
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 06 June 2022
Sec. People and Forests
Volume 5 - 2022 | https://doi.org/10.3389/ffgc.2022.696757
This article is part of the Research Topic Genetic Approaches and Conservation of the Atlantic Forest View all 4 articles
The biocultural heritage of a region is developed and adapted after centuries of interrelationships between humans and nature. The endangered brazilwood [Paubrasilia echinata (Lam.), E. Gagnon, H.C. Lima, and G.P. Lewis] is a flagship species with cultural and economic importance that is directly affected by the history of human occupation on the Brazilian coast from the Portuguese colonization beginning in the 16th century onward. Despite its historical–cultural relevance, the lack of effective management and the anthropic pressure in coastal areas of the Brazilian Atlantic Forest (BAF; one of the terrestrial hotspots for biodiversity conservation) has led to the fragmentation of landscape connectivity and drastic degradation of the knowledge associated with biodiversity, including that of brazilwood, which also affects the biocultural diversity of the region. In southeastern Brazil, in the region of Cabo Frio, state of Rio de Janeiro, there are fragments of forest remnants with genetically ancient populations of brazilwood (possibly from the colonization period). The recognition of this valuable natural and cultural heritage induced the establishment of protected areas (PAs) in this region in 1986. Here, we studied how the increase in urbanization can affect the biocultural heritage of a flagship species even those close to protected areas. In nearby areas with populations of brazilwood, we interviewed residents from three communities, namely, Peró and Jacaré (municipality of Cabo Frio), and José Gonçalves (municipality of Armação dos Búzios). We conducted semi-structured interviews and free lists using visual stimuli to understand the current knowledge on this species. Thus, we verified socio-ecological dissociation, both inside and outside the PAs. This indicates a possible threat of losing the local knowledge network and the native populations of brazilwood in these areas, which consequently reveals the weaknesses of the current management models of the local PAs. Despite this, we showed that residents are interested in increasing local mobilization, awareness, environmental education, and reforestation actions aimed at the biocultural conservation of the species that gives the country its name, as well as that of the Atlantic Forest biome.
The increasing loss of biodiversity on the planet has been accompanied by the decline of local ecological knowledge (LEK) (Diaz et al., 2006; Cardinale et al., 2012; Aswani et al., 2018), which affects the biocultural heritage. The biocultural heritage of a region was developed and adapted after centuries of interrelationships between humans and nature (Rotherham, 2015; Bridgewater and Rotherham, 2019; Lindholm and Ekblom, 2019).
In coastal areas, one of the main causes of habitat loss and landscape change is the growth of urbanization. The migratory aspect of globalization has led to the intensification of plant circulation, hybridization, and/or LEK loss (Santos, 2007; Gesteira, 2013; Reyes-García et al., 2013; Emery and Hurley, 2016; Aswani et al., 2018). This has changed the way of life for traditional populations (Gandolfo and Hanazaki, 2011), through increased migrations, urban and peri-urban neighborhood establishment (Diegues, 2004), intensification of real estate speculation, and large-scale tourism (Pereira, 2010; Christóvão, 2011).
Since the beginning of European colonization (16th century) in Brazil, the Brazilian Atlantic Forest (BAF) has consistently been highly pressured by deforestation for economic development. Currently, the BAF houses the largest urban centers in the country (Dean, 1997; WWF, 2019). The BAF has covered more than 1.5 million km2 (Dean, 1997; Tabarelli et al., 2005; Rezende et al., 2018), which remains at 12.4% of its original area (SOS Mata Atlântica - SOSMA, 2019).
From this perspective, the exploitation of brazilwood [Paubrasilia echinata (Lam.) E. Gagnon, H.C. Lima, and G.P. Lewis], which is endemic to the BAF, is an emblematic case of a plant near extinction due to human pressure (Souza, 1939; Dean, 1997; Lima, 2009). Brazilwood is now a Brazilian flagship species (Heywood, 1995) with historical, cultural, and economic importance as a source of red dye (Ferraz, 1939; Souza, 1939; Dean, 1997).
The local indigenous people used brazilwood for firewood and for making weapons (JBRJ, 2003). Currently, brazilwood is used to produce high-quality bows for musical instruments, such as violins, violas, and cellos (Angyalossy et al., 2005; Muralt, 2006; Alves et al., 2008; Dapson and Bain, 2015). Recent studies in ethnopharmacology have also demonstrated the potential of brazilwood, whose bark is used in cases of dysentery and diarrhea, since the seeds have astringent and tonic properties, the stems show a potential for the development of natural products against leishmaniasis, and the wood has antioxidant and antiangiogenic properties, with an emphasis on wood flavonoids (brazilin and brazilein), lignans, coumarins, and tannins (Cota et al., 2011; Gomes et al., 2014; Siqueira et al., 2014).
Some of the remaining brazilwood areas are in the Cabo Frio region, which has a distinct population of this species (Souza, 1939; Beranger, 1968; Dean, 1997; JBRJ, 2003; Lima, 2009), but also strong anthropic pressure (Strohaecker, 2008). The Cabo Frio region has environmental (e.g., geomorphological aspects and climatic diversity) and biodiversity peculiarities (Bohrer et al., 2009), being recognized worldwide as the Center of Vegetal Diversity of Cabo Frio (CVDCF) (Araújo, 1997), which is one of the priority areas for Brazilian biodiversity conservation within BAF (Ministério do Meio Ambiente, 2007).
Based on the State of Rio de Janeiro's Brazilwood Conservation Plan (JBRJ, 2003), it was possible to delimit the 13 remaining areas of brazilwood in the Rio de Janeiro State, nine of which were in the Cabo Frio region, which led to the constitution of several protected areas (PAs) in the region. Some are located between the municipalities of Cabo Frio and Armação dos Búzios, which consist of Atlantic Forest remnants, human settlements, important brazilwood populations, mangrove forests, islands, reefs, dunes, and areas of geological interest (RJ, 2002). The establishment of PAs demonstrated that even though these areas are strongly impacted by real estate speculation and, consequently, suppression of native vegetation cover (JBRJ, 2003), and considering the major socioeconomic and cultural transformations of the region, there are still places for conservation.
The main objective of this study was to investigate how this scenario affected the brazilwood biocultural heritage, even that near PAs. In addition, we aimed to analyze possible changes in the local perception of brazilwood over time.
Brazilwood exploration was a determining factor for the foundation of the first colonial settlements on the Brazilian coast (Almeida, 2013; Freire and Malheiros, 2009). The resin from brazilwood timber had an important place in the European market because of its use as a red dye for clothes (Dean, 1997). Therefore, a massive exploration began in 1501 that lasted ~375 years (Table 1) (Dean, 1997; Lima, 2009; D'Agostini et al., 2013; Dodge, 2018). During the colonial period, brazilwood was a monopoly of the crown. Throughout the 19th century, with the increasingly intensive use of synthetic dyes, brazilwood lost commercial value and began to be treated in a manner similar to other construction woods and used for the production of fine furniture and musical instruments (Lima, 2009). By 1876, the population of brazilwood declined dramatically, and in 1992, the tree was officially included in the list of endangered species (JBRJ, 2003). Its trade was a historical point in the devastation of the BAF. It is estimated that approximately 70 million brazilwood trees populated the BAF (Dean, 1997; D'Agostini et al., 2013), and the loss of vegetation cover is predicted to be ~6,000 km2 in the first century of exploration (Dean, 1997; Lima, 2009). The association between the brazilwood tree and the territory of Brazil marks the identity of the Brazilian people, not only by providing the country a name but also by reinforcing its symbolic role in the construction of Brazilian society. Brazilwood was officially declared the “national tree” in 1978 (Brasil, 1978).
