- 1Department of Energy and Technology, Swedish University of Agricultural Sciences, Uppsala, Sweden
- 2Tampere University of Applied Sciences, Tampere, Finland
To complement innovations at the front- and back-ends of source-separating sanitation systems, this study demonstrates a novel approach for stabilising human urine using sparingly soluble fumaric acid. A reactor was developed to dose fumaric acid passively into freshly excreted urine and was operated to mimic more than 250 typical urination events over 15 days. Fumaric acid at a dose of 5.6 g L-1 effectively maintained urine pH below 4.0, inhibiting enzymatic urea hydrolysis and preventing the precipitation of alkaline earth metals and phosphates, thereby protecting downstream infrastructure from blockages. The stabilised urine retained all its constituents, except for 20% of the sulphate. Novel UV-Vis monitoring techniques were introduced to track fumaric acid depletion (ΔAbs221) and solids settling rate (ΔAbs660), and were demonstrated to be practical surrogates for assessing real-time reactor performance. With an estimated operating cost of less than US$ 5 per person per year, this reactor provides a simple, cost-effective, and scalable solution for stabilising urine in decentralised settings.
1 Introduction
In source-separating sanitation systems, different fractions of wastewater, such as human urine, are collected separately at the source so that they can be efficiently treated and safely recycled (Lehtoranta et al., 2022), for instance as biobased fertilisers (Perez-Mercado et al., 2024). These systems typically consist of a front-end (a toilet user-interface that separates urine), an intermediate stage (pipes for urine collection and a storage tank), and a back-end (technologies for treating collected urine and producing marketable products) (Larsen et al., 2021a). Urine-separating toilets have been around for decades and have been manufactured since the early 1990s (Winblad and Simpson-Hebert, 2004). The invention of the ‘urine trap’ in 2017 significantly improved the user-interface design, enabling efficient urine separation without requiring changes in user behaviour (Gundlach et al., 2021). Earlier toilet models relied on separate compartments and precise user positioning, whereas toilets integrated with a urine trap resemble typical mixed toilets that fit seamlessly into modern bathroom aesthetics (MAK, 2019), while allowing for effective, if not perfect, urine separation (Reuter et al., 2022).
Numerous technologies have been developed to treat urine at the back-end, many of which have undergone extensive research and are nearing industrialization (Harder et al., 2019; Larsen et al., 2021b). These technologies typically target two types of urine—hydrolysed and unhydrolysed—with the type processed at the back-end influenced by collection infrastructure and treatment. Freshly excreted urine undergoes an enzyme-catalysed reaction that converts urea, its main nitrogen compound, to ammonia, unless treatment inhibits enzyme activity (Udert et al., 2003a; McMillan, 2015; Chipako and Randall, 2020). The enzyme urease is pervasive in nature (Krajewska, 2009) and produced by microbial activity in traps, pipes, and storage tanks that receive source-separated urine (Udert et al., 2003a). Since urine is over 95% water (Friedler et al., 2013), technologies aiming to concentrate nutrients and produce liquid or solid fertilizers, with nitrogen predominantly in the form of urea, require treatment that inhibit urease activity (Simha et al., 2020). A simple approach to inhibit urease is to regulate the urine pH to levels where enzymatic urea hydrolysis is inhibited: pH < 4 (Ray et al., 2018; Simha et al., 2023) or pH > 10 (Randall et al., 2016; Simha et al., 2022). While much work has focused on alkalizing fresh urine, acidification has recently gained research interest. Acidifying urine prevents precipitation of most minerals, such as hydroxyapatite, struvite, and calcite, in collection pipes, where the alkaline pH of hydrolysed urine triggers their formation, leading to blockages that require regular cleaning (Udert et al., 2003b; Yan et al., 2021). While the use of organic and inorganic acids that are readily soluble in urine has been explored (Hellström et al., 1999; Andreev et al., 2017; Courtney and Randall, 2022; Simha et al., 2023; Crane et al., 2024), the potential of sparingly soluble acids remains largely unexamined. In building-scale systems, acids with high solubility in urine require active dosing (Crane et al., 2024), whereas in household or single toilet-scale systems with batchwise treatment, these acids can be added as a single dose at the start of the treatment. The latter approach maintains a consistently low pH throughout the urine load, as treated urine is removed only after the entire load has been processed. For this to be effective, the treatment system must be placed close to or integrated with the user interface, as higher degrees of urea hydrolysis during collection require higher acid doses to reduce urine pH below 4.0 (Hellström et al., 1999). In building-scale systems, stabilizing urine at source is necessary to connect multiple toilets to a single back-end treatment unit, typically located outside the building or in the basement. Passive dosing of sparingly soluble acids in a reactor, similar to the approach used for alkaline agents like calcium hydroxide (Randall et al., 2016) and magnesium hydroxide (Simha et al., 2022), could offer an alternative and effective approach to stabilise urine closer to the user-interface.
