
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 10 October 2024
Sec. Forest Management
Volume 7 - 2024 | https://doi.org/10.3389/ffgc.2024.1437954
This article is part of the Research Topic Land Degradation and Forest Management View all 17 articles
 Nandan Singh1*†
Nandan Singh1*† Ashish Tewari1†
Ashish Tewari1† Amit Mittal2
Amit Mittal2 Shruti Shah1
Shruti Shah1 Mamta Bisht3
Mamta Bisht3 Sazada Siddiqui4*
Sazada Siddiqui4* Mohammed O. Alshaharni4
Mohammed O. Alshaharni4 Ayesha Saddiqua5
Ayesha Saddiqua5Several high-elevation plant species would experience an increased risk of regional extinction due to various climatic and anthropogenic factors. Information about the effects of climate change is urgently needed for modeling vegetation dynamics because it influences the various seed parameters like seed germination, seed maturation, seed mass, and seed bank in the soil. The present study was conducted at an elevation of 3145–3560 m in the treeline area of the western Himalayan region of India. The change in seed color is correlated with other seed parameters such as seed moisture content, seed germination, seed mass, and seed fall density. A decline in moisture content in maturing seeds is closely related to seed maturity (p < 0.05). Quercus semecarpifolia contains the highest seed mass followed by Abies spectabilis. Reportedly, the species with higher seed mass have an advantage in light-restricted environments for seed germination and seedling development. In addition, the fruit mass was observed to be the highest for Rhododendron campanulatum, while both Betula utilis and R. arboreum had similar fruit mass. The seed fall density varied between 1.55 and 7.85 seeds m–2 and the maximum mortality of up to 32% of seedlings was observed during post-monsoon season from November to February. The potential disruption in the timing of seed fall, soil seed bank, and seed germination due to climatic irregularities has broader implications for forest ecosystems. Generally, the soil in treeline areas gets frozen during winter, resulting in seedlings facing severe water stress and a high rate of transpiration. The present study addresses the issue regarding the survival and proliferation of important treeline species in the western Himalayan region of India.
Treeline forms one of the most prominent ecologically significant boundaries in the Himalayan arc that marks the upper limit of the forest vegetation and represents an ecotone between the closed canopy forest and the alpine zone (Maletha et al., 2020; Singh et al., 2023). In the recent past, treeline zones have been identified as sensitive areas to environmental change and could be effectively modeled and monitored as an indicator for climate changes at regional and global levels (Maletha et al., 2020; Singh et al., 2019a). In the higher elevation, the impact of climate change is considered the most pronounced and severe risk to species regeneration, survival, diversity, distribution, phenology, physiology, and seed ecology (Singh et al., 2019b). Although, several studies have been conducted on vegetation structure and composition, plant communities along altitudinal gradients, resource utilization patterns, anthropogenic pressure, and the impact of climate change on Himalayan vegetation (Maletha et al., 2023), only a little attention paid to investigating the seed-related studies of treeline species in the Himalayan region.
In recent years several high-elevation or alpine plant species experienced an increased risk of regional extinction due to climate change (Dirnböck et al., 2003; Singh et al., 2021). Species differently response to warming either immediately or after some years. With the recent warming, several plant species showed early fruit ripening, particularly in the spring season, which has a significant impact on plant recruitment and population dynamics (Tewari et al., 2019). Information on regeneration under climate change is urgently needed for modeling vegetation dynamics (Leishman et al., 1992; Ibáñez et al., 2007; Morin and Thuiller, 2009) because it influences seed germination via seed maturation and/or seed mass and/or seed bank in the soil. High and low temperatures and lower amounts of water in soil imposed on parents influence the phenotypic expression of maturation, viability, seed mass, seed longevity, dormancy, germination percentage of seeds, and early growth in tree species, over more than one subsequent generation (Kochanek et al., 2010).
Climate has a dominant influence on several life-history traits of plant species (Bernareggi et al., 2016). Among plant reproductive phases, seed germination and seedling establishment are probably the most sensitive to variation in climate conditions (Walck et al., 2011). In seasonal climates, characterized by cyclic variations in temperature and precipitation, seed germination is usually synchronized with the changes in environmental conditions, being delayed until a favorable period occurs (Fenner and Thompson, 2005; Baskin and Baskin, 2014). Seed development and maturation is a process comprising a series of morphological, physical, physiological, and biochemical changes that occur from ovule fertilization to the time when seeds become physiologically independent of the parent plant (Delouche, 1971; Sripathy and Groot, 2023). Physiological maturity is identified as maximum seed dry mass accumulation. Seed maturation is one of the main factors of seed quality and a prerequisite for successful germination and emergence (Sripathy and Groot, 2023). After fertilization, the moisture content of seeds increases throughout the early stages of development before starting to decrease until environmental conditions are in balance (Sripathy and Groot, 2023).
