
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
POLICY AND PRACTICE REVIEWS article
Front. For. Glob. Change , 23 August 2023
Sec. Forest Disturbance
Volume 6 - 2023 | https://doi.org/10.3389/ffgc.2023.1239748
This article is part of the Research Topic Biotic Pest Disturbance - Risk, Evaluation, and Management in Forest Ecosystems View all 15 articles
Invasive bark beetles pose a threat to native biodiversity and to functional ecosystems and the economic productivity of forests, parks, and orchards. In the Czech Republic, there are six species of invasive ambrosia and bark beetles with a stable natural population, and it can be assumed that other invasive species that will be found. In the Czech Republic, there are no guidelines or methods for the early detection of invasive ambrosia and bark beetles. We propose monitoring at a total of 24 locations considering the following: (i) monitoring approaches used in other countries; (ii) identified entrance gates of invasive ambrosia and bark beetles found in the Czech Republic; (iii) presumed invasive species that occur in surrounding countries and are expanding their range; (iv) substances attractive to all the above mentioned species; (v) commonly available traps; and (vi) minimization of operating costs. Most of the chosen locations are located on the state borders and in river valleys, which are probably the entrance gates to the Czech Republic for invasive ambrosia and bark beetles. In addition, two large timber warehouses where international trade takes place, all international airports and three botanical gardens with tropical greenhouses were selected. Three Theysohn or Ecotrap impact traps should be installed every year at all locations. Traps should be baited with ethanol and exposed from mid-April to the end of July and should be checked every 2 weeks.
Invasive ambrosia and bark beetles (further BB) represent a threat to biodiversity, functional ecosystems, and the economic productivity of forestry (Brockerhoff et al., 2006; Aukema et al., 2011; Gohli et al., 2016), as well as to parks and orchards (Francardi et al., 2017; Branco et al., 2019; Fiala et al., 2022). BB are important vectors of fungal diseases that cause massive tree death. The simultaneous effect of several invasive species, their symbiotic fungi, and the subsequent interaction with climate change creates a situation in which it is difficult to predict the future impact of ambrosia and bark beetles on the environment (Lovett et al., 2013). Early detection is key to controlling BB because only then can a real integrated pest management (IPM) strategy be developed (Brockerhoff et al., 2006, 2010; Douglas et al., 2009; Samons, 2022).
Bark beetles spread in several ways, the most common being the global trade in wood material (treated and untreated wood), wooden packaging, and fruits or live seedlings of various non-native trees (Mathew, 1987; Meissner et al., 2008; Pombo et al., 2010; Augustin et al., 2012; Brockerhoff and Liebhold, 2017; Meurisse et al., 2019). It has also been confirmed that they can be introduced with wooden material that has been treated according to the international standard ISPM 15 (Haack and Petrice, 2009; Haack et al., 2014). In Europe, ports on the Atlantic and Mediterranean coasts are most often the gateway (Hagedorn, 1910; Hoffmann, 1942; Schedl, 1962; Cola, 1971, 1973; Faccoli, 2008; Moraal, 2010; Inghilesi et al., 2013; Rassati et al., 2015; Binazzi et al., 2019; Branco et al., 2019; Barnouin et al., 2020). Another entry point is botanical gardens, where non-native ambrosia and bark beetles may be introduced when expanding collections of exotic trees (Chobaut, 1897; Merkl and Tusnádi, 1992; Schuler et al., 2023).
Due to climate change, the host tree species are spreading northwards into areas where they did not originally occur (Ge et al., 2017). Even ambrosia and bark beetles, which are only found in southern Europe, may spread north; e.g., the bark beetle Phloeosinus aubei Perris, 1855 has spread to colder areas in Central Europe (Fiala and Holuša, 2019). Ambrosia and bark beetles not only spread through global trade but also naturally, as some are good flyers (Nilssen, 1984; Jones et al., 2019). Dry summers contribute to the appearance of ambrosia and bark beetles in alpine locations, even though they do not normally ascend to high altitudes, also (Marini et al., 2012).
However, the influence of humans on the spread of BB is far greater than the influence of climate (Gohli et al., 2016; Ward et al., 2019). Establishing plantations of non-native trees increases the risk of introducing non-native ambrosia and bark beetles (Lantschner et al., 2017). In Central Europe, this mainly concerns the cultivation of black pine (Pinus nigra) and bark beetles, which feed on it; Pityogenes bistridentatus Eichhoff, 1878 and Orthotomicus robustus Knotek, 1899 are found in several areas in the Czech Republic (Pfeffer and Knížek, 1996; Urban, 2000; Knížek, 2006; Knížek and Mertelík, 2017; Fiala et al., 2022). Climate change may help the maintenance of populations of BB on continents (Rassati et al., 2016a).
Most ambrosia and bark beetles are native to temperate and subtropical forests, so they represent the greatest danger for southern Europe due to a similar climate; hence, damage is most concentrated here (Pennacchio et al., 2004, 2012; Alfaro et al., 2007; Francardi et al., 2017; Leza et al., 2020). In the more northern countries of Europe, only damage by the ambrosia beetle Xylosandrus germanus Blandford, 1894 has been recorded (Maksymov, 1987; Graf and Manser, 2000; Galko et al., 2019).
Due to the economic and ecological damage caused by ambrosia and bark beetles, some governments perform regular monitoring of BB in their territory. This is helpful for identifying risk in a timely manner. There have been several monitoring attempts, of which baited traps are the most effective and least expensive method (Poland and Rassati, 2019).
Since BB are spreading increasingly around the world, there have also been efforts to introduce global monitoring. Observations were made on several continents at the same time to determine the abundance of ambrosia and bark beetles in the affected regions. The following semiochemicals were used in the traps: α-pinene + ethanol and α-pinene + ethanol + ipsdienol + ipsenol + Z-verbenol. The study is the first step toward the development of an international monitoring protocol based on trapping in traps baited with different types of substances (Faccoli et al., 2020).
There are six species of BB in the Czech Republic with a stable population in the wild (Knížek, 1988; Procházka et al., 2018; Fiala and Holuša, 2019; Fiala et al., 2020, 2021), and other species can be expected to occur in this territory (Gebhardt, 2014; Gebhardt and Doerfler, 2018). In the Czech Republic, there are no guidelines or methods for the early detection of BB. In addition, approximately half of the records of new species of ambrosia and bark beetles for the Czech Republic were accidental; the species were caught by amateur entomologists, and there was a delay of approximately 1–3 years between detection and publication (cf. Knížek, 2009a,b, 2011; Knížek and Kopecký, 2021). An extreme example is a report published 18 years after the species Pityophthorus balcanicus Pfeffer, 1940 was captured (Knížek and Liška, 2015). Therefore, it is necessary to create a stable network of traps for monitoring invasive species of ambrosia and bark beetles. To determine the methodology, several experiments were carried out in the Czech Republic, providing basic knowledge about the spread of BB and their bionomics in the Czech Republic (Fiala and Holuša, 2019, 2020; Fiala et al., 2020; Holuša et al., 2021; Fiala et al., 2023).
The aim of this work is to propose a methodology for monitoring BB based on the following:
(i) monitoring approaches in other countries;
(ii) the entrance gates of the existing species of BB found in the Czech Republic;
(iii) presumed species that occur in surrounding countries and are expanding their range;
(iv) substances attractive to all of the above;
(v) commonly available traps;
(vi) minimization of operating costs.
In Canada, the first attempts to detect BB were made at the end of the 1990s in the vicinity of Vancouver. The following substances were used for trapping: ethanol, α pinene, and attractants (cis-verbenol, ipsdienol, and methylbutenol) for Ips typographus Linnaeus, 1758 (Humble, 2001). Ethanol and α-pinene are kairomons for many ambrosia and bark beetles (Schroeder and Lindelöw, 1989). After that, long-term monitoring began, and was carried out in the period from 2000 to 2021. Each year between 2000 and 2011, six Lindgren funnel traps were installed at each of 63–80 locations (ports, industrial zones, and wood processing industries). Traps at each location included three baited with ethanol + α-pinene and cis-verbenol + ipsdienol + methylbutenol and three baited with ethanol alone. Since 2012, another trap baited with ethanol + C6-ketol + C8-ketol as aggregation pheromones have been added to longhorned beetles (see Hanks et al., 2019). Since 2015, traps for longhorned beetles have been baited with the combination of racemic (E,Z)-fuscumol + racemic (E,Z)-fuscumol acetate + ethanol and the combination of ipsenol + monochamol + α-pinene + ethanol. During the experiment, seven species of BB were captured, of which three species were new to Canada (Thurston et al., 2022).
The most sophisticated system of regular monitoring is carried out in the US, where monitoring has been ongoing for 20 years (Rabaglia et al., 2008). Even before the start of this program, BB were caught in ports and airports in the US (Rabaglia and Cavey, 1994; Haack, 2001, 2006; Mudge et al., 2001). The American system is based on a dense network of Lindgren funnel traps lured with ethanol, α-pinene + ethanol, and ipsdienol + cis-verbenol + methylbutenol, each separately. Traps are located mainly along both ocean coasts but also in the interior of the US. The US territory is divided into three parts, and each part is monitored once every 3 years. Even connected overseas territories such as Puerto Rico or Guam regularly participate in monitoring, where other volatile substances are also used for captures, such as manuka oil or ethanol + cubeb oil. Traps are located at seaports or at companies in the wood processing industry (Rabaglia et al., 2019). Data from this monitoring are used to determine the behavior of BB and to model their spread in the US (Rassati et al., 2016a). During the evaluation of this program (Rabaglia et al., 2019), ethanol was found to be the most suitable for trapping BB, while trapping with Ips lures was not effective for BB. Specific substances can be used to target selected BB (Hartshom et al., 2021).
Efforts to detect BB has also taken place in New Zealand. The first attempts to develop invasive species monitoring were in the 1980s (Hosking and Gadgil, 1987; Carter, 1989). Lindgren funnel traps with baits of α-pinene + ethanol, β-pinene + ethanol, frontalin + ethanol, and ipsdienol were also used in ports, international airports, and forests near these locations. This monitoring model has been proven to be successful in the early detection of BB, and it has, therefore, a good chance of eliminating these ambrosia and bark beetles (Brockerhoff et al., 2006). There was also an experimental trial to detect damage by invasive pests using field observations (car and walking) in New Zealand. Virtually no difference in results was found between these two methods (Bulman et al., 1999).
The monitoring of invasive species in Australia was broader; Lepidoptera was also caught. In sticky traps, Lindgren and Ecotrap. Ethanol, cineole, α-pinene, phellandrene, and a mixture of pinene, phellandrene, cineole, terpene, and cymene were used as bait. Traps were placed near ports and airports, and others were placed in a zone within 5 km of ports and airports (Bashford, 2012). The following baits were also tested in Brisbane harbor from 2006 to 2007: ipsenol, ipsdienol, frontalin, exo-brevicomin, and a combination of ethanol and α-pinene; a total of 29 species of ambrosia and bark beetles were caught (Wylie et al., 2008). In Tasmania, a method of static traps baited with a combination of α-pinene and ethanol was developed to monitor BB in Pinus radiata plantations (Bashford, 2008). These attempts subsequently developed into massive permanent monitoring throughout Australia (Carnegie et al., 2018, 2022; Carnegie and Nahrung, 2019).
