- 1Agroisolab UK Ltd., Welburn, United Kingdom
- 2Institute for Research in Tropical Ecology (IRET/CENAREST), Libreville, Gabon
- 3Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, United Kingdom
- 4Agroisolab GmbH, Jülich, Germany
Consumers are becoming increasingly aware of the environmental impacts caused by deforestation and illegal logging and there is an increasing demand for supply chain transparency and traceability of wood products. Many importing and exporting nations have implemented regulations which aim to control the origin and species of traded timbers of high ecological importance and economic value. However, despite growing interest in method development for timber authentication purposes, many studies have been limited by insufficient numbers of authentic timber reference samples. Our aim was to address the differences in stable isotope ratio profile of bulk, homogenized wood samples collected from living or recently felled trees in two FSC concessions in Gabon, which are approximately 240 km apart, for the purposes of origin classification and protecting valuable forest commodities. Forty-seven timber samples comprising 10 genera of tropical trees were obtained using a Pickering Punch sampling device or chainsaw from two forest concessions in Gabon (Precious Woods Group and Compagnie des Bois du Gabon) during July 2019. Samples were subject to δ18O, δ2H, δ13C, δ15N, and δ34S stable isotope analysis using elemental analysis-isotope ratio mass spectrometry (EA-IRMS). Results show that significant differences are evident in the stable isotope ratios of Aucoumea klaineana between Precious Woods Group and Compagnie des Bois du Gabon forest concessions. Relationships are evident between climatic and geological variables and the stable isotope ratios of the samples suggesting that further degrees of origin classification may be achievable in Gabon. For other species, insufficient numbers meant the possibility to determine discriminating factors between the two concessions was limited though data from these samples may prove useful to contribute to the understanding of stable isotope variability in tropical timber. The data presented establish a basis for evaluating origin claims of forest products and timber from the Compagnie des Bois du Gabon and Precious Woods Group concessions and lay a foundation for future development of timber tracking technologies in Gabon. The technique can be used for purposes of due diligence or forensic investigation by law enforcement as part of demand-side regulations such as the EU Timber Regulation, Illegal Logging Prevention Act, or the Lacey Act.
Introduction
Consumers are becoming increasingly aware of the environmental impacts caused by deforestation and illegal logging and there is an increasing demand for supply chain transparency and traceability of wood products. Many importing and exporting nations have implemented regulations which aim to control the origin and species of traded timbers of high ecological importance and economic value. One of the earliest regulations (US Lacey Act, 2008) saw the world’s first ban on trade in illegally sourced wood products, and in March 2013, the European Union (EU) implemented the EU timber regulation (EUTR) which prohibits illegally sourced timber from entering EU markets. A handful of scientific methods ranging from light microscopy to DNA analysis and mass spectrometry, are being developed in laboratories around the world to help verify the legal status of traded timbers. However, despite growing interest in method development for timber authentication purposes, many studies have been limited by insufficient numbers of authentic timber reference samples. To overcome these limitations, World Forest ID was established through the collaboration of several key organizations including Defra, Royal Botanic Gardens, Kew, Forest Stewardship Council (FSC), Agroisolab and the United States Forest Service, with the aim of carrying out large scale collections of timber reference samples from some of the world’s most endangered forests (Gasson et al., 2020)1.
Gabon is a West African nation located on the Gulf of Guinea and Atlantic Ocean, bordering Cameroon, Equatorial Guinea, and the Republic of Congo. Its land mass is approximately 85% forested with deforestation rates of approximately 0.1% occurring annually (NEPcon, 2017). The timber industry in Gabon accounts for nearly 20,000 jobs (EIA, 2019) and is the country’s most important export (5% of GDP) after oil (Bayol, 2002; NEPcon, 2017). Of the 22–23 million ha of forested area, about 4 million ha is protected and 14 million ha are allocated for forestry (EIA, 2019). All Gabon’s forests are managed by the Ministry of Forest, Environment and Natural Resource Protection which oversees the monitoring of forest resources, including the allocation of forest concessions. Two types of permits are issued by the ministry: Concession Forestière sous Aménagement Durable (CFAD), and Permis Forestier Associé (PFA). CFAD, allows for logging by corporations in land areas of between 50,000 and 200,000 ha, whereas PFA – (which is reserved exclusively for Gabonese nationals) has a maximum size of 50,000 ha (NEPcon, 2017). As of 2017, China, followed by France, Belgium, and Italy were the largest export markets for timber sourced from Gabon (EIA, 2019).
The most important species for the Gabonese timber industry is Okoumé (Aucoumea klaineana) which accounts for most of the country’s timber exports. The timber is commonly manufactured into plywood and veneers at plants within Gabon and Asia (EIA, 2019). A 4-year investigation by the EIA established that illegally sourced timber from Gabon has routinely entered the United States (US) for over a decade and made its way to thousands of United States consumers. An in-depth analysis of the Okoumé (Aucoumea klaineana) veneer imported directly into the United States from Gabon highlights the lax attitude toward legality and the complicity of United States importers (EIA, 2019). Following a corruption scandal in 2010, Gabon moved to ban the export of logs and switched to the export of sawn logs and plywood as a means of stimulating the domestic economy (Karsenty, 2019). Other economically important species for the Gabonese timber industry include Azobé, Bongossi (Lophira alata), Okan (Cylicodiscus gabunensis), Padouk d’Afrique (Pterocarpus soyauxii), Beli (Julbernardia pellegriniana), Tali (Erythrophleum ivorense), Missanda (Erythrophleum suaveolens). These timber species, and forest products derivatives, are in great need of protection.
Aucoumea klaineana is a long-lived pioneer species capable of converting savannah into rainforest (White et al., 1996; Born et al., 2006) and is the only species in its genus. It grows relatively quickly and makes up a large proportion of the trees within Gabon. The natural range of Aucoumea klaineana includes Gabon but also extends into Equatorial Guinea, southern Cameroon, and parts of the Republic of Congo (Born et al., 2010). Despite its relatively wide distribution in Gabon, Aucoumea klaineana can be difficult to grow in plantations. One study into the silviculture of young Aucoumea klaineana trees found that saplings grew best in the soil of their original population. The growth of the saplings could be described in terms of a function of the distance from the sites where their seeds were harvested to the research site (Koumba Zaou et al., 1998). This means that if Aucoumea klaineana is harvested to the point of exhaustion in one region of Gabon, it will not be a straightforward task to simply replant the trees from another region. Genotypic mechanisms were proposed as a potential explanation for this phenomenon. However, phenotypes are ultimately the product of genetic expression, of which, the available genetic sequences are only part of the story; the mechanism phenotypic variation can also be considered in terms of epigenetics or other genetic regulatory mechanisms. Even so, there are clear examples of distinct genetic populations of Aucoumea klaineana within Gabon; Muloko-Ntoutoume et al. (2000) revealed population differences in chloroplast DNA (cDNA), and differences in polymorphic microsatellites have also been identified (Born et al., 2006, 2008).
Humans are not the only great apes that rely on Aucoumea klaineana for survival; western lowland Gorillas have been observed eating the flowers of on Aucoumea klaineana. Though it is not a main food source for Gorillas, the availability of Aucoumea klaineana during times of famine caused by shortages of other food sources may ensure that Gorillas survive (Williamson et al., 1990). Nevertheless, in research where Gorillas have been observed, logging did not appear to have affected the local population at the time meaning that logging the trees can still be acceptable if done in a sustainable manner. Although Gabon constitutes a fraction of the Congo Basin, the country also shelters approximately 45,000 forest elephants, representing nearly 60% of Africa’s remaining population (EIA, 2019). Protection of natural forest is vital for the survival of forest elephants.
Aucoumea klaineana is most commonly traded as plywood and is often used for boatbuilding due to its excellent properties (Negro et al., 2011). Plywood is produced from the layering of multiple sheets (veneers) of rotary-peeled logs which are held together with thermosetting formaldehyde-urea-based resins (Desch and Dinwoodie, 1996; Negro et al., 2011). Typically, heartwood is used for veneer making. Heartwood can be defined as “the inner layers of the wood, which, in the growing tree, have ceased to contain living cells, and in which the reserve materials (e.g., starch) have been removed or converted into heartwood substance” (Hillis, 1987). The process of heartwood formation does not always lend itself to the preservation of genetic material either in plant cell nuclei, mitochondria, or chloroplasts. The process of veneer-making involves boiling the wood, applying steam and hot-pressing at over 100°C to cure the thermosetting resin. Though population genetics is an excellent method for timber tracking (Jolivet and Degen, 2012) and is highly suitable for verifying the origin of logs, the manufacturing process of plywood does not lend itself to ideal preservation of genetic material for later analysis and may present limitations for this technique in demand-side regulatory scenarios. Therefore, there needs to be a method capable of evaluating the origin of the veneers in plywood so supply chain stakeholders, enforcement and concerned parties can be assured of its legal origin as part of demand-side authentication. Nevertheless, population genetics is a vital technique and is suitable for addressing the origin of timber within Gabon where genetic material can be accessed for analysis.
Stable isotope ratio analysis (SIRA) is a widely accepted analytical technique, and since the beginning of the 21st century, has become established as a means of verifying the origin of food and drink (Kelly et al., 2002; Boner and Förstel, 2004; Heaton et al., 2008; Pilgrim et al., 2010; Li et al., 2015). The same principles used to authenticate food were later applied to timber provenance research (Boner et al., 2007; Keppler et al., 2007; Kagawa et al., 2008; Horacek et al., 2009; Kagawa and Leavitt, 2010; Gori et al., 2013, 2018; Rees, 2015; Watkinson et al., 2020, 2021). Verifying the origin of timber involves the comparison of an unknown sample against an authentic reference database for a region or territory. The technique is used routinely to assess legality, compliance with labeling legislation, and its use to conduct due diligence is advocated by EUTR (Regulation (EU) No 995/2010, 2010).
The ambitions of this project were to define the ranges of stable isotope ratios from multiple species of trees from two FSC forest concessions in Gabon by analyzing timber samples extracted from living trees. Our aims were to:
1. Collect and perform stable isotope analysis of authentic geo-referenced timber such as: Okoumé/Aucoumea klaineana (Burseraceae), Moabi/Baillonella toxisperma (Sapotaceae), Ebiara/Berlinia confusa (Leguminosae-Caesalpinioideae), Okan/Cylicodiscus gabunensis (Leguminosae-Caesalpinioideae), Ozigo/Dacryodes buettneri (Burseraceae; synonym of Pachylobus buettneri), Gombe/Didelotia africana (Leguminosae-Detarioideae), Kevazingo/Guibourtia tessmannii (Leguminosae-Detarioideae), Azobe/Lophira alata (Ochnaceae), Bilinga/Nauclea diderrichii (Rubiaceae), and Padouk d’Afrique/Pterocarpus soyauxii (Leguminosae-Papilionoideae).
2. Assess what differences occur in the stable isotope ratios of trees between and within different forest concessions within Gabon.
3. Use the data produced from samples as a baseline for evaluating the origin of timber from the two sampled concessions if the data characterize the concession well. In cases where data are insufficient to characterize the concession, the data will remain available to build data for later use.
If significant differences in the stable isotope ratios of trees from within different concessions in Gabon are evident, this may be of great benefit to audit teams wanting to meet due diligence requirements and demonstrate sustainable practices by using analysis to verify origin declarations. If the isotope ratios of trees from within different forest concessions in Gabon are relatively homogenous, this would mean that it may not be necessary to collect reference samples from all concessions to verify that the timber is from Gabon.
