- 1Centro per le Malattie dell’Apparato Digerente (CEMAD), Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Roma, Italy
- 2Dipartimento Universitario di Medicina e Chirurgia Traslazionale, Università Cattolica del Sacro Cuore, Roma, Italy
- 3Dipartimento di Biotecnologie e Bioscenze, Università degli Studi di Milano-Bicocca, Milano, Italy
- 4Dipartimento di Medicina e Scienze dell’Invecchiamento, Università degli studi di “G. D’Annunzio” Chieti-Pescara, Chieti, Italy
- 5Center for Advanced Studies and Technology (CAST), Università degli studi di “G. D’Annunzio” Chieti-Pescara, Chieti, Italy
- 6Dipartimento di Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Roma, Italy
- 7Nutrizione Clinica, Dipartimento di Scienze Mediche e Chirurgiche, Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Roma, Italy
- 8IBD Center, IRCCS Humanitas Research Hospital, Rozzano, Italy
Inflammatory bowel diseases (IBD) are chronic disabling conditions with a complex and multifactorial etiology, which is still not completely understood. In the last 20 years, anti-TNF-α antagonists have revolutionized the treatment of IBD, but many patients still do not respond or experience adverse events. Therefore, new biological therapies and small molecules, targeting several different pathways of gut inflammation, have been developed of which some have already been introduced in clinical practice while many others are currently investigated. Moreover, therapeutic procedures such as leukocytapheresis, fecal microbiota transplant and stem cell transplantation are currently being investigated for treating IBD. Lastly, complementary and alternative medicine has become a field of interest for gastroenterologist to reduce symptom burden in IBD patients. In this comprehensive and updated review, a novel classification of current and developing drugs is provided.
Introduction
Inflammatory bowel diseases (IBD), mainly Crohn’s disease (CD) and ulcerative colitis (UC), are lifelong conditions with a relapsing and remitting course that results from dysregulated immune responses against gut microbiota antigens. Despite many treatments for IBD, a number of patients fail to respond to long-term treatment, which has a negative impact on their quality of life. The etiology of IBD is still not completely understood and multifactorial, involving many susceptibility genes and environmental factors (1, 2).
In the last two decades, the introduction in clinical practice of monoclonal antibodies directed against pro-inflammatory cytokine tumour necrosis factor alpha (TNFα), e.g., infliximab, adalimumab, golimumab, certolizumab pegol, revolutionized IBD management, because anti-TNFα drugs induce and maintain clinical remission and mucosal healing (3), which reduce the need for corticosteroids, hospitalization and surgery; this improved health related quality of life (HRQOL) of IBD patients (4–13). Unfortunately, anti-TNFα therapy has been associated with infectious complications (14), and clinical efficacy is limited by a high rate of primary and secondary non-responsiveness (30%-40%). Loss of response could be a consequence of antibody production against the TNFα antagonists that can neutralize the drug, accelerate its clearance or induce infusion reactions (15, 16). For these reasons, new therapies with a better safety profile targeting different working mechanisms of inflammation, e.g., vedolizumab and ustekinumab, were approved for clinical practice and many others are presently studied (17, 18).
Small-molecule drugs (SMDs) are investigated to treat IBD, because of the high rate of non-response or loss of response to marketed drugs, and due to their invasive route of administration. In particular, SMDs have a low molecular weight (< 1 kDa) enabling them to diffuse through cell membranes to reach their intracellular targets (19). These drugs carry several advantages including lack of immunogenicity, short half-lives and oral administration (20). For every mechanism, SMDs are discussed in the second part of each paragraph.
Following our increasing knowledge about IBD pathogenesis, and specifically the role of an abnormal immune response against gut microbiota antigens, procedures like leukocytapheresis, fecal microbiota transplant and stem cell transplantation, which already proved to be effective, are also proposed as a therapeutic strategy in IBD (21–23).
Moreover, the use of complementary and alternative medicine (CAM) by gastroenterologists has become a common adjuvant to conventional medicine in the management of IBD patients (24–26).
This review aims to provide a broad overview of complementary and alternative therapies in IBD and to summarize the scientific evidence of currently used and developing drugs, providing a new comprehensive classification.
Methods
Search strategy
The literature search was conducted in PubMed (https://pubmed.ncbi.nlm.nih.gov) and Scopus (https://www.scopus.com) databases without restriction on publication period, using the following search string: (“IBD”, “ulcerative colitis”, Crohn’s disease) AND (“innovative therapy”, “complementary therapy”, “alternative therapy”). The “AND” operator was used to create all possible combinations of selected terms. ClinicalTrials.gov (https://clinicaltrials.gov) contains information about medical studies in human volunteer and was used to search for ongoing clinical trials.
Study selection
The initial screening of documents, using abstract and titles, was carried out including only English-language research articles, while articles without full text and abstract, duplicates studies, conferences, review articles, and editorial reports were excluded.
The selected clinical trials included in this review considered new small molecules or antibodies still in the experimental phase II, II or III used in IBD (UC and CD) patients.
In addition, the efficacy and safety of complementary and alternative therapies and how these could be a supplement in the treatment of IBD were evaluated.
Following the initial internet search, a total of 96 studies were retrieved by databases and following elimination of duplicates 88 articles remained. Out of the remaining studies, 11 records were excluded after review of their titles and abstracts and because not available in English language. At the end, 80 studies were included in this review, as 3 articles from other sources have been added.
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) was used as a guideline for including and reporting the studies and meta-analysis that we have included in this review (Figure 1).
Figure 1 PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Current drug targets and innovative therapies
Pro-inflammatory cytokine neutralization
IL-23 and IL-12 are proinflammatory cytokines that promote differentiation of naive CD4+ precursor T cells into T-helper 1 and T-helper 17; their inhibition has become a novel therapeutic target in IBD (27). Ustekinumab, a fully human immunoglobulin G1 kappa monoclonal antibody against the p40 subunit of IL-12 and IL-23 (28), has been approved for both induction and maintenance of clinical response and the remission in moderate-to-severe CD in patients with anti-TNF-α therapy failure (18). In 2019, a phase III study demonstrated that ustekinumab reached the same clinical response in UC that led to the approval to treat moderate to severe UC (29).
A new class of antibodies, including risankizumab, brazikumab, mirikizumab and guselkumab, that target solely IL-23 by linking its p19 subunit and that appears to have a potentially greater anti-inflammatory activity associated with an even safer profile, are currently investigated in phase II/III trials and showed promising results as induction and maintaining therapy in both CD and UC (30–34). One of them, Risankizumab, has been approved for the treatment of moderate to severe Crohn’s Disease (35–38). Guselkumab instead has been approved for the treatment of psoriasis and psoriatic arthritis (39).
PTG-200 is a new SMDs that antagonizes the IL-23 receptor (IL-23R). A phase I study has shown its efficacy in ameliorating colitis in rat models and a phase II trial for CD is currently recruiting patients (40).
Together with IL-12 and IL-23 overexpression, higher levels of IL-18 and IL-36 family members were described within the inflamed mucosa of IBD patients (41, 42). The IL-18 antibody GSK1070806 passed safety evaluation in a phase I clinical trial and is currently being investigated in phase I/II clinical trial to treat CD (43). Spesolimab, a humanized monoclonal IgG1 antibody, directed against the IL-36R, has shown clinical activity in patients with moderate-to-severe active UC, who failed previous biologic treatments (44). The long-term efficacy and safety of the IL-36R blockade with spesolimab are studied in a subsequent open-label phase II trial with UC patients who completed previous trials (45). Furthermore, spesolimab is undergoing phase II evaluation in patients with fistulising CD (46, 47).
Anti-inflammatory cytokines/pathway stimulation
Interleukin 10 and interleukin 22 agonists
Interleukin 10 (IL-10) is a cytokine with anti-inflammatory effects that inhibits both the antigen presentation and the subsequent release of pro-inflammatory cytokines and IL-22 attenuates intestinal inflammation, inducing the production of a mucine membrane by goblet cells (48). AMT-101 is a novel, GI-selective, investigational oral recombinant biologic fusion protein of human interleukin 10 (hIL-10), which is under development to treat IBD. AMT-101 will be evaluated in two phase II clinical trials covering a wide spectrum of UC patients: a monotherapy study to evaluate the efficacy and safety of the drug compared with a placebo (49) and a randomized, double-blind study in UC patients with an ileal pouch-anal anastomosis (IPAA) post-colectomy who have developed pouchitis (50). UTTR1147A is a recombinant fusion protein, targeting intestinal epithelial IL-22 receptors to promote mucosal repair. Promising results from a phase I clinical study showed improved clinical and pharmacodynamic responses in patients with UC treated with UTTR1147A compared with placebo (51). Currently, a phase II study is investigating the long-term safety and tolerability of UTTR1147A in participants with moderate to severe UC or CD (52).
STAT3 pathway stimulation
Over the past decades, scientists have investigated the role of microRNAs as key players in regulating immune responses.
microRNA showed the stimulation of anti-inflammatory pathways; in particular, miR-124, which can dampen inflammation, was previously found to be reduced in patients with certain autoimmune diseases. Importantly, increasing levels of miR-124 were found to inhibit intestinal inflammation by suppressing the activity of a main regulator of the inflammatory response, called STAT3 (53). ABX464, a small molecule, originally developed as an inhibitor of HIV replication, was shown to promote the production of miR-124 in immune cells. This drug has demonstrated safety and profound anti-inflammatory activity in preclinical trials. More specifically, in a phase IIa clinical trial, ABX464-101 showed effectiveness to treat UC (54). Based on the good safety profile and promising efficacy results obtained for ABX464 in UC, the initiation of a pivotal phase II trial for the treatment of subjects with moderate to severe active CD has been encouraged (55).
Blocking leukocytes trafficking
Anti-integrins
Another mechanism of IBD pathogenesis is leukocyte migration to gut mucosa mediated by the interaction between integrins on leukocyte surfaces and their ligands on endothelial and epithelial cells (56). Natalizumab, a humanized IgG4 anti-α4-integrin monoclonal antibody that inhibits both α4β7-integrin/mucosal addressin-cell adhesion molecule-1 (MadCAM-1) interaction and α4β1/vascular-cell adhesion molecule-1 (VCAM-1) binding, was the first drug targeting leukocyte trafficking in IBD. Despite natalizumab inducing clinical response and remission in CD, it is associated with an increased risk of progressive multifocal leukoencephalopathy (PML) due to its non-selective nature (57, 58). Vedolizumab, a fully humanized IgG1 monoclonal antibody binding α4β7 integrin, which ligand MadCAM-1expression is mainly restricted to the intestine, has shown to be effective and safe in moderate to severe UC and CD, especially in anti-TNFα naive patients and its good safety profile led to its approval in clinical practice in 2014 (59–61). Several new antibodies directed against integrins are currently being studied. Abrilumab, an anti-α4β7 antibody, induced clinical remission in UC, but did not reach statistical significance in CD in phase II trials (62, 63). Etrolizumab targets the β7 subunit of integrins α4β7 and αEβ7 that bind respectively MadCAM-1 and E-cadherin. In phase III trials, it has been more effective than placebo inducing clinical and endoscopic improvements in IBD (64). PN-943, an oral α4β7 integrin antagonist, is currently in a phase II placebo-controlled study to evaluate its safety, tolerability, and clinical efficacy in patients with moderate to severe active UC (65). Ontamalimab, a monoclonal antibody directed against MadCAM-1, a ligand of integrins, has been demonstrated to be effective and safe as induction therapy in UC, but the response and remission rates were not statistically significant in CD (66, 67). Currently, ontamalimab is undergoing phase III evaluation in IBD.
In addition, a new oral small molecule targeting integrins has been developed and investigated. AJM300 is an α4 integrin subunit antagonist that was more effective in a phase II study than placebo to achieve clinical remission and mucosa healing in moderate to severe UC, with no cases of PML (68). A phase III trial on efficacy and safety of an oral dose of AJM300 is active in Japan.
In addition to integrins, other adhesion molecules became therapeutic targets to suppress gut inflammation. Another SMD, with a similar mechanism is alicaforsen, which is an oligonucleotide that binds the mRNA of ICAM-1 (Intercellular Cell Adhesion Molecule-1), another ligand of integrins. Its action results in a reduced translation and expression of ICAM-1 by endothelial cells (69). Alicaforsen presents ICAM-1 mRNA to an RNAse, which enzymatically hydrolyse this mRNA (69). Despite the promising perspective (69–71), results emerging from the trials showed alicaforsen ineffectiveness to induce clinical remission in CD (72, 73). In UC, studies evidenced that alicaforsen enema induced acute and long-term topical improvement in patients with mild to moderate disease with a modest safety profile, but no significant differences were highlighted in clinical outcomes between patients treated with alicaforsen and placebo or mesalazine enemas (74–76). An open-label trial showed positive results in the treatment of pouchitis, and a phase III study on refractory pouchitis has just been completed (77).
Sphingosine-1 Phosphate Receptor modulation
Sphingosine-1 Phosphate Receptor modulators (S1PRs) are a class of small molecules that, after the interaction with their ligand, i.e. Sphingosine-1 Phosphate (S1P), cause internalization and degradation of S1P receptors, resulting in lymphocyte sequestration in lymph nodes and blocking their migration to the gut. S1P regulates the migration of naïve and central memory CCR7-positive T cells from the secondary lymphatic organs to the periphery. Fingolimod, a potent non-selective S1PR agonist used in multiple sclerosis, was effective in reducing gut inflammation in rats (78, 79). However, its clinical use in IBD was never been tested for its association with severe adverse events and cases of PML (80, 81). This led to the development of a more selective S1PR with a better safety profile. Ozanimod, an S1P1 and S1P5 modulator, and etrasimod, an oral S1P1, S1P4 and S1P55 modulator, produced positive results in terms of clinical and endoscopic improvement in IBD in phase II and III trials with an acceptable safety profile, since the adverse events reported, as bradycardia or conduction abnormalities, were mostly from mild to moderate. Several phase II and III trials on these selective S1PR agonists in both CD and UC are already ongoing (82–84). Ozanimod received approval for the treatment of moderate to severe UC/CD, because of the results obtained in phase III trials, such as improvement in clinical remission (primary end point) and in all key secondary end point (clinical response, endoscopic gain and mucosal healing) (85). Amiselimod/MT-1303 is a selective S1P1 modulator that has been tested in CD and UC and dose-finding studies have been completed (86, 87).
Anti-chemokine
Fractalkine (FKN)/CX3CL1 is a membrane-bound chemokine possessing a chemokine/mucin hybrid structure and a transmembrane domain and has a dual function as an adhesion molecule and a chemoattractant (88). It represents a new type of leukocyte trafficking regulator, which, signaling via its receptor CX3CR1 on leukocytes, allows them to migrate directly to effector sites during inflammation (88). E6011, a humanized anti-FKN monoclonal antibody, was evaluated for safety, tolerability, pharmacokinetics, and pharmacodynamics in a phase Ib randomized, double-blind, placebo-controlled trial in patients with moderate to severe CD (89).
