- 1Forest Entomology, Swiss Federal Institute for Forest, Snow and Landscape Research WSL, Birmensdorf, Switzerland
- 2Department of Environmental Systems Science, Institute of Terrestrial Ecosystems, ETH Zürich, Zurich, Switzerland
- 3Department of Biosciences, University of Salzburg, Salzburg, Austria
Forest ecosystems have a distinct vertical dimension, but the structuring of communities in this three-dimensional space is not well understood. Water-filled tree holes are natural microcosms structured in metacommunities. Here, we used these microcosms as model systems to analyze how insect communities and the occurrence and abundance of individual species are influenced by biotic and abiotic microhabitat characteristics, the vertical position of the tree hole, and stand-scale habitat availability. We found that both the characteristics of water-filled tree holes and their insect communities differ significantly between canopy and ground level. Individual insect species showed contrasting responses to the vertical position of the tree holes when important environmental factors at the stand and the tree-hole scale were considered. While some species, such as the mosquito Aedes geniculatus and the beetle Prionocyphon serricornis, decreased in abundance with increasing tree-hole height, the biting midge Dasyhelea sp., the non-biting midge Metriocnemus cavicola and the hoverfly Myiatropa florea increased in abundance. Our results suggest that vertical stratification in forests is most likely driven not only by variation in tree-hole microhabitat properties, i.e., niche separation, but also by individual species traits, such as adult dispersal propensity, food preferences and mating behavior of adult stages, and interspecific competition of larval stages. Therefore, communities of insect species developing in tree holes are likely structured by competition–colonization trade-offs predicted by metacommunity theory.
Introduction
Forest ecosystems have a distinct vertical dimension, covering a height of up to >100 m and forming a complex and heterogeneous structure (Parker, 1995). In tropical forests in particular, the canopy contributes significantly to overall diversity (Basset et al., 2015). Although they were identified as one of the last biotic frontiers by Terry Erwin (1983) almost 40 years ago, canopy communities and the drivers that structure these communities are still not well understood. In temperate forests, several studies have demonstrated that canopy assemblages are not just nested subsets of ground assemblages (Bouget et al., 2011) and might show contrasting responses to forest management (Leidinger et al., 2019) and forest health (Sallé et al., 2021). This stratification is likely driven by differences in species resource requirements related to microhabitat and food, but also to microclimate preferences, species interactions, dispersal and mating behavior (Bouget et al., 2011; Ulyshen, 2011).
Tree-related microhabitats (TreM) have been shown to be crucial for promoting biodiversity in forests and have therefore become an important measure for forest conservation and management (Asbeck et al., 2021). One type of TreM that occurs throughout the vertical extent of trees is the water-filled tree hole or dendrotelma (Kitching, 2000; Larrieu et al., 2018). Gossner et al. (2016) showed that water-filled tree holes can occur in large numbers in the canopy but also near the ground. Thus, these microhabitats are ideal study systems to investigate the vertical stratification of species occurrence.
Spatial niche separation due to different microhabitat preferences of species could be important for structuring local communities across scales (species sorting perspective; Leibold et al., 2004). In general, microclimate shows a gradient along the vertical extent of trees (Parker, 1995), and this might affect the occurrence, development and phenology of species in these microhabitats (Gossner, 2018). Tree-hole properties such as size, amount of detritus (the basic resource in these microhabitats), and water chemistry are likely additionally important niche parameters (Gossner et al., 2016). Independent of the vertical position, stand-scale variables, such as the density of suitable microhabitats, might also affect the communities in water-filled tree holes (Gossner et al., 2016; Petermann et al., 2020). A likely reason for this is that communities in these microhabitats are organized as metacommunities, with local communities forming as a result of limited dispersal (Leibold et al., 2004). However, besides environmental and spatial filters, the outcome of biotic interactions also needs to be considered (Kraft et al., 2015; Cadotte and Tucker, 2017). According to the patch dynamics paradigm, there might be a competition–colonization trade-off in species, as some species are likely superior competitors and others superior colonists (Leibold et al., 2004). This might lead to a higher abundance of superior competitors in situations of high microhabitat density, while superior colonists might dominate under low microhabitat density. The relative importance of these different factors in driving the occurrence and abundance of single species is, however, still poorly understood.
In this study, we used data from two large-scale projects on the insect communities of natural tree holes occurring across vertical strata in differently managed forests. Previous publications based on these data focused on the consequences of forest management for communities in water-filled tree holes (Gossner et al., 2016; Petermann et al., 2020). Here, we address the importance of the vertical position of tree holes, as a proxy for microclimatic conditions, relative to other environmental drivers at the tree-hole scale (e.g., tree-hole size and chemistry, which likely also change with height) and stand scale (tree-hole density) in structuring insect communities developing in tree holes. In particular, we focus on the abundance and occurrence of the most frequently observed species. We expect that species have preferences regarding the vertical stratum when accounting for differences in microhabitat properties with height, with some species preferring tree holes at a lower and other species at a higher vertical position on the tree. This is expected because of differences in microclimatic preference, availability of resources for adults (e.g., flowers, vertebrate blood), and mating behavior (e.g., mating swarms of dipterans above tree tops; Downes, 1969; Ulyshen, 2011). In addition, we expect that niche separation, due to variation in height-independent tree-hole properties, and competition–colonization trade-offs, due to dispersal limitations, drive the abundance and occurrence of species, independent of the vertical position of the tree holes.
Materials and Methods
Study Sites
The study was conducted within the Biodiversity Exploratories project1 (Fischer et al., 2010) in three different regions of Germany: the Biosphere Reserve Schwäbische Alb (ALB) in the southwest (48°34′–48°53′N; 9°18′–9°60′E; 460–860 m a.s.l.), the National Park Hainich and the surrounding Hainich-Dün region (HAI) in the center (50°94′–51°38′N; 10°17′–10°78′E; 285–550 m a.s.l.), and the Biosphere Reserve Schorfheide-Chorin (SCH) in the northeast (52°51′–53°11′N, 13°36′–14°01′E; 3–140 m). The distance between ALB and HAI and between HAI and SCH is approx. 300 km and between ALB and SCH 600 km. ALB is located in a low mountain range with a mean annual precipitation of 700–1000 mm and a mean temperature of 6–7°C. The forest landscape is fragmented by agriculture and rural settlements. HAI is a forested hill chain and comprises the largest coherent area of deciduous trees in Germany. It has a mean annual precipitation of 500–800 mm and a mean annual temperature of 6.5–8°C. SCH is a post-glacial, flat landscape and one of the driest regions in Germany, with a mean annual precipitation of 500–600 mm and a mean annual temperature of 8–8.5°C. Forests in all regions would naturally be dominated by European beech, Fagus sylvatica, but differ greatly in their past and present management intensity.
Study Plots
This study is based on a compiled dataset from two projects, one focusing on ALB (29 plots) and HAI (24) in 2009 and 2011, respectively (Gossner et al., 2016), and one conducted in ALB (25), HAI (25) and SCH (25) in 2014 (Petermann et al., 2020). The experimental plots were 100 m × 100 m (1 ha) in size and covered the specific range of forest management intensities found in the region, from unmanaged beech forests to managed beech and conifer forests [spruce (Picea abies) in ALB und HAI; pine (Pinus sylvestris) in SCH]. Unmanaged forests were set aside 20–70 years ago, but all forests were influenced by humans at some point.
Sampling and Assessment of Plot and Tree-Hole Parameters
All water-filled tree holes were mapped in each 1-ha plot in spring and early summer after rain filled up the tree holes in 2009, 2011, and 2014. The number of tree holes per 1 ha (TH density) was used as a predictor of species abundance or occurrence. In both projects pan holes (maintaining an unbroken bark lining) and rot holes (penetrating through to the wood of the tree) (Kitching R. L., 1971) were considered, but sampling followed a slightly different approach. Tree holes at ≥2 m height were defined as “canopy holes” and those at <2 m height as “ground holes”, following the classification of Kitching R. L. (1971) and Yanoviak and Fincke (2005). On each plot, either all available pan tree holes with a volume ≥50 ml plus all available rot holes were sampled (Gossner et al., 2016), or two canopy and two ground holes with a volume of 200–1000 ml were sampled, including both pan and rot holes (Petermann et al., 2020). Canopy tree holes were reached by using the single-rope climbing technique (Perry, 1978). In total, 318 tree holes were sampled.
