- 1School of Biological Sciences, University of Nebraska-Lincoln, Lincoln, NE, United States
- 2Laboratorio de Biología Reproductiva y Evolución, Instituto de Diversidad y Ecología Animal, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) – Universidad Nacional de Córdoba (UNC), Universidad Nacional de Córdoba, Córdoba, Argentina
- 3Department of Biology, Nebraska Wesleyan, Lincoln, NE, United States
- 4Laboratory for Animal Behavior, Smithsonian Tropical Research Institute, Ancón, Panama
- 5Department of Entomology and Nematology, University of California, Davis, Davis, CA, United States
- 6Science Focus Program, Lincoln Public Schools, Lincoln, NE, United States
Mate choice is posited to explain the evolution and maintenance of numerous secondary sexual traits, including ornamentation. This study explores the role of ornamentation in the mating success of two sister-species of wolf spider with divergent ornamentation. Mature male Schizocosa crassipalpata lack ornamentation while males of its closest living relative, S. bilineata, express both dark pigmentation and foreleg brushes. Following phenotypic manipulations of foreleg ornamentation – i.e. adding ornamentation in the form of dark pigment to non-ornamented males (S. crassipalpata, Aim 1) and removing ornamentation in varying degrees from highly ornamented males (S. bilineata, Aim 2 – shaving brushes; Aim 3 – shaving brushes and painting over dark pigment in vibration present/absent environments) – we found no evidence that ornamentation alone improves male mating success in either species, regardless of the vibratory signaling environment. In both S. bilineata experiments, however, higher courtship rates resulted in higher mating success, suggesting selection for courtship performance. Furthermore, females were more likely to turn, a presumed receptivity display, in response to males that courted at a higher rate. Also, similar to findings in another relative (S. stridulans), we found indications that ornamentation may function to ease a male’s reliance on courtship performance – i.e., at low courtship rates, only ornamented males can secure a mating. Our phenotypic manipulations also influenced courtship behavior in S. bilineata. Shaved males began courting earlier and courted more often over a longer time than intact males, yet ultimately acquired similar matings. This increased courtship effort likely compensated for reduced ornamentation. Finally, the vibratory environment appears crucial for female–male dialogue in S. bilineata, as vibratory absent environments resulted in increased female attacks and decreased male courtship rates. Together, our data suggest that S. crassipalpata females do not possess a preference for ornamentation and that S. bilineata females do not use ornamentation alone in mating decisions. Instead, our results are consistent with a hypothesis that ornamentation in Schizocosa evolved, and is likely maintained, due to its interactions with dynamic movement displays (i.e. leg movements), which can themselves be plastically altered based on the signaler’s phenotype as well as the signaling environment.
1 Introduction
Animal lineages comprising numerous species with elaborate and diverse displays and associated ornaments have attracted the attention of scientists, as well as non-scientists, for centuries (for examples, see Darwin, 1871). Significant empirical as well as theoretical research has focused on understanding the evolution and function of these elaborate displays (reviewed in Andersson, 1994; Rosenthal, 2017). Currently, we presume that sexual selection plays a large role in the divergence of secondary sexual traits (including courtship displays) among closely related lineages and populations, leading to behavioral isolation and ultimately speciation (West-Eberhard, 1983; Mendelson and Shaw, 2005; Rodriguez et al., 2013; Uy et al., 2018). Sexual selection has been implicated, for example, as having influenced the evolution of male plumage across manakins (Doucet et al., 2007); color pattern complexity across Australian dragon lizards (Agamidae) (Chen et al., 2012); and multimodal courtship displays in the Habronattus coecatus group of jumping spiders (Elias et al., 2012), among others. Although a variety of hypotheses exist regarding why choosers might enact trait-specific preferences (reviewed in Andersson, 1994; Rosenthal, 2017; Patricelli et al., 2019), regardless of the underlying reason or mechanism, mate choice is posited to explain the evolution and maintenance of numerous secondary sexual traits, including ornamentation. Multiple studies have found support for a role of ornamentation in the reproductive success of animals across a range of taxonomic groups. Familiar examples include the tail feathers of the male long-tailed widowbird, Euplectes progne (Andersson, 1982), the eye stalks of stalk-eyed flies, Cyrtodiopsis dalmanni (Cotton et al., 2004), and the elongated tail fin of male swordtail fish, Xiphophorus helleri (Basolo, 1990b).
Notably, multiple studies exist that fail to find support for a role of ornaments in reproductive success. In a moon moth, Actias luna, for example, females do not appear to discriminate among males based on their seemingly elaborate hindwing tail (Rubin and Kawahara, 2023). In great snipe, Gallinago media, the whiteness of a male’s tail shows no correlation with his mating success (Saether et al., 2000), and no evidence of directional or stabilizing selection was found for presumed ornamental traits (i.e. epaulet size) in red-winged blackbirds, Agelaius phoeniceus (Westneat, 2006). Additionally, though not ornamentation per se, frigatebirds, Fregata minor, produce vocalizations as part of their courtship display presumed to be important for reproductive success. An in-depth study exploring male vocalizations found that despite some condition-dependent expression, none of the analyzed vocal measures predicted pairing success (Juola and Searcy, 2011). Finally, although again not ornamentation, nuptial gifts are often considered a sexually selected trait, but in the spider Pisaura mirabilis, gift-wrapping did not influence chooser mating decisions (Albo et al., 2012). Thus, though it is often assumed that observed elaboration in ornaments or other sex-specific reproductive traits evolved due to chooser preferences, these assumptions require empirical testing.
One, of many, potential explanations for non-existent relationships between chooser preferences and degree of ornamentation is that selection may act differently on distinct signal components – e.g., selection for efficacy versus content (Guilford and Dawkins, 1991; Hebets and Papaj, 2005). Elaboration in animal displays is often obligately linked to an increased number of display components; and these components may experience different selection pressure (Rowe, 1999; Rowe and Guilford, 1999; Hebets and Papaj, 2005; Rowe, 2013; Hebets and McGinley, 2019). The observed patterns of nuptial coloration across 17 species of freshwater fish (darters, genus Etheostoma) (Gumm and Mendelson, 2011), for example, as well as a lack of correlation between song and plumage elaboration across 301 species of tanagers (Thraupidae) (Mason et al., 2014) suggests that individual components/signals evolved independently and under different selection pressure. Similarly, the evolutionary patterns of visual and vibratory signal complexity and mating success of Schizocosa wolf spiders show different relationships across multimodal signaling species (Hebets et al., 2013; Starrett et al., 2022). If color components in fishes, or elaboration in song and ornamentation in tanagers and wolf spiders evolved solely due to chooser preferences, we would expect to see correlated traits across species. A lack of correlation suggests distinct selection and function – e.g., some components may attract a chooser’s attention under certain environmental conditions while another is preferred due to its function as an indicator trait (Hebets and Papaj, 2005). Furthermore, in addition to distinct selection pressures acting on different display components, selection can act on interactions between display components (Hebets, 2005; reviewed in Hebets and Papaj, 2005; Wilgers and Hebets, 2012; Stafstrom and Hebets, 2013), making it even more challenging to identify possible selective pressures acting on ornamentation and display elaboration.
Animal taxa for which there is evidence for repeated gains and losses of secondary sexual traits offer an exceptional opportunity to improve evolutionary insight into their history of selection and putative function – e.g., gulls (Minias and Janiszewski, 2020); dragon lizards (Ord and Stuart-Fox, 2006); Sceloporous lizards (Martins et al., 2015), etc (Wiens, 2001). The present study focuses on Schizocosa wolf spiders, a powerful emerging system for exploring these evolutionary patterns. Among the 23 described Nearctic Schizocosa wolf spiders (Dondale and Redner, 1978), we observe species with (i) no secondary sexual traits or ornamentation, (ii) dark pigmentation on distinct segments of male forelegs, or first pair of walking legs, and (iii) both dark pigmentation and large dark bristles on foreleg segments (Dondale and Redner, 1978; Stratton, 2005; Starrett et al., 2022). In a molecular phylogenetic study with comprehensive species sampling and genome-wide markers (Starrett et al., 2022) this relatively recently radiated group (~10 million years ago; Piacentini and Ramírez, 2019) was shown to have undergone repeated gains and losses of male foreleg ‘ornamentation’ – i.e. dark pigmentation and/or bristles. According to this most recent evolutionary hypothesis, full or partial dark foreleg pigmentation has been lost seven times and gained 13 times across the genus, whereas dark foreleg bristles have evolved independently in six separate lineages (Starrett et al., 2022).
Schizocosa wolf spiders have been the focus of decades of research exploring the form and function of their courtship displays (reviewed in Stratton, 2005; Hebets et al., 2013; Uetz et al., 2016; Hebets and McGinley, 2019; Starrett et al., 2022). This body of research has been instrumental in guiding theory regarding signal/display complexity, especially as it relates to multimodal signaling (Hebets and Papaj, 2005; Hebets, 2011; Hebets and Rundus, 2011; Herberstein et al., 2013; Higham and Hebets, 2013; Hebets et al., 2016; Uetz et al., 2016; Rosenthal et al., 2018; Hebets and McGinley, 2019). Adding to the genus’ potential for advancing our understanding of the evolution and function of complex signaling, the recent comparative phylogenetic study confirms a previously observed pattern in which closely related species pairs appear divergent in their degree of ornamentation, suggesting strong selection on ornamentation (Starrett et al., 2022). One clear example of such a species pair is S. crassipalpata and S. bilineata.
Schizocosa crassipalpata and S. bilineata are sister-species (Stratton, 2005; Hebets et al., 2021; Starrett et al., 2022) that overlap in much of their range and microhabitat use, and differ in their secondary sexual traits and courtship displays. Mature S. crassipalpata males lack ornamentation and court females with a combination of substrate-borne vibratory song and asymmetrical tapping of the forelegs (Dondale and Redner, 1978; Stratton, 2005; Hebets et al., 2021; Starrett et al., 2022) (see video in Supplemental Material of Hebets et al., 2021). The vibratory display of S. crassipalpata is diet-dependent and the presence of vibratory signaling is known to be important for mating success (Hebets et al., 2021) similar to numerous other Schizocosa species (Hebets et al., 2013). In contrast, mature S. bilineata males possess ornamentation in the form of dark pigmentation and bristles on the tibiae of their forelegs (Stratton, 2005; Vaccaro et al., 2010; Hebets et al., 2021; Starrett et al., 2022). The courtship display of S. bilineata involves quick foreleg taps and incremental leg descends (see Table 1 in Vaccaro et al., 2010 for behavior descriptions; see video in Supplemental Material of Hebets et al., 2021). Brush size in male S. bilineata is diet-dependent, but vibratory signaling is not (Hebets et al., 2021). Additionally, S. bilineata pairs are more likely to mate in the light versus dark, suggesting a role of visual ornamentation (Hebets et al., 2021). Schizocosa bilineata vibratory signaling, however, becomes important for mating success in dark environments (Hebets et al., 2021). Thus, these two sister-species appear to have diverged in their sensory-reliance, with S. crassipalpata relying more on vibratory signaling for mating success and S. bilineata relying more on visual signaling. Given their overlap in space, prior research suggested that this divergence may have been influenced by competition for signaling space (Hebets et al., 2021).
Based on ancestral state reconstructions of ornamentation on tibia 1 (i.e. foreleg pigmentation), the common ancestor of S crassipalpata and S. bilineata is hypothesized to have likely had tibial ornamentation, with S. crassipalpata representing one of five to six losses of full tibial pigmentation across the genus (Starrett et al., 2022, Figure 7 therein). In contrast, their common ancestor was hypothesized to lack tibial and metatarsal bristles, meaning that S. bilineata independently evolved bristles (Starrett et al., 2022, Figure 8 therein). Due to the combined (a) loss of pigmentation in S. crassipalpata and (b) gain of bristles in S. bilineata, S. crassipalpata is noted as one of the species that exhibit the largest difference in ornamentation level compared to their sister species (Starrett et al., 2022). Such deviation in secondary sexual traits in closely related species provides an excellent opportunity to gain insight into evolutionary mechanisms of divergence and speciation. This study focuses explicitly on the putative role of ornamentation in the mating success of both sister-species, S. crassipalpata and S. bilineata.
Numerous hypotheses exist for the evolutionary origin of novel ornamentation (Andersson, 1994; Ord and Stuart-Fox, 2006; Charles and Ord, 2012; Hill and Yasukawa, 2014; Rosenthal, 2017; Fitzpatrick and Servedio, 2018; Broder et al., 2021). One of the more compelling and well-supported hypotheses posits that choosers have evolved stimulus-specific biases in their sensory system due to selection for sensory reception in other, non-reproductive contexts (Basolo, 1990a, b; Ryan et al., 1990; Endler, 1992; Endler and Basolo, 1998; reviewed in Cummings and Endler, 2018). We first (Aim 1) test the hypothesis that S. crassipalpata females have retained a preference for ornamentation. We test this by adding ornamentation in the form of dark pigment to males and assessing mating success. Next (Aim 2), we directly test the function of bristles (hereafter ‘brushes’) in S. bilineata courtship. We do this by shaving brushes in a subset of males and comparing mating success between shaved and unshaved males. Finally (Aim 3), again in S. bilineata, we explore potential interactions between the presence/absence of ornamentation and the presence/absence of vibratory signaling. We manipulate male ornamentation phenotypes using a combination of shaving and painting over existing pigment to create unornamented versus control males. We simultaneously manipulate the vibratory signaling environment to create a vibration present/absent environment by altering the substrate upon which female–male pairs interact. Understanding the importance of different signal components, in this case ornamentation and its potential interaction with vibratory signaling, for mating success in these two sister-species will provide insight into their function and the selection pressures involved in their divergence and maintenance.
