- 1Chair of Silviculture, Institute of Silviculture and Forest Protection, TU Dresden, Tharandt, Germany
- 2Internal Audit, Operations Management, HessenForst, Kassel, Germany
About 100 years ago, groups of European beech were reintroduced into Norway spruce stands in some parts of Germany as a restoration approach. The aim of this approach was to maintain or rehabilitate the ecological function of soil fertility and the regeneration option of beech for the next generation. The effect of beech groups on surrounding spruce stands was studied in the Ore Mountains by recording natural regeneration of beech and humus layer thickness and form. Point pattern statistics were used to analyse the spread of beech regeneration and to determine factors influencing its establishment. It was found that the density of regeneration decreases with increasing distance from the beech group. However, beech regeneration was found up to distances of 69 m. Furthermore, it becomes evident that PAR radiation (maximum regeneration densities at PAR values of 35 W/m2) and fencing (3.41 times higher regeneration density compared to unfenced areas) against deer have a positive influence on beech regeneration density. Ordered categorical models were used to model humus form and non-linear models were used to model humus layer thickness. It could be proven that the most bioactive humus forms and lowest humus layer thicknesses were found within the beech group. With increasing distance to the beech group, the total humus layer thickness and the proportion of mormoder in the spruce stand increased. The positive influence of the beech group on the humus composition extends to about 40 m from the centre of the beech group. Due to the former arrangement of the beech groups in the terrain, the effects can also extend to the spruce stands in between. The hypotheses on the restoration approach of reintroducing groups of beech into spruce stands formulated by foresters 100 years ago can thus be confirmed. For future restoration approaches of spruce stands, groupwise mixtures of beech should be established with a distance of 40–50 m.
Introduction
Forests are of great importance for multiple ecosystem services such as provisioning services, cultural services, regulating and maintaining services (Haines-Young and Potschin, 2011). Within the 18th and 19th centuries, exploitive harvesting, the removal of litter and grazing led to severe deforestation and degradation in Germany (Schmidt-Vogt, 1987; Hasel and Schwartz, 2006). These forests could only very inadequately fulfil the full range of ecosystem services. At the same time, the demand for wood increased rapidly due to the beginning of industrialisation (Kandler, 1992; Hasel and Schwartz, 2006). However, in order to satisfy the great demand for timber, wood production was unilaterally promoted through extensive reforestation with fast-growing conifers (Schmidt-Vogt, 1991). As a result, European beech (Fagus sylvatica L.) was largely replaced by secondary Norway spruce (Picea abies [L.] Karst) or Scots pine (Pinus sylvestris L.) forests (Johann et al., 2004; Zerbe, 2019). The main reasons for the strong decline of beech in the 19th century were the change from selective harvesting to clear-cutting, the easier artificial regeneration of coniferous trees on clear-cuts, the difficult natural regeneration of beech due to frost, soil compaction and deer browsing, as well as higher economic yields of coniferous stands.
Although the large, even-aged pure coniferous stands initially produced high yields, they were also the basis and starting point for widespread calamities caused by storms or insect pests (de Groot et al., 2019). Massive and increasingly frequent large-scale damage events caused foresters and scientists alike to think about alternative silvicultural methods (Weidenbach, 1895; Gayer, 1897; Wagner, 1905; Martin, 1919; Rebel, 1922; Graser, 1935). In addition, numerous studies showed the degradation of topsoils under spruce and pine, which becomes evident in topsoil acidification, accumulation of humus and loss of available fractions of some nutrient elements (Krauss et al., 1939; Worrell and Hampson, 1997). As a result of soil degradation, declines in spruce growth were observed after repeated rotations (Wiedemann, 1923).
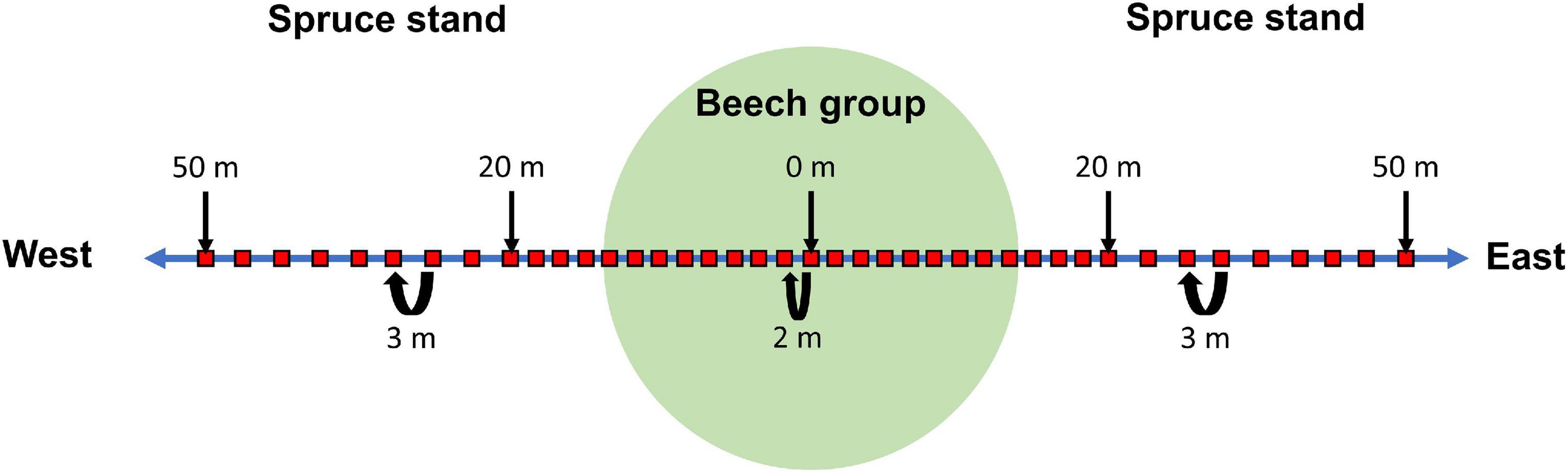
In order to combat further forest degradation, ecosystem restoration was necessary. However, this requires innovative rehabilitation approaches that encompass ecological, social and economic aspects (Stanturf, 2016). Appropriate rehabilitation approaches were already field tested by some German foresters in the 1920s in the Ore mountains (Clemens, 1930; Graser, 1935). The objectives of the concepts were the preservation of site sustainability, an increase in the resilience of spruce stands to abiotic and biotic damage, the enhancement of forest aesthetics, the creation of natural regeneration options, an improvement of the water regime as well as the protection of cultural values (Weidenbach, 1895; Gayer, 1897; Hübsch, 1898; Ranfft, 1913; Bernhard, 1922; Clemens, 1930; Graser, 1935). However, it was not the objective to produce high quality wood within the beech groups, as plant density and aggregate sizes were too small (Tiebel et al., 2016; Weidig and Wagner, 2021). The rehabilitation approaches in the pure spruce stands were realised by the following silvicultural techniques: Beech were planted in circular groups of 10 to 25 m diameter in the spruce stands (Graser, 1916, 1935; Clemens, 1930, 1931). The distance of 40-50 m between the beech groups should take into account the spread of beech litter as well as the spread of beech natural regeneration (Rebel, 1922; Clemens, 1930). The number of beech groups per hectare is given in the literature studied as between 5 and 7, thus an admixture proportion of 10 to 30 % should develop.
Today, after almost 100 years, we take the opportunity to evaluate the success of this restoration approach. For this purpose, the following hypotheses formulated by Clemens (1930) and Graser (1935) were to be examined: (1) By planting beech groups in spruce stands, initials for the natural regeneration of beech will serve to create mixed spruce and beech stands in the following generation. It is hypothesised that beech will disperse and establish in the neighbouring spruce stand. The chosen distances between the beech groups take into account the dispersal distance of beech. (2) Planting groups of beech in spruce stands is intended to preserve soil fertility or to rehabilitate soil degradation that has already occurred. It is hypothesised that beech litter has a positive influence on the humus composition of the site. The humus layer thickness increases with increasing distance to the beech trees. At the same time, humus quality decreases with increasing distance to the beeches.
Materials and Methods
Study Area
The main area, where small-scale restoration of beech at the beginning of the 20th century took place, was in the Ore mountains in Saxony, Germany (Ranfft, 1913; Graser, 1916; Bernhard, 1922; Clemens, 1930). Therefore, the study was conducted on six sites containing beech groups and surrounding spruce stand: Two beech groups were used to survey the natural regeneration. Additionally, four beech groups were selected to record the humus composition (Table 1). Based on representative stand and site characteristics, three sites are located in the central Ore Mountains in Zöblitz (50°41’09.0′N and 13°13’14.4′E). The other three sites are located in the eastern upper Ore Mountains in Holzhau (50°45’14.7′N and 13°35’02.3′E). The altitude in Zöblitz is 545 m while Holzhau is at 749 m. The altitude influences the average annual temperature and rainfall within the Ore Mountains (Gauer and Kroiher, 2012). The mean annual precipitation ranges from 950 mm in Zöblitz to 1100 mm in Holzhau. The annual average temperature varies between 8°C in Zöblitz and 6.5°C in Holzhau (Spekat et al., 2020). All sites were selected so that their sites had similar characteristics in terms of nutrient content and water supply. The dominant soil types of the sites are Cambisols with medium nutrient content and an average water supply. The selection of the humus sites was based on the fact that there was not too much natural regeneration, which itself already has a litterfall. In the selection process, importance was also attached to intact spruce stands with a closed canopy. Although Luzulo-Fagetum (high-montane) are the dominant potential natural vegetation types, even-aged pure spruce stands dominate the study area.
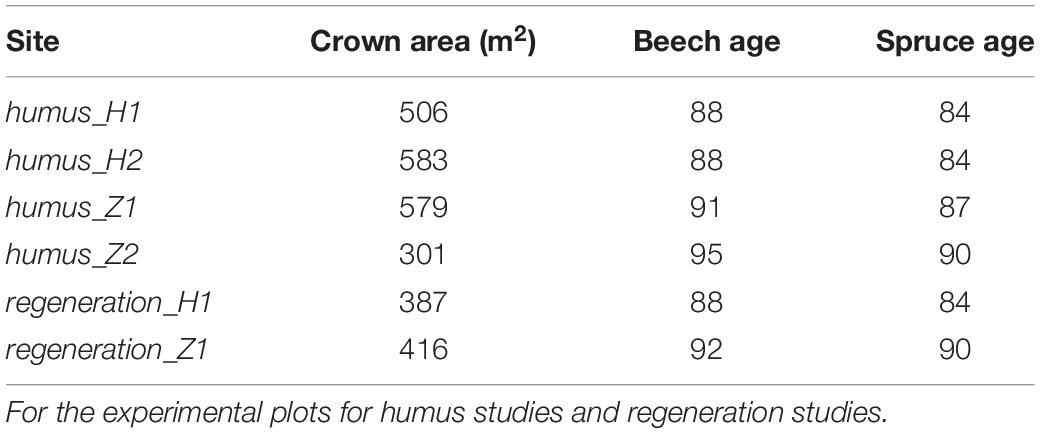
Table 1. Characteristics of the experimental plots with regard to size of the beech group, age of the beech and age of the surrounding spruce stand.
The age of the beech groups, the crown area of the beech group and the surrounding spruce stand age are characterised in Table 1. The distribution of the dbh of bech seed trees on the regeneration plots is given in Supplementary Figure 1. Through Table 1, the technique of restoration becomes clear: 2–3-year-old nursery beech trees are planted in existing or artificially created gaps with a density of approx. 10,000 plants per ha. With some age advance of the beech planted, the remaining spruce in the overstorey were harvested and spruce was naturally regenerated or planted.
