
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet. , 10 January 2017
Sec. Nutrigenomics
Volume 7 - 2016 | https://doi.org/10.3389/fgene.2016.00224
The “westernization” of global eating and lifestyle habits is associated with the growing rate of chronic diseases, mainly cardiovascular diseases, cancer, type 2 diabetes mellitus, and respiratory diseases. The primary prevention approach is to make nutritional and behavioral changes, however, there is another important determinant of our health that only recently has been considered and is the presence of beneficial microorganisms and their products in our gastrointestinal tract. Microorganisms living in our body can alter the fate of food, drugs, hormones, and xenobiotics, and recent studies point to the use of microorganisms that can counteract the harmful effects of certain compounds introduced or produced endogenously in our body. This review considers the effects of the western lifestyle on adiposity, glucose metabolism, oxidative markers and inflammation profile, emphasizes on the studies that have investigated bacterial strains and products of their metabolism that are beneficial under this lifestyle, and examines the screening strategies that recent studies are using to select the most promising probiotic isolates. In addition, we consider the relevance of studying the microbiota of metabolically healthy people under a western lifestyle for the understanding of the key components that delay the development of chronic diseases.
Our modern societies have settled on large urban arrangements that have changed behavior and alimentary patterns. The establishment of new alimentary habits has been influenced by industrialization and technological advances which have minimized the time for preparing and consuming meals, reduced the cost of livestock, dairy, and sugar-sweetened products, vegetable oils, and flours, and increased the availability of these foods, especially, to low-income families (Drewnowski and Popkin, 1997). Nowadays, the trend in nutritional epidemiology is the analysis of dietary patterns (i.e., through food-frequency questionnaires) to assess habits in food consumption. In this line, several studies have focused on identifying the main dietary factors that are common to the modern diet. For instance, Hu et al. (1999) identified a “western dietary pattern” through factor analysis of dietary patterns among cohorts in the United States. The authors described this dietary pattern as a diet with a “higher intake of processed meat, red meat, butter, high-fat dairy products, eggs, and refined grains”. Likewise, Slattery et al. identified a similar dietary pattern with “high levels of red meat, processed meat, fast food, refined grains, and sugar- containing foods, and low levels of vegetables (other than potatoes) and fruits, with the predominant fruit being canned fruit” (Slattery et al., 1998; Hu et al., 1999). Importantly, the “western diet” is no longer restricted to western societies, globalization and urbanization are increasing the worldwide exposition to this dietary pattern. For example, a Japanese study found a “westernized Japanese pattern” associated with “high intakes of bread, meat, processed meat, fruit juice, coffee, black tea, soft drinks, sauces, mayonnaise, and dressing” (Nanri et al., 2012). Overall, the “western diet” can be understood as a dietary pattern with a high intake of refined sugars, refined vegetable oils, and livestock products, and low intake of fresh fruits and vegetables (Cordain et al., 2005).
Concerns with the modern alimentary pattern can be traced back to the scientific literature of 1939, when Weston Price published his findings on modern degeneration related to the modernized diet (Price, 1939). Currently, not only the modern dietary habits are of concern but also low-activity high-stress occupations, sedentarism, alcohol binge drinking, and smoking. These behavior and alimentary patterns will be defined from now on as the “western lifestyle” (CDC, 2012; Parry and Straker, 2013; WHO, 2014). This lifestyle is increasingly being associated with several conditions, including: obesity, Alzheimer's disease (Kanoski and Davidson, 2011), cardiovascular disease, type 2 diabetes mellitus (Bazzano, 2005), non-alcoholic fatty liver disease (NAFLD) (Trovato et al., 2015), hypertension (Geleijnse et al., 2004), osteoporosis (Jehle et al., 2006), autoimmunity (Manzel et al., 2014), and cancer (Adlercreutz, 1990). There are several risk factors for developing chronic diseases, including genetic, environmental, demographic, social, and other factors that are not the scope of this review, instead the objective of this review is to relate the research made in the fields of microbiology, immunology, and nutrition to explain the role of gut microbiota as a risk factor of “western lifestyle”-related chronic conditions, and then the strategies that are being developed to shift the gut microbiota from a risk factor toward a more protective state that helps ameliorate the effects of this lifestyle.
The concept of the human microbiota, as first described by Joshua Lederberg, is defined as “the ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space” (Lederberg and McCray, 2001). Major efforts are being made worldwide in order to understand the composition and functional states of the healthy gut microbiota. So far, projects like the Human Microbiome Project Consortium among others (Eckburg et al., 2005; Qin et al., 2010; Huttenhower et al., 2012) have found that the gut microbiome of healthy individuals varies significantly and only dominant bacterial phyla have been consistently described, these are Firmicutes, Bacteroidetes and Actinobacteria, with Proteobacteria and Verrucomicrobia also present in lower abundance. Other studies also evidence that the microbiome of healthy and non-healthy states can be distinguished, as it is the case for ulcerative colitis, Crohn's disease (Qin et al., 2010), chronic fatigue syndrome (Frémont et al., 2013), rheumatoid arthritis (Zhang et al., 2015), type I diabetes (Brown et al., 2011), and type II diabetes (Larsen et al., 2010); nevertheless, one study warns that the patient's treatment can exert changes in the microbiota (Forslund et al., 2015).
Although not a single marker can be identified as representative of a healthy gut microbiome, a higher proportion of butyrate-producing and mucin-degrading bacteria has been mentioned in some studies (Brown et al., 2011; Joossens et al., 2011; Erickson et al., 2012). Butyrate is a short chain fatty acid produced mainly by bacterial fermentation of non-digestible fiber in the colon, and a correct balance of a butyrate-producing microbiota may induce the synthesis of mucin in the gut epithelium thus maintaining gut integrity (Finnie et al., 1995; Brown et al., 2011). Studies have shown that butyrate can enhance the assembly of tight junction proteins through regulation of AMP-activated protein kinase (AMPK), however the mechanism of AMPK activation is unknown (Peng et al., 2009). Butyrate also has anti-inflammatory and anti-carcinogenesis effects, mainly by two mechanisms: activation of GPCRs (GPR41 and GPR43) and inhibiton of histone deacetylase (HDAC). Some of the effects of butyrate that have been observed are enhancement of the expression of certain pro-apoptotic genes in malignant cells and suppression of the pro-inflammatory pathway of Nuclear Factor kappa beta (NF-κβ) (Vinolo et al., 2011). It is estimated that butyrate producers represent approximately 25% of all human fecal bacteria (Louis et al., 2010). Meanwhile, Bifidobacteria is another important group for colon health, they represent about <5% of the microbiota in adult subjects. In disease states like Clostridium difficile associated diarrhea, a 3 log10 reduction of this group of bacteria can occur (Hopkins et al., 2001). Bifidobacteria contributes to colon health through the production of organic acids, like acetate and lactate, that are then used by butyrate-producing bacteria. Thus, a high abundance of butyrate-producers, mucin-degraders, and Bifidobacteria could be an indicator of good health.
Another common feature in some studies is greater gut diversity in healthy states. In lean twins, a greater bacterial diversity has been observed compared to their obese twins (Turnbaugh et al., 2009), in patients with morbid obesity subjected to a gastric bypass an increased richness of gut microbiota was also observed after the surgery along with positive health outcomes (Kong et al., 2013), and another study analyzed the microbiota of non-colic and colic infants finding a higher microbiota diversity in non-colic infants during the first weeks after birth (de Weerth et al., 2013). Hence, a high bacterial diversity can be another indicator of a healthy gut microbiota.
In terms of western diet, Yatsunenko et al. observed that American microbiomes were enriched with genes degrading simple sugars and amino acids (Yatsunenko et al., 2012). As mentioned earlier, a western diet is characterized by a higher intake of processed meat and red meat, thus individuals following this diet may benefit from a Bacteroides-rich microbiota instead of a Prevotella-rich microbiota. This last type of microorganisms produces more trimethylamine from L-carnitine, a nutrient in red meat, which is then converted to pro-atherosclerotic trimethylamine-N-oxide (TMAO), increasing the risk of atherosclerosis. A study by Lozupone et al. evidenced that the immune dysfunction of HIV-infected individuals compromises their ability to select for bacteria that match their diet, thus HIV-positive individuals following a western diet, instead of having a Bacteroides-rich microbiota have a Prevotella-rich microbiota, which is normally present in individuals consuming a plant-based diet, in consequence, these HIV-positive subjects have an increased incidence of several health risks, including cardiovascular disease (Koeth et al., 2013; Tang et al., 2013; Lozupone et al., 2014). Therefore, a Bacteroides-rich microbiota is of benefit under a western diet, as it has been associated with reduced cardiovascular risk.
In order to reveal the key components that make a healthy western microbiota, studies at the strain-level are needed. Evidence points that different strains have distinct effects, as it is the case with strains belonging to a genus enriched in people following a western diet, Lactobacillus (Armougom et al., 2009; Million et al., 2012; Poutahidis et al., 2013). For example, the administration of Lactobacillus reuteri ATCC PTA 4659 was associated with weight decrease in mice, whereas the administration of L. reuteri L6798 was associated with weight gain (Fåk and Bäckhed, 2012). Differential effects have also been observed on the type of immunological response that the strain elicits, for example, Lactobacillus salivarius CECT5713 induced the anti-inflammatory cytokine IL-10, while Lactobacillus fermentum CECT5716 induced pro-inflammatory cytokines (Díaz-Ropero et al., 2007). Studying the intestinal microbiota at the strain-level has been proved challenging due to the great variability at this taxonomic level among individuals and the lack of reliable, easy to use tools for accurate identification of bacteria at strain level, however, these studies indicate that the insights obtained at the phylum-level are limited and that the understanding of the functionality of strains can help delineate the boundaries of a healthy gut microbiota.
