
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 28 September 2023
Sec. Pests, Pathogens and Invasions
Volume 6 - 2023 | https://doi.org/10.3389/ffgc.2023.1258729
This article is part of the Research Topic Herbivores and Pathogens in Forest Ecosystems in a Rapidly Changing World View all 4 articles
Bark and ambrosia beetles pose significant threats to the stability of forest stands worldwide, making their control crucial. Among these pests, Gnathotrichus materiarius, a polyphagous invasive ambrosia beetle living on conifers, has successfully established itself in Europe. Early identification of these pests plays a fundamental role in designing effective pest control strategies. The work aimed to assess the efficacy of different lures in Ecotrap® for capturing of invasive ambrosia bark beetles. The lures tested included Wood Stainers Lure (containing the potential pheromone sulcatol for capturing Gnathotrichus materiarius adults), α-pinene, ethanol UHR, and Cembräwit. The objective was to determine the most suitable lure for use in traps. In four locations in western Bohemia, a total of 7,410 individuals from 46 species of ambrosia and bark beetleswere captured. The abundance of invasive ambrosia beetles (Gnathotrichus materiarius, Cyclorhipidion bodoanum, Xyleborinus attenuatus, and Xylosandrus germanus) primarily depended on the day of the season and secondarily on the lure used. Although their population density was low, more beetles were caught using ethanol as the lure. Notably, these invasive ambrosia beetles accounted for less than 3 % of the total number of ambrosia and bark beetles detected (187 individuals). Ethanol was found to be a universal lure for attracting ambrosia beetles, with the majority of Scolytinae species being captured in traps baited with ethanol.
Bark and ambrosia beetles are among the most serious pests that threaten the stability of forest stands worldwide (Hulcr and Dunn, 2011; Hlásny et al., 2021). While bark beetles feed on the phloem, interrupting the transport of substances produced by photosynthesis and utilizing transferred fungi to overcome the defensive reactions of the host tree, ambrosia beetles feed on fungi carried in their mycangia and actively cultivate them on gallery walls within the wood (Hulcr et al., 2007). Invasive bark and ambrosia beetles pose threats not only to native biodiversity and functional ecosystems but also to the economic productivity of forest management (Brockerhoff et al., 2006a; Aukema et al., 2011; Gohli et al., 2016). Early identification of pests is a fundamental step in invasive bark and ambrosia beetle control, as it aids in the design of pest management strategies (Douglas et al., 2009).
Implementing integrated control measures proves more cost-effective than the loss of timber (Franjević et al., 2016). For example, failure to monitor the invasive ambrosia beetle in Pinus taeda L. forests in the southern USA has resulted in an estimated economic loss amounting to several hundred dollars per hectare for landowners (Susaeta et al., 2016). At the same time, lures require smaller financial expenditure than the human labor associated with the control of traps (Šramel et al., 2021).
Bark and ambrosia beetles use a complex chemical communication system (utilizing pheromones, allomones, kairomones, synomones) to locate and infest new host trees for feeding, mating, and reproduction (Wood, 1982; Hulcr et al., 2007; Seybold et al., 2018). Trees play a crucial role as producers of kairomones. When trees experience stress due to factors like drought, frost, floods, fire, or human-induced damage, they release ethanol, which is the most important volatile compound attractive to ambrosia beetles (Kühnholz et al., 2001; Kelsey and Joseph, 2003; Ranger et al., 2013, 2019, 2021). Different combinations of volatile compounds have been employed to monitor and detect bark and ambrosia beetles at an early stage. One of the most used approaches involves the simultaneous use of ethanol and α-pinene (Borden et al., 1980; Schroeder and Lindelöw, 1989; Miller and Rabaglia, 2009; Burbano et al., 2012; Flaherty et al., 2019).
In Europe, most invasive ambrosia beetles originate from temperate or subtropical regions. When these invasive beetles colonize new territories with suitable conditions, their population numbers can increase dramatically over time (Galko et al., 2014; Boland, 2016). Conversely, under unsuitable conditions, the abundance of ambrosia beetles remains low, or the population will even disappear (Fiala et al., 2020). Gnathotrichus materiarius Fitch, 1858 a polyphagous invasive ambrosia beetle that primarily infests conifers, has successfully spread throughout Europe (Kamp, 1970). Despite its presence in European forests for nearly a century, no significant damage has been reported from G. materiarius infestations (Mazur et al., 2018). Although we can lure this ambrosia beetle to host tree logs (Fiala et al. in prep.), it is often caught in traps baited with lures designed for capturing bark beetles of the Ips genus (Valkama et al., 1997; Fiala, 2019). Commonly used lures for this purpose include ID Ecolure®, IT Ecolure®, Cembräwit®, Amitinuswit®, Hostowit®, and ethanol combined with other lures (Schneider, 1985; Knížek, 2009; Mazur et al., 2018). It is worth noting that sulcatol, a potential aggregation pheromone, has also been identified (Flechtmann and Berisford, 2003) and for other member of Corthylini ambrosia beetles, Monarthrum mali Fitch, 1856 (Miller and Crowe, 2020). All mentioned lures are alcohol-based, aligning with the use of ethanol for capturing ambrosia beetles (Lingren and Fraser, 1994; Rassati et al., 2015; Rabaglia et al., 2019).
We aimed to: (i) Analyze the effectiveness of various lure types on capturing invasive ambrosia beetles in endemic populations in central Europe; (ii) Analyze the impact of various lure types on species richness and abundance of both bark and ambrosia beetles, identifying their distinct responses to lure treatments; (iii) Examine the proportions of invasive ambrosia beetles and native coniferous bark beetles in relation to lure attractiveness, emphasizing the lure preferences of each group; (iv) Assess the potential interaction effects between season and lure type, elucidating how these factors jointly influence beetle behavior and their distribution across the study period.
This work aimed to compare the attractiveness of different lures and determine which one is most effective in attracting G. materiarius. The lures tested included Wood Stainers Lure, containing the pheromone sulcatol (Flechtmann and Berisford, 2003); α-pinene due to the preference of coniferous bark beetles (Schroeder and Lindelöw, 1989); ethanol as G. materiarius is an ambrosia beetle known to respond to ethanol (Kelsey and Joseph, 2003), and Cembräwit since G. materiarius has shown a positive response also to this lure (Schneider, 1985). We predicted greater catches of invasive ambrosia beetles, coniferous bark beetles, and Ips spp., in traps baited, respectively, with ethanol, α-pinene, and the Cembräwit lure.
The experiments were conducted in four localities in western Bohemia (Czech Republic), in 2022. The invasive ambrosia beetle species, G. materiarius, Xylosandrus germanus, Cyclorhipidion bodoanum Reitter, 1913, and Xyleborinus attenuatus Blandford, 1894, were found to occur in the studied areas. All the localities are coniferous forests, namely Úbočí (50.0259°N, 12.5859°E, 750 m asl), Kladská (50.0116°N, 12.6746°E, 865 m asl), Žihle (50.0391°N, 13.3520°E, 510 m asl), and Kdyně (GPS 49.4024°N, 13.0995°E, 600 m asl) (Figure 1).
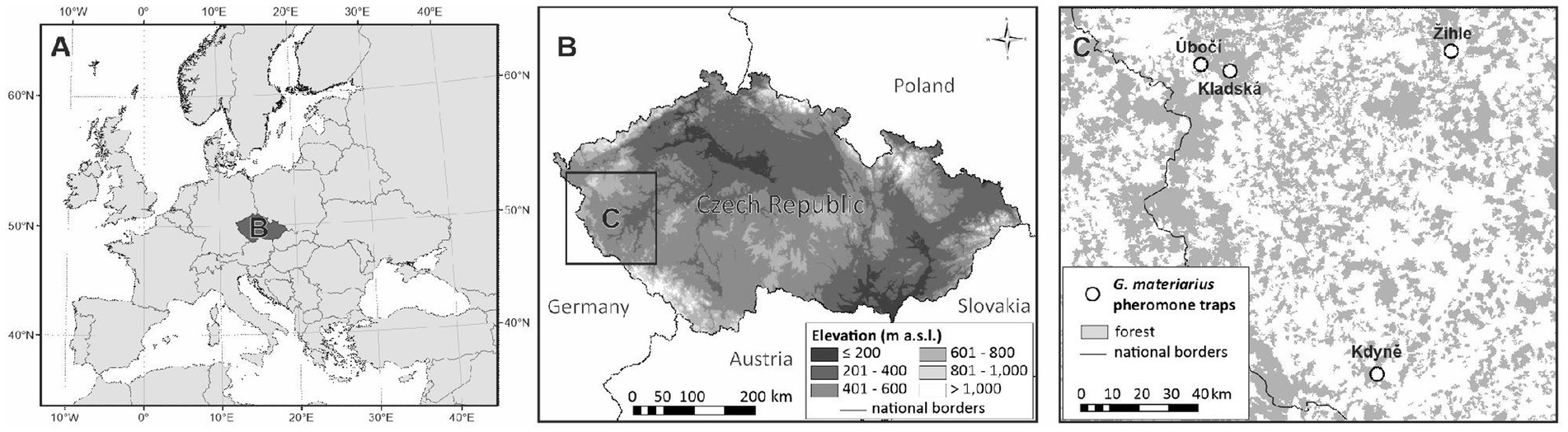
Figure 1. The location of the Czech Republic in Europe (A), the geography of the Czech Republic (B), and the localities where lures for their ability to attract Gnathotrichus materiarius were compared (C).
In 2022, four lures were compared for their ability to attract and trap G. materiarius: Cembräwit (as the control, Witasek GmbH, Austria), α-pinene, ethanol UHR (all from Synergy Semiochemicals Corp., United States), and Wood Stainers Lure (a mixture of α-pinene, ethanol, and sulcatol) (abbr. as WSL) (Alpha Scents, Inc., United States) (Table 1). All four lures were deployed in traps across all four localities. Each locality had 20 Ecotrap® traps (4 treatments × 5 replicates per treatment) (Fytofarm spol. s r.o., Slovakia) arranged in a linear transect at 10-meter intervals. This interval is sufficient because the effectiveness of traps on flying bark beetles can be calculated in units of meters (Duelli et al., 1997). The traps were placed 130 cm above the ground using wooden stakes along an east-west transect, with one lure assigned per trap. Randomly placed in the trap row, there were five replicates per lure treatment per locality.
The traps, baited with the lures, were deployed at the end of April, and checked every 14 days until mid-August. The collected beetles were frozen and subsequently identified by the first author according to Pfeffer (1989). Even if the beetles exceptionally had been damaged by predators, it was possible to study the remains and determine the number of specimens and species.