Experimental Design – Beech Natural Regeneration
In order to determine the dispersal of natural beech regeneration into the surrounding spruce stand, two experimental plots were surveyed and the position of beech seed trees and beech regeneration, i.e., beech seedlings and saplings (< 7 cm dbh), were mapped (regeneration_H1 and regeneration_Z1). For this purpose, it is necessary to measure the coordinates of the beech seed trees as well as the beech natural regeneration. The experimental plot was designed with a size of 100 × 100 m in Holzhau and 60 × 80 m in Zöblitz (Figure 1). The coordinates are defined relatively to lower left corner. The exact coordinates were measured by compass and laser distance measurement with the Haglöfs® Vertex IV.
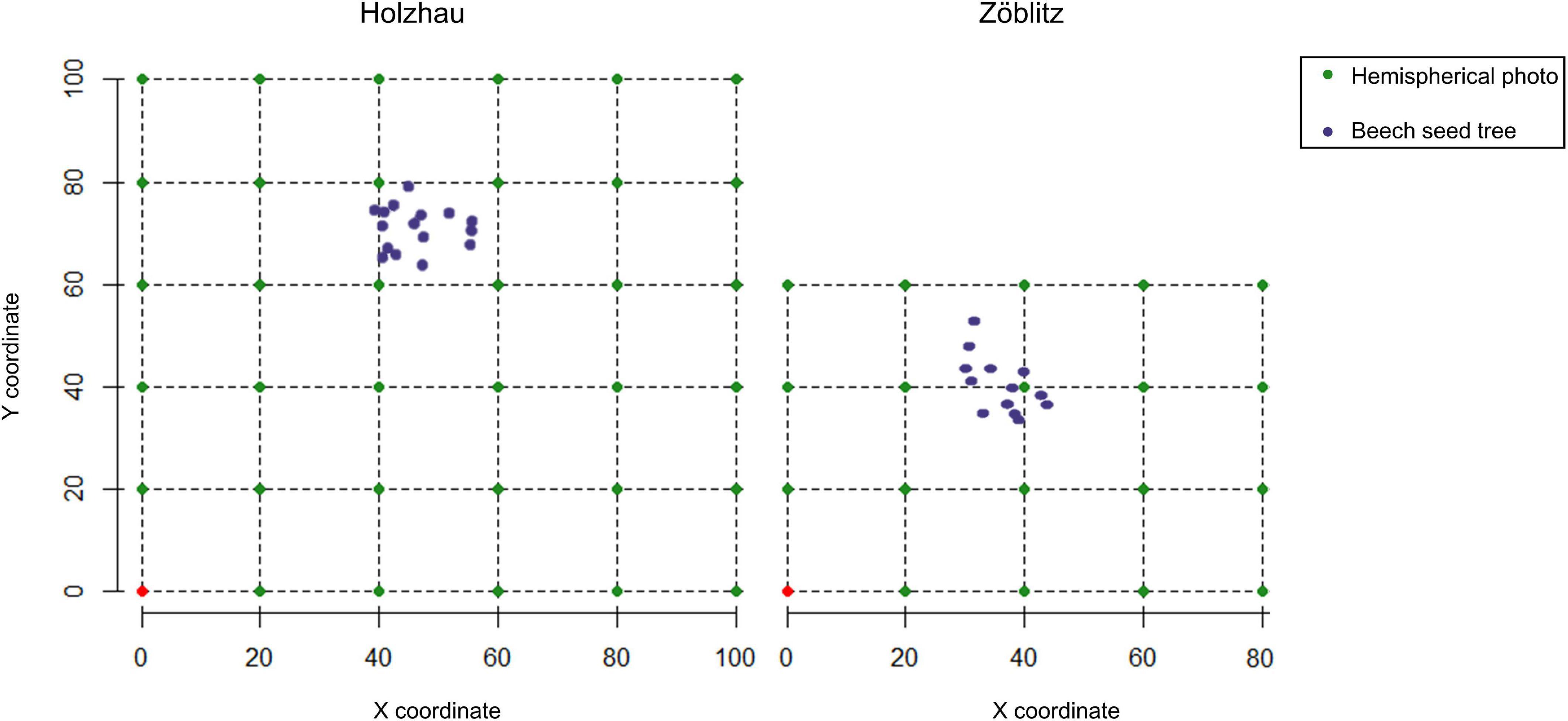
Figure 1. Experimental design for the recording of the position of beech seed trees and beech natural regeneration. The position of the radiation measurements and beech seed trees is illustrated.
In order to determine the dispersal distances of beech regeneration from beech seed trees, the distance to the nearest beech seed tree was calculated for the study area. The covariate distance (D) was created using the distmap function from the package spatstat. The distance map of the beech seed trees is a pixel image in which the value of the pixel u represents the shortest distance to the beech seed trees (Baddeley et al., 2015) (Supplementary Figure 2).
Radiation is of great importance for the success of regeneration and an essential variable for the establishment of beech regeneration. To assess the radiation situation on the sites, hemispherical photos were taken at the previously marked, measured corner points of the grid cells (Figure 1) using a Nikon® Coolpix 995 with a Nikon NIKKOR® 180° fisheye lens with 8 mm focal length. The photos were taken under standard overcast sky conditions. Immediately before taking the picture, an exposure measurement was carried out with a LUNASIX® 3S to determine the zenith luminance in order to derive the optimum exposure as a combination of aperture number and shutter speed. The adjusted exposure has great importance for accurate radiation estimation from the hemispherical images (Wagner, 1998; Beckschäfer et al., 2013). The photography was followed by automatic pixel segmentation of the hemispherical photos. Using the segmented images, the solar radiation was estimated by using the programme developed by Schwalbe et al. (2009), which incorporates radiation and solar trajectory models. The photosynthetically active radiation (PAR), which consists of diffuse and direct radiation, was derived. In addition, the percentage of diffuse radiation compared to open field conditions was derived (DIFFSF).
In order to obtain radiation values for the entire area, a kernel-smoothed spatial interpolation was carried out from the radiation measurements at the grid points (Baddeley et al., 2016). The Smooth function is used to smooth spatial data using Gaussian kernel and was used to generate radiation maps for PAR and DIFFSF (Supplementary Figure 2). The bandwith to control the kernel smoothing was selected by least-squares cross-validation using bw.smoothppp function.
In addition, parts of the experimental plot in Zöblitz were fenced against browsing by game. The coordinates of the fence were recorded and a factorial image of the categorical variable ‘unfenced‘ and ‘fenced‘ was created (Supplementary Figure 2).
Experimental Design – Humus Composition
In order to evaluate the influence of the distance to the beech group on the humus form and humus layer thickness, four 100 m long line transects were created. The beech group was located in the middle of the transect (Figure 2). The orientation of the transect was east-west, as the main wind direction is west (Bernhofer et al., 2008). When creating the transects, care was taken to ensure that neighbouring beech groups had no influence on the humus composition on the transect. For humus studies, a sufficient number of sampling points is required for the experimental set-up due to the spatial variability of humus properties (Ilvesniemi, 1991; Liski, 1995; Garten et al., 2007). Therefore, a stratified arrangement of the sample points on the transect was planned: From the centre point of the beech group up to a distance of 20 m, humus measurements was carried out at 2 m intervals. From 20 m up to a distance of 50 m, a humus measurement was carried out every 3 m. According to this, 41 humus measurements were carried out per line transect. The recordings were made in September before the new leaf fall.
Measurements of the humus composition were carried out on the plots according to the German guidance for soil surveying and mapping (Eckelmann et al., 2005). On an 18 cm humus cuboid, the different horizons were identified, and their thickness measured. A distinction is made between the L horizon, the Of and Oh horizon and the Ah horizon. The litter layer (L horizon) is characterised by undecomposed and poorly decomposed plant residues (Scheffer et al., 2016). The organic horizon (O horizon) consists of highly decomposed plant remains. Depending on the proportion of organic fine matter, a distinction is made between Of and Oh horizons. The Oh horizon has a proportion of fine material > 70 vol-%. The Of horizon consists of fermented plant residues and has fine material between 10 and 70 vol-% (Eckelmann et al., 2005). The Ah horizon is characterised as a mineral soil horizon of a high humus content.
After the measurement, the terrestrial humus forms are identified corresponding to Blume et al. (2011). According to the presence of the different humus horizons, their thickness, their type of storage and their transitions, a distinction is made between:
- mullmoder
- moder poor in fine organic material (mopfom)
- moder rich in fine organic material (morfom)
- mormoder
- mor
Point Process Statistics for Beech Regeneration
The spatial distribution of beech natural regeneration is the result of numerous ecological processes such as seed dispersal, storage, germination and establishment (Fischer et al., 2016). A point process is a random mechanism whose result is a point pattern (Baddeley et al., 2016). The aim of point pattern statistics is to analyse spatial structures of points (Illian et al., 2008) and to infer underlying ecological processes. Each point (natural regeneration and seed tree) is defined by its Cartesian coordinates (Baddeley et al., 2016) (Figure 1 and Supplementary Figure 3).
Point process models can be used to test hypotheses about the spatial distribution of observed point patterns. The response variable beech regeneration density consists of replicated point patterns i on both experimental plots (regeneration_H1 and regeneration_Z1) whose intensity is defined as a function of various covariates. Distance to the nearest beech seed tree, radiation and fence against browsing were included as covariates that can explain the variability in the point pattern (Supplementary Figure 2). Our inhomogeneous Poisson process model for multiple point patterns was then fitted simultaneously using the function mppm with R SPATSTAT (Baddeley et al., 2016) to both point pattern i using the following formula
where β0, β1, β2, β3 and β4 are coefficients to be estimated, and D(u)i, PAR(u)i, DIFFSF(u)i and Fence(u)i are the values of the spatial covariates at location u for the ith point pattern (Baddeley, 2015). For inhomogeneous Poisson processes, the beech regeneration density is described as point process intensity λ(u) of the spatial position u (Wiegand and Moloney, 2014; Baddeley et al., 2016). The inhomogeneous Poisson point process model was gradually adjusted by adding further covariates beginning with a minimum model (Baddeley et al., 2016) to verify the hypotheses on the effect and strength of covariates on the density of beech regeneration. Using the function anova.mppm the significance (p < 0.05) of the effect of each predictor was estimated using likelihood ratio tests until the best model was found (Baddeley, 2015; Baddeley et al., 2016).
Ordered Categorical Logistic Regression for Humus Forms
The humus form was classified from the horizon characteristics (Blume et al., 2011). It represents a response variable that falls in an ordered finite set of categories. Accordingly, ordinal-multinomial data are given (Venables and Ripley, 2002; Faraway, 2006). A generalised regression model with logit link for ordered categorical data (Christensen, 2019) was used to model humus form as a function of sample site distance from the center of the beech group, taking into account the group as a random variable.
The logit link function is applied and a parameter is estimated that is an expression of the probability that quality takes on a higher rather than a lower category when the explanatory variables change by one unit (Moutinho and Hutcheson, 2011).
with i = 1,….n, j = 1,…. J-1
This is a model for the cumulative probability of the ith humus form assessment falling in the jth category or below, where i index all observations (n = 161), j = 1,…, J index the response categories (J = 4) and {θj}is the intercept or threshold, i.e, cut-point, for the jth cumulative logit: logit(P(Yi ≤ j)).
We take the group effects to be random, and assume that the group effects are two dimensional normal:
The function clmm2 from the package ordinal was used for the generalised regression model with logit link for ordered categorical data (Christensen, 2019).
Non-linear Regression for Humus Thickness
We investigated the effect of distance to the beech group on the thickness of the Of plus Oh-layer by a non-linear mixed model approach. The self-starting function SSasymp from the package nlme was used for the asymptotic regression (Pinheiro and Bates, 2000). We used standard techniques, taking beech group as a random variable. However, when establishing confidence bands, we followed (Gsteiger et al., 2011).