In China, an IPM plan has been created and monitoring is carried out in designated areas using various methods, from baited traps with different types of semiochemicals to light traps to simply patrolling the area (Anonymus, 2009). At the same time, ambrosia and bark beetles are caught in ports (Lin et al., 2021). China also has an IPM standard for P. aubei, which causes serious damage to cypress trees there (Anonymus, 2017).
Other maritime countries also monitor BB in ports. In Japan, BB have been monitored in ports since the 1950s (Murayama, 1957; Schedl, 1966, 1969, 1970; Browne, 1980a,b; Ohno, 1989). In South Korea, BB were also monitored in harbors as early as the late 1970s (Choo et al., 1981; Choo and Woo, 1983; Choi et al., 2003).
In Italy, BB have long been monitored in ports (Cola, 1971, 1973). In total, 15 international ports and their adjacent forest stands are monitored; for trapping, Lindgren funnel traps and semiochemicals similar to those in the USA, ethanol, α pinene + ethanol, and ipsdienol + ipsenol + methylbutenol, are applied. Three traps were placed in the harbor, and three traps were placed in the adjacent forests. More species were found in deciduous forests than in coniferous stands. Invasive species richness was higher in forests than in harbors. The ambrosia and bark beetles were caught in the harbors, and were not yet able to establish a permanent population in the surrounding forests (Rassati et al., 2015). At Malpensa International Airport, the capture of invasive beetles in PET bottles was successfully tested using the following baits: apple cider vinegar, red wine, and 80% ethanol (Ruzzier et al., 2021).
Monitoring of invasive longhorned beetles (Cerambycidae) was launched in France, where they also tested trapping with α pinene + ethanol in Ecotrap traps. The traps were placed in natural forests and then in ports, airports, and orchards (Fan et al., 2019).
In Lithuania, as part of prevention, the bark beetle Dendroctonus rufipennis Kirby, 1837 was monitored in 2000 in the port of Klaipeda, near the Vaidotai railway station and along forest roads. D. rufipennis was not detected (Ostrauskas and Ferenca, 2010). In the period from 2002 to 2005, further monitoring was carried out at the borders, again in the port of Klaipeda, and at temporary wood warehouses, but no BB were caught. Lures α-pinene, myrcene, and cis-verbenol were used in Lindgren funnel traps (Ostrauskas and Tamutis, 2012).
Extensive monitoring of invasive species took place in Great Britain between 2013 and 2017. Lindgren funnel traps and cross-vane panel traps were placed in different types of forests near the ports. Ethanol and ethanol + α-pinene were used as bait. A total of three species of BB, Cyclorhipidion bodoanum, Gnathotrichus materiarius, and X. germanus, were captured (Inward, 2020).
In the Czech Republic, there are six species of BB with a stable natural population: C. bodoanum Reitter, 1913, Dryocoetes himalayensis Strohmeyer, 1908, G. materiarius Fitch, 1858, P. aubei, Xyleborinus attenuatus Blandford, 1894, and X. germanus (Knížek, 1988; Procházka et al., 2018; Fiala and Holuša, 2019; Fiala et al., 2020, 2021, 2023). Furthermore, several introduced species that could not form a stable population due to an unfavorable climate or absence of host plants were found in the territory of the Czech Republic: Coccotrypes dactyliperda Fabricius, 1801, Hypothenemus areccae Hornung, 1842, Hypothenemus hampei Ferrari, 1867, Hypothenemus setosus Eichhoff, 1868, Xyleborus affinis Eichhoff, 1868, Xyleborus volvulus Fabricius, 1794, and Xylosandrus morigerus Blandford, 1894 (Reitter, 1913; Fleischer, 1927–1930; Pfeffer and Knížek, 1989).
New invasive species of ambrosia and bark beetles which are already present in Germany may be expected to invade the Czech Republic. These include, Xyloterinus politus Say, 1826, which was detected in Bavaria in 2014 (Gebhardt and Doerfler, 2018), and Cyclorhipidion pelliculosum Eichhoff, 1878, which was found in Baden-Württemberg in 2013 (Gebhardt, 2014). The greatest economic danger to tree species in the Czech Republic is the bark beetle Pityophthorus juglandis Blackman, 1928, which has been spreading in Italy since 2013 and is a carrier of the serious fungal disease, thousand cankers disease (Montecchio and Faccoli, 2014). From the east, we can expect an invasion of the bark beetle Polygraphus proximus Blandford, 1894, which spreads from Siberia toward the west, and its harmfulness is comparable to that of I. typographus (Peña et al., 2020). Therefore, a pest risk analysis was developed for both species (EPPO, 2014, 2015).
The MaxEnt algorithm can be used to model the spread of invasive species around the world. For the invasive ambrosia beetle Xylosandrus compactus Eichhoff, 1876, which occurs in southern Europe (Pennacchio et al., 2012; Barnouin et al., 2020; Leza et al., 2020; Riba-Flinch et al., 2021), with the continuation of average climatic values from 1970 to 2000, X. compactus is predicted to find suitable ecological conditions for development in southern Moravia (which is the warmest region of the Czech Republic) by 2050 (Urvois et al., 2021).
Since 2020, efforts have been underway to determine the possible entry gates and directions of expansions of BB in the Czech Republic (Figure 1; Fiala and Holuša, 2019; Fiala et al., 2020, 2021, 2022, 2023). Potential types of volatile substances that could be used for monitoring were compared to find the simplest monitoring method (Fiala and Holuša, 2020; Fiala et al., 2023).
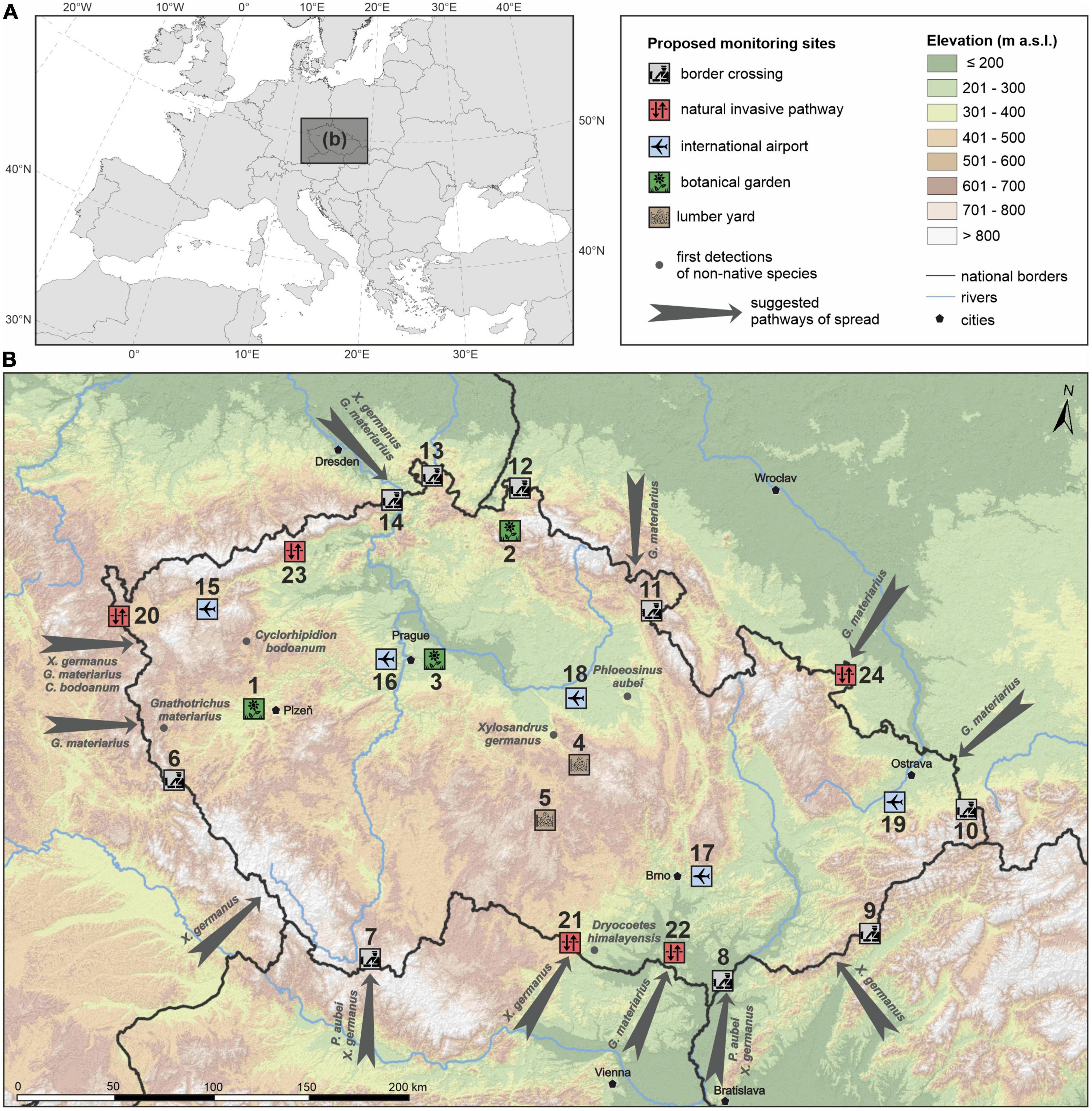
Figure 1. The position of the Czech Republic in Europe (A) and the possible entry gates, places of first detections, and a proposal for monitoring locations for invasive ambrosia and bark beetles in the Czech Republic (B).
The Czech Republic has no seaports, but has five international airports (Prague, Brno, Ostrava, Pardubice, and Karlovy Vary; Table 1) and many road and rail border crossings with foreign countries. Therefore, global trade is a possible reason for the flight activity of individual invasive species when entering the Czech Republic. In 2022, 302,640 tons of wood materials with a size larger than 6 mm were imported from all over the world into the Czech Republic, of which 4,993 tons were tropical wood of all kinds (ČSÚ, 2023).