2 Materials and methods
2.1 Spider collection and maintenance
Schizocosa crassipalpata is known to occur from the north-eastern USA and south-eastern Canada as far west as Colorado, USA, while the range of S. bilineata overlaps with that of S. crassipalpata but extends south to Mississippi, USA (Comstock, 1940; Kaston, 1948; Dondale and Redner, 1978; Sierwald et al., 2005; Vaccaro et al., 2010). Where their distributions overlap, we know of both sympatric and allopatric populations (Bern, 2011). Both species tend to be found in similar microhabitats – open grassy habitats with canopy heights of 20–30cm – and both have similar maturation times (beginning in late April and lasting until early June) (Bern, 2011).
For Aim 1, we collected immature individuals of S. crassipalpata from an open grassy habitat (canopy height < 25.4cm) at Bath Nature Preserve in Summit County, OH, USA (41° 10’ 35.7414” N, 81° 38’ 52.7928” W) from 17 to 21 March 2009. For Aim 2, we collected immature individuals of S. bilineata in an open grassy habitat (canopy height < 20.32cm) along a riparian zone near the north end of the Ohio State University campus in Newark, Licking County, OH, USA (40° 4’ 30.9144” N, 82° 26’ 32.8272” W) from 29 to 31 March 2010. Notably, both of these collection locations had sympatric S. crassipalpata and S. bilineata. While both species were present at both sites, each site had only one species that was present in high numbers. Finally, to increase our sample size for Aim 2, we collected an additional set of immature and subadult S. bilineata in Lancaster County, NE, USA (40° 45’ 1.2132” N; −96° 49’ 22.4718” W) in mid-May 2021. For Aim 3, we collected subadults of S. bilineata from this latter location in Lancaster County, NE, USA from 14 to 16 May 2023.
We transported all spiders to the University of Nebraska-Lincoln and housed them individually in 5.9 cm × 5.9 cm × 7.7 cm clear plastic containers (Amac Plastic Products, Petaluma, CA) with visual barriers between containers. We fed all individuals collected in 2009 and 2010 a cricket (Acheta domesticus, Bassetts cricket ranch, CA, USA) twice weekly. We fed all spiders collected in 2021 and 2023 two to three crickets (Gryllodes sigillatus, Ghann’s Crickets, Augusta, GA, USA) once per week. All spiders, regardless of their year of collection, were maintained on a 12:12 light:dark cycle and provided with a constant source of water.
2.2 Aim 1 – Role of ornamentation in S. crassipalata mating success
Given that S. crassipalpata’s sister-species, S. bilineata, is ornamented and overlaps in habitat type with S. crassipalata (Hebets et al., 2021), we wanted to know how S. crassipalpata females respond to ornamentation. Specifically, would they attend to ornaments and use them in mate choice decisions? To answer this question, we tested whether adding ornamentation to the unornamented forelegs of S. crassipalpata males influences male mating success and/or courtship behavior. Finding a preference for ornamentation might reflect a retained preference, given the hypothesis that this species has secondarily lost ornamentation.
2.2.1 Manipulating ornamentation
Once mature, we randomly assigned S. crassipalpata males to one of two experimental treatment groups – (i) Ornamentation present (O+; tibial forelegs painted, n = 16) and (ii) Ornamentation absent/control (O−; naturally unornamented males, n = 15). We added ornamentation in the O+ treatment by painting the tibia and patella of each assigned male’s forelegs with black acrylic paint (Anita’s All Purpose Acrylic Craft Paint – 11002 Black/Noir Negro, Synta Inc., Clarkston, GA, USA) using methods detailed below. This manipulation created a phenotype similar to other closely related species of Schizocosa (e.g., S. uetzi, S. stridulans) and is a method that has been used successfully in studies exploring the role of secondary sexual traits in Schizocosa and other wolf spiders (Hebets, 2003; Hebets et al., 2006; Rutledge et al., 2010; Hebets et al., 2011; Wilgers et al., 2022). To control for the experimental manipulation, we ‘painted’ the forelegs of the O− males with water. Finally, to ensure equivalent chemical/odor environments across mating trials, we placed a small spot of the black paint on a wall of the mating arena at the same time we painted males. This ensured that all trials took place in the presence of residual paint odor.
To facilitate the foreleg painting process, we placed males in a quart-sized Ziploc bag (SC Johnson, Racine, WI, USA) with a corner cut off, creating a small hole. We gently guided each male to the cut corner and carefully pulled their forelegs through the opening using soft tip forceps. While we gently restrained the male’s body with pressure from the experimenter’s fingers, we painted all sides of the relevant segments on the forelegs. We allowed the legs to dry before placing the male back in his home cage. All males experienced their manipulation (paint/water) approximately two hours prior to their mating trials. This allowed sufficient time for the spiders to recover, but not enough time for them to groom off the paint.
2.2.2 Mating trials
We ran all mating trials in arenas consisting of circular plastic enclosures measuring 12.5cm in diameter (Pioneer Plastics Inc., Dixon, KY). We lined the floor of all arenas with filter paper and surrounded the walls with a printed image of grass. The filter paper substrate allowed courtship vibrations to freely transmit throughout the arena (Hebets, 2005; Elias and Mason, 2014) while the surrounding photo of grass (taken at a spider’s eye view) both prevented interference from visual cues outside the arena and also simulated the natural backdrop of S. crassipalpata courtship. We assumed that adding complexity to the background would increase the putative impact of any visual signal elaboration (i.e. increased ornamentation), thus increasing the likelihood of detecting an effect.
We fed females one size-matched cricket approximately 24 hours before the trial. We did this to standardize hunger level and to minimize the potential for pre-copulatory cannibalism. On the day of the mating trial, we weighed females and males (Ohaus Corporation, Model: Adventurer Pro AV64; Pine Brook, NJ, USA) and placed females in their respective mating arena at least one hour prior to the start of the trial. This allowed the females acclimation time to the new environment and increased the likelihood of the deposition of silk and other cues that might facilitate male courtship (Roberts and Uetz, 2005; Hebets et al., 2021). Following the female’s acclimation time, we introduced the males into the arena and allowed the pair to interact for up to 30 minutes.
We illuminated individuals from above with two Lumina fiber-optic lights (Chiu Technical Corp., Kings Park, NY, USA) and recorded trials from above with a digital video camera (Sony Handycam 4.0 mega pixels). We quantified courtship and copulation behavior in real-time and went back to videos when necessary to confirm observations and/or to obtain more detail. For both species (all Aims), we considered the male to be courting when he performed ‘leg waves’ (Stratton, 2005; Framenau and Hebets, 2007; Vaccaro et al., 2010; Hebets et al., 2021). We analyzed the latency to the appearance of the first male courtship and recorded each leg wave (hereafter ‘courtship bout’). We also recorded the total time the male was courting. We computed this as the time from the beginning of courtship until (a) mounting, or the start of mating, or (b) the end of the recording, in cases where copulation did not occur. We calculated courtship rate by taking the total number of courtship bouts divided by the courtship duration. We also quantified the latency to mounting, or to successful mating. Thus, in total we had the following parameters for each mating trial: latency to courtship, number of courtship bouts, total courtship time, courtship rate, and latency to mounting. Following each trial, we sacrificed spiders using freezing and preserved them in 70% ethyl alcohol. We stored them in the Hebets’ Laboratory at the University of Nebraska-Lincoln.
2.3 Aim 2 – Role of brushes in S. bilineata mating success
Mature male S. bilineata develop diet-dependent black brushes on the tibiae of their forelegs at maturation; the same legs that they use in dynamic movements associated with courtship (Vaccaro et al., 2010; Hebets et al., 2021). To determine if choosing female S. bilineata attend to brushes during courtship assessment, we artificially removed male brushes and evaluated mating success. We also quantified potential impacts of brush removal on the male courtship behavior.
2.3.1 Manipulating brushes
Upon maturation, we randomly assigned males of S. bilineata to one of two experimental treatment groups – (i) Brushes intact (B+; with natural full ornamentation, n = 14) and (ii) Brushes shaved (B−; with brushes shaved off but natural ornamentation/pigmentation remaining, n = 15). To manipulate the brushes, we used the same method as described for S. crassipalpata of restraining males and accessing their forelegs – i.e. a Ziploc bag (SC Johnson, Racine, WI, USA) with a cut corner. Once the male’s forelegs were pulled through the cut corner of the Ziploc bag, we used the sharp edge of a hypodermic needle to gently shave off the brush while viewing it under a dissecting scope. Each leg was shaved from the dorsal and ventral surface multiple times until we determined ~70% of the brush to be removed. This is a technique that has been used previously in experiments with Schizocosa aimed at determining the role of foreleg brushes (Scheffer et al., 1996; Stafstrom and Hebets, 2013). For the sham-shaved, or control, treatment we simply used the rounded edge of the needle so that no bristles were removed during rubbing. We shaved or sham shaved all males 2–24 hours prior to the start of their mate choice trials.
2.3.2 Mating trials
We used the same arenas, the same design, and collected the same data for S. bilineata as we did for S. crassipalpata (Aim 1).
2.3.3 Aims 1 and 2 statistical analyses
We use the same analyses for both Aims 1 (S. crassipalpata) and 2 (S. bilineata brushes) and thus, report them both together here.
Before running full analyses, we verified that female and male weights for both species were equal between experimental treatments using linear models in R software (R Core Team, 2020). Because there were no differences between treatments (S. crassipalpata females: F = 0.366, df = 1, p-value = 0.550; males: F = 0.545, df = 1, p-value = 0.467/S. bilineata females: F = 0.078, df = 1, p-value = 0.782; males: F = 0.081, df = 1, p-value = 0.779), we did not include individual weights as covariates in the main models. We found the same results with t-tests (analyses not shown).
Before unifying the databases of the groups of S. bilineata collected in different years (2009 and 2021), we used Generalized Linear Models (GLMs) to verify that there were no differences between years in mating probability and sexual behaviors. Because we found no statistical differences between years (mating success: Chisq = 0.844, df = 1, p-value = 0.358; latency to courtship: Chisq = 1.064, df = 1, p-value = 0.302; total courtship time: Chisq = 0.645, df = 1, p-value = 0.422; latency to mounting: Chisq = 0.107, df = 1, p-value = 0.743) we pooled both datasets and did not include year as a factor in subsequent models.
To determine the potential influence of experimental treatment on reproductive behavior, we used GLMs in R software. First, we compared the proportions of successful matings between experimental treatments using a Proportions Test from the stats package. Next, we used GLM models including mating success as a response variable (coded binomially – 1: mating occurs, 0: mating does not occur), experimental treatment as a fixed effect (O+/− or B+/−), courtship rate as a covariate, and the interaction between these variables. In the models, we considered the experimental treatments as a fixed effect (S. crassipalpata: O+ added ornamentation; O− control without ornamentation; S. bilineata: B+ control with intact brushes; B− shaved brushes). We used ‘glm’ and ‘glm.nb’ functions of the stats (R Core Team, 2020) and MASS (Venables and Ripley, 2013) packages respectively. We performed a posteriori analysis with ‘emmeans’ function and package (Lenith, 2021). Because we found a broken relationship between courtship rate and mating success for B− (see Section 3.3) we applied Receiver Operating Characteristic (ROC) curves to identify an optimal threshold for courtship rate that segregates the data within this group with the pROC package (Robin et al., 2011).
In addition to exploring mating success, we ran independent models in which we considered male mating behaviors – latency to courtship, number of courtship bouts, total courtship time, courtship rate, latency to mounting – as response variables. In these models, we only include the experimental treatment (O+/− or B+/−) as a fixed effect. We assessed normality, heteroscedasticity and overdispersion of all courtship variables using the package ‘fitdistrplus’ (Delignette-Muller and Dutang, 2015). If the normality assumptions were not met, we modeled the variable according to the best distribution. To determine the statistical significance of all the GLMs, we utilized the ‘Anova’ function from the car package (Fox and Weisberg, 2019), and we set the significance level α at 0.05. We created graphical representations of the data and fitted models using the ggplot2 (Wickham, 2011) and visreg packages (Breheny and Burchett, 2017).
2.4 Aim 3 – Potential interacting role of ornamentation and vibratory signaling in S. bilineata mating success
Despite research demonstrating condition-dependence of S. bilineata brushes (Hebets et al., 2021), results of Aim 2 found no evidence that brush presence/absence influences male mating success (see Section 3.2). Past research in S. bilineata has, however, found interacting effects of modality-specific signaling and the signaling environment on mating success. Specifically, vibratory signaling was shown to increase in importance in the absence of visual signaling (Hebets et al., 2021). This prior study did not, however, explore whether visual signaling, or attention to ornamentation, might also increase in the absence of vibratory signaling. Thus, our final experiment explored the potential combined and interactive effects of visual signaling with ornamentation and vibratory signaling by running mating trials across a 2 × 2 full design of ornamentation (brushes plus pigmentation) present/absent (O+/−) and vibratory signaling present/absent (V+/−).
2.4.1 Manipulating ornamentation and vibration
Once mature, we randomly assigned S. bilineata males to one of two treatments – (i) Ornamentation present (O+; brushes intact, sham shaved with 2nd pair of legs painted brown) and (ii) Ornamentation absent (O−; brushes shaved and pigment covered with brown paint). We then further divided these males into two vibratory treatments – (a) Vibration present (V+; males could transmit vibrations through the substrate) and (b) Vibration absent (V−; males could not transmit vibrations through the substrate). Thus, we created four experimental groups: O+/V+ (n = 15), O+/V− (n = 14); O−/V+ (n = 14); and O−/V− (n = 13).