Materials and Methods
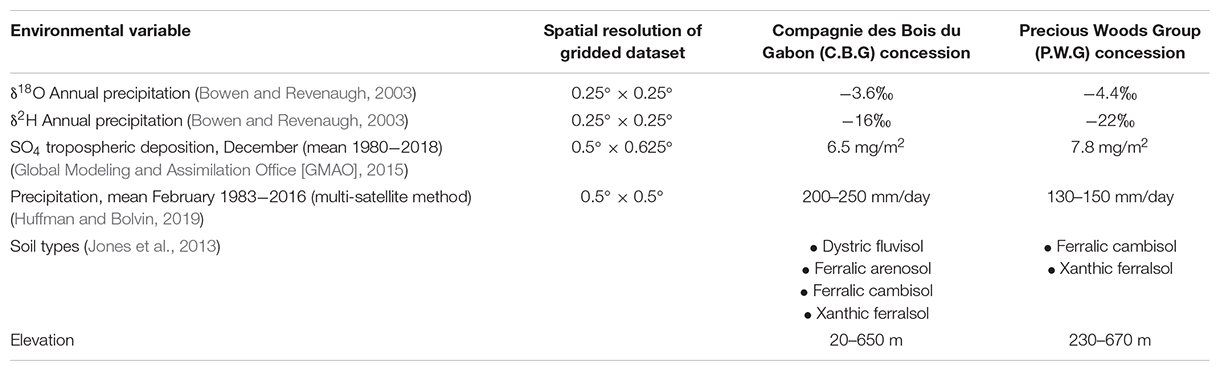
During planning, Agroisolab liaised with several organizations including FSC, Royal Botanic Gardens, Kew, United States Fish and Wildlife Service, World Resource Institute (WRI) and United States Forest Service to establish a taxa priority list and reach a consensus on which locations within Gabon should be sampled. It was agreed that two forest concessions; Compagnie des Bois du Gabon (C.B.G) and Precious Woods (P.W.G) would be selected for reference sample collection. The concessions are separated by approximately 240 km and presented an opportunity to assess the variability in stable isotope ratios at higher resolution and the viability of stable isotope data to differentiate several taxa at concession level resolution.
Samples were taken from 47 trees in two forest concessions (C.B.G, n = 33 and P.W.G, n = 14) in Gabon during June 2019 (Figure 1). Between 2 and 4 pieces of timber (heartwood and sapwood) were collected from each tree. In most cases three samples were taken per tree as well as leaf and twig samples to act as herbarium vouchers. Samples of heartwood/sapwood were submitted to Agroisolab for stable isotope ratio analysis whereas the remaining material was distributed between Conservation of Biodiversity at IRET/CENAREST in Gabon and the World Forest ID collection at the Royal Botanic Gardens, Kew. A Pickering Punch (Agroisolab UK, Welburn, United Kingdom), a type of hammer-driven bore, was used to collect 45 of the 47 cores of timber 9–11 cm in length and 1.5 cm wide. One sample (Berlinia confusa) was smaller than the recommended size due to the hardness of the tree. Samples were then transferred from the Pickering Punch into cardboard tubes which were sealed inside 500 mL evacuated bags with silica gel to aid drying. Additionally, 2 of the 47 samples were collected with a chainsaw. Samples were dried in-transit to Conservation of Biodiversity at IRET/CENAREST using 30 g silica gel per plug/core. No perforations were made in the acid-free cardboard tubes; field tests found this did not adequately facilitate drying and some cores presented with mold growth upon receipt at the Plant Quarantine Unit, Royal Botanic Gardens, Kew. To eliminate the risk of pathogenic fungi entering the United Kingdom, samples were subjected to 121°C heat at 15 psi in an autoclave for 30 min before they were dried and released into the WFID collection for analysis and storage. Prior to routine preparation mold was manually removed as it is considered that it may be source of variability for stable isotope analysis (Horacek et al., 2018; Beeckman et al., 2020).
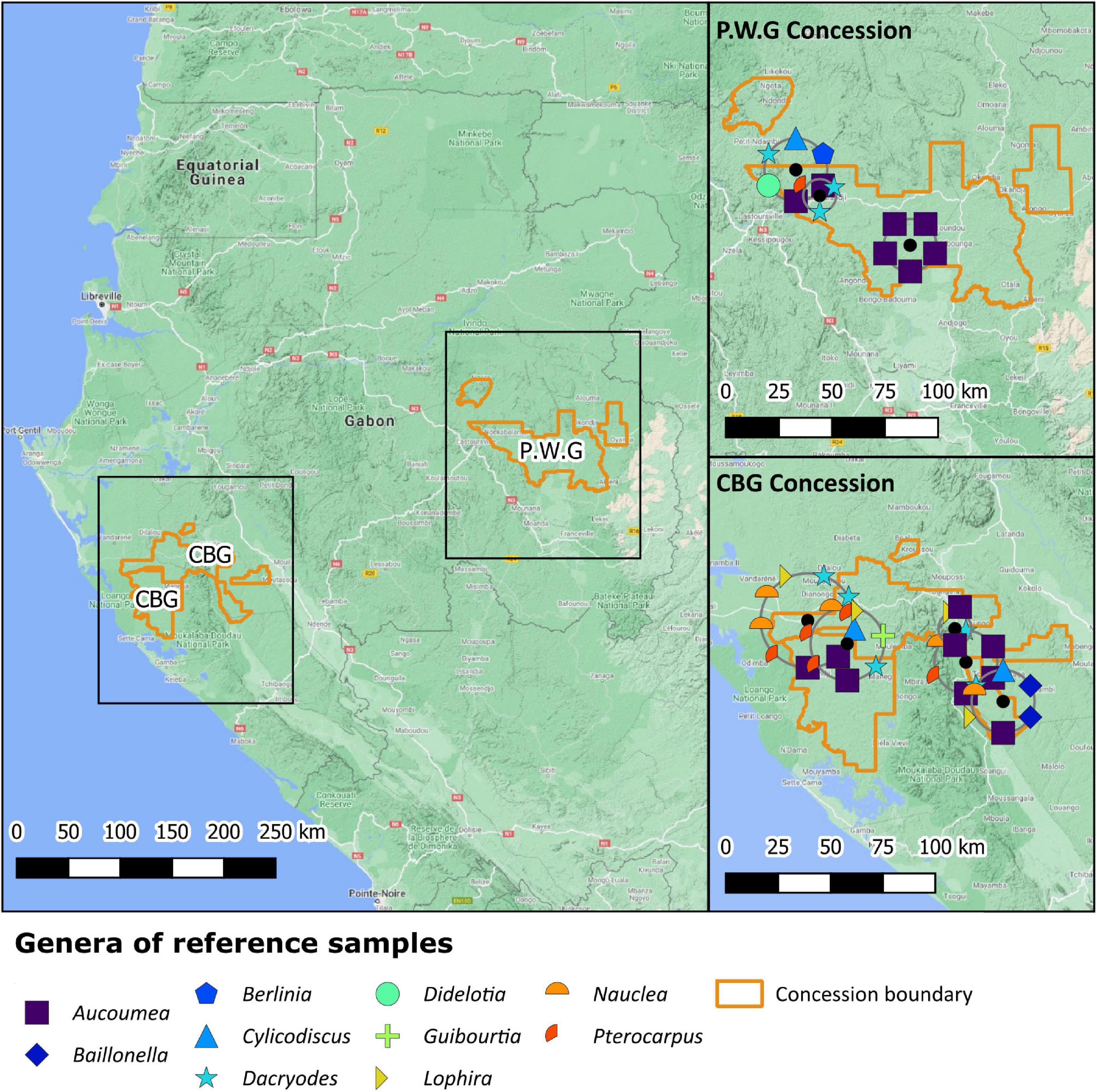
Figure 1. Locations and taxa of samples collected in the C.B.G and P.W.G concessions in Gabon. The positioning of location markers is displayed using point displacement. The black dots represent the centroid of the sample cluster.
GPS data, photographs of the trees and leaves, descriptions and comments about the sampled trees were recorded in a mobile phone application named TreeSnap (Crocker et al., 2019; TreeSnap version 1.15.3 Department of Entomology and Plant Pathology, University of Tennessee, Institute of Agriculture, Knoxville, TN, United States). The data from this collection have since been moved to the World Forest ID application (World Forest ID version 1.2.0, Knoxville, TN, United States).
There are clear environmental differences between the two concessions (Table 1) that may influence isotope ratios in timber. These are in addition to differences in elevation and distance to the sea. The C.B.G concession is situated next to a lagoon and approximately 20 km from the Atlantic Ocean at its closest point. P.W.G concession is approximately 380 km from the ocean at its nearest. The two concessions are approximately 240 km apart.
Measurement
Samples that presented with hyphae upon receipt at Kew had any mold physically removed as part of preparation in accordance with advice from Beeckman et al. (2020). Samples were dried at 103°C before being coarsely ground and placed into a ball-mill (Retsch MM220, Haan, Germany). The resulting fine powder was extracted in a soxhlet apparatus for 6 h with non-polar (dichloromethane) and polar (methanol) solvents which were then dried in a laboratory drying cabinet for at least 1 h. Finally, the samples were stored in air-tight sample vials and weighed for analysis.
The method chosen to isolate non-exchangeable hydrogen in cellulose is outlined by Cheung (2014). Homogenized samples were nitrated using 4 mL HNO3 (90%) and 4 mL of glacial H2SO4 (96%) in falcon tubes with 35 mL distilled water. As the reaction is exothermic, the solution was refrigerated for 2 h during the digest. The sample solution was agitated using an automatic shaker for 2 h allowing the cellulose to precipitate. Precipitated cellulose was then separated from the solution with an initial centrifugation for 1 min at 3,000 rpm. The supernatant was then discarded, and the precipitate was resuspended using 40 mL distilled water and subjected to another centrifugation for 1 min at 3,000 rpm. This process was repeated until a pH of 6–7 was achieved. Finally, precipitated cellulose was resuspended in 2–3 mL of distilled water, transferred to a glass vial and water decanted following centrifugation. Residual water was removed by gentle heating.
To avoid equilibration or humidity effects in the oxygen and hydrogen analysis, the weighed-in samples were equilibrated overnight in desiccators with a defined humidity of 10.6%. Afterward the samples were vacuum dried for at least 2 h.
Sample measurements were corrected against in-house standards at the beginning, middle and end of each measurement run. In-house standards are traceable back to certified reference materials enabling measurements to be reported relative to an internationally defined standard; for hydrogen and oxygen isotope ratio analysis, Vienna Standard Mean Ocean Water (VSMOW) is used. For carbon isotope ratio analysis, Vienna Pee Dee Belemnite (VPDB) is used. For sulfur isotope ratio analysis Vienna Canyon Diablo Troilite (VCDT) is used. The in-house standards used were 1,4-dihydroxyanthraquinone (Merck Schuchardt) for δ18O and δ2H, L-leucine (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) for δ13C and δ15N, and L-cysteine (Carl Roth GmbH + Co. KG, Karlsruhe, Germany) for δ34S. In-house standards are also controlled in Agroisolab’s free intercomparison testing platform “Agroisolab-KPT” which routinely operates with over 20 isotope laboratories. Measurements are reported in per mil (‰) and were made in accordance with processes outlined in Boner et al. (2007).
δ18O and δ2H measurement was performed using two Isotope Ratio Mass Spectrometers (IRMS) in a master/slave configuration (Isoprime, Elementar, Cheadle, United Kingdom) with each IRMS measuring one isotope ratio; δ18O or δ2H. This configuration provides excellent stability because the magnetic field and accelerating voltage remain constant on each IRMS. The working temperature for pyrolysis is >1530°C and HT-PyrOH is performed with a covalently bonded silicon carbide tube (patented by Agroisolab GmbH, Jülich, Germany) filled with glassy carbon chips and coal powder.
