
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Gastroenterol., 01 April 2025
Sec. Hepatology
Volume 4 - 2025 | https://doi.org/10.3389/fgstr.2025.1506032
 Emmanuel M. Sindato1†
Emmanuel M. Sindato1† Violet Dismas Kajogoo2*†
Violet Dismas Kajogoo2*† Gloria Ngajilo3
Gloria Ngajilo3 Wondwossen Amogne Degu4†
Wondwossen Amogne Degu4† Zahid Khan5
Zahid Khan5 Gideon Mlawa6
Gideon Mlawa6Background: Sub-Saharan Africa (SSA) is undergoing an epidemiological transition with a steady rise in non-communicable diseases. Among these diseases, metabolic dysfunction-associated fatty liver disease (MAFLD) has emerged as a rapidly increasing public health burden, but is inaccurately documented. We characterized the MAFLD prevalence and identified associated risk factors among adults in SSA.
Methods: We searched PubMed/Medline, Cochrane, Embase, Web of Science, Google Scholar, and African Journals Online for studies looking into the prevalence of and the risk factors for MAFLD in SSA. Studies from 1990 in the English language were included, and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used for reporting. The quality of the studies was assessed using the Newcastle–Ottawa Scale. A random-effects model was used to estimate the prevalence and the risk factors with 95% confidence intervals (CIs). Meta-regression was used for the subgroup analyses to account for heterogeneity. Stata version 17 software was used for the analysis. This study was registered with PROSPERO (registration no. CRD42024506067).
Results: A total of 538 studies were identified, with 22 included in the analysis. The overall prevalence of MAFLD was 29·21% (95%CI = 22.09–36.88, p < 0.05). Regionally, the results were: West, 34.36%; South, 26.92%; and East, 24.56%. The prevalence of MAFLD among people living with HIV was 13.02%, with diabetes was 37.06%, with hypertension was 36.75%, and with a body mass index above 25 kg/m2 was 46.05%. The prevalence was higher in women than in men (27.13% vs. 23.01%), as shown in studies conducted from 2000 onwards compared with those conducted between 2009 and 2019 (30.23% vs. 28.4%) and in studies with small sample sizes <500 than in studies with large sample sizes >500 (32.42% vs. 12.17%).
Interpretation: MAFLD is highly prevalent in SSA, with a steady increasing magnitude. The significant risk factors included diabetes, hypertension, obesity, and female sex. This study underscores the emerging need of clinicians in SSA to screen MAFLD among patients at high risk and to instigate tailored care.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42024506067.
Metabolic dysfunction-associated fatty liver disease (MAFLD) is a common type of chronic liver disease characterized by cardiometabolic risk factors and the hepatic steatosis affecting up to 30% of the population (1–7), with the highest burden in South America and the Middle East, followed by Asia, the USA, and Europe. In Europe and America, it is the second cause of end-stage liver disease, including liver cirrhosis and hepatocellular carcinoma (HCC), and the second cause of liver transplantation (8–10). Epidemiological studies have shown that Africa has the lowest burden of MAFLD, with a prevalence ranging from 10% to 28% (3). This proportion is projected to rise, as the African region is undergoing epidemiological transition with a particular steady rise in non-communicable diseases. Type 2 diabetes mellitus (DM), metabolic syndrome, and hypertension (HTN) are important risk factors for MAFLD (11–13).
MAFLD, also known as metabolic dysfunction-associated steatotic liver disease (MASLD), is the new nomenclature of the previous classification of nonalcoholic fatty liver disease (NAFLD). The new nomenclature was proposed around 2020 and was endorsed in 2023 by over 70 major liver societies and organizations, including the Society on Liver Disease in Africa (SOLDA), the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), the American Gastroenterology Associates (AGA), and the Asian Pacific Association for the Study of the Liver (APASL), among others, following the multi-society Delphi consensus statement on the new fatty liver disease nomenclature (14–17). The new nomenclature addressed the stigma associated with the use of the word “alcohol” in the NAFLD classification. The new nomenclature uses a simplified criterion for the diagnosis based on simple and practical parameters. The new criteria require the presence of steatohepatitis plus any one of the following five cardiometabolic risks: body mass index (BMI) ≥25 kg/m2 or waist circumference (WC) >94 cm; fasting blood glucose (FBG) ≥5.6 mmol/L or HbA1c >5.7% or type 2 diabetes; blood pressure (BP) ≥130/85 mmHg or anti-hypertensive drug treatment; and plasma triglycerides ≥1.70 mmol/L, plasma high-density lipoprotein (HDL) cholesterol ≤1, or lipid-lowering treatment (14, 16). Furthermore, the overlap of steatohepatitis with alcohol consumption was addressed, in which a new terminology, “metabolic and alcohol-related/associated liver disease (MetALD),” evolved for moderate drinkers with cardiometabolic risks. Under this regard, moderate drinkers are classified based on the amount of alcohol consumed per week, as follows: 140–350 g/week for women and 210–420 g/week for men (14–17). Taking the new nomenclature into context, studies have shown that it has a comparable prevalence with NAFLD, but has a better identification pattern for patients with high cardiometabolic risks, improved patient awareness, and hepatic fibrosis diagnosis (15, 17, 18).
