
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 21 September 2023
Sec. Forest Management
Volume 6 - 2023 | https://doi.org/10.3389/ffgc.2023.1257418
This article is part of the Research Topic Sustainable forest management under climate change conditions- A focus on biodiversity conservation and forest restoration View all 7 articles
 Asad Aslam1
Asad Aslam1 De-Fu Chi1*
De-Fu Chi1* Asim Abbasi2,3
Asim Abbasi2,3 Muhammad Arshad4
Muhammad Arshad4 Faisal Hafeez5
Faisal Hafeez5 Amna Fayyaz6
Amna Fayyaz6 Ashraf Atef Hatamleh7
Ashraf Atef Hatamleh7 Munirah Abdullah Al-Dosary7
Munirah Abdullah Al-Dosary7Termites have become a global concern, and their effective management has remained a challenge since time immemorial. Certain microbial and botanical agents have been used for their management, but their efficacy has been compromised, particularly in field conditions. Hence, the current study was designed to check the efficacy of low doses of different pesticides, such as chlorpyrifos, fipronil, bifenthrin, and chlorantraniliprole, against mortality and behavioral responses of Odontotermes obesus at two different temperatures (16 ± 1 and 26 ± 1°C). The discrete behavioral symptoms included intoxication, ataxia, moribundity, and death. Laboratory-maintained termite workers were exposed to different concentrations of pesticides through a filter paper bioassay. All tested pesticides and their concentrations differed significantly regarding their lethal time (LT50) values compared to the mortality of termite workers. Moreover, the LT50 values of pesticides gradually decreased with increased pesticidal concentrations. Temperature also had a significant effect on the efficacy of tested pesticides as all pesticides showed better results at higher temperatures. At both tested temperatures, chlorantraniliprole (5 ppm) proved to be the most effective pesticide against termite workers. Similarly, the behavioral symptoms also varied depending on pesticides and their administered concentrations and existed for a relatively longer time span at lower temperatures. In most cases, the order of responses was moribundity, followed by intoxication and ataxia. Moribundity and intoxication were the most frequently observed symptoms for chlorpyriphos and bifenthrin-treated termite workers. In the case of fipronil, intoxication was the most pronounced symptom. Similarly, the maximum value of ataxia was recorded in the case of chlorantraniliprole. However, moribund symptoms lasted longer in all tested concentrations of chlorantraniliprole, followed by ataxia and intoxication. The overall order of toxicity was chlorantraniliprole > bifenthrin > fipronil > chlorpyrifos. These pesticides, at their low doses, did not exhibit any repellent action and were not detected by the foraging termite workers. Moreover, their slow action mechanism makes them a suitable candidate for infecting whole colonies away from treated surfaces. Therefore, these pesticides can be successfully incorporated into different integrated termite management programs to keep the plantation free from threatening underground pests.
Forests are an important component of human's natural environment and cover approximately 30% of the total land area. They provide a score of useful ecosystem services that are crucial for the functioning of the Earth and human society (Keenan et al., 2015). They play vital roles in different biogeochemical cycles, sequester carbon, support fauna, and produce huge amounts of organic matter, which sustain different terrestrial organisms, including humans (Klapwijk and Björkman, 2018). However, the current global climatic variations may pose severe threats to forests ranging from different abiotic to biotic sources (Balla et al., 2021). Among biotic threats, insect pests are of prime importance as they damage a large proportion of forest trees every year in both commercial and natural settings. Non-indigenous insect pests pose a severe threat to forest ecosystems because of their rapid establishment and limited or no natural control in new localities (Prospero et al., 2021).
Termites, with 2,000 known species belonging to 8 sub-families and 250 genera, are found liberally everywhere, from cultivated land to wild plantations. Termites can easily adapt to a new locality owing to their cryptic feeding behavior and the formation of nests, which not only provide ideal conditions for population maintenance but also serve as a food storage site (Manzoor et al., 2011; Zanuncio et al., 2016). Among the different termite species, Odontotermes obesus (Blattodea: Termitidae) is the most damaging subterranean termite, which causes major economic losses to humans through feeding on stored timbers, wooden structures and buildings, agricultural crops, standing tress, and forests (Ravan et al., 2015).
A number of studies proved that this particular termite species has crossed all boundaries and can be found in forests as well as rural and urban areas, inflicting damage on multiple hosts (Rathour et al., 2014; Lin et al., 2015). In forest ecosystems, mature trees are usually more susceptible to termite attack, but damage is also being inflicted on young seedlings (Aslam et al., 2023). The infestation usually starts at the roots and later spreads to the upper plant tissues. Termites affect tree plants either through direct feeding on under and aboveground plant tissues or indirectly, which involves increased susceptibility of damaged plants to pathogenic attack (Rust and Su, 2012; Paul et al., 2018). It is anticipated that termites cause a notable economic loss of more than US$40 billion annually worldwide (Subekti et al., 2015; Ahmad et al., 2021).
Although substantial progress has been made in devising sustainable termite management strategies that mostly include biological, cultural, botanical, and physical barriers and bait applications, their efficacy in field conditions is questioned due to the cryptic feeding behavior of termites (Potter, 2007; Iqbal and Saeed, 2013). Termite management with pesticides has become a global phenomenon, and various pesticide application strategies have been practiced in different parts of the globe (Verma et al., 2009).
The soil application of slow-action non-repellent pesticides has been recommended for managing termites, particularly their subterranean species. These pesticides mainly counter termite infestations through remedial control (Spomer et al., 2008). Remedial control usually involves the application of non-repellent/odorless slow-action pesticides so that the infested termites may not be killed immediately and may easily transfer toxicants in lethal amounts from the application site to unexposed nestmates, ultimately infecting the whole colony through social grooming and trophallaxis (Kard, 2003; Tsunoda, 2006; Bagneres et al., 2009; Quarcoo et al., 2010). The objective of using these pesticides is to suppress colony populations rather than target termite workers at the point source. Another advantage of using odorless pesticides is that the target insects are unable to differentiate between pesticide-treated and untreated soil, even if pesticides are used at higher concentrations, and continue to forage on contaminated soils (Saran and Rust, 2007; Quarcoo et al., 2019).
The toxicity of pesticides is also greatly influenced by changing temperature, but the direction and extent of the effect, either positive or negative, depend on the target insect, tested pesticide, and temperatures (Satpute et al., 2007). In field conditions, increased temperature is associated with intense tunneling, foraging, and feeding activity, which ultimately enhance the uptake and transfer of toxicants from point source to termite colony (Spomer et al., 2008). Moreover, research regarding temperature-induced variations in pesticidal toxicities becomes critical due to differences in temperature at altered soil depths and climatic seasons. Hence, studies regarding the efficacy of specific termiticides must be conducted under a varying set of ecological conditions (Spomer et al., 2008). Keeping in mind the above realities, this study was designed to check the efficacy of low doses of different pesticides such as chlorpyrifos, fipronil, bifenthrin, and chlorantraniliprole against mortality and behavioral responses of O. obesus at two different temperatures (16 ± 1 and 26 ± 1°C). Previously, information on the specific effects of varying temperatures on the toxicity of these pesticides against O. obesus was missing from the literature.
A worker caste of termites (Odontotermes obesus) was collected from standing and infested fallen trees of Dalbergia sissoo plants present in Chichawatni Reserved Forest (30.5311° N, 72.6329° E), Punjab, Pakistan. The infested plants selected for collection were not exposed to any chemical or microbial treatment for managing termites. The collected specimens were brought to the Insect Biodiversity and Biosystematics Laboratory (IBBL), Department of Entomology, University of Agriculture, Faisalabad, Pakistan, for rearing purposes. A termite colony was maintained in plastic containers (80 cm × 70 cm × 70 cm) and offered pieces of wood in a dark chamber under controlled environmental conditions (25 ± 1°C and 65–70% RH) (Aslam et al., 2023). Only the worker caste of O. obesus was selected for the bioassay because of their voracious feeding nature and potential to distribute the termiticide to untreated colony nestmates through different social behaviors.