δ13C measurement was performed using an Elemental Analyzer (EA3000, Eurovector, Milan, Italy) in combination with IRMS (Nu Horizon, NU-Instruments, Wrexham, United Kingdom). The working temperatures are 1,021°C for oxidation and 600°C for reduction. Reduction is carried out in the presence of copper.
δ34S measurement was performed using an Elemental Analyzer (EA3000, Eurovector, Milan, Italy) with IRMS (Isoprime, Cheadle, United Kingdom). A one tube combustion (oxidation and reduction in one tube) is used to solve issues caused by SO3. Combustion water is directly trapped with magnesium perchlorate at the end of the tube. The working temperature is 1,000°C.
Typical values for Combined Uncertainty (uc) measurement in timber is 0.2‰ for δ18O, 1.3‰ for δ2H, 0.1‰ for δ13C, and 0.2 for δ34S.
Data Analysis
Due to the low number of samples representing many taxa, multivariate analysis methods were not considered ideal to draw conclusions from the data but were still performed to at least make inferences. Particular attention was given to Aucoumea klaineana which had high enough sample numbers from both concessions. Univariate analyses were performed to show differences between distributions of means such as Student’s t-test and ANOVA, or to assess co-variance such as regression as well as boxplot visualizations (Orange 3.24, University of Ljubljana, Slovenia; Microsoft Excel 2019 ver. 16.0.6742.2048, Redmond, WA, United States). Aucoumea klaineana and Dacryodes buettneri were assessed in SAGA GIS 2.3.2 (Departments for Physical Geography, Hamburg and Göttingen, Germany) for spatially related patterns using Inverse Distance Weighting (IDW) to the 2nd order power and weighting was applied using all sampling points in the area. IDW is an exact spatial interpolator, therefore, isoscapes generated in this way are useful to visualize consistencies, differences, and outliers within defined areas such as forest concessions. However, IDW is not an ideal method to generate isoscapes as statistical models due to the fact it always generates an “overfit” model. We do not recommend IDW’s use as a method of evaluating the authenticity of test samples.
Results
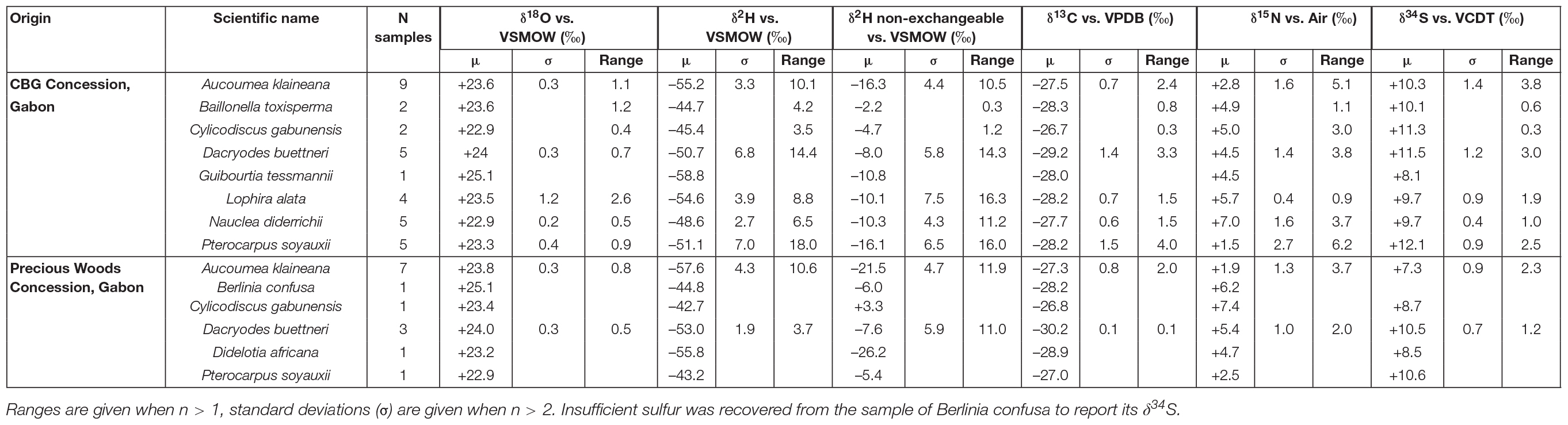
Nauclea diderrichii (n = 5) was remarkably consistent in terms of its δ18O isotopic composition relative to other sampled taxa, and it also has the least positive δ18O on average (mean) by group. Lophira alata (n = 4) has the most positive and widest range of δ18O (2.6‰). This seems unusual because the range δ18O for Aucoumea klaineana was 1.1‰ in the C.B.G concession and 0.8‰ in the P.W.G concession, and the range of Dacryodes buettneri was 0.7‰ in C.B.G and 0.5‰ in P.W.G, yet these species were sampled in greater quantities (Table 2). The reason for the wide range in δ18O for Lophira alata can be attributed to a single sample which was far more depleted in 18O than others. Of the four samples of Lophira alata that were collected, the sample in question was the only one collected with a chainsaw as opposed to a Pickering Punch. The samplers were contacted about the result and were able to provide further corroborating information about the authenticity of the sample. We cannot explain this outlier result, but outliers exist in the dataset and must be included. There are significant differences between the δ18O isotope ratios of the different taxa across the two locations (p = 0.002) as judged by ANOVA. For the interpretation of this result, it should be considered that the Aucoumea and Dacryodes were collected in two sites, whereas the other taxa were collected in just one. Based on the data from samples collected in this study, there is no strong relationship between the oxygen stable isotope ratios and the elevation of each sample. Aucoumea klaineana has the strongest relationship with elevation (R2 = 0.2) although this value lacks explanatory power to be used as a sole predictor of δ18O isotopic composition in Aucoumea klaineana.
Nauclea diderrichii has the narrowest range of δ2H isotope ratios in comparison to other taxa that were analyzed in this study. Contrary to the trend between taxa having the most positive δ18O, Nauclea shows the least positive δ2H (Table 2). The differences in the mean δ2H of the taxa are significant (ANOVA, p = 0.009), however, there is a lot of overlap in the ranges. The range of all taxa is approximately 20‰ (between −62 and −42‰). The typical range of δ2H in each concession is around 10‰, however, Pterocarpus (five samples collected in C.B.G concession, one sample collected in P.W.G concession) shows a range of 18‰ (Table 2). Nauclea diderrichii and Pterocarpus soyauxii appear to have strong, positive relationships with elevation (R2 s of 0.79 and 0.55, respectively). This positive proportionality suggests that their δ2H should increase with increasing elevation if the trend can be extrapolated. Dacryodes buettneri, Lophira alata, and Aucoumea klaineana do not show strong relationships between their δ2H and elevation.
Relative to the δ2H ratios of the extracted wood, analysis of the non-exchangeable δ2H isotope ratios show some interesting patterns: Aucoumea klaineana has the most negative δ2H isotope ratios in both cases, the remainder of the sampled taxa seemed to have switched places entirely. Table 2 shows that the typical ranges observed in the taxa are approximately 10‰ for δ2H from extracted wood, whereas the ranges in the δ2H isotope ratios for the non-exchangeable δ2H are all greater than 10‰ for all well-sampled taxa. Nauclea diderrichii, Pterocarpus soyauxii, and Lophira alata appear to have positive relationships between their non-exchangeable δ2H from cellulose and elevation with R2 s of 0.26, 0.73, and 0.31, respectively. This positive proportionality suggests that their δ2H isotope ratios should increase with increasing elevation if this trend persists. Aucoumea klaineana has a negative relationship between its non-exchangeable δ2H from cellulose and elevation with an R of −0.43. Dacryodes buettneri was the only taxon without a strong relationship between its δ2H and elevation.
Carbon isotope ratios have a range that varies between 1.5 and 4.0‰. The carbon isotope ratios of all species vary between −27 and −30.2‰ (Table 2). The widest range evident in this study exists in Pterocarpus (4‰ range).
The range of nitrogen isotope ratios in all sampled taxa was approximately between −2‰ to +8.5‰. The least positive δ15N belongs to a Pterocarpus sample, whereas the most positive δ15N belong to a Nauclea sample. The nitrogen isotope ratios of each taxa had an individual range of around 1–4‰. There are significant differences in the mean nitrogen isotope ratios of the taxa sampled (ANOVA, p = 0.000) implying a strong taxonomically related effect in the data for δ15N.
All taxa were enriched in 34S with δ34S greater than +6‰. The range of sulfur isotope ratios in all taxa range between +6 and +13.5‰ and have per-taxon ranges of approximately 2–3‰ in each concession (Table 2). The broadest range per taxon was evident in the Aucoumea samples which were collected in two concessions and were the most well-sampled timbers in this project. There are significant differences between the taxa (ANOVA, p = 0.001) suggesting a strong taxonomically related effect in sulfur stable isotope ratio profile. All analyzed taxa show negative relationships between sulfur isotope ratio and elevation meaning that, with increasing elevation, a decreasing sulfur isotope ratio would be expected if this trend can be extrapolated. The strongest relationship was evident in Aucoumea klaineana with an R of −0.79 (Figure 2).
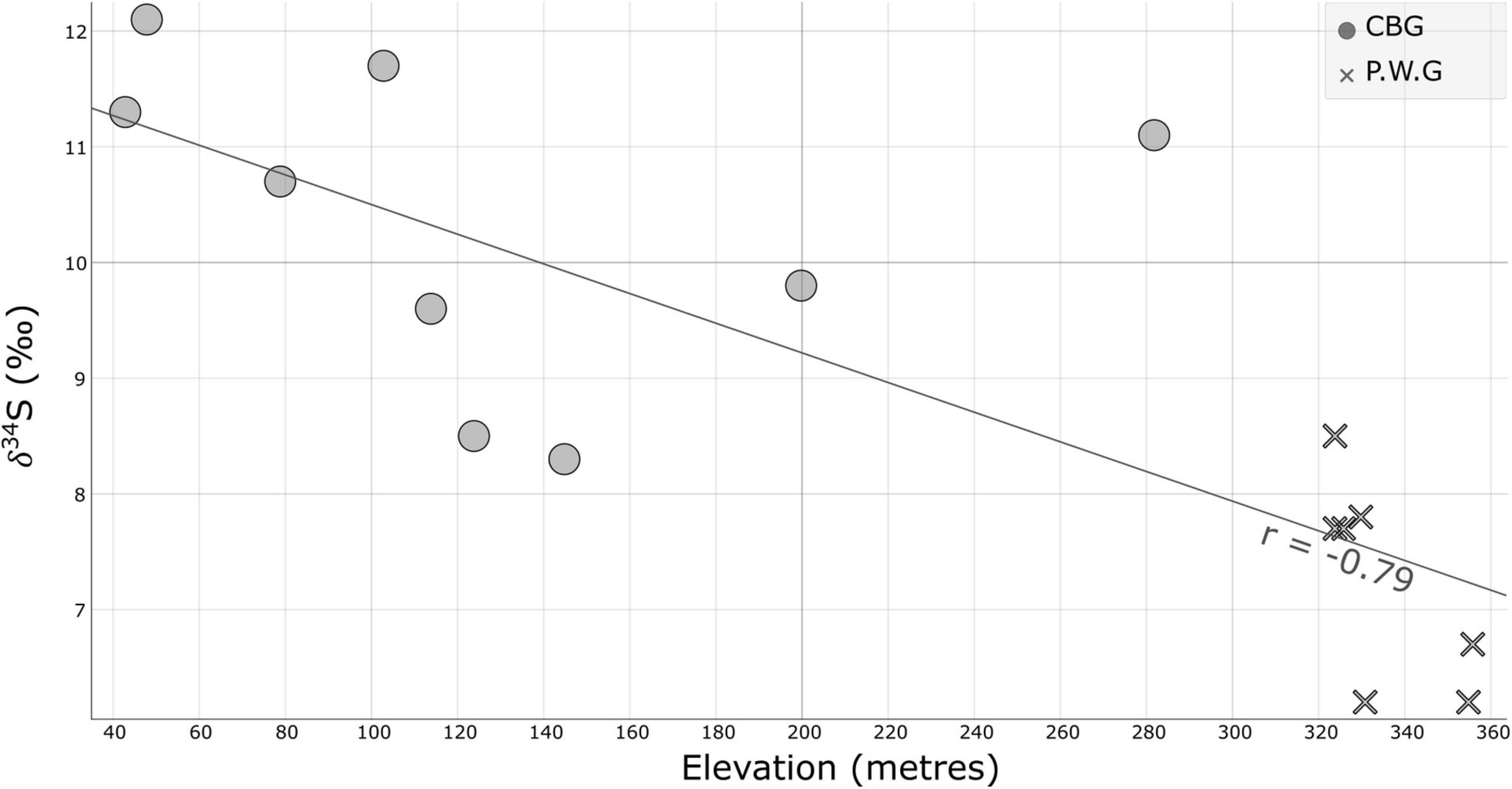
Figure 2. Relationship between elevation and the δ34S of Aucoumea klaineana sampled in the P.W.G and C.B.G concessions from Gabon.
