
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change, 25 August 2023
Sec. Pests, Pathogens and Invasions
Volume 6 - 2023 | https://doi.org/10.3389/ffgc.2023.1253022
This article is part of the Research TopicFoliar, Shoot, Stem and Rust Diseases of Trees IUFRO 2022View all 11 articles
 Alessandra Benigno1*
Alessandra Benigno1* Carlo Bregant2
Carlo Bregant2 Chiara Aglietti1
Chiara Aglietti1 Giovanni Rossetto2
Giovanni Rossetto2 Beatrice Tolio2,3,4
Beatrice Tolio2,3,4 Salvatore Moricca1*
Salvatore Moricca1* Benedetto T. Linaldeddu2
Benedetto T. Linaldeddu2Environmental changes are occurring on a global scale, but their effects are most pronounced in climate change hotspot zones, such as the Mediterranean basin. Within this area Italy, extending from its southern coasts in the core of the Mediterranean Sea to its northernmost pre-Alpine and Alpine regions, is characterized by a variety of climatic conditions and vegetation types. Surveys conducted in 2018–2022 in forest formations of Central-Northern Italy revealed that the enhanced warming trend and irregular distribution of precipitations are strongly impacting the health of Fraxinus species, with some pathogenic fungi and oomycetes being important contributing factors to the decline of the three main ash species growing there: common ash (Fraxinus excelsior), flowering ash (Fraxinus ornus), and narrow-leaved ash (Fraxinus angustifolia). Isolation from symptomatic plant material collected countrywide under different site conditions and pathogenicity tests revealed a complex phytopathological framework, with several pathogenic species in addition to Hymenoscyphus fraxineus involved with a prominent role in the ash dieback etiology. Key microbial taxa included the fungal and oomycete pathogens Botryosphaeria dothidea, Diplodia fraxini, Diplodia subglobosa, Phytophthora acerina, and Phytophthora plurivora. The disease impact was higher on sites where ash trees grew under environmental stress (i.e., areas characterized by mild dry winters, hot summers with intense and prolonged drought) and exhibited reduced vigor, also as a consequence of anthropogenic interference (i.e., silvicultural management and fires). The identified causative agents are emerging pathogens that thrive under warmer conditions, their impact in the investigated areas being prevalent compared to H. fraxineus, which appears to be restricted on the Italian peninsula to the cooler and wetter valleys of the Alps and Central-Northern Apennines.
The Mediterranean basin lies in a transition zone between the semi-arid climate of North Africa and the temperate and rainy conditions of central Europe, affected by interactions between temperate and tropical processes (Giorgi and Lionello, 2008). Due to their particularly favorable geographical and climatic features, Mediterranean regions are characterized by an enormous floral and faunistic diversity, recognized as the second most important biodiversity hotspot on the planet (Myers et al., 2000). The vastness and heterogeneity of this large geographical area allows the survival of over 25000 species, distributed in countless habitats, ranging from the coastal areas, islands and typical low-altitude formations to the closely subalpine and alpine regions; this high biodiversity is mostly linked to intense processes of speciation and extinction during the Quaternary age (Cowling et al., 1996; Myers et al., 2000).
Despite their relative integrity, Mediterranean forests show a high fragility and vulnerability to several natural and human-induced threats such as fires, pests and pathogens, habitat destruction and deforestation (Linaldeddu et al., 2014; Nunes et al., 2022). Climate change has also threatened the survival of these habitats in recent decades; due to its features and geographic position, the Mediterranean basin is considered one of the most prominent climatic hotspots on the planet, representing one of the areas more vulnerable to the impact of climate change in the future (Giorgi, 2006; Giorgi and Lionello, 2008).
Some authors have investigated the profound changes taking place in these regions, correlating them directly to the global climate change; the main factor is a much higher increase in temperatures than the rest of the planet (Ulbrich et al., 2012; Lionello and Scarascia, 2018). In addition, an irregular distribution of the rainfall regime characterizes the Mediterranean area, with increasing and anomalous episodes of drought alternating with extreme events and brief very rainy periods (Valdes-Abellan et al., 2017).
In this scenario of radical change for natural environments, trees are often under conditions of accentuated stress, exposing them more to diseases and pests and posing the potential for forest decline phenomena. These involve a complex group of abiotic and biotic elements and contributors to the losses in tree health and increasing mortality (Manion, 1981; Brasier et al., 1993). During the last three decades, extensive dieback and mortality phenomena have been affecting many European forests, with a greater incidence in the Mediterranean basin (Scanu et al., 2015; Bregant et al., 2020, 2023). One of the most significant examples characterizing the Mediterranean areas with particular incidence is certainly oak decline; recently, many studies have investigated the causes of these widespread phenomena, confirming the direct correlation between climate change as a predisposing factor and pathogens as primary cause of death (Moricca and Ragazzi, 2008; Moricca et al., 2016).
Unlike vast forests involved in decline phenomena, like oak-dominated forests, less widespread but ecologically important Fraxinus formations have received relatively little attention. However, in various regions of the Mediterranean in recent years there has been a progressive decline and dieback of the three species of the genus Fraxinus that are the main representatives of the genus, namely common ash (Fraxinus excelsior L.), flowering ash (Fraxinus ornus L.), and narrow-leaved ash (Fraxinus angustifolia Vahl.). The damage was particularly serious in some areas, where high mortality, especially in young seedlings, caused a strong limitation to natural regeneration. The attacked trees exhibited a variety of symptoms, the most typical being: sunken cankers on the stem and branches, with a characteristic wedge-shaped necrotic sector in cross section; leaf and shoot blight, resulting in a progressive dieback of the canopy; production of tarry exudates on the lower stem; root and collar rot; in response to the bark and root damages, the canopy evidenced non-specific symptoms of progressive or sudden decline (Orlikowski et al., 2011; Linaldeddu et al., 2020a; Peters et al., 2023).
All these variable symptoms represent a complex syndrome that substantially differ in its etiology and pattern from the simple pathosystem model ash dieback–Hymenoscyphus fraxineus. Regarding this latter fungus, it has being expanding since 2009 in various Italian regions starting from the North-Eastern Alps to some areas in the center of the country along the Apennines (Ogris et al., 2010; Luchi et al., 2016; Migliorini et al., 2022). The disease involves all the three above-named ash species, with particular incidence on common ash (Fraxinus excelsior) (Panconesi et al., 2014; Rigling et al., 2018). This helotiaceous fungus prefers the cold and humid valleys of the mountain areas of North Italy and North-Central Apennines, its current southern range being some scattered sites in mountain areas with Mediterranean climatic conditions, characterized by cold and snowy winters and cool summers with absence of drought (Migliorini et al., 2022). However, it is unlikely that it will succeed in expanding southward, being limited by the unfavorable conditions of the Mediterranean climate, characterized by mild-dry winters and hot summers with prolonged droughts even in mountainous areas.
This different and more complex phytopathological framework of ash decline in the Mediterranean region emerged in some recent studies and observations in Italy (Benigno et al., 2019; Linaldeddu et al., 2020a) and, paralleled by similar findings in Slovenia, (Linaldeddu et al., 2022), prompted the present investigation, aimed at clarifying the possible role of the new causal agents involved. There is compelling evidence that the rapid changing of climatic conditions occurring in the Mediterranean region is altering the ecology, biogeography and above all infection biology of plant pathogens, creating conditions conducive to new disease emergence and spread (Dukes et al., 2009; Sturrock et al., 2011). These changes markedly alter the relationship between pathogens and their hosts (Sturrock et al., 2011). Some groups of pathogens in particular, like some members of the Botryosphaeriaceae family, seem to gain advantage from and thrive under warmer conditions, spreading pervasively over new hosts and areas (Hansen, 2008; Rehfeldt et al., 2009; Venette, 2009). Furthermore, the increasing temperature and altered precipitation regimes also affect the physiology of trees while, at the same time, drought conditions may compromise the fine roots, making trees more susceptible to water stress and attack by root oomycete pathogens (Ginetti et al., 2014; Haavik et al., 2015; Moricca et al., 2016; Colangelo et al., 2018).
In this study, we present new insight into the infection and aggressive colonization of Fraxinus species by several emerging pathogens in Central-Northern Italy, with identification of the fungal (endophytic and canker-associated Botryosphaeriaceae) and oomycete (Phytophthora) species involved, proof of pathogenicity, and elucidation of the key role of some of these pathogens in the dieback of ash species in the investigated areas.
Investigations were conducted in 40 ash formations distributed from the plains to the mountainous areas in four regions of Central and North-eastern Italy: Toscana, Emilia Romagna, Veneto, and Friuli Venezia Giulia. Survey areas involved the natural ecological range of all three Italian spontaneous ash species: Fraxinus angustifolia, F. excelsior, and F. ornus (Supplementary Table 1). Study sites ranged from 0 to 1424 m. a.s.l, covering the entire altitude range for Fraxinus spp. in these regions and including the natural reserve of the lowland forest Boscone della Mesola (site 40). Forest sites were characterized by very different meteorological and climate conditions (Supplementary Table 1).
