- 1Department of Forest Growth, Silviculture & Genetics, Austrian Research Centre for Forests BFW, Vienna, Austria
- 2Department of Ecosystem Management, Climate and Biodiversity, Institute of Forest Growth, University of Natural Resources and Life Sciences, Vienna, Austria
Understanding tree growth in relation to environmental conditions is essential, particularly in the context of climate change, where rising temperatures, frequent droughts, and disturbances threaten forest health and productivity. This study uses high-resolution data from four intensively monitored Picea abies stands in Austria (2010–2020), with dendrometers recording hourly stem increments on 10 trees per site, allowing for detailed analysis of growth responses to environmental changes. For this purpose we tested different generalized additive mixed models (GAMs) using environmental data collected on site. The best model consisted of combinations of soil moisture (SM) and soil temperature (ST) data. Furthermore we analyzed how the relationships established differ for three different times during the growing season. We found that high SM consistently had a positive effect on tree growth, wheras the effect of ST varied depending on the timing. Our findings underscore the importance of monitoring soil conditions, particularly for species like Picea abies, which are known for their sensitivity to environmental changes due to their shallow rooting systems and vulnerability to drought.
1 Introduction
Forests present an important carbon sink, allowing for natural carbon dioxide removal from the atmosphere (Pan et al., 2011) and thereby aiding in the mitigation of climate change and its consequences. Climate change has a significant impact on forests: higher temperatures, more intensive and frequent drought spells and an increase in disturbances were shown to have a significant long-term impact on forest ecosystems and pose major challenges for forest conservation and management (Grossiord et al., 2020; Zhang et al., 2021). The warming climate is expected to result in irreversible effects on forests in terms of productivity, species distribution, increased pest vulnerability, and higher mortality (Arend et al., 2021; Dyderski et al., 2018; Grossiord et al., 2020; Lombardero et al., 2000; Oberleitner et al., 2022; Romeiro et al., 2022; Thurm et al., 2018; Zweifel et al., 2021).
However the response of productivity to climate change shows diverging trends (Migliavacca et al., 2021; Pretzsch et al., 2023). Large proportions of central European woodland comprise mountainous forests. Due to climate change temperature is expected to increase whilst precipitation levels are expected to stay unchanged (Riahi et al., 2017). Therefore, in these mountainous regions, where precipitation is abundant and growth is mostly limited by temperature, climate change is expected to have a positive effect on Picea abies (Norway spruce) and Fagus sylvatica (European beech) growth: According to D'Andrea et al. (2023) stem increment increased by 2.5%–7.9% in the past 50 years.
Picea abies is one of the economically most valuable tree species in many European countries (D'Andrea et al., 2023; Popa et al., 2024; Spiecker et al., 2000), simultaneously it is a tree species with high regional variety (Jansson et al., 2013). This might be caused by the high sensitivity of spruce to environmental conditions as the environmental restrictions of its productivity, physiological processes, and health condition are often related to temperature extremes and variations in precipitation distribution (Kunert, 2020; Leštianska et al., 2015; Mensah et al., 2021). As an evergreen species, Picea abies maintains some level of physiological activity throughout the year, except during freezing temperatures. During dry conditions, the transpiration rates of Picea abies are reduced, which impacts its growth kinetics by limiting carbon assimilation and thus slowing down growth. Picea abies begins its radial growth shortly before budburst, which is a critical phase in its annual growth cycle (Zweifel et al., 2006).
As a useful indicator for tree health and carbon sequestration, radial stem growth is a parameter investigated in numerous scientific studies (Makinen et al., 2003; Oberhuber et al., 2014; Schäfer et al., 2019). Stem radius (SR) changes composite of two factors: irreversible growth and reversible swelling/shrinking. The necessary high resolution (hourly) data can be obtained from automatic dendrometers. Dendrometer studies are particularly useful, as they provide detailed information on SR throughout the year, capturing not only total increment at the end of the growing season, but moreover the timing and rate of SR changes (Leštianska et al., 2015). In the temperate zone, cell formation follows distinct temporal patterns, both diurnal and seasonal. Diurnally, stem radius exhibits pronounced fluctuations linked to water dynamics: Trees typically reach their maximum circumference soon before dawn, at which point their stomata open and when they begin to lose more water to the atmosphere than they are absorbing from the soil water. The amplitude of the following shrinkage is a function of water loss through leaves and water uptake by roots (Herrmann et al., 2016). Seasonally, cell formation starts in spring, when temperatures rise and the cambium resumes the production of new xylem cells. Cambial activity in the temperate zone is at a maximum in May-June and begins to cease in August following the cycle of the season (Rathgeber et al., 2016). Intra-annual SR of Picea abies is tightly correlated with atmospheric and soil climatic variables which show seasonal patterns linked to solar radiation changes through the year such as daylength and solar angle (Kašpar et al., 2024). Temperature significantly influences the radial growth of Picea abies, with varying effects depending on local environmental conditions and temporal factors (Schurman et al., 2019). Another key determinant for the radial growth of Picea abies is water availability (Gričar et al., 2024). Vapor pressure deficit (VPD), which is a measure of the atmospheric demand for water, correlates strongly with xylem sap flux density in Picea abies, indicating that higher VPD increases the rate at which water is transported through the tree's xylem tissue (Smirnakou et al., 2017). The correlation between SR and VPD is typically negative hence tree growth occurs under low VPD conditions.
Soil parameters such as soil temperature (ST) and soil moisture (SM) were found to have a significant impact on intra-annual growth (Fleurial et al., 2022; Gričar et al., 2019; Jiao et al., 2024). ST is an important parameter in analyzing energy and mass exchange with the atmosphere as it strongly affects the water balance and ecohydrological processes like evapotranspiration and water uptake by plants. Decreased ST leads to a decreased water absorption rate hence influencing the water and nutrient uptake of plants (Onwuka and Mang, 2018). In boreal forests, soil warming has been shown to induce earlier growth cessation, resulting in a shorter growing season but higher mean growth rates and total annual growth. This is because higher ST early in the growing season provides better growth conditions when soil water content is elevated due to snow melt, although the critical soil temperature is reached earlier, leading to earlier growth cessation (Oogathoo et al., 2022).
The soil water conditions can also have a significant impact on the energy balance since they affect how incoming solar radiation is divided into sensible and latent heat (Li et al., 2020). Research also indicates that Picea abies strongly reduces sap flow during drought to conserve water (Zavadilová et al., 2023) which underscores the species' sensitivity to water availability (Ge et al., 2013). Klein et al. (2014) emphazised that understanding how soil water dynamics and tree water use interact is essential to comprehending how forests in water-limited habitats respond to environmental change.
The presented literature indicates that temperature related factors are found to be most influential in colder sites and early in the growing season while water related factors were found to be more important at dry sites. At intermediate sites both factors play an important role however the importance shifts throughout the year. Due to the shallow rooting system and consequently reduced access to water in deeper soil layers, the impacts are particularly pronounced for Picea abies.
Consequently, most modeling approaches incorporate a combined effect of atmospheric and soil parameters to better capture these dynamics (Oberhuber, 2017; Zweifel et al., 2005). Modeling tree growth in relation to environmental factors reveals complex interactions that vary by species, site, and temporal resolution (Kašpar et al., 2021; Montagnani et al., 2018).