For the O+/O− manipulations, we shaved and sham-shaved males using the same protocols as Aim 2. We added an additional step, however, to fully remove ornamentation: we painted over the dark pigmentation with brown paint matching the male’s body color (Anita’s All Purpose Acrylic Craft Paint – 11044 coffee/cafe, Synta Inc., Clarkston, GA, USA). Thus, our O− treatment fully removed all male ornamentation, not just the brushes as in Aim 2. We used the same Ziploc technique to both shave/sham-shave and paint the forelegs. As the control for painting in the O+ treatment, and to control for the presence and potential odor of the paint, we painted the tibiae and patellae of the 2nd pair of legs in O+ males brown. Both ornament treatments, then, had males with brown paint on their legs: O+ on legs 2 and O− on the forelegs.
To manipulate the presence/absence of vibratory signaling, we altered the signaling environment of the mating arenas. Instead of using mating arenas with an intact floor, we used arenas with the floor removed. This allowed us to alter the substrate upon which the pairs interacted. In the V+ treatments, we ran mating trials as in Aims 1 and 2 with filter paper lining the enclosure floor. Filter paper transmits vibratory signals well, ensuring that females and males could effectively send/receive vibratory communication (Hebets, 2005, 2008; Rundus et al., 2011; Sullivan-Beckers and Hebets, 2011, 2014; Hebets et al., 2021). In the V− treatments, however, the enclosures were placed on a granite slab. Research has shown that granite cannot transmit spider vibrations well (Elias et al., 2004) thus enabling us to remove vibratory signaling without manipulating signalers. To control for the visual background of the substrate, the granite slab was painted white to match the filter paper. Furthermore, to control for any potential effect of the white paint odor, both treatments were run on white-painted granite slabs, but the V+ treatment just had a piece of filter paper placed on top.
2.4.2 Mating trials
Similar to Aims 1 and 2, the diameter of our mating arenas was 12.5 cm in diameter (Pioneer Plastics Inc., Dixon, KY with the floor removed). Approximately 24 hours before the start of trials we fed females one size-matched cricket to minimize the potential for pre-copulatory cannibalism. On the day of their mating trial, we weighed females and males and placed females in their respective arenas at least 1 hour prior to the start of the trial to acclimate and deposit silk cues. Trials began when we introduced males into the arena, and we allowed each pair to interact for up to 30 minutes. Following each trial, we sacrificed all individuals (all were at near the end of their life) using freezing and subsequently preserved them in 70% ethyl alcohol. We recorded the same behavior in the same manner as previously described. Additionally, in this experiment we evaluated the following female behavior: (i) occurrence of turns (the female slowly turns in place) and (ii) occurrence and number of attacks (the female jumps at the male). The female turn is considered a behavioral proxy for receptivity, where the female performs a pivot display (~45–360 degree rotations) that produces vibratory cues (presumably through footfalls on the substrate) (see Supplementary Movie S2 of Sullivan-Beckers and Hebets, 2011). The female turn was readily observable in S. bilineata, which is why it is one of our focal female traits.
2.4.3 Aim 3 statistical analyses
As before, we verified by LMs that female and male weights were equal between experimental treatments. Because there were no differences between treatments (S. bilineata females: F = 0.049, df = 3, p-value = 0.985; males: F = 1.811, df = 3, p-value = 0.157), we did not include individual weights as covariates in the main models.
We statistically compared the proportions of mating success between categories using a Multiple Proportions Test from the stats package. Next, we coded mating success as binomial (1: occurrence, 0: non-occurrence) and considered the response variable of models including both experimental treatments as fixed effects (2 × 2 factorial design), courtship rate as a covariate, and the interaction between all of these variables. In the models, we consider the interaction of both experimental treatments as fixed effects (Ornamentation with 2 factors: Ornamentation present O+/Ornamentation absent O−; Vibration with 2 factors: Vibrations present V+/Vibrations absent V−), yielding four possible combinations (O+/V+, O+/V−, O−/V+, O−/V−). Because some treatments had almost complete data separation (almost all 0) we corrected the models with the ‘brglm’ method of the brglm2 package (Kosmidis, 2020) to provide reliable estimates.
We also performed binomial models with the same fixed effects for the occurrence of female turns and attacks. In addition, we performed GLMs where we considered sexual behaviors (latency to courtship, number of courtship bouts, total courtship time, courtship rate, latency to mounting, number of female attacks) as response variables in independent models. After evaluating the normality, heteroscedasticity and overdispersion of all variables, we constructed models according to the best distribution. We used the functions, packages and procedures described for Aims 1 and 2.
3 Results
3.1 Aim 1: Role of ornamentation in S. crassipalpata mating success
We found no statistical differences between the proportion of pairs that mated in the treatment with ornamentation (O+, 11/16; 69%) as compared to without ornamentation (O−, 9/15; 60%) (Chisq = 0.018, df = 1, p-value = 0.894; Table 1). Asking the same question in a slightly different way, the addition of ornamentation did not affect mating probability (Chisq = 0.557, df = 1, p-value = 0.455) (Figure 1A). Visual courtship rate, quantified as the ratio of the number of courtship bouts by the total courtship time, had no significant effect on the likelihood of mating (Chisq = 2.315, df = 1, p-value = 0.128) (Figure 1B), and the interaction term between courtship rate and experimental treatment showed no significant effect on the probability of mating (Chisq = 0.096, df = 1, p-value = 0.757).
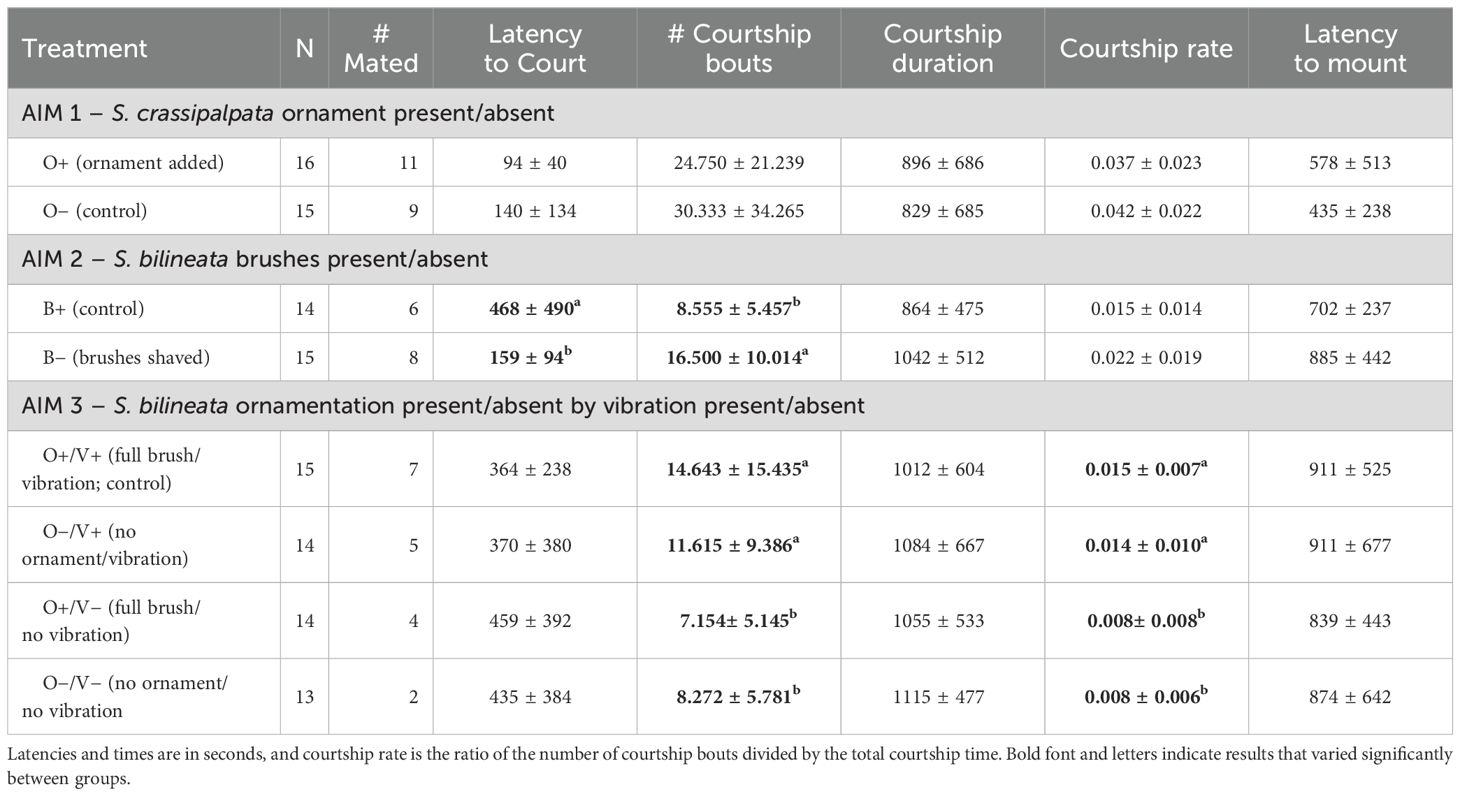
Table 1. Mean and Standard Deviation (X¯ ± SD) of sexual parameters measured in reproductive interactions in Schizocosa crassipalpata (Aim 1) and S. bilineata (Aims 2 and 3).
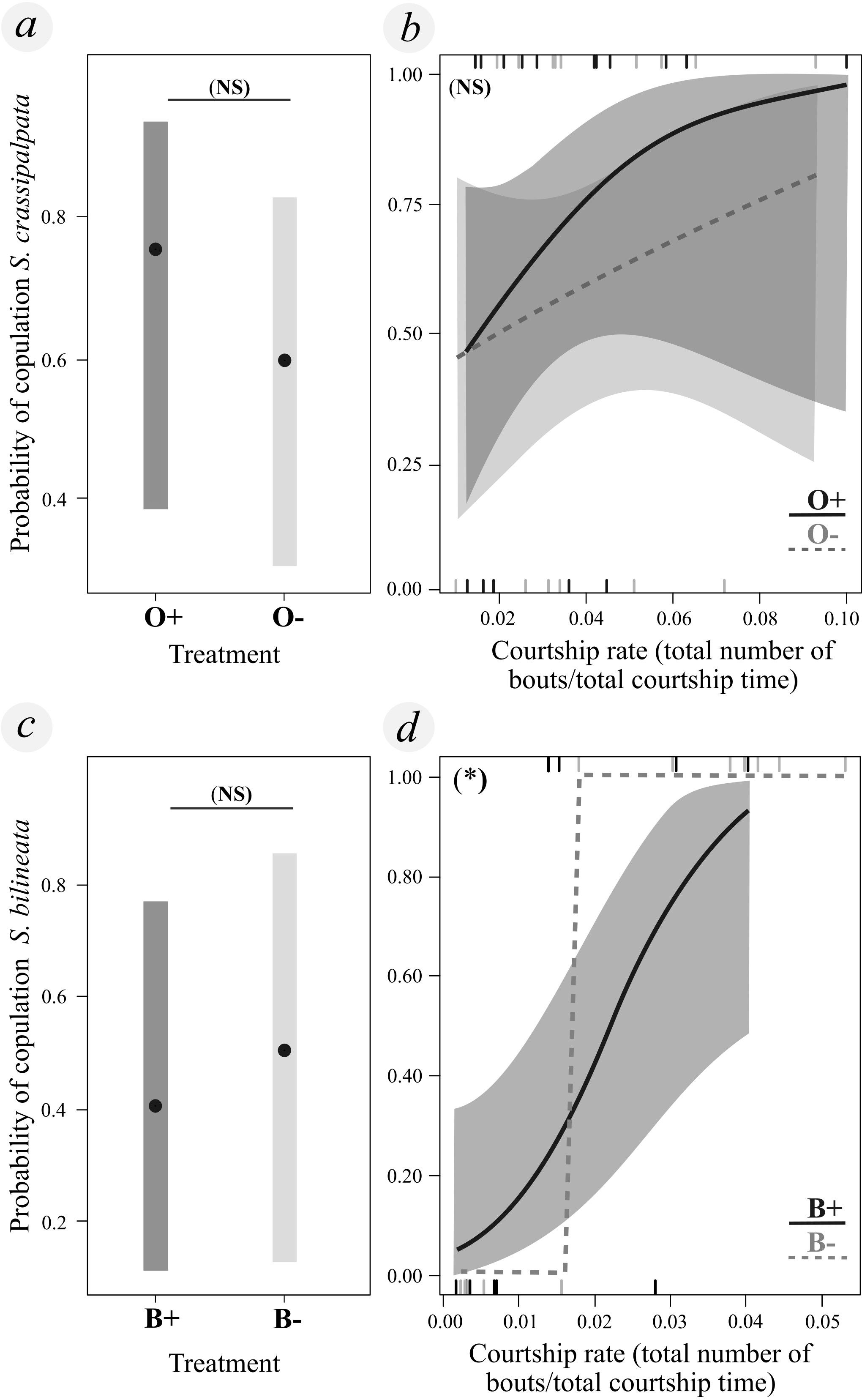
Figure 1. Ornamentation did not influence mating success (binomially coded mating probability) in either S. crassipalpata (A) or S. bilineata (B), but faster courting male S. bilineata were more likely to acquire matings (D). (A) Mating success in S. crassipalpata did not differ between different experimental treatments (O−: control without ornamentation, O+: ornamentation added experimentally). (B) Mating success was not significantly correlated with courtship rate [# bouts/total courtship time (sec)] in S. crassipalpata in any of the groups (O+: dark gray/solid line, O−: light gray/dashed line). (C) Mating success did not differ between S. bilineata male treatments (B+: control with brushes, B−: shaved, brushes removed experimentally). (D) Mating success was positively correlated with visual courtship rate [# bouts/total courtship time (sec)] in S. bilineata in both groups, with a marginally significant difference between groups (B+: dark gray/solid line, B−: light gray/dashed line). Only model (D) was statistically significant (p-value < 0.005). (A, C) Estimated marginal means (black points) of mating success for each treatment group, with 95% confidence intervals (gray areas). (B, D) Predicted probability of mating success as a function of courtship rate, shaded gray area indicates the 95% confidence interval (due to wide amplitude, the confidence intervals for B− are not shown). * Statistically significant difference between the response and predictor variable (p-value < 0.05).
