- 1Institute of Science and Technology, Niigata University, Niigata, Japan
- 2Institute for Space–Earth Environmental Research (ISEE), Nagoya University, Nagoya, Japan
- 3Nuclear Science and Engineering Center, Japan Atomic Energy Agency, Ibaraki, Japan
- 4Faculty of Bioresource Sciences, Akita Prefectural University, Akita, Japan
- 5Advanced Science Research Center, Japan Atomic Energy Agency, Ibaraki, Japan
Stable carbon (C) and nitrogen (N) isotopes (13C and 15N) in water-extractable organic matter (WEOM) derived from air-dried soils may be applicable to elucidate the microbial decomposition of soil organic matter (SOM), which is crucial in terrestrial C cycles. A total of 40 soil samples were collected from a depth of 0–6 cm from a temperate broadleaved forest in Japan with vegetation succession from grassland approximately 150 years ago. Those soil samples were air-dried before the water extraction process and organic matter analysis. The C and N concentrations of WEOM were <3.6% of those of the bulk soil and were positively correlated with those of the bulk soil at a p-value of < 0.01. A positive correlation between the two fractions (i.e., WEOM and bulk soils) was also found for natural 13C and 15N abundances (δ13C and δ15N; p < 0.01). However, the C/N ratio of WEOM was slightly correlated with that of bulk soils, exhibiting a narrow range of values of ~10. Thus, those features of the WEOM were similar to the well-known features of microbial biomass. The δ13C and δ15N enrichments in WEOM relative to bulk soil, the difference in stable isotope abundances between bulk SOM and WEOM were negatively and positively correlated, respectively, with the concentrations of organo-mineral complexes and short-range order minerals (non-crystalline oxyhydroxides of aluminum and iron, allophane, imogolite, and allophane-like constituents), which play significant roles in SOM stabilization in soils. These relationships suggest that the stable isotopic enrichments in WEOM can be a good indicator of the microbial utilization of soil C and N under different substrate availabilities, which are crucial to SOM decomposition and decomposability substantially varying from local to global scales.
1. Introduction
An analysis of soil microbial biomass may shed light on which carbon (C) substrates are practically used in soils (Wieder et al., 2013; Wei et al., 2016), which are crucial components driving the major carbon dioxide (CO2) efflux in terrestrial ecosystems (Davidson and Janssens, 2006; Bond-Lamberty et al., 2016, 2018; Luo et al., 2016). This is because soil microbes utilize organic substrates to develop their bodies (assimilation) and maintain their biotic metabolization (dissimilation) for survival (Dijkstra et al., 2008; Coyle et al., 2009; Makarov et al., 2015; Wang et al., 2021). Especially, the abundances of stable C and nitrogen (N) isotopes (i.e., δ13C and δ15N) in microbial biomass can be used to characterize the properties of the microbial utilization of SOM under different substrate availability conditions (Dijkstra et al., 2008; Coyle et al., 2009; Makarov et al., 2015; Wang et al., 2021). For example, isotope abundances in microbial biomass can represent the metabolized levels of microbially utilized substrates because both C and N in the substrate organic matter are expected to be enriched in heavier isotopes (i.e., 13C and 15N) during microbial metabolization, stimulating the loss of lighter isotopes (i.e., 12C and 14N) from the ecosystem (Dijkstra et al., 2008; Coyle et al., 2009; Craine et al., 2015; Makarov et al., 2015; Wang et al., 2021). If the interested ecosystem has experienced vegetation succession from C4 plants producing 13C-enriched organic matter to C3 plants producing 13C-depleted organic matter (Hobbie and Werner, 2004), 13C abundances in microbial bodies would be a proxy of the age of C substrates (Yoneyama et al., 2001). Another possible implication for isotope abundances in microbial biomass comes from the observation that δ15N enrichment in the microbial biomass relative to SOM in the surrounding soil environment was negatively correlated with the soil C/N ratio (Dijkstra et al., 2008). This relationship between microbial δ15N enrichment and the soil C/N ratio indicated that C-limited conditions (i.e., a low C/N ratio) stimulated N dissimilation, resulting in the heavier 15N remaining in microbial bodies while the lighter 14N was depleted. Nitrogen-limited conditions (i.e., a high C/N ratio) stimulated N assimilation, resulting in both 14N and 15N remaining in microbial bodies (Dijkstra et al., 2008; Coyle et al., 2009; Makarov et al., 2015; Wang et al., 2021).
However, the analysis of microbial bodies is not generally applicable to air-dried soil samples from the major soil forms in long-term storage worldwide (Kaiser et al., 2015). Conversely, obtaining information about the microbial utilization of SOM from air-dried soil samples could provide the opportunity to achieve sufficient resolution and spatial coverage of soil analysis data. This would then be practically useful for predicting the C substrates used in soils through microbial decomposition.
Water-extractable organic matter (WEOM) from air-dried soil samples could be used to investigate the microbial activities and processes of SOM decomposition because WEOM is likely derived from microbial cells (Marumoto et al., 1977, 1982; Marumoto, 1984; Unger et al., 2010, 2012; Kaiser et al., 2015). According to Unger et al. (2010, 2012), the rewetting of dry soil releases 13C-enriched CO2 from soils, demonstrating the stimulation of the decomposition of microbially derived 13C-enriched C substrates. In Japanese paddy field soils, the mineralized C and N observed after the rewetting of air-dried soils are likely derived from microbial cells (Marumoto et al., 1977, 1982; Marumoto, 1984). Given that air-dried soils are the major storage form of soil samples and are well archived by researchers (Kaiser et al., 2015), analyzing the properties of WEOM from air-dried soil samples would be advantageous in characterizing SOM decomposition, which varies spatially from local to global scales (Li et al., 2019; Tang et al., 2020; Zhang et al., 2020).