The primary objective of this study is to examine the effects of environmental conditions on stem radius, with a particular emphasis on growth dynamics rather than swelling and shrinking processes. The dataset used in this study encompasses ten years of dendrometer data collected at four Picea abies stands in differing altitudes and climate conditions. Since the sites are located in mountainous regions we expect growth to be strongly modulated by energy availability (e.g. air temperature, soil temperature). Even though all research sites are believed to be water saturated most times we further expect to see a clear response of growth to moisture availability due to the drought sensitivity of Picea abies. Therefore we tested different modeling approaches taking into account combinations of energy and moisture availability indicators. Furthermore we adapted six models from literature to our modeling approach and compared the results to our best model.
2 Materials and methods
2.1 Site descriptions and data preparation
The intensive plots of the European Forest Monitoring Programme (ICP-Forests) provide high quality data on tree vitality and adaptability, nutrient cycling, critical loads, and water relations (de Vries et al., 2003). For this study we used data from four ICP-Forests core sites in Austria (Neumann and Kindermann, 2016), namely four Picea abies stands located between 715m and 1,540 m above sea level with a area of 2,500m2 per site (Figure 1, Table 1). According to Köppen climate classification the sites are located in temperate and continental climatic regions. To give an overview of the site conditions climate diagrams for each sites are included in the (Supplementary Figure 1). The data used in this study were collected directly at the measurement sites in the period 2010-2020. Information on measurement sensors can be found in Supplementary Table 1. At an interval of 15 minutes open land parameters, namely temperature, relative humidity, wind speed, wind direction, solar radiation and precipitation were measured. Within the stand, ST and SM were measured at hourly intervals in 15cm depth. To ensure representativeness of the measurement, SM was measured in parallel at three different pits. The average value of these three pits is used for this analysis. At each site 10 trees are equipped with automatic band dendrometers manufactured by UMS München.
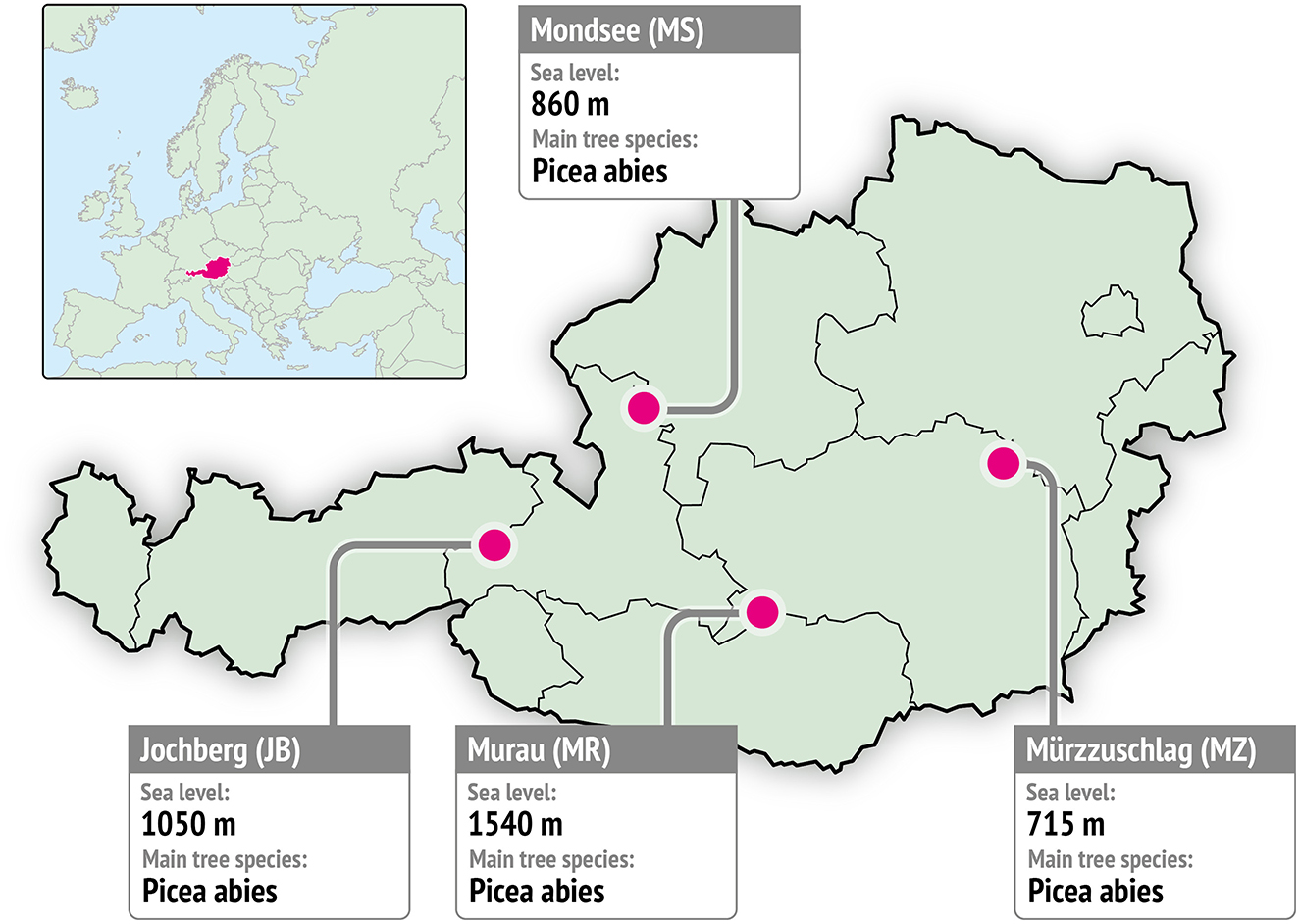
Figure 1. Overview of the research area and stations. Red markers showcase the position of the four measurement sites.
Dendrometers have a high measurement accuracy of ≤ 1 μm, which allows them to detect even small changes in tree girth, but this also means that the device reacts very sensitive to disturbances (e.g. litter, disturbances by animals) that can distort the measurement. Dendrometer data were cleaned using the R package treenetproc (Haeni et al., 2020; Knüsel et al., 2021; Wickham et al., 2019). Additional manual corrections were made where necessary, and in some cases years had to be removed completely because the amount of missing or corrupted data was too high. In total, we used 254 individual years from 40 tree individuals. The dendrometer value of day 91 (1. April) was subtracted from each individual year and individual tree in order to be able to better compare stem radius increments from individual years.
