- 1Department of Agricultural, Forest and Food Sciences, University of Torino, Grugliasco, Italy
- 2Department of Agrifood, Environmental and Animal Sciences. University of Udine, Udine, Italy
- 3Faculty of Forestry, University of Banja Luka, Banja Luka, Bosnia and Herzegovina
- 4Biotechnical Faculty, University of Montenegro, Podgorica, Montenegro
- 5ISEM, University of Montpellier, CNRS, IRD, Montpellier, France
- 6Faculty of Forestry, University of Agriculture in Krakow, Krakow, Poland
- 7Department of Earth and Environmental Sciences University of Pavia, Pavia, Italy
Introduction: According to various censuses, Europe has less than 1.5 million ha of old-growth forests (OGF). Most of them are in the boreal zone, while their presence in the temperate zone is residual and fragmented.In the framework of the EU biodiversity strategy, it has been adopted a broad definition of OGF which includes late-seral forests and forests with some management legacies. However, research purposes need to identify strictly defined OGFs characterized by structure, disturbance history, and processes typical and exclusive of the last stage of the forest dynamic.
Methods: The present paper wants to contribute to this debate by presenting a research network of four mixed (Fagus-Abies-Picea) montane OGFs in the Dinaric Alps (Lom, BiH; Janj, BiH; Perućica, BiH; Biogradska Gora, MNE), summarizing 20 years of multidisciplinary research by focusing on the structural characteristics and the disturbance history of the whole network and their coherency with strict OGF indicators. These sites were selected in relatively structurally uniform study areas, where 142 permanent plots have been established since 2002.
Results and discussion: The study sites have a high living (747–1,201 m3 ha−1) and coarse woody debris (CWD) biomass (304–410 m3 ha−1), resulting in the highest forest carbon sink at the continental level (398–484 Mg C ha−1). The presence of large and old trees is one of the critical characteristics of the old-growth stage: in Lom and Perućica, there are 19 trees and 14 ha−1 larger than 1 m at breast height, respectively, and 14 trees and 15 trees ha−1 older than 400 years. In the last three centuries, continuous small-scale disturbances have driven forest dynamics, developing stands characterized by gap-phase dynamics and quasi-equilibrium structure. The Dinaric OGF network presents robust indicators of old-growthness, similar structural characteristics, and dynamic processes across all four sites. Identifying this sub-set of OGF using strict criteria is critical for recognizing conservation priorities and for quantifying, along an old-growthness chronosequence, the current structural differences of managed or recently abandoned forests. Besides, only OGF selected with rigorous criteria can act as a reliable reference for ecological restoration and sustainable forest management as a benchmark for carbon sink and for quantifying the impact of climate change on forests.
1 Introduction
The first studies about old-growth forests (OGF) were developed in the Pacific Northwest in the last decades of the 20th century using structural and dynamic features (Franklin et al., 1981). Simultaneously, studies about the role of natural disturbances and natural disturbance regimes, the ecological role of coarse woody debris (CWD), and the old-growth stage have been started (Pickett and White, 1985; Harmon et al., 1986; Spies and Franklin, 1991). These studies have been developed in primary forests hosting a full range of stand development stages, from disturbance and cohort establishment to old-growth, describing structural features, age, and disturbance history (Franklin et al., 2002). The researchers have identified old-growth structural indicators, highlighted the importance of the OGF biodiversity, and delineated a major paradigm shift from one where equilibrium processes predominated in ecological systems (the Clementsian view) to one where forest systems are dynamic and are structured mainly by natural or anthropogenic disturbances (Clements, 1936; Oliver and Larson, 1996; Lindenmayer and Franklin, 2002). These studies were seminal in recognizing the critical role of OGF in biodiversity and conservation (Kormos et al., 2018; Watson et al., 2018).
Consequently, the term OGF became popular in other continents even where the primary forests are absent or rare (Jacobson et al., 2019) as OGF are not exclusively related to primary forests. However, secondary forests can also recover from human disturbances to develop secondary old-growth (O’Brien et al., 2021).
Due to the extreme variability in forest structures, disturbance regimes, land-use history, and ecological characteristics, a standard definition of the “old-growth” stage has always been challenging. A first attempt for a shared definition was made in 2001 during an FAO expert meeting where an OGF was defined as “primary or a secondary forest which has achieved an age at which structures and species normally associated with old primary forests of that type have sufficiently accumulated to act as a forest ecosystem distinct from any younger age class” (UNEP/CBD/SBSTTA, 2001).
In the late 20th century, the concept and interest in OGF also spread rapidly in Europe, where primary forests (undisturbed by man) were absent. Human interference during the past millennia led to intense and widespread forest and land use (Birks and Tinner, 2016) also in places that are currently identified as “natural” (Hejcman et al., 2013; Cagliero et al., 2023). The lack of primary forests is particularly evident in the temperate zone, one of the world’s most heavily populated and long-term developed regions (Silander, 2001; Burrascano et al., 2013).
According to available different country censuses, mainly based on extensive and not homogeneous definitions of OGF, Europe currently has less than 1.5 million ha of OGF, about 0.9% of the total forest cover, mainly in the boreal zone (Sabatini et al., 2018; Barredo Cano et al., 2021). The distribution of European OGF in the temperate zone is residual, being less than 0.1% of the forest cover, and these forests are scarce or barely existent for many EU forest types (Meyer et al., 2021).
For this reason, the EU Commission has adopted a broad, even though less rigorous, definition of OGF: “A forest stand or area consisting of native tree species that have developed, predominantly through natural processes, structures, and dynamics normally associated with late-seral developmental phases in primary or undisturbed forests of the same type. Signs of former human activities may be visible. Still, they are gradually disappearing or are too limited to significantly disturb natural processes,” including even “forest stands that originate not only from natural regeneration but also from planted or sown native tree species” (European Union Commission, 2023a). This broad definition includes not only primary and secondary OGF (which are very rare) but also late seral stands with some old-growth features that could potentially become OGF in the future. Thus the EU definition delineates a critical forest cover that could develop in “future old-growth” (Spies and Franklin, 1996), but it does not allow a realistic quantification of the current residual OGF such as those defined with more rigorous criteria that are crucial for protection and research purposes.
One of the European regions that still hosts OGF stands are the Dinaric Alps. This mountain range was a border region between European Kingdoms and the Ottoman Empire, and it experienced relatively low population densities for centuries. This translated into a history of less intensive and less widespread deforestation and land use than in other European mountain regions (Kaplan et al., 2009; Cagliero et al., 2023) with the conservation of large, well-preserved patches of forest that have been capturing the attention of European foresters, ecologists, and botanists since the beginning of the 20th century (Fröhlich, 1930; Tregubov, 1941; Jones, 1945; Susmel, 1956; Leibundgut, 1982).
A research network of four mixed (Fagus-Abies-Picea) montane OGF in the Dinaric Alps (Lom, BiH; Janj, BiH; Perućica, BiH; Biogradska Gora, MNE) was established in 2004. This forest type is typical of the mountain chains in central-southern Europe, covers more than 10 million hectares, is highly ecologically and socioeconomically significant, and provides various ecosystem goods and services (Hilmers et al., 2019). The selection of the study sites was based on strict structural criteria (Table 1) in order to identify forests that have a structure and a disturbance history that are not affected by past and present human disturbances and that can reliably identify the old-growth stage sensu Oliver and Larson (1996). The used criteria are based on the seminal studies in the Pacific-North-West (Franklin and Spies, 1991; Franklin et al., 2005) and have been tailored to mixed, uneven-aged montane forests belonging to the Abieti-Fagetum forest type ecology and land-use history.
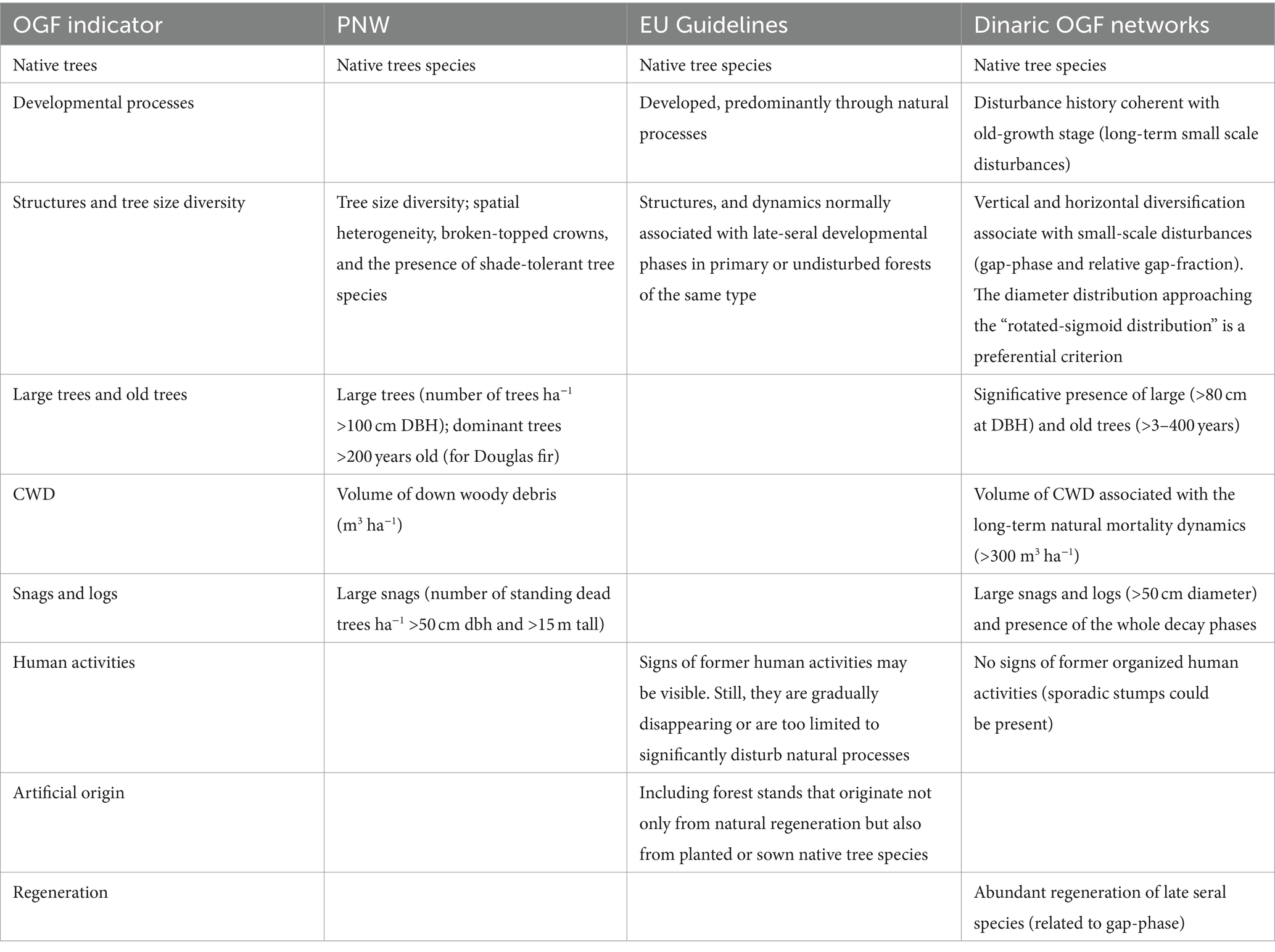
Table 1. Structural definition/indicators used in the Pacific Northwest (Franklin and Spies, 1991; Franklin et al., 2005), in the EU OGF Guidelines (European Union Commission, 2023a) and in the Dinaric OGF networks.
This paper presents the whole research Dinaric network coherently by summarizing 20 years of multidisciplinary research. It specifically aims to: (i) describe the structural characteristics and the disturbance history of the whole network and its coherency with the adopted OGF indicators; (ii) analyze structural commonalities and variabilities among these forests to delineate a common development process; (iii) compare structural and ecological differences at the studies sites with OGF criteria adopted by EU OGF Guidelines and discuss the scientific importance of rigorously characterizing OGF when used as a reference or benchmark for old-growthness, closer to nature forest management, ecological restoration, forest carbon policies, and climate change forest impacts; (iiii) supports the identification of a sub-set of OGF selected with more rigorous criteria, in the framework of the current OGF definition guidelines, for a better identification of conservation priorities and to be used as a reference or benchmark for research purposes.
