
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change , 11 February 2021
Sec. Forest Soils
Volume 3 - 2020 | https://doi.org/10.3389/ffgc.2020.621231
This article is part of the Research Topic Vegetation Effects on Soil Organic Matter in Forested Ecosystems View all 11 articles
 Speranza Claudia Panico1
Speranza Claudia Panico1 Valeria Memoli1
Valeria Memoli1 Lucia Santorufo1*
Lucia Santorufo1* Francesco Esposito1
Francesco Esposito1 Anna De Marco2
Anna De Marco2 Rossella Barile3
Rossella Barile3 Giulia Maisto1
Giulia Maisto1Altitude, exposure, and plant cover may have a significant impact on the soil system, affecting its abiotic characteristics and, in turn, soil microbial composition and activity. In the Mediterranean area, the relationships among environmental features and soil characteristics are still scarcely investigated. The present study aimed to evaluate the effects of altitude, slope exposure, and plant cover on soil abiotic characteristics and the responses of the soil microbial community. Surface soil was sampled at 32 field points of the Vesuvius Mountain (Southern Italy) at two slope exposures (North and South), two altitudes (600 and 900 m a.s.l), and under two different plant covers (pines and shrubs), and it was analyzed for soil abiotic and biotic characteristics. The results showed that soil characteristics mainly differed according to site altitude, but some characteristics also changed according to site exposure and plant cover. The soil organic carbon (Corg) showed significant high values at low altitude, south exposure, and under pines and played a role in influencing the soil microbial community. In soil covered by pines, the greatest soil Corg amount matched with the highest values of C/N ratio and fungal biomass. Finally, high Corg and water availability significantly enhanced the microbial activities.
Soil is defined as a complex mixture of eroded rock, mineral nutrients, organic matter, water, air, and billions of living organisms, whose combination is difficult to predict and depends on multiple environmental features (Miller, 2007). Among these features, altitude and slope exposure influence the local climate, which, in turn, affects soil characteristics (Griffiths et al., 2009).
Particularly in the Mediterranean area, moderately wet and cold winters are coupled with dry and hot summers, but the intensity of the drier periods, increasing from high to low latitudes, can vary widely and directly influence different soil characteristics (Tsui et al., 2004; Sardans and Peñuelas, 2013).
The temperature and moisture generated from the elevation and the exposition may affect soil nutrient availability, erodibility, moisture content, infiltration capacity, leaching and deposition processes, cation exchange capacity, soil organic matter dynamics, and stabilization and pH (Lemenih and Itanna, 2004; Griffiths et al., 2009; Saeed et al., 2014).
Furthermore, different altitude and slope exposure have an impact on plant community distribution as vegetation types and thus strictly interact with soil processes or function, modifying its chemical and physical characteristics (Griffiths et al., 2009; Thakur et al., 2015). In addition, vegetation density and type, affecting the litter quantity and quality, soil pH, and chemical composition (Menyailo et al., 2002; Mendes et al., 2014; Cline and Zak, 2015), directly or indirectly play important roles on soil microbial composition and activity (Rajala et al., 2012; Bardelli et al., 2018). For example, McCulley and Burke (2004) observed, in soils covered by grass, that altitude directly impacted microbial biomass amount, whereas, in boreal forest soils, topographic gradient changed thick forest floors, pH, and C/N ratio and the microbial community structures (Högberg et al., 2007; Seibert et al., 2007). So, differences in soil abiotic characteristics due to altitude, exposure (Swallow et al., 2009; Bach et al., 2010), and plant cover (Panico et al., 2020) have a significant impact on ecosystem dynamics and may lead to differences in soil microbial communities (Tajika et al., 2020).
Particularly interesting are the soils formed along the slopes of a volcano for their capacity to store the highest amount of organic C among all mineral soil types (Lilienfein et al., 2003; Egli et al., 2008; De Marco et al., 2013a). In fact, these soils are generally characterized by high litter input due to primary productivity (Dahlgren et al., 2004) but have also a high stabilization of soil organic matter by non-crystalline inorganic soil components (Torn et al., 1997). On the other hand, volcanic soils are characterized by the natural pedo-geochemical background with high heavy metal concentrations inherited from the parent rock material (Adamo and Zampella, 2007).
Although the impacts of the environmental features (i.e., altitude, exposure, and plant cover) on soil formation and evolution are known, the specific relationships between these and soil abiotic and biotic characteristics have been poorly studied (Liu et al., 2003; Brockett et al., 2012; Wang et al., 2016). In fact, the few studies performed in the Mediterranean area (Rutigliano et al., 2009; Iovieno et al., 2010; Lucas-Borja et al., 2012) are not interested at soil–vegetation relationships along an altitudinal gradient and among different slope exposures. Anyway, the low water and nutrient availability in soils of Mediterranean area affect plant cover and soil biological activity and diversity which, in turn, are related to soil hydrological and erosion behavior throughout the slopes (Ruiz-Sinoga et al., 2010, 2011). Moreover, the assessment of altitude and slope exposure incidence and their prevailing role as driving features for changes in soil characteristics is becoming fundamental especially in Mediterranean ecosystems due to their fragility and exposure to climate change conditions (Sardans and Peñuelas, 2013).
Therefore, the aims of this research were (i) to assess the effects of altitude, slope exposure, and plant cover (the main site features) on soil abiotic and biotic characteristics within a Mediterranean volcanic area and (ii) to analyze the responses of soil microbial community to soil abiotic characteristics at different altitude, slope exposure, and plant cover. The hypotheses behind the research were that sites of distinct altitude/exposure/vegetation directly affect the soil abiotic characteristics and indirectly the soil microbial community and activities. To achieve the aims, soils were sampled at the Vesuvius National Park (Southern Italy), a volcanic mountain covered by typical Mediterranean plants. The study was performed along two slope exposures (North and South) and two altitudes (600 and 900 m a.s.l.) characterized by two representatives (Vacchiano et al., 2012) plant covers (pines and shrubs). The soil samplings were carried out during spring, i.e., a growing season with non-restrictive temperatures and water availability, in three sampling campaigns (2015, 2016, and 2017). The Vesuvius National Park is a volcanic area located not so far from densely populated urban areas and characterized by intensive human activities. The investigated soils are particularly rich in Cr, Cu, Ni, and Pb because of both their pedogenetic origin and air deposition deriving by the surroundings with high human density and activity (De Nicola et al., 2003; Maisto et al., 2006; Memoli et al., 2018a). Besides, in previous researches performed in the same investigated area, many nutrients showed low ready availability (namely, acid-soluble, reducible, and oxidizable fractions) and did not exert meaningful effects on soil microbial biomass and activity (Memoli et al., 2018b). By contrast, ecotoxicological effects on soil biota were due to trace elements, particularly Cr, Cu, Ni, and Pb (Maisto et al., 2011; Memoli et al., 2018b).
The study was performed on the Vesuvius Mountain (Naples, Southern Italy), characterized by Mediterranean climatic conditions with dry summers and rainy autumns and winters; mean annual temperature is 9.8°C, and annual precipitation is 940 mm according to long-term averages (years 1961 to 1990) from the closest meteorological station at Osservatorio Vesuviano (605 m a.s.l.; 40°49′N; 14°24′E). The monthly mean of temperature (°C) and precipitation (mm) of the north and south slopes of the study area in the sampling period (2015–2017) is reported in Figure 1.