In the majority of patients, MAFLD is asymptomatic; however, some patients may present with a broad array of nonspecific symptoms, including exhaustion, malaise, and right upper abdominal discomfort (19, 20). Most patients will seek medical attention following an incidental hepatic steatosis finding on abdominal imaging and elevation of aminotransferase levels. Elevated transaminase levels in a patient with cardiometabolic risk should raise clinical suspicion for MALFD, although a normal transaminase level does not exclude MAFLD. Patients with MAFLD may have mild or moderate elevations of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). It is important to note that the extent of transaminase elevation does not reflect the degree of hepatic inflammation or fibrosis (21). Imaging findings in patients with MAFLD include increased echogenicity on ultrasound, decreased hepatic attenuation on computed tomography (CT), and an increased fat signal on magnetic resonance imaging (MRI) (22). The disease spectrum of MAFLD ranges in severity from steatosis (hepatic fat accumulation without inflammation), steatohepatitis (hepatic fat accumulation with inflammation and hepatic injury), hepatic fibrosis, liver cirrhosis, and, consequently, HCC (1, 16, 21, 23).
The SSA region is undergoing epidemiological transition with the steady increase in the rates of cardiometabolic diseases, which are strongly associated with the increased prevalence of MAFLD, which could lead to complications such as end-stage liver disease primarily, liver cirrhosis, and HCC. However, to date, no systematic reviews of the prevalence of and the risk factors for MAFLD in the region have been published. One meta-analysis on chronic liver disease in Ethiopia, with a particular focus on the etiological spectra, was published in 2021. Therefore, this study aimed to characterize the burden by looking at the prevalence and the risk factors associated with MAFLD in SSA.
In this systematic review and meta-analysis, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used, and studies that reported on the prevalence of and risk factors for MAFLD in SSA in the English language were included. This study was registered with PROSPERO, with registration number CRD42024506067.
Electronic databases such as PubMed/Medline, Cochrane, Embase, Web of Science, and African Journals Online (AJOL) were used to search and retrieve articles published in English from 1990 to February 2024 using the following key terms: “Prevalence, Risk Factors, Nonalcoholic fatty liver disease, Metabolic dysfunction-associated fatty liver disease, metabolic dysfunction-associated steatotic liver disease, “metabolic and alcohol related/associated liver disease and SSA.” For PubMed, the Medical Subject Headings (MeSH) terms were used based on the research question. Boolean operators were also utilized to fine-tune the search terms. Furthermore, the authors manually searched the reference lists of the included studies and relevant review articles.
Eligibility was formulated using the PICOS (participants, interventions, comparisons, outcomes, study design) format, as detailed in Table 1.
MAFLD was defined as steatosis plus any one of the following: BMI ≥25 kg/m2 or WC >94 cm; fasting serum glucose ≥5.6 mmol/L or HbA1c >5.7% or type 2 diabetes; blood pressure ≥130/85 mmHg or anti-hypertensive drug treatment; and plasma triglycerides ≥1.70 mmol/L, plasma HDL cholesterol ≤1, or lipid-lowering treatment. MetSLD was defined using the MASLD criteria plus moderate or greater alcohol consumption.
The articles were imported from electronic databases into the Mendeley Reference Manager. Duplicates were removed and the titles, abstracts, and full texts were screened. Two authors performed the screening independently, and discrepancies between authors was discussed with a third reviewer and resolved by consensus.
Two reviewers independently extracted data from the included studies using a standardized Excel data extraction form developed for the study. Discrepancies were resolved through discussion. The extracted data included author names, article publication year, country, study design, sample size, prevalence, and risk factors (e.g., categorical BMI, DM, HTN, and dyslipidemia) for MAFLD/NAFLD/MASLD in SSA.
The quality of the included studies was assessed using the Newcastle–Ottawa Scale for observational studies. Each study was graded as having a low, high, or unclear risk of bias for each domain and was color coded.
A random-effects model was used to estimate the pooled prevalence and the risk factors with 95% confidence intervals (CIs) from the studies. Heterogeneity between studies was estimated using Cochran’s Q statistic (p < 0.05 indicates low heterogeneity) and the I2 statistic (<50% indicates low heterogeneity). Subgroup analyses to account for heterogeneity were performed, which examined associations between sex, BMI, study period, sample size, and region. A pooled prevalence was also determined among people living with HIV (PLHIV) and those who are diabetic and hypertensive. Stata version 17 software package was used for the analysis.