From spring 2018 to summer 2022 trees in each site were visually checked for the presence of disease symptoms on canopy (shoot blight, branch canker, bleeding canker), collar and roots (bark necrosis, exudates and root rot). A roughly 50 m long transect was established to evaluate disease incidence and mortality rate, estimated as the number of symptomatic individuals out of the total number of trees (DI = n/N × 100) and the number of dead trees out of the total number of trees (M = d/N × 100), respectively (Moricca et al., 2012a; Linaldeddu et al., 2020a). An amount of 362 samples representative of all symptoms observed on roots including rhizosphere (R = 75 samples), at the collar (TC = 21) and on main stem and branches (C = 262) (Supplementary Table 1). The highest number of samples was collected from flowering ash (211), followed by common ash (116 samples) and narrow-leaved ash (35).
All branch, stem and collar samples were taken to the laboratory to be visually examined and the outer bark surface was initially disinfected with 90% ethanol and then removed with a sterile scalpel. Isolations were performed from about 5 mm2 fragments of inner bark and xylem cut aseptically from the margin of necrotic lesions (Panzavolta et al., 2018; Linaldeddu et al., 2020a). All fragments were placed on 90 mm Petri dishes containing potato dextrose agar (PDA, Oxoid Ltd., UK). After incubation at 25°C for 5–7 days in the dark, hyphal tips from the margin of emerging fungal colonies were sub-cultured onto half-strength PDA and incubated at room temperature under natural daylight to enhance sporulation.
Isolation of root rot agents was performed as reported by Bregant et al. (2020). In the laboratory root and rhizosphere samples were placed in a plastic box and flooded with 2 L of distilled water. After 24 h, young cork oak and elder leaves were placed on the water surface and used as baits. Boxes were kept at 20°C under natural daylight and after 5 days, leaves showing necrotic lesions were cut in small pieces (2–3 mm2) and placed on 90 mm Petri dishes containing PDA supplemented with 100 ml/L of carrot juice, 0.015 g/L of pimaricin and 0.05 g L–1 of hymexazol (PDA +) (Linaldeddu et al., 2020b). Isolation of root rot agents was also directly attempted from roots. Necrotic root tissues were cut in 2 cm long samples, externally disinfected with 90% ethanol, rinsed in distilled water, blotted dry on filter paper and then placed onto PDA + . Petri dishes were incubated in the dark at 20°C and examined every 12 h. Hyphal tips from the emerging colonies were sub-cultured on carrot agar (CA) (Erwin and Ribeiro, 1996) and PDA and incubated at 20°C in the dark. To enhance sporangia production, CA plugs (5 mm diameter) of each isolate were placed in Petri dishes containing unsterile pond water. Sporangial production was assessed every 12 h for 7 days by microscopic observation.
Molecular analysis was used to confirm the identification of all isolates at species level. Instagene Matrix (BioRad Laboratories, Hercules, CA, USA) was used to extract genomic DNA from mycelium of 5-day-old colonies grown on PDA and incubated at 20°C in the dark. The universal primers ITS1 and ITS4 were used to amplify the internal transcribed spacer regions (ITS), including the complete 5.8S gene (White et al., 1990). Polymerase chain reaction (PCR) mixtures and amplification conditions were as described by Linaldeddu et al. (2020a). The PCR products were purified using a EUROGOLD gel extraction kit (EuroClone S.p.A., Pero, Italy) following the manufacturer’s instructions. ITS regions were sequenced in both directions with the primers used for amplification by the BMR Genomics DNA sequencing service (BMR Genomics, Padua, Italy) and by the CIBIACI University service (Florence, Italy). The nucleotide sequences were read and edited with FinchTV 1.4.0 (Geospiza, Inc., Seattle, WA, USA) and then compared with reference sequences (ex-type culture or representative strains) available in GenBank (NCBI/EMBL) using the BLAST search function (Altschul et al., 2010). Isolates were assigned to a species when their sequences were at least 99.9% homologous to the sequence of type material or representative isolates. ITS sequences from representative isolates obtained in this study were deposited in GenBank (Table 1).
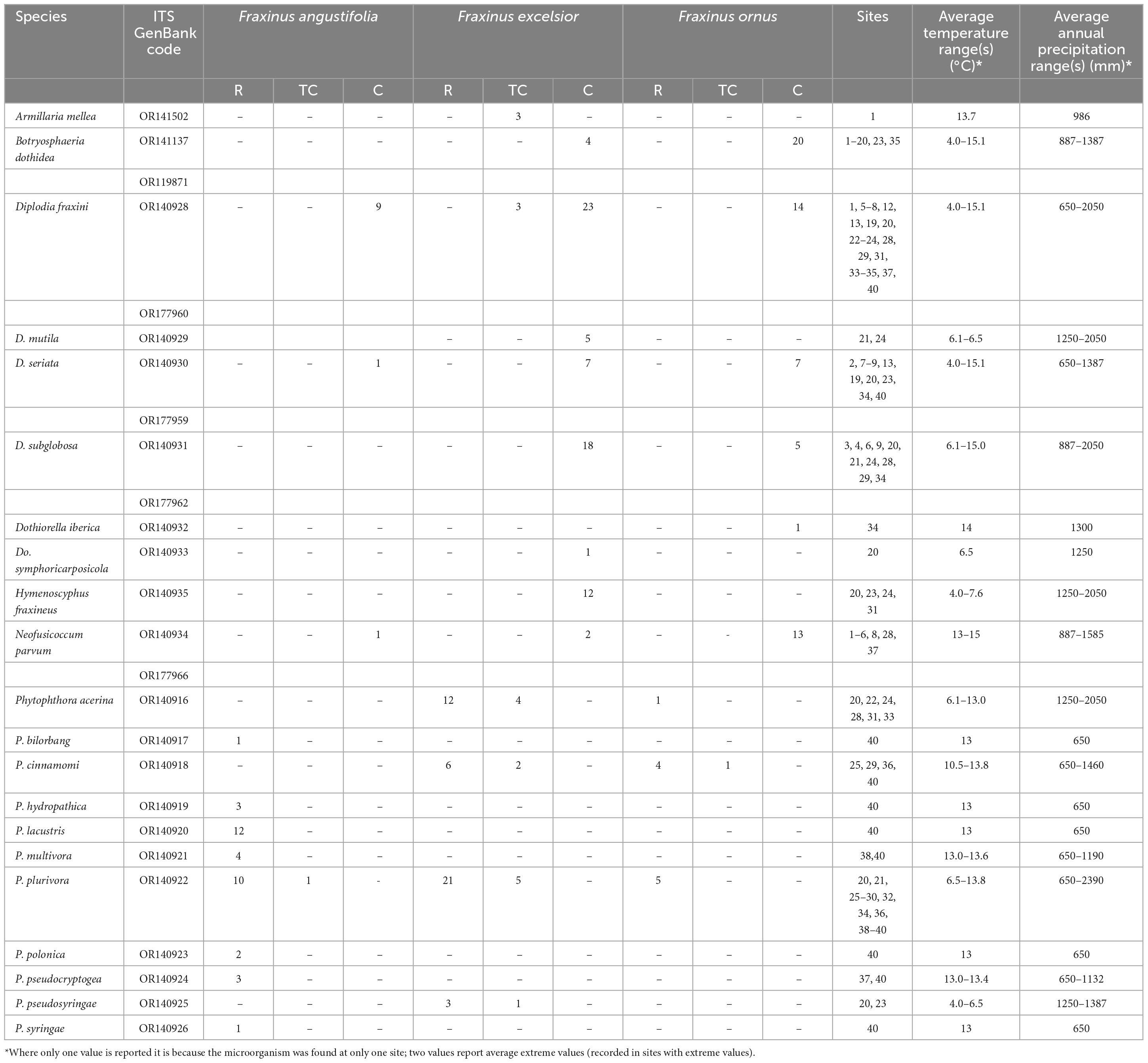
Table 1. Number of isolates obtained from each ash species from rhizosphere (R), collar tissue (TC), and canker (C) samples in the investigated sites.
For Botryosphaeriaceae species ITS sequences of eight representative isolates obtained in this study were compiled in a dataset together with sequences of other 15 isolates belonging to the genera Botryosphaeria, Diplodia, Dothiorella, and Neofusicoccum. For Phytophthora species ITS sequences of 11 representative isolates obtained in this study were compiled in a dataset together with sequences of other 19 isolates belonging to the phylogenetic clades 2, 3, 6, 7, 8, and 9.
Sequences were aligned with ClustalX v. 1.83 (Thompson et al., 1997), using the parameters reported by Bregant et al. (2020). Maximum likelihood (ML) analyses were performed with MEGA-X 10.1.8, including all gaps in the analyses. The best model of DNA sequence evolution was determined automatically by the software (Kumar et al., 2018).
