAS: that's not the question MIDD evolving into MELAS: a severe phenotype of the m.3243A>G mutation due to paternal co-inheritance of type 2 diabetes and a high heteroplasmy level. Neth. J. Med. 70, 460–462. doi: 10.1007/s12467-011-0033-4
Fan, H., Civalier, C., Booker, J. K., Gulley, M. L., Prior, T. W., and Farber, R. A. (2006). Detection of common disease-causing mutations in mitochondrial DNA (mitochondrial encephalomyopathy, lactic acidosis with stroke-like episodes MTTL1 3243 A>G and myoclonic epilepsy associated with ragged-red fibers MTTK 8344A>G) by real-time polymerase chain reaction. J. Mol. Diagn. 8, 277–281. doi: 10.2353/jmoldx.2006.050066
Finsterer, J., Harbo, H. F., Baets, J., Van Broeckhoven, C., Di Donato, S., Fontaine, B., et al. (2009). EFNS guidelines on the molecular diagnosis of mitochondrial disorders. Eur. J. Neurol. 16, 1255–1264. doi: 10.1111/j.1468-1331.2009.02811.x
Freyer, C., Stranneheim, H., Naess, K., Mourier, A., Felser, A., Maffezzini, C., et al. (2015). Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid. J. Med. Genet. 52, 779–783. doi: 10.1136/jmedgenet-2015-102986
Gerards, M., Kamps, R., van Oevelen, J., Boesten, I., Jongen, E., de Koning, B., et al. (2013). Exome sequencing reveals a novel Moroccan founder mutation in SLC19A3 as a new cause of early-childhood fatal Leigh syndrome. Brain 136, 882–890. doi: 10.1093/brain/awt013
Ghezzi, D., Baruffini, E., Haack, T. B., Invernizzi, F., Melchionda, L., Dallabona, C., et al. (2012). Mutations of the mitochondrial-tRNA modifier MTO1 cause hypertrophic cardiomyopathy and lactic acidosis. Am. J. Hum. Genet. 90, 1079–1087. doi: 10.1016/j.ajhg.2012.04.011
Gorman, G. S., Schaefer, A. M., Ng, Y., Gomez, N., Blakely, E. L., Alston, C. L., et al. (2015). Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann. Neurol. 77, 753–759. doi: 10.1002/ana.24362
Götz, A., Tyynismaa, H., Euro, L., Ellonen, P., Hyötyläinen, T., Ojala, T., et al. (2011). Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am. J. Hum. Genet. 88, 635–642. doi: 10.1016/j.ajhg.2011.04.006
Haitina, T., Lindblom, J., Renstrom, T., and Fredriksson, R. (2006). Fourteen novel human members of mitochondrial solute carrier family 25 (SLC25) widely expressed in the central nervous system. Genomics 88, 779–790. doi: 10.1016/j.ygeno.2006.06.016
He, L., Chinnery, P. F., Durham, S. E., Blakely, E. L., Wardell, T. M., Borthwick, G. M., et al. (2002). Detection and quantification of mitochondrial DNA deletions in individual cells by real-time PCR. Nucleic Acids Res. 30:e68. doi: 10.1093/nar/gnf067
Hellebrekers, D. M. E. I., Sallevelt, S. C. E. H., Theunissen, T. E. J., Hendrickx, A. T. M., Gottschalk, R. W., Hoeijmakers, J. G. J., et al. (2017). Novel SLC25A32 mutation in a patient with a severe neuromuscular phenotype. Eur. J. Hum. Genet. 25, 886–888. doi: 10.1038/ejhg.2017.62