Aucoumea klaineana (Okoumé)
Significant differences are evident in the mean values of non-exchangeable δ2H (Student’s t, p = 0.036), and the δ34S (Student’s t, p = 0.000) of the Aucoumea klaineana samples between the C.B.G and P.W.G concessions. Figure 3 show that there is very little overlap between the two concessions in the sulfur isotope ratios from the two concessions.
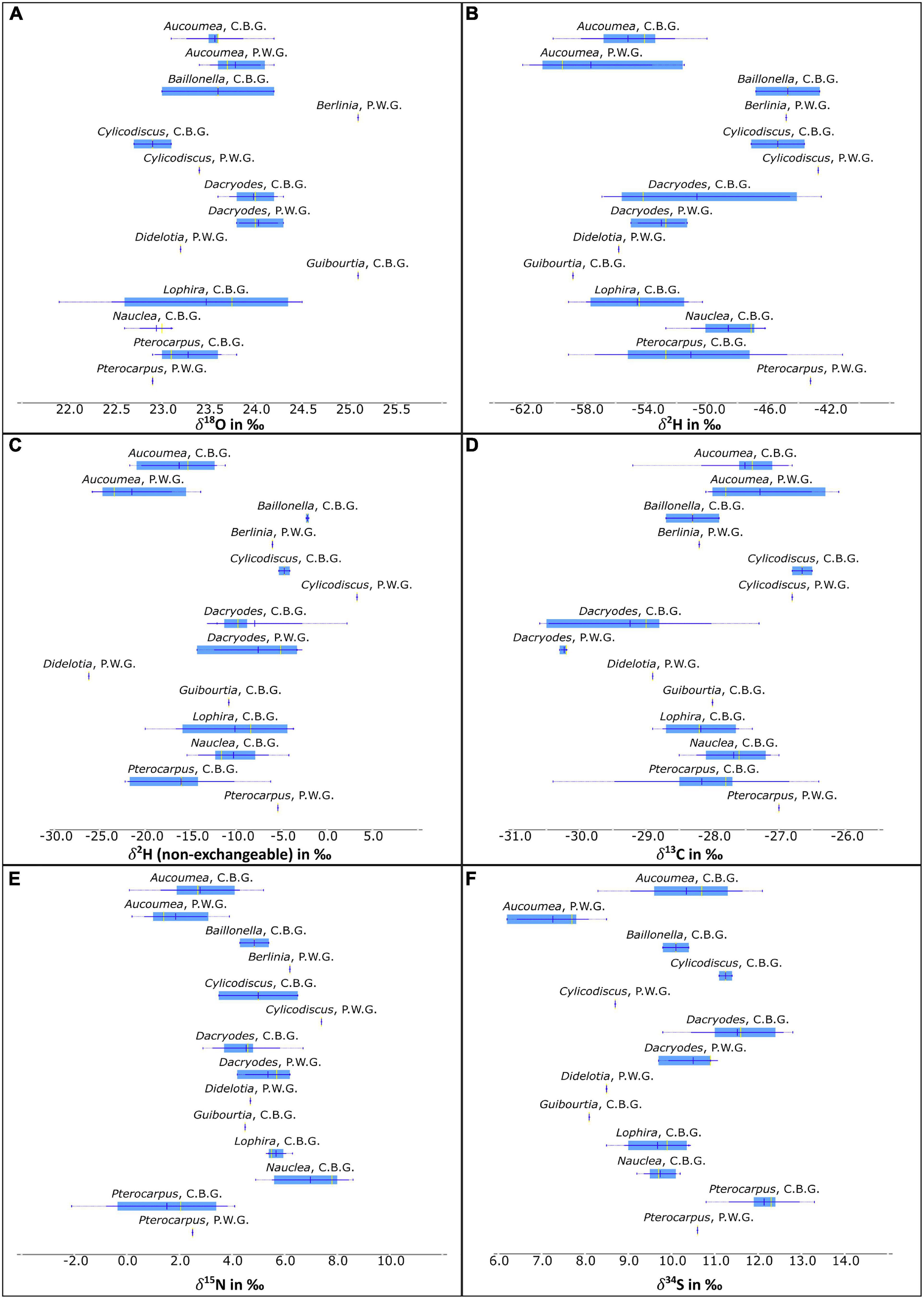
Figure 3. Boxplots of (A) δ18O, (B) δ2H, (C) δ2H (non-exchangeable), (D) δ13C, (E) δ15N, and (F) δ34S of the different genera from each concession (C.B.G. and P.W.G.).
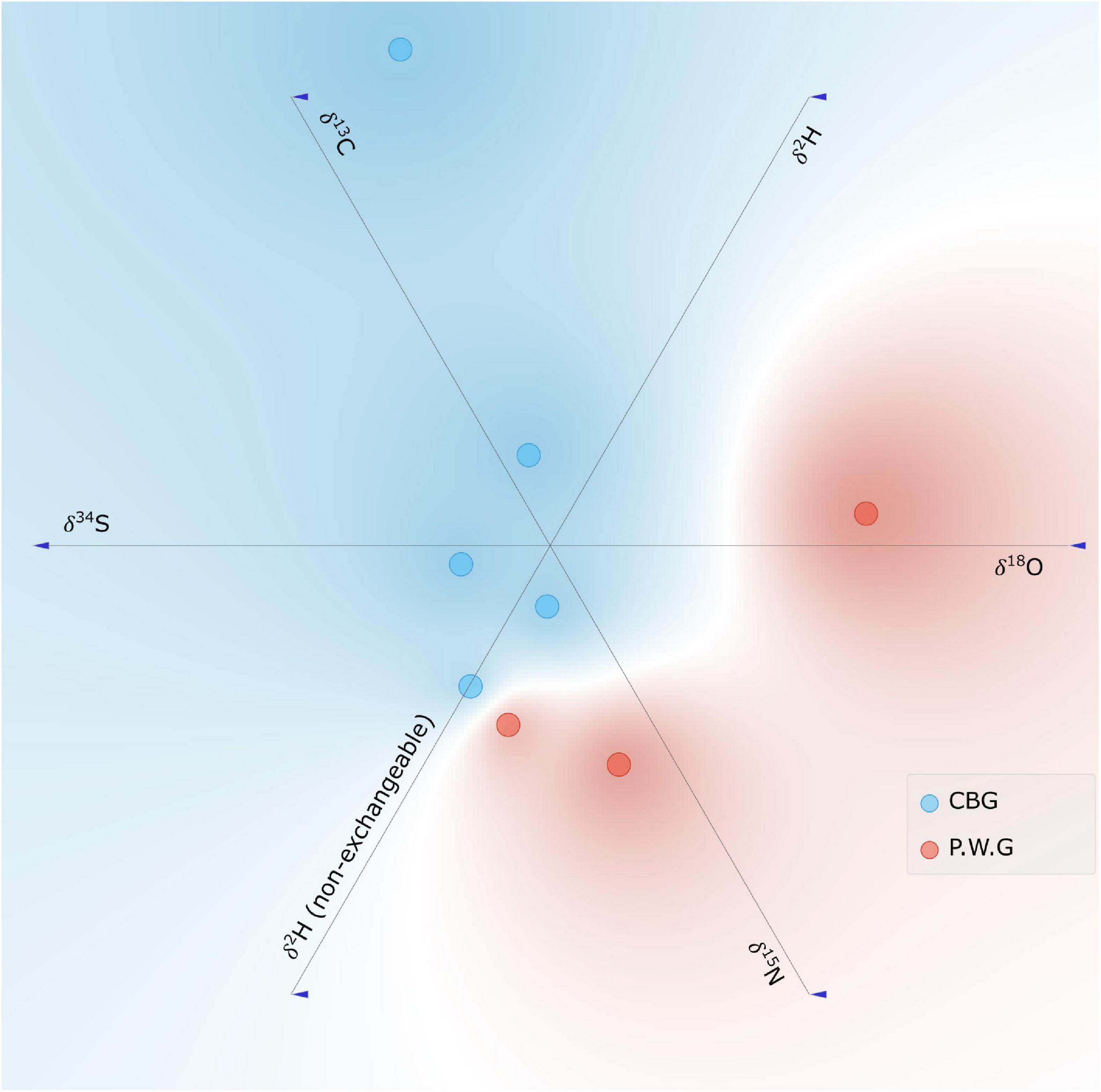
Linear Projection (Figure 4) of the stable isotope ratios of Aucoumea from the two concession shows the two concessions are well-separated. The k-means silhouette scores from the Principal Components of the stable isotope data show that it is most likely that the data have two clusters (48% probability), but it is also possible that there are more (3 clusters = 36.5%, 4 clusters 37.3%). There is one sample of Aucoumea from the C.B.G concession that has stable isotope ratios that are in the range of reference samples from the P.W.G concession.