The pathogenicity of four Phytophthora species, P. acerina Ginetti, Jung, Cooke and Moricca, P. cinnamomi Rands, P. plurivora Jung and Burgess and P. pseudosyringae Jung and Delatour, was tested on 3-year-old common ash seedlings grown in plastic pots (10 cm diameter, 1 L volume). The four species of Phytophthora were chosen taking into account: (a) their high isolation frequency; (b) the severity of symptoms observed in nature; and (c) the absence of information as common ash pathogens. Eight common ash seedlings were inoculated with a representative isolate of each species, and six were used as control. Inoculation point at the collar was surface-disinfected with 70% ethanol and a small piece of outer and inner bark (5 mm diameter) was removed with a flamed cork borer and replaced with an agar-mycelium plug, of the same size, taken from the margin of an actively growing pure culture colony. The inoculation point was covered with cotton wool soaked in sterile water and wrapped in a piece of aluminum foil. Controls were inoculated with a sterile PDA plug applied as described above. All inoculated seedlings were kept in field conditions at 10 to 34°C and watered regularly for 30 days. At the end of the experimental period, seedlings were checked for the presence of disease symptoms, the outer bark was carefully removed with a scalpel and the length of necrotic lesion surrounding each inoculation point was measured. Re-isolation of fungal isolates was attempted by transferring 5 pieces of inner bark taken around the margin of the necrotic lesions onto PDA + . Growing colonies were sub-cultured onto CA and PDA, incubated in the dark at 20°C and identified by morphological and molecular analysis (ITS region).
Pathogenicity assay data were checked for normality, then subjected to analysis of variance (ANOVA). Significant differences among mean values were determined using Fisher’s least significant differences (LSD) multiple range test (P = 0.05) using XLSTAT software (Addinsoft SARL, New York, NY, USA).
Symptoms of ash decline with high mortality were common in some hilly areas of Central Italy on flowering ash and everywhere in North-East Italy on common ash.
In Central Italy, disease severity was high on flowering ash, with typical Botryosphaeria cankers and dieback on natural regeneration. Cankers initials appeared as small, sunken, brown-purplish necroses; lesions then extended longitudinally, giving rise to narrow, elongated cankers. Cankers were often multiple along the axis, causing wilting and dieback of young trees. Leaf and shoot blight with crown dieback were also frequently observed (Figure 1).

Figure 1. Overview of symptoms detected on the ash species monitored in the study: extensive canopy dieback of Fraxinus excelsior (A), F. angustifolia (B,C) and F. ornus (D); bleeding canker (E), Phytophthora flame necrotic lesions at the collar of F. angustifolia (F), F. excelsior (G,H) and F. ornus (I); shoot blight (J), sunken canker (K) and inner bark discoloration (L) of F. excelsior and F. angustifolia (M); cross-section of branches showing wedge-shaped necrotic sector (N). Red arrow indicates the white mycelium of Armillaria mellea on a necrotic lesion caused by Phytophthora plurivora.
In North-East Italy, Fraxinus excelsior was severely affected, exhibiting a range of aerial symptoms including partial or complete dieback of the crown, abnormal production of epicormic shoots, bark discolorations and sunken cankers. The same symptoms but with a lower incidence were observed on the other two species of ash, in particular on F. angustifolia.
Disease incidence was very high, ranging between 70 and 100% among the sites, with a mortality range of 30–70%; the disease symptoms were observed on trees of all ages, with particular virulence and mortality on young seedlings, often reaching 100% of sudden death.
In addition to these common canopy symptoms, the same plants often exhibited root and collar rot, necrosis of inner bark and wood tissues in the basal part of the stem and in some cases bleeding cankers with production of blackish exudates. Root symptoms were often associated with forms of sudden death on young and mature trees. The complex symptomatology was also accentuated by the presence of some secondary pathogens and wood decay fungi, such as Armillaria spp. (Figure 1).
Isolation performed on 362 ash samples yielded a total of 251 isolates belonging to 21 different species of oomycetes (102 isolates), ascomycetes (146) and basidiomycetes (3). Of these, 132 isolates were obtained from Fraxinus excelsior, 71 from F. ornus and 48 from F. angustifolia (Table 1). With respect to the type of sample, 143 isolates were obtained from cankers, 83 from roots and rhizosphere and 20 from necrotic inner bark tissues collected at the collar level. Based on morphology, colony appearance and ITS sequence data the 102 isolates of oomycetes were identified as Phytophthora plurivora Jung and Burgess (42 isolates), Phytophthora acerina Ginetti, Jung, Cooke and Moricca (17), Phytophthora cinnamomi Rands (13), Phytophthora lacustris Brasier, Cacciola, Nechw., Jung and Bakonyi (13), Phytophthora multivora Scott and Jung (4), Phytophthora pseudosyringae Jung and Delatour (4), Phytophthora hydropathica Hong and Gallegly (3), Phytophthora pseudocryptogea Safaief., Mostowf., Hardy and Burgess (3), Phytophthora polonica Belbahri, Moralejo and Lefort (2), Phytophthora bilorbang Aghighi, Hardy, Scott and Burgess (1) and Phytophthora syringae Kleb. (1) (Table 1). The 146 fungal isolates belonged to 9 species of ascomycetes in the families Botryosphaeriaceae (134) and Helotiaceae (12). In particular, colonies were identified as Diplodia fraxini (Fr.) Fr. (49), Botryosphaeria dothidea (Moug.) Ces. and De Not. (24), Diplodia subglobosa A.J.L. Phillips, Deidda and Linald. (23), Neofusicoccum parvum (Pennycook and Samuels) Crous, Slippers and A.J.L. Phillips (16), Diplodia seriata De Not. (15), Hymenoscyphus fraxineus (T. Kowalski) Baral, Queloz and Hosoya (12), Diplodia mutila (Fr.) Mont. (5), Dothiorella iberica A.J.L. Phillips, J. Luque and A. Alves (1) and Dothiorella symphoricarposicola W.J. Li, Jian K. Liu and K.D. Hyde(1). Finally, 3 isolates from F. excelsior were identified as Armillaria mellea (Vahl) P. Kumm. (Physalacriaceae, Basidiomycota).
In the phylogenetic analysis of Botryosphaeriaceae species 15 terminal clades were resolved. The isolates obtained in this study clustered in eight well-supported clades (ML bootstrap >90%) together with sequences of ex-type cultures (Supplementary Figure 1). Phylogenetic relationships among the Phytophthora isolates were elucidated using ITS sequences. In particular, the 11 isolates included in the phylogenetic analysis were distributed in 11 terminal clades, which belong to formally described species (Supplementary Figure 2).
Phytophthora plurivora and Diplodia fraxini were the most commonly detected species. The isolates of these two species were obtained from all three investigated ash species (Figure 2). Phytophthora plurivora was isolated from root and collar tissues while D. fraxini from branch cankers and necrotic lesions at the collar. Also, D. seriata was detected on all host species, albeit less frequently than the other two species.
At the end of the experimental trial, all common ash seedlings inoculated with Phytophthora spp. displayed dark brown inner bark lesions that spread up and down from the inoculation point (Figure 3). Phytophthora plurivora and P. acerina were the most aggressive species, causing the longest necrotic lesions (Table 2). Control plants did not show any disease symptoms and exhibited faster growth; only a small light brown discoloration restricted to the inoculation point was observed. All Phytophthora isolates were successfully re-isolated from the margin on the necrotic inner bark lesions of all seedlings, thus fulfilling Koch’s postulates. No Phytophthora isolates or other pathogens were recovered from control seedlings.

Figure 3. Symptoms caused by Phytophthora acerina (A,B), P. cinnamomi (C,D), P. plurivora (E,F) and P. pseudosyringae (G,H) on common ash seedlings. Control seedling (I,N). Colony morphology of P. acerina (J), P. cinnamomi (K), P. plurivora (L), and P. pseudosyringae (M) on carrot agar after 7 days at 20°C in the dark.