2 Materials and methods
2.1 Study areas
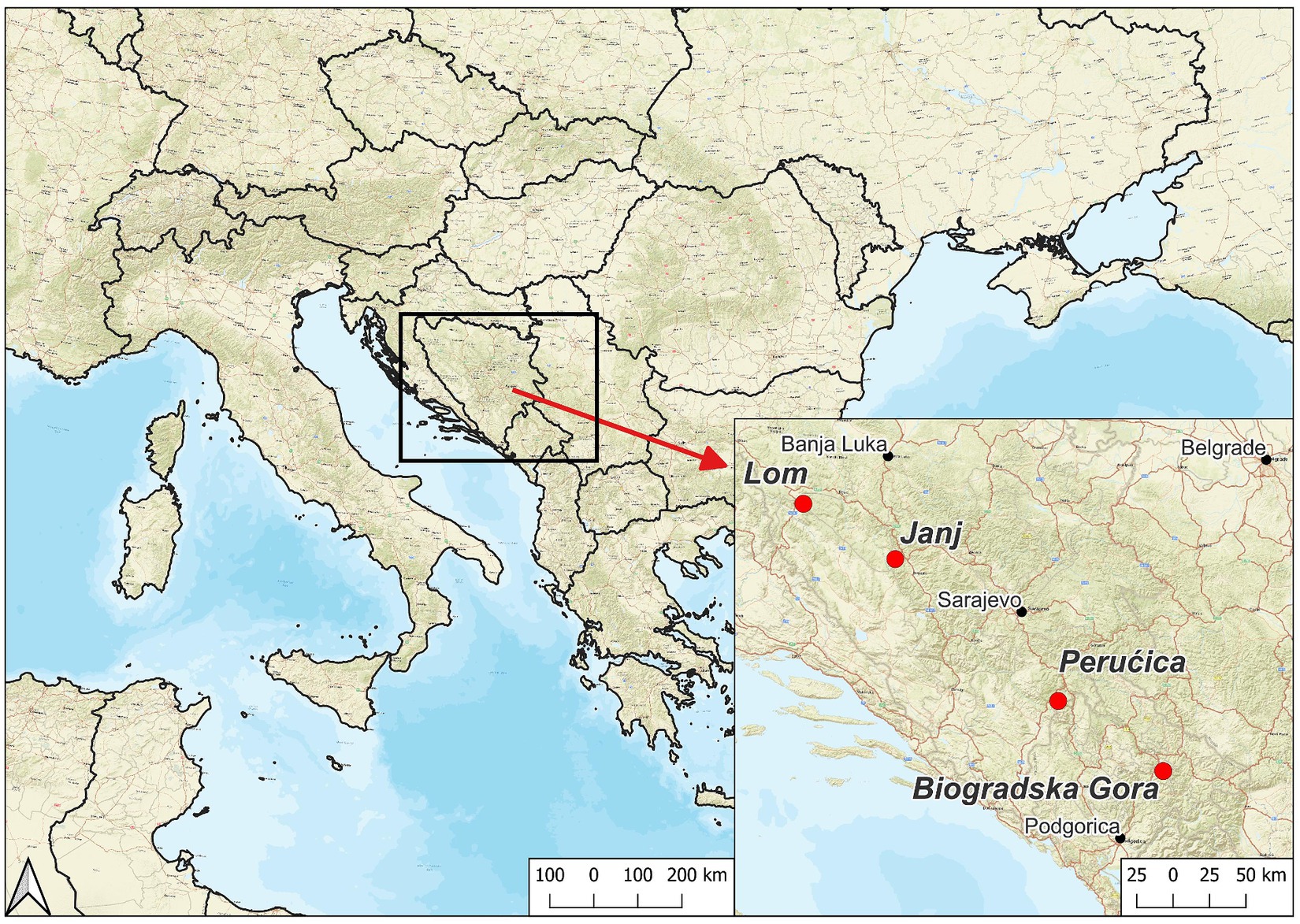
Since 2004, we have selected four study sites in the Dinaric Alps by following strict criteria (Table 1): Lom (LOM), Janj (JAN), and Perućica (PER) in Bosnia-Herzegovina and Biogradska Gora (BGO) in Montenegro (Figure 1; Table 2). We have focused our selection on mixed, uneven-aged montane forests belonging to the Abieti-Fagetum forest type (Figure 2). During the last 20 years, more than 40 researchers from 7 countries have been studying forest dynamics and ecological processes at different scales and according to a multidisciplinary approach (Table 3).

Figure 2. The four study sites of the Dinaric Alps in Bosnia-Herzegovina and Montenegro: (A) Biogradska Gora (MNE), (B) Perućica (BiH), (C) Janj (BiH) and (D) Lom (BiH). The forests are mixed with beech, fir, Norway spruce and sporadic broadleaves. The study sites are characterized by high living and CWD biomass, large and old trees, vertical multilayered structure and horizontal structure shaped by a small scale gap disturbance regime.
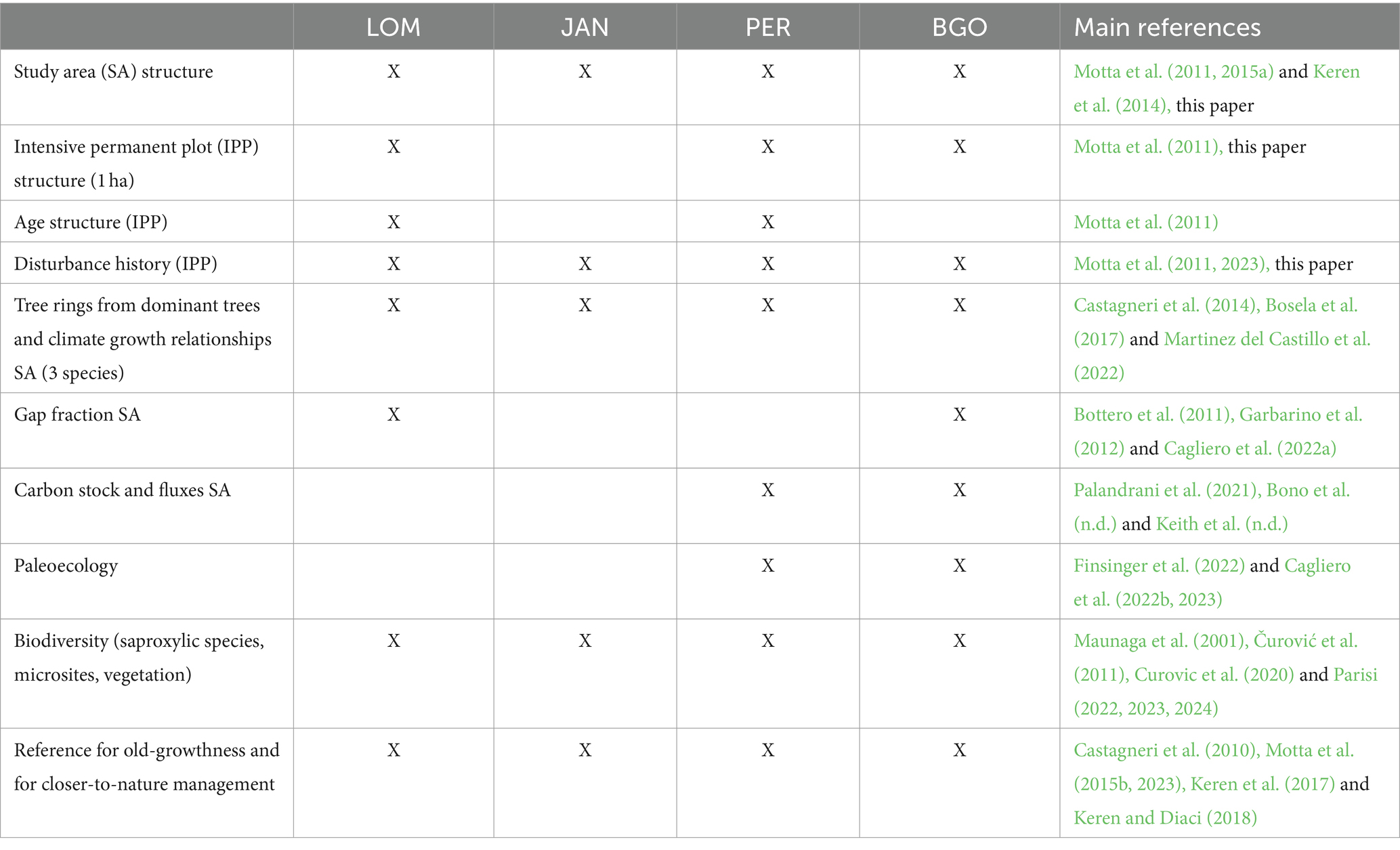
Table 3. Research infrastructures and main fields of research developed in the last 20 years at the four old-growth forests in the Dinaric Alps in Bosnia-Herzegovina and Montenegro.
LOM and JAN are Forest Reserves established by the State Institute for Protection of Cultural Monuments and Natural Rarities in Bosnia and Herzegovina (Maunaga et al., 2001). LOM reserve was set in 1956 (Pintarić, 1999; Motta et al., 2011). Initially, the Reserve was split between a core area of 55.8 ha under a strict protection and a buffer zone of 242 ha with low-intensity management. More recently (2021), strict protection was extended to the whole LOM reserve. JAN reserve was established in 1954 (Pintarić, 1999; Keren et al., 2017). As LOM, JAN was initially split between a strict protection core area (57.2 ha) surrounded by a buffer zone (237.8 ha) that were merged in 2021. In the same year, JAN was named a UNESCO World Heritage Site as an extension to the Ancient and Primeval Beech Forests of the Carpathians and Other Regions of Europe, and a new 295 ha buffer zone was delineated.
PER and BGO are among Europe’s most significant and best-preserved OGF (Sabatini et al., 2018). At both sites, the OGF covers more than 1,000 ha, including different forest types along an altitudinal gradient. The Abieti-Fagetum is the most represented one, covering more than 500–600 ha at both sites. The first official “protection” measures for these forests date back to 1893 for PER (Fukarek and Stefanović, 1958; Pintarić, 1978) and 1878 for BGO (Motta et al., 2015a; Stupar and Milanović, 2017), respectively. In both cases, the primary protection goal was hunting and game protection. BGO was declared a National Park (Biogradska Gora National Park) in 1952, while PER acquired the same status in 1962 (Sutjeska National Park).
All the sites are characterized by high fertility (Drinic, 1957), are among the highest carbon reserves in Europe (Keith et al., n.d.), and host some of the tallest European native trees, reaching more than 60 m height in PER and BGO and more than 55 m in LOM and JAN (Leibundgut, 1976; Diaci, 1999).
2.2 Dendrometric characteristics
2.2.1 Network of plots (forest inventory)
We identified a structurally uniform and representative study area at each site ranging from 33.1 to 57.2 ha. These areas were selected in the original reserve core area in LOM and JAN and after an intensive survey in the core area of Abieti-fagetum OGF in PER and BGO (Motta et al., 2011, 2015a; Keren et al., 2014). In each area, a regular grid (100 m for LOM, JAN, and PER and 120 m for BGO) was superimposed on the 1:10.000 raster map (see Supplementary material for further details).
In each plot, four types of measurement approaches were applied (Castagneri et al., 2010): (a1) in circular plots of 12 m of radius (14 m in LOM), we recorded species diameter at breast height (DBH) and height (to the closest 0.5 m) for all living trees with DBH >7.5 cm; (a2) in a 113.1 m2 round plot (radius = 6 m), species and height of each regeneration individual (h > 10 cm and DBH <7.5 cm) were recorded; (a3) on two 50 m intersecting lines oriented northward and eastward from the center of the sampling point, we measured the logs crossing the line (Van Wagner, 1968); and (a4) in a 50 × 8 m rectangular plot centered on the previous line we measured stumps (diameter at the ground and the top) and snags (DBH). For each element of coarse woody debris (CWD), size, species (when possible), and decay class were recorded (Maser et al., 1979). Volume for living trees and snags was calculated according to local volume tables. Volume for snags, logs, and stumps was calculated according to methods described in Motta et al. (2015b). Total deadwood carbon (C) stock was calculated using basic wood densities and C content for different decay stages derived from field sampling (Bono et al., n.d.). Litter and soil (down to 30 cm) were estimated through the Global Soil Organic Carbon Maps (FAO and ITPS, 2018), but also directly sampled (down to 90 cm) in PER and BGO (Bono et al., n.d.). The other C stocks were estimated using IPCC allometric algorithms (IPCC, 2006).
2.2.2 Intensive permanent plots
At three of the four study sites (LOM, PER, BGO), we have established a 1 ha intensive permanent plot (IPP) where all the trees with DBH >7.5 cm and CWD have been measured (DBH, height, and four canopy projections on the ground for the trees, diameters, height, and decay stage for the CWD) and mapped (Motta et al., 2011). The cores, due to the restrictions posed by the Biogradska Gora National Park Administration, were collected only in two sites (LOM and PER) that were analyzed for tree age and growth patterns.
2.3 Growth pattern and age
We collected one core from the living trees with DBH >10 cm from all the trees in the IPP of LOM and from 75% of the trees, randomly selected, in the IPP of PER. The cores were collected at 50 cm height from the ground to better estimate tree age and reconstruct the stand age structure. They were also used to analyze growth patterns and reconstruct disturbance history at the stand level. In addition, in each research sites, 12–30 dominant trees randomly selected in the study areas were cored (two cores for each tree) at DBH to analyze climate growth relationships (Castagneri et al., 2014; Bosela et al., 2017; Martinez del Castillo et al., 2022) and to estimate the maximum age reached by dominant trees of each species. In BGO, due to the restrictions posed by the National Park Administration, only standing or lying dead trees were cored, resulting in an under-representation of dominant Norway spruces.
All cores were fixed to wooden supports in the laboratory and prepared with a razor until an optimal surface resolution was achieved. Tree ring widths were measured to the nearest 0.01 mm using the LINTAB device and TSAP-Win software package (Rinntech, Heidelberg, Germany). The tree-ring series were visually and statistically cross-dated by CooRecorder software (Larsson and Larsson, 2018).
