
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. For. Glob. Change, 23 June 2020
Sec. Fire and Forests
Volume 3 - 2020 | https://doi.org/10.3389/ffgc.2020.00067
This article is part of the Research TopicImpact of Fire on BiodiversityView all 4 articles
Biotic homogenization—the erosion of biological differences among ecosystems due to human disturbance—is a pervasive threat to forest landscapes given the current global biodiversity crisis. In Mediterranean forests, wildfire is a particularly common disturbance that affects biodiversity at local, regional, and global scales. However, little is known about how fire influences biotic homogenization. We analyzed the taxonomic, functional, and phylogenetic β-diversity of ground-dwelling ant communities across a large region in the Mediterranean. We tested the hypothesis that fire leads to community heterogenization at the local landscape scale and community homogenization at the regional landscape scale. We sampled ant communities in five pairs of burned and unburned plots at 21 study sites (n = 210 plots) representing seven forest types in Catalonia (northeastern Iberian Peninsula). Ant species were characterized based on 10 functional traits and phylogenetic relatedness. We then calculated taxonomic, functional, and phylogenetic community dissimilarity indices at local and regional landscapes scales focusing on common vs. rare species. Our results show that fire strongly affected community dissimilarity at the local landscape scale. Regardless of diversity type or species type, community dissimilarity was always greater among burned and unburned plots combined than among unburned plots alone, a pattern that was largely driven by Pinus nigra forests, Quercus ilex forests, and shrublands composed of resprouter species. At the regional landscape scale, community dissimilarity was the same among burned and unburned plots and among unburned plots. However, when we examined taxonomic data for common species and phylogenetic data for rare species, community dissimilarity was slightly lower among burned plots than it was among unburned plots and among burned and unburned plots. Fire clearly affected the degree of biotic homogenization within ant communities, and these effects depended on spatial scale, local vegetation type, diversity type, and species type. Overall, our results suggest that, in Mediterranean regions composed of diverse forest types, wildfire generates local environmental heterogeneity and, as a consequence, locally heterogeneous ant communities. These effects are more dramatic in forest types where fire increases habitat openness. At the regional scale, fire does not create heterogeneity and might even render ant communities more homogeneous.
Understanding how disturbances affect natural forest communities is key to conserving multiple facets of biodiversity: It will help us sustain the integrity of terrestrial ecosystems, manage biotic communities, and anticipate how biotic communities will respond to changes in disturbance regimes (Ponge, 2013; Brockerhoff et al., 2017). Biotic homogenization is one of the most prominent forms of biotic impoverishment induced by current global changes (e.g., climate change, invasive species, agricultural intensification, urbanization, and habitat destruction) (Olden et al., 2004; Zwiener et al., 2018), and it can have effects at different spatial scales. In biotic homogenization, it is most common for one species—most often a generalist—to increase in abundance while specialist and/or rare species decrease in abundance or go locally extinct (McKinney and Lockwood, 1999; Olden, 2006). The result is a decrease in dissimilarity among species communities (or community β-diversity) at the landscape scale considered, whereby ecosystems lose their biological uniqueness (Olden and Rooney, 2006); unsurprisingly, there may be major ecological and evolutionary consequences (Olden et al., 2004; Olden, 2006). To date, biotic homogenization has mainly been explored from a taxonomic perspective, which neglects the functional and phylogenetic facets of diversity. It is important to consider all types of diversity if we wish to have a more detailed understanding of potential ecosystem resilience in the face of future disturbances (Mouillot et al., 2013).
Fire is an important and common form of disturbance that is also a major ecological and evolutionary force: It promotes and maintains biodiversity at local, regional, and global scales and is, therefore, one of the main drivers of landscape dynamics in terrestrial ecosystems (He et al., 2019). Although it is generally assumed that fire creates environmental heterogeneity that drives biodiversity (sensu He et al., 2019), the effects of fire on biotic homogenization have been poorly explored. In Mediterranean areas, most wildfires are intense canopy fires that tend to increase habitat openness by clearing away vegetation (Lloret, 1998; Rodrigo et al., 2004; Arnan et al., 2007a). Newly opened areas are then repopulated by species that resprout after fire, germinate from fire-resistant seeds, or disperse into the burned area from the unburned edges (Turner et al., 1999; Pausas et al., 2004; Rodrigo et al., 2012). In any case, secondary succession can take decades, and in the interim, species adapted to open conditions may colonize the habitat while species adapted to dense and complex vegetation may decline in abundance (Taylor and Fox, 2001; Brotons et al., 2005; Pérez-Granados et al., 2018; Andersen, 2019). Consequently, although burned and unburned areas often have similar levels of species richness and diversity, they frequently display marked differences in community composition due to species turnover (Rodrigo and Retana, 2006; Rodrigo et al., 2008; Jacquet and Prodon, 2009; Sasal et al., 2010). Moreover, greater differences in plant cover between burned and unburned areas lead to greater differences in the species composition of plant and animal communities (Arnan et al., 2006, 2007a).
These differences in community composition between individual burned and unburned areas are expected to scale up and increase species richness and diversity at the level of the entire ecosystem that experienced the disturbance. In other words, within this ecosystem, community dissimilarity (i.e., community β-diversity) should be greater after the disturbance than before. Fire may, therefore, render the local landscape more heterogeneous as it contains pools of species adapted to forests and pools of species adapted to open areas (Pons and Bas, 2005; Rodrigo et al., 2008). Such heterogeneity may be compounded because, first, community dissimilarity is more pronounced when unburned areas are compared to burned and unburned areas combined, and second, communities in burned areas can vary greatly in their responses to disturbance (i.e., there can also be great heterogeneity among burned areas; Laurance et al., 2007; Arroyo-Rodriguez et al., 2013).
However, local landscape biotic heterogenization might not translate into differences at broader landscape scales. During the fire and the post-fire succession period, burned habitats are characterized by high temperatures, which can act as a major environmental filter and limit species occurrence (Arnan et al., 2013). Because fire tends to impose similar environmental filters at broader landscape scales (Velle et al., 2014; Li and Waller, 2015; Trauernicht et al., 2015; He et al., 2019), species communities may be more similar between distant burned areas than between neighboring burned and unburned areas, especially if the unburned areas display a variety of vegetation types. This pattern results because the biotic communities associated with these different vegetation types are the product of filters that act locally, such as those related to climatic conditions, soil type, exposure to the wind or sun, and microhabitat type (Götzenberger et al., 2012). In this context, at a more regional landscape scale, we would expect to see greater β-diversity among unburned areas alone than among unburned areas and burned areas combined. Therefore, when a regional landscape contains a larger relative proportion of burned habitat, there should be fewer species that are forest specialists and more species that are open habitat specialists, leading to regional biotic homogenization (Velle et al., 2014). In short, in Mediterranean forest ecosystems, fire might increase species richness and diversity within the local landscape but cause biotic homogenization within the regional landscape, especially if the region encompasses contrasting vegetation types.
Mediterranean ant communities are an excellent study system for testing the effects of fire on biotic homogenization at local and regional landscape scales. Ants are among the most diverse and abundant terrestrial organisms on earth, and they help provide many basic ecosystem services (Hölldobler and Wilson, 1990; Del Toro et al., 2012). For instance, they make crucial contributions to soil cycling and aeration, organic matter decomposition, seed dispersal, and plant protection (Del Toro et al., 2012). Ants are extremely phylogenetically diverse (Hölldobler and Wilson, 1990) and display a broad range of morphological and life-history traits (Arnan et al., 2014; Parr et al., 2017). Ant communities are sensitive to environmental change and especially to habitat openness (Andersen, 2019). In Mediterranean forest ecosystems, habitat openness resulting from wildfire leads to large changes in ground temperature and food resource availability (Arnan et al., 2007b; Lázaro-González et al., 2013), which are two key factors structuring ant communities (Retana and Cerdá, 2000; Arnan et al., 2007b). Consequently, fire can cause dramatic changes in ant community composition (Arnan et al., 2006; Rodrigo and Retana, 2006; Vasconcelos et al., 2017), which is strongly associated with changes in several functional traits (Arnan et al., 2013) that display strong phylogenetic signals (Arnan et al., 2015, 2017).
