- 1Department of Environmental Studies, Prescott College, Prescott, AZ, United States
- 2Dorena Genetic Resource Center, USDA Forest Service, Cottage Grove, OR, United States
- 3School of Forestry, Northern Arizona University, Flagstaff, AZ, United States
White pine blister rust, caused by the non-native, invasive fungal pathogen Cronartium ribicola, is a significant cause of mortality in white pines (Pinus subgenus Strobus) in North America. Along with climate-driven range contraction, mortality from blister rust can seriously impact the abundance and distribution of the nine white pine species native to the United States and Canada. Very little evaluation of this disease in southwestern white pine (Pinus strobiformis) has been previously undertaken, but genetic resistance to the disease has been documented, including major gene resistance (MGR) conferred by a dominant R gene. Data is emerging suggesting that the species also has quantitative disease resistance (QR). Our results suggest QR occurs at low frequency, with perhaps 10% of trees having a moderate level (> 35% survival). We assessed progeny arrays from 40 P. strobiformis families (1873 seedlings), originating from three populations, inoculated with C. ribicola. Subsequently, the seedlings were assessed for signs, symptoms and resulting impact in a common garden trial over a 7.5-year period to determine the types and frequency of resistance in a portion of this species’ range. There was a high incidence of both stem symptoms and mortality in the P. strobiformis families tested, and families ranged in survival from 0 to 84.6%. Three families had > 70% survival, representing perhaps the highest documented QR to date in a North American white pine species. Approximately 29.1% of the 441 surviving seedlings showed no stem symptoms, and of the approximately 70.8% of seedlings surviving with infections only few (24 of 316) had infections of moderate to high severity. QR traits associated with improved survival were primarily related to lower severity of infection, a reduced number of stem symptoms, and an increased number of bark reactions. Despite the high overall susceptibility, the presence of QR appears to be at a frequency and level useful to forest managers involved in restoration and reforestation efforts.
Introduction
Non-native invasive pathogens and pests have had a substantial negative impact on tree species and their associated ecosystems. More recently, because of climate change and changing patterns of transient disturbances, there are increased concerns about how these pests and pathogens will affect the distribution and stability of forest host species. Cronartium ribicola J.C. Fisch, the invasive non-native fungal pathogen responsible for the disease white pine blister rust, has caused high mortality to both economically and ecologically important white pine species since its introduction in North America in the early 20th century (Kinloch, 2003). For example, the pathogen, along with early 20th century logging and harvest practices, has resulted in extensive forest loss in western white pine (Pinus monticola ex. Don) stands in the Interior West United States where it once comprised 25–50% of the forests in the region (Fins et al., 2002; Tomback and Achuff, 2010). There are eight species of Pinus subgenus Strobus, known as the white pines or five-needle pines, in the western United States (and three of these also occur in Canada). The white pines broadly are keystone species where they are found, are important components of the hydrological cycle, providing erosion control, and wildlife habitat as well as a component of temperate forest biodiversity (Schoettle, 2004; Tomback and Achuff, 2010). All of the North American white pine species are extremely susceptible to white pine blister rust (Hoff et al., 1980; Kinloch, 2003; Sniezko et al., 2008) and all but Pinus longaeva have C. ribicola within their native range in the United States and Canada, but the disease has not yet been documented in Mexico or Central America.
Pinus strobiformis Engelm. (Southwestern white pine) is a large, long-lived conifer native to the southwestern United States and Mexico (Looney and Waring, 2012). The species thrives in mixed-conifer stands at mid to high elevations (Looney and Waring, 2012; Shirk et al., 2018) where it is moderately shade-tolerant (Goodrich and Waring, 2017) and drought-tolerant (Bucholz et al., 2020), often occurring in sky islands. Within the northern extent of its range, P. strobiformis is part of a moving hybrid zone with Pinus flexilis (Menon et al., 2020) with some evidence of increased fitness due to introgressed alleles from P. flexilis (Menon et al., 2021).
Like all other North American white pines, P. strobiformis is very susceptible to the disease white pine blister rust (Conklin et al., 2009; Sniezko et al., 2011). However, the fungal pathogen is a more recent invader of the southwestern United States, detected in the Sacramento Mountains of New Mexico in 1990 (Hawksworth, 1990) with a likely earlier arrival in the 1970s (Jacobi et al., 2018), and has not yet been identified within the pine’s core range in Mexico. Unease about the potential impacts of increasing incidence of rust in P. strobiformis forests, along with projected increasing aridity and range contraction in the southern portion of the species range (Seager and Vecchi, 2010; Shirk et al., 2018), have led to an increased interest in what type and frequency of genetic resistance may naturally occur in the species. Characterizing patterns of genetic resistance will prove valuable, especially in the context of identifying how the most affected stands of P. strobiformis will be impacted, and will inform the conservation, restoration, and management actions that are likely to be the most beneficial. To be successful for management and mitigation, genetic resistance must be stable, durable and usable at an appropriate frequency and level (Sniezko et al., 2020).
Despite the ubiquitous susceptibility of white pines to white pine blister rust, and the lack of co-evolution with this non-native disease, white pines generally do have a low frequency of natural genetic resistance (Hoff et al., 1980; King et al., 2010; Sniezko et al., 2014), often referred to as exapted resistance (Gould and Vrba, 1982; Bartholomé et al., 2020). Genetic resistance in white pine species is generally associated with having either a dominant major R gene (MGR), Cr3 in P. strobiformis (Kinloch and Dupper, 2002), conveying complete resistance, or quantitative disease resistance (QR), with an array of phenotypic traits and more complex patterns of inheritance (Hoff et al., 1980; Sniezko et al., 2014; Weiss et al., 2020; Liu et al., 2021). Quantitative disease resistance can prevent some trees from developing cankers, but in other cases the trees do develop cankers, but respond via bark reactions and other mechanisms (Box 1) which reduce the rate of disease development and in some cases halt the spread of the fungus (Hoff, 1986; Sniezko et al., 2014; Vázquez-Lobo et al., 2017). Previous studies have acknowledged several traits potentially associated with QR in white pines including fewer needle spots (Hoff and McDonald, 1980), reduced number of stem symptoms (cankers and bark reactions) (Kegley and Sniezko, 2004; Sniezko et al., 2014), slow fungus growth in the needles (Hoff, 1988), the occurrence of partial or complete bark reactions (Hoff, 1986), slow canker growth (Hunt, 1997), delayed onset of symptom development (Kegley and Sniezko, 2004), and ultimately increased survival (Sniezko et al., 2014, 2020). The aforementioned traits are not mutually exclusive, and often occur together. Within and between families there is variation in the expression of different resistance components (Sniezko et al., 2014).
BOX 1 | Resistance continuum in Pinus strobiformis.
Genetic resistance to the disease white pine blister rust is generally split between major gene resistance (MGR) and quantitative disease resistance (QR). Each type of resistance is associated with different traits and frequencies. Infection of pine hosts with Cronartium ribicola occurs when (A) basidiospores of C. ribicola disperse from the alternate host, mostly notably species of Ribes, and enter the stomata of the pine needles. (B) The fungal hyphae spread through the needle tissue and forms needle spots. These needle spots may be diagnostic for MGR if a hypersensitive-like (HR-like) reaction occurs and arrests the further spread of the fungus. This results in trees that are stem symptom free. However, if MGR is not present than the fungal hyphae progress through the plant tissue and usually enter the bole of the tree. (C) If QR is present than several traits may manifest including necrotic bark lesions that wall off the spread of the fungus – known as bark reactions. Additional traits include slow fungal growth, decreased numbers of stem symptoms and decreased severity of infection. (D) If the tree is susceptible than the fungus will form a normal canker disrupt normal vascular processes eventually girdling and killing the tree. Eventually the fungus will proceed to develop aeciospores spores that will erupt from the canker and disperse to reinfect the alternate Ribes host. (E) The best methods for identifying and tracking the progression of the disease and the development of different QR traits requires growing seedlings from open-pollinated cones in common gardens (Photo of USDA Forest Service, Dorena Genetic Resource Center, Cottage Grove, OR. (F) As phenotypes develop in the inoculated seedling families a continuous range of variation in traits may become obvious, where, for example, family 1 may have a higher number of stem symptoms compared to another family 2. These differences are often associated with QR. (G) The most useful indicator of QR is survival even in the presence of infection. In inoculation trials, susceptible families (orange) will usually reach 100% stem symptoms and 100% mortality quickly compared to QR families (low QR black and high QR dark brown) that might range between 0 and 100% survival but mortality is delayed or, in the case of top QR families, avoided. The seedlings in the top QR families usually have some seedlings that have no stem symptoms, and some that have bark reactions or slow growing cankers, as well as some seedlings that died from rust infection. MGR, in contrast, is characterized by a family that segregates 1:1 or 3:1 for a SS-Free phenotype and 50% or greater survival (purple), with the progression of the fungus being stopped in the needles. Obvious HR-like spot phenotypes, like those common in P. lambertiana or P. monticola MGR seedlings, may or may not always clearly appear in Pinus strobiformis. Needle shed can also sometimes be notably present in MGR seedling families. MGR, when present, may mask the expression of QR and current resistance trials based on common garden approaches are generally unable to determine if families have both MGR and QR traits, unless a virulent strain of rust is used to overcome MGR. (H) Progression of the disease following infection of 2 year old seedlings occurs over several years with needle spots first appearing approximately 7–9 months after infection with canker and stem symptom formation occurring approximately 12 months after infection and continuing with some families having delayed symptom development. Mortality follows the expansion of cankers eventually girdling the tree. The timing of inspections will attempt to follow the natural progression of the disease.
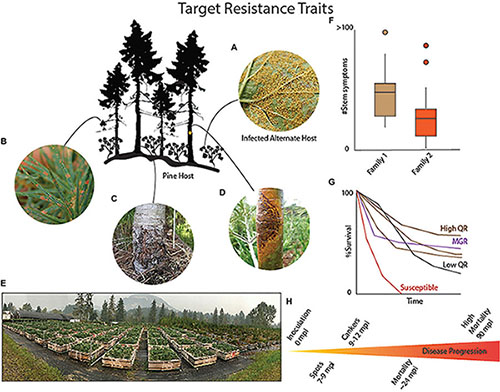
Some white pines with MGR exhibit a hypersensitive-like reaction that occurs within the needle and stops the pathogen from spreading into the stem of the tree. While MGR seems to be the best option for stabilizing forests with blister rust hazard, this type of resistance is often overcome by virulent strains of the disease (Kinloch and Comstock, 1981; Kinloch et al., 1999, 2004; Kinloch and Dupper, 2002) making it less durable than QR (Sniezko et al., 2020). As such QR represents a more usable form of resistance, especially in combination with MGR, in the face of an evolving white pine blister rust pathosystem.
