- 1Department of Biology, Grand Valley State University, Allendale, MI, United States
- 2Mary Griggs Burke Center for Freshwater Innovation, Northland College, Ashland, WI, United States
- 3Wisconsin Department of Natural Resources, Madison, WI, United States
Introduction: Identifying patterns in the primary limiting nutrients of basal trophic levels such as benthic algae can inform the prediction of potential ecological responses to anthropogenic nutrient loading. In coastal wetlands of the Laurentian Great Lakes, reduced concentrations of reactive nitrogen species such as ammonium and nitrate may limit algal growth, especially when nutrient loading is minimal. However, the response of benthic algae to macronutrient inputs remains understudied, especially in Lake Superior coastal wetlands.
Methods: We conducted nutrient amendment assays using nutrient diffusing substrate devices in 25 coastal wetlands along the southwestern shore of Lake Superior in the spring, summer, and fall. These assays allowed us to investigate seasonal and regional variation in nutrient limitation status and the relationship between nutrient limitation, in situ water quality (dissolved and total nitrogen and phosphorus, water temperature, dissolved oxygen, specific conductivity, and total suspended solids), and watershed land use.
Results: We found that nitrogen limitation was common, particularly during summer, with 60% of wetlands exhibiting this condition, while phosphorus limitation was not observed in any wetland during any season. The strongest N limitation was found in wetlands of the Apostle Islands National Lakeshore where watershed land cover was almost entirely natural. Wetlands with more developed watersheds, including those of the St. Louis River Estuary, had a lower degree of N limitation (p = 0.003). Nitrogen limitation was observed in spring, summer, and fall, but was most pronounced in the summer.
Discussion: These findings suggest that N limitation predominates in these Lake Superior coastal wetlands, contrasting with the well-documented phosphorus limitation of the lake's pelagic zone. Our study also highlights the potential for anthropogenic N loading to stimulate excessive benthic algal growth in Lake Superior coastal wetlands, particularly in more developed regions. These findings are consistent with those for coastal wetlands in other regions of the Great Lakes and support the need for continued monitoring and targeted mitigation of both nitrogen and phosphorus loading to shoreline habitats of large lakes.
Introduction
The response of primary producers to nutrient availability is a fundamental aspect of aquatic ecosystem function. The rate at which organic matter is produced is often regulated by the availability of a particular macronutrient such as nitrogen (N), phosphorus (P), or some other critical component of growth such as light or space (Vadeboncoeur et al., 2008; Harpole et al., 2011). Identifying key limiting resources for specific functions such as primary production can, therefore, enhance our understanding of ecological responses to nutrient loading from surrounding watersheds. Anthropogenic nutrient loading that results from altered watershed land use or point sources of pollution has become the primary cause of eutrophication and ecosystem degradation in coastal marine and freshwater ecosystems globally (Paerl et al., 2014; Le Moal et al., 2019; Malone and Newton, 2020). Like other freshwater and marine systems, wetlands are also subject to deleterious effects of anthropogenic nutrient loading, which can include loss of habitat area (Deegan et al., 2012), altered floral and faunal communities (Martínez-Megías and Rico, 2022), and altered biogeochemical cycling (Chen et al., 2020).
Phosphorus commonly limits phytoplankton growth in freshwater ecosystems (Schindler, 1977; Guildford and Hecky, 2000) and in the Laurentian Great Lakes, primary productivity in the pelagic zone is most often limited by P availability (Lin and Schelske, 1981). The pelagic zone of Lake Superior is particularly P-limited (Sterner et al., 2004). Accordingly, a steadily increasing concentration of nitrate in the water column of Lake Superior since the early 20th Century suggests that excess reactive N remains unused in the system due to P limitation of phytoplankton growth (Sterner, 2011; Dove and Chapra, 2015). In contrast, growth of shallow nearshore primary producers, especially benthic algae, has been shown to be limited by N availability in Lake Michigan, Lake Huron (Cooper et al., 2016), and other coastal wetlands throughout the Great Lakes (Hill et al., 2006; Eberhard et al., 2023). Thus, the different habitat types within these large lakes appear to respond in markedly different ways to nutrient inputs.
Coastal wetlands throughout the Great Lakes provide critical habitat for numerous plant and animal species (Sierszen et al., 2012) and play a vital role in nearshore nutrient cycling (Bellinger et al., 2014; Eberhard et al., 2023). These wetlands are often the first habitats to receive nutrient-laden runoff from the surrounding landscape and nutrient loading can reduce coastal wetland capacity to provide these beneficial functions (Sierszen et al., 2006; Schock et al., 2014). Nearly half of the coastal wetland area in the Great Lakes has been converted to other uses and many of the remaining wetlands have been altered (Sierszen et al., 2012). Since 2011, a basin-wide monitoring effort has been underway to quantify the overall ecological status of coastal wetlands in the Laurentian Great Lakes using standardized, quantitative methods (Uzarski et al., 2017). This program has shown that coastal wetlands in Lake Superior are among the highest quality in the Great Lakes, particularly in terms of water quality (Harrison et al., 2020), fish communities (Sierszen et al., 2012), and vegetation (Dybiec et al., 2020).
The relatively high ecological integrity of Lake Superior coastal wetlands compared to other regions in the basin is not surprising given the less altered watershed land use and cover of Lake Superior wetlands (Harrison et al., 2020). Others have reported lower watershed nutrient loading to Lake Superior compared to the other Laurentian Great Lakes (Dove and Chapra, 2015). We expect that nutrient loading is especially low for wetlands occurring on islands in Lake Superior, especially those protected from development such as the Apostle Islands in the southwest of the lake, which are part of the Apostle Islands National Lakeshore (hereafter, AINL). Understanding the response of benthic algae to nutrient loading in coastal wetlands of Lake Superior, including those occurring on remote islands as well as those associated with more manipulated watersheds, could provide valuable insights on potential impacts of anthropogenic nutrient loading to freshwater coastal wetlands.
To elucidate patterns in nutrient limitation in coastal wetlands of southwestern Lake Superior, we conducted nutrient amendment experiments using nutrient diffusing substrate (NDS) devices in the spring, summer, and fall of 2016 and the summer of 2017. Our primary hypothesis was that N limitation would be more common than P limitation given prior work on Lakes Michigan and Huron, which showed N limitation was greatest in coastal wetlands occurring in areas with more forested land cover and lower nutrient loads (Cooper et al., 2016). We also predicted that N limitation would be greater in summer than spring or fall due to greater nutrient uptake by primary producers and higher rates of denitrification in summer (Bellinger et al., 2014). Lastly, we predicted that N limitation would be greatest in coastal wetlands of AINL due to the natural land cover and presumably low watershed nutrient loading in these systems while limitation would be lowest in the wetlands of the St. Louis River Estuary (SLRE) due to the greater proportion of developed and agricultural land cover in that region.
Methods
We assessed nutrient limitation by measuring chlorophyll a accrual on NDS devices at 25 coastal wetlands of southwest Lake Superior (Figure 1). The NDS devices allowed us to experimentally manipulate the N and P available to biofilms growing on the artificial substrates (Tank and Dodds, 2003). The 25 sites included most of the major coastal wetlands located along the Wisconsin shoreline, including those located on islands within AINL. In 2016 we focused on seasonal sampling (spring, summer, and fall) at eight wetlands on the Bayfield Peninsula (BP) and in 2017 we focused on the summer season only and included all 25 wetlands (Figure 1).
