- 1Agroforestry Study Group, Faculty of Agriculture, Brawijaya University, Malang, Indonesia
- 2World Agroforestry (ICRAF), Bogor, Indonesia
- 3Plant Production Systems, Wageningen University and Research, Wageningen, Netherlands
Volcanic eruptions disturb vegetation at a time it is needed for preventing mudflows. A resilient indigenous non-legume nitrogen-fixing tree that is adapted to the ash and spreads rapidly protects areas downstream in a volcanic landscape in Indonesia. Within the volcanic ring of fire both the long-term benefits (including densely populated, fertile agricultural soils) and short-term ecological disturbance of volcanic ash deposition are clear. Mount Kelud in East Java has erupted on a 15–37-years cycle for the past centuries, most recently in 2014, causing damage to settlements, agricultural land, agroforestry, and watershed protection forests, as the ash deposits caused tree mortality, restricted infiltration, and led to ash flows. Rapid “restoration” or recovery of tree-based vegetation with planted Legume trees (such as Calliandra spp.) has been attempted but is not very effective. However, the non-legume nitrogen-fixing Parasponia rigida, symbiotic with rhizobium bacteria, contrasted to its non-symbiotic sibling (Trema orientalis) has been studied in laboratory conditions, but not in its native environment. We mapped and sampled P. rigida in various locations (upper, middle, and lower elevation positions in ridge-slope-valley toposequences) on the Kelud complex starting 1 year after the latest eruption, estimated biomass development, and quantified P. rigida root nodules in relation to N availability in the ash/soil mixtures in these locations. P. rigida was found as a pioneer tree at elevations between 600 and 1,700 m a.s.l. (above sea level) along ridges, in slope, and valley positions. At lower elevations T. orientalis dominated. Within 3 years of the eruption, stem diameters were 3–10 cm. Up to 93% of P. rigida root nodules were found to be effective, based on the hemoglobin color on cross-sections. Rhizobium bacteria were found in root nodule tissue at densities of two to a hundred times higher than in rhizosphere soil. Between a total soil N content from 0.01 to 0.04% the density of effective nodules decreased from 1,200 to 200 m−2. P. rigida stands in the area, especially at ridges close to the crater deserve to be managed proactively as future seed sources, given the high frequency of eruption episodes, while recovery after eruptions on similar volcanoes can likely be facilitated by tactical assisted seed dispersal if effective seed collection and storage methods can be established.
Introduction
Indonesia has more than 100 active volcanoes, and many more that are dormant, as it is part of the global ring of fire linked to tectonic plate subduction zones. Densely populated Java and Bali reflect both the long-term soil fertility benefits of volcanic ash and a large number of people at risk during fresh eruptions, ash deposition, and lahar flows (Van Ranst et al., 2004; Achmad and Hadi, 2015). Volcanoes are regularly resetting the clock on vegetation succession and create a need for “restoration” when downstream impacts such as mudflows of unconsolidated ash is to be controlled (van Noordwijk et al., 2020). Beyond scenic beauty, volcanoes are also home to a limited, but specialized flora, contributing to the overall biodiversity of the country. The short-term damage of eruptions involves settlements, access roads, agricultural lands, agroforestry, and watershed protection forests, and calls for remedial disaster responses. Human resilience is challenged by damaged infrastructure, loss of boundaries of land ownership, destruction of forests, disturbed water catchment areas, and springs (Rahayu et al., 2014). Can a resilient local tree, adapted to volcanic ash environments be of help? Current emergency preparedness documents in Indonesia are not aware of any such tree.
Atmospheric deposits after a volcanic eruption can consist of ash, sand, gravel, or stones with the lighter materials traveling further. Ash deposits change the physical, chemical, and biological properties of the soil surface (Achmad and Hadi, 2015). Thick layers of ash can cause a dense, cement-like soil surface after rainfall (Suriadikarta et al., 2010). Emergent hydrophobicity decreases infiltration below what is expected given the substrate's porosity and leads to a dry soil environment (Rudianto et al., 2017), with low levels of organic C and N and a soil pH that can be acidic, neutral, or alkaline depending on SiO2 levels in the volcano's substrate (McGeary et al., 2002). Regrowth of vegetation is hindered by a challenging soil environment (Sinaga et al., 2015), but also by a lack of viable seed supply, an absence of biological dispersal agents and shelter for seedlings; regrowth from stumps is possible, however, outside of the primary deposition zones. Lack of vegetation regrowth leads to the high mobility of the ash within the landscape and accumulations in the riverbeds, causing further problems downstream, but also opportunities for collecting “volcanic sands” that are a preferred resource for the building industry and allow former farmers a temporary source of income. Rapid recovery of vegetation, especially on the higher slopes is desirable to support the recovery of the village economy, however.
The Kelud eruption (East Java, also commonly spelled as Kelut) on 13 February 2014 was one in a long series of recorded eruptions and ash deposits with return times of 15–37 years (with ash deposits in intervening years as well): 1826, 1848, 1864, 1901, 1919, 1951, 1966, 1990 (Thouret et al., 1998) and a relatively minor event in 2007. These relatively short return times may be part of the local selection of species that rapidly recover. In 2014 the top of the plume reached to a height of nearly 30 km and the umbrella cloud spread radially at 17−20 km high; ash was recorded up to Bogor, West Java; due to the prevailing wind, there were large deposits on the north and northeast area of the volcano (Suzuki et al., 2014; Kristiansen et al., 2015). The 2014 eruption deposited about 50 × 106 m3 of material on the upper slope of Kelud Volcano. A considerable part of this was washed down by rainfall in mudflows along the rivers and deposited downstream where the riverbed widened (Dibyosaputro et al., 2015). This was not the first time this happened, and part of the vegetation may be adapted to these circumstances. The German explorer Junghuhn had in 1,844 collected a tree specimen on Mt Kelud from which Miquel (1859) described the species as belonging to the genus Parasponia, as it closely resembled Sponia, now known as Trema. Local names confirm the close resemblance, with Trema known as “anggrung”, and Parasponia as its greener sibling “anggrung hijau”. Clason (1935) exploring the vegetation of the mountain slopes and valleys after the Mount Kelud eruption of 1919, reported Parasponia especially from the volcanic ash and lahar valley, while Trema grew more frequently in places where the original soil, “although probably more or less sterilized” had persisted after the eruption. Clason (1935) mentioned in passing that “Parasponia possesses root nodules, nitrogenous food being possibly obtained in this way so that Parasponia is thus adapted as a pioneer type to virgin soil.” However, it took another 40 years before this observation was noticed, although Smiet (1992) described Parasponia as a common part of Java's mountain flora. Trinick (1973), Akkermans et al. (1978), Trinick (1979), and Becking (1979) established the exception to the rule that only the Leguminosae can associate with rhizobium bacteria. The genus Parasponia (in the Cannabaceae family, formerly seen as part of the Ulmaceae) that is known from volcanic ash environments in Indonesia and the Philippines can form effective nodules with rhizobium bacteria (Bradyrhizobium species according to Trinick and Hadobas, 1989). Due to its more open canopy, Parasponia stands allow the development of a dense layer of the grass Saccharum, which in turns prevents other late-successional trees to establish. “It would seem that the Parasponia-socion has already attained its greatest development and that it does not hold its own; in any case, I saw very few young trees and on the other hand several old trees which had died off. It seems as if Parasponia became established before the edaphic conditions allowed the development of the Saccharum-socion.” This description suggests that Parasponia occupies the “regeneration niche” (Grubb, 1977; Pickett and White, 2013), being able to establish itself rapidly in the extreme conditions that prevail after a recent ash deposition event (given the short return period of eruptions), but by enriching the ash deposits with nitrogen, paves the way for grasses to take over, which in turn delay succession to other woody vegetation. A deeper understanding of these ecological relations in the field and aspects of the “regeneration niche” is warranted, as the unique volcanic ash environment in which Parasponia is evolving may account for its unique properties.