We also found no statistical differences between treatments (O+/O−) in any of our measures of male reproductive behavior (latency to courtship: Chisq = 2.124, df = 1, p-value = 0.145; number of courtship bouts: Chisq = 0.397, df = 1, p-value = 0.528; total courtship time: Chisq = 0.074, df = 1, p-value = 0.785; Courtship rate: Chisq = 0.482, df = 1, p-value = 0.487; Latency to mounting: Chisq = 0.685, df = 1, p-value = 0.408) (Figures 2A–C; Table 1).
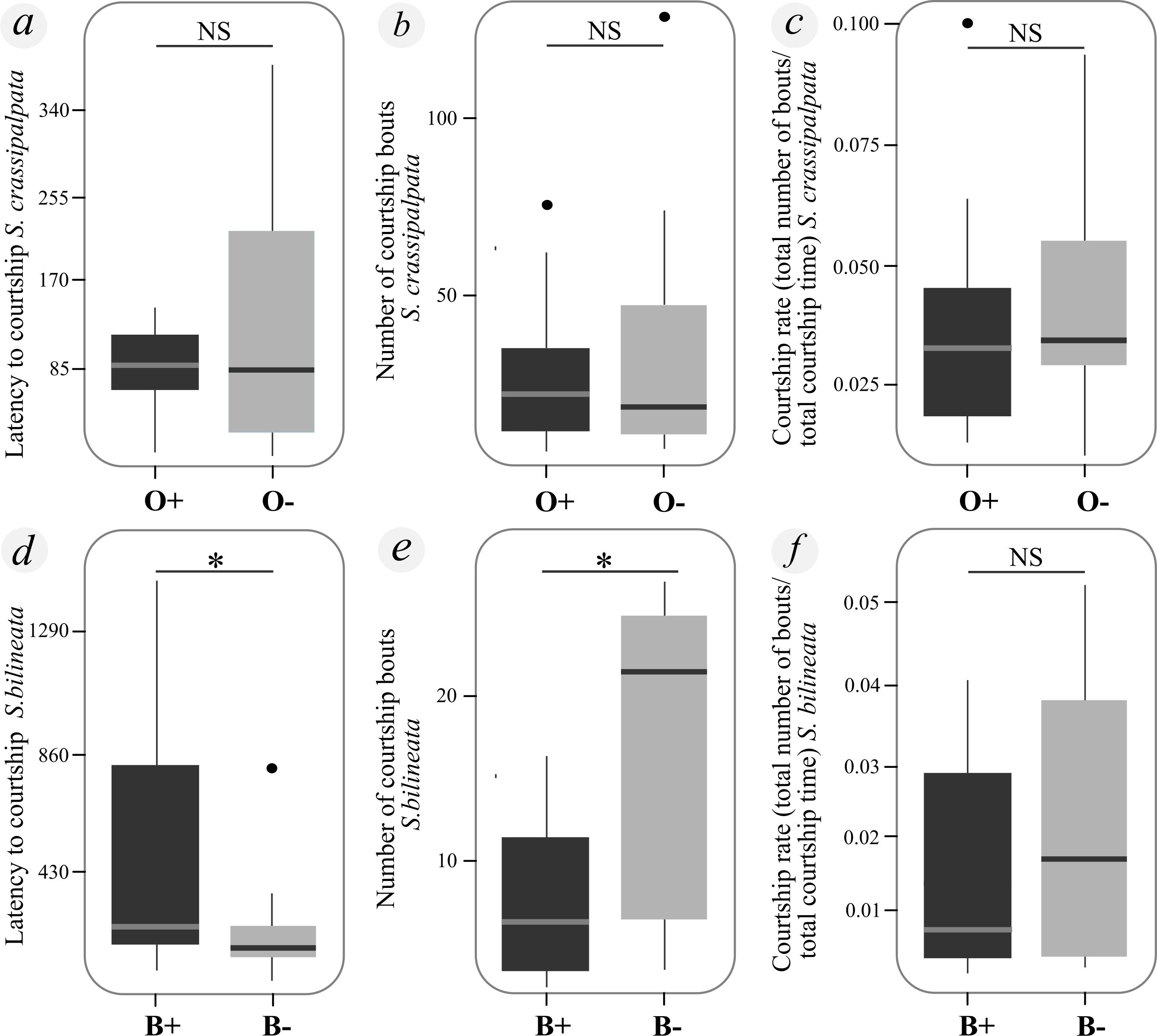
Figure 2. Male S. crassipalpata (top row) did not differ in courtship behavior between treatment groups (S. crassipalpata O+/−) while S. bilineata (bottom row) shaved males (B−) courted sooner and faster than intact (B+) males. (A) Courtship latency (seconds) of S. crassipalpata males did not differ between experimental groups (O−: control without ornamentation, O+: ornamentation added experimentally). (B) Number of courtship bouts of S. crassipalpata did not differ between experimental groups (idem A). (C) Courtship rate did not differ between experimental groups of S. crassipalpata (idem A). (D) Courtship latency (seconds) was shorter for B− (brushes removed experimentally) than B+ (control with brushes) male S. bilineata. (E) Number of courtship bouts was higher in B− than B+ S. bilineata males (idem D). (F) Courtship rate did not differ between experimental groups of S. bilineata (idem D). * Statistically significant difference between groups (p-value < 0.05); NS: Statistically significant differences not found. Box plots show 25% and 75% quartiles (boxes), medians (lines in the boxes), outermost values within the range of 1.5 times the respective quartiles (whiskers) and outliers (circles).
3.2 Aim 2: Role of brushes in S. bilineata mating success
Similar to our results for S. crassipalpata, we found no statistical differences between the proportion of pairs that mated in our treatment with brushes (B+, 6/14; 43%) as compared to with brushes shaved (B−, 8/15; 53%) (Chisq = 0.037, df = 1, p-value = 0.848; Table 1). Again, asking the question in a slightly different way, brush removal did not affect mating probability (Chisq = 0.194, df = 1, p-value = 0.659) (Figure 1C). Unlike S. crassipalpata, however, we found that courtship rate did affect the likelihood of mating (Chisq = 15.533, df = 1, p-value < 0.005; Figure 1D). Males that had a higher courtship rate (more bouts in less time) exhibited a higher probability of mating regardless of whether or not they had ornamentation (Figure 1D). The interaction between courtship rate and experimental treatment was marginally significant (Chisq = 3.351, df = 1, p-value = 0.067), suggesting that there may be differences in the significance of courtship rate for B+ and B− males. Explicitly, the courtship rate of B+ males scaled linearly and smoothly with the probability of mating, whereas the courtship rate of B− males scaled more steeply, reaching a threshold courtship rate (ROC = 0.01679) after which males mated successfully (Figure 1D). Beyond that point both B+ and B− have a higher likelihood of copulation if they court at a higher rate, but there is no difference between B− and B+ males at these higher courtship rates. Below that point, however, B+ males have an advantage (11% of males below this threshold were able to copulate successfully). In terms of courtship behavior, shaved males (B−) started courting faster (Chisq = 10.773, df = 1, p-value = 0.001) and performed more courtship bouts (Chisq = 4.295, df = 1, p-value = 0.038) than control males (B+). However, the total courtship time (Chisq = 1.264, df = 1, p-value = 0.342), courtship rate (Chisq = 0.673, df = 1, p-value = 0.412), and latency to mounting (Chisq = 0.943, df = 1, p-value = 0.331) were equal between experimental treatments (Figures 2D–F; Table 1).
3.3 Aim 3: Role of ornamentation and vibratory signaling in S. bilineata mating success
Control males with full ornamentation in environments where they could transmit vibrations (O+/V+) mated 47% (7/15) of the time, while males in the treatment with ornamentation removed (O−/V+) mated 36% of the time (5/14). Males with full ornamentation in environments where they could not transmit vibrations (O+/V−) mated 28% (4/14) of the time, while the smallest number of mating males (15% of males, 2/13) belonged to the group without ornamentation or vibration (O−/V−). We found no statistical difference between these proportions (Chisq = 3.288, df = 3, p-value = 0.349; Table 1). Overall, we found no effect of the experimental treatments of ornament present/absent (Chisq = 0.059, df = 1, p-value = 0.807) or vibratory signal present/absent (Chisq = 0.743, df = 1, p-value = 0.389) on mating success (Figure 3A). We also found no interaction between these treatments (Chisq = 0.211, df = 1, p-value = 0.646).
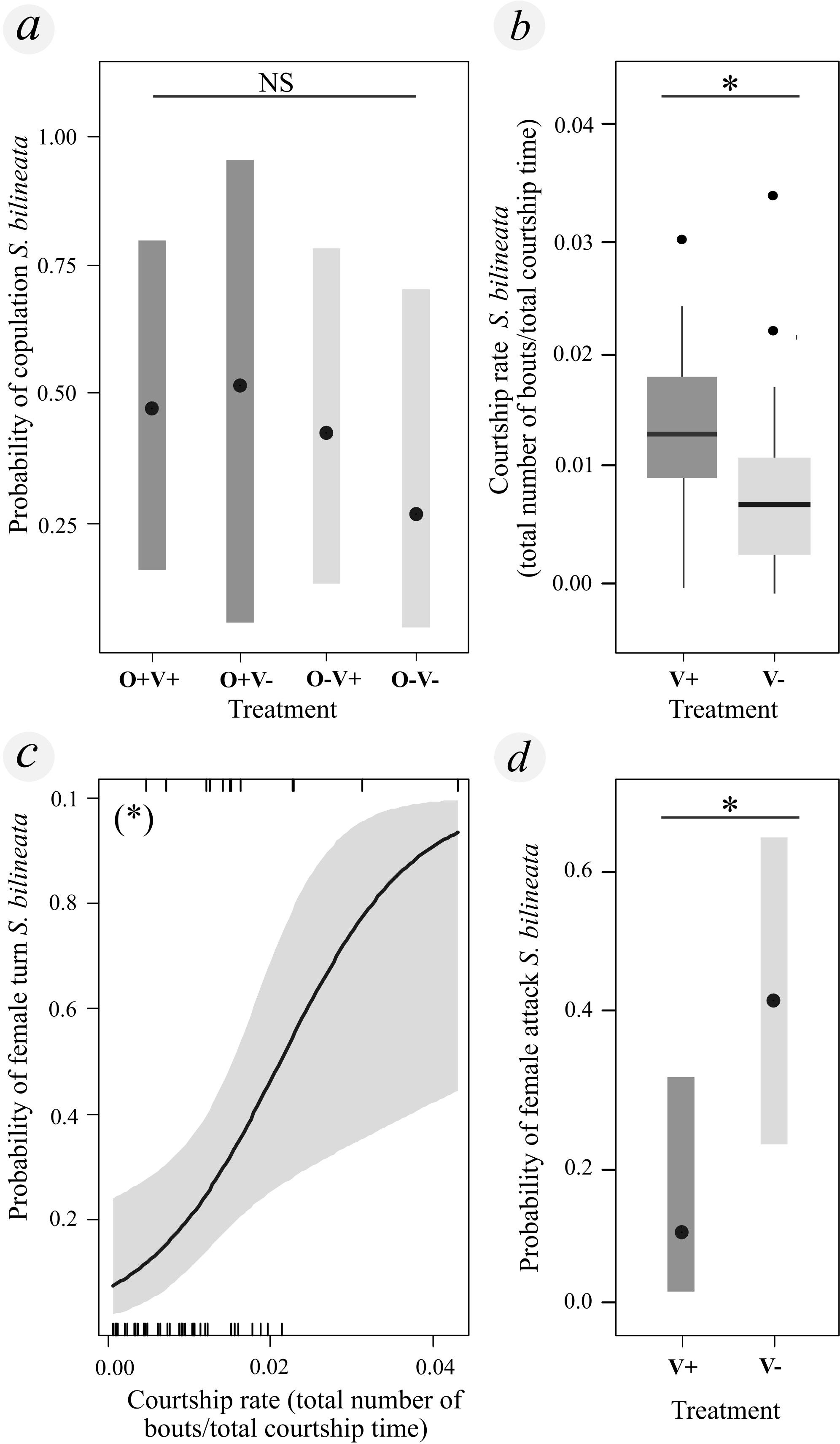
Figure 3. Neither the presence/absence of ornamentation (brushes and dark pigment) nor the presence/absence of vibration influences mating success (binomially coded mating probability) of S. bilineata, but males that courted faster were more likely to copulate. (A) Mating success did not differ across experimental treatments (O+/V+: full ornamentation/vibratory signaling allowed; O−/V+: no ornamentation/vibratory signaling allowed; O+/V−: full ornamentation/vibratory signaling not allowed; O−/V−: no ornamentation/vibratory signaling not allowed). (B) Males in environments in which vibrations could travel (V+) courted at a faster rate than V− males (V+: vibratory signaling allowed; V−: vibratory signaling not allowed). (C) The probability of a female engaging in a presumed receptivity display (slow turn) increased with increasing male courtship rates [# bouts/total courtship time (sec)]. (D) Females were more likely to attack males in the absence of vibratory signaling (V− versus V+) (idem B). *: Statistically significant difference between groups or between the response and predictor variable (p-value < 0.05). NS: Statistically significant differences not found. Box plot shows 25% and 75% quartiles (boxes), medians (lines in the boxes), outermost values within the range of 1.5 times the respective quartiles (whiskers) and outliers (circles) [# bouts/total courtship time (sec)].
