
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Fish Sci. , 19 February 2025
Sec. Fish Experimental Biology
Volume 3 - 2025 | https://doi.org/10.3389/frish.2025.1525181
This article is part of the Research Topic Patterns, causes and consequences of intraspecific variation in environmental tolerance in fishes View all 4 articles
This review revisits 20 years of collaborative studies that were pursued with Louis Bernatchez who sadly passed away in October 2023. With him, we explored the phenotypic plasticity of brook charr by combining ecophysiology, genetics, genomics, and more recently epigenetics. Over the years, we conducted extensive studies on brook charr, focusing on metabolism, stress response, growth regulation, and temperature tolerance across various strains. Our research highlighted the remarkable diversity in physiological responses to temperature and salinity conditions, along with significant differences in the heritability of key traits across different strains and life stages. We studied stages from yolk-sac fry to reproductive adults, compared freshwater residents and anadromous fish, and recently showed how epigenetics affects the physiological and transcriptomic responses of progeny to temperature conditions. This review highlights the incredible physiological plasticity of brook charr and presents future research avenues that will lead to a better understanding of how the species may face challenges related to global changes.
This review is a tribute to the collaborative work we accomplished with Louis Bernatchez on brook charr Salvelinus fontinalis. His passion for biological sciences, in particular for fish biology, his drive to always be at the forefront of technological developments, and his incredible capacity to understand complex questions made him the best collaborator we could have wished for. His mentorship and his friendship have enriched and inspired us, and he has left a strong legacy on this work and will continue to do so for many years.
The objective of this special issue is to highlight intraspecific variations in the functional traits of organisms. One of the main questions about intraspecific variations is whether there is a link between phenotypic variation and underlying genetic diversity. In other words, is there enough genetic variation for selection in the presence of environmental changes. This is what we addressed over the years using brook charr. While we do not yet have the final answer, we have clearly shown through different research projects how phenotype—genomic architecture relationships are incredibly complex and how the genetic influence on different traits varies during the life history of brook charr.
One way that organisms can cope with environmental fluctuations is through phenotypic plasticity. Phenotypic plasticity is a fast-acting mechanism that refers to the ability of an organism to alter its phenotype, including physiological, behavioral, and morphological traits, in response to changes in environmental conditions (1–3). Phenotypic plasticity provides immediate flexibility to changing environments, while genetic variation within a population ensures that selection can act to stabilize advantageous traits over generations, providing a long-term mechanism for adaptation to persistent environmental changes. The presence of phenotypic plasticity has long been recognized in fishes (4–8). The time of maximal plasticity in the life of an organism is during its developmental phase. The concept of developmental plasticity therefore implies a controlled disruptive process of the developmental program by the environment, and this process is controlled by inherited genes in early stages (egg, embryo, larvae), rather than random variations or “noise” in their developmental program. The effects of a changing environment occurring during these early stages can sometimes be observed later, so the environment experienced during early stages can affect the juvenile or adult phenotypes through carry-over effects, programming, or conditioning (9, 10).
Salmonidae display a tremendous diversity in terms of phenotypic traits both at the interspecific and intraspecific levels (11). As phenotypic variations arise not only from variations in genomic sequences but also from the genetic architecture, defined as the sum of the interacting genetic dimensions that leads to a given phenotype (12), our collaborative work combined phenotypic and genetic approaches in different brook charr populations and their hybrid crosses. Indeed, hybridization can provide information about parental genetic architecture. For instance, while parental populations are genetically divergent and adapted to their own environments, hybrids may express non-additive genetic effects due to complex genetic associations (13–15). These non-additive genetic effects are either called heterosis, when the hybrids express a phenotype better than both parents, or outbreeding depression, when the hybrids express a phenotype worse than both parents.
Through our work, we thus investigated phenotype–genotype–environment relationships on traits related to early development, growth, metabolism, climate change tolerance, sex-linked factors, and anadromy (Figure 1). We found strong variability across these relationships, even using only a limited number of populations. We suggest that an extensive overview of different brook charr populations would reveal an astonishing phenotypic diversity and adaptability on which brook charr could rely to ensure their continued existence.
The brook charr populations we used all presented specific features. First, the anadromous brook charr population from Laval River (L) was central to most of our work. The Laval River is located on the north shore of the St. Lawrence Estuary (48° 44′ N; 69° 05′ W); the migratory movements of its anadromous population were described in Curry et al. (16).
A freshwater resident population (Lr) is also present in this river. The freshwater residents originated from Adam Brook in the Laval River drainage, and the spawning sites of both populations in the Adam Brook are parapatric (17, 18). Analyses of neutral genetic markers have shown that these two populations (L and Lr) were partially reproductively isolated (19). This was further confirmed by the pronounced genetic divergence between these two populations based on allele frequencies at nine microsatellite loci [FST = 0.15; (17)].
While we did not maintain the Lr population for more than two generations in captivity (Station aquicole, ISMER, Rimouski, QC, Canada), we did initiate a selective program using the wild Laval anadromous population with the objective of developing a new strain (Ls) characterized by fast growth and reduced precocious sexual maturation (20, 21). A control anadromous strain (L) was continuously maintained at ISMER for seven generations, fusing the random within-family selection of breeders; this control anadromous strain was used in many of the studies cited hereafter.
The Rupert strain (R), which originates from a strictly freshwater resident population from the Rupert River (51° 05′ N; 73° 41′ W) near Lake Nemiscau (near James Bay in north-western Québec), was also used in our work. The Rupert strain was maintained at the Laboratoire de recherche en sciences aquatiques (LARSA, U. Laval, Québec, QC, Canada) for three generations. The R population was highly genetically distinct from the L population [FST = 0. 43 ± 0.02 using microsatellites markers; see (22)].
Finally, we also worked with a domestic strain (D) that has been used for aquaculture production in Québec, Canada, for more than a 100 years. These domestic fish show different morphological and ecological characteristics compared to wild brook charr populations (see (23) for example). Domestic breeders were obtained from local fish producers. The D strain was also genetically distinct from the R and L populations (22). It should be mentioned that rearing conditions were always the same for all cross-types compared (common garden type experiments) in the studies presented hereafter.
As mentioned earlier, plasticity may be highly expressed in early developmental stages. While studying traits related to early development, we focussed on three specific stages: the yolk-sac stage, during which fry depend on the quality of reserves deposited in the eggs; the yolk-sac resorption stage, with the beginning of external feeding; and the juvenile stage. According to classical quantitative genetic theory, early offspring phenotypes are partially controlled by the common environmental effects related to mothers and the genetic variance in reproductive investment by the mother to their offspring (24–26). In salmonids, resource accumulations in the yolk sac are critical for the transmission of maternal genetic variance since no behavioral parental care occurs. Maternal variance may also affect the progeny's phenotype via maternal–offspring genetic covariance [covariation between the effects of genes expressed by mothers and those expressed within their progeny; (25, 27, 28)].
Using L fish, we showed the abrupt transition from high maternal genetic control on embryonic fork length and yolk sac volume (maternal effects > 0.5) to sharp decrease in maternal variance for length following resorption of the yolk sac (29). The Perry et al. (29) study suggested that yolk-sac resorption was the major turning point, where maternal genetic contributions were replaced by those of individual progeny. Whether the presence of strong maternal effects early in development may be advantageous for local adaptation to the spawning habitat [see (29)], challenges met later on by fry may require more phenotypic flexibility. Such flexibility would be facilitated by the disappearance of strong maternal effects. From this point, these stages of development became our reference to study phenotypic variations during development.
After five generations, selection for absence of early sexual maturation combined with better growth performance (selected Ls) influenced early development compared to controls [L; (30)]. At hatching, dam identity significantly explained half of the variance for the standard length with low heritability in Ls fry, while dam identity explained 28% compared to the control strain (L fish; Figure 2). At the yolk-sac resorption stage, the proportion of variance in standard length that was explained by dam identity was lower and heritability was higher in both groups, indicating again the presence of differences in genetic contributions at this boundary.
