- 1Analytical Epidemiology and Health Impact Unit, Department of Epidemiology and Data Science, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
- 2Department of Epidemiology and Biostatistics, National Institute for Health Development, Tallinn, Estonia
- 3Consortium for Biomedical Research in Epidemiology and Public Health (CIBERESP), Madrid, Spain
- 4Epidemiology Unit and Girona Cancer Registry, Oncology Coordination Plan, Department of Health, Autonomous Government of Catalonia, Catalan Institute of Oncology (ICO), Girona, Spain
- 5Biomedical Research Institute (IdiBGi), Girona, Spain
- 6Belgium Cancer Registry, Bruxelles, Belgium
- 7Granada Cancer Registry, Granada, Spain
- 8Andalusian School of Public Health (EASP), Granada, Spain
- 9Descriptive Epidemiology, Genetics and Cancer Prevention Group, Biomedical Research Institute (IDIBGI), Girona, Spain
- 10Girona Cancer Registry, Girona, Spain
- 11Radiation Oncology Department, Catalan Institute of Oncology Hospital Trueta, Girona, Spain
Objectives: To investigate differences in lung cancer (LC) management and survival using data from European population cancer registries.
Methods: We analysed 4,602 lung cancer cases diagnosed in 2010–2013, followed-up to 2019 in five countries. Multivariable logistic regression was used to calculate the Odds Ratio (OR) of surgery for stages I–II LC or chemo- or radiotherapy for stages III–IV LC. Relative survival (RS) was estimated by the actuarial method; Relative Excess Risk of death (RER), with 95% CI, was calculated by generalized linear models.
Results: Diagnostic work-up was extensive for 65.9% patients (range 57%, Estonia, Portugal - 85% (Belgium). Sixty-six percent of stages I–II patients underwent surgery; compared to non-operated, their adjusted OR decreased with age and was associated with main bronchus cancer (OR vs. lobes 0.25, CI, 0.08–0.82), stage II (OR vs. stage I: 0.42, CI, 0.29–0.60), comorbidity (OR vs. absent: 0.55, CI, 0.33–0.93), country (ORs: Estonia 1.82, CI, 1.28–2.60; Belgium 0.62, CI, 0.42–0.91; Portugal 0.69, CI, 0.52–0.93).
Almost half of stages III–IV patients received chemo- or radiotherapy only; the adjusted OR vs. non receiving decreased with age and was associated with unspecified cancer topography or morphology. The adjusted five-year RER increased with age and stage and was lower for women (0.78, CI, 0.72–0.86), above the reference for main bronchus cancer (1.37, CI, 1.21–1.54) and unspecified morphology (1.17, CI, 1.05–1.30). Surgery carried the lowest mortality (RS 56.9; RER 0.13, CI, 0.11–0.15) with RER above the mean in Estonia (1.20, CI, 1.10–1.30), below it in Portugal (0.88, CI, 0.82–0.93) and Switzerland (0.91, CI, 0.84–0.99). Comorbidity (1.21, CI, 1.09–1.35) and not smoking (0.68, CI, 0.57–0.81) were associated with RER.
Conclusions: The survival benefit of early diagnosis, allowing curative surgery, was evident at the population level. Screening for subjects at risk and adhesion to standard care should be incremented across the EU by funding better equipment and training health personnel.
1. Introduction
Lung cancer (LC) is the leading cause of cancer mortality in the world, accounting for 2.1 million new cases and 1.8 million deaths in 2018. LC constitutes 11.6% of total cancer cases and 18.4% of total cancer deaths. In males, LC is the most commonly diagnosed cancer and the leading cause of cancer death. Among women LC is the third (after breast and colorectal cancers) most commonly diagnosed cancer and the second (after breast cancer) leading cause of cancer death (1).
Despite the advent of targeted therapies and immunotherapy in non-small-cell LC (NSCLC) (2), the average five-year relative survival (RS) of European LC patients diagnosed in 2000–07 remains poor, around 13%, with figures ranging from less than 10% (UK, Lithuania, Bulgaria) to 15% or higher (Belgium, Germany, Switzerland) (3). The over-time survival improvements reported from population-based studies (4, 5) can be attributed to the higher proportions of cases diagnosed at early stages, which can benefit from curative surgery (5).
EUROCARE, the widest cancer registry based study on survival of European cancer patients, active since the early 1990s (http://www.eurocare.it/), highlighted the between-country differences in lung cancer survival for patients diagnosed in 2000–2007, were more evident for patients with localised and regional stages than for those with an advanced stage tumour at diagnosis (4); this might be ascribed to availability and access to early diagnosis and treatment facilities (3–7).
The European High-Resolution (HR) studies (http://hrstudies.it/) on samples of cancer cases archived in European population-based cancer registries (CR) collect more clinical information than is routinely provided by population CRs, according to standardised protocols, making it possible to investigate disparities in care for early stage LC that could be corrected to improve survival and reduce inequalities.
Clinical guidelines indicate surgery with curative intent should be offered to stage I–II LC patients and chemo- or radiotherapy is recommended for stage III–IV LC (8, 9); pathological confirmation is essential in deciding the appropriate treatment plan for all LC types, and chest imaging (positron emission tomography (PET), computed tomography scan (CT), spiral CT) permits precise disease staging and is essential when curative treatment is intended.
Comorbidity (particularly smoke-related, e.g., chronic obstructive pulmonary disease - COPD), smoking habits and performance status guide treatment decisions and are prognostic factors (10–13). Continued smoking in a LC patient is associated with mortality, development of a second primary, or recurrence in both non-small and small-cell LC (14).
The above indications derive from controlled clinical studies and hospital-based studies, however their impact in populations are scarcely studied. We used HR data of incident patients in 2010–2013, provided by European population CRs, to investigate:
- Adhesion to selected clinical guidelines, i.e., surgery for stage I–II LC, and chemo- or radiotherapy for stage III–IV LC, also assessing the impact of treatment on survival.
- Across-country differences in diagnostic work-up, namely the use of diagnostic examinations and the proportion of microscopically verified (MV) cases with ICD-O code subtype specification (e.g., different from not-otherwise-specified - NOS).
- Five-year survival and relative excess risk of death (RER), adjusted for clinical and demographic variables under study, also taking into account comorbidity and smoking
2. Materials and methods
2.1. Study characteristics
The HR study protocol required each participating CR to provide at least 300 cases of malignant LC [International Classification of Diseases for Oncology topography codes C34.0–9 (15), with morphologies as defined in the study protocol], diagnosed in 2010–13 and followed up to 2019 in Belgium and Spain, up to 2018 in Estonia and Switzerland and up to 2016 in Portugal (16).
The staff of each CR accessed clinical records and abstracted information on tumour stage and morphology, diagnostic work-up, treatments, smoking habits, comorbidity and life status, according to the study protocol.
Five countries with national (Belgium, Estonia) or regional coverage (Portugal, Spain, and Switzerland) contributed sufficiently complete data (i.e., most of each variable analysed for ≥70% of cases) and were included in the study. Data from regional CR were pooled in order to consider the corresponding countries as a whole.
All patients were followed up at least 5 years, according to the CR specific methods; 42 cases (less than 1%) were lost (1 in Belgium, 29 in Estonia, 4 in Portugal, 3 in Spain and 5 in Switzerland).
Comorbidity was not available for 97% of cases in Switzerland, smoking status was unavailable for 61.5% cases in Estonia (Table 1) and for 100% cases in South Portugal: these countries and this registry were therefore excluded from the relevant analyses.