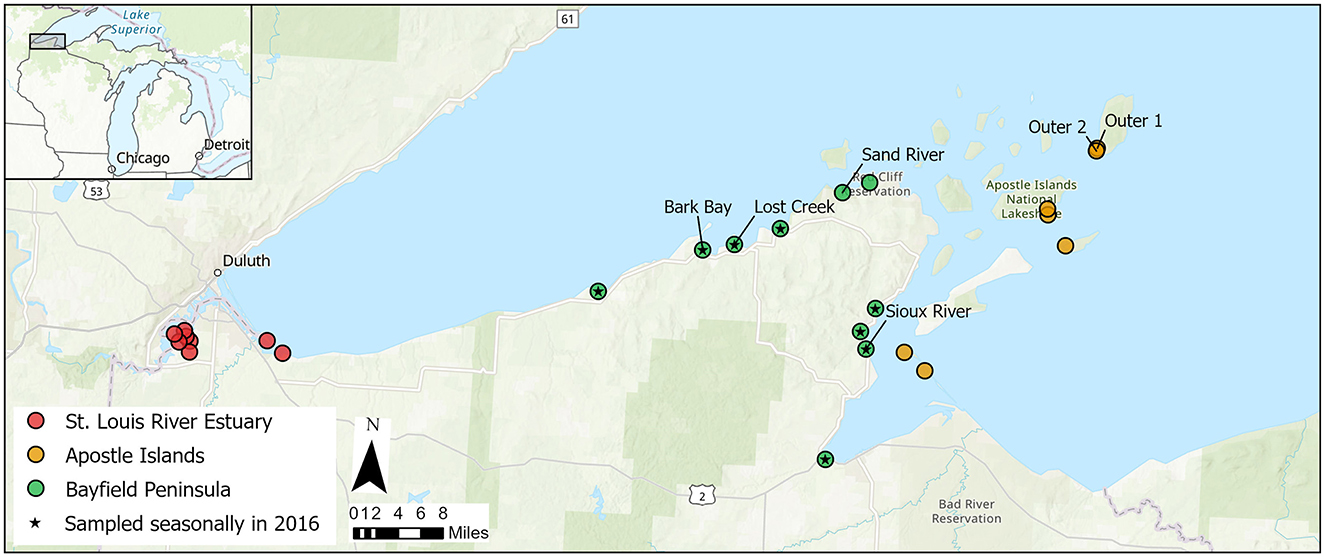
Figure 1. Coastal wetland study locations in southwestern Lake Superior. Eight wetlands on the Bayfield Peninsula were sampled in the spring, summer, and fall of 2016. These eight wetlands along with two additional Bayfield Peninsula wetlands, eight St. Louis River Estuary, and seven Apostle Islands wetlands were sampled during the summer of 2017.
Nutrient diffusing substrate devices were constructed using 60-mL plastic cups filled with a 2% agar solution, amended with either 0.5 M NaNO3 (N treatment), 0.1 M KH2PO4 (P treatment), both (N+P treatment), or no amendment (control) as described by Tank et al. (2017) and Cooper et al. (2016). Each NDS cup was topped with a 2.2 cm diameter permeable glass disk (Leco Corporation, St. Joseph, Michigan). The permeable glass disks provided a substrate for algal biofilm growth that allowed dissolved nutrients to permeate from the agar (Tank et al., 2017). We deployed five replicates of each treatment for each assay at every wetland (20 NDS cups per assay at each wetland). The NDS cups were attached to steel L-bars and staked to the sediment surface in each wetland at a location with representative vegetation and approximately 30 cm of water depth. Each NDS assay lasted 28 days. Upon retrieval, glass disks were separated from the agar, individually wrapped in aluminum foil, transported on ice to the laboratory, and frozen until analysis. Chlorophyll a accumulation was used as the metric for biomass accrual (Tank and Dodds, 2003; Johnson et al., 2009; Cooper et al., 2016).
To extract chlorophyll a, we placed each disk in 20 mL of 95% ethanol for 24 h at room temperature in the dark. We measured extracted chlorophyll a with a non-acidification method using a fluorometer (Turner Designs model 700, Sunnyvale, California) set to avoid interference from phaeophytin (Welschmeyer, 1994). This nutrient-amendment technique integrated conditions over the 28-day deployment, so it is robust to short-term fluctuations in ambient concentrations.
We measured water-column nutrients and other abiotic variables at each wetland prior to NDS deployment and again upon retrieval in the immediate vicinity of where NDS arrays were set. Water temperature, dissolved oxygen (DO), pH, and specific conductance (SpC) were measured at mid-water column depth using a Yellow Springs Instrument Professional Plus multiparameter sonde (YSI Environmental, Yellow Springs, Ohio). Water samples were collected at approximately mid-water column depth near the NDS arrays. We immediately filtered samples (100 mL) through 0.45-μm membrane filters for measuring soluble reactive P (SRP) and NO3-N. These samples were frozen prior to analysis. Samples for NH4-N were acidified with hydrochloric acid and stored at 4°C. Samples for total N (TN) and total P (TP) analysis were not filtered but were frozen. Soluble reactive phosphorus was measured using the molybdate-blue method (APHA, 2005), while TP was measured as SRP after persulfate digestion (USGS, 2003). NO3-N was analyzed by ion chromatography (APHA, 2005) and NH4-N by the phenol–hypochlorite method (Solórzano, 1969; APHA, 2005). All nutrient analyses were conducted by the Wisconsin State Lab of Hygiene at the University of Wisconsin (Madison, Wisconsin). Dissolved inorganic nitrogen (DIN) was approximated as the sum of NO3-N and NH4-N. Total nitrogen was measured as NO3-N after persulfate digestion (USGS, 2003). We averaged the water quality data from NDS deployment and retrieval to represent conditions over the deployment period. Nutrient concentrations below analytical detection limits (DLs) were assigned a value of one-half DL for statistical analyses. Detection limits were based on APHA (2005) methods and were consistent with those found in the literature (2 μg/L for SRP, 5 μg/L for NH4-N, and 10 μg/L for TP, NO3-N, and TN).
Contributing watersheds for each of the 25 sampling locations were delineated using the PRESTO tool (Diebel et al., 2013). To characterize land cover within each contributing watershed, we utilized Wiscland 2 data (Wisconsin Department of Natural Resources, 2016). Wiscland 2 is a 30-meter resolution raster dataset representing land cover across Wisconsin, derived primarily from remote sensing imagery captured by Landsat 5, 7, and 8 satellites between 2010 and 2014. The overall accuracy of the Wiscland classification is estimated at 93% across all classes. In our analysis, we focused on the Level 1 classes “urban/developed” and “agriculture” to estimate anthropogenic land use within each watershed.