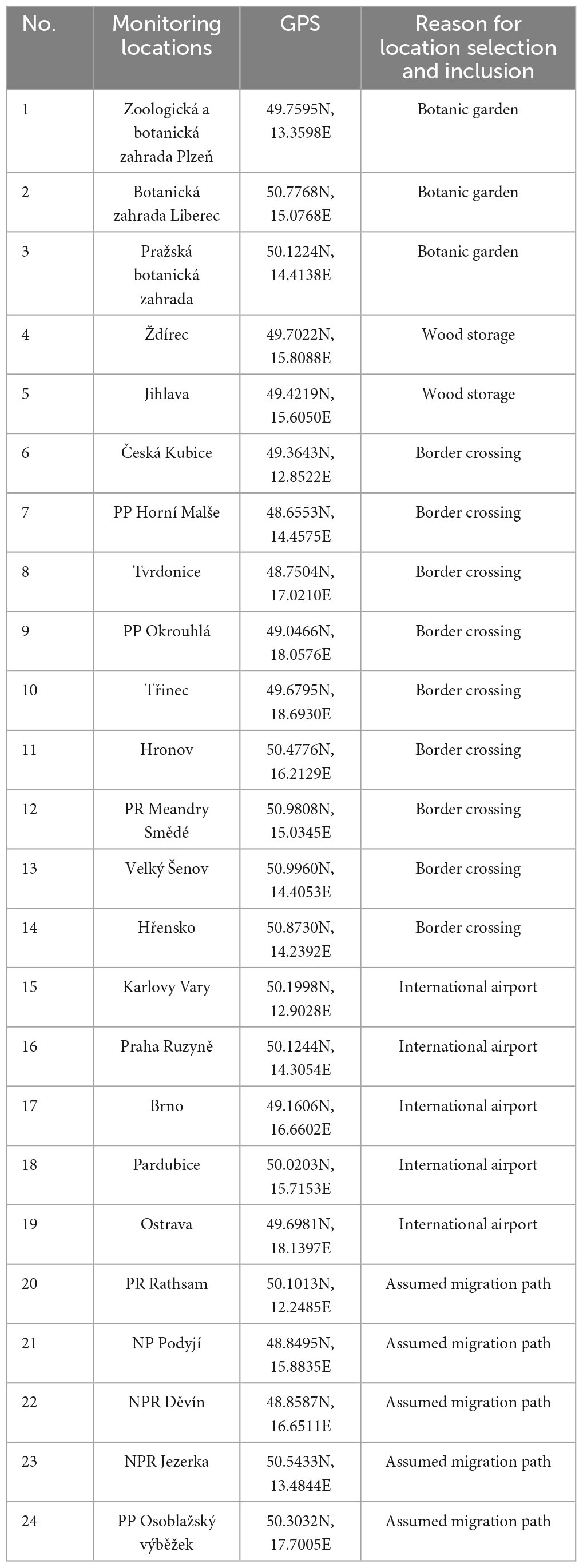
Table 1. Proposed localities for permanent monitoring of invasive ambrosia and bark beetles (types of protected areas of the Czech Republic: NP, National Park; NPR, National Nature Reserve; PP, Nature Monument; PR, Nature Reserve).
The invasive ambrosia beetle X. germanus in the middle of the Czech Republic in 2007 (Knížek, 2009a) was first found near the largest wood warehouse of Stora Enso in Ždírec nad Doubravou, similar to the invasive sawfly Urocerus albicornis Fabricius, 1781, was found on the grounds of the Kronospan wood processing plant in Jihlava (Háva and Holuša, 2019). The occurrence in botanical gardens through the importation of live exotic plants has only been demonstrated once in the Czech Republic, in the case of X. morigerus (Reitter, 1913); however, this does not mean that other introductions have not occurred and escaped notice. The ambrosia beetle G. materiarius was first found through flight monitoring near the border with Bavaria in western Bohemia (Knížek, 2009a). Likewise, the spreading of X. germanus in northern Bohemia and southern Moravia (Fiala et al., 2020) or D. himalayensis in southern Moravia (Procházka et al., 2018) is a result flight of beetles.
Most of the BB were found near the borders with Germany and Austria (cf. Fiala et al., 2021; Figure 1). This is logical because most of the BB in Europe have been detected near seaports in western and southern Europe. The main entry points were clearly identified as river valleys and border crossings (Fiala et al., 2020, 2023).
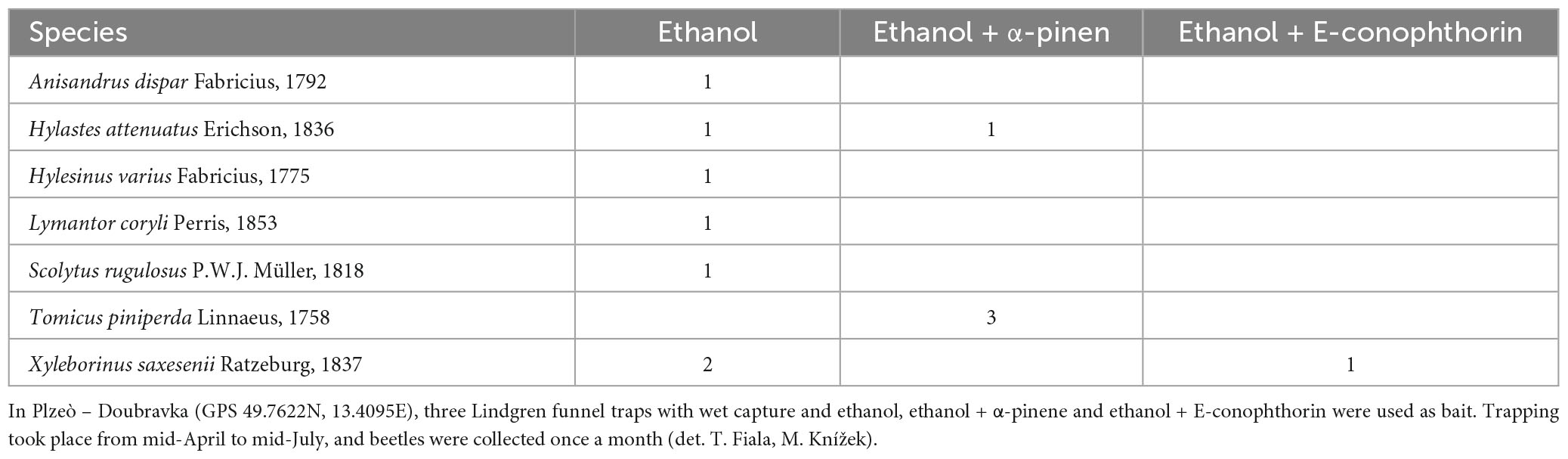
In 2021, two experiments were conducted to detect BB: (i) the capture of ambrosia and bark beetles at a warehouse of tropical wood imported from Central Africa in Pilsen – Doubravka town1 and (ii) the capture of ambrosia and bark beetles in the Botanical Garden in Prague – Troja with a tropical greenhouse, where tropical trees are brought in every year. This botanical garden is the largest in the Czech Republic, and its tropical greenhouse offers vegetation of dry tropics and subtropics, lowland rainforest, and tropical forests of high mountains.2
No invasive bark beetle was caught near Pilsen (Appendix Table 1); only the bark beetle Lymantor coryli Perris, 1855, which is rarely found throughout Europe, was detected (Fiala, 2021). No bark beetles were caught in the tropical greenhouse, but the two BB, X. germanus and D. himalayensis, were caught at the edge of oak forests (Appendix Table 2).
At the same time, at the end of 2021, 13 companies involved in the coffee trade in the Czech Republic were asked to cooperate to detect the occurrence of introduced species of ambrosia and bark beetles damaging coffee beans. Several samples of damaged beans were obtained, and the bark beetle H. hampei (Figure 2) from Brazil, Colombia, and India (Appendix Table 3) was detected by the occurrence several dead individuals in the Czech Republic. However, H. hampei does not pose a danger, even to undamaged coffee stocks, as its stages do not survive the Central European climate (Jaramillo et al., 2009). It can be speculated that beetles may, however, introduce various fungal and bacterial infections into uninfected beans (Damon, 2000; Jaramillo et al., 2006).

Figure 2. Dead individual of bark beetle H. hampei found in damaged coffee bean introduced to the Czech Republic.
The selection of locations is based on possible entry points such as border crossings, border river valleys, international airports, large timber warehouses, and botanical gardens; at the same time, these points will be used to monitor already established species whose abundance is still very low (Procházka et al., 2018; Fiala and Holuša, 2019, 2020; Fiala et al., 2020, 2021, 2022; Holuša et al., 2021). For the purposes of regular and permanent monitoring of BB, we therefore propose the following locations (Table 1 and Figure 1). A quarter of the locations are in protected areas; there is sufficient dead wood, and there are overgrown stands that provide a suitable environment for the development of ambrosia and bark beetles (Lee et al., 2019; Fiala et al., 2021).
Some invasive bark beetles are polyphagous, such as X. germanus (Weber and McPherson, 1983) and X. politus (MacLean and Giese, 1967), and can attack both coniferous and deciduous trees; some attack only deciduous trees, such as X. attenuatus (Kvamme et al., 2020), or only conifers, such as G. materiarius (Kamp, 1970). The representation of tree species is not significant for ambrosia and bark beetle monitoring because the type of forest has no effect on the abundance of beetles (Bouget et al., 2008). Therefore, the type of forest in which the trap is placed is not important, although a mixed forest with different tree species is preferable. We prefer oak forests, in the vicinity of which there are also conifers. In the Czech Republic, almost all forests are cultural, and conifers grow even at low altitudes. Therefore, choosing a combination of forests at the different locations was straightforward (Table 1).
Most BB in Europe are ambrosia species (Alonso-Zarazaga et al., 2023), and in our study in oak forests in western Bohemia, we found that ambrosia beetles had a higher abundance with a greater canopy cover, due to the wetter microclimate and greater amount of dead wood (Holuša et al., 2021). The influence of the close canopy on the abundance of ambrosia and bark beetles was also confirmed by Menocal et al. (2022). Therefore, forests with close canopy is generally preferred, although we are aware that C. bodoanum seems to prefer open forests (Fiala et al., 2021).
We also tested substances suitable for trapping BB. Factory-produced pheromones were suitable for trapping ambrosia and bark beetles of the genus Trypodendron; we found one specimen of X. germanus (Fiala and Holuša, 2020). Among volatile substances, we found the best combination of ethanol and juniper twigs suitable for trapping bark beetles P. aubei (Fiala et al., 2023). We found ethanol to be the most suitable for G. materiarius (Fiala et al., 2023). Likewise, C. bodoanum was captured in ethanol (Fiala et al., 2021), and although D. himalayensis and X. germanus were captured in impact traps as such, they were also captured in ethanol (Procházka et al., 2018; Hauptman et al., 2019a; Fiala et al., 2020; Appendix Table 2). X. attenuatus, like the ambrosia bark beetle, was attracted to ethanol (Galko et al., 2014).
Although sulcatol, which is considered a potential aggregation pheromone of G. materiarius, was expected to be successful (Flechtmann and Berisford, 2003), it was not the best lure tested in Central European conditions. The combination of sulcatol and ethanol resulted in the capture of a significantly greater number of beetles of Gnathotrichus sp. (McLean and Borden, 1977). However, in our case, ethanol alone captured more beetles than the combination of baits. Ethanol also significantly attracted other invasive ambrosia beetles, C. bodoanum, X. germanus, X. attenuatus, and other species of native ambrosia and bark beetles. Ethanol attracts both ambrosia and bark beetles X. politus and C. pelliculosum, which are already present in Germany (Ranger et al., 2011, 2014). Ethanol generally has a better capture ratio of invasive ambrosia beetles than the other substances (Fiala et al., 2023). Ethanol has long been known to be the main volatile substance on ambrosia and bark beetles (Kelsey and Joseph, 2003; Ranger et al., 2013, 2019).
