- Marine Biology Research Division, Scripps Institution of Oceanography, University of California San Diego, La Jolla, CA, United States
The evolution of conspicuous morphology and related displays is often ascribed to their role in sexual selection. The context of displays together with the phylogenetic sequence of morphological and behavioral innovations provides insight into their evolution. Do conspicuous features function in mate attraction and/or aggression and does a behavioral display evolve before or after the morphological feature most evident in the display? These questions were explored for a unique display and dorsal fin feature in a clade of blenniiform fishes for which both courtship and aggressive displays are known. The anterior dorsal-fin spines of the Spikefin Blenny, Coralliozetus rosenblatti, are elongate. It has a unique courtship display but the fin is held statically erect similar to congeners. This and other species of Coralliozetus, perform a unique aggressive display, the “fin flag”, in which the anterior dorsal fin is waved laterally when encountering conspecifics. The spike-like dorsal fin of C. rosenblatti and its exaggerated lateral movements render this display especially conspicuous. In addition, it performs the fin flag more than twice as often as congeners. Thus, the dorsal fin of the Spikefin Blenny evolved to amplify an extant aggressive display consistent with the behavioral drive hypothesis that posits behavior leads to subsequent morphological evolution making displays more effective.
Introduction
Conspicuous morphological features of animals and the behaviors displaying them are often attributed to their role in sexual selection, either for attracting mates, aggressive signaling in intraspecific interactions, or both (Andersson, 1994; Berglund et al., 1996; Laidre and Johnstone, 2013). Identifying which of these is the proper context of conspicuous displays can be inferred by observing the frequency of such displays during aggressive versus courtship interactions, but the origin of such displays and the morphology upon which they are based is more elusive. Comparison of morphology and behavior across species sharing a recent common ancestor (clades) is a valuable approach to understanding the origin and modification of both morphology and associated behaviors displaying that morphology (McLennan et al., 1988; Wcislo, 1989; Prum, 1990; Martins, 1996; Ord and Martins, 2010; Johnson et al., 2019).
A phylogenetic approach also provides promise for understanding the coevolution of morphology and behavior. Do unique morphological features evolve before behaviors displaying the feature (morphological drive) or do behaviors evolve first followed by morphology featuring or amplifying those displays (behavioral drive; Bateson, 1988; Wcislo, 1989; Bateson, 2004; Duckworth, 2009; Wcislo, 2021)? These questions can be addressed for groups in which both morphology and behavioral displays are known, and whose species-level phylogeny has been hypothesized (Wcislo, 1989). Behavioral drive is supported when associated morphological features evolve after the origin of a particular behavior, i.e., when the behavior is present in ancestors that do not share the morphology. Morphological drive is supported if ancestors possess a morphological feature used in displays by descendant species. Evolution of both behavior and morphology at the same point on a phylogenetic hypothesis cannot discriminate between the two (Wcislo, 1989). This study addresses these questions in a small clade of blenniiform fishes for which a behavioral inventory of aggressive and courtship displays and a species-level phylogenetic hypothesis are available.
Tube blennies of the genus Coralliozetus (Teleostei, Blenniiformes, Chaenopsidae) are a morphologically and genetically distinctive lineage found on shallow reefs in the tropical eastern Pacific (six species) and Caribbean (one species; Hastings, 1997; Lin and Hastings, 2011; Hastings, 2021). Typical of tube blennies, the species of Coralliozetus occupy vacant tests of invertebrates that are essential for survival (Hastings and Galland, 2010) and are necessary resources for male reproductive success (Hastings, 1986; Hastings and Petersen, 2010). They have a polygynous mating system in which females deposit demersal eggs in the shelters of males and eggs are guarded by the resident male until hatching (Hastings, 1986; Hastings, 1988; Hastings and Petersen, 2010). Males must defend shelters from competing males and also attract females to their shelters for mating. Consequently, shelters are highly contested by males and a surprising variety of aggressive displays and conspicuous courtship displays characterize the group (Hastings, in prep.). As a consequence, these fishes provide a convenient group to study the co-evolution of morphology and display behavior.