Calculations and analyses
We determined nutrient limitation status (i.e., N, P, N+P co-limitation, or no limitation) with 2-way factorial analyses of variance (ANOVAs) for each NDS assay. For ANOVAs, N and P amendment were main factors, and significant interaction terms indicated possible co-limitation (Tank and Dodds, 2003; Cooper et al., 2016). When a significant main effect or interaction was found, the response of chlorophyll a to the nutrient treatment(s) was analyzed graphically (Figure 2) and post hoc Tukey tests were conducted to confirm the response. Shapiro–Wilks tests for normality were conducted with each ANOVA and chlorophyll a data were log(x)-transformed for ~25% of nutrient-limitation ANOVAs based on significant (p < 0.05) Shapiro-Wilks tests. The N Response Ratio (NRR) was used as a measure of the magnitude of N limitation and was calculated for each assay as the log of the ratio of mean chlorophyll a on N-amended substrates to mean chlorophyll a on control substrates (Tank and Dodds, 2003; Tank et al., 2017). Thus, a NRR greater than zero indicated a positive response of algal chlorophyll a to nitrogen amendment.
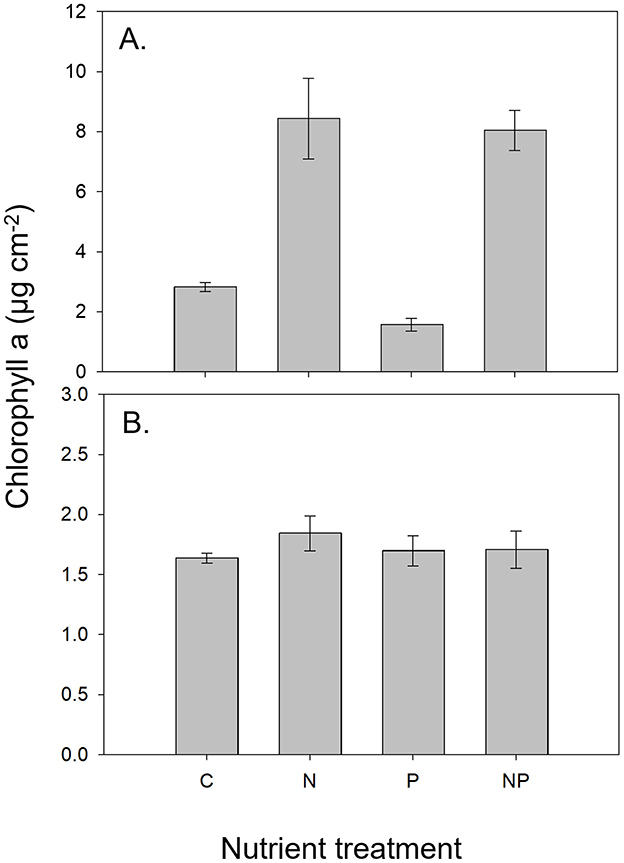
Figure 2. Representative chlorophyll a responses to nutrient treatments. (A) (Michigan Island, summer 2016) shows a strong response to nitrogen amendment (Nitrogen Response Ratio: 0.475). (B) (Onion River, spring 2016) shows no response to nutrient amendments (Nitrogen Response Ratio: 0.051).
Using both nutrient limitation status and NRR allowed us to compare nutrient limitation across seasons and regions and to relate benthic chlorophyll a response to nutrient amendments to predictor variables such as water-column nutrient concentrations and watershed land cover. We compared NRR among seasons in 2016 using a general linear model for repeated measures with Type III sum of squares and post-hoc Tukey tests. We compared NRR among regions (AINL, BP, and SLRE) in 2017 using 1-way ANOVA with post-hoc Tukey tests. We calculated Pearson correlation coefficients between NRR, water-column nutrients, and land cover variables for the 25 wetlands sampled in the summer of 2017. We also combined the full suite of 11 water quality and land cover variables in a principal components analysis (PCA) and interpreted the first 2 principal components (PCs) by identifying variables that loaded most heavily on each PC. We compared NRR to PC scores using Pearson correlations to identify potential relationships between nutrient limitation and overall wetland condition as represented by the PCs. We calculated molar ratios of DIN to TP as another proxy for potential nutrient limitation. This ratio has been found to be a reliable indicator of nutrient limitation in lakes (Bergström, 2010; Ptacnik et al., 2010). We used R (version 4.3; R Project for Statistical Computing, Vienna, Austria) for all statistical analyses.
Results
Of all nutrients, nitrogen limitation was most commonly observed, especially during the summer. During the summer of 2016, six of the eight BP wetlands were found to be N-limited (Figure 3). During the summer of 2017, 15 of the 25 wetlands sampled across all three regions were found to be N limited (Figure 4). Phosphorus limitation was not observed at any of the wetlands in any season.
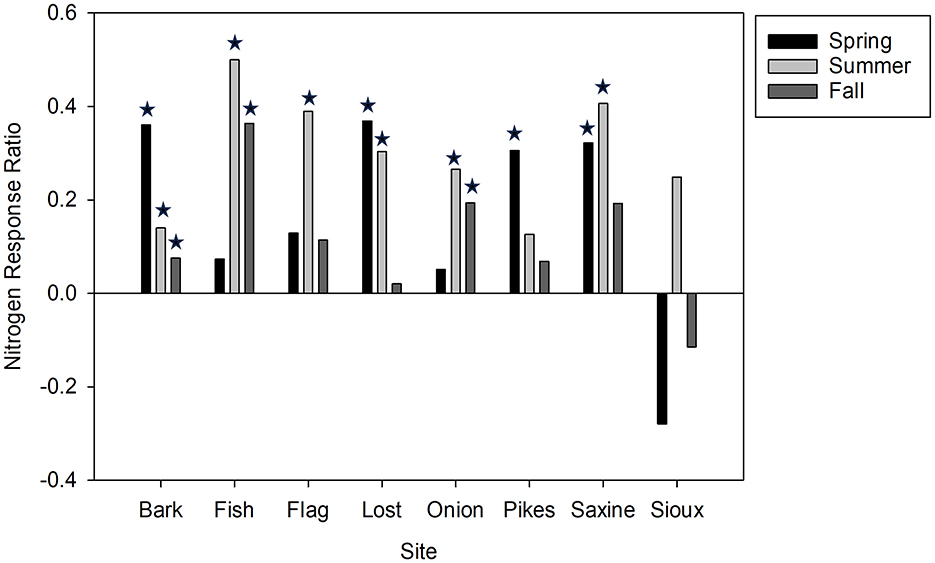
Figure 3. Nitrogen Response Ratio (NRR) across seasons in 2016 for Bayfield Peninsula wetlands. Stars indicate statistically significant N limitation (i.e., chlorophyll a accumulation on N-amended substrates was greater than control substrates, p < 0.05).