Here, we study the β-diversity of ground-dwelling ant communities across a large region in the Mediterranean. The objective was to explore the idea developed above: that fire promotes biotic heterogeneity at the local landscape scale (a mosaic of burned and unburned areas from one locality and same vegetation type) and, simultaneously, biotic homogenization at the regional landscape scale (a mosaic of burned and unburned areas from different localities and vegetation types). To this end, we analyzed the local and regional taxonomic, functional, and phylogenetic β-diversity of ant communities by collecting data at 21 study sites affected by canopy wildfires. These sites represented seven of the vegetation types found in the Mediterranean and sub-Mediterranean climate zones of Catalonia (northeastern Iberian Peninsula). More specifically, we tested the following hypotheses: (1) Fire increases heterogeneity among ant communities at the local landscape scale; (2) specific patterns of local landscape heterogeneity will depend on vegetation type (i.e., there will be greater local landscape heterogeneity when there are greater contrasts in habitat openness between the burned areas and the unburned areas due to vegetation type); (3) fire increases homogeneity among ant communities at the regional landscape scale; and (4) taxonomic, functional, and phylogenetic diversity will all display the patterns predicted in the first three hypotheses.
We sampled ant communities in 2002 in Catalonia (northeastern Iberian Peninsula) at 21 sites that had experienced a summer canopy wildfire (highest fire severity category; Keeley, 2009) in 1994 (Figure 1). These fires could be human ignited or not but not prescribed (or controlled) fires. Information obtained from aerial photographs from 1957, the Land Use Cover Map of Catalonia (1980), and the Forest Fire Map of Catalonia (1987–1994) confirmed that no prior fires had affected the vegetation of these sites during the 50 years previous to the one considered in this study. Thus, all study sites were similarly affected by fire in terms of season, recurrence, severity, and time since disturbance. The sites were distributed across seven Mediterranean vegetation types that represented a broad range of post-fire successional patterns (Rodrigo et al., 2004; Arnan et al., 2007a): shrublands dominated by seeding species, shrublands dominated by resprouter species, Pinus halepensis Miller forests with an understory, Pinus halepensis forests without an understory, Pinus nigra Arnold forests, Quercus ilex Linnaeus forests, and Quercus suber Linnaeus forests. There were three study sites within each vegetation type. Eight years after the fire, the percentage cover of the main tree or shrub species was similar for burned and unburned areas in the two types of shrubland, the two types of P. halepensis forest, and the Q. suber forests but remained different for the P. nigra forests. The pattern was intermediate for the Q. ilex forest. Herbaceous cover and shrub cover were generally similar between the burned and unburned areas, or they were higher in the burned areas, and tree cover was drastically lower in the burned areas. None of these areas were exposed to post-fire management practices (thinning or grazing) in the period between the fire and ant sampling. For more details on the study sites, see Arnan et al. (2006, 2007a) and Table S1.
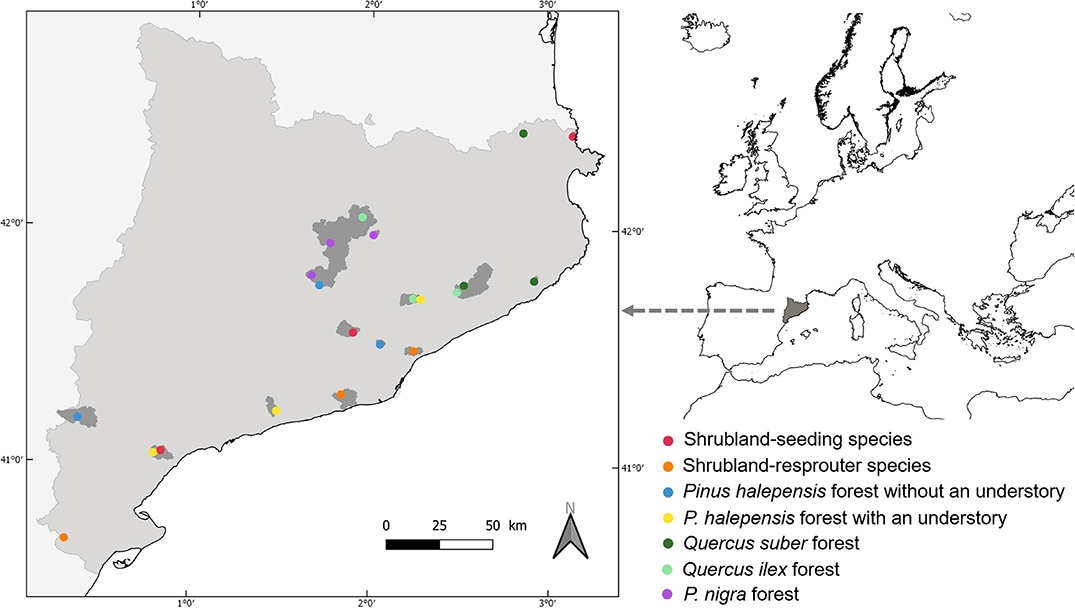
Figure 1. Map of Catalonia (left) in the northeast Iberian Peninsula showing the area affected by the fire (in gray) and the 21 study sites (circles). Circle color indicates vegetation type.
Five pairs of plots, each approximately 600 m2 in size, were established at each site. Within the pairs, one plot was located in a burned area, and the other was located in a nearby unburned area. We, therefore, carried out sampling on a total of 210 plots. All the plots were located more than 100 m from the boundary of the fire (either within the burned area or within the unburned area) so that the minimum distance between plots within a pair was 200 m. Paired plots faced in the same direction and were located at the same elevation. Within each site, the distance between the pairs of plots ranged from 30 to 5,600 m. For a detailed plot characterization, see Table S2. Each group of 10 plots established at each site was considered a local landscape, and the 21 local landscapes made up the regional landscape.
We sampled ground-dwelling ant communities using pitfall trapping. We placed 10 pitfall traps along two 25-m transects (the traps and transects were separated from each other by 5 m). The traps were plastic vials (diameter: 6.5 cm, depth: 9.5 cm) partially filled with water, ethanol, and soap. Traps were operated for 7 days during two contrasting seasons that fell within the normal activity period of most Mediterranean ant species (Cros et al., 1997): in mid-May (spring) and in mid-July (summer). The contents of the 10 traps on each plot were combined to obtain a single sample per plot. In the analyses, we pooled the samples from the two seasons for each plot so that we had a single set of data representing the ants' normal activity period (i.e., total number of ants per 10 traps across 7 days × 2 seasons). The ants were sorted in the laboratory and identified to species. For more details on ant sampling, see Arnan et al. (2006).
Each ant species was scored for 10 traits that reflect different functional niche dimensions (i.e., morphology, life history, and behavior). Because ants are social insects, functional traits may be quantified at the level of the individual worker and at the level of the colony. The following traits were used: worker size, worker polymorphism, colony size, food resource type exploited (i.e., relative consumption of seeds, insects, or liquid food), daily period of activity, position in the behavioral dominance hierarchy, ratio between queen and worker size, number of queens per colony, number of nests per colony, and colony foundation type. Note that these traits may be continuous, ordinal, or binary (Table S3). They are also traits considered to be important in ants because they help define ant autecology and influence ecosystem functioning (Hölldobler and Wilson, 1990; Arnan et al., 2014) (Table S3). The trait data were obtained from our past studies (Arnan et al., 2012, 2013, 2014, 2015, 2017).
We used a recently published ultrametric phylogeny of 156 extant European ant species (Arnan et al., 2017), which is the most complete species-level phylogeny for European ants to date. This phylogeny was then pruned to retain only the 47 ant species collected in this study (Figure S1).
We used the Rao quadratic entropy index (Rao, 1982; Pavoine et al., 2004), which provides a standardized methodology for comparing the taxonomic, functional, and phylogenetic components of diversity within a single mathematical framework (Pavoine et al., 2004; De Bello et al., 2010; Devictor et al., 2010). Moreover, this index can be used to calculate functional diversity for combinations of traits, and it can handle quantitative, categorical, and binary traits (e.g., Rao, 1982; Leps et al., 2006). Furthermore, its estimates of functional and phylogenetic diversity are relatively independent of taxonomic diversity (e.g., Mouchet et al., 2010). Thus, the beta component of this index focuses on taxonomic, functional, and phylogenetic compositional differences in communities that are independent of species richness gradients (De Bello et al., 2010; Arnan et al., 2015, 2017). The use of an index that only consider the turnover component and not the nestedness component of β-diversity is justified because previous research in the same study areas demonstrated that fire drove strong changes in species composition but did not drive changes in species richness (Arnan et al., 2006).
