
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 14 May 2024
Sec. Soil Processes
Volume 12 - 2024 | https://doi.org/10.3389/fenvs.2024.1414010
This article is part of the Research Topic Advanced Technologies for Remedying Environmental Pollution in Agricultural Systems View all 6 articles
The use of organic fertilizers instead of chemical fertilizers can improve soil pH, help to maintain soil health and enable landowners to achieve organic or ecological-status agriculture. Rapeseed cake, sheep manure, and biofungal fertilizer are considered to be effective amendments to improve soil quality. However, there have been few studies on the effects of the three fertilizers on strawberry production, soil physicochemical properties, and inter-root soil microbial community structure. In this study, field experiments were conducted to investigate the differences in strawberry growth, quality, yield, and the structure and diversity of strawberry soil bacterial and fungal communities under four treatments: no organic fertilizer (CK), rapeseed cake organic fertilizer (T1), sheep manure organic fertilizer (T2) and bio-organic fertilizer (algae-optimized bacteria) (T3), the relationship between soil physicochemical properties and soil microbial diversity were analyzed. Our results have shown that these three amendments promoted the growth of strawberry to some extent. The effects of available phosphorus, ammonium nitrogen, sucrase, protease and urease under T2 treatment were significantly increased by 50.62%, 54.14%, 276.50%, 129.47%, 232.61%, and 232.00%, respectively, compared with the control. The soil bacterial and fungi community were the most abundant and diversified under the T2 treatment. Soil physicochemical properties and soil key enzyme activities varied significantly under different fertilizer treatments, with the soil nutrient content and soil carbon and nitrogen metabolizing enzyme activities being highest under T2 treatment. A Pearson correlation analysis showed that soil organic matter was closely related to the diversity of soil microbial communities. A redundancy analysis (RDA) showed that the main variables of the bacterial community included nitrate nitrogen (NN) and rapidly available potassium (RAP), while the main variables of the fungal community included alkaline dissolved nitrogen (ADN) and ammonium nitrogen (AN). Overall, different fertilizers promoted the release and transformation of soil nutrients by affecting the structure and diversity of bacterial and fungal communities in strawberry soils, which was beneficial to the supply of soil nutrients and the improvement of soil quality. The application of sheep manure organic fertilizer had the best soil improvement effect.
Strawberry (Fragaria x ananassa) is a perennial herb whose fruit has a distinctive aroma, bright red color, and juicy flesh. It is an excellent source of natural antioxidants, including carotenoids, vitamins, phenolics, and flavonoids, and has high levels of antioxidant capacity against free radical species (Gramza-Michalowska et al., 2019). These characteristics make strawberries one of the most popular fruits in the world. China is the world’s largest producer, accounting for more than one-third of the total global strawberry production (Yan et al., 2018). In 2018, China produced about five million tons of strawberries (Zhang et al., 2022). Strawberry is a short-cycle crop, with shallow root systems, and it has a large demand for various nutrients. The reasonable and scientific use of soil fertilizers plays a key role in the development of strawberry cultivation.
Inorganic or organic fertilizers are one of the most important sources of nutrient inputs to soils. Fertilizers are applied to provide essential nutrients for plant growth and to maximize yields. However, in many cases, the overuse of inorganic fertilizers does not solve the problem of low soil fertility, but rather leads to problems such as the deterioration of soil quality, pollution of surface and groundwater, and increased greenhouse gas emissions (Kandulu et al., 2018). The use of organic fertilizers instead of chemical fertilizers is of great significance in improving soil acidification, maintaining soil health, and realizing organic ecological agriculture. Organic fertilizers, which are mainly derived from plants or animals, not only provide a comprehensive range of nutrients for crops, but also have long-term fertilization effects; they increase and renew soil organic matter, promote microbial reproduction, improve soil physicochemical properties and biological activity (Li X. Q. et al., 2022; Yan et al., 2023). Previous studies have shown that organically produced strawberries yield more than conventionally produced strawberries (Loyd et al., 2016). Therefore, in order to maximize the potential of organic strawberry production systems, it is important to improve soil nutrient effectiveness as well as soil microbial abundance and activity (Hassan, 2015; Yogesh et al., 2021).
Animal manure is one of the most common, cheapest, and easy-to-use organic fertilizers. Manure from different animal sources can be used as a soil conditioner. It can be decomposed through fermentation and made into environmentally friendly organic fertilizers, which can increase soil organic carbon and enhance nutrient uptake, and also benefit plant growth (Guo et al., 2019; Mostafa et al., 2022). Therefore, several studies have been conducted to determine the sheep (Jia et al., 2013; Lal et al., 2020), cow (Zhang S. N. et al., 2020), and pig organic fertilizers (Sui et al., 2023) on soil acidification and property improvement. These studies have shown that livestock manure fertilizers can effectively improve the soil, promote crop growth, and increase crop yield and quality. Rapeseed is commonly grown in southern China, and the rapeseed cake that remains after rapeseed is pressed for oil is a high-quality green soil fertilizer. Rapeseed cake is rich in protein and it is a good natural fertilizer for crops. It can improve the physical and chemical properties of soil, increase the soil nutrient (e.g., phosphorous, potassium, and nitrogen: PKN) contents, improve the activity of soil microorganisms, and significantly improve the yield and quality of melons and fruit crops (Zhou et al., 2022). In recent years, microbial fertilizers as a new class of fertilizers, have been found to improve the soil inter-root micro-ecology to promote plant growth, maintain soil fertility, and control diseases, and they are becoming widely used (Spanoghe et al., 2020). These microorganisms are known to convert the complex inorganic and organic nutrients available in the soil into bioavailable forms through solubilization, oxidation or reduction, chelation, and mineralization, thereby enhancing their availability to the host plant (Shen et al., 2011). Kumar et al. (2019) reported that replacing 25% of a typical mineral fertilizer application with nitrogen fixing bacteria or Azospirillum not only promoted straw-berry growth and yield, but also increase soil nutrient effectiveness (Kumar et al., 2019). Singhalage et al. (2019) showed that a significant increase in essential amino acids and other nutrients in mustard was promoted by the application of Bacillus subtilis and Bacillus shortis (Singhalage et al., 2019). Therefore, the rational application of organic and bio-organic fertilizers can be an effective means to maintain soil health and improve crop quality and yield.
The city of Jiande City, Hangzhou, China, began to promote strawberry cultivation in 1982 and after 40 years of development it has become known as the “Hometown of Strawberry in China”. The irrational application of inorganic fertilizers has caused problems such as alteration of the soil acid-base balance, reduced soil biological activity, and decreased soil fertility, which have seriously affected the soil health of the strawberry fields and the sustainable development of the strawberry crop in the region. Therefore, there is a need to find alternative sources of safe fertilizers to improve the sustainability of crop yields and reduce the cost of cultivation. In recent years, rape-seed cake, sheep manure, and bio-organic fertilizers have been widely used in agricultural production. However, there is little information available regarding the effects of organic fertilizers and biofertilizers on strawberry growth and inter-root soil proper-ties under humid subtropical conditions. In this study, the effects of rapeseed cake, sheep manure, and bio-fertilizers on strawberry growth, fruit quality, soil physicochemical properties, and the soil microbial community structure were investigated. Changes in soil microbial community function were analyzed, to provide a theoretical basis for the reasonable use of organic fertilizers to improve strawberry soils in Jiande City.
This study was carried out in demonstration base strawberry greenhouse, Yangchun bridge town, Hangzhou Jiande City, at a latitude 119°25‘49″of north and 29°34‘4”East meridian. The soil in greenhouses that have been continuously growing strawberries for many years was selected as the object of amendment, and the strawberry greenhouses were sterilized with a solar treatment before the experiment. The greenhouses were used to grow a new variety of early-maturing strawberry with pink fruit, which is an early-ripening, disease-resistant and pink-peel strawberry cultivar (Fragaria × ananassa Duchesne) derived from a cross between Kaorino as a female parent and 2012-W-02 as a male parent.