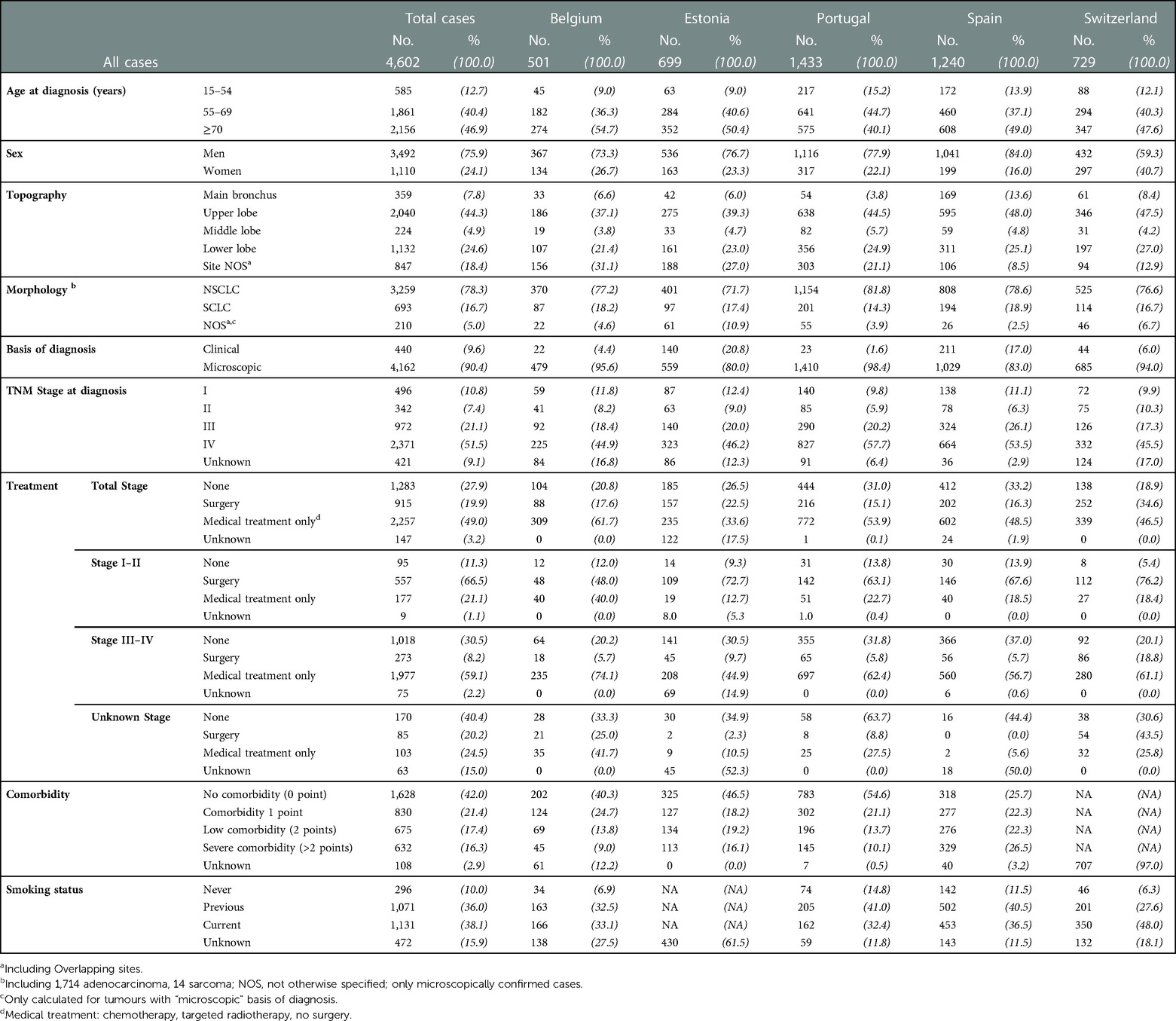
Table 1. Number of lung cancer cases (No.) diagnosed in 2009–2013 in five European countries and distributions (%) of patients and tumour characteristic by country.
Most CRs provided all cases incident in one or more years of the study period. Registries covering large areas sampled cases from a defined incidence period using a randomized procedure. The majority of CRs provided one complete year of incidence (CRs of Estonia, Girona, Granada and Geneva) or a random selection of at least 500 LC cases (CR of Northern Portugal) diagnosed in 2010–13. Supplementary Table S1 shows the Total number of lung cancer cases submitted for inclusion in the HR study by country and registry, with criteria for selection of cases.
Age at diagnosis was grouped as 15–54, 55–69, 70 years or over. Tumour stage at diagnosis was coded according to the TNM 7th edition (17) and classified in stage I - IV, and unknown. When available, T and N pathological stage was preferred to the clinical stage. In the present analysis, to maximise the availability of data on stage we used the combined information from clinical and pathological stage. In case of discordance between them the most advanced stage figures were chosen. M stage was established only clinically in 92.2% of cases.
The anatomical site of the primary LC was coded according to ICD-O-3 topography and classified as main bronchus (C34.0), upper lobe (C34.1) middle lobe (C34.2) lower lobe (C34.3), overlapping (C34.8) and NOS (C34.9).
Morphology was coded according to ICD-O-3 morphology codes and grouped into NSCLC, small-cell lung cancer (SCLC), not otherwise specified carcinoma (NOS); its distribution is only presented for tumours with a “microscopic” basis for diagnosis, as defined in the (Supplementary Table S2).
Information on surgery, type of surgery and other treatments (chemotherapy, radiotherapy, or targeted treatment), was analysed as done, not done or not known whether done or not.
A score from 1 to 6 was assigned to each Charlson Comorbidity Index (CCI) item (12, 18), and the total was calculated as the sum of the scores for the 19 items. The sum was then rated as 0 point, 1 point, 2 points, >2 points and unknown. Smoking habit was grouped as current, previous, never and unknown.
For countries with information on diagnostic work-up (Belgium, Estonia, Portugal and Spain), we analysed the frequency distributions of diagnostic examinations on the lung within three months (after or before) from diagnosis and coded as done, not done and not known whether done or not.
Chest radiography (Rx), computerized tomography (CT), or pulmonary stratigraphy was classified as “conventional chest imaging”; in addition, the distributions of the following examinations were analysed individually: spiral CT, positron emission tomography (PET), magnetic resonance imaging (MRI), bronchoscopy, mediastinoscopy, endobronchial ultrasound (Table 2). All diagnostic examinations were grouped in four categories: (1) conventional chest imaging only; (2) spiral CT or PET or MRI plus any endoscopy; (3) spiral CT or PET with no endoscopy; (4) not done and unknown diagnostic examinations.