Hellebrekers, D. M., Wolfe, R., Hendrickx, A. T., de Coo, I. F., de Die, C. E., Geraedts, J. P., et al. (2012). PGD and heteroplasmic mitochondrial DNA point mutations: a systematic review estimating the chance of healthy offspring. Hum. Reprod. Update 18, 341–349. doi: 10.1093/humupd/dms008
Huang, T. (2011). Next generation sequencing to characterize mitochondrial genomic DNA heteroplasmy. Curr. Protoc. Hum. Genet. Chapter 19:18. doi: 10.1002/0471142905.hg1908s71
Hui, J., Kirby, D. M., Thorburn, D. R., and Boneh, A. (2006). Decreased activities of mitochondrial respiratory chain complexes in non-mitochondrial respiratory chain diseases. Dev. Med. Child Neurol. 48, 132–136. doi: 10.1017/s0012162206000284
Janer, A., Prudent, J., Paupe, V., Fahiminiya, S., Majewski, J., Sgarioto, N., et al. (2016). SLC25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO Mol. Med. 8, 1019–1038. doi: 10.15252/emmm.201506159
Jenkinson, E. M., Rehman, A. U., Walsh, T., Clayton-Smith, J., Lee, K., Morell, R. J., et al. (2013). Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am. J. Hum. Genet. 92, 605–613. doi: 10.1016/j.ajhg.2013.02.013
Kamps, R., Szklarczyk, R., Theunissen, T. E., Hellebrekers, D. M. E. I., Sallevelt, S. C. E. H., Boesten, I. B., et al. (2018). Genetic defects in mtDNA-encoded protein translation cause pediatric, mitochondrial cardiomyopathy with early-onset brain disease. Eur. J. Hum. Genet. 26, 537–551. doi: 10.1038/s41431-017-0058-2
Karp, C. M., Pan, H., Zhang, M., Buckley, D. J., Schuler, L. A., and Buckley, A. R. (2004). Identification of HRPAP20: a novel phosphoprotein that enhances growth and survival in hormone-responsive tumor cells. Cancer Res. 64, 1016–1025. doi: 10.1158/0008-5472.CAN-03-0023
Koenig, M. K. (2008). Presentation and diagnosis of mitochondrial disorders in children. Pediatr. Neurol. 38, 305–313. doi: 10.1016/j.pediatrneurol.2007.12.001
Kopajtich, R., Murayama, K., Janecke, A. R., Haack, T. B., Breuer, M., Knisely, A. S., et al. (2016). Biallelic IARS mutations cause growth retardation with prenatal onset, intellectual disability, muscular hypotonia, and infantile hepatopathy. Am. J. Hum. Genet. 99, 414–422. doi: 10.1016/j.ajhg.2016.05.027
Kuo, M. L., Lee, M. B., Tang, M., den Besten, W., Hu, S., Sweredoski, M. J., et al. (2016). PYCR1 and PYCR2 interact and collaborate with RRM2B to protect cells from overt oxidative stress. Sci. Rep. 6:18846. doi: 10.1038/srep18846
Landoure, G., Zhu, P. P., Lourenco, C. M., Johnson, J. O., Toro, C., Bricceno, K. V., et al. (2013). Hereditary spastic paraplegia type 43 (SPG43) is caused by mutation in C19orf12. Hum. Mutat. 34, 1357–1360. doi: 10.1002/humu.22378
Lestienne, P. (1987). Evidence for a direct role of the DNA polymerase gamma in the replication of the human mitochondrial DNA in vitro. Biochem. Biophys. Res. Commun. 146, 1146–1153.