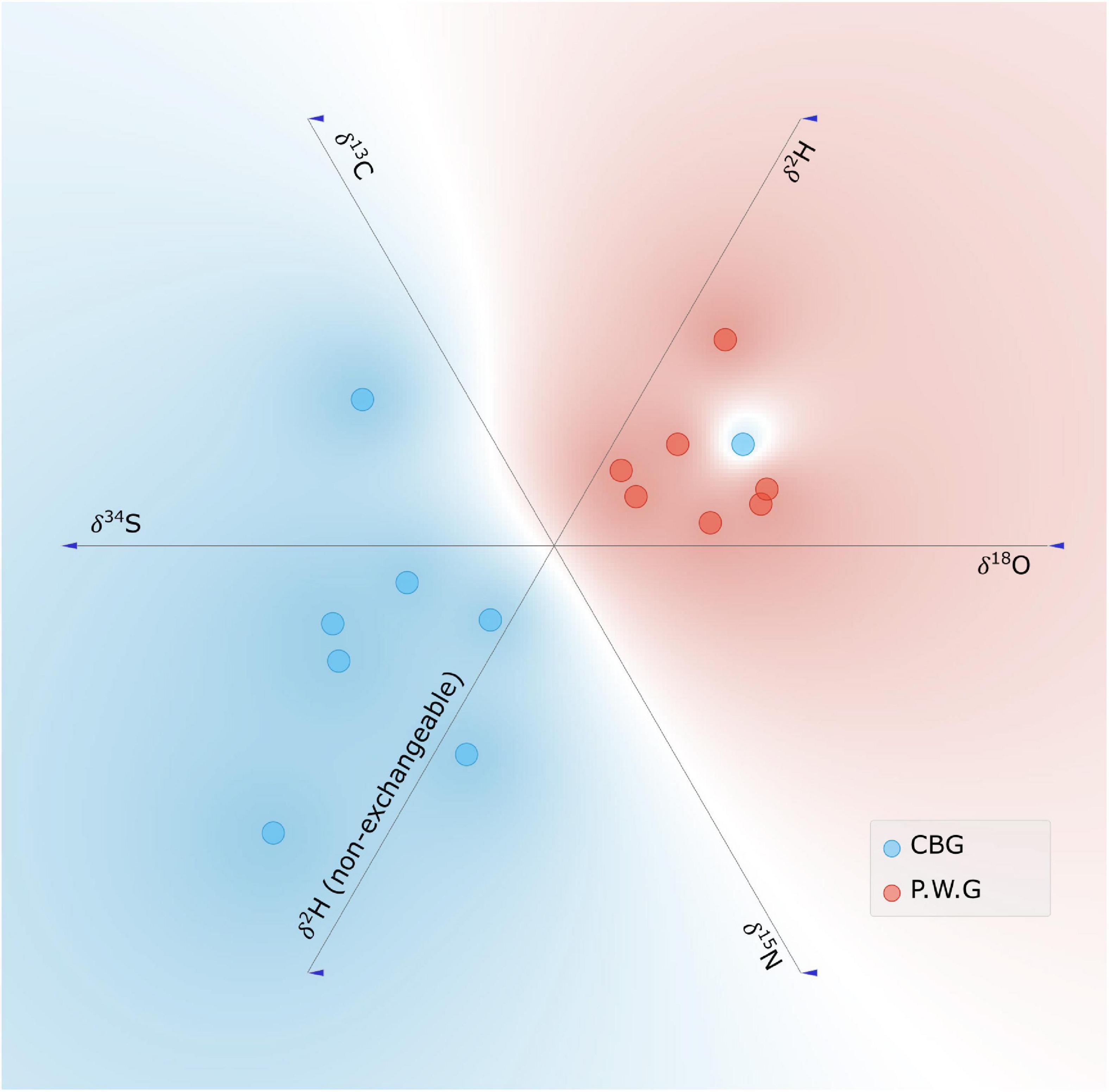
Figure 4. Linear projection of the stable isotope ratios of Aucoumea klaineana samples from the C.B.G (n = 9) and P.W.G (n = 7) concessions.
The Inverse Distance Weighting isoscape (Figure 5) highlights the spatial patterns in the stable isotope ratios in Aucoumea between and within the C.B.G and Precious Woods concessions. The color scales of the figures are set to maximize the visual differentiation in values rather than to reflect whether differences are significant or not. For example, there are no significant differences between the oxygen, hydrogen (extracted), carbon and nitrogen stable isotope ratios of Aucoumea between the two concessions. The scale for Figure 5A shows insignificant differentiation in the oxygen isotope ratios, however, the scales of the isotope ratios of carbon (B), extracted hydrogen (C), non-exchangeable δ2H from cellulose (D), nitrogen (E), and sulfur (F) all show good ranges in their respective values. Figure 5B shows that carbon isotope ratios may have been a more useful classifier to each concession were it not for a single sample in the northwest of the main portion of the P.W.G concession having such an enriched carbon isotope ratio. Figures 5C,D do not show comparable spatial patterns in hydrogen isotope ratios, Figure 5C shows that, generally, the hydrogen isotope ratios of Aucoumea are enriched in the C.B.G concession and depleted in the Precious Woods Group concession, whereas Figure 5D seems to show the opposite, discounting one sample in the C.B.G concession that has an enriched hydrogen isotope ratio in both situations. Figure 5E shows that the C.B.G concession has some populations of δ15N enriched Aucoumea close to δ15N depleted Aucoumea, whereas most of the samples collected in the Precious Woods Group concession are relatively depleted in δ15N with the exception of a sample in the northwest of the main body of the concession. This same sample had an enriched carbon isotope ratio. Figure 5F shows that the sulfur isotope ratios of Aucoumea within each concession are consistent. The C.B.G concession shows enriched sulfur isotope ratios whereas the P.W.G shows depleted. Only one sample in the C.B.G concession shows a relatively depleted sulfur isotope ratio.
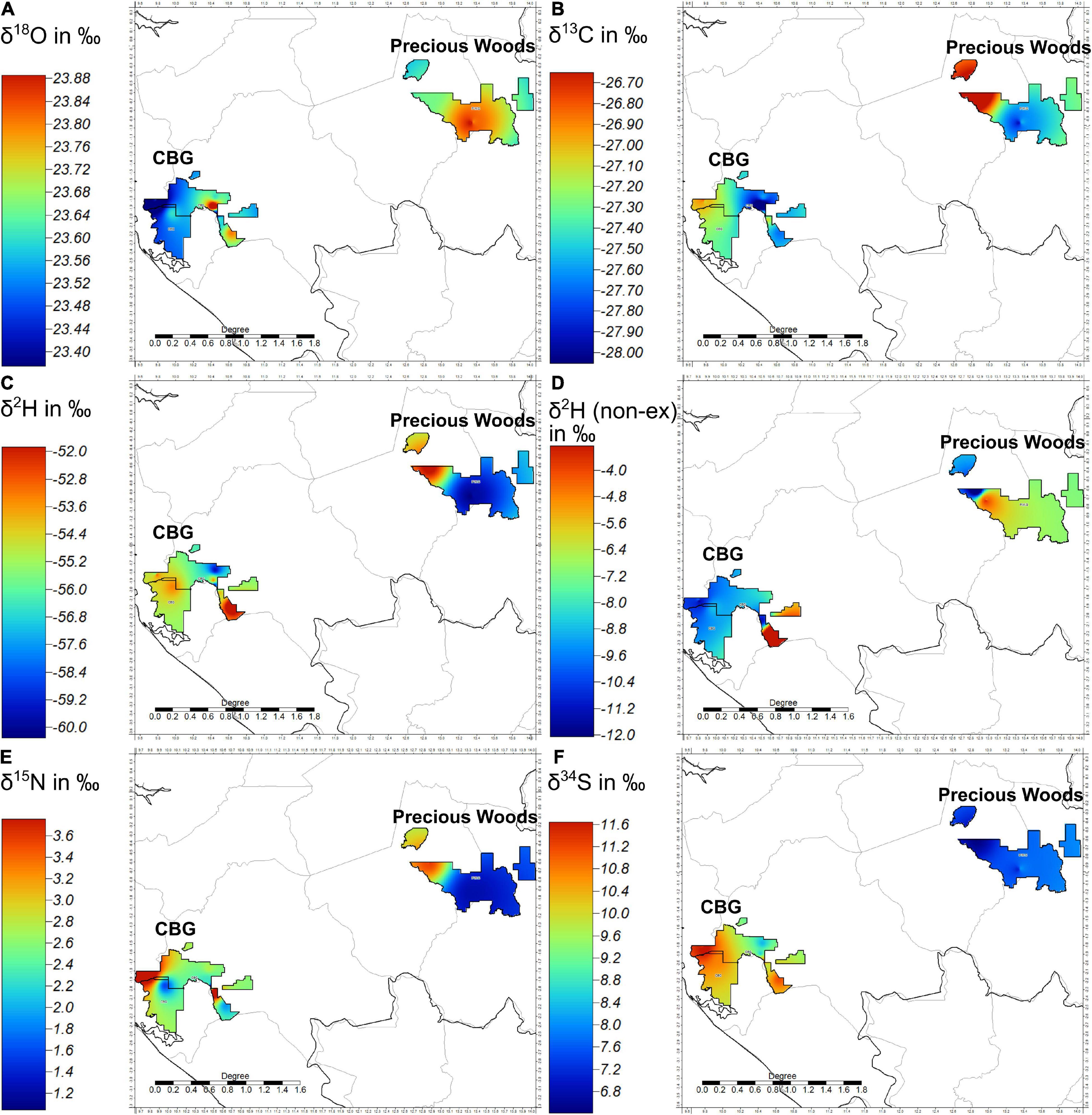
Figure 5. Inverse distance weighting (global) isoscapes of Aucoumea klaineana stable isotope data from samples collected in the C.B.G and P.W.G concessions. (A) δ18O, (B) δ13C, (C) δ2H, (D) non-exchangeable δ2H from cellulose, (E) δ15N, (F) δ34S.
Dacryodes buettneri (Synonym of Pachylobus buettneri)
Significant differences in the oxygen, hydrogen, non-exchangeable hydrogen, and nitrogen stable isotope ratios were not evident in Dacryodes buettneri between the C.B.G and Precious Woods concessions, however, differences between the mean values of the carbon and sulfur stable isotope ratios between the two concessions were observed. These differences were close to being significant (p = 0.143 for carbon isotope ratios, p = 0.127 for sulfur isotope ratios Figure 3).
Linear projection of the stable isotope ratios of the samples of Dacryodes buettneri (Figure 6) further demonstrate that there is some apparent separation of the stable isotope data between the two concessions, but these differences are not well-defined. This is also evident when observing the k-means silhouette scores of the PCA, there is a 24% probability that there are two clusters in the data, however, there is 24.7% probability that there are 3 clusters or 4 clusters within the data.
The inverse distance weighted (IDW) isoscapes (Figure 7) give insight into the spatial patterns of the Dacryodes data between and within the two concessions. Figure 7A shows that there is more variation of the oxygen stable isotopes of the Dacryodes within each concession than there is between the two. Figure 7B shows that there would be good segregation of the two concessions by the carbon isotope ratios of the Dacryodes were it not for the high variance in the C.B.G concession perhaps explaining why the Student’s t-test shows a nearly significant difference in carbon isotope ratios (P = 0.127). Figures 7C,D show that there are similar spatial patterns in the hydrogen stable isotopes of the extracted wood and of the non-exchangeable hydrogen from cellulose of the Dacryodes. The relatively high variation of hydrogen isotope ratios in the C.B.G concession limit the statistical separation of Dacryodes using the hydrogen isotope ratios alone. Figure 7E shows that Dacryodes buettneri have lower nitrogen isotope ratios in the C.B.G concession relative to the Precious Woods Group concession save for a single sample in C.B.G that was particularly enriched in δ15N. Figure 7F shows that Dacryodes follows a similar pattern to Aucoumea in its sulfur isotope ratios; the samples from the C.B.G concession are more enriched in δ34S than in the Precious Woods concession save for one sample in C.B.G that was depleted in 34S.