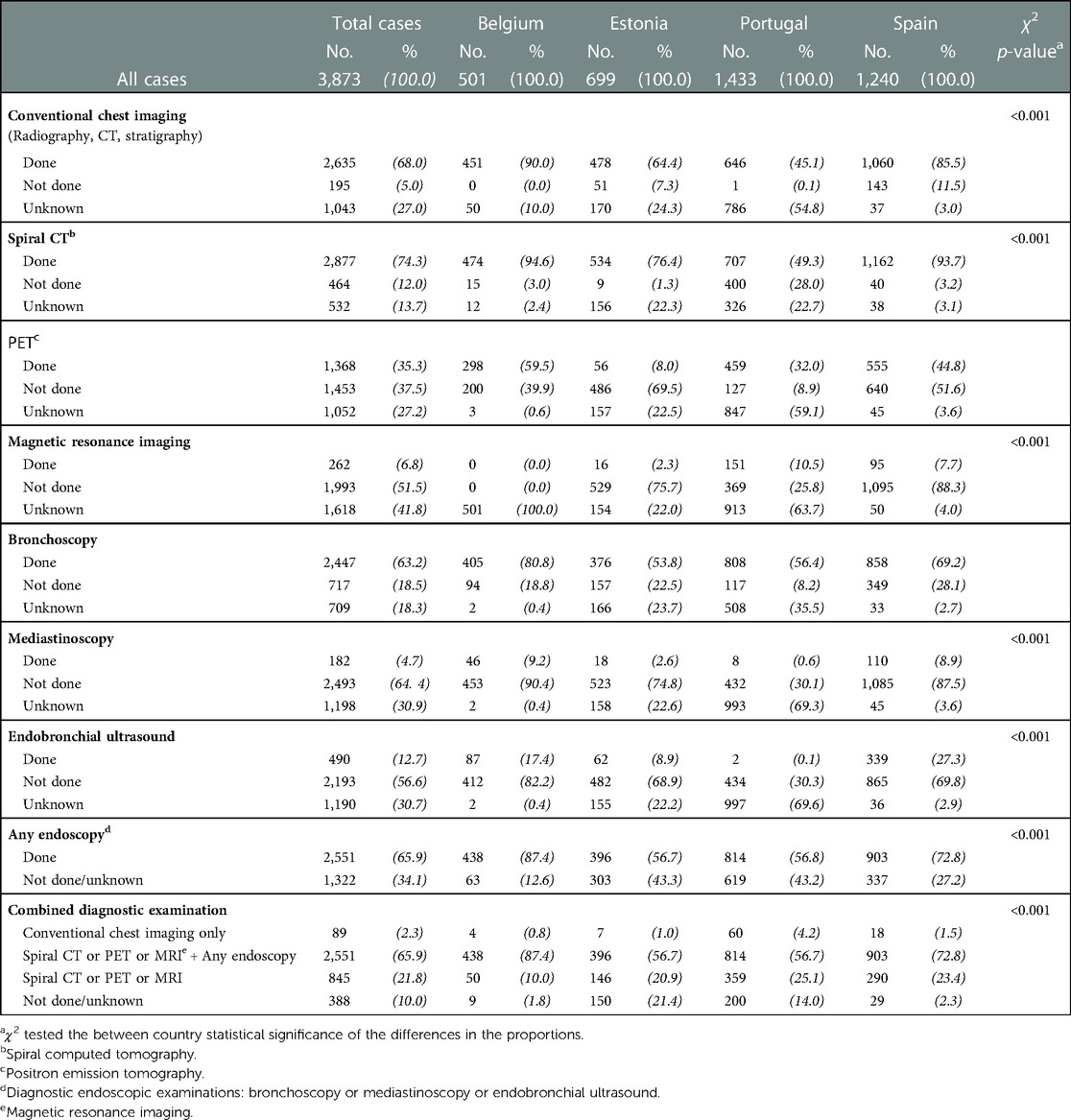
Table 2. Number of lung cancer cases (No.) and distributions (%) of diagnostic examinations on the lung within three months (after or before) from diagnosis in the four countries with information on diagnostic work-up.
2.2. Statistical analysis
To describe variability related to the patient's LC and their characteristics between countries, we used counts and proportions. Differences between countries in the proportions of LC diagnostic examinations were tested with a Chi-square test.
The odds (with 95% confidence intervals (CI) of receiving surgery (for patients with TNM stage I–II at diagnosis) and the odds of receiving chemo- or radiotherapy only (for patients with TNM stage III–IV at diagnosis) or not receiving the treatment, were calculated with multivariable logistic regression models (19) adjusted by age, sex, ICD-O codes for topography, morphology, TNM stage, comorbidity and country. Countries' ORs were based on the differences from the balanced grand mean; the common reference for the areas is therefore their grand mean (20).
Relative survival (RS), i.e., the ratio of the observed to the expected survival in the general underlying population, was calculated by the actuarial method (21). We estimated expected survival by the Ederer II method (22) using CR population life tables stratified by sex, age and year of diagnosis. Country-specific RS figures were standardised to the age structure of the study population.
To assess the impact on five-year risk of death of the variables under study (age and sex, tumour topography, morphology and stage, treatment, comorbidity, smoking status and country of residence), the Relative Excess of Risk of death (RER) five years after diagnosis, with 95% CI, was calculated with generalized linear models, using five-year RS as the dependent variable (23).
Three distinct models were fitted: Model 1) including all countries (4,449 cases) adjusted for all clinical-demographic variables in study (age at diagnosis, sex, topography, morphology, stage at diagnosis, treatment, country); Model 2), adding comorbidity to model 1, including the four countries with data on comorbidity (Belgium, Estonia, Portugal, Spain: 3,720 cases); Model 3) adding to model 1 the adjustment for both comorbidity and smoking, hence including the three countries with data on both covariates (Belgium, Portugal, Spain: 2,214 cases).
Subjects with unknown treatment (153, 3.3%) were excluded from these analyses.
In order to describe the percentage of total between-country variation due to heterogeneity rather than chance, we applied the I2 statistics to the distribution by country of the estimated RERs and to the Odds of surgical intervention in stage I–II patients or of medical treatments only in stage III–IV patients; the statistical significance threshold of the index was defined at p < 0.05 (24, 25).
3. Results
3.1. Study population characteristics
After excluding 63 cases known only from their death certificate (DCO), we analysed 4,602 LC cases. Table 1 shows the number of cases and the percentages of patients and tumour characteristics by country.
In all countries the percentage of patients aged over 70 was the highest, range 47.6 (Switzerland) to 54.7 (Belgium), except in Portugal, where patients aged 55–69 were the majority (44.7%). The majority of cases were men (75.9%) with similar between country proportions, while Switzerland had the highest percentage of women (40.7%).
In the majority of cases the cancer was located in the lobes, most frequently in the upper lobe (44.3%), while 7.8% were in the main bronchus. Ill-defined or overlapping topographies were 18.4% overall, ranging from 12.9 (Switzerland) to 31.1% (Belgium).
For more than 90% cases the LC diagnosis was microscopically verified (MV), with lower percentages in Estonia (80%) and highest in Belgium (95.6%).
Seventy-eight percent of cases were NSCLC and 16.7% were SCLC. In Portugal there were the highest percentages of NSCLC (81.8%) and the lowest of SCLC (14.3%). In all, NOS morphology cases were 5%, with the highest percentages in Estonia (10.9%) and Switzerland (6.7%).
Fifty-one percent of patients were diagnosed with TNM stage IV; those with stage I or II were 10.8% and 7.4%, respectively. The highest percentages of cases with unknown tumour stage at diagnosis were in Belgium (16.8%) and Switzerland (17%).
Approximately 30% of patients (27.9%) did not receive any anti-cancer treatment or received only palliative care, with the lowest percentage in Switzerland (18.9%). This was 11.3% for stage I–II, 30.5% for stage III–IV and 40.4% for patients with unknown stage at diagnosis.
Surgery, with or without adjuvant treatment (chemotherapy, radiotherapy, or target drugs) was done for 19.9% of patients and 49.0% received only radiotherapy or Chemo- or radiotherapy only, ranging from 33.6 (Estonia) to 61.7 (Belgium).
For patients with stage I–II at diagnosis, the most frequent therapy was surgery (66.5%), ranging from 48.0 (Belgium) to 76.2 (Switzerland); most patients (59.1%) with stage III–IV at diagnosis were given chemo- or radiotherapy only, ranging from 44.9% (Estonia) to 74.1% (Belgium).