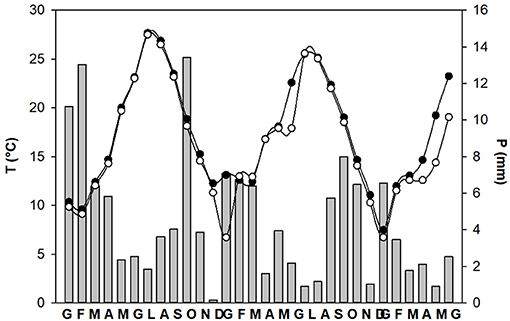
Figure 1. Monthly mean of temperature (T, expressed as °C) and precipitation (P, expressed as mm) values of north (filled symbols and bars) and south (empty symbols and bars) slopes related to the study area from January 2015 to June 2017.
The slopes of Mount Vesuvius are a complex mosaic of areas afforested mainly by Pinus pinea and sites characterized by shrubs (such as Myrtus communis L., Laurus nobilis L., Viburnum tinus L., Cistus sp., Ginesta sp.) of the Mediterranean maquis (De Nicola et al., 2003; De Marco et al., 2013b). The soils are Lepti-Vitric Andosols according to the FAO soil classification (Gennaro and Terribile, 1999; IUSS Working Group WRB, 2014); in the studied areas, soils derived by volcanic deposits of the recent cycles (1804–1906). These deposits were Leucititic Tephrite-Phonolites similar in chemical composition (Santacroce, 1987; Santacroce and Sbrana, 2003).
The soil samplings were carried out during three sampling campaigns conducted during spring in 2015, 2016, and 2017. Surface soils (0–10 cm) were collected at 32 sites: 16 along the south-exposed (S) and 16 north-exposed (N) slopes. Along each slope, 8 sites were chosen at low altitudes (L-600 m a.s.l.) and 8 at high altitudes (H-900 m a.s.l.), and for each altitude 4 soils were sampling under pines (P) and 4 soils under shrubs (S). At each site, 12 subsamples of soils were collected and mixed together in order to obtain a homogeneous sample to perform the analyses.
In the laboratory, the soil samples were sieved (2 mm) and divided in portions to measure, in triplicates: pH, water holding capacity (WHC), bulk density (BD), water content (WC), organic C (Corg) and N concentrations, Cr, Cu, Ni, and Pb total concentrations, and available fractions.
Soil pH was measured in water suspension (1:2.5 = v:v = soil:water) by the electrometric method (Colombo and Miano, 2015). WC was determined by drying fresh soil at 105°C until reaching a constant weight, and the water holding capacity (WHC) was determined by the gravimetric method according to Aceves et al. (1994). Bulk density (BD) was assayed on undisturbed soil cores of known volume after drying for 48 h at 105°C. Soil Corg, in samples previously treated with HCl (10%), and N concentrations were evaluated by a CN elemental analyzer (Thermo Finnigan).
Total concentrations of Cr, Cu, Ni, and Pb were measured and oven-dried (105°C until constant weight) and grounded (Fritsch Analysette Spartan 3 Pulverisette 0) after acidic digestion (HF 50% and HNO3 65% at 1:2=v: v) in a microwave oven (Milestone mls 1200—Microwave Laboratory Systems). The available metal fractions were extracted with diethylenetriamine pentaacetic acid, CaCl2, and triethanolamine at pH 7.3 ± 0.05 (Lindsay and Norvell, 1978). Metal concentrations were measured by atomic absorption spectrometry, via graphite furnace (SpectrAA 220 FS; Varian, Sidney, Australia).
The biological analyses, performed within a week after sampling, in triplicates, on fresh samples stored at 4°C, were microbial and fungal biomasses and soil basal respiration (BR). The microbial biomass (MB) was evaluated as microbial carbon, according to Anderson and Domsch (1978), by the method of substrate-induced respiration (SIR). SIR was determined using glucose 1% as the substrate and the evolved CO2 in 72 h incubation at 25°C in the dark (Anderson and Domsch, 1978). The evolved CO2 was adsorbed in NaOH and measured by two-phase titration with HCl (Froment, 1972). The fungal biomass (FB) was assayed by membrane filter technique (Sundman and Sivelä, 1978), after staining with Aniline Blue, determining hypha length by the intersection method (Olson, 1950) with an optical microscope (Optika, B-252).
BR was determined by measuring the CO2 evolved in the 10-day incubation at 25°C in the dark (Anderson and Domsch, 1993) and expressed as mg CO2 g−1 d.w.
The soil metabolic quotient, qCO2, was calculated as ratio between the C-CO2 obtained by basal respiration and Cmic (Cheng et al., 1996), and the coefficient of endogenous mineralization, CEM, was calculated as ratio between the CO2 obtained by basal respiration and Corg (Rutigliano et al., 2002).
To test the normality of the data distribution, the Shapiro–Wilk test was performed.
In order to highlight the direct influences of altitude, slope exposure, plant cover, and sampling time on soil abiotic (pH, WC, O.M., C/N, BD, WHC, total and available metal contents) and biotic (MB, FB, BR, qCO2, CEM) characteristics, linear mixed effect models (LME) were performed. For each soil characteristic, the influence of altitude, slope exposure, and plant cover, considered as fixed effects, and of sampling time, considered as random effect, was calculated using restricted maximum likelihood (REML), the better estimation of variance components for the present dataset. The significant impacts and interactions among altitude, slope exposure, plant cover, and sampling time on soil characteristics were calculated with the comparison of models, using the likelihood ratio test with the Anova function (for α = 0.05).
In order to assess the significant impacts of soil abiotic characteristics on soil biotic ones, multiple linear regressions were carried out. The responses of soil biotic properties (MB, FB, BR, qCO2, CEM), defined as dependent variables, to soil abiotic properties (pH, WC, Corg, C/N, BD, WHC, total and available metal contents), defined as independent variables, were tested. Before the linear mixed effect models and the multiple linear regressions' performance, the linearity of the data, the independence, the homogeneity, and the normality of residuals were tested. Only the normality was not always satisfied for some variables, and in that case a logarithmic transformation was done.
The R 3.6.2 programming environment (R Core Team 2016) was used to perform the statistical analyses, considered significant at least for P < 0.05, the linear mixed effect models (lme4 package), and the multiple linear regressions.
The results showed that soil characteristics were mainly dependent on altitude, partially dependent on slope exposure and plant cover, and to a lesser to sampling time (Tables 1–3). In particular, soil pH was high at high altitude (Table 1); WC at low altitude (Table 1); WHC at low altitude, along south exposure and under pine cover (Table 1); and BD at high altitude, along north exposure and under pine cover (Table 1).