Similar to results of Aim 2, S. bilineata males with higher courtship rates were more likely to mate regardless of the treatment (Chisq = 10.714, df = 1, p-value = 0.001). In Aim 3, however, the signaling environment also influenced courtship rate. Males in environments without vibrations (O+/V− and O−/V−) performed fewer courtship bouts (Chisq = 5.393, df = 1, p-value = 0.020) and performed them at a lower rate (Chisq = 6.771, df = 1, p-value = 0.009) than males run in the presence of vibration (O+/V+ and O−/V+) regardless of whether ornamented or not (Figure 3B). We found no effect of experimental treatments on latency to court (Ornamentation: Chisq = 0.099, df = 1, p-value = 0.753; Vibratory environment: Chisq = 1.110, df = 1, p-value = 0.292), total courtship time (Ornamentation: F = 0.035, df = 1, p-value = 0.853; Vibratory environment: F = 0.195, df = 1, p-value = 0.661) or latency to mounting (Ornamentation: Chisq = 0.016, df = 1, p-value = 0.899; Vibratory environment: Chisq = 0.006, df = 1, p-value = 0.980; Table 1).
Regarding female behavior, the probability of observing a female receptivity turn was higher when males had a higher courtship rate (Chisq = 10.220, df = 1, p-value = 0.001) regardless of treatment (Ornamentation: Chisq = 0.692, df = 1, p-value = 0.405; Vibratory environment: Chisq = 0.215, df = 1, p-value = 0.643) (Figure 3C). On the other hand, the probability that the female attacked the male (Chisq = 10.862, df = 1, p-value = 0.001) and the number of attacks (Chisq = 8.250, df = 1, p-value = 0.004) was higher with males in environments where vibratory signaling was not allowed (O+/V− & O−/V−) (Figure 3D).
4 Discussion
By first adding ornamentation to males of a naturally unornamented species (S. crassipalpata), we aimed to test whether females might choose ornamented males if given the opportunity to exercise such a preference. Using video playbacks of courting males with manipulated ornamentation, earlier research suggested that ornamentation may act to amplify dynamic movement displays, such as leg waves (Hebets and Uetz, 2000). Given that the courtship display of S. crassipalpata involves foreleg movements, we hypothesized that ornamentation might enhance these movements, resulting in a higher mating success for ornamented males. Further, given that elaborate ornamentation in the form of bristles appears to have evolved independently in S. bilineata, we reasoned that females of the sister-species, S. crassipalpata, might possess a pre-existing preference for increased ornamentation. A similar hypothesis of a pre-existing sensory bias for increased ornamentation was proposed for the independent evolution of black pigmentation and brushes of S. ocreata (McClintock and Uetz, 1996). In that study, S. rovneri females (an unornamented species that was considered to be the sister-species to S. ocreata) showed a preference for video playback males with brushes added, and the authors interpreted this as a pre-existing bias for brushes (McClintock and Uetz, 1996). Recent phylogenetic findings, however, indicate that brushes were secondarily lost in S. rovneri, suggesting that the prior findings are indicative of a retained, as opposed to pre-existing, preference for brushes in S. rovneri (Starrett et al., 2022). Unlike in S. rovneri, however, we found no evidence for either a pre-existing bias or a retained preference for ornamentation in S. crassipalpata, as mating success was equivalent across male phenotypes.
Across Schizocosa species, we know that ornamentation is diet-dependent, suggesting a cost of production (reviewed in Starrett et al., 2022) which could influence its evolutionary loss. Future empirical work, however, is now required to understand the observed evolutionary patterns of (i) ornament loss and preference retention (S. rovneri, McClintock and Uetz, 1996) versus (ii) ornament and preference loss (S. crassipalpata, current study). Notably, our results in both S. crassipalpata and S. bilineata could be considered in the context of sexually antagonistic coevolution, or a build-up of female resistance (e.g., to ornamentation) (Holland and Rice, 1998; Hebets and Maddison, 2005). It is possible, for example, that females of both species have built up resistance to ornamentation, effectively removing its influence on mating success and relaxing selection. Future research is required to test this hypothesis.
In addition to no influence of the presence versus absence of ornamentation on mating success, the visual courtship rate of S. crassipalpata males was also not a predictor of mating success for either phenotype (O+/O−). We know from prior experiments, however, that the vibratory courtship rate of S. crassipalpata males does matter. An earlier study found a relationship between faster, diet-dependent vibratory courtship rates and an increased likelihood of male S. crassipalpata mating (Hebets et al., 2021). In the present study, unfortunately, we did not quantify vibratory courtship rate, but we expect that it would have been a good predictor of male mating success. We also expect that the visual and vibratory courtship rate in S. crassipalpata are weakly correlated, as Figure 1B shows a non-significant pattern of increasing mating success with increased visual courtship rate. Future studies exploring the relationship between visual and vibratory courtship rates would likely provide more insight into this observation. In the end, however, none of our quantified phenotypes or behavior predicted mating success in S. crassipalpata, supporting prior findings of a very strong role of vibratory, but not visual, courtship signaling in this species (Hebets et al., 2021).
Similar to our findings for S. crassipalpata, across multiple experiments we found no evidence to suggest a strong role of ornamentation alone in the mating success of the naturally ornamented S. bilineata. Removing just the tibial bristles (Aim 2), or the tibial bristles plus pigmentation (Aim 3), of S. bilineata did not influence mating success, regardless of whether vibratory signals could transmit or not. At face value, this suggests that ornamentation in male S. bilineata is not under selection from mate choice. Interestingly, research on other Schizocosa species has similarly found no predictive power of diet-dependent ornamentation on mating success – e.g., S. floridana (Rundus et al., 2011; Rosenthal and Hebets, 2012), S. stridulans (Rosenthal and Hebets, 2015), S. uetzi (Hebets et al., 2006; Shamble et al., 2009). In S. uetzi, higher receptivity to increased ornamentation was only observed in the presence of vibratory signaling, suggesting an interactive role of the signaling modalities – e.g., vibration focuses visual attention (Hebets, 2005). Our Aim 3 tested for this possibility, but we saw no evidence for amplifying or attention-altering interactions between vibratory signaling and the presence/absence of ornamentation. We did, however, find a potential interaction between ornamentation and courtship rate that we will discuss shortly. In summary, regardless of the presence/absence of vibratory signaling, ornamentation alone does not appear to play a role in S. bilineata male mating success.
Though ornamentation itself did not predict mating success, the movement of the ornamented legs was important. Male S. bilineata that produced more courtship bouts, counted as leg waves, across a similar amount of time were more likely to successfully mate. This increased courtship rate (i.e. faster courtship) could indicate higher male vigor and/or skill. A mating advantage based on courtship performance (i.e., courtship rate) is common across this genus (S. stridulans, Hebets et al., 2011; S. uetzi, Shamble et al., 2009; S. retrorsa, Rundus et al., 2010; S. ocreata, Delaney et al., 2007, Gibson and Uetz, 2008; S. floridana, Rosenthal and Hebets, 2012, 2015). It is common across other animal taxa as well – e.g., orthopterans, homopterans and anurans (reviewed in Gerhardt and Huber, 2002), fiddler crabs (Backwell et al., 1999; Murai and Backwell, 2006), birds and mammals (reviewed in Byers et al., 2010). Unfortunately, studies quantifying Schizocosa motor skills or agility are lacking, making it challenging to determine whether or not higher courtship rate is actually indicative of high quality signaling.
Nonetheless, our results suggest that female S. bilineata are assessing dynamic visual signaling; consistent with a scenario in which females assess and choose a male based on motor performance (Byers et al., 2010). While it is too early to determine whether it is the quantity or quality of the performance (or both) that is most important, courtship movements are clearly under selection. A hypothesis of mate choice for motor performance or behavioral skills has received support from a number of empirical studies across a wide range of animal taxa. Among birds, female choice for agility is proposed to explain the small size and elaborate courtship displays of ‘bee’ hummingbirds (Mellisugini) (Wilcox and Clark, 2022) as well as the aerial displays of other passerines (Mikula et al., 2022). Female manakins appear to choose males based on their motor skills (reviewed in Fusani et al., 2014) and in the Diamond Firetail Finch (Stagonopleura guttata), the intensity of male courtship is correlated with both spot number and pairing success (Zanollo et al., 2013). These seemingly athletic displays are also often associated with special adaptations for performance. Pectoral sandpipers (Calidris melanotos), for example, are shown to maintain an incredibly high aerobic capacity during the breeding period with a seemingly related extraordinarily high sex-specific oxygen carrying capacity that is hypothesized to be the result of selection from female choice (Santema et al., 2023). Schizocosa wolf spiders appear to be another group in which selection for performance has influenced the evolution of courtship displays. In addition to quantifying and comparing motor skills or agility, future comparative work exploring the relationships between aerobic capacity and courtship complexity would be interesting to determine whether any physiological adaptations might accompany selection for performance.
Though not significant, we found a trend that the brushes of S. bilineata might interact with courtship rate to explain the probability of copulation (Aim 2 interaction term, p = 0.067). This interaction can be seen graphically in the different shapes of the plotted relationship between courtship rate and mating probability of (i) brushed males (linear with a proportional increase) versus (ii) experimentally removed brushed males (stepwise with a low probability of mating that increased abruptly when a threshold courtship rate was exceeded) (Figure 1D). We devote some discussion to this nonsignificant trend because surprisingly, our results look very similar to those of a study exploring the role of ornamentation in S. stridulans. Specifically, following artificial manipulations and assessments of natural ornamentation levels, Hebets et al. (2011) found that the least ornamented S. stridulans males had a very low probability of mating until a threshold of courtship rate was reached (see Figure 3 of Hebets et al., 2011). Below this threshold, more ornamented males remained able to acquire matings. These results are nearly identical to our findings in S. bilineata. Hebets et al. (2011) interpreted this as ornamentation having a function in easing a male’s reliance on courtship rate – below a certain threshold of courtship performance; only ornamented males could successfully acquire a mating. Again, similar to our results, in S. stridulans, at higher courtship rates, foreleg ornamentation offered no mating advantage or even a reduced mating advantage (Hebets et al., 2011). Our results are consistent with the proposed hypothesis that ornamentation reduces a male’s reliance on courtship rate for mating success and further supports the idea that females assess the interaction between these traits (Hebets et al., 2011). Since S. crassipalpata females do not appear to rely on visual courtship rate for mating decisions, it is not surprising that we did not find a similar result in S. crassipalpata (i.e. there was no trend towards courtship rate and ornamentation +/− interacting to influence mating success). Nonetheless, identifying a similar relationship between courtship rate and ornamentation across two distinct species of Schizocosa (S. stridulans and S. bilineata) supports a hypothesis that ornamentation in Schizocosa evolved, and is likely maintained, due to its interactions with dynamic movement displays (i.e. leg movements). This interaction is not as straightforward as a simple amplifier (Hasson, 1989, 1991), however, as the relationship between ornamentation and performance (i.e. courtship rate) and its influence on mating success is non-linear, suggesting more sophisticated assessment and mating decisions by females.
Performance displays such as courtship rate often have the potential to reveal something about the performer, making them potentially good traits upon which to make mating decisions. Abundant food, for example, increases the number of courtship waves produced by fiddler crabs (Takeshita et al., 2018); elaborate manakin displays are known to be androgen-dependent (Fuxjager and Schlinger, 2015; Fuxjager et al., 2018); and the vigor of male signaling in field crickets declines with age (Bertram et al., 2022). Numerous studies have also documented costs associated with courtship displays – e.g., in great snipe (Höglundi et al., 1992), fiddler crabs (Matsumasa and Murai, 2005), mole crickets (Prestwich and O’Sullivan, 2005), and wolf spiders (Mappes et al., 1996; Kotiaho et al., 1998), including Schizocosa (S. ocreata and S. rovneri, Cady et al., 2011), among others. The costliness of performance displays further supports the notion that performance can reflect quality in some way and thus, should influence choosers. In Schizocosa, however, no studies yet have found a relationship between courtship rate and any potential quality indicator (e.g., condition, nutritional history, etc.) (Rosenthal and Hebets, 2012, 2015; Gilbert et al., 2016; Gilbert and Uetz, 2016). In contrast, ornamentation has consistently been documented as condition-dependent across Schizocosa species, yet has been shown to have limited to no role in predicting mating success (S. bilineata, Hebets et al., 2021; S. floridana, Rundus et al., 2011, Rosenthal and Hebets, 2012; S. stridulans, Rosenthal and Hebets, 2015, S. uetzi, Shamble et al., 2009; S. ocreata, Uetz et al., 2002). These contradictory patterns – predictor of mating success but not condition-dependent (courtship rate) and non-predictors of mating success yet condition-dependent (ornamentation) – reveal a complicated scenario of selection that might involve choosers weighing behavioral performance against ornamentation and doing so differently across contexts or environments. Notably, as we will discuss shortly, performance traits can be dynamically altered, while morphological traits like ornamentation, especially in Schizocosa wolf spiders, are fixed. This variation in plasticity across display components affords choosers unique opportunities to assess multiple display components that may function differently across time and space. Additionally, or alternatively, the detectability of performance traits may vary with ornamentation. Under more natural conditions, for example, ornamentation may indeed amplify the dynamic visual displays of S. bilineata, especially in environments or contexts in which movement might be challenging to assess (e.g., wind, dark, etc., McGinley et al., 2023). Future studies exploring the detectability and assessability of dynamic movement across varying courtship rates, with and without ornamentation, and across environmental contexts, would help untangle some of these possibilities.
Our study did not test other potential functions of ornamentation, yet many exist. Schizocosa bilineata foreleg ornamentation may, for example, be useful in agonistic interactions between signalers. Despite a wealth of studies on this genus, however, there is no indication that ornamentation plays any role in intraspecific interactions (reviewed in Hebets and McGinley, 2019; Starrett et al., 2022). There is, however, evidence that different display components may function at different time points and/or distances during reproductive interactions. Some, for example, may help attract a female’s attention from a distance. Schizocosa floridana males incorporate specific display components in their courtship more often when females are absent versus present, suggesting that these components may play a specific role in mate attraction (Rosenthal et al., 2018). Ornamentation in S. bilineata may play a similar role. Indeed, field studies examining modality-specific signal transmission in S. ocreata suggest that visual signals can increase the active space of a display (Uetz et al., 2013). It remains entirely possible that ornamentation in S. bilineata functions to attract females from a distance, but that females then use other traits (e.g., courtship rate) to make mating decisions. This possibility requires testing.
