
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet. , 01 May 2015
Sec. Evolutionary and Population Genetics
Volume 6 - 2015 | https://doi.org/10.3389/fgene.2015.00154
This article is part of the Research Topic Origin of Tropical Diversity: From Clades to Communities View all 23 articles
 Lisa Pokorny1*
Lisa Pokorny1* Ricarda Riina1
Ricarda Riina1 Mario Mairal1
Mario Mairal1 Andrea S. Meseguer2
Andrea S. Meseguer2 Victoria Culshaw1
Victoria Culshaw1 Jon Cendoya1
Jon Cendoya1 Miguel Serrano3
Miguel Serrano3 Rodrigo Carbajal3
Rodrigo Carbajal3 Santiago Ortiz3
Santiago Ortiz3 Myriam Heuertz4,5,6
Myriam Heuertz4,5,6 Isabel Sanmartín1*
Isabel Sanmartín1*The Rand Flora is a well-known floristic pattern in which unrelated plant lineages show similar disjunct distributions in the continental margins of Africa and adjacent islands—Macaronesia-northwest Africa, Horn of Africa-Southern Arabia, Eastern Africa, and Southern Africa. These lineages are now separated by environmental barriers such as the arid regions of the Sahara and Kalahari Deserts or the tropical lowlands of Central Africa. Alternative explanations for the Rand Flora pattern range from vicariance and climate-driven extinction of a widespread pan-African flora to independent dispersal events and speciation in situ. To provide a temporal framework for this pattern, we used published data from nuclear and chloroplast DNA to estimate the age of disjunction of 17 lineages that span 12 families and nine orders of angiosperms. We further used these estimates to infer diversification rates for Rand Flora disjunct clades in relation to their higher-level encompassing lineages. Our results indicate that most disjunctions fall within the Miocene and Pliocene periods, coinciding with the onset of a major aridification trend, still ongoing, in Africa. Age of disjunctions seemed to be related to the climatic affinities of each Rand Flora lineage, with sub-humid taxa dated earlier (e.g., Sideroxylon) and those with more xeric affinities (e.g., Campylanthus) diverging later. We did not find support for significant decreases in diversification rates in most groups, with the exception of older subtropical lineages (e.g., Sideroxylon, Hypericum, or Canarina), but some lineages (e.g., Cicer, Campylanthus) showed a long temporal gap between stem and crown ages, suggestive of extinction. In all, the Rand Flora pattern seems to fit the definition of biogeographic pseudocongruence, with the pattern arising at different times in response to the increasing aridity of the African continent, with interspersed periods of humidity allowing range expansions.
Large-scale biodiversity patterns have intrigued naturalists since the eighteenth century (Forster, 1778; von Humboldt and Bonpland, 1805; Wallace, 1878; Fischer, 1960; Stevens, 1989; Lomolino et al., 2010). Recognizing that spatial variation in environmental variables such as temperature or precipitation is insufficient to explain such patterns, more integrative explanations that emphasize the role of both environmental and evolutionary factors have recently been advanced (Qian and Ricklefs, 2000; Wiens and Donoghue, 2004; Jablonski et al., 2006). As Wiens and Donoghue (2004) state “environmental variables cannot by themselves increase or decrease local or regional species richness”; only evolutionary processes such as dispersal, speciation and extinction can. Therefore, reconstructing rates of dispersal, speciation, and extinction across the component lineages of a biota might help us understand how assembly took place across space and through time (Pennington et al., 2004; Ricklefs, 2007; Wiens, 2011). Moreover, understanding patterns of biotic assembly is a pressing goal in biodiversity research at a time when nearly one tenth of species on Earth are projected to disappear in the next hundred years (Maclean and Wilson, 2011).
Africa is a continent especially interesting to study patterns of biotic assembly. On one hand, African tropical regions are comparatively species-poorer than regions situated in the same equatorial latitudes in the Neotropics and Southeast Asia (Lavin et al., 2001; Couvreur, 2015), which has led to the continent being referred to as the “odd man out” (Richards, 1973). On the other, Africa offers some extraordinary examples of continent-wide disjunctions. For example, tropical rainforests in Africa appear in two main blocks, the West-Central Guineo-Congolian region and the coastal and montane regions of East Africa, now separated by a 1000 Km-wide arid corridor (Couvreur et al., 2008). Another prime example is the so called Rand Flora (RF), a biogeographic pattern in which unrelated plant lineages show comparable disjunct distributions with sister taxa occurring on now distantly located regions in the continental margins of Africa: Macaronesia-northwest Africa, Western African mountains, Horn of Africa-South Arabia (including the Island of Socotra), Eastern Africa (incl. Madagascar), and Southern Africa (Christ, 1892; Lebrun, 1947, 1961; Quézel, 1978; Andrus et al., 2004; Sanmartín et al., 2010; Figure 1). All RF lineages share sub-humid to xerophilic affinities, so that the tropical lowlands of Central Africa and the large Sahara and Arabian deserts in the north or the Namib and Kalahari deserts in the south presumably constitute effective climatic barriers to their dispersal.
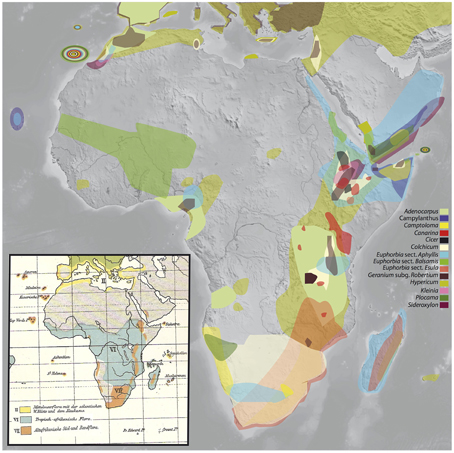
Figure 1. Rand Flora disjunction pattern as evidenced by angiosperm plant lineages analyzed for this study. The inset shows K.H.H. Christ's (1910) depiction of “cette flore marginale de l'Afrique” or “Randflora” (in orange color), note their similar geographic limits. Taxa: Adenocarpus (Fabaceae), Camptoloma (Scrophulariaceae), Campylanthus (Plantaginaceae), Canarina (Platycodoneae, Campanulaceae), Cicer (Fabaceae), Colchicum (Colchicaceae), Euphorbia subgen. Athymalus (sects. Anthacanthae and Balsamis; Euphorbiaceae), Euphorbia subgen. Esula (sect. Aphyllis), Euphorbia subgen. Esula (African clade of sect. Esula), Geranium subgen. Robertium (Geraniaceae), Hypericum (Hypericaceae), Kleinia (Asteraceae), Plocama (Rubiaceae), and Sideroxylon (Sideroxyleae, Sapotaceae).
Swiss botanist K. H. H. Christ (1892) first referred to “cette flore marginale de l'Afrique,” that is “this marginal African flora,” in a note addressing the role the so called ancient African flora played on European floras, with emphasis on the Mediterranean biome. Later, in his “Die Geographie der Farne” (i.e., “The Geography of Ferns”; Christ, 1910), he very aptly named this geographic pattern “Randflora” (see pp. 259–275), where the Germanic word “Rand” stands for rim, edge, border, margin (see Figure 1 inset), noting its similarities with Engler's “afrikanisch-makaronesische Element” (Engler, 1879, 1910; see pp. 76 in the former and pp. 983–984 and 1010 in the latter), that is, an “Afro-Macaronesian element” linking disjunct xerophilic taxa found in the continental margins of Africa and its adjacent islands (e.g., Canary Islands, Cape Verde, etc.).
Historical explanations for this pattern and, in particular, its temporal framework, its exact boundaries, and the ecology of the plants involved have varied through these past two centuries. The early view (Engler, 1879, 1910; Christ, 1892, 1910) was one of a pan-African flora found throughout the continent that became restricted to its margins as a result of major climate changes (i.e., increasing aridification) throughout the Tertiary (i.e., the Cenozoic Period, 66.0–2.58 Ma). Lebrun (1947; see pp. 134–137), and later Monod (1971, p. 377) and Quézel (1978, p. 511), interpreted Christ's ancient African flora as a complex ensemble that had experienced alternating expansions and contractions through time, having had a chance to spread across northern Africa during favorable moments in the Miocene and needing to retract at the end of the Neogene (i.e., Pliocene): a further increase in aridity at the beginning of Pleistocene glaciations would have confined relictual or vicariant taxa to Macaronesia, northwest Africa and Arabia. Axelrod and Raven (1978) explained some of these disjunctions in relation to a more ancient, widespread Paleogene flora of subtropical origin that covered the entire African continent at the beginning of the Cenozoic, and that was decimated by successive events of aridification, of which the relict floras of Macaronesia, the Cape Region, and the Afromontane forests in eastern and western Africa would be remnants. Bramwell (1985) explains this pattern in terms of pan-biogeographic “general tracks” that connect what would be the remains of an ancient flora that extended across the Mediterranean and Northern Africa in the Miocene, and whose vestiges could be found in the Macaronesian laurisilva and a few enclaves in the island of Socotra, the Ethiopian Highlands and southern Yemen.
These authors share a vicariant perspective and presume RF lineages were part of a widespread pan-African Tertiary flora that became fragmented by the appearance of climatic barriers (i.e., aridification), leaving relictual lineages with reduced distributions at “refugia” in the margins of Africa (i.e., “continental” islands). This “refugium” idea rests on the fact that many of these RF regions—Macaronesia, the South African Cape region, and the semi-arid regions of Eastern Africa and Southern Arabia (e.g., Ethiopia, Yemen, Socotra)—harbor a large number of endemic species, when compared to neighboring areas. Moreover, the “fragmentation-refugium” hypothesis implies the disappearance, possibly by extinction, of RF lineages from part of their distributional range (e.g., across the Sahara in central Northern Africa), which is consonant with the “climatic vicariance” concept (Wiens, 2004): an environmental change creates conditions within a species' geographic range that are outside the ancestral climatic tolerances; individuals are unable to persist and the species' geographic range becomes fragmented.
The alternative explanation is one of independent dispersal (immigration) events among geographically isolated regions and subsequent speciation in situ. In this framework, divergence events need not be congruent across lineages, since long-distance dispersal (LDD) events are highly stochastic in nature (Nathan, 2006). Asides from transoceanic dispersal—which has been postulated in the case of Aeonium (Kim et al., 2008), Geranium (Fiz et al., 2008), and other RF lineages (Andrus et al., 2004) based on molecular phylogenetic evidence—, cross-continent LDD dispersal is also possible: published examples favoring cross-continent LDD include Senecio, with a disjunct distribution between Macaronesia-Northern Africa and South Africa (Coleman et al., 2003; Pelser et al., 2012). Moreover, dispersal does not necessarily imply long-distance migration events. In some cases, dispersal across intermediate areas that act as “stepping stones” or “land bridges” could have been possible. For example, the presence of isolated mountain ranges (offering suitable habitats) throughout the Sahara, such as the Tibesti and Hoggar massifs, could have allowed this short or medium-range dispersal in Campanula (Alarcón et al., pers. comm.). Correspondingly, some RF lineages might have used the Arabian Plate as a land bridge to reach East Africa (Campanula, Roquet et al., 2009; Hypericum, Meseguer et al., 2013), and others may have benefited from the new habitats offered by the Pliocene uplift of the Eastern Arc Mountains to migrate to or from South Africa (Meseguer et al., 2013).