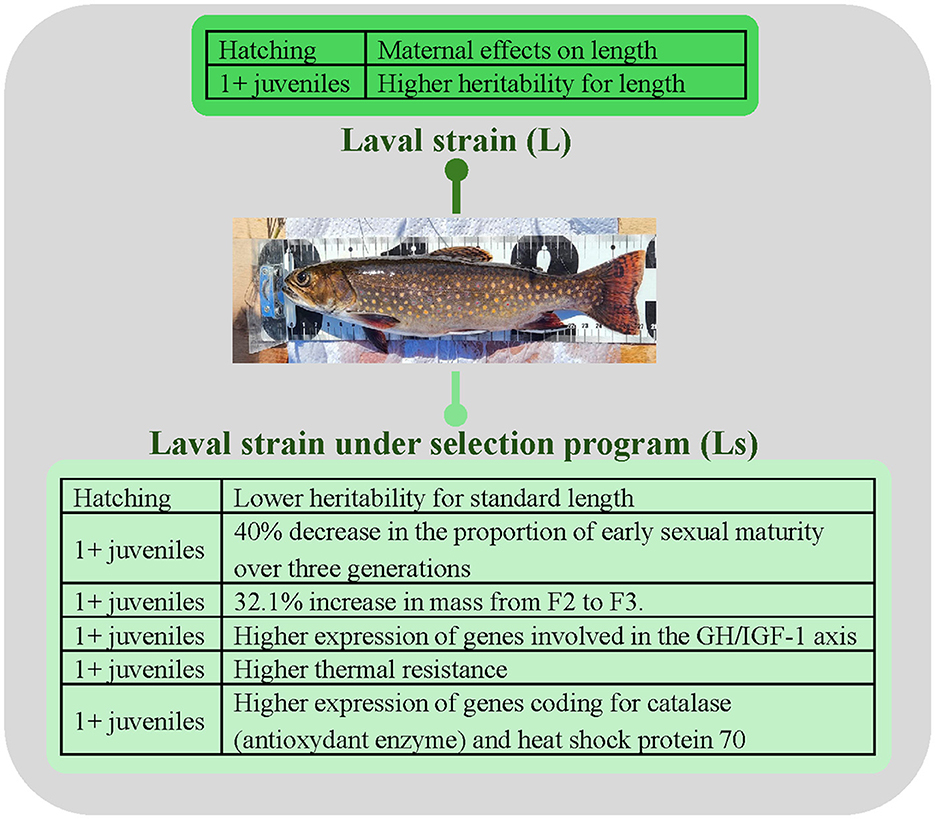
Figure 2. Highlights of differences between the Laval control strain (L) and the Laval selected strain (Ls; absence of early sexual maturation combined with better growth performance) at different stages of development. Brook charr photo credit: Julie Viana.
We were also interested in approaching phenotypic variability and its underlying genetic architecture through the response of hybrids between populations and the expression of heterosis. When we undertook this work, there was a general belief that heterosis was rare in salmonids (31). While it was previously suggested that there was a positive relationship in the genetic divergence between parents and the performance of their hybrid offspring, we compared the three strains (L, R, and D) and hypothesized that crossing the two most genetically distant ones (L × R) would increase the potential of a positive heterosis outcome (32). Using pure and reciprocal hybrid crosses for a total of 72 families among the three populations, we showed strong phenotypic differentiation in the early life stages of the three parental populations. The L fry had the biggest yolk-sac volume at hatching (85.5 ± 18.9 mm3) and the highest mass (0.12 ± 0.02 g) and length (25.7 ± 1.0 mm) at yolk-sac resorption (Figure 3). Ater 15 weeks of exogenous feeding, the D and R fry were bigger, with D fry exhibiting the highest increase in mass (0.00045 g degree-days−1). We were also able to show a complex pattern for both heterosis and outbreeding depression that varied according to developmental stages, with higher occurrences of heterosis (10 depending on different growth traits and crosses) than outbreeding depression (only two occurrences). Overall, the occurrence of heterosis seemed to be unpredictable and not necessarily related to the genetic distance of the parental populations.

Figure 3. Highlights of differences among the Domestic strain (D), the Rupert strain (R), and the Laval strain (L). See the section on our model brook charr populations for more information. Brook charr photo credit: Julie Viana.
Using the same experimental framework but only on the pure populations, Bougas et al. (33) focussed on the yolk-sac stage using microarray technology. They found 72 genes that were differentially expressed between the R and L fry (the two most genetically distant populations), but 178 genes that were differentially expressed between the R and D fry and 191 genes between L and D fry (Figure 3), indicating that divergence among populations was present not only at neutral loci (22) but also in coding areas. Interestingly, while R and L were the most genetically distant populations from a transcriptome standpoint, the D strain was the most differentiated and the R and L fish more similar to each other. Functional categories that were affected were detailed in Bougas et al. (33, 34), but a clear relationship with measured phenotypic traits was not obvious (except for metabolism as described below). However, the results of Bougas et al. (33) clearly showed how production under similar conditions enhances different transcriptome responses, and undoubtedly different phenotypic traits.
Growth is one of the most important fitness traits—not only when pursuing a more efficient production of livestock species, but also for ecological reasons, since it could determine the survival success of individuals (35). Over the years, we thus assessed growth potential through studies on domestication, genetics, endocrine regulation, and environmental contributions. Growth is a complex trait that relies on a network of genes (e.g., pleiotropy), as well as on many different types of environmental inputs (36). However, although many non-genetic factors shape growth variability, most studies investigating its heritability still revealed moderate-to-high levels of genetic variance/heritability (37).
When we started our selection program on the L population, growth in sexually immature 1+ fish was a trait that we focussed on (20, 21). We obtained an increase of 24 g from the F1 to the F2 generation (23.1%) and of 41 g (32.1%) from F2 to F3 (Figure 3). As expected, the phenotypic variance in weight was higher in L than in selected Ls individuals (21). Heritability estimates for the F3 generation were generally high (h2 > 0.4) for this trait (21). When we examined the transcriptomic response in the F4, we found that three generations of selective breeding resulted in significant changes in the regulation of gene transcription between L and Ls (20). Genes involved in growth pathways were generally expressed at higher levels in the Ls strain, whereas genes associated with other biological functions were generally expressed at lower levels relative to the control strain [L; (20)]. A cluster analysis performed on a subset of 52 genes associated with one of five under- or overrepresented biological processes almost completely separated the fish into two groups (controls/selected) except for eight of the 40 individuals. All these results suggested that selection on growth was indeed possible, and therefore that growth was a trait with high genetic regulation in brook charr.
Using the same experimental framework as described above for Bougas et al. (33) and Granier et al. (32), Crespel et al. (38–40) pursued the experiment until 21 months of age, adding comparisons with three different rearing environments: controlled rearing conditions and constant temperature environment (at LARSA), controlled rearing conditions and natural temperature variations (at ISMER), and fish farm conditions (outdoor pond). When considering the three strains (L, R, and D), we found that body mass was a heritable trait in all populations (h2 from 0.1 to 0.6), but that the level of heritability greatly differed among populations (2 fold greater in D than in L and R). In addition, heritability differed not only among strains, but also according to environmental conditions, with population-specific patterns of genotype–environment interactions (40). Indeed, D fish showed no change in heritability related to the environment while there was a decrease for L fish and an increase for R fish between the constant and variable temperature environments (Figure 3). Such heritability differences among populations and environments indicated divergent genetic architecture and therefore different potentials for evolutionary response among populations. From these results, we hypothesized that the high heritability in the D strain could provide an advantage since it could evolve faster than the two other strains when exposed to environmental selective constraints. We also postulated that the plasticity expressed by L and R fish could be a major asset in terms of adaptations to new environmental conditions. Using only three strains, we showed the presence of pronounced divergence in gene × environment interactions indicating that we must expect a large variability among brook charr populations. Such variability may represent different adaptive potential in presence of environmental variations (conservation, management), but it could also suggest differences in potential to respond to selection [fish production; e.g., (41)].