Simulation of Regeneration Density and Humus Layer Thickness
In a final step, a simulation of the spread of natural beech regeneration as well as the change in humus layer thickness depending on the distance to the beech groups were calculated. The location of beech groups in a forest district was determined using false-colour infrared aerial photographs provided by GeoSN (2021). This was used to calculate dispersal distances to the nearest beech groups and to predict the overall model.
Results
Modelling Beech Natural Regeneration Density
In Holzhau, 16 beech seed trees and 816 naturally regenerated beech saplings were found. On the experimental plot in Zöblitz, 2820 saplings and 13 beech seed trees were recorded (Figure 1 and Supplementary Figures 3, 4). This results in an average regeneration density of 816 plants/ha in Holzhau and 6,520 plants/ha in Zöblitz. Based on the point pattern (Supplementary Figure 3) and on preliminary investigations, it was evident that the density of regeneration varies strongly spatially within the experimental plot. The inhomogeneous replicated Poisson point process model incorporates the inhomogeneity of the point pattern. The results of the model which were used to test the influence of different covariates on density can be seen in Figure 3 and Table 2.
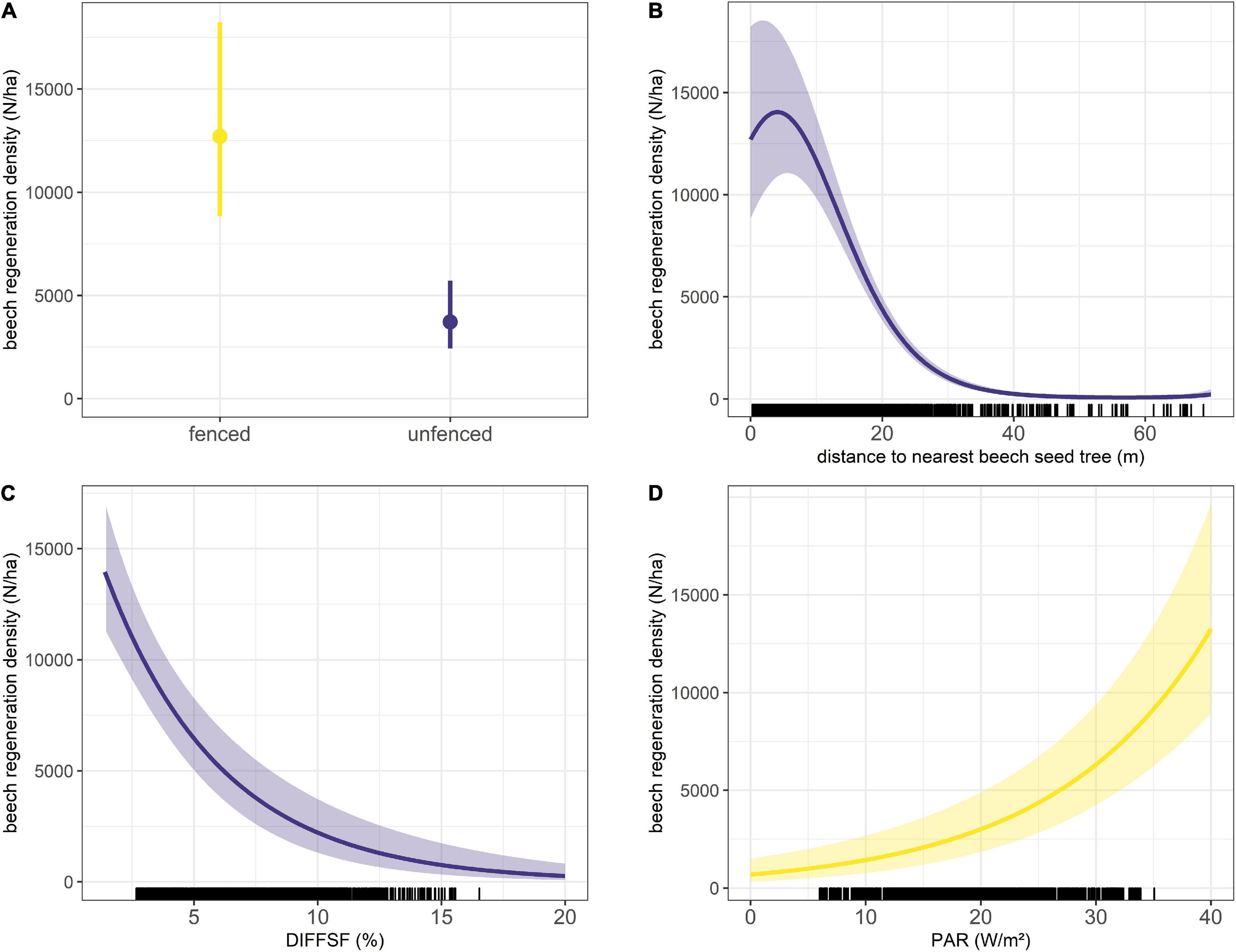
Figure 3. Effect of significant covariates on beech natural regeneration density: (A) influence of the fence, (B) influence of the distance to the nearest beech seed tree, (C) influence of the proportion of diffuse radiation and (D) influence of photosynthetically active radiation. The transparent polygons for the continuous covariates and the error bars for the categorical covariates show the 95% confidence interval. All radiation variables were set to their mean; the factor fence was set on “fenced.” The distance to nearest seed tree was set 0. The figure also includes the representation of the raw data, i.e., beech regeneration that has been recorded at different distances and radiation levels.
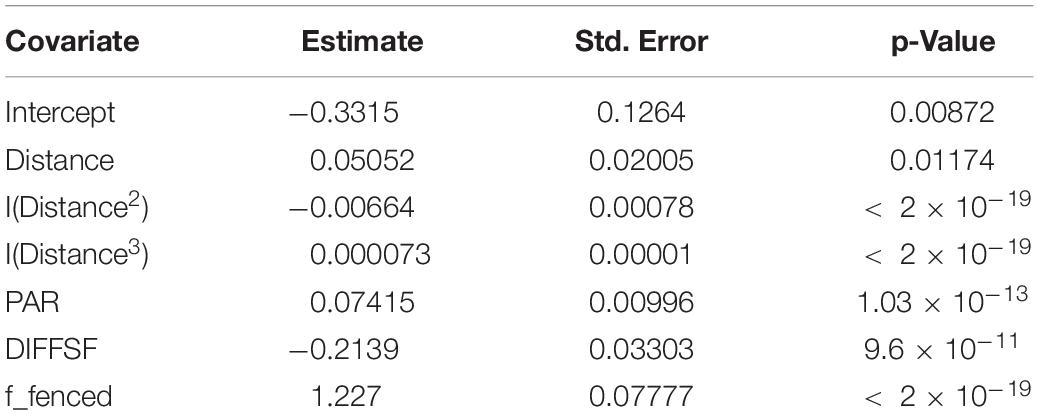
Table 2. Results of the inhomogeneous replicated Poisson point process model with coefficients, standard errors, and p-values.
It is obvious that the model predicts a 3.41 times higher regeneration density in areas that are fenced against browsing by deer (Figure 3 and Table 2). Furthermore, the model shows that the distance to the nearest beech seed tree has a significant influence on beech regeneration density. As the distance to the nearest beech seed tree increases, the regeneration density decreases strongly and approaches zero at 60 m. The highest regeneration densities of 14,000 per hectare are predicted near the beech seed trees (Figure 3). Opposing effects can be observed with the two radiation variables. While a negative effect is predicted for diffuse radiation, PAR radiation shows a positive influence (Table 2). With increasing PAR radiation, the regeneration density increases.
Modelling of the Humus Form Along the Transect
Modelling the humus form with an ordered categorical model revealed significant relationships between the distance to the centre of the beech group and the proportion of humus forms (Figure 4 and Table 3). Within the beech group, mullmoder and moder poor in fine organic material (mopfom) forms are predicted most frequently. At 50 m, the predicted proportion of mullmoder decreases to 10 %. Within the beech group, almost no mormoder forms are predicted. With increasing distance to the beech group, the proportion of mormoder increases to 10 % at a distance of 50 m. In parallel, the proportion of moder rich in fine organic material (morfom) also increases and reaches proportions of 15 % in the surrounding spruce stand.
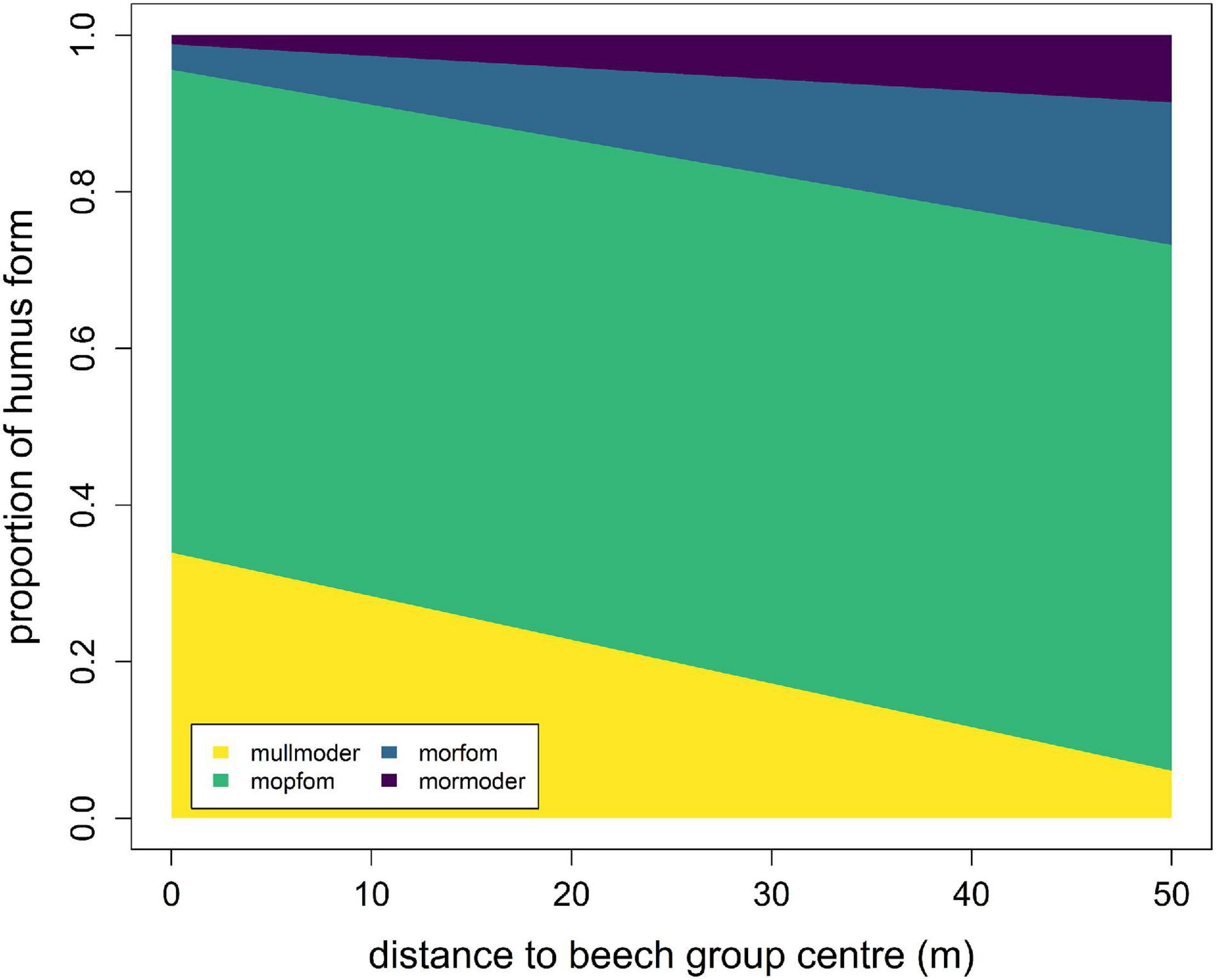
Figure 4. Results of the ordered categorical model for modelling humus form as a function of distance from the centre of the beech group.