In this study, we analyzed stable isotope abundances in WEOM from 40 air-dried soil samples gathered from the surfaces of a temperate broadleaved forest in Japan that had experienced a vegetation shift from C4 grassland to C3 tree vegetation. These soils were originally sampled to investigate the environmental distribution of radioactive cesium that was affected by the Fukushima nuclear accident (Atarashi-Andoh et al., 2021) and have been stored in air-dried conditions. The soils were heterogeneously affected by volcanic ash during their development, with various physiochemical and mineralogical properties and C dynamics within a single water catchment (Suzuki, 2002; Nagano et al., 2019). Assuming that WEOM from air-dried soils was derived from soil microbial bodies (Marumoto et al., 1977, 1982; Marumoto, 1984; Unger et al., 2010, 2012; Kaiser et al., 2015), we expected that the enrichments of 13C and 15N in WEOM relative to the bulk soils would be correlated with the concentrations of organo-mineral complexes and short-range order minerals (non-crystalline oxyhydroxides of aluminum and iron, allophane, imogolite, and allophane-like constituents; Imaya et al., 2007), which likely control the SOM stability and thus their availability to soil microbes (Johnson et al., 1995; Baldock and Skjemstad, 2000; Asano and Wagai, 2014; Takahashi and Dahlgren, 2016; Rasmussen et al., 2018; Wagai et al., 2018). We also investigated the similarity between WEOM features and the well-known features of microbial biomass, such as their narrow C/N ratio range being near 10 due to the significant N demand of bacteria (Strickland and Rousk, 2010), a low contribution to bulk SOM (e.g.,1.2% as average Inubushi et al., 2005; Xu et al., 2013), and higher δ15N than bulk soil (Dijkstra et al., 2008; Coyle et al., 2009; Makarov et al., 2015; Wang et al., 2021) to confirm the possibility of WEOM deriving from microbial biomass.
2. Materials and methods
2.1. Study site and soil sampling
The study forest was a deciduous broadleaved forest in the southern part of the Abukuma Mountains, Ibaraki, Japan (Figure 1). The dominant trees were Fagus crenata, Fagus japonica, and Quercus serrata (C3 vegetation). This forest is an old secondary forest that has not received significant disturbance since the Meiji era (ca. 150 years ago). Some surface soils in this forest have Andic properties, which implies a significant contribution of volcanic ash to the parent material of the soils. Before the Meiji era, Poaceae grasslands (C4 vegetation) dominated this area at a time when many artificial fires were set for agricultural purposes (Suzuki, 2002). The mean annual temperature and precipitation were 11°C and 1,900 mm, respectively (Mizoguchi et al., 2002). The soils in the region have been affected by volcanic ash, mainly from the Abukuma Mountains, which extends over a large area of the northern part of the forest. The soil characteristics in this area are highly heterogeneous in terms of specific landforms and amounts of volcanic ash. Detailed information related to the study forest is available in the studies by Mizoguchi et al. (2002), Yoshinaga et al. (2002), and Suzuki (2002) and chapters in a book by Nakashizuka and Matsumoto (2002).
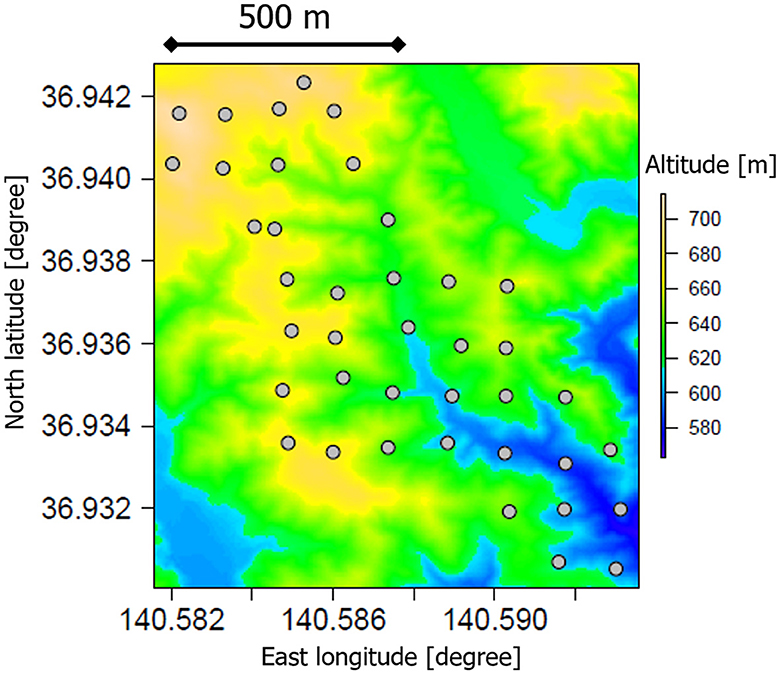
Figure 1. A map of soil-sampling locations (dots) in a deciduous broad-leaved forest in Ibaraki, Japan. The colors of the map represent the elevations visualized from a digital elevation model publicly distributed by the Geospatial Information Authority of Japan (http://www.gsi.go.jp/kiban/index.html).
In August and September 2013, soil samples were collected from a depth of 0–6 cm at 40 locations in the forest, originally to investigate the environmental distribution of radioactive cesium affected by the Fukushima nuclear accident (Figure 1; Atarashi-Andoh et al., 2021). Each sampling point was roughly located in the center of a rectangular grid with a length of 150 m north-south and 120 m east-west. A cylindrical soil core sampler was used to collect soil after removing the organic litter layers (Koarashi et al., 2016). The collected soil samples were gently passed through a 2-mm sieve to remove gravel and plant tissues, followed by fine root removal using tweezers. These soil samples were air-dried to a constant weight at room temperature (ca. 20°C) and were used for analysis.
2.2. Soil analysis
Organic matter extracted from air-dried soil by water (i.e., WEOM from air-dried soil) was analyzed to determine the C and N concentrations, C/N ratio, δ13C, and δ15N. The organic matter was extracted from a 1-g soil sample and 20 mL of deionized water by horizontal shaking for 1 h at 120 rpm. A membrane filter (0.45 μm pore size, HAWP04700, Merck Millipore) equipped with a filter unit (Thermo Scientific 300–4,050, Thermo Fisher Scientific) was used to remove solid materials in the solution. The C concentration in the extracted solution was determined using a total organic C analyzer (TOC-L, Shimadzu). The solution was freeze-dried into a powder. The powder was placed in a tin cup, and their N concentration, C/N ratio, δ13C, and δ15N were determined using an isotope ratio mass spectrometer (IsoPrime100, Isoprime Ltd.) connected to an elemental analyzer (Vario PYRO cube, Elementar). Using these data and the soil and water mixing ratio, we calculated the C and N concentrations, δ13C, and δ15N of WEOM.