One species, the so-called Spikefin Blenny (Coralliozetus rosenblatti), is endemic to the Gulf of California and possesses a unique (for the genus) dorsal fin in which the anterior three spines of males are prolonged (Figure 1), the longest being at least twice as long as more posterior spines (Stephens, 1963; Hastings, 2019). The anterior dorsal fin of males of most congeners is even in shape in that all spines are similar in length (in C. angelicus, C. boehlkei, C. cardonae, C. springeri and C. clausus), while the dorsal fin of C. micropes is sail-like with all spines prolonged (Figure 1). This study addresses two questions regarding the evolution of this autapomorphy in C. rosenblatti. First, what is the context of displays revealing the unique spike-like dorsal fin of C. rosenblatti? Is the dorsal fin presented in a unique or exaggerated way during courtship displays, aggressive displays or both? Second, did the evolution of the spike-like dorsal fin predate the evolution of any associated display, consistent with morphological drive, or did the behavior evolve before the morphology, consistent with the behavioral drive hypothesis? In order to address these questions both courtship and aggressive displays were recorded for the species of Coralliozetus and the outgroup species Protemblemaria bicirris and observed character states were mapped on a species-level phylogenetic hypothesis.

Figure 1 Dorsal fins of males of selected species of Coralliozetus. (A) C. rosenblatti with elongate anterior dorsal-fin spines; (B) C. micropes with sail-like dorsal fin; (C) C. angelicus and (D) C. cardonae with even dorsal fins. Photos reprinted with permission from (A, B) G. R. Allen (Source: D. R. Robertson and G. R. Allen. 2015. Shorefishes of the Tropical Eastern Pacific: online information system); (C) R. Woheau (Source: Mexican-fish.com); (D) N. DeLoach.
Methods
Behavioral recordings
A video inventory of displays by six species of Coralliozetus and the outgroup Protemblemaria bicirris was assembled from a larger survey of display behaviors in chaenopsids (Hastings, in prep.). This was accomplished during visits to field stations within the range of each species. This included ITESM - Guaymas, Sonora, Mexico for C. angelicus, C. micropes, C. rosenblatti, and P. bicirris, CIAD, Mazatlán, Mexico for C. boehlkei, and STRI - Naos, Panama for C. springeri, and Bocas del Toro, Panama for C. cardonae (specimens transported to and videos recorded at STRI - Naos). At each site, males and females were captured alive using plastic bags while snorkeling and returned to the lab were they were established in aquaria with substrates and shelters similar to those inhabited by the species in the wild. Shelters included barnacle tests for C. angelicus, C. cardonae, C. springeri and P. bicirris, and worm and vermetid gastropod tubes for C. boehlkei, C. micropes and C. rosenblatti. Study animals were fed live or frozen brine shrimp or flake food. After acclimation of several hours to a day individuals were video recorded for varying lengths of time. Solitary males were allowed to occupy vacant shelters and, in order to elicit aggressive displays, one or more additional males were added to aquaria, while courtship was elicited by adding a female. Video sequences of interactions were obtained using a Canon analog video system at 30 frames per second and later converted to digital format. Voucher specimens were deposited in the Marine Vertebrate Collection at Scripps Institution of Oceanography (SIO). Videos of a recently described species of Coralliozetus endemic to Isla del Coco, Costa Rica (Hastings, 2021) are unavailable.
Character scoring and mapping
The shape of the anterior dorsal fin, form of courtship displays, position of the dorsal fin during courtship displays, and all aggressive displays were described for the species of Coralliozetus and P. bicirris. The presence/absence of one notable aggressive display, the “fin flag”, known only within the genus Coralliozetus and P. bicirris, was mapped on a phylogenetic hypothesis for the family Chaenopsidae (Hastings, 2019) using Mesquite (Maddison and Maddison, 2017). For P. bicirris and six species of Coralliozetus, the number of separate male/male interactions where one male closely approached another and the number of these interactions that elicited a fin-flag display by either of the males were recorded.
Results
Dorsal-fin shape of male chaenopids
The shape of the dorsal fin of males of most species of chaenopsids is more-or-less even with all spines similar in length (Hastings, 2019). This includes P. bicirris and four species of Coralliozetus (Table 1; Figure 1). In C. micropes the dorsal fin of males is large and sail-like, similar to that in several members of Emblemaria and Chaenopsis (Hastings, 2019). In C. rosenblatti the dorsal fin of males is spike-like with the anterior three spines longer than more posterior spines (Figures 1, 2; Stephens, 1963). A somewhat similar dorsal fin with elongate anterior spines evolved independently in selected species of Emblemariopsis and one species of Emblemaria (Hastings, 2019).