Despite no clear evidence that male ornamentation alone influences mating success in S. bilineata, in these populations studied, we did find a relationship between ornamentation and male courtship behavior. Males with brushes removed (B−) began courtship more quickly and produced more courtship bouts than males with brushes intact (B+). They also tended to have higher courtship rates, but this was not significant. Interestingly, this fits with our earlier hypothesis that ornamentation eases a male’s reliance on courtship rate. In this scenario, unornamented males would need to court at a higher rate to attract a female than ornamented males until a threshold of performance was reached, after which ornamentation offers no obvious advantages. If males can assess their level of ornamentation by recognizing the size of their brushes, or by diminished reactions from females, they may increase their courtship performance to compensate for this reduced ornamentation. Across different taxa, males are known to exhibit behavioral flexibility to adapt their courtship behavior to unpredictable environmental changes (wolf spiders, Wilgers and Hebets, 2011; manakins, Janisch et al., 2020) and to changes in their own condition (garter snakes, Shine and Mason, 2005; guppies, Rahman et al., 2013). When the ground-dwelling wolf spider Pardosa milvina lost their signaling appendages, for example, they performed more pedipalp raises presumably to compensate for the inability to perform leg-waving displays (Brautigam and Persons, 2003). In S. ocreata, males lacking signaling appendages perform more jerky tapping than intact males (Taylor et al., 2006). Although these examples are more severe, since there is a loss of an appendage, S. bilineata males still experienced the loss of a courtship component. Given the numerous sensory sensilla on their legs (Foelix, 2011), it is entirely plausible that males were able to recognize the removal or diminishment of their brushes. Waving legs with and without brushes presumably alters the drag that male’s experience, thereby providing a mechanism by which to self-assess brush size. Alternatively, or additionally, the removal of brushes may decrease the cost of courtship, enabling B− males to court faster and produce more bouts. Ultimately, by beginning courtship earlier and producing more courtship bouts over a longer time, shaved males were able to achieve an equal mating probability to intact males. These compensatory abilities appear to occur in several species of wolf spiders. In the future, it will be valuable to understand in detail the cognitive or sensory mechanisms (e.g., if/how males can recognize their degree of ornamentation) and fitness consequences of this behavioral flexibility.
Numerous additional factors might also explain variation in male courtship investment, such as the individual’s own condition and the energy available for mating at each matting attempt (Kotiaho et al., 1996; Brandt and Greenfield, 2004). In our study, however, we found no differences in male weight between treatment groups, making differences in condition an unlikely explanation. Feedback from the female, as we will discuss shortly, is another factor that can influence male courtship behavior. The earlier start to courtship, however, is difficult to explain from a female-influence viewpoint, as females typically react to males following courtship. It is possible, however, that females responded more quickly in some way to shaved males, causing them to initiate courtship sooner; though we did not notice any such behavior. Ultimately, our data suggest that males can adjust their courtship to compensate for decreased ornamentation, but whether that compensation is due to self-assessment of brush size (e.g., from feedback with drag), decreased cost of courtship, response to female feedback cues, and/or something else, remains to be determined.
Regarding the importance of vibrational signals for S. bilineata, our results agree in part with those reported by Hebets et al. (2021), as the probability and latency to mating was the same in environments with and without vibrations. Our study, however, explored male courtship and female responses in more detail, uncovering additional male behavioral plasticity associated with the vibratory environment. In vibratory absent environments (V−), S. bilineata males performed fewer courtship bouts and courted at a lower rate. Females also showed a greater propensity to attack males in this environment (V−). These results highlight the importance of vibratory signaling for the female–male dialogue in S. bilineata and indicate that its importance is not only limited to poorly lit environments (Hebets et al., 2013, 2021). During courtship, both sexes exchange information assessing the tempo of courtship, the proximity of other individuals, and potentially quality and identity indicators through multiple sensory channels (Herberstein et al., 2014; Rodríguez, 2015). This dialogue sometimes requires fine adjustment and coordination between the sexes (Balsby and Dabelsteen, 2002; Patricelli et al., 2002, 2006) and in Schizocosa, we know that both visual and vibratory signaling can be involved in this dialogue (Uetz et al., 2009; Choi et al., 2022). In S. rovneri, males are known to attend to female vibratory feedback cues and to subsequently adjust and optimize their courtship signaling (Sullivan-Beckers and Hebets, 2011). We suspect that our V− treatment disrupted this dialogue. It is possible, for example, that females increased their attacks on males because of the missing vibratory signal. If vibratory signaling contains the password for species recognition (Hauber et al., 2001; Hebets, 2007), females might have been unable to readily identify males as conspecifics. Such female aggression then could reduce the male’s motivation to court, resulting in reduced courtship rates. In this scenario, the missing courtship led to increased female aggression and ultimately to decreased male courtship. Alternatively, because of missing vibratory cues from the female, males may have reduced their courtship rate, thereby increasing the likelihood of female rejection through attacks. Additional research is required to tease apart the cause and effect of these observations, but we are confident in our assertion that it all relates to vibratory-based female–male dialogue and cues.
5 Conclusion
Despite two sister-species demonstrating strong divergence in secondary sexual characters – i.e. ornamentation – we found no evidence that ornaments alone were under selection in a mate choice context. Ornaments, however, may interact with courtship rate to influence mating success, suggesting a more complex assessment strategy of females. We also failed to find support for a hypothesis of divergent ornamentation playing a role in species recognition (Starrett et al., 2022), as ornament present/absent did not influence mating success in either species. Nonetheless, results are consistent with prior findings that these species have diverged in their reliance on modality-specific courtship signaling with S. crassipalpata relying on vibratory signaling and S. bilineata relying on visual signaling (Hebets et al., 2021).
Although the most recent common ancestor of S. crassipalpata and S. bilineata was inferred to be ornamented (see Starrett et al., 2022), preferences for ornamentation may have been lost in the lineage leading to the common ancestor of the two species. Alternatively, females may have built up a resistance to this trait, and/or ornament evolution and maintenance may have been selected through its functional interaction with other traits. One potential explanation for the current day divergence is that S. crassipalpata diverged to rely more on vibratory signaling, losing the putatively costly ornamentation, while S. bilineata retained ornamentation due to its potential interaction with courtship performance. Future studies testing hypotheses regarding selection for sensory divergence between S. crassipalpata and S. bilineata (e.g., signal space partitioning due to competition; Hebets et al., 2021) as well as the cost(s) of ornamentation in S. bilineata would provide additional support for these hypotheses. Alternatively, we cannot discount the hypothesis that ornamentation plays a role in species recognition where S. crassipalpata and S. bilineata co-occur in higher frequencies. Though both species were present at each of our collecting sites, each site had one clearly dominant species. It remains possible that we would see a different pattern of behavioral response for individuals in populations with a more equal distribution of both species, if such a population exists. In such a scenario, behavioral responses would potentially have high plasticity, yet gene flow across populations sufficient to maintain the S. bilineata phenotype. Future studies testing this alternative hypothesis would include evaluations of gene flow and phylogeographic patterns across both species as well as similarly designed behavioral experiments testing the role of ornamentation on individuals sampled from populations where they co-occur in large numbers. With few exceptions – e.g., S. crassipes (Miller et al., 1998; Watts et al., 2019), S. ocreata (Stratton, 2005) – we simply have little data characterizing the extent of geographic variation in many of these behavioral traits where species are widespread. Nevertheless, rigorous comparative studies like the one reported herein, that compare sister taxa, show interesting behavioral responses that are integral to generating new and expanded hypotheses.
In terms of visual signaling, we confirm that it is the dynamic movement aspect of courtship that is crucial for male mating success in S. bilineata. We also uncovered an interesting pattern potentially indicating an interaction between ornamentation and courtship rate that may help us understand how S. bilineata brushes are maintained and how they may function. Specifically, our pattern matches earlier research suggesting that ornamentation may ease a male’s reliance on courtship performance at low courtship rates and across contexts. Whether this happens in nature and under what circumstances remains to be determined. We also uncovered significant plasticity in S. bilineata visual courtship displays. Males lowered their courtship rate in environments that did not transmit vibratory signals while females simultaneously increased their attack rates. The cause and effect of these changes was impossible to determine given our experimental design, but this pattern leads to many testable hypotheses regarding the role of vibratory signaling in species recognition and the role of female feedback in influencing male courtship rates.
Finally, this study again highlights the need to directly test commonly presumed functions of secondary sexual traits and the selection pressure(s) that maintains, or fails to maintain, them. We found zero support for any hypothesis related to female choice for ornamentation alone in either focal species, yet found strong support for selection for performance. Simultaneously, we found evidence of plasticity in performance, calling into question its reliability across contexts and potentially highlighting why choosers may assess interactions between display traits.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
EH: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal Analysis, Data curation, Conceptualization. MO-D: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal Analysis, Data curation. AA: Writing – review & editing, Investigation. SG: Writing – review & editing, Investigation. RM: Writing – review & editing, Supervision, Methodology, Data curation, Conceptualization. JS: Writing – review & editing, Data curation, Conceptualization. JB: Writing – review & editing, Supervision, Resources, Funding acquisition, Data curation, Conceptualization. MB: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant to EH (1556153) and JB (1836984) from the National Science Foundation.
Acknowledgments
We thank Jay Stafstrom, Brandi Pessman, and Laura Segura-Hernandez for help collecting spiders, and Andy Roberts and Todd Blackledge for housing MB during spider collecting trips. Thanks to Madison Hays for help with spider maintenance. We thank all members of the Hebets laboratory and the Basolo and Wagner laboratories for comments on early experimental design, analyses, and interpretations. Aims 1 and 2 were part of MB’s Master’s Thesis and we thank his committee members Alex Basolo and William Wagner for their help, support, and encouragement. We also thank the INBRE Scholars Program for funding SG and the National Science Foundation funded STEM-POWER program at UNL for funding AA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albo M. J., Toft S., Bilde T. (2012). Female spiders ignore condition-dependent information from nuptial gift wrapping when choosing mates. Anim. Behav. 84, 907–912. doi: 10.1016/j.anbehav.2012.07.014
Andersson M. (1982). Female choice selects for extreme tail length in a widowbird. Nature 299, 818–820. doi: 10.1038/299818a0
Backwell P. R. Y., Jennions M. D., Christy J. H., Passmore N. I. (1999). Female choice in the synchronously waving fiddler crab Uca annulipes. Ethology 105, 415–421. doi: 10.1046/j.1439-0310.1999.00387.x
Balsby T. J. S., Dabelsteen T. (2002). Female behaviour affects male courtship in whitethroats, Sylvia communis: an interactive experiment using visual and acoustic cues. Anim. Behav. 63, 251–257. doi: 10.1006/anbe.2001.1920
Basolo A. (1990a). Female preference for male sword length in the green swordtail, Xiphophorus helleri (Pisces, Poeciliidae). Anim. Behav. 40, 339–349. doi: 10.1016/S0003-3472(05)80928-5
Basolo A. L. (1990b). Female preference predates the evolution of the sword in swordtail fish. Science 250, 808–810. doi: 10.1126/science.250.4982.808
Bern M. (2011). Exploring sources of selection on the multimodal courtship displays of two sister species of wolf spiders: Schizocosa crassipalpata and Schizocosa bilineata.
Bertram S. M., Dakin R., Harrison S. J., Tremblay D. T., Reifer M. L., Kolluru G. R. (2022). Acoustic signalling performance: Variation in vigour at multiple scales. Anim. Behav. 184, 157–171. doi: 10.1016/j.anbehav.2021.08.001
Brandt L., Greenfield M. (2004). Condition-dependent traits and the capture of genetic variance in male advertisement song. J. Evolutionary Biol. 17, 821–828. doi: 10.1111/j.1420-9101.2004.00716.x
Brautigam S. E., Persons M. H. (2003). The effect of limb loss on the courtship and mating behavior of the wolf spider Pardosa milvina (Araneae: Lycosidae). J. Insect Behav. 16, 571–587. doi: 10.1023/A:1027311625059
Breheny P., Burchett W. (2017). Visualization of regression models using visreg. R J. 9, 56. doi: 10.32614/RJ-2017-046
Broder E. D., Elias D. O., Rodríguez R. L., Rosenthal G. G., Seymoure B. M., Tinghitella R. M. (2021). Evolutionary novelty in communication between the sexes. Biol. Lett. 17, 20200733. doi: 10.1098/rsbl.2020.0733
Byers J., Hebets E., Podos J. (2010). Female mate choice based upon male motor performance. Anim. Behav. 79, 771–778. doi: 10.1016/j.anbehav.2010.01.009
Cady A. B., Delaney K. J., Uetz G. W. (2011). Contrasting energetic costs of courtship signaling in two solf spiders having divergent courtship behaviors. J. Arachnology 39, 161–165. doi: 10.1636/Hi09-70.1
Charles G. K., Ord T. J. (2012). Factors leading to the evolution and maintenance of a male ornament in territorial species. Behav. Ecol. Sociobiology 66, 231–239. doi: 10.1007/s00265-011-1271-6
Chen I., Stuart-Fox D. M., Hugall A. F., Symonds M. R. E. (2012). Sexual selection and the evolution of complex color patterns in dragon lizards. Evolution 66, 3605–3614. doi: 10.1111/evo.2012.66.issue-11
Choi N., Adams M., Fowler-Finn K., Knowlton E., Rosenthal M., Rundus A., et al. (2022). Increased signal complexity is associated with increased mating success. Biol. Lett. 18, 20220052. doi: 10.1098/rsbl.2022.0052
Comstock J. H. (1940). The Spider Book: A Manual for the Study of the Spider and their Near Relatives, the Scorpions, Pseudoscorpions, Whip-scorpions, Harvestmen, and Other Members of the Class Aracnida, Found in America North of Mexico with Analytical Keys for their Classification and Popular Accounts of their Habits (Garden City, New York: Doubleday Press).