Fumaric acid is an organic acid produced by various species of the filamentous fungus Rhizopus (Roa Engel et al., 2008). It is commonly used as a safe additive in the food and beverage industry, liquid pharmaceutical formulations, and in manufacturing of unsaturated polyester resin, with a reported global production volume of 300,000 tonnes (ChemAnalyst, 2024a) and bulk price of US$ 1.24 kg-1 in 2024 (ChemAnalyst, 2024b). With a solubility of 6 g L-1 in water at 25°C (Goldberg and Rokem, 2009), fumaric acid is considered poorly soluble in aqueous solutions, such as human urine. Like other organic acids, fumaric acid (pKa = 3.03 at 25°C, according to O'Neil (2006)) exhibits strong antimicrobial activity at low pH. When protonated, it becomes lipophilic and can diffuse across cell membranes (Skřivanová and Marounek, 2007). Inside the microbial cell, it dissociates at cytoplasmic pH, causing metabolic uncoupling (Kashket, 1987). Moreover, fumaric acid has a high buffering capacity, which supports it to maintain a stable pH and inhibit urease activity in urine. It has a long shelf life under typical storage conditions, existing as a solid with a high melting point of 287°C (Lide and Milne, 1964). Given these properties, this study evaluated the feasibility of using fumaric acid to stabilize urine in an on-site reactor for building-scale sanitation systems. The specific objectives included evaluating the effectiveness of passively dosed fumaric acid in regulating urine pH, monitoring fumaric acid consumption using UV-Vis spectrophotometry, evaluating solids settling behaviour in the reactor, analysing the fate of major constituents and physicochemical properties of urine, and assessing the operational and practical implications of implementing such a system. By addressing these objectives, this study contributes to the development of effective technologies that complement innovations at both the front and back ends of source-separating sanitation systems.
2 Methods
2.1 Materials
Fresh urine was anonymously collected in polypropylene flasks (0.5 L and 1 L) from approximately 20 adults (male and female, aged 20–66 years). Urine was collected over the first half of a working day and used in the experiments during the second half. Unless stated otherwise, the collected urine was pooled and mixed before use. Fumaric acid (>99%) was purchased from Sigma-Aldrich.
2.2 Experimental procedure
The treatment of fresh urine with fumaric acid was studied in duplicate polypropylene reactors. The reactors had a truncated cone design with a maximum capacity of 5 L, marked with 0.5 L graduations. At the start of the experiment, 250 g of fumaric acid was loaded into each reactor, followed by fresh urine added at a rate of 10–20 mL s−1 using a specially designed funnel that mimicked the urination rate of an average individual. Urine was added in batches ranging from 200 to 800 mL to reflect daily variations in urine volume typically excreted by an average person, as reported by Rose et al. (2015), and based on preliminary data collected within our research group. Immediately after each urine addition, the mixture was stirred using a propeller (R 1345, IKA, Germany) attached to a digital overhead stirrer (Ministar 20 control, IKA, Germany). Initially, the urine was stirred at 350 rpm for 5 min, but this was later reduced to 250 rpm for 2 min, which was deemed sufficient for stabilizing urine. Urine addition was randomized throughout the day until each reactor accumulated a total of 4 L of treated urine, typically simulating 6-8 urination events per day. After treatment, the urine was left undisturbed, either overnight or over the weekend. Once settling was complete, 3 L of treated and settled urine was removed using a peristaltic pump (Schenchen LabS3/UD15, China). This procedure was repeated daily with the addition of 3 L of fresh urine to each reactor, continuing the treatment cycle for 15 days. Throughout the experiment, each reactor simulated 263 urination events and treated 46 L of fresh urine. Samples of untreated fresh urine and treated urine collected after solids settling were taken for further analysis.
2.3 Determining settling velocity of solids
Initially, the study aimed to determine the settling velocity of solids in the reactor using a visual space-time registration approach, as described by Shaddel et al. (2019). This method involved thoroughly mixing 4 L of treated urine in the reactor with an overhead stirrer at 250 rpm for 2 min, followed by monitoring the time required for solids to reach various marked levels within the reactor and form a distinct separation front, using a digital stopwatch. However, this approach proved ineffective due to the difficulty in discerning distinct solid-liquid interfaces, as the solids were loosely aggregated, leading to irregular settling and resuspension in the liquid. Consequently, an alternative method was adopted. On days 1, 5, 10, and 15 of the experiment, the urine in the reactor was stirred at 250 rpm for 2 min using the overhead stirrer. Subsequently, 15 mL urine samples were withdrawn at 15-min intervals for the first hour, and then after 2 h, from a depth corresponding to the 1-L graduation level of the reactor. These samples were vigorously mixed, immediately transferred into cuvettes, and the light transmittance was measured at 660 nm using a PerkinElmer LAMBDA 365 UV/Vis spectrophotometer (Gómez et al., 2007).
2.4 Determining fumaric acid depletion through UV-Vis absorbance
To determine the depletion of fumaric acid in the reactor, the UV absorbance of urine samples before and after treatment were measured on a spectrophotometer (LAMBDA 365, PerkinElmer, USA) in the wavelength range of 200–400 nm. Urine samples were diluted 100-fold and filtered (0.45 µm; Filtropur S, Sarstedt, Germany) before analysis. Untreated urine was used as a blank, and its spectrum was subtracted from treated urine spectra to isolate the contribution of fumaric acid. Fumaric acid exhibits maximum absorbance at ∼221 nm (Avendaño and Briceño, 2009), and can be detected spectrophotometrically in complex biological solutions at low concentrations (Sokullu et al., 2010). Thus, the change in absorbance at this wavelength (ΔAbs221) was used as a surrogate parameter to monitor fumaric acid depletion in the reactor, rather than as a method for absolute quantification.
2.5 Determining solubility of fumaric acid
A batch study was conducted using three different urine compositions to determine the apparent solubility of fumaric acid in real urine. These included first-morning urine and two urine samples collected during a working day. For each test, fumaric acid was incrementally added in 0.1 g doses to 100 mL of urine, mixed over a magnetic stirrer for 5 min, and then left undisturbed for 24 h before measuring pH and conductivity. We defined supersaturation as the point at which further additions of fumaric acid resulted in less than a 1% change in pH.
