
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Epidemiol., 19 April 2023
Sec. Occupational and Environmental Epidemiology
Volume 3 - 2023 | https://doi.org/10.3389/fepid.2023.1166174
This article is part of the Research TopicMultilevel Social Determinants of Individual and Family Well-being: National and International PerspectivesView all 10 articles
 Melissa A. Herrin1*
Melissa A. Herrin1* Allison R. Sherris2
Allison R. Sherris2 Logan C. Dearborn2
Logan C. Dearborn2 Christine T. Loftus2
Christine T. Loftus2 Adam A. Szpiro3
Adam A. Szpiro3 Paul E. Moore4
Paul E. Moore4 Margaret A. Adgent5
Margaret A. Adgent5 Emily S. Barrett6,7
Emily S. Barrett6,7 Ruby H. N. Nguyen8
Ruby H. N. Nguyen8 Kecia N. Carroll9,10
Kecia N. Carroll9,10 Catherine J. Karr2,11,12
Catherine J. Karr2,11,12
Background: Asthma is a leading cause of childhood morbidity in the U.S. and a significant public health concern. The prenatal period is a critical window during which environmental influences, including maternal occupational exposures, can shape child respiratory health. Cleaning chemicals are commonly encountered in occupational settings, yet few studies have examined the potential link between prenatal occupational exposures to cleaning chemicals and risk of childhood wheeze and asthma.
Methods: We evaluated the potential influence of maternal occupational exposure to cleaning chemicals during pregnancy on pediatric asthma and wheeze at child age 4–6 years in 453 mother-child pairs from two longitudinal pregnancy cohorts, TIDES and GAPPS, part of the ECHO prenatal and early childhood pathways to health (ECHO-PATHWAYS) consortium. Maternal occupational exposure to cleaning chemicals was defined based on reported occupation and frequency of occupational use of chemicals during pregnancy. Child current wheeze and asthma outcomes were defined by parental responses to a widely-used, standardized respiratory outcomes questionnaire administered at child age 4–6 years. Multivariable Poisson regression with robust standard errors was used to estimate relative risk (RR) of asthma in models adjusted for confounding. Effect modification by child sex was assessed using product interaction terms.
Results: Overall, 116 mothers (25.6%) reported occupational exposure to cleaning chemicals during pregnancy, 11.7% of children had current wheeze, and 10.2% had current asthma. We did not identify associations between prenatal exposure to cleaning chemicals and current wheeze [RRadjusted 1.03, 95% confidence interval (CI): 0.56, 1.90] or current asthma (RRadjusted 0.89, CI: 0.46, 1.74) in the overall sample. Analyses of effect modification suggested an adverse association among females for current wheeze (RR 1.82, CI: 0.76, 4.37), compared to males (RR 0.68, CI: 0.29, 1.58), though the interaction p-value was >0.05.
Conclusion: We did not observe evidence of associations between maternal prenatal occupational exposure to cleaning chemicals and childhood wheeze or asthma in the multi-site ECHO-PATHWAYS consortium. We leveraged longitudinal U.S. pregnancy cohorts with rich data characterization to expand on limited and mixed literature. Ongoing research is needed to more precisely characterize maternal occupational chemical exposures and impacts on child health in larger studies.
Childhood asthma affects approximately 8% of children in the U.S. and represents a significant public health concern (1). Asthma is a complex, chronic inflammatory respiratory disease, characterized by airway hyperresponsiveness, inflammation, and obstruction, and is often triggered by environmental factors (2). Symptoms include episodes of breathlessness, coughing, and wheezing, and asthma that develops in childhood has a profound impact on lifelong lung health, including airway remodeling and increased risk for adult asthma (3, 4). The prenatal period is a significant window during which interactions between genetics and environmental exposures, including environmental toxicants, modulate fetal lung development and immunologic responses that influence the risk, incidence, and severity of allergic diseases and asthma (3, 5–8).
Cleaning and disinfectant products are complex mixtures of chemicals, including irritants and potential sensitizers (9–11). Prior investigations have found robust, consistent epidemiological evidence that both home and occupational exposures to chemicals involved in cleaning and janitorial tasks, including disinfectants, fragrances, and solvents, increase risk for respiratory symptoms and asthma in adults (9, 11–18). In children, evidence also suggests a link between use of cleaning chemicals and sprays in the home with airway inflammation, persistent wheeze, lung function abnormalities, and increased risk of asthma (19–21).
By contrast, maternal environmental exposures during pregnancy and preconception, including occupational exposures to cleaning chemicals, have been found to be associated with childhood wheeze and asthma, though mechanisms are not yet fully understood (3, 10, 22–24). Several parental occupations are associated with higher risk of childhood respiratory outcomes, including jobs involving cleaning and chemical disinfection (22, 23, 25). Thus far, few studies have examined the potential link between maternal occupational exposures to cleaning agents specifically in the prenatal period and childhood asthma. A recent European cohort study found that both asthma and a related atopic condition in childhood (e.g., allergic rhinitis) were linked to prenatal exposure to cleaning agents (10). Finally, pre-adolescent boys have an increased prevalence of asthma, and child sex has been found to modify the relationship between prenatal environmental exposures and child airway outcomes (26–28), but few studies have specifically focused on the modifying role of child sex in prenatal occupational exposure to cleaning chemicals (22).
We contribute to this limited evidence base by evaluating the potential influence of maternal occupational exposure to cleaning chemicals during the prenatal period on pediatric asthma and wheeze outcomes at child age 4–6 years. Furthermore, we evaluate whether there is evidence of sex-specific associations. We utilize asthma and wheeze data collected in middle childhood in the ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS), a multi-site longitudinal study combining three U.S. pregnancy cohorts with extensive pregnancy exposure, child outcome and covariate characterization. We hypothesize that maternal exposure to cleaning chemicals during pregnancy will be associated with increased risk of asthma and wheeze at age 4–6 years and that effects will vary by child sex.
