- 1Menzies Institute for Medical Research, College of Health and Medicine, The University of Tasmania, Hobart, TAS, Australia
- 2Wicking Dementia Research and Education Center, College of Health and Medicine, The University of Tasmania, Hobart, TAS, Australia
- 3Pregnancy Research Centre, Department of Maternal-Fetal Medicine, The Royal Women’s Hospital, Melbourne, VIC, Australia
- 4Department of Obstetrics and Gynaecology, The Royal Women’s Hospital, The University of Melbourne, Melbourne, VIC, Australia
- 5School of Global and Population Health, The University of Western Australia, Crawley, WA, Australia
Background: Women with a history of preeclampsia (PE) have been shown to have up to five times the risk of developing later-life cardiovascular disease (CVD). While PE and CVD are known to share clinical and molecular characteristics, there are limited studies investigating their shared genomics (genetics, epigenetics or transcriptomics) variation over time. Therefore, we sought to systematically review the literature to identify longitudinal studies focused on the genomic progression to CVD following PE.
Methods: A literature search of primary sources through PubMed, Scopus, Web of Science and Embase via OVID was performed. Studies published from January 1, 1980, to July 28, 2023, that investigated genomics in PE and CVD were eligible for inclusion. Included studies were screened based on Cochrane systematic review guidelines in conjunction with the PRISMA 2020 checklist. Eligible articles were further assessed for quality using the Newcastle-Ottawa scale.
Results: A total of 9,231 articles were screened, with 14 studies subjected to quality assessment. Following further evaluation, six studies were included for the final review. All six of these studies were heterogeneous in regard to CVD/risk factor as outcome, gene mapping approach, and in different targeted genes. The associated genes were RGS2, LPA, and AQP3, alongside microRNAs miR-122-5p, miR-126-3p, miR-146a-5p, and miR-206. Additionally, 12 differentially methylated regions potentially linked to later-life CVD following PE were identified. The only common variable across all six studies was the use of a case-control study design.
Conclusions: Our results provide critical insight into the heterogeneous nature of genomic studies investigating CVD following PE and highlight the urgent need for longitudinal studies to further investigate the genetic variation underlying the progression to CVD following PE.
1. Introduction
Cardiovascular disease (CVD) is the global leading cause of morbidity and mortality in women (1). Recent studies have shown biological sex disparities in the CVD pathophysiology, clinical diagnosis and responsiveness to management (2). Dyslipidaemia, hypertension, smoking, obesity, and diabetes are some of the major CVD risk factors common between men and women (3). In addition, women can present with additional medical concerns which make CVD more challenging to identify (1, 4). Pregnancy complications are now considered an important later-life CVD risk factor in women (5, 6). This is especially apparent in women who have had the hypertensive disorder of pregnancy, preeclampsia (PE). PE is characterised by new-onset hypertension along with proteinuria or other maternal organ dysfunction including liver dysfunction, renal insufficiency, neurological complications or haematological complications, after 20 weeks of gestation (7, 8). PE, which affects 2% to 8% of pregnancies worldwide, can cause disruptions to the maternal endothelium, leading to a decrease in angiogenesis and reduced blood flow to organs and tissues (9, 10). Women with a history of PE have been shown to have up to five times the risk of developing later-life CVD when compared to their normotensive counterparts (11).
PE and CVD are known to share molecular pathological features, including endothelial dysfunction, metabolic abnormalities, inflammatory response, oxidative stress and hypercoagulability (12, 13). For example, increased levels of systemic inflammation in PE have been linked to a higher risk of atherosclerosis, endothelial dysfunction and early onset of arterial stiffness (14). Moreover, the adipocyte-derived hormone leptin, a marker of increased CVD and obesity risk, has also been found to be elevated in women with PE (13). Maternal pre-pregnancy BMI is strongly associated with increased risk of PE (15, 16) and elevated BMI is a hallmark risk factor for increased CVD risk.
To model the complex biological relationship between CVD and PE, we and others have undertaken several studies using a wide array of gene-mapping techniques (17–20). These range from candidate gene studies to whole-genome sequencing. These studies seek to identify specific loci within the human genome that are associated with either a PE or CVD-specific outcomes/traits that may underly both conditions. A common approach in these genetic studies is to look for overlap between CVD and PE using the concept of pleiotropy, which refers to when a single gene or loci influences two seemingly unrelated traits. For example, our genetic dissection of the PE susceptibility loci on chromosome 2q22 identified variants in four genes (LCT, LRP1B, GCA, RND3) that were associated with PE in an Australian family cohort (20). These variants were also associated with cardio-metabolic traits in both the San Antonio Family Heart Study and Australian adolescents from the Raine Study (19). While these previous genetic studies provide important information on the shared genetic susceptibility loci for PE and CVD they do not inform on specific genes or loci that are involved in the progression from PE to later-life CVD.
In addition to above referenced genetic studies, there are also several studies that used an array of recent genomic technologies to investigate the biological relationship between PE and CVD. These studies focus on gene regulation (epigenetics) and gene expression (transcriptomics). For example, a meta-analysis investigated differential expression of PE in placental tissue and whole blood from CVD patients and observed 22 genes common to both PE and CVD, from 925 PE and 181 CVD differentially expressed genes. This study also identified common biological pathways including oxidative stress, interleukin signalling, inflammation-mediated chemokines and cytokines that are known to play a role in the complex pathogenesis of both disorders (21–23). Other studies have focused on differential DNA methylation, a key epigenetic mechanism that regulates gene expression, and microRNAs (miRNAs), small non-coding RNA molecules that regulate gene expression by binding to messenger RNA (mRNA) of target genes (24, 25). Both DNA methylation and miRNAs are known to have an impact on both PE and CVD. However, there has been limited research into the progression of these genomic loci from PE to later-life CVD in women (23). The causal association between PE and CVD can be examined using Mendelian randomisation (MR), a technique that employs genetic variants as instrumental variables to evaluate causal effects (26, 27). MR was utilised in one of the latest studies to evaluate the risk of PE and its relationship between lipid levels and drug targets across four different ancestry groups. From the study, it was observed that higher levels of high-density lipoprotein cholesterol (HDL-C) may lower the risk of developing PE, while reducing the dose of low-density lipoprotein cholesterol (LDL-C) altering drugs does not have a significant impact (28). In addition, polygenic risk scores (PRS) can be used to evaluate the genetic risk of both PE and CVD, assisting in the identification of individuals at higher risk of developing later-life CVD following PE (29). In a recent study using PRS, a high genetic predisposition for hypertensive disorders during pregnancy was linked to an elevated risk of atherosclerotic CVD. This study underscores the utility of PRS for hypertensive disorders during pregnancy in estimating long-term CVD outcomes in later stages of life (30). However, these studies have not exclusively focused on PE or have not examined the progression from PE to later-life CVD.
The aim of our study was to systematically review the literature to identify genomic loci involved in the progression to CVD following PE in the same women over time. Findings from this review may inform more impactful research strategies for identifying genomic loci influencing increased CVD risk following PE.
2. Methods
An examination of primary research literature was performed to identify genomic variation associated with the progression to CVD following PE. The Cochrane handbook for systematic reviews of interventions (2nd edition) was followed (31) along with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (Supplementary Table S1) to enhance the study protocol and ensure comprehensive reporting of findings (32).