2.6 Physicochemical analyses
The pH was measured using a glass electrode (Metrohm iUnitrode with Pt1000, Switzerland) connected to a Metrohm 914 pH/Conductometer. The electrical conductivity was recorded using a 4-wire conductivity measuring cell with integrated Pt1000 temperature sensor (6.0917.080, Metrohm, Switzerland) connected to a measuring instrument (Metrohm 914 pH/Conductometer, 2.914.0020, Switzerland).
The concentration of urea, total ammonia, phosphate, sulphate, calcium, magnesium and potassium were measured daily using a Thermo Scientific™ Gallery™ discrete analyser. Further details on specific analytical methods are found in Supplementary Table S1 of the Supplementary Information (SI). Composite samples of untreated urine and treated urine were also analysed by inductively coupled plasma-optical emission spectrometry (ICP-OES, Optima Avio 200, PerkinElmer, United States) for concentrations of calcium, potassium, magnesium, sodium, phosphorus and sulphur. Before ICP-OES, samples were digested in 65% HNO3 and diluted with Milli-Q water.
Total solids in urine were determined by drying samples at 40°C for 72 h in a CO2-free drier (DC4600HPWR, Electrolux, Sweden). Volatile solids were determined by incinerating the dried urine samples at 550°C for 6 h.
3 Results and discussion
3.1 Urine stabilisation
In batch experiments, between 2 and 11 g L-1 of fumaric acid was needed to acidify urine to pH 3.0, depending on urine composition (Figure 1). The solubility of fumaric acid in urine ranged from 6–11 g L-1 at 20°C (Supplementary Figure S1 in SI), which is higher than its reported solubility in water (6 g L-1 at 25°C, Goldberg and Rokem (2009)). In long-term reactor experiments, fumaric acid dosing lowered urine pH from 6.4 (±0.4) to 3.0 (±0.5) (Figure 2A). The fumaric acid dose required to maintain urine pH below 3.0 was 7.93 g L-1, while 5.67 g L-1 was sufficient to maintain pH below 4.0. Acidification of urine to pH of less than 4.0 is sufficient to inhibit urease-catalysed hydrolysis of urea in human urine (Ray et al., 2018). Previous work with readily soluble organic acids showed that dosing with 2.53 g L−1 oxalic acid dihydrate and 5.9 g citric acid effectively maintains pH below 3.0 (Simha et al., 2023).
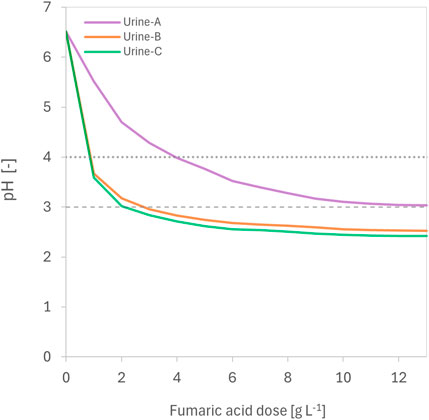
Figure 1. The pH (−) of three different urine compositions (Urine-A, Urine-B, Urine-C) at various fumaric acid doses (g L-1) during batch experiments. Urine-A was first-morning urine, whereas Urine-B and Urine-C were collected during a working day. Horizontal dotted lines indicate the fumaric dose required for urine pH to reach 3.0 and pH 4.0. See Supplementary Figure S1 in SI for further information.
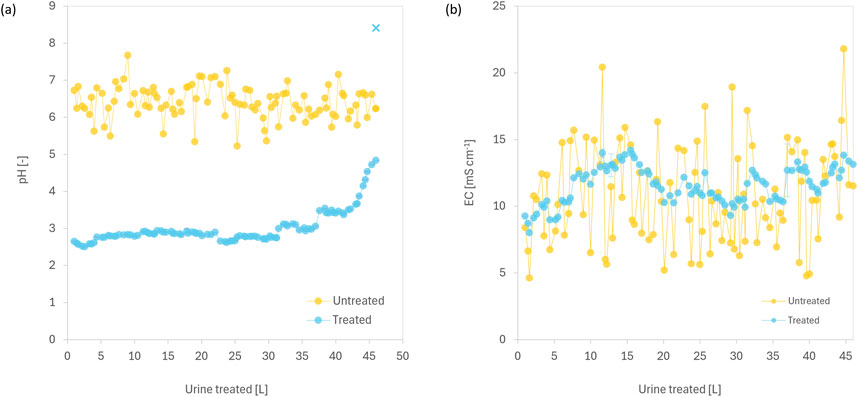
Figure 2. The (A) pH and (B) electrical conductivity (EC) of human urine, before and after treatment with fumaric acid, in a stirred reactor. The reactor initially contained 250 g of fumaric acid. Unhydrolysed urine was cumulatively added to reactor in batches of 200–800 mL at 10–20 mL s-1, simulating typical urination events. Following each event, the reactor was stirred at 250 rpm for 2 min. Once the volume of treated urine reached 4 L, the reactor was drained to remove 3 L after overnight settling, before more untreated urine was added. The cross mark ( × ) in panel (A) represents the final pH of 8.4, which was measured after fumaric acid was fully consumed and urea hydrolysis had proceeded over the weekend. Error bars show standard deviation (n = 2).