The data were processed using R 4.2.2 (R Core Team, 2022). Negative Binomial Zero-Inflated Mixed Models from the NBZIMM library (Yi, 2020) were fitted to analyze the richness and abundance of bark and ambrosia beetles. The response variables included the abundance of all beetles, the richness of all beetles, the overall abundance of invasive ambrosia beetles (C. bodoanum, G. materiarius, X. germanus, and X. attenuatus), their richness, and their individual abundances (except for X. attenuatus, where the model was omitted due to limited data). As potential explanatory variables, we considered the type of lure, the day of the season (dos), the quadratic polynomial of the dos, and the interaction between dos and the type of lure as this analysis focused on investigating the season-part-dependent effect of individual lures. The model incorporated sampling location, individual plots nested within the sampling location, and individual traps nested in plots as random terms. These components simultaneously defined the autocorrelation structure as “ar1” in the model.
For the second part of the analysis, Generalized Linear Mixed Models with binomial distribution from the lme4 library (Bates et al., 2015) were employed. Since the focus shifted away from the season, the catches from individual traps were aggregated across the season. The explanatory variable considered was the type of substance, while the random terms included sampling location and individual plots nested within the sampling location. The response variables were the proportions of individuals of invasive ambrosia beetles in all ambrosia beetles, the genus Ips, and native coniferous bark beetles (excluding Ips) in all individuals. The resulting models were compared with the respective null models to assess their fit. To monitor potential autocorrelation, auto- and cross-covariance and -correlation function estimation was performed on the residuals of the model. Given the interdependence of proportions, the resulting p-values were adjusted based on the false discovery rate. Potential convergence issues in the algorithm were addressed by setting the maximum number of iterations for the model to 105, establishing a tolPwrss tolerance level of 10−3 for declaring convergence in the penalized iteratively weighted residual sum-of-squares, and utilizing the Gauss-Hermite algorithm. Furthermore, in the model examining the ratio of individuals of invasive ambrosia beetles to all individuals, the sampling location Kladská and α-pinene lure were excluded from the analysis. This decision was made due to the absence of recorded invasive ambrosia beetles. In the analyses, WSL was chosen as the control group due to the absence of invasive ambrosia beetles on α-pinene and their limited abundance on Cembräwit.
Partial Canonical Correspondence Analysis (p-CCA) from Canoco 5.01 (ter Braak and Šmilauer, 2012) was utilized to investigate whether the dos and lure had a significant impact on the composition of beetle communities, with sampling locations included as a covariate. Before the analysis, rare species were down-weighted, and the results were assessed using the Monte-Carlo permutation test with 999 permutations. To identify indicator species for each lure, the IndVal method was employed, which considers the frequency and relative abundance of the beetles (Dufrêne and Legendre, 1997). This was followed by multilevel pattern analysis using “indicspecies” (De Cáceres and Legendre, 2009) and “labdsv” (Roberts, 2019) libraries. Most of the plots were generated using the sciplot library (Morales, 2020).
In total, 46 species of bark and ambrosia beetleswere caught amounting to a total of 7,410 individuals (Supplementary Table S1).
The numbers of G. materiarius captured were primarily dependent on the day of the season (dfnum = 1, dfden = 556, F = 119.65, p < 0.001), with higher numbers observed at the beginning of the season. The lure type also had an effect (dfnum = 3, dfden = 57, F = 4.95, p = 0.004), where most beetles were caught using ethanol (Figure 2A). The interaction between the day of the season and the lure was not found to be significant (dfnum = 3, dfden = 556, F = 1.69, p = 0.168). When comparing the capture of G. materiarius in WSL-baited traps with other lure treatments, no beetles were captured with α-pinene, fewer beetles were captured with Cembräwit, and more beetles were captured with ethanol.
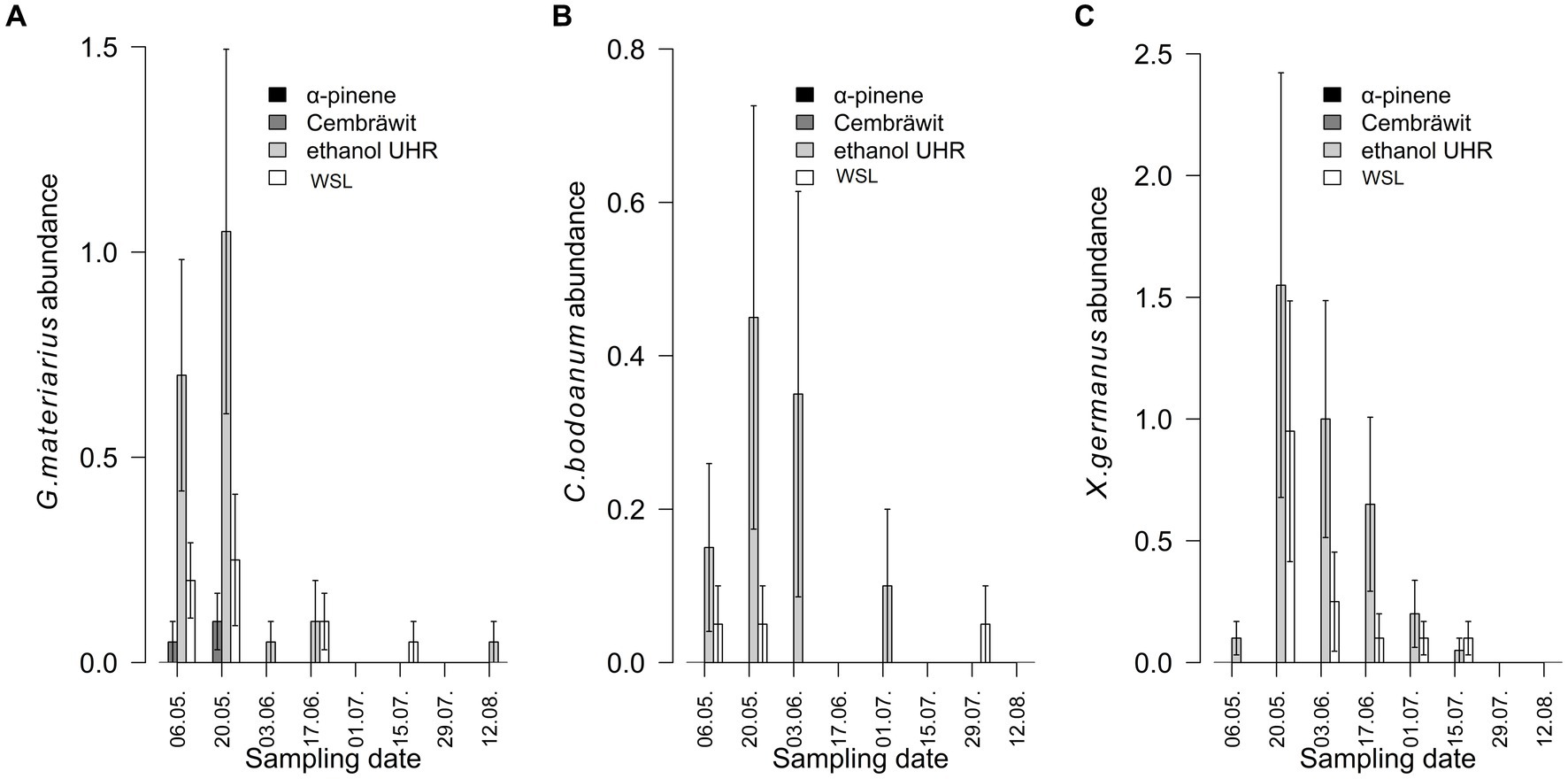
Figure 2. The numbers of ambrosia beetles caught per trap, including Gnathotrichus materiarius (A), Cyclorhipidion bodoanum (B), and Xylosandrus germanus (C), varied depending on the type of lure and the date of checking in year 2022 (mean ± SE) (WSL, Wood Stainers lure).
The numbers of C. bodoanum captured were significantly influenced by the day of the season (dfnum = 1, dfden = 556, F = 405.82, p < 0.001) with higher numbers observed at the beginning of the season. The lure type, on the other hand, had an insignificant effect (dfnum = 3, dfden = 57, F = 0.80, p = 0.497). However, there was a strong interaction between the day of the season and the type of lure (dfnum = 3, dfden = 556, F = 35.80, p < 0.001). The highest number of beetles was found in traps baited with ethanol at the beginning of the season (Figure 2B).
The numbers of X. germanus were significantly influenced by the day of the season (dfnum = 1, dfden = 556, F = 633.56, p < 0.001) with higher numbers observed at the beginning of the season. The lure type, however, had an insignificant effect (dfnum = 3, dfden = 57, F = 0.81, p = 0.491). Nevertheless, there was a strong interaction between the day of the season and the type of lure (dfnum = 3, dfden = 556, F = 8.00, p < 0.001). When comparing the catch with WSL-baited traps, no beetles were found on α-pinene or Cembräwit, while a higher number of beetles were found on ethanol at the beginning of the season (Figure 2C).
The average species richness of ambrosia beetles per trap depended on the day of the season (dfnum = 2, dfden = 552, F = 115.26, p < 0.001) with higher richness at the beginning of the season around the end of the May, and on the type of lure (dfnum = 3, dfden = 57, F = 39.96, p < 0.001), the interaction between them was not significant (dfnum = 3, dfden = 552, F = 1.36, p = 0.228, Figure 3A). In comparison to WSL, lower species richness was captured on α-pinene and Cembräwit, but higher species richness was captured on ethanol UHR.
