
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 05 April 2022
Sec. Tropical Forests
Volume 5 - 2022 | https://doi.org/10.3389/ffgc.2022.836247
This article is part of the Research Topic Lianas, Ecosystems, and Global Change View all 11 articles
Climbing plants need to reach supports and position their leaves for light capture. Vines and lianas develop a large diversity of self-supporting shoots among diverse species and different kinds of attachment. A searcher’s reach is a crucial trait for colonising supports in complex three-dimensional spaces. We explore the reach capacity and diversity of searcher shoots among representative temperate and tropical climbing plants. We investigate the overall range of variation between short- and long-reach searchers; the mechanical and anatomical organisations underlying reach capacities; how searcher architectures are linked to different climbing strategies such as stem twining, tendril climbing, root climbing, and branch-angle-hook climbing. We investigated reach and mechanical and anatomical organisations (stem rigidity and stiffness, stem and tissue geometry) in 29 climbing plant species from temperate and tropical habitats. Searchers show a wide range of maximal reach per species from 0.1 to 2.5 m. Flexural rigidity (EI) at the base of searchers increased with reach length; overall this increase was proportional although some longest-reaching shoots develop proportionally thinner searcher bases with higher stiffness [structural Young’s modulus (Estr)] than shorter-reach shoots. Bases of short-reach searchers rely more on primary tissues compared to long-reach shoots, which rely more on wood production. We identified different mechanical architectures for a given reach capacity across all species. These are linked to different kinds of attachment mechanisms, support foraging, and possibly leaf display. Plants attaching by twining of the main stem showed a wide range of reach capacity. They also developed lighter, more slender, less rigid, but generally relatively stiff (higher Estr) shoots compared with tendril climbers and branch-angle-hook climbers. Differences in the mechanical architecture of searcher shoots in climbing plants are informative for understanding how diverse climbing plant species explore and colonise different kinds of three-dimensional spaces. This is a key feature that distinguishes different habitat preferences. We discuss how such knowledge is not only important for understanding functional biology and ecology of climbing plants but is also of interest for developing new technologies in soft robotics that mimic climbing plants that can navigate through unstructured environments.
Many land plants such as trees mechanically sustain their entire aerial system for their entire life history (Givnish, 1995; Niklas, 1999b; Moulia et al., 2006; Read and Stokes, 2006). Climbing plants are well known for their highly flexible older stems and their reliance on host trees for support. Vines and lianas are also becoming increasingly better understood in terms of the functional biology of young stages of growth as self-supporting shoots and searchers. Searcher shoots or “searchers” represent a key developmental phase of climbing plants. In many natural conditions, young shoots of climbing plants must navigate an obstacle course of gaps, hindrances, potential supports, movements caused by wind and rain as well as moving, oscillating leaves and stems. Such shoots have been referred to as: rising shoots (Darwin, 1875), searcher shoots (Putz, 1984), erect leaders shoots (Hegarty, 1991), searchers (Rowe and Speck, 1996), stiff young shoots (Rowe et al., 2006) and erect leafless shoots (Gianoli, 2015). The climbing habit potentially saves energy by reducing investment in mechanical support. It is often characterised by slender stems and high leaf productivity and turn-over compared with self-supporting plants of similar stem biomass (Wyka et al., 2013). Slender stems, rapid growth, searching mechanisms, and movements combined with reliable attachment are all key adaptations for the climbing habit. These features facilitate exploration, and occupation of the forest understory, the canopy, and disturbed environments such as treefall gaps and forest margins (Putz, 1984; Nabe-Nielsen and Hall, 2002; Ledo and Schnitzer, 2014; Mori et al., 2018). Self-supporting phases of growth may be prolonged under certain environmental conditions such as water, light, or support availability (Gartner, 1991; Cai et al., 2008). In some species, young stages of self-supporting growth can even develop as small treelets and reach sexual maturity (Ménard et al., 2013). Self-supporting phases of growth are most often expressed during younger phases of development (Caballé, 1998). But are also expressed during episodes of vegetative growth and renewal of shoots (Peñalosa, 1984; Rowe and Speck, 1996; Baret et al., 2003; Gianoli, 2003; Rowe et al., 2006). We explore the functional significance of self-supporting properties in climbing plants in terms of three interrelated properties that are determined by bending experiments in the field. The reader is referred to two excellent sources that discuss these parameters in detail (Ennos, 2011; Niklas and Spatz, 2012) and a further source that details a more hands-on application of the experimental protocols (Rowe et al., 2006).
(i) stem rigidity, this is a measure of the resistance of a stem to a bending force (EI) in units of (N.mm2). It is a measure of the combined influence of the stem’s combined tissue’s stiffness (E) and the stem’s second moment of area (I), which is a measure of the stem’s size and geometry. Stem rigidity and the properties, which contribute to it are crucial traits for functional and ecological studies. A plant stem can change its flexural rigidity during development by either changing the stiffness of the material comprising the stem or increasing or decreasing the diameter of the stem or both.
(ii) Stem stiffness, this is a measure of the elastic mechanical resistance of a tissue or a combination of tissues that comprise a plant stem. It is measured by placing the material under compression, tension or bending and determining how much deformation is observed for a given force applied. It is normally referred to as Young’s modulus (E) in units of MNm–2 (units of force per units of area). A “soft” tissue that is low in stiffness such as parenchyma typically has a “low” Young’s modulus. “hard” tissue that is high in stiffness such as dense lignified wood typically has a high Young’s modulus. In some studies, the term “structural” Young’s modulus Estr is used to emphasise that we are referring to a combination of tissues comprising the materials under study. In this case, most searcher shoots comprise a mixture of different tissues with different Young’s moduli.
(iii) Stem second moment of area, this is a measure of the cross-sectional size and shape of a stem when under bending. It refers to the distribution of material(s) (tissues) in the stem cross-section relative to the neutral axis. This exists at the centroid and along the length of a structure under bending and at 90° to the direction of the applied force. It is referred to as second moment of area (I) and is measured in units of mm or m to the power of four (m4 or mm4). We refer to second moment of area in terms of a whole stem as well as in terms of single tissue areas. A large tree trunk will have an exponentially larger second moment of area than a small sapling. In terms of the organisation of tissues in a cross section, a centrally positioned pith will have a low second moment of area. A peripherally placed layer of sclerenchyma, for example, will have a high second moment of area.
For a given stem the product of stem stiffness (E) and stem second moment of area (I) result in the flexural rigidity of a stem in bending (EI). Approaches that measure and explore these three terms are an excellent way of revealing how different plant stems develop and optimise rigidity and stiffness and have a great potential for comparative studies in ecology. In this study, we explore the patterns of stiffness, rigidity, and second moment of area for diverse searcher shoots and their abilities to cross gaps between supports. In climbing plants these mechanical and geometrical features are linked to other complex attributes. These include circumnutatory movements, nastic movements, and a wide range of attachment mechanisms. All of these must work together to cross gaps and find and attach to supports especially in unstructured, unpredictable three-dimensional spaces. Rigid self-supporting stems and their length and reach capacity are possibly linked to complex movements and anchorage mechanisms. This combination of “static properties” and “movements” — what we might call “searcher behaviour” is much less well known but is of considerable interest.
Different species of searcher shoots can locate new hosts in different ways. In many species, there is a basal rigid component of the searcher shoot constructed of stiff tissues that ensures mechanical stability. More apical parts of the searcher shoot are often capable of movement. These can be either “active” such as circumnutations or nastic movements or more “passive” mechanisms such as off-vertical leaning and swaying toward potential supports. Some searcher shoots undergo extreme elastic buckling and collapsing under their own weight onto host branches below. Many species undergo what can be referred to as scrambling and roving forward by apical growth. Such forms can grow through complex three-dimensional environments without “targeting” specific host structures. Most climbers develop searchers that deploy attachment organs. These can comprise highly modified leaves, stems, petioles, branches and roots as well as smaller scale textured surfaces that stick or hook onto host surfaces. Searcher shoots and their attachment organs are often highly adapted for attaching to different supports in many different “active” and “passive” ways. These include twining on supports and tendril attachment to twigs and leaves. Hooks, branches, and petiole angle attachments can anchor more passively to host structures but need to remain in tension to stay reliably attached. Searchers can also attach and anchor themselves to supports via growth processes and thigmomorphogenetic responses induced by proximity or continuous or repeated contacts. Uninduced mechanisms exist such as preformed coiling and hook differentiation and deployment prior to any direct contact with a support (Gerbode et al., 2012; Guerra et al., 2019; Sousa-Baena et al., 2021). Support foraging behaviours can involve different mechanisms acting in concert such as stem elongation, self-supporting mechanics, searching by active and/or passive movement, attachment organ deployment, and anchorage (Baillaud, 1962; Millet et al., 1988; Stolarz, 2009; Simonetti et al., 2021). Searching and attaching mechanisms have been popularly studied in terms of the attachment organ itself. Rather less is known about how the searchers deploy combined searching and attachment behaviours. Furthermore, little is known about how deployment mechanisms vary according to the distance between supports, which is a crucial factor in colonising different habitats.
Searcher shoots are well known for developing relatively high stiffness compared to the older more compliant stems (Rowe and Speck, 1996; Isnard et al., 2003; Rowe et al., 2006). This allows them to span distances up to several metres in length (Putz, 1984). The reach capacity of searcher shoots has been shown to vary considerably between species. This is likely linked to the developmental constraints that limit rigidity for sustaining mechanical stability (Coudurier, 1992; Lahaye et al., 2005). Some authors have suggested that tendril-climbers span shorter distances than climbers with other modes of attachment such as twiners or scramblers. This difference might be related to tendril climbers being restricted mostly to only slender supports (Darwin, 1875; Putz, 1984; Gianoli, 2015). According to optimal foraging theory (e.g., Charnov, 1976; Pyke, 1984; Bartumeus and Catalan, 2009; Gianoli, 2015), selection should favour economising biomass investment for self-supporting searchers. This would potentially enhance support location and light acquisition at the level of the whole plant (Gianoli, 2015). Reaching a longer distance potentially comes at the expense of proportionally higher constructional costs via stem thickening and stiffening. This will likely influence different biomass and tissue development patterns of the stem. Furthermore, different climbing behaviours are likely adapted to differences in three-dimensional distances and support geometries. There is therefore a large potential for understanding climbing performances between different species and different climbing habits by considering how mechanical properties, anatomical organisation, and supported biomass are related to the gap spanning capacity of searcher stems.
Attachment to a support is believed to influence a change in shoot development from being stiff (high Young’s modulus) to being flexible (capable of deforming-bending or twisting-a lot without breaking). Flexible stems that are resistant to breaking are typical of many old stages of growth (Ewers et al., 1991; Putz and Holbrook, 1991). Transitions from stiff to flexible properties can involve changes in tissue organisation at several hierarchical levels. These can include the proportions and radial organisations of stiff versus compliant tissues, to changes in cell wall thickness and lumen diameter (Rowe and Speck, 1996; Rowe et al., 2004; Isnard et al., 2005) to changes in microfibril angle (Ménard et al., 2009) and cell wall chemistry (Chabbert et al., 1997; Hoffmann et al., 2003). The stiff to flexible transition also involves profound changes in biomass allocation, hydraulic conductivity, light capture as well as stem toughness (the resistance to fracture). One highly visible trait among shoots of many vines and lianas is delayed leaf expansion. This has been considered to be important for stem twiners and tendril climbers, since the presence of large foliage leaves are believed to physically hinder foraging for supports (Raciborski, 1900; French, 1977). Some architectural shifts known as “axialization” (growth and development of stems) and “foliarization” (growth and development of leaves) are well known for trees (Lauri, 1988; Lauri and Kelner, 2001) and also play important strategic roles in the growth of climbing plants (Peñalosa, 1982, 1984; Baret et al., 2003). Despite knowledge of many morphological strategies during the development of searcher shoots, the anatomical and mechanical features underlying stem development during leaf display, stem movements, and diverse support-foraging strategies remain little known.
We investigate how reach capacity in climbing plants varies between species and how it is linked to different attachment mechanisms. Second, we explore how reach capacities of different species are related to stem properties of the searcher base – the part that mechanically supports the entire searcher shoot. This includes searcher rigidity (EI), searcher stiffness structural Young’s modulus (Estr), cross-sectional area (A), and second moment of area and (I). We measure all these parameters for the basal whole stem cross section, and furthermore, we explored values of (A) and (I) for the different tissues of the load-bearing searcher base. For example, some searcher stem bases might employ a wide central pith as a geometrical spacer to optimise the contribution of stiff tissues at the outside. Other species rely on a central wood cylinder and others on an outer band of stiff primary fibre tissues. Some species might integrate all of these patterns for optimising stem properties and reach. Third, we discuss how divergent mechanical and anatomical organisations vary between short- and long-reach searcher shoots and how such differences might vary with overall fresh mass and dry mass of searcher shoots. In other words, how might climbing plants optimise the trade-off between maintaining long reaches but minimising mass and construction costs. Fourth, we analyse how divergent organisations vary for a given reach capacity and how these are related to attachment modes and leaf development.
In this paper, we use a working definition for a searcher shoot as an axial self-supporting part of a climbing plant having a growing apex that may show one or more characteristics linked to support foraging and attachment. These latter can include elongated internodes, attachment organs, and commonly, a segment of the stem (normally distal to median) that undergoes circumnutatory or nastic movement. This definition is not that straightforward across diverse climbers. However, it is necessary to identify in the field “true searcher shoots” that functionally locate and attach to supports. This is not always easy since some species of climber do not develop noticeably specialised climbing organs but achieve true climbing via modified branch angles (Gallenmüller et al., 2004, 2009; Ménard et al., 2009). Furthermore, vines and lianas can develop different kinds of self-supporting stems other than searcher shoots. For example, shoots of the same individual or species that are adapted for light capture and which differ markedly from self-supporting, support foraging (truly searching) searchers. The latter can differ in the presence of fully expanded leaves, shorter internodes, and absence of climbing organs and specialised circumnutatory movements.
In this study our approach was to include species from both tropical and temperate regions, thus including diverse searcher shoots from different climatic zones. We collected the longest searcher shoots for each species that still maintained an erect or self-supporting posture from a vertical to horizontal orientation but not downward pointing orientation and that were still unattached to a support. Our aim was to analyse how this maximal reach of a given species was related to the mechanical properties and anatomy of the crucial load bearing segment at the base of the searcher long- and short-reach searchers.