2.1. Information sources and search strategies
The primary literature search was conducted on August 27, 2021, and two updated searches on February 2 and July 28, 2023, using four electronic databases: PubMed, Scopus, Web of Science and Embase via OVID. Databases were searched using the title, abstract and full-text fields to find all significant articles. The Patient, Population or Problem, Intervention, Comparison and Outcome (PICO) search strategy was used to categorise the relevant keywords (Supplementary Table S2) (33). The search keywords used included “cardiovascular disease”, “heart arrhythmias”, “stroke”, “cardiometabolic risk”, “hypertension”, “dyslipidaemia”, “cardiomyopathy”, “atherosclerotic heart diseases”, “rheumatic heart disease”, “cerebrovascular disease”, “coronary artery disease”, “heart failure”, “heart valve disease”, “ischaemic heart disease”, “inflammatory heart disease”, “heart disorder”, “cardiac arrest”, “hypertensive heart disease”, “carditis”, “peripheral artery disease”, “myocardial infarction”, “acute coronary syndrome”, “cardiac failure”, “left ventricular systolic dysfunction”, “preeclampsia”, “toxaemia”, “maternal syndrome”, “pregnancy complication”, “pregnancy-specific disorder”, “pregnancy-induced hypertension”, “maternal hypertension”, “eclampsia”, “HELPP syndrome”, “genetic”, “candidate gene studies”, “association analysis”, “linkage studies”, “Genome-wide association studies”, “Genome-wide linkage studies”, “gene-gene interactions”, “gene-environmental interactions”, “epistasis”, “heritability”, “DNA methylation”, “epigenetics”, “microRNA”, “histone modification”, “chromatin modification”, “epigenetic modification”, “Epigenome-wide association studies”, “posttranslational regulation”, “transcriptional gene silencing”, “nucleosome remodelling”, “non-coding RNA regulation” and “RNA editing”. Detailed search keywords are mentioned in the data Supplementary Tables S2–S5.
2.2. Inclusion and exclusion criteria
This systematic review included original articles published in English from-1980 until July 28, 2023, in case-control, cohort, or cross-sectional study designs. We chose post-1980s due to significant advancements in genomic research and mapping techniques. Publications relevant to progression of PE to CVD endpoints (e.g., coronary artery disease, stroke, etc) or CVD risk factors (e.g., systolic blood pressure, cholesterol level) in women based on genetic, epigenetic or transcriptomic factors were included. PE was defined either based on the clinical outcome presented during pregnancy or as a history of PE from self-reported questionnaires answered by the participants. PE is clinically defined by the presence of new-onset hypertension, with blood pressure readings of 140/90 mmHg or higher, taken at least twice, four hours apart, after 20 weeks of gestation. The diagnosis of PE can be further confirmed with the presence of additional criteria such as proteinuria, identified by an excess of proteins in the urine, or maternal organ dysfunction. Maternal organ dysfunction encompasses liver dysfunction, renal insufficiency, neurological complications, or haematological complications (8). The self-reported responses obtained through questionnaires were crosschecked with the medical records of the participants by an Obstetrician/Gynaecologist to ensure the accuracy of the data.
The articles were restricted to humans and peer-reviewed empirical studies. Studies were excluded if: (i) CVD was not evaluated as the outcome; (ii) they were on behavioural CVD risk factors, including diet, physical activity, alcohol consumption, and tobacco use; (iii) they were solely aimed at other pregnancy complications other than PE, such as gestational hypertension, gestational diabetes, stillbirth, small-for-gestational-age; (iv) focused on offspring rather than mothers; (v) based on animal models; (vi) there was a lack of genetic, epigenetic or transcriptomic evidence; (vii) they were systematic reviews, discussion papers, case reports, case series, editorials, or conference abstracts; (viii) focused on a topic not related to CVD or PE; and (ix) different women or different cohorts of CVD and PE were used to study the association between both diseases.
2.3. Data screening, selection and extraction
The research articles from all four databases were imported to EndNote 20 to remove duplicates (34). The remaining articles were exported to Rayyan Systematic Review software for further assessment, screening, selection and extraction of data (35). Three reviewers (G.K., T.N., and N.T.) collaborated to screen and select relevant studies. First, reviewers independently conducted a blind assessment of the titles and abstracts of all studies. The eligible articles were then subjected to full-text screening based on the inclusion and exclusion criteria. Finally, disagreements or inconsistencies between the three reviewers were addressed through discussion and consultation with a fourth reviewer (P.M.). The reasons for exclusion were clearly stated at the end of each screening stage.
Data were retrieved from the included studies and recorded on a data extraction table. The significant extracted characteristics included study designs, gene identification approaches, number of variants from genes, polymorphisms or miRNAs, participant demographic data, CVD follow-up time after first index pregnancy, and the definition of PE and CVD outcome measures.
2.4. Risk of bias and methodological quality assessment
The quality level of each study that reached the final screening stage was independently reviewed by three reviewers (G.K., T.N., and N.T.). The evaluation was conducted using the Newcastle-Ottawa Quality Assessment Scale for case-control, cohort and cross-sectional studies (36). The articles were ranked on a scale of 0–10 and have been further customised for this review (See Supplementary Tables S6–S8). The quality assessment criteria were based on case selection, comparability between cases and controls and outcome or exposure. Most studies had more than one study population with different study designs. Hence, the quality of each of these cohorts has been individually assessed, as they could not be categorised into one study design. The reasons for excluding studies despite having a good quality score were mentioned in the Quality Assessment Supplementary Tables (see Supplementary Tables S6–S11). Any difference of opinion between the three reviewers was resolved through discussion with the fourth reviewer (P.M.).
2.5. Data analysis
Three reviewers (G.K., T.N., and N.T.) performed the data extraction using Microsoft Word based on the Cochrane handbook for systematic reviews (2nd edition) (31). Included articles were thoroughly read and classified into two study types: CVD endpoints and CVD risk factors. However, the study design, methodologies, results, and the CVD outcomes analysed differed in each included study. Hence, a meta-analysis was not performed due to cross study heterogeneity.
3. Results
3.1. Study selection
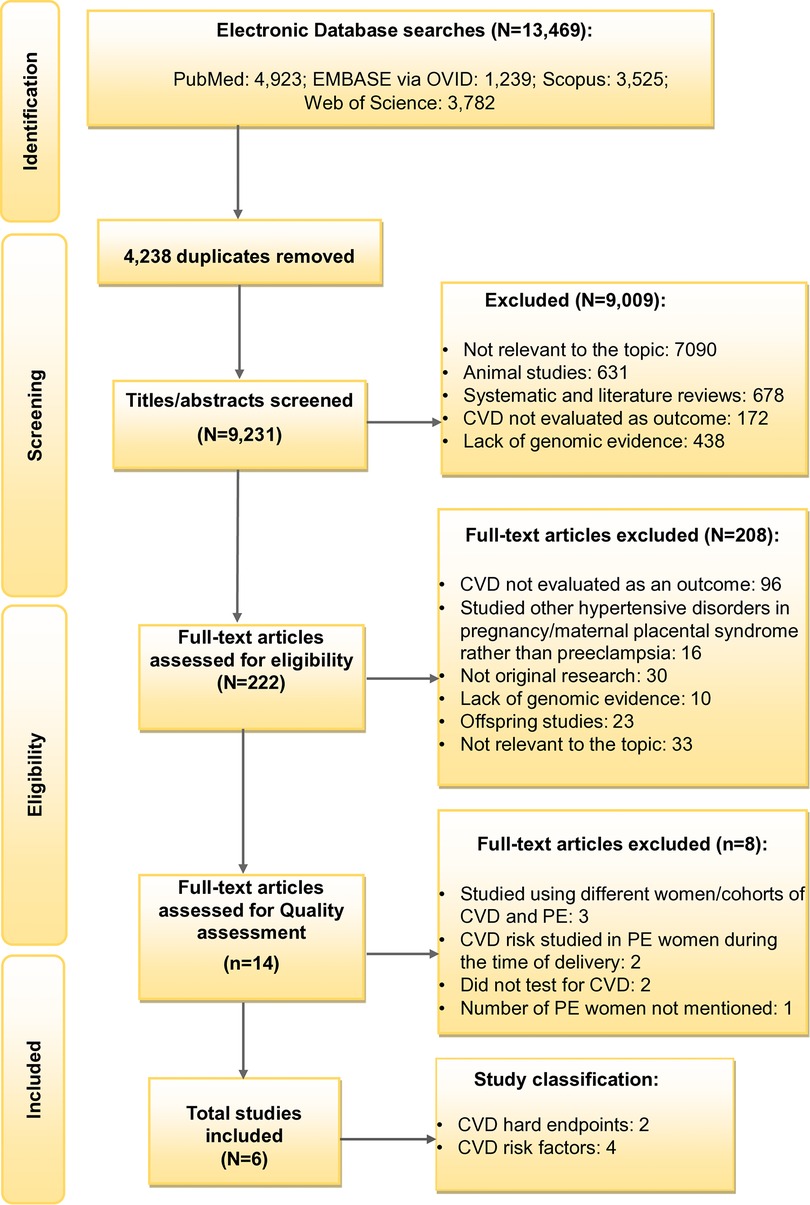
The Systematic Review Flowchart provides a detailed overview of the selection process (Figure 1). The primary search generated 13,469 records including the articles from the updated search. A total of 9,231 studies were then subjected to the title/abstract screening after eliminating duplicates (N = 4,238). Further, 9,009 additional studies were removed after the eligibility criteria were narrowed to be more specific to the study aim, excluding studies that: (i) were not focusing on both PE and CVD; (ii) were on animal studies; (iii) were systematic and literature reviews; (iv) did not evaluate CVD as the outcome; and (v) lacking genomic evidence. This resulted in the full-text screening of 222 studies. Following the full-text screening, 14 articles were included for quality assessment, excluding 208 studies with any of the above reasons. In addition, the articles which studied other hypertensive disorders of pregnancy and not PE, studies on offspring and those which were not original research were also excluded in the full-text screening stage. After further refinement, eight articles were removed for the following reasons: (i) two studies for using different cohorts for CVD and PE (20, 37); (ii) one article for investigating different women with CVD and a history of PE, although from the same cohort (38); (iii) two for making conclusions on the shared risk of PE and CVD using genes predisposed to CVD from another study (39, 40) and (iv) for not testing for CVD risk (39, 40); (v) two for studying CVD risk in women with PE during delivery, but not conducting any follow-up research on CVD risk (41, 42); and (vi) one study for not mentioning the number of women with PE (19). The included studies consisted of two studies on CVD endpoints following PE (35, 36) and four on CVD risk factors following PE (37–40) (see Supplementary Tables S6–S11). Ultimately, six articles met the study selection criteria.