The strong correlation between climate and plant regeneration from seeds has resulted in the evolution of specific germination requirements across many species (Fenner and Thompson, 2005; Baskin et al., 2000), which play a key role in plant distribution and vegetation dynamics (Thuiller et al., 2008). The effects of climate change on the early life-history stages of plants from high altitude and high latitude environments have recently become a focus of researchers (Briceño et al., 2015). Historically, it was believed that sexual reproduction in these ecosystems was rare and less important than clonal reproduction because of the harsh environmental conditions (Billings and Mooney, 1968; Hoyle et al., 2013). However, recent studies have found important persistent soil seed banks (Hoyle et al., 2013; Venn and Morgan, 2010), high rates of natural seedling recruitment (Venn and Morgan, 2010; Forbis, 2003), and considerable gene flow among populations (Jonsson et al., 1996; Pluess and Stöcklin, 2004), which suggests that recruitment from seed is common and plays an essential role in high altitude and high latitude community dynamics (Briceño et al., 2015). This study aims to investigate the physiological maturity of fruits and seeds, soil seed banks, and seedling dynamics of five major treeline-forming species in the western Himalayan region which somewhere divulges directly or indirectly into the seed ecology-related issues of treeline species that are rarely investigated such as; how global warming affects treeline species germination and survival, their seed maturation timing, what temperatures are needed for seed development, dormancy break and how does precipitation affect germination.
The present study was conducted at Tungnath treeline areas, situated in the western Indian Himalayas. The investigated site is located between 3145 and 3360 m above sea level at 30029′45½N latitude and 79013′24½E longitude and falls within the sub-alpine and alpine zones (Figure 1). The soil in these regions has a characteristic brown hue and possesses a sandy loam texture, characterized by a high concentration of sand and silt particles, and exhibits acidity, as indicated by pH values ranging from 4.0 to 5.0. The climate of the research region is impacted by the monsoon, which is characterized by extended periods of harsh winters and brief periods of mild summers. In the study sites, the monthly temperature varied between −6.02 ± 0.23 and 13.71 ± 1.03 C and the monthly precipitation was 13.0 ± 1.16 and 541.0 ± 4.37 mm during at study period between 2017 and 2020 (IHTP, 2021). The study was conducted in five major treeline-forming species, A. spectabilis, B. utilis, Q. semecarpifolia, R. arboreum, and R. campanulatum.

Figure 1. Map of the study site (Map of India source: Survey of India, Satellite Map of the study site source: Google Earth).
A designated area of 100 × 100 m was demarcated at the study site. Within this area, a total of 25 mature and healthy trees of each studied species were randomly selected and marked of approximately similar height and diameter, having a sufficient number of fruits and seeds. The fruits/seeds were directly collected manually from the previously marked trees. The color of fruits/seeds was manually observed and analyzed by Pantone color chart. The physical parameters of fruits and seeds, size (mm2) were measured using a digital vernier caliper, weight, and mass (g) were measured using a digital electronic balance (Singh et al., 2023; Mittal et al., 2020). The moisture content of fruits/seeds was calculated by comparing their actual weight to their dry weight, dried at 103 ± 2°C for 16 ± 1 h (International Seed Testing Association, 1981). The germination experiment was conducted in a dual chamber seed germinator for each collection date (Singh et al., 2021). The petri-dish and germination paper were sterilized at a high temperature (130°C) for 4 h to make it free from fungal infection. The germination of seeds was carried out at 25 ± 1°C on the top of the seed germinator paper under dark conditions in the seed germinator. Water was added as required during the experiment. After completion of the experiment, germination percent was calculated as the total number of germinated seeds out of tested seeds within the test period. The seed fall density, soil seed bank, and seedling dynamics assessment were carried out in different locations such as, under the canopy of trees, between the canopy of trees, and inside the canopy of trees at the study sites (Singh, 2019). The seed fall density assessment was observed in 20 seed traps of 1 × 1 m size from the different selected locations at the study site. Further, any loss of fruits/seeds due to any reason was neglected at the study site. For seed fall density assessment, the seeds were collected from the trap every week (Q. semecarpifolia and B. utilis) and fortnightly (A. spectabilis, R. arboreum, and R. campanulatum) during the seed fall season (Mittal, 2018), the fallen seeds were counted to calculate for seed fall density. For assessment of the soil seed bank, five soil samples from each 1 m2 in each transect sample, soil monoliths 25 × 25 × 15 cm3 were extracted from three depth classes 0–5 cm, 5–10 cm, and 10–15 cm in 20 quadrats of 5 × 5 m with a sub-plot of 1 m2 located in the middle of each quadrate from different locations at the study sites, after the seed fall season. From each soil depth class larger-sized seeds could be separated manually from the soil and soil excluding large seeds was kept depth-wise in a germination tray. To assess the seed density per m2 seed germination experiment was conducted. Newly germinated seedlings were identified and recorded (Mittal et al., 2021). To assess the seedling dynamics, newly recruited seedlings were tagged individually in the field on a 1 m2 plot from 20 different locations at the study sites, and the survival/mortality of the tagged seedling was subsequently monitored (Singh et al., 2021). The data were subjected to analysis of variance with a 95% confidence level using SPSS version 2016. The correlation coefficient (r) was used to express the strength of the relationship between variables.
