
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change, 25 August 2022
Sec. Forest Management
Volume 5 - 2022 | https://doi.org/10.3389/ffgc.2022.995427
This article is part of the Research TopicMountainous Forest Ecosystems: Challenges and Management ImplicationsView all 16 articles
A correction has been applied to this article in:
Corrigendum: Conservation Priority Index of species, communities, and habitats for biodiversity conservation and their management planning: a case study in Gulmarg Wildlife Sanctuary, Kashmir Himalaya
The present study is an attempt to evaluate the Conservation Priority Index (CPI) of species, habitats, and communities for their conservation and management planning in the Kashmir Himalayas in India. The present study is an attempt to prioritize 361 plant species, 18 plant communities (10 within the forest zone and 08 within the alpine zone), and 07 habitats for conservation planning. Out of the total plant species recorded, 06 species were categorized as critically endangered, 20 endangered, 28 vulnerable, and 98 species to be near threatened. Amongst the forest and alpine communities, Abies pindrow community and Juniperus squamata-Rhododendron anthopogon mixed community showed the maximum CPI values. Amongst the habitats, dry habitats showed the maximum CPI in the sanctuary. The study found that the threatened species positively correlated with the native and endemic species indicating that these species were rigorously affected due to biotic and abiotic stresses. Based on the results of the present study, we propose a practical method for biodiversity conservation and management of protected areas. The approach employs a variety of qualitative and quantitative features to compute CPI in conjunction with phytosociological data. This kind of study will be immensely helpful to forest officials, policy makers, conservators, and researchers for planning better strategies to conserve and manage particular species, communities, and even habitats in protected areas.
Asia is home to one of the largest, most elevated, and most highly populated mountain ranges in the world. This mountain range is called the Himalayas (Rana et al., 2021). The Himalayas designated as a global biodiversity hotspot underlines their significance as a storehouse for unique flora and fauna (Allen et al., 2010). A high degree of endemism in the area suggests the presence of numerous important eco-regions and ecosystems with global significance (Dhar, 2002). Himalayan forests serve as a significant “sink” for carbon dioxide and further provide a livelihood to a sizable population both upstream and downstream (Reddy et al., 2016; Chakraborty et al., 2017; Negi et al., 2018a). Therefore, India's National Action Plan on Climate Change (NAPCC) considered this region vital for preserving the ecological security of India (Negi et al., 2019). However, the Himalayan ecosystems are highly vulnerable and are degrading due to various biotic and abiotic stresses (Wani et al., 2022). Overall, climate change is a leading cause of ecological changes and biodiversity loss, although species invasions are also one of the important factors contributing to changes in forest productivity and biodiversity loss, especially native and endemic species (Behera et al., 2019; Negi et al., 2021; Wani et al., 2021). Besides these factors, the forests of the Himalayan region are now becoming vulnerable due to increasing anthropogenic disturbances like deforestation, unsustainable fuel wood, timber collection, and agricultural expansion (Negi and Maikhuri, 2017; Thakur et al., 2022). Therefore, detailed information on species, communities, and habitats is necessary to create effective conservation and management plans for these biodiversity-rich places.
Protected Areas (PA) are recognized as areas with high biodiversity, and as a result, receive special consideration and increased focus on biodiversity conservation and management (Rawat and Adhikari, 2015). A number of studies have, therefore, highlighted the contribution of PAs in the conservation of biodiversity and also in preventing threatened species from extinction risk (Karanth et al., 2010; Oldekop et al., 2016). However, recent studies conducted around the world have shown that biodiversity and other ecosystem products and services continue to be lost despite constant growth in the number of areas covered under PAs (Jenkins and Joppa, 2009; McDonald and Boucher, 2011). According to IPBES (2019), up to one million species of plants and animals are threatened by the risk of extinction. The most recent update of the IUCN Red List includes 142, 577 species [International Union for Conservation of Nature (IUCN), 2022] representing ~7% of the biodiversity of the planet (Ronsted et al., 2022). The irony is that most plant species lack global risk assessment, and the lack of knowledge of which plant species are at risk of extinction limits our ability to frame conservation and management policies (Nic Lughadha et al., 2020). Therefore, the first stage in conservation planning in every region is to establish priorities, criteria, and indicators (Mirtl et al., 2018; Negi et al., 2019). The criteria and indicators approach offers a mechanism for evaluating changes in specific forestry circumstances, and this knowledge helps forest managers plan pertinent conservation policies (Rawat et al., 2008). Additionally, vulnerability mapping of certain species and forests is needed, especially in the Himalayan region, for the implementation of ground-level management plans (Thakur et al., 2020, 2022). Further, to manage and conserve biodiversity in the face of ongoing global change, it is necessary to have enough data to evaluate the status and trends of species ranges, their population, community makeup, and habitat types (Oliver et al., 2021). Species richness has been the main focus of conservation studies and is still widely used (Bano et al., 2018). For instance, conservation planning has historically given priority to some regions over others using the information on richness mixed with various metrics (such as endemism or rarity) (Cadotte and Tucker, 2018). However, to assess the state and trends of species distributions and manage biodiversity in the face of continuing global change, sufficient evidence is required (Oliver et al., 2021). The status and trends of species populations and distributions, communities, and different types of habitats directly linked to the ecological value of those variables are crucial for conservation and management (Bland et al., 2015).
To prevent species extinction, broad, ecosystem-based conservation strategies are also required, which do not rely on taxonomic information or the determination of individual species but on the community composition and habitat types. Thus, developing a Conservation Priority Index of unique species, communities, and habitats at local, regional, national, and global levels is an important step in conservation and management planning. Species richness, nativity, and endemism are a few of the qualities that help prioritize species, communities, and habitats (Pant and Samant, 2007). In Kashmir Himalaya, few efforts have been made to prioritize species for conservation, but most of these studies primarily focused to prioritize medicinal or economically important plants (Dar and Naqshi, 2001; Baig et al., 2014; Tali et al., 2015, 2018; Haq et al., 2019, 2021). However, no attempt has been made to prioritize species, habitats, and communities for conservation and management within the protected area networks of Kashmir Himalaya. Keeping these research gaps in consideration, the present study attempts to evaluate the Conservation Priority Index (CPI) of species, habitats, and communities in Gulmarg Wildlife Sanctuary (GWLS), in order to find out which species, communities, and habitats should be prioritized for conservation and management planning within the sanctuary.