In order to better understand the healthy western microbiota, metabolic healthy individuals following a western lifestyle must be investigated. One potential group of people to be examined is metabolically healthy obeses. The prevalence of obesity in the United States has increased by 75% since 1980 along with the acquisition of a western diet, and is associated with an increased incidence of cardiovascular disease, type 2 diabetes mellitus, hypertension, stroke, dyslipidemia, osteoarthritis, and some cancers (Burton and Foster, 1985; Ogden et al., 2007). However, ~10–30% of obese individuals are metabolically healthy and even have a lifelong health (van Vliet-Ostaptchouk et al., 2014). The physiological factors that characterized a metabolically healthy obese are decreased visceral and liver fat, number of macrophages in adipose tissue, mean adipocyte size, circulating C-reactive protein; while having an increase in serum adiponectin, and adipocyte insulin sensitivity (Klöting et al., 2010). The genetic background might play an important part in this scenario, as it has been observed that some ethnic groups at a higher body mass index (BMI) accumulate less liver fat, a factor that affects the metabolic outcome of the individual (Naukkarinen et al., 2014). A study revealed that liver fat content is higher among Japanese than non-Hispanic whites despite a lower mean BMI, and the difference becomes more robust with a small increase in BMI; this might explain why obesity-related complications in Asians occur at a lower BMI (Azuma et al., 2009).
In African Americans, high rates of fructose malabsorption have been associated with reduced liver fat (Walker et al., 2012). African-Americans also appear to be more resistant to hypertriglyceridemia (high blood levels of triglycerides) associated with insulin resistance (Guerrero et al., 2009). Geographical factors might be also involved; migrants from lower-to-higher chronic disease areas (i.e., Japaneses that migrate to the United States) acquire a higher risk of developing a chronic disease (Marmot et al., 1975). But even under a similar background, differences are observed. Naukkarinen et al. studied 16 Finnish pairs of identical twins in which one twin was obese and the other lean, they found that despite all twin pairs being of the same age, had similar age of onset of obesity and weight difference, half of the obese co-twins were metabolically as healthy as their lean co-twins while the other half of the obese co-twins exhibited a typical response to obesity, this was increased insulin production and resistance, dyslipidaemia, fatty liver, and higher blood pressure; they also observed that the one factor that best predicted the metabolic outcome was the level of liver fat (Naukkarinen et al., 2014).
It is now recognized that the gut microbiota can influence liver fat in the host, thus the microbiota might be one of the factors modulating the individual susceptibility to chronic disease. A study that observed an association of microbiota and liver fat accumulation demonstrated that gut microbiota directly induced NAFLD in mice. The authors performed fecal transplantations from mice that developed, or not, liver steatosis (responders and non-responders, respectively) during a 16 week period of high-fat diet (HFAD) to receiver mice. The responder-receiver mice developed a higher level of liver steatosis and had higher levels of branched-chain fatty acids from bacterial amino acid fermentation than non-responder-receiver mice (Newgard, 2012). Similarly, non-alcoholic steatohepatitis (NASH) (severe hepatic steatosis and liver inflammation) patients had an increase in ester compounds and endogenous alcohol most likely produced from bacterial metabolism compared to patients with simple steatosis (fatty liver) and healthy volunteers. It is worth mentioning that healthy subjects and obese non-NASH patients had similar blood-ethanol concentrations (Raman et al., 2013; Zhu et al., 2013; for a review see Boursier and Diehl, 2016), thus indicating that even under an obese state, non-NASH patients may harbor a microbiota whose functionality resembles the one on a healthy state. In addition, the administration of probiotics can exert a positive effect on liver fat accumulation, which will be mentioned later. One potential mechanism for liver fat accumulation is that bacteria can suppress the expression of a lipoprotein lipase inhibitor, the fasting-induced adipose factor (Fiaf), thus increasing the lipase activity in the gut and affecting the outflow of free fatty acids to the liver (Bäckhed et al., 2007). In order to advance our understanding of the factors influencing metabolic health during a western diet, it is important to explore the microbiome of metabolically healthy individuals following a western diet which stay healthy at an advanced age, such studies might reveal components of the microbiome that can counteract the accumulation of liver fat, protecting the host from further health outcomes.
Nowadays in the modern societies, an unbalanced diet, stress, and smoking can onset the inflammatory response daily, leading to a chronic low-grade systemic inflammation. Inflammation is the process through which the body limits pathogen invasion and controls tissue damage after injury. It is mediated by many soluble factors essential to signal immune cells to eliminate the aggressor and initiate tissue repair. Among these factors are secreted polypeptides called cytokines, which include tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), IL-1 receptor antagonist (IL-1ra), and soluble TNF-α receptor (sTNF-R). TNF-α and IL-1β are pro-inflammatory cytokines, IL-6 has both anti- and pro-inflammatory properties, while IL-10, IL-1ra, and sTNF-R are anti-inflammatory cytokines. In acute inflammation, the levels of cytokines rapidly increase several fold and decrease when the infection is controlled or the injury is healed. However, acute inflammation does not always subside, and can become a chronic low-grade inflammation characterized by a two- to three-fold increase in the concentrations of cytokines and C-reactive protein, a molecule produced by the liver in response to inflammation (Petersen and Pedersen, 2005).
One way the western lifestyle can cause inflammation is by increasing the number of compounds and microbial products with inflammatory capability (Figure 1). Among these are: lipopolysaccharides (LPS) or endotoxins, D-lactate, acetaldehyde, hydrogen sulfide, toxic products of bacterial protein metabolism, and oxidative radicals, as described below. A role of antibiotics is also discussed.
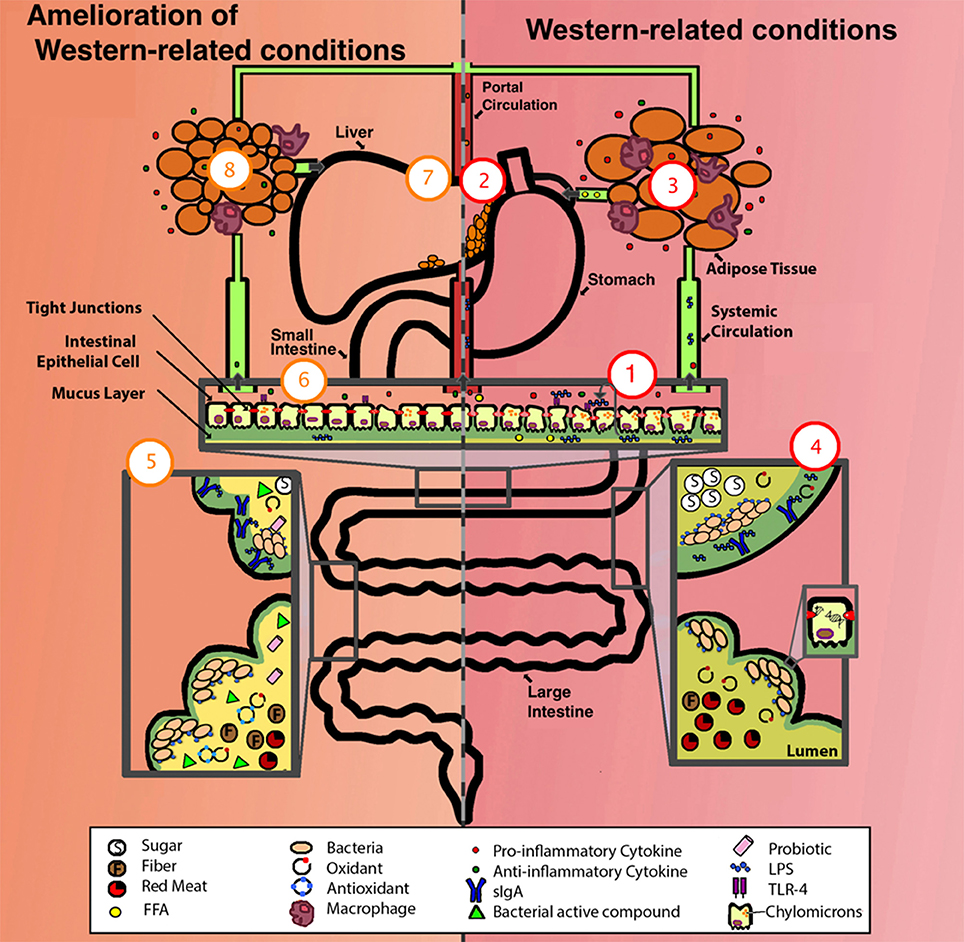
Figure 1. Comparison of a system suffering from western-related conditions (right, in red) and a system with amelioration of western-related conditions (left, in orange). One aspect of a western lifestyle is the higher intake of ω-6 PUFA (depicted as FFA), this enhances the formation of chylomicrons allowing the translocation of LPS, these then activate basolateral TLR which initiates a pro-inflammatory response, one overall consequence is the alteration of the gut epithelium and its permeability (depicted as deteriorated epithelium and compromised tight junctions), exacerbating inflammation by allowing the translocation of more LPS, pro-inflammatory cytokines, FFA, among other luminal compounds (1). LPS/ pro-inflammatory cytokines/ FFA can enter portal and systemic circulation, one consequence is the alteration of fat metabolism, thus enhancing fat accumulation in liver (2), and in adipose tissue, adipocytes increase in size, FFA synthesis is enhanced (depicted as FFA in circulation), and an elevated pro-inflammatory state occurs (depicted as increased infiltration of macrophages and production of pro-inflammatory cytokines) (3). The western lifestyle includes higher intake of simple sugars and red-meat, lower intake of antioxidants (depicted as presence of oxidants), and sedentarism (depicted as low production of sIgA), some of the consequences are lower-capacity for antigen neutralization (depicted as LPS not bound to sIgA) and damage to the DNA of epithelial cells (depicted as DNA strand breakage) (4). For the amelioration of these conditions, a person can take different approaches, these include exercise (depicted as high production of sIgA), intake of dietary nutrients (i.e., polyphenols and ω-3 PUFAs) (depicted as antioxidants), probiotics, prebiotics, and SCFA (depicted as fiber, bacterial active compounds and probiotics) (5). Some of the effects of these approaches include the reestablishment of gut epithelium permeability and a decrease in LPS translocation, TLR activation, chylomicron formation, presence of LPS/cytokines/FFA in portal and systemic circulation (6), liver fat (7), adipocyte size, FFA synthesis, macrophage infiltration in adipose tissue (8), and an overall amelioration of the inflammatory state (depicted as a higher concentration of anti-inflammatory cytokines compared to pro-inflammatory cytokines [6 and 8]). FFA, free fatty acids. For more details see the text.