Blocking intracellular pathways
Jak Inhibitors
Many cytokines, binding their receptors, induce intracellular phosphorylation and activation of Janus Kinases, a group of receptor-associated tyrosine kinases that convert extracellular stimuli into cellular processes involved in innate and adaptive immunity, through the activation of Signal Transducers and Activators of Transcription (STATs) (90). Given the role of the JAK/STAT pathway also in IBD pathogenesis, a new class of SMDs called JAK inhibitors, is being developed (90). Tofacitinib inhibits preferentially JAK1 and JAK3, which proved to be safe and effective as induction and maintenance therapy in UC, but not in CD in phase II and III clinical trials (91–95). In 2018, tofacitinib was approved in the US for the treatment of moderate to severe UC (90). In a phase II evaluation, filgotinib, a JAK inhibitor especially active against JAK1, and upadacitinib, a JAK1-selective inhibitor, showed good results in terms of clinical and endoscopic remission in CD and UC patients. Filgotinib is now undergoing phase III studies (96); filgotinib and updacitinib have been approved for the treatment of moderate to severe UC (97, 98).
A JAK1 and JAK3 inhibitor called peficitinib showed non-statistically significant efficacy in phase II trials in UC; therefore, further investigation was suspended (90, 99).
SHR0302 is a potent and highly selective JAK1 inhibitor and several late-stage clinical studies are ongoing with both oral and topical dosage forms for several immune-inflammatory diseases including UC and CD (100, 101).
Other drugs belonging to this category, but with different targets, like a JAK3 inhibitor (Pf-06651600) and a TYK2/JAK1 inhibitor (Pf-06700841), are being tested in phase II clinical trials in UC (90).
OST-122 is an oral, gut-restricted and subtype-selective JAK3/TYK2 inhibitor for the local treatment of IBD including UC, CD and, potentially, fibrotic lesions in CD patients. A phase I/II is currently underway to evaluate the safety and tolerability of OST-122 in patients with moderate to severe UC over 28 days. This trial will also explore the OST-122 pharmacokinetics (PK) profile and preliminary therapeutic efficacy through biomarker analysis and clinical, endoscopic and histologic assessments (102).
Deucravacitinib (BMS-986165), an oral selective TYK2 inhibitor is being studied in a wide spectrum of immune-mediated diseases, including psoriasis, psoriatic arthritis, lupus and inflammatory bowel disease (103). A phase II randomized, double-blind, placebo-controlled study is ongoing to establish the safety, efficacy, and biomarker response of BMS-986165 in subjects with moderate to severe UC and CD (104–106).
Unfortunately, JAK inhibitors seem to increase the risk of viral infections, particularly herpes zoster, but an increased risk of malignancy has not been reported (90). TD-1473, is a new pan-JAK inhibitor with the advantage of distribution limited to the gastrointestinal tract, minimizing systemic side effect, as demonstrated in a phase I study on healthy volunteers (107). In another phase I study, TD-1473 also proved clinical and biomarker activity in patients with moderate to severe UC (108). Phase II/III studies are ongoing in IBD.
PDE4 and NFκB modulation
Among second messengers, cyclic adenosine monophosphate (cAMP) is fundamental in inhibiting the pro-inflammatory process. Phosphodiesterase 4 (PDE4) is the predominant enzyme that metabolizes and degrades cAMP in inflammatory cells, resulting in the production of pro-inflammatory mediators, such as TNF-α, interferon-γ, and IL-23 (109).
The anti-inflammatory and immunomodulatory potential of PDE4 inhibitors in human leukocytes, endothelium and epithelium are now well documented (110–112).
Apremilast is a PDE4 inhibitor that can induce clinical remission, endoscopic and histological response in UC with an acceptable safety profile (113), although not statistically significant when compared to a placebo.
Laquinimod is another small molecule immunomodulator that, acting on the NF-κB pathway of dendritic cells, causes a reduction of pro-inflammatory cytokine and chemokine production and leucocyte migration. In a phase II trial, laquinimod leads to clinical response and remission in patients with moderate to severe UC (114).
Oligonucleotides messenger RNA inhibition
Oligonucleotides represent an emerging category of highly selective targeted agents, which action is mediated by the inhibition/degradation of messenger RNA of different pro-inflammatory cytokines or by bacterial antigen simulation triggering cellular targets for immunomodulation (115). Aside from alicaforsen, already discussed for its anti-trafficking effect, other oligonucleotides that determine the disruption of specific messenger RNA (mRNA) are currently undergoing preliminary investigation. SB012 is a GATA3-specific DNAzyme that can mediate the cleavage of the mRNA of the transcription factor GATA3, responsible for Th2 differentiation and that has proven to be over-expressed in the colonic mucosa of UC patients (116). A phase I/II study on efficacy, pharmacokinetics, tolerability and safety of SB012 enema in patients with active UC is completed but results are still unpublished (115). STNM01 is a double-strand RNA that is directed against the mRNA coding for carbohydrate sulfotransferase 15 (CHST15), an enzyme that mediates the biosynthesis of glycosaminoglycans (GAG), a process involved in the fibrotic evolution of IBD. In a phase I study, a submucosal injection of STNM01 in CD patients with treatment refractory mucosal lesions was safe and able to reduce Simple Endoscopic Score-CD and fibrosis (117). Another drug that deserves a mention is mongersen/GED-0301, an antisense oligonucleotide that mediates the degradation of Smad7 mRNA (118–120). Smad7 is an intracellular protein that inhibits the immunosuppressive cytokine TGF-β and was found to be overexpressed in IBD (121). In phase I and II trials, mongersen had shown safety, tolerability and efficacy in inducing endoscopic response and clinical remission in CD, but a phase III trial was discontinued because the drug was found to be non-effective (115, 118–120).
Innate immunity modulators
As mentioned above, oligonucleotide-based agents can elicit the innate immunity response linking a member of the Toll-Like Receptor (TLR) family, a group of receptors expressed on the surface of immune cells that are usually activated after the interaction with pathogen-associated molecular patterns (PAMPs) expressed by infectious agents (115). Cobitolimod/DIMS0150 is a single-strand oligonucleotide that mimics bacterial DNA as it is recognized by TLR9, mediating the induction of anti-inflammatory cytokines like IL-10 and IFNα (122). A small study, conducted in 2013, showed that topical administration of cobitolimod determined reduction in colitis activity index and clinical, endoscopic and histologic improvements in UC patients otherwise candidates for colectomy (122). In a randomized, placebo-controlled phase III trial with cobitolimod, a statistically significant improvement in clinical remission with mucosal healing and symptomatic remission was reported, together with a good safety profile at week 4. On the other side, it did not reach statistical significance in inducing remission in UC patients treatment-refractory at week 12 (123). Furthermore, a post-hoc analysis showed statistical significance in the rate of clinical remission using patient-reported outcome (PRO) measures (124). Similarly, BL7040 (monarsen) is an orally applied oligonucleotide that modulates the TLR9 that achieved clinical response in a phase II study in patients with moderately active UC (125). FimH, a novel microbiome-derived therapeutic target, is a TLR4 receptor, expressed on Escherichia coli and other Enterobacteriaceae in a host with CD. The inhibition of FimH adhesion, and consequently intracellular replication of adherent-invasive E.coli in epithelial cells, may prevent the establishment of a sub-mucosal infection leading to mucosal inflammation and epithelial barrier disruption (126).
Sibofimloc is a small molecule specifically designed to reduce the inflammatory cascade underlying CD and remain gut-restricted to minimize absorption into the bloodstream. This drug was included in a randomized, double-blind, placebo-controlled, multicenter, phase II study to evaluate its safety and tolerability to prevent the recurrence of intestinal inflammation in up to 96 postoperative participants with CD and the results are under discussion (127).
There is growing evidence that a variety of immune-related diseases, including UC, have an underlying defect or suppression of the innate immune system. QBECO, Site Specific Immunomodulator (SSI) derived from components of inactivated E.coli, has been approved in UC and designed to restore innate immune function in the colon and reverse the chronic inflammation and dysbiosis associated with the disease (128). Promising early clinical experience and a translational study with QBECO in UC, showing improved GI barrier function, have led to exploring the safety, efficacy, and tolerability of this novel immunotherapy in subjects with moderate to severe CD (129, 130).
The immune-activating receptor Natural Killer Group 2D (NKG2D) has been implicated in the pathogenesis of IBD through its presence on intestinal cytotoxic lymphocytes and the increased expression of activating ligands on inflamed tissue (131).
In IBD, the expression and function of NKG2D have not been fully characterized, and a recent phase II clinical trial tested the efficacy and safety of tesnatilimab (JNJ-64304500) in patients with active CD but not remission to the standard of care biologic therapy (132, 133).
Table 1 shows a list of current and emerging targeted therapies for IBD with their main characteristics.
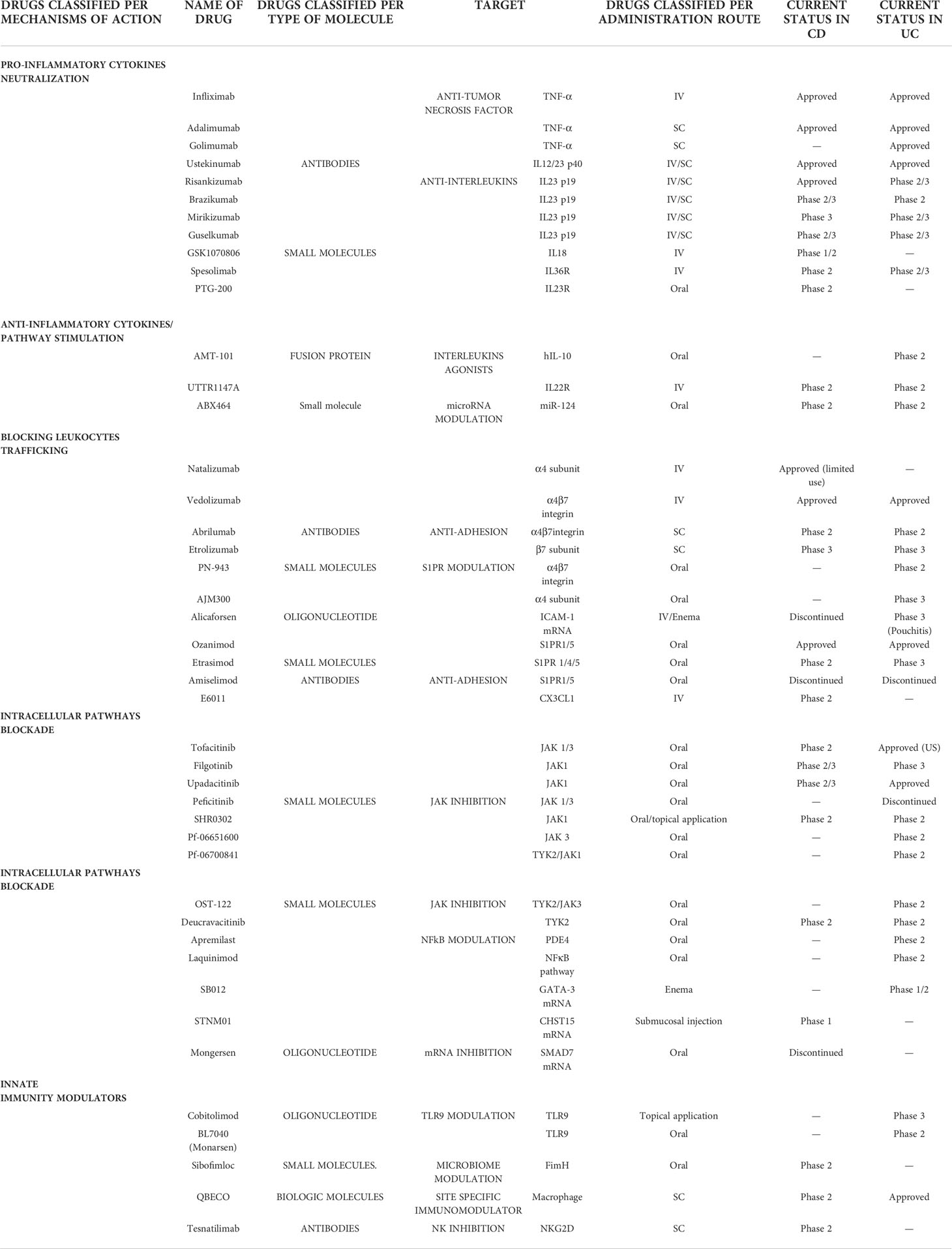
Table 1 Current and future therapies in IBD based on mechanisms of action, name of drug and type of molecule (table continues on the following page).
Leukocytapheresis
Leukocytapheresis is a non-pharmacologic approach to the treatment of IBD that works by removing activated circulating leukocytes in the colonic mucosa using beads or filters. A selective apheresis filter currently available is the Adacolumn® system (JIMRO, Japan), which contains cellulose diacetate beads that adhere to Fc-gamma and complement receptors on granulocytes and monocytes, selectively removing them without significantly affecting lymphocytes or platelets (21). Adacolumn® significantly suppresses proinflammatory cytokines, reduces neutrophil chemotaxis, causes down-regulation of leukocyte adhesion molecules and reduces neutrophil adhesion to endothelial cells activated by IL-1β; it also causes an adsorptive-dependent increase in anti-inflammatory cytokines such as IL-1Ra, growth factor of hepatocytes and soluble receptors I and II for TNF (134). Randomized phase III trials failed to demonstrate any efficacy of the Adacolumn® for induction therapy in patients with moderate to severe UC or active CD (135, 136). Conversely, two recent trials have shown efficacy in inducing remission for steroid-dependent ulcerative colitis demonstrating a potential role in this subclass of patients (137, 138).
Fecal microbiota transplant (FMT)
The gut microbiome has a mutual symbiotic relationship with the host, providing, for example, the production of carbohydrates, short-chain fatty acids and vitamins and the maintenance of immune homeostasis. Dysbiosis in IBD, most commonly causes a decrease in commensal bacteria diversity and an increase in bacterial species belonging to Enterobacteriaceae; this is generally accepted even though a clear causal link between cause and effect has not yet been established (139). Fecal microbiota transplantation (FMT) is a revolutionary therapeutic strategy to restore the normal gut microbiota that has proven to be extremely effective in the treatment of Clostridium Difficile infection (140). It consists in one or more infusions of feces from a healthy donor into the gastrointestinal tract of the host via nasogastric or nasojejunal tube, upper or lower endoscopy or enema (141). According to a 2017 meta-analysis of 53 studies, FMT with multiple infusions administered through the lower gastrointestinal tract achieved a clinical remission rate of 36% in UC, 50.5% in CD and 21.5% in pouchitis and appeared safe with just transient gastrointestinal symptoms as adverse events (142). Data on the long-term effectiveness of FMT are controversial, but the response seems to decrease over 3 months; however, some patients exhibit long-term remission (143–146), pointing out that the therapeutic effect of FMT, particularly in patients with UC, is very promising, especially in those patients who received multiple transfusions. The effects of FMT in CD patients are currently being tested in several active trials. Some successful strategies to improve efficacy of FMT were identified and suggest that donor microbiota stability and species evenness, were highly relevant to efficacy and patient outcomes as well as specific microbial species could improve donor selection and build artificial microbiota for FMT (147). FMT seemed to be effective against some symptoms; however, further research needs to be conducted to improve the quality of data (148).