Brazilwood exploration continued for years, whereby indigenous peoples were used to cut and transport brazilwood trees (Fernandes, 2008). Until 1960, the economy of the Cabo Frio region was based on fishing, salt exploration, and, to a lesser extent, livestock (Beranger, 1968). In the 1970s, the construction of the Rio-Niterói bridge and the paving of the RJ-106 highway improved the connection between the State capital to the Cabo Frio region, which accelerated the urbanization and the tourism industry in the region, with the consequent weakening or destruction of the remaining forest areas (Pereira, 2010). In the last 50 years, the Cabo Frio region had an exponential demographic growth due to the incentive to tourism but also the development of the oil industry (Figure 1).
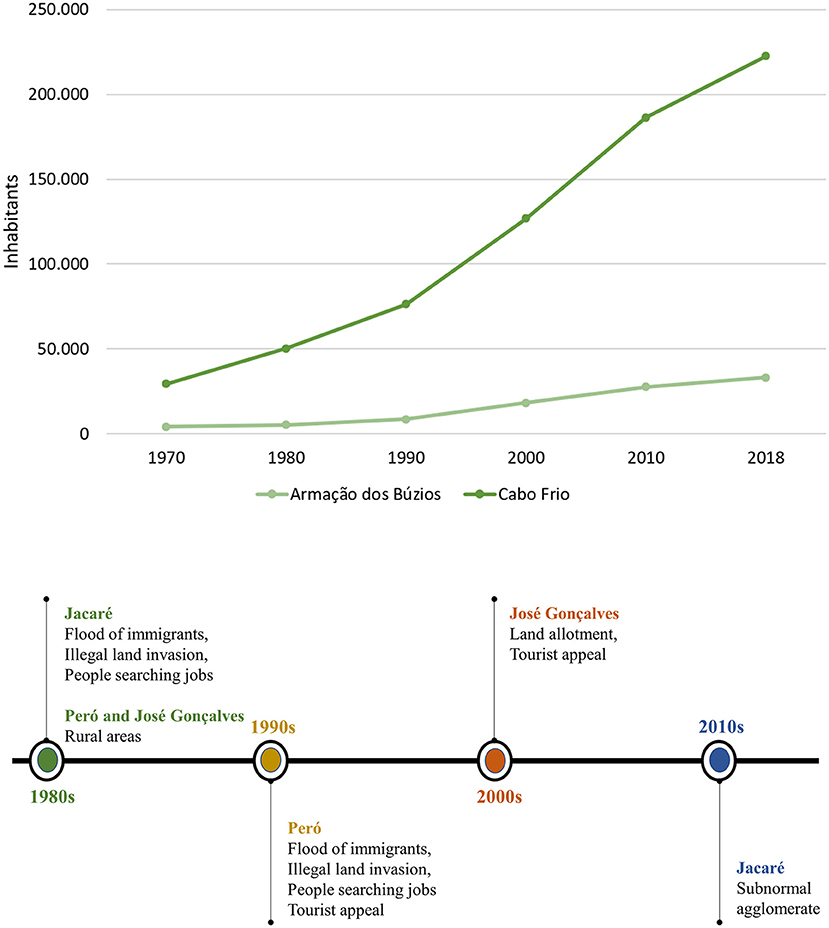
Figure 1. Demographic variation in the Cabo Frio and Armação dos Búzios municipalities (Fundação CEPERJ., 2013; IBGE, 2010), and summarizing demographic and urban growth in the studied communities.
Currently, the economy of the Cabo Frio region is based on the tourism industry (Christóvão, 2011). The tourism, coupled with the benefits of a safer region compared to metropolis, has attracted a greater number of people (Machado and Mello, 2015). Many communities established in the Cabo Frio region have less connection with their surroundings, which affects local conservation (Christóvão, 2011). It is estimated that up to 70% of the area has undergone some type of changes (CBLSJ, 2005).
Demographic growth has brought the establishment of peripheral communities in the region, such as Jacaré. In the 1980s, the Jacaré community grew due to the arrival of migratory people seeking jobs in the oil industry. Eventually, it began showing the characteristics of a subnormal agglomerate, according to the Brazilian Institute of Geography and Statistics (IBGE, 2010), commonly known as a “favela.” In the Peró community, the demographic growth process began in the 1990s. The human occupation in Peró was induced by the development of condominiums and second homes that were associated with tourism. The Peró community is located near the beautiful beaches of Peró and Conchas (Machado and Mello, 2015).
Demographic growth in the community of José Gonçalves (Armação do Búzios) is more recent than the other communities studied. Until the 2000s, the community was considered a rural area (Armação dos Búzios, 2006), which began dividing its properties into small lots, attracting migrants. The José Gonçalves community is the home of touristic points, such as José Gonçalves beach and Serra das Emerências.
To avoid the destruction of the remaining BAF and show the presence of the public authority, some PAs have been established in the region over the years. In the Cabo Frio coastal zone, there are five PAs, namely, Massambaba Environmental Protection Area (APAMAS), established in 1986, Serra de Sapiatiba Environmental Protection Area (APASES), established in 1990, Arraial do Cabo Marine Extractive Reserve (RESEX Arraial), established in 1997, Pau Brasil Environmental Protection Area (APABR), and Costa do Sol State Park (PECS). The Brazilian System of Conservation Units (SNUC) has established APAs that allow human settlements with the aim of the sustainable use of the environment, while parks, such as PECS, have the goal of total protection of the environment, while allowing tourist visitation but no human settlements. Here, we focus particularly on the APABR and PECS, due to their common areas of brazilwood populations, in the three human communities, namely, Peró, Jacaré, and José Gonçalves (Figure 2).
