
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Epidemiol., 07 April 2025
Sec. Infectious Disease Epidemiology
Volume 5 - 2025 | https://doi.org/10.3389/fepid.2025.1517476
This article is part of the Research TopicPrevalence of Urinary Tract Infection, Antimicrobial Resistance and Molecular Epidemiology of Uropathogenic Escherichia coli among Pregnant WomenView all articles
Background: Urinary tract infections (UTIs) remain one of the most common diseases worldwide that occur both in the community and in healthcare settings. Thus, this study aimed to compare the burden of nosocomial and community-acquired bacterial UTIs among patients attending Hiwot Fana Comprehensive Specialized University Hospital, Eastern Ethiopia.
Method: A hospital-based cross-sectional study was conducted using a convenient sampling technique from January 2024 to April 2024. Descriptive statistics were employed, and bivariate and multivariable logistic regression analyses were used to identify associated factors at p < 0.05 with a 95% confidence interval (CI) considered statistically significant.
Results: The rate of hospital-acquired UTIs was 42% (95% CI: 35–50), while the rate of community-acquired UTIs was 28% (95% CI: 22–36). The predominant bacterial isolates were Escherichia coli (37%), Staphylococcus aureus (7.8%), and Klebsiella pneumoniae (7.8%). The overall multidrug resistance rate was 91 (77.8%). Lack of formal education [adjusted odds ratio (AOR), 0.02; 95% CI: 0.001–0.6], surgery during admission (AOR, 0.02; 95% CI: 0.002–0.3), delay in voiding urine (AOR, 0.01; 95% CI: 0.005–0.1), previous UTIs (AOR, 0.04; 95% CI: 0.004–0.4), and previous admission (AOR, 0.07; 95% CI: 0.01–0.5) were the main factors significantly associated with bacterial UTIs.
Conclusions: A significantly higher prevalence of hospital-acquired bacterial UTIs was observed compared to community-acquired bacterial UTIs. The commonest isolates were E.coli, S. aureus, and K. pneumoniae. The drug resistance rate was very high. Modifiable individual-level factors were the major significant factors of UTIs. Thus, health workers and other stakeholders should tackle UTIs by increasing community awareness, promoting personal hygiene, and improving healthcare service quality.
Urinary tract infection (UTI) is the most common bacterial infection, and some groups of the population were at increased risk of acquiring the disease, resulting in nearly 7 million hospital visits and 100,000 hospitalizations (1). Urinary tract infections can be classified as complicated and uncomplicated based on the severity of the disease. Complicated urinary tract infection starts from the bladder and extends to the kidneys and is associated with abnormalities, immunosuppression, and previous exposure to antibiotics. Uncomplicated urinary tract infection involves the lower urinary tract, specifically the bladder and urethra (2). UTIs can be categorized based on their site of acquisition: nosocomial urinary tract infection is acquired in a healthcare facility or during patient care, and normal flora residing in the body are related to risk factors (3), whereas community-acquired urinary tract infection (CAUTI) is acquired outside of a healthcare facility or within 48 h of admission (4).
According to a global study conducted, ∼404.4 million people were diagnosed with urinary tract infections, resulting in 236,786 deaths (5). Similar global and regional studies showed E. coli and K. pneumoniae accounted for 40% and 10% of global antimicrobial resistance-related and attributed deaths in UTIs, respectively (6). A study conducted in Kuwait showed significant bacteriuria was detected in 26.6% of urine samples. Of these, the common isolates identified in catheter-associated urinary tract infection (CAUTI) and hospital-acquired urinary tract infection (HAUTI) were highly resistant to both first-line and second-line antibiotics (7). The lack of advanced diagnostic facilities and a trained workforce, a poor drug regulation system, and weak infection control mechanisms fuel urinary tract infection prevalence in developing countries (8). A similar study conducted in sub-Saharan African countries showed a 32% prevalence rate of UTI among nine countries. South Africa recorded a high UTI prevalence rate (67.7%), and the lowest UTI prevalence rate was recorded in Senegal at 5.1%. Ethiopia was found to be the fifth rank with a pooled UTI prevalence rate of 37% (9). Another study conducted on antimicrobial resistance in the East Africa region showed ampicillin, gentamicin, and ceftriaxone were highly resistant to the isolated bacteria, which puts the commonly used antibiotics at risk (10).
A study conducted in Ethiopia revealed that uropathogens with high multidrug resistance (MDR) were isolated with a prevalence rate of 88% (11). Similarly, the prevalence of multidrug-resistant bacteria in Ethiopia is high compared with much of the rest of the world, and bacterial isolates from urinary tract infections were multidrug-resistant with a prevalence rate of 67.6% (12). A study conducted among pediatric patients in Ethiopia showed that most bacterial isolates were highly resistant to commonly prescribed antibiotics. Of these, ampicillin and trimethoprim–sulfamethoxazole (TS) exhibited the highest resistance rate for tested antibiotics (13). Similarly, a study conducted in Eastern Ethiopia showed a high degree of MDR observed among the commonly used antibiotics in the area, which creates a room to revise treatment guidelines used and focus on AMR surveillance activities (14).
Urinary tract infection remains one of the most common causes of morbidity, and early diagnosis of the disease has become a crucial part of its management, as it is strongly associated with bad health outcomes and the commonly used antibiotics to treat UTI empirically are no longer functional due to sharply increasing multidrug-resistant bacterial isolates (15). In addition, the highly increasing rate of multidrug-resistant bacterial isolates in Ethiopia puts existing antibiotic treatment at risk (11). The lack of diagnostic facilities to early detect resistance types of isolates and the highly increasing resistance of bacterial isolates against antibiotics used to empirically treat UTI were growing challenges (13).
It is a known fact that disease prevalence varies in accordance with geographical settings, and it is too difficult to give the exact prevalence of both community-acquired and hospital-acquired urinary tract infections, bacterial etiologic agents, and antimicrobial resistance rates of isolates (16). It is very important to have knowledge of hospital-acquired and community-acquired bacterial isolates and antimicrobial susceptibility profiles to make first choices of antibiotics to treat the infection and, based on identified risk factors, to plan intervention measures. Even if many studies are done in the area, a comparative study on community-acquired and hospital-acquired urinary tract infections is lacking. Therefore, this study aimed to assess the comparative burden of nosocomial and community-acquired bacterial UTIs and their associated factors in patients attending Hiwot Fana Comprehensive University Hospital.
The study was conducted at Hiwot Fana Comprehensive University Hospital, Harar, Ethiopia. Hiwot Fana Comprehensive Specialized University Hospital (HFCSUH) was established in 1941 and became a university-specialized hospital for Haramaya University's College of Health and Medical Sciences in 2010. The hospital is the largest teaching and referral hospital in Eastern Ethiopia, serving many people coming from the surrounding zones and nearby regions, both for inpatient and outpatient services. It is located 525 km from the capital city, Addis Ababa, and serves as the only cancer treatment center in Eastern Ethiopia and consists of an operating room, intensive care unit (ICU), pediatric ward, gynecology and obstetrics ward, orthopedics ward, medical ward, recovery ward, and trauma and cancer centers.
A hospital-based comparative cross-sectional study was conducted from January to April 2024.
All inpatient and outpatient department attendants visiting Hiwot Fana Specialized University Hospital during the study period were the source population.
Inpatient and outpatient attendants who were clinically suspected of urinary tract infection during the study period were the study population.
Symptomatic UTI patients who took antibiotics within the previous 2 weeks and who were not volunteers to participate were excluded from the study.
The sample size was determined using the Epi Info Version 7 sample size calculator with the double population proportion that was used in a previous study. The calculation resulted in a sample size of 154. Then, we assumed a 10% non-response rate. Finally, by considering the stratified analysis, we doubled the sample size to 338 (169 from each group). P1, the prevalence of community-acquired UTI, was 19.3% (17) from a previous study conducted in Dessie and P2, the prevalence of hospital-acquired UTI, was 41.5% (18) from another study conducted in Northeast Ethiopia. At a 95% confidence interval and power at 80%, n1 (number of community-acquired UTI patients) and n2 (number of hospital-acquired UTI patients) = 1:1. The final sample size was 338 (159 hospital-acquired and 159 community-acquired UTI patients). A convenient sampling technique was employed to recruit study participants consecutively until the required sample size was reached because of time constraints and the lack of a readily available sampling frame. Nosocomial UTI was determined if a patient had a clinical urinary tract infection 48 h after hospital admission, and it was not the initial reason for admission. Community-acquired UTI was assumed when a patient developed clinical urinary tract infections during his hospital visit or before 48 h of hospital admission. Clinical UTI is a condition when a patient has one or more of the following symptoms: dysuria, frequency, urgency, hematuria, back pain, nocturia, costovertebral angle tenderness, and the absence of vaginal discharge or irritation (19).
Initially, patients with clinical UTIs were identified and recruited by physicians who were assigned and working in the respective inpatient and outpatient departments during the study period. A structured questionnaire-based patient interview was conducted, and the questionnaire was adopted from similar studies (15, 20) with some amendments. It was administered to collect information related to potential UTI-associated factors in the study. The questionnaires contained three parts: sociodemographic characteristics and clinical and behavioral risk factors of UTI. Two trained nurses and two laboratory technologists participated in data collection, and supervision was conducted by a health officer and senior medical microbiologist working in the hospital.
Study participants were oriented to cleansing the urethral opening with water and collecting freshly voided midstream urine samples of ∼10–15 ml using a sterile, wide-mouthed, screw-capped plastic container to avoid the risk of contamination. After collection, urine samples are labeled with identification number and date of collection. In catheterized patients, the soft rubber connector between the catheter and the collecting tubes is cleaned with 70% ethanol to avoid contamination. In case of delays in processing, samples were refrigerated to prevent cell decomposition and overgrowth.