The study participants were mother-child pairs from two ECHO-PATHWAYS consortium pregnancy cohorts: the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) and The Infant Development and Environment Study (TIDES) (29).
GAPPS participants were enrolled between 2011 and 2014 from three hospitals in Seattle, WA and Yakima, WA. Inclusion criteria included being 18 years or older, able to speak and write English, and planning to deliver at the study hospital in which they were enrolled. Eligible mother-child dyads were recruited into ECHO-PATHWAYS when the children were 4–6 years old and attended clinic visits at age 4–6 years and age 8–9 years. TIDES participants were recruited during the first trimester of pregnancy from participating academic medical centers, from 2010 to 2012: San Francisco, CA; Minneapolis, MN; Rochester, NY; and Seattle, WA. Women were eligible if they were 18 years old or older, planning to deliver at one of the participating study hospitals, and having a low-risk singleton pregnancy at enrollment. Mother-child pairs were administered questionnaires and/or attended clinic visits at ages 4–5 years, 6 years and 7 years (30, 31). This analysis includes participants who completed both the occupational exposure questionnaire and the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire (32, 33) at child age 4–6 years. Because visits varied in composition and not all surveys were administered at all visits, only a subset of the participants who attended visits have both exposure and outcome data required for the main study question and are included in these analyses.
Participants provided informed consent. Data were analyzed by the University of Washington (UW) ECHO-PATHWAYS team and study protocols were approved by the UW Institutional Review Board (IRB).
Our primary exposure of interest, maternal occupational exposure to cleaning chemicals during the prenatal period, was assessed using questionnaires administered to primary caregivers regarding job titles, occupational activities, and exposures during the prenatal period. Questionnaires were administered at the GAPPS child age 4–6 visit (GAPPS 4–6), GAPPS child age 8–9 visit (GAPPS 8–9) or TIDES child age 7 visit (TIDES 7). In both GAPPS and TIDES, prenatal exposure to cleaning chemicals was defined as meeting any of the following: (1) answered “Yes” to “Did the biological mother work in any of the following industries during pregnancy: Janitor or house cleaner?”; (2) answered “Yes” to “Did the biological mother do any of the following activities at her job during pregnancy: Clean floors, sinks, or toilets?”; (3) answered “Some days” or “Every day” to “How often did the biological mother use janitorial chemicals or cleaners at her job during pregnancy?”.
We defined our primary outcomes as current wheeze and current asthma and our secondary outcome as strict asthma as reported between child ages 4–6 using the ISAAC questionnaire. The categorization of outcomes is similar to that used previously in ECHO-PATHWAYS consortium research (27–29, 34, 35). Current wheeze was defined as a positive response to “Has the child had wheezing or whistling in the chest in the past 12 months?” Current asthma was defined as positive responses to two of the following: current wheeze, ever asthma (defined as positive response to the question “Has your child ever had asthma?”), and asthma medication use (“Does the child use any medications for treatment of recurrent cough, recurrent wheezing or asthma?”). Strict asthma was defined as positive response to the question “Has your child ever had asthma?” as well as either current wheeze or asthma medication use (35, 36).
Demographic and behavioral characteristics of mother-child pairs were summarized overall and by cohort.
Modified multivariable Poisson regression with robust standard errors was used to estimate associations [adjusted risk ratios (RR)] between exposure and outcomes. The primary analyses investigated the association between prenatal exposure to cleaning chemicals (yes/no) and primary outcomes (current wheeze and current asthma) using separate models for each outcome. Secondary analyses investigated the association between prenatal exposure to cleaning chemicals and strict asthma.
We used a staged modeling approach for covariate adjustment by fitting minimally adjusted, fully adjusted (main model), and extended models. Covariates were selected a priori based on a literature search to identify asthma and wheeze risk factors that may be correlated with the exposure and included maternal, child, and household demographic, health, and socioeconomic factors. Minimally adjusted models were adjusted for child age, child sex, and study site. Main models further adjusted for self-identified maternal race (White, Asian, or other) and maternal ethnicity (Hispanic/Latino or non-Hispanic/Latino), education at enrollment (less than high school, high school completion, graduated college/technical school, or any graduate school/professional), maternal history of asthma (yes/no), maternal age at delivery (years, continuous), maternal self-report of smoking status at enrollment (yes/no), household size category (<4, 4, 5, >5), region-and inflation-adjusted household income (continuous, $USD), postnatal second-hand smoke exposure (yes/no), season of birth (warm [April through September]/cold [October through March]), and firstborn status (yes/no). Extended models additionally adjusted for two potential confounders that may also act as mediators: preterm birth at less than 37 weeks (binary) and birthweight (continuous). To evaluate whether the association between prenatal exposure to cleaning chemicals and childhood asthma is modified by child sex, we tested for a statistical interaction using multiplicative interaction terms. The primary models, effect modification analysis, and sensitivity analyses utilized multiple imputation by chained equations (MICE) to impute missing covariates (37).
We conducted multiple sensitivity analyses to assess the robustness of findings to modeling approach. In all cases, the sensitivity analyses were compared to the main model. Demographic and behavioral characteristics were also summarized for participants included in this study and those who were excluded but still attended the age 4–6 visit. To explore whether results were influenced by site- and cohort- specific associations, leave-one-out analyses were conducted in which the main analysis was repeated with one cohort or site removed in each iteration. To assess whether bias was introduced due to variation in ability to recall exposures at different child ages, we additionally adjusted for visit of exposure questionnaire completion [age 4–6 or 7/8–9 visit (binary)]. We performed additional sensitivity analyses in which we adjusted for urinary cotinine (continuous) as a marker of maternal smoking and exposure to environmental tobacco smoke during pregnancy (38), measured during the second trimester visit, and whether the child had ever been diagnosed with bronchiolitis (yes/no). Maternal pregnancy tobacco smoke exposure and early childhood bronchiolitis are both associated with development of childhood asthma (1, 39); however, we did not include these in the main models because they were not collected for the GAPPS participants who completed the exposure recall at age 4–6 (N = 96) per study protocols. To more precisely capture clinically relevant exposure to cleaning chemicals, prenatal cleaning practices and prenatal cleaning frequency were assessed independently. Separate analyses were performed defining prenatal exposure as either (1) answered “Yes” to “Did the biological mother do any of the following activities at her job during pregnancy—Clean floors, sinks, or toilets?” or (2) answered “Some days” or “Every day” to “How often did the biological mother use janitorial chemicals or cleaners at her job during pregnancy?” Finally, we repeated the primary analysis using complete cases only.