A productive line of research has explored the molecular biology of Parasponia-rhizobium interactions and the evolutionary interpretation of such interactions as either independently (re)discovered in multiple plant families or lost from a large number of plant families that are otherwise related to both rhizobium hosts (Geurts et al., 2012; van Velzen et al., 2018). While many Leguminosae are not only able to initiate N2-fixation by rhizobium but also down-regulate it when there is sufficient nitrogen in its internal circulation system, there still is debate about the ability of Parasponia to do the same in laboratory test conditions (Vassey et al., 2005; Op den Camp et al., 2012; Yulia, 2013). Dupin et al. (2020) recently showed that in lab conditions exogenous fixed-nitrogen inhibits nodulation on P. andersonii. Much less is known on the ecology of the species in its native environment. From the existing evidence and literature, it appears that Parasponia evolved in the specific “regeneration niche” of volcanic ash where access to a nitrogen source is essential for early establishment but may pave the way, by enriching the soil, for more competitive non-nodulating sibling (or unrelated) species.
The specific research questions for our exploration of vegetation on Mount Kelud after the 2014 eruption were:
1) Has the most recent eruption had different effects on the populations of Trema and Parasponia? We quantified the composition of the recovering vegetation across the slope, with specific attention to the relative share of the two sibling species.
2) Which aspects of landscape position were related to biomass recovery? We compared seedling, sapling, pole, tree populations and biomass estimates with soil properties in toposequences at three elevation zones
3) Is (effective) nodulation of P. rigida in the regrowth stage related to soil nitrogen levels? We tested the hypothesis that down-regulation of N2 fixation is absent in this early stage of an evolving symbiosis with Rhizobium, leading to soil enrichment.
In relation to options for enhancing natural regeneration to restore landscapes, we will discuss the opportunities Parasponia provides to existing disaster preparedness plans for the area (and similar volcanoes elsewhere in Indonesia) to embrace a more pro-active vegetation management protecting seed sources in the highest zone.
Materials and Methods
Study Area
The research focused on the northeast side of Mount Kelud (Figure 1), where most of the ash of the 2014 eruption was deposited. The satellite imagery for the area (Figure 1) compares the land cover before the eruption (peaking 14 Feb 2014, BNPB, 2014) that covered an area at 5–10 km from the crater with ash. Vegetation at a larger distance from the crater recovered, despite some tree mortality, while regrowth closer to the crater had to start from either seeds or stumps.
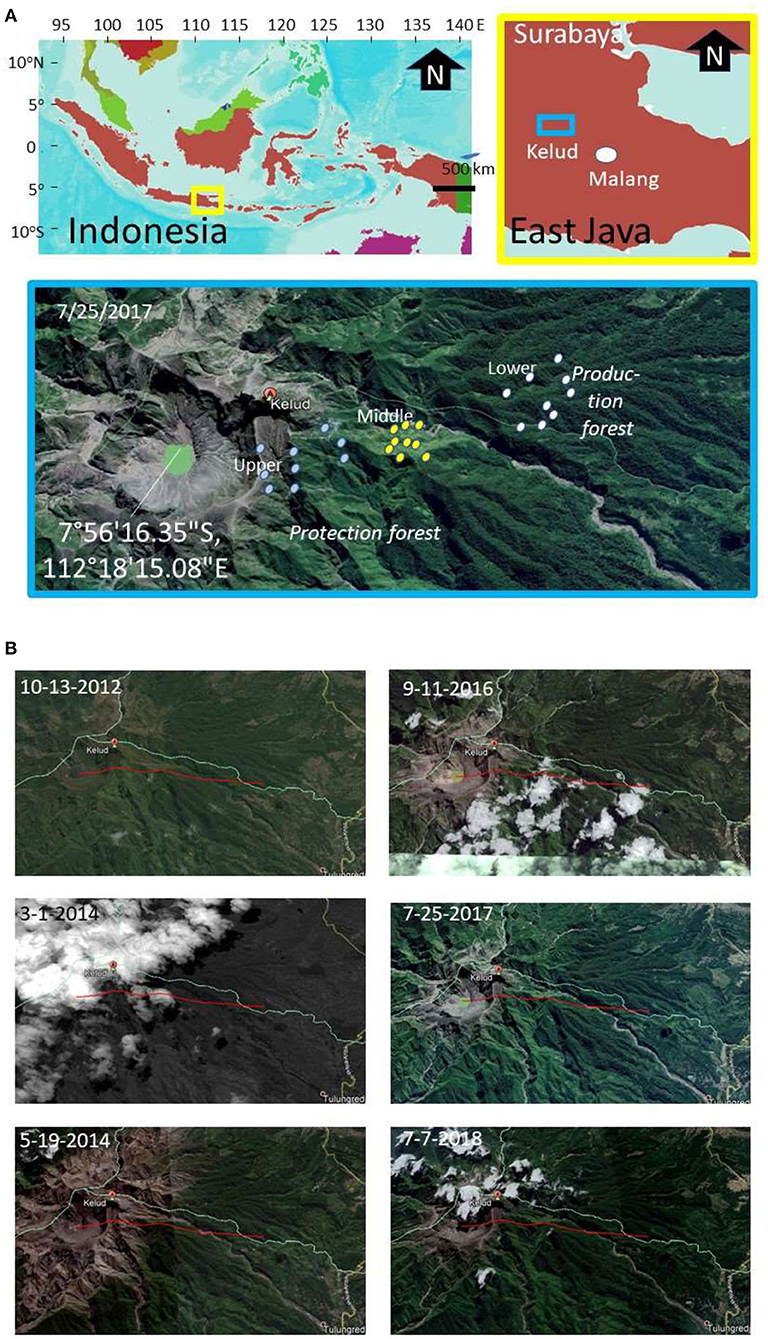
Figure 1. (A) Location of Mount Kelud (East Java, Indonesia), (B) with satellite imagery before (2012), directly after (March 2014), and in recovery phase from the 2014 eruption and the position of sampling points in lower, middle and upper elevational zone; the red line indicates a 5 km transect.
The main access to the mountain slopes we used was via the Kutut Pandansari village (7°54'35.74”S, 112°22'0189”E) in the area downstream of the Selorejo reservoir in Ngantang district (Malang Regency). A path toward the crater passes through three elevation zones: lower (700–800 m a.s.l.), middle (900–1,000 m a.s.l.), and upper (>1,000 m a.s.l.) (Figure 1). The steeper slopes of Mount Kelud are classified as “watershed protection” forest, with lower parts classified as production forest, both managed by the State Forest Company (Perum Perhutani).
The research activity was carried out in 2 stages: Phase 1 focused on vegetation analysis 1 year after the eruption (July—December 2015), and Phase 2 on Parasponia nodulation was carried out 2 years after the eruption (July—November 2017), with some follow-up observations at later dates.