The study emphasizes the unique ecological context of the Himalayan treeline region, where seed maturation is synchronized with the onset of the monsoon. This synchronization is critical for the reproductive success of many forest tree species in the region. The relationship between climate and the maturation process of seeds and fruits is a critical aspect of plant biology and ecological dynamics. Climate change, characterized by shifts in temperature and precipitation patterns, has profound effects on the timing and success of plant reproduction (Totland, 1997). Various mature and immature fruits/seeds can be distinguished in various ways e.g., by color difference, increased firmness, decreased moisture content, specific gravity, and change in physical dimensions (Tamta and Singh, 2018; Jyotnsa et al., 2020). Distinct fruit/seed color changes have been associated with seed maturity in several species (Singh et al., 2020a). In this study, the color change from greenish brown to brown of A. spectabilis seeds, from dark green to brown of B. utilis catkins, from light green to dark greenish brown of Q. semecarpifolia acorn and from green to dark green of R. arboreum and R. campanulatum capsules serves as a reliable indicator of maturity (Table 1). The changes in fruit/seed color from its appearance to maturity showed a significant relationship with other seed parameters such as seed moisture content, seed germination (Tewari et al., 2019; Tamta and Singh, 2018; Jyotnsa et al., 2020), seed mass, and seed fall density. Seeds may mature earlier or later than usual in response to changes in temperature, impacting the overall reproductive success of plant species.
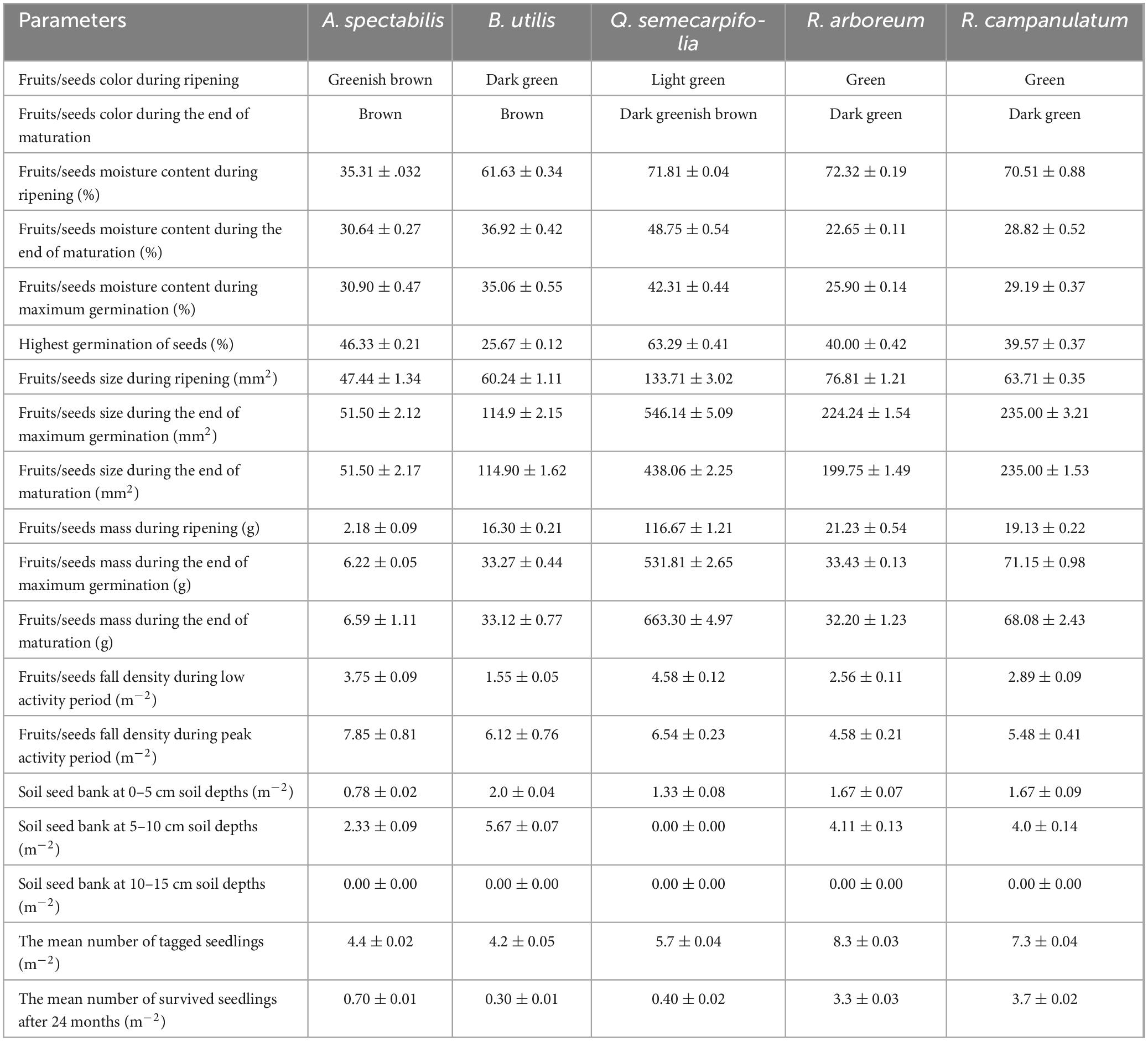
Table 1. Physical parameters of fruits/seeds, seed fall density (m–2), soil seed bank (m–2), and seedling survival (m–2) of studied treeline species.