We sampled 190 searcher shoots in 29 species from 21 families to represent a wide range of climbing habits (Table 1). For most species, we were able to sample searcher shoots derived from mature individuals in wooded, marginal to open habitats. A few species were only accessible in early stages of growth as young individuals (genets) because they were difficult to find free of any support as searcher shoots of older stages (i.e., Machaerium quinquinatum and Bauhinia guianensis). One species of a climbing Cactaceae was sampled from a common garden collection at the AMAP lab, Montpellier. Tropical species from natural habitats were sampled during a 2-month field season at the beginning of the rainy season from November 2020 to January 2021. Shoots were sampled in French Guiana from their natural habitats in two sites in the vicinity of Sinnamary. One site included the Paracou experimental station (5°16′26″ N, 52°55′26″ W) and the second included stations in forest margins accessible along the road known as the “Piste de St-Elie” (5°16′59″ N, 53°03′15″ W). Temperate species were sampled during the summer season from July to August 2020. Shoots were sampled in urban and natural habitats in the vicinity of Montpellier, South of France (43°39′7″ N, 3°51′41″ E).
Numerous approaches exist for categorising climbing plants based on (i) the degree of wood development of the stem (Gentry, 1991); (ii) the degree of strength of attachment with supports (Caballé, 1986); (iii) the kind of attachment mechanism (e.g., Darwin, 1875; Schenck, 1892; Menninger, 1970; Putz, 1984) and (iv) the morphological origin of the organ involved in the attachment process (Sousa-Baena et al., 2018). We sampled searchers of different species grouping them by their main kind of deployment and attachment. We identified four broad working categories for the study: (i) Stem twiners: searchers that attach by twining of the main searcher stem. (ii) Tendril climbers: searchers that attach by modified, determinate growth of lateral organs (leaves, stems, petioles) of the searcher stem that are highly modified into sensitive tendrillar organs. (iii) Branch-hook-angle climbers: searchers that attach by branch angles, petiole angles, preformed open hooks, epidermal spines, and prehensile branches. (iv) Root climbers: searchers that attach by adventitious roots deploying active adhesive and attachment development.
Twelve main traits were selected to investigate functional attributes of different searcher shoots (Table 2).
In order to estimate the reach of a searcher shoot, we measured in situ the distance in a straight line from the base to the apex of the searcher shoot (here called the “reach”). Searcher shoots were cut at the base and temporary stored in a plastic bag before measurements. In our field laboratory, all the leaves were removed by cutting the petiole/rachis as close as possible to the insertion point with the lamina(s). Laminae were digitised using a 300 dpi-imaging scanner (Epson Perfection V800; Epson America Inc., Long Beach, CA, United States and Canon CanoScan LiDE 400; Canon Japan Inc., Tokyo, Japan), and areas were extracted using the leafarea R package (Katabuchi, 2015). Fresh masses of the searcher stem and all appendages (branches, petioles, and laminae) were measured within 3 h after the shoot was sampled. Dry masses of each were measured using a precision balance (to 0.1 mg) after at least 72 h in the oven at 70° and constant mass. Stem diameters were measured using digital callipers with a stated precision of 0.01 mm.
Bending properties of the basal part of the searcher shoot were measured using four-point bending tests (Ennos, 2011). Flexural rigidity (EI) and Young’s modulus (E) were calculated from regression lines resulting from plots of the applied bending forces plotted against the maximum deflections (Rowe et al., 2006). Up to five weights were applied in sequence at 30 s intervals and deflections were measured with a dissecting microscope equipped with a calibrated eyepiece graticule. Weight increments were selected according to the bending resistance of each sample. Weights were made up of stainless-steel nuts and machined brass weights ranging from 1 g to 200 g. The exact mass of weights was measured to 0.001 gram before field work using a precision balance in the lab. Span distances were defined as proportional to the mean elliptical diameter of the stem segment. The span support was 40 times greater than the diameter and ranged from 30 mm to 447 mm for the longest stems. The load span was set at between one-half and two-thirds of the span support and ranged from 11 mm to 255 mm. For the shortest stem segments, we used customised panniers made of wood and aluminium profiles, and for the longer stems, we used customised panniers made of stainless steel.
Flexural rigidity EI (N.mm2) was calculated via the formula present in Table 2, where l is the load support (i.e., the distance between two internal supports), L is the support span (i.e., the distance between the two outside supports) and b is the slope of the force-deflection curve (N/mm). Stem diameters were measured at three positions along the searcher shoot (basal, medial, apical) in the vertical direction of the applied load and that orthogonal to it in order to calculate the second moment of area. We used the different formulas depending on whether the stem was ellipsoidal (e.g., Condylocarpon guianense and Trachelospermum jasminoides) or rectangular (i.e., Cissus haematantha). Some searcher stems had star-shaped or undulating cross-sectional shape (i.e., Serjania membraceae, Rubus ulmifolius, and Clematis vitalba). These were approximated as an ellipse. Since flexural rigidity (EI) is the product of stiffness (E) and second moment of area (I) we calculated the structural Young’s modulus at the base of the searcher shoot (Estr) (MNm–2) from measured values of EI and calculated values of I (Niklas, 1999b; Rowe et al., 2006).
Anatomical samples were taken from the segment used for the bending test from near the base of the shoot. 190 cross sections ranging from 0.59 to 13.74 mm of diameter were embedded in paraffin before sectioning at a thickness of 8 to 10 μm with a semi-automated rotary microtome (Leica Biosystems Rm2245). Sections were stained with Safranin/Astra blue to distinguish unlignified cells (blue) and lignified cells (red). Images were taken with a digital microscope (Keyence VHX-700F) with a magnification of X100 to X300. Multiple images were taken at different depths of focus and position to obtain a panorama of stacked pictures of the cross-section. Thick-walled lignified cells observed in cross section might represent elongated fibre cells or foreshortened sclereid tissue (Niklas, 1999a; Clair et al., 2019). We therefore prepared longitudinal sections on one individual per species to distinguish fibres from sclereids (Lehnebach et al., 2020) and thus interpret their putative mechanical significance to stem stiffness.
To characterise anatomical organisations of different species, we distinguished up to 9 types of tissue per species from the following tissue categories: (i) medullary parenchyma, (ii) medullary fibres, (iii) xylem (including primary and secondary xylem), (iv) phloem (including primary and secondary phloem), (v) cortical parenchyma, (vi) cortical sclereids, (vii) cortical fibres, (viii) cortical collenchyma and (iv) periderm. We manually delineated tissues from digitised cross-sections with a graphics tablet and created layers with the GIMP software (version 2.10.121). Masks of layers were used to calculate cross-sectional areas and second moments of the entire stem and each area of each tissue with a customised macro using ImageJ software (v.1.43u2).
The orientation of the cross-section is critical for calculating the second moment of area of the entire stem and tissue proportions. An oval cross-section positioned with its long axis in the vertical plane would have a higher second moment of area than if it would be placed in the horizontal plane. We therefore aligned each cross section and its masks with the long-axis of the stem arranged vertically. We then measured the second moment of area in this vertical position (Ix) and at 90° (Iy) to it for both the entire section and the individual tissues. We then calculated proportions of each tissue in terms of second moment of area based on the mean values calculated from the vertical and horizontal orientations.
All statistical analyses and data graphics were performed with R software3. We applied a one-way ANOVA on a subset data frame with 170 searcher shoots accounting for 22 species represented by at least 3 replicates to test how reach capacity varies between species.
We used standardised major axis regression (SMA) via the SMATR package (Warton et al., 2012) to observe absolute and relative changes of mechanical properties, anatomical organisations, and functional traits with reach capacity. Since power functions were linked to most traits and reach capacity, we used log-log graphs to plot the data and log-transformed the data via the sma function. The assumptions of linearity and equal variance of all fitted values and assumption of normally distributed residuals were graphically checked for all variables. We interpreted correlation coefficients to estimate the strength linking functional features with reach distance. We also compared regression slopes (beta) to interpret whether there were proportional or disproportional changes with increasing reach distance. Proportionality was defined according to an expected slope defined by the power function linking both variable units. If the observed slope is statistically equivalent to the expected one, we assume that both variables are proportionally related. If the observed slopes differ from the expected one, we assume that both variables are disproportionally related (Warton et al., 2012).
For exploring the different patterns of tissue organisation across different searchers, we grouped tissues into four categories according to their developmental origin and mechanical properties. These included pith, xylem, stiff bark, and soft bark. From a mechanical perspective, delimiting the pith is important since it maximises second moment of area of lignified tissues for petioles and stems of self-supporting plants (Mahley et al., 2018; Olson et al., 2018; Pittermann and Olson, 2018; Levionnois et al., 2020, 2021). Medullary fibres were not considered separately as a mechanical tissue for the regression analyses. This was because of their scarcity among species and low contributions to second moment of area (central positioning close to the neutral axis in bending) of the whole cross section. We considered the “xylem” component as a single tissue combining xylem fibres, vessels, and rays without regard to their primary or secondary origins. For more peripheral tissues, for simplicity, we defined outer tissues as “bark” (following the definition of Esau, See Evert, 2006) which included all tissues outside the xylem cylinder in both young and older developmental stages. For this category we differentiated the main supportive bark tissues (cortical fibres and collenchyma; Leroux, 2012; Mahley et al., 2018; Lehnebach et al., 2020) from the compliant ones (phloem, cortical parenchyma and periderm) and none-supportive one (cortical sclereids).
All sampled species could be grouped into one of four broad groups of attachment mode. These included stem twiners, tendril climbers, root climbers, and branch-hook angle climbers (Figure 1 and Table 1). There was a large morphological diversity within these groups apart from the root climbers which included only English Ivy (Hedera helix) (Figure 1). All searchers included a main axis, but species varied in terms of branching, presence of leaves, and secondary axes. The main axis of some searchers developed branches with tendrillar structures (e.g., Cissus haematantha and Serjania membranaceae) or prehensile branches (e.g., Machaerium quinata and floribundum). Other species developed branches producing attachment via wide angled-branches (e.g., Gouania blanchetiana) or developed photosynthetic leafy branches (e.g., Uncaria guianensis).

Figure 1. Morphological diversity of searcher shoots: (A) Elaeagnus umbellata; (B) Cissus haematantha; (C) Condylocarpon guianense; (D) Fallopia dumetorum; (E) Croton pullei; (F) Davilla nitida; (G) Gouania blanchatiana; (H) Machaerium quinata; (I) Rubus ulmifolius; (J) Stigmaphyllon sinuatum; (K) Hedera helix; (L) Uncaria guianensis; (M) Sabicea cinerea; (N) Mesechites trifidus; (O) Smilax aspera; (P) Bauhinia guianensis; (Q) Aegiphila laevis; (R) Dioscorea sagittata; (S) Serjania membranacea; (T) Styzophyllum riparium; (U) Vitis vinifera. Different searcher shoots vary significantly in terms of stem shape, degree of branching, kind of attachment organ and presence of leaves in addition to the length of the gap across which a species can reach. Many species show straight searcher stems (A,G,P,Q), but others, especially twiners develop conspicuously curved apical portions that undergo circumnutatory movements (C,D,F,J,N,M). Some searchers produce woody branches capable of attachment as sensitive prehensile structures (H), others develop sensitive, tendrillar attachment organs (B,G,O,S,T,U), while still others develop recurved spines, hooks and prickles acting as grappling attachment structures (P,I,L). Presence of leaves on searchers is highly variable; developmental patterns vary considerably from no leaves at all to basally developed leaves and to leaves all along the searcher stem. In some species leaf petioles can act as hook like anchoring structures (E).
Stem twiners often exhibited a hook-shaped distal part of the searcher, which underwent circumnutatory movements (e.g., Stigmaphyllon sinuatum), particularly among tropical species (Figures 1D,F,J,N). Hook-shaped apices, were not present in all twining species (e.g., Condylocarpon guianense or Aegiphila laevis). Different species possessed tendrillar attachment structures that are derived from different morphological organs. These included modifications of second-order axillary axes (e.g., Paullinia caloptera), 3rd order axilary axes (e.g., Gouania blanchetiana), as well as terminal axes resulting from sympodial branching at the phytomer level (e.g., Vitis vinifera), leaf sheath (e.g., Smilax aspera), rachis (e.g., Clematis vitalba), or leaflet level (e.g., Styzophyllum riparium). Branch-hook angle attachment mechanisms included attachment by reflexed lateral branches (e.g., Gouania blanchetiana; Uncaria guianense) petiole angles (e.g., Croton pullei, Dioscorea sagittata, Byttneria cordifolia). Hooks, grapnels and prickles were observed as modified 3rd order axes (e.g., Uncaria guianensis), modified stipules (e.g., Machaerium quinata and M. floribundum) and epicuticular structures (e.g., Rubus ulmifolius). Uncaria guianensis is commonly considered to be a hook climber. We observed that hooks are modified 3rd order stems borne by 2nd order lateral branches and that these may also function as angle attachment devices. Moreover, the 3rd order stems take the shape of spines when they are initiated in the adaxial position of the bearing branch. Different degrees of branching and branch modification can therefore produce combinations of different attachment mechanisms. Uncaria guianensis can be seen as hook climber, spine climber, and branch angle climber. The brief morphological survey underlined the fact that many climbing plants deploy more than one attachment mechanism. Even further attachment organ complexity was seen in Smilax aspera which has epicuticular structures modified into prickles, stipules modified into tendrils as well as weak stem-twining behaviour.
Leaf development on searcher shoots was extremely variable across species. Searcher shoots of some species developed fully expanded leaves from the beginning of the self-supporting phase such as Croton pullei, Hedera helix, and Aegiphila laevis (Figures 1E,K,Q). Other species such as Davilla nitida, Machaerium quinnata, and Odontadenia perotteti did not bear expanded leaves at all (Figures 1H,J). Leaf development on self-supporting searcher shoots was variable among species. It varied from leaves that were fully expanded only at the base (e.g., Styzophyllum riparium) (Figure 1T) to being fully expanded all along the searcher shoot during the self-supporting phase (e.g., Aegiphila laevis) (Figure 1Q). Some species showed delayed leaf development where only leaf primordia are present with small unexpanded laminas during the self-supporting phase (e.g., Fallopia dumetorum; Stigmaphyllon sinuatum; Condylocarpon guianense; Serjania membranaceae). Following the retention of leaves as primordia several developmental variations were seen: leaf expansion occurred following attachment to a support or also when a support was not found and the searcher became pendulous (Figure 2A). In other species, leaf primordia are rapidly aborted if the internode bearing them does not come in contact with a support (e.g., Odontadenia perotteti). In other species, expanded leaves are only produced via short leafy shoots from axilary or terminal meristems in growth episodes after the searching and support foraging phase (e.g., Davilla nitida and Machaerium quinata) (Figure 2B).