3.2. Quality assessment
The quality scores of six included studies (43–48) and the excluded eight studies (19, 20, 37–42) that reached the final screening stage are shown in Supplementary Tables S6–S8. The six studies that passed the final screening were all case-control design. Hence, the New-Castle Ottawa scale for case-control studies was used (maximum score: nine); three studies scored 7.5 (44, 45, 48), one received 8.5 (43) and another obtained 5.5 (47). The final included study consisted of two case-control cohorts, and both were assessed for quality separately (46). Among these, cohort 1 scored 6 and cohort 2 scored 6.5 (46). Four studies had reliable methods of ascertaining PE from medical records or databases (43–45, 48). However, two studies based their PE diagnosis on self-reported questionnaires (46, 47). All studies investigated a variety of genomic factors involved in the progression to CVD or CVD risk factors following PE in the same women from the same cohorts of PE and CVD.
3.3. Study characteristics
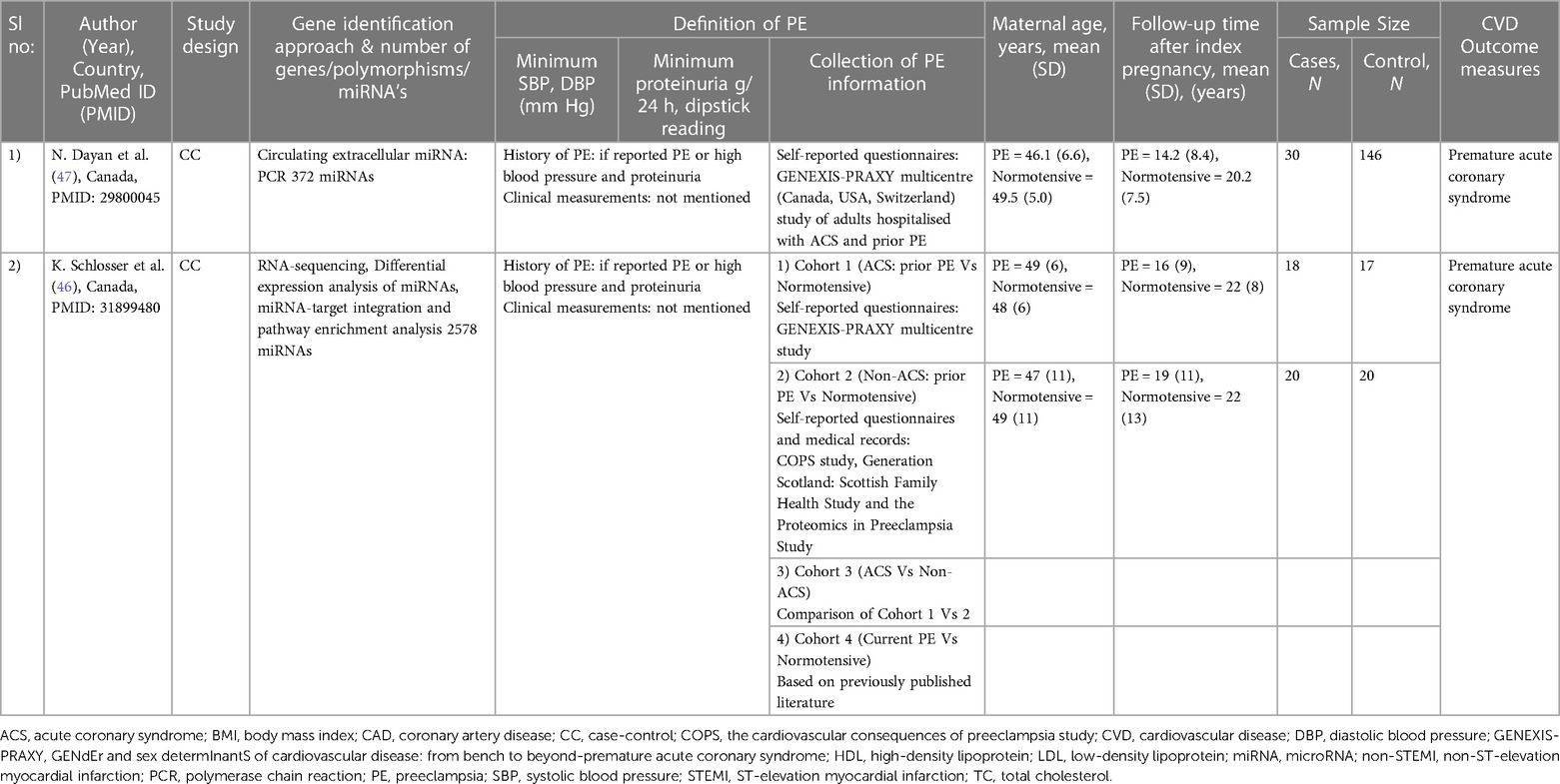
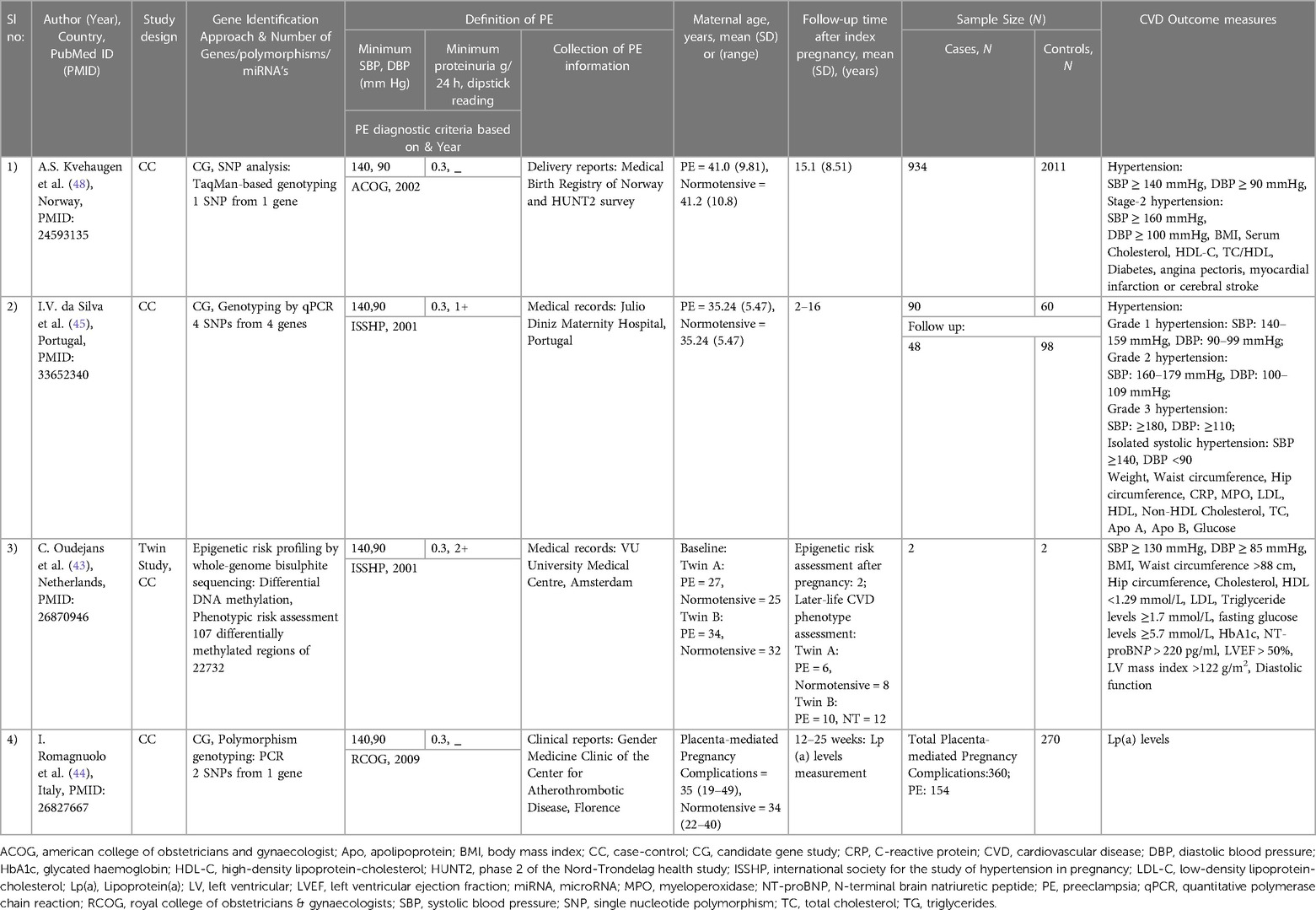
The study characteristics for the CVD endpoints and CVD risk factors studies are summarised in Tables 1, 2, respectively. The articles included consisted of three candidate gene studies (44, 45, 48), two miRNA studies (46, 47), and one epigenetic study investigating whole-genome bisulphite sequencing (43).