For capturing and monitoring the dangerous invasive species P. juglandis, ethanol is also a suitable substance (Roling and Kearby, 1975). However, in acute situations, the monitoring network can be extended by adding a trap with the aggregation pheromone prenol, which was detected in this bark beetle (Seybold et al., 2015). Ethanol can also be used to detect P. proximus, although the beetles will most likely be caught in small quantities, as it reacts mainly to cis-verbenol, ipsdienol, and ipsenol (EPPO, 2014), like I. typographus (Schlyter et al., 1987). If the occurrence of P. proximus in the vicinity of the Czech Republic has already been predicted, the monitoring network can be expanded by adding another trap to the monitoring location with one of the industrial attractants containing cis-verbenol.
We propose total of 24 monitoring locations. Most of them are located at the border crossings of the Czech Republic and in river valleys, which are probably the entrance gates to the Czech Republic of BB (Figure 1). In addition, two large timber warehouses in which international trade takes place were selected (Žemlička, 2012), along with all international airports and three botanical gardens with tropical greenhouses. The latter locations cover a variety of modes of invasion by ambrosia and bark beetles: natural dispersal by the flight abilities of ambrosia and bark beetles and spread by global trade (Table 1).
We designed specific locations so that they were easily accessible in forests and were warmer locations of southern exposures. We selected overgrown forests near state borders or places that represent a “steppingstone,” as in the case of point 22, NPR Děvín (a woven area in an agricultural landscape), and point 23, NPR Jezerka (located on the migration route along the Ohøe River valley). From airports and large timber warehouses, we assume that bark beetles will fly to the nearest forest stands. Botanical gardens have the character of open forests and are mostly surrounded by forests, so localities in the territory of the garden have been suggested.
Three traps at each location is sufficient (Rassati et al., 2015; Thurston et al., 2022). In the Czech Republic, two types of impact traps are used; both are inexpensive and commonly available. They are easy to install and do not catch large numbers of non-target insects (Lubojacký and Holuša, 2014; Galko et al., 2016). The traps can be a Theysohn slot type, which is the most widely used in forestry in the Czech Republic (Zahradník and Zahradníková, 2016), or impact type Ecotrap, from which it is easier to extract the caught beetles. They can be disassembled after each season and stored in a much smaller space than the Theysohn traps.
These types of traps are primarily intended for catching economically important bark beetles that are attracted by specific pheromones (Flechtmann et al., 2000; Šramel et al., 2021); however, they can also be used to capture invasive species without any problems (Holuša et al., 2021; Fiala et al., 2023). Different species of ambrosia and bark beetles are found to prefer different types of traps. Dryoxylon onoharaense Murayama, 1934, an invasive species also found in Europe (Marchioro et al., 2022), or G. materiarius prefer the Ecotrap type. In contrast, bark beetles X. affinis and Premnobius cavipennis Eichhoff, 1878 prefer the Theysohn type (Flechtmann et al., 2000; Dodds et al., 2010; Miller and Crowe, 2011).
Each trap is baited with ethanol, which is universal for catching ambrosia and bark beetles (Rassati et al., 2016b; Chen et al., 2021). Traps should be placed between 30 and 50 m apart (Niemeyer, 1997; Rassati et al., 2014). Ethanol is also partly attractive to common species of ambrosia and bark beetles that live on conifers (Fiala et al., 2023). Traps should be operated from mid-April to the end of July, as the flight activity of ambrosia and bark beetles decreases in August (Fiala et al., 2023). Traps are checked once every 14 days, and the collected samples are then stored in the freezer for later determination. Ethanol should be changed in early June since the evaporators are active for approximately 60 days.3
In total, there are only 72 traps (e.g., three traps at 24 locations), which represent 144 ethanol lures per year (Appendix 4). Given that the Czech Republic is a small country, the number of locations is small, and monitoring should be carried out annually. Since most of the locations are forested, we suggest, if agreeable, partnering with the local forest administration of Forest of the Czech Republic (LČR, s.p., in Czech), a company that manages more than 50% of the Czech Republic’s forest stands and has cooperation with the Forest Advisory Service (Lesní ochranná služba in Czech) of Forestry and Game Management Research Institute (FGMRI, VÚLHM in Czech) Jíloviště at Prague, capital of the Czech Republic. In total, the LČR manages thousands of trappers throughout the country every year. The traps that we suggest, slightly more than 70 traps, are not difficult to manage because foresters move around the forests every day. Similarly, workers at the botanical gardens and timber warehouses move around daily and can send samples for determination. The average catch per trap in the world varies between 200 and 500 specimens, similarly in the Czech Republic it is between 50 and 500 specimens (Appendix Table 5).
The entire organization of monitoring corresponds to the activity and assignment of the Forest Advisory Service. The Forest Advisory Service deals with research, expert, and monitoring activities in forest protection against biotic pests. It monitors the occurrence of the bark beetle Ips duplicatus Sahlberg, 1836, every year. This monitoring has been ongoing for a total of 25 years, and during this period, a total of approximately 400 traps baited with I. duplicatus were placed around the country (Holuša et al., 2010; Knížek and Liška, 2022). The traps were checked by foresters, and beetles were collected and sent to FGMRI for determination. In Central Europe, other forest research institutes have also been involved in monitoring BB, e.g., in Slovenia (see Hauptman et al., 2019a), Slovakia (see Galko et al., 2014), and Latvia (see Ostrauskas and Tamutis, 2012); however, these were one-time events.
Our proposed monitoring of BB can be easily merged with the existing monitoring of I. duplicatus. It involves incorporating only 72 traps. The Forest Advisory Service would purchase ethanol vaporizers for cooperating entities and provide basic operator training; however, it is also possible to use a recorded instructional video. The total volume of all samples from the three traps does not exceed 1 dm3, so workers can place it in closed cans in any freezer where the insects will be frozen. It is necessary to determine the entire material of beetles into species by a specialist because data will be obtained on several species of ambrosia and bark beetles, especially rare ones (Fiala, 2021; Holuša et al., 2021; Fiala and Nakládal, 2022; Fiala et al., 2023).
Due to the importance of early detection of invasive species of ambrosia and bark beetles, the economic costs are minimal (Table 2) compared to the damage that can occur. In the US, the annual loss associated with all invasive species is estimated at $120 billion (Pimentel et al., 2005). In Europe, the loss caused by all invasive species is estimated to be hundreds of millions of € per year (Vilà et al., 2010); e.g., for invasive longhorned beetles of the genus Anoplophora, the cost of eliminating one infested hectare of vegetation is $25,000 (Anonymus, 2014). Estimated economic loss to landowners exceeded hundreds of dollars per hectare for invasive pests in Pinus taeda Linnaeus, 1753 stands in the southern US when no monitoring was performed (Susaeta et al., 2016). When carrying out integrated protection, the cost is less than the loss of value of the wood (Franjević et al., 2016). At the same time, lures require smaller financial expenditure than the human labor associated with the control of traps (Šramel et al., 2021).
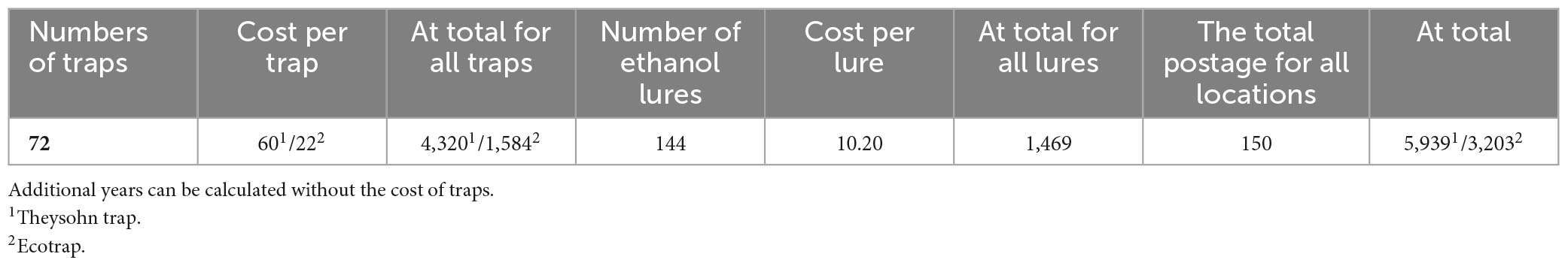
Table 2. Basic costs of operating the proposed monitoring network of invasive species of ambrosia and bark beetles in the Czech Republic (prices for the year 2023 in €) [energy costs (freezer), human fieldwork and labor costs, and determination costs are not included].
The proposed monitoring method based on commonly used traps in selected locations (entrance gates at borders, wood warehouses, tropical greenhouses, and airports) is necessary because we BB have already been detected in the Czech Republic. Therefore, it is necessary to monitor these species and be able to detect new ones. Ethanol is effective for capturing the species that have already been detected, and the method is inexpensive. The method can be implemented by the research institute for monitoring pests. The monitoring results can inform the professional actions of the Central Institute for Supervising and Testing in Agriculture and for the targeted eradication of invasive species, as required by EU regulations.
TF and JH contributed to the conception and design of the study and wrote the first draft of the manuscript. Both authors contributed to manuscript revision, read, and approved the submitted version.
We authors thank Jiøí Samek for support with the fieldwork and Zbynìk Kejval (Domažlice) for photographs of H. hampei specimen.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alfaro, R. I., Humble, L. M., Gonzalez, P., Villaverde, R., and Allegro, G. (2007). The threat of the ambrosia beetle Megaplatypus mutatus (Chapuis) (=Platypus mutatus Chapuis) to world poplar resources. Forestry 80, 471–479. doi: 10.1093/forestry/cpm029
Alonso-Zarazaga, M. A., Barrios, H., Borovec, R., Caldara, R., Colonnelli, E., Gültekin, L., et al. (2023). Cooperative catalogue of palaearctic coleoptera curculionoidea, 2nd Edn. Zaragoza: Sociedad Entomológica Aragonesa S.E.A.
Anonymus, (2009). Integrated forestry development project. Integrated pest management plan. Washington, DC: World Bank Loan Project Management Center.
Anonymus (2014). Risk management for the EC listed Anoplophora species, A. chinensis nad A. glabripennis. Final Draft Anoplorisk Report. Rotterdam: Euphresco.
Anonymus (2017). Technical regulations for controlling Phloeosinus aubei Perris. Beijing: State Forestry Administration.
