
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 10 June 2022
Sec. Forest Disturbance
Volume 5 - 2022 | https://doi.org/10.3389/ffgc.2022.868237
This article is part of the Research Topic Variation in Plant Strategies with Levels of Forest Disturbance View all 8 articles
The forest environment represents a unique ecosystem for medicinal plants and provides congenial growth and development conditions. Overexploitation of these medicinal flora has negatively affected biodiversity in these areas; some of the important plant species are facing local extinction. Seed is the essential source of regeneration in forests that demands specific growing conditions. Thus, understanding seeds can be linked to conserving forests and their resources. Medicinal plants' seeds degrade fast and lose their viability after a few months of harvests and storage. In protecting the genetic integrity of stored samples, seed viability must be retained for prolonged periods. The study deals with the influence of different seed packaging/storage materials (polythene bags, jute bags, cloth bags, aluminum foil, unburned earthen pot, and burned earthen-pots), storage duration (1, 6, and 12 months), and temperature (room, 4°C temperature) on seed germination and biochemical activities of seven medicinal plant species viz. Abelmoschus moschatus, Andrographis paniculata, Bixa orellana, Ocimum basilicum, Plumbago zeylanica, Psoralea corylifolia, and Withania somnifera. The germination ability of A. moschatus was maximum when stored in polythene bags, while unburned earthen pots favored the germination of W. somnifera. Out of seven species studied, seeds of four species showed maximum germination ability when stored at room temperature while a low-temperature environment was beneficial for another 3 species studied. The mean difference in total phenolic and flavonoid content was 4.69 and 8.38% higher, respectively, in low temperature than room temperature conditions. This study concluded that species-specific requirement of storage materials with adjustment of storage duration and temperature for higher germination and longer seed viability in medicinal plant species. Experiments using more medicinal plant species would be essential to test such potential effects of storage material, storage duration, temperature, and via changes in seed germination and biochemical activities; our findings provide important insights that can help to guide management plans that aim to preserve seeds of important medicinal plant species for a longer period.
Forests are home to a majority of medicinal plants. However, human-induced biodiversity losses affect medicinal plants occurring in the natural habitats (Sharma et al., 2018; Perinchery, 2020). Seeds are an essential component required for plant propagation, but due to the overexploitation of valued medicinal plants, many medicinal plants have vanished from their natural sources. As seeds of medicinal plants degrade fast during storage and packaging, understanding the impact of the same on seeds during storage and the suitability of different packaging materials seems critical. Medicinal plants have been used for therapeutic purposes since the dawn of humankind and are indispensable for human wellbeing as they play a significant role in human welfare. These plants enable building a bridge between different medicinal systems and provide a better way to cure even a complicated pandemic such as COVID 19 (Benarba and Pandiella, 2020) besides being helpful in common health problems. More than 80% of people worldwide rely on the plant-based medicines (Ekor, 2013) and utilize ~3,50,000 plant species as medicines and food supplements (Joppa et al., 2011). The high cost of allopathic treatments and their side effects have led to increased emphasis on plant medicines.
Notwithstanding, our changing lifestyle and altering food habits are causing stress, anxiety, and allergies to our body, resulting in a paradigm shift to traditional health systems. Globally, the herbal medicine market was valued at USD 71.19 billion in 2016, and it is expected to reach USD 44.6 billion by 2024 (MRR, 2017), which also indicates people's interest in herbal products. It has opened new vistas to cultivate valued medicinal plants for domestic and commercial use (Biswas, 2010; Chandra, 2020). However, the limited availability of quality seeds to grow medicinal plants restricts these plants' large-scale cultivation and incurs a high cost.
Seed quality is essential for producing and conserving the genetic resources of medicinal plants (Pradhan and Badola, 2012). Seeds of medicinal plants degrade faster and lose their viability after a few months of harvests and storage, and therefore, the high economic loss is reported when aged and stored (Tiwari and Das, 2014). In a study, Himangini and Thakur (2018) found a significantly lower germination percentage in seeds of Withania somnifera stored for 2 months. To protect the genetic integrity of stored samples, seed viability must be retained for a prolonged period. Since the beginning, simple techniques have been used to maintain seed viability in wild and domesticated sources (Roberts, 1973; Vertucci and Roos, 1990; Pradhan and Badola, 2008). Since farmers use these seeds in the next growing season, it is required to store them appropriately to avoid low germination because of the variation in the storage environment. Low seed germination, seed degradation, and loss of viability are all-natural phenomena that occur during storage when using improper storage conditions, such as room temperature (Schmidt, 2000) and moisture Afzal et al., 2020. Afzal et al. (2020) reported that moisture condition during storage of Moringa oleifera seeds is a major factor in their deterioration and suggested seed storage in hermetically sealed superbags at 8% of seed moisture content. Several factors, include temperature, seed nature, seed moisture content, and relative influence on seed longevity and viability during storage (Roberts, 1972b; Gordon, 1992; Yang et al., 2005; Pradhan and Badola, 2008). Manna et al. (2020) have observed a better germination percentage in onion seeds when stored in cold storage and glass containers. Juhari and Petersen (2018) suggested that storage of Hibiscus sabdariffa L. seeds under flushed nitrogen and darkness can improve germination percentage upto 3 months. In a study on Desmodium gangeticum, Gayathri and Kanakamany (2018) observed that seed storage at 8% moisture level, packed in polythene bags, and kept in a refrigerated environment ensures higher germination. Furthermore, the occurrence of genetic damage in the surviving seeds is closely related to the loss of seed viability during storage (Ellis and Roberts, 1981; Rao et al., 1987). The main factors influencing the aforementioned relationship are seed moisture content, temperature, and storage periods (Roberts, 1988; Pradhan and Badola, 2012; Manna et al., 2020). A slight rise in temperature and humidity may support fungal growth (Roberts, 1972b) and insect development in seeds (Christensen, 1972). Further, drying and long-term storage can significantly reduce germination or even the death of seeds, depending upon the period and method applied. On the other hand, appropriate storage conditions may enable seeds to retain significant vitality over a prolonged period (Chen et al., 2007; Pradhan and Badola, 2008, 2012). Recently, the use of storage containers has been tested for seeds of agricultural importance and reported significant effects on seeds viability and protection from degrading agents Saeed et al., 2020.
Various biochemicals of the seed, such as phenol and flavonoid compounds, act as a protectant but degrade after exposure to the storage environment, resulting in no or poor germination and loss in viability (Pradhan and Badola, 2012; Watson et al., 2013; Chaaban et al., 2017; Ali et al., 2018; Awolu et al., 2022). Therefore, understanding the stability of phenolic and flavonoids content in seeds during storage can be beneficial for maintaining the germination and viability of seeds (Awolu et al., 2022). The reduction in seed germination and vigor is a consequence of alteration in seed enzyme metabolism, the ribosome's inability to disassociate, starvation of meristematic cells, and subsequent accumulation of toxic compounds (Rai, 1999; Saisanthosh and Patil, 2018). The storage techniques for agricultural and other important crops have been established and used extensively (Silva and Peiris, 1997; Padma and Reddy, 2002), but similar reports on valued medicinal species are warranted. Studies on the germination of medicinal plants are now receiving wide attention with the increasing demand for these plants for industry and their production (Chandra et al., 2010; Elhindi et al., 2016; Khalaki et al., 2021). Therefore, maintaining high seed viability during storage is critical for better seedling establishment and sustainable production (Finch-Savage, 1995; Belmehdi et al., 2018). Therefore, this study was undertaken with seven important medicinal plants too, (i) test and standardize the seed storage techniques under different storage materials, (ii) assess the impact of temperature and time duration on seed germination, phenol, and flavonoids compounds of the seeds.