Stem cell therapies
Stem cell therapy using hematopoietic stem cells (HSC) and mesenchymal stromal cells (MSC) is a promising strategy to improve IBD control, especially in refractory patients.
HSCs are defined as progenitor cells that are capable of unlimited self-renewal. HSC autologous transplantation (HSCT) has been suggested to reset the immune system, attenuating the abnormal inflammatory immune response in IBD patients. Based on this, several studies have been conducted on CD and UC patients, some of them using autologous HSCT on refractory CD patients. After one year of autologous HSCT, almost 40% of patients who received transplantation stopped immunosuppressive therapy compared with the control group (149). Despite this, HSCT has been discouraged as a treatment option for autoimmune conditions, in particular for patients with refractory CD, due to high mortality rates, complications and serious associated adverse events (150).
MSCs are a type of immature and undifferentiated stem cells and have a tissue regenerative role. They are pluripotent and able to transform into adipocytes, osteoblasts, myocytes, and chondroblasts. The immunosuppressive effect of MSCs on IBD is mainly due to the paracrine function that secretes different cytokines to inhibit inflammation. MSCs can also secrete exosomes to the intestinal mucosa to inhibit inflammation (151). The first animal experiment to study the role of MSCs in the treatment of a UC mouse model was conducted in 2006. The results demonstrated that bone marrow-derived MSCs played a role in repairing injured intestinal mucosa and in downregulating the immune function of T cells (152). In a phase III study, using adipose-derived MSCs, ASC transplantation (ASCT) for CD patients with complex perianal fistulas showed that 59.2% of ASCT-treated patients achieved remission compared to the placebo group and that adverse events were similar in both groups (153).
All these current and future therapies in IBD are shown in Figure 2.
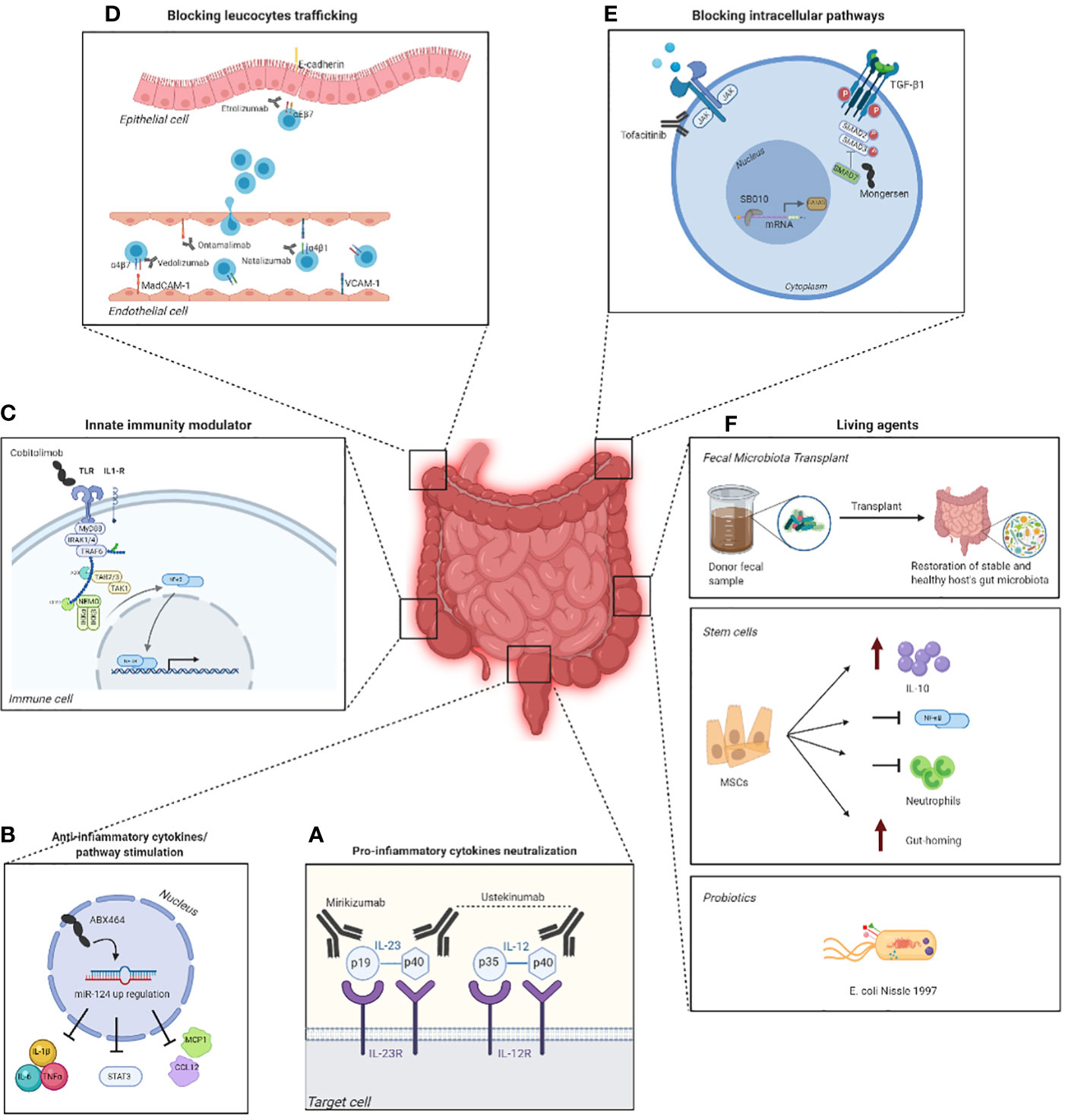
Figure 2 Emerging targeted therapies in IBD and their mechanism of action. Some therapies exploit monoclonal antibodies, such as mirikimumab and ustekinumab (A), that inhibit the binding between pro-inflammatory cytokines and their respective receptor on the surface of the target cell, which in consequence blocks the development of the inflammation. However, there are molecules, such as ABX464, capable of modulating the production of anti-inflammatory targets to regulate the immune response (B). Other therapies, on the other hand, aim to activate the innate immunity using drugs such as cobitolimod (C) that, by binding to the toll-like receptor 9 (TLR9) on the surface of the immune cell, leads to the suppression of Th17 cells and the induction IL-10+ macrophages and Treg cells. Blocking the trafficking of the leucocytes in the epithelial cell is another valid therapeutic strategy and can be accomplished by a multitude of drugs like estrolizumab, vedolizumab, ontamalimab and natalizumab (D), each of them designed to target a specific receptor. Another strategy is blocking intracellular pathways with drugs such as tofacitinib, that inhibits the JAK/STAT signaling pathway (panel E), and mongersen, which contrasts SMAD7 (E). One last therapy revolves around the use of living agents as probiotics, fecal microbiota transplant (FMT) and stem cells that aim to increase the levels of IL-10 and the gut-homing and block neutrophils and the NFĸB pathway (F). The figure was created with Biorender.com.
Complementary and alternative medicine
Complementary and alternative medicine (CAM) includes a very extensive and heterogeneous set of diagnostic-therapeutic practices that are not presently considered to be part of modern scientific medicine (24). Furthermore, the term ‘alternative medicine’ implies its use instead of, and the term ‘complementary medicine’ its use integrated with conventional medicine. CAMs have already been used extensively as coadjuvant in anti-cancer treatment; however, many publications show that CAMs are routinely used by patients affected by other categories of disease (154).
The percentage of IBD patients using CAM is high, ranging between 21% and 60% (23, 24). Most CAM therapies fall into one of the following main categories: herbal/botanical or dietary supplements; mind-body practices such as hypnosis, yoga/exercise, mindfulness and stress reduction. The most used CAM therapies are probiotics, herbs (e.g. curcumin, Chinese medicine, and cannabis) and fish oil (24). Other therapies include the use of traditional Chinese practices such as acupuncture and moxibustion.
Recent researches highlight that dietary therapy has a great implication for the treatment of IBD and suggest that the use of probiotics, dietary fibres and fat-soluble vitamins can substantially reduce the symptoms of IBD through their anti-inflammatory functions (155).
Nutraceutical approaches
Non-starch polysaccharides
Non-starch polysaccharides (NPS), classified as dietary fibres and prebiotics, are obtained from various natural sources that have been studied extensively as therapeutics against inflammation and other immune-related problems. All of the major components of NPS, including cellulose, glucomannan, glucan, pectin, inulin, and oligosaccharides have exhibited anti-inflammatory and immunomodulatory functions (156). It has been hypothesized that the NPS components are fermented by probiotics in the healthy large intestine where they act by carrying out their anti-inflammatory function (157).
Konjac glucomannan is a plant-derived polysaccharide that has been used to treat gastrointestinal inflammatory disorders. For example, supplementation in IBD patients with konjac glucomannan hydrolysate for fourteen days resulted in improved bowel movement, fecal consistency and reduced abdominal pain, resulting in a better lifestyle (158).
Specific carbohydrate diets (SCD) and FODMAP
The specific carbohydrate diet (SCD) allows carbohydrate foods consisting of monosaccharides, and excludes foods containing disaccharides and most polysaccharides. Large-scale clinical trial data on SCD in IBD is lacking, but several studies have explored the role of SCD in IBD, particularly in paediatric patients (159).
In a clinical study, children with CD under this dietary plan for 12 and 52 weeks had remarkably reduced mucosal damage and improved clinical symptoms (160). Suskind and colleagues conducted a retrospective study of seven paediatric patients with CD and found that all patients experienced symptom remission within three months of initiating SCD, with either normalization or improvement in CRP, haemoglobin, albumin, and fecal calprotectin (161). Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) is a term used to indicate sugars present in some highly fermentable foods, which can cause the onset of symptoms typical of an irritable bowel, e.g., abdominal swelling and pain, flatulence, alteration of the alvus or digestive difficulties. FODMAPs are short-chain carbohydrates that are not completely absorbed from the gastrointestinal tract, which can promote a state of excessive fermentation in the intestine that can compromise the quality of life in predisposed individuals. Evidence shows that a low FODMAP diet for 6 weeks helps to relieve symptoms of irritable bowel syndrome but there is currently no evidence to suggest that it works in IBD, although some patients find it helpful to control some of their symptoms (162).
Vitamins
It is also of interest to discuss the roles of fat-soluble vitamins such as A, D, E, and K, which are significantly decreased in patients with CD. Particularly, vitamin D deficiency in UC patients has been found to be associated with mucosal inflammation, and disease activity, which can increase the risk of clinical relapse of IBD (163, 164). The reasons for vitamin D deficiency are multifactorial but include lack of sun exposure due to immuno-suppressive treatments, dietary restrictions, and impaired nutrient absorption (165). Therefore, vitamin D supplements in IBD patients can result in positive outcomes. It has been demonstrated that vitamin D, by downregulating proinflammatory cytokines, IL-6, IL-21, TNF-α, and IFN-γ and by stabilizing the intestinal barrier, can contribute to the maintenance of the gastrointestinal barrier integrity and surveillance of the gut microbiota and inflammatory immune responses (166, 167).
Phytochemicals
Phytochemicals are substances naturally present in foods of plant origin and carry out important bioactive functions against oxidative and inflammatory disorders. Resveratrol is a polyphenol abundant in peanuts, berries and red grapes and exhibits biological functions that influence oxidative processes and inflammatory pathways. A randomized controlled study revealed that CD patients with resveratrol supplements had lower inflammation and reduced levels of TNF-α and NF-κB compared to the placebo group (168). The use of resveratrol efficaciously increased the activities of the antioxidant enzymes superoxide dismutase and glutathione peroxidase, protecting the cell from ROS damage. It can also reduce lipid peroxide and nitric oxide levels in colitis mice (169).
Acute colitis in an animal model was also partially inhibited by two other flavonoids, eupatilin and quercetin, which were orally administered 48 hours before colitis induction. The group of animals that received flavonoid extracts showed fewer mucosal lesions, nitric oxide and TNF-α production (170).
Prebiotics
Prebiotics are non-digestible organic substances, capable of selectively stimulating the growth and/or activity of one or a limited number of beneficial bacteria present in the colon. The best known and studied prebiotics are oligosaccharides and in particular inulin and fructo-oligosaccharides (FOS). Giving FOS to colitic mice resulted in increased lactic acid bacteria in the gut and decreased pro-inflammatory cytokines, such as IFN-γ, IL-17, and TNF-α (171). In addition, FOS treatment proved to have a protective effect on the epithelium, because both alkaline phosphatase (AP) enzyme activity and sensitivity to levamisole are decreased by FOS, which is consistent with a reduced AP isoform shift in enterocytes, a known feature of epithelial cells under inflammatory conditions.
Probiotics
Probiotics are live microorganisms (in most cases, bacteria) that are similarly beneficial to microorganisms present in the human gut and are used to restore gut bacterial flora. Probiotics ameliorate inflammation via several mechanisms, including alteration of the mucosal immune system, competitive exclusion of proinflammatory pathogens and production of antimicrobial factors such as bacteriocins and other metabolites (172). They also affect immune-modulatory pathways, such as downregulating the expression of Toll-like receptors and inflammatory cytokines as well as inhibiting the phosphoinositide 3-kinase/Akt pathway and the nuclear factor κ–light chain enhancer of activated B cells (NF-κB) pathway (173). They are also called “friendly bacteria” or “good bacteria”. Probiotics are available to consumers mainly in the form of dietary supplements and foods. Probiotics are among the most popular and most widely used CAM therapies and are available as single or multiple strains of bacteria or yeasts (24).
Escherichia coli Nissle (EcN) 1917 is a non-pathogenic Gram-negative strain used in many gastrointestinal disorders including diarrhoea, uncomplicated diverticular disease and IBD, in particular UC (174). Mechanisms of actions of this compound include immune-modulatory properties, reinforcement of intestinal barrier and inhibitory effect towards other pathogenic E. coli (175).
Lacticaseibacillus rhamnosus GG proved to be effective and safe for maintaining remission and preventing relapse in patients with ulcerative colitis in a clinical trial from our group (176).
Other probiotics displayed interesting data in preclinical and in clinical settings, however, although we acknowledge several limitations to recent AGA guidelines on probiotics, more structured clinical trials are necessary to better position probiotics in therapeutical work up in IBD (177).
Herbaceous medications
Natural products, especially herbal medications and herbal extracts, have a relevant effect on the treatment of IBD (178) with numerous biological potencies, including anti-inflammatory activity (179).