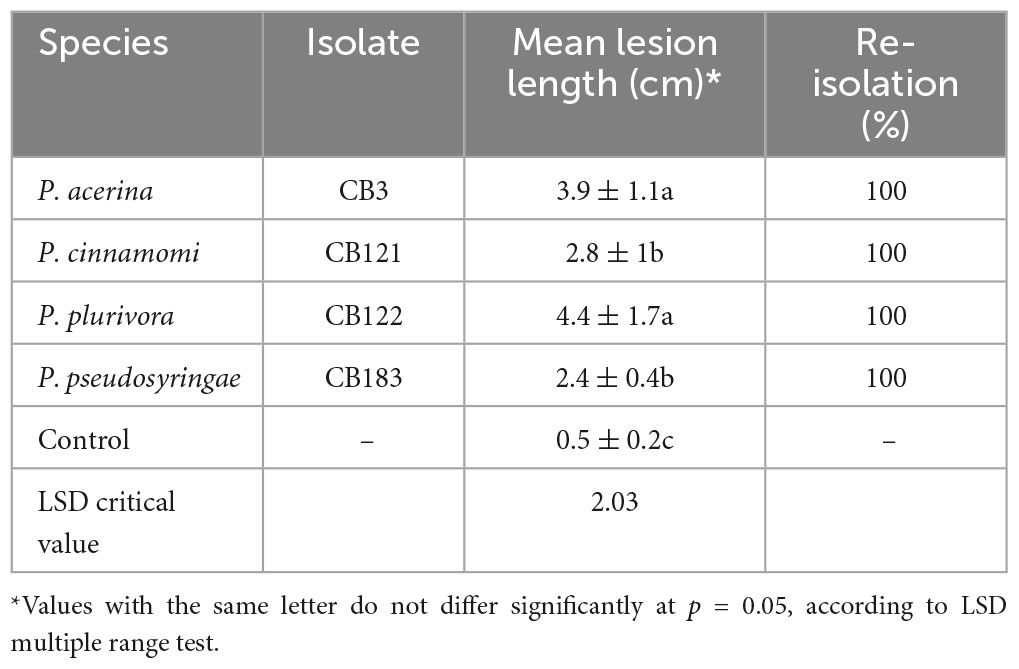
Table 2. Mean lesion length ± standard deviation caused by each Phytophthora species inoculated on common ash seedlings.
A complex of pathogenic fungi and oomycetes resulted associated with stem and branch cankers, leaf and shoot blights, collar necroses and root rot symptoms on common ash, flowering ash and narrow-leaved ash trees. From a phytopathological perspective, it emerged that ash trees in the investigated forest ecosystems live under significant pathogenic constraints, to which at least two distinct groups of major pathogens concur: prominent members of the Botryosphaeriaceae family and aggressive and emerging species of the Phytophthora genus.
Other opportunistic pathogens such as Armillaria spp., were often found coinfecting declining/dying ash trees. Hymenoscyphus fraxineus was found on F. excelsior at a few sites but its impact on infected trees appeared less than that caused by the other pathogens. Some species prevailed at one site or another, depending on the particular context and microclimatic conditions. H. fraxineus was isolated in the coldest sites (mean annual temperature ranging from 4 to 7.6°C) and with an average annual precipitation ranging from 1250 to 2050 mm.
The Botryosphaeriaceae (Botryosphaeriales, Ascomycetes) are an emerging family of plant pathogenic fungi affecting various tree and shrub species typical of the Mediterranean area (Ragazzi et al., 1997; Moricca et al., 2008, 2010; Linaldeddu et al., 2016; Moricca and Linaldeddu, 2017). This family encompasses 22 genera, of which Botryosphaeria, Diplodia, Dothiorella, and Neofusicoccum are the most common in forest ecosystems (Moricca et al., 2012b; Phillips et al., 2013; Batista et al., 2021). Affected plants can show a wide range of symptoms, the most typical of which are cankers on the stem and branches with a characteristic wedge-shaped necrotic sector in cross section resulting in a progressive dieback of the canopy (Ragazzi et al., 1999a; Linaldeddu et al., 2016; Manca et al., 2020).
The relevance of Botryosphaeriaceae as pathogens stands out by taking into consideration the number of disease reports caused by members of this family, that has undergone an exponential increase worldwide, rising from around 100 in the period 1960–2000 to over 1500 in the last 20 years (Scopus, June 2023); this is mainly due to a greater scientific interest in these diseases, the development of new molecular and bioinformatic diagnostic techniques, enabling more accurate identification of fungal taxa, and the ongoing climate change (Batista et al., 2021). The stressful conditions for host species triggered by global warming have provoked the manifestation of epidemic diseases by endophytic and latent species of Botryosphaeriaceae (Ragazzi et al., 1999b,c); therefore, the introduction and establishment of invasive Botryosphaeriaceae in new areas of the planet driven by the rising temperatures, caused the occurrence of new emerging diseases incited by these fungi in regions previously considered unsuitable to many of them (Batista et al., 2020, 2021).
Diseases caused by Botryosphaeriaceae are drawing the attention of researchers especially in the Mediterranean region, consider one of the most striking examples regarding the emerging diseases (Ragazzi et al., 1996; Linaldeddu et al., 2017; Panzavolta et al., 2017; Smahi et al., 2017). Italy is certainly one of the most affected countries within the Mediterranean region. Indeed, countless studies have reported about 60 species of Botryosphaeriaceae in this country, resulting in over 250 host-pathogen interactions threatening diverse sectors of primary production (Moral et al., 2010; Urbez-Torres, 2011; Batista et al., 2021; Fiorenza et al., 2023). Although the economic impact is higher in crop production, the diseases caused by Botryosphaeriaceae species can also be devastating in forestry, causing losses of biodiversity and impairing ecosystem integrity (Slippers and Wingfield, 2007; Marsberg et al., 2017).
Prominent examples are the decline of Mediterranean vegetation caused by several Diplodia and Neofusicoccum species (Moricca et al., 2012b), pine shoot blight due to the invasive species Diplodia sapinea, oak canker disease due to D. corticola and ash dieback caused by D. fraxini (Luchi et al., 2014; Cimmino et al., 2016; Manca et al., 2020).
The dieback of ash formations has been associated for a long time, especially in Eastern Europe, to the ascomycete fungus H. fraxineus (Kowalski, 2006). Recent investigations in Germany, Italy and Slovenia, as well as this study, better clarify the key role of Botryosphaeriaceae species in the etiology of ash dieback (Linaldeddu et al., 2020a,2022; Peters et al., 2023). In this study, B. dothidea was isolated at very high frequency from cankered tree tissues of F. ornus individuals in hilly areas of Central Italy. This cosmopolitan pathogen has been isolated worldwide from sites characterized by very different climatic conditions (mean annual temperature between 4 and 15.1°C) and mean annual rainfall between 887 and 1387 mm (Marsberg et al., 2017; Xue et al., 2021). In central Italy, the impact of the disease was greater on poor soils rich in gravel, on slopes facing south or south-west, at sites exposed to high daily temperatures and heat waves during the growing season and generally in stands suffering from drought stress. Mortality was high on young seedlings, especially following long dry periods. Diplodia fraxini was the most constantly isolated species from symptomatic ash trees and its virulence was confirmed by independent pathogenicity assays (Elena et al., 2018; Linaldeddu et al., 2020a). This fungus seems to manifest a particular host-specificity for the genus Fraxinus, including the capacity to produce selective phytotoxic secondary metabolites (Cimmino et al., 2017).
Diplodia fraxini, included for a long time in the Diplodia mutila complex, was recently re-instated; therefore, many isolates from Fraxinus that were assigned in GenBank to Diplodia mutila belong in reality to D. fraxini, a fact that demonstrates a wider distribution of this species in Europe (Alves et al., 2014; Linaldeddu et al., 2020a). The current distribution of D. fraxini is still unknown; however, the impact of ash dieback, that appear to be growing in central Europe and new areas of the Mediterranean region such as Central Italy, the Balkans and Iberian Peninsula, underline the strong adaptation of this fungus to changing environmental conditions (Alves et al., 2014; Elena et al., 2018; Peters et al., 2023). The plasticity of this pathogen is confirmed by its discovery in numerous sites with very different climatic conditions: average temperature ranging from 4 to 15.1°C and average annual precipitation ranging from 650 to 2050 mm.
Phytophthora spp. are another important group of lethal pathogens that are overbearingly emerging in many forest areas globally (Jung et al., 2016). Most Phytophthora species have a soilborne lifestyle, causing primarily root and collar rot on thousands of plant species, but some of them are endowed with an aerial dispersal lifestyle. These oomycetes cause a broad range of symptoms on affected hosts; in response to the bark and root damages, the canopy evidences non-specific symptoms of progressive or sudden decline (Bregant et al., 2020).
Over the past two decades, countless studies have demonstrated the involvement of a large number of soilborne Phytophthora species in the widespread declines of forest ecosystems dominated by the Fagaceae and the Betulaceae in Europe, especially in the Mediterranean basin (Brasier et al., 1993; Jung et al., 2000; Vettraino et al., 2005; Linaldeddu et al., 2014; Scanu et al., 2015; Corcobado et al., 2020; Riolo et al., 2020; Bregant et al., 2023).
In some cases, attacks were a matter of re-emergence of old-known Phytophthora species which, after decades of infrequent, sporadic outbreaks, in the past 20 years have come back to cause major epidemic outbreaks. A key to understanding the resurgence of diseases caused by old-known Phytophthora related diseases (e.g., the “ink disease” on chestnut) is the current climate trend. It must be considered that the Mediterranean-type climate ecosystems present today conditions particularly conducive to the lifestyle and development of this group of pathogens: the relatively warm and wet winter period can induce an easy zoospore production for host infection in combination with the subsequent long and dry summers that cause severe stress in tree populations (Serrano et al., 2022). In this scenario, an increasing number of Phytophthora species are emerging or re-emerging from the Mediterranean areas of the planet, such as SW Australia, California, South Africa and the classic Mediterranean Basin (Burgess et al., 2017).