LPS are part of the outer membrane of Gram-negative bacteria and are one of the most important compounds that can induce a low-grade inflammation. LPS is bound by Toll like receptors (TLR) in cell surfaces, specifically TLR-4. Particularly in intestinal epithelial cells, these receptors mediate the inflammatory response triggering different mechanisms depending on its membrane location, apical or basolateral. Apical TLR are normally exposed to luminal antigens, including bacteria and their LPS, and their stimulation results in a homeostatic response and tolerance but not inflammation. In contrast, basolateral TLR are exposed to antigens only if these have crossed important epithelial barriers, and are potentially infectious. Therefore, basolateral TLR stimulation triggers the activation of the transcription factor NF-kβ, one of the most important mediators of the pro-inflammatory response (Wells et al., 2011). Extra-luminal LPS can also reach the bloodstream, and subsequently, bind TLR on the surface of other cells, like blood vessel, muscle, joint, adipose, and hepatic Kupffer cells. Their activation affects processes like insulin signaling, adipose tissue differentiation, lipogenesis, and it has been suggested that the interaction LPS-adipocyte–macrophage can amplify the low-grade inflammation to the level of influencing metabolic disorders (Muccioli et al., 2010). Thus, inflammation is a mechanism vital to set a prompt response to pathogens, however, LPS can onset a low-grade inflammatory response that may alter the metabolic status of the host by unknown molecular mechanisms (Nakarai et al., 2012). LPS are being increasingly associated with a number of conditions summarized in Table 1.
Several behaviors associated with the western lifestyle can affect the levels of plasmatic LPS. Among these are sedentarism, smoking, stress, and an unhealthy diet. Lira et al. showed that sedentary people had higher levels of plasmatic LPS than highly trained people at rest (Lira et al., 2010). Pace et al. observed that cigarette smoke increased the expression of TLR-4 and LPS binding (Pace et al., 2008). Furthermore, it has been demonstrated that stress hormones stimulate the growth of LPS-containing bacteria such as Yersinia enterocolitica and Escherichia coli, and indeed, stress hormones achieved a 100,000-fold increase in viable E. coli in the cecum of mice within 24 h and promote the synthesis of an autoinducer of bacterial growth (Lyte and Ernst, 1992; Lyte et al., 1996; Lyte and Bailey, 1997; Freestone et al., 2008). Meanwhile, Cani et al. observed that a 4-week HFAD chronically increased plasma LPS concentration two to three times (Cani et al., 2007).
Among the factors that increase the abundance of plasmatic LPS, diet is the best studied. It is recognized that HFADs induce high levels of LPS in the blood through the stimulation of chylomicron (droplets of fat) formation in intestinal epithelial cells, this facilitates LPS transcellular transport across the gut epithelium and subsequently, LPS reach the bloodstream (Ghoshal et al., 2009). However, several investigations point that not every type of HFADs increases the concentration of plasmatic LPS. HFADs consisting of oils rich in ω-6 polyunsaturated fatty acids (ω-6 PUFA), like safflower oil, cause a markedly increase in the concentration of plasmatic LPS and pro-inflammatory cytokines compared to diets rich in coconut oil or fish oil, which instead are protective against a LPS challenge (Mascioli et al., 1988; Sadeghi et al., 1999). Meanwhile, a high-fructose diet (HFUD) promotes a more pronounced increase in plasmatic LPS concentration than diets rich in glucose. The mechanism for this is unknown, but evidence suggests that HFUD effects are related to the gut microbiota, since observations that oral non-absorbable antibiotics (antibiotics that act locally in the gut) can prevent the increase of plasmatic LPS, while the knockout of the LPS receptor TLR-4 greatly decreases lipid peroxidation, expression of TNF-α, and accumulation of fat in the liver that occurs in fructose-fed mice (Di Luccia et al., 2015). In humans, HFUD is also associated with NAFLD (Bergheim et al., 2008; Ouyang et al., 2008). The distinct effects of different fats and sugars might explain some of the variability of diet response among studies.
Bacterial products of metabolism released in our gut depend heavily on diet, host secretions and digestive enzymes, local conditions of pH, oxygen, and hydrogen, gut transit time, and the composition and activity of the microbiota, among other factors (Salonen and de Vos, 2014). Undigested dietary residues that arrive to the large intestine are the main substrates of bacterial metabolism, along with diet-independent substrates like endogenous host secretions. Undigested carbohydrates are fermented mainly to short-chain fatty acids (SCFAs) (such as butyrate, acetate, and propionate) and gases (mainly carbon dioxide, hydrogen, and methane) (Flint et al., 2012). However, an excessive consumption of carbohydrates can also increase the concentration of toxic compounds derived from microbial metabolism, as it is the case of D-lactate, which is produced during carbohydrate fermentation by D-lactic acid bacteria. This compound inhibits the transport of L-lactate and pyruvate, both essential for mitochondrial energy production (Ling et al., 2012). Several conditions have been associated with high concentration of D-lactate, among these are chronic fatigue syndrome, diarrhea, short bowel syndrome, and diabetes (Uribarri et al., 1998; Ewaschuk et al., 2005; Sheedy et al., 2009). Another toxic compound that has been associated with the excessive consumption of carbohydrates and alcoholic drinks is acetaldehyde. This compound is produced by ethanol-oxidizing bacteria and yeast, and is formed during ethanol metabolism. When acetaldehyde is metabolized, oxidative radicals are generated, altering the permeability of the intestinal epithelium facilitating the translocations of luminal contents to the bloodstream (Atkinson and Rao, 2001). Acetaldehyde is also a known carcinogenic compound (Salaspuro, 1996; Wright et al., 1999).
While carbohydrates are fermented in the proximal colon, amino acids are fermented in the distal colon and this results in branched-chain fatty acids and potentially toxic metabolites such as ammonia, phenols, indoles, amines, TMAO, and volatile sulfur compounds (den Besten et al., 2013), some of which are associated with the increased incidence of colorectal cancer (Hughes et al., 2000; Russell et al., 2011) and atherosclerosis (Koeth et al., 2013) in high-red meat diets, fresh or processed. In the case of ammonia, higher levels of this compound in the blood can enter the brain and cause conditions like hepatic encephalopathy. Ammonia is a concern in subjects with chronic diseases in the thyroid gland, kidneys, lungs, and liver, and it is been increasingly associated with diabetes, extreme obesity (Bengmark, 2009), and tumor promotion (Hughes et al., 2000). Interestingly, the evidence suggests that white meat (poultry and fish) do not have the same detrimental effects of red meat. A possible explanation is the higher content of dietary haem in red meat, which will provide a source of iron for some proteins that can form toxic nitrosating agents from nitric oxide under anaerobic conditions (Wade and Castro, 1990).
Another toxic compound of bacterial protein or carbohydrate metabolism is hydrogen sulfide (H2S). This is produced by sulfate-reducing bacteria during the oxidation of a wide range of substrates found in the large intestine (Gibson et al., 1991). In western countries there is a high incidence of people with a sulfate-reducing bacteria, 50–70% compared to 10–20% of rural black Africans. The H2S produced by this group of bacteria can cause DNA damage in susceptible subjects with genetic predisposition that compromises DNA repair, as it is observed in patients suffering from ulcerative colitis and colorectal cancer (Attene-Ramos et al., 2006). The concentration of these toxic compounds of bacterial protein and carbohydrate metabolism in the intestinal lumen might be the result of interplay between the microbiota capacity to produce them and the host capacity to clear them up; in addition, the metabolic effect of these compounds would depend on the host susceptibility.
Oxidative radicals are normally produced in high concentrations during food digestion and are also generated during cigarette smoking. Ingestion of food with antioxidants can control the exposure to these compounds, consequently diets with low levels of antioxidants will not subside the constant oxidative stress that occur in the gut and lung epitheliums (van der Vaart et al., 2004; Bloomer and Fisher-Wellman, 2010; Caesar et al., 2010). Overnutrition also increases the oxidative stress in the endoplasmatic reticulum, this activates a mediator of inflammation normally inactive in the hypothalamus, the kinase IKKβ, which regulates NF-κβ through the phosphorylation of its inhibitor IkBα (Zhang et al., 2008). Oxidative stress also increases the activity of the PI 3-kinase and the myosin light chain kinase promoter that regulate the opening of the intestinal tight junction barrier. Thus, oxidative stress mediates the enlargement of the spaces in the gut epithelium allowing the translocation of normally non-invasive bacteria or their toxic products and components, which will induce the activation of NF-κβ perpetuating a vicious cycle of NF-κβ activation and impairment of the tight junction barrier (Sheth et al., 2003; Maes and Leunis, 2008).
In summary, the NF-κβ pathway that mediates inflammation can be activated by several cellular stresses, including LPS and compounds that generate cellular damage, like D-lactate, acetaldehyde, and H2S. Importantly, the activation of NF-κβ in parts of the body different from the gastrointestinal tract might eventually alter the permeability of the intestinal epithelium, facilitating the translocation of luminal materials, including LPS, which will exacerbate the low-grade inflammation state.
The use of antibiotics in the modern era, including the extensive and inappropriate use in humans and animals, has changed the gut microbiota and this has diverse health implications. Broad-spectrum antibiotics can impact the gut microbiota causing a dysbiosis (“a pathological imbalance in a microbial ecological niche” Jones et al., 2014) which can alter the microbiota capacity to prevent the colonization and growth of pathogens and pathobionts with inflammatory capability. Two meta-analyses, one in >56,000 patients with C. difficile infection and the other in >7000 inflammatory bowel disease (IBD) patients showed that antibiotics were a high risk factor for the development of these diseases (Furuya-Kanamori et al., 2014; Ungaro et al., 2014). Depending on the class of antibiotic, the dosage, time of administration, and other antibiotic-independent factors, like genetic predisposition, sex, diet, physical activity, disease, and environmental toxicants, antibiotics can exert effects on the weight (underweight and overweight states) and metabolic profile (pro-diabetic and anti-diabetic effect) of an individual (for a review see Cox and Blaser, 2015). Antibiotic use carries other risks, like the dissemination of bacterial resistant genes and the alteration of the well-established host-microbiota symbiosis through the eradication of important susceptible strains (Blaser and Falkow, 2009). Recently, Moeller et al. demonstrated the cospeciation of certain symbiotic bacterial strains with hominids, including humans (Moeller et al., 2016). This unique set of symbionts might provide beneficial health effects to the host and could be under selective pressure by the modern use of antibiotics.
