- 1School of Medical Laboratory Sciences, Faculty of Health Science, Institute of Health, Jimma University, Jimma, Ethiopia
- 2College of Medical and Health Science, Department of Medical Laboratory Sciences, Mizan-Tepi University, Mizan Teferi, Ethiopia
- 3College of Medical and Health Science, Department of Medicine, Mizan-Tepi University, Mizan Teferi, Ethiopia
Background: Acute febrile illnesses such as typhoid fever, typhus, and malaria are still major causes of hospital admission in many parts of Ethiopia. However, there are substantial gaps in the monitoring systems, which result in a lack of knowledge about the geographic distribution and role of common pathogens, particularly in rural areas. Thus, this study was aimed at assessing the seroprevalence of typhoid fever, typhus, and malaria among suspected acute febrile patients at the MTU Teaching Hospital and Mizan-Aman Health Center, Southwest region of Ethiopia.
Method: A health facility-based cross-sectional study was carried out from July to October 2022. Blood samples were collected from a total of 384 individuals. Widal and Weilfelix direct card agglutination and tube agglutination test methods were used for the Salmonella enterica serotype Typhi (S. typhi) and Rickettsia infections. The diagnosis of malaria was made using thick and thin blood smears. Questionnaires given by interviewers were used to gather information on risk factors and other sociodemographic factors. The data was analyzed using STATA/SE 14.0.
Result: A total of 371 patients were tested for S. Typhi and Rickettsia infections using direct card agglutination and tube agglutination methods. Using the screening test, 20.5% (76/371) patients were reactive either for O or H antigens or both, of which 55.3% (42/76) were reactive by the titration test at the cutoff value ≥ 1:80. About 17.5% (65/371) were reactive to OX19 antigen by card agglutination test, and of which 58.5% (38/65) were reactive by the titration test at the cutoff value ≥ 1:80. The overall seroprevalence of S. Typhi and Rickettsia infections using combined direct card and tube agglutination techniques was 11.3% (42/371) and 10.2% (38/371), respectively. Out of 384 suspected malaria patients, 43 (11.2%) were found positive either for P. falciparum, 27 (7.03%), or P. vivax, 16 (4.2%).
Conclusion: In this study, typhoid fever, typhus, and malaria were found among symptomatic acute febrile patients. To increase disease awareness, it is necessary to provide sustainable health education about risk factor behaviors, disease transmission, and prevention strategies. In addition, improving laboratory diagnosis services and early treatment may also lower the likelihood of potentially fatal consequences.
Background
Although there has been tremendous progress over the past decade, febrile illnesses such as malaria and acute bacterial infections such as pneumonia, typhoid fever, typhus, and relapsing fever are the major causes of seeking healthcare and may be responsible for varying degrees of morbidity and mortality, especially in sub-Saharan Africa (1). A study in East Africa reported that the pooled prevalence of febrile cases with unidentified etiology was 64%, with a prevalence of 69% in Ethiopia, 67% in Kenya, and 61% in Tanzania (2). These diseases are often known by non-specific clinical signs and symptoms, and the scarcity of the proper diagnostic tools and limited laboratory capacity are the major challenges for health professionals in the diagnosis and treatment of patients (1–3).
The incidence of febrile illness and the prevalence of various pathogens vary by factors such as location, season, and urban or rural setting. Such infections like malaria, typhoid fever, and typhus share similar predisposing factors such as poverty, poor sanitation, and public health services, and in the same way, individuals in areas endemic for these infections are being put at substantial risk of contracting concurrently (4–6). In addition, although early and accurate diagnosis of fevers is essential for effective case management, due to the limited availability of diagnostics in low- and middle-income countries, most cases of acute febrile illnesses with symptoms such as fever, headache, joint pain, and back pain are often misdiagnosed as malaria, or as typhoid fever, or typhus (3, 6–8), and management remains challenging, especially for non-malarial fever (3, 9). Consequently, treatable bacterial infections were missed, and antibiotics are overused or poorly targeted, that may be resulted for poor patient outcomes.
In Ethiopia, the data from the nationwide inventory of sanitation facilities reported that, more than half of the population still used unimproved sanitation facilities (10). However, few studies have been done to determine the prevalence and etiology of febrile illness among people who present with typical symptoms in some parts of the country (11–13), which emphasizes the significant knowledge gaps regarding the geographic distribution and role of common pathogens of most febrile illnesses, including typhoid fever, typhus, and malaria. The recommendations from the research output are very important for ensuring that every patient receives the right care at the right time and for proposing appropriate services or interventions. Therefore, in the selected population, we evaluated the prevalence of typhoid fever, typhus, and malaria in those who were complaining of symptoms like fever, headache, joint pain, and back pain in the selected health facilities in the Southwest region of Ethiopia.
Methods and materials
Study area and period
This study was conducted at Mizan-Tepi University (MTU) teaching hospital and Mizan-Aman health center from July to October 2022. The teaching hospital and the health center are found in Mizan-Aman town, at 591 Km in the Southwest direction, from Addis Ababa, the capital city of Ethiopia. These health facilities were providing services to approximately 5 million people coming from four catchment zones, such as BENCH SHEKO, KEFA, SHEKA and MAJANG.
