
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Epidemiol., 04 February 2025
Sec. Cardiovascular Epidemiology
Volume 4 - 2024 | https://doi.org/10.3389/fepid.2024.1503261
Background: Obesity is closely associated with lipid metabolism, and the accumulation of lipids leads to low-level inflammation in the body, which can trigger cardiovascular disease. This study aimed to explore the association between a novel marker of lipid accumulation, the abdominal volume index (AVI), inflammatory parameters, and mortality.
Methods: This study enrolled 2,109 older adult senior citizens (aged over 60 years) with hypertension from the National Health and Nutrition Examination Survey. The primary endpoints included all-cause mortality and cardiovascular mortality, which were assessed by linking the data to the National Death Index records. Cox regression model and subgroup analysis were constructed to investigate the associations between AVI and both all-cause and cardiovascular mortality. Restricted cubic splines were employed to further explore the relationships among AVI, inflammatory parameters, and mortality. By considering inflammatory factors as mediators, we investigate the mediating effects of AVI on mortality.
Results: After a median follow-up of 69 months, there were 1,260 deaths, with 337 attributed to cardiovascular causes within the older adult population studied. In the multivariable-adjusted model, AVI was positively associated with both all-cause and cardiovascular mortality [Hazard Ratio (HR) = 1.09, 95% CI = 1.06–1.11 for all-cause mortality; HR = 1.07, 95% CI = 1.03–1.12 for cardiovascular mortality]. Kaplan-Meier survival plots indicated an overall median survival time of 144 months. Mediation analysis revealed that Systemic Inflammatory Response Index (SIRI), Monocyte-to-HDL ratio (MHR), and Neutrophil-to-Lymphocyte ratio (NLR) mediated 27.15%, 35.15%, and 16.55%, respectively, of the association between AVI and all-cause mortality.
Conclusion: AVI is positively associated with all-cause mortality in older adults with hypertension, and this association appears to be partially mediated by inflammatory parameters.
As the global population ages, the mortality associated with cardiovascular disease (CVD) continues to rise, presenting a significant public health challenge (1). CVD remains the leading cause of death among older adults, with nearly half of all cardiovascular deaths attributable to high blood pressure (2). The prevalence of hypertensive (defined as blood pressure ≥140/90 mmHg) has increased from 5.1% in 1958–1959 to 23.2% in 2012–2015 (3, 4). Cohort studies have demonstrated that hypertension is linked to a higher risk of all-cause mortality among the oldest segments of the population in China, showing a U-shaped association between systolic blood pressure and mortality (5), which indicates a need for increased healthcare resources to address the needs of hypertensive older adult individuals.
Obesity is significantly associated with increased all-cause mortality (6). It is characterized by excessive lipid accumulation and often accompanies metabolic abnormalities that trigger insulin resistance and related complications, including diabetes, CVD, and metabolic syndrome (MetS) (7, 8). There is growing concern over the adverse outcomes inked to central obesity, particularly abdominal fat. Although computed tomography (CT) and magnetic resonance imaging (MRI) are considered the gold standards for assessing abdominal fat distribution (9), commonly used anthropometric measures such as body mass index (BMI), waist circumference, and waist-to-hip ratio also provide valuable evaluations (10). The abdominal volume index (AVI), a novel parameter, measures overall abdominal volume and assesses fat distribution. Research indicates that AVI may be a superior predictor of MetS, particularly in women (11). This study aims to investigate the association between AVI and mortality and to further analyze whether this association is mediated through the inflammatory response.
National Health and Nutrition Examination Survey (NHANES) is a population-based representative survey performed by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) (12). Its primary goal is to assess the health status and disease risk factors in the American population (13). NHANES collects comprehensive information, including demographic data, disease-related questionnaire responses, examination results, and laboratory data, through structured home interviews, physical examinations performed at mobile centers, and laboratory tests conducted at analytical facilities. The datasets analyzed in this study can be accessed through the NHANES website (https://www.cdc.gov/nchs/nhanes/index.htm). The NHANES protocol was approved by the research ethics review board of NCHS, and informed consent was obtained from all participants (14).
In brief, we analyzed 6 cycles of NHANES data spanning 11 years, from 2007 to 2018 (2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, 2017–2018). A total of 59,842 participants were identified from the NHANES 2007–2018 data. We excluded individuals under the age of 60 (n = 47,932) and those without hypertension (n = 4,585). After further excluding participants with missing data on all-cause mortality (n = 14), BMI (n = 157), waist circumference (n = 420), triglycerides (n = 152), complete blood count (n = 672), medical history (n = 458), the final analysis included 5,433 individuals (see Supplementary Documents for screening process plots).
The NHANES public-use linked mortality file as of December 31, 2018, correlated with NCHS with the National Death Index, was used to determine mortality status in the follow-up individuals (15). The main outcomes in this study were all-cause mortality and cardiovascular mortality.
The parameters involving AVI, SIRI, NLR, and MHR were calculated using the below formulas:
The SIRI (systemic inflammatory response index) was defined as a neutrophil count × monocyte count/lymphocyte count and expressed as × 10^9 cells/µl (16).
The NLR (neutrophil-to-lymphocyte ratio) was defined as neutrophil count/lymphocyte count (17).
The MHR (monocyte to high-density lipoprotein) was defined as monocyte count/high-density lipoprotein (18, 19).
WC waist circumference, HC height circumference. CM centimeter (the unit).