To describe past disturbance events, we analyzed the growth pattern in cores from permanent plots to identify releases from suppression. The detection of growth releases was obtained with Jolt software (version 6.01P by R. L. Holmes, University of Arizona, Tucson, Arizona), using the running mean radial-averaging method (Rubino and McCarthy, 2004; Fraver and White, 2005) and taking into account abrupt growth increment over 166% of the previous years in a window of 10 years. Only the growth increments that last at least 4 years were classified as releases from suppression (Schweingruber et al., 1990). A chronology of the releases found in the analyzed trees, all the species together, was constructed for each site. As the time to respond to stand opening is highly variable among species and individuals, the results were grouped at 10-year intervals (Altman, 2020; Izworska et al., 2022). The percentage of trees showing a growth release in a given decade was then plotted against time (Motta et al., 1999) starting from 1700 when at least 15 trees were available in both sites.
For age estimation, tree age from cross-dated cores was taken as the number of rings between the pith and the cambium (age at the coring height) when cores included the pith. In the other cases, if possible, the pith location was geometrically estimated (Motta and Nola, 2001). The missing radius was measured as the distance from the estimated pith location and the innermost ring. Then, missing rings to the pith were estimated by counting the number of rings in a segment equivalent to the length of the missing radius in the innermost part of the core. This number was added to the number of rings counted on the core to obtain the estimated tree age at the coring height. When it was impossible to estimate the age, e.g., for the decay or because the missing part could not be estimated reliably, we used the number of rings counted, indicating the presence of a missing part not counted (e.g., >480 years).
2.4 Patterns of forest structure
Between-plot and between-forest variability was explored using a multivariate approach based on PCA free ordination analysis employing a single matrix (142 plots and 20 structural variables). Forest structure variables for living trees (basal area, density, volume, quadratic mean diameter, standard deviation of diameter, Shannon diameter diversity), coarse woody debris (overall CWD volume, volume of stumps, logs, snags having a diameter >47.5 cm), and regeneration (overall density of saplings and divided by species) were used to assess the between-forest and the between-plots variability. Species composition was measured as a proportion of single species (beech, silver fir, Norway spruce, and others) on the plot basal area. A particular focus on large (DBH >80 cm) and medium-size (DBH >50 cm) trees was adopted by measuring the densities of these two diameter classes. PCA was performed to explore the correlation structure among living trees, regeneration, and CWD attributes. The average structures of old-growth forests were indicated by centroids, and convex hulls indicated their range of variability. PCA was performed using the PC-ORD 7.10 (McCune and Mefford 1999) statistical package. The axes’ statistical significance was tested using the Monte Carlo permutation method based on 10,000 runs with randomized data.
3 Results
3.1 Dendrometric characteristics at the forest inventory plots and intensive permanent plots
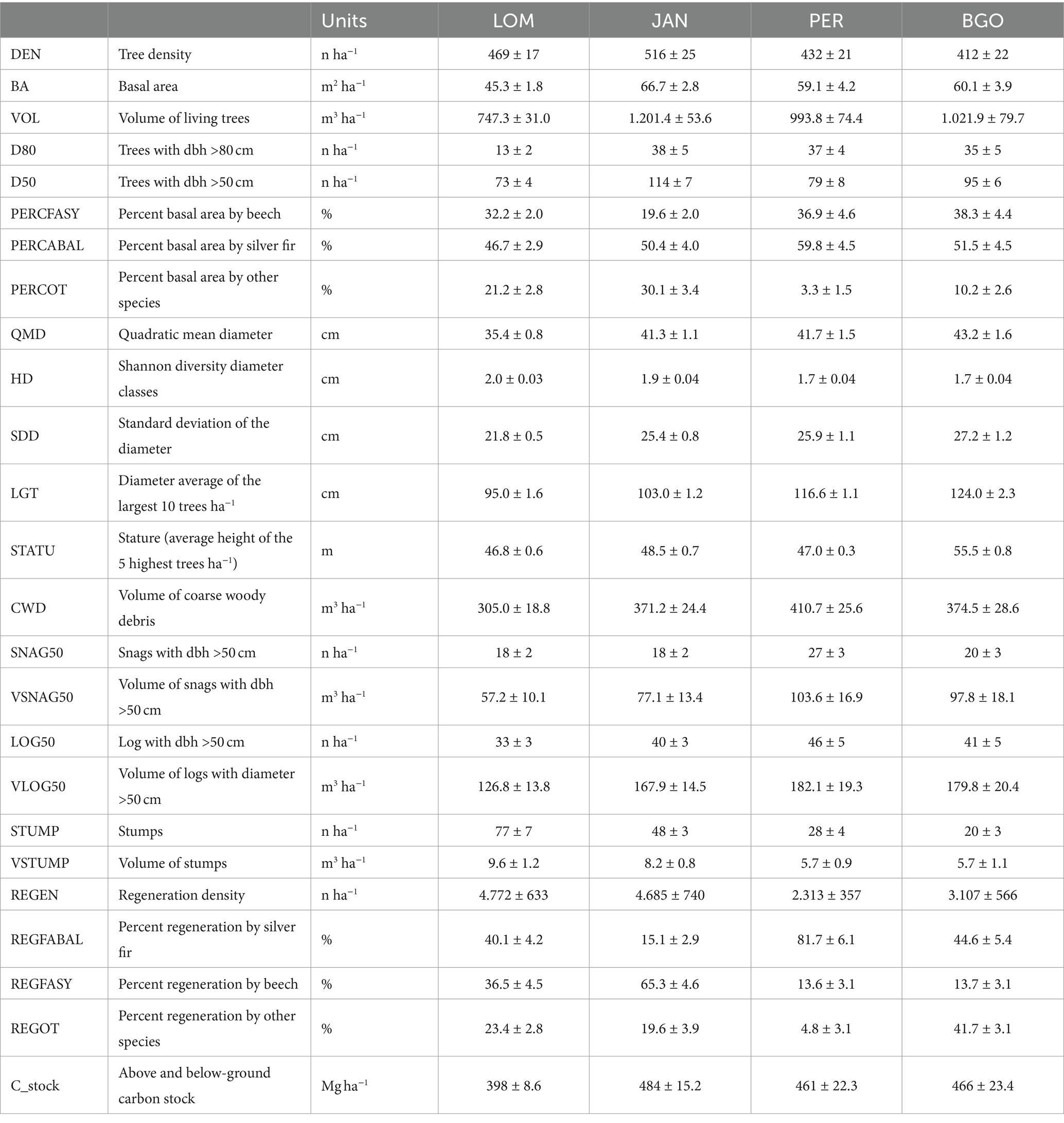
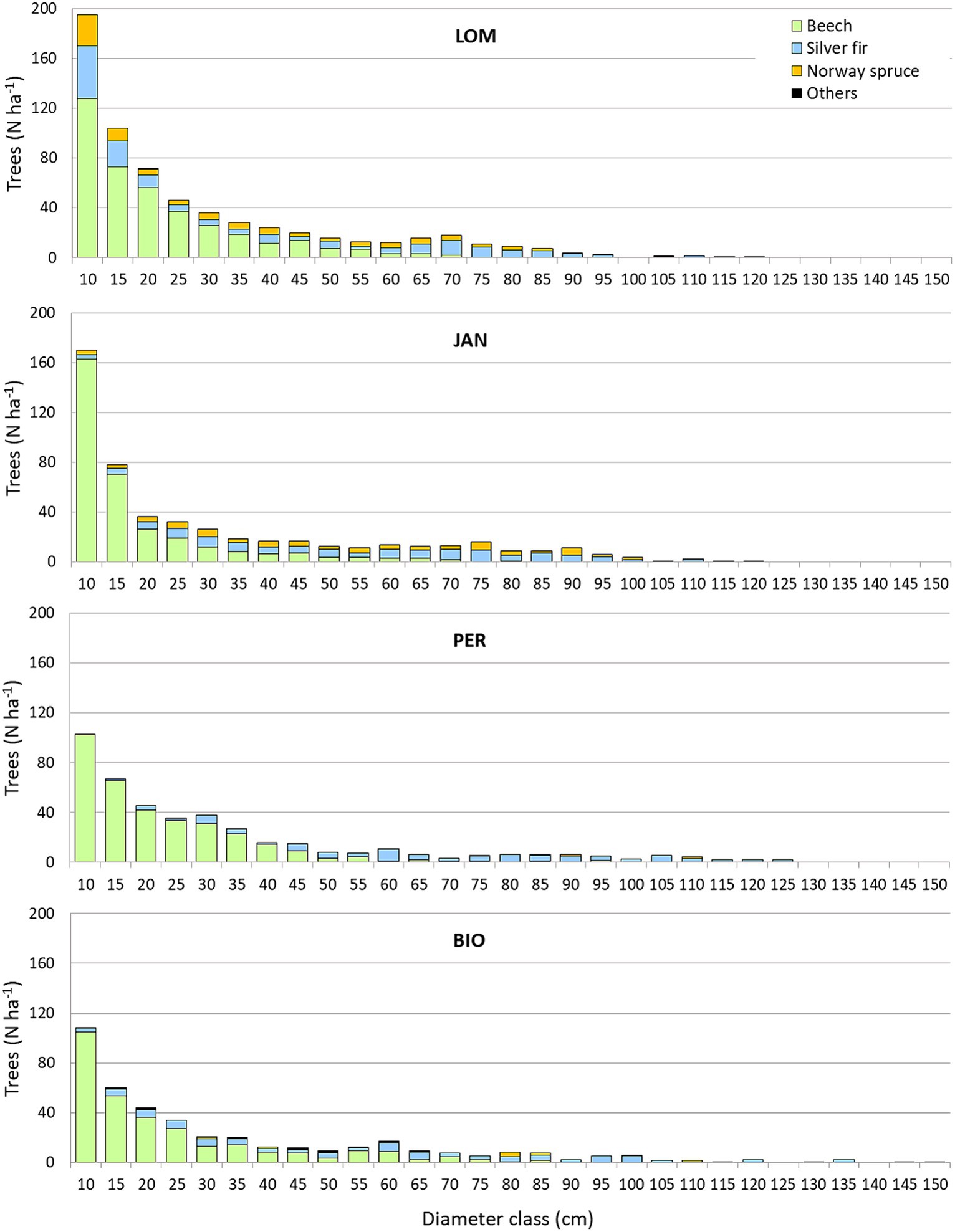
The density of living trees with a diameter greater than or equal to 7.5 cm was between 412 and 516 trees per hectare in BGO and JAN, respectively (Table 4). The basal area and volume ranged from 45.3 to 66.7 m2 ha−1 and from 747.3 to 1201.9 m3 ha−1 in LOM and JAN, respectively, with JAN having the highest values and LOM having the lowest. It is worth noting that the average volume data from LOM is influenced by the karstic morphology with sinkholes that limit the occupancy of the forest cover. In fact, the 1 hectare permanent plot in LOM, situated on a relatively uniform slope, has a volume of 1,116 m3, which is the same order as the other three sites (Motta et al., 2011). The beech accounts for 19.6 to 38.3% of the basal area in JAN and BGO, respectively. Silver fir is the dominant species at all sites, ranging from 46.6% in LOM to 59.8% in PER. The incidence of Norway spruce is higher in the northern sites (LOM and JAN) than in the southern ones (PER and BGO). Additionally, all sites have an impressive amount of CWD, ranging from 305.0 m3 ha−1 in LOM to 410.7 m3 ha−1 in BGO. Despite having high densities and volumes, all sites are relatively rich in regeneration, with LOM having the highest density with 4,772 individuals per hectare and PER having the lowest with 2,312 individuals per hectare. The carbon stocks range from 398.0 MgC ha−1 in LOM to 484.0 MgC ha−1 in JAN. The diameter distribution (Figure 3) has the structure of the uneven-aged forests and has a rotated sigmoid shape (Motta et al., 2015b) which is the typical distribution of the old-growth stage (Goff and West, 1975; Lorimer et al., 2001). The stature (i.e., the average of the five highest trees) a fertility index for uneven-aged and multilayered forests (Susmel, 1980) ranges between 55.8 m in BGO and 46.8 m in LOM (see Supplementary material for further details).
The in-depth study of the two IPP in LOM and PER has shown a density of large trees (DBH >1 m) of 19 trees per hectare in LOM (11 silver firs and 8 Norway spruce) and 14 trees in PER (11 silver firs and 3 Norway spruce). The largest beech has a DBH of 82 cm and 90 cm in LOM and PER, respectively (see Supplementary material for further details).
3.2 Growth patterns and age
We detected 564 releases in the IPP of LOM (493 trees analyzed) and 469 releases in PER (296 trees analyzed). The mean percentage of release per tree and the percentage of trees with release are slightly lower in LOM (about one release/tree and 64% of trees with release) than in PER (about 1.5 release/tree and 80% of trees with release).
Eighty-seven percentage of the decades in LOM and 77% of the decades in PER have less than 10% of the trees showing a release (Figure 4). At both sites, 13% of the decades have between 11 and 20% of trees with releases. In LOM, there is a low and regular distribution of the percentage of released trees, with a maximum percentage of about 13%. In PER, the pattern is characterized by slightly higher inter-decadal variability. However, the percentage of trees with release is over 20% (maximum 26%) only in two decades: 1880 and 1960. These conditions of low and quite regular % of releases for each decade have been relatively stable in both forests for the last three centuries.