All analyses were conducted in R version 4.2.2 and significance was assessed at an α level of 0.05.
Overall, 453 pregnancy exposure and occupation recall questionnaires were completed, 239 in GAPPS and 214 in TIDES (Table 1). Of these, 116 mothers (25.6%) were classified as having been exposed to cleaning chemicals at their job during pregnancy. There was overlap among classification of exposure: ten mothers (2.2%) worked as a janitor or house cleaner, 77 (17%) cleaned floors, sinks, or toilets as part of their job, and 88 (19.4%) used janitorial chemicals or cleaners in their job some days or every day (Table 2). Mean child age at outcome assessment was 5.8 years [standard deviation (SD) 0.7] with an interquartile range (IQR) of 5.3–6.2 (Table 1). The child participants were 54.3% male and 45.7% female. The overall prevalences of current wheeze, current asthma, and strict asthma were 11.7% (N = 53), 10.8% (N = 49), and 7.7% (N = 35), respectively (Table 3).
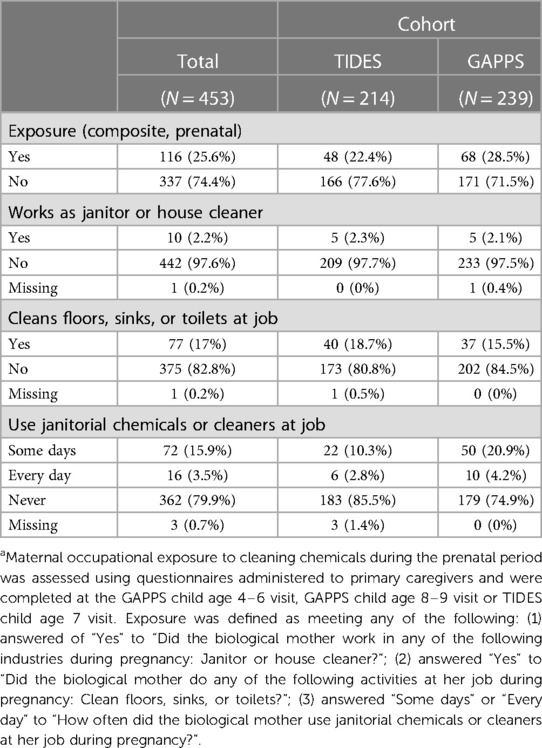
Table 2. Occupational exposure to cleaning chemicalsa among pregnant individuals in the study population.
In our primary analysis, we did not observe associations between the composite measure of prenatal cleaning chemical exposure and current wheeze in the main model (RR 1.03, CI: 0.56, 1.90) or current asthma (RR 0.89, CI: 0.46, 1.74) (Table 4). Results were similar in the minimally adjusted and extended models. Similarly, our secondary analysis found no association between prenatal exposure to cleaning chemicals and strict asthma (RR 0.82, CI: 0.33, 2.02) (Table 4).
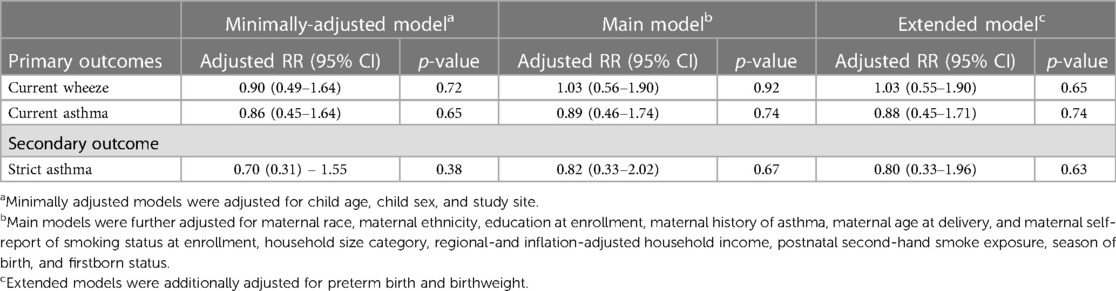
Table 4. Association between maternal occupation exposure to cleaning chemicals during pregnancy and airway outcomes.
We did not observe statistical evidence of an interaction between prenatal exposure to cleaning chemicals and sex on development of current wheeze and current asthma. Results suggest an adverse association limited to females for the current wheeze outcome (RR 1.82, CI: 0.76, 4.37, pinteraction = 0.13) compared to males (RR 0.68, CI: 0.29, 1.58); however, the confidence interval was wide and included the null. Effect estimates for current asthma were less than one and did not meet statistical significance in stratified analyses by child sex (Figure 1).