We downloaded various data from NHANES, including demographic information [age, gender, race, education levels, family income-to-poverty ratio (PIR)], smoking status, alcohol consumption, and medical history [heart failure (HF), coronary artery disease (CAD), angina, stroke, diabetes, and hypercholesterolemia]. At a mobile examination center, participants' waist circumference (WC), hip circumference (HC), and body mass index (BMI) were measured. BMI was calculated as the ratio of weight (kg) to height (cm) squared. Additionally, Serum specimens were collected as part of the NHANES laboratory examination component for 2007–2018, processed, stored, and sent to Collaborative Laboratory Services for analysis. At baseline, we measured complete blood count, plasma albumin (g/L), blood urea nitrogen (mg/dl), creatinine (µmol/L), uric acid (UA) (µmol/L), alanine aminotransferase (AST) (U/L), aspartate aminotransferase (U/L), glycohemoglobin (%), glucose (mmol/L), cholesterol (mmol/L), HDL-cholesterol (mmol/L), and triglyceride (mmol/L) levels. Detailed information regarding these methods is publicly available on the NHANES website (20). Race was classified into categories including Mexican American, other Hispanic, non-Hispanic White, non-Hispanic Black, or another race (21). Education level was categorized into three groups: less than high school, high school, and higher than high school. The family income-to-poverty ratio (PIR) was divided into three categories: less than 1.3 for low-income, between 1.3 and 3.5 for middle-income, and above 3.5 for high-income households (22). Smoker was defined as participants who had smoked more than 100 cigarettes in their lifetime (23), regardless of whether they had quit smoking at the time of the interview (24). Alcohol drinkers were defined as individuals who consumed at least 12 drinks in the year preceding the survey (25). Questionnaires were used to diagnose hypertension (BPD035), heart failure (MCQ160b), CAD (MCQ160c), angina (MCQ160d), heart attack (MCQ160e), and stroke (MCQ160f) (26).
All data were analyzed using R statistics (version 4.2.2, https://cran.r-project.org/). The Kolmogorov–Smirnov test indicated that all continuous variables did not follow a normal distribution; therefore, these variables are presented as medians with interquartile ranges. Categorical variables are expressed as frequencies and percentages. To examine differences in variables across different levels of the Atrial Volume Index (AVI), we categorized AVI into tertiles, from the lowest to the highest. The Chi-squared test or the Kruskal-Wallis H-test was utilized to analyze differences in AVI across these four categories. The one-way ANOVA test, Kruskal-Wallis H-test, or Chi-squared test were employed to compare continuous or categorical variables within the tertile groups of AVI (27). A p-value of <0.05 was considered statistically significant.
Multivariate Cox regression models were used to estimate the associations between AVI and all-cause as well as cardiovascular mortality, calculating hazard ratios (HRs) and 95% confidence intervals (CIs). Model 1 was unadjusted, while Model 2 was adjusted for gender, age, race, and education level. Model 3 included further adjustments for poverty income ratio (PIR), body mass index (BMI), smoking status, alcohol consumption, diabetes, hypercholesterolemia, cholesterol, and triglycerides. Subgroup analyses were conducted to explore potential differences among specific populations based on gender, race, education, PIR, diabetes, coronary artery disease (CAD), angina, stroke, smoking, and drinking status. Kaplan-Meier curves were plotted to estimate survival probabilities over time across different AVI levels, with the log-rank test employed to assess disparities among the tertile curves. A restricted cubic spline (RCS) model was applied to investigate the non-linear relationships between AVI, inflammatory parameters, and all-cause or cardiovascular mortality. Additionally, multivariate logistic regression was conducted to assess the associations between inflammatory parameters and AVI. The receiver operating characteristic (ROC) curve was utilized to determine the cutoff value of AVI for identifying mortality. The same statistical methods were applied in multivariate Cox regression to explore the associations between inflammatory parameters and mortality. Subsequently, mediation analysis was performed to assess whether the relationship between AVI and mortality was partially mediated by inflammatory parameters. The presence of a mediating effect was defined by significant indirect, direct, and total effects (26).
Eventually, we developed a predictive model for AVI to assess mortality risk. To eliminate collinearity among variables, we employed the least absolute shrinkage and selection operator (LASSO) regression model to exclude certain variables (28). The model's evaluation was conducted using 10-fold cross-validation, and we plotted a curve concerning lambda values. A risk prediction nomogram plot was created based on key mortality-related variables, including AVI and inflammatory parameters (SIRI and MHR). The model's forecasting performance was quantified by the area under the curve (AUC) with a 95% confidence interval from the ROC curve. The clinical applicability of the model was assessed through decision curve analysis.
Table 1 presents the baseline characteristics of the individuals enrolled in this study across different AVI tertiles. Among the participants, the median AVI values were 23.93, with ranges from 19.91 in T1, 23.93 in T2, to 29.49 in T3. The median age of the 5,433 senior citizens was 70 years, with approximately 47.78% being male. The incidence of all-cause and cardiovascular mortality among these participants during a median follow-up of 69 months was 23.19% and 6.20%, respectively. Compared to those in the low AVI group, participants with elevated AVI levels exhibited a higher prevalence of smoking, alcohol consumption, lower education levels, and PIR. Additionally, in terms of medical history, participants in higher quartiles reported a greater prevalence of diabetes, hypercholesterolemia, heart failure (HF), CAD, angina, and heart attacks. Regarding physical examination metrics, the T3 group demonstrated significantly higher levels of weight, height, BMI, and WC. Participants with higher AVI levels also showed significantly elevated inflammatory parameters (including SIR, MHR, and NLR), complete blood cell counts (including leukocyte, lymphocyte, and monocyte counts), as well as higher levels of ALT, creatinine, uric acid (UA), glucose, triglycerides, and HbA1c.