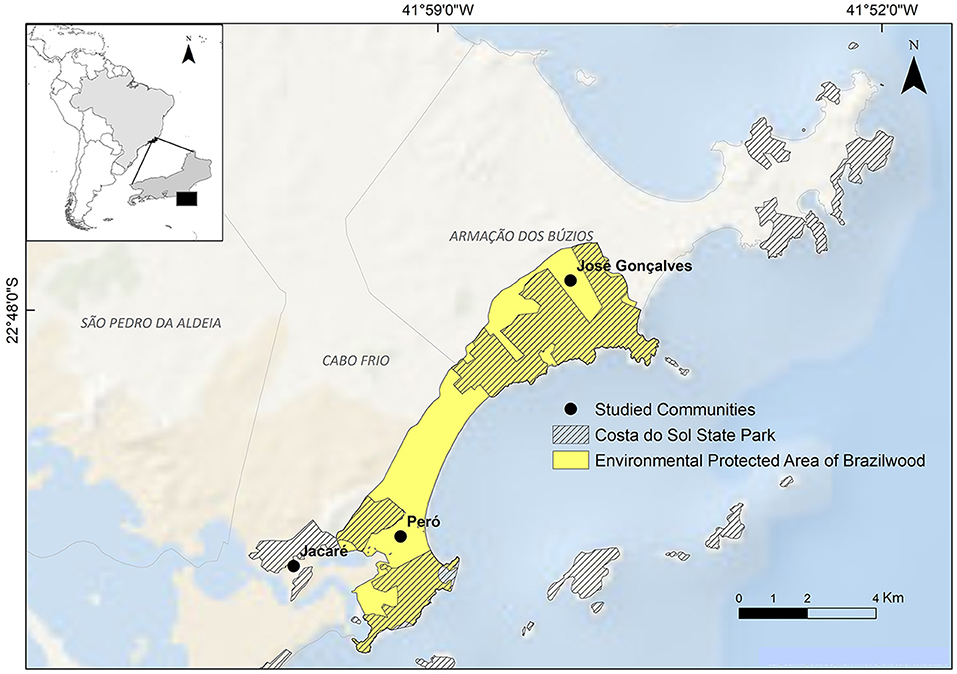
Figure 2. Study area: local communities studied and the local protected areas: PECS (Costa do Sol State Park) hatched and APABR (Pau Brasil Environmental Protected Area) in yellow.
We interviewed residents from three communities, namely, Peró (22°51′34.07″S, 41°59′35.17″W) and Jacaré (22°52′1.83″S, 42°1′16.31″W) in Cabo Frio municipality, and José Gonçalves (22°47′31.70″S, 41°56′54.54″W) in Armação dos Búzios municipality (refer to Figure 2).
In 2003, the Peró community had ~1,086 families (JBRJ, 2003) and 2,443 families in 2018 (data from neighborhood's family health facility, Supplementary Materials). It is located 3.5 km from the center of Cabo Frio, between Peró and Conchas beaches, and Morro da Piaçava. This community has three schools (i.e., two public schools and one private school), two health posts, a small shopping center, small stores in various segments, and a sub-mayor's office of the municipality of Cabo Frio.
In 2003, the Jacaré community had ~2,000 families (JBRJ, 2003) and 2,434 families in 2018 (data from neighborhood's family health facility, Supplementary Materials). It is located 1.5 km from the center of Cabo Frio in an area between Morro do Mico and Morro do Telégrafo. There are five public schools, health post, and shops.
The approximate number of families in the José Gonçalves community grew from 600 in 2003 (JBRJ, 2003) to 982 in 2018 (data from neighborhood's family health facility, Supplementary Materials). It is located 8.5 km from the center of Armação dos Búzios and 5 km far from the other communities studied. It has a public school, health post, and shops. Armação dos Búzios is a municipality that borders the west with the municipality of Cabo Frio, from which it became autonomous in 1995. José Gonçalves is located between Serra das Emerências, José Gonçalves Beach, and Baía Formosa. Historically, José Gonçalves was a slave trader, who lived and used the region for the landing of slaves until the end of the 19th century (Accioli, 2012).
We combined bibliographic research to map the main historical events and socioeconomic changes of the region considering the brazilwood trade, with local residents' interviews regarding their knowledge on this tree. The 2018 research was registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen), under the number A238919, and each interviewee signed the Free and Informed Consent Form (TCLE). There was no such system in 2003, which resulted in a bigger participation of respondents.
We interviewed one representative of each household, either an adult, male or female, either natural (born) or migrant in the Cabo Frio region. Data were collected through semi-structured interviews, including questions about socioeconomic data (e.g., age, gender, birthplace, economic income, and schooling), free lists (Weller and Romney, 1988), and open-ended questions for content analysis (Bardin, 1977).
The main interview questions were: Could you list the five most common trees in the region? Do you know any Protected Areas in the region? Do you know brazilwood? Would you know how to inform others about the importance of brazilwood? For those who were familiar with brazilwood, we also asked: Over the years, have you noticed an increase or decrease in brazilwood trees? Why? Is it easy or difficult to find brazilwood in the region? From this set of images of trees, could you indicate if there is a photo of a brazilwood? What would need to be done to conserve native brazilwood trees?
We used visual stimuli (Albuquerque et al., 2010) through photographs of the following trees common in the region: a. Brazilwood (correct species); b. Schinus terebinthifolia Raddi (species abundant in the area, and used in local medicine and as an aromatic compound); c. Tabebuia roseo-alba (Ridl.) Sand. (common tree, and appreciated for the flowering season); and d. Casuarina equisetifolia L. (an exotic species intensively introduced for at least 60 years and widely distributed in the region) (Figure 3). This method aimed to verify the ability to visually recognize brazilwood, showing possible inconsistencies between what was observed and what was relayed.

Figure 3. (A) Brazilwood, Paubrasilia echinata (Lam.) E. Gagnon, H.C. Lima & G.P. Lewis, (B) Schinus terebinthifolia Raddi, (C) Tabebuia roseo-alba (Ridl.) S, and (D) Casuarina equisetifolia L.
In 2018, we interviewed 50 residents in Peró and 28 residents in Jacaré, in the Cabo Frio municipality, and 40 residents in José Gonçalves, in the Armação dos Búzios municipality.
A comparative analysis with a diachronic perspective (Reyes-García et al., 2013) was carried out based on a set of interviews collected in 2003 by Viviane Fonseca-Kruel, who performed an ethnobotanical inventory on the local perception of brazilwood in the Cabo Frio region (JBRJ, 2003). This previous study has interviewed 577 residents, namely, 381 in the Jacaré community, 156 in Peró, and 40 in the José Gonçalves community. We checked the names of people interviewed in 2003 with the names of people interviewed in 2018 and found that none of the interviewees participated in both surveys.
We analyzed the changes in the biocultural heritage of brazilwood through socioeconomic and land-use changes over time and related them to the importance of conserving the biocultural heritage of brazilwood. Historical analysis was conducted considering the 1970s as a demographic growth milestone for the Cabo Frio region (Pereira, 2010). We used the chi-square test for independence to test the proportions of men and women interviewed in each period, using p < 0.01 as the reference for significant differences. We used the ANTHROPAC version 4.0 software (Borgatti, 1996) to analyze the free lists to verify the cultural salience index (CSI) of brazilwood (Smith, 1993). CSI varied from 0 to 1, where 1 indicated very prominent. For the systematization and contextualization of the qualitative data, we used the technique of content analysis (Bardin, 1977).
Table 2 highlights the differences between the studied communities and how they evolved throughout 15 years. We found no significant differences in the proportions of women and men interviewed in both periods as a whole (for interviews in 2003: chi-square = 7.7067, p = 0.021208; for interviews in 2018: chi-square = 2.6987, p = 0.259403) and at the community level (Jacaré chi-square = 1.9302, p = 0.164731; José Gonçalves chi-square = 0.8184, p = 0.365644), except for Peró where these differences were significant (chi-square = 9.5085, p = 0.002045).