Study design and subjects
A cross-sectional study was conducted to determine the prevalence of typhoid fever, typhus, and malaria among individuals who were complaining of a range of symptoms. All adults and children ≥2 years old and presenting with acute febrile illness at MTU Teaching Hospital and Mizan-Aman Health Center outpatients and emergency departments and willing to provide blood and gave informed consent and/or assents were included in the study. The acute fever may be measured when the patient presents, or it can be a recent history of fever (a body temperature ≥ 37.5°C with in the last 7 days), and the fever can be with or without other many possible symptoms, but generally not a localized infection. We didn't include inpatients, chronic fever, and new-borns or very young children (e. g., <2 years).
Sample size and sampling techniques
The required sample size is calculated using Cochran's general formula for a single population proportion by assuming a confidence interval of 95%, a margin error of 5%, and a prevalence of 0.5. Hence, the minimum number of study participants that were enrolled in the study was 384, and the required data were collected from consecutive patients during the study period.
Data collection process
Socio-demographic and other risk factor data
An interviewer administered questionnaires was used to collect the data. The questioner was developed after reviewing similar research conducted in Ethiopia and abroad. Socio-demographic data such as age, sex, educational status, occupational status, and clinical-related data such as body temperature, headache, joint pain, back pain, illness duration, and contact history with the same symptoms, and environmental related data such as source of water, availability of toilet facilities, hand washing habit, and raw meat and raw vegetable consumption habit, were collected. Knowledge of study participants of age ≥ 15 years on the causes, symptoms, ways of transmission, and prevention methods Typhoid fever, typhus, and malaria diseases were assessed. Three data collectors were assigned to collect the data after a briefing on the objective and purpose of the study. Once the appropriate history and information were collected, laboratory samples were collected. The questionnaire was checked daily for completeness, accuracy, clarity, and consistency, and necessary corrections were made.
Collection and processing of blood samples
The blood samples were collected from all febrile individuals after informed consent was obtained. Three milliliters (3 ml) of venous blood were collected from each patient into an EDTA® vacutainer test tube by the skilled laboratory technologist. But 13 patients were provided finger prick blood samples due to the obscurity of veins. First, thin, and thick blood smears were prepared with a small drop of blood for the detection of malaria. Then, following the manufacturer's instructions and as previously described (14, 15), the serum was separated, and on the same days, screening tests for Salmonella and Rickettsia infections were conducted using the Widal and Weil-felix direct card agglutination test techniques. All serum samples that were found to be reactive by the direct card agglutination tests were transported to the MTU microbiology laboratory and further tested by the tube agglutination test method. In detail, the serum samples were serially diluted using a fresh 0.95% saline preparation from 1:20 to 1:5,120 for anti-O, anti-H, and anti-OX19 separately in 10 test tubes. After adding one drop of O, H, and OX19 antigens to each test tube, it was incubated for 2–4 h at 50°C. An antibody titter of 1:80 for anti-O, for anti-H and anti-OX19 antibodies was taken as a cut-off value to indicate recent typhoid and Rickettsia infections. For quality assurance, positive and negative control of Widal and Weilfelix direct card agglutination tests were performed each day before taking to do patient's sample according to manufacturer instructions.
Data analysis and interpretation
The data was entered and analyzed using STATA/SE 14.0. Descriptive analysis, such as frequencies and percentages of variables, was used to summarize the data. Pearson chi-square test was performed to evaluate the statistically significant difference in the prevalence of typhoid fever, typhus, and malaria between the demographics of the study participants and according to their reported clinical and environmental features. Bivariate and multivariate logistic regression analyses were performed to assess the associations of socio-demographic, clinical characteristics, and hygienic or environmental conditions of the study participants with increased odds of having a higher prevalence of typhoid fever, typhus, and malaria. A p-value below 5% was considered as indicator of statistical significance.
Results
Socio-demographic characteristics
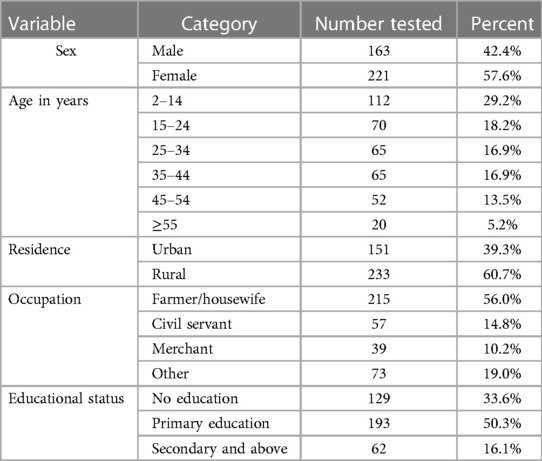
A total of 384 individuals who fulfilled acute febrile illness (AFI) criteria participated in the study. Of these, female patients account for 57.6% (n = 221), and male account for 42.4% (n = 163). The age range was 2–75 years, with a mean age of 27.64 years. All individuals were volunteers to consent to provide a venous blood sample, but 13 patients were provided a finger- prick blood sample due to the invisibility of their veins. The majority (65.2%) were from rural area and had only attained primary school. and were farmers/housewives in their occupations (Table 1).