We used additive partitioning to break down overall γ-diversity into within (α) and among (β) community diversity. Within each community k, α-diversity was calculated using Rao's coefficient of diversity (Rao, 1982; Pavoine et al., 2004):
where dij is the distance between species i and j, which can be taxonomic, functional, or phylogenetic, and pik and pjk are the relative abundance of species i and j in community k, respectively. This index represents the expected dissimilarity between two individuals chosen at random from the community.
Between communities k and l, β-diversity was then calculated using Rao's dissimilarity index (Rao, 1982; Pavoine et al., 2004). This index is the expected distance (taxonomic, functional, or phylogenetic) between two individuals chosen randomly from two distinct communities:
where γ (k+l) is the gamma diversity of the two communities (calculated with the same equation as for alpha diversity but taking into account all the species included in the two communities) and α (k, l) is the mean α-diversity of the two communities.
Prior to performing the calculations, we applied Jost's correction (Jost, 2007) to γ and α to properly quantify β-diversity independently of α-diversity (De Bello et al., 2010). To carry out these calculations, we used the function “rao” (De Bello et al., 2010) in R.
To calculate the Rao quadratic entropy index, different distance measures were used depending on diversity type. Taxonomic distances between species were measured as dij = 1 when i ≠ j and as dij = 0 when i = j. To calculate functional distances between species, we first conducted a principal component analysis (PCA) on the standardized (mean = 0, SD = 1) trait data to correct for dominance in the distance matrix of highly correlated traits (Devictor et al., 2010; Purschke et al., 2013). The resulting PCA axes were used to calculate Euclidean distances. Phylogenetic distances between species were measured using the cophenetic distances from the phylogenetic tree. We scaled all the distances to fall between 0 and 1 by dividing each type of distance by its maximum value in order to make the taxonomic, functional, and phylogenetic distances comparable.
The dissimilarity indices were calculated differently at the two landscape scales (i.e., local and regional) based on how the community, the unit of analysis, was defined. At the local landscape scale, the community was the plot. For a given site, RaoQ dissimilarity indices were calculated among burned plots (the “burned” index value was based on the 10 pairwise comparisons between the site's burned plots), among unburned plots (the “unburned” index value was based on the 10 pairwise comparisons between the site's unburned plots), and among the burned and unburned plots combined (the “both” index values were based on the 45 pairwise comparisons between all the site's plots). At the regional landscape scale, the community was the habitat. For a given site, ant abundance was averaged for the five burned plots and for the five unburned plots; we, thus, obtained two paired estimates of ant community composition (burned and unburned) per site. The RaoQ dissimilarity indices were then calculated among burned habitats (the “burned” index value was based on the 210 pairwise comparisons between the burned habitats), among unburned habitats (the “unburned” index value was based on the 210 pairwise comparisons between unburned habitats), and among the burned and unburned habitats combined (the “both” index value was based on the 861 pairwise comparisons between all the habitats).
Finally, to account for the effects of common vs. rare species, the dissimilarity indices were calculated twice: once using abundance data (as explained above) and once using occurrence data (where pik and pjk were 0 or 1 if the species was absent or present, respectively).
We analyzed whether fire heterogenized or homogenized ant communities at two landscape scales. At the local landscape scale, we were interested in testing whether the effect of fire depended on vegetation type; we then performed general linear mixed models where the RaoQ dissimilarity indices (taxonomic, functional, and phylogenetic) were the response variable, and fire index type (burned, unburned, and both), vegetation type, and their interaction were fixed factors. Site was included as a random factor. At the regional landscape scale, we conducted general linear models where, once again, the RaoQ dissimilarity indices were the response variable, and fire index type (burned, unburned, and both) was the fixed factor. All these analyses were carried out twice: once with the dissimilarity indices calculated from abundance data and once with the dissimilarity indices calculated from the occurrence data.
Overall, we collected 234,398 ant workers belonging to 49 species and 18 genera. However, this group included a parasitic species (Lasius affinis Schenk) and a slave-making species (Polyergus rufescens Latreille), which were excluded from the analyses because they have unique lifestyles, and some of their functional traits are likely shaped by their host species. It, thus, made no sense to include them in the analyses of functional diversity; therefore, for consistency's sake, they were also excluded from the analyses of taxonomic and phylogenetic diversity. The most abundant species were Lasius niger Linnaeus, Iberoformica subrufa Roger, Formica gagates Latreille, and Pheidole pallidula Nylander, and the least abundant species were Formica cunicularia Latreille, Linepithema humile Mayr, and Temnothorax parvulus Schenck. Nine species occurred in more than 100 plots, and only 12 species occurred in <10 plots. These ant species had a wide range of functional roles (Table S3) and represented diverse phylogenetic lineages (Figure S1).
At the local landscape scale, fire appeared to strongly affect ant community β-diversity (Table 1). When abundance-based indices of taxonomic, functional, and phylogenetic diversity were analyzed, community dissimilarity was greater among burned plots and among burned and unburned plots combined than among unburned plots (Figure 2). When occurrence-based indices of taxonomic, functional, and phylogenetic diversity were examined, community dissimilarity was greater among burned and unburned plots combined than among burned plots and among unburned plots (Figure 2). Vegetation type by itself only affected community dissimilarity when occurrence data were used (Table 1): β-diversity was highest in P. nigra forests and lowest in Q. suber forests and P. halepensis forests with an understory (Figure 3). Interestingly, our analyses revealed a strong interaction between fire index type and vegetation type; this interaction was significant in all the analyses (Table 1). More specifically, in the analyses using the abundance data, the overall patterns of all three diversity types were strongly driven by the patterns in the P. nigra forests and shrublands of resprouter species (Figure 3). Fire did not appear to affect β-diversity for any of the other vegetation types. An exception to this general pattern was seen in the Q. ilex forests, where taxonomic diversity was highest among burned and unburned plots combined and lowest among burned plots and functional diversity was lowest among unburned plots. In shrublands of seeding species, phylogenetic diversity was highest among burned plots and lowest among unburned plots (Figure 3). In the analyses using the occurrence data, all three types of diversity were higher among burned and unburned plots combined than among unburned plots in P. nigra and Q. ilex forests. This pattern was also seen for taxonomic diversity in P. halepensis forests without an understory and shrublands of resprouter species. None of the other vegetation types showed any significant differences associated with fire index type (Figure 3).
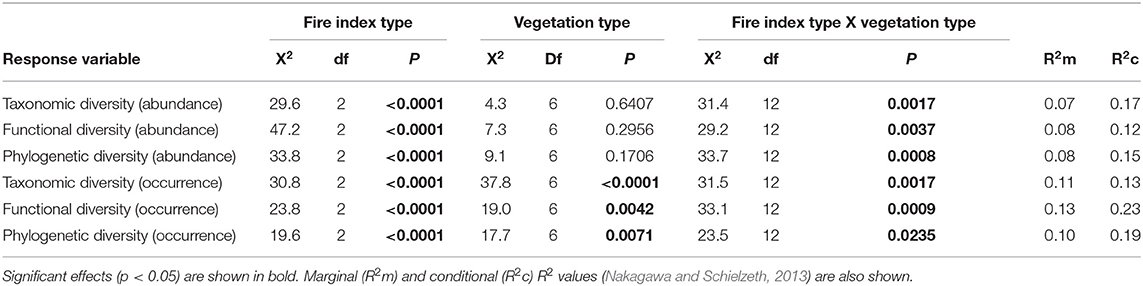
Table 1. Results of the general linear mixed models used to analyze the effects of fire index type, vegetation type, and their interaction on taxonomic, functional, and phylogenetic β-diversity at the local landscape scale for the abundance and occurrence data.