Cotton S., Fowler K., Pomiankowski A. (2004). Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046. doi: 10.1111/j.0014-3820.2004.tb00437.x
Cummings M. E., Endler J. A. (2018). 25 Years of sensory drive: the evidence and its watery bias. Curr. Zoology 64, 471–484. doi: 10.1093/cz/zoy043
Delaney K. J., Roberts J. A., Uetz G. W. (2007). Male signaling behavior and sexual selection in a wolf spider (Araneae: Lycosidae): a test for dual functions. Behav. Ecol. Sociobiology 62, 67–75. doi: 10.1007/s00265-007-0438-7
Delignette-Muller M. L., Dutang C. (2015). fitdistrplus: An R package for fitting distributions. J. Stat. software 64, 1–34. doi: 10.18637/jss.v064.i04
Dondale C. D., Redner J. H. (1978). Revision of nearctic wolf spider genus Schizocosa (Arachneida Lycosidae). Can. Entomologist 110, 143–181. doi: 10.4039/Ent110143-2
Doucet S. M., Mennill D. J., Hill G. E. (2007). The evolution of signal design in manakin plumage ornaments. Am. Nat. 169, S62–S80. doi: 10.1086/510162
Elias D. O., Maddison W. P., Peckmezian C., Girard M. B., Mason A. C. (2012). Orchestrating the score: complex multimodal courtship in the Habronattus coecatus group of Habronattus jumping spiders (Araneae: Salticidae). Biol. J. Linn. Soc. 105, 522–547. doi: 10.1111/bij.2012.105.issue-3
Elias D. O., Mason A. C. (2014). The role of wave and substrate heterogeneity in vibratory communication: practical issues in studying the effect of vibratory environments in communication. Studying vibrational communication, 215–247.
Elias D. O., Mason A. C., Hoy R. R. (2004). The effect of substrate on the efficacy of seismic courtship signal transmission in the jumping spider Habronattus dossenus (Araneae: Salticidae). J. Exp. Biol. 207, 4105–4110. doi: 10.1242/jeb.01261
Endler J. A. (1992). Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. doi: 10.1086/285308
Endler J. A., Basolo A. L. (1998). Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415–420. doi: 10.1016/S0169-5347(98)01471-2
Fitzpatrick C. L., Servedio M. R. (2018). The evolution of male mate choice and female ornamentation: a review of mathematical models. Curr. zoology 64, 323–333. doi: 10.1093/cz/zoy029
Fox J., Weisberg S. (2019). An R Companion to Applied Regression, Third edition (Sage, Thousand Oaks CA). https://socialsciences.mcmaster.ca/jfox/Books/Companion.
Framenau V. W., Hebets E. A. (2007). A review of leg ornamentation in male wolf spiders, with the description of a new species from Australia, Artoria schizocoides (Araneae, Lycosidae). J. Arachnology 35, 89–101. doi: 10.1636/ST06-15.1
Fusani L., Barske J., Day L. D., Fuxjager M. J., Schlinger B. A. (2014). Physiological control of elaborate male courtship: female choice for neuromuscular systems. Neurosci. Biobehav. Rev. 46, 534–546. doi: 10.1016/j.neubiorev.2014.07.017
Fuxjager M. J., Miles M. C., Schlinger B. A. (2018). Evolution of the androgen-induced male phenotype. J. Comp. Physiol. A 204, 81–92. doi: 10.1007/s00359-017-1215-3
Fuxjager M. J., Schlinger B. A. (2015). Perspectives on the evolution of animal dancing: a case study of manakins. Curr. Opin. Behav. Sci. 6, 7–12. doi: 10.1016/j.cobeha.2015.06.007
Gerhardt H. C., Huber F. (2002). Acoustic communication in insects and anurans (Chicago: University of Chicago Press).
Gibson J. S., Uetz G. W. (2008). Seismic communication and mate choice in wolf spiders: Components of male seismic signals and mating success. Anim. Behav. 75, 1253–1262. doi: 10.1016/j.anbehav.2007.09.026
Gilbert R., Karp R. D., Uetz G. W. (2016). Effects of juvenile infection on adult immunity and secondary sexual characters in a wolf spider. Behav. Ecol. 27, 946–954. doi: 10.1093/beheco/arv241
Gilbert R., Uetz G. W. (2016). Courtship and male ornaments as honest indicators of immune function. Anim. Behav. 117, 97–103. doi: 10.1016/j.anbehav.2016.04.013
Guilford T., Dawkins M. S. (1991). Receiver psychology and the evolution of animal signals. Anim. Behav. 42, 1–14. doi: 10.1016/S0003-3472(05)80600-1
Gumm J. M., Mendelson T. C. (2011). The evolution of multi-component visual signals in darters (genus Etheostoma). Curr. Zoology 57, 125–139. doi: 10.1093/czoolo/51.2.125
Hasson O. (1989). Amplifiers and the handicap principle in sexual selection: a different emphasis. Proc. R. Soc. London Ser. B-Biological Sci. 235, 383–406. doi: 10.1098/rspb.1989.0006
Hasson O. (1991). Sexual displays as amplifiers - practical examples with an emphasis on feather decorations. Behav. Ecol. 2, 189–197. doi: 10.1093/beheco/2.3.189
Hauber M. E., Russo S. A., Sherman P. W. (2001). A password for species recognition in a brood-parasitic bird. Proc. R. Soc. London Ser. B-Biological Sci. 268, 1041–1048. doi: 10.1098/rspb.2001.1617
Hebets E. (2003). Subadult experience influences adult mate choice in an arthropod: Exposed female wolf spiders prefer males of a familiar phenotype. Proc. Natl. Acad. Sci. United States America 100, 13390–13395. doi: 10.1073/pnas.2333262100
Hebets E. A. (2005). Attention-altering signal interactions in the multimodal courtship display of the wolf spider Schizocosa uetzi. Behav. Ecol. 16, 75–82. doi: 10.1093/beheco/arh133
Hebets E. A. (2007). Subadult female experience does not influence species recognition in the wolf spider Schizocosa uetzi Stratton 1997. J. Arachnology 35, 1–10. doi: 10.1636/S05-76.1
Hebets E. A. (2008). Seismic signal dominance in the multimodal courtship display of the wolf spider Schizocosa stridulans Stratton 1991. Behav. Ecol. 19, 1250–1257. doi: 10.1093/beheco/arn080
Hebets E. A. (2011). Current status and future directions of research in complex signaling. Curr. Zoology 57, i–v. doi: 10.1093/czoolo/57.2.i
Hebets E. A., Barron A. B., Balakrishnan C. N., Hauber M. E., Mason P. H., Hoke K. L. (2016). A systems approach to animal communication. Proc. Biol. sciences/The R. Soc. 283, 20152889. doi: 10.1098/rspb.2015.2889
Hebets E. A., Bern M., McGinley R. H., Roberts A., Kershenbaum A., Starrett J., et al. (2021). Sister species diverge in modality-specific courtship signal form and function. Ecol. Evol. 11, 852–871. doi: 10.1002/ece3.7089
Hebets E. A., Cuasay K., Rivlin P. K. (2006). The role of visual ornamentation in female choice of a multimodal male courtship display. Ethology 112, 1062–1070. doi: 10.1111/j.1439-0310.2006.01274.x
Hebets E. A., Maddison W. P. (2005). Xenophilic mating preferences among populations of the jumping spider Habronattus pugillis Griswold. Behav. Ecol. 16, 981–988. doi: 10.1093/beheco/ari079
Hebets E. A., McGinley R. H. (2019). “Multimodal signaling,” in Encyclopedia of Animal Behaviour, 2nd Edition. Ed. Choe J. C. (Amsterdam: Elsevier Academic Press), 487–499.
Hebets E. A., Papaj D. R. (2005). Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiology 57, 197–214. doi: 10.1007/s00265-004-0865-7
Hebets E. A., Rundus A. (2011). Chemical Communication in a Multimodal Context (New York, NY: Springer New York).
Hebets E. A., Stafstrom J. A., Rodriguez R. L., Wilgers D. J. (2011). Enigmatic ornamentation eases male reliance on courtship performance for mating success. Anim. Behav. 81, 963–972. doi: 10.1016/j.anbehav.2011.01.023
Hebets E. A., Uetz G. W. (2000). Leg ornamentation and the efficacy of courtship display in four species of wolf spider (Araneae: Lycosidae). Behav. Ecol. Sociobiology 47, 280–286. doi: 10.1007/s002650050667
Hebets E. A., Vink C. J., Sullivan-Beckers L., Rosenthal M. (2013). The dominance of seismic signaling and selection for signal complexity in Schizocosa multimodal courtship displays. Behav. Ecol. Sociobiology 67, 1483–1498. doi: 10.1007/s00265-013-1519-4
Herberstein M. E., Hebets E., Penney D. (2013). Why are spiders good models for research. Penney D, 230–251.
Herberstein M. E., Wignall A. E., Hebets E. A., Schneider J. M. (2014). Dangerous mating systems: Signal complexity, signal content and neural capacity in spiders. Neurosci. Biobehav. Rev. 46, 509–518. doi: 10.1016/j.neubiorev.2014.07.018
Higham J. P., Hebets E. A. (2013). An introduction to multimodal communication. Behav. Ecol. Sociobiology 67, 1381–1388. doi: 10.1007/s00265-013-1590-x
Hill G. E., Yasukawa K. (2014). The evolution of ornaments and armaments. Anim. behavior: how why Anim. do things they do 2, 145–172.
Höglundi J., Kålås J. A., Fiske P. (1992). The costs of secondary sexual characters in the lekking great snipe (Gallinago media). Behav. Ecol. Sociobiology 30, 309–315. doi: 10.1007/BF00170596
Holland B., Rice W. R. (1998). Perspective: Chase-away sexual selection: Antagonistic seduction versus resistance. Evolution 52, 1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x
Janisch J., Perinot E., Fusani L. (2020). Behavioural flexibility in the courtship dance of golden-collared manakins, manacus vitellinus. Anim. Behav. 166, 61–71. doi: 10.1016/j.anbehav.2020.06.002
Juola F. A., Searcy W. A. (2011). Vocalizations reveal body condition and are associated with visual display traits in great frigatebirds (Fregata minor). Behav. Ecol. Sociobiology 65, 2297–2303. doi: 10.1007/s00265-011-1240-0
Kaston B. J. (1948). Check-list of the spiders of Connecticut (Connecticut: State Geological and Natural History Survey).
Kosmidis I. (2020). brglm2: Bias reduction in generalized linear models. R package version 0.6, 2. 635. https://CRAN.R-project.org/package=brglm2.
Kotiaho J. S., Alatalo R. V., Mappes J., Nielsen M. G., Parri S., Rivero A. (1998). Energetic costs of size and sexual signalling in a wolf spider. Proc. R. Soc. London Ser. B-Biological Sci. 265, 2203–2209. doi: 10.1098/rspb.1998.0560
Kotiaho J., Alatalo R. V., Mappes J., Parri S. (1996). Sexual selection in a wolf spider: Male drumming activity, body size, and viability. Evolution 50, 1977–1981. doi: 10.2307/2410755
Mappes J., Alatalo R. V., Kotiaho J., Parri S. (1996). Viability costs of condition-dependent sexual male display in a drumming wolf spider. Proc. R. Soc. London Ser. B-Biological Sci. 263, 785–789. doi: 10.1098/rspb.1996.0117
Martins E. P., Ossip-Klein A. G., Jaime Zuniga-Vega J., Vital Garcia C., Campos S. M., Hews D. K. (2015). Evolving from static to dynamic signals: evolutionary compensation between two communicative signals. Anim. Behav. 102, 223–229. doi: 10.1016/j.anbehav.2015.01.028
Mason N. A., Shultz A. J., Burns K. J. (2014). Elaborate visual and acoustic signals evolve independently in large, phenotypically diverse radiation of songbirds. Proc. R. Soc. B-Biological Sci. 281, 20140967. doi: 10.1098/rspb.2014.0967
Matsumasa M., Murai M. (2005). Changes in blood glucose and lactate levels of male fiddler crabs: effects of aggression and claw waving. Anim. Behav. 69, 569–577. doi: 10.1016/j.anbehav.2004.06.017
McClintock W. J., Uetz G. W. (1996). Female choice and pre-existing bias: Visual cues during courtship in two Schizocosa wolf spiders (Araneae: Lycosidae). Anim. Behav. 52, 167–181. doi: 10.1006/anbe.1996.0162
McGinley R. H., Starrett J., Bond J. E., Hebets E. A. (2023). Light environment interacts with visual displays in a species-specific manner in multimodal-signaling wolf spiders. Am. Nat. 201, 472–490. doi: 10.1086/722830
Mendelson T. C., Shaw K. L. (2005). Sexual behaviour: Rapid speciation in an arthropod. Nature 433, 375–376. doi: 10.1038/433375a
Mikula P., Toszogyova A., Albrecht T. (2022). A global analysis of aerial displays in passerines revealed an effect of habitat, mating system and migratory traits. Proc. R. Soc. B 289, 20220370. doi: 10.1098/rspb.2022.0370
Miller G. L., Stratton G. E., Miller P. R., Hebets E. (1998). Geographical variation in male courtship behaviour and sexual isolation in wolf spiders of the genus Schizocosa. Anim. Behav. 56, 937–951. doi: 10.1006/anbe.1998.0851
Minias P., Janiszewski T. (2020). Evolution of a conspicuous melanin-based ornament in gulls Laridae. J. OF EVOLUTIONARY Biol. 33, 682–693. doi: 10.1111/jeb.13604
Murai M., Backwell P. R. Y. (2006). A conspicuous courtship signal in the fiddler crab Uca perplexa: female choice based on display structure. Behav. Ecol. Sociobiology 60, 736–741. doi: 10.1007/s00265-006-0217-x
Ord T. J., Stuart-Fox D. (2006). Ornament evolution in dragon lizards: multiple gains and widespread losses reveal a complex history of evolutionary change. J. Evolutionary Biol. 19, 797–808. doi: 10.1111/j.1420-9101.2005.01050.x
Patricelli G. L., Coleman S. W., Borgia G. (2006). Male satin bowerbirds, Ptilonorhynchus violaceus, adjust their display intensity in response to female startling: an experiment with robotic females. Anim. Behav. 71, 49–59. doi: 10.1016/j.anbehav.2005.03.029
Patricelli G. L., Hebets E. A., Mendelson T. C. (2019). Book review of Prum, RO 2018. The evolution of beauty: How Darwin’s forgotten theory of mate choice shapes the animal world—and us, (2017), Doubleday, 428 pages, ISBN: 9780385537216. Evolution 73, 115–124. doi: 10.1111/evo.2019.73.issue-1
Patricelli G. L., Uy J. A. C., Walsh G., Borgia G. (2002). Male displays adjusted to female’s response - Macho courtship by the satin bowerbird is tempered to avoid frightening the female. Nature 415, 279–280. doi: 10.1038/415279a
Piacentini L. N., Ramírez M. J. (2019). Hunting the wolf: a molecular phylogeny of the wolf spiders (Araneae, Lycosidae). Mol. Phylogenet. Evol. 136, 227–240. doi: 10.1016/j.ympev.2019.04.004
Prestwich K. N., O’Sullivan K. (2005). Simultaneous measurement of metabolic and acoustic power and the efficiency of sound production in two mole cricket species (Orthoptera: Gryllotalpidae). J. Exp. Biol. 208, 1495–1512. doi: 10.1242/jeb.01550
Rahman M. M., Kelley J. L., Evans J. P. (2013). Condition-dependent expression of pre-and postcopulatory sexual traits in guppies. Ecol. Evol. 3, 2197–2213. doi: 10.1002/ece3.632
R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://urldefense.com/v3/__https:/www.R-project.org/.
