
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 27 May 2024
Sec. Forest Management
Volume 7 - 2024 | https://doi.org/10.3389/ffgc.2024.1395394
This article is part of the Research Topic Land Degradation and Forest Management View all 17 articles
 Kamlesh Verma1,2
Kamlesh Verma1,2 Ashwani Kumar1*
Ashwani Kumar1* Raj Kumar1*
Raj Kumar1* Ajay Kumar Bhardwaj1
Ajay Kumar Bhardwaj1 Sunita Devi1
Sunita Devi1 Aarju Sharma1
Aarju Sharma1 Prashant Sharma3
Prashant Sharma3Introduction: Sandalwood (Santalum album L.) is categorized as vulnerable in the IUCN Red list and is also an industrially important tree species valued for its heartwood and aromatic oil. Sandalwood is a semi-root parasite tree that relies on its host plants for its water and nutrient requirements. Therefore, there is need to understand the growth and physiological interactions between sandalwood and its hosts.
Methods: Sandalwood were planted with ten different host species viz., Syzygium cumini, Punica granatum, Phyllanthus emblica, Melia dubia, Leucaena leucocephala, Dalbergia sissoo, Casuarina equisetifolia, Citrus aurantium, Azadirachta indica and Acacia ampliceps to assess the interactive effect on the change in growth and physiology of both sandalwood and host tree species.
Results: The findings revealed that sandalwood grown with hosts D. sissoo and C. equisetifolia showed higher growth performance, while among hosts, S. cumini, followed by C. aurantium and L. leucocephala, showed better growth and physiobiochemical traits. The stepwise regression analysis and trait modeling indicated that the six traits, namely, plant height, photosynthetic rate, relative water content, water potential, intercellular CO2 concentration, and total soluble protein, contributed greater growth in the sandalwood, while four traits, namely, water potential, osmotic potential, leaf area, and total soluble protein, contributed greater growth in the host species. The traits modeling study predicted greater growth of sandalwood with the hosts D. sissoo and C. equisetifolia, whereas among host species, prediction revealed greater growth of S. cumini and C. aurantium.
Discussion: The study concluded that host–parasite interaction modulated the growth and physiological processes in both sandalwood and hosts and sandalwood plantations can be successfully developed with the hosts D. sissoo and C. equisetifolia.
Sandalwood (Santalum album L.) is the world’s second most expensive tree that holds immense cultural, religious, and economic importance across the Asian sub-continent. Globally, a total of 18 species of sandalwood have been documented that belong to the genus Santalum, of which Indian sandalwood (Santalum album L.) is the commercially most valuable species, which is well known for aromatic oil derived from its heartwood. The demand for sandalwood products, including oil, is increasing annually in both the international and domestic markets (Kumar et al., 2012; Ramanan Suresh et al., 2020), and the future projection indicates an increase in the global demand for sandalwood to 6,000 and 7,000 metric tons/annum (Viswanath and Chakraborty, 2022). Moreover, at present, a significant disparity exists between the demand and supply of sandalwood, which creates tremendous pressure on harvesting of this species from natural stands. Simultaneously, illegal felling, smuggling, poor seedling establishment due to its parasitic nature, lack of knowledge on the host–parasite relationship, and other abiotic and biotic factors, etc., have greatly affected the natural plantation of sandalwood, resulting in species being classified as Vulnerable and included in IUCN Red list category (Arunkumar et al., 2019). Therefore, to fulfill the global demand for sandalwood products and to preserve its precious natural reserves, gradually more area needs to be brought under the sandalwood plantations.
In the past, sandalwood plantations were limited to natural forests; however, high demand-fostered greater price of sandalwood has led to species farming gaining huge popularity among the farming communities across Asia, Africa, and Australia, especially in India (Mishra et al., 2018). Since the last two decades, a huge expansion in area under sandalwood cultivation has been reported due to its high demand across the globe. Species can thrive in diverse climatic conditions and can be integrated into agroforestry as they can provide higher economic returns and conserve the natural environment (Mishra et al., 2018; Srikantaprasad et al., 2021; Kumar et al., 2022a,b; Verma et al., 2023a,b). However, the cultivation of semi-parasitic sandalwood is more challenging compared to monoculture plantations (Radomiljac et al., 1998). It obtains water, nutrients, and minerals from the host plant via haustorium (Kuijt, 1969). Haustorium is a complex set of physiological and structural linkages that connects the conductive system of phloem, xylem, or both, assisting parasitic plants by supplying water and minerals from the host (Yoshida et al., 2016). Moreover, the growth performance of sandalwood is influenced by the physiological activity of its hosts. Therefore, it is crucial to have a comprehensive understanding of parasitism ecology; particularly, the understanding between parasite and host is paramount to identify the suitable host, which is the most important strategy for the successful establishment of sandalwood plantations (Surendran et al., 1998).
Sandalwood has been found parasitizing on a wide range of plants (300 species) ranging from grasses to trees (Nagaveni and Vijayalakshmi, 2003). Among the host species, Azadirachta indica (Nagaveni and Vijayalakshmi, 2003), Dalbergia latifolia and Syzygium cumini (Guleria, 2013), Acacia nilotica and Melia dubia (Padmanabha et al., 1988), Leucaena leucocephala (Guleria, 2013), Casuarina equisetifolia (Nagaveni and Vijayalakshmi, 2003; Rocha et al., 2014), and Acacia hemignosta, A. ampliceps, and Melia azedarach (Radomiljac et al., 1998) were observed to be the most suitable host tree species in terms of maintaining the growth as well as biomass accumulation of sandalwood, but there is a dearth of knowledge regarding the physiological responses of sandalwood to diverse host species. Simultaneously, during the parasitism process, several growth and physiological alterations occur in the host species (Rocha et al., 2014), and at present, information about such is completely lacking. Moreover, parasite sandalwood induces several growth and physiological alterations in host species, which is one of the main hindrances in the successful establishment of sandalwood plantations; however, no systematic information on such interaction is available. Interaction inducing a favorable change in both sandalwood and host could be helpful in devising cultivation and management practices as well as could provide insight into the potential host trees that could support sandalwood throughout its lifespan (Lion, 2017; Rocha and Santhoshkumar, 2022).
Most of the previous studies were limited to screening of host species and assessing the influence of diverse tree host species on the growth and morphology of sandalwood. However, rarely any investigation is available that has reported the physiological response of both sandalwood and host species during their interaction process. We hypothesized that the parasite network process induces several changes in the growth and physiological response of hosts, thereby potentially influencing the growth and physiological processes of the sandalwood. The specific objectives of the study were (i) to systematically evaluate the response of growth and physiological processes involved in the complex interactions between host species and sandalwood and (ii) to identify potential traits that can greatly affect the performance of both sandalwood and host. Overall, this investigation aimed to explore host-specific compatibility by analysing alterations in growth, biochemical, and physiological traits of both host plants and sandalwood, which will aid in devising the best possible cultivation and management practices for the sandalwood.
The present experiment was carried out at the ICAR-CSSRI in Karnal, Haryana, India (29° 4230` N and 76° 5712` E, with an elevation of 282 m above mean sea level). The study area exhibits a semi-arid, subtropical, monsoonal climate marked by significant temperature variations that range between 32.7°C and 42.8°C during summer and 3.4°C and 10.8°C during winter. The region receives annual rainfall ranging between 700 and 800 mm. The seeds of sandalwood were obtained from the Institute of Wood Science and Technology (IWST), Bangalore, and sown in germination beds during May 2020. The germinating seedlings were pricked from germination beds and transferred to polybags (6″ × 3″) containing soil: FYM: sand in the ratio of 1:1:1. The planting material of host species was brought from the local private nurseries. The healthy plants of both sandalwood and host species were considered for the present investigation. Furthermore, 6-month-old seedlings of sandalwood were planted with ten host species, i.e., Syzygium cumini, Punica granatum, Phyllanthus emblica, Melia dubia, Leucaena leucocephala, Dalbergia sissoo, Casuarina equisetifolia, Citrus aurantium, Azadirachta indica and Acacia ampliceps, and control (sandalwood alone) during October 2020. The selection of the host species in the study was evidenced from the previous empirical investigations, indicating their efficacy as suitable hosts for sandalwood (Table 1). After 2 months, the nursery host was completely removed to allow haustorial connections of sandalwood with the hosts except for the control. The experiment was conducted under pothouse conditions to exclude the effect of rainfall. Pothouse has optimum growing conditions with light intensity (PAR) of 600 flux, relative humidity >60%, temperature of 25–30°C, and CO2 concentration of 400 ppm. Surprisingly, except for control (sandalwood alone), sandalwood grown with selected host plants had a 100% survival rate.