Discriminating between climate-driven vicariance vs. independent dispersal events between geographically isolated regions requires framing the evolution of disjunct lineages on a temporal scale (Sanmartín, 2014). On the other hand, to unravel the origin of a biota or biome, a meta-analysis across dated phylogenies of multiple non-nested clades is needed (Pennington et al., 2010; Wiens, 2011; Couvreur, 2015). Sanmartín et al. (2010) carried out a meta-analysis of 13 lineages to infer relative rates of historical dispersal among RF regions (Macaronesia, Eastern Africa-Southern Arabia, and Southern Africa) and found the highest rate of biotic exchange between east and west Northern Africa, across the Sahara. However, they did not integrate absolute estimates of lineage divergences in their inference, since very few RF lineages (e.g., Roquet et al., 2009) had been dated at the time.
In this study, we estimate time divergences for up to 13 plant lineages (Table 1) displaying RF disjunct distributions (Figure 1), and use published divergence times for four other lineages (see Materials and Methods), in order to provide a much-needed temporal framework for this pattern. An extensive description of each of these lineages, geographic distributions and phylogenetic relationships is provided in Supplementary Materials. We also frame these disjunctions in the context of major climatic and geological events in the history of Africa (see summary below) and estimate net diversification rates in an attempt to address the role that evolutionary processes, such as climate-driven extinction, may have played in the formation of the African RF pattern.
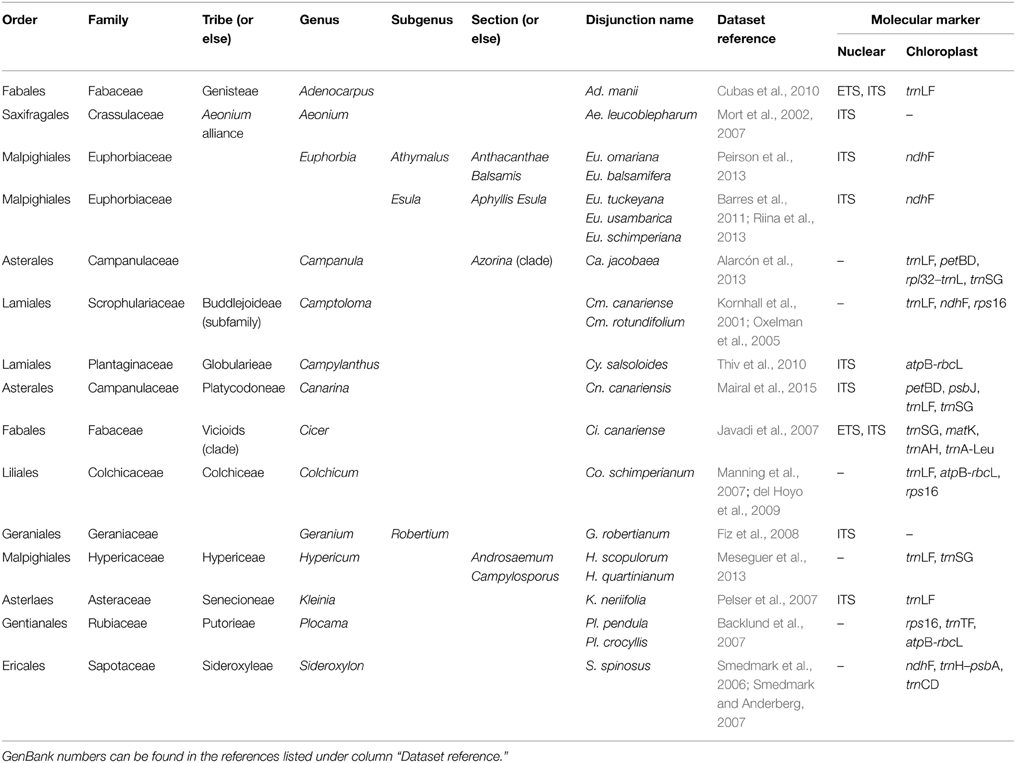
Table 1. Rand Flora disjunctions, encompassing (higher level) lineages, recent molecular phylogenetic studies, and molecular markers used in here.
To understand biogeographic patterns in the African flora, it is necessary to briefly review the climatic and geological history that might have influenced the evolution of African plant lineages. Extensive reviews of African climatic and vegetation history can be found in Axelrod and Raven (1978); van Zinderen Bakker (1978); Maley (1996, 2000); Morley (2000); Jacobs et al. (2010), Plana (2004), and Bonnefille (2011), among others.
During the Late Mesozoic, Africa was part of the supercontinent Gondwana, located in the southern hemisphere, and enjoyed a relatively humid and temperate climate (Raven and Axelrod, 1974). After breaking up from South America ca. 95 Ma, Africa started moving northwards toward the equatorial zone (Figure 2A). The result was a general trend toward continental aridification in which different regions became arid or wet at alternative times (Figure 2B, Senut et al., 2009). Paleocene Africa (66–56 Ma) was mainly wet and warm, characterized by a major diversification in the West African flora (Plana, 2004). A global increase in temperatures in the Eocene (56–33.9 Ma) led to increased aridity in Central Africa, with a rainforest-savannah mosaic in the Congo region. This was followed by a global cooling event at the Eocene-Oligocene boundary (33.9 Ma), which led again to aridification and major extinction but did not change biome composition (Axelrod and Raven, 1978).
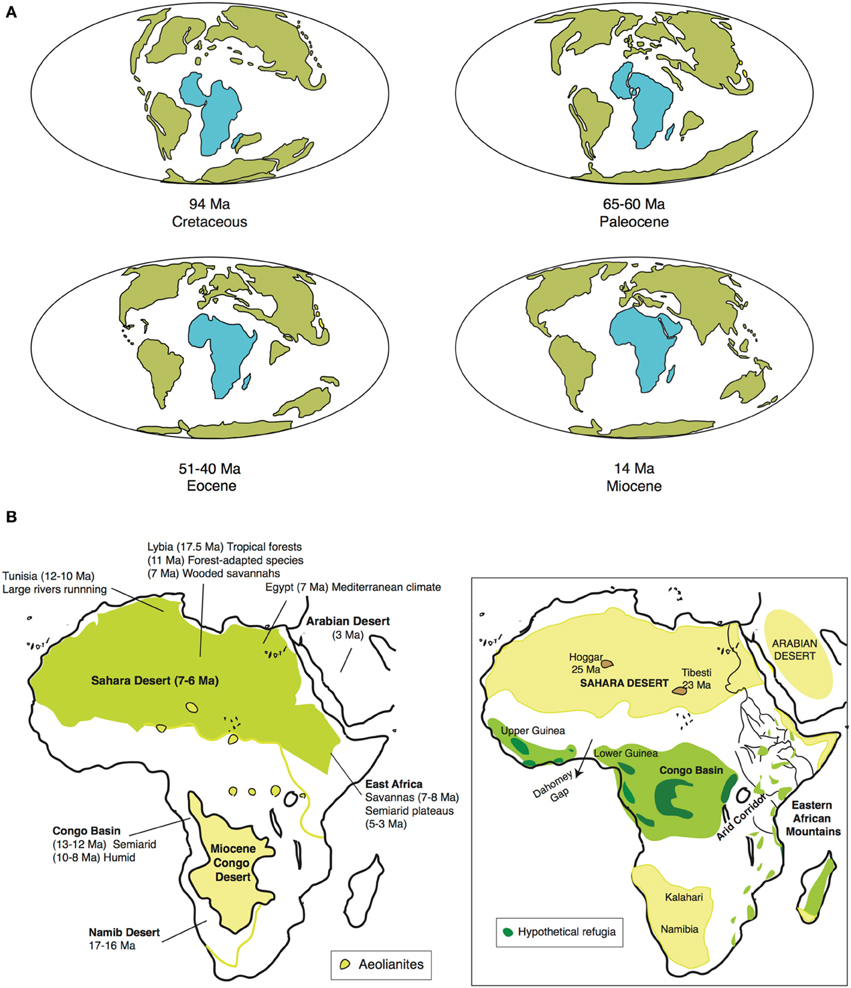
Figure 2. (A) Tectonic fragmentation of the supercontinent Gondwana through time, showing Africa's drift northwards; and (B) main climatic events in Africa during Neogene (adapted from Senut et al., 2009): (B-left) Early Neogene Central Africa was more arid than North Africa, with a desert, semiarid region in the Congo Basin. Desertification started in southwest Africa in the Mid-Miocene, proceeding eastward and northward, and finalizing with the formation of the Sahara Desert. Conversely, Central Africa became tropical due to subsidence and Eastern African uplift. (B-right) Schematic representation of present-day vegetation belts, showing position of main deserts and rainforest refugia (Eastern Arc Mountains/Guineo-Congolian region (the latter fragmented into smaller refugia). Rand Flora lineages occupy the regions in the margin that are not deserts or rainforests, rarely some find refuge in mountain areas of North African Sahara (e.g., Tibesti and Hoggar Massifs).
The Early Miocene (23–16 Ma) was warm and humid, with wide extension of rainforests, from the northern Sahara to parts of Southern Africa. The Mid Miocene (16–11.6 Ma) was a period of major changes in climate and topography. A combination of factors, including the gradual uplift of Eastern Africa, the successive closure of the Tethys seaway in the north, and the expansion of the East Antarctic ice sheet in the south (Trauth et al., 2009), led to a general intensification of the aridification process, though it was not homogeneous across the continent. Geological and paleontological evidence suggest that now arid regions (e.g., northern Africa, Horn of Africa, Namib Desert) were during this period more humid than they are today, whereas other now humid regions (e.g., Congo Basin) were much drier (Figure 2B). Desertification started in the southwest (Namib Desert) around 17–16 Ma ago, and proceeded eastward and northward. In Southern Africa, tropical to subtropical vegetation was replaced by wooded savannah during the lower Mid-Miocene (Senut et al., 2009). In Northern Africa, the earliest evidence of aridity in the Sahara region is from the Late Miocene (11.6–5.3 Ma), ca. 7–6 Ma (Senut et al., 2009; Figure 2B). In Central Africa, a semiarid desert (“Miocene Congo Desert,” Figure 2B) occupied the region until the Mid Miocene, 13–12 Ma ago, when the Eastern African uplift and subsequent subsidence led to the establishment of the Congo River drainage and a general increase in humidity (“tropicalization”). Also in the Late Miocene, ca. 7–8 Ma, a new period of tectonic activity in Eastern Africa led to the uplift of the Eastern Arc Mountains and the uplands of West Central Africa (Cameroon volcanic line), which led to increasing aridity and the expansion of savannahs and grasslands in these regions (Sepulchre et al., 2006). Uplifting reached a maximum during the Plio-Pleistocene and led to the formation of the Ethiopian Highlands and the desertification of low-lying areas in the Horn of Africa (Senut et al., 2009). From the Late Pliocene to the Holocene, the alternation of glacial-and interglacial periods seems to have led to repeated contractions and expansions of distributional ranges across both subtropical and tropical taxa (Maley, 2000; Bonnefille, 2011). Some areas like the Saharan massifs of Tibesti and Hoggar or the Ennedi Mountains could have served as refuges during arid periods for subtropical taxa (Osborne et al., 2008), whereas the uplands of Upper and Lower Guinea and the east of the Congo Basin, the Albertine Rift, or the Eastern Arc Mountains could have played the same role for tropical plant taxa (Maley, 1996; Figure 2B).