Among the countries providing comorbidity data, 42% presented no comorbidity at diagnosis (range 25.7% in Spain to 54.6% in Portugal). Respectively 17.4% and 16.3% patients had low or severe comorbidity. A larger proportion of women than men presented no comorbidity at diagnosis (51% vs. 31%) (data not shown).
Smoking data were available for Belgium, Spain, North Portugal CR and Switzerland, for a total of 2,970 cases. Among these, 38% were current smokers (38.1%), ranging from 33.1 (Belgium) to 48.0 (Spain). A lower percentage of women than men were current or past smokers (39% vs. 57%) and 19% women vs. 3% men were never smokers (data not shown).
3.2. Diagnostic work-up
Table 2 lists the numbers of cases and distributions (%) of diagnostic examinations on the lung within three months (after or before) from diagnosis in the four countries with information on diagnostic work-up. Conventional chest imaging (radiography, CT scan or pulmonary stratigraphy) was scheduled for 68.0% of patients (range: 45.1 Portugal to 90.0 Belgium). Seventy-four percent of patients received spiral CT, the most frequent examination in all countries (range: 49.3 Portugal to 94.6 Belgium) and PET was carried out for 35.3% of patients (range: 8% Estonia to 59.5 Belgium); MRI was used for less than 10% in all countries except Portugal (10.5%).
Bronchoscopy was done in 63% cases (range: 53.8 Estonia to 80.8 Belgium); mediastinoscopy was done for 5% (range: 0.6 Portugal to 9.2 Belgium); while in 13% endobronchial ultrasound was done (range: 0.1 Portugal to 27.3 Spain). At least one endoscopic examination was scheduled for 65.9% of the patients, with the highest percentages in Belgium (87.4).
The combination of diagnostic examinations indicated that the majority of patients (2,551, 65.9%) received a complete diagnostic work-up (at least one imaging examination—spiral CT or PET or MRI, plus at least one endoscopic diagnostic examination (bronchoscopy, mediastinoscopy or endobronchial ultrasound).
For 21.8% patients' diagnosis was based on spiral CT or PET or MRI and for 2.3% the diagnosis was based only on conventional chest imaging.
3.3. Adhesion to clinical guidelines
Table 3 shows the odds ratios and 95% confidence intervals of undergoing surgery or receiving only chemo- or radiotherapy, by tumour stage group (I–II and III–IV) adjusted by age at diagnosis, sex, topography, morphology, stage, comorbidity and country.
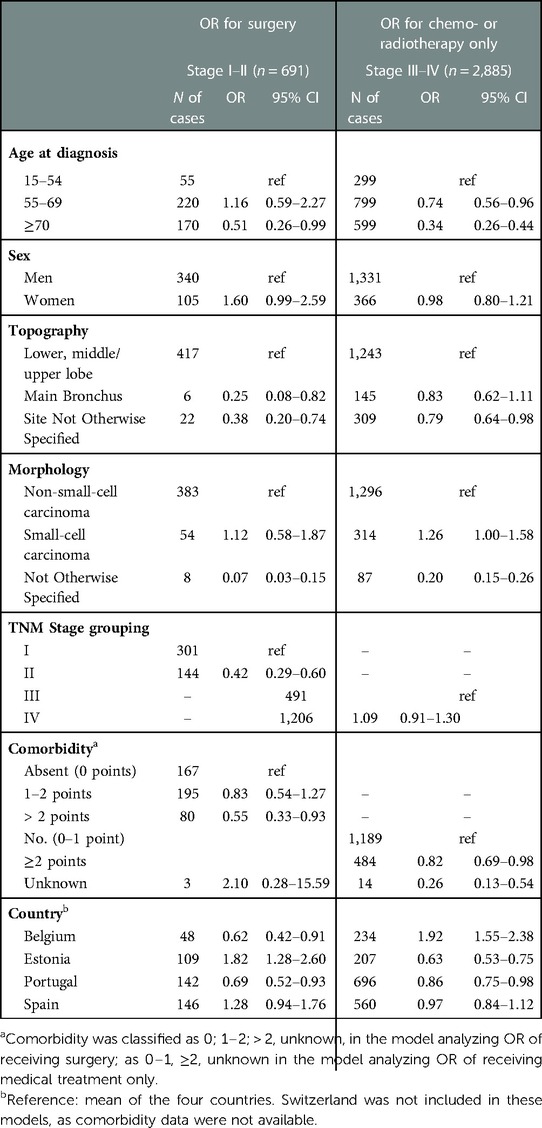
Table 3. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of surgery for lung cancer patients with tumour stage I–II, or receiving chemo- or radiotherapy only for patients with tumour stage III–IV at diagnosis.
Surgery was scheduled for 20% of total patients, but considering patients who had tumour stage I–II at diagnosis, for whom surgery is indicated, this percentage rose to 66% overall. Approximately 30% of patients had no specific cancer treatment, with lower percentages in Belgium and Switzerland.
Among the 281 patients with stage I–II who did not have surgery, medical contraindications were reported for 70 cases, patient's refusal for 21, but for 190 cases the reasons were unknown or unspecified.
For stages I–II, the adjusted OR of surgery decreased with advancing age; it was lower for cancer located in the main bronchus (OR 0.25, CI, 0.08–0.82) and not specified site (OR 0.38, CI, 0.20–0.74) than in other subsites; for TNM stage II (OR 0.42, CI, 0.29–0.60) than stage I and for patients with severe comorbidity (OR 0.55, CI, 0.33–0.93) than those with no comorbidity. The adjusted OR for surgery was higher than the mean in Estonia (OR1.82, CI, 1.28–2.60) and lower than the mean in Belgium (OR 0.62, CI, 0.42–0.91) and Portugal (OR 0.69, CI, 0.52–0.93).
Considering patients with tumour stages III–IV, the OR of receiving chemo- or radiotherapy decreased significantly with age; it was lower for cancers of ill-defined topography (OR 0.79, CI, 0.64–0.98) than other subsites. With reference to NSCLC, the OR was lower for NOS morphology (OR 0.20, CI, 0.15–0.26) and higher for SCLC (OR 1.26, CI, 1.00–1.58). The adjusted OR was higher than the mean in Belgium (OR 1.92, CI, 1.55–2.38) and lower in Estonia (OR 0.63, CI, 0.53–0.75) and Portugal (OR 0.86, CI, 0.75–0.98).
The I2 statistics for ORs of surgical intervention in stage I–II patients was 97.44% and 99.70% for ORs of chemo or radiotherapy in stage III–IV.
3.4. Survival
Figure 1 shows relative survival for patients with tumour stages I–II and III–IV, by sex at 1, and 5 years after diagnosis, with 95% CI. In all tumour stage groups, women had longer survival than men but in stage III–IV the difference by sex decreased over time.