Notwithstanding its promise, QR can be difficult to characterize, in part owed to the time commitment needed to monitor trials for several years and the challenge associated with interpreting the many interacting resistance phenotypes and the quantitative genetic contributions. Because of these difficulties, the underlying genetic control of QR in white pines is not well understood (Vázquez-Lobo et al., 2017). There are some emerging examples in the literature, mostly centered on Pinus lambertiana and P. monticola (Kegley and Sniezko, 2004; Kolpak et al., 2008; Kinloch et al., 2012; Liu et al., 2013; Sniezko et al., 2014, 2020; Vázquez-Lobo et al., 2017; Weiss et al., 2020), which have begun to characterize QR in white pines, and in some cases the genomic loci that partially contribute to this form of resistance (Liu et al., 2020, 2021; Weiss et al., 2020). However, the most detailed characterization of QR traits is when progeny arrays (families) grown in a common garden are assessed for variation in inheritance of resistance traits and by the co-occurrence of one or more of these traits allowing individuals, on average, to survive longer (Sniezko, 2006; Sniezko et al., 2014, 2020).
The objective of this study is to assess the phenotypes associated with increased survivorship in P. strobiformis and begin to characterize QR in the species from several populations in the New Mexico portion of the species range. We monitored the development of disease symptoms in P. strobiformis for 7.5 years post-inoculation. To characterize the components of QR to white pine blister rust, we collected measurements both within and between families on (1) disease incidence (the percentage of plants infected, both in the needle and the stem, in a seed family), (2) the number of stem symptoms, (3) the percentage of individuals within a family with stem symptoms, (4) the percentage of individuals within a family with bark reactions, (5) the timing of stem symptom development, (6) the severity of infection, and (7) temporal patterns of survival. We hypothesize that P. strobiformis families with a reduced number of stem symptoms (including some seedlings that have none), higher occurrence of either complete or incomplete bark reactions, and later appearance of stem symptoms, will lead to higher survival, and will indicate the presence of QR.
Materials and Methods
Experimental Design
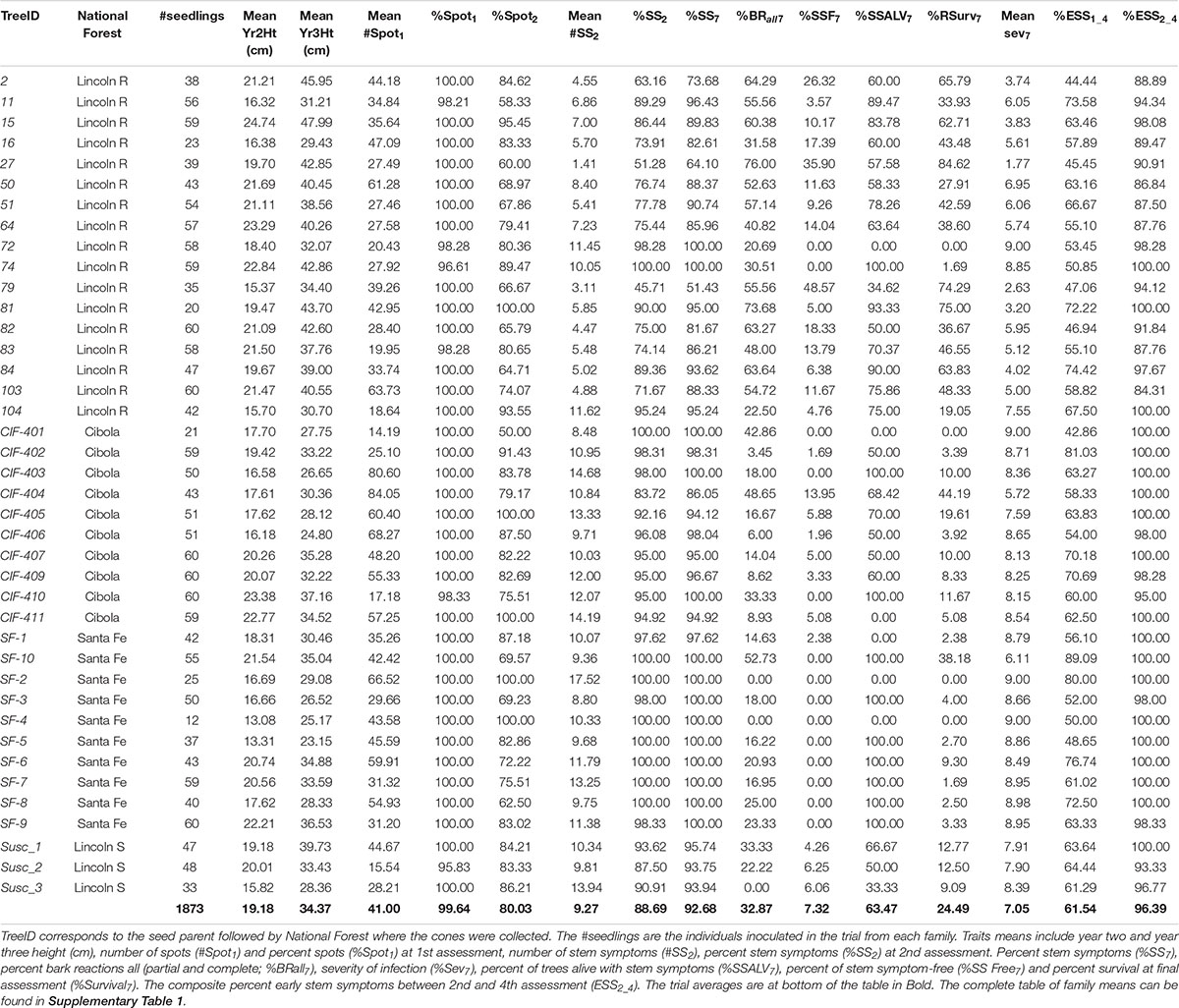
Cones from 40 open-pollinated, P. strobiformis trees were collected in 2008 from three populations, the Lincoln (n = 20), Santa Fe (n = 10), and Cibola (n = 10) National Forests in New Mexico, United States (Figure 1). Seed from the 40 parent trees were sown in March 2009 at the U.S. Department of Agriculture, Forest Service’s Dorena Genetic Resource Center (Cottage Grove, Oregon, United States). Bradford Canyon, on the Lincoln NF, had previously been documented with very high incidence of white pine blister rust, in some stands approximately 90% of the trees have been infected (Conklin, 2004; Conklin et al., 2009). Seventeen parent trees selected from the Lincoln NF, the site where the first P. strobiformis trees with MGR were documented, were canker-free, suggesting putative resistance (designated here as Lincoln R), in addition three trees with moderate-to-heavy cankering were selected to serve as susceptible controls (Lincoln S). The Lincoln R parent trees had been previously screened for MGR at the USDA Institute of Forest Genetics in Placerville, CA and they did not segregate in ratios that would be expected if they carried the Cr3 allele conveying MGR (D. Conklin Personal Communication). Parent trees selected from the Santa Fe NF and Cibola NF were selected from locations with little or no rust present at the time of cone collection and represent random genotype selections (Table 1). Seedlings were grown for 2 years, 1 year in 164 cm3 supercell Cone-tainersTM (Ray Leach, Canby, OR, United States) in family blocks in an unheated greenhouse and then transplanted to 0.9 m × 1.2 m × 0.3 m boxes outside for the second growing season. Twelve to 60 seedlings were available for each family (mean = 47), and seedlings were transplanted into family row plots in randomized complete block design with six blocks and up to 10 seedlings per family per block. Seedlings were inoculated in September of 2010 with basidiospores of C. ribicola. Details of the standard Dorena Genetic Resource Center (GRC) inoculation procedures are outlined elsewhere (Kegley and Sniezko, 2004; Sniezko et al., 2008, 2011). Mean inoculums density was 4,527 spores/cm2; basidiospore germination was 98.7%. Both primary and secondary needles were present on seedlings at the time of inoculation.
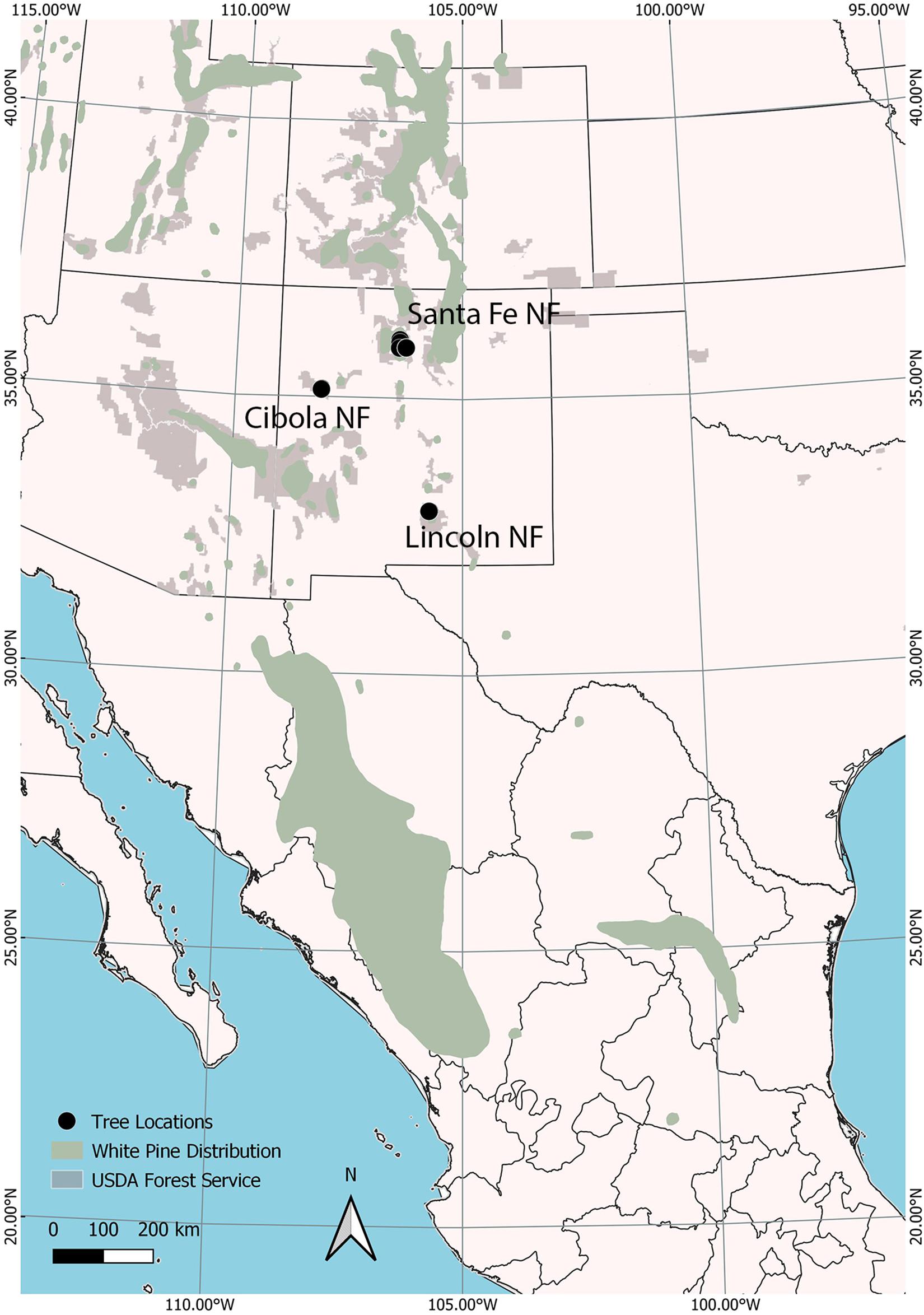
Figure 1. Open-pollinated cones from 40 trees were selected from three national forests (black dots): The Santa Fe in northern New Mexico, Cibola in western New Mexico, and the Lincoln in southern New Mexico. These population represent a portion of the disjunct northern periphery of the Pinus strobiformis range (extending south into Mexico) within the range of white pines (green). USDA Forest Service unit boundaries are shown in light gray.
