- Department of Life Sciences and Systems Biology, University of Torino, Turin, Italy
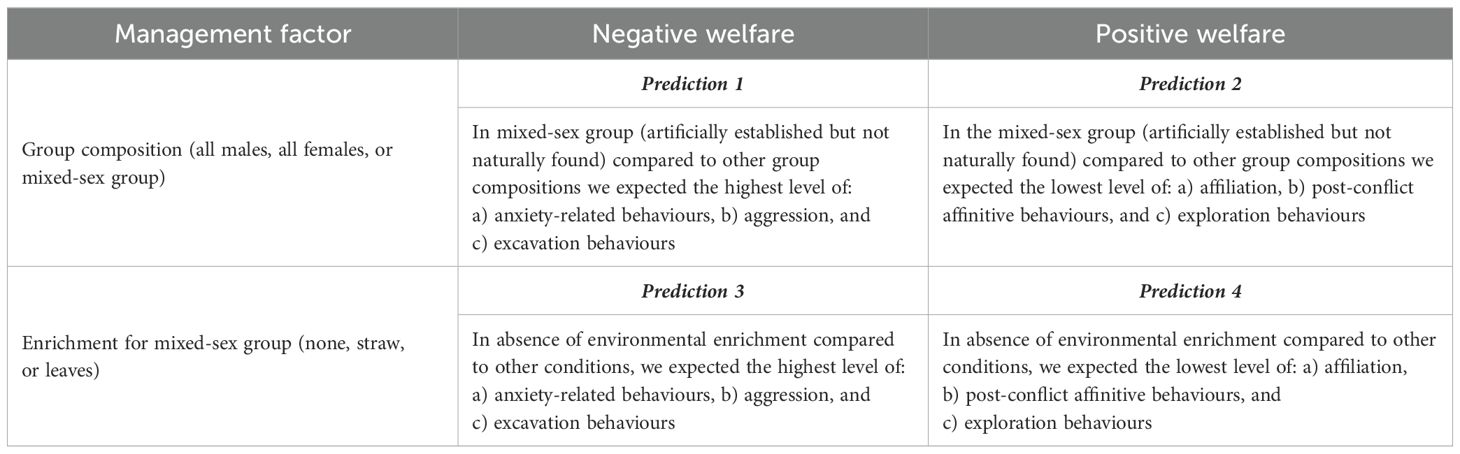
According to the modern perspective on evaluating animal welfare, it is important to consider both negative and positive experiences. This study investigated the impact of group composition and environmental enrichments on the behaviours of free-ranging pigs, focusing on anxiety-related behaviours, aggression, affiliation, post-conflict affiliation, excavation, and non-invasive exploration activities. Data were collected on three different groups (all-female: N=10; all-male: N=13; mixed-sex: N=12) of free-ranging pigs raised in a natural woodland habitat at the ethical farm “Parva Domus” (Turin, Italy). To evaluate the impact of environmental enrichment, further data collection was carried out on the mixed-sex group under three different enrichment conditions (absence; dry leaves; straw) provided in a rotational scheme. Group composition did not affect levels of anxiety-related behaviours, aggression, and non-invasive exploration. However, it did impact other social aspects (affiliation: One-way ANOVA: p=0.003; post-conflict affiliation: Kruskal–Wallis: p=0.005). In particular, the mixed-sex and the all-male groups showed higher levels of affiliation than the all-female group and the mixed-sex group showed higher levels of post-conflict affiliation than the all-male group. Moreover, we found differences in excavation behaviour levels (Kruskal–Wallis: p=0.001), with higher levels of excavation behaviour in the mixed-sex group compared to the all-female group. Regarding the impact of enrichments, we found differences in levels of anxiety-related behaviours (One-way ANOVA: p = 0.046), affiliation (One-way ANOVA: p = 0.006), excavation (One-way ANOVA: p<0.001), and non-invasive exploration activities (One-way ANOVA: p<0.001). In enrichment conditions with straw, we found a significant decrease in anxiety-related, affiliation, and excavation behaviours levels, and a significant increase in non-invasive exploration behaviours. A trend with lower levels of aggression was observed in straw enrichment condition compared to the absence of enrichment, although the difference was not significant. Moreover, there were no differences in post-conflict affiliation frequencies. Our findings suggest that forming mixed-sex groups and providing environmental enrichments such as the straw could be suitable solutions to effectively reduce invasive excavation behaviours without preventing pigs from expressing their natural behavioural repertoire, thus maintaining high standards of animal welfare. This study highlights behavioural aspects to be considered in extensive farming, confirming the importance of ethology as a tool for assessing pig welfare.
1 Introduction
The ethology plays a significant role in the increase of animal welfare. Understanding the behavioural repertoire of a given species permits to enhance its well-being as in the case of farm-raised animals (Kokocińska and Kaleta, 2016). By knowing animal behavioural norms, at least their basic needs can be addressed. Ethological studies represent the primary step in evaluating animal physical, social, and behavioural conditions (Dellmeier, 1989; Temple et al., 2011). According to the modern definition, animal welfare does not simply rely on the absence of negative events, but also on the presence of positive experiences (Mellor, 2015; Mellor and Beausoleil, 2015; Špinka, 2017; Lawrence et al., 2018). Therefore, both negative and positive behaviours have to be considered when assessing animal welfare. In case of farm-raised animals, a good management taking into account animal welfare can enhance and ameliorate food production and sustainability (Appleby and Mitchell, 2018).
Domestic pigs are social animals (Stolba and Wood-Gush, 1989) and, under extensive farming conditions, they can behaviourally express anxiety (not related to stereotypical behaviours) via a set of displacement activities, which can be considered as a proxy of stress (e.g., vacuum chewing, self-scratching/rubbing, yawning, body shaking; Norscia et al., 2021; Collarini et al., under review1). However, pigs are also able to cope with anxiety by exchanging affinitive contacts such as body contact and grooming (social buffering; Norscia et al., 2021, 2024). After an aggression occurring over a resource, pigs may engage in post-conflict affinitive behaviours (i.e. reconciliation, solicited and unsolicited triadic contacts and quadratic contacts) to possibly restore group homeostasis (Cordoni et al., 2023; Norscia et al., 2024). In a welfare assessment perspective, it is important to consider that positive social behaviour promotes cohesion and affiliation among conspecifics, as well as reduces the negative effects of stress (Temple et al., 2011). Excavation and exploration are other activities that can improve pig welfare, even though these behaviours cause damage to the natural habitat (Day et al., 1995; Edge et al., 2005; Studnitz et al., 2007).
Environmental enrichments are a widely employed solution to enhance the welfare of pigs, and previous studies confirm the benefits derived from the use of environmental enrichment (Ernst et al., 2005; Godyń et al., 2019). Specifically, enriching the pig environment is a way to reduce pathological behaviours (including aggression and stereotypies), promoting the expression of positive behaviours (EFSA Panel on Animal Health and Welfare (AHAW), 2014; Godyń et al., 2019). Furthermore, group composition and/or environmental enrichments (e.g. straw, vegetation parts) can mitigate negative situations and enhance positive behaviours (Day et al., 2001; Van de Weerd et al., 2003; Studnitz et al., 2007).
In this study, we took into account behavioural parameters to assess which conditions might buffer negative events and possibly enhance positive states. Specifically, we investigated whether different group compositions (all males, all females, and mixed-sex groups) and enrichments (none, dry leaves or straw, for the mixed-sex group) could influence levels of negative (anxiety-related behaviours, aggression, and excavation) and positive (affinitive behaviours, post-conflict strategies, and non-invasive exploration) behaviours. Here below we describe our predictions in detail.
1.1 Predictions
Under natural conditions, pig groups are composed by either female or young male associations and can freely interact with vegetation (Stolba and Wood-Gush, 1989; Gabor et al., 1999; D’Eath and Turner, 2008; Gonyou, 2001). Feral pigs form strong social bonds, particularly with related individuals, and aggression levels are very limited (Podgórski et al., 2014). In domestic pigs, establishing a stable social hierarchy could help avoid future conflicts and promote good social cohesion (Arey and Edwards, 1998; D’Eath and Turner, 2008). Moreover, an unstable social environment can lead to increased stress levels (Cunha et al., 2018). Because pigs do not naturally form mixed-sex groups, we expected that in the mixed-sex group (artificially established and possibly less stable) compared to other group compositions we would find the highest level of negative events (anxiety-related behaviours, aggression, and excavation; prediction 1, Table 1) and the lowest level of buffering behaviours (affinitive behaviours, post-conflict strategies, and non-invasive exploration; prediction 2, Table 1). Previous studies in pigs have shown that a sterile environment increases the risk of aggressive behaviours and stress (Petersen et al., 1995; Moinard et al., 2003; Brown et al., 2018). Furthermore, the presence of enrichments can promote exploratory behaviours and positive social interactions among individuals (Vanheukelom et al., 2012). Therefore, we expected that in the absence of the environmental enrichment compared to other conditions we would find the highest level of negative events (anxiety-related behaviours, aggression, and excavation; prediction 3, Table 1) and the lowest level of buffering behaviours (affinitive behaviours, post-conflict strategies, and non-invasive exploration; prediction 4, Table 1).