The pH and EC profiles of untreated urine displayed significant fluctuations, reflecting the natural variability in urine composition (Figures 2A, B). The pH of treated urine remained relatively stable until ∼40 L of urine had been treated, after which a significant increase was observed, likely due to depletion of fumaric acid in the reactor (Supplementary Figure S2A in SI). In contrast, the relative change in conductivity showed no clear pattern, with significant fluctuations throughout the treatment (Supplementary Figure S2B in SI). On the last day of treatment, the concentration of total ammonia in the treated urine increased 15-fold compared to untreated urine, with a simultaneous decrease in phosphate and sulphate concentrations, as well as the removal of all calcium and magnesium from the solution (Supplementary Table S2 in SI), indicative of urea hydrolysis (Udert et al., 2003c; Ray et al., 2018). The inactivation of urease by acidification is reversible, as urease activity resumes when pH rises above 4.0 (Yang et al., 2021). At the end of day 15, the pH of treated urine was 4.8, but after leaving the urine undisturbed in the reactor over the weekend, the pH increased to above 8.4 (Figure 2A). These findings indicate that pH monitoring is more effective than conductivity for detecting the onset of urea hydrolysis in urine stabilized with organic acids (Figure 2B).
The dissolution of fumaric acid in urine could be monitored using UV-Vis spectrophotometry, as there was a positive difference in light absorbance spectra between untreated and treated urine in the 200–230 nm wavelength range (Figure 3A). Fumaric acid has peak absorbance at 221 nm (Avendaño and Briceño, 2009) and can be detected spectrophotometrically at concentrations as low as 0.005 g/L in biological solutions such as fermentation broths (Sokullu et al., 2010). Given the concentration of fumaric acid in treated urine is significantly higher than that of other UV-absorbing organics, UV-spectral overlap is expected to be minimal. We observed only minor variations (±0.1) in ΔAbs221 until approximately 30 L of urine had been treated, after which ΔAbs221 rapidly decreased, reaching zero by the final day of the study (Figure 3B). This suggests that the first 30 L of urine treated in the reactor were supersaturated with fumaric acid, while subsequent treatments were undersaturated. The 250 g of fumaric acid in the reactor were fully depleted (ΔAbs221 = 0) after 46 L of urine had been treated. Notably, ΔAbs221 provided an earlier and more sensitive indication of fumaric acid depletion and the onset of urine instability than pH (Figure 2A). Thus, ΔAbs221 is an effective surrogate for predicting fumaric acid saturation in urine and monitoring its depletion in on-site reactors.
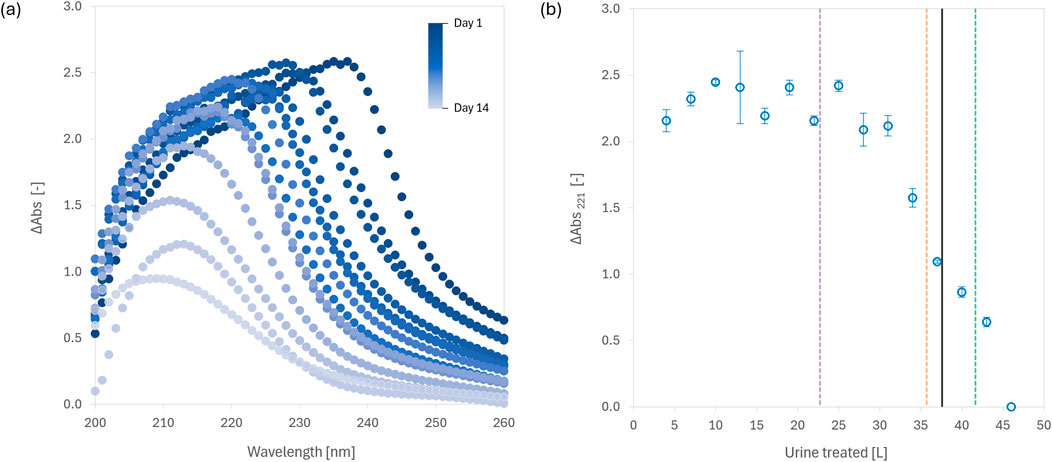
Figure 3. (A) Change in UV absorbance of urine in the 200–260 nm wavelength range after treatment with fumaric acid in an on-site reactor. The spectra for the last treatment day are not shown since treated urine hydrolysed following the consumption of fumaric acid and reactivation of urease. (B) Change in UV absorbance at 221 nm (ΔAbs221) in urine cumulatively added and treated with fumaric acid. Dashed vertical lines indicate the volume of urine that could be treated by 250 g of fumaric acid based on its solubility in batch experiments with three different urine compositions (See Supplementary Figure S1 in SI for further details). The solid vertical line shows solubility determined from the long-term reactor study. Error bars represent standard deviation (n = 2).
3.2 Reactor performance and impact on urine composition
Treatment with fumaric acid increased the concentration of total solids in urine by 5.2 ± 0.1 g L-1. To evaluate the settling behavior of solids in the reactor, the normalized change in light absorbance of urine was recorded at 660 nm at different time intervals and on various treatment days (Figure 4). During the first 10 days, a rapid initial decrease in normalized absorbance within 15 min indicated efficient initial settling of solids. The time for 90% of the solids to settle increased from 45 min on Day 1, to 60 min on Day 5, and to over 120 min on Day 10. By Day 15, the settling rate was poor, likely due to the consumption of fumaric acid in the reactor (Figure 3). An increase in light absorbance after the initial drop on this day suggests that fine solid particles were resuspended. Given that fumaric acid is poorly soluble in aqueous solutions (Goldberg and Rokem, 2009), and the initial 30 L of urine added to the reactor were supersaturated with fumaric acid (Figure 3B), undissolved fumaric acid was likely the dominant solid that settled in the reactor.
