- Department of Biology, University of San Francisco, San Francisco, CA, United States
Play behavior is common in a range of mammalian species and can have important influences on early development. We evaluated possible influences of social play on the development of behavior along the caution/boldness continuum in juvenile Belding’s ground squirrels (Urocitellus beldingi). We disrupted juvenile squirrels engaged in play by walking toward them until their play interactions stopped. We used undisrupted juveniles and juveniles disrupted while not engaged in play as controls. Caution was measured with behavioral tests during which a human intruder walked toward a squirrel and recorded the distances at which the squirrel first noticed and then fled from the intruder. Rates of social play were lower and play bouts were shorter in play-disrupted juveniles than in undisrupted and nonplay-disrupted juveniles. The distances at which juveniles noticed and fled from an intruder increased across the developmental period in which play primarily occurs, suggesting increases in caution across this interval. Increases in the distances to notice and flee from an intruder did not differ between undisrupted and nonplay-disrupted juveniles, but were greater in these groups than in play-disrupted juveniles. These results are consistent with social play behavior contributing to the development of cautious responses in juvenile U. beldingi.
Introduction
Play behavior occurs during the juvenile period in a broad range of mammalian species, is inherently rewarding, and can importantly influence development (Bekoff and Byers, 1998; Burghardt, 2005; Trezza et al., 2010; Pellis et al., 2014). We evaluated the relationship between social play in juvenile Belding’s ground squirrels (Urocitellus beldingi) and the development of temperament along the caution/boldness continuum. Young animals are often vulnerable to predation and in some cases infanticide by conspecifics, and caution should thus generally be favored among young animals (Hrdy, 1979; Sherman, 1981; Lima and Dill, 1990; Nunes and Monroy Montemayor, 2023).
Temperament includes behavioral traits of an individual that are consistent over time and across situations. Temperaments are expressed along continua that can include caution-boldness, exploration-avoidance, and ranges of docility (Réale et al., 2007; Petelle et al., 2013). The caution-boldness continuum specifically encompasses responses to danger or threats, whereas exploration-avoidance refers to responses to novel objects or unfamiliar situations, and docility refers to the degree to which responses are passive or active. Elements of temperament can exhibit plasticity in young animals during periods of behavioral development, and social, physiological, and ecological factors in early life can have important and long-lasting effects on temperament (Sinn et al., 2008; Rödel and von Hulst, 2009; Rödel and Monclús, 2011; Petelle et al., 2017; DiRienzo et al., 2018; Cabrera et al., 2021). Temperament in turn can affect a range of ecologically important variables including physiological responses to stress, reproductive success, and survival (Carere et al., 2001; Dingemanse et al., 2004; Both et al., 2005; Boon et al., 2008; Réale et al., 2010; Baugh et al., 2013; Colchester and Harrison, 2016; Rasmussen and Belk, 2017; Vetter et al., 2016). Temperament during the juvenile period can provide a foundation for development of adult behavioral and personality traits. Factors that influence juvenile temperament might thus have an important impact of the expression of behavior in adulthood (Rothbart and Ahadi, 1994; Eisenberg, 2012; Wright and Jackson, 2022).
Temperament can be an element of coping styles, which include correlated behavioral and physiological responses that help animals navigate ecological and social challenges. For example, proactive coping styles tend to be characterized by active responses and quick action, greater agression, lower behavioral flexibility, and lower responsiveness along the hypothalamus-pituitary-adrenal (HPA) axis involving release of glucocorticoids after exposure to a physical or psychological stressor. By contrast, reactive coping styles tend to be characterized by passive responses, information gathering, lower aggression, greater behavioral flexibility, and greater responsiveness along the HPA axis (Koolhaas et al., 1999; Coppens et al., 2010). Coping styles can vary according to ecological and social variables, with different coping styles being adaptive under different conditions (Duckworth et al., 2023).
Play behavior can appear frivolous with no immediate or obvious benefits for animals, creating challenges for defining or quantifying play. Nevertheless, various adaptive functions have been linked to play behavior (Palagi, 2018). The expression of play behavior can vary greatly across species; however, within a species play is typically characterized by specific behavior patterns that are exhibited in varied sequences (Bekoff, 1974; Burghardt, 2005). Moreover, Burghardt (2005) identified five elements common to play across species. Play behaviors are 1) voluntary and spontaneous, 2) characteristic of a species and performed repeatedly but not invariantly, 3) functionless in the context in which they occur but may resemble functional behaviors, 4) distinct in their expression from functional behaviors, and 5) expressed in healthy, unstressed individuals. Play behavior can be categorized as non-social, involving manipulation of objects or locomotor activity, or as social, involving interaction with other individuals (Burghardt, 2005; Pellis et al., 2014; Pellis and Pellis, 2017). Play most commonly occurs among juveniles, but in some cases may continue beyond the juvenile period into adulthood (Bekoff and Byers, 1998; Palagi, 2018, 2023).
A diverse range of adaptive benefits have been suggested to result from juvenile play behavior across different species (Nunes and Monroy Montemayor, 2023). In some cases, these benefits are experienced early in the life of individuals. For example, juvenile play can contribute to motor development in young animals. Play behavior boosts acquisition of motor skill and coordination in Assamese macaques (Macaca assamensis; Berghänel et al., 2015) and U. beldingi (Nunes et al., 2004a). Juvenile play can also contribute to social development. Play facilitates establishment and maintenance of dominance relationships in juvenile red foxes (Vulpes vulpes; Meyer and Weber, 1996), helps young seals (Phoca vitulina) integrate into their social groups (Wilson, 1974). Moreover, play enhances survival in young brown bears (Ursus arctos) by reducing stress-related mortality (Fagen and Fagen, 2004).
Play can also have longer-term benefits that extend beyond the juvenile period in a wide array of animals. Long-term benefits of play can be related to motor skill. For example, locomotor play in juvenile mule deer (Odocoileus hemionus) and white-tailed deer (O. virginianus) promotes development of motor skills important in evading predators (Carter et al., 2019). In some cases, juvenile play appears to enhance the competence of adult behavior. For example, rough and tumble play during the juvenile period in American minks (Neovison vison) is associated with increased proficiency of copulatory behavior in adulthood (Ahloy Dallaire and Mason, 2017). In female U. beldingi juvenile play is associated with greater intensity of maternal territorial behavior and increased likelihood of weaning a litter as a yearling (Nunes, 2014). In many animals, long-term benefits of juvenile play are related to social behavior and social organization. In horses (Equus caballus; Rho et al., 2007), domestic dogs (Canis lupus familiaris; Ward et al., 2008), female African elephants (Loxodonta africana; Lee and Moss, 2014), and geladas (Theropithcus gelada; Gallo et al., 2021), play interactions help young animals forge long-term social relationships. Play behavior in yellow-bellied marmots (Marmota flaviventris) helps to establish hierarchical relationships that persist into adulthood (Blumstein et al., 2013), and in baboons (Papio papio, P. hamadryas) and mandrills (Mandrillus leucophaeus, M. sphinx) juvenile play fighting is related to the structural rigidity of long-term dominance relationships (Kraus et al., 2019). In spotted hyenas (Crocuta crocuta) and male African elephants play facilitates social assessment by providing young animals with information about conspecifics with whom they will interact throughout their lives (Lee and Moss, 2014; Nolfo et al., 2021). In rats and an array of primate species play behavior is critical for development of socially competent adult behavior (Himmler et al., 2016; Pellis et al., 2017; Palagi, 2018; Stark and Pellis, 2020; Stark et al., 2021; Bijlsma et al., 2022, Bijlsma et al., 2024).
We evaluated the relationship between juvenile social play and development of temperament in U. beldingi, a species that is a well-established model system for studies of play (Holmes, 1994; Nunes and Monroy Montemayor, 2023). We used an experimental approach to build on prior studies reporting correlations between social play and development of temperament in U. beldingi (Marks et al., 2017; Hurst-Hopf et al., 2023; Shehan et al., 2023). Because U. beldingi is diurnal, it is well-suited for studies involving observation of behavior. The habitat of U. beldingi extends across high elevation meadows in the western United States. Squirrels live together in groups but do not have a complex social system. The active period of U. beldingi occurs during a 3–4 month period in late spring and summer coinciding with the local growing season. Squirrels hibernate in underground burrows between active seasons, subsisting on stored fat. Mating begins soon after squirrels emerge from hibernation. Because of the short active period, females bear at most one litter each year (Michener, 1983; Jenkins and Eshelman, 1984; Sherman and Morton, 1984). Young squirrels remain underground in natal burrows during lactation and first appear above ground between 25–28 days of age near the time of weaning (Holekamp et al., 1984). Juvenile U. beldingi engage in play behavior primarily during their first two weeks above ground. Most play interactions (> 97%) occur between littermates (Nunes et al., 1999). The short, well-defined play interval for U. beldingi makes the species especially amenable to studies involving experimental manipulation of play behavior.
Prior studies of juvenile U. beldingi suggested juvenile social play may contribute to development of temperament in this species. Social play in young squirrels was associated with increased exploration in an unfamiliar environment, a shift from docile to more active behavioral responses, and increased caution in response to a potential threat (Marks et al., 2017; Hurst-Hopf et al., 2023: Shehan et al., 2023). These prior studies were correlational and provided preliminary evidence supporting a relationship between social play and development of temperament in young animals. Here we expanded on this prior work and used an experimental approach to evaluate the hypothesis that social play behavior promotes development of cautious responses in juvenile U. beldingi. We disrupted the play behavior of juvenile U. beldingi engaged in social play by walking toward them until the play interactions were halted, and used undisrupted juveniles and juveniles disrupted while not engaged in play as controls. To evaluate caution, we conducted behavioral tests in which a human intruder approached a squirrel and noted the distances at which the squirrel first noticed and then fled from the intruder. We reasoned that if social play promotes development of cautious responses in U. beldingi, then play-disrupted juveniles should have smaller increases in caution across the developmental period in which play primarily occurs than undisrupted and nonplay-disrupted juveniles.