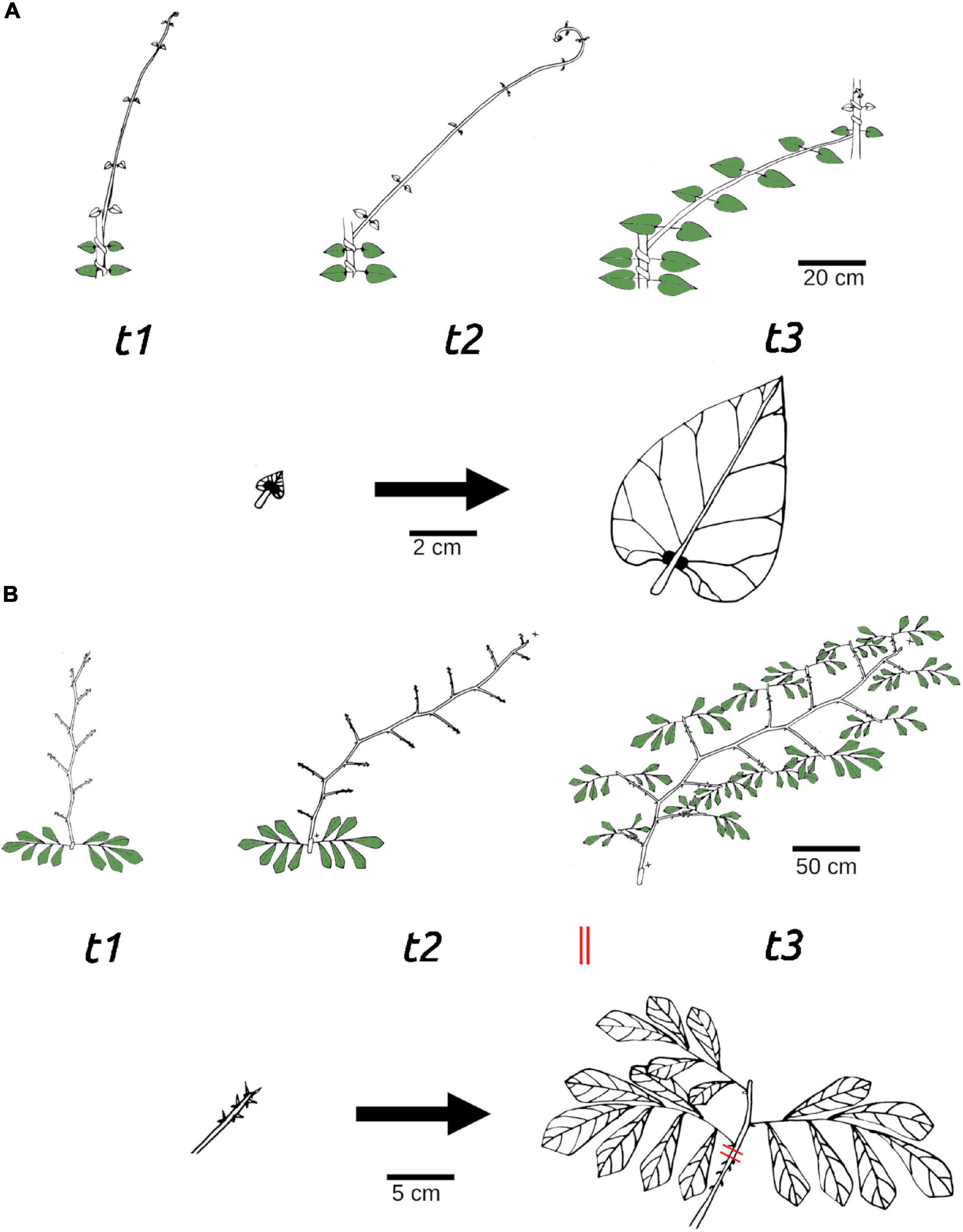
Figure 2. Leaf display patterns on searcher shoots: (A) Delayed leaf development in the stem twiner Stigmaphyllon sinuatum (Malpighiaceae). The searcher maintains leaves at a young stage of development, apart from at the very base during the entire self-supporting phase (t1). Leaves develop and fully expand only when a support is attached (t2) or if the searcher does not find a support and becomes pendulous (t3). (B) Continuous leaf development in Machaerium quinata (Fabaceae) a branch-hook angle climber. The searcher shoot remains self-supporting during multiple growth episodes. Leaves are developed initially at the searcher base and are fully expanded (t1). While still self-supporting, further axial branches are developed (t2) and leaf development continues with fully expanded leaves developing along the searcher from lateral branches (t3).
Among 190 searcher shoots sampled we observed a maximal reach capacity that varied from 10.5 cm in the stem twiner Fallopia dumetorum to 268 cm in the branch-hook angle climber Uncaria guianensis (Figure 3). About half of all species showed maximal reaches of up to a metre and the remaining half between approximately 1 m to more than two and a half metres. The analysis of variance (ANOVA) on a subsample of 181 shoots belonging to 22 species represented by at least 3 shoots per species showed significant differences between species (p-Value = 1.62e-81) with 91% of the variation being attributed to species identity. Tendril-climbers and root-climbers (the latter represented by only Hedera helix) spanned the shortest range of distances from 14 to 91 cm. Twining species showed the widest range of distances from 10 cm to 225 cm. Branch-hook angle climbers species showed the longest distances ranging from 72 to 268 cm.
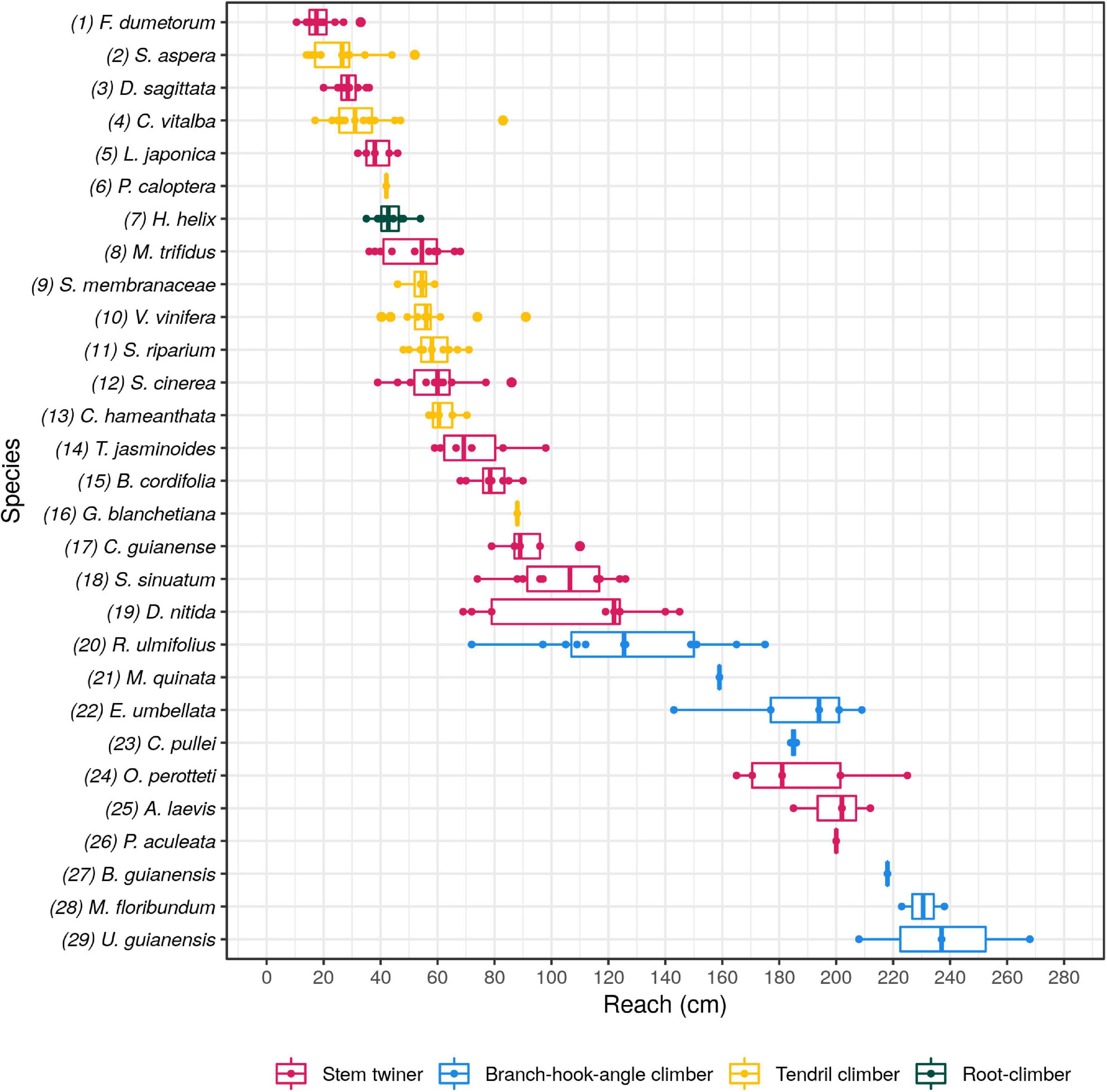
Figure 3. Boxplots showing the maximal reach per species. Species are ordered by mean maximal reach capacity from the top and as four functional groups based broadly on attachment type including: stem twiners (red), tendril climbers (yellow), root climbers (green), branch-hook-angle climbers (blue). (box plots: line = median, box = 1st and 3rd quartiles).
Basal diameter varied by more than an order of magnitude from 0.59 mm (the stem twiner Fallopia dumetorum) to 13.74 mm (the branch-hook angle climber Uncaria guianensis) (Figure 4A). Overall, maximal reach increased with increasing basal shoot diameter indicating that in general wider stems develop longer reaches (R2 = 0.738; p-value = 1.5e-56) (Figure 4A). There was a strong tendency for twiners to have smaller diameters than tendril climbers for a given reach, particularly for short and medium reaches. Branch-hook angle climbers tended to have the broadest basal stem and the longest reach (Figure 4A).
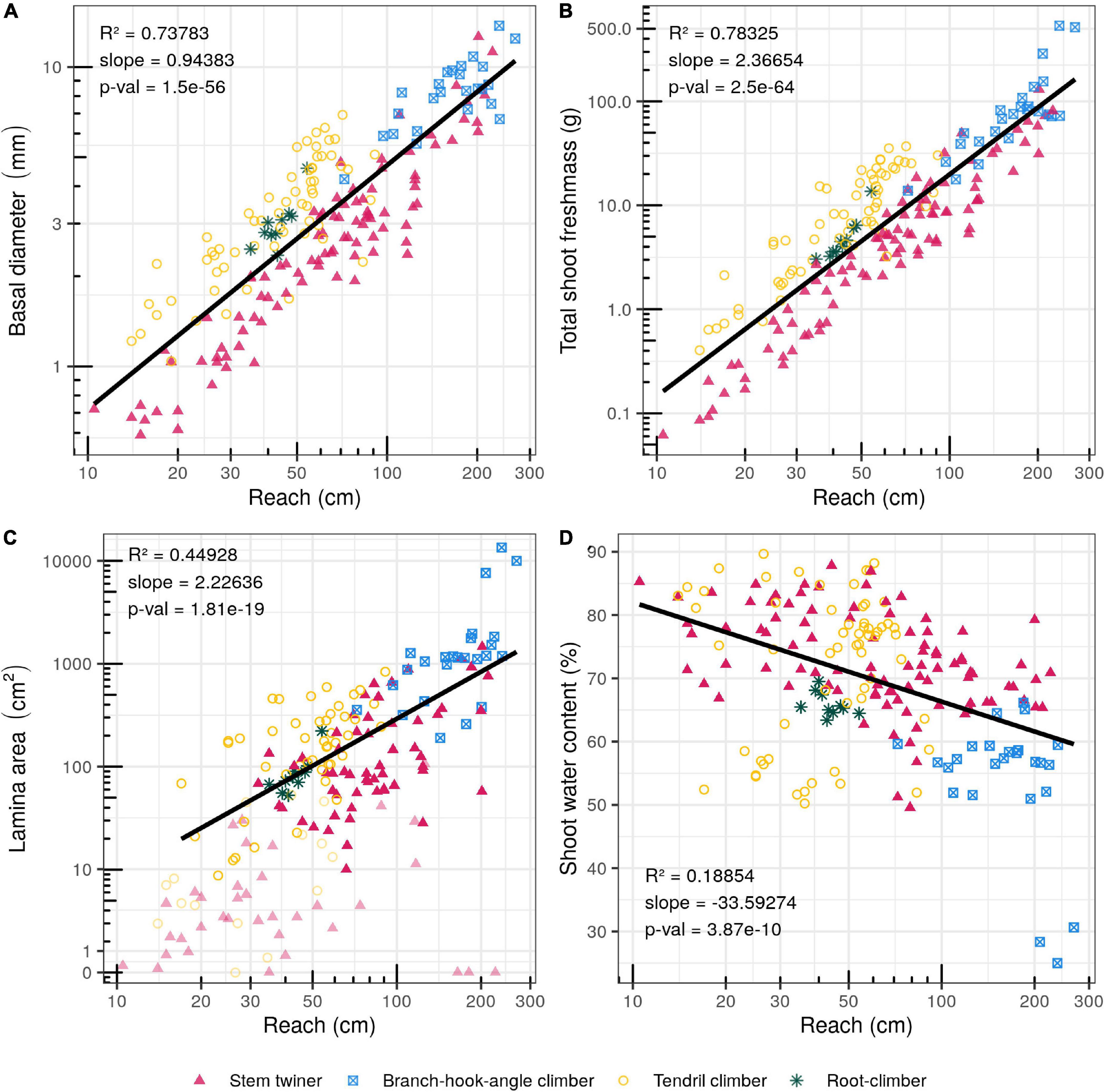
Figure 4. Bivariate plots (log scale) of searcher reach (cm) and morphological traits. (A) Basal diameter (mm). (B) Total shoot fresh mass (g). (C) Lamina area (cm2) (For searchers not producing leaves and for searchers bearing only leaf primordia or non-green leaves, the values were not included in the slope calculations and symbols are depicted as lighter shades). (D) Shoot water content (%) (fresh mass).