From the bulk soil samples, in addition to WEOM, we also measured the C/N ratio, δ13C, δ15N, and concentrations of organo-mineral complexes and short-range order minerals. The C and N concentrations, δ13C, and δ15N were determined using the same instruments as described above. The concentrations of organo-mineral complexes and short-range order minerals were expressed as the sum of aluminum (Al) and half-weighted iron (Fe) selectively dissolved with 2.0 M acid oxalate ammonium (Alo+1/2Feo) (Asano and Wagai, 2014; Takahashi and Dahlgren, 2016; Rasmussen et al., 2018; Wagai et al., 2018). The mineral extraction with 2.0 M acid oxalate ammonium was conducted for 4 h with horizontal shaking at 140 rpm in the dark. The supernatant of the extracted solution after centrifugation at 3,000 G for 10 min was filtered using a centrifugal filter unit (0.1 μm pore size, UFC30VV, Merck Millipore) and analyzed using inductively coupled plasma–optical emission spectrometry (ICP-OES: 5110, Agilent) to measure the selectively dissolved mineral concentrations. We also determined the concentrations of Al and half-weighted Fe dissolved with 0.1 M pyrophosphate sodium solution (Alp+1/2Fep) as minerals specifically associated with organo-mineral complexes. The extraction with a 0.1-M pyrophosphate sodium solution was conducted for 16 h. After centrifugation at 3,000 G for 10 min, the supernatant was centrifugated again at 30,000 G for 60 min. Then, centrifugal filtration and ICP-OES measurements were conducted. The difference between Alo+1/2Feo and Alp+1/2Fep (here represented as Alo-p+1/2Feo-p) represented the concentration of short-range order minerals (Courchesne and Turmel, 2008). The enrichments of δ13C and δ15N in WEOM were calculated as the differences in the δ13C and δ15N values between WEOM and bulk soil samples. The results of the soil analysis are presented in Supplementary Tables S1–S3 in Supplementary Information.
2.3. Data analysis
The data were analyzed with the R software 4.1.1 (R Core Team, 2021). The correlations among metrics were statistically evaluated using the cor.test function. Then, these correlations were compared between WEOM and bulk soil samples from the viewpoint of whether there were any apparent differences in correlation directions (i.e., positive, negative, or insignificant). Statistical differences in metrics between WEOM and bulk soils were determined using a t-test by the t.test function, and a p-value of < 0.05 was considered statistically significant.
3. Results
The C and N concentrations, δ13C, and δ15N showed positive correlations between WEOM and bulk soils, but the C/N ratio did not (Figure 2). The C and N concentrations in bulk soils were 46–230 mg C g−1 dry soil and 2–14 mg N g−1 dry soil, respectively; whereas, the corresponding values in WEOM were <3.6% of those in bulk soils (1.0% and 1.9% as averages for C and N, respectively). The correlation coefficients (r) for the C and N concentrations between WEOM and bulk soils were 0.80 or higher (p < 0.01). The δ13C and δ15N values ranged from −27.5 to −21.3‰ and 1.2 to 5.0‰ in bulk soils, respectively, while these values ranged from −26.7 to −23.0‰ and 3.5 to 9.2‰ in WEOM, respectively. These isotope abundances were significantly higher in WEOM than in bulk soils (p < 0.01), showing significant correlations between WEOM and bulk soils (r ≥ 0.59, p < 0.01). However, for the C/N ratio, there was a non-significant correlation between WEOM and bulk soils (r = −0.06, p = 0.73), showing narrow ranges of values around 10 in the WEOM. The C/N ratio in WEOM, ranging from 7.9 to 11.9, was significantly lower than that in bulk soil samples (13.9–25.6) (p < 0.05).
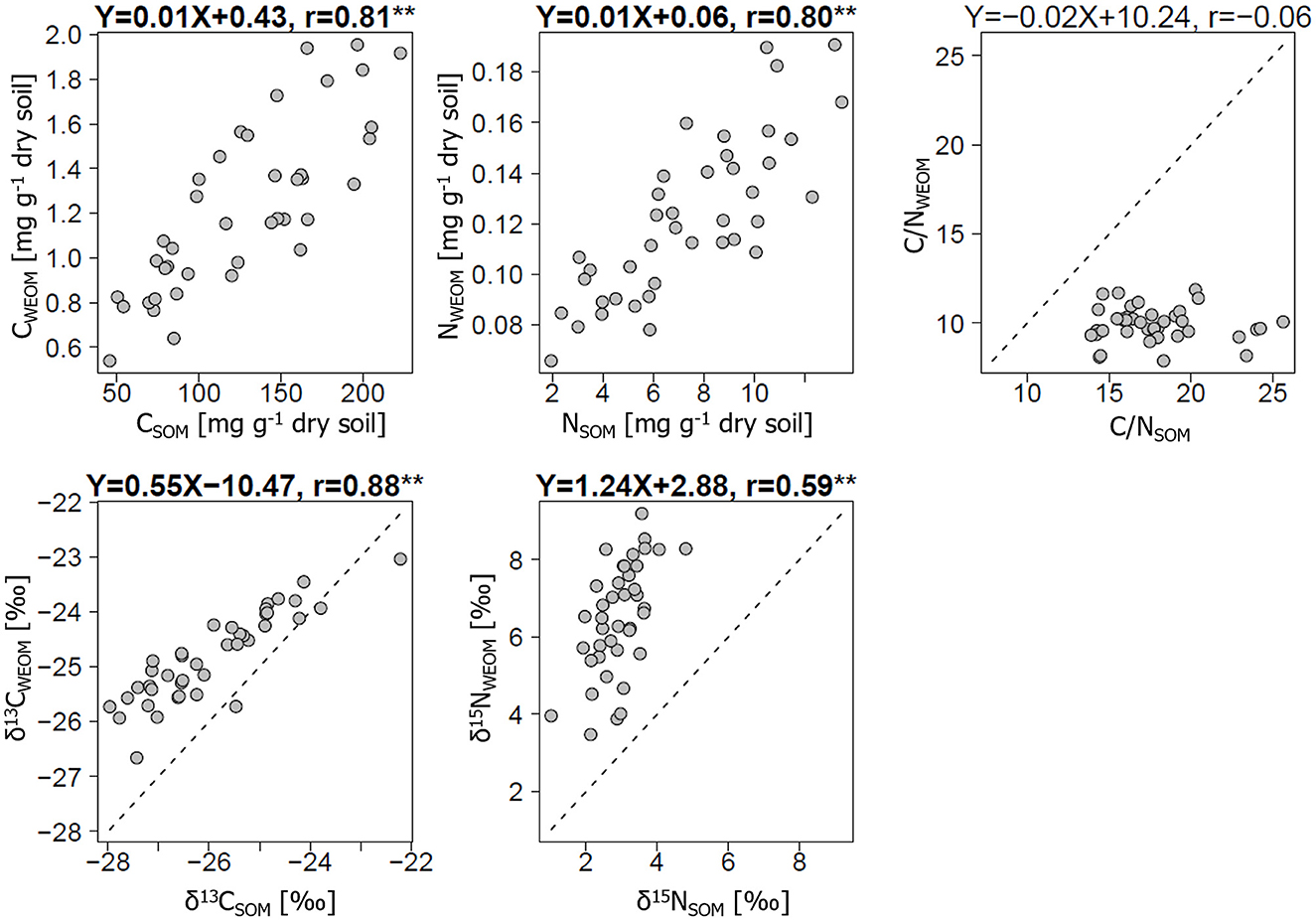
Figure 2. Relationships of carbon (C) and nitrogen (N) metrics [C and N contents, C/N ratio, and stable C and N isotope abundances (δ13C, δ15N)] between bulk soils and water-extractable organic matter (WEOM) from air-dried soils. All metrics were significantly different between the bulk soils and WEOM at a p-value of < 0.01. Linear regression equations are presented with correlation coefficients (r), suggesting their statistical significance by *p < 0.05 and **p < 0.01.