The urine samples were taken using a standardized calibrated wire loop (0.001 ml) and inoculated onto blood agar medium (Oxoid, Hampshire, UK) and MacConkey agar medium. The streaked culture plates were incubated aerobically for 24 h at 35°C–37°C. After overnight incubation, cultured media showing significant bacterial growth were further identified by their colony morphology, gram reaction, and biochemical tests. A 0.001 ml capacity loop was used for inoculation of urine, and then the number of colony-forming units (CFU) on blood agar was multiplied by 1,000 to get organisms per milliliter. Quantification of a single bacterium >105 CFU/ml growth was indicative of UTI (75).
The antimicrobial susceptibility test was carried out by the Kirby–Bauer disc diffusion method as per the Clinical Laboratory Standards Institute (21) guidelines on Mueller–Hinton agar (Oxoid, Basingstoke, England). A bacterial suspension of each isolate was prepared in 0.5 ml of nutrient broth medium, and the turbidity was adjusted to match the 0.5 McFarland standards to obtain approximately the organism number of 1 × 106 CFU/ml. A sterile cotton swab was immersed into the suspension. Then, the swab was applied to the center of the Mueller–Hinton agar plate and evenly spread on the medium (Oxoid, Basingstoke, UK). After 5 min, selected antimicrobial disks were aseptically placed on Mueller–Hinton agar plates and allowed to stand at room temperature for 15 min. The diameter of the zone of inhibition around the disk was measured using a ruler and compared to reference points stated in clinical laboratory guidelines (21), and the results were interpreted as sensitive, intermediate, and resistant.
The following antibiotics were used for this study includes as follows: gentamicin (GN) 10 µg, nitrofurantoin (F) 300 µg, tetracycline (T) 30 µg, cefazolin (Cz) 30 µg, cefoxitin (CXT) 30 µg, meropenem (MEM) 10 µg, tobramycin (TN) 10 µg, aztreonam (ATM) 30 µg, clindamycin (CN) 2 µg, rifampin (RF) 5 µg, chloramphenicol (C) 30 µg, ampicillin (AMP) 10 µg, amoxicillin–clavulanic acid (AMC) 10 µg, ceftazidime (CAZ) 30 µg, ciprofloxacin (CIP) 5 µg, trimethoprim–sulfamethoxazole (TS) 23.75 µg, erythromycin (E) 15 µg (obtained from Oxoid, Hampshire, UK) (21), and ceftriaxone (CRO).
Community-acquired urinary tract infection: when patients with urinary tract infection symptoms visit the HFSUH outpatient unit or when they become symptomatic within the previous 48 h of admission (4).
Multidrug resistance (MDR): refers to bacteria that are resistant to at least one agent in three or more antibiotic categories (22).
Nosocomial urinary tract infection: when patients develop urinary tract infections in the hospital after 48 h of admission, which are not present at the time of admission (3).
History of use of antibiotics: UTI patients who took any antibiotic within 2 weeks before the study (23).
The structured questionnaire was translated into the local languages (Afan Oromo and Amharic) and English language to check the consistency of the questions. A pilot study was conducted by pretesting translated questionnaires in the local language to make them suitable for 5% of the total sample size to ensure the validity and reliability of the data collection tools and the needed modification for the final data collection prior to the actual data collection at police hospital to assess its clarity, understandability, and simplicity. All the culture media were prepared by following the manufacturer's instructions, checked for sterility by incubating 5% of the prepared media for 24 h, observed for supporting the growth of organisms, and checked by inoculating control strains. Standard reference strains of E. coli (ATCC-25922), S. aureus (ATCC-25923), and P. aeruginosa (ATCC-27853) were used during culture and antimicrobial susceptibility testing.
Data collected were cleaned for completeness and consistency before data entry. Data were entered into EpiData 4.6 and then exported to STATA Version 16 software for analysis. During analysis, descriptive statistics, including mean, frequency, and percentage, were calculated to summarize the data as appropriate. Potential multicollinearity was also considered and tested using the variance inflation factor (VIF). Bivariable logistic regression was performed, and variables with p < 0.25 were eligible for the final model. The Hosmer–Lemshow goodness-of-fit test was applied for multivariable fitness. In multivariable logistic regression analysis, p < 0.05 adjusted odds ratio (AOR) with a 95% confidence interval (CI) was used to declare a significant association. Then, the findings of this study were interpreted and presented in the form of texts, tables, and graphs as appropriate.
Ethical clearance was obtained from the Institutional Health Research Review Committee (Ref. No. IHRERC/174/2023) of Haramaya University College of Health and Medical Sciences. Permission from HFCSUH was obtained through communicating with a letter of support that was written by the college. The objective, significance, benefit, risk, and procedural details of the study were explained, and informed, voluntarily, written, and signed consent was obtained from the head of the hospital and study participants. No identification or names were recorded to maintain confidentiality. Finally, data were collected after obtaining written informed consent from the participants. The culture-positive cases should be linked to the hospital for further management and care.
A total of 332 symptomatic UTI study participants were included in this study, of which 166 were hospital-acquired and 166 were community-acquired clinical UTIs. Of these, 174 (53.6%) were females, and 158 (47.4%) were males. The age of study participants ranges from 1 to 85 years old, with a mean age of 36.33. Out of the total study participants, 103 (62%) inpatient attendants and 91 (54.8%) outpatient attendants did not attend formal education. The mean family size of study participants was 4.42, +/− 1.76498 SD (range, 1–12). Regarding the income status of study participants, the average monthly income was 3725.27 (±SD, 1755.756 ETB). The mean daily water intake of study participants was 1.59 L (SD, ±0.513) (Table 1).
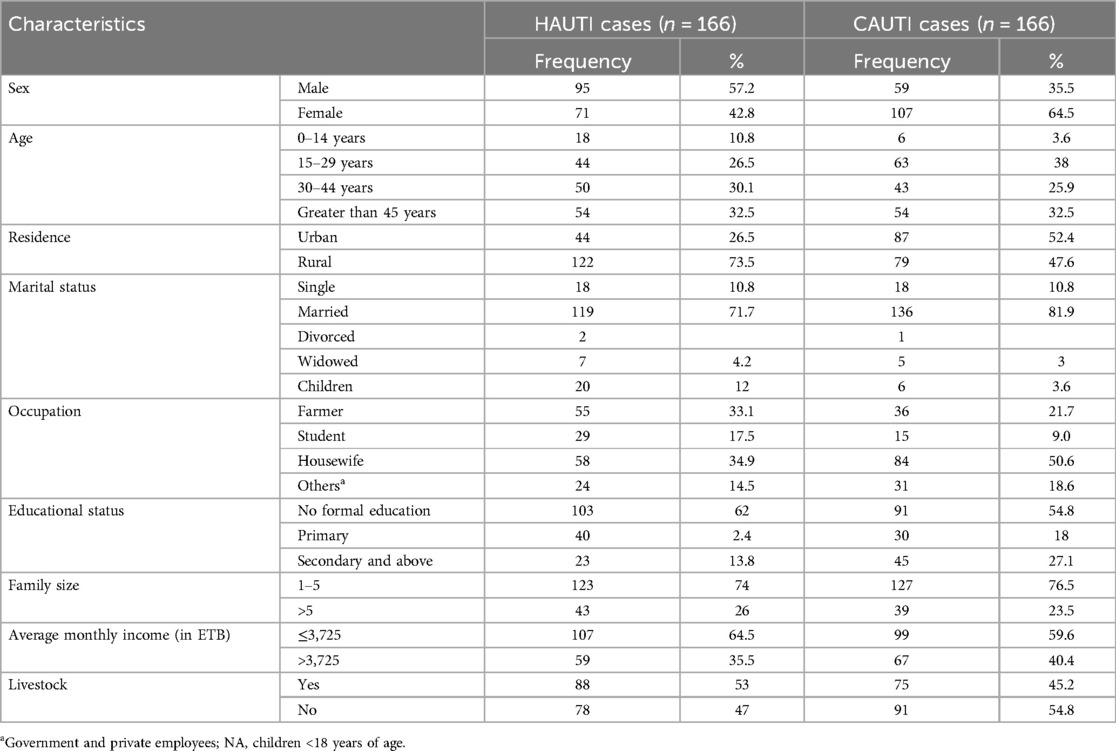
Table 1. Sociodemographic characteristics of patients with clinical urinary tract infection in HFCSUH, Eastern Ethiopia, 2024 (N = 332).
Out of the 322 symptomatic UTI patients, 281 (87%) had improved water sources for domestic use, and 51 (15.8%) had unimproved water sources for domestic use. Less than half (42%) of the study participants had a daily water intake of ≤2 L/day. Regarding behavioral factors, 69 (21.4%) of study participants had a habit of delaying voiding urine voluntarily. Of the total symptomatic UTI patients, 97 (30%) had habits of shower frequency less than or equal to three times per week, and 235 (70%) of the study participants had habits of shower frequency greater than three times per week (Table 2).