Methods
Study animals
We studied a population of U. beldingi from June-August in 2021 and 2022 at Tioga Pass (latitude 37.9, longitude -119.2, elevation 2950 meters) in Mono County, California, USA. In this population, between 25–40 reproductive females typically wean a litter during the active season each year (Little, 2022). We trapped a total of 71 juveniles from 12 litters in 2021, and 55 juveniles from 9 litters in 2022 to include in the study. All litters in the study ranged in size from 5–7 juveniles. We randomly assigned litters to one of three groups: control/undisrupted (7 litters, 40 juveniles), nonplay-disrupted (7 litters, 44 juveniles), or play-disrupted (7 litters, 42 juveniles). We note that a total of 8 juveniles disappeared during their first two weeks above ground (3 undisrupted, 2 nonplay-disrupted, 3 play-disrupted), and were omitted from the study. We were unable to complete follow-up behavioral tests (see below) for three juveniles in the play-disrupted group, and these juveniles were also omitted from the study. The final sample consisted of 115 squirrels, including 37 undisrupted juveniles (20 female, 17 male), 42 nonplay-disrupted juveniles (24 female, 18 male), and 36 play-disrupted juveniles (20 female, 16 male).
Animal handling
We captured adult and juvenile squirrels with live-traps (Tomahawk Live-Trap, Hazelhurst, WI) baited with peanut butter. When trapping adults, we checked traps every thirty minutes or less during trapping sessions, and released squirrels at their sites of capture after handling. We observed adults to identify reproductive females, and observed the maternal territories of these females daily to determine the dates that their litters first emerged from the natal burrow (Nunes et al., 1999). We trapped juveniles within two days of their first being observed above ground. When trapping juveniles, we observed traps continuously, handled juveniles immediately after they were captured, and released them at their natal burrows after handling. At juveniles’ first capture, we weighed them to the nearest gram with spring balance scales (Avinet Research Suppliers, Portland, ME). We fitted adult and juvenile squirrels with numbered metal ear tags for long-term identification (National Band and Tag, Newport, KY). We painted ear tags of juveniles different colors with nail polish to aid in identification of individuals during observations (Nunes et al., 2015). We also marked adult and juvenile squirrels with symbols using hair dye (Revlon, New York, NY) to aid in visual identification. Trapping and other methods in the study followed guidelines published by the American Society of Mammalogists (Sikes and The Animal Care and Use Committee of the American Society of Mammalogists, 2016).
Observation of behavior
We regularly observed the behavior of juvenile U. beldingi throughout the day between 0730–1400 hours from elevated posts such as boulders or hillsides. Observation sessions lasted from 30–120 minutes and were terminated when juveniles became scattered throughout the natal area and were no longer interacting with each other. Most observation sessions occurred in the morning between 0730 and 0930 after juveniles first emerged from the natal burrow for the day and all littermates were present and active. During observations, we recorded all occurrences of social play behavior for individual U. beldingi along with the duration of the interactions. Specific play behaviors are described in Nunes et al. (1999) and are listed in Table 1. We calculated rates of social play for individual U. beldingi as the number of interactions observed per hour of observation. We observed juveniles in the study for an average of 338.2 ± 15.2 (SE) minutes per individual on an average of 4.9 ± 0.2 (SE) different days from 2–11 days after their first appearance above ground.
Disruption of behavior
At their first emergence from the natal burrow, we randomly assigned litters to one of three groups: control/undisrupted, nonplay-disrupted, or play-disrupted. We observed juveniles in the undisrupted group without interference during observation sessions. We disrupted juveniles in the nonplay-disrupted group during observation sessions by walking at a pace of one meter per second every five minutes through areas in which juveniles were present. If any juveniles were engaged in play interactions at the time of a walk-through, the observer waited until the interactions were completed before walking through the area. We disrupted juveniles in the play-disrupted group by walking at a pace of one meter per second toward juveniles whenever they were observed to engage in play until the play bout halted. We also observed juveniles in the nonplay-disrupted and play-disrupted groups without interruption for at least one observation session to determine baseline rates of play and baseline durations of play bouts. We conducted uninterrupted observations sessions for juveniles in the nonplay-disrupted and play-disrupted groups on days following disrupted sessions.
Behavioral tests
To evaluate caution in response to an intruder in the natal area, we conducted behavioral tests toward the beginning of the play period within 1–3 days after individuals first appeared above ground, and again toward the end of the play period within 12–14 days after first emergence from the natal burrow. Behavioral tests were a modified version of flight-initiation distance tests (Runyan and Blumstein, 2004; Nunes, 2023), which measure the distance at which an individual first flees from an approaching human (Ydenberg and Dill, 1986; Blumstein, 2003). Flight is an anti-predatory behavior, and flight-initiation distance is commonly used to measure responses along the caution-boldness continuum (Cooper, 2009; Petelle et al., 2013). During tests, a human intruder identified a test subject and took a position approximately 20 meters from the subject. At the initiation of tests, the juveniles being tested (1) had been facing away from the intruder and for at least two minutes, (2) was not within two meters of any other squirrels, and (3) was within 1.5 meters of a burrow entrance that could be used for escape. The intruder walked toward the subject at a pace of one meter per second and dropped a marker flag (1) when the squirrel first noticed the intruder, as indicated by the squirrel turning toward the intruder, lifting its head or body, or standing on its hind legs, (2) when the squirrel fled from its original location, and (3) at the squirrel’s original location. We used measuring tapes to determine distances from their original location at which squirrels noticed and fled from intruders. We expressed changes in these distances across the play period as proportions by dividing distances at which squirrels noticed and fled from an intruder at the end of the play period by corresponding distances at the beginning of the play interval.
We conducted tests after juveniles had emerged from the natal burrow in the morning, had become scattered throughout the natal area, and were not interacting with other squirrels. In rare occurrences during which another squirrel responded to the intruder before the focal squirrel did, we terminated the test trial and conducted a new trial for the focal squirrel on the following day (Shehan et al., 2023).
Data analysis
We evaluated whether the size of litters or sex ratio within litters differed among the three groups of juveniles in our study (undisrupted, nonplay-disrupted, play disrupted). These variables did not follow the normal distribution, so we evaluated them with nonparametric Kruskal-Wallis tests. Distances at which juveniles noticed and fled from intruders also did not follow the normal distribution, so we used nonparametric Wilcoxon signed-rank tests to evaluate changes in these distances among individual squirrels across the play interval.
We used linear mixed effects models to evaluate whether rates and durations of play interactions and the distances at which juveniles noticed and fled from intruders during behavioral tests varied among groups of juveniles in our study. In these models, we included the sex of juveniles and treatment groups as fixed effects. We included litter as a random effect to account for possible effects of similarities among juveniles from the same litter. We included body mass as a random effect to account for differences in mass among juveniles. Assumptions of normal distribution of residuals were met in linear mixed effects models. We made pairwise post hoc comparisons of groups (undisrupted, nonplay-disrupted, play-disrupted) with Tukey’s tests, which adjust probabilities for multiple comparisons. We used linear mixed effects models first to verify that 1) tendencies to play during undisturbed observation session and 2) initial distances to notice and flee from an intruder did not differ between the sexes or among groups of juveniles. We then used linear mixed effects models to identify possible differences in 1) play behavior and 2) changes in distances to notice and flee from an intruder between the sexes and among groups of juveniles. To reduce the likelihood of detecting false positives in the latter analysis with linear mixed effects models, we adjusted P values using the Benjamini-Hochberg correction (Benjamini and Hochberg, 1995). We performed statistical tests with SYSTAT 13 (Systat Software, Inc., Chicago, Illinois). We considered relationships indicated by statistical tests to be significant when P ≤ 0.05.
Results
Initial play and cautious responses
We evaluated whether any differences that might influence the outcome of experiments existed between the sexes or among groups of juvenile U. beldingi (undisrupted, nonplay-disrupted, play-disrupted) in our study. There were no differences in litter size (H = 0.72, P = 0.699) or sex ratio within litters (H = 0.58, P = 0.747) among groups of juveniles at first emergence from the natal burrow, suggesting parity among groups with regard to these variables. We observed the behavior of all juveniles in the study during at least one observation session without experimental disruptions. There were no differences between the sexes or among groups in rates of play (Table 2; Figure 1A) or durations of play bouts (Table 2; Figure 2A) during these undisrupted observation sessions, and no interactions between treatment group and sex (Table 2), suggesting no difference in tendencies to play among juveniles in the study. We also evaluated cautious responses among juveniles at first emergence from the natal burrow. We saw no differences between the sexes or among groups and no interaction between treatment group and sex in the distances at which juveniles first noticed an intruder during behavioral tests (Table 2; Figure 3A), or in the distances at which juveniles fled from an intruder (Table 2; Figure 4A), suggesting parity in cautious responses among juveniles at the onset of the study.