Besides color, the other physical parameter that is interrelated with maturity is moisture content. Changes in precipitation patterns, including alterations in the frequency and intensity of rainfall, can directly affect plant water availability. Adequate water is crucial for seed ripening and development, and variations in precipitation can influence seed moisture content (Khaeim et al., 2022). The maximum germination rates under laboratory conditions were observed for Q. semecarpifolia followed by A. spectabilis, R. arboreum, R. campanulatum and B. utilis (Table 1). Generally, fleshy fruits typically increase in moisture content during ripening, while dry fruits experience a decline. The decline in moisture content in maturing seeds in the study serves as a strong indicator of ripeness and maturity (Singh et al., 2023; Singh et al., 2021; Tewari et al., 2019; Tamta and Singh, 2018; Jyotnsa et al., 2020; Singh et al., 2020a). The observation from the present study suggests a negative correlation between seed germination and moisture content of A. spectabilis (r = −0.13), B. utilis (r = −0.71), Q. semecarpifolia (r = −0.88), R. arboreum (r = −0.93) and R. campanulatum (r = −0.74) at 0.05% significant level (p < 0.05). This suggests that as seeds mature and moisture content decreases, germination potential increases. This relationship is important for understanding the optimal conditions for seed germination and how moisture content serves as a regulatory factor. In the Himalayan treeline region, there are many forest tree species in which seed maturation is synchronized with the commencement of monsoon, and their seed viability is very low (Tewari et al., 2019). Climatic irregularities like rising temperatures and irregular patterns of rainfall may impact the synchronization between the timing of seed fall and monsoon rains, particularly in Q. semecarpifolia which is a viviparous species and coincides with its seed maturation with monsoon rains (Tewari et al., 2019).
The physical parameters of fruit/seed such as size, weight, number, and mass have also been associated with maturation time (Singh et al., 2023). The study showed that the fruits/seed’s size and weight continuously increased from ripening to maturation. During maturation, the mean fruits/seeds size and the mean mass of 100 fruits/seeds were calculated for seeds of A. spectabilis, catkins of B. utilis, acorn of Q. semecarpifolia, and capsules of R. arboreum and R. campanulatum (Table 1). Species attain maximum weight and mass at the time the end of maturation and increase seed quality, including seed longevity (Ramtekey et al., 2022). The continuous increase in fruit and seed size and weight from ripening to maturation, suggests a strategic allocation of resources by plants. Larger and heavier seeds may have advantages in terms of nutrient reserves, potentially increasing the chances of successful germination and seedling establishment (Domic et al., 2020). Among the studied species, Q. semecarpifolia showed a higher seed size and seed mass (Table 1). The species that contain higher seed mass may be better equipped to withstand certain environmental stresses and provide a competitive advantage in light-restricted environments for seed germination and seedling development and also seeds with higher quality and longevity may have a better chance of persistence in the soil seed bank, contributing to the long-term resilience of plant populations (Chazdon, 2013). On the other hand, species like B. utilis, R. arboreum, and R. campanulatum, with smaller and minute seeds, may face challenges during the transition from germination to seedling emergence. Small-seeded species often experience higher mortality rates in this critical stage (Singh et al., 2020a).