Total shoot fresh mass varied from 0.062 g in the stem twiner Fallopia dumetorum to 534.5 g in the branch-hook angle climber Uncaria guianensis (Figure 4B). Overall total shoot fresh mass also increased with reach (R2 = 0.783; p-value = 2.5e-64) (Figure 4B). In general, longer searchers are also necessarily heavier than shorter ones. For a given reach, twiners are again distinguished by showing less fresh mass for a given reach compared with other groups. However, the longer and more weakly twining species, Pereskia aculeata and Aegiphila laevis did not follow this trend. Each showed a rather divergent habit with succulent stems (Pereskia) and expanded leaves (Aegiphila) possibly explaining their higher searcher fresh mass. Overall, the longest reaching species in the study were the heaviest.
Among searchers bearing leaves, leaf lamina area varied by more than two orders of magnitude from 0.18 cm2 (Fallopia dumetorum) to 13455.43 cm2 (the branch-hook angle climber Uncaria guianensis). Not all species presented leaf primordia or expanded leaves on the searcher stem (e.g., Dioscorea sagittata; Odontadenia perotteti, Smilax aspera) (R2 = 0.449; p-Value = 1.81e-19) (Figure 4C). Leaf lamina area increased with reach length, but lamina area was highly variable across species and functional groups especially in the middle part of the reach range (Figure 4B). Interestingly, some medium and high reach species developed no leaf laminae. Only three twining stem species and one tendril climber produced strictly no leaves on the searcher stem. Leaf fresh mass, dry mass and lamina area generally increased with reach length across all species bearing leaf lamina at varying stages of expansion (Supplementary Figure 3). Twining stems with lower reaches stand out from other climbing types in showing less leaf development.
Overall stem water content varied from c. 50 to 90% apart from the predominantly woody searchers of the angle climber Uncaria guianensis c. 20–30%. Overall, these values decreased with searcher reach (R2 = 0.189; p-Value = 3.87e-10) (Figure 4D). Longer reaching branch-hook angle climbers showed noticeably lower water content whereas, stem twiners and tendril climbers tended to show higher water content.
Flexural rigidity (EI) at the base of searcher shoots increased with increasing reach (R2 = 0.827; p-Value = 6.49e-71) (Figure 5A) varying by 7 orders of magnitude across all species, from 3.20 N.mm2 (Fallopia dumetorum) to 2.34 × 107 N.mm2 (Uncaria guianensis). Stem twiners tended to show a lower flexural rigidity for a given reach for all reach distances compared with other groups.
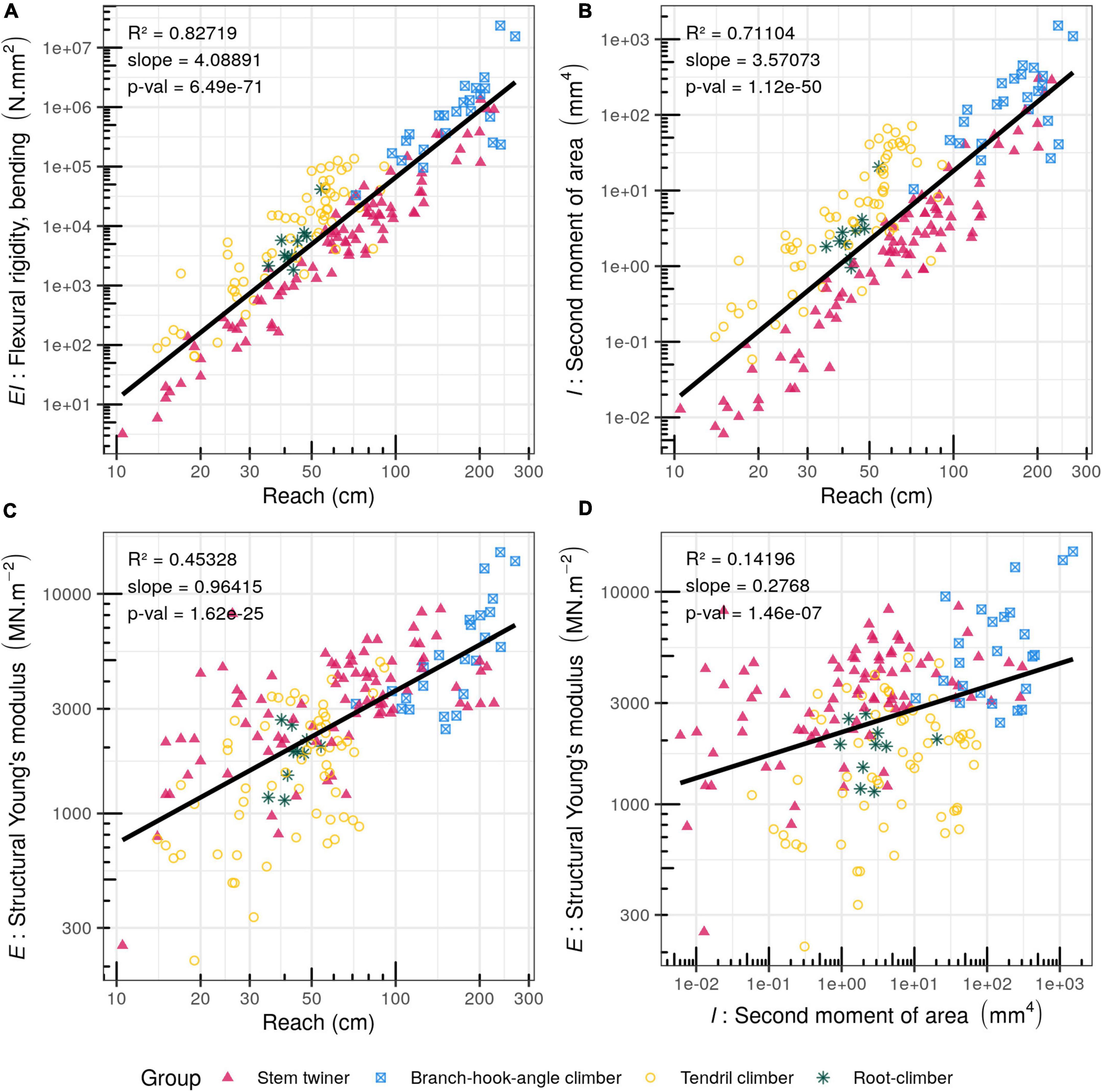
Figure 5. Bivariate plots (log scale) of searcher reach (cm) and mechanical traits. (A) Flexural rigidity, EI, (N.mm2). (B) Structural Young’s modulus Estr (MN.m–2). (C) Second moment of area, I (mm4). (D) Bivariate plot of Structural Young’s modulus Estr (MN.m–2) against stem second moment of area I (mm4).
A similar overall pattern was seen in terms of second moment of area of the basal searcher shoot stem segment (I) increased with reach (R2 = 0.711; p-Value = 1.12e-50) (Figure 5B) varying by more than 5 orders of magnitude: from 6.05 × 10–3 mm4 (Fallopia dumetorum) to 1517.048 mm4 (Uncaria guianensis) (Figure 5B). Stem twiners tended to have smaller second moments than most other functional groups even among medium to longer reaching species.
Overall, structural Young’s modulus (stem stiffness) (Estr) increased with reach (R2 = 0.453; p-Value = 1.62e-25) (Figure 5C) and varied by two orders of magnitude, from 212.95 MN.m–2 in the monocotyledonous spine and tendril-climbing Smilax aspera to 15479.08 MN.m–2 in the branch angle climber Uncaria guianensis.
When stiffness was plotted against second moment of area, a much lower prediction value was found compared with stem rigidity (R2 = 0.142; p-Value = 1.46e-07) (Figure 5D). Visual inspection of the data suggested that stem stiffness is not closely correlated with basal stem size across all species of searcher. In other words, for a given searcher basal size, there is a very wide range of structural Young’s modulus values. This implies a large range of organisational and structural anatomical patterns. Among smaller diameter stems, twining stems tended to develop relatively higher stiffness for a given reach and for a given basal stem size. Larger diameter stems representing the longest reaching angle climbers represented by Uncaria guianensis and Machaerium floribundum showed the highest stiffness. The data also pointed to the fact at least among the searchers studied, that searchers or all kinds require a structural Young’s modulus of c. 3000 MNm–2 to maintain a reach of over c. 80 cm (Figure 5C).
Tests were carried out on how mechanical parameters were scaled (slope) and constrained (R2) from short to long reach capacities (Figure 5 and Table 3). Flexural rigidity (EI) was expected to show a 104 relationship with reach capacity and did not differ significantly from the observed slope (slope = 4.089). This means that EI tends to remain proportional to the increase in reach. Second moment of area (I) was also expected to show a 104 function with reach, but the estimated slope was significantly lower (slope = 3.571). This means that second moment of area (I) tended to be proportionally lower with increasing reach distance. The slope of stem stiffness (Estr) against reach was expected to show a 100 relationship was observed to be significantly higher than the expected one (slope = 0.964). This means that basal stiffness tended to be proportionally higher with increasing reach.
Anatomical organisations of the basal segments of searcher stems are remarkably diverse (Figure 6). They vary from short reaching, small cross-sections with relatively high proportions of primary tissues to longer reaching, larger sections with noticeably higher proportions of secondary tissues (Figure 7A). Stem twiners and tendril climbers are mostly represented by smaller cross-sections with large proportions of primary tissue although stem twiners are also present as medium and long reaching larger stem sections with well-developed wood cylinders (Figures 6, 7A). Branch-hook angle climbers mostly include well-developed wood cylinders among larger, longer reaching searchers.
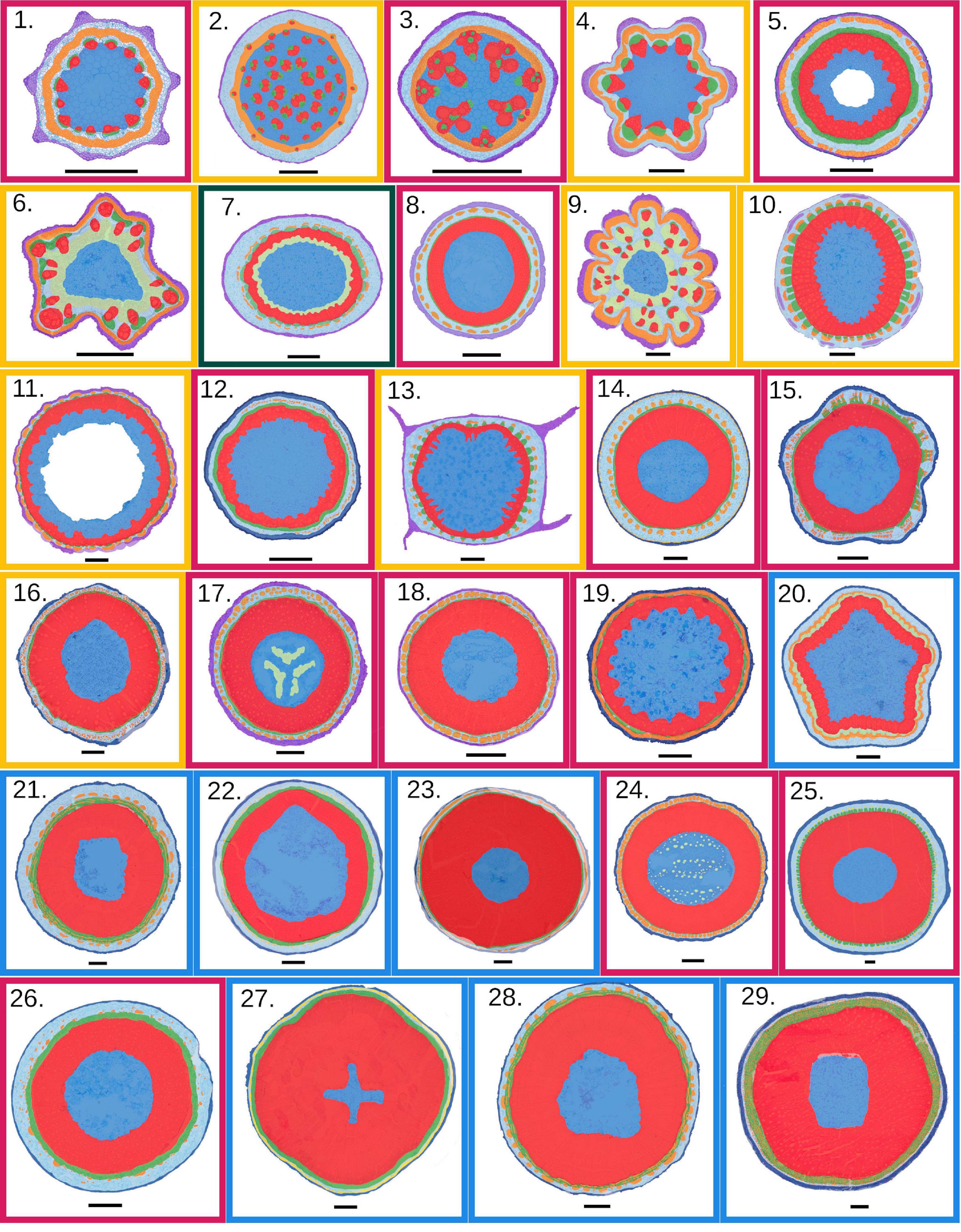
Figure 6. Diversity of anatomical organisations organised by increasing mean reach per species (scale bar = 500 μm for all specimens). Colours distinguishing different tissues are the same than choose for Figure 3. (1) Fallopia dumetorum; (2) Smilax aspera; (3) Dioscorea sagittata; (4) Clematis vitalba; (5) Lonicera japonica; (6) Paullinia caloptera; (7) Hedera helix; (8) Mesechites trifidus; (9) Serjania membranaceae; (10) V. Vitifera; (11) Styzophyllum riparium; (12) Sabicea cinerea; (13) Cissus haematantha; (14) T. jasminoïdes; (15) Byttneria cordifoli; (16) Gouania blanchatiana; (17) Condylocarpon guianense; (18) Stigmaphyllon sinuatum; (19) Davilla nitida; (20) Rubus ulmifolius; (21) Machaerium quinata; (22) Elaeagnus umbellata; (23) Croton pullei; (24) Odontadenia perotteti; (25) Aegiphila laevis; (26) Pereskia aculeata; (27) Bauhinia guianensis; (28) Machaerium floribundum; (29) Uncaria guianensis. Functional groups are indicated by different coloured frames: stem twiners (red), tendril climbers (yellow), root climbers (green), branch-hook-angle climbers (blue).