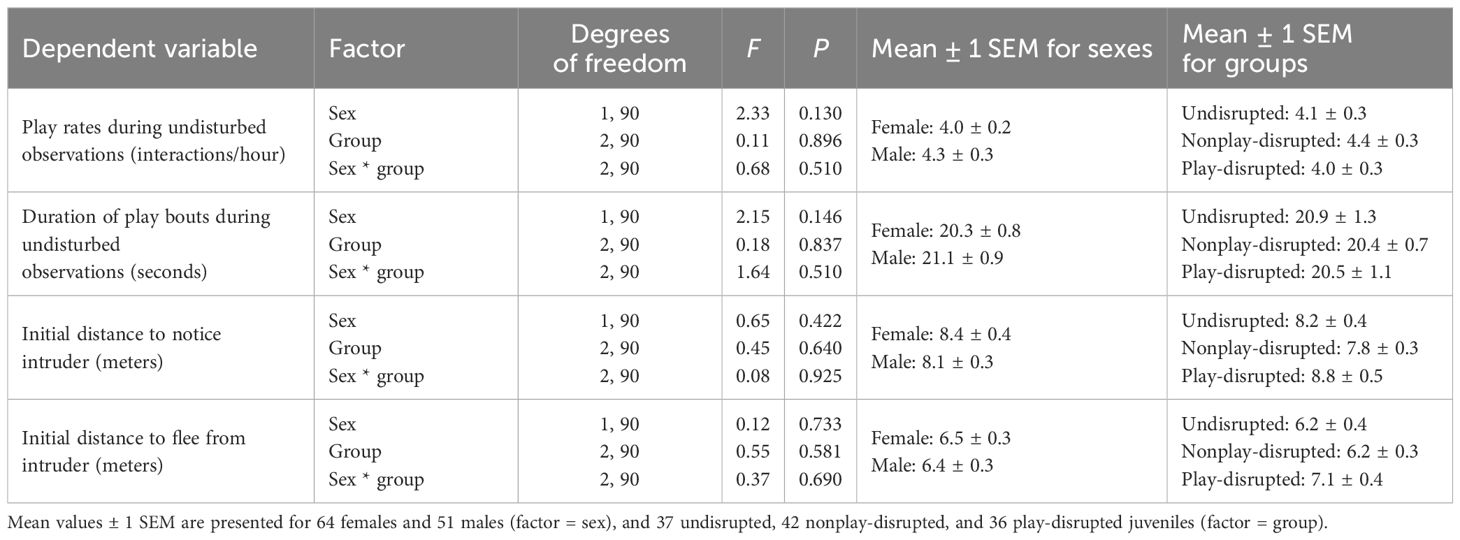
Table 2 Results of analysis with linear mixed effects models assessing possible differences in tendencies to play or initial cautious responses at first emergence from the natal burrow between juvenile female and male U. beldingi and among treatment groups (undisrupted, nonplay-disrupted, play-disrupted) in the study.
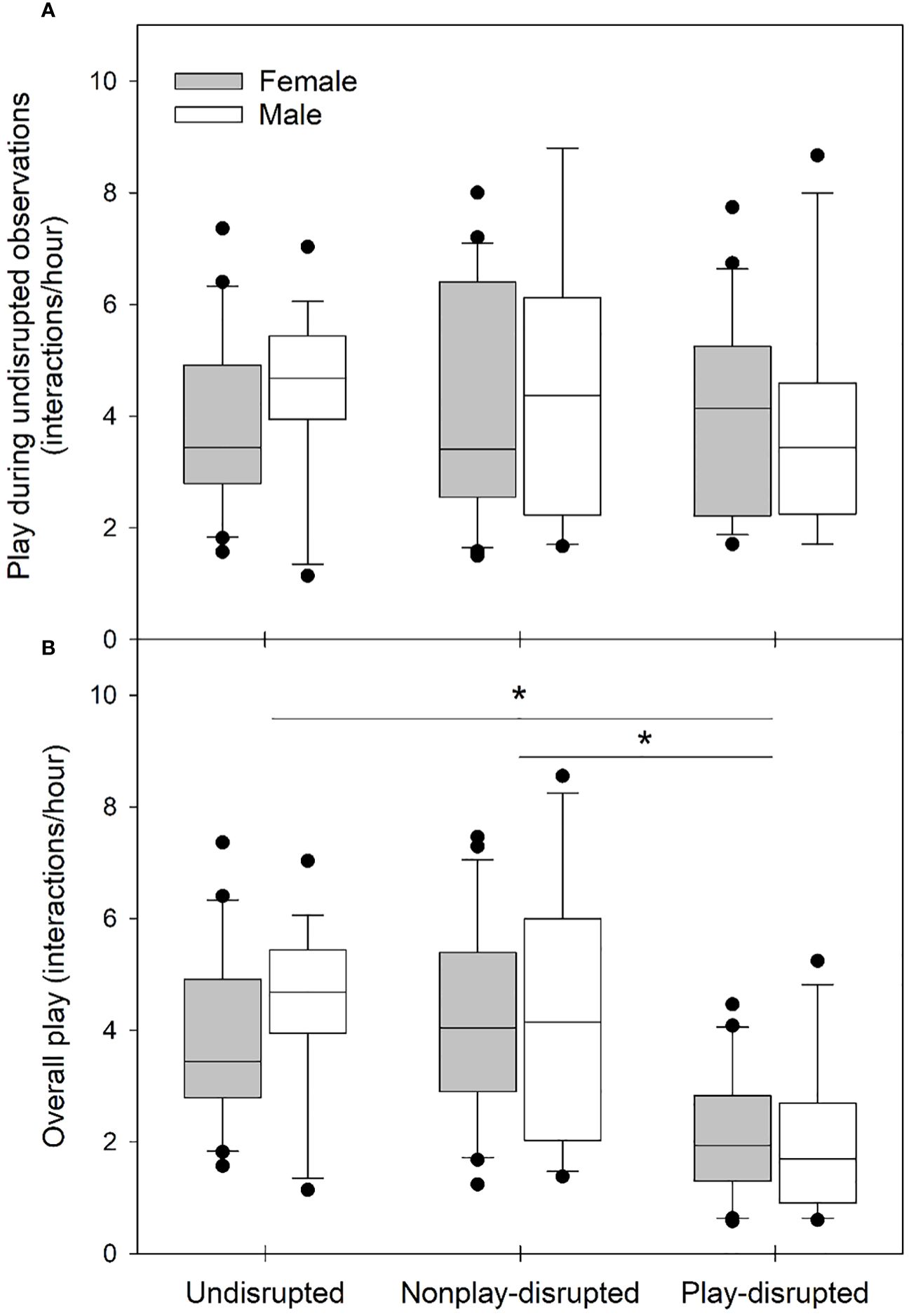
Figure 1 Box and whisker plots showing rates of play among juvenile U. beldingi during (A) observation sessions in which juveniles were not disrupted, and (B) all observations including disrupted and undisrupted sessions. Boxes delimit the 0.25 and 0.75 quantiles, horizontal lines indicate medians, whiskers extend to 1.5 inter-quartile range, and outliers are plotted as individual points. Asterisks indicate significant differences between groups of juveniles at P < 0.05, evaluated with Tukey’s tests. Sample sizes are as follows: undisrupted control—20 female, 17 male; nonplay-disrupted—24 female, 18 male; play-disrupted—20 female, 16 male.
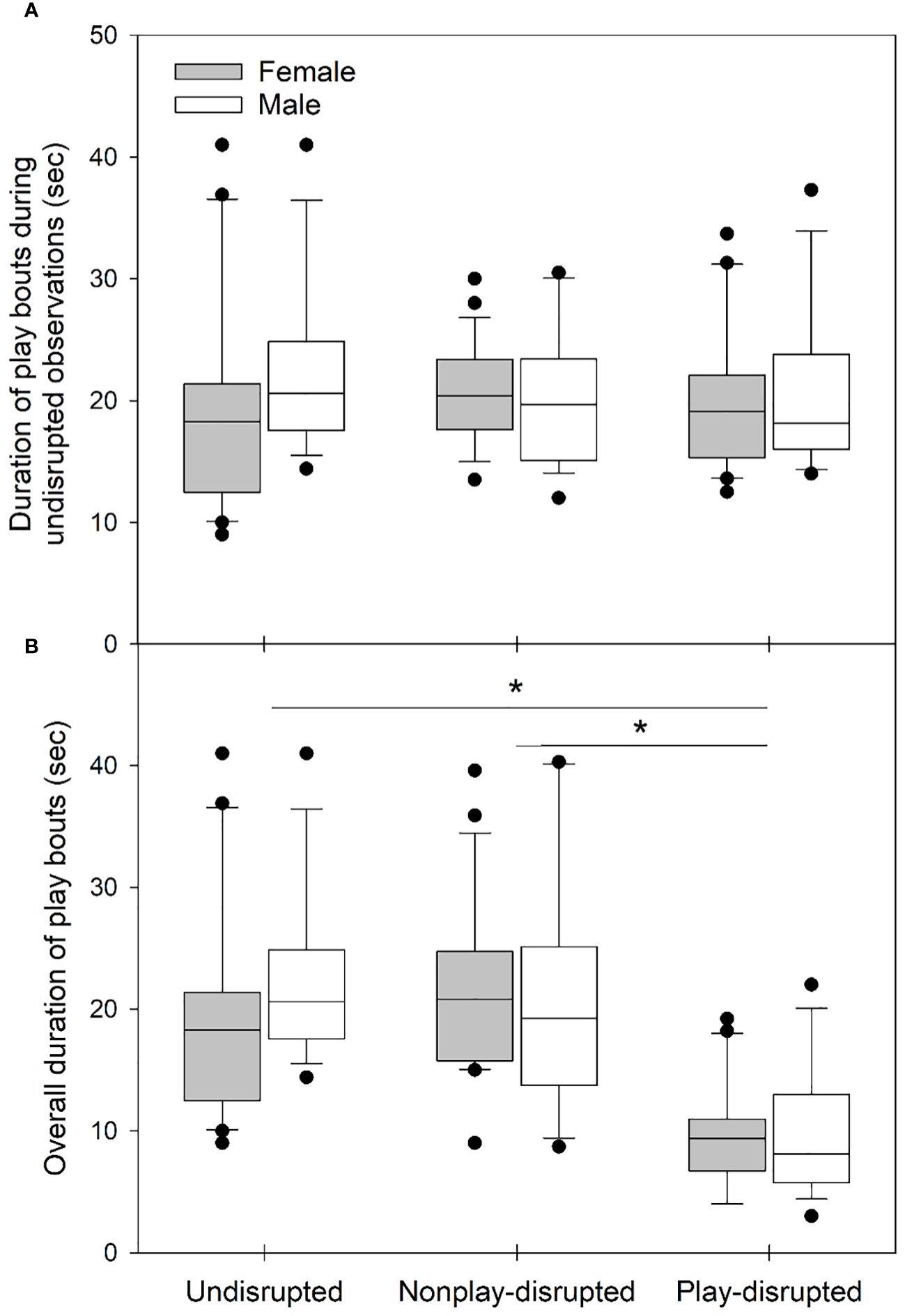
Figure 2 Box and whisker plots showing mean duration of play bouts of juvenile U. beldingi during (A) observation sessions in which juveniles were not disrupted, and (B) all observations including disrupted and undisrupted sessions. Boxes delimit the 0.25 and 0.75 quantiles, horizontal lines indicate medians, whiskers extend to 1.5 inter-quartile range, and outliers are plotted as individual points. Asterisks indicate significant differences between groups of juveniles at P < 0.05, evaluated with Tukey’s tests. Sample sizes are as follows: undisrupted control—20 female, 17 male; nonplay-disrupted—24 female, 18 male; play-disrupted—20 female, 16 male.