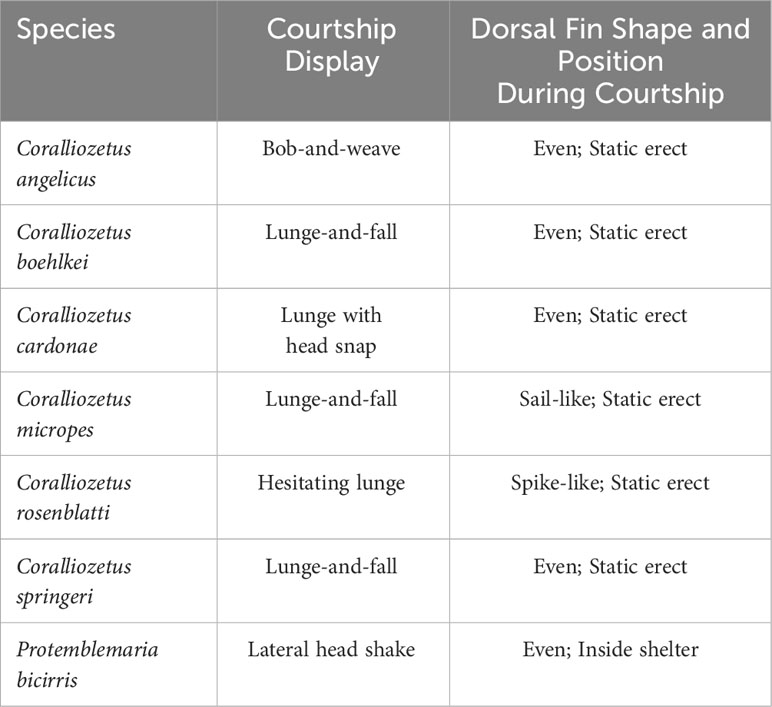
Table 1 Form of courtship displays and shape and position of the dorsal fin during courtship lunges in Coralliozetus species and P. bicirris.

Figure 2 Frame grabs from an approximate three-second interval of a fin-flag display in Coralliozetus rosenblatti (see Supplementary Video 2 for source of these images). Time proceeds down the left column, then down the right column of images. The last two elements of the time code represent seconds and frames (30 frames per second). The focal male is facing the camera and displaying to an intruder in the upper left of each image. Note left and right lateral (transverse) movements of the anterior dorsal fin are nearly 180 degrees.
Courtship displays
Male chaenopsids often court when they see a female near their shelter and when they see surrounding males courting (Hastings, 1988). Courtship displays typically involve a conspicuously colored resident lunging in and out of their shelter. The most common pattern in the family is the “jack-in-the-box” display in which males lunge directly outward and immediately withdraw backwards, directly into the shelter. Variation across species is seen in the speed of individual lunges and thus the duration of a single lunge, the frequency of lunges, the outward extent of each lunge, movement of the head during lunges, and movement and position of the dorsal and pelvic fins during lunges (Hastings, in prep.).
In three of the species of Coralliozetus the male lunges outward from the shelter approximately two thirds of his body length (i.e., the tail region remains in the shelter) and the anterior body drops downward before being withdrawn into the shelter, here termed “lunge-and-fall” (Table 1). Coralliozetus angelicus extends the amount of time outside of the shelter and with each lunge does a “bob-and-weave” movement in which the head and anterior body are moved side to side and up and down while the posterior body maintains contact with the shelter. Courtship in C. cardonae is unique in that males rapidly lunge partially outward from the shelter and snap the head to the side upon retreat (“lunge with head snap”). Courtship in C. rosenblatti is also unique in that the male lunges a half body length outward, pauses, and lunges further, pausing again before fully retracting into the shelter (‘hesitating lunge”; Supplementary Video 1). Courtship in P. bicirris differs from all of these and involves a short outward lunge with only the head protruding from the shelter and a rapid side-to-side shaking of the head. In these six species of Coralliozetus, including C. rosenblatti, the dorsal fin is held statically erect during each lunge and folded downward when retracting into the shelter (Table 1).