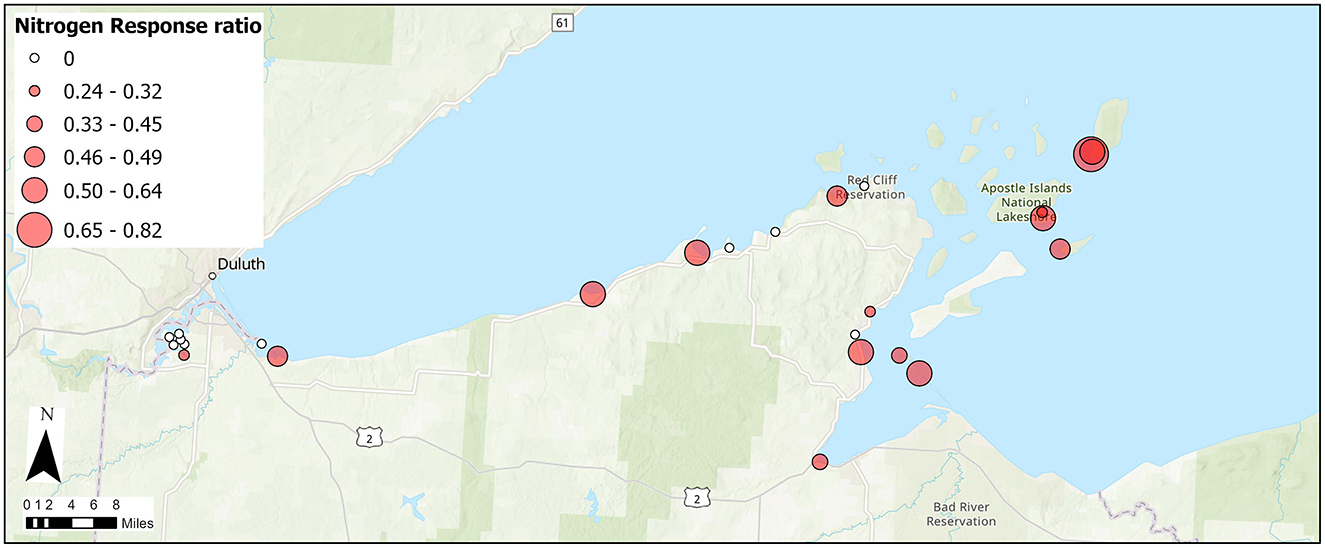
Figure 4. Significant N limitation (filled symbols) and Nitrogen Response Ratio (size of filled symbol) for 25 Lake Superior coastal wetlands sampled during the summer of 2017.
Nitrogen limitation was observed at four of the eight BP wetlands during the spring of 2016 and three of the eight wetlands during the fall (Figure 3). The Bark Bay wetland was the only location where N limitation occurred in all three seasons in 2016 and again during the summer of 2017. The Sioux River wetland was the only location where N limitation did not occur in any of the three seasons in 2016; N limitation did occur in the Sioux River wetland during summer 2017. Nitrogen response ratio varied seasonally (F: 6.02, DF: 14, p = 0.013) with Fall NRR being significantly lower than summer NRR (p = 0.034).
Nitrogen limitation varied among the three regions in 2017 and was most common in the AINL wetlands where all seven wetlands were N limited (Figure 4). Six of the 10 BP wetlands were N limited in 2017 while only two of the eight SLRE sites were N limited. Nitrogen response ratios were found to differ among the regions (p < 0.001) as well with NRR significantly greater in AINL than SLRE wetlands (p = 0.003, Figure 5).
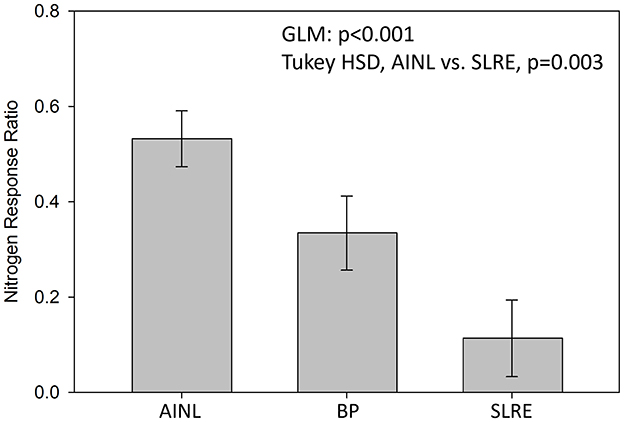
Figure 5. Regional differences in nitrogen response ratio of benthic algae in coastal wetlands of the Apostle Islands (AINL), Bayfield Peninsula (BP), and St. Louis River Estuary (SLRE) assessed during the summer of 2017.
Apostle Islands wetlands had much smaller contributing watersheds than BP wetlands or those of the SLRE (Table 1). Wetlands in all three regions had very little agriculture or developed lands within their contributing watersheds. The watersheds of AINL wetlands contained 100% natural land cover with no agriculture or developed land detected.
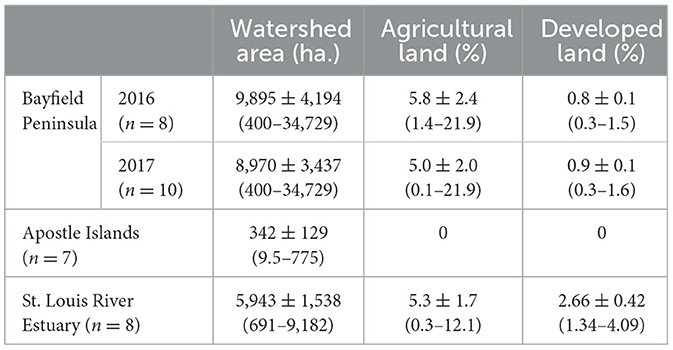
Table 1. Contributing watershed area and land cover for coastal wetlands on the Bayfield Peninsula, Apostle Islands, and St. Louis River Estuary.
In 2016, at the BP sites, TSS and TP tended to be higher in the spring than the other seasons (Table 2). Except for water temperature, other abiotic variables did not differ markedly among seasons (Table 2). In the summer of 2017, the SLRE wetlands tended to be warmer and have higher SpC, NH4-N, TP, TSS, and TN than wetlands in the other regions (Table 3). Consequently, the first principal component for the PCA combining chemical, physical, and land cover variables revealed a gradient in conditions from AINL and BP wetlands (low PC1 scores) to the SLRE wetlands (higher PC1 scores; Figure 6). The SLRE wetlands were also associated with higher watershed development and agriculture (Figure 6). The second principal component largely represented a gradient of temperature and natural land cover (low PC 2 scores) to nitrate and watershed agriculture (high PC 2 scores). For the 25 wetlands sampled in 2017, NRR was negatively correlated with SpC (R = −0.449, p = 0.025), watershed developed lands (R = −0.443, p = 0.027), and PC 1 (R = −0.406, p = 0.044).