Crown and root diseases related to Fraxinus spp. dieback are to date still little studied. Current knowledge about the association between Phytophthora spp. and ash is limited. Until a few years ago, the Fraxinus genus was considered resistant to Phytophthora attacks, as also ascertained by some pathogenicity tests (Jung and Nechwatal, 2008; Mràzkovà et al., 2013). The first study to report attacks of Phytophthora spp. on natural Fraxinus formations took place in 2011 in Poland and Denmark, highlighting root and collar root symptoms, although the Phytophthora presence had been ascertained a few years earlier in areas of Central and Eastern Europe such as Germany and the Czech Republic (Mràzkovà et al., 2010, 2013; Orlikowski et al., 2011; Langer, 2017).
A study conducted by Tkaczyk et al. (2016) found five Phytophthora species from common ash in a nature reserve in Poland. In this study, P. cactorum and P. plurivora appeared to be the most frequent and virulent species associated with common ash (Orlikowski et al., 2011; Tkaczyk et al., 2016). Recent studies have also demonstrated a high susceptibility to Phytophthora species of natural riparian formations of Fraxinus angustifolia in Sicily and Türkiye (Akilli et al., 2013; Jung et al., 2019). Some studies conducted in Northern and Eastern Europe highlighted a possible synergistic action between canopy and root pathogens (Orlikowski et al., 2011; Tkaczyk et al., 2016; Milenković et al., 2017, 2018; Peters et al., 2023). Other studies have shown a frequent association of declining ash trees with secondary root pathogens such as Armillaria spp. (Lygis et al., 2005; Skovsgaard et al., 2010; Langer, 2017; Kranjec Orlović et al., 2020; Peters et al., 2023).
Given the above, this study stands as the most complete census of Phytophthora species attacking members of the genus Fraxinus, with 11 different species, namely P. acerina, P. bilorbang, P. cinnamomi, P. hydropathica, P. lacustris, P. multivora, P. plurivora, P. polonica, P. pseudocryptogea, P. pseudosyringae, and P. syringae that are reported for the first time on these hosts in Italy.
The 4 Phytophthora species used in the pathogenicity tests, P. acerina, P. cinnamomi, P. plurivora, and P. pseudosyringae, selected on the basis of their high isolation frequencies and the severity of symptoms caused on ash trees, confirmed to be aggressive pathogens on ash and provided important information about their impact and pervasiveness in forest ecosystems. In particular, Phytophthora plurivora and P. acerina proved to be the most virulent species in the artificial inoculation tests, producing the longest lesions on inoculated seedlings. It is noteworthy that both species are members of the former P. citricola complex (Ginetti et al., 2014).
Phytophthora plurivora is a root and stem pathogen very widespread in Europe, known to occur in different settings and environments (plantations, nurseries, ornamental green, streams, primary forests) and to cause widespread declines on alder (Alnus spp.), beech (Fagus sylvatica), and oak (Quercus spp.) ecosystems (Jung, 2009; Jung and Burgess, 2009; Lilja et al., 2011; Prospero et al., 2013; Schoebel et al., 2014; Bregant et al., 2023). Having been found at very high frequencies even in ash formations confirms its widespread presence in forest ecosystems and suggests it might have a major role also in the decline of ash formations.
Phytophthora acerina was first reported and described about 10 years ago from a wooded area in a peri-urban park on the Lombardy plain (Northern Italy). Here, it infected and killed thousands of individuals of Acer pseudoplatanus, making this tree species literally disappear from the area (Ginetti et al., 2014). The pathogen was able to spread pervasively among water sources, the whole area being rich in water (some plots were in the past cultivated with rice). Phytophthora acerina was in fact recovered from streams, ponds, canals, reservoirs, runoffs, irrigation waters, as well as—of course—from bleeding cankers on the stem, root tissues and soil samples taken under the crown of dead/dying trees. The pathogen was reported a few years later, in 2018, causing sudden death to olive trees in the nearby Veneto region, a few hundred kms from the site of its first report. On olive trees P. acerina exhibited high virulence as well, causing a range of symptoms such as partial or complete crown dieback, reddening of drying foliage, loss of rootlets and collar rot (Linaldeddu et al., 2020c). These literature data and the high virulence displayed by P. acerina on inoculated seedlings as well as on ash trees in the woods prove it to be an extremely dangerous and highly pervasive pathogen, whose host and geographic range is still largely unexplored, but which certainly includes the Fraxinus species.
Phytophthora cinnamomi was isolated from ash trees at many sites and this confirms this generalist pathogen to be rather ubiquitous and to become invasive in some areas, often causing enormous damage to natural ecosystems (Hardham and Blackman, 2018). This oomycete shows a rather accentuated seasonality and is favored, in particular, by frequent rains in the spring and by mild winters, a condition typical of the Mediterranean climate; current climate anomalies could significantly modify these conditions, further favoring the epidemiological spread of this pathogen (Serrano et al., 2022). The recent description in the Mediterranean of two species close to P. cinnamomi with a further adaptation to even higher temperatures is proof of the vulnerability of natural ecosystems to this group of pathogens in the era of climate change (Scanu et al., 2014; Yang et al., 2017; Bregant et al., 2021).
Global climate change is altering site factors, biochemical processes and biotic interactions in natural plant communities (Sturrock et al., 2011). The impact of climate change is exacerbated in climate change hotspots. Italy, a peninsula jutting out into the center of the Mediterranean climate change hotspot region, is experiencing unprecedented mild dry winters, hot summers and prolonged droughts that are seriously impacting forest vegetation and changing the landscape. As the scientific community debates if species migration will be able to keep pace with climate change, with projections about climate-driven range shifts in tree species, forest pathologists tirelessly record extended forest diebacks, invasions by new pathogens and altered densities and distribution of forest tree species within natural plant communities (Singh et al., 2023). That’s what seems to be going on with ash formations: common ash, flowering ash and narrow-leaved ash stands are under unprecedented attack by new pathogens and the almost total loss of natural regeneration observed at some locations indicates these species might be retreating from the less favorable sites.
Another reason for concern arises from knowledge on the contrasting infection biologies and lifestyles of the two groups of causative agents involved. The fungal and oomycete pathogens reported here, being endowed with different thermo-hygrometric requirements, could be vicariant throughout the year in their pathogenetic action, with Phytophthora spp. being more active in mild and moist seasons and Botryosphaeriaceae that become more aggressive during hot and dry periods. If this pattern of vicariance, already observed in oak forests (Moricca et al., 2012c), would also be reproduced in ash stands, it could catch infected ash trees in a deadly spiral, as the two groups of pathogens would act synergistically in weakening ash trees and lead to their death.
The large number of pathogenic species found in this study agrees with the results of a recent research conducted in Germany (Peters et al., 2023), and underlines that while the litter species H. fraxineus has been getting the most attention in the last decades, the most common species associated with Fraxinus spp. with ash dieback symptoms are Diplodia fraxini and P. plurivora. The overall framework indicates that multi-trophic interactions are common in ash stands, representing an important and matter-of-fact aspect of tree-pathogen relationships, and this provides a more realistic picture of what’s going on in forests (Pillay et al., 2013; Smahi et al., 2017). Clarifying this complex etiology is critical to assess if, and to what extent, ash disease management and prevention measures can be effectively applied.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
AB, CB, SM, and BL contributed to conception and design of the study. AB and CB conducted the field and laboratory trials, analyzed the data, and wrote the original manuscript. CA, GR, and BT helped with laboratory investigations, data curation and preparation of the first draft of the manuscript. SM and BL supervised and funding acquisition. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was partially funded by the Regione Toscana—Servizio Fitosanitario, within the project “Accordo di collaborazione scientifica tra Regione Toscana—Servizio Fitosanitario e Università di Firenze—Dipartimento di Scienze e Tecnologie Agrarie, Alimentari, Ambientali e Forestali (DAGRI), per la realizzazione di attività congiunte in materia di organismi nocivi da quarantena e di interesse fitosanitario per le principali colture agrarie regionali (cereali, olivo, vite, vivaismo ornamentale e frutticolo) e in campo forestale” and by grant number DOR2305524/2023 “Monitoraggio dei marciumi radicali da Phytophthora negli ecosistemi forestali Italiani.”