Data Collection
Observation Plots for Vegetation Analysis
Observation plots were selected for exploring the distribution of pioneer plant vegetation in various landscape positions on the slopes of Mount Kelud starting from Mahogany plantations in the lower zone (700–800 m a.s.l.), mixed vegetation at the middle (900–1,000 m a.s.l.), and upper zones (>1,000 m a.s.l.) using a survey scheme of plants on the slopes of Kelud mountain (Smiet, 1992). In each toposequence an East-West transect was sampled, with ridge (8–15% slope), mid-slope (40–60% slope), and valley (0–3% slope) positions. Stand density was determined within patches of vegetation, not randomly sampling the landscape.
Starting 2 years after the eruption, stem diameter and tree biomass were measured for P. rigida in various landscape positions: zones (upper, middle, and lower) and for a toposequence of ridge, slope, and valley, as before.
Soil Analysis
In phase 1, soil samples were collected from each observation plot from soil depths of 0–10, 10–20, and 20–30 cm, respectively; soil samples per layer were composited from five points per plot. Dried soil samples were used for the analysis of total organic carbon (Corg) using the Walkley and Black (1934) method, total nitrogen (Ntot) with a Kjeldahl procedure (Bremner, 1960), pH(H2O), and soil texture as standard t the Chemical Laboratory, Soil Science Department, Faculty of Agriculture, Brawijaya University, Malang. Separate samples were taken for the determination of the soil bulk density (Hairiah et al., 2011).
In phase 2, soil samples were taken at 50 cm distance from the main stem of P. rigida at a depth of 0–10, 10–20, and 20–30 cm were taken and analyzed for Corg, Ntot (Kjeldahl), and mineral NH4 and NO3 concentrations in extracted soil solution using a colorimetric flow-injection autoanalyzer.
Vegetation
Observation of vegetation was carried out on all types of vegetation in various growth stages (seedlings, saplings, poles, and trees). Sample observation plots were nested within a 100 × 20 m main plot (Kusmana, 1997), with five plots of 20 × 20 m used to measure trees (D > 10 cm; D = diameter at 1.3 m above the ground, also known as diameter at breast height) (trees), subplots of 10 m × 10 m used for poles (5 < D <10 cm), sub-sub plots of 5 × 5 m for saplings (D < 5 cm, H > 2 m), and sub-sub-sub plots of 2 × 2 m for seedlings (H < 2 m).
Assessment of the Effectiveness of Root Nodules
Root nodules were sampled by sorting through all soil in a 50 × 50 × 10 cm depth sample adjacent to 10 trees that were considered representative of the stand (avoiding the largest and smallest 20% of the distribution as found in the stand). All root nodules were separated from the roots, counted, and stored in 70% alcohol. All nodules were observed under a dissecting microscope for hemoglobin color on a cross-section. Red hemoglobin was taken as an indicator of effectiveness, white nodules were classified as non-effective (Sarasawati, 2007).
Additional fresh nodule samples and rhizosphere soil adherent to roots was also collected for quantification of rhizobium density, via a dilution series and plating on petri dishes kept at room temperature (around 20°C) for 7 days to estimate the number of Colony Forming Units (CFU). Details of the method are described by Sarasawati (2007). The (autoclaved at 250°C) growth medium consisted of Potato Dextrose Agar (7.8 g), Mannitol (2 g), Yeast extract (0.2 g), K2HPO4 (0.1 g), MgSO4 (0.04 g), NaCl (0,1 g) in 200 ml distilled water, with Congo Red (0.25 g) added as indicator. The highest dilution that still showed bacterial colony formation was taken as an indicator of the rhizobium concentration in the original sample, with calculations specified in Sarasawati (2007).
Parasponia's Taxonomic Position
The GlobalTreeSearch (Beech et al., 2017) mentions only one Parasponia species for Indonesia P. rigida Merr. & Perry (Hassler, 2019). In the Kew Garden plant list, all species in the genus Parasponia are indicated as “unresolved.” Part of the literature on Indonesia [including Ishaq et al. (2020) refers to P. andersonii Planch. which has been confirmed for islands in the Pacific. Part of recent taxonomic interpretation places the species into the genus Trema (which has Sponia, to which Parasponia refers, as a synonym), as T. rigida (Merr. & L.M. Perry) Byng & Christenh. Pending this taxonomic debate, we will here use P. rigida as the botanically correct name for the tree species found on Mount Kelud.
Data Analyses
Pedotransfer as Reference Values for Soil Parameters
A reference value Cref for the Corg concentration expected under long-term forest conditions for a soil of the same texture and pH at the same elevation (with additional factors for Andisol and Wetland conditions) was used, based on van Noordwijk et al. (1998) and Hairiah et al. (2020), including a depth correction for a soil layer from ZH to ZL cm depth:
with elevation expressed in m a.s.l. and “Andisol?” and “Wetland?” are zero unless the specific soil condition (with higher Corg) applies and the value is 1.
Vegetation Analysis
The diversity of vegetation was characterized by identifying all woody plants in the observation plots and calculating several indices from the results: density (number of individuals per unit area), frequency (fraction of plots in which a species was found), dominance (relative abundance) and the INP or Index of Importance Values (Soerianegara and Indrawan, 1978).
with basal area (BA) derived as Σ π DBH2/4 from measurements of DBH or stem diameter at breast height (1.3 m).
Diversity Index (Shannon and Wiener, 1949):
where H′ = Diversity index, N = Total number of individuals sampled, ni = Number of species i.
Evenness index (with values between 0 and 1):
where Pi are the frequencies of the m species observed.
Tree Biomass
Measured stem diameters of poles and trees (for D > 5 cm) were converted to aboveground biomass (AGB) estimates using allometric equations for wet tropics (average rainfall 1,500–4,000 mm y−1) from Chave et al. (2005):
Where ρ = wood density (g cm−3), derived from http://db.worldagroforestry.org/wd. A locally developed (Alfian, 2017) species-specific allometric equation was used: AGB = 0.4992 D1.0012.
Statistical Analysis
The results of observations and measurements of biophysical data were analyzed by Analysis of Variance using Genstat (18th edition) software to test possible rejection of a null-hypothesis of no effects of toposequence and landscape positions as main factors, along with a simple interaction term. Where statistically significant differences (p < 0.05) were noted, a Duncan test was used to compare means at the combination of the two factors. Correlation and linear regression analysis (in the MS-Excel software) were used to describe relationships between response (Y) and explanatory (X) parameters, as Y = b Ym + (a Ym /Xm) X, after normalizing or rescaling data sets relative to their means, Ym and Xm, respectively.
Results
Vegetation and Soil Properties
Soil profiles in the area showed evidence of multiple ash deposition events, with topsoils buried by the 14 February 2014 and earlier Kelud eruptions at various depths (Figure 2). Soil analysis (Table 1) showed buried topsoil (with relatively high Corg) in the 20–30 cm depth layer, and slightly higher pH values. Lower elevations had higher silt content, but the sand fraction dominated in all samples.

Figure 2. Soil profile on the ridge position at three elevations, with soil layers as identified in the field indicated by dashed lines and topsoil recent ash deposits of close to 20, 12, and 7 cm, respectively; the measuring tape indicates depth increments of 10 cm (Photo courtesy from Nugraha, 2015).