The study populations were primarily of European ancestry, with the total number of participants ranging from 4 to 2,945 and maternal ages ranging from 27 to 49.5 years. The follow-up time after the index pregnancy varied from 12 weeks to 22 years. The International Society for the Study of Hypertension in Pregnancy (49), the American College of Obstetricians and Gynaecologists (50) and the Royal College of Obstetricians and Gynaecologists' (51, 52) diagnostic criteria were used in four studies to identify women with PE. The CVD outcome measures included premature acute coronary syndrome (ACS) (46, 47), angina pectoris (48), myocardial infarction (48), cerebral stroke (48) and cardiometabolic risk traits such as blood pressure (43, 45, 48), glucose levels (43, 45, 48), weight (45), BMI (43, 48), waist circumference (43, 45), hip circumference (43, 45), left ventricular ejection fraction (LVEF) (43), left ventricular mass index (43), diastolic function (43), apolipoprotein A (Apo A) (45), apolipoprotein B (Apo B) (45), LDL-C (43, 45), HDL-C (43, 45, 48), total cholesterol (43, 45, 48), triglyceride levels (43) and lipoprotein (a) [Lp(a)] levels (44).
3.4. CVD endpoints
Two studies were included on the progression of PE to CVD endpoints (Tables 1, 3) (46, 47). Both studies focused on circulating miRNAs, their association with PE, and premature ACS, the outcome of the studies (46, 47).
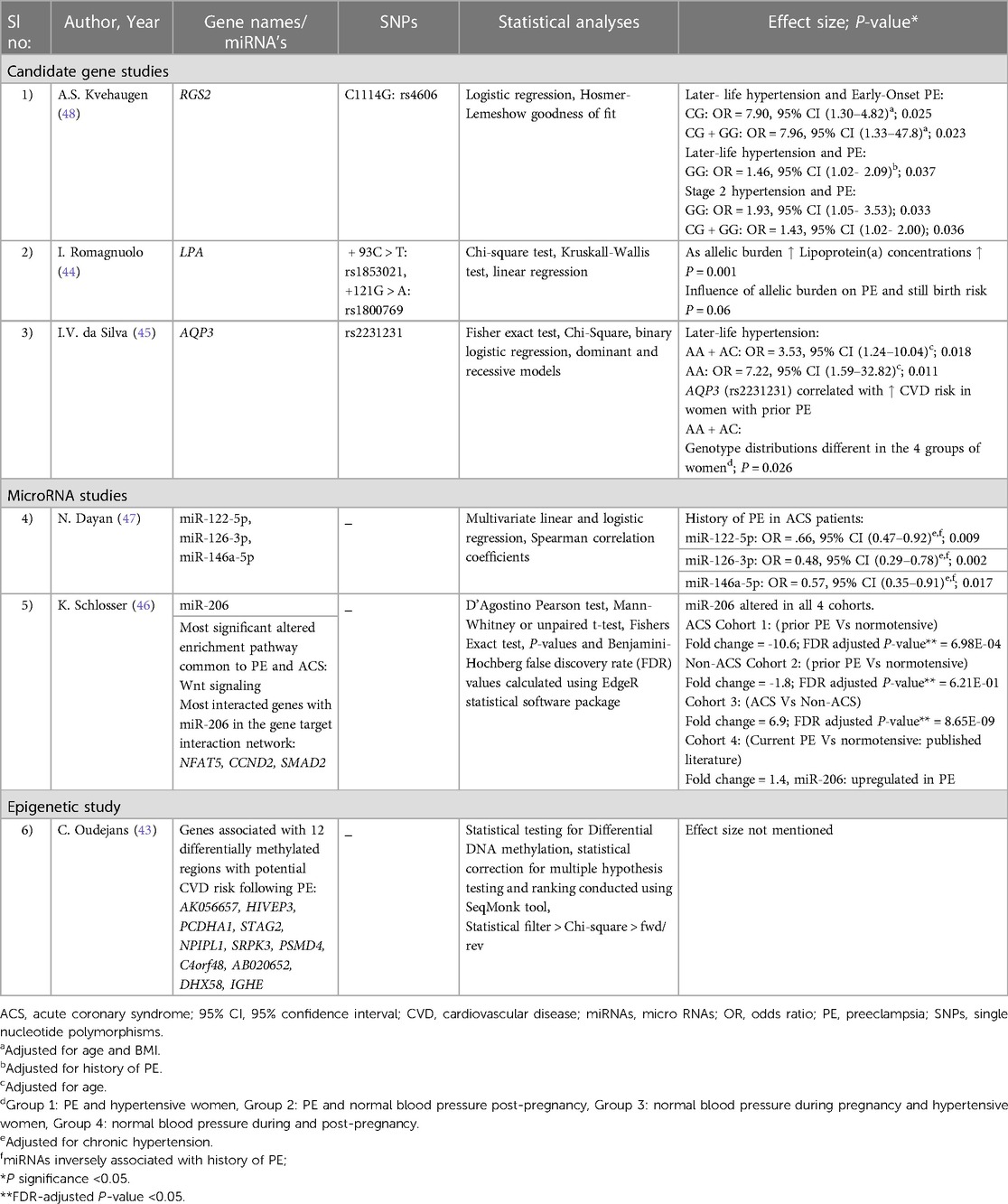
In the first study, Dayan et al. investigated 372 miRNAs in women who developed premature ACS 14.2 years after PE (N = 30) vs. those who had normotensive pregnancies (N = 146) (47). This led to the identification of 16 differentially expressed miRNAs in PE, which consisted of: (i) an increase of 10 miRNAs with a fold change of 1.3–2.0; and (ii) a decrease of 6 miRNAs with a fold change of 1.3–2.8. However, of the 16 miRNAs, only three miRNAs that met the eligibility requirements for evaluations in larger validation cohorts were selected in the study. Moreover, the three chosen miRNAs were also associated with various biological mechanisms involved in CVD risk (47, 53). The mir-1225p was previously associated with hepatic lipid metabolism, miR-126-3p with angiogenesis and miR-146a-5p with anti-inflammation (47, 53). However, in this study, all three miRNAs (miR-122-5; miR-126-3p; and miR-146a-5p) significantly lowered in women with premature ACS and a history of PE, even after adjusted for chronic hypertension (47) (See Tables 1, 3 for more details).