Li, H., and Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Li, M., Schonberg, A., Schaefer, M., Schroeder, R., Nasidze, I., and Stoneking, M. (2010). Detecting heteroplasmy from high-throughput sequencing of complete human mitochondrial DNA genomes. Am. J. Hum. Genet. 87, 237–249. doi: 10.1016/j.ajhg.2010.07.014
Lieber, D. S., Calvo, S. E., Shanahan, K., Slate, N. G., Liu, S., Hershman, S. G., et al. (2013). Targeted exome sequencing of suspected mitochondrial disorders. Neurology 80, 1762–1770. doi: 10.1212/WNL.0b013e3182918c40
Martina, J. A., Moriyama, K., and Bonifacino, J. S. (2003). BLOC-3, a protein complex containing the Hermansky-Pudlak syndrome gene products HPS1 and HPS4. J. Biol. Chem. 278, 29376–29384. doi: 10.1074/jbc.M301294200
McCormick, E., Place, E., and Falk, M. J. (2013). Molecular genetic testing for mitochondrial disease: from one generation to the next. Neurotherapeutics 10, 251–261. doi: 10.1007/s13311-012-0174-1
McFarland, R., Taylor, R. W., and Turnbull, D. M. (2010). A neurological perspective on mitochondrial disease. Lancet Neurol. 9, 829–840. doi: 10.1016/s1474-4422(10)70116-2
Morava, E., van den Heuvel, L., Hol, F., de Vries, M. C., Hogeveen, M., Rodenburg, R. J., et al. (2006). Mitochondrial disease criteria: diagnostic applications in children. Neurology 67, 1823–1826. doi: 10.1212/01.wnl.0000244435.27645.54
Mubiru, J. N., Shen-Ong, G. L., Valente, A. J., and Troyer, D. A. (2004). Alternative spliced variants of the alpha-methylacyl-CoA racemase gene and their expression in prostate cancer. Gene 327, 89–98. doi: 10.1016/j.gene.2003.11.009
Nagao, A., Suzuki, T., Katoh, T., Sakaguchi, Y., and Suzuki, T. (2009). Biogenesis of glutaminyl-mt tRNAGln in human mitochondria. Proc. Natl. Acad. Sci. U. S. A. 106, 16209–16214. doi: 10.1073/pnas.0907602106
Neveling, K., Feenstra, I., Gilissen, C., Hoefsloot, L. H., Kamsteeg, E. J., Mensenkamp, A. R., et al. (2013a). A post-hoc comparison of the utility of sanger sequencing and exome sequencing for the diagnosis of heterogeneous diseases. Hum. Mutat. 34, 1721–1726. doi: 10.1002/humu.22450
Neveling, K., Martinez-Carrera, L. A., Holker, I., Heister, A., Verrips, A., Hosseini-Barkooie, S. M., et al. (2013b). Mutations in BICD2, which encodes a golgin and important motor adaptor, cause congenital autosomal-dominant spinal muscular atrophy. Am. J. Hum. Genet. 92, 946–954. doi: 10.1016/j.ajhg.2013.04.011
Nguyen, M., Boesten, I., Hellebrekers, D. M., Mulder-den Hartog, N. M., de Coo, I. F., Smeets, H. J., et al. (2017). Novel pathogenic SLC25A46 splice-site mutation causes an optic atrophy spectrum disorder. Clin. Genet. 91, 121–125. doi: 10.1111/cge.12774
Nguyen, T. V., Andresen, B. S., Corydon, T. J., Ghisla, S., Abd-El Razik, N., Mohsen, A. W., et al. (2002). Identification of isobutyryl-CoA dehydrogenase and its deficiency in humans. Mol Genet Metab. 77, 68–79. doi: 10.1016/S1096-7192(02)00152-X
Niyazov, D. M., Kahler, S. G., and Frye, R. E. (2016). Primary mitochondrial disease and secondary mitochondrial dysfunction: importance of distinction for diagnosis and treatment. Mol. Syndromol. 7, 122–137. doi: 10.1159/000446586
Ortigoza-Escobar, J. D., Serrano, M., Molero, M., Oyarzabal, A., Rebollo, M., Muchart, J., et al. (2014). Thiamine transporter-2 deficiency: outcome and treatment monitoring. Orphanet. J. Rare Dis. 9:92. doi: 10.1186/1750-1172-9-92
Prokisch, H., and Ahting, U. (2007). MitoP2, an integrated database for mitochondrial proteins. Methods Mol. Biol. 372, 573–586. doi: 10.1007/978-1-59745-365-3_39
Pronicka, E., Piekutowska-Abramczuk, D., Ciara, E., Trubicka, J., Rokicki, D., Karkucinska-Wieckowska, A., et al. (2016). New perspective in diagnostics of mitochondrial disorders: two years' experience with whole-exome sequencing at a national paediatric centre. J. Transl. Med. 14:174. doi: 10.1186/s12967-016-0930-9
Pyle, A., Nightingale, H. J., Griffin, H., Abicht, A., Kirschner, J., Baric, I., et al. (2015). Respiratory chain deficiency in nonmitochondrial disease. Neurol. Genet. 1:e6. doi: 10.1212/nxg.0000000000000006
Rahman, S., and Thorburn, D. (1993). “Nuclear gene-encoded leigh syndrome overview,” in GeneReviews(R), eds R. A. Pagon, M. P. Adam, H. H. Ardinger, S. E. Wallace, A. Amemiya, L. J. H. Bean, T. D. Bird, N. Ledbetter, H. C. Mefford, R. J. H. Smith, and K. Stephens. (Seattle, WA: University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle).