Positive correlations were also found between most of the C and N metrics (in both bulk soil and WEOM) and selectively dissolved mineral concentrations (Alo+1/2Feo, Alp+1/2Fep, and Alo-p+1/2Feo-p) (Table 1). The ranges of Alo+1/2Feo, Alp+1/2Fep, and Alo-p+1/2Feo-p were 3.7–27.2, 2.0–16.3, and 1.0–12.0 mg g−1 dry soil, respectively (Supplementary Table S3). The correlation coefficients for the relationship between these minerals and the C and N concentrations, δ13C, and δ15N in the two fractions were 0.46 or greater (p < 0.01), except for the non-significant correlations of the WEOM C and N concentrations with Alo-p+1/2Feo-p (r = 0.09 to 0.14, p > 0.05).
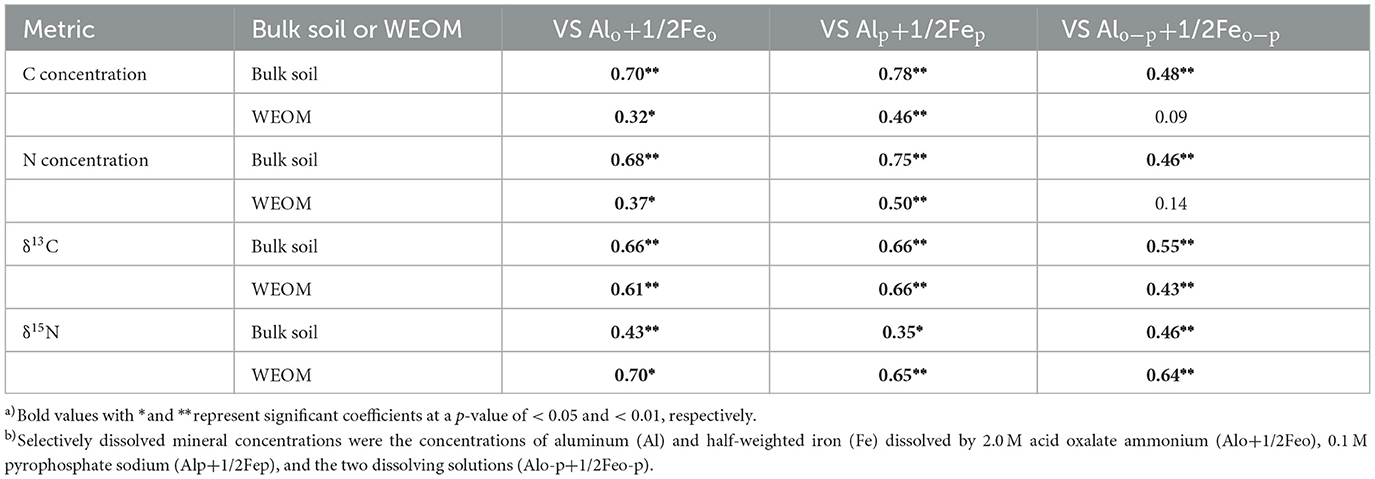
Table 1. Correlation coefficient a) between C and N metrics and selectively dissolved mineral concentrations b).
Conversely, the relationships between the stable isotope enrichments in WEOM and the concentrations of selectively dissolved minerals showed the opposite trend between 13C and 15N (Figure 3). The δ13C enrichments were negatively correlated with Alo+1/2Feo (r = −0.66, p < 0.01), whereas the δ15N enrichments were positively correlated with Alo+1/2Feo (r = 0.51, p < 0.01). Similarly, there were negative correlations for Alp+1/2Fep and Alo-p+1/2Feo-p with the δ13C enrichments (r < −0.62, p < 0.01) but positive correlations with the δ15N enrichments (r > 0.51, p < 0.01).
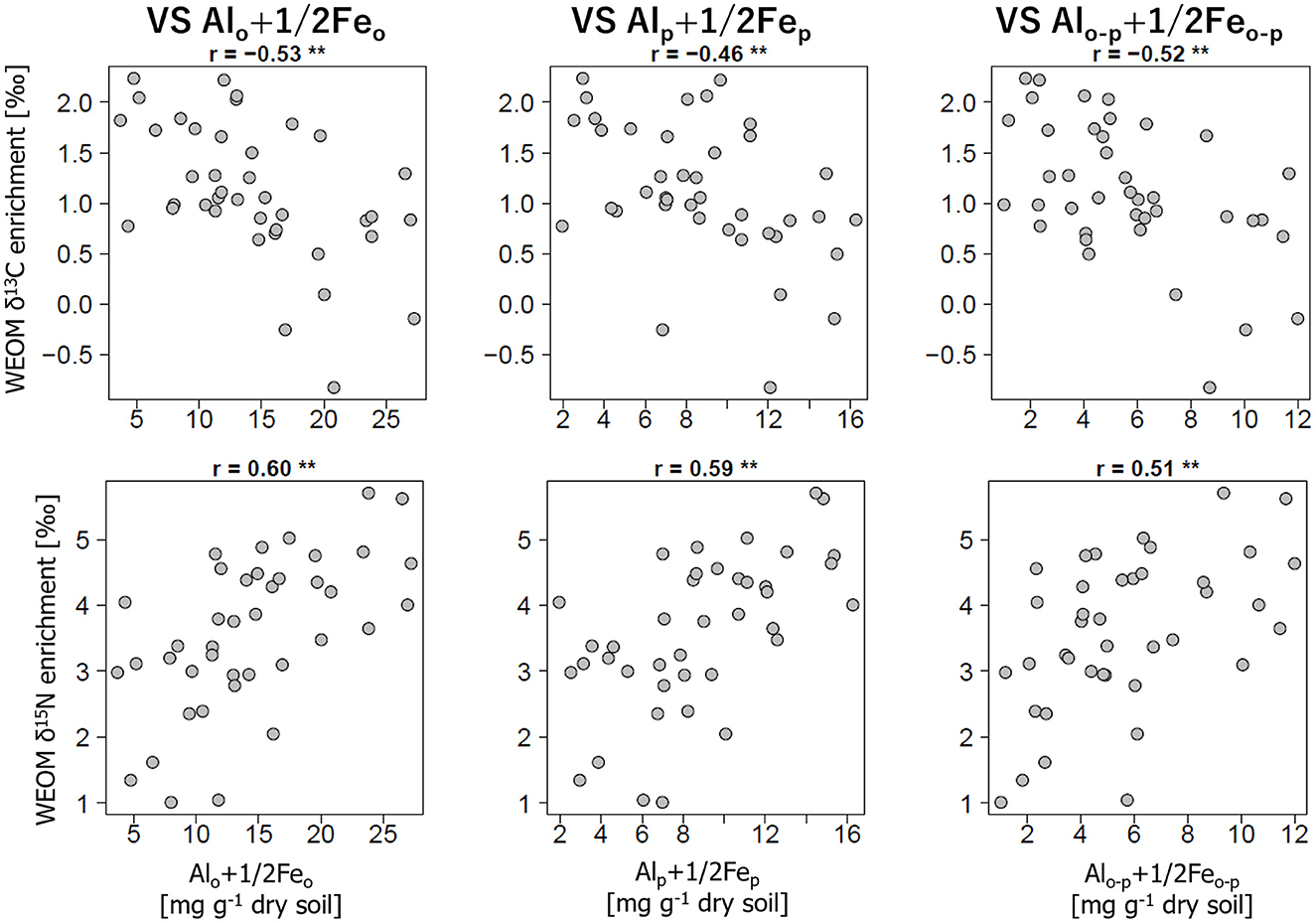
Figure 3. Relationships of the δ13C and δ15N enrichment in WEOM compared to bulk soils with the concentrations of Alo+1/2Feo (left panels), Alp+1/2Fep (center panels), and Alo-p+1/2Feo-p (right panels). Correlation coefficients (r) are presented above the panels with their statistical significance (*p < 0.05, **p < 0.01).
4. Discussion
As we expected, the lower variation in C/N ratio (values around 10) was the feature of WEOM from air-dried soil, rather than that of bulk SOM (Figure 2). This WEOM feature partially supports the significant potential that the WEOM was derived from microbial cells. This suggestion especially relies on the well-known features of microbial ecology, in which microbial communities, particularly bacterial communities dominating in surface mineral soils, have bodies with a C/N ratio near 10 due to the significant N demand of bacteria (Strickland and Rousk, 2010). Additionally, <3.6% of the contribution of WEOM to bulk SOM is of the same magnitude as the globally estimated contributions (1.2% as an average) of microbial biomass C to bulk soil organic C (Inubushi et al., 2005; Xu et al., 2013). A possible implication of the observed higher δ15N in WEOM (3.5–9.2‰) than in bulk soils (1.2–5.0‰) (Figure 2) is the isotope fractionation of N resulting from the metabolization of N substrates (Dijkstra