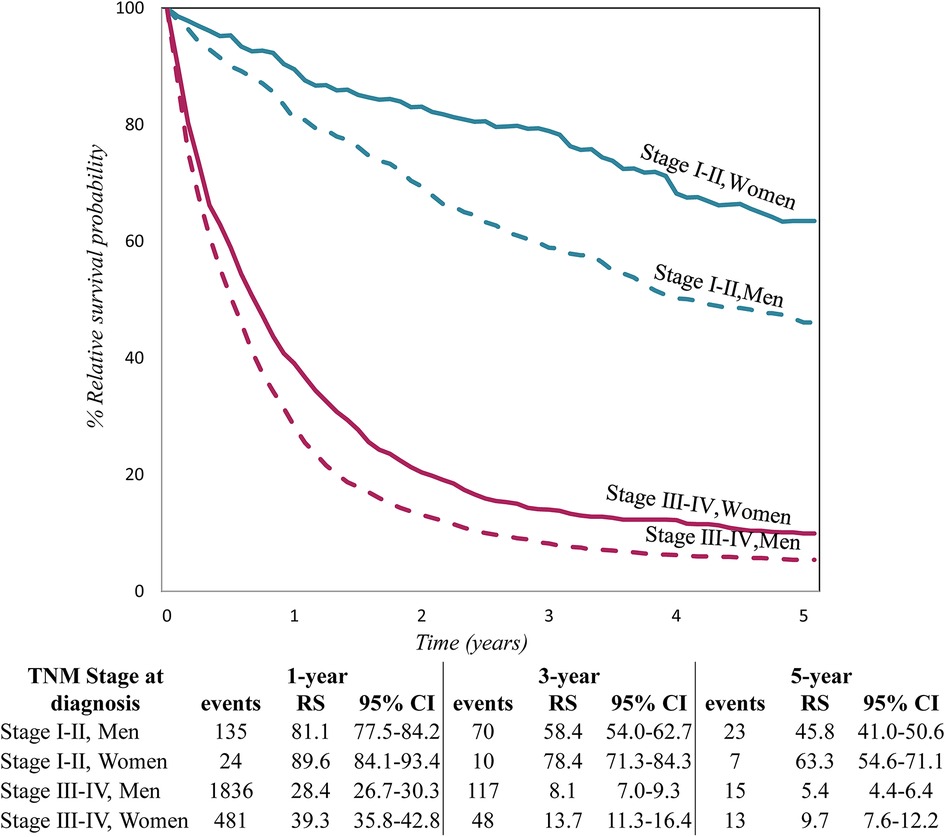
Figure 1. Relative survival by TNM stage at diagnosis and sex, for lung cancer patients diagnosed in 2009–2013 in five European countries.
Figure 2 shows age-standardised relative survival (RS) with 95% confidence intervals by country for operated and not operated cases. In all, 87.2% of operated cases were alive one year after diagnosis, ranging from 84.1% in Switzerland to 91.6% in Portugal; three years after diagnosis the overall RS had fallen to 67.2%. The 5-year RS of total operated cases was 56.9, ranging from to 47.7 in Portugal to 62.7 in Belgium. For non-operated cases, the overall 1-, 3- and 5-year RS figures were respectively 29.7, 8.4, 4.7, with little across-country differences. Table 4 shows the results of the multivariable analysis to assess the impact of the factors under study on five-year risk of death.
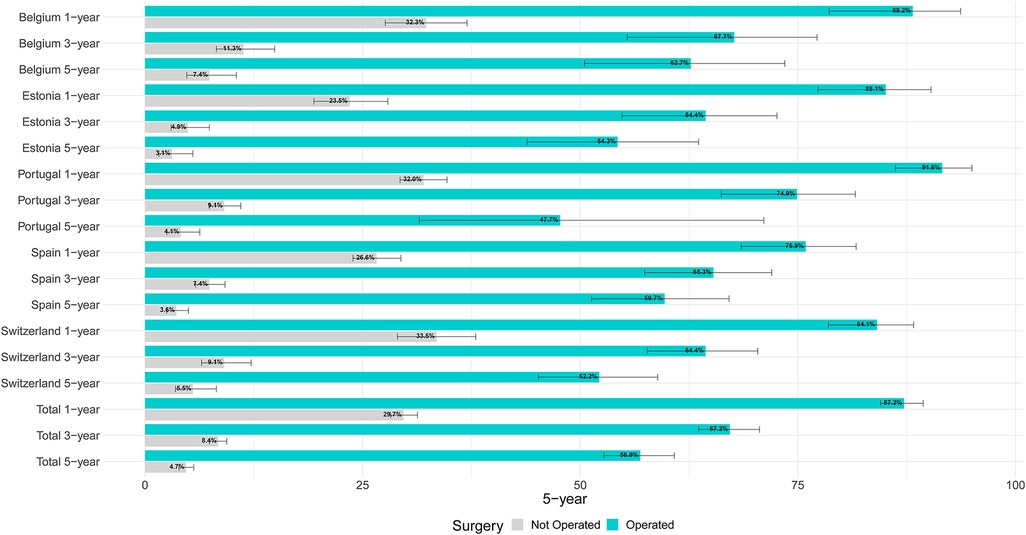
Figure 2. Age-adjusted relative survival by country and treatment for lung cancer patients diagnosed in 2009–2013 in five European countries.
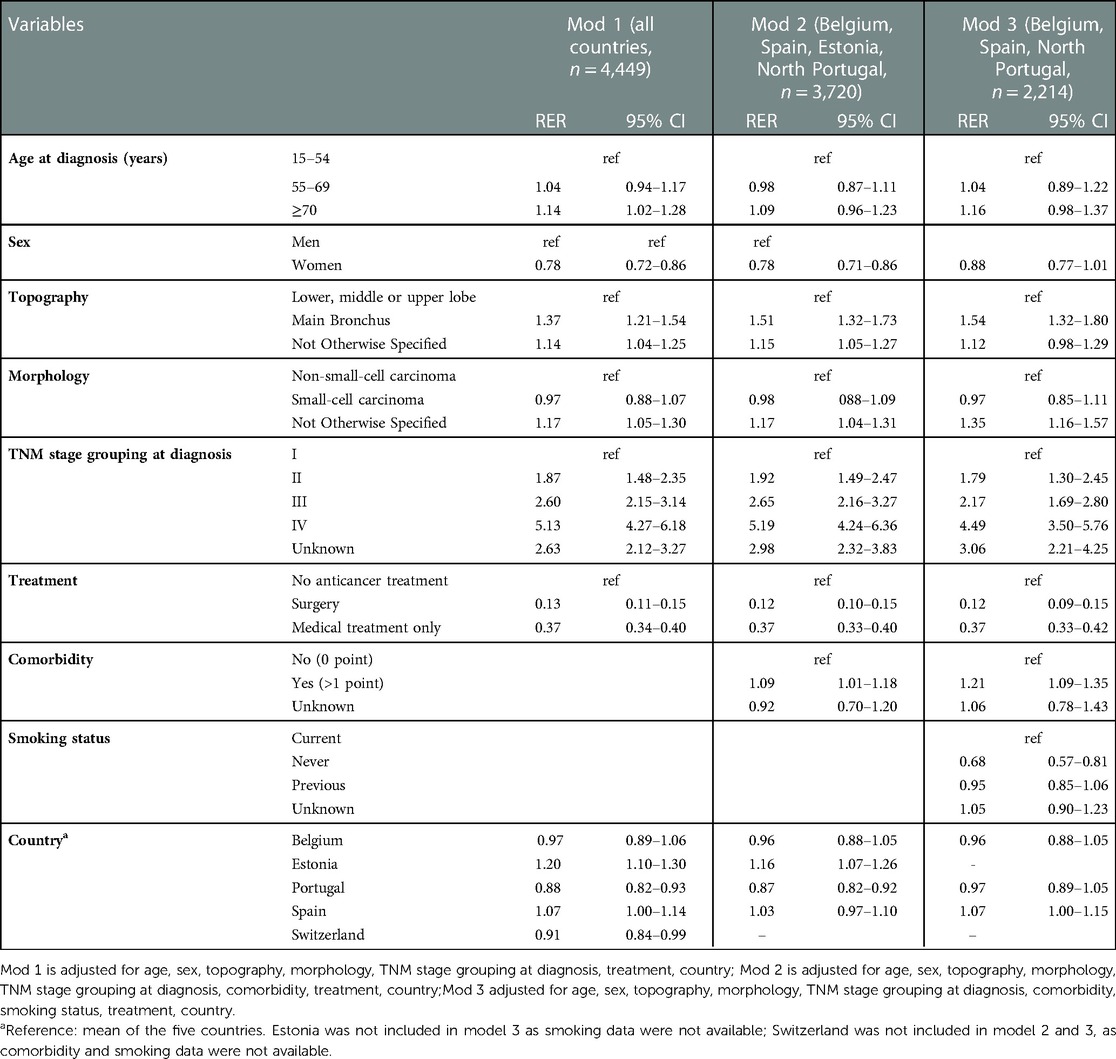
Table 4. Adjusted five-year relative excess of risk of death (RER) with 95% confidence intervals (CI) for lung cancer patients diagnosed in 2009–2013 in five European countries.