Gulmarg Wildlife Sanctuary (GWLS) stretches between 74°17′ to 74°79′ N latitude and 34°55′ to 34°60′ E longitude in District of Baramulla situated in Jammu and Kashmir (Figure 1). It covers an area of 180 km2, out of which 60 and 120 km2 of the areas are under forest and alpine zones, respectively, with an altitudinal range of 2,300–4,200m asl. GWLS has two administrative units: Ferozpora/Tangmarg and Block Gulmarg and are divided into 20 compartments numbering 31–41 (Lower elevation compartments) and 50–58 (Upper elevation compartments), respectively. The mountains in this Himalayan region maintain their glaciation above 3,700 m almost all year round, and the sanctuary has a wet temperate climate with a well-balanced supply of moisture. As a result, the area above 3,700 m asl represents the nival zone and fits well with the climatological concept of the snowline developed for mesic mountains (Gottfried et al., 2011). Geologically, the region is made up of Triassic Limestone rocks, Panjal Traps volcanic rocks, Salkhala Series metamorphic rocks associated with granitic intrusions, and Karewa Group sediments (Dar et al., 2014). Since the area is entirely mountainous, the topography is very uneven. The vegetation is affected at all levels by a number of topographic elements, including altitude, slope steepness, exposure to light and wind, and the direction of mountain chains (Nanda et al., 2019). Coniferous temperate mountain forests of Blue Pine (Pinus wallichiana), sub-alpine forests of Silver Fir (Abies pindrow), and Himalayan Birch (Betula utilis), then alpine scrub and meadows, make up the majority of the vegetation of Gulmarg (Khuroo et al., 2015). Additionally, the sanctuary is home to numerous medicinal plants and nomadic groups like the Gujjars and Bakerwals (Wani and Pant, 2020).
Field visits and surveys were carried out for the qualitative and quantitative assessment of the plant species within the forest, as well as the alpine zone, of the study area in different seasons from May 2018 to June 2022. A total of 123 sites (63 in the forest zone and 60 in the alpine zone) were selected to cover all the possible aspects and habitats along the altitudinal gradient (2,300–4,200m asl). Habitats were identified on the basis of physical features and the dominant vegetation of the sites. The sites having closed canopy along with a high proportion of organic matter and moisture were considered shady moist habitats. Sites having an open canopy with a low proportion of organic matter and moisture were classified as dry habitats whereas sites having an open canopy with high moisture and humus were classified as moist habitats. The sites with high anthropogenic pressures (assessed in terms of trampling, the quantity of animal and human wastes, and distance from the residential areas) were considered exposed habitats. Quantitative assessment was carried out using a stratified random sampling method after laying out a plot of 50 × 50 m at each site. For the assessment of plant species within each plot, 10 random quadrats of 10 × 10 m were laid for trees, 10 quadrats of 5 × 5 m for shrubs, and 20 quadrats of 1 × 1 m for herbs nested in the same plot following standard sampling methods (Curtis and Intosh, 1950; Mishra, 1968; Samant et al., 2002; Rawal et al., 2018). The plant specimens from each plot were collected and relevant information (altitudinal range, habitat, and growth forms) were recorded in a field book. Each collected specimen was later identified up to the species level with the help of local and regional floras (Dhar and Kachroo, 1983; Bhat, 1984; Naqshi et al., 1984; Singh and Kachroo, 1994; Singh et al., 2002). Accepted nomenclature and families of the species were retrieved from the Plants of the World Online database (https://powo.science.kew.org). Forest communities were delineated on the basis of the Importance Value Index (IVI) following the study of Mishra (1991). Sites having ≥50% of the total IVI contributed by a single species were categorized as pure communities of that species whereas sites having ≥50% of the total IVI contributed by two or more species were categorized as mixed communities of those species. Using the same criteria, communities in the alpine zone were identified on the basis of relative density instead of IVI following Rana and Samant (2009).
The nativity and endemism of the species were determined (Dhar and Samant, 1993; Samant et al., 1996; Samant and Dhar, 1997; Khuroo et al., 2007, 2010). Plant species having their origins in the Himalayan region were considered native and the rest were considered non-natives, while the plant species having their distribution limited to the Himalayan region were considered endemics (Samant et al., 1998). Furthermore, the threat status of the plant species was assessed following the study of Rana and Samant (2010) using the parameters viz., population size, habitat specificity, altitudinal range, nativity, and endemism. Furthermore, information regarding the use values of the plant species was collected through interviews and discussions with knowledgeable persons from the surrounding villages of the sanctuary.
CPI was calculated to assess the site-specific threat status at species, habitat, and community levels (Rana and Samant, 2009, 2010; Singh and Samant, 2010; Rana et al., 2020). The CPI value of plant species was calculated as the cumulative values of six attributes viz., altitudinal range, habitat specificities, use values, population size, nativity and endemism, and extraction trend following Rana and Samant (2010). The attributes used for each species were given grades/marks, with a maximum of 10 points, moderate of 6 points, and minimum of 2 points (Table 1). The species having ≥75% of total CPI (i.e., ≥45 out of 60) were categorized as Critically Endangered; 65–74% as Endangered; 55–64% as Vulnerable; 45–54% as Near Threatened, and ≤44% as Least Concerned, following Rana and Samant (2010) with some modifications. Based on the representative sites, range of altitudes, species richness, native species, endemic species, useful species, and threatened species, the CPI of habitats and communities was calculated (Rana and Samant, 2009). The CPI of habitats and communities is the aggregate value of all these attributes, the value of which ranges from 2 to 10 (Table 2). The species, habitats, and communities satisfying all the attributes in the highest grade resulted in the highest aggregate values while attributes with the lowest grade resulted in the lowest aggregate values. Correlation of the total species with a number of native and endemic species, number of users with threatened species, and number of native and endemic species with threatened species in the forest, as well as the alpine zone, was calculated using Microsoft Excel 2010.
A total of 361 plant species belonging to 73 families and 210 genera were recorded (Supplementary Table S1). Asteraceae was the dominant family with 56 species followed by Lamiaceae (22 species), Rosaceae (21 species), and Ranunculaceae (19 species). Ten most dominant families are presented in Figure 2. A total of 184 plant species were found native and 81 species endemic to the Himalayan region. Non-native species have different phytogeographic affinities with major contributions from Asia, Eurasia, and Europe (Figure 3). A total of 158 plant species were found to be utilized by the local people for various purposes i.e., food, fodder, medicine, fuel wood, and timber. Among the unique and high-value medicinal plants were Achillea millifolium, Angelica glauca, Artemisia absinthium, Bergenia ciliata, Colchicum luteum, Dolomiaea costus, Fritillaria roylei, Hyoscyamus niger, Inula racemosa, Lamium album, Picrorhiza kurroa, Podophyllum hexandrum, Trillium govanianum, and Thymus linearis. Medicinal plants like Aconitum heterophyllum, Angelica glauca, Dolomiaea costus, Fritillaria roylei, Picrorhiza kurroa, Rheum webbiana, and Trillium govanianum are used highly for commercial purposes. Prominent wild edibles used by the local inhabitants include Taraxacum sect. Taraxacum, Berberis lycium, Capsella bursa-pastrosis, Dipsacus innermis, Dioscorea deltoidea, Rheum webbiana, Malva neglecta, Fragaria nubicola, and Viburnum grandiflorum.