2.2 Data analysis and modeling
The target variable for modeling in this study was the stem radius (SR). To model SR we employed generalized additive models (GAMs) developed using the mgcv package framework in R (Wood, 2011; Wood et al., 2016). This framework allows to include smooth terms, which capture non-linear relationships between predictors and the response variable; parametric terms, which model linear effects; and random effects, which account for variability within hierarchical or grouped data structures. The smooth terms are composed by k simpler basis functions, which are summed to represent the non-linear function. Different types of basis functions are available such as thin plate regression splines, cubic regression splines and cyclic splines. The thin plate regression splines are the default splines in the mgcv package for one-dimensional smooth terms and the default number of basis functions is k=10. Both isotropic and anisotropic smooths can be used to include interactions in generalized additive models. The later, so called tensor interactions, can be used to model interactions between climate effects. Interaction terms use cubic regression splines as default. Moreover, the following random effects were included in all models:
Here TreeID is the individual and unique tree identification number, year is the current year and DOY is a variable containing the day of the year and the nearest hour (calculated by: DOY = Day of the year + Hour/24). The first random effect, s(TreeID, year, bs = “re”), accounts for variation between trees and years (nested structure where tree-specific responses are measured within each year). The second term, s(TreeID, year, DOY, bs = “re”), further captures any nested variation in tree responses within specific days of the year. In our modeling approach different climatic covariates were tested. As a significant amount of SM data was missing (3%), we imputed the data using also a GAM that contains the site ID and year as a random effect and the day of the year and precipitation (hourly cumulative sum, 12 hour cumulative sum, 24 hour cumulative sum, 48 hour cumulative sum). In addition to the parameters obtained directly at the ICP Forests sites, we included the VPD calculated from the measured parameters. We obtained ST and SM values at a depth of 15 cm as deeper layers might not respond as quickly to environmental changes.
As different meteorological parameters are measured at the sites, we tested different model configurations, each containing a parameter representing energy availability combined with a parameter describing moisture availability. Additionally we implemented models that contained both atmospheric and soil climate information to depict wether the combination yields better results. Furthermore, we adapted six models based on findings of recent studies from Etzold et al. (2022); Oogathoo et al. (2022); Rossi et al. (2006); Vospernik et al. (2020) and Zweifel et al. (2021) to our GAM framework and explored the results (Supplementary Table 2). Since soil water potential (SWP) data were not available at the measurement sites we used SM instead. The best fitting model was selected on the basis of AIC. The analysis of the model residuals indicated hourly autocorrelation and the final model was fit including an AR1 process.
To investigate the interaction between SM and ST, we created an artificial dataset representing various combinations of SM and ST values. These values were based on the observed 5%, 25%, 50%, 75%, and 95% percentiles for a specific day within the measurement period. To illustrate particularly extreme scenarios, we augmented the 95% percentile of ST by 1°C and 2°C to represent extremely warm conditions, and reduced the 5% percentile of SM by 2% and 4% to represent extremely dry conditions. These extreme values however remain within the range of historical climate. This artificial dataset was then used as input for the selected GAM to visualize how different combinations of SM and ST influence the prediction of SR. To showacse how these interactions might vary depending on the season we included visualizations for three different times during the growing season: the beginning of the growing season (May 25th), the middle of the growing season (June, 21st) and toward the end of the growing season (August, 10th). The days were selected based on the average curves of stem radius. For the early growing season a day was selected where growth at all four stands has certainly started. The middle of the growing season was selected to be summer solstice. The selected day for the end of tree growing season was chosen when when stem radius reaches a stable value.
3 Results
3.1 General growth conditions
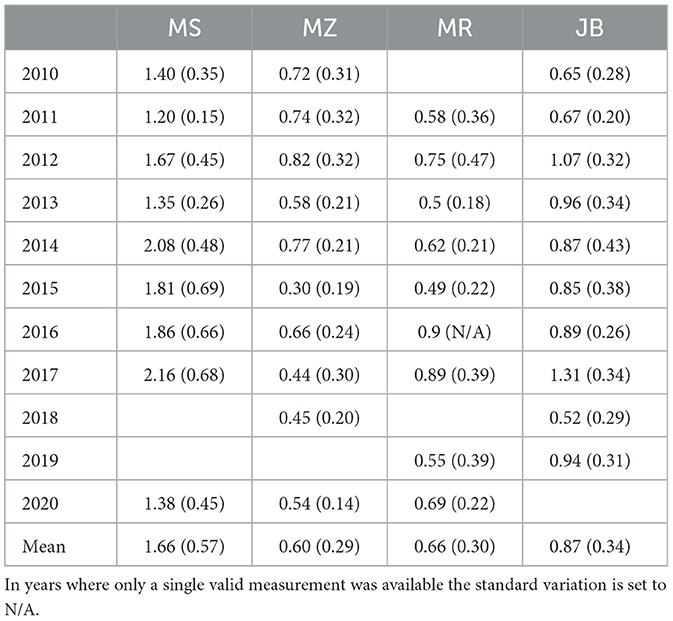
Given that the four measurement sites are spread throughout Austria with varying stand ages and growing conditions, in order to characterize the variations in annual radial increment between sites as well as between years, annual growth increment was extracted from the dendrometer timeseries (Table 2). On average, the annual growth increment across the four measurement sites ranged from 0.60 mm to 1.66 mm. Compared to the average site specific growth increment, deviations ranged from -50% (MZ in 2015) to +50% (JB in 2017). On average the first site to exceed 5% of annual growth was MR on April, 17th, followed by MS and JB on May, 2nd and May, 3rd respectively, and latest for MZ on May, 11th. The first site to exceed 95% of annual growth was JB on July, 29th, followed by MR on August, 5th and MS on August, 6th, with MZ again being the last site on August, 17th (Figure 2).
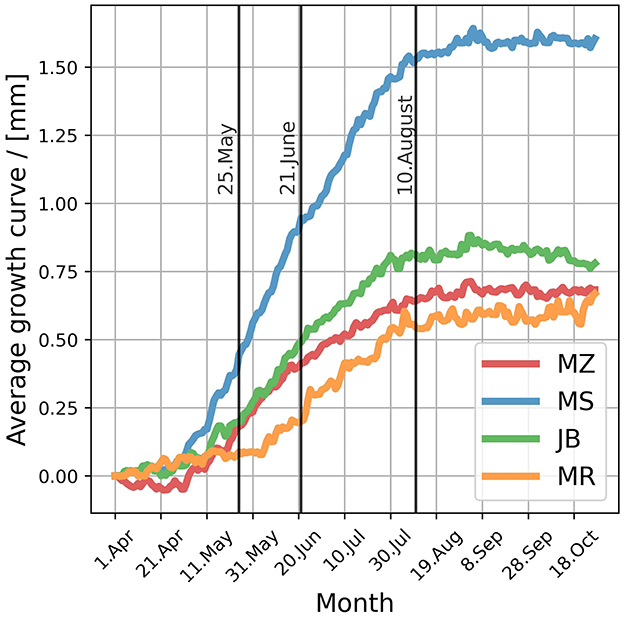
Figure 2. Comparison of the average radial growth of the four sites within the measurement period. Colored lines represent the average growth curves for the four different stands. Vertical black lines mark days selected for analysis of effects in the early growing season, middle of the growing season and end of the growing season.
3.2 Model performance
Overall all models consisting of combinations of different energy and moisture limitating parameters yielded a good R2 ranging from 0.97 to 0.98. R2 of models based on literature varied between 0.85 and 0.98 (Table 3). The best performing model, P1, was selected based on AIC and consisted of the random effects TreeID, year and DOY (Section 2.2) and the following fixed effects:
and the tensor interaction:
The smooth terms in Equation 2 model non-linear relationships. s(DOY) captures the overall effect of the day of the year (DOY), while s(DOY, SM) and s(DOY, ST) allow for interactions between DOY and SM as well as between DOY and ST, respectively. They account for seasonal dynamics and how they interact with environmental conditions. The tensor product interaction ti(DOY, SM, ST) models the combined, non-linear effect of DOY, SM, and ST. The tensor interaction allows for flexible modeling of three-way interactions without assuming that the effects are strictly additive, enabling us to capture complex relationships between these variables. The variable DOY appears as both random effect (see Equation 1) and a fixed effect in all tested models to capture different aspects of its influence on tree growth. As a random effect, it accounts for the specific deviations in growth patterns for individual trees and years, allowing the model to capture tree-specific and year-specific variations in growth dynamics. As a fixed effect, DOY models the overall seasonal trend in growth across all trees and years. This formulation further helps in accurately capturing how the effects of SM and ST levels may vary depending on timing. A comparison of observed and predicted SR for the year and individual with the highest and lowest growth can be found in the (Supplementary Figures 2, 3).