Disease Trait Assessment
Following inoculation with C. ribicola, infected seedlings were periodically assessed for the presence of rust symptoms, with data collection ending in February 2018 (7.42 years post-inoculation) (Table 2). Seedlings that died from causes other than white pine blister rust were removed from analysis (n = 10) and a total of 1883 seedlings were included in the study. The first assessment occurred approximately 0.75 years post-inoculation and seven assessments were conducted over the course of the trial. The timing of early assessments is calibrated to capture the peak of trait development (e.g., spots and canker emergence). All seedlings were assessed for a core set of traits. Specifically, number of needle spots at first assessment, the presence/absence of needle spots at second assessment, number of cankers, number of bole infections, number of bark reactions, number of partial bark reactions, overall severity of infection, and survival. Full counts of the number and type of stem symptoms were completed at second assessment and the presence of additional stem symptoms were noted at subsequent inspections, since the growth and merging of cankers made later counts more problematic. The counts represent one point in time, and some seedlings showed stem symptoms at later assessments. Additionally, both pre-inoculation height (recorded after inoculation but before 3rd year growth; April 2011) and height present one growing season post-inoculation, recorded during the 2nd assessment (February 2012; 1.42 years post-inoculation).
Based on the level and severity of infection with white pine blister rust, each tree was also assigned a severity classification at each assessment. The classification assigns a seedling a numeric value that assesses the severity of damage from 0, no infection, to 9, dead from rust with classes designated by the degree to which a canker has encircled the bole of the seedling and expanded vertically. For example, a tree that is infected with blister rust (presence of needle spots and a canker) with intermediate severity, a normal canker encircling > 50% but < 100% of the bole but little vertical expansion, would receive a rating of 4. The severity rating and the disease trait phenotypes are standard measurements recorded as part of rust inspections at the USDA Dorena GRC (Supplementary Table 1). The severity rating for each seedling is dynamic and can change (increase or decrease) with each subsequent assessment, reflecting the degree of rust progression or resistance response. Seedlings can have one or many stem symptoms and the severity provides a composite look at the progression of all infections present at each point in time.
Infection Means
Percentages and means were calculated from the measured rust phenotypes at the family, and forest scale. For final tally of needle spots, bole infections, complete bark reactions, partial bark reactions, all bark reactions (BRall), stem symptoms (normal cankers + bark reactions + partial bark reactions), and survival, percentages were calculated by categorically assigning individuals as either symptomatic or not (binary) for a rust phenotype. #Spots, #BI, #BRall, and severity was assessed as the family mean of the individual scored values. Forest means were assessed as the mean of family means for each population. Number of bole infections and normal cankers were assessed at the 2nd assessment following inoculation. Existence of stem symptoms was assessed at all following inspections along with mortality, %survival and severity allowing us to assess the temporal progression of the disease and delayed development of traits (survival with rust), a potential characteristic of QR. Percent bark reaction was calculated using only individuals within a family that developed stem symptoms to avoid underestimating the trait by including those individuals that remained stem symptom free.
Timing and Development of Resistance Traits
The temporal pattern, or timing of symptom development can result as a form of QR. If the spread of fungal hyphae through live tissue can be slowed, known as slow fungal growth (SFUG) in the needle (Hoff, 1988) or slow canker growth (SCANK) if in the stem (Hoff and McDonald, 1980; Hunt, 1997), the overall survival can be prolonged. We interpret this delay in symptom development from the percentage of early stem symptoms (%ESS2_4), where the maximum value is 100% when all seedlings in a family with stem symptoms at 2nd assessment and the minimum is 0% when no trees have stem symptoms at 2nd assessment. The focus is on delayed stem symptom development. %ESS is calculated between 2nd (1.42 ypi) and 4th (3.25 ypi) assessment. Families that had a lower %ESS may suggest a form of QR. Additionally, the percentage of seedlings that developed stem symptoms but survived (SSALV) was calculated from the subset of individuals that did develop stem symptoms. Those families with a higher SSALV percentage may also suggest a form of QR.
Test of Major Gene Resistance
The presence of stem or branch cankers were aggregated into a cumulative binary measure of the phenotype stem symptom-free (SS-free) or stem symptom (SS) at the final assessment. Segregation ratios of SS-free:SS were tested to identify the potential presence of MGR. We used the Mendelian segregation ratios 1:1 (Rr x rr) and 3:1 (Rr x Rr) for SS-free:SS seedlings and tested the hypothesis that each family did not differ significantly from a probability of 0.5 or 0.75, respectively using an exact binomial test. Maternal source trees whose families failed to differ significantly from expected 1:1 or 3:1 ratios are inferred to potentially be heterozygous for the Cr3 allele and possess MGR.
Frequency of Quantitative Disease Resistance
Variation in family performance was used to assess the level of QR. On an individual basis, the most susceptible seedlings were those that were cankered earliest and had the earliest mortality. The most susceptible families (and presumably most susceptible parent trees) were those in which 100% of seedlings were cankered by 2nd assessment and 100% mortality by 3rd assessment. The most resistant families were those with highest survival (lowest rust related mortality) at the final assessment. No one trait fully encompasses the full gamut of resistance between the families, but perhaps the most important is survival which conceivably could vary from 0 to 100 percent for QR families. Survival potentially includes both canker-free seedlings as well as seedlings with stem symptoms that are alive (SSALV) at later assessments (severity of stem symptoms on these living seedlings also may vary). Within the group of highest susceptible families described above, the most susceptible might be those with the highest number of Spots1 and/or number of stem symptoms early. Classification of QR in P. strobiformis families was based on seedling families that survived for the duration of the trial even in the presence of rust infection. The percentage of surviving individuals within a family at the end of the trial was used as a proxy for the frequency of QR that may be found in the field under high rust conditions. Survival was further partitioned into all survivors (RSurv), survivors with infection (SSALV), and survivors that were stem symptom free (SS-free). An additional point is the timing and progression of stem symptoms and mortality. By contrast, MGR is typically defined using a single trait, cankered vs. non-cankered.
Differences in mean disease symptom between both families and National Forests was assessed using analysis of variance (ANOVA) at 95% confidence. Regression analysis on traits was carried out using linear mixed effects models fit by REML with random factors of parent trees nested within Forest Stand. All statistical analyses were carried out in the R statistical environment (R Core Team, 2020).
Results
Characterizing QR Resistance Traits
Overall susceptibility to white pine blister rust in progenies from native stands was high. Inoculation was successful and indicated by nearly all seedlings developing needle spots on secondary needles (99.64% at 0.75 years post-inoculation). Family variation in needle spots ranged between 95.83 to 100%, and the number of spots varied dramatically by family (overall mean number of needle spots per family was 41.00 with family means ranging from 14.19 to 84.05 (Table 3). By 1.42 years-inoculation (2nd assessment) the percent needle spots per family was somewhat lower, at 80.03% with a family range between 50.0 to 100% (Supplementary Table 2), suggesting possible resistance related needle shed (sensu Hoff and McDonald, 1980) in some families. Mean percent stem symptoms at 1.42 years post-inoculation varied among families ranging from 45.71 to 100% with an overall mean of 88.69%, and nine of the 40 families having 100%. The number of stem symptoms averaged 9.27 over all families, with family means varying from 1.41 to 17.52. On a population basis, families from Lincoln R stands had a lower percentage of stem symptoms (86.07%) at the end of the trial (7.42 years post-inoculation) and lower mean number of stem symptoms per tree at 2nd assessment (6.38) compared to the random tree selections from stands with no rust presence in Cibola (11.63), Santa Fe (11.19) and the susceptible controls in Lincoln S (11.36).
At final inspection (February 2018, 7.42 years post-inoculation) 24.49% of trees had survived (Figure 2). There was a total of 446 living individuals and 130 of them were stem symptom free, and of the 316 with stem symptoms, only 24 have a severity rating > 4 and a number of those appeared to have inactive cankers. Family SF-10 had 38.18% survival and 100% of surviving trees had stem symptoms present at early assessments but generally low severity. Putatively resistant families in Lincoln R had higher survival and ranged from 0 to 84.62% (45.00%), while families in Cibola, Santa Fe, and Lincoln S ranged in survival from 0 to 44% (9.33%).
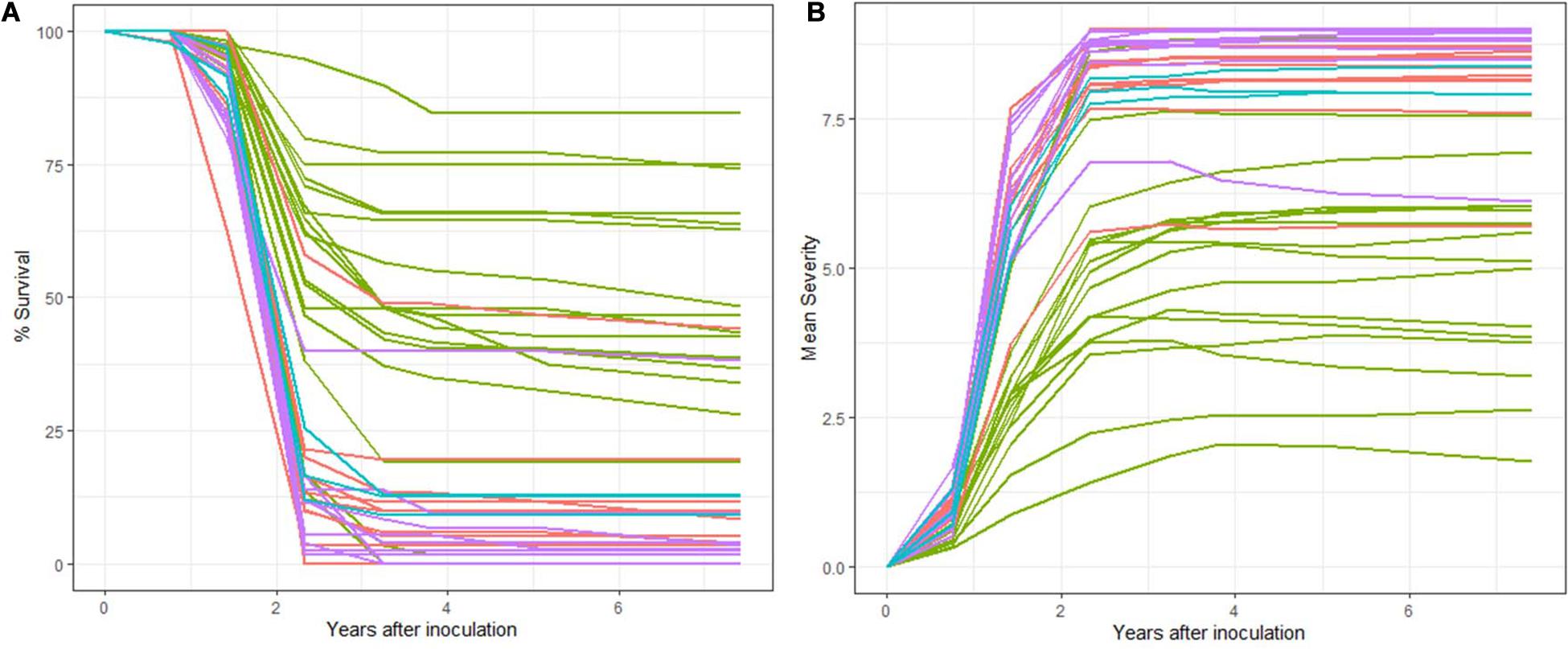
Figure 2. (A) Percent family survival. Families ranged widely in percent survival with a greater distribution of survival occurring within Lincoln R (green). Both Cibola NF (pink) and Santa Fe NF (purple) each had one family that had moderate survival: 44.19 and 38.18% respectively. (B) Mean family severity of infection also had a wide range. Almost a continuous distribution of mean values from low (< 2.5) to 9 (all trees dead) on the 0 to 9 scale. Note that both Cibola and Santa Fe NF have one family each with lower mean severity.