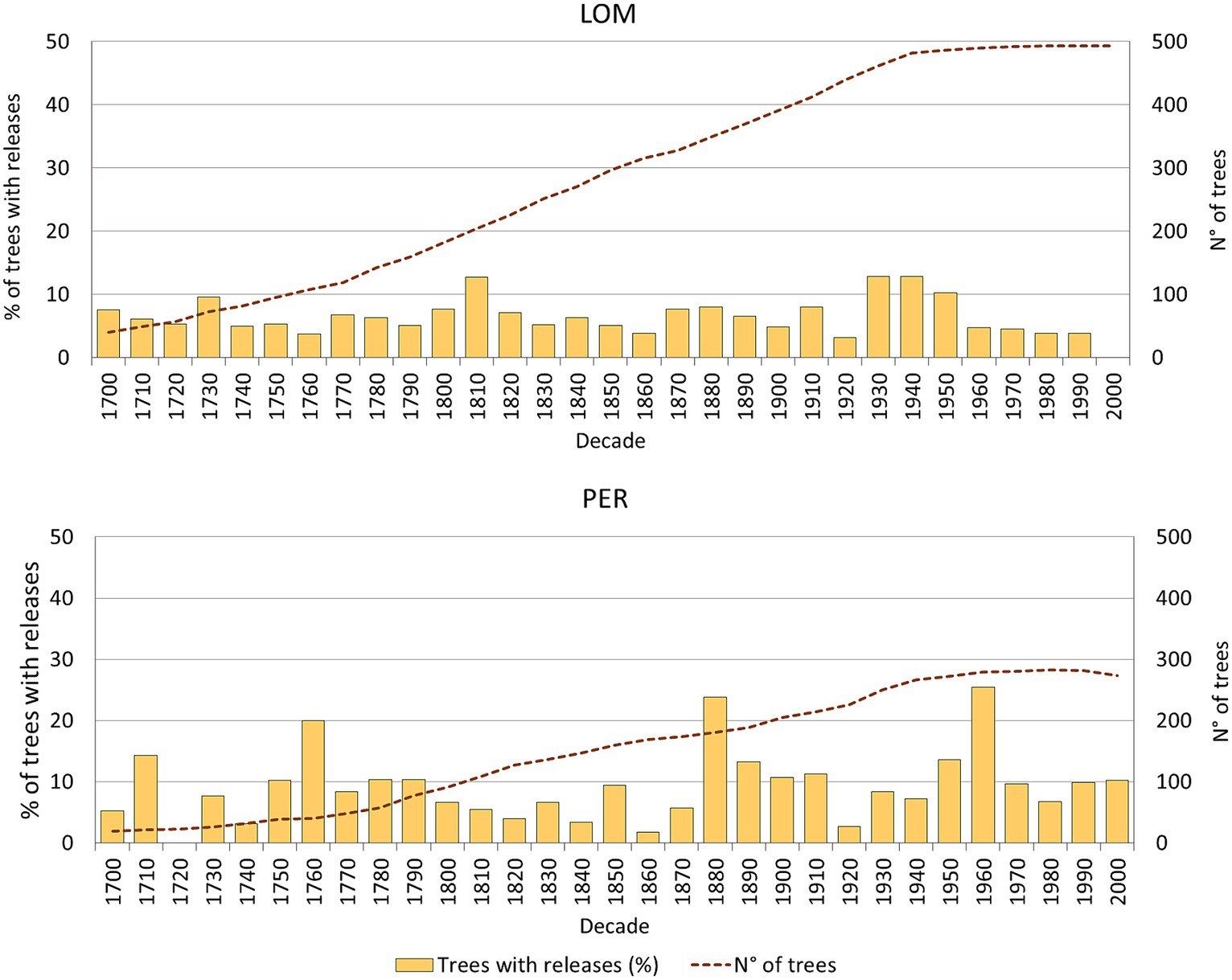
Figure 4. Release from suppression chronologies for the two 1 ha permanent plots analyzed (percent of trees showing abrupt growth release in a decade), including sample depth.
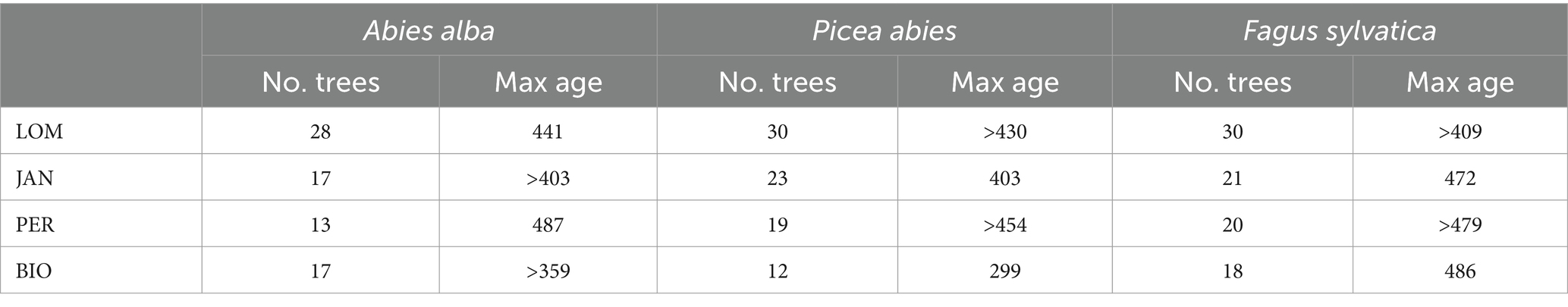
The age of dominant trees by species and site is summarized in Table 5. The three species showed similar maximum ages (except for Norway spruce in BGO affected by sampling limitations), and the age of oldest trees are near to the maximum biological age of the species (Castagneri et al., 2013; Piovesan and Biondi, 2020). Maximum age is achieved by silver fir in PER (487 years) and by beech in BIO (486 years). For Norway spruce, we cannot estimate the age of the oldest trees, neither in LOM nor in PER, due to the decay, but the maximum age is of the same order (>454 years in PER). As the estimated age is related to the coring height (130 cm above the ground), we can suppose that, taking into account the years employed by the tree to reach the sampling height, the oldest dominant trees should have an age >500 years. The two 1 ha sampling plots (see Supplementary material for further details) have a density of 15 trees ha−1 having >400 years at the sampling height (50 cm) in LOM (2 beeches, 7 silver firs, and 7 Norway spruces) and 11 trees ha−1 in PER (4 beeches, 5 silver firs, and 2 Norway spruces).
3.3 Patterns of forest structure
The forest structure of the four old-growth forests of the Dinaric Alps suggests some similarities and differences among sites. The difference is expressed by the distance among their centroids in the ordination environment and the similarities are expressed by the overlap among convex hulls polylines indicating the maximum surface area occupied by plots belonging to the same site: blue JAN, yellow LOM, grey BGO, and green PER (Figure 5). Considering the whole surface area occupied by the convex hulls, we observed that overlapping sites occupied 61.1% of this surface area and 15.3% was the proportion occupied by all the 4 sites. Instead, only 38.9% of this surface area was occupied by non-overlapping convex hulls. PER and BGO emerged as being quite similar regarding forest structure expressed by many structural data but particularly by a higher quantity of CWD and a lower proportion of Norway spruce. LOM and JAN were characterized by denser (De) and diverse (HD) stands with a higher density of regeneration (R-tot) and a higher volume of stumps (most of the stumps are of natural origin but in JAN and LOM there are also a few signs of human activity at the borders between the former core area and the buffer zone). JAN resulted separated from LOM along a gradient of overall basal area (BA), volume (Vol) and a lower proportion of beech. Therefore, the structural variability is PER > BGO > JAN > LOM. The overlapping is very high between PER and BGO and decreases following a latitudinal gradient towards JAN and LOM. The surface area proportion occupied by the convex hulls of the 4 study sites was 12.3% (LOM), 22.1% (JAN), 26.3% (PER), and 39.4% (BGO).
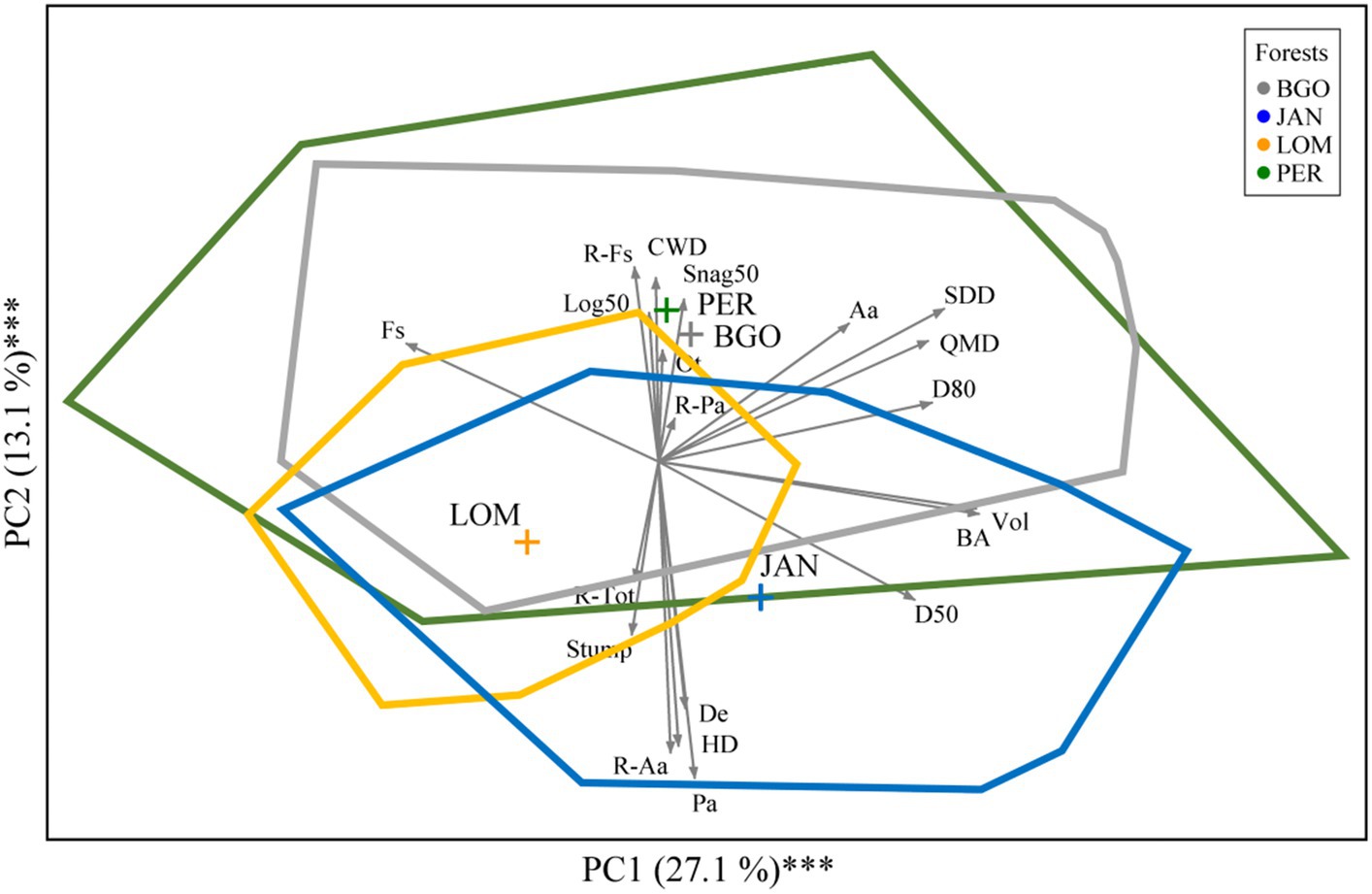
Figure 5. Principal component analysis of 142 plots of 4 old-growth forests of the Dinaric Alps indicated by colored lines with (blue for JAN, yellow for LOM, grey for BGO, and green for PER). Gray arrows indicate forest structure variables (n = 20) and colored polygons indicate convex hulls.
4 Discussion
4.1 Old-growthness
All the selected study sites fit in the strict old-growth structural criteria reported in Table 1. The four sites have the highest volume of living trees for the mixed montane forests of the central-southern part of Europe (Table 6). The high quantity of living biomass is typical of the old-growth stage. However, this characteristic is not an indicator of naturalness itself since high levels of biomass can also be found in managed forests or in forests that are in development stages (e.g., mature or transition) earlier than the old-growth one (Castagneri et al., 2008; Molina-Valero et al., 2021). The four sites have a stratified vertical structure, an uneven-aged age structure with low relationships between age and diameter (Motta et al., 2011), a rotated sigmoid diameter distribution (Motta et al., 2015b), and a clumped horizontal structure (Lingua et al., 2011). All these structural characteristics are peculiar of the old-growth developmental stage. The tree species approach their maximum age expectancy not with sporadic individuals but with many dominant trees (15 ha−1 older than 400 years in LOM and 11 ha−1 PER) and are richer in large and old trees (Table 5) compared to other OGF of the Carpathians (Pavlin et al., 2021).