For each tree hole, we measured the height above ground, opening area (Area, cm2), potential volume (Vmax, ml) and amount of detritus (mass in g). The proportion of total detritus relative to the maximal water volume (PropDetritustot; g/ml) was used as an independent predictor variable.
Among water properties, dissolved oxygen (O2 mg/l) and pH were measured in the field with mobile electrodes (Oxi 330, pH 330, WTW GmbH; Gossner et al., 2016) or multiprobes (Hach lange HQ40D.99.101301, field meter; Petermann et al., 2020). Nitrate (NO3– in mg/L), ammonium (NH4+ in mg/l) and phosphate (PO34– in mg/l) concentrations were measured using photometric determination (Gossner et al., 2016; Petermann et al., 2020).
In the laboratory, all insect eggs, larvae and pupae, and all other invertebrates in each sample were transferred to 70% ethanol. Subsequently, insect larvae and pupae were identified using a stereo microscope and insect larval identification keys. We identified all specimens to either the species or morphospecies level. For details on sampling and measurements, see Gossner et al. (2016) and Petermann et al. (2020).
Statistical Analyses
All analyses were performed in R version 4.0.2 (R Core Team, 2020). In a first step, we tested whether insect communities and environmental parameters differed between ground and canopy holes. To visualize differences in community composition, we performed non-metric multidimensional scaling (NMDS) based on Bray–Curtis dissimilarities using the “metaMDS” function in the vegan package (Oksanen et al., 2020) with 1000 permutations. Twenty-eight tree holes were excluded because they did not contain insect larvae, and another five were excluded because they contained only exclusive species and NMDS did not converge when these tree holes were included, resulting in 285 tree holes. We tested for differences in communities between regions, years and strata (ground vs. canopy) based on the same 285 tree holes using the “adonis” function in vegan.
To test for differences in total insect abundance, species richness, and plot- and tree-hole-scale parameters between ground and canopy holes, all 318 tree holes were included. We used generalized mixed effects models to analyze abundance (Poisson family, “glmer” function) and linear mixed effects models for all other response variables (“lmer” function), with stratum (ground vs. canopy) as a predictor and plot nested within region as the random effects structure, using the glmmTMB package (Brooks et al., 2017). Results are shown as histograms, separated into ground and canopy holes, created using the ggplot2 package (Wickham, 2016).
In a second step, we tested the importance of different plot- and tree-hole-scale predictors, including tree-hole height as a continuous predictor variable, for the abundance or occurrence of particular insect species. We again used generalized linear mixed effects models implemented in glmmTMB, with a Poisson (abundance) or binomial (presence/absence) distribution, with all environmental variables as predictors and, as above, plot nested within region as the random effects structure. All environmental variables were standardized to zero mean and unit variance using the “decostand” function in the vegan package (Oksanen et al., 2020) to allow comparisons of effect sizes. We performed two models for all abundance-based models, one with tree-hole density based on all tree holes and one using canopy tree-hole density for canopy tree holes and ground hole density for ground holes. We did that, because we expected different communities in canopy and ground holes and thus different importance of canopy and ground hole densities for some species. For occurrence-based models we were facing problems with model convergence and thus only used pooled tree holes density as predictor. Residual diagnostics were calculated using the DHARMa package (Hartig, 2020) and a test for multicollinearity was completed using the “check_collinearity” function in the performance package (Lüdecke et al., 2021). Neither significant zero inflation nor problems with dispersion or multicollinearity were observed. We then plotted the standardized estimates using the “plot_model” function in the sjPlot package (Lüdecke, 2021). Significant effects of tree-hole height above ground on either the abundance or occurrence of particular species were plotted using the “predictorEffect” function in the effects package (Fox, 2003; Fox and Weisberg, 2018, 2019).
Results
In the 318 tree holes studied (ground: 194, canopy: 124), we found 43 (morpho)species with a total of 13,456 individuals. The most abundant species were Metriocnemus cavicola (Diptera: Chironomidae; 5909 individuals, 194 tree holes), Myiatropa florea (Diptera: Syrphidae; 747, 139), Prionocyphon serricornis (Coleoptera: Scirtidae; 3099, 131), Dasyhelea spec. (Diptera: Ceratopogonidae; 1646, 109), Aedes geniculatus (Diptera: Culicidae; 1027, 88), Chironomidae spec. (Diptera: Chironomidae; 114, 32), Muscidae spec. (Diptera: Muscidae; 509, 26), Culicidae spec. (Diptera: Culicidae; 42, 15), Dolichopodidae spec. (Diptera: Dolichopodidae; 22, 15), and Pericoma spec. (Diptera: Psychodidae; 29, 14).
Community composition differed significantly between regions (F2,279 = 3.588, P < 0.001), years (F2,279 = 3.434, P < 0.001), and height strata (F1,279 = 5.757, P < 0.001), but the explained variance (R2) was low (region: 0.024; year: 0.023; stratum: 0.019; Figure 1).
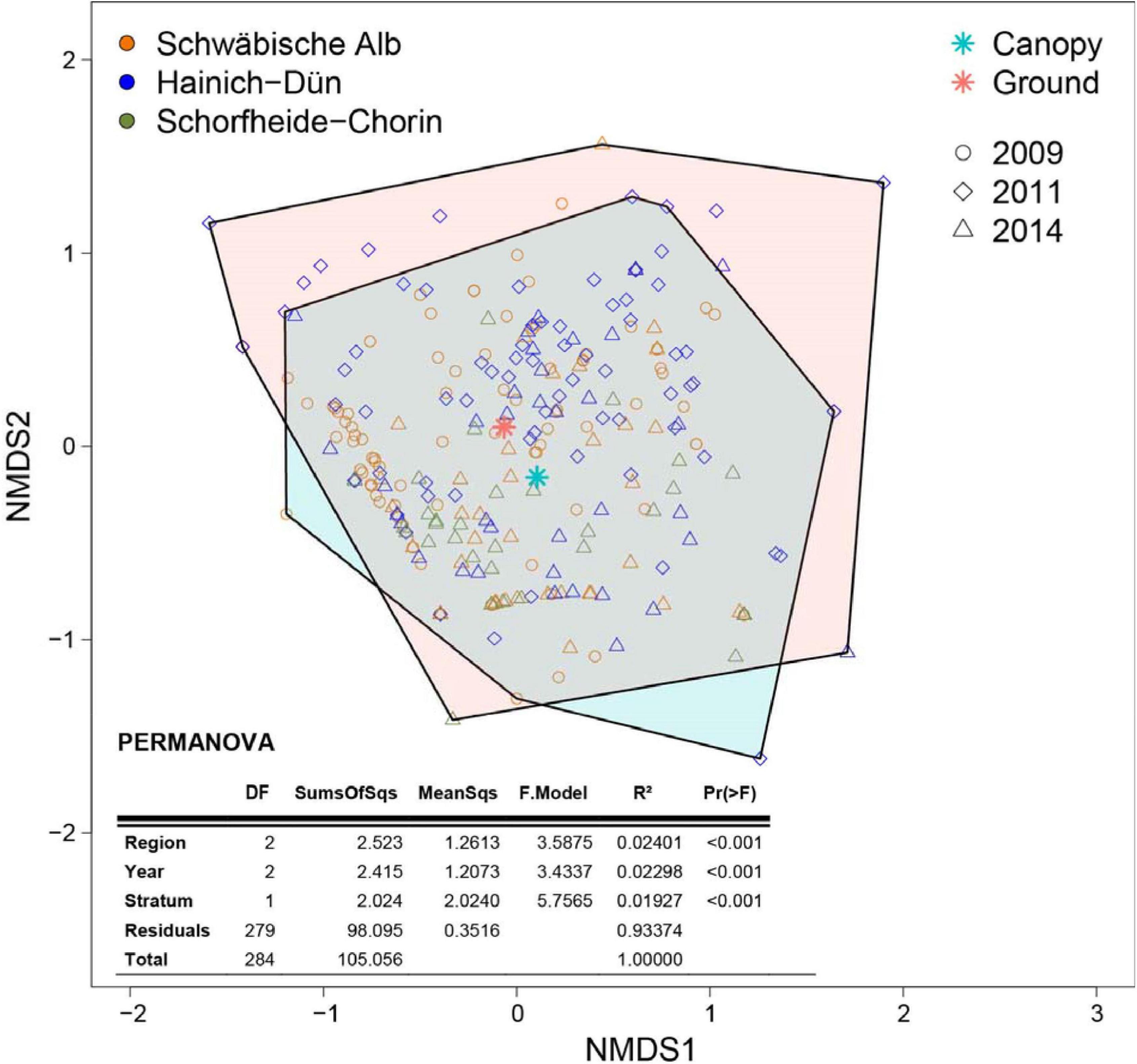
Figure 1. Non-metric multidimensional scaling (NMDS) based on Bray-Curtis distances of insect communities in water-filled tree holes sampled in the canopy and near the ground in three regions of Germany between 2009 and 2014 (stress = 0.142). Colored hulls indicate the space covered by the communities in the canopy (at ≥2 m height; blue) and near the ground (at <2 m; red). The table shows the results of a PERMANOVA, testing for differences in community composition between region, year and height stratum. Please note that the R2 value for stratum shows the effect strength after controlling for differences between individual regions and between individual years (Type I sum of squares, sequential).