In this study, all patients with an age group of ≥15 years were assessed for their knowledge about the causes of infections, symptoms of the disease, ways of transmission, and prevention methods of typhoid fever, typhus, and malaria. The patients were categorized as having good knowledge if he/she got ≥50% of the answer from each category of the questions. Accordingly, only 63 (24.1%) and 42 (16.1%) of individuals have good knowledge about typhoid fever and typhus infections, respectively. Only 78 (28.7%) had good knowledge about malaria infection.
The prevalence of typhoid, typhus, and malaria
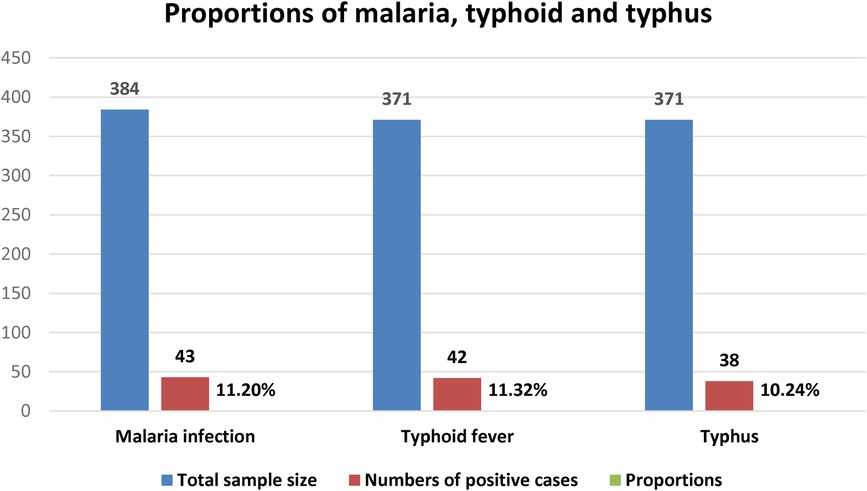
Only 43 (11.2%) of the 384 febrile patients tested microscopically positive for both P. falciparum and P. vivax. Among a total of 371 febrile patients, 20.5% (76/371) and 17.5% (65/371) were reactive for S. Typhi and Rickettsia infection by using the screening Widal and Weilfelix direct card agglutination test. Of these, 42 (11.3%) and 38 (10.2%) were positive for S. Typhi and Rickettsia infections as tested by titration techniques, respectively (Figure 1).
Prevalence of S. typhi infection
A total of 371 patients were tested for S. typhi infection using direct card agglutination and tube agglutination methods. During the screening test, 20.5% (76/371) of the patients were reactive against somatic (O) or flagella (H) antigens or both. In detail, 29/76 (38.2%) were reactive only against O antigen, 24 (31.6%) against flagella (H) antigen and 23 (30.3%) against both H, and O antigens. The total sera reactive either against O and/or H antigen by the tube agglutination test was 55.3% (42/76) at the cut off value of 1:80. Of these, 13 (17.1%), 7 (9.20%), and 3 (3.9%) of the reactive sera to O antigen were reactive at a titration of 1:80, 1:160, and 1:320, respectively. Among the reactive sera to H antigen, 11 (14.5%), 5 (6.6%), and 3 (3.9%) were reactive at the titration of 1:80, 1:160 and 1:320, respectively. Hence, the total seroprevalence of S. Typhi infection using direct card agglutination and tube agglutination technique was 11.3% (42/371) (Figure 1).