The study revealed a total of seven habitats including shady, moist, dry, bouldary, rocky, riverine, moist, and exposed throughout GWLS. A total of 18 plant communities were identified including 10 forest communities and 8 alpine communities. Ten plant communities delineated from the forest zone include Abies pindrow community, Pinus wallichiana community, A. pindrow-P. wallichiana mixed community, P. wallichiana-Cedrus deodara mixed community, A. pindrow-Picea smithiana mixed community, A. pindrow-Picea smithiana mixed community, Aesculus indica-Taxus wallichiana mixed community, Taxus wallichiana-Prunus cornuta-Aesculus indica mixed community, Abies pindrow-Acer cesium mixed community, and Betula utilis community. Eight plant communities delineated from the alpine zone include Viburnum grandiflorum community, Salix denticulata community, Rhododendron campanulatum community, Juniperus squamata community, Juniperus squamata-Rhododendron anthopogon mixed community, Bistorta affinis- Saussurea acktinsonii-Bergenia stracheyi mixed community, Rhododendron anthopogon community, and Bistorta affinis-Swertia petiolata mixed community.
Amongst habitats, dry habitats represented maximum sites (27) followed by bouldary (22), shady moist (20), exposed (17), riverine (13), rocky (16), and moist (6). Amongst forest communities, Abies pindrow community was represented at maximum sites (16 sites) followed by Pinus wallichiana community (9 sites), Betula utilis community (7 sites each) Abies pindrow-Pinus wallichiana mixed, Pinus wallichiana–Cedrus deodara mixed (6 each). The rest of the communities were represented at <6 sites. Amongst alpine communities, Juniperus squamata-Rhododendron anthopogon mixed community represented maximum sites (21) followed Bistorta affinis- Saussurea acktinsonii-Bergenia stracheyi mixed community (13) and Bistorta affinis-Swertia petiolata mixed and Rhododendron anthopogon community (7 sites each) and Viburnum grandiflorum (6 sites). Juniperus squamata, Salix denticulata, and Rhododendron campanulatum communities were represented at <4 sites.
Amongst the habitats, bouldary habitats showed a wide distribution range followed by dry riverine and rocky habitats. Amongst the forest communities, the distribution range was maximum (2,300–3,000) for Abies pindrow community followed by Pinus wallichiana-Abies pindrow mixed (2,400–2,700) and Pinus wallichiana (2,300–2,600) and Betula utilis (2,300–3,500). Amongst alpine communities, the altitudinal range was maximum for the Juniperus squamata-Rhododendron anthopogon mixed community (3,400–3,900) followed by Bistorta affinis- Saussurea acktinsonii- Bergenia stracheyi mixed (3,350–3,700) community. Rhododendron campanulatum and Salix denticulata communities were having the least altitudinal ranges.
Amongst habitats, species richness was highest (34.1%) in a dry habitat, followed by shady moist (32.7%), bouldary (29.7%), rocky (27.8%), riverine (22.3%), exposed (20.3%), and moist (13.4%). Amongst the forest communities, species richness was found maximum in Abies pindrow community (46.8%), followed by Pinus wallichiana community (28.9%), Pinus wallichiana-Abies pindrow mixed community (27.5%), and Abies pindrow-Picea smithiana mixed community (24.7%). Amongst the alpine communities, species richness was maximum in Juniperus squamata-Rhododendron anthopogon mixed community (23.4%) followed by Viburnum grandiflorum community (16.5%), Juniperus squamata community (10.1%) and Bistorta affinis- Saussurea acktinsonii- Bergenia stracheyi mixed community (9.6%).
Amongst the habitats, dry habitats exhibit maximum native species (35.5%) followed by bouldary (35.1%), shady moist (31.1%), rocky (25.6%), exposed (19.5%), riverine (13.5%), and moist (12.8%). Dry habitats exhibit maximum endemic species (44.4%) followed by shady moist (41.9%), bouldary (35.8%), exposed (29.6%), rocky (22.2%), riverine (16.1%), and moist (12.8%). Amongst the forest communities, maximum native species were found in the Abies pindrow community (40.52%) followed by Abies pindrow-Picea smithiana mixed community and Betula utilis (23.6% each), Abies pindrow-Acer cesium mixed community (22.9%), and Pinus wallichiana community (20.9%). Maximum endemic species were found in Abies pindrow community (40.7%) followed by Abies pindrow-Acer cesium mixed (28.3%), and Abies pindrow-Pinus wallichiana mixed (24.6%). Amongst alpine communities, maximum native, as well as endemic species (33.7% native and 41.9% endemic), were found in Juniperus squamata-Rhododendron anthopogon mixed community followed by Viburnum grandiflorum community (18.9% native and 14.8% endemic), and Rhododendron anthopogon community (14.8% native and 22.2% endemic).
Maximum useful species were distributed in bouldary habitats (38.5%) followed by dry (37.9%), shady moist (34.8%), rocky (34.1%), exposed (29.5%), riverine (22.7%), and moist habitats (19.6%). Amongst the forest communities, the maximum number of useful species were distributed in Abies pindrow community (46.2%) followed by Pinus wallichiana communities (35.4%), Abies pindrow-Pinus wallichiana mixed communities (32.9%), and Abies pindrow-Picea smithiana mixed community (25.9%). Amongst alpine communities, the maximum number of useful species were distributed in Viburnum grandiflorum community (16.4%) followed by Juniperus squamata-Rhododendron anthopogon mixed and Salix denticulata communities (11.3%), and Juniperus squamata community (8.8%).
Amongst habitats, maximum threatened species were distributed in rocky habitats (34.6%) followed by bouldary (32.6%), shady moist (30.7%), dry (25.0%), exposed (23.0%), moist (15.3), and riverine (13.4%). Amongst forest communities, maximum threatened species were distributed in Abies pindrow community (34.8%) followed by Abies pindrow-Pinus wallichiana mixed (21.7%), Abies pindrow-Acer cesium mixed (19.0%), Abies pindrow-Picea smithiana mixed and Betula utilis community (17.7% each). Amongst alpine communities, maximum threatened species were distributed in Juniperus squamata-Rhododendron anthopogon mixed community (26.3%) followed by Viburnum grandiflorum, Juniperus squamata and Rhododendron anthopogon communities (9.8% each). A significant positive correlation is found between the total species and native species in the forest zone (0.87, p < 0.01, R2 0.75) and alpine zone (0.94, p < 0.01, R2 = 0.87) (Figure 4) and there is also a significant positive correlation between the total species and endemic species in the forest (0.90, p < 0.01, R2 = 0.73) and alpine zones (0.75, p < 0.01, R2 = 0.45) (Figure 5). A significant positive correlation is found between the number of useful species and the number of threatened species in the forest (r = 0.85, p < 0.01, R2 = 0.70), as well in the alpine zone (r = 0.830, p < 0.01, R2 = −0.15) (Figure 6). Further, a significant positive correlation is found between the number of threatened species and native species in the forest zone (r = 0.96, p < 0.01, R2 = 0.91) and in alpine zone (r = 0.94, p < 0.01, R2 = 0.87) (Figure 7) and endemic species in forest zone (r = 0.95, p < 0.01, R2 = 0.90) and in the alpine zone (r = 0.95, p < 0.01, R2 = 0.87) (Figure 8).