2 Materials and methods
2.1 Study groups
This study was conducted from January to March 2022 on three different groups of free-ranging domestic pigs (Sus scrofa) at the Ethical Farm “Parva Domus” located at Cavagnolo (Turin, Italy). Each group was housed in a 3-ha fenced enclosure. The enclosures were located within an area covered by natural woodland habitat, and the groups were not in visual or acoustic contact with each other, despite sharing the same habitat conditions. The study groups consisted of i) a group of sexually mature females composed by 10 adult females (7–15 months; all-female group; hereafter AFg); ii) a group composed by 13 castrated young males (all-male group; hereafter AMg; 7–10 months of age) and iii) a mixed-sex group composed by 12 individuals of similar age (mixed-sex group; hereafter MSg) including 6 sexual mature females (7–10 months of age) and 6 castrated young males (7–10 months of age). All individuals were of three mixed-breeds (not pure-breed as at least one grandparent belonged to a different breed): Parma Black, Large White, and Piedmont Black. Moreover, all individuals had the same sire (mixed-breed), thus they could be either full siblings or half siblings. Males had been castrated via the removal of testes within their first days of life.
Each study group was formed 2 weeks before the beginning of the study to ensure group stability by the beginning of data collection. The pigs were provided with food (Ciclo Unico P, SILDAMIN®) every day from 9:30 to 10:30 and water was available ad libitum. The subjects could supplement their food intake with roots, leaves, fruits, seeds, and bark naturally available in the environment. For individual recognition, the pigs were marked with spray Raidex© for livestock, used by the farmers to mark animals, with no manipulation because the pigs were habituated to the human presence.
2.2 Data collection and operational definitions
Data were gathered via focal animal sampling (Altmann, 1974), which consisted of collecting all behaviours (audio recording) performed by one individual at a time within a predetermined interval (15 minutes), recording their behaviours using continuous recording (all occurrences within the interval). Focal samplings were carried out on all individuals in each group (NAFg = 10; NAMg = 13; NMSg = 12) and following a rotational scheme, we blindly selected the focal pig from a pool of pig identification codes. Data collection was conducted on a daily basis from 08:30 am to 05:30 pm.
2.2.1 Effects of group composition
Data collection followed a rotation of time slots (randomly selected, excluding those already covered) during which the behaviour of the focal animal was sampled. To ensure a balance in observations, multiple focal samplings were conducted for each individual throughout the data collection period at different times of day and each individual in each group was observed for 6 hr. We collected data on anxiety-related (vacuum-chewing, scratching/body-rubbing, head/body-shaking, and yawning; Norscia et al., 2021), affinitive and aggressive behaviours, post-conflict affiliation (affinitive contacts following in the three minutes after an aggressive event; Cordoni et al., 2023; Norscia et al., 2024), excavation (rooting plus rasping and pawing), and non-invasive exploration (grazing/browsing plus foraging). The behaviours were categorised following the ethogram used by Studnitz et al. (2003), Norscia et al. (2021, 2024) and Cordoni et al. (2023). Audio recordings were carried out by E.C., A.P. and L.C by voice recording application for smartphone (O.S. Android). Before starting systematic data collection I.N. and G.C. supervised E.C., A.P. and L.C. in a training period during which inter-observer reliability on Cohen’s k was determined for anxiety-related, aggression, affinitive, post-conflict, excavation, and exploratory behaviours (Cohen’s 0.87 ≤ k ≤ 0.92).
To reduce possible biases due to changes in temperature, humidity, and climatic conditions, observations were carried out during the same period.
2.2.2 Effects of enrichment conditions
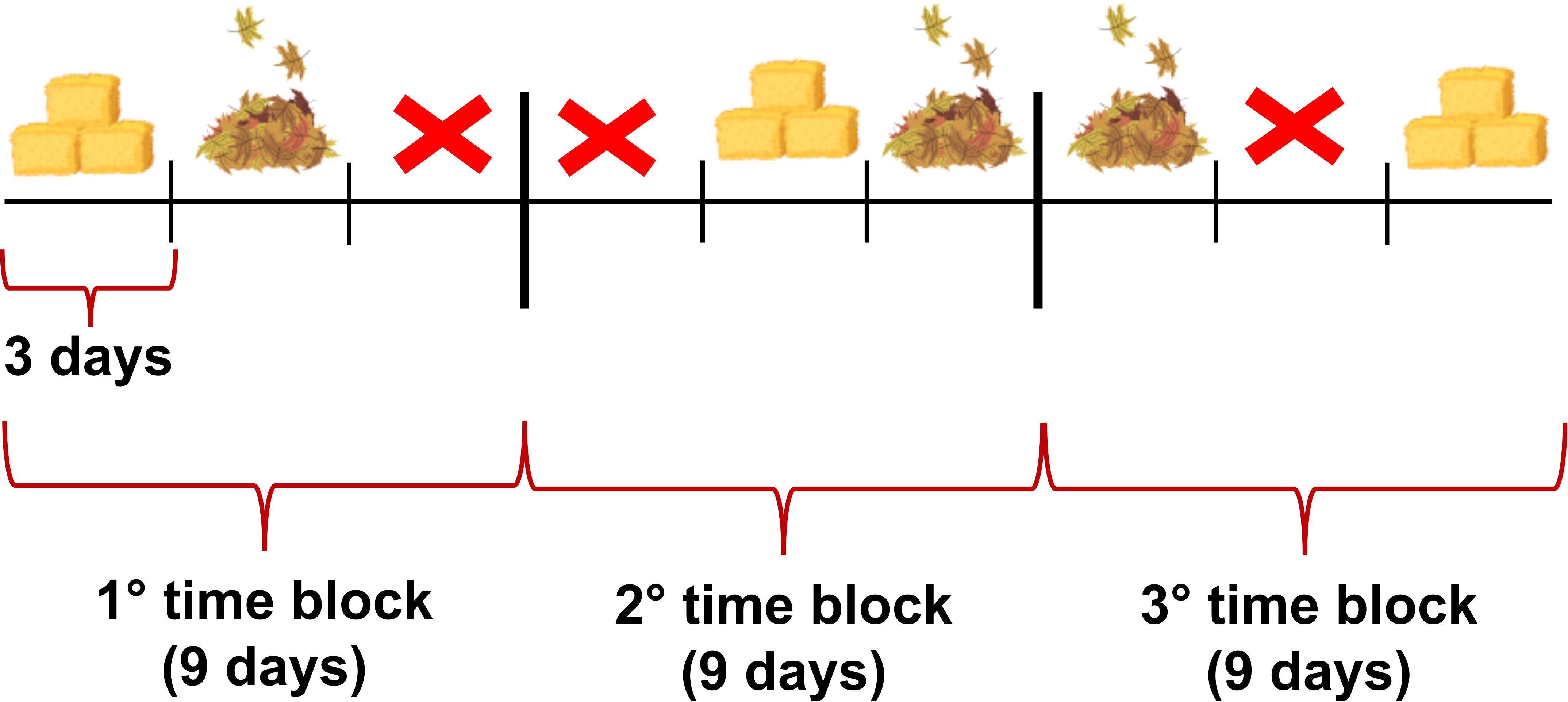
Further data collection (27 days) was carried out by E.C. on the MSg to assess the effect of different enrichment conditions on the frequencies of the behaviours described above (aggression, affiliation, anxiety behaviours, post-conflict affiliation, excavation; exploration). During this period of data collection, three different conditions of environmental enrichments were provided to the group: no enrichment (hereafter NE; Figure 1A); dry leaves (Quercus petraea, Quercus pubescens, Carpinus betulus, Sambucus nigra, Robinia pseudoacacia; hereafter LE; Figure 1B) and straw (hereafter ST; Figure 1C).