The approaches that can effectively counteract the effects of the western lifestyle are the ones that mitigate the translocation of LPS, prevent toxic microbial metabolism, and modulate the pro-inflammatory response and oxidative stress. Among these approaches are: exercise, dietary compounds, probiotics, prebiotics, and short chain fatty acids (SCFAs) (Figure 1 and Table 2), as described below.
Regular moderate doses of physical activity can ameliorate the effect of an LPS insult. In addition, it has been shown that exercise: controls the levels of pro-inflammatory cytokines, is associated with less liver fat (Starkie et al., 2003; Nichol et al., 2008), protects against insulin resistance (Schmitz et al., 2002), and increases the levels of SCFAs. The main SCFAs are butyrate, acetate and propionate, and these have anti-carcinogenic as well as anti-inflammatory properties and are essential for colon health (Matsumoto et al., 2012). Exercise can also modulate the microbiota, mice who exercised had lower intestinal and systemic bacterial loads than the group of sedentary mice, and had higher total and specific intestinal secretory immunoglobulin A (sIgA) which are the antibodies that control luminal antigens (Campos-Rodríguez et al., 2016).
Dietary compounds can also be introduced to prevent the negative effects of a western lifestyle. Among these are: dietary polyphenols and ω-3 PUFAs. Polyphenols can be found in wine, cocoa, cranberry, grape, curcumin, propolis, coffee, and tea; they function as antioxidants (Sies et al., 2005), strengthen intestinal barrier function (Wang et al., 2016), prevent endotoxemia (presence of LPS in the blood), the loss of some beneficial bacterial strains, and the development of diabetes (He et al., 2012; Cowan et al., 2013; Anhê et al., 2015). The other compounds are ω-3 PUFA, which are found in fish and olive oil, their addition to a high ω-6 PUFA diet can reverse some of the inflammatory effects of ω-6 PUFA, like immune cell infiltration and NF-kβ activation (Ghosh et al., 2013). It is possible that the beneficial effects of some of these dietary compounds are exerted through the modulation of the microbiota. For example, the administration of cranberry extract and grape polyphenols is associated with an increased abundance of the beneficial genus Akkermansia even under a high sucrose and/or HFAD, while ω-3 PUFA have been shown to enrich Lactobacillus and Bifidobacteria (Thompson and Spiller, 1995; Ghosh et al., 2013; Anhê et al., 2015; Roopchand et al., 2015).
A probiotic can be defined as “a live microorganism that, when administered in adequate amounts, confers a health benefit on the host” (FAO/WHO, 2002). Several recent studies have evaluated the benefits of probiotic supplementation in the absence of lifestyle changes (Doron and Gorbach, 2006). One example is the study of Park et al. who observed that mice following a HFAD for 8 weeks and supplemented with Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 for another 10 weeks gained 38% less weight than the unsupplemented controls (Park et al., 2013a). The same group also demonstrated that L. curvatus HY7601 and L. plantarum KY1032 at high (1010 cfu/d) or low dosage (109 cfu/d) lowered plasma glucose, insulin, triglycerides, and oxidative stress levels in rodents fed a HFUD, while only at high doses lower liver mass and liver cholesterol were achieved (Park et al., 2013b).
The bacteria Akkermansia muciniphila and Bacteroides uniformis have also been evaluated under a HFAD. Everard et al. showed that A. muciniphila reduced plasma levels of LPS, adiposity, insulin resistance, body weight (without changing food intake), hyperglycemia, increased adipocyte differentiation and lipid oxidation. The supplementation of live cells of A. muciniphila also prevented the thinning of the mucus layer that occurred when mice were fed a HFAD (Everard et al., 2013). Meanwhile, Gauffin Cano et al. (2012) showed that an oral administration of B. uniformis CECT 7771 significantly reduced total body weight gain, liver fat, levels of cholesterol and triglycerides. Furthermore, B. uniformis CECT 7771 improved glucose metabolism, insulin and leptin sensitivity, and immune function of macrophages and dendritic cells. The authors also measured the number of fat micelles per enterocyte as an indicator of intestinal lipid absorption which contributes to adiposity, and B. uniformis CECT 7771 also achieved a significant reduction in this aspect (Gauffin Cano et al., 2012). It was also demonstrated, in meat-fed rats, that Lactobacillus acidophilus strains NCFM and N-2 promoted a significantly lower production of free amines (Goldin and Gorbach, 1984a) and lowered significantly, in rats and subjects, the activity of cecal bacterial ß-glucuronidase, nitro-reductase, and azoreductase enzymes which are responsible for the generation of potential precarcinogenic compounds (Goldin and Gorbach, 1984b).
Studies have not only evaluated probiotic effects under a particular nutritional environment but have examined their effect on the treatment of alcohol-drinking and smoking induced diseases. Several studies have shown an improvement of alcohol-induced liver injury in mice and human subjects. For example, Kirpich et al. performed a pilot study evaluating the effect of a 5-day probiotic supplementation consisting of Bifidobacterium bifidum and L. plantarum 8PA3 on 66 alcoholic individuals, the subjects under the probiotic treatment had significantly lower alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity than those treated with standard therapy (abstinence plus vitamins) (Kirpich et al., 2008). Then again, the same group demonstrated that a Lactobacillus rhamnosus GG supplementation during the last 2-weeks of a 8-week diet containing 5% alcohol significantly improved liver function and reduced alcohol-induced endotoxemia, and hepatic steatosis in mice (Wang et al., 2011). Meanwhile, Naruszewicz et al. determined that the administration of L. plantarum 299v to heavy smokers for 6 weeks with no changes in lifestyle led to the significant reduction in systolic blood pressure, leptin, fibrinogen, IL-6, and monocytes adhesion to vein endothelial cells, thus reducing their risk of cardiovascular disease (Naruszewicz et al., 2002).
Prebiotics are non-digestible fiber that promotes the growth of beneficial microorganisms in the gastrointestinal tract. Some examples are: inulin, fructooligosaccharides, resistant starch, pectin, among others. These are metabolized to SCFAs, mainly propionate, acetate and butyrate, which as mentioned earlier exert many beneficial health outcomes. SCFAs activate the SCFA receptor GPR43 that reduces insulin sensitivity in adipose tissue and hence its fat accumulation, thereby reducing the uptake, synthesis, and oxidation of toxic fatty acids in other tissues (Kimura et al., 2013; Park et al., 2015). They also increase proliferation and inhibit apoptosis of intestinal cells (Tappenden et al., 2003), hinders intestinal secretion of chylomicron into the circulation (Marcil et al., 2003), and limits inflammation perhaps through inhibition of the NF-κβ pathway (Delzenne et al., 2013). Galisteo et al. analyzed several studies that showed that prebiotics reduce all the abnormalities clustered in the metabolic syndrome, including: body weight gain, dyslipidemia, inflammation, hypertension, and insulin resistance (Galisteo et al., 2008).
One drawback of prebiotics is that they can cause intestinal tract discomfort in individuals with limited microbial capacity to ferment the prebiotic. Thus, novel approaches to deliver the benefits of prebiotics have been developed. Chambers et al. (2015) designed an improved prebiotic compound linked to a SCFA, propionate, which exploits the benefits of prebiotics while reducing the amount to be administrated. SCFAs have many health benefits, but if they are supplemented orally, they will be absorbed in the upper part of the small intestine where their benefits are limited. In contrast, this new compound ensures the delivery of the SCFA directly to the colon and at the same time, reduces the patient complains about prebiotics (e.g., gas production and bloating). Only 10 g of the inulin-propionate ester achieved a 2.5-fold increase in colonic propionate, this in consequence, prevented weight gain, abdominal adiposity, liver fat, and reduced insulin resistance significantly more than in the prebiotic-only control group (Frost et al., 2013; Chambers et al., 2015).
Probiotics and prebiotics are already been used clinically for the improvement of fatty liver (Ma et al., 2013), minimal hepatic encephalopathy (Liu et al., 2004), diabetes (Asemi et al., 2014), abdominal adiposity (Kadooka et al., 2010), chronic fatigue syndrome (Maes and Leunis, 2008), diarrhea, and Clostridium difficile disease (McFarland, 2006), among others. Their supplementation is one alternative that is simple, safe, and that improves several health parameters simultaneously.
There is great interest in developing commercial probiotic formulations that include new beneficial strains. Thus, several studies focus on different screening strategies to find promising strains that can favorably shape host pathways. These strains can act directly or indirectly on the cells of the immune system, epithelial cells, adipocytes, beta pancreatic cells, and can also control pathobionts. For instance, Gauffin Cano et al. screened for the immunomodulation capabilities among different strains of Bacteroides spp., they carefully selected for a specific strain that had the lowest inflammatory potential on macrophages in vitro, specifically, low TNF-α and high IL-10 production (Gauffin Cano et al., 2012). Poutahidis et al. (2013) also demonstrated that L. reuteri protected the host from obesity through an immunomodulatory mechanism, specifically, L. reuteri had an effect on the IL-10-dependent function of CD4+ T cells. Interestingly, the researchers could replicate the phenotype of the probiotic-supplemented mice in naïve recipient rodents by transferring only the purified CD4+ T cells (Poutahidis et al., 2013). Meanwhile, Ito et al. screened the inhibitory activity of 49 lactic bacterial strains on lipid peroxidation in vivo and in vitro (Ito et al., 2003). While Kullisaar et al. measured the capacity of L. fermentum ME-3 to reduce oxidative stress, blood triglyceride levels, and lipoprotein status post-prandially (2 h after a meal) in a randomized double-blind placebo-controlled study with 100 healthy subjects (Kullisaar et al., 2011). Lastly, Chung et al. (2016) screened for a FFAs-absorbing strain, L. reuteri JBD30 l, in a fecal sample of a healthy lean subject. The administration of this strain to experimental animals and human subjects under a clinical trial lowered the concentration of FFAs in the fluid of the small intestine thus increasing fecal fat excretion, the efficacy was comparable to the one obtained for orlistat, a FDA-approved pharmaceutical that also increases the content of fat in feces (Chung et al., 2016).