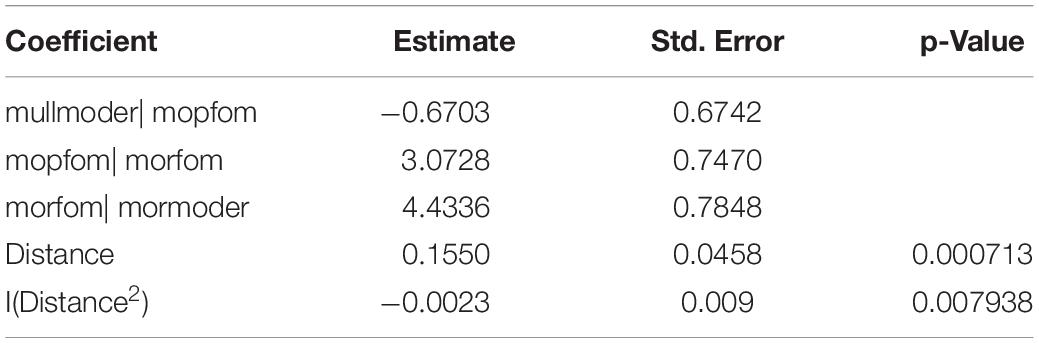
Table 3. Results of the ordered categorical logistic regression for estimating the relative proportions of the four humus forms as a function of the distance to the beech group.
Modelling of the Humus Layer Thickness Along the Transect
The results of the non-linear modelling of the sum of the Of- and Oh-horizon thickness show a significant influence of the distance to the centre of the beech group on the thickness (Table 4 and Figure 5). The humus layer thickness is lowest in the centre of the beech group and is predicted to be 2.5 cm. The humus layer thickness increases with increasing distance from the beech group. At a distance of 50 m, the highest humus layer thickness of 3.8 cm is predicted.
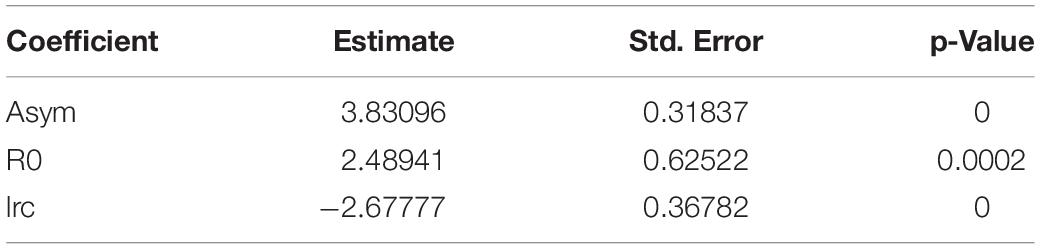
Table 4. Results of the non-linear model on the influence of the distance to the centre of the beech group on the sum of Oh and Of thickness.
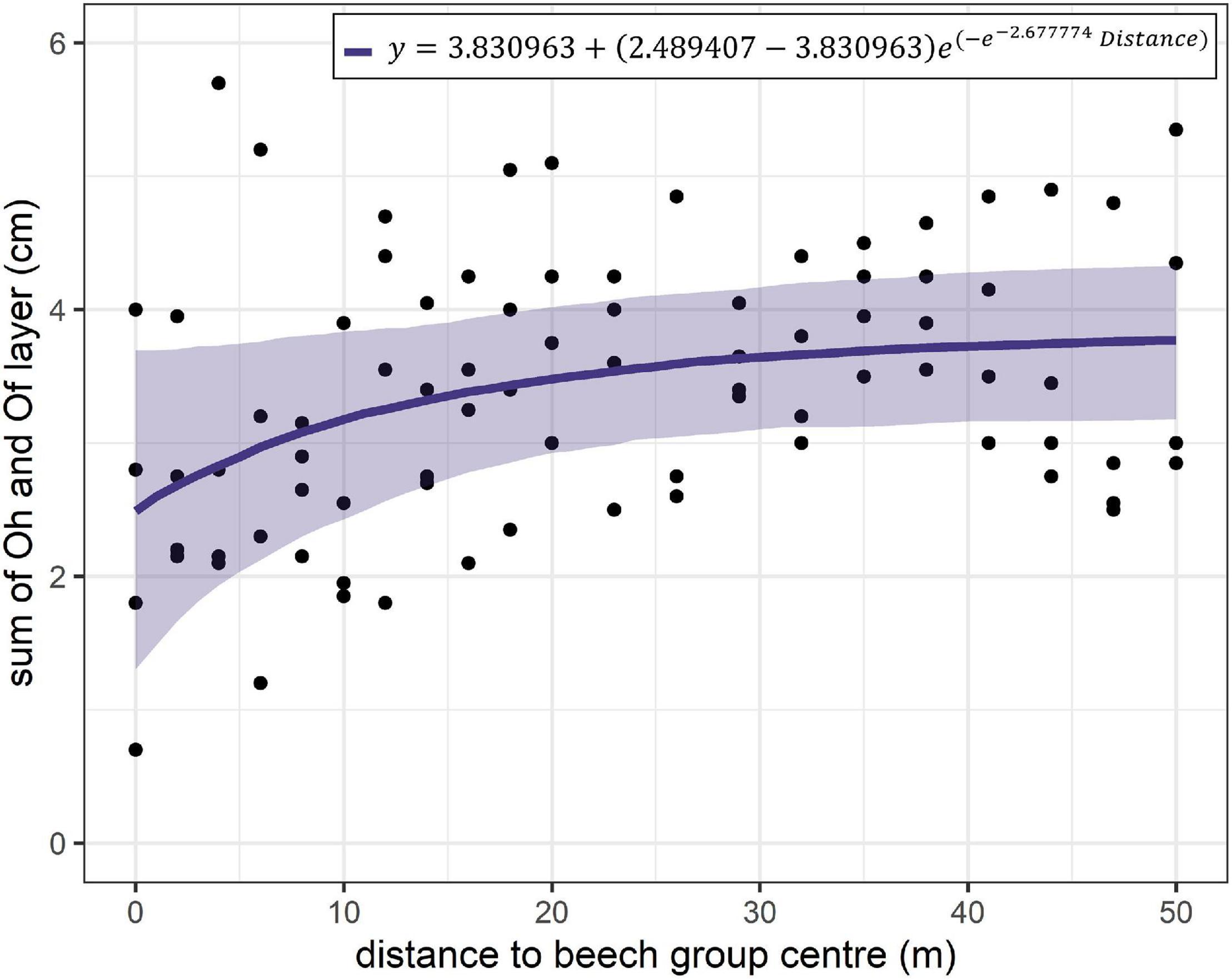
Figure 5. Result of the non-linear model for modelling the sum of Oh and Of horizon thickness as a function of the distance to the centre of the beech group. The transparent polygons show the 95 % confidence interval.
Simulation of the Restoration Approach at Forest District Level
According to the hypotheses on the establishment of beech groups in spruce stands for restoration, their effects were tested for a part of the forest district in Holzhau. Using the functions determined for the dispersal of beech regeneration and for humus layer thickness as a function of the distance to the beech groups, both effects were simulated on a large scale.
Figure 6 shows the current location of the beech groups. This is a result of the historical restoration of the beech groups. The average distances between the beech groups within a row are about 50 m. However, larger distances were also determined in the study area.
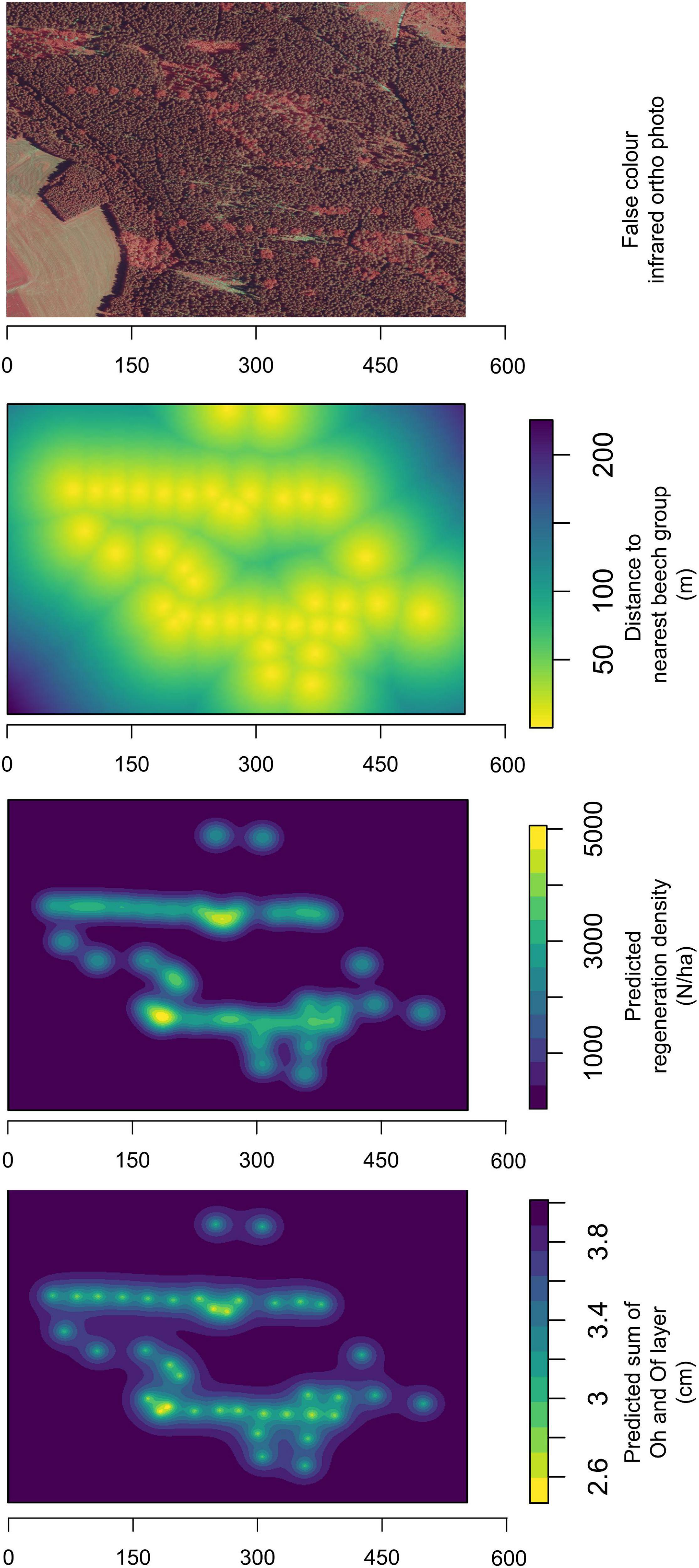
Figure 6. Position of the beech groups in the spruce stands in the false-colour infrared aerial photograph in the Holzhau forest district [Map with data from GeoSN (2021)]. Distance to the nearest beech group and simulated beech regeneration density and humus thickness.
The simulated regeneration density illustrates that natural regeneration is highest close to beech groups (Figure 6). At the same time, it can be seen that beech regeneration is also spreading into the surrounding spruce stand. However, areas with spruce stands exist, that are too far away for the dispersal of beech regeneration. The distances between the beech groups are designed to allow the beech regeneration to spread in densities of about 3000 per hectare.