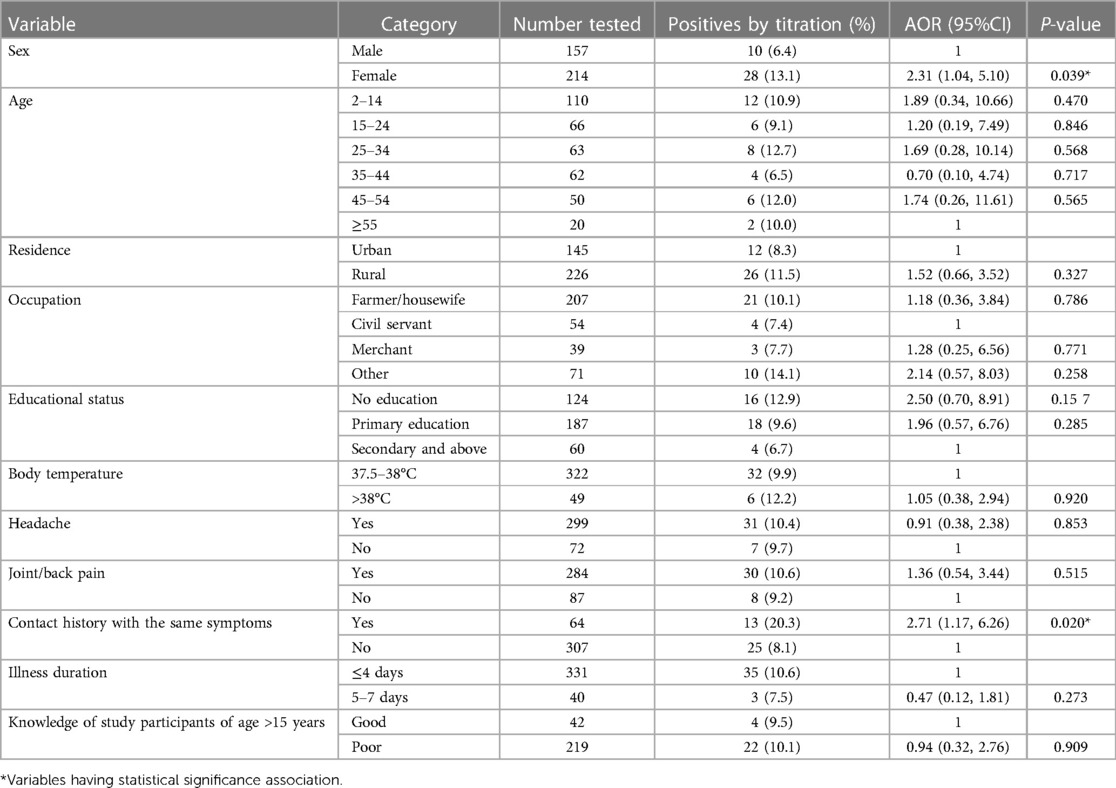
The prevalence of S. typhi infection was significantly higher among females (14.0%; AOR = 2.25; 95% CI: 1.01–5.05, P = 0.049) than among males (7.6%), as detected by the titration tests. Patients with no formal education (AOR = 3.82, 95%CI: 1.06–13.78, P = 0.041) and those with clinical symptoms of joint and/back pain (AOR = 3.89, 95%CI: 1.05–14.36, P = 0.042) were found to be associated with a higher rate of S. Typhi infection (Table 2).
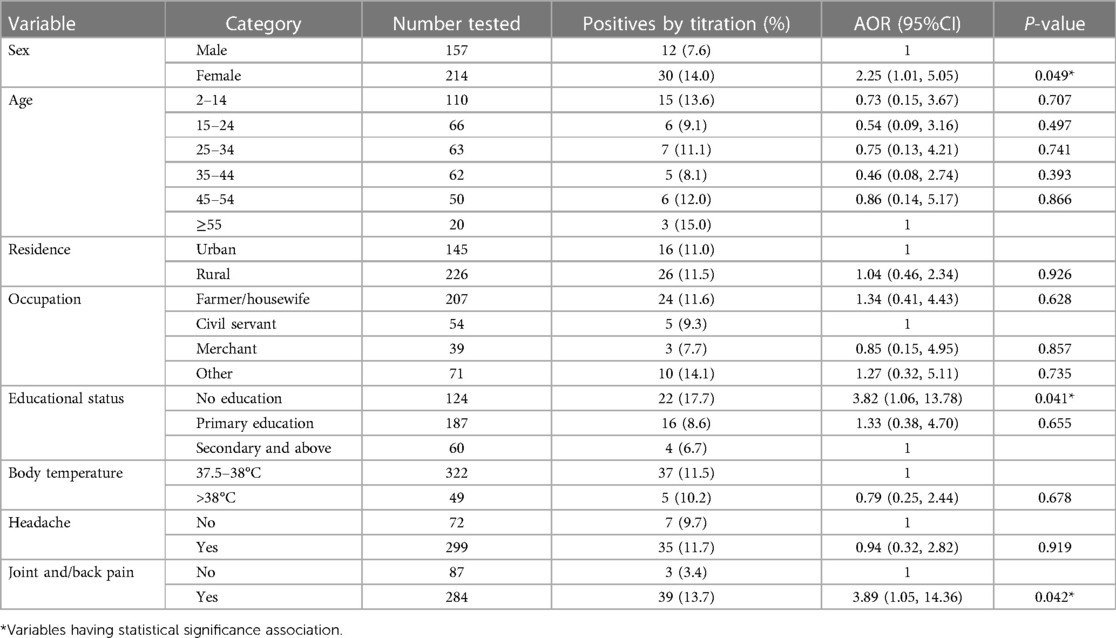
Table 2 Socio-demographic, clinical characteristics and hygienic conditions of the study participants and prevalence of typhoid fever infection.
Regarding the hygienic conditions of the study participants and S. typhi infection, patients who used unprotected well/spring water as a water source were 3.30 times (AOR = 3.30, 95%CI: 1.01–10.83, P = 0.049) more likely to get infection than those who used public/private tap water. Those patients with raw meat consumption habits are 3.22 times (AOR = 3.22, 95% CI: 1.09–9.48, P = 0.034) more likely to get S. typhi infection than non- consumers (Table 2).