In the present experiment, 50 plants each of sandalwood and 10 different host species were transplanted in 15-kg capacity plastic pots during October 2020. The growing media of plastic pots contained Soil: FYM: sand in the ratio of 6:3:1. A spacing of 10 cm was maintained between the host plant and sandalwood to allow the formation of haustorial connections. During the nursery stage, sandalwood necessitates a primary host, transitioning to a long-term secondary host under field conditions. Therefore, while transplanting, nursery host Alternanthera spp. was also planted with secondary hosts to ensure proper establishment of the sandalwood; 500 mL of irrigation water was provided to pots every day during October–November and February–March and alternatively during December–January. The irrigation requirement was calculated based on the soil volume and characteristics, and uniform irrigation was provided to all the plants during the entire experiment period. A standard dose of fertilizers (1 g of NPK per plant) along with a Hoagland solution for micronutrients was given to maintain the growth of plants (Bose et al., 2022). The weeding operations were carried out weekly. The suitability of the host for sandalwood was assessed by comparing the growth and physiological attributes of sandalwood as well as the host species. The data were recorded during March 2021, i.e., after 6 months of the transplantation.
The plant height (cm) of both the sandalwood and host plants was recorded from the base to the apical shoot using a measurement scale. The collar diameter of both sandalwood and host plants was assessed using a vernier caliper. Leaf area measurements were conducted using a “Portable Laser leaf area meter—CI-202” (Verma et al., 2023a).
The plant–water relation parameters, namely, osmotic potential, water potential, and relative water content (RWC), were assessed in both sandalwood and host plants. For estimation of the RWC, fully expanded leaves derived from the middle portion of the plant were harvested during the morning timeframe between 9:00 am and 10:00 am and were then promptly conveyed to the laboratory in a sealed polythene bag. The leaves were cut into five-leaf disks of 1 cm diameter each, and fresh leaf weight (FLW) was recorded. After that, leaf disks underwent a 4-h immersion in distilled water to estimate the turgid leaf weight (TLW). After this hydration period, the leaf dry leaf weight (DLW) was recorded post-drying and RWC was executed using the formula (Turner, 1981):
For the determination of water potential (ψw), the fresh leaves (1 g) were finely chopped and ψw was measured on WP4C Dewpoint Potentiometer (METER Group, Inc., United States) (Haghverdi et al., 2020) and expressed as –MPa. Furthermore, the osmotic potential (ψs) was ascertained utilizing the methodology outlined by Cuin et al. (2009), which measures osmolality (c) using the Vapor Pressure Osmometer (Model 5,600, ELITech Group, Belgium). The fresh leaves (1 g) were frozen at −20°C, crushed and squeezed to extract the sap. A 5 μL aliquot of the sap was taken to measure the osmolality on the osmometer and subsequently transformed to osmotic potential using the Van’t Hoff equation (Hessini et al., 2019).
ψs(MPa)=−c×2.58×10−3
For the determination of chlorophyll content, a 200 mg of leaf sample was placed in a test tube containing 10 mL acetone and incubated overnight, and on the subsequent day, the optical density was measured at 645 and 663 nm utilizing UV spectrophotometer (double beam) (Yoshida et al., 1976) and expressed in milligrams per gram fresh weight (FW). The LI-6800 portable photosynthesis system with a standard 6 cm2 leaf chamber (LI-COR, Inc., Lincoln, NE, United States) was used for the measurement of net rates of leaf photosynthesis, intercellular CO2 concentration, transpiration, stomatal conductance, and vapor pressure deficit (VPD, KPa) during 09:00 am to 11:00 am on 2 consecutive sunny days. Cuvette conditions were regulated at an ambient CO2 concentration of 400 ppm, relative humidity >60%, a photosynthetic photon flux density of 1,000 μmol m−2 s−1, and leaf temperature of 25°C (Kumar et al., 2018, 2019). The uniform and fully expanded leaves of host plants and sandalwood were considered for measuring the gas exchange attributes.
Various osmoprotectants, including total soluble sugars (TSS; Yemn and Willis, 1954), proline content (Bates et al., 1973), and protein content (Bradford, 1976), were measured. For estimation of TSS, a 100 mg sample was extracted in 2.5 mL of 80% ethanol, followed by centrifugation at 10,000 rpm for 10 min at 4°C. The resulting supernatant (10 μL) was mixed with anthrone reagent (5 mL), incubated at 100° C for 10 min and absorbance (using a UV spectrophotometer) was recorded at 620 nm. For proline determination, 200 mg of fresh leaves were homogenized in 3% sulphosalicylic acid (5 mL) and centrifuged (10,000 rpm for 10 min at 4°C). Subsequent supernatant mixed with glacial acetic acid and acid ninhydrin reagent (2 mL each), incubated (100°C for 60 min), and subjected to cooling after which toluene (4 mL) was added and vortexed and absorbance (using upper phase on UV spectrophotometer) recorded at 520 nm. Using a standard curve drawn with various L-proline concentrations, the proline content was determined. For protein estimation, the 100 μL supernatant was taken from TSS estimation and added to Bradford reagent (3 mL) taken in a test tube. The sample was then mixed thoroughly by Vortex and absorbance (using a spectrophotometer) was recorded at 595 nm (Bradford, 1976).
The present study was conducted in a randomized complete block design with five replicates to find out the best suitable host plant species for sandalwood. The normality of each variable was assessed through the Shapiro–Wilk (W) test using Q-Q plots of residuals. Variables found to deviate from normality underwent appropriate transformations. Subsequently, multiple comparison analysis was performed using Tukey’s HSD test to discern the significant differences in various parameters of sandalwood and hosts at a 5% significance level. Crucial morpho-physiological traits were individually prioritized for both hosts as well as sandalwood, and predicted responses of growth diameter of host–sandalwood associations and host species were modeled through a stepwise regression approach (backward elimination) in STAR statistical software (IRRI, 2022).
where α is indicate the intercept.
βi, (i = 1,… k), = partial regression coefficients.
Thus, in this multiple linear functional equation with k independent variables (traits), the presence of βi, (i.e., ≠ 0) indicates the dependence of Y on X i . The test of significance of each βi for respective variables was performed through a t-test (Gomez and Gomez, 1984). The modeled equations were fitted to select the best host–parasite interaction, in which regression coefficients (βs) of individual traits significantly associated with higher diameter growth of sandalwood diameter were considered as weighted coefficients.