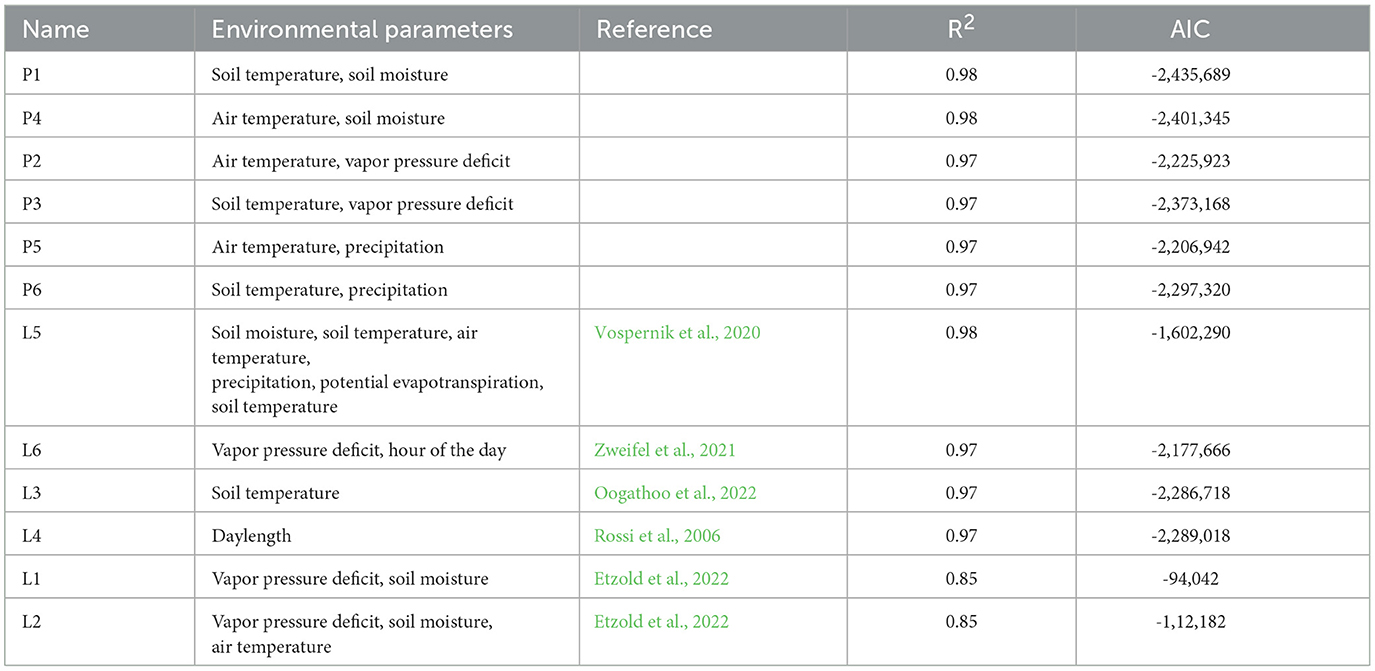
Table 3. Model configurations for models with different combinations of energy and moisture limiting parameters (P1–P6) and models adapted from literature (L1-L6) with corresponding R2 and AIC values.
3.3 Environmental conditions
In order to investigate the effects of different SM and ST levels on growth and how these effects may change seasonally we created conditional dependence plots for May 25th, June 21st, and August 10th (Figure 3). It is important to note that interpretation of the warm/dry conditions (shown in the graphs as either red shaded areas or dashed lines) should be done with caution, as there is little observational data available in this range. The overall distribution of SM and ST records can be found in Supplementary Figure 4.
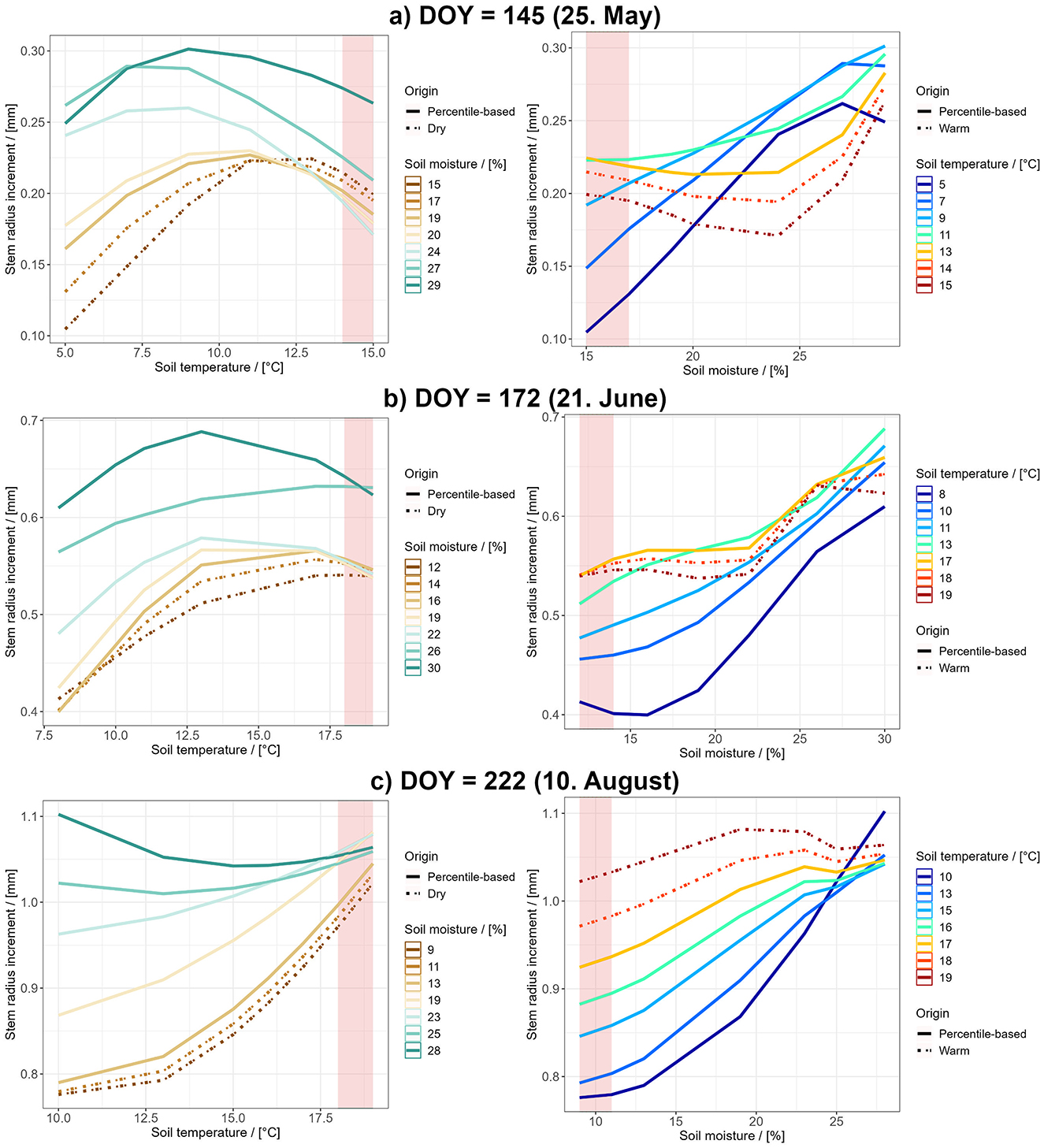
Figure 3. Deviation of growth under different SM and ST conditions from average growth at three different times during the growing season: (A) Early in the growing season, (B) in the middle of the growing season and (C) toward the end of the growing season. In the left plots particularly dry conditions are depicted by dashed lines, the red shaded area marks particularly high ST. In the right plot particularly warm conditions are depicted by dashed lines, the red shaded area marks particularly low SM values.