Infection Development
Across all families 92.68% of seedlings eventually developed stem symptoms. Several families experienced a delay in stem symptom development (lower %ESS2_4). In general, the degree of ESS2_4 was high 96.39 (Table 3) with a range of 84.31–100%. There was a significant and negative relationship between the %Survival and %ESS2_4 (DF = 1,38, R2 = 0.204, F = 11.0, P < 0.01) (Figure 3A). There was also a significant difference in %ESS2_4 between Lincoln R and the other three populations (DF = 1,38, R2 = 0.40, F = 19.25, P < 0.01) (Figure 3B). When Lincoln R was removed from the analysis there is still a significant difference of means between Lincoln S with both Cibola (P = 0.008) and Santa Fe National Forests (P = 0.005) for %ESS2_4 (full ANOVA: DF = 2,20, R2 = 0.0.35, F = 6.98, P < 0.005) but no significant difference between the Cibola and Santa Fe forests (P = 0.94) based on Tukey’s HSD post hoc test.
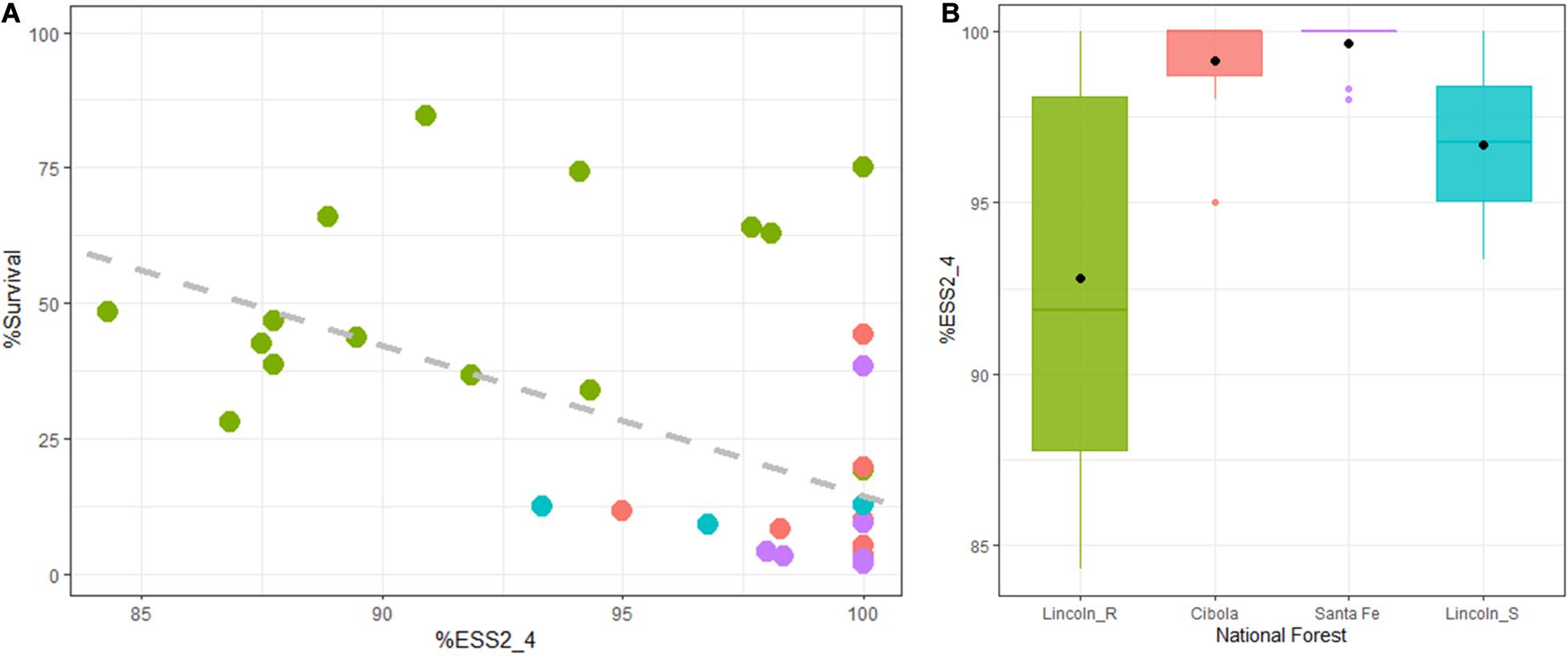
Figure 3. %ESS2_4: (A) There is a significant and negative relationship between the %Survival and %ESS2_4 (y = 291.99 × –2.78, P < 0.001, r = 0.532). (B) Most families in the Cibola (pink) and Santa Fe (purple) National Forests had 100% stem symptom development at 1.42 years post-inoculation (2nd assessment). Both Lincoln R (green) and Lincoln S (blue) had a lower %Early Stem Symptom development suggesting a level of QR.
Only one family (CIF-401) had no survival 2.33 years post-inoculation (3rd assessment), but six other families had very low survival at this early stage, approximately 5% survival all within Cibola or Santa Fe NFs (Supplementary Table 2). Across all families 67.14% of seedlings died from rust by 2.33 years post-inoculation (Figure 2). By 3.83 years post-inoculation (5th assessment) mortality was 73.88% and by the final assessment mortality reached 75.51% (Figure 2). The six families with the highest survival (> 60%) were all from Lincoln R. One family from both the Santa Fe NF (SF-10) and Cibola NF (CIF-404) each had moderately high rust survival at 38.18 and 44.19%, respectively despite having 100 and 83% stem symptoms at 1.42 years post-inoculation.
Survival tracked severity across inspections with an overall severity mean of 7.05 (Table 3) and a family range of 1.77 to 9. Lincoln R families had significantly lower mean severity ratings than the other sites at 5.36 (DF = 1,38, R2 = 0.49, F = 39.26, P < 0.01). Both Cibola NF and Santa Fe NF had one family each with a notable lower mean severity than the other nine families in their populations (Figure 2). Trees alive with stem symptoms (SSALV7) at final assessment (families that only included stem symptom free or 100% mortality were removed) had a mean severity rating of 1.48 with a range of 1 to 8 (Figure 4A). This is notable as survivors with low severity stem symptoms have little chance of dying later and in many cases stem symptoms were bark reactions or inactive cankers. For SSALV7 individuals, there was no significant difference of means between populations for Sev7 rating at 95% confidence. However, several families did differ significantly between each other for severity (Figure 4B).
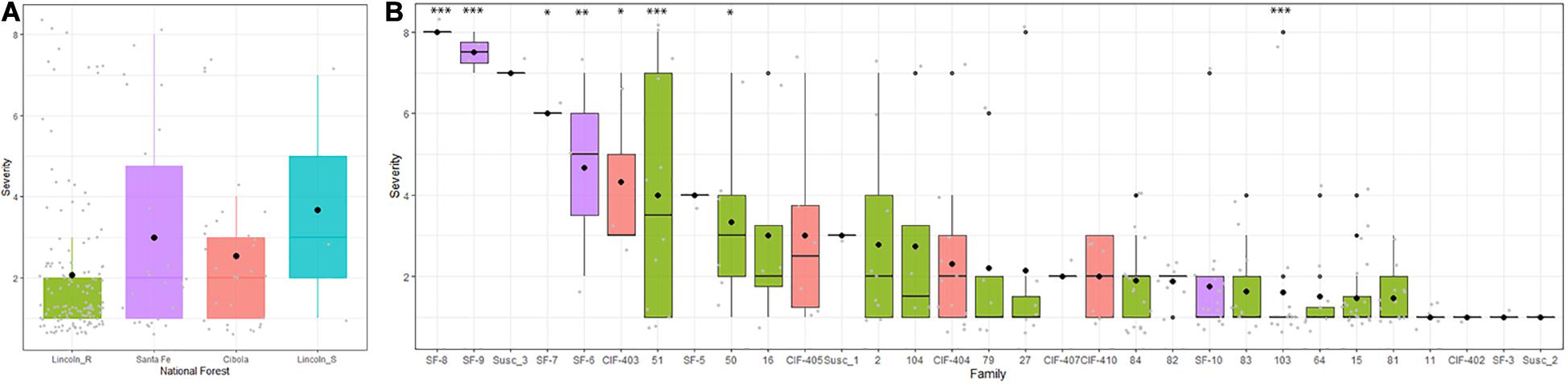
Figure 4. For trees that remained alive (stem symptom free and 100% mortality families removed) with stem symptoms (SSALV7) at the final assessment (7.42 years post-inoculation) the mean severity remained relatively low (1.48). (A) There was no significant difference at 95% confidence in the mean severity for SSALV7 at the forest population scale. There was a range of severity suggesting that several trees may have quantitative resistance. (B) There were some significant differences between families (∗∗∗P < 0.001, ∗∗P < 0.01, and ∗P < 0.05). Gray points reflect jittered individual seedling values for severity within forest stands (A) or families (B).
Pattern of Genetic Resistance
There was a significant difference in the mean number of stem symptoms (DF = 3,36, R2 = 0.52, F = 13.05, P < 0.001) among the sampled forests (Supplementary Table 3). Lincoln R a had much lower average number of stem symptoms at 2nd assessment (12.59) relative to Cibola, Lincoln S, and Santa Fe NF (22.39). When Lincoln R families were excluded from analysis there was no significant difference between forests and the mean number of stem symptoms (Figure 5A).