When looking for potential hybrid and heterosis effects on mass later in development, we noted the highest occurrence of heterosis in L♀R♂ hybrids (across all sampling times from 9 to 21 months and in the three tested environments), with masses from 9 to 20% greater than the average mass of the parental strains (for example: LARSA, 21 months, 155.7 ± 9.7 g compared to 106.3 ± 6.4 and 126.9 ± 7.7 g for the L and R fry). In contrast to previous results obtained on younger developmental stages (see above), this temporal heterosis appeared in only one of the reciprocal crosses involving parental populations with the highest level of genetic differentiation. Interestingly, heterosis was associated with cross direction (e.g., only R♀L♂ showed heterosis at the fish farm site), suggesting some sex-specific genetic architecture in these strains. In addition, heterosis in the L♀D♂ hybrid was only present in the constant temperature environment, suggesting phenotypic plasticity in the expression of heterosis (for example, LARSA, 21 months, 241.1 ± 27.3 g compared to 106.3 ± 6.4 and 217.6 ± 15.5 for the L and D fry). Because environmental interactions were not observed in all hybrid crosses, we hypothesized that environmental variability influenced different genomes in different ways, again confirming divergent genetic architecture for this trait among strains.
It is well known that growth can be impaired by stress. We examined stress response in 16-month-old fish issued from this same experimental framework, but only using fish reared at ISMER [controlled conditions, natural temperature variations; (38)]. We used a simulation of fish transfer procedures in transport bags as the stress exposure. The R population displayed a less pronounced response to transport stress, while the L population seemed to be the most sensitive (Figure 3). Correlations of body mass with both the primary and the secondary stress responses were weak, indicating a limited effect of body mass on stress resistance. The heritability estimates for cortisol (primary stress response), and plasma glucose (secondary stress response) following stress exposure were high (h2 > 0.6) in contrast to other indicators of the secondary response (plasma osmolality and haematocrit). Both maternal and paternal effects were significant when looking at the primary response (cortisol) and maternal effects were also observed on secondary response indicators (plasma osmolality and haematocrit). Again, these results indicated how physiological responses may differ among different populations.
More recently, we looked at growth regulation. We compared fifth generation Ls to the L strain as well as among- and within-families variability according to the averaged whole family performance and slow- and fast-growing fish within each family (42). Between strains—one under selection, the other not—we used “family performance” to indicate families expressing differences in average growth phenotypes; individuals within families that expressed extreme growth phenotypes were termed slow or fast growing. Not surprisingly, fish from the selected strain were 37.3% heavier and 11.5% longer than fish from the L strain (11.95 g ± 4.57 vs. 8.71 g ± 3.36; 10.76 cm ± 1.38 vs. 9.65 cm ± 1.26). However, the mean condition factor of L fish was significantly (even though very closed) higher than that of Ls fish (0.93 ± 0.14 vs 0.92 ± 0.12). The most interesting feature was how growth was regulated at the genetic level. Indeed, we found selection enhanced differential expressions of target genes involved in the GH/IGF-1 axis (Figure 3). Growth performance in the Ls strain was associated with an upregulation of liver igf-1 and muscle igf1-r gene expressions, indicating an effect of selection on the axis starting with pituitary ifg-1r and followed by liver ghr-1, liver igf-1, muscle ghr-1, and muscle igf-1r. Looking at among-family variability, the mass of the best- and the least-performing families in the Ls strain differed by 49.4%, while the difference was 14.8% in L fish. The family with the lowest mass in the Ls strain was significantly different from the rest of the Ls families, but not from the L families, which had average or low performance. At the genetic level, familial and individual phenotypes (slow growing vs. fast growing) were associated with upregulations of the lepr and npy genes related to appetite regulation. This again illustrates the complexity of the underlying mechanisms governing phenotype expression, with phenotype being the results of complex physiological/regulation mechanisms with genetic differences at the individual level not necessarily translated into population selection responses.
Quantitative trait loci (QTL) control portions of a species' genome that affect the variation of heritable phenotypic traits. They are revealed by the association of phenotypes with molecular markers (43). In a first attempt to identify QTL in brook charr, we developed a long-term survey of juveniles to measure many different phenotypes. Sauvage et al. (44, 45) found 63 QTL that were linked with growth and could be classified into two groups; 1) those composed of numerous, small-effect QTL that may be under the control of a large number of genes or pleiotropic genes, and 2) a group of fewers QTL associated with growth (i.e., gene expression) that displayed a larger effect, suggesting that they were under the control of a limited number of genes that had a major effect. Four QTL were also associated with the stress response to an acute stress challenge (45).
Metabolism is another complex but important fitness-related trait determining the capacity of individuals to survive in their environments because it determines the energy available for the fish to perform its physiological and behavioral activities. Metabolism is then a key determinant of fitness and plays a crucial role in mediating phenotypic plasticity, enabling organisms to adapt to environmental changes [e.g., (46)]. At the yolk-sac resorption stage, Bougas et al. (33) found transcriptional differences related to metabolism between the R and L populations and suggested that they may partially reflect adaptive responses to their distinct native environments (local adaptation). Thus, some genes related to carbohydrate metabolism were under expressed, whereas genes related to lipid metabolism (apolipoprotein A, fatty acid-binding protein) were over expressed in the L population. This could reflect a reduced basal metabolism and an increased growth-related resource allocation. At the time, we suggested that this could result in better growth performance (with L fry bigger than R fry at this life stage). This observed metabolic differences may suggest that the L population would prioritize growth-related processes over basal metabolic maintenance during early stages of development, potentially conferring an advantage in resource-rich environments, but this would need further investigation.
At a later stage of development, Crespel et al. (47) investigated intraspecific strategies of energy mobilization in the R, L, and D strains related to the accumulation of energy reserves during the fall and their use during the first winter of life. We found strong metabolic differences and strategies to cope with winter conditions among the three populations. Briefly, D fish accumulated high energy reserves before winter and kept accumulating liver glycogen during winter; L fish had low energy reserves at the onset of winter, mobilized liver glycogen and lipids during winter, and showed a reduced condition factor by March; and R fish had relatively low energy reserves accumulated at the onset of winter and mobilized visceral fat during winter (Figure 3). It is well known that the condition factor of anadromous fish is low during spring, and these results corresponded to those of Bastien et al. (21), who showed that genetic correlation between weight and condition factor was high and positive in L fish, except in April. In D and L strains, energy reserves patterns had heritable genetic bases while this was not found for R individuals. The three strains also showed distinct patterns of genetic correlations among the heritable traits measured, which again suggested differences in their genetic architecture related to energy mobilization. The genetic basis for energy strategy thus seemed to be population-specific and cannot be extrapolated to other populations. However, these distinct strategies may reflect evolutionary trade-offs that balance energy reserves with adaptive responses to overwintering conditions. This underlines how, in brook charr, conservation and management issues should not be considered as being species-specific, but rather as being population-specific.
With global warming, a species like brook charr, which inhabits temperate/cold and highly oxygenated water, may be at risk. In general, fishes—as ectotherms—are vulnerable to temperature changes because their physiology is determined by the thermodynamic effects of the surrounding water temperature, which sets their body temperature (48–50). Under global warming, genetic variation may not be sufficient to allow rapid adaptation to new selection pressures (51), and phenotypic plasticity may represent a rapid response mechanism to cope with these changes (52). We thus began a series of experiments to study temperature tolerance in this species. Because brook charr in Québec are essentially produced for stocking enhancement, we first tested whether selection for the absence of early sexual maturation combined with better growth performance in brook charr may have affected their thermal resistance and the gene expression response in the presence of thermal stress (30). Contrary to our expectations, Ls juveniles (under the selection process for five generations) had a higher thermal resistance than L fish (control population). In addition, once submitted to an acute thermal stress challenge, their loss of equilibrium occurred on average 1°C higher than what was observed for L individuals (30). The selected fish also showed higher relative liver cat (coding for catalase, an antioxidant enzyme) and hsp 70 (coding for heat shock protein HSP 70) gene expressions (Figure 3). These results provided support for adaptive differences between the Ls and L strains of brook charr in their potential for gene expression-mediated phenotypic plasticity in response to temperature changes. They also support that selection for greater thermal tolerance may be possible. As Ls juveniles were bigger than L ones, the relationship between weight and thermal tolerance certainly deserves to be studied further at the physiological level [see (53)].