In the second study, 2,578 miRNAs were screened comparing circulating miRNA levels between four cohorts: (i) an ACS cohort with a history of PE (N = 18) vs. normotensive (N = 17); (ii) a non-ACS cohort with a history of PE (N = 20) vs. normotensive (N = 20); (iii) an ACS vs. non-ACS cohort; and (iv) women with PE vs. normotensive without ACS (46). The development of premature ACS varied from 16- and 19-years post PE across the ACS and non-ACS cohorts respectively. Among the 2,578 miRNAs screened, only one miRNA (miR-206) was altered in all four cohorts. However, a history of PE was linked to approximately ten-fold lower plasma levels of miR-206 in women with ACS compared to a history of normotensive pregnancy. This was confirmed in a second cohort of women without ACS, but the change was more moderate at 1.8-fold. Moreover, through miRNA pathway enrichment analysis, Wnt-signalling was identified as the most significantly modified pathway common to PE and ACS. Besides, the most interacting genes with miR-206 in the gene target interaction network were identified as Nuclear Factor of Activated T-cells 5 (NFAT5), Cyclin D2 (CCND2) and Mothers Against Decapentaplegic homolog 2 (SMAD2) (46) (See Tables 1, 3 for more details).
3.5. CVD risk factors
Four case-control studies were included that investigated CVD risk factors (Tables 2, 3) (43–45, 48). Two were candidate gene studies on PE and later-life hypertension (45, 48). The later-life hypertension was considered as the outcome in both studies.
In the first study, four genetic variants from four genes: Aquaporin-3 (AQP3; rs2231231), Aquaporin-7 (AQP7; rs2989924), Nitric oxide synthase 3 (NOS3; 4B/A intron) and Cytochrome B-245 Alpha Chain (CYBA; rs4673) were investigated for their association with later-life hypertension, in a cohort of women with prior PE (N = 48) or who had a previous normotensive pregnancy (N = 98) (45). Previous studies have identified that all four genes included in the analysis play an essential role in redox homeostasis and oxidative stress, which are major components of a metabolic syndrome (a group of risk factors specific to CVD) (45, 54, 55). However, among the four single nucleotide polymorphisms (SNPs), only one intronic SNP rs2231231 from the AQP3 gene, dominant and recessive model of A allele [AA + AC] and [AA] respectively, was associated with PE and the development of hypertension in women 2–16 years post-pregnancy. Moreover, in the study, the AQP3 (rs2231231), [AA + AC] was also linked to a greater risk of CVD in women with a history of PE, as it showed different genotype distributions in four different groups of women. Group 1 included preeclamptic and hypertensive women, group 2 included preeclamptic women and those with normal blood pressure post-pregnancy, group 3 included women with normal blood pressure during pregnancy and hypertensive women, and group 4 included women with normal blood pressure during and post-pregnancy(45) (See Tables 2, 3 for more details).
The second study investigated a single genetic variant (rs4606) in the Regulator of G Protein Signaling 2 (RGS2) gene to check its association with later-life hypertension 15-years following PE. This analysis was conducted in Norwegian PE cases (N = 934) and controls (N = 2,011) (48). Past studies identified that numerous vasoconstrictors are negatively regulated by the regulator of G protein signaling 2 (56). Moreover, CG or GG genotypes of rs4606 in the RGS2 gene have been previously found to be associated with PE women (57). However, this study identified the association of rs4606 [CG, CG + GG] polymorphism with the risk of later-life hypertension and a history of early-onset PE after adjusting for age and BMI. In addition, associations were also identified with the SNP, rs4606 [GG], later-life hypertension and a history of PE and between rs4606[GG, CG + GG], later-life stage 2 hypertension and a history of PE (48) (See Tables 2, 3 for more details).
The third CVD risk factor study is a candidate gene study on PE and Lp(a) levels (44). The Lp(a) levels were considered the study's outcome. Lp(a), which is associated with the plasminogen-like glycoprotein, is a significant risk factor for atherosclerotic CVD, mainly in those with LDL-C or HDL-C (58, 59). Moreover, from previous studies, Lp(a) levels were also observed to be increased in women with a history of PE (60, 61). However, this research was conducted in a cohort with a history of various placenta-mediated pregnancy complications (N = 360), including PE (N = 154), stillbirth (N = 121), and small-for-gestational-age (N = 85) as cases. Healthy women with no history of vascular disorders (conditions that affects blood vessels, e.g., venous thrombosis, Aneurysm) and pregnancy complications were included as control groups. The study investigated the involvement of the two polymorphisms (rs1853021: + 93C > T and rs1800769: 121G > A) in the Lipoprotein A (LPA) gene in modifying Lp(a) levels and placenta-mediated pregnancy complications risk. The Lp(a) levels were analysed 12 weeks post-pregnancy, and it was observed that women with a history of PE and stillbirth had elevated Lp(a) levels. Moreover, as the unfavourable allelic burden of LPA gene elevated, Lp(a) concentrations gradually increased. A similar association of increased risk with PE and stillbirth with Lp(a) levels was identified, although it was not significant (P = 0.06) (See Tables 2, 3 for more details).
The last included article used whole-genome bisulphite DNA methylation study with two identical twin sister pairs discordant for PE (43). This study investigated the epigenomic alterations associated with CVD risk following PE. The twin sister pairs were examined for epigenetic risk two years post-pregnancy. Furthermore, 18 CVD markers were tested between the twin sisters to understand the phenotypic risk 6–12 years post-pregnancy; no differences were observed. However, a genome-wide methylC-sequencing of 22,732 differentially methylated regions (DMRs) revealed 107 DMRs significantly altered in all individuals. Among these, 12 DMRs were found to be shared by the affected twin sisters, with at least half a difference in their methylation percentage and having the same up or down-regulation. These findings were vastly different from those of their unaffected twins (Table 3). These 12 DMRs may be potentially linked to CVD risk following PE, and the genes associated with the regions can be found in Table 3. One of these genes DHX58 was found to be associated with coronary artery disease in another study (62). However, the remaining genes associated with DMRs were linked to CVD mainly in animal studies (63, 64). The authors of this epigenetic study concluded that the ongoing long-term CVD risk in the affected twin sister might be due to the changes in her DNA methylation caused by PE.
4. Discussion
4.1. Main findings
In this systematic review, we surveyed the published literature to identify common evidence on shared genomic factors associated with CVD endpoints or risk factors following PE available as of July 28, 2023. Following quality control and screening, we identified six case-control studies longitudinally testing for CVD endpoints or CVD risk factors following PE (43–48). All the included studies were of case-control design and European ancestry. Both studies investigating CVD endpoints focussed on premature ACS following PE using miRNA markers (46, 47). These studies identified four miRNAs (miR-122-5p, -126-3p, -146a-5p, -206) differentially expressed in women with premature ACS following PE and concluded that these findings might offer better insights into biological mechanisms that could be responsible for the elevated risk of CVD post-PE (46, 47).
The CVD risk factors category consisted of: (i) two candidate gene studies (45, 48) on PE and later-life hypertension (ii) one candidate gene study(44) on PE and Lp(a) levels and (iii) an epigenetic study on PE and later-life CVD risk factors (43). The first candidate gene study identified one SNP rs2231231 from the AQP3 gene associated with PE and later-life hypertension (45). The study concluded that as the AQP3 gene was only associated with hypertension post-pregnancy, the role of the gene might be linked to later-life hypertension risk factors including oxidative stress and metabolic syndrome (45). The second candidate gene study also identified another SNP rs4606 from the RGS2 gene associated with PE and later-life hypertension (48). In this analysis, even after accounting for rs4606 SNP and other CVD risk factors, PE continued to be a standalone risk factor for future hypertension (48). Moreover, from another candidate gene study on PE and lipoprotein(a) [Lp(a)] levels, two polymorphisms (rs1853021: + 93C > T and rs1800769: 121G > A) in the LPA gene were observed in modifying Lp(a) levels and placenta-mediated pregnancy complications risk (44). The study detected that, those women with a history of PE and stillbirth had an increased concentration of Lp(a). This research helped in confirming the relationship between pregnancy complications and the atherothrombotic marker, Lp(a) (44). Finally, from the epigenetic study, 12 DMRs associated with CVD risk following PE were identified (43). The study concluded that the whole-genome bisulfite DNA methylation sequencing approach used in this study would help in identifying biomarkers that can be used for early CVD risk stratification for women after a complicated pregnancy (43).