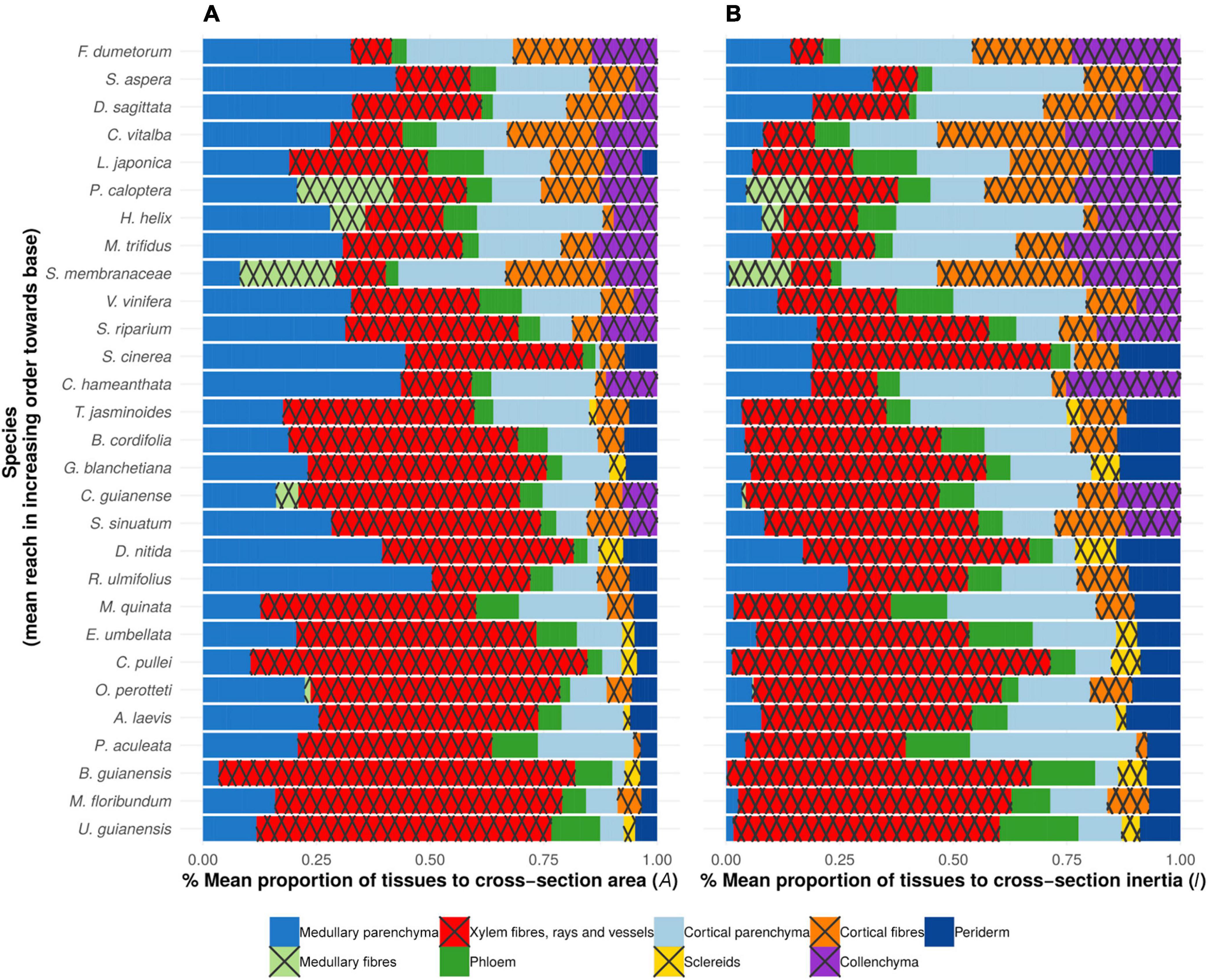
Figure 7. Proportions of main tissues at base of searcher shoot (A) mean proportion of tissues to cross-sectional area (A%) (B) mean% contribution of tissues to second moment of area (I%).
Overall, outlines of searcher bases were mostly circular to ellipsoidal and close to radially symmetrical, with the possible exception of Paullinia caloptera, a tendril climber (Figure 6(6)). Some of the smaller reach stems developed lobed outer stem outlines, often containing mechanical tissue packed into the distal extremities of the lobes (Figures 6(1,4,10)). Stem geometries of longer reach species were nearly all ellipsoid to rounded. This suggests that secondary growth has a “rounding” effect on stem geometry when wood and periderm replace outer primary tissues (Figures 6(16-29)). Searchers of the tendril climber Cissus haematantha showed a distinctly square external outline, with pointed wing-like extensions packed with collenchyma cells.
Cross-sectional area ranged from 0.30 mm2 in the small twiner Fallopia dumetorum to 149.9 mm2 in the large, long reaching branch-hook angle climber Uncaria guianensis (Supplementary Table 1).
Cross-sectional areas of mechanical tissues (xylem, cortical fibres and collenchyma) varied from 0.15 mm2 (Fallopia dumetorum) to 103.6 mm2 (Uncaria guianensis) and from 18% (Hedera helix) to 84% (Bauhinia guianensis). Second moment of area of all mechanical tissues combined varied from 0.0048 mm4 (Fallopia dumetorum) to 1182.73 mm4 (Uncaria guianensis) (Supplementary Table 1). The relative contribution of all mechanical tissues combined to the second moment of area (I) varied from 28.4% (Smilax aspera) to 80.8% (Stigmaphyllon sinuatum) (Figure 7A).
Cross sectional area of pith (medullary parenchyma and fibres combined) varied from 0.06 mm2 (Fallopia dumetorum) to 33.8 mm2 (Rubus ulmifolius) (Figures 7A, 8A). Relative proportions varied from 2.97% (Elaeagnus umbellata) to 59% (Rubus ulmifolius) of the cross-sectional area and were not observed to vary significantly with the reach. Absolute values of pith area increased with reach (R2 = 0.54; p-value = 1.5e-33) indicating that the pith is a potentially key trait linked to searcher length. Absolute second moment of area of pith varied from 0.00028 mm4 (Fallopia dumetorum) to 93.74 mm4 (Rubus ulmifolius). Pith contribution to second moment of area varied from 0.098% (Elaeagnus umbellata) to 43.6% (Smilax aspera) (Figure 7B). Some searcher stems containing a relatively large pith showed evidence of tissue voiding and the development of a pith cavity. Pith voids were not included in the calculations of the cross-sectional area but directly influenced values of second moment of area of the remaining pith and other tissues (Figures 6(5,11)).
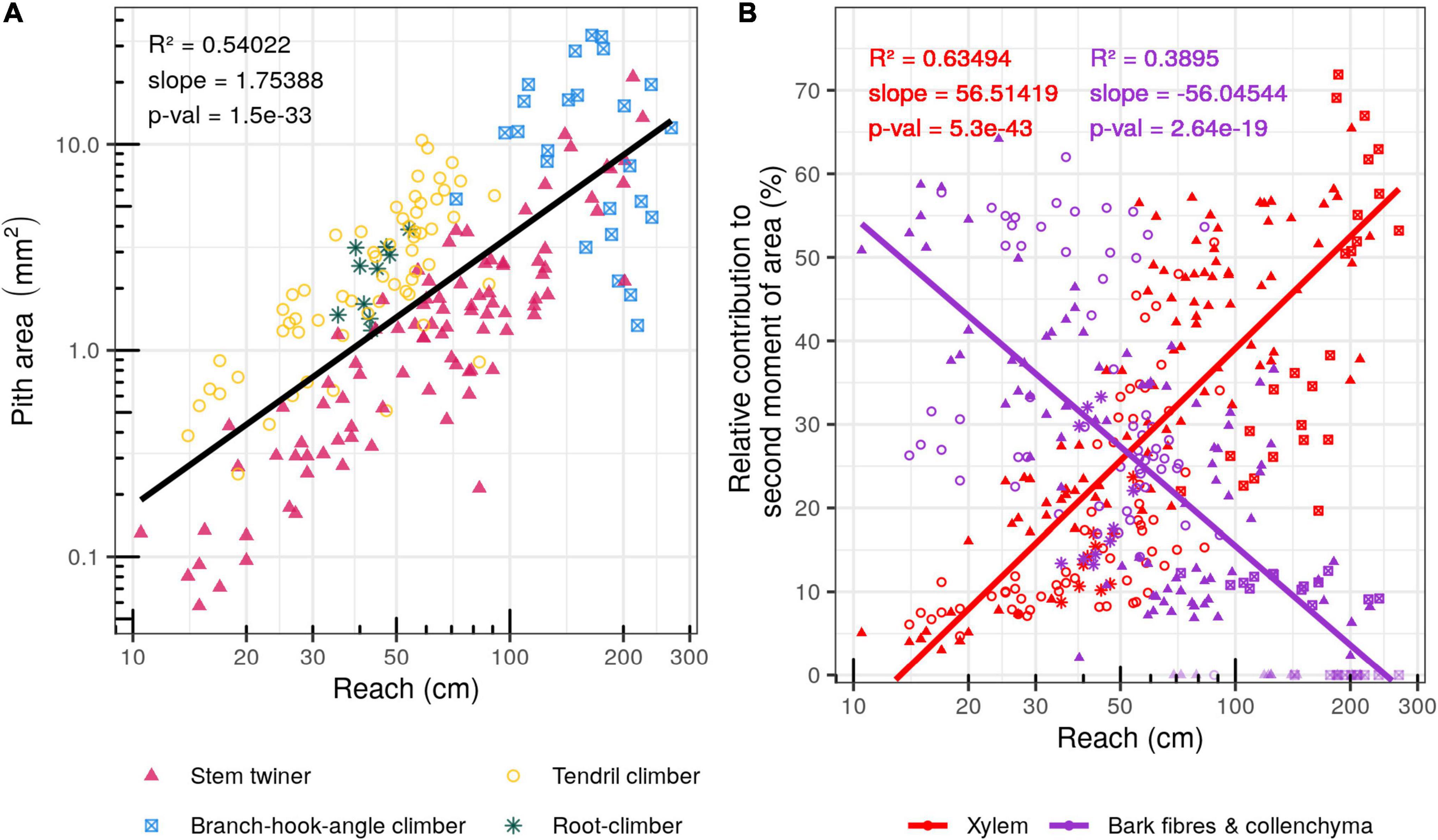
Figure 8. Bivariate scatter plots of reach (cm) against main tissue types. (A) Plot of Reach (cm) against pith area (mm2). (B) Plot of Reach (cm) against% contribution of xylem and bark to second moment of are of the stem (Values for searchers not encounting for cortical fibers and collenchyma in their stem anatomy were not included in the slope calculations and symbols are depicted as lighter shades).
Xylem cross-sectional area (cross-sectional area of primary xylem bundles for monocots and cross-sectional area of primary and secondary xylem for dicots) varied from 0.028 mm2 (Fallopia dumetorum) to 103.59 mm2 (Uncaria guianensis). Xylem relative proportion of cross-sectional area ranged from 6.46% (Fallopia dumetorum) to 81.2% (Bauhinia guianensis) of the cross-sectional area. Absolute second moment of area of xylem varied from 0.00033 mm4 (Fallopia dumetorum) to 1182.73 mm4 (Uncaria guianensis). Xylem relative contribution to the second moment of area varied from 3.02% (Fallopia dumetorum) to 71.9% (Croton pullei) (Figure 8B). Xylem relative contribution to the second moment of area (I) was significantly and positively correlated with the reach (R2 = 0.635; p-Value = 5.3e-43).
Cross-sectional areas of bark supportive tissues (cortical fibers and collenchyma) were not observed in Gouania blanchetiana, Elaeagnus umbellata, Davilla nitida, Croton pullei, Bauhinia guianensis, Aegiphila laevis and Uncaria guianensis. But when present, it varied from 0.026 mm2 (Sabicea cinerea) to 5.026 mm2 (Rubus ulmifolius). In such case, stiff bark relative proportion of cross-sectional area ranged from 1.29% (Sabicea cinerea) to 42.86% (Fallopia dumetorum) of the cross-section area. Second moment of area of bark supportive tissues varied from 0.0045 mm4 (Fallopia dumetorum) to 43.3054 mm4 (Rubus ulmifolius). Contribution of bark supportive tissues to the second moment of area varied from 2.09% (Sabicea cinerea) to 64.17% (Fallopia dumetorum) (Figure 8B). Their relative contribution to the second moment of area (I) was significantly and negatively correlated with the reach (R2 = 0.389; p-Value = 2.64e-19).
Overall, smaller reach species developed relatively less wood and notably produced higher proportions of peripheral extraxylary mechanical tissues (Figure 7A), especially cortical fibres and hypodermal collenchyma (Supplementary Figures 1A–L). Analyses of second moment of area indicate that for a number of small reach species including stem twiners and tendril climbers the peripheral placement of mechanical tissue occurs outside relatively large areas of cortical tissue. A variety of thickened fibre tissue was observed across species including lignified cells which represented either longitudinally elongated fibres (Supplementary Figure 2B) or longitudinally foreshortened sclereids (Supplementary Figure 2A). Primary fibre organisations included entire rings of interconnected fibres (Supplementary Figure 2F), separated perivascular bundles (Supplementary Figure 2E), and also, notably in twining species of Apocynaceae and Malpighiaceae islets of cortical fibres with g-layer walls (Supplementary Figures 2G–I). Basal organisations of longer reach searchers overall showed a decreasing amount of primary fibre tissue in terms of both cross-sectional area and second moment of area concomitant with increasing amounts of vascular tissue (Figures 7A,B and Supplementary Figures 2U–X).