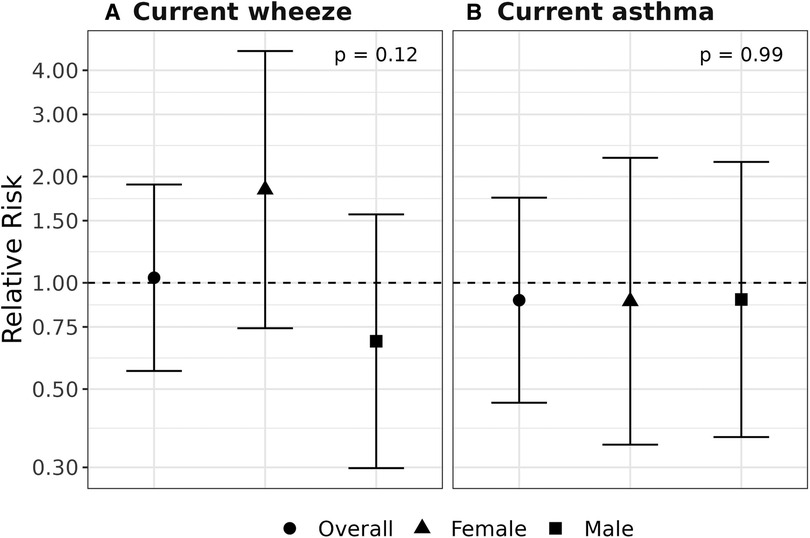
Figure 1. Assessment of effect modification by child sex. Relative risks (95% confidence intervals) are shown for overall, male- and female-specific associations between prenatal exposure to cleaning chemicals and development of current wheeze (A) and current asthma (B) at age 4–6 years. Models were adjusted for child age, study site, maternal race, maternal ethnicity, education at enrollment, maternal history of asthma, maternal age at delivery, maternal self-report of smoking status at enrollment, household size category, region-and inflation-adjusted household income, postnatal second-hand smoke exposure, season of birth, firstborn status, child sex, and a multiplicative interaction term for exposure to cleaning chemicals by child sex.
The participants included in our study population were similar in all characteristics except maternal education and household income, which were both somewhat higher in the analytic sample, on average, than those excluded (Supplementary Table S1). We did not observe any statistically significant associations between our primary exposure and current wheeze or current asthma in the sensitivity analyses described above (see Supplementary Material for results). Specifically, when excluding the GAPPS 4–6 exposure recalls, we observed no significant association between the exposure and current wheeze (RR 0.94, CI: 0.50, 1.79) or current asthma (RR 0.92, CI: 0.48, 1.75) (Supplementary Table S2). However, additional adjustment for cotinine and bronchiolitis in a subpopulation in which these data elements were collected led to elevated effect estimates, though with 95% confidence intervals that included the null (RR 1.88, CI: 0.78–4.55) (Supplementary Table S1).
Additionally, upon disaggregation of the exposure classification by question, we did not observe a significant association between exposure and current wheeze (RR 0.81, CI: 0.42, 1.54) or current asthma (RR 0.88, CI: 0.42, 1.85) among mothers who cleaned floors, sinks, or toilets at their job during pregnancy. Similarly, there was not a significant association between exposure and current wheeze (RR 1.39, CI: 0.71, 2.73) or current asthma (RR 1.03, CI: 0.49, 2.15) among mothers who used janitorial chemicals or cleaners some days or every day at their job during pregnancy (Supplementary Table S3).
This study investigated the association between occupationally related maternal exposure to cleaning chemicals during pregnancy and childhood respiratory outcomes in a combined U.S. pregnancy cohort. We found no evidence of an association between prenatal exposure to cleaning chemicals and childhood wheeze or asthma. Our findings suggested a possible sex-specific adverse association between exposure and current wheeze in females, although this result was not statistically significant.
Prior studies have investigated the link between maternal occupational exposures to cleaning products in the prenatal period and childhood respiratory outcomes. Our findings are consistent with results reported by Pape et al., who studied the association between parental occupational pre- and post-conception exposure and asthma in 3,985 offspring participating in the Respiratory Health in Northern Europe, Spain and Australia generation study (24). The authors found that parental occupational exposure to reactive chemicals, including disinfectants and cleaning chemicals, in pre- and post-conception (including the prenatal period) was not related to offspring asthma at 0–15 years of age. While maternal exposure to reactive chemicals increased the odds for early-onset asthma (0–3 years) [odds ratio (OR) 1.65, CI: 0.98, 2.77], no association was found for maternal chemical exposures and late-onset asthma (4–15 years) (OR 1.03, CI: 0.73, 1.45). The latter corresponded to a more similar child age group to our study population. Tagiyeva et al. found that maternal prenatal occupational exposure to biocides/fungicides was associated with wheeze at medium/high intensity exposure (OR 1.23, CI: 1.07, 1.40), but not with wheeze at low exposure intensity (OR 1.06, CI: 0.93, 1.20), asthma at low exposure intensity (OR 0.96, CI 0.79, 1.17) or asthma at medium/high exposure intensity (OR 1.20, CI: 0.98–1.47) in 7,088 children at 7 years of age (23). Christensen et al. found that prenatal exposure to low molecular weight (LMW) agents, identified as an exposure based on job codes for cleaners, had a borderline non-significant adverse association with asthma in 7-year-old children in the Danish National Birth Cohort (22). However, both maternal postnatal exposure to LMW agents and the combined effects of prenatal and postnatal exposure were associated with asthma. In contrast, Tjalvin et al. found that maternal occupational exposure to cleaning agents starting before conception and continuing through pregnancy were associated with childhood asthma: (OR 1.56, CI: 1.05, 2.31), childhood asthma with nasal allergies (OR 1.77, CI: 1.13, 2.77), and childhood wheeze and/or asthma (OR 1.71, CI: 1.19, 2.44) before 10 years of age among 3,318 children in two multi-national cohorts (10).
Previous studies investigating non-occupational exposures to cleaning products during pregnancy and childhood asthma and allergic disorders offer useful paradigms for comparison when evaluating our results. These studies also yielded mixed results. Bably et al. analyzed 400 children with a mean age of 6 years (SD 2.9) from Pakistan and demonstrated an association between prenatal exposure to scented cleaning products or perfume in the home with nocturnal cough among children, but not current asthma status or nocturnal symptoms of wheezing, shortness of breath, and chest tightness (40). In a study investigating household use of cleaning products during pregnancy, Casas et al. found that use of sprays or air fresheners was associated with higher prevalence of lower respiratory tract infections (LRTI) and use of sprays or solvents during pregnancy was associated with a higher prevalence of wheezing in the first year of life (41). Sherriff and colleagues reported a dose-dependent relationship between prenatal domestic use of chemical products, including disinfectants, cleaners, and fragrances, and persistent wheezing in the first 3.5 years of life, though significance differed by wheeze phenotype (42).