Figure 1. Photos representing the three conditions of environmental enrichment: (A) absence of environmental enrichment, (B) dry leaves and (C) straw. Individuals of the mixed-sex group are visible.
We applied a study design by dividing a period of 27 days into three different time blocks of 9 days each. Following a rotation scheme, all three different enrichment conditions were provided for each block (9 days). Each condition was maintained for three consecutive days. To avoid potential biases and to assess that the condition of the forest floor did not influence the interactions with the added enrichments, we included the no enrichment condition as a control in the experimental design. To rotate the different environmental enrichments, we randomly picked the conditions for each 9-day time block by blindly selecting the name from a conditions pool, using three cards (each one with a different enrichment condition) to be extracted manually from a bag. The random extraction of conditions followed a non-replacement sampling technique, thereby excluding conditions that had already been drawn within the 9-day block (Figure 2). Environmental enrichment was provided every morning at 8:30 A.M. The amount of daily enrichment was always the same (10kg divided into 4 bags; each bag contained 2.5kg of environmental enrichment; bag capacity = 56l; Figure 3) and was supplied each time in the same way at the same points (Figure 1). Distribution was not clumped to avoid conflict over concentrated material spots. Moreover, data collection followed a rotation of time slots (randomly selected, excluding those already covered) during which the behaviour of the focal animal was sampled. As for comparison across groups, to ensure a balance in observations, multiple focal samplings were conducted for each individual throughout the data collection period at different times of day and each individual was observed for a total of 18 hr (6 hr in NE condition; 6 hr in LE condition and 6 hr in ST condition).

Figure 3. Photos representing (A) a bag containing dry leaves and (B) a bag containing straw. Each bag contained 2.5kg of environmental enrichment; bag volume = 0.056m3.
2.2.3 Anxiety-related behaviours
We considered as anxiety-related behaviours the patterns described by Norscia et al. (2021): yawning, head/body shaking, scratching/body rubbing, and vacuum chewing. These behavioural patterns are associated with stressful events (under natural conditions; Norscia et al., 2021) and their fluctuation can follow the fluctuation of cortisol levels (Collarini et al., under review1). Moreover, to compare individual levels of anxiety-related behaviours across groups and across environmental enrichment conditions, we calculated the individual absolute frequencies.
2.2.4 Aggression
We considered as aggression all the agonistic interactions in which one or more individuals perform one or more aggressive patterns (aggressive bite, aggressive chase, aggressive push, aggressive head knocking, Norscia et al., 2021; Cordoni et al., 2023) towards another individual. For each individual of each group, we calculated the number of aggression in which the selected individual was involved. Moreover, to compare the individual levels of aggression across groups, we normalised the number of aggression per individual on the number of group members (N − 1 for each group). To compare individual levels of aggression across environmental enrichment conditions, we calculated the individual absolute frequencies.
2.2.5 Affinitive interactions
In the analyses we considered the following affinitive behaviours: rest in contact, grooming, contacts with nose (e.g. nose–nose contacts; nose–body contacts) and head-over (Norscia et al., 2021, 2024; Cordoni et al., 2023). To compare the individual levels of affiliation across groups, we normalised the number of affinitive interactions per individual on the number of group members (N − 1 for each group). To compare individual levels of affiliation across environmental enrichment conditions, we calculated the individual absolute frequencies.
2.2.6 Post-conflict affinitive interactions
We considered as post-conflict affinitive contacts all the affinitive contacts occurring within three minutes following an aggression (Cordoni et al., 2023; Norscia et al., 2024). In particular, within a 3-min time window following an aggression, we recorded the first post-conflict affinitive contact that occurred between i) the aggressor and the aggressee (i.e. reconciliation; de Waal and van Roosmalen, 1979), ii) a third individual not involved in the previous conflict and the aggressor/aggressee (i.e. solicited and unsolicited triadic contacts; Cordoni et al., 2023), and iii) two or more individuals not involved in the conflict (i.e. quadratic contacts; Norscia et al., 2024). In the analyses we considered the following affinitive behaviours: rest in contact, grooming, contacts with nose (e.g. nose–nose contacts; nose–body contacts) and head-over (Norscia et al., 2021, 2024; Cordoni et al., 2023). For group comparison purpose, we normalised the total number of post-conflict affinitive contacts in which a selected individual was involved on the total number of aggression occurring in its group. Furthermore, to compare individual post-conflict affiliation levels across the three different conditions of environmental enrichment (no enrichment: NE; dry leaves: LE; straw: ST), we normalised the total number of post-conflict affinitive contacts in which a selected individual was involved on the total number of aggression occurring in each condition of environmental enrichment.
2.2.7 Excavation and exploratory activities
We included rooting, rasping with the snout, and pawing as part of the excavation activity. For exploratory activity, we considered grazing, browsing, and foraging (we excluded those cases in which the food was provided by the farmer). Moreover, to compare individual levels of excavation and exploration behaviours across groups and across environmental enrichment conditions, we calculated the individual absolute frequencies.
2.3 Statistical analyses
2.3.1 Comparison across groups
As a first step, we performed a preliminary analysis to verify that the groups were comparable in terms of age. Owing to the non-normal data distributions (Lilliefors-corrected Kolmogorov–Smirnov test: for ages, p ≤ 0.005; for post-conflict, excavation and exploratory behaviours, 0.001≤ p ≤ 0.200), we applied non-parametric statistics (Siegel and Castellan, 1988). In particular, we applied Kruskal–Wallis test for k-independent samples to compare the age of individuals between the three groups (AFg; AMg; MSg) and the individual frequencies of post-conflict affinitive contacts and excavation and exploratory behaviours between the study groups. We applied the Dunn post hoc test for pairwise comparisons, with the significance level of probability adjusted downward using the Bonferroni correction. Owing to the normal data distributions (Lilliefors-corrected Kolmogorov–Smirnov test: p ≥ 0.05), we applied the parametric one-way ANOVA test for k-independent samples, to compare the individual frequencies of anxiety-related behaviours and affinitive and aggressive interactions between the three groups (AFg; AMg; MSg). We did not include post-conflict affinitive contacts in this analysis (see below). We applied the Bonferroni post hoc test for pairwise comparisons.
2.3.2 Comparison across enrichment conditions
Owing to the non-normal data distributions (Lilliefors-corrected Kolmogorov–Smirnov test: 0.001≤ p ≤ 0.200), we applied non-parametric statistics (Siegel and Castellan, 1988). In particular, we applied the non-parametric Friedman test for k-dependent samples to compare the individual frequencies of aggression and post-conflict affinitive interactions across the different enrichment conditions (NE, LE, ST). We applied the Dunn post hoc test for pairwise comparisons, with the significance level of probability adjusted downward using the Bonferroni correction.
For all the analyses, the statistical significance was set at p < 0.05. A trend of significance was considered for discussion for 0.05 ≤ p <0.1.
3 Results
3.1 Comparison across groups
Preliminary analysis showed that the individuals of the all-female (AFg), all-male (AMg), and mixed-sex (MSg) groups had similar ages (Kruskal–Wallis test: χ2 = 5.131, df = 2, p = 0.077; mean age ± SE: AFg = 10.90 ± 1.12, AMg = 8.61 ± 0.43; MSg = 8.00 ± 0.43).
The frequencies of anxiety-related behaviours did not show any significant difference between the three groups (One-way ANOVA test: F = 1.014; df = 2; p = 0.374; mean value ± SE: AFg = 16.70 ± 3.46, AMg = 18.38 ± 2.66; MSg = 13.25 ± 1.91; Figure 4A). Similarly, the frequencies of aggression did not show any significant difference between the groups (One-way ANOVA test: χ2 = 0.738; df = 2; p = 0.486; mean value ± SE: AFg = 0.39 ± 0.09, AMg = 0.50 ± 0.05; MSg = 0.41 ± 0.08; Figure 4B). Hence, group composition affected neither anxiety behaviours nor aggression levels.