The result demonstrated that the host species induced variability in the growth of sandalwood. Sandalwood exhibited significant (p ≤ 0.05) differences in plant height, collar diameter, and leaf area of the sandalwood. Results explained that the diameter increment of sandalwood was recorded maximum (p ≤ 0.05) with host D. sissoo (4.04 mm), followed by C. equisetifolia (4.01 mm) and S. cumini (3.64 mm) hosts, and minimum with the host C. aurantium (3.02 mm) (Figure 1A). However, among the host plant species, the highest (p ≤ 0.05) collar diameter was recorded in S. cumini (8.64 mm), followed by C. aurantium (6.83 mm), whereas the lowest collar diameter was recorded in C. equisetifolia (4.18 mm). Results further showed the significant (p ≤ 0.05) effect of host plant species on plant height in sandalwood which ranged between 42.25 cm (A. ampliceps) and 61.95 cm (P. emblica) (Figure 1B). Among the host species (Figure 1B), the maximum (p ≤ 0.05) plant height was observed in D. sissoo (97.95 cm) followed by P. granatum (94.00 cm), L. leucocephala (90.50 cm), and the minimum plant height was recorded in M. dubia (43.55 cm). Results further explained the non-significant (p ≤ 0.05) effect of different host species on the leaf area of sandalwood, whereas host plant species displayed significant variations (p ≤ 0.05) in leaf area, which ranged between 0.48 (C. equisetifolia) and 27.70 cm2 (S. cumini) with a mean value of 10.52 cm2 (Figure 1C).
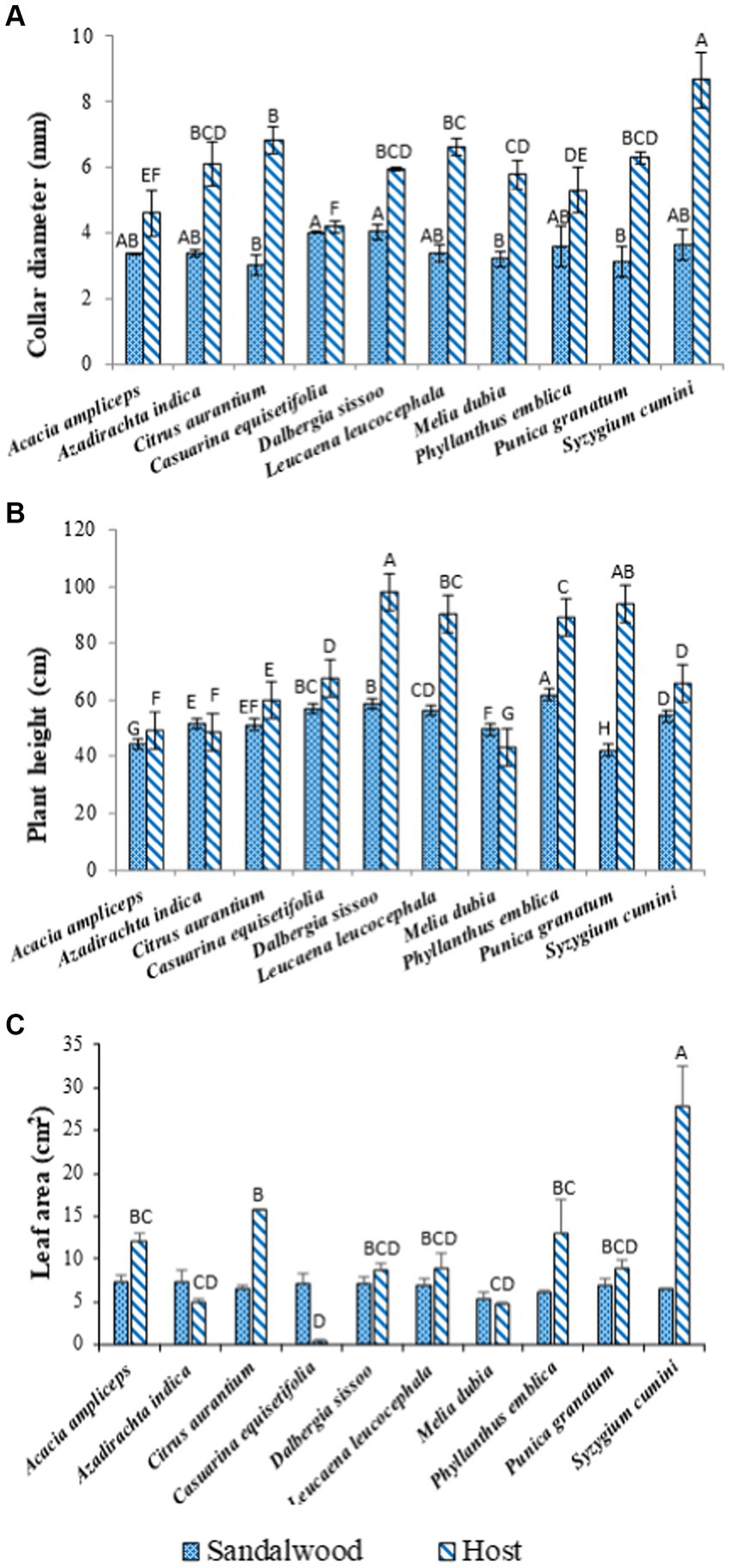
Figure 1. Collar diameter (A), plant height (B), and leaf area (C) of sandalwood and host species. Bars followed by different letters (A,B,C… etc.) indicate statistically significant differences.
The result showed that the host species induced differences in plant water relation of sandalwood. The highest (p ≤ 0.05) relative water content (RWC) in sandalwood was recorded with host L. leucocephala (89.57), whereas its lowest value was observed with host A. ampliceps (73.85) (Table 2). Among the host species, A. indica (89.00) possessed the highest (p ≤ 0.05) RWC, whereas C. aurantium (71.49) and C. equisetifolia (67.30) possessed the lowest value to RWC. In contrast, the host did not display any significant (p ≥ 0.05) impact on the water potential (ψw) of sandalwood while the water potential of host species varied from −0.97 MPa (A. ampliceps) to −1.26 MPa (A. indica). The osmotic potential (ψs) of sandalwood is affected by different host species was ranged from a maximum of −1.78 MPa (P. granatum) to a minimum of −1.30 MPa (C. aurantium), whereas in host plants the maximum value of ψs was observed in P. emblica (−1.57 MPa) and minimum in D. sissoo (−1.08 MPa). Furthermore, host plants affected (p ≤ 0.05) the vapor pressure deficit (VPD) in the sandalwood, which was observed highest with host P. granatum (1.39 MPa) followed by S. cumini (1.36 MPa), P. emblica (1.33 MPa), L. leucocephala (1.15 MPa), and C. equisetifolia (1.09 MPa) and minimum with host D. sissoo (0.12 MPa) (Table 2). Similarly, in host plant species, significantly (p ≤ 0.05) highest VPD was noted in C. equisetifolia (3.43 MPa) followed by C. aurantium (3.33 MPa), L. leucocephala (3.16 MPa), and S. cumini (3.08 MPa), and minimum VPD was observed in D. sissoo (2.00 MPa).
The findings indicated that the host species induced variations in chlorophyll and gas exchange characteristics of sandalwood while no change in photosynthetic rate and stomatal conductance of sandalwood were observed. Particularly, there were significant (p ≤ 0.05) differences in the chlorophyll content in sandalwood leaves ranging from 0.91 to 1.43 mg g−1 (Table 3), which was observed in the order of C. aurantium > D. sissoo > S. cumini > P. granatum > C. equisetifolia > M. dubia = P. emblica > A. ampliceps > A. indica. Similarly, chlorophyll content in host plant leaves was recorded maximum (p ≤ 0.05) in C. aurantium (2.04 mg g−1), followed by C. equisetifolia (1.94 mg g−1), P. granatum (1.81 mg g−1), P. emblica (1.78 mg g−1), A. indica (1.78 mg g−1), and S. cumini (1.70 mg g−1) host species and minimum in D. sissoo (0.92 mg g−1). Furthermore, the present results explained that the host plant exerted a non-significant (p ≥ 0.05) impact on the stomatal conductance and photosynthetic rate of sandalwood (Table 3). In contrast, host plant species displayed a significant (p ≤ 0.05) difference in photosynthetic rate, which was recorded highest in A. ampliceps (8.56 μmol m−2 s−1) followed by A. indica, M. dubia, and S. cumini and lowest in C. equisetifolia (1.69 μmol m−2 s−1). Similarly, host D. sissoo showed maximum (p ≤ 0.05) stomatal conductance of 9.27 mol H2O m−2 s−1 followed by A. ampliceps, M. dubia, L. leucocephala, A. indica, S. cumini, and P. granatum, in decreasing order. Results further showed that the host plant contributed significantly to variations in the transpiration rate of sandalwood, which was observed at maximum (p ≤ 0.05) with host D. sissoo (4.09 mmol m−2 s−1) and P. granatum (4.09 mmol m−2 s−1) and minimum (p ≤ 0.05) with P. emblica (2.47 mmol m−2 s−1) (Table 3). In host plants, the transpiration rate remained non-significant (p ≥ 0.05) ranging from 0.70 mmol m−2 s−1 (D. sissoo) to 2.73 mmol m−2 s−1 (M. dubia). Similarly, the intercellular CO2 concentration in sandalwood plants was observed to be highest (p ≤ 0.05) with A. ampliceps (328.51 μmol mol−1), followed by A. indica (307.32 μmol mol−1), C. aurantium (296.41 μmol mol−1), D. sissoo (273.17 μmol mol−1), P. emblica (263.01 μmol mol−1), M. dubia (255.06 μmol mol−1) hosts, and minimum with host S. cumini (249.89 μmol mol−1). In host plant species, the intercellular CO2 concentration was recorded as maximum (p ≤ 0.05) in C. aurantium (338.66 μmol mol−1) and minimum (p ≤ 0.05) in S. cumini (222.62 μmol mol−1).