At the beginning of the growing season SR increases with increasing soil temperatures up to 7-13°C, a further increase in ST leads to a decline in SR prediction (left graph in Figure 3A). An increase in SM yielded higher results in SR prediction, this increase was more pronounced when soil temperature was lower (right graph in Figure 3A).
During the middle of the growing season SR increased with increasing ST and SM respectively. Within lower temperature ranges an increase in SM has a bigger positive impact on SR in comparison to higher temperature ranges above 12°C. Strong positive effects of ST on SR are found under drier conditions whereas predictions under conditions with high moisture vary only minor in comparison with changes in ST (Figure 3B).
Toward the end of the growing season increasing ST has a constant or positive effect on growth however a strong variation with SM can be found. In general SM has a strong positive effect on growth which is more pronounced in the lower temperature ranges (Figure 3C).
A more detailed look into how variation of the two parameters (SM and ST) can be given when keeping one of the two constant (at the median value for the given day) and assessing the change in growth prediction when the other varies between the 5% and 95% percentile. Starting with constant SM, early in the growing season growth decreased by 0.03mm when ST increased, in the middle of the growing season it increases by 0.08mm and later in the growing season it increases by 0.08mm. If ST is fixed and SM increased, early in the growing season growth increased by 0.08mm, in the middle of the growing season it increases by 0.17mm and toward the end of the growing season it increases by 0.17mm. Hence both an increase in ST and an increase in SM has the biggest overall positive effect in the middle and toward the end of the growing season. When comparing the effect of variation of either ST or SM on the SR (-0.03mm vs. 0.08mm early in the growing season, 0.08mm vs. 0.17mm in the middle of the growing season, 0.08mm vs. 0.17mm toward the end of the growing season) it can be seen that SM variation has a stronger impact on the growth prediction than ST variation.
4 Discussion
Understanding the responses of SR to environmental factors is important in times of climate change where environmental conditions are expected to change severely in the coming years. Our analysis indicates that radial stem changes are closely linked to soil climate variables, with the impact varying depending on the timing within the growing season. Our study utilizes an exceptional dataset that encompasses four different Picea abies stands in temperate and continental regions, with ten trees equipped with dendrometers per site and a measurement period of ten years.
4.1 The influence of environmental conditions on growth
The derived model effectively captures the overall annual increments, including annual patterns; however, its ability to represent swelling and shrinking remains limited (Supplementary Figures 2, 3). We explored several approaches to better capture the processes of swelling and shrinking, including the incorporation of temperature moving averages and lagged precipitation sums into the model. However, these modifications did not lead to significant improvements in the model's ability to represent these dynamics. One possible explanation for this is that, while SM and ST are good predictors of SR increment, other environmental factors cloud be more closely related to the processes driving shrinking and swelling. Another possible explanation is the substantial variation in the magnitude of shrinking and swelling observed across the four research sites. This variability may cause the model to adopt a more conservative representation of these processes, particularly at sites where they are more pronounced. When evaluating the average RMSE throughout the course of the year, we observed seasonal changes. RMSE fluctuates around 0.09 mm at the start of the year, rises around the beginning of July (fluctuating around 0.11 mm), declines in the middle of August (down to 0.04 mm), and then increases once more at the start of September (up to 0.15 mm) (Supplementary Figure 5).
Previous studies reported that atmospheric conditions had a greater influence on tree growth than soil climate conditions (Oberhuber et al., 2014; Lupi et al., 2011). In contrast, our model, which incorporates both SM and ST, outperformed those relying solely on atmospheric parameters, as indicated by AIC. ST can be highly correlated with air temperature (which is used to calculate VPD), though the variability in soil temperature is typically dampened. This raises the possibility that the effects we observe might also be influenced indirectly by VPD. However, the correlation between ST and air temperature can vary considerably depending on site-specific factors and measurement depth of the soil temperature sensor (Zhang et al., 2022; Islam et al., 2015). At our study sites, we found a moderate Pearson correlation of 0.6 between ST and air temperature. Additionally, the correlation between VPD and ST was only weak (0.2), suggesting that the observed effects of ST may not primarily operate through VPD.
Furthermore, we observed that the effect of increasing ST on SR shifted early in the growing season from being beneficial to being detrimental (left graph in Figure 3A). This is in contrast to previous findings on effects of elevated air temperature (Deslauriers et al., 2008; Lupi et al., 2010). In principle, higher temperatures can enhance metabolic activity and radial stem growth (Mäkinen et al., 2001, 2003). Especially at the beginning of the growing season when SM levels are high due to snow melt. However, our findings suggest that other factors may modulate this response when ST gets to high. One possible explanation is that trees allocate more resources to root development at the expense of radial stem growth during this period. This hypothesis aligns with Lahti et al. (2005) who found that diameter growth in five-year-old Picea abies peaked at approximately 9°C and declined with higher ST.
Later in the season, increasing ST appears to have mostly positive effects on growth, as indicated by the model's higher predictions under moist conditions (left graph in Figures 3B, C). This is consistent with studies that report temperature-induced enhancement of photosynthesis and growth when soil moisture is adequate. Overall, our findings highlight the complexity of the relationship between ST and SR and suggest that local environmental factors, such as soil properties, seasonal dynamics, or tree physiological strategies, may play a significant role. While further research is required to fully elucidate the mechanisms behind these observations, our results underline the need to understand SR responses to temperature variations. Overall, our findings highlight the complexity of the relationship between ST and SR and suggest that local environmental factors, such as soil properties, seasonal dynamics, or tree physiological strategies, may play a significant role. While further research is required to fully explain the mechanisms behind these observations.
4.2 Effect of drought
Growth declines observed during the study period correspond to years identified as dry in Europe, even though the yearly climate diagrams for the study sites do not indicate evidence of drought during these periods. This suggests that drought effects at the study sites are visible in the form of growth declines, but a finer temporal resolution is needed to fully capture these dynamics. This supports the hypothesis of Zweifel et al. (2021) that drought should be assessed at the seasonal and daily level and is in line with Oberleitner et al. (2022) who report drought limitation also for sub-alpine sites. The observed growth decreases are however smaller than on more xeric sites (Table 2).