et al., 2008; Coyle et al., 2009; Makarov et al., 2015; Wang et al., 2021), supporting our inference on the origins of WEOM from air-dried soils. While there are other possible organic origins of WEOM, such as fine plant debris and root exudates, the suggested possibility of significant linkage between WEOM and soil microbial bodies enables us to expect a significant linkage of variation in stable isotope enrichments of WEOM (Figure 3) to the microbial utilization of soil C and N substrates, as described below.
A possible implication for the observed variations in δ13C and δ15N enrichments of WEOM along with selectively dissolved mineral concentrations (Figure 3) is that the age and metabolized levels of SOM utilized by soil microbes depend on the selectively dissolved mineral concentrations (Figure 4), which likely control SOM stability (and thus availability) (Shirato et al., 2004; Asano and Wagai, 2014; Takahashi and Dahlgren, 2016; Rasmussen et al., 2018; Wagai et al., 2018). The age of utilized substrates determining the variation in δ13C of substrate organic matter and microbial bodies (Yoneyama et al., 2001) is likely reliable in soils with a succession history from C4 to C3 vegetation, such as the investigated forest and many other Japanese forests (Yoneyama et al., 2001; Suzuki, 2002; Hiradate et al., 2004; Takahashi and Dahlgren, 2016), where the δ13C values of photosynthesized organic matter apparently differ between the two plant types (~14‰ for C4 and −27‰ for C3; Hobbie and Werner, 2004). No evidence of vegetation succession fluctuating the substrate δ15N was available in the study forest. Thus, if microbial bodies are the major sources of WEOM derived from air-dried soils, the δ13C and δ15N enrichments in WEOM can be used to deduce the age and metabolized levels of the substrate organic matter utilized by soil microbes, respectively (Figure 4). This analysis takes into account the heterogeneous distribution of microbially available substrates (Koarashi et al., 2009; Mueller et al., 2012). Under this statement, microbes in soils with large concentrations of organo-mineral complexes and short-range order minerals, resulting in a low availability of organic substrates, are likely to incorporate relatively young (added by C3 vegetation since the Meiji era) but well-metabolized organic matter, which has been depleted in 13C but enriched in 15N compared to bulk soils. In other soils with lower concentrations of organo-mineral complexes and short-range order minerals, and consequently a high availability of organic substrates, microbes likely incorporate older (added by C4 vegetation before the Meiji era) but less-metabolized organic matter, which is enriched in 13C but not in 15N compared to bulk soils.