Considering model 1, the adjusted five-year RER increased with age at diagnosis, significantly for patients aged 70 and over (RER 1.14, CI, 1.02–1.28). The RER was lower for women (RER 0.78, CI, 0.72–0.86) than men. It was higher for cancers in the main bronchus (RER 1.37, CI, 1.21–1.54) than other subsites, for NOS morphology (RER 1.17, CI, 1.05–1.30) than NSCLC. The RER rose significantly with worse stage at diagnosis.
Compared to patients receiving no anti-cancer treatment, those treated surgically had the lowest mortality (RER 0.13, CI, 0.11–0.15), but those receiving chemo- or radiotherapy only also had lower than reference RER (RER 0.37, CI, 0.34–0.40). The fully adjusted five-year RER was higher than the mean in Estonia (RER 1.20, CI, 1.10–1.30) and lower in Portugal (RER 0.88, CI, 0.82–0.93) and Switzerland (RER 0.91, CI, 0.84–0.99).
The analyses restricted to the subsets of CRs with information on comorbidity (model 2) and both comorbidity and smoking (model 3) provided RERs and CI very similar to those of model 1 for age, sex, topography, morphology, stage, treatment and country.
In addition, these models indicated that patients with any comorbidity at diagnosis had significantly higher RER than those with no comorbidity (RER 1.09, CI, 1.01–1.18 in model 2; RER 1.21, CI, 1.09–1.35 in model 3). Model 3 also showed that the adjusted RER of never smokers was lower than that of current smokers (RER 0.68, CI, 0.57–0.81).
A larger proportion of women than men presented no comorbidity at diagnosis (51% vs. 31%), 29% vs. 36% was current, 17% vs. 45% was past smoker and 37% vs. 5% was never smoker). Adjustment for these factors and for clinical-pathological characteristics in the multivariable models confirmed the independent effects of sex on survival.
The I2 statistics for the distribution in the RERs across countries varied from 0.00 to 0.01, indicating small between country heterogeneity in the RER (i.e., heterogeneity is mostly due to the other model covariates).
4. Discussion
Consistently with previous studies (4–7). In our study approximately 20% (or less) LC patients were diagnosed with early stage tumours potentially amenable to curative surgery. More than half, however, had advanced tumours, a condition that precludes the possibility of curative treatment. These results point to the need to improve access to timely diagnosis.
We uncovered geographical differences in adhering to the clinical guidelines under study (surgery in stage I–II, medical treatment in stage II–IV); the I2 high values in the ORs models indicate these variations are attributable to real heterogeneity across countries.
Although LC survival was uniformly low in all countries, surgery was independently and significantly associated with lower mortality and compared to patients receiving no anticancer treatment, chemo- or radiotherapy also offered a significant protective effect.
Our protocol did not investigate the intent to treat; however, for of 82% operated patients, radical surgery was possible and was highly protective of mortality risk, so we maintain that surgery was scheduled with curative intent. In addition to the factors captured by our study, survival of operated patients may also be related with the expertise and training of the surgeons, the number of cases performed by individual surgeons or the case load of the hospitals within the registry. Likewise, difference in experience and training among medical and radio oncologists could account for difference in outcomes.
The geographical differences in LC survival evident in our univariate analyses, and also reported in previous studies (3, 4), were attenuated by adjustment for clinical characteristics (morphology, topography), sex, age, treatment, comorbidity or smoking. In fact, the low values for I2 in the RER distribution across countries, suggest the clinical pathological covariates included in the models are main determinants of survival, while variations by country are less important.
Accurate diagnostic investigation is essential for guiding therapy and for selecting patients who can benefit from surgery. The selection of patients for surgery affects its outcome: in countries with higher proportions of surgically treated patients, more frail patients may have been treated and the outcomes therefore might be worse.
In our study more than 65% of patients received imaging combined with an endoscopy. In Belgium, where this percentage was the highest, the proportion of stage I–II patients treated surgically was lower and the proportion of patients receiving medical treatment (chemo- or radiotherapy) was higher than in the other countries, and five-year relative survival of the operated cases was among the highest in the countries analysed. By contrast, in Estonia where the percentage of extensive diagnostic work-up was lower than average, the odds of surgery for stage I–II cases was higher than average and survival lower.
Although in Estonia the probability of surgical treatment for localised lung cancer increased markedly from the late 1990s to the 2010s, even for SCLC, correlating with increasing survival rates (5), in the present study survival was lower than in other countries, for both surgically and medically treated LC patients. In Estonia, the limited availability of radiotherapy, particularly stereotactic body radiation therapy (SBRT), could have contributed to the worse survival of patients who were not operated. Estonia was among the countries with the lowest availability of radiotherapy equipment in Europe until 2012 (26). SBRT was not available in 2011, molecular targeted therapies for NSCLC were reimbursed from 2010, and immunotherapy became available after 2014.
Data on the diagnostic work-up was not available for Switzerland, where five-year survival of operated cases was lower than in other countries, and the corresponding figure for non-operated cases was among the highest.
These findings confirm the importance of accurate diagnostic profiling to select patients who will benefit from surgery.
In line with other studies reporting that the frequency of surgery decreased with age (5, 27), we found that the odds of undergoing surgery for stage I–II LC patients did in fact decrease with age, stage, comorbidity and location of the cancer in the main bronchus. The worse survival with greater age at diagnosis explained the decreases in the frequency of surgery with later age.
Although surgery is mainly recommended for early stage NSCLC (9), we found the odds of receiving surgery in stage I–II did not differ between SCLC and NSCLC and the ratio was reduced only for ill-defined histotypes (NOS). Coherently with clinical guidelines (8), among stage III–IV cases SCLC were more likely than NSCLC to receive medical treatment (chemo- or radiotherapy).
Our results are in line with studies suggesting resection might be indicated also for very early SCLC (28–30) and with population-based studies reporting that from 1% to 25.1% SCLC received surgery in the UK (31). According to many studies, SCLC have a worse prognosis than NSCLC (3, 5). In our study three-year survival of SCLC cases was 27.9%, vs. 33.4% for NSCLC, but once all the factors in the multivariable model were adjusted, only patients with ill-defined histology (NOS) had higher than the reference RER. This is in line with reports that the survival of operated SCLC can be comparable to that of NSCLC patients (29).
Probably on account of the problematic anatomic suitability for resection and reconstruction, and the technical complexity of surgery for tumours of the main bronchus and carina (32, 33), only 7 of the 358 patients with cancer in the main bronchus were treated surgically, while 53% of total surgeries involved lobectomy to resect peripheral cancers in the lobes. Coherently with the low frequency of surgery, location in the main bronchus carried higher RER than other sites, while there were no RER differences amongst lobectomy, segmentectomy or pneumectomy. We have no data on minimally invasive surgery, which has advanced rapidly, making surgery an acceptable treatment option even for elderly and frail patients and those with comorbidities. The use of thoracoscopic surgery may have differed between countries at the time of data collection.