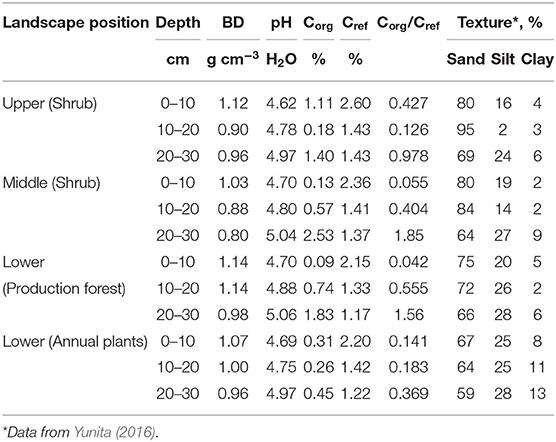
Table 1. Soil properties for three soil layers in various landscape positions (BD, bulk density; Cref, reference Corg concentration based on pedotransfer function; averages for three replicate plots).
Most of P. rigida plants were found in clusters on sites with a slope of 30–80%, spread over an altitude of 600–1,700 m a.s.l. at the lower, middle, and upper landscape positions, with sandy and relatively wet soil conditions. Usually, P. rigida was found in open shrubland, but it also developed well in the lava flow deposits (layers of sand and gravel) in the riverbeds (Figure 3D).
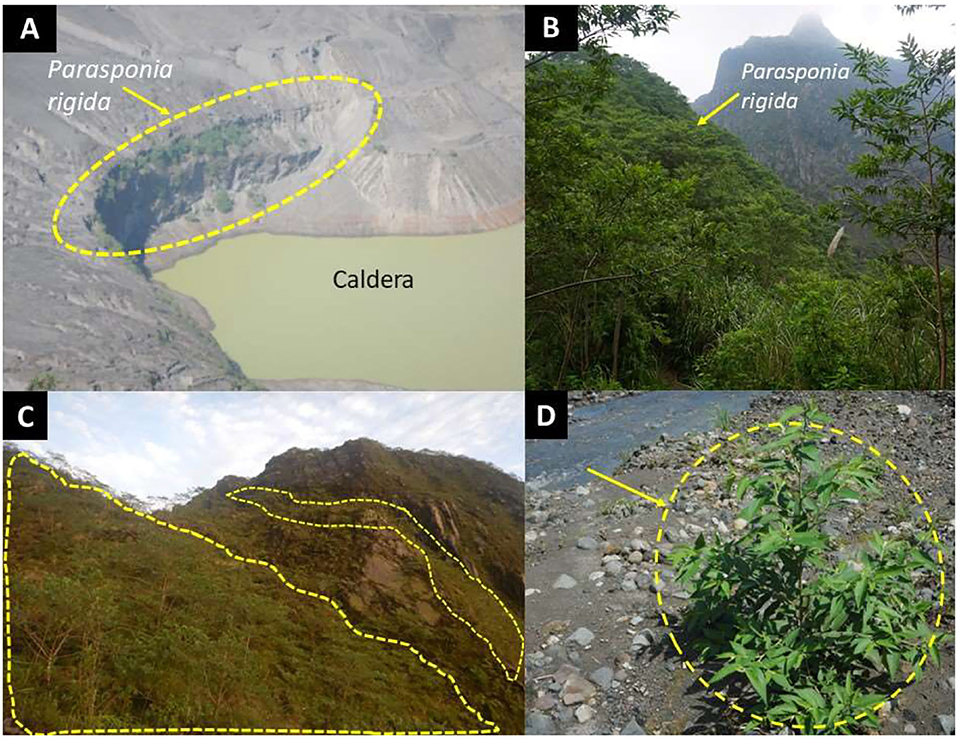
Figure 3. P. rigida (dashed yellow lines) stands at various landscape locations: (A) Caldera, (B,C) high ridges, (D) Ash deposits in a river valley (Photo credits: first author).
Basal area, biomass, and necromass of trees differed significantly between landscape positions (Table 2). Necromass was 34 and 26% of biomass at upper and middle landscape positions, indicative of eruption-related tree mortality, but no necromass was observed in the lower zone, while biomass there was highest. In the middle and upper zones, several trees had survived, as indicated by the biomass of trees above 30 cm diameter. Please note that the vegetation sampling referred to existing stands, not to their frequency within the wider landscape. The absence of necromass at lower elevation can be an indication of firewood collection by the neighboring village.
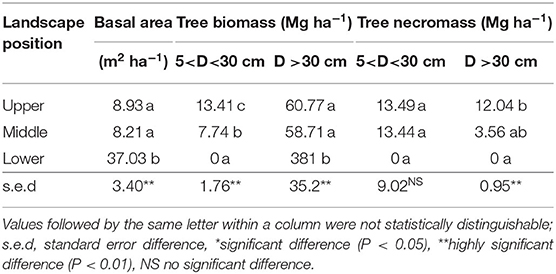
Table 2. Tree biomass and necromass differentiated by stem diameter in stands in three landscape positions (based on three replicates in each of three elevational zones).
Tree population data (Table 3) matched the basal area (Table 2) data, with the high biomass but low tree diversity (and near-absence of internal regeneration) in the Mahogany stands in the lower zone). Tree diversity in the middle zone was significantly (p < 0.01) higher than that in the upper zone for trees (D > 10 cm) and poles (5 < D < 10 cm) but was not distinguishable between these two zones for the sapling and seedling stages. In the latter two categories, however, sapling and seedling diversity reflected that in poles and trees at the high landscape position.
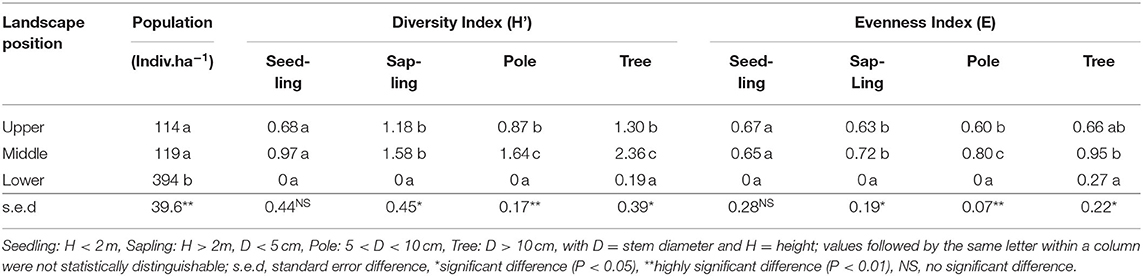
Table 3. Tree populations in various growth stages and biodiversity indices in three landscape positions (based on three replicates in each of three elevational zones).
For further analysis of the differences between the zones, the Importance Value Index combines relative density, relative frequency, and relative domination for the commonest species. In the middle zone the seedling and sapling stage was dominated by Ageratum conyzoides (babadotan, locally called “Tropos”) with INP = 177% and INP = 116%, respectively, and the pole stage by T. orientalis (INP = 61%). In the upper zone seedling and sapling, stages were dominated by Begonia multangular (“Mencok”) with INP = 100% and INP = 63%, respectively, while the pole stage was dominated P. rigida and T. orientalis, both?? with INP = 226%.
Biomass Production and Root Nodule Density of P. rigida
Results for the second survey showed that for each of the three elevational zones, the P. rigida population density, as well as average stem diameter, varied with position along the local toposequence (Figure 4). While population density was highest (80–200 trees/ha) in the valley positions in the upper and middle elevational zone, respectively. Tree diameter was highest in the ridge. Please note that in this second survey the lower zone toposequence did not include the Mahogany plantation, but P. rigida numbers were relatively low while other vegetation dominated.