Successful management of natural regeneration must use the natural patterns and timing of seed production to maximize the available seed supply to a given site during the period of greatest site receptivity (Nixon and Worrell, 1998). Seed production can vary considerably as a result of tree species, tree age, woodland management, and climate conditions in a particular area (Broome et al., 2016). In this study, the fruits/seeds fall density varied between 3.75 ± 0.09 and 7.85 ± 0.81 seeds m–2 in A. spectabilis and 1.55 ± 0.05 to 6.12 ± 0.76 seeds m–2 in Q. semecarpifolia, 4.58 ± 0.12 to 6.54 ± 0.23 catkin m–2 in B. utilis, 2.56 ± 0.11 to 4.58 ± 0.21 capsule m–2 in R. arboreum and 2.89 ± 0.09 to 5.48 ± 0.41 capsule m–2 in R. campanulatum (Table 1). The seed fall density of these species was found very low, as a report on Q. semecarpifolia suggests that the species can produce a good seed crop after a few years of gap (Verma et al., 2015). Further, a study on conifers reported the first large cone crop at the age of 15 to 30 years (Broome et al., 2016), as A. spectabilis is also a conifer species. While B. utilis, R. arboreum, and R. campanulatum produce very minute seeds, the germination rate of minute seeds becomes very low (Martínez-Garza et al., 2013). Climate change-induced irregularities, such as rising temperatures and unpredictable rainfall patterns, pose a threat to the synchronization between seed maturation and monsoon rains. The delay or prolonged breaks in monsoons can adversely affect the timing of seed fall and, consequently, the regeneration ecology of forests in monsoonal climates (Negi and Rawal, 2019). Climatic factors such as temperature and precipitation, along with local variations in elevation, aspect, and exposure, play a crucial role in seed production. Favorable climatic conditions support the development of seeds and contribute to higher seed viability. At higher altitudes, cones tend to be smaller, with fewer and lighter seeds that have lower average viability levels. Increasing elevation can also influence the periodicity of good seed years with the intervals between good seed years being longer and more irregular at higher elevations compared with lower elevations (Nixon and Worrell, 1998). The potential disruption in the timing of seed fall and germination due to climatic irregularities has broader implications for the overall dynamics of forest ecosystems, whereas, lower seed fall density and irregular seed production patterns can pose challenges for natural regeneration. These challenges may lead to fluctuations in the abundance and distribution of plant species within forest ecosystems.
A soil seed bank is a major initiator of regeneration in a natural forest, especially in treeline areas where several anthropogenic and grazing pressures are commonly observed (Singh et al., 2019a). Across the treeline sites and species, the number of viable seeds of all species declined with increasing soil depth. The maximum number of viable seeds of all species were present in the topsoil layer while it was completely absent on 10–15 cm soil depths of all species (Table 1). The topsoil layer contains the highest number of viable seeds because the topsoil layer has the maximum number of seeds of the current year and they maintain their viability while at the deeper layer, they lose their viability with time. The overall, soil seed bank of studied species was very low and ranged between 0.78 ± 0.02 and 2.33 ± 0.09 seed m–2 of A. spectabilis, 2.0 ± 0.04 and 5.67 ± 0.07 seed m–2 of B. utilis, 1.33 ± 0.08 seed m–2 of Q. semecarpifolia, 1.67 ± 0.07 and 4.11 ± 0.13 seed m–2 of R. arboreum and 1.67 ± 0.09 and 4.0 ± 0.14 seed m–2 of R. campanulatum (Table 1). Several factors such as low density of trees, immature or over mature trees, low seed production, gap in the mast seed year, collection of fruit/seed from the tree, lopping, and grazing could be responsible for minimal soil seed banks. Increased frequency and intensity of extreme weather events, such as storms or droughts, can disturb the soil and affect seed viability and germination.
The regeneration of a forest is a vital process in which old trees die and are replaced by young ones in perpetuity (Malik and Bhatt, 2016). Harsh climatic conditions in the alpine zone might restrict the regeneration and survival of treeline tree species (Tewari et al., 2018). Changes in winter temperatures may impact the freezing and thawing of soil, affecting seedling survival. Over the study period, the seedlings number continuously declined from initial to final observation. The seedling survival percentage (m–2) after 24 months of observation, was 15.91% seedling m–2, 7.14% seedling m–2, 7.02% seedling m–2, 39.76% seedling m–2 and 50.68% seedling m–2 respectively of A. spectabilis, B. utilis, Q. semecarpifolia, R. arboreum and in R. campanulatum (Figure 2). The transformation from seedlings to adults is important and therefore the regeneration dynamics is a major thrust area of the study in terms of regeneration and management of forests (Miranda et al., 2018). The maximum 20−32% mortality of seedlings was observed during the winter season from November to February (Figure 2). Generally, the soil in treeline areas gets frozen during winter and the transpiration rate of seedlings becomes high and faces severe water stress this may be the main cause of maximum mortality during winter (Singh et al., 2019b). Periods of low water or drought, intensified by climate change, can induce stress on plants, potentially affecting seed development and maturation. Drought stress might lead to premature maturation, impacting the quality, viability, and survival of seeds and seedlings (Zhang et al., 2009). Severe biotic and high anthropogenic pressure was also responsible for high seedling mortality in treeline areas (Singh et al., 2020b). Across all the study sites heavy grazing was observed during snow snow-free period from May to October during the daytime which can directly or indirectly impact the overall life cycle of tree species.