A total of 538 studies were identified. After removal of the duplicates, 349 records were retained for screening. The titles and abstracts were then screened, which excluded 307 records. The full texts of the remaining 42 records were then assessed for eligibility, from which 20 records were excluded. In the end, a total of 22 articles from 10 countries—Nigeria = 5, Sudan = 4, Ghana = 4, South Africa = 2, Kenya = 2, Ethiopia = 1, Zambia = 1, Côte d’Ivoire = 1, Uganda = 1, and Tanzania = 1—were included in the meta-analysis (Figure 1). Data from all the 22 (24–45) studies were included for the overall prevalence of NAFLD, 11 studies for the subgroup prevalence in patients with DM, 7 studies for HTN cases, 11 studies for sex, 9 studies for the risk factor BMI, and 3 studies for prevalence in people living with HIV (Supplementary Table S1).
The quality of the studies assessed according to the Newcastle–Ottawa Quality Assessment Scale (46) is shown in Supplementary Table S1. Overall, one study had moderate quality (Afolabi et al.), while the other studies had high quality.
The overall prevalence analysis included all of the 22 studies, with a total sample size of 5,813 individuals. The sample size range was from 80 to 1,463 individuals, within a study period from 2009 to 2024. The characteristics of the studies are shown in Supplementary Table S2.
The overall prevalence of MAFLD was 29.21% (95%CI = 22.09–36.88). The study by Afolabi et al. had the highest prevalence (68.75%), while that by Onyekwere et al. had the lowest prevalence (8.67%). Both studies were conducted in Nigeria (Figure 2).
The regional prevalence was as follows: West, 34.36% (95%CI = 22.58–47.18), with a total of 10 studies and 1,406 participants; South, 26.92% (95%CI = 6.2–55.22), with a total of three studies and 722 participants; and East, 24.56% (95%CI = 16.08–34.16), with a total of nine studies and 3,685 participants (Table 2).
The prevalence of MAFLD among people with HIV was 13.02% (95%CI = 2.19–30.45), with a total of three studies and a sample size of 373 participants (Table 2). In diabetics, this was 37.06% (95%CI = 23.8–51.36), with a total of 11 studies and 1,066 participants (Table 2). The prevalence in hypertensives was 36.75% (95%CI = 25.86–48.33), with a total of seven studies and 569 participants (Table 2), while that in people with a BMI above 25 kg/m2 was 46.05% (95%CI = 30.12–62.38), with a total of nine studies and 999 participants (Table 2).
The prevalence of MAFLD was higher in females than males, 27.13% (95% CI 16.91 – 38.71) (Table 2) vs 23.01% (95% CI 14.77 – 32.4) (Table 2), higher in studies conducted from 2000+ as compared to those conducted between 2009-2019, 30.23% (95% CI 20.49 – 40.95) (Table 2) vs 28.4% (95% CI 17.52 – 40.7) (Table 2), and higher in studies with small sample sizes ≤500 than in studies with large sample sizes >500; 32.42% (23.78 – 41.7) (Table 2) vs 12.17% (10.58 – 13.14) (Table 2).
Considerable heterogeneity was observed overall between the prevalence studies and the subgroup analyses.
There was also an unequal scatter of the funnel plot, which could be due to small study bias or publication bias (Figure 3).