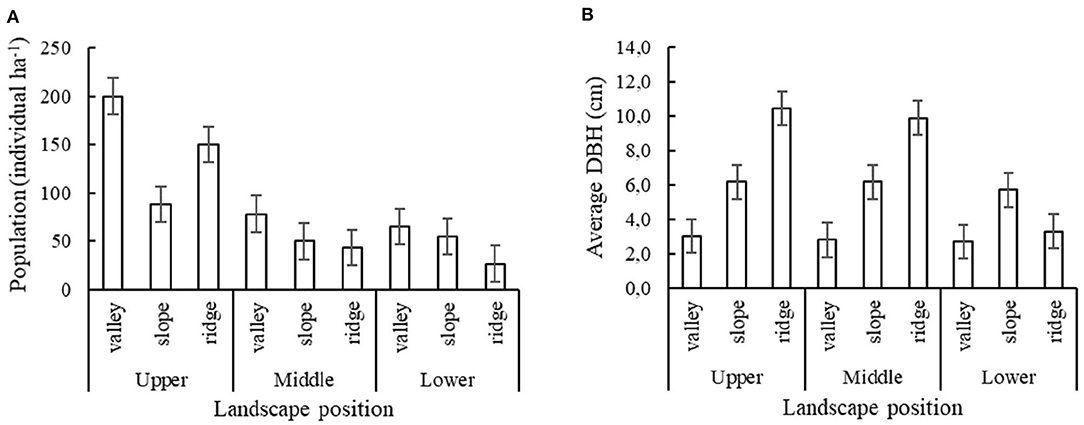
Figure 4. P. rigida performance in various toposequence and elevational positions on the slopes of Mount Kelud (three sample plots per elevation * landscape position). (A) Total population of P. rigida with standard error of differences, (B) Average stem diameter (DBH).
Plot-level biomass estimates (Figure 5A) show by far the highest P. rigida biomass (164 kg plot−1) on the ridges in the upper zone. The relative share of P. rigida and T. orientalis in their combined tree population showed that T. orientalis was absent at the highest elevation and dominated at the middle elevation (Figure 5B).
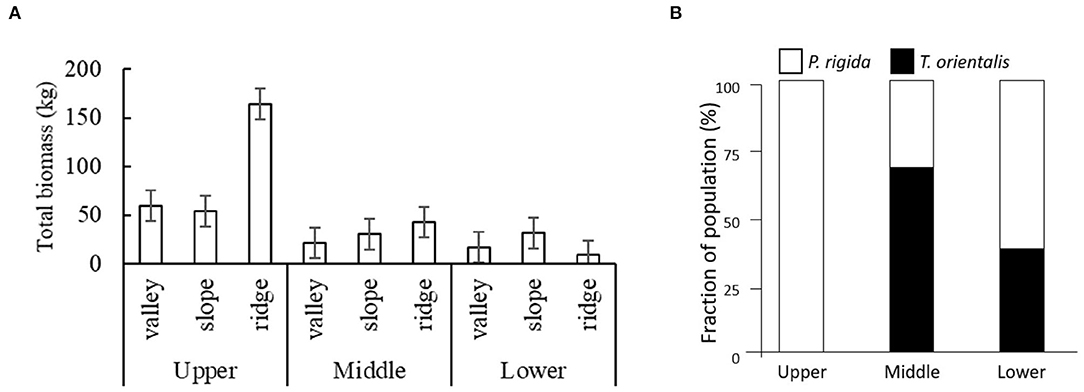
Figure 5. (A) Total biomass production of P. rigida (per 20 × 20 m2 plot) in the various landscape positions and elevation zones, with standard error of differences; (B) relative share of P. rigida and T. orientalis in their combined tree population.
Soil samples of the rhizosphere soil in existing P. rigida stands (Table 4) showed domination by the sand-sized fraction (fresh ash), with silt and clay at slope and ridge positions in the middle and lower zone. Bulk density was high (1.2 g cm−3 to 1.6 g cm−3, Ntot (0.012–0.040%), and Corg (0.13 to 0.26%) concentrations very low in all stands.
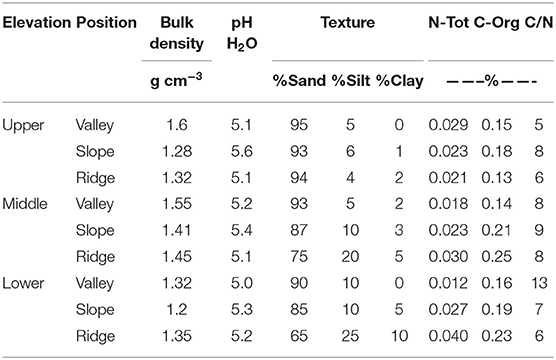
Table 4. Characteristics of soil physico-chemical properties in the P. rigida rhizosphere (three replicates).
Based on the hemoglobin color assessment 79–93% of nodules were classified as “effective,” while nodule densities per m2 of soil surface were highest in the valley positions at low and middle zone, and in the ridge position in the upper zone (Table 5).
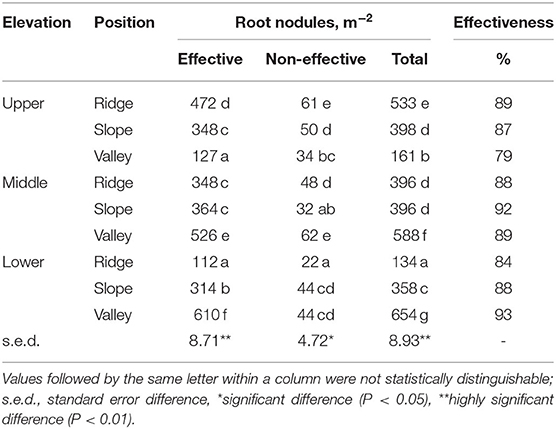
Table 5. Root nodules of P. rigida in various locations, distinguished by effectiveness (hemoglobin color) (averages for three replicates).
Regression analysis of nodules on the three indicators of soil nitrogen supply in P. rigida stands, Ntot, mineral and , was based on normalized parameters to allow direct comparison between these indicators. The regression of the density of effective nodules on soil nitrogen indicators accounted for at least 85% of the observed variation (Figure 6). Nodulation was most abundant and effective on the poorest sites.