We retrieved sequences from GenBank from existing studies (Table 1) for the following 13 lineages exhibiting a distribution congruent with the RF pattern (Andrus et al., 2004; Sanmartín et al., 2010): Adenocarpus, Aeonium, Camptoloma, Campylanthus, Cicer, Colchicum, Euphorbia sects. Antachanthae, Aphyllis, Balsamis, and Esula, Geranium, Kleinia, and Plocama (Figure 3). We chose these lineages because sampling is nearly complete in most cases with very few to no missing taxa. Most of these RF taxa have been sequenced for several markers from the nuclear and chloroplast DNA regions. For each group we selected the markers with most sequences and tried representing both genomic compartments whenever possible. The sequences were aligned using the Opalescent package (Opal v2.1.0; Wheeler and Kececioglu, 2007) in Mesquite v3.01 (Maddison and Maddison, 2014) and manually adjusted in SE-AL v2.0a11 (Rambaut, 2002) using a similarity criterion, as recommended by Simmons (2004). For four other RF lineages —Campanula (Alarcón et al., 2013), Canarina (Mairal et al., 2015), Hypericum (Meseguer et al., 2013), and Sideroxylon (Stride et al., 2014)—we used recently published time estimates by our research team (except for Sideroxylon, which nonetheless used a dating approach similar to ours). Approximately 1600 sequences from ca. 675 taxa from 12 families and 9 orders of angiosperms were included in our study (Table 1).
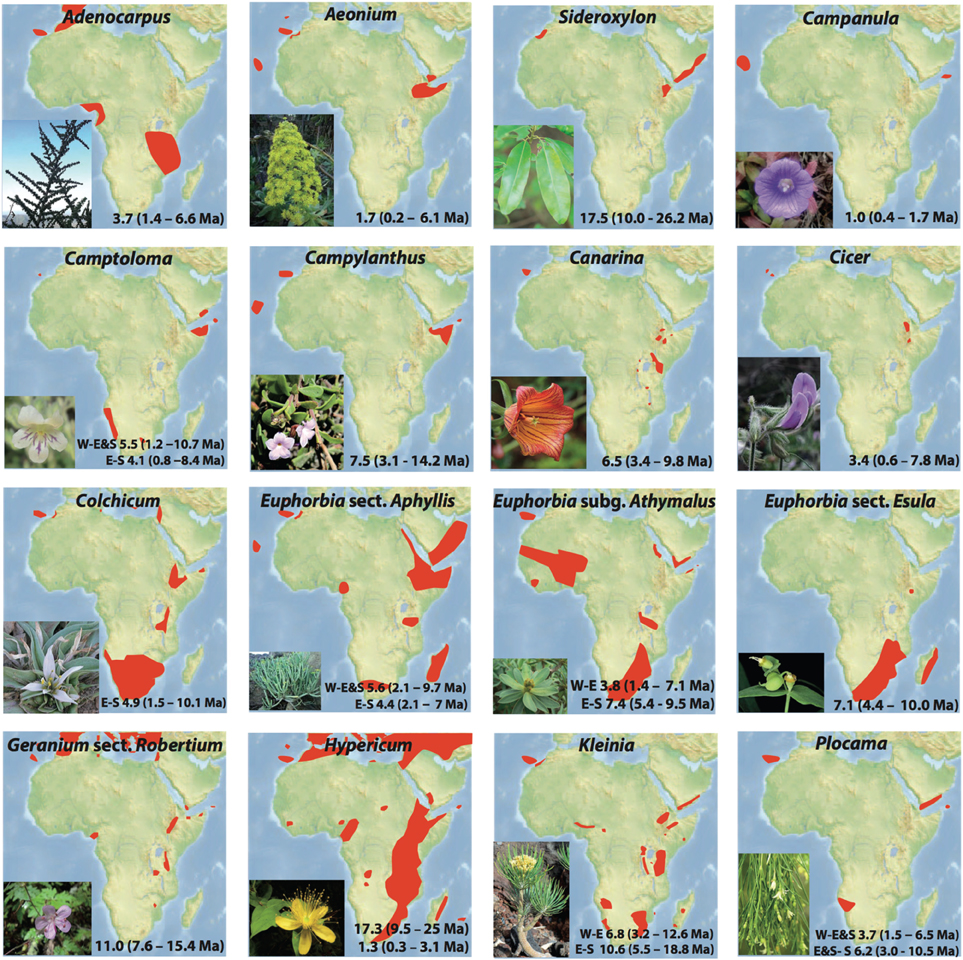
Figure 3. Individual distributions and habit illustrations for 16 plant lineages exhibiting Rand Flora disjunctions. Estimated divergence times within each lineage correspond to the disjunctions represented in Figures 4, 5 and indicated in the MCC chronograms shown in Figures S1–S16. Taxa names correspond to those in Table 1.
Divergence times were estimated under a Bayesian framework in BEAST v1.8 (Drummond et al., 2012). For each lineage, we constructed a dataset including the markers listed in Table 1, which were partitioned by genome (chloroplast vs. nuclear), whenever possible. The best-fitting substitution model for each partition was selected using the Akaike Information Criterion implemented in MrModeltest v2.2 (Nylander, 2004) and run in PAUP* v4.0b (Swofford, 2002). The relaxed uncorrelated lognormal clock model (UCLD, Drummond et al., 2006) and a Yule speciation process as tree model were selected for all datasets based on preliminary explorations. MCMC searches were run 5 × 107 generations and sampled and logged every 2500th generation. We used Tracer v1.6 (Rambaut et al., 2013) to determine stationarity of the Markov chain and to verify that all parameters had large enough effective sampling sizes (ESS>200). TreeAnnotator v1.8.0 (Drummond et al., 2012) and FigTree v. 1.4.2 (Rambaut, 2009) were used respectively to generate and visualize the resulting maximum clade credibility (MCC) chronograms.
Calibration points for obtaining absolute divergence times were based on either the fossil record or on published secondary calibration constraints (Table 2). The latter were obtained from published dated phylogenies of datasets including our study groups (e.g., the family to which the genus belongs), and were assigned normal distribution priors (Ho and Phillips, 2009) in the BEAST analysis that encompassed the mean and the 95% highest posterior density (HPD) confidence interval (CI) from these studies [except in the case of time constrains from Bell et al. (2010), for which a lognormal distribution was used, since posterior estimates for a normal prior were not available]. For fossil calibration points we used a lognormal prior, since this distribution better represents the stratigraphic uncertainty associated to the fossil record (Ho and Phillips, 2009). The offset of the lognormal distribution was set to the upper bound of the stratigraphic period where the fossil was found, and the standard deviation (SD) and mean were set so that the 95% CI encompassed the lower and upper bound of the period (e.g., for Late Eocene Hypericum antiquum a lognormal distribution offset at 33.9 Myr, with mean = 1.0 and SD = 0.7, was used to cover the length of the period where the fossil was found, that is 33.9–37.2 Ma). A summary of time constraints used for each dataset and their provenance can be found in Table 2.
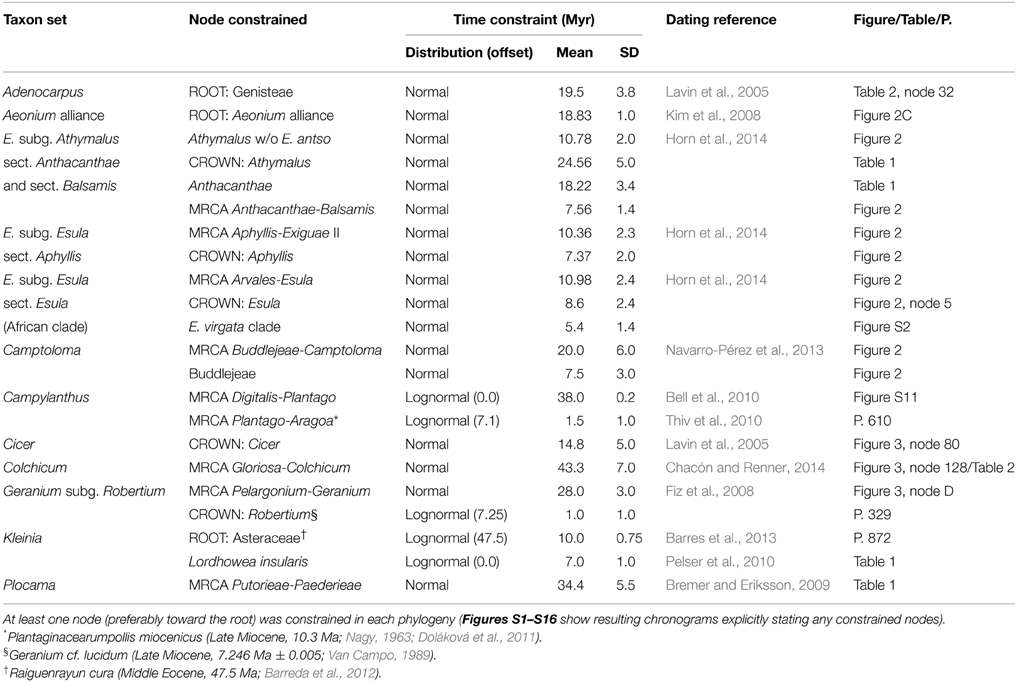
Table 2. Time constraints and prior probability distributions imposed on constrained nodes to estimate divergence times in RF lineages.
We used divergence times estimated above to calculate absolute diversification rates in the aforementioned lineages. There have been numerous developments in macroevolutionary birth-death models that allow a more accurate estimation of extinction and speciation rates from dated molecular phylogenies, including episodic time-variable models and trait-dependent diversification models (Stadler, 2013; Morlon, 2014; Rabosky et al., 2014). However, these methods usually require both very large phylogenies (e.g., ≥100 tips) and a fairly complete sampling. We here chose a simpler approach, the “method-of-moments” estimator (Magallón and Sanderson, 2001), implemented in the R package GEIGER (Harmon et al., 2008). This method uses clade size (extant species number) and clade age (either crown or stem) to estimate net diversification rates (r = speciation minus extinction), under different values of background extinction or turnover rate (ε = extinction/speciation = 0.0, 0.5, and 0.9). Net diversification rates (bd.ms function in GEIGER) were here estimated for all RF disjunctions and for a series of successively encompassing clades (e.g., section, genus, tribe, subfamily, and so on) to detect possible rate shifts. Crown diversification rates could not be estimated for clades containing only two taxa because Magallón and Sanderson's formula (r = [log(n)–log 2]/t in its simplest version, that is, with no extinction; for ε > 0 see formula number 7 in Magallón and Sanderson, 2001) results in zero in this case. In an attempt to counter this problem, clades containing two taxa were assigned a diversity value of 2.01, which permitted the estimation of net diversification rates (r).
Additionally, the probability of obtaining a clade with the same size and age as the RF disjunction, given the background diversification rate of the encompassing clade/s and at increasing extinction fractions (ε = 0, 0.5, and 0.9), was estimated with the crown.p function in GEIGER. We also estimated the 95% confidence interval of expected diversity through time (crown.limits function, GEIGER, ε = 0, 0.5, and 0.9) for a clade that diversifies with a rate equal to that of the family containing a RF disjunction with the highest diversification rate (i.e., Asteraceae); we then mapped RF lineages according to their crown or stem age and standing species diversity to assess which RF disjunct clades are significantly less diverse than expected given their stem and crown age in relation to the highest rate calculated for a RF family (Magallón and Sanderson, 2001; Warren and Hawkins, 2006).