Temperature and precipitation play a significant role in various aspects of plant reproductive and vegetative processes, including seed maturation, seed fall, soil seed banks, and seedling survival in treeline areas. The visible indicators such as color changes in fruits and seeds highlight the complexity of ecological processes influenced by climatic factors. The challenges observed in seedling survival are exacerbated by heavy anthropogenic pressure, particularly grazing, and stressful winter conditions. The low regeneration of seeds and seedling survival of treeline species emphasize the need for adaptive management approaches that consider both natural and human-induced factors. Knowledge of the exact time of seed maturation is essential that help land managers prescribe site treatments that produce desired vegetation conditions for future multiplication. Furthermore, to authenticate the above conclusion, a more extensive period will be required.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
NS: Writing – review and editing, Writing – original draft, Visualization, Validation, Supervision, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. AT: Writing – review and editing, Writing – original draft, Validation, Supervision, Methodology, Conceptualization. AM: Writing – review and editing, Validation, Methodology, Data curation, Conceptualization. SS: Writing – review and editing, Validation, Methodology. MB: Writing – review and editing. SS: Validation, Writing – review and editing, Funding acquisition. MA: Validation, Writing – review and editing. AS: Validation, Writing – review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Group Project under grant number RGP2/49/45.
We are thankful to the Head, Department of Forestry, Kumaun University, Nainital for providing laboratory facilities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Baskin, C. C., and Baskin, J. M. (2014). Seeds: Ecology, biogeography, and evolution of dormancy and germination, 2nd Edn. London: Academic Press.
Baskin, J. M., Baskin, C. C., and Li, X. (2000). Taxonomy, ecology, and evolution of physical dormancy in seeds. Plant Species Biol. 15, 139–152.
Bernareggi, G., Carbognani, M., Mondoni, A., and Petraglia, A. (2016). Seed dormancy and germination changes of snowbed species under climate warming: The role of pre- and post-dispersal temperatures. Ann. Bot. 118, 529–539. doi: 10.1093/aob/mcw125
Billings, W. D., and Mooney, H. A. (1968). The ecology of artic and alpine plants. Biol. Rev. 43, 481–529.
Briceño, V. F., Hoyle, G. L., and Nicotra, A. B. (2015). Seeds at risk: How will a changing alpine climate affect regeneration from seeds in alpine areas? Alp. Bot. 125, 59–68.
Broome, A., Summers, R. W., and Vanhala, T. (2016). Understanding the provision of conifer seed for woodland species. Forest Res. 28, 1–12.
Chazdon, R. L. (2013). “Tropical forest regeneration,” in Encyclopedia of biodiversity, 2nd Edn, ed. S. A. Levin (Cambridge, MA: Academic Press), 277–286. doi: 10.1016/B978-0-12-384719-5.00377-4
Delouche, J. C. (1971). “Determinants of seed quality,” in Proceedings of the short course for seedsmen, (New York, NY). doi: 10.3390/plants9111493
Dirnböck, T., Dullinger, S., and Grabherr, G. (2003). A regional impact assessment of climate and land-use change on alpine vegetation. J. Biogeogr. 30, 401–417.
Domic, A. I., Capriles, J. M., and Camilo, G. R. (2020). Evaluating the fitness effects of seed size and maternal tree size on Polylepis tomentella (Rosaceae) seed germination and seedling performance. J. Trop Ecol 36, 115–122. doi: 10.1017/S0266467420000061
Forbis, T. A. (2003). Seedling demography in an alpine ecosystem. Am. J. Bot. 90, 1197–1206. doi: 10.3732/ajb.90.8.1197
Hoyle, G. L., Venn, S. E., Steadman, K. J., Good, R. B., Mcauliffe, E. J., Williams, E. R., et al. (2013). Soil warming increases plant species richness but decreases germination from the alpine soil seed bank. Glob. Chang Biol. 19, 1549–1561. doi: 10.1111/gcb.12135
Ibáñez, I., Clark, J. S., LaDeau, S., and Hille Ris Lambers, J. (2007). Exploiting temporal variability to understand tree recruitment response to climate change. Ecol. Monogr. 77, 163–177.