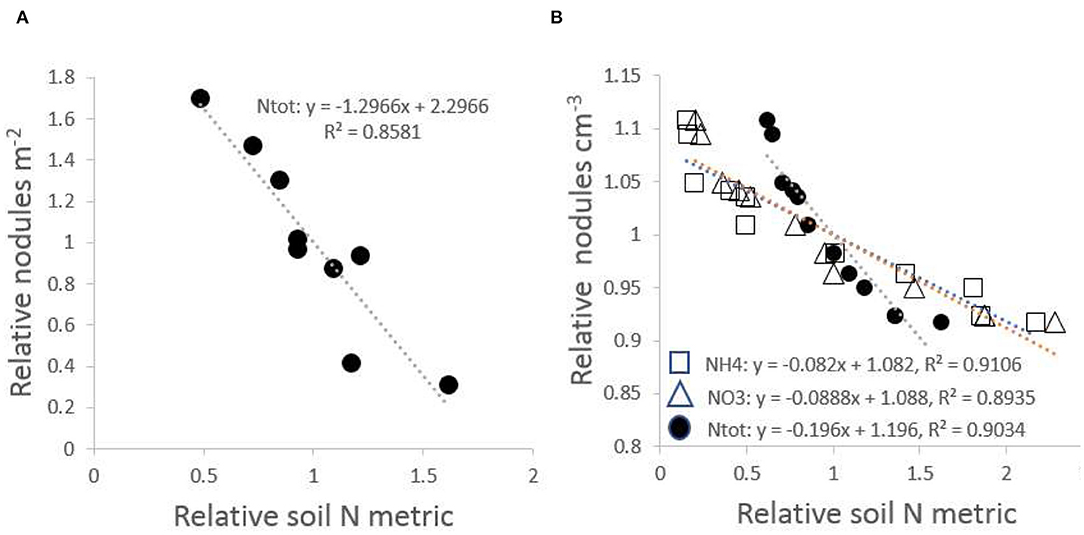
Figure 6. Relationship of effective root nodules with total N-content of the soil, with both X and Y variates normalized and expressed relative to the mean; (A) nodules per unit soil area vs. Ntot; (B) nodules per unit soil volume vs. Ntot, nitrate and ammonium levels in soil solution.
Discussion
We found P. rigida populations over a considerable elevational range, from 600 to 1,700 m a.s.l., on around Mount Kelud after the recent eruption. A comparison of satellite imagery before (2013) and after (2014) the eruption (Nuzulah, 2016) indicated a decrease in vegetation density up to a radius of 10 km from the caldera, with a vegetation density index decreasing from 0.64 to 0.34 in 2013 to 0.52–0.01 in 2014; despite a partial recovery in 2016, vegetation was affected until a radius of 5 km. The observations of dominance by P. rigida in the highest zone (>1,000 m a.s.l.) demonstrate the remarkable adaptation of this species to the extreme environment of frequently erupting volcanoes and abundant ash deposition. Similar observations in 2018 in about 2 km from the active crater of Mount Merapi (Central Java) after an eruption in 2010 showed low levels of plant diversity, but P. rigida was observed in dense monospecific stands with some patches of Acacia decurrens (Dr. Subekti Rahayu, pers. comm. 2020). According to local informants on Mount Merapi P. rigida only occurred on the ridge and along riverbanks before the recent eruption, but it spread out throughout the landscape after the eruption. The literature on the vegetation of Mount Kelud in the past and the high frequency of eruptions match an interpretation that the species combines the effective colonization of T. orientalis (Mangopang, 2016) a pantropical pioneer species with the ability to thrive on soils of very low nitrogen content. The distribution of P. rigida observed suggests that its seeds may be carried by overland and river flows from trees on higher ridges, including the slopes of the caldera itself. P. rigida appeared to develop well in nutrient-poor and open soil conditions where the plants produce root nodules and the fresh green of leaves (Figure 3).
Our observations suggest that the stands on ridges in the highest zone (with populations of around 150 trees ha−1) had the highest biomass and were the likely seed source for abundant regeneration in the valley positions at the middle and lower elevation. Seeds can be produced within 1 year of a regrowing stand. The trees also contribute to soil formation, with a half-life time of litter of around 20 weeks (Ishaq et al., 2020). The sibling species T. orientalis and P. rigida co-occurred at lower and middle elevation, while only P. rigida was found in the highest zone. These findings suggest that the selective advantage of the nodulated P. rigida over its non-nodulated sibling species T. orientalis is most pronounced in the most extreme and N-poor parts of the landscape. In the lusher vegetation at lower elevations, T. orientalis can maintain a presence among grasses and other trees, P. rigida is abundant as a pioneer on the ash deposits in the valley but appears to lose out from other vegetation later in the succession. From the absence, at this stage of the recovery after the most recent eruption, of T. orientalis in the highest zone we cannot distinguish between lack of seed sources or lack of ability to grow as direct explanation, while P. rigida clearly meets both requirements for restoration success. Successful seed sources that can reach the rest of the landscape, however, do depend on the ability of pioneer plants to grow in the relatively harsh and nitrogen-poor soil conditions. The reduced role of P. rigida beyond the pioneer zone may suggest that the ability of Parasponia species to nodulate has had negative consequences, relative to their Trema siblings, for competitiveness under less extreme soil conditions. From these observations, it appears that explanations of the distributions of P. rigida and T. on the slopes of Mount Kelud will consist of the position of seed sources that survive the eruption, supported by the successful colonization of N-poor substrates by P. rigida. In more sheltered and N-rich landscape positions, T. orientalis appears to have an edge in growth rates—but a direct test of competitive ability across levels of soil N availability has yet to be performed. Styger et al. (2009) reported from secondary forest regeneration sites in Madagascar that 3-years old T. orientalis had a biomass of 8.5 Mg ha−1 and 5-years old stands 24.7 Mg ha−1. These biomass data, in a less extreme environment, are higher than what T. orientalis and P. rigida achieved on Mt Kelud, but effective soil cover was achieved and ash deposits along the riverbed were stabilized by P. rigida seedlings.
Our findings of a negative response of nodule formation to external nitrogen supply in the field match the laboratory results reported recently by Dupin et al. (2020). The density of effective nodules per unit soil surface area was associated with below-average soil nitrogen indicators, with rhizobium populations in the nodules (153 × 104 to 112 × 106 CFU g−1) up to a 100-fold increase above their concentrations in rhizosphere soil (average 82 × 104 CFU g−1). For comparison, Widawati (2015) reported a rhizobium density in the legume kaliandra (Calliandra tetragona) nodules of 2.2 × 106 CFU g−1.
Current emergency management plans for active volcanoes like Mount Kelud rely on nursery-produced legume trees as planting material to stabilize ash and reduce downstream mudflow risks. Three years after the eruption of Mount Kelud other surveys in the restoration area (Tanjungsari et al., 2018) found the introduced legume tree Calliandra [both the red (C. tetragona calothyrsus) and white (C. tetragona) species to have been planted, along with naturally dispersed mahang (Macaranga hispida), and anggrung (T. orientalis)]. Relative to such plans and practice, our findings of a key role for P. rigida stands on the ridges at high elevation are highly relevant. These trees can recover rapidly after an eruption and probably are the main seed source for successful natural regeneration of tree-based vegetation in the post-eruption landscape. P. rigida is not only a very interesting biological object of study, suggesting the conditions under which association between higher plants and rhizobium bacteria can evolve, it also deserves a key role in emergency preparedness plans for the area. Some investment in securing local P. rigida seed sources for assisted seed rains could likely speed up the recovery process in next steps of the eruption cycle.
Conclusions
1. P. rigida populations survived the recent eruption of Mount Kelud at an elevation of 600–1,700 m a.s.l. The sibling species T. orientalis and P. rigida (differing in their ability to nodulate) co-occurred at lower and middle elevation, while only P. rigida was found in the highest zone.
2. The rate of biomass recovery was related to landscape position: Stands on ridges in the highest zone (around 150 trees ha−1) had the highest biomass and were the likely seed source for abundant regeneration in the valley positions at middle and lower elevation.
3. The density of effective nodules per unit soil surface area was associated with below-average soil nitrogen indicators.
4. A practical implication of these findings is that existing P. rigida stands on the ridges at high elevation are key to the successful natural regeneration of tree-based vegetation in the post-eruption landscape and deserve protection and a key role in emergency preparedness plans for the area.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
The field and laboratory research was carried out by RM and IA as part of their Master of Science program at Brawijaya University, under direct supervision of KH. RM and IA collected and analyzed the data. RM, KH, and MN designed the research and drafted the manuscript, for which all authors agree to be accountable for the content of the work. All authors contributed to the article and approved the submitted version.