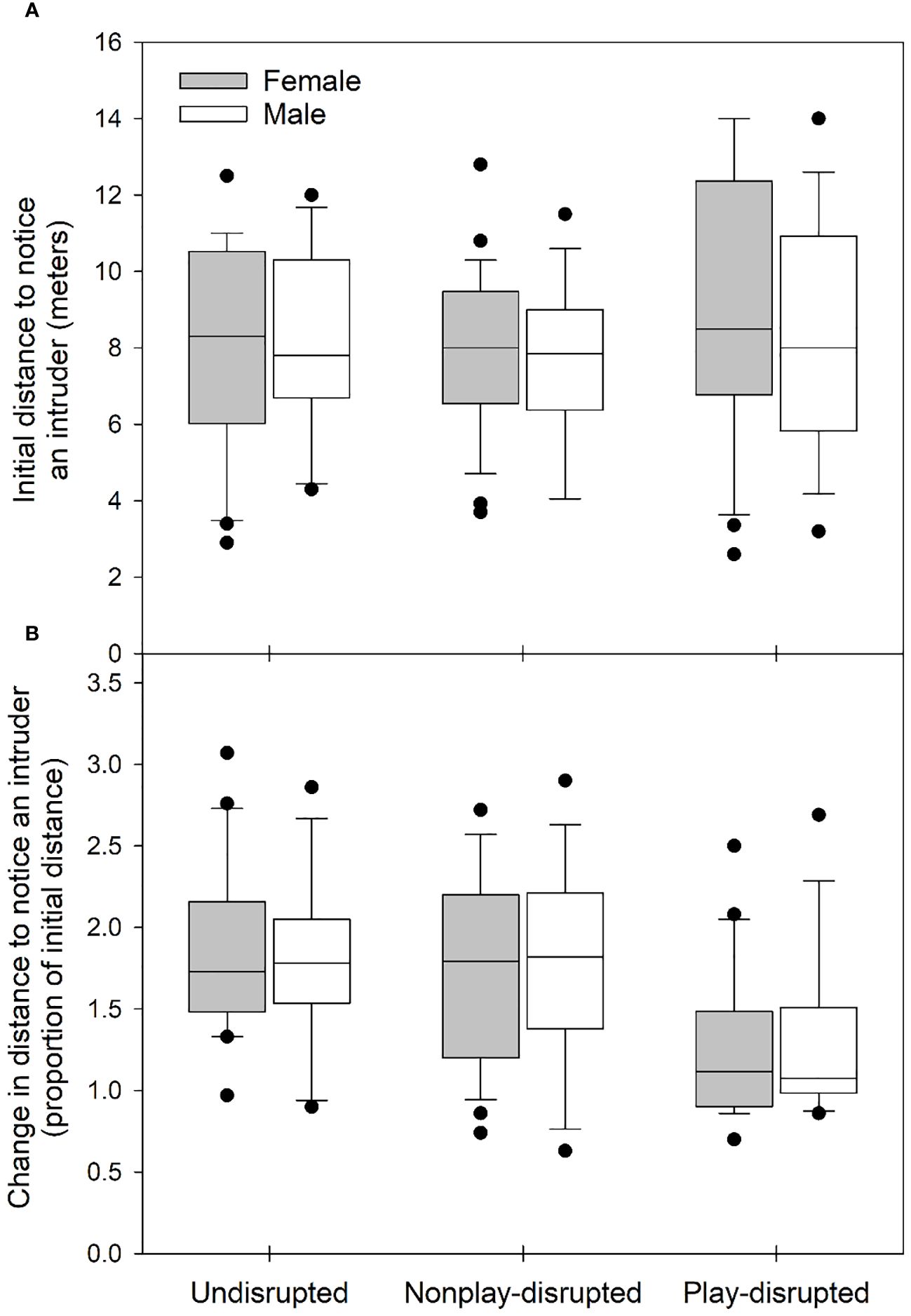
Figure 3 Box and whisker plots showing (A) the distance at which juvenile U. beldingi first noticed an intruder during behavioral tests at first emergence from the natal burrow and (B) changes across the play interval in the distance at which the intruder was noticed, expressed at a proportion of the initial distance (a proportion of 1.0 indicates no change). Boxes delimit the 0.25 and 0.75 quantiles, horizontal lines indicate medians, whiskers extend to 1.5 inter-quartile range, and outliers are plotted as individual points. Sample sizes are as follows: undisrupted control—20 female, 17 male; nonplay-disrupted—24 female, 18 male; play-disrupted—20 female, 16 male.
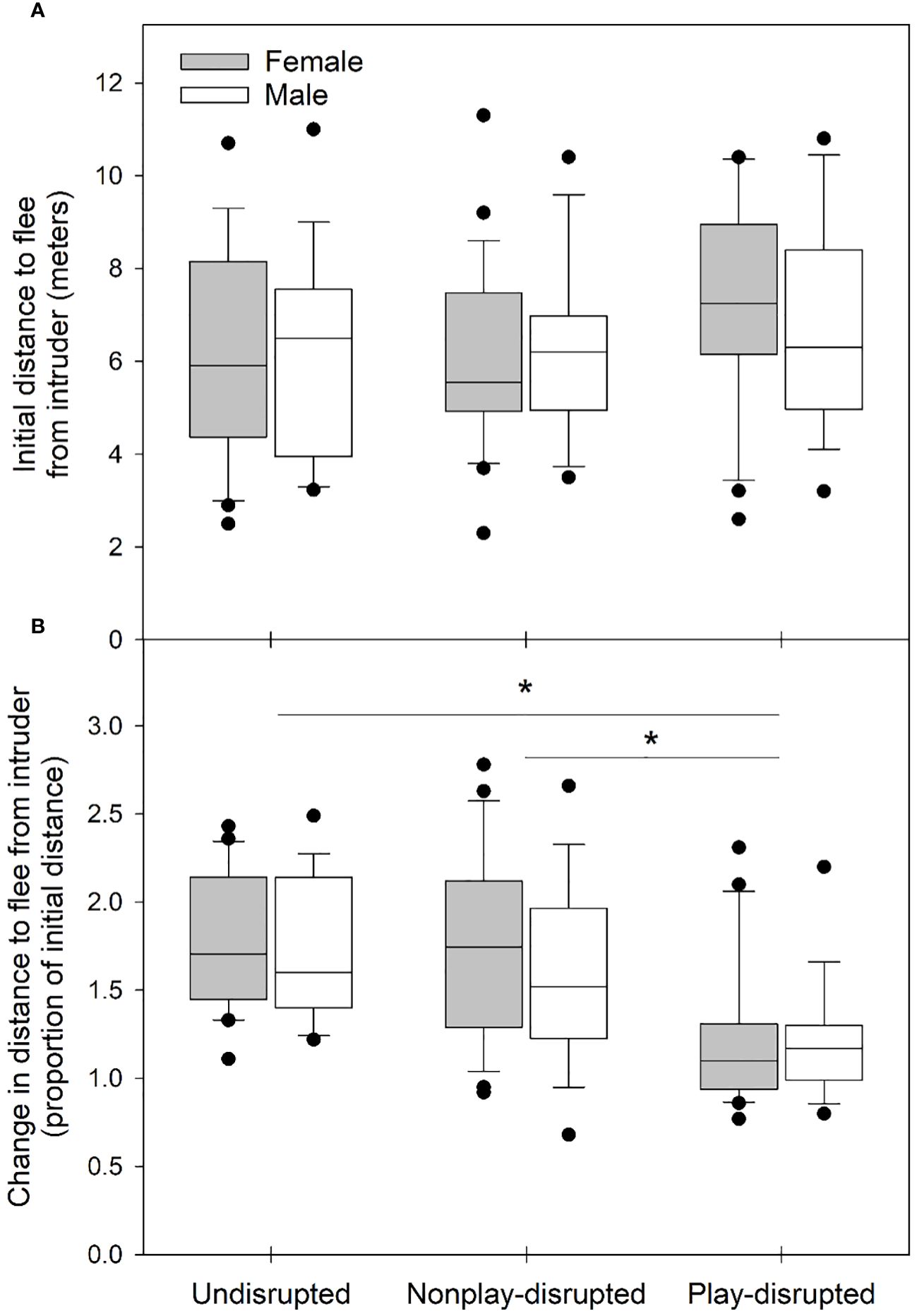
Figure 4 Box and whisker plots showing (A) the distance at which juvenile U. beldingi fled from an intruder during behavioral tests at first emergence from the natal burrow and (B) changes across the play interval in flight distances, expressed at a proportion of the initial distance (a proportion of 1.0 indicates no change). Boxes delimit the 0.25 and 0.75 quantiles, horizontal lines indicate medians, whiskers extend to 1.5 inter-quartile range, and outliers are plotted as individual points. Asterisks indicate significant differences between groups of juveniles at P < 0.05, evaluated with Tukey’s tests. Sample sizes are as follows: undisrupted control—20 female, 17 male; nonplay-disrupted—24 female, 18 male; play-disrupted—20 female, 16 male.