The greenhouse was divided into 12 plots with four replications for each treat-ment, and all plots had a completely randomized design. The area of each plot was 22 m2. Treatments included (1) blank control: no organic fertilizer (single application of chemical fertilizer (150 kg hm−2) (CK). (2) rotted rapeseed cake 150 kg hm−2+ chemical fertilizer 150 kg hm−2 (T1) (3) sheep manure 1 t hm−1 + chemical fertilizer 150 kg hm−2 (T2) treatment 3: bio-organic fertilizer-algae superior bacteria 150 kg hm−2 + chemical fertilizer 150 kg hm−2 (T3). The test sheep manure organic fertilizer (organic matter 497.0 g kg−1, nitrogen 14.2 g kg−1, phosphorus 13.2 g kg−1, potassium 24.4 g kg−1) was produced by Henan Zhenghui Fertilizer Co. The rotted rapeseed cake fertilizer (Organic matter ≥60%, containing more than 35% of crude protein ≥ 35%, nitrogen ≥ 4.64%, phosphorus ≥ 2.48%, potassium ≥ 1.40%) was produced by Changsha Telai Biotechnology Co., LTD. The test bio-organic fertilizer (Ca ≥ 5%, organic matter ≥ 40%, seaweed polysaccharide ≥ 500 ppm, alginic acid ≥1,000 ppm, effective strains: Bacillus subtilis, Bacillus amyloliquefaciens, the effective number of live bacteria ≥5.0 billion·g−1) was produced by Qingdao Haida Bio Group Co. Ltd; inorganic fertilizer was compound fertilizer (N: P2O5: K2O = 18:18:18), calcium superphosphate (P2O5, 160 g kg−1) and potassium sulfate (K2O, 500 g kg−1).
The ground was plowed again with a rotary tiller, then leveled and borders were constructed. The spacing of the beds was 90–100 cm, the width of the beds was 40 cm, the width of the bottom of the beds was 60 cm, the height of the beds was 35 cm, and the width of the furrows in the beds was about 35 cm. The beds were bow-back shaped with a high center and low sides to avoid waterlogging. The experiment commenced on 18 September 2022, by selecting robust and uniformly sized strawberry seedlings for planting, with a spacing of 20 cm between plants, and two rows planted in each row. The experiment was conducted using the film drip irrigation cultivation mode. Two drip irrigation tubes were laid close to the strawberry plants in each bed, with the drip heads facing upwards and spaced 20 cm apart, and the distance between the two bands was 15–20 cm. In early October, the soil surface was covered with a new mulch, thus preventing the soil from losing water and lowering the temperature. In the middle and late stages, according to the growth of female seedlings and progeny, the reasonable control of fertilizer and water is crucial. In late October to early mid-November when the night temperature drops to about 10°C, the greenhouse was covered with film. When the night temperature was forecast to be below 5°C, an inner membrane cover was applied. Pink Jade one is resistant to anthracnose, powdery mildew, and gray mold, and can be controlled by conventional methods. Sixty-five days after the strawberry plants were transplanted, strawberry inter-root soil was collected by the root-shaking method, mixed evenly, and air-dried for the determination of soil physicochemical properties and soil enzyme activity. Some of the soil was loaded into sterile centrifuge tubes and stored in liquid nitrogen for DNA extraction. During the fruiting period, 10 freshly ripened fruits were randomly harvested on the same day in each treatment, and the weight of individual fruits was determined using an electronic balance. All fruits in each plot were collected and weighed at fruit maturity, and the average plot yield was calculated and converted to a yield per hectare. Twenty consecutive strawberry plants were selected near the center of each plot during the first generation of fruit ripening to determine the growth trait indexes of strawberries. Strawberry fruits with an intact and uniform shape were selected for the determination of fruit quality.
Soil samples were taken from each sample plot soil using the five-step soil sampling method. Each soil sample was screened through a 2 mm sieve and the first soil sample was stored at 4°C for determination of soil physicochemical properties. The second soil sample was refrigerated at −80°C for a subsequent soil DNA analysis to determine microbial community in the soil.
To assess the soil physicochemical properties, ammonium nitrogen (NH4+-N), and nitrate nitrogen (NO3-N) were quantified with a Futura continuous flow analysis system (KPM Analytics, Westborough, MA, United States of America), alkali-hydrolyzed nitrogen, sucrase, protease, urease and available potassium, refer to Bao description (Bao, 2005). Soil available phosphorus was extracted with 0.5 M NaHCO3 and determined by uv2102-PC spectrophotometer (UNICO, New Jersey, United States of America) (Cao et al., 2014). Organic matter was determined by K2Cr2O7-H2SO4 REDOX method (Schinner et al., 1995).
After 120 days of transplanting, 40 strawberry plants were randomly selected from each plot and stem thickness and plant height were measured using vernier calipers and a ruler. Ten strawberry plants were randomly selected from each plot for recording the number of flowers, total number of flowers and fruits, and leaf area was deter-mined using a leaf area meter. Two rows of strawberries were randomly selected in each plot to collect yield data during the harvest season, and for each harvest the yield of each row was recorded.
Soluble solids were determined using a handheld digital refractometer (30PX, Mettler Toledo, Columbus, OH, United States of America) and the results were expressed as Brix. Strawberry fruit soluble sugars were determined by anthrone colorimetry, following the method of Cao et al. (2014) (Cao et al., 2014). The method described by Nielsen (1998) was used (Nielsen, 1998). Fruit vitamin C content was determined by phosphomolybdic acid microplate method and titratable acidity was determined by titrimetric method and the results were expressed as percentage of malic acid per 100 g of fruit weight (FW) (Rangana, 1987).
Soil DNA extraction was performed using a FastDNA spin kit (FastDNA Spin Kit for soil (MP Biomedicals, Irvine. CA, United States of America) for soil, and the purified genomic DNA was used as a template in a polymerase chain reaction (PCR). The bacterial amplification primer 515F (5′-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′) were used for bacterial amplification, while the fungal ITS1 segment amplification primers ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCC GCTTATTGA-TATGC-3′) were also used. The PCR was performed using a 25 μL reaction PCR system with 10-fold PCR buffer (5 μ) and dNTP (0.5 μL). Tapase 0.25 μL (250 U), DNA template 1 μL, dH2O make-up to 25 μL. PCR reaction strategy: pre-denaturation at 98°C for 3 min, 98°C for 15 s, 50°C for 30 s and 72°C for 30 s for a total of 27 cycles; extension at 72°C for 10 min and storage at 4°C.
The PCR products from the same sample were mixed and detected by 2% agarose gel electrophoresis, and the PCR products were cut and recovered using an Axy-PrepDNA gel recovery kit (Axygen Inc. Union City, CA, United States of America), eluted by Tris-HCl, and detected by 2% agarose electrophoresis. A QuantiFluorTM-ST Blue Fluorescence Quantification System (Promega, Madison, WI, United States of America) was used for detection and quantification. The library was constructed using an Illumina NovaSeq PE250 sequencing plat-form (Illumina, San Diego, CA, United States of America), with ligation of the “Y” junction; screening with magnetic beads to remove the junction self-associated fragments; enrichment of the library template using PCR amplification; and denaturation with sodium hydroxide to produce single-stranded DNA fragments. Sequencing was performed using the Illumina PE250 platform (Hangzhou Kinko Technology Co.).
The raw sequences were quality-controlled using fastp software, and spliced using FLASH software. The bases with quality values below 20 bp at the end of the READ were filtered out, and a window of 50 bp was set. If the average quality value within the window was below 20 bp, the back-end bases were truncated starting from the window, and the bases after 50 bp were filtered out after quality control. According to the overlap relationship between paired-end (PE) reads, the PE reads were merged into a single sequence, and the minimum overlap length was 10 bp. The maximum mismatch ratio permitted for the overlap regions of spliced sequences was 0.2, and the non-compliant sequences were filtered out. Samples were distinguished based on the barcodes at the first and last ends of the sequences, and the sequence orientation was adjusted. The maximum number of mismatches allowed in the overlap region of spliced sequences was 0.2, and the maximum number of primer mismatches was 2. Then, all the sequences were classified into operational taxonomic units (OTUs) at the 97% similarity level using the Usearch software, and the representative sequences of OTUs at the 97% similarity level were analyzed by the Bayesian algorithm using the Ribosomal Database Project (RDP) classifier to perform the classification analysis. The sequences were calculated by the bioinformatic tool in MOTHUR software to calculate the OTUs of bacteria and fungi using the RDP classifier. The alpha diversity indices of bacterial and fungal communities were calculated using the bioinformatics tool MOTHUR software. Differences in the inter-root soil microbiota between groups were determined by a similarity analysis, and a linear discriminant analysis (LDA) effect size (linear discriminant analysis effect size, LEfSe) was performed using default parameters to observe taxa rich in differences between groups (Caporaso et al., 2010). To investigate the effects of environmental factors on microbial community structure, a redundancy analysis (RDA) was performed using Origin software (v.2021). To study the relationship between soil microbial community diversity and soil environmental factors, Origin software was used to conduct a correlation analysis.