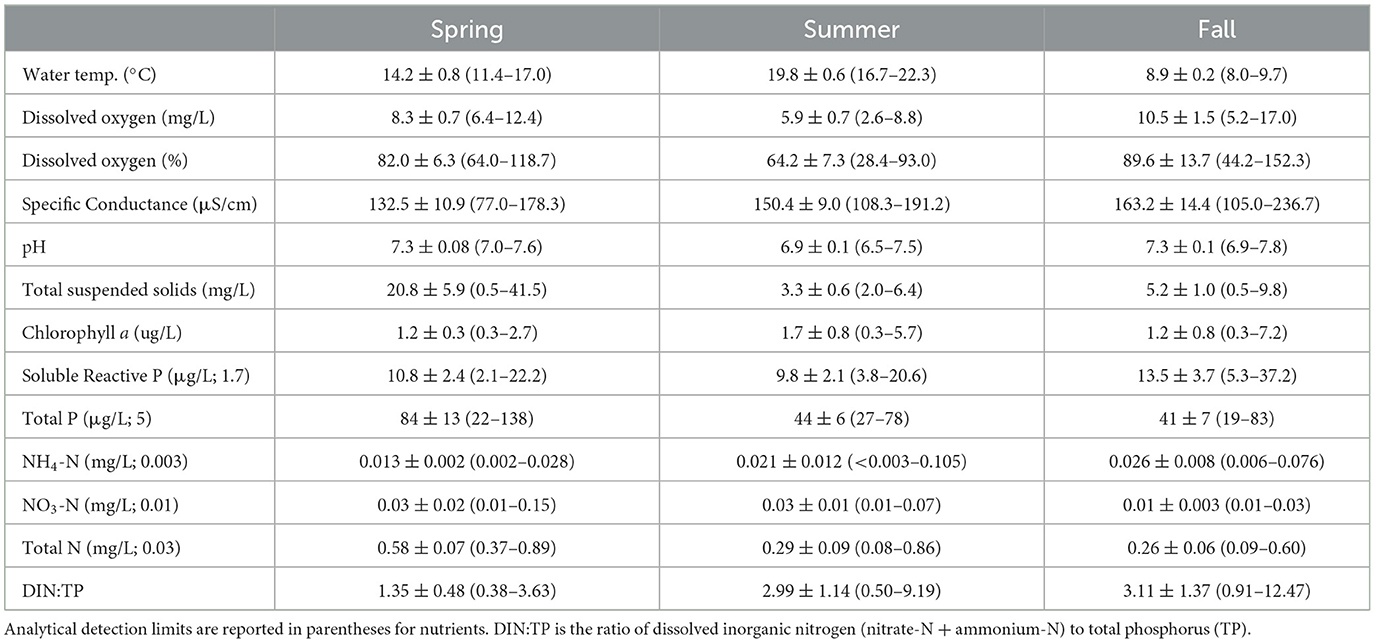
Table 2. Mean ± SE (range) of seasonal water quality variables for eight coastal wetlands sampled on the Bayfield Peninsula in 2016.
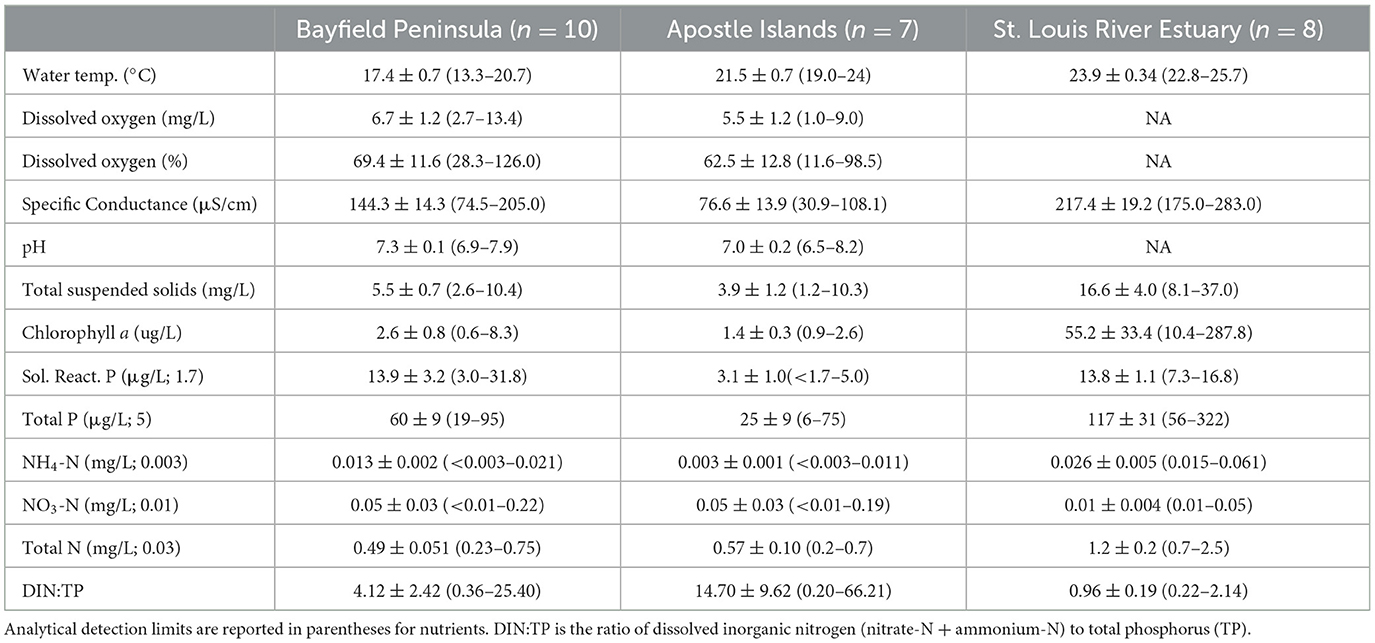
Table 3. Mean ± SE (range) of water quality variables for coastal wetlands sampled in three regions during the summer of 2017.
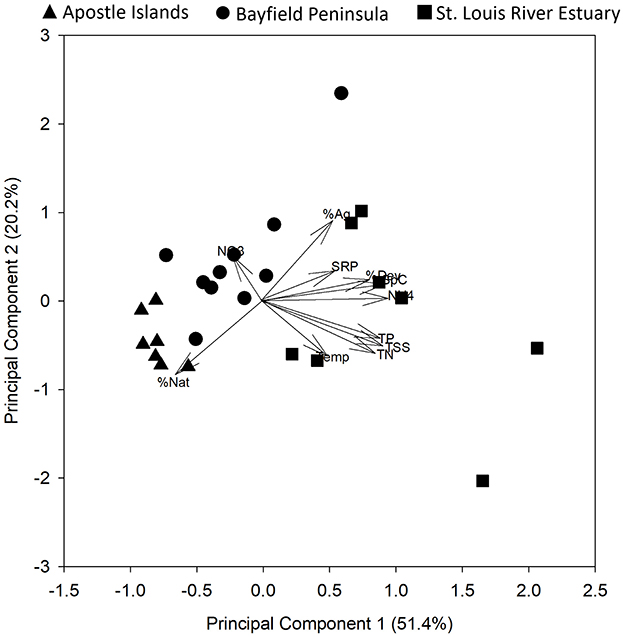
Figure 6. Principal components analysis of chemical, physical, and watershed land cover variables for 25 coastal wetlands of Lake Superior sampled during the summer of 2017. Symbol shape denotes region. Variables included watershed percent agriculture (%Ag), developed land (%Dev), and natural land cover (%Nat) and water temperature (Temp), specific conductivity (SpC), total suspended solids (TSS), soluble reactive P (SRP), ammonium-N (NH4), nitrate-N (NO3), and total N (TN).