Biological mechanisms by which cleaning chemical exposure during the prenatal period affect respiratory health in children are not fully understood. Many cleaning products contain both irritants and sensitizers (9). The main sensitizers contained in cleaning products are disinfectants, quaternary ammonium compounds, amine compounds, and fragrances, whereas airway irritants in cleaning products include bleach, solvents, hydrochloric acid, alkaline agents, and phthalates all of which are commonly mixed together (9, 43). Many cleaning agents are LMW chemicals and are lipophilic, so transplacental diffusion may alter fetal airway development (10, 44, 45). Several human studies suggest maternal cytokines, specifically cytokines released from CD4+ Th2 T helper cells and type 2 innate lymphoid cells, mediate childhood asthma risk; however, whether this association is due to maternal cytokines passing through the placenta from maternal to fetal circulation or by modulating placental cytokine release is not clear (3). Another review found that prenatal exposure to common environmental allergens and chemicals, including tobacco smoke, organic pollutants, metals and outdoor air pollutants, may alter distributions of immune system cells, immunoglobulins and cytokine patterns in neonate cord blood (8). This derangement was postulated to result in predisposition of infants to respiratory infections during the early postnatal period and potentially an increased risk of wheeze and asthma in childhood.
Sex-dependent biological mechanisms have been implicated in asthma development (26). Prior findings have been mixed regarding effect modification by sex in the relationship between prenatal environmental exposures and child airway outcomes (27, 28) but few studies have specifically focused on prenatal occupational exposure to cleaning chemicals. Similar to our results, Christensen et al. did not find effect modification by child sex in the association between occupational exposure to LMW agents and childhood asthma (22). The prevalence of asthma is higher in boys than in girls in pre-adolescence, though the mechanism by which sex hormones regulate asthma pathophysiology is complex and requires further investigation (21, 46).
Excluding GAPPS 4–6 recall survey data and adjusting the main model for cotinine and/or bronchiolitis altered the effect estimates from less than one to greater than one. The greatest change was in the association between exposure and current asthma; after adjusting for bronchiolitis, the estimated risk ratios approached 2, though the CIs widened significantly potentially due to reduced sample size. Acute LRTI such as bronchiolitis during infancy has been found to be a strong risk factor for childhood asthma (1, 47, 48). More research on the relationship between environmental factors, including prenatal occupational cleaning product exposures, and early childhood LRTI and asthma is warranted.
Because our characterization of mothers’ cleaning chemical exposure was based on a composite measure that included job category, specific task, and frequency of chemical use, we separately examined two classifications of exposure, defined by single survey questions, as a sensitivity analysis. While the questionnaire did allow for more granular and comprehensive ascertainment of mothers’ exposure by including specific tasks and frequency rather than a single job title or category, mothers may have been misclassified as not exposed based on the wording of the questions. As in studies defining exposure through job exposure matrices, in which job titles constitute a proxy for exposure to specific agents and average exposures are often based on expert evaluation of job category, any exposure misclassification is likely to be non-differential, biasing the association towards the null (10, 24). There were very few (N = 10) respondents who worked as a janitor or house cleaner, suggesting that we were underpowered to investigate routine, intense occupational exposure. Furthermore, this group exhibited complete overlap with those who cleaned floors, sinks or toilets at her job, so they could not be disaggregated for separate analysis. While none of the associations reached significance, the effect estimates for those who cleaned floors, sinks or toilets at their job were less than one, whereas the effect estimates for those who used janitorial chemicals or cleaners at their jobs some days or every day were greater than one. This suggests that improved exposure classification that better approximates “dose” through frequency of use and more specific chemical data vs. more crude measures based on job duties or job type are important considerations for future research.
Our study had several strengths. Our findings contribute to a very limited and mixed literature on maternal occupational exposures, specifically during the prenatal period, and child airway health. We were able to examine the association between prenatal exposure to occupationally associated cleaning chemicals and risk of developing childhood wheeze and asthma in a U.S. based cohort comprising several cities with robust adjustment for mother and child demographic, behavioral and socioeconomic covariates and potential confounders (29).
Several limitations should be considered. Maternal occupational exposures during pregnancy were assessed retrospectively at visits with existing knowledge about whether the respiratory outcomes had occurred, which may introduce recall bias. Despite having a robust set of covariates known to influence asthma risk, we did not have data on other chemicals and products that mothers could have been exposed to associated with occupational use of cleaning chemicals or outside of work. The specific wording of the job information could include a myriad of job tasks, and occupational cleaning tasks could also confer more or less exposure to other agents, such as dust, animal dander, microbes, indoor air pollutants, all of which may impact risk of childhood asthma. Our exposures of interest in the prenatal period were highly correlated with those in the preconception and postnatal period; however, we did not have the power in this study to differentiate exposure periods to perform separate analyses. Thus, despite inclusion of numerous covariates in our models, we cannot rule out residual confounding by other factors. Furthermore, we were unable to control for application method, dose, job duration, or use of protective equipment. The sample size and number of outcomes in our final study population limited our statistical power to detect differences among exposed compared to non-exposed. The outcomes of interest were only observed among mothers who identified as White, Asian and other race and these race categories along with median income of the overall sample (>$100,000) limit generalizability of this analysis, especially given that asthma has been found to be more prevalent among Black and Hispanic children and among children living in households with lower income (49). Finally, diagnosis of asthma in children can be challenging especially in younger ages, though our study population was comprised of children at or near school age where clinical history allows more confident detection despite lack of more objective measures such as spirometry. Child airway outcome definitions may be influenced by caregiver recognition of symptoms in their children, healthcare access and utilization, and accurate recall of symptoms, medications, and diagnoses at the time of ISAAC survey administration. However, any outcome misclassification would have likely been non-differential with regards to exposure. Furthermore, symptom-based history is a broadly accepted approach to childhood asthma diagnosis and our questions were derived from the validated ISAAC questionnaire (32, 33) which remains the most widely used across the globe standardized survey to assess asthma in children (27, 28, 35, 50–54).