Part of this study was conducted by AB within her Ph.D. doctoral project at the University of Florence, Italy, Ph.D. doctoral program in Agricultural and Environmental Sciences and by C.B. within by the Land Environment Resources and Health (L.E.R.H.) doctoral course (University of Padova). We are grateful to the Unione dei Comuni della Val di Merse (SI), Ten. Col. Giovanni Nobili and to Carabinieri of the Bosco della Mesola (FE), for their technical and logistic support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1253022/full#supplementary-material
Akilli, S., Ulubaş Serçe, Ç, Katırcıoğlu, Y. Z., and Maden, S. (2013). Phytophthora dieback on narrow leaved ash in the black sea region of Turkey. For. Pathol. 43, 252–256. doi: 10.1111/efp.12024
Altschul, S. F., Wootton, J. C., Zaslavsky, E., and Yu, Y. K. (2010). The construction and use of log-odds substitution scores for multiple sequence alignment. PLoS Comput. Biol. 6, e1000852. doi: 10.1371/journal.pcbi.1000852
Alves, A., Linaldeddu, B. T., Deidda, A., Scanu, B., and Phillips, A. J. L. (2014). The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Diver. 67, 143–156. doi: 10.1007/s13225-014-0282-9
Batista, E., Lopes, A., and Alves, A. (2020). Botryosphaeriaceae species on forest trees in Portugal: Diversity, distribution and pathogenicity. Eur. J. Plant Pathol. 158, 693–720. doi: 10.1007/s10658-020-02112-8
Batista, E., Lopes, A., and Alves, A. (2021). What do we know about Botryosphaeriaceae? An overview of a worldwide cured dataset. Forests 12:313. doi: 10.3390/f12030313
Benigno, A., Cerboneschi, M., and Moricca, S. (2019). “Dieback of natural regeneration of Flowering ash (Fraxinus ornus) in a hilly area of central Tuscany,” in Paper Presented at the Joint Meeting IUFRO WP 7.02. 02 and 7.02. 03, Figline Valdarno, Florence, Italy, (Florence), 28.
Brasier, C. M., Robredo, F., and Ferraz, J. F. P. (1993). Evidence for Phytophthora cinnamomi involvement in Iberian oak decline. Plant Pathol. 42, 140–145.
Bregant, C., Batista, E., Hilário, S., Linaldeddu, B. T., and Alves, A. (2023). Phytophthora species involved in Alnus glutinosa decline in Portugal. Pathogens 12:276. doi: 10.3390/pathogens12020276
Bregant, C., Mulas, A. A., Rossetto, G., Deidda, A., Maddau, L., Piras, G., et al. (2021). Phytophthora mediterranea sp. nov., a new species closely related to Phytophthora cinnamomi from nursery plants of Myrtus communis in Italy. Forests 12:682. doi: 10.3390/f12060682
Bregant, C., Sanna, G. P., Bottos, A., Maddau, L., Montecchio, L., and Linaldeddu, B. T. (2020). Diversity and pathogenicity of Phytophthora species associated with declining alder trees in Italy and description of Phytophthora alpina sp. nov. Forests 11:848. doi: 10.3390/f11080848
Burgess, T. I., Scott, J. K., Mcdougall, K. L., Stukely, M. J., Crane, C., Dunstan, W. A., et al. (2017). Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Glob. Change Biol. 23, 1661–1674. doi: 10.1111/gcb.13492
Cimmino, A., Maddau, L., Masi, M., Evidente, M., Linaldeddu, B. T., and Evidente, A. (2016). Further secondary metabolites produced by Diplodia corticola, a fungal pathogen involved in cork oak decline. Tetrahedron 72, 6788–6793. doi: 10.1016/j.tet.2016.09.008
Cimmino, A., Maddau, L., Masi, M., Linaldeddu, B. T., Pescitelli, G., and Evidente, A. (2017). Fraxitoxin, a new isochromanone isolated from Diplodia fraxini. Chem. Biodivers. 14:e1700325. doi: 10.1002/cbdv.201700325
Colangelo, M., Camarero, J. J., Borghetti, M., Gentilesca, T., Oliva, J., Redondo, M. A., et al. (2018). Drought and Phytophthora are associated with the decline of oak species in southern Italy. Front Plant Sci. 9:1595. doi: 10.3389/fpls.2018.01595
Corcobado, T., Cech, T. L., Brandstetter, M., Daxer, A., Hüttler, C., Kudláček, T., et al. (2020). Decline of European beech in Austria: Involvement of Phytophthora spp. and contributing biotic and abiotic factors. Forests 11:895. doi: 10.3390/f11080895
Cowling, R. M., Rundel, P. W., Lamont, B. B., Arroyo, M. K., and Arianoutsou, M. (1996). Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. 11, 362–366. doi: 10.1016/0169-5347(96)10044-6
Dukes, J. S., Pontius, J., Orwig, D., Garnas, J. R., Rodgers, V. L., Brazee, N., et al. (2009). Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 39, 231–248. doi: 10.1139/X08-171
Elena, G., León, M., Abad-Campos, P., Armengol, J., Mateu-Andrés, I., and Güemes-Heras, J. (2018). First report of Diplodia fraxini causing dieback of Fraxinus angustifolia in Spain. Plant Dis. 102:2645. doi: 10.1094/PDIS-05-18-0792-PDN
Fiorenza, A., Gusella, G., Vecchio, L., Aiello, D., and Polizzi, G. (2023). Diversity of Botryosphaeriaceae species associated with canker and dieback of avocado (Persea americana) in Italy. Phytopathol. Mediterr. 62, 47–63. doi: 10.36253/phyto-14057
Ginetti, B., Moricca, S., Squires, J. N., Cooke, D. E. L., Ragazzi, A., and Jung, T. (2014). Phytophthora acerina sp. nov., a new species causing bleeding cankers and dieback of acer pseudoplatanus trees in planted forests in Northern Italy. Plant Pathol. 63, 858–876. doi: 10.1111/ppa.12153
Giorgi, F., and Lionello, P. (2008). Climate change projections for the Mediterranean region. Glob. Planet Change 63, 90–104. doi: 10.1016/j.gloplacha.2007.09.005
Haavik, L. J., Billings, S. A., Guldin, J. M., and Stephen, F. M. (2015). Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 354, 190–205. doi: 10.1016/j.foreco.2015.06.019
Hansen, E. M. (2008). Alien forest pathogens: Phytophthora species are changing world forests. Boreal Environ. Res. 13, 33–41.
Hardham, A. R., and Blackman, L. M. (2018). Phytophthora cinnamomi. Mol. Plant Pathol. 19, 260–285. doi: 10.1111/mpp.12568
Jung, T. (2009). Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. For. Pathol. 39, 73–94. doi: 10.1111/j.1439-0329.2008.00566.x
Jung, T., and Burgess, T. I. (2009). Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov. Persoonia 22, 95–110. doi: 10.3767/003158509X442612
Jung, T., and Nechwatal, J. (2008). Phytophthora gallica sp. nov., a new species from rhizosphere soil of declining oak and reed stands in France and Germany. Mycol. Res. 112, 1195–1205. doi: 10.1016/j.mycres.2008.04.007
Jung, T., Blaschke, H., and Osswald, W. (2000). Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 49, 706–718. doi: 10.1046/j.1365-3059.2000.00521.x
Jung, T., La Spada, F., Pane, A., Aloi, F., Evoli, M., Horta Jung, M., et al. (2019). Diversity and distribution of Phytophthora species in protected natural areas in Sicily. Forests 10:259. doi: 10.3390/f10030259
Jung, T., Orlikowski, L., Henricot, B., Abad Campos, P., Aday, A. G., Aguín Casal, O., et al. (2016). Widespread Phytophthora infestations in European nurseries put forest, semi-natural and horticultural ecosystems at high risk of Phytophthora diseases. For. Pathol. 46, 134–163. doi: 10.1111/efp.12239
Kowalski, T. (2006). Chalara fraxinea sp. nov. associated with dieback of ash (Fraxinus excelsior) in Poland. For. Pathol. 36, 264–270. doi: 10.1111/j.1439-0329.2006.00453.x
Kranjec Orlović, J., Moro, M., and Diminić, D. (2020). Role of root and stem base fungi in Fraxinus angustifolia (Vahl) dieback in croatian floodplain forests. Forests 11:607. doi: 10.3390/f11060607
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549.
Langer, G. (2017). Collar rots in forests of Northwest Germany affected by ash dieback. Bal. For. 23, 4–19.