For the humus layer thickness, Figure 6 shows that the lowest thickness is predicted within the beech groups. Within a row of beech groups, a reduction in humus layer thickness was predicted compared to the surrounding spruce stand. However, the effects on total humus layer thickness did not extend as far as the effects on the beech regeneration spreading.
Discussion
Beech Groups as Initials for Natural Regeneration
From modelling of the point patterns, it appears that there is a density trend of beech regeneration on a larger scale, which is explained by distance to the nearest seed trees, as well as by radiation variables and fencing against browsing. The effects of these variables will be discussed subsequently:
By planting groups of beech in spruce stands, seed sources should be created for the natural regeneration of beech, so that spruce-beech mixed stands can unfold in the next generation. The beech groups were planted at distances of approx. 50 m. Maximum barochorous seed dispersal distance of beech is 19–20 m (Wagner, 1999; Millerón et al., 2013). However, almost 25 % of the observed beech saplings are at distances above 20 m, and the dispersal curve of natural regeneration predicted by the model as a function of the distance to the nearest seed tree (Figure 3) exceeds observed barochorous dispersal curves determined by Wagner (1999) and Sagnard et al. (2007). The results indicate that a considerable number of seed is removed from the seed tree by zoochorous vectors. The seed dispersal of the zoochorous vectors is essentially dependent on the seed hoarding behaviour of the animals and the habitat preference (Löf et al., 2018): While small mammals, especially mice, are able to transport beechnut over short to medium distances of up to about 130 m (Jensen, 1985; Jensen and Nielsen, 1986; Den Ouden et al., 2005; Perea et al., 2011a), birds are able to transport them over longer distances (Nilsson, 1985). Compared to the European jay (Garrulus glandarius L.), the nuthatch (Sitta europaea L.) has smaller territories and places seeds mostly near the source and only rarely at distances above 40 m (Moreno et al., 1981; Perea et al., 2011b). The present results, however, show in part significant greater dispersal distances up to 69 m. Irmscher (2009) even found maximum dispersal distances of established beech regeneration of 254 m. While previous studies generally describe a preference of the jay for acorns over beechnuts, Perea et al. (2011b) were able to show that dispersal depends on the presence of other fruits and their masting frequency. From the foregoing, it can be deduced that the jay is also a potential vector of the observed spread of beech regeneration, in addition to various mice and nuthatches. This is particularly true, as in the surrounding of the beech patches at 749 m elevation, no oak trees are known to exist.
The predicted dispersal curve (Figure 3) is in accordance with studies of Dobrovolny and Tesař (2010), Mirschel et al. (2011), and Dobrovolny (2016). Dobrovolny and Tesař (2010) also observed a slight increase of density at 70 m with a second maximum at 110 m for one of their research sites. The frequency distribution of flight distances of the jay might give the explanation for the observed densities of beech seedlings (Bossema, 1979; Gómez, 2003). Alternatively, this behaviour may be due to the use of the distance as a 3rd degree polynomial in the model (Motulsky, 2004).
While the barochorous dispersal curve shows its maximum at the seed tree, the dispersal curve in Figure 3 shows its maximum at a distance of 5 m to the nearest old beech. This result is in line with previous studies (Ganz, 2004; Dobrovolny and Tesař, 2010; Dobrovolny, 2016) and is dependent on the combination of barochorous seed dispersal, zoochorous seed dispersal and the different establishment probability (Akashi, 1997; Topoliantz and Ponge, 2000; Millerón et al., 2013). Further evidence on the decrease of survival probability of beech regeneration near old beech can be found in previous studies (Augustin et al., 2005; Shimatani et al., 2007; Ramage and Mangana, 2017). The results of the radiation measurement indicate that the lowest radiation values are found within the beech group (Supplementary Figure 2). Previous studies showed weaker effects of solar radiation on the survival of beech seedlings (Kunstler et al., 2005; Petritan et al., 2007). Although beech seedlings survive strong shade (Szwagrzyk et al., 2001), there are indications that shading has an influence on the root development of beech trees, so that survival under water stress may be at risk (Burschel et al., 1964; Ammer et al., 2002).
While very low radiation values prevail within the beech group (Leuchner et al., 2011), it can be deduced that there is a sufficient radiation supply for beech regeneration in the surrounding spruce stand (Supplementary Figure 2) (Madsen, 1994; Emborg, 1998). Thinning in old spruce stands creates gap sizes and radiation levels that are suitable for beech regeneration establishment (Courbaud et al., 2003; Huth and Wagner, 2006; Čater et al., 2013). The positive influence of PAR radiation indicates this (Figure 3). However, there is evidence that competition from spruce regeneration exceeds beech regeneration if radiation availability is too high (Dobrovolný and Cháb, 2013; Dobrovolny, 2016). While beech regeneration is clearly superior to spruce regeneration in both plant density and height growth at low radiation availability, the competitive relationship changes from a relative light availability of 20 % (Kühne and Bartsch, 2003). Other studies found a diffuse site factor greater than 12 or 17 %, where beech regeneration density decreases in favour of spruce (Unkrig, 1997; von Lüpke and Spellmann, 1997; Dobrovolny, 2016). Undisturbed spruce stands were selected as study sites and the majority of the diffuse site factor values lies below these threshold values (Supplementary Figure 2), at which the competition shifts in favour of spruce.
There are significant differences in regeneration density between the two sites (Supplementary Figure 3). The factor “fence” shows a clear influence on the regeneration density. Fenced areas are expected to have a 3.41 times higher regeneration density compared to unfenced areas, holding the other variables in the model constant (Figure 3). Dobrovolny and Tesař (2010) also found large differences in plant density of beech regeneration between fenced and unfenced areas. The differences highlighted in Figure 3 are reflected in the differences of that study. Compared to other tree species, beech usually does not show such a high risk of being browsed (Boulanger et al., 2009; Bobrowski et al., 2015; Orman et al., 2018). Compared to spruce regeneration, however, it is preferred by deer (Motta, 2003; Vacek et al., 2014). In mixed spruce-beech stands, a decline in beech regeneration is therefore to be expected as a result of indirect resource competition due to the selectivity of game browsing (Madsen, 1995; Olesen and Madsen, 2008). In addition to deer, however, feeding activity of wild boar (Sus scrofa L.) can also lead to a considerable reduction in regeneration density (Gómez and Hódar, 2008; Bisi et al., 2018). As possible countermeasures to facilitate beech natural regeneration, fencing or intensive hunting can therefore be derived as a sensible management measure (Kamler et al., 2010; Chevrier et al., 2012). Despite fencing, small mammals, insects and fungi can cause significant losses while the beechnuts are stored on the forest floor. As various studies have shown, mice contribute to a high degree to the predation of beechnuts (Akashi, 1997; Ida et al., 2004; Perea et al., 2012).
The preceding discussion showed that there are various influencing variables that affect beech regeneration density. Residuals in the model indicate that there are also influencing variables that were not captured by the model (Supplementary Figure 3). Small site differences or ground vegetation as competition could be decisive here and should be included in future studies. However, it is evident that mixing beech groups into spruce stands creates the potential for mixed beech spruce stands to develop due to beech seed dispersal and establishment conditions within surrounding spruce stands. The extent of the beech group depends on the desired mixture and required plant density, according to the functionality of the future stands.
Beech Groups for Preserving Soil Fertility
The modelling of the humus forms and the total humus layer thickness showed that the distance to the centre of the beech group has a significant influence on humus composition (Figures 4, 5). Previous studies confirm the influence of beech admixture on humus layer thickness in spruce stands (Rothe et al., 2002; Hojjati, 2008; Achilles et al., 2021b). Differences in humus thickness between beech groups and the spruce stands were found by Achilles et al. (2021b) in the range of 0.9 to 2.3 cm and by Rothe et al. (2002) of 3.5 cm (including L horizon) confirming our results (Figure 5). These results will be discussed below in the context of humus ecology: In addition to the site variables (Vesterdal, 1999; Moore et al., 2007), tree species especially determine the thickness and composition of humus through stand microclimate, litter volume and litter composition (Bradford et al., 2016). The composition of tree species thus has an effect on chemical, physical and biological (edaphon) properties of the topsoil (Augusto et al., 2002; Frouz, 2018). With the restoration approach, the microclimate was changed by the beech groups, as indicated for example by the radiation patterns (Supplementary Figure 2) and supported by further studies (Benecke, 1984; Schume et al., 2004; Frischbier and Wagner, 2015). At the same time, beech litter was brought into the spruce-dominated stands by planting the beech groups. Beech leaves show a better decomposition rate compared to spruce needles, which is due to a closer C/N ratio and a higher pH-value (Berg, 2000; Augusto et al., 2002; Albers et al., 2004; Zhong and Makeschin, 2004; Wälder et al., 2008; Jacob et al., 2010). More favourable micro-environmental conditions for decomposer communities in forest floor of beech stands in comparison to spruce are repeatedly described. Equally Hojjati et al. (2009) point out the connection to pH-values. And this finding in turn is strongly related to the pattern of water fluxes via throughfall. Compared with the spruce canopy they are higher under beech. This is in agreement with the investigation results of earlier comparative studies between pure beech and pure spruce stands (Albers et al., 2004; Sariyildiz et al., 2005). Augusto and Ranger (2001) also claimed that greater throughfall can be linked to higher pH values in the upper soil. Other studies indicate that rather unfavourable environmental conditions in spruce stands, delay decomposition (Mardulyn et al., 1993; Augusto et al., 2002; Berger and Berger, 2014). Albers et al. (2004) conclude that the adverse conditions for litter decomposition and microorganisms in spruce forests are effectively improved by the admixture of beech to spruce pure stands. Like most ecological effects, the influence of beech groups on the humus composition of the surrounding spruce stand is also spatially limited (Wu et al., 1985). Non-linear modelling of humus thickness revealed that this effect decreased up to distances of 40 m and is only weakly visible thereafter (Figure 5). This effect can be attributed to the dispersal of beech leaves, as dispersal models on leaf litterfall by Ferrari and Sugita (1996), Staelens et al. (2003), and Nickmans et al. (2019) demonstrate. From these studies, it can be seen that 50% of the leaf mass falls in a range of 10 m and 90% of the leaf mass falls in the range of up to 40 m. Considering the size of the beech groups (diameters of 20 to 27 m) (Table 1), it can be seen that the effect of leaf dispersal extends from the edge of the beech group into the spruce stand. The effect of the beech group on humus layer thickness decreases as the amount of leaves decreases (Wälder et al., 2008). Non-linear relationships between tree species composition and humus layer thickness were also found by Rothe et al. (2002).
Simultaneously with the modelling of the humus layer thickness, there are also the most bioactive humus forms, i.e., mullmoder within the beech group, as confirmed by the results of the ordered categorical model (Figure 4). These results are in line with the pairwise comparisons of humus form of beech groups and spruce stands by Achilles et al. (2021b). In spruce stands, fewer biologically active humus forms were found compared to beech (Nihlgård and Nihlgard, 1971). The results also show that the proportion of mormoder is highest in the spruce stand which is far away from the beech group (Figure 4). Previous studies also confirm significant improvement in humus forms through the restoration of beech, which is associated with a significant increase in pH and base saturation as well as a significant reduction in the C:N ratio (Heinze et al., 2001; Prietzel, 2004). Due to the deeper rooting of beech compared to spruce, it serves as a base pump and increases base saturation via litterfall (Berger et al., 2006; Achilles et al., 2021a).
From the preceding discussion it becomes apparent, that the forest restoration approach thus leads to a reduction in humus layer thickness and to a change in humus form due to the change in litter quality, with effects on the decomposing soil organisms (Fischer et al., 2002; Bens et al., 2006; Fischer and Fischer, 2012). From the distance between the beech groups (Figure 6), it is evident that the positive effect of the beech groups on the humus composition of the surrounding spruce stand becomes effective.