IHTP (2021). “Meteorological data were collected from Tungnath,” in Under the project “Timberline and altitudinal gradient ecology of Himalayas, and human use sustenance in a warming climate”, MoEF&CC, Government of India, ed. R. Joshi (Paris: IHTP).
International Seed Testing Association (1981). Moisture content and equipment Wkg Group: Report of the forest tree seed committee. Seed Sci. Technol. 9, 101–108.
Jonsson, B. O., Jonsdottir, I. S., and Cronberg, N. (1996). Clonal diversity and allozyme variation in populations of the arctic sedge Carex bigelowii (Cyperaceae). J. Ecol. 84:449.
Jyotnsa, Tewari, A., Shah, S., Tamta, K. K., and Singh, N. (2020). Fruit maturation and germination in Ficus auriculata Lour. A Lesser-known multipurpose tree species in Kumaun Himalayan Region. Ecol. Environ. Conserv. 26, 142–147.
Khaeim, H., Kende, Z., Jolánkai, M., Kovács, G. P., Gyuricza, C., and Tarnawa, Á (2022). Impact of temperature and water on seed germination and seedling growth of maize (Zea mays L.). Agronomy 12:397. doi: 10.3390/agronomy12020397
Kochanek, J., Buckley, Y. M., Probert, R. J., Adkins, S. W., and Steadman, K. J. (2010). Pre-zygotic parental environment modulates seed longevity. Aust. Ecol. 35, 837–848. doi: 10.1111/j.1469-8137.2011.03681.x
Leishman, M. R., Hughes, L., French, K., Armstrong, D., and Westoby, M. (1992). Seed and seedling biology in relation to modelling vegetation dynamics under global climate change. Aust. J. Bot. 40, 599–613.
Maletha, A., Maikhuri, R. K., and Bargali, S. S. (2020). Criteria and indicator for assessing threat on Himalayan birch (B. utilis) at timberline ecotone of Nanda Devi biosphere reserve: A world heritage site, Western Himalaya, India. Environ. Sustain. Indicat. 8, 1–7.
Maletha, A., Maikhuri, R. K., and Bargali, S. S. (2023). Population structure and regeneration pattern of Himalayan birch (Betula utilis D. Don) in the timberline zone of Nanda Devi biosphere reserve, Western Himalaya, India. Geol. Ecol. Landsc. 7, 248–257.
Malik, A. Z., and Bhatt, A. B. (2016). Regeneration status of tree species and survival of their seedlings in Kedarnath wildlife sanctuary and its adjoining areas in Western Himalaya, India. Trop. Ecol. 57, 677–690.
Martínez-Garza, C., Bongers, F., and Poorter, L. (2013). Are functional traits good predictors of species performance in restoration plantings in tropical abandoned pastures? Forest Ecol. Manag. 303, 35–45.
Miranda, Z. P., Guedes, M. C., Batista, A. P. B., and da Silva, D. A. S. (2018). Natural regeneration dynamics of Mora paraensis (Ducke) in estuarine floodplain forests of the Amazon River. Forests 9, 1–14.
Mittal, A. (2018). Impact of tree water relations and environmental drivers on phenology and regeneration in forests of Kumaun central Himalaya. [Ph. D. Thesis]. Nainital: Kumaun University.
Mittal, A., Singh, N., Tewari, A., and Shah, S. (2020). Cone maturation timing and seed germination in Pinus roxburghii (Serg.) in the central Himalayan region of Uttarakhand, India. Ecol. Envi. Conserv. 26, 286–290.
Mittal, A., Tewari, A., Singh, N., and Shah, S. (2021). Assessment of soil seed bank on three different vegetation types in Kumaun Central Himalayan Forest. Indian J. Ecol. 48, 381–386.
Morin, X., and Thuiller, W. (2009). Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90, 1301–1313. doi: 10.1890/08-0134.1
Negi, M., and Rawal, R. S. (2019). Desiccation response of seeds of Himalayan oak, Quercus floribunda Lindl. ex A. Camus. Natl. Acad. Sci. Lett. 42, 291–294.
Nixon, C. N., and Worrell, R. (1998). The potential for the natural regeneration of conifers in britain. Bull. For. Commission 174:7. doi: 10.1371/journal.pone.0222936
Pluess, A. R., and Stöcklin, J. (2004). Population genetic diversity of the clonal plant Geum reptans (Rosaceae) in the swiss alps. Am. J. Bot. 91, 2013–2021. doi: 10.3732/ajb.91.12.2013
Ramtekey, V., Cherukuri, S., Kumar, S., Kudekallu, V. S., Sheoran, S., Bhaskar, U. K., et al. (2022). Seed longevity in legumes: Deeper insights into mechanisms and molecular perspectives. Front. Plant Sci. 13:918206. doi: 10.3389/fpls.2022.918206
Singh, N. (2019). Phenological events and water relations of major tree species in treeline areas of Uttarakhand. [Ph. D. thesis]. Nainital: Kumaun University.