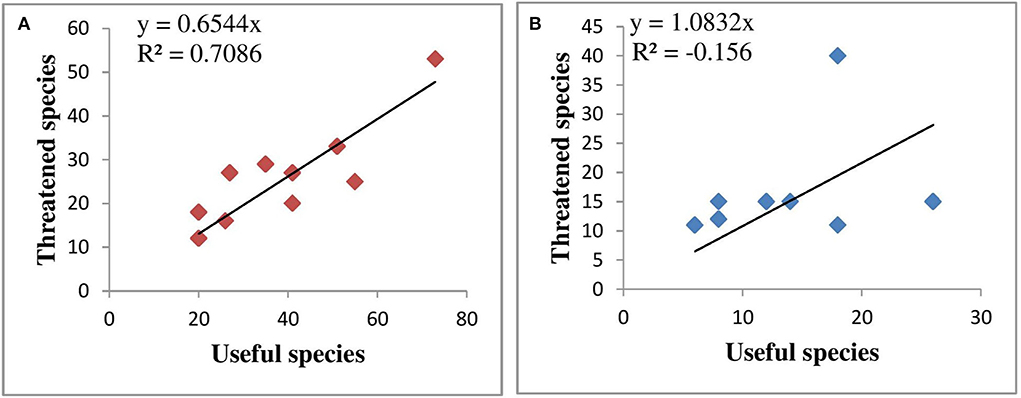
Figure 6. Correlation between useful species and threatened species (A) forest zone (B) alpine zone.
Based on the threat assessment of the species, 6 plant species were assessed to be critically endangered, 20 endangered, 28 vulnerable, and 98 plant species were assessed to be near threatened. Rests of the species were assessed to be the least concern in the study area (Supplementary Table S1). Amongst the habitats, dry habitats showed the maximum CPI (50) followed by bouldary (48), shady moist (46), and rocky (36) habitats (Table 3). Amongst forest communities, Abies pindrow community showed maximum CPI value (56), followed by Abies pindrow-Pinus wallichiana mixed community (42), Abies pindrow-Picea smithiana mixed community (40) (Table 4). Amongst alpine communities, Juniperus squamata-Rhododendron anthopogon mixed community showed maximum CPI value (44), followed by Salix denticulata (40), Rhododendron campanulatum (38), and Juniperus squamata community (36) (Table 4).
Species richness recorded during the present study was higher than in earlier reports from the Indian Himalayan region (Singh et al., 2007; Chawla et al., 2008; Dar and Sundarapandian, 2016; Pandey et al., 2018; Haq et al., 2019, 2021; Ahmad et al., 2020). Asteraceae, Lamiaceae, Rosaceae, and Ranunculaceae were reported to be dominant contributing maximum species. These families have been reported as dominant in Indian Himalayas in earlier studies also (Samant et al., 2007; Dar and Khuroo, 2012; Haq et al., 2019; Ahmad et al., 2021; Altaf et al., 2021). As a large number of species has become threatened in Kashmir Himalaya over the decades due to various anthropogenic disturbances (Hamid et al., 2020), the rich floristic diversity of the native, endemic, economically important, and threatened species in the GWLS signifies the enormous conservation and socio-economic values of the sanctuary.
A significant positive correlation was found between the total species and native species in the forest and alpine zones. There is also a significant positive correlation between the total species and endemic species in the forest and alpine zones which indicate that as the number of species increases there is an increase in the number of native and endemic species. The correlation between the number of useful species and the number of threatened species in the forest was much strong than in the alpine zone indicating that the number of threatened species was higher in forest communities having biotic/abiotic pressures. It is obvious that an increased number of useful species in a community will lead to a burden on the unique species, resulting in their declining population. Further, a significant positive correlation between the number of threatened species and native species in the forest zone and in the alpine zone and endemic species in the forest zone and in the alpine zone indicated that the native and endemic plant species were rigorously affected due to biotic and abiotic stresses (Rana et al., 2020). Anthropogenic disturbances coupled with climate change promote biological invasions in mountain ecosystems and thus pose threat to native and endemic species and are a global concern (Ahmad et al., 2021; Li et al., 2021). Endemic species are specialist species and have narrow niche widths and thus narrow environmental tolerance (Mills et al., 2020).
Conservation prioritization of species, habitats, and communities is a prerequisite for biodiversity conservation and management planning in the protected area (Singh and Samant, 2010). Based on the CPI value of species in the present study, 41.8% of species were categorized as threatened and near threatened in the study area. Some of the plants assessed to be threatened in GWLS are given in Figure 9. Some of these plant species have been categorized as threatened in some previous studies also (Table 5). However, Hamid et al. (2020) pointed out that many plant species like Betula utilis, Colchicum luteum, Podophyllum hexandrum, Rheum webbianum, Taxus wallichiana, and Valeriana jatamansii have not been included in the threatened lists despite of their dwindling populations and thus recommended their immediate categorization as per the IUCN criteria.

Figure 9. Notable threatened plants of GWLS (A) Podophyllum hexandrum (B) Aconitum heterophyllum (C) Trillium govanianum (D) Phytolacca acinosa (E) Arisaema jacquemontii (F) Dioscorea deltoidea (G) Rhododendron campanulatum (H) Arisaema propinquum (I) Hyoscyamus niger.
Among the forest community, Abies pindrow community and Abies pindrow-Pinus wallichiana mixed community, whereas among alpine communities, Juniperus squamata-Rhododendron anthopogon mixed and Salix denticulata community with high CPI values indicated the need for conservation (Figure 10). Furthermore, dry, bouldary, and shady moist habitats with high CPI value reveal that the proper management of these habitats would help in maintaining the natural ecosystems and conservation of species of the area, as the habitat fragmentation and degradation are considered as one of the primary drivers of species extinction (Tilman et al., 2001; Ganie et al., 2019; Quan et al., 2021; Su et al., 2022). The prioritized habitats and communities not only have higher species richness, but also possess the premier number of native, endemic, economically important, and threatened species. Thus, any alteration in these communities and habitats will lead to an alteration in their species composition; the consequences of which may be the loss of native, endemic, and threatened species (Mehta et al., 2020). Further, the communities were identified on the basis of the dominance of one or more species pointing out that any alteration in such dominant species will alter the composition of these communities. Therefore, the regeneration dynamics of such dominant species need to be analyzed thoroughly for conservation and management planning. Further, regular monitoring of the prioritized habitats and communities is desirable to comprehend the structural and functional alterations in the natural vegetation due to various biotic and abiotic stresses as suggested by previous studies (Negi et al., 2018b, 2019).