Tree holes were observed at heights of up to 34 m in the canopy, although median height was 0.58 m and mean height was 4.13 m, indicating that most of the sampled canopy holes occurred at rather low heights (Figure 2A). Tree holes in the canopy (≥2 m) were smaller overall, but contained more detritus relative to their size and had a higher water pH and ammonium concentration than ground holes (Figure 2). Insect abundance and species richness, did not differ between ground and canopy holes, nor did hole opening area or dissolved oxygen, nitrate and phosphate concentration at the tree-hole scale (Figure 2). At the plot scale, tree-hole density tended to be higher at ground level (P = 0.051; Figure 2).
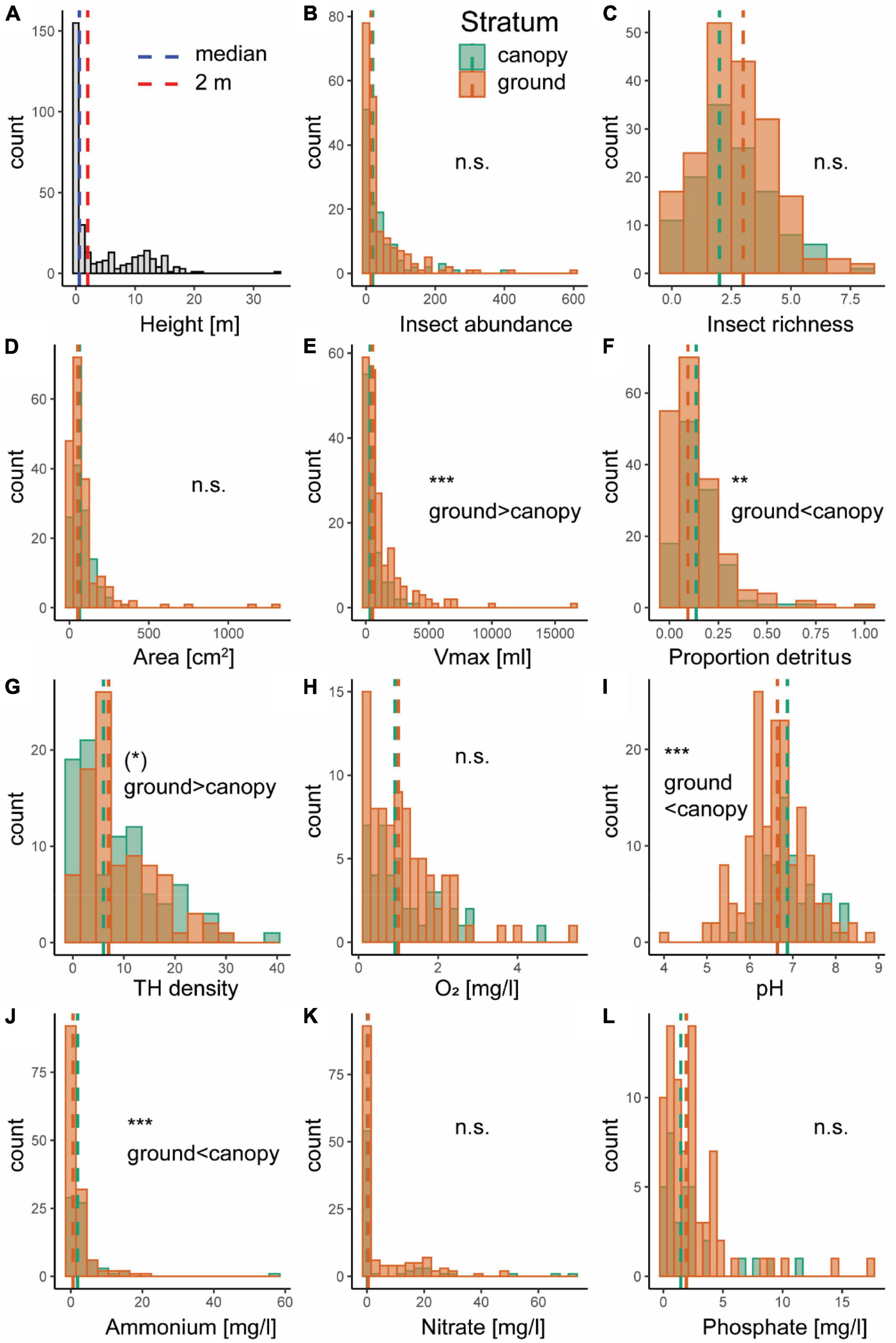
Figure 2. Histograms of tree-hole height (A), insect abundance (B), insect species richness (C), tree-hole opening area (D), tree-hole maximum volume (Vmax; E), total amount of detritus relative to tree-hole Vmax (proportion detritus; F), plot-level tree-hole density (G), and tree-hole water chemistry: dissolved oxygen concentration (O2) (H), pH (I), ammonium concentration (J), nitrate concentration (K), and phosphate concentration (L). Values for ground holes (<2 m height; orange) and canopy holes (>2 m height; green) in panels (B–L) are indicated by different colors, with median values shown as dashed lines. Asterisks indicate significance based on generalized (for insect abundance; Poisson distribution) or linear (all other variables) mixed effects models [(*) P = 0.051, ** P < 0.01, *** P < 0.001, n.s. not significant].
The abundances of the most frequent species, Metriocnemus cavicola, Myiatropa florea, P. serricornis, Dasyhelea sp. and A. geniculatus, were significantly affected by tree-hole height above ground, with P. serricornis and A. geniculatus negatively and Metriocnemus cavicola, Myiatropa florea and Dasyhelea sp. positively affected (Figures 3, 4; for separation between canopy and ground holes, see Supplementary Figure 2 in the Supplementary Material). In addition, Metriocnemus cavicola was positively affected by overall tree-hole density at the plot scale (Figure 3). When tree-hole density was separated into canopy and ground holes, P. serricornis also responded positively to tree-hole density (Supplementary Figure 1 in the Supplementary Material). This was also the only model in which the marginal R2 slightly increased by separating between canopy and ground holes. All other models showed higher marginal R2 when using overall tree-hole density. Water chemistry parameters had contrasting responses in the most frequently found species (Figure 3). While the abundance of A. geniculatus was positively affected by dissolved oxygen concentration, Dasyhelea sp. and P. serricornis showed negative responses. Higher water pH affected Metriocnemus cavicola and Dasyhelea sp. positively, but Myiatropa florea and P. serricornis negatively. Ammonium and nitrate concentration affected A. geniculatus (only nitrate) and Dasyhelea sp. negatively, whereas Myiatropa florea (only nitrate) and P. serricornis showed a positive response. Only Metriocnemus cavicola showed a response to phosphate concentration and this was positive. Regarding the size of the tree holes, A. geniculatus preferred holes with large and Myiatropa florea with small opening areas (Figure 3). While Dasyhelea sp. was more abundant in small holes (low Vmax), Myiatropa florea and P. serricornis were more abundant in large holes. The total amount of detritus relative to tree-hole size affected the abundance of all analyzed species, with positive effects for Metriocnemus cavicola, Dasyhelea sp. and Myiatropa florea, and negative effects for A. geniculatus and P. serricornis.