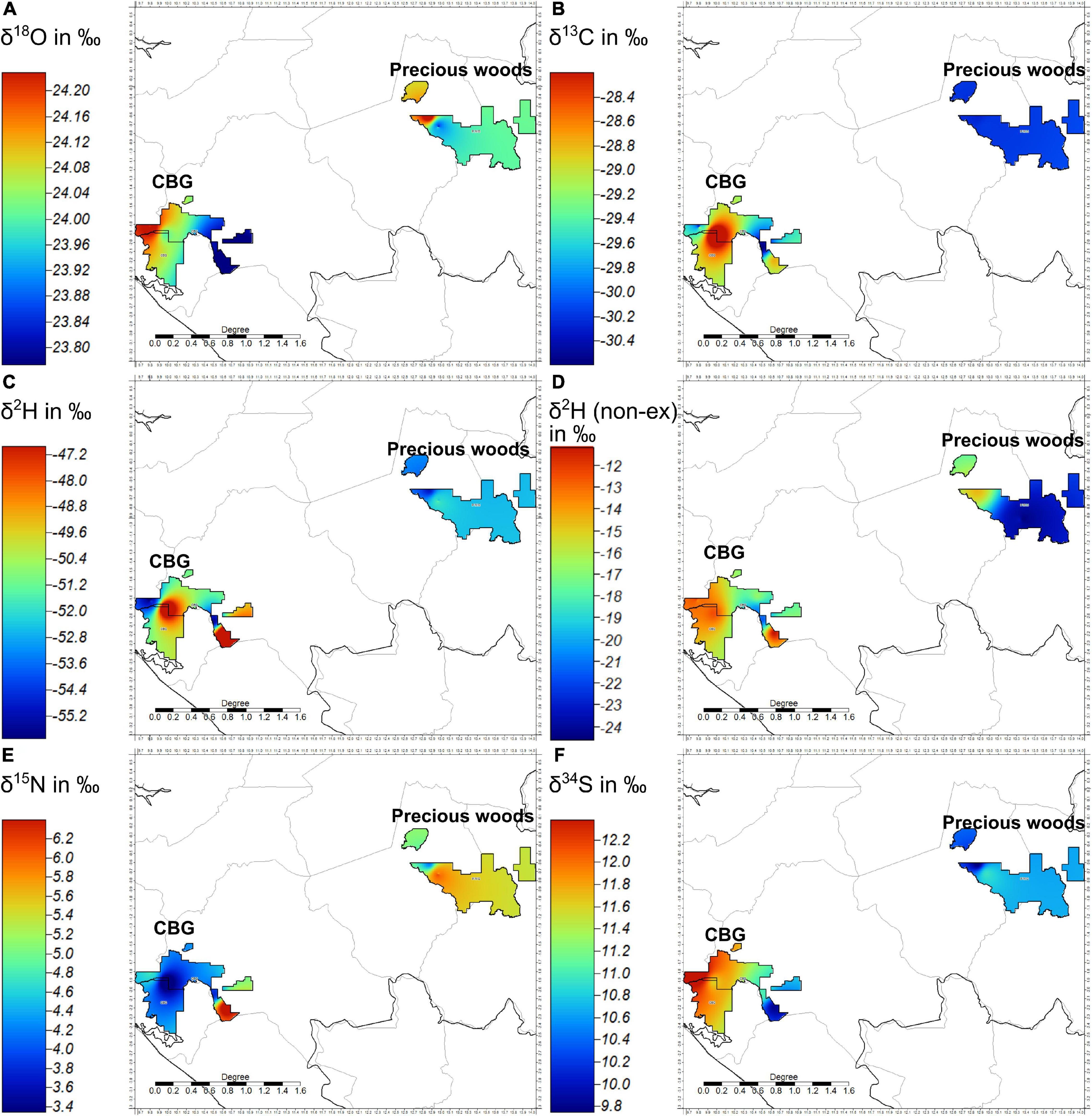
Figure 7. Inverse distance weighting (global) isoscapes of Dacryodes buettneri stable isotope data (A) δ18O, (B) δ13C, (C) δ2H, (D) non-exchangeable δ2H from cellulose, (E) δ15N, (F) δ34S.
Discussion
Aucoumea klaineana
Aucoumea klaineana (Okoumé) is a fast-growing, light-demanding pioneer tree (Koumba Zaou et al., 1998). In growing conditions with favorable light, Aucoumea klaineana can develop rapidly. In terms of photosynthesis, it can be considered that the main source of water for incorporation into sugars is likely to be primarily precipitation (Ohashi et al., 2016). It is therefore difficult to understand why there is no strong relationship between the hydrogen and oxygen isotope ratios of Okoumé, and very little trend with respect to elevation and its water hydrogen and oxygen isotope ratios. The lack of a trend may be summarized by the fact there are only relatively small differences in δ18O and δ2H in annual precipitation. This may be due to phenomena that work against each other such as the rainout effect and the continental effect (Ohashi et al., 2016), or that despite the fact there are differences in rainfall and elevation they are not sufficient to make a significant difference. Perhaps there is little meaningful difference in relative humidity between the P.W.G and C.B.G concessions that may also counter the anticipated relationship between δ18O and δ2H (Fritts et al., 1971; Roden et al., 2000).
One of the most important activities of rainforests is regulating the temperature of the atmosphere by removing CO2 and moderating rainfall. Provided that excessive evaporation does not occur, precipitation can be expected to follow the Global Meteoric Water Line (Craig and Boato, 1955; Craig, 1961). Evapotranspiration in rainforests produces rain for the rainforest and contributes to atmospheric cooling. However, this cyclical evapotranspiration may be considered as “excessive evaporation” meaning that the precipitation tropical trees receive is effectively not meteoric precipitation (Marryanna et al., 2017). One idea to investigate this further would be to sample Aucoumea klaineana under very different conditions (i.e., not just within Gabon, but elsewhere in the world), however, this is marred by the fact that Aucoumea klaineana primarily grows in Gabon and Equatorial Guinea with a small extent in Republic of Congo (Born et al., 2010). There are few plantations outside this range. Differences are evident in the nitrogen isotope ratios of the Aucoumea klaineana samples from the P.W.G and C.B.G concessions. Nitrogen isotope ratios are expected to increase with the age of a tree. Hietz et al. (2011) showed that a 2‰ increase in nitrogen isotope ratio was evident over an 80-year chronology of three tropical timbers. Variation in age of trees that were sampled may have played a factor in the nitrogen isotope ratio that was measured. Nitrogen deposition is increasing in the tropics (Högberg and Johannisson, 1993; Hietz et al., 2010), one of the suggested means is by tropospheric NOx deposition. It is not inconceivable that the differences between the two concessions may be partially explained by this, however, there are insufficient samples and conditions to be able to assess if this was the case in this study. Adding nitrogen to trees is associated with losses of NO32– from a tree and is a mechanism by which trees can become more enriched in 15N (Högberg and Johannisson, 1993), this phenomenon is also related to cation loss from soil and increase in soil acidity. Different soils can be more resistant to change perhaps explaining why significant differences were observed in the nitrogen isotope ratios of Aucoumea klaineana on the three different soil types. It also must be considered that segregating the Aucoumea klaineana samples by soil type rather than by concession may, on one hand, suggest that there is a predictable overall pattern within Gabon that may aid the origin classification of unknown samples of Okoumé; on the other, it may be an arbitrary segregation and the significant differences that were observed are the result of the fact that very few samples of Aucoumea klaineana have been examined in this study (n = 16). Though Aucoumea klaineana was the best-sampled species the data only portray a snapshot of reality. Greater sampling of Aucoumea klaineana in the Precious Woods concession would give spatial models more certainty. Sampling other concessions and countries will give a better idea of the differentiation and classification that is possible with Okoumé. One challenge to using nitrogen isotope ratios as an origin classifier is that Aucoumea klaineana exists as plywood in western markets. Veneers in plywood are typically glued using formaldehyde-urea-based resins (Desch and Dinwoodie, 1996; Negro et al., 2011), some also include melamine. Urea and melamine contain nitrogen, this exogenous nitrogen must be removed for the natural nitrogen to be accessed for comparison. The method of sample preparation posed in this study is also intended to mitigate the effect of resins and other secondary metabolic compounds and therefore is a practical solution to the problems that glues present in plywood.
Sulfur isotope ratios were the best discriminator of Aucoumea klaineana between the two concessions. There are clear differences between C.B.G and P.W.G concessions in terms of distance from the sea, tropospheric sulfate deposition (Novák et al., 1996; Global Modeling and Assimilation Office [GMAO], 2015) and elevation that may explain the differences in observed values either individually or in combination. Soil type may also be important in separating ranges of sulfur isotope ratios in Aucoumea klaineana as different soils contain varying concentrations of sulfate (Jones et al., 2013). If this is the case patterns in δ34S in Gabon would follow the patterns in soil type. All these potential predictors suggest that there is a higher spatial structure of sulfur isotope ratios in Aucoumea klaineana across Gabon that is likely to be useful at identifying the origin of Okoumé.
Based on the results, we believe that the ranges of stable isotope ratios for Aucoumea klaineana for the two concessions are defined well-enough. Leavitt (2010) shows that temperate trees growing on the same site may vary between 1 and 4‰ for δ18O, 5 and 30‰ for δ2H, and 1 and 3‰ for δ13C. In the C.B.G concession, Aucoumea klaineana ranged 1.1‰ for δ18O, 10.1‰ for δ2H, 10.5‰ for δ2H (non-exchangeable), and 2.4‰ for δ13C. In the P.W.G concession, Aucoumea klaineana ranged 0.8‰ for δ18O, 10.6‰ for δ2H, 11.9‰ for δ2H (non-exchangeable), and 2.0‰ for δ13C. It is not clear what proportion of sites had greater ranges in δ18O, δ2H, and δ13C in the Leavitt (2010) review or what the concession ranges from Aucoumea klaineana mean in this context, the isotopic ranges recorded in this study are on the lower end of the variability scale. Perhaps this means that Aucoumea klaineana is relatively homogenous in its isotopic composition in its growing sites, and the comparison of tropical trees with temperate trees is not ideal. Van der Sleen et al. (2017) conducted a review of tropical timber stable isotope variability. The review mainly focuses on inter-ring stable isotope variability rather than inter-tree (on the same site) stable isotope variability. The review discusses that inter-ring variation of 1–2‰ for δ13C, in some cases up to 3‰ is often found, and ranges of 1–5‰ for δ18O are not uncommon. The review also demonstrated one extreme case of 9‰δ18O variability within a tree ring. Similar values for inter-ring variability are discussed by Leavitt (2010). Though it may be a bit of leap to try to infer that because inter-ring stable isotope variability is similar in tropical and temperate trees, inter-tree variability should be similar too, the lack of a good comparison shows the importance of publishing stable isotope data for tropical trees so that Reviews and comparisons can be made in future. It is recommended that sample quantity per site should be compared to the range of stable isotope ratios for many species and many origins to observe the relationship between the two variables. We hypothesize that this relationship is horizontal asymptotic where having a small quantity of samples per site gives the greatest uplift in knowledge (from no knowledge) about the variability or range of site initially with the benefit of increased sampling yielding diminishing returns after a certain threshold is reached. This is one of the reasons for analyzing opportunistically collected samples in low quantities (e.g., Baillonella toxisperma, Cylicodiscus gabunensis, Guibourtia tessmannii, Lophira alata, Nauclea diderrichii, Pterocarpus soyauxii, Berlinia confusa, and Didelotia africana).