Aggressive displays
As far as known, all chaenopsids perform two basic displays in intraspecific aggressive interactions. The first is “branchial flare” in which the branchial chamber is expanded ventrally and laterally by lowering the urohyal region and flaring the opercular series (Lindquist, 1971). The second is “dorsal-fin erection” in which the anterior dorsal fin is held fully and statically erect and presented toward an intruder. Variations of these ubiquitous displays account for a surprising array of aggressive displays in these fishes (Hastings, in prep.).
One of the variations of “dorsal-fin erection” is “fin flag” in which the anterior dorsal-fin spines are repeatedly moved laterally, both to the left and to the right, when encountering a conspecific (Figure 2; Supplementary Videos 2–4). This display is performed by non-resident (those outside of a shelter) and resident males (inside a shelter) when closely approached by a conspecific. Among the 25 chaenopsid species surveyed (Hastings, in prep.), this fin-flag display was observed only in P. bicirris and five of the species of Coralliozetus (Table 2). This display was not observed in C. angelicus creating uncertainty of the precise evolutionary history of this display although evidence indicates that the display was present in the ancestor of the four species clade that includes C. rosenblatti (Figure 3).
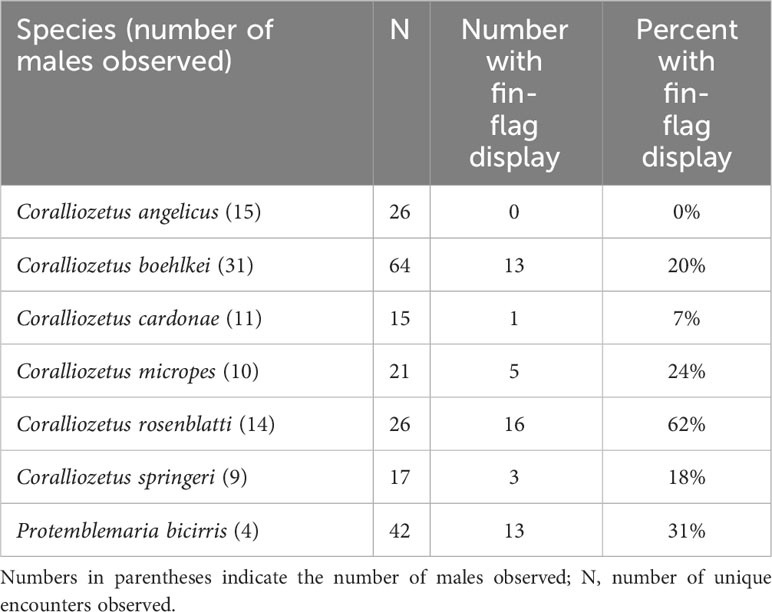
Table 2 Proportion of male-male encounters that involved an aggressive fin-flag display by one of the participants.
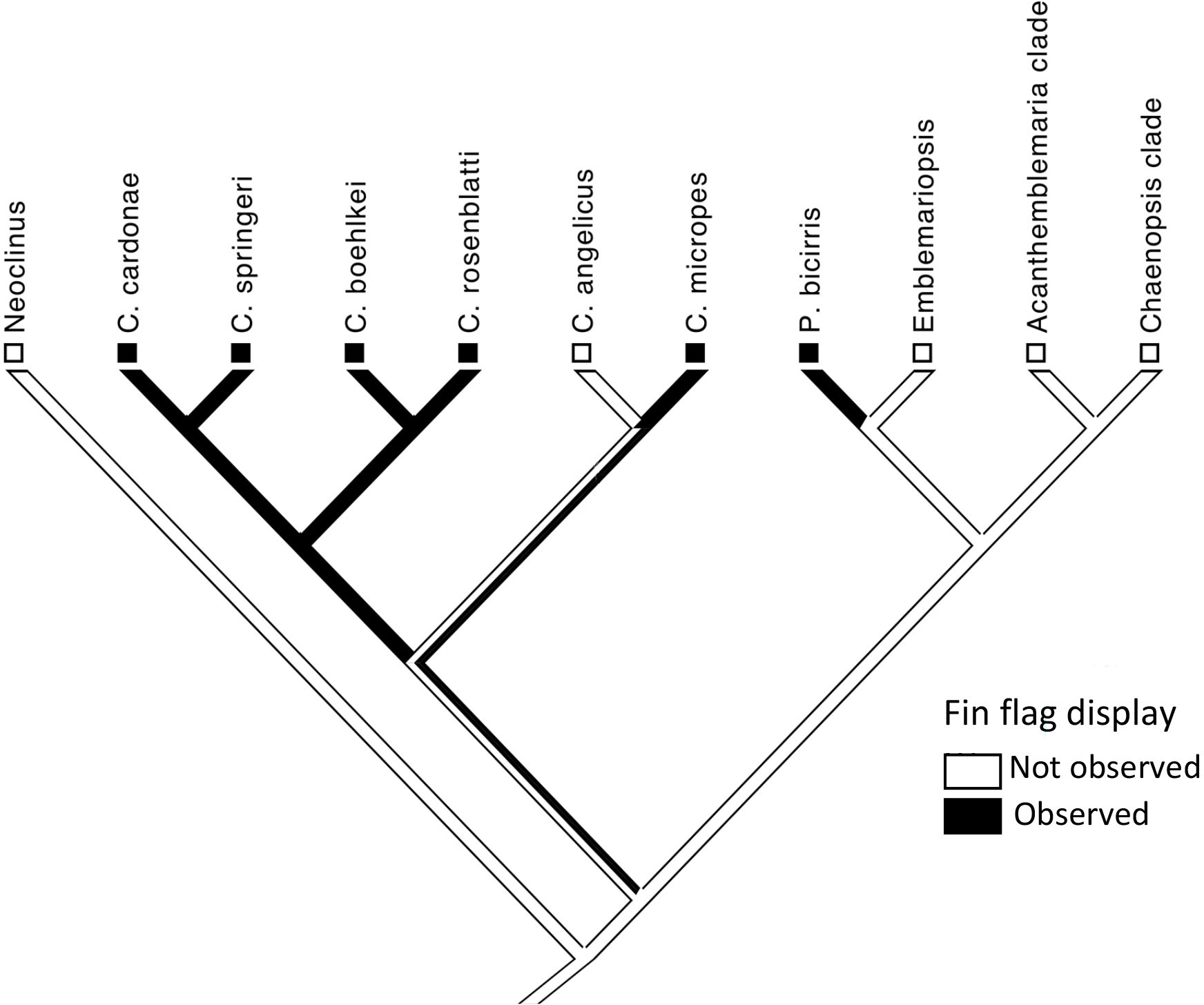
Figure 3 Character map for presence of the fin-flag display in chaenopsid blennies. Phylogeny is after Hastings (2019).
In the species with an even dorsal fin and in C. micropes with a sail-like fin (i.e., all except the Spikefin Blenny), lateral movements resemble a waving flag with movement greatest anteriorly and increasingly dampened more posteriorly through the first several spines (Supplementary Videos 3, 4). In the Spikefin Blenny the lateral movement is largely restricted to the elongate anterior spines. Also, the extent of lateral movement of the anterior spines is greatest in C. rosenblatti and may be as much as 180 degrees (Figure 2; Supplementary Video 2).