Effects of disruptions on juvenile play
We evaluated rates of play and durations of play bouts during both disrupted and undisrupted observation sessions to verify that disruption of play reduced overall play behavior among juvenile U. beldingi. Rates of play and durations of play bouts did not differ between juvenile male and female U. beldingi, and we saw no interaction between sex and treatment group. However, we observed significant variation in both overall rates of play (Table 3; Figure 1B) and durations of play bouts (Table 3; Figure 2B). In particular, play behavior occurred at lower rates among play-disrupted juveniles compared to undisrupted controls and juveniles whose nonplay behavior was disrupted (Table 3; Figure 1B). The mean duration of play bouts was similarly lower among play-disrupted juveniles compared to undisrupted controls and nonplay-disrupted juveniles (Table 3; Figure 2B).
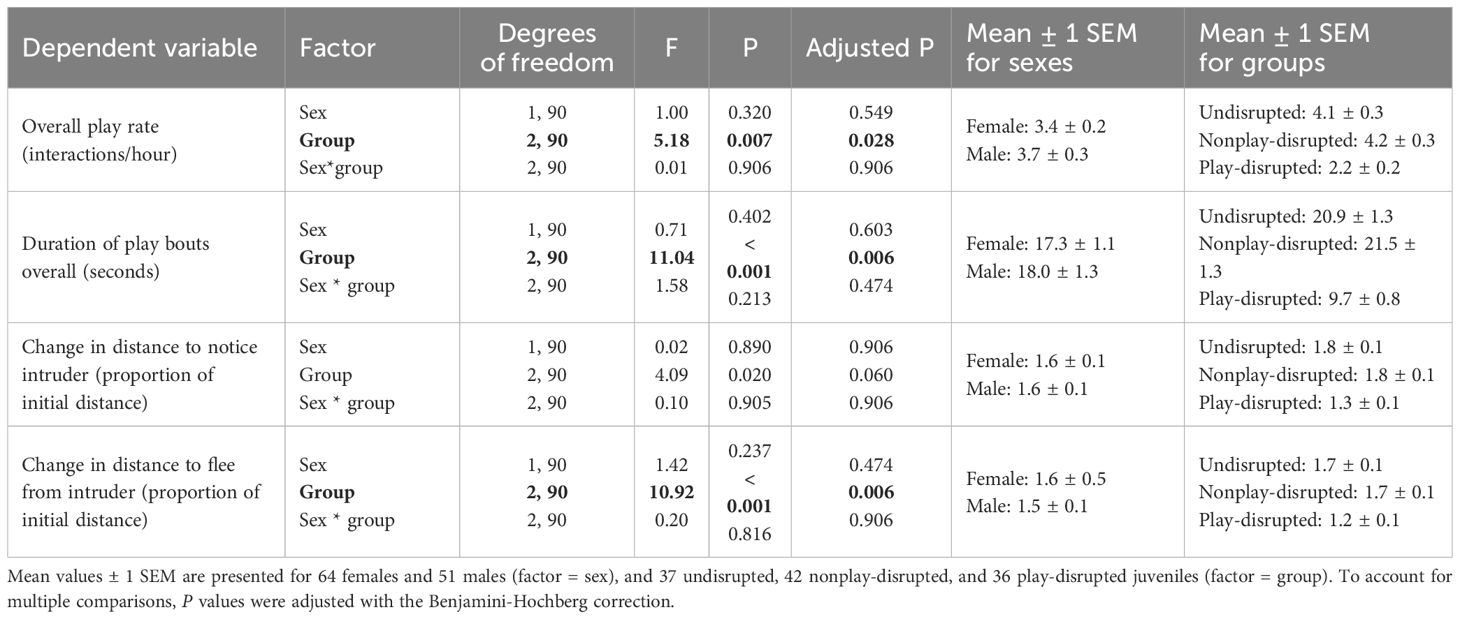
Table 3 Results of analysis with linear mixed effects models evaluating effects of sex (male, female) and treatment group (undisrupted, nonplay-disrupted, play-disrupted) on the play and cautious behavior of juvenile U. beldingi.
Effects of disruptions on cautious responses
We evaluated the effects of disrupting play behavior on cautious responses in juvenile U. beldingi. The distances at which juveniles first noticed an intruder during behavioral tests increased significantly across the play interval (Z = 8.2, P < 0.001). Changes in these distances, expressed as a proportion of the initial distance, did not differ between the sexes, and there was no interaction between sex and treatment group (Table 3; Figure 3B). We note, however, that there was a trend approaching significance for changes in distances to notice an intruder to vary among groups of juveniles, with increases in these distances tending to be smaller among play-disrupted juveniles than among undisrupted controls or nonplay-disrupted juveniles (Table 3; Figure 3B).
Distances at which juveniles fled from an intruder during behavioral tests similarly increased significantly across the play interval (Z = 8.6, P < 0.001). Changes in theses distances, expressed as a proportion of the initial distance, did not differ significantly between juvenile males and females, and there was no interaction between sex and treatment group (Table 3; Figure 4B). However, changes in distances to flee from an intruder varied significantly among groups, and in particular were significantly smaller among play-disrupted juveniles than among undisrupted controls and nonplay-disrupted juveniles (Table 3; Figure 4B).
Discussion
The distances at which juvenile U. beldingi in the study noticed and fled from intruders during behavioral tests increased across the primary play period. Increases in distances to flee from an intruder were significantly greater in juveniles whose play behavior was not disrupted compared to play-disrupted juveniles. We further observed a trend approaching significance for increases in distances to first notice an intruder to be greater in juveniles whose play behavior was not disrupted. These results are consistent with social play behavior in juvenile U. beldingi contributing to development of cautious responses. Shehan et al. (2023) similarly observed that higher rates of social play in juvenile U. beldingi were correlated with greater increases in cautious responses across the play interval. Together with the results of Shehan et al. (2023), our findings here provide strong support for the hypothesis that juvenile social play in U. beldingi influences the development of temperament along the caution-boldness continuum, promoting shifts toward more cautious behavior in response to a potential threat.
Marks et al. (2017) observed that juvenile U. beldingi that engaged in social play at higher rates tended to have greater increases in exploratory behavior in a novel environment. Hurst-Hopf et al. (2023) further observed that greater social play among juvenile U. beldingi was associated with greater shifts from passive to active behavioral responses across the play interval. Thus, temperament appears to be plastic in U. beldingi during the juvenile period, and experiences associated with social play may promote refinement of various temperament traits. These refinements might better equip young squirrels to express adaptive responses across a diverse array of situations they may encounter as they interact with the environment. For example, as young squirrels begin to venture farther from the natal area, they may shift to a more proactive coping style accompanied by greater caution to better equip them to explore unfamiliar surroundings and interact with unfamiliar conspecifics.
A range of variables are associated with development of temperament in young animals (Cabrera et al., 2021). For example, in European rabbits (Oryctolagus cuniculus) greater body mass among juvenile males is associated with greater aggressiveness in agonistic interactions, as well as greater exploration in an unfamiliar environment (Rödel and von Hulst, 2009; Rödel and Monclús, 2011). Moreover, maternal effects on the development of temperament and other variables have been suggested to prepare offspring to be successful under prevailing environmental conditions (Storm and Lima, 2010; Kapheim et al., 2011; Dantzer et al., 2013). Environmental stresses experienced by mothers can directly affect the way they raise their young, which in turn can affect the behavior and other characteristics of offspring (Mousseau and Fox, 1998; Weinstock, 2001; Hayward and Wingfield, 2004). For example, environmental stressors and prior experience raising young have been observed to influence glucocorticoid concentrations in mothers, and glucocorticoids passed to offspring through milk during lactation can influence development of behavior and elements of temperament such as boldness and docility (Hinde et al., 2015; Petelle et al., 2017).
Possible influences of social play on the development of temperament in U. beldingi might be mediated by effects of play on the developing brain. Pellis et al. (2023) noted that juvenile play behavior in rats promotes increased behavioral skill and social competency during adulthood, and suggested that a general benefit of play may be to refine or improve skills and behaviors that can be applied adaptively across a variety of social and non-social contexts. Various aspects of brain development exhibit plasticity during neonatal and juvenile periods, and a range of experiences after birth can direct elements of neural and behavioral development (Johnson, 2001; Stiles and Jernigan, 2010; Kolb and Gibb, 2011; Sakai and Sugiyama, 2018). Various brain regions help to modulate the expression of play behavior in mammals (Pellis and Iwaniuk, 2004; Siviy, 2016; Achterberg and Vanderschuren, 2023). Moreover, juvenile play in rats and hamsters (Mesocricetus auratus) has been shown to modify development of areas in the frontal cortex associated with motor and social behavior as well as behavioral flexibility. These modifications have been suggested to facilitate more nuanced neural responses and more adaptable behavior later in life as well as context-appropriate behavioral responses (Bell et al., 2010; Pellis et al., 2010; Himmler et al., 2013; Burleson et al., 2016; Himmler et al., 2017; Bijlsma et al., 2022; Cooper et al., 2023; Pellis et al., 2023; Bijlsma et al., 2024).