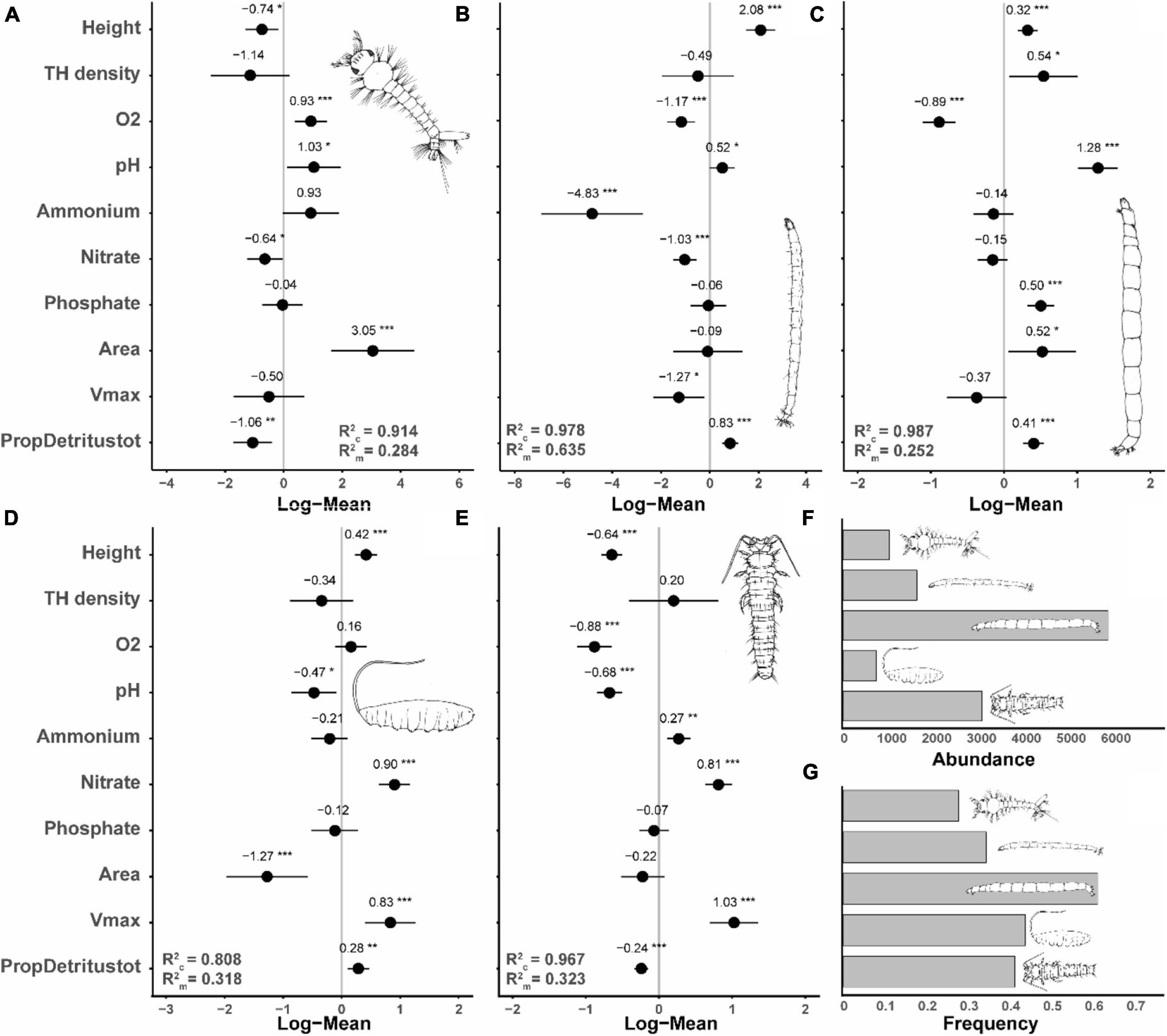
Figure 3. Standardized model coefficients (including conditional and marginal R2-values) of generalized linear mixed effects models with a Poisson distribution, showing the importance of tree-hole height above ground, tree-hole density (canopy and ground holes combined), tree-hole opening area, tree-hole maximum water volume (Vmax), water chemistry [dissolved oxygen concentration (O2), pH, and ammonium, nitrate and phosphate concentrations], and total amount of detritus relative to Vmax (PropDetritustot) in determining the abundance of the five most frequently observed insect species. (A) Diptera, Culicidae: Aedes geniculatus, occurrence in n = 88 tree holes; (B) Diptera, Ceratopogonidae: Dasyhelea sp., n = 109; (C) Diptera, Chironomidae: Metriocnemus cavicola, n = 194; (D) Diptera, Syrphidae: Myiatropa florea, n = 139; (E) Coleoptera, Scirtidae: Prionocyphon serricornis, n = 131. (F,G) show the total abundance and the frequencies of occurrence of the five species, respectively. *p < 0.05, **p < 0.01, ***p < 0.001.
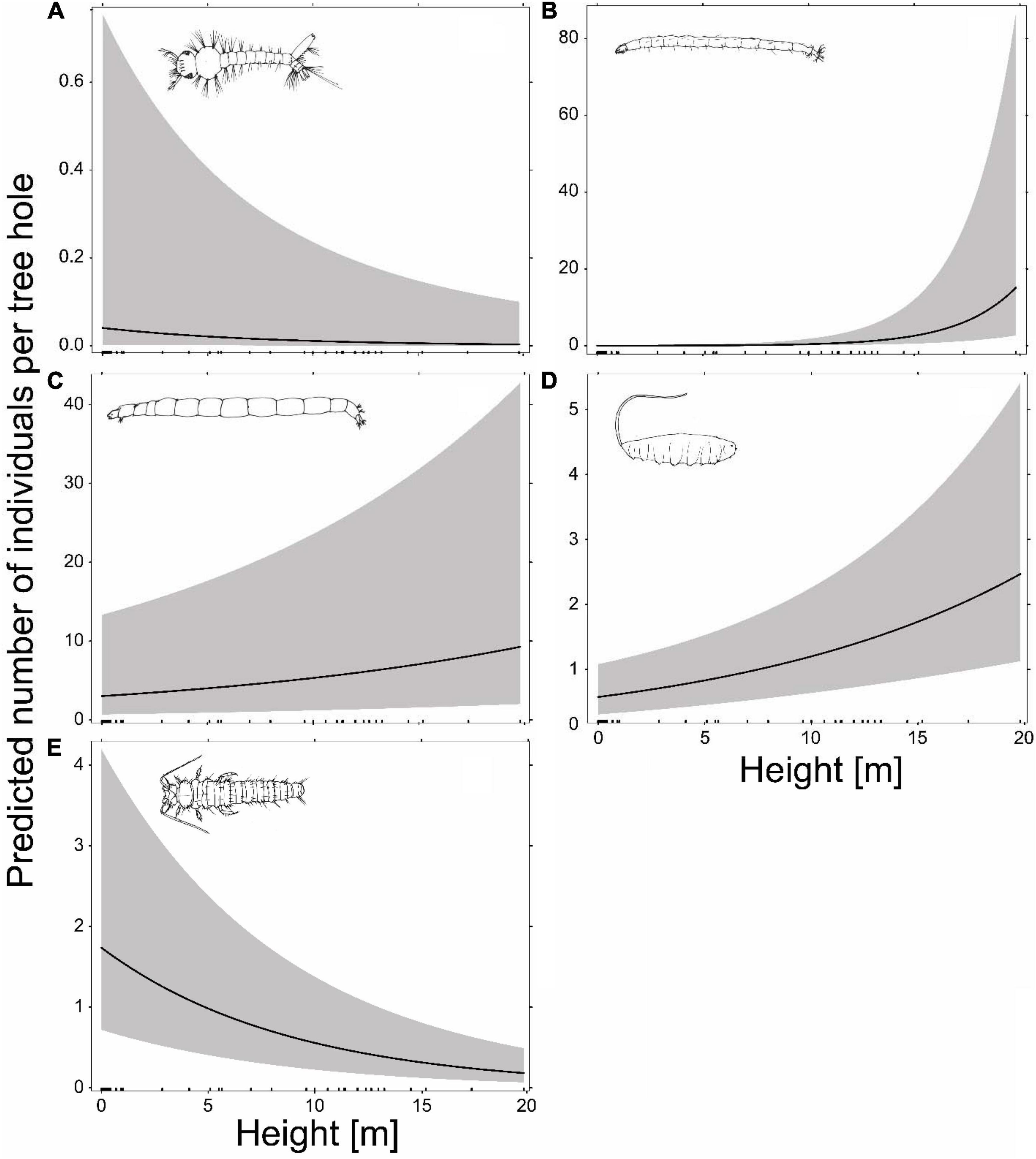
Figure 4. Prediction of the abundance of the most abundant species in our study as a function of tree-hole height above ground, based on generalized linear mixed effects models with a Poisson distribution, including tree-hole-scale and plot-scale variables (for tree-hole density canopy and ground holes were combined) as additional predictors. The gray bands show 95% confidence intervals. The bars on the x-axis indicate the tree holes sampled along the vertical gradient. (A) Diptera, Culicidae: Aedes geniculatus occurrence in n = 88 tree holes; (B) Diptera, Ceratopogonidae: Dasyhelea sp., n = 109; (C) Diptera, Chironomidae: Metriocnemus cavicola, n = 194; (D) Diptera, Syrphidae: Myiatropa florea, n = 139; (E) Coleoptera, Scirtidae: Prionocyphon serricornis, n = 131.