Using data of six CRs in the four countries that provided less than 12% of cases with unknown comorbidity, we found severe comorbidity (CCI >2) was associated with omission of surgery for stage I–II cancers, as well as with the odds of receiving chemo- or radiotherapy for stage III–IV patients. We cannot exclude that for some cases for whom CCI was coded as “no comorbidity” (CCI = 0), the relevant information was actually unknown, this bias could have led to underestimating the effect of comorbidity on RERs and ORs.
Our findings are in line with reports suggesting that between 24% and 70% of cancer patients with comorbidity are not treated according to guidelines (34). The probability of successful surgery is reduced with advanced age and the presence of comorbidities, also due to the expected higher incidence of postoperative complications (18).
In accordance with other studies (14, 35), in the multivariable analysis restricted to the CRs providing the relevant data, comorbidity and smoking habit were independently and positively associated with RER.
Coherently with other studies (14), LC patients who are current smokers had a higher risk of death than non-smokers. The most frequent comorbidities in our study population were COPD and CVD, both related to smoking, operatory risk and mortality. Severity of comorbidity was also associated to mortality (data not shown).
While in many western countries LC incidence is decreasing for men, an increase over the recent decades is evident for women (1, 36), so the numbers of cases among women can be expected to rise in the next few years, with specific health needs. Identifying predictive prognostic factors can help tailor sex-specific disease management (37–40).
The survival advantage of women also found in other studies has been attributed to earlier tumour stage at diagnosis, better health behaviour, lower comorbidity (41), all factors related to the possibility of curative treatment. Contrary to a report from England (31), in our study surgery did not differ by sex; nor did the stage distribution (stage I–II was 17% in women and 18% in men; stage III–IV was 69% and 72%, respectively). In each stage category survival was higher for women than men (Figure 1).
Although a larger proportion of women than men presented no comorbidity at diagnosis and were non-smokers, adjustment for these factors and for clinical-pathological characteristics in the multivariable models confirmed the independent effects of sex on survival.
4.1. Strengths and limits
A major strength of our study consists in the large number of cases and in its population basis with recruitment of all incident cases in the study period or registry sub-area, with collection of ad hoc data additional to those routinely available to cancer registries, using a common protocol and analysis methods. Thus, our study provides information concerning standard care and treatment effectiveness in everyday clinical practice without any patient or outcome selection, and is therefore representative of what happens in real-life.
A limit of the study is its retrospective observational design, with variables abstracted from clinical records, which are not structured nor standardised across hospitals and countries. Hence, a part of differences in care and outcomes found by our study may depend on data availability and clinicians' attitudes to reporting.
Epidermal growth factor receptor (EGFR) testing was done in 6% of NSCLC, with small across-registry differences. In a similar study period, other population data report 45% of cases tested (42), so we have to consider the percentage found in our study unreliable. However, clinical guidelines (43) concluded that EGFR-targeted agents were not associated with better survival in the subgroup with EGFR-mutated LC (43).
5. Conclusions
Although the prognosis for LC is uniformly poor in all countries, the survival benefit of diagnosis at early stages, allowing curative surgery, was confirmed by multivariable analyses adjusted by clinic, pathological and demographic factors, comorbidity and smoking. Our findings support the importance of accurate diagnostic investigation for selecting patients who can benefit from surgery, and of screening for subjects at risk, to detect lung cancer at a very early stage when curative treatment is applicable.
Early diagnosis should be increased through screening for subjects at risk and public health campaigns on awareness of LC symptoms; adhesion to standard care should be incremented by funding better equipment across the EU, and better training of health personnel.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics
All procedures performed in these studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
MS: conceptualisation; Writing Original draft, Supervising. CD: Data curation; Formal analysis; Visualization. RL: Data curation Formal analysis, conceptualisation; Validation; All authors and Working Group contributed to Data curation; Validation; Writing Review & editing. All authors contributed to the article and approved the submitted version.
Funding
This work was carried out in the framework of the EUROPEAN HIGH RESOLUTION CANCER STUDIES and partially funded by Italian Ministry of Health “Ricerca Corrente” funds. The work of Kaire Innos was supported by Estonian Research Council (grant no PRG722).
Acknowledgments
Simone Bonfarnuzzo provided technical and administrative support and Judith Baggott reviewed the English language. All the participating cancer registries collected and prepared the data as part of their essential role in cancer control. This work was carried out as part of the European High Resolution project on prognosis and care of cancer patients.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fepid.2023.1109853/full#supplementary-material.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Cortinovis D, Abbate M, Bidoli P, Capici S, Canova S. Targeted therapies and immunotherapy in non-small-cell lung cancer. Ecancermedicalscience. (2016) 10:648. doi: 10.3332/ecancer.2016.648
3. Francisci S, Minicozzi P, Pierannunzio D, Ardanaz E, Eberle A, Grimsrud TK, et al. Survival patterns in lung and pleural cancer in Europe 1999–2007: results from the EUROCARE-5 study. Eur J Cancer. (2015) 51:2242–53. doi: 10.1016/j.ejca.2015.07.033
4. Minicozzi P, Walsh PM, Sánchez MJ, Trama A, Innos K, Marcos-Gragera R, et al. Is low survival for cancer in Eastern Europe due principally to late stage at diagnosis? Eur J Cancer. (2018) 93:127–37. doi: 10.1016/j.ejca.2018.01.084
5. Innos K, Oselin K, Laisaar T, Aareleid T. Patterns of survival and surgical treatment in lung cancer patients in Estonia by histologic type and stage, 1996–2016. Acta Oncol. (2019) 58(11):1549–56. doi: 10.1080/0284186X.2019.1637539
6. Rodríguez-Barranco M, Salamanca-Fernández E, Fajardo M, Bayo E, Chang-Chan YL, Expósito J, et al. Patient, tumor, and healthcare factors associated with regional variability in lung cancer survival: a Spanish high-resolution population-based study. Clin Transl Oncol. (2019) 21:621–9. doi: 10.1007/s12094-018-1962-9
7. Mangone L, Minicozzi P, Vicentini M, Giacomin A, Caldarella A, Cirilli C, et al. Key factors influencing lung cancer survival in northern Italy. Cancer Epidemiol. (2013) 37:226–32. doi: 10.1016/j.canep.2013.02.005
8. Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E. Small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol. (2013) 24:vi99–vi105. doi: 10.1093/annonc/mdt178
9. Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early-stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO clinical practice guidelines. Ann Oncol. (2017) 28:iv1–iv21. doi: 10.1093/annonc/mdx222
10. Pallis AG, Syrigos KN. Lung cancer in never smokers: disease characteristics and risk factors. Crit Rev Oncol Hematol. (2013) 88:494–503. doi: 10.1016/j.critrevonc.2013.06.011
11. Tabchi S, Kassouf E, Florescu M, Tehfe M, Blais N. Factors influencing treatment selection and survival in advanced lung cancer. Curr Oncol. (2017) 24(2):e115–22. doi: 10.3747/co.24.3355
12. Tammemagi CM, Neslund-Dudas C, Simoff M, Kvale P. Impact of comorbidity on lung cancer survival. Int J Cancer. (2003) 103(6):792–802. doi: 10.1002/ijc.10882
13. Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. (2002) 54(2):357–64. doi: 10.1016/s0360-3016(02)02939-5
14. Parsons A, Daley R, Begh P. Aveyard influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. Br Med J. (2010) 340:b5569. doi: 10.1136/bmj.b5569
15. Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al., editors. International classification of diseases for oncology (ICD-O). 3rd ed. Geneva, Switzerland: World Health Organisation (2000). 247 p.