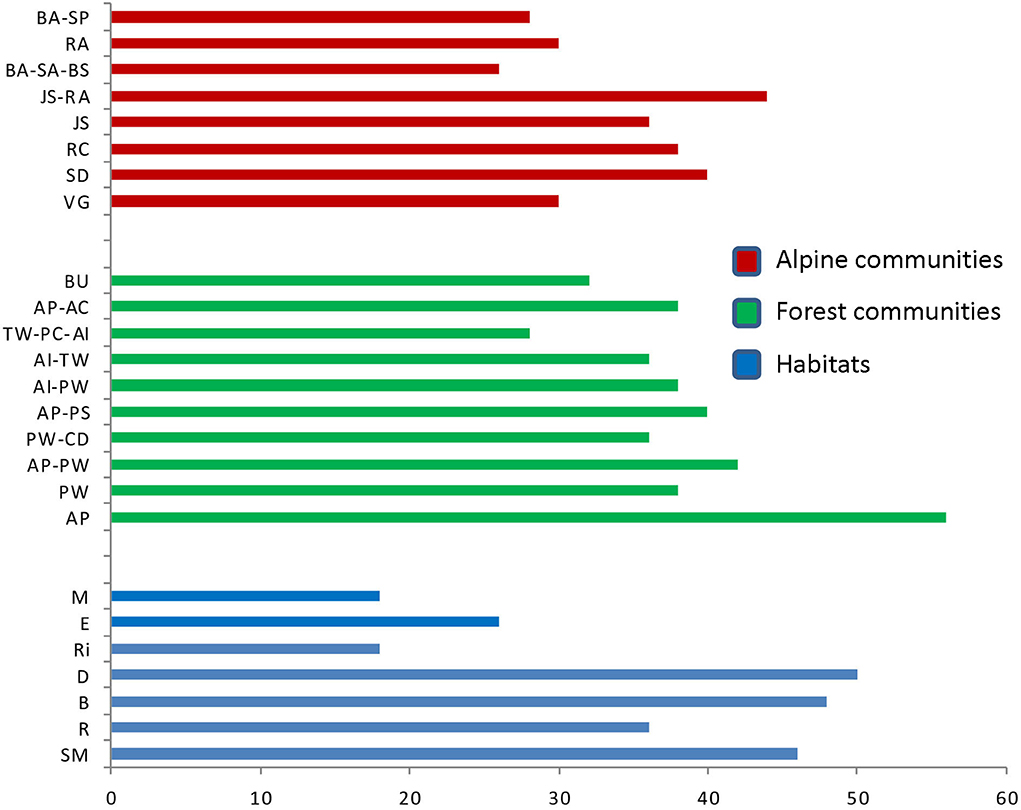
Figure 10. CPI of delineated alpine and forest communities and habitats; BA-SP, Bistorta affinis-Swertia petiolata community; RA, Rhododendron anthopogon community; BA-SA-BS, Bistorta affinis-Saussurea acktinsonii-Bergenia stracheyi community; Juniperus squamata-Rhododendron anthopogon community; Juniperus squamata community; Rhododendron campanulatum community; Salix denticulata community; Viburnum grandiflorum community; AP, Abies pindrow community; PW, Pinus wallichiana community; AP-PW, Abies pindrow-Pinus wallichiana community; PW-CD, Pinus wallichiana-Cedrus deodara community; AP-PS, Abies pindrow-Picea smithiana community; AI-PW, Aesculus indica-Pinus wallichiana community; AI-TW, Aesculus indica-Taxus wallichiana community; TW-PC-AI, Taxus wallichiana-Prunus cornuta-Aesculus indica community; AP-AC, Abies pindrow-Acer cesium community; BU, Betula utilis community; M, Moist; E, Exposed; Ri, Riverine; D, Dry; B, Bouldary; R, Rocky; SM, Shady Moist.
At the local level, proper application of the IUCN criteria is necessary for assessing the conservation status of taxonomic units (Rodrigues et al., 2006; Miller et al., 2007; Abeli et al., 2009). The comprehensive method used in this study includes IUCN criteria that are suitable in the local context (Dunn et al., 1999; Keller and Bollmann, 2004; Gauthier et al., 2010). The current work proposes a practical method for managing protected areas based on scientific site-specific techniques (species, habitats, and communities), which employs a variety of qualitative and quantitative features to compute CPI in conjunction with phytosociological data. However, it is well-established that such research is useless unless the findings of these studies are used by forest managers and policymakers for implementing biodiversity conservation and management policies.
The present study attempts to assess the floristic diversity of forest and alpine communities in GWLS. Further, the study attempts to prioritize the species, habitats, and communities for conservation and management strategies. A number of species were assessed to be threatened within the study area and out of which many species are on the IUCN's Red List of species. Further, the prioritized habitats and communities in GWLS are not only rich in species number, but also possess a greater number of native, endemic, and threatened species. Therefore, any type of negative impact on such habitats and communities, in turn will affect the survival of such precious species. Long-term monitoring using the quadrat method for all the growth forms of the georeferenced plots representing the prioritized habitats and communities is required. Further, tourist influx should be limited as per the carrying capacity of the sanctuary, and guidelines for the visitors should be framed in order to reduce the trampling of herbaceous flora inside the sanctuary. Thus, the present study has developed baseline information for initiating conservation and management planning.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
ZW: conceptualization, methodology, data collection, curation and analysis, investigation, and writing original draft. JB and VN: writing the review and validation. KS: writing the review. SS: writing the review and funding acquisition. SP: conceptualization, supervision, validation, and writing the review. All authors contributed to the article and approved the submitted version.
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a Large Group Research Project under grant number (RGP2/90/44).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.995427/full#supplementary-material
Abeli, T., Gentili, R., Rossi, G., Bedini, G., and Foggi, B. (2009). Can the IUCN criteria be effectively applied to peripheral isolated plant populations? Biodivers. Conserv. 18, 3877–3890. doi: 10.1007/s10531-009-9685-4
Ahmad, M., Sharma, P., Rathee, S., Singh, H. P., Batish, D. R., Lone, G. R., et al. (2021). Niche width analyses facilitate identification of high-risk endemic species at high altitudes in western Himalayas. Ecol. Indcat. 126, 107653. doi: 10.1016/j.ecolind.2021.107653
Ahmad, M., Uniyal, S. K., Batish, D. R., Singh, H. P., Jaryan, V., Rathee, S., et al. (2020). Patterns of plant communities along vertical gradient in Dhauladhar Mountains in Lesser Himalayas in North-Western India. Sci. Tot. Environ. 716, 136919. doi: 10.1016/j.scitotenv.2020.136919
Allen, D. J., Molur, S., and Daniel, B. A. (2010). The Status and Distribution of Freshwater Biodiversity in the eastern Himalaya. International Union for Conservation of Nature and Natural Resources. Cambridge; Gland: IUCN.
Altaf, A., Haq, S. M., Shabnum, N., and Jan, H. A. (2021). Comparative assessment of Phyto diversity in Tangmarg Forest division in Kashmir Himalaya, India. Acta Ecol. Sin. doi: 10.1016/j.chnaes.2021.04.009
Baig, B. A., Ramamoorthy, D., and Wani, B. A. (2014). Population status and conservation prioritization of some threatened medicinal plants of Kashmir Himalayas. Int. J. Appl. Biol. Pharm. Technol. 5.