Prevalence of Rickettsia infection
In this study, a total of 371 patients’ serum was screened for Rickettsia infection by direct card agglutination, and 17.5% (65/371) were reactive against the OX19 antigen. Out of these reactive sera, 22 (33.8%), 10 (15.4%), and 6 (9.2%) were reactive at a titration of 1:80, 1:160, and 1:320, respectively. Hence, the total seroprevalence of Rickettsia infection using direct card agglutination and tube agglutination technique, was 10.2% (38/371).
The rate of infections was more significant among females (AOR = 2.31; 95% CI: 1.04–5.10, P = 0.039) and those who had contact history of the same symptoms (AOR = 2.71; 95% CI: 1.17–6.26, P = 0.020) as compared to males and those who do not have contact history as done by titration test (Table 3).
Prevalence of malaria infection
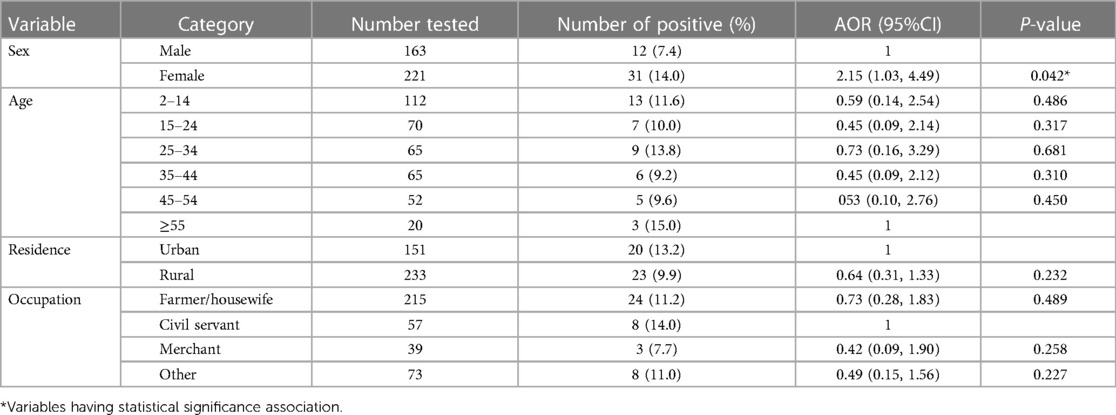
In this study, a total of 384 suspected malaria patients were microscopically tested for plasmodium infections, and 43 (11.2%) were found positive for P. falciparum, 27 (7.03%), and P. vivax [16 (4.2%)]. The rate of infection was significantly higher among females (14.0%) and among patients with body temperature > 38°C (20.4%). On binary regression analysis, being female (AOR = 2.15, 95% CI: 1.03–4.49, P = 0.042) and having fever > 38°C (AOR = 2.84, 95% CI: 1.17–6.87, P = 0.021) were independently associated with increased odds of having Plasmodium infection (Table 4).
Discussion
A health facility-based study was conducted to assess the prevalence of typhoid fever, typhus, and malaria among individuals who reported the sign/symptoms of fever, headache, joint pain, and back pain in the Southwest region of Ethiopia. In our study, of the total individuals suspected of acute febrile illness, 11.3% and 10.2% were confirmed to be sero-positive for S. typhi, and Rickettsia infection by confirmatory tests. The sero-prevalence of S. typhi infection in this study was slightly higher than the previous reports in Ethiopia (15, 16), and India (17), but lower than other findings in Ethiopia (18, 19) and Sudan (20). The sero-prevalence of Rickettsia infection was in line with other reported findings in different developing countries (21–25), but it is lower than a finding in Ethiopia (15, 18), Malaysia (26), and India (27). This divergence in the results may rest on the differences in investigation methods, the study populations, and the awareness of the community about the transmission and prevention of S. typhi, and Rickettsia infections.
The distribution of typhoid risk factors is uneven within the sub-national boundary level and is geographically heterogeneous (4). In this study, the seroprevalence for S. typhi infection was significantly higher among females. In addition, level of education, use of unprotected well/spring water as their water sources and consumption of raw meat were significantly associated with a high rate of S. typhi infection. Similar results were reported in other previous studies in Ethiopia (4, 15, 28). Geographical location, inadequate food and personal hygiene, lack of potable water, and awareness of the community on the transmission and prevention of typhoid fever have been frequently cited as the major risk factors contributing to the high burden of S. typhi infection (29–31). As a result, the high seroprevalence of S. typhi infection in females may be related to their level of education and daily activities, such as the frequency of exposure to contaminated water while fetching or washing clothes. To lessen the burden of these diseases, it is crucial to provide access to safe water, create a solid healthcare system for the diagnosis and treatment of typhoid fever, and raise community awareness.
The sero-prevalence of Rickettsia infection was also significantly high among females and in patients who had contact history with family members who had the same sign/symptoms. Similar results were reported in other previous studies in other developing countries (22, 27, 32). Diagnostic services for non-malaria febrile cases are complex and not widely available, and with a few exceptions, preventive measures for specific infections are poorly developed or difficult to implement (33). Thus, implementation of an effective health care system for the diagnosis and treatment of Rickettsial infections and increasing community awareness on modes of acquisition and prevention strategies are very important to reduce the burden of these diseases.