In this study, after a median follow-up of 69 months, we observed 1,260 deaths, including 337 attributed to cardiovascular causes, within the older adult population. Table 2 illustrates the associations between the Atherogenic Index of Plasma (AVI) and both all-cause and cardiovascular mortality. In the crude model, AVI demonstrated a significant association with an increased risk of all-cause mortality (HR = 1.03, 95% CI = 1.02–1.04) and cardiovascular mortality (HR = 1.04, 95% CI = 1.02–1.06). These associations remained robust and statistically significant after multivariable adjustments, with Model 2 showing HRs of 1.04 (95% CI = 1.03–1.05) for all-cause mortality and 1.06 (95% CI = 1.03–1.08) for cardiovascular mortality, and Model 3 showing HRs of 1.09 (95% CI = 1.06–1.11) and 1.07 (95% CI = 1.03–1.12) respectively. Compared to the first tertile of AVI, the hazard ratios for participants in the second, third, and fourth tertiles were consistently higher, regardless of variable adjustments. The Kaplan-Meier survival plots presented in Figure 1 indicate significant differences in all-cause and cardiovascular mortality across AVI tertiles (P < 0.01), with an overall median survival time of 144 months in tertile 3 for all-cause mortality.
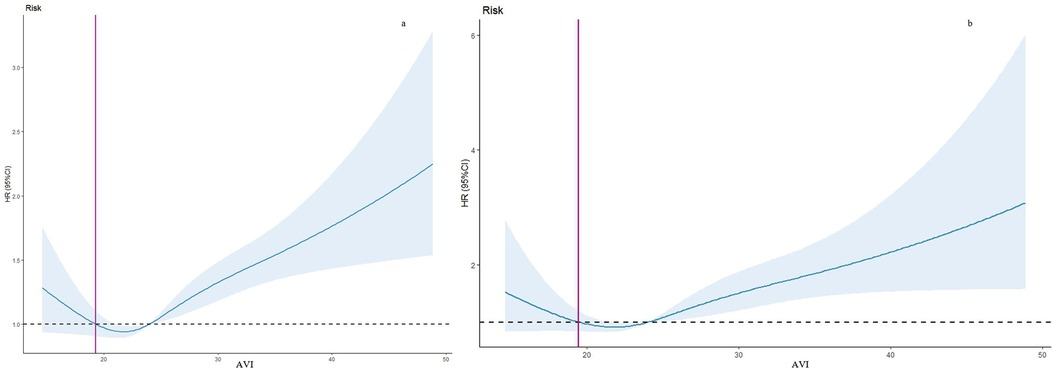
Figure 1. Kaplan–Meier curves of the survival rate of participants with abdominal volume index (AVI) tertiles. (a) All-cause mortality. (b) Cardiovascular mortality.
Using restricted cubic spline regression (RCS) models adjusted for the aforementioned confounders, we identified an L-shaped association between Dietary Inflammatory Index (DII) and both all-cause and cardiovascular mortality (Figure 2). The cut-off values were determined to be 19.26 for all-cause mortality and 19.43 for cardiovascular mortality.
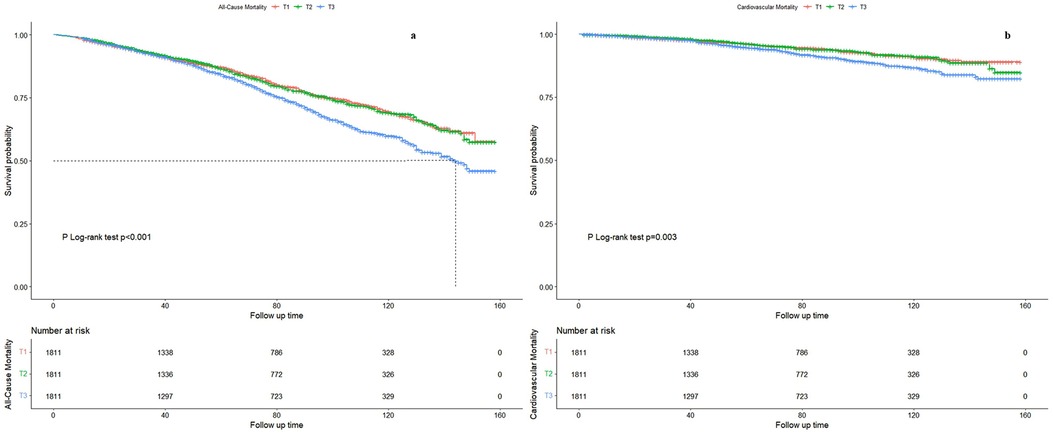
Figure 2. Restricted cubic spline regression curve of the association between abdominal volume index (AVI) and mortality. (a) All-cause mortality. (b) Cardiovascular mortality. The value corresponding to the red vertical line is the cutoff value in the x-axis. HR, hazard ratio; CI, confidence interval.
We conducted subgroup analyses to further explore the relationships between AVI and mortality, with detailed results presented in Figure 3. Most analyses revealed differences within groups; however, the association between AVI and all-cause mortality was not significant among participants of other races, those with a PIR ≥ 3.5, individuals with only a high school diploma, and those with coronary artery disease (CAD) or angina. For cardiovascular mortality, significant differences were mostly absent across subgroup analyses, except for female participants, Hispanic participants, those with a PIR < 3.5, individuals with a high school education or less, and non-diabetics who did not have CAD, angina, or a smoking and drinking history.