Up to 21 disjunctions were identified and divergence times were estimated for 17 lineages exhibiting a geographic distribution consistent with the RF pattern (Figures 3, 4 and Figures S1–S17). These disjunctions represent two possible geographic splits: I) Eastern Africa (including the Eastern Arc Mountains, the Horn of Africa, and Southern Arabia) vs. Southern Africa (including southern Angola and Namibia and the Cape Flora region up to the Drakensberg Mountains), hereafter E-S, and II) Western Africa (including Macaronesia and NW Africa south to the Cameroon volcanic line) vs. Eastern Africa, (with or without S Africa), hereafter W-E(&S).
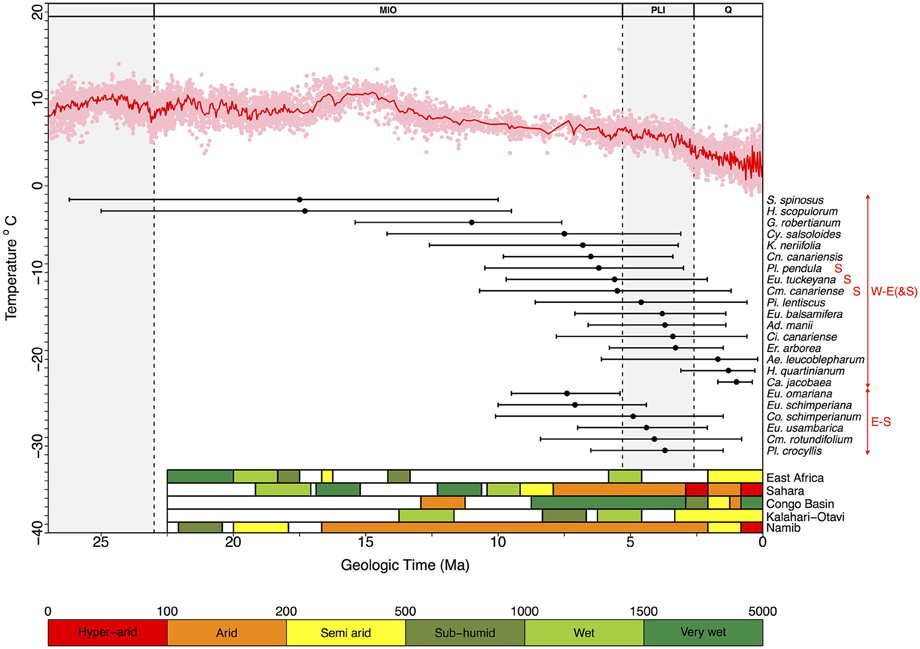
Figure 4. Diagram showing estimated lineage divergence times (mean and 95% HPD confidence intervals) for Rand Flora disjunctions dated in this study and indicated in the MCC chronograms depicted in Figures S1–S17. W-E(&S): divergence times estimated between disjunct taxa distributed in Macaronesia-NW-W Africa vs. Eastern Africa (a red S indicates presence in Southern Africa); E-S: estimated divergence times between disjunct taxa distributed in southern Arabia-Eastern Africa vs. southern Africa. The red line above represents the change in global temperatures over the Cenozoic as reflected by global-deep-sea oxygen records compiled from Zachos et al. (2008); colored bars in the right bottom corner indicate climatic conditions in five regions that underwent major climate changes—either desertification or tropicalization—during the Neogene (adapted from Senut et al., 2009). Taxa names correspond to those in Table 1, plus two groups from the literature: Pistacia lentiscus and Erica arborea (see Discussion).
From youngest to oldest, E-S disjunctions (Figure 4) occur in Plocama (ca. 4 Ma between S African Pl. crocyllis on one side and, among other E African-S Arabian species, Pl. yemenensis and Pl. tinctoria on the other; Figure 3 and Figure S15), Camptoloma (ca. 4 Ma between E African Cm. lyperiiflorum and S African Cm. rotundifolium; Figure 3 and Figure S4), Colchicum (ca. 5 Ma between E African Co. schimperianum and S African Co. albanense and Co. longipes, Figure 3 and Figure S8), the African clade of Euphorbia sect. Esula (ca. 7 Ma between S African and E African taxa; Figure 3 and Figure S10), and E. sect. Anthacanthae (ca. 7.5 Ma separate subsects. Platycephalae and Florispinae; Figure 3 and Figure S11).
Also from youngest to oldest, W-E disjunctions (Figure 4) can be found in the Azorina clade of Campanula (ca. 1 Ma between Cape Verdean Ca. jacobaea and Socotran Ca. balfouri; Figure 3 and Figure S3), in Hypericum sect. Campylosporus (ca. 1.5 Ma within H. quartinianum; Figure 3 and Figure S13), in Aeonium (1.7 Ma between E African Ae. leucoblepharum and a number of Macaronesian species; Figure 3 and Figure S2), in Cicer (ca. 3.5 Ma between Canarian Ci. canariense and E African Ci. cuneatum; Figure 3 and Figure S7), in Adenocarpus (ca. 4 Ma between E African Ad. mannii and a number of species in the Ad. complicatus complex; Figure 3 and Figure S1), in Euphorbia sect. Balsamis (ca. 4 Ma between W African Eu. balsamifera subsp. balsamifera and E African-S Arabian Eu. balsamifera subsp. adenensis; Figure 3 and Figure S11), in Camptoloma (ca. 5.5 Ma between Canarian Cm. canariense, on one hand, and E African Cm. lyperiiflorum and S African Cm. rotundifolium, on the other; Figure 3 and Figure S4), Eu. sect. Aphyllis (ca. 5.5 Ma between Cape Verdean Eu. tuckeyana and all E African and S African species in this section; Figure 3 and Figure S9), Plocama (ca. 6 Ma between Canarian Pl. pendula and S African Pl. crocyllis plus a number of E African/S Arabian Plocama species, Figure 3 and Figure S16), in Canarina (6.5 Ma between Canarian Cn. canariensis and E African Cn. eminii; Figure 3 and Figure S6), in Kleinia (ca. 7 Ma between the Macaronesian species, on one hand, and a clade of several E African species, on the other; Figure 3 and Figure S14), in Campylanthus (ca. 7.5 Ma between the Macaronesian and the E African-S Arabian species in the genus; Figure 3 and Figure S5), in Geranium subgen. Robertium (ca. 11 Ma between all E African species in this subgenus and a clade formed by W African taxa and a number of broadly distributed circum-Mediterranean and E Asian taxa; Figure 3 and Figure S12), in the Androsaemum clade of Hypericum (ca. 17 Ma between Socotran H. scopulorum, H. tortuosum and Turkish H. pamphylicum, on one hand, and a number of Macaronesian and W Mediterranean species, on the other; Figure 3 and Figure S13), and in Sideroxylon (ca. 17 Ma between Moroccan S. spinosus and E African S. mascatense; Figure 3 and Figure S16).
Figure 5 and Table S1 show results from net diversification rate analyses. Most lineages fall within the 95% CI of expected diversity under a no-extinction scenario (ε = 0) in the context of the RF family showing the highest rate of diversification (i.e., Asteraceae). However, some RF disjunct clades were significantly less diverse: W-E disjunctions in Sideroxylon (S. spinosus vs. S. mascatense), Canarina (C. canariensis vs. C. eminii), and Hypericum (H. canariense clade vs. H. scopulorum and H. pamphylicum). Other RF disjunct taxa were above the upper bound of the 95% CI: W-E(&S) disjunction in Euphorbia sect. Aphyllis (S), Adenocarpus, Aeonium, and Campanula; and E-S disjunction in Plocama. Otherwise, all taxa fell within the 95% CI with increasing ε values 0.5 and 0.9, except for Sideroxylon.
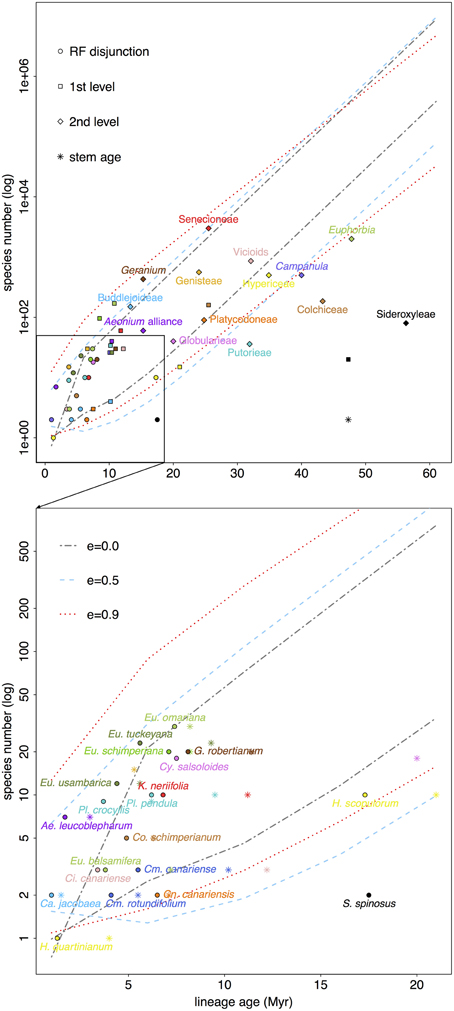
Figure 5. RF lineages (names as in Table 1) are plotted according to their standing diversity (n) and age of the node (circle, crown; star, stem) corresponding to their disjunction (below). Successive encompassing lineages (above) also plotted (squares indicate the clade, section, subgenus the RF disjunct clade falls in; diamonds go one level above indicating genus, tribe, subfamily). Ninety five percent confidence intervals show expected diversity through time for a RF lineage that diversifies at the highest rate estimated (i.e., Asteraceae) given three possible scenarios: no extinction (ε = 0), turnover at equilibrium (ε = 0.5), and high extinction (ε = 0.9). See Table S1 for associated net diversification rate estimates.
Interestingly these trends are generally repeated in the more encompassing lineages of the least diverse RF disjunct clades (e.g., Canarina, Hypericum, Sideroxylon). Notably, though Camptoloma has a low extant diversity given its age (three species diverging in the last 6 Myr), the subfamily it belongs to, that is Buddlejoideae, stands above the 95% CI for ε = 0 (Figure 5). Something similar can be observed in the case of Kleinia, which shows lower diversity than its encompassing lineage, tribe Senecioneae. Another example of potential diversification shift, though in the opposite direction, is that of Euphorbia, where the genus is significantly less diverse than expected given its age (for all ε values) but RF disjunct clades are species-richer than expected (i.e., E. sect. Aphyllis), except for those that fall within the 95% CI limits (e.g., E. sect. Balsamis, Figure 5).
When comparing crown vs. stem age it is noticeable that in some RF disjunct clades crown and stem ages are far apart: Cicer canariensis vs. Ci. cuneatum (crown age = 3.4 Ma, stem age = 12.2 Ma, with the stem age falling below the lower bound of 95% CIs when ε = 0.0 and 0.9; Figure 5). Other examples include, Camptoloma (crown age= 5.5 Ma, stem age = 10.2 Ma), Campylanthus (crown age = 7.5 Ma, stem age = 20.0 Ma), and most notably Sideroxylon (crown age = 17.4 Ma, stem age = 47.3 Ma, Figure 5).