Excel 2012 software was used for data processing, with the mean ± standard error used as data points in graphs. Field trial data were analyzed by ANOVA using the SPSS statistical software. Yield and soil physicochemical property data were analyzed directly without normalization. Tukey’s HSD test was used to evaluate significant differences between means, with p = 0.05 indicating significance. Drawing using Origin (v2021) (OriginLab Corp., United States of America) and Adobe IllustratorCS6 (Adobe Systems Inc., United States of America) drawing software.
In order to examine the three kinds of fertilizer on strawberry agronomic trait and production, the plant height, leaf area, peduncle number, total number of flowers and fruits, weight of single fruit and production was evaluated about 68 days after trans-planting. As shown in Table 1, there were differences in agronomic traits and yield of strawberries under three organic fertilizer applications. Plant height and production under T1 and T2 treatments were significantly higher than CK. In detail, compared to the control, there was a significant increase in the plant height by T1 (5.39%) and T2 (7.67%), respectively. Leaf area (26.60%), total number of flowers (29.05%) and fruits and weight of single fruit (33.46%) under T2 treatment was significantly higher than those under CK. However, there was no significant differences in peduncle number. The various effects of three different kinds of fertilizer on the agronomic trait and production may be due to the difference in their components.
There were differences in the quality of strawberry fruits under the different fertilization methods (Figure 1), and the soluble solid content of strawberries under the T2 and T3 treatments differed significantly from that of the control (CK), with significant increases of 50.80% and 64.41%, respectively, compared to the CK (p < 0.05). The vitamin C content was significantly increased by 5.16% under the T2 treatment compared to the CK, while the T3 treatment had the lowest titratable acid content, which was significantly decreased by 22.45% under the T3 treatment compared to the CK (p < 0.05). Compared with CK, the soluble sugar content under T2 and T3 treatment was significantly increased by 44.14% and 29.37%, respectively (p < 0.05).
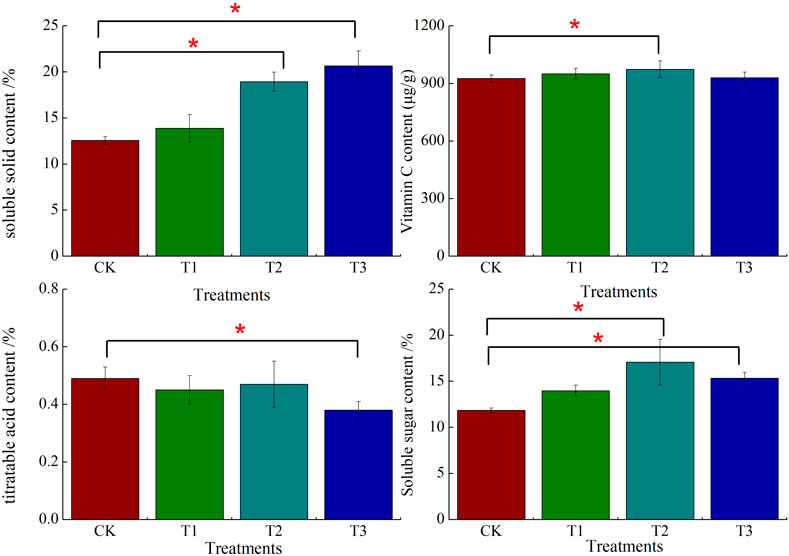
Figure 1. The effect of three kinds of fertilizers on the ACE, Chaol, Shannon, and Simpson index distribution of bacterial and fungi. Different letters on the bars demonstrate the significant differences between treatments at p ≤ 0.05. In figures, ns = non-significant; * = significant at 0.05, and *** = significant at 0.001 levels. The same below.
The results from this study indicated that there was a significant difference in soil organic, hydrolyzed nitrogen, available phosphorus, rapidly available potassium, nitrate nitrogen, sucrose and urease between the three kinds of fertilizer treatments and the control (Table 2). In detail, organic matter. The effects of available phosphorus, ammonium nitrogen, sucrase, protease and urease under T2 treatment were significantly increased by 50.62%, 54.14%, 276.50%, 129.47%, 232.61%, and 232.00%, respectively, compared with the control. The effect of avaliable nitrogen and available potassium was the best under T1 treatment, which significantly increased by 52.30% and 52.66% compared with the control, respectively. The content of nitrate nitrogen under T3 treatment was the highest, which significantly increased by 129.61% compared with the control.
The abundance of soil microorganisms was characterized by abundance-based coverage estimators (ACE), and diversity was characterized by the Shannon and Simpson indices. As shown in Figure 2, the T2 treatment had the highest bacterial and fungal ACE, Chao1, and Shannon index values and the lowest Simpson index value. Both the ACE and Shannon indices of soil bacteria followed the order of T2 > T3 > T1 > CK (Figure 2A). Soil ACE and Chao1 indices were significantly higher in T2 and T3 treatments by 9.48%, 7.12% and 4.52% and 3.93%, respectively, compared to CK treatment; Simpson’s index was significantly lower in T1 and T2 treatments by 26.15% and 38.30%, respectively, compared to CK. The ACE and Shannon index of soil fungi followed the order of T2 > T1 > T3 > CK (Figure 2B), and the soil ACE and Chaol index of the T1, T2, and T3 treatments significantly increased by 17.19%, 19.68%, and 12.96%, respectively, compared with the CK. The Chao1 index of soil fungi followed the order of T1 > T2 > T3 > CK, and for the T2 treatment the value was significantly increased by 7.13% compared with that of the CK. However, the different treatments had no significant effect on the Shannon index, but all of them significantly decreased the Simpson index of soil fungi. All of them significantly reduced the Simpson index, which was significantly reduced by 20.89% and 21.12% for T2 and T3 treatments, respectively, compared to CK.
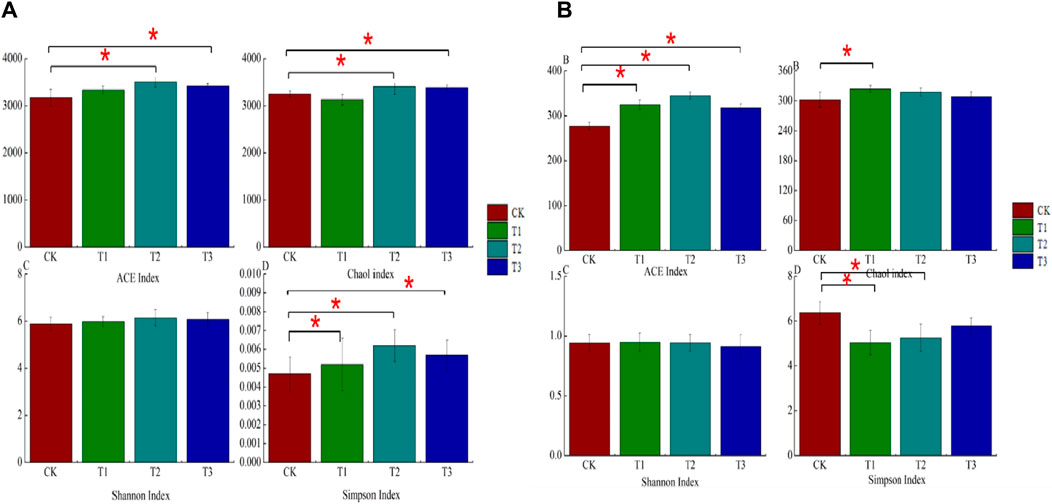
Figure 2. The effect of three kinds of fertilizers on the ACE, Chaol, Shannon, and Simpson index distribution of bacterial and fungi. Different letters on the bars demonstrate the significant differences between treatments at p ≤ 0.05. In figures, ns = non-significant; * = significant at 0.05, and *** = significant at 0.001 levels. The same below. (A): Bacterial, (B): Fungi.