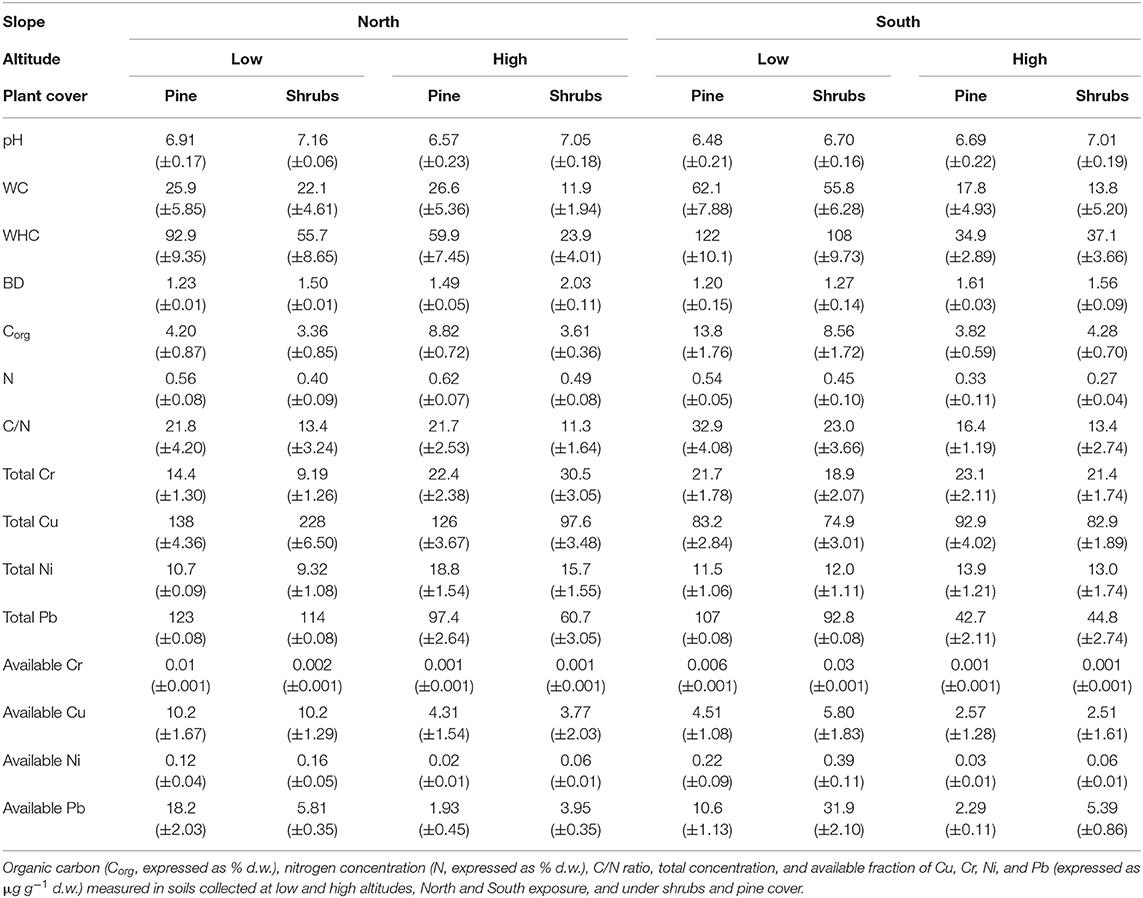
Table 1. Mean values (±s.e.) of pH, water content (WC, expressed as % d.w.), water holding capacity (WHC, expressed as % d.w.), and bulk density (BD, expressed as mg cm−3).
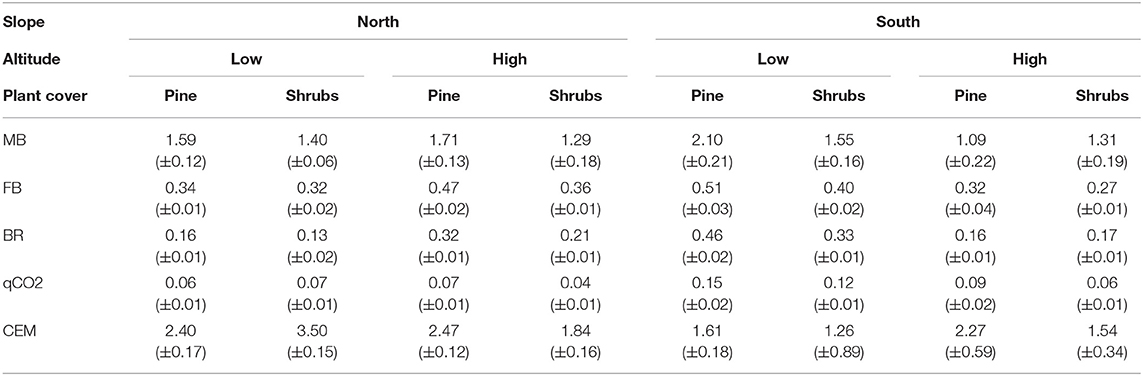
Table 2. Mean values (±s.e.) of microbial biomass (MB, expressed as mg C g−1 d.w.), fungal biomass (FB, expressed as mg g−1 d.w.), basal respiration (BR, expressed as mg CO2 g−1 d.w.), metabolic quotient (qCO2, expressed as mg C-CO2 mg−1 Cmic), and coefficient of endogenous mineralization (CEM, expressed as mg C-CO2 g−1 Corg) measured in soils collected at low and high altitudes, North and South exposure, and under shrubs and pine cover.
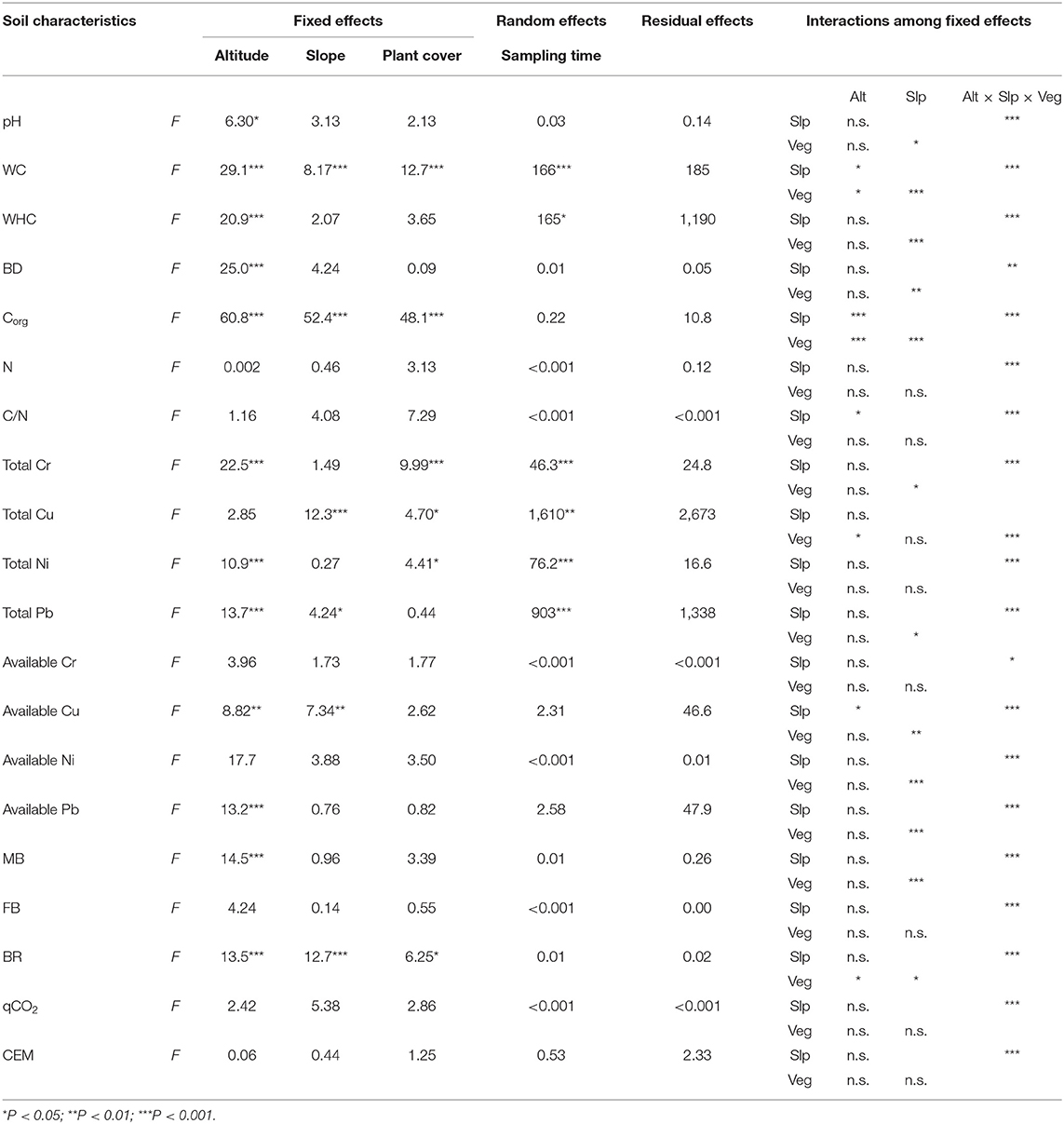
Table 3. Summary of mixed-effect model analyses (F-value: F) among altitude (Alt), slope exposure (Slp), and plant cover (Veg), as fixed effects, and sampling time, as random effects, on abiotic (pH, water content—WC, water holding capacity—WHC, bulk density—BD, organic carbon content—Corg, nitrogen concentration—N, C/N ratio, total concentration and available fraction of Cu, Cr, Ni, and Pb) and biotic (microbial biomass—MB, fungal biomass—FB, basal respiration—BR, metabolic quotient—qCO2, coefficient of endogenous mineralization—CEM) characteristics of soils collected at the Vesuvius National Park. Asterisks indicate significant impacts of fixed effects and their interactions on soil characteristics (Anova test—model comparison).