Funding
We acknowledge funding from BOPTN 2015 (State university operational assistance) of Brawijaya University in 2015 and cooperation with farmers of Kutut and Pandansari village, Ngantang sub-district (East Java Province) that were affected by the Mount Kelud eruption, for their helped to carry out this research and for their kindness to share their ecological knowledge of the area. Publication was financially supported by the WCU program /UN10/PN/2020.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer RG declared a shared affiliation, with no collaboration, with one of the authors, MN, to the handling editor.
Acknowledgments
We thank Dr. Rene Geurts (Wageningen University) for putting us on the Parasponia trail. We acknowledge cooperation with the people of Kutut and Pandansari village who, despite being affected by the Mount Kelud eruption, assisted with the research and shared their ecological knowledge of the area.
References
Achmad, S. R., and Hadi, H. (2015). Identifikasi sifat kimia abu vulkanik dan upaya pemulihan tanaman karet terdampak letusan gunung kelud. Studi kasus kebun ngrangkah pawon jawa timur. Balai Penelitian GETAS. Salatiga. Warta Perkaretan. 34, 19–30. doi: 10.22302/ppk.wp.v34i1.60
Akkermans, A. D. L., Abdulkadir, S., and Trinick, M. J. (1978). N2-fixing root nodules in ulmaceae: parasponia or (and) trema spp? Plant Soil 49, 711–715. doi: 10.1007/BF02183301
Alfian, I. (2017). Reklamasi Lahan Pasca Erupsi Gunung Kelud Secara Biologi: Evaluasi Ketersediaan N Dalam Tanah dan Kerapatan Bintil Akar Parasponia Andersonii. Skripsi. Malang: Universitas Brawijaya.
Becking, J. H. (1979). Root-nodule symbiosis between rhizobium and parasponia (Ulmaceae). Plant Soil 51, 289–296. doi: 10.1007/BF02232892
Beech, E., Rivers, M., Oldfield, S., and Smith, P. P. (2017). GlobalTreeSearch: the first complete global database of tree species and country distributions. J. Sustain. For. 36, 454–489. doi: 10.1080/10549811.2017.1310049
BNPB. (2014). Kelud Telah Meletus [Kelud has Erupted]. https://bnpb.go.id/berita/keludtelah-meletus (accessed November 23, 2020).
Bremner, J. M. (1960). Determination of nitrogen in soil by the kjeldahl method. J. Agric. Sci. 55, 11–33. doi: 10.1017/S0021859600021572
Chave, J., Andalo, C., Brown, S., Cairns, M. A., Chambers, J. Q., Eamus, D., et al. (2005). Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145, 87–99. doi: 10.1007/s00442-005-0100-x
Clason, E. W. (1935). The vegetation of the upper-badak region of mount kelut [East Java]. Bull. Jard. Bot. Buitenzorg 13, 509–518.
Dibyosaputro, S., Dipayana, G. A., Nugraha, H., Pratiwi, K., and Valeda, H. P. (2015). Lahar at kali konto after the 2014 eruption of kelud volcano, east java: impacts and risk. Forum Geografi 29, 59–72. doi: 10.23917/forgeo.v29i1.793
Dupin, S. E., Geurts, R., and Kiers, E. T. (2020). The non-legume Parasponia andersonii mediates the fitness of nitrogen-fixing rhizobial symbionts under high nitrogen conditions. Front. Plant Sci. 10:1779. doi: 10.3389/fpls.2019.01779
Geurts, R., Lillo, A., and Bisseling, T. (2012). Exploiting an ancient signalling machinery to enjoy a nitrogen fixing symbiosis. Curr. Opin. Plant Biol. 15, 438–443. doi: 10.1016/j.pbi.2012.04.004
Grubb, P. J. (1977). The maintenance of species-richness plant communities: the importance of the regeneration niche. Biol. Rev. 52, 107–145. doi: 10.1111/j.1469-185X.1977.tb01347.x
Hairiah, K., Dewi, S., Agus, F., Velarde, S. J., Ekadinata, A., Rahayu, S., and van Noordwijk, M. (2011). Measuring Carbon Stocks Across Land Use Systems: A Manual. Bogor: World Agroforestry Centre (ICRAF) Southeast Asia Regional Program, 154.
Hairiah, K., van Noordwijk, M., Sari, R. R., Saputra, D. D., Suprayogo, D., Kurniawan, S., et al. (2020). Soil carbon stocks in indonesian (agro) forest transitions: compaction conceals lower carbon concentrations in standard accounting. Agric. Ecosyst. Environ. 294:106879. doi: 10.1016/j.agee.2020.106879
Hassler, M. (2019). “Parasponia rigida,” in World Plants: Synonymic Checklists of the Vascular Plants of the World, eds. Y. Roskov, L. Abucay, T. Orrell, D. Nicolson, N. Bailly, P. Kirk et al. Available online at: http://www.catalogueoflife.org/ (accessed May 3, 2020).
Ishaq, R. M., Saputra, D. D., Sari, R. R., Suprayogo, D., Widianto, W., Prayogo, C., et al. (2020). Turning volcanic ash into fertile soil: farmers' options in coffee agroforestry after the 2014 mount kelud eruption. AGRIVITA. J. Agric. Sci. 42, 78–91. doi: 10.17503/agrivita.v42i1.2494
Kristiansen, N. I., Prata, A. J., Stohl, A., and Carn, S. A. (2015). Stratospheric volcanic ash emissions from the 13 February 2014 kelut eruption. Geophys. Res. Lett. 42, 588–596. doi: 10.1002/2014GL062307
Mangopang, A. D. (2016). Morfologi Trema orientalis (L.) Blume dan Manfaatnya Sebagai Tanaman Pionir Restorasi Tambang Nikel. Makasar: Prosiding Seminar Nasional from Basic Science to Comprehensive Education.
McGeary, D., Plummer, C. C., and Carlson, D. H. (2002). Physical Geology Earth Revealed. Boston: McGraw Hill Higher Education, 574.
Nuzulah, S. N. (2016). Kajian dinamika suksesi vegetasi di kawasan terdampak erupsi gunung api kelud berbasis data penginderaan Jauh Tahun 2013-2016. 17(1) Thesis, Study Program Geography, Concentration of Potential and Mapping Areas,Department of Geography, Faculty of Social Sciences, Universitas Negeri Malang.
Op den Camp, R. H., Polone, E., Fedorova, E., Roelofsen, W., Squartini, A., Op den Camp, H. J., et al. (2012). Nonlegume Parasponia andersonii deploys a broad rhizobium host range strategy resulting in largely variable symbiotic effectiveness. Mol. Plant Microbe Interact. 25, 954–63. doi: 10.1094/MPMI-11-11-0304
Pickett, S. T., and White, P. S. (2013). The Ecology of Natural Disturbance and Patch Dynamics. Amsterdam: Elsevier.