Augustin, S., Boonham, N., De Kogel, W. J., Donner, P., Faccoli, M., Lees, D. C., et al. (2012). A review of pest surveillance techniques for detecting quarantine pests in Europe. EPPO Bull. 42, 515–551. doi: 10.1111/epp.2600
Aukema, J. E., Leung, B., Kovacs, K., Chivers, C., Britton, K. O., Englin, J., et al. (2011). Economic impacts of non-native forest insects in the continental United States. PLoS One 6:e24587. doi: 10.1371/journal.pone.0024587
Barnouin, T., Soldati, F., Roques, A., Faccoli, M., Kirkendall, L. R., Mouttet, R., et al. (2020). Bark beetles and pinhole borers recently or newly introduced to France (Coleoptera: Curculionidae, Scolytinae and Platypodinae). Zootaxa 4877, 051–074. doi: 10.11646/zootaxa.4877.1.2
Bashford, R. (2008). The development of static trapping systems to monitor for wood-boring insects in forestry plantations. Austr. For. 71, 236–241.
Bashford, R. (2012). “The development of a port surrounds trapping systém for the detection of exotic forest insect pests in Australia,” in New advances and contributions to forestry research, ed. A. A. Oteng-Amoako (London: InTechOpen), doi: 10.5772/35068
Binazzi, F., Del Nista, D., Peverieri, G. S., Marianelli, L., Roversi, P. F., and Pennacchio, F. (2019). Saperda tridentata Olivier (Coleoptera Cerambycidae Lamiinae): continuous interceptions at the italian port of Livorno represent a growing challenge for phytosanitary services. Redia 102, 171–176. doi: 10.19263/REDIA-102.19.24
Bouget, C., Brustel, H., Brin, A., and Noblecourt, T. (2008). Sampling saproxylic beetles with window flight traps: methodological insights. Revue Ecol. Terre et Vie 10, 21–32.
Branco, M., Nunes, P., Roques, A., Fernandes, M. R., Orazio, C., and Jactel, H. (2019). Urban trees facilitate the establishment of non-native forest insects. NeoBiota 52, 25–46. doi: 10.3897/neobiota.52.36358
Brockerhoff, E. G., Jones, D. C., Kimberley, M. O., Suckling, D. M., and Donaldson, T. (2006). Nationwide survey for invasive wood-boring and bark beetles (Coleoptera) using traps baited with pheromones and kairomones. For. Ecol. Manage. 228, 234–240. doi: 10.1016/j.foreco.2006.02.046
Brockerhoff, E. G., and Liebhold, A. M. (2017). Ecology of forest insect invasions. Biol. Invas. 19, 3141–3159. doi: 10.1007/s10530-017-1514-1
Brockerhoff, E. G., Liebhold, A. M., Richardson, B., and Suckling, D. M. (2010). Eradication of invasive forest insects: concepts, methods, costs and benefits. N. Zeal. J. For. Sci. 40, S117–S135.
Browne, F. G. (1980a). Bark beetles and ambrosia beetles (Coleoptera, Scolytidae and Platypodidae) intercepted at Japanese ports, with descriptions of new species, II. Kontyû 48, 380–389.
Browne, F. G. (1980b). Bark beetles and ambrosia beetles (Coleoptera, Scolytidae and Platypodidae) intercepted at Japanese ports, with descriptions of new species, III. Kontyû 48, 482–489.
Bulman, L. S., Kimberley, M. O., and Gadgil, P. D. (1999). Estimation of the efficiency of pest detection surveys. N. Zeal. J. For. Sci. 29, 102–115.
Carnegie, A. J., Lawson, S., Wardlaw, T., Cameron, N., and Venn, T. (2018). Benchmarking forest health surveillance and biosecurity activities for managing Australia’s exotic forest pest and pathogen risks. Austr. For. 81, 14–23. doi: 10.1080/00049158.2018.1433271
Carnegie, A. J., and Nahrung, H. F. (2019). Post-border forest biosecurity in Australia: response to recent exotic detections, current surveillance and ongoing needs. Forests 10, 336. doi: 10.3390/f10040336
Carnegie, A. J., Tovar, F., Collins, S., Lawson, S. A., and Nahrung, H. F. (2022). A coordinated, risk-based, National Forest Biosecurity Surveillance Program for Australian forests. Front. For. Glob. Change 4:756885. doi: 10.3389/ffgc.2021.756885
Carter, P. C. S. (1989). Risk assessment and pest detection surveys for exotic pests and diseases which threaten commercial forestry in New Zealand. N. Zeal. J. For. Sci. 19, 353–374.
Chen, Y., Coleman, T. W., Ranger, C. M., and Seybold, S. J. (2021). Differential flight responses of two ambrosia beetles to ethanol as indicators of invasion biology: the case with Kuroshio shot hole borer (Euwallacea kuroshio) and fruit-tree pinhole borer (Xyleborinus saxesenii). Ecol. Entomol. 46, 651–667. doi: 10.1111/een.13013
Chobaut, A. (1897). Sur un Xyleborus parasite. D’une orchidée des serres européennes. Ann. Soc. Entomol. France 66, 261–264.
Choi, E. J., Choo, H. Y., Lee, D. W., Lee, S. M., and Park, J. K. (2003). Scolytidae, Platypodidae, Bostrichidae and Lyctidae intercepted from imported timbers at Busan port entry. Kor. J. Appl. Entomol. 42, 173–184.
Choo, H. Y., and Woo, K. S. (1983). Classification of the Scolytidae and Platypodidae intercepted from imported timbers III. Kor. J. Plant Prot. 22, 35–41.
Choo, H. Y., Woo, K. S., and Kim, B. H. (1981). Classification of the Scolytidae and Platypodidae intercepted from imported timbers I. Kor. J. Plant Prot. 20, 196–206.
Cola, L. (1971). Mit fremden Hölzern eingeschleppte Insekten, insbesondere Scolytidae und Platypodidae. Anz. Schädlingsk. Pflanzensch. 44, 65–68. doi: 10.1007/BF02027387
Cola, L. (1973). Mit fremden Hölzern eingeschleppte Insekten, insbesondere Scolytidae und Platypodidae (2. Beitrag). Anz. Schädlingsk. Pflanzen Umweltsch. 46, 7–11. doi: 10.1007/BF01992961
Damon, A. (2000). A review of the biology and control of the coffee berry borer Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Entomol. Res. 90, 453–465. doi: 10.1017/S0007485300000584
Dodds, K. J., Dubois, G. D., and Hoebeke, E. R. (2010). Trap type, lure placement, and habitat effects on Cerambycidae and Scolytinae (Coleoptera) catches in the northeastern United States. J. Econ. Entomol. 103, 698–707. doi: 10.1603/EC09395
Douglas, H., Dang, P. T., Gill, B. D., Huber, J., Mason, P. G., Parker, D. J., et al. (2009). The importance of taxonomy in responses to invasive alien species. Biodiversity 10, 92–99. doi: 10.1080/14888386.2009.9712850
EPPO (2015). Pest risk analysis for Thousand cankers disease (Geosmithia morbida and Pityophthorus juglandis). Paris: EPPO.
Faccoli, M. (2008). First record of Xyleborus atratus Eichhoff from Europe, with an illustrated key to the European Xyleborini (Coleoptera: Curculionidae: Scolytinae). Zootaxa 1772, 55–62. doi: 10.11646/zootaxa.1772.1.2
Faccoli, M., Gallego, D., Branco, M., Brockerhoff, E. G., Corley, J., Coyle, D. R., et al. (2020). A first worldwide multispecies survey of invasive mediterranean pine bark beetles (Coleoptera: Curculionidae, Scolytinae). Biol. Invas. 22, 1785–1799. doi: 10.1007/s10530-020-02219-3
Fan, J.-T., Denux, O., Courtin, C., Bernard, A., Javal, M., Millar, J. G., et al. (2019). Multi-component blends for trapping native and exotic longhorn beetles at potential points-of-entry and in forests. J. Pest Sci. 92, 281–297. doi: 10.1007/s10340-018-0997-6
Fiala, T. (2019). Kůrovci (Coleoptera: Curculionidae: Scolytinae) v národní přírodní památce Komorní hůrka. Západ. Entomol. Listy 10, 34–39.
Fiala, T. (2021). Výskyt kůrovce Lymantor coryli (Coleoptera: Curculionidae: Scolytinae) v České republice. Západ. Entomol. Listy 12, 80–83.
Fiala, T., and Holuša, J. (2019). Occurrence of the invasive bark beetle Phloeosinus aubei on common juniper trees in the Czech Republic. Forests 10, 12. doi: 10.3390/f10010012
Fiala, T., and Holuša, J. (2020). Trapping ambrosia beetles by artificially produced lures in an oak forest. Plant Protect. Sci. 56, 226–230. doi: 10.17221/133/2019-PPS
Fiala, T., Holuša, J., Procházka, J., Čížek, L., Dzurenko, M., Foit, J., et al. (2020). Xylosandrus germanus in central Europe: Spread into and within the Czech Republic. J. Appl. Entomol. 144, 423–433. doi: 10.1111/jen.12759
Fiala, T., Holuša, J., and Véle, A. (2022). Both native and invasive bark beetles threaten exotic conifers within the spa towns in the Czech part of,, The Great Spas of Europe“. Urban For. Urban Green. 67, 127417. doi: 10.1016/j.ufug.2021.127417
Fiala, T., Knížek, M., and Holuša, J. (2021). Continued eastward spread of the invasive ambrosia Cyclorhipidion bodoanum (Reitter, 1913) in Europe and its distribution in the world. BioInvas. Records 10, 65–73. doi: 10.3391/bir.2021.10.1.08
Fiala, T., and Nakládal, O. (2022). Výskyt kůrovce Kissophagus novaki (Coleoptera: Curculionidae: Scolytinae) v Česku. Západ. Entomol. Listy 13, 75–77.
Fiala, T., Pyszko, P., and Holusa, J. (2023). Efficacy of different lures for Phloeosinus aubei and other native and exotic bark and ambrosia beetles. Ann. Appl. Biol. 2023, 1–12. doi: 10.1111/aab.12860
Flechtmann, C. A. H., and Berisford, C. W. (2003). Identification of sulcatol, a potential pheromone of the ambrosia beetle Gnathotrichus materiarius (Col., Scolytidae). J. Appl. Entomol. 127, 189–194. doi: 10.1046/j.1439-0418.2003.00743.x
Flechtmann, C. A. H., Ottati, A. L. T., and Berisford, C. W. (2000). Comparison of four trap types for ambrosia beetles (Coleoptera, Scolytidae) in brazilian Eucalyptus stands. J. Econ. Entomol. 93, 1701–1707. doi: 10.1603/0022-0493-93.6.1701
Fleischer, A. (1927–1930). Pøehled broukù fauny Československé republiky. Brno: Moravské zemské museum.