We also explored the potential for multigenerational plasticity, when the environment of previous generations shapes the physiological and behavioral phenotypes of offspring (54, 55). Intergenerational plasticity occurs when plastic phenotypes are passed on from a parent (56), based on their environmental experience, to offspring and influence their fitness (57, 58) and fitness-related traits (59, 60). Such intergenerational plasticity is generally supported by non-genetic inheritance, including, among others, parental effects and provisioning as well as epigenetic inheritance. Epigenetic inheritance refers to the transmission of epigenetic markers (e.g., DNA methylation) that can modify gene expression, ultimately affecting the phenotype and fitness in offspring (55, 61). To achieve our goal, we exposed breeders to autumnal temperature decreases differing by 2°C over the whole period during which final gonad maturation occurs (producing cold and warm breeder groups). We then raised half of the offspring from each group at 5°C and the other half at 8°C (62, 63). Using whole-genome bisulfite sequencing, we showed that parental sexual maturation temperature—but not offspring rearing temperature—had considerable effects on DNA methylation in juveniles [2 months after the beginning of exogenous feeding; (62)]. The negligible effects of offspring rearing temperature on DNA methylation was unexpected due to the overwhelming evidence that thermal regime drives within-generation plastic changes in DNA methylation in fish. However, these results were supported by the subsequent study conducted on brain transcriptome (63). Hierarchical cluster analysis of the 500 most expressed genes resulted in a clear separation of brain gene–transcriptional expression in fry based on parental temperatures during final gonad maturation. As for DNA methylation, offspring rearing temperatures did not have a significant impact on gene expression. Neuroplasticity and processes related to thermal tolerance, such as those involved in energy production, immunity, and cellular stress response, were the main categories of functions affected by parental temperature increase, with upregulation of the most salient processes related to thermal stress compensation and downregulation of several functions related to neuronal and synaptic activity. This suggested that even though brook charr may have some ability to cope with warmer conditions due to intergenerational plasticity, their behavior may still be affected. Studies on the transcriptome of other fry organs are presently in progress and should generate more information on the epigenetic inheritance related to temperature.
Finally, we also assessed how parental thermal conditions during final gonad maturation (cold and warm breeders described above) influenced offspring growth and survival 1 year after stocking in natural lakes (64). Interestingly—but contrary to our expectations—our results revealed that offspring from cold breeders showed lower survival than those from warm breeders. A potential explanation for this observation is that lakes where fish were stocked were unusually warm during our experiment and, thus, that beneficial intergenerational plasticity may have favored and increased the survival of fish from the warm-breeder temperature group. Such transmitted plasticity could thus be highly beneficial when the parental environments match offspring environments, which is expected to happen more and more often in climate change scenarios. If such plasticity can be transmitted over several generations, it could also help to buffer environmental effects until genetic accommodation can occur.
Understanding how sex bias in gene expression contributes to sexually dimorphic phenotypes, and how this affects fitness, is important for understanding genotype–phenotype interactions (65). Working with the L strain, Bastien et al. (21) showed that selection led to a 40% decrease in the proportion of early sexual maturity at 22 months over three generations (from 67.5% of early sexual maturation to 38.6%) (Figure 3). This result revealed that both growth and lower early maturation could be simultaneously selected in these fish, reflecting different underlying genetic regulations. The analysis of genetic variance–covariance of precocious maturation and weight at 22 months in the F3 population indicated moderate to high heritability (h2 > 0.30) for both traits in both the Ls and L strains (21). In addition, Crespel et al. (39) compared the L, R, and D strains and as well as their hybrids and showed a greater proportion of early sexual maturation in 1+ juveniles of the D population (more than 25%) than in the other pure populations (<10%), but there was no heterosis for environmental effect related to this trait (Figure 3). Because cross direction in the hybrids still played a role on the expression of some phenotypes, a possible linkage between sex genes and genes associated with some traits of performance may be present. Such a linkage might influence the predominance of a specific population as female or male (66–69).
With Sutherland et al. (70, 71), we looked at the genetic architecture in relation to antagonistic selection and sex determination. Some alleles may benefit sex differently (43, 72), while allelic dominance may also depends on the individual's (73). Moreover, the salmonid genome remains in a residually tetraploid state, with some chromosomal telomeric regions continuing to recombine between homologous chromosomes while others have rediploidized (74–77). A sex-linked chromosome was identified in brook charr (70) that was different from those linked to sex in any other species characterized by high-density genetic maps.
Sutherland et al. (71) constructed a high-density genetic map with 3,826 markers [details in (78)] from the same phenotypic data set used by Sauvage et al. (44, 45) and were able to characterize co-expression networks for both female and male liver transcriptomes and compare them. Sexual dimorphism in body size and secondary sexual characteristics is associated with reproductive success in brook charr and other salmonids (79). In our results, we showed that females displayed higher inter-individual variance in gene expression than males. The liver tissue was used for this work and many phenotypic associations in females, but not in males, were related to sexual maturation. As liver function is crucial to the vitellogenesis process, this may partly explain the difference observed in inter-individual variance between sexes. A network comprises modules, each of which comprises a group of genes with correlated expression patterns. Unlike the female network, a large proportion of the male data could not be assigned to a module. Our observations confirmed previous findings that co-expression patterns are often preserved between sexes (80, 81). The most preserved modules between the sexes were involved in basic cellular processes, such as translation machinery. Immunity-related modules were also preserved between the sexes.
A total of 29 QTL were found to be significant at the chromosome-wide level, and these included QTL for egg and sperm diameter; changes in cortisol, chloride, and osmolality after an acute handling stress; growth hormone receptor gene expression; and hematocrit (71). Several traits showed sexual dimorphism and therefore required sex as a model covariate; these included weight, length, liver weight, hepatosomatic index, hematocrit, change in osmolality and cortisol from a stressor, resting plasma chloride, hepatic glycogen, and the expressions of insulin-like growth factor 1 and ifg receptor genes. However, growth, condition factor, change in chloride, resting plasma osmolality and glucose, and growth hormone receptor gene expression did not show sexual dimorphism. Changes in cortisol from acute handling stress were highly dependent on sex: the identified QTL explained 9% of the trait variation whereas sex explained 43%. To a lesser extent, osmolality change was also affected by sex, with the identified QTL explaining 14% of the trait variance and sex explaining 14.6%. Sex thus seemed to have a huge implication in the expression of many growth- and stress-related traits in brook charr. QTL are primary viewed as efficient tools for marker-assisted selection and mapping of QTL may help avoiding negative correlations with other traits. More importantly, enhanced understanding of the genetic architecture is essential to better understand genotype-phenotype interactions and therefore phenotypic plasticity.
The phenotype–genotype relationship goes far beyond the expression of specific traits. Complex assemblages of phenotypes characterize different ecotypes. In coastal areas, where there is access to a marine environment, two main ecotypes of brook charr are observed: the anadromous ecotype, which undertakes migration from freshwater lakes and rivers to estuaries and returns to freshwater habitats for spawning, and the resident (non-anadromous) ecotype, which permanently resides in fresh water (16). The two ecotypes may live in sympatry, either as alternative life history tactics within a single gene pool (82) or as locally adapted populations and can result in varied trajectories for growth and maturation across sizes and ages [e.g., (83)]. Moreover, the characteristics of anadromy seem to be related to river-specific conditions in which the ecotype occurs (16). For example, comparing three feral anadromous populations [the Laval River population located on the north shore of the St. Lawrence estuary, the Petite Cascapédia River population located in the Baie-des-Chaleurs, and the Kennebecasis River population located in southern New-Brunswick; (16)], the Laval River fish were the most “anadromous” as they traveled to the estuary and remained at near full salinity (salinity up to 27‰, 10°C) for the majority of summer (84). The direct comparison between the L and Lr ecotypes in the field did not show a significant relationship between length or weight and any of the variables measured, which ensures that our results were not biased by size differences among groups (19). Physiological differences in osmoregulation traits once captured in sea water, gill Na+-K+-ATPase activity (osmo-iono-regulation), thyroid hormones (silvering, homing), and stress levels (reactivity) were observed between Lr and anadromous fish, with these traits being more elevated in the L population (Figure 4). These physiological differences were also associated with significant divergences at microsatellite markers. Indeed, genetic data strongly supported the hypothesis that L fish collected at different time periods during this study belonged to a single gene pool, while Lr fish were strongly genetically differentiated, with a clear signal of genetic divergence between L and Lr fish (19). We also compared wild L fish to the L fish maintained in our laboratory and found that the wild and domestic anadromous fish were much more similar to each other than were the wild L and Lr fish (19).