This study was conducted in the laboratory of the agricultural pathology, TCB College of Agriculture and Research Station, Bilaspur Chhattisgarh India (22°06'17.48″N, 82°.08'26.13″ E) during 2017–2018. The seeds of seven medicinal plants are based on industrial, commercial, and medicinal significance, namely, Abelmoschus moschatus Medik, Andrographis paniculata (Burn. F.) Nees, Bixa Orellana L., Ocimum basilicum L., Plumbago zeylanica L., Psoralea corylifolia L., and Withania somnifera L. Dunal (see Table 1) were chosen for this study. Seeds of the selected medicinal plants were collected from the medicinal plant nursery of TCB College of Agriculture and Research Station, Bilaspur, India, and brought to the laboratory, cleaned thoroughly for impurities, and dried at room temperature for 15 days. The seeds were desiccated to the moisture content of 3.5% using 0.5 kg of zeolite beads. A total of one kg of desiccated seeds of individual species was stored for 3 storage duration viz. 1, 6, and 12 months in six packaging materials (Tre) (polythene bag, jute bag, cloth bag, aluminum foil, unburned earthen pot, and burned earthen pot) under 2 two different temperatures (Tmp) regimes [i.e., room temperature, (ambient), and low temperature (4°C)]. Each packaging material stored one kg of physically purified seeds of individual medicinal plant species. For storing the seeds, we included six packaging materials viz. polythene bags (the mouth of polythene bags was tightly sealed by the rubber bands); jute bags (gunny, stitched jute bags); cloth bags (stitched cloth bags); aluminum foil (seeds were wrapped with three layers of aluminum foil); unburned earthen pot (air tighten by taps) and burned earthen pots (air tighten by taps). A total of two sets of seed bags were prepared for each storage material, and one set was kept at both temperature conditions.
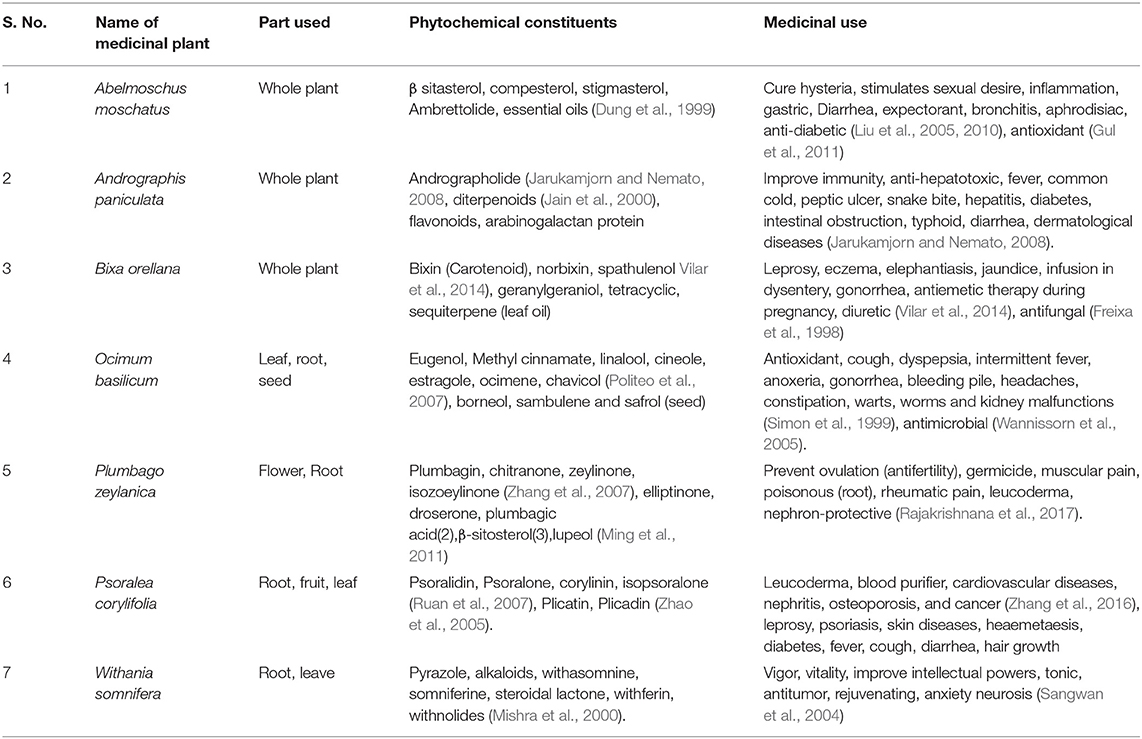
Table 1. Summary of the phytochemical constituents and medicinal use of plants selected for the study.
Germination was evaluated according to the standard procedures of ISTA (2007). Seeds stored at low temperature (4°C) and room temperature and stored with six materials were taken separately after 1, 6, and 12 months of storage. Seeds were surface sterilized with sodium hypochlorite solution (4% w/v available chlorine) for 5 s to reduce the incidence of fungal attack and soaked in double-distilled water for 24 h. The soaked seeds were placed in sterilized plastic Petri plates (90 mm dia.) lined with filter paper soaked with double distilled water (Whatman No. 1). Each experiment consists of three replication containing 100 seeds per replicate. The Petri plated seeds were placed in seed germinator at 25 ± 1°C and 95 ± 1% RH for 15 days. At the end of the 15th day, germination percentage was determined as follows:
It was calculated based on the fresh seed weight after different months of storage. The weight loss from the initial weight of seed was determined on a percentage (%) basis.
SWL- seed weight loss, M1- the initial weight of 100 g seeds, and M2- the weight of seed after given months.
In total, five-gram seeds were taken from different storage materials (bags) and powdered. Seed powder was dissolved in a 50 ml of methanol solution @ 1:10 ratio (g/ml) then kept for 24 h in a rotary shaker followed by filtering by Whatman filter No. 1. This extract was stored at 4°C and was used for biochemical analysis using the standard procedures of Azwanida (2015).
Total phenolic content in seed was determined following the method prescribed by Singleton and Rossi (1965) using folin-ciocalteu reagent and the phenolic content in seed extracts was expressed in terms of gallic acid equivalent (GAE) in μg/g dry mass. Total flavonoid content was analyzed using a colorimetric method (Zou et al., 2004) expressed as quercetin equivalents (QUE) in μg/g dry mass.
To assess the seed mycoflora (seed-borne fungi) in different medicinal plants, 100 seeds were taken from different storage materials on three occasions 1, 6, and 12 months of storage. These seeds were grown in a Petri plate and germinated directly without any seed treatment. After 30 days, the seed mycoflora grown in seeds were examined and identified.