The pesticides procured during the current trial were chlorpyrifos (40%, Kanzo AG, Evyol Group), fipronil (5%, Kanzo AG, Evyol Group), bifenthrin (20%, Kanzo AG, Evyol Group), and chlorantraniliprole (20%, FMC). A stock solution of each pesticide was prepared by diluting them in 500 ml of distilled water. The details of the serial dilutions of pesticides used are given in Table 1.
The workers of O. obesus were collected from the laboratory-maintained termite colony and carefully transferred to Petri dishes (9.1 cm internal diameter and 1.7 cm height) provided with Whatman No. 1 filter papers of the same diameter treated with each tested concentration of pesticides. However, Petri dishes containing filter papers treated with only deionized water served as untreated controls. The Petri dishes were then sealed with Parafilm® strips to avoid moisture loss and placed in a large plastic container covered with aluminum foil to maintain a dark environment. The plastic containers containing Petri dishes were subsequently placed in growth chambers (NK System Biotron, Model 03E-D3P, Japan) maintained at two different temperatures, i.e., 16 ± 1 and 26 ± 1°C with 65% RH. The treated termite workers were observed after every hour (behavioral symptoms) for the first 2 days and later after a 6-h interval until 100% mortality was achieved. The behavioral symptoms associated with pesticide exposure were video recorded. The discrete behavioral symptoms included intoxication, ataxia, and moribundity. Intoxication usually includes disorientation, horizontal oscillatory movements, and frequent changes in walking speed and direction. Termites showing ataxia symptoms showed frequent circling, frequent falling, reverse walking, drooping antennae, and often the release of proctodeal or stomodeal fluids. However, termites defined as moribund were unable to move a distance equivalent to the length of their body, remained stationary on their tarsi or dorsum, and the antennae remained bent and motionless (Quarcoo et al., 2010).
The percentage of individuals exhibiting each behavior/condition was calculated using the following formula:
The experimental setup was laid out in a completely randomized design with five replications per treatment (20 workers per replication). The mortality and behavioral responses were recorded at both tested temperatures, i.e., 16 ± 1 and 26 ± 1°C. These temperatures were selected based on soil temperature data reported by Hu and Appel (2004).
An analysis of variance (ANOVA) test was used on mortality data, and further means were separated by using Tukey's post-hoc HSD test (p ≤ 0.05). The data also analyzed a probit regression model using the likelihood computer-based program “POLO-PC” to obtain medium exposure time to kill 50% (LT50) test termites, 95% fiducial limits, slope, and chi-square values. Significant differences in LT50 were determined on a non-overlapping basis by keeping 95% confidence intervals in view. The behavioral data were subjected to SigmaPlot 12.5 to record the response of worker termites to different sublethal and low-lethal concentrations.
The tested pesticides and their concentrations differed significantly (p ≤ 0.05) regarding their LT50 values recorded against the mortality of termite workers (O. obesus). The results further showed that lethal time (LT50) values of tested pesticides gradually decreased with increased pesticidal concentrations (Tables 2, 3). Temperature also had a significant effect on the efficacy of tested pesticides as all pesticides exhibited better results at higher temperature (26 ± 1°C). The results of the current study further revealed that at both tested temperatures (16 ± 1°C and 26 ± 1°C), chlorantraniliprole (5 ppm) proved to be the most effective pesticide against termite workers with LT50 values (3.01 and 2.08 h, respectively). However, LT50 values rapidly increased from (3.01–23.14 h at 16 ± 1°C) to (2.08–13.77 h at 26 ± 1°C) when the tested pesticidal concentrations of chlorantraniliprole decreased from 5 to 0.15 ppm, respectively. Similarly, bifenthrin also proved to be more toxic at its highest concentration (5 ppm) with recorded LT50 values of 3.25 and 2.43 h at 16 ± 1°C and 26 ± 1°C, respectively. Among all the tested pesticides, chlorpyrifos was the least toxic with the highest LT50 values (Tables 2, 3).
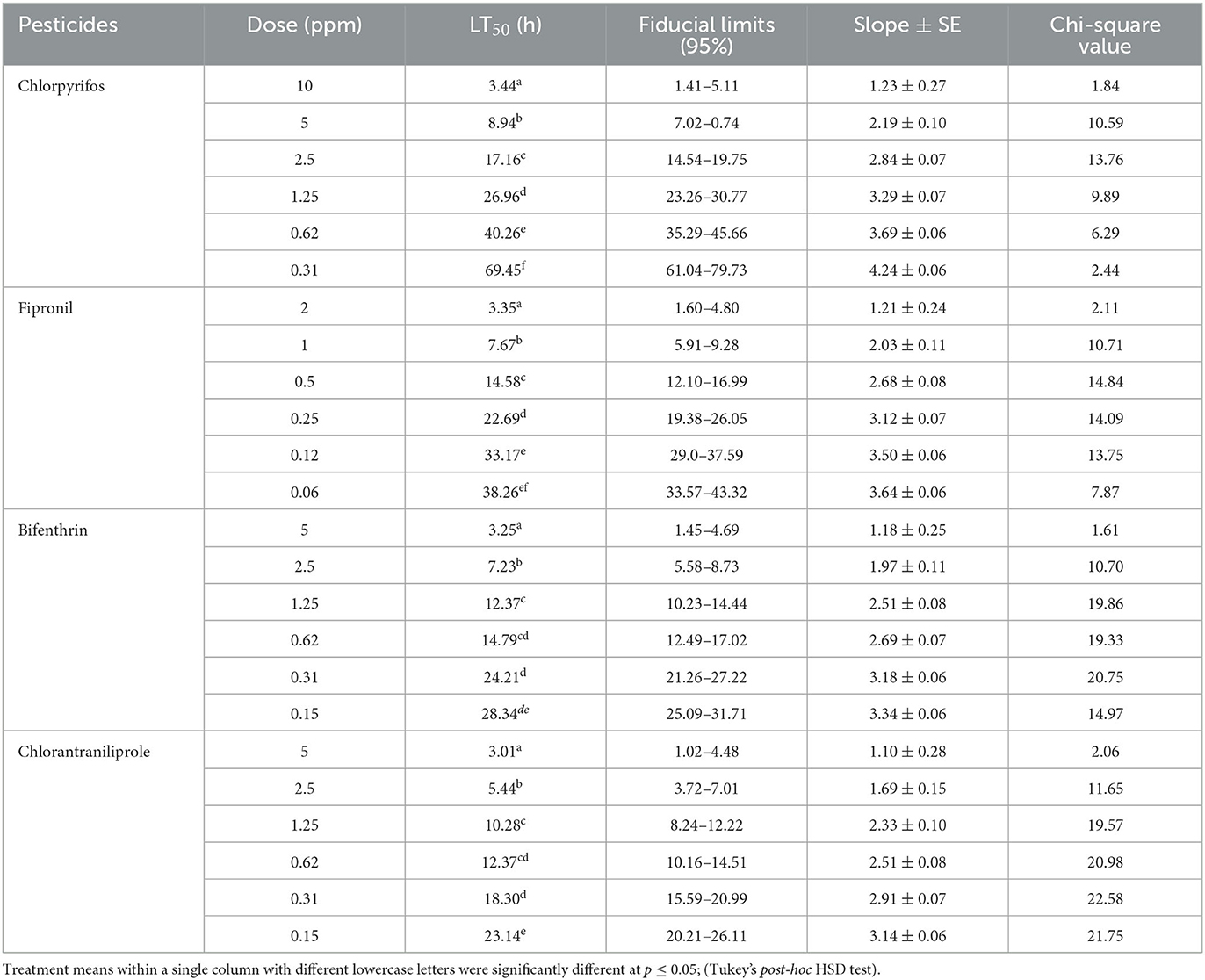
Table 2. Effect of pesticides on time-dependent mortality response of O. obesus workers at 16 ± 1°C.