Average DIN:TP molar ratios tended to be quite low and well below the Redfield ratio of 16:1 in all seasons in 2016 and all regions in the summer of 2017 (Tables 2, 3). Exceptions included a relatively high DIN:TP ratio of 9.19 in the Bark Bay wetland (BP) in the summer of 2016, which was driven by an NH4-N concentration of 0.11 mg/L. This wetland also had a relatively high NH4-N concentration of 0.07 mg/L in the fall of 2016, which contributed to a high DIN:TP ratio of 12.47 in the fall. Elevated DIN:TP ratios also occurred during the summer of 2017 at two wetland sites on Outer Island (AINL) where we observed NO3-N concentrations of 0.09 and 0.19 mg/L and at the Sand River wetland (BP) where we measured a NO3-N concentration of 0.22 mg/L. DIN concentrations and DIN:TP ratios were low (≤ 0.07 mg/L and < 2.2, respectively) in the SLRE wetlands.
Discussion
The common N limitation of benthic algal growth that we observed in coastal wetlands of Lake Superior differs from the strong P limitation reported for algal production in Lake Superior pelagic habitats (Sterner et al., 2004; Sterner, 2011; Guildford et al., 2000). This was further supported by the relatively low DIN concentrations that we measured in a majority of the 25 wetlands. Lake Superior open water nitrate concentration averages approximately 0.3 mg/L in the western portion of the lake (Sterner, 2021; Dove and Chapra, 2015), and is similar in shallow non-wetland coastal habitats of Lake Superior (Ozersky and Camilleri, 2021). This is 10-fold higher than the average wetland nitrate concentration we observed in Lake Superior coastal wetlands. Consistent with our nutrient assay results, DIN:TP ratios were generally much lower than Redfield ratio and many coastal wetlands had DIN:TP ratios below 2:1; the ratio that others have reported for N-limited conditions in lakes (Bergström, 2010; Ptacnik et al., 2010). The markedly higher DIN:TP ratios that we observed at the Sand River and Outer Island wetlands were most likely the result of high-NO3 Lake Superior nearshore water being driven into the wetlands by seiche activity at the time of our sampling. Such lake-wetland mixing, including flow reversals at the wetland mouth, are frequently observed in Lake Superior lagoonal wetlands that have a surface water connection to Lake Superior (Trebitz et al., 2002). Interestingly, the high DIN:TP ratio at the Outer Island and Sand River wetlands and the high DIN:TP ratio at the Bark Bay wetland, which was driven by elevated NH4-N concentration did not appear to alleviate the N limitation at these wetlands. This suggests that even episodic pulses of reactive N can be drawn down rapidly so that N limitation of benthic algae persists.
While our use of nutrient amendment assays allowed for direct measurement of nutrient limitation, other studies have reported potential N limitation of algal productivity in Lake Superior coastal wetlands using other techniques. In the Lost Creek wetland, one of our BP sites, Morrice et al. (2004) found N:P ratios to be relatively low, especially during the summer and fall, which suggests the potential for N limitation. Similarly, Hill et al. (2006) used both N:P ratios and extracellular enzyme activity and found a majority of the Lake Superior coastal wetlands surveyed were likely N-limited. Additionally, Eberhard et al. (2023) used NDS arrays similar to ours at the Sioux River wetland and a coastal wetland on the Keweenaw Peninsula (approximately 150 km east of our AINL sites) and found N limitation of benthic algae to predominate. These previous studies, combined with our direct nutrient assay approach support the hypothesis that Lake Superior coastal wetland benthic algal productivity is most often N limited.
Our hypothesis that N limitation would be greater in summer than spring or fall was only partially supported. Uptake of nutrients by macrophytes in the early to middle growing season likely contributes to the drawdown of reactive N and resulting N limitation. However, N limitation also occurred at over half of the wetlands in the fall of 2016 suggesting that a combination of N uptake by primary producers and microbial denitrification (Eberhard et al., 2023) may be keeping reactive N availability low throughout the fall. Notably, our fall NO3-N concentrations remained at or near the detection limit of 0.01 mg/L at six of the eight wetlands sampled in the fall and NH4-N concentrations were similar among seasons. While our fall NDS assays occurred after the typical growing season, it is possible that nutrient availability and nutrient limitation would have differed later in the fall after more of the year's vegetative biomass had senesced. Thus, the principal mechanisms leading to N drawdown remains unclear and both the high uptake rates by macrophytes and algae and microbial denitrification are likely important (Cooper et al., 2016; Nikolakopoulou et al., 2020; Ozersky and Camilleri, 2021). Eberhard et al. (2023) found that denitrification rates tended to exceed N2 fixation rates in Great Lakes coastal wetlands suggesting denitrification as a net sink of reactive N. These authors also found no significant relationship between denitrification rates and biofilm nutrient limitation suggesting that other mechanisms of N removal may outweigh denitrification. We also acknowledge that our study did not account for within-wetland variability in nutrient limitation. For example, in a eutrophic lake connected to Lake Michigan, benthic algae were found to be co-limited by N and P, while phytoplankton were found to be P-limited (Steinman et al., 2016), suggesting that nutrient drawdown leading to limitation can be spatially complex. We recommend future studies work to clarify the mechanisms responsible for N limitation and its spatial and temporal dynamics in Great Lakes coastal ecosystems.
Our results and others (Morrice et al., 2004; Hill et al., 2006; Cooper et al., 2016) suggest that anthropogenic N loading has the potential to counteract the natural N limitation that occurs in Great Lakes coastal wetlands. We observed the strongest N limitation in AINL wetlands where little to no human land uses or other potential anthropogenic N sources occur and a significantly lower magnitude of N limitation in wetlands of the more developed SLRE. While other factors such as differences in wetland hydrogeomorphology between the regions may have influenced N limitation, it is likely that anthropogenic N loading to the SLRE was an important factor. Thus, N loading via tributary streams draining agricultural lands or delivery of other anthropogenically derived N (lawn fertilizers, wastewater treatment plant effluent, etc.) may cause impacts in Great Lakes coastal wetlands. While such impacts associated with overabundance of primary production are difficult to discern in naturally eutrophic ecosystems like wetlands, there is ample evidence that watershed land use and elevated in situ nutrient concentrations elicit biotic responses in Great Lakes coastal wetlands. For example, algal chlorophyll a has been related to watershed agriculture and nutrient inputs (Gentine et al., 2022) and macroinvertebrate and fish community structure has been shown to shift as a result of poor water quality and surrounding agricultural land use (Uzarski et al., 2005; Trebitz et al., 2009; Cooper et al., 2014; Kovalenko et al., 2014). The influence of anthropogenic N loading on basal trophic levels is likely to play an important role in these relationships. Management practices aimed at improving ecological conditions in both freshwater and marine wetlands should focus on strategies to limit N inputs.
To date, N inputs to the Great Lakes have been only moderately regulated. The Great Lakes Water Quality Agreement, for example, provides a binational framework for nutrient management and establishes P loading targets for each of the Great Lakes but does not directly address N inputs. Future management strategies should consider a dual nutrient approach, particularly in light of climate change, which is likely to cause increased nutrient loading to coastal ecosystems via more frequent, intense, and altered seasonal patterns of precipitation (Zhang et al., 2020). Such an approach is especially important for coastal wetlands given that these habitats appear vulnerable to nutrient loading in ways that other habitats in the Laurentian Great Lakes are not.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://dnr.wisconsin.gov/topic/SurfaceWater/SWIMS.