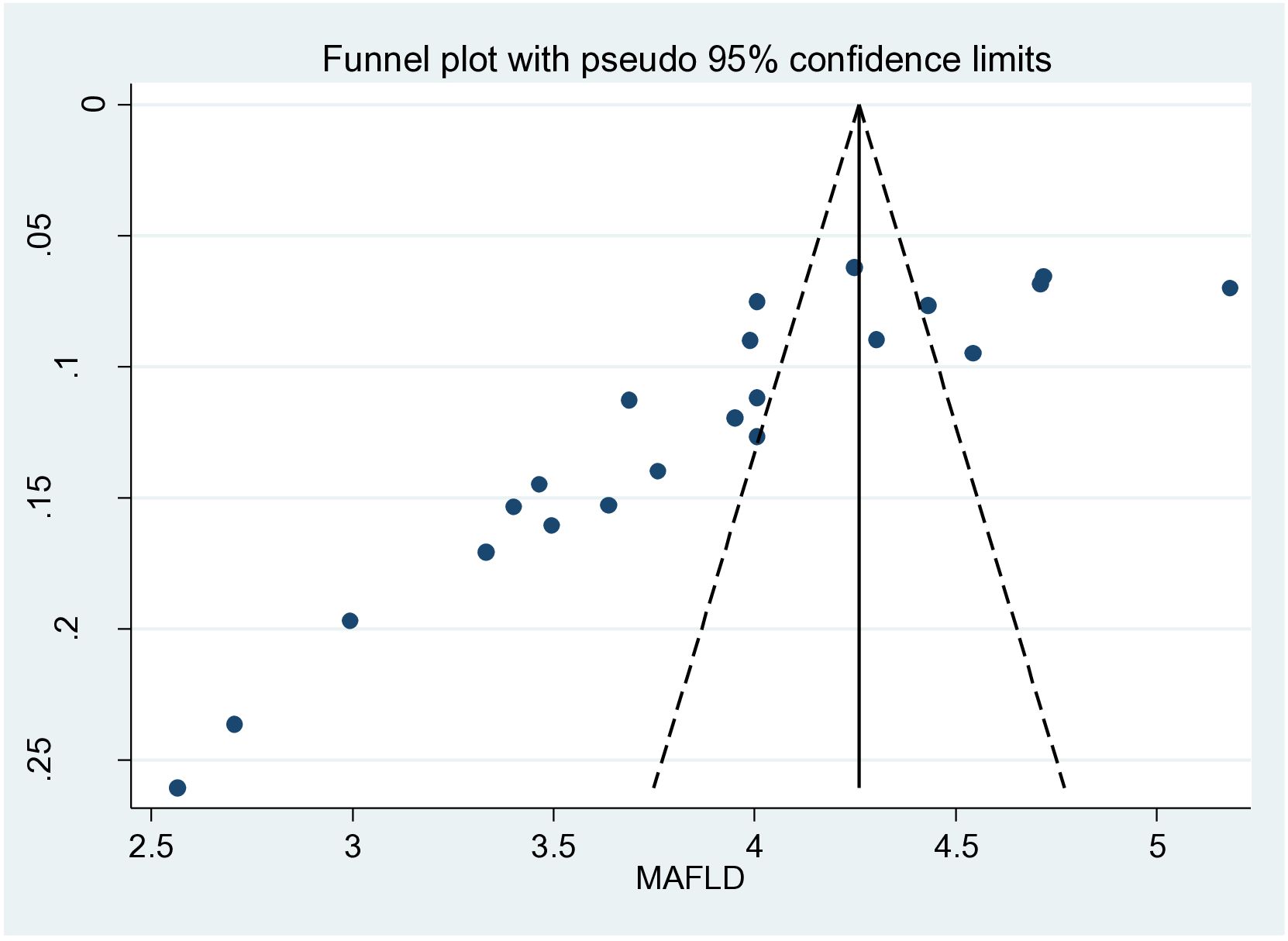
Figure 3. Funnel plot of the overall metabolic dysfunction-associated fatty liver disease (MAFLD) studies showing publication bias.
This review determined the prevalence of MAFLD in SSA, which was found to be 29.21%. Regionally, there was a higher prevalence in the West (34.36%) compared with the South (26.92%) and the East (24.54%). This was slightly more than the global estimates, where it was estimated to affect approximately 25% of the world’s population (47), but lower than the prevalence in Iran (33%) as of 2024 (48). The overall prevalence of MAFLD in the region was challenging to assess because, in February 2020, consensus by an international panel recommended the adoption of MAFLD as a more appropriate term; however, perspectives as to whether or not these changes are needed can vary substantially between different regions and healthcare systems (49). Nevertheless, the increase might be ascribed to the increased availability of healthcare and the concomitant rise in accurate NAFLD diagnosis, as well as to changes in lifestyle (50).
There were considerable variations in ethnicities, lifestyles, economic conditions, and disease epidemiology, which might have contributed to the wide variation in the prevalence of NAFLD (47). In a study conducted in different ethnic groups of South African women, the prevalence of metabolic syndrome was highest in the Indian subjects, being significantly higher than that observed in the Caucasian cohort, while the total energy expenditure was significantly higher in the African subjects compared with the Indian and Caucasian subjects (51). In a study done in the United States, the NAFLD prevalence was highest in Hispanic people, intermediate in Caucasian people, and lowest in African American people, although the differences between groups were smaller in the high-risk cohorts (range, 47.6%–55.5%) than in the population-based cohorts (range, 13.0%–22.9%) (52).