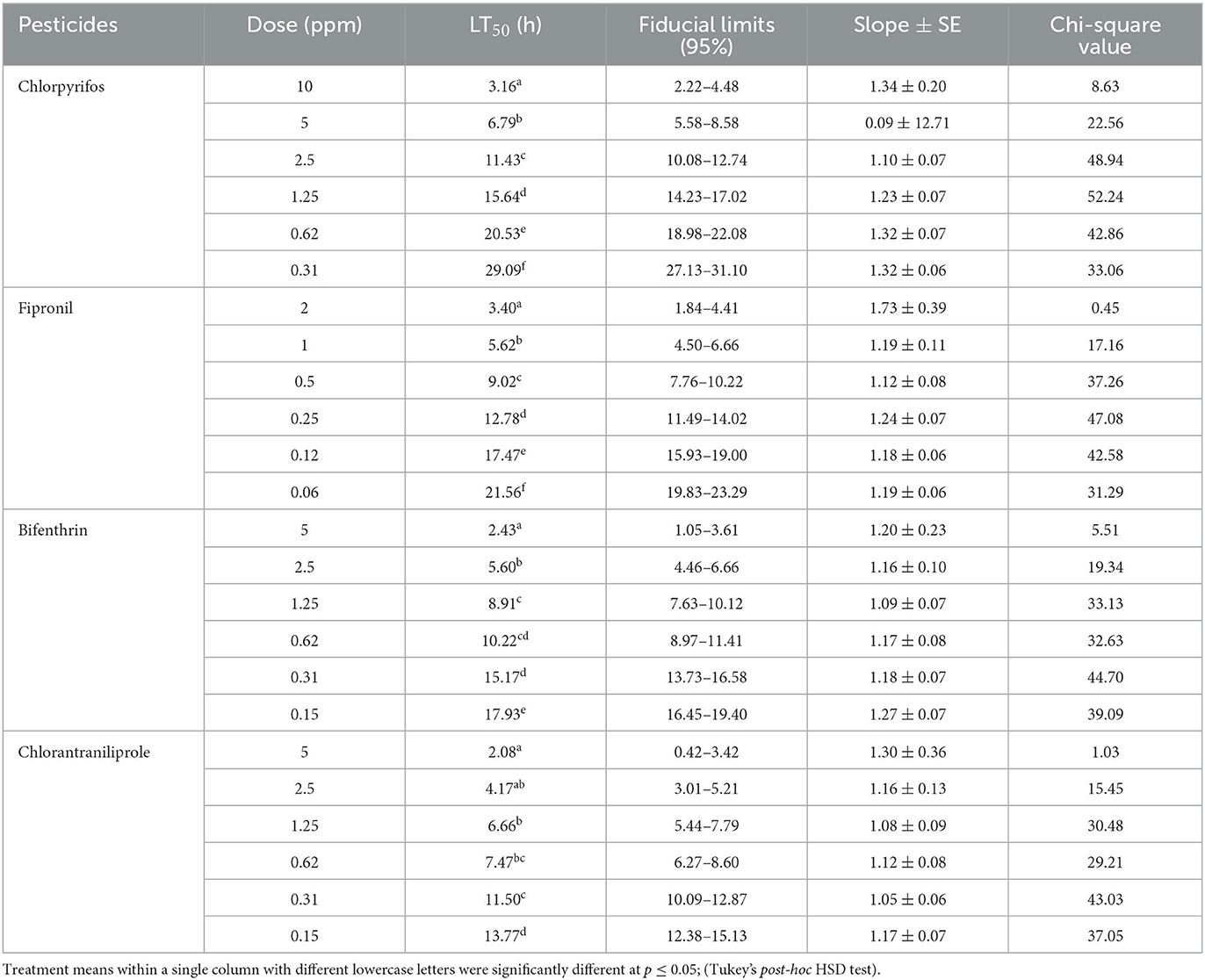
Table 3. Effect of pesticides on time-dependent mortality response of O. obesus workers at 26 ± 1°C.
The worker termites exposed to most of the pesticidal concentrations exhibited distinct behavioral responses, including intoxication, ataxia, moribundity, and death. The behavioral symptoms varied depending on the pesticides and their administered concentrations. The behavioral response was observed at lower doses of each pesticide at both temperatures (16 ± 1 and 26 ± 1°C), owing to the fact that these dose rates provided ample time for workers to exhibit behavioral symptoms before dying. Moreover, as the tested dose rates of pesticides increased, the time span to show behavioral symptoms decreased in both tested temperatures. The results further showed that every behavioral symptom of termite workers associated with pesticidal exposure existed for a relatively longer time span at lower temperature (16 ± 1°C) as compared to higher temperature (26 ± 1°C). In most cases, the order of responses was moribundity followed by intoxication and ataxia (Figures 1–4).
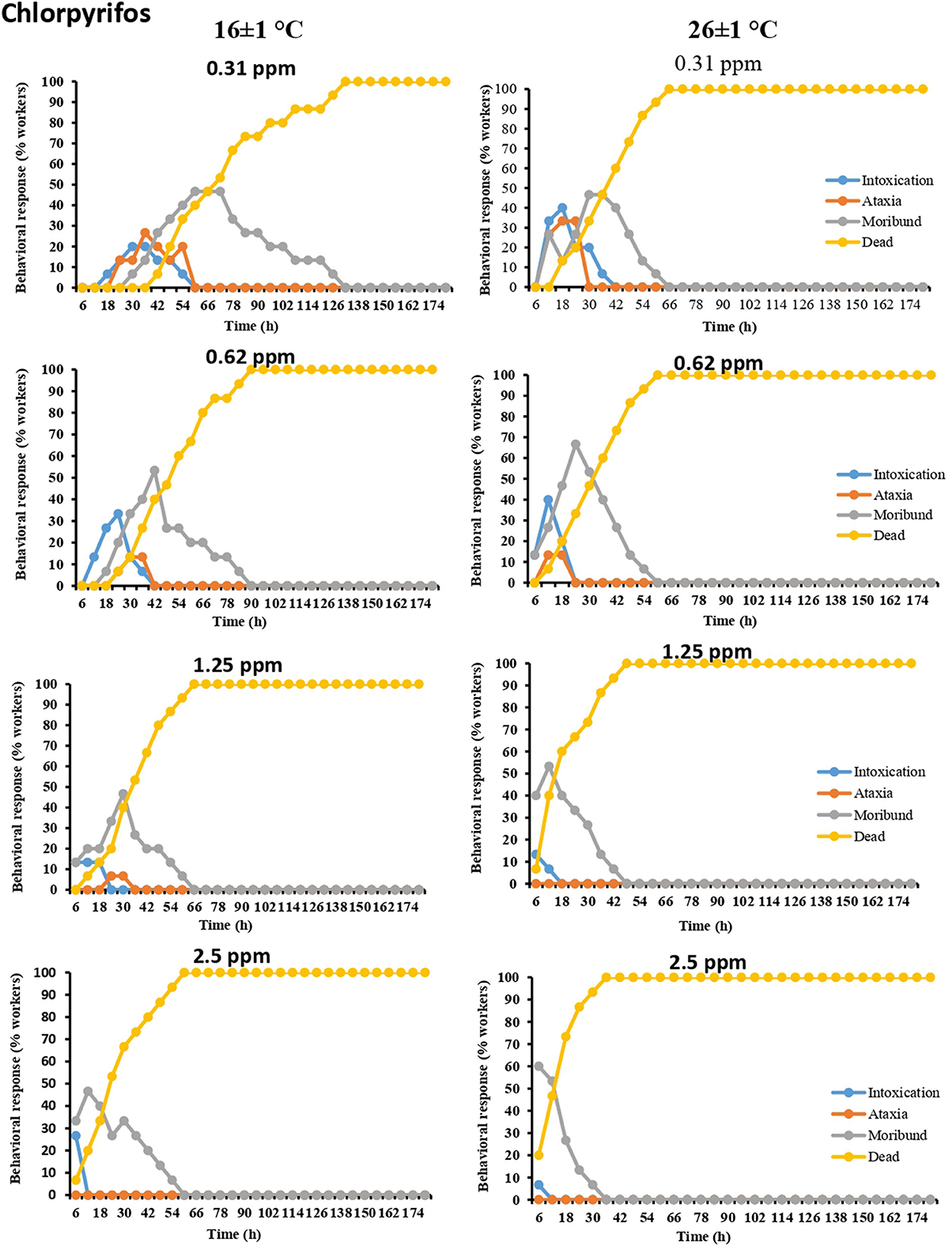
Figure 1. Time-dependent behavioral and mortality response of O. obesus exposed to different concentrations of chlorpyrifos at 16 ± 1°C and 26 ± 1°C.