Author contributions
MC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MW: Conceptualization, Funding acquisition, Investigation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by funding from the Great Lakes Restoration Initiative, the Wisconsin Department of Natural Resources, and the National Park Service (collaborative agreement P16AC01387).
Acknowledgments
We thank Aaron Marti, Joe Graham, Tom Simmons, and Cherie Hagen from the Wisconsin Department of Natural Resources for assistance in data collection, data synthesis, and review of an earlier version of this manuscript. Aletha Hefko from Northland College assisted in preparing NDS equipment and with field work. We are grateful for the support of NPS staff including J. Van Stappen, P. Burkman, K. Pemble and others who assisted with boat transports and other logistics within AINL. Lelia Sigmon from Grand Valley State University provided GIS support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
APHA (2005). Standard Methods for the Evaluation of Water and Wastewater. 21st edition. American Public Health Association, Washington, DC.
Bellinger, B. J., Jicha, T. M., Lehto, L. P., Seifert-Monson, L. R., Bolgrien, D. W., Starry, M. A., et al. (2014). Sediment nitrification and denitrification in a Lake Superior estuary. J. Great Lakes Res.40, 392–403. doi: 10.1016/j.jglr.2014.03.012
Bergström, A. K. (2010). The use of TN: TP and DIN: TP ratios as indicators for phytoplankton nutrient limitation in oligotrophic lakes affected by N deposition. Aquat. Sci. 72, 277–281. doi: 10.1007/s00027-010-0132-0
Chen, M., Chang, L., Zhang, J., Guo, F., Vymazal, J., He, Q., et al. (2020). Global nitrogen input on wetland ecosystem: the driving mechanism of soil labile carbon and nitrogen on greenhouse gas emissions. Environ. Sci. Ecotechnol. 4:100063. doi: 10.1016/j.ese.2020.100063
Cooper, M. J., Costello, G. M., Francoeur, S. N., and Lamberti, G. A. (2016). Nitrogen limitation of algal biofilms in coastal wetlands of Lakes Michigan and Huron. Freshwater Sci. 35, 25–40. doi: 10.1086/684646
Cooper, M. J., Lamberti, G. A., and Uzarski, D. G. (2014). Spatial and temporal trends in invertebrate communities of Great Lakes coastal wetlands, with emphasis on Saginaw Bay of Lake Huron. J. Great Lakes Res. 40, 168–182. doi: 10.1016/j.jglr.2013.12.003
Deegan, L. A., Johnson, D. S., Warren, R. S., Peterson, B. J., Fleeger, J. W., Fagherazzi, S., et al. (2012). Coastal eutrophication as a driver of salt marsh loss. Nature 490, 388–392. doi: 10.1038/nature11533
Diebel, M., Kirsch, K., Freihoefer, A., Nelson, T., Hook, T., Zhang, X., et al. (2013). PRESTO Pollutant Load Ratio Estimation Tool: Documentation, Validation and Analysis, Version 1.1. Madison, WI: Wisconsin Department of Natural Resources, 82.
Dove, A., and Chapra, S. C. (2015). Long-term trends of nutrients and trophic response variables for the Great Lakes. Limnol. Oceanogr. 60, 696–721. doi: 10.1002/lno.10055
Dybiec, J. M., Albert, D. A., Danz, N. P., Wilcox, D. A., and Uzarski, D. G. (2020). Development of a preliminary vegetation-based indicator of ecosystem health for coastal wetlands of the laurentian great lakes. Ecol. Indic. 119:106768. doi: 10.1016/j.ecolind.2020.106768
Eberhard, E. K., Kane, E. S., and Marcarelli, A. M. (2023). Heterogeneity in habitat and nutrient availability facilitate the co-occurrence of N2 fixation and denitrification across wetland–stream–lake ecotones of Lakes Superior and Huron. Biogeochemistry 166, 169–189. doi: 10.1007/s10533-023-01090-3
Gentine, J. A., Conard, W. M., O'Reilly, K. E., Cooper, M. J., Fiorino, G. E., Harrison, A. M., et al. (2022). Environmental predictors of phytoplankton chlorophyll-a in great lakes coastal wetlands. J. Great Lakes Res. 48, 927–934. doi: 10.1016/j.jglr.2022.04.015
Guildford, S. J., Bootsma, H. A., Fee, E. J., Hecky, R. E., and Patterson, G. (2000). Phytoplankton nutrient status and mean water column irradiance in lakes malawi and superior. Aquat. Ecosyst. Health Manag. 3, 35–45. doi: 10.1080/14634980008656989
Guildford, S. J., and Hecky, R. E. (2000). Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: is there a common relationship? Limnol. Oceanogr. 45, 1213–1223. doi: 10.4319/lo.2000.45.6.1213
Harpole, W. S., Ngai, J. T., Cleland, E. E., Seabloom, E. W., Borer, E. T., Bracken, M. E., et al. (2011). Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862. doi: 10.1111/j.1461-0248.2011.01651.x
Harrison, A. M., Reisinger, A. J., Cooper, M. J., Brady, V. J., Ciborowski, J. J., O'Reilly, K. E., et al. (2020). A basin-wide survey of coastal wetlands of the laurentian great lakes: development and comparison of water quality indices. Wetlands 40, 465–477. doi: 10.1007/s13157-019-01198-z
Hill, B. H., Elonen, C. M., Jicha, T. M., Cotter, A. M., Trebitz, A. S., Danz, N. P., et al. (2006). Sediment microbial enzyme activity as an indicator of nutrient limitation in Great Lakes coastal wetlands. Freshwater Biol. 51, 1670–1683. doi: 10.1111/j.1365-2427.2006.01606.x
Johnson, L. T., Tank, J. L., and Dodds, W. K. (2009). The influence of land use on stream biofilm nutrient limitation across eight North American ecoregions. Can. J. Fish. Aquat. Sci. 66, 1081–1094. doi: 10.1139/F09-065
Kovalenko, K. E., Brady, V. J., Brown, T. N., Ciborowski, J. J., Danz, N. P., Gathman, J. P., et al. (2014). Congruence of community thresholds in response to anthropogenic stress in great lakes coastal wetlands. Freshwater Sci. 33, 958–971. doi: 10.1086/676913
Le Moal, M., Gascuel-Odoux, C., Ménesguen, A., Souchon, Y., Étrillard, C., Levain, A., et al. (2019). Eutrophication: a new wine in an old bottle? Sci. Total Environ. 651, 1–11. doi: 10.1016/j.scitotenv.2018.09.139
Lin, C. K., and Schelske, C. L. (1981). Seasonal variation of potential nutrient limitation to chlorophyll production in southern lake huron. Can. J. Fish. Aquat. Sci. 38, 1–9. doi: 10.1139/f81-001
Malone, T. C., and Newton, A. (2020). The globalization of cultural eutrophication in the coastal ocean: causes and consequences. Front. Mar. Sci. 7:670. doi: 10.3389/fmars.2020.00670