Currently, the Peró community had the highest level of education among the communities studied, while the Jacaré community had the lowest. The proportion of native residents (born in the region) was higher in the community of José Gonçalves than in the other two communities.
The semi-structured interviews showed that 99% of the residents stated that they were aware of brazilwood, but when we deepened the analysis through the free listing and visual stimulus, the results differed considerably.
Figure 4 shows that brazilwood was visually recognized by 55% of the inhabitants of the Cabo Frio region in 2018, mainly in the communities of Peró (60%) and José Gonçalves (66%). In the Jacaré community, the choice of the unique exotic species used in the research (Casuarina equisetifolia) was identified as brazilwood in 46% of the recognitions. This widespread alien species is directly linked to the old saltpans in the region (Rosa Carvalho, 2014; Zimmermann, 2016).
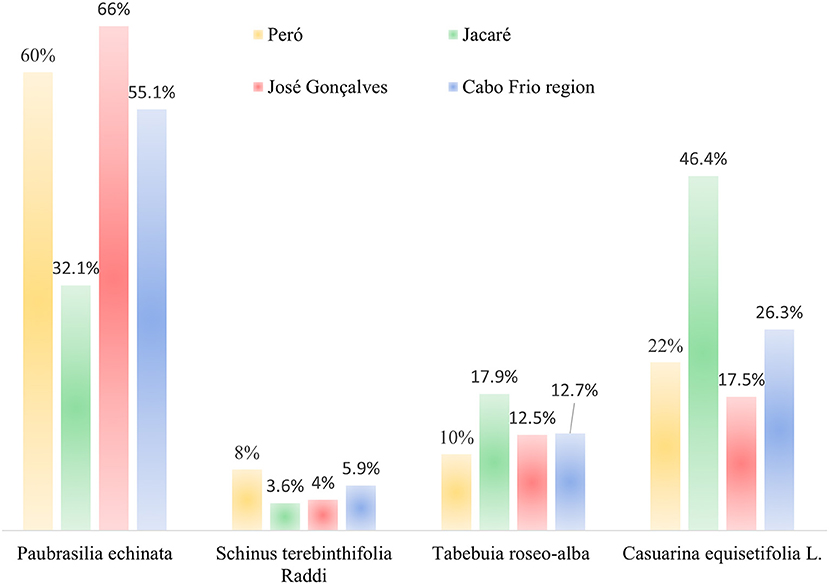
Figure 4. Results of the visual stimuli technique among the interviewees in the municipalities of Cabo Frio (Peró and Jacaré) and Armação dos Búzios (José Gonçalves) in the state of Rio de Janeiro, Brazil.
In 2018, respondents listed a total of 104 plants, 31 of which were native and 73 were exotic to the region. In José Gonçalves, native species appeared in 38% of the interviews, while in Peró and Jacaré, they were included in 23 and 17%, respectively. In the Jacaré community, exotic species were mentioned in 83% of the interviews. This result suggests how the urbanization process can influence the conservation of biocultural heritage, thereby threatening a loss of the knowledge on native species and the conservation of the brazilwood in the region.
Through the free listings, we analyzed the CSI of brazilwood, which reflects a cognitive understanding of the plants of the region (Bernard, 1988). Brazilwood was the most salient tree species mentioned in José Gonçalves and the third in Peró, but in Jacaré, it figured out to be in the 13th place (Table 3). The results highlight the differences between the LEK over this flagship species among the studied communities and can reflect the status of conservation of the biocultural heritage of brazilwood among the studied communities.
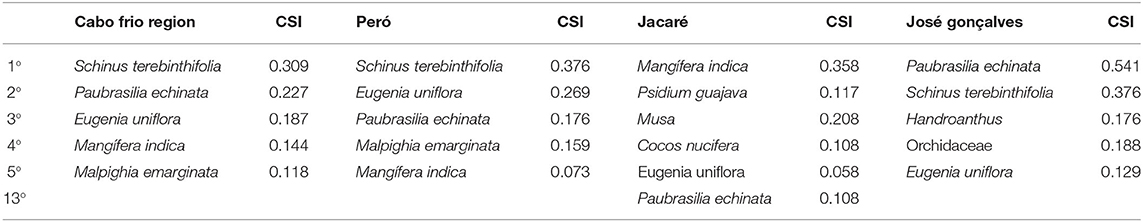
Table 3. Cultural salience index (CSI) results among the interviewees in the municipalities of Cabo Frio (Peró and Jacaré) and Armação dos Búzios (José Gonçalves) in the state of Rio de Janeiro, Brazil.
The multiple factors affecting the region increase the necessity of public authorities to find the methods of managing territory while attempting to satisfy the environmental legal parameters of conservation. However, existence is not an indication of effectiveness. In the Cabo Frio region, our research showed that this management model may be fragile, because only 11% of all interviewees were aware of PECS and 12% of APABR. In the Jacaré community, 64% did not know any PAs.
Nonetheless, 35% of the interviewees of the José Gonçalves community showed an encouraging connection with APABR, indicating a greater possibility of interaction between the community and PAs in future activities focusing on nature conservation in the region.
The content analysis (Table 4) showed that the communities had an interest in promoting activities aimed at the preservation of nature in the region, demonstrating a predisposition for the effectiveness of future conservationist actions. However, the creation of PAs in Brazil may be less effective due to the lack of knowledge on the concept for most of the interviewees. It can also be inferred that the main concern and risk that these areas endure currently is deforestation, which is usually associated with the lack of awareness, illegal construction, and real estate speculation.
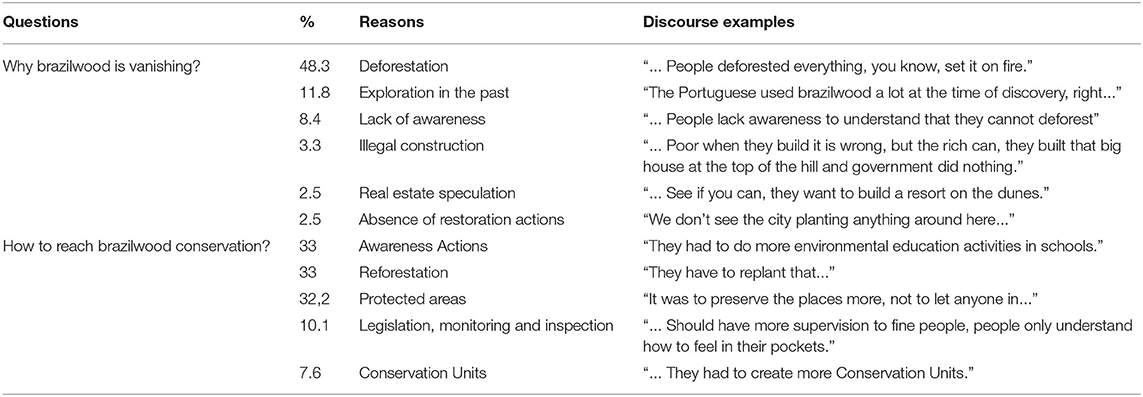
Table 4. Content analysis technique results among the interviewees in the municipalities of Cabo Frio (Peró and Jacaré) and Armação dos Búzios (José Gonçalves) in the state of Rio de Janeiro, Brazil.