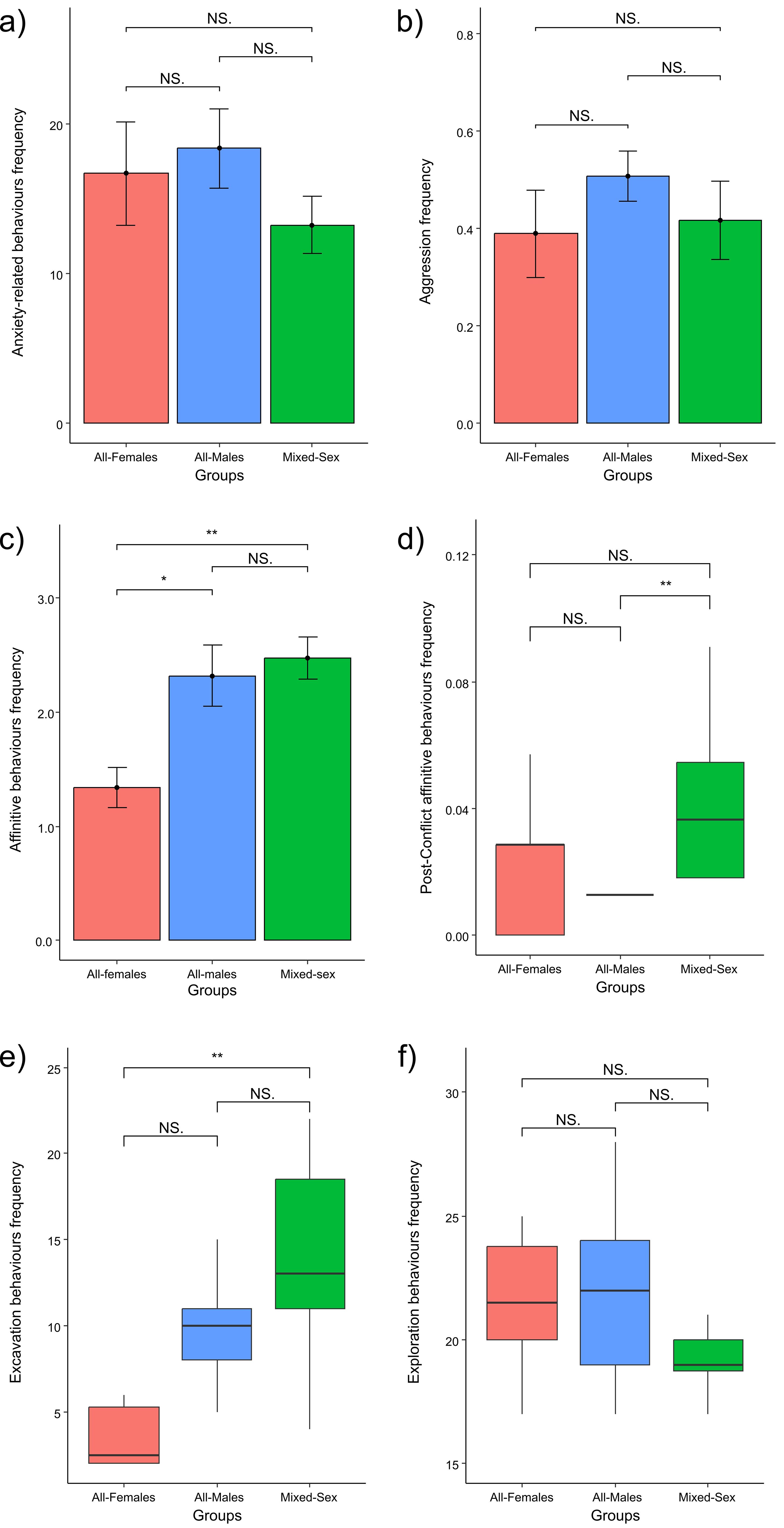
Figure 4. Comparisons across groups: (A) error bar plots showing that there were no differences in levels of anxiety-related behaviours across groups (One-way ANOVA test; p = 0.374); (B) error bar plots showing that there were no differences in levels of aggression (normalised on the number of group members) across groups (One-way ANOVA test; p = 0.486); (C) error bar plots showing differences in levels of affinitive behaviours (normalised on the number of group members) across groups (One-way ANOVA test, p = 0.007; Bonferroni post-hoc test, AFg vs. AMg: p = 0.009; AFg vs. MSg: p = 0.026; AFg vs. MSg: p = 1.000); (D) boxplots showing differences in levels of post-conflict affinitive behaviours (normalised on the total number of aggression occurred in the group) across groups (Kruskal–Wallis test, p = 0.005; Dunn post-hoc test, AMg vs. MSg: p = 0.004; AFg vs. AMg: p = 0.970; AFg vs. MSg: p = 0.128); (E) boxplots showing differences in levels of excavation behaviours across groups (Kruskal–Wallis test, p = 0.001; Dunn post-hoc test, MSg vs. AFg: p = 0.001; AFg vs. AMg: p = 0.183; AMg vs. MSg: p = 0.137); (F) boxplots showing that there were no differences in levels of exploration behaviours across groups (Kruskal–Wallis test, p = 0.114). Error bars: 95% confidence interval (bars) around the mean (dots); Boxplot: median value (horizontal line), interquartile range (box); minimum and maximum values in the data (vertical line). NS = non-significant, * = p < 0.05; ** = p < 0.01.
The frequencies of affinitive interactions varied significantly across groups (One-way ANOVA test: F = 6.993; df = 2; p = 0.003; mean value ± SE: AFg = 1.34 ± 0.18, AMg = 2.32 ± 0.26; MSg = 2.48 ± 0.19, Figure 4C). In particular, we found that the AFg showed lower affinitive interaction frequencies than AMg (Bonferroni post-hoc test: AFg vs. AMg; p = 0.013) and MSg (Bonferroni post-hoc test: AFg vs. MSg; p = 0.004), whereas we found no difference between AMg and MSg (Bonferroni post-hoc test: AFg vs. MSg; p = 1.000). Hence, the mixed-sex and all-male groups showed higher affiliation levels than the all-female group.
The frequencies of post-conflict affinitive contacts varied significantly across groups (Kruskal-Wallis test: χ2 = 10.569, df = 2, p = 0.005; mean value ± SE: AFg = 0.03 ± 0.01, AMg = 0.01 ± 0.00; MSg = 0.05 ± 0.01; Figure 4D). In particular, we found that the AMg showed lower post-conflict affinitive contact frequencies than MSg (Dunn post-hoc test: AMg vs. MSg; p = 0.004), whereas we found no difference between AFg and AMg (Dunn post-hoc test: AFg vs. AMg; p = 0.970) and between AFg and MSg (Dunn post-hoc test: AFg vs. MSg; p = 0.128). Thus, the mixed-sex group showed higher levels of post-conflict affiliation compared to all-male group.
The frequencies of excavation behaviours varied significantly across the groups (Kruskal-Wallis test: χ2 = 13.805, df = 2, p = 0.001; mean value ± SE: AFg = 4.90 ± 1.48, AMg = 9.38 ± 0.90; MSg = 14.25 ± 1.55, Figure 4E). In particular, we found that the MSg showed higher excavation behaviour frequencies than AFg (Dunn post-hoc test: MSg vs. AFg; p = 0.001), whereas we found no difference between AFg and AMg (Dunn post-hoc test: AFg vs. AMg; p = 0.183) and AMg and MSg (Dunn post-hoc test; AMg vs. MSg; p = 0.137). Hence, the mixed-sex group showed higher excavation frequencies, compared to the all-female group.
The frequencies of non-invasive exploration behaviours did not show any significant difference between the three groups (Kruskal–Wallis test: χ2 = 4.339, df = 2, p = 0.114; mean value ± SE: AFg = 21.40 ± 0.93, AMg = 21.69 ± 0.94; MSg = 19.33 ± 0.45; Figure 4F). Hence, the level of non-invasive exploration was not affected by group composition.
3.2 Comparison of different environmental enrichment conditions
The frequencies of anxiety-related behaviours varied significantly across the different conditions of environmental enrichments (One-way ANOVA test: F = 3.385; df = 2; p = 0.046; mean value ± SE: NE = 13.08 ± 2.07, LE = 11.25 ± 1.83; ST = 7.00 ± 1.01; Figure 5A). In particular, we found lower frequencies of anxiety related-behaviours in ST than in NE condition (Bonferroni post-hoc test: ST vs. NE; p = 0.048), whereas we found no difference between LE and ST conditions (Bonferroni post-hoc test: LE vs. ST; p = 0.257) and LE and NE conditions (Bonferroni post-hoc test; LE vs. NE; p = 1.000). Thus, only ST condition was associated with reduced anxiety behaviours compared to NE.