There are other reported mechanisms that can guide screening studies, among these are the increment in the expression of lectins against Gram-positive bacteria, e.g., A. muciniphila produces RegIIIγ (Everard et al., 2013); the inhibition of T cell activation, e.g., S. boulardii produces a <3 kDa protein that has this effect (Thomas et al., 2011); production of phosphatases that can dephosphorylate LPS, as it has been observed also in S. boulardii (Buts et al., 2006); upregulation of the expression of cytoprotective heat shock proteins that increase the protection against oxidative damage and gut barrier loss in intestinal cells, e.g., Bacillus subtilis produces a quorum-sensing signal molecule, the competence- and sporulation-stimulating factor, which induces the heat shock protein Hsp27 (Fujiya et al., 2007); inhibition of the hydrogen peroxide-induced epithelial barrier disruption, e.g., L. rhamnosus GG produces two soluble proteins, p40 and p75, that control this aspect (Seth et al., 2008); inhibition of NF-kβ pathway (Petrof et al., 2004); and enhancement of SCFA production (Kimura et al., 2013; Park et al., 2015).
The western lifestyle causes the overproduction of inflammation signals and underprovides the means to block them, driving the body into a chronic low inflammation state. To avoid some negative consequences, people can introduce light exercise, simple dietary compounds, probiotics, prebiotics and/or SCFAs into their daily routine. Interestingly, several recent studies have proved that the effects of probiotics and prebiotics can even be exploited under a HFAD and smoking conditions, providing a way to extend the health of a person with a western lifestyle. The presence of probiotics in dairy products has made them well accepted and recognized by their health benefits on the gastrointestinal tract, and given that the clinical evidence points that they also have benefits on the lipid and glucose metabolism, gut permeability, mood, and immune system, it is foresighted that this field will keep introducing new probiotic strains to the market, perhaps specific formulations depending on the desired benefit. We proposed that for the advancement of this field, it is important to understand if there is a microbiological component that is extending the health of asymptomatic lean, overweight and obese people following a western diet, and the factors that increase the fitness of these strains in the western microbiome. Ultimately, considering that in western countries the most prevalent diseases are inflammatory in nature, it will be important that in the near future, inflammation markers would be routinely screened in the clinical setup and anti-inflammatory probiotics administered as an alternative preventive measure.
GR conceived the work, GR, AC, AR, and FL wrote the paper and revised it critically.
Research supported by Direction of Research (DIN), from Universidad de La Sabana (MED-198-2015), GR is supported by a grant from Colciencias, 647 2015. AR is supported by proyecto FAPA, P14.160422.001, Universidad de los Andes.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
BMI, Body mass index; HFAD, high-fat diet; HFUD, high-fructose diet; H2S, hydrogen sulfide; IL-1ra, IL-1 receptor antagonist; IL-10, interleukin-10; IL-1β, interleukin-1β; IL-6, interleukin-6; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; LPS, lipopolysaccharides; NASH, Non-alcoholic steatohepatitis; NF-κβ, Nuclear Factor kappa beta; SCFA, short chain fatty acid; sTNF-R, soluble TNF-α receptor; TLR, Toll like receptors; TNF-α, tumor necrosis factor-α; TMAO, trimethylamine-N-oxide; ω-3 PUFAs, ω-3 polyunsaturated fatty acids; ω-6 PUFA, ω-6 polyunsaturated fatty acids.
Adlercreutz, H. (1990). Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Invest. Suppl. 50(Suppl. 201), 3–23.
Anhê, F. F., Roy, D., Pilon, G., Dudonné, S., Matamoros, S., Varin, T. V., et al. (2015). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64, 872–883. doi: 10.1136/gutjnl-2014-307142
Armougom, F., Henry, M., Vialettes, B., Raccah, D., and Raoult, D. (2009). Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 4:e7125. doi: 10.1371/journal.pone.0007125
Asemi, Z., Khorrami-Rad, A., and Alizadeh, S. (2014). Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin. Nutr. 33, 198–203. doi: 10.1016/j.clnu.2013.05.015
Atkinson, K. J., and Rao, R. K. (2001). Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am. J. Physiol. Gastrointest Liver Physiol. 280, G1280–G1288.
Attene-Ramos, M. S., Wagner, E. D., Plewa, M. J., and Gaskins, H. R. (2006). Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 4, 9–14. doi: 10.1158/1541-7786.MCR-05-0126
Azuma, K., Kadowaki, T., Cetinel, C., Kadota, A., El-Saed, A., Kadowaki, S., et al. (2009). Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism. 58, 1200–1207. doi: 10.1016/j.metabol.2009.03.021
Bäckhed, F., Manchester, J. K., Semenkovich, C. F., and Gordon, J. I. (2007). Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. U.S.A. 104, 979–984. doi: 10.1073/pnas.0605374104
Bazzano, L. (2005). Dietary Intake of Fruit and Vegetables and Risk of Diabetes Mellitus and Cardiovascular Diseases. Geneva: WHO.
Bengmark, S. (2009). Bio-ecological control of chronic liver disease and encephalopathy. Metab. Brain Dis. 24, 223–236. doi: 10.1007/s11011-008-9128-z
Bergheim, I., Weber, S., Vos, M., Krämer, S., Volynets, V., Kaserouni, S., et al. (2008). Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J. Hepatol. 48, 983–992. doi: 10.1016/j.jhep.2008.01.035
Blaser, M. J., and Falkow, S. (2009). What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 7, 887–894. doi: 10.1038/nrmicro2245
Bloomer, R. J., and Fisher-Wellman, K. H. (2010). Lower postprandial oxidative stress in women compared with men. Gend. Med. 7, 340–349. doi: 10.1016/j.genm.2010.07.001
Boursier, J., and Diehl, A. M. (2016). Nonalcoholic fatty liver disease and the gut microbiome. Clin. Liver Dis. 20, 263–275. doi: 10.1016/j.cld.2015.10.012
Brown, C. T., Davis-Richardson, A. G., Giongo, A., Gano, K. A., Crabb, D. B., Mukherjee, N., et al. (2011). Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 6:e25792. doi: 10.1371/journal.pone.0025792
Burton, B. T., and Foster, W. R. (1985). Health implications of obesity: an NIH consensus development conference. J. Am. Diet. Assoc. 85, 1117–1121.
Buts, J. P., Dekeyser, N., Stilmant, C., Delem, E., Smets, F., and Sokal, E. (2006). Saccharomyces boulardii produces in rat small intestine a novel protein phosphatase that inhibits Escherichia coli endotoxin by dephosphorylation. Pediatr Res. 60, 24–29. doi: 10.1203/01.pdr.0000220322.31940.29
Caesar, R., Fåk, F., and Bäckhed, F. (2010). Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism: review. J. Intern. Med. 268, 320–328. doi: 10.1111/j.1365-2796.2010.02270.x
Campos-Rodríguez, R., Godínez-Victoria, M., Arciniega-Martínez, I. M., Resendiz-Albor, A. A., Reyna-Garfias, H., Cruz-Hernández, T. R., et al. (2016). Protective effect of moderate exercise for BALB/c mice with salmonella typhimurium infection. Int. J. Sports Med. 37, 63–70. doi: 10.1055/s-0035-1559697
Cani, P. D., Amar, J., Iglesias, M. A., Poggi, M., Knauf, C., Bastelica, D., et al. (2007). Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772. doi: 10.2337/db06-1491
CDC (2012). Vital Signs: Binge Drinking Prevalence, Frequency, and Intensity Among Adults - United States, 2010. Morbidity and mortality weekly report 61. Atlanta: CDC.
Chambers, E., Viardot, A., Psichas, A., Morrison, D. J., Murphy, K. G., Zac-Varghese, S. E., et al. (2015). Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754. doi: 10.1136/gutjnl-2014-307913
Chung, H. J., Yu, J. G., Lee, I. A., and Liu, M. J. (2016). Intestinal removal of free fatty acids from hosts by Lactobacilli for the treatment of obesity. FEBS Open Bio. 6, 64–76. doi: 10.1002/2211-5463.12024
Cordain, L., Eaton, S. B., Sebastian, A., Mann, N., Lindeberg, S., Watkins, B. A., et al. (2005). Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 81, 341–354.
Coussens, L. M., and Werb, Z. (2002). Inflammation and cancer. Nature 420, 860–867. doi: 10.1038/nature01322
Cowan, T. E., Palmnas, M., Ardell, K., Yang, J. J., Reimer, R., Vogel, H., et al. (2013). Chronic coffee consumption alters gut microbiome: potential mechanism to explain the protective effects of coffee on type 2 diabetes? FASEB J. 27, 951–951.