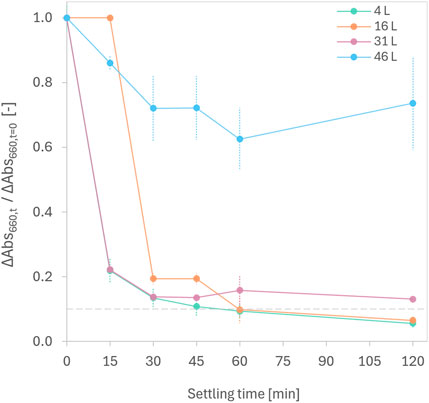
Figure 4. Change in normalized absorbance at 660 nm (ΔAbs660,t/ΔAbs660,t=0) as a function of settling time for urine treated with fumaric acid in an on-site reactor. Measurements were taken at a depth corresponding to the 1-L graduation level of the reactor at specified time intervals after 4 L (Day 1), 16 L (Day 5), 31 L (Day 10), and 46 L (Day 15) of urine were treated. The dashed line indicates settling time required for >90% of the solids. Error bars show standard deviation (n = 2).
Treatment with fumaric acid did not affect the concentration of urea or major cations and anions in urine, except for sulphate (Figure 5). The mechanism underlying this apparent sulphate removal is not fully understood and may involve complexation or co-precipitation with metabolites in urine, such as carboxylate-containing compounds, rather than precipitation with inorganic cations. For example, hippuric acid is known to precipitate at low pH (Kohlstaedt and Helmer, 1936). Recent work by Courtney and Randall (2022) has also shown that uric acid dihydrate crystals can form in unhydrolysed urine acidified with citric acid.
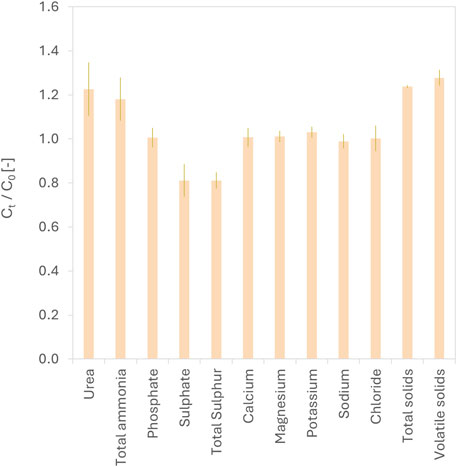
Figure 5. Concentration ratios (Ct/C0) of various constituents in urine, before and after treatment, with fumaric acid. The horizontal dashed line at Ct/C0 = 1 indicates the baseline concentration. Values above or below this line represent increases or decreases in concentration post-treatment. For all ionic constituents except total sulphur, sodium, total solids, and volatile solids, data represent the daily average over the 14-day reactor operation period (n = 28). The remaining parameters were measured from composite samples of untreated and treated urine collected at the end of the experiment (n = 6). Error bars represent standard deviation.
These results suggest that the change in normalized absorbance at 660 nm is an effective surrogate parameter for monitoring solid settling rates in on-site urine treatment reactors. Real-time turbidity monitoring is widely used in various applications, including decentralized water purification, centralized wastewater treatment, and urban stormwater management (Metadier and Bertrand-Krajewski, 2012; Leow et al., 2017). Installing turbidity sensors near the reactor outlet, where treated and settled urine is drained, could provide continuous data on settling efficiency and reactor performance (Supplementary Figure S4 in SI). This monitoring would be particularly valuable in settings handling large urine loads (e.g., public building toilets) where timely reactor drainage is essential to prevent overflow and ensure efficient acidification. In settings with stable, low urine loads, such as households, reactors could be programmed to drain settled urine twice daily based on average settling time. Since urination events typically peak in the morning and late evening, draining treated urine in the late mid-day and after midnight could be an effective approach. Using Wi-Fi activity as a surrogate for building occupancy (Aden and Boyer, 2022) could further optimize toilet usage estimates, urine load to the reactor, and consequently, reactor drainage schedules.
3.3 Practical implications
Acidifying urine as close as possible to the front-end inhibits urease-catalysed hydrolysis (Hellström et al., 1999), which is essential for retaining nitrogen when urine requires back-end treatment to reduce water content and facilitate transportation (Simha et al., 2020). In building-scale systems, citric acid is commonly used for cleaning urine-separating toilets and removing phosphate precipitates that block urine collection pipes (Lienert and Larsen, 2007). Adding fumaric acid lowers urine pH (Figures 1, 2), inhibiting the precipitation of alkaline earth metal phosphates (Figure 5), which may help prevent pipe blockages (Crane et al., 2024). Unlike readily water-soluble acids such as citric or acetic acid, fumaric acid can be added to on-site reactors in amounts exceeding the saturation point, allowing for passive dosing at the source. This passive dosing, combined with automated reactor drainage, can protect pipes and reduce the need for manual interventions, enhancing the acceptance of such sanitation systems.