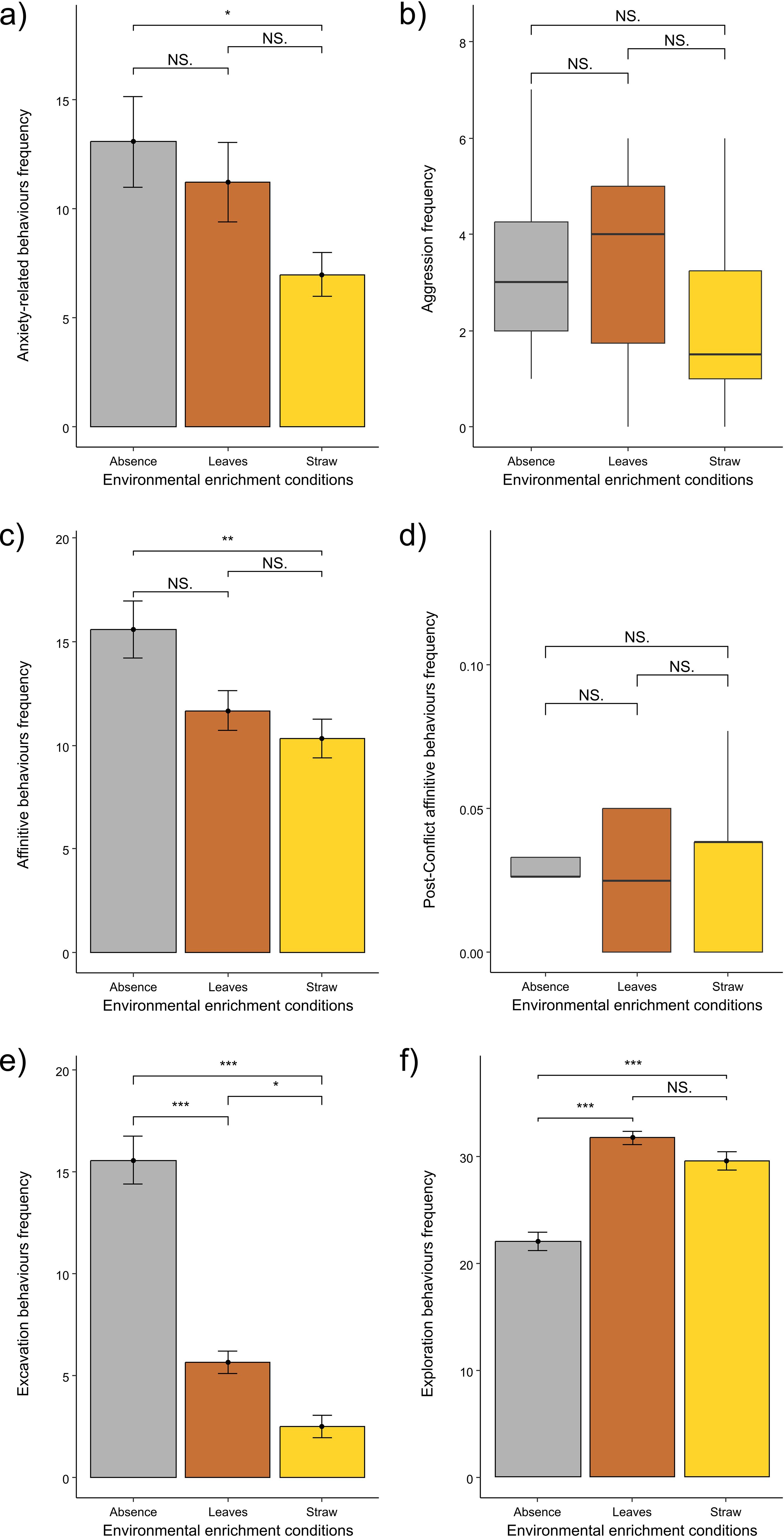
Figure 5. Comparison across environmental enrichment conditions: (A) error bar plots showing differences in levels of anxiety-related behaviours across environmental conditions (One-way ANOVA test, p = 0.046; Bonferroni post-hoc test; ST vs. NE; p = 0.048; LE vs. ST; p = 0.257; LE vs. NE; p = 1.000); (B) boxplots showing differences in levels of aggression across environmental conditions (Friedman test p = 0.040; Dunn post-hoc test, ST vs. LE: p = 0.124; LE vs. NE: p = 1.000; ST vs. NE: p = 0.074); (C) error bar plots showing differences in levels of affinitive behaviours across environmental conditions (One-way ANOVA test, p = 0.006; Bonferroni post-hoc test, ST vs. NE: p = 0.006; LE vs. ST: p = 1.000; LE vs. NE: p = 0.053); (D) boxplots showing that there were no differences in levels of post-conflict affinitive behaviours (normalised on the total number of attacks occurred in the same condition of environmental enrichment) across environmental conditions (Friedman test: p = 0.929); (E) error bar plots showing differences in levels of excavation behaviours across environmental conditions (One-way ANOVA test, p < 0.001; Bonferroni post-hoc test, ST vs. LE: p = 0.030; ST vs. NE: p < 0.001; LE vs. NE: p < 0.001); (F) error bar plots showing differences in levels of exploration behaviours across environmental conditions (One-way ANOVA test, p < 0.001; Bonferroni post-hoc test, ST vs. NE: p < 0.001; LE vs. NE; p < 0.001; LE vs. ST; p = 0.178). Error bars: 95% confidence interval (bars) around the mean (dots); Boxplot: median value (horizontal line), interquartile range (box); minimum and maximum values in the data (vertical line). NS = non-significant, * = p < 0.05; ** = p < 0.01; *** = p < 0.001.
The frequencies of aggression varied significantly across conditions (Friedman test: χ2 = 6.435, df = 2, p = 0.040; mean value ± SE: NE = 3.16 ± 0.52, LE = 3.33 ± 0.62; ST = 2.17 ± 0.51; Figure 5B). However, the pairwise comparisons showed any significant difference (Dunn post-hoc test: ST vs. LE: Q = 2.041, p = 0.124; LE vs. NE: Q = 0.204, p = 1.000; ST vs. NE: Q = 2.245, p = 0.074) Hence, none enrichment condition had a significant effect on aggression frequency, although straw tended to be associated with reduced conflict levels.
The frequencies of affinitive interactions showed significant difference across conditions (One-way ANOVA test: F = 6.065; df = 2; p = 0.006; mean value ± SE: NE = 15.59 ± 1.38, LE = 11.67 ± 0.96; ST = 10.33 ± 0.93; Figure 5C). In particular, we found lower frequencies of affinitive behaviours in ST than in NE condition (Bonferroni post-hoc test: ST vs. NE; p = 0.006), whereas we found no difference between LE and ST (Bonferroni post-hoc test: LE vs. ST; p = 1.000) and a statistical trend between LE and NE conditions (Bonferroni post-hoc test: LE vs. NE; p = 0.053). Hence, straw was associated with reduced affiliation compared to no enrichment condition.
The frequencies of post-conflict affinitive contacts did not show any significant difference across conditions (Friedman test: χ2 = 0.146, df = 2, p = 0.929; mean value ± SE: NE = 0.04 ± 0.01, LE = 0.02 ± 0.01; ST = 0.03 ± 0.01; Figure 5D).
The frequencies of excavation behaviours showed significant variation across conditions (One-way ANOVA test: F = 69.731; df = 2; p < 0.001; mean value ± SE: NE = 15.58 ± 1.17, LE = 5.67 ± 0.57; ST = 2.50 ± 0.56; Figure 5E). In particular, we found lower frequencies of excavation behaviours in ST than in both LE (Bonferroni post-hoc test: ST vs. LE; p = 0.030) and NE (Bonferroni post-hoc test: ST vs. NE; p < 0.001) and in LE than in NE conditions (Bonferroni post-hoc test: LE vs. NE; p < 0.001). Thus, both straw and leaves were associated with reduced excavation frequencies but straw was the most effective enrichment in this respect.
The frequencies of non-invasive exploration behaviours significantly differed across conditions (One-way ANOVA test: F = 41.818; df = 2; p < 0.001; mean value ± SE: NE = 22.00 ± 0.87, LE = 31.67 ± 0.61; ST = 29.50 ± 0.85; Figure 5F). In particular, we found higher frequencies of exploration behaviours in ST than in NE (Bonferroni post-hoc test: ST vs. NE; p < 0.001) and in LE than in NE conditions (Bonferroni post-hoc test: LE vs. NE; p < 0.001), whereas we found no difference between LE and ST conditions (Bonferroni post-hoc test: LE vs. ST; p = 0.178). Hence, both straw and leaves were associated with an increase in non-invasive exploratory behaviours.