Childhood asthma is a chronic disease and serious public health problem that can have significant lifelong health implications. The prenatal period is a crucial period during which environmental influences, including maternal occupational exposures, can shape child respiratory health. Given the widespread use of cleaning products, amplified during the COVID epidemic, research is needed to address the role of maternal occupational exposure to specific compounds found in cleaning chemicals on offspring respiratory outcomes. Future studies should investigate more diverse and representative U.S. populations and larger sample sizes to inform our understanding. Better characterization of exposure to include ingredients of cleaning products are needed, and future studies should include more quantitative assessment of exposure, including dose, timing, and duration. Such data can better inform appropriate strategies for protecting pregnant individuals from potentially hazardous occupational exposures.
In conclusion, we did not find support for our hypothesis that maternal report of occupations using cleaning chemicals or use of cleaning chemicals at work is associated with childhood wheeze or asthma in the ECHO-PATHWAYS combined cohort, nor did we find statistically significant evidence of sex-specific associations. However, our results provide support for needed further investigation in other cohorts. Our study contributes to the emerging body of literature of prenatal occupational exposures and risk of adverse child health outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Washington Institutional Review Board. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
ARS, CK, CL, MH, and LD contributed to conception and design of the study. ARS and LD obtained and organized the data and performed statistical analysis. MH wrote the first draft of the manuscript. AAS, ARS, CK, CL, EB, KC, LD, MA, MH, PM, and RN revised the manuscript and/or contributed critical content expertise and/or study design knowledge. All authors contributed to the article and approved the submitted version.
Research reported in this publication was supported by National Institutes of Health (NIH) grants: UG3/UH3OD023271, UG3/UH3OD023305, R01ES016863, R01ES25169, UL1 TR002319, P30ES005022 and P30ES007033. Research reported in this publication was also supported by the National Institute for Occupational Safety and Health (NIOSH) under Federal Training Grant T42OH008433. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NIOSH. This research was conducted using data collected on behalf of the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) Repository. This manuscript has been reviewed by ECHO-PATHWAYS for scientific content and consistency of data interpretation with previous ECHO-PATHWAYS publications.
We acknowledge the contributions of the GAPPS and TIDES participants and families and the study research staff.
AAS received funding from the National Institutes of Health (NIH) and the Health Effects Institute (HEI). Neither the NIH nor HEI were involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fepid.2023.1166174/full#supplementary-material.
1. Nanishi M, Chandran A, Li X, Stanford JB, Alshawabkeh AN, Aschner JL, et al. Association of severe bronchiolitis during infancy with childhood asthma development: an analysis of the ECHO consortium. Biomedicines. (2023) 11(1):23. doi: 10.3390/biomedicines11010023
2. Perry R, Braileanu G, Palmer T, Stevens P. The economic burden of pediatric asthma in the United States: literature review of current evidence. Pharmacoeconomics (2019) 37(2):155–67. doi: 10.1007/s40273-018-0726-2
3. Lebold KM, Jacoby DB, Drake MG. Inflammatory mechanisms linking maternal and childhood asthma. J Leukoc Biol. (2020) 108(1):113–21. doi: 10.1002/JLB.3MR1219-338R
4. Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet Lond Engl. (2008) 372(9643):1058–64. doi: 10.1016/S0140-6736(08)61447-6
5. Li MC, Chen CH, Guo YL. Phthalate esters and childhood asthma: a systematic review and congener-specific meta-analysis. Environ Pollut. (2017) 229:655–60. doi: 10.1016/j.envpol.2017.06.083
6. Shao J, Wheeler AJ, Zosky GR, Johnston FH. Long-term impacts of prenatal and infant exposure to fine particulate matter on wheezing and asthma. Environ Epidemiol. (2019) 3(2):e042. doi: 10.1097/EE9.0000000000000042
7. Hernández CD, Casanello P, Harris PR, Castro-Rodríguez JA, Iturriaga C, Perez-Mateluna G, et al. Early origins of allergy and asthma (ARIES): study protocol for a prospective prenatal birth cohort in Chile. BMC Pediatr. (2020) 20:164. doi: 10.1186/s12887-020-02077-x
8. García-Serna AM, Martín-Orozco E, Hernández-Caselles T, Morales E. Prenatal and perinatal environmental influences shaping the neonatal immune system: a focus on asthma and allergy origins. Int J Environ Res Public Health. (2021) 18(8):3962. doi: 10.3390/ijerph18083962
9. Quirce S, Barranco P. Cleaning agents and asthma. J Investig Allergol Clin Immunol. (2010) 20(7):542–50. Available at: https://www.jiaci.org/summary/vol20-issue7-num660 21313993
10. Tjalvin G, Svanes Ø, Igland J, Bertelsen RJ, Benediktsdóttir B, Dharmage S, et al. Maternal preconception occupational exposure to cleaning products and disinfectants and offspring asthma. J Allergy Clin Immunol. (2022) 149(1):422–431.e5. doi: 10.1016/j.jaci.2021.08.025