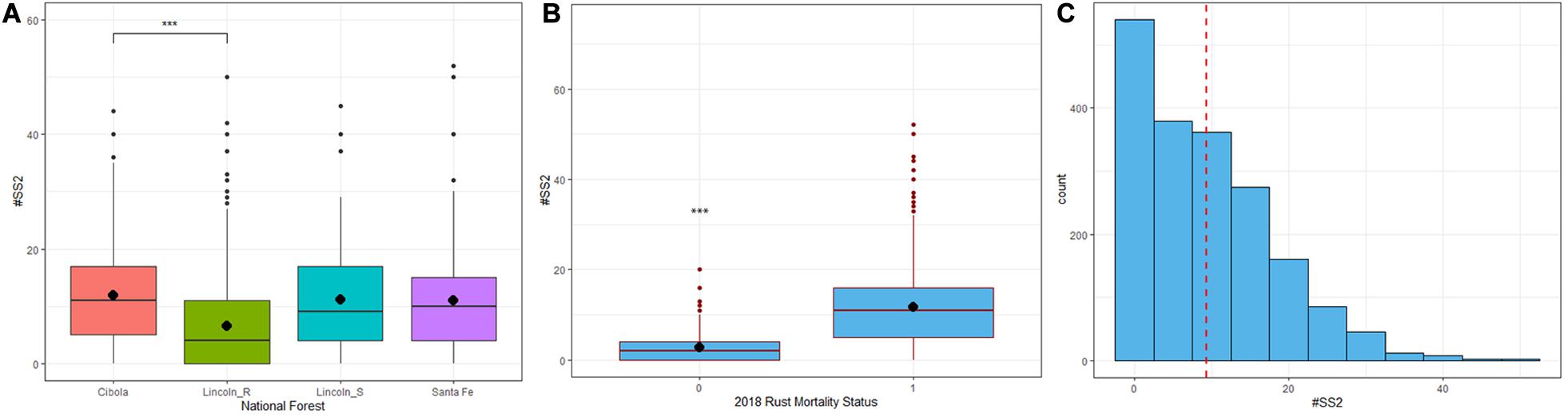
Figure 5. (A) Lincoln R (green) had significantly fewer (P < 0.01) stem symptoms than the Cibola (pink), Santa Fe (purple) and Lincoln S (blue) National Forests. ∗∗∗P < 0.01 (B) The difference in mean number of stem symptoms at 2nd assessment (1.42 years post-inoculation) between trees that remained alive with stem symptoms (SSALV7) at final assessment and those that died of rust. SSALV7 trees had significantly fewer stem symptoms than their counterparts that died of rust (P < 0.01). (C) The frequency distribution of all stem symptoms (normal cankers + complete bark reactions + partial bark reactions) at 2nd assessment had a mean number of stem symptoms of 9.27.
Trees that survived the duration of the trial with stem symptoms (n = 316 SSALV7) had significantly fewer stem symptoms at 2nd assessment (DF = 1,1741, R2 = 0.16, F = 329.6, P < 0.001) compared to those that eventually died from blister rust. The mean number of stem symptoms at 2nd assessment in trees that ultimately survived (SSALV7) was 3.32 compared to those that died from rust 11.39 at final assessment (Figure 5B). The overall mean number of stem symptoms for all families at 2nd assessment was 9.27 (Figure 5C).
Trees from Lincoln R and Lincoln S were significantly taller 1.42 years post-inoculation (3rd year height; F-value = 237.06, DF = 1,1868, P < 0.001) than those found in Cibola and Santa Fe (both sites shorter on average by approximately 8 and 3 cm, respectively). Lincoln S seedlings were shorter than Lincoln R by approximately 5 cm.
Linear mixed effects models found that on an individual tree basis (parent trees nested within forests) the probability of dying from blister rust was positively and statistically associated with the number of stem symptoms at 2nd assessment (t-value = 18.95, P < 0.001). Additionally, there was a significant relationship between the number of normal cankers (nc2) and 2nd year (pre-inoculation) height (t-value = 6.78, P < 0.001). When individuals from the Lincoln NF were removed there was still a positive relationship between nc2 and 2nd year height (t-value = 5.69, P < 0.001). The number of normal cankers at 2nd assessment was positively associated with the number of spots at 1st assessment (t-value = 5.84, P < 0.001) as was the overall number of stem symptoms at 2nd assessment (t-value = 6.01, P < 0.001). Dying from blister rust was negatively associated with the number of complete bark reactions at 2nd assessment (t-value = −7.834, P < 0.001). There was no significant association with the number of partial bark reactions at the same assessment (t-value = −1.798, P = 0.07). At the final assessment (7.42 years post-inoculation) there was a negative significant association with the number of complete bark reactions (t-value = −2.086, P = 0.037), and total bark reactions (BRall; t-value = −15.465, P < 0.001).
As expected, percent survival in a family is significantly and negatively associated with percent of seedlings in the family with stem symptoms (t-value = −8.448, P < 0.001). However, there is quite a bit of variation with some families exhibiting a high percentage of stem symptoms at 2nd assessment but lower final percent rust mortality (Figure 6A). On a family basis there was a significant effect of percent bark reactions (BRall) on percent survival (DF = 1,38, R2 = 0.45, F-value = 115.6, P < 0.001) (Figure 6B). At the final assessment there was a negative relationship between BRall and normal cankers at 2nd assessment (t-value = −6.918, P < 0.001). The appearance of BRall culminated around the 5th assessment (3.83 years post-inoculation) (Figure 7).
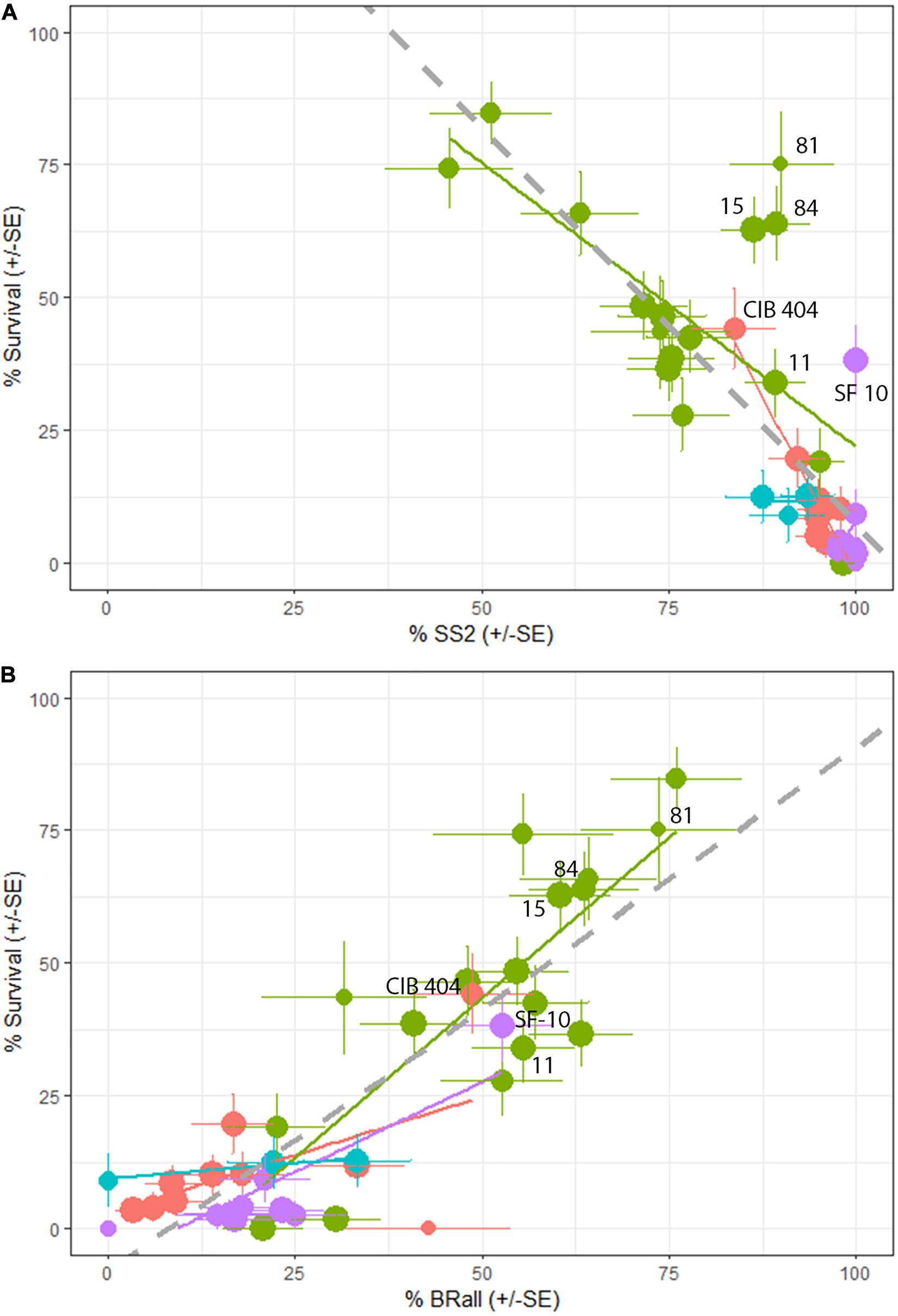
Figure 6. (A) Final family percent survival is negatively associated with percent stem symptoms at 2nd assessment (%SS2; P < 0.001). Lincoln R (green) had significantly lower percent stem symptoms and higher overall survival compared to Lincoln S (blue), Cibola (pink) and Santa Fe (purple) National Forests. (B) Family percent bark reactions + partial bark reactions (BRall7) were positively and significantly related to overall survival (P < 0.001). Lincoln R had significantly more BRall than either Cibola or Santa Fe forests. Error bars ± SE point size increases with the number of seedlings in a family. Seed families that have % SS2 > 80% and % Survival > 25% are labeled in both panels.