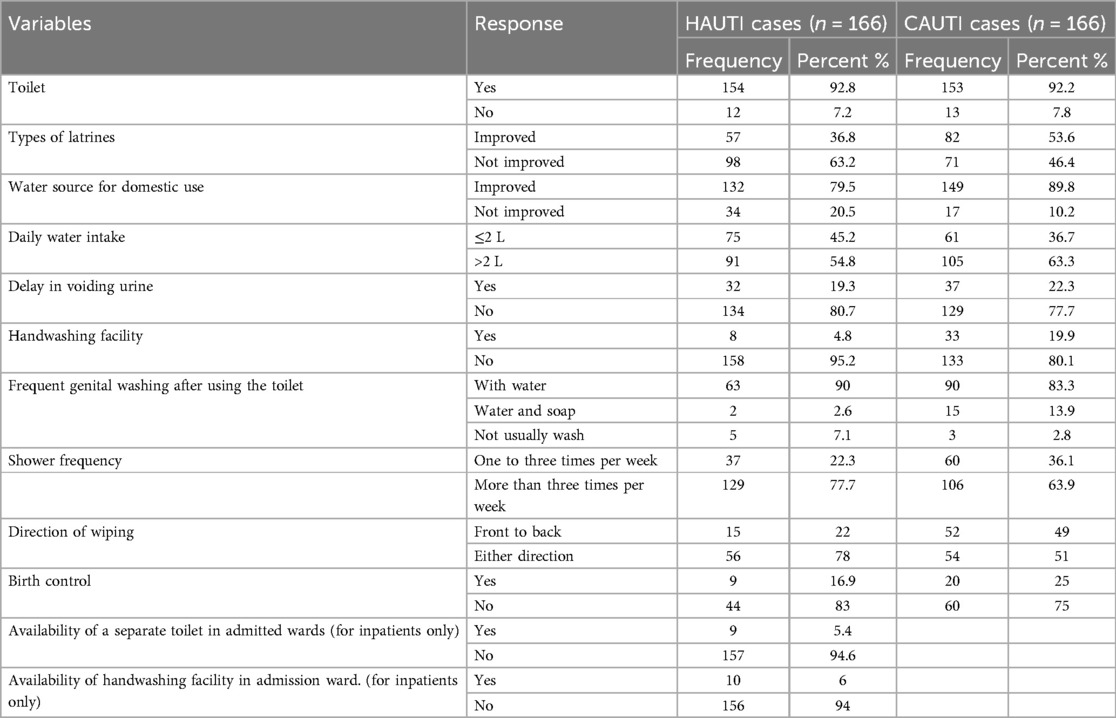
Table 2. Environmental and behavioral factors of patients with clinical urinary tract infection in HFCSUH, Eastern Ethiopia, 2024 (N = 332).
Out of the 332 patients with clinical UTIs, 35 (21.7%), 23 (13.8%), 22 (13.3%), and 65 (39.2%) had a history of admission in the last 2 months, a history of UTI in the last 6 months, a history of catheterization in the past 1 year, and chronic disease among inpatient study participants, respectively. Correspondingly, 36 (22%), 34 (21%), 10 (6%), and 35 (21.1%) had a history of admission in the last 2 months, a history of UTI in the last 6 months, a history of catheterization in the past 1 year, and chronic disease among outpatient study participants, respectively. Moreover, 45 (27.1%), 5 (3%), and 43 (25.9%) inpatient study participants had a history of catheterization, history of intubation, and surgery during the current admission, respectively. Approximately 30% (100) of the study participants had chronic diseases, of whom 49 (50%) had diabetes mellitus, followed by 15 (15%) with hypertension, 12 (11%) with cancer, and the remaining 25 (24%) with other chronic diseases. The risk factors associated with culture-confirmed bacterial UTI were history of admission in the last 2 months, history of UTI in the last 6 months, and surgery during admission, which were significantly associated with culture-confirmed bacterial UTI at p < 0.05 (Table 3).
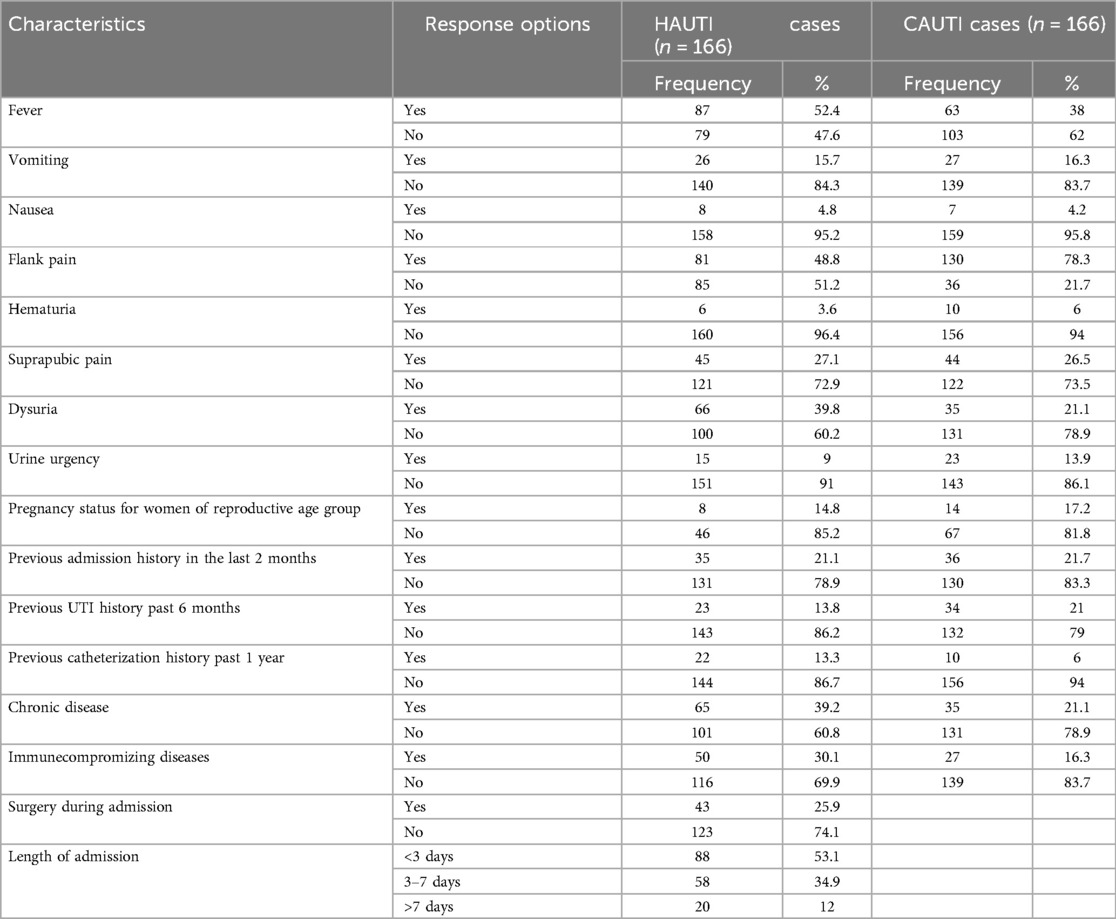
Table 3. Clinical characteristics of symptomatic UTI patients in HFCSUH, Eastern Ethiopia, 2024 (n = 332).
The overall prevalence of urinary tract infection was 35% (117/332) (95% CI: 22–50). Of these, the prevalence of hospital-acquired urinary tract infection was 42% (70/166) (95% CI: 35–50), and community-acquired urinary tract infection was 28% (47/166) (95% CI: 22–36). High culture-confirmed bacterial uropathogens were isolated from rural attendants (79/201 or 39%) (Figure 1).
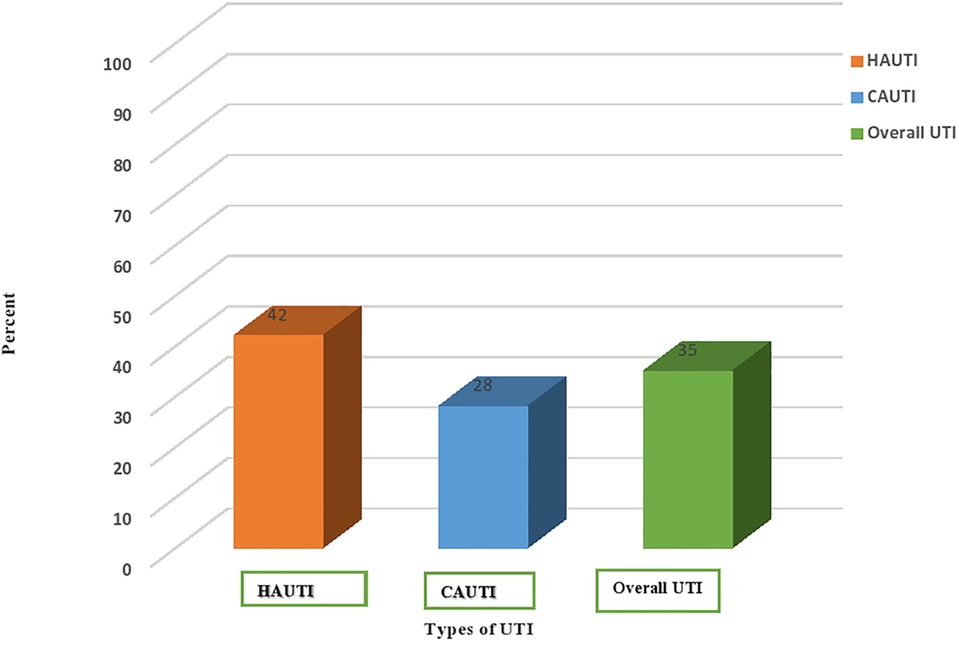
Figure 1. Prevalence of culture-confirmed bacterial urinary tract infection among community and nosocomial UTI cases in HFCSUH, Eastern Ethiopia, 2024. HAUTI, hospital-acquired urinary tract infections. CAUTI, community-acquired urinary tract infections.
The overall bacterial uropathogen culture-positive rate was 35%. The prevalence of hospital-acquired bacterial UTIs was 42%, whereas that of community-acquired UTIs was 28%. The majority (87%) of the isolated uropathogens responsible for UTIs were gram-negative bacteria. The predominant bacterial species isolated from hospital-acquired UTI were E. coli (21/71 or 29.7%) and K. pneumoniae (6/71 or 8.5%), whereas E. coli (22/47 or 46.8%) and P. mirabilis (4/47 or 8.5%) were the predominant bacterial species isolated from community-acquired UTIs. Among gram-positive bacteria, the predominant isolate in hospital and community-acquired UTIs were Staphylococcus aureus, followed by Staphylococcus saprophyticus, with an isolation rate of 9/118 (7.6%) and 4/118 (3.4%), respectively. It was to be noted that mixed bacterial growth was observed in one patient with an isolation rate of 1/118 (0.85%) (Figure 2).