Possible effects of play on the development of temperament might also be mediated in some capacity by play-related modulation of glucocorticoid secretion. Social play behavior in dogs is associated with decreased concentrations of salivary cortisol and increased performance on learning-related memory tests (Horváth et al., 2008; Affenzeller et al., 2017). Moreover, glucocorticoids early in life can have important effects on the course of brain development (Gunnar and Barr, 1998; Davis et al., 2013). Thus, one possibility is that social play alters glucocorticoid secretion in ways that influence behavioral learning or brain development during the juvenile period, resulting in refinement in the development of temperament.
Experimental disruption of play in this study decreased the rates of play and duration of play bouts among juvenile U. beldingi. Thus, the effects of play that we observed on the cautious responses of young squirrels might have been related to the amount of play in which squirrels engaged. Various studies have suggested that reduced play can influence development of the brain in young animals and have long-term consequences on the expression of behavior (e.g., Stark and Pellis, 2020; Stark et al., 2021; Bijlsma et al., 2023; Pellis et al., 2023). Leca (2023) and Pellis et al. (2024) noted that the structure of play behavior is related to its adaptive functions, and Bijlsma et al. (2024) observed that the environment in which play occurs can affect the way in which it influences development of the brain and behavior. Thus, results in our study might also be related to the disruptions that occurred while juveniles were engaged in play. Disruption of play behavior under natural conditions might provide information about the density of conspecifics or predators, and might alter play in ways that promote development of coping styles that are adaptive under prevailing environmental conditions. For example, disruption of play interactions might have shaped the ways in which juvenile U. beldingi in our study assessed their play partners or surroundings, or gauged potential risk or danger during play interactions. Ecological conditions can influence temperament in young animals (Sinn et al., 2008; DiRienzo et al., 2018; Cabrera et al., 2021). In yellow-bellied marmots, for example, environmental conditions influence the glucocorticoid content of milk in mothers, which in turn influences the temperament of their offspring (Petelle et al., 2017). We note that currently little is known about how the specific structure of play behavior or context in which play occurs might influence the development of behavior in free-living animals.
Human disturbance can influence the behavior of animals. For example, elk (Cervus elaphus) have been observed to exhibit greater vigilance at the cost of foraging in response to human activity (Ciuti et al., 2012). Similarly, in yellow-eyed penguins increased vigilance at the cost of preening is associated with human activity (French et al., 2019). Moreover, increased aggressive behavior has been observed in little penguins (Eudyptula minor) living on islands that have greater human activity (Colombelli-Négrel and Katsis, 2021), and increased avoidance behavior associated with human activity has been observed in zoo populations of E. minor (Sherwen et al., 2015). In this study, disruption of juvenile U. beldingi when they were not engaged in play interactions did not influence their cautious responses relative to undisrupted juveniles. We note that disruption of the nonplay behavior of juvenile U. beldingi during observation sessions in our study was relatively infrequent and low intensity, suggesting that low-levels of human activity may not have a notable impact on development of cautious responses in juvenile U. beldingi.
Field studies of play behavior allow for evaluation of play in the context in which it evolved and under conditions in which it is naturally expressed, but tend to be observational or correlational (Palagi, 2018; Nunes, 2023). Here we conducted a simple manipulation of play behavior in the field that did not appear to be overly disruptive for the squirrels. Our results suggest that play behavior in young U. beldingi contributes to development of caution across the play interval. Play in young U. beldingi promotes development of motor skill and coordination (Nunes et al., 2004a; Nunes et al., 2004b), and our results further suggest that play behavior might also promote or refine development of nonplay elements of behavior.
Data availability statement
The dataset evaluated in this study is available through Zenodo: https://doi.org/10.5281/zenodo.10667044.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the University of San Francisco does not have an Animal Care and Use Committee and does not require review of research using animals. We followed guidelines for the use of mammals in field studies published by the American Society of Mammalogists.
Author contributions
WR: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. MK: Data curation, Investigation, Writing – original draft, Writing – review & editing. SN: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided to SN by the Faculty Development Fund at the University of San Francisco.
Acknowledgments
We thank Matt Greer for his excellent assistance with fieldwork. We thank Naupaka Zimmerman and Tracy Benning for insightful comments throughout the study. We thank the reviewers for their perceptive suggestions for interpreting the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achterberg E. J. M., Vanderschuren L. J. M. J. (2023). The neurobiology of social play behavior: Past, present and future. Neurosci. Biobehav. Rev. 152, 105319. doi: 10.1016/j.neubiorev.2023.105319
Affenzeller N., Palme R., Zulch H. (2017). Playful activity post-learning improves training performance in Labrador Retriever dogs (Canis lupus familiaris). Physiol. Behav. 168, 62–73. doi: 10.1016/j.physbeh.2016.10.014
Ahloy Dallaire J., Mason G. M. (2017). Rough-and-tumble play predicts adult sexual behavior in American mink. Anim. Behav. 123, 81–89. doi: 10.1016/j.anbehav.2016.10.023
Baugh A. T., Van Oers K., Naguib M., Hau M. (2013). Initial reactivity and magnitude of the acute stress response associated with personality in wild great tits (Parus major). Gen. Comp. Endocrinol. 189, 96–104. doi: 10.1016/j.ygcen.2013.04.030
Bekoff M. (1974). Social play and play-soliciting by infant canids. Amer. Zool. 14, 323–340. doi: 10.1093/icb/14.1.323
Bekoff M., Byers J. A. (Eds.) (1998). Animal play: evolutionary, comparative, and ecological processes (Cambridge, United Kingdom: Cambridge University Press).
Bell H. C., Pellis S. M., Kolb B. (2010). Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav. Brain Res. 2017, 7–13. doi: 10.1016/j.bbr.2009.09.029
Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Berghänel A., Schülke O., Ostner J. (2015). Locomotor play drives motor skill acquisition at the expense of growth: A life history trade-off. Sci. Adv. 1, e1500451. doi: 10.1126/sciadv.1500451
Bijlsma A., Birza E. E., Pimentel T. C., Maranus J. P. M., van Gaans M. J. J. M., Lozeman-van t Klooster J. G., et al. (2024). Opportunities for risk-taking alters cognitive performance and prefrontal inhibitory signaling in rats of both sexes. Eur. J. Neurosci. 2024, 1–18. doi: 10.1111/ejn.16313
Bijlsma A., Omrani A., Spoelder M., Verharen J. P. H., Bauer L., Cornelis C., et al. (2022). Social play behavior is critical for the development of prefrontal inhibitory synapses and cognitive flexibility in rats. J. Neurosci. 42, 8716–8728. doi: 10.1523/JNEUROSCI.0524-22.2022
Bijlsma A., Vanderschuren L. J. M. J., Wierenga C. J. (2023). Social play behavior shapes the development of prefrontal inhibition in a region-specific manner. Cereb. Cortex 33, 9399–9408. doi: 10.1093/cercor/bhad212
Blumstein D. T. (2003). Flight-initiation distance in birds is dependent on intruder starting distance. J. Wildl. Manage. 67, 852–857. doi: 10.2307/3802692
Blumstein D. T., Chung L. K., Smith J. E. (2013). Early play may predict later dominance relationships in yellow-bellied marmots (Marmota flaviventris). Proc. R. Soc B 280, 1–7. doi: 10.1098/rspb.2013.0485
Boon A. K., Réale D., Boutin S. (2008). Personality, habitat use, and their consequences for survival in North American red squirrels Tamiasciurus hudsonicus. Oikos 117, 1321–1328. doi: 10.1111/j.0030-1299.2008.16567.x
Both C., Dingemanse N. J., Drent P. J., Tinbergen J. M. (2005). Pairs of extreme avian personalities have highest reproductive success. J. Anim. Ecol. 74, 667–674. doi: 10.1111/j.1365-2656.2005.00962.x
Burghardt G. M. (2005). The genesis of animal play: testing the limits (Cambridge, Massachusetts: MIT Press). doi: 10.7551/mitpress/3229.001.0001
Burleson C. A., Pedersen R. W., Seddighi S., DeBusk L. E., Burghardt G. M., Cooper M. A. (2016). Social play in juvenile hamsters alters dendritic morphology in the medial prefrontal cortex and attenuates effects of social stress in adulthood. Behav. Neurosci. 130, 437–447. doi: 10.1037/bne0000148
Cabrera D., Nilsson J. R., Griffen B. D. (2021). The development of animal personality across ontogeny: a cross-species review. Anim. Behav. 173, 137–144. doi: 10.1016/j.anbehav.2021.01.003
Carere C., Welink D., Drent P. J., Koolhass J. M., Groothuis T. G. G. (2001). Effect of social defeat in a territorial bird (Parus major) selected for different coping styles. Physiol. Behav. 73, 427–433. doi: 10.1016/S0031-9384(01)00492-9
Carter R. N., Romanow C. A., Pellis S. M., Lingle S. (2019). Play for prey: do deer fawns play to develop species-typical tactics or to prepare for the unexpected. Anim. Behav. 156, 31–40. doi: 10.1016/j.anbehav.2019.06.032
Ciuti S., Northrup J. M., Muhly T. B., Simi S., Musiani M., Pitt J. A., et al. (2012). Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PloS One 7, e50611. doi: 10.1371/journal.pone.0050611
Colchester C., Harrison N. M. (2016). Personality in blue tits (Cyanistes caeruleus) and its effect on their breeding success. Ethology 122, 695–701. doi: 10.1111/eth.12516
Colombelli-Négrel D., Katsis A. C. (2021). Little penguins are more aggressive on islands that experience greater unregulated human disturbance. Anim. Behav. 182, 195–202. doi: 10.1016/j.anbehav.2021.10.012
Cooper W. E. Jr. (2009). Variation in escape behavior among individuals of the striped plateau lizard Sceloporous virgatus may reflect differences in boldness. J. Herpetol. 43, 495–502. doi: 10.1670/08-197R1.1
Cooper M. A., Grizzell J. A., Whitten C. J., Burghardt G. M. (2023). Comparing the ontogeny, neurobiology, and function of social play in hamsters and rats. Neurosci. Biobehav. Rev. 147, 105102. doi: 10.1016/j.neubiorev.2023.105102
Coppens C. M., de Boer S. F., Koolhaas J. M. (2010). Coping styles and behavioral flexibility: towards underlying mechanisms. Phil. Trans. R. Soc B 365, 4021–4028. doi: 10.1098/rstb.2010.0217
Dantzer B., Newman A. E. M., Boonstra R., Palme R., Boutin S., Humphries M. M., et al. (2013). Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science 340, 1215–1217. doi: 10.1126/science.1235765
Davis E. P., Sandman C. A., Buss C., Wing D. A., Head K. (2013). Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol. Psychiatry 74, 647–655. doi: 10.1016/j.biopsych.2013.03.009
Dingemanse N. J., Both C., Drent P. J., Tinbergen J. M. (2004). Fitness consequences of avian personalities in a fluctuating environment. Proc. R. Soc Lond. B 271, 847–852. doi: 10.1098/rspb.2004.2680
DiRienzo N., Johnson J. C., Dornhaus A. (2018). Juvenile social experience generates differences in behavioral variation but not averages. Behav. Ecol. 30, 455–464. doi: 10.1093/beheco/ary185
Duckworth R. A., Chenard K. C., Meza L., Beiriz M. C. (2023). Coping styles vary with species’ sociality and life history: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 151, 105241. doi: 10.1016/j.neubiorev.2023.105241
Eisenberg N. (2012). Gauging what we know and don’t know about temperament. Hum. Dev. 55, 30–34. doi: 10.1159/000336042
Fagen R., Fagen J. (2004). Juvenile survival and benefits of play behaviour in brown bears, Ursus arctos. Evol. Ecol. Res. 6, 89–102.