Bano, S., Khan, S. M., Alam, J., Alqarawi, A. A., Abd Allah, E. F., Ahmad, Z., et al. (2018). Eco-Floristic studies of native plants of the Beer Hills along the Indus River in the districts Haripur and Abbottabad, Pakistan. Saudi J. Biol. Sci. 25, 801–810. doi: 10.1016/j.sjbs.2017.02.009
Behera, M. D., Behera, S. K., and Sharma, S. (2019). Recent advances in biodiversity and climate change studies in India. Biodivers. Conserv. 28, 1943–1951. doi: 10.1007/s10531-019-01781-0
Bhat, N. A. (1984). Floristic Composition of Gulmarg (Baramulla) (Ph. D. Thesis). University of Kashmir, Srinagar.
Bland, L. M., Collen, B., Orme, C. D. L., and Bielby, J. (2015). Predicting the conservation status of data- deficient species. Conserv. Biol. 29, 250–259. doi: 10.1111/cobi.12372
Cadotte, M. W., and Tucker, C. M. (2018). Difficult decisions: strategies for conservation prioritization when taxonomic, phylogenetic and functional diversity are not spatially congruent. Biol. Conserv. 225, 128–133. doi: 10.1016/j.biocon.2018.06.014
Chakraborty, A., Joshi, P. K., and Sachdeva, K. (2017). Capturing forest dependency in the central Himalayan region: variations between Oak (Quercus spp.) and Pine (Pinus spp.) dominated forest landscapes. Ambio. 47, 504–522. doi: 10.1007/s13280-017-0947-1
Chawla, A., Rajkumar, S., Singh, K. N., Lal, B., and Singh, R. D. (2008). Plant Species Diversity along an Altitudinal Gradient of Bhabha Valley in Western Himalaya. J. Moun. Sci. 5, 157–177. doi: 10.1007/s11629-008-0079-y
Curtis, J. T., and Intosh, M. C. (1950). The Inter relation of certain analytic and phytosociological character. Ecology 31, 434–445. doi: 10.2307/1931497
Dar, G. H., and Khuroo, A. A. (2012). Floristic diversity in the Kashmir Himalaya: progress, problems and prospects. Sains Malay. 42, 1377–1386.
Dar, G. H., and Naqshi, A. R. (2001). Threatened flowering plants of the Kashmir Himalaya – a checklist. Orien. Sci. 6, 23–53.
Dar, J. A., and Sundarapandian, S. (2016). Patterns of plant diversity in seven temperate forest types of Western Himalaya, India. J. Asia Pac. Biodivers. 9, 280–292. doi: 10.1016/j.japb.2016.03.018
Dar, R. A., Rashid, I., Romshoo, S. A., and Marazi, A. (2014). Sustainability of winter tourism in a changing climate over Kashmir Himalaya. Environ. Mon. Asses. 186, 2549–2562. doi: 10.1007/s10661-013-3559-7
Dhar, U. (2002). Conservation implications of plant endemism in high-altitude Himalaya. Curr. Sci. 82, 141–148.
Dhar, U., and Kachroo, P. (1983). Alpine flora of Kashmir Himalaya, Scientific Publishers, Jodhpur. India. doi: 10.2307/2805987
Dhar, U., and Samant, S. S. (1993). Endemic diversity of Indian Himalaya I. Ranunculaceae and II. Paeoniaceae. J, Biogeogra. 20, 659–668. doi: 10.2307/2845521
Dunn, E. H., Hussell, D. J. T., and Welsh, D. A. (1999). Priority-setting tool applied to Canada's land birds based on concern and responsibility of species. Conserv. Biol. 13, 1404–1415. doi: 10.1046/j.1523-1739.1999.98400.x
Ganie, A. H., Tali, B. A., Khuroo, A. A., Reshi, Z. A., and Nawchoo, I. A. (2019). Impact assessment of anthropogenic threats to high-valued medicinal plants of Kashmir Himalaya, India. J. Nat. Conserv 50, 125715. doi: 10.1016/j.jnc.2019.125715
Gauthier, P., Debussche, M., and Thompson, J. D. (2010). Regional priority setting for rare species based on a method combining three criteria. Biol. Conserv. 143, 1501–1509. doi: 10.1016/j.biocon.2010.03.032
Gottfried, M., Hantel, M., Maurer, C., Toechterle, R., Pauli, H., and Grabherr, G. (2011). Coincidence of the alpine–nival ecotone with the summer snowline. Environ. Res. Lett. 6, 014013. doi: 10.1088/1748-9326/6/1/014013
Hamid, M., Khuroo, A. A., Ahmad, R., Rasheed, S., Malik, A. H., and Dar, G. H. (2020). “Threatened flora of Jammu and Kashmir state,” in Biodiversity of the Himalaya: Jammu and Kashmir state, eds G. H. Dar and A. A. Khuroo (Singapore: Springer Nature). 957–995. doi: 10.1007/978-981-32-9174-4_37
Haq, S. M., Malik, A. H., Khuroo, A. A., and Rashid, I. (2019). Floristic composition and biological spectrum of Keran-a remote valley of northwestern Himalaya. Acta Ecol. Sin. 39, 372–379. doi: 10.1016/j.chnaes.2018.12.001
Haq, S. M., Shah, A. A., Yaqoob, U., and Hassan, M. (2021). Floristic quality assessment index of the Dagwan stream in Dachigam National Park of Kashmir Himalaya. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 91, 657–664. doi: 10.1007/s40011-021-01247-w
International Union for Conservation of Nature (IUCN) (2022). The IUCN Red List of threatened species (Version 2021-3). Available online at: https://www.iucnredlist.org. (accessed May 18, 2021–November 23, 2021).
IPBES (2019). Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES Secretariat.
Jenkins, C. N., and Joppa, L. (2009). Expansion of the global terrestrial protected area system. Biol. Conserv. 142, 2166–2174. doi: 10.1016/j.biocon.2009.04.016
Karanth, K. K., Nichols, J. D., Ullas, Karanth, K., Hines, J. E., and Christensen, N. L. (2010). The shrinking ark: patterns of large mammal extinctions in India. Proc. R. Soc. B Biol. Sci. 277, 1971–1979. doi: 10.1098/rspb.2010.0171
Keller, V., and Bollmann, K. (2004). From red lists to species of conservation concern. Conserv. Biol. 18, 1636–1644. doi: 10.1111/j.1523-1739.2004.00464.x
Khuroo, A. A., Hamid, M., and Malik, A. H. (2015). Himadri site in Kashmir Himalaya. ENVIS Newsl. Him. Ecol. 12, 4.