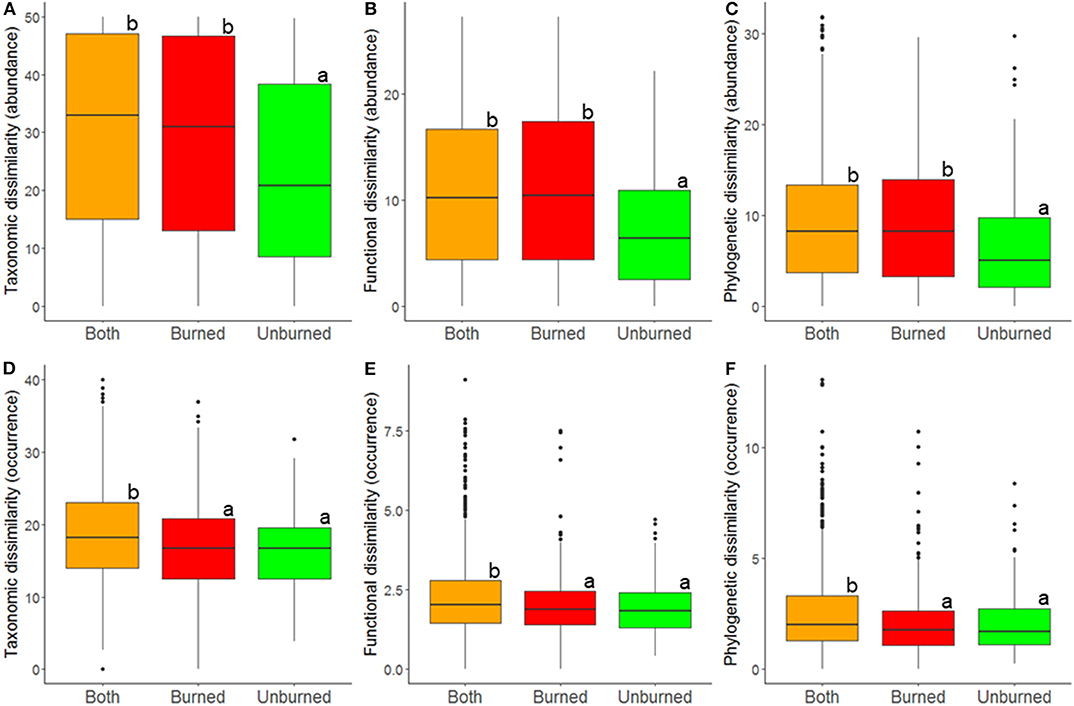
Figure 2. Relationship between fire index type and β-diversity at the local landscape scale; estimates of taxonomic (A,D), functional (B,E), and phylogenetic (C,F) b-diversity were calculated using abundance (A–C) and occurrence data (D–F). Dissimilarity was determined among burned plots, among unburned plots, and among both burned and unburned plots. A difference in letters indicates that there were significant differences (p < 0.05) based on Tukey post-hoc tests.
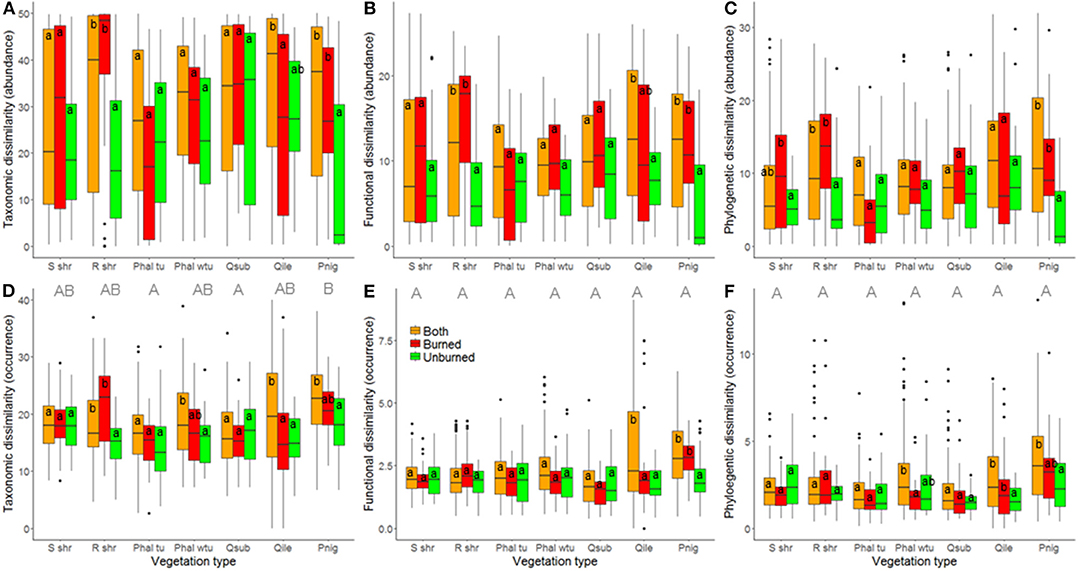
Figure 3. Relationship of fire index type and vegetation type with community dissimilarity at the local landscape scale; estimates of taxonomic (A,D), functional (B,E), and phylogenetic (C,F) β-diversity were calculated using abundance (A–C) and occurrence data (D–F). A difference in lowercase letters indicates that there were significant differences (p < 0.05) among fire index types within vegetation types while a difference in uppercase letters indicates that there were significant differences (p < 0.05) among vegetation types; in both cases, Tukey post-hoc tests were employed. S shr, shrublands of seeding species; R shr, shrublands of resprouter species; Phal tu, Pinus halepensis forests with an understory; Phal wtu, P. halepensis forests without an understory; Qsub, Quercus suber forests; Qile, Q. ilex forests; and Pnig, Pinus nigra forests.
At the regional landscape scale, fire did not general affect ant community β-diversity. However, albeit weakly, fire affected abundance-based taxonomic diversity and occurrence-based phylogenetic diversity (Table 2, Figure 4). Contrary to what was seen at the local scale, both types of diversity were lower among burned plots than among unburned plots and among burned and unburned plots combined (Figure 4).
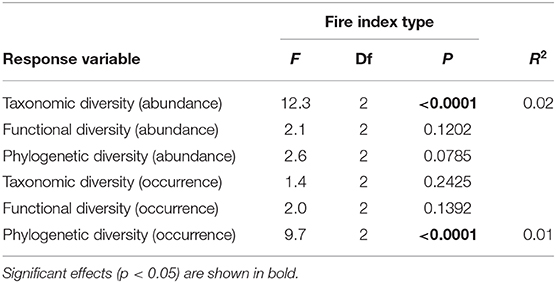
Table 2. Results of the general linear models used to analyze the effects of fire index type on taxonomic, functional, and phylogenetic β-diversity at the regional landscape scale for the abundance and occurrence data.
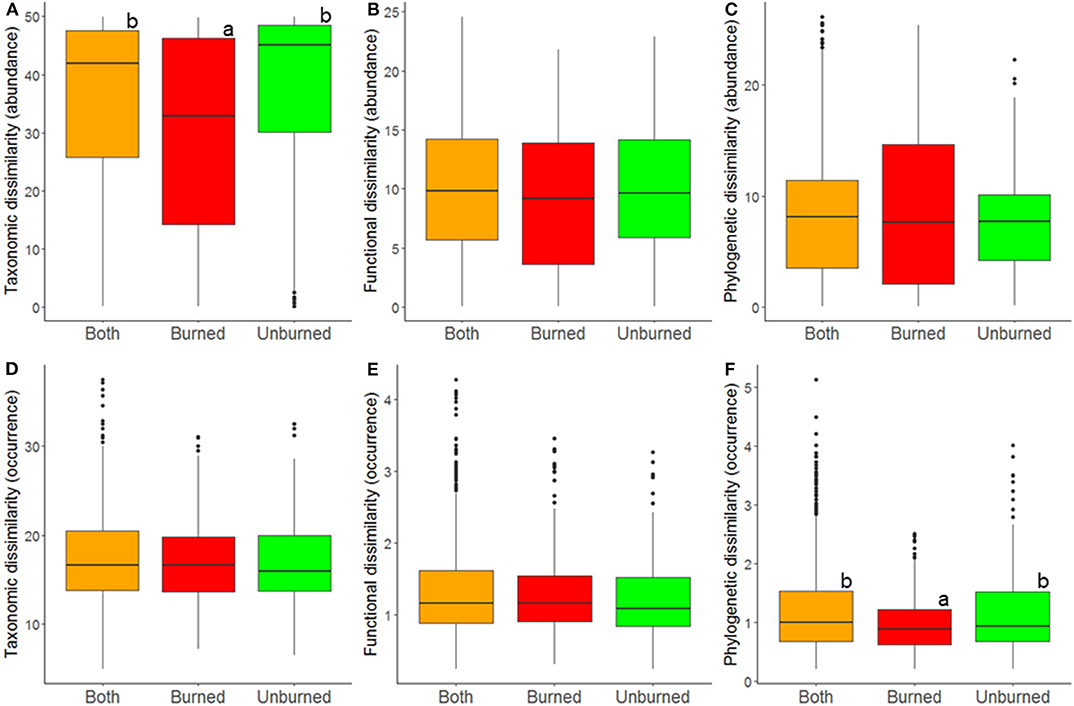
Figure 4. Relationship between fire index type and comunity dissimilarity at the regionall landscape scale; estimates of taxonomic (A,D), functional (B,E), and phylogenetic (C,F) β-diversity were calculated using abundance (A–C) and occurrence data (D–F). A difference in letters indicates that there were significant differences (p < 0.05) based on Tukey post-hoc tests.