Dacryodes buettneri (Synonym of Pachylobus buettneri)
Eight samples of Dacryodes buettneri were sampled in the Precious Woods (n = 3) and C.B.G (n = 5) concessions. The range of stable isotope variability is well-defined enough for the C.B.G concession, but more samples are needed for the P.W.G concession. Dacryodes ranged 0.7‰ for δ18O, 14.4‰ for δ2H, 14.3‰ for δ2H (non-exchangeable), and 3.3‰ for δ13C in the C.B.G concession which is comparable to the ranges expected on a site as discussed by Leavitt (2010). It is likely collecting more samples of Dacryodes would elucidate the true differentiation possibilities between the two origins. Though multiple multivariate models were attempted with the Dacryodes data, it is statistically inappropriate to attempt to use three to six variables to classify eight samples from two origins. The Principal Component Analysis and the silhouette scores of the data show that there is not any real clustering of the data, this is because there aren’t enough samples to define clusters. Further interpretation of the differentiation possibilities of Dacryodes buettneri using stable isotope ratio measurements is beyond the scope of this paper. Nonetheless, it is interesting that the sulfur isotope ratios of Dacryodes buettneri appear to follow a similar trend to those of the Aucoumea klaineana reference samples. Further collection of this timber should reveal a higher spatial structure within Gabon that can be used to classify the origin of Dacryodes timber.
Baillonella toxisperma, Cylicodiscus gabunensis, Guibourtia tessmannii, Lophira alata, Nauclea diderrichii, Pterocarpus soyauxii, Berlinia confusa, and Didelotia africana
Data presented in this report provide a first glimpse at the stable isotopic variation of the species from the concessions where they were collected from within Gabon. However, little can be stated about what overtly the data means for collections with an n < 3 until a point where collections are large enough to permit thorough analysis and interpretation. Ideally, collections of species/genera from concession areas should be at least five samples and as many as 10–20, perhaps more depending on the size of the concession. There are clear, significant differences between these species even though they were collected mostly on the same sites, suggesting that data from one species cannot easily be applied to another at this stage. As collections of samples and data grow, it is likely that higher-order patterns will become evident and this may permit interpretation of the origin of various species using data from another species such as using Dunbar lines (Dunbar and Wilson, 1983) to convert between datasets, or using mechanistic models to forecast data (Roden et al., 2000; Cueni et al., 2021).
Of all sampled timbers, Pterocarpus soyauxii showed the most negative nitrogen stable isotope ratios. This was expected as Pterocarpus soyauxii is a nitrogen-fixing tree and perhaps acts as a primary nitrogen source in the areas where it was sampled. The results are supported by Hietz et al. (2011) who demonstrated that leaves of leguminous trees had more negative δ15N relative to non-leguminous trees. Variability in nitrogen isotope composition was also demonstrated by Hietz et al. (2011) to be influenced by sun/shade. Results from nitrogen fixers are important from an ecological perspective as they are one of the nitrogen sources for other nearby trees due to the nitrogen they fix in the soil and the distribution of the nitrogen by mycorrhiza. Mycorrhizal type varies with climate, such as arbuscular mycorrhiza which are more abundant in tropical areas such as Gabon. Steidinger et al. (2019) show that varying proportions of these arbuscular mycorrhiza exist across Gabon and between the two concessions that were sampled in this project. This may give rise to geographic variation in nitrogen isotope ratios of Pterocarpus and other flora and fauna that source nitrogen through mycorrhizal networks (Williamson et al., 1990). However, Berlinia confusa and Cylicodiscus gabunensis are also from the Fabaceae family yet have much more positive δ15N than the reference samples of Pterocarpus soyauxii (Figure 3). If the explanation for the δ15N in Fabaceae timbers is a simple as nitrogen fixing trees have lower δ15N then the results from the Berlinia confusa and Cylicodiscus gabunensis suggest there is more to δ15N variance in tropical timber than simply local nitrogen fixation.
Conclusion
Despite the limited quantity of samples and species, the data acquired establish a basis of evaluation for assessing geographic origin claims of certain forest products including plywood and veneers from Gabon. The differences in the sulfur isotope ratios of Aucoumea and Dacryodes reference samples from Precious Woods Group (P.W.G) and Compagnie des Bois du Gabon (C.B.G) forest concessions suggest that regional scale origin classification may be realized with high enough frequency of reference sample collection. Furthermore, higher resolution sampling of target species including Aucoumea and Dacryodes will address this much needed comparison as it may enable more efficient allocation of reference sampling resources. Further sampling of Burseraceae family timbers in the tropics may permit a global model for verifying their geographic origin.
Future Work
Expanding the collection of reference samples will be necessary to investigate regional classification further. More sample data will improve discrimination between concessions or regions, as well as allowing for comparison of stable isotopes both within a single sampling site and single taxa. A higher frequency of reference sample collection will also enable assessment of the suitability of specific taxa to act as isotopic proxies for other species. Natural variability of isotopic distribution within a site is still not fully understood, and it is anticipated that large scale sample collections for specific taxa will also enable a better understanding of this. So far, the study has focused on whole wood and cellulose components of timber reference samples. Several alternative analytical methods detail procedures for analyzing alternative metabolic fractions including lignin and proteins in the form of amino acids. Furthermore, isotope methods are already being used to measure the stable isotopes of position-specific atoms within a selection of molecules such as ethanol, providing higher resolution information on the source water incorporated during metabolism. Analysis of alternative fractions and position-specific isotope ratios within a molecule such as glucose or lignin may yield higher quality results and aid the discrimination of reference samples taken from concessions within the same country.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
CW was repsonsible for sample planning and co-ordination with FSC. CW and GR were responsible for interpretation and production of manuscript. MG was responsible for sample collection and organization in Gabon as well as contribution of contextual information. PG was responsible for co-ordinating curation of samples as well as editing and advising on content and contributed significantly to disussions and content related to the relation of phylogenetics, wood structure and stable isotope results. SH, LM, and MB were responsible for sample preparation and analysis and contributed the analytical method section.
Conflict of Interest
CW and GR were employed by company Agroisolab UK Ltd. SH, LM, and MB were employed by company Agroisolab GmbH.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks go out to the following people and organizations, as this research would not have been made possible without their input, collaboration, and expertise: The United States Department of State and United States Department of Agriculture for funding this research. Elizabeth Lebow, Alex Moad, and their respective colleagues at the United States Forest Service for overseeing the World Forest ID project. This work published here has been made possible by RBG Kew’s Defra Plant Health License 2149/194627/5 which enables the safe import, keeping and use of plant materials which are normally prohibited due to biosecurity concerns. FSC, World Forest ID, Phil Guillery, Emily Crumley, and Nathalie Bouville organizing and collecting samples in Gabon. Staff of Precious Woods Group and Compagnie des Bois du Gabon for permitting and facilitating the collection of reference samples. Meaghan Parker-Forney and her respective colleagues at the World Resources Institute for overseeing the World Forest ID project. Jade Saunders of Forest Trends for her advisory and coordination support to the World Forest ID project. Isabella Miles-Bunch and Sara Redstone at the Royal Botanic Gardens, Kew for assisting with sample shipment documentation, receipt of samples in quarantine and their expert help in verifying declared information of field collectors. Tim Fox, Jackie Borrows, and Matt Watkinson for spending 100 s of collective hours assembling sample collection packs. Agroisolab GmbH staff for spending 100 s of collective hours preparing and measuring samples. Meg Staton, Abdullah Almsaeed et al. for developing the World Forest ID app which tracks samples from the field through Kew to analysis labs. We gratefully acknowledge funding via Defra-ICF: Building and Enhancing the Evidence Base for Nature Based Solutions to Climate Change. Project 29084. Driving innovation in forest protection and enforcement monitoring. Tackling illegal logging: creating a timber reference library to support enforcement (World Forest ID at Kew). Google Maps Terrain basemap (accessed 21.06.2021) was used to help create Figure 1.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2021.650257/full#supplementary-material
Footnotes
References
Bayol, N. (2002). Etude de cas D’ameìnagement Forestier Exemplaire en Afrique : La Concession Forestière sous Aménagement Durable (CFAD), Forest Management Working Papers, Working Paper FM/15F. Forest Resources Development Service, Forest Resources Division. Rome: FAO.
Beeckman, H., Blanc-Jolivet, C., Boeschoten, L., Braga, J. W. B., Cabezas, J. A., Chaix, G., et al. (2020). Tech. Ed. Schmitz, N., Overview of Current Practices in Data Analysis for Wood Identification A Guide for the Different Timber Tracking Methods. Global Timber Tracking Network, GTTN Secretariat. Joensuu: European Forest Institute.
Boner, M., and Förstel, H. (2004). Stable isotope variation as a tool to trace the authenticity of beef. Anal. Bioanal. Chem. 378, 301–310. doi: 10.1007/s00216-003-2347-6
Boner, M., Somner, T. H., Erven, C., and Förstel, H. (2007). “Stable Isotopes as a Tool to Trace Back the Origin of Wood,” in Proceedings of the International Workshop “Fingerprinting Methods for the Identification of Timber Origins, Bonn, 47–57.
Born, C., Alvarez, N., McKey, D., Ossari, S., Wickings, E. J., Hossaert-McKey, M., et al. (2010). Insights into the biogeographical history of the lower guinea forest domain: evidence for the role of refugia in the intraspecific differentiation of Aucoumea klaineana. Mol. Ecol. 20, 131–142. doi: 10.1111/j.1365-294x.2010.04919.x
Born, C., Hardy, O. J., Chevallier, M. H., Oossari, S., Attéké, C., Wickings, E. J., et al. (2008). Small-scale spatial genetic structure in the central African rainforest tree Species Aucoumea klaineana: a stepwise approach to infer the impact of limited gene dispersal, population history and habitat fragmentation. Mol. Ecol. 17, 2041–2050. doi: 10.1111/j.1365-294x.2007.03685.x
Born, C., Vignes, H., Muloko, N., Wickings, E. J., Hossaert-McKey, M., and Chevallier, M. H. (2006). Isolation and characterization of polymorphic microsatellite loci from Aucoumea klaineana Pierre (Burseraceae), a tropical rainforest tree of Central Africa. Mol. Ecol. Notes 6, 1054–1056. doi: 10.1111/j.1471-8286.2006.01431.x
Bowen, G. J., and Revenaugh, J. (2003). Interpolating the isotopic composition of modern meteoric precipitation. Water Resour. Res. 39:1299. doi: 10.1029/2003wr002086
Cheung, C. (2014). Studies of the Nitration of Cellulose - Application in New Membrane Materials. Ph.D. thesis. Vancouver: University of British Columbia. doi: 10.14288/1.0072160
Craig, H. (1961). Isotopic variations in meteoric waters. Science 133, 1702–1703. doi: 10.1126/science.133.3465.1702
Craig, H., and Boato, G. (1955). Isotopes. Annu. Rev. Phys. Chem. 6, 403–432. doi: 10.1146/annurev.pc.06.100155.002155
Crocker, E., Condon, B., Almsaeed, A., Jarret, B., Nelson, C. D., Abbott, A. G., et al. (2019). TreeSnap: a citizen science app connecting tree enthusiasts and forest scientists. Plants People Planet 2, 47–52. doi: 10.1002/ppp3.41
Cueni, F., Nelson, D. B., Boner, M., and Kahmen, A. (2021). Using plant physiological stable oxygen isotope models to counter food fraud. Sci. Rep. 11. doi: 10.1038/s41598-021-96722-9
Desch, H. E., and Dinwoodie, J. M. (1996). Timber: Structure, Properties, Conversion and Use. Houndmills: MacMillan Press. doi: 10.1007/978-1-349-13427-4
Dunbar, J., and Wilson, A. T. (1983). Oxygen and hydrogen isotopes in fruit and vegetable juices. Plant Physiol. 72, 725–727. doi: 10.1104/pp.72.3.725
EIA (2019). Toxic Trade. Forest Crime in Gabon and the Republic of Congo and contamination of the US market. Available online at: https://content.eia-global.org/assets/2019/03/Toxic_Trade_Executive_Summary-web.pdf (accessed April 21, 2020).