Soil Corg content was high at low altitude along south exposure and under pine cover (Figure 1); finally, the C/N ratio was high at low altitude and showed wide variability among the soils along south and north exposure and under different plant covers (Figure 1).
Also, soil total heavy metal (Cr, Cu, Ni, and Pb) concentrations highlighted wide variability among all investigated soils (Table 1). The high Cu concentrations were observed in soils collected along south exposure (Table 1). Concerning the heavy metal available fractions, all of them showed high concentrations at low altitude (Table 1); additionally, Cu showed high concentrations in soil collected along south exposure (Table 1).
Among the soil biotic characteristics, MB was high at low altitude (Table 2), FB at low altitude and under pine cover (Table 2), and BR at low altitude and along south exposure (Table 2).
The variability of FB and MB was comparable according to different altitudes, slope exposures, and plant covers (Table 2); instead, BR, qCO2, and CEM mainly showed wide variability particularly in soils collected at low altitude, north exposure, and under pines as compared to those collected, respectively, at high altitude, south exposure, and under shrubs (Table 2).
The linear mixed-effect model showed that soil characteristics were mainly dependent on altitude, partially dependent on slope exposure and plant cover (fixed effects) and to a lesser extent on sampling time (random effects) (Table 3). In particular, altitude significantly influenced the great part of the investigated soil characteristics, with the exception of N, C/N, total Cu concentration, available Cr and Ni concentrations, FB, qCO2, and CEM (Table 3); slope exposure significantly influenced the WC, Corg, total Cu and Pb concentrations, available Cu concentration, and BR (Table 3); and plant cover significantly influenced the WC, Corg, total Cr, Cu, and Ni concentrations, and BR (Table 3). A great part of the WC, WHC, and total Cr, Cu, Ni, and Pb concentration variabilities were also due to the sampling time (Table 3). In addition, the interactions among the altitude, slope exposure, and plant cover significantly influenced all the soil characteristics (Table 3), whereas the interaction between altitude and slope significantly influenced WC, Corg, C/N, and available Cu fraction (Table 3). The interaction between altitude and plant cover significantly influenced WC, Corg, total Cu concentration, and BR (Table 3), whereas the interaction between slope and plant cover significantly influenced pH, WC, WHC, BD, Corg, total Cr and Pb concentration, available Cu, Ni, and Pb fractions, MB, and BR (Table 3). Overall, the influence of altitude, slope exposure, and plant cover on soil abiotic characteristics was higher than that on the biotic ones (Table 3).
The multiple linear regressions highlighted that Cmic was positively correlated with soil N and Corg content and negatively with C/N ratio (Table 4); FB was negatively correlated with soil Cr available fraction and positively with C/N ratio and Ni available fraction (Table 4); BR was positively correlated with soil WHC (Table 4); and qCO2 was positively correlated with soil C/N ratio and negatively to Ni total content (Table 4).
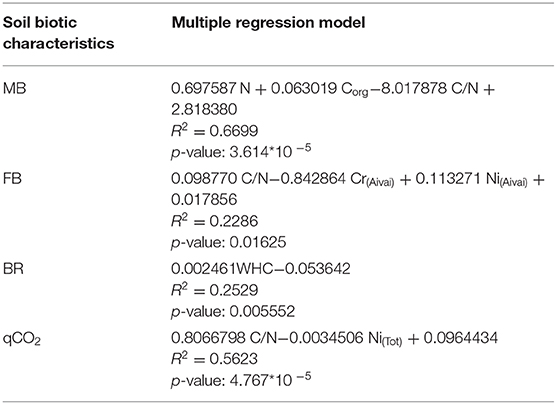
Table 4. Significant results of multiple linear regression analyses of soil biotic (microbial biomass: MB, fungal biomass: FB, basal respiration: BR, metabolic quotient: qCO2) characteristics in relationship with soil abiotic (water holding capacity: WHC, organic carbon: Corg, nitrogen concentration: N, C/N ratio, total and available concentration of Cu, Cr, Ni, and Pb) characteristics.
In the studied area, altitude more than slope exposure and plant cover seems the main discriminating feature in defining directly the characteristics of the investigated soils. In fact, as shown by the linear mixed effect analyses, a significant influence on soil abiotic properties was highlighted according to the different altitudes. In fact, altitude seemed to affect the soil structure and porosity as shown by the highest values of WHC and the lowest values of BD measured in soil at low altitude. The lower BD in soil indicates a higher degree of soil organic matter, good granulation, aeration, and higher infiltration (Dar and Somaiah, 2015; Saeed et al., 2019). The high Corg, observed at low altitude and south exposure, could be due to the greater plant canopy which will be responsible for the falling of a high amount of leaves on soil floor (Hutchins et al., 1976; Kao and Chang, 2001) as well as to the inputs of litter deriving by phenomena of leaching along the slope (Mukai et al., 2016). The Corg observed in soil at low altitude could enhance the soil stabilization (Ruiz-Sinoga et al., 2012) and be the main responsible for the high soil water content and C/N ratio (Tipping et al., 2016). By contrast, the high pH detected at high altitude could be also due to the low values of Corg and humic acids, known to lead to a decrease of soil pH (Finzi et al., 1998).
The observed variability of total and available element concentrations suggested the direct impact of altitude and slope exposure on their distribution. This finding confirms previous studies hypothesizing that the content of elements in the Vesuvius area was influenced by lithogenic factors and microclimatic conditions due to site features (Memoli et al., 2018a, 2019a). However, the high Ni and Cr availability found at low altitude and south exposure could be due also to the high Corg content and the low pH, as the capability of organic compounds to retain soil elements (Vega et al., 2004; Nunes et al., 2014) and the role of low soil pH to enhance element availability are known (Acosta et al., 2010). In addition, the high element availability at low altitude could be also due to the accumulation of soil components, produced at high altitude, which were transported along the slope through leaching phenomena (Acosta et al., 2010). Nevertheless, it cannot be overlooked that sites at low altitude, being nearer suburban areas, could receive by air depositions pollutants rich in potential toxic elements as Pb and Cu (Memoli et al., 2019b), which form weakly binds to soil particles and can become readily available (Massas et al., 2009). The high bulk density observed at high altitudes disagrees with other authors (Saeed et al., 2014, 2019), and it could be due to the low amount of soil Corg (Athira et al., 2019).