We found support for our first hypothesis: There was evidence that ground-dwelling ant communities were experiencing heterogenization at the local landscape scale 8 years after a wildfire. It has previously been shown that fire plays a key role in shaping ant community composition but not necessarily species richness in the Mediterranean (Rodrigo and Retana, 2006; Vasconcelos et al., 2017; for the same study areas in which this study was conducted: Arnan et al., 2006) and many other parts of the world (Parr et al., 2004; Andersen et al., 2007; Frizzo et al., 2012). These changes in community composition are the result of species extinction, persistence, and colonization during the post-fire period (Arnan et al., 2006, 2013). Fire directly causes the death of ants that nest in the vegetation or leaf litter (Frizzo et al., 2012). However, most ground-dwelling species can survive fires because temperature effects are essentially limited to the ground surface (temperature differences are minimal just a few centimeters below the surface; Cane and Neff, 2011; Frizzo et al., 2012). That said, any survivors then face the more open habitat created by changes in the physiognomy and composition of the vegetation (Andersen, 2019); fire, thus, has significant indirect consequences on ground-dwelling ants in the intermediate and long term (Arnan et al., 2006; Rodrigo and Retana, 2006). For instance, when fire removes plant cover, ground temperatures may increase (Ordoñez et al., 2004; Arnan et al., 2011), and the availability of food resources may change (Arnan et al., 2007b; Lázaro-González et al., 2013; Caut et al., 2014), which can affect habitat suitability. Furthermore, new species or species that did not survive the fire can colonize the newly created habitat if their dispersal abilities allow them to reach burned areas from neighboring unburned areas (Arnan et al., 2006, 2013). The degree of habitat openness and the magnitude of the differences between the burned and unburned areas both influence the succession process and community composition (Andersen, 2019). There are two main factors underlying the heterogenization of ground-dwelling ant communities by fire at the local landscape scale. First, these changes in community composition clearly increase ant diversity at the local landscape scale: Fire creates a mosaic of burned and unburned areas (Parr and Andersen, 2006; Maravalhas and Vasconcelos, 2014) whose communities share fewer common and rare species than before the fire. Such heterogenization has also been observed for other taxa (Pons and Bas, 2005; Moretti et al., 2006; Rodrigo et al., 2008; Santos and Cheylan, 2013). Second, when focusing exclusively on common species, there was more heterogeneity in the ant communities found between burned plots than between unburned plots. This pattern may result from the fact that unburned areas harbor stable communities while burned areas harbor unstable communities, whose species composition is likely to vary because of differences in the effects of disturbance or in the ways in which disturbance processes interact with underlying differences in microenvironmental heterogeneity (Laurance et al., 2007; Arroyo-Rodriguez et al., 2013). Although a study in the same plots showed the abundance of the most abundant species increases in burned plots (Arnan et al., 2006), our results suggests that these dominant species are not the same across the burned plots that compose the local landscape.
Our second hypothesis was partially supported. We found that the heterogenization of ground-dwelling ant communities at the local landscape scale was driven by certain vegetation types, namely P. nigra and Q. ilex forests. Among the vegetation types that we studied here, these forests displayed the greatest difference in habitat openness between burned and unburned areas (Rodrigo et al., 2004; Arnan et al., 2006, 2007a). However, heterogenization was also seen in the ant communities in shrublands of resprouter species, where differences in habitat openness were limited between burned and unburned areas. Therefore, although the degree of habitat openness clearly played an important role in heterogenization, other factors related to vegetation type, such as food resource availability, are also evidently involved. For instance, obligate seeding species were found to be more common in burned than in unburned shrublands of resprouters (Arnan et al., 2007a), which implies that seeds, a key food resource for ants, should have been more abundant.
Our third hypothesis stated that fire should increase homogeneity among ground-dwelling ant communities at the regional landscape scale. We found some evidence (although weak) to support this idea. Whatever the case, and contrary to what we saw at the local landscape scale, at the regional landscape scale, β-diversity was not greater among burned and unburned habitats combined than among unburned habitats. Furthermore, ant communities in burned habitats were slightly more similar in taxonomic composition than were ant communities in unburned habitats. In other words, we found a trend that ant communities on burned plots at a given site are more similar to ant communities on burned plots at other sites than they are to ant communities from unburned plots at the same site. In short, fire might homogenize ant communities at the regional landscape scale. At the local regional scale, by creating greater habitat openness and by changing the vegetation, fire generates environmental conditions that differ from those in the neighboring forest matrix, leading to more dramatic environmental heterogeneity (He et al., 2019) and, consequently, higher local diversity and more pronounced dissimilarity among communities (Stein et al., 2014). In contrast, at the regional landscape scale, fire generates environmental (and biotic) homogeneity because similar habitats (burned habitats) occur in very different vegetation types. Moreover, these newly burned areas may have similar levels of habitat openness to those of certain types of unburned habitats, such as grasslands or shrublands. In contrast to the general assumption that fire increases environmental heterogeneity and, therefore, diversity at the regional landscape scale (Pausas and Ribeiro, 2017; He et al., 2019), we show here that this pattern is not universal and might be highly dependent on landscape structure. For instance, in a habitat-diverse landscape such as our study region, fire might even promote environmental homogenization by creating new habitats that are highly similar to each other and that might also be similar to certain unburned habitats. In such cases, fire may promote biotic homogenization instead of biotic heterogenization. It is important to recognize that the effects of fire may be context dependent. For example, fire may drive environmental and biotic heterogeneity (Pausas and Ribeiro, 2017) within large homogeneous and continuous landscapes: In tropical and subtropical rainforests, fire creates patches of savanna within the otherwise homogeneous forest matrix. In our study system, it seems like this type of effect occurred at the local landscape scale. Interestingly, our results at the regional scale might fit with the worldwide trend of disturbance resulting in biotic homogenization (Olden, 2006; Clavel et al., 2011). More specifically, it appears that less tolerant specialist species are being progressively replaced by more tolerant generalist species (McKinney and Lockwood, 1999; Devictor et al., 2008). Biotic homogenization has been seen at large spatial scales for different types of diversity and taxa (ants: Ribeiro-Neto et al., 2016; Heuss et al., 2019; birds: Barnagaud et al., 2017; White et al., 2018; Liang et al., 2019; fishes: Nowakowski et al., 2018; and multitrophic organisms: Gossner et al., 2016) even when there is also evidence of local-scale heterogenization (White et al., 2018).
With regards to the fourth hypothesis, taxonomic, functional, and phylogenetic diversity all reflected that communities were being heterogenized at the local landscape scale; in contrast, there was mixed support for community homogenization at the regional scale. Other studies have also found that there is correlated turnover in the different facets of diversity at smaller, but not larger, spatial scales (White et al., 2018). Thus, at the local landscape scale, fire promoted the heterogenization of ant communities regardless of diversity type or data type (i.e., abundance vs. occurrence); furthermore, the same patterns were seen across vegetation types. Previous research (Arnan et al., 2006, 2013) in this same study system revealed that fire did not affect species richness but has a negative impact on some species (e.g., Aphaenogaster subterranea Latreille, Camponotus lateralis Olivier, Crematogaster scutellaris Olivier, Dolichoderus quadripunctatus Linnaeus, and several Temnothorax Mayr species) that are strongly associated with vegetation. It has a positive impact on others (e.g., Aphaenogaster gibbosa Emery, Iberoformica subrufa, Lasius niger, Messor capitatus Latreille, Pheidole pallidula, Plagiolepis pygmaea Santschi, Tapinoma nigerrimum Nylander, and several Camponotus Mayr species). These species have functional traits that allow them to survive fire (e.g., they nest in the ground rather than in the vegetation), persist during the post-fire period (e.g., larger colony size, more pronounced worker polymorphism, a diet based more on seeds and liquid foods than on insects), or colonize the burned area (e.g., better dispersal abilities as reflected by a higher ratio between queen and worker size). In western European ants, these functional traits all have a strong phylogenetic signal (Arnan et al., 2015, 2017). Taken together, these relationships explain why local landscape-scale biotic heterogenization was seen for taxonomic, functional, and phylogenetic diversity. The consequence is that fire increases the range of ecosystem functions and enhances the ecosystem's ability to adapt and respond to future environmental change (Olden et al., 2004; Olden, 2006) at the local scale.