Curcumin, or rather, Curcuma longa is a spice from the Zingiberaceae family. It has anti-inflammatory properties, which inhibit the two important mediators COX2 and TNF-α. Curcuma longa can reduce pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β and IL-12, modulate signal transduction pathways such as p38MAPK and NF-kB, and reduce the expression of enzymes involved in the pathogenesis of inflammation (COX2) (180). Wang et al. found that different concentrations of curcumin could increase transepithelial electrical resistance by increasing the expression of occludins and maintaining the integrity of the tight junction (181). Curcumin, as a complementary therapy to 5-aminosalicylic acid (5-ASA), may be effective in inducing remission in mild to moderately active UC. In fact, a double-blinded, randomized pilot study documented the efficacy of curcumin in patients who were already on standard therapy and demonstrated a possible beneficial outcome in patients with distal UC and mild to moderate disease activity without significant side effects. Authors speculate that the use of curcumin can be a potential and safe therapy for the management of patients with UC (182).
Cannabis, a drug produced from the flowers and buds of the Cannabis sativa plant, has been therapeutically used for centuries (183). Cannabis contains dozens of terpene phenols known as cannabinoids many of which are pharmacologically active and induce their effects by binding to their specific CB1 and CB2 receptors. CB1 receptors are expressed in not only neurons of the central and peripheral nervous system but also in the GI gastrointestinal tract and are involved in various GI functions, such as: visceral pain, motility and sensitivity (184). CB2 receptors are mainly expressed in epithelial cells and cells of both innate and adaptive immunity, lymphocytes, macrophages, NK cells and neutrophils. Therefore, CB2 receptors are involved in the modulation of immune responses (180). Cannabinoids act on the endocannabinoid system, and have demonstrated analgesic and antinociceptive activity in several animal models and human (185). Cannabis has anti-inflammatory effects, and therefore may be useful in the treatment of several chronic inflammatory conditions including IBD (186). An unpublished meta-analysis of 100 patients from nonrandomized studies and randomized controlled trials until July 2018, considering the safety and efficacy of cannabis/cannabinoids in IBD was presented at the ECCO Congress 2019 (187). The authors found significant differences in disease activity scores in the treated group compared to the control group and an improvement in quality of life (QoL) and symptoms in cannabis-treated patients. However, data suggest a limited efficacy of cannabis in inducing remission of disease activity. In summary, cannabis may improve clinical symptoms but has not demonstrated any improvement in IBD activity. Aside from the lack of objective evidence that cannabis decreases inflammation in IBD, there are also legal and psychosocial risks involved with its use, particularly among younger patients (188). Side effects of cannabis use include confusion, ataxia, dizziness, nausea, and vomiting. Chronic use is associated with cognitive impairments and deficits in motivation, learning, and memory, as well as increased risk of motor vehicle crashes and decreased fertility (189). However, a recent study by Cocetta et al., showed that the non-psychotropic phytocannabinoid, cannabidiol isolated from Cannabis sativa, could represent the most promising candidate for clinical utilization due to its lack of psychoactive actions (190). The authors highlighted the role of cannabidiol as a potential modulator of markers of gut inflammation, being able to counteract the overproduction of reactive oxygen species and to prevent tight junction alterations allowing better maintenance of the intestinal epithelial barrier.
Fish oil is a dietary supplement derived from fish. It contains omega-3 fatty acids (n-3 PUFAs), which are one of the essential fatty acids in the human diet, along with omega-6 and omega-9. Oxidative stress occurs in several chronic inflammatory conditions, including IBD. Increasing the level of antioxidants could reduce tissue damage and the inflammatory process. Fish and fish proteins can have such antioxidant potential (191). Recently, a few studies completed in adults showed some common changes in the gut microbiota after omega-3 PUFAs supplementation. In particular, a decrease in Faecalibacterium, often associated with an increase in the Bacteroidetes has been shown. However, many more studies need to be conducted to demonstrate the effective relationship between omega-3 PUFAs consumption and gut microbiota changes (192).
Chinese herbal medicine
Herbal medicine (or phytotherapy) is a form of alternative medicine practiced for centuries, especially in China, where it is considered traditional medicine. For instance, pomegranate peel decoction can inhibit the growth of pathogenic intestinal bacteria (e.g. C. difficile, Staphylococcus aureus and Pseudomonas ceruminous), induce intestinal probiotics bacteria and regulate intestinal flora, improving its related disorders (193). The extract of pomegranate seeds is an oil that could inhibit proinflammatory cytokines’ expression (such as IL-6, IL-8, IL-23, IL-12, and TNF-α), preventing gastrointestinal inflammation (194). Andrographis paniculata (AP) is a traditional Chinese medicine that belongs to the Acanthaceae family and has anti-inflammatory properties on innate and adaptive immunity cells (e.g., dendritic cells, macrophages, T-cells, pro-inflammatory enzymes, cytokines and signal transduction pathways) (195). Andrographolide, an active ingredient isolated from the stem and leaves of AP, could have a down-regulating effect on the levels of TNF-α, IL-1β and IL-6 (196). AP extract (HMPL-004) and andrographolide sulfonate, an andrographolide derivative, could be employed respectively in the treatment of UC and experimental colitis (197). A recent study by Qin Zhu et al. investigated the andrographolide’s therapeutic effect in 2,4,6-Trinitrobenzene Sulfonic Acid Colitis (TNBS)-induced experimental colitis. Treatment with andrographolide showed a decrease in the percentages of Th17 cells in CD4+ cells and in pro-inflammatory cytokines’ levels (198).
Indigo naturalis (also referred to as Qing-Dai) is an herbal preparation extracted from leaves and stems of Baphicacanthus cusia (Nees) Bremek, which has been employed as a blue dye since ancient times (199). Its anti-inflammatory effect is supposed to be due to inhibition of TNF-α, IL-1 and IL-6, and NF-κB, as well as promotion of IL-22 production (200). In rat models with sodium dextran sulfate-induced colitis, Indigo reduced the expression of inflammatory cytokines while increasing the expression of proteins linked to the repair of the intestinal epithelium and colon mucosa (201). In a randomized, placebo-controlled trial, it was found that 8 weeks of Indigo was effective in inducing a clinical response in patients with UC. However, it should not yet be used because of the potential adverse effects, including pulmonary arterial hypertension (199).
Pogostone (PO) is one of the major chemical constituents of Pogostemon cablin (Blanco) Benth. In a study by Su et al., PO improved the inflammatory state, reducing effects on the infiltration of total Th cells into the inflammatory colon, especially pro-inflammatory Th1, and pro-inflammatory cytokine levels (202).
Cognitive-physical approaches
Chinese practices (body-based interventions)
Acupuncture and moxibustion are therapies that have been used for over three millennia, representing the oldest practices of traditional Chinese medicine (203). Acupuncture consists in inserting thin needles into the skin at specific acupoints to attain various effects. Moxibustion consists in applying burning hot material on certain body areas, causing relief by the generated heat (203). A moxibustion’s variation is the herb-partitioned moxibustion (HPM), in which several herbs are added to burning cones to achieve the desired benefit (203). HPM turned out to promote the repair of damaged colonic tissue in ulcerative colitis rats and the secretion of mucin, which was correlated with an increased frequency of therapy (204). These clinical benefits were examined also in IBD patients in a meta-analysis, which assessed the clinical efficacy of acupuncture and/or moxibustion compared with sulphasalazine (SASP) for the treatment of UC (205). The overall efficacy of acupuncture alone, moxibustion alone or acupuncture and moxibustion combined was superior to the effectiveness of SASP.
Mind-body interventions
The role played by mood disorders and emotional distress on the microbiota is of growing interest. Studies in humans show that depression is associated with fecal microbiota alterations, with increased Bacteroidetes, Proteobacteria and Actinobacteria, and reduced Firmicutes (206, 207). Furthermore, studies in animals proved that a fecal microbial transfer from depressed humans to non-depressed rats could trigger mood disorders in rats (208). The bi-directional relationship between mood and disease activity might evidence benefits IBD patients could obtain by integrating standard medical treatments with novel interventions focused on mental wellness (209). Since IBD and psychological stress are related, mind-body interventions (MBI) e.g., psychotherapy, relaxation, mindfulness, biofeedback, yoga and clinical hypnosis, as complement therapies, which target inflammatory disease activity, with safe, economic and non-pharmacological interventions might decrease stress response and increase the relaxation response of the autonomic nervous system (209).
Clinical hypnosis is a common MBI and refers to the specific ability to focus the patient’s attention narrowly deepening concentration, while simultaneously diminishing awareness of external stimuli. The mechanism of action of hypnotherapy is believed to be via the ‘brain–gut axis’, through the modulation of vagal visceral afferent signals. The strongest evidence of hypnotherapy’s effectiveness in IBD is its association with reduced IBD-related inflammation and improved QoL. Indeed, convincing studies have been performed suggesting that hypnotherapy can reduce inflammatory responses of the rectal mucosa (IL-6, IL-13, TNF-α, substance P and histamine) in patients with UC after just one hypnotherapy session (210).
Mindfulness is a type of meditation practiced to focus on the present moment, to relax in the “here” and “now”. It involves mind-body exercises where the patient tries to be fully engaged with a particular sensation, such as taste or smell, while focusing on breathing (211). In one trial, 60 patients with UC and CD were randomized to receive a mindfulness-based stress reduction intervention. The mindfulness intervention group for IBD patients reported significantly greater improvements in anxiety, QoL and mindfulness, with a significant reduction in depression compared to the control group (211).
Meditation is a practice of turning the patient’s attention inward calming the mind. There are many different forms of meditation, some including meditative movements such as Tai Chi or yoga.
Tai Chi is a martial art that has several beneficial effects on health, which is not only guaranteed by physical activity, but also by deep breathing and meditation exercises. Estaki et al. showed that cardiorespiratory fitness is associated with increased microbial diversity and butyrate production (212). Even though the association between Tai Chi and gut microbiota is still unknown, Tai Chi is supposed to modulate gut microbiota by improving cardiorespiratory fitness, which benefits the gut microbiota by reducing stress; it may also improve gut immune function and inflammation mediating the hypothalamic-pituitary-adrenal (HPA) axis and vagal modulation (213).
Yoga is an ancient Indian discipline, which affects the person physically, mentally and spiritually. In a RCT study, patients with UC in remission who performed 12 yoga sessions had an improvement in QoL, anxiety and abdominal pain, but no difference in their immune response markers was demonstrated (214).
Physical activity in wider terms has been found to reduce some symptoms and complications of IBD, improving the QoL in patients (215). A study by Jones et al. demonstrated that patients with inactive CD who performed higher levels of exercise were less likely to develop active CD at 6 months. The study was replicated in UC patients, but the association was not statistically significant (216). In fact, strict exercise routines could be counter-productive in patients with active disease, since its interference with absorption could promote bleeding. In patients with chronic inflammation, exercise exerts an anti-inflammatory effect when IL-6 is released by the muscles, which inhibits the production of TNF and stimulates the release of IL-10. The release of myokine after physical activity has also been important in improving the anti-inflammatory effect (217). Physical activity has also counteracted bone mineral loss and targeted pain management and fatigue in patients with IBD. Other studies investigated the effects of exercise on inflammatory biomarkers like faecal calprotectin (FCP), C-reactive protein (CRP) and TNF-α, with merely one study showing a significant reduction in CRP (218). Despite the importance of TNF-α in IBD, only one study explored this parameter; however, no significant effect on the TNF-α basal level was observed within the treatment group that conducted mind-body therapy (219). Figure 3 summarizes complementary and alternative medicine in IBD treatment.
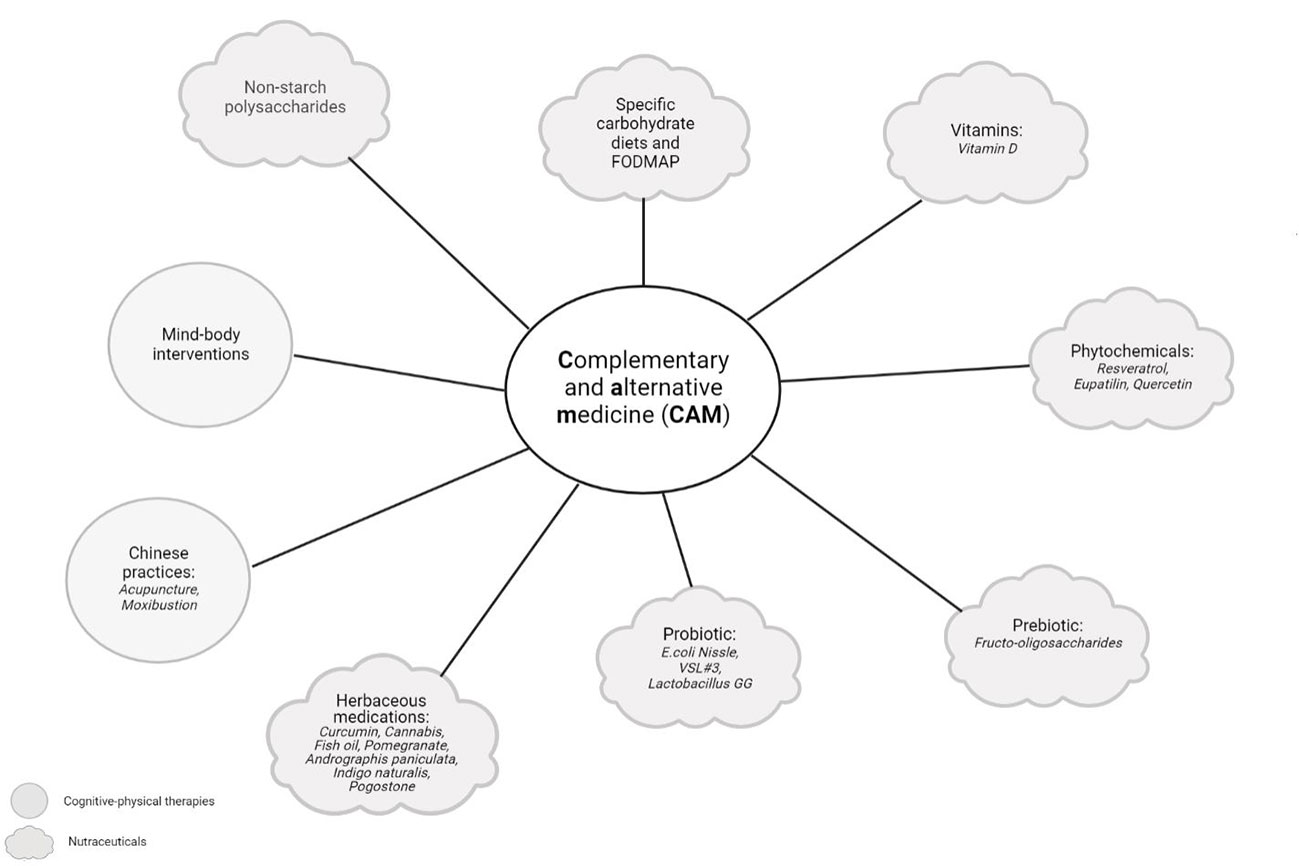
Figure 3 Complementary and alternative medicine (CAM) in IBD treatment. Complementary and alternative medicine (CAM) includes products or medical practices that encompass herbal and dietary supplements, probiotics, traditional Chinese medicines, and a variety of mind-body techniques. The figure was created with Biorender.com.