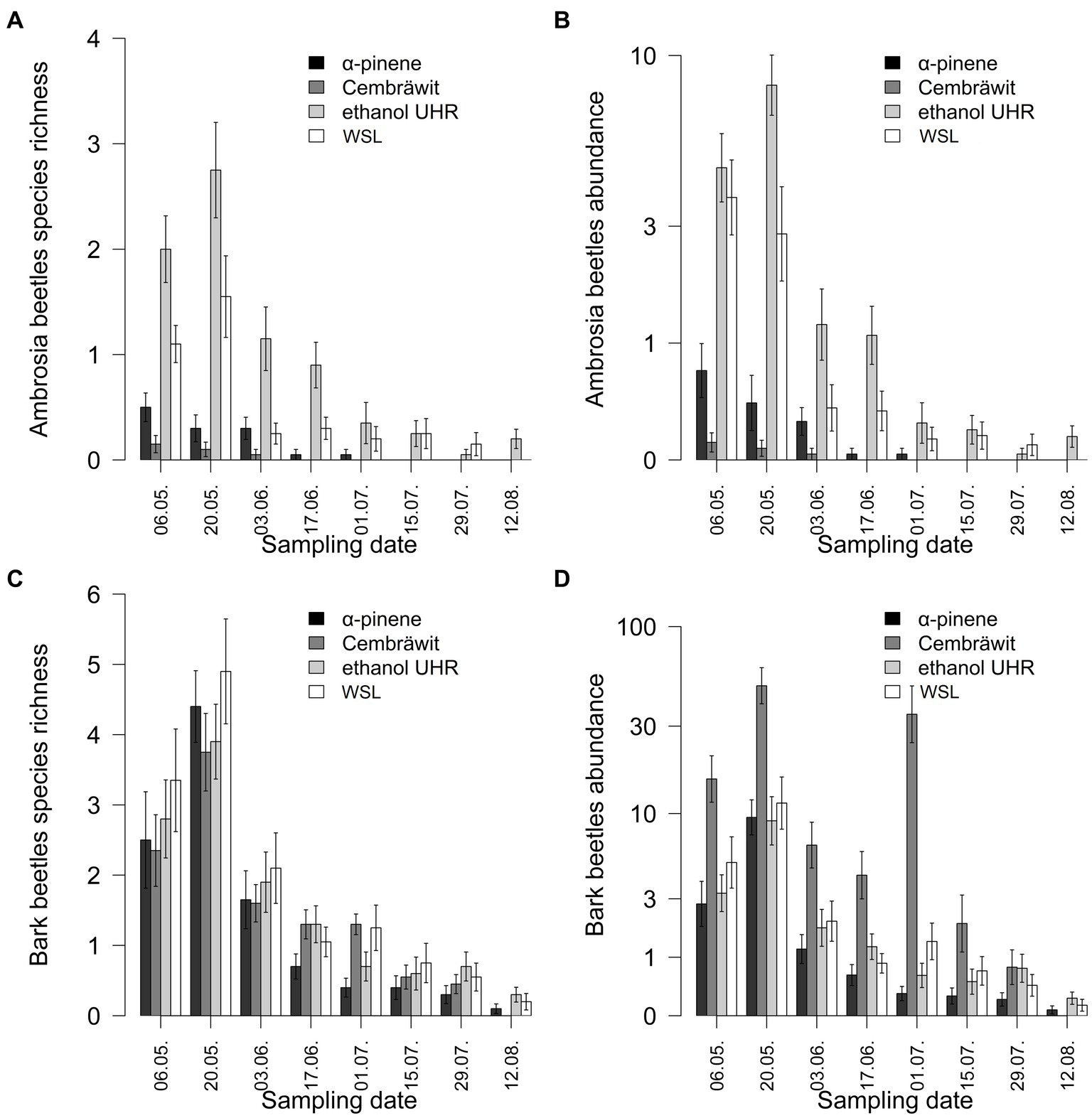
Figure 3. The richness (A,C) and abundance (B,D) of ambrosia and bark beetles varied depending on the type of lure and the date of checking in year 2022 (mean ± SE) (WSL, Wood Stainers lure).
The average abundance of ambrosia beetles per trap depended on the day of the season (dfnum = 2, dfden = 552, F = 274.99, p < 0.001) with higher abundance at the beginning of the season around the end of the May, and on the type of lure (dfnum = 3, dfden = 57, F = 46.98, p < 0.001) with their interaction (dfnum = 6, dfden = 552, F = 2.20, p = 0.041, Figure 3B). In comparison to WSL, lower abundance was captured on α-pinene and Cembräwit, and similar abundance was captured on ethanol UHR with except of period from end of May till end of June, where ethanol UHR was most effective lure.
The average species richness of bark beetles per trap depended on the day of the season (dfnum = 2, dfden = 552, F = 156.16, p < 0.001) with higher richness at the beginning of the season around the end of the May, and tended to depend on the type of lure (dfnum = 3, dfden = 57, F = 2.76, p = 0.050), the interaction between them tended to be significant (dfnum = 3, dfden = 552, F = 1.87, p = 0.084, Figure 3C). WSL was the most effective lure at the beginning of the season, later in the season, its efficacy became comparable to other compounds, leading to a diminishing distinction in attractant preferences among bark beetles.
The average abundance of bark beetles per trap depended on the day of the season (dfnum = 2, dfden = 552, F = 187.65, p < 0.001) with higher abundance at the beginning of the season around the end of the May, and on the type of lure (dfnum = 3, dfden = 57, F = 52.86, p < 0.001) with their interaction (dfnum = 6, dfden = 552, F = 12.55, p < 0.001, Figure 3D). In comparison to WSL, lower abundance was captured on α-pinene, higher abundance was captured on Cembräwit, and similar abundance was captured on ethanol UHR. Cembräwit had a different progression of the season than the other types of lure—a second peak during the season and a much faster decline after that.
The average species richness of bark and ambrosia beetles per trap was significantly influenced by the day of the season (dfnum = 2, dfden = 552, F = 195.32, p < 0.001), with higher richness observed at the beginning of the season, particularly around the end of the May. Additionally, the type of lure also had a significant effect (dfnum = 3, dfden = 57, F = 15.30, p < 0.001). However, the interaction between them was not found to be significant (dfnum = 3, dfden = 552, F = 1.72, p = 0.114, Figure 4A). When comparing the species richness captured in WSL-baited traps with other lure treatments, lower species richness was observed on α-pinene and Cembräwit, while higher species richness was observed on ethanol.
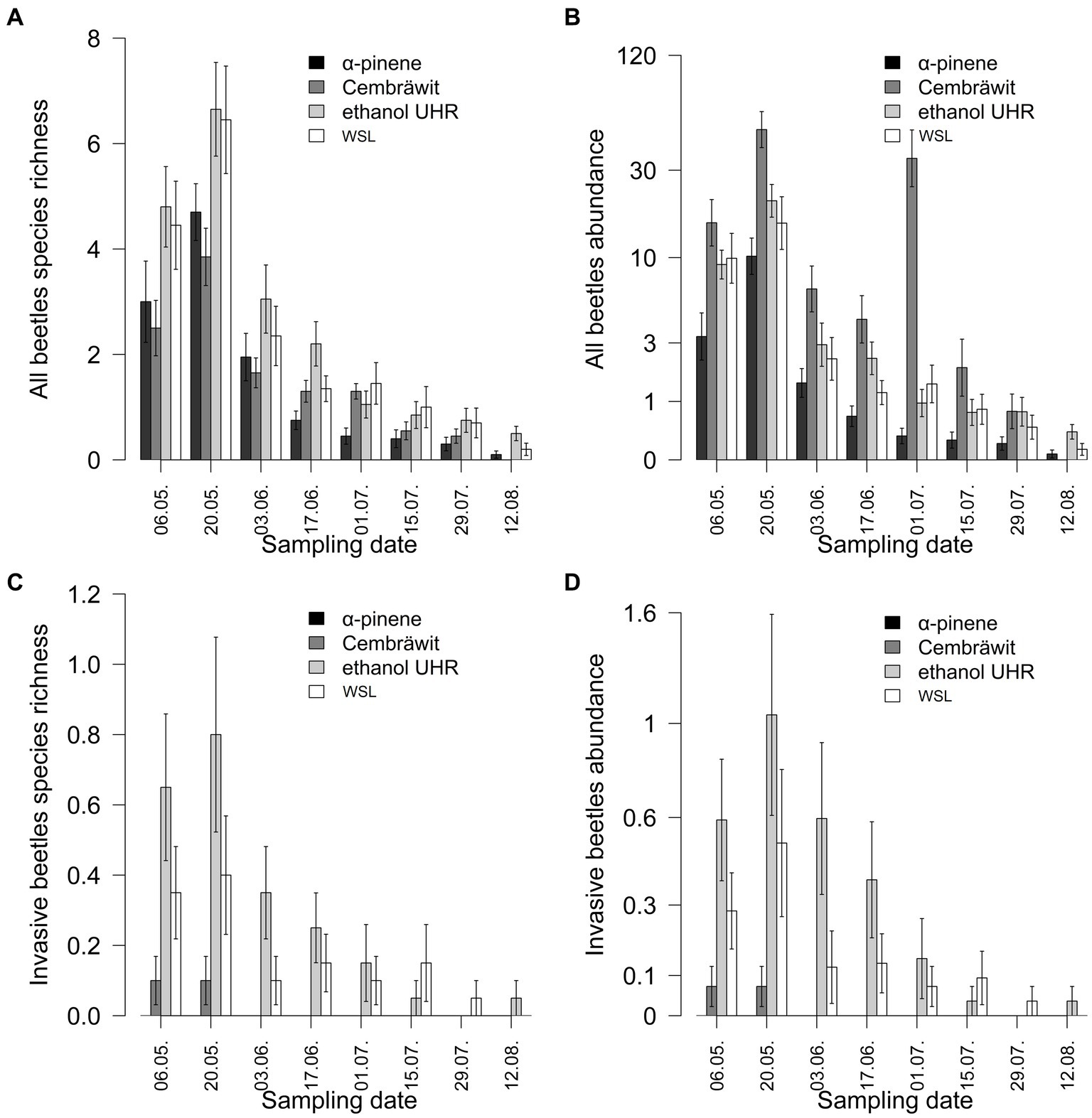
Figure 4. The richness (A,C) and abundance (B,D) of both invasive and all bark and ambrosia beetles varied depending on the type of lure and the date of checking in year 2022 (mean ± SE) (WSL, Wood Stainers lure).
The average abundance of bark and ambrosia beetles per trap was significantly influenced by the day of the season (dfnum = 2, dfden = 552, F = 226.86, p < 0.001), with higher abundance observed at the beginning of the season, particularly around the end of the May. The type of lure also had a significant effect (dfnum = 3, dfden = 57, F = 63.26, p < 0.001), and there was a significant interaction between them (dfnum = 6, dfden = 552, F = 14.49, p < 0.001, Figure 4B). When comparing the abundance captured in WSL-baited traps with other lure treatments, lower abundance was observed on α-pinene, higher abundance was observed on Cembräwit, and similar abundance was observed on ethanol. Cembräwit showed a different progression throughout the season compared to the other lure types, with a second peak during the season and a faster decline afterward.
The average species richness of invasive beetles per trap was significantly influenced by the day of the season (dfnum = 2, dfden = 552, F = 91.80, p < 0.001), with higher richness observed at the beginning of the season, particularly around the end of the May. The type of lure also had a significant effect (dfnum = 3, dfden = 57, F = 2.99, p = 0.038, Figure 4C). However, the interaction between them was not found to be significant (dfnum = 3, dfden = 552, F = 1.13, p = 0.339). When comparing the species richness captured in WSL-baited traps with other lure treatments, no beetles were captured on α-pinene, lower species richness was observed on Cembräwit, and higher species richness was observed on ethanol.
The average abundance of invasive beetles per trap is significantly influenced by the day of the season (dfnum = 2, dfden = 558, F = 98.05, p < 0.001), with higher abundance observed at the beginning of the season, particularly around the end of the May. The type of lure also had a significant effect (dfnum = 3, dfden = 57, F = 5.89, p = 0.001, Figure 4D). When comparing the abundance captured in WSL-baited traps with other lure treatments, no beetles were captured on α-pinene, lower abundance was observed on Cembräwit, and higher abundance was observed on ethanol.