The data collected in 2003 in the same communities revealed interesting results regarding changes to the local population over the years and their effect on the relationship with brazilwood. When asked regarding the source of their knowledge on brazilwood (Table 5), a significant change occurred, reinforcing the idea that current schools are emphasizing the symbolic role of brazilwood.
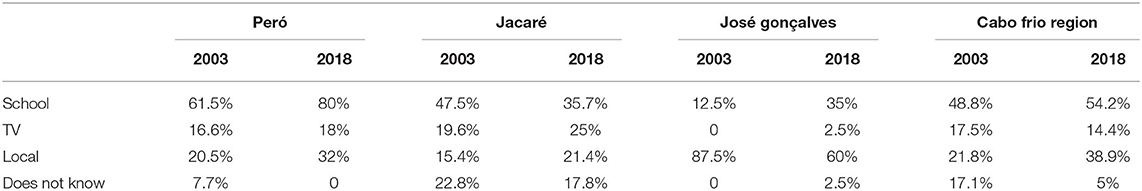
Table 5. Diachronic analysis comparing the changes in the origin of the knowledge between Cabo Frio region residents over 15 years.
In the Peró community, there has been an increase in the percentage of respondents recognizing brazilwood, mainly related to schooling over the years. In the Jacaré community, this knowledge acquired from schools was reduced, as was the number of people who were unaware of how they attained their knowledge on brazilwood. In the José Gonçalves community, the less urbanized community among those surveyed, there was a marked reduction in the number of interviewees who stated that their knowledge about the brazilwood was related to their own community, while knowledge received from school was more prevalent. For the first two communities, we must consider that the context of accelerated growth and the current social scenario did not allow the same sampling effort adopted in 2003 to be maintained in 2018. These differences in the sampling effort may have reflections on the most recent data for these two communities. However, in José Gonçalves, a similar sampling effort was feasible (6.7% of the estimated population in 2003 and 4.1% in 2018). For this community, in particular, diachronic comparisons can be made with greater confidence.
Understanding the perception of the importance of brazilwood (Figure 5) showed us that the historical relevance is the main importance given to brazilwood according to the residents. However, there was a commonality in the results obtained. The education has been standardized over the years, as well the perception of brazilwood importance.
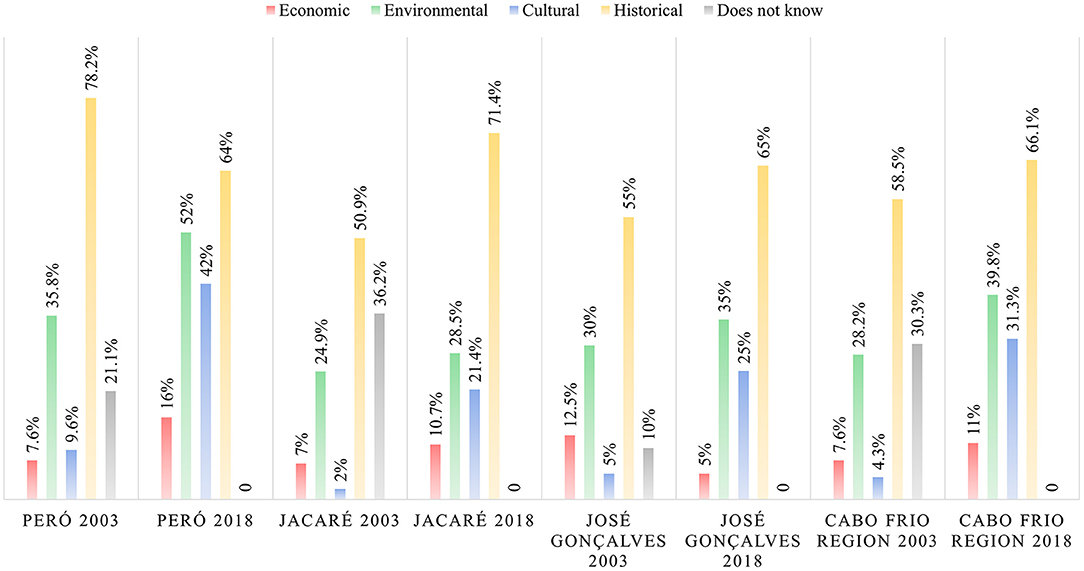
Figure 5. Diachronic analysis comparing the changes in brazilwood importance perception between Cabo Frio region residents over 15 years.
Regarding the question of whether residents found it was easy or difficult to find brazilwood in the region (Figure 6), there was a clear difference in the perceptions of brazilwood abundance between the Cabo Frio communities (Peró and Jacaré) and the Armação dos Búzios community (José Gonçalves). According to the José Gonçalves community, brazilwood was readily found in the region, whereas both Cabo Frio communities stated that it was difficult to locate brazilwood in these areas.
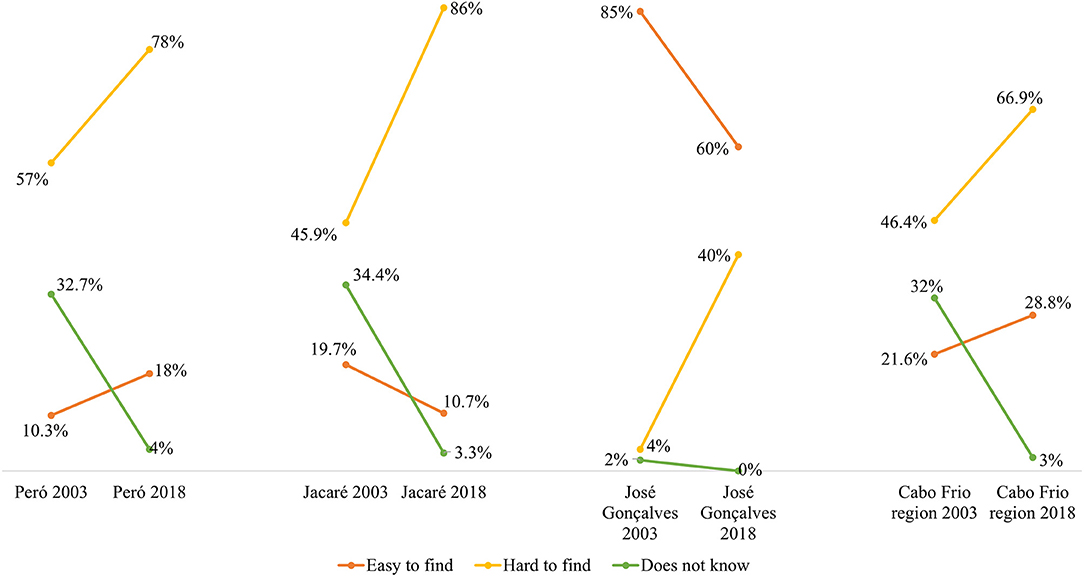
Figure 6. Diachronic analysis comparing the changes in brazilwood abundance perception between Cabo Frio region residents over 15 years.