Besides altitude and slope exposure, plant covers (namely, shrubs and pines) also play a role in influencing some soil characteristics, such as Corg, WHC, and C/N. Particularly, the soil under pine was richer in Corg than soil under shrubs, as observed in soil at low altitude and at south exposed sites as compared to those at high altitude and north exposure. In addition, pine cover was responsible, as compared to shrub cover, for greater litter accumulation and higher soil water retention. Under pines, the greatest soil Corg corresponded to the highest values of C/N ratio, which indicated an increase of organic matter recalcitrance. In fact, the trend of Corg in soils with different altitudes, exposure, and plant cover was similar to that of the C/N ratio, although this parameter showed significant differences only for soils at different altitudes.
The altitude, slope exposure, and plant cover partially explain the variability of some soil abiotic characteristics, as they were also influenced by the sampling time. In particular, the content of water in soil varied with the sampling time as the precipitation rate could influence the quantity of water in soil. Metal concentrations also can vary with time, as they are influenced by anthropic activities, such as tourism (Memoli et al., 2019b), and by climatic factors, such as wind intensity and direction.
The direct influence of altitude and slope exposure on soil abiotic characteristics indirectly affect the composition and activity of soil microorganisms. In fact, the high soil WC and Corg in the soil at low altitude favored the abundance and activity of microorganisms (both bacteria and fungi), conversely to what occurred at high altitude. The close dependence between the soil microbial biomass and the soil Corg was also confirmed by the multiple linear regressions that showed the positive correlations between MB and Corg and N contents. The findings agreed with those reported by numerous studies that highlighted the fundamental role of organic compounds and N as resources for microorganisms (Aneja et al., 2004; McMahon et al., 2005; Williams et al., 2006). In addition, soil microorganisms are involved in C and N cycles (Zeraatpishe and Khormali, 2012; Aislabie and Deslippe, 2013; Wang et al., 2013) and contribute to organic matter stabilization (Six et al., 2000). Moreover, the results also highlighted that soil water availability and capacity to retain water significantly affected microbial respiration (Wang et al., 2013).
Anyway, although in soil collected at low altitude the microbial biomass was abundant and Corg was available, an inadequate mineralization occurred (i.e., CEM values were similar in the soils both at low and high altitudes). This could be probably due to both the dominance of organic compounds difficult to degrade, as suggested by the high C/N (Yüksek et al., 2013), and the presence of high Ni and Cr available concentrations (Chu, 2018). The increase of the organic matter complexity and recalcitrance (high values of C/N ratio) as well as the availability of potential toxic elements could create stress conditions for the microbial community, measured by high qCO2 values (Mataix-Solera et al., 2002; Panico et al., 2020; Zhao et al., 2020). Although this parameter did not significantly differ in soil at different altitudes, slope exposures, and plant cover, because of the high variability of the collected data, a positive correlation was found between qCO2 and C/N ratio. In addition, the results of multiple linear regressions highlighted that the variations of the soil organic matter quality significantly affected the microbial and fungal biomass. So, the quality of organic matter negatively affected the microbial biomass (Li et al., 2012) and positively the fungal one. The selective role of organic matter quality was particularly evident in soils under pine where its quality affected the composition of microorganisms (Bardgett and van der Putten, 2014; Panico et al., 2020). This was confirmed by the statistically higher FB in soils under pines than under shrubs. Pine litter, in fact, constituted by complex compounds and waxes, favored fungi rather than bacteria (Virzo De Santo et al., 2002), as the former are particularly able to feed on recalcitrant substrates (De Marco et al., 2013b, 2016).
Additionally, as microorganisms are known to be sensitive to the variations of temperature (Kirschbaum, 2006), the higher microbial biomass observed at low altitude could be also due to the occurrence of favorable microclimatic conditions (Memoli et al., 2019a). The results of RDA performed using the soil abiotic and biotic characteristics highlighted similar trends in soil at low altitude and at south exposure. The similar amount of Corg observed between soils at low altitude (15.5% d.w.) and south exposure (15.1% d.w.) could be due to the better microclimatic conditions. In fact, at these sites, the expected warmer conditions together with higher water soil availability (Tamai, 2010; He et al., 2016) could enhance plant productivity and, in turn, litter fall. Soil organic matter and water availability affected the soil microbial community, as it was always significantly correlated with microbial biomass (Hackl et al., 2005) and microbial activity (Wang et al., 2013). In addition, water availability seemed to drive soil community composition (Stefan et al., 2014) as fungi and bacteria differently responded to soil moisture according to other studies (De Vries et al., 2006; Bapiri et al., 2010). These findings could suggest that bacteria and fungi occupy different niches, avoiding competition in using resources (Panico et al., 2020).
The soil fungal biomass was also negatively affected by high Cr availability, showing more sensitivity to this metal as compared to the other ones (Marzaioli et al., 2010). Anyway, the heavy metal effects on the soil microbial community can often be minimized or masked by fluctuations in soil characteristics, mainly Corg, which may contribute to counterbalance the negative effects of heavy metal on the soil microbial community (D'Ascoli et al., 2006).
In the investigated Mediterranean volcanic area, altitude appeared the main factor in influencing soil characteristics. In fact, numerous soil characteristics significantly differed between soils at low and high altitudes. However, also site exposure and plant cover affected some abiotic characteristics.
Organic matter quantity and quality were the main soil abiotic characteristics affected by site altitudes, exposures, and plant covers. These organic matter characteristics associated with high water availability enhanced the fungal rather than the bacterial component of the soil microbial community. However, the content of metals, impacted by site features, played a role in influencing negatively the activity of microorganisms.
An overall evaluation highlighted that, in the studied Mediterranean volcanic area, the altitude and slope exposure have a crucial role in affecting directly soil abiotic characteristics and indirectly the biotic ones.
Finally, the resultant soil–plant cover and soil–environmental feature interrelationships could be more complex than either of the two considered separately and other studies are necessary to expand knowledge especially in Mediterranean ecosystems.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
GM: conceptualization. SP, LS, and AD: writing. SP and LS: statistical analyses. SP, FE, LS, AD, VM, and GM: writing revision. GM, AD, and RB: validation. All authors contributed to the article and approved the submitted version.
This research was funded by the collaboration of the Biology Department of University Federico II of Naples and the Vesuvius National Park within the Azione di Sistema - Impatto antropico da pressione turistica nelle aree protette: interferenze su territorio e biodiversità funded by Ministero dell'Ambiente e della Tutela del Territorio e del Mare, Direttiva Conservazione della Biodiversità Vesuvius.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aceves, M. B., Grace, C., Hart, M., Lin, Q., and Brookes, P. C. (1994). Laboratory Manual of the Soil Microbial Biomass Group. Harpenden: Rothamsted Experimental Station. Soil Science Department, 8–9.
Acosta, J., Faz, A., and Martínez-Martínez, S. (2010). Identification of heavy metal sources by multivariable analysis in a typical Mediterranean city (SE Spain). Environ. Monit. Assess. 169, 519–530. doi: 10.1007/s10661-009-1194-0
Adamo, P., and Zampella, M. (2007). “Trace elements in polluted Italian volcanic soils,” in Soils of Volcanic Regions in Europe, eds Ó. Arnalds, H. Óskarsson, F. Bartoli, P. Buurman, G. Stoops, and E. García-Rodeja (Berlin; Heidelberg: Springer), 51–67
Aislabie, J., and Deslippe, J. R. (2013). “Soil microbes and their contribution to soil services,” in Ecosystem Services in New Zealand-Conditions and Trends, ed J. R. Dymond (Lincoln: Manaaki Whenua Press), 143–161.