Martínez-Megías, C., and Rico, A. (2022). Biodiversity impacts by multiple anthropogenic stressors in Mediterranean coastal wetlands. Sci. Total Environ. 818:151712. doi: 10.1016/j.scitotenv.2021.151712
Morrice, J. A., Kelly, J. R., Trebitz, A. S., Cotter, A. M., and Knuth, M. L. (2004). Temporal dynamics of nutrients (N and P) and hydrology in a lake superior coastal wetland. J. Great Lakes Res. 30, 82–96. doi: 10.1016/S0380-1330(04)70379-2
Nikolakopoulou, M., Argerich, A., Bernal, S., Gacia, E., Ribot, M., Martí, E., et al. (2020). Effect of three emergent macrophyte species on nutrient retention in aquatic environments under excess nutrient loading. Environ. Sci. Technol. 54, 15376–15384. doi: 10.1021/acs.est.0c03216
Ozersky, T., and Camilleri, A. (2021). Factors regulating lake periphyton biomass and nutrient limitation status across a large trophic gradient. Freshwater Biol. 66, 2338–2350. doi: 10.1111/fwb.13836
Paerl, H. W., Hall, N. S., Peierls, B. L., and Rossignol, K. L. (2014). Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuaries Coasts. 37, 243–258. doi: 10.1007/s12237-014-9773-x
Ptacnik, R., Andersen, T., and Tamminen, T. (2010). Performance of the redfield ratio and a family of nutrient limitation indicators as thresholds for phytoplankton N vs. P limitation. Ecosystems 13, 1201–1214. doi: 10.1007/s10021-010-9380-z
Schindler, D. W. (1977). Evolution of phosphorus limitation in lakes: natural mechanisms compensate for deficiencies of nitrogen and carbon in eutrophied lakes. Science 195, 260–262. doi: 10.1126/science.195.4275.260
Schock, N. T., Murry, B. A., and Uzarski, D. G. (2014). Impacts of agricultural drainage outlets on great lakes coastal wetlands. Wetlands 34, 297–307. doi: 10.1007/s13157-013-0486-x
Sierszen, M. E., Morrice, J. A., Trebitz, A. S., and Hoffman, J. C. (2012). A review of selected ecosystem services provided by coastal wetlands of the laurentian great lakes. Aquat. Ecosyst. Health Manag. 15, 92–106. doi: 10.1080/14634988.2011.624970
Sierszen, M. E., Peterson, G. S., Trebitz, A. S., Brazner, J. C., and West, C. W. (2006). Hydrology and nutrient effects on food-web structure in ten lake superior coastal wetlands. Wetlands 26, 951–964. doi: 10.1672/0277-5212(2006)26[951:HANEOF]2.0.CO;2
Solórzano, L. (1969). Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol. Oceanogr. 14, 799–801.
Steinman, A., Abdimalik, M., Ogdahl, M. E., and Oudsema, M. (2016). Understanding planktonic vs. benthic algal response to manipulation of nutrients and light in a eutrophic lake. Lake Reserv. Manag. 32, 402–409. doi: 10.1080/10402381.2016.1235065
Sterner, R. W. (2011). C:N stoichiometry in lake superior: freshwater sea as end member. Inland Waters 1, 29–46. doi: 10.5268/IW-1.1.365
Sterner, R. W. (2021). The laurentian great lakes: a biogeochemical test bed. Annu. Rev. Earth Planet. Sci. 49, 201–229. doi: 10.1146/annurev-earth-071420-051746
Sterner, R. W., Smutka, T. M., McKay, R. M. L., Xiaoming, Q., Brown, E. T., Sherrell, R. M., et al. (2004). Phosphorus and trace metal limitation of algae and bacteria in lake superior. Limnol. Oceanogr. 49, 495–507. doi: 10.4319/lo.2004.49.2.0495
Tank, J. L., and Dodds, W. K. (2003). Nutrient limitation of epilithic and epixylic biofilms in ten North American streams. Freshwater Biol. 48, 1031–1049. doi: 10.1046/j.1365-2427.2003.01067.x
Tank, J. L., Reisinger, A. J., and Rosi, E. J. (2017). Nutrient Limitation and Uptake. In: Methods in Stream Ecology. Academic Press, 147–171. doi: 10.1016/B978-0-12-813047-6.00009-7
Trebitz, A. S., Brazner, J. C., Danz, N. P., Pearson, M. S., Peterson, G. S., Tanner, D. K., et al. (2009). Geographic, anthropogenic, and habitat influences on Great Lakes coastal wetland fish assemblages. Can. J. Fish. Aquat. Sci. 66, 1328–1342. doi: 10.1139/F09-089
Trebitz, A. S., Morrice, J. A., and Cotter, A. M. (2002). Relative role of lake and tributary in hydrology of lake superior coastal wetlands. J. Great Lakes Res. 28, 212–227. doi: 10.1016/S0380-1330(02)70578-9
USGS (2003). Methods of Analysis by the U.S. Geological Survey National Water Quality Laboratory—Evaluation of Alkaline Persulfate Digestion as an Alternative to Kjeldahl Digestion for Determination of Total and Dissolved Nitrogen and Phosphorus in Water. Water-Resources Investigations Report 03–4174. U.S. Department of the Interior, U.S. Geological Survey, Denver, CO.
Uzarski, D. G., Brady, V. J., Cooper, M. J., Wilcox, D. A., Albert, D. A., Axler, R. P., et al. (2017). Standardized measures of coastal wetland condition: implementation at a laurentian great lakes basin-wide scale. Wetlands 37, 15–32. doi: 10.1007/s13157-016-0835-7
Uzarski, D. G., Burton, T. M., Cooper, M. J., Ingram, J. W., and Timmermans, S. T. (2005). Fish habitat use within and across wetland classes in coastal wetlands of the five great lakes: development of a fish-based index of biotic integrity. J. Great Lakes Res. 31, 171–187. doi: 10.1016/S0380-1330(05)70297-5
Vadeboncoeur, Y., Peterson, G., Vander Zanden, M. J., and Kalff, J. (2008). Benthic algal production across lake size gradients: interactions among morphometry, nutrients, and light. Ecology 89, 2542–2552. doi: 10.1890/07-1058.1
Welschmeyer, N. A. (1994). Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnol. Oceanogr. 39, 1985–1992. doi: 10.4319/lo.1994.39.8.1985
Wisconsin Department of Natural Resources (2016). WISCLAND 2 Land Cover (Level 1). Available at: https://geodata.wisc.edu/catalog/650C42AF-B720-41DA-BDB8-F6CEA69CE792 (accessed June 6, 2024).
Keywords: Laurentian Great Lakes, Lake Superior, eutrophication, phosphorus, nutrients
Citation: Cooper MJ and Wheeler MC (2025) Watershed land use decreases the nitrogen limitation of benthic algal growth in coastal wetlands of a large oligotrophic lake. Front. Freshw. Sci. 3:1513130. doi: 10.3389/ffwsc.2025.1513130
Received: 17 October 2024; Accepted: 04 February 2025;
Published: 19 February 2025.
Edited by:
Paul Kemp, University of Southampton, United KingdomCopyright © 2025 Cooper and Wheeler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthew J. Cooper, Y29vcG1hdEBndnN1LmVkdQ==
 Matthew J. Cooper
Matthew J. Cooper Michele C. Wheeler3
Michele C. Wheeler3