This study compared the attachment and reach characteristics of 29 species of climber from tropical and temperate environments. The sampling included most of the well-known kinds of attachment strategies that characterise climbing plants, including stem twiners, tendril climbers, and branch-hook angle climbers. Our sample only included one root climbing species from temperate environments and this category is perhaps underrepresented. Despite this, the sampling overall reflects climbing mechanisms among many woody vines and lianas (Gallagher and Leishman, 2012).
One of the aims of this analysis was to find out how different reach capabilities might be linked to different attachment modes, climbing strategies, and stem structure and mechanics (Putz, 1984; Rowe et al., 2006; Wyka et al., 2013; Gianoli, 2015). Our field observations underlined the difficulty of attributing a single kind of attachment strategy or attachment mode. Instead, many temperate and tropical climbers rely on combinations of attachment mode during searching and climbing. For example, Smilax aspera has epicuticular structures modified into prickles as well as stipules modified into tendrils. Both of these are observed to anchor the plant to host plants in different ways and on different kinds of supports. Both Dioscorea sagittata and Byttneria cordifolia twine around supports via the main searcher axis but these plants also relied on relatively stiff and angled or reflexed-petioles that act as hooks to attach to host branches. These multi-attaching mechanisms underline the difficulty of attributing species to a functional group based on attachment. It is clear that many species possess a combination of attachment and climbing methods (e.g., Cabanillas and Hurrell, 2012; Soffiatti and Rowe, 2020) that can operate at different scales and attachment forces (Steinbrecher et al., 2010). This likely influences the range of habitats and kinds of three-dimensional space they can exploit. The notion of “complex attachments” (Caballé, 1986), also supports the idea that attachment strategies cannot always be categorised in regard of a single organ. Studies attempting to integrate attachment mechanisms and ecological patterns possibly require more detailed methods of comparing complex combinations of growth and attachment mechanisms.
Maximal reach varied markedly between species and was not strictly linked to mode of attachment. This was particularly the case in stem twining species where both short and long reach species are represented. Our measurements seeking to identify the maximal reaches for a given plant individual in the field also suggested that maximal reach can vary in amplitude among longer reach species. The implication is that some longer reach species might be able to exploit smaller gaps as self-supporting shoots foraging for supports, however a more detailed sampling including maximum and minimum reach would be necessary to explore this further.
Our study showed a highest reach limit of approximately 2.5 m, this is less than previous measurements of reach in tropical climbers (Putz, 1984), with lengths reported up to 3 m. However, sampling here focussed on searcher stems derived mostly from branches of mature individuals rather than young individuals in a juvenile self-supporting phase of growth (Caballé, 1998; Speck and Rowe, 1999). Some tropical climbing species can reach heights of well over 2.5 m as young self-supporting individuals, as can be regularly seen with species of Strychnos (Loganiaceae) as well as Croton and Manihot (Euphorbiaceae) (Gallenmüller et al., 2004; Rowe et al., 2006; Ménard et al., 2013). These can develop self-supporting statures comparable with treelets. Furthermore, our survey did not include some of the larger bodied monocot climbers, the rattans (Calamoideae) and genus Desmoncus (Arecaceae) (Isnard and Rowe, 2008). These non-woody palms can produce self-supporting attachment organs (cirri) that can also have reaches longer than 2.5 m. Clearly, the reach capabilities between different species and different life histories can also depend on the state of development as young “treelet”-sized individuals or as branches developing on individuals that are already attached and climbing. There are probably functional differences as well as risk differences between attachment strategies of juvenile plants and searcher branches of adults. For example, the collapse of searchers on adult branches is potentially an advantage representing a shift to alternative functions such as light capture. However, a young individual that collapse to the ground in the dark understory possibly faces a setback to the plants’ development.
Reach capacity is strongly linked to flexural rigidity at the base of the searcher shoot. Longer searchers have higher stem rigidity at their bases than shorter ones. Values of Young’s modulus showed a huge range of values for a given reach and also for a given basal stem second moment of area. This suggests that different species have evolved many different ways of developing mechanical properties to enable a given rigidity and reach. This is consistent with the anatomical observations indicating a wide array of anatomical architectures.
The scaling relationships suggested that none of the searcher shoots were mechanically “over-designed.” Instead, we found that they were just as rigid as necessary to sustain their total shoot fresh mass and traverse the gap distances consistent that they are putatively adapted for. This is consistent with the long-held belief that vines and lianas have diverse habitat preferences (Gentry, 1991), such as transitions from disturbed to stable environments (Laurance et al., 2014; Ledo and Schnitzer, 2014; Campbell et al., 2018); attachment to large- to small-diameter supports (Putz, 1984; Hegarty, 1991; Goriely and Neukirch, 2006; Carrasco-Urra and Gianoli, 2009) and establishment in early to late successional settings (Letcher and Chazdon, 2012; Letcher, 2015). We suggest that habitat preference also involves selection in terms of reach implying that different climbing plants are adapted for different three-dimensional habitats in terms of interspacing of supports.
The scaling relationship also indicated that longest reaching stems had proportionally smaller stem cross-sections with proportionally smaller second moments of area for their long reach. These longest reaching stems also developed the stiffest mechanical properties in terms of structural Young’s modulus (Estr). The result is interesting because it suggests that even the longest reaching, broadest searcher stems remain proportionally narrow compared to short-reaching searchers.
Development of a wood cylinder with a significant contribution to rigidity is eventually necessary for sustaining a searcher shoot beyond a certain reach. The study suggests that searcher shoots of vines and lianas (at least those included in this study) do not cross a potential allometric divide separating them from trees. The study suggests that climbers do not grow like trees with indeterminate secondary growth of the stiff early growth-phase wood of the juvenile phase.
The mechanical data indicated that stem twiners potentially develop a different mechanical architecture compared with other tested groups. Overall, twiners developed less rigid, smaller cross-sections but apparently compensated for a small diameter by developing a relatively high Young’s modulus (high stiffness of the tissues comprising the stem). For a given reach, stem twiners were also lighter than other climbing categories and at the same time also tended to have a higher shoot water content. We suspect that this reflects the need for turgor driven movements and circumnutation needed to deploy attachment and twining. Oscillatory movements are controlled by active water transport in cells, generating elongation on one side of the stem that is actively regulated by signals emitted on the compression side (Millet et al., 1988; Care et al., 1998; Rivière et al., 2017). Searcher organisation reflects the developmental compromise between being stiff and rigid enough at the base to mechanically support and orientate the searcher but at the same time retain flexibility and mobility nearer the apex. The presence of stem twining across such a large range of reaches is a striking result of this study. It contrasts with the narrower ranges of reach seen among tendril climbers and branch-angle climbers. Twiners seem able to do all of the reaches.
Overall, narrow searchers of short-reach species develop small cross-sectional areas and second moments of area of wood. Instead, they rely on peripheral primary tissues for stem stiffness. Long-reach searchers almost all have well-developed wood cylinders with large wood cross-sections and second moments of area.
The data also indicate that there is a threshold of maximum reach, which is limited by stem stiffness. Searchers generally develop a relatively high stiffness with a structural Youngs modulus of at least 3000 MNm–2 in order to cross spans of more than c. 80 cm. In other words, whatever the interplay of stem second moment of area (I) and stem stiffness (E) across all species, it appears that searchers must have tissues with high stiffness contributing to the rigidity in order to traverse gaps longer than 80 cm. It appears, at least among those tested, that climbing plants do not produce long reaches with wide diameters (high second moments of area) and with tissues that are low in stiffness (compliant). Short-reach searchers limit development of stiff wood whereas most long-reach species develop significant amounts of wood. However long reach searchers must develop stiff tissues to exceed gaps of 80 cm.
The study emphasised that pith size is a key developmental feature behind searcher stem diameter and rigidity. Measurements of second moment of area indicate that the pith can act as a geometrical spacer around which stiff tissues can be placed and provide relatively high rigidity for relatively little material (Mahley et al., 2018; Olson et al., 2018; Pittermann and Olson, 2018; Levionnois et al., 2020, 2021). A large pith occurred across different reach lengths and also between different attachment strategies. Short-reach twiners, in particular, developed a large pith with stiff primary fibre tissues positioned to the outside of the stem cross-section thus raising their second moment of area and contribution to rigidity. The principal is not just seen in short-reach twiners but is also well known in species of Rubus, a branch-hook climber and medium to long reach searcher. Stems produced a very large pith but with relatively little wood in the cross-sectional area producing a long reach and high rigidity. The pith acting as a central spacer is well known for fern and vascular plant petioles (Mahley et al., 2018; Levionnois et al., 2020) and self-supporting stems of plants in general (Borchert and Pockman, 2005; Rosell and Olson, 2014; Plavcová and Jansen, 2015). In the vines and lianas studied here, most short-reach species bear significant proportions of parenchyma tissues but this was also observed in a long-reach species (i.e., Pereskia acuelata). Abundant parenchyma cells may be linked to other functions. (i) fast growing systems that require tissues with a high volume:weight ratio to produce relatively high moments of inertia (e.g., the case of many short-reach species, vine-like and fast-growing lianas). (ii) searchers with active searching movements may require water capacitance to sustain growth in environments with high evapotranspiration (Borchert and Pockman, 2005).
G-fibres have been viewed as playing a role in attachment mechanisms via thigmomorphogenetic development (Meloche et al., 2006; Bowling and Vaughn, 2009). Extraxylary fibres with gelatinous wall layers are present in the bark tissues of many searcher shoots and these have been recently linked to stem development in the climbing habit (Chery et al., 2020). The presence of G-fibres was identified in Stigmaphyllon sinuatum and four species within the Apocynaceae. They were present as bundles of thick-walled gelatinous fibres in the outer part of the cross-stem section. These were mostly observed in twining species in which the searcher shoot needs to extend across gaps but also attach to supports via movement and twining. We suspect that G-fibres possibly facilitate small changes in motor tissue properties in some searchers to generate rapid postural responses. In contrast searcher shoots adapted for crossing large gaps between supports, such as some of the branch-hook angle climbers and tendril climbers may maximise second moment of area and stem stiffness but possibly at the risk of limiting stem mobility and active movements for finding and attaching actively to supports.
To our knowledge, leaf development on searcher shoots has not been studied in any detail across different functional groups of climbers and in relation to searching, attaching, and climbing. Our survey showed a highly variable leaf development pattern. Previous observers have noted that leaf expansion during twining and attachment would hinder circumnutatory movements and connection with host supports (Raciborski, 1900; French, 1977). To some extent, this is borne out by our observations where stem twining species deploying long, curved, circumnutatory shoots are leafless or bear only leaf primordia or undeveloped leaves.
Some of the tendril climbers, especially Clematis vitalba, develop many leaves during searcher deployment representing a large proportion of the fresh mass that the searcher base must support. We suspect that in some searcher deployment mechanisms, early deployment of fully developed leaves would mean that searcher shoots would become elastically unstable. In Clematis, we have observed that this results in the collapse of the leafy searcher on top of host branches with leaves fully expanded thus ensuring that the climbers’ leaves lie on top of the host leaves. The outcome would be similar to other species which accomplish a “leaves on top” strategy via a leaf ratcheting mechanism of micro hooks on the leaf surfaces (Bauer et al., 2011).
Elastic instability and flopping of searchers that do not reach a support are generally widespread in climbing plants. Some climbing life histories might take advantage of elastic instability for deploying leaf-ready stems that can cover host leaves before attachment. This kind of mechanism might be especially relevant among long range branch-hook angle climbers that deploy leaves early during the searcher self-supporting phase. Elastic instability and early leaf expansion might also be consistent with tendril and hook climbing mechanisms that attach to narrow branches on contact. It is perhaps less consistent with circumnutatory movements and twining attachment. In summary, some of the leaf deployment strategies are consistent with the idea that reach and leaf deployment on top of host leafy shoots might be coordinated.
Overall, most searcher shoots developed leaves to some extent but the development and positioning of leaves vary a lot between individual species and functional groups. Our observations of leafy searchers suggest that leaf development probably varies according to different searcher attachment mechanisms.
In the long reaching stem twiner Odontadenia leaf expansion occurs close to the basal attachment point of the searcher but leaf primordia along the rest of the searcher do not continue development if the searcher remains in an open self-supporting state. Among twiners, it appears that searchers can grow autonomously without leaves up to around 1 m in length but after this most species need to develop leaves to maintain growth. The longest reaching species Uncaria guianensis developed an order of magnitude more of leaf area than other species. This is consistent with its high rigidity and an attachment mode that does not rely on rapid movements and circumnutation. The example highlights the fact that some species can sacrifice active searching and circumnutatory movements for long reach with high rigidity, high leaf surface area (autonomy), and passive attachment mechanism via open hook-like organs.
Our survey of liana searchers and their reach has highlighted some important principles on how climbing plants cross gaps to attach to supports. First, at the risk of generalisation numerous studies have highlighted the huge diversity of cambial variants across many liana groups and its functional convergence to promote flexibility and toughness. We suggest that the diversity and convergence of the self-supporting and attaching phases of the life history are no less important for understanding the climbing growth habit, but have perhaps not been as fully studied. Our findings highlighted the fact that the reach of searchers is integrated with other essential functional traits. A key finding is that long reach requires sufficient rigidity in the basal part of the searcher. However, long-reach and high rigidity might limit active circumnutational and nutational searching movements to more distal parts of the searcher. We suspect that this basic underlying requirement and limit on searching movement is a key feature of liana growth strategies, from short range highly mobile searcher behaviours to long range less mobile searching and foraging behaviours. Our study suggests that this trade-off between reach length and searching behaviour also influences the strength and reliability of attachment. Most long-range searchers attach by branch-hook-angle mechanisms, which only need passive or swaying movements of the searcher to engage. Most shorter-range searching mechanisms rely on less rigid mechanical organisations and could depend on stem twining and tendril climbing. Long range branch-hook-angle attachments require that the searcher stays in tension to remain engaged whereas short range twining and tendril mechanisms can form irreversible attachments to the appropriate supports.