The proportion of invasive ambrosia beetles to all ambrosia beetles depended on the type of the lure (df = 40, χ2 = 10.52, p = 0.005, Figure 5A). In comparison to WSL, no beetles were captured on α-pinene, the higher proportion was captured on Cembräwit (z = 2.96, p = 0.003), and the lower proportion tended to be captured on ethanol UHR (z = 1.69, p = 0.092). The proportion of Ips beetles to all individuals was significantly influenced by the type of the lure (df = 74, χ2 = 5267.30, p < 0.001, Figure 5B). When comparing the proportion in WSL-baited traps with other lure treatments, there was a tendency for a higher proportion to be captured on α-pinene (z = 1.78, p = 0.075), a higher proportion was captured on Cembräwit (z = 37.51, p < 0.001), and there was a tendency for a lower proportion to be captured on ethanol (z = −1.73, p = 0.083).
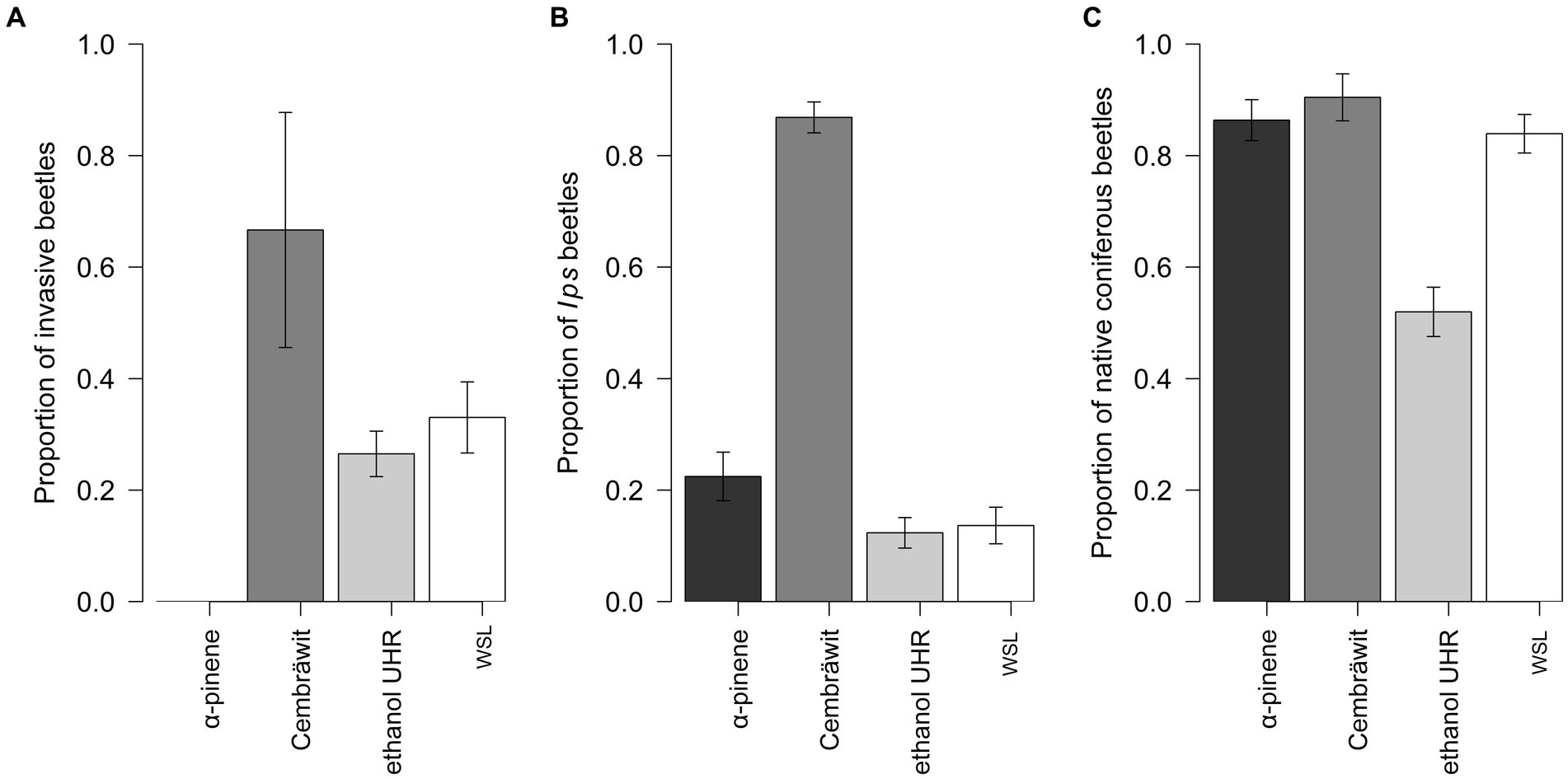
Figure 5. The proportion of invasive ambrosia beetles to all ambrosia beetles (A), the proportion of Ips beetles to all individuals (B), and the proportion of native coniferous beetles to all individuals (excluding Ips) in relation to the type of lure (C) (mean ± SE) (WSL, Wood Stainers lure; Dos, day of the season).
The proportion of native coniferous beetles (excluding Ips) to all individuals was significantly influenced by the type of the lure (df = 74, χ2 = 33.65, p < 0.001, Figure 5C). When comparing the proportion in WSL-baited traps with other lure treatments, a similar proportion was captured on α-pinene (z = 0.19, p = 0.847) and Cembräwit (z = 0.10, p = 0.532), while a lower proportion was captured on ethanol (z = −5.03, p < 0.001).
Of the 46 species of bark and ambrosia beetles identified, 16 were found to significantly respond to one or more lures. Table 2 presents the species that were associated with some lure (i.e., they were caught on this lure at higher frequencies and abundances).
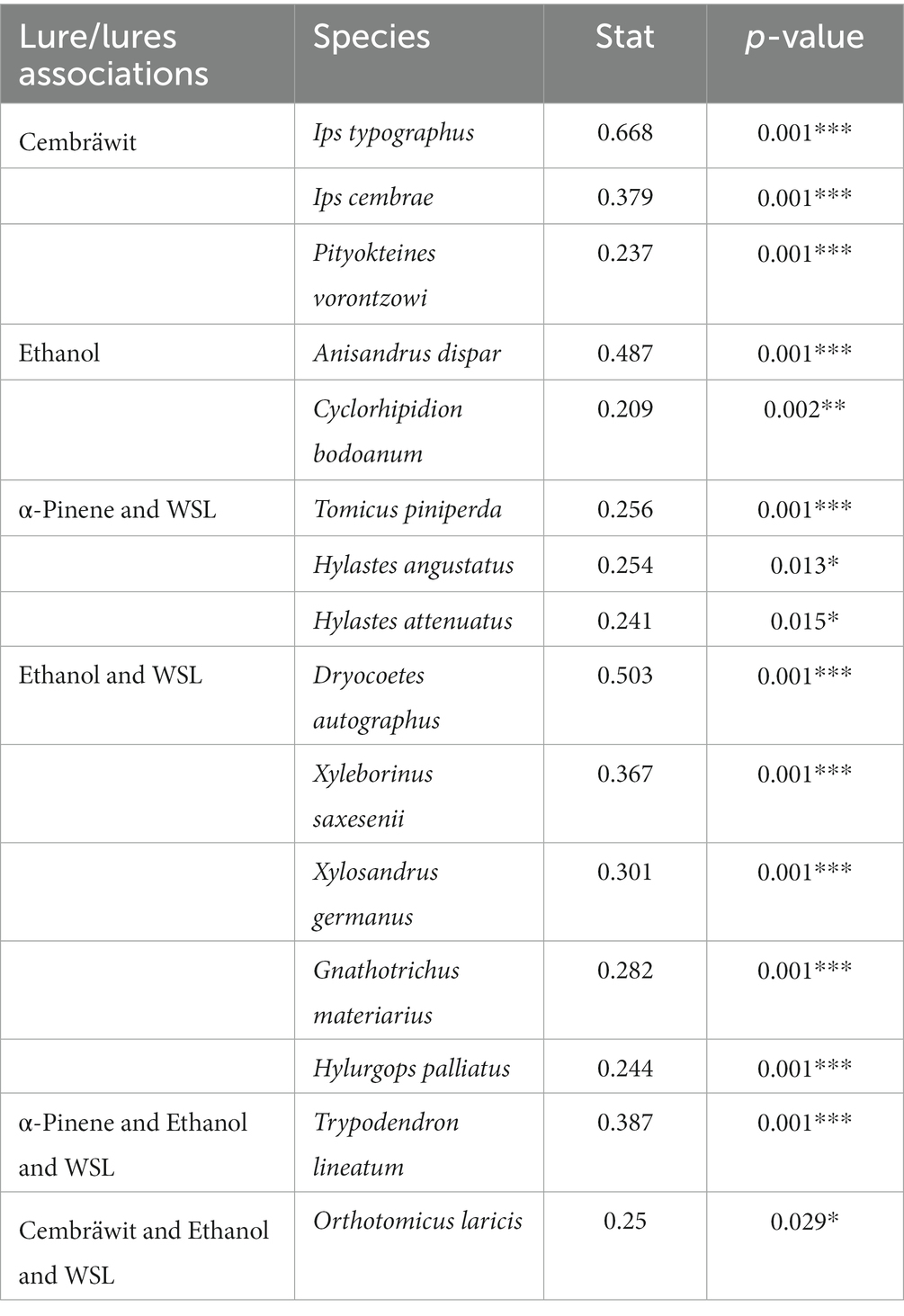
Table 2. Species of bark and ambrosia beetles significantly associated with studied lures or their associations (WSL, Wood Stainers Lure).
Furthermore, p-CCA analysis (after removing the effect of locality) revealed that the species spectrum of bark and ambrosia beetles was primarily influenced by the lure (df = 381, F = 20.30, p = 0.002) and secondarily by the day of the season (df = 381, F = 6.10, p = 0.025; Figures 6A,B).