The occurrence of the five next most frequent species showed only three significant responses to our predictor variables. The occurrence of a Culicidae species that could not be identified to the genus or species level showed a positive response to tree-hole opening area, Chironomidae species occurred more frequently with higher nitrate concentration, and Pericoma sp. at lower pH (Figure 5).
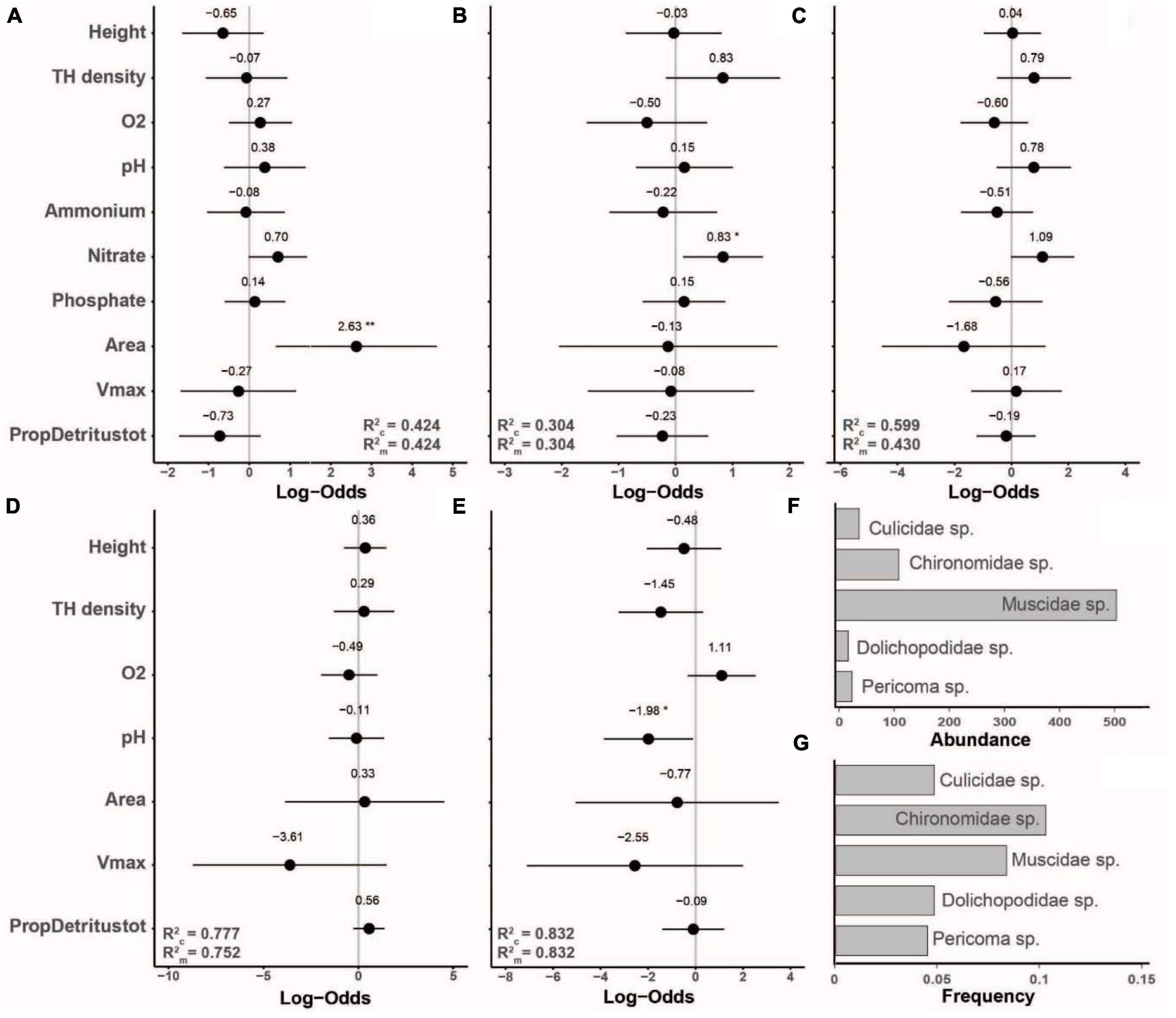
Figure 5. Standardized model coefficients (including conditional and marginal R2-values) of generalized linear mixed effects models with binomial distribution based on occurrence data, showing the importance of tree-hole height above ground, tree-hole density, tree-hole opening area, tree-hole maximum water volume (Vmax), water chemistry [dissolved oxygen concentration (O2), pH, and ammonium, nitrate and phosphate concentrations] and total amount of detritus relative to Vmax (PropDetritustot) in determining the occurrence of less frequently observed insect species that were observed in >10 but <88 tree holes. (A) Diptera, Culicidae sp., occurrence in n = 15 tree holes; (B) Diptera, Chironomidae sp., n = 32; (C) Diptera, Muscidae sp., n = 26; (D) Diptera, Dolichopodidae sp., n = 15; (E) Diptera, Psychodidae, Pericoma sp., n = 14. Please note that in (D,E) a reduced model was used because there were many missing values. (F,G) show the total abundance and the frequencies of occurrence of the five species, respectively. *p < 0.05, **p < 0.01.
Discussion
Our study provides novel insight into the importance of the vertical position of microhabitats, relative to other abiotic and biotic drivers, in determining insect community structure and abundance of individual species. By using water-filled tree holes as a model system, we showed that canopy communities differ significantly from those near ground level. Our analyses of individual species revealed that this pattern is due to contrasting responses of individual species to the vertical position of the tree holes, when controlling for important environmental factors at the plot and tree-hole scale. Our results suggest that vertical stratification in forests is most likely driven not only by changes in tree-hole microhabitat properties (niche separation) with height above ground, but also by other factors such as adult species traits (e.g., dispersal propensity, food, mating behavior) and interspecific competition. Therefore, communities of insect species developing in tree holes are likely structured by competition–colonization trade-offs predicted by metacommunity theory.
We found significant differences in the composition of insect communities developing in water-filled tree holes. This confirms findings from previous studies that height above ground is an important factor structuring insect communities of these microhabitats in temperate (Blakely and Didham, 2010; Gossner et al., 2016; Petermann et al., 2020) and tropical forests (Yanoviak, 1999). However, the underlying mechanisms might be manifold.