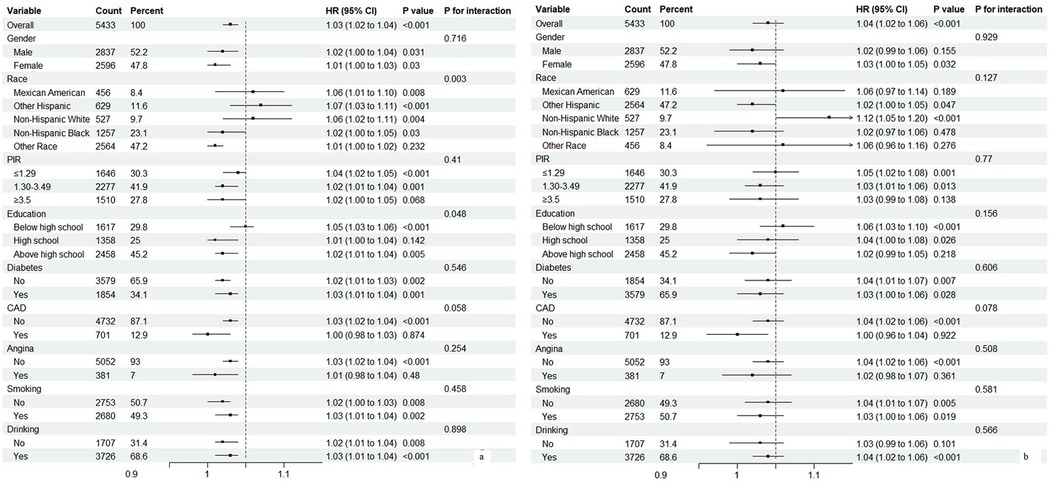
Figure 3. Subgroup analysis of associations between abdominal volume index (AVI) and mortality. (a) All-cause mortality. (b) Cardiovascular mortality.
Table 3 displays the associations between AVI and inflammatory parameters as determined by multivariate logistic regression. The results indicate that AVI is positively associated with Systemic Inflammation Response Index (SIRI) (OR = 1.12, 95% CI = 1.07–1.18), Monocyte to High-Density Lipoprotein Ratio (MHR) (OR = 5.69, 95% CI = 4.47–7.24), and Neutrophil to Lymphocyte Ratio (NLR) (OR = 1.04, 95% CI = 1.01–1.08). We employed RCS models with three knots (10th, 50th, and 90th percentiles) to assess the nonlinearity of these associations, confirming an L-shaped relationship (see Supplementary Documents).
Cox regression results examining the impact of inflammatory parameters on all-cause and cardiovascular mortality are summarized in Table 4. All indicators were positively associated with all-cause mortality, and most factors also correlated with cardiovascular mortality, with the exception of MHR in Model 3. Additionally, RCS models adjusted for confounders revealed an anhaped association between the two (see supplementary documents.
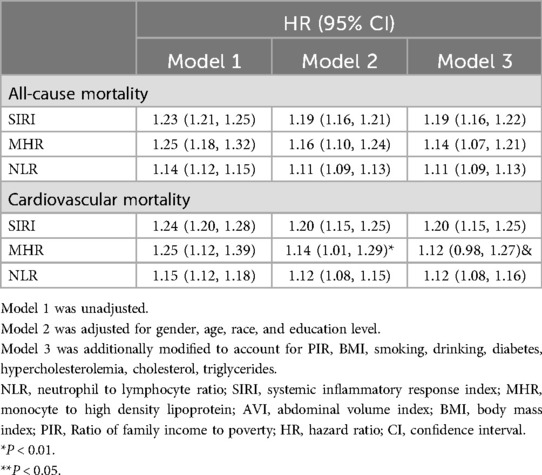
Table 4. The associations of inflammatory parameters with all-cause mortality and cardiovascular mortality.
Figure 4(a) illustrates the mediation effects of SIRI, MHR, and NLR on the relationship between AVI and all-cause mortality, accounting for 27.15%, 35.15%, and 16.55% of the association, respectively. Figure 4(b) shows that these parameters mediated 14.08%, 9.71%, and 8.17% of the association between AVI and cardiovascular mortality.
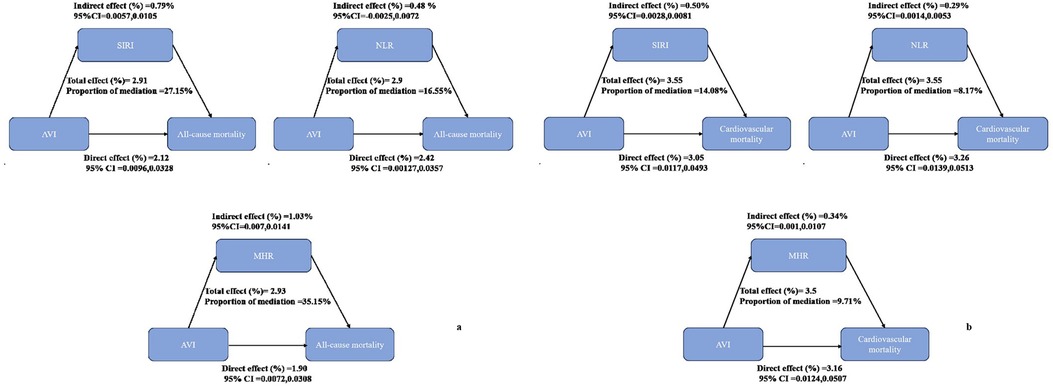
Figure 4. Analysis of the mediation by inflammatory parameters including systemic inflammatory response index (SIRI), neutrophil-to-lymphocyte ratio (NLR), and monocyte-to-high-density lipoprotein (MHR). (a) All-cause mortality. (b) Cardiovascular mortality.