All the statistical tests were conducted with SPSS 21 for Windows (SPSS Inc., 2012, Chicago, IL USA). Multivariate ANOVA was employed to demonstrate the treatment effect on seed parameters. Tukey's post-hoc test was performed to observe the significant differences for the studied parameters between the treatment combinations. Compared t-test was performed to analyze the impact of temperature on various parameters for individual species.
Seed storage materials significantly affect the germination of medicinal plants (Table 2). Seed germination, seed weight loss, seed mycoflora, total phenolic content, and total flavonoid content were significantly affected by species (S), duration of storage (M), temperature (Tem), and storage material (Tre). The interactions such as S × M, S × Tem, S × Tre, M × Tem, M × Tre, Tem × Tre, S × M × Tem, S × Tem × Tre also showed a significant effect on the seed germination, seed weight loss, seed mycoflora, total phenolic content and total flavonoid contents (Table 2). The storage material did not affect seed germination of studied medicinal plant species (except A. moschatus and W. somnifera) (Figure 1). Seed weight loss was also not affected by storage material (Figure 1). Seeds of A. moschatus stored in polythene bags attained significantly higher seed germination as compared to other storage materials (Figure 1). In the case of W. somnifera the maximum seed germination was observed for the seeds stored in unburned earthen pots; while minimum seed germination was observed when seeds were stored in the jute bags (Figure 1).
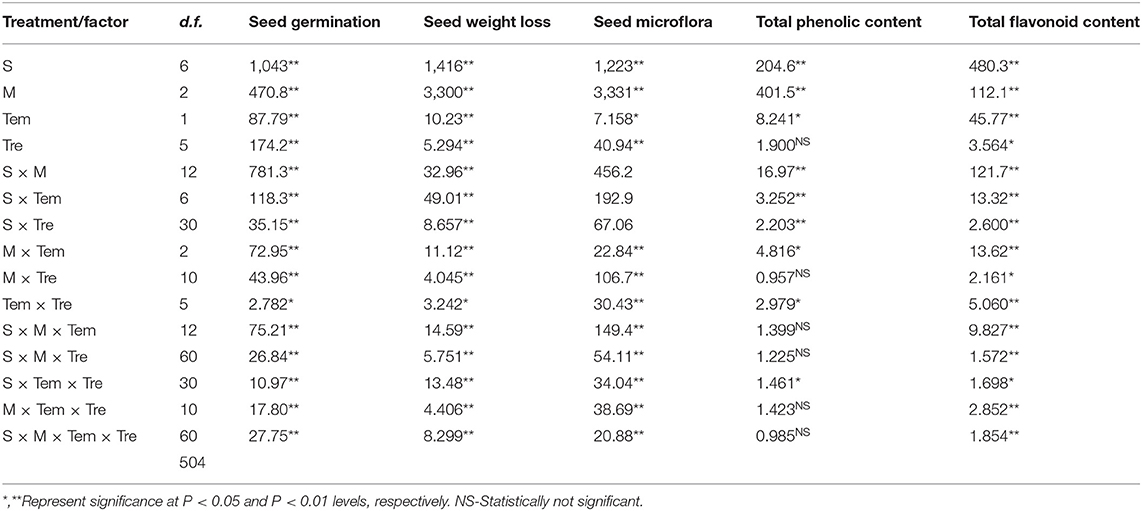
Table 2. Summary of MANOVA for various parameters; seed germination (%), seed weight loss (%), seed microflora (%), total phenolic content (ppm dry weight gm−1), total flavonoid content (ppm dry weight gm−1) for seeds of seven medicinal species with factors species (S), month (M), temperature (Tem), treatment (Tre).

Figure 1. Effect of different types of seed packaging materials on seed germination (%), seed weight loss (%), mycoflora (%), phenolic content (μg dry weight gm−1), flavonoid content (μg dry weight gm−1) in seven commercially important medicinal plants. Bars with different letters are significantly different according to the Tukey's test (P < 0.05).
The significant effect of storage material on seed-borne mycoflora was observed for the species Psoralea corylifolia, W. somnifera, and B. olerina. The seeds of P. corylifolia stored in a cloth bag attained maximum seed-borne mycoflora while seeds stored in burned the earthen pots showed the lowest value of mycoflora. The seeds of W. somnifera and B. olerina, stored in a burned earthen pot, showed the highest value of mycoflora (Figure 1).
Total phenolic content and total flavonoid content of the seeds of studied species were not affected by storage material (Figure 1).
The storage duration significantly affects the studied parameters (Table 2). All the studied parameters of the present investigation significantly differ due to the storage duration of the seeds for individual species (Figure 2). Seed of P. corylifolia and B. orelina germinated highest when they were stored for only 1 month, while, the medium-term storage (i.e., 6 months) rendered the highest germination in W. somnifera and A. paniculata, and longtime storage was best for A. moschatus, O. basilicum, and P. zeylanica. However, the percent germination decreased up to 160% in P. corylifolia when stored for the medium term compared to the 1-month seed storage. Interestingly, germination of A. paniculata was increased by 84.73% if stored for the long term (i.e., 12 months) than 1-month seed storage. The increasing duration of seed storage causes seed weight loss substantially and consistently in studied medicinal plants (Figure 2). The maximum of 4 times weight loss was observed in O. basilicum and a minimum of 45.26% seed weight loss in A. moschatus. Further, the one-month stored seeds were the most resistant to mycoflora than the seeds stored for a long time. Seeds of O. basilicum showed the highest resistance to mycoflora across storage durations, while P. corylifolia and A. moschatus were found to be the most vulnerable to seed mycoflora particularly when the duration of storage was increased (Figure 2). Similarly, seed mycoflora increased maximum by 25-folds in W. somnifera, 2.84-folds in B. orelina, 2.69-folds in P. zeylanica, and 2-folds in A. paniculata when storage duration increases from medium to long term (Figure 2).