Cox, L. M., and Blaser, M. J. (2015). Antibiotics in early life and obesity. Nat. Rev. Endocrinol. 11, 182–190. doi: 10.1038/nrendo.2014.210
Delzenne, N. M., Neyrinck, A. M., and Cani, P. D. (2013). Gut microbiota and metabolic disorders: how prebiotic can work? Br. J. Nutr. 109(Suppl.), S81–S85. doi: 10.1017/S0007114512004047
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D. J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid. Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
de Weerth, C., Fuentes, S., Puylaert, P., and de Vos, W. M. (2013). Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 131, e550–e558. doi: 10.1542/peds.2012-1449
Díaz-Ropero, M. P., Martín, R., Sierra, S., Lara-Villoslada, F., Rodríguez, J. M., Xaus, J., et al. (2007). Two Lactobacillus strains, isolated from breast milk, differently modulate the immune response. J. Appl. Microbiol. 102, 337–343. doi: 10.1111/j.1365-2672.2006.03102.x
Di Luccia, B., Crescenzo, R., Mazzoli, A., Cigliano, L., Venditti, P., Walser, J. C., et al. (2015). Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS ONE 10:e0134893. doi: 10.1371/journal.pone.0134893
Dohgu, S., and Banks, W. A. (2008). Lipopolysaccharide-enhanced transcellular transport of HIV-1 across the blood-brain barrier is mediated by the p38 mitogen-activated protein kinase pathway. Exp. Neurol. 210, 740–749. doi: 10.1016/j.expneurol.2007.12.028
Doron, S., and Gorbach, S. L. (2006). Probiotics: their role in the treatment and prevention of disease. Expert Rev. Anti. Infect. Ther. 4, 261–275. doi: 10.1586/14787210.4.2.261
Drewnowski, A., and Popkin, B. M. (1997). The nutrition transition: new trends in the global diet. Nutr Rev. 55, 31–43. doi: 10.1111/j.1753-4887.1997.tb01593.x
Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., et al. (2005). Diversity of the human intestinal microbial flora. Science 308, 1635–1638. doi: 10.1126/science.1110591
Emanuele, E., Orsi, P., Boso, M., Broglia, D., Brondino, N., Barale, F., et al. (2010). Low-grade endotoxemia in patients with severe autism. Neurosci. Lett. 471, 162–165. doi: 10.1016/j.neulet.2010.01.033
Erickson, A. R., Cantarel, B. L., Lamendella, R., Darzi, Y., Mongodin, E. F., Pan, C., et al. (2012). Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of crohn's disease. PLoS ONE 7:e49138. doi: 10.1371/journal.pone.0049138
Everard, A., Belzer, C., Geurts, L., Ouwerkerk, J. P., Druart, C., Bindels, L. B., et al. (2013). Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 110, 9066–9071. doi: 10.1073/pnas.1219451110
Ewaschuk, J. B., Naylor, J. M., and Zello, G. A. (2005). D-lactate in human and ruminant metabolism. J. Nutr. 135, 1619–1625.
Fåk, F., and Bäckhed, F. (2012). Lactobacillus reuteri prevents diet-induced obesity, but not atherosclerosis, in a strain dependent fashion in Apoe−/− mice. PLoS ONE 7:e46837. doi: 10.1371/journal.pone.0046837
FAO/WHO (2002). Guidelines for the Evaluation of Probiotics in Food. London: London World Health Organization; Canada Food Agric Organ.
Finnie, I. A., Dwarakanath, A. D., Taylor, B. A., and Rhodes, J. M. (1995). Colonic mucin synthesis is increased by sodium butyrate. Gut 36, 93–99. doi: 10.1136/gut.36.1.93
Flint, H. J., Scott, K. P., Louis, P., and Duncan, S. J. (2012). The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9, 577–589. doi: 10.1038/nrgastro.2012.156
Forslund, K., Hildebrand, F., Nielsen, T., Falony, G., Le Chatelier, E., Sunagawa, S., et al. (2015). Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266. doi: 10.1038/nature15766
Freestone, P. P., Sandrini, S. M., Haigh, R. D., and Lyte, M. (2008). Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 16, 55–64. doi: 10.1016/j.tim.2007.11.005
Frémont, M., Coomans, D., Massart, S., and De Meirleir, K. (2013). High-throughput 16S rRNA gene sequencing reveals alterations of intestinal microbiota in myalgic encephalomyelitis/chronic fatigue syndrome patients. Anaerobe 22, 50–56. doi: 10.1016/j.anaerobe.2013.06.002
Frost, G., Morrison, D., and Preston, T. (2013). Compounds and Their Effects on Appetite Control and Insulin Sensitivity. US Pat App 14/418,448.
Fujiya, M., Musch, M. W., Nakagawa, Y., Hu, S., Alverdy, J., Kohgo, Y., et al. (2007). The Bacillus subtilis quorum-sensing molecule CSF contributes to intestinal homeostasis via OCTN2, a host cell membrane transporter. Cell Host Microbe. 1, 299–308. doi: 10.1016/j.chom.2007.05.004
Furuya-Kanamori, L., Stone, J. C., Clark, J., Mckenzie, S. J., Yakob, L., Paterson, D. L., et al. (2014). Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 36, 132–141. doi: 10.1017/ice.2014.39
Galisteo, M., Duarte, J., and Zarzuelo, A. (2008). Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J. Nutr. Biochem. 19, 71–84. doi: 10.1016/j.jnutbio.2007.02.009
Gauffin Cano, P., Santacruz, A., Moya, A., and Sanz, Y. (2012). Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE 7:e41079. doi: 10.1371/journal.pone.0041079
Geleijnse, J. M., Kok, F. J., and Grobbee, D. E. (2004). Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations. Eur. J. Public Health. 14, 235–239. doi: 10.1093/eurpub/14.3.235
Ghosh, S., DeCoffe, D., Brown, K., Rajendiran, E., Estaki, M., Dai, C., et al. (2013). Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 8:e55468. doi: 10.1371/journal.pone.0055468
Ghoshal, S., Witta, J., Zhong, J., de Villiers, W., and Eckhardt, E. (2009). Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 50, 90–97. doi: 10.1194/jlr.M800156-JLR200
Gibson, G. R., Cummings, J. H., and Macfarlane, G. T. (1991). Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol Lett. 86, 103–111. doi: 10.1111/j.1574-6968.1991.tb04799.x
Gleeson, M., Bishop, N. C., Stensel, D. J., Lindley, M. R., Mastana, S. S., and Nimmo, M. A. (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11, 607–615. doi: 10.1038/nri3041
Goldin, B. R., and Gorbach, S. L. (1984a). Alterations of the intestinal microflora by diet, oral antibiotics, and Lactobacillus: decreased production of free amines from aromatic nitro compounds, azo dyes, and glucuronides. J. Natl. Cancer Inst. 73, 689–695.
Goldin, B. R., and Gorbach, S. L. (1984b). The effect of milk and Lactobacillus feeding on human intestinal bacterial enzyme activity. Am. J. Clin. Nutr. 39, 756–761.
Guerrero, R., Vega, G. L., Grundy, S. M., and Browning, J. D. (2009). Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology 49, 791–801. doi: 10.1002/hep.22726
He, H.-J., Wang, G.-Y., Gao, Y., Ling, W.-H., Yu, Z.-W., and Jin, T.-R. (2012). Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 3:94. doi: 10.4239/wjd.v3.i5.94
Hopkins, M. J., Sharp, R., and Macfarlane, G. T. (2001). Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 48, 198–205. doi: 10.1136/gut.48.2.198
Hsu, R. Y., Chan, C. H., Spicer, J. D., Rousseau, M. C., Giannias, B., Rousseau, S., et al. (2011). LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 71, 1989–1998. doi: 10.1158/0008-5472.CAN-10-2833
Hu, F. B., Rimm, E., Smith-Warner, S. A., Feskanich, D., Stampfer, M. J., Ascherio, A., et al. (1999). Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 69, 243–249.
Hughes, R., Magee, E. A., and Bingham, S. (2000). Protein degradation in the large intestine: relevance to colorectal cancer. Curr. Issues Intest. Microbiol. 1, 51–58.
Huttenhower, C., Gevers, D., Knight, R., Abubucker, S., Badger, J. H., Chinwalla, A. T., et al. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi: 10.1038/nature11234
Ito, M., Ohishi, K., Yoshida, Y., Yokoi, W., and Sawada, H. (2003). Antioxidative effects of lactic acid bacteria on the colonic mucosa of iron-overloaded mice. J. Agric. Food Chem. 51, 4456–4460. doi: 10.1021/jf0261957
Jehle, S., Zanetti, A., Muser, J., Hulter, H. N., and Krapf, R. (2006). Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J. Am. Soc. Nephrol. 17, 3213–3222. doi: 10.1681/ASN.2006030233
Jones, M. L., Martoni, C. J., Ganopolsky, J. G., Labbé, A., and Prakash, S. (2014). The human microbiome and bile acid metabolism: dysbiosis, dysmetabolism, disease and intervention. Expert Opin. Biol. Ther. 14, 467–482. doi: 10.1517/14712598.2014.880420
Joossens, M., Huys, G., Cnockaert, M., De Preter, V., Verbeke, K., Rutgeerts, P., et al. (2011). Dysbiosis of the faecal microbiota in patients with Crohn's disease and their unaffected relatives. Gut 60, 631–637. doi: 10.1136/gut.2010.223263
Kadooka, Y., Sato, M., Imaizumi, K., Ogawa, A., Ikuyama, K., Akai, Y., et al. (2010). Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 64, 636–643. doi: 10.1038/ejcn.2010.19
Kanoski, S. E., and Davidson, T. L. (2011). Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol. Behav. 103, 59–68. doi: 10.1016/j.physbeh.2010.12.003
Kimura, I., Ozawa, K., Inoue, D., Imamura, T., Kimura, K., Maeda, T., et al. (2013). The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 4:1829. doi: 10.1038/ncomms2852
Kirpich, I. A., Solovieva, N. V., Leikhter, S. N., Shidakova, N. A., Lebedeva, O. V., Sidorov, P. I., et al. (2008). Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol 42, 675–682. doi: 10.1016/j.alcohol.2008.08.006
Klöting, N., Fasshauer, M., Dietrich, A., Kovacs, P., Schön, M. R., Kern, M., et al. (2010). Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 299, 506–515. doi: 10.1152/ajpendo.00586.2009
Koeth, R. A., Wang, Z., Levison, B. S., Buffa, J. A., Org, E., Sheehy, B. T., et al. (2013). Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585. doi: 10.1038/nm.3145
Kong, L. C., Tap, J., Aron-Wisnewsky, J., Pelloux, V., Basdevant, A., Bouillot, J. L., et al. (2013). Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am. J. Clin. Nutr. 98, 16–24. doi: 10.3945/ajcn.113.058743
Kullisaar, T., Shepetova, J., and Zilmer, K. (2011). An antioxidant probiotic reduces postprandial lipemia and oxidative stress. Cent. Eur. J. Biol. 6, 32–40. doi: 10.2478/s11535-010-0103-4
Larsen, N., Vogensen, F. K., van Den Berg, F. W., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., et al. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 5:e9085. doi: 10.1371/journal.pone.0009085
Lederberg, J., and McCray, A. (2001). Ome SweetOmics–a genealogical treasury of words. Scientist. 15, 8.