Richards, S., Aziz, N., Bale, S., Bick, D., Das, S., Gastier-Foster, J., et al. (2015). Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424. doi: 10.1038/gim.2015.30
Ronchi, D., Di Fonzo, A., Lin, W., Bordoni, A., Liu, C., Fassone, E., et al. (2013). Mutations in DNA2 link progressive myopathy to mitochondrial DNA instability. Am. J. Hum. Genet. 92, 293–300. doi: 10.1016/j.ajhg.2012.12.014
Rotig, A., and Munnich, A. (2003). Genetic features of mitochondrial respiratory chain disorders. J Am Soc Nephrol. 14, 2995–3007. doi: 10.1097/01.ASN.0000095481.24091.C9
Santel, A., and Fuller, M. T. (2001). Control of mitochondrial morphology by a human mitofusin. J Cell Sci. 114, 867–874. Available online at: http://jcs.biologists.org/content/114/5/867
Schaefer, A. M., McFarland, R., Blakely, E. L., He, L., Whittaker, R. G., Taylor, R. W., et al. (2008). Prevalence of mitochondrial DNA disease in adults. Ann. Neurol. 63, 35–39. doi: 10.1002/ana.21217
Seelow, D., Schuelke, M., Hildebrandt, F., and Nurnberg, P. (2009). HomozygosityMapper–an interactive approach to homozygosity mapping. Nucleic Acids Res. 37, W593–599. doi: 10.1093/nar/gkp369
Spelbrink, J. N., Li, F. Y., Tiranti, V., Nikali, K., Yuan, Q. P., Tariq, M., et al. (2001). Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat. Genet. 28, 223–231. doi: 10.1038/90058
Sugiana, C., Pagliarini, D. J., McKenzie, M., Kirby, D. M., Salemi, R., Abu-Amero, K. K., et al. (2008). Mutation of C20orf7 disrupts complex I assembly and causes lethal neonatal mitochondrial disease. Am. J. Hum. Genet. 83, 468–478. doi: 10.1016/j.ajhg.2008.09.009
Sun, J., and Ren, D. (2017). IER3IP1 deficiency leads to increased beta-cell death and decreased beta-cell proliferation. Oncotarget 8, 56768–56779. doi: 10.18632/oncotarget.18179
Takeuchi, N., Kawakami, M., Omori, A., Ueda, T., Spremulli, L. L., and Watanabe, K. (1998). Mammalian mitochondrial methionyl-tRNA transformylase from bovine liver. purification, characterization, and gene structure. J. Biol. Chem. 273, 15085–15090.