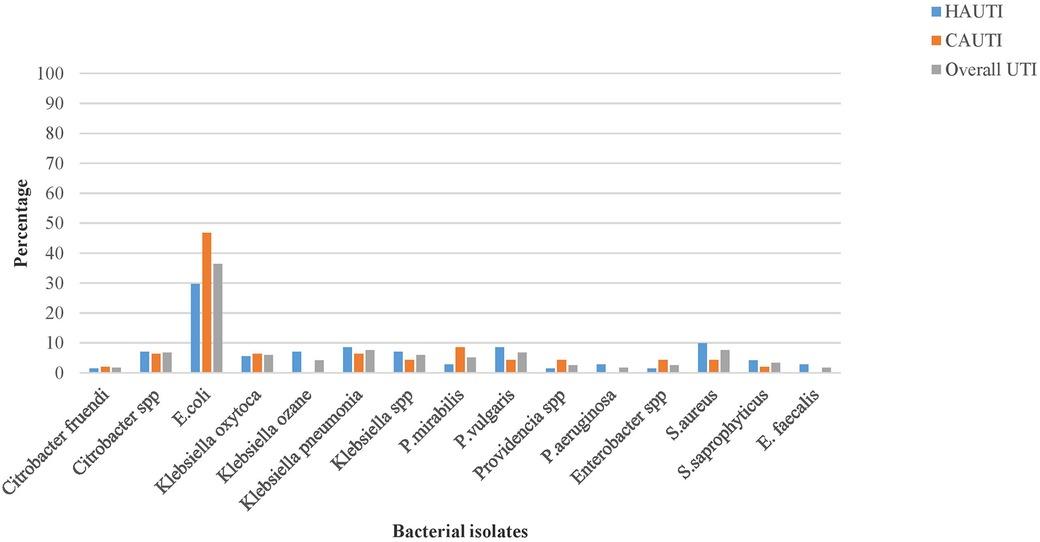
Figure 2. The pathogenic bacterial isolates of urine cultured among patients with clinical urinary tract infection in HFCSUH, Eastern Ethiopia, 2024.
Antimicrobial susceptibility tests were performed for each bacterial isolate on Muller–Hinton agar, and the susceptibility results were interpreted based on CLSI guidelines 2022. Gram-negative bacterial species isolated from hospital-acquired UTI patients showed susceptibility rates of 52 (88%), 36 (61%), and 32 (54%) to meropenem, ciprofloxacin, and gentamicin and gram-negative bacterial species isolated from community-acquired UTI patients showed susceptibility rates of 43 (98%), 29 (66%), and 24 (58%) to meropenem, ciprofloxacin, and augmentin, respectively.
Among the predominant bacterial uropathogens isolated from hospital and community-acquired UTI patients, E. coli showed higher resistance rates of 90.7% and 88.4% to tetracycline and ampicillin, respectively. In contrast, E. coli demonstrated the highest susceptibility rates to meropenem and ciprofloxacin with 88.4% and 67%, respectively. K. pneumoniae is the second most predominant bacterial isolate and showed resistance rates of 88.9% and 77.7% to ceftriaxone and cotrimoxazole, respectively. It also presented higher susceptibility rates or 77.8% to meropenem and 66.7% to augumentin. Gram-positive bacteria species showed the highest susceptibility rates of 86.7% and 66.7% to nitrofurantoin and ciprofloxacin, respectively, whereas gram-positive bacterial species showed the highest resistance rates of 80% and 73% to penicillin and tetracycline, respectively. At the species level, Staphylococcus aureus demonstrated the highest resistance rates of 100% and 88.8% to penicillin and tetracycline and the highest susceptibility rates of 77.8% and 55.6% to nitrofurantoin and gentamicin, respectively (Tables 4, 5).
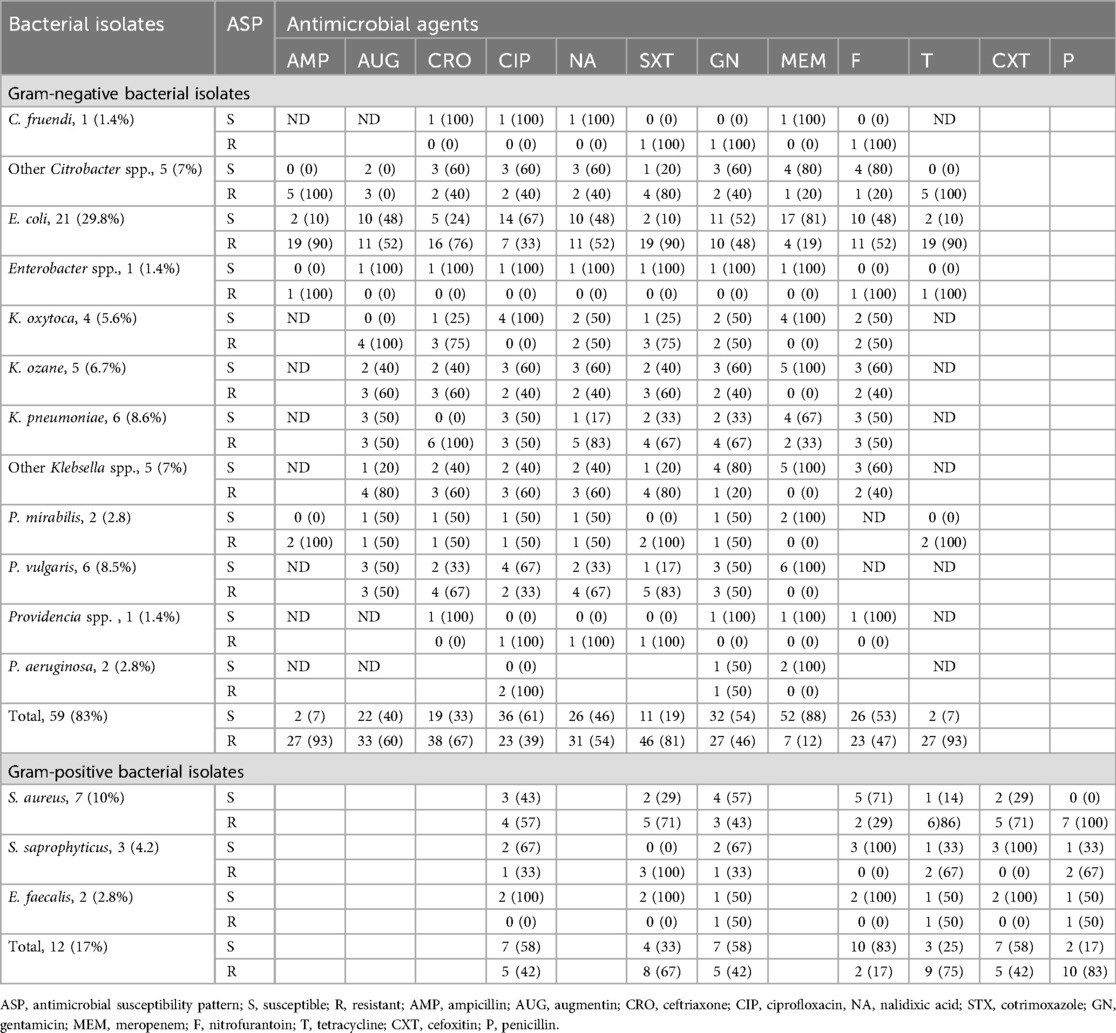
Table 4. Antimicrobial susceptibility patterns of bacterial isolates from urine cultures among patients with nosocomial UTIs in HFCSUH, Eastern Ethiopia, 2024.
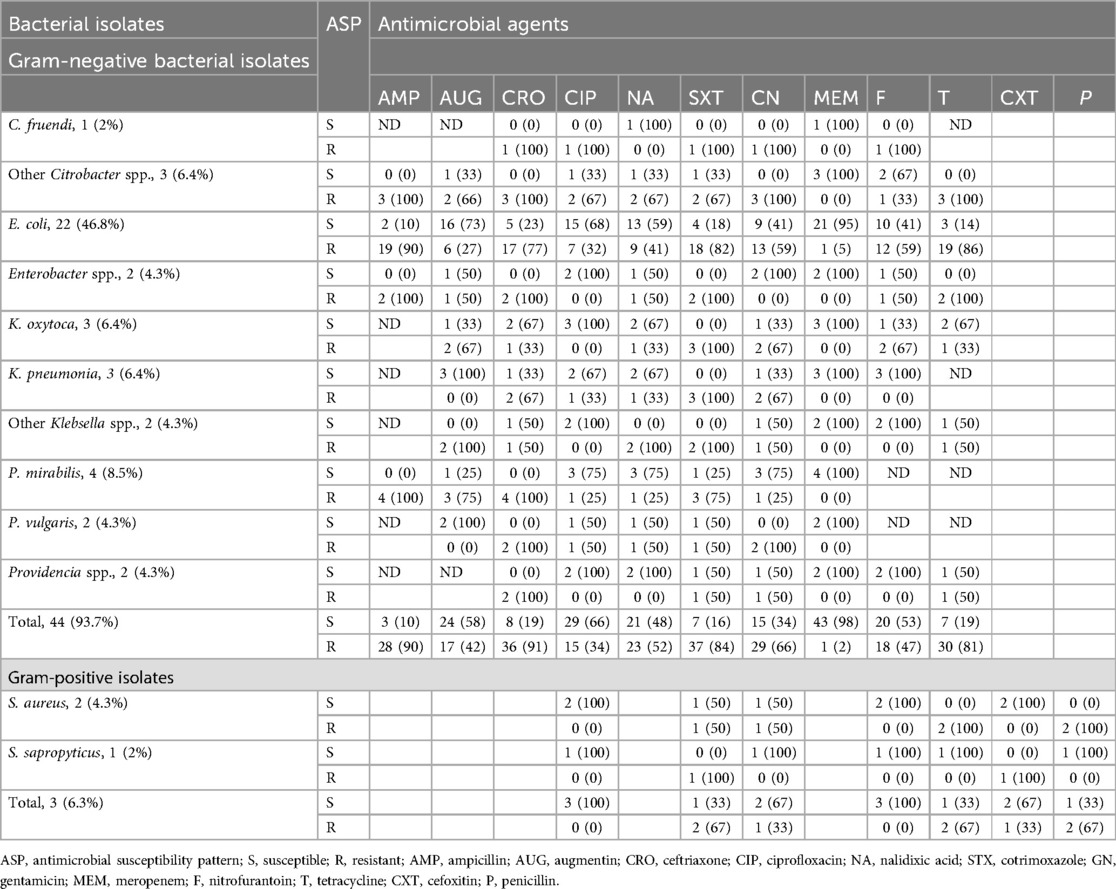
Table 5. Antimicrobial susceptibility patterns of bacterial isolates from urine cultures among patients with community-acquired UTIs in HFCSUH, Eastern Ethiopia, 2024.