In the Cabo Frio region, brazilwood is no longer threatened by its exploration to produce dye or high-quality wood, but by deforestation. In the beginning of the 20th century, the deforestation threat came mainly from agricultural expansion and, at present, deforestation is driven by the predatory tourism industry and its correlates. Coastal areas undergo subdivision processes for the construction of condominiums for vacation homes, hotels, and real estate developments. The changes in land use are intensified as the city grows in a disorderly way, and the seasonality of the occupation (during holidays and weekends) creates moments of hyper-intensive use of public services and local natural attractions. The population that permanently inhabits Cabo Frio and Armação dos Búzios lives on the basis of the provision of services for the structure of tourism. As the municipalities' growth was not planned, the working population often occupies the often occupies public areas and often destroys the remaining forest for small swiddens and subsistence activities. The tourism industry dictated the recent urbanization process.
This history of occupation, deceived from exogenous activities (Rodrigues, 2005) based on the arrival of migrants from other regions of Brazil, tends to negatively affect the conservation of the biocultural heritage of the region (Santos, 2007). It also allows us to observe that the communities in the municipality of Cabo Frio, where these processes began earlier, had less knowledge on brazilwood in relation to the José Gonçalves community.
The knowledge regarding the tree comes basically from basic education, mainly from history classes (Macedo et al., 2018). Although brazilwood is currently symbolically recognized for having given the country name, it is less known by the general population.
In the case of the Cabo Frio region, which is the main region in the state of Rio de Janeiro to shelter native areas and remnants of brazilwood, the tree is less known. In the communities studied, the situation is also a relative ignorance. It is likely that populations with a higher level of education have learned to identify the species based on its symbolic relevance. The more traditional and rural community of José Gonçalves possibly conserves the knowledge on the forest that passes through generations. In the case of the Jacaré community, which is more recent and socially vulnerable, the low level of education and less organic relationship with the region can explain the results in the interview.
The presence of exotic species in the free list, especially in the Jacaré community (83% of exotic species mentioned), highlights the distance of communities from the environment in which they live, in addition to an evident transformation in their LEK as noticed by Diaz et al. (2006), Cardinale et al. (2012), and Aswani et al. (2018). Lack of knowledge on local flora among the residents of the Jacaré community indicates that the remnants of brazilwood found in the surroundings of this community are at the greatest conservation risk in the region.
Another concerning data are the fact that 45% of the interviewees did not visually recognize brazilwood, although 99% said they knew about it. This scenario highlights the urgency to change this discrepancy and to avoid further conservation challenges.
Although less is known and is generally ineffective to date, the PAs in the region are important tools for the conservation. The results obtained support the ideas of Begossi (1998), Alves and Hanazaki (2015), Vandebroek and Balick (2012), and Silva (2013), who showed that even the establishment of PAs, which changes environmental perceptions, is not sufficient to generate a feeling of belonging in the local population. Instead, it could harm the relationship between humans and local nature. Mcpherson et al. (2016) stated that the involvement of the community in nature conservation issues allows for reconciliation between culture and conservation, which can work to prevent the extinction of species, or at least delay this outcome through risk reduction.
Diachronic analysis showed a tendency toward standardization of knowledge and perceptions in the region, especially for José Gonçalves where our sampling effort was similar in 2018 and 2003, and which is common in urbanized and interconnected societies (Santos, 2007). However, among the communities studied, José Gonçalves reported the most instances of the community region itself being identified as the source of knowledge, indicating the presence of the species in the daily lives of this population. The José Gonçalves community also maintained the largest number of native inhabitants among the studied communities, which suggests a relationship with the history and temporality of brazilwood. This is corroborated by the fact that the historical importance of the species was the most cited in this community in 2018, which may indicate a greater proximity of the community to nature and, thus, an improved perception of the importance of biocultural preservation. Considering the intense demographic growth in the region, we suggested that future samplings prioritize the saturation of responses over new information on knowledge on brazilwood, as well as the representation of gender and age groups and levels of education.
Socioeconomic characteristics appear to play a fundamental role in the relationship between communities in the municipality of Cabo Frio and the surrounding nature. In the Jacaré community, which is the lowest level of education between the studied communities and is considered a subnormal agglomerate, we observed the largest number of people who could not identify how they were introduced to brazilwood. This community also showed a decrease in those who stated that they learned about brazilwood in school when compared to 15 years ago. The Peró community had higher levels of education, and 80% of the knowledge on the species was attained through schooling, which indicates that LEK was superseded by school knowledge, where brazilwood was identified more as a symbol than a living being that needs to be conserved and to have its habitat protected. For further studies, we recommend an awareness of gender balance among interviewees, especially in this community where we had an unbalanced sample.
The ability to recognize and perceive the species was another indicator of a better understanding of brazilwood in the José Gonçalves community than in the other studied communities. The maintenance of this biocultural heritage can indicate a greater involvement in the conservation of the species and the environment in which it occurs. Such characteristics suggest that this community has a stronger connection to the nature surrounding it when compared to the other communities studied, although it does not exempt them from the risks of biocultural loss related to this species.
Despite the historical and economic importance of brazilwood, over 500 years of Brazilian history, in the studied region, there were no reports of the current use of brazilwood for dyeing or medicinal purposes. The species has a symbolic role as a flagship species, which is taught in schools relating to the history of colonization in Brazil.
This research highlighted the gap between the disciplines of history and biology in the context of Brazilian education. This socio-ecological dissociation is both inside and outside PAs, which threatens the network of local knowledge on native populations of brazilwood. Consequently, this reveals weaknesses in the current management models of local PAs, which has space to improve the local people approach.
Overall, the results suggest that in the Cabo Frio region the conservation of nature and the biocultural heritage of brazilwood is in decline, either due to the loss or transformation of LEK or the intense migration in the region, and that there is a loss of natural habitat for populations of native species under anthropogenic pressure and alteration of the local landscape. However, there is scope for biocultural conservation actions including social participation and mobilization and awareness-raising among PAs managers and politicians to increase the effectiveness of decision-making regarding the local environment.
The specific case of brazilwood shows the importance that awareness of history could provide in environmental preservation policies, when coupled with discussions regarding biocultural heritage. Furthermore, the approximation with nature and the recognition of local plant species can also reinforce the symbolic connections between nature and national identity, and thereby contribute to the improvement of environmental protection policies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen), under the number A238919. The participants provided their written informed consent to participate in this study.
JB made the 2018 interviews, analyzed, and wrote the article. LK and RC historical survey consultant and historical research. NH ethnobiological survey consultant and contributed to the text discussion and writing. VF-K guided all this research, as well as conducted the research carried out in the same area 15 years ago and part of this data was used and analyzed here and also collaborated in the writing and analysis of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
To the staff of the Scientific Computing Center and GIS Board of Research of the Rio de Janeiro Botanical Garden, especially Ernani Bellon for the preparation of maps. To all participants from the communities where this research took place, who made their time and knowledge available to carry out the research. To Fernanda Terra, from PPGHIS/UFRJ, for helping us with the translation. NH thanks to CNPq for a research productivity scholarship (304515/2019-1). LK thanks to CNPq for a research productivity scholarship (310255-2019-8). VF-K is grateful to researchers Tania Sampaio and Haroldo Lima (JBRJ) for the opportunity to participate and to have collected ethnobotanical data during the elaboration of the Brazilwood Conservation Action Plan, Caesalpinia echinata, in State of Rio de Janeiro, Brazil (Fauna & Flora International, 2006).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2022.696757/full#supplementary-material
Accioli, N. T. (2012). José Gonçalves da Silva à Nação brasileira: O tráfico ilegal de escravos no antigo Cabo Frio. Niterói: FUNARJ.