Meanwhile, according to the distribution and relative abundances of fungi in strawberry rhizosphere soil at the phylum level. Soil bacteria dominated the soil microbial community, and the dominant phyla (relative abundance > 5%) in the soil bacterial community under the four treatments was mainly Proteobacteria (19.53%–36.99%), Acidobacteriota (15.94%–25.03%) and Chloroflexi (7.68%–16.32%) (Figure 3A). The effect of the T2 treatment on the abundance of soil bacterial phyla with a relative abundance >1% was mainly manifested as an increase in the relative abundance of Proteobacteria, Acidobacteriota, Bacteroidota, Gemmatimonadota, and other phyla compared to the CK. The effect of the T1 treatment on the abundance of soil bacterial phyla with relative abundance >1% was mainly manifested as an increase in the relative abundance of Proteobacteria and Patescibacteria. The effect of the T3 treatment on the abundance of soil bacterial phyla with a relative abundance >1% was mainly manifested as an increase in the relative abundance of Chloroflexi, Bacteroidota, Actinobacteriota, Gemmatimonadota, Unclassified unclassified, and other bacterial phyla. In detail, Bacteroidota, Gemmatimonadota, Myxococcota and other abundance in-creased by 184.50%, 20.84%, 15.74%, 9.76% and 144.48%, 21.94%, 23.49% under T2 and T3 treatments, respectively, as compared to control and 45.09%, Firmicutes and Patescibacteria abundance decreased on average by 82.36%, 46.72% and 67.15%, and 62.04%, respectively; T1 treatment significantly increased the abundance of Proteobacteria (35.56%).
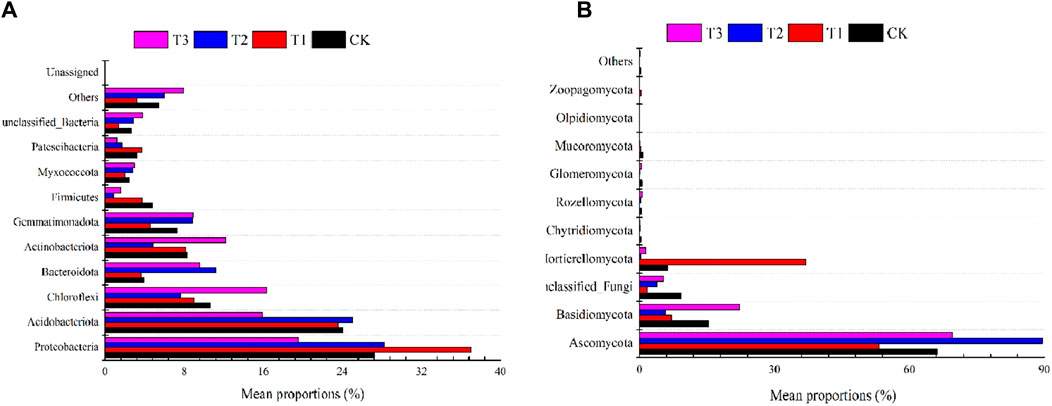
Figure 3. Effects of Three Kinds of Fertilizer on the Soil Microbial Community at Phylum Level (A), bacterial (B), fungi.
Soil fungi accounted for a very low percentage of the soil microbial community, with 10 fungal phyla and one unclassified detected in the samples (Figure 3B). Ascomycota dominated the fungal taxa under different treatments (53.20%–89.47%), the abundance of Ascomyota phylum in soil increased by 35.13% and 5.08% after T2 and T3 treatments, respectively, and decreased by 19.66% after T1 treatment. The percentage of Basidiomycota in the fungal taxa ranged from 5.69% to 22.21% under different treatments, the abundance of Basidiomycota phylum in the soil decreased by 53.36% and 62.93% after T1 and T2 treatments, respectively, and the abundance of Mortierellomycota in the soil increased by 487.15% after T2 treatment. The abundance of Chytridiomycota, Glomeromycota and Mucoromycota phylum in soil decreased after different fertilization treatments.
As Furthermore, at the genus level, unclassified_Gemmatimonadaceae, unclassified_Acidobacteriales, Bryobacter, Sphingomonas, unclassified_JG30_KF_AS9, unclassified_Bacteria were the main fungal genera (average relative abundance >1%) (Figure 4A). Compared with the control, T1 treatment increased an Mizugakiibacter (140.39%), unclassified_Acidobacteriales (7.98%), Bryobacter (28.13%), Sphingomonas (76.13%) and unclassified_Saccharimonadales (5.87%). The relative abundance of Thermoflavifilum, unclassified_JG30_KF_AS9 and Thermomonas were reduced by 92.61%, 77.15%, 59.35%, 26.02%, 63.07%,51.15%,76.06%, 77.47% and 97.48% under different fertilizer application.
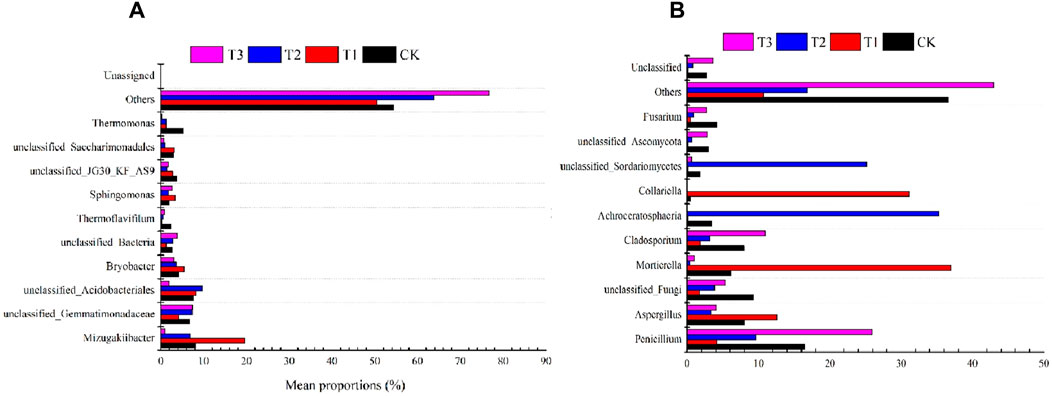
Figure 4. Effects of Three Kinds of Fertilizer on the Soil Microbial Community at Genus Level (A), bacterial (B), fungi.
Different fertilization treatments had a significant effect on the composition of the strawberry soil microbial community at the fungal genus level, with different fertilization treatments affecting the Penicillium genus abundance differently (Figure 4B). T1 treatment decreased the Penicillium and Cladosporium abundance. In detail, T1 and T2 decreased Penicillium (75.05%) and Cladosporium (41.38%) genus abundance, respectively, whereas T3 treatment increased Penicillium (57.35%) and Cladosporium (37.44%) genus abundance, respectively. Interestingly, the different fertilization treatments decreased the genus abundance of unclassified-Fungi, unclassified-Asocomycota, Fusarium, and other phyla, with the T1 treatment increasing the genus abundance of Aspergillus (56.98%) and Mortierella (497.38%). Both T2 and T3 reduced Aspergillus (57.62% and 49.82%, respectively) and Mortierella (93.82% and 84.62%, respectively) genus abundance.
Using the LEfSe analysis of variance to study the biomarkers with significant differences among treatments at different taxonomic levels (LDA> 4, p < 0.05) (Figure 5), a total of 66 bacterial biomarkers were identified in the three organic fertilizer treatments and the CK treatment, with 17 bacterial species identified in the T1 treatment. Figure 5A shows the bacterial biomarkers at the LDA>4.5 level, In detail, T1 treatments is enriched with Mi-zugakiibacter_sediminis, Rhodanobacteraccea, Rhodanobacteraceae and Xanthomonadales. The enriched taxa in T2 were incultured_bacterium_GR_296_II_11, BSV26 and Kyptoniales. The distinctly abundant taxa in T3 was the Ignavibacteriales. A total of 56 abundant fungal taxa significantly differed across CK, T1, T2 and T3 treatments. Figure 5B shows the bacterial biomarkers at the LDA> 4.5 level, T1 treatments is enriched with Collariella_gracilis, Chae-tomiaceae, Sordariales, Mortierella_wolfii, Mortierellaceae and Mortierellales. The enriched taxa in T2 were Achroceratosphaeria_potamia, Pisorisporiaceae, Posorisporiales, and Unclassified_Sordariomycetes.

Figure 5. Linear discriminant analysis (LDA) effect size (LEfSe) of the bacterial (A) and fungal taxa (B), which identifies the most differentially abundant taxa among the two kinds of commercial organic fertilizers at different treatments. Only taxa with LDA values greater than four (p < 0.05) are shown.