Rahayu, R., Ariyanto, D. P., Komariah, K., Hartati, S., Syamsiyah, J., and Dewi, W. S. (2014). Dampak erupsi gunung merapi terhadap lahan dan upaya-upaya pemulihannya [Impacts of the eruption of Mount Merapi on land and livelihoods]. Caraka Tani 29, 61–72. doi: 10.20961/carakatani.v29i1.13320
Rudianto, G., Indradewa, D., and Utami, S. N. H. (2017). The influence of volcanic ash thickness in the soil surface that fell various of growth phase to the growth and result of maize (Zea mays L.). Vegetalika 6, 1–11. doi: 10.22146/veg.27959
Sarasawati, R. (2007). Bakteri Pembentuk Bintil Akar dalam Metode Analisis Biologi Tanah. Bogor: Balai Penelitian dan Pengembangan Sumberdaya Lahan Pertanian, 27–38.
Shannon, C. E., and Wiener, W. (1949). The Mathematical Theory of (USA) Communication: Unknown Distance Function. Urbana, IL: Illinois Press.
Sinaga, B. I. L. J., Sembiring, M., and Lubis, A. (2015). Dampak ketebalan abu vulkanik erupsi gunung sinabung terhadap sifat biologi tanah di kecamatan naman teran kabupaten karo. J. Online Agroekoteknolgi 3, 1159–1163. Available online at: https://media.neliti.com/media/publications/105600-ID-dampak-ketebalan-abu-vulkanikerupsi-gun.pdf (accessed November 24, 2020).
Smiet, A. C. (1992). Forest ecology on java. Human impact and vegetation of montane forest. J. Trop. Ecol. 8, 129–152. doi: 10.1017/S026646740000626X
Soerianegara, I., and Indrawan, A. (1978). Indonesian Forest Ecology. Bogor: Departemen Manajemen hutan, Fakultas Kehutanan Institut Pertanian Bogor.
Styger, E., Fernandes, E. C. M., Rakotondramasy, H. M., and Rajaobelinirina, E. (2009). Degrading uplands in the rainforest region of madagascar: fallow biomass, nutrient stocks, and soil nutrient availability. Agrofor. Syst. 77, 107–122. doi: 10.1007/s10457-009-9225-y
Suriadikarta, D. A., Id, A. A., Sutono, D. E., Santoso, E., and Kasno, A. (2010). Identifikasi sifat kimia abu volkan, tanah dan air di lokasi dampak letusan gunung Merapi [Chemical properties of volcanic ash erupted from Mount Merapi]. Bogor: Balai Penelitian Tanah.
Suzuki, Y., Iguchi, M., Maeno, F., Nakada, S., Hashimoto, A., Shimbori, T., and Ishii, K. (2014). “3D numerical simulations of volcanic plume and tephra dispersal: reconstruction of the 2014 kelud eruption,” in AGU Fall Meeting Abstracts V53E-02 (San Francisco, CA). Available online at: https://ui.adsabs.harvard.edu/abs/2014AGUFM.V53E.02S/abstract (accessed November 24, 2020).
Tanjungsari, A., Hakim, L., and Retnaningdyah. (2018). Provisioning and cultural services of restored ecosystem in mount kelud after 2014 eruption. J PAL 9:336. doi: 10.21776/ub.jpal.2018.009.02.01
Thouret, J. C., Abdurachman, K. E., Bourdier, J. L., and Bronto, S. (1998). Origin, characteristics, and behaviour of lahars following the 1990 eruption of Kelud volcano, eastern Java (Indonesia). Bull. Volcanol. 59, 460–480. doi: 10.1007/s004450050204
Trinick, M. J. (1973). Symbiosis between rhizobium and the non-legume, trema aspera. Nature 244, 459–460. doi: 10.1038/244459a0
Trinick, M. J. (1979). Structure of nitrogen-fixing nodules formed by Rhizobium on roots of Parasponia andersonii planch. Can. J. Microbiol. 25, 565–578. doi: 10.1139/m79-082
Trinick, M. J., and Hadobas, P. A. (1989). Competition by Bradyrhizobium strains for nodulation of the nonlegume Parasponia andersonii. Appl. Environ. Microbiol. 55, 1242–1248. doi: 10.1128/AEM.55.5.1242-1248.1989
van Noordwijk, M., Cerri, C., Woomer, P. L., Nugroho, K., and Bernoux, M. (1998). Soil carbon dynamics in the humid tropical forest zone. Geoderma 79, 187–225.
van Noordwijk, M., Ekadinata, A., Leimona, B., Catacutan, D., Martini, E., Tata, H. L., et al. (2020). “Agroforestry options for degraded landscapes in southeast Asia,” in Agroforestry for Degraded Landscapes: Recent Advances and Emerging Challenges, eds. J. C. Dagar, S. R. Gupta and D. Teketay (Springer: Singapore), 307–347. doi: 10.1007/978-981-15-4136-0_11
Van Ranst, E., Utami, S. R., Vanderdeelen, J., and Shamshuddin, J. (2004). Surface reactivity of Andisols on volcanic ash along the Sunda arc crossing Java Island, Indonesia. Geoderma 123, 193–203. doi: 10.1016/j.geoderma.2004.02.005
van Velzen, R., Holmer, R., Bu, F., Rutten, L., van Zeijl, A., Liu, W., et al. (2018). Comparative genomics of the nonlegume Parasponia reveals insights into evolution of nitrogen-fixing rhizobium symbioses. Proc. Natl. Acad. Sci. U.S.A. 115, E4700–E4709. doi: 10.1073/pnas.1721395115
Vassey, J. K., Pawlowski, K., and Bergman, B. (2005). Root-based N2-fixing symbioses: Legumes, actinorhizal plants, Parasponia sp. and cycads. Plant Soil 274, 51–78. doi: 10.1007/s11104-005-5881-5
Walkley, A., and Black, I. A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi: 10.1097/00010694-193401000-00003
Widawati, S. (2015). Isolasi dan aktivitas plant growth promoting rhizobacteria (Rhizobium, Azospirillum, Azotobacter, Pseudomonas) dari tanah perkebunan Karet, Lampung [Isolation and activity of plant-growth promoting Rhizobacteria (Rhizobium, Azospirillum, Azotobacter, Pseudomonas) from a rubber garden in Lampung]. Berita Biol. 14, 77–88.
Yulia, E. (2013). Pertumbuhan Hasil Kacang Hijau (Vigna Radiata L.) Pada Beberapa Konsentrasi Limbah Cair Pabrik Kelapa Sawit. Available online at: http://www.journal.unitaspdg.ac.id/downlotfilemh.php?file=Endang%20Yulia.pdf (accessed Januari 12, 2017).
Keywords: disaster preparedness, Indonesia, Mount Kelud, N2-fixation, restoration, rhizobium, trema orientalis, volcanic ash
Citation: Ishaq RM, Hairiah K, Alfian I and van Noordwijk M (2020) Natural Regeneration After Volcanic Eruptions: Resilience of the Non-legume Nitrogen-Fixing Tree Parasponia rigida. Front. For. Glob. Change 3:562303. doi: 10.3389/ffgc.2020.562303
Received: 15 May 2020; Accepted: 17 November 2020;
Published: 10 December 2020.
Edited by:
Debora Cristina Rother, University of São Paulo, BrazilReviewed by:
Nino Tavares Amazonas, Federal University of Rio de Janeiro, BrazilRene Geurts, Wageningen University and Research, Netherlands
Copyright © 2020 Ishaq, Hairiah, Alfian and van Noordwijk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kurniatun Hairiah, a3VybmlhdHVuX2hAdWIuYWMuaWQ=
 Rizki M. Ishaq1
Rizki M. Ishaq1 Meine van Noordwijk
Meine van Noordwijk