Albuquerque, U. D., Lucena, R. D., and Cunha, L. V. F. C. (2010). Métodos e técnicas na pesquisa etnobiológica e etnoecológica. Recife: Nupeea.
Almeida, M. R. C. (2013). Metamorfoses indígenas. Identidade e cultura nas aldeias coloniais do Rio de Janeiro. Rio de Janeiro, FGV, 2ª edição.
Alves, E. S., Amano, E., and Longui, E. L. (2008). Pernambuco wood (Caesalpinia Echinata) used in the manufacture of bows for string instruments. IAWA J. 29, 323–335. doi: 10.1163/22941932-90000190
Alves, R. P., and Hanazaki, N. (2015). Áreas protegidas marinho-costeiras de Santa Catarina sob a perspectiva das populações locais: contribuições da literatura. Ambiente Soc. 18, 97–118. doi: 10.1590/1809-4422ASOC974V1842015
Angyalossy, V., Amano, E., and Alves, E. S. (2005). Madeiras utilizadas na fabricação de arcos para instrumentos de corda: aspectos anatômicos. Acta Bot. Brasil. 19, 819–834, doi: 10.1590/S0102-33062005000400018
Araújo, D. S. D. (1997). “Cabo Frio region, southeastern Brazil,” in Centers of Plant Diversity: A Guide and Strategy for Their Conservation, eds S. D. Davis, V. H. Heywood, O. Herrera-Macbryde, J. Villa-Lobos, and A. C. Hamilton (Washington, DC: The Americas).
Armação dos Búzios (2006). Plano Diretor de Desenvolvimento Sustentável de Armação dos Búzios – Perfil do Município. Armação dos Búzios.
Aswani, S., Lemahieu, A., and Sauer, W. H. H. (2018). Global trends of local ecological knowledge and future implications. PLoS ONE 4, e0195440. doi: 10.1371/journal.pone.0195440
Begossi, A. (1998). Resilience and neo-traditional populations: the caiçaras (Atlantic Forest) and caboclos (Amazon, Brazil). Link. Soc. Ecol. Syst. 1998, 129–157.
Bernard, H. R. (1988). Research Methods in Anthropology: Qualitative and Quantitative Approaches. Oxford: Altamira Press.
Bohrer, C. B. A., Dantas, H. G. R., Cronemberger, F. M., Vicens, R. S., and Andrade, S. F. (2009). Mapeamento da vegetação e do uso do solo no Centro de Diversidade Vegetal de Cabo Frio, Rio de Janeiro, Brasil. Rodriguésia 60, 1–23. doi: 10.1590/2175-7860200960101
Brasil. (1978). Lei 6607, 7 de dezembro de 1978. Available online at: https://www.planalto.gov.br/ccivil_03/Leis/L6607.htm
Bridgewater, P., and Rotherham, I. D. (2019). A critical perspective on the concept of biocultural diversity and its emerging role in nature and heritage conservation. People Nat. 1, 291–304. doi: 10.1002/pan3.10040
Cardinale, B. J., Duffy, J. E., Gonzalez, A., Hooper, D. U., Perrings, C., Venail, P., et al. (2012). Corrigendum: Biodiversity loss and its impact on humanity. Nature 2012, 59–87. doi: 10.1038/nature11373
Christóvão, J. H. O. (2011). Do Sal ao Sol: A construção social da imagem do turismo em Cabo Frio. São Gonçalo: UERJ.
Comitê das Bacias Hidrográficas da Região dos Lagos e do rio São João, - CBLSJ. (2005). Plano da Bacia Hidrográfica da Região dos Lagos e do Rio São João; Consórcio Intermunicipal para Gestão das Bacias Hidrográficas da Região dos Lagos, Rio São João e Zona Costeira.
Cota, B. B., de Oliveira, D. M., de Siqueira, E. P., Souza-Fagundes, E. M., Pimenta, A. M. C., Santos, D. M., et al. (2011). New cassane diterpenes from Caesalpinia echinata. Fitoterapia 82, 969–975. doi: 10.1016/j.fitote.2011.05.014
D'Agostini, S., Bacilieri, S., Hojo, H., Vitiello, N., Bilynskyj, M. C. V., Filho, A. B., et al. (2013). Ciclo econômico do Pau-Brasil: Cesalpinia Echinata Lam., 1785. Rev. Páginas Inst. Biol. 9:1785.
Dapson, R., and Bain, C. (2015). Brazilwood, sappanwood, brazilin and the red dye brazilein: from textile dyeing and folk medicine to biological staining and musical instruments. Biotechnic. Histochem. 90, 401–423. doi: 10.3109/10520295.2015.1021381
Dean, W. (1997). A Ferro e fogo a história da devastação da mata atlântica brasileira. São Paulo: Companhia das Letras.
Diaz, S., Fargione, J., Chapin, F. S., and Tilman, D. (2006). Biodiversity loss threatens human well-being. PLoS Biol. 4:e277. doi: 10.1371/journal.pbio.0040277
Diegues, A. C. (2004). “A mudança como modelo cultural: o caso da cultura caiçara e a urbanização,” in Enciclopédia caiçara: o olhar do pesquisador, ed A. C. Diegues (São Paulo: Editora Hucitec).
Dodge, C. J. G. (2018). A forgotten century of brazilwood: the brazilwood trade from the mid-sixteenth to mid-seventeenth century. e-JPH 16, 1–27.
Emery, M., and Hurley, P. T. (2016). Ethnobiology in the city: Embracing the urban ecological moment. J. Ethnobiol. 36, 807–819. doi: 10.2993/0278-0771-36.4.807
Fernandes, F. L. (2008). A Feitoria Portuguesa do Rio de Janeiro. Revista de História 27, 155–194. doi: 10.1590/S0101-90742008000100010
Freire, J. R. B., and Malheiros, M. F. (2009). Aldeamentos indígenas do Rio de Janeiro. Rio de Janeiro, EdUerj.
Fundação CEPERJ. (2013). Anuário Estatístico do Estado do Rio de Janeiro. Available online at: http://www.ceperj.rj.gov.br/Conteudo.asp?ident=75 (Accessed April 11, 2021).
Gandolfo, E. S., and Hanazaki, N. (2011). Etnobotânica e urbanização: conhecimento e utilização de plantas de restinga pela comunidade nativa do distrito do Campeche (Florianópolis, SC). Acta Botanica Brasilica 25, 168–177. doi: 10.1590/S0102-33062011000100020
Gesteira, H. M. (2013). A América portuguesa e a circulação de plantas. Séculos XVI-XVIII. Usos e Circulação de plantas. Brasil Séculos.