We developed a risk prediction model utilizing LASSO penalized regression, incorporating 33 additional covariates (including age, gender, PIR, BMI, and laboratory examination results) to assess the impact of AVI on all-cause mortality. Through LASSO regression and a 10-fold cross-validation method for model evaluation, we identified 12 significant variables for inclusion in the nomogram. The predictive performance for AVI was validated using the ROC curve (AUC = 0.76 for the total), with performance metrics for 2, 3, and 4 years being 0.62, 0.56, and 0.52, respectively (see Supplementary Documents).
In this study, we identified a positive association between Abdominal Volume Index (AVI) and mortality among older adults with hypertension. Notably, this association remained significant even after applying a comprehensive adjustment model. Our findings demonstrated positive correlations among inflammatory parameters, mortality, and AVI, as indicated by Cox and logistic regression analyses. To further explore the potential inflammatory mechanisms linking AVI to mortality, we employed a mediation effect model to examine the significant roles of the Systemic Inflammation Response Index (SIRI), Monocyte to High-Density Lipoprotein Ratio (MHR), and Neutrophil to Lymphocyte Ratio (NLR). Given that both AVI and inflammatory parameters serve as risk factors for mortality, we developed a prognostic nomogram model, which was validated to effectively predict all-cause mortality.
Obesity, characterized by excessive body fat accumulation, has become a major global public health challenge. The prevalence of obesity in adults has risen dramatically, increasing from 3% in 1975 to 11% among men and from 6% to 15% among women by 2016 (29). A larger-scale study indicated that obesity throughout adulthood, as well as weight gain from youth to middle age, correlates with higher mortality risk (29). Defined as a chronic and complex disease, obesity can lead to various adverse cardiovascular events (30, 31). Body Mass Index (BMI) is a widely recognized indicator of obesity, with a well-established J-shaped association with mortality (32). However, many previous cohort studies relied on a single BMI measurement, neglecting other parameters that assess lipid accumulation. Indicators like AVI, which are easy to calculate and readily accessible, serve as proxies for visceral fat accumulation and are linked to impaired glucose tolerance and insulin resistance (IR). Fernando Guerrero-Romero highlighted AVI as a reliable tool for estimating overall abdominal volume, showing a strong relationship with IR (OR = 1.6, 95% CI: 1.1–9.1) (33). By better assessing abdominal fat accumulation, AVI offers improved predictions for the development of metabolic syndrome (MetS) (34). Given the interrelationship among IR, MetS, and diabetes, evaluating abdominal fat is crucial for assessing adiposity and predicting the risks of Type 2 Diabetes (T2D) and MetS (35). These metabolic diseases can impair vascular function, leading to atherosclerosis and cardiovascular events. A study found that AVI is an optimal predictor of cardiometabolic abnormalities in a cohort of Lebanese adults, with a cutoff value of 19.61 (36). Furthermore, abdominal fat deposition correlates with various cardiometabolic risk factors, including blood pressure, triglycerides, and cholesterol levels (37). A prospective cohort to investigate the impact of computed tomography (CT)-measured abdominal fat levels on mortality in undergoing hemodialysis patients, reported that high abdominal fat distribution has a high risk of death (38).
Moreover, numerous inflammatory parameters have been consistently linked to both obesity and the risk of adverse outcomes associated with obesity-related diseases (39). A meta-analysis encompassing 51 cross-sectional studies supports a positive correlation between C-reactive protein (CRP) and various obesity indicators, including BMI, waist circumference (WC), and waist-to-hip ratio (40). Increases in various inflammatory parameters have also been associated with an increased risk of obesity-related diseases, including cardiovascular disease (41, 42). Furthermore, several studies have indicated that elevated circulating levels of inflammatory cytokines, such as tumor necrosis factor (TNF)α, interleukin (IL)-6, or CRP, have been documented in overweight adults (43–45). Interestingly, other markers of abdominal obesity (e.g., WC) appear to be more strongly associated with inflammatory parameters than BMI (46), indicating a greater impact of central obesity on inflammation. Similarly, VAI as a novel marker of abdominal fat can be applied to characterize lipid accumulation, and our study confirms this perspective. Specifically, AVI and inflammatory parameters involving SIRI, MHR, and NLR exhibit an L-shaped association. Moreover, the relationship between lipid accumulation and inflammatory parameters has been described in previous studies. For instance, Hermsdorff revealed that indices of abdominal fat accumulation were associated with CRP, IL-6, and retinol-binding protein 4 concentrations (47). Even in non-obese subjects, CRP has a positive association with abdominal fat (48). Some studies have reported that adipocytes can secrete substantial bioactive molecules with immuno-modulatory actions, such as leptin and adiponectin (49, 50). Leptin can stimulate monocyte proliferation and differentiation into macrophages, which induces the production of pro-inflammatory cytokines such as TNFα, IL-6, or IL-12 (51). Elevated blood lipid levels in obese adults may produce a toxic effect on their adipose tissue according to the “adipose tissue expandability” hypothesis (52). Additionally, fatty acids can trigger the inflammatory response by modulating adipokine production or secretion. NEFA can cause an inflammatory response by modulating adipokine production or activating Toll-like receptors (53).