The results revealed that the host species induced significant (p ≤ 0.05) variations in proline content accumulation in sandalwood among the different osmolytes studied. Sandalwood showed non-significantly (p ≥ 0.05) maximum total soluble sugar with host species A. indica (1.37 mg g−1) whereas minimum with A. ampliceps (0.81 mg g−1). Conversely among the host species, significantly (p ≤ 0.05) higher total soluble sugar was found in A. ampliceps (1.63 mg g−1), followed by C. aurantium (1.21 mg g−1) and P. granatum (1.21 mg g−1) and minimum in C. equisetifolia (0.72 mg g−1). Furthermore, the proline content in sandalwood leaves was observed highest (p ≤ 0.05) with host L. leucocephala (37.49 μg g−1) followed by M. dubia (34.57 μg g−1) and lowest (p ≤ 0.05) with host C. equisetifolia (14.93 μg g−1). Similarly, among the host plant species, M. dubia (24.73 μg g−1) possessed the highest (p ≤ 0.05) proline content followed by C. aurantium (17.54 μg g−1) and C. equisetifolia (16.22 μg g−1), and the lowest (p ≤ 0.05) proline was recorded in P. emblica (7.1 μg g−1). On the other hand, host plants did not (p ≥ 0.05) affect protein content in sandalwood, which ranged from 7.70 mg g−1 (P. emblica) to 12.85 mg g−1 (A. indica), whereas in host plant the significantly (p ≤ 0.05) highest protein content was recorded in A. ampliceps (15.75 mg g−1) and minimum with P. emblica (6.86 mg g−1) (Table 4).
Plant water traits, gas exchange attributes, and osmolytes play a significant role in determining the host response to sandalwood. Magnitude of traits toward the host–parasitic relationship was identified through multiple regression approach. Analysis showed that six traits (plant height, total soluble protein, intercellular CO2 concentration, relative water content, photosynthetic rate, and water potential) of sandalwood; while, four traits (water potential, osmotic potential, leaf area, and total soluble protein) of host plants induced significant differences in the growth performance of sandalwood (Tables 5, 6). Consequently, these traits were included in sandalwood diameter growth modeling to predict the sandalwood response with respect to host species in different host plant associations. Furthermore, on the basis of diameter growth modeling, host species were prioritized on the basis of predicted growth response of growth diameters.
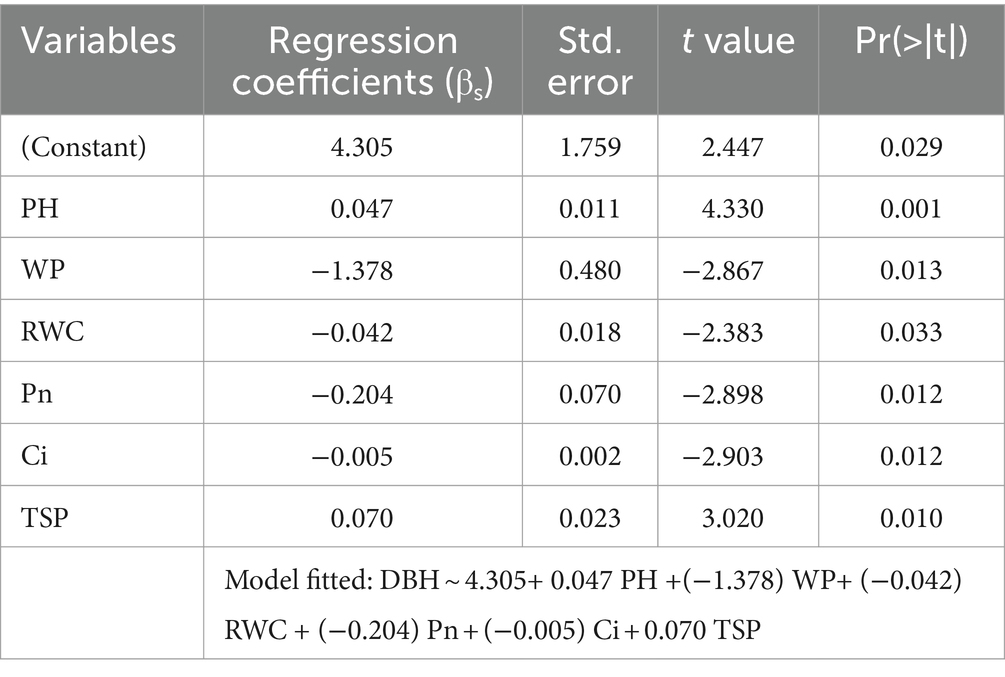
Table 5. Significance of traits magnitude toward diameter growth of sandalwood associated with different host plants.
Overall, results showed that Casuarina equisetifolia followed by Dalbergia sissoo is the best host for sandalwood. However, among the host species Syzygium cumini followed by Citrus aurantium is best with respect to their growth performance (Tables 7, 8).
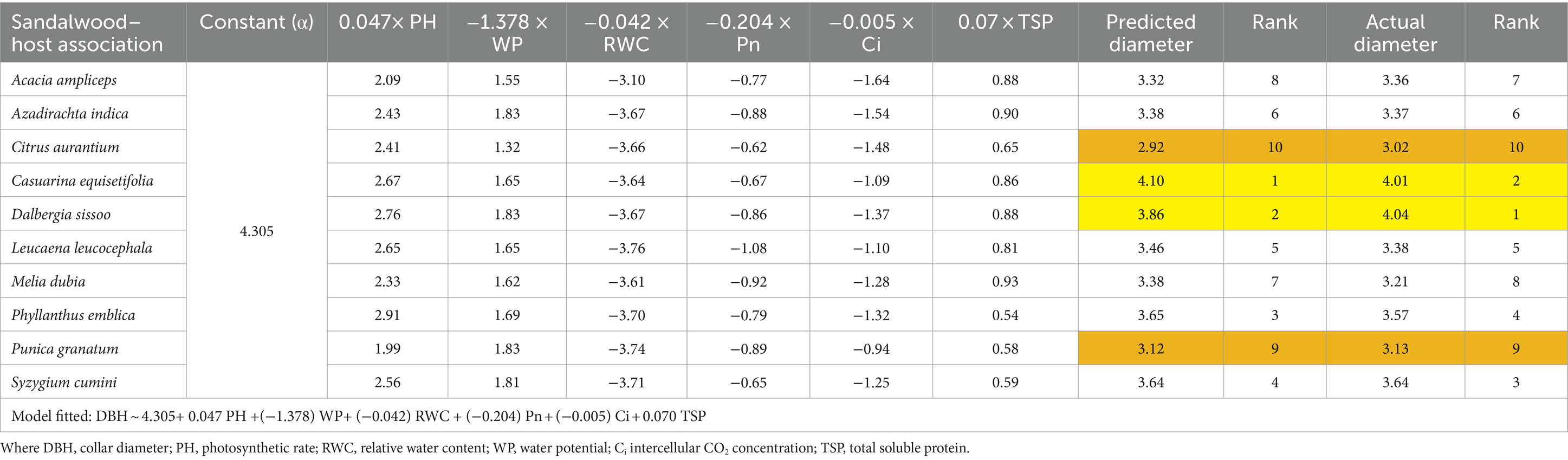
Table 7. Prioritization of host–sandalwood associations through the predicted response of sandalwood diameter growth.