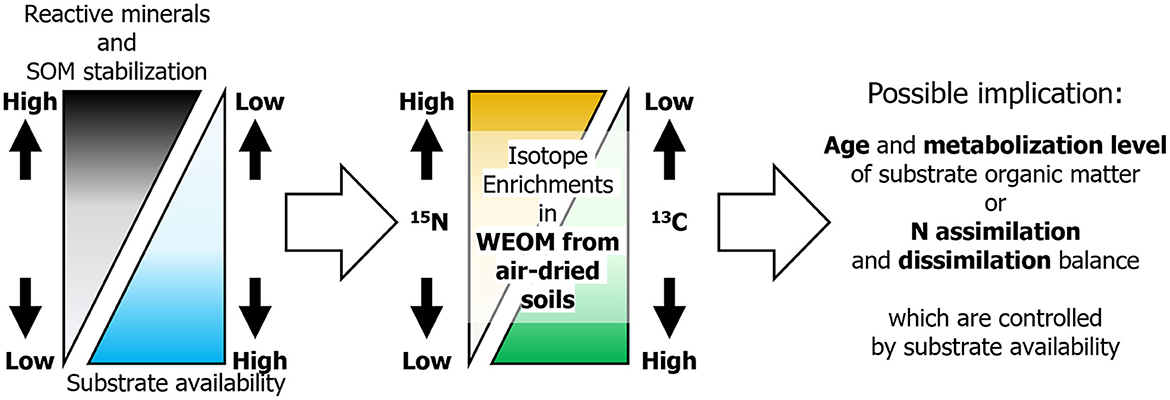
Figure 4. Proposed relationships among selectively dissolved minerals, SOM stabilization, substrate C availability, microbial 13C and 15N enrichments, and microbial SOM utilization (i.e., the C and N assimilation and dissimilation, and the ages and metabolized levels of utilized SOM) in forest soils that had experienced vegetation changes from C4 to C3 plants since the Meiji era (ca. 150 years ago). Here, we designated the 13C depleted SOM added by the C3 vegetation since the Meiji era as young.
Another implication is possible as substrate availability controls the balance of microbial N assimilation and dissimilation (Figure 4; Dijkstra et al., 2008; Coyle et al., 2009; Wang et al., 2021), with particular emphasis on the positive correlation between the δ15N enrichments in WEOM and selectively dissolved mineral concentrations (Figure 3). Specifically, in soils where C availability is relatively limited compared to N availability, microbes are likely to promote nitrogen dissimilation, resulting in the retention of 15N while depleting 14N in their bodies (Dijkstra et al., 2008). However, in soils with a relatively limited N availability compared to C availability, microbes stimulate N assimilation, resulting in 15N-depleted bodies (Dijkstra et al., 2008). In a different vein, substrate availability-controlling microbial assimilation and dissimilation have been presumed with soil and substrate C/N ratio (Dijkstra et al., 2008; Coyle et al., 2009; Wang et al., 2021). The negligible relationship between the δ15N enrichment in WEOM and the soil C/N ratio in the investigated soils (r = −0.23, p > 0.05; Supplementary Figure S1) indicated that the selectively dissolved mineral concentration rather than soil C/N ratio is a better index for substrate availability for soil microbes in these soils.
Regardless of which implication is a representative of the relationship between microbial stable isotope enrichments and selectively dissolved mineral concentrations in the study forest, characterizing the properties of substrate organic matter for soil microbes and their metabolization processes are the keys to reliably inferring the microbial decomposition of SOM and the consequent CO2 release from soils under changing environments. For example, well-metabolized organic matter is sometimes recalcitrant to microbial decomposition (Six et al., 2002; Lützow et al., 2006; Wagai et al., 2018), and its decomposition is expected to be sensitive to temperature, according to the Arrhenius theory, which describes the response of C release from the soil through microbial SOM decomposition to increasing temperature (Davidson and Janssens, 2006). The relationships between the age of SOM and its decomposability are also a global concern for predicting the future of the global C cycle (Lawrence et al., 2020). Thus, the δ13C and δ15N signatures, particularly their enrichments in WEOM relative to bulk soils, which can be obtained from air-dried soils, have a significant potential to obtain a better understanding for microbial C and N utilization in soils.
5. Conclusions
Our findings indicate that WEOM obtained from air-dried soil exhibiting relatively consistent C/N ratios close to a value of 10 is present in relatively small amounts in soils, and displays more enriched δ13C and δ15N values compared to bulk SOM. These findings suggest that the WEOM is likely derived from microbial biomass. However, it is possible to note that the possibility of other organic sources, such as fine plant debris and root exudates, also requires further investigation. Assuming the WEOM consists of microbial biomass, the observed variations in δ13C and δ15N enrichments of the WEOM and selectively dissolved mineral concentrations indicated a significant linkage between microbial substrate utilization and substrate availability. Thus, δ13C and δ15N enrichments in WEOM from air-dried soils have significant potential for investigating the microbial utilization of substrate organic matter in a Japanese temperate broadleaved forest. Since this method is easily applicable to air-dried soils, the primary storage form of soils for researchers, analyzing stable isotope abundances of WEOM from air-dried soils would have a significant advantage in determining SOM decomposition and decomposability, which vary substantially from local to global scales. It should be noted that the findings of the present study are based on the insufficient types of soil collected at one site and the correlation analysis between metrics. Therefore, further study is required to address the generality of the findings from this study.
Data availability statement
The primary datasets supporting this study's findings are available in Supplementary Tables S1–S3. Additional data and R scripts for data analysis are also available from the authors upon reasonable request.
Author contributions
HN established the basic research design, conducted all analyses, including software preparation, validation, visualization, and wrote the manuscript. MA-A and JK conducted the soil sampling. MA-A, ST, TY, NK, and JK contributed to the detailed research design, soil analysis, data validation, interpretation of the results, and editing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant numbers 18K14497, 21H02231, 21H05313, and 22H05717), the Kurita Water and Environment Foundation (grant number 21E019), the Nagoya University External Funding Challenge Promotion Grant (KAKENHI Challenge Safety Net), and the Joint Research Program of Arid Land Research Center, Tottori University (04B2010).
Acknowledgments
The authors thank Ayako Tamaki of Niigata University for her editing support in preparing the manuscript. The authors also thank Misuzu Kaminaga, Kikuko Yoshigaki, Taro Ishii, Makiko Ishihara, and Kazumi Matsumura of the Japan Atomic Energy Agency for their support with the laboratory work. A preliminary discussion with Syuntaro Hiradate helped develop the analytical strategy of the present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2023.1228053/full#supplementary-material
References
Asano, M., and Wagai, R. (2014). Evidence of aggregate hierarchy at micro- to submicron scales in an allophanic Andisol. Geoderma 216, 62–74. doi: 10.1016/j.geoderma.10005
Atarashi-Andoh, M., Koarashi, J., Tsuduki, K., Takeuchi, E., Nishimura, S., Muto, K., et al. (2021). Spatial variations in radiocesium deposition and litter–soil distribution in a mountainous forest catchment affected by the Fukushima nuclear accident. J. Environ. Radioact. 238–239, 106725. doi: 10.1016/J.JENVRAD.2021.106725
Baldock, J., and Skjemstad, J. (2000). Role of the soil matrix and minerals in protecting natural organic materials against biological attack. Org. Geochem. 31, 697–710. doi: 10.1016/S0146-6380(00)00049-8
Bond-Lamberty, B., Bailey, V. L., Chen, M., Gough, C. M., and Vargas, R. (2018). Globally rising soil heterotrophic respiration over recent decades. Nature 560, 80–83. doi: 10.1038/s41586-018-0358-x
Bond-Lamberty, B., Epron, D., Harden, J., Harmon, M. E., Hoffman, F., Kumar, J., et al. (2016). Estimating heterotrophic respiration at large scales: challenges, approaches, and next steps. Ecosphere 7, e01380. doi: 10.1002/ECS2.1380
Courchesne, F., and Turmel, M-. C. (2008). “Extractable Al, Fe, Mn, and Si,” in Soil Sampling and Methods of Analysis, eds. M. R. Carter and E. G. Gregorich (CRC Press), 307–316.