16. European High-Resolution studies. Available at: http://hrstudies.it (last visit on 30th January 2023).
17. Sobin LH, Gospodarowicz MK, Wittekind CH, editors. TNM Classification of malignant tumours. 7th ed. Chichester: Wiley (2010). 310 p.
18. Birim O, Maat AP, Kappetein AP, van Meerbeeck JP, Damhuis RA, Bogers AJ. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg. (2003) 23(1):30–4. doi: 10.1016/s1010-7940(02)00721-2
19. Menard SW. Applied logistic regression analysis. 2nd ed. Thousand Oaks, CA, United States: Sage (2002). 111 p.
20. Te Grotenhuis M, Pelzer B, Eisinga R, Nieuwenhuis R, Schmidt-Catran A, Konig R. When size matters: advantages of weighted effect coding in observational studies. Int J Public Health. (2017) 62:163–7. doi: 10.1007/s00038-016-0901-1
21. Dickman PW, Coviello E. Estimating and modeling relative survival. Stata J. (2015) 15(1):186–215. doi: 10.1177/1536867X1501500112
22. Ederer F, Axtell LM, Cutler SJ. The relative survival: a statistical methodology. J Natl Cancer Inst Monogr. (1961) 6:101–21. PMID: 13889176
23. Dickman PW, Sloggett A, Hills M, Hakulinen T. Regression models for relative survival. Stat Med. (2004) 23:51–64. doi: 10.1002/sim.1597
24. Botta L, Gatta G, Capocaccia R, Stiller C, Cañete A, Dal Maso L, et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): results from a population-based study. Lancet Oncol. (2022) 23(12):1525–36. doi: 10.1016/S1470-2045(22)00637-4
25. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Grau C, Defourny N, Malicki J, Dunscombe P, Borras JM, et al. Radiotherapy equipment and departments in the European countries: final results from the ESTRO-HERO survey. Radiother Oncol. (2014) 112:155–64. doi: 10.1016/j.radonc.2014.08.029
27. Veronesi G, Bottoni E, Finocchiaro G, Alloisio M. When is surgery indicated for small-cell lung cancer? Lung Cancer. (2015) 90(3):582–9. doi: 10.1016/j.lungcan.2015.10.019
28. Weksler B, Nason KS, Shende M, Landreneau RJ, Pennathur A. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg. (2012) 94(3):889–93. doi: 10.1016/j.athoracsur.2012.01.015
29. Wei S, Wei B, Tian J, Song X, Wu B, Hu P. Propensity score matching analysis for the role of surgery in small cell lung cancer. Oncol Res Treat. (2020) 43(6):276–88. doi: 10.1159/000506867
30. Lüchtenborg M, Riaz SP, Lim E, Page R, Baldwin DR, Jakobsen E, et al. Survival of patients with small cell lung cancer undergoing lung resection in England, 1998-2009. Thorax. (2014) 69(3):269–73. doi: 10.1136/thoraxjnl-2013-203884
31. Belot A, Fowler H, Njagi EN, Luque-Fernandez MA, Maringe C, Magadi W, et al. Association between age, deprivation and specific comorbid conditions and the receipt of major surgery in patients with non-small cell lung cancer in England: a population-based study. Thorax. (2019) 74(1):51–9. doi: 10.1136/thoraxjnl-2017-211395. Epub 2018 Aug 12. PMID: 30100577
32. Chen L, Campisi A, Wang Z, Dell’Amore A, Ciarrocchi AP, Zhao H, et al. Left secondary carinal resection and reconstruction for low-grade bronchial malignancies. JTCVS Tech. (2021) 8:196–201. doi: 10.1016/j.xjtc.2021.05.013
33. Matsuura N, Igai H, Kamiyoshihara M. Carinal resection and reconstruction: now and in the future. Transl Lung Cancer Res. (2021) 10(10):4039–42. doi: 10.21037/tlcr-21-731
34. Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. (2013) 5(Suppl 1):3–29. doi: 10.2147/CLEP.S47150
35. Niksic M, Redondo-Sanchez D, Chang YL, Rodriguez-Barranco M, Exposito-Hernandez J, Marcos-Gragera R, et al. The role of multimorbidity in short-term mortality of lung cancer patients in Spain: a population-based cohort study. BMC Cancer. (2021) 21(1):1048. doi: 10.1186/s12885-021-08801-9
36. Lortet-Tieulent J, Renteria E, Sharp L, Weiderpass E, Comber H, Baas P, et al. Convergence of decreasing male and increasing female incidence rates in major tobacco-related cancers in Europe in 1988–2010. Eur J Cancer. (2015) 51:1144–63. doi: 10.1016/j.ejca.2013.10.014
37. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. (2019) 85(1):8. doi: 10.5334/aogh.2419
38. Li Z, Ma Y, Xu Y. Burden of lung cancer attributable to household air pollution in the Chinese female population: trend analysis from 1990 to 2019 and future predictions. Cad Saude Publica. (2022) 38(9):e00050622. doi: 10.1590/0102-311XEN050622
39. Schabath MB, Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. (2019) 28(10):1563–79. doi: 10.1158/1055-9965.EPI-19-0221
40. Wang S, Zhang J, He Z, Wu K, Liu XS. The predictive power of tumor mutational burden in lung cancer immunotherapy response is influenced by patients’ sex. Int J Cancer. (2019) 145(10):2840–9. doi: 10.1002/ijc.32327
41. Innos K, Padrik P, Valvere V, Aareleid T. Sex differences in cancer survival in Estonia: a population-based study. BMC Cancer. (2015) 15:72. doi: 10.1186/s12885-015-1080-9
42. Aye PS, McKeage MJ, Tin S, Khwaounjoo P, Elwood JM. Population-based incidence rates and increased risk of EGFR mutated non-small cell lung cancer in Māori and Pacifica in New Zealand. PLoS One. (2021) 16(5):e0251357. doi: 10.1371/journal.pone.0251357
Keywords: lung cancer, survival, population registries, comorbidity, smoking
Citation: Sant M, Daidone C, Innos K, Marcos-Gragera R, Vanschoenbeek K, Barranco MR, Poch EO and Lillini R (2023) Patterns of care and survival for lung cancer: Results of the European population-based high-resolution study. Front. Epidemiol. 3:1109853. doi: 10.3389/fepid.2023.1109853
Received: 28 November 2022; Accepted: 8 February 2023;
Published: 3 March 2023.
Edited by:
Juan Manuel Mejia-Arangure, Universidad Nacional Autonoma de Mexico, MexicoReviewed by:
Mark Utell, University of Rochester, United StatesSarah Lucht, University of Minnesota Twin Cities, United States
© 2023 Sant, Daidone, Innos, Marcos-Gragera, Vanschoenbeek, Barranco, Poch and Lillini and The Lung Cancer HR Study Working Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caterina Daidone Y2F0ZXJpbmEuZGFpZG9uZUBpc3RpdHV0b3R1bW9yaS5taS5pdA==
†Lung Cancer High Resolution Study Working Group: Belgium: E. Van Eycken (Belgium- Cancer Registry); K. Vanschoenbeek (Hasselt- University); Estonia: K. Innos, M. Magi¸ K. Paapsi (Estonia Cancer Registry); Iceland: L. Tryggvadóttir (Iceland- Cancer Registry); Italy:
Specialty Section: This article was submitted to Clinical Epidemiology, a section of the journal Frontiers in Epidemiology
 Milena Sant
Milena Sant Caterina Daidone
Caterina Daidone Kaire Innos
Kaire Innos Rafael Marcos-Gragera
Rafael Marcos-Gragera Katrijn Vanschoenbeek6
Katrijn Vanschoenbeek6