Our study only analysed the maximal reach of searcher shoots, in other words, the longest reaches a given species can span. It would be interesting for further studies to see, in particular, whether woody, long-reaching, branch-hook-angle climbers can attach and exploit short range supports in cluttered 3-D spaces or whether they are more restricted to spanning longer distances. The whole question of the ability of a given species to effectively reach and attach across short or long distances is an important ecological consideration.
This study is an unashamedly detailed exploration of reach, stem mechanics, tissue distributions, and attachment mechanisms in climbing plant searchers. Broad-based comparisons of many species at an ecological scale would probably require a shorter task list of nevertheless informative traits providing information on vine and liana dynamics. What are the key traits among the traits? We think that the shift from “stiff” to “flexible” is central to vine and liana biology and a major axis of the developmental spectrum that can potentially explain many patterns of functional diversity at an ecological level. The relationship between stem rigidity (EI), stem stiffness (E) and stem second moment of area (I) pioneered for climbing plants by Speck (1991) is a powerful approach and can also be relatively easily carried out in the field with minimal logistics. Conserving stem segments measured in basic storage media (alcohol) can enable further measurements of density, tissue development as well as chemical and ultrastructural traits at a later date if desired. Of course, without necessarily going into the same level of tissue mapping detail that we demonstrate in this paper. However, the opportunity to identify tissue patterns in relation to mechanical properties and measures of performance such as reach offer higher levels of interpretation than for example measurements restricted to stem or wood density.
Our study on searcher biology highlighted the interest of observing and measuring liana searchers in field conditions, where functional traits can be measured in the context of the demands and constraints acting on them in the environment. These not only generate information on the functional biology and potential ecological implications of complex functional traits but can at the same time generate new information for bioinspired technologies. Plant stems have been of interest over recent years as potential models for new technological innovations (Milwich et al., 2006). Climbing plants have come under increasing scrutiny as biological role models for new technological applications, particularly in soft robotics (Fiorello et al., 2020). The light structured organisation of searcher shoots—the use of a light central pith as a mechanical spacer; externally placed rings, columns, ribs and struts of stiff, light, geometrically optimised tissues are all present in the limited sample we studied. All are of potential interest as transferable blueprints for new kinds of technical artefacts. Searcher-like artefacts for difficult-to-reach applications in cluttered, cramped, and unpredictable 3-D spaces have been developed from climbing plants (Walker, 2015; Wooten and Walker, 2018; Wooten et al., 2018). Other studies have explored climbing plant searchers for technical innovations in terms of new bio-inspired actuating mechanisms based on changes in stem orientation, stem shape, and swellability of their hydrogel-like tissues (Soffiatti and Rowe, 2020; Bastola et al., 2021a,b).
At the beginning of this paper, we mentioned that the presence of multi-attachment systems seemed “complex” and “difficult to suitably classify or group.” This attachment organ dilemma highlights the difficulty and usefulness of trying to “group” climbing plant life histories or functional groups via a single “key syndrome.” The issue is probably widespread in functional biology and ecology. In fact, detailed in situ-field observations indicate that multi-attachment systems are common and can show a meaningful and consistent overall functionality when observed with respect to the plant development (in time) and the spatial and three-dimensional contingencies in the environment (in space). Notions such as two-step attachment mechanisms in the spines and root-climbing attachment of a climbing cactus (Soffiatti and Rowe, 2020) and active, coordinated, multi-step attachment processes in the humble English ivy (Melzer et al., 2010) are the kinds of dynamic trait combination that when understood and measured in the field and lab can potentially resolve (i) the problem of “attempting to group climbing plant life histories by attachment type, (ii) demonstrate and resolve a detailed functionality and (iii) provide novel concepts for bioinspired technologies based on trait behaviours under real-world situations.
Data are available at https://zenodo.org/record/6347468.
NR, PH, and TH conceived and designed the study and performed the data analysis. TH, LP-B, PH, and NR collected the field samples and measured the morphological and biomechanical traits. TH, LP-B, and CH performed the anatomical sections. TH, PH, NR, and CH performed image interpretation and analysis. TH and NR wrote the manuscript with contribution of PH. All authors discussed the results and contributed valuable comments on the manuscript.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 824074 (GrowBot) and an “Investissement d’Avenir” grant from the Agence Nationale de la Recherche (CEBA: ANR-10-LABX-0025; ARBRE, ANR-11-LABX-0002-01). TH was supported by a doctoral fellowship from the CEBA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Chantal Geniez (IRD, UMR AMAP) and Maryline Harroué (INRAE, UMR SILVA) for performing a significant part of the anatomical sections. We also thank Quentin Le Blaye and Stéphane Fourtier (INRAe, UMR AMAP) for their contribution to the technical development of the study as well as Tancrède Alméras (CNRS, UMR LMGC) for sharing a script for analysis of second moment of area. We thank many colleagues of UMR ECOFOG: Geraldine Derroire and Laetitia Plaisance (CIRAD) for facilities on field work. In particular, we also thank Jacques Beauchêne (CIRAD) for hosting us at the wood lab of Pariacabo and the loan of equipment. We also thank Clément Stahl (INRAe) and Sabrina Coste (French Guiana University), from the ecophysiology lab (INRAe) for technical assistance. We would also like to thank SILVATECH (Silvatech, INRAE, 2018; Structural and functional analysis of tree and wood Facility, doi: 10.15454/1.5572400113627854E12) from UMR 1434 SILVA, 1136 IAM, 1138 BEF, and 4370 EA LERMAB from the research center INRAE Grand-Est Nancy for its contribution to stem cross-section production. SILVATECH facility was supported by the French National Research Agency through the Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.836247/full#supplementary-material
Baillaud, L. (1962). “Les mouvements d’exploration et d’enroulement des plantes volubiles,” in Physiology of Movements / Physiologie der Bewegungen, eds L. Aletsee, L. Anker, L. Baillaud, G. H. Banbury, L. Brauner, W. M. L. Crombie, et al. (Berlin: Springer Berlin Heidelberg), 635–715. doi: 10.1007/978-3-642-94852-7_18
Baret, S., Nicolini, E., Le Bourgeois, T., and Strasberg, D. (2003). Developmental patterns of the invasive bramble (Rubus alceifolius Poiret, Rosaceae) in Réunion Island: an architectural and morphometric analysis. Ann. Bot. 91, 39–48. doi: 10.1093/aob/mcg006
Bartumeus, F., and Catalan, J. (2009). Optimal search behavior and classic foraging theory. J. Phys. Math. Theor. 42:434002. doi: 10.1088/1751-8113/42/43/434002
Bastola, A. K., Rodriguez, N., Behl, M., Soffiatti, P., Rowe, N. P., and Lendlein, A. (2021a). Cactus-inspired design principles for soft robotics based on 3D printed hydrogel-elastomer systems. Mater. Des. 202:109515. doi: 10.1016/j.matdes.2021.109515
Bastola, A. K., Soffiatti, P., Behl, M., Lendlein, A., and Rowe, N. P. (2021b). Structural performance of a climbing cactus: making the most of softness. J. R. Soc. Interface 18:20210040. doi: 10.1098/rsif.2021.0040
Bauer, G., Klein, M.-C., Gorb, S. N., Speck, T., Voigt, D., and Gallenmüller, F. (2011). Always on the bright side: the climbing mechanism of Galium aparine. Proc. R. Soc. B Biol. Sci. 278, 2233–2239. doi: 10.1098/rspb.2010.2038
Borchert, R., and Pockman, W. T. (2005). Water storage capacitance and xylem tension in isolated branches of temperate and tropical trees. Tree Physiol. 25, 457–466. doi: 10.1093/treephys/25.4.457
Bowling, A. J., and Vaughn, K. C. (2009). Gelatinous fibers are widespread in coiling tendrils and twining vines. Am. J. Bot. 96, 719–727. doi: 10.3732/ajb.0800373
Caballé, G. (1986). Sur la biologie des lianes ligneuses en forêt gabonaise. Ph. D. thesis. Montpellier: Université des Sciences et Techniques du Languedoc.
Caballé, G. (1998). Le port autoportant des lianes tropicales : une synthèse des stratégies de croissance. Can. J. Bot. 76, 1703–1716.
Cabanillas, P. A., and Hurrell, J. A. (2012). Plantas trepadoras: tipo biologico y clasificacion. Cienc. Morfol. 14:95.
Cai, Z.-Q., Poorter, L., Han, Q., and Bongers, F. (2008). Effects of light and nutrients on seedlings of tropical Bauhinia lianas and trees. Tree Physiol. 28, 1277–1285. doi: 10.1093/treephys/28.8.1277
Campbell, M. J., Edwards, W., Magrach, A., Alamgir, M., Porolak, G., Mohandass, D., et al. (2018). Edge disturbance drives liana abundance increase and alteration of liana–host tree interactions in tropical forest fragments. Ecol. Evol. 8, 4237–4251. doi: 10.1002/ece3.3959
Care, A.-F., Nefed’ev, L., Bonnet, B., Millet, B., and Badot, P.-M. (1998). Cell elongation and revolving movement in Phaseolus vulgaris L. twining shoots. Plant Cell Physiol. 39, 914–921. doi: 10.1093/oxfordjournals.pcp.a029454
Carrasco-Urra, F., and Gianoli, E. (2009). Abundance of climbing plants in a southern temperate rain forest: host tree characteristics or light availability? J. Veg. Sci. 20, 1155–1162. doi: 10.1111/j.1654-1103.2009.01115.x
Chabbert, B., Monties, B., Rowe, N. P., and Speck, T. (1997). “Variability of lignin composition and lignification pattern in the lianescent and self-supporting growth phase of the liana Condylocarponn guianense,” in Plant Biomechanics: Conference Proceedings, Vol. 1, eds G. J. Jeronimidis and J. F. V. Vincent (Reading: Centre for Biomimetics, The University of Reading), 73–78.
Charnov, E. L. (1976). Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129–136. doi: 10.1016/0040-5809(76)90040-X
Chery, J. G., Glos, R. A. E., and Anderson, C. T. (2020). Do woody vines use gelatinous fibers to climb? New Phytol. 2020:17576. doi: 10.1111/nph.17576
Clair, B., Ghislain, B., Prunier, J., Lehnebach, R., Beauchêne, J., and Alméras, T. (2019). Mechanical contribution of secondary phloem to postural control in trees: the bark side of the force. New Phytol. 221, 209–217. doi: 10.1111/nph.15375
Coudurier, T. (1992). Sur la place des lianes dans la forêt guyanaise : une approche qui utilise l’architecture végétale. Ph. D. thesis. Montpellier: Université des Sciences et Techniques du Languedoc.
Ennos, R. (2011). Solid Biomechanics. Princeton: Princeton University Press, doi: 10.1515/9781400840649
Evert, R. F. (2006). Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development. Hoboken, NJ: John Wiley & Sons.
Ewers, F. W., Fisher, J. B., and Fichtner, K. (1991). “Water flux and xylem structure in vines,” in The Biology of Vines, eds F. E. Putz and H. A. Mooney (Cambridge: Cambridge University Press), 127–160.
Fiorello, I., Del Dottore, E., Tramacere, F., and Mazzolai, B. (2020). Taking inspiration from climbing plants: methodologies and benchmarks—a review. Bioinspir. Biomim. 15:031001. doi: 10.1088/1748-3190/ab7416
French, J. C. (1977). Growth relationships of leaves and internodes in viny angiosperms with different modes of attachment. Am. J. Bot. 64, 292–304. doi: 10.1002/j.1537-2197.1977.tb15730.x
Gallagher, R. V., and Leishman, M. R. (2012). A global analysis of trait variation and evolution in climbing plants. J. Biogeogr. 39, 1757–1771. doi: 10.1111/j.1365-2699.2012.02773.x
Gallenmüller, F., Bauer, G., Kubinski, K.-R., Voigt, D., Gorb, S., and Speck, T. (2009). Plant leaves as attachment devices: an experimental approach. Sixth Plant Biomech. Conf. Novemb. 16th 21st Cayenne Fr. Guyana Fr. 2009:194.
Gallenmüller, F., Rowe, N. P., and Speck, T. (2004). Development and growth form of the neotropical liana Croton nuntians: the effect of light and mode of attachment on the biomechanics of the stem. J. Plant Growth Regul. 23, 83–97. doi: 10.1007/s00344-004-0045-z
Gartner, B. L. (1991). Is the climbing habit of poison oak ecotypic? Funct. Ecol. 5, 696–704. doi: 10.2307/2389490
Gentry, A. G. (1991). “The distribution and evolution of climbing plants,” in The Biology of Vines, eds F. E. Putz and H. A. Mooney (Cambridge: Cambridge University Press), 3–42.
Gerbode, S. J., Puzey, J. R., McCormick, A. G., and Mahadevan, L. (2012). How the cucumber tendril coils and overwinds. Science 337, 1087–1091. doi: 10.1126/science.1223304
Gianoli, E. (2003). Phenotypic responses of the twining vine Ipomoea purpurea (Convolvulaceae) to physical support availability in sun and shade. Plant Ecol. 165, 21–26. doi: 10.1023/A:1021412030897
Gianoli, E. (2015). The behavioural ecology of climbing plants. AoB Plants 7:lv013. doi: 10.1093/aobpla/plv013
Givnish, T. J. (1995). “Plant Stems: biomechanical adaptation for energy capture and influence on species distributions,” in Plant Stems: Physiology and Functional Morphology Physiological Ecology, ed. B. L. Gartner (San Diego: Academic Press), 3–49. doi: 10.1016/B978-012276460-8/50003-5
Goriely, A., and Neukirch, S. (2006). Mechanics of climbing and attachment in twining plants. Phys. Rev. Lett. 97:184302. doi: 10.1103/PhysRevLett.97.184302
Guerra, S., Peressotti, A., Peressotti, F., Bulgheroni, M., Baccinelli, W., D’Amico, E., et al. (2019). Flexible control of movement in plants. Sci. Rep. 9:16570. doi: 10.1038/s41598-019-53118-0
Hegarty, E. E. (1991). “Vine-host interactions,” in The Biology of Vines, eds F. E. Putz and H. A. Mooney (Cambridge: Cambridge University Press), 357–375.