The enriched tax The OUT count, ACE and Chaol index of soil bacteria were found to be highly significantly positively correlated with soil OM, AP, sucrase, proteases and urease by Pearson’s analysis (p < 0.01). Simpson’s index was found to be highly significantly positively correlated with HN (p < 0.01) and significantly negatively correlated with AK and NN (p < 0.05). Shannon index showed highly significant positive correlation (p < 0.01) with OM and NN, and significant correlation (p < 0.05) with AP and proteases (Figure 6A). The number of soil fungi OUT was positively correlated with OM, and ACE and Chaol indices were highly significant positively correlated with OM; the number of fungi OUT, ACE and Chaol indices were highly significant positively correlated (p < 0.01) with soil AP, sucrase and urease. Chaol and Ace indices were significantly correlated with AN, and Ace was significantly positively correlated (p < 0.05) with proteases were significantly positively correlated (p < 0.01). Simpson index was highly significantly positively correlated (p < 0.01) with NN and significantly negatively correlated (p < 0.05) with proteases (Figure 6B). It indicated that changes in soil environmental factors had significant effects on the abundance and diversity of soil bacteria and fungi, among which changes in soil OM, AP, sucrase, proteases and urease were the important environmental factors affecting the diversity of soil microbial communities.

Figure 6. Pearson correlation analysis of soil microbial community diversity and soil environmental factors. HN, hydrolyzed nitrogen; AP, available phosphorus; NN, nitrate nitrogen; OR, Organic matter; AN, ammonium nitrogen; AP, rapidly available potassium. (A): Bacterial, (B): Fungi.
In order to further investigate the relationship between different types of organic fertilizer application on the dominant species in the strawberry inter-root microbial community and with soil physicochemical factors, the dominant species of soil microorganisms were subjected to redundancy analysis with environmental factors. The results showed that the RDA1 and RDA2 axes explained 84.76% and 9.04% of the soil bacterial community in the samples treated with different types of organic fertilizers, respectively (Figure 7). For the genus level of bacterial community, the two main variables were nitrate nitrogen contributing 62.90% and rapidly available potassium content contributing 22.80% (Table 3); for the genus level of fungi, the RDA1 and RDA2 axes explained 50.10% and 35.45% of the soil bacterial community in samples from different types of organic fertilizer treatments, respectively (Figure 7). 50.10% and 35.45%, respectively, and the three main variables were contributed by hydrolyzed nitrogen 41.60%, ammonium nitrogen 35.90% and available phosphorus 9.6% (Table 3).

Figure 7. Redundancy discriminant analysis (RDA) of the rhizosphere bacterial (A) and fungal (B) community compositions at genus levels with soil physicochemical properties. SUC, sucrose; URE, urease; PTE, protease; HN, hydrolyzed nitrogen; AP, available phosphorus; NN, nitrate nitrogen; OR, Organic matter; AN, ammonium nitrogen; RAP, rapidly available potassium; Miz, Mizugakiibacter; Uge, Unclassified-Gemmatimonadaceae; Uac, Unclassified-Acidobacteriales; Bry, Bryobacter; Uba, Unclassified_Bacteria; Thr, Thermoflavifilum; Sph, Sphingomonas; Ujg, Unclassified_JG30_KF_AS9; Tha, Thermomonas; Pen, Penicillium; Asp, Aspergillus; Ufu, Unclassfied_Fungi; Mor, Morticrclla; Cla, Cladosporium; Acha, Achroccratophacria; Col, Collariclla; Uso, Unclassified_Sordariomycetes; Fus, Fusarium.
Strawberry fruit weight, vitamin C, total sugar, soluble solids and titratable acidity are fruit quality characteristics are important indicators of its cultivation and production. Muty and Taha applied seaweed fertilizer and humic acid to Albion and Rubyfen varieties of strawberries, respectively, and found that the yields of both species of strawberries were significantly improved (Mufty and Taha, 2021). The application of compost to plots significantly increased strawberry leaf area, flower number, branch weight and yield compared to plots with only inorganic fertilizers (Arancon et al., 2004). Application of organic and bio-fertilizers led to an increase in the number of leaves and leaf area of the plants (Amos et al., 2013). In addition, different types of organic fertilizers were able to contribute to crop growth and increase crop yield, e.g., growth parameters of vegetable and maize plants significantly increased with an increasing amount of chicken manure applied in the field (Li X. et al., 2022), organic fertilizer from sheep manure significantly increased maize yield (Nedunchezhiyan et al., 2014), rice straw and farmyard manure applications contributed to sweet potato growth and root yield increase (Tripathi et al., 2010). Kai and Adhikari in a study conducted on apples, reported that the sugar content of fruit grown with organic fertilizer applications is higher than those grown with chemical fertilizer applications (Kai and Adhikari, 2021). Wang et al. found that chicken manure treatment increased the soluble content of tomato and improved the nutritional quality and flavor of tomato at harvest (Wang L. Y. et al., 2011). Similarly, our data showed that different organic fertilizer applications increased the soluble solids and soluble sugars content and decreased titratable acid content in strawberry fruits (Figure 2), which may be attributed to nutrient effectiveness and the improved uptake of inter-root nutrients by the plant. The nutrients are converted to sugars and their derivatives through reaction involving the reversal of glycolysis pathways (Tripathi et al., 2010). The increase in the soluble sugar content in fruits (Figure 2D) may be attributed to the rapid metabolic conversion of starch and pectin into soluble compounds, the rapid translocation of sugars from leaves to developing fruits and conversion of complex polysaccharides into polysaccharides. Moreover, numerically, sheep manure application performed better in terms of fruit weight, which was attributed to the rich nitrogen (N), phosphorus (P), potassium (K) and calcium (Ca) content of sheep manure organic fertilizer. This finding was supported by the results of previous studies (Preciado-Rangel et al., 2020).
The effect of different organic fertilizers on soil improvement was more significant, for example, chicken manure applied as fertilizer significantly increased organic matter, PKN and trace elements in soil (Wang B. et al., 2011). Criquet and Braud showed that in degraded Mediterranean soils, sludge and sludge composts can increase phosphorus effectiveness (Criquet and Braud, 2008; Baran et al., 2019). Chicken manure applied as fertilizer significantly increased the amount of organic matter, PKN, and trace elements in soil (Peng et al., 2019). Commercial organic fertilizer, sheep manure and mushroom slag significantly increased quick-acting potassium concentration, but the effect was related to the type of organic fertilizer, its concentration and application time (Li Q. J. et al., 2022). Application of organic fertilizers can effectively increase OMC, quick-acting phosphorus and total nitrogen content of newly reclaimed land (Li et al., 2021).
Sucrase plays an important role in increasing the soluble nutrients in soil. Studies have proved that sucrase is correlated with many soil factors. For example, soil organic matter, phosphorus content and microbial population are related; proteases are involved in the conversion of amino acids, proteins and other organic compounds containing protein nitrogen present in the soil. Urease is widely present in the soil and free urease is also present in organic fertilizers, meanwhile, urease is closely related to the organic matter content and the number of microorganisms in the soil. In the present study, it was found that there were differences in the effect of different fertilizers on soil physicochemical properties. The highest activities of sucrase, urease and protease were observed in sheep manure treated soil. This was because sheep manure organic fertilizer enables the initiating of soil microorganisms by inorganic nitrogen sources, while the application of rotted sheep manure organic fertilizer to the soil provides a large carbon source for microorganisms, which facilitates microbial value-added, thus increasing soil enzyme activities (Wu et al., 2019; Li X. Q. et al., 2022). Moreover, the increase in soil effectiveness under different treatments may be attributed to the fact that organic fertilizers provide a good source of nutrients for both plants and microorganisms (Negi et al., 2021).
Soil microorganisms are important components of the soil microhabitat. They are key drivers of soil nutrient cycling, are important for maintaining soil quality and promoting plant growth, and are extremely sensitive to fertilizer applications. More than 90% of the micro-organisms in the soil are usually bacteria and fungi, and they are the main dynamic soil nutrient regulators (Ramirez et al., 2012). Some studies have shown that organic and inorganic fertilizers can significantly increase the Chaol and Shannon indices of bacteria in the soil when they are applied in a certain proportion, and at the same time they can effectively improve the structure of fungal communities (Dong et al., 2014). Luo et al. reported that a mixture of swine manure, mineral nitrogen, phosphate, and potash fertilizers applied to the soil could significantly increase soil fungal diversity (Luo et al., 2015). In the present study, soil bacterial richness increased after the different organic fertilizer treatments, while fungal diversity decreased (Figure 2). There is competition between soil microbial communities for nutrients, and certain groups of bacteria will consume resources such as nutrients or occupy reproductive sites in a limited way. This may be because the application of organic fertilizers increases the competition for nutrients, including carbon, by soil bacteria, inhibiting the growth of such fungi (Liu et al., 2019).