Leung, K. W., Barnstable, C. J., and Tombran-Tink, J. (2009). Bacterial endotoxin activates retinal pigment epithelial cells and induces their degeneration through IL-6 and IL-8 autocrine signaling. Mol. Immunol. 46, 1374–1386. doi: 10.1016/j.molimm.2008.12.001
Licht, R., Van Bruggen, M., Oppers-Walgreen, B., Rijke, T., and Berden, G. (2001). Plasma levels of nucleosomes and nucleosome–autoantibody complexes in murine lupus: effects of disease progression and lipopolysaccharide administration. Arthritis Rheum. 44, 1320–1330. doi: 10.1002/1529-0131(200106)44:6<1320::AID-ART224>3.0.CO;2-X
Ling, B., Peng, F., Alcorn, J., Lohmann, K., Bandy, B., and Zello, G. A. (2012). D-Lactate altered mitochondrial energy production in rat brain and heart but not liver. Nutr. Metab. (Lond). 9:6. doi: 10.1186/1743-7075-9-6
Lira, F. S., Rosa, J. C., Pimentel, G. D., Souza, H. A., Caperuto, E. C., Carnevali, L. C., et al. (2010). Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 9:82. doi: 10.1186/1476-511X-9-82
Liu, Q., Duan, Z. P., Ha, D. K., Bengmark, S., Kurtovic, J., and Riordan, S. M. (2004). Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 39, 1441–1449. doi: 10.1002/hep.20194
Louis, P., Young, P., Holtrop, G., and Flint, H. J. (2010). Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 12, 304–314. doi: 10.1111/j.1462-2920.2009.02066.x
Lozupone, C. A., Rhodes, M. E., Neff, C. P., Fontenot, A. P., Campbell, T. B., and Palmer, B. E. (2014). HIV-induced alteration in gut microbiota. Gut Microbes. 5, 562–570. doi: 10.4161/gmic.32132
Lyte, M., and Bailey, M. T. (1997). Neuroendocrine-bacterial interactions in a neurotoxin-induced model of trauma. J. Surg. Res. 70, 195–201. doi: 10.1006/jsre.1997.5130
Lyte, M., and Ernst, S. (1992). Catecholamine induced growth of gram negative bacteria. Life Sci. 50, 203–212. doi: 10.1016/0024-3205(92)90273-R
Lyte, M., Frank, C. D., and Green, B. T. (1996). Production of an autoinducer of growth by norepinephrine cultured Escherichia coli O157:H7. FEMS Microbiol. Lett. 139, 155–159. doi: 10.1111/j.1574-6968.1996.tb08196.x
Ma, Y.-Y., Li, L., Yu, C. H., Shen, Z., Chen, L. H., and Li, Y. (2013). Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J. Gastroenterol. 19, 6911–6918. doi: 10.3748/wjg.v19.i40.6911
Maes, M., Kubera, M., Leunis, J. C., Berk, M., Geffard, M., and Bosmans, E. (2013). In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr. Scand. 127, 344–354. doi: 10.1111/j.1600-0447.2012.01908.x
Maes, M., and Leunis, J. (2008). Normalization of leaky gut in chronic fatigue syndrome (CFS) is accompanied by a clinical improvement: effects of age, duration of illness and the translocation. Neuroendocrinol. Lett. 29, 902.
Manach, C., Williamson, G., Morand, C., Scalbert, A., and Rémésy, C. (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 81(1 Suppl), 230S–242S.
Manzel, A., Muller, D. N., Hafler, D. A., Erdman, S. E., Linker, R. A., and Kleinewietfeld, M. (2014). Role of “western diet” in inflammatory autoimmune diseases. Curr. Allergy Asthma Rep. 14, 1–8. doi: 10.1007/s11882-013-0404-6
Marcil, V., Delvin, E., Garofalo, C., and Levy, E. (2003). Butyrate impairs lipid transport by inhibiting microsomal triglyceride transfer protein in Caco-2 cells. J. Nutr. 133, 2180–2183.
Marmot, M. G., Syme, S. L., Kagan, A., Kato, H., Cohen, J. B., and Belsky, J. (1975). Epidemiologic studies of coronary heart disease and stroke in Japanese men living in Japan, Hawaii and California: prevalence of coronary and hypertensive heart disease and associated risk factors. Am. J. Epidemiol. 102, 514–525.
Mascioli, E., Leader, L., Flores, E., Trimbo, S., Bistrian, B., and Blackburn, G. (1988). Enhanced survival to endotoxin in guinea pigs fed IV fish oil emulsion. Lipids 23, 623–625. doi: 10.1007/BF02535609
Matsumoto, M., Inoue, R., Tsukahara, T., Ushida, K., Chiji, H., Matsubara, N., et al. (2008). Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 72, 572–576. doi: 10.1271/bbb.70474
Matsumoto, M., Kibe, R., Ooga, T., Aiba, Y., Kurihara, S., Sawaki, E., et al. (2012). Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2:233. doi: 10.1038/srep00233
McFarland, L. V. (2006). Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am. J. Gastroenterol. 101, 812–822. doi: 10.1111/j.1572-0241.2006.00465.x
Mehta, N. N., McGillicuddy, F. C., Anderson, P. D., Hinkle, C. C., Shah, R., Pruscino, L., et al. (2010). Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59, 172–181. doi: 10.2337/db09-0367
Million, M., Maraninchi, M., Henry, M., Armougom, F., Richet, H., Carrieri, P., et al. (2012). Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int. J. Obes. (Lond). 36, 817–825. doi: 10.1038/ijo.2011.153
Moeller, A. H., Caro-Quintero, A., Mjungu, D., Georgiev, A. V., Lonsdorf, E. V., Muller, M. N., et al. (2016). Cospeciation of gut microbiota with hominids. Science 353, 380–382. doi: 10.1126/science.aaf3951
Muccioli, G. G., Naslain, D., Bäckhed, F., Reigstad, C. S., Lambert, D. M., Delzenne, N. M., et al. (2010). The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 6, 392. doi: 10.1038/msb.2010.46
Nakarai, H., Yamashita, A., Nagayasu, S., Iwashita, M., Kumamoto, S., Ohyama, H., et al. (2012). Adipocyte-macrophage interaction may mediate LPS-induced low-grade inflammation: potential link with metabolic complications. Innate Immun. 18, 164–170. doi: 10.1177/1753425910393370
Nanri, A., Shimazu, T., Ishihara, J., Takachi, R., Mizoue, T., Inoue, M., et al. (2012). Reproducibility and validity of dietary patterns assessed by a food frequency questionnaire used in the 5-year follow-up survey of the Japan Public Health Center-Based Prospective Study. J. Epidemiol. 22, 205–215. doi: 10.2188/jea.JE20110087
Naruszewicz, M., Johansson, M.-L., Zapolska-Downar, D., and Bukowska, H. (2002). Effect of Lactobacillus plantarum 299v on cardiovascular disease risk factors in smokers. Am. J. Clin. Nutr. 76, 1249–1255.
Naukkarinen, J., Heinonen, S., Hakkarainen, A., Lundbom, J., Vuolteenaho, K., Saarinen, L., et al. (2014). Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia 57, 167–176. doi: 10.1007/s00125-013-3066-y
Newgard, C. B. (2012). Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614. doi: 10.1016/j.cmet.2012.01.024
Nichol, K. E., Poon, W. W., Parachikova, A. I., Cribbs, D. H., Glabe, C. G., and Cotman, C. W. (2008). Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflammation 5:13. doi: 10.1186/1742-2094-5-13
Ogden, C., Yanovski, S., Carroll, M., and Flegal, K. (2007). The epidemiology of obesity. Gastroenterology 132, 2087–2102. doi: 10.1053/j.gastro.2007.03.052
Ouyang, X., Cirillo, P., Sautin, Y., McCall, S., Bruchette, J. L., Diehl, A. M., et al. (2008). Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 48, 993–999. doi: 10.1016/j.jhep.2008.02.011
Pace, E., Ferraro, M., Siena, L., Melis, M., Montalbano, A. M., Johnson, M., et al. (2008). Cigarette smoke increases Toll-like receptor 4 and modifies lipopolysaccharide-mediated responses in airway epithelial cells. Immunology 124, 401–411. doi: 10.1111/j.1365-2567.2007.02788.x
Park, D. Y., Ahn, Y. T., Huh, C. S., McGregor, R. A., and Choi, M. S. (2013b). Dual probiotic strains suppress high fructose-induced metabolic syndrome. World J. Gastroenterol. 19, 274–283. doi: 10.3748/wjg.v19.i2.274
Park, D. Y., Ahn, Y. T., Park, S. H., Huh, C. S., Yoo, S. R., Yu, R., et al. (2013a). Supplementation of Lactobacillus curvatus KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 8:59470. doi: 10.1371/journal.pone.0059470
Park, K.-Y., Kim, B., and Hyun, C.-K. (2015). Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. J. Clin. Biochem. Nutr. 56, 240–246. doi: 10.3164/jcbn.14-116
Parry, S., and Straker, L. (2013). The contribution of office work to sedentary behaviour associated risk. BMC Public Health. 13:296. doi: 10.1186/1471-2458-13-296
Peng, L., Li, Z.-R., Green, R. S., Holzman, I. R., and Lin, J. (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139, 1619–1625. doi: 10.3945/jn.109.104638
Petersen, A. M., and Pedersen, B. K. (2005). The anti-inflammatory effect of exercise. J. Appl. Physiol. 98, 1154–1162. doi: 10.1152/japplphysiol.00164.2004
Petrof, E. O., Kojima, K., Ropeleski, M. J., Musch, M. W., Tao, Y., De Simone, C., et al. (2004). Probiotics inhibit nuclear factor-kB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127, 1474–1487. doi: 10.1053/j.gastro.2004.09.001
Poutahidis, T., Kleinewietfeld, M., Smillie, C., Levkovich, T., Perrotta, A., Bhela, S., et al. (2013). Microbial reprogramming inhibits western diet-associated obesity. PLoS ONE 8:e68596. doi: 10.1371/journal.pone.0068596
Qin, J., Li, R., Raes, J., and Arumugam, M. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. doi: 10.1038/nature08821
Qin, L., Wu, X., Block, M. L., Liu, Y., Breese, G. R., Hong, J. S., et al. (2007). Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462. doi: 10.1002/glia.20467
Raman, M., Ahmed, I., Gillevet, P. M., Probert, C. S., Ratcliffe, N. M., Smith, S., et al. (2013). Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 11, 868–875. doi: 10.1016/j.cgh.2013.02.015
Roopchand, D. E., Carmody, R. N., and Kuhn, P. (2015). Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes 64, 2847–2858. doi: 10.2337/db14-1916
Russell, W. R., Gratz, S. W., Duncan, S. H., Holtrop, G., Ince, J., Scobbie, L., et al. (2011). High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 93, 1062–1072. doi: 10.3945/ajcn.110.002188
Sadeghi, S., Wallace, F. A., and Calder, P. C. (1999). Dietary lipids modify the cytokine response to bacterial lipopolysaccharide in mice. Immunology 96, 404–410. doi: 10.1046/j.1365-2567.1999.00701.x
Salaspuro, M. (1996). Bacteriocolonic pathway for ethanol oxidation: characteristics and implications. Ann. Med. 28, 195–200. doi: 10.3109/07853899609033120
Salonen, A., and de Vos, W. M. (2014). Impact of diet on human intestinal microbiota and health. Annu. Rev. Food Sci. Technol. 5, 239–262. doi: 10.1146/annurev-food-030212-182554
Schmitz, K. H., Jacobs, D. R. Jr., Hong, C.-P., Steinberger, J., Moran, A., and Sinaiko, A. R. (2002). Association of physical activity with insulin sensitivity in children. Int. J. Obes. Relat. Metab. Disord. 26, 1310–1316. doi: 10.1038/sj.ijo.0802137
Segain, J. P., Raingeard de la Blétière, D., Bourreille, A., Leray, V., Gervois, N., Rosales, C., et al. (2000). Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut 47, 397–403. doi: 10.1136/gut.47.3.397
Seth, A., Yan, F., Polk, D. B., and Rao, R. K. (2008). Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC-and MAP kinase-dependent mechanism. Am. J. Physiol. Liver Physiol. 294, G1060–G1069. doi: 10.1152/ajpgi.00202.2007
Sheedy, J. R., Wettenhall, R. E., Scanlon, D., Gooley, P. R., Lewis, D. P., McGregor, N., et al. (2009). Increased D-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo 23, 621–628.