The overall MDR rate of the bacterial isolate was 77.8%, and a higher MDR rate was observed among gram-negative bacterial isolates, with a rate of 78.6%. E. coli, K. pneumoniae, and Citrobacter spp. isolates showed MDR rates of 39 (37.8%), 8 (7.8%), and 7 (6.8%), respectively, whereas Pseudomonas aeruginosa demonstrated the lowest MDR rate of 0%. Among gram-positive bacterial isolates, Staphylococcus aureus revealed a higher MDR rate of 7 (40%) for tested antibiotics in the panel, and Enterococcus faecalis isolates had the lowest MDR rate of 1 (6.7%) (Table 6).
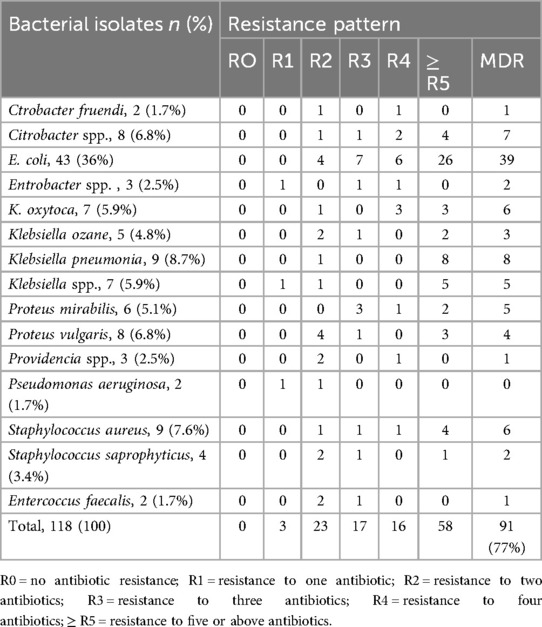
Table 6. Multidrug resistance patterns of bacterial isolates from urine cultures of patients with clinical UTIs in HFCSUH, Eastern Ethiopia, 2024.
In bivariate analysis, those variables with p < 0.25 were selected as candidates for the multivariable analysis. In multivariable logistic regression analysis, surgery during admission, delay in voiding urine, previous UTI history, and previous admission history were significantly associated with culture-confirmed bacterial UTI at p < 0.05.
Patients who had not undergone surgery during admission had a 98% (AOR, 0.02; 95% CI: 0.00002–0.3) lower risk of developing culture-confirmed bacterial UTI compared to their counterparts. Patients who had no previous history of UTI had a 96% (AOR, 0.04; 95% CI: 0.004–0.4) lower risk of developing culture-confirmed bacterial UTI compared to those with a previous history of UTI in the last 6 months, and patients who had no previous admission history in the last 2 months had a 93% (AOR, 0.07; CI: 0.01–0.5) lower risk of developing culture-confirmed bacterial UTI. Patients with educational status of secondary and above had a 98% (AOR, 0.02; 95% CI: 0.001– 0.6) lower risk of developing bacterial UTI compared to patients who had primary or no formal education (Table 7).
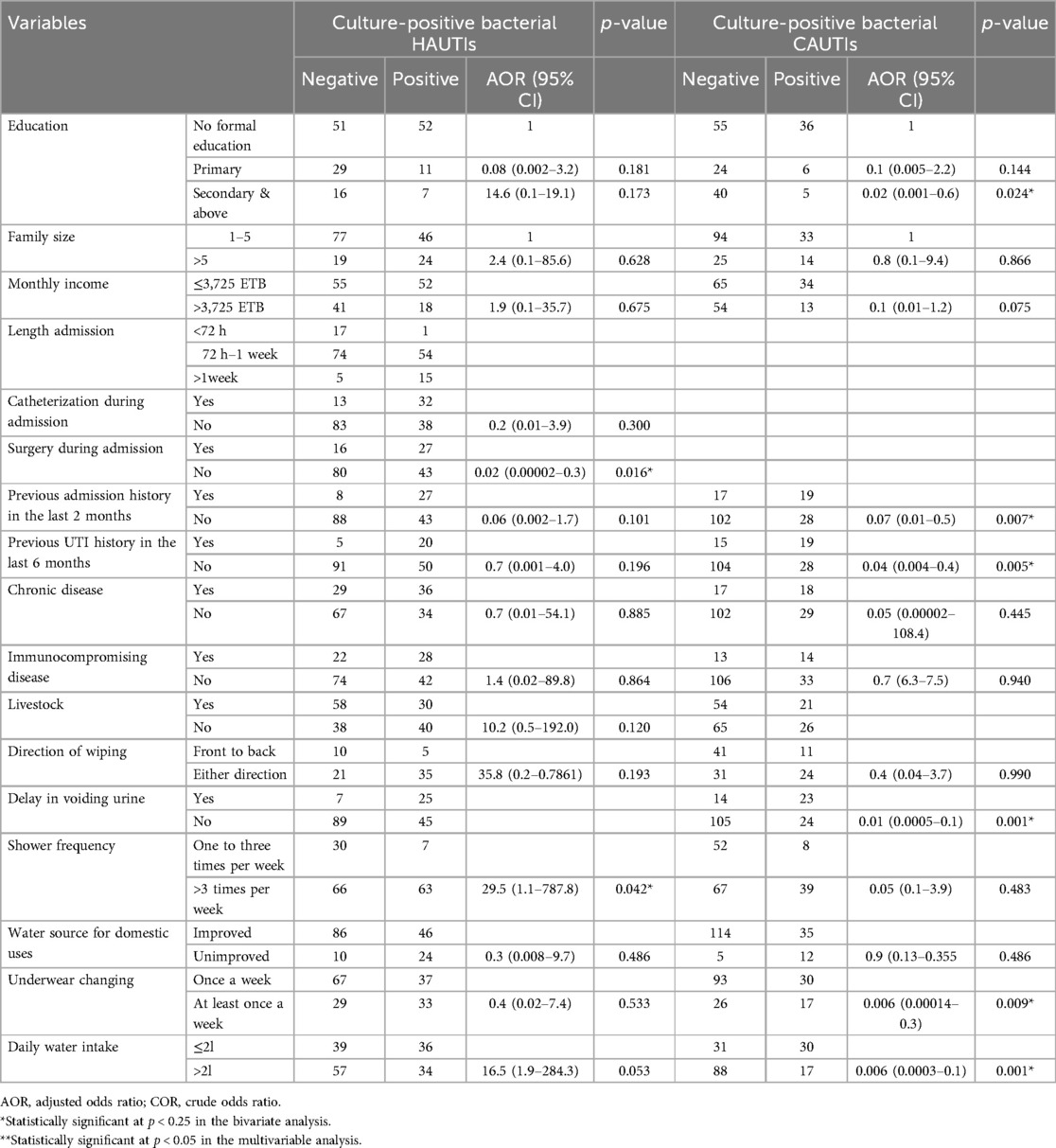
Table 7. Bivariable and multivariable logistic regression analysis of risk factors of bacterial UTI among patients with clinical UTIs in HFCSUH, Eastern Ethiopia, from January 19 to April 19, 2024.
This study showed an overall UTI prevalence rate of 35% (95% CI: 22–50), which was in line with a study conducted in local areas showing prevalence rates of 31% (20) and 31.8% (24). This finding was lower than those of the previous local studies, with UTI prevalence rates of 90% (25) and 65% (26). Although it was not statistically significant, the current study found a higher prevalence rate of hospital-acquired UTIs, 42% (95% CI: 35–50), compared to community-acquired bacterial UTIs, 28% (95% CI: 22–36). These findings are concordant with the results of other similar studies (27, 28). This could be due to several factors, including the fact that hospitalized patients are usually catheterized, have delayed urine voiding, lack urine meter drainage, and have underlying diseases such as diabetes mellitus. Additionally, factors such as microbial contamination of the drainage bag, intrahospital transfers, cross-infections in overcrowded patient rooms, immunosuppressive therapy, and surgical operations contribute to the risk (29–31).
The current prevalence of nosocomial UTI was concurrent with the previous studies conducted in Northeast Ethiopia (41.5%) (18) and Somalia (42.9%) (32). However, this finding is higher than those of the studies conducted in Jimma, Ethiopia (25.4%) (33), and Egypt (18.9%) (34). This high prevalence of hospital-acquired urinary tract infection might be due to deprived infection control measures implemented in the hospital settings of the study (35). Our study also pointed out a community-acquired UTI prevalence rate of 28% (95% CI: 22–36). This finding is comparable with those of the studies conducted in Dar es Salaam, Tanzania (27.4%) (36), and Iraq (27.3%) (37). Our finding is higher than those of most previous studies conducted in Northwest Ethiopia (19.3%) (27) and Dhaka (20%) (38). This high prevalence of community-acquired urinary tract infections might be due to poor environmental and personal hygiene, variation in the study population, and geographical locations (39).
The study also revealed the predominant bacterial uropathogen responsible for urinary tract infections was gram-negative bacteria, accounting for a rate of 103 (87%). Our study was consistent with studies conducted in other parts of the world that showed the dominant causative agents were gram-negative bacteria (15, 38, 40, 41). Of the isolates, the commonest bacterial uropathogen recovered in both hospital and community-acquired UTIs was E. coli, with rates of 21 (30.4%) and 22 (46.8%), respectively. This finding was in agreement with the findings of studies conducted in different countries revealing that E. coli remains the leading causative agent of bacterial uropathogens in both community and hospital-acquired UTIs (27, 42, 43).