Francardi, V., Noal, A., Francescato, S., Pinto, R., Bruni, A., Loffredi, L., et al. (2017). Coexistence of Xylosandrus crassiusculus (Motschulsky) and X. compactus (Eichhoff) (Coleoptera Curculionidae Scolytinae) in the National park of Circeo (Lazio, Italy). Redia 100, 149–155. doi: 10.19263/REDIA-100.17.19
Franjević, M., Poršinsky, T., and Ðuka, A. (2016). Integrated oak timber protection from ambrosia bark beetles: Economic and ecological importance in harvesting operations. Croatian J. For. Eng. 37, 353–364.
Galko, J., Dzurenko, M., Ranger, C. M., Kulfan, J., Kula, E., Nikolov, C., et al. (2019). Distribution, habitat preference, and management of the invasive ambrosia beetle, Xylosandrus germanus (Coleoptera: Curculionida, Scolytinae) in european forests with an emphasis on the West Carpathians. Forests 10, 10. doi: 10.3390/f10010010
Galko, J., Nikolov, C., Kimoto, T., Kunca, A., Gubka, A., Vakula, J., et al. (2014). Attraction of ambrosia beetles to ethanol baited traps in a Slovakian oak forest. Biologia 69, 1376–1383. doi: 10.2478/s11756-014-0443-z
Galko, J., Nikolov, C., Kunca, A., Vakula, J., Gubka, A., Zúbrik, M., et al. (2016). Effectiveness of pheromone traps for the European spruce bark beetle: a comparative study of four commercial products and two new models. For. J. 62, 207–215. doi: 10.1515/forj-2016-0027
Ge, X., Jiang, C., Chen, L., Qiu, S., Zhao, Y., Wang, T., et al. (2017). Predicting the potential distribution in China of Euwallacea fornicates (Eichhoff) under current and future climate conditions. Sci. Rep. 7, 1–13. doi: 10.1038/s41598-017-01014-w
Gebhardt, H. (2014). Erstfund des Ambrosiakäfers Cyclorhipidion pelliculosum (Eichhoff) in Deutschland (Coleoptera, Curculionidae, Scolytinae). Mitteilung. Entomol. Vereins Stuttg. 49, 67–69.
Gebhardt, H., and Doerfler, I. (2018). Erster Nachweis von Xyloterinus politus (Say 1826) (Coleoptera, Curculionidae, Scolytinae) in Deutschland. Mitteilung. Entomol. Vereins Stuttg. 53, 61–63.
Gohli, J., Selvarajah, T., Kirkendall, L. R., and Jordal, B. H. (2016). Globally distributed Xyleborus species reveal recurrent intercontinental dispersal in a landscape of ancient worldwide distributions. BMC Evol. Biol. 16:37. doi: 10.1186/s12862-016-0610-7
Graf, E., and Manser, P. (2000). Beitrag zum eingeschleppten Schwarzen Nutzholzborkenkäfer Xylosandus germanus. Biologie und Schadenpotential an im Wald gelagertem Rundholz im Vergleich zu Xyloterus lineatus und Hylecoetus dermestoides. Schweiz. Zeitsch. Forstwes. 151, 271–281.
Haack, R. A. (2001). Intercepted Scolytidae (Coleoptera) at U.S. ports of entry: 1985–2000. Integr. Pest Manage. Rev. 6, 253–282. doi: 10.1023/A:1025715200538
Haack, R. A. (2006). Exotic bark- and wood-boring Coleoptera in the United States: recent establishments and interceptions. Can. J. For. Res. 36, 269–288. doi: 10.1139/X05-249
Haack, R. A., Britton, K. O., Brockerhoff, E. G., Cavey, J. F., Garrett, L. J., Kimberley, M., et al. (2014). Effectiveness of the International Phytosanitary Standard ISPM No. 15 on reducing wood borer infestation rates in wood packaging material entering the United States. PLoS One 9:e96611. doi: 10.1371/journal.pone.0096611
Haack, R. A., and Petrice, T. R. (2009). Bark- and wood-borer colonization of logs and lumber after heat treatment to ISPM 15 specifications: the role of residual bark. J. Econ. Entomol. 102, 1075–1084. doi: 10.1603/029.102.0328
Hanks, L. M., Mongold-Diers, J. A., Mitchell, R. F., Zou, Y., Wong, J. C. H., Meier, L. R., et al. (2019). The role of minor pheromone components in segregating 14 species of longhorned beetles (Coleoptera: Cerambycidae) of the subfamily Cerambycinae. J. Econ. Entomol. 112, 2236–2252. doi: 10.1093/jee/toz141
Hartshom, J. A., Coyle, D. R., and Rabaglia, R. J. (2021). Responses of native and non-native bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to different chemical attractants: Insights from the USDA Forest Service Early Detection and Rapid Response program data analysis. J. Economic Entomol. 114, 776–783. doi: 10.1093/jee/toaa309
Hauptman, T., Piškur, B., Faccoli, M., Rekanje, B., Marinč, A., and Jurc, M. (2019a). The first record of two non-native ambrosia beetles in Slovenia: Ambrosiodmus rubricollis (Eichhoff, 1875) and Ambrosiophilus atratus (Eichhoff, 1875) (Coleoptera: Curculionidae, Scolytinae). Zootaxa 4657, 397–400. doi: 10.11646/zootaxa.4657.2.13
Hauptman, T., Pavlin, R., Grošelj, P., and Jurc, M. (2019b). Distribution and abundance of the alien Xylosandrus germanus and other ambrosia beetles (Coleoptera: Curculionidae, Scolytinae) in different forest stands in central Slovenia. iForest – Biogeosci. For. 12, 451–458. doi: 10.3832/ifor3114-012
Háva, J., and Holuša, J. (2019). First record of the siricid Urocerus albicornis, an invasive alien pest, in the Czech Republic. J. Appl. Entomol. 143, 487–491. doi: 10.1111/jen.12596
Hoffmann, A. (1942). Description d’un genre nouveau et observations diverses sur plusieurs especes de Scolytidae [Col.] de la faune francaise. Bull. Soc. Entomol. France 47, 72–74.
Holuša, J., Fiala, T., and Foit, J. (2021). Ambrosia beetles prefer closed canopies: A case study in oak forests in central Europe. Forests 12, 1223. doi: 10.3390/f12091223
Holuša, J., Lubojacký, J., and Knížek, M. (2010). Distribution of the double-spined spruce bark beetle Ips duplicatus in the Czech Republic: spreading in 1997–2009. Phytoparasitica 38, 435–443. doi: 10.1007/s12600-010-0121-9
Hosking, G. P., and Gadgil, P. D. (1987). Development of contingency plans for use against exotic pests and diseases of trees and timber. Austr. For. 50, 37–39.
Humble, L. M. (2001). “Invasive bark and wood-boring beetles in British Columbia, Canada,” in Protection of World Forests: Advances in Research, Proceedings: XXI IUFRO World Congress. August 7-12, 2001, eds R. I. Alfaro, K. R. Day, S. M. Salom, K. S. S. Nair, H. F. Evans, A. M. Liebhold, et al. (Kuala Lumpur: IUFRO), 69–77.
Inghilesi, A. F., Mazza, G., Cervo, R., Gherardi, F., Sposimo, P., Tricarico, E., et al. (2013). Alien insects in Italy: Comparing patterns from the regional to European level. J. Insect Sci. 13, 73. doi: 10.1673/031.013.7301
Inward, D. J. G. (2020). Three new species of ambrosia beetles established in Great Britain illustrate unresolved risks from imported wood. J. Pest Sci. 93, 117–126. doi: 10.1007/s10340-019-01137-1
Jaramillo, J., Borgemeister, C., and Baker, P. (2006). Coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): searching for sustainable control strategies. Bull. Entomol. Res. 96, 223–233. doi: 10.1079/BER2006434
Jaramillo, J., Chabi-Olaye, A., Kamonjo, C., Jaramillo, A., Vega, F. E., Poehling, H.-M., et al. (2009). Thermal tolerance of the Coffee berry borer Hypothenemus hampei: Predictions of climate change impact on a tropical insect pest. PLoS One 4:e6487. doi: 10.1371/journal.pone.0006487
Jones, K. L., Shegelski, V. A., Marculis, N. G., Wijerathna, A. N., and Evenden, M. L. (2019). Factors influencing dispersal by flight in bark beetles (Coleoptera: Curculionidae: Scolytinae): from genes to landscapes. Can. J. For. Res. 49, 1024–1041. doi: 10.1139/cjfr-2018-0304
Kamp, H. J. (1970). Zur Biologie und derzeitigen Verbreitung von Gnathotrichus materiarius Fitch und Xylosandrus germanus Blandf. in der Bundesrepublik Deutschland. Verein Entomol. 5, 34–40.
Kelsey, R. G., and Joseph, G. (2003). Ethanol in ponderosa pine as an indicator of physiological injury from fire and its relationship to secondary beetles. Can. J. For. Res. 33, 870–884. doi: 10.1139/x03-007
Knížek, M. (1988). Coleoptera, Scolytidae: Xyleborus alni Niijima, 1909. Acta Entomol. Bohemosl. 85, 396.
Knížek, M. (2006). “Nepůvodní druhy kůrovcovitých v Česku,” in Zoologické dny Brno 2006. Sborník abstraktů z konference 9.-10. února 2006, eds J. Bryja and J. Zukal (Brno: Ústav biologie obratlovců AV ČR), 98–99.
Knížek, M., and Kopecký, T. (2021). Faunistic records from the Czech Republic – 505. Klapalekiana 57, 157–158.
Knížek, M., and Liška, J. (2015). Faunistic records from the Czech Republic – 381. Klapalekiana 51, 92.
Knížek, M., and Liška, J. (2022). Výskyt lesních škodlivých činitelù v roce 2021 a jejich očekávaný stav v roce 2022. Zprav. Ochrany Lesa 2022, 1–86.
Knížek, M., and Mertelík, J. (2017). Faunistic records from the Czech Republic – 411. Klapalekiana 53, 26.
Kvamme, T., Lindelöw, Å, and Knížek, M. (2020). Xyleborinus attenuatus (Blandford, 1894) (Coleoptera, Curculionidae, Scolytinae) in Scandinavia. Norw. J. Entomol. 67, 19–30.
Lantschner, M. V., Atkinson, T. H., Corley, J. C., and Liebhold, A. M. (2017). Predicting North American Scolytinae invasions in the Southern Hemisphere. Ecol. Applic. 27, 66–77. doi: 10.1002/eap.1451
Lee, J., Mendel, H., Knížek, M., and Barclay, M. V. L. (2019). Cyclorhipidion bodoanum (Reitter, 1913) (Curculionidae: Scolytinae: Xyleborini) new to Britain. Coleopterist 28, 65–70.