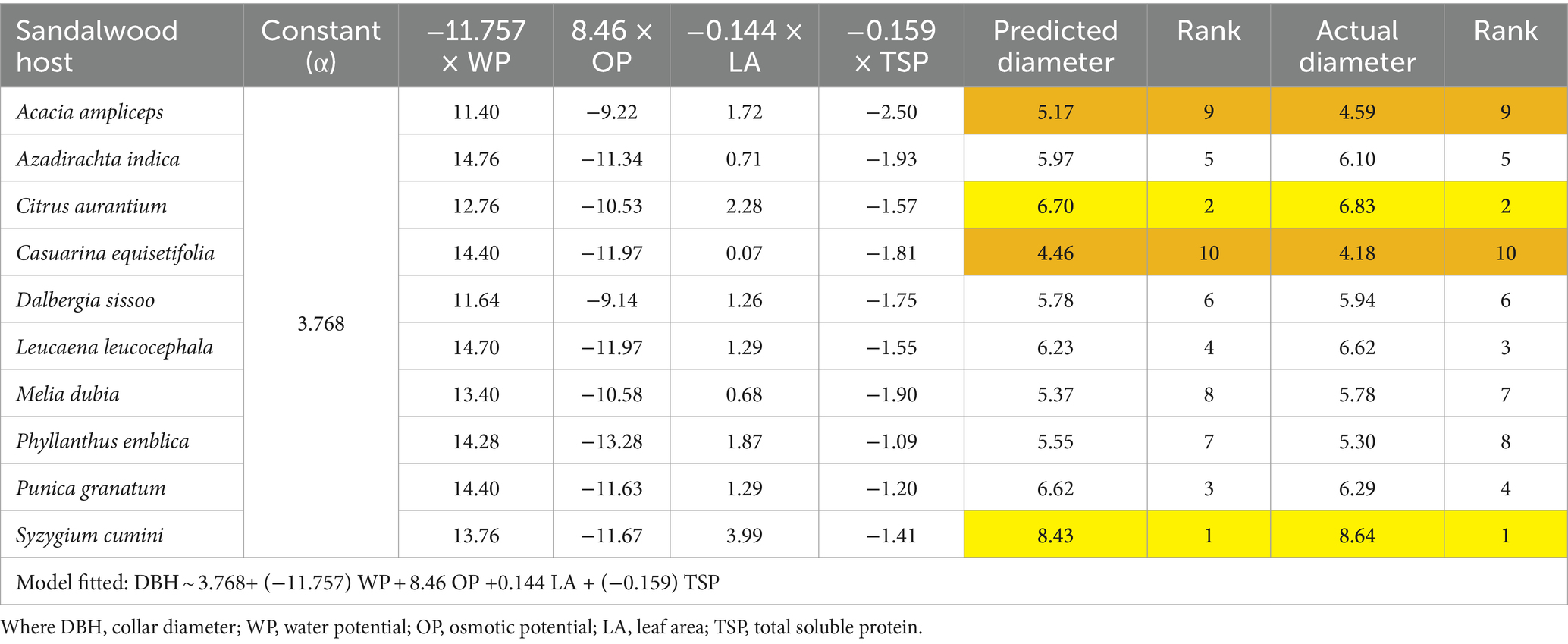
Table 8. Prioritization of host species through the predicted response of the diameter growth of the host.
Sandalwood is a semi-root parasite plant that depends on the host plants for water and nutrient requirements. Consequently, a large number of growth and physiological process occurring in both sandalwood and host species govern their performance under a particular set of conditions. The present results revealed significant differences in plant height, collar diameter, and leaf area of the sandalwood. The highest growth of sandalwood with P. emblica followed by D. sissoo and C. equisetifolia might have resulted from the better association of sandalwood with these hosts. The varied growth pattern of sandalwood with different host have been extensively studied (Rocha et al., 2014; Doddabasawa et al., 2020; Sahu et al., 2021), which suggested that the performance of sandalwood is governed by the characteristics of the different host plants (translocation of mineral nutrients and water, slow growth rate, and lateral root system) and the competition for above-ground resources, including light (Rocha et al., 2014). Specifically, the sandalwood roots not only absorb water and nutrients from the host plants, but also control the host plant roots and effectively regulate the supply of water and nutrients (Verma et al., 2023a,b), thereby regulating various growth processes in the sandalwood. Simultaneously, host requirement and the growth stage of host introduction have also a substantial impact on the development of sandalwood (Ramya, 2010), which indicates that sandalwood growth and development are regulated by the selected host species.
In the current investigation, the plant water traits, such as RWC, osmotic potential (ψs), and vapor pressure deficit (VPD) of sandalwood, were higher with hosts L. leucocephala and P. granatum. The host-wise variation in plant water traits of sandalwood suggests the difference in the water absorption by both sandalwood and host plants. Rocha et al. (2014) also reported that host species induced variation in plant water of sandalwood, and they showed that the host Casuarina equisetifolia has a higher water potential than the sandalwood (without host). However, sandalwood could adjust its water and osmotic potential as well as relative water content either by accumulating osmotically active chemicals or by maintaining the xylem-to-xylem connection with host plants. Such processes could lead to the enhancement in turgor to facilitate the water uptake in plants (Dhaniklal, 2006; Rocha et al., 2014; Kumar et al., 2021). Results further revealed that among different host species, A. indica, L. leucocephala, and C. equisetifolia showed the highest value for RWC, ψs, and VPD, respectively. The species-specific differences in morphology of total number of leave and roots biomass, etc., might be responsible for variation in plant–water relations in these species. Mielke et al. (2005), Johnson et al. (2009), Klein (2014), and Leuschner et al. (2019) have provided substantial evidence supporting the existence of species-specific variations in plant–water relations, which indicates that plant–water relations in sandalwood are governed by the type of host species as latter is responsible for maintaining the plant water status of the former.
Furthermore, results revealed that, C. aurantium as a host of sandalwood as well as individually showed the maximum chlorophyll content in the leaves, indicating that citrus might be absorbing a greater quantity of minerals responsible for the formation of higher chlorophyll content on its own as well as in sandalwood leaves (Rocha et al., 2014; Doddabasawa et al., 2020). The host-wise difference in chlorophyll content of sandalwood suggests species-wise differences in the absorption of substances responsible for initiating the synthesis of chlorophyll in the leaves (Coste et al., 2010; Li et al., 2018; Leuschner et al., 2019). Furthermore, the non-significant (p ≥ 0.05) effect of host plant on both the photosynthetic rate and stomatal conductance of sandalwood suggests species ability to maintain both these processes equally, irrespective of types of host species. Moreover, despite influence of the hosts, sandalwood can adjust activities of chlorophyll, RuBPase, and stomata under the prevailing environmental conditions (Balasubramanian et al., 2021). Furthermore, Rocha et al. (2014) showed that the lower content of nitrogen and chlorophyll and the altered RuBPase activities were responsible for decline in the photosynthetic rate of sandalwood. The species (host) wise differences in light utilization efficiency, stomata closure, and CO2 absorption in chloroplasts were major causes of differences in their photosynthetic rate (Kumar et al., 2016; Sheoran et al., 2021; Soni et al., 2021).