4 Discussion
Our results show that group composition (all-male, all-female, and mixed-sex groups) did not affect the level of anxiety, aggression, and non-invasive exploration. However, group composition seemed to affect other aspects of social life. Particularly, the mixed-sex group was associated with an increase in the levels of affiliation (as it occurred with the all-male group, compared to the all-female group), post-conflict affinitive contact (especially, compared to all-male group), and excavation activity (especially, compared with the all-female group). Focusing on the mixed-sex group and different enrichment conditions, we found no effect on post-conflict affiliation levels but straw was associated with i) reduced anxiety and affinitive behaviour levels (compared to when no enrichment was provided) and possibly with reduced aggression levels (we obtained a statistical trend); ii) lowest levels of excavation, and iii) (as it occurred with leaves) enhanced non-invasive exploration activity.
4.1 Comparison across groups
The lack of effect of group composition on aggression levels may be due to different factors including group stability and farming conditions. In the wild, pig groups have a relatively stable composition and they are composed by related adult females with their offspring or by young males (Stolba and Wood-Gush, 1989; Gabor et al., 1999; D’Eath and Turner, 2008; Gonyou, 2001). In our case, the three study groups were formed well before the beginning of the observations and no individual was replaced during data collection. From this perspective, we may assume that our groups were stable. The stability of group composition can reduce aggression; indeed, high levels of aggression can be observed in groups where individuals are regularly replaced (Simmins, 1993; Lagoda et al., 2021; Jowett and Amory, 2021). Moreover, it has been observed that pigs can develop preferred social relationships, and such social preferences are weakly influenced by individual attributes, including sex, but are predominantly influenced by the behavioural context (Goumon et al., 2020). Furthermore, we have to take into account the space available to pigs. Increased space availability may promote a reduction in aggressive event frequencies in the long-term; where space is virtually unlimited the levels of aggression are very low (Jensen and Wood-Gush, 1984; Arey and Edwards, 1998). Moreover, since castration may reduce aggression in males (Fredriksen et al., 2008), another aspect to consider is that males were castrated (both in the all males and mixed-sex groups) and this may have helped to keep the levels of aggression under control in groups where males were present. Hence, extensive farming conditions, group stability and male castration may help keeping aggression under control in all study groups regardless of group composition.
Intra-group aggression is one of the major sources of anxiety in social mammals (Aureli et al., 2002), and no difference in aggression levels between the three groups may also account for the absence of differences in anxiety-related behaviours. Moreover, the extensive farming conditions – rather than the composition of the groups- could play an important role in keeping anxiety levels under control in all groups. Indeed, a previous study on another pig group housed in the same environmental condition as the present research, showed that levels of anxiety-related behaviours in free-ranging pigs were low, with an increase related to aggression, and did not differ between castrated males and females (Norscia et al., 2021).
When studying animal welfare, it is necessary to consider not only the absence of negative behaviours/events (such as anxiety and aggressive interactions) but also – and predominantly – the presence of positive interactions, such as affinitive behaviours (Bradburn, 1969; Seligman and Csikszentmihalyi, 2000). In pigs, affinitive and exploratory behaviours are considered as indicators of a positive welfare (Špinka, 2017; Lawrence et al., 2018). Regarding non-invasive exploratory behaviours, our results showed no differences across groups. In line with previous studies that have shown that pigs raised in semi-natural conditions spend most of their time in exploratory activities (Stolba and Wood-Gush, 1989; Day et al., 1995; Studnitz et al., 2007), extensive farming conditions could play an important role in maintaining high levels of exploratory behaviour, regardless of group composition. Affinitive behaviours levels (including post-conflict interactions) were higher in the mixed-sex group than in the all-male group. In social mammals, affinitive behaviours play an important role in maintaining group homeostasis (de Waal, 2000) and in domestic pigs, these behaviours can be used to buffer anxiety (Norscia et al., 2021). Unlike the groups formed by related females and by young males that show a stable composition (Stolba and Wood-Gush, 1989; Gabor et al., 1999; D’Eath and Turner, 2008; Gonyou, 2001), the mixed groups, composed of females and castrated males, could be less stable from a social point of view, as these groups are artificially established and not found naturally. In this regard, the higher levels of affinitive behaviours observed in the mixed group may be necessary due to the potential lower stability of the composition and contribute effectively to maintaining the social cohesion of the group, promoting the maintenance of group homeostasis. Moreover, the absence of variations in the levels of anxiety-related behaviours across groups may suggest that high levels of affinitive behaviours could help keep anxiety under control especially in the mixed group, where males and females share space and resources.
From a welfare point of view, it is important to consider not only the frequency of aggression but even if the aggression are managed with efficient post-conflict strategies and our results showed that the levels of post-conflict affinitive interactions were higher in the mixed-sex group than in the all-male group. Aggressive events are an integral part of social life (de Waal, 2000) and previous studies showed that domestic pigs can effectively engage in post-conflict strategies (e.g. reconciliation, triadic and quadratic contacts; Cordoni et al., 2023; Norscia et al., 2024). Differences in agonistic behaviour between sexes have been observed in domestic pigs, with males differing significantly from females in their fighting ability, competitive behaviour and related costs (Camerlink et al., 2022) and castrated males may also engage in aggressive behaviours for dominance (Foister et al., 2018), although we cannot exclude that castration may help reduce levels of aggression. However, aggressive interactions between castrated males and females could still be unbalanced, even though the males are castrated. Therefore, there may be a greater need for social buffering to restore social homeostasis within the mixed-sex group. Furthermore, to explain the levels of post-conflict affinitive behaviour exhibited by individuals in the mixed-sex group, it may be necessary to consider the potential social instability that could characterise the mixed-sex group, as we hypothesised for affinitive behaviours in general. In this regard, both affinitive behaviours and post-conflict affinitive behaviours could contribute to maintaining social cohesion in the mixed-sex group more than in groups formed by females or castrated males. The fact that the mixed-sex group showed the same level of aggression but more solved conflicts and higher levels of affinitive behaviours compared to the other groups could represent a positive welfare trait that should be considered in the management of free-ranging pigs.
Finally, the mixed-sex group showed higher excavation levels than the all-female group. In this regard, the presence of males within the group could play an important role, although they are castrated males. Taking into account the deep differences in agonistic behaviour between the sexes and that aggression between males are costly (Camerlink et al., 2022), indirect forms of competition could emerge to avoid conflicts. Moreover, it is worth considering that castrated males can maintain competition, although in a less aggressive way (Fredriksen et al., 2008; Foister et al., 2018). The reduction of aggression over resources (contest competition) may be associated with an increase in indirect forms of competition over food, such as scramble competition, which occurs when an individual reduces the quantity of food available to others by consuming it (Koenig, 2002). Thus, excavation may be a way to prevent others to take food resource (e.g, roots, tubers), avoiding aggressive interaction.
4.2 Comparison of different environmental enrichment conditions
Pigs have an innate propensity for socialization, exploration and chewing and poorly enriched environments can cause stress and induce abnormal, stereotypical and aggressive behaviours (Day et al., 2001; Studnitz et al., 2007). Environmental enrichment is one mean to reduce such behaviours, since the lack of a substrate or object to be manipulated in the environment in which pigs live can lead to an increased frequency of pathological patterns (EFSA Panel on Animal Health and Welfare (AHAW), 2014). It has been suggested that environmental enrichment for pigs to be effective should be manipulable, chewable and edible (Van de Weerd et al., 2003).
Environmental enrichment is effective in lowering levels of aggressive behaviours (Van de Weerd et al., 2003; Bolhuis et al., 2005). Consistently, our results showed that the presence of straw tended to decrease levels of aggression compared to the no enrichment condition, although we did not observe significant variations. Extensive farming conditions could play an important role, keeping levels of aggression under control regardless of enrichment conditions. Moreover, low levels of aggression could ensure that no differences emerge in post-conflict affiliation levels, as in our case.