Table 6. Stand characteristics of some of the most important mixed Abies-Fagus-Picea old-growth forests in central and south-east Europe.
All the study sites have impressive amounts of CWD ranging between 305.0 and 410.7 m3 ha−1 (Table 4). The high quantity of CWD is peculiar to the old-growth stage and is the legacy of the long-term small-scale disturbance history that has characterized the dynamics in the last centuries (Lombardi et al., 2012; Mikolas et al., 2021; Motta et al., 2023). Besides, all sites have a high density of large (>50 cm DBH) snags (ranging between 18 and 27 ha−1) and logs (between 33 and 46 ha−1 with largest diameter >50 cm) (Table 4).
The high regeneration density is related to the fact that all the dominant species are shade-tolerant and can regenerate and establish below the forest cover and to the small-scale disturbances that create gaps that allow the regeneration to establish and grow continuously in the stand. The canopy gap fraction in the studied sites is 19.3% in LOM, 17.2% in PER, and, in extended gaps fraction, 41.4% in LOM, and 39.7% in PER (Nagel and Svoboda, 2008; Bottero et al., 2011).
Tree rings provided detailed insight into temporal patterns of forest disturbance history in LOM and PER. In both forests, most decades in the last three centuries experienced less than 10% of canopy opening signs (release from suppression). We have observed in one decade a maximum of 16% of trees showing a release in LOM and 26% in PER, with only two decades with >20% of trees showing a release in PER. Thus, the dynamics of these stands have been strongly controlled by small-scale disturbances that have maintained a typical small-gap structure for a relatively long time. According to this small level of disturbances, these stands can be classified at the limit of the range of dynamics, from disturbance-structured to near steady-state (Antos and Parish, 2002; Halpin and Lorimer, 2016).
The methodological approach used here has the potential to reconstruct the disturbance history carefully, but conversely, this can be done at a relatively small scale, in this case one hectare (Nagel et al., 2014). Thus, these results have to be taken with caution as, if we had expanded the structural analysis at the landscape scale, we could have detected some legacies of past infrequent intermediate severity-disturbances from fire (PER), wind (PER, BGO, LOM) and from small landslides and snow avalanches (BGO), large enough to favor the regeneration of more light-demanding tree species like maple and other broadleaves (Nagel et al., 2014; Motta et al., 2015a). Besides, more intensive past land use by the local population has been observed locally and at the regional scale during the middle ages (Finsinger et al., 2022; Cagliero et al., 2022b). The extensive overlap between convex hulls (Figure 5) confirms the commonality of structures and processes already observed in the structural data and the disturbance regime.
4.2 Old-growth forests as reference or benchmark
The current forest cover of OGF in Europe is residual (or even absent for many forest types, particularly in temperate and Mediterranean forests), since most European forests have been intensively managed and, even if withdrawn from regular management, show structural legacies of the past land use. After the cessation of the management, some structural elements can recover faster, and, in contrast, others recover only in the long term. For example, large dead trees, old trees, rotated sigmoid diameter distribution, gap fraction, development of microhabitats (von Oheimb et al., 2005; Paillet et al., 2010; Meyer and Schmidt, 2011; Albrich et al., 2021; Kõrkjas et al., 2021) and the time framework of the restoration (Frelich et al., 2005; Nagel et al., 2013) is often measured not by decades but by centuries (Motta et al., 2015b; Albrich et al., 2021; Meyer et al., 2021; Martin-Benito et al., 2022; Larrieu et al., 2023).
The criteria adopted by the recent EU Guidelines (European Union Commission, 2023a) “Defining, Mapping, Monitoring and Strictly Protecting EU Primary and Old-Growth Forests” are relatively broad and not well-defined. Consequently, most of the selected and mapped OGF according to the EU guidelines could have a low rate of old-growthness and could not adequately represent the final stage of the forest dynamics. The EU approach considers as OGF those stands “relatively old and relatively undisturbed by humans.” As such, it identifies a critical amount of forests mostly represented by “future potential old-growth” (Spies, 2004), but, even if it is an appropriate approach to protect the residual biodiversity related to late seral developmental stages, has limits both for strict protection of current OGF and for those scientific goals that need an absolute reference of the final stage of the forest dynamic processes. The priority of biodiversity conservation would be to accurately select the “current” old-growth stands, analyze their distribution, and protect them at the continental scale. These forests represent a residual fraction of the continental forest cover and deserve a different recognition and designation among the massive selection made by the EU Guidelines. A fundamental issue is related to the scientific purposes connected to the old-growth designation. Indeed, for scientific research, only OGF selected with strict criteria can be reliably used as a reference or benchmark (Table 7). Another issue to be taken into account when research and benchmarking are involved is the need to assure long-term potential conservation of the whole range of variability (Hansen et al., 1991) inherent to the complete forest dynamics, including stand-replacing disturbances and early seral stages. For this purpose, it would be necessary to define a minimal critical size of old-growth sites (Landres et al., 1999; Veblen, 2003; Peck et al., 2015). The Abieti-fagetum is not characterized by high severity and large disturbance events (Senf and Seidl, 2021), and in the studied Dinaric forests, we have not observed stand-replacing disturbances larger than 1 ha, even at the landscape scale (Garbarino et al., 2012; Motta et al., 2015a). However, considering a comprehensive variability covering the European range of the montane forest types, we have to take into account the occurrence in this forest type of high-severity stand-replacing events larger than 15 ha (Maroschek et al., 2023). In a temperate primary forest, depending on the longevity of the trees and on the persistence of each developmental stage, the percentage of the forest covered with old-growth stage can vary approximately from 25 to 60% (Swanson et al., 1994; Oliver and Larson, 1996). As a consequence, we can hypothesize, in a prudential way and according to the information currently available, a size of at least 25–30 ha to host the current and future entire range of variability of the different developmental stages of the forest dynamics (Landres et al., 1999; Keane et al., 2009) and to guarantee long-term conservation of the whole biodiversity associated to the forest type. A more detailed study will be necessary to explore the range of variability in the European forest types as natural disturbance regimes and interactions among disturbances at different spatial and temporal scales are poorly understood (Landres et al., 1999). Besides, future climate change impacts can significantly impact the current framework (Seidl et al., 2017; Thom et al., 2017).
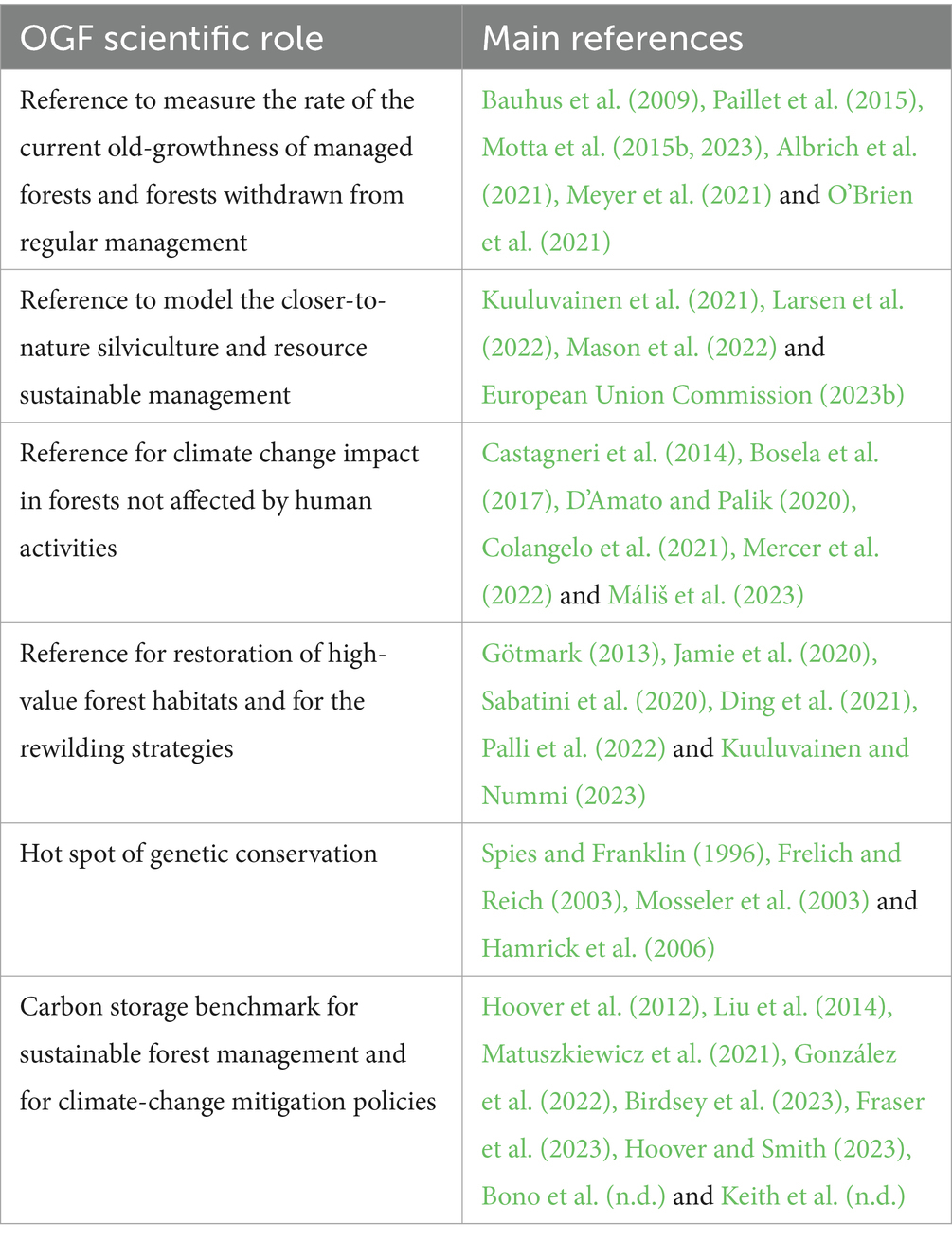
Table 7. Reference and benchmark roles played by old-growth forests for which a strict definition is necessary.
5 Conclusion
Old-growthness is a dynamic process and the development of the old-growth stage takes a long time (Oliver and Larson, 1996). This time depends on forest type and on-site characteristics, but the old-growth stage’s structural characteristics are similar among forests belonging to the same forest type (Franklin et al., 1981).
The four studied Dinaric forests have all the basic and seminal structural indicators of an OGF: large trees (>10 trees per ha with DBH above 100 cm), old trees (more than 10 trees per ha older than 400 years old), a huge amount of CWD (>300 m3 ha−1) and a diversified vertical and horizontal structure (Franklin et al., 1981, 2005). The structure shows the same structural patterns and developmental processes across all the studied sites. All three main species are approaching their maximum age expectancy. The four studied forests share a set of most refined structural and functional indicators (e.g., rotated sigmoid diameter distribution, gap fraction, disturbance history) that are critical to defining the threshold between late seral and over-mature forests and strict old-growth (Whitman and Hagan, 2007; Motta et al., 2015b; Meyer et al., 2021; Price et al., 2023).
In Europe, there are very few forests that fit strict OGF parameters. Most of the research and the governance guidelines currently focus on protecting the residual OGF biodiversity using broad definitions of old-growth and including late-seral stands and forests that have been withdrawn from management for a few decades. This policy is commendable for identifying a critical amount of forest cover at the continental scale. Besides this, we need to define a sub-set of forests using a “robust” and “reliable” definition of strict OGF to be considered a priority for biodiversity conservation against late seral, transition, and future OGF (Woodall et al., 2023). We must recognize the irreplaceable conservation and scientific value of the current OGF (selected with strict criteria), and if we did not take apart the strict OGF from the other forests, any use of them as a reference or benchmark would be impossible or misleading (Table 7).
As it is not possible to define a threshold that covers different forest types, fertility classes, and regional-local peculiarities, basic structural parameters associated with a “combined provisions” made by experts and supported by field, remote sensing data, adjusted to criteria at local scales, and incorporating social and traditional knowledge could be used to define the old-growthness of each forest. We can expect that it will not be possible to identify stands with these characteristics in all the European forest types, but this information is currently missing and will be relevant for future European policies regarding forest conservation. Finally, the definition and mapping of strict OGF, given the importance of these references also for EU forests, should be expanded on sites currently outside the EU’s borders, like most of the OGF in the Dinaric mountains.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. GA: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. DA: Data curation, Investigation, Writing – original draft, Writing – review & editing. RB: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. SB: Data curation, Investigation, Writing – original draft, Writing – review & editing. AB: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. CM: Investigation, Writing – original draft, Writing – review & editing. DV: Investigation, Writing – original draft, Writing – review & editing. WF: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MG: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. ZG: Data curation, Investigation, Writing – original draft, Writing – review & editing. SK: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. FM: Investigation, Writing – original draft, Writing – review & editing. FR: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. PN: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The data analysis was supported by the WILDCARD project funded by the European Climate, Infrastructure and Environment Executive Agency (CINEA) of the European Union under Grant Number 101081177. Views and opinions expresses are however those of the authors only and do not necessarily reflect those of the European Union of CINEA. Neither the European Union nor the granting authority can be held responsible for them.