Tang, S., and Huang, T. (2010). Characterization of mitochondrial DNA heteroplasmy using a parallel sequencing system. Biotechniques 48, 287–296. doi: 10.2144/000113389
Targoff, I. N., Trieu, E. P., and Miller, F. W. (1993). Reaction of anti-OJ autoantibodies with components of the multi-enzyme complex of aminoacyl-tRNA synthetases in addition to isoleucyl-tRNA synthetase. J. Clin. Invest. 91, 2556–2564. doi: 10.1172/jci116493
Theunissen, T. E., Szklarczyk, R., Gerards, M., Hellebrekers, D. M., Mulder-Den Hartog, E. N., Vanoevelen, J., et al. (2016). Specific MRI abnormalities reveal severe perrault syndrome due to CLPP defects. Front. Neurol. 7:203. doi: 10.3389/fneur.2016.00203
Theunissen, T. E. J., Gerards, M., Hellebrekers, D. M. E. I., van Tienen, F. H., Kamps, R., Sallevelt, S. C. E. H., et al. (2017a). Selection and characterization of palmitic acid responsive patients with an OXPHOS complex I defect. Front. Mol. Neurosci. 10:336. doi: 10.3389/fnmol.2017.00336
Theunissen, T. E. J., Sallevelt, S. C. E. H., Hellebrekers, D. M. E. I., de Koning, B., Hendrickx, A. T. M., van den Bosch, B. J. C., et al. (2017b). Rapid resolution of blended or composite multigenic disease in infants by whole-exome sequencing. J. Pediatr. 182:e372. doi: 10.1016/j.jpeds.2016.12.032
Thorburn, D. R., and Dahl, H. H. (2001). Mitochondrial disorders: genetics, counseling, prenatal diagnosis and reproductive options. Am. J. Med. Genet. 106, 102–114. doi: 10.1002/ajmg.1380
Triepels, R., Smeitink, J., Loeffen, J., Smeets, R., Trijbels, F., and van den Heuvel, L. (2000). Characterization of the human complex I NDUFB7 and 17.2-kDa cDNAs and mutational analysis of 19 genes of the HP fraction in complex I-deficient-patients. Hum. Genet. 106, 385–391. doi: 10.1007/s004390000278
van Eijsden, R. G., Gerards, M., Eijssen, L. M., Hendrickx, A. T., Jongbloed, R. J., Wokke, J. H., et al. (2006). Chip-based mtDNA mutation screening enables fast and reliable genetic diagnosis of OXPHOS patients. Genet. Med. 8, 620–627.
van Rij, M. C., Jansen, F. A., Hellebrekers, D. M., Onkenhout, W., Smeets, H. J., Hendrickx, A. T., et al. (2016). Polyhydramnios and cerebellar atrophy: a prenatal presentation of mitochondrial encephalomyopathy caused by mutations in the FBXL4 gene. Clin. Case Rep. 4, 425–428. doi: 10.1002/ccr3.511
Vernau, K., Napoli, E., Wong, S., Ross-Inta, C., Cameron, J., Bannasch, D., et al. (2015). Thiamine deficiency-mediated brain mitochondrial pathology in alaskan huskies with mutation in SLC19A3.1. Brain Pathol. 25, 441–453. doi: 10.1111/bpa.12188
Visch, H. J., Rutter, G. A., Koopman, W. J., Koenderink, J. B., Verkaart, S., de Groot, T., et al. (2004). Inhibition of mitochondrial Na+-Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human complex I deficiency. J. Biol. Chem. 279, 40328–40336. doi: 10.1074/jbc.M408068200
Wang, J., Venegas, V., Li, F., and Wong, L. J. (2011). Analysis of mitochondrial DNA point mutation heteroplasmy by ARMS quantitative PCR. Curr. Protoc. Hum. Genet. 19:116. doi: 10.1002/0471142905.hg1906s68
Wang, Z. G., White, P. S., and Ackerman, S. H. (2001). Atp11p and Atp12p are assembly factors for the F(1)-ATPase in human mitochondria. J. Biol. Chem. 276, 30773–30778. doi: 10.1074/jbc.M104133200
Warnecke, T., Duning, T., Schwan, A., Lohmann, H., Epplen, J. T., and Young, P. (2007). A novel form of autosomal recessive hereditary spastic paraplegia caused by a new SPG7 mutation. Neurology 69, 368–375. doi: 10.1212/01.wnl.0000266667.91074.fe
Wessels, H. J., Vogel, R. O., Lightowlers, R. N., Spelbrink, J. N., Rodenburg, R. J., van den Heuvel, L. P., et al. (2013). Analysis of 953 human proteins from a mitochondrial HEK293 fraction by complexome profiling. PLoS ONE 8:e68340. doi: 10.1371/journal.pone.0068340
Witzemann, V., Schwarz, H., Koenen, M., Berberich, C., Villarroel, A., Wernig, A., et al. (1996). Acetylcholine receptor epsilon-subunit deletion causes muscle weakness and atrophy in juvenile and adult mice. Proc. Natl. Acad. Sci. U. S. A. 93, 13286–13291.