Dosing urine with 6 g L-1 of fumaric acid, considering that an average person excretes 1.5 L of urine per day (Vinnerås et al., 2006), and given the global bulk price of fumaric acid at US$ 1.24 kg-1 (ChemAnalyst, 2024b), results in an annual per capita cost of approximately US$ 4. Assuming an average European electricity mix and a price of US$ 0.26 kWh-1, the energy demand for mixing urine with fumaric acid (∼3.69 kWh cap−1 y-1) would result in additional operating cost of US$ 0.96 cap−1 y-1. Thus, the overall operating cost for stabilising urine is calculated to be less than US$ 5 cap−1 y-1. This is significantly below the Bill and Melinda Gates Foundation’s Reinvent the Toilet Challenge target of US$ 18.25 cap−1 y-1 (US$ 0.05 cap−1 d-1), leaving room for additional processing, such as converting stabilised urine into concentrated fertilisers (Perez-Mercado et al., 2024). At a dose of 6 g L-1, a household-scale reactor treating urine from four regular occupants would require replenishing fumaric acid once every week, assuming the same reactor dimensions as used in our study. If the reactor is integrated with urine-separating toilets having a urine trap, where only 70%–80% of the urine is collected (Reuter et al., 2022) and diluted with flush water at a 1:1 ratio (Gundlach et al., 2021), the acid replenishment rate would need to be increased, as the solubility of fumaric acid in urine and water is similar. While the reactor size could be increased, especially in settings where space is not a constraint, a 5 L reactor is compact enough to be integrated with the front-end interface, such as by utilising unused space below wall-hung toilets or water cisterns.
4 Conclusion
• Passively added fumaric acid effectively maintained urine pH below 3.0 at a dose of 7.93 g L-1 and below 4.0 at a dose of 5.67 g L-1. This pH regulation resulted in reversible inactivation of urease and inhibition of phosphate precipitation, both of which are critical for protecting downstream pipes and reducing the need for manual interventions to address pipe blockages. Fumaric acid is therefore a viable alternative to alkaline chemicals like Ca(OH)2 for stabilising freshly excreted urine.
• The difference in UV absorbance at 221 nm (ΔAbs221) was demonstrated to be an effective surrogate for predicting the degree of fumaric acid saturation in urine and its consumption in the reactor.
• Analysing the change in normalized absorbance of urine at 660 nm (ΔAbs660,t/ΔAbs660,t=0) near the reactor outlet was shown to be suitable for monitoring solid settling rates. Incorporating turbidity monitoring at this wavelength could provide real-time data on settling efficiency and reactor performance, facilitating better management and servicing of urine separating toilets in building-scale sanitation systems.
• Fumaric acid-treated urine retained nitrogen and all major cations and anions, except for 20% of the sulphate, a finding that warrants further investigation.
• The estimated operating cost of an on-site reactor dosing urine with fumaric acid was less than US$ 5 cap−1 y-1, making it a feasible option for implementation in a wide range of global settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because urine was collected anonymously and pooled together, ensuring that no identifiable personal data were recorded or stored. The study did not involve any invasive procedures or health interventions. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because participation was entirely voluntary.
Author contributions
PS: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing–original draft, Writing–review and editing. NA: Data curation, Formal Analysis, Investigation, Methodology, Writing–review and editing. OP: Data curation, Formal Analysis, Investigation, Methodology, Writing–review and editing. JJ: Formal Analysis, Supervision, Writing–review and editing. AV: Conceptualization, Formal Analysis, Investigation, Methodology, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by a grant from Familjen Kamprads Stiftelse for the Urine Diversion 3.0 project (Grant 20230107).
Acknowledgments
We thank Björn Vinnerås for helping conceptualise this study and Dyllon Randall for feedback on the manuscript, particularly regarding the design of experiments to determine fumaric acid solubility in urine. We are grateful to Cecilia Lalander and Marcus Korvella for their valuable advice on UV-Vis spectrophotometry. Finally, we acknowledge the support of colleagues at SLU who donated urine and contributed to our research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1546396/full#supplementary-material
References
Aden, K., and Boyer, T. H. (2022). Shift to remote learning degrades water quality in buildings. AWWA Water Sci. 4 (6). doi:10.1002/aws2.1316
Andreev, N., Ronteltap, M., Boincean, B., Wernli, M., Zubcov, E., Bagrin, N., et al. (2017). Lactic acid fermentation of human urine to improve its fertilizing value and reduce odour emissions. J. Environ. Manag. 198 (Pt 1), 63–69. doi:10.1016/j.jenvman.2017.04.059
Avendaño, C., and Briceño, A. (2009). Concomitant [2 + 2] cycloaddition solid state reactions from co-crystals self-assembled via mechanochemistry. CrystEngComm 11 (3), 408–411. doi:10.1039/b820463b
ChemAnalyst (2024a). Fumaric acid market analysis 2015-2035. Available at: https://www.chemanalyst.com/industry-report/fumaric-acid-market-3063 (Accessed August 04, 2024).
ChemAnalyst (2024b). Fumaric acid price trend and forecast. Available at: https://www.chemanalyst.com/Pricing-data/fumaric-acid-1134 (Accessed August 03, 2024).