11. Clausen PA, Frederiksen M, Sejbæk CS, Sørli JB, Hougaard KS, Frydendall KB, et al. Chemicals inhaled from spray cleaning and disinfection products and their respiratory effects. A comprehensive review. Int J Hyg Environ Health. (2020) 229:113592. doi: 10.1016/j.ijheh.2020.113592
12. Svanes Ø, Bertelsen RJ, Lygre SH, Carsin AE, Antó JM, Forsberg B, et al. Cleaning at home and at work in relation to lung function decline and airway obstruction. Am J Respir Crit Care Med. (2018) 197(9):1157–63. doi: 10.1164/rccm.201706-1311OC
13. Zock JP, Plana E, Jarvis D, Antó JM, Kromhout H, Kennedy SM, et al. The use of household cleaning sprays and adult asthma: an international longitudinal study. Am J Respir Crit Care Med. (2007) 176(8):735–41. doi: 10.1164/rccm.200612-1793OC
14. Weinmann T, Gerlich J, Heinrich S, Nowak D, Von Mutius E, Vogelberg C, et al. Association of household cleaning agents and disinfectants with asthma in young German adults. Occup Environ Med. (2017) 74(9):684–90. doi: 10.1136/oemed-2016-104086
15. Medina-Ramon M, Zock J, Kogevinas M, Sunyer J, Anto J. Asthma symptoms in women employed in domestic cleaning: a community based study. Thorax. (2003) 58(11):950–4. doi: 10.1136/thorax.58.11.950
16. Folletti I, Zock JP, Moscato G, Siracusa A. Asthma and rhinitis in cleaning workers: a systematic review of epidemiological studies. J Asthma. (2014) 51(1):18–28. doi: 10.3109/02770903.2013.833217
17. Jaakkola JJ, Jaakkola MS. Professional cleaning and asthma. Curr Opin Allergy Clin Immunol. (2006) 6(2):85–90. doi: 10.1097/01.all.0000216849.64828.55
18. Zock JP, Vizcaya D, Le Moual N. Update on asthma and cleaners. Curr Opin Allergy Clin Immunol. (2010) 10(2):114–20. doi: 10.1097/ACI.0b013e32833733fe
19. Casas L, Zock JP, Torrent M, García-Esteban R, Gracia-Lavedan E, Hyvärinen A, et al. Use of household cleaning products, exhaled nitric oxide and lung function in children. Eur Respir J. (2013) 42(5):1415–8. doi: 10.1183/09031936.00066313
20. Henderson J, Sherriff A, Farrow A, Ayres JG. Household chemicals, persistent wheezing and lung function: effect modification by atopy? Eur Respir J. (2008) 31(3):547–54. doi: 10.1183/09031936.00086807
21. Parks J, McCandless L, Dharma C, Brook J, Turvey SE, Mandhane P, et al. Association of use of cleaning products with respiratory health in a Canadian birth cohort. CMAJ. (2020) 192(7):E154–61. doi: 10.1503/cmaj.190819
22. Christensen BH, Thulstrup AM, Hougaard KS, Skadhauge LR, Hansen KS, Frydenberg M, et al. Maternal occupational exposure to asthmogens during pregnancy and risk of asthma in 7-year-old children: a cohort study. BMJ Open. (2013) 3(4):e002401. doi: 10.1136/bmjopen-2012-002401
23. Tagiyeva N, Devereux G, Semple S, Sherriff A, Henderson J, Elias P, et al. Parental occupation is a risk factor for childhood wheeze and asthma. Eur Respir J. (2010) 35(5):987–93. doi: 10.1183/09031936.00050009
24. Pape K, Svanes C, Sejbæk CS, Malinovschi A, Benediktsdottir B, Forsberg B, et al. Parental occupational exposure pre- and post-conception and development of asthma in offspring. Int J Epidemiol. (2020) 49(6):1856–69. doi: 10.1093/ije/dyaa085
25. Li X, Sundquist K, Sundquist J. Parental occupation and risk of hospitalization for asthma in children and adolescents. J Asthma Off J Assoc Care Asthma. (2009) 46(8):815–21. doi: 10.1080/02770900903141260
26. Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. (2017) 17(3):19. doi: 10.1007/s11882-017-0686-1
27. Hazlehurst MF, Carroll KN, Loftus CT, Szpiro AA, Moore PE, Kaufman JD, et al. Maternal exposure to PM2.5 during pregnancy and asthma risk in early childhood. Environ Epidemiol. (2021) 5(2):e130. doi: 10.1097/EE9.0000000000000130
28. Loftus CT, Szpiro AA, Workman T, Wallace ER, Hazlehurst MF, Day DB, et al. Maternal exposure to urinary polycyclic aromatic hydrocarbons (PAH) in pregnancy and childhood asthma in a pooled multi-cohort study. Environ Int. (2022) 170:107494. doi: 10.1016/j.envint.2022.107494
29. LeWinn KZ, Karr CJ, Hazlehurst M, Carroll K, Loftus C, Nguyen R, et al. Cohort profile: the ECHO prenatal and early childhood pathways to health consortium (ECHO-PATHWAYS). BMJ Open. (2022) 12(10):e064288. doi: 10.1136/bmjopen-2022-064288
30. Ni Y, Loftus CT, Szpiro AA, Young MT, Hazlehurst MF, Murphy LE, et al. Associations of Pre- and postnatal air pollution exposures with child behavioral problems and cognitive performance: a U. S. Multi-cohort study. Environ Health Perspect. (2022) 130(6):67008. doi: 10.1289/EHP10248
31. Wallace ER, Buth E, Szpiro AA, Ni Y, Loftus CT, Masterson E, et al. Prenatal exposure to polycyclic aromatic hydrocarbons is not associated with behavior problems in preschool and early school-aged children: a prospective multi-cohort study. Environ Res. (2023) 216:114759. doi: 10.1016/j.envres.2022.114759
32. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur Respir J. (1995) 8(3):483–91. doi: 10.1183/09031936.95.08030483
33. National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. (2007). Available at: https://www.nhlbi.nih.gov/sites/default/files/media/docs/EPR-3_Asthma_Full_Report_2007.pdf (Accessed March 11, 2023).