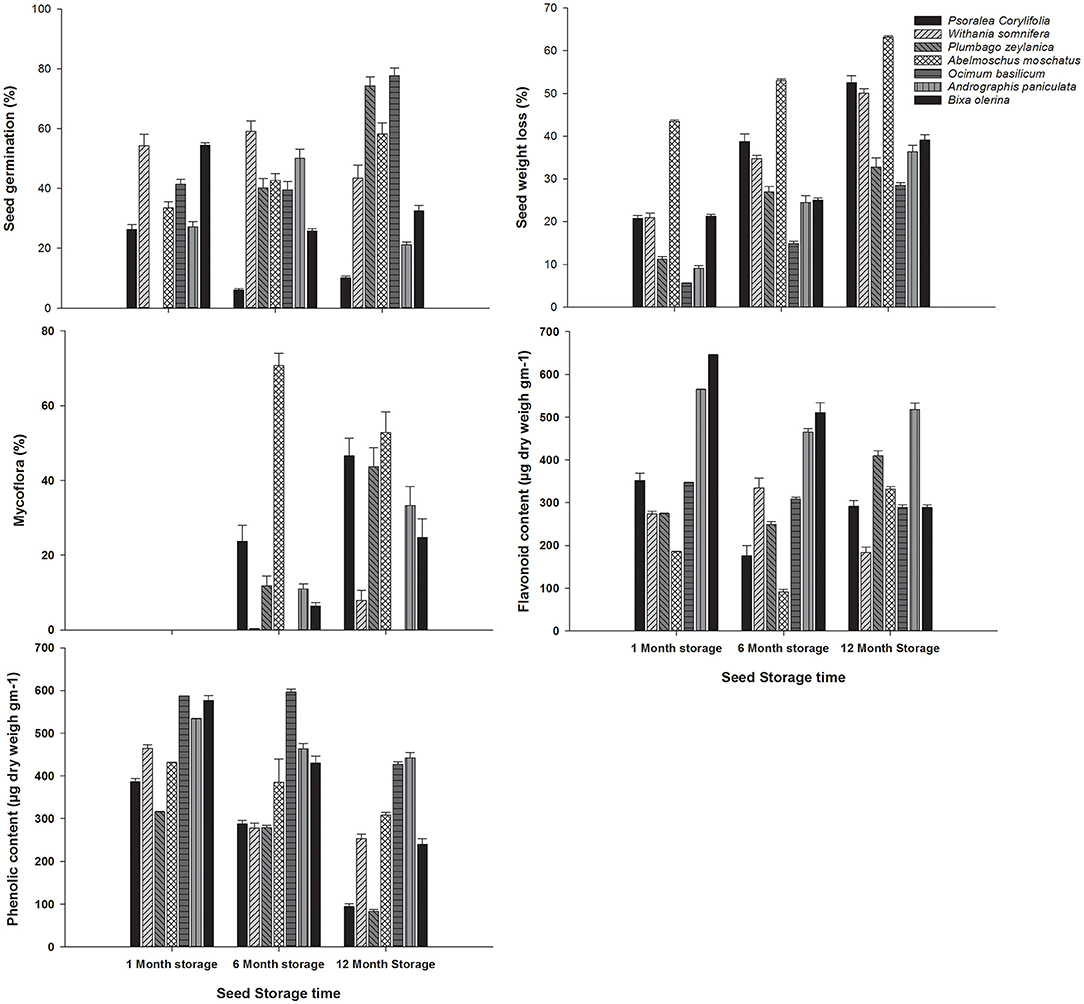
Figure 2. Impact of storage durations on seed germination (%), seed weight loss (%), mycoflora (%), phenolic content (μg dry weight gm−1), flavonoid content (μg Q14 dry weight gm−1) in seven commercially important medicinal plants. Bars with different letters are significantly different according to the Tukey's test (P < 0.05).
A total of 1 month stored seeds of studied medicinal plants contained the highest TPC and degrade with the increment in the duration of seed storage (Figure 2). This degradation was exponential in P. corylifolia (309%), followed in P. zeylanica (281%), and lowest in O. basilicum (37.54%). TFC also followed a similar trend in O. basilicum and B. orelina but unlikely P. corylifolia, P. zeylanica, A. moschatus, and A. paniculata exhibited a high-low-high trend in TFC with short-, medium-, and long-term storage.
The storage temperature significantly affects seed germination, seed weight loss, seed mycoflora, total phenolic content, and total flavonoid content of the studied medicinal plant species (Table 2). Storage temperature affects seed germination (except P. zeylanica), significantly at P < 0.05. In this study, ambient temperature (room temperature) was best-suited for obtaining higher germination in A. moschatus, P. corylifolia, B. olerina while, low-temperature storage favored germination in A. paniculata, O. basilicum, and W. somnifera (Figure 3). The maximum seed germination was observed in W. somnifera with low temperature while the minimum seed germination was attained by P. corylifolia under low temperature. The seed weight loss also varied with the temperature during storage and the loss was found higher under low temperature but exceptionally in A. paniculata and P. corylifolia, the trend was opposite (Figure 3). Seed mycoflora was significantly affected by storage temperature for individual species (Figure 3; Supplementary Table 1). Seed mycoflora was found higher in A. paniculata, B. orelina, P. corylifolia, and W. somnifera with ambient temperature, while in A. moschatus and P. zeylanica mycoflora flourished higher in a low-temperature environment (Figure 3; Supplementary Table 1). The total phenolic content of the studied species was not affected by variation in storage temperature. Furthermore, the TFC of W. somnifera, A. moschatus, and A. paniculata were significantly higher when seeds were stored at low temperatures.

Figure 3. Influence of temperature gradients during seed storage on seed germination (%), seed weight loss (%), mycoflora (%), phenolic content (μg dry weight gm−1), flavonoid content (μg dry weight gm−1) in the seven commercially important medicinal plants. Bars with different letters are significantly different according to the Tukey's test (P < 0.05).
Seed weight loss was positively correlated with seed mycoflora. Seed mycoflora and seed weight loss negatively correlate with TPC and TFC (Table 3). Our study shows the positive relationship between TPC and TFC (Table 3).
At the outbreak of COVID 19, the demand for plant-based medicine and food supplements has been increased worldwide as immunity boosters and/or as to cure disease. Numerous studies confined on novel compounds of different medicinally valued plants and their effects on various diseases and health worldwide (Jain et al., 2000; Mishra et al., 2000; Wannissorn et al., 2005; Politeo et al., 2007; Ruan et al., 2007; Liu et al., 2010; Vilar et al., 2014; Zhang et al., 2016; Rajakrishnana et al., 2017). However, the understanding of aspects of seed storage, seed degradation on these plants is very limited. Indeed, there are problems in seed germination, and the storability of these medicinal plants results in delays in commercialization. The strengthening of basic knowledge on medicinal plants for individual species would strengthen the cultivation aspect (Chandra, 2020) using seeds and be exploited for the conservation of genetic resources. Among constraints, the unavailability of seeds in the market is a major restriction to bring these medicinally valued species outside the forest. So far, the storage practices of these species have not been standardized, and often receive complaints of poor germination (Abdusalaam and Shenge, 2011; Saisanthosh and Patil, 2018). In this study has made efforts to determine the appropriate storage materials, temperature, and storage durations for some important medicinally valued plants with potential for commercial cultivation.