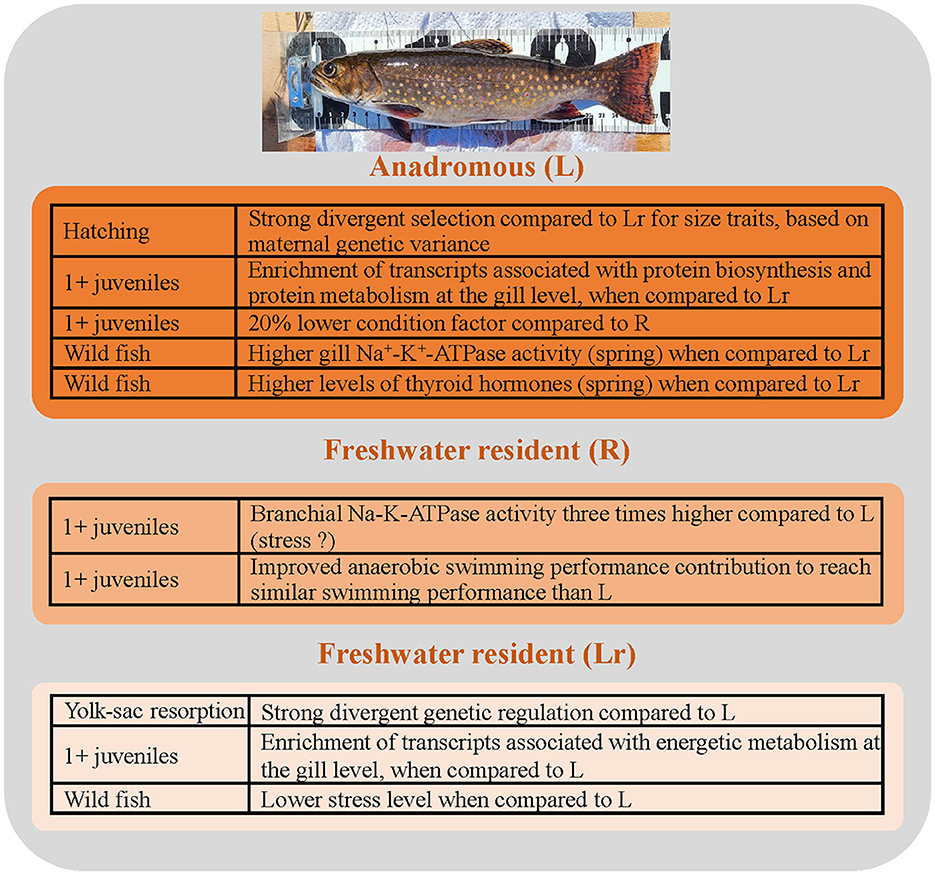
Figure 4. Highlights of differences among anadromous (L strain) and freshwater resident fish from the Laval River drainage system (Lr strain) or from the Rupert system (R strain). See the section on our model brook charr populations for more information. Brook charr photo credit: Julie Viana.
Over the years, we looked at different phenotypes related to anadromy. Early development was again thoroughly investigated. Adaptive divergence between migratory and nonmigratory forms of salmonids for early size and/or developmental characteristics might be possible if the different ecotypes used alternate reproductive strategies for reasons of local adaptations [i.e., phenotype–habitat matching; (85)]. One of our first attempts was to compare offspring from the two parapatric populations from Laval River, the freshwater resident (Lr) and anadromous (L) populations (17). In an extension of the work of Martin et al. (22), we showed that allele frequencies in fresh water Lr and L fish were significantly different at all nine microsatellite loci, confirming that they comprise genetically distinct populations in this system (17). Maternal quantitative genetic differentiation between embryonic Lr and L fish was also high [phenotypic divergence, (QST) > 0.5] and was greater than neutral genetic divergence for specific embryonic traits including length, yolk-sac volume, and growth rate for length (Figure 4). These results indicated that divergent natural selection has played a role in driving the differentiation between these two populations at the embryonic stage. However, there was no evidence for the role of directional selection acting on the post yolk-sac resorption stage (length and growth rate) since maternal between-population genetic variance was effectively zero for both traits.
Mavarez et al. (18) compared L and Lr fry (yolk-sac resorption) as well as the L♀Lr♂ hybrids using microarray technology. The results of this transcriptomic study clearly indicated that hybridization between anadromous and resident brook charr was accompanied by a massive breakdown of gene regulation in the F1 individuals, with most transcripts exhibiting low levels of expression relative to pure ecotypes. In summary, there was a 5-fold increase in the number of expression differences when the hybrid vs. Lr comparison was contrasted to the Lr vs. L (94 vs. 18; with 83% of the genes down-regulated in the hybrids) and there was an even higher−12-fold—increase (227 vs. 18, with 80% of the genes down-regulated in the hybrids) when the hybrids vs. L comparison was contrasted to the Lr vs. L (18). Disrupted genes in the F1 hybrids represented 23 different biological processes, including several categories associated with energy metabolism (oxidative phosphorylation, electron transport, lipid metabolism). It seemed that some failures in the interaction between the transcription factors of one population and their binding sites in the regulatory modules of enhancers of the other population were present, illustrating how different energetic phenotypes could be even at this very young age. This is in line with the results of Morinville and Rasmussen (86), who showed that juvenile future-migrant anadromous brook charr have consumption rates 1.4 times higher than resident charr of the same age, but a smaller ratio of growth to consumption, which indicates that they have higher metabolic costs. Overall, these results revealed that the L and Lr ecotypes had strong divergent genetic regulations (Figure 4), additionally supporting the conclusions of Perry et al. (17) for divergent natural selection.
Using these two same populations, we also compared their transcriptomics regulations before their first saltwater transition (age 1+). Using a 16,000 cDNA microarray chip, we contrasted gene expression in muscle (locomotion—an activity that should be enhanced during migration) and gills [osmo-iono-regulation; (87)]. The transcriptome differences were exclusively observed in gills. Compared to Mavarez et al. (18), who studied the first-feeding stage, 1+ L and Lr appeared more distinct. Briefly, an upregulation of the gill transcriptome was observed in L fish with a significant enrichment of transcripts associated with protein biosynthesis and protein metabolism, development, and immunity (Figure 4). Twelve transcripts involved in energetic metabolism were downregulated (three cytochrome oxidase c subunits and a pyruvate kinase). Overall, differences in the gill transcriptome supported a reorganization of the gill tissue prior to life in salt water (87).