Khuroo, A. A., Rashid, I., Reshi, Z., Dar, G. H., and Wafai, B. A. (2007). The alien flora of Kashmir Himalaya. Biol. Inv. 9, 269–292. doi: 10.1007/s10530-006-9032-6
Khuroo, A. A., Weber, E., Malik, A. H., Dar, G. H., and Reshi, Z. A. (2010). Taxonomic and biogeographic patterns in the native and alien woody flora of Kashmir Himalaya, India. Nord. J. Bot. 28, 685–696. doi: 10.1111/j.1756-1051.2010.00750.x
Li, W., Shi, Y., Zhu, D., Wang, W., Liu, H., Li, J., et al. (2021). Fine root biomass and morphology in a temperate forest are influenced more by the nitrogen treatment approach than the rate. Ecol. Indicat. 130, 108031. doi: 10.1016/j.ecolind.2021.108031
McDonald, R. I., and Boucher, T. M. (2011). Global development and the future of the protected area strategy. Biol. Conserv. 144, 383–392. doi: 10.1016/j.biocon.2010.09.016
Mehta, P., Sekar, K. C., Bhatt, D., Tewari, A., Bisht, K., Upadhyay, S., et al. (2020). Conservation and prioritization of threatened plants in Indian Himalayan Region. Biodivers. Conserv. 29, 1723–1745. doi: 10.1007/s10531-020-01959-x
Miller, R. M., Rodríguez, J. P., Aniskowicz-Fowler, T., Bambaradeniya, C., Boles, R., Eaton, M. A., et al. (2007). National threatened species listing based on IUCN criteria and regional guidelines: current status and future perspectives. Conserv. Biol. 21, 684–686. doi: 10.1111/j.1523-1739.2007.00656.x
Mills, C. G., Allen, R. J., and Blythe, R. A. (2020). Resource spectrum engineering by specialist species can shift the specialist-generalist balance. Theor. Ecol. 13, 149–163. doi: 10.1007/s12080-019-00436-8
Mirtl, M., Borer, E. T., Djukic, I., Forsius, M., Haubold, H., Hugo, W., et al. (2018). Genesis, goals and achievements of long-term ecological research at the global scale: a critical review of ILTER and future directions. Sci. Tot. Environ. 626, 1439–1462. doi: 10.1016/j.scitotenv.2017.12.001
Molur, S., and Walker, S. (1998). Report of the workshop “conservation, assessment and management plan for selected medicinal plants of northern, northeastern and central India” (BCPP Endangered Species Project) Zoo Outreach Organisation. Conservation Breeding Specialist Group, India, Coimbatore, India, 62.
Nanda, A. B., Nelofar, and Lone, R. A. (2019). Study of phenology of woody flora of Gulmarg and its neighbourhood for landscape use. J. Sci. Res. Rep. 22, 1–7. doi: 10.9734/jsrr/2019/v22i430095
Naqshi, A. R., Malla, M. Y., and Dar, G. H. (1984). Plants of Gulmarg. J. Ecol. Tax. Bot. 5, 709–741.
Negi, V. S., Giri, L., and Sekar, K. C. (2018b). Floristic diversity, community composition and structure in Nanda Devi National Park after prohibition of human activities, Western Himalaya, India. Curr. Sci. 115, 1056–1064. doi: 10.18520/cs/v115/i6/1056-1064
Negi, V. S., Joshi, B. C., Pathak, R., Rawal, R. S., and Sekar, K. C. (2018a). Assessment of fuel wood diversity and consumption patterns in cold desert part of Indian Himalaya: implication for conservation and quality of life. J. Clean Prod. 196, 23–31. doi: 10.1016/j.jclepro.2018.05.237
Negi, V. S., and Maikhuri, R. K. (2017). Forest resources consumption pattern in Govind wildlife sanctuary, western Himalaya, India. J. Environ. Plan Manag. 60, 1235–1252. doi: 10.1080/09640568.2016.1213707
Negi, V. S., Pathak, R., Rawal, R. S., Bhatt, I. D., and Sharma, S. (2019). Long-term ecological monitoring on forest ecosystems in Indian Himalayan Region: criteria and indicator approach. Ecol. Ind. 102, 374–381. doi: 10.1016/j.ecolind.2019.02.035
Negi, V. S., Tiwari, D. C., Singh, L., Thakur, S., and Bhatt, I. D. (2021). Review and synthesis of climate change studies in the Himalayan region. Environ. Dev. Sust. 10471–10502. doi: 10.1007/s10668-021-01880-5
Nic Lughadha, E., Bachman, S. P., Leao, T. C., Forest, F., Halley, J. M., Moat, J., et al. (2020). Extinction risk and threats to plants and fungi. Plants Peo Plan 2, 389–408. doi: 10.1002/ppp3.10146
Oldekop, J. A., Holmes, G., Harris, W. E., and Evans, K. L. (2016). A global assessment of the social and conservation outcomes of protected areas. Conserv. Biol. 30, 133–141. doi: 10.1111/cobi.12568
Oliver, R. Y., Meyer, C., Ranipeta, A., Winner, K., and Jetz, W. (2021). Global and national trends, gaps, and opportunities in documenting and monitoring species distributions. PLoS Biol. 19, e3001336. doi: 10.1371/journal.pbio.3001336
Pandey, A., Rai, S., and Kumar, D. (2018). Changes in vegetation attributes along an elevation gradient towards timberline in Khangchendzonga National Park, Sikkim. Trop. Ecol. 59, 259–271.
Pant, S., and Samant, S. S. (2007). Assessment of plant diversity and prioritization of communities for conservation in Mornaula Reserve Forest. Appl. Ecol. Environ. Res. 5, 123–138. doi: 10.15666/aeer/0502_123138
Quan, Q., Gao, S., Shang, Y., and Wang, B. (2021). Assessment of the sustainability of Gymnocypris eckloni habitat under river damming in the source region of the Yellow River. Sci. Total Environ. 778, 146312. doi: 10.1016/j.scitotenv.2021.146312
Rana, D., Kapoor, K. S., Samant, S. S., and Bhatt, A. (2020). Plant species conservation priority index for preparing management strategies: a case study from the Western Himalayas of India. Small Scale For. 19, 461–481. doi: 10.1007/s11842-020-09447-4
Rana, M. S., and Samant, S. S. (2009). Prioritization of habitats and communities for conservation in the Indian Himalayan Region: a state-of-the-art approach from Manali Wildlife Sanctuary. Curr. Sci. 97, 326–335.
Rana, M. S., and Samant, S. S. (2010). Threat categorisation and conservation prioritisation of floristic diversity in the Indian Himalayan region: a state of art approach from Manali Wildlife Sanctuary. J. Natl. Conserv. 18, 159–168. doi: 10.1016/j.jnc.2009.08.004
Rana, S. K., Rawal, R. S., Dangwal, B., Bhatt, I. D., and Price, T. D. (2021). 200 years of research on Himalayan biodiversity: trends, gaps, and policy implications. Front. Ecol. Evol. 8, 603422. doi: 10.3389/fevo.2020.603422
Rao, C. S., Suresh, B. L., and Suresh, G. (2003). Red list of threatened Vascular Plant Species in India. ENVIS, Botanical Survey of India, Howrah, Ministry of Environment and Forests, Government of India.
Rawal, R. S., Rawal, R., Rawat, B., Negi, V. S., and Pathak, R. (2018). Plant species diversity and rarity patterns along elevation range covering treeline ecotone in Uttarakhand: conservation implications. Trop. Ecol. 59, 1–15.