The other finding of this study was a malaria prevalence of 11.2%, which is lower than the previous reported findings in Ethiopia (19, 34–38) and Nigeria (39). P. falciparum in this study was the most prevalent species that causes severe forms of malaria, as reported in previous studies (15, 34–36). Moreover, the prevalence of Plasmodium infection was significant among females and among patients with clinical signs of fever ≥ 37.5°C, as reported in previously conducted studies (2, 15, 34). Although Ethiopia has implemented a comprehensive program to control and prevent malaria, the prevalence of the disease has been rising in various regions of the nation since a few years ago. Hence, to achieve the plan for malaria elimination, it is crucial to improve case detection of malaria in this region and raise community understanding of the transmission and prevention of malaria, for instance through community health extension workers.
Serological agglutination assays are still used in developing countries because of their speed and technical ease of application, as well as their low cost (40). In this study, we also used Widal and Weil-felix tests to determine the seroprevalence of S. typhi and Rickettsia infections in those who had sign/symptoms of a febrile illness. Hence, one of the limitations of the current study may be that these serological-based tests do not provide conclusive evidence for the diagnosis of current infection because of flaws like false positivity due to prior exposure or false negativity for those living in an endemic setting (28, 40). Additionally, the selection of research participants may have been biased because it was based on clinical signs and symptoms that were reported by the participants rather than those that were necessarily brought on by an infectious agent that causes acute illness. Hence, the results of this study cannot be applied to other populations since, in an endemic area, people may have these diseases without exhibiting symptoms.
Conclusion
Typhoid fever, typhus, and malaria were important infections observed among symptomatic acute febrile individuals in the study area. The sero-prevalence of each infection varies in relation to the socio-demographic, clinical characteristics, and hygienic or environmental conditions of the study participants. Therefore, efforts are needed to improve disease awareness by providing enhanced and sustainable health education about typhoid fever, typhus, and malaria infection, its transmission, prevention strategies, and identifying risky behaviours with focusing on households, housewives, and students. In addition, early diagnosis and treatment for these non-specific febrile illnesses is essential to reduce the incidence of potentially fatal complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Mizan-Tepi University, Mizan, Ethiopia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MeA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. MiA: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. YH: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. YA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank Mizan- Tepi University for permitting the laboratory setup with the necessary supplies. We also thank all the data collectors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maze MJ, Bassat Q, Feasey NA, Mandomando I, Musicha P, Crump JA. The epidemiology of febrile illness in Sub-Saharan Africa: implications for diagnosis and management. Clin Microbiol Infect. (2018) 24 (8):808–14. doi: 10.1016/j.cmi.2018.02.011
2. Nooh F, Chernet A, Reither K, Okuma J, Brattig NW, Utzinger J, et al. Prevalence of fever of unidentified aetiology in East African adolescents and adults: a systematic review and meta-analysis. Infect Dis Poverty. (2023) 12:55. doi: 10.1186/s40249-023-01105-z
3. Shimelis T, Vaz Nery S, Tadesse BT, Bartlett AW, Belay FWG, Schierhout G, et al. Clinical management and outcomes of acute febrile illness in children attending a tertiary hospital in southern Ethiopia. BMC Infect Dis. (2022) 22:434. doi: 10.1186/s12879-022-07424-0
4. Lee JS, Mogasale VV, Mogasale V, Lee K. Geographical distribution of typhoid risk factors in low- and middle-income countries. BMC Infect Dis. (2016) 16(1):732. doi: 10.1186/s12879-016-2074-1
5. Gelaw NB, Tessema GA, Gelaye KA, Tessema ZT, Ferede TA, Tewelde AW/S. Exploring the spatial variation and associated factors of childhood febrile illness among under-five children in Ethiopia: geographically weighted regression analysis. PLoS One. (2022) 17(12):e0277565. doi: 10.1371/journal.pone.0277565
6. Prasad N, Murdoch DR, Reyburn H, Crump JA. Etiology of severe febrile illness in low-and middle-income countries: a systematic review. PLoS One. (2015) 10(6):e0127962. doi: 10.1371/journal.pone.0127962
7. World Health Organization. Biomarkers for Acute Febrile Illness at the Point of Care in Low-Resource Settings. Meeting Report. Geneva, Switzerland: WHO (2021).
8. Chandna A, Chew R, Shwe Nwe Htun N, Peto TJ, Zhang M, Liverani M, et al. Defining the burden of febrile illness in rural South and Southeast Asia: an open letter to announce the launch of the rural febrile illness project. Wellcome Open Res. (2022) 6:64. doi: 10.12688/wellcomeopenres.16393.2
9. Dolan-Branton L, Stash S, Moyo T, Mwandama D, Mbeye N, Faramand TH, et al. Health Facility Guide for Assessing Treatment of Febrile Illness. Published by the USAID Assist Project. Bethesda, MD: University Research Co., LLC (2016).