Hoffmann, B., Chabbert, B., Monties, B., and Speck, T. (2003). Mechanical, chemical and X-ray analysis of wood in the two tropical lianas Bauhinia guianensis and Condylocarpon guianense: variations during ontogeny. Planta 217, 32–40. doi: 10.1007/s00425-002-0967-2
Isnard, S., and Rowe, N. P. (2008). Mechanical role of the leaf sheath in rattans. New Phytol. 177, 643–652. doi: 10.1111/j.1469-8137.2007.02308.x
Isnard, S., Speck, T., and Rowe, N. P. (2003). Mechanical architecture and development in Clematis: implications for canalised evolution of growth forms. New Phytol. 158, 543–559. doi: 10.1046/j.1469-8137.2003.00771.x
Isnard, S., Speck, T., and Rowe, N. P. (2005). Biomechanics and development of the climbing habit in two species of the South American palm genus Desmoncus (Arecaceae). Am. J. Bot. 92, 1444–1456. doi: 10.3732/ajb.92.9.1444
Katabuchi, M. (2015). LeafArea: an R package for rapid digital image analysis of leaf area. Ecol. Res. 30, 1073–1077. doi: 10.1007/s11284-015-1307-x
Lahaye, R., Civeyrel, L., Speck, T., and Rowe, N. P. (2005). Evolution of shrub-like growth forms in the lianoid subfamily Secamonoideae (Apocynaceae s.l.) of Madagascar: phylogeny, biomechanics, and development. Am. J. Bot. 92, 1381–1396. doi: 10.3732/ajb.92.8.1381
Laurance, W. F., Andrade, A. S., Magrach, A., Camargo, J. L. C., Valsko, J. J., Campbell, M., et al. (2014). Long-term changes in liana abundance and forest dynamics in undisturbed Amazonian forests. Ecology 95, 1604–1611. doi: 10.1890/13-1571.1
Lauri, P. -É (1988). Le mouvement morphogénétique : approche morphométrique et restitution graphique. L’exemple de quelques plantes tropicales. Ph. D. thesis. Montpellier: Université de Montpellier, 565.
Lauri, P. -É, and Kelner, J.-J. (2001). Shoot type demography and dry matter partitioning: a morphometric approach in apple (Malus × domestica). Can. J. Bot. 79, 1270–1273. doi: 10.1139/b01-113
Ledo, A., and Schnitzer, S. A. (2014). Disturbance and clonal reproduction determine liana distribution and maintain liana diversity in a tropical forest. Ecology 95, 2169–2178. doi: 10.1890/13-1775.1
Lehnebach, R., Alméras, T., and Clair, B. (2020). How does bark contribution to postural control change during tree ontogeny? A study of six Amazonian tree species. J. Exp. Bot. 71, 2641–2649. doi: 10.1093/jxb/eraa070
Leroux, O. (2012). Collenchyma: a versatile mechanical tissue with dynamic cell walls. Ann. Bot. 110, 1083–1098. doi: 10.1093/aob/mcs186
Letcher, S. G. (2015). Patterns of liana succession in tropical forests. Ecol. Lianas 2015, 116–130. doi: 10.1002/9781118392409.ch10
Letcher, S. G., and Chazdon, R. L. (2012). Life history traits of lianas during tropical forest succession. Biotropica 44, 720–727. doi: 10.1111/j.1744-7429.2012.00865.x
Levionnois, S., Coste, S., Nicolini, E., Stahl, C., Morel, H., and Heuret, P. (2020). Scaling of petiole anatomies, mechanics and vasculatures with leaf size in the widespread Neotropical pioneer tree species Cecropia obtusa Trécul (Urticaceae). Tree Physiol. 40, 245–258. doi: 10.1093/treephys/tpz136
Levionnois, S., Salmon, C., Alméras, T., Clair, B., Ziegler, C., Coste, S., et al. (2021). Anatomies, vascular architectures, and mechanics underlying the leaf size-stem size spectrum in 42 Neotropical tree species. J. Exp. Bot. 72, 7957–7969. doi: 10.1093/jxb/erab379
Mahley, J. N., Pittermann, J., Rowe, N. P., Baer, A., Watkins, J. E., Schuettpelz, E., et al. (2018). Geometry, allometry and biomechanics of fern leaf petioles: their significance for the evolution of functional and ecological diversity within the pteridaceae. Front. Plant Sci. 9:197. doi: 10.3389/fpls.2018.00197
Meloche, C. G., Knox, J. P., and Vaughn, K. C. (2006). A cortical band of gelatinous fibers causes the coiling of redvine tendrils: a model based upon cytochemical and immunocytochemical studies. Planta 225, 485–498. doi: 10.1007/s00425-006-0363-4
Melzer, B., Steinbrecher, T., Seidel, R., Kraft, O., Schwaiger, R., and Speck, T. (2010). The attachment strategy of English ivy: a complex mechanism acting on several hierarchical levels. J. R. Soc. Interface 7, 1383–1389. doi: 10.1098/rsif.2010.0140
Ménard, L., McKey, D., and Rowe, N. P. (2009). Developmental plasticity and biomechanics of treelets and lianas in Manihot aff. quinquepartita (Euphorbiaceae): a branch-angle climber of French Guiana. Ann. Bot. 103, 1249–1259. doi: 10.1093/aob/mcp078
Ménard, L., McKey, D., Mühlen, G. S., Clair, B., and Rowe, N. P. (2013). The evolutionary fate of phenotypic plasticity and functional traits under domestication in manioc: changes in stem biomechanics and the appearance of stem brittleness. PLoS One 8:e74727. doi: 10.1371/journal.pone.0074727
Menninger, E. A. (1970). Flowering vines of the world: an encyclopedia of climbing plants. Downers Grove, IL: Hearthside Press.
Millet, B., Melin, D., and Badot, P.-M. (1988). Circumnutation in Phaseolus vulgaris. I. Growth, osmotic potential and cell ultrastructure in the free-moving part of the shoot. Physiol. Plant. 72, 133–138. doi: 10.1111/j.1399-3054.1988.tb06634.x
Milwich, M., Speck, T., Speck, O., Stegmaier, T., and Planck, H. (2006). Biomimetics and technical textiles: solving engineering problems with the help of nature’s wisdom. Am. J. Bot. 93, 1455–1465. doi: 10.3732/ajb.93.10.1455
Mori, H., Ueno, S., Matsumoto, A., Kamijo, T., Tsumura, Y., and Masaki, T. (2018). Large contribution of clonal reproduction to the distribution of deciduous liana species (Wisteria floribunda) in an old-growth cool temperate forest: evidence from genetic analysis. Ann. Bot. 121, 359–365. doi: 10.1093/aob/mcx153
Moulia, B., Coutand, C., and Lenne, C. (2006). Posture control and skeletal mechanical acclimation in terrestrial plants: implications for mechanical modeling of plant architecture. Am. J. Bot. 93, 1477–1489. doi: 10.3732/ajb.93.10.1477
Nabe-Nielsen, J., and Hall, P. (2002). Environmentally induced clonal reproduction and life history traits of the liana Machaerium cuspidatum in an Amazonian rain forest, Ecuador. Plant Ecol. 12, 215–226.
Niklas, K. J. (1999b). “The mechanical stability of vertical stems,” in The evolution of plant architecture, eds M. H. Kurmann and A. R. Hemsley (Kew: Royal Botanic Gardens), 377–397.
Olson, M. E., Rosell, J. A., Muñoz, S. Z., and Castorena, M. (2018). Carbon limitation, stem growth rate and the biomechanical cause of Corner’s rules. Ann. Bot. 122, 583–592. doi: 10.1093/aob/mcy089
Peñalosa, J. (1982). Morphological specialization and attachment success in two twining lianas. Am. J. Bot. 69, 1043–1045. doi: 10.1002/j.1537-2197.1982.tb13348.x
Peñalosa, J. (1984). Basal branching and vegetative spread in two tropical rain forest lianas. Biotropica 16:1. doi: 10.2307/2387886
Pittermann, J., and Olson, M. E. (2018). Transport efficiency and cavitation resistance in developing shoots: a risk worth taking. Tree Physiol. 38, 1085–1087. doi: 10.1093/treephys/tpy094
Plavcová, L., and Jansen, S. (2015). “The role of xylem parenchyma in the storage and utilization of nonstructural carbohydrates,” in Functional and Ecological Xylem Anatomy, ed. U. Hacke (Cham: Springer International Publishing), 209–234. doi: 10.1007/978-3-319-15783-2_8
Putz, F. E. (1984). The natural history of lianas on Barro Colorado Island, Panama. Ecology 65, 1713–1724. doi: 10.2307/1937767
Putz, F. E., and Holbrook, N. M. (1991). “Biomechanical studies of vines,” in The Biology of Vines, eds F. E. Putz and H. A. Mooney (Cambridge: Cambridge University Press), 73–97.
Pyke, G. H. (1984). Optimal foraging theory: a critical review. Annu. Rev. Ecol. Syst. 15, 523–575. doi: 10.1146/annurev.es.15.110184.002515
Read, J., and Stokes, A. (2006). Plant biomechanics in an ecological context. Am. J. Bot. 93, 1546–1565. doi: 10.3732/ajb.93.10.1546
Rivière, M., Derr, J., and Douady, S. (2017). Motions of leaves and stems, from growth to potential use. Phys. Biol. 14:051001. doi: 10.1088/1478-3975/aa5945
Rosell, J. A., and Olson, M. E. (2014). The evolution of bark mechanics and storage across habitats in a clade of tropical trees. Am. J. Bot. 101, 764–777. doi: 10.3732/ajb.1400109
Rowe, N. P., and Speck, T. (1996). Biomechanical characteristics of the ontogeny and growth habit of the tropical liana Condylocarpon guianense (Apocynaceae). Int. J. Plant Sci. 157, 406–417. doi: 10.1086/297357
Rowe, N. P., Isnard, S., and Speck, T. (2006). “Diversity of mechanical architectures in climbing plants: an ecological perspective,” in Ecology and biomechanics: a mechanical approach to the ecology of animals and plants, eds A. Herrel, T. Speck, and N. P. Rowe (Boca Raton, FL: CRC Press), 35–59. doi: 10.1111/j.1469-8137.2004.01309.x
Rowe, N., Isnard, S., and Speck, T. (2004). Diversity of mechanical architectures in climbing plants: an evolutionary perspective. J. Plant Growth Regul. 23, 108–128. doi: 10.1007/s00344-004-0044-0
Schenck, H. (1892). Beiträge zur Biologie und Anatomie der Lianen im besonderen der in Brasilien einheimischen Arten. Frankfurt am Main: G. Fischer.
Simonetti, V., Bulgheroni, M., Guerra, S., Peressotti, A., Peressotti, F., Baccinelli, W., et al. (2021). Can plants move like animals? A three-dimensional stereovision analysis of movement in plants. Animals 11:1854. doi: 10.3390/ani11071854
Soffiatti, P., and Rowe, N. P. (2020). Mechanical innovations of a climbing cactus: functional insights for a new generation of growing robots. Front. Robot. AI 7:64. doi: 10.3389/frobt.2020.00064
Sousa-Baena, M. S., Hernandes-Lopes, J., and Van Sluys, M.-A. (2021). Reaching the top through a tortuous path: helical growth in climbing plants. Curr. Opin. Plant Biol. 59:101982. doi: 10.1016/j.pbi.2020.101982
Sousa-Baena, M. S., Sinha, N. R., Hernandes-Lopes, J., and Lohmann, L. G. (2018). Convergent evolution and the diverse ontogenetic origins of tendrils in angiosperms. Front. Plant Sci. 9:403. doi: 10.3389/fpls.2018.00403
Speck, T. (1991). “Changes of the bending mechanics of lianas and self-supporting taxa during ontogeny”, in: Natural structures. principles, Strategies and models in Architecture and Nature, Proceedings of the II International Symposium Sonderforschungsbereich 230, part I. Mitteilungen des SFB 230 Heft 6, 89–95.
Speck, T., and Rowe, N. P. (1999). “A quantitative approach for analytically defining size, growth form and habit in living and fossil plants,” in The evolution of plant architecture, eds M. H. Kurmann and A. R. Hemsley (Kew: Royal Botanic Gardens), 447–479.
Steinbrecher, T., Danninger, E., Harder, D., Speck, T., Kraft, O., and Schwaiger, R. (2010). Quantifying the attachment strength of climbing plants: A new approach. Acta Biomater. 6, 1497–1504. doi: 10.1016/j.actbio.2009.10.003
Stolarz, M. (2009). Circumnutation as a visible plant action and reaction: physiological, cellular and molecular basis for circumnutations. Plant Signal. Behav. 4, 380–387. doi: 10.4161/psb.4.5.8293
Walker, I. D. (2015). Biologically inspired vine-like and tendril-like robots. SAI 2015, 714–720. doi: 10.1109/SAI.2015.7237221
Warton, D. I., Duursma, R. A., Falster, D. S., and Taskinen, S. (2012). smatr 3– an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 3, 257–259. doi: 10.1111/j.2041-210X.2011.00153.x
Wooten, M., and Walker, I. (2018). Vine-inspired continuum tendril robots and circumnutations. Robotics 7:58. doi: 10.3390/robotics7030058
Wooten, M., Frazelle, C., Walker, I. D., Kapadia, A., and Lee, J. H. (2018). Exploration and inspection with vine-inspired continuum robots. ICRA 2018, 5526–5533. doi: 10.1109/ICRA.2018.8461132
Keywords: biomechanics, climbing plants, liana, searcher shoot, self-supporting anatomy, support foraging, three-dimensional space
Citation: Hattermann T, Petit-Bagnard L, Heinz C, Heuret P and Rowe NP (2022) Mind the Gap: Reach and Mechanical Diversity of Searcher Shoots in Climbing Plants. Front. For. Glob. Change 5:836247. doi: 10.3389/ffgc.2022.836247
Received: 15 December 2021; Accepted: 21 February 2022;
Published: 05 April 2022.
Edited by:
Félicien Meunier, Ghent University, BelgiumReviewed by:
Hao Ran Lai, University of Canterbury, New ZealandCopyright © 2022 Hattermann, Petit-Bagnard, Heinz, Heuret and Rowe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tom Hattermann, dG9tLmhhdHRlcm1hbm5AZ21haWwuY29t
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.