Lilja, A., Rytkönen, A., and Hantula, J. (2011). Introduced pathogens found on ornamentals, strawberry and trees in Finland over the past 20 years. Agric. Food Sci. 20, 74–85. doi: 10.2137/145960611795163051
Linaldeddu, B. T., Bottecchia, F., Bregant, C., Maddau, L., and Montecchio, L. (2020a). Diplodia fraxini and Diplodia subglobosa: The main species associated with cankers and dieback of Fraxinus excelsior in north-eastern Italy. Forests 11:883. doi: 10.3390/f11080883
Linaldeddu, B. T., Bregant, C., Montecchio, L., Brglez, A., Piškur, B., and Ogris, N. (2022). First report of Diplodia fraxini and Diplodia subglobosa causing canker and dieback of Fraxinus excelsior in Slovenia. Plant Dis. 106, 26–29. doi: 10.1094/PDIS-06-21-1204-SC
Linaldeddu, B. T., Bregant, C., Montecchio, L., Favaron, F., and Sella, L. (2020c). First report of Phytophthora acerina, P. pini, and P. plurivora causing root rot and sudden death of olive trees in Italy. Plant Dis. 104, 996–996. doi: 10.1094/PDIS-10-19-2080-PDN
Linaldeddu, B. T., Bregant, C., Ruzzon, B., and Montecchio, L. (2020b). Coniella granati and Phytophthora palmivora: The main pathogens involved in pomegranate dieback and mortality in north-eastern Italy. Italian J. Mycol. 49, 92–100. doi: 10.6092/issn.2531-7342/11170
Linaldeddu, B. T., Maddau, L., and Franceschini, A. (2017). First report of Diplodia corticola causing canker and dieback of Quercus ilex, Q. petraea, and Q. suber in Corsica (France). Plant Dis. 101:256. doi: 10.1094/PDIS-07-16-1076-PDN
Linaldeddu, B. T., Maddau, L., Franceschini, A., Alves, A., and Phillips, A. J. L. (2016). Botryosphaeriaceae species associated with lentisk dieback in Italy and description of Diplodia insularis sp. nov. Mycosphere 7, 962–977. doi: 10.5943/mycosphere/si/1b/8
Linaldeddu, B. T., Scanu, B., Maddau, L., and Franceschini, A. (2014). Diplodia corticola and Phytophthora cinnamomi: The main pathogens involved in holm oak decline on Caprera Island (Italy). For. Pathol. 44, 191–200. doi: 10.1111/efp.12081
Lionello, P., and Scarascia, L. (2018). The relation between climate change in the Mediterranean region and global warming. Reg. Environ. Change 18, 1481–1493. doi: 10.1007/s10113-018-1290-1
Luchi, N., Ghelardini, L., Santini, A., Migliorini, D., and Capretti, P. (2016). First record of ash dieback caused by Hymenoscyphus fraxineus on Fraxinus excelsior in the Apennines (Tuscany, Italy). Plant Dis. 100:535. doi: 10.1094/PDIS-09-15-0975-PDN
Luchi, N., Oliveira Longa, C. M., Danti, R., Capretti, P., and Maresi, G. (2014). Diplodia sapinea: The main fungal species involved in the colonization of pine shoots in Italy. For. Pathol. 44, 372–381. doi: 10.1111/efp.12109
Lygis, V., Vasiliauskas, R., Larsson, K. H., and Stenlid, J. (2005). Wood-inhabiting fungi in stems of Fraxinus excelsior in declining ash stands of northern Lithuania, with particular reference to Armillaria cepistipes. Scand. J. For. Res. 20, 337–346. doi: 10.1080/02827580510036238
Manca, D., Bregant, C., Maddau, L., Pinna, C., Montecchio, L., and Linaldeddu, B. T. (2020). First report of canker and dieback caused by Neofusicoccum parvum and Diplodia olivarum on oleaster in Italy. Italian J. Mycol. 49, 85–91. doi: 10.6092/issn.2531-7342/11048
Marsberg, A., Kemler, M., Jami, F., Nagel, J. H., Postma-Smidt, A., Naidoo, S., et al. (2017). Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 18, 477–488. doi: 10.1111/mpp.12495
Migliorini, D., Luchi, N., Nigrone, E., Pecori, F., Pepori, A. L., and Santini, A. (2022). Expansion of ash dieback towards the scattered Fraxinus excelsior range of the Italian Peninsula. Biol. Invas. 24, 1359–1373.
Milenković, I., Jung, T., Stanivuković, Z., and Karadžić, D. (2017). First report of Hymenoscyphus fraxineus on Fraxinus excelsior in Montenegro. For Pathol. 47:e12359. doi: 10.1111/efp.12359
Milenković, I., Keèa, N., Karadžić, D., Nowakowska, J. A., Oszako, T., Sikora, K., et al. (2018). Interaction between Hymenoscyphus fraxineus and Phytophthora species on young Fraxinus excelsior seedlings. For. Chron. 94, 135–139. doi: 10.5558/tfc2018-020
Moral, J., Muñoz-Díez, C., González, N., Trapero, A., and Michailides, T. J. (2010). Characterization and pathogenicity of Botryosphaeriaceae species collected from olive and other hosts in Spain and California. Phytopathology 100, 1340–1351. doi: 10.1094/PHYTO-12-09-0343
Moricca, S., and Linaldeddu, B. (2017). “Climate change triggers the pervasive spread of botryosphaeriaceous fungi in the Mediterranean region,” in Proceedings of the Invasive Forest Pathogens and Implications for Biology and Policy IUFRO Working Party 7.02. 02, May 7-11, 2017, Niagara Falls, Ontario, Niagara Falls, ON, 34.
Moricca, S., and Ragazzi, A. (2008). Fungal endophytes in Mediterranean oak forests: A lesson from Discula quercina. Phytopathology 98, 380–386. doi: 10.1094/phyto-98-4-0380
Moricca, S., Ginetti, B., and Ragazzi, A. (2012a). Species- and organ-specificity in endophytes colonizing healthy and declining Mediterranean Oaks. Phytopathol. Mediterr. 51, 587–598.
Moricca, S., Linaldeddu, B. T., Ginetti, B., Scanu, B., Franceschini, A., and Ragazzi, A. (2016). Endemic and emerging pathogens threatening cork oak trees: Management options for conserving a unique forest ecosystem. Plant Dis. 100, 2184–2193. doi: 10.1094/PDIS-03-16-0408
Moricca, S., Uccello, A., Ginetti, B., and Ragazzi, A. (2012b). First report of Neofusicoccum parvum associated with bark canker and dieback of acer pseudoplatanus and Quercus robur in Italy. Plant Dis. 96:1699. doi: 10.1094/PDIS-06-12-0543-PDN
Moricca, S., Uccello, A., Ginetti, B., and Ragazzi, A. (2012c). Isolation and growth temperature requirements of oomycetes and Botryosphaeriaceae from the same oak hosts: Evidence for a vicariant pathogenic action? IOBC/WPRS Bull. 76, 79–84.
Moricca, S., Uccello, A., Turco, E., Ginetti, B., and Ragazzi, A. (2010). Multiple Botryosphaeriaceae infection in forest trees: Synergistic or antagonistic interaction? J. Plant Pathol. 92:91.
Moricca, S., Uccello, A., Zini, E., Campana, F., Gini, R., Selleri, B., et al. (2008). Spread and virulence of Botryosphaeria dothidea on broadleaved trees in urban parks of northern Italy. J. Plant Pathol. 90:452.
Mràzkovà, M., Černy, K., Tomšovský, M., Holub, V., Strnadova, V., Zlatohlavek, A., et al. (2010). First report of root rot of pedunculate oak and other forest tree species caused by Phytophthora plurivora in the Czech Republic. Plant Dis. 94, 272–272. doi: 10.1094/PDIS-94-2-0272B
Mràzkovà, M., Černý, K., Tomšovský, M., Strnadova, V., Gregorová, B., Holub, V., et al. (2013). Occurrence of Phytophthora multivora and Phytophthora plurivora in the Czech Republic. Plant Prot. Sci. 49, 155–164. doi: 10.17221/74/2012-PPS
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858.
Nunes, L. J., Meireles, C. I., Gomes, C. J. P., Ribeiro, N., and Almeida, M. C. (2022). The impact of climate change on forest development: A sustainable approach to management models applied to Mediterranean-type climate regions. Plants 11:69. doi: 10.3390/plants11010069
Ogris, N., Hauptman, T., Jurc, D., Floreancig, V., Marsich, F., and Montecchio, L. (2010). First report of Chalara fraxinea on common ash in Italy. Plant Dis. 94:133. doi: 10.1094/PDIS-94-1-0133A
Orlikowski, L. B., Ptaszek, M., Rodziewicz, A., Nechwatal, J., Thinggaard, K., and Jung, T. (2011). Phytophthora root and collar rot of mature Fraxinus excelsior in forest stands in Poland and Denmark. For. Pathol. 41, 510–519. doi: 10.1111/j.1439-0329.2011.00714.x
Panconesi, A., Moricca, S., Ragazzi, A., Dellavalle, I., and Tiberi, R. (2014). Parassiti delle piante arboree forestali ed ornamentali: Specie introdotte e di temuta introduzione. Bologna: Pàtron Editore.
Panzavolta, T., Panichi, A., Bracalini, M., Croci, F., Benigno, A., Ragazzi, A., et al. (2018). Tree pathogens and their insect-mediated transport: Implications for oak tree die-off in a natural park area. Glob. Ecol. Conserv. 15:e00437.