Coyle, J. S., Dijkstra, P., Doucett, R. R., Schwartz, E., Hart, S. C., Hungate, B. A., et al. (2009). (2009). Relationships between C and N availability, substrate age, and natural abundance 13C and 15N signatures of soil microbial biomass in a semiarid climate. Soil Biol. Biochem. 41, 1605–1611. doi: 10.1016/J.SOILBIO.04022
Craine, J. M., Brookshire, E. N. J., Cramer, M. D., Hasselquist, N. J., Koba, K., Marin-Spiotta, E., et al. (2015). Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 396, 1–26. doi: 10.1007/s11104-015-2542-1
Davidson, E. A., and Janssens, I. A. (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173. doi: 10.1038/nature04514
Dijkstra, P., LaViolette, C. M., Coyle, J. S., Doucett, R. R., Schwartz, E., and Hart, S. C. et al. (2008), 15. N enrichment as an integrator of the effects of C and N on microbial metabolism and ecosystem function. Ecol. Lett. 11, 389–397. doi: 10.1111/j.1461-0248.2008.01154.x.
Hiradate, S., Nakadai, T., Shindo, H., and Yoneyama, T. (2004). Carbon source of humic substances in some Japanese volcanic ash soils determined by carbon stable isotopic ratio, δ13C. Geoderma 119, 133–141. doi: 10.1016/S0016-7061(03)00257-X
Hobbie, E. A., and Werner, R. A. (2004). Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol. 161, 371–385. doi: 10.1111/J.1469-8137.2004.00970.X
Imaya, A., Inagaki, Y., Tanaka, N., and Ohta, S. (2007). Free oxides and short-range ordered mineral properties of brown forest soils developed from different parent materials in the submontane zone of the Kanto and Chubu districts, Japan. Soil Sci. Plant Nutr. 53, 621–633. doi: 10.1111/j.1747-0765.2007.00175.x
Inubushi, K., Sakamoto, K., and Sawamoto, T. (2005). Properties of microbial biomass in acid soils and their turnover. Soil Sci. Plant Nutr. 51, 605–608. doi: 10.1111/j.1747-0765.2005.tb00073.x
Johnson, M. G., Levine, E. R., and Kern, J. S. (1995). Soil organic matter: distribution, genesis, and management to reduce greenhouse gas emissions. Water, Air, Soil Pollut. 823 82, 593–615. doi: 10.1007/BF00479414
Kaiser, M., Kleber, M., and Berhe, A. A. (2015). How air-drying and rewetting modify soil organic matter characteristics: an assessment to improve data interpretation and inference. Soil Biol. Biochem. 80, 324–340. doi: 10.1016/j.soilbio.10018
Koarashi, J., Atarashi-Andoh, M., Ishizuka, S., Miura, S., Saito, T., Hirai, K., et al. (2009). Quantitative aspects of heterogeneity in soil organic matter dynamics in a cool-temperate Japanese beech forest: aA radiocarbon-based approach. Glob. Chang. Biol. 15, 631–642. doi: 10.1111/j.1365-200801745.x
Koarashi, J., Nishimura, S., Nakanishi, T., Atarashi-Andoh, M., Takeuchi, E., Muto, K., et al. (2016). Post-deposition early-phase migration and retention behavior of radiocesium in a litter–mineral soil system in a Japanese deciduous forest affected by the Fukushima nuclear accident. Chemosphere 165, 335–341. doi: 10.1016/j.chemosphere.09043
Lawrence, C. R., Beem-Miller, J., Hoyt, A. M., Monroe, G., Sierra, C. A., Stoner, S., et al. (2020). An open-source database for the synthesis of soil radiocarbon data: international soil radiocarbon database (ISRaD) version 1.0. Earth Syst. Sci. Data 12, 61–76. doi: 10.5194/essd-12-61-2020
Li, J., Nie, M., Pendall, E., Reich, P. B., Pei, J., Noh, N. J., et al. (2019). Biogeographic variation in temperature sensitivity of decomposition in forest soils. Glob. Chang. Biol. 3, 1873–1885. doi: 10.1111./gcb.14838
Luo, Y., Ahlström, A., Allison, S. D., Batjes, N. H., Brovkin, V., Carvalhais, N., et al. (2016). Toward more realistic projections of soil carbon dynamics by Earth system models. Global Biogeochem. Cycles 30, 40–56. doi: 10.1002/2015GB005239
Lützow, M. V., Kögel-Knabner, I., Ekschmitt, K., Matzner, E., Guggenberger, G., Marschner, B., et al. (2006). Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 57, 426–445. doi: 10.1111/j.1365-200600809.x
Makarov, M. I., Malysheva, T. I., Menyailo, O. V., Soudzilovskaia, N. A., Van Logtestijn, R. S. P., Cornelissen, J. H. C., et al. (2015). Effect of K2SO4 concentration on extractability and isotope signature (δ13C and δ15N) of soil C and N fractions. Eur. J. Soil Sci. 66, 417–426. doi: 10.1111/ejss.12243
Marumoto, T. (1984). Mineralization of C and N from microbial biomass in paddy soil. Plant Soil 76, 165–173. doi: 10.1007/BF02205577
Marumoto, T., Anderson, J. P. E., and Domsch, K. H. (1982). Decomposition of 14C- and 15N-labelled microbial cells in soil. Soil Biol. Biochem. 14, 461–467. doi: 10.1016/0038-0717(82)90105-5
Marumoto, T., Kai, H., Yoshida, T., and Harada, T. (1977). Drying effect on mineralizations of microbial cells and their cell walls in soil and contribution of microbial cell walls as a source of decomposable soil organic matter due to drying. Soil Sci. Plant Nutr. 23, 9–19. doi: 10.1080/00380768.1977.10433017
Mizoguchi, Y., Morisawa, T., and Ohtani, Y. (2002). “Climate in Ogawa Forest Reserve,” in Diversity and Interaction in a Temperate Forest Community—Ogawa Forest Reserve of Japan, eds. T. Nakashizuka and Y. Matsumoto (Springer, Tokyo), 11–18. doi: 10.1007./978-4-431-67879-3_2
Mueller, C. W., Kölbl, A., Hoeschen, C., Hillion, F., Heister, K., Herrmann, A. M., et al. (2012). Submicron scale imaging of soil organic matter dynamics using NanoSIMS—From single particles to intact aggregates. Org. Geochem. 42, 1476–1488. doi: 10.1016/j.orggeochem.06003