We further continued our investigation on phenotypic traits a few years later, when Crespel et al. (88) again compared anadromous (L strain) and resident (R population this time) fish, as well as their hybrids, in 1+ juveniles. These results confirmed the presence of a 20% lower condition factor in L compared to R fish (Figure 4). In this study, Crespel et al. (88) compared swimming performance (critical swimming speed) and contrasted metabolic phenotypes of the populations in fresh and saltwater. They showed that swimming values were similar in pure strains (L and R), but that it was 18% lower in L♀R♂ hybrids compared to R♀L♂ hybrids. The branchial Na-K-ATPase activity was three times higher in R individuals than in L and both hybrids, which was unexpected, due to the adaptation of L fish to saltwater. However, the most interesting feature was the phenotypic differences at the metabolic level supporting the swimming. Indeed, the measure of different metabolic enzymatic activities, hepatic and muscle energy reserves, and lactate and pyruvate levels in both heart and muscle illustrated how similar swimming performances were achieved using different metabolic strategies. The results showed that anaerobic swimming performance contributed more to the swimming performance of R fish (fish are less streamlined and thus need to compensate for the advantage that body shape conferred to anadromous fish; Figure 4). This higher anaerobic capacity of the R fish was associated with higher energy reserves, suggesting that R fish may be less adapted for sustained swimming. Overall, these metabolic differences suggest that anadromous populations are better suited for prolonged migratory activity, while resident populations optimize energy use for local conditions. The performance of hybrids depended on cross direction which could be explained by different factors, such as maternal or paternal effects, or genetic linkage between sex genes and performance genes. There was no evidence of heterosis or outbreeding depression in the hybrids, which suggests that the extent of the genetic differences that have accumulated between these two populations has not been sufficient to cause genomic incompatibilities between the parental genomes (89, 90). This differs from what was previously obtained when comparing L and Lr fish originating from the same water system (18).
Maintaining population biodiversity (at the phenotypic and genetic level) is crucial for the resilience and adaptability of brook charr, particularly considering the differences in phenotypic and gene × environment interactions observed in our research. This will increase the ability of populations to cope with environmental changes and stressors, ensuring that various traits can emerge in response to shifting conditions. By preserving diverse populations of brook charr, we can thus enhance their potential for adaptive responses to stressful variations in their environments, such as climate change.
CA: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. DG: Conceptualization, Investigation, Writing – review & editing. AC: Conceptualization, Investigation, Writing – review & editing. MV: Conceptualization, Investigation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This review was funded by a Discovery Grant to C. Audet by the Conseil de Recherche en Sciences Naturelles et en Génie du Canada (CRSNG, Discovery Grant RGPIN-2019-05739).
The authors are grateful to all colleagues, students, friends who contributed along the years to this brook charr adventure.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Thompson JD. Phenotypic plasticity as a component of evolutionary change. Trends Ecol Evol. (1991) 6:246–9. doi: 10.1016/0169-5347(91)90070-E
2. West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford Academic (2003). p. 1–794. doi: 10.1093/oso/9780195122343.001.0001
3. Pigliucci M. Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol. (2005) 20:481–6. doi: 10.1016/j.tree.2005.06.001
4. Hutchings JA. Adaptive phenotypic plasticity in brook trout, Salvelinus fontinalis, life histories. Écoscience. (1996) 3:25–32. doi: 10.1080/11956860.1996.11682311
5. Grabowski TB, Young SP, Libungan LA, Steinarsson A, Marteinsdóttir G. Evidence of phenotypic plasticity and local adaption in metabolic rates between components of the icelandic cod (Gadus morhua L.) stock. Env Biol Fishes. (2009) 86:361–70. doi: 10.1007/s10641-009-9534-z
6. Koumoundouros GC, Ashton DG, Sfakianakis P, Divanach P, Kentouri M, Anthwal N, et al. Thermally induced phenotypic plasticity of swimming performance in European sea bass Dicentrarchus labrax juveniles. J Fish Biol. (2009) 74:1309–22. doi: 10.1111/j.1095-8649.2009.02206.x
7. Karjalainen J, Urpanen O, Keskinen T, Huuskonen H, Sarvala J, Valkeajärvi P, et al. Phenotypic plasticity in growth and fecundity induced by strong population fluctuations affects reproductive traits of female fish. Ecol Evol. (2016) 6:779–90. doi: 10.1002/ece3.1936
8. Potts LB, Mandrak NE, Chapman LJ. Coping with climate change: phenotypic plasticity in an imperilled freshwater fish in response to elevated water temperature. Aquat Conserv Mar Freshw Ecosyst. (2021) 31:2726–36. doi: 10.1002/aqc.3620
9. Burton T, Metcalfe NB. Can environmental conditions experienced in early life influence future generations? Proc Roy Soc B Biol Sci. (2014) 281:20140311. doi: 10.1098/rspb.2014.0311
10. Jonsson B, Jonsson N. Early environment influences later performance in fishes: effects of early experiences. J Fish Biol. (2014) 85:151–88. doi: 10.1111/jfb.12432
11. Hendry AP, Stearns SC. Evolution Illuminated: Salmon and Their Relatives. Oxford: Oxford University Press (2004). doi: 10.1093/oso/9780195143850.001.0001
13. Edmands S. Heterosis and outbreeding depression in interpopulation crosses spanning a wide range of divergence. Evol. (1999) 53:1757–68. doi: 10.2307/2640438
14. Cooke SJ, Kassler TW, Phillipp DP. Physiological performance of largemouth bass related to local adaptation and interstock hybridization: implications for conservation and management. J Fish Biol. (2001) 59:248–68. doi: 10.1111/j.1095-8649.2001.tb01389.x
15. Landry CR, Hartl DL, Ranz JM. Genome clashes in hybrids: insights from gene expression. Heredity. (2007) 99:483–93. doi: 10.1038/sj.hdy.6801045
16. Curry RA, Bernatchez L, Whoriskey F Jr, Audet C. The origins and persistence of anadromy in brook charr. Rev Fish Biol Fisheries. (2010) 20:557–70. doi: 10.1007/s11160-010-9160-z
17. Perry GML, Audet C, Bernatchez L. Maternal genetic effects on adaptive divergence between anadromous and resident brook charr during early life history. J Evol Biol. (2005) 18:1348–61. doi: 10.1111/j.1420-9101.2005.00954.x
18. Mavárez J, Audet C, Bernatchez L. Major disruption of gene expression in hybrids between young sympatric anadromous and resident populations of brook charr (Salvelinus fontinalis Mitchill). J Evol Biol. (2009) 22:1708–20. doi: 10.1111/j.1420-9101.2009.01785.x
19. Boula D, Castric V, Bernatchez L, Audet C. Physiological, endocrine, and genetic bases of anadromy in the brook charr, Salvelinus fontinalis, of the Laval River (Québec, Canada). Environ Biol Fishes. (2002) 64:229–42. doi: 10.1023/A:1016054119783
20. Sauvage C, Derôme N, Normandeau E, St-Cyr J, Audet C, Bernatchez L. Fast transcriptional responses to domestication in the brook charr Salvelinus fontinalis. Genetics. (2010) 185:105–12. doi: 10.1534/genetics.110.115071
21. Bastien A, Perry GML, Savaria J-Y, Bernatchez L, Audet C. Genetic gain for growth and delayed sexual maturation using a feral strain of anadromous brook trout. N Am J Aquac. (2011) 73:24–33. doi: 10.1080/15222055.2011.544609
22. Martin S, Savaria J-Y, Audet C, Bernatchez L. Microsatellites reveal no evidence for inbreeding effects but low inter-stock genetic diversity among brook charr stocks used for production in Québec. Bull Aquacul Assoc Canada. (1997) 97:21–3.
23. Gossieaux P, Lavoie E, Sirois P, Thibault I, Bernatchez L, Garant D. Effects of genetic origin on phenotypic divergence in brook trout populations stocked with domestic fish. Ecosphere. (2020) 11:e03119. doi: 10.1002/ecs2.3119
24. FalconerDS, Mackay TFC. Introduction to Quantitative Genetics. 4th ed. Longman Group, Harlow, U.K. (1996).
25. Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sinauer Associates, Sunderland, MA (1998).