At the regional landscape scale, in contrast, fire's ability to homogenize ant communities was only reflected (although weakly) in taxonomic diversity when the abundance data were used and in phylogenetic diversity when the occurrence data were used. Thus, fire might induce homogeneity in ant communities among common, but not rare, species and among rare, but not common, phylogenetic lineages. The most common/abundant species in local communities are usually those that have larger distribution ranges. Thus, our results support the idea that fire's biotic homogenization of communities at the regional landscape scale might be driven by generalist species with broad geographical and environmental distributions (Clavel et al., 2011). However, it also appears to be driven by rare phylogenetic lineages. One explanation for this pattern may be that the lineages representing the greatest numbers of individuals, such as Camponotus and Aphaenogaster Mayr, are composed of several closely related species that are differently (positively or negatively) influenced by fire (Figure S1). Meanwhile, the lineages representing the fewest number of individuals are composed of either a few (e.g., Dolichoderinae and Plagiolepis Mayr) or many species (e.g., Temnothorax) that tend to respond in the same way to fire. Whatever the case, taxonomic and phylogenetic homogenization was not accompanied by functional homogenization. This finding suggests that even though the burned areas became more taxonomically and phylogenetically similar, trait diversity was maintained (White et al., 2018). Different studies have found that changes in taxonomic β-diversity were not necessarily accompanied by corresponding changes in either functional or phylogenetic β-diversity (e.g., Purschke et al., 2013; Monnet et al., 2014; White et al., 2018), including in ants (Bishop et al., 2015; Liu et al., 2016; Arnan et al., 2017).
In short, we have demonstrated that wildfire's effects on the β-diversity of ground-dwelling ant communities may be influenced by spatial scale, landscape structure, vegetation type, and diversity type. In particular, we showed that, in a region composed of diverse vegetation types, wildfire consistently heterogenized local landscape ant communities. These effects were clearly related to vegetation type and, more specifically, to vegetation types where fire serves to increase habitat openness. In contrast, fire did not promote heterogenization at the regional landscape scale. Instead, it might homogenize ant communities but only in specific and limited ways. The effects of fire on biodiversity have been broadly addressed in the scientific literature (for a review, see He et al., 2019), but studies examining how fire specifically results in biotic homogenization or heterogenization remain scarce. Our results indicate that many responses are possible. Because biotic homogenization plays an important role in the biodiversity crisis (Olden et al., 2018), we need more research analyzing how fire drives biotic homogenization across multiple taxa and ecosystems if we wish to adopt appropriate conservation strategies. Indeed, fire is the most natural form of disturbance in Mediterranean ecosystems (Pausas et al., 2008; Pausas and Parr, 2018) and a key factor explaining biodiversity around the world (Pausas and Ribeiro, 2017; He et al., 2019). It is important to consider whether the positive effects of fire at the local landscape scale—namely an increased range of ecosystem functions and ability to cope with environmental changes—could be counteracted by the potential negative effects of fire at the regional landscape scale. Large-scale disturbances are more and more common in natural systems (Seidl et al., 2017; IPCC, 2019; Newman, 2019; Ripple et al., 2020), and it is possible that, in the near future, fire might have more negative than positive consequences in Mediterranean ecosystems (but see Pausas and Keeley, 2019). It is clear that wildfire is a key force driving biotic homogenization in Mediterranean regions worldwide and that Mediterranean forests are likely to face significant ecological adversity in the future.
Publicly available datasets were analyzed in this study. This data can be found here: http://www.ecography.org/sites/ecography.org/files/appendix/ecog-01938.pdf, https://peerj.com/articles/1241/#supp-8.
XA and AR came up with the idea, design for the study, interpreted the results, and drafted the manuscript. XA, XC, and AR contributed to data collection. XA analyzed the data. XC contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
XA was supported by a Ramón y Cajal research contract from the Spanish Ministry of Economy and Competitiveness (RYC-2015-18448).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are very grateful to Javier Retana for providing data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2020.00067/full#supplementary-material
Andersen, A. N. (2019). Responses of ant communities to disturbance: five principles for understanding the disturbance dynamics of a globally dominant faunal group. J. Anim. Ecol. 88, 350–362. doi: 10.1111/1365-2656.12907
Andersen, A. N., Parr, C. L., Lowe, L. M., and Müller, W. J. (2007). Contrasting fire-related resilience of ecologically dominant ants in tropical savannas of northern Australia. Divers. Distrib. 13, 438–446. doi: 10.1111/j.1472-4642.2007.00353.x
Arnan, X., Cerdá, X., and Retana, J. (2015). Partitioning the impact of environment and spatial structure on alpha and beta components of taxonomic, functional, and phylogenetic diversity in European ants. PeerJ 3:e1241. doi: 10.7717/peerj.1241
Arnan, X., Cerdá, X., and Retana, J. (2012). Distinctive life traits and distribution along environmental gradients of dominant and subordinate Mediterranean ant species. Oecologia 170, 489–500. doi: 10.1007/s00442-012-2315-y
Arnan, X., Cerdá, X., and Retana, J. (2014). Ant functional responses along environmental gradients. J. Anim. Ecol. 83, 1398–1408. doi: 10.1111/1365-2656.12227
Arnan, X., Cerdá, X., and Retana, J. (2017). Relationships among taxonomic, functional, and phylogenetic ant diversity across the biogeographic regions of Europe. Ecography 40, 448–457. doi: 10.1111/ecog.01938
Arnan, X., Cerdá, X., Rodrigo, A., and Retana, J. (2013). Response of ant functional composition to fire. Ecography 36, 1182–1192. doi: 10.1111/j.1600-0587.2013.00155.x
Arnan, X., Rodrigo, A., and Retana, J. (2006). Post-fire recovery of Mediterranean ground ant communities follows vegetation and dryness gradients. J. Biogeogr. 33, 1246–1258. doi: 10.1111/j.1365-2699.2006.01506.x
Arnan, X., Rodrigo, A., and Retana, J. (2007a). Post-fire regeneration of Mediterranean plant communities at a regional scale is dependent on vegetation type and dryness. J. Veg. Sci. 18, 111–122. doi: 10.1111/j.1654-1103.2007.tb02521.x
Arnan, X., Rodrigo, A., and Retana, J. (2007b). Uncoupling the effects of shade and food resources of vegetation on Mediterranean ants: an experimental approach at the community level. Ecography 30, 161–172. doi: 10.1111/j.0906-7590.2007.04796.x
Arnan, X., Rodrigo, A., and Retana, J. (2011). What are the consequences of ant–seed interactions on the abundance of two dry-fruited shrubs in a Mediterranean scrub? Oecologia 167, 1027–1039. doi: 10.1007/s00442-011-2034-9
Arroyo-Rodriguez, V., Roes, M., Escobar, F., Melo, F. P. L., Santos, B. A., Tabarelli, M., et al. (2013). Plant beta-diversity in fragmented rain forests: testing floristic homogenization and differentiation Hypotheses. J. Ecol. 101, 1449–1458. doi: 10.1111/1365-2745.12153
Barnagaud, J. Y., Kissling, W. D., Tsirogiannis, C., Fisikopoulos, V., Villéger, S., Sekercioglu, C. H., et al. (2017). Biogeographical, environmental and anthropogenic determinants of global patterns in bird taxonomic and trait turnover. Glob. Ecol. Biogeogr. 26, 1190–1200. doi: 10.1111/geb.12629
Bishop, T. R., Robertson, M. P., van Rensburg, B. J., and Parr, C. L. (2015). Contrasting species and functional beta diversity in montane ant assemblages. J. Biogeogr. 42, 1776–1786. doi: 10.1111/jbi.12537
Brockerhoff, E., Barbaro, L., Castagneyrol, B., Forrester, D. I., Gardiner, B., González-Olabarria, J. R., et al. (2017). Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 26, 3005–3035. doi: 10.1007/s10531-017-1453-2
Brotons, L., Pons, P., and Herrando, S. (2005). Colonization of dynamic Mediterranean landscapes: where do birds come from after fire? J. Biogeogr. 32, 789–798. doi: 10.1111/j.1365-2699.2004.01195.x
Cane, J. H., and Neff, J. L. (2011). Predicted fates of ground-nesting bees in soil heated by wildfire: thermal tolerances of life stages and a survey of nesting depths. Biol. Conserv. 144, 2631–2636. doi: 10.1016/j.biocon.2011.07.019
Caut, S., Jowers, M. J., Arnan, X., Pearce-Duvet, J., Rodrigo, A., Cerdá, X., et al. (2014). The effect of fire on ant trophic assemblage and sex allocation. Ecol. Evol. 4, 35–49. doi: 10.1002/ece3.714
Clavel, J., Julliard, R., and Devictor, V. (2011). Worldwide decline of specialist species: towards a global functional homogenization? Front. Ecol. Environ. 9, 222–228. doi: 10.1890/080216
Cros, S., Cerdá, X., and Retana, J. (1997). Spatial and temporal variations in the activity patterns of Mediterranean ant communities. Ecoscience 4, 269–278. doi: 10.1080/11956860.1997.11682405
De Bello, F., Lavergne, S., Meynard, C. N., Leps, J., and Thuiller, W. (2010). The partitioning of diversity: showing Theseus a way out of the labyrinth. J. Veg. Sci. 21, 992–1000. doi: 10.1111/j.1654-1103.2010.01195.x
Del Toro, I., Ribbons, R. R., and Pelini, S. L. (2012). The little things that run the world revisited: a review of ant-mediated ecosystem services and disservices (Hymenoptera: Formicidae). Myrmecol. News 17, 133–146.