French R. K., Muller C. G., Chilvers B. L., Battley P. F. (2019). Behavioural consequences of human disturbance on subantarctic Yellow-eyed Penguins Megadyptes antipodes. Bird Conserv. Int. 29, 277–290. doi: 10.1017/S0959270918000096
Gallo A., Caselli M., Norscia I., Palagi E. (2021). Let’s unite in play! Play modality and group membership in wild geladas. Behav. Processes 184, 104338. doi: 10.1016/j.beproc.2021.104338
Gunnar M. R., Barr R. G. (1998). Stress, early brain development, and behavior. Infants Young Child. 11, 1–14. doi: 10.1097/00001163-199807000-00004
Hayward L. S., Wingfield J. C. (2004). Maternal corticosterone is transferred to yolk and may alter offspring growth and phenotype. Gen. Comp. Endocrinol. 135, 365–371. doi: 10.1016/j.ygcen.2003.11.002
Himmler S. M., Himmler B. T., Pellis V. C., Pellis S. M. (2016). Play, variation in play and the development of socially competent rats. Behaviour 153, 1103–1137. doi: 10.1163/1568539X-00003307
Himmler B. T., Mychasiuk R., Nakahashi A., Himmler S. M., Pellis S. M., Kolb B. (2017). Juvenile social experience and differential age-related changes in the dendritic morphologies of subareas of the prefrontal cortex in rats. Synapse 72, e22022. doi: 10.1002/syn.22022
Himmler B. T., Pellis S. M., Kolb B. (2013). Juvenile play experience primes neurons in the medial prefrontal cortex to be more responsive to later experiences. Neurosci. Lett. 556, 42–45. doi: 10.1016/j.neulet.2013.09.061
Hinde K., Skibiel A. L., Foster A. B., Del Rosso L., Mendoza S. P., Capitanio J. P. (2015). Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav. Ecol. 26, 269–281. doi: 10.1093/beheco/aru186
Holekamp K. E., Smale L., Simpson H. B., Holekamp N. A. (1984). Hormonal influences on natal dispersal in free-living Belding’s ground squirrels (Spermophilus beldingi). Horm. Behav. 18, 465–483. doi: 10.1016/0018-506X(84)90031-X
Holmes W. G. (1994). The development of littermate preferences in juvenile Belding’s ground squirrels. Anim. Behav. 48, 1071–1084. doi: 10.1006/anbe.1994.1341
Horváth Z., Dóka A., Miklósi Á. (2008). Affiliative and disciplinary behavior of human handlers during play with their dog affects cortisol concentrations in opposite directions. Horm. Behav. 54, 107–114. doi: 10.1016/j.yhbeh.2008.02.002
Hrdy S. B. (1979). Infanticide among animals: A review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1, 13–40. doi: 10.1016/0162-3095(79)90004-9
Hurst-Hopf J. S., Monroy Montemayor M. P., Leonardi N. N., Nunes S. (2023). Juvenile social play predicts docility in Belding’s ground squirrels. Behav. Ecol. Sociobiol. 77, 62. doi: 10.1007/s00265-023-03341-7
Jenkins S. H., Eshelman B. D. (1984). Spermophilus beldingi . Mamm. Species 221, 1–8. doi: 10.2307/3503911
Johnson M. H. (2001). Functional brain development in humans. Nat. Rev. Neurosci. 2, 475–483. doi: 10.1038/35081509
Kapheim K. M., Bernal S. P., Smith A. R., Nonacs P., Wcislo W. T. (2011). Support for maternal manipulation of developmental nutrition in a facultatively eusocial bee, Megalopta genalis (Halictidae). Behav. Ecol. Sociobiol. 65, 1179–1190. doi: 10.1007/s00265-010-1131-9
Kolb B., Gibb R. (2011). Brain plasticity and behavior in the developing brain. J. Can. Acad. Child Adolesc. Psychiatry 20, 256–276. doi: 10.1016/B978–0-444–63327-9.00005–9
Koolhaas J. M., Korte S. M., de Boer S. F., van der Vegt B. J., van Reenen C. G., Hopster H., et al. (1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. doi: 10.1016/S0149-7634(99)00026-3
Kraus K. L., Pellis V., Pellsi S. M. (2019). Targets, tactics, and cooperation in the play fighting of two genera of old world monkeys (Mandrillus and Papio): accounting for similarities and differences. Int. J. Comp. Psychol. 32, 1–31. doi: 10.46867/ijcp.2019.32.00.10
Leca J.-B. (2023). Towards a three-level neo-Tinbergenian approach to object play: Structure, causes, and consequences of a behavioral puzzle. Neurosci. Biobehav. Rev. 152, 105290. doi: 10.1016/j.neubiorev.2023.105290
Lee P. C., Moss C. J. (2014). African elephant play, competence, and social complexity. Anim. Behav. Cogn. 1, 144–156. doi: 10.12966/abc.05.05.2014
Lima S. L., Dill L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Little K. (2022). Climate-related declines in reproductive output in a high-elevation population of ground squirrels. B.S. thesis, University of San Francisco, San Francisco, USA. Available at: https://repository.usfca.edu/honors/42.
Marks K. A., Vizconde D. L., Gibson E. S., Rodriguez J. R., Nunes S. (2017). Play behavior and responses to novel situations in juvenile ground squirrels. J. Mammal. 98, 1202–1210. doi: 10.1093/jmammal/gyx049
Meyer S., Weber J.-M. (1996). Ontogeny of dominance in free-living red foxes. Ethology 102, 1008–1019. doi: 10.1111/j.1439-0310.1996.tb01178.x
Michener G. R. (1983). “Kin identification, matriarchies, and the evolution of sociality in ground-dwelling sciurids,” in Advances in the study of mammalian behavior. Eds. Eisenberg J. F., Kleiman D. G. (Stillwater, Oklahoma, USA: American Society of Mammalogists Special Publication No. 7), 528–572.
Mousseau T. A., Fox C. W. (1998). The adaptive significance of maternal effects. Trends. Ecol. Evol. 13, 403–407. doi: 10.1016/S0169-5347(98)01472-4
Nolfo A. P., Casetta G., Palagi E. (2021). Play fighting in wild spotted hyenas: like a bridge over the troubled water of a hierarchical society. Anim. Behav. 180, 363–373. doi: 10.1016/j.anbehav.2021.07.012
Nunes S. (2014). Juvenile social play and yearling behavior and reproductive success in female Belding’s ground squirrels. J. Ethol. 32, 145–153. doi: 10.1007/s10164-014-0403-7
Nunes S. (2023). Animal-friendly behavioral testing in field studies: examples from ground squirrels. Front. Behav. Neurosci. 17. doi: 10.3389/fnbeh.2023.1239774
Nunes S., Monroy Montemayor M. P. (2023). Multiple benefits of juvenile play: A ground squirrel’s perspective. Neurosci. Biobehav. Rev. 147, 105099. doi: 10.1016/j.neubiorev.2023.105099
Nunes S., Muecke E.-M., Anthony J. A., Batterbee A. S. (1999). Endocrine and energetic mediation of play behavior in free-living ground squirrels. Horm. Behav. 36, 153–165. doi: 10.1006/hbeh.1999.1538
Nunes S., Muecke E.-M., Lancaster L. T., Miller N. A., Mueller M. A., Muelhaus J., et al. (2004a). Functions and consequences of play behaviour in juvenile Belding’s ground squirrels. Anim. Behav. 68, 27–37. doi: 10.1016/j.anbehav.2003.06.024
Nunes S., Muecke E.-M., Sanchez Z., Hoffmeier R. R., Lancaster L. T. (2004b). Play behavior and motor development in juvenile Belding’s ground squirrels (Spermophilus beldingi). Behav. Ecol. Sociobiol. 56, 97–105. doi: 10.1007/s00265-004-0765-x
Nunes S., Weidenbach J. N., Lafler M. R., Dever J. A. (2015). Sibling relatedness and social play in juvenile ground squirrels. Behav. Ecol. Sociobiol. 69, 357–369. doi: 10.1007/s00265-014-1848-y
Palagi E. (2018). Not just for fun! Social play as a springboard for adult social competence in human and non-human primates. Behav. Ecol. Sociobiol. 72, 90. doi: 10.1007/s00265-018-2506-6
Palagi E. (2023). Adult play and the evolution of tolerant and cooperative societies. Neurosci. Biobehav. Rev. 148, 105124. doi: 10.1016/j.neubiorev.2023.105124
Pellis S. M., Iwaniuk A. N. (2004). Evolving a playful brain: a levels of control approach. Int. J. Comp. Psych. 17, 90–118. doi: 10.46867/IJCP.2004.17.01.01
Pellis S. M., Pellis V. C. (2017). What is play fighting and what is it good for? Learn. Behav. 45, 355–366. doi: 10.3758/s13420–017-0264–3
Pellis S. M., Pellis V. C., Bell H. C. (2010). The function of play in the development of the social brain. Am. J. Play 2, 278–296.