Anderson, T. H., and Domsch, K. H. (1978). A physiological method for the quantitative measurements of microbial biomass in soil. Soil Biol. Biochem. 10, 215–221. doi: 10.1016/0038-0717(78)90099-8
Anderson, T. H., and Domsch, K. H. (1993). The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of the soil. Soil Biol. Biochem. 25, 393–395. doi: 10.1016/0038-0717(93)90140-7
Aneja, M. K., Sharma, S., Munch, J. C., and Schloter, M. (2004). RNA fingerprinting - a new method to screen for differences in plant litter degrading microbial communities. J. Microbiol. Methods 59, 223–231. doi: 10.1016/j.mimet.2004.07.005
Athira, M., Jagadeeswaran, R., and Kumaraperumal, R. (2019). Influence of soil organic matter on bulk density in Coimbatore soils. Int. J. Chem. Stud. 7, 3520–3523.
Bach, E. M., Baer, S. G., Meyer, C. K., and Six, J. (2010). Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 42, 2182–2191. doi: 10.1016/j.soilbio.2010.08.014
Bapiri, A., Bååth, E., and Rousk, J. (2010). Drying–rewetting cycles affect fungal and bacterial growth differently in an arable soil. Microb. Ecol. 60, 419–428. doi: 10.1007/s00248-010-9723-5
Bardelli, T., Ascher-Jenull, J., Stocker, E. B., Fornasier, F., Arfaioli, P., Fravolini, G., et al. (2018). Impact of slope exposure on chemical and microbiological properties of Norway spruce deadwood and underlying soil during early stages of decomposition in the Italian Alps. Catena 167, 100–115. doi: 10.1016/j.catena.2018.04.031
Bardgett, R. D., and van der Putten, W. H. (2014). Belowground biodiversity and ecosystem functioning. Nature 515, 505–511. doi: 10.1038/nature13855
Brockett, B. F. T., Prescott, C. E., and Grayston, S. J. (2012). Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 44, 9–20. doi: 10.1016/j.soilbio.2011.09.003
Cheng, W., Zhang, Q., Coleman, D. C., Carroll, C. R., and Hoffman, C. A. (1996). Is available carbon limiting microbial respiration in the rhizosphere? Soil Biol. Biochem. 28, 1283–1288. doi: 10.1016/S0038-0717(96)00138-1
Chu, D. (2018). Effects of heavy metals on soil microbial community. IOP Conf. Ser. Earth Environ. Sci. 113:012009. doi: 10.1088/1755-1315/113/1/012009
Cline, L. C., and Zak, D. R. (2015). Soil microbial communities are shaped by plant-driven changes in resource availability during secondary succession. Ecology 96, 3374–3385. doi: 10.1890/15-0184.1
Colombo, C., and Miano, T. (2015). Metodi di analisi chimica del suolo. Bari: Pubblicità and Stampa Ed.
Dahlgren, R. A., Saigusa, M., and Ugolini, F. C. (2004). The nature, properties and management of volcanic soils. Adv. Agron. 82, 113–182. doi: 10.1016/S0065-2113(03)82003-5
Dar, J. A., and Somaiah, S. (2015). Altitudinal variation of soil organic carbon stocks in temperate forests of Kashmir Himalayas, India. Environ. Monit. Assess. 187:11. doi: 10.1007/s10661-015-4299-7
D'Ascoli, R., Rao, M. A., Adamo, P., Renella, G., Landi, L., Rutigliano, F. A., et al. (2006). Impact of river overflowing on trace element contamination of volcanic soils in south Italy: part II. Soil biological and biochemical properties in relation to trace element speciation. Environ. Pollut. 144, 317–326. doi: 10.1016/j.envpol.2005.11.017
De Marco, A., Arena, C., Giordano, M., and Virzo De Santo, A. (2013a). Impact of the invasive tree Black locust on soil properties of Mediterranean stone pine-holm oak forests. Plant Soil 372, 473–486. doi: 10.1007/s11104-013-1753-6
De Marco, A., Esposito, F., Berg, B., Giordano, M., and Virzo De Santo, A. (2013b). Soil C and N sequestration in organic and mineral layers of two coeval forest stands implanted on pyroclastic material (Mount Vesuvius, South Italy). Geoderma 209–210, 128–135. doi: 10.1016/j.geoderma.2013.06.011
De Marco, A., Fioretto, A., Giordano, M., Innangi, M., Menta, C., Papa, S., et al. (2016). C Stocks in forest floor and mineral soil of two Mediterranean Beech Forests. Forests 7:181. doi: 10.3390/f7080181
De Nicola, F., Maisto, G., and Alfani, A. (2003). Assessment of nutritional status and trace element contamination of holm oak woodlands through analyses of leaves and surrounding soils. Sci. Total Environ. 311, 191–203. doi: 10.1016/S0048-9697(03)00132-3
De Vries, F. T., Hoffland, E., Van Eekeren, N., Brussaard, L., and Bloem, J. (2006). Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 38, 2092–2103. doi: 10.1016/j.soilbio.2006.01.008
Di Gennaro, A., and Terribile, F. (1999). I suoli della provincia di Napoli. Carta 1:75.000. Camera di Commercio Industria Artigianato e Agricoltura di Napoli. GE.PRO.TER.
Egli, M., Natera, M., Mirabella, A., Raimondi, S., Plötze, M., and Alioth, L. (2008). Clay minerals, oxyhydroxide formation, element leaching and humus development in volcanic soils. Geoderma 143, 101–114. doi: 10.1016/j.geoderma.2007.10.020
Finzi, A. C., Canham, C. D., and Van Breemen, N. (1998). Canopy tree-soil interactions within temperate forests: species effects on pH and cations. Ecol. Appl. 8, 447–454. doi: 10.1890/1051-0761(1998)008[0447:CTSIWT]2.0.CO;2
Griffiths, R. P., Madritch, M. D., and Swanson, A. K. (2009). The effects of topography on forest soil properties in the Oregon Cascade Mountains (USA): implications for the effects of climate change on soil properties. For. Ecol. Manage. 257, 1–7. doi: 10.1016/j.foreco.2008.08.010
Hackl, E., Pfeffer, M., Donat, C., Bachmann, G., and Zechmeister-Boltenstern, S. (2005). Composition of the microbial communities in the mineral soil under different types of natural forest. Soil Biol. Biochem. 37, 661–671. doi: 10.1016/j.soilbio.2004.08.023
He, X., Hou, E., Liu, Y., and Wen, D. (2016). Altitudinal patterns and controls of plant and soil nutrient concentrations and stoichiometry in subtropical China. Sci. Rep. 6:24261. doi: 10.1038/srep24261
Högberg, M. N., Högberg, P., and Myrold, D. D. (2007). Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees, or all three? Oecologia 150, 590–601. doi: 10.1007/s00442-006-0562-5
Hutchins, R. L., Hill, J. D., and White, E. H. (1976). The influence of soil and microclimate on vegetation of forested slopes in eastern Kentucky. Soil Sci. 121, 234–241. doi: 10.1097/00010694-197604000-00008
Iovieno, P., Alfani, A., and Bååth, E. (2010). Soil microbial community structure as affected by Pinus pinea plantation into Mediterranean areas. Appl. Soil Ecol. 45, 56–63. doi: 10.1016/j.apsoil.2010.02.001
IUSS Working Group WRB (2014). World Reference Base for Soil Resources 2014. Update 2015. World Soil Resources Reports No. 106. Rome: FAO. Available online at: http://www.fao.org/3/i3794en/I3794en
Kao, W. Y., and Chang, K. W. (2001). Altitudinal trends in photosynthetic rate and leaf properties of miscanthus populations from central Taiwan. Aust. J. Bot. 49:, 509–514. doi: 10.1071/BT00028
Kirschbaum, M. U. F. (2006). The temperature dependence of organic-matter decomposition - still a topic of debate. Soil Biol. Biochem. 38, 2510–2518. doi: 10.1016/j.soilbio.2006.01.030