Moribundity and intoxication were the most frequently observed behavioral symptoms for chlorpyriphos-treated termite workers. The maximum value of moribundity (66.66%) was recorded in the case of 0.62 ppm concentration of chlorpyriphos at 26 ± 1°C. Similarly, the maximum value of intoxication (40%) was recorded at the same temperature at two dose rates of chlorpyriphos (0.31 and 0.62 ppm) (Figure 1).
In the case of fipronil, all termite workers exposed to low concentrations of pesticide (0.06 ppm at 16 ± 1°C) exhibited intoxication symptoms (46.66% to 100%) after 6- to 24-h intervals. However, after 24 h, the number of termite workers exhibiting intoxication rapidly declined. The results further showed that intoxication followed by moribundity was more pronounced at both temperatures in the case of the two lowest doses (0.06 and 0.12 ppm) of fipronil. However, at the highest concentration (0.5 ppm) of fipronil, moribundity was more pronounced (46.66% and 73.33%) at both temperatures (16 ± 1 and 26 ± 1°C) (Figure 2).
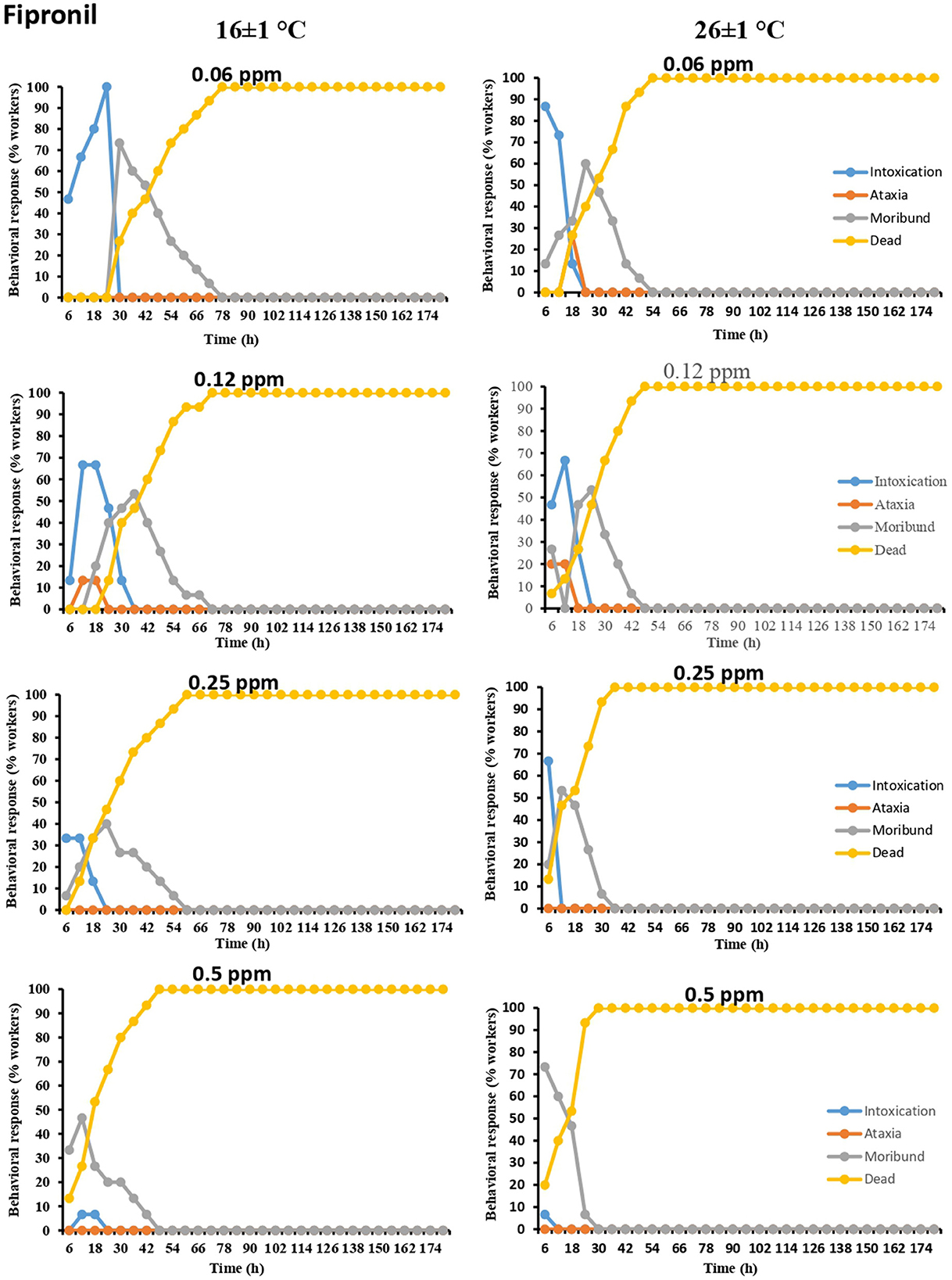
Figure 2. Time-dependent behavioral and mortality response of O. obesus exposed to different concentrations of fipronil at 16 ± 1°C and 26 ± 1°C.
Similarly, moribundity followed by intoxication was the most commonly exhibited symptom of termite workers exposed to bifenthrin at both temperatures. In the case of the lowest concentration (0.15 ppm) at lower temperature, the number of workers exhibiting moribundity ranged from 13.33 to 93.33%. However, after a 24-h interval, this percentage rapidly declined. Ataxia symptoms were only noted at the lowest concentration (0.15 ppm) of the tested pesticide at both temperatures. Moreover, 100% mortality was achieved relatively earlier at higher temperatures, thus obscuring sublethal behaviors as compared to lower temperatures (Figure 3).
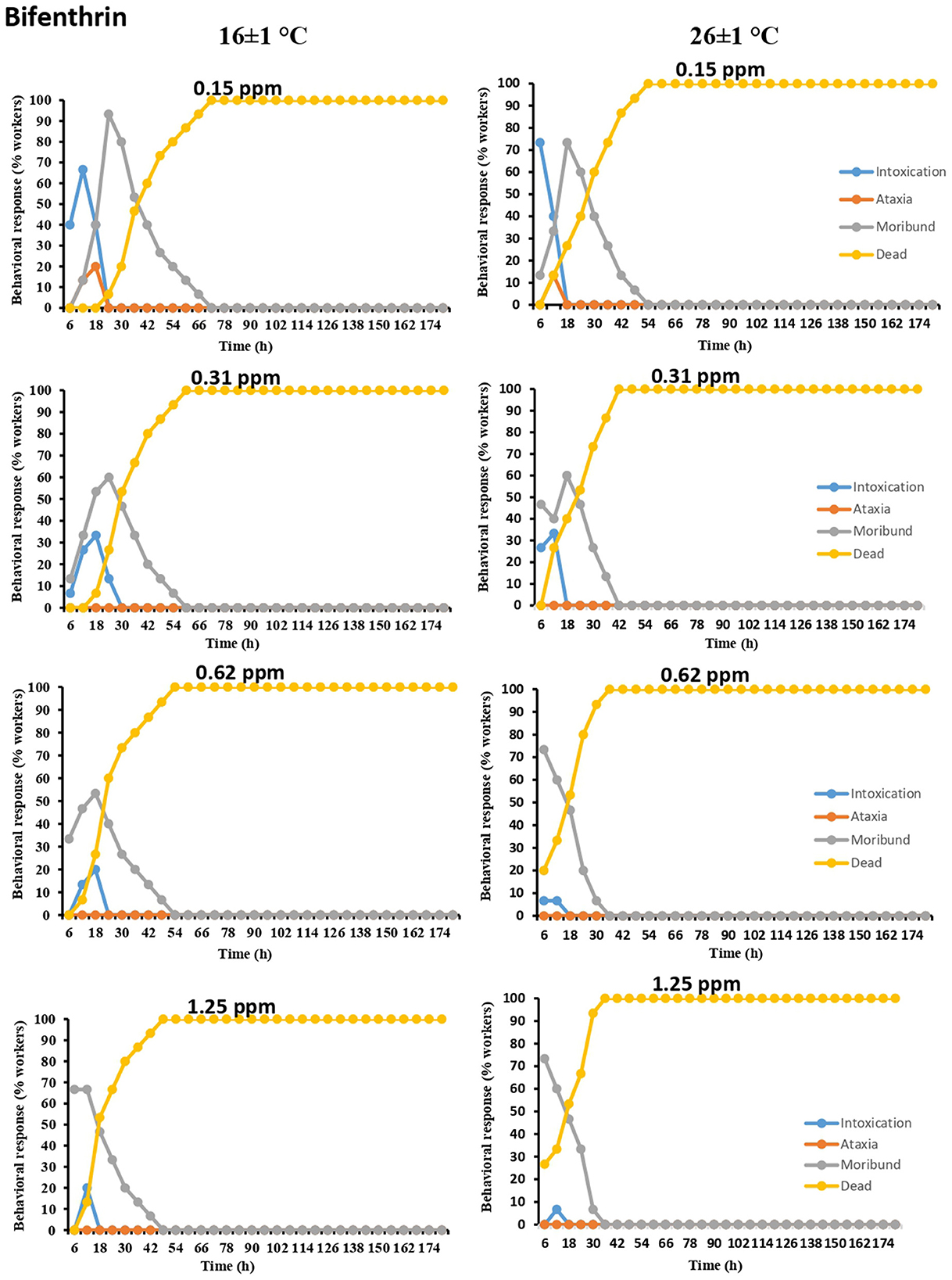
Figure 3. Time-dependent behavioral and mortality response of O. obesus exposed to different concentrations of bifenthrin at 16 ± 1°C and 26 ± 1°C.