The host plant also induced differences in transpiration rate of sandalwood, which was observed maximum (p ≤ 0.05) with host D. sissoo, suggesting that despite providing uniform irrigation to all plants, the host induced differences in the transpiration rate of sandalwood, which can be attributed to the substantial disparity in driving forces responsible for the movement of water from the soil-to-leaf system and leaf–atmosphere interface. Moreover, from the physiological point of view, vaporpressure is sought to play a key role in the rate of water flow and transpiration (Zhang et al., 2017). It has also been reported that sandalwood for its survival produces high transpiration rates through uncontrolled stomata to maximize water loss and generate a water potential gradient and sink strength even larger than the host to extract the crucial resources from it (Liu et al., 2003; Grewell, 2008). Furthermore, the ‘resistance of host to xylem solute transfer, as well as its stomatal response, also influences the maintenance of solute flow from host to parasite (Jiang et al., 2003). The highest intercellular CO2 concentration in sandalwood plants with the host A. ampliceps and individually in C. aurantium resulted from species-specific differences in the absorption of CO2. Similarly, Annapurna et al. (2006) also observed host species effects on physiological processes of sandalwood and reported that hosts D. sissoo maintained higher RWC along with the higher photosynthetic rate, stomatal conductance, and transpiration, compared to rest of the host species, which might have contributed to the higher growth of sandalwood. Therefore, the type of relationship between parasite and host is one of the most important aspects in terms of intake and translocation of mineral nutrient, as well as maintenance of physiological efficiency (Shen et al., 2006; Kumar et al., 2016).
Moreover, in the current study, sandalwood showed maximum (p ≤ 0.05) production of total soluble sugar with host A. ampliceps. The greater production of specific organic solutes (osmolytes) aids in maintaining the plant water status and photosynthesis as well as safeguarding the cellular machinery against harmful substances (Lata et al., 2019; Kumar et al., 2021). Moreover, during the transfer of nutrients from host to the parasite, potentially hazardous chemicals and disease infections may pass through the haustoria, causing stress in the parasitic plants that contribute to an increase in the production of osmolytes (Zagorchev et al., 2021). Among the host species, A. indica showed maximum total soluble sugar, suggesting an increase in total sugar might contribute to greater production of biomass in the species. Similarly, Zhou et al. (2021) reported that the soluble carbohydrate accumulation acts as a source of energy for branch sprouting, elongation, and thickening. Moreover, the soluble carbohydrate is sought to have a key role in the acquisition of solute by reducing the ψw of hemi- parasite and facilitating the flow of salute from the host that might have substantial ramifications for the host–parasite system. Furthermore, these might help to counterbalance the excessive inorganic ion concentrations in vacuoles and improve the stability of membranes and enzymes (Karakas et al., 1997; Pooja Nandwal et al., 2019). The higher soluble sugar concentrations have been reported to also alter the nutrient availability in hemiparasitic plants (Gomes and Adnyana, 2017).
Proline accumulation is a key physiological indicator of signaling regulatory molecules in plants that trigger a variety of responses during the adaptation process. Our result suggested that sandalwood accumulates higher proline content with host L. leucocephala, compared to the rest of the host species, indicating the better adaptability of sandalwood with this host. Moreover, an increase in proline content will eventually promote the synthesis of protein and the development of cells and will provide nitrogen and carbon needed for them to expand and energy use (Bell and Adams, 2011; Christgen and Becker, 2019). However, results showed non-significant influence of host plants on the protein content in sandalwood. This was due to the greater change in cellular soluble protein content occurring only under stress conditions. Moreover, the increased protein accumulation indicates the preservation of nontoxic useable forms of cellular N that aids in the re-establishment of C and N balance by utilizing carbon from photosynthesis and glycolysis (Chen et al., 2017). Physiologically, changes in metabolite concentrations typically occur in trees before the appearance of any visible symptoms, and the changes in nitrogen metabolism are inextricably linked to changes in carbon metabolism (Minocha et al., 2019).
The current investigation provided detailed insight into the simultaneous changes in the growth and physiology of both sandalwood and different host species during the host–parasite interaction process, thereby enhancing our understanding of the physiological and biochemical aspects involved in this complex interaction. In conclusion, sandalwood exhibited higher growth performance when grown with hosts D. sissoo and C. equisetifolia, while among host species, S. cumini, C. aurantium, and L. leucocephala showed superior growth and physiobiochemical traits. Moreover, plant height, water potential, relative water content, photosynthetic rate, intercellular CO2 concentration, and total soluble protein were the major traits influencing the growth of the sandalwood while only four traits, namely, water potential, osmotic potential, leaf area, and total soluble protein favored the growth of the host plants. The present finding will aid the manipulation of physio-biochemical traits during host–parasite interaction and based on the performance of traits during the interaction process, the site-specific cultivation and management practices could be devised to successfully establish and manage the sandalwood plantations across the globe.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
KV: Writing – original draft, Visualization, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. AK: Writing – original draft, Validation, Supervision, Software, Project administration, Data curation, Conceptualization. RK: Writing – original draft, Validation, Supervision, Software, Project administration, Data curation, Conceptualization. AB: Writing – review & editing, Validation, Supervision, Formal analysis. SD: Writing – review & editing, Visualization, Validation, Formal analysis. AS: Writing – review & editing, Visualization, Resources, Investigation. PS: Writing – review & editing, Visualization, Resources, Funding acquisition, Formal analysis, Data curation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors are grateful to the director of ICAR–CSSRI and CCS HAU for providing the necessary logistics/facilities. The authors thank Arvind Kumar and Ram Kishor Fagodiya from ICAR-Central Soil Salinity Research Institute, Karnal, India, and Sneha Dobhal from VCSGUUHF, College of Forestry, Tehri, India, for their heartfelt support during the investigation and revision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Annapurna, D., Rathore, T. S., and Joshi, G. (2006). Modern nursery practices in the production of quality seedlings of Indian sandalwood (Santalum album L.) stage of host requirement and screening of primary host species. J. Sust. For. 22:3355. doi: 10.1300/J091v22n03_03
Arunkumar, A. N., Dhyani, A., and Joshi, G. (2019). Santalum album; in the IUCN red list of threatened species 2019. Gland: International Union for Conservation of Nature and Natural Resources. doi: 10.2305/IUCN.UK.2019-1.RLTS.T31852A2807668.en
Balasubramanian, A., Prasath, C. H., Radhakrishnan, S., and Sivaprakash, M. (2021). Host-specific influence on early growth and physi-ological attributes of sandal (Santalum album) grown in farmlands. J. Environ. Biol. 42, 1162–1167. doi: 10.22438/jeb/42/4(SI)/MRN-1542a
Bates, L. S., Waldren, R. A., and Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207. doi: 10.1007/BF00018060
Bell, T. L., and Adams, M. A. (2011). Attack on all fronts: functional relationships between aerial and root parasitic plants and their woody hosts and consequences for ecosystems. Tree Physiol. 31, 3–15. doi: 10.1093/treephys/tpq108
Bose, G., Prajapati, V. M., Tandel, M. B., Pathak, J. G., and Parmar, M. R. (2022). Seedling quality and growth of sandalwood in response to integrated nutrient management. Pharm. Innov. SP-11, 1220–1224.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Brand, J. E., Crombie, D. S., and Mitchell, M. D. (2000). Establishment and growth of sandalwood (Santalum spicatum) in South-Western Australia: the influence of host species. Aust. For. 63, 60–65. doi: 10.1080/00049158.2000.10674814
Chen, H., Zheng, Y., Zhan, J., He, C., and Wang, Q. (2017). Comparative metabolic profiling of the lipid-producing green microalga Chlorella reveals that nitrogen and carbon metabolic pathways contribute to lipid metabolism. Biotechnol. Biofuels 10, 153–120. doi: 10.1186/s13068-017-0839-4
Christgen, S. L., and Becker, D. F. (2019). Role of proline in pathogen and host interactions. Antioxid. Redox Signal. 30, 683–709. doi: 10.1089/ars.2017.7335
Coste, S., Baraloto, C., Leroy, C., Marcon, É., Renaud, A., Richardson, A. D., et al. (2010). Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: a calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 67:607. doi: 10.1051/forest/2010020
Cuin, T. A., Tian, Y., Betts, S. A., Chalmandrier, R., and Shabala, S. (2009). Ionic relations and osmotic adjustment in durum and bread wheat under saline conditions. Funct. Plant Biol. 36, 1110–1119. doi: 10.1071/FP09051
Dhaniklal, G. (2006). Influence of host plants on soil moisture stress on the water relations in sandal, MSc thesis. Thrissur: Kerala Agricultural University.