Discussion
IBD, with its unknown and multifactorial pathogenesis, has prevented the development of a unique therapeutic intervention that can be considered valid and effective for all affected patients. Furthermore, data from basic science suggest that IBD is an ensemble of several different diseases, which may require different therapeutic approaches depending on pathogenic features that are to date unknown.
For this reason, the 2020s “IBDologist” need to approach IBD more comprehensively, considering classic, innovative and complementary medicine.
In this comprehensive review, we have conferred a synthesis of current and investigational agents that are already enjoying or might play a very important role in the current and future treatment of IBD.
For many treatments, the results of RCTs or different varieties of clinical trials are the summit of several basic analysis studies that grow out of, contribute to, and improve our understanding of basic biological illness mechanisms. There is a crucial activity between basic analysis and clinical analysis. The basic analysis aims to advance data and understanding of the biological mechanisms of illness and treatment. A lot of clinical analysis builds on the results of the basic analysis to work out whether treatments supported new illnesses and whether treatment ideas will give measurable edges in specific patient populations. Findings from clinical analysis might support insights from basic analysis or reveal shocking results that cause new queries or hypotheses that are tested in laboratory studies. CAM come back from a range of sources and embrace seasoning therapies, organic process supplements, probiotics, and different physical or religious practices for the body and mind. though the majority contemplate these therapies as natural and safe, adverse effects will occur, and potential interactions with commonplace therapies ought to even be thought about. Patients usually address complementary and various medicines (CAM), which are medications or practices that are not a part of thought medication, to regulate their symptoms and manage their chronic ill health. Recent studies show patients with IBD use some variety of CAM to manage their illness (220–224). Given its increasing use in patients with IBD, physicians must treat these patients and remember current proof to raised counsel patients concerning the role of CAM in the management of IBD.
This review therefore highlights novel and emerging therapies for UC and CD and the most up-to-date results from clinical trials. We also report the data about unpublished paper related to undergoing clinical trials. However, there are a few limitations. The data from each study cannot be compared as all of the trials differ in design with varying inclusion and exclusion criteria, induction and maintenance periods, re-randomisation strategies following induction and outcomes. Limited data have been reported from negative and potential negative trials. No indications have been provided on where such treatments can be positioned into the treatments approach of active IBD patients.
Despite these limitations, our review provides an updated guide for gastroenterologists in classifying treatments, in order to facilitate decision making for IBD patients based on innovative, complementary and alternative therapy.
Conclusion
The world of IBD is rapidly evolving while improving understanding of disease pathogenesis as well as increasing targets to different pathways.
In parallel to pharmacological therapies other complementary approaches (CAM) have also been developed, encountering the interest of a vast majority of patients, perhaps because of reported ‘natural’ (non -synthetic) origin and safety profile, whose use has also been assessed in clinical trials. We believe it is important that gastroenterologists take into consideration both pharmacological and CAM, in order to engage positively all patients. Furthermore, innovative therapeutic approaches coming from combination of CAM to other pharmacological approach are more than welcome, in order to maximise results believing in a perhaps synergic role of it.
Understanding impact and potential of combination therapies could drive the future of research in IBD to create a more personalised approach to the treatment of patients and improve outcomes.
Author contributions
LMa, CC, and VP gave substantial contributions to the conception and design of the work. LMa, CC, and VP wrote the manuscript with the support of DP, CG, and LLo. PP, FdV, GP, MP, DN, LT, VA, and ES performed literature review. LP, LLa, LMi, MM, and AP have revised the work critically for important intellectual content. AA, AG, and FS made critical revisions related to the important intellectual content of the manuscript and gave the final approval of the article to be published.
Acknowledgments
Thanks to Fondazione Roma for the continuous support to our scientific research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
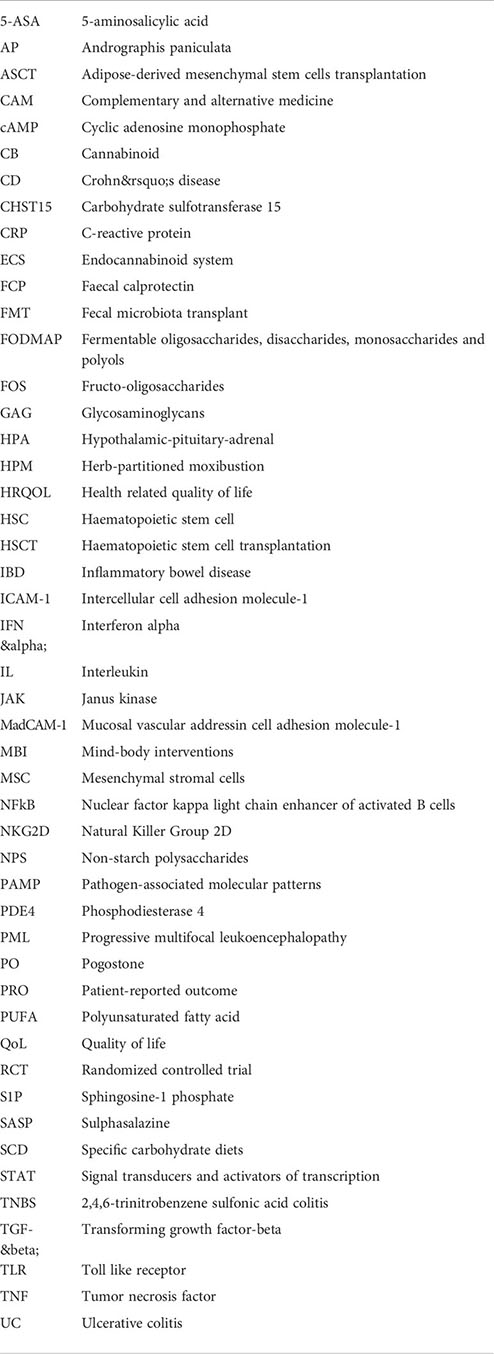
Glossary
References
1. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in crohn's disease: Medical treatment. J Crohns Colitis (2019) 14:4–22. doi: 10.1093/ecco-jcc/jjz180
2. Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third european evidence-based consensus on diagnosis and management of ulcerative colitis. part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis (2017) 11:649–70. doi: 10.1093/ecco-jcc/jjx008
3. Papi C, Fascì-Spurio F, Rogai F, Settesoldi A, Margagnoni G, Annese V. Mucosal healing in inflammatory bowel disease: treatment efficacy and predictive factors. Dig Liver Dis (2013) 45:978–85. doi: 10.1016/j.dld.2013.07.006
4. Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology (2011) 141:1194–201. doi: 10.1053/j.gastro.2011.06.054
5. Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in crohn's disease. Inflammation Bowel Dis (2009) 15:1295–301. doi: 10.1002/ibd.20927
6. Colombel J, Rutgeerts PJ, Sandborn WJ, Yang M, Camez A, Pollack PF, et al. Adalimumab induces deep remission in patients with crohn’s disease. Clin Gastroenterol Hepatol (2014) 12:414–42. doi: 10.1016/j.cgh.2013.06.019
7. Colombel J, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with crohn’s disease: The CHARM trial. Gastroenterology (2007) 132:52–65. doi: 10.1053/j.gastro.2006.11.041
8. Sandborn WJ, van Assche G, Reinisch W, Colombel J, D’Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology (2012) 142:257–65. doi: 10.1053/j.gastro.2011.10.032
9. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology (2014) 146:85–95. doi: 10.1053/j.gastro.2013.05.048
10. Sandborn WJ, Feagan BG, Marano C, Zhang H, Strauss R, Johanns J, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology (2014) 146:96–109. doi: 10.1053/j.gastro.2013.06.010
11. Deeks ED. Certolizumab pegol: A review in inflammatory autoimmune diseases. BioDrugs (2016) 30:607–17. doi: 10.1007/s40259-016-0197-y
12. Sokol H, Seksik P, Cosnes J. Complications and surgery in the inflammatory bowel diseases biological era. Curr Opin Gastroenterol (2014) 30:378–84. doi: 10.1097/MOG.0000000000000078
13. Sherman M, Tsynman DN, Kim A, Arora J, Pietras T, Messing S, et al. Sustained improvement in health-related quality of life measures in patients with inflammatory bowel disease receiving prolonged anti-tumor necrosis factor therapy. J Dig Dis (2014) 15:174–9. doi: 10.1111/1751-2980.12125
14. Dulai PS, Sandborn WJ, Gupta S. Colorectal cancer and dysplasia in inflammatory bowel disease: a review of disease epidemiology, patho-physiology, and management. Cancer Prev Res (2016) 9:887–94. doi: 10.1158/1940-6207.CAPR-16-0124
15. Sprakes MB, Ford AC, Warren L, Greer D, Hamlin J. Efficacy, tolerability, and predictors of response to infliximab therapy for crohn’s disease: A large single centre experience. J Crohns Colitis (2012) 6:143–53. doi: 10.1016/j.crohns.2011.07.011
16. Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: Definition, epidemiology, and management. Clin Transl Gastroenterol (2016) 7:135. doi: 10.1038/ctg.2015.63
17. Engel T, Ungar B, Yung DE, Ben-Horin S, Eliakim R, Kopylov U. Vedolizumab in IBD–lessons from real-world experience; a systematic review and pooled analysis. J Crohns Colitis (2017) 12:245–57. doi: 10.1093/ecco-jcc/jjx143
18. Engel T, Yung DE, Ma C, Pariente B, WIls P, Eliakim R, et al. Effectiveness and safety of ustekinumab for crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig Liver Dis (2019) 51:1232–40. doi: 10.1016/j.dld.2019.05.002
19. Danese S, Furfaro F, Vetrano S. Targeting S1P in inflammatory bowel disease: New avenues for modulating intestinal leukocyte migration. J Crohns Colitis (2018) 12:S678–86. doi: 10.1093/ecco-jcc/jjx107
20. Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut (2016) 66:199–209. doi: 10.1136/gutjnl-2016-312912
21. Saniabadi AR, Tanaka T, Ohmori T, Sawada K, Yamamoto T, Hanai H. Treating inflammatory bowel disease by adsorptive leucocytapheresis: a desire to treat without drugs. World J Gastroenterol (2014) 20:9699–715. doi: 10.3748/wjg.v20.i29.9699
22. Paramsothy S, Paramsothy R, Rubin DT, Kamm MA, Kaakoush NO, Mitchell HM, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis (2017) 11:1180–99. doi: 10.1093/ecco-jcc/jjx063
23. Martínez-Montiel Mdel P, Gómez-Gómez GJ, Flores AI. Therapy with stem cells in inflammatory bowel disease. World J Gastroenterol (2014) 20:1211–27. doi: 10.3748/wjg.v20.i5.1211
24. Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and alternative medicines used by patients with inflammatory bowel diseases. Gastroenterology (2017) 152:415–29. doi: 10.1053/j.gastro.2016.10.004
25. Yanai H, Salomon N, Lahat A. Complementary therapies in inflammatory bowel diseases. Curr Gastroenterol Rep (2016) 18:62. doi: 10.1007/s11894-016-0537-6
26. Nguyen GC, Croitoru K, Silverberg MS, Steinhart AH, Weizman AV. Use of complementary and alternative medicine for inflammatory bowel disease is associated with worse adherence to conventional therapy: the COMPLIANT study. Inflammation Bowel Dis (2016) 22:1412–7. doi: 10.1097/MIB.0000000000000773
27. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol (2019) 16:185–96. doi: 10.1038/s41575-018-0084-8
28. Deepak P, Loftus EV Jr. Ustekinumab in treatment of crohn's disease: design, development, and potential place in therapy. Drug Des Devel Ther (2016) 10:3685–98. doi: 10.2147/DDDT.S102141
29. Sands BE, Sandborn WJ, Panaccione R, O’Brien CD, Zhang H, Johanns J, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med (2019) 381:1201–14. doi: 10.1056/NEJMoa1900750
30. Feagan BG, Sandborn WJ, D’Haens G, Panés J, Kaser A, Ferrante M, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet (2017) 389:1699–709. doi: 10.1016/S0140-6736(17)30570-6
31. Feagan BG, Panés J, Ferrante M, Kaser A, D’Haens GR, Sandborn WJ, et al. Risankizumab in patients with moderate to severe crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol (2018) 3:671–80. doi: 10.1016/S2468-1253(18)30233-4
32. Sands BE, Chen J, Feagan BG, Penney M, Rees WA, Danese S, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe crohn’s disease: A phase 2a study. Gastroenterology (2017) 153:77–86. doi: 10.1053/j.gastro.2017.03.049
33. Sandborn WJ, Ferrante M, Bhandari BR, Berliba E, Hibi T, D’Haens GR, et al. Extended treatment with mirikizumab in patients with moderately-To-Severely active ulcerative colitis: Results from a phase 2 trial. Gastroenterology (2019) 156:1094.
34. Sands BE, Sandborn WJ, Peyrin-Biroulet L, Higgins PD, Hirai F, Belin R, et al. Efficacy and safety of mirikizumab (LY3074828) in a phase 2 study of patients with crohn’s disease. Gastroenterology (2019) 156:216. doi: 10.1016/S0016-5085(19)37335-4
35. Ferrante M, Feagan BG, Panés J, Baert F, Louis E, Dewit O, et al. Long-term safety and efficacy of risankizumab treatment in patients with crohn's disease: Results from the phase 2 open-label extension study. J Crohns Colitis (2021) 15:2001–10. doi: 10.1093/ecco-jcc/jjab093
36. D'Haens G, Panaccione R, Baert F, Bossuyt P, Colombel JF, Danese S, et al. Risankizumab as induction therapy for crohn's disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet (2022) 399:2015–30. doi: 10.1016/S0140-6736(22)00467-6
37. Ferrante M, Panaccione R, Baert F, Bossuyt P, Colombel JF, Danese S, et al. Risankizumab as maintenance therapy for moderately to severely active crohn's disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet (2022) 399:2031–46. doi: 10.1016/S0140-6736(22)00466-4
38. Visvanathan S, Baum P, Salas A, Vinisko R, Schmid R, Grebe KM, et al. Selective IL-23 inhibition by risankizumab modulates the molecular profile in the colon and ileum of patients with active crohn's disease: Results from a randomised phase II biopsy Sub-study. J Crohns Colitis (2018) 12:1170–9. doi: 10.1093/ecco-jcc/jjy099
39. Deodhar A, Helliwell PS, Boehncke WH, Kollmeier AP, Hsia EC, Subramanian RA, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet (2020) 395:1115–25. doi: 10.1016/S0140-6736(20)30265-8
40. Cheng X, Taranath R, Mattheakis L, Bhandari A, Liu D. P001 the biomarker profile of PTG-200, an oral peptide antagonist of IL-23 receptor, tracks with efficacy in a preclinical model of IBD. J Crohns Colitis (2017) 11:S80. doi: 10.1093/ecco-jcc/jjx002.128
41. Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell (2015) 163:1444–56. doi: 10.1016/j.cell.2015.10.072
42. Scheibe K, Kersten C, Schmied A, Vieth M, Primbs T, Carlé B, et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology (2019) 156:1082–97. doi: 10.1053/j.gastro.2018.11.029
43. A clinical trial of antibody GSK1070806 in the treatment of patients with moderate to severe crohn's disease (CDAID) . Available at: https://clinicaltrials.gov/ct2/show/study/NCT03681067.