In line with previous studies which showed that pigs raised in sterile environments experienced more stress than in enriched environments (Van de Weerd et al., 2003), we found a reduction in anxiety-related behaviours in straw enrichment condition compared to no enrichment condition. In addition, since affiliation is an important tool to buffer anxiety (Norscia et al., 2021; Cordoni et al., 2023; Norscia et al., 2024), we could assume a cascade effect that could lead to reduced affiliation levels when anxiety levels are low. In this regard, low levels of affinitive behaviours in the straw enrichment condition may be associated with low levels of anxiety observed in the same condition. In our study, straw was an effective enrichment in lowering excavation levels and increasing levels of non-invasive exploratory behaviours. Outdoor pigs often perform rooting or foraging behaviour; rooting is an important part of the behavioural repertoire, constituting a rewarding experience and satisfying a behavioural need for pigs (Day et al., 1995; Studnitz et al., 2003, 2007). Consistent with our findings, pigs can adapt their feeding behaviour to the prevailing conditions (Gustafsson et al., 1999) and environmental enrichment can increase the time spent in explorative behaviours (Beattie et al., 2000). In our case, individuals could spend more time chewing and manipulating straw, engaging in straw exploratory behaviours rather than in excavation behaviours with negative consequences on environment.
5 Conclusion
The group composition and the type of environmental enrichment can represent two important elements for improving pig welfare while reducing animal impact on the natural habitat. In this regard, ethological studies represent an important tool for evaluating the welfare of farm-raised animals such as domestic pigs. Our study provides insight into behavioural aspects worthy of consideration for the management of extensive farming in terms of animal welfare. In particular, the mixed-sex group showed higher levels of behaviours that may indicate positive animal welfare states, such as affinitive and post-conflict affinitive behaviours, although it also exhibited higher levels of excavation behaviours compared to both all-female and all-male groups. In this regard, affinitive and post-conflict affinitive contacts could contribute to the cohesion and stability of mixed-sex groups. Moreover, this aspect could be supported by the absence of differences regarding negative welfare aspects such as aggression and anxiety-related behaviours. From this perspective, the formation of mixed groups by farmers could be a management strategy that could enhance animal welfare. Under straw enrichment conditions, individuals exhibited lower levels of anxiety-related behaviours compared to the no enrichment condition. Conversely, in the absence of enrichment, we recorded higher levels of affinitive behaviours, which could indicate a greater need to alleviate stress compared to the straw enrichment condition. Specifically, providing straw to free-ranging pigs raised in semi-natural conditions can represent an environmental enrichment strategy that reduces negative welfare aspects while also decreasing invasive excavation behaviours, leading to an increase in non-invasive exploratory behaviours. Furthermore, our findings suggest that forming mixed-sex groups and providing them with environmental enrichment such as straw could be a suitable solution to effectively reduce invasive excavation behaviours without preventing pigs from showing their natural behavioural repertoire. Indeed, the combination of these two measures could ensure high standards of welfare, considering both the negative and positive effects, and a reduction of the impact on the natural environment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by the Bioethics Committee of the University of Turin (Italy). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EC: Data curation, Writing – original draft, Writing – review & editing. LC: Data curation, Writing – review & editing. AP: Data curation, Writing – review & editing. GC: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – review & editing. IN: Conceptualization, Data curation, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Fundings
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was funded by the University of Torino, Department of Life Sciences and System Biology (@DBIOSUnito), via research funds granted to IN (code: NORI_RILO_18_01; NORI_RILO_19_01; NORI_RILO_20_01; NORI_RILO_21_01) and PON Green oriented PhD grant to EC (Action IV.5; Ministry Decree 1061/2021; FSE-REACT-EU funds).
Acknowledgments
The authors wish to thank Cristina Desdera and Davide Lovera, who own and manage the ethical farm “Parva Domus” (Turin, Italy) for their interest, encouragement and availability since the study start. This study is part of the broader projects “So.Pig” and “GreenPig” (Department of Life Sciences and Systems Biology, University of Turin).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fetho.2024.1450585/full#supplementary-material
Footnotes
- ^ Collarini, E., Cordoni, G., Dal Monte, O., Traversa, M., Medana, C., Visentin, S., et al. Nose-clip use in semi-free ranging pigs reduces rooting without disrupting affiliative behaviour or causing prolonged stress. Under review.
References
Altmann J. (1974). Observational study of behavior: Sampling methods. Behaviour 49, 227–266. doi: 10.1163/156853974x00534
Appleby M. C., Mitchell L. A. (2018). Understanding human and other animal behaviour: Ethology, welfare and food policy. Appl. Anim. Behav. Sci. 205, 126–131. doi: 10.1016/j.applanim.2018.05.032
Arey D. S., Edwards S. A. (1998). Factors influencing aggression between sows after mixing and the consequences for welfare and production. Livest. Prod. Sci. 56, 61–70. doi: 10.1016/s0301-6226(98)00144-4
Aureli F., Cords M., van Schaik C. P. (2002). Conflict resolution following aggression in gregarious animals: a predictive framework. Anim. Behav. 64, 325–343. doi: 10.1006/anbe.2002.3071
Beattie V. E., O’Connell N. E., Moss B. W. (2000). Influence of environmental enrichment on the behaviour, performance and meat quality of domestic pigs. Livest. Prod. Sci. 65, 71–79. doi: 10.1016/s0301-6226(99)00179-7
Bolhuis J. E., Schouten W. G. P., Schrama J. W., Wiegant V. M. (2005). Behavioural development of pigs with different coping characteristics in barren and substrate-enriched housing conditions. Appl. Anim. Behav. Sci. 93, 213–228. doi: 10.1016/j.applanim.2005.01.006
Bradburn N. M. (1969). The structure of psychological well-being (Chicago, Illinois, USA: Aldine publishing).
Brown S. M., Bush S. J., Summers K. M., Hume D. A., Lawrence A. B. (2018). Environmentally enriched pigs have transcriptional profiles consistent with neuroprotective effects and reduced microglial activity. Behav. Brain Res. 350, 6–15. doi: 10.1016/j.bbr.2018.05.015
Camerlink I., Farish M., Arnott G., Turner S. P. (2022). Sexual dimorphism in ritualized agonistic behaviour, fighting ability and contest costs of Sus scrofa. Front. Zool. 19. doi: 10.1186/s12983-022-00458-9
Cordoni G., Comin M., Collarini E., Robino C., Chierto E., Norscia I. (2023). Domestic pigs (Sus scrofa) engage in non-random post-conflict affiliation with third parties: cognitive and functional implications. Anim. Cogn. 26, 687–701. doi: 10.1007/s10071-022-01688-4
Cunha E. C. P., de Alcantara Menezes T., Bernardi M. L., Mellagi A. P. G., da Rosa Ulguim R., Wentz I., et al. (2018). Reproductive performance, offspring characteristics, and injury scores according to the housing system of gestating gilts. Livest. Sci. 210, 59–67. doi: 10.1016/j.livsci.2018.02.008
D’Eath R. B., Turner S. P. (2008). “The natural behaviour of the pig,” in The Welfare of Pigs (Springer Netherlands, Dordrecht), 13–45.
Day J. E. L., Kyriazakis I., Lawrence A. B. (1995). The effect of food deprivation on the expression of foraging and exploratory behaviour in the growing pig. Appl. Anim. Behav. Sci. 42, 193–206. doi: 10.1016/0168-1591(95)93889-9
Day J. E. L., Spoolder H. A. M., Edwards S. A. (2001). “Straw as environmental enrichment: which properties do growing pigs find behaviourally rewarding?,” in Proceedings of the International Symposium of the C.I.G.R. Animal Welfare Considerations in Livestock Housing Systems, (Newcastle, UK: Newcastle University 157–167.