Devictor, V., Julliard, R., Clavel, J., Jiguet, F., Lee, A., and Couvet, D. (2008). Functional biotic homogenization of bird communities in disturbed landscapes. Glob. Ecol. Biogeogr. 17, 252–261. doi: 10.1111/j.1466-8238.2007.00364.x
Devictor, V., Mouillot, D., Meynard, C., Jiguet, F., Thuiller, W., and Mouquet, N. (2010). Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecol. Lett. 13, 1030–1040. doi: 10.1111/j.1461-0248.2010.01493.x
Frizzo, L. M., Campos, R. I., and Vasconcelos, H. (2012). Contrasting effects of fire on arboreal and ground-dwelling ant communities of a Neotropical savanna. Biotropica 44, 254–261. doi: 10.1111/j.1744-7429.2011.00797.x
Gossner, M. M., Lewinsohn, T. M., Kahl, T., Grassein, F., Boch, S., Prati, D., et al. (2016). Land-use intensification causes multitrophic homogenization of grassland communities. Nature 540, 266–269. doi: 10.1038/nature20575
Götzenberger, L., de Bello, F., Brathen, K. A., Davison, J., Dubuis, J., Guisan, A., et al. (2012). Ecological assembly rules in plant communities—approaches, patterns and prospects. Biol. Rev. 87, 111–127. doi: 10.1111/j.1469-185X.2011.00187.x
He, T., Lamont, B. B., and Pausas, J. G. (2019). Fire as a key driver of Earth's biodiversity. Biol. Rev. 94, 1983–2010. doi: 10.1111/brv.12544
Heuss, L., Grevé, M. E., Schäfer, D., Busch, V., and Feldhaar, H. (2019). Direct and indirect effects of land-use intensification on ant communities in temperate grasslands. Ecol. Evol. 9, 4013–4024. doi: 10.1002/ece3.5030
IPCC (2019). Climate Change and Land: an IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems, Summary for Policymakers (Approved Draft). IPCC. Available online at: https://www.ipcc.ch/site/assets/uploads/2019/08/Fullreport.pdf (accessed November 18, 2019).
Jacquet, K., and Prodon, R. (2009). Measuring the postfire resilience of a bird-vegetation system: a 28-year study in a Mediterranean oak woodland. Oecologia 161, 801–811. doi: 10.1007/s00442-009-1422-x
Jost, L. (2007). Partitioning diversity into independent alpha and beta components. Ecology 88, 2427–2439. doi: 10.1890/06-1736.1
Keeley, J. E. (2009). Fire intensity, fire severity and burn severity: a brief review and suggested usage. Int. J. Wildl. Fire 18, 116–126. doi: 10.1071/WF07049
Laurance, W. F., Nascimento, H. E. M., Laurance, S. G., Andrade, A., Ewers, R. M., Harms, K. E., et al. (2007). Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2:e1017. doi: 10.1371/journal.pone.0001017
Lázaro-González, A., Arnan, X., Boulay, R., Cerdá, X., and Rodrigo, A. (2013). Short-term ecological and behavioural responses to wildfire of a Mediterranean ant species, Aphaenogaster gibbosa (Latr. 1798). Insect Conserv. Divers. 6, 627–638. doi: 10.1111/icad.12018
Leps, J., De Bello, F., Lavorel, S., and Berman, S. (2006). Quantifying and interpreting functional diversity of natural communities: practical considerations matter. Preslia 78, 481–501.
Li, D., and Waller, D. (2015). Drivers of observed biotic homogenization in pine barrens of central Wisconsin. Ecology 96, 1030–1041. doi: 10.1890/14-0893.1
Liang, C., Yang, G., Wang, N., Feng, G., Yang, F., Svenning, J. C., et al. (2019). Taxonomic, phylogenetic and functional homogenization of bird communities due to land use change. Biol. Conserv. 236, 37–43. doi: 10.1016/j.biocon.2019.05.036
Liu, C., Guénard, B., Blanchard, B., Peng, Y.-Q., and Economo, E. P. (2016). Reorganization of taxonomic, functional, and phylogenetic ant biodiversity after conversion to rubber plantation. Ecol. Monogr. 86, 215–227. doi: 10.1890/15-1464.1
Lloret, F. (1998). Fire, canopy cover and seedling dynamics in Mediterranean shrubland of northeastern Spain. J. Veg. Sci. 9, 417–430. doi: 10.2307/3237106
Maravalhas, J., and Vasconcelos, H. L. (2014). Revisiting the pyrodiversity-biodiversity hypothesis: long-term fire regimes and the structure of ant communities in a Neotropical savanna hotspot. J. Appl. Ecol. 51, 1661–1668. doi: 10.1111/1365-2664.12338
McKinney, M. L., and Lockwood, J. L. (1999). Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453. doi: 10.1016/S0169-5347(99)01679-1
Monnet, A. C., Jiguet, F., Meynard, C. N., Mouillot, D., Mouquet, N., Thuiller, W., and Devictor, V. (2014) Asynchrony of taxonomic, functional phylogenetic diversity in birds. Glob. Ecol. Biogeogr. 23, 780–788. doi: 10.1111/geb.12179
Moretti, M., Duelli, P., and Obrist, M. K. (2006). Biodiversity and resilience of arthropod communities after fire disturbance in temperate forests. Oecologia 149, 312–327. doi: 10.1007/s00442-006-0450-z
Mouchet, M. A., Villéger, S., Mason, N. W. H., and Mouillot, D. (2010). Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct. Ecol. 24, 867–876. doi: 10.1111/j.1365-2435.2010.01695.x
Mouillot, D., Graham, N. A. J., Villéger, S., Mason, N. W. H., and Bellwood, D. R. (2013). A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. doi: 10.1016/j.tree.2012.10.004
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Newman, E. A. (2019). Disturbance ecology in the anthropocene. Front. Ecol. Evol 7:147. doi: 10.3389/fevo.2019.00147
Nowakowski, A. J., Frishkoff, L. O., Thompson, M. E., Smith, T. M., and Todd, B. D. (2018). Phylogenetic homogenization of amphibian assemblages in human-altered habitats across the globe. Proc. Natl. Acad. Sci. U.S.A. 115, E3454–E3462. doi: 10.1073/pnas.1714891115
Olden, J. D. (2006). Biotic homogenization: a new research agenda for conservation biogeography. J. Biogeogr. 33, 2027–2039. doi: 10.1111/j.1365-2699.2006.01572.x
Olden, J. D., Comte, L., and Giam, X. (2018). The Homogocene: a research prospectus for the study of biotic homogenization. NeoBiota 37, 23–36. doi: 10.3897/neobiota.37.22552
Olden, J. D., Poff, N. L.-R., Douglas, M. R., Douglas, M. E., and Fausch, K. D. (2004). Ecological and evolutionary consequences of biotic homogenization. Trends Ecol. Evol. 19, 18–24. doi: 10.1016/j.tree.2003.09.010
Olden, J. D., and Rooney, T. P. (2006). On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 15, 113–120. doi: 10.1111/j.1466-822X.2006.00214.x
Ordoñez, J. L., Franco, S., and Retana, J. (2004). Limitation of the recruitment of Pinus nigra in a gradient of post-fire environmental conditions. Ecoscience 11, 296–304. doi: 10.1080/11956860.2004.11682836
Parr, C. L., and Andersen, A. N. (2006). Patch mosaic burning for biodiversity conservation: a critique of the pyrodiversity paradigm. Conserv. Biol. 20, 1601–1619. doi: 10.1111/j.1523-1739.2006.00492.x
Parr, C. L., Dunn, R. R., Sanders, N. J., Weiser, M. D., Photakis, M., Fitzpatrick, M. C., et al. (2017). GLobal ants trait database (GLAD): a new database on the geography of ant traits (Hymenoptera: Formicidae). Insect Conserv. Divers. 10, 5–20. doi: 10.1111/icad.12211