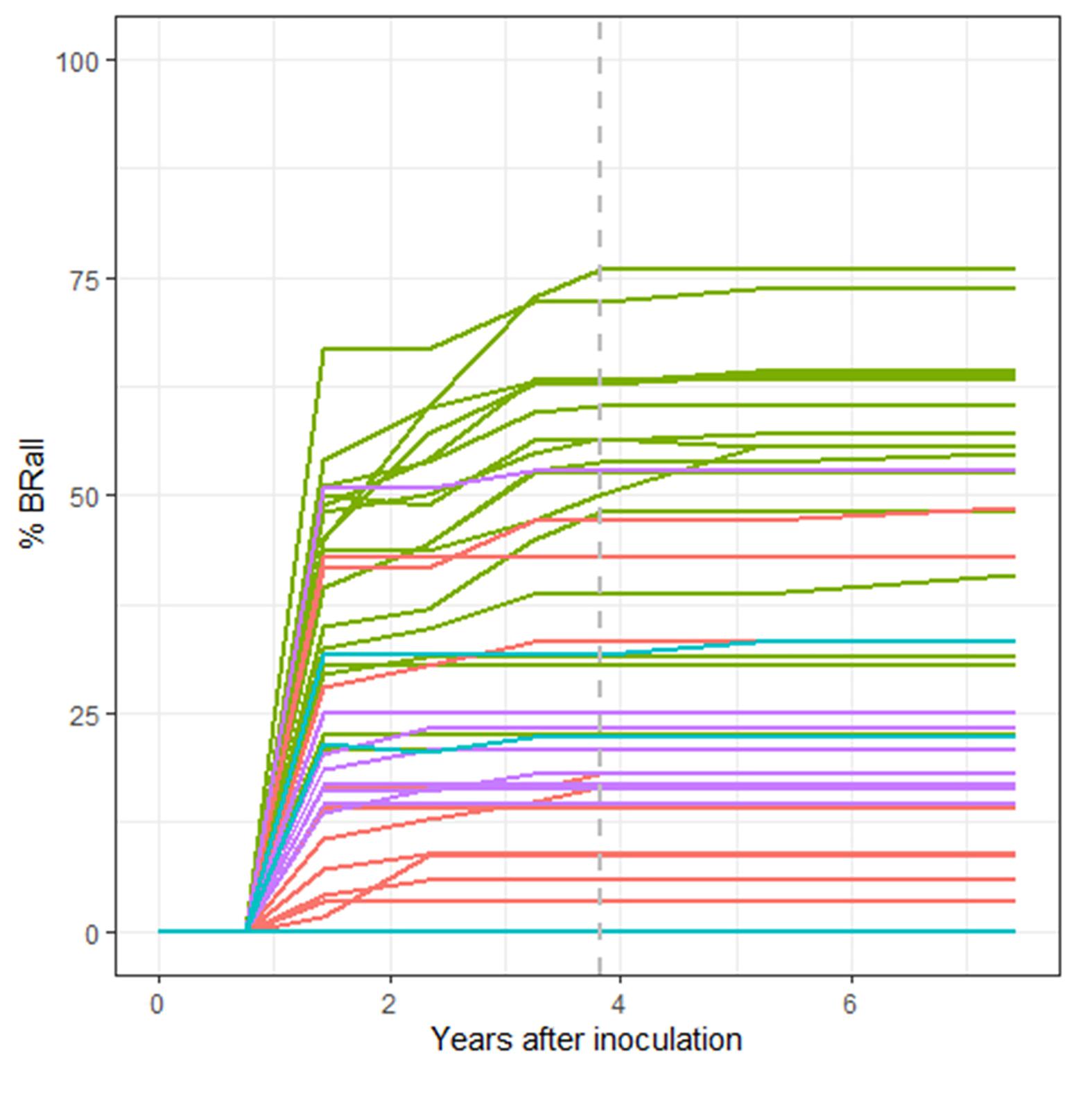
Figure 7. Temporal trend in family percent complete bark reactions and partial bark reactions (%BRall). Lincoln R (green), Lincoln S (blue), Cibola (pink) and Santa Fe (purple) %BRall continued to rise throughout the trial with families reaching a maximum mean %BRall near 5th assessment (3.83 years post-inoculation: gray dashed line), although most bark reactions were noted less than 2 years post-inoculation.
MGR vs. QR
Exact binomial tests of Mendelian segregation ratios 1:1 (Rr x rr) and 3:1 (Rr x Rr) for SSf:SS seedlings identified two families (Lincoln R Id 27 and 79) that failed to differ significantly from 1:1 and there were no families that failed to differ significantly from 3:1 (Supplementary Table 4). The two potential MGR families (Rr x Rr or Rr x rr) occurred in the Lincoln R stand. However, the range of stem symptom free trees in the 17 Lincoln R parents is relatively continuous and the families also generally have moderate to high bark reactions (BRall7), suggesting the two families that are candidates for MGR are more likely at the upper spectrum in this population for QR. Support for QR is provided by an earlier test where the families included in this trial had previously been tested for MGR and were presumed not to carry the Cr3 allele (did not segregate 1:1 in that test). High levels of QR could either mask MGR or express similar to MGR, and the two resistance types may occur in combination in some families since MGR is known to be present at low frequency in this stand.
Discussion
Quantitative Disease Resistance in Pinus strobiformis
Many tree species in North America are susceptible to non-native pathogens and pests (National Academies of Sciences, Engineering, and Medicine, 2019). The level of susceptibility can be extremely high, with local extirpation to potential species level extinction possible. The classic forest example is of the American chestnut (Castanea dentata) which has been functionally extirpated on the landscape due to the occurrence of two diseases, a root rot and the chestnut blight, but other pests and pathogens are increasing and are greatly impacting forests across much of North America (Fei et al., 2019). Several successful programs are in place to identify the type and frequency of resistance to disease and produce seed from resistant populations to aid in restoration or reforestation (Sniezko and Koch, 2017). These programs must first identify the type(s) of genetic resistance, second determine whether to enhance the level of resistance through tree breeding so that it is more usable and lastly determine its durability through field validation trials (Sniezko et al., 2020). Trees tend to be long-lived, and because of this resistance must persist for decades or centuries. Although there are different types of resistance to white pine blister rust, including MGR, the most durable type looks to be QR due to the reduced likelihood of C. ribicola developing virulence to it (Sniezko et al., 2020). Identifying QR can be challenging because of the time and costs associated with screening seedling families for disease symptoms and discerning the different phenotypes of the seedlings. As further advances in biotechnologies progress, it may be possible in the future to accelerate the process of resistance detection by combining QR trials with genomics (Weiss et al., 2020; Liu et al., 2021), genetic field detection (Aglietti et al., 2021) and phenomics (Conrad et al., 2020; Haagsma et al., 2020). Then, field validation trials under natural rust hazard must be installed to better characterize durability and stability of resistance to ensure healthy forests for the long-term following restoration or reforestation with the resistant populations (Sniezko and Koch, 2017). Field validation is one of the best ways to assess whether identified QR in seedlings is representative in adult trees.
Most QR screening trials for resistance to white pine blister rust typically last from 3 to 5 years. However, there is a risk of overestimating survival because some families may exhibit slow rusting and delayed symptom development which ultimately results in seedling mortality (Sniezko et al., 2011). In this study we have monitored infected P. strobiformis seedlings for 7.5 years post-inoculation, providing higher temporal resolution enabling us to be more confident in our early characterization of QR and associated traits in the species, and that in the most resistant families, individuals may survive with non-lethal stem infections. Additional observations in August 2021 of the trees remaining in the trial (after a thinning) indicate that the resistant trees with stem symptoms survive at least 12 years post-inoculation (Sniezko, personal communication). Our analysis suggests that there is a usable level of QR in P. strobiformis, but that there is only a low frequency, ∼10 percent, of parent trees that will have a moderate level of high resistant (surviving) progeny in natural populations. As with most white pine species native to North America, susceptibility of the species in native forests can be very high, for example, greater than 90% of trees cankered. The families with the most effective QR showed levels of survival ranging from 1 to 85%. The Lincoln R population averaged 45% survival, which is the among the highest of any population with QR currently reported for a North American species tested at the Dorena GRC, and very similar to results of testing of canker-free parent trees of P. albicaulis that were selected in stands with 90 percent blister rust mortality (Hoff et al., 2001). Additionally, the level of survival (> 70%) in the top P. strobiformis families far exceeds what has been found in much more extensive screening of > 4000 seedling families over many decades in both P. monticola or P. lambertiana at Dorena GRC and is similar to some of the advance-generation families of P. monticola from the breeding program. Trials of wild open pollinated P. monticola have recorded survival between 3.4 and 9% while P. lambertiana was noted as having survival between 1.6 and 14% (Kegley and Sniezko, 2004).
From the samples in this study, parent trees with a moderate level of survival and QR appear, at a frequency of approximately 10% in this portion of the species range. This frequency stems primarily from the assessment of the Santa Fe and Cibola NF sites which, at the time of cone collection, had little rust present and represented random genotype selections compared to cone collections in the high infection Bradford Canyon population in the Lincoln NF. On balance, the Lincoln R families provide a better look at the range of potential levels of QR. In the Lincoln NF near the Bradford Canyon site 85–90% of trees are cankered. If we assume that the cankered trees are susceptible then we can begin to see the range of QR based on our results. Moreover, because the families from Lincoln R were canker-free trees in a stand with ∼ 90% susceptibility we can extrapolate overall frequency of QR to about 10% of the stand but noticing that the level of QR varied substantially among the Lincoln R parents with 15 of 17 parents the survival ranged from 9.5 to 84.6%. It can also be difficult to identify bark reactions in the field, so some parent tree selections may have had stem symptoms in the past but appeared canker-free when cones were selected. The Lincoln S families were cankered trees that were selected as susceptible controls. However, even the Lincoln S trees tended to fare slightly better in terms of the production of bark reactions. This is probably due to pollen gene flow of resistant genes from the trees with QR in the vicinity.
When assessing the Lincoln NF families, we find an overall higher level of QR. These findings are supported by an earlier smaller trial of P. strobiformis from the same Bradford Canyon collection site where families had only 45.83% mortality after nearly 14 years of monitoring (personal communication R. Sniezko; Sniezko et al., 2008). Since both MGR and QR have now been documented in Bradford Canyon of the Lincoln NF there is potential for at least some of the SS-free trees to be the result of pollen from MGR parents – however, this is expected to be low because 1.) there is a low frequency of MGR; and 2.) greater than 85% of trees alive in Bradford Canyon (Lincoln R) have stem symptoms, which also contribute to the pollen cloud.
Survival and Slow Fungus Growth/Slow Canker Growth
Interestingly, seedling mortality in Cibola and Santa Fe families peaked about 2 years post-inoculation with very little additional mortality occurring at subsequent assessments. In contrast, the Lincoln R families experience more rust mortality at later stages with peak mortality occurring around 4 years post-inoculation. The trees from Bradford Canyon were on average taller than trees from seed collected in the Cibola and Santa Fe so it might take longer to die, or the resistance may be higher (e.g., fewer stem symptoms per tree). Mortality in Lincoln R also occurred at lower frequencies and with higher between family variation. Survival, however, did decrease slowly over the intervening years (Figure 2A). Despite the strong selection pressure in Lincoln R due to the high rust hazard, two of the 17 families from this collection were among the most susceptible in the trial. The two seed parents are approximately 61 meters apart and are on the eastern edge of the sampled population. It’s possible that they are not receiving as much pollen flow from the QR trees in the vicinity. Other possibilities for their low survival include (1) they could be in a microenvironment of lower infection and thus ‘escapes,’ (2) show ontogenetic resistance that would not be conveyed to young progeny, or (3) be homozygous for a recessive gene for resistance. We suspect they are likely escapes but further investigation would be needed to resolve their status.
Two families in the trial had percent stem symptoms consistent with 1:1 segregation expected of MGR parent trees. Yet, based on previous testing, the parent trees from Lincoln NF whose progeny were tested in our study were not MGR candidates, however, at least a low frequency of the canker-free seedling may be the result of pollen contribution from the low frequency of MGR parents in the stand, as noted in a small earlier trial (Sniezko et al., 2008). Thus, surviving seedlings in these families may be a mix of QR, MGR, and QR + MGR genotypes. Approximately 63% of surviving seedlings had stem symptom at the end of the trial and, in ten families, 100% of living seedlings had stem symptoms. The two parents from Bradford Canyon with relatively low percent stem symptoms (< 65%) are likely near the top of the continuum for QR which can provide resistance at levels like MGR. It is still unclear if the Bradford Canyon location is a hot spot for genetic resistance (not likely), or if it only represents a high hazard site where all the susceptible trees are infected, and the efficacy for resistance selection is greatly enhance by focusing on canker-free parent trees which has been noted in trials of P. monticola (Kinloch et al., 1999) and P. albicaulis (Hoff et al., 2001). It is notable that both MGR and high level of QR have now been documented in the Bradford Canyon stand (Sniezko et al., 2008, 2011), and if so, some naturally pyramiding of resistance may already be present. The low degree of QR in the susceptible control families selected from Bradford Canyon, Lincoln S, matched that of the Cibola NF families and were somewhat better performers than the Santa Fe NF families which provides additional support for the likely frequency of QR that can be expected across the range of the species, however, this still must be verified.