Lemenih, M., and Itanna, F. (2004). Soil carbon stocks and turnovers in various vegetation type and arable lands along an elevation gradient in Southern Ethiopia. Geoderma 123, 177–188. doi: 10.1016/j.geoderma.2004.02.004
Li, D., Sharp, J. O., Saikaly, P. E., Ali, S., Alidina, M., Alarawi, M. S., et al. (2012). Composition and diversity in managed aquifer recharge systems. Appl. Environ. Microbiol. 78, 6819–6828. doi: 10.1128/AEM.01223-12
Lilienfein, J., Qualls, R. G., Uselman, S. M., and Bridgham, S. D. (2003). Soil formation and organic matter accretion in a young andesitic chronosequence at Mt. Shasta, California. Geoderma 116, 249–264. doi: 10.1016/S0016-7061(03)00086-7
Lindsay, W. L., and Norvell, W. A. (1978). Development of a DTPA soil test for zinc, iron, manganese, and copper. SSSAJ 42, 421–428. doi: 10.2136/sssaj1978.03615995004200030009x
Liu, S., Ke-Ming, M. A., Bo-Jie, F., Yong-Xiang, K., Jie-Yu, Z., and Yu-Xin, Z. (2003). The Relationship between landform, soil properties and plant community structure in the Donglingshan Mountain Region, Beijing. Chin. J. Plant Ecol. 27, 496–502. doi: 10.17521/cjpe.2003.0072
Lucas-Borja, M. E., Candel, D., Jindo, K., Moreno, J., Andrés, M., and Bastida, F. (2012). Soil microbial community structure and activity in monospecific and mixed forest stands, under Mediterranean humid conditions. Plant Soil 354, 359–370. doi: 10.1007/s11104-011-1072-8
Maisto, G., De Nicola, F., Iovieno, P., Prati, M. V., and Alfani, A. (2006). PAHs and trace elements in volcanic urban and natural soils. Geoderma 136, 20–27. doi: 10.1016/j.geoderma.2006.01.009
Maisto, G., Manzo, S., De Nicola, F., Carotenuto, R., Rocco, A., and Alfani, A. (2011). Assessment of the effects of Cr, Cu, Ni and Pb soil contamination by ecotoxicological tests. J. Environ. Monit. 13, 3049–3056. doi: 10.1039/c1em10496a
Marzaioli, R., D'Ascoli, R., De Pascale, R. A., and Rutigliano, F. A. (2010). Soil microbial community as affected by heavy metal pollution in a Mediterranean area of Southern Italy. Freshw. Environ. Bull. 19, 2411–2419.
Massas, I., Ehaliotis, C., Gerontidis, S., and Sarris, E. (2009). Elevated heavy metal concentrations in top soils of an Aegean island town (Greece): total and available forms, origin and distribution. Environ. Monit. Assess. 151, 105–116. doi: 10.1007/s10661-008-0253-2
Mataix-Solera, J., Gòmez, I., Navarro-Pedreño, J., Guerrero, C., and Moral, R. (2002). Soil organic matter and aggregates affected by wildfire in a Pinus halepensis forest in a Mediterranean environment. Int. J. Wildland Fire 11, 107–114. doi: 10.1071/WF02020
McCulley, R. L., and Burke, I. C. (2004). Microbial community composition across the great plains: landscape vs. regional variability. SSSAJ 68, 106–115. doi: 10.2136/sssaj2004.1060
McMahon, S. K., Williams, M. A., Bottomley, P. J., and Myrold, D. D. (2005). Dynamics of microbial communities during decomposition of carbon-13 labeled ryegrass fractions in soil. SSSAJ 69, 1238–1247. doi: 10.2136/sssaj2004.0289
Memoli, V., De Marco, A., Esposito, F., Panico, S. C., Barile, R., and Maisto, G. (2019a). Seasonality, altitude and human activities control soil quality in a national park surrounded by an urban area. Geoderma 337, 1–10. doi: 10.1016/j.geoderma.2018.09.009
Memoli, V., Esposito, F., Panico, S. C., De Marco, A., Barile, R., and Maisto, G. (2019b). Evaluation of tourism impact on soil metal accumulation through single and integrated indices. Sci. Total Environ. 682, 685–691. doi: 10.1016/j.scitotenv.2019.05.211
Memoli, V., Eymar, E., García-Delgado, C., Esposito, F., Panico, S. C., De Marco, A., et al. (2018b). Soil element fractions affect phytotoxicity, microbial biomass and activity in volcanic areas. Sci. Total Environ. 636, 1099–1108. doi: 10.1016/j.scitotenv.2018.04.327
Memoli, V., Eymar, E., García-Delgado, C., Esposito, F., Santorufo, L., De Marco, A., et al. (2018a). Total and fraction content of elements in volcanic soil: natural or anthropogenic derivation. Sci. Total Environ. 625, 16–26. doi: 10.1016/j.scitotenv.2017.12.223
Mendes, L. W., Kuramae, E. E., Navarrete, A. A., van Veen, J. A., and Tsai, S. M. (2014). Taxonomical and functional microbial community selection in soybean rhizosphere. ISME J. 8, 1577–1587. doi: 10.1038/ismej.2014.17
Menyailo, O. V., Hungate, B. A., and Zech, W. (2002). Tree species mediated soil chemical changes in a Siberian artificial afforestation experiment. Plant Soil 242, 171–182. doi: 10.1023/A:1016290802518
Mukai, M., Aiba, S., and Kitayama, K. (2016). Soil-nutrient availability and the nutrient-use efficiencies of forests along an altitudinal gradient on Yakushima Island, Japan. Ecol. Res. 31, 719–730. doi: 10.1007/s11284-016-1381-8
Nunes, J. R., Ramos-Miras, J., Lopez-Piñeiro, A., Loures, L., Gil, C., Coelho, J., et al. (2014). Concentrations of available heavy metals in mediterranean agricultural soils and their relation with some soil selected properties: a case study in typical Mediterranean soils. Sustainability 6, 9124–9138. doi: 10.3390/su6129124
Olson, F. C. W. (1950). Quantitative estimates of filamentous algae. Trans. Am. Microsc. Soc. 69, 272–279. doi: 10.2307/3223098
Panico, S. C., Ceccherini, M. T., Memoli, V., Maisto, G., Pietramellara, G., Barile, R., et al. (2020). Effects of different vegetation types on burnt soil properties and microbial communities. Int. J. Wildland Fire. 29, 628–636. doi: 10.1071/WF19081
Rajala, T., Peltoniemi, M., Pennanen, T., and Mäkipää, R. (2012). Fungal community dynamics in relation to susbstrate quality of decaying Norway spruce (Picea abies [L.] Karst.) logs in boreal forests. FEMS Microbiol. Ecol. 81, 494–505. doi: 10.1111/j.1574-6941.2012.01376.x
Ruiz-Sinoga, J. D., Diaz, A. R., Bueno, E. F., and Martínez-Murillo, J. F. (2010). The role of soil surface conditions in regulating runoff and erosion processes on a metamosphic hillslope (Southern Spain). Soil surface conditions, runoff and erosion in Southern Spain. Catena 80, 131–139 doi: 10.1016/j.catena.2009.09.007
Ruiz-Sinoga, J. D., Gabarón Galeote, M. A., Martínez Murillo, J. F., and Garcia Marín, R. (2011). Vegetation strategies for soil consumption along a pluviometric gradient in southern Spain. Catena 84, 12–20. doi: 10.1016/j.catena.2010.08.011
Ruiz-Sinoga, J. D., Pariente, S., Romero Díaz, A., and Martínez Murillo, J. F. (2012). Variability of relationships between soil organic carbon and some soil properties in Mediterranean rangelands under different climatic conditions (South of Spain). Catena 94, 17–25. doi: 10.1016/j.catena.2011.06.004
Rutigliano, F. A., Castaldi, S., D'Ascoli, R., Papa, S., Carfora, A., Marzaioli, R., et al. (2009). Soil activities related to nitrogen cycle under 3 plant cover types in Mediterranean environment. Appl. Soil Ecol. 43, 40–46. doi: 10.1016/j.apsoil.2009.05.010
Rutigliano, F. A., D'Ascoli, R., De Marco, A., and De Santo, A. V. (2002). “Soil microbial community as influenced by experimental fires of different intensities,” in Fire and Biological Processes, eds L. Trabaud and R. Prodon (Leiden: Backhuys Publishers), 137–150.