When studying sites located at lower altitudes (between 319m and 425m) D'Andrea et al. (2023) found that Picea abies is affected more by precipitation (and therefore water availability) than temperature. This is in accordance to our findings as it was also evident that SM had a bigger impact on SR than ST. However Alavi (2002) reported that even in areas with high annual precipitation, water availability significantly affects tree development, with drier sites experiencing greater growth variation. Our study is consistent with these observations, as none of our sites were water limited, yet we still observed significant SR variation associated with changing SM conditions (Figure 3). Furthermore, Ježík et al. (2015) showed that non-irrigated trees had significantly lower growth compared to irrigated trees, highlighting the positive influence of adequate SM on growth. Our results echo these findings, as increased SM generally led to increased predictions in our model.
4.3 Technical constraints of the model
It is important to acknowledge constraints given by the selected modeling approach. Among these is the model's exclusion of legacy effects. Previous research found that especially precipitation from the previous year had a strong impact on the growth of the radial stem (Miller et al., 2023; Zweifel et al., 2020). Moreover age effects on growth are only taken into account indirectly by the random effects implemented within the model. The higher average growth for MS, seen in Figure 2, is likely caused by such effects as the stand age of 26 is lower than for the other three stands (ranging from 76 and 133 years).
Despite these limitations, we observed a strong relationship between SR of Picea abies and both SM and ST. This relationship may be stronger compared to other tree species, as previous research has shown that Picea abies tends to react more sensitively to environmental conditions (Vacek et al., 2019). Our findings underscore the importance of incorporating SM and ST measurements into future study designs.
5 Conclusions
Our study investigates the effects of environmental conditions on the SR of Picea abies across four different sites in Austria over a ten-year period. Using high-frequency dendrometer data and generalized additive mixed models, we found that SM and ST significantly influence tree growth, with their impacts varying throughout the growing season.
Our results indicate that both SM and ST are critical factors for SR, even in regions with adequate rainfall. Early in the growing season, SR increases with rising ST up to a threshold, beyond which further increases in ST lead to a decline. Conversely, SM consistently promotes SR, with its effects being more pronounced at lower ST. During the middle and toward the end of the growing season, both SM and ST positively influence SR, with SM having a stronger impact toward the end of the season.
The results of our study underscore the importance of incorporating soil parameters into forest monitoring, particularly for species like Picea abies, which exhibit high sensitivity to environmental changes. Our findings suggest that future research initiatives should include detailed monitoring of soil conditions to better predict and mitigate the impacts of climate change on forest ecosystems.
Overall, our research highlights the complex interactions between environmental parameters in determining SR and emphasizes the need for high-resolution, long-term data and large scale monitoring programmes to capture climatological gradients more accurately. We also emphasize the need to incorporate a broader range of soil variables into climate change projections, moving beyond a focus solely on atmospheric parameters.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: data can be requested from the corresponding author. Requests to access these datasets should be directed to Anita Zolles, YW5pdGEuem9sbGVzQGJmdy5ndi5hdA==.
Author contributions
AZ: Writing – original draft, Writing – review & editing. SV: Writing – original draft, Writing – review & editing. SS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors gratefully acknowledge the support of the DocSchool Hazards and Risks in Alpine Regions under Global Change (HADRIAN) for covering the publication fees associated with this manuscript. The authors also thank Karl Gartner for setting up and maintaining the measurement system.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1523834/full#supplementary-material
References
Alavi, G. (2002). The impact of soil moisture on stem growth of spruce forest during a 22-year period. For. Ecol. Manage. 166, 17–33. doi: 10.1016/S0378-1127(01)00661-2
Arend, M., Link, R. M., Patthey, R., Hoch, G., Schuldt, B., and Kahmen, A. (2021). Rapid hydraulic collapse as cause of drought-induced mortality in conifers. Proc. Nat. Acad. Sci. 118:e2025251118. doi: 10.1073/pnas.2025251118
D'Andrea, G., Šimŭnek, V., Pericolo, O., Vacek, Z., Vacek, S., Corleto, R., et al. (2023). Growth response of norway spruce (Picea abies [l.] karst.) in central bohemia (Czech Republic) to climate change. Forests 14, 1215–1215. doi: 10.3390/f14061215
de Vries, W., Reinds, G. J., Posch, M., Sanz, M. J., Krause, G. H. M., Calatayud, V., et al. (2003). Intensive Monitoring of Forest Ecosystems in Europe. Technical Report.
Deslauriers, A., Rossi, S., Anfodillo, T., and Saracino, A. (2008). Cambial phenology, wood formation and temperature thresholds in two contrasting years at high altitude in southern italy. Tree Physiol. 28, 863–871. doi: 10.1093/treephys/28.6.863
Dyderski, M. K., Pa, S., Frelich, L. E., and Jagodziński, A. M. (2018). How much does climate change threaten european forest tree species distributions? Glob. Chang. Biol. 24, 1150–1163. doi: 10.1111/gcb.13925
Etzold, S., Sterck, F., Bose, A. K., Braun, S., Buchmann, N., Eugster, W., et al. (2022). Number of growth days and not length of the growth period determines radial stem growth of temperate trees. Ecol. Lett. 25, 427–439. doi: 10.1111/ele.13933
Fleurial, K., Vaziriyeganeh, M., and Zwiazek, J. J. (2022). Getting cold feet: tree productivity at the mercy of soil temperature. Tree Physiol. 42, 1695–1699. doi: 10.1093/treephys/tpac077
Ge, Z.-M., Ge, Z.-M., Kellomäki, S., Zhou, X., Wang, K.-Y., Wang, K.-Y., et al. (2013). Effects of climate change on evapotranspiration and soil water availability in norway spruce forests in southern Finland: an ecosystem model based approach. Ecohydrology 6, 51–63. doi: 10.1002/eco.276
Gričar, J., Jevšenak, J., Giagli, K., Eler, K., Tsalagkas, D., Gryc, V., et al. (2024). Temporal and spatial variability of phloem structure in picea abies and fagus sylvatica and its link to climate. Plant Cell Environ. 47, 1285–1299. doi: 10.1111/pce.14811
Gričar, J., Zavadlav, S., Jyske, T., Lavrič, M., Laakso, T., Hafner, P., et al. (2019). Effect of soil water availability on intra-annual xylem and phloem formation and non-structural carbohydrate pools in stem of Quercus pubescens. Tree Physiol. 39, 222–233. doi: 10.1093/treephys/tpy101
Grossiord, C., Buckley, T. N., Cernusak, L. A., Novick, K. A., Poulter, B., Siegwolf, R. T., et al. (2020). Plant responses to rising vapor pressure deficit. New Phytol. 226, 1550–1566. doi: 10.1111/nph.16485
Haeni, M., Knüsel, S., Wilhelm, M., Peters, R. L., and Zweifel, R. (2020). treenetproc - Clean, process and visualise dendrometer data.