There is considerable evidence supporting the correlation between inflammation and mortality. Yiyuan Xia reported that adults with SIRI levels greater than 1.43 had a higher risk of all-cause mortality (HR: 1.39, 95%CI: 1.26–1.52) and cardiovascular death (HR: 1.39, 95%CI: 1.14–1.68) (54). This study also provides evidence for these novel inflammation indices in predicting mortality. Notably, inflammation indicators may correspond to various signaling pathways, such as mitogen-activated protein kinase (MAPK) pathways, PI3 K/Akt pathways, and NF-κB signaling pathways (55). The PI3 K/Akt pathway can promote cell survival by inhibiting apoptotic processes through mediating responses to chemokines and other inflammatory stimuli (56). TNFR1 activates the MAPK and NF-κB pathways to promote inflammation leading to apoptosis (57). In this study, we found that inflammatory parameters mediated the association between AVI and mortality. Xiaoqi Deng also indicated a significant association between SII and both all-cause and cardiovascular mortality (58).
This study was a prospective cohort study with an explicit causal association. We provided additional evidence supporting the positive associations of AVI with all-cause mortality in older adults with hypertension. We also highlight the mediating role of inflammation in the associations of AVI with mortality. In summary, we underscore that obesity may cause lipid accumulation, further triggering low-grade inflammation in the body and related poor prognosis of obesity. The main limitation of this study is that diagnoses of hypertension, DM, heart failure, etc., were determined by questionnaires, which may introduce recall bias, potentially affecting the accuracy of covariates.
This study provided evidence for the positive associations of AVI with all-cause mortality in the older population with hypertension, while also highlighting the significant mediating role of inflammatory indices in this association.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
The studies involving humans were approved by The NHANES has been approved by the National Center for Health Statistics Ethics Review Board, and all participants were provided informed written consent at enrollment. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YC: Software, Conceptualization, Investigation, Methodology, Writing – original draft. YZ: Conceptualization, Investigation, Methodology, Software, Writing – original draft. HL: Investigation, Writing – original draft, Data curation. SZ: Investigation, Visualization, Writing – original draft. GJ: Investigation, Visualization, Writing – original draft. WW: Visualization, Software, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We sincerely thank the participants of the NHANES for their contributions and all members who contributed to this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fepid.2024.1503261/full#supplementary-material
1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. (2020) 141(9):e139–596. doi: 10.1161/CIR.0000000000000757
2. Pokharel Y, Karmacharya BM, Neupane D. Hypertension-A silent killer without global bounds: what next? J Am Coll Cardiol. (2022) 80(8):818–20. doi: 10.1016/j.jacc.2022.05.043
3. Wang JG, Zhang W, Li Y, Liu L. Hypertension in China: epidemiology and treatment initiatives. Nat Rev Cardiol. (2023) 20(8):531–45. doi: 10.1038/s41569-022-00829-z
4. Zhang M, Shi Y, Zhou B, Huang Z, Zhao Z, Li C, et al. Prevalence, awareness, treatment, and control of hypertension in China, 2004–18: findings from six rounds of a national survey. Br Med J. (2023) 380:e071952. doi: 10.1136/bmj-2022-071952
5. Lv YB, Gao X, Yin ZX, Chen HS, Luo JS, Brasher MS, et al. Revisiting the association of blood pressure with mortality in oldest old people in China: community based, longitudinal prospective study. Br Med J. (2018) 361:k2158. doi: 10.1136/bmj.k2158
6. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. (2013) 309(1):71–82. doi: 10.1001/jama.2012.113905
7. Goossens GH. The metabolic phenotype in obesity: fat mass, body fat distribution, and adipose tissue function. Obes Facts. (2017) 10(3):207–15. doi: 10.1159/000471488
8. Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. (2012) 85(1009):1–10. doi: 10.1259/bjr/38447238
9. Finch P. Intra-abdominal fat: comparison of computed tomography fat segmentation and bioimpedance spectroscopy. Malawi Med J. (2017) 29(2):155–9. doi: 10.4314/mmj.v29i2.15
10. Quaye L, Owiredu WKBA, Amidu N, Dapare PPM, Adams Y. Comparative abilities of body mass Index, Waist circumference, abdominal volume Index, body adiposity Index, and conicity index as predictive screening tools for metabolic syndrome among apparently healthy Ghanaian adults. J Obes. (2019) 2019:8143179. doi: 10.1155/2019/8143179
11. Witarto BS, Witarto AP, Visuddho V, Wungu CDK, Maimunah U, Rejeki PS, et al. Gender-specific accuracy of lipid accumulation product index for the screening of metabolic syndrome in general adults: a meta-analysis and comparative analysis with other adiposity indicators. Lipids Health Dis. (2024) 23(1):198. doi: 10.1186/s12944-024-02190-1
12. Cetners for disease control and prevention. About the National health and nutrition examination survey. NHANES. Available online at: https://www.cdc.gov/nchs/nhanes/index.htm (accessed January 8 2022).
13. Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National health and nutrition examination survey: sample design, 2007–2010. Vital Health Stat 2. (2013) 160:1–23.
14. National Center for Health Statistics, Centers for Disease Control and Prevention NCHS research ethics review board (ERB) approval. Available online at: https://www.cdc.gov/nchs/nhanes/irba98.htm (accessed January 8 2022).