These species vary in the frequency of this display, performing it rarely, to in about one in four or five male-male encounters, to one in three in P. bicirris (Table 2). The greatest frequency of this display is seen in the Spike-fin Blenny that performs the fin-flag display in nearly two out of every three male-male encounters. Species that perform this display often have distinctive coloration on the anterior fin spines that are moved laterally (see Figure 1).
Discussion
The dorsal fin of the Spikefin Blenny appears to have evolved in the context of aggression rather than courtship. While this species performs a unique courtship display (“hesitating lunge”; Supplementary Video 1), during these lunges the dorsal fin is held rigidly erect as it is in all other species of Coralliozetus (Table 1). Although evident during courtship the elevated dorsal fin is not displayed in a unique way and does not involve a unique movement of the fin. While it may function in species recognition, it does not contribute to a unique form of courtship display.
Instead, the spike-like fin appears to have evolved in context of aggression. The fin of this species is especially evident in an aggressive display common to four other species of Coralliozetus and P. bicirris. The fin-flag display reaches its highest expression in terms of both frequency and extent of lateral fin movement in C. rosenblatti. This species’ elongate anterior dorsal-fin spines make this display especially conspicuous and the extent of lateral movement of the dorsal fin is both unique and extraordinary. In addition, this display is performed in the majority of male-male encounters (Table 2). These observations are consistent with the hypothesis that the elongation of the anterior spines of this species evolved to amplify (sensu Hasson, 1990) the fin-flag display.