Several properties differed between the ground and canopy holes studied here and could have caused niche separation of species. While the volume of tree holes was on average larger near the ground than in the canopy (Figure 2E), the proportion of detritus and thus the resource concentration was higher in the canopy (Figure 2F). Because of the branching pattern of beech canopies, the main tree species on which we found tree holes in our study (see also, von Brandt, 1934; Gossner et al., 2016), the size of tree holes decreases with height. In addition, stem holes, which can be quite large, become less abundant in the canopy. However, leaf biomass is greatest in the upper canopy of closed forests, causing high leaf input into canopy holes and thus a higher resource concentration in the canopy (Figure 6A). Moreover, the pH and ammonium concentration of the water in tree holes were significantly higher in the canopy than near the ground, which might be caused by greater drought disturbance in the canopy (Blakely and Didham, 2010). These changes with greater tree height are generally accompanied by increases in temperature and conductivity (both not measured), as well as larger temporal fluctuations in environmental parameters (Yanoviak, 1999; Blakely and Didham, 2010; Gossner, 2018). These changes are most likely due to windier conditions and increased exposure to sunlight the closer the tree holes are to the upper boundary of the canopy (Parker, 1995).

Figure 6. (A) Water-filled tree hole in a branch fork of a beech tree at 20 m height. At this height these microcosms receive considerable leaf litter input, resulting in a large amount of detritus relative to the tree-hole volume. (B) The abundance of the hoverfly Myiatropa florea (Diptera: Syrphidae) increases with tree-hole height. These rat-tailed maggots are characterized by a long, tube-like, telescoping breathing siphon that acts like a snorkel, allowing the larvae to breathe air while submerged. (C) Near ground level, very large water-filled tree holes with a volume >10 l are sometimes found. (D) The larvae of the mosquito Aedes geniculatus (Diptera: Culicidae) prefers holes with wide openings near ground level. Larvae breathe atmospheric air through a siphon and filter algae, bacteria, and other microbes from the surface microlayer. (E) The beetle Prionocyphon serricornis (Coleoptera: Scirtidae) depends on large holes that do not dry out frequently. They survive freezing of the water in tree holes (most of the water column in the photo is frozen) by staying in bark crevices at the upper water level and become active as soon as the top layer melts.
Various microhabitat properties significantly affected the insect communities developing in the water-filled tree holes in our three study regions, confirming results from previous studies (Blakely and Didham, 2010; Gossner et al., 2016). Although we found no significant differences in overall insect abundance and species richness between our coarse categories canopy vs. ground, either a decrease (e.g., in Nothofagus forest of New Zealand and tropical forests in Panama; Yanoviak, 1999; Blakely et al., 2008) or an increase (e.g., in Fagus forests of Germany; Gossner et al., 2016; Petermann et al., 2020) in species richness with height has been observed in previous studies. These differences might be due to different adaptations of the species to drought in the different ecosystems. It has been shown that environmental filters such as drought disturbance can eliminate many species that survive in wetter environments (Chase, 2007). The combination of effective drought resistance strategies and faster development under high temperatures in the canopy might have positive effects on the species richness of insect communities in water-filled tree holes in the canopies of Central European forests.
We observed clear contrasting effects of individual species to the microhabitat properties, which likely relates to species-specific traits. The general importance of species traits in determining the responses of communities in water-filled tree holes to environmental drivers has already been shown by Petermann et al. (2020). While the mosquito Aedes geniculatus and another non-identified mosquito species preferred holes with a larger opening area (Figures 6C,D), independent of tree-hole volume, the hoverfly Myiatropa florea (Figure 6B) and the beetle Prionocyphon serricornis (Figure 6E) preferred large holes with a rather small opening area. In contrast, the biting midge Dasyhelea sp. was more abundant in smaller holes. This suggests that mosquitoes prefer large opening areas for oviposition (Bentley and Day, 1989; Clements, 1999), whereas hoverflies and beetles, which have longer developmental times, need a larger water volume and more stable water levels so that tree holes do not dry out during their development. Small biting midges such as Dasyhelea sp. might have a competitive advantage in small holes due to their fast development.
Species such as the non-biting midge Metriocnemus cavicola and the beetle P. serricornis seem to do well under low dissolved oxygen concentrations, while mosquitoes depend on high concentrations. As mosquito larvae are air breathers (Figure 6D), they do not directly depend on oxygen in the water. Thus, the positive relationship with dissolved oxygen concentration might be indirect via a higher abundance of microorganismic food species such as bacteria and ciliates (Schmidl et al., 2008). In contrast, P. serricornis, which lives saprophagously by shredding leaves and detritus, can store air in its tracheal system (Klausnitzer, 1984) and thus tolerates relatively anoxic conditions (Schmidl et al., 2008). The negative relationship with oxygen supply that we observed for saprophagous Metriocnemus cavicola is in contrast to results from previous studies (von Brandt, 1934; Schmidl et al., 2008) and might be caused by the fact that the canopy stratum was not considered in the previous investigations.
Moreover, biting (Dasyhelea sp.) and non-biting (Metriocnemus cavicola) midges, as well as hoverflies (Myiatropa florea), seem to reach higher densities under high resource concentrations (see also, Schmidl et al., 2008), whereas high resource concentrations might reduce densities of mosquitoes and P. serricornis due to inhibition by leachates (Blakely, 2008; Schmidl et al., 2008). Water chemistry, including nutrient concentrations, might also be important cues for oviposition (Bentley and Day, 1989; Clements, 1999), which could explain the observed responses of all abundant species to water chemistry.
The strong response of Myiatropa florea to microhabitat properties is surprising, as this species is known to be a habitat generalist, also occurring in other water bodies, and showed no clear preferred physicochemical conditions in a previous study on water-filled tree holes (Schmidl et al., 2008). Because of its relatively large size, however, it might depend on properties, e.g., tree-hole volume, that support complete development in tree holes, given the rather ephemeral character of this microhabitat in terms of water availability.
There was still a significant effect of height after accounting for changes in microhabitat properties with height, which might be related to shifts in unmeasured variables such as temperature. In addition, many insects have flight height preferences (Basset et al., 2003), which likely influences where they feed, mate and oviposit (Scholl and Defoliart, 1977; Sinsko and Craig, 1979; Copeland and Craig, 1990). Moreover, the availability of food might play a role in height effects. Mosquitoes, for instance, need vertebrate blood for egg maturation and thus prefer lower forest strata where vertebrates are overall more abundant in temperate forests (Shaw, 2004).
Dispersal limitation could be an additional driver of species occurrence and abundance. Competition–colonization trade-offs most likely help to structure insect communities of water-filled tree holes, as predicted by the metacommunity patch-dynamics paradigm (Leibold et al., 2004). Good colonizers might be poor competitors and thus might be pushed to less suitable marginal habitats. In our study, positive responses to tree-hole density might characterize species that are limited by a low colonization ability, requiring high densities of suitable habitats to be able to colonize from source habitats, but that have a high competitive ability, giving them an advantage under high tree-hole density. The non-biting midges (Metriocnemus cavicola) and the marsh beetle (Prionocyphon serricornis) might be examples of this dispersal guild. In contrast, the syrphid Myiatropa florea and the mosquito Aedes geniculatus seem to be good colonizers (strong negative effect of overall tree-hole density although not significant) but poor competitors, so they can reach high densities even when habitat availability is low. This is supported by the observation that non-biting midges are weak, slow flyers, whereas the syrphid Myiatropa florea and the mosquito Aedes geniculatus are strong, fast flyers leading to differences in colonization potential (Kitching R., 1971; Verdonschot and Besse-Lototskaya, 2014). The midges seem to compensate for the lower dispersal success by higher fecundity, laying large clutches of up to 100 eggs (Kitching R., 1971; Kitching, 1972). This high fecundity combined with high drought and/or freezing tolerance may make midges (drought and freezing tolerance) and marsh beetles (freezing tolerance) (see Figure 6; Gossner, 2018) superior competitors under the harsh conditions that prevail in tree holes.
Conclusion
Water-filled tree holes are important tree-related microhabitats promoting forest biodiversity. Our study clearly shows that communities in these microhabitats are structured by complex and potentially interacting mechanisms proposed by metacommunity theory, such as niche separation, competition and dispersal limitations. Besides larval requirements, traits of adult life stages, such as food preferences and behavioral traits related to mating and oviposition, likely also shape these communities. Our study therefore clearly supports the conclusion of Srivastava et al. (2004) that these microcosms serve as a useful model system in ecology. These microhabitats host complex interactions involving different trophic levels, yet they are discrete, small enough to measure abiotic properties and collect all insect species present, and easy to manipulate. Water-filled tree holes thus open multiple possibilities to study responses of whole communities to environmental perturbation.