15. National Center for Health Statistics. National Health and Nutrition Examination Survey. Atlanta: Centers for Disease Control and Prevention; 2018. Available online at: https://www.cdc.gov/nchs/nhanes/index.htm (cited November 29, 2018).
16. Wang RH, Wen WX, Jiang ZP, Du ZP, Ma ZH, Lu AL, et al. The clinical value of neutrophil-to-lymphocyte ratio (NLR), systemic immune-inflammation index (SII), platelet-to-lymphocyte ratio (PLR) and systemic inflammation response index (SIRI) for predicting the occurrence and severity of pneumonia in patients with intracerebral hemorrhage. Front Immunol. (2023) 14:1115031. doi: 10.3389/fimmu.2023.1115031
17. Sakurai A, Yamaguchi K, Ishida K, Horikawa N, Kawai E, Kotani Y, et al. Prognostic significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio in uterine carcinosarcoma. Int J Clin Oncol. (2025). doi: 10.1007/s10147-024-02687-w
18. Berna-Rico E, Abbad-Jaime de Aragon C, Ballester-Martinez A, Perez-Bootello J, Solis J, Fernandez-Friera L, et al. Monocyte-to-high-density lipoprotein ratio is associated with systemic inflammation, insulin resistance, and coronary subclinical atherosclerosis in psoriasis: results from 2 observational cohorts. J Invest Dermatol. (2024) 144(9):2002-2012.e2. doi: 10.1016/j.jid.2024.02.015
19. Li H, Zhang Y, Luo H, Lin R. The lipid accumulation product is a powerful tool to diagnose metabolic dysfunction-associated fatty liver disease in the United States adults. Front Endocrinol (Lausanne). (2022) 13:977625. doi: 10.3389/fendo.2022.977625
20. Bailey RL, Durazo-Arvizu RA, Carmel R, Green R, Pfeiffer CM, Sempos CT, et al. Modeling a methylmalonic acid-derived change point for serum vitamin B-12 for adults in NHANES. Am J Clin Nutr. (2013) 98(2):460–7. doi: 10.3945/ajcn.113.061234
21. Patel JS, Oh Y, Rand KL, Wu W, Cyders MA, Kroenke K, et al. Measurement invariance of the patient health questionnaire-9 (PHQ-9) depression screener in U.S. Adults across sex, race/ethnicity, and education level: nHANES 2005–2016. Depress Anxiety. (2019) 36(9):813–23. doi: 10.1002/da.22940
22. Okosun IS, Annor FB, Seale JP, Eriksen MP. Abdominal adiposity and family income-to-poverty ratio in American women. Obes Res Clin Pract. (2014) 8(3):e201–98. doi: 10.1016/j.orcp.2012.12.002
23. Zhu S, Ji L, He Z, Zhang W, Tong Y, Luo J, et al. Association of smoking and osteoarthritis in US (NHANES 1999–2018). Sci Rep. (2023) 13(1):3911. doi: 10.1038/s41598-023-30644-6
24. Liu C, Zhao M, Zhao Y, Hu Y. Association between serum total testosterone levels and metabolic syndrome among adult women in the United States, NHANES 2011–2016. Front Endocrinol. (2023) 14:1053665. doi: 10.3389/fendo.2023.1053665
25. Chen X, Wei G, Jalili T, Metos J, Giri A, Cho ME, et al. The associations of plant protein intake with all-cause mortality in CKD. Am J Kidney Dis. (2016) 67(3):423–30. doi: 10.1053/j.ajkd.2015.10.018
26. Xu B, Wu Q, La R, Lu L, Abdu FA, Yin G, et al. Is systemic inflammation a missing link between cardiometabolic index with mortality? Evidence from a large population-based study. Cardiovasc Diabetol. (2024) 23(1):212. doi: 10.1186/s12933-024-02251-w
27. Cao Y, Li P, Zhang Y, Qiu M, Li J, Ma S, et al. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: results from NHANES. Front Immunol. (2023 ) 14:1087345. doi: 10.3389/fimmu.2023.1087345
28. Rajaratnam B, Roberts S, Sparks D, Dalal O. Lasso regression: estimation and shrinkage via the limit of gibbs sampling. J Royal Stat Soc Ser B: Stat Methodol. (2015) 78(1):153–74. doi: 10.1111/rssb.12106
29. Chen C, Ye Y, Zhang Y, Pan XF, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. Br Med J. (2019) 367:l5584. doi: 10.1136/bmj.l5584
30. Huang Y, Zhou Y, Xu Y, Wang X, Zhou Z, Wu K, et al. Inflammatory markers link triglyceride-glucose index and obesity indicators with adverse cardiovascular events in patients with hypertension: insights from three cohorts. Cardiovasc Diabetol. (2025) 24(1):11. doi: 10.1186/s12933-024-02571-x
31. Liang W, Ouyang H. The association between triglyceride-glucose index combined with obesity indicators and stroke risk: a longitudinal study based on CHARLS data. BMC Endocr Disord. (2024) 24(1):234. doi: 10.1186/s12902-024-01729-8
32. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. (2017) 390(10113):2627–42. doi: 10.1016/S0140-6736(17)32129-3
33. Guerrero-Romero F, Rodríguez-Morán M. Abdominal volume index. An anthropometry-based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch Med Res. (2003) 34(5):428–32. doi: 10.1016/S0188-4409(03)00073-0
34. Ozturk EE, Yildiz H. Evaluation of different anthropometric indices for predicting metabolic syndrome. Eur Rev Med Pharmacol Sci. (2022) 26:8317–25. doi: 10.26355/eurrev_202211_30364
35. Garg UK, Mathur N, Sahlot R, Tiwari P, Sharma B, Saxena A, et al. Abdominal fat depots and their association with insulin resistance in patients with type 2 diabetes. PLoS One. (2023) 18(12):e0295492. doi: 10.1371/journal.pone.0295492