Herrmann, V., McMahon, S. M., Detto, M., Lutz, J. A., Davies, S. J., Chang-Yang, C.-H., et al. (2016). Tree circumference dynamics in four forests characterized using automated dendrometer bands. PLoS ONE 11:e0169020. doi: 10.1371/journal.pone.0169020
Islam, K. I., Khan, A., and Islam, T. (2015). Correlation between atmospheric temperature and soil temperature: a case study for dhaka, bangladesh. Appl. Categor. Struct. 05, 200–208. doi: 10.4236/acs.2015.53014
Jansson, G., Danusevičius, D., Grotehusman, H., Kowalczyk, J., Krajmerova, D., Skroppa, T., et al. (2013). Norway Spruce (Picea abies (L.) H.Karst.). Dordrecht: Springer Netherlands, 123–176. doi: 10.1007/978-94-007-6146-9_3
Ježík, M., Blaženec, M., Letts, M. G., Ditmarová, L., Sitková, Z., and Střelcová, K. (2015). Assessing seasonal drought stress response in norway spruce (picea abies (l.) karst.) by monitoring stem circumference and sap flow. Ecohydrology 8, 378–386. doi: 10.1002/eco.1536
Jiao, L., Xue, R., Che, X., and Wang, X. (2024). Seasonal and depth dynamics of soil moisture affect trees on the Tibetan plateau. Forests 15:752. doi: 10.3390/f15050752
Kašpar, J., Krček, M., and Král, K. (2024). The effects of solar radiation on daily and seasonal stem increment of canopy trees in European temperate old-growth forests. New Phytol. 243, 662–673. doi: 10.1111/nph.19852
Kašpar, J., Tumajer, J., Tumajer, J., Šamonil, P., and Vašíčková, I. (2021). Species-specific climate-growth interactions determine tree species dynamics in mixed central european mountain forests. Environm. Res. Lett. 16:034039. doi: 10.1088/1748-9326/abd8fb
Klein, T., Rotenberg, E., Cohen-Hilaleh, E., Raz-Yaseef, N., Tatarinov, F., Preisler, Y., et al. (2014). Quantifying transpirable soil water and its relations to tree water use dynamics in a water-limited pine forest. Ecohydrology 7, 409–419. doi: 10.1002/eco.1360
Knüsel, S., Peters, R. L., Haeni, M., Wilhelm, M., and Zweifel, R. (2021). Processing and extraction of seasonal tree physiological parameters from stem radius time series. Forests 12:765. doi: 10.3390/f12060765
Kunert, N. (2020). Preliminary indications for diverging heat and drought sensitivities in norway spruce and scots pine in central europe. iForest-Biogeosci. Forestry 13:89. doi: 10.3832/ifor3216-012
Lahti, M., Aphalo, P., Finér, L., Ryyppö, A., Lehto, T., and Mannerkoski, H. (2005). Effects of soil temperature on shoot and root growth and nutrient uptake of 5-year-old norway spruce seedlings. Tree Physiol. 25, 115–122. doi: 10.1093/treephys/25.1.115
Leštianska, A., Merganičová, K., Merganič, J., and Střelcová, K. (2015). Intra-annual patterns of weather and daily radial growth changes of norway spruce and their relationship in the western carpathian mountain region over a period of 2008-2012. J. Forest Sci. 61, 315–324. doi: 10.17221/24/2015-JFS
Li, M., Wu, P., and Ma, Z. (2020). A comprehensive evaluation of soil moisture and soil temperature from third-generation atmospheric and land reanalysis data sets. Int. J. Climatol. 40, 5744–5766. doi: 10.1002/joc.6549
Lombardero, M., Ayres, M. P., Lorio Jr, P. L., and Ruel, J. J. (2000). Environmental effects on constitutive and inducible resin defences of pinus taeda. Ecol. Lett. 3, 329–339. doi: 10.1046/j.1461-0248.2000.00163.x
Lupi, C., Morin, H., Deslauriers, A., and Rossi, S. (2010). Xylem phenology and wood production: resolving the chicken-or-egg dilemma. Plant, Cell Environ. 33, 1721–1730. doi: 10.1111/j.1365-3040.2010.02176.x
Lupi, C., Morin, H., Deslauriers, A., and Rossi, S. (2011). Xylogenesis in black spruce: does soil temperature matter? Tree Physiol. 32, 74–82. doi: 10.1093/treephys/tpr132
Mäkinen, H., Nöjd, P., Kahle, H., Neumann, U., Tveite, B., Mielikäinen, K., et al. (2003). Large-scale climatic variability and radial increment variation of Picea abies (l.) karst. in central and northern Europe. Trees 17, 173–184. doi: 10.1007/s00468-002-0220-4
Mäkinen, H., Nöjd, P., and Mielikäinen, K. (2001). Climatic signal in annual growth variation in damaged and healthy stands of Norway spruce [Picea abies (l.) karst.] in southern Finland. Trees 15, 177–185. doi: 10.1007/s004680100089
Makinen, H., Nojd, P., and Saranpaa, P. (2003). Seasonal changes in stem radius and production of new tracheids in Norway spruce. Tree Physiol. 23, 959–968. doi: 10.1093/treephys/23.14.959
Mensah, C., Šigut, L., Fischer, M., Foltỳnová, L., Jocher, G., Acosta, M., et al. (2021). Assessing the contrasting effects of the exceptional 2015 drought on the carbon dynamics in two norway spruce forest ecosystems. Atmosphere 12:988. doi: 10.3390/atmos12080988
Migliavacca, G., Rossi, M., Siface, D., Marzoli, M., Ergun, H., Rodríguez-Sánchez, R., et al. (2021). The innovative flexplan grid-planning methodology: how storage and flexible resources could help in de-bottlenecking the european system. Energies 14:1194. doi: 10.3390/en14041194
Miller, T. W., Stangler, D. F., Larysch, E., Honer, H., Puhlmann, H., Schindler, D., et al. (2023). Later growth onsets or reduced growth rates: What characterises legacy effects at the tree-ring level in conifers after the severe 2018 drought? Sci. Total Environm. 854:158703. doi: 10.1016/j.scitotenv.2022.158703
Montagnani, L., Zanotelli, D., Tagliavini, M., and Tomelleri, E. (2018). Timescale effects on the environmental control of carbon and water fluxes of an apple orchard. Ecol. Evol. 8, 416–434. doi: 10.1002/ece3.3633
Neumann, M., and Kindermann, G. (2016). Waldzustandsmonitoring in Österreich 20 Jahre Intensivbeobachtungsflächen (Level II). Technical Report.