The predominance of Escherichia coli as causative agents of UTI might be due to its structural flagella and pili, which enabled it to move, and dominating normal flora in the epithelium of the intestine resulted in major UTI occurrence (44). The study also revealed that Klebsiella pneumonia was the second most common (6; 8.7%) bacterial pathogen in hospital-acquired UTIs while Proteus mirabilis (4; 8.5%) was common in community-acquired UTIs. This was in agreement with studies conducted in Addis Ababa (11; 7.8%) (20) and Tigray (22.4%) (45). This study contradicted some of the local studies where Staphylococcus aureus was the second most predominant bacterial isolate recovered in hospital and community-acquired UTIs (27). These could be due to the fact that most gram-negative bacteria (GNB) are gut residents that colonize the urethra and subsequently the bladder through the action of specific adhesins and then begin to multiply, producing toxins and enzymes that promote their survival and could have significant clinical importance that lead to high morbidity and mortality (46).
The present study revealed that gram-negative bacterial species isolated from both groups showed a high level of resistance to ampicillin and tetracycline with rates of 91.6% and 86% and a high level of susceptibility to meropenem and ciprofloxacin with rates of 92.2% and 63%. This high resistance rate of gram-negative bacterial isolates to ampicillin was comparable with other similar studies (15, 47). These highest resistance rates of gram-negative bacteria isolates to ampicillin and tetracycline might be due to the easy accessibility of the drugs at the community and facility levels, which are prone to misuse by patients and health workers. On the other hand, the gram-negative uropathogens isolated in our study were more susceptible (92%) to meropenem, and it was congruent to the previous studies conducted in Addis Ababa, 91% (13), and in Jimma, 96% (33). E. coli showed the highest levels of susceptibility to meropenem with a rate of 88% and ciprofloxacin with a rate of 67%. In contrast, E. coli showed a higher resistance level (88%) to ampicillin and tetracycline. This high resistance rate of E. coli to tetracycline was comparable with a study (45), and the extrusion of drugs from the cytoplasm via specific efflux helped pump tetracycline (48).
The present study showed that the overall MDR prevalence rate is 77.8%, which is higher than those of the studies in Gondar (70.4%) (49), Eastern Ethiopia (61.6%) (47), Iran (56%) (41), and Bangladesh (72%) (50), and Northwest Ethiopia (55.7%) (24). These variations could be due to the irrational use of drugs at community levels (36). The current high MDR rate could resulted from the muddled use of antimicrobial agents in the study area (51), and it emphasizes the need for a strong antimicrobial stewardship implementation program that will lead to effective AMR prevention and control. This study also indicated nearly 3/4 of the MDR prevalence rate among gram-negative bacteria isolates, and this finding was in agreement with previous studies done elsewhere (38, 52). The reason for the high MDR prevalence among gram-negative bacteria might be the majority of the groups had intrinsic resistance and most of the bacterial isolates were acquired in hospital settings (53). Of the gram-positive bacterial isolates, Staphylococcus aureus showed the highest MDR rate of 40%, whereas E. faecalis from gram-positive and P. aeruginosa from gram-negative bacterial isolates showed the lowest MDR rate. These findings were discordant with similar studies due to variations in study areas and community.
In the current study of outpatients who had secondary or above educational status by far, 98% had a lower risk of UTIs compared to those who never went to formal education. The finding is in line with other similar studies in Ethiopia (14), confirming that education increases knowledge and improves most modifiable individual-level factors with a reduction in the urinary tract infection rate (54). Similar results were observed in other studies (55–57). This study showed that patients who had previous UTI history within the last 6 months were 96% at higher risk of developing UTI compared to those who had no UTI history in the timeline, which was supported by other studies (20, 24, 58, 59). A history of previous UTIs increases the risk of developing a new active UTI because it can alter the bladder lining, making it more susceptible to bacterial colonization, potentially due to changes in the immune response triggered by the initial infection. This allows bacteria to more easily adhere and establish themselves, leading to recurrent infections (60). In our study, patients who had no previous admission history within the previous 2 months had a 93% lower risk of developing bacterial UTI compared to their counterparts. The study corresponds to studies conducted in southern Ethiopia (15), Pakistan (26), and Uganda (4). The epidemiology and presentation of UTIs are changing over time in patients with frequent hospitalization (61).
The study also showed that outpatients who were delayed in voiding urine habits had a 99% higher risk of contracting bacterial UTIs than their counterparts. This finding is in agreement with similar studies conducted in different areas (15, 62), and delaying or holding urine for a long time increases the chances of UTIs because it allows bacteria to multiply in the bladder due to urine stasis, which is when urine remains in the bladder for too long; essentially, the longer you hold your urine, the higher the chance of developing a UTI (63). Moreover, this study also indicated that outpatients who changed their underwear at least once a week were 94% less likely to have a UTI than those who did not change their underwear within a week. This result was in agreement with a similar study (64). Infrequent underwear changing often deprives personal hygiene and favors the ascending of bacterial uropathogens into upper urinary tracts from the genital area, resulting in microbial colonization and multiplication, ending up in a complicated urinary tract infection (65).
The current study also showed that patients whose daily water intake was >2 L per day were 96% less likely to develop UTI compared to those who took ≤2 L of daily water. This is a fundamental established truth supported by various studies (66, 67), and high daily water intake reduces invasion of the urinary tract by flushing the urinary tract and makes an unfavorable environment for multiplication (68).
Inpatients who had not undergone surgery during admission had a 98% lower likely risk of developing bacterial UTIs compared to those who had surgery during admission. This finding is consistent with other studies conducted in different areas (69–71). Routine catheterization has been employed in many surgical centers to avoid postoperative urinary retention, something for which patients undergoing total joint arthroplasty are known to be at increased risk and which itself is associated with UTIs (72). This study also indicated that inpatients who took showers greater than three times per week had ∼30 times lower risk of getting a UTI compared to those who took showers three or fewer times per week. This finding was also comparable with another study (73). That is why lack of access to the necessary hygiene facilities resulted in inadvertent health outcomes and UTIs (74). In our study, some sociodemographic and behavioral risk factors that commonly had significant associations with bacterial UTIs in many other studies did not demonstrate statistically significant associations with either the hospital- or community-acquired UTIs. The potential reasons could be the differences in study population type, socioeconomic status, and geography.
The study employed a non-probability sampling method.
The present study revealed that the rate of hospital-acquired UTIs is relatively higher than that of community-acquired ones. E. coli, S. aureus, K. pneumoniae, and P. mirabilis were the prominent uropathogens isolated from hospital-acquired and community-acquired urinary tract infections. E. coli showed the highest resistance rates to tetracycline and ampicillin and the highest susceptibility rates to meropenem and ciprofloxacin. The study also revealed that there was a very high MDR rate in bacterial UTIs in the study area. Generally, modifiable individual-level factors such as educational status, delay in voiding urine habits, previous UTI history within the last 6 months, admission history in the previous 2 months, and infrequent changing of underwear were the significantly associated factors that play a role in community-acquired UTIs, whereas surgery during admission and taking infrequent showers at the time of admission were the main risk factors for hospital-acquired UTIs. Therefore, increasing community awareness and knowledge about UTIs and their treatment through health education is mandatory to reduce both types of UTIs. Strengthening the infection prevention and control implementation strategies regularly could have an immense and indispensable impact in reducing hospital-acquired UTIs. Clinicians and other stakeholders should advocate for a conservative and proper use of antibiotics. The MDR challenge demands regular surveillance, and the application of updated antibiograms is crucial to monitor the AMR situation in the country. Finally, the government and non-government organizations should collaborate to put in place an integrated AMR surveillance system at all levels of hospitals in Ethiopia.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethical clearance was obtained from the Institutional Health Research Review Committee (Ref. No. IHRERC/174/2023) of Haramaya University College of Health and Medical Sciences. Written informed consent was obtained from the participants.