A few other species in other lineages of chaenopsids have independently evolved elongate anterior dorsal-fin spines (Hastings, 2019), but as far as known these species do not perform the aggressive fin-flag display. For example, Emblemariopsis signifera has elongate anterior dorsal-fin spines that appear to function in crypsis. This species moves the anterior dorsal fin side to side and in a circular motion after short swimming movements when outside of shelters (Hastings, in prep.). These movements of the fin resemble the motion of algae and other filamentous items in the benthic reef environment and may function in crypsis.
Aggressive displays in fishes often involve static erection of the dorsal fin for example in poeciliids (Goldberg et al., 2019) or repeated flashing of the dorsal fin such as in the blenniid Alticus (Ord and Hsieh, 2011). However, the dorsal-fin spines of most acanthopterygian fishes have limited lateral movement and the extent of lateral movement seen in these tube blennies is extraordinary. This appears to be facilitated by the interlocking ring-like structures between the base of the dorsal-fin spines and each supporting pterygiophore in species of Coralliozetus (Hastings, 1997: see character 18, Figure 2E). This permits movement of the spines in both the medial and transverse planes.
The fin-flag was not observed in C. angelicus. While not observing a display behavior in a particular species is not strong evidence of its absence (Foster, 2013; Foster and Baker, 2019), the possible absence of this display may be related to this species’ microhabitat. The Angel Blenny occupies vacant barnacle tests in high surge zones (Hastings, 1988) where intentional lateral movement of the fin may be constrained.
Consistent with the behavioral drive hypothesis, evolution of the spike-like dorsal fin in C. rosenblatti occurred after the origin of the fin-flag display. The unique shape of the fin together with its exaggerated lateral movements serve to amplify (sensu Hasson, 1990) this extant aggressive display.
Behavioral drive posits that individuals with phenotypic variation in a morphological feature that makes an extant behavior more effective or more efficient gain a selective advantage and over time the feature becomes fixed (Bateson, 1988; Bateson, 2004; Duckworth, 2009; Wcislo, 2021). While some behaviors may be a constraint (Huey et al., 2003), others are hypothesized to have led to subsequent morphological evolution (reviewed in Wcislo, 1989; Wcislo, 2021). Although relatively few studies have explicitly invoked behavioral drive as a mechanism of display evolution it is likely to have played a significant role in amplification of a variety sexually selected characters (Hasson, 1991). This includes amplifying or making more conspicuous the courtship plumage of male manakins (Prum, 1990) and increased nuptial coloration in male sticklebacks (McLennan, 1990). Behavioral drive may also account for the evolution of aggressive display features such as the increased head width in Hawaiian Drosophila species that first evolved a unique head-to-head fighting posture (Spieth, 1981). Similarly, the elongation and conspicuous coloration of the jaws in the Sarcastic Fringehead blenny (Neoclinus blanchardi) have been shown to amplify an extant aggressive gaping display (Hongjamrassilp et al., 2018). Additional phylogenetic studies on the evolutionary sequence of display behaviors and their revealed structures are needed to more fully document the role of behavioral drive in display evolution.
Data availability statement
The datasets presented in this article are not readily available because all data are included in the article. Requests to access the datasets should be directed tocGhhc3RpbmdzQHVjc2QuZWR1.
Ethics statement
The animal study was approved by UCSD Institutional Animal Care and Use Committee (IACUC). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PH: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Science Foundation (BSR 9001173 and IBN 9407993), the Link Family Foundation, and the UCSD Academic Senate.
Acknowledgments
Ed Pfeiler, Lloyd Findley, Albert van der Heiden and Ross Robertson are thanked for hosting research visits. Some behavioral observations were made prior to implementation of institutional animal care requirements, while more recent observations were made under UCSD IACUC S-00080.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fetho.2023.1325273/full#supplementary-material
Supplementary Video 1 | Courtship in Coralliozetus rosenblatti. The ‘hesitating lunge’ is unique to this species. As in other tube blennies, the dorsal fin is held erect during the courtship display but not moved in a unique way in this species. Recorded at ITESM of a specimen collected in the Guaymas, Sonora, Mexico region (voucher: SIO 04-172).
Supplementary Video 2 | Fin-flag display in Coralliozetus rosenblatti. Focal male is displaying to intruder in the upper left of images. Recorded at ITESM of specimens collected in the Guaymas, Sonora, Mexico region (vouchers: SIO 04-172 and SIO 04-173).