Higher prevalence rates were found in people with diabetes (37.06%), HTN (36.75%), and BMI >25 kg/m2 (46.05%), these being risk factors. Central obesity, a higher blood pressure, and a raised FBG were the predominant components that contributed to the syndrome in Ghanaian women (53). There was also a high prevalence of liver function test abnormalities in the group of patients with type 2 diabetes, and particularly so in the morbidly obese subjects in South Africa (54). A meta-analysis conducted in Mainland China had similar results, where there was a higher prevalence than the overall in those with diabetes (51.83% vs. 30.76% in non-diabetics) and in obese participants (66.21% vs. 11.72% in lean) (55). Similarly, a meta-analysis conducted in Latin America found type 2 DM or obesity to have a higher mean prevalence that reached 68% (56), as well as in a global epidemiology meta-analysis where the metabolic comorbidities associated with NAFLD included obesity (51.34%, 95%CI = 41.38–61.20), type 2 diabetes (22.51%, 95%CI = 17.92–27.89), hyperlipidemia (69.16%, 95%CI = 49.91–83.46), HTN (39.34%, 95%CI = 33.15–45.88), and metabolic syndrome (42.54%, 95%CI = 30.06–56.05) (1).
Our meta-analysis found a higher prevalence in women (27.13%) than in men (23.01%). Similar findings were observed where men accounted for approximately two-thirds of all cases; however, women experienced a greater increase in NAFLD on a global level (50).
A strength of this study is the fact that it is one of the most updated meta-analyses revealing the status of MAFLD in SSA, with prevalence data that represent the general adult population. The included studies had high to moderate quality.
A limitation of this study is the high heterogeneity among studies. This could be due to the populations and areas used in the included studies and the inclusion of studies deemed of lower quality, which was all that was available. Another limitation is the possible selection bias in some of the analyses due to the small number of published studies, as seen in the funnel plot. If the MAFLD in the individuals included was diagnosed by ultrasound, which can have limited sensitivity, the prevalence of MAFLD may have been underestimated. The authors also understand that there are people with BMI <25 kg/m2 who have MAFLD. This was not included in the meta-analysis as it was a missing finding in the studies.
In conclusion, MAFLD is a common liver disease affecting all of the three regions in SSA at a prevalence of 29.21%. There are regional variations, but also variations in ethnic groups, gender, and other comorbidities that are risk factors. These risk factors (i.e., diabetes, HTN, and obesity) highlight the significance of having metabolic control measures in place, including policies such as screening for patients with cardiometabolic risks, health education such as a change in lifestyle and increased awareness, and diagnosis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
ES: Conceptualization, Data curation, Formal analysis, Investigation, Resources, Software, Validation, Writing – original draft, Writing – review & editing. VK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GN: Validation, Visualization, Writing – review & editing. WD: Project administration, Supervision, Visualization, Writing – review & editing. ZK: Data curation, Supervision, Validation, Writing – review & editing. GM: Software, Supervision, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
We thank the team of University of Dodoma, Benjamin Mkapa Hospital and Tanzania Diabetes Association for their enormous support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2025.1506032/full#supplementary-material
AASLD, American Association for the Study of Liver Diseases; AGA, Atlanta Gastroenterology Associates; ALT, alanine aminotransferase; APASL, The Asian Pacific Association for the Study of the Liver; AST, aspartate aminotransferase; BMI, body mass index; CT, computed tomography; DM, diabetes mellitus; EASL, European Association for the Study of the Liver; HCC, hepatocellular carcinoma; HTN, hypertension; MAFLD, metabolic dysfunction-associated fatty liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease; MeSH, Medical Subject Headings; MetALD, metabolic and alcohol-related/associated liver disease; MRI, magnetic resonance imaging; NAFLD, non-alcoholic fatty liver disease; PLHIV, people living with HIV; SOLDA, Society on Liver Disease in Africa; SSA, Sub-Saharan Africa.
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
2. Teng MLP, Ng CH, Huang DQ, Chan KE, Tan DJH, Lim WH, et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin Mol Hepatol. (2023) 29:. doi: 10.3350/cmh.2022.0365
3. Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD prevalence: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2022) 20:2809–2817.e28. doi: 10.1016/j.cgh.2021.12.002
4. Amarapurkar DN, Hashimoto E, Lesmana LA, Sollano JD, Chen PJ, Goh KL. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol. (2007) 22:788–93. doi: 10.1111/j.1440-1746.2007.05042.x
5. Goh SC, Ho ELM, Goh KL. Prevalence and risk factors of non-alcoholic fatty liver disease in a multiracial suburban Asian population in Malaysia. Hepatol Int. (2013) 7:548–54. doi: 10.1007/s12072-012-9359-2
6. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2017) 15:11–20. doi: 10.1038/nrgastro.2017.109
7. Im HJ, Ahn YC, Wang JH, Lee MM, Son CG. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin Res Hepatol Gastroenterol. (2021) 45:101526. doi: 10.1016/j.clinre.2020.06.022
8. Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. (2018) 113:1649–59. doi: 10.1038/s41395-018-0088-6
9. Wang S, Toy M, Hang Pham TT, So S. Causes and trends in liver disease and hepatocellular carcinoma among men and women who received liver transplants in the U.S., 2010-2019. PloS One. (2020) 15:1–14. doi: 10.1371/journal.pone.0239393
10. Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. (2023) 79:516–37. doi: 10.1016/j.jhep.2023.03.017
11. National Research Council. The Continuing Epidemiological Transition in Sub-Saharan Africa A Workshop Summary-Health Financing in sub-Saharan Africa. National Research Council (US) (2012).