36. Abboud M, Haidar S, Mahboub N, Papandreou D, Rizk R. Abdominal volume index, waist-to-height ratio, and waist circumference are optimal predictors of cardiometabolic abnormalities in a sample of Lebanese adults: a cross-sectional study. PLOS Glob Public Health. (2023) 3(12):e0002726. doi: 10.1371/journal.pgph.0002726
37. Zhang QH, Xie LH, Zhang HN, Liu JH, Zhao Y, Chen LH, et al. Magnetic resonance imaging assessment of abdominal ectopic fat deposition in correlation with cardiometabolic risk factors. Front Endocrinol (Lausanne). (2022) 13:820023. doi: 10.3389/fendo.2022.820023
38. Iida T, Morimoto S, Okuda H, Amari Y, Yurugi T, Nakajima F, et al. Impact of abdominal fat distribution on mortality and its changes over time in patients undergoing hemodialysis: a prospective cohort study. J Ren Nutr. (2023) 33(4):575–83. doi: 10.1053/j.jrn.2023.03.004
39. Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. (2015) 3(3):207–15. doi: 10.1016/S2213-8587(14)70134-2
40. Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. (2013) 14(3):232–44. doi: 10.1111/obr.12003
41. Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, et al. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler Thromb Vasc Biol. (2013) 33(7):1728–33. doi: 10.1161/ATVBAHA.112.301174
42. Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. (2012) 367(14):1310–20. doi: 10.1056/NEJMoa1107477
43. Li G, Wu HK, Wu XW, Cao Z, Tu YC, Ma Y, et al. The feasibility of two anthropometric indices to identify metabolic syndrome, insulin resistance and inflammatory factors in obese and overweight adults. Nutrition. (2019) 57:194–201. doi: 10.1016/j.nut.2018.05.004
44. Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. (2005) 69:29–35. doi: 10.1016/j.diabres.2004.11.007
45. Bulló M, García-Lorda P, Megias I, Salas-Salvadó J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obes Res. (2003) 11(4):525–31. doi: 10.1038/oby.2003.74
46. Festa A, D'Agostino R Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, et al. The relation of body fat mass and distribution to markers of chronic inflammation. Int J Obes Relat Metab Disord. (2001) 25(10):1407–15. doi: 10.1038/sj.ijo.0801792
47. Hermsdorff HH, Zulet MÁ, Abete I, Martínez JA. Discriminated benefits of a Mediterranean dietary pattern within a hypocaloric diet program on plasma RBP4 concentrations and other inflammatory markers in obese subjects. Endocrine. (2009) 36(3):445–51. doi: 10.1007/s12020-009-9248-1
48. Lapice E, Maione S, Patti L, Cipriano P, Rivellese AA, Riccardi G, et al. Abdominal adiposity is associated with elevated C-reactive protein independent of BMI in healthy nonobese people. Diabetes Care. (2009) 32(9):1734–6. doi: 10.2337/dc09-0176
49. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. (2004) 92:347–55. doi: 10.1079/BJN20041213
50. Martí A, Marcos A, Martínez JA. Obesity and immune function relationships. Obes Rev. (2001) 2:131–40. doi: 10.1046/j.1467-789x.2001.00025.x
51. Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. (2006) 6(10):772–83. doi: 10.1038/nri1937
52. Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the metabolic syndrome—an allostatic perspective. Biochim Biophys Acta. (2010) 1801:338–49. doi: 10.1016/j.bbalip.2009.12.006
53. de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. (2012) 71(2):332–8. doi: 10.1017/S0029665112000092
54. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation Index (SII), system inflammation response Index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12(3):1128. doi: 10.3390/jcm12031128
55. Negishi I, Motoyama N, Nakayama K, Nakayama K, Senju S, Hatakeyama S, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. (1995) 376(6539):435–8. doi: 10.1038/376435a0
56. Wang X, Cai J, Lin B, Ma M, Tao Y, Zhou Y, et al. GPR34-mediated Sensing of lysophosphatidylserine released by apoptotic neutrophils activates type 3 innate lymphoid cells to mediate tissue repair. Immunity. (2021) 54(6):1123–36.e8. doi: 10.1016/j.immuni.2021.05.007
57. van Loo G, Bertrand MJM. Death by TNF: a road to inflammation. Nat Rev Immunol. (2023) 23(5):289–303. doi: 10.1038/s41577-022-00792-3
Keywords: abdominal volume index, all-cause mortality, cardiovascular mortality, inflammatory parameters, nomogram
Citation: Chi Y, Zhang Y, Lin H, Zhou S, Jia G and Wen W (2025) The association of lipid accumulation product with inflammatory parameters and mortality: evidence from a large population-based study. Front. Epidemiol. 4:1503261. doi: 10.3389/fepid.2024.1503261
Received: 4 October 2024; Accepted: 27 December 2024;
Published: 4 February 2025.
Edited by:
He He, Sichuan University, ChinaReviewed by:
Lei Zhou, Zhejiang University, ChinaCopyright: © 2025 Chi, Zhang, Lin, Zhou, Jia and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wen, d2Vud2VpOTQwNDA0QHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.