26. Heath DD, Fox CW, Heath JW. Maternal effects on offspring size: variation through early development of chinook salmon. Evolution. (1999) 53:1605–11. doi: 10.2307/2640906
27. Wolf JB, Brodie Iii ED, Cheverud JM, Moore AJ, Wade MJ. Evolutionary consequences of indirect genetic effects. Trends Ecol Evol. (1998) 13:64–9. doi: 10.1016/S0169-5347(97)01233-0
28. Wolf JB. Gene interactions from maternal effects. Evolution. (2000) 54:1882–98. doi: 10.1111/j.0014-3820.2000.tb01235.x
29. Perry GM, Audet C, Laplatte B, Bernatchez L. Shifting patterns in genetic control at the embryo-alevin boundary in brook charr. Evolution. (2004) 58:2002–12. doi: 10.1111/j.0014-3820.2004.tb00485.x
30. Gourtay C, Rivolet M, Ghinter L, Bernatchez L, Garant D, Audet C. Selection effects on early life history traits and thermal resistance in brook charr Salvelinus fontinalis. Can J Zool. (2024) 102:429–42. doi: 10.1139/cjz-2023-0086
31. Bryden CA, Heath JW, Heath DD. Performance and heterosis in farmed and wild chinook salmon (Oncorhynchus tshawytscha) hybrid and purebred crosses. Aquaculture. (2004) 235:249–61. doi: 10.1016/j.aquaculture.2004.01.027
32. Granier S, Audet C, Bernatchez L. Heterosis and outbreeding depression between strains of young-of-the-year brook trout (Salvelinus fontinalis). Can J Zool. (2011) 89:190–8. doi: 10.1139/Z10-108
33. Bougas B, Granier S, Audet C, Bernatchez L. The transcriptional landscape of cross-specific hybrids and its possible link with growth in brook charr (Salvelinus fontinalis Mitchill). Genetics. (2010) 186:97–107. doi: 10.1534/genetics.110.118158
34. Bougas B, Audet C, Bernatchez L. The influence of parental effects on transcriptomic landscape during early development in brook charr. Heredity. (2013) 110:481–91. doi: 10.1038/hdy.2012.113
35. Garcia de Leaniz C, Fleming IA, Einum S, Verspoor E, Jordan WC, Consuegra S, et al. A critical review of adaptive genetic variation in Atlantic salmon: implications for conservation. Biol Rev. (2007) 82, 173–211. doi: 10.1111/j.1469-185X.2006.00004.x
36. Johnston IA. Environment and plasticity of myogenesis in teleost fish. J Exp Biol. (2006) 209:2249–64. doi: 10.1242/jeb.02153
37. Wringe BF, Devlin RH, Ferguson MM, Moghadam HK, Sakhrani D, et al. Growth-related quantitative trait loci in domestic and wild rainbow trout (Oncorhynchus mykiss). BMC Genet. (2010) 11:63. doi: 10.1186/1471-2156-11-63
38. Crespel A, Audet C, Bernatchez L, Garant D. Quantitative genetic analysis of the physiological stress response in three strains of brook charr Salvelinus fontinalis and their hybrids. J Fish Biol. (2011) 79:2019–33. doi: 10.1111/j.1095-8649.2011.03149.x
39. Crespel A, Audet C, Bernatchez L, Garant D. Effects of rearing environment and strain combination on heterosis in brook trout. N Am J Aquacul. (2012) 74:188–98. doi: 10.1080/15222055.2012.672884
40. Crespel A, Bernatchez L, Audet C, Garant D. Strain specific genotype – environment interactions and evolutionary potential for body mass in brook charr (Salvelinus fontinalis). G3. (2013) 3:379–86. doi: 10.1534/g3.112.005017
41. Vandeputte M, Gagnaire PA, Allal F. The European sea bass: a key marine fish model in the wild and in aquaculture. Anim Genet. (2019) 50:195–206. doi: 10.1111/age.12779
42. Martinez-Silva MA, Dupont-Prinet A, Houle C, Vagner M, Garant D, Bernatchez L, et al. Growth regulation in brook charr Salvelinus fontinalis. Gen Comp Endocrinol. (2023) 331:114160. doi: 10.1016/j.ygcen.2022.114160
43. Mackay TF. The genetic architecture of quantitative traits. Annu Rev Genet. (2001) 35:303–39. doi: 10.1146/annurev.genet.35.102401.090633
44. Sauvage C, Vagner M, Derôme N, Audet C, Bernatchez L. Coding gene single nucleotide polymorphism mapping and quantitative trait loci detection for physiological reproductive traits in brook charr, Salvelinus fontinalis. G3. (2012) 2:379–92. doi: 10.1534/g3.111.001867
45. Sauvage C, Vagner M, Derôme N, Audet C, Bernatchez L. Coding gene SNP mapping reveals QTL linked to growth and stress response in brook charr (Salvelinus fontinalis). G3. (2012) 2:707–20. doi: 10.1534/g3.112.001990
46. Middleton EK, Gilbert MJ, Landry T, Lamarre SG, Speers-Roesch B. Environmental variation associated with overwintering elicits marked metabolic plasticity in a temperate salmonid, Salvelinus fontinalis. J Exp Biol. (2024) 227:jeb246743. doi: 10.1242/jeb.246743
47. Crespel A, Bernatchez L, Garant D, Audet C. Genetically based population divergence in overwintering energy mobilization in brook charr (Salvelinus fontinalis). Genetica. (2013) 141:51–64. doi: 10.1007/s10709-013-9705-x
48. Fry FEJ. The effect of environmental factors on the physiology of fish. Fish Physiol. (1971). 6:1–98. doi: 10.1016/S1546-5098(08)60146-6
49. Schulte PM, Healy TM, Fangue NA. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol. (2011) 51:691–702. doi: 10.1093/icb/icr097
50. Currie S, Schulte PM. Thermal stress. In:Evans DH, Claiborne J, Currie S, , editor. The Physiology of Fishes, 4th ed. CRC Press, Boca Raton (2014). p. 257–79.
51. Møller AP, Merilä J. Analysis and interpretation of long-term studies investigating responses to climate change. Adv Ecol Res. (2004) 35:111–130. doi: 10.1016/S0065-2504(04)35006-3
52. Merilä J, Hendry AP. Climate change, adaptation, and phenotypic plasticity: the problem and the evidence. Evol Appl. (2014) 7:1–14. doi: 10.1111/eva.12137
53. Jonsson B. Thermal effects on ecological traits of salmonids. Fishes. (2023) 8:337. doi: 10.3390/fishes8070337
54. Toyota K, Cambronero Cuenca M, Dhandapani V, Suppa A, Rossi V, Colbourne JK, et al. Transgenerational response to early spring warming in Daphnia. Sci Rep. (2019) 9:4449. doi: 10.1038/s41598-019-40946-3
55. Anastasiadi D, Venney CJ, Bernatchez L, Wellenreuther M. Epigenetic inheritance and reproductive mode in plants and animals. Trends Ecol Evol. (2021) 36:1124–40. doi: 10.1016/j.tree.2021.08.006
56. Jonsson B, Jonsson N. Trans-generational maternal effect: temperature influences egg size of the offspring in Atlantic salmon Salmo salar. J Fish Biol. (2016) 89:1482–7. doi: 10.1111/jfb.13040
57. Doyle CM, Leberg PL, Klerks PL. Heritability of heat tolerance in a small livebearing fish, Heterandria formosa. Ecotoxicol. (2011) 20:535–42. doi: 10.1007/s10646-011-0624-2
58. Houde A, Fraser DJ, Reilly PO, Hutchings JA. Maternal and paternal effects on fitness correlates in outbred and inbred Atlantic salmon (Salmo salar). Can J Fish Aquat Sci. (2011) 68:534–49. doi: 10.1139/f2011-001
59. Eilertsen EM, Bårdsen B-J, Liljedal S, Rudolfsen G, Folstad I. Experimental evidence for paternal effects on offspring growth rate in Arctic charr (Salvelinus alpinus). Proc R Soc B. (2009) 276:129–36. doi: 10.1098/rspb.2008.0884
60. Janhunen M, Piironen J, Peuhkuri N. Parental effects on embryonic viability and growth in Arctic charr Salvelinus alpinus at two incubation temperatures. J Fish Biol. (2010) 76:2558–70. doi: 10.1111/j.1095-8649.2010.02648.x