Engler's (1910) intuition on the Tertiary origins of the Afro-Macaronesian floristic element, aka Christ's (1910) Rand Flora, very much hit the mark on the timing of its assembly. Our divergence estimates for Rand Flora disjunctions span five successive time frames (Figure 4): Burdigalian, Tortonian, and Messinian Stages (within the Miocene), the Pliocene, and the Pleistocene. The two earliest disjunctions happen on genera Sideroxylon and Hypericum and date back to the Early Miocene (Burdigalian; 17.5 and 17.3 Ma, respectively), coinciding with the longest warming period of the Miocene (the Miocene Climatic Optimum; Zachos et al., 2008) and with the start of desertification in south-central Africa (Senut et al., 2009). Couvreur et al. (2008) also dated divergences in Annonaceae back to this time period and explained them in terms of a once-continuous Early Miocene rainforest that became fragmented by decreasing moisture brought by the closure of the Tethys Sea. The fact that Sideroxylon and Hypericum exhibit less xeric affinities than other RF lineages, and that their crown diversification dates back to the Paleogene (Meseguer et al., 2013; Stride et al., 2014), suggests these taxa could be relicts of an earlier megathermal flora (sensu Morley, 2000, 2003).
The next disjunction is that of Geranium subgen. Robertium and it dates back to the Late Miocene (Tortonian, 11.0 Ma). This disjunction follows a drastic decline in global temperatures (Late Miocene cooling, 11.6–5.3 Ma; Beerling et al., 2012) and coincides with the temporary closing of the Panama isthmus in America and a moist “washhouse” climate period in Europe (Böhme et al., 2008). This disjunction marks the separation of Macaronesian (e.g., G. maderense) and circum-Mediterranean taxa (e.g., G. robertianum), on one side, and E African species (e.g., G. mascatense), on the other, leaving open the possibility of a colonization of Macaronesia by a Mediterranean ancestor (Figure 4 and Figure S12). Since the disjunction in Geranium subgen. Robertium is linked to a more humid period, rather than an increase on aridity, and because the possible Mediterranean origin of its Macaronesian taxa, this lineage does not exactly match the RF pattern.
Most other Neogene disjunctions seem to concentrate around the Miocene-Pliocene border (Figure 4). Messinian disjunctions can be observed in Camptoloma, Campylanthus, Canarina, Euphorbia sects. Anthacanthae and Aphyllis, Kleinia, and Plocama. Pliocene disjunctions are found in Adenocarpus, Camptoloma, Cicer, Colchicum, Euphorbia. sects. Balsamis and Aphyllis, and Plocama. These disjunctions follow two different geographic splits, W-E(&S) Africa and E-S Africa. W-E(&S) disjunctions present the widest temporal (as well as spatial) range. Besides the lineages dated here, other examples can be found in the literature of this W-E(&S) disjunction, e.g., according to Xie et al. (2014), in the Anacardiaceae Pistacia lentiscus and P. aethiopica diverged 4.55 Ma (see Figure S17). E-S disjunctions link South Africa and adjacent areas to the East African Rift Mountains, the Ethiopian Highlands, and the Arabian Peninsula. The timing of these E-S disjunctions (Mio-Pliocene) matches the uplift of the Eastern Arc Mountains (Sepulchre et al., 2006). The absence of W-S disjunctions is notable and probably results from African aridification having started in the early Miocene (some 17–16 Ma) in the region where the current Namib Desert stands. This aridification not only persisted through time in this area but also intensified and resulted in the formation of the Kalahari Desert (Senut et al., 2009), effectively limiting range expansions in this direction (W-S), in the absence of successful colonization following LDD. Even in the case of genus Colchicum (Figure S8), were S African species appear closely related to NW African ones, W Mediterranean species are always sister to E Mediterranean ones. These leaves open the possibility of a colonization of NW Africa (from S Africa) via E Africa and W Mediterranean populations with subsequent extinction in E Africa. An alternative colonization from Central-West Asia into South Africa and NW Africa seems unlikely given the phylogeny of this genus (Figure S8), though proper biogeographic inference to test either possibility remains to be done. Indeed, Sanmartín et al. (2010) found a higher frequency of biotic exchange between NW-E African elements than with either E-S African or W-S African ones, where the latter elements were hardly connected, if at all, confirming our observations. We further argue that the magnitude of observed biotic exchange follows the history of desertification in Africa.
In all, the sequential timing of Neogene disjunctions in RF lineages, which is nonetheless concentrated in certain time intervals (e.g., Late Miocene-Pliocene), is in agreement with a scenario of range expansions (dispersal) in favorable times (windows of opportunity) and range contractions (extinction) as aridification flared up. Extinction results in absence (of a population, species, clade, or lineage) and thus leaves hard to track traces in phylogenies in the absence of fossil data (Meseguer et al., 2015). If repeated cycles of speciation, dispersal, and extinction take place in the same area over time, only taxa that optimize any (or a combination) of these processes (e.g., increased speciation, higher dispersal, lower extinction rates) will persist. It is to be expected that more recent populations, species, clades, or lineages show traces of these processes when compared to ancient ones.
On the other hand, our net diversification rate estimates (Figure 5) do no fully support an extinction explanation since, in the context of the family with the highest diversification rate among RF lineages, i.e., Asteraceae, most of the taxa fall inside the 95% CI under a no-extinction scenario (ε = 0.0). However, the method chosen to estimate net diversification rates (Magallón and Sanderson, 2001), though more appropriate given phylogeny size and sampling effort, is still limited. Crown diversification rates cannot be estimated for clades with 2 terminal taxa (see Materials and Methods), which is the case for several RF lineages (e.g., Sideroxylon). Additionally, the “method-of-moments” estimator performs well detecting declining diversity for old groups in exceedingly species-poor clades (Magallón and Sanderson, 2001; Warren and Hawkins, 2006) or young groups notably species-rich (recent radiations, Magallón and Sanderson, 2001), but we observed that statistical power is low to detect declines in diversity for young species-poor groups (e.g., Camptoloma). Most RF disjunct clades dated comprise less than 10 species—e.g., Aeonium, Campanula, Camptoloma, Cicer, Colchicum, Euphorbia sect. Balsamis, Kleinia, and Plocama—, limiting our ability to effectively detect the effects of extinction.
Nonetheless, if we focus on crown ages, disjunct clades in Canarina, Hypericum, and Sideroxylon are less diverse than expected, and given that their encompassing lineages (Table 1, Figure 5) also follow this trend, it would be safe to assume these lineages have indeed experienced high levels of extinction through time. Likewise, if we were to focus on stem ages, a few other groups fall below the no-extinction scenario (ε = 0.0), notably, Camptoloma, Campylanthus, and Cicer. Moreover, these groups exhibit wide-spanning (often >10 Ma) stem-crown intervals (see Sideroxylon or Cicer in Figure 5), an observation that has been tied to historically high extinction rates in recent diversification studies (Antonelli and Sanmartín, 2011; Nagalingum et al., 2011). This would further support the hypothesis that lower diversification rates in RF lineages could be explained in terms of increased extinction rather than a decrease in speciation rates.
Additionally, and given the aforementioned limitations of our diversification method of choice, it would also be safe to conclude that, within Euphorbia, sects. Anthacanthae (sect. Balsamis included), sect. Esula, and sect. Aphyllis, present higher diversity than expected (above the CI for ε = 0.0 in all cases, and also above the CI for ε = 0.5 for the former two clades), which is exceptional in the context of the genus, since Euphorbia is significantly poorer than expected for all ε values. Horn et al. (2014) also detected increased diversification rates in these sections of Euphorbia. Desertification-tropicalization cycles in Africa (Senut et al., 2009) suggest repeated reconnections between now disjunct RF regions since the Neogene, which would have permitted biotic exchange in favorable periods, whereas the isolation of these regions at unfavorable times would have induced speciation through vicariance, enhancing endemicity in these sub-humid/sub-xeric lineages. Molecular dating in tropical trees from the genus Acridocapus (Malpighiaceae; Davis et al., 2002) and the Annonaceae family (Couvreur et al., 2008) shows a similar pattern of connection phases between East African and Guineo-Congolian rainforest regions since the Oligocene following major climate shifts.
The youngest disjunctions, those of Aeonium, Campanula, and Hypericum sect. Campylosporus, are Pleistocene in age (Figure 4) and far too recent to result from the Neogene aridification of the African continent. Either rare LDD (i.e., Aeonium; Kim et al., 2008) or stepping-stone dispersal events (i.e., Campanula, Alarcón et al., pers. comm.), perhaps favored by Pleistocene cool and drier glacial cycles, could explain these more recent disjunct geographic patterns, as previously observed in other African taxa, e.g., Convolvulus (Carine, 2005), Moraea (Galley et al., 2007), or the tree heath (Erica arborea). Désamoré et al. (2011) took notice of successive range expansions of Er. arborea from an Eastern African center of diversity toward Northwest Africa, Southwest Europe, and Macaronesia, first during the Late Pliocene (ca. 3 Ma; Figure 4) and subsequently in the Pleistocene (ca. 1 Ma).
In a recent review, Linder (2014) synthesized the individual histories of numerous African lineages by recognizing five different “floras,” which he defined as “groups of clades, which: (a) are largely found in the same area, (b) have largely the same extra-African geographical affinities, (c) share a diversification history, and (d) have a common maximum age.” The “Rand Flora” does not fit well this definition. This flora does group a number of lineages that share the same geographic range (even if discontinuous), but they have slightly different climatic tolerances, i.e., sub-humid to sub-xeric or xerophilic, and they do not necessarily share the same extra-African geographical affinities. Some RF lineages fall within what Linder (2014) terms “tropic-montane flora” (e.g., Hypericum, Canarina), others within the “arid flora” (e.g., Kleinia, Campylanthus). Some RF lineages are better connected with the Mediterranean Region (e.g., Adenocarpus), others with Asia and the Indo-Pacific Region (e.g., Plocama). Moreover, RF taxa on either side of any given disjunction (i.e., W-E or E-S) do no longer share a “diversification history,” though they do share the same fate as other RF lineages with similar distribution. In fact, the different ages estimated here for the various RF disjunctions agree well with what has been termed biogeographic pseudocongruence (Donoghue and Moore, 2003), a phenomenon whereby two or more lineages display the same biogeographic pattern but with different temporal origins (Sanmartín, 2014). What is shared by all RF lineages is the nature of the climatic (ecological) barriers separating the taxa at either side of any given disjunction: arid regions such as the Sahara, the Kalahari or the Namib deserts, or the tropical lowlands in Central Africa. The congruence between RF disjunction ages and successive major climatic events in Africa during the Neogene (Figure 4) suggest that the ongoing aridification of the continent (or the “tropicalization” of Central Africa) affected RF lineages according to their different physiological (climatic) tolerances: more sub-humid lineages diverged first (e.g., Sideroxylon), more xeric later (e.g., Campylanthus).
One point of contention in the literature has been the limits of the Rand Flora with respect to the “Arid Corridor” or “Arid Track” (hereafter AC), a path repeatedly connecting south-west to north-east arid regions in Africa (and henceforth to central and southwest Asia) first proposed by Winterbottom (1967) and later expanded by de Winter (1966, 1971) and Verdcourt (1969). Bellstedt et al. (2012) defined the AC pattern as the disjunction occurring between Southern Africa and Eastern African-Southern Arabian xeric floristic elements. Linder (2014) considered the RF as an expansion of the AC to the west, in agreement with Jürgens' (1997) view. However, we consider that the RF and AC patterns are different. AC elements have more xeric preferences than the sub-humid to sub-xeric ones exhibited by RF elements. AC elements often extend into deserts (e.g., Namib, Kalahari, Sahara)—see studies by Beier et al. (2004) on Fagonia (Zygophyllaceae), Bellstedt et al. (2012) on Zygophyllum (also Zygophyllaceae), Carlson et al. (2012) on Scabiosa (Dipsacaceae), or Bruyns et al. (2014) on Ceropegieae— and have broader, more continuous distributions, plus they tend to be younger in age (often Pleistocene, coincident with Quaternary glaciation cycles). Our understanding is that this younger xeric AC elements move in parallel to RF taxa webbing with them in areas favorable to either, and thus confusing their limits. Something similar could have happened with Afromontane elements migrating south to north as the Eastern African mountains rose through the Mio-Pliocene; these elements are not part of the RF (e.g., Iris, Moraea, Galley et al., 2007).