Sheth, P., Basuroy, S., Li, C., Naren, A. P., and Rao, R. K. (2003). Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J. Biol. Chem. 278, 49239–49245. doi: 10.1074/jbc.M305654200
Sies, H., Stahl, W., and Sevanian, A. (2005). Nutritional, dietary and postprandial oxidative stress. J. Nutr. 135, 969–972.
Slattery, M. L., Boucher, K. M., Caan, B. J., Potter, J. D., and Ma, K. N. (1998). Eating patterns and risk of colon cancer. Am. J. Epidemiol. 148, 4–16. doi: 10.1093/aje/148.1.4-a
Starkie, R., Ostrowski, S. R., Jauffred, S., Febbraio, M., and Pedersen, B. K. (2003). Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 17, 884–886. doi: 10.1096/fj.02-0670fje
Suffredini, A. F., and Noveck, R. J. (2014). Human endotoxin administration as an experimental model in drug development. Clin. Pharmacol. Ther. 96, 418–422. doi: 10.1038/clpt.2014.146
Tang, W. H., Wang, Z., Levison, B. S., Koeth, R. A., Britt, E. B., Fu, X., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 368, 1575–1584. doi: 10.1056/NEJMoa1109400
Tappenden, K. A., Albin, D. M., Bartholome, A. L., and Mangian, H. F. (2003). Glucagon-like peptide-2 and short-chain fatty acids: a new twist to an old story. J. Nutr. 133, 3717–3720.
Thomas, S., Metzke, D., Schmitz, J., Dörffel, Y., and Baumgart, D. C. (2011). Anti-inflammatory effects of Saccharomyces boulardii mediated by myeloid dendritic cells from patients with Crohn's disease and ulcerative colitis. Am. J. Physiol. Gastrointest Liver Physiol. 301, G1083–G1092. doi: 10.1152/ajpgi.00217.2011
Thompson, L., and Spiller, R. C. (1995). Impact of polyunsaturated fatty acids on human colonic bacterial metabolism: an in vitro and in vivo study. Br. J. Nutr. 74, 733–741. doi: 10.1079/BJN19950176
Trovato, F. M., Catalano, D., Martines, G. F., Pace, P., and Trovato, G. M. (2015). Mediterranean diet and non-alcoholic fatty liver disease. The need of extended and comprehensive interventions. Clin. Nutr. 34, 86–88. doi: 10.1016/j.clnu.2014.01.018
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi: 10.1038/nature07540
Ungaro, R., Bernstein, C. N., Gearry, R., Hviid, A., Kolho, K.-L., Kronman, M. P., et al. (2014). Antibiotics associated with increased risk of new-onset Crohn's disease but not ulcerative colitis: a meta-analysis. Am. J. Gastroenterol. 109, 1728–1738. doi: 10.1038/ajg.2014.246
Uribarri, J., Oh, M. S., and Carroll, H. J. (1998). D-lactic acidosis. A review of clinical presentation, biochemical features, and pathophysiologic mechanisms. Medicine (Baltimore). 77, 73–82. doi: 10.1097/00005792-199803000-00001
van der Vaart, H., Postma, D. S., Timens, W., and ten Hacken, N. H. (2004). Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax 59, 713–721. doi: 10.1136/thx.2003.012468
van Vliet-Ostaptchouk, J. V., Nuotio, M.-L., Slagter, S. N., Doiron, D., Fischer, K., Foco, L., et al. (2014). The prevalence of Metabolic Syndrome and metabolically healthy obesity in Europe: a collaborative analysis of ten large cohort studies. BMC Endocr Disord. 14:9. doi: 10.1186/1472-6823-14-9
Vinolo, M. A., Rodrigues, H. G., Nachbar, R. T., and Curi, R. (2011). Regulation of inflammation by short chain fatty acids. Nutrients 3, 858–876. doi: 10.3390/nu3100858
Wade, R. S., and Castro, C. E. (1990). Redox reactivity of iron (III) porphyrins and heme proteins with nitric oxide. Nitrosyl transfer to carbon, oxygen, nitrogen and sulfur. Chem. Res. Toxicol. 3, 289–291. doi: 10.1021/tx00016a002
Walker, R. W., Lê, K.-A., Davis, J., Alderete, T. L., Cherry, R., Lebel, S., et al. (2012). High rates of fructose malabsorption are associated with reduced liver fat in obese African Americans. J. Am. Coll. Nutr. 31, 369–374. doi: 10.1080/07315724.2012.10720445
Wang, K., Jin, X., Chen, Y., Song, Z., and Jiang, X. (2016). Polyphenol-rich propolis extracts strengthen intestinal barrier function by activating AMPK and ERK signaling. Nutrients 8:272. doi: 10.3390/nu8050272
Wang, Y., Kirpich, I., Liu, Y., Ma, Z., Barve, S., McClain, C. J., et al. (2011). Lactobacillus rhamnosus GG treatment potentiates intestinal hypoxia-inducible factor, promotes intestinal integrity and ameliorates alcohol-induced liver injury. Am. J. Pathol. 179, 2866–2875. doi: 10.1016/j.ajpath.2011.08.039
Wells, J. M., Rossi, O., Meijerink, M., and van Baarlen, P. (2011). Epithelial crosstalk at the microbiota-mucosal interface. Proc. Natl. Acad. Sci. U.S.A. 108(Suppl.), 4607–4614. doi: 10.1073/pnas.1000092107
WHO (2014). Global Status Report on Noncommunicable Diseases 2014. Geneva: World Health Organization.
Wiedermann, C. (1999). Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J. Am. Coll Cardiol. 34, 1975–1981. doi: 10.1016/S0735-1097(99)00448-9
Wright, R. M., McManaman, J. L., and Repine, J. E. (1999). Alcohol-induced breast cancer: a proposed mechanism. Free Radic. Biol. Med. 26, 348–354. doi: 10.1016/S0891-5849(98)00204-4
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., et al. (2012). Human gut microbiome viewed across age and geography. Nature 486, 222–227. doi: 10.1038/nature11053
Yoshino, S., Sasatomi, E., Mori, Y., and Sagai, M. (1999). Oral administration of lipopolysaccharide exacerbates collagen-induced arthritis in mice. J. Immunol. 163, 3417–3422.
Zhang, X., Zhang, D., Jia, H., Feng, Q., Wang, D., Liang, D., et al. (2015). The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 21, 895–905. doi: 10.1038/nm.3914
Zhang, X., Zhang, G., Zhang, H., Karin, M., Bai, H., and Cai, D. (2008). Hypothalamic IKKa/NF-kB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73. doi: 10.1016/j.cell.2008.07.043
Keywords: nutrition, microbiome, obesity, lifestyle, inflammation
Citation: Rodriguez-Castaño GP, Caro-Quintero A, Reyes A and Lizcano F (2017) Advances in Gut Microbiome Research, Opening New Strategies to Cope with a Western Lifestyle. Front. Genet. 7:224. doi: 10.3389/fgene.2016.00224
Received: 01 June 2016; Accepted: 14 December 2016;
Published: 10 January 2017.
Edited by:
Steven H. Zeisel, University of North Carolina at Chapel Hill, USAReviewed by:
Matthew Lange, University of California, Davis, USACopyright © 2017 Rodriguez-Castaño, Caro-Quintero, Reyes and Lizcano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fernando Lizcano, ZmVybmFuZG8ubGl6Y2Fub0B1bmlzYWJhbmEuZWR1LmNv
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.