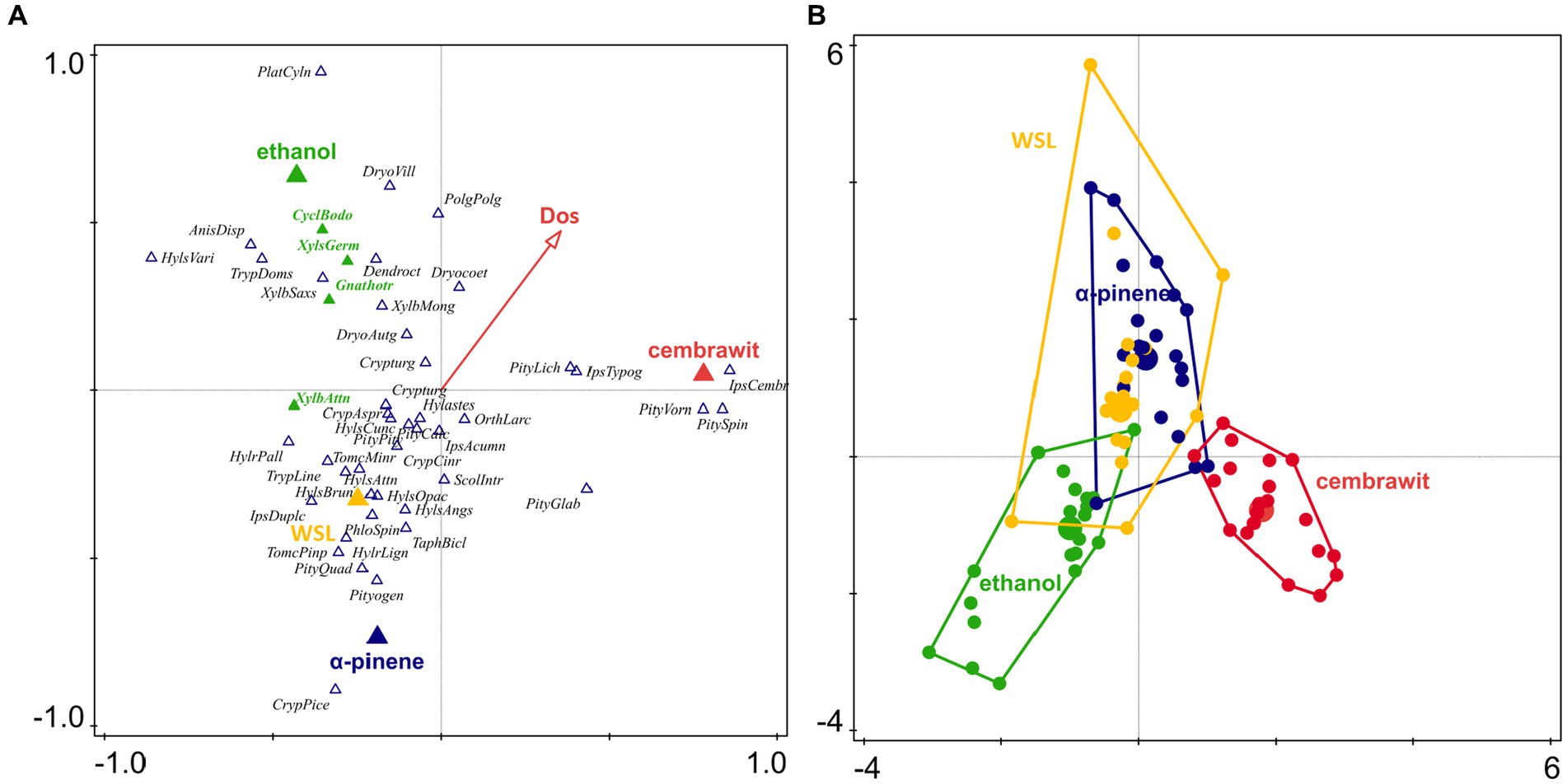
Figure 6. Ordination diagram of the p-CCA method showing the different compositions of the bark and ambrosia beetle assemblages depending on the lure (A) and PCoA diagram based on the p-CCA (B) analysis showing the different communities of bark and ambrosia beetles in individual lures (Dos, day of season; WSL, Wood Stainers Lure).
Sulcatol has been reported as a potential aggregation pheromone of G. materiarius (Flechtmann and Berisford, 2003). This is likely because sulcatol has been detected in the guts of males that initiate attacks on the host tree and ceases production after mating with females (Flechtmann and Berisford, 2003). However, we captured more G. materiarius in ethanol-baited traps than in WSL-baited traps (Table 2). The details and chiral ratio of sulcatol in WSL are unknown to us, while the chiral ratio of the pheromone observed from the headspace of G. materiarius was 31% (S)-(+)- and 69% (R)-(−)-sulcatol (Flechtmann and Berisford, 2003). No G. materiarius has been caught on sulcatol in the USA (Miller and Crowe, 2020).
Ethanol alone significantly attracted all ambrosia beetles. It is well-established that ethanol is the primary volatile lure for ambrosia beetles (Kühnholz et al., 2001; Kelsey and Joseph, 2003; Ranger et al., 2013, 2019, 2021; Supplementary Table S2). Therefore, it was expected that ethanol would have a higher capture ratio of invasive ambrosia beetles compared to the other lures.
Among the 46 bark and ambrosia beetle species detected, C. bodoanum, G. materiarius, X. attenuatus, and X. germanus are the invasive ambrosia beetle species that were expected to be found. These species were identified in a total of 187 individuals, which accounts for less than 3 % of the total number of bark and ambrosia beetles collected. This suggests that the population densities of these beetles are still relatively low. In comparison, Slovakia and Slovenia have reported higher captures of the invasive ambrosia beetle X. germanus (Galko et al., 2018; Franjević et al., 2019), suggesting that the Czech Republic is on the edge of the range of the species’ range. Similar observations apply to C. bodoanum (Fiala et al., 2021) and G. materiarius (Fiala, observ.). X. attenuatus, on the other hand, is widespread throughout the Czech Republic but is not abundant (Fiala and Holuša, 2024), which explains its relatively low number of individuals in the current study.
Europe is home to approximately 30 species of invasive Scolytinae, with most of them belonging to the ambrosia beetle group (Marchioro et al., 2022; Alonso-Zarazaga et al., 2023). These ambrosia beetles are predominantly native to temperate or subtropical forests, making them particularly concerning for southern Europe regions due to similar climatic conditions (Francardi et al., 2017; Leza et al., 2020). In the Central and Northern European countries, damage has been recorded only for X. germanus, while other species have not caused significant harm (Maksymov, 1987; Galko et al., 2019). X. attenuatus is considered a secondary pest (Borowski et al., 2012; Skrylnik et al., 2019). As for C. bodoanum, no damage has been documented in Europe; and G. materiarius is also not known to cause damage in the region (Mazur et al., 2018). Nevertheless, it is important to note that all these ambrosia beetles can serve as vectors for fungi that can be detrimental to trees, so their occurrence should not be ignored (Batra, 1963; Nakashima et al., 1992; Kawasaki et al., 2010; McPherson et al., 2013; Moore et al., 2019).
The occurrence of all species of invasive ambrosia beetles, as well as most of the other bark and ambrosia beetles, was observed at a single locality (Kdyně). This can be probably attributed to the presence of diverse range of tree species, both deciduous and coniferous, in the surrounding area [e.g., Quercus robur L., 1753, Larix decidua Mill., 1768, Fagus sylvatica L., 1753, Abies alba Mill., 1768, Populus tremulae L., 1753, Sorbus aucuparia L., 1753, Acer pseudoplatanus L., 1753, Pinus sylvestris L., 1753, Corylus avellana L., 1753, Picea abies (L.) H. Karst, 1881].
In all conducted analyses, the day of season emerged as a significant factor, with the highest number of captured beetles observed in May during the study (as shown in Figures 2, 3). This pattern can be attributed to the increased flight activity of the most abundant beetle species during the period (Pfeffer, 1989). Notably, traps baited with Cembräwit exhibited a significant peak in summer, which can be attributed to the captures of Ips cembrae, with most often two generations per year in Central Europe (Holuša et al., 2014). The observed variation in species richness among the different localities can be attributed to the variations in tree species composition across the study area.
A total of 10 species of bark and ambrosia beetles were captured across all locations (Anisandrus dispar, Crypturgus cinereus, Dryocoetes autographus, Hylastes attenuatus, Hylastes cunicularius, Ips typographus, Pityogenes chalcographus, Pityophthorus pityographus, Taphrorychus bicolor, and Xyleborinus saxesenii). The species are commonly found in Central Europe (Pfeffer, 1989). Notably, two of these species, I. typographus and P. chalcographus, have recently experienced severe outbreaks and have become epidemic in the studied area (Fiala and Holuša, 2022). The occurrence of most of the species was observed in two or three localities, depending on the presence of their respective host trees. For instance, Pityokteines spinidens and Pityokteines vorontzowi were predominantly found in sites with Abies alba Mill. Another group of species was present in several localities but was captured in only a few specimens. An example is Phloeotribus spinulosus, which primarily develops on thin dead spruce twigs and is exclusively found on them (Pfeffer, 1989). In our experiment, this bark beetle was detected in three localities, with a total of 5 specimens caught. It can be speculated whether this occurrence is influenced by the I. typographus outbreak and the availability of abundant suitable breeding material, as observed in other species (Fiala and Holuša, 2021, 2022), or if it represents a long-term trend.
Relatively low catches of certain bark beetle species can be attributed to specific factors. Cryphalus piceae is generally a rare inhabitant of fir forests (Procházka and Schlaghamerský, 2019), and the occurrence of fir trees is limited in the Czech Republic (Kozáková et al., 2011). Dryocoetes hectographus primarily occurs in mountain spruce forests (Pfeffer, 1989), where traps were not placed. Only two specimens of the bark beetle Dendroctonus micans were caught in the Kladská locality because this species is not attracted to traps (Procházka et al., 2014). Although substances such as E-conophthorin, exo-brevicomin, and ipsdienol elicit a positive response from D. micans, they are most likely not its pheromones (Tømmerås et al., 1984; Zhang et al., 2002). Contrarily, the substance exo-brevicomin has shown effectiveness in trapping American and Asian species of the Dendroctonus genus (Barclay et al., 1998; Greenwood and Borden, 2000; Zhao et al., 2017). However, the fertilized females of Dendroctonus emerge from galleries and primarily rely on visual detection during host tree attacks, suggesting that pheromones may not play a significant role for them, except during the larval stage (Lukášová and Holuša, 2011).
An association between α-pinene and WSL had a significant effect on attracting conifer species of bark beetles that typically inhabit areas where pine bark meets the soil, such as Hylastes angustatus and Hylastes attenuatus (Schroeder and Lindelöw, 1989; Erasmus and Chown, 1994). While aggregation pheromones cis-verbenol and trans-verbenol have been reported for T. piniperda (Kangas et al., 1970), it has been suggested that T. piniperda relies on monoterpenes, including kairomones derived from its host, pine, for host and mate location (Lanne et al., 1987). This explains the high attractiveness of α-pinene observed in our experiments, as all the species live on pine trees (Byers et al., 1985; Schroeder and Lindelöw, 1989; Czokajlo and Teale, 1999; Poland et al., 2004).