Nagano, H., Atarashi-Andoh, M., and Koarashi, J. (2019). Effect of dry-wet cycles on carbon dioxide release from two different volcanic ash soils in a Japanese temperate forest. Soil Sci. Plant Nutr. 65, 525–533. doi: 10.1080/00380768.2019.1649976
Nakashizuka, T., and Matsumoto, Y. (2002). Diversity and Interaction in a Temperate Forest Community: Ogawa Forest Reserve of Japan. Tokyo, Japan: Springer Japan
R Core Team. (2021). R: A Language and Environment for Statistical Computing. Available online at: https://www.r-project.org/
Rasmussen, C., Heckman, K., Wieder, W. R., Keiluweit, M., Lawrence, C. R., Berhe, A. A., et al. (2018). Beyond clay: toward an improved set of variables for predicting soil organic matter content. Biogeochemistry. 25, 3. doi: 10.1007./s10533-018-0424-3
Shirato, Y., Hakamata, T., and Taniyama, I. (2004). Modified rothamsted carbon model for andosols and its validation: changing humus decomposition rate constant with pyrophosphate-extractable Al. Soil Sci. Plant Nutr. 50, 149–158. doi: 10.1080/00380768.2004.10408463
Six, J., Conant, R. T., Paul, E. A., and Paustian, K. (2002). Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241, 155–176. doi: 10.1023/A:1016125726789
Strickland, M. S., and Rousk, J. (2010). (2010). Considering fungal: bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 42, 1385–1395. doi: 10.1016/j.soilbio.05007
Suzuki, W. (2002). “Forest vegetation in and around ogawa forest reserve in relation to human impact,” in Diversity and Interaction in a Temperate Forest Community—Ogawa Forest Reserve of Japan, eds. T. Nakashizuka and Y. Matsumoto (Springer, Tokyo), 27–41. doi: 10.1007./978-4-431-67879-3_4
Takahashi, T., and Dahlgren, R. A. (2016). Nature, properties and function of aluminum-humus complexes in volcanic soils. Geoderma 263, 110–121. doi: 10.1016/j.geoderma.08032
Tang, X., Fan, S., Du, M., Zhang, W., Gao, S., Liu, S., et al. (2020). Spatial and temporal patterns of global soil heterotrophic respiration in terrestrial ecosystems. Earth Syst. Sci. Data 12, 1037–1051. doi: 10.5194/essd-12-1037-2020
Unger, S., Máguas, C., Pereira, J. S., David, T. S., and Werner, C. (2010). (2010). The influence of precipitation pulses on soil respiration—Assessing the “Birch effect” by stable carbon isotopes. Soil Biol. Biochem. 42, 1800–1810. doi: 10.1016/j.soilbio.06019
Unger, S., Máguas, C., Pereira, J. S., David, T. S., and Werner, C. (2012). Interpreting post-drought rewetting effects on soil and ecosystem carbon dynamics in a Mediterranean oak savannah. Agric. For. Meteorol. 154–155, 9–18. doi: 10.1016/j.agrformet.10007
Wagai, R., Kajiura, M., Uchida, M., and Asano, M. (2018). Distinctive roles of two aggregate binding agents in allophanic andisols: young carbon and poorly-crystalline metal phases with old carbon. Soil Syst. 2, 29. doi: 10.3390/soilsystems2020029
Wang, R., Peñuelas, J., Li, T., Liu, H., Wu, H., Zhang, Y., et al. (2021). Natural abundance of 13C and 15N provides evidence for plant–soil carbon and nitrogen dynamics in a N-fertilized meadow. Ecology 102, e03348. doi: 10.1002/ecy.3348
Wei, H., Chen, X., Xiao, G., Guenet, B., Vicca, S., Shen, W., et al. (2016). Are variations in heterotrophic soil respiration related to changes in substrate availability and microbial biomass carbon in the subtropical forests? Sci. Rep. 5, 18370. doi: 10.1038/srep18370
Wieder, W. R., Bonan, G. B., and Allison, S. D. (2013). Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Chang. 3, 909–912. doi: 10.1038/nclimate1951
Xu, X., Thornton, P. E., and Post, W. M. (2013). A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 22, 737–749. doi: 10.1111/geb.12029
Yoneyama, T., Nakanishi, Y., Morita, A., and Liyanage, B. C. (2001). δ13C values of organic carbon in cropland and forest soils in Japan. Soil Sci. Plant Nutr. 47, 17–26. doi: 10.1080/00380768.2001.10408364
Yoshinaga, S., Takahashi, M., and Aizawa, S. (2002). “Landforms and soil characteristics in ogawa forest reserve,” in Diversity and Interaction in a Temperate Forest Community: Ogawa Forest Reserve of Japan, eds. T. Nakashizuka and Y. Matsumoto (Springer, Tokyo), 19–26. doi: 10.1007./978-4-431-67879-3_3
Keywords: microbial decomposition, soil organic matter, stable isotopic signatures, substrate availability, water-extractable organic matter
Citation: Nagano H, Atarashi-Andoh M, Tanaka S, Yomogida T, Kozai N and Koarashi J (2023) Stable C and N isotope abundances in water-extractable organic matter from air-dried soils as potential indices of microbially utilized organic matter. Front. For. Glob. Change 6:1228053. doi: 10.3389/ffgc.2023.1228053
Received: 24 May 2023; Accepted: 13 June 2023;
Published: 11 July 2023.
Edited by:
Rudong Zhao, Wuhan Botanical Garden, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Min Wang, Zhongkai University of Agriculture and Engineering, ChinaCanlan Jiang, Nanjing Agricultural University, China
Copyright © 2023 Nagano, Atarashi-Andoh, Tanaka, Yomogida, Kozai and Koarashi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hirohiko Nagano, aG5hZ2Fub0BhZ3IubmlpZ2F0YS11LmFjLmpw
 Hirohiko Nagano
Hirohiko Nagano Mariko Atarashi-Andoh
Mariko Atarashi-Andoh Sota Tanaka
Sota Tanaka Takumi Yomogida3
Takumi Yomogida3 Naofumi Kozai
Naofumi Kozai