Fritts, H. C., Blasing, T. J., Hayden, B. P., and Kutzbach, J. E. (1971). Multivariate techniques for specifying tree-growth and climate relationships and for reconstructing anomalies in paleoclimate. J. Appl. Meteorol. 10, 845–864.
Gasson, P. E., Lancaster, C. A., Young, R., Redstone, S., Miles-Bunch, I. A., Rees, G., et al. (2020). WorldForestID: addressing the need for standardized wood reference collections to support authentication analysis technologies; a way forward for checking the origin and identity of traded timber. Plants People Planet 3, 130–141. doi: 10.1002/ppp3.10164
Global Modeling and Assimilation Office [GMAO] (2015). MERRA-2 tavgM_2d_aer_Nx: 2d, Monthly Mean, Time-Averaged, Single-Level, Assimilation, Aerosol Diagnostics V5. 12. 4. Greenbelt, MD: GMAO.
Gori, Y., Stradiotti, A., and Camin, F. (2018). Timber Isoscapes. A case study in a mountain area in the Italian Alps. PLoS One 13, e0192970. doi: 10.1371/journal.pone.0192970
Gori, Y., Wehrens, R., Greule, M., Keppler, F., Ziller, L., La Porta, N., et al. (2013). Carbon, hydrogen and oxygen stable isotope ratios of whole wood, cellulose and lignin methoxyl groups of Picea abies as Climate Proxies. Rapid Commun. Mass Spectrom. 27, 265–275. doi: 10.1002/rcm.6446
Heaton, K., Kelly, S. D., Hoogewerff, J., and Woolfe, M. (2008). Verifying the geographical origin of beef: the application of multi-element isotope and trace element analysis. Food Chem. 107, 506–515. doi: 10.1016/j.foodchem.2007.08.010
Hietz, P., Dünisch, O., and Wanek, W. (2010). Long-term trends in nitrogen isotope composition and nitrogen concentration in brazilian rainforest trees suggest changes in nitrogen cycle. Environ. Sci. Technol. 44, 1191–1196. doi: 10.1021/es901383g
Hietz, P., Turner, B. L., Wanek, W., Richter, A., Nock, C. A., and Wright, S. J. (2011). Long-term change in the nitrogen cycle of tropical forests. Science 334, 664–666. doi: 10.1126/science.1211979
Hillis, W. E. (1987). Heartwood and Tree Exudates, 1st Edn. (Berlin: Springer), 21. doi: 10.007/978-3-642-72534-0
Högberg, P., and Johannisson, C. (1993). 15N abundance of forests is correlated with losses of nitrogen. Plant Soil 157, 147–150. doi: 10.1007/bf02390237
Horacek, M., Jakusch, M., and Krehan, H. (2009). Control of Origin of Larch Wood: discrimination between European (Austrian) and Siberian Origin by Stable Isotope Analysis. Rapid Commun. Mass Spectrom. 23, 3688–3692. doi: 10.1002/rcm.4309
Horacek, M., Rees, G., Boner, M., and Zahnen, J. (2018). Comment on: developing Forensic Tools for an African Timber: […], By Vlam et al., 2018. Biol. Conserv. 226, 333–334. doi: 10.1016/j.biocon.2018.06.037
Huffman, G. J., and Bolvin, D. T. (2019). GPCP Precipitation Level 3 Monthly 0.5-Degree V3.0 Beta. Greenbelt, MD: NASA GES DISC.
Jolivet, C., and Degen, B. (2012). Use of DNA Fingerprints to Control the Origin of Sapelli Timber (Entandrophragma Cylindricum) at the Forest Concession Level in Cameroon. Forensic Sci. Int. Genet. 6, 487–493. doi: 10.1016/j.fsigen.2011.11.002
Jones, A., Breuning-Madsen, H., Brossard, M., Dampha, A., Deckers, J., Dewitte, O., et al. (2013). Soil Atlas of Africa. Luxembourg: European Commission. doi: 10.2788/52319
Kagawa, A., Abe, H., Fuji, T., and Itoh, Y. (2008). “Stable isotopes and inorganic elements as potential indicators of timber geographic origin,” in Proceedings of the American Geophysical Union, Fall Meeting, San Francisco, CA.
Kagawa, A., and Leavitt, S. W. (2010). Stable carbon isotopes of tree rings as a tool to pinpoint the geographic origin of timber. J. Wood Sci. 56, 175–183. doi: 10.1007/s10086-009-1085-6
Karsenty, A. (2019). Certification of tropical forests: a private instrument of public interest? A focus on the congo basin. For. Policy Econ. 106:101974. doi: 10.1016/j.forpol.2019.101974
Kelly, S., Baxter, M., Chapman, S., Rhodes, C., Dennis, J., and Brereton, P. (2002). The application of isotopic, and elemental analysis to determine the geographical origin of premium long grain rice. Eur. Food Res. Technol. 214, 72–78. doi: 10.1007/s002170100400
Keppler, F., Harper, D. B., Kalin, R. M., Meier-Augenstein, W., Farmer, N., Davis, S., et al. (2007). Stable hydrogen isotope ratios of lignin methoxyl groups as a paleoclimate proxy and constraint of the geographical origin of wood. New Phytol. 176, 600–609. doi: 10.1111/j.1469-8137.2007.02213.x
Koumba Zaou, P., Mapaga, D., and Verkaar, H. J. (1998). Effect of shade on young Aucoumea klaineana Pierre trees of various Provenance under field conditions. For. Ecol. Manage. 106, 107–114. doi: 10.1016/s0378-1127(97)00301-0
Leavitt, S. W. (2010). Tree-Ring C–H–O isotope variability and sampling. Sci. Total Environ. 408, 5244–5253. doi: 10.1016/j.scitotenv.2010.07.057
Li, A., Keely, B., Chan, S. H., Baxter, M., Rees, G., and Kelly, S. (2015). Verifying the provenance of rice using stable isotope ratio and multi-element analyses: a feasibility study. Qual. Assur. Saf. Crops Foods 7, 343–354. doi: 10.3920/qas2013.0378
Marryanna, L., Kosugi, Y., Itoh, M., Noguchi, S., Takanashi, S., Katsuyama, M., et al. (2017). Temporal variation in the stable isotopes in precipitation related to the rainfall pattern in a tropical rainforest in Peninsular Malaysia. J. Trop. For. Sci. 29, 349–362. doi: 10.26525/jtfs2017.29.3.349362
Muloko-Ntoutoume, N., Petit, R. J., White, L., and Abernathy, K. (2000). Chloroplast DNA Variation in a Rainforest Tree (Aucoumea klaineana, Burseraceae) in Gabon. Mol. Ecol. 9, 359–363. doi: 10.1046/j.1365-294x.2000.00859.x
Negro, F., Cremonini, C., and Zanuttini, R. (2011). A new wood-based lightweight composite for boatbuilding. Wood Res. 56, 257–266.
NEPcon (2017). Timber Risk Assessments. Available online at: https://www.nepcon.org/sourcinghub/timber/timber-gabon (accessed April 21, 2020).
Novák, M., Bottrell, S. H., Fottová, D., Buzek, F., Groscheová, H., and Žák, K. (1996). Sulfur isotope signals in forest soils of central europe along an air pollution gradient. Environ. Sci. Technol. 30, 3473–3476. doi: 10.1021/es960106n
Ohashi, S., Durgante, F. M., Kagawa, A., Kajimoto, T., Trumbore, S. E., Xu, X., et al. (2016). Seasonal variations in the stable oxygen isotope ratio of wood cellulose reveal annual rings of trees in a central Amazon Terra Firme Forest. Oecologia 180, 685–696. doi: 10.1007/s00442-015-3509-x
Pilgrim, T. S., Watling, R. J., and Grice, K. (2010). Application of trace element and stable isotope signatures to determine the provenance of Tea (Camellia sinensis) samples. Food Chem. 118, 921–926. doi: 10.1016/j.foodchem.2008.08.077
Rees, G. (2015). Verifying the Declared Origin of Timber Using Stable Isotope Ratio and Multi-Element Analyses. Ph.D. thesis. Heslington: University of York.
Regulation (EU) No 995/2010 (2010). Guidance Document for the EU. (Timber) Regulation. 12.2.2016 C 755 final. Brussels: EU.
Roden, J. S., Lin, G., and Ehleringer, J. R. (2000). A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in tree-ring cellulose. Geochim. Cosmochim. Acta 64, 21–35. doi: 10.1016/s0016-7037(99)00195-7
Steidinger, B. S., Crowther, T. W., Liang, J., Van Nuland, M. E., Werner, G. D., Reich, P. B., et al. (2019). Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569, 404–408. doi: 10.1038/s41586-019-1128-0
US Lacey Act (2008). 16 U.S.C. §§3371-3378. https://uscode.house.gov/view.xhtml?path=/prelim@title16/chapter53&edition=prelim (accessed April 30, 2021).
Van der Sleen, P., Zuidema, P. A., and Pons, L. T. (2017). Stable isotopes in tropical tree rings: theory, methods and applications. Funct. Ecol. 31, 1674–1689. doi: 10.1111/1365-2435.12889
Watkinson, C. J., Gasson, P., Rees, G., and Boner, M. (2020). The development and use of isoscapes to determine the geographical origin of Quercus Spp. in the United States. Forests 11:862. doi: 10.3390/f11080862
Watkinson, C. J., Rees, G. O., Hofem, S., Gasson, P., and Boner, M. (2021). A case study to establish a basis for evaluating geographic origin claims of timber from the Solomon Islands using stable isotope ratio analysis. Front. Forests Glob. Change Forest Ecophysiol. (in press).
White, L., Abernethy, K., Oslisly, R., and Maley, J. (1996). “L’okoumé (Aucoumea klaineana): expansion et déclin d’un arbre pionnier en Afrique Centrale au cours de l’Holocène,” in Dynamique à Long Terme des Écosystèmes Forestiers Intertropicaux: Résumés, eds M. Servant and S. Servant-Vildary (Paris: UNESCO), 195–198.
Keywords: IRMS, Aucoumea klaineana, Gabon, 2H/1H isotope ratio analysis, illegal logging and timber trade, Lacey Act, EUTR
Citation: Watkinson CJ, Rees GO, Gwenael MC, Gasson P, Hofem S, Michely L and Boner M (2022) Stable Isotope Ratio Analysis for the Comparison of Timber From Two Forest Concessions in Gabon. Front. For. Glob. Change 4:650257. doi: 10.3389/ffgc.2021.650257
Received: 06 January 2021; Accepted: 27 September 2021;
Published: 27 January 2022.
Edited by:
Micha Horacek, HBLFA Francisco Josephinum, BLT Wieselburg, AustriaReviewed by:
Dana Alina Magdas, National Institute for Research and Development of Isotopic and Molecular Technologies, RomaniaCristina Maria Máguas, University of Lisbon, Portugal
Nives Ogrinc, Institut “Jožef Stefan” (IJS), Slovenia
Copyright © 2022 Watkinson, Rees, Gwenael, Gasson, Hofem, Michely and Boner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charles J. Watkinson, Y2hhcmxpZS53YXRraW5zb25AYWdyb2lzb2xhYi5jb20=
 Charles J. Watkinson
Charles J. Watkinson Gareth O. Rees
Gareth O. Rees Moundounga Cynel Gwenael
Moundounga Cynel Gwenael Peter Gasson
Peter Gasson Sabine Hofem4
Sabine Hofem4