Rawat, G. S., and Adhikari, B. S., (2015). Ecology and Management of Grassland Habitats in India, ENVIS Bulletin: Wildlife and Protected Areas. Vol. 17, Wildlife Institute of India, Dehradun, India
Rawat, T. S., Menaria, B. L., Dugaya, D., and Kotwal, P. C. (2008). Sustainable forest management in India. Curr. Sci. 94, 996–1002.
Reddy, C. S., Jha, C. S., Dadhwal, V. K., Krishna, P. H., Pasha, S. V., Satish, K. V., et al. (2016). Quantification and monitoring of deforestation in India over eight decades (1990-2013). Biodivers. Conserv. 25, 93–116. doi: 10.1007/s10531-015-1033-2
Rodrigues, A. S. L., Pilgrim, J. D., Lamoreux, J. F., Hofman, M., and Brooks, T. M. (2006). The value of the IUCN Red List for conservation. Trend Ecol. Evol. 21, 71–76. doi: 10.1016/j.tree.2005.10.010
Ronsted, N., Walsh, S. K., Clark, M., Edmonds, M., Flynn, T., Heintzman, S., et al. (2022). Extinction risk of the endemic vascular flora of Kauai, Hawaii, based on IUCN assessments. Conserv. Biol. e13896. doi: 10.1111/cobi.13896
Samant, S. S., Butola, J. S., and Sharma, A. (2007). Assessment of diversity, distribution, conservation status and preparation of management plan for medicinal plants in the catchment areas of Parbati Hydroelectric Power Project Stage –III in Northwestern Himalaya. J. Moun Sci. 4, 34–56. doi: 10.1007/s11629-007-0034-3
Samant, S. S., and Dhar, U. (1997). Diversity, endemism and economic potential of wild edible plants of Indian Himalaya. Int. J. Sust. Dev. World Ecol. 4, 179–191. doi: 10.1080/13504509709469953
Samant, S. S., Dhar, U., and Palni, L. M. S. (1998). Medicinal Plants of Indian Himalaya. Almora, India, Gyanodaya Prakashan.
Samant, S. S., Dhar, U., and Rawal, R. S. (1996). “Conservation of rare endangered plants. The context of Nanda Devi Biosphere Reserve,” in Conservation and Management of Biological Resources in Himalaya, eds P. S. Ramakrishnan, A. N. Purohit, K. G. Saxena, K.S. Rao, and R. K. Maikhuri (New Delhi: Oxford and IBH Publishing Company Private Limited), 521–545.
Samant, S. S., Joshi, H. C., Arya, S. C., and Pant, S. (2002). Studies on the structure, composition and changes of vegetation in Nanda Devi Biosphere Reserve, West Himalaya. New Delhi: Final Technique submitted to Ministry of Environment and Forests.
Singh, A., and Samant, S. S. (2010). Conservation prioritization of habitats and forest communities in the Lahaul valley of proposed cold desert biosphere reserve, north western Himalaya, India. Appl. Ecol. Environ. Res. 8, 101–107. doi: 10.15666/aeer/0802_101117
Singh, J. B., and Kachroo, P. (1994). Forest Flora of Pir Panjal Range (North Western Himalaya). Dehradun: Bishen Singh Mahendra Pal Singh.
Singh, K. N., Lal, B., Singh, R. D., Todaria, N. P., and Ahuja, P. S. (2007). Species richness, distribution pattern and conservation status of higher plants in the Spiti cold desert of trans-Himalaya, India. I. J. Biodivers. Sci. Manag. 3, 223–233. doi: 10.1080/17451590709618176
Singh, N. P., Singh, D. K., and Uniyal, B. P. (2002). Flora of Jammu and Kashmir. Calcutta: Botanical Survey of India.
Su, N., Jarvie, S., Yan, Y., Gong, X., Li, F., Han, P., et al. (2022). Landscape context determines soil fungal diversity in a fragmented habitat. Catena 213. doi: 10.1016/j.catena.2022.106163
Tali, B. A., Ganie, A. H., Nawchoo, I. A., Wani, A. A., and Reshi, Z. A. (2015). Assessment of threat status of selected endemic medicinal plants using IUCN regional guidelines: a case study from Kashmir Himalaya. J. Natl. Conserv. 23, 80–89. doi: 10.1016/j.jnc.2014.06.004
Tali, B. A., Khuroo, A. A., Nawchoo, I. A., and Ganie, A. H. (2018). Prioritizing conservation of medicinal flora in the Himalayan biodiversity hotspot: an integrated ecological and socioeconomic approach. Environ. Conserv 46, 147–154. doi: 10.1017/S0376892918000425
Thakur, S., Negi, V. S., Dhyani, R., Bhatt, I. D., and Yadava, A. K. (2022). Influence of environmental factors on tree species diversity and composition in the Indian western Himalaya. For. Ecol. Manag. 503, 119746. doi: 10.1016/j.foreco.2021.119746
Thakur, S., Negi, V. S., Pathak, R., Dhyani, R., Durgapal, K., and Rawal, R. S. (2020). Indicator based integrated vulnerability assessment of community forests in Indian west Himalaya. For. Ecol. Manag. 457, 117674. doi: 10.1016/j.foreco.2019.117674
Tilman, D., Fargione, J., Wolff, B., Antonio, C., Dobson, A., Howarth, R., et al. (2001). Forecasting agriculturally driven global environmental change. Science 292, 281–284 doi: 10.1126/science.1057544
Walter, K. S., and Gillett, H. J. (1998). IUCN Red List of Threatened Plants. Compiled by the World Conservation Monitoring Centre. Gland/Cambridge: IUCN – The World Conservation Union.
Wani, Z. A., and Pant, S. (2020). Ethnomedicinal study of plants used to cure skin diseases and healing of wounds in Gulmarg Wildlife Sanctuary (GWLS), Jammu and Kashmir. Ind. J. Trad. Knowl. 19, 327–334.
Wani, Z. A., Pant, S., and Sharma, V. (2021). “Diversity, distribution and status of weed species of Northwest Himalaya,” in Bioremediation using weeds, Energy, Environment and Sustainability, eds D. Pant, Bhatia, S. K., Patel, A. K., and Giri, A. (Singapore: Springer), 3–36. doi: 10.1007/978-981-33-6552-0_1
Keywords: Himalayan region, plant community, Conservation Priority Index, endemism, threatened species
Citation: Wani ZA, Bhat JA, Negi VS, Satish KV, Siddiqui S and Pant S (2022) Conservation Priority Index of species, communities, and habitats for biodiversity conservation and their management planning: A case study in Gulmarg Wildlife Sanctuary, Kashmir Himalaya. Front. For. Glob. Change 5:995427. doi: 10.3389/ffgc.2022.995427
Received: 15 July 2022; Accepted: 28 July 2022;
Published: 25 August 2022.
Edited by:
Yashwant Singh Rawat, Federal Technical and Vocational Education and Training Institute (FTVETI), EthiopiaCopyright © 2022 Wani, Bhat, Negi, Satish, Siddiqui and Pant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shreekar Pant, c2hyZWVrYXJwYW50LjJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.