Conclusion
These nearly 100-year-old experiments offer a rare opportunity to contrast the initiators’ expectations of restoration with the actual effects in temperate conditions. Specifically, we have studied the long-term effects on soil fertility and natural regeneration. The ecological functions of the nutrient cycle and the natural regeneration ability have been restored in general. However, while after 100 years an increased proportion of biologically active humus forms can already be detected in the beech groups and their surroundings, the natural beech regeneration is rather in its initial stage. Because the target dimension of spruce has not yet been reached, the planned transfer of beech regeneration to the next generation would still take several decades. However, against the background of the continuing bark beetle calamities in spruce after drought, the possibility of increasing the proportion of beech in the next few years via natural regeneration is very pleasing.
The hypotheses formulated by foresters 100 years ago regarding the restoration approach of reintroducing beech groups into spruce stands can thus be confirmed. For future restoration approaches, groupwise mixtures should be included in the planning. The size and spacing of the groups should be chosen according to the ecology of the tree species and the effect they should achieve. Despite the successes of the restoration approach, it should be noted in conclusion how long these renaturation efforts actually take in temperate forests.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MA, FK, and SW: conceptualisation and methodology. MA and SW: software, validation, formal analysis, writing—original draft preparation, writing—review and editing, and visualisation. MA and FK: investigation and data curation. SW: resources and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was done in the course of two master’s theses. No funding was granted.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would especially like to thank Sven Irrgang, Maik Stachowiak, Gunter Haase, and Tino Kermer for finding suitable study sites and for the work permits in the field. We thank Jörg Wollmerstädt for his support in analysing the hemispherical images for radiation estimation. Furthermore, we thank Holger Fischer for introducing us to humus ecological measurements in the field. We are also grateful for his proofreading. Finally, we would like to thank Robert Schlicht, who gave us valuable advice on statistical issues.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.826186/full#supplementary-material
References
Achilles, F., Tischer, A., Bernhardt-Römermann, M., Heinze, M., Reinhardt, F., Makeschin, F., et al. (2021b). European beech leads to more bioactive humus forms but stronger mineral soil acidification as Norway spruce and Scots pine – results of a repeated site assessment after 63 and 82 years of forest conversion in Central Germany. For. Ecol. Manage. 483:118769. doi: 10.1016/j.foreco.2020.118769
Achilles, F., Tischer, A., Bernhardt-Römermann, M., Chmara, I., Achilles, M., and Michalzik, B. (2021a). Effects of moderate nitrate and low sulphate depositions on the status of soil base cation pools and recent mineral soil acidification at forest conversion sites with European beech (“Green Eyes”) embedded in Norway spruce and Scots Pine stands. Forests 12:573. doi: 10.3390/f12050573
Akashi, N. (1997). Dispersion pattern and mortality of seeds and seedlings of Fagus crenata Blume in a cool temperate forest in western Japan. Ecol. Res. 12, 159–165. doi: 10.1007/BF02523781
Albers, D., Migge, S., Schaefer, M., and Scheu, S. (2004). Decomposition of beech leaves (Fagus sylvatica) and spruce needles (Picea abies) in pure and mixed stands of beech and spruce. Soil Biol. Biochem. 36, 155–164. doi: 10.1016/j.soilbio.2003.09.002
Ammer, C., Mosandl, R., and El Kateb, H. (2002). Direct seeding of beech (Fagus sylvatica L.) in Norway spruce (Picea abies [L.] Karst.) stands – effects of canopy density and fine root biomass on seed germination. For. Ecol. Manage. 159, 59–72. doi: 10.1016/S0378-1127(01)00710-1
Augustin, N. H., Kublin, E., Metzler, B., Meierjohann, E., and von Wühlisch, G. (2005). Analyzing the spread of beech canker. For. Sci. 51, 438–448. doi: 10.1093/forestscience/51.5.438
Augusto, L., and Ranger, J. (2001). Impact of tree species on soil solutions in acidic conditions. Ann. For. Sci. 58, 47–58. doi: 10.1051/forest:2001102
Augusto, L., Ranger, J., Binkley, D., and Rothe, A. (2002). Impact of several common tree species of European temperate forests on soil fertility. Ann. For. Sci. 59, 233–253. doi: 10.1051/forest:2002020
Baddeley, A., Rubak, E., and Turner, R. (2015). Spatial Point Patterns: Methodology and Applications with R. Boca Raton, FL: CRC Press.
Baddeley, A., Turner, R., and Rubak, E. (2016). Adjusted composite likelihood ratio test for spatial Gibbs point processes. J. Stat. Comput. Simul. 86, 922–941. doi: 10.1080/00949655.2015.1044530
Beckschäfer, P., Seidel, D., Kleinn, C., and Xu, J. (2013). On the exposure of hemispherical photographs in forests. iForest 6, 228–237.
Benecke, P. (1984). Der Wasserumsatz eines Buchen- und Eines Fichtenwaldökosystems im Hochsolling. Frankfurt am Main: Sauerländer.
Bens, O., Buczko, U., Sieber, S., and Hüttl, R. F. (2006). Spatial variability of O layer thickness and humus forms under different pine beech–forest transformation stages in NE Germany. Z. Pflanzenernähr. Bodenk. 169, 5–15. doi: 10.1002/jpln.200521734
Berg, B. (2000). Litter decomposition and organic matter turnover in northern forest soils. For. Ecol. Manage. 133, 13–22. doi: 10.1016/S0378-1127(99)00294-7
Berger, T. W., and Berger, P. (2014). Does mixing of beech (Fagus sylvatica) and spruce (Picea abies) litter hasten decomposition? Plant Soil 377, 217–234. doi: 10.1007/s11104-013-2001-9
Berger, T. W., Swoboda, S., Prohaska, T., and Glatzel, G. (2006). The role of calcium uptake from deep soils for spruce (Picea abies) and beech (Fagus sylvatica). For. Ecol. Manage. 229, 234–246. doi: 10.1016/j.foreco.2006.04.004
Bernhard, R. (1922). Erhaltung und Wiedereinbringung des Laubholzes in den Sächsischen Forsten. Bericht über die 60. Versammlung des Forstvereins. 14–37.
Bernhofer, C., Goldberg, V., Franke, J., Häntzschel, J., Harmansa, S., Pluntke, T., et al. (2008). Sachsen im Klimawandel: Eine Analyse. Dresden: Freistaat Sachsen Staatsministerium für Umwelt und Landwirtschaft.
Bisi, F., Chirichella, R., Chianucci, F., Von Hardenberg, J., Cutini, A., Martinoli, A., et al. (2018). Climate, tree masting and spatial behaviour in wild boar (Sus scrofa L.): insight from a long-term study. Ann. For. Sci. 75:46. doi: 10.1007/s13595-018-0726-6
Blume, H.-P., Stahr, K., and Leinweber, P. (2011). Bodenkundliches Praktikum: Eine Einfüh-rung in Pedologisches Arbeiten für Ökologen, Land- und Forstwirte, Geo- und Umweltwissenschaftler. Heidelberg: Spektrum Akademischer Verlag.
Bobrowski, M., Gillich, B., and Stolter, C. (2015). Modelling browsing of deer on beech and birch in northern Germany. For. Ecol. Manage. 358, 212–221. doi: 10.1016/j.foreco.2015.08.031
Bossema, I. (1979). Jays and oaks: an eco-ethological study of a symbiosis. Behaviour 70, 1–116. doi: 10.1163/156853979X00016
Boulanger, V., Baltzinger, C., Saïd, S., Ballon, P., Picard, J.-F., and Dupouey, J.-L. (2009). Ranking temperate woody species along a gradient of browsing by deer. For. Ecol. Manage. 258, 1397–1406. doi: 10.1016/j.foreco.2009.06.055
Bradford, M. A., Berg, B., Maynard, D. S., Wieder, W. R., and Wood, S. A. (2016). Under-standing the dominant controls on litter decomposition. J. Ecol. 104, 229–238. doi: 10.1111/1365-2745.12507
Burschel, P., Huss, J., and Kalbhenn, R. (1964). Die natürliche verjüngung der buche. Schriftenr. Forstl. Fak. Univ. Göttingen 34:186.
Čater, M., Schmid, I., and Kazda, M. (2013). Instantaneous and potential radiation effect on underplanted European beech below Norway spruce canopy. Eur. J. For. Res. 132, 23–32. doi: 10.1007/s10342-012-0651-4
Chevrier, T., Said, S., Widmer, O., Hamard, J.-P., Saint-Andrieux, C., and Gaillard, J.-M. (2012). The oak browsing index correlates linearly with roe deer density: a new indicator for deer management? Eur. J. Wildl. Res. 58, 17–22. doi: 10.1007/s10344-011-0535-9
Christensen, R. H. B. (2019). Regression Models for Ordinal Data [R Package Ordinal Version 2019.12-10]. Comprehensive R Archive Network (CRAN).
Clemens, R. (1930). Anbau von Buchenhorsten in Einem Sächsischen Fichtenrevier. Der Deutsche Forstwirt 12. 505–507, 513–515.
Clemens, R. (1931). Führer für den Waldbegang auf Bienenmühler Revier : am 23.6.1931. Freiberg: Mauckisch.
Courbaud, B., de Coligny, F., and Cordonnier, T. (2003). Simulating radiation distribution in a heterogeneous Norway spruce forest on a slope. Agric. For. Meteorol. 116, 1–18. doi: 10.1016/S0168-1923(02)00254-X
de Groot, M., Diaci, J., and Ogris, N. (2019). Forest management history is an important factor in bark beetle outbreaks: lessons for the future. For. Ecol. Manage. 433, 467–474. doi: 10.1016/j.foreco.2018.11.025
Den Ouden, J., Jansen, P. A., and Smit, R. (2005). “Jays, mice and oaks: predation and dispersal of Quercus robur and Q. petraea in north-western Europe,” in Seed Fate. Predation, Dispersal and Seedling Establishment, eds P. M. Forget, J. Lambert, and S. B. Vander Wall (Wallingford: CABI Publishing), 223–240. doi: 10.1079/9780851998060.0223
Dobrovolny, L. (2016). Density and spatial distribution of beech (Fagus sylvatica L.) regeneration in Norway spruce (Picea abies (L.) Karsten) stands in the central part of the Czech Republic. iForest 9, 666–672. doi: 10.3832/ifor1581-008
Dobrovolný, L., and Cháb, M. (2013). Ecology of beech regeneration in the allochthonous spruce stands – a case study. Acta Univ. Agric. Silvic. Mendelianae Brun. 61, 1261–1268. doi: 10.11118/actaun201361051261
Dobrovolny, L., and Tesař, V. (2010). Extent and distribution of beech (Fagus sylvatica L.) regeneration by adult trees individually dispersed over a spruce monoculture. J. For. Sci. 56, 589–599. doi: 10.17221/12/2010-JFS
Eckelmann, W., Sponagel, H., Grottenthaler, W., Hartmann, K. J., Hartwich, R., Janetzko, P., et al. (2005). Bodenkundliche Kartieranleitung: Mit …103 Tabellen. Stuttgart: Schweizerbart i. Komm.
Emborg, J. (1998). Understorey light conditions and regeneration with respect to the structural dynamics of a near-natural temperate deciduous forest in Denmark. For. Ecol. Manage. 106, 83–95. doi: 10.1016/S0378-1127(97)00299-5
Faraway, J. J. (2006). Extending the Linear Model with R: Generalized Linear. Mixed Effects and Nonparametric Regression Models, 1 Edn. Boca Raton, FL: Chapman & Hall/CRC.