Dellmeier G. R. (1989). Motivation in relation to the welfare of enclosed livestock. Appl. Anim. Behav. Sci. 22, 129–138. doi: 10.1016/0168-1591(89)90049-x
de Waal F. B. M. (2000). Primates-a natural heritage of conflict resolution. Science 289, 586–590. doi: 10.1126/science.289.5479.586
de Waal F. B. M., van Roosmalen A. (1979). Reconciliation and consolation among chimpanzees. Behav. Ecol. Sociobiol. 5, 55–66. doi: 10.1007/bf00302695
Edge H. L., Bulman C. A., Edwards S. A. (2005). Alternatives to nose-ringing in outdoor sows: the provision of root crops. Appl. Anim. Behav. Sci. 92, 15–26. doi: 10.1016/j.applanim.2004.10.021
EFSA Panel on Animal Health and Welfare (AHAW) (2014). Scientific Opinion concerning a Multifactorial approach on the use of animal and non-animal-based measures to assess the welfare of pigs. Eur. Food Saf. Auth. J. 12, 1–101. doi: 10.2903/j.efsa.2014.3702
Ernst K., Puppe B., Schön P. C., Manteuffel G. (2005). A complex automatic feeding system for pigs aimed to induce successful behavioural coping by cognitive adaptation. Appl. Anim. Behav. Sci. 91, 205–218. doi: 10.1016/j.applanim.2004.10.010
Foister S., Doeschl-Wilson A., Roehe R., Arnott G., Boyle L., Turner S. (2018). Social network properties predict chronic aggression in commercial pig systems. PloS One 13, e0205122. doi: 10.1371/journal.pone.0205122
Fredriksen B., Lium B. M., Marka C. H., Mosveen B., Nafstad O. (2008). Entire male pigs in farrow-to-finish pens—Effects on animal welfare. Appl. Anim. Behav. Sci. 110, 258–268. doi: 10.1016/j.applanim.2007.04.007
Gabor T. M., Hellgren E. C., Van Den Bussche R. A., Silvy N. J. (1999). Demography, sociospatial behaviour and genetics of feral pigs (Sus scrofa) in a semi-arid environment. J. Zool. (1987) 247, 311–322. doi: 10.1111/j.1469-7998.1999.tb00994.x
Godyń D., Nowicki J., Herbut P. (2019). Effects of environmental enrichment on pig welfare—A review. Animals 9, 383. doi: 10.3390/ani9060383
Gonyou H. W. (2001). “The social behaviour of pigs,” in Social behaviour in farm animals (CABI Publishing, UK), 147–176.
Goumon S., Illmann G., Leszkowová I., Dostalová A., Cantor M. (2020). Dyadic affiliative preferences in a stable group of domestic pigs. Appl. Anim. Behav. Sci. 230, 105045. doi: 10.1016/j.applanim.2020.105045
Gustafsson M., Jensen P., de Jonge F. H., Schuurman T. (1999). Domestication effects on foraging strategies in pigs (Sus scrofa). Appl. Anim. Behav. Sci. 62, 305–317. doi: 10.1016/s0168-1591(98)00236-6
Jensen P., Wood-Gush D. G. M. (1984). Social interactions in a group of free-ranging sows. Appl. Anim. Behav. Sci. 12, 327–337. doi: 10.1016/0168-1591(84)90125-4
Jowett S., Amory J. (2021). The stability of social prominence and influence in a dynamic sow herd: A social network analysis approach. Appl. Anim. Behav. Sci. 238, 105320. doi: 10.1016/j.applanim.2021.105320
Koenig A. (2002). Competition for resources and its behavioral consequences among female primates. Int. J. Primatol. 23, 759–783. doi: 10.1023/a:1015524931226
Kokocińska A., Kaleta T. (2016). The role of ethology in animal welfare. Sci. Ann. Polish Soc. Anim. Prod. 12, 49–62. doi: 10.5604/01.3001.0013.6981
Lagoda M. E., Boyle L. A., Marchewka J., Calderón Díaz J. A. (2021). Mixing aggression intensity is associated with age at first service and floor type during gestation, with implications for sow reproductive performance. Animal 15, 100158. doi: 10.1016/j.animal.2020.100158
Lawrence A. B., Newberry R. C., Špinka M. (2018). “Positive welfare,” in Advances in Pig Welfare (Amsterdam, The Netherlands: Elsevier), 415–444.
Mellor D. J. (2015). Enhancing animal welfare by creating opportunities for positive affective engagement. N. Z. Vet. J. 63, 3–8. doi: 10.1080/00480169.2014.926799
Mellor D. J., Beausoleil N. J. (2015). Extending the ‘Five Domains’ model for animal welfare assessment to incorporate positive welfare states. Anim. Welf. 24, 241–253. doi: 10.7120/09627286.24.3.241
Moinard C., Mendl M., Nicol C. J., Green L. E. (2003). A case control study of on-farm risk factors for tail biting in pigs. Appl. Anim. Behav. Sci. 81, 333–355. doi: 10.1016/s0168-1591(02)00276-9
Norscia I., Collarini E., Cordoni G. (2021). Anxiety behavior in pigs (Sus scrofa) decreases through affiliation and may anticipate threat. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.630164
Norscia I., Collarini E., Robino C., Chierto E., Cordoni G. (2024). Witness for resolution: Post-conflict quadratic affiliation in semi-free ranging pigs. Curr. Zool. 70, 233–243. doi: 10.1093/cz/zoad016
Petersen V., Simonsen H. B., Lawson L. G. (1995). The effect of environmental stimulation on the development of behaviour in pigs. Appl. Anim. Behav. Sci. 45, 215–224. doi: 10.1016/0168-1591(95)00631-2
Podgórski T., Lusseau D., Scandura M., Sönnichsen L., Jędrzejewska B. (2014). Long-lasting, kin-directed female interactions in a spatially structured wild boar social network. PloS One 9, e99875. doi: 10.1371/journal.pone.0099875
Seligman M. E., Csikszentmihalyi M. (2000). Positive psychology: an introduction. Am. Psychol. 55, 5–14. doi: 10.1037/0003-066X.55.1.5
Siegel S., Castellan N. J. J. (1988). Nonparametric Statistics for the Behavioral Sciences (New York: McGraw Hill).
Simmins P. H. (1993). Reproductive performance of sows entering stable and dynamic groups after mating. Anim. Sci. 57, 293–298. doi: 10.1017/s0003356100006917
Špinka M. (2017). Behaviour of pigs. In the ethology of domestic animals: an introductory text, (Wallington, UK: CABI), 214–227.
Stolba A., Wood-Gush D. G. M. (1989). The behaviour of pigs in a semi-natural environment. Anim. Sci. 48, 419–425. doi: 10.1017/s0003356100040411
Studnitz M., Jensen K. H., Jørgensen E. (2003). The effect of nose rings on the exploratory behaviour of outdoor gilts exposed to different tests. Appl. Anim. Behav. Sci. 84, 41–57. doi: 10.1016/s0168-1591(03)00144-8
Studnitz M., Jensen M. B., Pedersen L. J. (2007). Why do pigs root and in what will they root? Appl. Anim. Behav. Sci. 107, 183–197. doi: 10.1016/j.applanim.2006.11.013
Temple D., Manteca X., Velarde A., Dalmau A. (2011). Assessment of animal welfare through behavioural parameters in Iberian pigs in intensive and extensive conditions. Appl. Anim. Behav. Sci. 131, 29–39. doi: 10.1016/j.applanim.2011.01.013
Van de Weerd H. A., Docking C. M., Day J. E. L., Avery P. J., Edwards S. A. (2003). A systematic approach towards developing environmental enrichment for pigs. Appl. Anim. Behav. Sci. 84, 101–118. doi: 10.1016/s0168-1591(03)00150-3
Keywords: Sus scrofa, animal welfare, social behaviour, extensive farming, habitat destruction
Citation: Collarini E, Capponcelli L, Pierdomenico A, Cordoni G and Norscia I (2024) Balancing welfare and habitat damage in pigs (Sus scrofa) under extensive farming: an ethological approach for determining the effects of group composition and environmental enrichment. Front. Ethol. 3:1450585. doi: 10.3389/fetho.2024.1450585
Received: 17 June 2024; Accepted: 23 August 2024;
Published: 20 September 2024.
Edited by:
Cemil Tölü, Çanakkale Onsekiz Mart University, TürkiyeReviewed by:
Temple Grandin, Colorado State University, United StatesTürker Savaş, Çanakkale Onsekiz Mart University, Türkiye
Copyright © 2024 Collarini, Capponcelli, Pierdomenico, Cordoni and Norscia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Edoardo Collarini, ZWRvYXJkby5jb2xsYXJpbmlAdW5pdG8uaXQ=; Giada Cordoni, Z2lhZGEuY29yZG9uaUB1bml0by5pdA==; Ivan Norscia, aXZhbi5ub3JzY2lhQHVuaXRvLml0
†These authors share senior authorship
‡ORCID: Edoardo Collarini, orcid.org/0000-0003-0789-4089
Giada Cordoni, orcid.org/0000-0001-7093-0025
Ivan Norscia, orcid.org/0000-0002-1618-7717
 Edoardo Collarini
Edoardo Collarini Luca Capponcelli
Luca Capponcelli Giada Cordoni
Giada Cordoni Ivan Norscia
Ivan Norscia