Acknowledgments
Thanks to the Public Forestry Enterprise “Forests of the Republic of Srpska” and the Forest Estates “Oštrelj-Drinić (Drinić, Republic of Srpska/BB.H.) and “Gorica” (Šipovo, Republic of Srpska/BB.H.) for providing data, support, transportation and for the fruitful discussions. Thanks to the National Parks Biogradska Gora (Kolašin, Montenegro) and Sutjeska (Tjentište, Bosnia and Herzegovina) for providing information, data, and logistic support. The data set is part of the European Forest Reserves Initiative (EuFoRIa, www.euforia-project.org).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2024.1371144/full#supplementary-material
References
Albrich, K., Thom, D., Rammer, W., and Seidl, R. (2021). The long way back: development of Central European mountain forests towards old-growth conditions after cessation of management. J. Veg. Sci. 32:e13052. doi: 10.1111/jvs.13052
Altman, J. (2020). Tree-ring-based disturbance reconstruction in interdisciplinary research: current state and future directions. Dendrochronologia 63:125733. doi: 10.1016/j.dendro.2020.125733
Antos, J. A., and Parish, R. (2002). Dynamics of an old-growth, fire-initiated, subalpine forest in southern interior British Columbia: tree size, age, and spatial structure. Can. J. For. Res. 32, 1935–1946. doi: 10.1139/x02-116
Barredo Cano, J. I., Brailescu, C., Teller, A., Sabatini, F. M., Mauri, A., and Janouskova, K. (2021). Mapping and assessment of primary and old-growth forests in Europe. Publications Office of the European Union: Luxembourg.
Bauhus, J., Puettmann, K., and Messier, C. (2009). Silviculture for old-growth attributes. For. Ecol. Manag. 258, 525–537. doi: 10.1016/j.foreco.2009.01.053
Birdsey, R. A., DellaSala, D. A., Walker, W. S., Gorelik, S. R., Rose, G., and Ramírez, C. E. (2023). Assessing carbon stocks and accumulation potential of mature forests and larger trees in U.S. federal lands. Front. For. Glob. Change 5:1074508. doi: 10.3389/ffgc.2022.1074508
Birks, H. J. B., and Tinner, W. (2016). Past forests of Europe. J. San-Miguel-Ayanz, D. Rigode, G. Caudullo, T. Houston Durrant, and A. Mauri, European atlas of forest tree species. Publications Office of the European Union, Luxembourg.
Bono, A., Alberti, G., Berretti, R., Curovic, M., Dukic, V., and Motta, R.. (n.d.). The largest European forest carbon sinks are in the Dinaric Alps: direct measurements and standardized approaches comparison. (Submitted)
Bosela, M., Lukac, M., Castagneri, D., Sedmák, R., Biber, P., Carrer, M., et al. (2017). Contrasting effects of environmental change on the radial growth of co-occurring beech and fir trees across Europe. Sci. Total Environ. 615, 1460–1469. doi: 10.1016/j.scitotenv.2017.09.092
Bottero, A., Garbarino, M., Dukic, V., Govedar, Z., Lingua, E., Nagel, T. A., et al. (2011). Gap-phase dynamics in the old-growth forest of Lom, Bosnia and Herzegovina. Silva Fenn. 45, 875–887. doi: 10.14214/sf.76
Burrascano, S., Keeton, W. S., Sabatini, F. M., and Blasi, C. (2013). Commonality and variability in the structural attributes of moist temperate old-growth forests: a global review. For. Ecol. Manag. 291, 458–479. doi: 10.1016/j.foreco.2012.11.020
Cagliero, E., Morresi, D., Marchi, N., Paradis, L., Finsinger, W., Garbarino, M., et al. (2022a). “Land-cover mapping in the Biogradska Gora National Park with very-high-resolution Pléiades images” in Geomatics and geospatial technologies. eds. E. Borgogno-Mondino and P. Zamperlin (Cham: Springer International Publishing), 15–27.
Cagliero, E., Morresi, D., Paradis, L., Curovic, M., Spalevic, V., Marchi, N., et al. (2022b). Legacies of past human activities on one of the largest old-growth forests in the south-east European mountains. Veg. Hist. Archaeobot. 31, 415–430. doi: 10.1007/s00334-021-00862-x
Cagliero, E., Paradis, L., Marchi, N., Lisztes-Szabó, Z., Braun, M., Hubay, K., et al. (2023). The role of fire disturbances, human activities and climate change for long-term forest dynamics in upper-montane forests of the central Dinaric Alps. Holocene 33, 827–841. doi: 10.1177/09596836231163515
Castagneri, D., Garbarino, M., Berretti, R., and Motta, R. (2010). Site and stand effects on coarse woody debris in montane mixed forests of Eastern Italian Alps. For. Ecol. Manag. 260, 1592–1598. doi: 10.1016/j.foreco.2010.08.008
Castagneri, D., Nola, P., Motta, R., and Carrer, M. (2014). Summer climate variability over the last 250 years differently affected tree species radial growth in a mesic Fagus-Abies-Picea old-growth forest. For. Ecol. Manag. 320, 21–29. doi: 10.1016/j.foreco.2014.02.023
Castagneri, D., Storaunet, K. O., and Rolstad, J. (2013). Age and growth patterns of old Norway spruce trees in Trillemarka forest, Norway. Scand. J. For. Res. 28, 232–240. doi: 10.1080/02827581.2012.724082
Castagneri, D., Vacchiano, G., Lingua, E., and Motta, R. (2008). Analysis of intraspecific competition in two subalpine Norway spruce (Picea abies (L.) Karst.) stands in Paneveggio (Trento, Italy). For. Ecol. Manag. 255, 651–659. doi: 10.1016/j.foreco.2007.09.041
Clements, F. E. (1936). Nature and structure of the climax. J. Ecol. 24, 252–284. doi: 10.2307/2256278
Colangelo, M., Camarero, J. J., Gazol, A., Piovesan, G., Borghetti, M., Baliva, M., et al. (2021). Mediterranean old-growth forests exhibit resistance to climate warming. Sci. Total Environ. 801:149684. doi: 10.1016/j.scitotenv.2021.149684
Čurović, M., Medarević, M., Cvjetićanin, R., and Knežević, M. (2011). Major characteristics of mixed fir and beech virgin forests in the National Park Biogradska Gora in Montenegro. Bull. Fac. For. 103, 157–172. doi: 10.2298/GSF1103157C
Curovic, M., Spalevic, V., Sestras, P., Motta, R., Dan, C., Garbarino, M., et al. (2020). Structural and ecological characteristics of mixed broadleaved old-growth forest (Biogradska Gora—Montenegro). Turk. J. Agric. For. 44, 428–438. doi: 10.3906/tar-2003-103
D’Amato, A. W., and Palik, B. J. (2020). Building on the last “new” thing: exploring the compatibility of ecological and adaptation silviculture. Can. J. For. Res. 51, 172–180. doi: 10.1139/cjfr-2020-0306
Diaci, J. (Ed.) (1999). Virgin forests and forest reserves in Central and East European countries, Ljubljana, Slovenia: University of Ljubljana.
Ding, Z., Li, R., O'Connor, P., Zheng, H., Huang, B., Kong, L., et al. (2021). An improved quality assessment framework to better inform large-scale forest restoration management. Ecol. Indic. 123:107370. doi: 10.1016/j.ecolind.2021.107370
Drinic, P. (1957). Taksacioni elementi bukovih sastojina prasumskog tipa u Donjoj Drinjaci. Radovi Šumarskog Fakulteta Univerziteta U Sarajevu 5, 105–140. doi: 10.54652/rsf.1957.v2.i2.394
Duduman, G., Duduman, M.-L., Daniel, A., Barnoaiea, I., Barbu, C.-O., Ciornei, I., et al. (2020). A permanent research platform for ecological studies in intact temperate mountainous forests from Slătioara UNESCO site and its surroundings, Romania. Forests 11, 1–20. doi: 10.3390/f11091004
European Union Commission . (2023a). Commission guidelines for defining, mapping, monitoring and strictly protecting EU primary and old-growth forests, Publications Office of the European Union. Bruxelles.
European Union Commission . (2023b). Guidelines on closer-to-nature forest management. Publications Office of the European Union, Bruxelles.
FAO and ITPS (2018). Global Soil Organic Carbon Map (GSOCmap). Rome, Italy: Food and Agriculture Organization of the United Nations.
Finsinger, W., Cagliero, E., Morresi, D., Paradis, L., Čurović, M., Garbarino, M., et al. (2022). The value of long-term history of small and fragmented old-growth forests for restoration ecology. Past Global Changes Magazine 30, 8–9. doi: 10.22498/pages.30.1.8
Franklin, J. F., Cromack, K. J., Denison, W., McKee, A., Maser, C., Sedell, J., et al. (1981). Ecological characteristics of old-growth Douglas-fir forests. U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR.
Franklin, J. F., and Spies, T. A. (1991). “Ecological definitions of old-growth Douglas-fir forests” in Wildlife and vegetation of unmanaged Douglas-fir forests. General technical report PNW-GTR-85. eds. L. F. Ruggiero, K. B. Aubry, A. B. Carey, and M. H. Huff (Portland: USDA Forest Service, Pacific Northwest Research Station), 61–69.
Franklin, J., Spies, T. A., Pelt, R. V., Carey, A. B., Thornburgh, D. A., Berg, D. R., et al. (2002). Disturbance and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fire forests as an example. For. Ecol. Manag. 155, 399–423. doi: 10.1016/S0378-1127(01)00575-8
Franklin, J. F., Spies, T. A., and Van Pelt, R.. (2005). Definition and inventory of old-growth forests on DNR-managed state lands. Washington State Department of Natural Resources, Olympia, WA, p. 74.
Fraser, J. S., Pile Knapp, L. S., Graham, B., Jenkins, M. A., Kabrick, J., Saunders, M., et al. (2023). Carbon dynamics in old-growth forests of the Central Hardwoods Region, USA. For. Ecol. Manag. 537:120958. doi: 10.1016/j.foreco.2023.120958
Fraver, S., and White, A. S. (2005). Disturbance dynamics of old-growth Picea rubens forests of northern Maine. J. Veg. Sci. 16, 597–610. doi: 10.1111/j.1654-1103.2005.tb02401.x
Frelich, L. E., Cornett, M. W., and White, M. A. (2005). Controls and reference conditions in forestry: the role of old-growth and retrospective studies. J. For. 103, 339–344. doi: 10.1093/jof/103.7.339
Frelich, L. E., and Reich, P. B. (2003). Perspectives on development of definitions and values related to old-growth forests. Environ. Rev. 11, S9–S22. doi: 10.1139/a03-011
Fröhlich, J. (1930). Der südosteuropäische Urwald und seine Überführung in Wirtschaftswald. Centralblatt für das Gesamte Forstwesen 52, 65–74. doi: 10.1007/BF01773690
Fukarek, P., and Stefanović, V. (1958). Virgin Perucica and its vegetation. Pap. Agric. For. Fac. Sarajevo 6, 93–146. doi: 10.54652/rsf.1958.v6.i3.399
Garbarino, M., Mondino, E. B., Lingua, E., Nagel, T. A., Dukic, V., Govedar, Z., et al. (2012). Gap disturbances and regeneration patterns in a Bosnian old-growth forest: a multispectral remote sensing and ground-based approach. Ann. For. Sci. 69, 617–625. doi: 10.1007/s13595-011-0177-9
Goff, F. G., and West, D. (1975). Canopy-understory interaction effects on forest population structure. For. Sci. 21, 98–108. doi: 10.1093/forestscience/21.2.98
González, M. E., Lara, A., Urrutia-Jalabert, R., Bustos-Salazar, A., Ruiz-Gómez, C., and Aravena, J. C. (2022). Carbon stocks across different environments, disturbance regimes, and stand age in Fitzroya cupressoides forests, the longest-lived species of the southern hemisphere. Front. For. Glob. Change 5:960429. doi: 10.3389/ffgc.2022.960429
Götmark, F. (2013). Habitat management alternatives for conservation forests in the temperate zone: review, synthesis, and implications. For. Ecol. Manag. 306, 292–307. doi: 10.1016/j.foreco.2013.06.014
Halpin, C. R., and Lorimer, C. G. (2016). Trajectories and resilience of stand structure in response to variable disturbance severities in northern hardwoods. For. Ecol. Manag. 365, 69–82. doi: 10.1016/j.foreco.2016.01.016
Hamrick, J. L., Godt, M. J. W., and Gonzales, E. (2006). Conservation of genetic diversity in old-growth forest communities of the southeastern United States. Appl. Veg. Sci. 9, 51–58. doi: 10.1111/j.1654-109X.2006.tb00655.x
Hansen, A. J., Spies, T. A., Swanson, F. J., and Ohmann, J. L. (1991). Conserving biodiversity in managed forests: lesson from natural forests. BioScience 41, 382–392. doi: 10.2307/1311745
Harmon, M. E., Franklin, J. F., Swanson, F. J., Sollins, P., Gregory, S. V., Lattin, J. D., et al. (1986). “Ecology of coarse woody debris in temperate ecosystems” in Advances in ecological research. eds. A. MacFadyen and E. D. Ford (Academic Press), 133–282.