None of the included studies reached the maximum score on the Newcastle-Ottawa Scale, as most studies did not include their non-response rate (Supplementary Tables S6–S11). Thus, the non-response bias that may have existed in the studies could not be calculated. The limited amount of primary research demonstrates a critical gap in the literature that needs to be addressed. Hence, despite the increasing number of publications on PE and its relationship with later-life CVD (19, 20, 65–67), more empirical research is required to identify the genomic factors involved in the progression to CVD after a history of PE using a longitudinal study framework.
While suggestive evidence has also been found using MR and PRS with PE and later-life CVD, more comprehensive research is required to fully understand the causal genetics (30, 68, 69). A genome-wide association study using MR found that hypertensive disorders of pregnancy were associated with an increased risk of coronary artery disease and stroke (68). Conversely, the causal genetic variants associated with PE and later-life CVD remain unclear in the study. In addition, a recent investigation using PRS found a heightened risk of atherosclerotic CVD associated with significant genetic susceptibility for hypertensive disorders during pregnancy. This study highlights the benefit of PRS in predicting long-term CVD outcomes in later life for hypertensive disorders during pregnancy (30). However, this study did not solely focus on PE and its associated genetic risk with later-life CVD.
4.2. Interpretation
Several systematic reviews and meta-analyses discuss CVD risk following PE (70–73), but only a small number consider the shared genomic risk factors associated with both diseases (74, 75). In addition, many of these previous systematic reviews make conclusions combining the results between animal and human studies (75). While animal models on PE are well established across different species, the focus on CVD in vivo models is heterogeneous in nature and does not always capture the complexity of how biological processes may evolve. Hence, a standard animal model does not work for CVD, and several animal models or a personalised model would be required for a better understanding, increasing the complexity (76). Thus, this systematic review focused on including studies on genetic, epigenetic or transcriptomic factors associated with women with a history of PE and later-life CVD risk.
4.3. Strengths
A comprehensive analysis was conducted to systematically identify up-to-date studies on genomic loci associated with the progression of PE to CVD. Only studies conducted using the same cohorts of women with CVD and PE, and articles on CVD risk following PE, were included. This would help better identify the overlapping genomic risk loci of both diseases over time without any bias. The scores were given based on the checklist relevant to their study design. Moreover, the criteria for ascertainment of exposure in the Newcastle-Ottawa scale were tailored to incorporate genetic or epigenetic assessments. These customisations in quality assessments have helped thoroughly analyse the studies, irrespective of their study type or design.
4.4. Limitations
This study has certain drawbacks that need to be addressed. First, each included literature had different methodologies, study designs, findings, and analyses of the CVD outcomes. Thus, a meta-analysis could not be performed because of the significant heterogeneity between studies. Second, the sample size of each included study was comparatively small. Third, only articles published in English were included. Fourth, as all included studies were of European ancestry, there was no data relevant to other ethnicities. Finally, we recognise that there are various other women-specific CVD risk factors, such as foetal growth restriction, polycystic ovarian syndrome, menopause, and premature ovarian failure, that can influence the development of CVD and may contribute to the onset of metabolic syndrome (77). However, the primary focus of our review is to analyse the genomic variations associated with the progression of CVD in later life following an incidence of PE. While we acknowledge the significance of other contributing factors, our current review focuses solely on this relationship. Our goal is to provide a comprehensive analysis that delves into the genomic risk association between PE and its progression to later-life CVD.
5. Recommendation
Identifying the genomic risk loci associated with the progression of PE to CVD would provide a better understanding of the underlying biology of both diseases. Therefore, more comprehensive longitudinal research is required directed to this aim. Moreover, the direction of causality needs to be determined using MR. Also, the self-reported surveys need to be further validated using electronic health records, and study populations with larger sample sizes should be included. Future studies may also consider examining the impact of additional women-specific risk factors associated with the progression to CVD.
6. Conclusion
We conducted an extensive systematic review of the literature that demonstrates limited publications regarding genomic risk loci associated with the progression of PE to later-life CVD. This review provides critical insight into the heterogeneous nature of genomic studies investigating CVD following PE and highlights the urgent need for large scale longitudinal studies that investigate the genetic risk underlying the progression to CVD following PE.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
PM, EM, and SB conceived the project idea, while GK and HP designed the analysis workflow. GK, TN, and NT screened all the relevant articles and performed quality assessment analysis. GK wrote the manuscript, with supervision from PM. All authors contributed to the article and approved the submitted version.
Funding
All the funds required for this systematic review were covered by National Health and Medical Research Council (NHMRC), Australia, research grant (APP2001203) and Tasmania Graduate Research Scholarship (TGRS) from the University of Tasmania, Hobart, Australia.
Acknowledgments
We would like to acknowledge NHMRC Australia and TGRS for their generous financial support of this research. We are also grateful to Menzies Institute for Medical Research, the University of Tasmania, for providing access to the research facilities and resources.
Conflict of interest
The authors PM and HP declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fepid.2023.1221222/full#supplementary-material
Abbreviations
PE, Preeclampsia; CVD, Cardiovascular disease; PICO, P: Patient, Population or Problem, I: Intervention, C: Comparison, O: Outcome; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; BMI, Body mass index;
References
1. Saeed A, Kampangkaew J, Nambi V. Prevention of cardiovascular disease in women. Methodist Debakey Cardiovasc J. (2017) 13(4):185–92. doi: 10.14797/mdcj-13-4-185
2. Gongora MC, Wenger NK. Cardiovascular complications of pregnancy. Int J Mol Sci. (2015) 16(10):23905–28. doi: 10.3390/ijms161023905
3. Mehta LS, Beckie TM, Devon HA, Grines CL, Krumholz HM, Johnson MN, et al. Acute myocardial infarction in women: a scientific statement from the American heart association. Circulation. (2016) 133(9):916–47. doi: 10.1161/CIR.0000000000000351
4. Bennett AL, Lavie CJ, Grace SL. Cardiac rehabilitation following acute coronary syndrome in women. Curr Treat Options Cardiovasc Med. (2017) 19(8):57. doi: 10.1007/s11936-017-0559-x
5. Brown HL, Warner JJ, Gianos E, Gulati M, Hill AJ, Hollier LM, et al. Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists: a presidential advisory from the American heart association and the American college of obstetricians and gynecologists. Circulation. (2018) 137(24):e843–52. doi: 10.1161/CIR.0000000000000582
6. Parikh NI, Gonzalez JM, Anderson CAM, Judd SE, Rexrode KM, Hlatky MA, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American heart association. Circulation. (2021) 143(18):e902–16. doi: 10.1161/CIR.0000000000000961
7. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803
8. Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, et al. The 2021 international society for the study of hypertension in pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. (2022) 27:148–69. doi: 10.1016/j.preghy.2021.09.008
9. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. (2009) 33(3):130–7. doi: 10.1053/j.semperi.2009.02.010
10. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, De Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. (2016) 387(10022):999–1011. doi: 10.1016/S0140-6736(15)00070-7
11. Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG. (2018) 125(13):1642–54. doi: 10.1111/1471-0528.15394
12. Berends AL, de Groot CJM, Sijbrands EJ, Sie MPS, Benneheij SH, Pal R, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. (2008) 51(4):1034–41. doi: 10.1161/HYPERTENSIONAHA.107.101873
13. Craici I, Wagner S, Garovic VD. Preeclampsia and future cardiovascular risk: formal risk factor or failed stress test? Ther Adv Cardiovasc Dis. (2008) 2(4):249–59. doi: 10.1177/1753944708094227
14. Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol. (2017) 8:1058. doi: 10.3389/fimmu.2017.01058
15. O'Brien TE, Ray JG, Chan W-S. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. (2003) 14(3):368–74. doi: 10.1097/00001648-200305000-00020
16. Motedayen M, Rafiei M, Rezaei Tavirani M, Sayehmiri K, Dousti M. The relationship between body mass index and preeclampsia: a systematic review and meta-analysis. Int J Reprod Biomed. (2019) 17(7):463–72. doi: 10.18502/ijrm.v17i7.4857
17. Yong HEJ, Murthi P, Brennecke SP, Moses EK. Genetic approaches in preeclampsia. Methods Mol Biol. (2018) 1710:53–72. doi: 10.1007/978-1-4939-7498-6_5
18. Kalayinia S, Goodarzynejad H, Maleki M, Mahdieh N. Next generation sequencing applications for cardiovascular disease. Ann Med. (2018) 50(2):91–109. doi: 10.1080/07853890.2017.139259519
19. Løset M, Johnson MP, Melton PE, Ang W, Huang R-C, Mori TA, et al. Preeclampsia and cardiovascular disease share genetic risk factors on chromosome 2q22. Pregnancy Hypertens. (2014) 4(2):178–85. doi: 10.1016/j.preghy.2014.03.005
20. Johnson MP, Brennecke SP, East CE, Dyer TD, Roten LT, Proffitt JM, et al. Genetic dissection of the pre-eclampsia susceptibility locus on chromosome 2q22 reveals shared novel risk factors for cardiovascular disease. Mol Hum Reprod. (2013) 19(7):423–37. doi: 10.1093/molehr/gat011
21. Valenzuela FJ, Pérez-Sepúlveda A, Torres MJ, Correa P, Repetto GM, Illanes SE. Pathogenesis of preeclampsia: the genetic component. J Pregnancy. (2012) 2012:632732. doi: 10.1155/2012/632732
22. Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. Cardiovasc Res. (2014) 101(4):579–86. doi: 10.1093/cvr/cvu018
23. Lisowska M, Pietrucha T, Sakowicz A. Preeclampsia and related cardiovascular risk: common genetic background. Curr Hypertens Rep. (2018) 20(8):71. doi: 10.1007/s11906-018-0869-8
24. Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. (2014) 15(8):509–24. doi: 10.1038/nrm3838
25. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. (2012) 13(7):484–92. doi: 10.1038/nrg3230
26. Sanderson E, Glymour MM, Holmes MV, Kang H, Morrison J, Munafò MR, et al. Mendelian Randomization. Nat Rev Methods Primers. (2022) 2:6. doi: 10.1038/s43586-021-00092-5
27. Bell KJL, Loy C, Cust AE, Teixeira-Pinto A. Mendelian randomization in cardiovascular research: establishing causality when there are unmeasured confounders. Circ Cardiovasc Qual Outcomes. (2021) 14(1):e005623. doi: 10.1161/CIRCOUTCOMES.119.005623
28. Hosier H, Lipkind HS, Rasheed H, Dewan AT, Rogne T. Dyslipidemia and risk of preeclampsia: a multiancestry mendelian randomization study. Hypertension. (2023) 80(5):1067–76. doi: 10.1161/HYPERTENSIONAHA.122.20426
29. Cross B, Turner R, Pirmohamed M. Polygenic risk scores: an overview from bench to bedside for personalised medicine. Front Genet. (2022) 13:1000667. doi: 10.3389/fgene.2022.1000667
30. Lee SM, Shivakumar M, Xiao B, Jung SH, Nam Y, Yun JS, et al. Genome-wide polygenic risk scores for hypertensive disease during pregnancy can also predict the risk for long-term cardiovascular disease. Am J Obstet Gynecol. (2023) 16:S0002-9378(23)00157-6. doi: 10.1016/j.ajog.2023.03.013
31. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester, UK: John Wiley & Sons (2019).
32. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
33. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club. (1995) 123(3):A12–3. doi: 10.7326/ACPJC-1995-123-3-A12
35. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5(1):210. doi: 10.1186/s13643-016-0384-4
36. Wells GA, Wells G, Shea B, Shea B, O’Connell D, Peterson J, et al. editors. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2014).
37. Johansson Å, Curran JE, Johnson MP, Freed KA, Fenstad MH, Bjørge L, et al. Identification of ACOX2 as a shared genetic risk factor for preeclampsia and cardiovascular disease. Eur J Hum Genet. (2011) 19(7):796–800. doi: 10.1038/ejhg.2011.19
38. Fatini C, Romagnuolo I, Sticchi E, Rossi L, Cellai AP, Rogolino A, et al. ACE Gene in pregnancy complications: insights into future vascular risk. Hypertens Pregnancy. (2016) 35(1):62–72. doi: 10.3109/10641955.2015.1115059
39. Gray KJ, Kovacheva VP, Mirzakhani H, Bjonnes AC, Almoguera B, Dewan AT, et al. Gene-centric analysis of preeclampsia identifies maternal association at PLEKHG1. Hypertension. (2018) 72(2):408–16. doi: 10.1161/HYPERTENSIONAHA.117.10688
40. Best LG, Saxena R, Anderson CM, Barnes MR, Hakonarson H, Falcon G, et al. Two variants of the C-reactive protein gene are associated with risk of pre-eclampsia in an American Indian population. PLoS One. (2013) 8(8):e71231. doi: 10.1371/journal.pone.0071231
41. Tuten A, Gungor Z, Ekmekci H, Ekmekci OB, Kucur M, Yilmaz N, et al. Relationship between LPA SNPs and inflammatory burden in patients with preeclampsia to address future cardiovascular risk. J Matern Fetal Neonatal Med. (2021) 34(6):898–906. doi: 10.1080/14767058.2019.1622667
42. Güngör ZB, Tüten A, Ekmekçi H, Ekmekçi ÖB, Kucur M, Öncül M, et al. Possible effects of lipoprotein-associated phospholipase A2 single-nucleotide polymorphisms on cardiovascular risk in patients with preeclampsia. J Matern Fetal Neonatal Med. (2018) 31(23):3119–27. doi: 10.1080/14767058.2017.1365125
43. Oudejans C, Poutsma A, Michel O, Mulders J, Visser A, Van Dijk M, et al. Genome-wide identification of epigenetic hotspots potentially related to cardiovascular risk in adult women after a complicated pregnancy. PLoS One. (2016) 11(2):e0148313. doi: 10.1371/journal.pone.0148313
44. Romagnuolo I, Sticchi E, Attanasio M, Grifoni E, Cioni G, Cellai AP, et al. Searching for a common mechanism for placenta-mediated pregnancy complications and cardiovascular disease: role of lipoprotein(a). Fertil Steril. (2016) 105(5):1287–93.e3. doi: 10.1016/j.fertnstert.2016.01.014
45. da Silva IV, Santos AC, Matos A, Pereira da Silva A, Soveral G, Rebelo I, et al. Association of Aquaporin-3, Aquaporin-7, NOS3 and CYBA polymorphisms with hypertensive disorders in women. Pregnancy Hypertens. (2021) 24:44–9. doi: 10.1016/j.preghy.2021.02.008
46. Schlosser K, Kaur A, Dayan N, Duncan PL, Delles C. Circulating miR-206 and Wnt-signaling are associated with cardiovascular complications and a history of preeclampsia in women. Clin Sci. (2020) 134(2):87–101. doi: 10.1042/CS20190920
47. Dayan N, Schlosser K, Stewart DJ, Delles C, Kaur A, Pilote L. Circulating MicroRNAs implicate multiple atherogenic abnormalities in the long-term cardiovascular sequelae of preeclampsia. Am J Hypertens. (2018) 31(10):1093–7. doi: 10.1093/ajh/hpy069
48. Kvehaugen AS, Melien Ø, Holmen OL, Laivuori H, Dechend R, Staff AC. Hypertension after preeclampsia and relation to the C1114G polymorphism (rs4606) in RGS2: data from the Norwegian HUNT2 study. BMC Med Genet. (2014) 15:28. doi: 10.1186/1471-2350-15-28
49. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the international society for the study of hypertension in pregnancy (ISSHP). Hypertens Pregnancy. (2001) 20(1):IX–XIV. doi: 10.1081/PRG-100104165
50. ACOG Committee on Practice Bulletins–Obstetrics. ACOG Practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. (2002) 99(1):159–67. doi: 10.1016/s0029-7844(01)01747-1
51. Royal College of Obstetricians & Gynaecologists. Severe Pre-eclampsia/Eclampsia, Management (Green-top Guideline No. 10A). https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/severe-pre-eclampsiaeclampsia-management-green-top-guideline-no-10a/ (Accessed May 10, 2023).