61. Ashe A, Colot V, Oldroyd BP. How does epigenetics influence the course of evolution? Phil Trans R Soc B. (2021) 376:20200111. doi: 10.1098/rstb.2020.0111
62. Venney CJ, Wellband KW, Normandeau E, Houle C, Garant D, Audet C, et al. Thermal regime during parental sexual maturation, but not during offspring rearing, modulates DNA methylation in brook charr (Salvelinus fontinalis). Proc Roy Soc B. (2022) 289:20220670. doi: 10.1098/rspb.2022.0670
63. Banousse G, Normandeau E, Semeniuk C, Bernatchez L, Audet C. Parental thermal environment controls the offspring phenotype in brook charr (Salvelinus fontinalis): insights from a transcriptomic study. G3. (2024) 14:jkae051. doi: 10.1093/g3journal/jkae051
64. Houle C, Gossieaux P, Bernatchez L, Audet C, Garant D. Transgenerational effects on body size and survival in Brook charr (Salvelinus fontinalis). Evolutionary Appl. (2023) 16:1061–70. doi: 10.1111/eva.13553
65. Parsch J, Ellegren H. The evolutionary causes and consequences of sex-biased gene expression. Nature Publish Gr. (2013) 14:83–7. doi: 10.1038/nrg3376
66. Nilsson J. Arctic charr strain crosses: effects on growth and sexual maturity. J Fish Biol. (1993) 43:163–71. doi: 10.1111/j.1095-8649.1993.tb00420.x
67. Bentsen HB, Eknath AE, Palada-de Vera MS, Danting JC, Bolivar HL, Reyes RA, et al. Genetic improvement of farmed tilapias: growth performance in a complete diallel cross experiment with eight strains of Oreochromis niloticus. Aquaculture. (1998) 160:145–173. doi: 10.1016/S0044-8486(97)00230-5
68. Ellegren H, Parsch J. The evolution of sex-biased genes and sex biased gene expression. Nature Rev Genet. (2007) 8:689–98. doi: 10.1038/nrg2167
69. Derome N, Bougas B, Rogers SM, Whiteley AR, Labbe A, Laroche J, et al. Pervasive sex-linked effects on transcription regulation as revealed by expression quantitative trait loci mapping in lake whitefish species pairs (Coregonus sp. Salmonidae). Genetics. (2008) 179:1903–17. doi: 10.1534/genetics.107.086306
70. Sutherland BJG, Rico C, Audet C, Bernatchez L. Sex, chromosome evolution, heterochiasmy, and physiological QTL in the Salmonid brook charr Salvelinus fontinalis. G3. (2017) 7:2749–62. doi: 10.1534/g3.117.040915
71. Sutherland BJG, Prokkola JM, Audet C, Bernatchez L. Sex-specific co-expression networks and sex-biaised gene expression in the salmonid brook charr Salvelinus fontinalis. G3. (2019) 9:955–68. doi: 10.1534/g3.118.200910
72. Charlesworth D, Charlesworth B, Marais G. Steps in the evolution of heteromorphic sex chromosomes. Heredity. (2005) 95:118–28. doi: 10.1038/sj.hdy.6800697
73. Barson NJ, Aykanat T, Hindar K, Baranski M. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature. (2015) 528:405–8. doi: 10.1038/nature16062
74. Allendorf FW, Thorgaard GH. Tetraploidy and the evolution of salmonid fishes. In:Turner BJ, , editor. Plenum Publishing Corporation: New York (1984). p. 1–53
75. Allendorf FW, Bassham S, Cresko WA, Limborg MT, Seeb LW, Seeb JE. Effects of crossovers between homeologs on inheritance and population genomics in polyploid-derived salmonid fishes. J Hered. (2015) 106:217–27. doi: 10.1093/jhered/esv015
76. May B, Delany ME. Meiotic models to explain classical linkage, pseudolinkage, and chromosomal pairing in tetraploid derivative salmonid genomes: II. Wright is still right. J Hered. (2015) 106:762–6. doi: 10.1093/jhered/esv056
77. Lien S, Koop BF, Sandve SR, Miller JR, Kent MP, et al. The Atlantic Salmon genome provides insights into rediploidization. Nature. (2016) 533:200–5. doi: 10.1038/nature17164
78. Sutherland BJG, Gosselin T, Normandeau E, Lamothe M, Isabel N, Audet C, et al. Salmonid chromosome evolution as revealed by a novel method for comparing RADseq linkage maps. Genome Biol Evol. (2016) 8:3600–17. doi: 10.1093/gbe/evw262
79. Quinn TP, Foote CJ. The effects of body size and sexual dimorphism on the reproductive behavior of sockeye salmon, Oncorhynchus nerka. Anim Behav. (1994) 48:751–61. doi: 10.1006/anbe.1994.1300
80. van Nas A, Guhathakurta D, Wang SS, Yehya N, Horvath S, Zhang B, et al. Elucidating the role of gonadal hormones in sexually dimorphic gene coexpression networks. Endocrinology. (2009) 150:1235–49. doi: 10.1210/en.2008-0563
81. Wong RY, McLeod MM, Godwin J. Limited sex-biased neural gene expression patterns across strains in Zebrafish (Danio rerio). BMC Genomics. (2014) 15:905–10. doi: 10.1186/1471-2164-15-905
82. Thériault V, Bernatchez L, Dodson JJ. Mating patterns and individual reproductive success of sympatric anadromous and resident brook charr Salvelinus fontinalis under natural conditions. Behav Ecol Sociobiol. (2007) 62:51–65. doi: 10.1007/s00265-007-0437-8
83. Power G. “The brook charr, Salvelinus fontinalis.” In:Balon EK, , editor. Charrs: Salmonid Fishes of the Genus Salvelinus. Dr. W. Junk, The Hague (1980). p. 141–203.
84. Curry RA, van de Sande J, Whoriskey FWJr. Temporal and spatial habitats of anadromous brook charr in the laval river and its estuary. Environ Biol Fish. (2006) 76:361–70. doi: 10.1007/s10641-006-9041-4
85. Hendry AP, Bohlin T, Jonsson B, Berg OK. “To sea or not to sea? Anadromy versus non-anadromy in salmonids.” In:Hendry AP, Stearns SC, , editors. Evolution Illuminated: Salmon and their Relatives. Oxford University Press, Oxford, UK (2004). p. 92–125.
86. Morinville GR, Rasmussen JB. Early juvenile bioenergetic differences between anadromous and resident brook charr (Salvelinus fontinalis). Can J Fish Aquat Sci. (2003) 60:401–10. doi: 10.1139/f03-036
87. Boulet M, Normandeau E, Bougas B, Audet C, Bernatchez L. Comparative transcriptomics of anadromous and resident brook charr (Salvelinus fontinalis) before their first salt water transition. Curr Zool. (2012) 58, 158–70. doi: 10.1093/czoolo/58.1.158
88. Crespel A, Dupont-Prinet A, Bernatchez L, Claireaux G, Tremblay R, Audet C. Divergence in physiological factors affecting migratory performance between anadromous and resident populations of brook charr Salvelinus fontinalis. J Fish Biol. (2017) 90:2170–93. doi: 10.1111/jfb.13300
89. Bieri J, Kawecki TJ. Genetic architecture of differences between populations of cowpea weevil (Callosobruchus maculatus) evolved in the same environment. Evolution. (2003) 57:274–87. doi: 10.1111/j.0014-3820.2003.tb00262.x
Keywords: phenotypic plasticity, development, growth, stress, metabolism, sex-linked traits, anadromy, adaptability
Citation: Audet C, Garant D, Crespel A and Vagner M (2025) How genomic and environmental relationships shape phenotypic plasticity in brook charr Salvelinus fontinalis: an historical review. Front. Fish Sci. 3:1525181. doi: 10.3389/frish.2025.1525181
Received: 08 November 2024; Accepted: 31 January 2025;
Published: 19 February 2025.
Edited by:
David J. McKenzie, Centre National de la Recherche Scientifique, FranceReviewed by:
Florian Mauduit, University of California, Davis, United StatesCopyright © 2025 Audet, Garant, Crespel and Vagner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Céline Audet, Y2VsaW5lX2F1ZGV0QHVxYXIuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.