44. BI655130 (SPESOLIMAB) induction treatment in patients with moderate-to-severe ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT03482635.
45. BI 655130 long-term treatment in patients with moderate-to severe ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT03648541.
46. A study to test long-term treatment with spesolimab in patients with fistulising crohn's disease who took part in previous trials . Available at: https://clinicaltrials.gov/ct2/show/NCT04362254.
47. A study testing how BI 655130 works in patients with fistulizing crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03752970.
48. Li LJ, Gong C, Zhao MH, Feng BS. Role of interleukin-22 in inflammatory bowel disease. World J Gastroenterol (2014) 20:18177–88. doi: 10.3748/wjg.v20.i48.18177
49. Study of the efficacy and safety of AMT-101 in subjects with ulcerative colitis (LOMBARD). Available at: https://clinicaltrials.gov/ct2/show/NCT04583358.
50. Study of the safety and efficacy of AMT-101 in subjects with pouchitis (FILLMORE). Available at: https://clinicaltrials.gov/ct2/show/NCT04741087.
51. Wagner F, Mansfield J, Geier C, Dash A, Wang Y, Li C, et al. P420 a randomised, observer-blinded phase ib multiple, ascending dose study of UTTR1147A, an IL-22Fc fusion protein, in healthy volunteers and ulcerative colitis patients. J Crohns Colitis (2020) 14:S382–3. doi: 10.1093/ecco-jcc/jjz203.549
52. An extension study to evaluate the long-term safety and tolerability of UTTR1147A in participants with moderate to severe ulcerative colitis or crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03650413.
53. Wang C, Chen J. microRNAs as therapeutic targets in intestinal diseases. ExRNA (2019) 1:23. doi: 10.1186/s41544-019-0026-9
54. Vermeire S, Hébuterne X, Tilg H, De Hertogh G, Gineste P, Steens JM. Induction and long-term follow-up with ABX464 for moderate-to-severe ulcerative colitis: Results of phase IIa trial. Gastroenterology (2021) 160(7): 2595–2598.e3. doi: 10.1053/j.gastro.2021.02.054
55. Safety evaluation of ABX464 in patients with moderate to severe active crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03905109.
56. Pérez-Jeldres T, Tyler CJ, Boyer JD, Karuppuchamy T, Bamias G, Dulai PS, et al. Cell trafficking interference in inflammatory bowel disease: Therapeutic interventions based on basic pathogenesis concepts. Inflammation Bowel Dis (2018) 25:270–82. doi: 10.1093/ibd/izy269
57. Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, et al. Natalizumab induction and maintenance therapy for crohn’s disease. N Engl J Med (2005) 353:1912–25. doi: 10.1056/NEJMoa043335
58. Targan SR, Feagan BG, Fedorak RN, Lashner BA, Panaccione R, Present DH, et al. Natalizumab for the treatment of active crohn’s disease: Results of the ENCORE trial. Gastroenterology (2007) 132:1672–83. doi: 10.1053/j.gastro.2007.03.024
59. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med (2013) 369:699–710. doi: 10.1056/NEJMoa1215734
60. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for crohn's disease. N Engl J Med (2013) 369:711–21. doi: 10.1056/NEJMoa1215739
61. Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D’Haens G, et al. The safety of vedolizumab for ulcerative colitis and crohn's disease. Gut (2017) 66:839–51. doi: 10.1136/gutjnl-2015-311079
62. Sandborn WJ, Cyrille M, Hansen MB, Feagan BG, Loftus EV, Rogler G, et al. Efficacy and safety of abrilumab in subjects with moderate to severe ulcerative colitis: Results of a phase 2b, randomized, double-blind, multiple-dose, placebo-controlled study. Gastroenterology (2017) 152:S198. doi: 10.1016/S0016-5085(17)30968-X
63. Sandborn WJ, Cyrille M, Hansen MB, Feagan BG, Loftus EV, Vermeire S, et al. Efficacy and safety of abrilumab (AMG 181/MEDI 7183) therapy for moderate to severe crohn’s disease. Gastroenterology (2017) 152:S598. DOI: 10.1053/j.gastro.2018.11.035
64. Sandborn WJ, Panés J, Jones J, Hassanali A, Jacob R, Sharafali Z, et al. Etrolizumab as induction therapy in moderate to severe crohn's disease: Results from BERGAMOT cohort 1. Am J Gastroenterol (2018) 113:S3.
65. PN-943 in adults with moderate to severe active ulcerative colitis (UC). Available at: https://clinicaltrials.gov/ct2/show/NCT04504383.
66. Vermeire S, Sandborn WJ, Danese S, Hébuterne X, Salzberg BA, Klopocka M, et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet (2017) 390:135–44. doi: 10.1016/S0140-6736(17)30930-3
67. Sandborn WJ, Lee SD, Tarabar D, Louis E, Klopocka M, Klaus J, et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of crohn’s disease: report of the OPERA study. Gut (2017) 67:1824–35. doi: 10.1136/gutjnl-2016-313457
68. Yoshimura N, Watanabe M, Motoya S, Tominaga K, Matsuoka K, Iwakiri R, et al. Safety and efficacy of AJM300, an oral antagonist of α4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology (2015) 149:1775–83. doi: 10.1053/j.gastro.2015.08.044
69. Yacyshyn BR, Barish C, Goff J, Dalke D, Gaspari M, Yu R, et al. Dose ranging pharmacokinetic trial of high-dose alicaforsen (intercellular adhesion molecule-1 antisense oligodeoxynucleotide) (ISIS 2302) in active crohn's disease. Aliment Pharmacol Ther (2002) 16:1761–70. doi: 10.1046/j.1365-2036.2002.01341.x
70. Yacyshyn BR1, Bowen-Yacyshyn MB, Jewell L, Tami JA, Bennett CF, Kisner DL, et al. A placebo-controlled trial of ICAM-1 antisense oligonucleotide in the treatment of crohn's disease. Gastroenterology (1998) 114:1133–42. doi: 10.1016/S0016-5085(98)70418-4
71. Yacyshyn BR, Chey WY, Goff J, Salzberg B, Baerg R, Buchman AL, et al. Double blind, placebo controlled trial of the remission inducing and steroid sparing properties of an ICAM-1 antisense oligodeoxynucleotide, alicaforsen (ISIS 2302), in active steroid dependent crohn's disease. Gut (2002) 51:30–6. doi: 10.1136/gut.51.1.30
72. Schreiber S, Nikolaus S, Malchow H, Kruis W, Lochs H, Raedler A, et al. Absence of efficacy of subcutaneous antisense ICAM-1 treatment of chronic active crohn's disease. Gastroenterology (2001) 120:1339–46. doi: 10.1053/gast.2001.24015
73. Yacyshyn B, Chey WY, Wedel MK, Yu RZ, Paul D, Chuang E. A randomized, double-masked, placebo-controlled study of alicaforsen, an antisense inhibitor of intercellular adhesion molecule 1, for the treatment of subjects with active crohn's disease. Clin Gastroenterol Hepatol (2007) 5:215–20. doi: 10.1016/j.cgh.2006.11.001
74. Miner PB Jr, Geary RS, Matson J, Chuang E, Xia S, Baker BF, et al. Bioavailability and therapeutic activity of alicaforsen (ISIS 2302) administered as a rectal retention enema to subjects with active ulcerative colitis. Aliment Pharmacol Ther (2006) 23:1427–34. doi: 10.1111/j.1365-2036.2006.02909.x
75. Van Deventer SJ, Tami JA, Wedel MK. A randomised, controlled, double blind, escalating dose study of alicaforsen enema in active ulcerative colitis. Gut (2004) 53:1646–51. doi: 10.1136/gut.2003.036160
76. Van Deventer SJ, Wedel MK, Baker BF, Xia S, Chuang E, Miner PB Jr. A phase II dose ranging, double-blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis. Aliment Pharmacol Ther (2006) 23:1415–25. doi: 10.1111/j.1365-2036.2006.02910.x
77. Miner P, Wedel M, Bane B, Bradley J. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment Pharmacol Ther (2004) 19:281–6. doi: 10.1111/j.1365-2036.2004.01863.x
78. Mizushima T, Ito T, Kishi D, Kai Y, Tamagawa H, Nezu R, et al. Therapeutic effects of a new lymphocyte homing reagent FTY720 in interleukin-10 gene-deficient mice with colitis. Inflammation Bowel Dis (2004) 10:182–92. doi: 10.1097/00054725-200405000-00002
79. Radi ZA, Heuvelman DM, Masferrer JL, Benson EL. Pharmacologic evaluation of sulfasalazine, FTY720, and anti-IL-12/23p40 in a TNBS-induced crohn's disease model. Dig Dis Sci (2011) 56:2283–91. doi: 10.1007/s10620-011-1628-8
80. Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med (2012) 366:339–47. doi: 10.1056/NEJMct1101691
81. Berger JR, Cree BA, Greenberg B, Hemmer B, Ward BJ, Dong VM, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology (2018) 90:1815–21. doi: 10.1212/WNL.0000000000005529
82. Sandborn WJ, Feagan BG, Wolf DC, D'Haens G, Vermeire S, Hanauer SB, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med (2016) 374:1754–62. doi: 10.1056/NEJMoa1513248
83. Feagan BG, Sandborn WJ, Danese S, D'Haens G, Levesque BG, Wolf DC, et al. Endoscopic and clinical efficacy demonstrated with oral ozanimod in moderately to severely active crohn's disease. Am J Gastroenterol (2017) 112:S371.
84. Sandborn WJ, Peyrin-Biroulet L, Zhang J, Chiorean M, Vermeire S, Lee SD, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology (2020) 158:550–61. doi: 10.1053/j.gastro.2019.10.035
85. Sandborn WJ, Feagan BG, D'Haens G, Wolf DC, Jovanovic I, Hanauer SB, et al. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med (2021) 385:1280–91. doi: 10.1056/NEJMoa2033617
86. Extension study of MT-1303 in subjects with crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT02389790.
87. Dose-finding study of MT-1303 in subjects with mod-erate to severe chronic plaque psoriasis. Available at: https://clinicaltrials.gov/ct2/show/NCT01987843.
88. Imai T, Yasuda N. Therapeutic intervention of inflammatory/immune diseases by inhibition of the fractalkine (CX3CL1)-CX3CR1 pathway. Inflammation Regener (2016) 36:9. doi: 10.1186/s41232-016-0017-2
89. A study of E6011 in participants with active crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03733314.
90. Soendergaard C, Bergenheim FH, Bjerrum JT, Nielsen OH. Targeting JAK-STAT signal transduction in IBD. Pharmacol Ther (2018) 192:100–11. doi: 10.1016/j.pharmthera.2018.07.003
91. Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral janus kinase inhibitor, in active ulcerative colitis. New Engl J Med (2012) 367:616–24. doi: 10.1056/NEJMoa1112168
92. Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. New Engl J Med (2017) 376:1723–36. doi: 10.1056/NEJMoa1606910
93. Sandborn WJ, Panés J, D’Haens GR, Sands BE, Su C, Moscariello M, et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin Gastroenterol Hepatol (2019) 17:1541–50. doi: 10.1016/j.cgh.2018.11.035
94. Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. A phase 2 study of tofacitinib, an oral janus kinase inhibitor, inpatients with crohn’s disease. Clin Gastroenterol Hepatol (2014) 12:1485–93. doi: 10.1016/j.cgh.2014.01.029
95. Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D’Haens G, et al. Tofacitinib for induction and maintenance therapy of crohn’s disease: Results of two phase IIb randomised placebo-controlled trials. Gut (2017) 66:1049–59. doi: 10.1136/gutjnl-2016-312735
96. Feagan BG, Danese S, Loftus EV Jr, Vermeire S, Schreiber S, Ritter T, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet (2021) 397:2372–84. doi: 10.1016/S0140-6736(21)00666-8
97. Ghosh S, Sanchez Gonzalez Y, Zhou W, Clark R, Xie W, Louis E, et al. Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis (2021) 15:2022–30. doi: 10.1093/ecco-jcc/jjab099
98. Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet (2022) 399:2113–28. doi: 10.1016/S0140-6736(22)00581-5
99. Sands BE, Sandborn WJ, Feagan BG, Lichtenstein GR, Zhang H, Strauss R, et al. Peficitinib, an oral janus kinase inhibitor, in moderate-to-severe ulcerative colitis: Results from a randomised, phase 2 study. J Crohns Colitis (2018) 12:1158–69. doi: 10.1093/ecco-jcc/jjy085
100. A phase II study in patients with moderate to severe active crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03677648.
101. A phase II study in patients with moderate to severe active ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT03675477.
102. Study of OST-122 in patients with moderate to severe ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT04353791.
103. Chimalakonda A, Burke J, Cheng L, Strnad J, Catlett I, Patel A, et al. AB0026 selective inhibition of tyrosine kinase 2 with an oral agent, BMS-986165, compared with janus kinase inhibitors. Ann Rheum Dis (2020) 79:1316. doi: 10.1136/annrheumdis-2020-eular.4598
104. Safety and efficacy of BMS-986165 in participants with moderate to severe ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT03934216.
105. A study of the safety, efficacy, and biomarker response of BMS-986165 in participants with moderate to severe ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT04613518.
106. An investigational study of experimental medication BMS-986165 in participants with moderate to severe crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT03599622.