Oberhuber, W. (2017). Soil water availability and evaporative demand affect seasonal growth dynamics and use of stored water in co-occurring saplings and mature conifers under drought. Trees 31, 467–478. doi: 10.1007/s00468-016-1468-4
Oberhuber, W., Gruber, A., Kofler, W., and Swidrak, I. (2014). Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner alpine site. Eur. J. For. Res. 133, 467–479. doi: 10.1007/s10342-013-0777-z
Oberleitner, F., Hartmann, H., Hasibeder, R., Huang, J., Losso, A., Mayr, S., et al. (2022). Amplifying effects of recurrent drought on the dynamics of tree growth and water use in a subalpine forest. Plant Cell Environm. 45, 2617–2635. doi: 10.1111/pce.14369
Onwuka, B., and Mang, B. (2018). Effects of soil temperature on some soil properties and plant growth. Adv. Plants Agric. Res 8, 34–37. doi: 10.15406/apar.2018.08.00288
Oogathoo, S., Duchesne, L., Houle, D., and Kneeshaw, D. (2022). Characterizing seasonal radial growth dynamics of balsam fir in a cold environment using continuous dendrometric data: A case study in a 12-year soil warming experiment. Sensors 22:5155. doi: 10.3390/s22145155
Pan, Y., Birdsey, R. A., Fang, J., Houghton, R., Kauppi, P. E., Kurz, W. A., et al. (2011). A large and persistent carbon sink in the world's forests. Science 333, 988–993. doi: 10.1126/science.1201609
Popa, A., van der Maaten, E., Popa, I., and van der Maaten-Theunissen, M. (2024). Early warning signals indicate climate change-induced stress in Norway spruce in the Eastern Carpathians. Sci. Total Environ. 912:169167. doi: 10.1016/j.scitotenv.2023.169167
Pretzsch, H., Del Río, M., Arcangeli, C., Bielak, K., Dudzinska, M., Forrester, D. I., et al. (2023). Forest growth in europe shows diverging large regional trends. Sci. Rep. 13:15373. doi: 10.1038/s41598-023-41077-6
Rathgeber, C. B., Cuny, H. E., and Fonti, P. (2016). Biological basis of tree-ring formation: a crash course. Front. Plant Sci. 7:734. doi: 10.3389/fpls.2016.00734
Riahi, K., van Vuuren, D. P., Kriegler, E., Edmonds, J., O'Neill, B. C., Fujimori, S., et al. (2017). The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Global Environm. Change 42, 153–168. doi: 10.1016/j.gloenvcha.2016.05.009
Romeiro, J. M. N., Eid, T., Antón-Fernández, C., Kangas, A., and Trømborg, E. (2022). Natural disturbances risks in european boreal and temperate forests and their links to climate change-a review of modelling approaches. For. Ecol. Manage. 509:120071. doi: 10.1016/j.foreco.2022.120071
Rossi, S., Deslauriers, A., Anfodillo, T., Morin, H., Saracino, A., Motta, R., et al. (2006). Conifers in cold environments synchronize maximum growth rate of tree-ring formation with day length. New Phytol. 170, 301–310. doi: 10.1111/j.1469-8137.2006.01660.x
Schäfer, C., Rötzer, T., Thurm, E. A., Biber, P., Kallenbach, C., and Pretzsch, H. (2019). Growth and tree water deficit of mixed norway spruce and european beech at different heights in a tree and under heavy drought. Forests 10:577. doi: 10.3390/f10070577
Schurman, J. S., Babst, F., Björklund, J., Rydval, M., Bače, R., Čada, V., et al. (2019). The climatic drivers of primary picea forest growth along the carpathian arc are changing under rising temperatures. Glob. Chang. Biol. 25, 3136–3150. doi: 10.1111/gcb.14721
Smirnakou, S., Ouzounis, T., and Radoglou, K. (2017). Effects of continuous spectrum leds used in indoor cultivation of two coniferous species Pinus sylvestris l. and Abies borisii-regis Mattf. Scand. J. Forest Res. 32, 115–122. doi: 10.1080/02827581.2016.1227470
Spiecker, H. (2000). “Growth of norway spruce (Picea abies [l.] karst.) under changing environmental conditions in Europe,” in EFI Proceedings (Joensuu: Finland European Forest Institute), 11–26.
Thurm, E. A., Hernandez, L., Baltensweiler, A., Ayan, S., Rasztovits, E., Bielak, K., et al. (2018). Alternative tree species under climate warming in managed european forests. For. Ecol. Manage. 430, 485–497. doi: 10.1016/j.foreco.2018.08.028
Vacek, Z., Vacek, S., Slanař, J., Bílek, L., Bulušek, D., Štefančík, I., et al. (2019). Adaption of norway spruce and european beech forests under climate change: from resistance to close-to-nature silviculture. Cent. Eur. Forestry J. 65, 129–144. doi: 10.2478/forj-2019-0013
Vospernik, S., Nothdurft, A., and Mehtätalo, L. (2020). Seasonal, medium-term and daily patterns of tree diameter growth in response to climate. Forestry 93, 133–149. doi: 10.1093/foresj/cpz059
Wickham, H., François, R., Henry, L., and Müller, K. (2019). dplyr: A Grammar of Data Manipulation. R Package Version 0.8.3.
Wood, S. N, Pya, N., and Säfken, B. (2016). Smoothing parameter and model selection for general smooth models (with discussion). J. Am. Stat. Assoc. 111, 1548–1575. doi: 10.1080/01621459.2016.1180986
Wood, S. N. (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society (B) 73, 3–36. doi: 10.1111/j.1467-9868.2010.00749.x
Zavadilová, I., Szatniewska, J., Petrík, P., Mauer, O., Pokorný, R., and Stojanovic, M. (2023). Sap flow and growth response of Norway spruce under long-term partial rainfall exclusion at low altitude. Front. Plant Sci. 14:1089706. doi: 10.3389/fpls.2023.1089706
Zhang, T., Huang, J.-C., Lei, Q., Liang, X., Lindsey, S., Luo, J., et al. (2022). Empirical estimation of soil temperature and its controlling factors in australia: Implication for interaction between geographic setting and air temperature. Catena 208:105696. doi: 10.1016/j.catena.2021.105696
Zhang, X., Li, X., Manzanedo, R. D., D'Orangeville, L., Lv, P., Wang, C., et al. (2021). High risk of growth cessation of planted larch under extreme drought. Environm. Res. Letters 16:014040. doi: 10.1088/1748-9326/abd214
Zweifel, R., Etzold, S., Sterck, F., Gessler, A., Anfodillo, T., Mencuccini, M., et al. (2020). Determinants of legacy effects in pine trees-implications from an irrigation-stop experiment. New Phytol. 227, 1081–1096. doi: 10.1111/nph.16582
Zweifel, R., Sterck, F., Braun, S., Buchmann, N., Eugster, W., Gessler, A., et al. (2021). Why trees grow at night. New Phytol. 231, 2174–2185. doi: 10.1111/nph.17552
Zweifel, R., Zimmermann, L., and Newbery, D. (2005). Modeling tree water deficit from microclimate: an approach to quantifying drought stress. Tree Physiol. 25, 147–156. doi: 10.1093/treephys/25.2.147
Keywords: dendrometer, tree growth, soil moisture, soil temperature, generalized additive mixed models
Citation: Zolles A, Vospernik S and Schüler S (2025) Effects of soil parameters of radial stem growth of four spruce stands in Austria. Front. For. Glob. Change 8:1523834. doi: 10.3389/ffgc.2025.1523834
Received: 06 November 2024; Accepted: 10 January 2025;
Published: 21 February 2025.
Edited by:
Gabriel Sangüesa-Barreda, University of Valladolid, SpainReviewed by:
Álvaro Rubio Cuadrado, Universidad Politécnica de Madrid, SpainJan Tumajer, Charles University, Czechia
Copyright © 2025 Zolles, Vospernik and Schüler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anita Zolles, YW5pdGEuem9sbGVzQGJmdy5ndi5hdA==
 Anita Zolles
Anita Zolles Sonja Vospernik
Sonja Vospernik Silvio Schüler
Silvio Schüler