12. Munseri PJ, Kimambo H, Pallangyo K. Diabetes mellitus among patients attending TB clinics in Dar es Salaam: A descriptive cross-sectional study. BMC Infect Dis. (2019) 19:4–11. doi: 10.1186/s12879-019-4539-5
13. Agyei-Mensah S, De-Graft Aikins A. Epidemiological transition and the double burden of disease in Accra, Ghana. J Urban Heal. (2010) 87:879–97. doi: 10.1007/s11524-010-9492-y
14. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. Annals of Hepatology Concise reviews A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatol, J Hepatol, Ann Hepatol. (2024) 29. doi: 10.1016/j.aohep.2023.101133
15. Marchesini G, Vettor R, Pinzani M. MASLD emerging from the fog of fatty liver. J Hepatology. Eur Assoc Study Liver. (2024) 80:178–80. doi: 10.1016/j.jhep.2023.10.011
16. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-gomez M, et al. Expert Opinion A new de fi nition for metabolic dysfunction-associated fatty liver disease : An international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
17. Alharthi J, Eslam M. Metabolic associated fatty liver disease (MAFLD): a milestone in the history of fatty liver disease. Hepatobiliary Surg Nutr. (2021) 10:696–8. doi: 10.21037/hbsn-21-269
18. Liu Q, Zhao G, Li Q, Wu W, Zhang Y, Bian H. A comparison of NAFLD and MAFLD diagnostic criteria in contemporary urban healthy adults in China : a cross − sectional study. BMC Gastroenterol. (2022) 22:471. doi: 10.1186/s12876-022-02576-4
19. Basaranoglu M, Neuschwander-tetri BA. Clinical features and pathogenesis. Gastroenterol Hepatol (New York). (2006) 2:282–91.
20. Khoonsari M, Mohammad M, Azar H, Ghavam R. Clinical manifestations and diagnosis of nonalcoholic fatty liver disease. (2017) 12:99–105. doi: 10.30699/ijp.2017.25038
21. Rinella ME. Nonalcoholic fatty liver disease a systematic review. JAMA - J Am Med Assoc. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
22. Delvin E, Patey N, Dubois J, Henderson M, Lévy É. Pediatric non-alcoholic fatty liver disease. J Med Biochem. (2014) 34:3–12. doi: 10.2478/jomb-2014-0059
23. Heintz EC, Axelrod CL. Obesity management in the primary prevention of hepatocellular carcinoma. (2022). doi: 10.3390/cancers14164051
24. Lesi OA, Soyebi KS, Eboh CN. Fatty liver and hyperlipidemia in a cohort of HIV-positive africans on highly active antiretroviral therapy. J Natl Med Assoc. (2009) 101:151–5. doi: 10.1016/S0027-9684(15)30828-2
25. Kruger FC, Daniels C, Kidd M, Swart G, Brundyn K, Van RC, et al. O RIGINAL A RTICLES Non-alcoholic fatty liver disease (NAFLD) in the Western Cape : A descriptive analysis. South Afr Med J. (2010) 100:168–71. doi: 10.7196/SAMJ.1422
26. Onyekwere CA, Ogbera AO, Balogun BO. Non-alcoholic fatty liver disease and the metabolic syndrome in an urban hospital serving an African community. Ann Hepatol. (2019) 10:119–24. doi: 10.1016/S1665-2681(19)31559-5
27. Bahaaedin A, Elkhader MZM. Prevalence of non-alcoholic fatty liver among adults in khartoum- Sudan: epidemiological survey. J Am Sci. (2013) 9:2–6.