In this study, we analyzed the composition of soil bacterial communities under the under different treatments and found that the dominant phyla of strawberry soil bacterial commu-nities were mainly Ascomycetes, Acidithiobacillus and Chloroflexi (Figure 4). It was found that the main function of Ascomycetes phylum was to increase soil nitrogen fertilizer utilization (Song et al., 2016), where the main function of Acidithiobacillus phylum was to participate in the carbon cycling process of humus decomposition and to maintain the health of the soil ecosystem (Wang et al., 2016; Li X. et al., 2022). In this study, the relative abundance of Ascomycetes phylum and Acidithiobacillus phylum were increased under the T2 treatment, which further corroborates the roles of sheep manure organic fertilizer in enhancing the nutrient supply for strawberry crops and in maintaining the soil carbon and nitrogen balance. Acidobacteria can degrade hemicellulose and xylan, especially in soils with high carbon content (Zhang et al., 2023). The phylum Ascomycetes and the most abundant phyla in the samples of this study, which may be related to the increase in soil nutrient content after the application of organic fertilizers. The changes in the Actinomycetes phylum may be related to soil aggregate size, and it has been shown that the combined ap-plication of organic fertilizers paired with chemical fertilizers can significantly increase soil agglomeration, whereas an increase in soil aggregate particle size leads to a decrease in the relative abundance of the actinomycetes phylum (Rong et al., 2018).
The T3 treatment increased the relative abundance of the phyla of Chloroflexi, Bac-teroidetes, Actinomyces and Bacillus phyla, and decreased the relative abundance of the phylum of Thick-walled Bacteria and the Patella Patescibacteria phylum relative abundance. It was found that the functional strain of the biofertilizer was Bacillus, and its application to the soil directly affected the relative abundance and gene function of soil Bacillus.
The Chloroflexi bacterial phylum is more stress-tolerant and suitable for propagation in nutrient-poor soil environment (Huang et al., 2019), indicating that biofertilizer is conducive to the growth of stress-tolerant bacterial flora. Long-term continuous cropping and crop rotation treatments significantly increased the relative abundance of the beneficial bacterium Gemmatimonassp and significantly decreased the relative abundance of the pathogenic fungus Fusariumsp (Soman et al., 2016), which was consistent with the findings of this study.
The results of this study showed that the inter-root soil fungi Ascomycetes was dominant in the soil of the strawberry crops. Further analysis revealed that the abundance of the as-comycetes phylum increased under the T2 and T3 treatments, and the main function of the ascomycetes phylum was to participate in soil organic matter decomposition, particularly due to their ability to decompose lignocellulose (Souza et al., 2016). The increased relative abundance of the ascomycetes phylum was therefore conducive to the mineralization of exogenous organic matter and nutrient release from strawberry soils. This suggests that the organic fertilizer based on goat manure and the biomicrobial fertilizer were more readily degraded than the canola cake, and therefore the relative abundance of the Ascomycota phylum was high under their application. Similarly to the Chloroflexi phylum, the Ascomycetes phylum occurs mainly in nutrient-poor environments (Sun et al., 2018; Wu et al., 2024). In the present study, the T1 and T2 treatments significantly increased soil fertility, resulting in a decrease in the relative abundance of ascomycetes. Xie found that Mortierella abundance among wheat roots was significantly correlated with crop growth and yield, and the increase in its relative abundance may promote plant growth (Xie et al., 2021). Consistent with the results of the present study, our previous study found that the T2 treatment significantly increased the strawberry yield and the Mortierellomycota abundance increased by 487.15% in the soil. This suggests that the improvement of soil quality by applying sheep manure organic fertilizers may change the distribution of specific soil microorganisms in the farmland (Li Q. J. et al., 2022).
The relative abundance of Sphingomonas, which are plant growth-promoting fungi, in-creased in the soil treated with canola cake fertilizer. This suggests that organic fertilizers can change the dominant bacterial species, which was consistent with the results reported by Ren (Ren et al., 2020). Sheep manure organic fertilizers promoted eutrophic microorganisms such as Ascomycetes and Acidobacteria, and the phosphorus-enhancing bacteria Gemmatimonas, and inhibited nutrient-poor microorganisms such as Chloroflexi, whereas the application of bio-microbial fertilizers was more conducive to the growth and reproduction of nutrient-poor or stress-tolerant microorganisms such as Anabacteria. The different fertilizers provided carbon sources for the soil while selectively shaping the structure of microbial communities, and ultimately enabling the corresponding microbial communities to gradually become dominant.
A Pearson correlation analysis found that the enzyme activity in the strawberry soil during the test were significantly positively correlated with the ACE, Chaol and Shannon index of soil bacteria, and significantly negatively correlated with the Simpson index of soil fungi, which indicated that the abundance and diversity of soil fungal communities were more susceptible to stress than those of soil bacteria. Soil bacterial communities are more susceptible to fertilizer application because soil fungi lie mainly on the surface of aggregates and are more susceptible to microenvironmental changes than bacteria (Zhang R. P. et al., 2020). This was consistent with Suzuki’s finding that the soil fungal community structure is more sensitive to the response of organic and inorganic fertilizers than the soil bacterial structure (Suzuki et al., 2009).
The application of different types of fertilizers to soils planted with strawberries for several consecutive years improved the Leaf area, total number of flowers and fruits, fruit weight and yield of strawberry, while the application of a sheep manure organic fertilizer resulted in the largest leaf area and more abundant soil fungal communities. The inter-root soil microbial diversity of strawberry was significantly affected by the organic matter and effective phosphorus contents, as well as protease and urease activities. The microbial community structure was improved under different fertilizer applications by regulating the soil organic matter content and microbial diversity, which in turn, affected the soil capacity of strawberry soil. Some specific microorganisms, such as the plant growth-promoting fungus Mortierella, the phosphorus-enhancing bacterium Gemmatimonas, and the plant growth-promoting bacterium Sphingomonas, were found to be closely associated with soil improvement by different fertilizers, suggesting a relationship between microorganisms, fertilizers, and soil. Overall, the results of this study revealed significant changes in soil microorganisms under different fertilizer applications. Sheep manure organic fertilizer can be used as a good ameliorator for soils in which strawberries are grown in successive years.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
YZ: Investigation, Methodology, Supervision, Writing–original draft. AL: Data curation, Validation, Writing–review and editing. WL: Software, Writing–review and editing. JW: Methodology, Writing–review and editing. XL: Formal Analysis, Methodology, Writing–review and editing. HY: Project administration, Writing–review and editing. WX: Methodology, Software, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work is supported by the Agriculture and Social Development Research Project of Hangzhou (202203A07). The Key Research and Development Program of Zhejiang Province (2022C02032). The Municipal Academy of Agricultural Sciences Alliance Regional Demonstration Project of Zhejiang Province (2023SJLM01). The Science and Technology Innovation and Promotion Demonstration project of Hangzhou Academy of Agricultural Sciences (2022HNCT-10).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1414010/full#supplementary-material
Amos, H., Obi, C. I., and Audu, I. (2013). Effect of chicken manure on the performance of vegetable maize (zea mays saccharata) varieties under irrigation. Discourse J. Agric. Food Sci. 1, 190–195.