In Cembräwit, the main component is ipsdienol, which serves as an aggregation pheromone for Ips typographus and Ips cembrae in low concentrations (Vité et al., 1972; Stoakley et al., 1978). Therefore, it is not surprising that most specimens of both bark beetles were caught in traps baited with Cembräwit (see also Grucmanová et al., 2014; Holuša et al., 2014). Pityokteines vorontzowi was also significantly attracted to Cembräwit, as it shares ipsdienol and ipsenol as its aggregation pheromones (Harring, 1978). The highest number of bark beetles caught was observed on Cembräwit® compared to other lures, which can be attributed to the aforementioned outbreak of I. typographus in the study area (Fiala et al., 2022).
Ethanol and WSL significantly attracted two species of ambrosia beetles, Xyleborinus saxesenii and X. germanus as well as two species of bark beetles, Dryocoetes autographus and Hylurgops palliatus. These species have been frequently observed in traps baited with ethanol (Schroeder and Lindelöw, 1989; Lindelöw et al., 1993). More species of bark and ambrosia beetles responded to these lures compared to any other lure.
The ambrosia beetle Trypodendron lineatum was significantly associated with three lures, α-pinene, ethanol, and WSL. This is consistent with previous studies that have shown α-pinene and ethanol to be attractants for this species (McLean and Borden, 1977; Schroeder and Lindelöw, 1989). Conversely, this ambrosia beetle exhibits weak attraction to the substances used in Cembrawit, such as ipsdienol and methylbutenol (Gavyalis et al., 1981). The association of Cembräwit, ethanol, and WSL significantly attracted only the bark beetle Orthotomicus laricis. Bark beetles of the entire genus Orthotomicus are primarily attracted to ipsdienol, cis-verbenol, and 2-methyl-3-buten-2-ol (Giesen et al., 1984; Valkama et al., 1997). Ipsdienol is the main component of Cembräwit®, which also contains ipsenol, methylbutenol, and amitinol (Zuhlke and Mueller, 2008). However, since O. laricis is found in the tops of uprooted spruces (Fiala, observ.), on pine stumps, and on felled spruce wood that is shaded (Holuša et al., 2017, 2019), it is evident that this bark beetle is also attracted to ethanol.
Overall, the results demonstrate that accounting for the influence of location and day of the season, the differences among lures were significant (see also Beaver and Löyttyniemi, 1991). Ethanol significantly attracted all ambrosia beetles in our experiments. In the USA and Italy, volatile lures such as ethanol, a combination of α-pinene and ethanol, and other combinations of ipsdienol+cis-verbenol+methylbutenol are used for monitoring invasive bark beetles (Rassati et al., 2015; Rabaglia et al., 2019). In Italy, these traps are placed in ports and adjacent forests; with higher capture success observed in deciduous forests compared to coniferous forests or ports (Rassati et al., 2015). Our experiment also shows the higher capture success of invasive ambrosia beetles was in deciduous forests, as evidenced by the capture of 162 specimens (e.g., 87%) at the Kdyně locality, probably due to the prevalence of deciduous trees in that area. A similar approach is employed in New Zealand, where traps are placed in ports, international airports, and adjacent forests using different lure combinations such as α-pinene+ethanol, β-pinene+ethanol, frontalin+ethanol, and ipsdienol. This monitoring model has proven successful in the early detection of invasive bark beetles, increasing the chances of eradication when infestations are still relatively small (Brockerhoff et al., 2006b). In Australia, the monitoring efforts have a broader focus and include the capture of Lepidoptera. Various lures such as ethanol, cineole, α-pinene, phellandrene, and a combination of pinene, phellandrene, cineole, terpene, and cymene as bait are used. Traps are positioned near ports and airports and within a 5 km radius of these areas (Bashford, 2012). In France, monitoring activities targeting invasive cerambycids include testing trapping methods using α-pinene+ethanol in Ecotrap traps. Traps were deployed in natural forests and later extended to ports, airports, and horticulture areas (Fan et al., 2019). In all these cases, the traps successfully captured invasive species.
There are several methods available for monitoring bark and ambrosia beetles, with baited traps being the most effective among them. Baited traps offer several advantages, including their ability to cover large areas, easy application in various locations, and cost-effectiveness. However, they also have some drawbacks, primarily related to the selection of bait types and the physical demands associated with checking numerous traps and subsequent determination in the laboratory (Poland and Rassati, 2019). While citizen science can help alleviate the physical demands of monitoring (Steininger et al., 2015), coordination is still necessary, and the expertise of bark beetle specialists will always be essential for accurate determination.
Ethanol serves as a universal lure that effectively attracts both bark and ambrosia beetles, with the majority of Scolytinae species being caught in traps baited with ethanol. Conversely, ethanol’s inability to attract economically important species in Central Europe, such as Ips typographus, is a significant advantage since these species are typically abundant. By not attracting them, the number of bark and ambrosia beetles captured is not needlessly increased, thus reducing the complexity and cost associated with sorting and determination. For the detection of invasive ambrosia beetles in Central Europe, it is recommended to use one bait of ethanol per year installed in April in any impact trap. Our preference lies with Ecotrap® due to its disassemblability, storage capacity, and ability to catch beetles in a dry state without the need for preservative liquid. Moreover, the material remains intact by predators even after a two-week period.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JH: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. TF: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. PP: Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Václav Týr and Martin Kacerovský for fieldwork support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1258729/full#supplementary-material
Alonso-Zarazaga, M. A., Barrios, H., Borovec, R., Caldara, R., Colonnelli, E., Gültekin, L., et al. (2023). Cooperative catalogue of Palaearctic Coleoptera Curculionoidea. 2. Available at: http://sea-entomologia.org/monoelec.html (Accessed March 1, 2023).
Aukema, J. E., Leung, B., Kovacs, K., Chivers, C., Britton, K. O., Englin, J., et al. (2011). Economic impacts of non-native forest insects in the continental United States. PLoS One 6:e24587. doi: 10.1371/journal.pone.0024587
Barclay, H. J., Safranyik, L., and Linton, D. (1998). Trapping mountain pine beetles Dendroctonus ponderosae (Coleoptera: Scolytidae) using pheromone-baited traps: effects of trapping distance. J. Entomol. Soc. Brit. Col. 95, 25–32.
Bashford, R. (2012). “The development of a port surrounds trapping system for the detection of exotic forest insect pests in Australia” in New advances and contributions to forestry research. ed. A. A. Oteng-Amoako (Rijeka: InTechOpen), 85–100.
Bates, D., Maechler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Batra, L. R. (1963). Contributions to our knowledge of ambrosia fungi. II. Endomycopsis fasciculata nom. Nov. (Ascomycetes). Am. J. Bot. 50, 481–487. doi: 10.1002/j.1537-2197.1963.tb07218.x
Beaver, R. A., and Löyttyniemi, K. (1991). Annual flight patterns and diversity of bark and ambrosia beetles (Col., Scolytidae, and Platypodidae) attracted to bait logs in Zambia. J. App. Entomol. 112, 505–511. doi: 10.1111/j.1439-0418.1991.tb01084.x
Boland, J. M. (2016). The impact of an invasive ambrosia beetle on the riparian habitats of the Tijuana River valley. California. PeerJ 4:e2141. doi: 10.7717/peerj.2141
Borden, J. H., Lindgren, B. S., and Chong, L. (1980). Ethanol and α-pinene as synergists for the aggregation pheromones of two Gnathotrichus species. Can. J. For. Res. 10, 290–292. doi: 10.1139/x80-049
Borowski, J., Piętka, J., and Szczepkowski, A. (2012). Insects found on black alder Alnus glutinosa (L.) Gaertn. When stands are dying back. For. Res. Pap. 73, 355–362. doi: 10.2478/v10111-012-0034-0
Brockerhoff, E. G., Bain, J., Kimberley, M., and Knížek, M. (2006a). Interception frequency of exotic bark and ambrosia beetles (Coleoptera: Scolytinae) and relationship with establishment in New Zealand and worldwide. Can. J. For. Res. 36, 289–298. doi: 10.1139/x05-250
Brockerhoff, E. G., Jones, D. C., Kimberley, M. O., Suckling, D. M., and Donaldson, T. (2006b). Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. For. Ecol. Manag. 228, 234–240. doi: 10.1016/j.foreco.2006.02.046
Burbano, E. G., Wright, M. G., Gillette, N. E., Mori, S., Dudley, N., Jones, T., et al. (2012). Efficacy of traps, lures, and repellents for Xylosandrus compactus (Coleoptera: Curculionidae) and other ambrosia beetles on Coffea arabica plantations and Acacia koa nurseries in Hawaii. Environ. Entomol. 41, 133–140. doi: 10.1603/EN11112
Byers, J. A., Lanne, B. S., Löfqvist, J., Schlyter, F., and Bergström, G. (1985). Olfactory recognition of host-tree susceptibility by pine shoot beetles. Naturwissenschaften 72, 324–326. doi: 10.1007/BF00454776
Czokajlo, D., and Teale, S. A. (1999). Synergistic effect of ethanol to α-pinene in primary attraction of the larger pine shoot beetle, Tomicus piniperda. J. Chem. Ecol. 25, 1121–1130. doi: 10.1023/A:1020838010648
De Cáceres, M., and Legendre, P. (2009). Associations between species and groups of sites: indices and statistical inference. Ecology 90, 3566–3574. doi: 10.1890/08-1823.1
Douglas, H., Dang, P. T., Gill, B. D., Huber, J., Mason, P. G., Parker, D. J., et al. (2009). The importance of taxonomy in responses to invasive alien species. Biodiversity 10, 92–99. doi: 10.1080/14888386.2009.9712850
Duelli, P., Zahradník, P., Knížek, M., and Kalinová, B. (1997). Migration in spruce bark beetles (Ips typographus L.) and the efficiency of pheromone traps. J. Appl. Entomol. 121, 297–303. doi: 10.1111/j.1439-0418.1997.tb01409.x
Dufrêne, M., and Legendre, P. (1997). Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol. Monogr. 67, 345–366. doi: 10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Erasmus, M. J., and Chown, S. L. (1994). Host location and aggregation behaviour in Hylastes angustatus (Herbst) (Coleoptera: Scolytidae). Afr. Entomol. 2, 7–11. doi: 10.10520/AJA10213589_125
Fan, J.-T., Denux, O., Courtin, C., Bernard, A., Javal, M., Millar, J. G., et al. (2019). Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J. Pest. Sci. 92, 281–297. doi: 10.1007/s10340-018-0997-6
Fiala, T. (2019). Kůrovci (Coleoptera: Curculionidae: Scolytinae) v národní přírodní památce Komorní hůrka. Zap. Entomol. Listy 10, 34–39.