Seed storage materials greatly influence seed germination of agricultural and horticultural crops, and therefore, the selection of suitable materials for seed storage has been advocated for Sorghum bicolor (Owalade et al., 2011), Allium cepa (Saisanthosh and Patil, 2018), Swertia chirayita (Pradhan and Badola, 2012), Pericopsis elata (Tandoh et al., 2017), Cotton and Wheat (Saeed et al., 2020). Our study shows that the storage material could not affect the seed germination of studied species (except W. somnifera, A. moschatus). This is in contrary to the earlier studies related to seed storage with various packaging materials. This result further indicates that seed storage with semi impervious material such as unburned earthen pot was best for W. somnifera while polythene bags were suitable for storage of A. moschatus. This study also reveals that the seeds of individual species need specific storage material. Most of the studies suggest that seeds using polythene during storage interact less with the storage atmosphere, absorb no or low moisture, and control weight loss and as such, they are unlikely to suffer because of higher oxidation (Netra et al., 2015). Numerous researchers reported higher seed germination in moisture-proof storage materials (Rahman and Rahman, 1997; Khalequzzaman et al., 2012; Lambat et al., 2015; Sultana et al., 2016; Saisanthosh and Patil, 2018). Further, high germination in seeds with low moisture and thereby reduced respiration rate was also reported (Doijode, 2007). Studies also reported the highest seed germination in cloth bags, a porous material that demonstrates the need for species-specific storage material for higher germinability of stored seed (Netra et al., 2015; Saisanthosh and Patil, 2018). Differences in seed germination in the medicinal plants could also occur because of these differences in solubility and polarity of genetic material in seed (Dapkevicius et al., 1998). Besides, the genetic components of species affects phenotypic expression and determines seed quality and seed vigor (Al-Yahya, 1995; Vieira et al., 2001), which may cause variation in seed germination. However, this study demonstrated a species-specific response to the storage material, which emphasize toward taking up more such studies to generate a comprehensive understating on the germination of the seeds subjected to such storage treatments. This study also demonstrated no significant effect on seed weight loss by using storage material. The polythene bag was again found effective against seed mycoflora for W. somnifera and A. paniculata as seeds stored in this material, reducing 18.44- and 2.54-folds mycoflora compared with the burned earthen pot where the highest mycoflora exhibited on the seed. However, variation exists in seed mycoflora with storage materials and species, resulting in O. basilicum being the most resistant among other species investigated. TPC and TFC have antimicrobial properties that exhibited a low but positive correlation with seed germination (R2 0.128 TPC, 0.008 TFC), indicating their significance in accelerating germination. Both the compounds act as seed preservatives and improve seed quality (Volf et al., 2014) showed a negative correlation with mycoflora (R2 −0.343 and −0.196 TPC and TFC, respectively) proves its role in the maintenance of higher seed germination through checking of mycoflora and seed deterioration. The degradation of these compounds varied significantly with the species, and storage materials resulted in varying percentages of germination and mycoflora. Fu et al. (2018) evaluated four storage materials for peanut and reported polythene material as best in reducing the oxidation of phenols, maintaining peanut quality, freshness for more than 1 year, and better germination. They also found this material suitable for controlling pests compared with the highly permeable woven bags that suffered serious invasion from pests was an agreement of the present result.
The periods of seed storage significantly change the germination of the medicinal plants. Fresh seeds of P. corylifolia and B. orelina germinated higher when fresh, but medium-term aging gave good results in W. somnifera and A. paniculata. On the contrary, the seeds of A. moschatus, O. basilicum, and P. zeylanica exhibited higher germination with long-term storage. This indicates the requirement of careful storage of seeds of medicinal plant species. Tiwari and Das (2014) also reported variable patterns in seed germination of different medicinal plants due to cofounding effect of factors such as storage materials, temperature, and plant species.
Similarly, based on varying storability of seeds, Roberts (1972a) and Ellis and Roberts (1981) classified seeds as an orthodox, recalcitrant, and intermediate category. The increasing duration of seed storage caused weight loss consistently in all the medicinal plants, irrespective of storage materials. In our results, the aged seeds degraded faster with a high mycofloral invasion and increased deterioration of TPC and TFC compared with the fresh seeds. This is evident that fresh seeds are usually more resistant to mycoflora due to a higher concentration of protective biochemicals than the stored seeds (Li et al., 2019). Therefore, species that retain a higher amount of biochemical during storage can prevent invasion of mycoflora and remain vigorous for a more extended period. When seeds deteriorate during storage, they lose vigor and ultimately become unable to germinate (Rajjou and Debeaujon, 2008). Walter et al. (2005) quoted that the length of storage time influence varyingly to species due to differences in genetic factors combined with storage atmosphere. Moreover, Morello et al. (2004) proved that rancidity development due to oxygen-dependent deterioration of lipids predominantly causes germination reduction during storage. Also, Suriyong (2007) and Genes and Nyomora (2018) also supported our results that aging affects the seed quality during storage due to the oxidation processes.
The storability of seed is a function of quality seed and the storage temperature (Fabrizius et al., 1999; Heatherly and Elmore, 2004). The intensity of germination and other attributes of stored seed was significantly varied with plant species concerning temperature gradients. The ambient temperature favor seed germination of A. moschatus, P. corylifolia, P. zeylanica, and B. olerina, while seeds stored in low-temperature condition germinated the highest in O. basilicum, W. somnifera, and A. paniculata (Figure 3). Such variations have been propounded majorly due to the result of genotype variability, implying the considerable influence of genetic components on phenotypic expression of traits that determine seed quality and other attributes (Al-Yahya, 1995; Vieira et al., 2001). Besides, there were significant differences (P < 0.05) in seed germination of medicinal plants for storage materials, duration, and temperature. This was also related to the weight loss pattern and seed mycoflora in the different plant species. Seed storage coupled with ambient temperature facilitated high-infection rates could lead to a substantial loss in seeds of medicinal plant, particularly, in A. paniculata, B. olerina, P. corylifolia, and W. somnifera therefore, needs to adjust the temperature of the store. Usually, the moisture of seed provides an opportunity for mycoflora which increases the rate of seed damage and thereby deterioration (Abdusalaam and Shenge, 2011; Owalade et al., 2011). TPC and TFC maintained high stability with a low temperature in all the species except B. olerina. Kalt (2005) and Kyi et al. (2005) also studied the degradation of TPC in Cherries with storage temperature and reported up to 25.4% loss of phenolic after 60 days of storage at a temperature higher than 50°C. The decrease of the TPC and TFC content of the investigated seeds at room temperature may be due to increased oxidation of these bioactive components (Moldovan et al., 2016). Similarly, high temperatures with light could degrade the polyphenols (Baiano et al., 2009; Del-Toro-Sanchez et al., 2015).
The result showed that adequate handling could retain the viability of seeds of specific species, which can help restore medicinal plants in forests. The interaction of multiple factors, such as moisture, temperature, seed quality, mycoflora, and specific genotype traits, determines the germinability of the plant species. Using appropriate storage materials followed by adjusting the time and temperature of storage can sustain germination in medicinal plants even after a long time. However, no general storage practice suffices the objective of maintaining seed quality for all the medicinal species but needs standardization of species-specific storage methods. As for storage duration was concerned, P. corylifolia and B. olerina need short-term storage time; A. paniculata and W. somnifera demand medium-term storage time, and other species of the study require long-term storage time.
Similarly, variation due to storage temperature is exerted with species and parameters of this study. Hence, it is of great significance to investigate the best storage material that could be used for ambient storage for each medicinal plant species. The results may be simply for conserving medicinal valued species and cultivators engaged in producing raw materials for pharmaceutical industries. Medicinal plants can also be utilized in reclamation of degraded land and disturbed forests. Hence, it is imperative to develop a comprehensive understanding of the conservation of the seeds of medicinal plants.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
RT conducted the experiment. KC prepared the manuscript. SD tabulated data. ST analyzed data and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. Rahul Bhadouria, Assistant Prof, Department of Environmental Science, Rajmata Vijayaraje Scindia Krishi Vishwavidyala, Gwalior, India for his statistical analysis and necessary modifications in the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.868237/full#supplementary-material
Abdusalaam, S., and Shenge, K. S. (2011). Seed-borne pathogens on farmer saved Sorghum (Sorghum bicolor L.) seed. J. Stored Prod. Post-harvest Res. 2, 24–28.