Bark Reactions and Normal Cankers
The necrotic bark reaction allows a tree to develop a wound-periderm on the branch or stem of the tree that can stop the spread of C. ribicola (Struckmeyer and Riker, 1951). Bark reactions in P. monticola have exhibited varying effectiveness, where some bark reactions stop the growth of the fungus quickly, while in other cases, bark reactions are partial or incomplete, and the fungus returns and continues to expand (Hoff, 1986) escaping the tree’s defenses. Either way, the occurrence of a bark reaction suggests a trait capable of slowing the spread of rust and at best stopping the spread of the fungus. Bark reactions have been documented in several white pine species, including P. strobus (Struckmeyer and Riker, 1951), P. lambertiana (Kinloch and Littlefield, 1977; Kegley and Sniezko, 2004), and P. monticola (Hoff, 1986; Kegley and Sniezko, 2004; Sniezko et al., 2014). An inoculated seedling can have from 0 to dozens of stem infections, so even when bark reactions do occur, they can appear in conjunction with normal cankers on the same tree. The incidence of bark reactions is positively associated with increased survival. We have shown here that the occurrence of bark reactions appears along a continuum that is associated with QR. Complete bark reactions were notable for this species in this trial, and many occurred early and were no longer visible by the final assessment or had a very low severity rating of 1 or 2.
Quantitative Disease Resistance in Other White Pines
In this study, we equate QR with overall survival. However, we also note that some further QR variation may exist among trees that did not survive. Compared with other five-needle pine species such as P. monticola and P. lambertiana, P. strobiformis has a higher frequency of QR, though still low (∼10%). Previous studies in P. monticola found that both the number of bark reactions and percent of a family with bark reactions is significantly and negatively correlated with percent stem symptoms as well as the percent of trees that are actively infected and alive (SSALV; Kolpak et al., 2008; Sniezko et al., 2020).
Variation in patterns of genetic resistance to white pine blister rust has been addressed in many of the white pine species. In particular, P. monticola, P. flexilis, and P. lambertiana have received a great deal of focus because they each carry a Cr allele for MGR. For example, in P. lambertiana MGR resistance was identified (Kinloch et al., 1970; Devey et al., 1995) at low frequencies across its range, typically less than 1% (Kinloch and Dupper, 1987; Kinloch, 1992; Kinloch et al., 2018). Similarly, P. monticola exhibits a HR-like phenotype in MGR trees and low frequency (0–8%) of resistance in natural populations though frequencies vary across its range (Kinloch et al., 1999, 2003). The overall frequency of MGR resistance in P. flexilis was higher than P. monticola or P. lambertiana, typically near 5% and as high as 14% in the portions of the range tested (Schoettle et al., 2013), and examination of much more of the geographic range is underway. Kinloch and Dupper (1987) reported an HR-like reaction on needles of young P. strobiformis, but similar to P. flexilis, subsequent trials with P. strobiformis have shown HR-like reactions to be less consistent and the use of needle phenotypes is often more difficult to utilize in identification of MGR and the presences of a stem symptom-free phenotype may prove to be a better trait for MGR characterization. Because stem symptom free phenotypes can occur in both MGR and QR progenies, molecular markers would be useful to distinguish the underlying type of resistance and its control as well or the use of virulent strains of rust (which are currently used in P. monticola and P. lambertiana).
An additional consideration for P. strobiformis and for the generalization of levels of genetic resistance across its range involves the position of its northern range within a moving hybrid zone with P. flexilis. Recent studies have found that the two species hybridize (Menon et al., 2018) and because P. flexilis is also known to have low levels of MGR (Schoettle et al., 2013), it is unclear if the somewhat higher level of resistance in P. strobiformis, relative to other white pines, is the product of introgression within this hybrid zone. One of the results of Menon et al. (2018) was that there was little ongoing gene flow between the periphery and core of the P. strobiformis range. The question then begs to be asked, will much of the species range outside of this hybrid zone be more susceptible to the advance of C. ribicola in the future? If, however, resistance is exapted (sensu Gould and Vrba, 1982) and has evolved in response to a different abiotic or biotic selection pressures, then we would hypothesis a more equitable distribution across its range. Efforts have begun to further characterize a range-wide baseline frequency and geographic pattern of genetic resistance to white pine blister rust in P. strobiformis, as well as identifying genomic controls of resistance, its effects on the host physiology, and its future distribution under climate change.
In this study we identified and characterized QR traits in P. strobiformis from a portion of its northern range limit. In families with highest resistance (generally highest survival), we identified (1) fewer early stem symptoms and a lower frequency of early stem symptoms (%ESS2_4), suggesting a slowing of fungal growth, (2) a higher frequencies of bark reactions, and (3) lower severity of infections over the duration of the trial.
The levels and frequency of QR found here are encouraging, and with a focused selection program more resistant parents can be identified. Field trials to validate the resistance from seedling trials have begun and grafting of resistant parents (or forward selections from resistant families) for placement into a clone bank or orchards have also been begun. The results from this trial and ensuing trials will provide land managers the first source of white pine blister rust resistant seed to use in reforestation efforts, but additional testing is needed to identify more resistant parents to ensure seedlots used for reforestation are genetically diverse. Periodic checks of the resistant parent trees on the Lincoln NF have shown no infections, providing cautious optimism that the resistance will be durable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JJ analyzed and interpreted the data, and led the writing of the manuscript. RS conceived of the manuscript and assisted with writing, analysis, and interpretation. Both authors reviewed and edited the manuscript and approved of the final version.
Funding
JJ was supported by NSF 1340852. Dave Conklin, USFS R3 FHP coordinated the cone collections with others in Region 3, and Region 3 FHP provided funding for the study.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the technicians and staff at Dorena Genetics Resource Center for their dedication and efforts in conducting the trial inspections over the many years, particularly the lead efforts of Bob Danchok and Angelia Kegley. We would also like to acknowledge the contributions of our late colleague Douglas Savin. The manuscript was improved thanks to the comments and suggestions from two reviewers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.765871/full#supplementary-material
References
Aglietti, C., Meinecke, C. D., Ghelardini, L., Barnes, I., van der Nest, A., and Villari, C. (2021). Rapid detection of pine pathogens Lecanosticta acicola, Dothistroma pini and D. septosporum on needles by probe-pased LAMP assays. Forests 12:479. doi: 10.3390/f12040479
Bartholomé, J., Brachi, B., Marçais, B., Mougou-Hamdane, A., Bodénès, C., Plomion, C., et al. (2020). The genetics of exapted resistance to two exotic pathogens in pedunculate oak. New Phytol. 226, 1088–1103. doi: 10.1111/nph.16319
Bucholz, E. R., Waring, K. M., Kolb, T. E., Swenson, J. K., and Whipple, A. V. (2020). Water relations and drought response of Pinus strobiformis. Can. J. Forest Res. 50, 905–916. doi: 10.1139/cjfr-2019-0423
Conklin, D. A. (2004). Development of the white pine blister rust outbreak in New Mexico. Albuquerque, NM: USDA Forest Service, Souwestern Region.
Conklin, D. A., Fairweather, M. L., Ryerson, D. E., Geils, B. W., and Vogler, D. R. (2009). White pines, blister rust, and management in the Southwest. Albuquerque, NM: USDA Forest Service Southwest Region.
Conrad, A. O., Villari, C., Sherwood, P., and Bonello, P. (2020). Phenotyping Austrian pine for resistance using fourier-transform infrared spectroscopy. Arboricul. Urban Forestry 46, 276–286. doi: 10.48044/jauf.2020.020
Devey, M. E., Delfino-Mix, A., Kinloch, B. B., and Neale, D. B. (1995). Random amplified polymorphic DNA markers tightly linked to a gene for resistance to white pine blister rust in sugar pine. Proc. Natl. Acad. Sci. 92, 2066–2070. doi: 10.1073/pnas.92.6.2066
Fei, S., Morin, R. S., Oswalt, C. M., and Liebhold, A. M. (2019). Biomass losses resulting from insect and disease invasions in US forests. Proc. Natl. Acad. Sci. 116:17371. doi: 10.1073/pnas.1820601116
Fins, L., Byler, J., Ferguson, D., Harvey, A., Mahalovich, M. F., McDonald, G., et al. (2002). Return of the giants: Restoring western white wine to the Inland Northwest. J. Forestry 100, 20–26. doi: 10.1093/jof/100.4.20
Goodrich, B. A., and Waring, K. M. (2017). Pinus strobiformis seedling growth in southwestern US mixed conifer forests in managed and non-managed stands. Forestry 90, 393–403. doi: 10.1093/forestry/cpw057
Gould, S. J., and Vrba, E. S. (1982). Exaptation—A missing term in the science of form. Paleobiology 8, 4–15. doi: 10.1017/S0094837300004310
Haagsma, M., Page, G. F. M., Johnson, J. S., Still, C., Waring, K. M., Sniezko, R. A., et al. (2020). Using hyperspectral imagery to detect an invasive fungal pathogen and symptom severity in Pinus strobiformis seedlings of different genotypes. Remote Sens. 12:4041. doi: 10.3390/rs12244041
Hawksworth, F. G. (1990). White pine blister rust in southern New Mexico. Plant Dis. 74:938. doi: 10.1094/pd-74-0938a
Hoff, R., Bingham, R. T., and McDonald, G. I. (1980). Relative blister rust resistance of white pines. Eur. J. Forest Pathol. 10, 307–316. doi: 10.1111/j.1439-0329.1980.tb00042.x
Hoff, R. J. (1986). Inheritance of the bark reaction mechanism in Pinus monticola infected by Cronartium ribicola. Collins, CO: USDA Forest Service, Intermount Research Station.
Hoff, R. J. (1988). “Blister rust resistance in western white pine for eastern Washington, Idaho, and western Montana,” in Proc. of a western white pine management symposium, ed. R. S. Hunt (Victoria, BC: Pacific Forestry Centre), 12–20.
Hoff, R. J., Ferguson, D., McDonald, G. I., and Keane, R. E. (2001). “Strategies for managing whitebark pine in the presence of white pine blister rust,” in Whitebark Pine Communities: Ecology and Restoration, eds D. Tomback, S. F. Arno, and R. E. Keane (Washington: Island Press), 346–366.
Hoff, R. J., and McDonald, G. I. (1980). Resistance to Cronartium ribicola in Pinus monticola: reduced needle-spot frequency. Can. J. Bot. 58, 574–577. doi: 10.1139/b80-071
Hunt, R. S. (1997). Relative value of slow-canker growth and bark reactions as resistance responses to white pine blister rust. Can. J. Plant Pathol. 19, 352–357. doi: 10.1080/07060669709501059
Jacobi, W. R., Kearns, H. S. J., Cleaver, C. M., Goodrich, B. A., and Burns, K. S. (2018). Epidemiology of white pine blister rust on limber pine in Colorado and Wyoming. Forest Pathol. 48:e12465. doi: 10.1111/efp.12465
Kegley, A. J., and Sniezko, R. A. (2004). “Variation in blister rust resistance among 226 Pinus monticola and 217 P. lambertiana seedling families in teh Pacific Noorthwest,” in Breeding and genetic resources of five needle pines: Genetics, breeding and adaptability, Proceedings of the IUFRO 2.02.15 Working Party Conference, eds R. A. Sniezko, S. Samman, S. E. Schlarbaum, and H. B. Kriebel (Fort Collins, CO: USDA Forest Service, Rocky Mountain Research Station RMPS-P-32), 209–225.