Supplementary Video 3 | Fin-flag display in Coralliozetus boehlkei. Focal male is displaying to male in shelter in lower right of images. Recorded at CIAD-Mazatlán of specimens collected in the Mazatlán, Sinaloa, Mexico region (vouchers: SIO 20-16).
Supplementary Video 4 | Fin-flag display in Protemblemaria bicirris. Focal male is displaying to a male in the barnacle in lower right of images. Recorded at ITESM of specimens collected in Guaymas, Sonora, Mexico region (vouchers: SIO 04-172 and SIO 04-173).
References
Andersson M. (1994). Sexual selection (Princeton (NJ: Princeton University Press). doi: 10.1515/9780691207278
Bateson P. (1988). “The active role of behaviour in evolution,” in Evolutionary processes and metaphors. Eds. Ho M.-W., Fox S. W. (New York: Wiley), 191–207.
Bateson P. (2004). The active role of behaviour in evolution. Biol. Philosophy 19, 283–298. doi: 10.1023/B:BIPH.0000024468.12161.83
Berglund A., Bisazza A., Pilastro A. (1996). Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399. doi: 10.1111/j.1095-8312.1996.tb01442.x
Duckworth R. A. (2009). The role of behavior in evolution: a search for mechanism. Evol. Ecol. 23, 513–531. doi: 10.1007/s10682-008-9252-6
Foster S. A. (2013). Evolution of behavioural phenotypes: influences of ancestry and expression. Anim. Behav. 85, 1061–1075. doi: 10.1016/j.anbehav.2013.02.008
Foster S. A., Baker J. A. (2019). Loss and re-emergence of plastic ancestral behavioural traits: Influences on phenotypic and evolutionary pattern. Anim. Behav. 155, 271–277. doi: 10.1016/j.anbehav.2019.06.013
Goldberg D. L., Landy J. A., Travis J., Springer M. S., Reznick D. N. (2019). In love and war: The morphometric and phylogenetic basis of ornamentation, and the evolution of male display behavior, in the livebearer genus Poecilia. Evolution 73, 360–377. doi: 10.1111/evo.13671
Hasson O. (1990). The role of amplifiers in sexual selection: An integration of the amplifying and the Fisherian mechanisms. Evol. Ecol. 4, 277–289. doi: 10.1007/BF02270927
Hasson O. (1991). Sexual displays as amplifiers: practical examples with emphasis on feather decorations. Behav. Ecol. 2, 189–197. doi: 10.1093/beheco/2.3.189
Hastings P. A. (1986). “Habitat selection, sex ratio, and sexual selection in Coralliozetus angelica (Blennioidea: Chaenopsidae),” in Indo-pacific fish biology. Eds. Uyeno T., Arai R., Taniuchi T., Matsuura K. (Tokyo: Ichthyological Soc. Japan), 785–793.
Hastings P. A. (1988). Female choice and male reproductive success in the angel blenny, Coralliozetus angelica (Chaenopsidae). Anim. Behav. 36, 115–124. doi: 10.1016/S0003-3472(88)80254-9
Hastings P. A. (1997). Phylogenetic relationships of the Coralliozetus clade of chaenopsid blennies, with description of a new genus (Teleostei, Blennioidei). Bull. Mar. Sci. 61, 743–761.
Hastings P. A. (2019). Evolution of sexual dimorphism in tube blennies (Teleostei: Chaenopsidae). Integr. Org. Biol. 1, 1–22. doi: 10.1093/IOB/obz003
Hastings P. A. (2021). The Pandemic Blenny, Coralliozetus clausus, a new species of tube blenny endemic to Isla del Coco, Costa Rica (Teleostei: Chaenopsidae). Zootaxa 4926, 296–300. doi: 10.11646/zootaxa.4926.2.10
Hastings P. A., Galland G. R. (2010). Ontogeny of microhabitat use and two-step recruitment in a specialist reef fish, the Browncheek Blenny (Chaenopsidae). Coral Reefs 29, 155–164. doi: 10.1007/s00338-009-0565-x
Hastings P. A., Petersen C. W. (2010). “Parental care, oviposition sites and mating systems of blennioid fishes,” in Reproduction in marine fishes: evolutionary patterns and innovations. Ed. Cole K. S. (Berkeley: University of California Press), 91–116.