10. Beyene A, Hailu T, Faris K, Kloos H. Current state and trends of access to sanitation in Ethiopia and the need to revise indicators to monitor progress in the post-2015 era. BMC Public Health. (2015) 15:451. doi: 10.1186/s12889-015-1804-4
11. Shimelis T, Tadesse BT, W/Gebriel F, Crump JA, Schierhout G, Dittrich S, et al. Aetiology of acute febrile illness among children attending a tertiary hospital in Southern Ethiopia. BMC Infect Dis. (2020) 20:903. doi: 10.1186/s12879-020-05635-x
12. Yemata GA, Yenew C, Mamuye M, Tiruneh M, Assfaw T, Mulatu S, et al. Descriptive analysis of typhoid fever surveillance data in the Jimma Zone, Southwest Ethiopia (2015–2019). Interdisciplinary Perspectives on Infectious Diseases. (2021) 2021:1255187. doi: 10.1155/2021/1255187
13. Birhanei M, Tesema B, Ferede G, Endris M, Enawgaw B. Malaria, typhoid fever, and their confection among febrile patients at a rural health center in Northwest Ethiopia: a cross-sectional study. Adv Med. (2014) 2014:531074. doi: 10.1155/2014/531074
14. Ley B, Mtove G, Thriemer K, Amos B, Seidlein L, Hendriksen I, et al. Evaluation of the widal tube agglutination test for the diagnosis of typhoid fever among children admitted to a rural hospital in Tanzania and a comparison with previous studies. BMC Infect Dis. (2010) 10:180. doi: 10.1186/1471-2334-10-180
15. Zerfu B, Medhin G, Mamo G, Getahun G, Tschopp R, Legesse M. Community-based prevalence of typhoid fever, typhus, brucellosis and malaria among symptomatic individuals in Afar Region, Ethiopia. PLoS Negl Trop Dis. (2018) 12(10):e0006749. doi: 10.1371/journal.pntd.0006749
16. Wasihun AG, Wlekidan LN, Gebremariam SA, Welderufael AL, Muthupandian S, Haile TD, et al. Diagnosis and treatment of typhoid fever and associated prevailing drug resistance in Northern Ethiopia. Int J Infect Dis. (2015) 35:96–102. doi: 10.1016/j.ijid.2015.04.014
17. Kavirayani V, Madiyal M, Aroor S, Chhabra S. Clinical profile and role of serology in pediatric acute febrile illness: experience from a tertiary care hospital in South India. Clin Epidemiol Glob Health. (2021) 12:100898. doi: 10.1016/j.cegh.2021.100898
18. Geteneh A, Tadesse S, Biset S, Girma L, Fissiha P. Rapid stool antigenic test for typhoid fever among suspected cases, Northeast, Ethiopia. Sci Rep. (2023) 13(1):1–6. doi: 10.1038/s41598-023-27909-5
19. Feleke SM, Animut A, Belay M. Prevalence of malaria among acute febrile patients clinically suspected of having malaria in the Zeway Health Center, Ethiopia. Jpn J Infect Dis. (2015) 68(1):55–9. doi: 10.7883/yoken.JJID.2013.062
20. Mohamed AAA, Ahmed MAI, IbnIdriss GEO, Husein AM, Abdirahman AA, Ali MA, et al. Typhoid fever: prevalence and risk factors among symptomatic Sudanese patients. J Bacteriol Mycol Open Access. (2023) 11(2):72–5. doi: 10.15406/jbmoa.2023.11.00347
21. Thapa S, Hamal P, Chaudhary NK, Sapkota LB, Singh JP. Burden of scrub typhus among patients with acute febrile illness attending tertiary care hospital in Chitwan, Nepal. BMJ Open. (2020) 10:e034727. doi: 10.1136/bmjopen-2019-034727
22. Pokhrel A, Rayamajhee B, Khadka S, Thapa S, Kapali S, Pun SB, et al. Seroprevalence and clinical features of scrub typhus among febrile patients attending a referral hospital in Kathmandu, Nepal. Trop Med Infect Dis. (2021) 6(2):78. doi: 10.3390/tropicalmed6020078
23. Mansoor T, Fomda BA, Koul AN, Bhat MA, Abdullah N, Bhattacharya S, et al. Rickettsial infections among the undifferentiated febrile patients attending a tertiary care teaching hospital of Northern India: a longitudinal study. Infect Chemother. (2021) 53(1):96–106. doi: 10.3947/ic.2020.0147
24. Fournier JB, Blanton LS, Nery N, Wunder EA, Costa F, Reis MG, et al.. Rickettsial infections causing acute febrile illness in Urban Slums, Brazil. Emerg Infect Dis. (2022) 28(10):2132–4. doi: 10.3201/eid2810.220497
25. Das P, Rahman MZ, Banu S, Rahman M, Chisti MJ, Chowdhury F, et al. Acute febrile illness among outpatients seeking health care in Bangladeshi hospitals prior to the COVID-19 pandemic. PLoS One. (2022) 17:9. doi: 10.1371/journal.pone.0273902