King, J. N., David, A., Noshad, D., and Smith, J. (2010). A review of genetic approaches to the management of blister rust in white pines. Forest Pathol. 40, 292–313. doi: 10.1111/j.1439-0329.2010.00659.x
Kinloch, B. B. (1992). Distribution and frequency of a gene for resistance to white pine blister rust in natural populations of sugar pine. Can. J. Bot. 70, 1319–1323. doi: 10.1139/b92-165
Kinloch, B. B. (2003). White pine blister rust in North America: Past and prognosis. Phytopathology 93, 1044–1047. doi: 10.1094/PHYTO.2003.93.8.1044
Kinloch, B. B., Burton, D., Davis, D. A., Westfall, R. D., Dunlap, J., and Vogler, D. R. (2012). “Strong partial resistance to white pine blister rust in sugar pine,” in Proceedings of the fourth international workshop on the genetics of host-parasite interactions in forestry: Disease and insect resistance in forest trees, eds R. A. Sniezko, A. D. Yanchuk, J. T. Kliejunas, K. M. Palmieri, J. M. Alexander, and S. J. Frankel (Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture), 80–91.
Kinloch, B. B., and Comstock, M. (1981). Race of Cronartium ribicola virulent to major gene resistance in sugar pine. Plant Dis. 65, 604–605. doi: 10.1094/pd-65-604
Kinloch, B. B., and Dupper, G. E. (1987). Restricted distribution of a virulent race of the white pine blister rust pathogen in the western United States. Can. J. Forest Res. 17, 448–451. doi: 10.1139/x87-077
Kinloch, B. B., and Dupper, G. E. (2002). Genetic specificity in the white pine-blister rust pathosystem. Phytopathology 92, 278–280. doi: 10.1094/phyto.2002.92.3.278
Kinloch, B. B., and Littlefield, J. L. (1977). White pine blister rust: hypersensitive resistance in sugar pine. Can. J. Bot. 55, 1148–1155. doi: 10.1139/b77-133
Kinloch, B. B., Parks, G. K., and Fowler, C. W. (1970). White pine blister rust: Simply inherited resistance in sugar pine. Science 167, 193–195.
Kinloch, B. B., Sniezko, R. A., Barnes, G. D., and Greathouse, T. E. (1999). A major gene for resistance to white pine blister rust in western white pine from the western Cascade range. Phytopathology 89, 861–867. doi: 10.1094/phyto.1999.89.10.861
Kinloch, B. B., Sniezko, R. A., and Dupper, G. E. (2003). Origin and distribution of Cr2, a gene for resistance to white pine blister rust in natural populations of western white pine. Phytopathology 93, 691–694. doi: 10.1094/PHYTO.2003.93.6.691
Kinloch, B. B., Sniezko, R. A., and Dupper, G. E. (2004). Virulence gene distribution and dynamics of the white pine blister rust pathogen in western North America. Phytopathology 94, 751–758. doi: 10.1094/PHYTO.2004.94.7.751
Kinloch, B. B., Sniezko, R. A., Savin, D. P., Danchok, R., Kegley, A., Burton, D., et al. (2018). “Patterns of variation in blister rust resistance in sugar pine (Pinus lambertiana),” in Proceedings of the IUFRO joint conference: Genetics of five-needle pines, rusts of forest trees, and Strobusphere, eds A. W. Schoettle, R. A. Sniezko, and J. T. Kliejunas (Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station), 124–128.
Kolpak, S. E., Sniezko, R. A., and Kegley, A. J. (2008). Rust infection and survival of 49 pinus monticola families at a field site six years after planting. Annal. Forest Res. 51, 67–80.
Liu, J.-J., Fernandes, H., Zamany, A., Sikorski, M., Jaskolski, M., and Sniezko, R. A. (2021). In-vitro anti-fungal assay and association analysis reveal a role for the Pinus monticola PR10 gene (PmPR10-3.1) in quantitative disease resistance to white pine blister rust. Genome 64, 693–704. doi: 10.1139/gen-2020-0080
Liu, J.-J., Hammett, C., and Sniezko, R. A. (2013). Pinus monticola pathogenesis-related gene PmPR10-2 alleles as defense candidates for stem quantitative disease resistance against white pine blister rust (Cronartium ribicola). Tree Genet. Genomes 9, 397–408. doi: 10.1007/s11295-012-0561-0
Liu, J.-J., Williams, H., Zamany, A., Li, X.-R., Gellner, S., and Sniezko, R. A. (2020). Development and application of marker-assisted selection (MAS) tools for breeding of western white pine (Pinus monticola Douglas ex D. Don) resistance to blister rust (Cronartium ribicola J.C. Fisch.) in British Columbia. Can. J. Plant Pathol. 42, 250–259. doi: 10.1080/07060661.2019.1638454
Looney, C. E., and Waring, K. M. (2012). Patterns of forest structure, competition and regeneration in southwestern white pine (Pinus strobiformis) forests. Forest Ecol. Manage. 286, 159–170. doi: 10.1016/j.foreco.2012.09.008
Menon, M., Bagley, J. C., Friedline, C. J., Whipple, A. V., Schoettle, A. W., Leal-Sàenz, A., et al. (2018). The role of hybridization during ecological divergence of southwestern white pine (Pinus strobiformis) and limber pine (P. flexilis). Mol. Ecol. 27, 1245–1260. doi: 10.1111/mec.14505
Menon, M., Bagley, J. C., Page, G. F. M., Whipple, A. V., Schoettle, A. W., Still, C. J., et al. (2021). Adaptive evolution in a conifer hybrid zone is driven by a mosaic of recently introgressed and background genetic variants. Commun. Biol. 4:160. doi: 10.1038/s42003-020-01632-7
Menon, M., Landguth, E., Leal-Saenz, A., Bagley, J. C., Schoettle, A. W., Wehenkel, C., et al. (2020). Tracing the footprints of a moving hybrid zone under a demographic history of speciation with gene flow. Evolut. Appl. 13, 195–209. doi: 10.1111/eva.12795
National Academies of Sciences, Engineering, and Medicine (2019). Forest health and biotechnology: Possibilities and considerations. Washington, DC: National Academies Press.
R Core Team (2020). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
Schoettle, A. W. (2004). “Ecological roles of five-needle pines in Colorado: Potential consequences of their loss,” in Breeding and Genetic Resources of Five-Needle Pines: Growth, Adaptability, and Pest Resistance, eds R. A. Sniezko, S. Samman, S. E. Schlarbaum, and H. B. Kriebel (Fort Collins, CO: USDA Forest Service, Rocky Mountain Research Station), 124–135.
Schoettle, A. W., Sniezko, R. A., Kegley, A., and Burns, K. S. (2013). White pine blister rust resistance in limber pine: Evidence for a major gene. Phytopathology 104, 163–173. doi: 10.1094/phyto-04-13-0092-r
Seager, R., and Vecchi, G. A. (2010). Greenhouse warming and the 21st century hydroclimate of southwestern North America. Proc. Natl. Acad. Sci. 107, 21277–21282. doi: 10.1073/pnas.0910856107
Shirk, A. J., Cushman, S. A., Waring, K. M., Wehenkel, C. A., Leal-Sáenz, A., Toney, C., et al. (2018). Southwestern white pine (Pinus strobiformis) species distribution models project a large range shift and contraction due to regional climatic changes. Forest Ecol. Manage. 411, 176–186. doi: 10.1016/j.foreco.2018.01.025
Sniezko, R. A. (2006). Resistance breeding against nonnative pathogens in forest trees — current successes in North America. Can. J. Plant Pathol. 28, S270–S279. doi: 10.1080/07060660609507384
Sniezko, R. A., Johnson, J. S., and Savin, D. P. (2020). Assessing the durability, stability, and usability of genetic resistance to a non-native fungal pathogen in two pine species. Plants People Planet 2, 57–68. doi: 10.1002/ppp3.49
Sniezko, R. A., Kegley, A., and Danchok, R. (2008). White pine blister rust resistance in North American, Asian and European species - results from artificial inoculation trials in Oregon. Annal. Forest Res. 51, 53–66.
Sniezko, R. A., and Koch, J. (2017). Breeding trees resistant to insects and diseases: putting theory into application. Biol. Invasions 19, 3377–3400. doi: 10.1007/s10530-017-1482-5
Sniezko, R. A., Mahalovich, M. F., Schoettle, A. W., and Vogler, D. R. (2011). “Past and current investigations of the genetic resistance to Cronartium ribicola in high-elevation five-needle pines,” in The future of hight-elevation, five-needle white pines in western North America. Proc High Five Symp RMRS-P-63, eds R. E. Keane, D. F. Tomback, M. P. Murray, and C. M. Smith (Fort Collins, CO: USDA Forest Service Rocky Mountain research Station), 246–264.
Sniezko, R. A., Smith, J., Liu, J.-J., and Hamelin, R. (2014). Genetic resistance to fusiform rust in southern pines and white pine blister rust in white pines—A contrasting tale of two rust pathosystems—current status and future prospects. Forests 5, 2050–2083. doi: 10.3390/f5092050
Struckmeyer, B. E., and Riker, A. J. (1951). Wound-periderm formation in white pine trees resistant to blister rust. Phytopathology 41, 276–281.
Tomback, D. F., and Achuff, P. (2010). Blister rust and western forest biodiversity: ecology, values and outlook for white pines. Forest Pathol. 40, 186–225. doi: 10.1111/j.1439-0329.2010.00655.x
Vázquez-Lobo, A., De La Torre, A. R., Martínez-García, P. J., Vangestel, C., Wegzryn, J. L., Ćalić, I., et al. (2017). Finding loci associated to partial resistance to white pine blister rust in sugar pine (Pinus lambertiana Dougl.). Tree Genet. Genomes 13:108. doi: 10.1007/s11295-017-1190-4
Keywords: Cronartium ribicola, Pinus strobiformis, bark reactions, five needle pines, quantitative disease resistance, white pine blister rust
Citation: Johnson JS and Sniezko RA (2021) Quantitative Disease Resistance to White Pine Blister Rust at Southwestern White Pine’s (Pinus strobiformis) Northern Range. Front. For. Glob. Change 4:765871. doi: 10.3389/ffgc.2021.765871
Received: 27 August 2021; Accepted: 11 October 2021;
Published: 11 November 2021.
Edited by:
Ahmed Najar, University of Alberta, CanadaReviewed by:
Louis Bernier, Laval University, CanadaBraham Dhillon, University of Florida, United States
Copyright © 2021 Johnson and Sniezko. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeremy S. Johnson, amVyZW15LmpvaG5zb25AcHJlc2NvdHQuZWR1
 Jeremy S. Johnson
Jeremy S. Johnson Richard A. Sniezko
Richard A. Sniezko