In this study, we have provided a temporal framework for the Rand Flora pattern and estimated net diversification rates for 17 RF lineages. Our results provide some support to the historical view of an ancient African flora, whose current disjunct distribution was probably modeled by the successive waves of aridification events that have affected the African continent starting in the Miocene, but whose origin predates the latest events of Pleistocene climate change. These patterns were probably formed by a combination of climate-driven extinction and vicariance within a formerly widespread distribution. Whether these lineages all had a continuous, never interrupted, distribution that occupied all the area that now lies in between the extremes of the disjunction, or they had a somewhat narrower distribution in the past and they expanded their range tracking their habitat across the landscape in response to changing climate (e.g., along a corridor), is difficult to say with the current evidence. Discerning between these hypotheses will require the integration of phylogenetic, biogeographic and ecological approaches to reconstruct the ancestral ranges and climatic preferences of ancestral lineages (Mairal et al., 2015; Meseguer et al., 2015). Compared to speciation, extinction has received far less attention in studies focusing on the assembly of tropical biotas. Disentangling extinction from other processes is particularly difficult because the biodiversity we observe today is only a small fraction of that of the past. The Rand Flora pattern might offer a prime study model to understand the effects of climate-driven extinction in the shaping of continent-wide biodiversity patterns.
IS and LP conceived and designed the study. LP analyzed the data with help from IS, RR, and MM. LP and IS co-wrote the text, with contributions from MH, RR, MM, and AM. All authors contributed with data compilation, figure preparation, or text comments. MM has copyright of all plant pictures, except for Cicer canariense.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This study was funded by the Spanish Ministry of Economy and Competitiveness (MINECO): Project AFFLORA, CGL2012-40129-C02-01 to IS. LP was funded by CSIC postdoctoral contract within AFFLORA. MH was funded by CGL2012-40129-C02-02, the Research Council of Norway (203822/E40) and a Ramón y Cajal Fellowship (RYC2009-04537). RR was supported by a JAE-DOC postdoctoral fellowship (MINECO) and the European Social Fund. MM and VC were supported by MINECO FPI predoctoral fellowships (BES-2010-037261 and BES-2013-065389 respectively). We thank Virginia Valcárcel (Department of Biology, UAM, Spain) for help with data compilation and literature revision during the earlier stages of the project, Andrea Briega (Department of Ecology, UAH, Spain) for help with data compilation, and Manuel Gil for providing a Cicer canariense picture.
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fgene.2015.00154/abstract
Supplementary Materials include descriptions of study groups with references, Table S1, and Figures S1–S17.
Table S1. Net diversification rates (bd.ms) for all RF disjunct clades and their encompassing lineages (bold = highest crown.p, red when n ≤ 2) under three possible scenarios: no extinction (ε = 0), turnover at equilibrium (ε = 0.5), and high extinction (ε = 0.9). Probability (crown.p) of obtaining a clade with the same size and age as the RF disjunction, given the background diversification rate of the encompassing clade/s and at increasing extinction fractions (bold = highest crown.p, italics p < 0.05). Stem and Crown ages in Myr.
Figures S1–S17. BEAST MCC chronograms showing mean estimates and 95% high posterior density (HPD) confidence intervals for those nodes receiving 50% support. Branch width is proportional to PP support. Red colored taxa indicate Eastern African provenance; Macaronesia/western African taxa and southern African taxa are colored in blue and green, respectively. Calibration points are indicated with stars; RF disjunctions within each lineage discussed in the text and represented in Figures 3–5 are indicated with arrows.
Alarcón, M., Roquet, C., García-Fernández, A., Vargas, P., and Aldasoro, J. J. (2013). Phylogenetic and phylogeographic evidence for a Pleistocene disjunction between Campanula jacobaea (Cape Verde Islands) and C. balfourii (Socotra). Mol. Phylogenet. Evol. 69, 828–836. doi: 10.1016/j.ympev.2013.06.021
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Andrus, N., Trusty, J., Santos-Guerra, A., Jansen, R. K., and Francisco-Ortega, J. (2004). Using molecular phylogenies to test phytogeographical links between East/South Africa–Southern Arabia and the Macaronesian islands—a review, and the case of Vierea and Pulicaria section Vieraeopsis (Asteraceae). Taxon 53, 333–333. doi: 10.2307/4135612
Antonelli, A., and Sanmartín, I. (2011). Mass extinction, gradual cooling, or rapid radiation? Reconstructing the spatiotemporal evolution of the ancient angiosperm genus Hedyosmum (Chloranthaceae) using empirical and simulated approaches. Syst. Biol. 60, 596–615. doi: 10.1093/sysbio/syr062
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Axelrod, D. I., and Raven, P. H. (1978). “Late Cretaceous and Tertiary vegetation history of Africa,” in Biogeography and Ecology of southern Africa, ed M. J. A. Werger (The Hague: Dr W. Junk bv Publishers), 77–130.
Backlund, M., Bremer, B., and Thulin, M. (2007). Paraphyly of Paederieae, recognition of Putorieae and expansion of Plocama (Rubiaceae-Rubioideae). Taxon 56, 315–328. Available online at: http://www.ingentaconnect.com/content/iapt/tax/2007/00000056/00000002/art00006
Barreda, V. D., Palazzesi, L., Katinas, L., Crisci, J. V., Tellería, M. C., Bremer, K., et al. (2012). An extinct Eocene taxon of the daisy family (Asteraceae): evolutionary, ecological and biogeographical implications. Ann. Bot. 109, 127–134. doi: 10.1093/aob/mcr240
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barres, L., Sanmartín, I., Anderson, C. L., Susanna, A., Buerki, S., Galbany-Casals, M., et al. (2013). Reconstructing the evolution and biogeographic history of tribe Cardueae (Compositae). Am. J. Bot. 100, 867–882. doi: 10.3732/ajb.1200058
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barres, L., Vilatersana, R., Molero, J., Susanna, A., and Galbany-Casals, M. (2011). Molecular phylogeny of Euphorbia subg. Esula sect. Aphyllis (Euphorbiaceae) inferred from nrDNA and cpDNA markers with biogeographic insights. Taxon 60, 705–720. Available online at: http://www.ingentaconnect.com/content/iapt/tax/2011/00000060/00000003/art00007
Beerling, D. J., Taylor, L. L., Bradshaw, C. D., Lunt, D. J., Valdes, P. J., Banwart, S. A., et al. (2012). Ecosystem CO2 starvation and terrestrial silicate weathering: mechanisms and global-scale quantification during the late Miocene. J. Ecol. 100, 31–41. doi: 10.1111/j.1365-2745.2011.01905.x
Beier, B. A., Nylander, J. A. A., Chase, M. W., and Thulin, M. (2004). Phylogenetic relationships and biogeography of the desert plant genus Fagonia (Zygophyllaceae), inferred by parsimony and Bayesian model averaging. Mol. Phylogenet. Evol. 33, 91–108. doi: 10.1016/j.ympev.2004.05.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bell, C. D., Soltis, D. E., and Soltis, P. S. (2010). The age and diversification of the angiosperms re-revisited. Am. J. Bot. 97, 1296–1303. doi: 10.3732/ajb.0900346
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bellstedt, D. U., Galley, C., Pirie, M. D., and Linder, H. P. (2012). The migration of the palaeotropical arid flora: zygophylloideae as an example. Syst. Bot. 37, 951–959. doi: 10.1600/036364412X656608
Böhme, M., Ilg, A., and Winklhofer, M. (2008). Late Miocene “washhouse” climate in Europe. Earth Planet. Sci. Lett. 275, 393–401. doi: 10.1016/j.epsl.2008.09.011
Bonnefille, R. (2011). “Rainforest responses to past climate changes in tropical Africa,” in Tropical Rainforest Responses to Climate Change, 2nd Edn, eds M. Bush, J. Flenley, and W. Gosling (Berlin; Heidelberg: Springer-Verlag), 125–184.
Bramwell, D. (1985). Contribución a la biogeografía de las Islas Canarias. Bot. Macaronésica 14, 3–34.
Bremer, B., and Eriksson, T. (2009). Time tree of Rubiaceae: phylogeny and dating the family, subfamilies, and tribes. Int. J. Plant Sci. 170, 766–793. doi: 10.1086/599077
Bruyns, P. V., Klak, C., and Hanáček, P. (2014). Evolution of the stapeliads (Apocynaceae–Asclepiadoideae)—repeated major radiation across Africa in an Old World group. Mol. Phylogenet. Evol. 77, 251–263. doi: 10.1016/j.ympev.2014.03.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carine, M. A. (2005). Spatio-temporal relationships of the Macaronesian endemic flora: a relictual series or window of opportunity? Taxon 54, 895–903. doi: 10.2307/25065476
Carlson, S. E., Linder, H. P., and Donoghue, M. J. (2012). The historical biogeography of Scabiosa (Dipsacaceae): implications for Old World plant disjunctions. J. Biogeogr. 39, 1086–1100. doi: 10.1111/j.1365-2699.2011.02669.x
Chacón, J., and Renner, S. S. (2014). Assessing model sensitivity in ancestral area reconstruction using LAGRANGE: a case study using the Colchicaceae family. J. Biogeogr. 41, 1414–1427. doi: 10.1111/jbi.12301
Christ, H. (1892). Exposé sur le rôle que joue dans le domaine de nos flores la flore dite ancienne africaine. Arch. Sci. Phys. Nat. Genève 3, 369–374.
Coleman, M., Liston, A., Kadereit, J. W., and Abbott, R. J. (2003). Repeat intercontinental dispersal and Pleistocene speciation in disjunct Mediterranean and desert Senecio (Asteraceae). Am. J. Bot. 90, 1446–1454. doi: 10.3732/ajb.90.10.1446
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Couvreur, T. L. (2015). Odd man out: why are there fewer plant species in African rain forests? Plant Syst. Evol. 301, 1299–1313. doi: 10.1007/s00606-014-1180-z
Couvreur, T. L., Chatrou, L. W., Sosef, M. S., and Richardson, J. E. (2008). Molecular phylogenetics reveal multiple tertiary vicariance origins of the African rain forest trees. BMC Biol. 6:54. doi: 10.1186/1741-7007-6-54
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cubas, P., Pardo, C., Tahiri, H., and Castroviejo, S. (2010). Phylogeny and evolutionary diversification of Adenocarpus DC. (Leguminosae). Taxon 59, 720–732. Available online at: http://www.ingentaconnect.com/content/iapt/tax/2010/00000059/00000003/art00005
Davis, C. C., Bell, C. D., Fritsch, P. W., and Mathews, S. (2002). Phylogeny of Acridocarpus-Brachylophon (Malpighiaceae): implications for Tertiary tropical floras and Afroasian biogeography. Evolution 56, 2395–2405. doi: 10.1111/j.0014-3820.2002.tb00165.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
del Hoyo, A., García-Marín, J. L., and Pedrola-Monfort, J. (2009). Temporal and spatial diversification of the African disjunct genus Androcymbium (Colchicaceae). Mol. Phylogenet. Evol. 53, 848–861. doi: 10.1016/j.ympev.2009.08.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Désamoré, A., Laenen, B., Devos, N., Popp, M., González-Mancebo, J. M., Carine, M. A., et al. (2011). Out of Africa: north-westwards Pleistocene expansions of the heather Erica arborea. J. Biogeogr. 38, 164–176. doi: 10.1111/j.1365-2699.2010.02387.x
de Winter, B. (1966). Remarks on the distribution of some desert plants in Africa. Palaeoecol. Afr. 1, 188–189.
de Winter, B. (1971). Floristic relationships between the northern and southern arid areas in Africa. Mitt. Bot. Staatssamml. Munch. 10, 424–437.