Arancon, N. Q., Edwards, C. A., Bierman, P., Welch, C., and Metzger, J. D. (2004). Influences of vermicomposts on field strawberries: 1. Effects on growth and yields. Bioresour. Technol. 93, 145–153. doi:10.1016/j.biortech.2003.10.014
Baran, A., Mierzwa-Hersztek, M., Gondek, K., Tarnawski, M., Szara, M., Gorczyca, O., et al. (2019). The influence of the quantity and quality of sediment organic matter on the potential mobility and toxicity of trace elements in bottom sediment. Environ. Geochem. Health. 41, 2893–2910. doi:10.1007/s10653-019-00359-7
Cao, A. C., Guo, M. X., Yan, D. D., Mao, L. G., Wang, Q. X., Li, Y., et al. (2014). Evaluation of sulfuryl fluoride as a soil fumigant in China. Pest Manag. Sci. 70 (2), 219–227. doi:10.1002/ps.3535
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods. 7, 335–336. doi:10.1038/nmeth.f.303
Criquet, S., and Braud, A. (2008). Effects of organic and mineral amendments on available P and phosphatase activities in a degraded Mediterranean soil under short-term incubation experiment. Soil till. Res. 98, 164–174. doi:10.1016/j.still.2007.11.001
Dong, W. Y., Zhang, X. Y., Dai, X. Q., Fu, X. L., Yang, F. T., Liu, X. Y., et al. (2014). Changes in soil microbial community composition in response to fertilization of paddy soils in subtropical China. Appl. Soil Ecol. 84, 140–147. doi:10.1016/j.apsoil.2014.06.007
Gramza-Michalowska, A., Bueschke, M., Kulczynski, B., Gliszczynska-Swiglo, A., Kmiecik, D., Bilska, A., et al. (2019). Phenolic compounds and multivariate analysis of antiradical properties of red fruits. J. Food Meas. Charact. 13, 1739–1747. doi:10.1007/s11694-019-00091-x
Guo, Z. C., Zhang, J. B., Fan, J., Yang, X. Y., Yi, Y. L., Han, X. R., et al. (2019). Does animal manure application improve soil aggregation? Insights from nine long-term fertilization experiments. Sci. Total Environ. 01, 1029–1037. doi:10.1016/j.scitotenv.2019.01.051
Hassan, A. H. (2015). Effect of nitrogen fertilizer levels in the form of organic, inorganic and bio fertilizer applications on growth, yield and quality of strawberry. Middle East J. Appl. Sci. 5, 604–617.
Huang, Q., Wang, J. L., and Wang, C. (2019). The 19-years inorganic fertilization increased bacterial diversity and altered bacterial community composition and potential functions in a paddy soil. Appl. Soil Ecol. 144, 60–67. doi:10.1016/j.apsoil.2019.07.009
Jia, X. L., Wang, Y. H., Zhang, Q., Lin, S. X., Zhang, Y., Du, M. R., et al. (2013). Reasonable deep application of sheep manure fertilizer to alleviate soil acidification to improve tea yield and quality. Front. Plant Sci. 14, 1179960. doi:10.3389/fpls.2023.1179960
Kai, T., and Adhikari, D. (2021). Effect of organic and chemical fertilizer application on apple nutrient content and orchard soil condition. Agriculture 11, 340. doi:10.3390/agriculture11040340
Kandulu, J., Thorburn, P., Biggs, J., and Verburg, K. (2018). Estimating economic and environmental trade-offs of managing nitrogen in Australian sugarcane systems taking agronomic risk into account. J. Environ. Manage 223, 264–274. doi:10.1016/j.jenvman.2018.06.023
Kumar, S., Kundu, M., Das, A., Rakshit, R., Siddiqui, Md.W., and Rani, R. (2019). Substitution of mineral fertilizers with biofertilizer: an alternate to improve the growth, yield and functional biochemical properties of strawberry (Fragaria×ananassa Duch.) cv. Camarosa. J. Pl. Nutr. 42, 1818–1837. doi:10.1080/01904167.2019.1643363
Lal, B., Sharma, S. C., Meena, R. L., Sarkar, S., Sahoo, A., Balai, R. C., et al. (2020). Utilization of byproducts of sheep farming as organic fertilizer for improving soil health and productivity of barley forage. J. Environ. Manage 269, 110765. doi:10.1016/j.jenvman.2020.110765
Li, Q. J., Zhang, D. Q., Cheng, H. Y., Ren, L. R., Jin, X., Fang, W. S., et al. (2022c). Organic fertilizers activate soil enzyme activities and promote the recovery of soil beneficial microorganisms after dazomet fumigation. J. Environ. Manage 309, 114666. doi:10.1016/j.jenvman.2022.114666
Li, X., Li, D., Lu, Q., Wang, D., Ren, X., Lv, L., et al. (2022b). Effects of different organic fertilizers on sweet potato growth and rhizosphere soil properties in newly reclaimed land. Agronomy 12, 1649. doi:10.3390/agronomy12071649
Li, X., Su, Y., Ahmed, T., Ren, H., Javed, M. R., Yao, Y., et al. (2021). Effects of different organic fertilizers on improving soil from newly reclaimed land to crop soil. Agriculture 11, 560. doi:10.3390/agriculture11060560
Li, X. Q., Lu, Q. J., Li, D. Y., Wang, D. Z., Ren, X. X., Yan, J. L., et al. (2022a). Effects of two kinds of commercial organic fertilizers on growth and rhizosphere soil properties of corn on new reclamation land. Plants 11, 2553. doi:10.3390/plants11192553
Liu, J. A., Shu, A. P., Liu, G. R., Zhang, L. Z., Liu, Z. B., and Gao, Z. (2019). Research progress on effect of fertilization on soil properties and microbiome. Biotechnol. Bull. 35 (9), 21–28. doi:10.13560/j.cnki.biotech.bull.1985.2019-0579
Loyd, M., Kluepfel, D., and Gordon, T. (2016). Evaluation of four commercial composts on strawberry plant productivity and soil characteristics in California. Int. J. Fruit. Sci. 16, 84–107. doi:10.1080/15538362.2016.1239562
Luo, P. Y., Han, X. R., Wang, Y., Han, M., Shi, H., Ning, H., et al. (2015). Influence of long-term fertilization on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 65 (1), 533–542. doi:10.1007/s13213-014-0889-9
Mostafa, S., Naglaa, K., Raghda, Z., Adel, M. G., Mahmoud, E. S., and Esawy, M. (2022). Effect of integration of poultry manure and vinasse on the abundance and diversity of soil fauna, soil fertility index, and barley (Hordeum aestivum L.) growth in calcareous soils. BMC Plant Biol. 22 (1), 492. doi:10.1186/s12870-022-03881-6
Mufty, R. K., and Taha, S. M. (2021). Response two strawberry cultivars (fragaria X ananassa duch.) for foliar application of two organic fertilizers. IOP Conf. Ser. Earth Environ. Sci. 910, 012033. doi:10.1088/1755-1315/910/1/012033
Nedunchezhiyan, M., Sinhababu, D. P., and Sahu, P. K. (2014). Effect of soil amendments and irrigation regimes on minimum tillage planted sweet potato (Ipomoea batatas) in rice (Oryza sativa) fallows under lowland conditions. Indian J. Agric. Sci. 84, 371–375. doi:10.56093/ijas.v84i3.38585
Negi, Y. K., Sajwan, P., Uniyal, S., and Mishra, A. C. (2021). Enhancement in yield and nutritive qualities of strawberry fruits by the application of organic manures and biofertilizers. Sci. Hortic. 2021, 110038. doi:10.1016/j.scienta.2021.110038
Peng, J., Gao, Y. Z., Gu, Y., Xu, Y. F., Wang, A. J., and Wang, K. (2019). Effects of chicken manure organic fertilizer on antibiotic resistance genes and integrase genes in soil. Chin. J. Environ. Eng. 13 (4), 984–991. doi:10.12030/j.cjee.201901192
Preciado-Rangel, P., Troyo-Diéguez, E., Valdez-Aguilar, L. A., García-Hernández, J. L., and Luna-Ortega, J. G. (2020). Interactive effects of the potassium and nitrogen relationship on yield and quality of strawberry grown under soilless conditions. Plants 9, 441. doi:10.3390/plants9040441
Ramirez, K. S., Craine, J. M., and Fierer, N. (2012). Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 18 (6), 1918–1927. doi:10.1111/j.1365-2486.2012.02639.x
Rangana, S. (1987) Handbook of analysis and quality control for fruit and vegetable products. New Delhi: Tata McGraw-Hill Ltd.
Ren, N., Wang, Y., Ye, Y. L., Zhao, Y. N., Chu, X., Fu, W., et al. (2020). Effects of continuous nitrogen fertilizer application on the diversity and composition of rhizosphere soil bacteria. Front. Microbiol. 11, 1948. doi:10.3389/fmicb.2020.01948
Rong, Q. L., Li, R. N., Huang, S. W., Tang, J. W., Zhang, Y. C., and Wang, L. Y. (2018). Soil microbial characteristics and yield response to partial substitution of chemical fertilizer with organic amendments in greenhouse vegetable production. J. Integr. Agric. 17 (6), 1432–1444. doi:10.1016/s2095-3119(18)61946-x
Schinner, F., Öhlinger, R., Kandeler, D. E., and Margesin, R. (1995). “Methods in soil biology,” in Methods in soil biology (Berlin, Germany: Springer), 386–389.