Among all tested pesticides and their concentrations, the maximum value of ataxia (53.33%) was recorded in the case of chlorantraniliprole-exposed workers (26 ± 1°C). Similarly, intoxication symptoms were more pronounced at the lowest pesticidal dose (0.15 ppm), with mean values of 46.66% and 73.33% at 16 ± 1°C and 26 ± 1°C, respectively. Moribund symptoms lasted longer at all tested concentrations of chlorantraniliprole, followed by ataxia and intoxication (Figure 4).
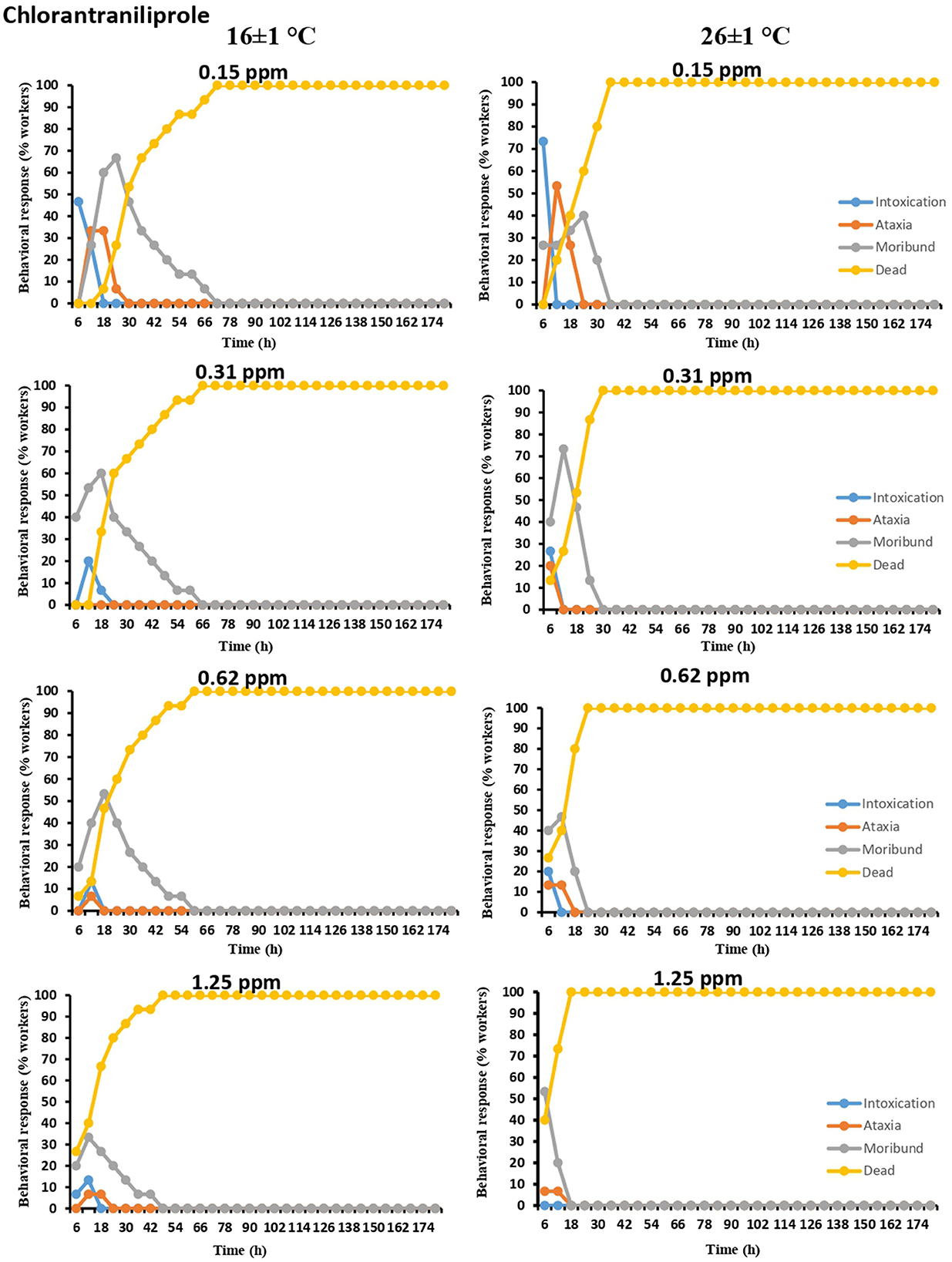
Figure 4. Time-dependent behavioral and mortality response of O. obesus exposed to different concentrations of chlorantraniliprole at 16 ± 1°C and 26 ± 1°C.
Termites are small detrimental creatures that cause severe economic losses to forests and agro-ecosystems by feeding plantations below the soil surface and destroying the internal root systems of plants by making tunnels (Vargo and Husseneder, 2009; Shelton et al., 2014). The efficacy of different integrated termite management approaches has been compromised primarily due to the cryptic feeding behavior of this pest. Effective termite management still remains a challenge for farming communities belonging to different parts of the globe (Peterson, 2010). However, the fate of termite management has changed dramatically with the advent of certain new pesticide molecules such as pyrethroids (bifenthrin), phenylpyrazole (fipronil), organophosphate (chlorpyrifos), and anthranilic diamide (chlorantraniliprole) (Davis and Kamble, 1992; Ahmed et al., 2007; Gunasekara et al., 2007; Saran et al., 2014). Termite workers fail to detect the presence of these pesticides due to their non-repellent nature and forage freely on the treated soil. During foraging, the worker termites carry lethal doses of toxicants from a point source and later transfer them to their untreated nestmates, thus affecting whole colony members (Quarcoo et al., 2010).
In the current trials, all the tested pesticides provided satisfactory control of termite workers, which was quite evident from their respective LT50 values. The results further revealed that the LT50 values of each pesticide were considerably low when used at higher dose rates. These results suggested the increased toxicity of pesticides toward termite workers at higher concentrations. The current results were in line with the findings of a number of researchers who proved the toxicity of different pesticides against different strains of termites both under laboratory and field conditions (Iqbal and Saeed, 2013; Bhagawati et al., 2014; Manzoor et al., 2014; Wang et al., 2014; Ahmed et al., 2015). Among the currently tested pesticides, the toxicity of Fipronil against termite workers had already been reported (Huang et al., 2006; Saljoqi et al., 2014; Chen et al., 2015). Similarly, Nisar et al. (2020) also proved the toxicity of bifenthrin against O. obesus. They also reported that the LT50 values of the tested pesticide decreased with increased pesticidal concentrations. In the current trials, chlorpyrifos also provided satisfactory control of termite workers, particularly at higher concentrations. The same had also been reported by Singh and Singh (2001) who used chlorpyrifos 15 G at 2.50 kg active ingredient/ha and chlorpyrifos 20 EC at 1 kg active ingredient/ha on sugarcane setts for controlling the termite infestations and got satisfactory results. Similarly, Rana and Dahiya (2001) also reported that wheat plots treated with chlorpyrifos were least affected by termite infestation.