Chipako, T. L., and Randall, D. G. (2020). Urine treatment technologies and the importance of pH. J. Environ. Chem. Eng. 8 (1), 103622. doi:10.1016/j.jece.2019.103622
Courtney, C., and Randall, D. G. (2022). Concentrating stabilized urine with reverse osmosis: how does stabilization method and pre-treatment affect nutrient recovery, flux, and scaling? Water Res. 209, 117970. doi:10.1016/j.watres.2021.117970
Crane, L., Saetta, D., and Boyer, T. H. (2024). Acid dosing increases recoverable phosphorus during different occupancy conditions in full-scale urine diversion system. ACS ES&T Eng. 4 (9), 2109–2120. doi:10.1021/acsestengg.4c00164
Friedler, E., Butler, D., and Alfiya, Y. (2013). “Wastewater composition,” in Source separation and decentralization for wastewater management. Editors T. Larsen, K. Udert, and J. Lienert (London: IWA Publishing), 241–257.
Goldberg, I., and Rokem, J. S. (2009). “Organic and fatty acid production, microbial,” in Encyclopedia of microbiology. Editor M. Schaechter, Third Edition ed (Academic Press), 421–442.
Gómez, M., Plaza, F., Garralón, G., Pérez, J., and Gómez, M. A. (2007). A comparative study of tertiary wastewater treatment by physico-chemical-UV process and macrofiltration–ultrafiltration technologies. Desalination 202 (1-3), 369–376. doi:10.1016/j.desal.2005.12.076
Gundlach, J., Bryla, M., Larsen, T. A., Kristoferitsch, L., Gründl, H., and Holzner, M. (2021). Novel NoMix toilet concept for efficient separation of urine and feces and its design optimization using computational fluid mechanics. J. Build. Eng. 33, 101500. doi:10.1016/j.jobe.2020.101500
Harder, R., Wielemaker, R., Larsen, T. A., Zeeman, G., and Öberg, G. (2019). Recycling nutrients contained in human excreta to agriculture: pathways, processes, and products. Crit. Rev. Environ. Sci. Technol. 49 (8), 695–743. doi:10.1080/10643389.2018.1558889
Hellström, D., Johansson, E., and Grennberg, K. (1999). Storage of human urine: acidification as a method to inhibit decomposition of urea. Ecol. Eng. 12 (3-4), 253–269. doi:10.1016/s0925-8574(98)00074-3
Kashket, E. R. (1987). Bioenergetics of lactic acid bacteria: cytoplasmic pH and osmotolerance. FEMS Microbiol. Rev. 46, 233–244. doi:10.1016/0378-1097(87)90110-8
Kohlstaedt, K. G., and Helmer, O. M. (1936). A study of the hippuric acid excretion as a test of hepatic function. Am. J. Dig. Dis. 3, 459–466. doi:10.1007/bf03000730
Krajewska, B. (2009). Ureases I. Functional, catalytic and kinetic properties: a review. J. Mol. Catal. B Enzym. 59 (1-3), 9–21. doi:10.1016/j.molcatb.2009.01.003
Larsen, T. A., Gruendl, H., and Binz, C. (2021a). The potential contribution of urine source separation to the SDG agenda – a review of the progress so far and future development options. Environ. Sci. Water Res. and Technol. 7 (7), 1161–1176. doi:10.1039/d0ew01064b
Larsen, T. A., Riechmann, M. E., and Udert, K. M. (2021b). State of the art of urine treatment technologies: a critical review. Water Res. X 13, 100114. doi:10.1016/j.wroa.2021.100114
Lehtoranta, S., Malila, R., Sarkilahti, M., and Viskari, E. L. (2022). To separate or not? A comparison of wastewater management systems for the new city district of Hiedanranta, Finland. Environ. Res. 208, 112764. doi:10.1016/j.envres.2022.112764
Leow, A., Burkhardt, J., Platten, W. E., Zimmerman, B., Brinkman, N. E., Turner, A., et al. (2017). Application of the CANARY event detection software for real-time performance monitoring of decentralized water reuse systems. Environ. Sci. Water Res. and Technol. 3 (2), 224–234. doi:10.1039/C6EW00226A
Lide, D. R., and Milne, G. W. A. (1964). Handbook of data on organic compounds. Boca Raton ,FL: CRC Press, Inc.
Lienert, J., and Larsen, T. A. (2007). Pilot projects in bathrooms: a new challenge for wastewater professionals. Water Pract., 2. doi:10.2166/wpt.2007.057
MAK (2019). Circular flows: the toilet revolution!. Available at: https://www.mak.at/en/program/exhibitions/circular_flows (Accessed August 4, 2024).
McMillan, M. (2015). Biological treatment of source separated urine in a sequencing batch reactor. MSc: Stellenbosch University.
Metadier, M., and Bertrand-Krajewski, J. L. (2012). The use of long-term on-line turbidity measurements for the calculation of urban stormwater pollutant concentrations, loads, pollutographs and intra-event fluxes. Water Res. 46 (20), 6836–6856. doi:10.1016/j.watres.2011.12.030
O'Neil, M. J. (2006). The merck index - an encyclopedia of chemicals, drugs, and biologicals. Whitehouse Station, NJ: Merck and Co., Inc.
Perez-Mercado, L. F., Simha, P., Moreira, A. P., Paulo, P. L., and Vinneras, B. (2024). Circular fertilisers combining dehydrated human urine and organic wastes can fulfil the macronutrient demand of 15 major crops. Sci. Total Environ. 951, 175655. doi:10.1016/j.scitotenv.2024.175655
Randall, D. G., Krahenbuhl, M., Kopping, I., Larsen, T. A., and Udert, K. M. (2016). A novel approach for stabilizing fresh urine by calcium hydroxide addition. Water Res. 95, 361–369. doi:10.1016/j.watres.2016.03.007
Ray, H., Saetta, D., and Boyer, T. H. (2018). Characterization of urea hydrolysis in fresh human urine and inhibition by chemical addition. Environ. Sci. Water Res. and Technol. 4 (1), 87–98. doi:10.1039/c7ew00271h
Reuter, S., Demant, D., Heredia, G., Lüthi, C., Reymond, P., Schertenleib, R., et al. (2022). Compendium of sanitation systems and technologies for the wider caribbean region. Bremen, Germany: Bremen Overseas Research and Development Association (BORDA).