Ferrari, J. B., and Sugita, S. (1996). A spatially explicit model of leaf litterfall in hemlock–hardwood forests. Can. J. For. Res. 26, 1905–1913. doi: 10.1139/x26-215
Fischer, A., and Fischer, H. (2012). “Restoration of temperate forests: an European approach,” in Restoration Ecology: The New Frontier, eds J. van Andel and J. Aronson (Chichester: John Wiley & Sons), 145–160. doi: 10.1016/j.ppees.2018.01.002
Fischer, H., Bens, O., and Huttl, R. (2002). Veränderung von humusform, -vorrat und -verteilung im zuge von waldumbau-maßnahmen im Nordostdeutschen tiefland [Changes in humus form, humus stock and soil organic matter distribution caused by forest transformation in the northeastern lowlands of Germany]. Forstw. Cbl. 121, 322–334. doi: 10.1046/j.1439-0337.2002.02037.x
Fischer, H., Huth, F., Hagemann, U., and Wagner, S. (2016). “Developing restoration strategies for temperate forests using natural regeneration processes,” in Restoration of Boreal and Temperate Forests, ed. J. A. Stanturf (Boca Raton, FL: CRC Press), 103–164.
Frischbier, N., and Wagner, S. (2015). Detection, quantification and modelling of small-scale lateral translocation of throughfall in tree crowns of European beech (Fagus sylvatica L.) and Norway spruce (Picea abies (L.) Karst.). J. Hydrol. 522, 228–238. doi: 10.1016/j.jhydrol.2014.12.034
Frouz, J. (2018). Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 332, 161–172. doi: 10.1016/j.geoderma.2017.08.039
Ganz, M. (2004). Entwicklung von Baumartenzusammensetzung und Struktur der Wälder vom Schwarzwald bis auf die Schwäbische Alb-mit Besonderer Berücksichtigung der Buche. Ph.D. dissertation. Freiburg im Breisgau: Albert-Ludwigs-Universität.
Garten, C. T. Jr., Kang, S., Brice, D. J., Schadt, C. W., and Zho, J. (2007). Variability in soil properties at different spatial scales (1m–1km) in a deciduous forest ecosystem. Soil Biol. Biochem. 39, 2621–2627. doi: 10.1016/j.soilbio.2007.04.033
Gauer, J., and Kroiher, F. (2012). Waldökologische Naturräume Deutschlands: Forstliche Wuchsgebiete und Wuchsbezirke. Digitale Topographische Grundlagen – Neubearbeitung Stand 2011. Braunschweig: Johann Heinrich von Thünen Institut.
Gayer, K. (1897). Über buchenmischung im nadelwald. For. Centralbl. 19, 486–492. doi: 10.1007/bf01842447
GeoSN (2021). Karte – WMS SN DOP-CIR: dl-de/by-2-0. Dresden: Staatsbetrieb Geobasisinformation und Vermessung Sachsen.
Gómez, J. M. (2003). Spatial patterns in long-distance dispersal of Quercus ilex acorns by jays in a heterogeneous landscape. Ecography 26, 573–584. doi: 10.1034/j.1600-0587.2003.03586.x
Gómez, J. M., and Hódar, J. A. (2008). Wild boars (Sus scrofa) affect the recruitment rate and spatial distribution of holm oak (Quercus ilex). For. Ecol. Manage. 256, 1384–1389. doi: 10.1016/j.foreco.2008.06.045
Graser, H. (1916). Zur frage der buchenanzucht im sächsischen Erzgebirge. Tharandter For. Jahrb. 67, 1–30. doi: 10.1515/9783486734362-003
Graser, H. (1935). Die Bewirtschaftung des Erzgebirgischen Fichtenwaldes. Mit 9 Anl. Einschl. 38 Abb, Vol. 2. Dresden: Burdach.
Gsteiger, S., Bretz, F., and Liu, W. (2011). Simultaneous confidence bands for nonlinear regression models with application to population pharmacokinetic analyses. J. Biopharm. Stat. 21, 708–725. doi: 10.1080/10543406.2011.551332
Haines-Young, R., and Potschin, M. (2011). Common International Classification of Ecosystem Services (CICES): 2011 Update. Nottingham: Report to the European Environmental Agency.
Hasel, K., and Schwartz, E. (2006). Forstgeschichte ein Grundriß für Studium und Praxis. Remagen: Verlag Dr. Kessel.
Heinze, M., Tomczyk, S., and Nicke, A. (2001). Vergleich von rot-buche (Fagus sylvatica L.) in sogennaten Grünen Augen mit benachbarten standortsgleichen Fichtenbeständen (Picea abies [L.] KARST.) des Thüringer Vogtlandes bezüglich eigenschaften und durchwurzelung des bodens sowie baumwachstum. Eur. J. For. Res. 120, 139–153. doi: 10.1007/BF02796088
Hojjati, S. M. (2008). The Impact of Canopy Composition on the Nutritional Status of an Admixed Spruce and Beech Forest at Solling, Central Germany. Ph.D. dissertation. Göttingen: Georg-August Göttingen University.
Hojjati, S. M., Hagen-Thorn, A., and Lamersdorf, N. P. (2009). Canopy composition as a measure to identify patterns of nutrient input in a mixed European beech and Norway spruce forest in central Europe. Eur. J. For. Res. 128, 13–25. doi: 10.1007/s10342-008-0235-5
Hübsch (1898). “Unter welchen verhältnissen und in welchem umfange ist eine künstliche einmischung der buche und anderer laubhölzer bei verjüngung von nadelholzbeständen angezeigt? Verhandlungen des bad,” in Proceeding of the Forstvereins bei Seiner 41. Versammlung, 91–102.
Huth, F., and Wagner, S. (2006). Gap structure and establishment of silver birch regeneration (Betula pendula Roth.) in Norway spruce stands (Picea abies L. Karst.). For. Ecol. Manage. 229, 314–324. doi: 10.1016/j.foreco.2006.04.010
Ida, H., Hotta, M., and Ezaki, Y. (2004). Predispersal predation by rodents to beechnuts (Fagus crenata Blume). Ecol. Res. 19, 503–509. doi: 10.1111/j.1440-1703.2004.00664.x
Illian, J., Penttinen, A., Stoyan, H., and Stoyan, D. (2008). Statistical Analysis and Modelling of Spatial Point Patterns. Chichester: John Wiley.
Ilvesniemi, H. (1991). Spatial and temporal variation of soil chemical characteristics in pine sites in southern Finland. Silva Fenn. 25:5446. doi: 10.14214/sf.a15600
Irmscher, T. (2009). Zoochores ausbreitungspotenzial der rotbuche (Fagus sylvatica L.) mit blick auf die minimierung der eingriffsintensität beim waldumbau in wäldern mit naturschutzstatus. Forstarchiv 80, 29–32.
Jacob, M., Viedenz, K., Polle, A., and Thomas, F. M. (2010). Leaf litter decomposition in temperate deciduous forest stands with a decreasing fraction of beech (Fagus sylvatica). Oecologia 164, 1083–1094. doi: 10.1007/s00442-010-1699-9
Jensen, T. S. (1985). Seed-seed predator interactions of European beech, Fagus sylvatica and forest rodents, Clethrionomys glareolus and Apodemus flavicollis. Oikos 44, 149–156. doi: 10.2307/3544056
Jensen, T. S., and Nielsen, O. F. (1986). Rodents as seed dispersers in a heath – oak wood succession. Oecologia 70, 214–221. doi: 10.1007/BF00379242
Johann, E., Agnoletti, M., Axelsson, A.-L., Bürgi, M., Östlund, L., Rochel, X., et al. (2004). “History of secondary Norway spruce forests in Europe,” in Norway Spruce Conversion: Options and Consequences, eds H. Spiecker, J. Hansen, E. Klimo, J. P. Skovsgaard, H. Sterba, and K. von Teuffel (Boston, MA: Brill), 25–62. doi: 10.1163/9789047412908_006
Kamler, J., Homolka, M., Baranèeková, M., and Krojerová-Prokešová, J. (2010). Reduction of herbivore density as a tool for reduction of herbivore browsing on palatable tree species. Eur. J. For. Res. 129, 155–162. doi: 10.1007/s10342-009-0309-z
Kandler, O. (1992). Historical declines and diebacks of central European forests and present conditions. Environ. Toxicol. Chem. 11, 1077–1093. doi: 10.1002/etc.5620110805
Krauss, G., Müller, K., Gärtner, G., Härtel, F., Schanz, H., and Blanckmeister, H. (1939). Standortsgemäße durchführung der abkehr von der fichtenwirtschaft im nordwestsächsischen Niederland. Tharandter For. Jahrb. 90, 481–716.
Kühne, C., and Bartsch, N. (2003). Zur Naturverjüngung von Fichten-Buchen-Mischbestanden im Solling. Forst. Holz 58, 3–7.
Kunstler, G., Curt, T., Bouchaud, M., and Lepart, J. (2005). Growth, mortality, and morphological response of European beech and downy oak along a light gradient in sub-Mediterranean forest. Can. J. For. Res. 35, 1657–1668. doi: 10.1139/x05-097
Leuchner, M., Hertel, C., and Menzel, A. (2011). Spatial variability of photosynthetically active radiation in European beech and Norway spruce. Agric. For. Meteorol. 151, 1226–1232. doi: 10.1016/j.agrformet.2011.04.014
Liski, J. (1995). Variation in soil organic carbon and thickness of soil horizons within a boreal forest stand – effect of trees and implications for sampling. Silva Fenn. 29:5561. doi: 10.14214/sf.a9212
Löf, M., Ammer, C., Coll, L., Drössler, L., Huth, F., Madsen, P., et al. (2018). “Regeneration patterns in mixed-species stands,” in Dynamics, Silviculture and Management of Mixed Forests, eds A. Bravo-Oviedo, H. Pretzsch, and M. del Río (Cham: Springer), 103–130. doi: 10.1007/978-3-319-91953-9_4
Madsen, P. (1994). Growth and survival of Fagus sylvatica seedlings in relation to light intensity and soil water content. Scand. J. For. Res. 9, 316–322. doi: 10.1080/02827589409382846
Madsen, P. (1995). Effects of seedbed type on wintering of beech nuts (Fagus sylvatica) and deer impact on sprouting seedlings in natural regeneration. For. Ecol. Manage. 73, 37–43. doi: 10.1016/0378-1127(94)03503-O
Mardulyn, P., Godden, B., Echezarreta, P., Penninckx, M., Gruber, W., and Herbauts, J. (1993). Changes in humus microbiological activity induced by the substitution of the natural beech forest by Norway spruce in the Belgian Ardennes. For. Ecol. Manage. 59, 15–27. doi: 10.1016/0378-1127(93)90068-X
Martin, H. (1919). Die erhaltung der buche in Sachsen, insbesondere in gemischten beständen. Tharandter Forst. Jahrb. 70, 83–110.
Millerón, M., Lopez de Heredia, U., Lorenzo, Z., Alonso, J., Dounavi, A., Gil, L., et al. (2013). Assessment of spatial discordance of primary and effective seed dispersal of European beech (Fagus sylvatica L.) by ecological and genetic methods. Mol. Ecol. 22, 1531–1545. doi: 10.1111/mec.12200
Mirschel, F., Zerbe, S., and Jansen, F. (2011). Driving factors for natural tree rejuvenation in anthropogenic pine (Pinus sylvestris L.) forests of NE Germany. For. Ecol. Manage. 261, 683–694. doi: 10.1016/j.foreco.2010.11.025
Moore, T. R., Bubier, J. L., and Bledzki, L. (2007). Litter decomposition in temperate peat-land ecosystems: the effect of substrate and site. Ecosystems 10, 949–963. doi: 10.1007/s10021-007-9064-5
Moreno, J., Lundberg, A., and Carlson, A. (1981). Hoarding of individual nuthatches Sitta europaea and march tits Parus palustris. Ecography 4, 263–269. doi: 10.1111/j.1600-0587.1981.tb01007.x
Motta, R. (2003). Ungulate impact on rowan (Sorbus aucuparia L.) and Norway spruce (Picea abies (L.) Karst.) height structure in mountain forests in the eastern Italian Alps. For. Ecol. Manage. 181, 139–150. doi: 10.1016/S0378-1127(03)00128-2
Motulsky, H. (2004). Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. Oxford, NY: Oxford University Press.