Panzavolta, T., Panichi, A., Bracalini, M., Croci, F., Ginetti, B., Ragazzi, A., et al. (2017). Dispersal and propagule pressure of Botryosphaeriaceae species in a declining oak stand is affected by insect vectors. Forests 8:228. doi: 10.3390/f8070228
Peters, S., Fuchs, S., Bien, S., Bußkamp, J., Langer, G. J., and Langer, E. J. (2023). Fungi associated with stem collar necroses of Fraxinus excelsior affected by ash dieback. Mycol. Prog. 22:52. doi: 10.1007/s11557-023-01897-2
Phillips, A. J., Alves, A., Abdollahzadeh, J., Slippers, B., Wingfield, M. J., Groenewald, J. Z., et al. (2013). The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 76, 51–167. doi: 10.3114/sim0021
Pillay, K., Slippers, B., Wingfield, M. J., and Gryzenhout, M. (2013). Diversity and distribution of co-infecting Botryosphaeriaceae from Eucalyptus grandis and Syzygium cordatum in South Africa. S. Afr. J. Bot. 84, 38–43. doi: 10.1016/j.sajb.2012.09.003
Prospero, S., Vercauteren, A., Heungens, K., Belbahri, L., and Rigling, D. (2013). Phytophthora diversity and population structure of Phytopthora ramorum in Swiss ornamental nurseries. Plant Pathol. 62, 1063–1071. doi: 10.1111/ppa.12027
Ragazzi, A., Moricca, S., and Dellavalle, I. (1997). Vegetative compatibility and pathogenicity of Diplodia mutila isolates on oak. Eur. J. Plant Pathol. 27, 391–396.
Ragazzi, A., Moricca, S., and Dellavalle, I. (1999a). Water stress and the development of cankers by Diplodia mutila on Quercus robur. J. Phytopathol. 147, 425–428.
Ragazzi, A., Moricca, S., and Dellavalle, I. (1999b). Interactions between Quercus spp. and Diplodia mutila under water stress conditions. Z. Pflanzenkr. Pflanzenschutz 106, 495–500.
Ragazzi, A., Moricca, S., Vagniluca, S., and Dellavalle, I. (1996). Antagonism of Acremonium mucronatum towards Diplodia mutila in tests in vitro and in situ. Eur. J. Plant Pathol. 26, 235–243.
Ragazzi, A., Moricca, S., Vagniluca, S., Comparini, C., and Dellavalle, I. (1999c). Leaf water potential and peroxidase activity in Quercus cerris and Quercus pubescens after inoculation with Diplodia mutila. J. Phytopathol. 147, 55–59.
Rehfeldt, G. E., Ferguson, D. E., and Crookston, N. L. (2009). Aspen, climate, and sudden decline in western USA. For. Ecol. Manag. 258, 2353–2364. doi: 10.1016/j.foreco.2009.06.005
Rigling, D., Hilfiker, S., Schöbel, C., Meier, F., Engesser, R., Scheidegger, C., et al. (2018). Il deperimento del frassino. Biologia, sintomi e raccomandazioni per la gestione. Notizie per la pratica 57. Birmensdorf: Istituto federale di ricerca WSL, 8.
Riolo, M., Aloi, F., La Spada, F., Sciandrello, S., Moricca, S., Santilli, E., et al. (2020). Diversity of Phytophthora communities across different types of Mediterranean vegetation in a nature reserve area. Forests 11, 853–873. doi: 10.3390/f11080853
Scanu, B., Hunter, G. C., Linaldeddu, B. T., Franceschini, A., Maddau, L., Jung, T., et al. (2014). A taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. For. Pathol. 44, 1–20. doi: 10.1111/efp.12064
Scanu, B., Linaldeddu, B. T., Deidda, A., and Jung, T. (2015). Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS One 10:e0143234. doi: 10.1371/journal.pone.0143234
Schoebel, C. N., Stewart, J., Gruenwald, N. J., Rigling, D., and Prospero, S. (2014). Population history and pathways of spread of the plant pathogen Phytophthora plurivora. PLoS One 9:e85368. doi: 10.1371/journal.pone.0085368
Serrano, M. S., Romero, M. Á, Homet, P., and Gómez-Aparicio, L. (2022). Climate change impact on the population dynamics of exotic pathogens: The case of the worldwide pathogen Phytophthora cinnamomi. Agric. For. Meteorol. 322:109002. doi: 10.1016/j.agrformet.2022.109002
Singh, S., Dalbehera, M. M., Maiti, S., Bisht, R. S., Balam, N. B., and Panigrahi, S. K. (2023). Investigation of agro-forestry and construction demolition wastes in alkali-activated fly ash bricks as sustainable building materials. J. Waste Manag. 159, 114–124. doi: 10.1016/j.wasman.2023.01.031
Skovsgaard, J. P., Thomsen, I. M., Skovgaard, I. M., and Martinussen, T. (2010). Associations among symptoms of dieback in even-aged stands of ash (Fraxinus excelsior L.). For. Pathol. 40, 7–18. doi: 10.1111/j.1439-0329.2009.00599.x
Slippers, B., and Wingfield, M. J. (2007). Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 21, 90–106. doi: 10.1016/j.fbr.2007.06.002
Smahi, H., Belhoucine-Guezouli, L., Berraf-Tebbal, A., Chouih, S., Arkam, M., Franceschini, A., et al. (2017). Molecular characterization and pathogenicity of Diplodia corticola and other Botryosphaeriaceae species associated with canker and dieback of Quercus suber in Algeria. Mycosphere 8, 1261–1272. doi: 10.5943/mycosphere/8/2/10
Sturrock, R. N., Frankel, S. J., Brown, A. V., Hennon, P. E., Kliejunas, J. T., Lewis, K. J., et al. (2011). Climate change and forest diseases. Plant Pathol. 60, 133–149. doi: 10.1111/j.1365-3059.2010.02406.x
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882.
Tkaczyk, M., Nowakowska, J. A., and Oszako, T. (2016). Phytophthora species isolated from ash stands in Białowieża Forest nature reserve. For. Pathol. 46, 660–662. doi: 10.1111/efp.12295
Ulbrich, U., Lionello, P., Belušić, D., Jacobeit, J., Knippertz, P., Kuglitsch, F. G., et al. (2012). “Climate of the Mediterranean: Synoptic patterns, temperature, precipitation, winds, and their extremes,” in The Climate of the Mediterranean Region, ed. P. Lionello (Amsterdam: Elsevier).
Urbez-Torres, J. R. (2011). The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 50, S5–S45. doi: 10.14601/Phytopathol_Mediterr-9316
Valdes-Abellan, J., Pardo, M. A., and Tenza-Abril, A. J. (2017). Observed precipitation trend changes in the western Mediterranean region. Int. J. Climatol. 37, 1285–1296. doi: 10.1002/joc.4984
Venette, R. C. (2009). “Implication of global climate change on the distribution and activity of Phytophthora ramorum,” in Proceedings of the 20th US Department of Agriculture Interagency Research Forum on Invasive Species, (Newtown Square, PA), 58–59.
Vettraino, A. M., Morel, O., Perlerou, C., Robin, C., Diamandis, S., and Vannini, A. (2005). Occurrence and distribution of Phytophthora species in European chestnut stands, and their association with ink disease and crown decline. Eur. J. Plant Pathol. 111, 169–180. doi: 10.1007/s10658-004-1882-0
White, T. J., Bruns, T., Lee, S. J. W. T., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols: A guide to methods and applications, Vol. 18, eds M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (New York, NY: Academic Press), 315–322.
Xue, D. S., Liu, J., Li, B. H., Xu, X. M., Liu, N., Lian, S., et al. (2021). Effect of rainfall and temperature on perithecium production of Botryosphaeria dothidea on cankered apple branches. Phytopathology 111, 982–989.
Keywords: ash-tree dieback, stem cankers, leaf and shoot blight, collar necrosis, root diseases, invasive pathogens, climate change
Citation: Benigno A, Bregant C, Aglietti C, Rossetto G, Tolio B, Moricca S and Linaldeddu BT (2023) Pathogenic fungi and oomycetes causing dieback on Fraxinus species in the Mediterranean climate change hotspot region. Front. For. Glob. Change 6:1253022. doi: 10.3389/ffgc.2023.1253022
Received: 04 July 2023; Accepted: 09 August 2023;
Published: 25 August 2023.
Edited by:
Nicolas Feau, Natural Resources Canada, CanadaReviewed by:
Ippolito Camele, University of Basilicata, ItalyCopyright © 2023 Benigno, Bregant, Aglietti, Rossetto, Tolio, Moricca and Linaldeddu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandra Benigno, YWxlc3NhbmRyYS5iZW5pZ25vQHVuaWZpLml0; Salvatore Moricca, c2FsdmF0b3JlLm1vcmljY2FAdW5pZmkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.