SF: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TS: Data curation, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. GM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The authors declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. (2002) 113:5–13. doi: 10.1016/S0002-9343(02)01054-9
2. Sharma K, Verma R. Urinary tract infections: a review. World J Biol Pharm Health Sci. (2023) 13:189–92. doi: 10.30574/wjbphs.2023.13.2.0099
3. Iacovelli V, Gaziev G, Topazio L, Bove P, Vespasiani G, Agrò EF. Nosocomial urinary tract infections: a review. Urol J. (2014) 81:222–7. doi: 10.5301/uro.5000092
4. Odoki M, Almustapha Aliero A, Tibyangye J, Nyabayo Maniga J, Wampande E, Drago Kato C, et al. Prevalence of bacterial urinary tract infections and associated factors among patients attending hospitals in Bushenyi district, Uganda. Int J Microbiol. (2019) 2019:4246780. doi: 10.1155/2019/4246780
5. Zeng Z, Zhan J, Zhang K, Chen H, Cheng S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: an analysis of the global burden of disease study 2019. World J Urol. (2022) 40:755–63. doi: 10.1007/s00345-021-03913-0
6. Li X, Fan H, Zi H, Hu H, Li B, Huang J, et al. Global and regional burden of bacterial antimicrobial resistance in urinary tract infections in 2019. J Clin Med. (2022) 11:2817. doi: 10.3390/jcm11102817
7. Al Benwan K, Al Sweih N, Rotimi VO. Etiology and antibiotic susceptibility patterns of community-and hospital-acquired urinary tract infections in a general hospital in Kuwait. Med Princ Pract. (2010) 19:440–6. doi: 10.1159/000320301
8. Samuel S, Kayode O, Musa O, Nwigwe G, Aboderin A, Salami T, et al. Nosocomial infections and the challenges of control in developing countries. Afr J Clin Exp Microbiol. (2010) 11(2). doi: 10.4314/ajcem.v11i2.53916
9. Mwang'onde BJ, Mchami JI. The aetiology and prevalence of urinary tract infections in sub-Saharan Africa: a systematic review. J Health Biol Sci. (2022) 10:1–7. doi: 10.12662/2317-3076jhbs.v10i1.4501.p1-7.2022
10. Ampaire L, Muhindo A, Orikiriza P, Mwanga-Amumpaire J, Boum Y, Bebell L. A review of antimicrobial resistance in east Africa. Afr J Lab Med. (2016) 5:1–6. doi: 10.4102/ajlm.v5i1.432
11. Oumer Y, Regasa Dadi B, Seid M, Biresaw G, Manilal A. Catheter-associated urinary tract infection: incidence, associated factors and drug resistance patterns of bacterial isolates in Southern Ethiopia. Infect Drug Resist. (2021) 14:2883–94. doi: 10.2147/IDR.S311229
12. Theuretzbacher U. Global antibacterial resistance: the never-ending story. J Glob Antimicrob Resist. (2013) 1:63–9. doi: 10.1016/j.jgar.2013.03.010
13. Bitew A, Zena N, Abdeta A. Bacterial and fungal profile, antibiotic susceptibility patterns of bacterial pathogens and associated risk factors of urinary tract infection among symptomatic pediatrics patients attending St. Paul’s Hospital Millennium Medical College: a cross-sectional study. Infect Drug Resist. (2022) 15:1613–24. doi: 10.2147/IDR.S358153
14. Tesfa T, Baye Y, Sisay M, Amare F, T G. Bacterial uropathogens and susceptibility testing among patients diagnosed with urinary tract infections at Hiwot Fana Specialized University Hospital, Eastern Ethiopia. SAGE Open Med. (2021) 9:20503121211001162. doi: 10.1177/20503121211001162
15. Seid M, Markos M, Aklilu A, Manilal A, Zakir A, Kebede T, et al. Community-acquired urinary tract infection among sexually active women: risk factors, bacterial profile and their antimicrobial susceptibility patterns, Arba Minch, Southern Ethiopia. Infect Drug Resist. (2023) 16:2297–310. doi: 10.2147/IDR.S407092
16. Bryce A, Hay AD, Lane IF, Thornton HV, Wootton M, Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. Br Med J. (2016) 352:i939. doi: 10.1136/bmj.i939
17. Adugna B, Sharew B, Jemal M. Bacterial profile, antimicrobial susceptibility pattern, and associated factors of community- and hospital-acquired urinary tract infection at Dessie Referral Hospital, Dessie, Northeast Ethiopia. Int J Microbiol. (2021) 2021:1–14. doi: 10.1155/2021/5553356
18. Shibabaw A, Sahle Z, Metaferia Y, Atlaw A, Adenew B, Gedefie A, et al. Epidemiology and prevention of hospital-acquired carbapenem-resistant Enterobacterales infection in hospitalized patients, Northeast Ethiopia. IJID Regions. (2023) 7:77–83. doi: 10.1016/j.ijregi.2023.02.008
19. Giesen LGM, Cousins G, Dimitrov BD, van de Laar FA, Fahey T. Predicting acute uncomplicated urinary tract infection in women: a systematic review of the diagnostic accuracy of symptoms and signs. BMC Fam Pract. (2010) 11:1–14. doi: 10.1186/1471-2296-11-1
20. Gebretensaie Y, Atnafu A, Girma S, Alemu Y, Desta K. Prevalence of bacterial urinary tract infection, associated risk factors, and antimicrobial resistance pattern in Addis Ababa, Ethiopia: a cross-sectional study. Infect Drug Resist. (2023) 16:3041–50. doi: 10.2147/IDR.S402279
21. CLSI. Performance Standards for Antimicrobial Susceptibility Testing, M100, 32nd. Wayne, PA: Clinical and Laboratory Standards Institute (2022).
22. WHO. Antimicrobial stewardship programmes in health-care facilities in low-and middle-income countries: a WHO practical toolkit. (2019). p. 1–71. Availlable at: https://www.who.int/publications/i/item/9789241515481 (Accessed August 20, 2024).
23. Lee DS, Lee CB, Lee S-J. Prevalence and risk factors for extended spectrum beta-lactamase-producing uropathogens in patients with urinary tract infection. Korean J Urol. (2010) 51:492–7. doi: 10.4111/kju.2010.51.7.492
24. Kiros T, Zeleke M, Eyayu T, Workineh L, Damtie S, Andualem T, et al. Bacterial etiology of urinary tract infection and antibiogram profile in children attending Debre Tabor Comprehensive Specialized Hospital, Northwest Ethiopia. Interdiscipl Perspect Infect Dis. (2023) 2023:1035113. doi: 10.1155/2023/1035113
25. Seifu WD, Gebissa AD. Prevalence and antibiotic susceptibility of uropathogens from cases of urinary tract infections (UTI) in Shashemene referral hospital, Ethiopia. BMC Infect Dis. (2018) 18:1–9. doi: 10.1186/s12879-017-2892-9
26. Khatoon I, Khanam S, Azam A, Qadeer S, Naz S, Hassan NU. Incidence pattern, antibiotic susceptibility pattern and associated risk factors of bacterial uropathogens among general population of Pakistan. Infect Drug Resist. (2023) 16:4995–5005. doi: 10.2147/IDR.S418045
27. Adugna B, Sharew B, Jemal M. Bacterial profile, antimicrobial susceptibility pattern, and associated factors of community- and hospital-acquired urinary tract infection at Dessie Referral Hospital, Dessie, Northeast Ethiopia. Int J Microbiol. (2021) 2021:5553356. doi: 10.1155/2021/5553356
28. Jombo GTA, Egah DZ, Banwat EB, Ayeni JA. Nosocomial and community acquired urinary tract infections at a teaching hospital in North Central Nigeria: findings from a study of 12,458 samples. Niger J Med. (2006) 15:230–6. doi: 10.4314/njm.v15i3.37219
29. Platt R, Polk BF, Murdock B, Rosner B. Risk factors for nosocomial urinary tract infection. Am J Epidemiol. (1986) 124:977–85. doi: 10.1093/oxfordjournals.aje.a114487
30. Isigi SS, Parsa AD, Alasqah I, Mahmud I, Kabir R. Predisposing factors of nosocomial infections in hospitalized patients in the United Kingdom: systematic review. JMIR Public Health Surveill. (2023) 9:e43743. doi: 10.2196/43743
31. Isikgoz Tasbakan M, Durusoy R, Pullukcu H, Sipahi OR, Ulusoy S, 2011 Turkish Nosocomial Urinary Tract Infection Study Group. Hospital-acquired urinary tract infection point prevalence in Turkey: differences in risk factors among patient groups. Ann Clin Microbiol Antimicrob. (2013) 12:1–8. doi: 10.1186/1476-0711-12-31
32. Mohamed AH, Mohamud MFY, Mohamud HA. Epidemiology and antimicrobial susceptibility pattern of uropathogens in patients with the community- and hospital-acquired urinary tract infections at a tertiary hospital in Somalia. Jundishapur J Microbiol. (2020) 13(9):e107453. doi: 10.5812/jjm.107453
33. Teferi S, Sahlemariam Z, Mekonnen M, Tamrat R, Bekana T, Adisu Y, et al. Uropathogenic bacterial profile and antibiotic susceptibility pattern of isolates among gynecological cases admitted to Jimma Medical Center, South West Ethiopia. Sci Rep. (2023) 13:7078. doi: 10.1038/s41598-023-34048-4
34. Shaker M, Zaki A, Asser SL, Sayed IE. Trends and predictors of antimicrobial resistance among patients with urinary tract infections at a tertiary hospital facility in Alexandria, Egypt: a retrospective record-based classification and regression tree analysis. BMC Infect Dis. (2024) 24:246. doi: 10.1186/s12879-024-09086-6
35. Stewart S, Robertson C, Pan J, Kennedy S, Dancer S, Haahr L, et al. Epidemiology of healthcare-associated infection reported from a hospital-wide incidence study: considerations for infection prevention and control planning. J Hosp Infect. (2021) 114:10–22. doi: 10.1016/j.jhin.2021.03.031
36. Silago V, Moremi N, Mtebe M, Komba E, Masoud S, Mgaya FX, et al. Multidrug-resistant uropathogens causing community acquired urinary tract infections among patients attending health facilities in Mwanza and Dar es Salaam, Tanzania. Antibiotics. (2022) 11:1718. doi: 10.3390/antibiotics11121718
37. Almukhtar SH. Urinary tract infection among women aged (18–40) years old in Kirkuk city, Iraq. Open Nurs J. (2018) 12(1). doi: 10.2174/1874434601812010248
38. Islam MA, Islam MR, Khan R, Amin MB, Rahman M, Hossain MI, et al. Prevalence, etiology and antibiotic resistance patterns of community-acquired urinary tract infections in Dhaka, Bangladesh. PLoS One. (2022) 17:e0274423. doi: 10.1371/journal.pone.0274423
39. Vasudevan R. Urinary tract infection: an overview of the infection and the associated risk factors. J Microbiol Exp. (2014) 1:00008. doi: 10.15406/jmen.2014.01.00008
40. Hamid MA, Bayoumi MA, Hamid O. Urinary tract infections: prevalence, risk factors, and antimicrobial susceptibility profile of associated bacterial pathogens among pregnant women visiting teaching hospitals, Khartoum, Sudan. Merit Res J Med Med Sci. (2022) 10:086–92. doi: 10.5281/zenodo.6393102
41. Noori M, Aghamohammad S, Ashrafian F, Gholami M, Dabiri H, Goudarzi H. A retrospective cross-sectional study on prevalence and susceptibility pattern of bacterial pathogens causing urinary tract infections at a teaching hospital in Tehran, Iran. Journal Arch Pediatr Infect Dis. (2023) 11. doi: 10.5812/pedinfect-120903
42. Donkor ES, Horlortu PZ, Dayie NT, Obeng-Nkrumah N, Labi A-K. Community acquired urinary tract infections among adults in Accra, Ghana. Infect Drug Resist. (2019) 12:2059–67. doi: 10.2147/IDR.S204880
43. Abate D, Kabew G, Urgessa F, Meaza D. Bacterial etiologies, antimicrobial susceptibility patterns and associated risk factors of urinary tract infection among diabetic patients attending diabetic clinics in Harar, Eastern Ethiopia. East Afr Health Biomed Sci. (2017) 1:11–20.