28. Almobarak AO, Barakat S, Khalifa MH, Elhoweris MH, Elhassan TM, Ahmed MH. Non alcoholic fatty liver disease (NAFLD) in a Sudanese population : What is the prevalence and risk factors? Arab J Gastroenterol. (2014) 15:12–5. doi: 10.1016/j.ajg.2014.01.008
29. Almobarak AO, Barakat S, Suliman EA, Elmadhoun WM, Mohamed NA, Abobaker IO, et al. Prevalence of and predictive factors for nonalcoholic fatty liver disease in Sudanese individuals with type 2 diabetes : Is metabolic syndrome the culprit? Arab J Gastroenterol. (2015) 16:54–8. doi: 10.1016/j.ajg.2015.06.001
30. Hoffmann CJ, Hoffmann JD, Kensler C, Van Der M. Tuberculosis and hepatic steatosis are prevalent liver pathology findings among HIV-infected patients in South Africa. PloS One. (2015) 10:1–8. doi: 10.1371/journal.pone.0117813
31. Olusanya TO, Lesi OA, Fasanmade OA. Non alcoholic fatty liver disease in a Nigerian population with type II diabetes mellitus. Pan Afr Med J. (2016) 8688:1–9. doi: 10.11604/pamj.2016.24.20.8181
32. Ishmael B, Ch AMB, Olubunmi B, Ch IMB, Temidayo R, Ch IMB, et al. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J Natl Med Assoc. (2017) 110:256–64. doi: 10.1016/j.jnma.2017.06.001
33. Karim NH, Adam R, Vinayak S. Prevalence of hepatic steatosis as diagnosed on unenhanced abdominal computed tomography. East Afr Med J. (2018) 95:1852–7.
34. Zawdie B, Tadesse S, Wolide AD, Nigatu TA, Bobasa EM. Non-alcoholic fatty liver disease and associated factors among type 2 diabetic patients in southwest Ethiopia. Ethiop J Health Sci. (2018) 28:19–30. doi: 10.4314/ejhs.v28i1.4
35. Abdalla R, Adam A, Abdalazeez E. Prevalence of non alcoholic fatty liver diseases in general population, khartoum – Sudan. Saudi J Med Pharm Sci. (2019) 4929:38–42. doi: 10.21276/sjmps.2019.5.1.7
36. Odenigbo CU, Oguejiofor OC, Ezejiofor OI, Jisieike Onuigbo NN, Nwaneli CU, Umeh EO, et al. Nonalcoholic fatty liver disease among adults attending medical outpatient clinic using ultrasound. Orient J Med. (2020) 32:67–75.
37. Setroame AM, Affrim PK, Abaka-yawson A, Kwadzokpui PK, Adrah FE, Bless H, et al. Prevalence of metabolic syndrome and nonalcoholic fatty liver disease among premenopausal and postmenopausal women in ho municipality : A cross-sectional study. BioMed Res Int. (2020) 2020, 1–10. doi: 10.1155/2020/2168381
38. Ssentongo A, Ssentongo P, Laura Keeney A, Ebenezer ,A, Amponsah-Manu F, David Soybel and J, et al. Opportunistic screening for nonalcoholic fatty liver disease (NAFLD) in Ghana: A prospective study. Curr Dev Nutr. (2020) 4:nzaa053_114. doi: 10.1093/cdn/nzaa053_114
39. Chihota BV, Riebensahm C, Muula G, Sinkala E, Chilengi R, Mulenga L, et al. Liver steatosis and metabolic associated fatty liver disease among HIV- positive and negative adults in urban Zambia. BMJ Open Gastroenterol. (2022) 9. doi: 10.1136/bmjgast-2022-000945
40. Bockarie AS, Nartey YA, Tuoyire D, Nkum B. Fatty liver biomarkers and insulin resistance indices in the prediction of non- alcoholic fatty liver disease in Ghanaian patients. Endocrinol Diabetes Metab Case Rep. (2023) :1–10. doi: 10.1002/edm2.v6.6
41. Hatrydt K, Dimitri G, Ya KAH, Patricia G, Demba BA, Adjeka DS, et al. Factors associated with hepatic steatosis in black african subjects with chronic viral hepatitis B in côte d ‘ Ivoire. Open J Gastroenterol. (2023) 12:328–37. doi: 10.4236/ojgas.2023.1310030
42. Lajeunesse-trempe F, Boit MK, Kaduka LU, Baass EDLA, Piché MPM. Validation of the Fatty Liver Index for identifying non-alcoholic fatty liver disease in a Kenyan population. Alimentary Pharmacology & Therapeutics. (2023) 59:1111–21. doi: 10.1111/tmi.13927
43. Wiafe YA, Afihene MY, Anto EO, Nmai RA, Amoah-kumi L, Frimpong J, et al. Non-alcoholic fatty liver disease and liver fibrosis in persons with type 2 diabetes mellitus in Ghana : A study of prevalence, severity, and contributing factors using transient elastography. J Clin Med. (2023) 12. doi: 10.3390/jcm12113741
44. Enriquez R, Homsi M, Ssekubugu R, Nabukalu D, Zeebari Z, Marrone G, et al. Prevalence and risk factors of metabolic dysfunction- associated steatotic liver disease in south Central Uganda : A sectional survey. Aliment Pharmacol Ther. (2024) :1–11. doi: 10.1111/apt.17931
45. Kilonzo SB, Kamala E, Jaka H, Ngoya P. Non − alcoholic fatty liver disease in Tanzania : prevalence, determinants, and diagnostic performance of triglycerides − glucose index and triglycerides − glucose index – body mass index compared to the hepatic ultrasound in overweight and obese indivi. BMC Gastroenterol. (2024) 24:96. doi: 10.1186/s12876-024-03164-4
46. Shea B, Robertson J, Peterson J, Welch V, Losos M. The newcastle-ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta- analysis bias and confounding newcastle-ottowa scale.