Data Availability Statement
The datasets are publicly available in the Biodiversity Exploratories Information System: http://doi.org/10.17616/R32P9Q (Gossner and Weisser, 2017; Petermann et al., 2018a, b), https://www.bexis.uni-jena.de/ddm/data/Showdata/19086, https://www.bexis.uni-jena.de/ddm/data/Showdata/24146, and https://www.bexis.uni-jena.de/ddm/data/Showdata/24148.
Author Contributions
MG conceived the ideas for this study, analyzed the data, and wrote the first draft of the manuscript. MG and JP led the two underlying projects. JP commented on all versions of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work has been funded by the DFG Priority Program 1374 “Biodiversity-Exploratories” (DFG-WE 3081/21-1, DFG21234_P).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the managers of the three Exploratories, K. Wells, S. Renner, K. Reichel-Jung, I. Steitz, S. Weithmann, S. Gockel, K. Wiesner, K. Lorenzen, J. Vogt, A. Hemp, M. Gorke and M. Teuscher, for their work in maintaining the plot and project infrastructures; S. Pfeifer, M. Gleisberg, C. Fischer and J. Mangels for giving support through the central office, J. Nieschulze, M. Owonibi and A. Ostrowski for managing the central database, and M. Fischer, E. Linsenmair, D. Hessenmöller, D. Prati, I. Schöning, F. Buscot, E.-D. Schulze, W. W. Weisser and the late E. Kalko for their roles in setting up the Biodiversity Exploratories project. We thank the administration of the Hainich National Park, the UNESCO Biosphere Reserve Swabian Alb and the UNESCO Biosphere Reserve Schorfheide-Chorin, as well as all landowners, for this excellent collaboration. Field work permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen and Brandenburg. We are grateful for fieldwork and lab support from P. Lade, A. Rohland, N. Sichardt and A. L. Roberts and thank M. Dawes for English editing and A. Sallé and A. Feest for the valuable suggestions they provided through their reviews.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.816570/full#supplementary-material
Supplementary Figure 1 | Standardized model coefficients (including conditional and marginal R2-values) of generalized linear mixed effects models with a Poisson distribution, showing the importance of tree-hole height above ground, plot-level tree-hole density (separated by canopy and ground), tree-hole opening area, tree-hole maximum water volume (Vmax), water chemistry (dissolved oxygen concentration (O2), pH, and ammonium, nitrate and phosphate concentrations), and total amount of detritus relative to Vmax (PropDetritustot) in determining the abundance of the five most frequently observed insect species. (A) Diptera, Culicidae: Aedes geniculatus, occurrence in n = 88 tree holes; (B) Diptera, Ceratopogonidae: Dasyhelea sp., n = 109; (C) Diptera, Chironomidae: Metriocnemus cavicola, n = 194; (D) Diptera, Syrphidae: Myiatropa florea, n = 139; (E) Coleoptera, Scirtidae: Prionocyphon serricornis, n = 131. (F,G) show the total abundance and the frequencies of occurrence of the five species, respectively. *p < 0.05, **p < 0.01, ***p < 0.001.
Supplementary Figure 2 | Prediction of the abundance of the most abundant species in our study as a function of tree-hole height above ground, based on generalized linear mixed effects models with a Poisson distribution, including tree-hole-scale and plot-scale variables (tree-hole density was assessed separately for canopy and ground holes) as additional predictors. The gray bands show 95% confidence intervals. The bars on the x-axis indicate the tree holes sampled along the vertical gradient. (A) Diptera, Culicidae: Aedes geniculatus occurrence in n = 88 tree holes; (B) Diptera, Ceratopogonidae: Dasyhelea sp., n = 109; (C) Diptera, Chironomidae: Metriocnemus cavicola, n = 194; (D) Diptera, Syrphidae: Myiatropa florea, n = 139; (E) Coleoptera, Scirtidae: Prionocyphon serricornis, n = 131.
Footnotes
References
Asbeck, T., Großmann, J., Paillet, Y., Winiger, N., and Bauhus, J. (2021). The use of tree-related microhabitats as forest biodiversity indicators and to guide integrated forest management. Curr. Forestry Rep. 7, 59–68. doi: 10.1007/s40725-020-00132-5
Basset, Y., Cizek, L., Cuénoud, P., Didham, R. K., Novotny, V., Ødegaard, F., et al. (2015). Arthropod distribution in a tropical rainforest: tackling a four dimensional puzzle. PLoS One 10:e0144110. doi: 10.1371/journal.pone.0144110
Basset, Y., Hammond, P. M., Barrios, H., Holloway, J. D., and Miller, S. E. (2003). “Vertical stratification of arthropod assemblages,” in Arthropods Of Tropical Forests: Spatio Temporal Dynamics And Resource Use In The Canopy, eds Y. Basset, V. Novotny, S. E. Miller, and R. L. Kitching (Cambridge: Cambridge University Press), 17–27.
Bentley, M. D., and Day, J. F. (1989). Chemical ecology and behavioral aspects of mosquito oviposition. Ann. Rev. Entomol. 34, 401–421. doi: 10.1146/annurev.en.34.010189.002153
Blakely, T. J. (2008). Tree Holes As Habitat For Aquatic And Terrestrial Invertebrates In Mixed Broadleaf-Podocarp Rainforest, New Zealand. Ph.D. thesis. Christchurch: University of Canterbury.
Blakely, T. J., and Didham, R. K. (2010). Disentangling the mechanistic drivers of ecosystem-size effects on species diversity. J. Anim. Ecol. 79, 1204–1214. doi: 10.1111/j.1365-2656.2010.01729.x
Blakely, T. J., Jellyman, P. G., Holdaway, R. J., Young, L., Ben, B., Duncan, P., et al. (2008). The abundance, distribution and structural characteristics of tree-holes in Nothofagus forest, New Zealand. Aust. Ecol. 33, 963–974. doi: 10.1111/j.1442-9993.2008.01867.x
Bouget, C., Brin, A., and Brustel, H. (2011). Exploring the “last biotic frontier”: are temperate forest canopies special for saproxylic beetles? For. Ecol. Manage. 261, 211–220. doi: 10.1016/j.foreco.2010.10.007
Brooks, M. E., Kristensen, K., Van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 9, 378–400. doi: 10.32614/RJ-2017-066
Cadotte, M. W., and Tucker, C. M. (2017). Should environmental filtering be abandoned? Trends Ecol. Evol. 32, 429–437. doi: 10.1016/j.tree.2017.03.004
Chase, J. M. (2007). Drought mediates the importance of stochastic community assembly. Proc. Natl. Acad. Sci. U. S. A. 104, 17430–17434. doi: 10.1073/pnas.0704350104
Clements, A. N. (1999). The Biology Of Mosquitoes. Volume 2: Sensory Reception And Behaviour. Egham: CABI publishing.
Copeland, R. S., and Craig, G. B. (1990). Habitat segregation among treehole mosquitos (Diptera, Culicidae) in the Great-Lakes region of the United-States. Ann. Entomol. Soc. Am. 83, 1063–1073. doi: 10.1093/aesa/83.6.1063
Downes, J. A. (1969). The Swarming and Mating Flight of Diptera. Ann. Rev. Entomol. 14, 271–298. doi: 10.1146/annurev.en.14.010169.001415
Erwin, T. L. (1983). Tropical forest canopies: the last biotic frontier. Bull. Entomol. Soc. Am. 29, 14–19. doi: 10.1093/besa/29.1.14
Fischer, M., Bossdorf, O., Gockel, S., Hansel, F., Hemp, A., Hessenmoller, D., et al. (2010). Implementing large-scale and long-term functional biodiversity research: the biodiversity exploratories. Basic Appl. Ecol. 11, 473–485. doi: 10.1016/j.baae.2010.07.009
Fox, J. (2003). Effect displays in R for generalised linear models. J. Stat. Softw. 8, 1–27. doi: 10.18637/jss.v008.i15
Fox, J., and Weisberg, S. (2018). Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. J. Stat. Softw. 87, 1–27. doi: 10.18637/jss.v087.i09
Fox, J., and Weisberg, S. (2019). An R Companion to Applied Regression, 3rd Edn. Thousand Oaks: Sage Publications.