Hejcman, M., Karlík, P., Ondráček, J., and Klír, T. (2013). Short-term medieval settlement activities irreversibly changed forest soils and vegetation in Central Europe. Ecosystems 16, 652–663. doi: 10.1007/s10021-013-9638-3
Hilmers, T., Avdagić, A., Bartkowicz, L., Bielak, K., Binder, F., Bončina, A., et al. (2019). The productivity of mixed mountain forests comprised of Fagus sylvatica, Picea abies, and Abies alba across Europe. Forestry, 92, 512–522. doi: 10.1093/forestry/cpz035
Holeksa, J., Saniga, M., Szwagrzyk, J., Czerniak, M., Staszynska, K., and Kapusta, P. (2009). A giant tree stand in the West Carpathians-an exception or a relic of formerly widespread mountain European forests? For. Ecol. Manag. 257, 1577–1585. doi: 10.1016/j.foreco.2009.01.008
Hoover, C. M., Leak, W. B., and Keel, B. G. (2012). Benchmark carbon stocks from old-growth forests in northern New England, USA. For. Ecol. Manag. 266, 108–114. doi: 10.1016/j.foreco.2011.11.010
Hoover, C. M., and Smith, J. E. (2023). Aboveground live tree carbon stock and change in forests of conterminous United States: influence of stand age. Carbon Balance Manag. 18:7. doi: 10.1186/s13021-023-00227-z
IPCC , (2006). IPCC guidelines for national greenhouse gas inventories. Institute for Global Environmental Strategies (IGES). Japan.
Izworska, K., Muter, E., Fleischer, P., and Zielonka, T. (2022). Delay of growth release after a windthrow event and climate response in a light-demanding species (European larch Larix decidua Mill.). Trees 36, 427–438. doi: 10.1007/s00468-021-02218-4
Jacobson, A. P., Riggio, J., Tait, M., and Baillie, J. E. M. (2019). Global areas of low human impact (‘low impact areas’) and fragmentation of the natural world. Sci. Rep. 9:14179. doi: 10.1038/s41598-019-50558-6
Jamie, M. W., Anthony, W. D. A., David, R. F., David, A. O., and Neil, P. (2020). Historic forest composition and structure across an old-growth landscape in New Hampshire, USA. J. Torrey Bot. Soc. 147, 291–303. doi: 10.3159/TORREY-D-18-00033.1
Jones, E. W. (1945). The structure and reproduction of the virgin forest of the north temperate zone. New Phytol. 44, 130–148. doi: 10.1111/j.1469-8137.1945.tb05026.x
Kaplan, J. O., Krumhardt, K. M., and Zimmermann, N. (2009). The prehistoric and preindustrial deforestation of Europe. Quat. Sci. Rev. 28, 3016–3034. doi: 10.1016/j.quascirev.2009.09.028
Keane, R. E., Hessburg, P. F., Landres, P. B., and Swanson, F. J. (2009). The use of historical range and variability (HRV) in landscape management. For. Ecol. Manag. 258, 1025–1037. doi: 10.1016/j.foreco.2009.05.035
Keith, H., Kun, Z., Hugh, S., Svoboda, M., Mikoláš, M., Adam, D., et al. (n.d.). Carbon carrying capacity of primary forests as reference levels for mitigation potential of European forests. (Submitted)
Keren, S., and Diaci, J. (2018). Comparing the quantity and structure of deadwood in selection managed and old-growth forests in South-East Europe. Forests 9:76. doi: 10.3390/f9020076
Keren, S., Diaci, J., Motta, R., and Govedar, Z. (2017). Stand structural complexity of mixed old-growth and adjacent selection forests in the Dinaric Mountains of Bosnia and Herzegovina. For. Ecol. Manag. 400, 531–541. doi: 10.1016/j.foreco.2017.06.009
Keren, S., Motta, R., Govedar, Z., Lucic, R., Medarevic, M., and Diaci, J. (2014). Comparative structural dynamics of the Janj mixed old-growth mountain forest in Bosnia and Herzegovina: are conifers in a long-term decline? Forests 5, 1243–1266. doi: 10.3390/f5061243
Kõrkjas, M., Remm, L., and Lõhmus, A. (2021). Development rates and persistence of the microhabitats initiated by disease and injuries in live trees: a review. For. Ecol. Manag. 482:118833. doi: 10.1016/j.foreco.2020.118833
Kormos, C. F., Mackey, B., DellaSala, D. A., Kumpe, N., Jaeger, T., Mittermeier, R. A., et al. (2018). “Primary forests: definition, status and future prospects for global conservation” in The encyclopedia of the anthropocene. eds. D. A. DellaSala and M. I. Goldstein (Oxford: Elsevier)
Kral, K., Janik, D., Vrska, T., Adam, D., Hort, L., Unar, P., et al. (2010). Local variability of stand structural features in beech dominated natural forests of Central Europe: implications for sampling. For. Ecol. Manag. 260, 2196–2203. doi: 10.1016/j.foreco.2010.09.020
Kucbel, S., Jaloviar, P., Saniga, M., Vencurik, J., and Klimaš, V. (2010). Canopy gaps in an old-growth fir-beech forest remnant of Western Carpathians. Eur. J. For. Res. 129, 249–259. doi: 10.1007/s10342-009-0322-2
Kuuluvainen, T., Angelstam, P., Frelich, L., Jõgiste, K., Koivula, M., Kubota, Y., et al. (2021). Natural disturbance-based forest management: moving beyond retention and continuous-cover forestry. Front. For. Glob. Change 4:629020. doi: 10.3389/ffgc.2021.629020
Kuuluvainen, T., and Nummi, P. (2023). “Strategies for the ecological restoration of the boreal forest facing climate change” in Boreal forests in the face of climate change: sustainable management. eds. M. M. Girona, H. Morin, S. Gauthier, and Y. Bergeron (Cham: Springer International Publishing), 443–466.
Landres, P. B., Morgan, P., and Swanson, F. J. (1999). Overview of the use of natural variability concepts in managing ecological systems. Ecol. Appl. 9, 1179–1188. doi: 10.1890/1051-0761(1999)009[1179:OOTUON]2.0.CO;2
Larrieu, L., Burri, S., Corriol, G., Gouix, N., Ladet, S., Laroche, F., et al. (2023). Are the remnants of old-growth mountain forests always relevant to inspire close-to-nature forest management and efficient biodiversity conservation? Biol. Conserv. 279:109954. doi: 10.1016/j.biocon.2023.109954
Larsen, J. B., Angelstam, P., Bauhus, J., Carvalho, J. F., Diaci, J., Dobrowolska, D., et al. (2022). Closer to nature forest management. From science to policy 12. Finland: European Forest Institute.
Larsson, L. A., and Larsson, P. O. (2018). CDendro and CooRecorder (v. 9.3. 1). Cybis Elektronik, Saltsjöbaden, Sweden
Leibundgut, H. (1976). The biggest silver firs and spruces [in Europe]. Schweiz. Z. Forstwes. 127:427.
Lindenmayer, D. B., and Franklin, J. F.. (2002). Conserving forest biodiversity: a comprehensive multiscaled approach. Island Press. Washington, DC
Lingua, E., Garbarino, M., Borgogno Mondino, E., and Motta, R. (2011). Natural disturbance dynamics in an old-growth forest: from tree to landscape. Proc. Environ. Sci. 7, 365–370. doi: 10.1016/j.proenv.2011.07.063
Liu, Y., Yu, G., Wang, Q., and Zhang, Y. (2014). How temperature, precipitation and stand age control the biomass carbon density of global mature forests. Glob. Ecol. Biogeogr. 23, 323–333. doi: 10.1111/geb.12113
Lombardi, F., Lasserre, B., Chirici, G., Tognetti, R., and Marchetti, M. (2012). Deadwood occurrence and forest structure as indicators of old-growth forest conditions in Mediterranean mountainous ecosystems. Ecoscience 19, 344–355. doi: 10.2980/19-4-3506
Lorimer, C. G., Dahir, S. E., and Nordheim, E. V. (2001). Tree mortality rates and longevity in mature and old-growth hemlock-hardwood forests. J. Ecol. 89, 960–971. doi: 10.1111/j.1365-2745.2001.00619.x
Máliš, F., Ujházy, K., Hederová, L., Ujházyová, M., Csölleová, L., Coomes, D. A., et al. (2023). Microclimate variation and recovery time in managed and old-growth temperate forests. Agric. For. Meteorol. 342:109722. doi: 10.1016/j.agrformet.2023.109722
Maroschek, M., Seidl, R., Poschlod, B., and Senf, C. (2023). Quantifying patch size distributions of forest disturbances in protected areas across the European Alps. J. Biogeogr. 51, 368–381. doi: 10.1111/jbi.14760
Martin-Benito, D., Molina-Valero, J. A., Pérez-Cruzado, C., Bigler, C., and Bugmann, H. (2022). Development and long-term dynamics of old-growth beech-fir forests in the pyrenees: evidence from dendroecology and dynamic vegetation modelling. For. Ecol. Manag. 524:120541. doi: 10.1016/j.foreco.2022.120541
Martinez del Castillo, E., Zang, C. S., Buras, A., Hacket-Pain, A., Esper, J., Serrano-Notivoli, R., et al. (2022). Climate-change-driven growth decline of European beech forests. Commun. Biol. 5:163. doi: 10.1038/s42003-022-03107-3
Maser, C., Anderson, R. G., Cromack, K. J., Williams, J. T., and Martin, R. E. (1979). “Dead and down woody material” in Wildlife habitats in managed forests: the Blue Mountains of Oregon and Washington. ed. J. W. Thomas (Portland: USDA), 78–95.
Mason, W. L., Diaci, J., Carvalho, J., and Valkonen, S. (2022). Continuous cover forestry in Europe: usage and the knowledge gaps and challenges to wider adoption. Forestry 95, 1–12. doi: 10.1093/forestry/cpab038
Matuszkiewicz, J. M., Affek, A. N., and Kowalska, A. (2021). Current and potential carbon stock in the forest communities of the Białowieża Biosphere Reserve. For. Ecol. Manag. 502:119702. doi: 10.1016/j.foreco.2021.119702
Maunaga, Z., Burlica, C., Stanivukovic, Z., Rapaic, Ž., Pavlovic, B., Kovacevic, Z., et al., (2001). Management plan for forests with special purpose in the strict natural reservations “Janj” and “Lom”. Bosnia and Herzegovina Forestry project, Republic of Srpska Banja Luka.
McCune, B., and Meford, M. J., (1999). PC-ORD. Multivariate Analysis of Ecological Data. Version 4. MjM Software Design. Gleneden Beach. Oregon. USA.
Mercer, C., Comeau, V. M., Daniels, L. D., and Carrer, M. (2022). Contrasting impacts of climate warming on coastal old-growth tree species reveal an early warning of forest decline. Front. For. Glob. Change 4:775301. doi: 10.3389/ffgc.2021.775301
Meyer, P., Aljes, M., Culmsee, H., Feldmann, E., Glatthorn, J., Leuschner, C., et al. (2021). Quantifying old-growthness of lowland European beech forests by a multivariate indicator for forest structure. Ecol. Indic. 125:107575. doi: 10.1016/j.ecolind.2021.107575
Meyer, P., and Schmidt, M. (2011). Accumulation of dead wood in abandoned beech (Fagus sylvatica L.) forests in northwestern Germany. For. Ecol. Manag. 261, 342–352. doi: 10.1016/j.foreco.2010.08.037
Mikolas, M., Svitok, M., Bace, R., Meigs, G. W., Keeton, W. S., Keith, H., et al. (2021). Natural disturbance impacts on trade-offs and co-benefits of forest biodiversity and carbon. Proc. Biol. Sci. 288:288. doi: 10.1098/rspb.2021.1631
Molina-Valero, J. A., Camarero, J. J., Álvarez-González, J. G., Cerioni, M., Hevia, A., Sánchez-Salguero, R., et al. (2021). Mature forests hold maximum live biomass stocks. For. Ecol. Manag. 480:118635. doi: 10.1016/j.foreco.2020.118635
Mosseler, A., Major, J. E., and Rajora, O. P. (2003). Old-growth red spruce forests as reservoirs of genetic diversity and reproductive fitness. Theor. Appl. Genet. 106, 931–937. doi: 10.1007/s00122-002-1156-1
Motta, R., Berretti, R., Castagneri, D., Dukic, V., Garbarino, M., Govedar, Z., et al. (2011). Toward a definition of the range of variability of central European mixed Fagus-Abies-Picea forests: the nearly steady-state forest of Lom (Bosnia and Herzegovina). Can. J. For. Res. 41, 1871–1884. doi: 10.1139/x11-098
Motta, R., Garbarino, M., Berretti, R., Bjelanovic, I., Borgogno Mondino, E., Čurović, M., et al. (2015a). Structure, spatio-temporal dynamics and disturbance regime of the mixed beech–silver fir–Norway spruce old-growth forest of Biogradska Gora (Montenegro). Plant Biosyst. 149, 966–975. doi: 10.1080/11263504.2014.945978
Motta, R., Garbarino, M., Berretti, R., Bono, A., Curovic, M., Dukić, V., et al. (2023). Monastic silviculture legacies and current old-growthness of silver fir (Abies alba) forests in the northern Apennines (Italy). Front. For. Glob. Change 6:1252462. doi: 10.3389/ffgc.2023.1252462
Motta, R., Garbarino, M., Berretti, R., Meloni, F., Nosenzo, A., and Vacchiano, G. (2015b). Development of old-growth characteristics in uneven-aged forests of the Italian Alps. Eur. J. For. Res. 134, 19–31. doi: 10.1007/s10342-014-0830-6
Motta, R., and Nola, P. (2001). Growth trends and dynamics in sub-alpine forest stands in the Varaita Valley (Piedmont, Italy) and their relationships with human activities and global change. J. Veg. Sci. 12, 219–230. doi: 10.2307/3236606
Motta, R., Nola, P., and Piussi, P. (1999). Structure and stand development in three subalpine Norway spruce (Picea abies (L.) Karst.) stands in Paneveggio (Trento, Italy). Glob. Ecol. Biogeogr. 8, 455–471. doi: 10.1046/j.1365-2699.1999.00165.x
Nagel, T. A., and Svoboda, M. (2008). Gap disturbance regime in an old-growth Fagus-Abies forest in the Dinaric Mountains, Bosnia-Herzegovina. Can. J. For. Res. 38, 2728–2737. doi: 10.1139/X08-110
Nagel, T. A., Svoboda, M., and Kobal, M. (2014). Disturbance, life history traits, and dynamics in an old-growth forest landscape of southeastern Europe. Ecol. Appl. 24, 663–679. doi: 10.1890/13-0632.1
Nagel, T., Zenner, E., and Brang, P. (2013). “Research in old-growth forests and forest reserves: implications for integrated forest management” in Integrative approaches as an opportunity for the conservation of forest biodiversity (Bonn, Germany: European Forest Institute), 44–50.