Shen, J., Yuan, L., Zhang, J., Li, H., Bai, Z., Chen, X., et al. (2011). Phosphorus dynamics: from soil to plant. Pl. Physiol. 156, 997–1005. doi:10.1104/PP.111.175232
Singhalage, I. D., Seneviratne, G., Madawala, H. M. S. P., and Wijepala, P. C. (2019). Profitability of strawberry (Fragaria ananassa) production with biofilmed biofertilizer application. Sci. Horticul. 243, 411–413. doi:10.1016/j.scienta.2018.08.033
Soman, C., Li, D., Wander, M. M., and Kent, A. (2016). Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant and Soil 413, 145–159. doi:10.1007/s11104-016-3083-y
Song, Z. Q., Wang, L., Liu, X. H., and Liang, F. (2016). The diversities of Proteobacteria in four acidic hot springs in Yunan. J. Henan Agric. Univ. 50 (3), 376–382. doi:10.16445/j.cnki.1000-2340.2016.03.015
Souza, R. C., Mendes, I. C., Reis-Junior, F. B., Carvalho, F. M., Nogueira, M. A., Vasconcelos, A. T. R., et al. (2016). Shifts in taxonomic and functional microbial diversity with agriculture: how fragile is the Brazilian Cerrado? BMC Microbiol. 16, 42. doi:10.1186/s12866-016-0657-z
Spanoghe, J., Grunert, O., Wambacq, E., Sakarika, M., Papini, G., Alloul, A., et al. (2020). Storage, fertilization and cost properties highlight the potential of dried microbial biomass as organic fertilizer. Microb. Biotechnol. 13 (5), 1377–1389. doi:10.1111/1751-7915.13554
Sui, F. F., Wang, M., Cui, L. Q., Quan, G. X., Yan, J. L., and Li, L. Q. (2023). Pig manure biochar for contaminated soil management: nutrient release, toxic metal immobilization, and Chinese cabbage cultivation. Ecotoxicol. Environ. Saf. 257, 114928. doi:10.1016/J.ECOENV.2023.114928
Sun, Q. Q., Wang, R., Hu, Y. X., Yao, L. G., and Gao, S. L. (2018). Spatial variations of soil respiration and temperature sensitivity along a steep slope of the semiarid Loess Plateau. PLoS One 13 (4), e0195400. doi:10.1371/journal.pone.0195400
Suzuki, C., Nagaoka, K., Shimada, A., and Takenaka, M. (2009). Bacterial communities are more dependent on soil type than fertilizer type, but the reverse is true for fungal communities. Soil Sci. Plant Nutr. 55 (1), 80–90. doi:10.1111/j.1747-0765.2008.00344.x
Tripathi, V. K., Kumar, N., Shukla, H. S., and Mishra, A. N. (2010). Influence of Azotobacter, Azospirillum and PSB on growth, yield and quality of strawberry cv. Chandler. Indian Journals 48, 198–199. doi:10.5958/2249-5258.2016.00009.9
Wang, B., Liu, G. B., Xue, S., and Zhu, B. B. (2011b). Changes in soil physico-chemical and microbiological properties during natural succession on abandoned farmland in the Loess Plateau. Environ. Earth Sci. 62 (5), 915–925. doi:10.1007/s12665-010-0577-4
Wang, G. H., Liu, J. J., Yu, Z. H., Wang, X. Z., Jin, J., and Liu, X. B. (2016). Research progress of Acidobacteria ecology in soils. Biotechnol. Bull. 32 (2), 14–20. doi:10.13560/j.cnki.biotech.bull.1985.2016.02.002
Wang, L. Y., Zhang, Y. C., Chen, L. L., Li, R. N., Zhai, C. X., and Li, Q. Y. (2011a). Effects of different kinds of manure combination with chemical fertilizer on yield, quality and soil nutrient content in greenhouse tomato. Acta Agric. boreali-sinica., 152–156. doi:10.7668/hbnxb.2011.s2.033
Wu, Z. N., Gao, G. H., and Wang, Y. Y. (2019). Effects of soil properties, heavy metals, and PBDEs on microbial community of e-waste contaminated soil. Ecotoxicol. Environ. Saf. 180, 705–714. doi:10.1016/j.ecoenv.2019.05.027
Wu, Z. N., Kang, L. H., Man, Q. L., Xu, X. Y., Zhu, F. J., and Lyu, H. H. (2024). Effects of hexabromocyclododecane and polyethylene microplastics on soil bacterial communities. Sci. Total Environ. 906, 167691. doi:10.1016/j.scitotenv.2023.167691
Xie, X., Xiang, Q., Wu, T., Zhu, M., Xu, F., Xu, Y., et al. (2021). Impacts of agricultural land reclamation on soil nutrient contents, pools, stoichiometry, and their relationship to oat growth on the East China coast. Land 10, 355. doi:10.3390/land10040355
Yan, B. J., Zhang, Y. P., Wang, Y. Z., Rong, X. M., Peng, J. W., Fei, J. C., et al. (2023). Biochar amendments combined with organic fertilizer improve maize productivity and mitigate nutrient loss by regulating the C-N-P stoichiometry of soil, microbiome, and enzymes. Chemosphere 324, 138293. doi:10.1016/J.CHEMOSPHERE.2023.138293
Yan, J., Ban, Z., Lu, H., Li, D., Poverenov, E., Luo, Z., et al. (2018). The aroma volatile repertoire in strawberry fruit: a review. J. Sci. Food Agric. 98 (12), 4395–4402. doi:10.1002/jsfa.9039
Yogesh, K. N., Paramjeet, S., Shweta, U., and Mishra, A. C. (2021). Enhancement in yield and nutritive qualities of strawberry fruits by the application of organic manures and biofertilizers. Sci. Hortic. 283, 110038. doi:10.1016/j.scienta.2021.110038
Zhang, C., Zhao, X., Liang, A. J., Li, Y. Y., Song, Q. Y., Li, X. Y., et al. (2023). Insight into the soil aggregate-mediated restoration mechanism of degraded black soil via biochar addition: emphasizing the driving role of core microbial communities and nutrient cycling. Environ. Res. 228, 115895. doi:10.1016/j.envres.2023.115895
Zhang, G. L., Bai, J. H., Zhao, Q. Q., Jia, J., Wang, W., and Wang, X. (2022). Bacterial succession in salt marsh soils along a short-term invasion chronosequence of spartina alterniflora in the Yellow River Estuary, China. Microb. Ecol. 79, 644–661. doi:10.1007/s00248-019-01430-7
Zhang, R. P., Gou, X. M., Zhang, Y., Cai, Y., Li, B., Ye, Q. X., et al. (2020b). Effects of bio-organic fertilizer combined with chemical fertilizer on nutrients and fungal community characteristics in tobacco-growing soil. J. Northwest A&F Univ. 48 (8), 85–92. doi:10.13207/j.cnki.jnwafu.2020.08.011
Zhang, S. N., Sun, L. T., Wang, Y., Fan, K., Xu, Q. S., Li, Y. S., et al. (2020a). Cow manure application effectively regulates the soil bacterial community in tea plantation. BMC Microbiol. 20 (1), 190. doi:10.1186/s12866-020-01871-y
Keywords: rapeseed cake, sheep manure, strawberry, bacteria, fungi, diversity and community composition
Citation: Zha Y, Liu A, Lai W, Wang J, Li X, Yu H and Xiao W (2024) Sheep manure organic fertilizer is an effective strategy to promote strawberry growth by improving soil physicochemical properties and microbiota. Front. Environ. Sci. 12:1414010. doi: 10.3389/fenvs.2024.1414010
Received: 08 April 2024; Accepted: 29 April 2024;
Published: 14 May 2024.
Edited by:
Meng Jiang, Zhejiang University, ChinaReviewed by:
Wu Zhineng, Hebei University of Technology, ChinaCopyright © 2024 Zha, Liu, Lai, Wang, Li, Yu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Yu, eWh0bHpqMTIzNDVAMTI2LmNvbQ==; Wenfei Xiao, eGlhb193ZW5mZWlAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.