Hongjamrassilp W., Summers A. P., Hastings P. A. (2018). Heterochrony in fringeheads (Neoclinus) and amplification of an extraordinary aggressive display in the Sarcastic Fringehead (Teleostei: Blenniiformes). J. Morph. 279, 626–635. doi: 10.1002/jmor.20798
Huey R. B., Hertz P. E., Sinervo B. (2003). Behavioral drive versus behavioral inertia in evolution: a null model approach. Amer. Nat. 161, 357–366. doi: 10.1086/346135
Johnson M. A., Cook E. G., Kircher B. K. (2019). “Phylogeny and ontogeny of display behavior,” in Behavior of lizards: evolutionary and mechanistic perspectives. Eds. Bels V. L., Russell A. P. (Boca Raton, Florida: CRC Press, Taylor and Francis Group), 259–287, ISBN-13: 978-1-4987-8272-2.
Laidre M. E., Johnstone R. A. (2013). Animal signals. Cur. Biol. 23, R829–R833. doi: 10.1016/j.cub.2013.07.070
Lin H. C., Hastings P. A. (2011). Evolution of a Neotropical marine fish lineage (Subfamily Chaenopsinae, Suborder Blennioidei) based on phylogenetic analysis of combined molecular and morphological data. Molec. Phylog. Evol. 60, 236–248. doi: 10.1016/j.ympev.2011.04.018
Lindquist D. G. (1971). The comparative behavior of three species of blennioid fishes (Hayward: California State College).
Maddison W. P., Maddison D. R. (2017). Mesquite: a modular system for evolutionary analysis. Version 3.13. Available at: http://mesquiteproject.org.
Martins E. P. (1996). Phylogenies and the comparative method in animal behaviour. (New York: Oxford Univ. Press).
McLennan D. A. (1990). Integrating phylogeny and experimental ethology: from pattern to process. Evolution 45, 1773–1789. doi: 10.1111/j.1558–5646.1991.tb02687.x
McLennan D. A., Brooks D. R., McPhail J. D. (1988). The benefits of communication between comparative ethology and phylogenetic systematics: a case study using gasterosteid fishes. Can. J. Zool. 66, 2177–2190. doi: 10.1139/z88-325
Ord T. J., Hsieh S. T. (2011). A highly social, land-dwelling fish defends territories in a constantly fluctuating environment. Ethology 117, 918–927. doi: 10.1111/j.1439-0310.2011.01949.x
Ord T. J., Martins E. P. (2010). “Evolution of behaviour: phylogeny and the origin of present-day diversity,” in Evolutionary behavioral ecology. Eds. Westneat D., Fox C. W. (Oxford, UK: Oxford University Press), 108–128. doi: 10.1111/j.14390310.2011.01949.x
Prum R. A. (1990). Phylogenetic analysis of the evolution of display behavior in Neotropical manakins (Aves: Pipridae). Ethology 84, 202–231. doi: 10.1111/j.1439-0310.1990.tb00798.x
Spieth H. T. (1981). Drosophila heteroneura and Drosophila silvestris: Head shapes, behavior and evolution. Evolution 35, 921–930. doi: 10.2307/2407863
Stephens J. S. Jr. (1963). A revised classification of the blennioid fishes of the American family Chaenopsidae. Univ. California Publications Zoology 68, 1–165.
Wcislo W. T. (1989). Behavioral environments and evolutionary change. Ann. Rev. Ecol. Evol. Syst. 20, 137–169. doi: 10.1146/annurev.es.20.110189.001033
Keywords: behavioral drive, displays, amplification, aggression, courtship, Chaenopsidae, Blenniiformes, Teleostei
Citation: Hastings PA (2023) Behavioral drive and morphological amplification of an aggressive display. Front. Ethol. 2:1325273. doi: 10.3389/fetho.2023.1325273
Received: 20 October 2023; Accepted: 30 November 2023;
Published: 18 December 2023.
Edited by:
Michel Baguette, Muséum National d’Histoire Naturelle, FranceReviewed by:
Vincent Bels, Muséum National d’Histoire Naturelle, FranceMatthew Lovern, Oklahoma State University, United States
Copyright © 2023 Hastings. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip A. Hastings, cGhhc3RpbmdzQHVjc2QuZWR1
†ORCID: Philip A. Hastings, https://orcid.org/0000-0002-9867-6601
 Philip A. Hastings
Philip A. Hastings