52. National Institute for Health and Care Excellence. Overview: Hypertension in Pregnancy: Diagnosis and Management: Guidance. https://www.nice.org.uk/guidance/ng133 (Accessed May 10, 2023).
53. Laffont B, Rayner KJ. MicroRNAs in the pathobiology and therapy of atherosclerosis. Can J Cardiol. (2017) 33(3):313–24. doi: 10.1016/j.cjca.2017.01.001
54. Brito R, Castillo G, González J, Valls N, Rodrigo R. Oxidative stress in hypertension: mechanisms and therapeutic opportunities. Exp Clin Endocrinol Diabetes. (2015) 123(6):325–35. doi: 10.1055/s-0035-1548765
55. da Silva IV, Rodrigues JS, Rebelo I, Miranda JPG, Soveral G. Revisiting the metabolic syndrome: the emerging role of aquaglyceroporins. Cell Mol Life Sci. (2018) 75(11):1973–88. doi: 10.1007/s00018-018-2781-4
56. Tsang S, Woo AY, Zhu W, Xiao R-P. Deregulation of RGS2 in cardiovascular diseases. Front Biosci. (2010) 2(2):547–57. doi: 10.2741/s84
57. Kvehaugen AS, Melien O, Holmen OL, Laivuori H, Oian P, Andersgaard AB, et al. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension. (2013) 61(3):655–61. doi: 10.1161/HYPERTENSIONAHA.111.00331
58. Jacobson TA. Lipoprotein(a), cardiovascular disease, and contemporary management. Mayo Clin Proc. (2013) 88(11):1294–311. doi: 10.1016/j.mayocp.2013.09.003
59. Kassner U, Schlabs T, Rosada A, Steinhagen-Thiessen E. Lipoprotein(a)—an independent causal risk factor for cardiovascular disease and current therapeutic options. Atheroscler Suppl. (2015) 18:263–7. doi: 10.1016/j.atherosclerosissup.2015.02.039
60. Manten GTR, Sikkema MJ, Voorbij HAM, Visser GHA, Bruinse HW, Franx A. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy. (2007) 26(1):39–50. doi: 10.1080/10641950601146574
61. van Pampus MG, Koopman MM, Wolf H, Büller HR, Prins MH, van den Ende A. Lipoprotein(a) concentrations in women with a history of severe preeclampsia–a case control study. Thromb Haemost. (1999) 82(1):10–3.10456446
62. van Der Harst P, Verweij N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res. (2018) 122(3):433–43. doi: 10.1161/CIRCRESAHA.117.312086
63. Zhou Y, Alimohamadi S, Wang L, Liu Z, Wall JB, Yin C, et al. A loss of function screen of epigenetic modifiers and splicing factors during early stage of cardiac reprogramming. Stem Cells Int. (2018) 2018:3814747. doi: 10.1155/2018/3814747
64. Thirunavukkarasu M, Adluri RS, Juhasz B, Samuel SM, Zhan L, Kaur A, et al. Novel role of NADPH oxidase in ischemic myocardium: a study with NOX2 knockout mice. Funct Integr Genomics. (2012) 12(3):501–14. doi: 10.1007/s10142-011-0256-x
65. Boom G, O’Sullivan JM, Schierding W. Spatially constrained gene regulation identifies key genetic contributions of preeclampsia, hypertension and proteinuria. Biol Reprod. (2023) 108(4):659–70. doi: 10.1093/biolre/ioad016
66. Suvakov S, Bonner E, Nikolic V, Jerotic D, Simic TP, Garovic VD, et al. Overlapping pathogenic signalling pathways and biomarkers in preeclampsia and cardiovascular disease. Pregnancy Hypertens. (2020) 20:131–6. doi: 10.1016/j.preghy.2020.03.011
67. Tyrmi JS, Kaartokallio T, Lokki AI, Jääskeläinen T, Kortelainen E, Ruotsalainen S, et al. Genetic risk factors associated with preeclampsia and hypertensive disorders of pregnancy. JAMA Cardiol. (2023) 8(7):674–83. doi: 10.1001/jamacardio.2023.1312
68. Rayes B, Ardissino M, Slob EAW, Patel KHK, Girling J, Ng FS. Association of hypertensive disorders of pregnancy with future cardiovascular disease. JAMA Netw Open. (2023) 6(2):e230034. doi: 10.1001/jamanetworkopen.2023.0034
69. Xiao B, Velez Edwards DR, Lucas A, Drivas T, Gray K, Keating B, et al. Inference of causal relationships between genetic risk factors for cardiometabolic phenotypes and female-specific health conditions. J Am Heart Assoc. (2023) 12(5):e026561. doi: 10.1161/JAHA.121.026561
70. Wu P, Haththotuwa R, Kwok CS, Babu A, Kotronias RA, Rushton C, et al. Preeclampsia and future cardiovascular health. Circ Cardiovasc Qual Outcomes. (2017) 10(2):e003497. doi: 10.1161/CIRCOUTCOMES.116.003497
71. Bellamy L, Casas J-P, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Br Med J. (2007) 335(7627):974. doi: 10.1136/bmj.39335.385301
72. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. (2013) 28(1):1–19. doi: 10.1007/s10654-013-9762-6
73. Zoet GA, Koster MPH, Velthuis BK, De Groot CJM, Maas AHEM, Fauser BCJM, et al. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas. (2015) 82(2):153–61. doi: 10.1016/j.maturitas.2015.07.004
74. Reddy M, Wright L, Rolnik DL, Li W, Mol BW, La Gerche A, et al. Evaluation of cardiac function in women with a history of preeclampsia: a systematic review and meta-analysis. J Am Heart Assoc. (2019) 8(22):e013545. doi: 10.1161/JAHA.119.013545
75. Mohseni Z, Spaanderman MEA, Oben J, Calore M, Derksen E, Al-Nasiry S, et al. Cardiac remodeling and pre-eclampsia: an overview of microRNA expression patterns. Ultrasound Obstet Gynecol. (2018) 52(3):310–7. doi: 10.1002/uog.17516
76. Liu Chung Ming C, Sesperez K, Ben-Sefer E, Arpon D, McGrath K, McClements L, et al. Considerations to model heart disease in women with preeclampsia and cardiovascular disease. Cells. (2021) 10(4):899. doi: 10.3390/cells10040899
Keywords: cardiovascular disease, preeclampsia, pregnancy hypertensive disorder, genomic, genetic, epigenetic, transcriptomic
Citation: Krishnamurthy G, Nguyen PT, Tran BN, Phan HT, Brennecke SP, Moses EK and Melton PE (2023) Genomic variation associated with cardiovascular disease progression following preeclampsia: a systematic review. Front. Epidemiol. 3:1221222. doi: 10.3389/fepid.2023.1221222
Received: 12 May 2023; Accepted: 14 September 2023;
Published: 16 October 2023.
Edited by:
Faith Osaretin Alele, James Cook University, AustraliaReviewed by:
Igor Victorovich Lakhno, Kharkiv National Medical University, UkraineIshag Adam, Qassim University, Saudi Arabia
© 2023 Krishnamurthy, Nguyen, Tran, Phan, Brennecke, Moses and Melton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gayathry Krishnamurthy Z2F5YXRocnkua3Jpc2huYW11cnRoeUB1dGFzLmVkdS5hdQ==
 Gayathry Krishnamurthy
Gayathry Krishnamurthy Phuong Tram Nguyen
Phuong Tram Nguyen Bao Ngoc Tran
Bao Ngoc Tran Hoang T. Phan
Hoang T. Phan Shaun P. Brennecke
Shaun P. Brennecke Eric K. Moses
Eric K. Moses Phillip E. Melton
Phillip E. Melton