Doláková, N., Kovaìcovaì, M., and Basistovaì, P. (2011). Badenian (Langhian-Early Serravallian) palynoflora for the Carpathian Foredeep and Vienna Basin (Czech and Slovak Republics). Acta Ent. Mus. Nat. Pra. 67, 63–71. Available online at: http://www.muni.cz/research/publications/955017
Donoghue, M. J., and Moore, B. R. (2003). Toward an integrative historical biogeography. Integr. Comp. Biol. 43, 261–270. doi: 10.1093/icb/43.2.261
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drummond, A. J., Ho, S. Y., Phillips, M. J., and Rambaut, A. (2006). Relaxed phylogenetics and dating with confidence. PLoS Biol. 4:e88. doi: 10.1371/journal.pbio.0040088
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drummond, A. J., Suchard, M. A., Xie, D., and Rambaut, A. (2012). Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973. doi: 10.1093/molbev/mss075
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Engler, A. (1879). “Die extratropischen Gebiete der nördlichen Hemisphäre. Mit einer chromolithographischen Karte,” in Versuch einer Entwicklungsgeschichte der Pflanzenwelt: insbesondere der Florengebiete seit der Tertiärperiode. Theil 1, ed A. Engler (Leipzig: Verlag von Wilhelm Engelmann), 1–202.
Engler, A. (1910). “Die Pflanzenwelt Afrikas inbesondere seiner tropischen Gebiete. Gründzuge der Pflanzenverbreitung in Afrika un die Characterpflanzen Afrikas,” in Die Vegetation der Erde, eds A. Engler and O. Drude (Leipzig: Verlag von Wilhelm Engelmann), 1030.
Fischer, A. G. (1960). Latitudinal variations in organic diversity. Evolution 14, 64–81. doi: 10.2307/2405923
Fiz, O., Vargas, P., Alarcón, M., Aedo, C., García, J. L., and Aldasoro, J. J. (2008). Phylogeny and historical biogeography of Geraniaceae in relation to climate changes and pollination ecology. Syst. Bot. 33, 326–342. doi: 10.1600/036364408784571482
Forster, J. R. (1778). Observations Made During a Voyage Round the World, on Physical Geography, Natural History, and Ethic Philosophy. London: G. Robinson.
Galley, C., Bytebier, B., Bellstedt, D. U., and Linder, H. P. (2007). The Cape element in the Afrotemperate flora: from Cape to Cairo? Proc. R. Soc. B 274, 535–543. doi: 10.1098/rspb.2006.0046
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Harmon, L. J., Weir, J. T., Brock, C. D., Glor, R. E., and Challenger, W. (2008). GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. doi: 10.1093/bioinformatics/btm538
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ho, S. Y., and Phillips, M. J. (2009). Accounting for calibration uncertainty in phylogenetic estimation of evolutionary divergence times. Syst. Biol. 58, 367–380. doi: 10.1093/sysbio/syp035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Horn, J. W., Xi, Z., Riina, R., Peirson, J. A., Yang, Y., Dorsey, B. L., et al. (2014). Evolutionary burst in Euphorbia (Euphorbiaceae) are linked with Photosynthetic pathway. Evolution 68, 3485–3504. doi: 10.1111/evo.12534
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jablonski, D., Roy, K., and Valentine, J. W. (2006). Out of the tropics: evolutionary dynamics of the latitudinal diversity gradient. Science 314, 102–106. doi: 10.1126/science.1130880
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jacobs, B. F., Pan, A. D., and Scotese, C. R. (2010). “A review of the Cenozoic vegetation history of Africa,” in Cenozoic Mammals of Africa, eds L. Werdelin and W. J. Sanders (Berkeley, CA: University of California Press), 57–72.
Javadi, F., Wojciechowski, M. F., and Yamaguchi, H. (2007). Geographical diversification of the genus Cicer (Leguminosae: Papilionoideae) inferred from molecular phylogenetic analyses of chloroplast and nuclear DNA sequences. Bot. J. Linn. Soc. 154, 175–186. doi: 10.1111/j.1095-8339.2007.00649.x
Jürgens, N. (1997). Floristic biodiversity and history of African arid regions. Biodiv. Conserv. 6, 495–514. doi: 10.1023/A:1018325026863
Kim, S. C., McGowen, M. R., Lubinsky, P., Barber, J. C., Mort, M. E., and Santos-Guerra, A. (2008). Timing and tempo of early and successive adaptive radiations in Macaronesia. PLoS ONE 3:e2139. doi: 10.1371/journal.pone.0002139
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kornhall, P., Heidari, N., and Bremer, B. (2001). Selagineae and Manuleeae, two tribes or one? Phylogenetic studies in the Scrophulariaceae. Plant Syst. Evol. 228, 199–218. doi: 10.1007/s006060170029
Lavin, M., Herendeen, P. S., and Wojciechowski, M. F. (2005). Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the Tertiary. Syst. Biol. 54, 575–594. doi: 10.1080/10635150590947131
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lavin, M., Wojciechowski, M. F., Richman, A., Rotella, J., Sanderson, M. J., and Matos, A. B. (2001). Identifying Tertiary radiations of Fabaceae in the Greater Antilles: alternatives to cladistic vicariance analysis. Int. J. Plant Sci. 162, S53–S76. doi: 10.1086/323474
Lebrun, J. (1947). “Essai sur l'origine et le développement de la flore,” in Exploration du Parc National Albert. Mission J. Lebrun (1937–1938). La végétation de la plaine alluviale au sud du Lac Édouard, ed J. Lebrun (Brussels: Inst. des Parcs Nationaux du Congo Belge). Fasc. 1. 2ème Part., 115–397.
Lebrun, J. (1961). Les deux flores d'Afrique tropicale. Acad. Roy. Belg. Cl. Sci. Mém. (coll. 8. 2éme sér.) 32, 1–82.
Linder, H. P. (2014). The evolution of African plant diversity. Front. Ecol. Evol. 2, 1–14. doi: 10.3389/fevo.2014.00038
Lomolino, M. V., Riddle, B. R., Whittaker, R. J., and Brown, J. H. (2010). Biogeography. 4th Edn. Sunderland, MA: Sinauer Associates, Inc. 764.
Maclean, I. M. D., and Wilson, R. J. (2011). Recent ecological responses to climate change support predictions of high extinction risk. Proc. Natl. Acad. Sci. U.S.A. 108, 12337–12342. doi: 10.1073/pnas.1017352108
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maddison, W. P., and Maddison, D. R. (2014). Mesquite: A Modular System for Evolutionary Analysis. Version 3.01. Available online at: http://mesquiteproject.org
Magallón, S., and Sanderson, M. J. (2001). Absolute diversification rates in angiosperm clades. Evolution 55, 1762–1780. doi: 10.1111/j.0014-3820.2001.tb00826.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mairal, M., Pokorny, L., Aldasoro, J. J., Alarcón, M., and Sanmartín, I. (2015). Ancient vicariance and climate-driven extinction explain continental-wide disjunctions in Africa: the case of the Rand Flora genus Canarina (Campanulaceae). Mol. Ecol. 24, 1335–1354. doi: 10.1111/mec.13114
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maley, J. (1996). The African rain forest—main characteristics of changes in vegetation and climate from the Upper Cretaceous to the Quaternary. Proc. Roy. Soc. Edinburgh Sect. B. Biol. Sci. 104, 31–73. doi: 10.1017/S0269727000006114
Maley, J. (2000). Last Glacial Maximum lacustrine and fluviatile formations in the Tibesti and other Saharan mountains, and large-scale climatic teleconnections linked to the activity of the Subtropical Jet Stream. Glob. Planet. Change 26, 121–136. doi: 10.1016/S0921-8181(00)00039-4
Manning, J., Forest, F., and Vinnersten, A. (2007). The genus Colchicum L. redefined to include Androcymbium Willd. based on molecular evidence. Taxon 56, 872–882. doi: 10.2307/25065868
Meseguer, A. S., Aldasoro, J. J., and Sanmartín, I. (2013). Bayesian inference of phylogeny, morphology and range evolution reveals a complex evolutionary history in St. John's wort (Hypericum). Mol. Phylogenet. Evol. 67, 379–403. doi: 10.1016/j.ympev.2013.02.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Meseguer, A. S., Lobo, J. M., Ree, R., Beerling, D. J., and Sanmartín, I. (2015). Integrating Fossils, Phylogenies, and Niche Models into Biogeography to reveal ancient evolutionary history: the Case of Hypericum (Hypericaceae). Syst. Biol. 64, 215–232. doi: 10.1093/sysbio/syu088
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Monod, T. (1971). Remarques sur les symétries floristiques des zones sèches nord et sud en Afrique. Mitt. Bot. Staatssamml. München 10, 375–423.
Morley, R. J. (2000). Origin and Evolution of Tropical Rain Forests. Chinchester: John Wiley and Sons Ltd. 1–362.
Morley, R. J. (2003). Interplate dispersal paths for megathermal angiosperms. Perspect. Plant Ecol. Evol. Syst. 6, 5–20. doi: 10.1078/1433-8319-00039
Morlon, H. (2014). Phylogenetic approaches for studying diversification. Ecol. Lett. 17, 508–525. doi: 10.1111/ele.12251
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mort, M. E., Soltis, D. E., Soltis, P. S., Francisco-Ortega, J., and Santos-Guerra, A. (2002). Phylogenetics and evolution of the Macaronesian clade of Crassulaceae inferred from nuclear and chloroplast sequence data. Syst. Bot. 27, 271–288. doi: 10.1043/0363-6445-27.2.271
Mort, M. E., Soltis, D. E., Soltis, P. S., Santos-Guerra, A., and Francisco-Ortega, J. (2007). Physiological evolution and association between physiology and growth form in Aeonium (Crassulaceae). Taxon 56, 453–464.
Nagalingum, N. S., Marshall, C. R., Quental, T. B., Rai, H. S., Little, D. P., and Mathews, S. (2011). Recent synchronous radiation of a living fossil. Science 334, 796–799. doi: 10.1126/science.1209926
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nagy, E. (1963). Some new spore and pollen species from the Neogene of the Mecsek Mountain. Acta Bot. Hung. 9, 387–404.
Nathan, R. (2006). Long-distance dispersal of plants. Science 313, 786–788. doi: 10.1126/science.1124975
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Navarro-Pérez, M. L., López, J., Fernández-Mazuecos, M., Rodríguez-Riaño, T., Vargas, P., and Ortega-Olivencia, A. (2013). The role of birds and insects in pollination shifts of Scrophularia (Scrophulariaceae). Mol. Phylogenet. Evol. 69, 239–254. doi: 10.1016/j.ympev.2013.05.027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nylander, J. A. A. (2004). MrModeltest v2.2 Program Distributed by the Author. Uppsala: Evolutionary biology centre, Uppsala University, 2.