Wong, L. J., Perng, C. L., Hsu, C. H., Bai, R. K., Schelley, S., Vladutiu, G. D., et al. (2003). Compensatory amplification of mtDNA in a patient with a novel deletion/duplication and high mutant load. J. Med. Genet. 40:e125. doi: 10.1136/jmg.40.11.e125
Wortmann, S. B., Koolen, D. A., Smeitink, J. A., van den Heuvel, L., and Rodenburg, R. J. (2015). Whole exome sequencing of suspected mitochondrial patients in clinical practice. J. Inherit. Metab. Dis. 38, 437–443. doi: 10.1007/s10545-015-9823-y
Wortmann, S. B., Vaz, F. M., Gardeitchik, T., Vissers, L. E., Renkema, G. H., Schuurs-Hoeijmakers, J. H., et al. (2012). Mutations in the phospholipid remodeling gene SERAC1 impair mitochondrial function and intracellular cholesterol trafficking and cause dystonia and deafness. Nat. Genet. 44, 797–802. doi: 10.1038/ng.2325
Wrutniak-Cabello, C., Casas, F., and Cabello, G. (2001). Thyroid hormone action in mitochondria. J. Mol. Endocrinol. 26, 67–77. doi: 10.1677/jme.0.0260067
Xie, H. M., Perin, J. C., Schurr, T. G., Dulik, M. C., Zhadanov, S. I., Baur, J. A., et al. (2011). Mitochondrial genome sequence analysis: a custom bioinformatics pipeline substantially improves Affymetrix MitoChip v2.0 call rate and accuracy. BMC Bioinformatics 12:402. doi: 10.1186/1471-2105-12-402
Yan, Q., and Guan, M. X. (2004). Identification and characterization of mouse TRMU gene encoding the mitochondrial 5-methylaminomethyl-2-thiouridylate-methyltransferase. Biochim. Biophys. Acta 1676, 119–126. doi: 10.1016/j.bbaexp.2003.11.010
Keywords: mitochondrial disease, next-generation sequencing, mtDNA sequencing, whole-exome sequencing, diagnostic yield
Citation: Theunissen TEJ, Nguyen M, Kamps R, Hendrickx AT, Sallevelt SCEH, Gottschalk RWH, Calis CM, Stassen APM, de Koning B, Mulder-Den Hartog ENM, Schoonderwoerd K, Fuchs SA, Hilhorst-Hofstee Y, de Visser M, Vanoevelen J, Szklarczyk R, Gerards M, de Coo IFM, Hellebrekers DMEI and Smeets HJM (2018) Whole Exome Sequencing Is the Preferred Strategy to Identify the Genetic Defect in Patients With a Probable or Possible Mitochondrial Cause. Front. Genet. 9:400. doi: 10.3389/fgene.2018.00400
Received: 03 April 2018; Accepted: 03 September 2018;
Published: 12 October 2018.
Edited by:
Enrico Baruffini, Università degli Studi di Parma, ItalyReviewed by:
Agnes Rotig, Institut National de la Santé et de la Recherche Médicale (INSERM), FranceCopyright © 2018 Theunissen, Nguyen, Kamps, Hendrickx, Sallevelt, Gottschalk, Calis, Stassen, de Koning, Mulder-Den Hartog, Schoonderwoerd, Fuchs, Hilhorst-Hofstee, de Visser, Vanoevelen, Szklarczyk, Gerards, de Coo, Hellebrekers and Smeets. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hubert J. M. Smeets, YmVydC5zbWVldHNAbWFhc3RyaWNodHVuaXZlcnNpdHkubmw=
†These authors share Joint senior-authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.