Afzal, I., Jaffar, I., Zahid, S., Ur Rehman, H., and Basr, S. M. A. B. (2020), Physiological and biochemical changes during hermetic storage of Moringa oleifera seeds, South African. J. Botany. 129, 435–441. doi: 10.1016/j.sajb.2019.11.011
Ali, A., Chong, C. H., Mah, S. H., Abdullah, L. C., Choong, T. S. Y., and Chua, B. L. (2018). Impact of storage conditions on the stability of predominant phenolic constituents and antioxidant activity of dried piper betle extracts. Molecules 23, 84. doi: 10.3390/molecules23020484
Awolu, O. O., Fole, E. T., Oladeji, O. A., Ayo-Omogie, H. N., and Olagunju, A. I. (2022). Microencapsulation of avocado pear seed (Persea Americana mill) bioactive-rich extracts and evaluation of its antioxidants, in vitro starch digestibility and storage stability. Bull. Natl. Res. Centre 46, 1–11. doi: 10.1186/s42269-022-00714-2
Azwanida, N. N. (2015). A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants. 4, 1000196. doi: 10.4172/2167-0412.1000323
Baiano, A., Terracone, C., Gambacorta, G., and Notte, E. L. (2009). Changes in quality indices, phenolic content and antioxidant activity of flavored olive oils during storage. J. Am. Oil Chem. Soc. 86, 1083–1092. doi: 10.1007/s11746-009-1446-8
Belmehdi, O., El Harsal, A., Benmoussi, M., Laghmouchi, Y., Senhaji, N. S., and Abrini, J. (2018). Effect of light, temperature, salt stress and pH on seed germination of medicinal plant Origanum elongatum (Bonnet) Emb. and Maire. Biocatal. Agric. Biotechnol. 16, 126–131. doi: 10.1016/j.bcab.2018.07.032
Benarba, B., and Pandiella, A. (2020). Medicinal plants as sources of active molecules against COVID-19. Front. Pharmacol. 11, e01189. doi: 10.3389/fphar.2020.01189
Biswas, B. C. (2010). Cultivation of medicinal plant, success stories of two farmers. Fertil. Market. News 41, 1–4.
Chaaban, H., Ioannou, I., Paris, C., Charbonne, C., and Ghoul, M. (2017). The photo stability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. A Chem., 336, 131–139. doi: 10.1016/j.jphotochem.2016.12.027
Chandra, K. K. (2020). Microbial inoculants responses on biomass, nutrients and biochemical constituents of Withania somnifera (L.) Dunal and Adhatoda vasica Nees. Int. J. Agricul. Animal Sci. Plant Arch. 20, 2061–2069.
Chandra, K. K., Kumar, N., and Chand, G. (2010). Studies on Mycorrhizal inoculation on dry matter yield and root colonization of some medicinal plants grown in different stress soils and forest soil. J. Environ. Biol. 3, 975–979.
Chen, S. Y., Kuo, S. R., and Chien, C. T. (2007). Storage behaviour of seeds of Cinnamomum osmophloeum and Neolitsea aciculate var. variabillima (Lauraceae). Seed Sci. Technol. 35, 237–243. doi: 10.15258/sst.2007.35.1.22
Christensen, C. M. (1972). “Micro flora and seed deterioration,” in Viability of Seeds, R. H. Roberts, ed. (London: Chapman and Hall), 59–93.
Dapkevicius, A., Venskutonis, R., Van Beek, T. A., and Linssen, J. P. H. (1998). Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agric. 77, 140–146.
Del-Toro-Sanchez, C. L., Gutierrez-Lomeli, M., Lugo-Cervantes, E., Zurita, F., Robles-García, M. A., Ruiz-Cruz, C., et al. (2015). Storage effect on phenols and on the antioxidant activity of extracts from Anemopsis californica and inhibition of elastase enzyme. J. Chem. 2015, 602136. doi: 10.1155/2015/602136
Doijode, S. D. (2007). Low cost seed storage techniques for certain hardy indigenous pod vegetables. Acta-Hort 752, 589–591. doi: 10.17660/ActaHortic.2007.752.111
Dung, N. X., Khien, P. V., Nhuan, D. D., Hoi, T. M., Ban, N. K., Leclercq, P. A., et al. (1999). Composition of the seed oil of Hibiscus abelmoschus L. (Malvaceae) growing in Vietnam. J. Essent. Oil Res. 11, 447–452. doi: 10.1080/10412905.1999.9701181
Ekor, M. (2013). The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4, 177. doi: 10.3389/fphar.2013.00177
Elhindi, K. M., Dewir, Y. H., Asrar, A., Abdel-Salam, E., El-Din, A. S., and Ali, M. (2016). Improvement of seed germination in three medicinal plant species by plant growth regulators. Hort Sci. Volume 51, 887–891. doi: 10.21273/HORTSCI.51.7.887
Ellis, R. H., and Roberts, E. H. (1981). The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 9, 373–409.
Fabrizius, E., Tekrony, D., Egli, D. B., and Rucker, M. (1999). Evaluation of a viability model for predicting soybean seed germination during warehouse storage. Crop Sci. 39, 194–201. doi: 10.2135/cropsci1999.0011183X003900010030x
Finch-Savage, W. E. (1995). “Influence of seed quality on crop establishment, growth and yield,” in Seed Quality: Basic Mechanisms and Agricultural Implications, ed. A. S. Basra (Binghamton, NY: Food Products Press), 470.
Freixa, B., Vila, R., Vargas, L., Lozano, N., Adzet, T., and Canigueral, S. (1998). Screening for antifungal activity of nineteen Latin American plants. Phytother. Res. 12, 427–430. doi: 10.1002/(SICI)1099-1573(199809)12:6<427::AID-PTR338>3.0.CO;2-X
Fu, X., Xing, S., Xiong, H., Min, H., Zhu, X., He, J., et al. (2018). Effects of packaging materials on storage quality of peanut kernels. PLoS ONE. 2018, e0190377. doi: 10.1371/journal.pone.0190377
Gayathri, P., and Kanakamany, M. T. (2018). Influence of storage environment and packing materials on seed germination and viability of Desmodium gangeticum (L.) DC. Open Access J. Med. Arom. Plants 9, 23.
Genes, F., and Nyomora, A. M. S. (2018). Effect of storage time and temperature on Germination ability of Escoecaria bussei. Tanzania J. Sci. 44, 123–133.
Gul, M. Z., Bhakshu, L. M., and Ahmad, F. (2011). Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement. Altern. Med. 11, 64. doi: 10.1186/1472-6882-11-64
Heatherly, L. G., and Elmore, R. W. (2004). “Managing inputs for peak production.” in: Soybeans: Improvement, Production and Uses, 3rd Edn, eds H. R. Boerma and J. E. Specht (Madison, WI: Agronomy N-16, ASA, CSSA, SSSA), 451–536.
Himangini, and Thakur, A. (2018). Effect of seed storage conditions on seed germination and vigor of Withania somnifera. J. Pharm. Phytochem. 7, 1409–1413.
ISTA (2007). International Rules for Seed Testing. Bassersdorf: International Seed Testing Association.