Osborne, A. H., Vance, D., Rohling, E. J., Barton, N., Rogerson, M., and Fello, N. (2008). A humid corridor across the Sahara for the migration of early modern humans out of Africa 120,000 years ago. Proc. Natl. Acad. Sci. U.S.A. 105, 16444–16447. doi: 10.1073/pnas.0804472105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oxelman, B., Kornhall, P., Olmstead, R. G., and Bremer, B. (2005). Further disintegration of Scrophulariaceae. Taxon 54, 411–425. doi: 10.2307/25065369
Peirson, J. A., Bruyns, P. V., Riina, R., Morawetz, J. J., and Berry, P. E. (2013). A molecular phylogeny and classification of the largely succulent and mainly African Euphorbia subg. Athymalus (Euphorbiaceae). Taxon 62, 1178–1199. doi: 10.12705/626.12
Pelser, P. B., Abbott, R. J., Comes, H. P., Milton, J. J., Moeller, M., Looseley, M. E., et al. (2012). The genetic ghost of an invasion past: colonization and extinction revealed by historical hybridization in Senecio. Mol. Ecol. 21, 369–387. doi: 10.1111/j.1365-294X.2011.05399.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pelser, P. B., Kennedy, A. H., Tepe, E. J., Shidler, J. B., Nordenstam, B., Kadereit, J. W., et al. (2010). Patterns and causes of incongruence between plastid and nuclear Senecioneae (Asteraceae) phylogenies. Am. J. Bot. 97, 856–873. doi: 10.3732/ajb.0900287
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pelser, P. B., Nordenstam, B., Kadereit, J. W., and Watson, L. E. (2007). An ITS phylogeny of tribe Senecioneae (Asteraceae) and a new delimitation of Senecio L. Taxon 56, 1077–1077. doi: 10.2307/25065905
Pennington, R. T., Cronk, Q. C., and Richardson, J. A. (2004). Introduction and synthesis: plant phylogeny and the origin of major biomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 359, 1455–1464. doi: 10.1098/rstb.2004.1539
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pennington, R. T., Lavin, M., Särkinen, T., Lewis, G. P., Klitgaard, B. B., and Hughes, C. E. (2010). Contrasting plant diversification histories within the Andean biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A. 107, 13783–13787. doi: 10.1073/pnas.1001317107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Plana, V. (2004). Mechanisms and tempo of evolution in the African Guineo–Congolian rainforest. Philos. Trans. R. Soc. Lond. B, Biol. Sci. 359, 1585–1594. doi: 10.1098/rstb.2004.1535
Qian, H., and Ricklefs, R. E. (2000). Large-scale processes and the Asian bias in species diversity of temperate plants. Nature 407, 180–182. doi: 10.1038/35025052
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Quézel, P. (1978). Analysis of the flora of mediterranean and saharan africa. Ann. Missouri Bot. Gard. 65, 479–534. doi: 10.2307/2398860
Rabosky, D. L., Grundler, M., Anderson, C., Shi, J. J., Brown, J. W., Huang, H., et al. (2014). BAMMtools: an R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol. Evol. 5, 701–707. doi: 10.1111/2041-210X.12199
Rambaut, A. (2002). Se-Al, Version 2.0 a11. Computer Program Distributed by the Author. Available online at: http://tree.bio.ed.ac.uk/software/seal/
Rambaut, A. (2009). FigTree, Version 1.4.2. Computer Program Distributed by the Author. http://tree.bio.ed.ac.uk/software/figtree/
Rambaut, A., Suchard, M. A., Xie, W., and Drummond, A. J. (2013). Tracer v1.6. Available online at: http://tree.bio.ed.ac.uk/soft-ware/tracer
Raven, P. H., and Axelrod, D. I. (1974). Angiosperm biogeography and past continental movements. Ann. Missouri Bot. Gard. 61, 539–673. doi: 10.2307/2395021
Richards, P. W. (1973). “Africa, the ‘Odd man out,’” in Tropical Forest Ecosystems of Africa and South America: A Comparative Review, eds B. J. Meggers, E. S. Ayensu, and W. D. Duckworth (Washington, DC: Smithsonian Institution Press), 21–26.
Ricklefs, R. E. (2007). Estimating diversification rates from phylogenetic information. Trends Ecol. Evol. 22, 601–610. doi: 10.1016/j.tree.2007.06.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Riina, R., Peirson, J. A., Geltman, D. V., Molero, J., Frajman, B., Pahlevani, A., et al. (2013). A worldwide molecular phylogeny and classification of the leafy spurges, Euphorbia subgenus Esula (Euphorbiaceae). Taxon 62, 316–342. doi: 10.12705/622.3
Roquet, C., Sanmartín, I., García-Jacas, N., Sáez, L., Susanna, A., Wikström, N., et al. (2009). Reconstructing the history of Campanulaceae with a Bayesian approach to molecular dating and dispersal–vicariance analyses. Mol. Phylogenet. Evol. 52, 575–587. doi: 10.1016/j.ympev.2009.05.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sanmartín, I. (2014). “Biogeography,” in The Tree of Life, eds P. Vargas and R. Zardoya (Sunderland, MA: Sinauer Associates, Inc.), 555–576.
Sanmartín, I., Anderson, C. L., Alarcon, M., Ronquist, F., and Aldasoro, J. J. (2010). Bayesian island biogeography in a continental setting: the Rand Flora case. Biol. Lett. 6, 703–707. doi: 10.1098/rsbl.2010.0095
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Senut, B., Pickford, M., and Ségalen, L. (2009). Neogene desertification of Africa. C. R. Geoscience 341, 591–602. doi: 10.1016/j.crte.2009.03.008
Sepulchre, P., Ramstein, G., Fluteau, F., Schuster, M., Tiercelin, J. J., and Brunet, M. (2006). Tectonic uplift and Eastern Africa aridification. Science 313, 1419–1423. doi: 10.1126/science.1129158
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Simmons, M. P. (2004). Independence of alignment and tree search. Mol. Phylogenet. Evol. 31, 874–879. doi: 10.1016/j.ympev.2003.10.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Smedmark, J. E., and Anderberg, A. A. (2007). Boreotropical migration explains hybridization between geographically distant lineages in the pantropical clade Sideroxyleae (Sapotaceae). Am. J. Bot. 94, 1491–1505. doi: 10.3732/ajb.94.9.1491
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Smedmark, J. E., Swenson, U., and Anderberg, A. A. (2006). Accounting for variation of substitution rates through time in Bayesian phylogeny reconstruction of Sapotoideae (Sapotaceae). Mol. Phylogenet. Evol. 39, 706–721. doi: 10.1016/j.ympev.2006.01.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stadler, T. (2013). Recovering speciation and extinction dynamics based on phylogenies. J. Evol. Biol. 26, 1203–1219. doi: 10.1111/jeb.12139
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stevens, G. C. (1989). The latitudinal gradient in geographical range: how so many species coexist in the tropics. Am. Nat. 133, 240–256. doi: 10.1086/284913
Stride, G., Nylinder, S., and Swenson, U. (2014). Revisiting the biogeography of Sideroxylon (Sapotaceae) and an evaluation of the taxonomic status of Argania and Spiniluma. Austral. Syst. Bot. 27, 104–118. doi: 10.1071/SB14010
Swofford, D. (2002). Phylogenetic Analysis Using Parsimony (PAUP* v4.0b). Sunderland, MA: Sinauer Associates.
Thiv, M., Thulin, M., Hjertson, M., Kropf, M., and Linder, H. P. (2010). Evidence for a vicariant origin of Macaronesian–Eritreo/Arabian disjunctions in Campylanthus Roth (Plantaginaceae). Mol. Phylogenet. Evol. 54, 607–616. doi: 10.1016/j.ympev.2009.10.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Trauth, M. H., Larrasoaña, J. C., and Mudelsee, M. (2009). Trends, rhythms and events in Plio-Pleistocene African climate. Quat. Sci. Rev. 28, 399–411. doi: 10.1016/j.quascirev.2008.11.003
Van Campo, E. (1989). Flore pollinique du Miocene superieur de Venta del Moro (Espagne). Acta Palynol. 1, 9–32.
van Zinderen Bakker, E. M. Sr. (1978). “Quaternary vegetation changes in southern Africa,” in Biogeography and Ecology of southern Africa, ed M. J. A. Werger (The Hague: Dr W. Junk bv Publishers), 131–143.
Verdcourt, B. (1969). The arid corridor between the northeast and southwest areas of Africa. Palaeoecol. Afr. 4, 140–144.
von Humboldt, A., and Bonpland, A. (1805). Essai sur la Geìographie des Plantes; Accompagneì d'un Tableau Physique des Reìgions Eìquinoxiales, Fondeì sur des Mesures exeìcuteìes, Depuis le DixieÌme Degreì de Latitude Boreìale Jusqu'au DixieÌme Degreì de Latitude Australe, Pendant les Anneìes 1799, 1800, 1801, 1802 et 1803. Paris: chez Levrault, Schoell et compagnie, libraries, 155.
Warren, B. H., and Hawkins, J. A. (2006). The distribution of species diversity across a flora's component lineages: dating the Cape's ‘relicts’. Proc. R. Soc. B 273, 2149–2158. doi: 10.1098/rspb.2006.3560
Wheeler, T. J., and Kececioglu, J. D. (2007). Multiple alignment by aligning alignments. Bioinformatics 23, i559–i568. doi: 10.1093/bioinformatics/btm226
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wiens, J. J. (2004). What is speciation and how should we study it? Am. Nat. 163, 914–922. doi: 10.1086/386552
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wiens, J. J. (2011). The causes of species richness patterns across space, time, and clades and the role of “ecological limits.” Q. Rev. Biol. 86, 75–96. doi: 10.1086/659883
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wiens, J. J., and Donoghue, M. J. (2004). Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644. doi: 10.1016/j.tree.2004.09.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Winterbottom, J. M. (1967). Climatological implications of avifaunal resemblances between South Western Africa and Somaliland. Palaeoecol. Afr. 2, 77–79.
Xie, L., Yang, Z. Y., Wen, J., Li, D. Z., and Yi, T. S. (2014). Biogeographic history of Pistacia (Anacardiaceae), emphasizing the evolution of the Madrean-Tethyan and the eastern Asian-Tethyan disjunctions. Mol. Phylogenet. Evol. 77, 136–146. doi: 10.1016/j.ympev.2014.04.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zachos, J. C., Dickens, G. R., and Zeebe, R. E. (2008). An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 451, 279–283. doi: 10.1038/nature06588
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: Africa, historical biogeography, climate change, diversification rates, long-distance dispersal, Rand Flora, vicariance
Citation: Pokorny L, Riina R, Mairal M, Meseguer AS, Culshaw V, Cendoya J, Serrano M, Carbajal R, Ortiz S, Heuertz M and Sanmartín I (2015) Living on the edge: timing of Rand Flora disjunctions congruent with ongoing aridification in Africa. Front. Genet. 6:154. doi: 10.3389/fgene.2015.00154
Received: 06 October 2014; Accepted: 05 April 2015;
Published: 01 May 2015.
Edited by:
James Edward Richardson, Royal Botanic Garden Edinburgh, UKReviewed by:
Thomas L. P. Couvreur, Institut de Recherche pour le Développement, CameroonCopyright © 2015 Pokorny, Riina, Mairal, Meseguer, Culshaw, Cendoya, Serrano, Carbajal, Ortiz, Heuertz and Sanmartín. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa Pokorny and Isabel Sanmartín, Real Jardín Botánico (RJB-CSIC), Plaza de Murillo 2, 28014 Madrid, SpaincG9rb3JueUByamIuY3NpYy5lcw==;aXNhbm1hcnRpbkByamIuY3NpYy5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.