In the current study, chlorantraniliprole provided the best results against termite workers with the least LT50 values at all concentrations compared with the rest of the tested pesticides. This was mainly attributed to its unique mode of action which involves targeting the ryanodine receptor, leading to impaired muscle regulation, paralysis, and eventually death of the targeted host (Cordova et al., 2006). This pesticide was recommended to manage termites due to its better binding potential with soil particles and minimal leaching losses (Spomer and Kamble, 2011; Wagner et al., 2011; Shelton et al., 2014). It was also reported that chlorantraniliprole (0.05%) applied to USDA Forest Service field plots provided 8 years of protection from termite infestation (Shelton et al., 2014).
All the tested pesticides used in the current study induced lethal and sublethal behavioral disorders among termite workers, and their ill effects are transmissible from poisoned termites to non-poisoned individuals in the colony, leading to substantial deaths (Haynes, 1988; Hu and Hickman, 2006). The results further revealed that these behavioral responses were more pronounced at lower pesticidal doses as compared to their higher concentrations. Previously, it had been recommended that evaluation of pesticides against termites could not be based on mortality alone, but behavioral responses must also be considered because termites could avoid or seal off treated areas and effectively protect themselves and their colony from lethal damages (Su et al., 1982). This normally happens in cases where pesticides possess repellent properties. However, in some cases, the target insects are unable to detect the pesticidal inoculum and continue to move around on the treated surface, which helps in the transfer of poison from the treated surface to unexposed colony members (Su et al., 1982; Soeprono and Rust, 2004). In the case of termites, the possible ways of transferring toxicants among nest mates might be grooming, cannibalism, necrophagy, and coprophagy (Haagsma and Rust, 2005; Neoh et al., 2012). Considering the LT50 values of pesticides in the current trial, it can be anticipated that the pesticidal inoculum can easily be transferred to naïve colony members, particularly in the case of lower pesticidal doses. The same had also been reported in a number of other studies, but the pesticide and their dose rates were different from the current trial (Valles and Woodson, 2002; Saran and Rust, 2007; Bagneres et al., 2009). However, in field trials, the donor/recipient ratios might play a crucial role in the successful transfer of pesticidal inoculum (Hu et al., 2005; Song and Hu, 2006; Spomer et al., 2008; Gautam et al., 2012). The same had also been reported by Huang and Lei (2005), who executed a study to explore the possibility of the transfer of fipronil from treated termite workers to unexposed colony members. The results of their study revealed that 15–20 donors subjected to 5 ppm pesticide and exposed to nestmates for a 6-h interval provided significant mortality in recipient workers. Moreover, Santos et al. (2004) also reported the phenomenon of social facilitation among termite workers when poisoned, which affects the median time for mortality.
In the current study, certain behavioral symptoms, such as intoxication, ataxia, and moribundity, were also observed, particularly at lower pesticidal doses at both tested temperatures. These pesticide-induced behavioral symptoms play a vital role in managing termite colonies (Su et al., 1982; Shelton, 2014). The time span related to the availability of pesticide-exposed termites to colony members was greatly affected by certain behavioral symptoms, particularly intoxication (Quarcoo et al., 2010). The behavioral symptoms, including moribundity and ataxia, associated with high pesticidal doses might hinder workers ability to move away from the treated area and affect pesticide uptake (Shelton and Grace, 2003; Saran and Rust, 2007), and they also possess the potential to transfer the pesticidal inoculum to other colony members far away from the treated area (Osbrink et al., 2005). However, sublethal behavioral symptoms, such as moribundity and ataxia, associated with exposure to pesticide-treated termites usually prompt caregiving and grooming from untreated termites and hence increase the spread of pesticidal inoculum within termite colonies through secondary and tertiary transfers (Hu et al., 2006; Quarcoo et al., 2010).
Temperature may affect the LT50 values of pesticides by altering target site interactions, distribution, metabolism, and penetration (Scott, 1995). Moreover, it can cause movement, decreased survival, rapid knockdown, and mortality in termite workers. The current study also revealed that temperature had a significant effect on the LT50 values of the tested pesticides. The LT50 values of pesticides significantly declined at higher temperatures as compared to lower temperatures. These results are in line with the findings of Smythe and Williams (1972) who conducted a laboratory bioassay and reported 90% mortality of eastern subterranean termites at 35°C. However, mortality was significantly lower (44%) at 15°C. Moreover, Quarcoo et al. (2019) also reported that LT50 values declined with increasing concentrations and temperatures for fipronil against eastern and Formosan subterranean termites. Besides instant physiological effects, temperature also influences the behavior of termite workers including their movement, foraging, tunneling, and feeding. The movements associated with food transportation and tunneling ultimately enhance the potential exposure of termite workers to non-repellent pesticides (Fei and Henderson, 2004; Cao and Su, 2014).
Chlorantraniliprole proved to be the most toxic pesticide, followed by bifenthrin, to manage termite workers, as evident from their respective LT50 values. The efficacy of each pesticide was more pronounced at high temperatures. This highlights the importance of management season, as during the summer, the temperature will be more favorable for pesticides to show their full potential. Moreover, termite workers showed significant behavioral symptoms at lower doses of pesticides, which also play a significant role in the transfer of pesticide inoculum from the point source to the rest of the colony nestmates. Termite management science has progressed greatly in the last few decades but still, the management approaches vary in different parts of the globe depending on the situation and cost incurred. Although some natural enemies of termites have been reported, reports regarding their successful use are still in their infancy. Certain botanicals have also been reported to possess anti-termite properties, but their viable application technologies, particularly in forests, are yet to be developed. Hence, no matter what termite management strategy we may adopt, chemical control still remains the backbone of termite management. Hence, more experimental work needs to be executed to work out strategies to avoid their damage in a more pragmatic way.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
AAs: Conceptualization, Formal analysis, Methodology, Writing—original draft. D–FC: Conceptualization, Project administration, Software, Supervision, Writing—original draft. AAb: Conceptualization, Methodology, Writing—original draft. MAr: Conceptualization, Data curation, Formal analysis, Investigation, Writing—review and editing. FH: Data curation, Formal analysis, Investigation, Software, Writing—review and editing. AF: Writing—review and editing. AH: Funding acquisition, Investigation, Resources, Writing—review and editing. MAb: Data curation, Formal analysis, Funding acquisition, Resources, Software, Writing—original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The current work was supported by Researchers Supporting Project Number (RSP2023R316) King Saud University, Riyadh, Saudi Arabia.
The authors extend their appreciation to the Researchers Supporting Project Number (RSP2023R316) King Saud University, Riyadh, Saudi Arabia.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, F., Fouad, H., Liang, S., Hu, Y., and Mo, J. (2021). Termites and Chinese agricultural system: applications and advances in integrated termite management and chemical control. Insect Sci. 28, 2–20. doi: 10.1111/1744-7917.12726
Ahmed, M. A. I., Eraky, E. S. A., Mohamed, M. F., and Soliman, A. A. S. (2015). Potential toxicity assessment of novel selected pesticides against sand termite, Psammotermes hypostoma (Desneux workers) (Isoptera: Rhinotermitidae) under field conditions in Egypt. J. Plant Prot. Res. 55, 193–197. doi: 10.1515/jppr-2015-0026
Ahmed, S., Riaz, M. A., and Hussain, A. (2007). Assessment of the damaged and population of termites (Odontotermes and Unicolor) under various methods of insecticide application. Int. J. Agric. Biol. 9, 125–128. Available online at: https://www.fspublishers.org/published_papers/18809_..pdf
Aslam, A., Chi, D.-F., Abbasi, A., and Arshad, M. (2023). Biocontrol potential of entomopathogenic nematodes against Odontotermes obesus (Blattodea: Termitidae) under laboratory and field conditions. Forests 14, 580. doi: 10.3390/f14030580
Bagneres, A. G., Pichon, A., Hope, J., and Davis, R. W., and Cle'ment, J. L. (2009). Contact versus feeding intoxication by fipronil in Reticulitermes termites (Isoptera: Rhinotermitidae): laboratory evaluation of toxicity, uptake, clearance, and transfer among individuals. J. Econ. Entomol. 102, 347–356. doi: 10.1603/029.102.0145
Balla, A., Silini, A., Cherif-Silini, H., Bouket, A. C., Moser, W. K., Nowakowska, J. A., et al. (2021). The threat of pests and pathogens and the potential for biological control in forest ecosystems. Forests 12, 1579. doi: 10.3390/f12111579
Bhagawati, S., Bhattacharyya, B., Mishra, H., and Gogoi, D. (2014). Chemical management of termites (Odontotermes obesus) in preserved setts of sugarcane (Saccharum officinarum). J. Entomol. Zool. Stud. 5, 856–859.