47. Spearman CW, Afihene M, Betiku O, Bobat B, Cunha L, Kassianides C, et al. Series Non-alcoholic fatty liver disease in sub-Saharan Africa 1 Epidemiology, risk factors, social determinants of health, and current management for non-alcoholic fatty liver disease in sub-Saharan Africa. Lancet Gastroenterol Hepatol. (2021) 1253:1–11. doi: 10.1016/S2468-1253(21)00275-2
48. Tabaeian SP, Rezapour A, Azari S, Martini M, Saran M, Behzadifar M, et al. Prevalence of non-alcoholic fatty liver disease in Iran: A systematic review and meta-analysis. J Clin Exp Hepatol. (2024) 14:101209. doi: 10.1016/j.jceh.2023.06.009
49. Shiha G, Alswat K, Al KM, AI S, Örmeci N, Waked I, et al. Review Nomenclature and definition of metabolic-associated fatty liver disease : a consensus from the Middle East and north Africa. Lancet Gastroenterol Hepatol. (2020) 1253, 57–64. doi: 10.1016/S2468-1253(20)30213-2
50. Ge X, Zheng L, Wang M, Du Y, Jiang J, Wang M. Prevalence trends in non- alcoholic fatty liver disease at the global, regional and national levels, 1990 – 2017 : a population- based observational study. BMJ Open. (2020) 10. doi: 10.1136/bmjopen-2019-036663
51. Naran NH, Haagensen M, Crowther NJ. Steatosis in South African women : How much and why? PloS One. (2018), 1–12. doi: 10.1371/journal.pone.0191388
52. Rich NE, Oji S, Mufti AR, Browning JD, Parikh ND, Odewole M, et al. Prevalence, severity, and outcomes in the United States. Clin Gastroenterol Hepatol. (2018) 16:198–210.e2. doi: 10.1016/j.cgh.2017.09.041
53. Kow F, Arthur N, Adu-frimpong M, Osei-yeboah J, Mensah FO, Owusu L. The prevalence of metabolic syndrome and its predominant components among pre-and postmenopausal Ghanaian women. BMC Res Notes. (2013) 6. doi: 10.1186/1756-0500-6-446
54. Paruk IM, Pirie FJ, Motala AKB. High prevalence of abnormal liver enzymes in South African patients with type 2 diabetes mellitus attending a diabetes clinic. JEMDSA. (2011) 16:43–7. doi: 10.1080/22201009.2011.10872250
55. Wu Y, Zheng Q, Zou B, Hui Y, Xiaohe Y, Jie L, et al. The epidemiology of NAFLD in Mainland China with analysis by adjusted gross regional domestic product : a meta − analysis. Hepatol Int. (2020) 0123456789), 151–5. doi: 10.1007/s12072-020-10023-3
Keywords: metabolic-associated fatty liver disease, Sub-Saharan Africa, non-alcoholic fatty liver disease, metabolic associated steatohepatitis, prevalence, risk factors
Citation: Sindato EM, Kajogoo VD, Ngajilo G, Degu WA, Khan Z and Mlawa G (2025) Prevalence and risk factors of metabolic-associated fatty liver disease in sub-Saharan Africa: a systematic review and meta-analysis. Front. Gastroenterol. 4:1506032. doi: 10.3389/fgstr.2025.1506032
Received: 04 October 2024; Accepted: 18 February 2025;
Published: 01 April 2025.
Edited by:
Colm Antoine O. Morain, Trinity College Dublin, IrelandReviewed by:
Monjur Ahmed, Thomas Jefferson University, United StatesCopyright © 2025 Sindato, Kajogoo, Ngajilo, Degu, Khan and Mlawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Violet Dismas Kajogoo, dmtham9nb29Acm9ja2V0bWFpbC5jb20=
†ORCID: Emmanuel M. Sindato, orcid.org/0000-0003-4640-5559
Violet Dismas Kajogoo, orcid.org/0000-0002-6122-3828
Wondwossen Amogne Degu, orcid.org/0000-0002-4950-3966
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.