Singh, N., Tewari, A., and Shah, S. (2019a). Tree regeneration pattern and size class distribution in anthropogenically disturbed sub-alpine treeline areas of indian Western Himalaya. Int. J. Sci. Technol. Res. 8, 537–546.
Singh, N., Tewari, A., and Shah, S. (2020a). Catkin maturation timing and seed germination in Betula utilis (D. Don) in the western Himalayan treeline area of Uttarakhand. J. Adv. Sci. Res. 11, 145–151.
Singh, N., Tewari, A., Shah, S., and Mittal, A. (2019b). Water relations and phenological events of two treeline Rhododendron species in Indian western Himalaya. Sylwan 163, 164–176.
Singh, N., Tewari, A., Shah, S., and Mittal, A. (2020b). Treeline species: Regeneration status and seedling dynamics in Western Himalayan Region. Environ. Ecol. 38, 725–732.
Singh, N., Tewari, A., Shah, S., and Mittal, A. (2021). Capsule ripening and seed germination in Rhododendron campanulatum (D. don) in Aali treeline area of Western Himalaya, India. Indian J. Ecol. 48, 398–403.
Singh, N., Tewari, A., Shah, S., and Mittal, A. (2023). Seasonal water relations and stress tolerance of Quercus semecarpifolia (Smith) in treeline areas of Western Himalaya, India. Vegetos 37, 1307–1318. doi: 10.1007/s42535-023-00665-7
Sripathy, K. V., and Groot, S. P. C. (2023). “Seed development and maturation,” in Seed science and technology, eds M. Dadlani and D. Yadava (Singapore: Springer), doi: 10.1007/978-981-19-5888-5_2
Tamta, K., and Singh, N. (2018). Seed ripening indication in Quercus leucotrichophora A. Camus, in Kumaun, Central Himalayan region. Int. J. Adv. Res. Dev. 3, 49–52.
Tewari, A., Shah, S., Singh, N., and Mittal, A. (2018). Treeline species in Western Himalaya are not water stressed: A comparison with low elevation species. Trop. Ecol. 59, 313–325.
Tewari, A., Shah, S., Singh, N., Tamta, K. K., and Mittal, A. (2019). Acorn maturation and regeneration problem in Quercus Semecarpifolia Sm. in Himalayan treeline. Int. J. Sci. Technol. Res. 8, 3781–3787.
Thuiller, W., Albert, C. H., Araújo, M. B., Berry, P. M., Cabeza, M., Guisan, A., et al. (2008). Predicting global change impacts of plant species’ distributions: Future challenges. Perspect. Plant Ecol. Evol. Syst. 9, 137–152. doi: 10.1016/j.ppees.2007.09.004
Totland, Ø (1997). Effects of flowering time and temperature on growth and reproduction in Leontodon autumnalis var. taraxaci, a late-flowering alpine plant. Arctic Alp. Res. 29, 285–290. doi: 10.2307/1552142
Venn, S. E., and Morgan, J. W. (2010). Soil seedbank composition and dynamics across alpine summits in south-eastern Australia. Aust. J. Bot. 58, 349–362.
Verma, A., Shah, S., and Tewari, A. (2015). Survival problem in regeneration of high altitude Kharsu oak (Quercus semecarpifolia Smith) Forests in Central Himalaya. Int. J. Bioassays 4, 3689–3692.
Walck, J. L., Hidayati, S. N., Dixon, K. W., Thompson, K., and Poschlod, P. (2011). Climate change and plant regeneration from seed. Glob. Change Biol. 17, 2145–2161.
Keywords: seed maturity, seedling dynamics, seed mass, soil seed bank, treeline
Citation: Singh N, Tewari A, Mittal A, Shah S, Bisht M, Siddiqui S, Alshaharni MO and Saddiqua A (2024) Seed ecology and seedling dynamics of western Himalayan treeline tree species. Front. For. Glob. Change 7:1437954. doi: 10.3389/ffgc.2024.1437954
Received: 24 May 2024; Accepted: 24 September 2024;
Published: 10 October 2024.
Edited by:
Gopal Shukla, North Eastern Hill University, IndiaReviewed by:
Krishna Kumar Tamta, Uttarakhand Open University, IndiaCopyright © 2024 Singh, Tewari, Mittal, Shah, Bisht, Siddiqui, Alshaharni and Saddiqua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nandan Singh, bmFuZGFuZm9yZXN0cnlAZ21haWwuY29t; Sazada Siddiqui, c2FzZGVreUBra3UuZWR1LnNh
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.