Moutinho, L., and Hutcheson, G. (2011). The Sage Dictionary of Quantitative Management Research. Los Angeles, CA: Sage.
Nickmans, H., Jonard, M., Verheyen, K., and Ponette, Q. (2019). Modelling leaf dispersal and nutrient return in tree species mixtures. For. Ecol. Manage. 436, 68–78. doi: 10.1016/j.foreco.2019.01.001
Nihlgård, B., and Nihlgard, B. (1971). Pedological influence of spruce planted on former beech forest soils in Scania, south Sweden. Oikos 22, 302–314. doi: 10.2307/3543854
Nilsson, S. G. (1985). Ecological and evolutionary interactions between reproduction of beech Fagus sylvatica and seed eating animals. Oikos 44, 157–164.
Olesen, C. R., and Madsen, P. (2008). The impact of roe deer (Capreolus capreolus), seedbed, light and seed fall on natural beech (Fagus sylvatica) regeneration. For. Ecol. Manage. 255, 3962–3972. doi: 10.1016/j.foreco.2008.03.050
Orman, O., Dobrowolska, D., and Szwagrzyk, J. (2018). Gap regeneration patterns in Carpathian old-growth mixed beech forests – interactive effects of spruce bark beetle canopy disturbance and deer herbivory. For. Ecol. Manage. 430, 451–459. doi: 10.1016/j.foreco.2018.08.031
Perea, R., Miguel, A. S., and Gil, L. (2011a). Acorn dispersal by rodents: the importance of re-dispersal and distance to shelter. Basic Appl. Ecol. 12, 432–439. doi: 10.1016/j.baae.2011.05.002
Perea, R., San Miguel, A., and Gil, L. (2011b). Flying vs. climbing: factors controlling arbore-al seed removal in oak-beech forests. For. Ecol. Manage. 262, 1251–1257. doi: 10.1016/j.foreco.2011.06.022
Perea, R., San Miguel, A., Martínez-Jauregui, M., Valbuena-Carabaña, M., and Gil, L. (2012). Effects of seed quality and seed location on the removal of acorns and beechnuts. Eur. J. For. Res. 131, 623–631. doi: 10.1007/s10342-011-0536-y
Petritan, A. M., von Lüpke, B., and Petritan, I. C. (2007). Effects of shade on growth and mortality of maple (Acer pseudoplatanus), ash (Fraxinus excelsior) and beech (Fagus sylvatica) saplings. Forestry 80, 397–412. doi: 10.1093/forestry/cpm030
Pinheiro, J. C., and Bates, D. M. (eds). (2000). “Nonlinear mixed-effects models: basic concepts and motivating examples,” in Statistics and Computing (New York, NY: Springer), 273–304. doi: 10.1007/0-387-22747-4_6
Prietzel, J. (2004). Humusveränderungen nach einbringung von buche und eiche in kiefern-reinbestände. J. Plant Nutr. Soil Sci. 167, 428–438. doi: 10.1002/jpln.200421363
Ramage, B. S., and Mangana, I. J. (2017). Conspecific negative density dependence in American beech. For. Ecosyst. 4:8. doi: 10.1186/s40663-017-0094-y
Rothe, A., Kreutzer, K., and Küchenhoff, H. (2002). Influence of tree species composition on soil and soil solution properties in two mixed spruce-beech stands with contrasting history in Southern Germany. Plant Soil 240, 47–56. doi: 10.1023/A:1015822620431
Sagnard, F., Pichot, C., Dreyfus, P., Jordano, P., and Fady, B. (2007). Modelling seed dispersal to predict seedling recruitment: recolonization dynamics in a plantation forest. Ecol. Model. 203, 464–474. doi: 10.1016/j.ecolmodel.2006.12.008
Sariyildiz, T., Tüfekçioðlu, A., and Küçük, M. (2005). Comaprison of decomposition rates of beech (Fagus orientalis Lipsky) and spruce (Picea abies (L.) litter in pure and mixed stands of both species in Artvin, Turkey. Turk. J. Agric. For. 29, 429–438.
Schmidt-Vogt, H. (1991). Die Fichte. 2,3, Waldbau, Ökosysteme, Urwald, Wirtschaftswald, Ernährung, Düngung, Ausblick/von Helmut Schmidt-Vogt. Hamburg: Parey.
Schume, H., Jost, G., and Hager, H. (2004). Soil water depletion and recharge patterns in mixed and pure forest stands of European beech and Norway spruce. J. Hydrol. 289, 258–274. doi: 10.1016/j.jhydrol.2003.11.036
Schwalbe, E., Maas, H.-G., Kenter, M., and Wagner, S. (2009). Hemispheric image modeling and analysis techniques for solar radiation determination in forest ecosystems. Photogramm. Eng. Remote Sens. 75, 375–384. doi: 10.14358/PERS.75.4.375
Shimatani, K., Kimura, M., Kitamura, K., Suyama, Y., Isagi, Y., and Sugita, H. (2007). Determining the location of a deceased mother tree and estimating forest regeneration variables by use of microsatellites and spatial genetic models. Popul. Ecol. 49, 317–330. doi: 10.1007/s10144-007-0050-8
Spekat, A., Enke, W., and Franke, J. (2020). Regionale Klimaprojektionen für Sachsen WMSax2.0. Dresden: Sächsisches Landesamt für Umwelt, Landwirtschaft und Geologie; Sächsische Landesbibliothek – Staats- und Universitätsbibliothek Dresden.
Staelens, J., Nachtergale, L., Luyssaert, S., and Lust, N. (2003). A model of wind-influenced leaf litterfall in a mixed hardwood forest. Can. J. For. Res. 33, 201–209. doi: 10.1139/x02-174
Szwagrzyk, J., Szewczyk, J., and Bodziarczyk, J. (2001). Dynamics of seedling banks in beech forest: results of a 10-year study on germination, growth and survival. For. Ecol. Manage. 141, 237–250. doi: 10.1016/S0378-1127(00)00332-7
Tiebel, K., Huth, F., and Wagner, S. (2016). Qualität von buchenvoranbauten (Fagus sylvatica L.) unterschiedlicher flächengröße unter fichtenschirm (Picea abies (L.) KARST.). Allg. For. Jagdzeitung 187, 103–120.
Topoliantz, S., and Ponge, J. F. (2000). Influence of site conditions on the survival and growth of Fagus sylvatica seedlings in an old-growth beech forest. J. Veg. Sci. 11, 369–374. doi: 10.2307/3236629
Unkrig, V. (1997). Zur verjüngung von buche und fichte im Naturwald Sonnenkopf. For. Holz 52, 538–543.
Vacek, Z., Vacek, S., Bílek, L., Král, J., Remeš, J., Bulušek, D., et al. (2014). Ungulate impact on natural regeneration in spruce-beech-fir stands in Černý důl Nature Reserve in the Orlické Hory Mountains, case study from Central Sudetes. Forests 5, 2929–2946. doi: 10.3390/f5112929
Venables, W. N., and Ripley, B. D. (eds). (2002). “Random and mixed effects,” in Modern Applied Statistics with S (New York, NY: Springer), 271–300.
Vesterdal, L. (1999). Influence of soil type on mass loss and nutrient release from decomposing foliage litter of beech and Norway spruce. Can. J. For. Res. 29, 95–105. doi: 10.1139/x98-182
von Lüpke, B., and Spellmann, H. (1997). Aspekte der stabilität und des wachstums von mischbeständen aus fichte und buche als grundlage für waldbauliche entscheidungen. Forstarchiv 68, 167–179.
Wagner, C. (1905). Ist es Angezeigt, auf einem Standort, auf dem die Fichte Erfahrungsgemäß hohe Erträge Liefert, Demungeachtet bei der Verjüngung auf Gemischte Bestände – z.B. Beimischung der Buche hinzuarbeiten, Selbst dann, Wenn dies nur auf Künstlichem Weg und mit Kosten Möglich ist? Bericht über die 20. Versammlung des Württ. Forstvereins in Craisheim. 39–54.
Wagner, S. (1998). Calibration of grey values of hemispherical photographs for image analysis. Agric. For. Meteorol. 90, 103–117. doi: 10.1016/S0168-1923(97)00073-7
Wagner, S. (1999). The Initial Phase of Natural Regeneration in Mixed Ash-Beech Stands – Ecological Aspects. Frankfurt am Main: Sauerländer.
Wälder, K., Frischbier, N., Bredemeier, M., Näther, W., and Wagner, S. (2008). Analysis of OF-layer humus mass variation in a mixed stand of European beech and Norway spruce: an application of structural equation modelling. Ecol. Model. 213, 319–330. doi: 10.1016/j.ecolmodel.2007.12.014
Weidenbach (1895). “Erscheint es notwendig und wieweit erfolg versprechend, die einmischung der buche in den fichtenbeständen zu befördern? Verhandlungen des bad,” in Proceedings of the Forstvereins bei Seiner 39. Versammlung, September 2, 1894, Heidelberg, 14–24.
Weidig, J., and Wagner, S. (2021). Growth response of advanced planted European beech (Fagus sylvatica L.) after storm-caused loss of shelterwood. Eur. J. For. Res. 140, 931–946. doi: 10.1007/s10342-021-01376-x
Wiedemann, E. (1923). Zuwachsrückgang und Wuchsstockungen der Fichte in den Mittleren und Unteren Höhenlagen der Sächsischen Staatsforsten. Tharandt: Laux.
Wiegand, T., and Moloney, K. A. (2014). Handbook of Spatial Point-Pattern Analysis in Ecology. Boca Raton, FL: Taylor & Francis.
Worrell, R., and Hampson, A. (1997). The influence of some forest operations on the sustain-able management of forest soils— a review. Forestry 70, 61–85. doi: 10.1093/forestry/70.1.61
Wu, H.-I., Sharpe, P. J., Walker, J., and Penridge, L. K. (1985). Ecological field theory: a spatial analysis of resource interference among plants. Ecol. Model. 29, 215–243. doi: 10.1016/0304-3800(85)90054-7
Zerbe, S. (2019). “Wälder,” in Renaturierung von Ökosystemen im Spannungsfeld von Mensch und Umwelt: Ein Interdisziplinäres Fachbuch, ed. S. Zerbe (Berlin: Springer Spektrum), 107–149. doi: 10.1055/s-0038-1645856
Keywords: forest restoration, European beech (Fagus sylvatical L.), Norway spruce (Picea abies [L.] H. Karst), humus form, natural regeneration, dispersal, humus layer thickness
Citation: Axer M, Kluckow F and Wagner S (2022) Evaluation of a Restoration Approach After One Century – Effects of Admixed European Beech on the Natural Regeneration Potential and Humus Condition in Spruce Stands. Front. For. Glob. Change 5:826186. doi: 10.3389/ffgc.2022.826186
Received: 30 November 2021; Accepted: 02 March 2022;
Published: 07 April 2022.
Edited by:
Jasmin Mantilla Contreras, University of Hildesheim, GermanyReviewed by:
Roque Rodríguez-Soalleiro, University of Santiago de Compostela, SpainMatthias Schmidt, Northwest German Forest Research Institute, Germany
Copyright © 2022 Axer, Kluckow and Wagner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maximilian Axer, bWF4aW1pbGlhbi5heGVyQHR1LWRyZXNkZW4uZGU=, orcid.org/0000-0003-1482-9613
 Maximilian Axer
Maximilian Axer Fabian Kluckow2
Fabian Kluckow2