44. Narang P, Narang UR. Urinary tract infection and bacterial virulence. Am J Curr Microbiol. (2018) 5:19–32. Available at: http://www.ivyunion.org
45. Gebremedhin MG, Weldu Y, Kahsay AG, Teame G, Adane K. Extended-spectrum β-lactamase and carbapenemase-producing gram-negative bacteria and associated factors among patients suspected of community and hospital-acquired urinary tract infections at Ayder Comprehensive Specialized Hospital, Tigrai, Ethiopia. Infect Drug Resist. (2023) 16:4025–37. doi: 10.2147/IDR.S412350
46. Mancuso G, Midiri A, Gerace E, Marra M, Zummo S, Biondo C. Urinary tract infections: the current scenario and future prospects. Pathogens. (2023) 2023(12):623. doi: 10.3390/pathogens12040623
47. Mekonnen S, Tesfa T, Shume T, Tebeje F, Urgesa K, Weldegebreal F. Bacterial profile, their antibiotic susceptibility pattern, and associated factors of urinary tract infections in children at Hiwot Fana Specialized University Hospital, Eastern Ethiopia. PLoS One. (2023) 18:e0283637. doi: 10.1371/journal.pone.0283637
48. Reuter A, Virolle C, Goldlust K, Berne-Dedieu A, Nolivos S, Lesterlin C. Direct visualisation of drug-efflux in live Escherichia coli cells. FEMS Microbiol Rev. (2020) 44:782–92. doi: 10.1093/femsre/fuaa031
49. Feleke T, Eshetie S, Dagnew M, Endris M, Abebe W, Tiruneh M, et al. Multidrug-resistant bacterial isolates from patients suspected of nosocomial infections at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Res Notes. (2018) 11:1–7. doi: 10.1186/s13104-018-3709-7
50. Sanjee S, Karim M, Akter T, Parvez M, Hossain M, Jannat B, et al. Prevalence and antibiogram of bacterial uropathogens of urinary tract infections from a tertiary care hospital of Bangladesh. J Sci Res. (2017) 9:317–28. doi: 10.3329/jsr.v9i3.31677
51. Edessa D, Asefa Kumsa F, Dinsa G, Oljira L. Inappropriate antibiotic access practices at the community level in Eastern Ethiopia. Sci Rep. (2024) 14:17751. doi: 10.1038/s41598-024-67688-1
52. Mechal T, Hussen S, Desta M. Bacterial profile, antibiotic susceptibility pattern and associated factors among patients attending adult OPD at Hawassa University Comprehensive Specialized Hospital, Hawassa, Ethiopia. Infect Drug Resist. (2021) 14:99–110. doi: 10.2147/IDR.S287374
53. Gomila A, Shaw E, Carratalà J, Leibovici L, Tebé C, Wiegand I, et al. Predictive factors for multidrug-resistant gram-negative bacteria among hospitalised patients with complicated urinary tract infections. Antimicrob Resist Infect Control. (2018) 7:1–11. doi: 10.1186/s13756-018-0401-6
54. Almeida da Silva JL, Ribeiro da Silva M, Ramos da Cruz Almeida TH, Barbosa DA. Educational interventions to prevent urinary infections in institutionalized elderly people. Quasi-experimental study. Invest Educ Enferm. (2024) 42. doi: 10.17533/udea.iee.v42n1e05
55. Kebamo S, Dabso R, Deressa A, Gebrie M. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors among diabetic patients attending Nekemte Referral Hospital, Ethiopia. Am J Curr Microbiol. (2017) 5(1):19–31. Available at: http://ivyunion.org/index.php/ajcmicrob/
56. Lee AC, Mullany LC, Koffi AK, Rafiqullah I, Khanam R, Folger LV, et al. Urinary tract infections in pregnancy in a rural population of Bangladesh: population-based prevalence, risk factors, etiology, and antibiotic resistance. BMC Pregnancy Childbirth. (2020) 20:1–11. doi: 10.1186/s12884-019-2665-0
57. Paudel L, Manandhar N, Sah S, Khadka S, Neupane S, Joshi SK. Prevalence of urinary tract infection and associated risk factors among women in Sindhupalchowk district, Nepal. Int J CommunMed Public Health. (2018) 5:2714. doi: 10.18203/2394-6040.ijcmph20182604
58. Belete MA, Saravanan M. A systematic review on drug resistant urinary tract infection among pregnant women in developing countries in Africa and Asia; 2005–2016. Infect Drug Resist. (2020) 13:1465–77. doi: 10.2147/IDR.S250654
59. Tandogdu Z, Wagenlehner FM. Global epidemiology of urinary tract infections. Curr Opin Infect Dis. (2016) 29:73–9. doi: 10.1097/QCO.0000000000000228
60. Franco AVM. Recurrent urinary tract infections. Best Pract Res Clin Obstet Gynaecol. (2005) 19:861–73. doi: 10.1016/j.bpobgyn.2005.08.003
61. Garcia-Bustos V, Escrig AIR, López CC, Estellés RA, Jerusalem K, Cabañero-Navalón MD, et al. Prospective cohort study on hospitalised patients with suspected urinary tract infection and risk factors por multidrug resistance. Sci Rep. (2021) 11:11927. doi: 10.1038/s41598-021-90949-2
62. Zhu M, Wang S, Zhu Y, Wang Z, Zhao M, Chen D, et al. Behavioral and dietary risk factors of recurrent urinary tract infection in Chinese postmenopausal women: a case–control study. J Int Med Res. (2020) 48:0300060519889448. doi: 10.1177/0300060519889448
63. Jagtap S, Harikumar S, Vinayagamoorthy V, Mukhopadhyay S, Dongre A. Comprehensive assessment of holding urine as a behavioral risk factor for UTI in women and reasons for delayed voiding. BMC Infect Dis. (2022) 22:521. doi: 10.1186/s12879-022-07501-4
64. Haghdoost S, Pazandeh F, Khabazkhoob M, Bhroozi R. Assocoation between sexual and genital hygiene habits with the urinary tact infectin during pregnancy: a case-control study. Int J Women’s Health Reprod Sci. (2020) 8:158–63. doi: 10.15296/ijwhr.2020.25
65. Badran YA, El-Kashef TA, Abdelaziz AS, Ali MM. Impact of genital hygiene and sexual activity on urinary tract infection during pregnancy. Urol Ann. (2015) 7:478–81. doi: 10.4103/0974-7796.157971
66. Gemechu MM, Tadesse TA, Takele GN, Bisetegn FS, Gesese YA, Zelelie TZ. Bacterial profile and their antimicrobial susceptibility patterns in patients admitted at MaddaWalabu University Goba Referral Hospital, Ethiopia: a cross sectional study. Afr Health Sci. (2021) 21:513–22. doi: 10.4314/ahs.v21i2.5
67. Kamal Abd Elkhalek N, Ezz Elregal Ibrahim Eisa E, Ahmed Mohammed Sabry F. Correlation between genital hygiene and sexual behavior with urinary tract infections in pregnant women. Egypt J Nurs Health Sci. (2021) 2:317–37. doi: 10.21608/ejnhs.2021.196032
68. Booth J, Agnew R. Evaluating a hydration intervention (DRInK Up) to prevent urinary tract infection in care home residents: a mixed methods exploratory study. J Frailty Sarcopenia Falls. (2019) 4:36. doi: 10.22540/JFSF-04-036
69. Yoshida B, Nguyen A, Formanek B, Alam M, Wang JC, Buser Z. Hypoalbuminemia and elevated CRP are risk factors for deep infections and urinary tract infections after lumbar spine surgery in a large retrospective patient population. Global Spine J. (2023) 13:33–44. doi: 10.1177/2192568221990647
70. Suen KFK, Low JXY, Charalambous CP. Urinary tract infection is associated with 2.4-fold increased risk of surgical site infection in hip fracture surgery: systematic review and meta-analysis. J Hosp Infect. (2023) 139:56–66. doi: 10.1016/j.jhin.2023.06.016
71. Sanaee MS, Pan K, Lee T, Koenig NA, Geoffrion R. Urinary tract infection after clean-contaminated pelvic surgery: a retrospective cohort study and prediction model. Int Urogynecol J. (2020) 31:1821–8. doi: 10.1007/s00192-019-04119-0
72. Hansen B, Søreide E, Warland A, Nilsen OB. Risk factors of post-operative urinary retention in hospitalised patients. Acta Anaesthesiol Scand. (2011) 55(5):545–8. doi: 10.1111/j.1399-6576.2011.02416.x
73. Burns R. A case study of eliminating urinary tract infections for an elderly woman with frequent recurring UTIs. Can J Med. (2023) 5:44–7. doi: 10.33844/cjm.2023.6031
74. Nava B. Hygiene is a human right: access to hygiene facilities in unsheltered individuals and its effects on health. (2023).
Keywords: nosocomial acquired UTI, antimicrobial susceptibility patterns, community-acquired UTI, Ethiopia, risk factors
Citation: Fekadu S, Weldegebreal F, Shumie T and Mekonnen GK (2025) A comparative study on nosocomial and community-acquired bacterial urinary tract infections: prevalence, antimicrobial susceptibility pattern, and associated risk factors among symptomatic patients attending Hiwot Fana Comprehensive Specialized University Hospital, Eastern Ethiopia. Front. Epidemiol. 5:1517476. doi: 10.3389/fepid.2025.1517476
Received: 26 October 2024; Accepted: 5 March 2025;
Published: 7 April 2025.
Edited by:
Shailendra Saxena, King George's Medical University, IndiaReviewed by:
Anna Benini, University of Verona, ItalyCopyright: © 2025 Fekadu, Weldegebreal, Shumie and Mekonnen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Getachew Kabew Mekonnen, Z2VjaG1pY3JvQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.