26. Grigg MJ, William T, Clemens EG, Patel K, Chandna A, Barber BE, et al. Rickettsioses as Major etiologies of unrecognized acute febrile illness, Sabah, east Malaysia. Emerg Infect Dis. (2020) 26(7):1409–19. doi: 10.3201/eid2607.191722
27. Trowbridge P, Divya P, Samuel P, Varghese G. The prevalence and risk factors for scrub typhus in South India. Open Forum Infect Dis. (2016) 3(suppl_1):615. doi: 10.1093/ofid/ofw172.478
28. Deksissa T, Gebremedhin EZ. A cross-sectional study of enteric fever among febrile patients at ambo hospital: prevalence, risk factors, comparison of widal test and stool culture and antimicrobials susceptibility pattern of isolates. BMC Infect Dis. (2019) 19(1):288. doi: 10.1186/s12879-019-3917-3
29. Brockett S, Wolfe MK, Hamot A, Appiah GD, Mintz ED, Lantagne D. Associations among water, sanitation, and hygiene, and food exposures and typhoid fever in case–control studies: a systematic review and meta-analysis. Am J Trop Med Hyg. (2020) 103(3):1020–31. doi: 10.4269/ajtmh.19-0479
30. Habte L, Tadesse E, Ferede G, Amsalu A. Typhoid fever: clinical presentation and associated factors in febrile patients visiting shashemene referral hospital, Southern Ethiopia. BMC Res Notes. (2018) 11:605. doi: 10.1186/s13104-018-3713-y
31. Mulu W, Akal CG, Ababu K, Getachew S, Tesfaye F, Wube A, et al. Sero-confirmed typhoid fever and knowledge, attitude, and practices among febrile patients attending at Injibara General Hospital, Northwest Ethiopia”. BioMed Res Int. (2021) 2021:8887266. doi: 10.1155/2021/8887266
32. Tran HTD, Hattendorf J, Do HM, Hoang TT, Hoang HTH, Lam HN, et al. Ecological and behavioral risk factors of scrub typhus in central Vietnam: a case-control study. Infect Dis Poverty. (2021) 10:110. doi: 10.1186/s40249-021-00893-6
33. Jude Prakash JA. Scrub typhus: risks, diagnostic issues, and management challenges. Res Rep Trop Med. (2017) 8:73–83. doi: 10.2147/RRTM.S105602
34. Kebede F, Kebede T. Malaria sero-survey among acute febrile patients come for health care seeking at the high malaria-endemic setting of North West Ethiopia. SAGE Open Med. (2022) 10:20503121221111709. doi: 10.1177/20503121221111709
35. Adugna F, Wale M, Nibret E. Prevalence of malaria and its risk factors in Lake Tana and surrounding areas, northwest Ethiopia. Malar J. (2022) 21:313. doi: 10.1186/s12936-022-04310-7
36. Haji Y, Fogarty AW, Deressa W. Prevalence and associated factors of malaria among febrile children in Ethiopia: a cross-sectional health facility-based study. Acta Trop. (2016) 155:63–70. doi: 10.1016/j.actatropica.2015.12.009
37. Debash H, Bisetegn H, Ebrahim H, Feleke DG, Gedefi A, Tilahun M, et al. Prevalence and associated risk factors of malaria among febrile under-five children visiting health facilities in Ziquala District, Northeast Ethiopia: a multicenter cross-sectional study. PLoS One. (2022) 17(10):e0276899. doi: 10.1371/journal.pone.0276899
38. Duguma T, Nuri A, Melaku Y. Prevalence of malaria and associated risk factors among the community of mizan-aman town and its catchment area in Southwest Ethiopia. J Parasitol Res. (2022) 2022:3503317. doi: 10.1155/2022/3503317
39. Awosolu OB, Yahaya ZS, Farah Haziqah MT, Simon-Oke IA, Fakunle C. A cross-sectional study of the prevalence, density, and risk factors associated with malaria transmission in urban communities of Ibadan, Southwestern Nigeria. Heliyon. (2021) 7(1):e05975. doi: 10.1016/j.heliyon.2021.e05975
Keywords: seroprevalence, typhoid fever, typhus, malaria, Southwest Ethiopia
Citation: Abayneh M, Aberad M, Habtemariam Y and Alemu Y (2024) Health facility-based prevalence of typhoid fever, typhus and malaria among individuals suspected of acute febrile illnesses in Southwest Region, Ethiopia. Front. Epidemiol. 4: 1391890. doi: 10.3389/fepid.2024.1391890
Received: 26 February 2024; Accepted: 24 June 2024;
Published: 18 July 2024.
Edited by:
Susanta Kumar Ghosh, National Institute of Malaria Research (ICMR), IndiaReviewed by:
Praveen K. Bharti, National Institute of Malaria Research (ICMR), IndiaLaurence Flevaud, Physicians Without Borders, Spain
© 2024 Abayneh, Aberad, Habtemariam and Alemu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengistu Abayneh, bWVuZ2lzdHUuYWJheW5laEBqdS5lZHUuZXQ=
 Mengistu Abayneh
Mengistu Abayneh Mitiku Aberad2,3
Mitiku Aberad2,3