Fiala, T., and Holuša, J. (2021). Infestation of Norway spruce seedlings by Cryphalus asperatus: new threat for planting of forests? Plant Prot. Sci. 57, 167–170. doi: 10.17221/112/2020-PPS
Fiala, T., and Holuša, J. (2022). Outbreak of Pityogenes chalcographus and Pityophthorus pityographus on spruce seedlings resulting from inappropriate Management in a Forest Nursery. Forests 13:987. doi: 10.3390/f13070987
Fiala, T., and Holuša, J. (2024). Distribution of the invasive ambrosia beetle Xyleborinus attenuatus Blandford, 1894 (Coleoptera: Curculionidae: Scolytinae) in the Czech Republic (Central Europe). Cent. Eur. For. J. 69. doi: 10.2478/forj-2023-0022
Fiala, T., Holuša, J., Procházka, J., Čížek, L., Dzurenko, M., Foit, J., et al. (2020). Xylosandrus germanus in Central Europe: spread into and within the Czech Republic. J. App. Entomol. 144, 423–433. doi: 10.1111/jen.12759
Fiala, T., Holuša, J., and Véle, A. (2022). Both native and invasive bark beetles threaten exotic conifers within the spa towns in the Czech part of “the great spas of Europe”. Urban Forest. Urban Green. 67:127417. doi: 10.1016/j.ufug.2021.127417
Fiala, T., Knížek, M., and Holuša, J. (2021). Continued eastward spread of the invasive ambrosia beetle Cyclorhipidion bodoanum (Reitter, 1913) in Europe and its distribution in the world. BioInv. Rec. 10, 65–73. doi: 10.3391/bir.2021.10.1.08
Flaherty, L., Gutowski, J. M. G., Hughes, C., Mayo, P., Mokrzycki, T., Pohl, G., et al. (2019). Pheromone-enhanced lure blends and multiple trap heights improve detection of bark and wood-boring beetles potentially moved in solid wood packaging. J. Pest Sci. 92, 309–325. doi: 10.1007/s10340-018-1019-4
Flechtmann, C. A. H., and Berisford, C. W. (2003). Identification of sulcatol, a potential pheromone of the ambrosia beetle Gnathotrichus materiarius (Col., Scolytidae). J. App. Entomol. 127, 189–194. doi: 10.1046/j.1439-0418.2003.00743.x
Francardi, V., Noal, A., Francescato, S., Pinto, R., Bruni, A., Loffredi, L., et al. (2017). Coexistence of Xylosandrus crassiusculus (Motschulsky) and X. compactus (Eichhoff) (Coleoptera Curculionidae Scolytinae) in the national park of Circeo (Lazio, Italy). Redia 100, 149–155. doi: 10.19263/REDIA-100.17.19
Franjević, M., Poršinsky, T., and Ðuka, A. (2016). Integrated oak timber protection from ambrosia bark beetles: economic and ecological importance in harvesting operations. Croat. J. For. Eng. 37, 353–364.
Franjević, M., Šikić, Z., and Hrašovec, B. (2019). First occurrence of Xylosandrus germanus (Blandford, 1894) – black steam borer in pheromone baited panel traps and population build up in croatian oak stands. Šumarski List 143:219. doi: 10.31298/sl.143.5-6.2
Galko, J., Dzurenko, M., Ranger, C. M., Kulfan, J., Kula, E., Nikolov, C., et al. (2019). Distribution, habitat preference, and management of the invasive ambrosia beetle, Xylosandrus germanus (Coleoptera: Curculionida, Scolytinae) in european forests with an emphasis on the West Carpathians. Forests 10:10. doi: 10.3390/f10010010
Galko, J., Dzurenko, M., Zach, P., Rell, S., Lalík, M., Vakula, J., et al. (2018). “Výskum a monitoring nepôvodného škodcu drvinárika čierneho na Slovensku” in Aktuálné problémy v ochrane lesa 2018: Zborník referátov z 27. ročníka medzinárodnej konferencie, ktorá sa konala 1. a 2. februára 2018 v Novom Smokovci. ed. A. Kunca (Zvolen: Národné lesnícke centrum), 120–123.
Galko, J., Nikolov, C., Kimoto, T., Kunca, A., Gubka, A., Vakula, J., et al. (2014). Attraction of ambrosia beetles to ethanol baited traps in a slovakian oak forest. Biologia 69, 1376–1383. doi: 10.2478/s11756-014-0443-z
Gavyalis, V. M., Yakaitis, B. Y., Gavelis, V., and Jakaitis, B. (1981). The attraction of various species of bark-beetles with methylbutenol, cis-verbenol, ipsdienol and mixtures of these pheromones. Khemo. Nasek. 6, 115–120.
Giesen, H., Kohnle, U., Vité, J. P., Pan, M.-L., and Francke, W. (1984). Das Aggregationspheromon des mediterranen Kieferborkenkäfres Ips (Orthotomicus) erosus. Zeit. Ang. Entomol. 98, 95–97. doi: 10.1111/j.1439-0418.1984.tb02688.x
Gohli, J., Selvarajah, T., Kirkendall, L. R., and Jordal, B. H. (2016). Globally distributed Xyleborus species reveal recurrent intercontinental dispersal in a landscape of ancient worldwide distributions. BMC Evol. Biol. 16:37. doi: 10.1186/s12862-016-0610-7
Greenwood, M. E., and Borden, J. H. (2000). Co-baiting for spruce beetles, Dendroctonus rufipennis, and western balsam bark beetles, Dryocoetes confusus (Coleoptera: Scolytidae). Can. J. For. Res. 30, 50–58. doi: 10.1139/x99-184
Grucmanová, Š., Holuša, J., Trombik, J., and Lukášová, K. (2014). Large larch bark beetle Ips cembrae (Coleoptera: Curculionidae, Scolytinae) in the Czech Republic: analysis of population development and catches in pheromone traps. Les. Čas. For. J. 60, 143–149. doi: 10.2478/forj-2014-0015
Harring, C. M. (1978). Aggregation pheromones of the European fir engraver beetles Pityokteines curvidens, P. spinidens and P. vorontzovi and the role of juvenile hormone in pheromone biosynthesis. Zeit. Ang. Entomol. 85, 281–317. doi: 10.1111/j.1439-0418.1978.tb04040.x
Hlásny, T., Zimová, S., Merganičová, K., Štěpánek, P., Modlinger, R., and Turčáni, M. (2021). Devastating outbreak of bark beetles in the Czech Republic: drivers, impacts, and management implications. For. Ecol. Manag. 490:119075. doi: 10.1016/j.foreco.2021.119075
Holuša, J., Foit, J., Knížek, M., Schovánková, J., Lukášová, K., Vanická, H., et al. (2019). The bark beetles Orthotomicus laricis and Orthotomicus longicollis are not pests in Central Europe: a case study from the Czech Republic. Bull. Insect. 72, 253–260.
Holuša, J., Kula, E., Wewiora, F., and Lukášová, K. (2014). Flight activity, within the trap tree abundance and overwintering of the larch bark beetle (Ips cembrae) in the Czech Republic. Šumarski List 138, 19–27.
Holuša, J., Lukášová, K., Hubáčková, J., Knížek, M., and Wegensteiner, R. (2017). Pathogens and nematodes associated to three bark beetle species of the genus Orthotomicus (Coleoptera Curculionidae) in central-South Europe. Bull. Insect. 70, 291–297.
Hulcr, J., and Dunn, R. R. (2011). The sudden emergence of pathogenicity in insect-fungus symbioses threatens naive forest ecosystems. Proc. Biol. Sci. 278, 2866–2873. doi: 10.1098/rspb.2011.1130
Hulcr, J., Mogia, M., Isua, B., and Novotný, V. (2007). Host specificity of ambrosia and bark beetles (Col., Curculionidae: Scolytinae and Platypodinae) in a New Guinea rainforest. Ecol. Entomol. 32, 762–772. doi: 10.1111/j.1365-2311.2007.00939.x
Kamp, H. J. (1970). Zur Biologie und derzeitigen Verbreitung von Gnathotrichus materiarius Fitch und Xylosandrus germanus Blandf. in der Bundesrepublik Deutschland. Ver. Entomol. 5, 34–40.
Kangas, E., Oksanen, H., and Perttunen, V. (1970). Responses of Blastophagus piniperda L. (Col., Scolytidae) to trans-verbenol, cis-verbenol, and verbenone, known to be population pheromones of some american bark beetles. Anna. Entomolog. Fenn. 36, 75–83.
Kawasaki, Y., Ito, M., Miura, K., and Kajimura, H. (2010). Superinfection of five Wolbachia in the alnus ambrosia beetle, Xylosandrus germanus (Blandford) (Coleoptera: Curculionidae). Bull. Entomolog. Res. 100, 231–239. doi: 10.1017/S000748530999023X
Kelsey, R. G., and Joseph, G. (2003). Ethanol in ponderosa pine as an indicator of physiological injury from fire and its relationship to secondary beetles. Can. J. For. Res. 33, 870–884. doi: 10.1139/x03-007
Kozáková, R., Šamonil, P., Kuneš, P., Novák, J., Kočár, P., and Kočárová, R. (2011). Contrasting local and regional holocene histories of Abies alba in the Czech Republic in relation to human impact: evidence from forestry, pollen and anthracological data. The Holocene 21, 431–444. doi: 10.1177/0959683610385721
Kühnholz, S., Borden, J. H., and Uzunovic, A. (2001). Secondary ambrosia beetles in apparently healthy trees: adaptations, potential causes and suggested research. Int. Pest Manage. Rev. 6, 209–219. doi: 10.1023/A:1025702930580
Lanne, B. S., Schlyter, F., Byers, J. A., Löfqvist, J., Leufvén, A., Bergström, G., et al. (1987). Differences in attraction to semiochemicals present in sympatric pine shoot beetles, Tomicus minor and T. piniperda. J. Chem. Ecol. 13, 1045–1067. doi: 10.1007/BF01020537
Leza, M., Nuñez, L., Riba, J. M., Comparini, C., Roca, A., and Gallego, D. (2020). First record of the black twig borer, Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) in Spain. Zootaxa 4767, 345–350. doi: 10.11646/zootaxa.4767.2.9
Lindelöw, Å., Eidmann, H. H., and Nordenhem, H. (1993). Response on the ground of bark beetle and weevil species colonizing conifer stumps and roots to terpenes and ethanol. J. Chem. Ecol. 19, 1393–1403. doi: 10.1007/BF00984884
Lingren, S. L., and Fraser, R. G. (1994). Control of ambrosia beetle damage by mass trapping at a dryland log sorting area in British Columbia. For. Chro. 70, 159–163. doi: 10.5558/tfc70159-2
Lukášová, K., and Holuša, J. (2011). Přirození nepřátelé a biologický boj s Dendroctonus micans: review. Zpr. Les. Výzk. 56, 15–23.