Leza, M., Nuñez, L., Riba, J. M., Comparini, C., Roca, Á, and Gallego, D. (2020). First record of the black twig borer, Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) in Spain. Zootaxa 4767, 345–350. doi: 10.11646/zootaxa.4767.2.9
Lin, W., Xu, M., Gao, L., Ruan, Y., Lai, S., Xu, Y., et al. (2021). New records of two invasive ambrosia beetles (Curculionidae: Scolytinae: Xyleborini) to mainland China. BioInvas. Records 10, 74–80. doi: 10.3391/bir.2021.10.1.09
Lovett, G. M., Arthur, M. A., Weathers, K. C., and Griffin, J. M. (2013). Effects of introduced insects and diseases on forest ecosystems in the Catskill Mountains of New York. Ann. N. Y. Acad. Sci. 1298, 66–77. doi: 10.1111/nyas.12215
Lubojacký, J., and Holuša, J. (2014). Effect of insecticide-treated trap logs and lure traps for Ips typographus (Coleoptera: Curculionidae) management on nontarget arthropods catching in Norway spruce stands. J. For. Sci. 60, 6–11. doi: 10.17221/62/2013-JFS
MacLean, D. B., and Giese, R. L. (1967). The life history of the ambrosia beetle Xyloterinus politus (Coleoptera: Scolytidae). Can. Entomol. 99, 285–299. doi: 10.4039/Ent99285-3
Maksymov, J. K. (1987). Erstmaliger Massenbefall des schwarzen Nutzholzborkenkäfers, Xylosandrus germanus Blandf., in der Schweiz. Schweiz. Zeitsch. Forstwesen 138, 215–227. doi: 10.5169/seals-766029
Marchioro, M., Faccoli, M., Cortivo, M. D., Branco, M., Roques, A., Garcia, A., et al. (2022). New species and new records of exotic Scolytinae (Coleoptera, Curculionidae) in Europe. Biodivers. Data J. 10, e93995. doi: 10.3897/BDJ.10.e93995
Marini, L., Ayres, M. P., Battisti, A., and Faccoli, M. (2012). Climate affects severity and altitudinal distribution of outbreaks in an eruptive bark beetle. Clim. Change 115, 327–341. doi: 10.1007/s10584-012-0463-z
Mathew, G. (1987). Insects borers of commercially important stored timber in the state of Kerala, India. J. Stored Prod. Res. 23, 185–190. doi: 10.1016/0022-474X(87)90001-4
McLean, J. A., and Borden, J. H. (1977). Attack by Gnathotrichus sulcatus (Coleoptera: Scolytidae) on stumps and felled trees baited with sulcatol and ethanol. Can. Entomol. 109, 675–686. doi: 10.4039/Ent109675-5
Meissner, H. E., Culliney, T. W., Lemay, A. V., Newton, L. P., and Bertone, C. A. (2008). Wood packaging material as a pathway for the movement of exotic insect pests into and within the Greater Caribbean Region. Proc. Caribb. Food Crops Soc. 44, 621–627.
Menocal, O., Kendra, P. E., Padilla, A., Chagas, P. C., Chagas, E. A., Crane, J. H., et al. (2022). Influence of canopy cover and meteorological factors on the abundance of bark and ambrosia beetles (Coleoptera: Curculionidae) in avocado orchards affected by Laurel Wilt. Agronomy 12, 547. doi: 10.3390/agronomy12030547
Merkl, O., and Tusnádi, C. K. (1992). First introduction of Xyleborus affinis (Coleoptera: Scolytidae), a pest of Dracaena fragrans ‘Massangeana’, to Hungary. Folia Entomol. Hung. 52, 67–72.
Meurisse, N., Rassati, D., Hurley, B. P., Brockerhoff, E. G., and Haack, R. A. (2019). Common pathways by which non-native forest insects move internationally and domestically. J. Pest Sci. 92, 13–27. doi: 10.1007/s10340-018-0990-0
Miller, D. R., and Crowe, C. M. (2011). Relative performance of lindgren multiple-funnel, intercept panel, and colossus pipe traps in catching Cerambycidae and associated species in the southeastern United States. J. Econ. Entomol. 104, 1934–1941. doi: 10.1603/EC11166
Montecchio, L., and Faccoli, M. (2014). First record of Thousand cankers disease Geosmithia morbida and Walnut twig beetle Pityophthorus juglandis on Juglans nigra in Europe. Plant Dis. 98, 696.
Moraal, L. G. (2010). Infestations of the cypress bark beetles Phloeosinus rudis, P. bicolor and P. thujae in The Netherlands (Coleoptera: Curculionidae: Scolytinae). Entomol. Berichten 70, 140–145.
Mudge, A. D., LaBonte, J. R., Johnson, K. J. R., and LaGasa, E. H. (2001). Exotic woodboring Coleoptera (Micromalthidae, Scolytidae) and Hymenoptera (Xiphydriidae) new to Oregon and Washington. Proc. Entomol. Soc. Washington 103, 1011–1019.
Murayama, J. J. (1957). Bark-beetles and pin-hole borers recently imported into Japan with timbers from the United States and other foreign countries. Pan-Pac. Entomol. 33, 35–37.
Niemeyer, H. (1997). “Integrated bark beetle control: experiences and problems in Northern Germany,” in Proceedings: Integrating cultural tactics into the management of bark beetle and reforestation pests. Vallombrosa, Italy, September 1-3, 1996, eds J. C. Grégoire, A. M. Liebhold, F. M. Stephen, K. R. Day, and S. M. Salom (Radnor: USDA Forest Service), 80–86.
Nilssen, A. C. (1984). Long-range aerial dispersal of bark beetles and bark weevils (Coleoptera, Scolytidae and Curculionidae) in northern Finland. Ann. Entomol. Fennici 50, 37–42.
Ohno, S. (1989). Studies on Scolytidae and Platypodidae (Coleoptera) found on imported logs at Japanese ports I. Res. Bull. Plant Prot. Serv. 25, 7–22.
Ostrauskas, H., and Ferenca, R. (2010). Beetles (Coleoptera) caught in traps baited with pheromones for Dendroctonus rufipennis (Kirby) (Curculionidae: Scolytinae) in Lithuania. Ekologija 56, 41–46. doi: 10.2478/v10055-010-0006-8
Ostrauskas, H., and Tamutis, V. (2012). Bark and longhorn beetles (Coleoptera: Curculionidae, Scolytinae et Cerambycidae) caught by multiple funnel traps at the temporary storages of timbers and wood in Lithuania. Baltic For. 18, 263–269.
Peña, E., Kinkar, M., and Vos, S. (2020). Pest survey card on Polygraphus proximus. EFSA Support. Public. 17, 1780E. doi: 10.2903/sp.efsa.2020.EN-1780
Pennacchio, F., Faggi, M., Gatti, E., Caronni, F., Colombo, M., and Roversi, P. F. (2004). First record of Phloeotribus liminaris (Harris) from Europe (Coleoptera Scolytidae). Redia 87, 85–89.
Pennacchio, F., Santini, L., and Francardi, V. (2012). Bioecological notes on Xylosandrus compactus (Eichhoff) (Coleoptera Curculionidae Scolytinae), a species recently recorded into Italy. Redia 95, 67–77.
Pfeffer, A., and Knížek, M. (1989). Problematika kůrovců introdukovaných do Evropy. Lesnic. Práce 68, 311–312.
Pfeffer, A., and Knížek, M. (1996). “Coleoptera: Curculionoidea 2,” in Terrestrial Invertebrates of the Pálava Biosphere Reserve of UNESCO III. Folia Facultatis Scientiarium Naturalium Universitatis Masarykianae Brunensis, Biologia 94, eds R. Rozkošný and J. Vaòhara (Brno: Masaryk University), 601–607.
Pimentel, D., Zuniga, R., and Morrison, D. (2005). Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 52, 273–288. doi: 10.1016/j.ecolecon.2004.10.002
Poland, T. M., and Rassati, D. (2019). Improved biosecurity surveillance of non-native forest insects: a review of current methods. J. Pest Sci. 92, 37–49. doi: 10.1007/s10340-018-1004-y
Pombo, D. A., Aguiar, A. M. F., and Nunes, É (2010). “Exotic arthropods in Macaronesia: vectors, pathways, control measures and global trade,” in Terrestrial arthropods of Macaronesia - Biodiversity, ecology and evolution, eds A. R. M. Serrano, P. A. V. Borges, M. Boieiro, and P. Oromí (Lisabon: Sociedade Portuguesa de Entomologia), 145–168.
Procházka, J., Stejskal, R., Čížek, L., Hauck, D., and Knížek, M. (2018). Dryocoetes himalayensis (Coleoptera: Curculionidae: Scolytinae), a new bark beetle species for Slovakia and Austria, and its occurrence in the Czech Republic. Klapalekiana 54, 117–121.
Rabaglia, R., Duerr, D., Acciavatti, R., and Ragenovich, I. (2008). Early detection and rapid response for non-native bark and ambrosia beetles. Washington, DC: United States Department of Agriculture.
Rabaglia, R. J., and Cavey, J. F. (1994). Note on the distribution of the immigrant bark beetle, Hylastes opacus, in North America (Coleoptera: Scolytidae). Entomol. News 105, 277–279.
Rabaglia, R. J., Cognato, A. I., Hoebeke, E. R., Johnson, C. W., Labonte, J. R., Carter, M. E., et al. (2019). Early detection and rapid response. A 10-year summary of the USDA Forest Service program of surveillance for non-native bark and ambrosia beetles. Am. Entomol. 65, 29–42. doi: 10.1093/ae/tmz015
Ranger, C. M., Gorzlancyk, A. M., Addesso, K. M., Oliver, J. B., Reding, M. E., Schultz, P. B., et al. (2014). Conophthorin enhances the electroantennogram and field behavioural response of Xylosandrus germanus (Coleoptera: Curculionidae) to ethanol. Agric. For. Entomol. 16, 327–334. doi: 10.1111/afe.12062
Ranger, C. M., Reding, M. E., Schultz, P. B., and Oliver, J. B. (2013). Influence of flood-stress on ambrosia beetle host-selection and implications for their management in a changing climate. Agric. For. Entomol. 15, 56–64. doi: 10.1111/j.1461-9563.2012.00591.x
Ranger, C. M., Reding, M. E., Gandhi, K. J. K., Oliver, J. B., Schultz, P. B., Cañas, L., et al. (2011). Species dependent influence of (-)-α-pinene on attraction of ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) to ethanol-baited traps in nursery agroecosystem. J. Econ. Entomol. 104, 574–579. doi: 10.1603/ec10243
Ranger, C. M., Schultz, P. B., Frank, S. D., and Reding, M. E. (2019). Freeze stress of deciduous trees induces attacks by opportunistic ambrosia beetles. Agric. For. Entomol. 21, 168–179. doi: 10.1111/afe.12317
Rassati, D., Faccoli, M., Haack, R. A., Rabaglia, R. J., Toffolo, E. P., Battisti, A., et al. (2016a). Bark and ambrosia beetles show different invasion patterns in the USA. PLoS One 11:e0158519. doi: 10.1371/journal.pone.0158519
Rassati, D., Faccoli, M., Battisti, A., and Marini, L. (2016b). Habitat and climatic preferences drive invasions of non-native ambrosia beetles in deciduous temperate forests. Biol. Invas. 18, 2809–2821. doi: 10.1007/s10530-016-1172-8
Rassati, D., Faccoli, M., Toffolo, E. P., Battisti, A., and Marini, L. (2015). Improving the early detection of alien wood-boring beetles in ports and surrounding forests. J. Appl. Ecol. 52, 50–58. doi: 10.1111/1365-2664.12347
Rassati, D., Toffolo, E. P., Roques, A., Battisti, A., and Faccoli, M. (2014). Trapping wood boring beetles in Italian ports: a pilot study. J. Pest Sci. 87, 61–69. doi: 10.1007/s10340-013-0499-5
Reitter, E. (1913). Bestimmungs-Tabelle der Borkenkäfer (Scolytidae) aus Europa und den Angrenzenden Ländern. Wien. Entomol. Zeitung 32, 1–116.