Saeed, S., Sun, Y., Beckline, M., Chen, L., Lai, Z., Mannan, A., et al. (2019). Altitudinal gradients and forest edge effect on soil organic carbon in Chinese fir (Cunninghamia lanceolata): a study rom southeastern China. Appl. Ecol. Environ. Res. 17, 745–757. doi: 10.15666/aeer/1701_745757
Saeed, S., Younus, M., Barozai, M. Y., Ahmed, A., and Shah, S. (2014). Impact of Altitude on soil physical and chemical properties in Sra Ghurgai (Takatu mountain range) Quetta, Balochistan. Int. J. Eng. Sci. 5, 730–735.
Santacroce, R. (1987). Somma-Vesuvius, Progetto Finalizzato Geodinamica, Monografie Finali. 1-251 CNR, Quaderni de la Ricerca Scientifica, 114.
Santacroce, R., and Sbrana, A. (2003). Geological Map of Vesuvius at Scale 1: 15,000. Florence: SELCA.
Sardans, J., and Peñuelas, J. (2013). Plant-soil interactions in Mediterranean forest and shrublands: impacts of climatic change. Plant Soil 365, 1–33. doi: 10.1007/s11104-013-1591-6
Seibert, J., Stendahl, J., and Sørensen, R. (2007). Topographical influences on soil properties in boreal forests. Geoderma 141, 139–148. doi: 10.1016/j.geoderma.2007.05.013
Six, J., Elliott, E. T., and Paustian, K. (2000). Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 32, 2099–2103. doi: 10.1016/S0038-0717(00)00179-6
Stefan, G., Cornelia, B., Jörg, R., and Michael, B. (2014). Soil water availability strongly alters the community composition of soil protists. Pedobiologia 57, 205–213. doi: 10.1016/j.pedobi.2014.10.001
Sundman, V., and Sivelä, S. (1978). A comment on the membrane filter technique for estimation of length of fungal hyphae in soil. Soil Biol. Biochem. 10, 399–401. doi: 10.1016/0038-0717(78)90065-2
Swallow, M., Quideau, S. A., MacKenzie, M. D., and Kishchuk, B. E. (2009). Microbial community structure and function: the effect of silvicultural burning and topographic variability in northern Alberta. Soil Biol. Biochem. 41, 770–777. doi: 10.1016/j.soilbio.2009.01.014
Tajika, S., Ayoubia, S., and Lorenz, N. (2020). Soil microbial communities affected by vegetation, topography and soil properties in a forest ecosystem. Appl. Soil Ecol. 149:103514. doi: 10.1016/j.apsoil.2020.103514
Tamai, K. (2010). Effects of environmental factors and soil properties on topographic variations of soil respiration. Biogeosciences 7, 1133–1142. doi: 10.5194/bg-7-1133-2010
Thakur, M. P., Milcu, A., Manning, P., Niklaus, P. A., Roscher, C., Power, S., et al. (2015). Plant diversity drives soil microbial biomass carbon in grasslands irrespective of global environmental change factors. Global Change Biol. 21, 4076–4085. doi: 10.1111/gcb.13011
Tipping, E., Somerville, C. J., and Luster, J. (2016). The C:N:P:S stoichiometry of soil organic matter. Biogeochemistry 130, 117–131. doi: 10.1007/s10533-016-0247-z
Torn, M. S., Trumbore, S. E., Chadwick, O. A., Vitousek, P. M., and Hendricks, D. M. (1997). Mineral control of soil organic carbon cycling. Nature 389, 170–173. doi: 10.1038/38260
Tsui, C. C., Chen, Z. S., and Hsie, C. F. (2004). Relationships between soil properties and slope position in a lowland rain forest of southern Taiwan. Geoderma 123, 131–142. doi: 10.1016/j.geoderma.2004.01.031
Vacchiano, G., Magnani, F., and Collati, A. (2012). Modelling Italian forests: state of the art and future challenges. IFOREST 5, 235–246. doi: 10.3832/ifor0614-005
Vega, F., Covelo, E., Andrade, M., and Marcet, P. (2004). Relationships between heavy metals content and soil properties in minesoils. Anal. Chim. Acta 524, 141–150. doi: 10.1016/j.aca.2004.06.073
Virzo De Santo, A., Rutigliano, F. A., Berg, B., Fioretto, A., Puppi, G., and Alfani, A. (2002). Fungal mycelium and decomposition of needle litter in three contrasting coniferous forests. Acta Oecologica 23, 247–259. doi: 10.1016/S1146-609X(02)01155-4
Wang, J., Wang, H., Cao, Y., Bai, Z., and Qin, Q. (2016). Effects of soil and topographic factors on vegetation restoration in opencast coal mine dumps located in a loess area. Sci. Rep. 6, 1–11. doi: 10.1038/srep22058
Wang, Q., He, T., Wang, S., and Liu, L. (2013). Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agric. For. Meteorol. 178–179, 152–160. doi: 10.1016/j.agrformet.2013.04.021
Williams, M. A., Myrold, D. D., and Bottomley, P. J. (2006). Carbon flow from 13C-labeled straw and root residues into the phospholipid fatty acids of a soil microbial community under field conditions. Soil Biol. Biochem. 38, 759–768. doi: 10.1016/j.soilbio.2005.07.001
Yüksek, F., Altun, L., Karaöz, Ö., Sengönül, K., Yüksek, T., and Küçük, M. (2013). “The effect of altitude on soil properties and leaf traits in wild Vaccinium arctostaphylos L. populations in the forest understory in Firtina river basin,” in International Caucasian Forestry Symposium (Artvin).
Zeraatpishe, M., and Khormali, F. (2012). Carbon stock and mineral factors controlling soil organic carbon in a climatic gradient, Golestan province. J. Soil Sci. Plant Nutr. 12, 637–654. doi: 10.4067/S0718-95162012005000022
Keywords: plant cover, altitude, exposure, volcanic soils, microbial community, microbial activity
Citation: Panico SC, Memoli V, Santorufo L, Esposito F, De Marco A, Barile R and Maisto G (2021) Linkage Between Site Features and Soil Characteristics Within a Mediterranean Volcanic Area. Front. For. Glob. Change 3:621231. doi: 10.3389/ffgc.2020.621231
Received: 25 October 2020; Accepted: 30 December 2020;
Published: 11 February 2021.
Edited by:
Jeff Allen Hatten, Oregon State University, United StatesReviewed by:
Jörg Luster, Swiss Federal Institute for Forest, Snow and Landscape Research (WSL), SwitzerlandCopyright © 2021 Panico, Memoli, Santorufo, Esposito, De Marco, Barile and Maisto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Santorufo, bHVjaWEuc2FudG9ydWZvQHVuaW5hLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.