Jain, D. C., Gupta, M. M., Saxena, S., and Kumar, S. (2000). LC analysis of hepatoprotective diterpenoids from Andrographis paniculata. J. Pharm. Biomed. Anal. 22, 705–709. doi: 10.1016/S0731-7085(99)00297-6
Jarukamjorn, K., and Nemato, N. (2008). Pharmacological aspect of A. paniculata on health and its major diterpenoid constituent andrographolide. J. Health Sci. 54, 370–384. doi: 10.1248/jhs.54.370
Joppa, L. N., Roberts, D. L., Myers, N., and Pimm, S. L. (2011). Biodiversity hotspots house most undiscovered plant species. Proc. Natl. Acad. Sci. USA 108, 13171–13176. doi: 10.1073/pnas.1109389108
Juhari, N. H., and Petersen, M. A. (2018). Physicochemical properties and oxidative storage stability of milled roselle (Hibiscus sabdariffa L.) Seeds. Molecules. 23, 385. doi: 10.3390/molecules23020385
Kalt, W. (2005). Effects of production and processing factors on major fruit and vegetable antioxidants. J. Food Sci. 70, 11–19. doi: 10.1111/j.1365-2621.2005.tb09053.x
Khalaki, M. A., Moameri, M., Asgari Lajayer, B., and Astatkie, T. (2021). Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul. 93, 13–28. doi: 10.1007/s10725-020-00670-9
Khalequzzaman, K. M., Rashid, M. M., Hasan, M. A., and Reza, M. M. A. (2012). Effect of storage containers and storage periods on the seed quality of French bean (Phaseolus valgaris). Bangladesh J. Agril. Res. 37, 195–205. doi: 10.3329/bjar.v37i2.11221
Kyi, T. M., Daud, W. R. M., Mohammad, A. B., Samsudin, M. W., Kadhum, A. A. H., and Talib, M. Z. M. (2005). The kinetics of polyphenol degradation during the drying of Malaysian cocoa beans. Int. J. Food Sci. Technol. 40, 323–331. doi: 10.1111/j.1365-2621.2005.00959.x
Lambat, A., Lambat, P., Gadewar, R., Charjan, S., and Charde, P. N. (2015). Effect of storage containers on mycoflora and germinability of til. Int. J Res. in Bio-Sci., Agril. Technol. 2, 10–12. doi: 10.29369/ijrbat.2015.03.II.0013
Li, Y. M., Shaffer, J. P., Hall, B., and Ko, H. (2019). Soil-borne fungi influence seed germination and mortality, with implications for coexistence of desert winter annual plants. PLoS ONE 14, e0224417. doi: 10.1371/journal.pone.0224417
Liu, I. M., Liou, S., Lan, T. W., Hsu, F. L., and Cheng, J. T. (2005). Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 71, 617–62. doi: 10.1055/s-2005-871266
Liu, I. M., Tzeng, T.-F., and Liou, S.-S. (2010). Abelmoschus moschatus (Malvaceae), an aromatic plant, suitable for medical or food uses to improve insulin sensitivity. Phytother. Res. 24, 233–239. doi: 10.1002/ptr.2918
Manna, D., Maity, T. K., and Basu, A. K. (2020). Comparison on condition and packaging materials for better storage of onion seeds. Asian J. Horticul. 15, 15–25. doi: 10.15740/HAS/TAJH/15.1/15-25
Ming, Y., Wang, Y. J, and Liu, W. (2011). Chemical constituents of Plumbago Zeylanica L. advanced materials research. Ed Gao. J. 308–310, 1662–1664. doi: 10.4028/www.scientific.net/AMR.308-310.1662
Mishra, L. C., Singh, B. B., and Dagenais, S. (2000). Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): a review. Altern. Med. Rev. 5, 334–346.
Moldovan, B., Popa, A., and David, L. (2016). Effects of storage temperature on the total phenolic content of Cornelian Cherry (Cornus mas L.) fruits extracts. J. Appl. Botany Food Qual. 89, 208–211. doi: 10.5073/JABFQ.2016.089.026
Morello, J. R., Motilva, M. J., Tovar, M. J., and Romero, M. P. (2004). Changes in commercial virgin olive oil (cv. Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem. 85, 357–364. doi: 10.1016/j.foodchem.2003.07.012
MRR (2017). Herbal Medicine Market Size and Forecast, By Product (Tablets and Capsules, Powders, Extracts), By Indication (Digestive Disorders, Respiratory Disorders, Blood Disorders), And Trend Analysis. 2014–2024. Available online at: https://www.hexaresearch.com/research-report/global-herbal-medicine-market (accessed January 16, 2022).
Netra, N., Uma Rani, K., Gowda, R., Rajendra Prasad, S., and Narayanaswamy, S. (2015). Effect of packaging material and desiccant on storability of soybean seeds. Seed Sci. Technol. 44, 207–211. doi: 10.15258/sst.2016.44.1.05
Owalade, O. F., Olasoji, J. O., and Afolabi, C. G. (2011). Effect of storage temperature and packaging materials on seed germination and seed borne fungi of sorghum (Sorghum bicolor) in South West Nigeria. Afr. J Plant Sci. 5, 873–877. doi: 10.5897/AJPS11.209
Padma, V., and Reddy, M. B. (2002). Storage of Brinjal seeds under ambient temperatures at two moisture levels. J. Res. Angrau. 30, 6–10.
Perinchery, A. (2020). Forest that Heal: Medicinal Plants as an Ecosystem Service. Mangabay Series: Environment and Health. The Indian Forest Story. Available online at: https://india.mongabay.com/2020/02/forests-that-heal-medicinal-plants-as-an-ecosystem-service/ (accessed January 13, 2022).
Politeo, O., Jukic, M., and Milos, M. (2007). Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 101, 379–385. doi: 10.1016/j.foodchem.2006.01.045
Pradhan, B. K., and Badola, H. K. (2008). Seed germination response of populations of Swertia chirayita following periodical storage. Seed Technol. 30, 63–69. Available online at: http://www.jstor.org/stable/23433371
Pradhan, B. K., and Badola, H. K. (2012). Effect of storage conditions and storage periods on seed germination in eleven populations of Swertia chirayita: a critically endangered medicinal herb in Himalaya. Sci World J. 2012, 128105. doi: 10.1100/2012/128105
Rahman, M. M. K., and Rahman, G. M. M. (1997). Effect of containers and length of storage on germination and seed-borne fungi associated with jute seed. Bangladesh J. Plant Pathol. 13, 13–16.
Rai, A. S. (1999). An Investigation into the problems of maintenance of seed vigour and viability under adverse climate conditions of Darjeeling Hills (Doctoral dissertation). University of North Bengal, Darjeeling.
Rajakrishnana, R., Lekshmi, R., Benil, P. B., Thomas, J., AlFarhana, A. H., Rakesh, V., et al. (2017). Phytochemical evaluation of roots of Plumbago zeylanica L. and assessment of its potential as a nephron-protective agent. Saudi J. Biol. Sci. 24, 760–767. doi: 10.1016/j.sjbs.2017.01.001
Rajjou, L., and Debeaujon, I. (2008). Seed longevity: survival and maintenance of high germination ability of dry seeds. Current. Rev. Biol., 331, 796–805. doi: 10.1016/j.crvi.2008.07.021
Rao, N. K., Roberts, E. H., and Ellis, R. H. (1987). Loss of viability in lettuce seeds and the accumulation of chromosome damage under different storage conditions. Ann. Botany 60, 85–96. doi: 10.1093/oxfordjournals.aob.a087425
Roberts, E. H. (1972b). “Storage environment and the control of viability,” in Viability of Seeds, ed E. H. Roberts (London: Chapman and Hall), 14–58.