Maksymov, J. K. (1987). Erstmaliger Massenbefall des schwarzen Nutzholzborkenkäfers, Xylosandrus germanus Blandf., in der Schweiz. Schweiz. Zeit. Forst. 138, 215–227.
Marchioro, M., Faccoli, M., Dal Cortivo, M., Branco, M., Roques, A., Garcia, A., et al. (2022). New species and new records of exotic Scolytinae (Coleoptera, Curculionidae) in Europe. Biodiv. Data J. 10:e93995. doi: 10.3897/BDJ.10.e93995
Mazur, A., Witkowsk, I. R., Góral, J., and Rogowski, G. (2018). Occurrence of Gnathotrichus materiarius (Fitch, 1858) (Coleoptera, Curculionidae, Scolytinae) in South-Western Poland. Fol. For. Pol. Ser. A For. 60, 154–160. doi: 10.2478/ffp-2018-0015
McLean, J. A., and Borden, J. H. (1977). Suppresion of Gnathotrichus sulcatus with sulcatol-baited traps in a commercial sawmill and notes on the occurrence of G. retusus and Trypodendron lineatum. Can. J. For. Res. 7, 348–356. doi: 10.1139/x77-044
McPherson, B. A., Erbilgin, N., Bonello, P., and Wood, D. L. (2013). Fungal species assemblages associated with Phytophthora ramorum-infected coast live oaks following bark and ambrosia beetle colonization in northern California. For. Ecol. Manag. 291, 30–42. doi: 10.1016/j.foreco.2012.11.010
Miller, D. R., and Crowe, C. M. (2020). Sulcatol: enantiospecific attractants for Monarthrum mali (Coleoptera: Curculionidae: Scolytinae), Leptostylus asperatus (Coleoptera: Cerambycidae) and associated predators. Env. Entomol. 49, 593–600. doi: 10.1093/ee/nvaa042
Miller, D. R., and Rabaglia, R. J. (2009). Ethanol and (−)-α-pinene: attractant kairomones for bark and ambrosia beetles in the southeastern US. J. Chem. Ecol. 35, 435–448. doi: 10.1007/s10886-009-9613-9
Moore, M., Juzwik, J., Miller, F., Roberts, L., and Ginzel, M. D. (2019). Detection of Geosmithia morbida on numerous insects species in four eastern states. Plant Health Prog. 20, 133–139. doi: 10.1094/PHP-02-19-0016-RS
Morales, M. (2020). Scientific graphing functions for factorial designs. Available at: https://cran.r-project.org/web/packages/sciplot/index.html (Accessed June 20, 2022).
Nakashima, T., Otomo, T., Owada, Y., and Iizuka, T. (1992). SEM observations on growing conditions of the fungi in the galleries of several ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). J. Fac. Agric. Hokk. Un. 65, 239–273.
Poland, T. M., de Groot, P., Haack, R. A., and Czokajlo, D. (2004). Evaluation of semiochemicals potentially synergistic to α-pinene for trapping the larger european pine shoot beetle, Tomicus piniperda (Col., Scolytidae). J. App. Entomol. 128, 639–644. doi: 10.1111/j.1439-0418.2004.00900.x
Poland, T. M., and Rassati, D. (2019). Improved biosecurity surveillance of non-native forest insects: a review of current methods. J. Pest Sci. 92, 37–49. doi: 10.1007/s10340-018-1004-y
Procházka, J., and Schlaghamerský, J. (2019). Does dead wood volume affect saproxylic beetles in montane beech-fir forests of Central Europe? J. Ins. Cons. 23, 157–173. doi: 10.1007/s10841-019-00130-4
Procházka, J., Schlaghamerský, J., and Knížek, M. (2014). Kůrovci (Coleoptera: Curculionidae: Scolytinae) jedlobukových lesů CHKO Beskydy. Zpr. Les. Výzk. 59, 126–132.
R Core Team. (2022). The R project for statistical computing. [https://www.R-project.org].
Rabaglia, R. J., Cognato, A. I., Hoebeke, E. R., Johnson, C. W., LaBonte, J. R., Carter, M. E., et al. (2019). Early detection and rapid response. A 10-year summary of the USDA forest service program of surveillance for non-native bark and ambrosia beetles. Am. Entomol. 65, 29–42. doi: 10.1093/ae/tmz015
Ranger, C. M., Reding, M. E., Addesso, K., Ginzel, M., and Rassati, D. (2021). Semiochemical-mediated host selection by Xylosandrus spp. Ambrosia beetles (Coleoptera: Curculionidae) attacking horticultural tree crops: a review of basic and applied science. Can. Entomol. 153, 103–120. doi: 10.4039/tce.2020.51
Ranger, C. M., Reding, M. E., Schultz, P. B., and Oliver, J. B. (2013). Influence of flood-stress on ambrosia beetle host-selection and imlications for their management in a changing climate. Agr. For. Entomol. 15, 56–64. doi: 10.1111/j.1461-9563.2012.00591.x
Ranger, C. M., Schultz, P. B., Frank, S. D., and Reding, M. E. (2019). Freeze stress of deciduous trees induces attacks by opportunistic ambrosia beetles. Agr. For. Entomol. 21, 168–179. doi: 10.1111/afe.12317
Rassati, D., Faccoli, M., Toffolo, E. P., Battisti, A., and Marini, L. (2015). Improving the early detection of alien wood-boring beetles in ports and surrounding forests. J. Appl. Ecol. 52, 50–58. doi: 10.1111/1365-2664.12347
Roberts, D. W. (2019). Labdsv: ordination and multivariate analysis for ecology. R package version 2.0-1. Available at: https://cran.r-project.org/web/packages/labdsv/index.html (Accessed June 20, 2022).
Schneider, I. (1985). Gnathotrichus materiarius Fitch (Col., Scolytidae) in Pheromonfallen von Ips cembrae (Heer) (Col., Scolytidae), ein neuer Fundort für NW-Deutschland. Anz. Sch. Pfl. Umwelt. 58, 50–51. doi: 10.1007/BF01903080
Schroeder, L. M., and Lindelöw, Å. (1989). Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J. Chem. Ecol. 15, 807–817. doi: 10.1007/BF01015179
Seybold, S. J., Bentz, B. J., Fettig, C. J., Lundquist, J. E., Progar, R., and Gillette, N. E. (2018). Management of western north american bark beetles with semiochemicals. Annu. Rev. Entomol. 63, 407–432. doi: 10.1146/annurev-ento-020117-043339
Skrylnik, Y., Koshelyaeva, Y., and Meshkova, V. (2019). Harmfulness of xylophagous insects for silver birch (Betula pendula Roth.) in the left-bank forest-steppe of Ukraine. Fol. For. Pol. Ser. A For. 61, 159–173. doi: 10.2478/ffp-2019-0016
Šramel, N., Kavčič, A., Kolšek, M., and de Groot, M. (2021). Estimating the most effective and economical pheromone for monitoring the European spruce bark beetle. J. App. Entomol. 145, 312–325. doi: 10.1111/jen.12853
Steininger, M. S., Hulcr, J., Šigut, M., and Lucky, A. (2015). Simple and efficient trap for bark and ambrosia beetles (Coleoptera: Curculionidae) to facilitate invasive species monitoring and citizen involvement. J. Econ. Entomol. 108, 1115–1123. doi: 10.1093/jee/tov014
Stoakley, J. T., Bakke, A., Renwick, J. A. A., and Vité, J. P. (1978). The aggregation pheromone system of the larch bark beetle, Ips cembrae Heer. Zeit. Ang. Entomol. 86, 174–177. doi: 10.1111/j.1439-0418.1978.tb01925.x
Susaeta, A., Soto, J. R., Adams, D. C., and Hulcr, J. (2016). Pre-invasion economic assessment of invasive species prevention: a putative ambrosia beetle in southeastern loblolly pine forests. J. Environ. Manag. 183, 875–881. doi: 10.1016/j.jenvman.2016.09.037
ter Braak, C. J. F., and Šmilauer, P. (2012). Canoco reference manual and CanoDraw for windows User’s guide: Software for canonical community ordination (version 5.01). Ithaca: Microcomputer Power.
Tømmerås, B. Å., Mustaparta, H., and Gregoire, J.-C. (1984). Receptor cells in Ips typographus and Dendroctonus micans specific to pheromones of the reciprocal genus. J. Chem. Ecol. 10, 759–769. doi: 10.1007/BF00988541
Valkama, H., Räty, M., and Niemelä, P. (1997). Catches of Ips duplicatus and other non-target Coleoptera by Ips typographus pheromone trapping. Entomol. Fenn. 8, 153–159. doi: 10.33338/ef.83934
Vité, J. P., Bakke, A., and Renwick, J. A. A. (1972). Pheromones in Ips (Coleoptera: Scolytidae): occurrence and production. Can. Entomol. 104, 1967–1975. doi: 10.4039/Ent1041967-12
Wood, D. L. (1982). The role of pheromones, kairomones, and allomones in the host selection and colonization behavior of bark beetles. Annu. Rev. Entomol. 27, 411–446. doi: 10.1146/annurev.en.27.010182.002211
Yi, N. (2020). NBZIMM: negative binomial and zero-inflated mixed models. R package version 1.0. Available at: https://rdrr.io/github/nyiuab/NBZIMM/ (Accessed June 20, 2022).
Zhang, Q.-H., Tolasch, T., Schlyter, F., and Francke, W. (2002). Enantiospecific antennal response of bark beetles to spiroacetal (E)-conophthorin. J. Chem. Ecol. 28, 1839–1852. doi: 10.1023/A:1020569303433
Zhao, M., Dai, L., Fu, D., Gao, J., and Chen, H. (2017). Electrophysiological and behavioral responses of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae) to two candidate pheromone components: frontalin and exo-brevicomin. Chemoecology 27, 91–99. doi: 10.1007/s00049-017-0235-3
Keywords: α-pinene, Cembräwit, Cyclorhipidion bodoanum, Gnathotrichus materiarius, sulcatol, Xylosandrus germanus, Xyleborinus attenuatus
Citation: Fiala T, Pyszko P and Holuša J (2023) Using ethanol and other lures to monitor invasive ambrosia beetles in endemic populations: case study from the Czech Republic. Front. For. Glob. Change. 6:1258729. doi: 10.3389/ffgc.2023.1258729
Received: 14 July 2023; Accepted: 11 September 2023;
Published: 28 September 2023.
Edited by:
Filipe Xavier Catry, University of Lisbon, PortugalReviewed by:
Diego Gallego, University of Alicante, SpainCopyright © 2023 Fiala, Pyszko and Holuša. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaroslav Holuša, aG9sdXNhQGZsZC5jenUuY3o=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.