34. Rosa MJ, Hartman TJ, Adgent M, Gardner K, Gebretsadik T, Moore PE, et al. Prenatal polyunsaturated fatty acids and child asthma: effect modification by maternal asthma and child sex. J Allergy Clin Immunol. (2020) 145(3):800–807.e4. doi: 10.1016/j.jaci.2019.10.039
35. Adgent MA, Carroll KN, Hazlehurst MF, Loftus CT, Szpiro AA, Karr CJ, et al. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ Int. (2020) 143:105970. doi: 10.1016/j.envint.2020.105970
36. Wilson K, Gebretsadik T, Adgent MA, Loftus C, Karr C, Moore PE, et al. The association between duration of breastfeeding and childhood asthma outcomes. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. (2022) 129(2):205–11. doi: 10.1016/j.anai.2022.04.034
37. Buuren Sv, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. (2011) 45:1–67. doi: 10.18637/jss.v045.i03
38. Schick SF, Blount BC, Jacob P, Saliba NA, Bernert JT, El Hellani A, et al. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol-Lung Cell Mol Physiol. (2017) 313(3):L425–52. doi: 10.1152/ajplung.00343.2016
39. McEvoy CT, Spindel ER. Pulmonary effects of maternal smoking on the Fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev. (2017) 21:27–33. doi: 10.1016/j.envint.2020.105970
40. Bably M, Arif AA, Post A. Prenatal use of cleaning and scented products and its association with childhood asthma, asthma symptoms, and mental health and developmental comorbidities. J Asthma. (2021) 58(1):46–51. doi: 10.1080/02770903.2019.1656229
41. Casas L, Zock JP, Carsin AE, Fernandez-Somoano A, Esplugues A, Santa-Marina L, et al. The use of household cleaning products during pregnancy and lower respiratory tract infections and wheezing during early life. Int J Public Health. (2013) 58(5):757–64. doi: 10.1007/s00038-012-0417-2
42. Sherriff A, Farrow A, Golding J, Henderson J. Frequent use of chemical household products is associated with persistent wheezing in pre-school age children. Thorax. (2005) 60(1):45–9. doi: 10.1136/thx.2004.021154
43. Parks J, Takaro TK. Exposure to cleaning products and childhood asthma: more than just a link? Expert Rev Respir Med. (2020) 14(12):1185–8. doi: 10.1080/17476348.2020.1813572
44. Capra L, Tezza G, Mazzei F, Boner AL. The origins of health and disease: the influence of maternal diseases and lifestyle during gestation. Ital J Pediatr. (2013) 39(1):7. doi: 10.1186/1824-7288-39-7
45. Griffiths SK, Campbell JP. Placental structure, function and drug transfer. Contin Educ Anaesth Crit Care Pain. (2015) 15(2):84–9. doi: 10.1093/bjaceaccp/mku013
46. Koper I, Hufnagl K, Ehmann R. Gender aspects and influence of hormones on bronchial asthma—secondary publication and update. World Allergy Organ J. (2017) 10(1):46. doi: 10.1186/s40413-017-0177-9
47. Abreo A, Gebretsadik T, Stone CA, Hartert TV. The impact of modifiable risk factor reduction on childhood asthma development. Clin Transl Med. (2018) 7(1):15. doi: 10.1186/s40169-018-0195-4
48. Balekian DS, Linnemann RW, Hasegawa K, Thadhani R, Camargo CA. Cohort study of severe bronchiolitis during infancy and risk of asthma by age 5 years. J Allergy Clin Immunol Pract. (2017) 5(1):92–6. doi: 10.1016/j.jaip.2016.07.004
49. Zanobetti A, Ryan PH, Coull B, Brokamp C, Datta S, Blossom J, et al. Childhood asthma incidence, early and persistent wheeze, and neighborhood socioeconomic factors in the ECHO/CREW consortium. JAMA Pediatr. (2022) 176(8):759–67. doi: 10.1001/jamapediatrics.2022.1446
50. Adgent MA, Gebretsadik T, Elaiho CR, Milne GL, Moore P, Hartman TJ, et al. The association between prenatal F2-isoprostanes and child wheeze/asthma and modification by maternal race. Free Radic Biol Med. (2022) 189:85–90. doi: 10.1016/j.freeradbiomed.2022.07.008
51. Anandan C, Nurmatov U, Van Schayck OCP, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. (2010) 65(2):152–67. doi: 10.1111/j.1398-9995.2009.02244.x
52. Mudiyanselage SIRR, Amarasiri WADL, Yasaratne BMGD, Warnasekara J, Agampodi S. Epidemiology of wheeze among preschool children: a population-based cross-sectional study from rural Sri Lanka. BMJ Open. (2021) 11(7):e046688. doi: 10.1136/bmjopen-2020-046688
53. Patel SP, Järvelin MR, Little MP. Systematic review of worldwide variations of the prevalence of wheezing symptoms in children. Environ Health. (2008) 7(1):57. doi: 10.1186/1476-069X-7-57
Keywords: cleaning chemicals, childhood wheeze, childhood asthma, occupational exposure, prenatal exposure, environmental exposure, respiratory outcomes
Citation: Herrin MA, Sherris AR, Dearborn LC, Loftus CT, Szpiro AA, Moore PE, Adgent MA, Barrett ES, Nguyen RHN, Carroll KN and Karr CJ (2023) Association between maternal occupational exposure to cleaning chemicals during pregnancy and childhood wheeze and asthma. Front. Epidemiol. 3:1166174. doi: 10.3389/fepid.2023.1166174
Received: 15 February 2023; Accepted: 30 March 2023;
Published: 19 April 2023.
Edited by:
Kristina Whitworth, Baylor College of Medicine, United StatesReviewed by:
Ahmed Arif, University of North Carolina at Charlotte, United States© 2023 Herrin, Sherris, Dearborn, Loftus, Szpiro, Moore, Adgent, Barrett, Nguyen, Carroll and Karr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melissa A. Herrin bWhhdXJ5QHV3LmVkdQ==
Specialty Section: This article was submitted to Occupational and Environmental Epidemiology, a section of the journal Frontiers in Epidemiology
Abbreviations ECHO-PATHWAYS, ECHO prenatal and early childhood pathways to health consortium; GAPPS, Global Alliance to Prevent Prematurity and Stillbirth; IQR, interquartile range; IRB, Institutional Review Board; ISAAC, International Study of Asthma and Allergies in Childhood; LMW, low molecular weight; LRTI, lower respiratory tract infection; MICE, multiple imputation by chained equations; OR, odds ratio; RR, risk ratio; SD, standard deviation; TIDES, The Infant Development and Environment Study (TIDES); U.S., United States, UW, University of Washington.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.