Parr, C. L., Robertson, H. G., Biggs, H. C., and Chown, S.L. (2004). Response of african savanna ants to long-term fire regimes. J. Appl. Ecol. 41, 630–642. doi: 10.1111/j.0021-8901.2004.00920.x
Pausas, J. G., Bradstock, R. A., Keith, D. A., Keeley, J. E., and GCTE (Global Change of Terrestrial Ecosystems) Fire Network (2004). Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 85, 1085–1100. doi: 10.1890/02-4094
Pausas, J. G., and Keeley, J. E. (2019). Wildfires as ecosystem services. Front. Ecol. Environ. 17, 289–295. doi: 10.1002/fee.2044
Pausas, J. G., Llovet, J., Rodrigo, A., and Vallejo, R. (2008) Are wildfires a disaster in the Mediterranean basin? - A review. Int. J. Wildland Fire 17, 713–723. doi: 10.1071/WF07151
Pausas, J. G., and Parr, C. L. (2018). Towards an understanding of the evolutionary role of fire in animals. Evol. Ecol. 32, 1–13. doi: 10.1007/s10682-018-9927-6
Pausas, J. G., and Ribeiro, E. (2017). Fire and plant diversity at the global scale. Glob. Ecol. Biogeogr. 26, 889–897. doi: 10.1111/geb.12596
Pavoine, S., Dufuor, A. B., and Chessel, D. (2004). From dissimilarities among species to dissimilarities among communities: a double principal coordinate analysis. J. Theor. Biol. 228, 523–537. doi: 10.1016/j.jtbi.2004.02.014
Pérez-Granados, C., Serrano-Davies, E., and Noguerales, V. (2018). Returning home after fire: how fire may help us manage the persistence of scrub-steppe specialist bird populations. Biodivers. Conserv. 27, 3087–3102. doi: 10.1007/s10531-018-1586-y
Ponge, J.-P. (2013). Disturbances, organisms and ecosystems: a global change perspective. Ecol. Evol. 3, 1113–1124. doi: 10.1002/ece3.505
Pons, P., and Bas, J. M. (2005). Open-habitat birds in recently burned areas: the role of the fire extent and species' habitat breadth. Ardeola 52, 119–131.
Purschke, O., Schmid, B. C., Sykes, M. T., Poschlod, P., Michalski, S. G., Durka, W., et al. (2013). Contrasting changes in taxonomic, phylogenetic and functional diversity during a long-term succession: insights into assembly processes. J. Ecol. 101, 857–866. doi: 10.1111/1365-2745.12098
Rao, C. R. (1982). Diversity and dissimilarity coefficients: a unified approach. Theor. Popul. Biol. 21, 24–43. doi: 10.1016/0040-5809(82)90004-1
Retana, J., and Cerdá, X. (2000). Patterns of diversity and composition of Mediterranean ground ant communities tracking spatial and temporal variability in the thermal environment. Oecologia 123, 436–444. doi: 10.1007/s004420051031
Ribeiro-Neto, J., Arnan, X., Tabarelli, M., and Leal, I. R. (2016). Chronic anthropogenic disturbance causes homogenization of plant and ant communities in the Brazilian Caatinga. Biodivers. Conserv. 25, 943–956. doi: 10.1007/s10531-016-1099-5
Ripple, W. J., Wolf, C., Newsome, T. M., Barnard, P., and Moomaw, W. R. (2020). World scientists' warning of a climate emergency. BioScience 70, 8–12. doi: 10.1093/biosci/biz088
Rodrigo, A., Arnan, X., and Retana, J. (2012). Relevance of soil seed bank and seed rain to immediate seed supply after a large wildfire. Int. J. Wildland Fire 21, 449–458. doi: 10.1071/WF11058
Rodrigo, A., and Retana, J. (2006). Post-fire recovery of ant communities in Submediterranean Pinus nigra forests. Ecography 29, 231–239. doi: 10.1111/j.2006.0906-7590.04272.x
Rodrigo, A., Retana, J., and Picó, F. X. (2004). Direct regeneration is not the only response of Mediterranean forests to large fires. Ecology 85, 716–729. doi: 10.1890/02-0492
Rodrigo, A., Sardà-Palomera, F., Bosch, J., and Retana, J. (2008). Changes of dominant ground beetles in black pine forests with fire severity and successional age. Ecoscience 15, 442–452. doi: 10.2980/15-4-3117
Santos, X., and Cheylan, M. (2013). Taxonomic and functional response of a Mediterranean reptile assemblage to a repeated fire regime. Biol. Conserv. 168, 90–98. doi: 10.1016/j.biocon.2013.09.008
Sasal, Y., Raffaele, E., and Farji-Brener, A. (2010). Succession of ground-dwelling beetle assemblages after fire in three habitat types in the Andean Forest of NW patagonia, argentina. J. Insect Sci. 10:37. doi: 10.1673/031.010.3701
Seidl, R., Thom, D., Kautz, M., et al. (2017). Forest disturbances under climate change. Nat. Clim. Change 7, 395–402. doi: 10.1038/nclimate3303
Stein, A., Gerstner, K., and Kreft, H. (2014). Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 17, 866–880. doi: 10.1111/ele.12277
Taylor, J. E., and Fox, B. J. (2001). Disturbance effects from fire and mining produce different lizard communities in eastern Australian forests. Austral. Ecol. 26, 193–204. doi: 10.1046/j.1442-9993.2001.01105.x
Trauernicht, C., Brook, B. W., Murphy, B. P., Williamson, G. J., and Bowman, D. M. J. S. (2015). Local and global pyrogeographic evidence that indigenous fire management creates pyrodiversity. Ecol. Evol. 5, 1908–1918. doi: 10.1002/ece3.1494
Turner, M. G., Romme, W. H., and Gardner, R. H. (1999). Prefire heterogeneity, fire severity, and early post-fire plant reestablishment in subalpine forests of Yellowstone National Park, Wyoming. Int. J. Wildland Fire 9, 21–36. doi: 10.1071/WF99003
Vasconcelos, H. L., Maravalhas, J. B., and Cornelissen, T. (2017). Effects of fire disturbance on ant abundance and diversity: a global meta-analysis. Biodivers. Conserv. 26, 177–188. doi: 10.1007/s10531-016-1234-3
Velle, L. G., Nilsen, L. S., Norderhaug, A., and Vandvik, V. (2014). Does prescribed burning result in biotic homogenization of coastal heathlands? Glob. Change Biol. 20, 1429–1440. doi: 10.1111/gcb.12448
White, H.J., Montgomery, W.I., Storchová, L., Horak, D., and Lennon, J.J. (2018). Does functional homogenization accompany taxonomic homogenization of British birds and how do biotic factors and climate affect these processes? Ecol. Evol. 8, 7365–7377. doi: 10.1002/ece3.4267
Keywords: ants, biotic homogenization, fire, forests, functional diversity, Mediterranean, spatial scale, phylogenetic diversity
Citation: Arnan X, Cerdá X and Rodrigo A (2020) Do Forest Fires Make Biotic Communities Homogeneous or Heterogeneous? Patterns of Taxonomic, Functional, and Phylogenetic Ant Beta Diversity at Local and Regional Landscape Scales. Front. For. Glob. Change 3:67. doi: 10.3389/ffgc.2020.00067
Received: 28 November 2019; Accepted: 06 May 2020;
Published: 23 June 2020.
Edited by:
Peter Fule, Northern Arizona University, United StatesReviewed by:
Daniel Moya, University of Castilla La Mancha, SpainCopyright © 2020 Arnan, Cerdá and Rodrigo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xavier Arnan, eGF2aS5hcm5hbkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.