DoddabasawaChittapur, B. M., and Lokesh, R. (2020). Parasitism ecology of sandalwood (Santalum album L.) for commercial production in the semi-arid tropics. Curr. Sci. 119, 699–703. doi: 10.18520/cs/v119/i4/699-703
Gomes, D.Adnyana (2017). The effect of legume and non-legume to the sandalwood (Santalum album L.) growth in Timor leste. Int. J. Sci. Basic Appl. 32, 207–237.
Gomez, K. A., and Gomez, A. A. (1984). Statistical procedures for agricultural research. 2nd Edn. New York: John Wiley and Sons, 680.
Grewell, B. J. (2008). Parasite facilitates plant species coexistence in a coastal wetland. Ecology 89, 1481–1488. doi: 10.1890/07-0896.1
Guleria, V. (2013). Analysis of plant, host and management relationships for sandalwood (Santalum album) cultivation in new sub-tropical locality of hill region of Indian Himalayas. Ind. For. 139, 53–57. doi: 10.36808/if%2F2013%2Fv139i1%2F29134
Haghverdi, A., Najarchi, M., Öztürk, H. S., and Durner, W. (2020). Studying unimodal, bimodal, PDI and bimodal-PDI variants of multiple soil water retention models: I. Direct model fit using the extended evaporation and Dewpoint methods. Water 12:900. doi: 10.3390/w12030900
Hessini, K., Issaoui, K., Ferchichi, S., Saif, T., Abdelly, C., Siddique, K. H., et al. (2019). Interactive effects of salinity and nitrogen forms on plant growth, photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 139, 171–178. doi: 10.1016/j.plaphy.2019.03.005
IRRI . (2022). International Rice research institute (IRRI), Philippines. Available at: http://bbi.irri.org/products. 387 (Accessed 21 May 2022).
Jiang, F., Jeschke, W. D., and Hartung, W. (2003). Water flows in the parasitic association Rhinanthus minor/Hordeum vulgare. J. Exp. Bot. 54, 1985–1993. doi: 10.1093/jxb/erg212
Johnson, D. M., Woodruff, D. R., Mcculloh, K., and Meinzer, F. C. (2009). Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiol. 29, 879–887. doi: 10.1093/treephys/tpp031
Karakas, B., Ozias-Akins, P., Stushnoff, C., Suefferheld, M., and Rieger, M. (1997). Salinity and drought tolerance of mannitol-accumulating transgenic tobacco. Plant Cell Environ. 20, 609–616. doi: 10.1111/j.1365-3040.1997.00132.x
Klein, T. (2014). The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 28, 1313–1320. doi: 10.1111/1365-2435.12289
Kuijt, J. (1969). The biology of parasitic flowering plants. Berkeley: University of California Press.
Kumar, R., Banyal, R., Singh, A., and Yadav, R. K. (2022a). Exploring the genetic variation for sodicity tolerance in Melia dubia evolved in Indian conditions. Land Degrad. Dev. 33, 41–54. doi: 10.1002/ldr.4126
Kumar, A. A., Joshi, G., and Ram, H. M. (2012). Sandalwood: history, uses, present status and the future. Curr. Sci. 103, 1408–1416.
Kumar, A., Krishnamurthy, S. L., Lata, C., Kumar, P., Devi, R., Kulshrestha, N., et al. (2016). Effect of dual stress (salinity and drought) on gas exchange attributes and chlorophyll fluorescence characteristics in rice. Ind. J. Agr. Sci. 86, 19–27. doi: 10.56093/ijas.v86i6.58833
Kumar, A., Mann, A., Kumar, A., Kumar, N., and Meena, B. L. (2021). Physiological response of diverse halophytes to high salinity through ionic accumulation and ROS scavenging. Internat. J. Phytorem. 23, 1041–1051. doi: 10.1080/15226514.2021.1874289
Kumar, A., Mishra, A. K., Singh, K., Lata, C., Kumar, A., Krishnamurty, S. L., et al. (2019). Diurnal changes and effect of elevated CO2 on gas exchange under individual and interactive salt and water stress in wheat (Triticum aestivum). Ind. J. Agr. Sci. 89:763. doi: 10.56093/ijas.v89i5.89644
Kumar, A., Sharma, S. K., Lata, C., Devi, R., Kulshrestha, N., Krishnamurthy, S. L., et al. (2018). Impact of water deficit (salt and drought) stress on physiological, biochemical and yield attributes on wheat (Triticum aestivum) varieties. Ind. J. Agr. Sci. 88, 1624–1632. doi: 10.56093/ijas.v88i10.84255
Kumar, R., Singh, A., Bhardwaj, A. K., Kumar, A., Yadav, R. K., and Sharma, P. C. (2022b). Reclamation of salt-affected soils in India: Progress, emerging challenges, and future strategies. Land Degrad. Dev. 33, 2169–2180. doi: 10.1002/ldr.4320
Lata, C., Soni, S., Kumar, N., Kumar, A., Pooja, M., and Rani, S. (2019). Adaptive mechanism of stress tolerance in Urochondra (grass halophyte) using roots study. Ind. J. Agr. Sci. 89:158. doi: 10.56093/ijas.v89i6.90834
Leuschner, C., Wedde, P., and Lübbe, T. (2019). The relation between pressure–volume curve traits and stomatal regulation of water potential in five temperate broadleaf tree species. Ann. For. Sci. 76, 1–14. doi: 10.1007/s13595-019-0838-7
Li, Y., He, N., Hou, J., Xu, L., Liu, C., Zhang, J., et al. (2018). Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 6:64. doi: 10.3389/fevo.2018.00064
Liu, M. Z., Jiang, G. M., Li, Y. G., Gao, L. M., Niu, S. L., Cui, H. X., et al. (2003). Gas exchange, photochemical efficiency, and leaf water potential in three Salix species. Photosynthetica 41, 393–398. doi: 10.1023/B:PHOT.0000015463.04706.f3
Mielke, M. S., Almeida, A. A. F. D., and Gomes, F. P. (2005). Photosynthetic traits of five neotropical rainforest tree species: interactions between light response curves and leaf-to-air vapour pressure deficit. Braz. Arch. Biol. Technol. 48, 815–824. doi: 10.1590/S1516-89132005000600018
Minocha, R., Long, S., Turlapati, S. A., and Fernandez, I. (2019). Dynamic species-specific metabolic changes in the trees exposed to chronic N+ S additions at the bear brook watershed in Maine, USA. Ann. For. Sci. 76, 1–13. doi: 10.1007/s13595-019-0808-0
Mishra, B., Chakraborty, S., Sandhya, M. C., and Viswanath, S. (2018). Sandalwood farming in India: problems and prospects. Ind. J. Trop. Biodiv. 26, 1–12.
Nagaveni, H. C., and Vijayalakshmi, G. (2003). Growth performance of sandal (Santalum album L.) with different host species. Sandalwood Res. 18, 1–4.