Cao, R., and Su, N. Y. (2014). Tunneling and food transportation activity of four subterranean termite species (Isoptera: Rhinotermitidae) at various temperatures. Ann. Entomol. Soc. Am. 107, 696–701. doi: 10.1603/AN13181
Chen, Z., Qu, Y. Y., Xiao, D., Song, L. F., Zhang, S. H., Gao, X. W., et al. (2015). Lethal and social-mediated effects of ten insecticides on the subterranean termite Reticulitermes speratus. J. Pest Sci. 88, 741–751. doi: 10.1007/s10340-015-0656-0
Cordova, D., Benner, E. A., Sacher, M. D., Rauh, J. J., Sopa, J. S., Lahm, G. P., et al. (2006). Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pesticide Biochem. Physiol. 84, 196–214. doi: 10.1016/j.pestbp.2005.07.005
Davis, R. W., and Kamble, S. T. (1992). Distribution of subslab injected Dursban TC (chlorpyrifos) in a loamy sand soil when used for subterranean termite control. Bull. Environ. Contam. Toxicol. 48, 585–591. doi: 10.1007/BF00199078
Fei, H., and Henderson, G. (2004). Effects of temperature, directional aspects, light conditions and termite species on subterranean termite activity (Isoptera: Rhinotermitidae). Environ. Entomol. 33, 242–248. doi: 10.1603/0046-225X-33.2.242
Gautam, B. K., Henderson, G., and Davis, R. W. (2012). Toxicity and horizontal transfer of 0.5 % fipronil dust against Formosan subterranean termites. J. Econ. Entomol. 105, 1766–1772. doi: 10.1603/EC12165
Gunasekara, A. S., Truong, T., Goh, K. S., Spurlock, F., and Tjeerdema, R. S. (2007). Environmental fate and toxicology of fipronil. J. Pesticide Sci. 32, 189–199. doi: 10.1584/jpestics.R07-02
Haagsma, K. A., and Rust, M. K. (2005). Effect of hexaflumuron on mortality of the western subterranean termite (Isoptera: Rhinotermitidae) during and following exposure and movement of hexaflumuron in termite groups. Pest Manag. Sci. 61, 517–531. doi: 10.1002/ps.1003
Haynes, K. F. (1988). Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 33, 149–168. doi: 10.1146/annurev.en.33.010188.001053
Hu, X. P., and Appel, A. G. (2004). Seasonal variation of critical thermal limits and temperature tolerance in two subterranean termites (Isoptera: Rhinotermitidae). Environ. Entomol. 33, 197–205. doi: 10.1603/0046-225X-33.2.197
Hu, X. P., and Hickman, B. (2006). Exterior perimeter plus limited interior treatments with fipronil as an IPM option for subterranean termite management. Int. Pest Contr. 48, 200–203.
Hu, X. P., Song, D., and Presley, W. (2006). “Horizontal and vertical transfer of fipronil within functional colonies,” in Proceedings of the 2006 National Conference on Urban Entomology, Raleigh, NC, 39–44.
Hu, X. P., Song, D. L., and Scherer, C. W. (2005). Transfer of indoxacarb among workers of Coptotermes formosanus (Isoptera: Rhinotermitidae): effects of dose, donor: recipient ratio and post-exposure time. Pest Manag. Sci. 61, 1209–1214. doi: 10.1002/ps.1124
Huang, Q. Y., and Lei, C. L. (2005). Transfer of fipronil from exposed workers of the subterranean termite Odontotermes formosanus (Isoptera: Termitidae) to unexposed nestmates. Sociobiology 46, 385–395.
Huang, Q. Y., Lei, C. L., and Xue, D. (2006). Field evaluation of a fipronil bait against subterranean termite Odontotermes formosanus (Isoptera: Termitidae). J. of Econ. Entomol. 99, 455–461. doi: 10.1093/jee/99.2.455
Iqbal, N., and Saeed, S. (2013). Toxicity of six new chemical insecticides against the termite, Microtermes mycophagus D. (Isoptera: Termitidae: Macrotermitinae). Pak. J. Zool. 45, 709–713. Available online at: http://zsp.com.pk/pdf45/709-713%20_18_%20PJZ-1258-13%2022-4-13%20Revised%20Manuscript%20of%20Page%20Proof.pdf
Kard, B. M. (2003). Integrated pest management of subterranean termites (Isoptera). J. Entomol. Sci. 38, 200–224. doi: 10.18474/0749-8004-38.2.200
Keenan, R. J., Reams, G. A., Achard, F., Freitas, d. e., and Grainger, J. V. A., and Lindquist, E. (2015). Dynamics of global forest area: Results from the FAO global forest resources assessment 2015. Forest Ecol. Manag. 352, 9–20. doi: 10.1016/j.foreco.2015.06.014
Klapwijk, M. J., and Björkman, C. (2018). Mixed forests to mitigate risk of insect outbreaks. Forestry 33, 772–780. doi: 10.1080/02827581.2018.1502805
Lin, Y., Fang, D., and Wang, L. (2015). Termites and microbial biological control strategies. South Asia J. Multidiscip. Stud. 1, 33–62.
Manzoor, F., Chaudhary, M., Sheikh, N., Khan, I. A., and Khan, T. (2011). Diversity and proportion of termite species in garden trees and wheat crop in District Bhakkar, Pakistan. Pak. J. Zool. 43, 537541. Available online at: https://www.zsp.com.pk/pdf/537-541%20(16)%20PJZ-456-10.doc
Manzoor, F., Saleem, S., and Abbas, M. (2014). Laboratory evaluation of imidacloprid against Microtermes obesi (Holmgren) (Isoptera: Macrotermitinae). Proc. Pak. Acad. Sci. 51, 43–48.
Neoh, K. B., Hu, J., Yeoh, B. H., and Lee, C. Y. (2012). Toxicity and horizontal transfer of chlorantraniliprole against the Asian subterranean termite Coptotermes gestroi (Wasmann): effects of donor: recipient ratio, exposure duration and soil type. Pest Manag. Sci. 68, 749–756. doi: 10.1002/ps.2322
Nisar, M. S., Bashir, M. A., Naz, H., and Ahmed, S. (2020). Comparative effect of termiticides and plant extracts on mortality and tunnel formation of Odontotermes obesus. Pure Appl. Biol. 9, 1903–1910. doi: 10.19045/bspab.2020.90203
Osbrink, W. L. A., Cornelius, M. L., and Lax, A. R. (2005). Effect of imidacloprid soil treatments on the occurrence of Formosan subterranean termites, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae), in independent monitors. J. Econ. Entomol. 98, 2160–2168. doi: 10.1093/jee/98.6.2160
Paul, B., Khan, M. A., Paul, S., Shankarganesh, K., and Chakravorty, S. (2018). Termites and Indian Agriculture. Termites and Sustainable Management. Cham: Springer, 51–96.