Gomes, E. C. B. S., Jimenez, G. C., da Silva, L. C. N., de Souza, K. P. C., Paiva, G. S., et al. (2014). Evaluation of Antioxidant and Antiangiogenic Properties of Caesalpinia Echinata Extracts. J. Cancer 5, 143–150. doi: 10.7150/jca.7439
IBGE (2010). Censo Cabo Frio 2010. Available online at: https://cidades.ibge.gov.br/brasil/rj/cabo-frio (Accessed April 12, 2021).
Jardim Botânico do Rio de Janeiro - JBRJ. (2003). Plano de Conservação para o Pau Brasil no Estado do Rio de Janeiro. Brasil: Rio de Janeiro.
Lima, J. S. (2009). Pau-brasil: os diferentes significados dos discursos de conservação séculos XIX e XX. UFRRJ.
Lindholm, K.-J., and Ekblom, A. (2019). A framework for exploring and managing biocultural heritage. Anthropocene 2019, 100195. doi: 10.1016/j.ancene.2019.100195
Macedo, T. M., Silva, A. V. S., Gonçalves, M. L., and Aguiar-Dias, A. C. A. (2018). Pau-brasil: como conservar sem conhecer?. Diversidade e Gestão 2, 189–197.
Machado, L. D., and Mello, D. S. (2015). O pleito judicial e o movimento SOS Dunas do Peró: um caso de participação popular envolvendo conselhos de Unidades de Conservação. UFF.
Mcpherson, J., Sammy, J., Sheppard, D., Mason, J., Brichieri-Colombi, T., and Moehrenschlager, A. (2016). Integrating traditional knowledge when it appears to conflict with conservation: lessons from the discovery and protection of sitatunga in Ghana. Ecol. Soc. 21, 24. doi: 10.5751/ES-08089-210124
Ministério do Meio Ambiente, – MMA. (2007). Portaria MMA n°9, de 23 de janeiro de 2007. Áreas Prioritárias para Conservação, Uso Sustentável e Repartição de Benefícios da Biodiversidade Brasileira. Brasília: Série Biodiversidade.
Muralt, M. (2006). A árvore que se tornou país. REVISTA USP 71, 171–198. doi: 10.11606/issn.2316-9036.v0i71p171-198
Pereira, W. L. C. (2010). Waves of modernity: the Companhia Nacional de Alcalis in Arraial do Cabo (1943-1964). Estudos Históricos 23, 321–343. doi: 10.1590/S0103-21862010000200006
Reyes-García, V., Guèze, M., Luz, A. C., Paneque-Gálvez, J., Macía, M. J., Orta-Martínez, M., et al. (2013). Evidence of traditional knowledge loss among a contemporary indigenous society. Evol. Hum. Behav. 34, 249–257. doi: 10.1016/j.evolhumbehav.2013.03.002
Rezende, C. L., Scarano, F. R., Assad, E. D., Joly, C. A., Metzger, J. P., Strassburg, B. B. N., et al. (2018). From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 16, 208–214. doi: 10.1016/j.pecon.2018.10.002
Rio de Janeiro - RJ (2002). Decreto Estadual n° 31.346, de 06 de junho de 2002. Institui a Área de Proteção Ambiental do Pau Brasil.
Rodrigues, F. M. (2005). Forma, imagem e significado em estruturas urbanas centrais. Niterói: EdUFF.
Rosa Carvalho, A. S. (2014). Processos de regeneração ‘via semente' em uma formação arbustiva aberta da Restinga de Massambaba, Arraial do Cabo, RJ. JBRJ.
Rotherham, I. D. (2015). Bio-cultural heritage and biodiversity: emerging paradigms in conservation and planning. Biodiversity Conserv. 24, 3405–3429. doi: 10.1007/s10531-015-1006-5
Santos, B. S. (2007). Para além do pensamento abissal: das linhas globais a uma ecologia de saberes. Novos Estudos – CEBRAP 79, 71–94. doi: 10.1590/S0101-33002007000300004
Silva, N. F. (2013). Contribuição do saber local na identificação de plantas medicinais prioritárias para a conservação in situ na floresta nacional do Araripe, nordeste do Brasil. UFRPE.
Siqueira, E. P., Zani, C. L., Alves, T. M. A., Parreiras, P. M., Martins Filho, O. A., Araújo, M. S. S., et al. (2014). Evaluation of the in vitro leishmanicidal and In vivo acute oral toxicity of the Caesalpinia echinata L. extracts as source of natural products against Leishmaniasis. J. Natural Product Plant Res. 4, 30–38.
Smith, J. J. (1993). Using Anthropac 3.5 and a spreadsheet to compute a free-list salience index. Cultu Anthropo Methods 9, 8–12. doi: 10.1177/1525822X9300500301
SOS Mata Atlântica - SOSMA (2019). Atlas dos Remanescentes Florestais da Mata Atlântica: Período 2017-2018, Relatório Técnico.
Souza, B. J. (1939). O Pau-brasil na História Nacional. São Paulo: Companhia Editora Nacional e MEC.
Strohaecker, T. M. (2008). Dinâmica populacional, Macrodiagnóstico da Zona Costeira e Marinha do Brasil. Brasília, IBAMA/MMA.
Tabarelli, M., Pinto, L. P., da Silva, J. M. C., and Bede, L. C. (2005). Desafios oportunidades para a conservação da biodiversidade na Mata Atlântica brasileira. Megadiversidade 1, 132–138.
Vandebroek, I., and Balick, M. J. (2012). Globalization and loss of plant knowledge: challenging the paradigm. PLoS ONE 7, e37643. doi: 10.1371/journal.pone.0037643
Weller, S. C., and Romney, A. K. (1988). Systematic Data Collection, Vol. 10. Newbury Park, CA: Sage Publications.
WWF (2019). Ameaças à Mata Atlântica. Available online at: https://www.wwf.org.br/natureza_brasileira/questoes_ambientais/biomas/bioma_mata_atl/bioma_mata_atl_ameacas (accessed April 10, 2021).
Keywords: human occupation history, protected areas, brazilwood, local ecological knowledge, Atlantic Forest, Ethnobotany, urbanization
Citation: Bastos JG, Kury L, Hanazaki N, Capozzi R and Fonseca-Kruel VSd (2022) A Biodiversity Hotspot Losing Its Biocultural Heritage: The Challenge to Biocultural Conservation of Brazilwood (Paubrasilia echinata). Front. For. Glob. Change 5:696757. doi: 10.3389/ffgc.2022.696757
Received: 17 April 2021; Accepted: 08 April 2022;
Published: 06 June 2022.
Edited by:
Narel Yaroslava Paniagua-Zambrana, Higher University of San Andrés, BoliviaReviewed by:
Swetha Peteru, Center for International Forestry Research (CIFOR), IndonesiaCopyright © 2022 Bastos, Kury, Hanazaki, Capozzi and Fonseca-Kruel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Viviane Stern da Fonseca-Kruel, dmZvbnNlY2FAamJyai5nb3YuYnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.