107. Beattie D, Tsuruda P, Shen F, Brassil P, Langrish C, Janc J, et al. TD-1473, a novel, potent, and orally administered, GI-targeted, pan-janus kinase (JAK) inhibitor. 2016 J Crohns Colitis (2016) 10:S123.1–S123. doi: 10.1093/ecco-jcc/jjw019.188
108. Sandborn WJ, Bhandari R, Leighton J, Ganeshappa R, Nguyen D, Ferslew B, et al. The gut selective, orally administered, pan-JAK inhibitor TD-1473 demonstrates favorable safety, tolerability, pharmacokinetic and signal for clinical activity in subjects with moderately to severely active ulcerative colitis. Gastroenterology (2019) 156:S29–30. doi: 10.1053/j.gastro.2019.01.093
109. Kumar N, Goldminz AM, Kim N, Gottlieb AB. Phosphodiesterase 4-targeted treatments for autoimmune diseases. BMC Med (2013) 11:96. doi: 10.1186/1741-7015-11-96
110. Wittmann M, Helliwell PS. Phosphodiesterase 4 inhibition in the treatment of psoriasis, psoriatic arthritis and other chronic inflammatory diseases. Dermatol Ther (Heidelb) (2013) 3:1–15. doi: 10.1007/s13555-013-0023-0
111. Sakkas LI, Mavropoulos A, Bogdanos DP. Phosphodiesterase 4 inhibitors in immune-mediated diseases: Mode of action, clinical applications, current and future perspectives. Curr Med Chem (2017) 24:3054–67. doi: 10.2174/0929867324666170530093902
112. Li H, Zuo J, Tang W. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol (2018) 9:1048. doi: 10.3389/fphar.2018.01048
113. Danese S, Neurath MF, Kopoń A, Zakko SF, Simmons TC, Fogel R, et al. Effects of apremilast, an oral inhibitor of phosphodiesterase 4, in a randomized trial of patients with active ulcerative colitis. Clin Gastroenterol Hepatol (2020) 18(11):2526–2534.e9. doi: 10.1016/j.cgh.2019.12.032
114. D'Haens G, Sandborn WJ, Colombel JF, Rutgeerts P, Brown K, Barkay H, et al. A phase II study of laquinimod in crohn's disease. Gut (2015) 64:1227–35. doi: 10.1136/gutjnl-2014-307118
115. Scarozza P, Schmitt H, Monteleone G, Neurath MF, Atreya R. Oligonucleotides-a novel promising therapeutic option for IBD. Front Pharmacol (2019) 10:314. doi: 10.3389/fphar.2019.00314
116. Atreya R, Kühbacher T, Schmitt H, Hirschmann S, Waldner MJ, Drvarov O, et al. DOP055 luminal application of a GATA3-specific DNAzyme ameliorates mucosal inflammation in a randomised trial with active ulcerative colitis patients. J Crohn Colitis (2018) 12:S069–70. doi: 10.1093/ecco-jcc/jjx180.092
117. Suzuki K, Yokoyama J, Kawauchi Y, Honda Y, Sato H, Aoyagi Y, et al. Phase 1 clinical study of siRNA targeting carbohydrate sulphotransferase 15 in crohn's disease patients with active mucosal lesions. J Crohn Colitis (2016) 11:221–8. doi: 10.1093/ecco-jcc/jjw143
118. Monteleone I, Biancone L, Pallone F. Phase I clinical trial of Smad7 knockdown using antisense oligonucleotide in patients with active crohn's disease. Mol Ther (2012) 20:870–6. doi: 10.1038/mt.2011.290
119. Monteleone G, Neurath MF, Ardizzone S, Di Sabatino A, Fantini MC, Castiglione F, et al. Mongersen, an oral SMAD7 antisense oligonucleotide, and crohn's disease. N Engl J Med (2015) 372:1104–13. doi: 10.1056/NEJMoa1407250
120. Feagan BG, Sands BE, Rossiter G, Li X, Usiskin K, Zhan X, et al. Effects of mongersen (GED-0301) on endoscopic and clinical outcomes in patients with active crohn's disease. Gastroenterology (2018) 154:61–4. doi: 10.1053/j.gastro.2017.08.035
121. Monteleone G, Boirivant M, Pallone F, MacDonald TT. TGF-beta1 and Smad7 in the regulation of IBD. Mucosal Immunol (2008) 1:50–3. doi: 10.1038/mi.2008.55
122. Musch E, Lutfi T, von Stein P, Zargari A, Admyre C, Malek M, et al. Topical treatment with the toll-like receptor agonist DIMS0150 has potential for lasting relief of symptoms in patients with chronic active ulcerative colitis by restoring glucocorticoid sensitivity. Inflammation Bowel Dis (2013) 19:283–92. doi: 10.1002/ibd.23019
123. Atreya R, Bloom S, Scaldaferri F, Gerardi V, Admyre C, Karlsson Å, et al. Clinical effects of a topically applied toll-like receptor 9 agonist in active moderate-to-Severe ulcerative colitis. J Crohns Colitis (2016) 10:1294–302. doi: 10.1093/ecco-jcc/jjw103
124. Atreya R, Reinisch W, Peyrin-Biroulet L, Scaldaferri F, Admyre C, Knittel T, et al. Clinical efficacy of the toll-like receptor 9 agonist cobitolimod using patient-reported-outcomes defined clinical endpoints in patients with ulcerative colitis. Dig Liver Dis (2018) 50:1019–29. doi: 10.1016/j.dld.2018.06.010
125. Dotan I, Levy-Nissenbaum E, Chowers Y, Fich A, Israeli E, Adar T, et al. Ameliorating active ulcerative colitis via an orally available toll-like receptor-9 modifier: a prospective open-label, multicenter phase II trial. Dig Dis Sci (2016) 61:3246–54. doi: 10.1007/s10620-016-4276-1
126. Sauer MM, Jakob RP, Eras J, Baday S, Eriş D, Navarra G, et al. Catch-bond mechanism of the bacterial adhesin FimH. Nat Commun (2016) 7:10738. doi: 10.1038/ncomms10738
127. TAK-018 for prevention of the recurrence of postoperative crohn's disease (CD) . Available at: https://clinicaltrials.gov/ct2/show/NCT03943446.
128. Safety and efficacy of QBECO in moderate to severe ulcerative colitis. Available at: https://clinicaltrials.gov/ct2/show/NCT02426372.
129. QBECO SSI for clinical and endoscopic remission in moderate to severe crohn's disease . Available at: https://clinicaltrials.gov/ct2/show/NCT03472690.
130. Safety and treatment effect of QBECO in moderate to severe crohn's disease. Available at: https://clinicaltrials.gov/ct2/show/NCT01809275.
131. Allez M, Tieng V, Nakazawa A, Treton X, Pacault V, Dulphy N, et al. CD4+NKG2D+ T cells in crohn's disease mediate inflammatory and cytotoxic responses through MICA interactions. Gastroenterology (2007) 132:2346–58. doi: 10.1053/j.gastro.2007.03.025
132. Safety and efficacy study of JNJ-64304500 in participants with moderately to severely active crohn's disease (TRIDENT). Available at: https://clinicaltrials.gov/ct2/show/NCT02877134.
133. A study of JNJ-64304500 as add-on therapy in participants with active crohn's disease (DUET). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04655807.
134. Habermalz B, Sauerland S. Clinical effectiveness of selective granulocyte, monocyte adsorptive apheresis with the adacolumn device in ulcerative colitis. Dig Dis Sci (2010) 55:1421–8. doi: 10.1007/s10620-009-0845-x
135. Sands BE, Sandborn WJ, Feagan B. A randomized, double-blind, sham-controlled study of granulocyte/monocyte apheresis for active ulcerative colitis. Gastroenterology (2008) 135:400–9. doi: 10.1053/j.gastro.2008.04.023
136. Sands BE, Katz S, Wolf DC. A randomised, double-blind, sham-controlled study of granulocyte/monocyte apheresis for moderate to severe crohn’s disease. Gut (2013) 62:1288–94. doi: 10.1136/gutjnl-2011-300995
137. Dignass A, Akbar A, Hart A. Safety and efficacy of Granulocyte/Monocyte apheresis in steroid-dependent active ulcerative colitis with insufficient response or intolerance to immunosuppressants and/or biologics (the ART trial): 12-week interim results. J Crohns Colitis (2016) 10:812–20. doi: 10.1093/ecco-jcc/jjw032
138. Imperiali G, Amato A, Terpin MM. Granulocyte-monocyte apheresis in steroid-dependent, azathioprine-Intolerant/Resistant moderate ulcerative colitis: A prospective multicenter study. Gastroenterol Res Pract (2017) 2017:1–5. doi: 10.1155/2017/9728324
139. Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens (2019) 8:126. doi: 10.3390/pathogens8030126
140. Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol (2013) 108:500–8. doi: 10.1038/ajg.2013.59
141. Borody TJ, Khoruts A. Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol (2011) 9:88–96. doi: 10.1038/nrgastro.2011.244
142. Costello SP, Soo W, Bryant RV, Jairath V, Hart AL, Andrews JM. Systematic review with meta-analysis: faecal microbiota transplantation for the induction of remission for active ulcerative colitis. Aliment Pharmacol Ther (2017) 46:213–24. doi: 10.1111/apt.14173
143. Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA (2019) 321:156–64. doi: 10.1001/jama.2018.20046
144. Wei Y, Gong J, Zhu W, Tian H, Ding C, Gu L, et al. Pectin enhances the effect of fecal microbiota transplantation in ulcerative colitis by delaying the loss of diversity of gut flora. BMC Microbiol (2016) 16:255. doi: 10.1186/s12866-016-0869-2
145. Damman CJ, Brittnacher MJ, Westerhoff M, Hayden HS, Radey M, Hager KR, et al. Low level engraftment and improvement following a single colonoscopic administration of fecal microbiota to patients with ulcerative colitis. PloS One (2015) 10:e0133925. doi: 10.1371/journal.pone.0133925
146. Vermeire S, Joossens M, Verbeke K, Wang J, Machiels K, Sabino J, et al. Donor species richness determines faecal microbiota transplantation success in inflammatory bowel disease. J Crohns Colitis (2016) 10:387–94. doi: 10.1093/ecco-jcc/jjv203
147. Haifer C, Luu LDW, Paramsothy S, Borody TJ, Leong RW, Kaakoush NO. Microbial determinants of effective donors in faecal microbiota transplantation for UC. Gut (2022) 327742. doi: 10.1136/gutjnl-2022-327742
148. Scaldaferri F, Pecere S, Petito V, Zambrano D, Fiore L, Lopetuso LR, et al. Efficacy and mechanisms of action of fecal microbiota transplantation in ulcerative colitis: Pitfalls and promises from a first meta-analysis. Transplant Proc (2016) 48:402–7. doi: 10.1016/j.transproceed.2015.12.040
149. López-García A, Rovira M, Jauregui-Amezaga A. Autologous haematopoietic stem cell transplantation for refractory crohn’s disease: Efficacy in a single-centre cohort. J Crohns Colitis (2017) 11:1161–8. doi: 10.1093/ecco-jcc/jjx054
150. Jauregui-Amezaga A, Rovira M, Marín P. Improving safety of autologous haematopoietic stem cell transplantation in patients with crohn’s disease. Gut (2016) 65:1456–62. doi: 10.1136/gutjnl-2015-309836
151. Shi Y, Wang Y, Li Q. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol (2018) 14:493–507. doi: 10.1038/s41581-018-0023-5
152. Gao WB. Effects of bone marrow-derived FLK1+CD31-CD34- mesenchymal stem cells on TNBS-induced ulcerative colitis in mice. Beijing Union Med Coll (2006).