Gossner, M. M. (2018). A three year study of the phenology of insect larvae (Coleoptera, Diptera) in water-filled tree holes in the canopy of a beech tree. Eur. J. Entomol. 115, 524–534.
Gossner, M. M., Lade, P., Rohland, A., Sichardt, N., Kahl, T., Bauhus, J., et al. (2016). Effects of management on aquatic tree-hole communities in temperate forests are mediated by detritus amount and water chemistry. J. Anim. Ecol. 85, 213–226. doi: 10.14411/eje.2018.052
Gossner, M. M., and Weisser, W. W. (2017). Arthropods in Tree Holes And Additional Parameters For Some Forest EPs, 2009-2011. Version 2. London: Department Of Business Energy And Industrial Strategy. doi: 10.1111/1365-2656.12437
Hartig, F. (2020). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.3.3.0. Available Online at: https://CRAN.R-project.org/package=DHARMa. (accessed August 07, 2021)
Kitching, R. (1971). A simple simulation model of dispersal of animals among units of discrete habitats. Oecologia 7, 95–116. doi: 10.1007/BF00346353
Kitching, R. (1972). Population Studies of the Immature Stages of the Tree-Hole Midge Metriocnemus martinii Thienemann (Diptera: Chironomidae). J. Anim. Ecol. 41, 53–62. doi: 10.2307/3504
Kitching, R. L. (1971). An ecological study of water-filled tree-holes and their position in the woodland ecosystem. J. Anim. Ecol. 40, 281–302. doi: 10.2307/3247
Kitching, R. L. (2000). Food Webs And Container Habitats: The Natural History And Ecology Of Phytotelmata. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511542107
Kraft, N. J. B., Adler, P. B., Godoy, O., James, E. C., Fuller, S., and Levine, J. M. (2015). Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 29, 592–599. doi: 10.1098/rsbl.2018.0460
Larrieu, L., Paillet, Y., Winter, S., Butler, R., Kraus, D., Krumm, F., et al. (2018). Tree related microhabitats in temperate and Mediterranean European forests: a hierarchical typology for inventory standardization. Ecol. Indic. 84, 194–207. doi: 10.1016/j.ecolind.2017.08.051
Leibold, M. A., Holyoak, M., Mouquet, N., Amarasekare, P., Chase, J. M., Hoopes, M. F., et al. (2004). The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613. doi: 10.1111/j.1461-0248.2004.00608.x
Leidinger, J., Seibold, S., Weisser, W. W., Lange, M., Schall, P., Turke, M., et al. (2019). Effects of forest management on herbivorous insects in temperate Europe. For. Ecol. Manage. 437, 232–245.
Lüdecke, D. (2021). sjPlot: Data Visualization for Statistics in Social Science. R Package Version 2.8.7. Available Online at: https://CRAN.R-project.org/package=sjPlot. doi: 10.1016/j.foreco.2019.01.013. (accessed August 07, 2021)
Lüdecke, D., Ben-Shachar, M. S., Patil, I., Waggoner, P., and Makowski, D. (2021). Performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 6:3139. doi: 10.21105/joss.03139
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., et al. (2020). vegan: Community Ecology Package. R Package Version 2.5-7. Available Online at: https://CRAN.R-project.org/package=vegan. (accessed August 07, 2021)
Parker, G. G. (1995). “Structure and microclimate of forest canopies,” in Forest Canopies, eds M. Lowman and N. M. Nadkarni (San Diego: Academic Press), 73–106.
Perry, D. R. (1978). A method of access into the crowns of emergent and canopy trees. Biotropica 10, 155–157. doi: 10.2307/2388019
Petermann, J. S., Roberts, A. L., Hemmerling, C., Bajerski, F., Pascual, J., Overmann, J., et al. (2020). Direct and indirect effects of forest management on tree-hole inhabiting aquatic organisms and their functional traits. Sci. Total Environ. 704:135418. doi: 10.1016/j.scitotenv.2019.135418
Petermann, J. S., Roberts, A. L., Weisser, W. W., and Gossner, M. M. (2018a). Tree Hole Sampling At 52 Forest Plots: Gerneral Information, 2014. Version 2. London: Department Of Business Energy And Industrial Strategy.
Petermann, J. S., Roberts, A. L., Weisser, W. W., and Gossner, M. M. (2018b). Tree Hole Sampling At 52 Forest Plots: Species Abundance, 2014. Version 2. London: Department Of Business Energy And Industrial Strategy.
R Core Team (2020). R: A Language and Environment For Statistical Computing, Version 4.0.2. R Foundation for Statistical Computing: Vienna.
Sallé, A., Cours, J., Le Souchu, E., Lopez-Vaamonde, C., Pincebourde, S., and Bouget, C. (2021). Climate change alters temperate forest canopies and indirectly reshapes arthropod communities. Front. For. Glob. Change 4:710854. doi: 10.3389/ffgc.2021.710854
Schmidl, J., Sulzer, P., and Kitching, R. L. (2008). The insect assemblage in water filled tree-holes in a European temperate deciduous forest: community composition reflects structural, trophic and physicochemical factors. Hydrobiologia 598, 285–303. doi: 10.1007/s10750-007-9163-5
Scholl, P. J., and Defoliart, G. R. (1977). Aedes triseriatus and Aedes hendersoni - (Diptera-Culicidae): vertical and temporal distribution as measured by oviposition. Environ. Entomol. 6, 355–358. doi: 10.1093/ee/6.3.355
Shaw, D. C. (2004). “Vertical organization of canopy biota,” in Forest Canopies, 2nd Edn, eds M. Lowman and B. Rinker (Cambridge: Academic Press). doi: 10.1016/B978-012457553-0/50008-3
Sinsko, M. J., and Craig, G. B. (1979). Dynamics of an isolated population of Aedes triseriatus (Diptera: Culicidae). I. Population size. J. Med. Entomol. 15, 89–98. doi: 10.1093/jmedent/15.2.89
Srivastava, D. S., Kolasa, J., Bengtsson, J., Gonzalez, A., Lawler, S. P., Miller, T. E., et al. (2004). Are natural microcosms useful model systems for ecology? Trends Ecol. Evol. 19, 379–384. doi: 10.1016/j.tree.2004.04.010
Ulyshen, M. D. (2011). Arthropod vertical stratification in temperate deciduous forests: implications for conservation-oriented management. For. Ecol. Manage. 261, 1479–1489. doi: 10.1016/j.foreco.2011.01.033
Verdonschot, P. F. M., and Besse-Lototskaya, A. A. (2014). Flight distance of mosquitoes (Culicidae): a metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 45, 69–79. doi: 10.1016/j.limno.2013.11.002
von Brandt, A. (1934). Untersuchungen in Baumhöhlengewässern auf Fagus sylvatica. Arch. Hydrobiol. 27, 546–563.
Wickham, H. (2016). ggplot2: Elegant Graphics for Data Analysis. New York: Springer-Verlag. doi: 10.1007/978-3-319-24277-4
Yanoviak, S. P. (1999). Community structure in water-filled tree holes of Panama: effects of hole height and size. Selbyana 20, 106–115.
Keywords: dendrotelmata, tree hole, canopy, vertical stratification, niche separation, metacommunity, dispersal, microcosm
Citation: Gossner MM and Petermann JS (2022) Vertical Stratification of Insect Species Developing in Water-Filled Tree Holes. Front. For. Glob. Change 4:816570. doi: 10.3389/ffgc.2021.816570
Received: 16 November 2021; Accepted: 21 December 2021;
Published: 14 January 2022.
Edited by:
Brett Scheffers, University of Florida, United StatesReviewed by:
Aurélien Sallé, Université d’Orléans, FranceAlan Feest, University of Bristol, United Kingdom
Copyright © 2022 Gossner and Petermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin M. Gossner, bWFydGluLmdvc3NuZXJAd3NsLmNo
 Martin M. Gossner
Martin M. Gossner Jana S. Petermann
Jana S. Petermann