Roa Engel, C. A., Straathof, A. J., Zijlmans, T. W., van Gulik, W. M., and van der Wielen, L. A. (2008). Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 78 (3), 379–389. doi:10.1007/s00253-007-1341-x
Rose, C., Parker, A., Jefferson, B., and Cartmell, E. (2015). The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 45 (17), 1827–1879. doi:10.1080/10643389.2014.1000761
Shaddel, S., Ucar, S., Andreassen, J.-P., and Østerhus, S. W. (2019). Engineering of struvite crystals by regulating supersaturation – correlation with phosphorus recovery, crystal morphology and process efficiency. J. Environ. Chem. Eng. 7 (1), 102918. doi:10.1016/j.jece.2019.102918
Simha, P., Deb, C., Randall, D., and Vinnerås, B. (2022). Thermodynamics and kinetics of pH-dependent dissolution of sparingly soluble alkaline earth hydroxides in source-separated human urine collected in decentralised sanitation systems. Front. Environ. Sci. 10, 889119. doi:10.3389/fenvs.2022.889119
Simha, P., Senecal, J., Gustavsson, D. J., and Vinnerås, B. (2020). “Resource recovery from wastewater: a new approach with alkaline dehydration of urine at source,” in Current developments in biotechnology and bioengineering (Elsevier), 205–221.
Simha, P., Vasiljev, A., Randall, D. G., and Vinnerås, B. (2023). Factors influencing the recovery of organic nitrogen from fresh human urine dosed with organic/inorganic acids and concentrated by evaporation in ambient conditions. Sci. Total Environ. 879, 163053. doi:10.1016/j.scitotenv.2023.163053
Skřivanová, E., and Marounek, M. (2007). Influence of pH on antimicrobial activity of organic acids against rabbit enteropathogenic strain of Escherichia coli. Folia Microbiol. 52 (1), 70–72. doi:10.1007/bf02932141
Sokullu, E., Palabıyık, İ. M., Onur, F., and Boyacı, İ. H. (2010). Chemometric methods for simultaneous quantification of lactic, malic and fumaric acids. Eng. Life Sci. 10 (4), 297–303. doi:10.1002/elsc.200900080
Udert, K. M., Larsen, T. A., Biebow, M., and Gujer, W. (2003a). Urea hydrolysis and precipitation dynamics in a urine-collecting system. Water Res. 37 (11), 2571–2582. doi:10.1016/S0043-1354(03)00065-4
Udert, K. M., Larsen, T. A., and Gujer, W. (2003b). Biologically induced precipitation in urine-collecting systems. Water Sci. Technol. Water Supply 3 (3), 71–78. doi:10.2166/ws.2003.0010
Udert, K. M., Larsen, T. A., and Gujer, W. (2003c). Estimating the precipitation potential in urine-collecting systems. Water Res. 37 (11), 2667–2677. doi:10.1016/S0043-1354(03)00071-X
Vinnerås, B., Palmquist, H., Balmér, P., and Jönsson, H. (2006). The characteristics of household wastewater and biodegradable solid waste—a proposal for new Swedish design values. Urban Water J. 3 (1), 3–11. doi:10.1080/15730620600578629
Winblad, U., and Simpson-Hebert, M. (2004). Ecological sanitation. revised and enlarged edition. Stockholm, Sweden: EcoSanRes Programme. Stocholm Environment Institute.
Yan, Z., Cheng, S., Zhang, J., Saroj, D. P., Mang, H.-P., Han, Y., et al. (2021). Precipitation in urine source separation systems: challenges for large-scale practical applications. Resour. Conservation Recycl. 169, 105479. doi:10.1016/j.resconrec.2021.105479
Keywords: decentralised sanitation, nutrient recovery, urine stabilisation, wastewater treatment, UV-Vis monitoring
Citation: Simha P, Ahopalo N, Pay O, Jermakka J and Vasiljev A (2025) On-site reactor for treating source-separated human urine with sparingly soluble fumaric acid in building-scale sanitation systems. Front. Environ. Sci. 13:1546396. doi: 10.3389/fenvs.2025.1546396
Received: 16 December 2024; Accepted: 20 February 2025;
Published: 12 March 2025.
Edited by:
Maria Elisa Magri, Federal University of Santa Catarina, BrazilReviewed by:
Maria Harja, Gheorghe Asachi Technical University of Iași, RomaniaKai M. Udert, Swiss Federal Institute of Aquatic Science and Technology, Switzerland
Copyright © 2025 Simha, Ahopalo, Pay, Jermakka and Vasiljev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prithvi Simha, UHJpdGh2aS5TaW1oYUBzbHUuc2U=, UHJpdGh2aS5TaW1oYUBtZXNwb20uZXU=
 Prithvi Simha
Prithvi Simha Nea Ahopalo2
Nea Ahopalo2 Oliver Pay
Oliver Pay Johannes Jermakka
Johannes Jermakka