153. Panés J, García-Olmo D, Van Assche G. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet (2016) 388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X
154. Berretta M, Della Pepa C, Tralongo P, Fulvi A, Martellotta F, Lleshi A, et al. Use of complementary and alternative medicine (CAM) in cancer patients: An Italian multicenter survey. Oncotarget (2017) 8:24401–14. doi: 10.18632/oncotarget.14224
155. Durchschein F, Petritsch W, Hammer HF. Diet therapy for inflammatory bowel diseases: The established and the new. World J Gastroenterol (2016) 22:2179–94. doi: 10.3748/wjg.v22.i7.2179
156. Liu X, Wu Y, Li F, Zhang D. Dietary fiber intake reduces risk of inflammatory bowel disease: Result from a meta-analysis. Nutr Res (2015) 35:753–8. doi: 10.1016/j.nutres.2015.05.021
157. Nie Y, Lin Q, Luo F. Effects of non-starch polysaccharides on inflammatory bowel disease. Int J Mol Sci (2017) 18:E1372. doi: 10.3390/ijms18071372
158. Suwannaporn P, Thepwong K, Tester R, Al-Ghazzewi F, Piggott J, Shen N, et al. Tolerance and nutritional therapy of dietary fibre from konjac glucomannan hydrolysates for patients with inflammatory bowel disease (IBD). Bioact Carbohydr Diet Fiber (2013) 2:93–8. doi: 10.1016/j.bcdf.2013.09.005
159. Burgis JC, Nguyen K, Park KT. Response to strict and liberalized specific carbohydrate diet in pediatric crohn’s disease. World J Gastroenterol (2016) 22:2111–7. doi: 10.3748/wjg.v22.i6.2111
160. Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch B, et al. Clinical and mucosal improvement with specific carbohydrate diet in pediatric crohn disease. J Pediatr Gastroenterol Nutr (2014) 59:516–21. doi: 10.1097/MPG.0000000000000449
161. Suskind DL, Wahbeh G, Gregory N. Nutritional therapy in pediatric crohn disease: the specific carbohydrate diet. J Pediatr Gastroenterol Nutr (2014) 58:87–91. doi: 10.1097/MPG.0000000000000103
162. Prince AC. Fermentable carbohydrate restriction (Low FODMAP diet) in clinical practice improves functional gastrointestinal symptoms in patients with inflammatory bowel disease. Inflammation Bowel Dis (2016) 22:1129–36. doi: 10.1097/MIB.0000000000000708
163. Uranga JA, López-Miranda V, Lombó F, Abalo R. Food, nutrients and nutraceuticals affecting the course of inflammatory bowel disease. Pharmacol Rep (2016) 68:816–26. doi: 10.1016/j.pharep.2016.05.002
164. Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GAD. The association of disease activity, BMI and phase angle with vitamin d deficiency in patients with IBD. Nutrients (2019) 11:2583. doi: 10.3390/nu11112583
165. Taylor L, Almutairdi A, Shommu N, Fedorak R, Ghosh S, Reimer RA, et al. Cross-sectional analysis of overall dietary intake and Mediterranean dietary pattern in patients with crohn’s disease. Nutrients (2018) 10:1761. doi: 10.3390/nu10111761
166. Chen SW, Wang PY, Zhu J, Chen GW, Zhang JL, Chen ZY, et al. Protective effect of 1,25-dihydroxyvitamin d3 on lipopolysaccharide-induced intestinal epithelial tight junction injury in caco-2 cell monolayers. Inflammation (2015) 38:375–83. doi: 10.1007/s10753-014-0041-9
167. Meeker S, Seamons A, Paik J, Treuting PM, Brabb T, Grady WM. Increased dietary vitamin d suppresses MAPK signaling, colitis, and colon cancer. Cancer Res (2014) 74:4398–408. doi: 10.1158/0008-5472.CAN-13-2820
168. Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: A randomized, double-blind, placebo-controlled pilot study. Arch Med Res (2015) 46:280–5. doi: 10.1016/j.arcmed.2015.05.005
169. Berretta M, Bignucolo A, Di Francia R, Comello F, Facchini G, Ceccarelli M, et al. Resveratrol in cancer patients: From bench to bedside. Int J Mol Sci (2020) 21:2945. doi: 10.3390/ijms21082945
170. Joo M, Kim HS, Kwon TH, Palikhe A, Zaw TS. Anti-inflammatory effects of flavonoids on TNBS-induced colitis of rats. Korean J Physiol Pharmacol (2015) 19:43–50. doi: 10.4196/kjpp.2015.19.1.43
171. Capitán-Cañadas F, Ocón B, Aranda CJ, Anzola A, Suárez MD, Zarzuelo A, et al. Fructooligosaccharides exert intestinal anti-inflammatory activity in the CD4+ CD62L+ T cell transfer model of colitis in C57BL/6J mice. Eur J Nutr (2016) 55:1445–54. doi: 10.1007/s00394-015-0962-6
172. Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology (2001) 121:580–91. doi: 10.1053/gast.2001.27224
173. Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflammation Bowel Dis (2014) 20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be
174. Schultz M. Clinical use of e. coli nissle 1917 in inflammatory bowel disease. Inflammation Bowel Dis (2008) 14:1012–8. doi: 10.1002/ibd.20377
175. Scaldaferri F, Gerardi V, Mangiola F, Lopetuso LR, Pizzoferrato M, Petito V, et al. Role and mechanisms of action of escherichia coli nissle 1917 in the maintenance of remission in ulcerative colitis patients: An update. World J Gastroenterol (2016) 22:5505–11. doi: 10.3748/wjg.v22.i24.5505
176. Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, et al. Efficacy of lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther (2006) 23:1567–74. doi: 10.1111/j.1365-2036.2006.02927.x
177. Preidis GA, Weizman AV, Kashyap PC, Morgan RL. AGA technical review on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology (2020) 159:708–738.e4. doi: 10.1053/j.gastro.2020.05.060
178. Lin Z, Wu H, Fu Y, Dai S. Application of herbaceous medications for inflammatory bowel disease as a complementary and alternative therapy. Inflammation Bowel Dis (2019) 25:1886–95. doi: 10.1093/ibd/izz190
179. Jalili C, Taghadosi M, Pazhouhi M, Bahrehmand F, Miraghaee SS, Pourmand D, et al. An overview of therapeutic potentials of taraxacum officinale (dandelion): a traditionally valuable herb with a reach historical background. WCRJ (2020) 7:e1679. doi: 10.32113/wcrj_20209_1679
180. Holleran G, Scaldaferri F, Gasbarrini A, Currò D. Herbal medicinal products for inflammatory bowel disease: A focus on those assessed in double-blind randomised controlled trials. Phytother Res (2020) 34:77–93. doi: 10.1002/ptr.6517
181. Wang N, Wang G, Hao J, Ma J, Wang Y, Jiang X, et al. Curcumin ameliorates hydrogen peroxide-induced epithelial barrier disruption by upregulating heme oxygenase-1 expression in human intestinal epithelial cells. Dig Dis Sci (2012) 57:1792–801. doi: 10.1007/s10620-012-2094-7
182. Singla V, Pratap Mouli V, Garg SK, Rai T, Choudhury BN, Verma P, et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study. J Crohns Colitis (2014) 8:208–14. doi: 10.1016/j.crohns.2013.08.006
183. Friedman D, Sirven JI. Historical perspective on the medical use of cannabis for epilepsy: ancient times to the 1980s. Epilepsy Behav (2017) 70:298–301. doi: 10.1016/j.yebeh.2016.11.033
184. Ligresti A, De Petrocellis L, Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev (2016) 96:1593–659. doi: 10.1152/physrev.00002.2016
185. Hasenoehrl C, Storr M, Schicho R. Cannabinoids for treating inflammatory bowel diseases: where are we and where do we go? Expert Rev Gastroenterol Hepatol (2017) 11:329–37. doi: 10.1080/17474124.2017.1292851
186. Naftali T, Mechulam R, Lev LB, Konikoff FM. Cannabis for inflammatory bowel disease. Dig Dis (2014) 32:468–74. doi: 10.1159/000358155
187. Doeve B, van Schaik F, van de Meeberg M. P448 cannabis and cannabinoids for the treatment of inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis (2019) 13:335–6. doi: 10.1093/ecco-jcc/jjy222.572
188. Leinwand KL, Gerich ME, Hoffenberg EJ, Collins CB. Manipulation of the endocannabinoid system in colitis: a comprehensive review. Inflammation Bowel Dis (2017) 23:192–9. doi: 10.1097/MIB.0000000000001004
189. Ahmed W, Katz S. Therapeutic use of cannabis in inflammatory bowel disease. Gastroenterol Hepatol (2016) 12:668–79.
190. Cocetta V, Governa P, Borgonetti V, Tinazzi M, Peron G, Catanzaro D, et al. Cannabidiol isolated from cannabis sativa l. protects intestinal barrier from In vitro inflammation and oxidative stress. Front Pharmacol (2021) 12:641210. doi: 10.3389/fphar.2021.641210
191. Grimstad T, Bjørndal B, Cacabelos D, Aasprong OG, Omdal R, Svardal A, et al. A salmon peptide diet alleviates experimental colitis as compared with fish oil. J Nutr Sci (2013) 2:e2. doi: 10.1017/jns.2012.23
192. Costantini L, Molinari R, Farinon B, Merendino N. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci (2017) 18:18. doi: 10.3390/ijms18122645
193. González-Sarrías A, Larrosa M, Tomás-Barberán FA, Dolara P, Espín JC. NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr (2010) 104:503–12. doi: 10.1017/S0007114510000826
194. Colombo E, Sangiovanni E, Dell’agli M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid Based Complement Alternat Med (2013) 2013:247145. doi: 10.1155/2013/247145
195. Tan WSD, Liao W, Zhou S, Wong WSF. Is there a future for andrographolide to be an anti-inflammatory drug? deciphering its major mechanisms of action. Biochem Pharmacol (2017) 139:71–81. doi: 10.1016/j.bcp.2017.03.024
196. Mohammadi M, Hayatbakhsh MM, Zahedi MJ, Jalalpour MR, Pakgohar A. Serum interleukin-23 levels in patients with ulcerative colitis. Iran J Immunol (2011) 8:183–8.
197. Sandborn WJ, Targan SR, Byers VS, Rutty DA, Mu H, Zhang X, et al. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am J Gastroenterol (2013) 108:90–8. doi: 10.1038/ajg.2012.340
198. Zhu Q, Zheng P, Chen X, Zhou F, He Q, Yang Y. Andrographolide presents therapeutic effect on ulcerative colitis through the inhibition of IL-23/IL-17 axis. Am J Transl Res (2018) 10:465–73.
199. Naganuma M, Sugimoto S, Mitsuyama K, Kobayashi T, Yoshimura N, Ohi H, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology (2018) 154:935–47. doi: 10.1053/j.gastro.2017.11.024
200. Sugimoto S, Naganuma M, Kiyohara H, Arai M, Ono K, Mori K, et al. Clinical efficacy and safety of oral Qing-dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion (2016) 93:193–201. doi: 10.1159/000444217
201. Wang Y, Liu L, Guo Y, Mao T, Shi R, Li J. Effects of indigo naturalis on colonic mucosal injuries and inflammation in rats with dextran sodium sulphate induced ulcerative colitis. Exp Ther Med (2017) 14:1327–36. doi: 10.3892/etm.2017.4701
202. Su J, Li C, Yu X, Yang G, Deng J, Su Z, et al. Protective effect of pogostone on 2,4,6-trinitrobenzenesulfonic acid-induced experimental colitis via inhibition of T helper cell. Front Pharmacol (2017) 8:829. doi: 10.3389/fphar.2017.00829
203. Stein DJ. Massage acupuncture, moxibustion, and other forms of complementary and alternative medicine in inflammatory bowel disease. Gastroenterol Clin North Am (2017) 46:875–80. doi: 10.1016/j.gtc.2017.08.015
204. Zhang D, Ren YB, Wu HG. Effect of different doses of herbal cakepartitioned moxibustion on histopathological changes of colon tissue in ulcerative colitis rats (in Chinese). Zhen Ci Yan Jiu (2018) 43:68–74. doi: 10.13702/j.1000-0607170843
205. Ji J, Lu Y, Liu H, Feng H, Zhang F, Wu L, et al. Acupuncture and moxibustion for inflammatory bowel diseases: A systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med (2013) 2013:158352. doi: 10.1155/2013/158352
206. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun (2015) 48:186–94. doi: 10.1016/j.bbi.2015.03.016
207. Ancona A, Petito C, Iavarone I, Petito V, Galasso L, Leonetti A, et al. The gut-brain axis in irritable bowel syndrome and inflammatory bowel disease. Dig Liver Dis (2021) 53:298–305. doi: 10.1016/j.dld.2020.11.026
208. Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res (2016) 82:109–18. doi: 10.1016/j.jpsychires.2016.07.019
209. Serpico M, Boyle B, Kemper KJ, Kim S. Complementary and alternative medicine use in children with inflammatory bowel diseases: A single center survey. J Pediatr Gastroenterol Nutr (2016) 63:651–7. doi: 10.1097/MPG.0000000000001187
210. Mawdsley JE, Jenkins DG, Macey MG. The effect of hypnosis on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Am J Gastroenterol (2008) 103:1460–9. doi: 10.1111/j.1572-0241.2008.01845.x
211. Neilson K, Ftanou M, Monshat K, Salzberg M, Bell S, Kamm MA, et al. A controlled study of a group mindfulness intervention for individuals living with inflammatory bowel disease. Inflammation Bowel Dis (2016) 22:694–701. doi: 10.1097/MIB.0000000000000629
212. Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S, et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome (2016) 4:42. doi: 10.1186/s40168-016-0189-7
213. Bonaz BL, Bernstein CN. Brain-gut interactions in inflammatory bowel disease. Gastroenterology (2013) 144:36–49. doi: 10.1053/j.gastro.2012.10.003
214. Cramer H, Schäfer M, Schöls M, Köcke J, Elsenbruch S, Lauche R, et al. Randomised clinical trial: Yoga vs written self-care advice for ulcerative colitis. Aliment Pharmacol Ther (2017) 45:1379–89. doi: 10.1111/apt.14062
215. Klare P, Nigg J, Nold J, Haller B, Krug AB, Mair S, et al. The impact of a ten-week physical exercise program on healthrelated quality of life in patients with inflammatory bowel disease: A prospective randomized controlled trial. Digestion (2015) 91:239–47. doi: 10.1159/000371795
216. Jones PD, Kappelman MD, Martin CF, Chen W, Sandler RS, Long MD. Exercise decreases risk of future active disease in patients with inflammatory bowel disease in remission. Inflammation Bowel Dis (2015) 21:1063–71. doi: 10.1097/MIB.0000000000000333
217. Pedersen BK, Saltin B. Exercise as medicine- evidence for prescribing exercise as therapy in 26 different chronic diseases. Scan J Med Sci Sports (2015) 3:1–72. doi: 10.1111/sms.12581
218. Gerbarg PL, Jacob VE, Stevens L, Bosworth BP, Chabouni F, DeFilippis EM, et al. The effect of breathing, movement, and meditation on psychological and physical symptoms and inflammatory biomarkers in inflammatory bowel disease: a randomized controlled trial. Inflammation Bowel Dis (2015) 21:2886–96. doi: 10.1097/MIB.0000000000000568
219. Elsenbruch S, Langhorst J, Popkirowa K, Müller T, Luedtke R, Franken U, et al. Effects of mind-body therapy on quality of life and neuroendocrine and cellular immune functions in patients with ulcerative colitis. Psychother Psychosom (2005) 74:277–87. doi: 10.1159/000086318
220. Hung A, Kang N, Bollom A, Wolf JL, Lembo A. Complementary and alternative medicine use is prevalent among patients with gastrointestinal diseases. Dig Dis Sci (2015) 60:1883–8. doi: 10.1007/s10620-014-3498-3
221. Mountifield R. Doctor communication quality and friends’ attitudes influence complementary medicine use in inflammatory bowel disease. World J Gastroenterol (2015) 21:3663–70. doi: 10.3748/wjg.v21.i12.3663
222. Yoon SL, Grundmann O, Smith KF, Mason SR. Dietary supplement and complementary and alternative medicine use are highly prevalent in patients with gastrointestinal disorders: results from an online survey. J Diet Suppl (2019) 16:635–48. doi: 10.1080/19390211.2018.1472712
223. Hilsden RJ, Verhoef MJ, Best A, Pocobelli G. Complementary and alternative medicine use by Canadian patients with inflammatory bowel disease: Results from a national survey. Am J Gastroenterol (2003) 98:1563–8. doi: 10.1111/j.1572-0241.2003.07519.x
Keywords: crohn’s disease, ulcerative colitis, innovative therapies, complementary and alternative medicine, small molecules, biologic drugs
Citation: Masi L, Ciuffini C, Petito V, Pisani LF, Lopetuso LR, Graziani C, Pugliese D, Laterza L, Puca P, Di Vincenzo F, Pizzoferrato M, Napolitano D, Turchini L, Amatucci V, Schiavoni E, Privitera G, Minordi LM, Mentella MC, Papa A, Armuzzi A, Gasbarrini A and Scaldaferri F (2022) Innovative, complementary and alternative therapy in inflammatory bowel diseases: A broad 2020s update. Front. Gastroenterol. 1:1022530. doi: 10.3389/fgstr.2022.1022530
Received: 18 August 2022; Accepted: 27 September 2022;
Published: 17 October 2022.
Edited by:
Thomas Klag, University of Tübingen, GermanyReviewed by:
Wolfgang Reindl, University Medical Centre Mannheim, University of Heidelberg, GermanyEndrit Shahini, National Institute of Gastroenterology S. de Bellis Research Hospital (IRCCS), Italy
Copyright © 2022 Masi, Ciuffini, Petito, Pisani, Lopetuso, Graziani, Pugliese, Laterza, Puca, Di Vincenzo, Pizzoferrato, Napolitano, Turchini, Amatucci, Schiavoni, Privitera, Minordi, Mentella, Papa, Armuzzi, Gasbarrini and Scaldaferri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Petito, dmFsZW50aW5hLnBldGl0b0Bwb2xpY2xpbmljb2dlbWVsbGkuaXQ=
†These authors have contributed equally to this work and share first authorship
 Letizia Masi1†
Letizia Masi1† Valentina Petito
Valentina Petito Laura Francesca Pisani
Laura Francesca Pisani Cristina Graziani
Cristina Graziani Pierluigi Puca
Pierluigi Puca Federica Di Vincenzo
Federica Di Vincenzo Laura Turchini
Laura Turchini Alfredo Papa
Alfredo Papa Antonio Gasbarrini
Antonio Gasbarrini