Roberts J. A., Uetz G. W. (2005). Information content of female chemical signals in the wolf spider, Schizocosa ocreata: male discrimination of reproductive state and receptivity. Anim. Behav. 70, 217–223. doi: 10.1016/j.anbehav.2004.09.026
Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J. C., et al. (2011). pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 12, 1–8. doi: 10.1186/1471-2105-12-77
Rodríguez R. L. (2015). Mating is a give-and-take of influence and communication between the sexes. Cryptic female choice arthropods: Patterns Mech. prospects, 479–496.
Rodriguez R. L., Boughman J. W., Gray D. A., Hebets E. A., Hobel G., Symes L. B. (2013). Diversification under sexual selection: the relative roles of mate preference strength and the degree of divergence in mate preferences. Ecol. Lett. 16, 964–974. doi: 10.1111/ele.12142
Rosenthal G. G. (2017). Mate choice: the evolution of sexual decision making from microbes to humans (Princeton: Princeton University Press). doi: 10.23943/princeton/9780691150673.001.0001
Rosenthal M. F., Hebets E. A. (2012). Resource heterogeneity interacts with courtship rate to influence mating success in the wolf spider Schizocosa floridana. Anim. Behav. 84, 1341–1346. doi: 10.1016/j.anbehav.2012.08.028
Rosenthal M. F., Hebets E. A. (2015). Temporal patterns of nutrition dependence in secondary sexual traits and their varying impacts on male mating success. Anim. Behav. 103, 75–82. doi: 10.1016/j.anbehav.2015.02.001
Rosenthal M. F., Wilkins M. R., Shizuka D., Hebets E. A. (2018). Dynamic changes in display architecture and function across environments revealed by a systems approach to animal communication*. Evolution 72, 1134–1145. doi: 10.1111/evo.13448
Rowe C. (1999). Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921–931. doi: 10.1006/anbe.1999.1242
Rowe C. (2013). Receiver psychology: a receiver’s perspective. Anim. Behav. 85, 517–523. doi: 10.1016/j.anbehav.2013.01.004
Rowe C., Guilford T. (1999). The evolution of multimodal warning displays. Evolutionary Ecol. 13, 655–671. doi: 10.1023/A:1011021630244
Rubin J. J., Kawahara A. Y. (2023). Sexual selection does not drive hindwing tail elaboration in a moon moth, Actias luna. Behav. Ecol. 34, 488–494. doi: 10.1093/beheco/arad019
Rundus A. S., Santer R. D., Hebets E. A. (2010). Multimodal courtship efficacy of Schizocosa retrorsa wolf spiders: implications of an additional signal modality. Behav. Ecol. 21, 701–707. doi: 10.1093/beheco/arq042
Rundus A. S., Sullivan-Beckers L., Wilgers D. J., Hebets E. A. (2011). Females are choosier in the dark: environment-dependent reliance on courtship components and its impact on fitness. Evolution 65, 268–282. doi: 10.1111/j.1558-5646.2010.01125.x
Rutledge J. M., Miller A., Uetz G. W. (2010). Exposure to multiple sensory cues as a juvenile affects adult female mate preferences in wolf spiders. Anim. Behav. 80, 419–426. doi: 10.1016/j.anbehav.2010.05.027
Ryan M. J., Fox J. H., Wilczynski W., Rand A. S. (1990). Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature 343, 66–67. doi: 10.1038/343066a0
Saether S. A., Fiske P., Kalas J. A., Gjul J. M. (2000). Females of the lekking great snipe do not prefer males with whiter tails. Anim. Behav. 59, 273–280. doi: 10.1006/anbe.1999.1301
Santema P., Eberhart-Hertel L., Valcu M., Kempenaers B. (2023). Sexual selection for extreme physical performance in a polygynous bird is associated with exceptional sex differences in oxygen carrying capacity. Biol. Lett. 19, 20230391. doi: 10.1098/rsbl.2023.0391
Scheffer S. J., Uetz G. W., Stratton G. E. (1996). Sexual selection, male morphology, and the efficacy of courtship signalling in two wolf spiders (Araneae: Lycosidae). Behav. Ecol. Sociobiology 38, 17–23. doi: 10.1007/s002650050212
Shamble P. S., Wilgers D. J., Swoboda K. A., Hebets E. A. (2009). Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav. Ecol. 20, 1242–1251. doi: 10.1093/beheco/arp116
Shine R., Mason R. T. (2005). Do a male garter snake’s energy stores limit his reproductive effort? Can. J. Zoology 83, 1265–1270. doi: 10.1139/z05-119
Sierwald P., Draney M. L., Prentice T., Pascoe F., Sandlin N., Lehman E. M., et al. (2005). “The spider species of the Great Lakes states,” in Proceedings of the Indiana Academy of Science, 111–206.
Stafstrom J. A., Hebets E. A. (2013). Female mate choice for multimodal courtship and the importance of the signaling background for selection on male ornamentation. Curr. Zoology 59, 200–209. doi: 10.1093/czoolo/59.2.200
Starrett J., McGinley R. H., Hebets E. A., Bond J. E. (2022). Phylogeny and secondary sexual trait evolution in Schizocosa wolf spiders (Araneae, Lycosidae) shows evidence for multiple gains and losses of ornamentation and species delimitation uncertainty. Mol. Phylogenet. Evol. 169, 107397. doi: 10.1016/j.ympev.2022.107397
Stratton G. E. (2005). Evolution of ornamentation and courtship behavior in Schizocosa: Insights from a phylogeny based on morphology (Araneae, Lycosidae). J. Arachnology 33, 347–376. doi: 10.1636/04-80.1
Sullivan-Beckers L., Hebets E. A. (2011). Modality-specific experience with female feedback increases the efficacy of courtship signalling in male wolf spiders. Anim. Behav. 82, 1051–1057. doi: 10.1016/j.anbehav.2011.07.040
Sullivan-Beckers L., Hebets E. A. (2014). Tactical adjustment of signaling leads to increased mating success and survival. Anim. Behav. 93, 111–117. doi: 10.1016/j.anbehav.2014.04.021
Takeshita F., Murai M., Matsumasa M., Henmi Y. (2018). Multimodal signaling in fiddler crab: waving to attract mates is condition-dependent but other sexual signals are not. Behav. Ecol. Sociobiology 72, 1–10. doi: 10.1007/s00265-018-2555-x
Taylor P. W., Roberts J. A., Uetz G. W. (2006). Mating in the absence of visual cues by Schizocosa ocreata (Hentz 1844) wolf spiders (Araneae, Lycosidae). J. Arachnology 34, 501–505. doi: 10.1636/S04-98.1
Uetz G. W., Clark D. L., Roberts J. A. (2016). “Multimodal communication in wolf spiders (Lycosidae)-an emerging model for study,” in Advances in the Study of Behavior, vol. 48 . Eds. Naguib M., Mitani J. C., Simmons L. W., Barrett L., Healy S., Zuk M., 117–159. doi: 10.1016/bs.asb.2016.03.003
Uetz G. W., Papke R., Kilinc B. (2002). Influence of feeding regime on body size, body condition and a male secondary sexual character in Schizocosa ocreata wolf spiders (Araneae, Lycosidae): Condition-dependence in a visual signaling trait. J. Arachnology 30, 461–469. doi: 10.1636/0161-8202(2002)030[0461:IOFROB]2.0.CO;2
Uetz G. W., Roberts J. A., Clark D. L., Gibson J. S., Gordon S. D. (2013). Multimodal signals increase active space of communication by wolf spiders in a complex litter environment. Behav. Ecol. Sociobiology 67, 1471–1482. doi: 10.1007/s00265-013-1557-y
Uetz G. W., Roberts J. A., Taylor P. W. (2009). Multimodal communication and mate choice in wolf spiders: female response to multimodal versus unimodal signals. Anim. Behav. 78, 299–305. doi: 10.1016/j.anbehav.2009.04.023
Uy J. A. C., Irwin D. E., Webster M. S. (2018). Behavioral isolation and incipient speciation in birds. Annu. Rev. Ecology Evolution Systematics 49, 1–24. doi: 10.1146/annurev-ecolsys-110617-062646
Vaccaro R., Uetz G. W., Roberts J. A. (2010). Courtship and mating behavior of the wolf spider Schizocosa bilineata (Araneae: Lycosidae). J. Arachnology 38, 452–459. doi: 10.1636/Hi09-115.1
Venables W. N., Ripley B. D. (2013). Modern applied statistics with S-PLUS (New York: Springer Science & Business Media).
Watts J. C., Flynn A., Tenhumberg B., Hebets E. A. (2019). Contemporary sexual selection does not explain variation in male display traits among populations. Evolution 73, 1927–1940. doi: 10.1111/evo.13808
West-Eberhard M. (1983). Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183. doi: 10.1086/413215
Westneat D. F. (2006). No evidence of current sexual selection on sexually dimorphic traits in a bird with high variance in mating success. Am. Nat. 167, E171–E189. doi: 10.1086/503385
Wickham H. (2011). ggplot2. Wiley Interdiscip. reviews: Comput. Stat 3, 180–185. doi: 10.1002/wics.147
Wiens J. J. (2001). Widespread loss of sexually selected traits: how the peacock lost its spots. Trends Ecol. Evol. 16, 517–523. doi: 10.1016/S0169-5347(01)02217-0
Wilcox S. C., Clark C. J. (2022). Sexual selection for flight performance in hummingbirds. Behav. Ecol. 33, 1093–1106. doi: 10.1093/beheco/arac075
Wilgers D. J., Colton Watts J., Hebets E. A. (2022). Habitat complexity and complex signal function–exploring the role of ornamentation. Behav. Ecol. 33, 307–317. doi: 10.1093/beheco/arab144
Wilgers D. J., Hebets E. A. (2011). Complex courtship displays facilitate male reproductive success and plasticity in signaling across variable environments. Curr. Zoology 57, 175–186. doi: 10.1093/czoolo/57.2.175
Wilgers D. J., Hebets E. A. (2012). Seismic signaling is crucial for female mate choice in a multimodal signaling wolf spider. Ethology 118, 387–397. doi: 10.1111/j.1439-0310.2012.02023.x
Keywords: behavioral skills, compensatory traits, mate choice, plasticity, sensory bias, sensory drive, sexual selection, signal interactions
Citation: Hebets EA, Oviedo-Diego M, Abdallah A, Griger S, McGinley R, Starrett J, Bond JE and Bern M (2024) Courtship performance, not ornamentation, predicts mating success in two sister-species of wolf spider with divergent phenotypes. Front. Ethol. 3:1460323. doi: 10.3389/fetho.2024.1460323
Received: 05 July 2024; Accepted: 24 July 2024;
Published: 14 August 2024.
Edited by:
John Swallow, University of Colorado Denver, United StatesReviewed by:
Sergio Castellano, University of Turin, ItalyRafael Lucas Rodriguez, University of Wisconsin–Milwaukee, United States
Copyright © 2024 Hebets, Oviedo-Diego, Abdallah, Griger, McGinley, Starrett, Bond and Bern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eileen A. Hebets, ZWhlYmV0czJAdW5sLmVkdQ==
†These authors have contributed equally to this work and share first authorship
 Eileen A. Hebets
Eileen A. Hebets Mariela Oviedo-Diego
Mariela Oviedo-Diego Abdallah Abdallah1
Abdallah Abdallah1 Rowan McGinley
Rowan McGinley James Starrett
James Starrett Jason E. Bond
Jason E. Bond