Peterson, C. (2010). Considerations of soil-applied insecticides for termite control. Outlooks Pest Manag. 21, 89–93. doi: 10.1564/21apr09
Prospero, S., Botella, L., Santini, A., and Robin, C. (2021). Biological control of emerging forest diseases: How can we move from dreams to reality? Forest Ecol. Manag. 496, 119377. doi: 10.1016/j.foreco.2021.119377
Quarcoo, F. Y., Appel, A. G., and Hu, X. P. (2010). Effects of indoxacarb concentration and exposure time on the onset of abnormal behaviors and death in the eastern subterranean termite. J. Econ. Entomol. 103, 762–769. doi: 10.1603/EC09345
Quarcoo, F. Y., Hu, X. P., and Appel, A. G. (2019). Temperature-mediated variations in behavior and mortality caused by non-repellent insecticides in subterranean termites (Blattodea: Rhinotermitidae). Insects 10, 37. doi: 10.3390/insects10020037
Rana, J. S., and Dahiya, K. K. (2001). Management of termite, Microtermes obesi (Holmgren) in wheat, Triticum aestivum through seed treatment. Ann. Biol. 17, 207–209.
Rathour, K. S., Sudershan, G., Das, T., Pargat, S., Anjani, K., Somvanshi, V. S., et al. (2014). Biological management of subterranean termites (Odontotermes obesus) infesting wheat and pearl millet crops by entomopathogenic nematodes. Ind. J. Nematol. 44, 97–100.
Ravan, S., Khan, I. A., Manzoor, F., and Khan, Z. (2015). Feeding habitats and wood preferences of termites in Iran. J. Entomol. Zool. Stud. 3, 20–23.
Rust, M. K., and Su, N. Y. (2012). Managing social insects of urban importance. Annu. Rev. Entomol. 57, 355–375. doi: 10.1146/annurev-ento-120710-100634
Saljoqi, A. U. R., Muhammad, N., Khan, I. A., Nadeem, M., and Salim, M. (2014). Effect of different insecticides against termites, Heterotermes indicola L. (Isoptera: Termitidae) as slow acting toxicants. Sarhad J. Agric. 30, 333–339.
Santos, C. A., DeSouza, O., and Guedes, R. N. C. (2004). Social facilitation attenuating insecticide driven stress in termites (Isoptera: Nasutitermitinae). Sociobiology 44, 1–7.
Saran, R. K., and Rust, M. K. (2007). Toxicity, uptake, and transfer efficiency of fipronil in western subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 100, 495–508. doi: 10.1093/jee/100.2.495
Saran, R. K., Ziegler, M., Kudlie, S., Harrison, D., Leva, D. M., Scherer, C., et al. (2014). Behavioral effects and tunneling responses of eastern subterranean termites (Isoptera: Rhinotermitidae) exposed to chlorantraniliprole-treated soils. J. Econ. Entomol. 107, 1878–1889. doi: 10.1603/EC11393
Satpute, N. S., Deshmukh, S. D., Rao, N. G. V., Tikar, S. N., Moharil, M. P., Nimbalkar, S. A., et al. (2007). Temperature-dependent variation in toxicity of insecticides against Earias vitella (Lepidoptera: Noctuidae). J. Econ. Entomol. 100, 357–360. doi: 10.1603/0022-0493(2007)100(357:TVITOI)2.0.CO;2
Scott, J. G. (1995). Effects of temperature on insecticides toxicity. Rev. Pesticide Toxicol. 3, 111–135.
Shelton, T. G. (2014). Distance of the repellency of dead Reticulitermes flavipes (Isoptera: Rhinotermitidae) nestmates. J. Entomol. Sci. 49, 221–227. doi: 10.18474/0749-8004-49.3.221
Shelton, T. G., Fye, D., and Ulyshen, M. (2014). U.S. Department of Agriculture-Forest Service Termiticide Report for 2013. Pest Manag. Profess. 82, 42–52.
Shelton, T. G., and Grace, J. K. (2003). Effects of exposure duration on transfer of nonrepellent termiticides among workers of Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). J. Econ. Entomol. 96, 456–460. doi: 10.1603/0022-0493-96.2.456
Singh, M., and Singh, N. B. (2001). Application of insecticide for termite control and its effect on yield contributing characters in sugarcane. Sugar Tech. 3, 146–153. doi: 10.1007/BF02956807
Smythe, R. V., and Williams, L. H. (1972). Feeding and survival of two subterranean termite species at constant temperatures. Ann. Entomol. Soc. Am. 65, 226–229. doi: 10.1093/aesa/65.1.226
Soeprono, A. M., and Rust, M. K. (2004). Effect of horizontal transfer of barrier insecticides to control Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 97, 1675–1681. doi: 10.1603/0022-0493-97.5.1675
Song, D. L., and Hu, X. P. (2006). Effects of dose, donor-recipient interaction time and ratio on fipronil transmission among the Formosan subterranean termite nestmates (Isoptera: Rhinotermitidae). Sociobiol. 48, 237–246.
Spomer, N. A., and Kamble, S. T. (2011). Temporal changes in chlorantraniliprole and indoxacarb in four midwestern soils and bioefficacy against the eastern subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 104, 990–1001. doi: 10.1603/EC10371
Spomer, N. A., Kamble, S. T., Warriner, R. A., and Davis, R. W. (2008). Influence of temperature on rate of uptake and subsequent horizontal transfer of [14C] fipronil by eastern subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 101, 902–908. doi: 10.1093/jee/101.3.902
Su, N. Y., Tamashiro, M., Yates, J. R., and Haverty, H. I. (1982). Effect of behaviour on the evaluation of insecticides for prevention of or remedial control of the formosan subterranean termite. J. Econ. Entomol. 75, 188–193. doi: 10.1093/jee/75.2.188
Subekti, N., Yoshimura, T., Rokhman, F., and Mastur, Z. (2015). Potential for subterranean termite attack against five bamboo speciesin correlation with chemical components. Proc. Environ. Sci. 28, 783–788. doi: 10.1016/j.proenv.2015.07.092
Tsunoda, K. (2006). Transfer of fipronil, a nonrepellent termiticide, from exposed workers of Coptotermes formosanus (Isoptera: Rhinotermitidae) to unexposed workers. Sociobiol. 47, 563–575.
Valles, S. M., and Woodson, W. D. (2002). Group effects on insecticide toxicity in workers of the Formosan subterranean termite, Coptotermes formosanus Shiraki. Pest Manag. Sci. 58, 769–774. doi: 10.1002/ps.528
Vargo, E. L., and Husseneder, C. (2009). Biology of subterranean termite: Insights from molecular studies of Reticulitermes and Coptotermes. Annu. Rev. Entomol. 54, 379–403. doi: 10.1146/annurev.ento.54.110807.090443
Verma, M., Sharma, S., and Prasad, R. (2009). Biological alternative for termite control: a review. Int. Biodeter. Biodegrad. 63, 959–972. doi: 10.1016/j.ibiod.2009.05.009
Wagner, T., Peterson, C., and Shelton, T. (2011). Termiticide efficacy reports 2010. Pest Manag. Profess. 2011, 28–34.
Wang, C., Henderson, G., Gautam, B. K., and Chen, X. (2014). Lethal and sublethal effects of lufenuron on the Formosan subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 107, 1573–1581. doi: 10.1603/EC14103
Zanuncio, J. C., Lemes, P. G., Antunes, L. R., Maia, J. L. S., Mendes, J. E. P. M., Tanganelli, K. M., et al. (2016). The impact of the Forest Stewardship Council (FSC) pesticide policy on the management of leaf-cutting ants and termites in certified forests in Brazil. Ann. Forest Sci. 73, 205–214. doi: 10.1007/s13595-016-0548-3
Keywords: behavioral symptoms, mortality, Odontotermes obesus, pesticides, temperature
Citation: Aslam A, Chi D-F, Abbasi A, Arshad M, Hafeez F, Fayyaz A, Hatamleh AA and Abdullah Al-Dosary M (2023) Time-dependent mortality and behavioral response of Odontotermes obesus (Blattodea: Termitidae) against different dose rates of pesticides for sustainable forest management. Front. For. Glob. Change 6:1257418. doi: 10.3389/ffgc.2023.1257418
Received: 12 July 2023; Accepted: 28 August 2023;
Published: 21 September 2023.
Edited by:
Muhammad Rafay, Islamia University of Bahawalpur, PakistanReviewed by:
Bilkess Salim, Sabha University, LibyaCopyright © 2023 Aslam, Chi, Abbasi, Arshad, Hafeez, Fayyaz, Hatamleh and Abdullah Al-Dosary. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Fu Chi, Y2hpZGVmdTYyMDkyOEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.