Roberts, E. H. (1988). “Seed ageing-the genome and its expression,” in Senescence and Ageing in Plants, eds L. D. Nooden and A. C. Leopold (New York, NY: Academic Press), 465–598.
Ruan, B., Kong, L. Y., and Takaya, N.iwa M. (2007). Studies on the chemical constituents of Psoralea corylifolia L. J. Asian Nat. Prod. Res. 9, 41–44. doi: 10.1080/10286020500289618
Saeed, M. F., Jamal, A., Ahmad, I., Ali, M., Shah, G. M., Husnain, S. K., et al. (2020). Storage conditions deteriorate cotton and wheat seeds quality: an assessment of farmers' awareness in Pakistan. Agronomy. 10, 91246. doi: 10.3390/agronomy10091246
Saisanthosh, K., and Patil, N. K. B. (2018). Effect of packaging materials and moisture content on seed storability of Onion. J. Pharm. Phytochem. 7, 1745–1750. Available online at: https://www.phytojournal.com/archives?year=2018&vol=7&issue=4&ArticleId=5183
Sangwan, R. S., Chaurasiya, N. D., Mishra, L. N., Lal, P., Uniyal, G. C., Sharma, R., et al. (2004). Phytochemical variability in commercial herbal products and preparations of Withania somnifera (Ashwagandha). Curr. Sci. 86, 461–465. Available online at: https://www.researchgate.net/publication/235733546_Phytochemical_variability_in_commercial_herbal_products_and_preparations_of_Withania_somnifera_Ashwagandha/link/00b7d52d612ac8ae2b000000/download
Schmidt, L. (2000). Guide to Handling of Tropical and Subtropical Forest Seed. Humlebaek: Danida Forest Seed Centre. p. 511.
Sharma, T. V. R. S., Abirami, K., and Punnam, C. M. (2018). Medicinal plants used by tribes of Andaman and Nicobar islands: a conservation appraisal. Indian J. Pl. Genet. Resour. 31, 125–133. doi: 10.5958/0976-1926.2018.00015.3
Silva, S. G. R., and Peiris, D. B. C. N. (1997). Effect of storage materials on the storability of chili seeds in Sri Lanka. Trop. Agric. Res. 6, 23–30.
Simon, J. E., Morales, M. R., Phippen, W. B., Vieira, R. F., and Hao, Z. (1999). “Perspectives on new corps and new uses,” in A Source of Aroma Compounds and a Popular Culinary and Ornamental Herb, ed J. Janick (Alexandria, VA: ASHS Press), 499–505.
Singleton, V. L., and Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Ame. J. Eno. Viticul. 16, 144–158.
Sultana, R., Chowdhury, M. S. M., Islam, M. R., and Akhter, K. (2016). Effect of container and duration of storage on the quality of okra (Abelmoschus esculentus) seeds. Agriculturists 14, 63–72. doi: 10.3329/agric.v14i1.29101
Suriyong, S. (2007). Studies About Mechanisms of Oil Seed Deterioration Under Different Storage Conditions in Oil Seed Rape (Brassica napus L.) Cuvillier verlag Gottingen. Göttingen: Cuvillier Verlag, 2.
Tandoh, P. K., Banful, B., Gaveh, E., and Amponsah, J. O. (2017). Effects of packaging materials and storage periods on seed quality and longevity dynamics of Pericopsis elata seeds. Environ. Earth Ecol. 1, 27–38. doi: 10.24051/eee/76919
Tiwari, R. K. S., and Das, K. (2014). Impact of different storage conditions on seed germination and viability of some medicinal plants. Afr. J. Agric. Res. 20, 1578–1585. doi: 10.5897/AJAR2012.1758
Vertucci, C. W., and Roos, E. E. (1990). Theoretical basis of protocols for seed storage. Plant Physiol. 94, 1019–1023. doi: 10.1104/pp.94.3.1019
Vieira, R. D., Te-Krony, D. M., Egli, D. B., and Rucker, M. (2001). Electrical conductivity of soybean seeds after storage in several environments. Seed Sci. Technol. 29, 599–608.
Vilar, D. A., Vilar, M. S. A., Moura, T. F. A. L., Raffin, F. N., Oliveira, M. R., Franco, C. F. O., et al. (2014). Traditional uses, chemical constituents, and biological activities of Bixa orellana L: a review. Sci. World J. 2014, 857292. doi: 10.1155/2014/857292
Volf, I., Ignat, I., Neamtu, M., and Popa, V. I. (2014). Thermal stability, antioxidant activity, and photo-oxidation of natural polyphenols. Chem. Papers 68, 121–129. doi: 10.2478/s11696-013-0417-6
Walter, L. M., Wheeler, J., and Grotenhuis, M. (2005). Longevity of seeds stored in a gene bank: species characteristics. Seed Sci. Res., 15, 1–20. doi: 10.1079/SSR2004195
Wannissorn, B., Jarikasem, S., Siriwangchai, T., and Thubthimthed, S. (2005). Antibacterial properties of essential oils from Thai medicinal plants. Fitoterapia 76, 233–236. doi: 10.1016/j.fitote.2004.12.009
Watson, R. R., Preedy, V. R., and Zibadi, S. (2013). Polyphenols in Human Health and Disease, First Edn. Cambridge, MA: Academic Press.
Yang, Q. H., Ye, W. H., Deng, X., Cao, H. L., Zhang, Y., and Xu, K. Y. (2005). Seed germination eco-physiology of Mikania micrantha HBK. Botanical Bull. Acad. Sin. 46, 293–299. Available online at: https://ejournal.sinica.edu.tw/bbas/content/2005/4/Bot464-02.html
Zhang, Q. R., Mei, Z. N., Yang, G. Z., and Xiao, Y. X. (2007). Chemical constituents from aerial parts of plumbago zeylanica Linn. Zhong yao cai 30, 558–560.
Zhang, X., Zhao, W., Wang, Y., Lu, J. J., and Chen, X. (2016). The chemical constituents and bioactivities of Psoralea corylifolia Linn: a review. Am. J. Chin. Med. 44, 35–60. doi: 10.1142/S0192415X16500038
Zhao, L. H., Huang, C. Y., Shan, Z., Xiang, B. G., and Mei, L. H. (2005). Fingerprint analysis of Psoralea corylifolia by HLPC and LC-MS. J. Chromatogr. B. 821, 67–74. doi: 10.1016/j.jchromb.2005.04.008
Keywords: biochemical, medicinal plants, mycoflora, seed, storage, temperature
Citation: Tiwari RS, Chandra KK, Dubey S and Tripathi S (2022) Influence of Packaging Materials and Storage Conditions on Seed Germination Ability and Biochemical Changes in Some Medicinal Plants of Indian Forests. Front. For. Glob. Change 5:868237. doi: 10.3389/ffgc.2022.868237
Received: 02 February 2022; Accepted: 14 March 2022;
Published: 10 June 2022.
Edited by:
Ravi Kant Chaturvedi, Xishuangbanna Tropical Botanical Garden (CAS), ChinaReviewed by:
Prasant Kumar Singh, Government Vijay Bhushan Singh Deo Girls College, IndiaCopyright © 2022 Tiwari, Chandra, Dubey and Tripathi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Krishna Kumar Chandra, a2tja3ZrQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.