Riba-Flinch, J. M., Leza, M., and Gallego, D. (2021). First records of Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) in the Iberian Peninsula: an expanding alein species. Zootaxa 4970, 161–170. doi: 10.11646/zootaxa.4970.1.8
Roling, M. P., and Kearby, W. H. (1975). Seasonal flight and vertical distribution of Scolytidae attracted to ethanol in an oak-hickory forest in Missouri. Can. Entomol. 107, 1315–1320. doi: 10.4039/Ent1071315-12
Ruzzier, E., Galli, A., and Bani, L. (2021). Monitoring exotic beetles with inexpensive attractants: A case study. Insects 12, 462. doi: 10.3390/insects12050462
Samons, M. (2022). The control and eradication of invasive species in urban area in terms of South African law: The city of Cape Town and polyphagous shot hole borer beetles. Potchefstr. Electron. Law J. 25, 1–17. doi: 10.17159/1727-3781/2022/v25i0a13012
Schedl, K. E. (1962). Scolytidae und Platypodidae Afrikas. Band II. Familie Scolytidae. Rev. Entomol. Moçambique 5, 1–594.
Schedl, K. E. (1966). Pin-hole borers and bark-beetles (Scolytidae and Platypodidae) intercepted from imported logs in japanese ports. Kontyû 34, 29–43.
Schedl, K. E. (1969). Pin-hole borers and bark-beetles (Scolytidae and Platypodidae) intercepted from imported logs in Japanese ports III. Kontyû 37, 202–219.
Schedl, K. E. (1970). Pin-hole borers and bark-beetles (Scolytidae and Platypodidae) intercepted from imported logs in Japanese ports IV. Kontyû 38, 353–370.
Schlyter, F., Birgersson, G., Byers, J. A., Löfqvist, J., and Bergström, G. (1987). Field response of spruce bark beetle, Ips typographus, to aggregation pheromone candidates. J. Chem. Ecol. 13, 701–716. doi: 10.1007/BF01020153
Schroeder, L. M., and Lindelöw, Å (1989). Attraction of scolytids and associated beetles by different absolute amounts and proportions of α-pinene and ethanol. J. Chem. Ecol. 15, 807–817. doi: 10.1007/BF01015179
Schuler, H., Witkowski, R., van de Vossenberg, B., Hoppe, B., Mittelbach, M., Bukovinszki, T., et al. (2023). Recent invasion and eradication of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae) from tropical greenhouses in Europe. Biol. Invas. 25, 299–307. doi: 10.1007/s10530-022-02929-w
Seybold, S. J., Dallara, P. L., Nelson, L. J., Graves, A. D., Hishinuma, S. M., and Gries, R. (2015). Methods of monitoring and controlling the walnut twig beetle, Pityophthorus juglandis. U. S. Patent No. US 9,137,990 B2. Washington, DC: U.S. Patent and Trademark Office.
Šramel, N., Kavčč, A., Kolšek, M., and de Groot, M. (2021). Estimating the most effective and economical pheromone for monitoring the European spruce bark beetle. J. Appl. Entomol. 145, 312–325. doi: 10.1111/jen.12853
Susaeta, A., Soto, J. R., Adams, D. C., and Hulcr, J. (2016). Pre-invasion economic assessment of invasive species prevention: A putative ambrosia beetle in Southeastern loblolly pine forests. J. Environ. Manage. 183, 875–881. doi: 10.1016/j.jenvman.2016.09.037
Thurston, G. S., Slater, A., Nei, I., Roberts, J., Hamilton, K. M., Sweeney, J. D., et al. (2022). New canadian and provincial records of Coleoptera resulting from annual Canadian Food Inspection Agency surveillance for detection of non-native, potentially invasive forest insects. Insects 13, 708. doi: 10.3390/insects13080708
Urvois, T., Auger-Rozenberg, M. A., Roques, A., Rossi, J. P., and Kerdelhue, C. (2021). Climate change impact on the potential geographical distribution of two invanding Xylosandrus ambrosia beetles. Sci. Rep. 11, 1339. doi: 10.1038/s41598-020-80157-9
Vilà, M., Basnou, C., Pyšek, P., Josefsson, M., Genovesi, P., Gollasch, S., et al. (2010). How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 8:135–144. doi: 10.1890/080083
Ward, S. F., Fei, S., and Liebhold, A. M. (2019). Spatial patterns of discovery points and invasion hotspots of non-native forest pests. Glob. Ecol. Biogeogr. 28, 1749–1762. doi: 10.1111/geb.12988
Weber, B. C., and McPherson, J. E. (1983). World list of host plants of Xylosandrus germanus (Blandford) (Coleoptera: Scolytidae). Coleopter. Bull. 37, 114–134.
Wylie, F. R., Griffiths, M., and King, J. (2008). Development of hazard site surveillance programs for forest invasive species: a case study from Brisbane, Australia. Austral. For. 71, 229–235.
Zahradník, P., and Zahradníková, M. (2016). Použití feromonových lapačů v ochranì lesa proti lýkožroutu smrkovému. Lesnická Práce 4, 50–51.
Žemlička, K. (2012). Analysis of selected branch of manufacturing industry. Ph.D. thesis. Prague: Západočeská univerzita v Plzni.
Appendix Table 1. Detection of ambrosia and bark beetles according to the type of bait at a tropical wood warehouse in Pilsen.
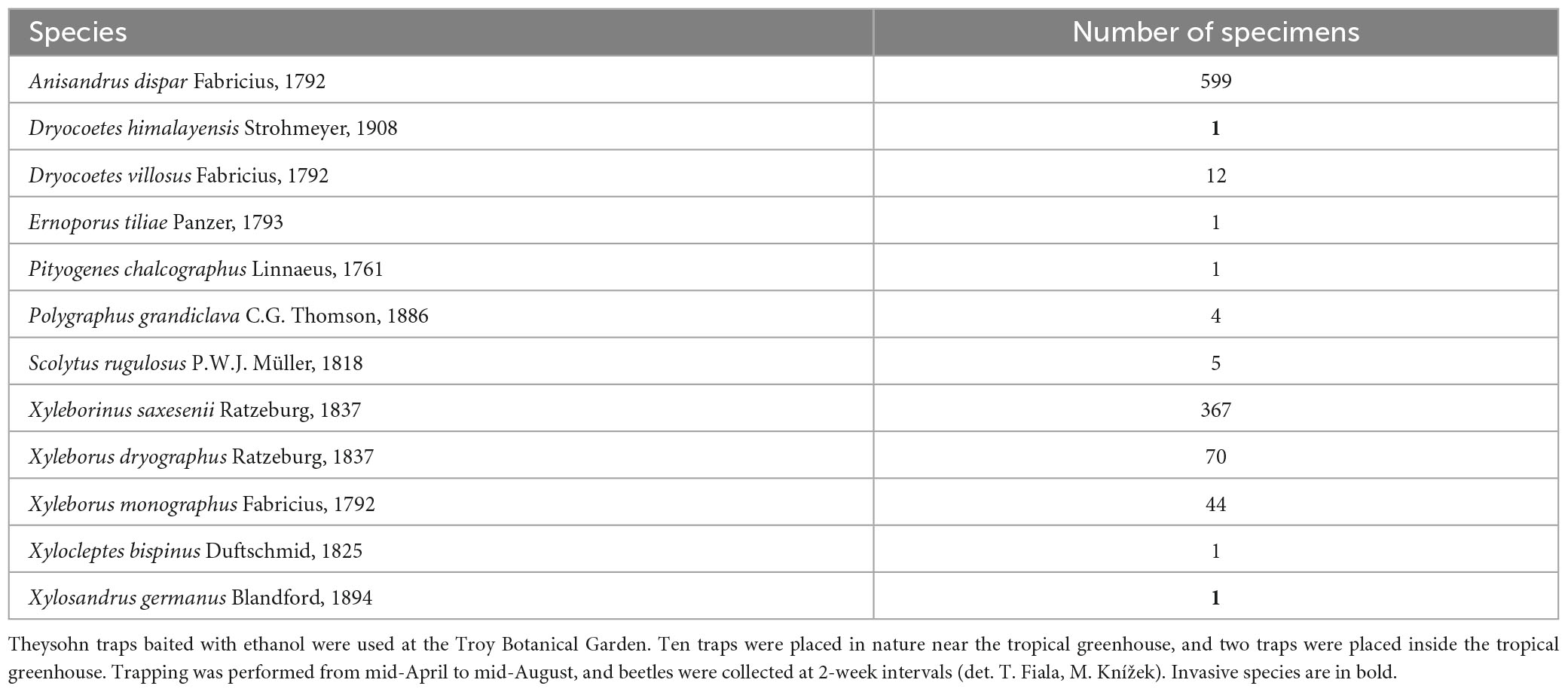
Appendix Table 2. Detected species of ambrosia and bark beetles in the Prague-Troja Botanical Garden (GPS 50.1224N, 14.4139E).

Appendix Table 3. The presence of feeding and the detected numbers of Hypothenemus hampei Ferrari, 1867 in samples of ten coffee beans imported to the Czech Republic from seven countries in 2021–2022 (det. T. Fiala).
Appendix 4 | Basic monitoring design.
• Twenty-four localities
• Three traps per locality, 30–50 m each other
• Each trap baited with ethanol
• Traps checked once every 14 days
Keywords: Cyclorhipidion bodoanum, Dryocoetes himalayensis, Gnathotrichus materiarius, Phloeosinus aubei, Xyleborinus attenuatus, Xylosandrus germanus
Citation: Fiala T and Holuša J (2023) A monitoring network for the detection of invasive ambrosia and bark beetles in the Czech Republic: principles and proposed design. Front. For. Glob. Change 6:1239748. doi: 10.3389/ffgc.2023.1239748
Received: 13 June 2023; Accepted: 07 August 2023;
Published: 23 August 2023.
Edited by:
Milica Zlatkovic, University of Novi Sad, SerbiaReviewed by:
Dimitrios N. Avtzis, Hellenic Agricultural Organization, GreeceCopyright © 2023 Fiala and Holuša. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaroslav Holuša, aG9sdXNhakBzZXpuYW0uY3o=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.