Ouyang, Y., Zhang, X., Chen, Y., Teixeira da Silva, J. A., and Ma, G. (2016). Growth, photosynthesis and haustorial development of sem-iparasitic Santalum album L. penetrating into roots of three hosts: a comparative study. Trees 30, 317–328. doi: 10.1007/s00468-015-1303-3
Padmanabha, H. A., Nagaveni, H. C., and Rai, S. N. (1988). Influence of host plants on growth of sandal. Myforest 24, 154–160.
Parthasarathi, K., Gupta, S. K., and Rao, P. S. (1974). Differential response in the cation exchange capacity of the host plants on parasitization on sandal (Santalum album). Curr. Sci. 43:20.
Pooja Nandwal, A. S., Chand, M., Singh, K., Mishra, A. K., Kumar, A., Kumar, A., et al. (2019). Varietal variation in physiological and biochemical attributes of sugarcane varieties under different soil moisture regimes. Ind. J. Exp. Biol. 57, 721–732.
Radomiljac, A. M., McCOMB, J. A., Pate, J. S., and Tennakoon, K. U. (1998). Xylem transfer of organic solutes in Santalum album L. (Indian sandalwood) in association with legume and non-legume hosts. Ann. Bot. 82, 675–682. doi: 10.1006/anbo.1998.0741
Ramanan Suresh, S., George, A. K., Chavan, S. B., and Kumar, S. (2020). Progress and future research trends on Santalum album: A bibliometric and science mapping approach. Ind. Crop. Prod. 158, 112972–112982. doi: 10.1016/j.indcrop.2020.112972
Ramya, R. (2010). Physiological and genetic diversity studies on regeneration of Santalum album L., PhD thesis,. Kerala: Cochin University of Science and Technology.
Rocha, D., Ashokan, P. K., Santhoshkumar, A. V., Anoop, E. V., and Sureshkumar, P. (2014). Influence of host plant on the physiological attributes of field-grown sandal tree (Santalum album). J. Trop. For. Sci. 26, 166–172.
Rocha, D., Ashokan, P. K., Santhoshkumar, A. V., Anoop, E. V., and Sureshkumar, P. (2017). Anatomy and functional status of haustoria in field grown sandalwood tree (Santalum album L.). Curr. Sci. 113, 130–133. doi: 10.18520/cs/v113/i01/130-133
Rocha, D., and Santhoshkumar, A. V. (2022). “Host plant influence on Haustorial growth and development of Indian sandalwood (Santalum album)” in Indian sandalwood. Materials horizons: From nature to nanomaterials. eds. A. N. Arunkumar, G. Joshi, R. R. Warrier, and N. N. Karaba (Singapore: Springer), 229–244.
Sahu, S. V., Maheswarappa, H., Kencharaddi, R. N., and Sathish, B. N. (2021). Effect of host plants and potting mixture on growth of sandalwood seedlings at nursery stage. Int. J. Curr. Microbiol. App. Sci. 10, 545–549. doi: 10.20546/ijcmas.2021.1009.063
Shen, H. W. Y. L., Ye, W., Hong, L., Huang, H., Wang, Z., Deng, X., et al. (2006). Progress in parasitic plant biology: host selection and nutrient transfer. Plant Bio. 8, 175–185. doi: 10.1055/s-2006-923796
Sheoran, P., Basak, N., Kumar, A., Yadav, R. K., Singh, R., Sharma, R., et al. (2021). Ameliorants and salt tolerant varieties improve rice wheat production in soils undergoing sodification with alkali water irrigation in indo-Gangetic Plains of India. Agr. Water Manage. 243:106492. doi: 10.1016/j.agwat.2020.106492
Singh, B., Singh, G., and Rathore, T. S. (2018). The effects of woody hosts on Santalum album L. tree growth under agroforestry in semi-arid North Gujarat, India. Ind. For. 144, 424–430. doi: 10.36808/if%2F2018%2Fv144i4%2F119104
Soni, S., Kumar, A., Sehrawat, N., Kumar, A., Kumar, N., Lata, C., et al. (2021). Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J. Biol. Sci. 28, 2510–2517. doi: 10.1016/j.sjbs.2021.01.052
Srikantaprasad, D., Mallikarjuna Gowda, A. P., Umesha, K., Thimmegowda, M. N., and Pusha, T. N. (2021). Influence of nursery hosts on physiology of sandalwood seedlings. Pharm. Innov. 10, 1055–1057.
Surendran, C., Parthiban, K. T., Bhuvaneswaran, C., and Murugesh, M. (1998). “Silvicultural strategies for augmentation of sandal regeneration” in Sandal and its products. eds. A. M. Radomiljac, H. S. Ananthapadmanabha, R. M. Welbourn, and K. Rao (Canberra: Australian Centre for International Agricultural Research), 69–73.
Turner, N. C. (1981). Techniques and experimental approaches for the measurement of plant water status. Plant Soil 58, 339–366. doi: 10.1007/BF02180062
Verma, K., Kumar, R., Kumar, A., Bhardwaj, A. K., and Verma, R. C. (2023a). Host plant regulates growth processes, ion homeostasis, and salinity tolerance of sandalwood (Santalum album L.). J. Plant Growth Regul. 42, 4423–4435. doi: 10.1007/s00344-023-10906-3
Verma, K., Kumar, R., Sharma, A., Devi, S., Sharma, P., Bhardwaj, A. K., et al. (2023b). “Sandalwood: A potential high-value tree species for salinity stress conditions” in Salinity and drought tolerance in plants: Physiological perspectives (Singapore: Springer Nature Singapore), 585–602.
Viswanath, S., and Chakraborty, S. (2022). “Indian sandalwood cultivation prospects in India” in Indian sandalwood. Materials horizons: From nature to nanomaterials. eds. A. N. Arunkumar, G. Joshi, R. R. Warrier, and N. N. Karaba (Springer: Singapore), 281–292.
Yemn, E. W., and Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 57, 508–514. doi: 10.1042/bj0570508
Yoshida, S., Cui, S., Ichihashi, Y., and Shirasu, K. (2016). The haustorium, a specialized invasive organ in parasitic plants. Annu. Rev. Plant Biol. 67, 643–667. doi: 10.1146/annurev-arplant-043015-111702
Yoshida, S., Forno, D. A., Cock, J. H., and Gomez, K. A. (1976). “Determination of chlorophyll in plant tissue” in Laboratory manual for Physi-ological studies of rice (Laguna: International Rice Research Institute), 43–45.
Zagorchev, L., Atanasova, A., Albanova, I., Traianova, A., Mladenov, P., Kouzmanova, M., et al. (2021). Functional characterization of the photosynthetic machinery in Smicronix galls on the parasitic plant Cuscuta campestris by JIP-test. Cells 10:1399. doi: 10.3390/cells10061399
Zhang, D., Du, Q., Zhang, Z., Jiao, X., Song, X., and Li, J. (2017). Vapour pressure deficit control in relation to water transport and water productivity in greenhouse tomato production during summer. Sci. Rep. 7, 1–11. doi: 10.1038/srep43461
Keywords: sandalwood, host species, plant-water relation, physiological processes, osmoprotectants
Citation: Verma K, Kumar A, Kumar R, Bhardwaj AK, Devi S, Sharma A and Sharma P (2024) Host–parasite interaction: an insight into the growth and physiological responses of sandalwood and associated host species. Front. For. Glob. Change. 7:1395394. doi: 10.3389/ffgc.2024.1395394
Received: 03 March 2024; Accepted: 03 May 2024;
Published: 27 May 2024.
Edited by:
Gopal Shukla, Uttar Banga Krishi Viswavidyalaya, IndiaReviewed by:
Pankaj Panwar, Indian Institute of Soil and Water Conservation (ICAR), IndiaCopyright © 2024 Verma, Kumar, Kumar, Bhardwaj, Devi, Sharma and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashwani Kumar, Ashwani.kumar1@icar.gov.in; Raj Kumar, rajcswcrti@gmail.com
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.