Pellis S. M., Pellis V. C., Ham J. R. (2024). Play fighting revisited: its design features and how they shape our understanding of its mechanisms and functions. Front. Ethol. 3, 1–13. doi: 10.3389/fetho.2024.1362052
Pellis S. M., Pellis V. C., Ham J. R., Stark R. A. (2023). Play fighting and the development of the social brain: The rat’s tale. Neurosci. Biobehav. Rev. 145, 105037. doi: 10.1016/j.neubiorev.2023.105037
Pellis S. M., Pellis V. C., Himmler B. T. (2014). How play makes for a more adaptable brain: A comparative and neural perspective. Am. J. Play 7, 73–98.
Pellis S. M., Williams L. A., Pellis V. C. (2017). Adult-juvenile play fighting in rats: insight into the experiences that facilitate the development of socio-cognitive skills. Int. J. Comp. Psychol. 30, 1–13. doi: 10.46867/ijcp.2017.30.00.14
Petelle M. B., Dang B. N., Blumstein D. T. (2017). The effect of maternal glucocorticoid levels on juvenile docility in yellow-bellied marmots. Horm. Behav. 89, 86–91. doi: 10.1016/j.yhbeh.2016.12.014
Petelle M. B., McCoy D. E., Alejandro V., Martin J. G. A., Blumstein D. T. (2013). Development of boldness and docility in yellow-bellied marmots. Anim. Behav. 86, 1147–1154. doi: 10.1016/j.anbehav.2013.09.016
Rödel H. G., Monclús R. (2011). Long-term consequences of early development on personality traits: a study in European rabbits. Behav. Ecol. 22, 1123–1130. doi: 10.1093/beheco/arr100
Rödel H. G., von Hulst D. (2009). Features of the early juvenile development predict competitive performance in male European rabbits. Physiol. Behav. 97, 495–502. doi: 10.1016/j.physbeh.2009.04.005
Rasmussen J. E., Belk M. C. (2017). Predation environment affects boldness temperament of neotropical livebearers. Ecol. Evol. 7, 3059–3066. doi: 10.1002/ece3.2886
Réale D., Dingemanse N. J., Kazem A. J. N., Wright J. (2010). Evolutionary and ecological approaches to the study of personality. Philos. Trans. R. Soc 365, 3937–3946. doi: 10.1098/rstb.2010.0222
Réale D., Reader S. M., Sol D., McDougall P. T., Dingemanse N. J. (2007). Integrating animal temperament within ecology and evolution. Biol. Rev. 82, 291–318. doi: 10.1111/j.1469-185X.2007.00010.x
Rho J. R., Srygley R. B., Choe J. C. (2007). Sex preferences in Jeju pony foals (Equus caballus) for mutual grooming and play-fighting behaviors. Zool. Sci. 24, 769–773. doi: 10.2108/zsj.24.769
Rothbart M. K., Ahadi S. A. (1994). Temperament and the development of personality. J. Abnorm. Psychol. 103, 55–66. doi: 10.1037/0021-843X.103.1.55
Runyan A., Blumstein D. T. (2004). Do individual differences influence flight initiation distance? J. Wildl. Manage. 68, 1124–1129. doi: 10.2193/0022-541X(2004)068[1124:DIDIFI]2.0.CO;2
Sakai A., Sugiyama S. (2018). Experience-dependent transcriptional regulation in juvenile brain development. Dev. Growth Differ. 60, 473–482. doi: 10.1111/dgd.12571
Shehan M. I., Hernandez M., Rodriguez J. D., Nunes S. (2023). Social play predicts caution in juvenile Belding’s ground squirrels (Urocitellus beldingi). J. Mammal. 104, 1408–1420. doi: 10.1093/jmammal/gyad082
Sherman P. W. (1981). “Reproductive competition and infanticide in Belding’s ground squirrels and other animals,” in Natural selection and social behavior: recent research and new theory. Eds. Alexander R. D., Tinkle D. W. (Chiron Press, New York), 311–333.
Sherman P. W., Morton M. L. (1984). Demography of Belding’s ground squirrels. Ecology 5, 1617–1628. doi: 10.2307/1939140
Sherwen S. L., Magrath M. J. L., Butler K., Hemsworth P. H. (2015). Little penguins, Eudyptula minor, show increased avoidance, aggression and vigilance in response to zoo visitors. Appl. Anim. Behav. Sci. 168, 71–76. doi: 10.1016/j.applanim.2015.04.007
Sikes R., The Animal Care and Use Committee of the American Society of Mammalogists (2016). 2016 Guidelines of the American Society of Mammalogists for the use of wild animals in research and education. J. Mammal 97, 663–688. doi: 10.1093/jmammal/gyw078
Sinn D. L., Gosling S. D., Moltschaniwskyj N. A. (2008). Development of shy/bold behaviour in squid: context-specific phenotypes associated with developmental plasticity. Anim. Behav. 75, 433–422. doi: 10.1016/j.anbehav.2007.05.008
Siviy S. M. (2016). A brain motivated to play: insights into the neurobiology of playfulness. Behaviour 153, 819–844. doi: 10.1163/1568539X-00003349
Stark R., Pellis S. M. (2020). Male Long Evans rats reared with a Fischer-344 peer during the juvenile period show deficits in social competency: a role for play. Int. J. Play 9, 76–91. doi: 10.1080/21594937.2020.1720142
Stark R. A., Ramkumar R., Pellis S. M. (2021). Deficient play-deprived experiences in juvenile Long Evans rats reared with a Fischer 344 partner: a deficiency shared by both sexes. Int. J. Comp. Psychol. 34, 1–19. doi: 10.46867/ijcp.2021.34.5592
Stiles J., Jernigan T. L. (2010). The basics of brain development. Neuropsychol. Rev. 20, 327–348. doi: 10.1007/s11065-010-9148-4
Storm J. J., Lima S. L. (2010). Mothers forewarn offspring about predators: a transgenerational maternal effect on behavior. Am. Nat. 175, 382–390. doi: 10.1086/650443
Trezza V., Baarendse P. J. J., Vanderschuren L. J. M. J. (2010). The pleasures of play: pharmocological insights into social reward mechanisms. Trends Pharmacol. Sci. 31, 463–469. doi: 10.1016/j.tips.2010.06.008
Vetter S. G., Brandstatter C., Macheiner M., Suchentrunk F., Gerritsmann H., Bieber C. (2016). Shy is sometimes better: personality and juvenile body mass affect adult reproductive success in wild boars, Sus scrofa. Anim. Behav. 115, 193–205. doi: 10.1016/j.anbehav.2016.03.026
Ward C., Bauer E. B., Smuts B. B. (2008). Partner preferences and asymmetries in social play among domestic dog, Canis lupus familiaris, littermates. Anim. Behav. 76, 1187–1199. doi: 10.1016/j.anbehav.2008.06.004
Weinstock M. (2001). Alterations induced by gestational stress in brain morphology and behavior of the offspring. Progr. Neurobiol. 65, 427–451. doi: 10.1016/S0301-0082(01)00018-1
Wilson S. (1974). Juvenile play of the common seal Phoca vitulina vitulina with comparative notes on the gray seal Halichoerus grypus. Behaviour 48, 37–60. doi: 10.1163/156853974X00246
Wright A. J., Jackson J. J. (2022). Childhood temperament and adulthood personality differentially predict life outcomes. Sci. Rep. 12, 10286. doi: 10.1038/s41598–022-14666–0
Keywords: behavioral development, caution, boldness, ground squirrel, play behavior, social play, temperament, Urocitellus beldingi
Citation: Ryan WJ, Kuan MB and Nunes S (2024) Disruption of social play influences development of caution in juvenile ground squirrels. Front. Ethol. 3:1410334. doi: 10.3389/fetho.2024.1410334
Received: 31 March 2024; Accepted: 04 June 2024;
Published: 20 June 2024.
Edited by:
Christine Drea, Duke University, United StatesReviewed by:
Michael Potegal, University of Minnesota Twin Cities, United StatesMarijke Achterberg, Utrecht University, Netherlands
Copyright © 2024 Ryan, Kuan and Nunes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott Nunes, bnVuZXNAdXNmY2EuZWR1
 William J. Ryan
William J. Ryan Scott Nunes
Scott Nunes