O’Brien, L., Schuck, A., Fraccaroli, C., Pötzelsberger, E., Winkel, G., and Lindner, M. (2021). Protecting old-growth forests in Europe—a review of scientific evidence to inform policy implementation. European Forest Institute, Bonn.
Paillet, Y., Bergès, L., Hjältén, J., Ódor, P., Avon, C., Bernhardt-Römermann, M., et al. (2010). Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conserv. Biol. 24, 101–112. doi: 10.1111/j.1523-1739.2009.01399.x
Paillet, Y., Pernot, C., Boulanger, V., Debaive, N., Fuhr, M., Gilg, O., et al. (2015). Quantifying the recovery of old-growth attributes in forest reserves: a first reference for France. For. Ecol. Manag. 346, 51–64. doi: 10.1016/j.foreco.2015.02.037
Palandrani, C., Motta, R., Cherubini, P., Curovic, M., Dukic, V., Tonon, G., et al. (2021). Role of photosynthesis and stomatal conductance on the long-term rising of intrinsic water use efficiency in dominant trees in three old-growth forests in Bosnia-Herzegovina and Montenegro. iForest 14, 53–60. doi: 10.3832/ifor3414-013
Palli, J., Mensing, S. A., Schoolman, E. M., Solano, F., and Piovesan, G. (2022). Historical ecology identifies long-term rewilding strategy for conserving Mediterranean mountain forests in South Italy. Ecol. Appl. 33:e2758. doi: 10.1002/eap.2758
Parisi, F. (2022). First record of the rare and threatened saproxylic beetle Rhysodes sulcatus (Fabricius, 1787) in Montenegro (Coleoptera Rhysodidae) and implication for habitat conservation. J. Zool. 105, 77–80. doi: 10.19263/REDIA-05.22.09
Parisi, F. (2023). Further finding of Bolitophagus reticulatus (Linnaeus, 1767) in Montenegro (Coleoptera: Tenebrionidae) with brief comments on its distribution and conservation. Ecol. Montenegrina 67, 40–44. doi: 10.37828/em.2023.67.6
Parisi, F. (2024). First record of saproxylic beetle Corticeus (= Hypophloeus) unicolor Piller & Mitterpacher, 1783 in Montenegro (Coleoptera: Tenebrionidae) with comments on old-growth forests conservation in the Country. Ecol. Montenegrina 71, 187–192. doi: 10.37828/em.2024.71.18
Parobeková, Z., Pittner, J., Kucbel, S., Saniga, M., Filípek, M., Sedmáková, D., et al. (2018). Structural diversity in a mixed spruce-fir-beech old-growth forest remnant of the Western Carpathians. Forests 9:379. doi: 10.3390/f9070379
Pavlin, J., Nagel, T., Svitok, M., Pettit, J., Begović, K., Mikac, S., et al. (2021). Disturbance history is a key driver of tree lifespan in temperate primary forests. J. Veg. Sci. 32:e13069. doi: 10.1111/jvs.13069
Peck, J. E., Commarmot, B., Hobi, M. L., and Zenner, E. K. (2015). Should reference conditions be drawn from a single 10 ha plot? Assessing representativeness in a 10,000 ha old-growth European beech forest. Restor. Ecol. 23, 927–935. doi: 10.1111/rec.12258
Petritan, I. C., Commarmot, B., Hobi, M. L., Petritan, A. M., Bigler, C., Abrudan, I. V., et al. (2015). Structural patterns of beech and silver fir suggest stability and resilience of the virgin forest Sinca in the Southern Carpathians, Romania. For. Ecol. Manag. 356, 184–195. doi: 10.1016/j.foreco.2015.07.015
Pickett, S. T. A., and White, P. S. (1985). The ecology of natural disturbance and patch dynamics. Academic Press, New York.
Pintarić, K. (1978). Urwald Peručica als natürliches Forschungslaboratorium. Allgemeine Forst-zeitschrift 24, 702–707.
Pintarić, K. (1999). Forestry and forest reserves in Bosnia and Herzegovina. Proceedings of the Invited Lecturers’ Reports presented at the COST E4 Management Committee and Working Groups Meeting. Ljubljana, Slovenia, 25–28 April, 1998
Piovesan, G., and Biondi, F. (2020). On tree longevity. New Phytol. 231, 1318–1337. doi: 10.1111/nph.17148
Price, K., Daust, D., Daust, K., and Holt, R. (2023). Estimating the amount of British Columbia’s “big-treed” old growth: navigating messy indicators. Front. For. Glob. Change 5:958719. doi: 10.3389/ffgc.2022.958719
Rubino, D. L., and McCarthy, B. C. (2004). Comparative analysis of dendroecological methods used to assess disturbance events. Dendrochronologia 21, 97–115. doi: 10.1078/1125.7865.00047
Sabatini, F. M., Burrascano, S., Keeton, W. S., Levers, C., Lindner, M., Potzschner, F., et al. (2018). Where are Europe’s last primary forests? Divers. Distrib. 24, 1426–1439. doi: 10.1111/ddi.12778
Sabatini, F. M., Keeton, W. S., Lindner, M., Svoboda, M., Verkerk, P. J., Bauhus, J., et al. (2020). Protection gaps and restoration opportunities for primary forests in Europe. Divers. Distrib. 26, 1646–1662. doi: 10.1111/ddi.13158
Saniga, M., Kucbel, S., Anié, I., Mikàc, S., and Prebeg, M. (2011). Structure, growing stock, coarse woody debris and regeneration processes in virgin forests Dobroc (Slovakia) and Corkova Uvala (Croatia). Beskydy 4, 39–50.
Schweingruber, F. H., Eckstein, D., Serre-Bachet, F., and Braker, O. U. (1990). Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia 8, 9–38.
Seidl, R., Thom, D., Kautz, M., Martin-Benito, D., Peltoniemi, M., Vacchiano, G., et al. (2017). Forest disturbances under climate change. Nat. Clim. Chang. 7, 395–402. doi: 10.1038/nclimate3303
Senf, C., and Seidl, R. (2021). Mapping the forest disturbance regimes of Europe. Nat. Sustain. 4, 63–70. doi: 10.1038/s41893-020-00609-y
Silander, J. A. (2001). “Temperate forests” in Encyclopedia of biodiversity. ed. S. A. Levin . 2nd ed (Waltham: Academic Press), 112–127.
Spies, T. A. (2004). Ecological concepts and diversity of old-growth forests. J. For. 102, 14–20. doi: 10.1093/jof/102.3.14
Spies, T. A., and Franklin, J. F. (1991). “The structure of natural young, mature, and old-growth Douglas-fir forests in Oregon and Washington” in Wildlife and vegetation of unmanaged Douglas-fir forests. General technical report PNW-GTR-85. eds. L. E. Ruggiero, K. B. Aubry, A. B. Carey, and M. H. Huff (Portland: U.S. Department of Agriculture Forest Service, Pacific Northwest Research Station), 91–111.
Spies, T. A., and Franklin, J. F. (1996). “The diversity and maintenance of old-growth forests” in Biodiversity in managed landscapes: Theory and practice. eds. R. C. Szaro and D. W. Johston (New York: Oxford University Press), 778.
Stupar, V., and Milanović, D. (2017). History of nature protection in the Sutjeska National Park. Bull. Fac. For. Univ. Banja Luka 26, 113–128. doi: 10.7251/GSF1726113S
Susmel, L. (1956). Caratteri comparati delle abetine primarie delle Alpi dinariche e delle abetine secondarie delle Alpi orientali italiane. Annali Accademia Italiana di Scienze Forestali 5, 115–146.
Swanson, F. J., Jones, J. A., Wallin, D. O., and Cissel, J. H. (1994). “Natural variability—implications for ecosystem management” in Ecosystem management: principles and applications. General technical report PNW-GTR-318. eds. M. E. Jensen and P. S. Bourgeron (Portland: U.S. Department of Agriculture Forest Service, Pacific Northwest Research Station), 80–94.
Thom, D., Rammer, W., and Seidl, R. (2017). The impact of future forest dynamics on climate: interactive effects of changing vegetation and disturbance regimes. Ecol. Monogr. 87, 665–684. doi: 10.1002/ecm.1272
Tregubov, V. S. (1941). Les forêts vierges montagnardes des Alpes Dinariques—Massif de Klekovatcha-Guermetch: Étude Botanique et Forestière. Causse Graille et Castelnau Montpellier.
UNEP/CBD/SBSTTA (2001). “Connecting biodiversity and climate change mitigation and adaptation” in Report of the ad hoc technical expert group on forest biological diversity. Subsidiary body for scientific, technical and technological advice (Montreal: Secretariat of the Convention on Biological Diversity)
Van Wagner, C. E. (1968). The line intersect method in forest fuel sampling. For. Sci. 14, 20–26. doi: 10.1093/forestscience/14.1.20
Veblen, T. T. (2003). Historic range of variability of mountains forest ecosystems: concepts and applications. For. Chron. 79, 223–226. doi: 10.5558/tfc79223-2
von Oheimb, G., Westphal, C., Tempel, H., and Hardtle, W. (2005). Structural pattern of a near-natural beech forest (Fagus sylvatica) (Serrahn, North-East Germany). For. Ecol. Manag. 212, 253–263. doi: 10.1016/j.foreco.2005.03.033
Watson, J. E. M., Evans, T., Venter, O., Williams, B., Tulloch, A., Stewart, C., et al. (2018). The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610. doi: 10.1038/s41559-018-0490-x
Whitman, A. A., and Hagan, J. M. (2007). An index to identify late-successional forest in temperate and boreal zones. For. Ecol. Manag. 246, 144–154. doi: 10.1016/j.foreco.2007.03.004
Keywords: forest structure, forest dynamics, CWD (coarse woody debris), carbon stock, tree rings, natural disturbance regime, natural range of variability, European forest and environmental policies
Citation: Motta R, Alberti G, Ascoli D, Berretti R, Bilic S, Bono A, Milic C, Vojislav D, Finsinger W, Garbarino M, Govedar Z, Keren S, Meloni F, Ruffinatto F and Nola P (2024) Old-growth forests in the Dinaric Alps of Bosnia-Herzegovina and Montenegro: a continental hot-spot for research and biodiversity. Front. For. Glob. Change. 7:1371144. doi: 10.3389/ffgc.2024.1371144
Edited by:
Isabella De Meo, Council for Agricultural and Economics Research (CREA), ItalyReviewed by:
Marcus Lindner, European Forest Institute, FinlandDamir Ugarkovic, University of Zagreb, Croatia
Copyright © 2024 Motta, Alberti, Ascoli, Berretti, Bilic, Bono, Milic, Vojislav, Finsinger, Garbarino, Govedar, Keren, Meloni, Ruffinatto and Nola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renzo Motta, cmVuem8ubW90dGFAdW5pdG8uaXQ=
 Renzo Motta
Renzo Motta Giorgio Alberti
Giorgio Alberti Davide Ascoli
Davide Ascoli Roberta Berretti1
Roberta Berretti1 Srdjan Bilic
Srdjan Bilic Alessia Bono
Alessia Bono Curovic Milic
Curovic Milic Dukić Vojislav
Dukić Vojislav Walter Finsinger
Walter Finsinger Matteo Garbarino
Matteo Garbarino Zoran Govedar
Zoran Govedar Srdjan Keren
Srdjan Keren Fabio Meloni
Fabio Meloni Flavio Ruffinatto
Flavio Ruffinatto Paola Nola
Paola Nola